Filed
pursuant to Rule 424(b)(3)
Registration
No. 333-276151
AGRIFORCE
GROWING SYSTEMS, LTD.
Common
Stock
This
prospectus related to the offer and sale from time to time of up to 8,717,454 shares of common stock of AgriFORCE Growing Systems,
Ltd. by the selling stockholders identified in this prospectus. The number of shares offered for sale by the selling stockholders consists
of up to 8,717,454 shares of our common stock. We are not selling any shares of our common stock in this offering and we will
not receive any of the proceeds from the sale of shares of our common stock by the selling stockholders. The selling stockholders will
receive all of the proceeds from any sales of the shares of our common stock offered hereby. However, we will incur expenses in connection
with the registration of the shares of our common stock offered hereby. The selling stockholders may sell these shares through public
or private transactions at market prices prevailing at the time of sale or at negotiated prices. The timing and amount of any sale are
within the sole discretion of the selling stockholders. The selling stockholders and any underwriters, dealers or agents that participate
in distribution of the securities may be deemed to be underwriters, and any profit on sale of the securities by them and any discounts,
commissions or concessions received by any underwriter, dealer or agent may be deemed to be underwriting discounts and commissions under
the Securities Act. There can be no assurances that the selling stockholders will sell any or all of the securities offered under this
prospectus. For further information regarding the possible methods by which the shares may be distributed, see the section titled “Plan
of Distribution” beginning on page 38 of this prospectus.
Our
common stock is listed on the Nasdaq Capital Market under the symbol “AGRI” and our Series A Warrants are listed on the Nasdaq
Capital Market under the symbol “AGRI”. On December 19, 2023, the last reported sale price of our common stock on
the Nasdaq Capital Market was $0.58 per share.
You
should read this prospectus, together with additional information described under the heading “Where You Can Find More Information,”
carefully before you invest in any of our securities.
Investing
in our securities involves a high degree of risk. See “Risk Factors” beginning on page 10 of this prospectus for a
discussion of information that should be considered in connection with an investment in our securities.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this prospectus is January 26, 2024.
TABLE
OF CONTENTS
We
have not, and the selling stockholders have not, authorized anyone to provide you with any information or to make any representations
other than those contained in this prospectus or in any free writing prospectus we have prepared and filed with the SEC. We and the selling
stockholders take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may
give you. This prospectus is an offer to sell only the shares offered hereby, but only under the circumstances and in jurisdictions where
it is lawful to do so. The information contained in this prospectus is current only as of its date, regardless of the time of delivery
of this prospectus or of any sale of our common stock. For investors outside of the United States: Neither we nor the selling stockholders
have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action
for that purpose is required, other than in the United States. Persons outside of the United States who come into possession of this
prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of our common stock and
the distribution of this prospectus outside of the United States.
No
person is authorized in connection with this prospectus to give any information or to make any representations about us, the securities
offered hereby or any matter discussed in this prospectus, other than the information and representations contained in this prospectus
or in any free writing prospectus we may authorize to be delivered or made available to you. If any other information or representation
is given or made, such information or representation may not be relied upon as having been authorized by us.
For
investors outside the United States: Neither we nor the underwriters have done anything that would permit this offering or possession
or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You
are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
Unless
otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including
our general expectations and market position, market opportunity and market share, is based on information from our own management estimates
and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Management
estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and
knowledge, which we believe to be reasonable. Our management’s estimates have not been verified by any independent source, and
we have not independently verified any third-party information. In addition, assumptions and estimates of our and our industry’s
future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described
in “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions
and estimates. See “Cautionary Note Regarding Forward-Looking Statements.”
SUMMARY
This
summary highlights selected information from this prospectus and does not contain all of the information that you should consider in
making your investment decision. You should carefully read the entire prospectus, the applicable prospectus supplement and any related
free writing prospectus, including the risks of investing in our securities discussed under the heading “Risk Factors” contained
in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the documents that are
incorporated by reference into this prospectus. You should also carefully read the information incorporated by reference into this prospectus,
including our financial statements, and the exhibits to the registration statement of which this prospectus is a component.
The
terms “AgriFORCE™,” the “Company,” “we,” “our” or “us” in this prospectus
refer to AgriFORCE Growing Systems, Ltd. and its wholly-owned subsidiaries, unless the context suggests otherwise.
PROSPECTUS
SUMMARY
This
summary highlights selected information from this prospectus and does not contain all of the information that you should consider in
making your investment decision. You should carefully read the entire prospectus and any related free writing prospectus, including the
risks of investing in our securities discussed under the heading “Risk Factors” contained in the prospectus supplement and
any related free writing prospectus.
The
terms “AgriFORCE,” the “Company,” “we,” “our” or “us” in this prospectus
refer to AgriFORCE Growing Systems, Ltd. and its wholly-owned subsidiaries, unless the context suggests otherwise.
OUR
BUSINESS
Overview
AgriFORCE™
was incorporated as a private company by Articles of Incorporation issued pursuant to the provisions of the Business Corporations Act
(British Columbia) on December 22, 2017. The Company’s registered and records office address is at 800-525 West 8th Avenue Vancouver,
BC V5Z 1C6.
Our
Business
AgriFORCE™
is dedicated to positively transforming farm, food, and family every day, everywhere. We aim to achieve this goal by providing novel
agriculturally focused consulting, facility solutions, and products & services through our Solutions division, and by leveraging
innovative technologies and processes to deliver healthier more nutritious food to consumers through our Brands division.
The
AgriFORCE™ Solutions division is dedicated to transforming modern agricultural development “Building from the Seed to Deliver
sustainable, Efficient, and Healthier crops” through our integrated Agtech platform 2.0 combining knowledge and IP with CEA equipment
solutions, including our FORCEGH+™” solution, implementing solutions that are best suited to the crops and environment chosen.
Our
AgriFORCE™ Brands division is focused on the development and commercialization of plant-based ingredients and products that deliver
more nutritious “Food to Table”. We will market and commercialize both branded consumer product offerings and ingredient
supplies.
AgriFORCE™
Solutions
Understanding
Our Approach – The AgriFORCE™ Precision Growth Method
Traditional
farming includes three fundamental approaches: outdoor, greenhouse and indoor. AgriFORCE™ introduces a unique fourth method, the
AgriFORCE™ precision growth method, which is informed by cutting-edge science and leveraging the latest advances in artificial
intelligence (AI) and Internet of Things (IoT).
With
a carefully optimized approach to facility design, IoT, AI utilization, nutrient delivery, and micro-propagation, we have devised an
intricate, scientific and high success-oriented approach designed to produce much greater efficacy yields using fewer resources. This
method is intended to outperform traditional growing methods using a specific combination of new and traditional techniques required
to attain this efficiency. We call it precision growth. The AgriFORCE™ precision growth method focuses on addressing some of the
most important legacy challenges in agriculture: environmental impact, operational efficiency and yield volumes.
The
AgriFORCE™ precision growth method presents a tremendous opportunity to positively disrupt all corners of the industry. The market
size of just the nutraceutical and plant-based pharmaceutical and vaccine/therapeutics market is over $500 billion. Including the traditional
hydroponics high value crops and controlled-environment food markets, the addressable market approaches nearly $1 trillion. (1)(2)(3).
The
AgriFORCE™ Model – Managing the Difficulties of Agricultural Verticals with Modern Technology and Innovation
Our
intellectual property combines a uniquely engineered facility design and automated growing system to provide a clear solution to the
biggest problems plaguing most high value crop agricultural verticals. It delivers a clean, self-contained environment that maximizes
natural sunlight and offers near ideal supplemental lighting. It also limits human intervention and – crucially – it was
designed to provide superior quality control. It was also created to drastically reduce environmental impact, substantially decrease
utility demands, as well as lower production costs, while delivering customers daily harvests and higher crop yields.
Plants
grow most robustly and flavorfully in full natural sunlight. While it may seem counterintuitive to some, even the clearest of glass greenhouses
inhibit the full light spectrum of the sun. However, new translucent and transparent membrane materials have emerged recently that enable
the near-full-transmission of the sun’s light spectrum.
Our
Position in the Ag-Tech Sector
The
Ag-Tech sector is severely underserved by the capital markets, and we see an opportunity to acquire global companies who have provided
solutions to the industry and are leading innovation moving forward. We are creating a separate corporate office to aggressively pursue
such acquisitions. The robustness of our engagement with potential targets has confirmed our belief and desire to be part of a larger
integrated Ag-Tech solutions provider, where each separate element of the business has its existing legacy business and can leverage
across areas of expertise to expand their business footprint. We believe that there is currently no one that we are aware of who is pursuing
this model in the US capital markets environment at this time.
(1)
https://home.kpmg/pl/en/home/insights/2015/04/nutraceuticals-the-future-of-intelligent-food.html
(2)
https://link.springer.com/article/10.1057/jcb.2010.37
(3)
https://medium.com/artemis/lets-talk-about-market-size-316842f1ab27
The
AgriFORCE™ Grow House
The
Company is an agriculture-focused technology company that delivers innovative and reliable, financially robust solutions for high value
crops through our proprietary facility design and automation IP to businesses and enterprises globally. The Company intends to operate
in the plant based pharmaceutical, nutraceutical, and high value crop markets using its unique patented facility design and hydroponics
based automated growing system that enable cultivators to effectively grow crops in a controlled environment (“FORCEGH+™”).
The Company has designed FORCEGH+™ facilities to produce in virtually any environmental condition and to optimize crop yields to
as near their full genetic potential possible while substantially eliminating the need for the use of pesticides, fungicides and/or irradiation.
The
Company continues to develop its solution for fruits and vegetables focusing on the integration of its current structure with a new form
of vertical grow technology.
BUSINESS
PLAN
PHASE
1 (COMPLETED):
| |
●
|
Conceptualization,
engineering, and design of facility and systems. (complete) |
| |
●
|
Completed
selection process of key environmental systems with preferred vendors. (complete) |
| |
●
|
Selection
and Land Purchase agreement in Coachella, CA subject to financing. (complete) |
| |
●
|
ForceFilm
material ordered. (complete) |
PHASE
2:
| |
●
|
Complete
the timing of financing for, and purchase of, the selected parcel in Coachella, CA, subject to market conditions, |
| |
●
|
Complete
feasibility study for new contracts’ structures for facilities with new independent operators. |
| |
●
|
Identify
procurement of AgriFORCE™ IP specific automated grow system, supplemental grow lighting and controls systems, and manufacture
of the building envelope materials. |
| |
●
|
Conceptualization
and design of vertical grow solutions. |
| |
●
|
Initiate
the design of an R&D facility for food solutions and plant-based pharma. |
PHASE
3:
| |
●
|
Complete
the delivery and installation of facilities. Proof of quantitative and qualitative benefits will drive both sales pipeline acceleration
for subsequent years. |
| |
●
|
Complete
the design of an R&D facility for food solutions and plant-based pharma. Commence engagement with universities and pharmaceutical
companies. |
| |
●
|
Review
potential licensing opportunities for the Solutions patent portfolio. |
PHASE
4:
| |
●
|
Focus
on delivery and installation of additional facilities. |
| |
●
|
Expand
geographic presence into other geographies by introducing the FORCEGH+™ to other international markets with a view to securing
additional locations and markets. |
AgriFORCE™
Brands
The
Company purchased Intellectual Property (“IP”) from Manna Nutritional Group, LLC (“Manna”), a privately held
firm based in Boise, Idaho on September 10, 2021. The IP encompasses a granted patent to naturally process and convert grain, pulses
and root vegetables, resulting in low-starch, low-sugar, high-protein, fiber-rich baking flour as well as produces a natural sweetener
juice. The core process is covered under the Patent Nr. 11,540,538 in the U.S. and key international markets. The all-natural process
is designed to unlock nutritional properties, flavors and other qualities in a range of modern, ancient and heritage grains, pulses and
root vegetables to create specialized all-natural baking and all-purpose flours, sweeteners, juices, naturally sweet cereals and other
valuation products, providing numerous opportunities for dietary nutritional, performance and culinary applications.
Wheat
and Flour Market
Modern
diet is believed to be a contributor to health risks such as heart disease, cancer, diabetes and obesity, due in part to the consumption
of highly processed foods that are low in natural fiber, protein and nutrition; and extremely high in simple starch, sugar and calories.
These “empty carbs” produce glycemic swings that may cause overeating by triggering cravings for food high in sugar, salt
and starch. As an example, conventional baking flour is low in natural fiber (~ 2-3%), low-to-average in protein (~ 9%), and very high
in starch (~ 75%)(4). Whole wheat flour is only marginally better.
(4)
Based on protein, fiber, and starch content figures from a nationally certified independent laboratory, as compared to standard all-purpose
flour.
In
contrast, foods high in fiber help to satiate hunger, suppress cravings and raise metabolism(5). They also assist in weight
loss, lower cholesterol, and may reduce the risk of cancer, heart disease and diabetes.
Advantages
of the UN(THINK)™ Foods IP
The
Controlled Enzymatic Reaction & Endothermic Saccharification with Managed Natural Germination (“CERES-MNG”)
patented process allows for the development and manufacturing of all-natural flours that are significantly higher in fibers, nutrients
and proteins and significantly lower in carbohydrates and calories than standard baking flour.
CERES-MNG
baking flour produced from soft white wheat has 40 times more fiber, three (3) times more protein and 75% less net carbohydrates than
regular all- purpose flour8 (6).
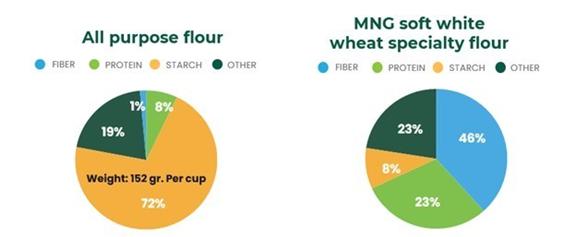
Source:
Independent analysis by Eurofins Food Chemistry Testing Madison, Inc, February 2022
The
CERES-MNG patent will help develop new flours and products from modern, ancient and heritage grains, seeds, legumes and tubers/root vegetables.
(5)
https://my.clevelandclinic.org/health/articles/14400-improving-your-health-with-fiber
(6)
Based on protein, fiber, and starch content figures from a nationally certified independent laboratory, as compared to standard all-purpose
flour.
Products
that AgriFORCE™ intends to develop for commercialization from the CERES-MNG patented process under the UN(THINK)™ foods brand:
| |
-
|
High
protein, high fiber, low carb modern, heritage and ancient grain flours (for use in breads, baked goods, doughs, pastry, snacks,
and pasta) |
| |
-
|
Protein
flours and protein additives |
| |
-
|
High
protein, high fiber, low carb cereals and snacks |
| |
-
|
High
protein, high fiber, low carb oat based dairy alternatives |
| |
-
|
Better
tasting, cleaner label high protein, high fiber, low carb nutrition bars |
| |
-
|
High
protein, high fiber low carb nutrition juices |
| |
-
|
Sweeteners
– liquid, granulated |
| |
-
|
High
protein, high fiber, low carb pet foods and snacks |
We
intend to commercialize these products behind three (3) main sales channels:
| |
-
|
Ingredients
|
| |
-
|
Branded
ingredients |
| |
-
|
Consumer
brand |
The
business opportunity for AgriFORCE™ to successfully commercialize premium specialized products from the UN(THINK)™ foods
IP – by capturing a conservatively very small percentage share of the category it is targeting to enter in the premium segments.
We estimate these revenues to be between $500 million and $1 billion by 2030 (excluding any potential revenues from the Maltose-Power
Juice applications).
| | |
Breads &Bakery | |
Functional Flours | |
Pulse Flours | |
Dairy Alternatives | |
Nutrition Bars | |
Total | |
| Global market size of target categories | |
$ | 222B | | |
$ | 48B | | |
$ | 17B | | |
$ | 6B | | |
$ | 45B | | |
|
| |
| Potential market share | |
| 0.1 | % | |
| 1 | % | |
| 1 | % | |
| 1 | % | |
| 0.1 | % | |
|
| |
| AgriFORCE™ potential net revenues | |
$ | 100-200M | | |
$ | 200-480M | | |
$ | 100- 170M | | |
$ | 30-60M | | |
$ | 20-40M | | |
$ |
450-950M | |
Sources:
Grand View Research Reports, San Francisco CA, 2018 Estimates.
While
we are working on setting up a pilot plant in Canada to produce the UN(THINK)™ power wheat flour for the end of 2023, our patented
process allows us to develop a gold-standard sprouted wheat flour, which we have qualified and have made available for sale through brokers
as of January 2023 in Canada and the USA, under the UN(THINK)™ Awakened Grains™ brand. This new Awakened Grains™ flour
will provide enhanced nutrition with over five times more fiber, up to two times more protein and 77% of net carbs versus conventional
all purpose flour (source: Eurofins Food Chemistry Madison, Inc, December 2022).
BUSINESS
PLAN
AgriFORCE™’s
organic growth plan is to actively establish and deploy the commercialization of products, following the acquisition of the Manna IP,
is focused on four distinct phases:
PHASE
1 (COMPLETED):
| |
●
|
Product
and process testing and validation. (completed) |
| |
●
|
Filing
of US and international patent. (completed) |
| |
●
|
Conceptual
engineering and preliminary budgeting on commercial pilot plant. (completed) |
| |
●
|
Creation
of the UN(THINK)™ foods brand. (completed) |
| |
●
|
Qualification
and operational and commercial set up of the Awakened Grains™ line of products (completed) |
PHASE
2:
| |
●
|
Launch
of the UN(THINK)™ Awakened Grains™ sprouted flour range of products in business to business (“B2B”) and direct
to consumers (“D2C”) channels. |
| |
●
|
Design,
build, start-up, and operation of the pilot plant for the fully processed and patented flours |
| |
●
|
Develop
range of finished products behind the wheat grain flours, qualify patented process for pulse/legume, and rice based protein flours.
|
| |
●
|
Collaborate
with Nutritional Flour Medical Research Institute (an IRS section 501(c)(3) Medical Research Organization) funded by private &
public research grants. |
PHASE
3:
| |
●
|
Launch
first range of fully patent processed products in US/Canada (UN(THINK)™ power wheat flour. |
| |
●
|
Drive
business with finished products in D2C, retail, food service. |
| |
●
|
Drive
business as ingredients for bakery, snack and plant based protein products manufacturers. |
| |
●
|
Develop
manufacturing base through partnerships and licensing. |
| |
●
|
Conceptual
engineering and preliminary budgeting on large-scale processing plant. |
PHASE
4:
| |
●
|
Expand
product range in US/Canada. |
| |
●
|
Expand
business to other geographies internationally. |
| |
●
|
Design,
build, start-up, and operation of large-scale processing plan. |
Merger
and Acquisition (“M&A”)
With
respect to M&A growth, the Company is aggressively pursuing acquisitions in the agriculture technology space. The Company believes
that a buy and build strategy will provide unique opportunities for innovation across each segment of the Ag-Tech market we serve. Our
unique IP combined with the know-how and IP of acquired companies will create additional value if the way we grow or produce crops. The
Company believes there is currently no other public traded publicly in the United States pursing this model.
Manna
Nutritional Group Asset Acquisition
On
September 10, 2021, the Company signed a definitive asset purchase agreement to acquire food production and processing IP from Manna.
On
May 10, 2022, the Company completed an amendment to its asset purchase agreement with Manna Nutritional Group LLC, dated September 10,
2021. The amendment required the issuance of prefunded warrants instead of shares over several tranches and contained covenants to obtain
shareholder approval of the acquisition transactions before the prefunded warrants can be exercised into Company common shares.
The
transaction was fully approved by the shareholders on December 15, 2022. The Company paid consideration of $1,475,000 in cash and issued
7,379,969 prefunded warrants valued at $12,106,677 adjusted for foreign exchange differences of $492,300. Subject to a 9.99% stopped
and SEC Rule 144 restrictions, the prefunded warrants will vest in tranches up until March 10, 2024. When vested the tranches of prefunded
warrants will be converted into an equal number of common shares.
Delphy
Groep BV Acquisition
On
February 10, 2022, the Company signed a definitive share purchase agreement (the “Delphy Agreement”) to acquire Delphy,
a Netherlands-based AgTech consultancy firm, for €23.5 million through a combination of cash and stock. The definitive agreement
follows the binding letter of intent as previously announced in the Company’s press release in October 2021. Delphy, which optimizes
production of plant-based foods and flowers, has multinational operations in Europe, Asia, and Africa, with approximately 200 employees
and consultants. Delphy’s client list includes agriculture companies, governments, universities, and leading AgTech suppliers,
who turn to the company to drive agricultural innovation, solutions, and operational expertise. The Delphy Agreement was negotiated at
arm’s length and is not a related party transaction.
On
September 22, 2022, the Company entered an amendment to the Delphy Agreement, pursuant to which the parties agreed to reduce the total
purchase from €$23.5 million to €17.7 million, plus a potential earnout of up to €6.0 million over two (2) years, based
on achieving future performance milestones. The Company also agreed to pay interest in the amount of €0.2 million on the purchase
price and additional interest from November 15, 2022 up to January 15, 2023 (the “Long Stop Date”).
Management
is currently in negotiating an amendment which will extend the Long Stop Date past January 15, 2023. Neither party has provided notice
to terminate the agreement.
Deroose
Plants NV Binding Letter of Intent
On
February 23, 2022, the Company signed a binding letter of intent (the “Deroose LOI”) with Deroose Plants NV (“Deroose”),
one of the largest tissue culture propagation companies in the world with a leadership position in horticulture, plantation crops, and
fruit and vegetables. Founded in 1980, Deroose has multi-national operations in Europe, North America, and Asia, and over 800 employees.
The
Deroose LOI is subject to completion of standard due diligence and entry into a definitive purchase agreement, which shall include commercially
standard terms and conditions, including, but not limited to, representations and warranties, covenants, events of default and conditions
to closing.
The
net purchase price by the Company is expected to be approximately €61 million. The purchase price represents approximately €41
million for the Deroose business on a cash and debt free basis and €20 million for the genetic IP portfolio.
The
parties are working through the Letter of Intent. Neither party has provided notice to terminate the agreement.
Stronghold
Land Acquisition
On
August 30, 2022, the Company entered into a Purchase and Sale Agreement (“PSA”) with Stronghold Power Systems, Inc. (“Stronghold”)
to purchase approximately 34 acres of land in Coachella California. The purchase price is $4,300,000, payable as follows: (i) $1,500,000
in cash and (ii) $2,800,000 in restricted shares of common stock of the Company. The stock is being issued in the form of prefunded warrants
in two tranches: (i) $1,700,000 (695,866 prefunded warrants) issued within five days of entry into the PSA, and (ii) $1,100,000 (450,266
prefunded warrants) at closing of the transaction. The first tranche shall be void if closing of the transaction does not occur by March
31, 2023. The prefunded warrants per share exercise price is $2.443 which is subject to certain adjustments. Issuance of all securities
in this transaction are exempt from registration under Section 4(a)(2) of the Securities Act of 1933, as amended. Under the terms of
the agreement, Stronghold must complete certain permitting, zoning, and infrastructure work by March 31, 2023, to close the transaction.
The
Company is currently reviewing the progress made by Stronghold and the market conditions to evaluate the feasibility of acquiring the
property.
Recent
Developments
Convertible
Debt Financing
On
January 17, 2023, the Convertible Debt Investors purchased an additional tranche of $5,076,923 in convertible debentures and received
2,661,289 warrants. The convertible debt and warrants were issued with an exercise price of $1.24. The issuance of the additional tranche
triggered the down round provision, adjusting the exercise prices of the Debentures and the Debenture Warrants to $1.24.
Berry
People LLC Binding Letter of Intent
On
January 24, the Company announced it has entered into a binding letter of intent (“BP LOI”) to acquire Berry People LLC,
(“Berry People”), a berry business with an increasingly international footprint and a scalable business model. The acquisition
bolsters the AgriFORCE™ Brands division and allows the Company to realize commercial synergies with UN(THINK)™.
Berry
People was founded in 2017 by berry industry veterans to create a new platform to meet market demand for a branded, year-round supply
of organic and conventional berries. Berry People quickly established a recognized global trade brand and scalable operations, comprised
of over 200 retail and foodservice clients and over 100 grower and exporter clients across the US, Canada, Mexico, and Peru. Berry People
had net revenues of USD $37 (unaudited) million for the year ended December 31, 2022.
The
LOI states, among other things that:
| |
●
|
the
transaction will be subject to completion of due diligence to the Company’s satisfaction and, after satisfactory due diligence,
the reaching of agreement on the terms of the purchase pursuant to a definitive purchase agreement, including conditions precedent
for closing of the transaction; |
| |
●
|
the
parties will sign the definitive purchase agreement no later than April 30, 2023, unless agreed to by both parties; and |
| |
●
|
Berry
People will not enter into any negotiations with other parties for a period of three months following the execution of the BP LOI.
|
The
BP LOI sets forth a purchase price of $28.0 million, consisting of $18.2 million in cash and $9.8 million in AgriFORCE™ restricted
shares, will be paid at closing to acquire 70% of Berry People’s equity interests. Berry People will have the opportunity for future
earnouts during the five years after closing based on future revenue and EBITDA targets associated with agreed upon growth targets.
In
collaboration with AgriFORCE™, Berry People aims to further develop backward integration into agricultural production via farming
joint ventures and deploy licensed and developed IP as part of a scalable franchising model. The berries market was $9.65 billion in
2021 in the U.S. alone7, with growth rates of around 10% or more each year since 2019— a trend that is expected to continue.
(7)
As per IRI Integrated Fresh, Latest 52 WE 3/20/2022
Manna
Patent Issuance
On
January 3, 2023, Manna satisfied all its contractual obligations when the patent was approved by the US Patents Office and title was
transferred to the Company. The Company issued 1,637,049 shares upon exercise of vested tranches of Manna’s prefunded warrants
in relation to this transaction on January 3, 2023.
Reverse
Split
On
October 11, 2023, the Company executed a one-for-fifty reverse stock split of the Company’s common shares (the “Reverse Split”).
As a result of the Reverse Split, every 50 shares of the Company’s old common shares were converted into one share of the Company’s
new common shares. Fractional shares resulting from the reverse split were rounded up to the nearest whole number. The Reverse Split
automatically and proportionately adjusted all issued and outstanding shares of the Company’s common shares, as well as convertible
debentures, convertible features, prefunded warrants, stock options and warrants outstanding at the time of the date of the Reverse Split.
The exercise price on outstanding equity based-grants was proportionately increased, while the number of shares available under the Company’s
equity-based plans was proportionately reduced. Share and per share data (except par value) for the periods presented reflect the effects
of the Reverse Split. References to numbers of common shares and per share data in the accompanying financial statements and notes thereto
for periods ended prior to October 11, 2023 have been adjusted to reflect the Reverse Split on a retroactive basis.
Delphy
Groep BV Acquisition
On
February 10, 2022, the Company signed a definitive share purchase agreement (the “Delphy Agreement”) to acquire Delphy, a
Netherlands-based AgTech consultancy firm, for €23.5 million through a combination of cash and stock. Delphy, which optimizes production
of plant-based foods and flowers, has multi-national operations in Europe, Asia, and Africa, with approximately 200 employees and consultants.
The Delphy Agreement was negotiated at arm’s length and was not a related party transaction.
On
May 25, 2023, the parties mutually terminated the share purchase agreement after extensive due diligence, an evaluation of the historical
and projected financial information, potential for impairment risk as well as current market conditions.
Deroose
Plants NV Binding Letter of Intent
On
February 23, 2022, the Company signed a binding letter of intent (the “Deroose LOI”) with Deroose Plants NV (“Deroose”),
one of the largest tissue culture propagation companies in the world with a leadership position in horticulture, plantation crops, and
fruit and vegetables. Founded in 1980, Deroose has multi-national operations in Europe, North America, and Asia, and over 800 employees.
The
Deroose LOI is subject to completion of standard due diligence and entry into a definitive purchase agreement, which is expected to include
commercially standard terms and conditions, including, but not limited to, representations and warranties, covenants, events of default
and conditions to closing.
Neither
party has provided notice to terminate the agreement.
Stronghold
Land Acquisition
On
August 30, 2022, the Company entered into a Purchase and Sale Agreement (“PSA”) with Stronghold Power Systems, Inc. (“Stronghold”)
to purchase approximately 34 acres of land in Coachella California. The purchase price was $4,300,000, payable as follows: (i) $1,500,000
in cash and (ii) $2,800,000 in restricted shares of common stock of the Company. The stock was issued in the form of prefunded warrants
in two tranches: (i) $1,700,000 (13,917 prefunded warrants) issued within five days of entry into the PSA, and (ii) $1,100,000 (9,005
prefunded warrants) at closing of the transaction. The first tranche, issued during the first quarter, was voided on March 31, 2023 when
closing of the transaction did not occur. The prefunded warrants per share exercise price was $122.15 which was subject to certain adjustments.
Issuance of all securities in this transaction were exempt from registration under Section 4(a)(2) of the Securities Act of 1933, as
amended. Under the terms of the agreement, Stronghold was to complete certain permitting, zoning, and infrastructure work by March 31,
2023, to close the transaction.
As
at March 31, 2023 the prefunded warrants issued were rescinded and the warrants were rendered null and void as the Company presented
termination notice to Stronghold.
On
October 12, 2023, the Company was served a complaint filed in the Superior Court of California from Stronghold for breach of contract
in relation to the PSA. The Company denies any liability, other than what is already recorded in the financial statements and will vigorously
defend the claims made against the Company.
Berry
People LLC Binding Letter of Intent
On
January 24, the Company announced it has entered a binding letter of intent (“BP LOI”) to acquire Berry People LLC, (“Berry
People”), a berry business with an increasingly international footprint and a scalable business model. The acquisition bolsters
the AgriFORCE™ Brands division and allows the Company to realize commercial synergies with UN(THINK)™.
Berry
People was founded in 2017 by berry industry veterans to create a new platform to meet market demand for a branded, year-round supply
of organic and conventional berries. Berry People quickly established a recognized global trade brand and scalable operations, comprised
of over 200 retail and foodservice clients and over 100 grower and exporter clients across the US, Canada, Mexico, and Peru. Berry People
had net revenues of USD $35.4 (unaudited) million for the year ended December 31, 2022.
The
BP LOI states, among other things that:
| |
● |
the
transaction will be subject to completion of due diligence to the Company’s satisfaction and, after satisfactory due diligence,
the reaching of agreement on the terms of the purchase pursuant to a definitive purchase agreement, including conditions precedent
for closing of the transaction; |
| |
● |
the
parties will sign the definitive purchase agreement no later than April 30, 2023(8), unless agreed to by both parties;
and |
| |
● |
Berry
People will not enter any negotiations with other parties for a period of three months following the execution of the BP LOI. |
(8)
Berry People and the Company mutually agreed to be amended the long stop date to August 31, 2023
The
BP LOI sets forth a proposed purchase price of $28.0 million, consisting of $18.2 million in cash and $9.8 million in AgriFORCE™
restricted shares, which will be paid at closing to acquire 70% of Berry People’s equity interests. Berry People will have the
opportunity for future earnouts during the five years after closing based on future revenue and EBITDA targets associated with agreed
upon growth targets.
In
collaboration with AgriFORCE™, Berry People aims to further develop backward integration into agricultural production via farming
joint ventures and deploy licensed and developed IP as part of a scalable franchising model. The berries market was $9.65 billion in
2021 in the U.S. alone, with growth rates of around 10% or more each year since 2019(9)— a trend that is expected to
continue.
(9)
As per IRI Integrated Fresh, Latest 52 WE 3/20/2022
Recent
Developments
On
July 18, 2023, the Company announced a restructuring of management. Ingo Mueller departed from his position as CEO and Chair of the Board.
Richard Wong was concurrently appointed as interim CEO, and David Welch and John Meekison each assumed the role of Co-Chair of the Board.
Ingo Mueller served as a director of the Company until the shareholder meeting dated September 27, 2023 at which time he was not re-elected
and ceased to serve as a director. The Company is currently evaluating options regarding the appointment of a fulltime CEO.
Employees
As
of December 19, 2023, we had approximately 8 employees and three consultants. We believe our employee relations to be good.
Corporate
Information
AgriFORCE™
Growing Systems Ltd. was incorporated as a private company by Articles of Incorporation issued pursuant to the provisions of the Business
Corporations Act (British Columbia) on December 22, 2017. The Company currently leases office space at 2233 Colombia Street, Suite 300,
Vancouver, B.C., V5Y 0M6 as its principal office. The Company believes the office is in good condition and satisfy its current operational
requirements. On February 13, 2018, the Company changed its name from 1146470 B.C. Ltd to Canivate Growing Systems Ltd. On November 22,
2019 the Company changed its name from Canivate Growing Systems Ltd. to AgriFORCE™ Growing Systems Ltd.
| Use
of proceeds |
|
We
are not selling any shares of our common stock in this offering and we will not receive any of the proceeds from the sale of shares
of our common stock by the selling stockholders. The selling stockholders will receive all of the proceeds from any sales of the
shares of our common stock offered hereby. |
| |
|
|
| Dividend
policy |
|
We
have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and future
earnings, if any, to fund the development and expansion of our business, and we do not anticipate declaring or paying any cash dividends
in the foreseeable future. See “Dividend Policy.” |
| |
|
|
| Risk
factors |
|
You
should read the “Risk Factors” section beginning on page 10 and the other information included in this prospectus for
a discussion of factors to consider before deciding to invest in shares of our Class A common stock |
| |
|
|
| Market
Symbol and trading |
|
Our
common stock is listed on the Nasdaq Capital Market under the symbol “AGRI” and our Series A Warrants under the symbol
“AGRIW”. |
RISK
FACTORS
Investing
in our securities involves a high degree of risk. Before making an investment decision, you should consider carefully the risks, uncertainties
and all risk factors set forth in the applicable prospectus supplement and the documents incorporated by reference in this prospectus,
including the risk factors discussed under the heading “Risk Factors” in our most recent Annual Report on Form 10-K for the
year ended December 31, 2022 and each subsequent filed quarterly report on Form 10-Q and current reports on Form 8-K, which may be amended,
supplemented or superseded from time to time by the other reports we file with the SEC in the future.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements. Such statements include statements regarding our expectations, hopes, beliefs or intentions
regarding the future, including but not limited to statements regarding our market, strategy, competition, development plans (including
acquisitions and expansion), financing, revenues, operations, and compliance with applicable laws. Forward-looking statements involve
certain risks and uncertainties, and actual results may differ materially from those discussed in any such statement. Factors that could
cause actual results to differ materially from such forward-looking statements include the risks described in greater detail in the following
paragraphs. All forward-looking statements in this document are made as of the date hereof, based on information available to us as of
the date hereof, and we assume no obligation to update any forward-looking statement. Market data used throughout this prospectus is
based on published third party reports or the good faith estimates of management, which estimates are based upon their review of internal
surveys, independent industry publications and other publicly available information.
You
should review carefully the section entitled “Risk Factors” within this prospectus for a discussion of these and other risks
that relate to our business and investing in shares of our Common Stock.
All
forward-looking statements speak only as of the date of this prospectus. We disclaim any obligation to update or revise these statements
unless required by law, and you should not place undue reliance on these forward-looking statements. Although we believe that our plans,
intentions and expectations reflected in or suggested by the forward-looking statements we make in this prospectus are reasonable, we
can give no assurance that these plans, intentions or expectations will be achieved. We disclose important factors that could cause our
actual results to differ materially from our expectations under “Risk Factors” and elsewhere in this prospectus. These cautionary
statements qualify all forward-looking statements attributable to us or persons acting on our behalf.
Risks
Relating to the Company’s Business
The
Company is an early stage company with little operating history, a history of losses and the Company cannot assure profitability.
The
Company currently has no revenues and does not have any history of revenue generating operations. The Company has been involved in the
design and development of its CEA FORCEGH+™ facility which incorporates the Company’s AgriFORCE™ micropropagation laboratories,
acquisition and advancement of the UN(THINK)™ foods IP, product base, development of its pilot plant, and transacting with potential
revenue generating acquirees. While the Company has invested considerably in these business plans, no FORCEGH+™ facility has been
constructed to date, the Company has not generated revenue from UN(THINK)™, nor has the Company completed any acquisition of revenue
generating companies. The commercial or operating viability of the Company’s business plans have not been proven. There is no assurance
that the revenue generated from its operations, and if those revenues, when and if generated, will be sufficient to sustain operations,
nonetheless achieve profitability.
There
is no assurance that the Company’s FORCEGH+™ facilities or micropropagation laboratories will operate as intended.
The
Company’s initial state of its business operations will be to construct and deploy its initial FORCEGH+™ facility and micropropagation
laboratories. However, the Company has yet to complete construction of any laboratories. Accordingly, this component of the Company’s
business plan is subject to considerable risks, including:
| |
● |
there
is no assurance that the laboratories will achieve the intended plantlet production rates; |
| |
● |
the
costs of constructing and operating the laboratories may be greater than anticipated; |
| |
● |
the
potential offtake partners who have indicated a willingness to deploy the laboratories at their existing cultivation operations may
withdraw and determine not to deploy the laboratories; |
| |
● |
there
is no assurance that the facilities will deliver the intended benefits of high production yields, lower crop losses and reduced operation
costs; |
| |
● |
if
the company is not able to fully develop the grow house or it does not operate as intended, it could prevent the company from realizing
any of its business goals or achieving profitability; |
| |
● |
the
costs of constructing the grow houses may be greater than anticipated and the Company may not be able to recover these greater costs
through increases in the lease rates, license fees and services fees that it charges to its customers; and |
| |
● |
the
costs of operating the grow house may be greater than anticipated. |
There
is no assurance that UN(THINK)™ will operate as intended.
The
Company’s plans for developing and advancing the UN(THINK)™ are in its preliminary stages. The Company has yet to fully launch
their range of products in either the B2B or D2C channels. Accordingly, this component of the Company’s business plan is subject
to considerable risks, including:
| |
● |
the
costs of constructing and operating the pilot plant may be greater than anticipated; |
| |
● |
the
potential B2B and D2C sales may not achieved the planned levels of sales; |
| |
● |
there
is no assurance that the pilot plant will deliver the planned production levels or scale; |
| |
● |
if
the company is not able to fully develop the pilot plan, it could prevent the company from realizing any of its business goals or
achieving profitability; |
| |
● |
the
costs of operating the AgriFORCE™ pilot plant may be greater than anticipated. |
| |
● |
the
quality of product from the co-manufacturing may not be sufficient. |
| |
● |
the
cost from co-manufacturing may be greater than anticipated. |
| |
● |
the
demand for the products may not be as high as predicted. |
| |
● |
the
pricing of the products may deter potential buyers and may not cover the cost of production. |
| |
● |
the
brand may not attract sufficient volume. |
We
may not realize the anticipated benefits of, and synergies from, acquisitions and may become responsible for certain liabilities and
integration costs as a result.
The
businesses we have proposed to acquire have previously operated independently from us. The proposed integrations of our operations with
the proposed businesses acquisitions are intended to result in financial and operational benefits, and business synergies. There can
be no assurance, however, regarding when or the extent to which we will be able to realize these and other benefits. Integration may
also be difficult, unpredictable, and subject to delay because of possible company culture conflicts, system integrations, regulatory
compliance, and other factors. Difficulties associated with the integration of the proposed business acquisitions could have a material
adverse effect on our business.
Fluctuations
in the exchange rate of foreign currencies could result in losses.
We
incur a portion of our operating expenses in Canadian dollars, and in the future, as we expand into other foreign countries, we expect
to incur operating expenses in other foreign currencies. We are exposed to foreign exchange rate fluctuations as the financial results
of our international operations are translated from the local functional currency into U.S. dollars upon consolidation. A decline in
the U.S. dollar relative to foreign functional currencies would increase our non-U.S. revenue and improve our operating results. Conversely,
if the U.S. dollar strengthens relative to foreign functional currencies, our revenue and operating results would be adversely affected.
We have not previously engaged in foreign currency hedging. If we decide to hedge our foreign currency exchange rate exposure, we may
not be able to hedge effectively due to lack of experience, unreasonable costs or illiquid markets.
The
Company will require additional financing and there is no assurance that additional financing will be available when required.
The
Company will require substantial additional capital in order to execute its business plan. Existing funds will not be sufficient and
additional financing will be needed for this purpose and for other purposes. The Company plans to achieve this additional financing through
equity and/or debt financing which will likely be dilutive to the position of then current shareholders. However, there is no assurance
that this financing will be available at favorable terms, if at all, when required, given the Company’s small asset base and current
lack of revenue.
The
Company had negative cash flow for the year ended December 31, 2022.
The
Company had negative cash flows from operating activities for year ended December 31, 2022. To the extent that the Company has negative
cash flows from operating activities in future periods, it may need to allocate a portion of its cash reserves to fund such negative
cash flow. The Company may also be required to raise additional funds through the issuance of equity or debt securities. There can be
no assurance that the Company will be able to generate a positive cash flow from operating activities, that additional capital or other
types of financing will be available when needed or that these financings will be on terms favorable to the Company. The Company’s
actual financial position and results of operations may differ materially from the expectations of the Company’s management.
The
Company’s actual financial position and results of operations may differ materially from the expectations of the Company’s
management.
The
Company’s actual financial position and results of operations may differ materially from management’s expectations. The process
for estimating the Company’s revenue, net income and cash flow requires the use of judgment in determining the appropriate assumptions
and estimates. These estimates and assumptions may be revised as additional information becomes available and as additional analyses
are performed. In addition, the assumptions used in planning may not prove to be accurate, and other factors may affect the Company’s
financial condition or results of operations. As a result, the Company’s revenue, net income and cash flow may differ materially
from the Company’s projected revenue, net income and cash flow.
The
Company expects to incur significant ongoing costs and obligations related to its investment in infrastructure, growth, regulatory compliance
and operations.
The
Company expects to incur significant ongoing costs and obligations related to its planned investments. To the extent that these costs
may be greater than anticipated or the Company may not be able to generate revenues or raise additional financing to cover these costs,
these operating expenses could have a material adverse impact on the Company’s results of operations, financial condition and cash
flows. In addition, future changes in regulations, more vigorous enforcement thereof or other unanticipated events could increase costs
and have a material adverse effect on the business, results of operations and financial condition of the Company. The Company may not
be able to recover sufficient revenues to offset its higher operating expenses or to recoup its initial capital investment. The Company
may incur significant losses in the future for a number of reasons, including, unforeseen expenses, difficulties, complications and delays,
and other unknown events. If the Company is unable to achieve and sustain profitability, the market price of our securities may significantly
decrease.
There
is no assurance the Company will be able to repatriate or distribute funds for investment from the United States to Canada or elsewhere.
In
the event that any of the Company’s investments, or any proceeds thereof, any dividends or distributions there from, or any profits
or revenues accruing from such investments in the United States were found to be in violation of money laundering legislation or otherwise,
such transactions may be viewed as proceeds of crime under applicable federal laws, rules and regulations or any other applicable legislation.
This could restrict or otherwise jeopardize the ability of the Company to declare or pay dividends, effect other distributions or subsequently
repatriate such funds back to Canada or elsewhere.
The
Company may not be able to effectively manage its growth and operations, which could materially and adversely affect its business.
If
the Company implements it business plan as intended, it may in the future experience rapid growth and development in a relatively short
period of time. The management of this growth will require, among other things, continued development of the Company’s financial
and management controls and management information systems, stringent control of costs, the ability to attract and retain qualified management
personnel and the training of new personnel. The Company intends to utilize outsourced resources, and hire additional personnel, to manage
its expected growth and expansion. Failure to successfully manage its possible growth and development could have a material adverse effect
on the Company’s business and the value of the shares.
The
Company may face significant competition from other facilities.
Many
other businesses in California engage in similar activities to the Company, leasing commercial space to agricultural producers generally,
and providing additional products and services to similar customers. The Company cannot assure you that it will be able to compete successfully
against current and future competitors. Competitive pressures faced by the Company could have a material adverse effect on its business,
operating results and financial condition.
The
Company may face significant competition from other nutritious food companies.
We
face significant competition from other nutritious food companies. Many of our competitions may have established brands, more experience
and competency in the industry, larger fulfillment infrastructure, significantly more marketing and other financial resources, and larger
customers bases than we do. These factors may allow our competitions to achieve greater net sales and profits. The significant competition
faced by the Company could have a material adverse effect on its business, operating results and financial condition.
If
we are unable to protect our intellectual property, our business may be adversely affected.
There
can be no assurance that trade secrets and other intellectual property will not be challenged, invalidated, misappropriated or circumvented
by third parties. Currently, our intellectual property includes provisional patents, patent applications, trademarks, trademark applications
and know-how related to business, product and technology development. We plan on taking the necessary steps, including but not limited
to the filing of additional patents as appropriate. There is no assurance any additional patents will issue or that when they do issue
they will include all of the claims currently included in the applications. Even if they do issue, those new patents and our existing
patents must be protected against possible infringement. Nonetheless, we currently rely on contractual obligations of our employees and
contractors to maintain the confidentiality of our products. To compete effectively, we need to develop and continue to maintain a proprietary
position with respect to our technologies, and business. The risks and uncertainties that we face with respect to intellectual property
rights principally include the following:
| |
● |
Provisional
protection may not result in full patents being granted, and any full patent applications that we file may not result in issued patents
or may take longer than expected to result in issued patents; |
| |
|
|
| |
● |
we
may be subject to interference proceedings; |
| |
|
|
| |
● |
other
companies may claim that patents applied for by, assigned or licensed to, us infringe upon their own intellectual property rights; |
| |
|
|
| |
● |
we
may be subject to trademark opposition proceedings in the U.S. and in foreign countries; |
| |
● |
any
patents that are issued to us may not provide meaningful protection; |
| |
|
|
| |
● |
we
may not be able to develop additional proprietary technologies that are patentable; |
| |
|
|
| |
● |
other
companies may challenge patents licensed or issued to us as invalid, unenforceable or not infringed; |
| |
|
|
| |
● |
other
companies may independently develop similar or alternative technologies, or duplicate our technologies; |
| |
|
|
| |
● |
other
companies may design around technologies that we have licensed or developed; |
| |
|
|
| |
● |
any
patents issued to us may expire and competitors may utilize the technology found in such patents to commercialize their own products;
and |
| |
|
|
| |
● |
enforcement
of patents is complex, uncertain and expensive. |
It
is also possible that others may obtain issued patents that could prevent us from commercializing certain aspects of our products or
require us to obtain licenses requiring the payment of significant fees or royalties in order to enable us to conduct our business. If
we license patents, our rights will depend on maintaining our obligations to the licensor under the applicable license agreement, and
we may be unable to do so. Furthermore, there can be no assurance that the work-for-hire, intellectual property assignment and confidentiality
agreements entered into by our employees and consultants, advisors and collaborators will provide meaningful protection for our trade
secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure of such trade secrets, know- how
or other proprietary information. The scope and enforceability of patent claims are not systematically predictable with absolute accuracy.
The strength of our own patent rights depends, in part, upon the breadth and scope of protection provided by the patent and the validity
of our patents, if any.
We
operate in an industry with the risk of intellectual property litigation. Claims of infringement against us may hurt our business.
Our
success depends, in part, upon non-infringement of intellectual property rights owned by others and being able to resolve claims of intellectual
property infringement without major financial expenditures or adverse consequences. Participants that own, or claim to own, intellectual
property may aggressively assert their rights. From time to time, we may be subject to legal proceedings and claims relating to the intellectual
property rights of others. Future litigation may be necessary to defend us or our clients by determining the scope, enforceability, and
validity of third-party proprietary rights or to establish its proprietary rights. Some competitors have substantially greater resources
and are able to sustain the costs of complex intellectual property litigation to a greater degree and for longer periods of time. In
addition, patent holding companies that focus solely on extracting royalties and settlements by enforcing patent rights may target us.
Regardless of whether claims that we are infringing patents or other intellectual property rights have any merit, these claims are time-consuming
and costly to evaluate and defend and could:
| |
● |
adversely
affect relationships with future clients; |
| |
|
|
| |
● |
cause
delays or stoppages in providing products; |
| |
|
|
| |
● |
divert
management’s attention and resources; |
| |
|
|
| |
● |
require
technology changes to our platform that would cause our Company to incur substantial cost; |
| |
|
|
| |
● |
subject
us to significant liabilities; and |
| |
|
|
| |
● |
require
us to cease some or all business activities. |
In
addition to liability for monetary damages, which may be tripled and may include attorneys’ fees, or, in some circumstances, damages
against clients, we may be prohibited from developing, commercializing, or continuing to provide some or all of our products unless we
obtain licenses from, and pay royalties to, the holders of the patents or other intellectual property rights, which may not be available
on commercially favorable terms, or at all.
We
have limited foreign intellectual property rights and may not be able to protect our intellectual property rights throughout the world.
We
have limited intellectual property rights outside the United States. Filing, prosecuting and defending patents on devices in all countries
throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States
can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property
to the same extent as laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions
in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States
or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patents to develop their own
products and further, may export otherwise infringing products to territories where we have patents, but enforcement is not as strong
as that in the United States.
Many
companies have encountered significant problems in protecting and defending intellectual property in foreign jurisdictions. The legal
systems of certain countries, particularly China and certain other developing countries, do not favor the enforcement of patents, trade
secrets and other intellectual property, which could make it difficult for us to stop the infringement of our patents or marketing of
competing products in violation of our proprietary rights generally. To date, we have not sought to enforce any issued patents in these
foreign jurisdictions. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert
our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly
and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in
any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. The requirements
for patentability may differ in certain countries, particularly developing countries. Certain countries in Europe and developing countries,
including China and India, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties.
In those countries, we and our licensors may have limited remedies if patents are infringed or if we or our licensors are compelled to
grant a license to a third party, which could materially diminish the value of those patents. This could limit our potential revenue
opportunities. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant
commercial advantage from the intellectual property that we develop or license.
If
we are unable to obtain or defend our patents, our business could be materially adversely affected.
Our
patent position is highly uncertain and involves complex legal and factual questions. Accordingly, we cannot predict the breadth of claims
that may be allowed or enforced under our patents or in third-party patents. For example, we might not have been the first to make the
inventions covered by each of our pending patent applications and provisional patents; we might not have been the first to file patent
applications for these inventions; others may independently develop similar or alternative technologies or duplicate any of our technologies;
it is possible that none of our pending patent applications will result in issued patents; our issued patents may not provide a basis
for commercially viable technologies, or may not provide us with any competitive advantages, or may be challenged and invalidated by
third parties; and, we may not develop additional proprietary technologies that are patentable.
As
a result, our owned and licensed patents may not be valid and we may not be able to obtain and enforce patents and to maintain trade
secret protection for the full commercial extent of our technology. The extent to which we are unable to do so could materially harm
our business.
We
have applied for and will continue to apply for patents for certain products. Such applications may not result in the issuance of any
patents, and any patents now held or that may be issued may not provide us with adequate protection from competition. Furthermore, it
is possible that patents issued or licensed to us may be challenged successfully. In that event, if we have a preferred competitive position
because of such patents, such preferred position would be lost. If we are unable to secure or to continue to maintain a preferred position,
we could become subject to competition from the sale of generic products. Failure to receive, inability to protect, or expiration of
our patents would adversely affect our business and operations.
Patents
issued or licensed to us may be infringed by the products or processes of others. The cost of enforcing our patent rights against infringers,
if such enforcement is required, could be significant, and we do not currently have the financial resources to fund such litigation.
Further, such litigation can go on for years and the time demands could interfere with our normal operations. We may become a party to
patent litigation and other proceedings. The cost to us of any patent litigation, even if resolved in our favor, could be substantial.
Many of our competitors may be able to sustain the costs of such litigation more effectively than we can because of their substantially
greater financial resources. Litigation may also absorb significant management time.
Unpatented
trade secrets, improvements, confidential know-how and continuing technological innovation are important to our scientific and commercial
success. Although we attempt to and will continue to attempt to protect our proprietary information through reliance on trade secret
laws and the use of confidentiality agreements with our partners, collaborators, employees and consultants, as well as through other
appropriate means, these measures may not effectively prevent disclosure of our proprietary information, and, in any event, others may
develop independently, or obtain access to, the same or similar information.
International
intellectual property protection is particularly uncertain, and if we are involved in opposition proceedings in foreign countries, we
may have to expend substantial sums and management resources.
Patent
and other intellectual property law outside the United States is more uncertain and is continually undergoing review and revisions in
many countries. Further, the laws of some foreign countries may not protect intellectual property rights to the same extent as the laws
of the United States. For example, certain countries do not grant patent claims that are directed to business methods and processes.
In addition, we may have to participate in opposition proceedings to determine the validity of its foreign patents or its competitors’
foreign patents, which could result in substantial costs and diversion of its efforts and loss of credibility with customers.
If
we are found to be infringing on patents or trade secrets owned by others, we may be forced to cease or alter our product development
efforts, obtain a license to continue the development or sale of our products, and/or pay damages.
Our
processes and potential products may violate proprietary rights of patents that have been or may be granted to competitors, universities
or others, or the trade secrets of those persons and entities. As our industry expands and more patents are issued, the risk increases
that our processes and potential products may give rise to claims that they infringe the patents or trade secrets of others. These other
persons could bring legal actions against us claiming damages and seeking to enjoin manufacturing and marketing of the affected product
or process. If any of these actions are successful, in addition to any potential liability for damages, we could be required to obtain
a license in order to continue to manufacture or market the affected product or use the affected process. Required licenses may not be
available on acceptable terms, if at all, and the results of litigation are uncertain. If we become involved in litigation or other proceedings,
it could consume a substantial portion of our financial resources and the efforts of our personnel.
We
rely on confidentiality agreements to protect our trade secrets. If these agreements are breached by our employees or other parties,
our trade secrets may become known to our competitors.
We
rely on trade secrets that we seek to protect through confidentiality agreements with our employees and other parties. If these agreements
are breached, our competitors may obtain and use our trade secrets to gain a competitive advantage over us. We may not have any remedies
against our competitors and any remedies that may be available to us may not be adequate to protect our business or compensate us for
the damaging disclosure. In addition, we may have to expend resources to protect our interests from possible infringement by others.
We
have a limited operating history on which to judge our business prospects and management.
Our
company was incorporated and commenced operations in 2017. Accordingly, we have only a limited operating history upon which to base an
evaluation of our business and prospects. Operating results for future periods are subject to numerous uncertainties and we cannot assure
you that we will achieve or sustain profitability. Our prospects must be considered in light of the risks encountered by companies in
the early stage of development, particularly companies in new and rapidly evolving markets. Future operating results will depend upon
many factors, including increasing the number of affiliates, our success in attracting and retaining motivated and qualified personnel,
our ability to establish short term credit lines, our ability to develop and market new products, control costs, and general economic
conditions. We cannot assure you that we will successfully address any of these risks.
We
may not be able to continue as a going concern.
The
Company has incurred substantial operating losses since its inception and expects to continue to incur significant operating losses for
the foreseeable future and may never become profitable. As reflected in the financial statements, the Company had an accumulated deficit
of approximately $32.8 million at December 31, 2022, a net loss of approximately $12.9 million, and approximately $12.1 million of net
cash used in operating activities for the year ended December 31, 2022. The accompanying financial statements have been prepared on a
going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The
financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the
amounts and classification of liabilities that might result from the outcome of this uncertainty. The Company anticipates incurring additional
losses until such time, if ever, that it can obtain marketing approval to sell, and then generate significant sales, of its technology
that is currently in development. As such it is likely that additional financing will be needed by the Company to fund its operations
and to develop and commercialize its technology. These factors raise substantial doubt about the Company’s ability to continue
as a going concern. The Company is seeking additional financing to support its growth plans. The sale of additional equity may dilute
existing shareholders and newly issued shares may contain senior rights and preferences compared to currently outstanding common shares.
Our
management team will be required to devote substantial time to regulatory compliance which may divert our attention from the day-to-day
management of our business.
Our
management team will require substantial attention from our senior management and could divert our attention away from the day-to-day
management of our business. Regulatory compliance is increasingly complex and management may not have experience in all areas of public
company compliance. The management team will seek assistance from external resources when appropriate for public company regulatory compliance
and tax regulatory compliance for applicable jurisdictions.
The
Company may become subject to litigation, which may have a material adverse effect on the Company’s reputation, business, results
from operations, and financial condition.
The
Company may be named as a defendant in a lawsuit or regulatory action. The Company may also incur uninsured losses for liabilities which
arise in the ordinary course of business, or which are unforeseen, including, but not limited to, employment liability and business loss
claims. Any such losses could have a material adverse effect on the Company’s business, results of operations, sales, cash flow
or financial condition.
If
the Company is unable to attract and retain key personnel, it may not be able to compete effectively.
The
Company’s success has depended and continues to depend upon its ability to attract and retain key management, including the Company’s
Chief Executive Officer and technical experts. The Company will attempt to enhance its management and technical expertise by continuing
to recruit qualified individuals who possess desired skills and experience in certain targeted areas. The Company’s inability to
retain employees and attract and retain sufficient additional employees or engineering and technical support resources could have a material
adverse effect on the Company’s business, results of operations, sales, cash flow or financial condition. Shortages in qualified
personnel or the loss of key personnel could adversely affect the financial condition of the Company, results of operations of the business
and could limit the Company’s ability to develop and market its intellectual property. The loss of any of the Company’s senior
management or key employees could materially adversely affect the Company’s ability to execute the Company’s business plan
and strategy, and the Company may not be able to find adequate replacements on a timely basis, or at all. The Company does not maintain
key person life insurance policies on any of the Company’s employees.
The
size of the Company’s initial target market is difficult to quantify and investors will be reliant on their own estimates on the
accuracy of market data.
Because
high growth crop technology is in an early stage with uncertain boundaries, there is a lack of information about comparable companies
available for potential investors to review in deciding about whether to invest in the Company and, few, if any, established companies
whose business model the Company can follow or upon whose success the Company can build. Accordingly, investors will have to rely on
their own estimates in deciding about whether to invest in the Company. There can be no assurance that the Company’s estimates
are accurate or that the market size is sufficiently large for its business to grow as projected, which may negatively impact its financial
results. The Company regularly follows market research.
The
Company’s industry is experiencing rapid growth and consolidation that may cause the Company to lose key relationships and intensify
competition.
The
agriculture industry and various verticals within it are undergoing rapid growth and substantial change, which has resulted in an increase
in competitors, consolidation and formation of strategic relationships. Acquisitions or other consolidating transactions could harm the
Company in a number of ways, including by losing strategic partners and or customers if they are acquired by or enter into relationships
with a competitor, losing customers, revenue and market share, or forcing the Company to expend greater resources to meet new or additional
competitive threats, all of which could harm the Company’s operating results. As competitors enter the market and become increasingly
sophisticated, competition in the Company’s industry may intensify which could negatively impact its profitability.
The
Company will be reliant on information technology systems and may be subject to damaging cyberattacks.
The
Company’s operations depend, in part, on how well it and its suppliers protect networks, equipment, information technology systems
and software against damage from a number of threats, including, but not limited to, cable cuts, damage to physical plants, natural disasters,
intentional damage and destruction, fire, power loss, hacking, computer viruses, vandalism and theft. The Company’s operations
also depend on the timely maintenance, upgrade and replacement of networks, equipment, IT systems and software, as well as pre-emptive
expenses to mitigate the risks of failures. Any of these and other events could result in information system failures, delays and/or
increase in capital expenses. The failure of information systems or a component of information systems could, depending on the nature
of any such failure, adversely impact the Company’s reputation and results of operations.
The
Company has not experienced any material losses to date relating to cyber-attacks or other information security breaches, but there can
be no assurance that the Company will not incur such losses in the future. The Company’s risk and exposure to these matters cannot
be fully mitigated because of, among other things, the evolving nature of these threats. As a result, cyber security and the continued
development and enhancement of controls, processes and practices designed to protect systems, computers, software, data and networks
from attack, damage or unauthorized access is a risk. As cyber threats continue to evolve, the Company may be required to expend additional
resources to continue to modify or enhance protective measures or to investigate and remediate any security vulnerabilities.
The
Company’s officers and directors may be engaged in a range of business activities resulting in conflicts of interest.
Although
certain officers and board members of the Company are expected to be bound by anti-circumvention agreements limiting their ability to
enter into competing and/or conflicting ventures or businesses, the Company may be subject to various potential conflicts of interest
because some of its officers and directors may be engaged in a range of business activities. In addition, the Company’s executive
officers and directors may devote time to their outside business interests, so long as such activities do not materially or adversely
interfere with their duties to the Company. In some cases, the Company’s executive officers and directors may have fiduciary obligations
associated with these business interests that interfere with their ability to devote time to the Company’s business and affairs
and that could adversely affect the Company’s operations. These business interests could require significant time and attention
of the Company’s executive officers and directors.
In
addition, the Company may also become involved in other transactions which conflict with the interests of its directors and the officers
who may from time to time deal with persons, firms, institutions or companies with which the Company may be dealing, or which may be
seeking investments similar to those desired by it. The interests of these persons could conflict with those of the Company. In addition,
from time to time, these persons may be competing with the Company for available investment opportunities. Conflicts of interest, if
any, will be subject to the procedures and remedies provided under applicable laws. In particular, if such a conflict of interest arises
at a meeting of the Company’s directors, a director who has such a conflict will abstain from voting for or against the approval
of such participation or such terms. In accordance with applicable laws, the directors of the Company are required to act honestly, in
good faith and in the best interests of the Company.
There
is no guarantee that how the Company uses its available funds will yield the expected results or returns which could impact the business
and financial condition of the Company.
The
Company cannot specify with certainty the particular uses of available funds. Management has broad discretion in the application of its
proceeds. Accordingly, a holder of shares will have to rely upon the judgment of management with respect to the use of available funds,
with only limited information concerning management’s specific intentions. The Company’s management may spend a portion or
all of the available funds in ways that the Company’s shareholders might not desire, that might not yield a favorable return and
that might not increase the value of a purchaser’s investment. The failure by management to apply these funds effectively could
harm the Company’s business. Pending use of such funds, the Company might invest the available funds in a manner that does not
produce income or that loses value.
Our
Articles of incorporation, by-laws and certain Canadian legislation, contain provisions that may have the effect of delaying or preventing
a change in control.
Certain
provisions of our by-laws, together or separately, could discourage potential acquisition proposals, delay or prevent a change in control
and limit the price that certain investors may be willing to pay for our common shares. For instance, our by-laws contain provisions
that establish certain advance notice procedures for nomination of candidates for election as directors at shareholders’ meetings.
The
Investment Canada Act requires any person that is non-Canadian (as defined in the Investment Canada Act) who acquires “control”
(as defined in the Investment Canada Act) of an existing Canadian business to file either a pre-closing application for review
or notification with Innovation, Science and Economic Development Canada. An acquisition of control is a reviewable transaction where
prescribed financial thresholds are exceeded. The Investment Canada Act generally prohibits the implementation of a reviewable
transaction unless, after review, the relevant Minister is satisfied that the acquisition is likely to be of net benefit to Canada. Under
the national security regime in the Investment Canada Act, the federal government may undertake a discretionary review of a broader
range of investments by a non-Canadian to determine whether such an investment by a non-Canadian could be “injurious to national
security.” Review on national security grounds is at the discretion of the federal government and may occur on a pre- or post-closing
basis.
Furthermore,
limitations on the ability to acquire and hold our common shares may be imposed by the Competition Act (Canada). This legislation
permits the Commissioner of Competition to review any acquisition or establishment, directly or indirectly, including through the acquisition
of shares, of control over or of a significant interest in us. This legislation grants the Commissioner of Competition jurisdiction,
for up to one year, to challenge this type of acquisition before the Canadian Competition Tribunal on the basis that it would, or would
be likely to, substantially prevent or lessen competition. This legislation also requires any person who intends to acquire our common
shares to file a notification with the Canadian Competition Bureau if (i) that person (and their affiliates) would hold, in the aggregate,
more than 20% of all of our outstanding voting shares, (ii) certain financial thresholds are exceeded, and (iii) no exemption applies.
Where a person (and their affiliates) already holds, in the aggregate, more than 20% of all of our outstanding voting shares, a notification
must be filed if (i) the acquisition of additional shares would bring that person’s (and their affiliates) holdings to over 50%,
(ii) certain financial thresholds are exceeded and (iii) no exemption applies. Where a notification is required, the legislation prohibits
completion of the acquisition until the expiration of the applicable statutory waiting period, unless compliance with the waiting period
has been waived or the Commissioner of Competition provides written notice that he does not intend to challenge the acquisition. The
Commissioner of Competition’s review of a notifiable transaction for substantive competition law considerations may take longer
than the statutory waiting period.
We
are governed by the corporate laws of British Columbia, Canada which in some cases have a different effect on shareholders than the corporate
laws of the United States.
We
are incorporated under the Business Corporations Act (British Columbia) (the “BC Act”) and other relevant laws, which
may affect the rights of shareholders differently than those of a company governed by the laws of a U.S. jurisdiction, and may, together
with our charter documents, have the effect of delaying, deferring or discouraging another party from acquiring control of our company
by means of a tender offer, a proxy contest or otherwise, or may affect the price an acquiring party would be willing to offer in such
an instance. The material differences between the BC Act and Delaware General Corporation Law (“DGCL”) that may have the
greatest such effect include, but are not limited to, the following: (i) for certain corporate transactions (such as mergers and amalgamations
or amendments to our articles) the BC Act generally requires the voting threshold to be a special resolution approved by 66 2/3% of shareholders,
or as set out in the articles, as applicable, whereas DGCL generally only requires a majority vote; and (ii) under the BC Act a holder
of 5% or more of our common shares can requisition a special meeting of shareholders, whereas such right does not exist under the DGCL.
We cannot predict whether investors will find our company and our common shares less attractive because we are governed by foreign laws.
Risks
Related to the Ownership of Our Common Shares
New
laws, regulations, and standards relating to corporate governance and public disclosure may create uncertainty for public companies,
increasing legal and financial compliance costs and making some activities more time consuming.
These
laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result,
may evolve over time as new guidance is provided by the courts and other bodies. This could result in continuing uncertainty regarding
compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. If our efforts to comply
with new laws, regulations, and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related
to their application and practice, regulatory authorities may initiate legal proceedings against us and our business may be adversely
affected.
As
a public company subject to these rules and regulations, we may find it more expensive for us to obtain director and officer liability
insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could
also make it more difficult for us to attract and retain qualified members of our Board of Directors, particularly to serve on its audit
committee and compensation committee, and qualified executive officers.
The
market price of our common shares and Series A Warrants may be volatile, and you may not be able to resell your common shares and Series
A Warrants at or above the acquisition price.
The
market price for our common shares and Series A Warrants may be volatile and subject to wide fluctuations in response to factors including
the following:
| |
● |
actual
or anticipated fluctuations in our quarterly or annual operating results; |
| |
|
|
| |
● |
changes
in financial or operational estimates or projections; |
| |
|
|
| |
● |
conditions
in markets generally; |
| |
|
|
| |
● |
changes
in the economic performance or market valuations of companies similar to ours; |
| |
|
|
| |
● |
general
economic or political conditions in the United States or elsewhere; |
| |
|
|
| |
● |
any
delay in development of our products or services; |
| |
|
|
| |
● |
failure
to comply with regulatory requirements; |
| |
|
|
| |
● |
inability
to commercially launch products and services and market and generate sales of our products and services, |
| |
|
|
| |
● |
developments
or disputes concerning intellectual property rights; |
| |
|
|
| |
● |
our
or our competitors’ technological innovations; |
| |
|
|
| |
● |
general
and industry-specific economic conditions that may affect our expenditures; |
| |
|
|
| |
● |
changes
in market valuations of similar companies; |
| |
|
|
| |
● |
announcements
by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures, capital commitments, new
technologies, or patents; |
| |
|
|
| |
● |
future
sales of our common shares or other securities, including shares issuable upon the exercise of outstanding warrants or convertible
securities or otherwise issued pursuant to certain contractual rights; |
| |
|
|
| |
● |
period-to-period
fluctuations in our financial results; and |
| |
|
|
| |
● |
low
or high trading volume of our common shares due to many factors, including the terms of our financing arrangements. |
In
addition, if we fail to reach an important research, development or commercialization milestone or result by a publicly expected deadline,
even if by only a small margin, there could be significant impact on the market price of our common shares. Additionally, as we approach
the announcement of anticipated significant information and as we announce such information, we expect the price of our common shares
to be particularly volatile and negative results would have a substantial negative impact on the price of our common shares and Series
A Warrants.
In
addition, in recent years, the stock market in general has experienced extreme price and volume fluctuations. This volatility has had
a significant effect on the market price of securities issued by many companies, including for reasons unrelated to their operating performance.
These broad market fluctuations may adversely affect our stock price, notwithstanding our operating results. The market price of our
common shares and Series A Warrants will fluctuate and there can be no assurances about the levels of the market prices for our common
shares and Series A Warrants.
In
some cases, following periods of volatility in the market price of a company’s securities, shareholders have often instituted class
action securities litigation against those companies. Such litigation, if instituted, could result in substantial costs and diversion
of management attention and resources, which could significantly harm our business operations and reputation.
As
an “emerging growth company” under applicable law, we will be subject to lessened disclosure requirements, which could leave
our shareholders without information or rights available to shareholders of more mature companies.
For
as long as we remain an “emerging growth company” as defined in the JOBS Act, we have elected to take advantage of certain
exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies”
including, but not limited to:
| |
● |
not
being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| |
|
|
| |
● |
being
permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements,
with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
disclosure; |
| |
|
|
| |
● |
reduced
disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; |
| |
|
|
| |
● |
taking
advantage of an extension of time to comply with new or revised financial accounting standard; and |
| |
|
|
| |
● |
exemptions
from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute
payments not previously approved. |
We
expect to take advantage of these reporting exemptions until we are no longer an “emerging growth company.” Because of these
lessened regulatory requirements, our shareholders would be left without information or rights available to shareholders of more mature
companies. We cannot predict whether investors will find our common shares less attractive if we rely on these exemptions. If some investors
find our common shares less attractive as a result, there may be a less active trading market for our common shares and our stock price
may be more volatile.
We
are also a “smaller reporting company” as defined in Rule 12b-2 of the Exchange Act and have elected to follow certain scaled
disclosure requirements available to smaller reporting companies.
Because
we have elected to use the extended transition period for complying with new or revised accounting standards for an “emerging growth
company” our financial statements may not be comparable to companies that comply with public company effective dates.
We
have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1) of
the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have different effective dates
for public and private companies until those standards apply to private companies. As a result of this election, our financial statements
may not be comparable to companies that comply with public company effective dates and may contain less or more modified disclosure than
those public companies. Because our financial statements may not be comparable to companies that comply with public company effective
dates, investors may have difficulty evaluating or comparing our business, performance or prospects in comparison to other public companies,
which may have a negative impact on the value and liquidity of our common shares.
FINRA
sales practice requirements may also limit your ability to buy and sell our common shares, which could depress the price of our shares.
Financial
Industry Regulatory Authority, Inc. (FINRA) rules require broker-dealers to have reasonable grounds for believing that an investment
is suitable for a customer before recommending that investment to the customer. Prior to recommending speculative low-priced securities
to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial
status, tax status and investment objectives, among other things. Under interpretations of these rules, FINRA believes that there is
a high probability such speculative low-priced securities will not be suitable for at least some customers. Thus, FINRA requirements
make it more difficult for broker-dealers to recommend that their customers buy our common shares, which may limit your ability to buy
and sell our shares, have an adverse effect on the market for our shares, and thereby depress our share price.
If
research analysts do not publish research about our business or if they issue unfavorable commentary or downgrade our common shares or
Series A Warrants, our securities’ price and trading volume could decline.
The
trading market for our securities may depend in part on the research and reports that research analysts publish about us and our business.
If we do not maintain adequate research coverage, or if any of the analysts who cover us downgrade our stock or publish inaccurate or
unfavorable research about our business, the price of our common shares and Series A Warrants could decline. If one or more of our research
analysts ceases to cover our business or fails to publish reports on us regularly, demand for our securities could decrease, which could
cause the price of our common shares and Series A Warrants or trading volume to decline.
We
may issue additional equity securities, or engage in other transactions that could dilute our book value or relative rights of our common
shares, which may adversely affect the market price of our common shares and Series A Warrants.
Our
Board of Directors may determine from time to time that it needs to raise additional capital by issuing additional shares of our common
shares or other securities. Except as otherwise described in this filing, we will not be restricted from issuing additional common shares,
including securities that are convertible into or exchangeable for, or that represent the right to receive common shares. Because our
decision to issue securities in any future offering will depend on market conditions and other factors beyond our control, we cannot
predict or estimate the amount, timing, or nature of any future offerings, or the prices at which such offerings may be affected. Additional
equity offerings may dilute the holdings of existing shareholders or reduce the market price of our common shares and Series A Warrants,
or all of them. Holders of our securities are not entitled to pre-emptive rights or other protections against dilution. New investors
also may have rights, preferences and privileges that are senior to, and that adversely affect, then-current holders of our securities.
Additionally, if we raise additional capital by making offerings of debt or preference shares, upon our liquidation, holders of our debt
securities and preference shares, and lenders with respect to other borrowings, may receive distributions of its available assets before
the holders of our common shares.
An
investment in our Series A Warrants is speculative in nature and could result in a loss of your investment therein.
The
Series A Warrants do not confer any rights of common share ownership on their holders, such as voting rights or the right to receive
dividends, but rather merely represent the right to acquire shares of our common shares at a fixed price for a limited period of time.
Specifically, commencing on the date of issuance, holders of the Series A Warrants may exercise their right to acquire the common shares
and pay an exercise price of $6.00 per share, prior to three years from the date of issuance, after which date any unexercised Series
A Warrants will expire and have no further value. Moreover, the market value of the Series A Warrants is uncertain and there can be no
assurance that the market value of the Series A Warrants will equal or exceed their initial price. There can be no assurance that the
market price of the common shares will ever equal or exceed the exercise price of the Series A Warrants, and consequently, whether it
will ever be profitable for holders of the Series A Warrants to exercise the Series A Warrants.
Our
Series A Warrants and contain a provision which only permits securities claims to be brought in federal court.
Section
11 of our Series A Warrants states in relevant part: “The Company hereby irrevocably submits to the exclusive jurisdiction of the
state and federal courts sitting in The City of New York, Borough of Manhattan (except for claims brought under the Securities Act of
1933, as amended, and the Securities Exchange Act of 1934, as amended, which must be brought in federal court)”. Therefore any
claims with respect to our Series A Warrants brought under the Securities Act of 1933 or the Securities Exchange Act must be brought
in federal court while all other claims may be brought in federal or state court. Proceedings in federal court may be more expensive
than in state court due to more comprehensive rules on how discovery and motion and trial practice are handled. This provision may have
a dampening effect on claims brought under these securities laws or limit the ability of the investor to bring a claim in the jurisdiction
it deems more favorable. This provision is likely enforceable as requirements regarding bringing securities claims have been met, but
it may have the overall effect of discouraging litigation due to the circumstances described herein.
We
do not currently intend to pay dividends on our common shares in the foreseeable future, and consequently, your ability to achieve a
return on your investment will depend on appreciation in the price of our common shares.
We
have never declared or paid cash dividends on our common shares and do not anticipate paying any cash dividends to holders of our common
shares in the foreseeable future. Consequently, investors must rely on sales of their common shares after price appreciation, which may
never occur, as the only way to realize any future gains on their investments. There is no guarantee that our common shares will appreciate
in value or even maintain the price at which our shareholders have purchased their shares.
USE
OF PROCEEDS
We
are not selling any shares of our common stock in this offering and we will not receive any of the proceeds from the sale of shares of
our common stock by the selling stockholders. The selling stockholders will receive all of the proceeds from any sales of the shares
of our common stock offered hereby. However, we will incur expenses in connection with the registration of the shares of our common stock
offered hereby.
MARKET
FOR OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Market
information
Our
common stock is currently quoted on Nasdaq Capital Market under the symbol “AGRI”, and warrants under the symbol “AGRIW”.
Trading in our common stock has historically lacked consistent volume, and the market price has been volatile.
On
December 19, 2023, the closing price for our common stock as reported on the Nasdaq Capital Market was $0.58 per share.
Securities
outstanding and holders of record
On
December 19, 2023, there were approximately 5467 shareholders of record for our common stock and AGRI shares of our common stock
issued and outstanding.
Dividend
Policy
We
have never paid any cash dividends on our common shares. However, we have paid common share dividends on our preferred stock. Our preferred
stock was retired and there were no preferred shares outstanding after the IPO. We anticipate that we will retain funds and future earnings
to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends on
our common shares in the foreseeable future following this offering. Any future determination to pay cash dividends on our common shares
will be at the discretion of our Board of Directors and will depend on our financial condition, results of operations, capital requirements
and other factors that our Board of Directors deems relevant. In addition, the terms of any future debt or credit financings may preclude
us from paying dividends.
Information
respecting equity compensation plans
The
Company adopted a stock option plan originally on December 12, 2018 (the “Option Plan”), as amended, under which the compensation
committee of the Board (the “Compensation Committee”) may from time to time in its discretion, recommend changes to the Option
Plan to grant to directors, officers, employees and consultants of the Company non-transferable options to purchase common shares (“Options”).
The Board of Directors review recommendations and approve changes. As of the date of this filling, the Company has 76,112 Options outstanding.
The Option Plan was approved by the shareholders of the Company on June 10, 2019.
The
following table provides information with respect to options outstanding under our Plan as at December 31, 2022:
| Plan category | |
Number of securities to be issued upon exercise of outstanding options | | |
Weighted- average exercise price of outstanding options | | |
Number of securities remaining available for future issuance | |
| | |
| | |
| | |
| |
| Equity compensation plans approved by security holders | |
| 27,653 | | |
$ | 165.00 | | |
| 8,640 | |
| Equity compensation plans not approved by security holders | |
| - | | |
| - | | |
| - | |
| Total | |
| 27,653 | | |
$ | 165.00 | | |
| 8,640 | |
Corporate
Structure
The
Company currently has the following wholly-owned subsidiaries, which perform the following functions – AgriFORCE Investments holds
the Company’s U.S. investments, West Pender Holdings retains real estate assets, West Pender Management is a management company,
AGI IP holds the Company’s intellectual property in the U.S., un(Think) Food Company will manufacture food products in the U.S.
and un(Think) Food Company Canada Ltd. manufactures food products in Canada:
| Name
of Subsidiary |
|
Jurisdiction
of Incorporation |
|
Date
of Incorporation |
| AgriFORCE
Investments Inc. (US) |
|
Delaware |
|
April
9, 2019 |
| West
Pender Holdings, Inc. |
|
Delaware |
|
September
1, 2018 |
| AGI
IP Co. |
|
Nevada |
|
March
5, 2020 |
| West
Pender Management Co. |
|
Nevada |
|
July
9, 2019 |
| un(Think)
Food Company |
|
Nevada |
|
June
20, 2022 |
| un(Think)
Food Company Canada Ltd.* |
|
British
Columbia |
|
December
4, 2019 |
| *
|
un(Think)
Food Company Canada Ltd. changed its name from Daybreak AG Systems Ltd. during the year ended December 31, 2022. |
Recent
Debt Financing
On
June 30, 2022, the Company entered into security purchase agreements with certain accredited investors (the “Convertible Debt Investors”)
for the purchase of $14,025,000 in convertible debentures (the “Debentures”) due December 31, 2024. The interest rates on
the Debentures are 5% for the first 12 months, 6% for the subsequent 12 months, and 8% per annum thereafter. Principal repayments will
be made in 25 equal monthly installments and began on September 1, 2022. The Debenture may be extended by six months at the election
of the Company by paying a sum equal to six months interest on the principal amount outstanding at the end of the 18th month,
at the rate of 8% per annum. The Debentures are convertible into common shares at $111.00 per share. The Convertible Debt Investors have
the right to purchase additional tranches of $5,000,000 each, up to a total additional principal amount of $33,000,000. In addition,
the Convertible Debt Investors received 82,128 warrants at a strike price of $122.10, which expire on December 31, 2025 (the “Debenture
Warrants”). The Debenture Warrants and Debentures each have down round provisions whereby the conversion and strike prices will
be adjusted downward if the Company issues equity instruments at lower prices.
On
January 17, 2023, the Convertible Debt Investors purchased an additional tranche of $5,076,923. The convertible debt and warrants were
issued with an exercise price of $62.00. The issuance of the additional tranche triggered the down round provision, adjusting the exercise
prices of the Debentures and the Debenture Warrants to $62.00.
Pioneer
provided notice on October 17, 2023 to purchase an additional Debenture and warrants in the amount of $2,750,000. The conversion price
of the new debenture and exercise price of the new warrants has been set at $2.62 (based on the Nasdaq Official Closing Price on October
16, 2023), and the conversion price of all existing debentures and warrants has been set at $2.62. The floor price has been set at $0.52.
Pioneer
provided notice on November 30, 2023 to purchase an additional Debenture and warrants in the amount of $2,750,000. Pursuant to the terms
of the June 30, 2022 Securities Purchase Agreement, the conversion price of the new debenture and exercise price of the new warrants
was then automatically reset at $0.90 (based on the Nasdaq Official Closing Price on November 29, 2023), and the conversion price of
all existing debentures and warrants has been set at $0.90. The floor price has been set at $0.18.
Intellectual
Property
The
Company’s intellectual property rights are important to its business. In accordance with industry practice, the Company protects
its proprietary products, technology and its competitive advantage through a combination of contractual provisions and trade secret,
copyright and trademark laws in Canada, the United States and in other jurisdictions in which it conducts its business. The Company also
has confidentiality agreements, assignment agreements and license agreements with employees and third parties, which limit access to
and use of its intellectual property.
Patent
Applications
| Patent
Application # | |
Application
Date | |
Expiry
Date | |
Title | |
Case
Status | |
Country |
| 2001/2096 | |
26-Aug-2020 | |
26-Aug-2040 | |
AUTOMATED
GROWING SYSTEMS | |
Pending | |
Barbados |
| 3151492 | |
26-Aug-2020 | |
26-Aug-2040 | |
AUTOMATED
GROWING SYSTEMS | |
Pending | |
Canada |
| 202080073940.7 | |
26-Aug-2020 | |
| |
AUTOMATED
GROWING SYSTEMS | |
Pending | |
China |
| 20858811.1 | |
26-Aug-2020 | |
26-Aug-2040 | |
AUTOMATED
GROWING SYSTEMS | |
Pending | |
European
Patent Office |
| TT/A/2022/00024 | |
26-Aug-2020 | |
| |
AUTOMATED
GROWING SYSTEMS | |
Abandoned
(p) | |
Trinidad
& Tobago |
| 11528859 | |
26-Aug-2020 | |
26-Aug-2040 | |
AUTOMATED
GROWING SYSTEMS | |
Registered | |
United
States |
| 17/983109 | |
08-Nov-2022 | |
| |
AUTOMATED
GROWING SYSTEMS | |
Pending | |
United
States |
| PCT/CA2023/051251 | |
21-Sep-2023 | |
| |
PROCESS
AND SYSTEM FOR GROWING PLANTS USING CLONE TO FLOWER MODEL | |
Pending | |
Patent
Cooperation Treaty |
| 2018215090 | |
31-Jan-2018 | |
31-Jan-2038 | |
HIGH
FIBER, HIGH PROTEIN, LOW CARBOHYDRATE FLOUR AND POWER JUICE AND METHODS FOR PRODUCTION THEREOF | |
Pending | |
Australia |
| 3051860 | |
31-Jan-2018 | |
31-Jan-2038 | |
HIGH
FIBER, HIGH PROTEIN, LOW CARBOHYDRATE FLOUR AND POWER JUICE AND METHODS FOR PRODUCTION THEREOF | |
Pending | |
Canada |
| 18747157.8 | |
31-Jan-2018 | |
| |
HIGH
FIBER, HIGH PROTEIN, LOW CARBOHYDRATE FLOUR AND POWER JUICE AND METHODS FOR PRODUCTION THEREOF | |
Pending | |
European
Patent Office |
| 201917032603 | |
31-Jan-2018 | |
| |
HIGH
FIBER, HIGH PROTEIN, LOW CARBOHYDRATE FLOUR AND POWER JUICE AND METHODS FOR PRODUCTION THEREOF | |
Pending | |
India |
| 755792 | |
31-Jan-2018 | |
31-Jan-2038 | |
HIGH
FIBER, HIGH PROTEIN, LOW CARBOHYDRATE FLOUR AND POWER JUICE AND METHODS FOR PRODUCTION THEREOF | |
Pending | |
New
Zealand |
| 11540538 | |
31-Jan-2018 | |
31-Jan-2038 | |
HIGH
FIBER, HIGH PROTEIN, LOW CARBOHYDRATE FLOUR, SWEETENED LIQUID, SWEETENERS, CEREALS, AND METHODS FOR PRODUCTION THEREOF | |
Registered | |
United
States |
| 17/963690 | |
11-Oct-2022 | |
| |
HIGH
FIBER, HIGH PROTEIN, LOW CARBOHYDRATE FLOUR, SWEETENED LIQUID, SWEETENERS, CEREALS, AND METHODS FOR PRODUCTION THEREOF | |
Application
filed | |
United
States |
| 2001/2057 | |
06-Mar-2020 | |
06-Mar-2040 | |
STRUCTURES
FOR GROWING
PLANTS | |
Pending | |
Barbados |
| 3132672 | |
06-Mar-2020 | |
06-Mar-2040 | |
STRUCTURES
FOR GROWING
PLANTS | |
Granted | |
Canada |
| CN202080033944.2 | |
06-Mar-2020 | |
| |
STRUCTURES
FOR GROWING
PLANTS | |
Pending | |
China |
| 20765629.9 | |
06-Mar-2020 | |
06-Mar-2040 | |
STRUCTURES
FOR GROWING
PLANTS | |
Pending | |
European
Patent Office |
| TT/A/2021/00093 | |
06-Mar-2020 | |
| |
STRUCTURES
FOR GROWING
PLANTS | |
Abandoned
(p) | |
Trinidad
& Tobago |
| 11582918 | |
06-Mar-2020 | |
06-Mar-2040 | |
STRUCTURES
FOR GROWING PLANTS | |
Registered | |
United
States |
| 18/096417 | |
12-Jan-2023 | |
| |
STRUCTURES
FOR GROWING PLANTS | |
Application
allowed | |
United
States |
Trademarks
| Application # | |
Application Date | |
Expiry Date | |
Title | |
Case Status | |
Country |
| 1997835 | |
26-Nov-2019 | |
| |
AGRIFORCE | |
In examination | |
Canada |
| 018243244 | |
22-May-2020 | |
| |
AGRIFORCE | |
Registered | |
European
Union Intellectual Property Office |
| UK00918243244 | |
22-May-2020 | |
| |
AGRIFORCE | |
Registered | |
United
Kingdom |
| 88/930218 | |
22-May-2020 | |
| |
AGRIFORCE | |
Suspended | |
United
States |
| 2044675 | |
07-Aug-2020 | |
| |
FORCEFILM | |
TM Application filed | |
Canada |
| 018389838 | |
04-Feb-2021 | |
| |
FORCEFILM | |
Registered | |
European
Union Intellectual Property Office |
| 90/124842 | |
19-Aug-2020 | |
| |
FORCEFILM | |
Suspended | |
United
States |
| 2127781 | |
18-Aug-2021 | |
| |
UN(THINK) | |
TM Application filed | |
Canada |
| 018572674 | |
06-Oct-2021 | |
| |
UN(THINK) | |
Application filed | |
European
Union Intellectual Property Office |
| 1669126 | |
18-Feb-2022 | |
| |
UN(THINK) | |
Pending | |
Madrid
Protocol (TM) |
| 90/897689 | |
23-Aug-2021 | |
| |
UN(THINK) | |
Suspended | |
United
States |
| 2196090 | |
06-Jul-2022 | |
| |
C2F | |
TM Application filed | |
Canada |
| 97/495313 | |
08-Jul-2022 | |
| |
C2F | |
Suspended | |
United
States |
| 2198964 | |
20-Jul-2022 | |
| |
AWAKENED GRAINS | |
TM Application filed | |
Canada |
| 97/527128 | |
29-Jul-2022 | |
| |
AWAKENED GRAINS | |
Suspended | |
United
States |
| 2207782 | |
02-Sep-2022 | |
| |
FORCEGH+ | |
TM Application filed | |
Canada |
| 97/605026 | |
23-Sep-2022 | |
| |
FORCEGH+ | |
Suspended | |
United
States |
| 2243222 | |
02-Mar-2023 | |
| |
AWAKENED FLOUR | |
TM Application filed | |
Canada |
| 1752858 | |
01-Sep-2023 | |
| |
AWAKENED FLOUR | |
Registered | |
Madrid
Protocol (TM) |
| 97/824500 | |
06-Mar-2023 | |
| |
AWAKENED FLOUR | |
Suspended | |
United
States |
| TMA1175334 | |
24-Jan-2019 | |
| |
PLANET LOVE | |
Registered | |
Canada |
| UK00801504091 | |
24-Jul-2019 | |
| |
PLANET LOVE | |
Registered | |
United
Kingdom |
| 1504091 | |
24-Jul-2019 | |
| |
PLANET LOVE | |
Registered | |
Madrid
Protocol (TM) |
| 6197554 | |
24-Jul-2019 | |
| |
PLANET LOVE | |
Registered | |
United
States |
| UK00801494234 | |
30-Aug-2019 | |
| |
CANIVATE | |
Registered | |
United
Kingdom |
| 1494234 | |
30-Aug-2019 | |
| |
CANIVATE | |
Registered | |
Madrid
Protocol (TM) |
| 6191972 | |
30-Aug-2019 | |
| |
CANIVATE | |
Registered | |
United
States |
| UK00801494231 | |
30-Aug-2019 | |
| |
THE CANIVATE WAY | |
Registered | |
United
Kingdom |
| 1494231 | |
30-Aug-2019 | |
| |
THE CANIVATE WAY | |
Registered | |
Madrid
Protocol (TM) |
| 6182017 | |
30-Aug-2019 | |
| |
THE CANIVATE WAY | |
Registered | |
United
States |
Operations
The
Company primary operating activities are in California, USA and Saskatoon, Canada. The Company’s head office is located in Vancouver,
Canada.
Description
of Property
The
Company currently leases office space at 800-525 West 8th Avenue Vancouver, BC V5Z 1C6 as its principal office. The Company believes
the office is in good condition and satisfy its current operational requirements.
Litigation
We
are subject to the legal proceeding and claims described in detail in “Note 17. Commitments and Contingencies” to the audited
financial statements included in this Annual Report on Form 10-K. Although the results of litigation and claims cannot be predicted with
certainty, as of the date of this Annual Report on Form 10-K, we do not believe the outcome of such legal proceeding and claims, if determined
adversely to us, would be reasonably expected to have a material adverse effect on our business. Regardless of the outcome, litigation
can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Directors,
Executive Officers and Corporate Governance
| Name
|
|
Age
|
|
Position |
|
Served
Since |
| |
|
|
|
|
|
|
| William
J. Meekison |
|
58
|
|
Director,
M&A Committee Chair, and Audit Committee |
|
June
2019 |
| David
Welch |
|
42
|
|
Board
Chair, Director, Compensation Committee Chair, and M&A Committee |
|
June
2019 |
| Richard
Levychin |
|
64
|
|
Director,
Audit Committee Chair, M&A Committee |
|
July
2021 |
| Amy
Griffith |
|
52
|
|
Director,
Governance Committee Chair, and Compensation Committee |
|
July
2021 |
| Richard
S. Wong |
|
58
|
|
Interim
Chief Executive Officer and Chief Financial Officer |
|
October
2018 |
| Troy
T. McClellan |
|
61
|
|
President,
AgriFORCE™ Solutions |
|
February
2018 |
| Mauro
Pennella |
|
58
|
|
Chief
Marketing Officer and President AgriFORCE™ Brands division. |
|
July
2021 |
| Elaine
Goldwater |
|
52 |
|
Director,
Audit and Governance Committee |
|
October
2023 |
| Margaret
A. Honey |
|
67 |
|
Director,
Compensation and Governance Committee |
|
October
2023 |
Directors
serve until the next annual meeting and until their successors are elected and qualified. Officers are appointed to serve for one year
until the meeting of the Board of Directors following the annual meeting of shareholders and until their successors have been elected
and qualified.
David
Welch, Director, Nominating and Governance Committee
Mr.
Welch is a founding partner at ENSO LAW, LLP, based in Los Angeles. He has a broad base of experience in representing US, Canadian and
Mexican corporate clients in the areas of litigation, intellectual property and government regulatory advisement and defense. Mr. Welch
has represented recognizable businesses in the agriculture and food services space in Federal Court, California state courts and before
the USPTO. Mr. Welch has also argued before the California Supreme Court and the US 9th Circuit Court of Appeals on constitutional issues
related to preemption and the application of US law to various companies. Mr. Welch obtained his Juris Doctorate degree from Loyola Law
School with an emphasis in international trade and has received various accolades for his work in intellectual property and regulatory
law, including Top 40 under 40 by the Daily Journal; National Law Journal Intellectual Property Trail Blazer, and Super Lawyers from
2013 until 2023. In his business ventures, Mr. Welch is a registered aquaculturist and farmer focusing on sustainable and regenerative
agricultural practices. He is suited to serve as a director due to his long standing experience in international intellectual property
and business.
William
John Meekison, Director, Audit Committee, Compensation Committee
Mr.
Meekison is a career Chief Financial Officer and former investment banker. He has spent the last fifteen years serving in a variety of
executive management and CFO roles with both private and public companies, currently as the CFO and Director of Exro Technologies Inc.
(since October 2017), a technology company that creates energy management system, and CFO and CFO of ArcWest Exploration Inc. (since
December 2010), a mining exploration company in British Columbia. He is currently on the board of Telo Genomics Corp. (since July 2018)
and Adven Inc. (since April 2021). Prior to his position at Exro Technologies Inc. and other CFO roles, Mr. Meekison spent fifteen years
in corporate finance with a focus on raising equity capital for North American technology companies, including nine years at Haywood
Securities Inc. Mr. Meekison received his Bachelor of Arts from the University of British Columbia and is a Chartered Professional Accountant,
Professional Logistician and Certified Investment Manager. He is suited to serve as a director due to his long time experience as a CFO.
Richard
Levychin, Director, Audit Committee Chair, Nominating and Governance Committee, Compensation Committee
Richard
Levychin, CPA, CGMA, is a Partner in Galleros Robinson’s Commercial Audit and Assurance practice where he focuses on both privately
and publicly held companies. Prior to taking this position in October 2018, Richard was the managing partner of KBL, LLP, a PCAOB certified
independent registered accounting firm, since 1994. Mr. Levychin has over 25 years of accounting, auditing, business advisory services
and tax experience working with both privately owned and public entities in various industries including media, entertainment, real estate,
manufacturing, not-for-profit, technology, retail, technology, and professional services. His experience also includes expertise with
SEC filings, initial public offerings, and compliance with regulatory bodies. As a business adviser, he advises companies, helping them
to identify and define their business and financial objectives, and then provides them with the on-going personal attention necessary
to help them achieve their established goals. Mr. Levychin is well suited to serve on our Board due to his decades of experience as the
managing partner of a PCAOB certified independent registered accounting firm, which included decades of expertise with SEC filings and
initial public offerings.
Amy
Griffith, Director, Audit Committee, Compensation Committee Chair, Nominating and Governance Committee
Ms.
Griffith currently serves as Group Director for the North America Operating unit of the Coca-Cola Company, in this capacity she oversees
public affairs, government relations, sustainability and communications in Canada and the Northeastern United States. Previously, she
served as Wells Fargo’s State & Local Government Relations Senior Vice President. She was recruited to Wells Fargo’s
Government Relations and Public Policy team in 2019. In this role, Griffith led Wells Fargo’s legislative and political agenda
in her region and managed relationships with state and local policymakers and community stakeholders. From 2008-2019, Ms. Griffith led
government relations for sixteen states in the Eastern United States for TIAA for over a decade. Prior to that, she worked in the aerospace,
high tech, education, private and public sectors, and has managed multiple high-profile political campaigns at the local, state and national
level. Griffith is active in her community and has co-chaired The Baldwin School Golf Outing to raise funds for girls’ athletics
programs. She is a graduate of Gwynedd-Mercy College and holds a Bachelor of Arts in History. Ms. Griffith is well qualified to serve
as a director due to her significant experience in government relations, policy and politics as well as decades of experience working
with companies in both the private and public sectors.
Richard
Wong, Interim Chief Executive Officer and Chief Financial Officer
Mr.
Wong, who works full time for the Company, has over 25 years of experience in both start-up and public companies in the consumer goods,
agricultural goods, manufacturing, and forest industries. Prior to joining the Company in 2018, he was a partner in First Choice Capital
Advisors from 2008-2016 and a partner in Lighthouse Advisors Ltd. from 2016-2018. Mr. Wong has also served as the CFO of Emerald Harvest
Co., Dan-D Foods, Ltd., and was the Director of Finance and CFO of SUGOI Performance Apparel and had served positions at Canfor, Canadian
Pacific & other Fortune 1000 companies. Mr. Wong is a Chartered Professional Accountant, and a member since 1999. Mr. Wong has a
Diploma in Technology and Financial Management from the British Columbia Institute of Technology.
Troy
McClellan, President AgriFORCE™ Solutions
Mr.
McClellan, who works full time for the Company, has focused on innovative design and construction technologies throughout his career.
Most recently, he was V.P. of Design and Development at WIGU City from 2015-2018, at which time he joined the Company. Mr. McClellan
was the VP Design and Development of MGM Macau. Previously, he was a Project Manager at Wynn Design & Development and a Design Manager
at Universal Studios (Japan). Mr. McClellan is a registered professional architect and received his Master’s Degree in Architecture
from Montana State University.
Mauro
Pennella, Chief Marketing Officer and President, AgriFORCE™ Brands
Mr.
Pennella, who works full time for the Company, is a consumer products veteran with more than 30 years of experience in the consumer-packaged
goods industry. From May 2018 until January 2021, he was Chief Growth & Sustainability Officer at McCain Foods, a Canadian multinational
frozen food company. In that role, he was responsible for global marketing, sales, research and development (R&D) and sustainability.
From October 2014 to April 2018, Mr. Pennella served as the President, International of Combe Incorporated, a personal care products
company where he oversaw the international division, R&D and the internal advertising agency. He was also a member of the Executive
Committee at Combe Incorporated, where he was responsible for the P&L - overseeing eight subsidiaries with more than 100 employees
around the world. Prior to that, Mr. Pennella led the Retail and International businesses at Conagra’s Lamb Weston division and
developed his career at Diageo and Procter & Gamble. Mr. Pennella received a Master of Business from Audencia, a premier European
business school, as well as an M.A.B.A. in Marketing and Finance from The Ohio State University Fisher College of Business.
Elaine
Goldwater is an executive in the Bio-Pharmaceutical Industry. She is the Senior Director of Marketing, Endocrinology at Recordati
Rare Diseases. Offering 20 plus years of experience creating and launching complex global marketing strategies in the competitive pharmaceutical
industry, she offers a talent for guiding informed decision-making, leading strategic planning and strategic operations, and delivering
double-digit growth and transform across high-value product portfolios. Most recently driving her business unit to over 50% growth in
6- months.
Her
expertise includes deep knowledge of the product lifecycle from pre-clinical/early-stage development through launch, loss of exclusivity
(LOE), line-extension, and late lifecycle products. In addition, Elaine’s mastery of country and global operations is leveraged
with a background in building market archetypes, shared best practices, and profitable strategy and execution models. She drives end
to end commercial strategy creation and execution through a collaborative cross functional process that delivers above brand performance
driving to growing net revenue and ensuring patient access. She has brought this strategic expertise into strategy development and into
market execution driving double digit growth and across multiple disease categories from Cushing Disease, Acromegaly, Infectious Disease
(antibiotic, anti-fungal, HIV, HPC); Contraception, hematology, oncology, respiratory, diabetes and urology. Additionally, Elaine has
expertise in orphan drug and rare disease filing, launching, and marketing execution.
She
is especially adept at motivating and uniting cross-functional headquarter global and country teams toward common goals. By gathering
input from internal and external customers and engaging with scientific leader, HCPs, Patients, patient advocacy, and payors, she innovates
solutions, shape and articulate our vision, and generate buy-in across an enterprise. From August 2019-August 2022, Ms. Goldwater was
a Director of Global Marketing for Merck & Co., Inc. (across two product lines), and from December 2022 to present, she has been
a USA Marketing, Senior Director of Marketing for Endocrinology for Recordati Rare Diseases, Inc. The Board and Company believe that
Ms. Goldwater is qualified to serve as a Director due to her long term experience as a high level marketing executive.
Margaret
A. Honey is an experienced board member and leader with extensive expertise in STEM learning and public engagement in science. She
has since 2008 been the President and CEO of the New York Hall of Science (“NYSCI”) where she has worked with local, state,
and federal elected officials to secure financial resources and ensure NYSCI is seen as an essential institution She has an extensive
background in strategic planning, program development, management, and administration with an established track record of successful
fundraising, including government grants, foundation support and individual doners.
She
is widely recognized for her work using digital technologies to support children’s learning across the disciplines of science,
mathematics, engineering and technology. Prior to joining NYSCI, she spent 15 years as vice president of the Education Development Center
(EDC) and director of EDC’s Center for Children and Technology. While at EDC, Dr. Honey was the architect and overseer of numerous
large-scale projects funded by organizations including the National Science Foundation, the Institute for Education Sciences, The Carnegie
Corporation, The Library of Congress, the U.S. Department of Education, and the U.S. Department of Energy.
Dr.
Honey has shared her expertise before Congress, state legislatures, and federal panels, and through numerous articles, chapters, and
books. She serves on several boards including the National Academies Division of Behavioral, Social Sciences and Education, Advisory
committee.
Dr.
Honey has led diverse teams to foster business transformation, building partnerships with government, not-for-profit, and commercial
entities, including a track record of successfully securing federal funds for STEM-focused R&D.
Combined
with her experience working with federal, state, and local policymakers, the Board and Company believe that Dr. Honey is qualified to
serve as a Director due to her long term experience in organizational transformation and excellence.
Corporate
Governance
The
business and affairs of our Company are managed under the direction of the Board of Directors.
Term
of Office
Directors
serve until the next annual meeting and until their successors are elected and qualified. Officers are appointed to serve until the Company
requires them to be replaced.
Director
Independence
We
use the definition of “independence” of The NASDAQ Stock Market to make this determination. We are not yet listed on NASDAQ,
and although we use its definition of “independence,” its rules are inapplicable to us until such time as we become listed
on NASDAQ. NASDAQ Listing Rule 5605(a)(2) provides that an “independent director” is a person other than an officer or employee
of our Company or any other individual having a relationship which, in the opinion of the Board of Directors, would interfere with the
exercise of independent judgment in carrying out the responsibilities of a director. The NASDAQ rules provide that a director cannot
be considered independent if:
| |
● |
the
director is, or at any time during the past three years was, an employee of our Company; |
| |
|
|
| |
● |
the
director or a family member of the director accepted any compensation from our Company in excess of $120,000 during any period of
12 consecutive months within the three years preceding the independence determination (subject to certain exclusions, including,
among other things, compensation for board or board committee service); |
| |
|
|
| |
● |
a
family member of the director is, or at any time during the past three years was, an executive officer of our Company; |
| |
|
|
| |
● |
the
director or a family member of the director is a partner in, controlling shareholder of, or an executive officer of an entity to
which our Company made, or from which our Company received, payments in the current or any of the past three fiscal years that exceed
5% of the recipient’s consolidated gross revenue for that year or $200,000, whichever is greater (subject to certain exclusions); |
| |
|
|
| |
● |
the
director or a family member of the director is employed as an executive officer of an entity where, at any time during the past three
years, any of the executive officers of our Company served on the compensation committee of such other entity; or |
| |
|
|
| |
● |
the
director or a family member of the director is a current partner of our Company’s outside auditor, or at any time during the
past three years was a partner or employee of our Company’s outside auditor, and who worked on our Company’s audit. |
Under
the following three NASDAQ director independence rules a director is not considered independent: (a) NASDAQ Rule 5605(a)(2)(A), a director
is not considered to be independent if he or she also is an executive officer or employee of the corporation, (b) NASDAQ Rule 5605(a)(2)(B),
a director is not consider independent if he or she accepted any compensation from our Company in excess of $120,000 during any period
of twelve consecutive months within the three years preceding the determination of independence, and (c) NASDAQ Rule 5605(a)(2)(D), a
director is not considered to be independent if he or she is a partner in, or a controlling shareholder or an executive officer of, any
organization to which our Company made, or from which our Company received, payments for property or services in the current or any of
the past three fiscal years that exceed 5% of the recipient’s consolidated gross revenues for that year, or $200,000. Under such
definitions, we have six independent directors.
Family
Relationships
There
are no family relationships among any of the directors and executive officers.
Board
Committees
Our
Board has established the following three standing committees: audit committee; compensation committee; and nominating and governance
committee, or nominating committee. Our board of directors has adopted written charters for each of these committees. Copies of the charters
will be available on our website. Our board of directors may establish other committees as it deems necessary or appropriate from time
to time.
Audit
Committee
Our
Audit Committee is comprised of at least three individuals, each of whom are independent director and at least one of whom will be an
“audit committee financial expert,” as defined in Item 407(d)(5)(ii) of Regulation S-K. Our audit committee is currently
comprised of Richard Levychin, John Meekison and Elaine Goldwater, who are independent, and Mr. Levychin is our financial expert.
Our
Audit Committee will oversee our corporate accounting, financial reporting practices and the audits of financial statements. For this
purpose, the Audit Committee will have a charter (which will be reviewed annually) and perform several functions. The Audit Committee
will:
| |
● |
evaluate
the independence and performance of, and assess the qualifications of, our independent auditor and engage such independent auditor; |
| |
|
|
| |
● |
approve
the plan and fees for the annual audit, quarterly reviews, tax and other audit-related services and approve in advance any non-audit
service to be provided by our independent auditor; |
| |
|
|
| |
● |
monitor
the independence of our independent auditor and the rotation of partners of the independent auditor on our engagement team as required
by law; |
| |
|
|
| |
● |
review
the financial statements to be included in our future Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q and review with
management and our independent auditor the results of the annual audit and reviews of our quarterly financial statements; and |
| |
|
|
| |
● |
oversee
all aspects our systems of internal accounting control and corporate governance functions on behalf of the Board of Directors. |
Compensation
Committee
Our
Compensation Committee comprises of at least three individuals, each of whom will be an independent director, Our Compensation committee
is currently comprised of David Welch (Chair), Amy Griffith and Margaret Honey and who are independent.
The
Compensation Committee will review or recommend the compensation arrangements for our management and employees and also assist our Board
of Directors in reviewing and approving matters such as company benefit and insurance plans, including monitoring the performance thereof.
The Compensation Committee will have a charter (which will be reviewed annually) and perform several functions.
The
Compensation Committee will have the authority to directly engage, at our expense, any compensation consultants or other advisers as
it deems necessary to carry out its responsibilities in determining the amount and form of employee, executive and director compensation.
Nominating
and Corporate Governance Committee
Our
Nominating and Corporate Governance Committee is comprised of at least three individuals, each of whom will be an independent director.
Currently Amy Griffith (Chair), Elaine Goldwater and Margaret Honey are members of the committee.
The
NC&G Committee is charged with the responsibility of reviewing our corporate governance policies and with proposing potential director
nominees to the Board of Directors for consideration. This committee also has the authority to oversee the hiring of potential executive
positions in our Company. The NC&G Committee also has a charter, which is to be reviewed annually.
Executive
Compensation
| Name & Principal Position | |
Year | | |
Salary | | |
Bonus | | |
Share-Based Awards | | |
Option-Based Awards | | |
All Other Compensation | | |
Total Compensation | |
| Ingo W. Mueller (resigned July 2023) | |
2022 | | |
| 392,464 | | |
| 375,718 | | |
| 359,881 | | |
| 6,866 | | |
| 1,741 | | |
| 1,136,670 | |
| Former Chief Executive Officer | |
2021 | | |
| 299,299 | | |
| 282,808 | | |
| 155,668 | | |
| 279,632 | | |
| 14,958 | | |
| 1,032,365 | |
| Richard S. Wong, | |
2022 | | |
| 295,216 | | |
| 134,696 | a | |
| 86,456 | | |
| 28,831 | | |
| 1,741 | | |
| 546,940 | |
| Interim Chief Executive Officer and Chief Financial Officer | |
2021 | | |
| 237,582 | | |
| 132,070 | | |
| 37,397 | | |
| 186,422 | | |
| - | | |
| 593,471 | |
| Troy T. McClellan, | |
2022 | | |
| 246,732 | | |
| 69,162 | b | |
| 76,846 | | |
| 30,132 | | |
| 1,741 | | |
| 424,613 | |
| President Design & Construction | |
2021 | | |
| 206,280 | | |
| 80,774 | | |
| 35,456 | | |
| 167,778 | | |
| - | | |
| 490,288 | |
| Mauro Pennella | |
2022 | | |
| 268,962 | | |
| - | | |
| 115,269 | | |
| 45,593 | | |
| 1,741 | | |
| 431,565 | |
| Chief Marketing Officer, President AgriFORCE™ Brands | |
2021 | | |
| 128,841 | | |
| - | | |
| 55,179 | | |
| 85,693 | | |
| - | | |
| 269,713 | |
| (a) |
Bonus
was paid out $101,022 in shares and $33,674 in cash. |
| (b) |
Bonus
was paid out $69,162 in shares |
| (c)
|
Some
share-based awards were issued net of income taxes. The Company repurchased shares on the issuance date to remit as income taxes
to the appropriate government revenue service agencies. |
Security
Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The
following table sets forth information known to us regarding the beneficial ownership of our common stock as of December 19, 2023
by:
| ● |
each
person known to us to be the beneficial owner of more than 5% of our outstanding common stock; |
| ● |
each
of our executive officers and directors; and |
| ● |
all
of our executive officers and directors as a group. |
| | |
Common shares | | |
Options Granted vested within 60 days of December
19, 2023 | | |
Warrants | | |
Total | | |
Percentage beneficially owned | |
| Directors and Officers: | |
| | | |
| | | |
| | | |
| | | |
| | |
| Richard Wong | |
| 18,900 | | |
| 11,580 | | |
| - | | |
| 30,570 | | |
| 0.6 | % |
| Troy McClellan | |
| 11,616 | | |
| 2,150 | | |
| - | | |
| 13,766 | | |
| 0.3 | % |
| Mauro Pennella | |
| 15,756 | | |
| 7,401 | | |
| - | | |
| 23,157 | | |
| 0.5 | % |
| John Meekison | |
| 865 | | |
| 2,541 | | |
| - | | |
| 3,406 | | |
| 0.1 | % |
| David Welch | |
| 1,049 | | |
| 2,507 | | |
| - | | |
| 3,556 | | |
| 0.1 | % |
| Amy Griffith | |
| - | | |
| 2,148 | | |
| - | | |
| 2,148 | | |
| 0.0 | % |
| Richard Levychin | |
| - | | |
| 2,148 | | |
| - | | |
| 2,148 | | |
| 0.0 | % |
| Elaine Goldwater | |
| - | | |
| - | | |
| - | | |
| - | | |
| 0.0 | % |
| Margaret Honey | |
| - | | |
| - | | |
| - | | |
| - | | |
| 0.0 | % |
| Total all officers and directors (9 persons) | |
| 48,276 | | |
| 30,475 | | |
| - | | |
| 78,751 | | |
| 1.6 | % |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| 5% or Greater Beneficial Owners | |
| | | |
| | | |
| | | |
| | | |
| | |
Item
13. Certain Relationships and Related Transactions, and Director Independence
We
have adopted a written related-person transactions policy that sets forth our policies and procedures regarding the identification, review,
consideration and oversight of “related-party transactions.” For purposes of our policy only, and not for purposes of required
disclosure, which will be all related party transactions, even if less than $120,000, a “related-party transaction” is a
transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any “related
party” are participants involving an amount that exceeds $120,000.
Transactions
involving compensation for services provided to us as an employee, consultant or director are not considered related-person transactions
under this policy. A related party is any executive officer, director or a holder of more than five percent of our common shares, including
any of their immediate family members and any entity owned or controlled by such persons.
At
present, we have appointed three independent directors to the Nominating and Corporate Governance Committee. As a result, our Chief Financial
Officer, Richard Wong, must present information regarding a proposed related-party transaction to the Nominating and Corporate Governance
Committee. Under the policy, where a transaction has been identified as a related-party transaction, Mr. Wong must present information
regarding the proposed related-party transaction to our Nominating and Corporate Governance Committee, once the same is established,
for review. The presentation must include a description of, among other things, the material facts, the direct and indirect interests
of the related parties, the benefits of the transaction to us and whether any alternative transactions are available. To identify related-party
transactions in advance, we rely on information supplied by our executive officers, directors and certain significant shareholders. In
considering related-party transactions, our Nominating and Corporate Governance Committee takes into account the relevant available facts
and circumstances including, but not limited to:
| |
● |
whether
the transaction was undertaken in the ordinary course of our business; |
| |
|
|
| |
● |
whether
the related party transaction was initiated by us or the related party; |
| |
|
|
| |
● |
whether
the transaction with the related party is proposed to be, or was, entered into on terms no less favorable to us than terms that could
have been reached with an unrelated third party; |
| |
|
|
| |
● |
the
purpose of, and the potential benefits to us from the related party transaction; |
| |
|
|
| |
● |
the
approximate dollar value of the amount involved in the related party transaction, particularly as it relates to the related party; |
| |
|
|
| |
● |
the
related party’s interest in the related party transaction, and |
| |
|
|
| |
● |
any
other information regarding the related party transaction or the related party that would be material to investors in light of the
circumstances of the particular transaction. |
The
Nominating and Corporate Governance Committee shall then make a recommendation to the Board, which will determine whether or not to approve
of the related party transaction, and if so, upon what terms and conditions. In the event a director has an interest in the proposed
transaction, the director must recuse himself or herself from the deliberations and approval.
Except
as set forth below, we have not had any related party transactions, regardless of dollar amount:
As
of December 31, 2022, $32,500 (December 31, 2021, $47,461) in total was owing to officers and directors or to companies owned by officers
and directors of the Company for services and expenses. These amounts owing have been included in accounts payable and accrued liabilities.
During
the year ended December 31, 2022 and 2021, the Company incurred $79,457 and $66,246, respectively, to our U.S. general counsel firm,
D R Welch against legal services, a corporation controlled by a director of the Company. No shares were issued in the year ended December
31, 2022 (an aggregate of 13,158 shares were issued – December 31, 2021) to David Welch as part of the payment.
DESCRIPTION
OF OUR SECURITIES
General
We
have authorized unlimited common shares and preferred shares.
Common
Shares
As
of December 19, 2023, we had 5,236,124 common shares issued and outstanding.
Voting
The
holders of the common shares are entitled to one vote for each share held at all meetings of shareholders (and written actions in lieu
of meeting). There is no cumulative voting. The holders of common shares are entitled to dividends when and as declared by the Board
of Directors from funds legally available therefor, and upon liquidation are entitled to share pro rata in any distribution to holders
of common shares. There are no preemptive, conversion or redemption privileges, nor sinking fund provisions with respect to the common
shares.
Warrants
As
of the date of this prospectus, the Company has issued and outstanding warrants to purchase 2,908,449 shares of the Company’s common
stock on the terms set forth below.
| Securities Class | |
Number of issuable shares upon exercise of warrants | | |
Expiry | |
Conversion feature |
| | |
| | |
| |
|
| $375.00 Common Share Warrants Tranche 1 | |
| 31,276 | | |
May 2, 2025 | |
Each warrants entitles holder to purchase One common share within 5 years, and is accelerated to 30 days expiry when stock trades for a minimum of $570.00 for 10 consecutive days |
| $375.00 Common Share Warrants Tranche 2 | |
| 19,645 | | |
May 10, 2025 | |
Each warrants entitles holder to purchase One common share within 5 years, and is accelerated to 30 days expiry when stock trades for a minimum of $570.00 for 10 consecutive days |
| $300.00 Common Share Warrants from IPO | |
| 64,486 | | |
July 12, 2024 | |
Each 50 public warrants entitles a holder to purchase one common share at
an aggregate price of $300.0 per each issuable common share within 3 years, and is accelerated to 30 days expiry when
stock trades for a minimum of $570.00 for 10 consecutive days |
| Prefunded warrants | |
| 31,346 | | |
No expiry date | |
Each warrant entitles holder to convert one warrant into common share upon a Patent being issued and transferred to the Company. The warrants will vest in four equal amounts commencing on the date of issuance of the Patent. |
| $0.90 Common Share Warrants from convertible debentures | |
| 82,128 | | |
December 31, 2025 | |
Each warrant entitles holder to purchase One common share within 42 months of the issuance date of the warrant (June 30, 2022). There is a down round provision that adjusts the strike price based on certain future events. |
| $0.90 Common Share Warrants from Convertible Debentures | |
| 53,226 | | |
July 17, 2026 | |
Each warrant entitles holder to purchase One common share within 42 months of the issuance date of the warrant (January 17, 2023). There is a down round provision that adjusts the strike price based on certain future events. |
| $25.00 Private Placement Warrants | |
| 20,000 | | |
June 20, 2023 | |
Each warrant entitles holder to purchase One common share within 42 months of the issuance date of the warrant (January 17, 2023). There is a down round provision that adjusts the strike price based on certain future events. |
| $0.90 Common Share Warrants from Convertible Debentures | |
| 620,230 | | |
April 18, 2027 | |
Each warrant entitles holder to purchase One common share within 42 months of the issuance date of the warrant (October 18, 2023). There is a down round provision that adjusts the strike price based on certain future events. |
| $0.90 Common Share Warrants from Convertible Debentures | |
| 1,986,112 | | |
May 30, 2027 | |
Each warrant entitles holder to purchase One common share within 42 months of the issuance date of the warrant (November 30, 2023). There is a down round provision that adjusts the strike price based on certain future events. |
| TOTAL | |
| 2,908,449 | | |
| |
|
JULY
2022 DEBENTURES AND SELLING STOCK HOLDERS TABLE
July
2022 Debt Financing
On
June 30, 2022, AgriFORCE™ Growing Systems, Ltd. (the “Company”) entered into a Securities Purchase Agreement (“SPA”)
with two institutional investors (“Investors”) with an initial purchase of $14.025 million principal amount of debentures
(“Debentures”) and accompanying warrants (“Warrants”) and up to an additional $33 million principal amount of
Debentures and accompanying Warrants. Under the SPA, the Company expects to receive an initial amount of $12.75 million (gross of fees
which will be deducted from that amount) on July 6, 2022 and has the right to receive up to an additional aggregate of $33.0 million
at the discretion of each of the purchasers hereunder (the “Investors”), in one or multiple tranches, subject to certain
conditions, at then-current market prices in minimum tranches of $5 million each. The SPA contains industry standard representations
and warranties and negative covenants, including, but not limited to, limitations upon the amounts of indebtedness and other securities
which may be incurred and issued by the Company under certain circumstances as set forth in the SPA.
The
initial conversion price of the Debentures is $2.22 per share. The Debentures are due in 2.5 years from June 30, 2022, which may be extended
for an additional six month period by the Company by paying, at the end of the 18th month of the term of the Debentures, six
months of interest at the rate of 8% per annum. The Debentures are subject to a 10% original issue discount and bear interest at 5% for
the first 12 months, 6% for the next 12 months and 8% until maturity. The Debentures amortize over a 25 month period commencing on September
1, 2022, and the monthly amortization of the Debentures are payable in cash only for the first 12 months of amortizations and in cash
or stock thereafter at the option of the Company. Once the monthly amortizations are payable in cash or stock, the Company can only elect
to pay the monthly amortization in stock if certain equity conditions, as set forth in the Debentures, are met, which include, but are
not limited to, for each Trading Day in a period of 20 consecutive Trading Days prior to the applicable date in question, the daily trading
volume for the Common Stock on the principal Trading Market exceeds $1,000,000 per Trading Day, the Company is not in default of any
of its obligations under the Debentures, there is an effective registration statement for the resale of shares issuable under the Debentures,
and the Company is in compliance with all Nasdaq listing requirements. The Debentures contain commercially standard events of default
and covenants and the like.
In
addition, the Investors have received 3.5-year Warrants with 65% warrant coverage at an initial exercise price of $122.10 per share,
subject to customary adjustments, including a price ratchet (to the price of the new issuance) if it issues its common shares at a price
less than the then in effect exercise price and are subject to standard pro rata dilution for reverse stock splits and the like. The
Debentures have the same dilution protection as the Warrants.
Both
the Debentures and Warrants contain exercise limitations upon an Investor beneficially owning more than either 4.99% or 9.99% of the
Company’s common shares and also contain caps upon the total amount of common shares issuable upon conversion of the Debentures
and exercise of the Warrants of 19.9% of the issued and outstanding shares of the Company at the time of the closing of the transactions,
until shareholder approval of both the financing transaction, including all subsequent tranches of the financing, and the Delphy acquisition
are received, consistent with Nasdaq rules.
The
Company has entered into a Registration Rights Agreement with the Investors to register the shares issuable upon conversion of the Debentures
and exercise of the Warrants with a registration statement to be filed on Form S-1 no later than 30 days from June 30, 2022 (or any subsequent
closing) and effective no later than 60 days from June 30, 2022 (or the date of any subsequent closing; or 90 days, if there is full
SEC review). Penalties for missing those deadlines are equal to 2% of the subscription amount per month up to 10% of the subscription
amount.
The
Company’s subsidiaries have also entered into subsidiary guarantees pursuant to which each guarantees the performance of the Company
of its obligations under the SPA and related instruments. Each of the officers and directors has also entered into a lockup agreement
to not sell any common shares of the Company owned by each such person for one year from June 30, 2022 (subject to the ability to sell
shares received by each as the result of an employment agreement at any time, which ability to sell shares commences on January 1, 2023).
All
of the Debentures and Warrants sold under the SPA are sold in private placement transactions exempt from registration under Section 4(a)(2)
of the Securities Act of 1933, as amended.
On
January 17, 2023, the two investors in the initial transaction purchased an additional $5,076,923.08 principal amount of Notes and 53,226
Warrants. At the same time the initial conversion price of the Notes and exercise price of the Warrants was reduced to $62.00.
Pioneer
provided notice on October 17, 2023 to purchase an additional Debenture and warrants in the amount of $2,750,000. The conversion price
of the new debenture and exercise price of the new warrants has been set at $2.62 (based on the Nasdaq Official Closing Price on October
16, 2023), and the conversion price of all existing debentures and warrants has been set at $2.62. The floor price has been set at $0.52.
Pioneer
provided notice on November 30, 2023 to purchase an additional Debenture and warrants in the amount of $2,750,000. Pursuant to the terms
of the June 30, 2022 Securities Purchase Agreement, the conversion price of the new debenture and exercise price of the new warrants
was then automatically reset at $0.90 (based on the Nasdaq Official Closing Price on November 29, 2023), and the conversion price of
all existing debentures and warrants has been set at $0.90. The floor price has been set at $0.18.
SELLING
STOCKHOLDERS
The
shares of common stock being offered by the selling stockholders are those issuable upon conversion of the Debentures and exercise of
the Warrant, see above. We are registering the shares of common stock in order to permit the selling stockholders to offer the shares
for resale from time to time. Except for the ownership of the common stock, Debentures and Warrants issued pursuant to the Securities
Purchase Agreement, the selling stockholders have not had any material relationship with us within the past three years.
The
table below lists the selling stockholders and other information regarding the beneficial ownership (as determined under Section 13(d)
of the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder) of the shares of common stock held by each
of the selling stockholders. The second column lists the number of shares of common stock beneficially owned by the selling stockholders,
based on their respective ownership of shares of Debentures and Warrants, as of July 27, 2022, assuming exercise of the warrants held
by each such selling stockholder on that date but taking account of any limitations on exercise set forth therein.
The
third column lists the shares of common stock being offered by this prospectus by the selling stockholders and does not take in account
any limitations on exercise of the Debentures and Warrants set forth therein.
In
accordance with the terms of a registration rights agreement with the holders of the Debentures and Warrants, this prospectus generally
covers the resale of the sum of (i) the number of shares of common stock into which the Debentures are convertible, and (ii) the maximum
number of shares of common stock issuable pursuant to the Warrants, in each case, determined as if the outstanding Debentures and Warrants
were converted/exercised in full (without regard to any limitations on exercise contained therein) (collectively, the “Registrable
Securities”) as of the trading day immediately preceding the date this registration statement was initially filed with the SEC.
Because the conversion price of the Debentures and exercise price of the Warrants may be adjusted, the number of shares that will actually
be issued may be more or less than the number of shares being offered by this prospectus. The fourth column assumes the sale of all of
the shares offered by the selling stockholders pursuant to this prospectus.
Under
the terms of the Debentures and Warrants, as applicable, a selling stockholder may not convert the Debentures and/or exercise the Warrants
to the extent (but only to the extent) such selling stockholder or any of its affiliates would beneficially own a number of shares of
our common stock which would exceed the applicable ownership percentage limitation (either 4.99% or 9.99%, which we refer to herein as
the “blocker”) of the outstanding shares of the Company. The number of shares in the second column reflects these limitations.
The selling stockholders may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
| Name of Selling Stockholder | |
Number of Shares of Common Stock Owned Prior to Offering | | |
Maximum Number of Shares of Common Stock to be Sold Pursuant to this Prospectus(2) | | |
Number of Shares of Common Stock of Owned After Offering | |
| Pioneer Capital Anstalt(1) | |
| - | | |
| 8,717,454 | | |
| - | |
| |
(1). |
Consists
of 6,111,112 shares issuable upon conversion of the Debentures and 2,606,342 shares issuable upon exercise of the Warrants. Each
of the Debentures and Warrants has a beneficial ownership blocker that precludes Pioneer from converting or exercising such instrument
if such conversion or exercise would cause Pioneer’s beneficial ownership of the Company’s common stock to exceed 9.99%.
Pioneer Capital Anstalt has an address at Drescheweg 2, 9490 Vaduz, Liechtenstein. Voting and dispositive control of securities owned
by Pioneer is shared by its two directors, Nicola Feuerstein and Lucas Mair. |
| |
|
|
| |
(2) |
Pursuant
to the terms of the registration rights agreement, we have agreed to register the sale of up to 8,717,454 shares of our common stock. |
PLAN
OF DISTRIBUTION
We
are registering the shares of common stock previously issued and the shares of common stock issuable upon conversion of the Debentures
and exercise of the warrants to permit the resale of these shares of common stock by the holders of the common stock, Debentures, and
warrants from time to time after the date of this prospectus. We will not receive any of the proceeds from the sale by the selling stockholders
of the shares of common stock, although we will receive the exercise price of any Warrants not exercised by the selling stockholders
on a cashless exercise basis. We will bear all fees and expenses incident to our obligation to register the shares of common stock.
The
selling stockholders may sell all or a portion of the shares of common stock held by them and offered hereby from time to time directly
or through one or more underwriters, broker-dealers or agents. If the shares of common stock are sold through underwriters or broker-dealers,
the selling stockholders will be responsible for underwriting discounts or commissions or agent’s commissions. The shares of common
stock may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices
determined at the time of sale or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block
transactions, pursuant to one or more of the following methods:
| |
● |
on
any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale; |
| |
|
|
| |
● |
in
the over-the-counter market; |
| |
|
|
| |
● |
in
transactions otherwise than on these exchanges or systems or in the over-the-counter market; |
| |
|
|
| |
● |
through
the writing or settlement of options, whether such options are listed on an options exchange or otherwise; |
| |
|
|
| |
● |
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| |
|
|
| |
● |
block
trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as
principal to facilitate the transaction; |
| |
|
|
| |
● |
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| |
|
|
| |
● |
an
exchange distribution in accordance with the rules of the applicable exchange; |
| |
|
|
| |
● |
privately
negotiated transactions; |
| |
|
|
| |
● |
short
sales made after the date the Registration Statement is declared effective by the SEC; |
| |
|
|
| |
● |
broker-dealers
may agree with a selling security holder to sell a specified number of such shares at a stipulated price per share; |
| |
|
|
| |
● |
a
combination of any such methods of sale; and |
| |
|
|
| |
● |
any
other method permitted pursuant to applicable law. |
The
selling stockholders may also sell shares of common stock under Rule 144 promulgated under the Securities Act of 1933, as amended, if
available, rather than under this prospectus. In addition, the selling stockholders may transfer the shares of common stock by other
means not described in this prospectus. If the selling stockholders effect such transactions by selling shares of common stock to or
through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts,
concessions or commissions from the selling stockholders or commissions from purchasers of the shares of common stock for whom they may
act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers
or agents may be in excess of those customary in the types of transactions involved). In connection with sales of the shares of common
stock or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short
sales of the shares of common stock in the course of hedging in positions they assume. The selling stockholders may also sell shares
of common stock short and deliver shares of common stock covered by this prospectus to close out short positions and to return borrowed
shares in connection with such short sales. The selling stockholders may also loan or pledge shares of common stock to broker-dealers
that in turn may sell such shares.
The
selling stockholders may pledge or grant a security interest in some or all of the warrants or shares of common stock owned by them and,
if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common
stock from time to time pursuant to this prospectus or any amendment to this prospectus under Rule 424(b)(3) or other applicable provision
of the Securities Act amending, if necessary, the list of selling stockholders to include the pledgee, transferee or other successors
in interest as selling stockholders under this prospectus. The selling stockholders also may transfer and donate the shares of common
stock in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial
owners for purposes of this prospectus.
To
the extent required by the Securities Act and the rules and regulations thereunder, the selling stockholders and any broker-dealer participating
in the distribution of the shares of common stock may be deemed to be “underwriters” within the meaning of the Securities
Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions
or discounts under the Securities Act. At the time a particular offering of the shares of common stock is made, a prospectus supplement,
if required, will be distributed, which will set forth the aggregate amount of shares of common stock being offered and the terms of
the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation
from the selling stockholders and any discounts, commissions or concessions allowed or re-allowed or paid to broker-dealers.
Under
the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers
or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified
for sale in such state or an exemption from registration or qualification is available and is complied with.
There
can be no assurance that any selling stockholder will sell any or all of the shares of common stock registered pursuant to the registration
statement, of which this prospectus forms a part.
The
selling stockholders and any other person participating in such distribution will be subject to applicable provisions of the Securities
Exchange Act of 1934, as amended, and the rules and regulations thereunder, including, without limitation, to the extent applicable,
Regulation M of the Exchange Act, which may limit the timing of purchases and sales of any of the shares of common stock by the selling
stockholders and any other participating person. To the extent applicable, Regulation M may also restrict the ability of any person engaged
in the distribution of the shares of common stock to engage in market-making activities with respect to the shares of common stock. All
of the foregoing may affect the marketability of the shares of common stock and the ability of any person or entity to engage in market-making
activities with respect to the shares of common stock.
We
will pay all expenses of the registration of the shares of common stock pursuant to the registration rights agreement, including, without
limitation, Securities and Exchange Commission filing fees and expenses of compliance with state securities or “blue sky”
laws; provided, however, a selling stockholder will pay all underwriting discounts and selling commissions, if any. We will indemnify
the selling stockholders against liabilities, including some liabilities under the Securities Act in accordance with the registration
rights agreements or the selling stockholders will be entitled to contribution. We may be indemnified by the selling stockholders against
civil liabilities, including liabilities under the Securities Act that may arise from any written information furnished to us by the
selling stockholder specifically for use in this prospectus, in accordance with the related registration rights agreements or we may
be entitled to contribution.
Once
sold under the registration statement, of which this prospectus forms a part, the shares of common stock will be freely tradable in the
hands of persons other than our affiliates.
Listing
Our
common shares and Series A warrants are traded on the Nasdaq Capital Market under the symbols “AGRI” and “AGRIW”,
respectively.
LEGAL
MATTERS
The
validity of the issuance of the securities offered by this prospectus will be passed upon for us by Jolie Kahn, Esq. of New York, NY.
EXPERTS
The
consolidated balance sheets of AgriFORCETM Growing Systems Ltd. as of December 31, 2022 and December 31, 2021, and the related
consolidated statements of operations stockholders’ equity, and cash flows for the years then ended have been audited by MARCUM
LLP, as stated in their report, which is incorporated herein by reference. Such consolidated financial statements are incorporated herein
by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
We
file annual, quarterly and special reports, along with other information with the SEC. Our SEC filings are available to the public over
the Internet at the SEC’s website at http://www.sec.gov. Our SEC filings are also available on our website, https://www.agriforcegs.com
under the heading “Investors.” The information on this website is expressly not incorporated by reference into, and does
not constitute a part of, this prospectus.
This
prospectus is part of a registration statement on Form S-1 that we filed with the SEC to register the securities offered hereby under
the Securities Act of 1933, as amended. This prospectus does not contain all of the information included in the registration statement,
including certain exhibits and schedules. You may obtain the registration statement and exhibits to the registration statement from the
SEC at the address listed above or from the SEC’s internet site.
INCORPORATION
BY REFERENCE
This
prospectus is part of a registration statement filed with the SEC. The SEC allows us to “incorporate by reference” into this
prospectus the information that we file with them, which means that we can disclose important information to you by referring you to
those documents. The information incorporated by reference is considered to be part of this prospectus, and information that we file
later with the SEC will automatically update and supersede this information. The following documents are incorporated by reference and
made a part of this prospectus:
| |
● |
Annual
Report on Form 10-K for the year ended December 31, 2022 filed on March 14, 2023 and Quarterly Reports on Form 10-Q for the quarters
ended March 31, 2023, June 30, 2023 and September 30, 2023 filed on May 9, 2023, August 4, 2023 and November 2, 2023, respectively;
|
| |
|
|
| |
● |
Current Reports on Form 8-K filed on January 17, 2023,
April 6, 2023, April 28, 2023, June 1, 2023, June 7, 2023, June 27, 2023, July 18, 2023, August 10, 2023, September 8, 2023, September 29, 2023, October 6, 2023, October 19, 2023, November 27, 2023 and December 8, 2023. |
| |
|
|
| |
● |
Our
Definitive Proxy Statement on Schedule 14A and accompanying additional proxy materials filed with the SEC on August 22, 2023; |
| |
|
|
| |
● |
Our
registration statement on Form 8-A filed on July 2, 2021. |
We
also incorporate by reference all additional documents that we file with the Securities and Exchange Commission under the terms of Sections
13(a), 13(c), 14 or 15(d) of the Exchange Act that are made after the date of the initial registration statement but prior to effectiveness
of the registration statement and after the date of this prospectus but prior to the termination of the offering of the securities covered
by this prospectus. We are not, however, incorporating, in each case, any documents or information that we are deemed to furnish and
not file in accordance with Securities and Exchange Commission rules.
You
may request, and we will provide you with, a copy of these filings, at no cost, by calling us at (604) 757-0952 or by writing to us at
the following address:
800-525
West 8th Avenue
Vancouver,
BC |
|
V5Z
1C6 |
| (Address
of principal executive offices) |
|
(Zip
Code) |
AGRIFORCE
GROWING SYSTEMS, LTD.
Common
Stock
PROSPECTUS
January
26, 2024
DEALER
PROSPECTUS DELIVERY OBLIGATION
Until
(insert date), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required
to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and
with respect to their unsold allotments or subscriptions.
AgriFORCE Growing Systems (NASDAQ:AGRI)
Historical Stock Chart
From Oct 2024 to Nov 2024

AgriFORCE Growing Systems (NASDAQ:AGRI)
Historical Stock Chart
From Nov 2023 to Nov 2024


