false
0001616543
0001616543
2024-11-19
2024-11-19
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES
EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported):
November 19, 2024
| SENSEONICS
HOLDINGS, INC. |
| (Exact Name of Registrant as Specified in its Charter) |
| Delaware |
|
001-37717 |
|
47-1210911 |
(State or Other
Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
20451 Seneca Meadows Parkway
Germantown, MD 20876-7005 |
| (Address of Principal Executive Office) (Zip Code) |
Registrant's telephone number, including
area code: (301) 515-7260
Not Applicable
Former name or former address, if changed
since last report
Check the appropriate box below if the
Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions
(see General Instruction A.2 below):
| ¨ | Written communications pursuant to Rule 425 under the Securities
Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange
Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under
the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under
the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
| Common Stock |
SENS |
NYSE American |
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities
Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the
registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01 Regulation FD Disclosure.
As previously announced, on November 19,
2024, members of the management of Senseonics Holdings, Inc. (the “Company”) presented at the Stifel 2024 Healthcare
Conference. An archive of the presentation, which was originally streamed live, is available on the Investor Relations section of
the Company's website. Additionally, on November 19, 2024, the Company updated its corporate presentation. A copy of the
presentation is also available on the Investor Relations section of the Company’s website and is furnished as Exhibit 99.1 to
this Current Report on Form 8-K.
The information set forth in this Item 7.01 and
contained in the presentation furnished as Exhibit 99.1 shall not be deemed "filed" for purposes of Section 18 of the Securities
Exchange Act of 1934, as amended (the “Exchange Act”), and is not incorporated by reference into any of the Company's filings
under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof, except as shall be expressly
set forth by specific reference in any such filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit |
|
|
| Number |
|
Description |
| |
|
|
| 99.1 |
|
Company Presentation |
| |
|
|
| 104 |
|
Cover Page Interactive Data (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
| Date: November 19, 2024 |
SENSEONICS HOLDINGS, INC. |
| |
| |
By: |
/s/ Rick Sullivan |
| |
Name: |
Rick Sullivan |
| |
Title: |
Chief Financial Officer |
Exhibit 99.1

1 Eversense ® CGM System THE WORLD’S FIRST AND ONLY LONG - TERM CONTINUOUS GLUCOSE MONITORING SYSTEM Corporate Overview November 2024
2 FORWARD - LOOKING STATEMENTS Any statements in this presentation about future expectations, plans and prospects for Senseonics and its business, including st atements regarding managements plans, objectives and goals for future operations, expectations for future financial or other performance, statements about the comm erc ial launch and growth of Eversense® 365 and Eversense RPM programs, statements regarding planned initiatives, investments or marketing or other programs of Senseonics or it s commercial partner, Ascensia Diabetes Care, statements regarding progress and timing of collaboration and rate of adoption or growth with respect to Eversense and any health system, or its patients and providers, or the potential to enhance patient outcomes or reduce healthcare costs or other benefits, statements regarding increasing pa tie nt access, adoption and market share, and the future growth of the CGM market, statements regarding advancing development programs and potential regulatory events and ava ilability, and other statements containing the words “believe,” “expect,” “intend,” “may,” “projects,” “will,” “planned” and similar expressions constitute f orw ard - looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. These forward - looking statements are based on management’s current expectation s and projections about future events, and such statements are, by their nature subject to risk and uncertainties. Actual results may differ materially from those indic ate d by such forward - looking statements as a result of various important factors, including: uncertainties inherent in the new product launch and ongoing commercialization of th e E versense product and the expansion of the Eversense product and a new RPM solution, uncertainties inherent in the reliance on the commercialization efforts and investm ent of Ascensia and its commercial initiatives, uncertainties inherent in reliance on and finalizing integration and commercial terms with partners or other third parties, u nce rtainties in user decisions and responses to new technology or initiatives, uncertainties in insurer, regulatory and administrative processes and decisions, uncertainties inh ere nt in the development and registration of new technology, uncertainties relating to the current economic environment, uncertainties in the development of the overall CGM m ark et, and such other factors as are set forth in the risk factors detailed in Senseonics’ Annual Report on Form 10 - K for the year ended December 31, 2023 and the Company’s Quart erly Report on Form 10 - Q for the quarter ended Septmeber 30, 2024, each as filed with the SEC, and Senseonics’ other filings with the SEC under the heading “Risk Factors.” In additio n, the forward - looking statements included in this presentation represent Senseonics’ views as of the date hereof. Senseonics anticipates that subse que nt events and developments will cause Senseonics’ views to change. However, while Senseonics may elect to update these forward - looking statements at some point in the future, Senseonics specifically disclaims any obligation to do so except as required by law. This presentation also presents management’s goals and vision for Senseoni cs development programs, including without limitation the Gemini and Freedom development programs. These products are not approved by the FDA and are not subject to an IDE or other investigational approval. Plans, timing, specifications and other details of these programs are subject to change based on the factors above. The forward - looking statements in this presentation should not be relied upon as representing Senseonics’ views as of any date subsequent to the date hereof. ABOUT EVERSENSE The Eversense® Continuous Glucose Monitoring (CGM) Systems are indicated for continually measuring glucose levels for up to 365 days for Eversense® 365 and 180 days for Eversense® E3 in persons with diabetes age 18 and older. The systems are indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time per week after day 14 for Eversense® 365 and one time per day after day 21 for Eversense® E3, and when symptoms do not match CGM information or when taking medications of the tetracycline class. T he sensor insertion and removal procedures are performed by a health care provider. The Eversense CGM Systems are prescription devices; patients should talk to their health care provider to learn more. For important safety information, see https://www.eversensediabetes.com/safety - info/ .

3 3 To transform lives in the global diabetes community with differentiated, long - term implantable glucose management technology. OUR MISSION

4 Senseonics has the first and only fully - implantable continuous glucose monitor (CGM) that lasts for one year The Eversense ® 365 CGM System was recently cleared by the FDA, with commercial launch now underway Addresses the top complaints with other CGMs including comfort, convenience and accuracy CGMs are the fastest growing diabetes tech segment and U.S. total addressable market is more than $20 billion Market remains significantly underpenetrated with ~1/4 eligible U.S. T1 and T2 diabetes patients utilizing CGMs Partnerships enable near - term market ramp - up Exclusive worldwide distribution agreement with Ascensia Diabetes Care leverages global commercial reach and experience Recently formed a multi - year collaboration with Mercy Health that anticipates up to 30,000 of their patients could benefit from CGM; treatments began in Q3 ‘24 with expansion expected throughout 2025 Third - and fourth - generation devices being developed in parallel Gemini System is designed to utilize a fully implantable self - powering system (IDE filing planned 2025) Freedom System is designed with no transmitter and to enable direct communication between sensor and smart phone Senseonics is targeting the large and growing Type 1 and Type 2 diabetes patient populations with the first - and - only, long - term, convenient and accurate continuous glucose monitoring systems

5 Timothy Goodnow, PhD President & Chief Executive Officer Mukul Jain, PhD Chief Operating Officer Francine Kaufman, M.D. Chief Medical Officer Rick Sullivan Chief Financial Officer MANAGEMENT
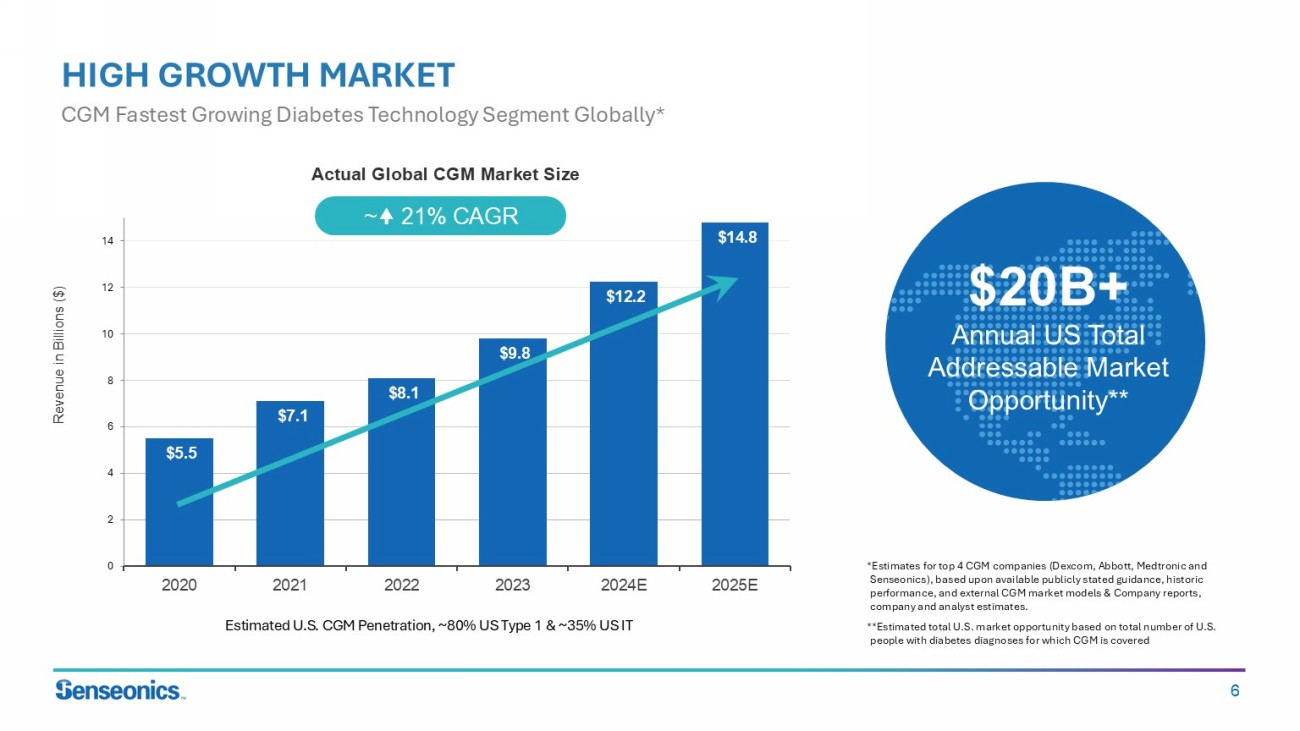
6 $5.5 $7.1 $8.1 $9.8 $12.2 $14.8 0 2 4 6 8 10 12 14 2020 2021 2022 2023 2024E 2025E Revenue in Billions ($) HIGH GROWTH MARKET CGM Fastest Growing Diabetes Technology Segment Globally* Estimated U.S. CGM Penetration, ~80% US Type 1 & ~35% US IT ~ 21% CAGR *Estimates for top 4 CGM companies (Dexcom, Abbott, Medtronic and Senseonics ), based upon available publicly stated guidance, historic performance, and external CGM market models & Company reports, company and analyst estimates. **E stimated total U.S. market opportunity based on total number of U.S. people with diabetes diagnoses for which CGM is covered $20B+ Annual US Total Addressable Market Opportunity** Actual Global CGM Market Size

7 Therapeutic indication for 18 yo and older. *In the ENHANCE study, Mean Absolute Relative Difference (MARD) of 8.7% was observed in the Eversense® 365 Sensor, and MARD of 9.1% was observed in primary Sensor. See “About Eversense” slide 2 for label and safety information. • CGM that lasts for 12 months vs. 10 - 14 days • Fully implantable 3rd generation sensor • CGM achieves 8.7% MARD* • Transmitter that is removable • System with on - body vibe alerts for hypo and hyper warnings • System with fresh, gentle - on skin adhesive patch • Device that does not require self - insertions • Sensor that has approved MRI conditional labeling EVERSENSE CGM SYSTEM ADDRESSES UNMET NEEDS AS THE FIRST AND ONLY 7
8 STRONG AND EXPANDING REIMBURSEMENT ACCESS FOR OVER 300 MILLION LIVES Recent eligibility expansion extends medicare coverage to all individuals with diabetes who use insulin and non - insulin - taking patients at risk of hypoglycemia Effectively doubling the total addressable patient population. Commercial payers transitioning as well Broad Commercial and Medicare coverage for both Product and Procedure
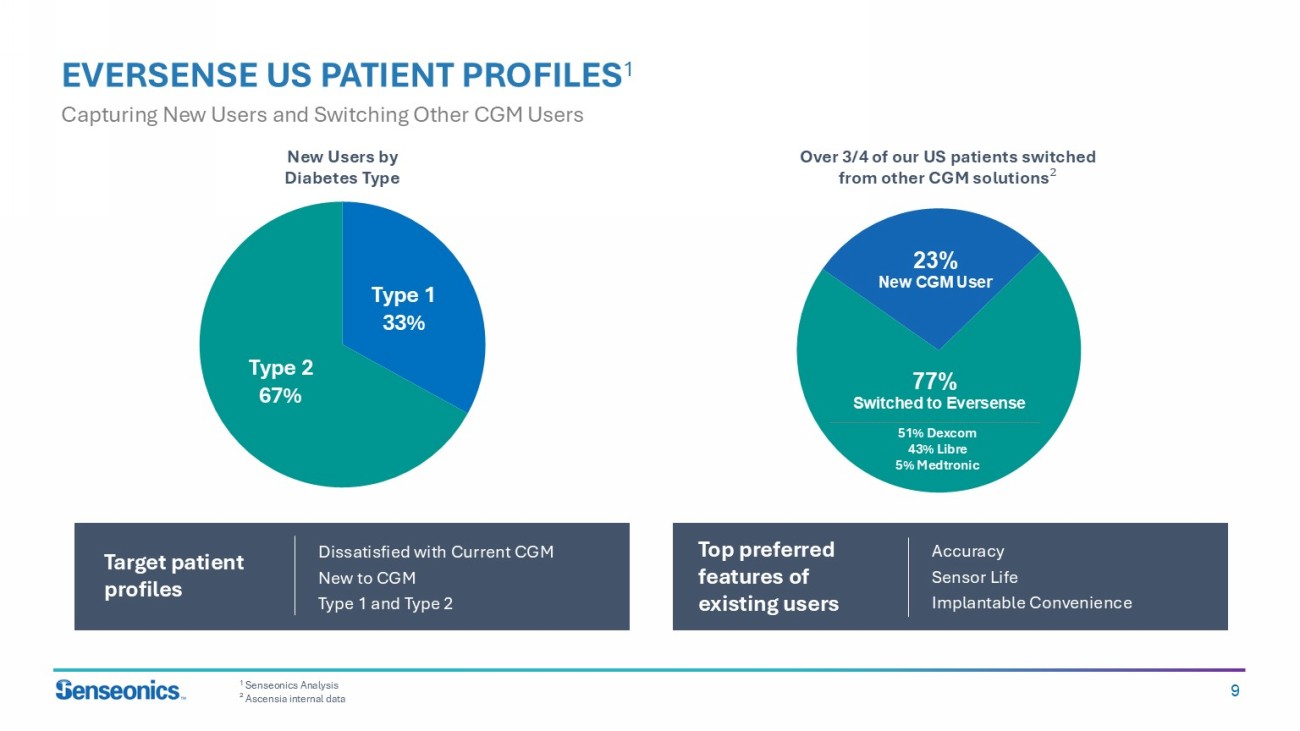
9 EVERSENSE US PATIENT PROFILES 1 Capturing New Users and Switching Other CGM Users Target patient profiles 1 Senseonics Analysis ² Ascensia internal data Over 3/4 of our US patients switched from other CGM solutions ; 23% New CGM User 77% Switched to Eversense 51% Dexcom 43% Libre 5% Medtronic Type 2 52% Type 1 48% New Users by Diabetes Type Type 1 33% Type 2 67% Dissatisfied with Current CGM New to CGM Type 1 and Type 2 Top preferred features of existing users Accuracy Sensor Life Implantable Convenience

10 • Broad Partnership Features Global Commercialization and Financing Arrangements with Leader in BGM • ASCENSIA is the Exclusive Worldwide Distribution Partner for Eversense ® 365 and Future Generation Products • Senseonics to Focus Streamlined Operations on Manufacturing, Research and Development of Next Generations of Eversense ® Systems • Revenue sharing arrangement - Ascensia’s share of revenue increases with volume and contract duration Leverage ADC commercial strength with current and new products Eversense® 365 CGM Focus Areas: • Build brand awareness and adoption • Protect and grow installed base WW Estimated Commercial Investment: ~ $60M in 2024* *Current estimated investment and headcount plans with ADC. Subject to risks and uncertainties COMMERCIAL COLLABORATION WITH ASCENSIA • A leader in BGM with 80 YEARS of innovative diabetes care products • Approximately $700M in sales in over 125 countries and covering 10 million people • Approximately 1700 employees working in 31 countries 10
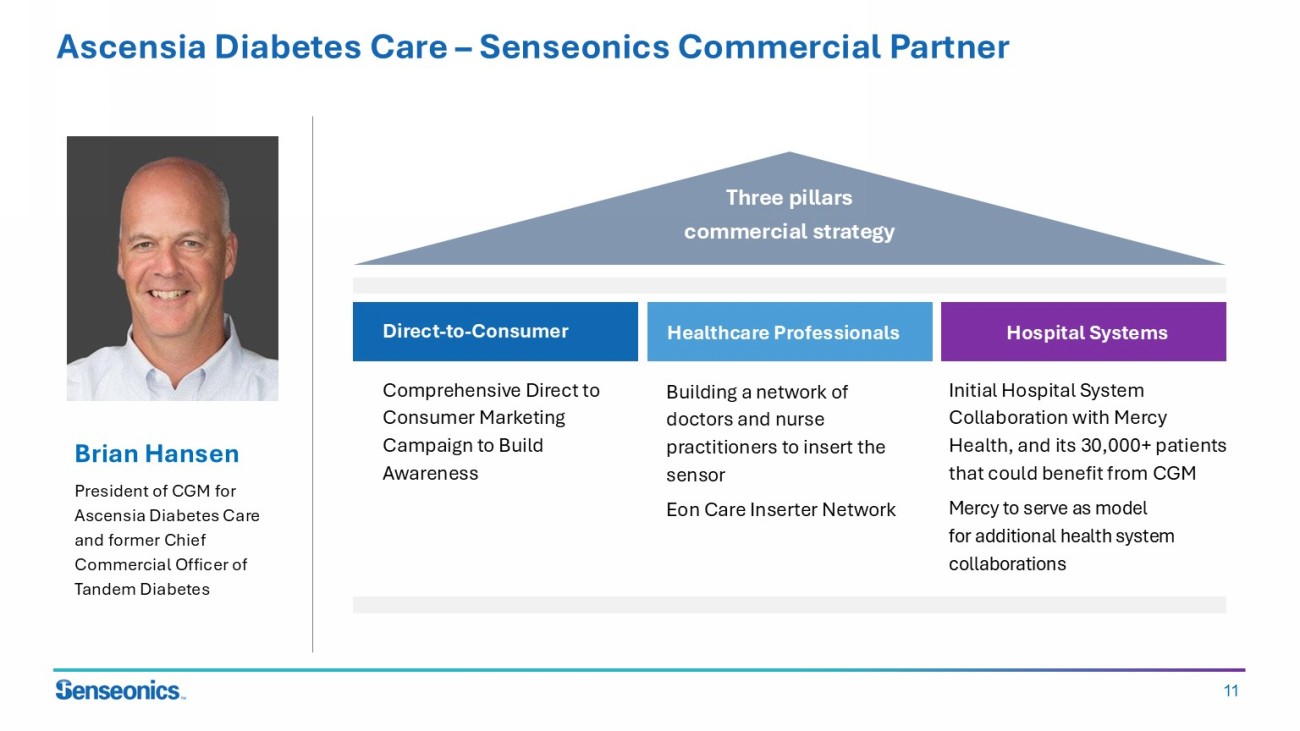
11 Ascensia Diabetes Care – Senseonics Commercial Partner Brian Hansen President of CGM for Ascensia Diabetes Care and former Chief Commercial Officer of Tandem Diabetes Three pillars commercial strategy Comprehensive Direct to Consumer Marketing Campaign to Build Awareness Direct - to - Consumer Healthcare Professionals Hospital Systems Building a network of doctors and nurse practitioners to insert the sensor Eon Care Inserter Network Initial Hospital System Collaboration with Mercy Health, and its 30,000+ patients that could benefit from CGM Mercy to serve as model for additional health system collaborations
12 Eversense 365 Commercial Launch
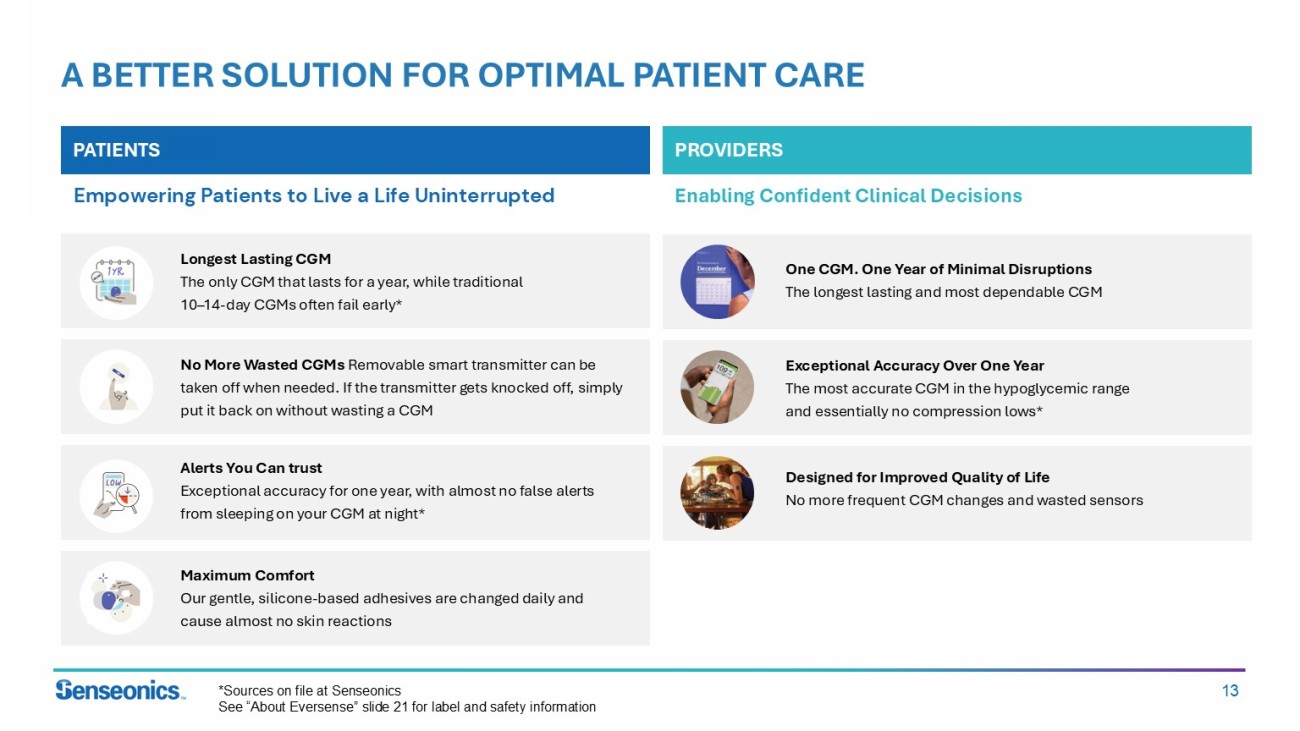
13 A BETTER SOLUTION FOR OPTIMAL PATIENT CARE PATIENTS PROVIDERS Empowering Patients to Live a Life Uninterrupted Enabling Confident Clinical Decisions Longest Lasting CGM The only CGM that lasts for a year, while traditional 10 – 14 - day CGMs often fail early* No More Wasted CGMs Removable smart transmitter can be taken off when needed. If the transmitter gets knocked off, simply put it back on without wasting a CGM Alerts You Can trust Exceptional accuracy for one year, with almost no false alerts from sleeping on your CGM at night* Maximum Comfort Our gentle, silicone - based adhesives are changed daily and cause almost no skin reactions One CGM. One Year of Minimal Disruptions The longest lasting and most dependable CGM Exceptional Accuracy Over One Year The most accurate CGM in the hypoglycemic range and essentially no compression lows* Designed for Improved Quality of Life No more frequent CGM changes and wasted sensors *Sources on file at Senseonics See “About Eversense” slide 21 for label and safety information
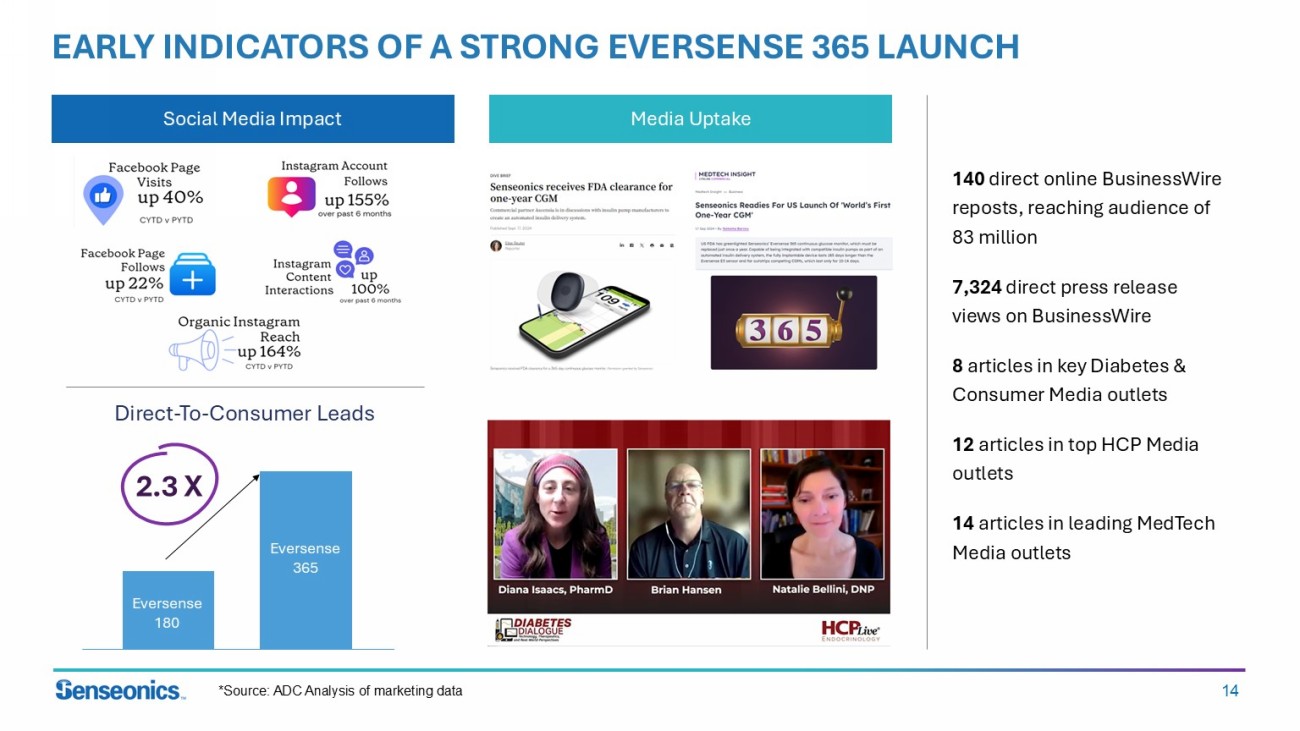
14 EARLY INDICATORS OF A STRONG EVERSENSE 365 LAUNCH Direct - To - Consumer Leads Eversense 180 Eversense 365 2.3 X 140 direct online BusinessWire reposts, reaching audience of 83 million 7,324 direct press release views on BusinessWire 8 articles in key Diabetes & Consumer Media outlets 12 articles in top HCP Media outlets 14 articles in leading MedTech Media outlets Media Uptake Social Media Impact *Source: ADC Analysis of marketing data
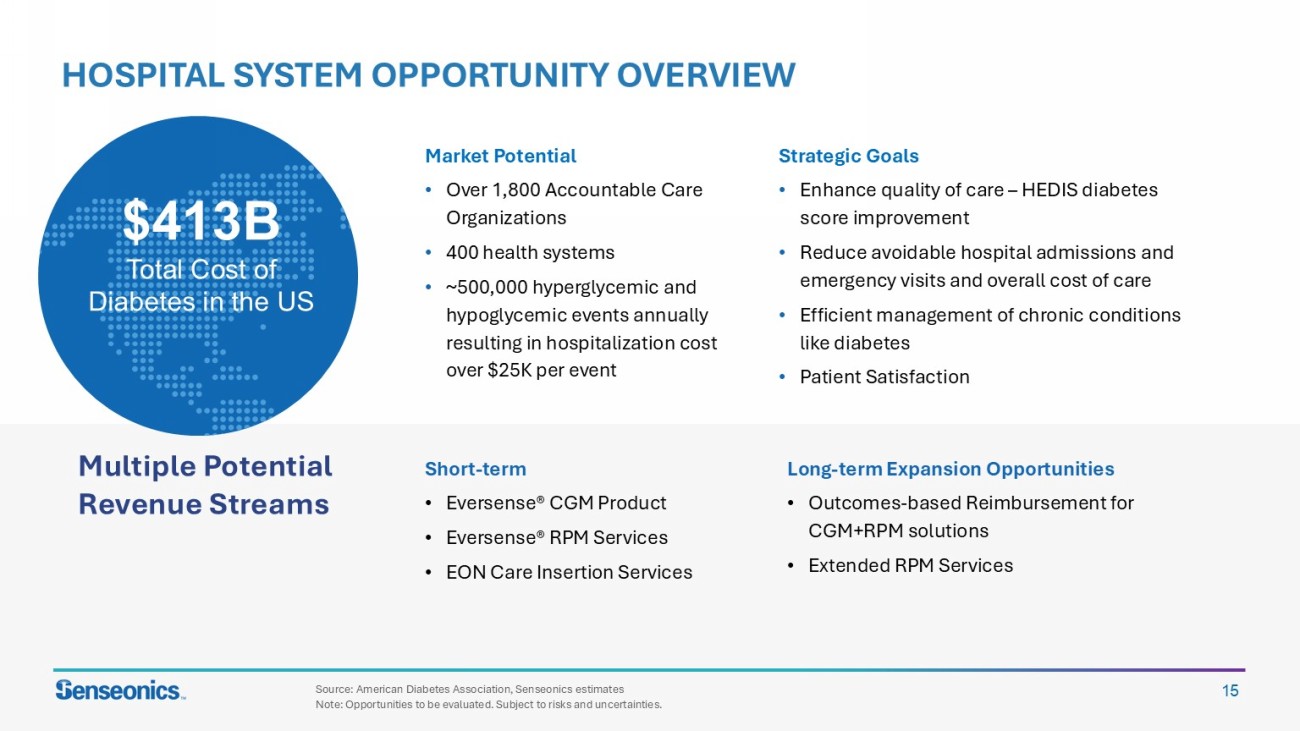
15 HOSPITAL SYSTEM OPPORTUNITY OVERVIEW Market Potential • Over 1,800 Accountable Care Organizations • 400 health systems • ~500,000 hyperglycemic and hypoglycemic events annually resulting in hospitalization cost over $25K per event Multiple Potential Revenue Streams $413B Total Cost of Diabetes In the US Source: American Diabetes Association, Senseonics estimates Note: Opportunities to be evaluated. Subject to risks and uncertainties. Long - term Expansion Opportunities • Outcomes - based Reimbursement for CGM+RPM solutions • Extended RPM Services Short - term • Eversense ® CGM Product • Eversense ® RPM Services • EON Care Insertion Services Strategic Goals • Enhance quality of care – HEDIS diabetes score improvement • Reduce avoidable hospital admissions and emergency visits and overall cost of care • Efficient management of chronic conditions like diabetes • Patient Satisfaction $413B Total Cost of Diabetes in the US
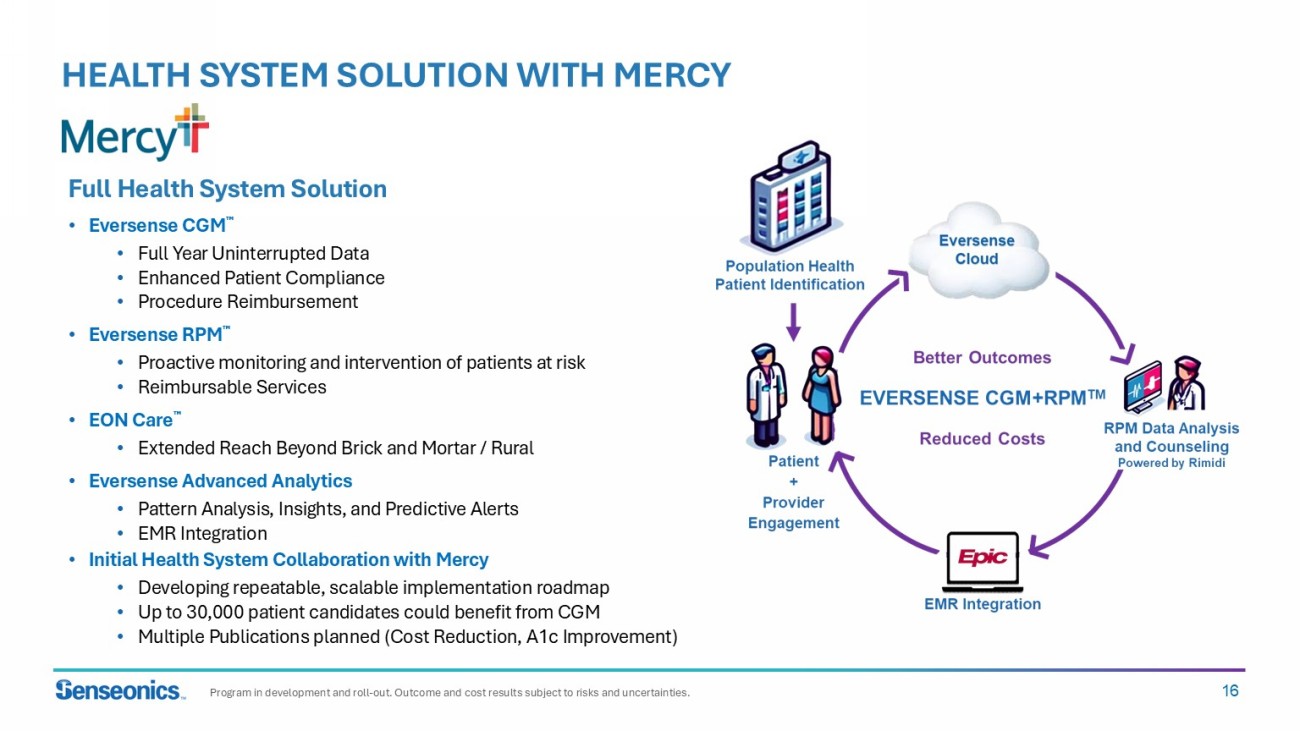
16 HEALTH SYSTEM SOLUTION WITH MERCY Full Health System Solution • Eversense CGM • Full Year Uninterrupted Data • Enhanced Patient Compliance • Procedure Reimbursement • Eversense RPM • Proactive monitoring and intervention of patients at risk • Reimbursable Services • EON Care • Extended Reach Beyond Brick and Mortar / Rural • Eversense Advanced Analytics • Pattern Analysis, Insights, and Predictive Alerts • EMR Integration • Initial Health System Collaboration with Mercy • Developing repeatable, scalable implementation roadmap • Up to 30,000 patient candidates could benefit from CGM • Multiple Publications planned (Cost Reduction, A1c Improvement) Program in development and roll - out. Outcome and cost results subject to risks and uncertainties.
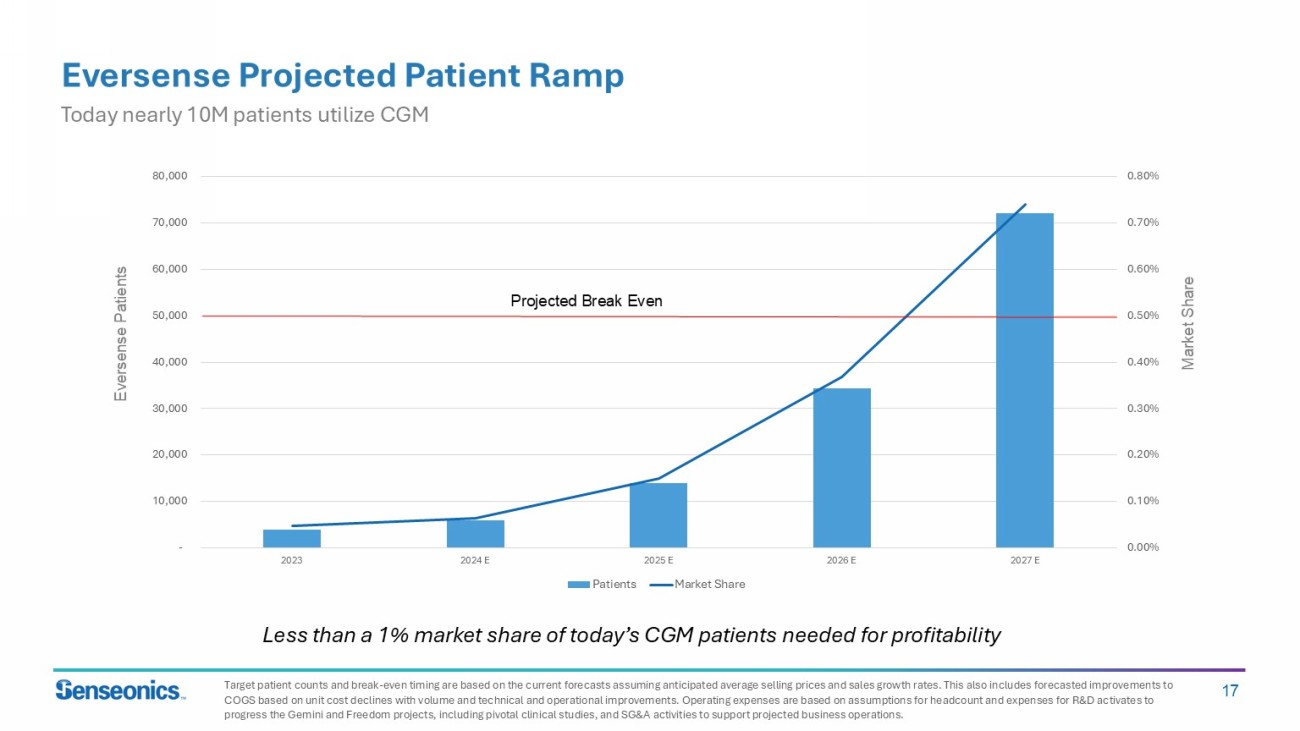
17 0.00% 0.10% 0.20% 0.30% 0.40% 0.50% 0.60% 0.70% 0.80% - 10,000 20,000 30,000 40,000 50,000 60,000 70,000 80,000 2023 2024 E 2025 E 2026 E 2027 E Patients Market Share Eversense Projected Patient Ramp Today nearly 10M patients utilize CGM Projected Break Even Less than a 1% market share of today’s CGM patients needed for profitability Eversense Patients Market Share Target patient counts and break - even timing are based on the current forecasts assuming anticipated average selling prices and s ales growth rates. This also includes forecasted improvements to COGS based on unit cost declines with volume and technical and operational improvements. Operating expenses are based on assu mpt ions for headcount and expenses for R&D activates to progress the Gemini and Freedom projects, including pivotal clinical studies, and SG&A activities to support projected busine ss operations.
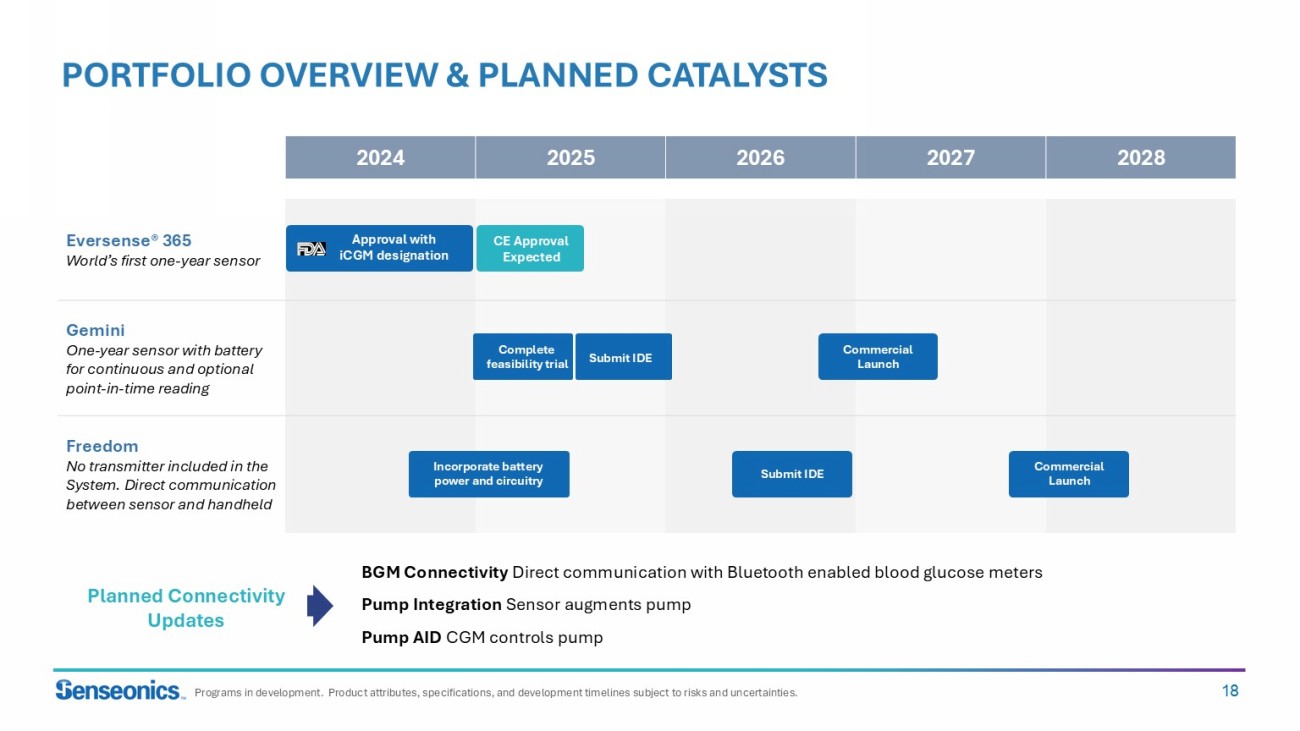
18 2028 2027 2026 2025 2024 Eversense ® 365 World’s first one - year sensor Gemini One - year sensor with battery for continuous and optional point - in - time reading Freedom No transmitter included in the System. Direct communication between sensor and handheld PORTFOLIO OVERVIEW & PLANNED CATALYSTS Programs in development. Product attributes, specifications, and development timelines subject to risks and uncertainties. BGM Connectivity Direct communication with Bluetooth enabled blood glucose meters Pump Integration Sensor augments pump Pump AID CGM controls pump Planned Connectivity Updates Incorporate battery power and circuitry Submit IDE Commercial Launch Complete feasibility trial Submit IDE Approval with iCGM designation CE Approval Expected Commercial Launch
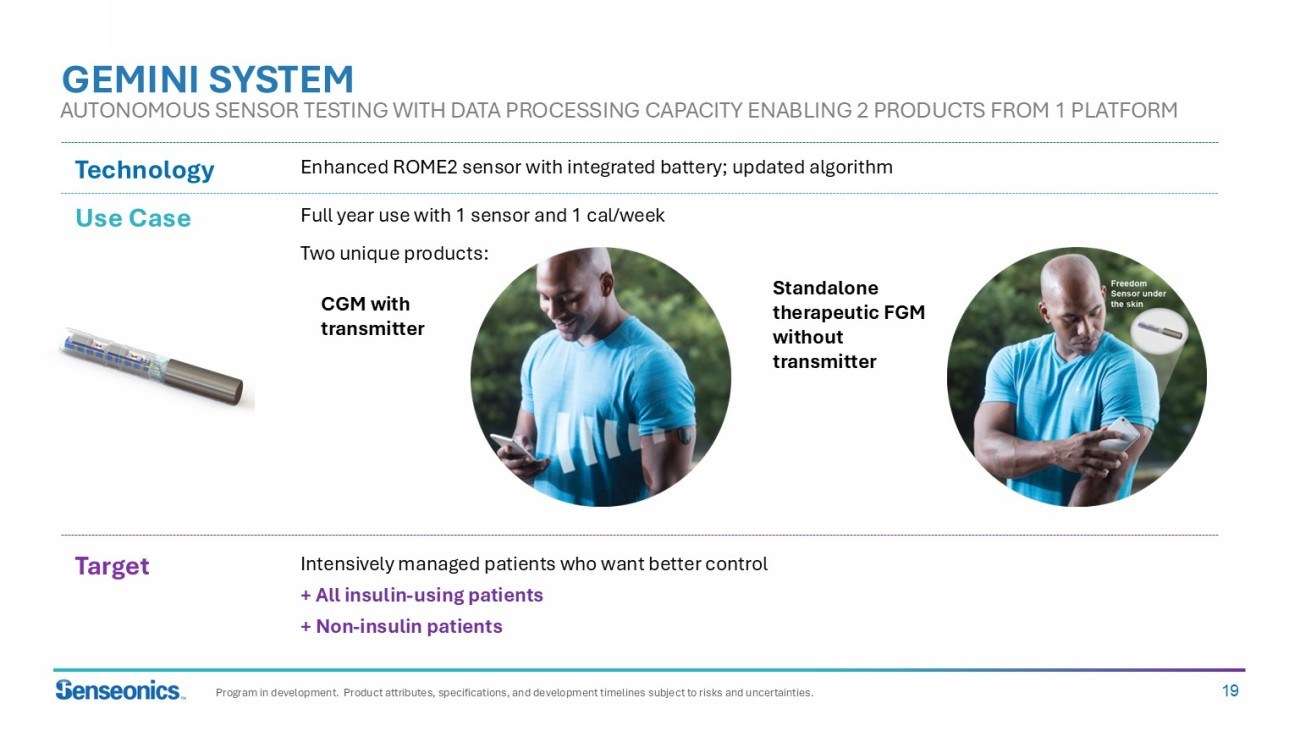
19 GEMINI SYSTEM Use Case Target Technology Enhanced ROME2 sensor with integrated battery; updated algorithm Intensively managed patients who want better control + All insulin - using patients + Non - insulin patients Full year use with 1 sensor and 1 cal /week Two unique products: CGM with transmitter Standalone therapeutic FGM without transmitter AUTONOMOUS SENSOR TESTING WITH DATA PROCESSING CAPACITY ENABLING 2 PRODUCTS FROM 1 PLATFORM Program in development. Product attributes, specifications, and development timelines subject to risks and uncertainties.
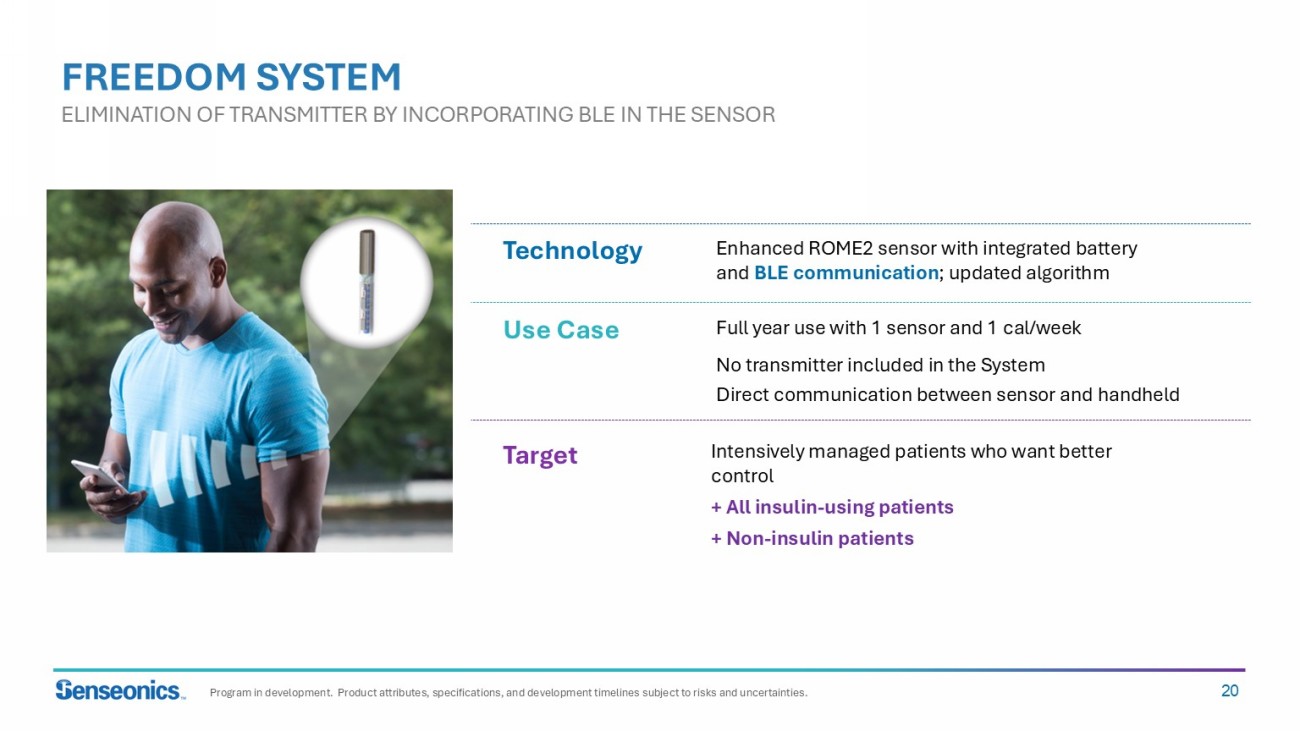
20 Enhanced ROME2 sensor with integrated battery and BLE communication ; updated algorithm Intensively managed patients who want better control + All insulin - using patients + Non - insulin patients Use Case Target Technology Full year use with 1 sensor and 1 cal /week No transmitter included in the System Direct communication between sensor and handheld FREEDOM SYSTEM ELIMINATION OF TRANSMITTER BY INCORPORATING BLE IN THE SENSOR Program in development. Product attributes, specifications, and development timelines subject to risks and uncertainties.

21 Thank You Corporate Overview November 2024
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Senseonics (AMEX:SENS)
Historical Stock Chart
From Nov 2024 to Dec 2024

Senseonics (AMEX:SENS)
Historical Stock Chart
From Dec 2023 to Dec 2024
