0001213037false00012130372024-12-102024-12-10
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): December 10, 2024 |
Cardiff Oncology, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-35558 |
27-2004382 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
11055 Flintkote Avenue |
|
San Diego, California |
|
92121 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (858) 952-7570 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock |
|
CRDF |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On December 10, 2024, Cardiff Oncology, Inc. (the “Company”) plans to present in a conference call presentation materials related to the announcement of positive initial data from CRDF-004, a randomized, Phase 2 clinical trial evaluating onvansertib in combination with standard-of-care (SoC) in patients with first-line RAS-mutated metastatic colorectal cancer (mCRC). A copy of the presentation materials is furnished as Exhibit 99.1 to this Form 8-K.
The information in this report, including the press release furnished as Exhibit 99.1 hereto, shall not be deemed to be “filed” for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, and shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing. In addition, the exhibit furnished herewith contain statements intended as “forward-looking statements” that are subject to the cautionary statements about forward-looking statements set forth in such exhibit.
Item 8.01 Other Events.
On December 10, 2024, the Company issued a press release (the “Press Release”) announcing positive initial data from CRDF-004, a randomized, Phase 2 clinical trial evaluating onvansertib in combination with standard-of-care (SoC) in patients with first-line RAS-mutated metastatic colorectal cancer (mCRC). A copy of the press release is attached as Exhibit 99.2 hereto. The information in the Press Release, except for the information set forth in the second paragraph of the Press Release containing a quote by Fairooz Kabbinavar, MD, FACP, Chief Medical Officer of the Company and the seventh paragraph of the Press Release containing a quote by Mark Erlander, Chief Executive Officer of the Company, is incorporated herein by reference.
Item 9.01. Financial Statements and Exhibits
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
CARDIFF ONCOLOGY, INC. |
|
|
|
|
Date: |
December 10, 2024 |
By: |
/s/ Mark Erlander |
|
|
|
Mark Erlander
Chief Executive Officer |
DECEMBER 10, 2024 CRDF-004 Trial 1st Line RAS-mutated mCRC Initial Data Release

Forward-looking statements Certain statements in this presentation are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of words such as "anticipate," "believe," "forecast," "estimated" and "intend" or other similar terms or expressions that concern our expectations, strategy, plans or intentions. These forward-looking statements are based on our current expectations and actual results could differ materially. There are several factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results; our clinical trials may be suspended or discontinued due to unexpected side effects or other safety risks that could preclude approval of our product candidate; results of preclinical studies or clinical trials for our product candidate could be unfavorable or delayed; early results from clinical trials may not be indicative of final results; our need for additional financing; risks related to business interruptions, including the outbreak of COVID-19 coronavirus and cyber-attacks on our information technology infrastructure, which could seriously harm our financial condition and increase our costs and expenses; uncertainties of government or third party payer reimbursement; dependence on key personnel; limited experience in marketing and sales; substantial competition; uncertainties of patent protection and litigation; dependence upon third parties; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. There are no guarantees that our product candidate will be utilized or prove to be commercially successful. Additionally, there are no guarantees that future clinical trials will be completed or successful or that our product candidate will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in our Form 10-K for the year ended December 31, 2023, and other periodic reports filed with the Securities and Exchange Commission. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and we do not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances.

Mark Erlander, PhD Chief Executive Officer

1st line RAS-mut mCRC trial data (CRDF-004) Commercial opportunity in 1st line mCRC The broader onvansertib opportunity AGENDA
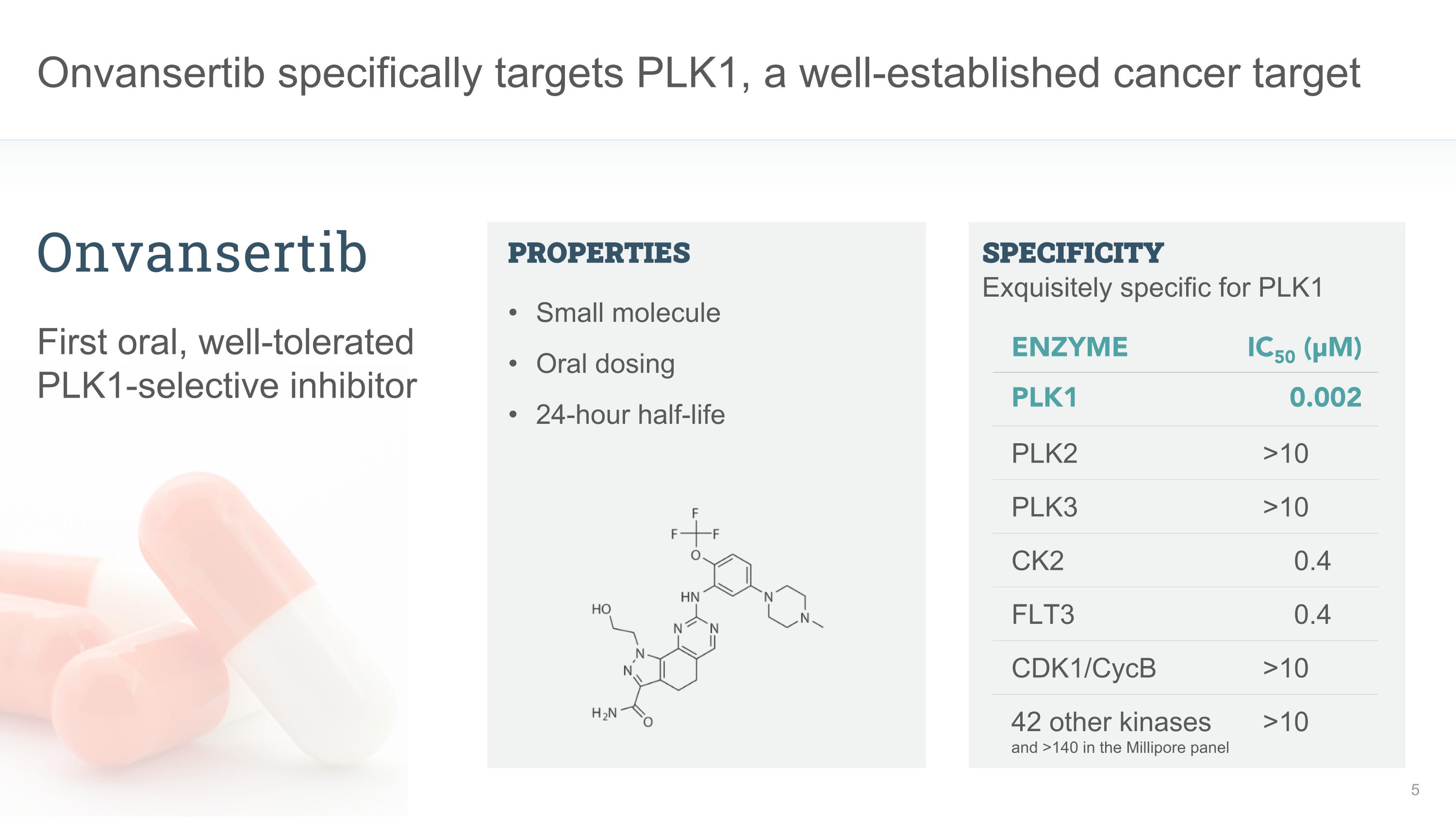
Onvansertib specifically targets PLK1, a well-established cancer target Onvansertib First oral, well-tolerated PLK1-selective inhibitor ENZYME IC50 (µM) PLK1 0.002 PLK2 >10.000 PLK3 >10.000 CK2 0.400 FLT3 0.400 CDK1/CycB >10.000 42 other kinases�and >140 in the Millipore panel >10.000 Exquisitely specific for PLK1 SPECIFICITY PROPERTIES Small molecule Oral dosing 24-hour half-life

1st line RAS-mut mCRC trial data (CRDF-004) AGENDA Fairooz Kabbinavar, MD, FACP Chief Medical Officer
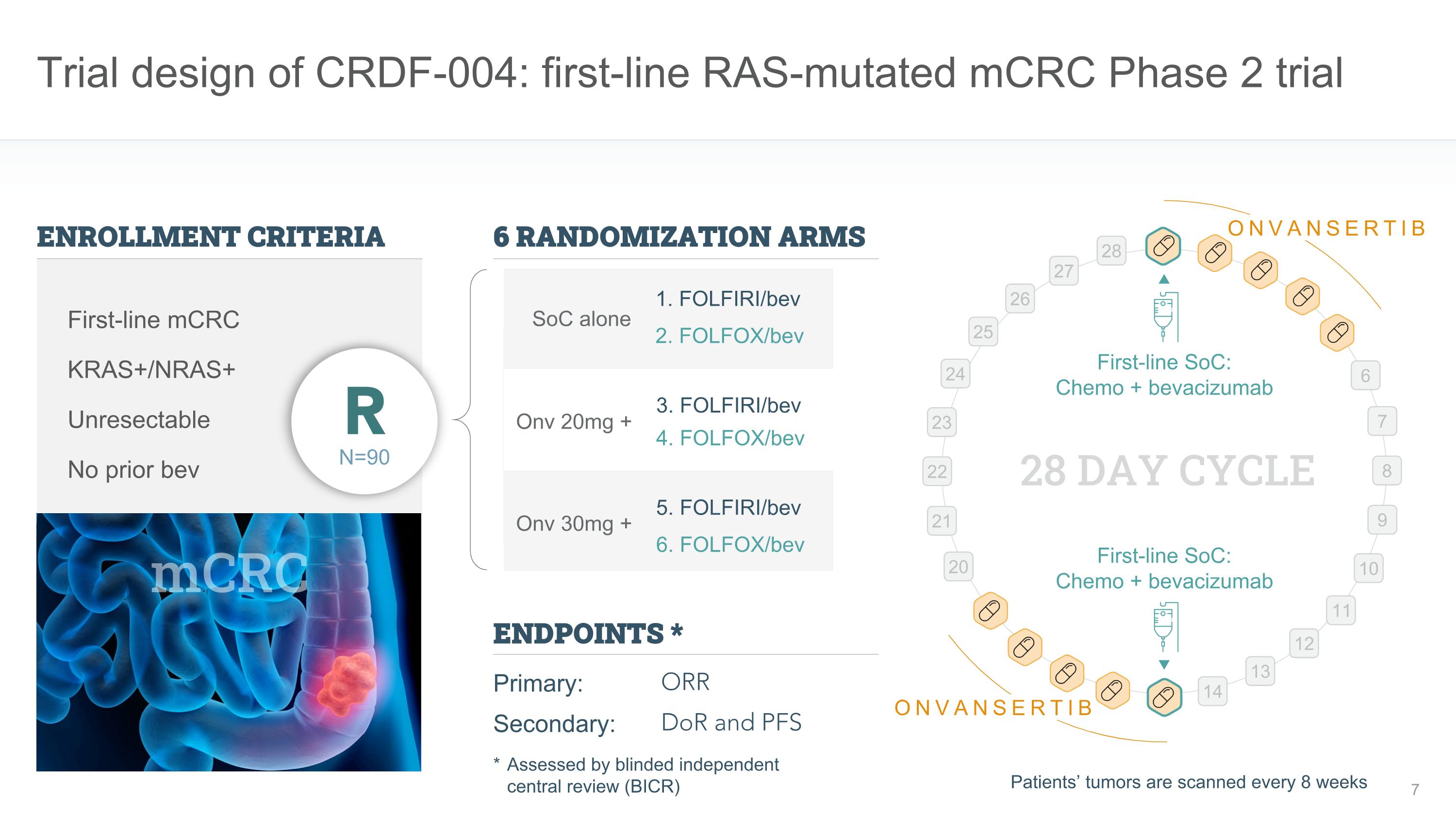
Trial design of CRDF-004: first-line RAS-mutated mCRC Phase 2 trial First-line SoC: �Chemo + bevacizumab First-line SoC: �Chemo + bevacizumab 28 DAY CYCLE 6 7 8 9 10 11 12 13 14 20 21 22 23 24 25 26 27 28 ONVANSERTIB ONVANSERTIB ENROLLMENT CRITERIA ENDPOINTS * Primary: Secondary: ORR DoR and PFS KRAS+/NRAS+ Unresectable First-line mCRC N=90 R No prior bev mCRC Onv 30mg + Onv 20mg + SoC alone 6 RANDOMIZATION ARMS * Assessed by blinded independent �central review (BICR) Patients’ tumors are scanned every 8 weeks 1. FOLFIRI/bev 2. FOLFOX/bev 3. FOLFIRI/bev 4. FOLFOX/bev 5. FOLFIRI/bev 6. FOLFOX/bev
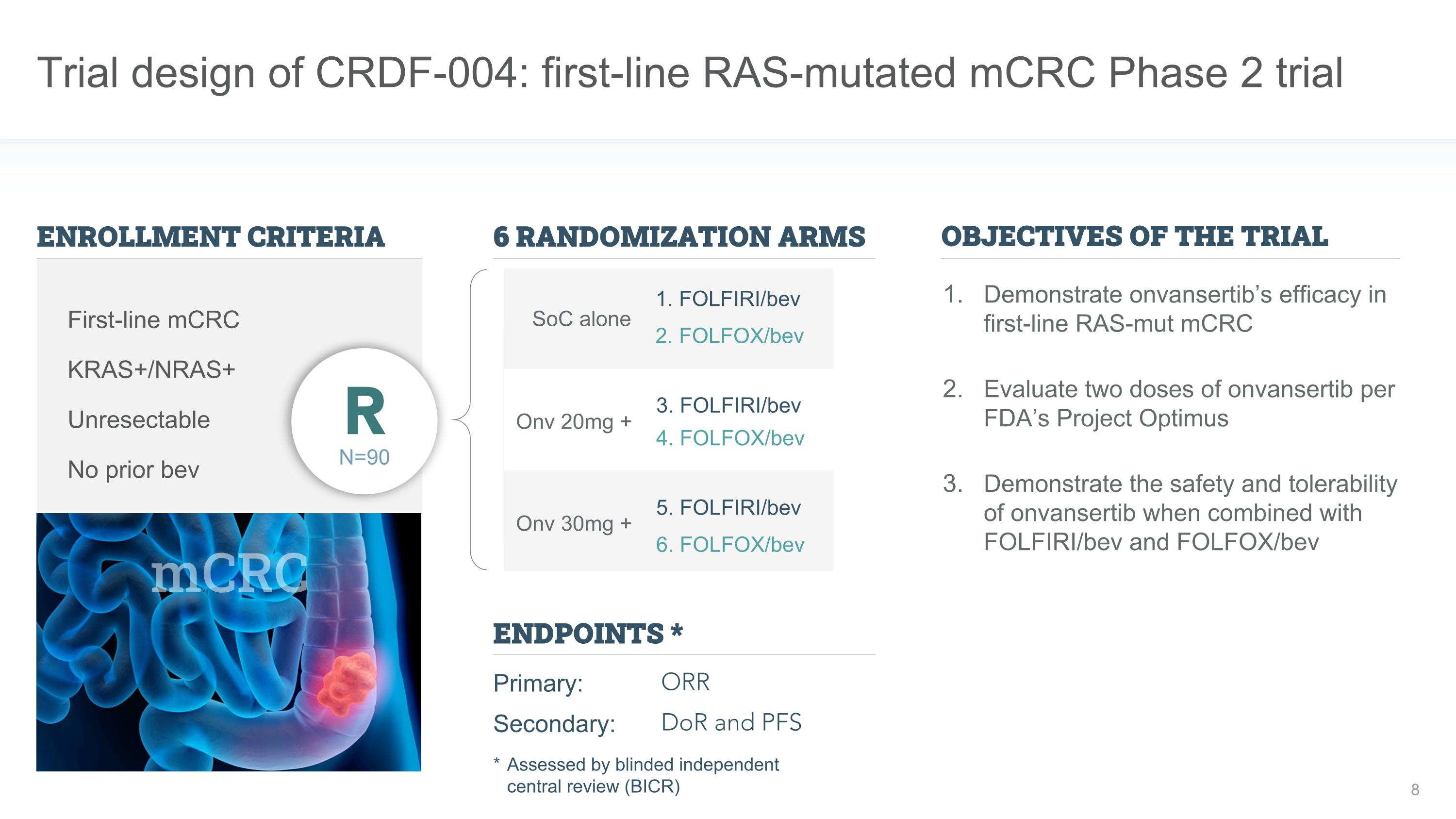
Trial design of CRDF-004: first-line RAS-mutated mCRC Phase 2 trial ENROLLMENT CRITERIA ENDPOINTS * Primary: Secondary: ORR DoR and PFS KRAS+/NRAS+ Unresectable First-line mCRC N=90 R No prior bev mCRC Onv 30mg + Onv 20mg + SoC alone 6 RANDOMIZATION ARMS Demonstrate onvansertib’s efficacy in first-line RAS-mut mCRC Evaluate two doses of onvansertib per FDA’s Project Optimus Demonstrate the safety and tolerability of onvansertib when combined with FOLFIRI/bev and FOLFOX/bev OBJECTIVES OF THE TRIAL 1. FOLFIRI/bev 2. FOLFOX/bev 3. FOLFIRI/bev 4. FOLFOX/bev 5. FOLFIRI/bev 6. FOLFOX/bev * Assessed by blinded independent �central review (BICR)
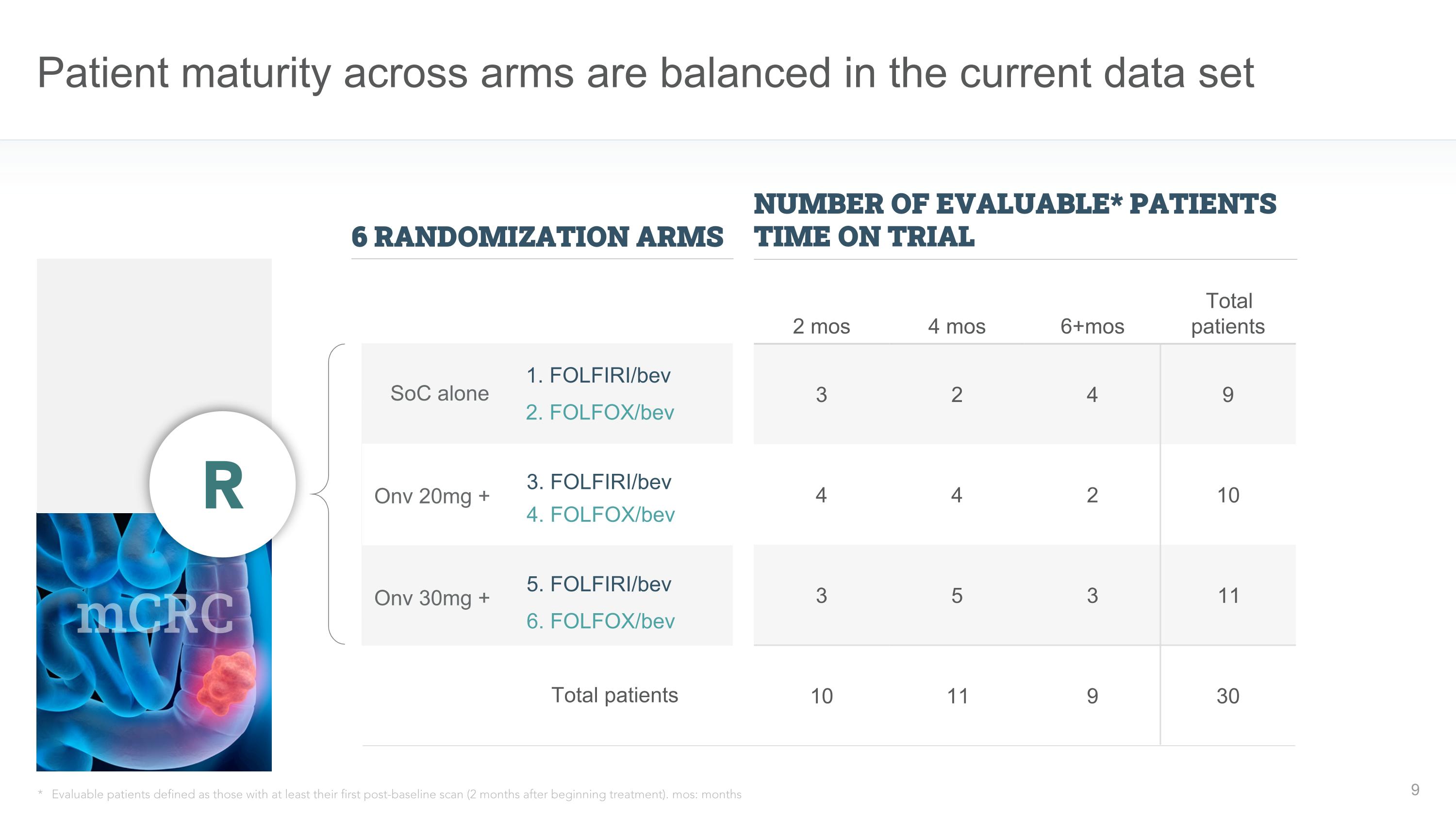
Patient maturity across arms are balanced in the current data set mCRC Onv 30mg + Onv 20mg + SoC alone 6 RANDOMIZATION ARMS NUMBER OF EVALUABLE* PATIENTS TIME ON TRIAL 2 mos 4 mos 6+mos Total patients 3 2 4 9 4 4 2 10 3 5 3 11 10 11 9 30 R * Evaluable patients defined as those with at least their first post-baseline scan (2 months after beginning treatment). mos: months 1. FOLFIRI/bev 2. FOLFOX/bev 3. FOLFIRI/bev 4. FOLFOX/bev 5. FOLFIRI/bev 6. FOLFOX/bev Total patients
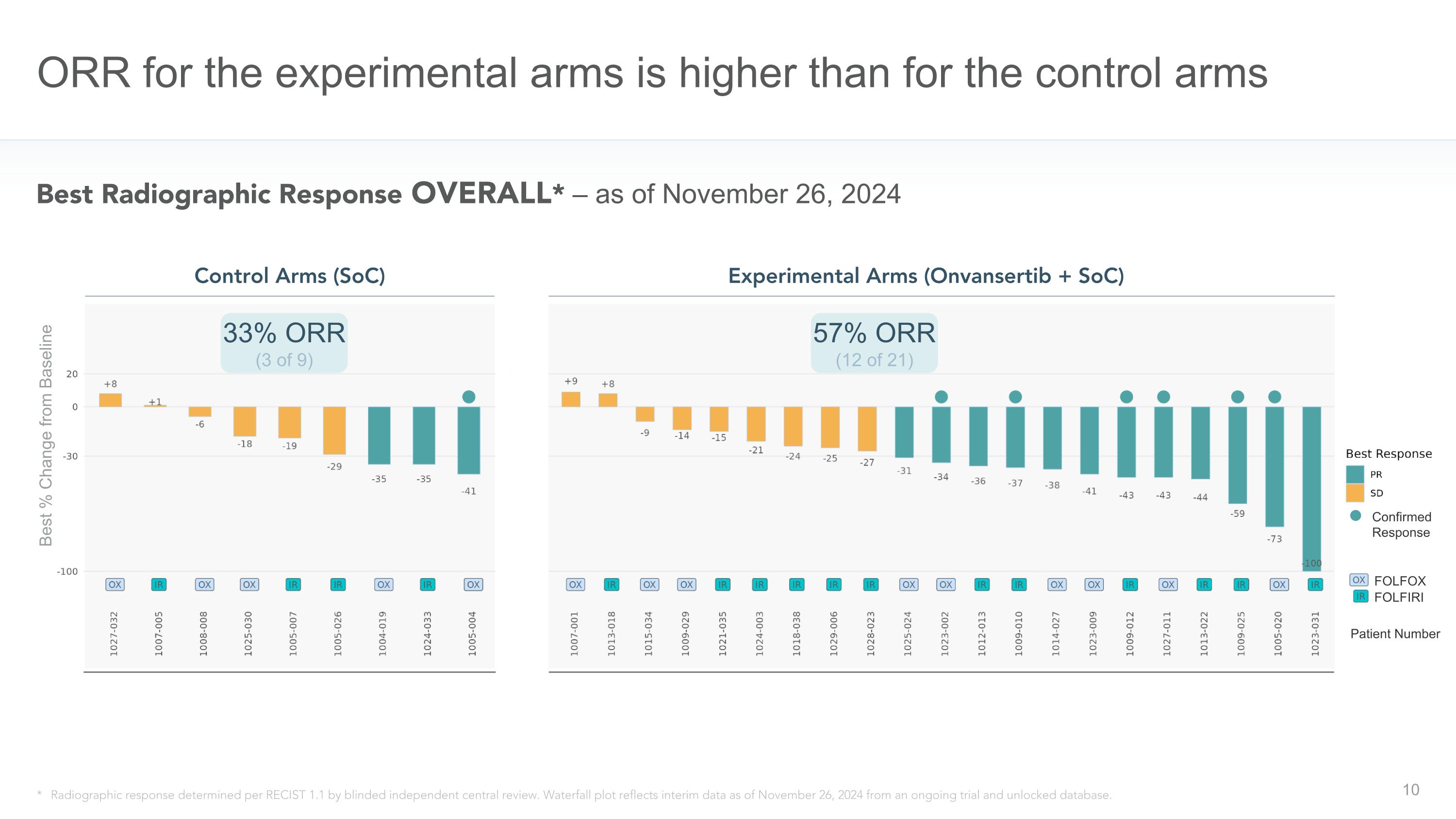
ORR for the experimental arms is higher than for the control arms * Date Labels: estimated date of 8-week scan * Radiographic response determined per RECIST 1.1 by blinded independent central review. Waterfall plot reflects interim data as of November 26, 2024 from an ongoing trial and unlocked database. 57% ORR (12 of 21) Control Arms (SoC) Experimental Arms (Onvansertib + SoC) Best Radiographic Response OVERALL* – as of November 26, 2024 Patient Number FOLFOX FOLFIRI Confirmed Response 33% ORR (3 of 9) Best % Change from Baseline
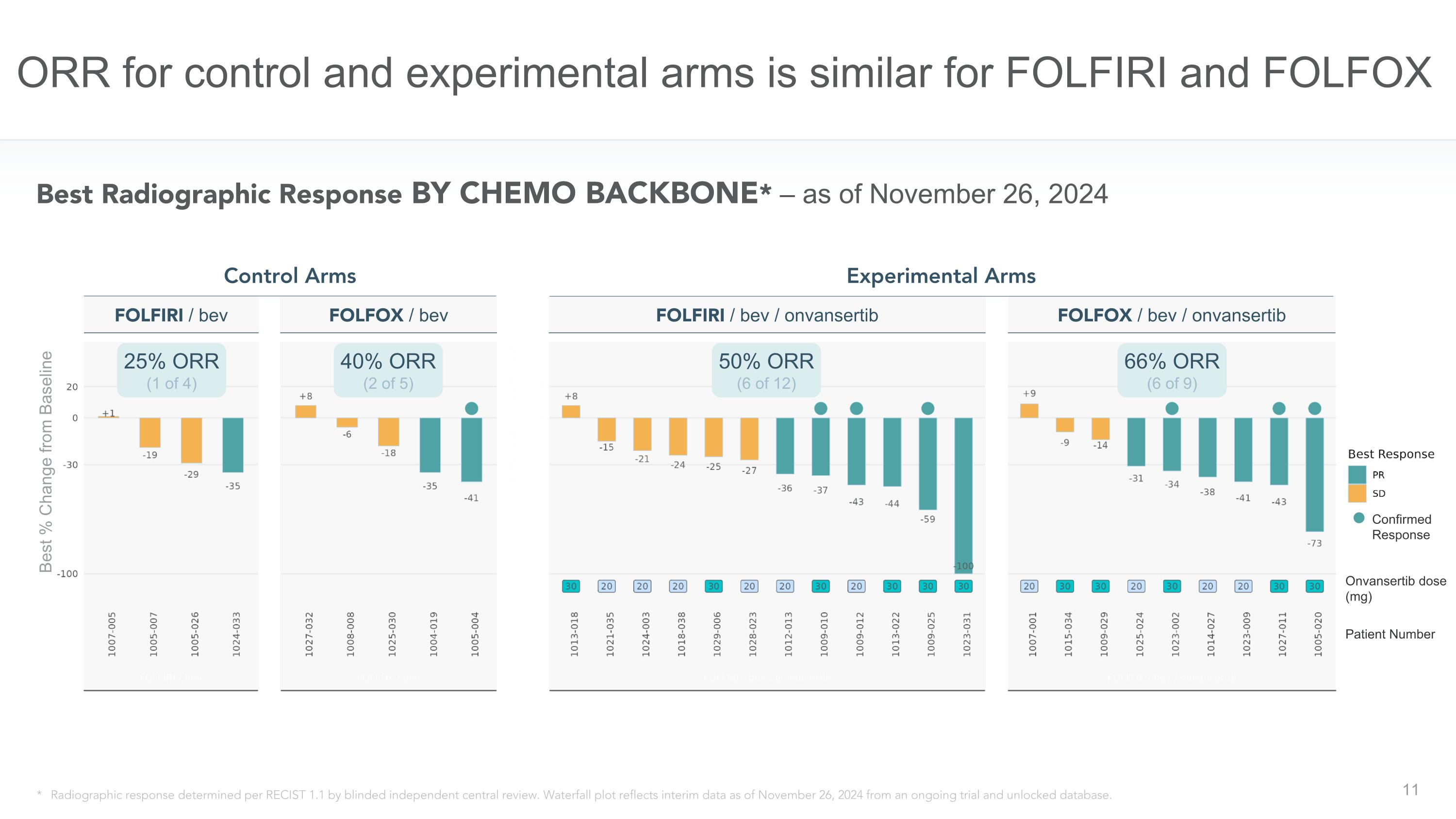
Patient Number FOLFIRI / bev ORR for control and experimental arms is similar for FOLFIRI and FOLFOX * Radiographic response determined per RECIST 1.1 by blinded independent central review. Waterfall plot reflects interim data as of November 26, 2024 from an ongoing trial and unlocked database. 25% ORR (1 of 4) 40% ORR (2 of 5) 66% ORR (6 of 9) Control Arms Experimental Arms FOLFOX / bev Best Radiographic Response BY CHEMO BACKBONE* – as of November 26, 2024 FOLFOX / bev / onvansertib 50% ORR (6 of 12) FOLFIRI / bev / onvansertib Confirmed Response Onvansertib dose (mg) Best % Change from Baseline
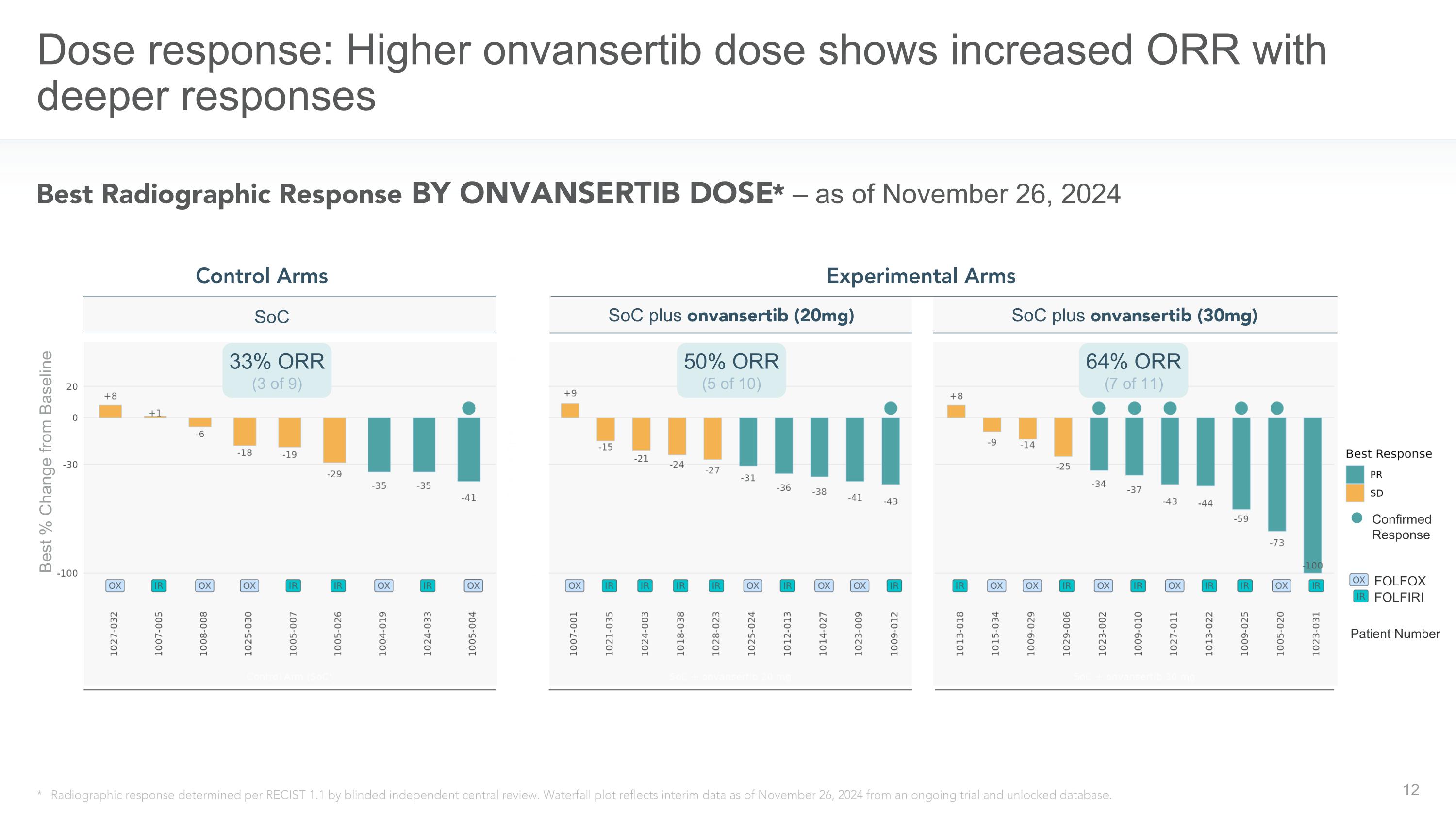
Dose response: Higher onvansertib dose shows increased ORR with deeper responses Best Radiographic Response BY ONVANSERTIB DOSE* – as of November 26, 2024 * Radiographic response determined per RECIST 1.1 by blinded independent central review. Waterfall plot reflects interim data as of November 26, 2024 from an ongoing trial and unlocked database. Control Arms Experimental Arms SoC plus onvansertib (20mg) SoC plus onvansertib (30mg) SoC Confirmed Response Patient Number FOLFOX FOLFIRI 50% ORR (5 of 10) 64% ORR (7 of 11) 33% ORR (3 of 9) Best % Change from Baseline
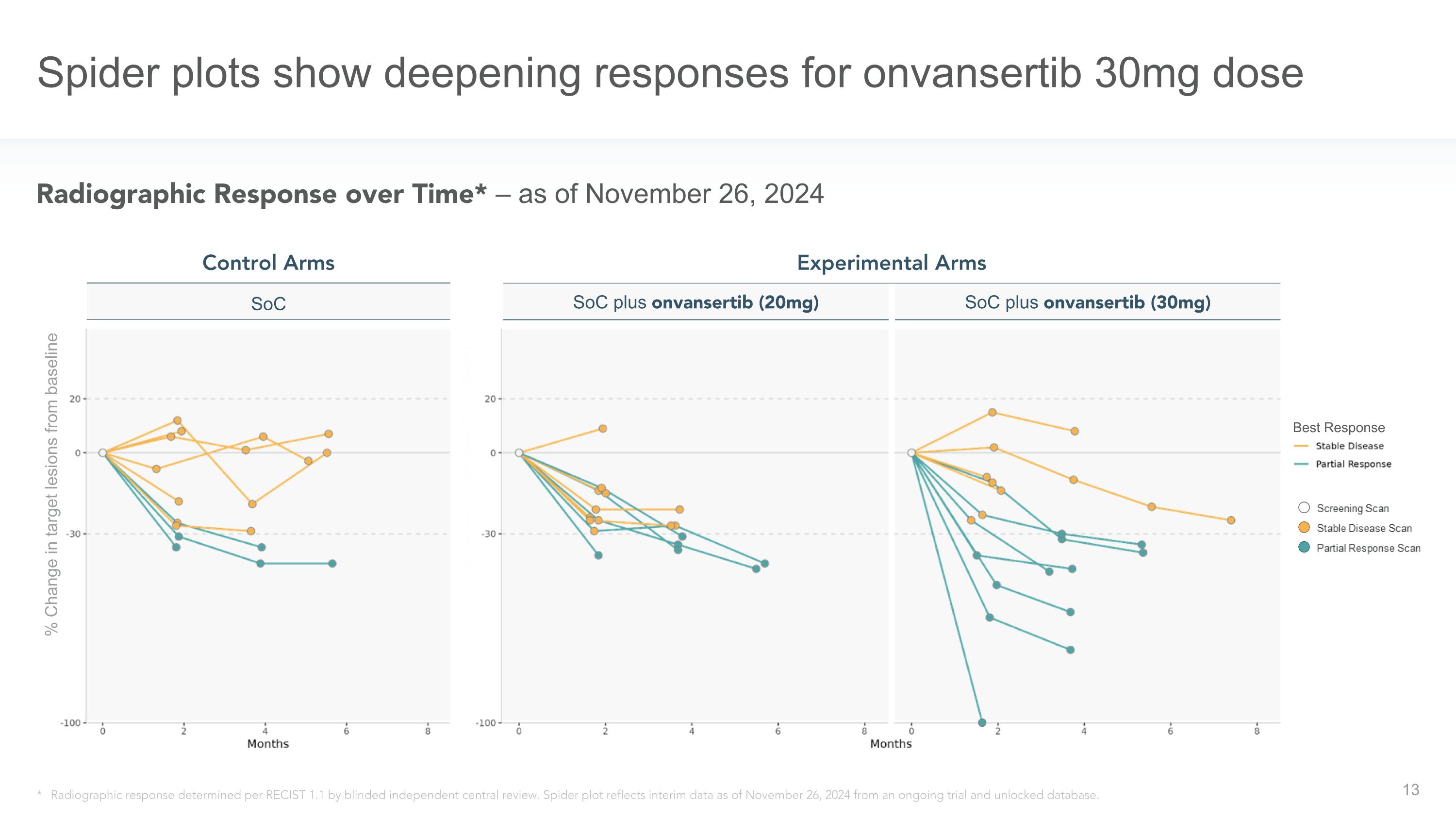
Spider plots show deepening responses for onvansertib 30mg dose * Radiographic response determined per RECIST 1.1 by blinded independent central review. Spider plot reflects interim data as of November 26, 2024 from an ongoing trial and unlocked database. Radiographic Response over Time* – as of November 26, 2024 Experimental Arms SoC plus onvansertib (20mg) SoC plus onvansertib (30mg) Control Arms SoC Best Response % Change in target lesions from baseline
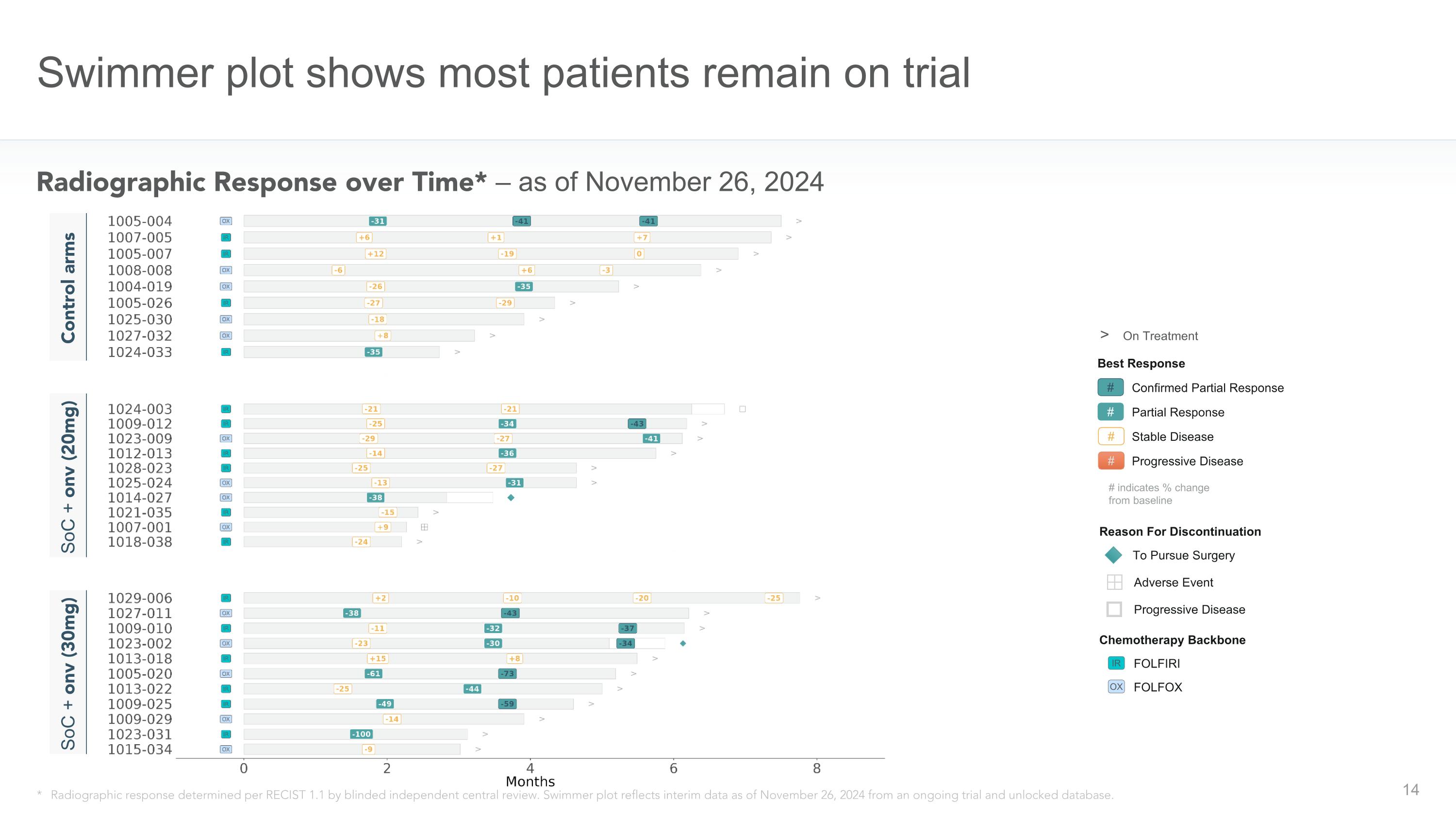
Swimmer plot shows most patients remain on trial Radiographic Response over Time* – as of November 26, 2024 Best Response # Confirmed Partial Response # Partial Response # Stable Disease Reason For Discontinuation # indicates % change from baseline # Progressive Disease > On Treatment To Pursue Surgery Adverse Event Progressive Disease * Radiographic response determined per RECIST 1.1 by blinded independent central review. Swimmer plot reflects interim data as of November 26, 2024 from an ongoing trial and unlocked database. SoC + onv (20mg) Control arms SoC + onv (30mg) Chemotherapy Backbone FOLFIRI FOLFOX IR OX
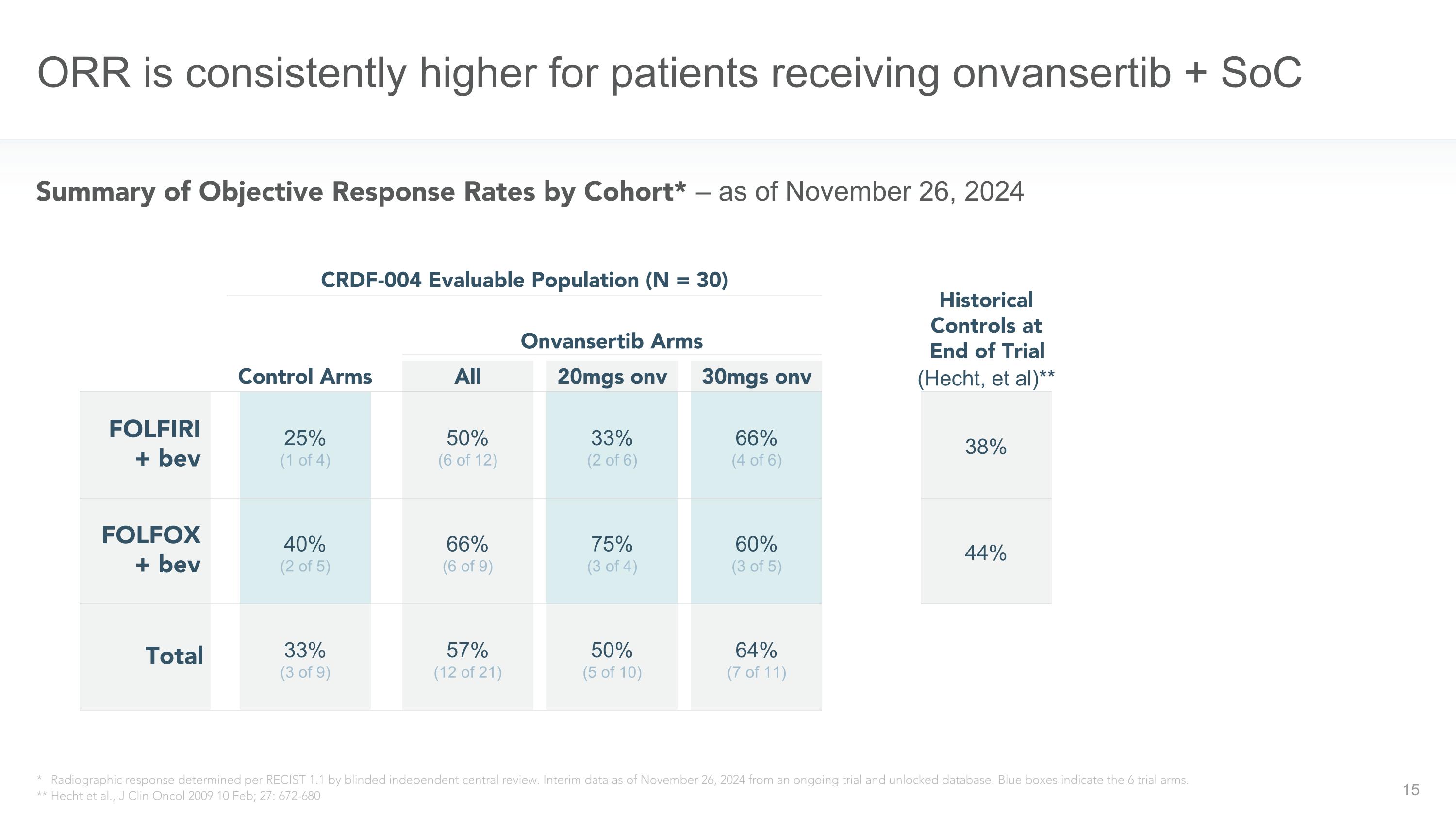
ORR is consistently higher for patients receiving onvansertib + SoC FOLFOX�+ bev FOLFIRI�+ bev 25% (1 of 4) Control Arms Onvansertib Arms Total 40% (2 of 5) 33% (3 of 9) All 30mgs onv 20mgs onv 50% (6 of 12) 66% (6 of 9) 57% (12 of 21) CRDF-004 Evaluable Population (N = 30) * Radiographic response determined per RECIST 1.1 by blinded independent central review. Interim data as of November 26, 2024 from an ongoing trial and unlocked database. Blue boxes indicate the 6 trial arms. ** Hecht et al., J Clin Oncol 2009 10 Feb; 27: 672-680 38% Historical Controls at�End of Trial (Hecht, et al)** 44% Summary of Objective Response Rates by Cohort* – as of November 26, 2024 66% (4 of 6) 60% (3 of 5) 64% (7 of 11) 33% (2 of 6) 75% (3 of 4) 50% (5 of 10)
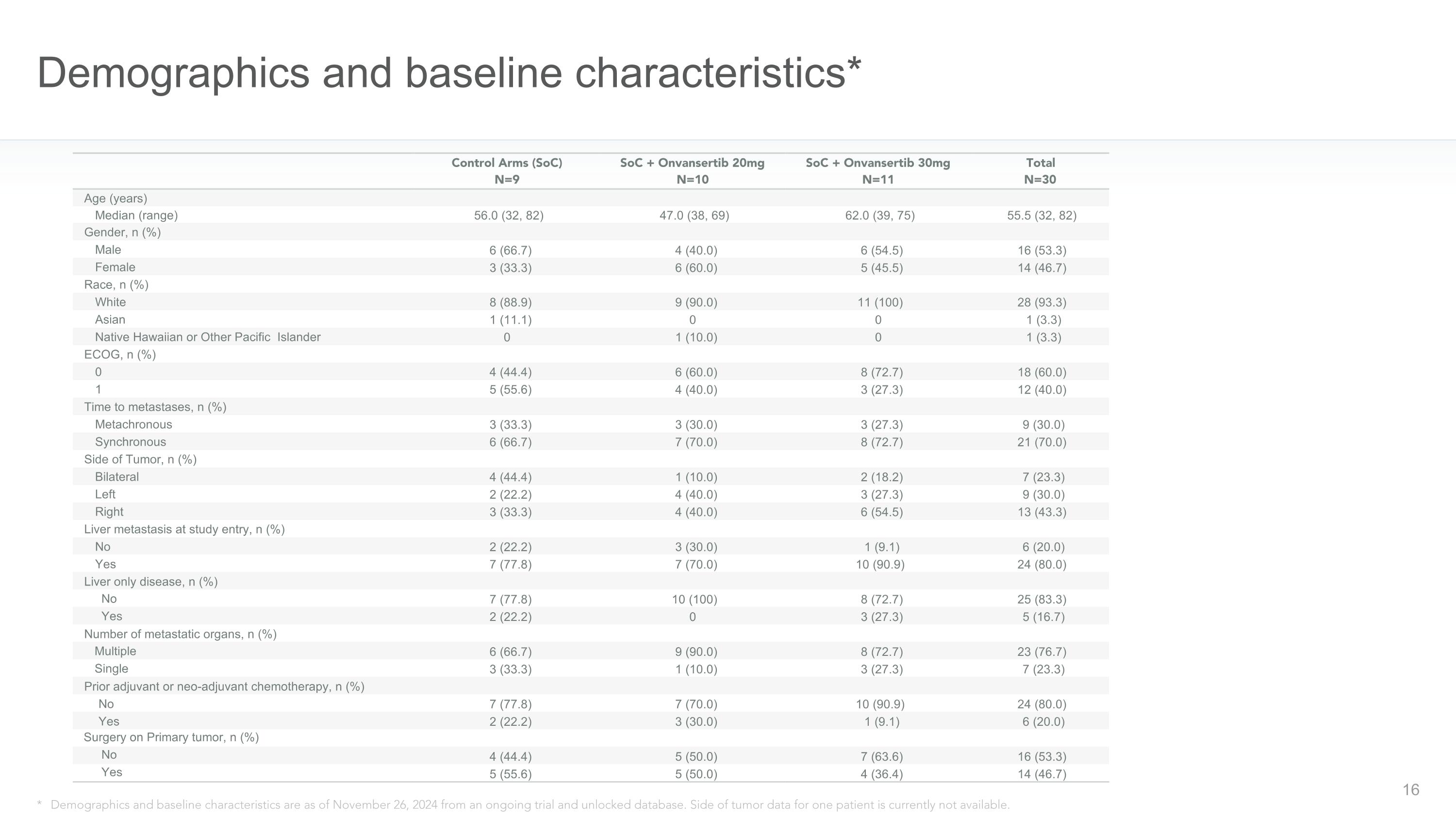
Demographics and baseline characteristics* * Demographics and baseline characteristics are as of November 26, 2024 from an ongoing trial and unlocked database. Side of tumor data for one patient is currently not available. Control Arms (SoC)�N=9 SoC + Onvansertib 20mg�N=10 SoC + Onvansertib 30mg�N=11 Total�N=30 Age (years) Median (range) 56.0 (32, 82) 47.0 (38, 69) 62.0 (39, 75) 55.5 (32, 82) Gender, n (%) Male 6 (66.7) 4 (40.0) 6 (54.5) 16 (53.3) Female 3 (33.3) 6 (60.0) 5 (45.5) 14 (46.7) Race, n (%) White 8 (88.9) 9 (90.0) 11 (100) 28 (93.3) Asian 1 (11.1) 0 0 1 (3.3) Native Hawaiian or Other Pacific Islander 0 1 (10.0) 0 1 (3.3) ECOG, n (%) 0 4 (44.4) 6 (60.0) 8 (72.7) 18 (60.0) 1 5 (55.6) 4 (40.0) 3 (27.3) 12 (40.0) Time to metastases, n (%) Metachronous 3 (33.3) 3 (30.0) 3 (27.3) 9 (30.0) Synchronous 6 (66.7) 7 (70.0) 8 (72.7) 21 (70.0) Side of Tumor, n (%) Bilateral 4 (44.4) 1 (10.0) 2 (18.2) 7 (23.3) Left 2 (22.2) 4 (40.0) 3 (27.3) 9 (30.0) Right 3 (33.3) 4 (40.0) 6 (54.5) 13 (43.3) Liver metastasis at study entry, n (%) No 2 (22.2) 3 (30.0) 1 (9.1) 6 (20.0) Yes 7 (77.8) 7 (70.0) 10 (90.9) 24 (80.0) Liver only disease, n (%) No 7 (77.8) 10 (100) 8 (72.7) 25 (83.3) Yes 2 (22.2) 0 3 (27.3) 5 (16.7) Number of metastatic organs, n (%) Multiple 6 (66.7) 9 (90.0) 8 (72.7) 23 (76.7) Single 3 (33.3) 1 (10.0) 3 (27.3) 7 (23.3) Prior adjuvant or neo-adjuvant chemotherapy, n (%) No 7 (77.8) 7 (70.0) 10 (90.9) 24 (80.0) Yes 2 (22.2) 3 (30.0) 1 (9.1) 6 (20.0) Surgery on Primary tumor, n (%) No 4 (44.4) 5 (50.0) 7 (63.6) 16 (53.3) Yes 5 (55.6) 5 (50.0) 4 (36.4) 14 (46.7)
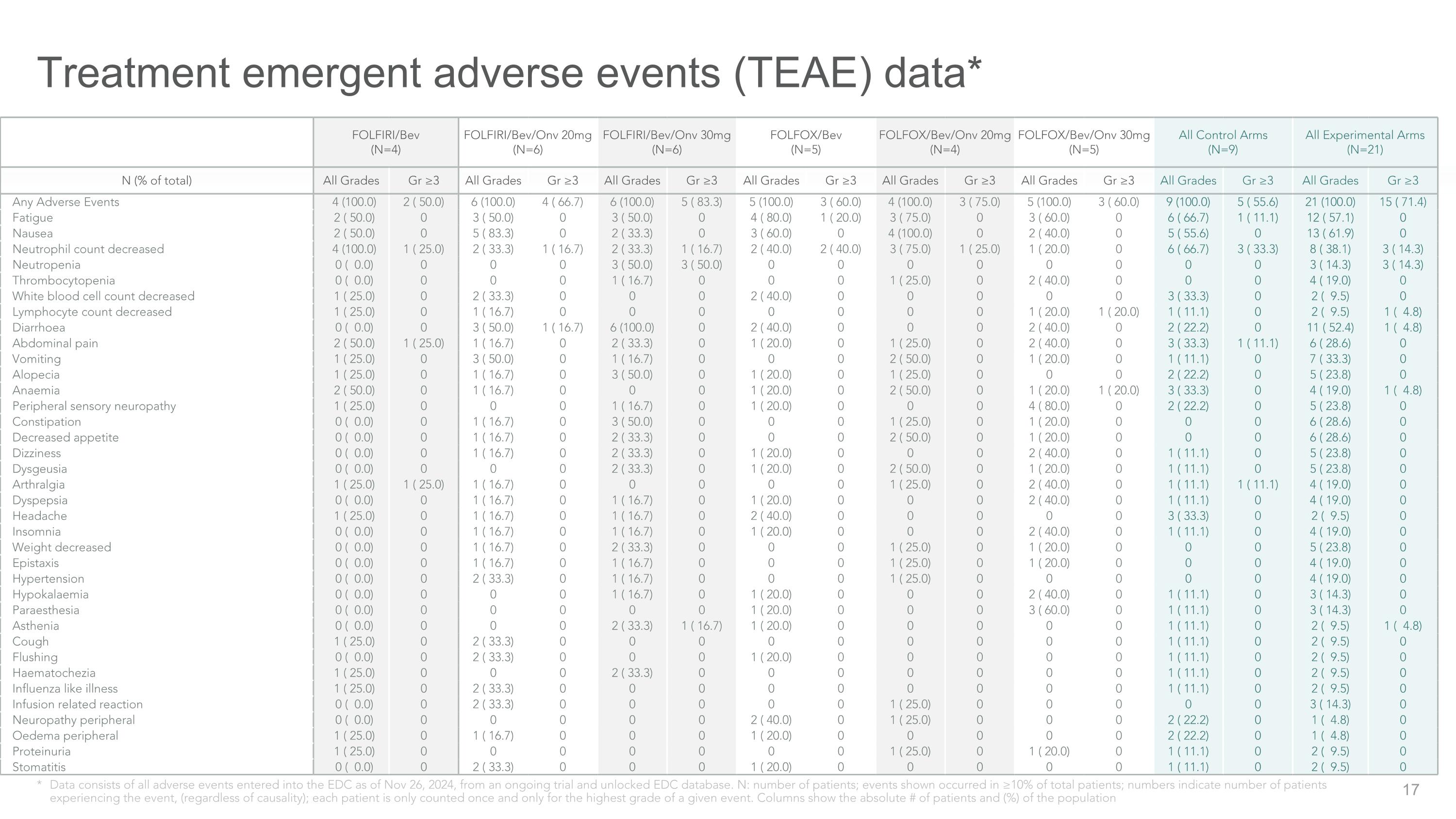
Treatment emergent adverse events (TEAE) data* * Data consists of all adverse events entered into the EDC as of Nov 26, 2024, from an ongoing trial and unlocked EDC database. N: number of patients; events shown occurred in ≥10% of total patients; numbers indicate number of patients experiencing the event, (regardless of causality); each patient is only counted once and only for the highest grade of a given event. Columns show the absolute # of patients and (%) of the population FOLFIRI/Bev �(N=4) FOLFIRI/Bev/Onv 20mg �(N=6) FOLFIRI/Bev/Onv 30mg �(N=6) FOLFOX/Bev �(N=5) FOLFOX/Bev/Onv 20mg �(N=4) FOLFOX/Bev/Onv 30mg �(N=5) All Control Arms�(N=9) All Experimental Arms�(N=21) N (% of total) All Grades Gr ≥3 All Grades Gr ≥3 All Grades Gr ≥3 All Grades Gr ≥3 All Grades Gr ≥3 All Grades Gr ≥3 All Grades Gr ≥3 All Grades Gr ≥3 Any Adverse Events 4 (100.0) 2 ( 50.0) 6 (100.0) 4 ( 66.7) 6 (100.0) 5 ( 83.3) 5 (100.0) 3 ( 60.0) 4 (100.0) 3 ( 75.0) 5 (100.0) 3 ( 60.0) 9 (100.0) 5 ( 55.6) 21 (100.0) 15 ( 71.4) Fatigue 2 ( 50.0) 0 3 ( 50.0) 0 3 ( 50.0) 0 4 ( 80.0) 1 ( 20.0) 3 ( 75.0) 0 3 ( 60.0) 0 6 ( 66.7) 1 ( 11.1) 12 ( 57.1) 0 Nausea 2 ( 50.0) 0 5 ( 83.3) 0 2 ( 33.3) 0 3 ( 60.0) 0 4 (100.0) 0 2 ( 40.0) 0 5 ( 55.6) 0 13 ( 61.9) 0 Neutrophil count decreased 4 (100.0) 1 ( 25.0) 2 ( 33.3) 1 ( 16.7) 2 ( 33.3) 1 ( 16.7) 2 ( 40.0) 2 ( 40.0) 3 ( 75.0) 1 ( 25.0) 1 ( 20.0) 0 6 ( 66.7) 3 ( 33.3) 8 ( 38.1) 3 ( 14.3) Neutropenia 0 ( 0.0) 0 0 0 3 ( 50.0) 3 ( 50.0) 0 0 0 0 0 0 0 0 3 ( 14.3) 3 ( 14.3) Thrombocytopenia 0 ( 0.0) 0 0 0 1 ( 16.7) 0 0 0 1 ( 25.0) 0 2 ( 40.0) 0 0 0 4 ( 19.0) 0 White blood cell count decreased 1 ( 25.0) 0 2 ( 33.3) 0 0 0 2 ( 40.0) 0 0 0 0 0 3 ( 33.3) 0 2 ( 9.5) 0 Lymphocyte count decreased 1 ( 25.0) 0 1 ( 16.7) 0 0 0 0 0 0 0 1 ( 20.0) 1 ( 20.0) 1 ( 11.1) 0 2 ( 9.5) 1 ( 4.8) Diarrhoea 0 ( 0.0) 0 3 ( 50.0) 1 ( 16.7) 6 (100.0) 0 2 ( 40.0) 0 0 0 2 ( 40.0) 0 2 ( 22.2) 0 11 ( 52.4) 1 ( 4.8) Abdominal pain 2 ( 50.0) 1 ( 25.0) 1 ( 16.7) 0 2 ( 33.3) 0 1 ( 20.0) 0 1 ( 25.0) 0 2 ( 40.0) 0 3 ( 33.3) 1 ( 11.1) 6 ( 28.6) 0 Vomiting 1 ( 25.0) 0 3 ( 50.0) 0 1 ( 16.7) 0 0 0 2 ( 50.0) 0 1 ( 20.0) 0 1 ( 11.1) 0 7 ( 33.3) 0 Alopecia 1 ( 25.0) 0 1 ( 16.7) 0 3 ( 50.0) 0 1 ( 20.0) 0 1 ( 25.0) 0 0 0 2 ( 22.2) 0 5 ( 23.8) 0 Anaemia 2 ( 50.0) 0 1 ( 16.7) 0 0 0 1 ( 20.0) 0 2 ( 50.0) 0 1 ( 20.0) 1 ( 20.0) 3 ( 33.3) 0 4 ( 19.0) 1 ( 4.8) Peripheral sensory neuropathy 1 ( 25.0) 0 0 0 1 ( 16.7) 0 1 ( 20.0) 0 0 0 4 ( 80.0) 0 2 ( 22.2) 0 5 ( 23.8) 0 Constipation 0 ( 0.0) 0 1 ( 16.7) 0 3 ( 50.0) 0 0 0 1 ( 25.0) 0 1 ( 20.0) 0 0 0 6 ( 28.6) 0 Decreased appetite 0 ( 0.0) 0 1 ( 16.7) 0 2 ( 33.3) 0 0 0 2 ( 50.0) 0 1 ( 20.0) 0 0 0 6 ( 28.6) 0 Dizziness 0 ( 0.0) 0 1 ( 16.7) 0 2 ( 33.3) 0 1 ( 20.0) 0 0 0 2 ( 40.0) 0 1 ( 11.1) 0 5 ( 23.8) 0 Dysgeusia 0 ( 0.0) 0 0 0 2 ( 33.3) 0 1 ( 20.0) 0 2 ( 50.0) 0 1 ( 20.0) 0 1 ( 11.1) 0 5 ( 23.8) 0 Arthralgia 1 ( 25.0) 1 ( 25.0) 1 ( 16.7) 0 0 0 0 0 1 ( 25.0) 0 2 ( 40.0) 0 1 ( 11.1) 1 ( 11.1) 4 ( 19.0) 0 Dyspepsia 0 ( 0.0) 0 1 ( 16.7) 0 1 ( 16.7) 0 1 ( 20.0) 0 0 0 2 ( 40.0) 0 1 ( 11.1) 0 4 ( 19.0) 0 Headache 1 ( 25.0) 0 1 ( 16.7) 0 1 ( 16.7) 0 2 ( 40.0) 0 0 0 0 0 3 ( 33.3) 0 2 ( 9.5) 0 Insomnia 0 ( 0.0) 0 1 ( 16.7) 0 1 ( 16.7) 0 1 ( 20.0) 0 0 0 2 ( 40.0) 0 1 ( 11.1) 0 4 ( 19.0) 0 Weight decreased 0 ( 0.0) 0 1 ( 16.7) 0 2 ( 33.3) 0 0 0 1 ( 25.0) 0 1 ( 20.0) 0 0 0 5 ( 23.8) 0 Epistaxis 0 ( 0.0) 0 1 ( 16.7) 0 1 ( 16.7) 0 0 0 1 ( 25.0) 0 1 ( 20.0) 0 0 0 4 ( 19.0) 0 Hypertension 0 ( 0.0) 0 2 ( 33.3) 0 1 ( 16.7) 0 0 0 1 ( 25.0) 0 0 0 0 0 4 ( 19.0) 0 Hypokalaemia 0 ( 0.0) 0 0 0 1 ( 16.7) 0 1 ( 20.0) 0 0 0 2 ( 40.0) 0 1 ( 11.1) 0 3 ( 14.3) 0 Paraesthesia 0 ( 0.0) 0 0 0 0 0 1 ( 20.0) 0 0 0 3 ( 60.0) 0 1 ( 11.1) 0 3 ( 14.3) 0 Asthenia 0 ( 0.0) 0 0 0 2 ( 33.3) 1 ( 16.7) 1 ( 20.0) 0 0 0 0 0 1 ( 11.1) 0 2 ( 9.5) 1 ( 4.8) Cough 1 ( 25.0) 0 2 ( 33.3) 0 0 0 0 0 0 0 0 0 1 ( 11.1) 0 2 ( 9.5) 0 Flushing 0 ( 0.0) 0 2 ( 33.3) 0 0 0 1 ( 20.0) 0 0 0 0 0 1 ( 11.1) 0 2 ( 9.5) 0 Haematochezia 1 ( 25.0) 0 0 0 2 ( 33.3) 0 0 0 0 0 0 0 1 ( 11.1) 0 2 ( 9.5) 0 Influenza like illness 1 ( 25.0) 0 2 ( 33.3) 0 0 0 0 0 0 0 0 0 1 ( 11.1) 0 2 ( 9.5) 0 Infusion related reaction 0 ( 0.0) 0 2 ( 33.3) 0 0 0 0 0 1 ( 25.0) 0 0 0 0 0 3 ( 14.3) 0 Neuropathy peripheral 0 ( 0.0) 0 0 0 0 0 2 ( 40.0) 0 1 ( 25.0) 0 0 0 2 ( 22.2) 0 1 ( 4.8) 0 Oedema peripheral 1 ( 25.0) 0 1 ( 16.7) 0 0 0 1 ( 20.0) 0 0 0 0 0 2 ( 22.2) 0 1 ( 4.8) 0 Proteinuria 1 ( 25.0) 0 0 0 0 0 0 0 1 ( 25.0) 0 1 ( 20.0) 0 1 ( 11.1) 0 2 ( 9.5) 0 Stomatitis 0 ( 0.0) 0 2 ( 33.3) 0 0 0 1 ( 20.0) 0 0 0 0 0 1 ( 11.1) 0 2 ( 9.5) 0
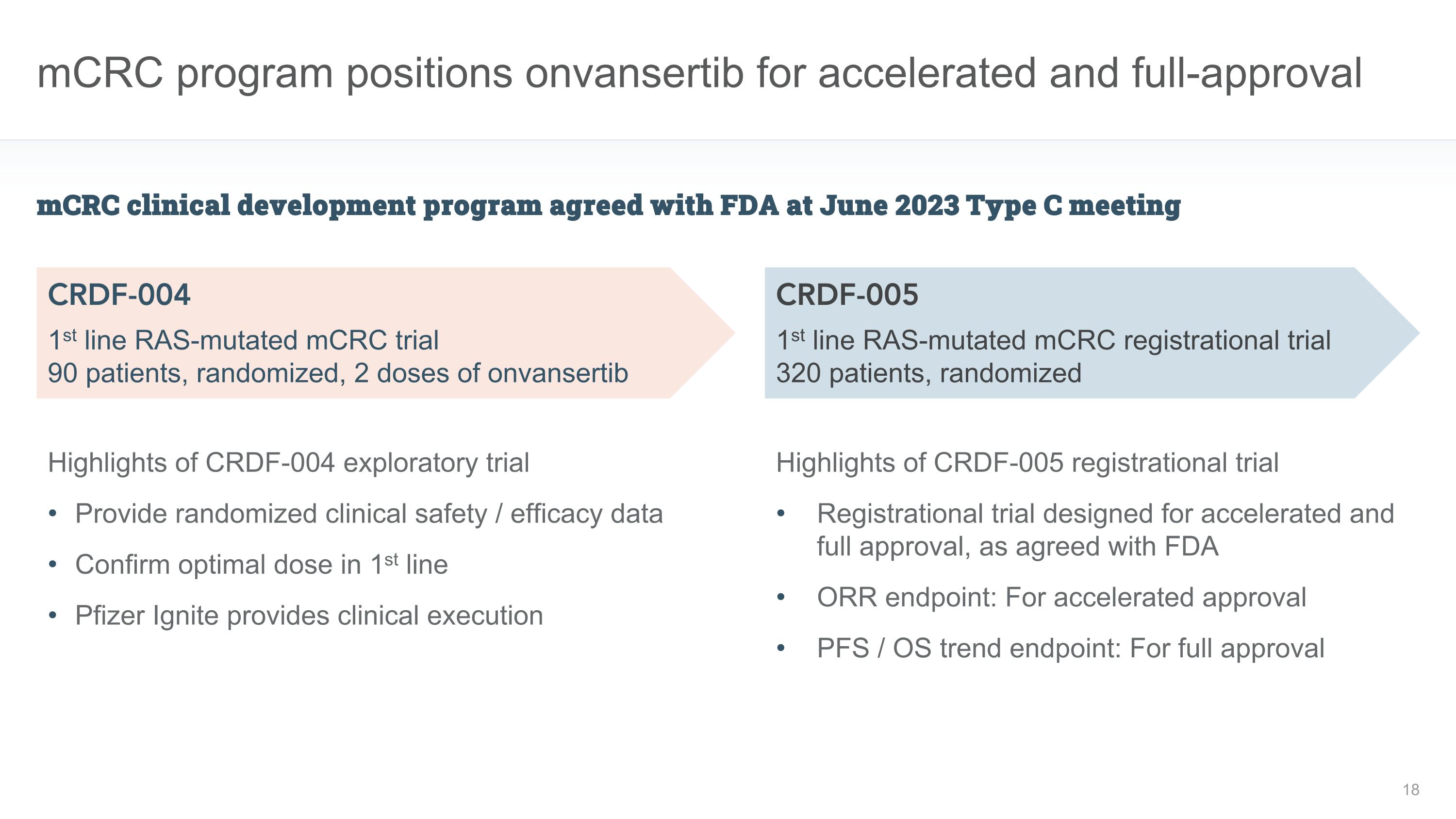
mCRC program positions onvansertib for accelerated and full-approval mCRC clinical development program agreed with FDA at June 2023 Type C meeting CRDF-004 1st line RAS-mutated mCRC trial 90 patients, randomized, 2 doses of onvansertib CRDF-005 1st line RAS-mutated mCRC registrational trial 320 patients, randomized Highlights of CRDF-005 registrational trial Registrational trial designed for accelerated and full approval, as agreed with FDA ORR endpoint: For accelerated approval PFS / OS trend endpoint: For full approval Highlights of CRDF-004 exploratory trial Provide randomized clinical safety / efficacy data Confirm optimal dose in 1st line Pfizer Ignite provides clinical execution

1st line RAS-mut mCRC trial data (CRDF-004) Commercial opportunity in 1st line mCRC The broader onvansertib opportunity AGENDA
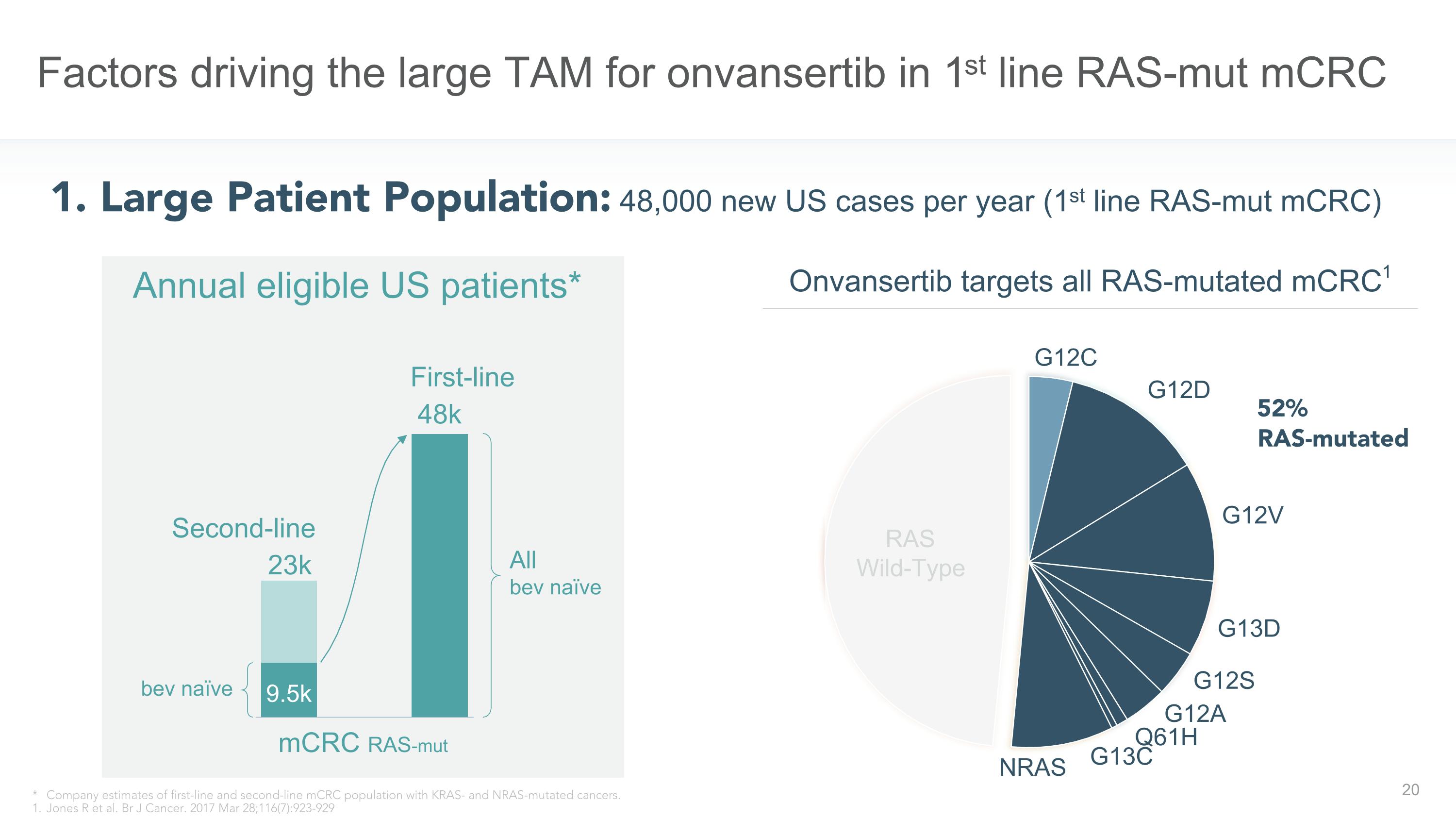
(TROV-054) Factors driving the large TAM for onvansertib in 1st line RAS-mut mCRC 1. Large Patient Population: 48,000 new US cases per year (1st line RAS-mut mCRC) Onvansertib targets all RAS-mutated mCRC1 52% RAS-mutated * Company estimates of first-line and second-line mCRC population with KRAS- and NRAS-mutated cancers. 1. Jones R et al. Br J Cancer. 2017 Mar 28;116(7):923-929 Ph 1b/2 COMPLETED 66 evaluable patients, single arm KRAS-mutated mCRC First-line Second-line 48k mCRC RAS-mut bev naïve Annual eligible US patients* All �bev naïve 23k 9.5k
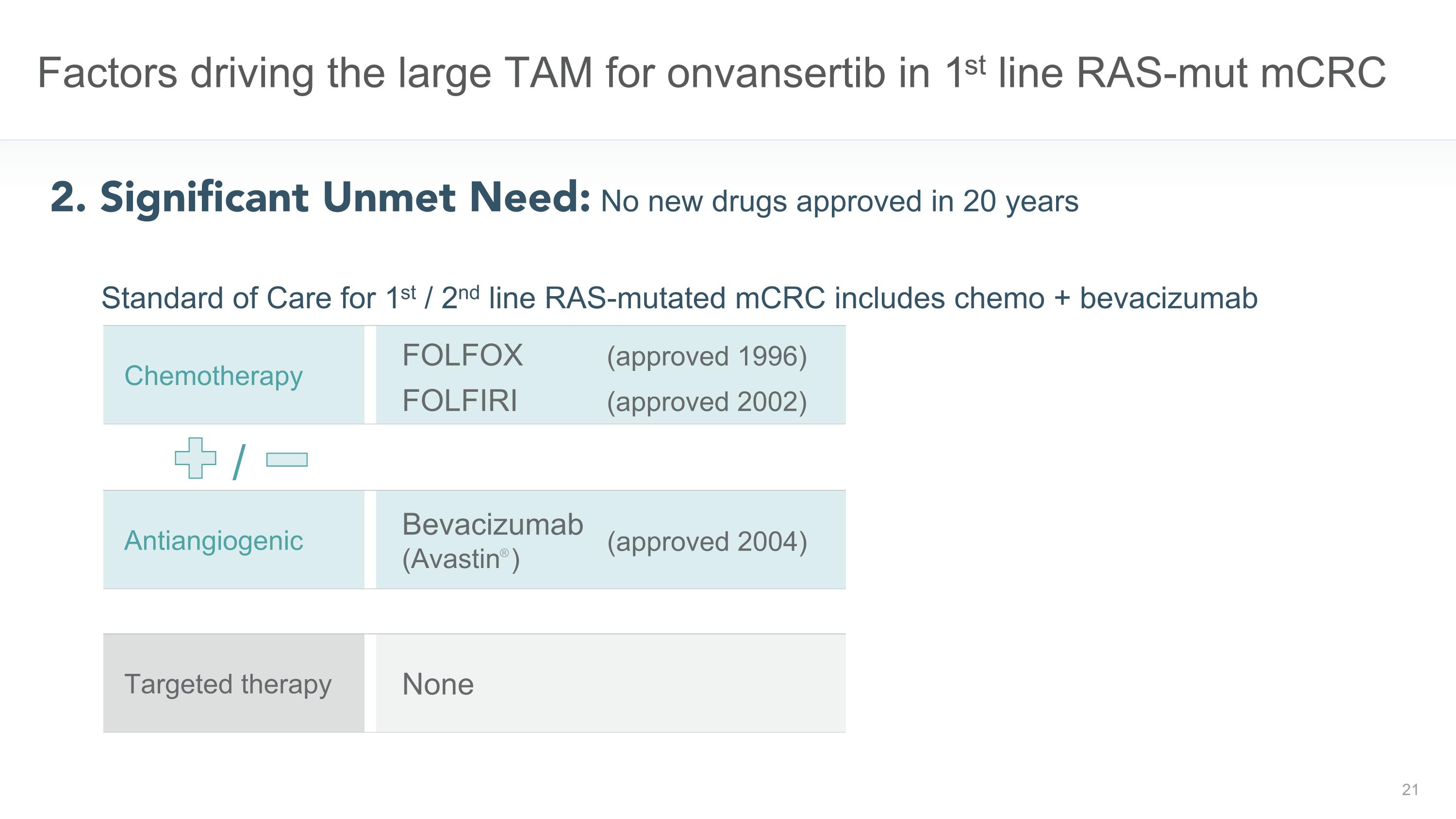
Factors driving the large TAM for onvansertib in 1st line RAS-mut mCRC 2. Significant Unmet Need: No new drugs approved in 20 years FOLFOX (approved 1996) FOLFIRI (approved 2002) Chemotherapy None Targeted therapy Bevacizumab (Avastin® ) Antiangiogenic (approved 2004) / Standard of Care for 1st / 2nd line RAS-mutated mCRC includes chemo + bevacizumab
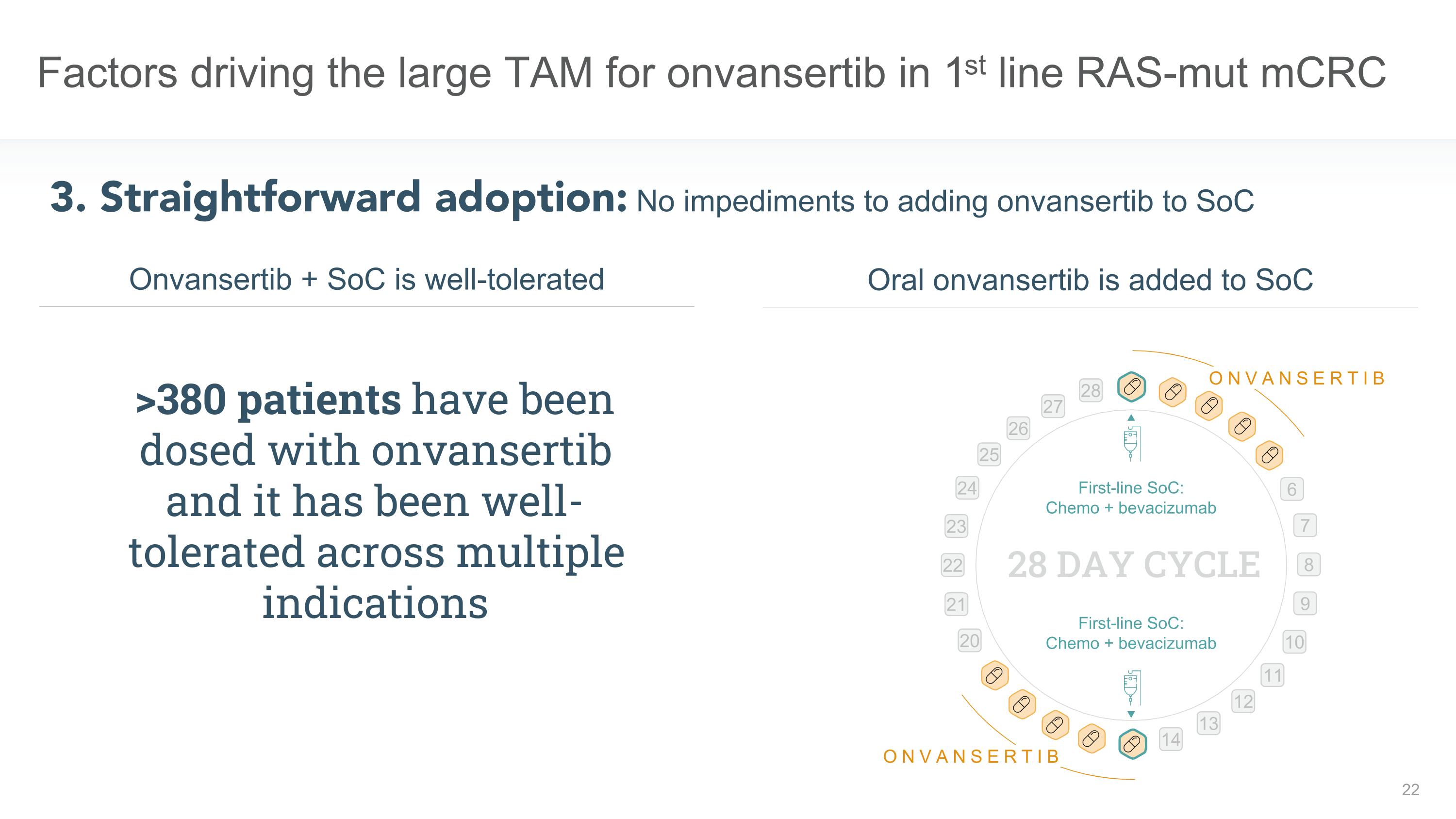
Factors driving the large TAM for onvansertib in 1st line RAS-mut mCRC 3. Straightforward adoption: No impediments to adding onvansertib to SoC Oral onvansertib is added to SoC Onvansertib + SoC is well-tolerated First-line SoC: �Chemo + bevacizumab First-line SoC: �Chemo + bevacizumab 28 DAY CYCLE 6 7 8 9 10 11 12 13 14 20 21 22 23 24 25 26 27 28 ONVANSERTIB ONVANSERTIB >380 patients have been dosed with onvansertib and it has been well-tolerated across multiple indications
1st line RAS-mut mCRC trial data (CRDF-004) Commercial opportunity in 1st line mCRC The broader onvansertib opportunity AGENDA
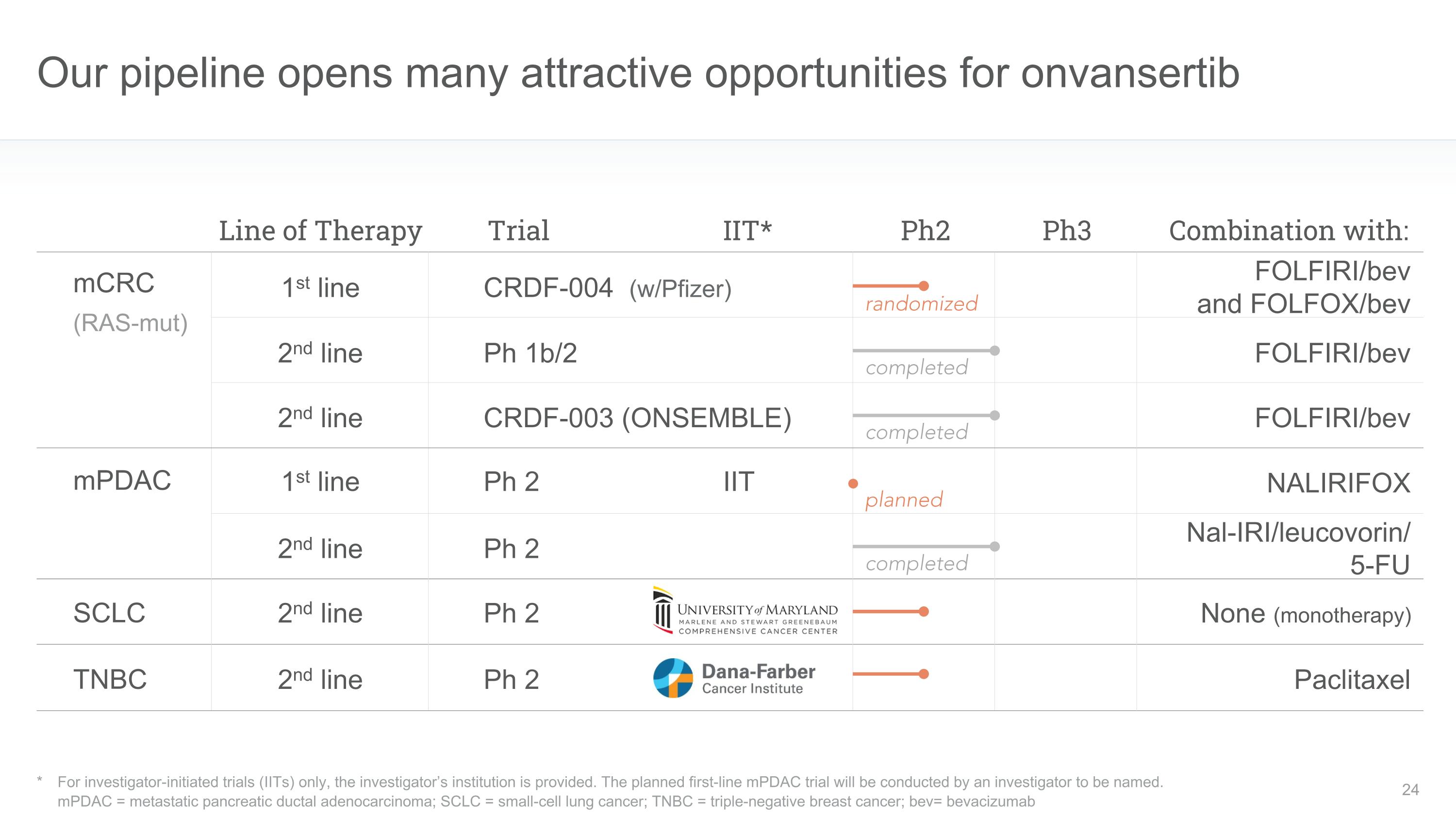
Our pipeline opens many attractive opportunities for onvansertib * For investigator-initiated trials (IITs) only, the investigator’s institution is provided. The planned first-line mPDAC trial will be conducted by an investigator to be named. mPDAC = metastatic pancreatic ductal adenocarcinoma; SCLC = small-cell lung cancer; TNBC = triple-negative breast cancer; bev= bevacizumab Line of Therapy Ph2 Ph3 mPDAC TNBC SCLC mCRC (RAS-mut) Nal-IRI/leucovorin/�5-FU Paclitaxel None (monotherapy) FOLFIRI/bev Combination with: 1st line 1st line 2nd line 2nd line Ph 1b/2 CRDF-004 (w/Pfizer) Ph 2 Trial Ph 2 Ph 2 randomized 2nd line FOLFIRI/bev�and FOLFOX/bev completed 2nd line Ph 2 IIT* NALIRIFOX FOLFIRI/bev CRDF-003 (ONSEMBLE) 2nd line completed planned completed IIT
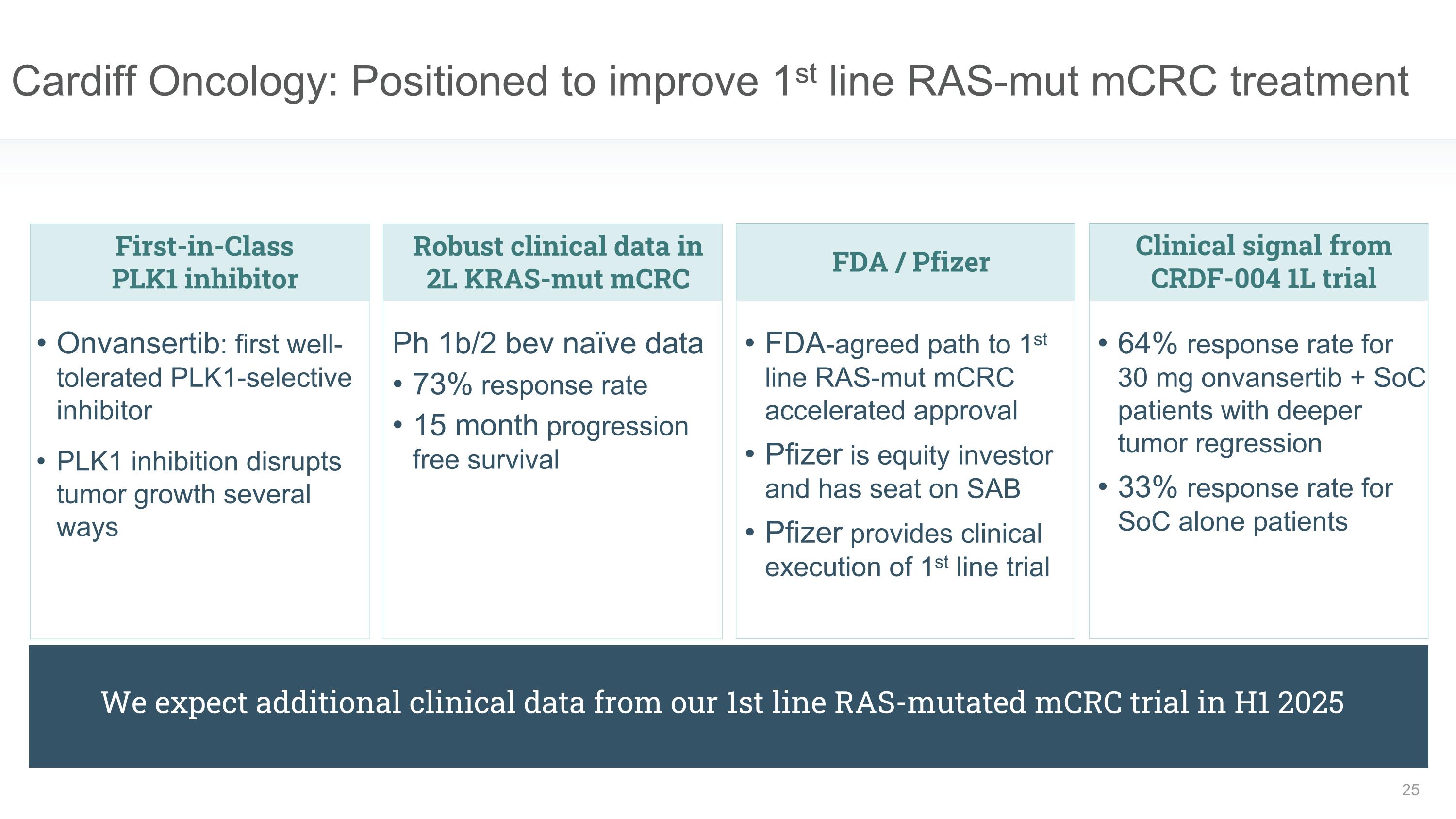
Cardiff Oncology: Positioned to improve 1st line RAS-mut mCRC treatment First-in-Class �PLK1 inhibitor Onvansertib: first well-tolerated PLK1-selective inhibitor PLK1 inhibition disrupts tumor growth several ways FDA / Pfizer FDA-agreed path to 1st line RAS-mut mCRC accelerated approval Pfizer is equity investor and has seat on SAB Pfizer provides clinical execution of 1st line trial Robust clinical data in 2L KRAS-mut mCRC Ph 1b/2 bev naïve data 73% response rate 15 month progression free survival Clinical signal from CRDF-004 1L trial 64% response rate for 30 mg onvansertib + SoC patients with deeper tumor regression 33% response rate for SoC alone patients We expect additional clinical data from our 1st line RAS-mutated mCRC trial in H1 2025
APPENDIX
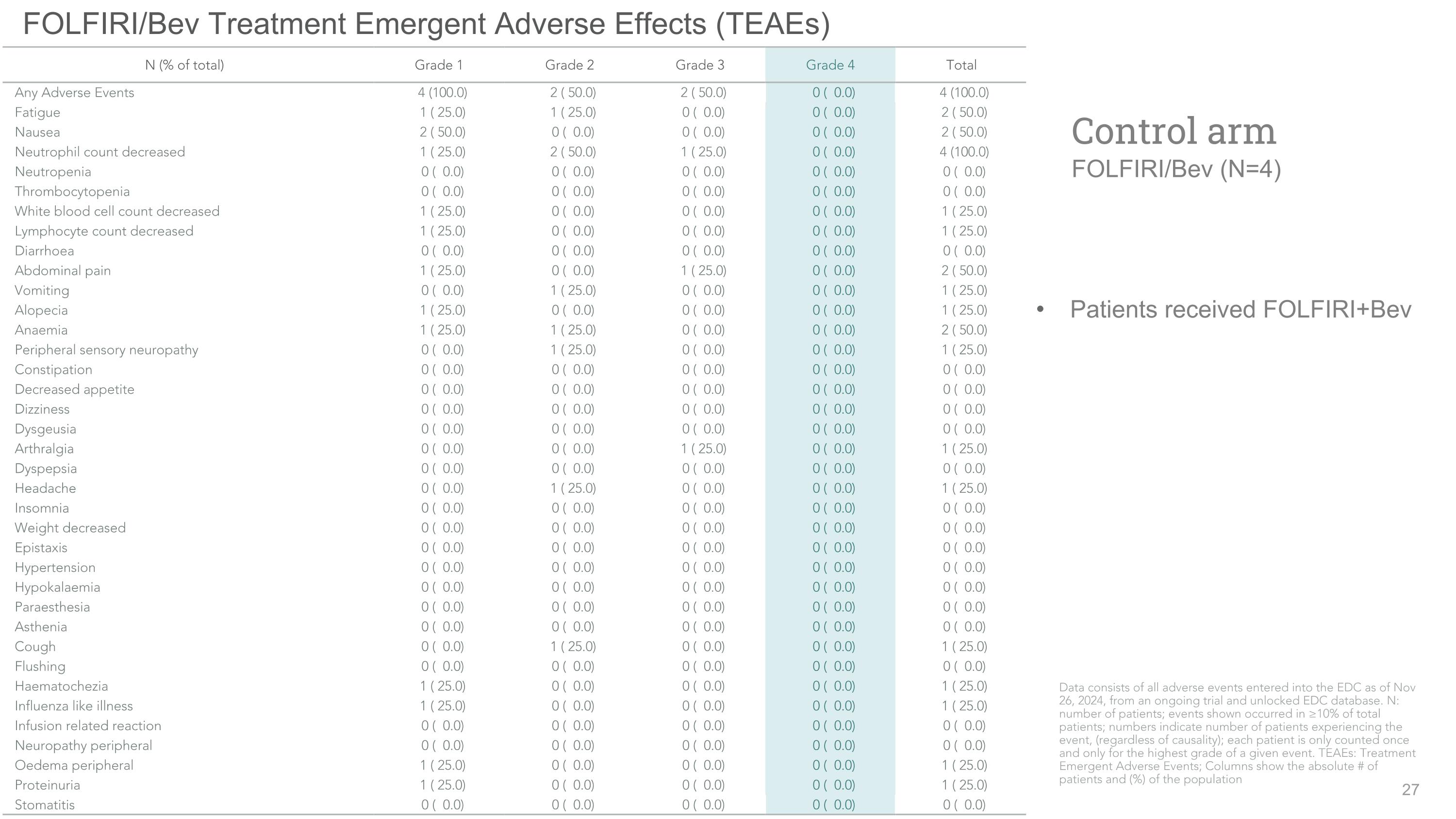
FOLFIRI/Bev Treatment Emergent Adverse Effects (TEAEs) Patients received FOLFIRI+Bev Control arm�FOLFIRI/Bev (N=4) N (% of total) Grade 1 Grade 2 Grade 3 Grade 4 Total Any Adverse Events 4 (100.0) 2 ( 50.0) 2 ( 50.0) 0 ( 0.0) 4 (100.0) Fatigue 1 ( 25.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 2 ( 50.0) Nausea 2 ( 50.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 50.0) Neutrophil count decreased 1 ( 25.0) 2 ( 50.0) 1 ( 25.0) 0 ( 0.0) 4 (100.0) Neutropenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Thrombocytopenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) White blood cell count decreased 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Lymphocyte count decreased 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Diarrhoea 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Abdominal pain 1 ( 25.0) 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 2 ( 50.0) Vomiting 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Alopecia 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Anaemia 1 ( 25.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 2 ( 50.0) Peripheral sensory neuropathy 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Constipation 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Decreased appetite 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Dizziness 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Dysgeusia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Arthralgia 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 1 ( 25.0) Dyspepsia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Headache 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Insomnia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Weight decreased 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Epistaxis 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Hypertension 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Hypokalaemia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Paraesthesia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Asthenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Cough 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Flushing 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Haematochezia 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Influenza like illness 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Infusion related reaction 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Neuropathy peripheral 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Oedema peripheral 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Proteinuria 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Stomatitis 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Data consists of all adverse events entered into the EDC as of Nov 26, 2024, from an ongoing trial and unlocked EDC database. N: number of patients; events shown occurred in ≥10% of total patients; numbers indicate number of patients experiencing the event, (regardless of causality); each patient is only counted once and only for the highest grade of a given event. TEAEs: Treatment Emergent Adverse Events; Columns show the absolute # of patients and (%) of the population
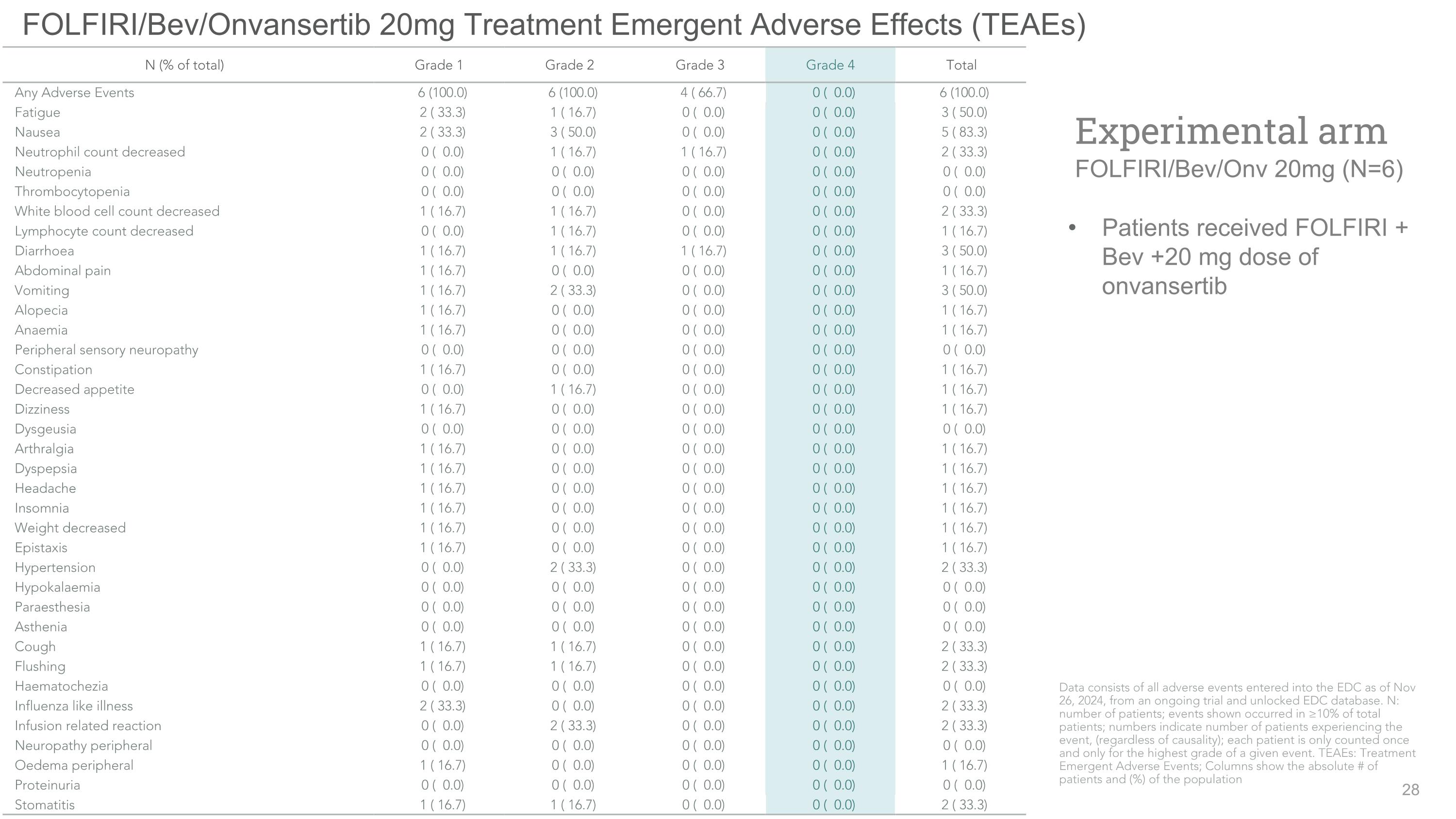
FOLFIRI/Bev/Onvansertib 20mg Treatment Emergent Adverse Effects (TEAEs) Patients received FOLFIRI + Bev +20 mg dose of onvansertib Experimental arm�FOLFIRI/Bev/Onv 20mg (N=6) N (% of total) Grade 1 Grade 2 Grade 3 Grade 4 Total Any Adverse Events 6 (100.0) 6 (100.0) 4 ( 66.7) 0 ( 0.0) 6 (100.0) Fatigue 2 ( 33.3) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 3 ( 50.0) Nausea 2 ( 33.3) 3 ( 50.0) 0 ( 0.0) 0 ( 0.0) 5 ( 83.3) Neutrophil count decreased 0 ( 0.0) 1 ( 16.7) 1 ( 16.7) 0 ( 0.0) 2 ( 33.3) Neutropenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Thrombocytopenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) White blood cell count decreased 1 ( 16.7) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Lymphocyte count decreased 0 ( 0.0) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Diarrhoea 1 ( 16.7) 1 ( 16.7) 1 ( 16.7) 0 ( 0.0) 3 ( 50.0) Abdominal pain 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Vomiting 1 ( 16.7) 2 ( 33.3) 0 ( 0.0) 0 ( 0.0) 3 ( 50.0) Alopecia 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Anaemia 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Peripheral sensory neuropathy 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Constipation 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Decreased appetite 0 ( 0.0) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Dizziness 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Dysgeusia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Arthralgia 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Dyspepsia 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Headache 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Insomnia 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Weight decreased 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Epistaxis 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Hypertension 0 ( 0.0) 2 ( 33.3) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Hypokalaemia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Paraesthesia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Asthenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Cough 1 ( 16.7) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Flushing 1 ( 16.7) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Haematochezia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Influenza like illness 2 ( 33.3) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Infusion related reaction 0 ( 0.0) 2 ( 33.3) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Neuropathy peripheral 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Oedema peripheral 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Proteinuria 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Stomatitis 1 ( 16.7) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Data consists of all adverse events entered into the EDC as of Nov 26, 2024, from an ongoing trial and unlocked EDC database. N: number of patients; events shown occurred in ≥10% of total patients; numbers indicate number of patients experiencing the event, (regardless of causality); each patient is only counted once and only for the highest grade of a given event. TEAEs: Treatment Emergent Adverse Events; Columns show the absolute # of patients and (%) of the population
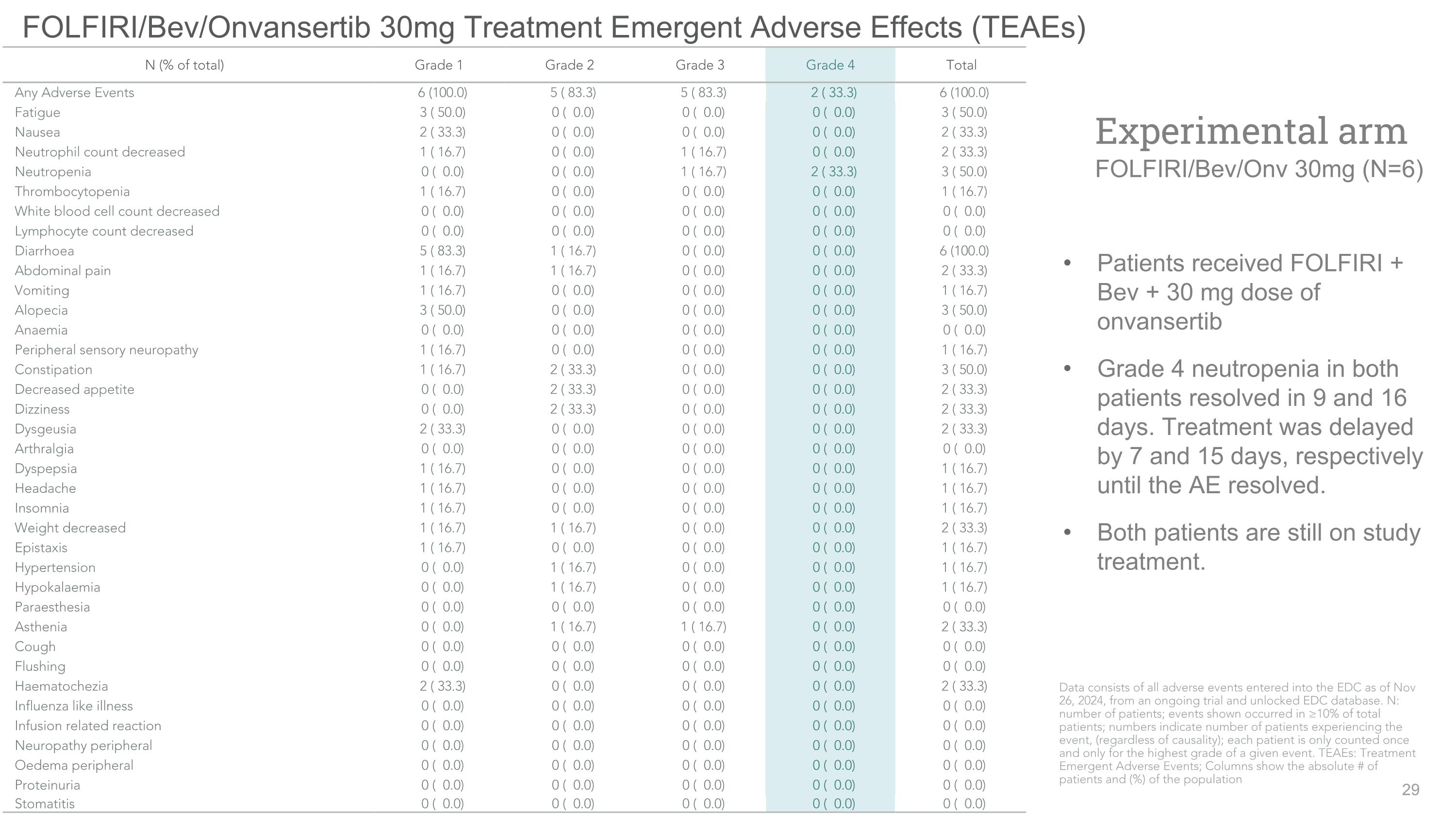
FOLFIRI/Bev/Onvansertib 30mg Treatment Emergent Adverse Effects (TEAEs) Patients received FOLFIRI + Bev + 30 mg dose of onvansertib Grade 4 neutropenia in both patients resolved in 9 and 16 days. Treatment was delayed by 7 and 15 days, respectively until the AE resolved. Both patients are still on study treatment. Experimental arm�FOLFIRI/Bev/Onv 30mg (N=6) N (% of total) Grade 1 Grade 2 Grade 3 Grade 4 Total Any Adverse Events 6 (100.0) 5 ( 83.3) 5 ( 83.3) 2 ( 33.3) 6 (100.0) Fatigue 3 ( 50.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 3 ( 50.0) Nausea 2 ( 33.3) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Neutrophil count decreased 1 ( 16.7) 0 ( 0.0) 1 ( 16.7) 0 ( 0.0) 2 ( 33.3) Neutropenia 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) 2 ( 33.3) 3 ( 50.0) Thrombocytopenia 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) White blood cell count decreased 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Lymphocyte count decreased 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Diarrhoea 5 ( 83.3) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 6 (100.0) Abdominal pain 1 ( 16.7) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Vomiting 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Alopecia 3 ( 50.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 3 ( 50.0) Anaemia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Peripheral sensory neuropathy 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Constipation 1 ( 16.7) 2 ( 33.3) 0 ( 0.0) 0 ( 0.0) 3 ( 50.0) Decreased appetite 0 ( 0.0) 2 ( 33.3) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Dizziness 0 ( 0.0) 2 ( 33.3) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Dysgeusia 2 ( 33.3) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Arthralgia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Dyspepsia 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Headache 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Insomnia 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Weight decreased 1 ( 16.7) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Epistaxis 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Hypertension 0 ( 0.0) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Hypokalaemia 0 ( 0.0) 1 ( 16.7) 0 ( 0.0) 0 ( 0.0) 1 ( 16.7) Paraesthesia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Asthenia 0 ( 0.0) 1 ( 16.7) 1 ( 16.7) 0 ( 0.0) 2 ( 33.3) Cough 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Flushing 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Haematochezia 2 ( 33.3) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 33.3) Influenza like illness 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Infusion related reaction 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Neuropathy peripheral 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Oedema peripheral 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Proteinuria 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Stomatitis 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Data consists of all adverse events entered into the EDC as of Nov 26, 2024, from an ongoing trial and unlocked EDC database. N: number of patients; events shown occurred in ≥10% of total patients; numbers indicate number of patients experiencing the event, (regardless of causality); each patient is only counted once and only for the highest grade of a given event. TEAEs: Treatment Emergent Adverse Events; Columns show the absolute # of patients and (%) of the population
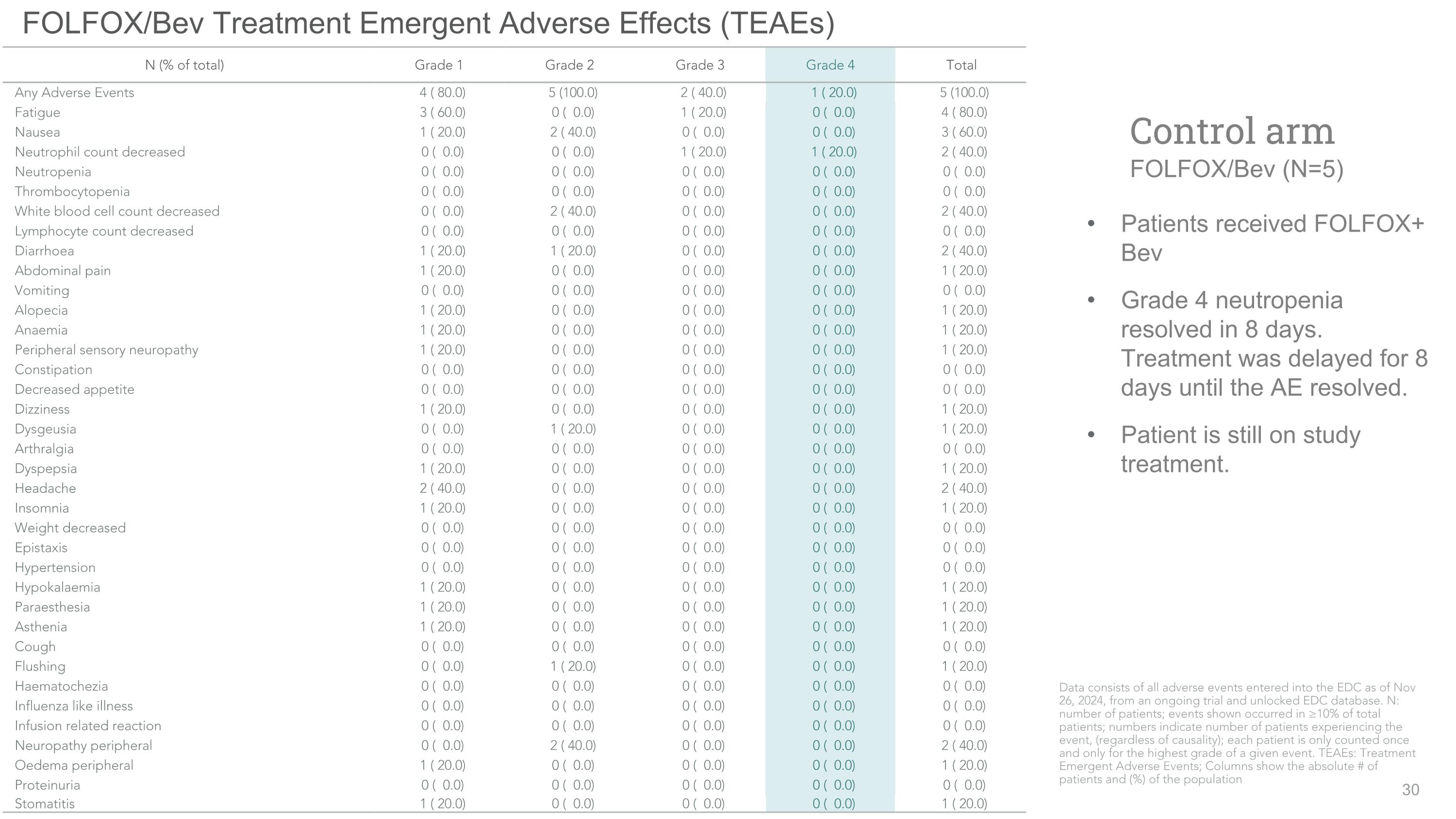
FOLFOX/Bev Treatment Emergent Adverse Effects (TEAEs) Patients received FOLFOX+ Bev Grade 4 neutropenia resolved in 8 days. Treatment was delayed for 8 days until the AE resolved. Patient is still on study treatment. Control arm�FOLFOX/Bev (N=5) N (% of total) Grade 1 Grade 2 Grade 3 Grade 4 Total Any Adverse Events 4 ( 80.0) 5 (100.0) 2 ( 40.0) 1 ( 20.0) 5 (100.0) Fatigue 3 ( 60.0) 0 ( 0.0) 1 ( 20.0) 0 ( 0.0) 4 ( 80.0) Nausea 1 ( 20.0) 2 ( 40.0) 0 ( 0.0) 0 ( 0.0) 3 ( 60.0) Neutrophil count decreased 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) 1 ( 20.0) 2 ( 40.0) Neutropenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Thrombocytopenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) White blood cell count decreased 0 ( 0.0) 2 ( 40.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Lymphocyte count decreased 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Diarrhoea 1 ( 20.0) 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Abdominal pain 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Vomiting 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Alopecia 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Anaemia 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Peripheral sensory neuropathy 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Constipation 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Decreased appetite 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Dizziness 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Dysgeusia 0 ( 0.0) 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Arthralgia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Dyspepsia 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Headache 2 ( 40.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Insomnia 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Weight decreased 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Epistaxis 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Hypertension 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Hypokalaemia 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Paraesthesia 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Asthenia 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Cough 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Flushing 0 ( 0.0) 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Haematochezia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Influenza like illness 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Infusion related reaction 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Neuropathy peripheral 0 ( 0.0) 2 ( 40.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Oedema peripheral 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Proteinuria 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Stomatitis 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Data consists of all adverse events entered into the EDC as of Nov 26, 2024, from an ongoing trial and unlocked EDC database. N: number of patients; events shown occurred in ≥10% of total patients; numbers indicate number of patients experiencing the event, (regardless of causality); each patient is only counted once and only for the highest grade of a given event. TEAEs: Treatment Emergent Adverse Events; Columns show the absolute # of patients and (%) of the population
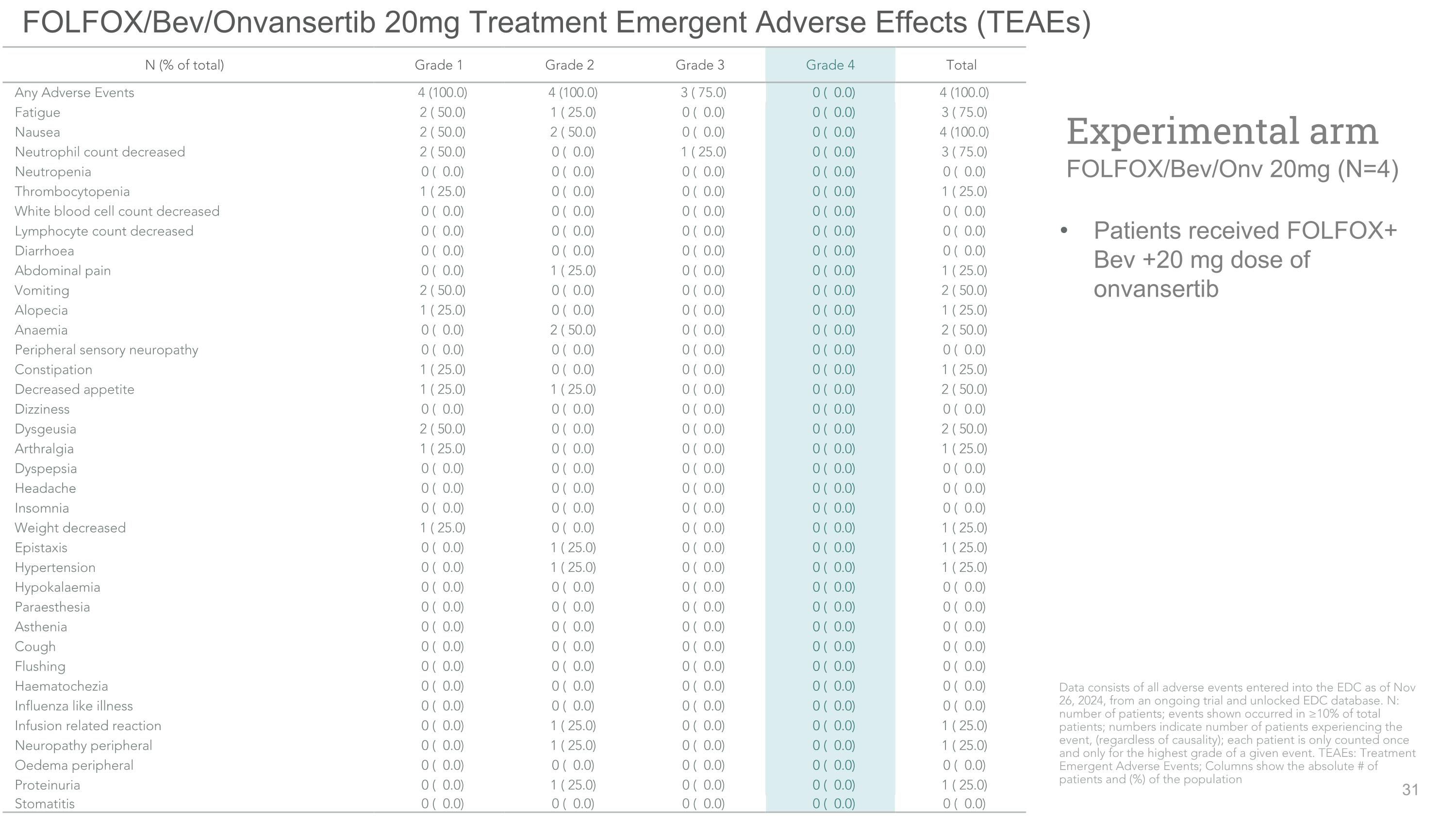
FOLFOX/Bev/Onvansertib 20mg Treatment Emergent Adverse Effects (TEAEs) Patients received FOLFOX+ Bev +20 mg dose of onvansertib Experimental arm�FOLFOX/Bev/Onv 20mg (N=4) N (% of total) Grade 1 Grade 2 Grade 3 Grade 4 Total Any Adverse Events 4 (100.0) 4 (100.0) 3 ( 75.0) 0 ( 0.0) 4 (100.0) Fatigue 2 ( 50.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 3 ( 75.0) Nausea 2 ( 50.0) 2 ( 50.0) 0 ( 0.0) 0 ( 0.0) 4 (100.0) Neutrophil count decreased 2 ( 50.0) 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 3 ( 75.0) Neutropenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Thrombocytopenia 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) White blood cell count decreased 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Lymphocyte count decreased 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Diarrhoea 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Abdominal pain 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Vomiting 2 ( 50.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 50.0) Alopecia 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Anaemia 0 ( 0.0) 2 ( 50.0) 0 ( 0.0) 0 ( 0.0) 2 ( 50.0) Peripheral sensory neuropathy 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Constipation 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Decreased appetite 1 ( 25.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 2 ( 50.0) Dizziness 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Dysgeusia 2 ( 50.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 50.0) Arthralgia 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Dyspepsia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Headache 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Insomnia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Weight decreased 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Epistaxis 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Hypertension 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Hypokalaemia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Paraesthesia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Asthenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Cough 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Flushing 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Haematochezia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Influenza like illness 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Infusion related reaction 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Neuropathy peripheral 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Oedema peripheral 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Proteinuria 0 ( 0.0) 1 ( 25.0) 0 ( 0.0) 0 ( 0.0) 1 ( 25.0) Stomatitis 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Data consists of all adverse events entered into the EDC as of Nov 26, 2024, from an ongoing trial and unlocked EDC database. N: number of patients; events shown occurred in ≥10% of total patients; numbers indicate number of patients experiencing the event, (regardless of causality); each patient is only counted once and only for the highest grade of a given event. TEAEs: Treatment Emergent Adverse Events; Columns show the absolute # of patients and (%) of the population
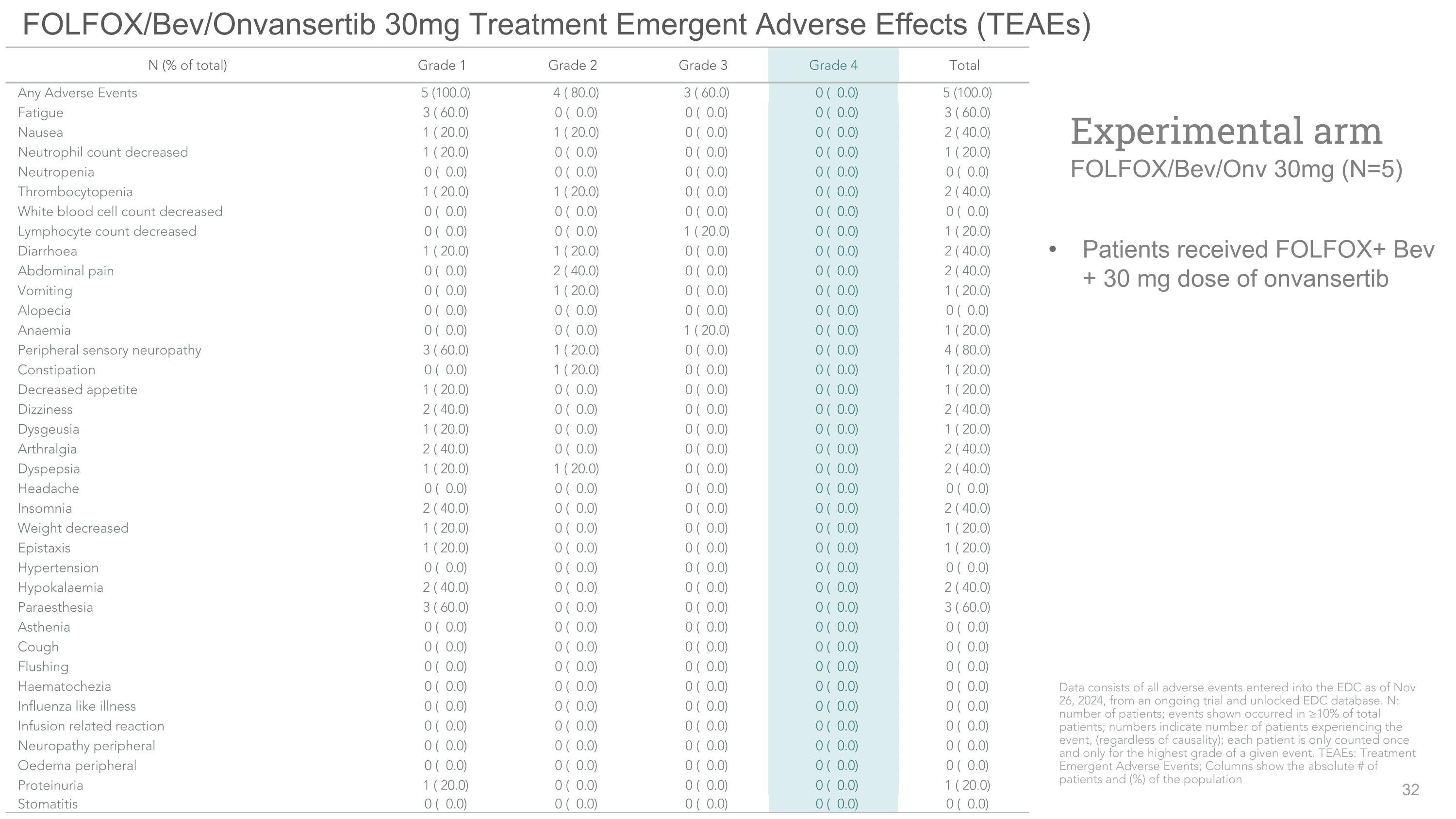
FOLFOX/Bev/Onvansertib 30mg Treatment Emergent Adverse Effects (TEAEs) Patients received FOLFOX+ Bev + 30 mg dose of onvansertib Experimental arm�FOLFOX/Bev/Onv 30mg (N=5) N (% of total) Grade 1 Grade 2 Grade 3 Grade 4 Total Any Adverse Events 5 (100.0) 4 ( 80.0) 3 ( 60.0) 0 ( 0.0) 5 (100.0) Fatigue 3 ( 60.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 3 ( 60.0) Nausea 1 ( 20.0) 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Neutrophil count decreased 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Neutropenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Thrombocytopenia 1 ( 20.0) 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) White blood cell count decreased 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Lymphocyte count decreased 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) 0 ( 0.0) 1 ( 20.0) Diarrhoea 1 ( 20.0) 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Abdominal pain 0 ( 0.0) 2 ( 40.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Vomiting 0 ( 0.0) 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Alopecia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Anaemia 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) 0 ( 0.0) 1 ( 20.0) Peripheral sensory neuropathy 3 ( 60.0) 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 4 ( 80.0) Constipation 0 ( 0.0) 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Decreased appetite 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Dizziness 2 ( 40.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Dysgeusia 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Arthralgia 2 ( 40.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Dyspepsia 1 ( 20.0) 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Headache 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Insomnia 2 ( 40.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Weight decreased 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Epistaxis 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Hypertension 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Hypokalaemia 2 ( 40.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 2 ( 40.0) Paraesthesia 3 ( 60.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 3 ( 60.0) Asthenia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Cough 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Flushing 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Haematochezia 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Influenza like illness 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Infusion related reaction 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Neuropathy peripheral 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Oedema peripheral 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Proteinuria 1 ( 20.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 1 ( 20.0) Stomatitis 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Data consists of all adverse events entered into the EDC as of Nov 26, 2024, from an ongoing trial and unlocked EDC database. N: number of patients; events shown occurred in ≥10% of total patients; numbers indicate number of patients experiencing the event, (regardless of causality); each patient is only counted once and only for the highest grade of a given event. TEAEs: Treatment Emergent Adverse Events; Columns show the absolute # of patients and (%) of the population

Cardiff Oncology Announces Positive Initial Data from First-line RAS-mutated mCRC Clinical Trial
- Initial results from randomized Phase 2 CRDF-004 trial evaluating onvansertib + standard of care in RAS-mut mCRC demonstrated 64% ORR in the 30mg onvansertib dose arm versus 33% ORR in the control arm -
- In the experimental arms, 30mg dose of onvansertib demonstrated a higher ORR compared to 20mg dose of onvansertib (64% vs. 50%) with deeper tumor regression in the 30mg arm -
- Onvansertib was well tolerated at both doses -
- Additional clinical data from CRDF-004 trial expected in 1H 2025 -
- Company will hold a conference call today at 8:00 a.m. ET / 5:00 a.m. PT -
SAN DIEGO, December 10, 2024 -- Cardiff Oncology, Inc. (Nasdaq: CRDF), a clinical-stage biotechnology company leveraging PLK1 inhibition to develop novel therapies across a range of cancers, today announced positive initial data from CRDF-004, a randomized, Phase 2 clinical trial evaluating onvansertib in combination with standard-of-care (SoC) in patients with first-line RAS-mutated metastatic colorectal cancer (mCRC). Efficacy and safety data are for all evaluable patients as of a November 26, 2024 data cut-off date, and all efficacy data are determined by a blinded, independent central review (BICR) of each patient’s tumor scan.
“We are highly encouraged by the robust efficacy signal and favorable safety profile observed with onvansertib plus standard-of-care from the first 30 evaluable patients in our randomized first-line RAS-mutated mCRC CRDF-004 trial,” said Fairooz Kabbinavar, MD, FACP, Chief Medical Officer of Cardiff Oncology. “Our data shows an objective response rate of 64% in patients receiving the 30 mg dose of onvansertib in combination with standard of care, significantly higher than the 33% objective response rate observed in the control arms of standard of care alone. In addition, as can be seen in the spider plots, we are observing deeper tumor response in patients receiving the 30mg dose of onvansertib compared to those receiving the 20mg dose with similar safety profiles for both doses.”
Study Design
The CRDF-004 phase 2 trial is currently enrolling patients with mCRC who have a documented KRAS or NRAS mutation. Onvansertib is added to SoC consisting of FOLFIRI plus bevacizumab or FOLFOX plus bevacizumab. Patients are being randomized in a 1:1:1 ratio to either 20mg of onvansertib plus SoC, 30mg of onvansertib plus SoC, or SoC alone. The primary endpoint is objective response rate (ORR), and the secondary endpoints include progression-free survival (PFS), duration of response (DOR) and safety.
Efficacy Data
Objective Response Rates observed in the CRDF-004 clinical trial, as of the data cut-off date of November 26, 2024, are shown below.
|
|
|
|
Control Arm (SoC alone) |
20mg dose of onvansertib + SoC |
30mg dose of onvansertib + SoC |
All onvansertib patients |
33% ORR (3 of 9) |
50% ORR (5 of 10) |
64% ORR (7 of 11) |
57% ORR (12 of 21) |
Spider Plots, displaying the change in tumor size from baseline for each patient over time, demonstrate deeper responses observed in patients receiving the 30mg dose of onvansertib in combination with the SoC compared to both the control arms and 20mg dose of onvansertib arms.
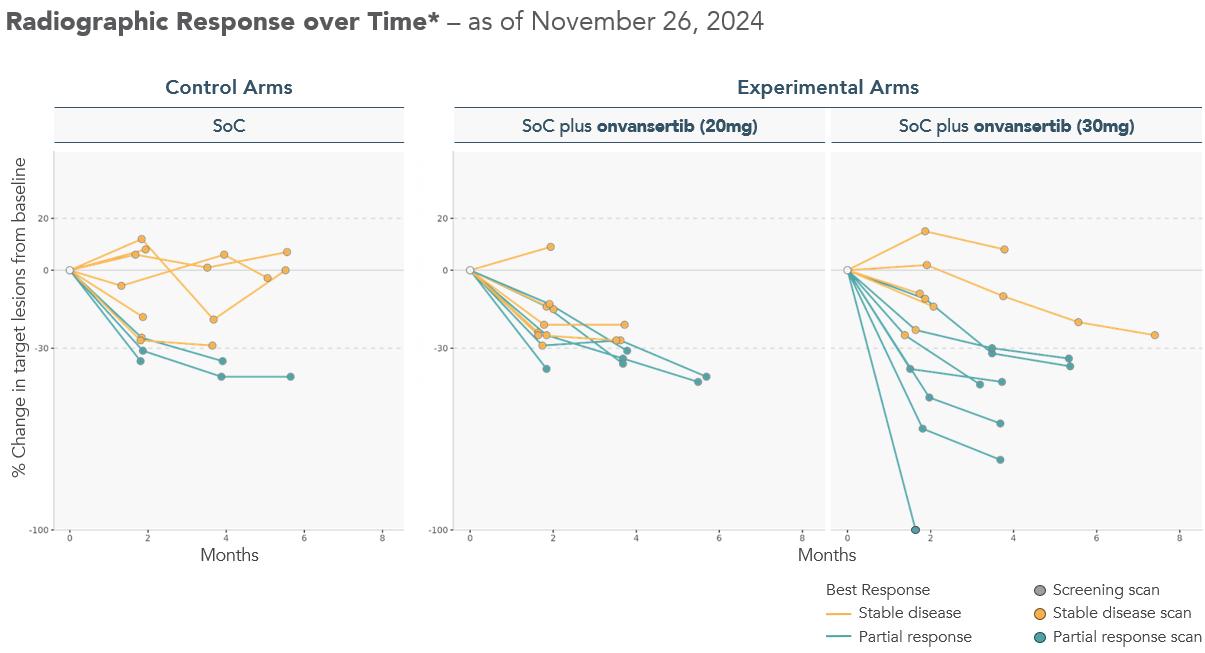
Note: Radiographic response determined per RECIST 1.1 by blinded independent central review. Spider plot reflects interim data as of November 26, 2024 from an ongoing trial and unlocked database.
Safety and Tolerability
Onvansertib in combination with chemo/bevacizumab was well-tolerated and there have been no major or unexpected toxicities observed.
“Overall, these data support our belief that onvansertib has potential to change the treatment paradigm for the entire first-line RAS-mutated mCRC patient population of almost 50,000 new patients diagnosed in the U.S. annually,” said Mark Erlander, Chief Executive Officer of Cardiff Oncology. “In addition to the efficacy signal observed, the data demonstrate that onvansertib
can safely be combined with the two different chemo backbones that are currently approved as standard of care in the first-line setting, thus providing a key differentiated profile over previous generation PLK1 inhibitors. We look forward to providing additional clinical updates from our CRDF-004 trial in the first half of 2025.”
Upcoming expected milestones
•Additional clinical data from CRDF-004 trial expected in 1H 2025
Conference Call and Webcast
Cardiff Oncology will host a conference call and live webcast at 8:00 a.m. ET / 5:00 a.m. PT on December 10, 2024. Individuals interested in listening to the live conference call may do so by using the webcast link in the "Events" section of the company's website. A webcast replay will be available in the investor relations section on the company's website following the completion of the call.
About Cardiff Oncology, Inc.
Cardiff Oncology is a clinical-stage biotechnology company leveraging PLK1 inhibition, a well-validated oncology drug target, to develop novel therapies across a range of cancers. The Company's lead asset is onvansertib, a PLK1 inhibitor being evaluated in combination with standard of care (SoC) therapeutics in clinical programs targeting indications such as RAS-mutated metastatic colorectal cancer (mCRC), as well as in ongoing and planned investigator-initiated trials in metastatic pancreatic ductal adenocarcinoma (mPDAC), small cell lung cancer (SCLC) and triple negative breast cancer (TNBC). These programs and the Company's broader development strategy are designed to target tumor vulnerabilities in order to overcome treatment resistance and deliver superior clinical benefit compared to SoC alone. For more information, please visit https://www.cardiffoncology.com.
Forward-Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified using words such as "anticipate," "believe," "forecast," "estimated" and "intend" or other similar terms or expressions that concern Cardiff Oncology's expectations, strategy, plans or intentions. These forward-looking statements, including statements regarding Cardiff Oncology’s plans to provide additional clinical updates from our CRDF-004 trial in the first half of 2025, are based on Cardiff Oncology's current expectations and actual results could differ materially. There are several factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results; our clinical trials may be suspended or discontinued due to unexpected side effects or other safety risks that could preclude approval of our product candidate; results of preclinical studies or clinical trials for our product candidate could be
unfavorable or delayed; our need for additional financing; risks related to business interruptions, including the outbreak of an epidemic or pandemic such as the COVID-19 coronavirus and cyber-attacks on our information technology infrastructure, which could seriously harm our financial condition and increase our costs and expenses; uncertainties of government or third party payer reimbursement; dependence on key personnel; limited experience in marketing and sales; substantial competition; uncertainties of patent protection and litigation; dependence upon third parties; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. There are no guarantees that our product candidate will be utilized or prove to be commercially successful. Additionally, there are no guarantees that future clinical trials will be completed or successful or that our product candidate will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in Cardiff Oncology's Form 10-K for the year ended December 31, 2023, and other periodic reports filed with the Securities and Exchange Commission. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and Cardiff Oncology does not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances.
Cardiff Oncology Contact:
James Levine
Chief Financial Officer
858-952-7670
jlevine@cardiffoncology.com
Investor Contact:
Kiki Patel, PharmD
Gilmartin Group
332-895-3225
Kiki@gilmartinir.com
Media Contact:
Grace Spencer
Taft Communications
609-583-1151
grace@taftcommunications.com
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Cardiff Oncology (NASDAQ:CRDF)
Historical Stock Chart
From Nov 2024 to Dec 2024

Cardiff Oncology (NASDAQ:CRDF)
Historical Stock Chart
From Dec 2023 to Dec 2024
