0001980845false00-000000000019808452024-09-052024-09-050001980845engn:WarrantsMember2024-09-052024-09-050001980845us-gaap:CommonStockMember2024-09-052024-09-05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): September 05, 2024 |
enGene Holdings Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
British Columbia |
001-41854 |
Not applicable |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
4868 Rue Levy, Suite 220 |
|
Saint-Laurent, Quebec, Canada |
|
H4R 2P1 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 514 332-4888 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Shares |
|
ENGN |
|
The Nasdaq Stock Market LLC |
Warrants, each exercisable for one Common Share, at an exercise price of $11.50 per Share |
|
ENGNW |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Item 7.01 Regulation FD Disclosure.
On September 5, 2024, enGene Holdings Inc. (the “Company”) updated its Corporate Presentation. The updated Corporate Presentation is being furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated herein by reference.
The information in this Current Report on Form 8-K (including Exhibit 99.1) is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
ENGENE HOLDINGS INC. |
|
|
|
|
Date: |
September 5, 2024 |
By: |
/s/ Ryan Daws |
|
|
Name: Title: |
Ryan Daws
Chief Financial Officer |

Non-Viral Genetic Medicine Corporate Presentation September 2024

Cautionary Statement Regarding Forward-Looking Statements This Presentation contains certain forward-looking statements within the meaning of the federal securities laws and "forward-looking information" within the meaning of Canadian securities laws (collectively, "forward-looking statements"). Forward-looking statements may be identified by the use of the words such as “plan”, “forecast”, “intend”, “development”, “expect”, “anticipate”, “become”, “believe”, “continue”, “could”, “estimate”, “expect”, “intends”, “may”, “might”, “plan”, “possible”, “project”, “should”, “would”, “strategy”, “future”, “potential”, “opportunity”, “target”, “term”, “will”, “would”, “will be” or similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding estimates and forecasts of financial and performance metrics, projections of market opportunity and market share, expectations and timing related to regulatory submissions and commercial product launches. These forward-looking statements are based on various estimates and assumptions, whether or not identified in this presentation, and on the current expectations of the management of enGene Holdings Inc. ("enGene"), are not predictions of annual performance, and are subject to risks and uncertainties. These forward-looking statements are subject to a number of risks and uncertainties, including but not limited to, those described in the “Risk Factors” sections of enGene’s Annual Report on Form 10-K for the fiscal year ended October 31, 2023, Quarterly Report on Form 10-Q for the fiscal quarter ended January 31, 2024 and Quarterly Report on Form 10-Q for the fiscal quarter ended April 30, 2024, each of which has been filed with the Securities and Exchange Commission (“SEC”) and Canadian Securities Regulators (copies of which may be obtained at www.sedarplus.ca or www.sec.gov). You should carefully consider the risks and uncertainties described in the “Risk Factors” section of such Annual Report and Quarterly Reports, as well as other documents if and when filed by enGene from time to time with the SEC and Canadian securities regulators. If any of these risks materialize or our assumptions prove incorrect, actual events and results could differ materially from those contained in the forward-looking statements. There may be additional risks that enGene presently knows or that enGene currently believes are immaterial that could also cause actual events and results to differ. In addition, forward-looking statements reflect enGene’s expectations, plans, or forecasts of future events and views as of the date of this presentation. enGene anticipates that subsequent events and developments will cause enGene’s assessments to change. While enGene may elect to update these forward-looking statements at some point in the future, enGene specifically disclaim any obligation to do so, unless required by applicable law. These forward-looking statements should not be relied upon as representing enGene’s assessments as of any date subsequent to the date of this presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements contained herein Intellectual Property This Presentation contains trademarks, service marks, trade names, copyrights, and products of enGene and other companies, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade names, copyrights, or products in this Presentation is not intended to, and does not, imply a relationship with enGene, or an endorsement of or sponsorship by enGene. Solely for convenience, the trademarks, service marks, and trade names referred to in this Presentation may appear without the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that enGene will not assert, to the fullest extent permitted under applicable law, their rights or the right of the applicable licensor in such trademarks, service marks and trade names. Industry and Market Data This Presentation relies on and refers to certain information and statistics based on estimates by enGene’s management and/or obtained from third party sources which enGene believes to be reliable. enGene has not independently verified the accuracy or completeness of any such third party information, which involves elements of subjective judgment and analysis that may or may not prove to be accurate. None enGene, or its affiliates or any third parties that provide information to enGene or its affiliates, such as market research firms, guarantees the accuracy, completeness, timeliness, or availability of any information. None enGene, or its affiliates, or any third parties that provide information to enGene, and its affiliates, such as market research firms, is responsible for any errors or omissions (negligent or otherwise), regardless of the cause, or the results obtained from the use of such content. enGene may have supplemented such information where necessary, taking into account publicly available information about other industry participants and enGene management’s best view as to information that is not publicly available. Neither enGene nor its affiliates give any express or implied warranties with respect to the information included herein, including, but not limited to, any warranties regarding its accuracy or of merchantability or fitness for a particular purpose or use, and they expressly disclaim any responsibility or liability for direct, indirect, incidental, exemplary, compensatory, punitive, special, or consequential damages, costs, expenses, legal fees, or losses (including lost income or profits and opportunity costs) in connection with the use of the information herein. Lead Program (detalimogene voraplasmid) The lead program described herein is an investigational drug therapy that has not been subject to testing designed to demonstrate that the therapy is effective in humans or to provide a basis to predict in advance whether an adequate level of efficacy in humans will be demonstrated in further testing. Although deemed sufficient to permit further testing, the limited, early Phase 1 testing to date is not a sufficient basis on which to predict efficacy or safety. Although the FDA has indicated that the Phase 2 portion of the current LEGEND study may potentially support BLA approval, that outcome will depend entirely on the results of Phase 2 clinical testing, which are not expected to be available until 2026. Disclaimers

Detalimogene Voraplasmid for NMIBC Designed to be a practice-changing therapy requiring no change in practice Detalimogene voraplasmid was formerly referred to as EG-70 NMIBC = Non-muscle invasive bladder cancer NMIBC Market Forecast based on internal estimates utilizing multiple source data Highly Differentiated Investigational Product Explosive Transformational Market Opportunity Near Term Commercial Opportunity Well-capitalized, with cash projected into 2027 BLA filing mid ‘26 (est.) Unique combination of clinical activity, tolerability, ease of use NMIBC market forecast >$20B

NMIBC Represents 75-80% of Bladder Cancer Diagnosis�Estimated Bladder Cancer US Prevalence: ~730,000 Patients Non-Muscle Invasive 80% of newly diagnosed �bladder cancer �(~65,000 cases in USA) Muscle-Invasive 20% of newly diagnosed bladder cancer �(~20,000 cases in USA) Metastatic Papillary tumors: non-invasive outgrowths from the bladder surface Carcinoma in situ (CIS): flat, aggressive cancer that is not easily removed 4 Sources: SEER database; Knowles et al., Nat Rev Cancer 15, 25–41 (2015); Isharwal and Konety, Indian Journal of Urology, 2015; TNM Classification, 8th Edition; Tan et al., Eur Urol Oncol (2022): https://doi.org/10.1016/j.euo.2022.05.005 Carcinoma in situ Non-invasive papillary tumors Papillary tumor that invades subepithelial connective tissue Outer muscle Inner muscle Lamina propria Bladder lumen Cis/Tis Ta T1

Bladder Cancer- High Incidence, High Prevalence, High Cost to Society Bladder Cancer is among the most expensive cancers to manage per patient due to: Ongoing cystoscopy visits, imaging visits, and urine testing every 3-6m Progression to muscle invasive disease (MIBC) Costly, complex treatments for MIBC (e.g., radical cystectomy) and for metastatic disease Estimated >$6.5B total annual cost to manage US bladder cancer patients Bladder is a Top-10 Cancer by US Incidence Rank by Incidence Indication 1 Breast 2 Prostate 3 Lung 4 Colorectal 5 Melanoma 6 Bladder 7 Kidney/Renal 8 Lymphoma (NHL) 9 Uterus 10 Pancreas Sources: Mossanen M, Gore JL, Curr Opin Urol. 2014 Sep;24(5):487-91; Clark O et al., Pharmacoecon Open. 2024 Aug 18; https://seer.cancer.gov/statfacts/html/common.html#comparison

High-Risk NMIBC: A Multi-year Journey Managed by Community Urologists Goal: Avoid Radical Cystectomy and Tumor Progression Beyond the Bladder Diagnosis of high-risk (HR) disease 1st line: Intravesical BCG and/or Chemo 2nd line and beyond: Exhaustion of additional non-surgical options Disease Progression MIBC Treatment (e.g., radical cystectomy) 50% 2-year recurrence Patients commonly treated in community urology clinics over 2-5 year period Patients commonly treated �in academic medical centers and/or by oncologists

Radical Cystectomy: Life-Transforming Surgery of Last Resort 6-8 hour inpatient surgery 10% mortality rate 75% complication rate 43% readmission rate Significant (and often lifelong) impact �on sexual function, body image, depression, anxiety, and QoL (e.g., urostomy required) Radical Cystectomy remains standard-of-care for treating High-Risk (HR) NMIBC with Cis Image of Radical Cystectomy Surgery PATIENT ABDOMEN Sources: Korkes et al. Urol Int (2023) 107 (1): 96–104; Stitzenberg et al. J Clin Onc, 33.5 (2015): 455-464.2015; Djaladat et al. World J Uro, 35 (2017): 907-911. 7
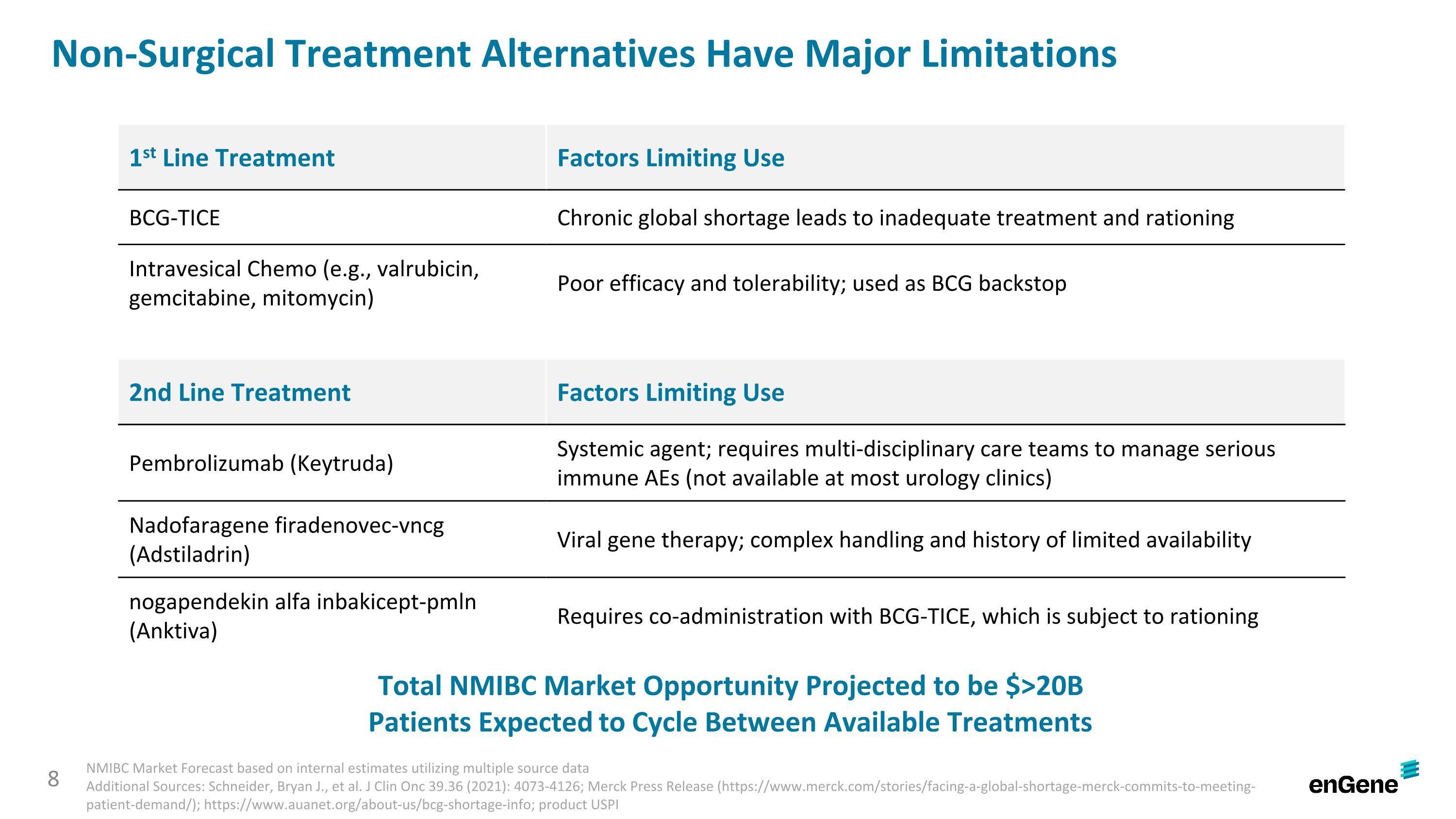
Non-Surgical Treatment Alternatives Have Major Limitations 1st Line Treatment Factors Limiting Use BCG-TICE Chronic global shortage leads to inadequate treatment and rationing Intravesical Chemo (e.g., valrubicin, gemcitabine, mitomycin) Poor efficacy and tolerability; used as BCG backstop 2nd Line Treatment Factors Limiting Use Pembrolizumab (Keytruda) Systemic agent; requires multi-disciplinary care teams to manage serious immune AEs (not available at most urology clinics) Nadofaragene firadenovec-vncg (Adstiladrin) Viral gene therapy; complex handling and history of limited availability nogapendekin alfa inbakicept-pmln (Anktiva) Requires co-administration with BCG-TICE, which is subject to rationing 8 Total NMIBC Market Opportunity Projected to be $>20B Patients Expected to Cycle Between Available Treatments NMIBC Market Forecast based on internal estimates utilizing multiple source data Additional Sources: Schneider, Bryan J., et al. J Clin Onc 39.36 (2021): 4073-4126; Merck Press Release (https://www.merck.com/stories/facing-a-global-shortage-merck-commits-to-meeting-patient-demand/); https://www.auanet.org/about-us/bcg-shortage-info; product USPI

Detalimogene: “first choice agent” in a multi-year, multi-drug treatment journey, if approved Clinical activity and durability demonstrated: Promising results from Ph1 LEGEND study Potential best-in-class ease-of-use: Non-viral genetic medicine; no specialized handling or cold-chain storage Favorable safety profile observed: Ph1 LEGEND TRAEs were mild, largely instrumentation-related Well-characterized, scalable manufacturing: Cost-effective process supports wide availability Detalimogene: Designed To Be the First-Choice Therapy TRAE = Treatment-related adverse event 9
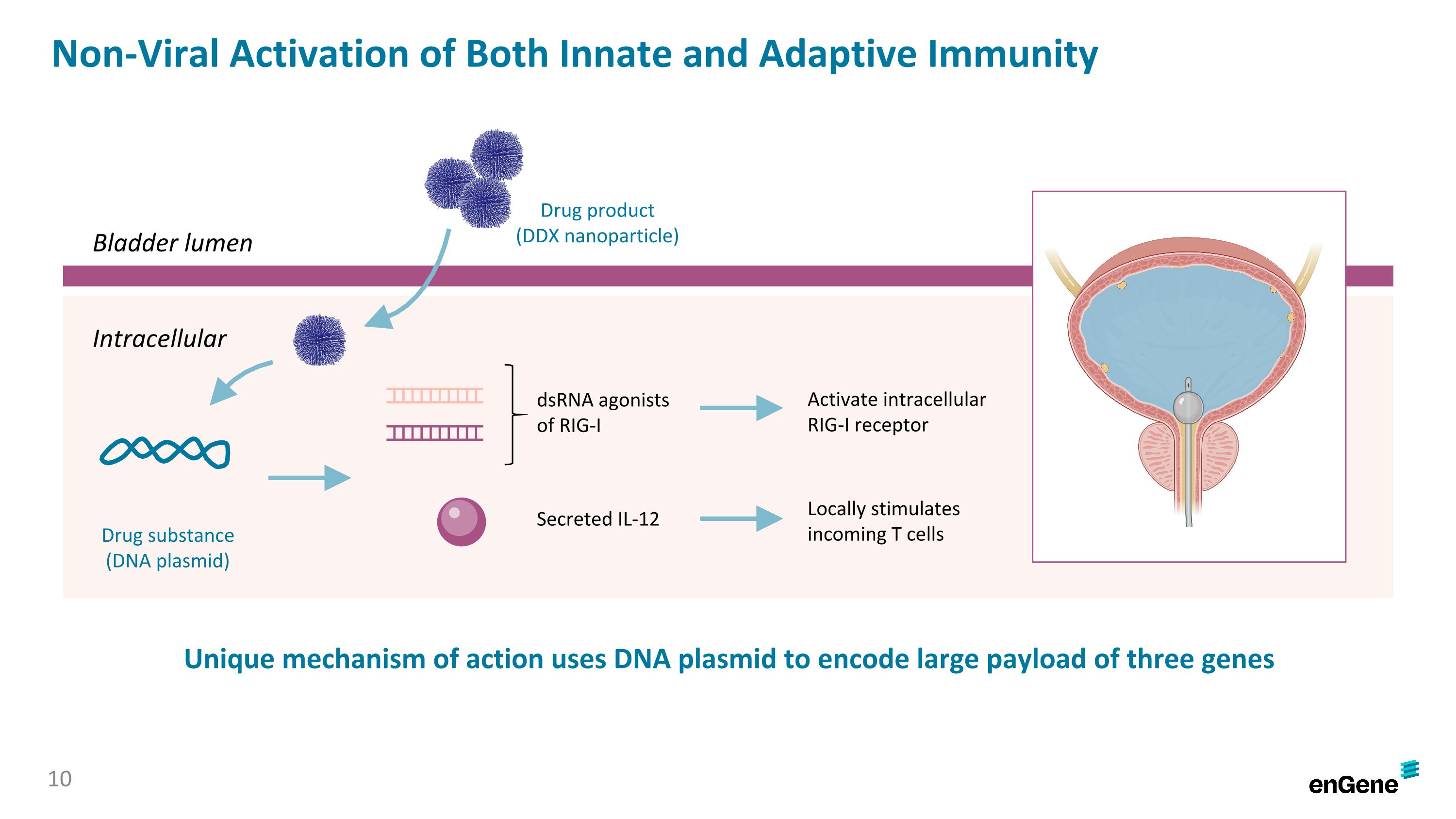
Non-Viral Activation of Both Innate and Adaptive Immunity Unique mechanism of action uses DNA plasmid to encode large payload of three genes 10 Bladder lumen Intracellular Drug product (DDX nanoparticle) Drug substance (DNA plasmid) dsRNA agonists of RIG-I Secreted IL-12 Activate intracellular RIG-I receptor Locally stimulates incoming T cells

e e e e e - - - - - (Excipient) PEG-PLE Bulk Mixing In-line Mixing PEGylated DDX/DNA Nanoparticle (Non-Viral �Drug Product) DDX/DNA Nanoparticle + + + + + + + Non-viral, lyophilized drug product Established at-scale manufacturing of all key components No special delivery or handling requirements + + + + + + + Dually Derivatized Oligochitosan (DDX) Plasmid DNA (Drug Substance) - - - - - - - - - + 2 4 3 1 Industrial-Scale, Well-Characterized Manufacturing Process 11

Phase 1 Demonstrated Promising Clinical Profile Phase 1: Study Design Phase 1 Results Patients: BCG-Unresponsive, High-risk NMIBC with Cis Dosing: 2 or 4 doses in 12-week cycle Cohorts: 3+3 dose escalation (4 dose levels) Endpoints: 1° - Safety; 2° - Efficacy All Dose Groups (N = 22) CR Rate (%) Anytime 73% 3 Months 68% 6 Months 45% Phase 2 Dose (N = 10) 3 Months 70% 6 Months 60%

Detalimogene Was Well Tolerated in Ph 1, No Grade 4/5 TRAEs Observed TRAE = Treatment-related adverse event *Patient had a history of renal failure and recurrent obstructive uropathy with presence of bilateral hydroureteronephrosis at screening – enrollment criteria later modified and excludes patients with a history of persistent or ongoing renal failure. Detalimogene (N = 24) Grade 1/2 Grade 3 Grade 4/5 Patients with ≥ 1 TRAE 13 (54.2%) 1 (4.2%)* 0 (0) TRAE Reported in >10% patients: Dysuria 3 (12.5%) 0 (0) 0 (0) Hematuria 3 (12.5%) 0 (0) 0 (0) Micturition Urgency 4 (16.7%) 0 (0) 0 (0) Urinary Tract Infection 3 (12.5%) 0 (0) 0 (0) No Grade 4/5 TRAEs No safety-related treatment discontinuations Only 1 Grade 3 �TRAE reported �(Grade 3 renal failure)* All AEs were reversible �and largely consistent �with catheterization

Pembrolizumab �(Keytruda) Nadofaragene firadenovec (Adstiladrin) Nogapendekin alfa inbakicep (Anktiva) + BCG Approval Date January 2020 December 2022 April 2024 Study Design Single Arm, Open label Single Arm, Open label Single Arm, Open label Efficacy Evaluable �Patients (N)** 96 98 77 12 Month Complete Response 19% 24% 45% August 2024 Draft Guidance reconfirms single arm open label study sufficient for approval Single-Arm, Open-Label Study Has Been Sufficient for FDA Approval* * For BCG-unresponsive High-Risk NMIBC with Cis ** Study sizes reported based on evaluable patients as describe on USPI Sources: Balar et al., Lancet Oncology 2021, 22:919-30; Boorjian et al. 2022 Lancent Oncology 2021 22(1) 107-17; Chamie, Karim, et al. NEJM evidence 2.1 (2022): EVIDoa2200167 14

Registrational Study Patients: BCG-Unresponsive High-risk NMIBC with Cis Design: Global, single-arm, open label N = 100 Dosing: 4x800ug intravesical at weeks 1,2,5,6 Q3M Endpoints: 1° - CR rate at 12-months; 2° - safety and durability Status: Enrolling, BLA filing planned for mid’26 15
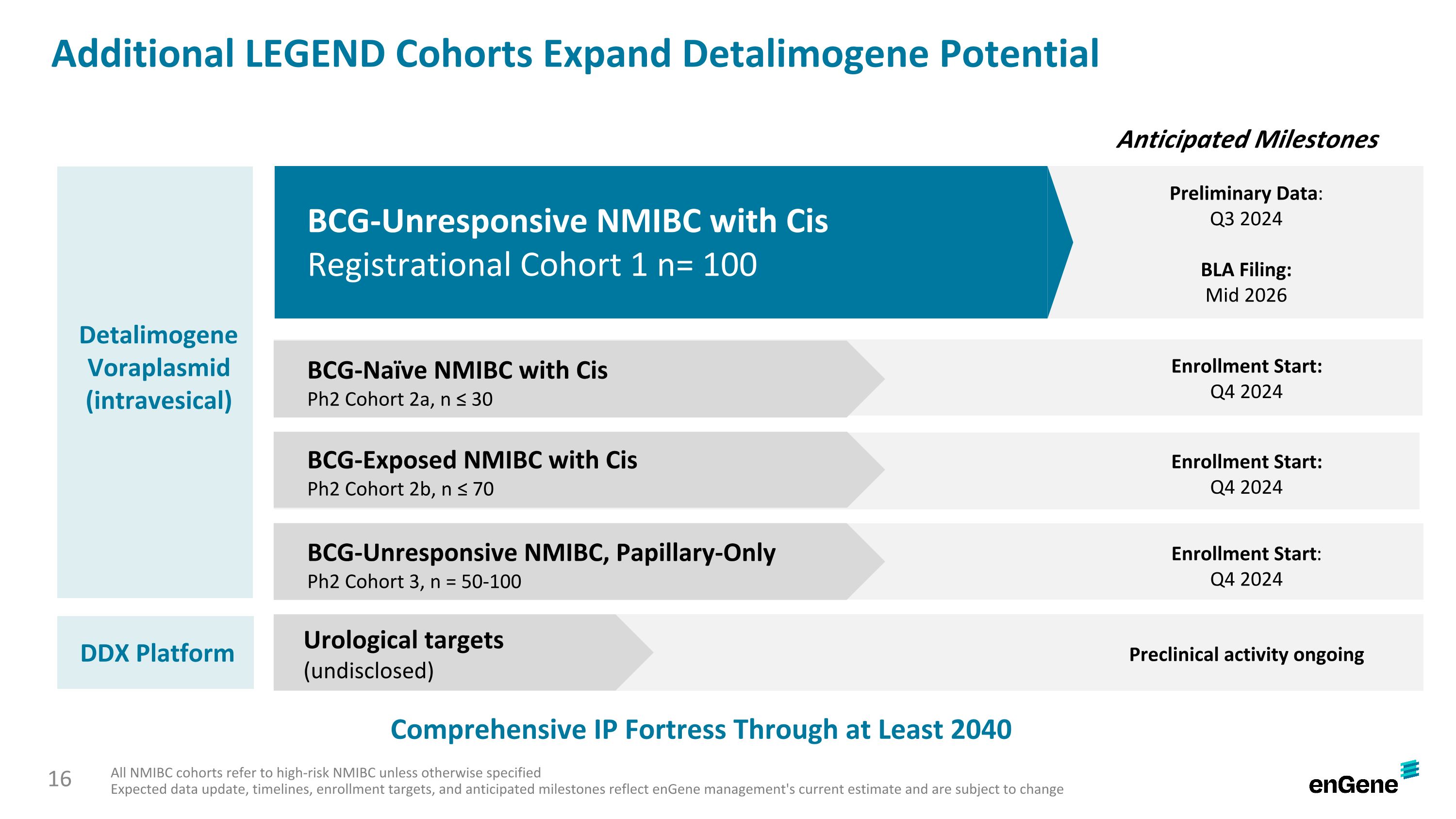
Additional LEGEND Cohorts Expand Detalimogene Potential All NMIBC cohorts refer to high-risk NMIBC unless otherwise specified Expected data update, timelines, enrollment targets, and anticipated milestones reflect enGene management's current estimate and are subject to change Anticipated Milestones Detalimogene Voraplasmid (intravesical) BCG-Unresponsive NMIBC with Cis Registrational Cohort 1 n= 100 BCG-Naïve NMIBC with Cis Ph2 Cohort 2a, n ≤ 30 BCG-Exposed NMIBC with Cis Ph2 Cohort 2b, n ≤ 70 BCG-Unresponsive NMIBC, Papillary-Only Ph2 Cohort 3, n = 50-100 Enrollment Start: Q4 2024 Preliminary Data: Q3 2024 BLA Filing: Mid 2026 Enrollment Start: Q4 2024 Enrollment Start: Q4 2024 DDX Platform Preclinical activity ongoing Urological targets (undisclosed) Comprehensive IP Fortress Through at Least 2040

Detalimogene: ‘Practical Therapy’ Ease of Administration for Patients Clinical Activity and Durability Observed* Ease of Administration For Clinicians Ease of Manufacture and Storage Favorable AE Profile Observed* Detalimogene is Uniquely Designed for Busy Urology Practices 17 *LEGEND Phase 1 reported data

Adstiladrin 3-10 hr vial thaw Use w/in 24hrs once thawed Anticholinergic premedication, biosafety precautions, urine bleaching TAR-200 Procedure room and cystoscopy likely required to place and remove ≥Grade 3 AEs Cretostimogene* Complex process Cold chain storage required Oncolytic virus biosafety Possible need for urine, facilities decontamination Detalimogene Clinical activity observed in Ph 1 Minimal adverse events (Ph 1) Easily administered Urologist-Friendly Design: Detalimogene Ideal For Early Use 18 4 prewashes + administration Insertion and removal every 3 weeks Estimated 4-10 hour total process * BSL2-like handling recommendations are likely based on precedent described in USPI for Imlygic, an FDA-approved, locally administered oncolytic virus, as well as handling recommendations for oncolytic viruses as reported by the Oncology Nursing Society. Detailed preparation and/or administration instructions have not yet been reported for cretostimogene. Other information based on CG Oncology’s non-confidential investor presentation. ** Based on USPI. Once thawed, Adstiladrin vial must be used within 24 hours. Adstiladrin prescribing information also advises that “persons who are immunocompromised or immunodeficient may be at risk for disseminated infection from ADSTILADRIN due to low levels of replication-competent adenovirus.” 4-10 hour total process estimated based on vial thaw requirement timing and estimated pharmacy preparation times.

Adstiladrin** Virus exposure precautions Extended clinic visit time, 48 hours of urine bleaching TAR-200 Device insertion and cystoscopic removal Potential foreign body sensation when urinating Cretostimogene* Virus exposure precautions likely Prewashes extend chair time Possible contact restrictions �with loved ones Detalimogene One hour administration time No post treatment action No washes Patient Friendly Design Makes for Ease of Experience 19 4 prewashes + administration Insertion and removal every 3 weeks Estimated 4-10 hour time in-clinic * BSL2-like handling recommendations are likely based on precedent described in USPI for Imlygic, an FDA-approved, locally administered oncolytic virus, as well as handling recommendations for oncolytic viruses as reported by the Oncology Nursing Society. Detailed preparation and/or administration instructions have not yet been reported for cretostimogene. Other information based on CG Oncology’s non-confidential investor presentation. ** Based on USPI. Once thawed, Adstiladrin vial must be used within 24 hours. Adstiladrin prescribing information also advises that “persons who are immunocompromised or immunodeficient may be at risk for disseminated infection from ADSTILADRIN due to low levels of replication-competent adenovirus.” 4-10 hour total in-clinic time estimated based on vial thaw requirement timing, estimated pharmacy preparation times, and administration timing.

Viral Gene Therapies Nadofaragene firadenovec (Adstiladrin) Cold-chain storage required Burdensome handling requirements Post-treatment biocontainment Similar limitations expected for cretostimogene grenadenorepvec Bacterial Immunotherapy Bacille Calmette-Guerin (BCG) Global supply chain challenges limit availability of critical �TICE strain for �foreseeable future Tolerability�(e.g., bladder spasms) Drug/Device Combos and Chemo Gemcitabine/TAR-200�(not approved) Burdensome placement Higher incidence of ≥Gr3 AEs (e.g., urosepsis) Recombinant Immunotherapy and CPIs Pembrolizumab (Keytruda): Substantial risk of severe, systemic AEs that urologists cannot manage Requires systemic IV infusion Nogapendekin alfa inbakicep + BCG (Anktiva) Supply-limited due to �BCG co-administration requirement Non-viral genetic medicine Detalimogene Complementary Investigational Therapies Should Transform and Expand NMIBC Market Shift in NMIBC management paradigm drives projected market growth to >$20B Patients expected to be sequentially treated with multiple therapies 20 Gr3 = Grade 3 NMIBC Market Forecast based on internal estimates utilizing multiple source data

Experience Developing and Commercializing Highly Successful Medicines RAJ PRUTHI, MD MHA FACS �Chief Medical Officer Global Medical Affairs Lead, TAR-210 Bladder Cancer Program, Johnson&Johnson Professor and Chair, Dept of Urology, UCSF Member, AUA/SUO NMIBC Guidelines RON COOPER�Chief Executive Officer Chief Executive Officer, Albireo Pharma President, Europe, Bristol Myers Squibb RYAN DAWS�Chief Financial Officer CFO Roles: Concert Therapeutics, Obsidian Therapeutics Investment Banker Roles: Cowen, Stifel, Baird ANTHONY CHEUNG, Ph.D�Chief Technology Officer Co-founder, enGene (CEO through 2018) Co-inventor on all key enGene patents Former Committee Member, ASGCT Industry Liaison Committee (2008-2014) EY Entrepreneur of the Year Finalist (2017) ALEX NICHOLS, Ph.D�President and Chief �Operating Officer Co-Founder and CEO, Mythic Therapeutics Co-Founder, Cogen Therapeutics Associate, Flagship Pioneering JAMES SULLIVAN, Ph.D�Chief Scientific Officer VP, Pulmonary Discovery, Translate Bio Executive Director, Sana Biotechnology Director, R&D, Vertex Pharmaceuticals LEE GIGUERE�Chief Legal Officer and Secretary CLO, Obsidian Therapeutics General Counsel, Chiasma Inc. Other legal roles: Karyopharm Therapeutics, Boston Scientific, Goodwin Procter

Detalimogene Voraplasmid for NMIBC Designed to be a practice-changing therapy requiring no change in practice Detalimogene voraplasmid was formerly referred to as EG-70 NMIBC = Non-muscle invasive bladder cancer NMIBC Market Forecast based on internal estimates utilizing multiple source data Highly Differentiated Investigational Product Explosive Transformational Market Opportunity Near Term Commercial Opportunity Well-capitalized, with cash projected into 2027 BLA filing mid ‘26 (est.) Unique combination of clinical activity, tolerability, ease of use NMIBC market forecast >$20B

Non-Viral Genetic Medicine Additional Slides

Data cutoff 8.22.2023; **E means ‘expansion’; †, Patient or physician declined to continue on investigational agent to pursue other modes of treatment (e.g., surgery) † †, One additional patient was dosed in this group but later deemed by the independent DSMB to be ineligible and excluded from ‘efficacy set’ First-in-Human Ph1 LEGEND Demonstrated Clinical Activity

Detalimogene is Designed to Streamline Administration and Ease the Experience for Patients and Clinicians †† Based on USPI. * Based on USPI. Note: Once thawed, Adstiladrin vial must be used within 24 hours. Adstiladrin prescribing information also advises that “persons who are immunocompromised or immunodeficient may be at risk for disseminated infection from ADSTILADRIN due to low levels of replication-competent adenovirus.” ** BSL2-like handling recommendations are likely based on precedent described in USPI for Imlygic, an FDA-approved, locally administered oncolytic virus. Detailed preparation and/or administration instructions have not yet been reported for cretostimogene. Other information based on CG Oncology’s non-confidential investor presentation. Cretostimogene dwell (45-60m) DDM Re-infusion and dwell (15m) Saline �Wash DDM �Wash Saline �Wash Preparation and Handling: BSL2-like handling likely recommended** Cretostimogene: (Based on publicly available data): Complex process with unclear vial thaw, biosafety, and urine decontamination requirements ~10m thaw Urine bleaching requirements not reported Adstiladrin instillation �and dwell (60m)* 3-10 hour �vial thaw* Adstiladrin: Vial thaw introduces preparation bottleneck; elevated biosafety procedures required; additional pre/post treatment burden for patients Anticholinergic premedication* 48-hour period of required urine bleaching* Preparation: “Universal biosafety precautions” required; USPI contains infection risk warning* 6-hour period of required urine bleaching †† BCG-TICE: Risk of infection for patients and caregivers; longer, uncomfortable dwell time; urine decontamination burden Preparation: mask/gown minimally required †† BCG instillation and dwell �(120m) †† Instillation �and dwell (60m) Detalimogene: As designed, no vial thaw, simple preparation; no pre/post treatment protocol Preparation: can be conducted on open table or benchtop; no special handling (No post-treatment urine bleaching required)
v3.24.2.u1
Document And Entity Information
|
Sep. 05, 2024 |
| Document Information [Line Items] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Sep. 05, 2024
|
| Entity Registrant Name |
enGene Holdings Inc.
|
| Entity Central Index Key |
0001980845
|
| Entity Emerging Growth Company |
true
|
| Entity File Number |
001-41854
|
| Entity Incorporation, State or Country Code |
A1
|
| Entity Tax Identification Number |
00-0000000
|
| Entity Address, Address Line One |
4868 Rue Levy, Suite 220
|
| Entity Address, City or Town |
Saint-Laurent
|
| Entity Address, State or Province |
QC
|
| Entity Address, Country |
CA
|
| Entity Address, Postal Zip Code |
H4R 2P1
|
| City Area Code |
514
|
| Local Phone Number |
332-4888
|
| Entity Information, Former Legal or Registered Name |
Not Applicable
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
true
|
| Common Stock [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Common Shares
|
| Trading Symbol |
ENGN
|
| Security Exchange Name |
NASDAQ
|
| Warrants [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Warrants, each exercisable for one Common Share, at an exercise price of $11.50 per Share
|
| Trading Symbol |
ENGNW
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=engn_WarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
enGene (NASDAQ:ENGNW)
Historical Stock Chart
From Oct 2024 to Nov 2024

enGene (NASDAQ:ENGNW)
Historical Stock Chart
From Nov 2023 to Nov 2024
