
August 2023 Corporate Presentation Advancing Medicines for Solid Tumors

Important Notice and Disclaimers Except for statements of historical fact, any information contained in this presentation may be a forward-looking statement that reflects the Company’s current views about future events and are subject to risks, uncertainties, assumptions and changes in circumstances that may cause events or the Company’s actual activities or results to differ significantly from those expressed in any forward-looking statement. In some cases, you can identify forward- looking statements by terminology such as “may”, “will”, “should”, “plan”, “predict”, “expect,” “estimate,” “anticipate,” “intend,” “goal,” “strategy,” “believe,” “could”, “would”, “potential”, “project”, “continue” and similar expressions and variations thereof. Forward-looking statements may include statements regarding the Company’s business strategy, cash flows and funding status, potential growth opportunities, clinical development activities, the timing and results of preclinical research, clinical trials and potential regulatory approval and commercialization of product candidates. Although the Company believes that the expectations reflected in such forward- looking statements are reasonable, the Company cannot guarantee future events, results, actions, levels of activity, performance or achievements. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under the heading “Risk Factors” in documents the Company has filed with the SEC. These forward-looking statements speak only as of the date of this presentation and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. 2 Context Therapeutics Inc. - August 2023 Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company's own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. This presentation discusses product candidates that are under preclinical and clinical study, and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. While the Company believes its internal research is reliable, such research has not been verified by any independent source. All the scientific, preclinical and clinical data presented within this presentation are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. Forward Looking Statement Company Overview
Lead Program: CTIM-76, a Claudin 6 x CD3 Bispecific Antibody Opportunity • Claudin 6 (CLDN6) is a tumor-specific protein that is present at high surface density across many adult cancers1 • CLDN6 is expressed at very low levels or absent in normal adult tissue Challenge • CLDN6 antigen is conformationally dependent, which limits access to antibody-antigen binding and antibody development • The CLDN6 antigen binding region is highly conserved with CLDN3, CLDN4, and CLDN9, which increases the risk of off-target binding and potential side effects associated with CLDN3 (pancreas), CLDN4 (kidney, pancreas), and CLDN9 (ear, gut) Target Validation • TORL’s TORL-1-23 ADC and BioNTech’s BNT211 CAR-T cell therapy establishes Proof of Concept2,3: ‒ Efficacy: TORL-1-23 demonstrated 75% ORR (3/4 pts) at 2.4 mg/kg; BNT211 demonstrated 75% ORR (6/8 pts) at DL2 ‒ Safety: TORL-1-23 exhibited MMAE-related toxicities; BNT211 exhibited CRS that was adequately managed with anti-IL6 Company Overview Context Therapeutics Inc. - August 20233 DL2 = dose level 2; CRS = cytokine release syndrome 1 Faber MS, et al. Bispecific claudin-6 x CD3 antibodies AACR Annual Meeting; 2021; Virtual. Abstract 1860 2 Sahin U, et al. TORL1-23: Initial results of a dose finding Phase 1 study. ASCO Annual Meeting; 2023; Chicago, IL. Abstract 3082 3 Haanen JB, et al. BNT211: A Phase I trial. ASCO Annual Meeting; 2023; Chicago, IL. Abstract 2518 CTIM-76 Claudin 6 x CD3 bispecific antibody • Selective for CLDN6: limited off-target effects • Potent: effective CLDN6-positive tumor killing at low doses • Wide therapeutic window: decreased risk of dangerous immune response • IND Filing: on track for Q1 2024

Claudin-targeted Therapeutics are Gaining Momentum $263 million raised in 2023 for early-stage programs from TORL and Alentis Company Overview TORL Biotherapeutics (Private) Alentis Therapeutics (Private) Description Funding to advance TORL-1-23, a first-in-class, clinical- stage ADC targeting Claudin 6 and other novel clinical and preclinical stage programs. $158 million Financing Participating Investors Description Funding to advance clinical programs ALE.F02 and ALE.C04 – two first-in-class anti-Claudin-1 (CLDN1) antibodies for organ fibrosis and CLDN1 positive tumors. $105 million Financing Participating Investors Selected information presented is for illustrative purposes onlyContext Therapeutics Inc. - August 20234
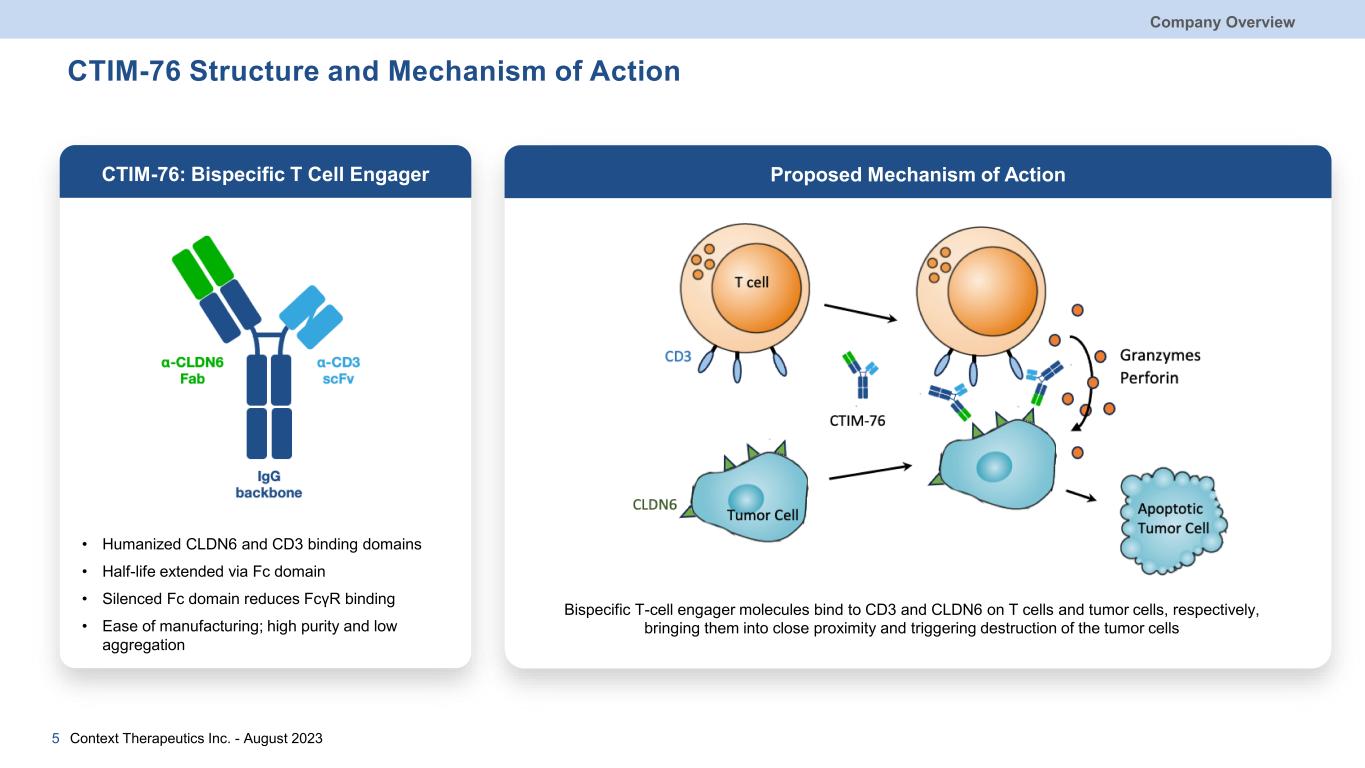
CTIM-76 Structure and Mechanism of Action Company Overview Context Therapeutics Inc. - August 20235 CTIM-76: Bispecific T Cell Engager Proposed Mechanism of Action • Humanized CLDN6 and CD3 binding domains • Half-life extended via Fc domain • Silenced Fc domain reduces FcγR binding • Ease of manufacturing; high purity and low aggregation Bispecific T-cell engager molecules bind to CD3 and CLDN6 on T cells and tumor cells, respectively, bringing them into close proximity and triggering destruction of the tumor cells

Bispecific Antibody T Cell Engagers (bsAb TCE) in Solid Tumors 2nd generation assets are addressing toxicity and dosing challenges associated with 1st generation products Company Overview Context Therapeutics Inc. - August 20236 1 Data as of August 1, 2023 Over 50 TCE in Clinical Development1Innovation Driving Clinical Success Limitations of 1st generation bsAb TCE: 1) Poor pharmacokinetics, continuous dosing 2) Cytokine release syndrome (CRS) 3) On-target/off-tumor toxicity Advantages of 2nd generation bsAb TCE: 1) Potential for dosing every 1-3 weeks 2) Improved TCE engineering to mitigate CRS 3) Better target selection and/or enhanced avidity 0 1 2 3 4 5 6 7 8 TROP2 B7H3 CLDN6 CLDN18.2 PSMA EGFR DLL3 HER2 CEA EpCAM Clinical Assets per Target Select Assets in Clinical Development

Claudin 6 (CLDN6) Target biology and therapeutic rationale Context Therapeutics Inc. - August 20237

CLDN6 is an Oncofetal Protein Oncofetal proteins are considered favorable candidates for immunotherapy • Normally present at higher levels during embryonic development • Turned off or have low levels of expression in adult tissues • Increased expression known to occur in some tumor cells, including non-small cell lung cancer (NSCLC), ovarian, and testicular CLDN6 Target Biology Context Therapeutics Inc. - August 20238 Oncofetal Characteristics of CLDN6 Huan, Mol Med Reports, 2021
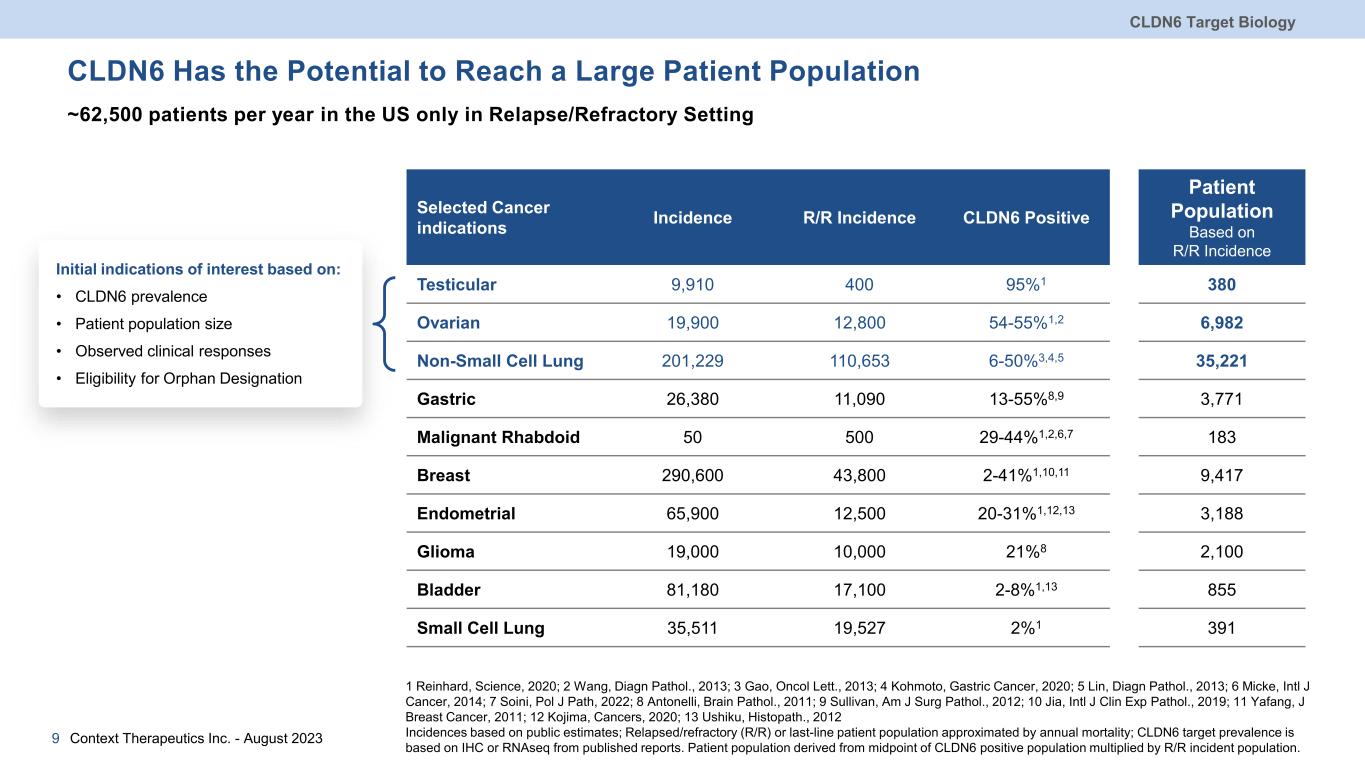
CLDN6 Has the Potential to Reach a Large Patient Population ~62,500 patients per year in the US only in Relapse/Refractory Setting CLDN6 Target Biology 9 Selected Cancer indications Incidence R/R Incidence CLDN6 Positive Patient Population Based on R/R Incidence Testicular 9,910 400 95%1 380 Ovarian 19,900 12,800 54-55%1,2 6,982 Non-Small Cell Lung 201,229 110,653 6-50%3,4,5 35,221 Gastric 26,380 11,090 13-55%8,9 3,771 Malignant Rhabdoid 50 500 29-44%1,2,6,7 183 Breast 290,600 43,800 2-41%1,10,11 9,417 Endometrial 65,900 12,500 20-31%1,12,13 3,188 Glioma 19,000 10,000 21%8 2,100 Bladder 81,180 17,100 2-8%1,13 855 Small Cell Lung 35,511 19,527 2%1 391 1 Reinhard, Science, 2020; 2 Wang, Diagn Pathol., 2013; 3 Gao, Oncol Lett., 2013; 4 Kohmoto, Gastric Cancer, 2020; 5 Lin, Diagn Pathol., 2013; 6 Micke, Intl J Cancer, 2014; 7 Soini, Pol J Path, 2022; 8 Antonelli, Brain Pathol., 2011; 9 Sullivan, Am J Surg Pathol., 2012; 10 Jia, Intl J Clin Exp Pathol., 2019; 11 Yafang, J Breast Cancer, 2011; 12 Kojima, Cancers, 2020; 13 Ushiku, Histopath., 2012 Incidences based on public estimates; Relapsed/refractory (R/R) or last-line patient population approximated by annual mortality; CLDN6 target prevalence is based on IHC or RNAseq from published reports. Patient population derived from midpoint of CLDN6 positive population multiplied by R/R incident population. Initial indications of interest based on: • CLDN6 prevalence • Patient population size • Observed clinical responses • Eligibility for Orphan Designation Context Therapeutics Inc. - August 2023

CLDN6 is Selectively Expressed on Cancer Cells CLDN6 Target Biology Context Therapeutics Inc. - August 202310 (a) adrenal gland, (b) fallopian tube, (c) kidney, (d) liver, (e) thyroid, (f) prostate, (g) esophagus, (h) stomach, (i) colon, (j) cerebrum, (k) cerebellum, (l) spinal cord. (m) thymus, (n) spleen, (o) bone marrow, (p) pancreas, (q) skin, (r) bladder, (s) placenta, (t) heart muscle, (u) striated muscle, (v) testis, (w) ovary, (x) lung (CA1) testicular cancer, (CA2) ovarian cancer, and (CA3) lung cancer Reinhard, Science, 2020 Normal TissueCancer Tissue

High CLDN6 Associated with a Worsened Prognosis in Cancer Patients CLDN6 Target Biology Context Therapeutics Inc. - August 202311 Endometrial Cancer1 Overexpression of CLDN6 is associated with worse overall survival in endometrial cancer patients 1 Kojima, Cancers, 2020 2 Zhang, Front. Cell Dev. Biol., 2021 3 Kohmoto, Gastric Cancer, 2020 Bladder Cancer2 Overexpression of CLDN6 is associated with worse overall survival and higher disease stage (more aggressive) in bladder cancer patients Stomach Cancer3 Overexpression of CLDN6 is associated with worse overall survival in stomach cancer patients

CLDN6 Has Limited Overlap with Competing Drug Targets in Solid Tumors CLDN6 Target Biology Context Therapeutics Inc. - August 202312 Bishop, AnalyzeR [Data Set] Accessed August 1, 2023 CLDN6 vs. TROP2 CLDN6 vs. NECTIN4 Respiratory Cancer Limited correlation with TROP2 and Nectin-4, no correlation with PD-L1 and EGFR Female Reproductive Cancer Limited correlation with PD-L1 and MUC16, no correlation with Mesothelin and Folate Receptor Alpha CLDN6 vs. PD-L1 CLDN6 vs. EGFR CLDN6 vs. PD-L1 CLDN6 vs. MUC16 CLDN6 vs. Mesothelin CLDN6 vs. Folate Receptor

CTIM-76 Claudin 6 x CD3 Development Candidate Context Therapeutics Inc. - August 202313
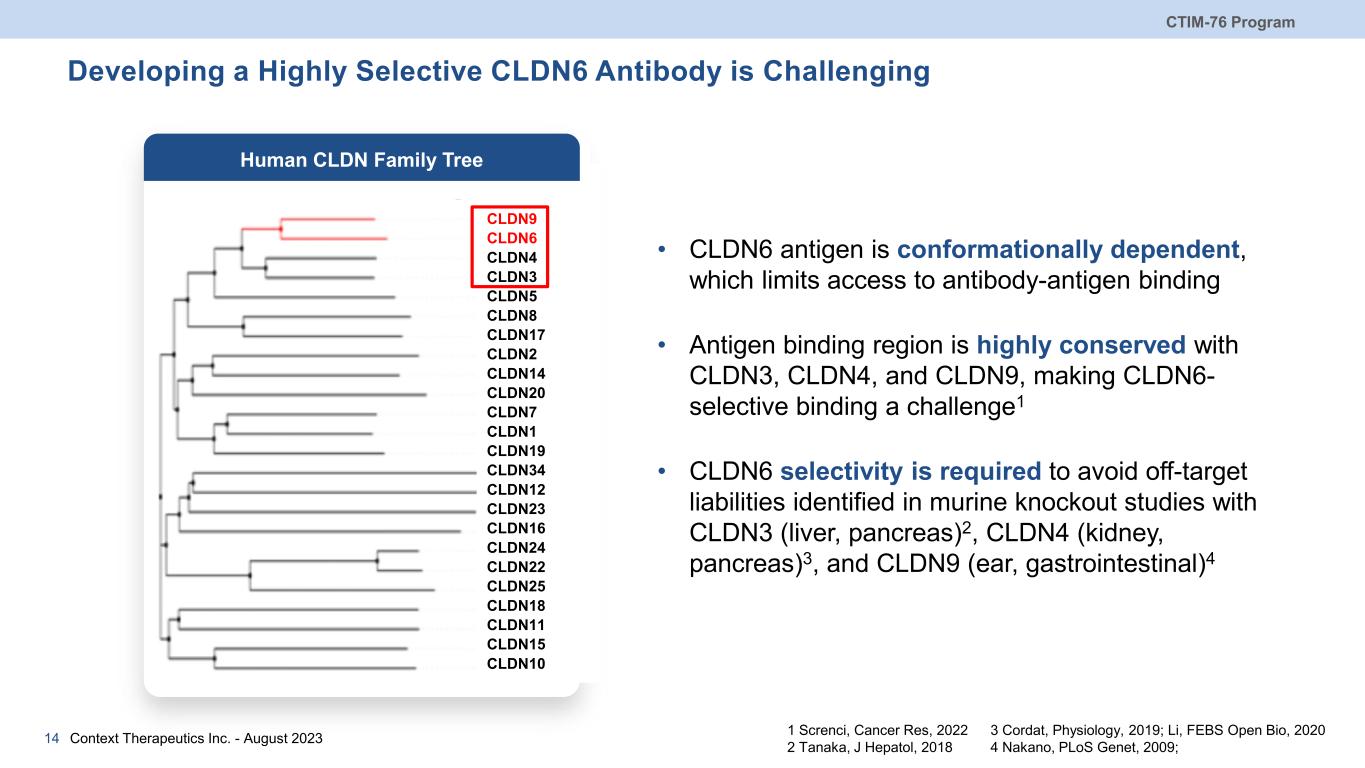
Developing a Highly Selective CLDN6 Antibody is Challenging CTIM-76 Program Context Therapeutics Inc. - August 202314 CLDN9 CLDN6 CLDN4 CLDN3 CLDN5 CLDN8 CLDN17 CLDN2 CLDN14 CLDN20 CLDN7 CLDN1 CLDN19 CLDN34 CLDN12 CLDN23 CLDN16 CLDN24 CLDN22 CLDN25 CLDN18 CLDN11 CLDN15 CLDN10 • CLDN6 antigen is conformationally dependent, which limits access to antibody-antigen binding • Antigen binding region is highly conserved with CLDN3, CLDN4, and CLDN9, making CLDN6- selective binding a challenge1 • CLDN6 selectivity is required to avoid off-target liabilities identified in murine knockout studies with CLDN3 (liver, pancreas)2, CLDN4 (kidney, pancreas)3, and CLDN9 (ear, gastrointestinal)4 3 Cordat, Physiology, 2019; Li, FEBS Open Bio, 2020 4 Nakano, PLoS Genet, 2009; 1 Screnci, Cancer Res, 2022 2 Tanaka, J Hepatol, 2018 Human CLDN Family Tree

CTIM-76 Exhibits Excellent Selectivity and Potency CTIM-76 Program Context Therapeutics Inc. - August 202315 • CTIM-76 CLDN6 EC50 of 3.41 nM (binding) • CTIM-76 preferentially binds to CLDN6 over CLDN3/4/9 • CLDN3/4/6/9 were transiently transfected in HEK-293F cells (4:1 Target:GFP) CLDN6 Selectivity -11 -10 -9 -8 -7 -6 -5 0 50,000 100,000 150,000 200,000 Log [Bispecific] (M) Ce ll Bi nd in g (M FI ) Human CLDN6 Human CLDN4 Human CLDN3 Human CLDN9 Negative CLDN6 Potency • Potency assay provides a better assessment than binding assays for off-target liabilities associated with CLDN3, CLDN4, or CLDN9 • CTIM-76 CLDN6 EC50 of 0.0004 nM (cytotoxicity) • CTIM-76 preferentially targets CLDN6, with minimal binding and cytotoxicity against CLDN9-expressing cells >10,000x >500x
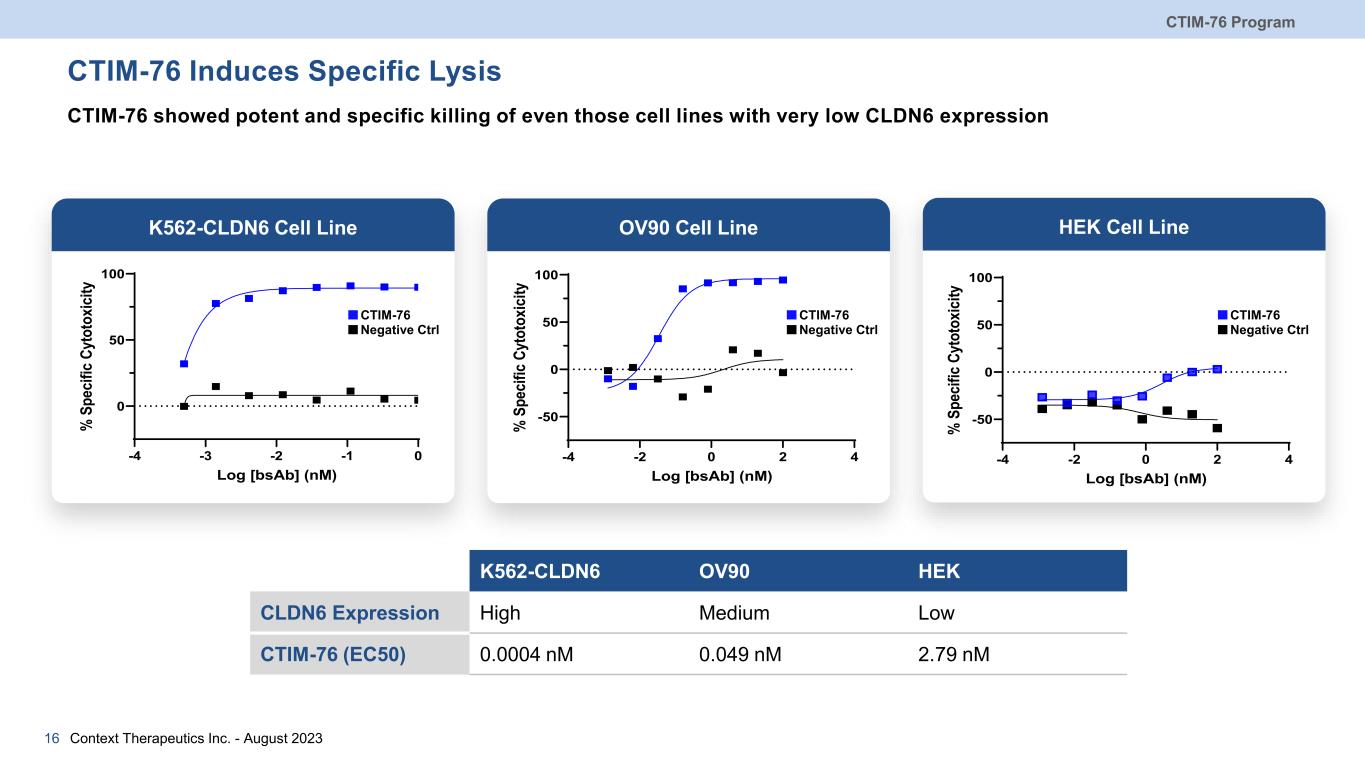
CTIM-76 Induces Specific Lysis CTIM-76 showed potent and specific killing of even those cell lines with very low CLDN6 expression Context Therapeutics Inc. - August 202316 K562-CLDN6 OV90 HEK CLDN6 Expression High Medium Low CTIM-76 (EC50) 0.0004 nM 0.049 nM 2.79 nM OV90 Cell LineK562-CLDN6 Cell Line HEK Cell Line -4 -3 -2 -1 0 0 50 100 Log [bsAb] (nM) % S pe ci fic C yt ot ox ic ity CTIM-76 Negative Ctrl -4 -2 0 2 4 -50 0 50 100 Log [bsAb] (nM) % S pe cif ic Cy to to xic ity -4 -2 0 2 4 -50 0 50 100 Log [bsAb] (nM) % S pe cif ic Cy to to xic ity CTIM-76 Negative Ctrl CTIM-76 Negative Ctrl CTIM-76 Program
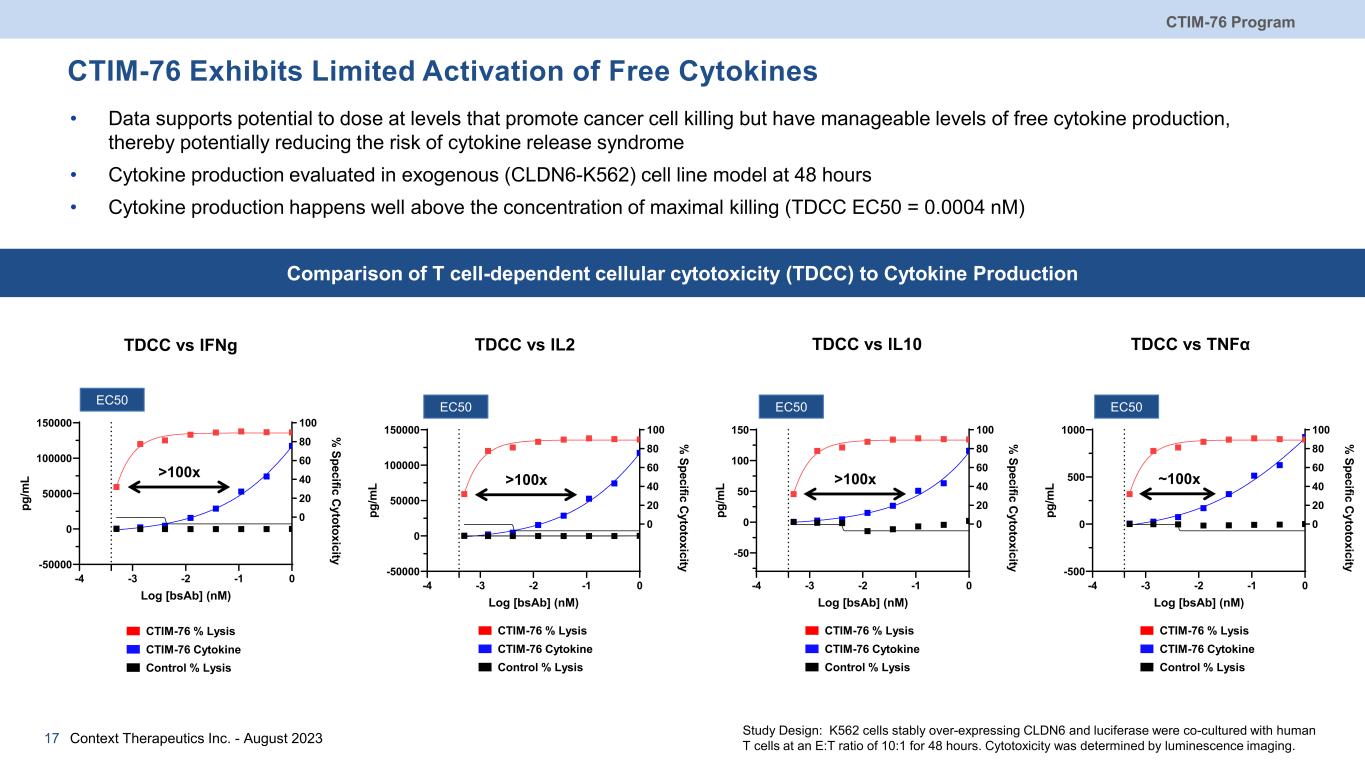
CTIM-76 Program Context Therapeutics Inc. - August 202317 CTIM-76 Exhibits Limited Activation of Free Cytokines • Data supports potential to dose at levels that promote cancer cell killing but have manageable levels of free cytokine production, thereby potentially reducing the risk of cytokine release syndrome • Cytokine production evaluated in exogenous (CLDN6-K562) cell line model at 48 hours • Cytokine production happens well above the concentration of maximal killing (TDCC EC50 = 0.0004 nM) CTIM-76 Cytokine Control % Lysis CTIM-76 % Lysis TDCC vs IL2 -4 -3 -2 -1 0 -50000 0 50000 100000 150000 0 20 40 60 80 100 Log [bsAb] (nM) pg /m L % Specific Cytotoxicity EC50 TDCC vs IFNg EC50 -4 -3 -2 -1 0 -50000 0 50000 100000 150000 0 20 40 60 80 100 Log [bsAb] (nM) pg /m L % Specific Cytotoxicity TDCC vs TNFα EC50 -4 -3 -2 -1 0 -500 0 500 1000 0 20 40 60 80 100 Log [bsAb] (nM) pg /m L % Specific Cytotoxicity TDCC vs IL10 EC50 -4 -3 -2 -1 0 -50 0 50 100 150 0 20 40 60 80 100 Log [bsAb] (nM) pg /m L % Specific Cytotoxicity Study Design: K562 cells stably over-expressing CLDN6 and luciferase were co-cultured with human T cells at an E:T ratio of 10:1 for 48 hours. Cytotoxicity was determined by luminescence imaging. CTIM-76 Cytokine Control % Lysis CTIM-76 % Lysis CTIM-76 Cytokine Control % Lysis CTIM-76 % Lysis CTIM-76 Cytokine Control % Lysis CTIM-76 % Lysis Comparison of T cell-dependent cellular cytotoxicity (TDCC) to Cytokine Production >100x >100x >100x ~100x
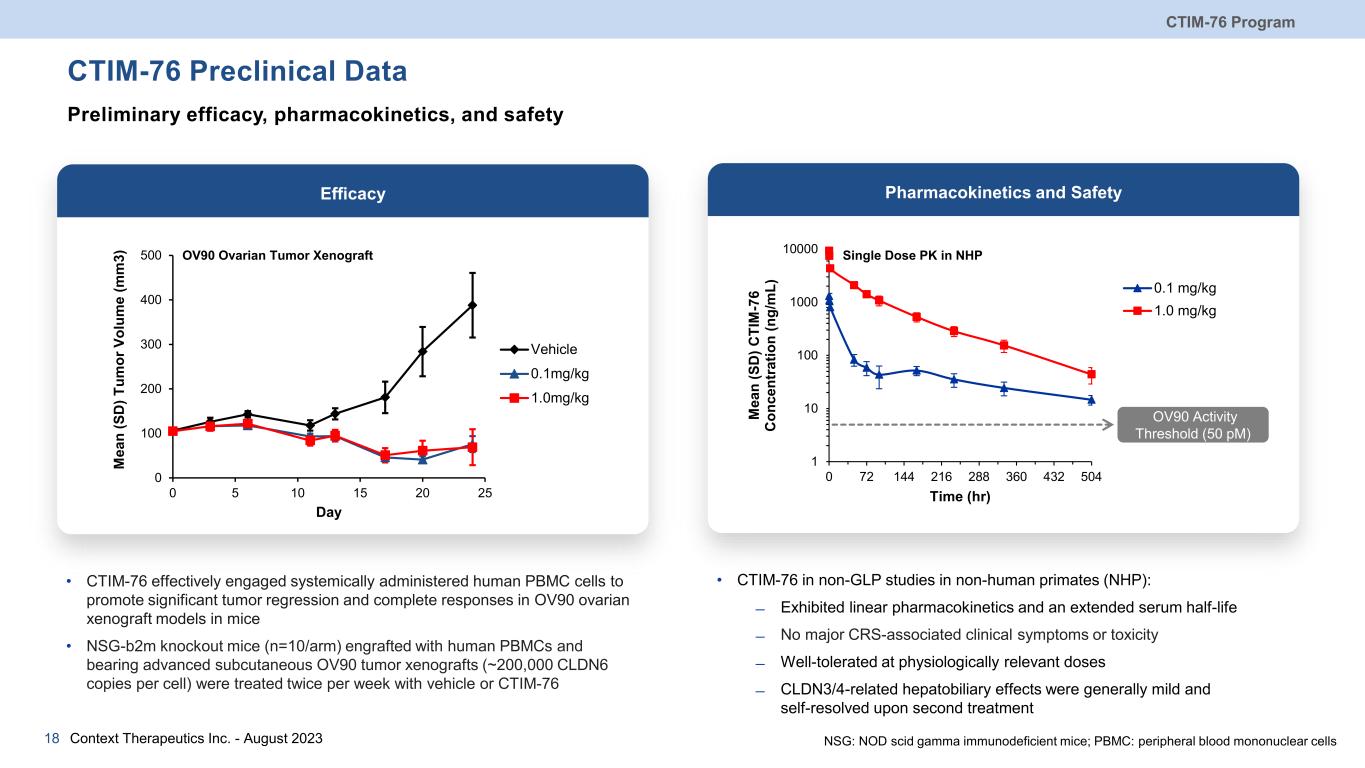
CTIM-76 Preclinical Data CTIM-76 Program Context Therapeutics Inc. - August 202318 Preliminary efficacy, pharmacokinetics, and safety • CTIM-76 effectively engaged systemically administered human PBMC cells to promote significant tumor regression and complete responses in OV90 ovarian xenograft models in mice • NSG-b2m knockout mice (n=10/arm) engrafted with human PBMCs and bearing advanced subcutaneous OV90 tumor xenografts (~200,000 CLDN6 copies per cell) were treated twice per week with vehicle or CTIM-76 Efficacy • CTIM-76 in non-GLP studies in non-human primates (NHP): ̶ Exhibited linear pharmacokinetics and an extended serum half-life ̶ No major CRS-associated clinical symptoms or toxicity ̶ Well-tolerated at physiologically relevant doses ̶ CLDN3/4-related hepatobiliary effects were generally mild and self-resolved upon second treatment Pharmacokinetics and Safety 0 100 200 300 400 500 0 5 10 15 20 25 M ea n (S D ) T um or V ol um e (m m 3) Day Vehicle 0.1mg/kg 1.0mg/kg OV90 Activity Threshold (50 pM) NSG: NOD scid gamma immunodeficient mice; PBMC: peripheral blood mononuclear cells 1 10 100 1000 10000 0 72 144 216 288 360 432 504 M ea n (S D ) C TI M -7 6 C on ce nt ra tio n (n g/ m L) Time (hr) 0.1 mg/kg 1.0 mg/kg OV90 Ovarian Tumor Xenograft Single Dose PK in NHP

Competitive Landscape 19 Context Therapeutics Inc. - August 2023

CLDN6 Competitive Landscape1 Context Therapeutics Inc. - August 202320 Preclinical Phase 1 Antibody Drug Conjugate (ADC) Bispecific Antibody Cell Therapy CLDN6-CAR-NK CAR-NK + IL7 BNT211 CAR-T + CARVac TJ-C64B 2+2 bsAb CLDN6x41BB TORL-1-23 CLDN6 + MMAE AMG794 BiTE CLDN6xCD3 BNT142 mRNA encoded BsAb CLDN6xCD3 XmAb541 2+1 bsAb CLDN6xCD3 SAIL66 bsAb CLDN6xCD3 DS-9606a CLDN6/CLDN9 + DXd GB-7008-01 CLDN6/CLDN9 + MMAE 1 Analysis based on current understanding of publicly available information compiled as of August 1, 2023 CTIM-76 bsAb CLDN6xCD3 Competitive Landscape Undisclosed CAR-NK

Phase 1 Data Presented by BioNTech and TORL Biotherapeutics at ASCO 2023 CLDN6 programs differentiated by product category, selectivity, potency, and manufacturability CTIM-76 BNT211 TORL-1-23 Category T cell Engager CAR-T + CARVac ADC Mechanism of Action T cell activation and recruitment to CLDN6+ tumor Ex vivo T cell activation and recruitment to CLDN6+ tumor with CLDN6 antigen primer to enhance T cell persistence Preclinical data suggests activity driven by a mix of ADCC-mediated and ADC bystander effect Side Effects n/a (preclinical) Liver enzyme elevations, CRS Alopecia, anemia, neuropathy, pneumonia Selectivity Potency Manufacturability Competitive Landscape Context Therapeutics Inc. - August 202321 Comparison based on publicly available information and represents a non-head-to-head summary comparison Analysis based on current understanding of publicly available information compiled as of August 1, 2023
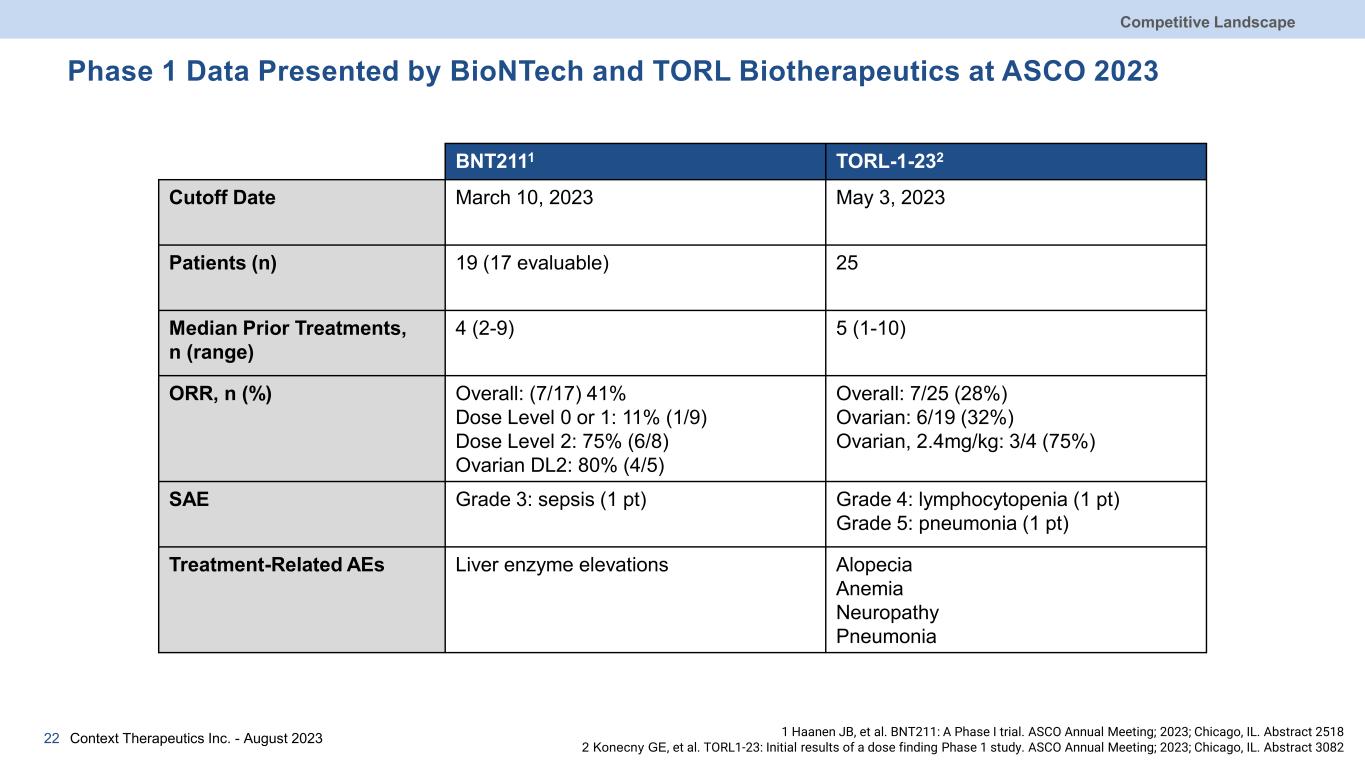
Phase 1 Data Presented by BioNTech and TORL Biotherapeutics at ASCO 2023 BNT2111 TORL-1-232 Cutoff Date March 10, 2023 May 3, 2023 Patients (n) 19 (17 evaluable) 25 Median Prior Treatments, n (range) 4 (2-9) 5 (1-10) ORR, n (%) Overall: (7/17) 41% Dose Level 0 or 1: 11% (1/9) Dose Level 2: 75% (6/8) Ovarian DL2: 80% (4/5) Overall: 7/25 (28%) Ovarian: 6/19 (32%) Ovarian, 2.4mg/kg: 3/4 (75%) SAE Grade 3: sepsis (1 pt) Grade 4: lymphocytopenia (1 pt) Grade 5: pneumonia (1 pt) Treatment-Related AEs Liver enzyme elevations Alopecia Anemia Neuropathy Pneumonia Context Therapeutics Inc. - August 202322 Competitive Landscape 1 Haanen JB, et al. BNT211: A Phase I trial. ASCO Annual Meeting; 2023; Chicago, IL. Abstract 2518 2 Konecny GE, et al. TORL1-23: Initial results of a dose finding Phase 1 study. ASCO Annual Meeting; 2023; Chicago, IL. Abstract 3082

Corporate 23 Context Therapeutics Inc. - August 2023

Corporate Experienced Leadership Team • Experienced team with deep oncology experience • Our CMO led the clinical development of multiple blockbuster drugs including Kisqali, Arimidex, and Afinitor • Our management team is supported by a Board with strong public company operating and governance experience Focus on Execution Martin Lehr CEO and Director Tarek Sahmoud, MD, PhD Chief Medical Officer Alex Levit, Esq Chief Legal Officer Jennifer Minai, CPA Chief Financial Officer Chris Beck, MBA SVP Operations Priya Marreddy, MS VP Clinical Operations Context Therapeutics Inc. - August 202324

Corporate Investment Highlights (Nasdaq: CNTX) Solid Tumors Large Unmet Need Claudin 6 High-Value Target Anticipated Q1 2024 IND filing Near-Term Milestones Deep Domain Experience, Track Record of Success Strong Team Expected Cash Runway into late 2024 Financial Strength Context Therapeutics Inc. - August 202325

Advancing Medicines for Solid Tumors © Context Therapeutics 2023