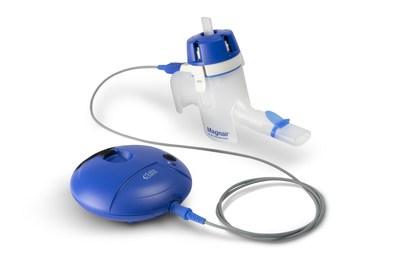
- LONHALA MAGNAIR Inhalation Solution is the first
nebulized long-acting muscarinic antagonist (LAMA) approved for the
treatment of COPD in the U.S. -
STARNBERG, Germany,
Dec. 7, 2017 /PRNewswire/
-- PARI Pharma GmbH, a company focused on the development and
commercialization of advanced aerosol delivery systems based on
eFlow® Technology, announces approval of its first eFlow closed
system nebulizer Magnair™ together with Sunovion's Lonhala™
(glycopyrrolate) Inhalation Solution. The drug/device combination
product received FDA approval on December
5th, 2017 under a New Drug Application (NDA).
"So far, eFlow Technology nebulizers like Altera®, Zirela®,
Tolero® or eRapid®/eFlow®rapid are available to patients suffering
from the orphan indication of cystic fibrosis. With the approval of
LONHALA MAGNAIR for COPD, we are bringing a new advancement in
nebulizers that are designed to be efficient, silent and fast to
people with COPD. It is great to see our technology addressing an
unmet medical need for a nebulized LAMA," states Dr. Martin Knoch, President of PARI Pharma. "MAGNAIR
is a great example of the flexibility of development and
optimization using the eFlow Technology platform," added
Geoff Hunziker, President of PARI
Pharma's US sister company eFlow LLC.
"Sunovion is pleased that the FDA has approved LONHALA MAGNAIR
as the first nebulized long-acting muscarinic antagonist treatment
option for people in the U.S. living with COPD," said Antony Loebel, M.D., Executive Vice President
and Chief Medical Officer at Sunovion. "We believe that LONHALA
MAGNAIR is an important advancement for patients who may prefer
nebulized medications and a therapy that fits into their everyday
lives."
About PARI Pharma and eFlow® Technology
PARI Pharma is a world leader in the development of aerosol
delivery devices optimized with drug products for nebulized
treatments using its proprietary technologies and expertise.
PARI's platform of nebulizer systems based on eFlow Technology
incorporate a vibrating, perforated membrane. eFlow® Technology
devices are designed to significantly improve upper and lower
respiratory tract deposition and reduce the burden of treatment for
patients with severe respiratory conditions. PARI Pharma, a
PARI Medical Holding company, is located near Munich, Germany with a major presence in
the United States. For additional
information, please visit www.paripharma.com.
About LONHALA MAGNAIR
LONHALA MAGNAIR (glycopyrrolate) Inhalation Solution, previously
known as SUN-101/eFlow®, is the first long-acting muscarinic
antagonist (LAMA) bronchodilator delivered via the innovative,
proprietary MAGNAIR closed system nebulizer based on eFlow®
Technology. The MAGNAIR nebulizer system is designed to deliver the
drug in two to three minutes and allows patients to breathe
normally while using the device. LONHALA MAGNAIR is approved for
the long-term, maintenance treatment of airflow obstruction in
patients with chronic obstructive pulmonary disease (COPD),
including chronic bronchitis and/or emphysema.
LONHALA is a trademark of Sunovion Pharmaceuticals Inc.
MAGNAIR is a trademark of PARI Pharma GmbH.
eFlow® is a registered trademark of PARI Pharma GmbH.
|
Contact:
|
Michael
Hahn
|
|
Director eFlow
Partnering
|
|
PARI Pharma
GmbH
|
|
+49 (0) 89 74 28 46
-831
|
|
michael.hahn@pari.com
|
Photo -
https://mma.prnewswire.com/media/616445/PARI_Pharma_Lonhala_Magnair_Inhalation_Solution.jpg