false
Q3
--12-31
0001873835
0001873835
2024-01-01
2024-09-30
0001873835
2024-11-12
0001873835
2024-09-30
0001873835
2023-12-31
0001873835
2024-07-01
2024-09-30
0001873835
2023-07-01
2023-09-30
0001873835
2023-01-01
2023-09-30
0001873835
us-gaap:CommonStockMember
2023-12-31
0001873835
us-gaap:AdditionalPaidInCapitalMember
2023-12-31
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-12-31
0001873835
us-gaap:RetainedEarningsMember
2023-12-31
0001873835
us-gaap:TreasuryStockCommonMember
2023-12-31
0001873835
us-gaap:NoncontrollingInterestMember
2023-12-31
0001873835
us-gaap:CommonStockMember
2024-03-31
0001873835
us-gaap:AdditionalPaidInCapitalMember
2024-03-31
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-03-31
0001873835
us-gaap:RetainedEarningsMember
2024-03-31
0001873835
us-gaap:TreasuryStockCommonMember
2024-03-31
0001873835
us-gaap:NoncontrollingInterestMember
2024-03-31
0001873835
2024-03-31
0001873835
us-gaap:CommonStockMember
2024-06-30
0001873835
us-gaap:AdditionalPaidInCapitalMember
2024-06-30
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-06-30
0001873835
us-gaap:RetainedEarningsMember
2024-06-30
0001873835
us-gaap:TreasuryStockCommonMember
2024-06-30
0001873835
us-gaap:NoncontrollingInterestMember
2024-06-30
0001873835
2024-06-30
0001873835
us-gaap:CommonStockMember
2022-12-31
0001873835
us-gaap:AdditionalPaidInCapitalMember
2022-12-31
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-12-31
0001873835
us-gaap:RetainedEarningsMember
2022-12-31
0001873835
us-gaap:TreasuryStockCommonMember
2022-12-31
0001873835
us-gaap:NoncontrollingInterestMember
2022-12-31
0001873835
2022-12-31
0001873835
us-gaap:CommonStockMember
2023-03-31
0001873835
us-gaap:AdditionalPaidInCapitalMember
2023-03-31
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-03-31
0001873835
us-gaap:RetainedEarningsMember
2023-03-31
0001873835
us-gaap:TreasuryStockCommonMember
2023-03-31
0001873835
us-gaap:NoncontrollingInterestMember
2023-03-31
0001873835
2023-03-31
0001873835
us-gaap:CommonStockMember
2023-06-30
0001873835
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-06-30
0001873835
us-gaap:RetainedEarningsMember
2023-06-30
0001873835
us-gaap:TreasuryStockCommonMember
2023-06-30
0001873835
us-gaap:NoncontrollingInterestMember
2023-06-30
0001873835
2023-06-30
0001873835
us-gaap:CommonStockMember
2024-01-01
2024-03-31
0001873835
us-gaap:AdditionalPaidInCapitalMember
2024-01-01
2024-03-31
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-01-01
2024-03-31
0001873835
us-gaap:RetainedEarningsMember
2024-01-01
2024-03-31
0001873835
us-gaap:TreasuryStockCommonMember
2024-01-01
2024-03-31
0001873835
us-gaap:NoncontrollingInterestMember
2024-01-01
2024-03-31
0001873835
2024-01-01
2024-03-31
0001873835
us-gaap:CommonStockMember
2024-04-01
2024-06-30
0001873835
us-gaap:AdditionalPaidInCapitalMember
2024-04-01
2024-06-30
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-04-01
2024-06-30
0001873835
us-gaap:RetainedEarningsMember
2024-04-01
2024-06-30
0001873835
us-gaap:TreasuryStockCommonMember
2024-04-01
2024-06-30
0001873835
us-gaap:NoncontrollingInterestMember
2024-04-01
2024-06-30
0001873835
2024-04-01
2024-06-30
0001873835
us-gaap:CommonStockMember
2024-07-01
2024-09-30
0001873835
us-gaap:AdditionalPaidInCapitalMember
2024-07-01
2024-09-30
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-07-01
2024-09-30
0001873835
us-gaap:RetainedEarningsMember
2024-07-01
2024-09-30
0001873835
us-gaap:TreasuryStockCommonMember
2024-07-01
2024-09-30
0001873835
us-gaap:NoncontrollingInterestMember
2024-07-01
2024-09-30
0001873835
us-gaap:CommonStockMember
2023-01-01
2023-03-31
0001873835
us-gaap:AdditionalPaidInCapitalMember
2023-01-01
2023-03-31
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-01-01
2023-03-31
0001873835
us-gaap:RetainedEarningsMember
2023-01-01
2023-03-31
0001873835
us-gaap:TreasuryStockCommonMember
2023-01-01
2023-03-31
0001873835
us-gaap:NoncontrollingInterestMember
2023-01-01
2023-03-31
0001873835
2023-01-01
2023-03-31
0001873835
us-gaap:CommonStockMember
2023-04-01
2023-06-30
0001873835
us-gaap:AdditionalPaidInCapitalMember
2023-04-01
2023-06-30
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-04-01
2023-06-30
0001873835
us-gaap:RetainedEarningsMember
2023-04-01
2023-06-30
0001873835
us-gaap:TreasuryStockCommonMember
2023-04-01
2023-06-30
0001873835
us-gaap:NoncontrollingInterestMember
2023-04-01
2023-06-30
0001873835
2023-04-01
2023-06-30
0001873835
us-gaap:CommonStockMember
2023-07-01
2023-09-30
0001873835
us-gaap:AdditionalPaidInCapitalMember
2023-07-01
2023-09-30
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-07-01
2023-09-30
0001873835
us-gaap:RetainedEarningsMember
2023-07-01
2023-09-30
0001873835
us-gaap:TreasuryStockCommonMember
2023-07-01
2023-09-30
0001873835
us-gaap:NoncontrollingInterestMember
2023-07-01
2023-09-30
0001873835
us-gaap:CommonStockMember
2024-09-30
0001873835
us-gaap:AdditionalPaidInCapitalMember
2024-09-30
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-09-30
0001873835
us-gaap:RetainedEarningsMember
2024-09-30
0001873835
us-gaap:TreasuryStockCommonMember
2024-09-30
0001873835
us-gaap:NoncontrollingInterestMember
2024-09-30
0001873835
us-gaap:CommonStockMember
2023-09-30
0001873835
us-gaap:AdditionalPaidInCapitalMember
2023-09-30
0001873835
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-09-30
0001873835
us-gaap:RetainedEarningsMember
2023-09-30
0001873835
us-gaap:TreasuryStockCommonMember
2023-09-30
0001873835
us-gaap:NoncontrollingInterestMember
2023-09-30
0001873835
2023-09-30
0001873835
IMMX:ImmixBiopharmaAustraliaPtyLtdMember
2024-09-30
0001873835
IMMX:JulySalesAgreementMember
2023-07-13
2023-07-14
0001873835
us-gaap:ScenarioAdjustmentMember
IMMX:JulyATMFacilityMember
2023-07-13
2024-02-05
0001873835
us-gaap:CommonStockMember
2024-02-01
2024-02-29
0001873835
us-gaap:CommonStockMember
2024-02-29
0001873835
us-gaap:CommonStockMember
2024-03-01
2024-03-01
0001873835
2024-07-25
0001873835
IMMX:AustralianTaxIncentiveMember
2024-07-01
2024-09-30
0001873835
IMMX:AustralianTaxIncentiveMember
2023-07-01
2023-09-30
0001873835
IMMX:AustralianTaxIncentiveMember
2024-01-01
2024-09-30
0001873835
IMMX:AustralianTaxIncentiveMember
2023-01-01
2023-09-30
0001873835
us-gaap:CommonStockMember
IMMX:PrefundedWarrantsMember
2024-01-01
2024-09-30
0001873835
us-gaap:CommonStockMember
IMMX:PrefundedWarrantsMember
2024-09-30
0001873835
IMMX:StockOptionsAndWarrantsExercisableMember
2024-01-01
2024-09-30
0001873835
IMMX:StockOptionsAndWarrantsExercisableMember
2023-01-01
2023-09-30
0001873835
us-gaap:FairValueInputsLevel1Member
2024-09-30
0001873835
us-gaap:FairValueInputsLevel2Member
2024-09-30
0001873835
us-gaap:FairValueInputsLevel3Member
2024-09-30
0001873835
us-gaap:FairValueInputsLevel1Member
2023-12-31
0001873835
us-gaap:FairValueInputsLevel2Member
2023-12-31
0001873835
us-gaap:FairValueInputsLevel3Member
2023-12-31
0001873835
IMMX:OperatingEquipmentMember
srt:MinimumMember
2024-09-30
0001873835
IMMX:OperatingEquipmentMember
srt:MaximumMember
2024-09-30
0001873835
IMMX:ElectronicEquipmentMember
srt:MinimumMember
2024-09-30
0001873835
IMMX:ElectronicEquipmentMember
srt:MaximumMember
2024-09-30
0001873835
us-gaap:OfficeEquipmentMember
srt:MinimumMember
2024-09-30
0001873835
us-gaap:OfficeEquipmentMember
srt:MaximumMember
2024-09-30
0001873835
IMMX:NexcellaAbsorptionMember
IMMX:NexcellaIncMember
2024-05-20
0001873835
IMMX:NexcellaAbsorptionMember
2024-05-20
0001873835
IMMX:NexcellaAbsorptionMember
2024-05-19
2024-05-20
0001873835
IMMX:Nexcella2022EquityIncentivePlanMember
2024-05-19
2024-05-20
0001873835
IMMX:Nexcella2022EquityIncentivePlanMember
srt:MaximumMember
2024-05-19
2024-05-20
0001873835
IMMX:Nexcella2022EquityIncentivePlanMember
2024-05-20
0001873835
IMMX:NexcellaFoundersAgreementMember
2022-12-08
0001873835
2022-12-07
2022-12-08
0001873835
IMMX:NexcellaFoundersAgreementMember
2022-12-07
2022-12-08
0001873835
IMMX:NexcellaFoundersAgreementMember
us-gaap:PreferredClassAMember
2022-12-07
2022-12-08
0001873835
IMMX:NexcellaFoundersAgreementMember
us-gaap:CommonClassAMember
2022-12-07
2022-12-08
0001873835
IMMX:NexcellaFoundersAgreementMember
us-gaap:CommonStockMember
2022-12-07
2022-12-08
0001873835
IMMX:NexcellaManagementServicesAgreementMember
2022-12-07
2022-12-08
0001873835
IMMX:OperatingEquipmentMember
2024-09-30
0001873835
IMMX:OperatingEquipmentMember
2023-12-31
0001873835
us-gaap:OfficeEquipmentMember
2024-09-30
0001873835
us-gaap:OfficeEquipmentMember
2023-12-31
0001873835
IMMX:JulyATMSalesAgreementMember
2023-07-14
0001873835
IMMX:JulyATMSalesAgreementMember
2023-07-13
2023-07-14
0001873835
IMMX:JulyATMSalesAgreementMember
2023-07-01
2023-09-30
0001873835
IMMX:JulyATMSalesAgreementMember
2023-10-01
2023-12-31
0001873835
IMMX:JulyATMFacilityMember
2024-01-01
2024-09-30
0001873835
IMMX:JulyATMFacilityMember
2024-09-30
0001873835
IMMX:UnderwritingAgreementMember
2024-02-05
2024-02-05
0001873835
IMMX:UnderwritingAgreementMember
2024-02-05
0001873835
2024-02-08
2024-02-08
0001873835
us-gaap:OverAllotmentOptionMember
2024-02-05
2024-02-05
0001873835
IMMX:MarketingServiceAgreementMember
IMMX:JulyTwentyFiveTwoThousandTwentyThreeMember
2024-01-01
2024-09-30
0001873835
IMMX:MarketingServiceAgreementMember
2024-01-01
2024-09-30
0001873835
IMMX:MarketingServicesAgreementMember
us-gaap:CommonStockMember
2024-01-01
2024-09-30
0001873835
us-gaap:CommonStockMember
IMMX:MarketingServicesAgreementMember
2023-01-01
2023-12-31
0001873835
us-gaap:CommonStockMember
IMMX:NexcellaTwoThousandTwentyTwoEquityIncentivePlanMember
2024-01-01
2024-09-30
0001873835
us-gaap:GeneralAndAdministrativeExpenseMember
us-gaap:RestrictedStockMember
2024-01-01
2024-09-30
0001873835
us-gaap:RestrictedStockMember
2024-09-30
0001873835
us-gaap:RestrictedStockMember
2024-01-01
2024-09-30
0001873835
IMMX:TwoThousandSixteenPlanMember
2016-12-31
0001873835
IMMX:TwoThousandSixteenPlanMember
2021-12-31
0001873835
IMMX:TwoThousandTwentyOnePlanMember
2021-09-10
0001873835
IMMX:AmendedAndRestatedTwoThousandTwentyOneOmnibusEquityIncentivePlanMember
2023-04-22
2023-04-24
0001873835
IMMX:AmendedAndRestatedTwoThousandTwentyTwoOmnibusEquityIncentivePlanMember
2023-04-22
2023-04-24
0001873835
IMMX:TwoThousandTwentyOnePlanMember
2024-09-30
0001873835
IMMX:NexcellaTwoThousandTwentyTwoEquityIncentivePlanMember
2024-01-01
2024-09-30
0001873835
IMMX:NonEmployeeMember
2024-01-01
2024-09-30
0001873835
srt:ManagementMember
2024-01-01
2024-09-30
0001873835
IMMX:ConsultantMember
2024-01-01
2024-09-30
0001873835
us-gaap:GeneralAndAdministrativeExpenseMember
2024-07-01
2024-09-30
0001873835
us-gaap:GeneralAndAdministrativeExpenseMember
2023-07-01
2023-09-30
0001873835
us-gaap:GeneralAndAdministrativeExpenseMember
2024-01-01
2024-09-30
0001873835
us-gaap:GeneralAndAdministrativeExpenseMember
2023-01-01
2023-09-30
0001873835
us-gaap:WarrantMember
2024-09-30
0001873835
us-gaap:StockOptionMember
IMMX:SecondAmendedAndRestatedNexcellaTwoThousandTwentyTwoEquityIncentivePlanMember
srt:MinimumMember
2024-01-01
2024-09-30
0001873835
us-gaap:StockOptionMember
IMMX:SecondAmendedAndRestatedNexcellaTwoThousandTwentyTwoEquityIncentivePlanMember
srt:MaximumMember
2024-01-01
2024-09-30
0001873835
us-gaap:StockOptionMember
IMMX:ThirdAmendedAndRestatedTwoThousandTwentyTwoEquityIncentivePlanMember
srt:MinimumMember
2024-01-01
2024-09-30
0001873835
us-gaap:StockOptionMember
IMMX:ThirdAmendedAndRestatedTwoThousandTwentyTwoEquityIncentivePlanMember
srt:MaximumMember
2024-01-01
2024-09-30
0001873835
IMMX:FoundersAgreementMember
2023-03-12
2023-03-13
0001873835
us-gaap:GeneralAndAdministrativeExpenseMember
us-gaap:RestrictedStockMember
2024-07-01
2024-09-30
0001873835
us-gaap:StockOptionMember
IMMX:NexcellaMember
2024-07-01
2024-09-30
0001873835
us-gaap:StockOptionMember
IMMX:NexcellaMember
2024-01-01
2024-09-30
0001873835
us-gaap:WarrantMember
2024-01-01
2024-09-30
0001873835
IMMX:NexcellaMember
2024-01-01
2024-09-30
0001873835
IMMX:NexcellaMember
2023-12-31
0001873835
IMMX:NexcellaMember
2024-09-30
0001873835
IMMX:RangeOneMember
2024-01-01
2024-09-30
0001873835
IMMX:RangeOneMember
2024-09-30
0001873835
IMMX:RangeTwoMember
2024-01-01
2024-09-30
0001873835
IMMX:RangeTwoMember
2024-09-30
0001873835
IMMX:RangeThreeMember
2024-01-01
2024-09-30
0001873835
IMMX:RangeThreeMember
2024-09-30
0001873835
IMMX:RangeFourMember
2024-01-01
2024-09-30
0001873835
IMMX:RangeFourMember
2024-09-30
0001873835
us-gaap:WarrantMember
IMMX:RangeOneMember
2024-09-30
0001873835
us-gaap:WarrantMember
IMMX:RangeTwoMember
2024-09-30
0001873835
us-gaap:WarrantMember
IMMX:RangeTwoMember
2024-01-01
2024-09-30
0001873835
IMMX:RangeThreeMember
us-gaap:WarrantMember
2024-09-30
0001873835
us-gaap:WarrantMember
IMMX:RangeThreeMember
2024-01-01
2024-09-30
0001873835
us-gaap:LicenseAgreementTermsMember
2024-01-01
2024-09-30
0001873835
us-gaap:LicenseAgreementTermsMember
2023-01-01
2023-09-30
0001873835
IMMX:LicenseAgreementMember
2024-08-01
2024-08-31
0001873835
IMMX:LicenseAgreementMember
IMMX:PhaseIIStudiesMember
2024-08-01
2024-08-31
0001873835
IMMX:LicenseAgreementMember
IMMX:PhaseIIIStudiesMember
2024-08-01
2024-08-31
0001873835
IMMX:LicenseAgreementMember
IMMX:LicensedProductMember
2024-08-01
2024-08-31
0001873835
2024-01-31
0001873835
srt:MinimumMember
2024-01-01
2024-01-31
0001873835
srt:MaximumMember
2024-01-01
2024-01-31
0001873835
IMMX:EmploymentAgreementMember
IMMX:DrRachmanMember
2021-06-17
2021-06-18
0001873835
IMMX:EmploymentAgreementMember
IMMX:DrRachmanMember
2022-11-08
2022-11-09
0001873835
IMMX:EmploymentAgreementMember
IMMX:DrRachmanMember
2023-05-11
2023-05-12
0001873835
IMMX:EmploymentAgreementMember
IMMX:DrRachmanMember
srt:MaximumMember
2023-11-09
2023-11-09
0001873835
IMMX:EmploymentAgreementMember
IMMX:DrRachmanMember
2023-03-06
2023-03-07
0001873835
IMMX:ManagementSevicesAgreementMember
IMMX:MrMorrisMember
2021-03-17
2021-03-18
0001873835
IMMX:ManagementSevicesAgreementMember
IMMX:MrMorrisMember
2021-12-01
2021-12-31
0001873835
IMMX:ManagementSevicesAgreementMember
IMMX:MrMorrisMember
2022-11-08
2022-11-09
0001873835
IMMX:ManagementSevicesAgreementMember
IMMX:MrMorrisMember
2023-05-11
2023-05-12
0001873835
IMMX:ManagementSevicesAgreementMember
IMMX:MrMorrisMember
2023-11-09
2023-11-09
0001873835
IMMX:ManagementSevicesAgreementMember
IMMX:MrMorrisMember
2023-03-06
2023-03-07
0001873835
IMMX:MarketingServiceAgreementMember
IMMX:JulyTwentyFiveTwoThousandTwentyThreeMember
2024-10-01
2024-10-01
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
iso4217:AUD
utr:acre
IMMX:Segment
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
DC 20549
FORM
10-Q
(Mark
One)
| ☒ |
QUARTERLY
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For
the quarterly period ended September 30, 2024
OR
| ☐ |
TRANSITION
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For
the transition period from _________to ___________
Commission
File Number: 001-41159
IMMIX
BIOPHARMA, INC.
(Exact
Name of Registrant as Specified in its Charter)
| Delaware |
|
45-4869378 |
(State
or other jurisdiction
of
incorporation or organization) |
|
(I.R.S.
Employer
Identification
No.) |
| |
|
|
| 11400
West Olympic Blvd., Suite 200, Los Angeles, CA |
|
90064 |
| (Address
of principal executive offices) |
|
(Zip
Code) |
(310)
651-8041
(Registrant’s
telephone number, including area code)
Not
applicable
(Former
name, former address and former fiscal year, if changed since last report)
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which registered |
| Common
stock, $0.0001 par value |
|
IMMX |
|
The
Nasdaq Stock Market LLC |
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files). Yes ☒ No ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer |
☐ |
|
Accelerated
filer |
☐ |
| |
|
|
|
| Non-accelerated
filer |
☒ |
|
Smaller
reporting company |
☒ |
| |
|
|
|
| |
|
|
Emerging
growth company |
☒ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Number
of shares of common stock outstanding as of November 12, 2024 was 27,507,637.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS AND INDUSTRY DATA
This
Quarterly Report on Form 10-Q contains forward-looking statements which are made pursuant to the safe harbor provisions of Section 27A
of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934,
as amended (the “Exchange Act”). These statements may be identified by such forward-looking terminology as “may,”
“should,” “expects,” “intends,” “plans,” “anticipates,” “believes,”
“estimates,” “predicts,” “potential,” “continue” or the negative of these terms or other
comparable terminology. Our forward-looking statements are based on a series of expectations, assumptions, estimates and projections
about our company, are not guarantees of future results or performance and involve substantial risks and uncertainty. We may not actually
achieve the plans, intentions or expectations disclosed in these forward-looking statements. Actual results or events could differ materially
from the plans, intentions and expectations disclosed in these forward-looking statements. Our business and our forward-looking statements
involve substantial known and unknown risks and uncertainties, including the risks and uncertainties inherent in our statements regarding:
| |
● |
our
projected financial position and estimated cash burn rate; |
| |
|
|
| |
● |
our
estimates regarding expenses, future revenues and capital requirements; |
| |
|
|
| |
● |
our
ability to continue as a going concern; |
| |
|
|
| |
● |
our
need to raise substantial additional capital to fund our operations, the availability and terms of such funding, and dilution caused
thereby; |
| |
|
|
| |
● |
the
success, cost and timing of our clinical trials; |
| |
|
|
| |
● |
our
dependence on third parties in the conduct of our clinical trials; |
| |
|
|
| |
● |
our
ability to obtain the necessary regulatory approvals to market and commercialize our product candidates; |
| |
|
|
| |
● |
the
ultimate impact of a health epidemic, on our business, our clinical trials, our research programs, healthcare systems or the global
economy as a whole; |
| |
|
|
| |
● |
the
potential that results of pre-clinical and clinical trials indicate our current product candidates or any future product candidates
we may seek to develop are unsafe or ineffective; |
| |
|
|
| |
● |
the
results of market research conducted by us or others; |
| |
|
|
| |
● |
our
ability to obtain and maintain intellectual property protection for our current and future product candidates; |
| |
|
|
| |
● |
our
ability to protect our intellectual property rights and the potential for us to incur substantial costs from lawsuits to enforce
or protect our intellectual property rights; |
| |
● |
the
possibility that a third party may claim we or our third-party licensors have infringed, misappropriated or otherwise violated their
intellectual property rights and that we may incur substantial costs and be required to devote substantial time defending against
claims against us; |
| |
|
|
| |
● |
our
reliance on third-party suppliers and manufacturers; |
| |
|
|
| |
● |
the
success of competing therapies and products that are or become available; |
| |
|
|
| |
● |
our
ability to expand our organization to accommodate potential growth and our ability to retain and attract key personnel; |
| |
|
|
| |
● |
the
potential for us to incur substantial costs resulting from product liability lawsuits against us and the potential for these product
liability lawsuits to cause us to limit our commercialization of our product candidates; |
| |
● |
market
acceptance of our product candidates, the size and growth of the potential markets for our current product candidates and any future
product candidates we may seek to develop, and our ability to serve those markets; and |
| |
|
|
| |
● |
the
successful development of our commercialization capabilities, including sales and marketing capabilities. |
All
of our forward-looking statements are as of the date of this Quarterly Report on Form 10-Q only. In each case, actual results may differ
materially from such forward-looking information. We can give no assurance that such expectations or forward-looking statements will
prove to be correct. An occurrence of, or any material adverse change in, one or more of the risk factors or risks and uncertainties
referred to in our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 29, 2024, this Quarterly
Report on Form 10-Q or included in our other public disclosures or our other periodic reports or other documents or filings filed with
or furnished to the U.S. Securities and Exchange Commission (the “SEC”) could materially and adversely affect our business,
prospects, financial condition and results of operations. Except as required by law, we do not undertake or plan to update or revise
any such forward-looking statements to reflect actual results, changes in plans, assumptions, estimates or projections or other circumstances
affecting such forward-looking statements occurring after the date of this Quarterly Report on Form 10-Q, even if such results, changes
or circumstances make it clear that any forward-looking information will not be realized. Any public statements or disclosures by us
following this Quarterly Report on Form 10-Q that modify or impact any of the forward-looking statements contained in this Quarterly
Report on Form 10-Q will be deemed to modify or supersede such statements in this Quarterly Report on Form 10-Q.
This
Quarterly Report on Form 10-Q may include market data and certain industry data and forecasts, which we may obtain from internal company
surveys, market research, consultant surveys, publicly available information, reports of governmental agencies and industry publications,
articles and surveys. Industry surveys, publications, consultant surveys and forecasts generally state that the information contained
therein has been obtained from sources believed to be reliable, but the accuracy and completeness of such information is not guaranteed.
While we believe that such studies and publications are reliable, we have not independently verified market and industry data from third-party
sources, and we have not commissioned any such information.
PART
I – FINANCIAL INFORMATION
ITEM
1. FINANCIAL STATEMENTS.
Immix
Biopharma, Inc.
Condensed
Consolidated Balance Sheets
| | |
September 30, 2024 | | |
December 31, 2023 | |
| | |
(Unaudited) | | |
| |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 19,690,431 | | |
$ | 17,509,791 | |
| Tax receivable | |
| 1,975,437 | | |
| 1,172,183 | |
| Prepaid expenses and other current assets | |
| 1,089,637 | | |
| 1,105,776 | |
| | |
| | | |
| | |
| Total current assets | |
| 22,755,505 | | |
| 19,787,750 | |
| | |
| | | |
| | |
| Other assets | |
| 20,418 | | |
| - | |
| Deferred offering cost | |
| - | | |
| 87,229 | |
| Right-of-use asset, net | |
| 1,010,205 | | |
| - | |
| Property and equipment, net | |
| 1,355,424 | | |
| 50,181 | |
| | |
| | | |
| | |
| Total assets | |
$ | 25,141,552 | | |
$ | 19,925,160 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable and accrued expenses | |
$ | 6,501,786 | | |
$ | 3,721,783 | |
| Operating lease liability - current | |
| 62,715 | | |
| - | |
| | |
| | | |
| | |
| Total current liabilities | |
| 6,564,501 | | |
| 3,721,783 | |
| | |
| | | |
| | |
| Operating lease liability – long term | |
| 1,026,340 | | |
| - | |
| Total liabilities | |
| 7,590,841 | | |
| 3,721,783 | |
| | |
| | | |
| | |
| Commitments and contingencies | |
| - | | |
| - | |
| | |
| | | |
| | |
| Stockholders’ equity: | |
| | | |
| | |
| Preferred stock, $0.0001 par value; 10,000,000 shares authorized; no shares issued and outstanding | |
| | | |
| - | |
| Common stock, $0.0001 par value; 200,000,000 shares authorized; 27,552,938 shares issued and 27,480,575 shares outstanding at September 30, 2024 and 19,994,719 shares issued and 19,922,356 shares outstanding at December 31, 2023 | |
| 2,757 | | |
| 2,000 | |
| Additional paid-in capital | |
| 87,668,483 | | |
| 69,779,706 | |
| Accumulated other comprehensive income | |
| 192,051 | | |
| 134,666 | |
| Accumulated deficit | |
| (70,212,617 | ) | |
| (53,411,295 | ) |
| Treasury stock at cost, 72,363 shares as of September 30, 2024 and December 31, 2023 | |
| (99,963 | ) | |
| (99,963 | ) |
| Total Immix Biopharma, Inc. stockholders’ equity | |
| 17,550,711 | | |
| 16,405,114 | |
| Non-controlling interests | |
| - | | |
| (201,737 | ) |
| Total stockholders’ equity | |
| 17,550,711 | | |
| 16,203,377 | |
| | |
| | | |
| | |
| Total liabilities and stockholders’ equity | |
$ | 25,141,552 | | |
$ | 19,925,160 | |
See
accompanying notes to the unaudited condensed consolidated financial statements.
Immix
Biopharma, Inc.
Condensed
Consolidated Statements of Operations and Comprehensive Loss
(Unaudited)
| | |
| | |
| | |
| | |
| |
| | |
For the Three Months Ended | | |
For the Nine Months Ended | |
| | |
September 30, | | |
September 30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| General and administrative expenses | |
$ | 2,949,403 | | |
$ | 2,417,776 | | |
$ | 7,769,224 | | |
$ | 5,130,977 | |
| Research and development | |
| 4,445,528 | | |
| 2,106,020 | | |
| 9,918,336 | | |
| 5,634,284 | |
| | |
| | | |
| | | |
| | | |
| | |
| Total operating expenses | |
| 7,394,931 | | |
| 4,523,796 | | |
| 17,687,560 | | |
| 10,765,261 | |
| | |
| | | |
| | | |
| | | |
| | |
| Loss from operations | |
| (7,394,931 | ) | |
| (4,523,796 | ) | |
| (17,687,560 | ) | |
| (10,765,261 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | |
| Interest income | |
| 256,680 | | |
| 186,691 | | |
| 831,503 | | |
| 343,431 | |
| Total other expense, net | |
| 256,680 | | |
| 186,691 | | |
| 831,503 | | |
| 343,431 | |
| | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Provision for income taxes | |
| 11,144 | | |
| 6,807 | | |
| 30,252 | | |
| 18,326 | |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| (7,149,395 | ) | |
| (4,343,912 | ) | |
| (16,886,309 | ) | |
| (10,440,156 | ) |
| Net loss attributable to non-controlling interests | |
| - | | |
| 63,248 | | |
| 84,987 | | |
| 103,612 | |
| Net loss attributable to Immix Biopharma, Inc. common stockholders | |
| (7,149,395 | ) | |
| (4,280,664 | ) | |
| (16,801,322 | ) | |
| (10,336,544 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Other comprehensive income (loss): | |
| | | |
| | | |
| | | |
| | |
| Foreign currency translation | |
| 75,079 | | |
| (34,147 | ) | |
| 57,385 | | |
| (40,286 | ) |
| Total other comprehensive loss | |
| 75,079 | | |
| (34,147 | ) | |
| 57,385 | | |
| (40,286 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Comprehensive loss | |
| (7,074,316 | ) | |
| (4,314,811 | ) | |
| (16,743,937 | ) | |
| (10,376,830 | ) |
| Less: comprehensive loss attributable to non-controlling interests | |
| - | | |
| - | | |
| - | | |
| - | |
| Comprehensive loss attributable to Immix Biopharma, Inc. common stockholders | |
$ | (7,074,316 | ) | |
$ | (4,314,811 | ) | |
$ | (16,743,937 | ) | |
$ | (10,376,830 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Loss per common share - basic and diluted | |
$ | (0.24 | ) | |
$ | (0.23 | ) | |
$ | (0.60 | ) | |
$ | (0.65 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding - basic and diluted | |
| 29,424,444 | | |
| 18,578,414 | | |
| 27,859,556 | | |
| 15,861,100 | |
See
accompanying notes to the unaudited condensed consolidated financial statements.
Immix
Biopharma, Inc.
Condensed
Consolidated Statements of Stockholders’ Equity
For
the Three and Nine Months Ended September 30, 2024 and 2023
(Unaudited)
| | |
| | |
Common | | |
Additional | | |
Accumulated Other | | |
| | |
| | |
Treasury | | |
Non- | | |
Total | |
| | |
Common | | |
Stock | | |
Paid-in | | |
Comprehensive | | |
Accumulated | | |
Treasury | | |
Stock | | |
Controlling | | |
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Income | | |
Deficit | | |
Shares | | |
Amount | | |
Interests | | |
Equity | |
| Balance December 31, 2023 | |
| 19,994,719 | | |
$ | 2,000 | | |
$ | 69,779,706 | | |
$ | 134,666 | | |
$ | (53,411,295 | ) | |
| (72,363 | ) | |
$ | (99,963 | ) | |
$ | (201,737 | ) | |
$ | 16,203,377 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued under ATM facility for cash proceeds, net of offering costs | |
| 68,302 | | |
| 7 | | |
| 338,488 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 338,495 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued under public offering for cash proceeds, net of offering costs | |
| 6,319,025 | | |
| 632 | | |
| 15,519,722 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 15,520,354 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued for exercise of stock options | |
| 1,251 | | |
| - | | |
| 2,489 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,489 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued for services | |
| 85,486 | | |
| 9 | | |
| 327,367 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 327,376 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based compensation | |
| - | | |
| - | | |
| 615,888 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 615,888 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Non-controlling interests in subsidiary | |
| - | | |
| - | | |
| 9,472 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (9,472 | ) | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (5,258,991 | ) | |
| - | | |
| - | | |
| (72,073 | ) | |
| (5,331,064 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Foreign currency translation adjustment | |
| - | | |
| - | | |
| - | | |
| (45,052 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (45,052 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance March 31, 2024 | |
| 26,468,783 | | |
| 2,648 | | |
| 86,593,132 | | |
| 89,614 | | |
$ | (58,670,286 | ) | |
| (72,363 | ) | |
| (99,963 | ) | |
| (283,282 | ) | |
| 27,631,863 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued for services | |
| 42,901 | | |
| 5 | | |
| 102,495 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 102,500 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based compensation | |
| - | | |
| - | | |
| 535,350 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 535,350 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Non-controlling interests in subsidiary | |
| - | | |
| - | | |
| 20,200 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (20,200 | ) | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Buyout of non-controlling interests in subsidiary | |
| 989,876 | | |
| 99 | | |
| (316,495 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| 316,396 | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (4,392,936 | ) | |
| - | | |
| - | | |
| (12,914 | ) | |
| (4,405,850 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Foreign currency translation adjustment | |
| - | | |
| - | | |
| - | | |
| 27,358 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 27,358 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance June 30, 2024 | |
| 27,501,560 | | |
| 2,752 | | |
| 86,934,682 | | |
| 116,972 | | |
| (63,063,222 | ) | |
| (72,363 | ) | |
| (99,963 | ) | |
| - | | |
| 23,891,221 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued for services | |
| 51,378 | | |
| 5 | | |
| 104,995 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 105,000 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based compensation | |
| - | | |
| - | | |
| 628,806 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 628,806 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (7,149,395 | ) | |
| - | | |
| - | | |
| - | | |
| (7,149,395 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Foreign currency translation adjustment | |
| - | | |
| - | | |
| - | | |
| 75,079 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 75,079 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance September 30, 2024 | |
| 27,552,938 | | |
$ | 2,757 | | |
$ | 87,668,483 | | |
$ | 192,051 | | |
$ | (70,212,617 | ) | |
| (72,363 | ) | |
$ | (99,963 | ) | |
$ | - | | |
$ | 17,550,711 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance December 31, 2022 | |
| 13,964,485 | | |
$ | 1,397 | | |
$ | 51,156,597 | | |
$ | 87,021 | | |
$ | (37,985,247 | ) | |
| (72,363 | ) | |
$ | (99,963 | ) | |
$ | - | | |
$ | 13,159,805 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued under ATM facility for cash proceeds, net of offering costs | |
| 50,000 | | |
| 5 | | |
| 101,318 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 101,323 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Nexcella shares issued for cash proceeds | |
| - | | |
| - | | |
| 650,000 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 650,000 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based compensation | |
| 6,700 | | |
| 1 | | |
| 329,918 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 329,919 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Non-controlling interests in subsidiary | |
| - | | |
| - | | |
| 13,990 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (13,990 | ) | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (2,479,664 | ) | |
| - | | |
| - | | |
| (18,368 | ) | |
| (2,498,032 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Foreign currency translation adjustment | |
| - | | |
| - | | |
| - | | |
| (4,474 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (4,474 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance March 31, 2023 | |
| 14,021,185 | | |
| 1,403 | | |
| 52,251,823 | | |
| 82,547 | | |
| (40,464,911 | ) | |
| (72,363 | ) | |
| (99,963 | ) | |
| (32,358 | ) | |
| 11,738,541 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued under ATM facility for cash proceeds, net of offering costs | |
| 2,213,868 | | |
| 221 | | |
| 4,584,032 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 4,584,253 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based compensation | |
| 99,128 | | |
| 10 | | |
| 447,646 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 447,656 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Non-controlling interests in subsidiary | |
| - | | |
| - | | |
| 2,416 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (2,416 | ) | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (3,576,216 | ) | |
| - | | |
| - | | |
| (21,996 | ) | |
| (3,598,212 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Foreign currency translation adjustment | |
| - | | |
| - | | |
| - | | |
| (1,665 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,665 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance June 30, 2023 | |
| 16,334,181 | | |
| 1,634 | | |
| 57,285,917 | | |
| 80,882 | | |
| (44,041,127 | ) | |
| (72,363 | ) | |
| (99,963 | ) | |
| (56,770 | ) | |
| 13,170,573 | |
| Balance | |
| 16,334,181 | | |
| 1,634 | | |
| 57,285,917 | | |
| 80,882 | | |
| (44,041,127 | ) | |
| (72,363 | ) | |
| (99,963 | ) | |
| (56,770 | ) | |
| 13,170,573 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares issued under ATM facility for cash proceeds, net of offering costs | |
| 105,834 | | |
| 10 | | |
| 185,272 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 185,282 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Shares and warrants issued under private placement for cash proceeds, net of offering costs | |
| 3,241,076 | | |
| 324 | | |
| 9,933,829 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 9,934,153 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based compensation | |
| - | | |
| - | | |
| 737,325 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 737,325 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Non-controlling interests in subsidiary | |
| - | | |
| - | | |
| 4,649 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (4,649 | ) | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (4,280,664 | ) | |
| - | | |
| - | | |
| (63,248 | ) | |
| (4,343,912 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Foreign currency translation adjustment | |
| - | | |
| - | | |
| - | | |
| (34,147 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (34,147 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance September 30, 2023 | |
| 19,681,091 | | |
$ | 1,968 | | |
$ | 68,146,992 | | |
$ | 46,735 | | |
$ | (48,321,791 | ) | |
| (72,363 | ) | |
$ | (99,963 | ) | |
$ | (124,667 | ) | |
$ | 19,649,274 | |
| Balance | |
| 19,681,091 | | |
$ | 1,968 | | |
$ | 68,146,992 | | |
$ | 46,735 | | |
$ | (48,321,791 | ) | |
| (72,363 | ) | |
$ | (99,963 | ) | |
$ | (124,667 | ) | |
$ | 19,649,274 | |
See
accompanying notes to the unaudited condensed consolidated financial statements.
Immix
Biopharma, Inc.
Condensed
Consolidated Statements of Cash Flows
(Unaudited)
| | |
| | |
| |
| | |
For the Nine Months Ended | |
| | |
September 30, | |
| | |
2024 | | |
2023 | |
| Operating Activities: | |
| | | |
| | |
| Net loss | |
$ | (16,886,309 | ) | |
$ | (10,440,156 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Stock-based compensation | |
| 2,314,920 | | |
| 1,514,900 | |
| Depreciation | |
| 12,338 | | |
| 2,392 | |
| Amortization of right of use asset | |
| 61,713 | | |
| - | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Tax receivable | |
| (746,748 | ) | |
| (427,476 | ) |
| Prepaid expenses and other current assets | |
| 7,932 | | |
| (923,909 | ) |
| Other assets | |
| (20,418 | ) | |
| - | |
| Accounts payable and accrued expenses | |
| 2,120,531 | | |
| 1,580,248 | |
| Operating lease liability | |
| 17,137 | | |
| - | |
| | |
| | | |
| | |
| Net cash used in operating activities | |
| (13,118,904 | ) | |
| (8,694,001 | ) |
| | |
| | | |
| | |
| Investing Activities: | |
| | | |
| | |
| Purchase of property and equipment | |
| (670,529 | ) | |
| (38,912 | ) |
| | |
| | | |
| | |
| Net cash used in investing activities | |
| (670,529 | ) | |
| (38,912 | ) |
| | |
| | | |
| | |
| Financing Activities: | |
| | | |
| | |
| Payments of deferred offering costs | |
| - | | |
| (234,617 | ) |
| Proceeds from sale of common stock, net of offering costs | |
| 15,946,078 | | |
| 14,936,437 | |
| Proceeds from exercise of stock options | |
| 2,489 | | |
| - | |
| Funds received for subsidiary private offering | |
| - | | |
| 175,000 | |
| | |
| | | |
| | |
| Net cash provided by financing activities | |
| 15,948,567 | | |
| 14,876,820 | |
| | |
| | | |
| | |
| Effect of foreign currency on cash | |
| 21,506 | | |
| 1,804 | |
| | |
| | | |
| | |
| Net change in cash and cash equivalents | |
| 2,180,640 | | |
| 6,145,711 | |
| Cash and cash equivalents – beginning of period | |
| 17,509,791 | | |
| 13,436,714 | |
| Cash and cash equivalents – end of period | |
$ | 19,690,431 | | |
$ | 19,582,425 | |
| | |
| | | |
| | |
| Supplemental Disclosures of Cash Flow Information: | |
| | | |
| | |
| Income taxes paid | |
$ | 30,252 | | |
$ | 18,326 | |
| | |
| | | |
| | |
| Supplemental Disclosures of Noncash Financing Information: | |
| | | |
| | |
| Establishment of right of use asset and liabilities | |
$ | 1,071,918 | | |
$ | - | |
| Purchases of property and equipment included in accounts payable and accrued liabilities | |
| 647,052 | | |
| - | |
| Deferred offering costs charged against proceeds from sale of common stock | |
$ | 87,229 | | |
$ | 131,426 | |
| Shares issued in subsidiary absorption | |
$ | 99 | | |
$ | - | |
| Nexcella shares issued for funds previously received | |
$ | - | | |
$ | 475,000 | |
See
accompanying notes to the unaudited condensed consolidated financial statements.
Immix
Biopharma, Inc.
Notes
to the Condensed Consolidated Financial Statements
(Unaudited)
Note
1 – Nature of Business
Immix
Biopharma, Inc. (the “Company”) is a clinical-stage biopharmaceutical pharmaceutical company organized as a Delaware corporation
on January 7, 2014, which is focused on developing cell therapies in AL Amyloidosis and select immune-mediated diseases. In August 2016,
the Company established a wholly-owned Australian subsidiary, Immix Biopharma Australia Pty Ltd. (“IBAPL”), in order to conduct
various preclinical and clinical activities for its development candidates. In November 2022, the Company established a majority-owned
subsidiary, Nexcella, Inc. (“Nexcella”), its cell therapy division, which subsequently merged into the Company in May 2024,
with the Company continuing as the surviving entity. To ensure continuity of operations, the Company re-established Nexcella in 2024
as a wholly-owned subsidiary
Note
2 – Summary of Significant Accounting Policies
Basis
of Presentation - The accompanying condensed consolidated financial statements and related notes have been prepared in accordance
with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and in accordance with the rules
and regulations of the United States Securities and Exchange Commission (the “SEC”). The Company’s fiscal year end
is December 31.
The
condensed consolidated financial statements and related disclosures as of September 30, 2024, and for the three and nine months ended
September 30, 2024 and 2023 are unaudited, pursuant to the rules and regulations of the SEC. Certain information and footnote disclosures
normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted pursuant to such rules
and regulations. In the Company’s opinion, these unaudited condensed consolidated financial statements include all adjustments
(consisting only of normal recurring adjustments) necessary for the fair statement of the results for the interim periods. These unaudited
condensed consolidated financial statements should be read in conjunction with the audited financial statements of the Company for the
years ended December 31, 2023 and 2022 which are included in the Company’s Annual Report on Form 10-K filed with the SEC on March
29, 2024. The results of operations for the three and nine months ended September 30, 2024, are not necessarily indicative of the results
to be expected for the full year ending December 31, 2024.
Risk
and Uncertainties - The Company operates in a dynamic and highly competitive industry and is subject to risks and uncertainties common
to early-stage companies in the biotechnology industry, including, but not limited to, development by competitors of new technological
innovations, protection of proprietary technology, dependence on key personnel, contract manufacturers and contract research organizations,
compliance with government regulations and the need to obtain additional financing to fund operations. Product candidates currently under
development will require significant additional research and development efforts, including extensive preclinical studies and clinical
trials and regulatory approval, prior to commercialization. These efforts require significant amounts of additional capital, adequate
personnel infrastructure and extensive compliance and reporting. The Company believes that changes in any of the following areas could
have a material adverse effect on the Company’s future financial position, results of operations, or cash flows; ability to obtain
future financing; advances and trends in new technologies and industry standards; results of clinical trials; regulatory approval and
market acceptance of the Company’s products; development of sales channels; certain strategic relationships; litigation or claims
against the Company based on intellectual property, patent, product, regulatory, or other factors; and the Company’s ability to
attract and retain employees necessary to support its growth.
Products
developed by the Company require approvals from the U.S. Food and Drug Administration (“FDA”) or other international regulatory
agencies prior to commercial sales. There can be no assurance that the Company’s research and development will be successfully
completed, that adequate protection for the Company’s intellectual property will be obtained or maintained, that the products will
receive the necessary approvals, or that any approved products will be commercially viable. If the Company is denied approval, approval
is delayed, or the Company is unable to maintain approval, it could have a material adverse impact on the Company. Even if the Company’s
product development efforts are successful, it is uncertain when, if ever, the Company will generate revenue from product sales. The
Company operates in an environment of rapid change in technology and substantial competition from other pharmaceutical and biotechnology
companies. In addition, the Company is dependent upon the services of its employees, consultants and other third parties.
The
Company has expended and plans to continue to expend substantial funds to complete the research, development and clinical testing of
product candidates. The Company also will be required to expend additional funds to establish commercial-scale manufacturing arrangements
and to provide for the marketing and distribution of products that receive regulatory approval. The Company may require additional funds
to commercialize its products. The Company is unable to entirely fund these efforts with its current financial resources and will need
to raise additional funding in the future. If adequate funds are unavailable on a timely basis from operations or additional sources
of financing, the Company may have to delay, reduce the scope of or eliminate one or more of its research or development programs which
may materially and adversely affect its business, financial condition and operations.
Use
of Estimates – The preparation of these condensed consolidated financial statements in conformity with U.S. GAAP requires management
to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements
and the reported amounts of revenues and expenses during the reporting periods. The Company uses significant judgments when making estimates
related to the valuation of deferred tax assets and related valuation allowances, accrual and prepayment of research and development
expenses, and the valuation of stock-based compensation. Actual results could differ from those estimates.
Principles
of Consolidation – The accompanying condensed consolidated financial statements include the accounts of Immix Biopharma, Inc.
and the accounts of its 100% owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation. For
previously consolidated entities where the Company owned less than 100% of the subsidiary, the Company recorded net loss attributable
to non-controlling interests in its condensed consolidated statements of operations and comprehensive loss equal to the percentage of
the economic or ownership interest retained in such entities by the respective non-controlling parties.
Segment
Reporting - The Company manages its operations as a single segment for the purposes of assessing performance and making operating
decisions. The Company’s Chief Operating Decision Maker (“CODM”) is its Chief Executive Officer. The CODM allocates
resources and evaluates the performance of the Company at the consolidated level using information about its revenues, gross profit,
income from operations, and other key financial data. All significant operating decisions are based upon an analysis of the Company as
one operating segment, which is the same as its reporting segment.
Liquidity
and Going Concern – These consolidated financial statements have been prepared on a going concern basis, which assumes the
Company will continue to realize its assets and discharge its liabilities in the normal course of business. Since the initial public
offering of its common stock in December 2021, the Company has financed its operations through various equity financing. On July 14,
2023, the Company entered into an additional ATM Sales Agreement (the “July Sales Agreement”) with ThinkEquity LLC (the “Sales
Agent”), pursuant to which the Company, could, from time to time, issue and sell through the Sales Agent shares of the Company’s
common stock in sales deemed to be “at-the-market offerings” as defined in Rule 415(a)(4) promulgated under the Securities
Act of 1933, as amended (the “July ATM Facility”) (see Note 7). Initially, the Company is eligible to sell up to $4,200,000
worth of shares of its common stock as the aggregate market value of the Company’s shares of common stock eligible for sale under
the July Sales Agreement is subject to the limitations of General Instruction I.B.6 of Form S-3 until such time that the Company’s
public float equals or exceeds $75.0 million. In the event the aggregate market value of the Company’s outstanding common stock
held by non-affiliates equals or exceeds $75.0 million, then the one-third limitation on sales set forth in General Instruction I.B.6
of Form S-3 shall not apply to additional sales made pursuant to the July Sales Agreement.
From
July 14, 2023 through February 5, 2024, the Company has sold 328,136 common shares pursuant to the July ATM Facility for net proceeds
of $1,091,887, after offering expenses. On February 5, 2024, the Company suspended, and is not offering any shares of its common stock
pursuant to, the prospectus supplement dated July 14, 2023, relating to the July Sales Agreement by and between the Company and the Sales
Agent. The Company will not make any sales of common stock pursuant to the July Sales Agreement unless and until a new prospectus supplement
is filed with the SEC; however, the July Sales Agreement remains in full force and effect.
In
February 2024, the Company conducted an underwritten public offering of 5,535,055 shares of its common stock at the public offering price
of $2.71 per share, for net proceeds of $13,565,760, after underwriter discounts and offering expenses (the “Offering”).
Pursuant to the underwriting agreement, the Company granted the underwriter a 30-day over-allotment option to purchase up to an additional
783,970 shares of the Company’s common stock, which was exercised in full on March 1, 2024, for net proceeds of $1,954,594, after
underwriting discounts and offering expenses (see Note 7).
On
July 25, 2024, the Company was awarded an $8
million grant from the California Institute for Regenerative Medicine to support the clinical development of chimeric antigen
receptor T-cell therapy NXC-201 for the treatment of relapsed/refractory AL Amyloidosis. The award is payable to the Company upon
achievement of milestones that are primarily based on patient enrollment in the Company’s clinical trials. Additionally, if
CIRM determines, in its sole discretion, that the Company has not complied with the terms and conditions of the grant, CIRM may
suspend or permanently cease disbursements. Funds received under this grant may only be used for allowable project costs
specifically identified with the CIRM-funded project. Such costs can include, but are not limited to, salary for personnel, itemized
supplies, consultants, and itemized clinical study costs. Under the terms of the grant, both CIRM and the Company will co-fund the
research project and the amount of the Company’s co-funding requirement is predetermined as a part of the award. The Company
signed the grant agreement in November 2024 and expects to begin receiving funds from the grant beginning in November of
2024.
The
Company has a history of, and expects to continue to report, negative cash flows from operations and net losses. We believe that our
existing cash, cash equivalents and restricted cash as of September 30, 2024 will enable us to fund our operating expenses and capital
expenditure requirements for at least the next 12 months.
Concentration
of Credit Risk – Periodically, the Company may carry cash and cash equivalents balances at financial institutions in excess
of the federally insured limit of $250,000, or the Australian insured limit of AUD 250,000. At times, deposits held with financial institutions
may exceed the amount of insurance provided. The Company has not experienced losses on these accounts and management believes that the
credit risk with regard to these deposits is not significant.
Cash
and Cash Equivalents – The Company’s cash equivalents include short-term highly liquid investments with an original maturity
of 90 days or less when purchased and are carried at fair value.
Fair
Value of Financial Instruments – The carrying value of short-term instruments, including cash and cash equivalents, tax receivable,
accounts payable and accrued expenses, approximate fair value due to the relatively short period to maturity for these instruments.
Fair
value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal
or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The
Company utilizes a three-level valuation hierarchy for disclosures of fair value measurements, defined as follows:
Level
1 – inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level
2 – inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs
that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
Level
3 – inputs to the valuation methodology are unobservable and significant to the fair value.
The
following fair value hierarchy table presents information about the Company’s asset measured at fair value on a recurring basis:
Schedule of Asset Measured at Fair Value on a Recurring Basis
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at September 30, 2024 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets: | |
| | | |
| | | |
| | |
| Cash equivalents (money market funds) | |
$ | 18,650,711 | | |
$ | - | | |
$ | - | |
As
of September 30, 2024, the Company had no liabilities required to be measured at fair value on a recurring basis.
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at December 31, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets: | |
| | | |
| | | |
| | |
| Cash equivalents (money market funds) | |
$ | 16,113,006 | | |
$ | - | | |
$ | - | |
As
of December 31, 2023, the Company had no liabilities required to be measured at fair value on a recurring basis.
Australian
Tax Incentive – IBAPL is eligible to receive a cash refund from the Australian Taxation Office for eligible research and development
(“R&D”) expenditures under the Australian R&D Tax Incentive Program (the “Australian Tax Incentive”).
The Australian Tax Incentive is recognized as a reduction to R&D expense when there is reasonable assurance that the relevant expenditure
has been incurred, the amount can be reliably measured and that the Australian Tax Incentive will be received. The Company recognized
additional R&D expense of $66,594 and reductions to R&D expense of $378,390 for the three months ended September 30, 2024 and
2023, respectively. The Company recognized reductions to R&D expense of $1,076,193 and $599,926 for the nine months ended September
30, 2024 and 2023, respectively.
Deferred
Offering Costs – The Company had capitalized qualified legal, accounting and other direct costs related to its efforts to raise
capital through the sale of its common stock under the July ATM Facility. Deferred offering costs were deferred and being amortized ratably
upon sales under the July ATM Facility to additional paid-in capital as a reduction of the July ATM proceeds. As a result of the Company
pausing the July ATM Facility, all of the remaining deferred offering costs were immediately amortized to additional paid-in capital
as a reduction to the proceeds received in the nine months ended September 30, 2024. As of September 30, 2024, no remaining amounts of
deferred offering costs were capitalized related to the July ATM Facility.
Stock-Based
Compensation – Stock-based compensation expense represents the estimated grant date fair value of the Company’s equity
awards, consisting of stock options issued under the Company’s stock option plan and restricted common stock (see Note 7). The
fair value of equity awards is recognized over the requisite service period of such awards (usually the vesting period) on a straight-line
basis. The Company estimates the fair value of stock options using the Black-Scholes option pricing model on the date of grant and recognizes
forfeitures as they occur. For stock awards for which vesting is subject to performance-based milestones, the expense is recorded over
the remaining service period after the point when the achievement of the milestone is probable, or the performance condition has been
achieved.
Research
and Development Costs – R&D costs are expensed as incurred. R&D costs consist primarily of clinical research fees paid
to consultants and outside service providers, other expenses relating to design, development and testing of the Company’s therapy
candidates, and for license and milestone costs related to in-licensed products and technology. Costs incurred in obtaining technology
licenses are charged to R&D expense if the technology licensed has not reached commercial feasibility and has no alternative future
use. Such licenses purchased by the Company require substantial completion of research and development, regulatory and marketing approval
efforts in order to reach commercial feasibility and have no alternative future use.
Clinical
trial costs are a component of R&D expenses. The Company estimates expenses incurred for clinical trials that are in process based
on services performed under contractual agreements with clinical research organizations and actual clinical investigators. Included in
the estimates are (1) the fee per patient enrolled as specified in the clinical trial contract with each institution participating in
the clinical trial and (2) progressive data on patient enrollments obtained from participating clinical trial sites and the actual services
performed. Changes in clinical trial assumptions, such as the length of time estimated to enroll all patients, rate of screening failures,
patient drop-out rates, number and nature of adverse event reports, and the total number of patients enrolled can impact the average
and expected cost per patient and the overall cost of the clinical trial. The Company monitors the progress of the trials and their related
activities and adjusts expense accruals, when applicable. Adjustments to accruals are charged to expense in the period in which the facts
give rise to the adjustments become known.
Other
Comprehensive Income (Loss) – Other comprehensive income (loss) includes foreign currency translation gains and losses. The
cumulative amount of translation gains and losses are reflected as a separate component of stockholders’ equity in the condensed
consolidated balance sheets, as accumulated other comprehensive income.
Foreign
Currency Translation and Transaction Gains (Losses) – The Company, and its wholly-owned subsidiary Nexcella, maintain their
accounting records in U.S. Dollars. The Company’s operating subsidiary, IBAPL, is located in Australia and maintains its accounting
records in Australian Dollars, which is its functional currency. Assets and liabilities of the subsidiary are translated into U.S. dollars
at exchange rates at the balance sheet date, equity accounts are translated at historical exchange rate and revenues and expenses are
translated by using the average exchange rates for the period. Translation adjustments are reported as a separate component of other
comprehensive income (loss) in the consolidated statements of operations and comprehensive loss. Foreign currency denominated transactions
are translated at exchange rates approximating those in effect at the transaction dates. Gains (losses) resulting from foreign currency
transactions are included in general and administrative expenses in the accompanying condensed consolidated statements of operations
and comprehensive loss and were $(2,849) and $8,095 for the three months ended September 30, 2024 and 2023, respectively, and $(22,326)
and $6,372 for the nine months ended September 30, 2024 and 2023, respectively.
Loss
Per Common Share - Basic loss per common share is computed by dividing net loss attributable to common stockholders by the weighted-average
number of common shares outstanding during the period. Diluted loss per common share is determined using the weighted-average number
of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents. In periods when losses
are reported, the weighted-average number of common shares outstanding excludes common stock equivalents because their inclusion would
be anti-dilutive. Basic weighted average shares outstanding for the three and nine months ended September 30, 2024 include 1,913,661
shares underlying Pre-Funded warrants to purchase common shares (See Note 7). As the shares underlying these Pre-Funded warrants can
be issued for nominal consideration (an exercise price per share equal to $0.0001 per share), these shares are deemed to be issued for
purposes of basic loss per common share. For the three and nine months ended September 30, 2024 and 2023, the Company’s potentially
dilutive shares, which were not included in the calculation of net loss per share, included stock options and warrants exercisable for
4,408,488 and 2,911,412 shares of common stock, respectively.
Property
and Equipment - Included in property and equipment is construction-in-progress which consists of manufacturing space improvements
and includes the costs of construction, machinery and equipment, and any interest charges arising from borrowings used to finance these
assets during the period of construction or installation of the assets. No provision for depreciation is made on construction-in-progress
until such time as the relevant assets are completed and ready for their intended use.
Estimated
useful lives of the Company’s assets are as follows:
Schedule
of Property and Equipment Useful Lives
| | |
Useful Life |
| Operating equipment | |
3-10 years |
| Electronic equipment | |
3-5 years |
| Office equipment | |
3-5 years |
The
cost and related accumulated depreciation of assets sold or otherwise retired are eliminated from the accounts, and any gain or loss
are included in the Company’s results of operations. The costs of maintenance and repairs are recognized to expenses as incurred;
significant renewals and betterments are capitalized.
Leases
- At the inception of a contract the Company determines if the arrangement is, or contains a lease. Operating lease right-of-use
(“ROU”) assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent
its obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement
date based on the present value of the lease payments over the lease term. Lease expense is recognized on a straight-line basis over
the lease term.
The
Company has made certain accounting policy elections whereby it (i) does not recognize ROU assets or lease liabilities for short-term
leases (those with original terms of 12-months or less) and (ii) separates lease and non-lease elements of its operating leases as separate
lease components. As of September 30, 2024 and December 31, 2023, the Company did not have any finance leases.
Recent
Accounting Pronouncements
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”)
2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires disclosure
of incremental segment information on an annual and interim basis. This ASU is effective for fiscal years beginning after December 15,
2023, and interim periods within fiscal years beginning after December 15, 2024, on a retrospective basis. The Company has implemented
this ASU effective January 1, 2024, and determined no retrospective changes were necessary.
In
December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures,
which expands the disclosures required for income taxes. This ASU is effective for fiscal years beginning after December 15, 2024,
with early adoption permitted. The amendment should be applied on a prospective basis while retrospective application is permitted. The
Company is currently evaluating the effect of this pronouncement on its disclosures.
Note
3 – Prior Agreements with Nexcella Subsidiary
Nexcella
Absorption
On
May 20, 2024, Nexcella, was merged (the “Merger”) with and into the Company, with the Company as the surviving corporation
(the “Nexcella Absorption”). The Merger was effected pursuant to Section 253 of the Delaware General Corporation Law (“DGCL”)
when the Company filed a Certificate of Ownership and Merger (“Certificate of Merger”) with the Secretary of State of the
State of Delaware. Immediately prior to the Merger, the Company owned greater than 95% of outstanding common stock on a fully diluted
basis of Nexcella, par value $0.0001 per share (the “Nexcella Shares”), and 100% of the outstanding shares of each other
class of capital stock of Nexcella. Under the DGCL, the only approval required was that of the Company’s Board of Directors for
the Merger to become effective. As a result of the Merger, Nexcella ceased to exist and all assets, operations and other property and
rights of Nexcella have been succeeded to by the Company. Pursuant to the terms of the Certificate of Merger, as a result of the Merger,
each of the outstanding Nexcella Shares (other than Nexcella Shares held by the Company) were converted into common stock of the Company
(“Company Merger Shares”). In connection with the Merger, the Company issued 989,876 shares of its common stock to the former
stockholders of Nexcella (other than shares held by the Company) (including Company common stock issued to third-party cash investors
in Nexcella) (the “Merger Shares”). The shares were issued on a pro-rata basis and as such resulted in no change in fair
value. In addition, the Company issued to the former participants in the Nexcella 2022 Equity Incentive Plan, 275,759 restricted stock
awards to receive common stock in the Company and options to purchase up to 595,676 shares of Company common stock at an exercise price
of $2.47 per share (the closing price on May 17, 2024), under the Company’s Amended and Restated 2021 Omnibus Equity Incentive
Plan. As such, as of May 20, 2024, the Founders Agreement and Management Services Agreement agreements listed below with Nexcella are
no longer in effect.
Founders
Agreement
Effective
December 8, 2022, the Company entered into a Founders Agreement with Nexcella (the “Nexcella Founders Agreement”).
The
Nexcella Founders Agreement provided that prior to a Qualified IPO (as defined in Nexcella’s Amended and Restated Certificate of
Incorporation, as amended (the “Nexcella COI”)) or Qualified Change in Control (as defined in the Nexcella COI), the Company
shall provide funds to Nexcella as requested by Nexcella, in good faith, to be evidenced by a senior unsecured promissory note. In exchange
for the time and capital expended in the formation of Nexcella and the identification of specific assets, the acquisition of which benefit
Nexcella, on December 21, 2022, the Company loaned Nexcella approximately $2.1 million, evidenced by a senior unsecured promissory note,
representing the up-front fee required to acquire Nexcella’s license agreement with Hadasit Medical Research Services & Development,
Ltd. (“HADASIT”) and BIRAD Research and Development Company Ltd. (“BIRAD”), and for use as working capital for
its research and development activities. The note, which had a maturity date of January 31, 2030, accrued interest at a rate of 7.875%
per annum and was convertible into shares of common stock of Nexcella at a conversion price of $2.00 per share, subject to adjustment;
provided, however, that such note shall automatically convert into shares of Nexcella common stock immediately prior to certain conversion
triggers set forth in the note. Nexcella may not prepay the note without the Company’s prior written consent. The note and accrued
interest were converted in full prior to the Nexcella Absorption. The Nexcella Founders Agreement had a term of 15 years, which, upon
expiration, would automatically renew for successive one-year periods unless terminated by the Company upon notice at least six months
prior to the end of the term or upon the occurrence of a Change of Control (as defined in the Nexcella Founders Agreement). In connection
with the Nexcella Founders Agreement, the Company was issued 250,000 shares of Nexcella’s Class A Preferred Stock, 1,000,000 shares
of Nexcella’s Class A Common Stock, and 5,000,000 shares of Nexcella’s common stock. The Class A Preferred Stock was identical
to the common stock other than as to conversion rights, the PIK Dividend right (as defined below) and voting rights.
Each
share of Class A Preferred Stock was convertible, at the Company’s option, into one fully paid and nonassessable share of Nexcella’s
common stock, subject to certain adjustments. As a holder of Nexcella’s Class A Preferred Stock, the Company received on each March
13 (each a “PIK Dividend Payment Date”) until the date all outstanding Class A Preferred Stock was converted into Nexcella’s
common stock or redeemed (and the purchase price is paid in full), pro rata per share dividends paid in additional fully paid and nonassessable
shares of Nexcella common stock (“PIK Dividends”) such that the aggregate number of shares of common stock issued pursuant
to such PIK Dividend was equal to 2.5% of Nexcella’s fully-diluted outstanding capitalization on the date that was one business
day prior to any PIK Dividend Payment Date. In addition, as a holder of Class A Preferred Stock, the Company was entitled to cast for
each share of Class A Preferred Stock held as of the record date for determining stockholders entitled to vote on matters presented to
the stockholders of Nexcella, the number of votes that was equal to 1.1 times a fraction, the numerator of which was the sum of (A) the
shares of outstanding Nexcella common stock and (B) the whole shares of Nexcella common stock into which the shares of outstanding Nexcella
Class A Common Stock and the Class A Preferred Stock were convertible and the denominator of which was the number of shares of outstanding
Nexcella Class A Preferred Stock.
Each
share of Class A Common Stock was convertible, at the Company’s option, into one fully paid and nonassessable share of Nexcella’s
common stock, subject to certain adjustments. In addition, upon a Qualified IPO (as defined in the Nexcella COI) or Qualified Change
in Control (as defined in the Nexcella COI), each share of Class A Common Stock would automatically convert into one fully paid and nonassessable
share of Nexcella’s common stock; provided however, if at that time, the Class A Common Stock was not then convertible into a number
of shares of Nexcella common stock (or such other capital stock or securities at the time issuable upon the conversion of the Class A
Common Stock) that have a value of: (a) in the case of a Qualified IPO, at least $5,000,000 based on the initial offering price in such
initial public offering, or (b) in the case of a Qualified Change in Control, at least $5,000,000 in cash or at least $5,000,000 of equity
based on the implied value of a share of Nexcella common stock resulting from the price paid upon the consummation of such Qualified
Change of Control, the Class A Common Stock would automatically convert into such number of shares of Nexcella common stock (or such
other capital stock or securities at the time issuable upon the conversion of the Class A Common Stock) that have a value of $5,000,000
based on the initial offering price in such initial public offering or the implied value of a share of Nexcella common stock resulting
from the price paid upon the consummation of such Qualified Change of Control (or if such Qualified Change of Control results in the
Class A Shares being exchanged solely for cash, then $5,000,000 in cash). The Company was entitled to cast such number of votes equal
to the number of whole shares of Nexcella common stock into which the Company’s Class A Common Stock was convertible as of the
record date for determining stockholders entitled to vote on matters presented to the stockholders of Nexcella.
In
addition to the foregoing, the Company was entitled to one vote for each share of Nexcella common stock held by it. Except as provided
by law or by the Nexcella COI, holders of Nexcella Class A Common Stock and Class A Preferred Stock shall vote together with the holders
of Nexcella common stock, as a single class.
As
additional consideration under the Nexcella Founders Agreement, Nexcella also agreed to: (i) pay an equity fee in shares of common stock,
payable within five business days of the closing of any equity or debt financing for Nexcella or any of its respective subsidiaries that
occurs after the effective date of the Nexcella Founders Agreement and ending on the date when the Company no longer has majority voting
control in Nexcella’s voting equity, equal to 2.5% of the gross amount of any such equity or debt financing; and (ii) pay a cash
fee equal to 4.5% of Nexcella’s annual Net Sales (as defined in the Nexcella Founders Agreement), payable on an annual basis, within
90 days of the end of each calendar year. In the event of a Change of Control, Nexcella agreed to pay a one-time change in control fee
equal to five times the product of (A) Net Sales for the 12 months immediately preceding the Change of Control and (B) 4.5%.
Management
Services Agreement
Effective
as of December 8, 2022, the Company entered into a Management Services Agreement (the “Nexcella MSA”) with Nexcella. Pursuant
to the terms of the Nexcella MSA, the Company rendered management, advisory and consulting services to Nexcella. Services provided under
the Nexcella MSA may include, without limitation, (i) advice and assistance concerning any and all aspects of Nexcella’s operations,
clinical trials, financial planning and strategic transactions and financings and (ii) conducting relations on behalf of Nexcella with
accountants, attorneys, financial advisors and other professionals (collectively, the “Services”). At the request of the
Company, Nexcella utilized clinical research services, medical education, communication and marketing services and investor relations/public
relation services of companies or individuals designated by the Company, provided those services are offered at market prices. In consideration
for the Services, Nexcella paid the Company an annual base management and consulting fee of $500,000 (the “Annual Consulting Fee”).
Notwithstanding the foregoing, the first Annual Consulting Fee payment was not due until the first business day of the calendar quarter
immediately following the completion of the first equity financing for Nexcella that was in excess of $10 million in gross proceeds,
which did not occur. Actual and direct out-of-pocket expenses reasonably incurred by the Company in performing the Services were reimbursed
to the Company by Nexcella.
The
Nexcella MSA was terminated on May 20, 2024 in connection with the Nexcella Absorption. In addition, as a result of the Nexcella Absorption,
the Class A Preferred Stock, Class A Common Stock, and the Founders Agreement cease to exist.
Note
4 – Prepaid Expenses and Other Current Assets
Prepaid
expenses and other current assets consist of the following as of September 30, 2024 and December 31, 2023:
Schedule of Prepaid Expenses and Other Current Assets
| | |
September 30, 2024 | | |
December 31, 2023 | |
| Prepaid research and development expenses | |
$ | 486,912 | | |
$ | 412,773 | |
| Prepaid insurance expense | |
| 73,813 | | |
| 263,927 | |
| Prepaid investor relations expense | |
| 492,391 | | |
| 384,494 | |
| Other current assets | |
| 36,521 | | |
| 44,582 | |
| Total prepaid expenses and other current assets | |
$ | 1,089,637 | | |
$ | 1,105,776 | |
Note
5 – Accounts Payable and Accrued Expenses
Accounts
payable and accrued expenses consist of the following as of September 30, 2024 and December 31, 2023:
Schedule of Accounts Payable and Accrued Expenses
| | |
September 30, 2024 | | |
December 31, 2023 | |
| Accounts payable | |
$ | 3,288,579 | | |
$ | 1,433,022 | |
| Accrued research and development expenses | |
| 2,878,386 | | |
| 1,571,261 | |
| Accrued professional services | |
| 93,385 | | |
| 38,639 | |
| Accrued compensation and related expenses | |
| 198,095 | | |
| 577,854 | |
| Other accrued expenses | |
| 43,341 | | |
| 101,007 | |
| Total accounts payable and accrued expenses | |
$ | 6,501,786 | | |
$ | 3,721,783 | |
Note
6 – Property and Equipment
Property
and equipment at September 30, 2024 and December 31, 2023 consisted of:
Schedule
of Property and Equipment
| | |
September 30, 2024 | | |
December 31, 2023 | |
| Operating equipment | |
$ | 534,162 | | |
$ | 60,599 | |
| Office equipment | |
| 3,895 | | |
| 3,896 | |
| Total property and equipment, gross | |
| 538,057 | | |
| 64,495 | |
| Less: Accumulated depreciation | |
| (26,652 | ) | |
| (14,314 | ) |
| Property and equipment
excluding construction in progress | |
| 511,405 | | |
| 50,181 | |
| Construction in progress | |
| 844,019 | | |
| - | |
| Total property and equipment | |
$ | 1,355,424 | | |
$ | 50,181 | |
For
the nine months ended September 30, 2024 and 2023, depreciation expense amounted to $12,338 and $2,392, respectively. Depreciation is
not taken during the period of construction or equipment installation. Upon completion of the installation of manufacturing equipment
or any construction in progress, balances will be classified to their respective property and equipment category.
The
construction in progress of $844,019 as of September 30, 2024, represents the investment in building a biopharmaceutical processing facility
inside the leased property. The Company expects to complete the processing facility by the end of 2025.
Note
7 – Stockholders’ Equity
The
Company has authorized 200,000,000 shares of common stock and 10,000,000 shares of preferred stock, each with a par value of $0.0001
per share.
July
2023 ATM Sales Agreement
On
July 14, 2023, the Company entered into the July Sales Agreement with the Sales Agent pursuant to which the Company may offer and sell,
from time to time, through the Sales Agent, shares (the “July Shares”) of the Company’s common stock, par value $0.0001
per share, subject to the terms and conditions set forth in the July Sales Agreement. Initially, the Company is eligible to sell up to
$4,200,000 worth of shares of its common stock as the aggregate market value of the Company’s shares of common stock eligible for
sale under the July Sales Agreement is subject to the limitations of General Instruction I.B.6 of Form S-3 until such time that the Company’s
public float equals or exceeds $75.0 million. In the event the aggregate market value of the Company’s outstanding common stock
held by non-affiliates equals or exceeds $75.0 million, then the one-third limitation on sales set forth in General Instruction I.B.6
of Form S-3 shall not apply to additional sales made pursuant to the July Sales Agreement. The July Shares will be offered and sold pursuant
to the Company’s prospectus supplement, dated July 14, 2023, filed by the Company with the SEC on July 14, 2023, including the
accompanying base prospectus forming a part of the Company’s Registration Statement on Form S-3 (File No. 333-269100) filed by
the Company with the SEC on January 3, 2023 and declared effective by the SEC on January 11, 2023.
Under
the July Sales Agreement, the Sales Agent may sell the July Shares in sales deemed to be “at-the-market offerings” as defined
in Rule 415(a)(4) promulgated under the Securities Act, including sales made directly on or through The Nasdaq Capital Market or any
other existing trading market for the Company’s common stock, in negotiated transactions at market prices prevailing at the time
of sale or at prices related to such prevailing market prices, and/or any other method permitted by law. The Company may instruct the
Sales Agent not to sell any July Shares if the sales cannot be effected at or above the price designated by the Company from time to
time.
The
Company will pay the Sales Agent a fixed commission rate of 3.75% of the aggregate gross proceeds from the sale of the July Shares pursuant
to the July Sales Agreement. The Company has paid an expense deposit of $15,000 to the Sales Agent, which will be applied against the
actual out-of-pocket accountable expenses that will be paid by the Company to the Sales Agent in connection with the offering. The Company
has agreed to reimburse the Sales Agent for all expenses related to the offering including, without limitation, the fees and expenses
of the Sales Agent’s legal counsel up to $50,000, and shall reimburse the Sales Agent, upon request, for such costs, fees and expenses
in an amount not to exceed $7,500 on a quarterly basis for the first three fiscal quarters of each year and $10,000 for the fiscal fourth
quarter of each year. The Company has also agreed to provide indemnification and contribution to the Sales Agent with respect to certain
liabilities, including liabilities under the Securities Act.
During
the nine months ended September 30, 2024, the Company sold a total of 68,302 shares of its common stock under the July ATM Facility for
aggregate net proceeds of $338,495 after deducting commissions and SEC fees, and charging $87,229 of deferred offering costs against
the proceeds. On February 5, 2024, the Company suspended, and is not offering any shares of its common stock pursuant to, the prospectus
supplement dated July 14, 2023, relating to the July Sales Agreement by and between the Company and ThinkEquity LLC. The Company will
not make any sales of common stock pursuant to the July Sales Agreement unless and until a new prospectus supplement is filed with the
SEC; however, the July Sales Agreement remains in full force and effect.
Common
Stock Issuance – Public Offering
On
February 5, 2024, the Company entered into an Underwriting Agreement (the “Agreement”) with Titan Partners Group LLC, a division
of American Capital Partners, LLC (the “Underwriter”), relating to an underwritten offering (the “Offering”)
of 5,535,055 shares of common stock of the Company. The public offering price was $2.71 per share of Common Stock and the Underwriter
agreed to purchase the Common Stock pursuant to the Underwriting Agreement at a price of $2.5203 per share. On February 8, 2024, the
Company closed the offering and received net proceeds of $13,565,760, after deducting underwriting discounts and commissions and estimated
offering expenses. Pursuant to the Agreement, the Company granted the Underwriter a 30-day over-allotment option to purchase up to an
additional 783,970 shares of Common Stock which was exercised in full on March 1, 2024, for net proceeds of $1,954,594, after deducting
underwriting discounts and offering expenses.
Other
Common Stock Issuances
During
the nine months ended September 30, 2024, the Company issued 75,007 shares of restricted common stock valued at $202,500 for investor
relations services based on the average closing price for the prior 10 trading days pursuant to a marketing services agreement entered
into on July 25, 2023.
During
the nine months ended September 30, 2024, the Company issued 104,758 shares of restricted common stock valued at $317,500 for investor
relations services based on the closing price pursuant to the extensions of marketing services agreements.
During
the nine months ended September 30, 2024, the Company issued 1,251 shares of common stock upon the exercise of certain common stock options
for cash proceeds of $2,489.
During
the year ended December 31, 2023, the Company entered into various marketing services agreements, whereby the Company agreed to issue
122,300 shares of its common stock, valued at $247,500, in exchange for future services. As of December 31, 2023, the Company has issued
122,300 shares of the Company’s common stock pursuant to the marketing services agreements. During the year ended December 31,
2023, the Company recorded stock-based compensation expense of $232,624 related to the fair value of the shares of common stock. During
the nine months ended September 30, 2024, the Company recorded stock-based compensation expense of $14,876 related to the amortization
of the fair value of the 122,300 shares of common stock issued in 2023. As of September 30, 2024, the Company has $0 of unamortized stock-based
compensation remaining to be amortized over the remaining service period.
Restricted
Stock Awards
Pursuant
to the Merger, the Company issued to the former participants in the Nexcella 2022 Equity Incentive Plan, 275,759 restricted stock awards
to receive common stock in the Company. The shares were issued on a pro-rata basis and resulted in no change in fair value.
During
the nine months ended September 30, 2024, the Company recorded stock-based compensation expense of $263,962 related to the total fair
value of the previously issued restricted stock awards, which was included in general and administrative expenses. The unrecognized stock-based
compensation expense of $417,163 related to unvested restricted common stock is expected to be recognized over the remaining vesting
period of 0.62 years. As of September 30, 2024, 93,895 shares of restricted common stock have vested with the remaining 181,864 restricted
shares to vest over the vesting period of 0.62 years.
Stock
Options
In
2016, the Board of Directors of the Company approved the Immix Biopharma, Inc. 2016 Equity Incentive Plan (the “2016 Plan”).
The 2016 Plan allows for the Board of Directors to grant various forms of incentive awards covering up to 417,120 shares of common stock.
During the year ended December 31, 2021, the Board of Directors amended the 2016 Plan to increase the aggregate number of shares available
for issuance under the 2016 Plan to 1,761,120 shares of common stock. On September 10, 2021, the Board of Directors approved the 2021
Equity Incentive Plan (as amended and restated, the “2021 Plan”) pursuant to which it initially reserved and made available
for future issuance under the 2021 Plan (i) 900,000 shares of common stock, plus (ii) the number of shares of common stock reserved,
but unissued under the 2016 Plan, and (iii) the number of shares of common stock underlying forfeited awards under the 2016 Plan, provided
that shares of common stock issued under the 2021 Plan with respect to an Exempt Award (as defined in the 2021 Plan) would not count
against such share limit. Subsequent to September 10, 2021, no further awards are to be issued under the 2016 Plan, but all awards under
the 2016 Plan which were outstanding as of September 10, 2021 (including any Grandfathered Arrangement (as defined in the 2021 Plan))
shall continue to be governed by the terms, conditions and procedures set forth in the 2016 Plan and any applicable award agreement.
On
April 24, 2023, the Company’s Board of Directors adopted the Immix Biopharma, Inc. Amended and Restated 2021 Omnibus Equity Incentive
Plan (the “Amended 2021 Plan”) which, among other things, increased the number of shares of common stock that may be issued
under such plan by 1,034,561 shares, subject to stockholder approval. On June 7, 2023, stockholders of the Company approved the Amended
2021 Plan. On April 18, 2024, our Board of Directors approved amendments to the 2021 Plan (the “2nd Amended 2021 Plan”)
to (i) increase the number of shares of common stock available for issuance under the 2021 Plan by 3,000,000 to a total share reserve
of 4,934,561 and (ii) the adoption of an evergreen provision to the 2021 Plan to provide for an automatic annual increase in the shares
of common stock available for issuance under the 2021 Plan over the next ten years (the “2021 Plan Amendments”). Pursuant
to the evergreen provision, the number of shares available for issuance under the 2021 Plan shall automatically increase on January 1st
of each year for a period of ten years, commencing on January 1, 2025 and ending on (and including) January 1, 2034, in an amount equal
to five percent (5%) of the total number of shares of Common Stock outstanding on December 31st of the preceding calendar year. On June
11, 2024, stockholders of the Company approved the 2nd Amended 2021 Plan. As of September 30, 2024, there were 2,265,757 shares
of the Company’s common stock remaining to be issued under the Amended 2021 Plan.
In
addition, the Company issued to the former participants in the Nexcella 2022 Equity Incentive Plan, options to purchase up to 595,676
shares of Company common stock at an exercise price of $2.47 per share (the closing price on May 17, 2024), under the Company’s
Amended and Restated 2021 Omnibus Equity Incentive Plan. The options were issued on a pro-rata basis and resulted in no change in fair
value.
During
the nine months ended September 30, 2024, the Compensation Committee of the Board of Directors approved the issuance of options to purchase
198,000 shares of the Company’s common stock to non-employee members of the Board of Directors of the Company and 680,000 shares
of the Company’s common stock to management of the Company. The options have a term of 10 years, an exercise price of $2.04 per
share and vest over periods of 12 to 48 equal monthly installments.
During
the nine months ended September 30, 2024, the Board of Directors approved the issuance of options to purchase 43,500 shares of the Company’s
common stock to employees of the Company with a term of 10 years and exercise prices ranging from $2.11 - $2.17 per share, which options
vest in 48 equal monthly installments.
The
Company recognized stock-based compensation of $450,299 and $171,454 related to stock options for the three months ended September 30,
2024 and 2023 and $965,600 and $507,017 related to stock options for the nine months ended September 30, 2024 and 2023, respectively,
which is included in general and administrative expenses.
As
of September 30, 2024, the Company had unrecognized stock-based compensation expense of $3,153,545, related to unvested stock options,
which is expected to be recognized over the weighted-average vesting period of 2.61 years.
The
following table summarizes the stock option activity for the nine months ended September 30, 2024:
Schedule of Stock Option Activity
| | |
Options | | |
Weighted- Average Exercise Price Per Share | |
| Outstanding, January 1, 2024 | |
| 2,512,561 | | |
$ | 1.92 | |
| Granted | |
| 1,517,176 | | |
$ | 2.21 | |
| Exercised | |
| (834 | ) | |
$ | 1.95 | |
| Forfeited | |
| (17,915 | ) | |
$ | 1.95 | |
| Expired | |
| - | | |
$ | - | |
| Outstanding and expected to vest, September 30, 2024 | |
| 4,010,988 | | |
$ | 2.03 | |
The
following table discloses information regarding outstanding and exercisable options at September 30, 2024:
Schedule of Stock Outstanding and Exercisable
| | | |
Outstanding | | |
Exercisable | |
| Exercise Price Range | | |
Number of Option Shares | | |
Weighted Average Exercise Price | | |
Weighted Average Remaining Life (Years) | | |
Number of Option Shares | | |
Weighted Average Exercise Price | |
| $ | 0.00-1.00 | | |
| 256,500 | | |
$ | 0.80 | | |
| 6.45 | | |
| 256,500 | | |
$ | 0.80 | |
| $ | 1.01-2.00 | | |
| 1,646,062 | | |
$ | 1.81 | | |
| 7.13 | | |
| 1,060,836 | | |
$ | 1.78 | |
| $ | 2.01-3.00 | | |
| 2,097,176 | | |
$ | 2.33 | | |
| 9.13 | | |
| 751,854 | | |
$ | 2.50 | |
| $ | 3.01-6.00 | | |
| 11,250 | | |
$ | 5.83 | | |
| 7.29 | | |
| 7,500 | | |
$ | 5.83 | |
| | | | |
| 4,010,988 | | |
$ | 2.03 | | |
| 8.13 | | |
| 2,076,690 | | |
$ | 1.93 | |
Aggregate
intrinsic value is calculated as the difference between the exercise price of the underlying stock option and the fair value of the Company’s
common stock for stock options that were in-the-money at period end. As of September 30, 2024, the aggregate intrinsic value for the
options vested and outstanding was $200,675.
The
total intrinsic value of stock options exercised during the nine months ended September 30, 2024, was $3,069.
Stock
Warrants
The
following table summarizes the stock warrant activity for the nine months ended September 30, 2024:
Schedule of Stock Warrant Activity
| | |
Warrants | | |
Weighted-Average
Exercise Price Per
Share | |
| Outstanding and exercisable, January 1, 2024 | |
| 2,311,161 | | |
$ | 0.71 | |
| Granted | |
| - | | |
$ | - | |
| Exercised | |
| - | | |
$ | - | |
| Forfeited | |
| - | | |
$ | - | |
| Expired | |
| - | | |
$ | - | |
| Outstanding and exercisable, September 30, 2024 | |
| 2,311,161 | | |
$ | 0.71 | |
The
following table discloses information regarding outstanding and exercisable warrants at September 30, 2024:
Schedule of Stock Outstanding and Exercisable
| | | |
Outstanding | | |
Exercisable | |
| Exercise Price | | |
Number of Warrant Shares | | |
Weighted Average Exercise Price | | |
Weighted Average Remaining Life (Years) | | |
Number of Warrant Shares | | |
Weighted Average Exercise Price | |
| $ | 0.0001 | | |
| 1,913,661 | | |
$ | 0.0001 | | |
| - | | |
| 1,913,661 | | |
$ | 0.0001 | |
| $ | 0.80 | | |
| 156,000 | | |
$ | 0.80 | | |
| 6.48 | | |
| 156,000 | | |
$ | 0.80 | |
| $ | 6.25 | | |
| 241,500 | | |
$ | 6.25 | | |
| 2.21 | | |
| 241,500 | | |
$ | 6.25 | |
| | | |
| 2,311,161 | | |
$ | 0.71 | | |
| 0.67 | | |
| 2,311,161 | | |
$ | 0.71 | |
Aggregate
intrinsic value is calculated as the difference between the exercise price of the underlying stock warrant and the fair value of the
Company’s common stock for stock warrants that were in-the-money at period end. As of September 30, 2024, the intrinsic value for
the warrants vested and outstanding was $2,958,804.
Nexcella
Equity Transactions
The
Nexcella 2022 Equity Incentive Plan (the “2022 Plan”) allows for Nexcella’s Board of Directors to grant various forms
of incentive awards initially covering up to 375,000 shares of common stock. On May 29, 2023, Nexcella’s Board of Directors approved
the Second Amended and Restated Nexcella 2022 Equity Incentive Plan, which increased to the number of shares of Nexcella common stock
issuable under the plan from 375,000 shares to 607,640 shares. On August 11, 2023, Nexcella’s Board of Directors requested the
Third Amended and Restated 2022 Equity Incentive Plan, which increased the number of shares of Nexcella common stock issuable under the
plan from 607,640 to 800,000 shares. The Nexcella shareholders subsequently approved the increase in Nexcella common stock issuable under
the plan to 800,000 shares. On May 17, 2024, upon absorption into the Company, the 2022 Plan ceased to exist.
Common
stock
On
March 13, 2024, pursuant to the terms of the Founders Agreement, Nexcella issued 238,220 shares of common stock to the Company as a PIK
Dividend based on the total dilutive shares of Nexcella outstanding as of March 12, 2024.
Restricted
Stock Awards
During
the three and nine months ended September 30, 2024, the Company recorded stock-based compensation expense of $0 and $402,163, respectively,
related to the total fair value of the previously issued restricted stock awards, which was included in general and administrative expenses.
Pursuant to the Merger, the Company issued to the former participants in the Nexcella 2022 Equity Incentive Plan, 275,759 restricted
stock awards to receive common stock in the Company. The shares were issued on a pro-rata basis and resulted in no change in fair value.
As a result, there was no remaining unvested stock-based compensation expense under Nexcella.
Stock
Options
The
Company recognized stock-based compensation of $0 and $148,319 related to stock options for the three and nine months ended September
30, 2024, respectively, which is included in general and administrative expenses. Pursuant to the Merger, the Company issued to the former
participants in the Nexcella 2022 Equity Incentive Plan, options to purchase up to 595,676 shares of Company common stock under the Company’s
Amended and Restated 2021 Omnibus Equity Incentive Plan. The options were issued on a pro-rata basis and resulted in no change in fair
value. As a result, there was no remaining unvested stock-based compensation expense under Nexcella.
The
following table summarizes the stock option activity for the nine months ended September 30, 2024 for Nexcella:
Schedule of Stock Option Activity
| | |
Options | | |
Weighted- Average Exercise Price Per Share | |
| Outstanding and exercisable, January 1, 2024 | |
| 186,528 | | |
$ | 6.49 | |
| Granted | |
| - | | |
$ | - | |
| Exercised | |
| - | | |
$ | - | |
| Forfeited | |
| (186,528 | ) | |
$ | 6.49 | |
| Expired | |
| - | | |
$ | - | |
| Outstanding and expected to vest, September 30, 2024 | |
| - | | |
$ | - | |
Note
8 – Licenses Acquired
Research
and License Agreement with HADASIT and BIRAD
On
December 8, 2022, Nexcella entered into a Research and License agreement with HADASIT and BIRAD (collectively, the “Licensors”)
to acquire intellectual property rights pertaining to CAR-T (the “H&B License”). Pursuant to the H&B License, Nexcella
paid the Licensors an upfront license fee of $1.5 million in December 2022 (included in research and development expenses on the consolidated
statements of operations and comprehensive loss). Additional quarterly payments totaling approximately $13 million related to the Company’s
ongoing support of the CAR-T clinical trials currently ongoing at HADASIT, are due through September 2026, along with an annual license
fee of $50,000. Future royalty payments of 5% are due on net sales of licensed products, combined with sales milestone payments in the
aggregate amount of up to $20 million when annual net sales reach certain thresholds for each licensed product. The royalties for each
licensed product on a country-to-country basis are to be paid through the latter of (a) the expiration of the last-to-expire valid claim
under a licensed patent (if any) in such country; (b) the date of expiration of any other Exclusivity Right (as defined in the H&B
License) or data protection period granted by a regulatory or other governmental authority with respect to a licensed product that provides
exclusivity in the relevant country; or (c) the end of a period of 15 years from the date of the First Commercial Sale (as defined in
the H&B License) of the applicable Licensed Product (as defined in the H&B License) in such country. The H&B License remains
with the Company after the Nexcella Absorption.
During
the nine months ended September 30, 2024 and 2023, the Company recorded R&D expenses of $2,393,063 and $1,929,601, respectively,
related to the license agreement.
Patent
License Agreement with U.S. Medical Research Foundation
In
August 2024, the Company entered into a Patent License Agreement (“License Agreement”) with a U.S. medical research foundation
pursuant to which the Company was granted certain exclusive and nonexclusive licenses and sublicenses to intellectual and tangible property
for the development and commercialization of cell therapy products (“Licensed Products”). Pursuant to the terms of the License
Agreement, the Company shall pay an up-front payment in three installments of $500,000, with the first installment due concurrent with
the signing of the agreement and the second and third installments due in January and July 2025, respectively. Under the license agreement,
the Company must also pay a mid-single-digit net licensed product sales royalty, and milestone payments corresponding with the initiation
and completion of Phase II studies in the amounts of $1.5 million and $2 million, respectively, as well as a $10 million milestone payment
at the initiation of Phase III studies and a $13.5 million dollar milestone payment in the event of first commercial sale of a licensed
product. To date, no amounts have been paid under this license agreement.
Note
9 - CIRM Grants
On
July 25, 2024, the Company was awarded an $8
million grant from the California Institute for Regenerative Medicine to support the clinical development of chimeric antigen
receptor T-cell therapy NXC-201 for the treatment of relapsed/refractory AL Amyloidosis. The award is payable to the Company upon
achievement of milestones that are primarily based on patient enrollment in the Company’s clinical trials. Additionally, if
CIRM determines, in its sole discretion, that the Company has not complied with the terms and conditions of the grant, CIRM may
suspend or permanently cease disbursements. Funds received under this grant may only be used for allowable project costs
specifically identified with the CIRM-funded project. Such costs can include, but are not limited to, salary for personnel, itemized
supplies, consultants, and itemized clinical study costs. Under the terms of the grant, both CIRM and the Company will co-fund the
research project and the amount of the Company’s co-funding requirement is predetermined as a part of the award. The Company
signed the grant agreement in November 2024 and expects to begin receiving funds from the grant beginning in November of
2024.
Note
10 – Leases
In
January 2024, the Company entered into a long-term operating lease agreement for 14,000 square feet of biopharmaceutical manufacturing
space in California under a non-cancelable operating lease that expires in December 2033. Under the terms of the lease, the Company is
required to pay monthly base rents ranging from $11,900 to $16,218, and pay its proportionate share of property taxes, insurance and
normal maintenance costs. The lease agreement includes two options to extend the lease for a term of five years each.
The
components of lease cost for operating leases, which are recorded in general and administrative expenses in the accompanying condensed
consolidated statement of operations, for the three and nine months ended September 30, 2024 were as follows:
Schedule of Lease Cost for Operating Leases
| | |
Three Months Ended September 30, 2024 | | |
Nine Months Ended September 30, 2024 | |
| Operating lease cost | |
$ | 42,150 | | |
$ | 126,450 | |
| Short-term lease cost | |
| 12,196 | | |
| 44,020 | |
| Total lease cost | |
$ | 54,346 | | |
$ | 170,470 | |
The
following table summarizes the lease-related assets and liabilities recorded in the consolidated balance sheets at September 30, 2024:
Schedule of Lease Related Assets and Liabilities
| | |
September 30, 2024 | |
| Operating Leases | |
| - | |
| Operating lease right-of-use assets | |
$ | 1,010,205 | |
| Right of use liability operating lease current portion | |
$ | 62,715 | |
| Right of use liability operating lease long term | |
| 1,026,340 | |
| Total operating lease liabilities | |
$ | 1,089,055 | |
The
Company utilizes the incremental borrowing rate in determining the present value of lease payments unless the implicit rate is readily
determinable. The Company estimated its incremental borrowing rate to be 8%. The lease has a remaining term of 9.25 years and an implicit
weighted average interest rate of 8%.
The
following table provides the maturities of lease liabilities at September 30, 2024:
Schedule of Maturity Lease Liability
| | |
Operating | |
| | |
Leases | |
| 2024 (remaining 3 months) | |
$ | 35,700 | |
| 2025 | |
| 147,798 | |
| 2026 | |
| 152,971 | |
| 2027 | |
| 158,325 | |
| 2028 and thereafter | |
| 1,073,349 | |
| Total future undiscounted lease payments | |
| 1,568,143 | |
| Less: Interest | |
| (479,088 | ) |
| Present value of lease liabilities | |
$ | 1,089,055 | |
Note
11 – Commitments and Contingencies
Indemnifications
In
the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties
and may provide for indemnification of the counterparty. The Company’s exposure under these agreements is unknown because it involves
claims that may be made against it in the future, but have not yet been made. To date, the Company has not been subject to any claims
or been required to defend any action related to its indemnification obligations.
The
Company indemnifies each of its directors and officers for certain events or occurrences, subject to certain limits, while the director
is or was serving at the Company’s request in such capacity, as permitted under Delaware law and in accordance with its certificate
of incorporation and bylaws. The term of the indemnification period lasts as long as the director or officer may be subject to any proceeding
arising out of acts or omissions of such individual in such capacity. The maximum amount of potential future indemnification is unlimited.
The Company believes that the fair value of these indemnification obligations is minimal. Accordingly, the Company has not recognized
any liabilities relating to these obligations as of September 30, 2024.
Legal
Proceedings
From
time to time the Company may be involved in claims that arise during the ordinary course of business. Although the results of litigation
and claims cannot be predicted with certainty, the Company does not currently have any pending litigation to which it is a party or to
which its property is subject that it believes to be material. Regardless of the outcome, litigation can be costly and time consuming,
and it can divert management’s attention from important business matters and initiatives, negatively impacting the Company’s
overall operations.
Employment
Agreements
On
June 18, 2021, the Company entered into an Employment Agreement with Ilya Rachman (as amended, the “Rachman Employment Agreement”),
effective for a three-year term, subject to the terms of the agreement which provide that unless the Company and Dr. Rachman have otherwise
agreed in writing, if Dr. Rachman continues to work for the Company after the expiration of the term (which he has), his employment shall
be under the same terms and conditions provided for in the Rachman Employment Agreement, except that his employment will be on an “at
will” basis and the provisions of the agreement allowing for Dr. Rachman to terminate the agreement for “good reason”
and for Dr. Rachman to be paid severance in the event his employment is terminated by the Company without cause or by Dr. Rachman for
good reason will no longer apply, and the Rachman Employment Agreement currently remains in effect pursuant to such terms. Pursuant to
the Rachman Employment Agreement, the Company employs Dr. Rachman as Chief Executive Officer and Dr. Rachman was entitled to a base salary
of $360,000 annually. Dr. Rachman was also entitled to a performance-based bonus of 100% of the base salary (subject to, and determined
by, the Board in its sole discretion) plus additional performance bonuses to be determined by the Board. On November 9, 2022 and May
12, 2023, the Company entered into amendments to the Rachman Employment Agreement dated as of June 18, 2021 pursuant to which (i) Dr.
Rachman’s annual base salary was increased to $425,000 and $446,000, retroactive as of January 1, 2022 and 2023, respectively and
on November 9, 2023, and (ii) the agreement was amended to entitle Dr. Rachman to a performance-based bonus of up to 50% of his base
salary (subject to, and determined by, the Board in its sole discretion) plus additional performance bonuses to be determined by the
Board. On February 6, 2024, the Compensation Committee of the Board of Directors approved an increase in the annual base salary and on
May 9, 2024, the Company entered into an amendment to the Rachman Employment Agreement pursuant to which Dr. Rachman’s annual base
salary was increased to $475,000, effective January 1, 2024. Dr. Rachman’s employment agreement contains provisions for the protection
of the Company’s intellectual property and contains non-compete restrictions in the event of his termination other than by the
Company without “cause” or by Dr. Rachman with “good reason” (generally imposing restrictions on (i) employment
or consultation with competing companies or customers, (ii) recruiting or hiring employees for a competing company and (iii) soliciting
or accepting business from our customers for a period of six months following termination). Pursuant to the Rachman Employment Agreement,
Dr. Rachman may serve as a consultant to, or on board of directors of, or in any other capacity to, other companies provided that they
will not interfere with the performance of his duties to the Company. The full amount of the base salary and any bonus payments are included
in general and administrative expenses.
On
March 18, 2021, the Company entered into a Management Services Agreement with Alwaysraise LLC, an entity which Gabriel Morris, the Company’s
Chief Financial Officer and a member of the Board, is sole member, which was amended effective June 18, 2021 (as amended, the “Morris
MSA”). The Morris MSA had an initial two-year term, automatically renewable thereafter for successive one year terms unless terminated
by either party, and currently has a term through March 18, 2025. Pursuant to the Morris MSA, the Company employs Mr. Morris as Chief
Financial Officer and Mr. Morris was entitled to a base salary of $240,000 annually beginning in December 2021 ($120,000 annually prior).
Mr. Morris was also entitled to a performance-based bonus of 100% of the base salary (subject to, and determined by, the Board in its
sole discretion) plus additional performance bonuses to be determined by the Board. On November 9, 2022 and May 12, 2023, the Company
entered into amendments to the Morris MSA dated as of March 24, 2021, pursuant to which (i) Mr. Morris’ annual base salary was
increased to $425,000 and $446,000, retroactive as of January 1, 2022 and 2023, respectively, and on November 9, 2023, and (ii) Mr. Morris
is entitled to a performance-based bonus of up to 50% of his base salary (subject to, and determined by, the Board in its sole discretion)
plus additional performance bonuses to be determined by the Board. Unless terminated by the Company without “cause” or by
Alwaysraise LLC (as such terms are defined in the Morris MSA), upon termination, Mr. Morris will be entitled only to his base salary
through the date of termination, valid expense reimbursements and unused vacation pay. If terminated by the Company without “cause,”
he is entitled to be paid his base salary through the end of the term at the rate of 150%, valid expense reimbursements and accrued but
unused vacation pay. On February 6, 2024, the Compensation Committee of the Board of Directors approved an increase in annual base salary,
and on May 9, 2024, the Company entered into an amendment to the Morris MSA pursuant to which Mr. Morris’ annual base salary was
increased to $475,000, effective January 1, 2024. The Morris MSA contains provisions for the protection of the Company’s intellectual
property and confidential information. The full amount of the base salary and any bonus payments are included in general and administrative
expenses.
On
June 24, 2021, the Company issued an offer letter to Graham Ross Oncology Consulting Services Ltd., a United Kingdom company, of which
Graham Ross, the Company’s Acting Chief Medical Officer and Head of Clinical Development is the sole member, regarding Dr. Ross’s
provision of consultative services to the Company (the “Offer Letter”). Pursuant to the Offer Letter (signed by Dr. Ross
on June 24, 2021), Dr. Ross is entitled to an hourly rate for his consulting services and an option grant. On June 24, 2021, the Company
also signed a mutual confidentiality and non-disclosure agreement with Graham Ross Oncology Consulting Services Ltd.
Collaboration
Agreement
In
August 2021, the Company entered into a Clinical Collaboration and Supply Agreement with BeiGene Ltd. (“BeiGene”) for a combination
Phase 1b clinical trial in solid tumors of IMX-110 and anti-PD-1 Tislelizumab (the subject of a collaboration and license agreement among
BeiGene and Novartis). Under the terms of the agreement, the Company will conduct the combination trial. The cost of Tislelizumab manufacture
and supply (including shipping, taxes and duty if applicable and any third-party license payments that may be due) will be solely borne
by BeiGene. To date, no amounts have been paid to BeiGene.
Note
12 – Subsequent Events
Common
Stock Issuance – Marketing Services Agreements
Subsequent
to September 30, 2024, the Company issued 27,062 shares of restricted common stock valued at $45,000 for investor relations services
based on the average closing price for the prior 10 trading days pursuant to a marketing services agreement entered into on July 25,
2023.
ITEM
2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
You
should read the following discussion and analysis of our financial condition and results of operations together with our unaudited interim
condensed consolidated financial statements and the related notes appearing elsewhere in this Quarterly Report on Form 10-Q. In addition
to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions.
Our actual results may differ materially from those discussed below. Factors that could cause or contribute to such differences include,
but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included in our
Annual Report on Form 10-K for the fiscal year ended December 31, 2023, as may be amended, supplemented or superseded from time to time
by other reports we file with the SEC. All amounts in this report are in U.S. dollars, unless otherwise noted.
Throughout
this Quarterly Report on Form 10-Q, references to “we,” “our,” “us,” the “Company,” “Immix,”
or “Immix Biopharma” refer to Immix Biopharma, Inc., individually, or as the context requires, collectively with its subsidiaries.
Our
logo and some of our trademarks and tradenames are used in this Report. This Report also includes trademarks, tradenames and service
marks that are the property of others. Solely for convenience, trademarks, tradenames and service marks referred to in this Report may
appear without the ®, ™ and SM symbols. References to our trademarks, tradenames and service marks are not intended to indicate
in any way that we will not assert to the fullest extent under applicable law our rights or the rights of the applicable licensors if
any, nor that respective owners to other intellectual property rights will not assert, to the fullest extent under applicable law, their
rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with,
or endorsement or sponsorship of us by, any other companies.
Certain
capitalized terms used below and otherwise defined below, have the meanings given to such terms in the footnotes to our unaudited consolidated
financial statements included above under “Part I – Financial Information” – “Item 1. Financial Statements”.
Unless
the context otherwise requires and for the purposes of this Report only:
●
“Exchange Act” refers to the Securities Exchange Act of 1934, as amended;
●
“SEC” or the “Commission” refers to the United States Securities and Exchange Commission; and
●
“Securities Act” refers to the Securities Act of 1933, as amended.
Available
Information
We
file annual, quarterly, and current reports, proxy statements and other information with the Securities and Exchange Commission. Our
SEC filings (reports, proxy information statements, and other information) are available to the public over the Internet at the SEC’s
website at www.sec.gov and are available for download, free of charge, soon after such reports are filed with or furnished to the SEC,
on the “Investor & News,” “SEC Filings” page of our website at www.immixbio.com. Copies of documents filed
by us with the SEC are also available from us without charge, upon oral or written request to our Secretary, who can be contacted at
the address and telephone number set forth on the cover page of this Report. The information contained on the websites referenced in
this Report is not incorporated by reference into this filing. Further, the Company’s references to website URLs are intended to
be inactive textual references only.
Overview
Immix
Biopharma, Inc. is a clinical-stage biopharmaceutical company focused on the application of chimeric antigen receptor cell therapy
(“CAR-T”) in light chain (AL) Amyloidosis and select immune-mediated diseases. Our lead cell therapy candidate is U.S.
Food and Drug Administration (“FDA”) investigational new drug (“IND”) cleared CAR-T NXC-201, currently being
evaluated in our ongoing United States Phase 1b/2 NEXICART-2 (NCT06097832) clinical trial and our ex-U.S. phase 1b/2a NEXICART-1
(NCT04720313) clinical trial.
NXC-201 has been awarded Orphan
Drug Designation (“ODD”) by both the FDA and European Commission (“EMA”) in AL Amyloidosis.
Our
mission is to harness the immune system through innovative cell therapies and other modalities to deliver widely accessible cures in
select immune-mediated diseases and other indications, as we believe patients are waiting.
Our
strategy is to:
| |
● |
Develop
our lead candidate CAR-T NXC-201 in AL Amyloidosis and select immune-mediated diseases; and |
| |
|
|
| |
● |
Pursue
development of NXC-201 and additional cell therapy candidates in other applicable indications where CAR-T is not an approved therapy
today. |
Our
N-GENIUS platform has produced our clinical-stage lead candidate NXC-201, a next-generation CAR-T for AL Amyloidosis and select immune-mediated
diseases.
Figure
1: ImmixBio Pipeline
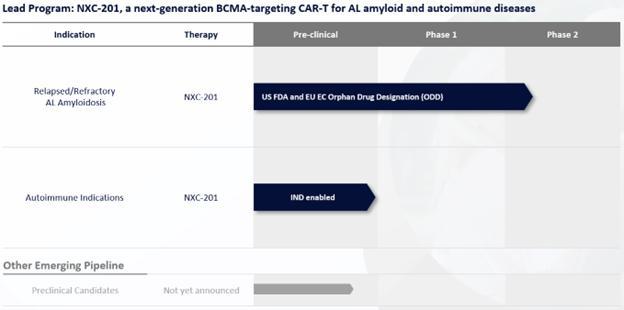
NXC-201
is in clinical trials to treat relapsed/refractory AL Amyloidosis.
AL
amyloidosis is a life-threatening immunological disorder in which an abnormal protein called amyloid builds up in tissues and organs.
This abnormal protein is produced by long-lived plasma cells (“LLPCs”), a type of immune B-cell. The signs and symptoms of
AL amyloidosis vary among patients because build-up may occur in the heart (most frequent cause of mortality), liver, kidneys, intestines,
muscles, joints, nerves, or spleen, according to the National Institutes of Health (“NIH”). Diagnosis is frequently delayed,
due to varied and non-specific symptoms including: fatigue, weight loss, shortness of breath, dizziness, and numbness in hands and feet.
Upon diagnosis, many patients already have late-stage disease, and are not aware of available treatment options and clinical trials.
As of September
2024, there are no FDA approved drugs for relapsed/refractory AL Amyloidosis.
The
U.S. observed prevalence of relapsed/refractory AL Amyloidosis is growing 12% per year according to Staron, et al Blood Cancer Journal
2021, estimated to reach 33,277 patients in 2024. Untreated patients with AL amyloidosis and cardiac involvement have a median survival
of less than 1 year, according to Quock, et al. Journal of Comparative Effective Research, 2023. The current market size for amyloidosis
therapies is estimated at $3.6 billion, expected to reach $6 billion in 2027, according to Grand View Research.
As
of September 2024, we have treated 3 relapsed/refractory AL Amyloidosis patients in the United States in our ongoing Phase 1b/2 multi-site NEXICART-2 (NCT06097832) U.S. clinical trial. Memorial
Sloan Kettering Cancer Center is the lead NEXICART-2 clinical site.
As
of September 30, 2024, we have treated 13 relapsed/refractory AL Amyloidosis patients in our ongoing Phase 1b/2a NEXICART-1
(NCT04720313) ex-U.S. clinical trial.
In
September 2023, the FDA granted ODD to NXC-201 for the treatment of AL Amyloidosis. If a product that has ODD subsequently receives the
first FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusive approval (or exclusivity),
which means that the FDA may not approve any other applications to market the same drug for the same indication for 7 years (except in
limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity).
In
November 2023, the FDA cleared an IND application for NXC-201 to enroll U.S. patients into NXC-201 clinical trials.
In
December 2023, NXC-201 clinical data in relapsed/refractory AL Amyloidosis was presented in an oral presentation at the 65th
annual American Society of Hematology (“ASH”) meeting, covering 10 relapsed/refractory AL Amyloidosis patients treated with
NXC-201, indicating an overall response rate of 100% (10/10) and a complete response rate of 70% (7/10).
In
February 2024, the European Commission (“EC”) granted orphan drug designation to NXC-201 for the treatment of AL Amyloidosis.
Benefits of European ODD include: 10 years of market exclusivity once authorized in the EU; Access to the EU centralized authorization
procedure; and reduced fees for EU protocol assistance, marketing authorization applications, inspections before authorization, applications
for changes to marketing authorizations made after approval, and reduced annual fees.
Our
Other Programs
Our
other programs include NXC-201 for select immune-mediated diseases, a $25 billion combined annual market size according to Grand View
Research and Fortune Business Insights and other preclinical candidates.
While
our focus is NXC-201 in AL Amyloidosis and select immune-mediated diseases, we continue to collect and organize IMX-110 data in monotherapy
for soft tissue sarcoma, a $3 billion market size according to Medgadget, and in combination with anti-PD-1 for colorectal cancer, a
$27 billion market size according to IndustryARC, to evaluate next steps.
Since
inception, we have devoted substantially all of our resources to developing product and technology rights, conducting research and development,
organizing and staffing our Company, business planning and raising capital. We operate as one business segment and have incurred recurring
losses, the majority of which are attributable to research and development activities and negative cash flows from operations. We have
funded our operations primarily through the sale of equity securities. Currently, our primary use of cash is to fund operating expenses,
which consist primarily of research and development expenditures, and to a lesser extent, general and administrative expenditures. We
expect to continue to incur significant expenses and operating losses for the foreseeable future as we advance our product candidates
through all stages of development and clinical trials and, ultimately, seek regulatory approval. In addition, if we obtain regulatory
approval for any of our product candidates, we expect to incur significant commercialization expenses related to product manufacturing,
marketing, sales and distribution. Furthermore, we incur costs associated with operating as a public company, including significant legal,
accounting, investor relations and other expenses. Our net losses may fluctuate significantly from quarter-to-quarter and year-to-year,
depending on the timing of our clinical trials and our expenses on other research and development activities.
Absorption
of Nexcella Subsidiary
On
May 20, 2024, Nexcella, was merged (the “Merger”) with and into the Company, with the Company as the surviving corporation.
The Merger was effected pursuant to Section 253 of the Delaware General Corporation Law (“DGCL”) when the Company filed a
Certificate of Ownership and Merger (“Certificate of Merger”) with the Secretary of State of the State of Delaware. Immediately
prior to the Merger, the Company owned greater than 95% of the outstanding common stock on a fully diluted basis of Nexcella, par value
$0.0001 per share (the “Nexcella Shares”), and 100% of the outstanding shares of each other class of capital stock of Nexcella.
Under the DGCL, the only approval required was that of the Company’s Board of Directors for the Merger to become effective. As
a result of the Merger, Nexcella ceased to exist and all assets, operations and other property and rights of Nexcella have been succeeded
to by the Company. Pursuant to the terms of the Certificate of Merger, as a result of the Merger, each of the outstanding Nexcella Shares
(other than Nexcella Shares held by the Company) were converted, into common stock of the Company (“Company Merger Shares”).
In connection with the Merger, the Company issued 989,876 shares of its common stock to the former stockholders of Nexcella (other than
shares held by the Company) (including Company common stock issued to third-party cash investors in Nexcella) (the “Merger Shares”).
In addition, the Company issued to the former participants in the Nexcella 2022 Equity Incentive Plan, 275,759 restricted stock awards
to receive common stock in the Company and options to purchase up to 595,676 shares of Company common stock at an exercise price of $2.47
per share (the closing price on May 17, 2024), under the Company’s Amended and Restated 2021 Omnibus Equity Incentive Plan.
Research
and License Agreement with Hadasit and BIRAD
On
December 8, 2022, our subsidiary Nexcella entered into a Research and License Agreement (the “Agreement”) with Hadasit Medical
Research Services & Development, Ltd. and BIRAD – Research and Development Company Ltd. (collectively, the “Licensors”)
pursuant to which the Licensors granted to Nexcella an exclusive, worldwide, royalty-bearing license throughout the world, except Israel,
Cyprus and other countries in the Middle East (the “Territory”) to an invention entitled “Anti-BCMA CAR-T cells to
target plasma cell” to develop, manufacture, have manufactured, use, market, offer for sale, sell, have sold, export and import
Licensed Product (as defined in the Agreement). Pursuant to the Agreement, Nexcella paid the Licensors an upfront fee of $1,500,000 in
December 2022. Additional quarterly payments totaling approximately $13.0 million are due through September 2026 along with an annual
license fee of $50,000. Nexcella has agreed to pay royalties to the Licensors equal to 5% of Net Sales (as defined in the Agreement)
during the Royalty Period. “Royalty Period” means for each Licensed Product, on a country-to-country basis, the period commencing
on December 8, 2022, and ending on the later of (a) the expiration of the last to expire Valid Claim (as defined in the Agreement) under
a Licensed Patent (as defined in the Agreement), if any, in such country, (b) the date of expiration of any other Exclusivity Right (as
defined in the Agreement) or data protection period granted by a regulatory or other governmental authority with respect to a Licensed
Product, or (c) 15 years from the date of First Commercial Sale (as defined in the Agreement) of a Licensed Product in such country.
In
addition, Nexcella is required to pay sales milestone payments of up to $20 million for Net Sales exceeding $700 million and Nexcella
has committed to funding NXC-201 clinical trials in Israel over four years for an estimated total cost of approximately $13 million,
spread out on a quarterly basis over that period, which Nexcella believes will generate clinical trial data owned by Nexcella. The term
of the Agreement commenced on December 8, 2022 and, unless earlier terminated pursuant to the terms thereof, will continue in full force
and effect until the later of the expiration of the last Valid Claim under a Licensed Patent or a Joint Patent (as defined in the Agreement)
or Exclusivity Right covering a Licensed Product or the expiration of a continuous period of 15 years during which there shall not have
been a First Commercial Sale of any Licensed Product in any country in the world. Licensors may terminate the Agreement immediately if
Nexcella or its affiliates or sublicensees commences an action in which it challenges the validity, enforceability or scope of any of
the Licensed Patents or Joint Patents. In addition, either party may terminate the Agreement if the other party materially breaches the
Agreement and fails to cure such breach within 30 days. Additionally, Licensors may terminate the Agreement if Nexcella becomes insolvent
or files for bankruptcy.
The
license remains with the Company after the Nexcella Absorption.
July
2023 ATM Offering
On
July 14, 2023, we entered into an ATM Sales Agreement (the “July Sales Agreement”) with the Sales Agent pursuant to which
we may offer and sell, from time to time, through the Sales Agent, shares of our common stock, subject to the terms and conditions set
forth in the July Sales Agreement. Initially, we are eligible to sell up to $4,200,000 worth of shares of our common stock as the aggregate
market value of our shares of common stock eligible for sale under the July Sales Agreement is subject to the limitations of General
Instruction I.B.6 of Form S-3 until such time that our public float equals or exceeds $75.0 million. In the event the aggregate market
value of our outstanding common stock held by non-affiliates equals or exceeds $75.0 million, then the one-third limitation on sales
set forth in General Instruction I.B.6 of Form S-3 will not apply to additional sales made pursuant to the July Sales Agreement. We agreed
to pay the Sales Agent a commission rate of 3.75% of the aggregate gross proceeds from the sale of the shares of our common stock pursuant
to the July Sales Agreement and have paid an expense deposit of $15,000 to the Sales Agent, which will be applied against the actual
out-of-pocket accountable expenses. In addition, we have agreed to reimburse the Sales Agent for all expenses related to the offering
including, without limitation, the fees and expenses of the Sales Agent’s legal counsel up to $50,000, and to reimburse the Sales
Agent, upon request, for such costs, fees and expenses in an amount not to exceed $7,500 on a quarterly basis for the first three fiscal
quarters of each year and $10,000 for the fiscal fourth quarter of each year. The offering pursuant to the July Sales Agreement will
terminate upon the earlier of (i) the sale of all of the shares of common stock subject to the July Sales Agreement and (ii) termination
of the July Sales Agreement as permitted therein. We may terminate the July Sales Agreement in our sole discretion at any time by giving
ten days’ prior notice to the Sales Agent. The Sales Agent may terminate the July Sales Agreement under the circumstances specified
in the July Sales Agreement and in its sole discretion at any time by giving ten days’ prior notice to us. In addition, the July
Sales Agreement may be terminated upon mutual agreement by us and the Sales Agent.
From
July 14, 2023 through February 5, 2024, the Company has sold 328,136 common shares pursuant to the July ATM Facility for net proceeds
of $1,091,887, after offering expenses. On February 5, 2024, the Company suspended, and is not offering any shares of its common stock
pursuant to, the prospectus supplement dated July 14, 2023, relating to the July Sales Agreement by and between the Company and ThinkEquity
LLC. The Company will not make any sales of common stock pursuant to the July Sales Agreement unless and until a new prospectus supplement
is filed with the SEC; however, the Sales Agreement remains in full force and effect.
Public
Offering
On
February 5, 2024, the Company entered into an Underwriting Agreement (the “Agreement”) with Titan Partners Group LLC, a division
of American Capital Partners, LLC (the “Underwriter”), relating to an underwritten offering (the “Offering”)
of 5,535,055 shares of common stock of the Company. The public offering price was $2.71 per share of Common Stock and the Underwriter
agreed to purchase the Common Stock pursuant to the Underwriting Agreement at a price of $2.5203 per share. On February 8, 2024, the
Company closed the offering and received net proceeds of $13,565,760, after deducting underwriting discounts and commissions and estimated
offering expenses. Pursuant to the Agreement, the Company granted the Underwriter a 30-day over-allotment option to purchase up to an
additional 783,970 shares of Common Stock which was exercised in full on March 1, 2024 for net proceeds of $1,954,594, after deducting
underwriting discounts and offering expenses.
Results
of Operations
Three
Months Ended September 30, 2024 compared to the Three Months Ended September 30, 2023
General
and Administrative Expense
General
and administrative expenses were $2,949,403 for the three months ended September 30, 2024, compared to $2,417,776 for the three months
ended September 30, 2023.
The
expenses incurred in both periods were related to salaries, patent maintenance costs and general accounting and other general consulting
expenses, which were higher for the three months ended September 30, 2024, due to increased investor relations services of $543,383,
increased compensation expense of $355,670, due to hiring of additional employees; and increased other general expenses of $11,227, offset
by decreased professional services of $309,561 primarily driven by a significant decrease in legal fees related to the Nexcella subsidiary
and decreased stock-based compensation of $69,092 from a reduction in equity awards issued.
Research
and Development Expense
Research
and development expense was $4,445,528 for the three months ended September 30, 2024, compared to $2,106,020 for the three months ended
September 30, 2023.
The
increased research and development expenses during the three months ended September 30, 2024, as compared to the three months ended September
30, 2023, were related to our ongoing Phase 1b/2a clinical trial and our CAR-T clinical trial, including, but not limited to, CRO and
related costs for maintaining and treating patients in the clinical trial, as well as site onboarding costs and license fees.
Interest
Income
Interest
income was $256,680 for the three months ended September 30, 2024, compared to $186,691 for the three months ended September 30, 2023.
Interest income in the current period was related to interest received on investments in a money market fund and increased from the prior
period as a result of the Company maintaining higher balances in money market funds during the current period.
Provision
for Income Taxes
Provision
for income taxes for the three months ended September 30, 2024 was $11,144, compared to $6,807 for the three months ended September 30,
2023, due to withholding taxes relating to our Australian subsidiary.
Net
Loss
Net
loss for the three months ended September 30, 2024 was $7,149,395, compared to $4,343,912 for the three months ended September 30, 2023,
which increase was due primarily to the increase in general and administrative expenses and research and development expenses, as discussed
in greater detail above.
Nine
Months Ended September 30, 2024 compared to the Nine Months Ended September 30, 2023
General
and Administrative Expense
General
and administrative expenses were $7,769,224 for the nine months ended September 30, 2024, compared to $5,130,977 for the nine months
ended September 30, 2023.
The
expenses incurred in both periods were related to salaries, patent maintenance costs and general accounting and other general consulting
expenses, which were higher for the nine months ended September 30, 2024, due to increased investor relations services of $1,199,230,
increased professional services of $65,764, both due to service scope expansion and price increases, increased compensation of $548,112
due to hiring additional employees, increased stock-based compensation of $462,645 from additional equity awards issued, and increased
other general expenses of $362,496.
Research
and Development Expense
Research
and development expense was $9,918,336 for the nine months ended September 30, 2024, compared to $5,634,284 for the nine months ended
September 30, 2023.
The
increased research and development expenses during the nine months ended September 30, 2024, as compared to the nine months ended September
30, 2023, were related to our ongoing Phase 1b/2a clinical trial and our CAR-T clinical trial, including, but not limited to, CRO and
related costs for maintaining and treating patients in the clinical trial, as well as site onboarding costs and license fees.
Interest
Income
Interest
income was $831,503 for the nine months ended September 30, 2024, compared to $343,431 for the nine months ended September 30, 2023.
Interest income in the current period was related to interest received on investments in a money market fund, which increased as a result
of the Company maintaining higher balances in money market funds during the current period.
Provision
for Income Taxes
Provision
for income taxes for the nine months ended September 30, 2024 was $30,252 compared to $18,326 for the nine months ended September 30,
2023, due to withholding taxes relating to our Australian subsidiary.
Net
Loss
Net
loss for the nine months ended September 30, 2024 was $16,886,309 compared to $10,440,156 for the nine months ended September 30, 2023,
which increase was due primarily to the increase in general and administrative expenses and research and development expenses, each as
discussed in greater detail above.
Liquidity
and Capital Resources
Our
primary use of cash is to fund operating expenses, which consist of research and development expenditures and various general and administrative
expenses. Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in
our outstanding accounts payable, accrued expenses and prepaid expenses.
Because
of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are
unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors,
including, but not limited to:
| |
● |
the
scope, timing, progress and results of discovery, pre-clinical development, laboratory testing and clinical trials for our product
candidates; |
| |
|
|
| |
● |
the
costs of manufacturing our product candidates for clinical trials and in preparation for regulatory approval and commercialization; |
| |
|
|
| |
● |
the
extent to which we enter into collaborations or other arrangements with additional third parties in order to further develop our
product candidates; |
| |
|
|
| |
● |
the
costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending
intellectual property-related claims; |
| |
|
|
| |
● |
the
costs and fees associated with the discovery, acquisition or in-license of additional product candidates or technologies; |
| |
|
|
| |
● |
expenses
needed to attract and retain skilled personnel; |
| |
|
|
| |
● |
the
costs associated with being a public company; |
| |
|
|
| |
● |
the
costs required to scale up our clinical, regulatory and manufacturing capabilities; |
| |
|
|
| |
● |
the
costs of future commercialization activities, if any, including establishing sales, marketing, manufacturing and distribution capabilities,
for any of our product candidates for which we receive regulatory approval; and |
| |
|
|
| |
● |
revenue,
if any, received from commercial sales of our product candidates, should any of our product candidates receive regulatory approval. |
As
of September 30, 2024, we had $16.2 million of working capital.
On
July 25, 2024, the Company was awarded an $8 million grant from the California Institute for Regenerative Medicine (CIRM) to support
the clinical development of chimeric antigen receptor T-cell therapy NXC-201 for the treatment of relapsed/refractory AL
Amyloidosis. The award is payable to the Company upon achievement of milestones that are primarily based on patient enrollment in
the Company’s clinical trials. Additionally, if CIRM determines, in its sole discretion, that the Company has not complied
with the terms and conditions of the grant, CIRM may suspend or permanently cease disbursements. Funds received under this grant may
only be used for allowable project costs specifically identified with the CIRM-funded project. Such costs can include, but are not
limited to, salary for personnel, itemized supplies, consultants, and itemized clinical study costs. Under the terms of the grant,
both CIRM and the Company will co-fund the research project and the amount of the Company’s co-funding requirement is
predetermined as a part of the award. The Company signed the grant agreement in November 2024 and expects to begin receiving funds
from the grant beginning in November of 2024.
We
believe that our existing cash and cash equivalents as of September 30, 2024 will enable us to fund our operating expenses and
capital expenditure requirements for at least the next 12 months. However, we may need additional funds depending on our operational
needs and capital requirements for clinical trials, other research and development expenditures, and general and administrative
expenses. We currently have no credit facility or committed sources of capital.
Until
such time, if ever, as we can generate substantial product revenue, we expect to finance our operations through a combination of equity
offerings, debt financings, government or other third-party funding, commercialization, marketing and distribution arrangements, other
collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity
or convertible debt securities, your ownership interest will be diluted, which dilution may be significant, and the terms of these securities
may include liquidation or other preferences that adversely affect your rights as a common stockholder. If we raise additional funds
through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish
valuable rights to our technologies, future revenue streams, research programs or product candidates, or grant licenses on terms that
may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed,
we may be required to delay, limit, reduce or terminate our research, product development or future commercialization efforts, or grant
rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Cash
used in operating activities
Net
cash used in operating activities was $13,118,904 for the nine months ended September 30, 2024 and $8,694,001 for the nine months ended
September 30, 2023. Net cash used in operating activities for the nine months ended September 30, 2024 was primarily related to our net
loss of $16,886,309, offset by non-cash items of stock-based compensation expense of $2,314,920, depreciation expense of $12,338 and
right of use asset amortization of $61,713. Operating activities also included an increase in accounts payable and accrued expenses of
$2,120,531, an increase in the tax receivable of $746,748, and an increase in prepaid expenses of $7,932. Net cash used in operating
activities for the nine months ended September 30, 2023, was primarily related to our net loss of $10,440,156, offset by non-cash items
of stock-based compensation expense of $1,514,900 and depreciation expense of $2,392. Operating activities also included an increase
in accounts payable of $1,580,248, an increase in the tax receivable of $427,476 and an increase in prepaid expenses of $923,909.
Cash
used in investing activities
Net
cash used in investing activities was $670,529 for the nine months ended September 30, 2024, consisting solely of purchase of
property and operating equipment, compared to $38,912 for the nine months ended September 30, 2023.
Cash
provided by financing activities
Net
cash provided by financing activities was $15,948,567 for the nine months ended September 30, 2024 and $14,876,820 for the nine months
ended September 30, 2023. Net cash provided by financing activities in 2024 was related to proceeds of $15,946,078 from the sale of common
shares through a public offering. Net cash provided by financing activities in 2023 was related to proceeds of $5,002,284 from the sale
of common shares through the at-the-market offerings, proceeds of $9,934,153 from the sale of common shares and pre-funded warrants in
a private placement offering, and proceeds of $175,000 from the sale of common shares of our former majority-owned (now wholly-owned)
subsidiary, Nexcella, offset by payments of deferred offering costs of $234,617.
Our
continuation as a going concern is dependent upon our ability to obtain necessary financing to continue operations and the attainment
of profitable operations. As of September 30, 2024, we have incurred an accumulated deficit of $70,212,617 and have not yet generated
any revenue from operations. Management anticipates that our cash on hand and funds that may be raised pursuant to the July Sales Agreement
and $8 million grant from CIRM will be sufficient to fund planned operations for at least 12 months from the filing date of this Quarterly
Report on Form 10-Q.
We
will have additional capital requirements going forward and may need to seek additional financing, which may or may not be available
to us on acceptable terms, if at all.
JOBS
Act
On
April 5, 2012, the Jumpstart Our Business Startups Act (the “JOBS Act”) was enacted. Section 107 of the JOBS Act provides
that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of
the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can
delay the adoption of certain accounting standards until those standards would otherwise apply to private companies.
We
have chosen to take advantage of the extended transition periods available to emerging growth companies under the JOBS Act for complying
with new or revised accounting standards until those standards would otherwise apply to private companies provided under the JOBS Act.
As a result, our financial statements may not be comparable to those of companies that comply with public company effective dates for
complying with new or revised accounting standards.
Subject
to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we intend to rely on certain of these exemptions,
including, without limitation, (i) providing an auditor’s attestation report on our internal controls over financial reporting
pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002, as amended, and (ii) complying with the requirement adopted by the Public
Company Accounting Oversight Board regarding the communication of critical audit matters in the auditor’s report on financial statements.
We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total
annual gross revenues of $1.235 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of
the completion of our initial public offering (December 31, 2026); (iii) the date on which we have issued more than $1 billion in nonconvertible
debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the
SEC.
Critical
Accounting Policies and Use of Estimates
Our
financial statements are prepared in accordance with U.S. GAAP. The preparation of these financial statements requires management to
make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. Management regularly evaluates
its estimates and judgments, including those related to revenue recognition, intangible assets, long-lived assets valuation, variable
interest entities, and legal matters. Actual results may differ from these estimates which may be material. “Note 2 – Summary
of Significant Accounting Policies” in Part I, Item 1 of this Quarterly Report on Form 10-Q and in the Notes to Consolidated Financial
Statements in Part II, Item 8 of our Annual Report on Form 10-K for the year ended December 31, 2023 (the “2022 Form 10-K”),
and “Critical Accounting Policies” in Part II, Item 7 of the 2023 Form 10-K describe the significant accounting policies
and methods used in the preparation of the Company’s financial statements. There have been no material changes to the Company’s
critical accounting policies and estimates since the 2023 Form 10-K.
ITEM
3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
We
are not required to provide the information required by this Item as we are a “smaller reporting company,” as defined in
Rule 12b-2 of the Exchange Act.
ITEM
4. CONTROLS AND PROCEDURES.
Evaluation
of Disclosure Controls and Procedures
Our
management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of
our “disclosure controls and procedures” (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of September 30, 2024,
the end of the period covered by this Quarterly Report on Form 10-Q. The term “disclosure controls and procedures” as defined
in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure
that information required to be disclosed by a company in the reports that it files under the Exchange Act is recorded, processed, summarized
and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without
limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it
files under the Exchange Act is accumulated and communicated to a company’s management, including its principal executive officer
and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. In designing and evaluating
the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated,
cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute
assurance that all control issues and instances of fraud, if any, within a company have been detected. Based on the evaluation of our
disclosure controls and procedures as of September 30, 2024, our management, with the participation of our principal executive officer
and principal financial officer has concluded that, based on such evaluation, as of the end of the period covered by this Quarterly Report
on Form 10-Q, our disclosure controls and procedures were not effective due to the material weakness described below.
Material
Weakness in Internal Controls Over Financial Reporting
We
identified a material weakness in our internal control over financial reporting that existed as of December 31, 2023. A material weakness
is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility
that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. We determined
that we had a material weakness because, due to our small size, and our limited number of personnel, we did not have in place an effective
internal control environment with formal processes and procedures, including adequate segregation of duties within account processes
and systems, and journal entry processing and review, to allow for a detailed review of accounting transactions that would identify errors
in a timely manner. Based on our assessment, our management concluded that, as of September 30, 2024, the material weakness still exists.
Notwithstanding
the material weaknesses in our internal control over financial reporting, we have concluded that the condensed consolidated financial
statements included in this Quarterly Report on Form 10-Q fairly present, in all material respects, our financial position, results of
operations and cash flows for the periods presented in conformity with accounting principles generally accepted in the United States
of America.
Management’s
Plan to Remediate the Material Weakness
We
are committed to continually improving our internal controls over financial reporting. Subsequent to December 31, 2023, we appointed
a new vice president of finance and accounting and director of corporate strategy, as part of our program to develop and implement effective
internal controls over financial reporting. Additionally, management is currently working on the plan to address the material weaknesses
noted above.
The
material weaknesses will not be considered remediated, however, until the applicable controls operate for a sufficient period and management
has concluded, through testing, that these controls are operating effectively. As we continue to evaluate and work to improve our internal
control over financial reporting, we may decide that additional measures are necessary to address these identified control deficiencies.
Changes
in Internal Control
Other
than the remediation actions noted above, there have been no changes in our internal control over financial reporting that occurred during
our last fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial
reporting.
PART
II — OTHER INFORMATION
ITEM
1. LEGAL PROCEEDINGS.
From
time to time, we may become involved in various lawsuits and legal proceedings, which arise in the ordinary course of business. Litigation
is subject to inherent uncertainties and an adverse result in these or other matters may arise from time to time that may harm our business.
We are currently not aware of any such legal proceedings or claims that will have, individually or in the aggregate, a material adverse
effect on our business, financial condition or operating results.
ITEM
1A. RISK FACTORS.
Risk
factors that affect our business and financial results are discussed in Part I, Item 1A “Risk Factors,” in our Annual Report
on Form 10-K for the year ended December 31, 2023 (“Annual Report”) as filed with the SEC on March 29, 2024 and below. There
have been no material changes in our risk factors from those previously disclosed in our Annual Report, except as set forth below. You
should carefully consider the risks described in our Annual Report and below, which could materially affect our business, financial condition
or future results. The risks described in our Annual Report are not the only risks we face. Additional risks and uncertainties not currently
known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition, and/or
operating results. If any of the risks actually occur, our business, financial condition, and/or results of operations could be negatively
affected.
Economic
uncertainty may affect our access to capital and/or increase the costs of such capital.
Global
economic conditions continue to be volatile and uncertain due to, among other things, consumer confidence in future economic conditions,
fears of recession and trade wars, the price of energy, fluctuating interest rates, the availability and cost of consumer credit, the
availability and timing of government stimulus programs, levels of unemployment, increased inflation, tax rates, and the war between
Ukraine and Russia which began in February 2022, and Israel and Hamas, which began in October 2023 and which threatens to spread to other
Middle Eastern countries. These conditions remain unpredictable and create uncertainties about our ability to raise capital in the future.
In the event required capital becomes unavailable in the future, or more costly, it could have a material adverse effect on our business,
future results of operations, and financial condition.
Our
outstanding options and warrants may adversely affect the trading price of our securities.
As
of September 30, 2024, we had (i) outstanding stock options to purchase an aggregate of 4,010,988 shares of common stock at a weighted
average exercise price of $2.03 per share; (ii) outstanding Pre-Funded warrants to purchase 1,913,661 shares of common stock with an
exercise price of $0.0001; and (iii) outstanding warrants to purchase 397,500 shares of common stock with a weighted average exercise
price of $4.11 per share (when not including the Pre-Funded warrants). For the life of the options and warrants, the holders have the
opportunity to profit from a rise in the market price of our common stock without assuming the risk of ownership. The issuance of shares
upon the exercise of outstanding securities will also dilute the ownership interests of our existing stockholders.
The
availability of these shares for public resale, as well as any actual resales of these shares, could adversely affect the trading price
of our common stock. We cannot predict the size of future issuances of our common stock pursuant to the exercise of outstanding options
or warrants or conversion of other securities, or the effect, if any, that future issuances and sales of shares of our common stock may
have on the market price of our common stock. Sales or distributions of substantial amounts of our common stock (including shares issued
in connection with an acquisition), or the perception that such sales could occur, may cause the market price of our common stock to
decline.
In
addition, the common stock issuable upon exercise/conversion of outstanding convertible securities may represent overhang that may also
adversely affect the market price of our common stock. Overhang occurs when there is a greater supply of a company’s stock in the
market than there is demand for that stock. When this happens the price of our stock will decrease, and any additional shares which stockholders
attempt to sell in the market will only further decrease the share price. If the share volume of our common stock cannot absorb shares
sold by holders of our outstanding convertible securities, then the value of our common stock will likely decrease.
A
significant number of our shares are eligible for sale and their sale or potential sale may depress the market price of our common stock.
Sales
of a significant number of shares of our common stock in the public market could harm the market price of our common stock. Most of our
common stock is available for resale in the public market, including (a) outstanding stock options to purchase an aggregate of 4,010,988
shares of common stock at a weighted average exercise price of $2.03 per share; (b) Pre-Funded warrants to purchase 1,913,661 shares
of common stock with an exercise price of $0.0001; and (c) 3,241,076 shares of common stock, the resale of which has been registered
under the Securities Act. We have also filed certain Form S-8 Registration Statements pursuant to which we can issue unregistered stock
in connection with awards under our equity plans. If a significant number of shares were sold, such sales would increase the supply of
our common stock, thereby potentially causing a decrease in its price. Some or all of our shares of common stock, including those discussed
above, may be offered from time to time in the open market pursuant to effective registration statements and/or compliance with Company
insider trading policy, Exchange Act Section 16 and/or Rule 144, which sales could have a depressive effect on the market for our shares
of common stock. Subject to certain restrictions, a person who has held restricted shares for a period of six months may generally sell
common stock into the market. The sale of a significant portion of such shares when such shares are eligible for public sale may cause
the value of our common stock to decline in value.
We
may not receive the $8 million which we recently learned was granted to us by the California Institute for Regenerative Medicine.
On
July 25, 2024, the Company learned that it was awarded an $8 million grant from the California Institute for Regenerative Medicine
(CIRM) to support the clinical development of chimeric antigen receptor T-cell therapy NXC-201 for the treatment of
relapsed/refractory AL Amyloidosis. The award is payable to the Company upon achievement of milestones that are primarily based on
patient enrollment in the Company’s clinical trials. Additionally, if CIRM determines, in its sole discretion, that the
Company has not complied with the terms and conditions of the grant, CIRM may suspend or permanently cease disbursements. Funds
received under this grant may only be used for allowable project costs specifically identified with the CIRM-funded project. Such
costs can include, but are not limited to, salary for personnel, itemized supplies, consultants, and itemized clinical study costs.
Under the terms of the grant, both CIRM and the Company will co-fund the research project and the amount of the Company’s
co-funding requirement is predetermined as a part of the award. The Company signed the grant agreement in November 2024 and expects
to begin receiving funds from the grant beginning in November of 2024. The Company has not yet received any funds in connection with
such award and may not receive funds on a timely basis, or at all, and such award may come with conditions. The Company is required
to complete certain requirements and agree to certain terms and conditions in connection with such grant, which have not been
completed or agreed to as of the date of this Report. In the event the award was not received on a timely basis, or at all, or
subject to conditions, the Company could be forced to seek out alternative funding which may not be on as favorable terms as such
currently expected grant.
ITEM
2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS.
Unregistered
Sales of Equity Securities
During
the three months ended September 30, 2024, the Company issued 19,737 shares of restricted common stock valued at $37,500 for investor
relations services based on the closing price on the date of the agreement pursuant to a marketing services agreement entered into on
September 20, 2024.
During
the three months ended September 30, 2024, the Company issued 31,641 shares of restricted common stock valued at $67,500 for investor
relations services based on the average closing price for the prior 10 trading days pursuant to a marketing services agreement entered
into on July 25, 2023.
Subsequent
to September 30, 2024, the Company issued 27,062 shares of restricted common stock valued at $45,000 for investor relations services
based on the average closing price for the prior 10 trading days pursuant to a marketing services agreement entered into on July 25,
2023.
The
issuances described above were exempt from registration pursuant to Section 4(a)(2), and/or Rule 506 of Regulation D of the Securities
Act, since the foregoing issuances did not involve a public offering, the recipient took the securities for investment and not resale,
we took take appropriate measures to restrict transfer, and the recipient was (a) an “accredited investor”; and/or
(b) had access to similar documentation and information as would be required in a Registration Statement under the Securities Act. The
securities are subject to transfer restrictions, and the securities contain an appropriate legend stating that such securities have not
been registered under the Securities Act and may not be offered or sold absent registration or pursuant to an exemption therefrom. The
securities were not registered under the Securities Act and such securities may not be offered or sold in the United States absent registration
or an exemption from registration under the Securities Act and any applicable state securities laws.
ITEM
5. OTHER INFORMATION.
Rule
10b5-1 Trading Plans. During the quarter ended September 30, 2024, none of the Company’s directors or officers (as defined
in Rule 16a-1(f)) adopted or terminated any contract, instruction or written plan for the purchase or sale of Company securities that
was intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) or any “non-Rule 10b5-1 trading arrangement”.
ITEM
6. EXHIBITS.
| Exhibit
No. |
|
Description |
| 3.1 |
|
Third Amended and Restated Certificate of Incorporation of Immix Biopharma, Inc. (Incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the SEC on December 20, 2021) |
| |
|
|
| 3.2 |
|
Amended and Restated Bylaws (Incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed with the SEC on December 20, 2021) |
| |
|
|
| 16.1 |
|
Letter from KMJ Corbin & Company LLP dated July 19, 2024 (filed as Exhibit 16.1 to the Company’s Current Report on Form 8-K filed with the SEC on July 22, 2024 and incorporated herein by reference) |
| |
|
|
| 31.1* |
|
Certification of Principal Executive Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
|
|
| 31.2* |
|
Certification of Principal Financial Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
|
|
| 32.1** |
|
Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
|
|
| 32.2** |
|
Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
|
|
| 101.INS* |
|
Inline
XBRL Instance Document |
| |
|
|
| 101.SCH* |
|
Inline
XBRL Taxonomy Extension Schema Document |
| |
|
|
| 101.CAL* |
|
Inline
XBRL Taxonomy Extension Calculation Linkbase Document |
| |
|
|
| 101.DEF* |
|
Inline
XBRL Taxonomy Extension Definition Linkbase Document |
| |
|
|
| 101.LAB* |
|
Inline
XBRL Taxonomy Extension Label Linkbase Document |
| |
|
|
| 101.PRE* |
|
Inline
XBRL Taxonomy Extension Presentation Linkbase Document |
| |
|
|
| 104* |
|
Cover
Page Interactive Data File - the cover page from the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September
30, 2023 is formatted in Inline XBRL and included in the Exhibit 101 Inline XBRL Document Set |
| * |
Filed
herewith. |
| ** |
Furnished
herewith. |
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by
the undersigned thereunto duly authorized.
| |
IMMIX
BIOPHARMA, INC. |
| |
|
| Date:
November 12, 2024 |
By: |
/s/
Ilya Rachman |
| |
|
Ilya
Rachman |
| |
|
Chief
Executive Officer |
| |
|
(Principal
Executive Officer) |
| |
|
|
| Date:
November 12, 2024 |
By: |
/s/
Gabriel Morris |
| |
|
Gabriel
Morris, |
| |
|
Chief
Financial Officer |
| |
|
(Principal
Financial and Accounting Officer) |
Exhibit
31.1
Certification
of Chief Executive Officer of Immix Biopharma, Inc.
Pursuant
to Section 302 of the Sarbanes-Oxley Act of 2002
I,
Ilya Rachman, certify that:
| 1. |
I
have reviewed this Quarterly Report on Form 10-Q of Immix Biopharma, Inc.; |
| |
|
| 2. |
Based
on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary
to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to
the period covered by this report; |
| |
|
| 3. |
Based
on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material
respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in
this report; |
| |
|
| 4. |
The
registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures
(as defined in Exchange Act Rules 13a-15(e) and 15d-15(e) and internal control over financial reporting (as defined in Exchange Act
Rules 13a-15(f) and 15(d)-15(f) for the registrant and have: |
| |
a. |
Designed
such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision,
to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others
within those entities, particularly during the period in which this report is being prepared; |
| |
|
|
| |
b. |
Designed
such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our
supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements
for external purposes in accordance with generally accepted accounting principles; |
| |
|
|
| |
c. |
Evaluated
the effectiveness of the registrant’s disclosure controls and procedures, and presented in this report our conclusions about
the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation;
and |
| |
|
|
| |
d. |
Disclosed
in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s
most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected,
or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. |
The
registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial
reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing
the equivalent functions): |
| |
a. |
All
significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are
reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information;
and |
| |
|
|
| |
b. |
Any
fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s
internal control over financial reporting. |
| Date:
November 12, 2024 |
/s/
Ilya Rachman |
| |
Ilya
Rachman |
| |
Chief
Executive Officer
(Principal
Executive Officer) |
Exhibit
31.2
Certification
of Chief Financial Officer of Immix Biopharma, Inc.
Pursuant
to Section 302 of the Sarbanes-Oxley Act of 2002
I,
Gabriel Morris, certify that:
| 1. |
I
have reviewed this Quarterly Report on Form 10-Q of Immix Biopharma, Inc.; |
| |
|
| 2. |
Based
on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary
to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to
the period covered by this report; |
| |
|
| 3. |
Based
on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material
respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in
this report; |
| |
|
| 4. |
The
registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures
(as defined in Exchange Act Rules 13a-15(e) and 15d-15(e) and internal control over financial reporting (as defined in Exchange Act
Rules 13a-15(f) and 15(d)-15(f) for the registrant and have: |
| |
a. |
Designed
such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision,
to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others
within those entities, particularly during the period in which this report is being prepared; |
| |
|
|
| |
b. |
Designed
such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our
supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements
for external purposes in accordance with generally accepted accounting principles; |
| |
|
|
| |
c. |
Evaluated
the effectiveness of the registrant’s disclosure controls and procedures, and presented in this report our conclusions about
the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation;
and |
| |
|
|
| |
d. |
Disclosed
in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s
most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected,
or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. |
The
registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial
reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing
the equivalent functions): |
| |
a. |
All
significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are
reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information;
and |
| |
|
|
| |
b. |
Any
fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s
internal control over financial reporting. |
| Date:
November 12, 2024 |
/s/
Gabriel Morris |
| |
Gabriel
Morris |
| |
Chief
Financial Officer
(Principal
Financial and Accounting Officer) |
Exhibit
32.1
Certification
of Chief Executive Officer
Pursuant
to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
Pursuant
to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, the undersigned, Ilya Rachman, of Immix
Biopharma, Inc. (the “Company”), hereby certifies that based on the undersigned’s knowledge:
| |
1. |
The
Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2024 (the “Report”) fully complies with
the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| |
|
|
| |
2. |
The
information contained in the Report fairly presents, in all material respects, the financial condition and results of operations
of the Company. |
| Date:
November 12, 2024 |
/s/
Ilya Rachman |
| |
Ilya
Rachman |
| |
Chief
Executive Officer |
| |
(Principal
Executive Officer) |
Exhibit
32.2
Certification
of Chief Financial Officer
Pursuant
to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
Pursuant
to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, the undersigned, Gabriel Morris, of
Immix Biopharma, Inc. (the “Company”), hereby certifies that based on the undersigned’s knowledge:
| |
1. |
The
Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2024 (the “Report”) fully complies with
the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| |
|
|
| |
2. |
The
information contained in the Report fairly presents, in all material respects, the financial condition and results of operations
of the Company. |
| Date:
November 12, 2024 |
/s/
Gabriel Morris |
| |
Gabriel
Morris |
| |
Chief
Financial Officer |
| |
(Principal
Financial and Accounting Officer) |
v3.24.3
Cover - shares
|
9 Months Ended |
|
Sep. 30, 2024 |
Nov. 12, 2024 |
| Cover [Abstract] |
|
|
| Document Type |
10-Q
|
|
| Amendment Flag |
false
|
|
| Document Quarterly Report |
true
|
|
| Document Transition Report |
false
|
|
| Document Period End Date |
Sep. 30, 2024
|
|
| Document Fiscal Period Focus |
Q3
|
|
| Document Fiscal Year Focus |
2024
|
|
| Current Fiscal Year End Date |
--12-31
|
|
| Entity File Number |
001-41159
|
|
| Entity Registrant Name |
IMMIX
BIOPHARMA, INC.
|
|
| Entity Central Index Key |
0001873835
|
|
| Entity Tax Identification Number |
45-4869378
|
|
| Entity Incorporation, State or Country Code |
DE
|
|
| Entity Address, Address Line One |
11400
West Olympic Blvd.
|
|
| Entity Address, Address Line Two |
Suite 200
|
|
| Entity Address, City or Town |
Los Angeles
|
|
| Entity Address, State or Province |
CA
|
|
| Entity Address, Postal Zip Code |
90064
|
|
| City Area Code |
(310)
|
|
| Local Phone Number |
651-8041
|
|
| Title of 12(b) Security |
Common
stock, $0.0001 par value
|
|
| Trading Symbol |
IMMX
|
|
| Security Exchange Name |
NASDAQ
|
|
| Entity Current Reporting Status |
Yes
|
|
| Entity Interactive Data Current |
Yes
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
| Entity Small Business |
true
|
|
| Entity Emerging Growth Company |
true
|
|
| Elected Not To Use the Extended Transition Period |
false
|
|
| Entity Shell Company |
false
|
|
| Entity Common Stock, Shares Outstanding |
|
27,507,637
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an quarterly report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-Q
-Number 240
-Section 308
-Subsection a
| Name: |
dei_DocumentQuarterlyReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Condensed Consolidated Balance Sheets - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 19,690,431
|
$ 17,509,791
|
| Tax receivable |
1,975,437
|
1,172,183
|
| Prepaid expenses and other current assets |
1,089,637
|
1,105,776
|
| Total current assets |
22,755,505
|
19,787,750
|
| Other assets |
20,418
|
|
| Deferred offering cost |
|
87,229
|
| Right-of-use asset, net |
1,010,205
|
|
| Property and equipment, net |
1,355,424
|
50,181
|
| Total assets |
25,141,552
|
19,925,160
|
| Current liabilities: |
|
|
| Accounts payable and accrued expenses |
6,501,786
|
3,721,783
|
| Operating lease liability - current |
62,715
|
|
| Total current liabilities |
6,564,501
|
3,721,783
|
| Operating lease liability – long term |
1,026,340
|
|
| Total liabilities |
7,590,841
|
3,721,783
|
| Commitments and contingencies |
|
|
| Stockholders’ equity: |
|
|
| Preferred stock, $0.0001 par value; 10,000,000 shares authorized; no shares issued and outstanding |
|
|
| Common stock, $0.0001 par value; 200,000,000 shares authorized; 27,552,938 shares issued and 27,480,575 shares outstanding at September 30, 2024 and 19,994,719 shares issued and 19,922,356 shares outstanding at December 31, 2023 |
2,757
|
2,000
|
| Additional paid-in capital |
87,668,483
|
69,779,706
|
| Accumulated other comprehensive income |
192,051
|
134,666
|
| Accumulated deficit |
(70,212,617)
|
(53,411,295)
|
| Treasury stock at cost, 72,363 shares as of September 30, 2024 and December 31, 2023 |
(99,963)
|
(99,963)
|
| Total Immix Biopharma, Inc. stockholders’ equity |
17,550,711
|
16,405,114
|
| Non-controlling interests |
|
(201,737)
|
| Total stockholders’ equity |
17,550,711
|
16,203,377
|
| Total liabilities and stockholders’ equity |
$ 25,141,552
|
$ 19,925,160
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 30: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred cost, excluding capitalized cost related to contract with customer; classified as noncurrent. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_DeferredCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liability recognized for present obligation requiring transfer or otherwise providing economic benefit to others. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to noncontrolling interest. Excludes temporary equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_MinorityInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent and noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483550/848-10-65-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479832/842-10-65-8
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483421/250-10-45-24
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483421/250-10-45-23
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483421/250-10-45-5
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479654/326-10-65-5
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479343/105-10-65-6
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479343/105-10-65-6
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482615/740-10-65-8
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482615/740-10-65-8
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479654/326-10-65-4
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-5
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-17
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 39: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 40: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 41: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 42: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 43: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 44: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 45: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 46: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-15
Reference 47: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-16
Reference 48: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4I
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4I
Reference 49: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476166/350-60-65-1
| Name: |
us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterestAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount allocated to treasury stock. Treasury stock is common and preferred shares of an entity that were issued, repurchased by the entity, and are held in its treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481520/505-30-50-4
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481549/505-30-45-1
| Name: |
us-gaap_TreasuryStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.3
Condensed Consolidated Balance Sheets (Parenthetical) - $ / shares
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Statement of Financial Position [Abstract] |
|
|
| Preferred stock, par value |
$ 0.0001
|
$ 0.0001
|
| Preferred stock, shares authorized |
10,000,000
|
10,000,000
|
| Preferred stock, shares issued |
0
|
0
|
| Preferred stock, shares outstanding |
0
|
0
|
| Common stock, par value |
$ 0.0001
|
$ 0.0001
|
| Common stock, shares authorized |
200,000,000
|
200,000,000
|
| Common stock, shares issued |
27,552,938
|
19,994,719
|
| Common stock, shares outstanding |
27,480,575
|
19,922,356
|
| Treasury stock, shares |
72,363
|
72,363
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued for nonredeemable preferred shares and preferred shares redeemable solely at option of issuer. Includes, but is not limited to, preferred shares issued, repurchased, and held as treasury shares. Excludes preferred shares classified as debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of previously issued common shares repurchased by the issuing entity and held in treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481549/505-30-45-1
| Name: |
us-gaap_TreasuryStockCommonShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.3
Condensed Consolidated Statements of Operations and Comprehensive Loss (Unaudited) - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Operating expenses: |
|
|
|
|
| General and administrative expenses |
$ 2,949,403
|
$ 2,417,776
|
$ 7,769,224
|
$ 5,130,977
|
| Research and development |
4,445,528
|
2,106,020
|
9,918,336
|
5,634,284
|
| Total operating expenses |
7,394,931
|
4,523,796
|
17,687,560
|
10,765,261
|
| Loss from operations |
(7,394,931)
|
(4,523,796)
|
(17,687,560)
|
(10,765,261)
|
| Other income (expense): |
|
|
|
|
| Interest income |
256,680
|
186,691
|
831,503
|
343,431
|
| Total other expense, net |
256,680
|
186,691
|
831,503
|
343,431
|
| Loss before provision for income taxes |
(7,138,251)
|
(4,337,105)
|
(16,856,057)
|
(10,421,830)
|
| Provision for income taxes |
11,144
|
6,807
|
30,252
|
18,326
|
| Net loss |
(7,149,395)
|
(4,343,912)
|
(16,886,309)
|
(10,440,156)
|
| Net loss attributable to non-controlling interests |
|
63,248
|
84,987
|
103,612
|
| Net loss attributable to Immix Biopharma, Inc. common stockholders |
(7,149,395)
|
(4,280,664)
|
(16,801,322)
|
(10,336,544)
|
| Other comprehensive income (loss): |
|
|
|
|
| Foreign currency translation |
75,079
|
(34,147)
|
57,385
|
(40,286)
|
| Total other comprehensive loss |
75,079
|
(34,147)
|
57,385
|
(40,286)
|
| Comprehensive loss |
(7,074,316)
|
(4,314,811)
|
(16,743,937)
|
(10,376,830)
|
| Less: comprehensive loss attributable to non-controlling interests |
|
|
|
|
| Comprehensive loss attributable to Immix Biopharma, Inc. common stockholders |
$ (7,074,316)
|
$ (4,314,811)
|
$ (16,743,937)
|
$ (10,376,830)
|
| Loss per common share - basic |
$ (0.24)
|
$ (0.23)
|
$ (0.60)
|
$ (0.65)
|
| Loss per common share - diluted |
$ (0.24)
|
$ (0.23)
|
$ (0.60)
|
$ (0.65)
|
| Weighted average shares outstanding - basic |
29,424,444
|
18,578,414
|
27,859,556
|
15,861,100
|
| Weighted average shares outstanding - diluted |
29,424,444
|
18,578,414
|
27,859,556
|
15,861,100
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4K
| Name: |
us-gaap_ComprehensiveIncomeNetOfTaxIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accretion (amortization) of purchase discount (premium) of interest income on nonoperating securities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
| Name: |
us-gaap_InvestmentIncomeInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of Net Income (Loss) attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4J
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
| Name: |
us-gaap_NetIncomeLossAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before tax of other comprehensive income (loss) attributable to noncontrolling interests. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossBeforeTaxPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherComprehensiveIncomeLossBeforeTaxPortionAttributableToParentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-10A
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
| Name: |
us-gaap_OtherComprehensiveIncomeLossForeignCurrencyTransactionAndTranslationAdjustmentNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of other comprehensive income (loss) attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-3
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477314/942-235-S99-1
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 31: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4J
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4K
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-2
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Condensed Consolidated Statements of Stockholders' Equity (Unaudited) - USD ($)
|
Common Stock [Member] |
Additional Paid-in Capital [Member] |
AOCI Attributable to Parent [Member] |
Retained Earnings [Member] |
Treasury Stock, Common [Member] |
Noncontrolling Interest [Member] |
Total |
| Balance at Dec. 31, 2022 |
$ 1,397
|
$ 51,156,597
|
$ 87,021
|
$ (37,985,247)
|
$ (99,963)
|
|
$ 13,159,805
|
| Balance, shares at Dec. 31, 2022 |
13,964,485
|
|
|
|
(72,363)
|
|
|
| Shares issued under ATM facility for cash proceeds, net of offering costs |
$ 5
|
101,318
|
|
|
|
|
101,323
|
| Shares issued under ATM facility for cash proceeds, net of offering costs, shares |
50,000
|
|
|
|
|
|
|
| Stock-based compensation |
$ 1
|
329,918
|
|
|
|
|
329,919
|
| Stock-based compensation, shares |
6,700
|
|
|
|
|
|
|
| Non-controlling interests in subsidiary |
|
13,990
|
|
|
|
(13,990)
|
|
| Net loss |
|
|
|
(2,479,664)
|
|
(18,368)
|
(2,498,032)
|
| Foreign currency translation adjustment |
|
|
(4,474)
|
|
|
|
(4,474)
|
| Nexcella shares issued for cash proceeds |
|
650,000
|
|
|
|
|
650,000
|
| Balance at Mar. 31, 2023 |
$ 1,403
|
52,251,823
|
82,547
|
(40,464,911)
|
$ (99,963)
|
(32,358)
|
11,738,541
|
| Balance, shares at Mar. 31, 2023 |
14,021,185
|
|
|
|
(72,363)
|
|
|
| Balance at Dec. 31, 2022 |
$ 1,397
|
51,156,597
|
87,021
|
(37,985,247)
|
$ (99,963)
|
|
13,159,805
|
| Balance, shares at Dec. 31, 2022 |
13,964,485
|
|
|
|
(72,363)
|
|
|
| Net loss |
|
|
|
|
|
|
(10,440,156)
|
| Balance at Sep. 30, 2023 |
$ 1,968
|
68,146,992
|
46,735
|
(48,321,791)
|
$ (99,963)
|
(124,667)
|
19,649,274
|
| Balance, shares at Sep. 30, 2023 |
19,681,091
|
|
|
|
(72,363)
|
|
|
| Balance at Dec. 31, 2022 |
$ 1,397
|
51,156,597
|
87,021
|
(37,985,247)
|
$ (99,963)
|
|
13,159,805
|
| Balance, shares at Dec. 31, 2022 |
13,964,485
|
|
|
|
(72,363)
|
|
|
| Balance at Dec. 31, 2023 |
$ 2,000
|
69,779,706
|
134,666
|
(53,411,295)
|
$ (99,963)
|
(201,737)
|
16,203,377
|
| Balance, shares at Dec. 31, 2023 |
19,994,719
|
|
|
|
(72,363)
|
|
|
| Balance at Mar. 31, 2023 |
$ 1,403
|
52,251,823
|
82,547
|
(40,464,911)
|
$ (99,963)
|
(32,358)
|
11,738,541
|
| Balance, shares at Mar. 31, 2023 |
14,021,185
|
|
|
|
(72,363)
|
|
|
| Shares issued under ATM facility for cash proceeds, net of offering costs |
$ 221
|
4,584,032
|
|
|
|
|
4,584,253
|
| Shares issued under ATM facility for cash proceeds, net of offering costs, shares |
2,213,868
|
|
|
|
|
|
|
| Stock-based compensation |
$ 10
|
447,646
|
|
|
|
|
447,656
|
| Stock-based compensation, shares |
99,128
|
|
|
|
|
|
|
| Non-controlling interests in subsidiary |
|
2,416
|
|
|
|
(2,416)
|
|
| Net loss |
|
|
|
(3,576,216)
|
|
(21,996)
|
(3,598,212)
|
| Foreign currency translation adjustment |
|
|
(1,665)
|
|
|
|
(1,665)
|
| Balance at Jun. 30, 2023 |
$ 1,634
|
57,285,917
|
80,882
|
(44,041,127)
|
$ (99,963)
|
(56,770)
|
13,170,573
|
| Balance, shares at Jun. 30, 2023 |
16,334,181
|
|
|
|
(72,363)
|
|
|
| Shares issued under ATM facility for cash proceeds, net of offering costs |
$ 10
|
185,272
|
|
|
|
|
185,282
|
| Shares issued under ATM facility for cash proceeds, net of offering costs, shares |
105,834
|
|
|
|
|
|
|
| Stock-based compensation |
|
737,325
|
|
|
|
|
737,325
|
| Non-controlling interests in subsidiary |
|
4,649
|
|
|
|
(4,649)
|
|
| Net loss |
|
|
|
(4,280,664)
|
|
(63,248)
|
(4,343,912)
|
| Foreign currency translation adjustment |
|
|
(34,147)
|
|
|
|
(34,147)
|
| Shares and warrants issued under private placement for cash proceeds, net of offering costs |
$ 324
|
9,933,829
|
|
|
|
|
9,934,153
|
| Shares and warrants issued under private placement for cash proceeds, net of offering costs, shares |
3,241,076
|
|
|
|
|
|
|
| Balance at Sep. 30, 2023 |
$ 1,968
|
68,146,992
|
46,735
|
(48,321,791)
|
$ (99,963)
|
(124,667)
|
19,649,274
|
| Balance, shares at Sep. 30, 2023 |
19,681,091
|
|
|
|
(72,363)
|
|
|
| Balance at Dec. 31, 2023 |
$ 2,000
|
69,779,706
|
134,666
|
(53,411,295)
|
$ (99,963)
|
(201,737)
|
16,203,377
|
| Balance, shares at Dec. 31, 2023 |
19,994,719
|
|
|
|
(72,363)
|
|
|
| Shares issued under ATM facility for cash proceeds, net of offering costs |
$ 7
|
338,488
|
|
|
|
|
338,495
|
| Shares issued under ATM facility for cash proceeds, net of offering costs, shares |
68,302
|
|
|
|
|
|
|
| Shares issued under public offering for cash proceeds, net of offering costs |
$ 632
|
15,519,722
|
|
|
|
|
15,520,354
|
| Shares issued under public offering for cash proceeds, net of offering costs, shares |
6,319,025
|
|
|
|
|
|
|
| Shares issued for exercise of stock options |
|
2,489
|
|
|
|
|
2,489
|
| Shares issued for exercise of stock options, shares |
1,251
|
|
|
|
|
|
|
| Shares issued for services |
$ 9
|
327,367
|
|
|
|
|
327,376
|
| Shares issued for services, shares |
85,486
|
|
|
|
|
|
|
| Stock-based compensation |
|
615,888
|
|
|
|
|
615,888
|
| Stock-based compensation, shares |
|
|
|
|
|
|
|
| Non-controlling interests in subsidiary |
|
9,472
|
|
|
|
(9,472)
|
|
| Net loss |
|
|
|
(5,258,991)
|
|
(72,073)
|
(5,331,064)
|
| Foreign currency translation adjustment |
|
|
(45,052)
|
|
|
|
(45,052)
|
| Balance at Mar. 31, 2024 |
$ 2,648
|
86,593,132
|
89,614
|
(58,670,286)
|
$ (99,963)
|
(283,282)
|
27,631,863
|
| Balance, shares at Mar. 31, 2024 |
26,468,783
|
|
|
|
(72,363)
|
|
|
| Balance at Dec. 31, 2023 |
$ 2,000
|
69,779,706
|
134,666
|
(53,411,295)
|
$ (99,963)
|
(201,737)
|
$ 16,203,377
|
| Balance, shares at Dec. 31, 2023 |
19,994,719
|
|
|
|
(72,363)
|
|
|
| Shares issued for exercise of stock options, shares |
|
|
|
|
|
|
834
|
| Net loss |
|
|
|
|
|
|
$ (16,886,309)
|
| Balance at Sep. 30, 2024 |
$ 2,757
|
87,668,483
|
192,051
|
(70,212,617)
|
$ (99,963)
|
|
17,550,711
|
| Balance, shares at Sep. 30, 2024 |
27,552,938
|
|
|
|
(72,363)
|
|
|
| Balance at Mar. 31, 2024 |
$ 2,648
|
86,593,132
|
89,614
|
(58,670,286)
|
$ (99,963)
|
(283,282)
|
27,631,863
|
| Balance, shares at Mar. 31, 2024 |
26,468,783
|
|
|
|
(72,363)
|
|
|
| Shares issued for services |
$ 5
|
102,495
|
|
|
|
|
102,500
|
| Shares issued for services, shares |
42,901
|
|
|
|
|
|
|
| Stock-based compensation |
|
535,350
|
|
|
|
|
535,350
|
| Non-controlling interests in subsidiary |
|
20,200
|
|
|
|
(20,200)
|
|
| Net loss |
|
|
|
(4,392,936)
|
|
(12,914)
|
(4,405,850)
|
| Foreign currency translation adjustment |
|
|
27,358
|
|
|
|
27,358
|
| Buyout of non-controlling interests in subsidiary |
$ 99
|
(316,495)
|
|
|
|
316,396
|
|
| Buyout of non-controlling interests in subsidiary, shares |
989,876
|
|
|
|
|
|
|
| Balance at Jun. 30, 2024 |
$ 2,752
|
86,934,682
|
116,972
|
(63,063,222)
|
$ (99,963)
|
|
23,891,221
|
| Balance, shares at Jun. 30, 2024 |
27,501,560
|
|
|
|
(72,363)
|
|
|
| Shares issued for services |
$ 5
|
104,995
|
|
|
|
|
105,000
|
| Shares issued for services, shares |
51,378
|
|
|
|
|
|
|
| Stock-based compensation |
|
628,806
|
|
|
|
|
628,806
|
| Net loss |
|
|
|
(7,149,395)
|
|
|
(7,149,395)
|
| Foreign currency translation adjustment |
|
|
75,079
|
|
|
|
75,079
|
| Balance at Sep. 30, 2024 |
$ 2,757
|
$ 87,668,483
|
$ 192,051
|
$ (70,212,617)
|
$ (99,963)
|
|
$ 17,550,711
|
| Balance, shares at Sep. 30, 2024 |
27,552,938
|
|
|
|
(72,363)
|
|
|
| X |
- DefinitionBuyout of noncontrolling interests in subsidiary.
| Name: |
IMMX_BuyoutOfNoncontrollingInterestsInSubsidiary |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionBuyout of noncontrolling interests in subsidiary shares.
| Name: |
IMMX_BuyoutOfNoncontrollingInterestsInSubsidiaryShares |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period shares shares and warrants issued under private placement for cash proceeds, net of offering costs.
| Name: |
IMMX_StockIssuedDuringPeriodSharesSharesAndWarrantIssuedUnderPrivatePlacement |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period shares under atm facility for cash proceeds net of offering costs.
| Name: |
IMMX_StockIssuedDuringPeriodSharesUnderAtmFacilityForCashProceedsNetOfOfferingCosts |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period value shares and warrants issued under private placement for cash proceeds, net of offering costs.
| Name: |
IMMX_StockIssuedDuringPeriodValueSharesAndWarrantIssuedUnderPrivatePlacement |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period value under atm facilities for cash proceeds net of offering costs.
| Name: |
IMMX_StockIssuedDuringPeriodValueUnderAtmFacilitiesForCashProceedsNetOfOfferingCosts |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in noncontrolling interest from subsidiary issuance of equity interests to noncontrolling interest holders. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-23
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section S99
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(2)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_NoncontrollingInterestIncreaseFromSubsidiaryEquityIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of tax expense (benefit), after reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-10A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 12
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481956/830-20-45-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-21
| Name: |
us-gaap_OtherComprehensiveIncomeLossForeignCurrencyTranslationAdjustmentTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-3
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477314/942-235-S99-1
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 31: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4J
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4K
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-2
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued in lieu of cash for services contributed to the entity. Number of shares includes, but is not limited to, shares issued for services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber, after forfeiture, of shares or units issued under share-based payment arrangement. Excludes shares or units issued under employee stock ownership plan (ESOP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued in lieu of cash for services contributed to the entity. Value of the stock issued includes, but is not limited to, services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodValueIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of shares of stock issued attributable to transactions classified as other.
| Name: |
us-gaap_StockIssuedDuringPeriodValueOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue, after forfeiture, of shares issued under share-based payment arrangement. Excludes employee stock ownership plan (ESOP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_StockIssuedDuringPeriodValueShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued as a result of the exercise of stock options. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent and noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483550/848-10-65-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479832/842-10-65-8
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483421/250-10-45-24
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483421/250-10-45-23
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483421/250-10-45-5
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479654/326-10-65-5
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479343/105-10-65-6
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479343/105-10-65-6
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482615/740-10-65-8
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482615/740-10-65-8
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479654/326-10-65-4
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-5
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-17
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 39: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 40: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 41: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 42: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 43: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 44: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 45: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 46: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-15
Reference 47: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-16
Reference 48: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4I
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4I
Reference 49: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476166/350-60-65-1
| Name: |
us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.3
Condensed Consolidated Statements of Cash Flows (Unaudited) - USD ($)
|
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Operating Activities: |
|
|
| Net loss |
$ (16,886,309)
|
$ (10,440,156)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
| Stock-based compensation |
2,314,920
|
1,514,900
|
| Depreciation |
12,338
|
2,392
|
| Amortization of right of use asset |
61,713
|
|
| Changes in operating assets and liabilities: |
|
|
| Tax receivable |
(746,748)
|
(427,476)
|
| Prepaid expenses and other current assets |
7,932
|
(923,909)
|
| Other assets |
(20,418)
|
|
| Accounts payable and accrued expenses |
2,120,531
|
1,580,248
|
| Operating lease liability |
17,137
|
|
| Net cash used in operating activities |
(13,118,904)
|
(8,694,001)
|
| Investing Activities: |
|
|
| Purchase of property and equipment |
(670,529)
|
(38,912)
|
| Net cash used in investing activities |
(670,529)
|
(38,912)
|
| Financing Activities: |
|
|
| Payments of deferred offering costs |
|
(234,617)
|
| Proceeds from sale of common stock, net of offering costs |
15,946,078
|
14,936,437
|
| Proceeds from exercise of stock options |
2,489
|
|
| Funds received for subsidiary private offering |
|
175,000
|
| Net cash provided by financing activities |
15,948,567
|
14,876,820
|
| Effect of foreign currency on cash |
21,506
|
1,804
|
| Net change in cash and cash equivalents |
2,180,640
|
6,145,711
|
| Cash and cash equivalents – beginning of period |
17,509,791
|
13,436,714
|
| Cash and cash equivalents – end of period |
19,690,431
|
19,582,425
|
| Supplemental Disclosures of Cash Flow Information: |
|
|
| Income taxes paid |
30,252
|
18,326
|
| Supplemental Disclosures of Noncash Financing Information: |
|
|
| Establishment of right of use asset and liabilities |
1,071,918
|
|
| Purchases of property and equipment included in accounts payable and accrued liabilities |
647,052
|
|
| Deferred offering costs charged against proceeds from sale of common stock |
87,229
|
131,426
|
| Shares issued in subsidiary absorption |
99
|
|
| Nexcella shares issued for funds previously received |
|
$ 475,000
|
| X |
- DefinitionDeferred offering costs charged against proceeds from sale of common stock.
| Name: |
IMMX_DeferredOfferingCostsChargedAgainstProceedsFromSaleOfCommonStock |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionPurchases of property and equipment included in accounts payable and accrued liabilities.
| Name: |
IMMX_PurchasesOfPropertyAndEquipmentIncludedInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionRight of use asset and liabilities.
| Name: |
IMMX_RightOfUseAssetAndLiabilities |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionShares issued for funds previously received.
| Name: |
IMMX_SharesIssuedForFundsPreviouslyReceived |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionShares issued in subsidiary absorption.
| Name: |
IMMX_SharesIssuedInSubsidiaryAbsorption |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CashFlowNoncashInvestingAndFinancingActivitiesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) from effect of exchange rate changes on cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; held in foreign currencies. Excludes amounts for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 230
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of amortization expense attributable to right-of-use asset from finance lease. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_FinanceLeaseRightOfUseAssetAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation for operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-SubTopic 20
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_IncreaseDecreaseInOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in other obligations or expenses incurred but not yet paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in current assets classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherCurrentAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe total of the cash outflow during the period which has been paid to third parties in connection with debt origination, which will be amortized over the remaining maturity period of the associated long-term debt and the cost incurred directly for the issuance of equity securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentOfFinancingAndStockIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's raising of capital via private rather than public placement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfPrivatePlacement |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-3
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477314/942-235-S99-1
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 31: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4J
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4K
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-2
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.3
Pay vs Performance Disclosure - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Pay vs Performance Disclosure [Table] |
|
|
|
|
| Net Income (Loss) |
$ (7,149,395)
|
$ (4,280,664)
|
$ (16,801,322)
|
$ (10,336,544)
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.3
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 2
-Subparagraph A
| Name: |
ecd_TradingArrByIndTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Nature of Business
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Nature of Business |
Note
1 – Nature of Business
Immix
Biopharma, Inc. (the “Company”) is a clinical-stage biopharmaceutical pharmaceutical company organized as a Delaware corporation
on January 7, 2014, which is focused on developing cell therapies in AL Amyloidosis and select immune-mediated diseases. In August 2016,
the Company established a wholly-owned Australian subsidiary, Immix Biopharma Australia Pty Ltd. (“IBAPL”), in order to conduct
various preclinical and clinical activities for its development candidates. In November 2022, the Company established a majority-owned
subsidiary, Nexcella, Inc. (“Nexcella”), its cell therapy division, which subsequently merged into the Company in May 2024,
with the Company continuing as the surviving entity. To ensure continuity of operations, the Company re-established Nexcella in 2024
as a wholly-owned subsidiary
|
| X |
- DefinitionThe entire disclosure for the nature of an entity's business, major products or services, principal markets including location, and the relative importance of its operations in each business and the basis for the determination, including but not limited to, assets, revenues, or earnings. For an entity that has not commenced principal operations, disclosures about the risks and uncertainties related to the activities in which the entity is currently engaged and an understanding of what those activities are being directed toward. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/275/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
| Name: |
us-gaap_NatureOfOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Summary of Significant Accounting Policies
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Summary of Significant Accounting Policies |
Note
2 – Summary of Significant Accounting Policies
Basis
of Presentation - The accompanying condensed consolidated financial statements and related notes have been prepared in accordance
with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and in accordance with the rules
and regulations of the United States Securities and Exchange Commission (the “SEC”). The Company’s fiscal year end
is December 31.
The
condensed consolidated financial statements and related disclosures as of September 30, 2024, and for the three and nine months ended
September 30, 2024 and 2023 are unaudited, pursuant to the rules and regulations of the SEC. Certain information and footnote disclosures
normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted pursuant to such rules
and regulations. In the Company’s opinion, these unaudited condensed consolidated financial statements include all adjustments
(consisting only of normal recurring adjustments) necessary for the fair statement of the results for the interim periods. These unaudited
condensed consolidated financial statements should be read in conjunction with the audited financial statements of the Company for the
years ended December 31, 2023 and 2022 which are included in the Company’s Annual Report on Form 10-K filed with the SEC on March
29, 2024. The results of operations for the three and nine months ended September 30, 2024, are not necessarily indicative of the results
to be expected for the full year ending December 31, 2024.
Risk
and Uncertainties - The Company operates in a dynamic and highly competitive industry and is subject to risks and uncertainties common
to early-stage companies in the biotechnology industry, including, but not limited to, development by competitors of new technological
innovations, protection of proprietary technology, dependence on key personnel, contract manufacturers and contract research organizations,
compliance with government regulations and the need to obtain additional financing to fund operations. Product candidates currently under
development will require significant additional research and development efforts, including extensive preclinical studies and clinical
trials and regulatory approval, prior to commercialization. These efforts require significant amounts of additional capital, adequate
personnel infrastructure and extensive compliance and reporting. The Company believes that changes in any of the following areas could
have a material adverse effect on the Company’s future financial position, results of operations, or cash flows; ability to obtain
future financing; advances and trends in new technologies and industry standards; results of clinical trials; regulatory approval and
market acceptance of the Company’s products; development of sales channels; certain strategic relationships; litigation or claims
against the Company based on intellectual property, patent, product, regulatory, or other factors; and the Company’s ability to
attract and retain employees necessary to support its growth.
Products
developed by the Company require approvals from the U.S. Food and Drug Administration (“FDA”) or other international regulatory
agencies prior to commercial sales. There can be no assurance that the Company’s research and development will be successfully
completed, that adequate protection for the Company’s intellectual property will be obtained or maintained, that the products will
receive the necessary approvals, or that any approved products will be commercially viable. If the Company is denied approval, approval
is delayed, or the Company is unable to maintain approval, it could have a material adverse impact on the Company. Even if the Company’s
product development efforts are successful, it is uncertain when, if ever, the Company will generate revenue from product sales. The
Company operates in an environment of rapid change in technology and substantial competition from other pharmaceutical and biotechnology
companies. In addition, the Company is dependent upon the services of its employees, consultants and other third parties.
The
Company has expended and plans to continue to expend substantial funds to complete the research, development and clinical testing of
product candidates. The Company also will be required to expend additional funds to establish commercial-scale manufacturing arrangements
and to provide for the marketing and distribution of products that receive regulatory approval. The Company may require additional funds
to commercialize its products. The Company is unable to entirely fund these efforts with its current financial resources and will need
to raise additional funding in the future. If adequate funds are unavailable on a timely basis from operations or additional sources
of financing, the Company may have to delay, reduce the scope of or eliminate one or more of its research or development programs which
may materially and adversely affect its business, financial condition and operations.
Use
of Estimates – The preparation of these condensed consolidated financial statements in conformity with U.S. GAAP requires management
to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements
and the reported amounts of revenues and expenses during the reporting periods. The Company uses significant judgments when making estimates
related to the valuation of deferred tax assets and related valuation allowances, accrual and prepayment of research and development
expenses, and the valuation of stock-based compensation. Actual results could differ from those estimates.
Principles
of Consolidation – The accompanying condensed consolidated financial statements include the accounts of Immix Biopharma, Inc.
and the accounts of its 100% owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation. For
previously consolidated entities where the Company owned less than 100% of the subsidiary, the Company recorded net loss attributable
to non-controlling interests in its condensed consolidated statements of operations and comprehensive loss equal to the percentage of
the economic or ownership interest retained in such entities by the respective non-controlling parties.
Segment
Reporting - The Company manages its operations as a single segment for the purposes of assessing performance and making operating
decisions. The Company’s Chief Operating Decision Maker (“CODM”) is its Chief Executive Officer. The CODM allocates
resources and evaluates the performance of the Company at the consolidated level using information about its revenues, gross profit,
income from operations, and other key financial data. All significant operating decisions are based upon an analysis of the Company as
one operating segment, which is the same as its reporting segment.
Liquidity
and Going Concern – These consolidated financial statements have been prepared on a going concern basis, which assumes the
Company will continue to realize its assets and discharge its liabilities in the normal course of business. Since the initial public
offering of its common stock in December 2021, the Company has financed its operations through various equity financing. On July 14,
2023, the Company entered into an additional ATM Sales Agreement (the “July Sales Agreement”) with ThinkEquity LLC (the “Sales
Agent”), pursuant to which the Company, could, from time to time, issue and sell through the Sales Agent shares of the Company’s
common stock in sales deemed to be “at-the-market offerings” as defined in Rule 415(a)(4) promulgated under the Securities
Act of 1933, as amended (the “July ATM Facility”) (see Note 7). Initially, the Company is eligible to sell up to $4,200,000
worth of shares of its common stock as the aggregate market value of the Company’s shares of common stock eligible for sale under
the July Sales Agreement is subject to the limitations of General Instruction I.B.6 of Form S-3 until such time that the Company’s
public float equals or exceeds $75.0 million. In the event the aggregate market value of the Company’s outstanding common stock
held by non-affiliates equals or exceeds $75.0 million, then the one-third limitation on sales set forth in General Instruction I.B.6
of Form S-3 shall not apply to additional sales made pursuant to the July Sales Agreement.
From
July 14, 2023 through February 5, 2024, the Company has sold 328,136 common shares pursuant to the July ATM Facility for net proceeds
of $1,091,887, after offering expenses. On February 5, 2024, the Company suspended, and is not offering any shares of its common stock
pursuant to, the prospectus supplement dated July 14, 2023, relating to the July Sales Agreement by and between the Company and the Sales
Agent. The Company will not make any sales of common stock pursuant to the July Sales Agreement unless and until a new prospectus supplement
is filed with the SEC; however, the July Sales Agreement remains in full force and effect.
In
February 2024, the Company conducted an underwritten public offering of 5,535,055 shares of its common stock at the public offering price
of $2.71 per share, for net proceeds of $13,565,760, after underwriter discounts and offering expenses (the “Offering”).
Pursuant to the underwriting agreement, the Company granted the underwriter a 30-day over-allotment option to purchase up to an additional
783,970 shares of the Company’s common stock, which was exercised in full on March 1, 2024, for net proceeds of $1,954,594, after
underwriting discounts and offering expenses (see Note 7).
On
July 25, 2024, the Company was awarded an $8
million grant from the California Institute for Regenerative Medicine to support the clinical development of chimeric antigen
receptor T-cell therapy NXC-201 for the treatment of relapsed/refractory AL Amyloidosis. The award is payable to the Company upon
achievement of milestones that are primarily based on patient enrollment in the Company’s clinical trials. Additionally, if
CIRM determines, in its sole discretion, that the Company has not complied with the terms and conditions of the grant, CIRM may
suspend or permanently cease disbursements. Funds received under this grant may only be used for allowable project costs
specifically identified with the CIRM-funded project. Such costs can include, but are not limited to, salary for personnel, itemized
supplies, consultants, and itemized clinical study costs. Under the terms of the grant, both CIRM and the Company will co-fund the
research project and the amount of the Company’s co-funding requirement is predetermined as a part of the award. The Company
signed the grant agreement in November 2024 and expects to begin receiving funds from the grant beginning in November of
2024.
The
Company has a history of, and expects to continue to report, negative cash flows from operations and net losses. We believe that our
existing cash, cash equivalents and restricted cash as of September 30, 2024 will enable us to fund our operating expenses and capital
expenditure requirements for at least the next 12 months.
Concentration
of Credit Risk – Periodically, the Company may carry cash and cash equivalents balances at financial institutions in excess
of the federally insured limit of $250,000, or the Australian insured limit of AUD 250,000. At times, deposits held with financial institutions
may exceed the amount of insurance provided. The Company has not experienced losses on these accounts and management believes that the
credit risk with regard to these deposits is not significant.
Cash
and Cash Equivalents – The Company’s cash equivalents include short-term highly liquid investments with an original maturity
of 90 days or less when purchased and are carried at fair value.
Fair
Value of Financial Instruments – The carrying value of short-term instruments, including cash and cash equivalents, tax receivable,
accounts payable and accrued expenses, approximate fair value due to the relatively short period to maturity for these instruments.
Fair
value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal
or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The
Company utilizes a three-level valuation hierarchy for disclosures of fair value measurements, defined as follows:
Level
1 – inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level
2 – inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs
that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
Level
3 – inputs to the valuation methodology are unobservable and significant to the fair value.
The
following fair value hierarchy table presents information about the Company’s asset measured at fair value on a recurring basis:
Schedule of Asset Measured at Fair Value on a Recurring Basis
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at September 30, 2024 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets: | |
| | | |
| | | |
| | |
| Cash equivalents (money market funds) | |
$ | 18,650,711 | | |
$ | - | | |
$ | - | |
As
of September 30, 2024, the Company had no liabilities required to be measured at fair value on a recurring basis.
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at December 31, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets: | |
| | | |
| | | |
| | |
| Cash equivalents (money market funds) | |
$ | 16,113,006 | | |
$ | - | | |
$ | - | |
As
of December 31, 2023, the Company had no liabilities required to be measured at fair value on a recurring basis.
Australian
Tax Incentive – IBAPL is eligible to receive a cash refund from the Australian Taxation Office for eligible research and development
(“R&D”) expenditures under the Australian R&D Tax Incentive Program (the “Australian Tax Incentive”).
The Australian Tax Incentive is recognized as a reduction to R&D expense when there is reasonable assurance that the relevant expenditure
has been incurred, the amount can be reliably measured and that the Australian Tax Incentive will be received. The Company recognized
additional R&D expense of $66,594 and reductions to R&D expense of $378,390 for the three months ended September 30, 2024 and
2023, respectively. The Company recognized reductions to R&D expense of $1,076,193 and $599,926 for the nine months ended September
30, 2024 and 2023, respectively.
Deferred
Offering Costs – The Company had capitalized qualified legal, accounting and other direct costs related to its efforts to raise
capital through the sale of its common stock under the July ATM Facility. Deferred offering costs were deferred and being amortized ratably
upon sales under the July ATM Facility to additional paid-in capital as a reduction of the July ATM proceeds. As a result of the Company
pausing the July ATM Facility, all of the remaining deferred offering costs were immediately amortized to additional paid-in capital
as a reduction to the proceeds received in the nine months ended September 30, 2024. As of September 30, 2024, no remaining amounts of
deferred offering costs were capitalized related to the July ATM Facility.
Stock-Based
Compensation – Stock-based compensation expense represents the estimated grant date fair value of the Company’s equity
awards, consisting of stock options issued under the Company’s stock option plan and restricted common stock (see Note 7). The
fair value of equity awards is recognized over the requisite service period of such awards (usually the vesting period) on a straight-line
basis. The Company estimates the fair value of stock options using the Black-Scholes option pricing model on the date of grant and recognizes
forfeitures as they occur. For stock awards for which vesting is subject to performance-based milestones, the expense is recorded over
the remaining service period after the point when the achievement of the milestone is probable, or the performance condition has been
achieved.
Research
and Development Costs – R&D costs are expensed as incurred. R&D costs consist primarily of clinical research fees paid
to consultants and outside service providers, other expenses relating to design, development and testing of the Company’s therapy
candidates, and for license and milestone costs related to in-licensed products and technology. Costs incurred in obtaining technology
licenses are charged to R&D expense if the technology licensed has not reached commercial feasibility and has no alternative future
use. Such licenses purchased by the Company require substantial completion of research and development, regulatory and marketing approval
efforts in order to reach commercial feasibility and have no alternative future use.
Clinical
trial costs are a component of R&D expenses. The Company estimates expenses incurred for clinical trials that are in process based
on services performed under contractual agreements with clinical research organizations and actual clinical investigators. Included in
the estimates are (1) the fee per patient enrolled as specified in the clinical trial contract with each institution participating in
the clinical trial and (2) progressive data on patient enrollments obtained from participating clinical trial sites and the actual services
performed. Changes in clinical trial assumptions, such as the length of time estimated to enroll all patients, rate of screening failures,
patient drop-out rates, number and nature of adverse event reports, and the total number of patients enrolled can impact the average
and expected cost per patient and the overall cost of the clinical trial. The Company monitors the progress of the trials and their related
activities and adjusts expense accruals, when applicable. Adjustments to accruals are charged to expense in the period in which the facts
give rise to the adjustments become known.
Other
Comprehensive Income (Loss) – Other comprehensive income (loss) includes foreign currency translation gains and losses. The
cumulative amount of translation gains and losses are reflected as a separate component of stockholders’ equity in the condensed
consolidated balance sheets, as accumulated other comprehensive income.
Foreign
Currency Translation and Transaction Gains (Losses) – The Company, and its wholly-owned subsidiary Nexcella, maintain their
accounting records in U.S. Dollars. The Company’s operating subsidiary, IBAPL, is located in Australia and maintains its accounting
records in Australian Dollars, which is its functional currency. Assets and liabilities of the subsidiary are translated into U.S. dollars
at exchange rates at the balance sheet date, equity accounts are translated at historical exchange rate and revenues and expenses are
translated by using the average exchange rates for the period. Translation adjustments are reported as a separate component of other
comprehensive income (loss) in the consolidated statements of operations and comprehensive loss. Foreign currency denominated transactions
are translated at exchange rates approximating those in effect at the transaction dates. Gains (losses) resulting from foreign currency
transactions are included in general and administrative expenses in the accompanying condensed consolidated statements of operations
and comprehensive loss and were $(2,849) and $8,095 for the three months ended September 30, 2024 and 2023, respectively, and $(22,326)
and $6,372 for the nine months ended September 30, 2024 and 2023, respectively.
Loss
Per Common Share - Basic loss per common share is computed by dividing net loss attributable to common stockholders by the weighted-average
number of common shares outstanding during the period. Diluted loss per common share is determined using the weighted-average number
of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents. In periods when losses
are reported, the weighted-average number of common shares outstanding excludes common stock equivalents because their inclusion would
be anti-dilutive. Basic weighted average shares outstanding for the three and nine months ended September 30, 2024 include 1,913,661
shares underlying Pre-Funded warrants to purchase common shares (See Note 7). As the shares underlying these Pre-Funded warrants can
be issued for nominal consideration (an exercise price per share equal to $0.0001 per share), these shares are deemed to be issued for
purposes of basic loss per common share. For the three and nine months ended September 30, 2024 and 2023, the Company’s potentially
dilutive shares, which were not included in the calculation of net loss per share, included stock options and warrants exercisable for
4,408,488 and 2,911,412 shares of common stock, respectively.
Property
and Equipment - Included in property and equipment is construction-in-progress which consists of manufacturing space improvements
and includes the costs of construction, machinery and equipment, and any interest charges arising from borrowings used to finance these
assets during the period of construction or installation of the assets. No provision for depreciation is made on construction-in-progress
until such time as the relevant assets are completed and ready for their intended use.
Estimated
useful lives of the Company’s assets are as follows:
Schedule
of Property and Equipment Useful Lives
| | |
Useful Life |
| Operating equipment | |
3-10 years |
| Electronic equipment | |
3-5 years |
| Office equipment | |
3-5 years |
The
cost and related accumulated depreciation of assets sold or otherwise retired are eliminated from the accounts, and any gain or loss
are included in the Company’s results of operations. The costs of maintenance and repairs are recognized to expenses as incurred;
significant renewals and betterments are capitalized.
Leases
- At the inception of a contract the Company determines if the arrangement is, or contains a lease. Operating lease right-of-use
(“ROU”) assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent
its obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement
date based on the present value of the lease payments over the lease term. Lease expense is recognized on a straight-line basis over
the lease term.
The
Company has made certain accounting policy elections whereby it (i) does not recognize ROU assets or lease liabilities for short-term
leases (those with original terms of 12-months or less) and (ii) separates lease and non-lease elements of its operating leases as separate
lease components. As of September 30, 2024 and December 31, 2023, the Company did not have any finance leases.
Recent
Accounting Pronouncements
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”)
2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires disclosure
of incremental segment information on an annual and interim basis. This ASU is effective for fiscal years beginning after December 15,
2023, and interim periods within fiscal years beginning after December 15, 2024, on a retrospective basis. The Company has implemented
this ASU effective January 1, 2024, and determined no retrospective changes were necessary.
In
December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures,
which expands the disclosures required for income taxes. This ASU is effective for fiscal years beginning after December 15, 2024,
with early adoption permitted. The amendment should be applied on a prospective basis while retrospective application is permitted. The
Company is currently evaluating the effect of this pronouncement on its disclosures.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Prior Agreements with Nexcella Subsidiary
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Prior Agreements With Nexcella Subsidiary |
|
| Prior Agreements with Nexcella Subsidiary |
Note
3 – Prior Agreements with Nexcella Subsidiary
Nexcella
Absorption
On
May 20, 2024, Nexcella, was merged (the “Merger”) with and into the Company, with the Company as the surviving corporation
(the “Nexcella Absorption”). The Merger was effected pursuant to Section 253 of the Delaware General Corporation Law (“DGCL”)
when the Company filed a Certificate of Ownership and Merger (“Certificate of Merger”) with the Secretary of State of the
State of Delaware. Immediately prior to the Merger, the Company owned greater than 95% of outstanding common stock on a fully diluted
basis of Nexcella, par value $0.0001 per share (the “Nexcella Shares”), and 100% of the outstanding shares of each other
class of capital stock of Nexcella. Under the DGCL, the only approval required was that of the Company’s Board of Directors for
the Merger to become effective. As a result of the Merger, Nexcella ceased to exist and all assets, operations and other property and
rights of Nexcella have been succeeded to by the Company. Pursuant to the terms of the Certificate of Merger, as a result of the Merger,
each of the outstanding Nexcella Shares (other than Nexcella Shares held by the Company) were converted into common stock of the Company
(“Company Merger Shares”). In connection with the Merger, the Company issued 989,876 shares of its common stock to the former
stockholders of Nexcella (other than shares held by the Company) (including Company common stock issued to third-party cash investors
in Nexcella) (the “Merger Shares”). The shares were issued on a pro-rata basis and as such resulted in no change in fair
value. In addition, the Company issued to the former participants in the Nexcella 2022 Equity Incentive Plan, 275,759 restricted stock
awards to receive common stock in the Company and options to purchase up to 595,676 shares of Company common stock at an exercise price
of $2.47 per share (the closing price on May 17, 2024), under the Company’s Amended and Restated 2021 Omnibus Equity Incentive
Plan. As such, as of May 20, 2024, the Founders Agreement and Management Services Agreement agreements listed below with Nexcella are
no longer in effect.
Founders
Agreement
Effective
December 8, 2022, the Company entered into a Founders Agreement with Nexcella (the “Nexcella Founders Agreement”).
The
Nexcella Founders Agreement provided that prior to a Qualified IPO (as defined in Nexcella’s Amended and Restated Certificate of
Incorporation, as amended (the “Nexcella COI”)) or Qualified Change in Control (as defined in the Nexcella COI), the Company
shall provide funds to Nexcella as requested by Nexcella, in good faith, to be evidenced by a senior unsecured promissory note. In exchange
for the time and capital expended in the formation of Nexcella and the identification of specific assets, the acquisition of which benefit
Nexcella, on December 21, 2022, the Company loaned Nexcella approximately $2.1 million, evidenced by a senior unsecured promissory note,
representing the up-front fee required to acquire Nexcella’s license agreement with Hadasit Medical Research Services & Development,
Ltd. (“HADASIT”) and BIRAD Research and Development Company Ltd. (“BIRAD”), and for use as working capital for
its research and development activities. The note, which had a maturity date of January 31, 2030, accrued interest at a rate of 7.875%
per annum and was convertible into shares of common stock of Nexcella at a conversion price of $2.00 per share, subject to adjustment;
provided, however, that such note shall automatically convert into shares of Nexcella common stock immediately prior to certain conversion
triggers set forth in the note. Nexcella may not prepay the note without the Company’s prior written consent. The note and accrued
interest were converted in full prior to the Nexcella Absorption. The Nexcella Founders Agreement had a term of 15 years, which, upon
expiration, would automatically renew for successive one-year periods unless terminated by the Company upon notice at least six months
prior to the end of the term or upon the occurrence of a Change of Control (as defined in the Nexcella Founders Agreement). In connection
with the Nexcella Founders Agreement, the Company was issued 250,000 shares of Nexcella’s Class A Preferred Stock, 1,000,000 shares
of Nexcella’s Class A Common Stock, and 5,000,000 shares of Nexcella’s common stock. The Class A Preferred Stock was identical
to the common stock other than as to conversion rights, the PIK Dividend right (as defined below) and voting rights.
Each
share of Class A Preferred Stock was convertible, at the Company’s option, into one fully paid and nonassessable share of Nexcella’s
common stock, subject to certain adjustments. As a holder of Nexcella’s Class A Preferred Stock, the Company received on each March
13 (each a “PIK Dividend Payment Date”) until the date all outstanding Class A Preferred Stock was converted into Nexcella’s
common stock or redeemed (and the purchase price is paid in full), pro rata per share dividends paid in additional fully paid and nonassessable
shares of Nexcella common stock (“PIK Dividends”) such that the aggregate number of shares of common stock issued pursuant
to such PIK Dividend was equal to 2.5% of Nexcella’s fully-diluted outstanding capitalization on the date that was one business
day prior to any PIK Dividend Payment Date. In addition, as a holder of Class A Preferred Stock, the Company was entitled to cast for
each share of Class A Preferred Stock held as of the record date for determining stockholders entitled to vote on matters presented to
the stockholders of Nexcella, the number of votes that was equal to 1.1 times a fraction, the numerator of which was the sum of (A) the
shares of outstanding Nexcella common stock and (B) the whole shares of Nexcella common stock into which the shares of outstanding Nexcella
Class A Common Stock and the Class A Preferred Stock were convertible and the denominator of which was the number of shares of outstanding
Nexcella Class A Preferred Stock.
Each
share of Class A Common Stock was convertible, at the Company’s option, into one fully paid and nonassessable share of Nexcella’s
common stock, subject to certain adjustments. In addition, upon a Qualified IPO (as defined in the Nexcella COI) or Qualified Change
in Control (as defined in the Nexcella COI), each share of Class A Common Stock would automatically convert into one fully paid and nonassessable
share of Nexcella’s common stock; provided however, if at that time, the Class A Common Stock was not then convertible into a number
of shares of Nexcella common stock (or such other capital stock or securities at the time issuable upon the conversion of the Class A
Common Stock) that have a value of: (a) in the case of a Qualified IPO, at least $5,000,000 based on the initial offering price in such
initial public offering, or (b) in the case of a Qualified Change in Control, at least $5,000,000 in cash or at least $5,000,000 of equity
based on the implied value of a share of Nexcella common stock resulting from the price paid upon the consummation of such Qualified
Change of Control, the Class A Common Stock would automatically convert into such number of shares of Nexcella common stock (or such
other capital stock or securities at the time issuable upon the conversion of the Class A Common Stock) that have a value of $5,000,000
based on the initial offering price in such initial public offering or the implied value of a share of Nexcella common stock resulting
from the price paid upon the consummation of such Qualified Change of Control (or if such Qualified Change of Control results in the
Class A Shares being exchanged solely for cash, then $5,000,000 in cash). The Company was entitled to cast such number of votes equal
to the number of whole shares of Nexcella common stock into which the Company’s Class A Common Stock was convertible as of the
record date for determining stockholders entitled to vote on matters presented to the stockholders of Nexcella.
In
addition to the foregoing, the Company was entitled to one vote for each share of Nexcella common stock held by it. Except as provided
by law or by the Nexcella COI, holders of Nexcella Class A Common Stock and Class A Preferred Stock shall vote together with the holders
of Nexcella common stock, as a single class.
As
additional consideration under the Nexcella Founders Agreement, Nexcella also agreed to: (i) pay an equity fee in shares of common stock,
payable within five business days of the closing of any equity or debt financing for Nexcella or any of its respective subsidiaries that
occurs after the effective date of the Nexcella Founders Agreement and ending on the date when the Company no longer has majority voting
control in Nexcella’s voting equity, equal to 2.5% of the gross amount of any such equity or debt financing; and (ii) pay a cash
fee equal to 4.5% of Nexcella’s annual Net Sales (as defined in the Nexcella Founders Agreement), payable on an annual basis, within
90 days of the end of each calendar year. In the event of a Change of Control, Nexcella agreed to pay a one-time change in control fee
equal to five times the product of (A) Net Sales for the 12 months immediately preceding the Change of Control and (B) 4.5%.
Management
Services Agreement
Effective
as of December 8, 2022, the Company entered into a Management Services Agreement (the “Nexcella MSA”) with Nexcella. Pursuant
to the terms of the Nexcella MSA, the Company rendered management, advisory and consulting services to Nexcella. Services provided under
the Nexcella MSA may include, without limitation, (i) advice and assistance concerning any and all aspects of Nexcella’s operations,
clinical trials, financial planning and strategic transactions and financings and (ii) conducting relations on behalf of Nexcella with
accountants, attorneys, financial advisors and other professionals (collectively, the “Services”). At the request of the
Company, Nexcella utilized clinical research services, medical education, communication and marketing services and investor relations/public
relation services of companies or individuals designated by the Company, provided those services are offered at market prices. In consideration
for the Services, Nexcella paid the Company an annual base management and consulting fee of $500,000 (the “Annual Consulting Fee”).
Notwithstanding the foregoing, the first Annual Consulting Fee payment was not due until the first business day of the calendar quarter
immediately following the completion of the first equity financing for Nexcella that was in excess of $10 million in gross proceeds,
which did not occur. Actual and direct out-of-pocket expenses reasonably incurred by the Company in performing the Services were reimbursed
to the Company by Nexcella.
The
Nexcella MSA was terminated on May 20, 2024 in connection with the Nexcella Absorption. In addition, as a result of the Nexcella Absorption,
the Class A Preferred Stock, Class A Common Stock, and the Founders Agreement cease to exist.
|
| X |
- DefinitionAgreements Disclosure [Text Block]
| Name: |
IMMX_AgreementsDisclosureTextBlock |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
IMMX_DisclosurePriorAgreementsWithNexcellaSubsidiaryAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Prepaid Expenses and Other Current Assets
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Prepaid Expenses And Other Current Assets |
|
| Prepaid Expenses and Other Current Assets |
Note
4 – Prepaid Expenses and Other Current Assets
Prepaid
expenses and other current assets consist of the following as of September 30, 2024 and December 31, 2023:
Schedule of Prepaid Expenses and Other Current Assets
| | |
September 30, 2024 | | |
December 31, 2023 | |
| Prepaid research and development expenses | |
$ | 486,912 | | |
$ | 412,773 | |
| Prepaid insurance expense | |
| 73,813 | | |
| 263,927 | |
| Prepaid investor relations expense | |
| 492,391 | | |
| 384,494 | |
| Other current assets | |
| 36,521 | | |
| 44,582 | |
| Total prepaid expenses and other current assets | |
$ | 1,089,637 | | |
$ | 1,105,776 | |
|
| X |
- References
+ Details
| Name: |
IMMX_DisclosurePrepaidExpensesAndOtherCurrentAssetsAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrepaid Expenses And Other Current Assets [Text Block]
| Name: |
IMMX_PrepaidExpensesAndOtherCurrentAssetsTextBlock |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Accounts Payable and Accrued Expenses
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Payables and Accruals [Abstract] |
|
| Accounts Payable and Accrued Expenses |
Note
5 – Accounts Payable and Accrued Expenses
Accounts
payable and accrued expenses consist of the following as of September 30, 2024 and December 31, 2023:
Schedule of Accounts Payable and Accrued Expenses
| | |
September 30, 2024 | | |
December 31, 2023 | |
| Accounts payable | |
$ | 3,288,579 | | |
$ | 1,433,022 | |
| Accrued research and development expenses | |
| 2,878,386 | | |
| 1,571,261 | |
| Accrued professional services | |
| 93,385 | | |
| 38,639 | |
| Accrued compensation and related expenses | |
| 198,095 | | |
| 577,854 | |
| Other accrued expenses | |
| 43,341 | | |
| 101,007 | |
| Total accounts payable and accrued expenses | |
$ | 6,501,786 | | |
$ | 3,721,783 | |
|
| X |
- DefinitionThe entire disclosure for accounts payable and accrued liabilities at the end of the reporting period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 720
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483384/720-30-45-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Property and Equipment
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Property, Plant and Equipment [Abstract] |
|
| Property and Equipment |
Note
6 – Property and Equipment
Property
and equipment at September 30, 2024 and December 31, 2023 consisted of:
Schedule
of Property and Equipment
| | |
September 30, 2024 | | |
December 31, 2023 | |
| Operating equipment | |
$ | 534,162 | | |
$ | 60,599 | |
| Office equipment | |
| 3,895 | | |
| 3,896 | |
| Total property and equipment, gross | |
| 538,057 | | |
| 64,495 | |
| Less: Accumulated depreciation | |
| (26,652 | ) | |
| (14,314 | ) |
| Property and equipment
excluding construction in progress | |
| 511,405 | | |
| 50,181 | |
| Construction in progress | |
| 844,019 | | |
| - | |
| Total property and equipment | |
$ | 1,355,424 | | |
$ | 50,181 | |
For
the nine months ended September 30, 2024 and 2023, depreciation expense amounted to $12,338 and $2,392, respectively. Depreciation is
not taken during the period of construction or equipment installation. Upon completion of the installation of manufacturing equipment
or any construction in progress, balances will be classified to their respective property and equipment category.
The
construction in progress of $844,019 as of September 30, 2024, represents the investment in building a biopharmaceutical processing facility
inside the leased property. The Company expects to complete the processing facility by the end of 2025.
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/360/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-7
| Name: |
us-gaap_PropertyPlantAndEquipmentDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Stockholders’ Equity
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Equity [Abstract] |
|
| Stockholders’ Equity |
Note
7 – Stockholders’ Equity
The
Company has authorized 200,000,000 shares of common stock and 10,000,000 shares of preferred stock, each with a par value of $0.0001
per share.
July
2023 ATM Sales Agreement
On
July 14, 2023, the Company entered into the July Sales Agreement with the Sales Agent pursuant to which the Company may offer and sell,
from time to time, through the Sales Agent, shares (the “July Shares”) of the Company’s common stock, par value $0.0001
per share, subject to the terms and conditions set forth in the July Sales Agreement. Initially, the Company is eligible to sell up to
$4,200,000 worth of shares of its common stock as the aggregate market value of the Company’s shares of common stock eligible for
sale under the July Sales Agreement is subject to the limitations of General Instruction I.B.6 of Form S-3 until such time that the Company’s
public float equals or exceeds $75.0 million. In the event the aggregate market value of the Company’s outstanding common stock
held by non-affiliates equals or exceeds $75.0 million, then the one-third limitation on sales set forth in General Instruction I.B.6
of Form S-3 shall not apply to additional sales made pursuant to the July Sales Agreement. The July Shares will be offered and sold pursuant
to the Company’s prospectus supplement, dated July 14, 2023, filed by the Company with the SEC on July 14, 2023, including the
accompanying base prospectus forming a part of the Company’s Registration Statement on Form S-3 (File No. 333-269100) filed by
the Company with the SEC on January 3, 2023 and declared effective by the SEC on January 11, 2023.
Under
the July Sales Agreement, the Sales Agent may sell the July Shares in sales deemed to be “at-the-market offerings” as defined
in Rule 415(a)(4) promulgated under the Securities Act, including sales made directly on or through The Nasdaq Capital Market or any
other existing trading market for the Company’s common stock, in negotiated transactions at market prices prevailing at the time
of sale or at prices related to such prevailing market prices, and/or any other method permitted by law. The Company may instruct the
Sales Agent not to sell any July Shares if the sales cannot be effected at or above the price designated by the Company from time to
time.
The
Company will pay the Sales Agent a fixed commission rate of 3.75% of the aggregate gross proceeds from the sale of the July Shares pursuant
to the July Sales Agreement. The Company has paid an expense deposit of $15,000 to the Sales Agent, which will be applied against the
actual out-of-pocket accountable expenses that will be paid by the Company to the Sales Agent in connection with the offering. The Company
has agreed to reimburse the Sales Agent for all expenses related to the offering including, without limitation, the fees and expenses
of the Sales Agent’s legal counsel up to $50,000, and shall reimburse the Sales Agent, upon request, for such costs, fees and expenses
in an amount not to exceed $7,500 on a quarterly basis for the first three fiscal quarters of each year and $10,000 for the fiscal fourth
quarter of each year. The Company has also agreed to provide indemnification and contribution to the Sales Agent with respect to certain
liabilities, including liabilities under the Securities Act.
During
the nine months ended September 30, 2024, the Company sold a total of 68,302 shares of its common stock under the July ATM Facility for
aggregate net proceeds of $338,495 after deducting commissions and SEC fees, and charging $87,229 of deferred offering costs against
the proceeds. On February 5, 2024, the Company suspended, and is not offering any shares of its common stock pursuant to, the prospectus
supplement dated July 14, 2023, relating to the July Sales Agreement by and between the Company and ThinkEquity LLC. The Company will
not make any sales of common stock pursuant to the July Sales Agreement unless and until a new prospectus supplement is filed with the
SEC; however, the July Sales Agreement remains in full force and effect.
Common
Stock Issuance – Public Offering
On
February 5, 2024, the Company entered into an Underwriting Agreement (the “Agreement”) with Titan Partners Group LLC, a division
of American Capital Partners, LLC (the “Underwriter”), relating to an underwritten offering (the “Offering”)
of 5,535,055 shares of common stock of the Company. The public offering price was $2.71 per share of Common Stock and the Underwriter
agreed to purchase the Common Stock pursuant to the Underwriting Agreement at a price of $2.5203 per share. On February 8, 2024, the
Company closed the offering and received net proceeds of $13,565,760, after deducting underwriting discounts and commissions and estimated
offering expenses. Pursuant to the Agreement, the Company granted the Underwriter a 30-day over-allotment option to purchase up to an
additional 783,970 shares of Common Stock which was exercised in full on March 1, 2024, for net proceeds of $1,954,594, after deducting
underwriting discounts and offering expenses.
Other
Common Stock Issuances
During
the nine months ended September 30, 2024, the Company issued 75,007 shares of restricted common stock valued at $202,500 for investor
relations services based on the average closing price for the prior 10 trading days pursuant to a marketing services agreement entered
into on July 25, 2023.
During
the nine months ended September 30, 2024, the Company issued 104,758 shares of restricted common stock valued at $317,500 for investor
relations services based on the closing price pursuant to the extensions of marketing services agreements.
During
the nine months ended September 30, 2024, the Company issued 1,251 shares of common stock upon the exercise of certain common stock options
for cash proceeds of $2,489.
During
the year ended December 31, 2023, the Company entered into various marketing services agreements, whereby the Company agreed to issue
122,300 shares of its common stock, valued at $247,500, in exchange for future services. As of December 31, 2023, the Company has issued
122,300 shares of the Company’s common stock pursuant to the marketing services agreements. During the year ended December 31,
2023, the Company recorded stock-based compensation expense of $232,624 related to the fair value of the shares of common stock. During
the nine months ended September 30, 2024, the Company recorded stock-based compensation expense of $14,876 related to the amortization
of the fair value of the 122,300 shares of common stock issued in 2023. As of September 30, 2024, the Company has $0 of unamortized stock-based
compensation remaining to be amortized over the remaining service period.
Restricted
Stock Awards
Pursuant
to the Merger, the Company issued to the former participants in the Nexcella 2022 Equity Incentive Plan, 275,759 restricted stock awards
to receive common stock in the Company. The shares were issued on a pro-rata basis and resulted in no change in fair value.
During
the nine months ended September 30, 2024, the Company recorded stock-based compensation expense of $263,962 related to the total fair
value of the previously issued restricted stock awards, which was included in general and administrative expenses. The unrecognized stock-based
compensation expense of $417,163 related to unvested restricted common stock is expected to be recognized over the remaining vesting
period of 0.62 years. As of September 30, 2024, 93,895 shares of restricted common stock have vested with the remaining 181,864 restricted
shares to vest over the vesting period of 0.62 years.
Stock
Options
In
2016, the Board of Directors of the Company approved the Immix Biopharma, Inc. 2016 Equity Incentive Plan (the “2016 Plan”).
The 2016 Plan allows for the Board of Directors to grant various forms of incentive awards covering up to 417,120 shares of common stock.
During the year ended December 31, 2021, the Board of Directors amended the 2016 Plan to increase the aggregate number of shares available
for issuance under the 2016 Plan to 1,761,120 shares of common stock. On September 10, 2021, the Board of Directors approved the 2021
Equity Incentive Plan (as amended and restated, the “2021 Plan”) pursuant to which it initially reserved and made available
for future issuance under the 2021 Plan (i) 900,000 shares of common stock, plus (ii) the number of shares of common stock reserved,
but unissued under the 2016 Plan, and (iii) the number of shares of common stock underlying forfeited awards under the 2016 Plan, provided
that shares of common stock issued under the 2021 Plan with respect to an Exempt Award (as defined in the 2021 Plan) would not count
against such share limit. Subsequent to September 10, 2021, no further awards are to be issued under the 2016 Plan, but all awards under
the 2016 Plan which were outstanding as of September 10, 2021 (including any Grandfathered Arrangement (as defined in the 2021 Plan))
shall continue to be governed by the terms, conditions and procedures set forth in the 2016 Plan and any applicable award agreement.
On
April 24, 2023, the Company’s Board of Directors adopted the Immix Biopharma, Inc. Amended and Restated 2021 Omnibus Equity Incentive
Plan (the “Amended 2021 Plan”) which, among other things, increased the number of shares of common stock that may be issued
under such plan by 1,034,561 shares, subject to stockholder approval. On June 7, 2023, stockholders of the Company approved the Amended
2021 Plan. On April 18, 2024, our Board of Directors approved amendments to the 2021 Plan (the “2nd Amended 2021 Plan”)
to (i) increase the number of shares of common stock available for issuance under the 2021 Plan by 3,000,000 to a total share reserve
of 4,934,561 and (ii) the adoption of an evergreen provision to the 2021 Plan to provide for an automatic annual increase in the shares
of common stock available for issuance under the 2021 Plan over the next ten years (the “2021 Plan Amendments”). Pursuant
to the evergreen provision, the number of shares available for issuance under the 2021 Plan shall automatically increase on January 1st
of each year for a period of ten years, commencing on January 1, 2025 and ending on (and including) January 1, 2034, in an amount equal
to five percent (5%) of the total number of shares of Common Stock outstanding on December 31st of the preceding calendar year. On June
11, 2024, stockholders of the Company approved the 2nd Amended 2021 Plan. As of September 30, 2024, there were 2,265,757 shares
of the Company’s common stock remaining to be issued under the Amended 2021 Plan.
In
addition, the Company issued to the former participants in the Nexcella 2022 Equity Incentive Plan, options to purchase up to 595,676
shares of Company common stock at an exercise price of $2.47 per share (the closing price on May 17, 2024), under the Company’s
Amended and Restated 2021 Omnibus Equity Incentive Plan. The options were issued on a pro-rata basis and resulted in no change in fair
value.
During
the nine months ended September 30, 2024, the Compensation Committee of the Board of Directors approved the issuance of options to purchase
198,000 shares of the Company’s common stock to non-employee members of the Board of Directors of the Company and 680,000 shares
of the Company’s common stock to management of the Company. The options have a term of 10 years, an exercise price of $2.04 per
share and vest over periods of 12 to 48 equal monthly installments.
During
the nine months ended September 30, 2024, the Board of Directors approved the issuance of options to purchase 43,500 shares of the Company’s
common stock to employees of the Company with a term of 10 years and exercise prices ranging from $2.11 - $2.17 per share, which options
vest in 48 equal monthly installments.
The
Company recognized stock-based compensation of $450,299 and $171,454 related to stock options for the three months ended September 30,
2024 and 2023 and $965,600 and $507,017 related to stock options for the nine months ended September 30, 2024 and 2023, respectively,
which is included in general and administrative expenses.
As
of September 30, 2024, the Company had unrecognized stock-based compensation expense of $3,153,545, related to unvested stock options,
which is expected to be recognized over the weighted-average vesting period of 2.61 years.
The
following table summarizes the stock option activity for the nine months ended September 30, 2024:
Schedule of Stock Option Activity
| | |
Options | | |
Weighted- Average Exercise Price Per Share | |
| Outstanding, January 1, 2024 | |
| 2,512,561 | | |
$ | 1.92 | |
| Granted | |
| 1,517,176 | | |
$ | 2.21 | |
| Exercised | |
| (834 | ) | |
$ | 1.95 | |
| Forfeited | |
| (17,915 | ) | |
$ | 1.95 | |
| Expired | |
| - | | |
$ | - | |
| Outstanding and expected to vest, September 30, 2024 | |
| 4,010,988 | | |
$ | 2.03 | |
The
following table discloses information regarding outstanding and exercisable options at September 30, 2024:
Schedule of Stock Outstanding and Exercisable
| | | |
Outstanding | | |
Exercisable | |
| Exercise Price Range | | |
Number of Option Shares | | |
Weighted Average Exercise Price | | |
Weighted Average Remaining Life (Years) | | |
Number of Option Shares | | |
Weighted Average Exercise Price | |
| $ | 0.00-1.00 | | |
| 256,500 | | |
$ | 0.80 | | |
| 6.45 | | |
| 256,500 | | |
$ | 0.80 | |
| $ | 1.01-2.00 | | |
| 1,646,062 | | |
$ | 1.81 | | |
| 7.13 | | |
| 1,060,836 | | |
$ | 1.78 | |
| $ | 2.01-3.00 | | |
| 2,097,176 | | |
$ | 2.33 | | |
| 9.13 | | |
| 751,854 | | |
$ | 2.50 | |
| $ | 3.01-6.00 | | |
| 11,250 | | |
$ | 5.83 | | |
| 7.29 | | |
| 7,500 | | |
$ | 5.83 | |
| | | | |
| 4,010,988 | | |
$ | 2.03 | | |
| 8.13 | | |
| 2,076,690 | | |
$ | 1.93 | |
Aggregate
intrinsic value is calculated as the difference between the exercise price of the underlying stock option and the fair value of the Company’s
common stock for stock options that were in-the-money at period end. As of September 30, 2024, the aggregate intrinsic value for the
options vested and outstanding was $200,675.
The
total intrinsic value of stock options exercised during the nine months ended September 30, 2024, was $3,069.
Stock
Warrants
The
following table summarizes the stock warrant activity for the nine months ended September 30, 2024:
Schedule of Stock Warrant Activity
| | |
Warrants | | |
Weighted-Average
Exercise Price Per
Share | |
| Outstanding and exercisable, January 1, 2024 | |
| 2,311,161 | | |
$ | 0.71 | |
| Granted | |
| - | | |
$ | - | |
| Exercised | |
| - | | |
$ | - | |
| Forfeited | |
| - | | |
$ | - | |
| Expired | |
| - | | |
$ | - | |
| Outstanding and exercisable, September 30, 2024 | |
| 2,311,161 | | |
$ | 0.71 | |
The
following table discloses information regarding outstanding and exercisable warrants at September 30, 2024:
Schedule of Stock Outstanding and Exercisable
| | | |
Outstanding | | |
Exercisable | |
| Exercise Price | | |
Number of Warrant Shares | | |
Weighted Average Exercise Price | | |
Weighted Average Remaining Life (Years) | | |
Number of Warrant Shares | | |
Weighted Average Exercise Price | |
| $ | 0.0001 | | |
| 1,913,661 | | |
$ | 0.0001 | | |
| - | | |
| 1,913,661 | | |
$ | 0.0001 | |
| $ | 0.80 | | |
| 156,000 | | |
$ | 0.80 | | |
| 6.48 | | |
| 156,000 | | |
$ | 0.80 | |
| $ | 6.25 | | |
| 241,500 | | |
$ | 6.25 | | |
| 2.21 | | |
| 241,500 | | |
$ | 6.25 | |
| | | |
| 2,311,161 | | |
$ | 0.71 | | |
| 0.67 | | |
| 2,311,161 | | |
$ | 0.71 | |
Aggregate
intrinsic value is calculated as the difference between the exercise price of the underlying stock warrant and the fair value of the
Company’s common stock for stock warrants that were in-the-money at period end. As of September 30, 2024, the intrinsic value for
the warrants vested and outstanding was $2,958,804.
Nexcella
Equity Transactions
The
Nexcella 2022 Equity Incentive Plan (the “2022 Plan”) allows for Nexcella’s Board of Directors to grant various forms
of incentive awards initially covering up to 375,000 shares of common stock. On May 29, 2023, Nexcella’s Board of Directors approved
the Second Amended and Restated Nexcella 2022 Equity Incentive Plan, which increased to the number of shares of Nexcella common stock
issuable under the plan from 375,000 shares to 607,640 shares. On August 11, 2023, Nexcella’s Board of Directors requested the
Third Amended and Restated 2022 Equity Incentive Plan, which increased the number of shares of Nexcella common stock issuable under the
plan from 607,640 to 800,000 shares. The Nexcella shareholders subsequently approved the increase in Nexcella common stock issuable under
the plan to 800,000 shares. On May 17, 2024, upon absorption into the Company, the 2022 Plan ceased to exist.
Common
stock
On
March 13, 2024, pursuant to the terms of the Founders Agreement, Nexcella issued 238,220 shares of common stock to the Company as a PIK
Dividend based on the total dilutive shares of Nexcella outstanding as of March 12, 2024.
Restricted
Stock Awards
During
the three and nine months ended September 30, 2024, the Company recorded stock-based compensation expense of $0 and $402,163, respectively,
related to the total fair value of the previously issued restricted stock awards, which was included in general and administrative expenses.
Pursuant to the Merger, the Company issued to the former participants in the Nexcella 2022 Equity Incentive Plan, 275,759 restricted
stock awards to receive common stock in the Company. The shares were issued on a pro-rata basis and resulted in no change in fair value.
As a result, there was no remaining unvested stock-based compensation expense under Nexcella.
Stock
Options
The
Company recognized stock-based compensation of $0 and $148,319 related to stock options for the three and nine months ended September
30, 2024, respectively, which is included in general and administrative expenses. Pursuant to the Merger, the Company issued to the former
participants in the Nexcella 2022 Equity Incentive Plan, options to purchase up to 595,676 shares of Company common stock under the Company’s
Amended and Restated 2021 Omnibus Equity Incentive Plan. The options were issued on a pro-rata basis and resulted in no change in fair
value. As a result, there was no remaining unvested stock-based compensation expense under Nexcella.
The
following table summarizes the stock option activity for the nine months ended September 30, 2024 for Nexcella:
Schedule of Stock Option Activity
| | |
Options | | |
Weighted- Average Exercise Price Per Share | |
| Outstanding and exercisable, January 1, 2024 | |
| 186,528 | | |
$ | 6.49 | |
| Granted | |
| - | | |
$ | - | |
| Exercised | |
| - | | |
$ | - | |
| Forfeited | |
| (186,528 | ) | |
$ | 6.49 | |
| Expired | |
| - | | |
$ | - | |
| Outstanding and expected to vest, September 30, 2024 | |
| - | | |
$ | - | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Licenses Acquired
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Licenses Acquired |
|
| Licenses Acquired |
Note
8 – Licenses Acquired
Research
and License Agreement with HADASIT and BIRAD
On
December 8, 2022, Nexcella entered into a Research and License agreement with HADASIT and BIRAD (collectively, the “Licensors”)
to acquire intellectual property rights pertaining to CAR-T (the “H&B License”). Pursuant to the H&B License, Nexcella
paid the Licensors an upfront license fee of $1.5 million in December 2022 (included in research and development expenses on the consolidated
statements of operations and comprehensive loss). Additional quarterly payments totaling approximately $13 million related to the Company’s
ongoing support of the CAR-T clinical trials currently ongoing at HADASIT, are due through September 2026, along with an annual license
fee of $50,000. Future royalty payments of 5% are due on net sales of licensed products, combined with sales milestone payments in the
aggregate amount of up to $20 million when annual net sales reach certain thresholds for each licensed product. The royalties for each
licensed product on a country-to-country basis are to be paid through the latter of (a) the expiration of the last-to-expire valid claim
under a licensed patent (if any) in such country; (b) the date of expiration of any other Exclusivity Right (as defined in the H&B
License) or data protection period granted by a regulatory or other governmental authority with respect to a licensed product that provides
exclusivity in the relevant country; or (c) the end of a period of 15 years from the date of the First Commercial Sale (as defined in
the H&B License) of the applicable Licensed Product (as defined in the H&B License) in such country. The H&B License remains
with the Company after the Nexcella Absorption.
During
the nine months ended September 30, 2024 and 2023, the Company recorded R&D expenses of $2,393,063 and $1,929,601, respectively,
related to the license agreement.
Patent
License Agreement with U.S. Medical Research Foundation
In
August 2024, the Company entered into a Patent License Agreement (“License Agreement”) with a U.S. medical research foundation
pursuant to which the Company was granted certain exclusive and nonexclusive licenses and sublicenses to intellectual and tangible property
for the development and commercialization of cell therapy products (“Licensed Products”). Pursuant to the terms of the License
Agreement, the Company shall pay an up-front payment in three installments of $500,000, with the first installment due concurrent with
the signing of the agreement and the second and third installments due in January and July 2025, respectively. Under the license agreement,
the Company must also pay a mid-single-digit net licensed product sales royalty, and milestone payments corresponding with the initiation
and completion of Phase II studies in the amounts of $1.5 million and $2 million, respectively, as well as a $10 million milestone payment
at the initiation of Phase III studies and a $13.5 million dollar milestone payment in the event of first commercial sale of a licensed
product. To date, no amounts have been paid under this license agreement.
|
| X |
- References
+ Details
| Name: |
IMMX_DisclosureLicensesAcquiredAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLicenses Acquired [Text Block]
| Name: |
IMMX_LicensesAcquiredTextBlock |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
CIRM Grants
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Cirm Grants |
|
| CIRM Grants |
Note
9 - CIRM Grants
On
July 25, 2024, the Company was awarded an $8
million grant from the California Institute for Regenerative Medicine to support the clinical development of chimeric antigen
receptor T-cell therapy NXC-201 for the treatment of relapsed/refractory AL Amyloidosis. The award is payable to the Company upon
achievement of milestones that are primarily based on patient enrollment in the Company’s clinical trials. Additionally, if
CIRM determines, in its sole discretion, that the Company has not complied with the terms and conditions of the grant, CIRM may
suspend or permanently cease disbursements. Funds received under this grant may only be used for allowable project costs
specifically identified with the CIRM-funded project. Such costs can include, but are not limited to, salary for personnel, itemized
supplies, consultants, and itemized clinical study costs. Under the terms of the grant, both CIRM and the Company will co-fund the
research project and the amount of the Company’s co-funding requirement is predetermined as a part of the award. The Company
signed the grant agreement in November 2024 and expects to begin receiving funds from the grant beginning in November of
2024.
|
| X |
- DefinitionCIRM Grants Disclosure [Text Block]
| Name: |
IMMX_CIRMGrantsDisclosureTextBlock |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
IMMX_DisclosureCirmGrantsAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Leases
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Leases |
|
| Leases |
Note
10 – Leases
In
January 2024, the Company entered into a long-term operating lease agreement for 14,000 square feet of biopharmaceutical manufacturing
space in California under a non-cancelable operating lease that expires in December 2033. Under the terms of the lease, the Company is
required to pay monthly base rents ranging from $11,900 to $16,218, and pay its proportionate share of property taxes, insurance and
normal maintenance costs. The lease agreement includes two options to extend the lease for a term of five years each.
The
components of lease cost for operating leases, which are recorded in general and administrative expenses in the accompanying condensed
consolidated statement of operations, for the three and nine months ended September 30, 2024 were as follows:
Schedule of Lease Cost for Operating Leases
| | |
Three Months Ended September 30, 2024 | | |
Nine Months Ended September 30, 2024 | |
| Operating lease cost | |
$ | 42,150 | | |
$ | 126,450 | |
| Short-term lease cost | |
| 12,196 | | |
| 44,020 | |
| Total lease cost | |
$ | 54,346 | | |
$ | 170,470 | |
The
following table summarizes the lease-related assets and liabilities recorded in the consolidated balance sheets at September 30, 2024:
Schedule of Lease Related Assets and Liabilities
| | |
September 30, 2024 | |
| Operating Leases | |
| - | |
| Operating lease right-of-use assets | |
$ | 1,010,205 | |
| Right of use liability operating lease current portion | |
$ | 62,715 | |
| Right of use liability operating lease long term | |
| 1,026,340 | |
| Total operating lease liabilities | |
$ | 1,089,055 | |
The
Company utilizes the incremental borrowing rate in determining the present value of lease payments unless the implicit rate is readily
determinable. The Company estimated its incremental borrowing rate to be 8%. The lease has a remaining term of 9.25 years and an implicit
weighted average interest rate of 8%.
The
following table provides the maturities of lease liabilities at September 30, 2024:
Schedule of Maturity Lease Liability
| | |
Operating | |
| | |
Leases | |
| 2024 (remaining 3 months) | |
$ | 35,700 | |
| 2025 | |
| 147,798 | |
| 2026 | |
| 152,971 | |
| 2027 | |
| 158,325 | |
| 2028 and thereafter | |
| 1,073,349 | |
| Total future undiscounted lease payments | |
| 1,568,143 | |
| Less: Interest | |
| (479,088 | ) |
| Present value of lease liabilities | |
$ | 1,089,055 | |
|
| X |
- References
+ Details
| Name: |
IMMX_DisclosureLeasesAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Commitments and Contingencies
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
Note
11 – Commitments and Contingencies
Indemnifications
In
the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties
and may provide for indemnification of the counterparty. The Company’s exposure under these agreements is unknown because it involves
claims that may be made against it in the future, but have not yet been made. To date, the Company has not been subject to any claims
or been required to defend any action related to its indemnification obligations.
The
Company indemnifies each of its directors and officers for certain events or occurrences, subject to certain limits, while the director
is or was serving at the Company’s request in such capacity, as permitted under Delaware law and in accordance with its certificate
of incorporation and bylaws. The term of the indemnification period lasts as long as the director or officer may be subject to any proceeding
arising out of acts or omissions of such individual in such capacity. The maximum amount of potential future indemnification is unlimited.
The Company believes that the fair value of these indemnification obligations is minimal. Accordingly, the Company has not recognized
any liabilities relating to these obligations as of September 30, 2024.
Legal
Proceedings
From
time to time the Company may be involved in claims that arise during the ordinary course of business. Although the results of litigation
and claims cannot be predicted with certainty, the Company does not currently have any pending litigation to which it is a party or to
which its property is subject that it believes to be material. Regardless of the outcome, litigation can be costly and time consuming,
and it can divert management’s attention from important business matters and initiatives, negatively impacting the Company’s
overall operations.
Employment
Agreements
On
June 18, 2021, the Company entered into an Employment Agreement with Ilya Rachman (as amended, the “Rachman Employment Agreement”),
effective for a three-year term, subject to the terms of the agreement which provide that unless the Company and Dr. Rachman have otherwise
agreed in writing, if Dr. Rachman continues to work for the Company after the expiration of the term (which he has), his employment shall
be under the same terms and conditions provided for in the Rachman Employment Agreement, except that his employment will be on an “at
will” basis and the provisions of the agreement allowing for Dr. Rachman to terminate the agreement for “good reason”
and for Dr. Rachman to be paid severance in the event his employment is terminated by the Company without cause or by Dr. Rachman for
good reason will no longer apply, and the Rachman Employment Agreement currently remains in effect pursuant to such terms. Pursuant to
the Rachman Employment Agreement, the Company employs Dr. Rachman as Chief Executive Officer and Dr. Rachman was entitled to a base salary
of $360,000 annually. Dr. Rachman was also entitled to a performance-based bonus of 100% of the base salary (subject to, and determined
by, the Board in its sole discretion) plus additional performance bonuses to be determined by the Board. On November 9, 2022 and May
12, 2023, the Company entered into amendments to the Rachman Employment Agreement dated as of June 18, 2021 pursuant to which (i) Dr.
Rachman’s annual base salary was increased to $425,000 and $446,000, retroactive as of January 1, 2022 and 2023, respectively and
on November 9, 2023, and (ii) the agreement was amended to entitle Dr. Rachman to a performance-based bonus of up to 50% of his base
salary (subject to, and determined by, the Board in its sole discretion) plus additional performance bonuses to be determined by the
Board. On February 6, 2024, the Compensation Committee of the Board of Directors approved an increase in the annual base salary and on
May 9, 2024, the Company entered into an amendment to the Rachman Employment Agreement pursuant to which Dr. Rachman’s annual base
salary was increased to $475,000, effective January 1, 2024. Dr. Rachman’s employment agreement contains provisions for the protection
of the Company’s intellectual property and contains non-compete restrictions in the event of his termination other than by the
Company without “cause” or by Dr. Rachman with “good reason” (generally imposing restrictions on (i) employment
or consultation with competing companies or customers, (ii) recruiting or hiring employees for a competing company and (iii) soliciting
or accepting business from our customers for a period of six months following termination). Pursuant to the Rachman Employment Agreement,
Dr. Rachman may serve as a consultant to, or on board of directors of, or in any other capacity to, other companies provided that they
will not interfere with the performance of his duties to the Company. The full amount of the base salary and any bonus payments are included
in general and administrative expenses.
On
March 18, 2021, the Company entered into a Management Services Agreement with Alwaysraise LLC, an entity which Gabriel Morris, the Company’s
Chief Financial Officer and a member of the Board, is sole member, which was amended effective June 18, 2021 (as amended, the “Morris
MSA”). The Morris MSA had an initial two-year term, automatically renewable thereafter for successive one year terms unless terminated
by either party, and currently has a term through March 18, 2025. Pursuant to the Morris MSA, the Company employs Mr. Morris as Chief
Financial Officer and Mr. Morris was entitled to a base salary of $240,000 annually beginning in December 2021 ($120,000 annually prior).
Mr. Morris was also entitled to a performance-based bonus of 100% of the base salary (subject to, and determined by, the Board in its
sole discretion) plus additional performance bonuses to be determined by the Board. On November 9, 2022 and May 12, 2023, the Company
entered into amendments to the Morris MSA dated as of March 24, 2021, pursuant to which (i) Mr. Morris’ annual base salary was
increased to $425,000 and $446,000, retroactive as of January 1, 2022 and 2023, respectively, and on November 9, 2023, and (ii) Mr. Morris
is entitled to a performance-based bonus of up to 50% of his base salary (subject to, and determined by, the Board in its sole discretion)
plus additional performance bonuses to be determined by the Board. Unless terminated by the Company without “cause” or by
Alwaysraise LLC (as such terms are defined in the Morris MSA), upon termination, Mr. Morris will be entitled only to his base salary
through the date of termination, valid expense reimbursements and unused vacation pay. If terminated by the Company without “cause,”
he is entitled to be paid his base salary through the end of the term at the rate of 150%, valid expense reimbursements and accrued but
unused vacation pay. On February 6, 2024, the Compensation Committee of the Board of Directors approved an increase in annual base salary,
and on May 9, 2024, the Company entered into an amendment to the Morris MSA pursuant to which Mr. Morris’ annual base salary was
increased to $475,000, effective January 1, 2024. The Morris MSA contains provisions for the protection of the Company’s intellectual
property and confidential information. The full amount of the base salary and any bonus payments are included in general and administrative
expenses.
On
June 24, 2021, the Company issued an offer letter to Graham Ross Oncology Consulting Services Ltd., a United Kingdom company, of which
Graham Ross, the Company’s Acting Chief Medical Officer and Head of Clinical Development is the sole member, regarding Dr. Ross’s
provision of consultative services to the Company (the “Offer Letter”). Pursuant to the Offer Letter (signed by Dr. Ross
on June 24, 2021), Dr. Ross is entitled to an hourly rate for his consulting services and an option grant. On June 24, 2021, the Company
also signed a mutual confidentiality and non-disclosure agreement with Graham Ross Oncology Consulting Services Ltd.
Collaboration
Agreement
In
August 2021, the Company entered into a Clinical Collaboration and Supply Agreement with BeiGene Ltd. (“BeiGene”) for a combination
Phase 1b clinical trial in solid tumors of IMX-110 and anti-PD-1 Tislelizumab (the subject of a collaboration and license agreement among
BeiGene and Novartis). Under the terms of the agreement, the Company will conduct the combination trial. The cost of Tislelizumab manufacture
and supply (including shipping, taxes and duty if applicable and any third-party license payments that may be due) will be solely borne
by BeiGene. To date, no amounts have been paid to BeiGene.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 405
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/405-30/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/450/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478522/954-440-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Subsequent Events
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent Events |
Note
12 – Subsequent Events
Common
Stock Issuance – Marketing Services Agreements
Subsequent
to September 30, 2024, the Company issued 27,062 shares of restricted common stock valued at $45,000 for investor relations services
based on the average closing price for the prior 10 trading days pursuant to a marketing services agreement entered into on July 25,
2023.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Summary of Significant Accounting Policies (Policies)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Basis of Presentation |
Basis
of Presentation - The accompanying condensed consolidated financial statements and related notes have been prepared in accordance
with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and in accordance with the rules
and regulations of the United States Securities and Exchange Commission (the “SEC”). The Company’s fiscal year end
is December 31.
The
condensed consolidated financial statements and related disclosures as of September 30, 2024, and for the three and nine months ended
September 30, 2024 and 2023 are unaudited, pursuant to the rules and regulations of the SEC. Certain information and footnote disclosures
normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted pursuant to such rules
and regulations. In the Company’s opinion, these unaudited condensed consolidated financial statements include all adjustments
(consisting only of normal recurring adjustments) necessary for the fair statement of the results for the interim periods. These unaudited
condensed consolidated financial statements should be read in conjunction with the audited financial statements of the Company for the
years ended December 31, 2023 and 2022 which are included in the Company’s Annual Report on Form 10-K filed with the SEC on March
29, 2024. The results of operations for the three and nine months ended September 30, 2024, are not necessarily indicative of the results
to be expected for the full year ending December 31, 2024.
|
| Risk and Uncertainties |
Risk
and Uncertainties - The Company operates in a dynamic and highly competitive industry and is subject to risks and uncertainties common
to early-stage companies in the biotechnology industry, including, but not limited to, development by competitors of new technological
innovations, protection of proprietary technology, dependence on key personnel, contract manufacturers and contract research organizations,
compliance with government regulations and the need to obtain additional financing to fund operations. Product candidates currently under
development will require significant additional research and development efforts, including extensive preclinical studies and clinical
trials and regulatory approval, prior to commercialization. These efforts require significant amounts of additional capital, adequate
personnel infrastructure and extensive compliance and reporting. The Company believes that changes in any of the following areas could
have a material adverse effect on the Company’s future financial position, results of operations, or cash flows; ability to obtain
future financing; advances and trends in new technologies and industry standards; results of clinical trials; regulatory approval and
market acceptance of the Company’s products; development of sales channels; certain strategic relationships; litigation or claims
against the Company based on intellectual property, patent, product, regulatory, or other factors; and the Company’s ability to
attract and retain employees necessary to support its growth.
Products
developed by the Company require approvals from the U.S. Food and Drug Administration (“FDA”) or other international regulatory
agencies prior to commercial sales. There can be no assurance that the Company’s research and development will be successfully
completed, that adequate protection for the Company’s intellectual property will be obtained or maintained, that the products will
receive the necessary approvals, or that any approved products will be commercially viable. If the Company is denied approval, approval
is delayed, or the Company is unable to maintain approval, it could have a material adverse impact on the Company. Even if the Company’s
product development efforts are successful, it is uncertain when, if ever, the Company will generate revenue from product sales. The
Company operates in an environment of rapid change in technology and substantial competition from other pharmaceutical and biotechnology
companies. In addition, the Company is dependent upon the services of its employees, consultants and other third parties.
The
Company has expended and plans to continue to expend substantial funds to complete the research, development and clinical testing of
product candidates. The Company also will be required to expend additional funds to establish commercial-scale manufacturing arrangements
and to provide for the marketing and distribution of products that receive regulatory approval. The Company may require additional funds
to commercialize its products. The Company is unable to entirely fund these efforts with its current financial resources and will need
to raise additional funding in the future. If adequate funds are unavailable on a timely basis from operations or additional sources
of financing, the Company may have to delay, reduce the scope of or eliminate one or more of its research or development programs which
may materially and adversely affect its business, financial condition and operations.
|
| Use of Estimates |
Use
of Estimates – The preparation of these condensed consolidated financial statements in conformity with U.S. GAAP requires management
to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements
and the reported amounts of revenues and expenses during the reporting periods. The Company uses significant judgments when making estimates
related to the valuation of deferred tax assets and related valuation allowances, accrual and prepayment of research and development
expenses, and the valuation of stock-based compensation. Actual results could differ from those estimates.
|
| Principles of Consolidation |
Principles
of Consolidation – The accompanying condensed consolidated financial statements include the accounts of Immix Biopharma, Inc.
and the accounts of its 100% owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation. For
previously consolidated entities where the Company owned less than 100% of the subsidiary, the Company recorded net loss attributable
to non-controlling interests in its condensed consolidated statements of operations and comprehensive loss equal to the percentage of
the economic or ownership interest retained in such entities by the respective non-controlling parties.
|
| Segment Reporting |
Segment
Reporting - The Company manages its operations as a single segment for the purposes of assessing performance and making operating
decisions. The Company’s Chief Operating Decision Maker (“CODM”) is its Chief Executive Officer. The CODM allocates
resources and evaluates the performance of the Company at the consolidated level using information about its revenues, gross profit,
income from operations, and other key financial data. All significant operating decisions are based upon an analysis of the Company as
one operating segment, which is the same as its reporting segment.
|
| Liquidity and Going Concern |
Liquidity
and Going Concern – These consolidated financial statements have been prepared on a going concern basis, which assumes the
Company will continue to realize its assets and discharge its liabilities in the normal course of business. Since the initial public
offering of its common stock in December 2021, the Company has financed its operations through various equity financing. On July 14,
2023, the Company entered into an additional ATM Sales Agreement (the “July Sales Agreement”) with ThinkEquity LLC (the “Sales
Agent”), pursuant to which the Company, could, from time to time, issue and sell through the Sales Agent shares of the Company’s
common stock in sales deemed to be “at-the-market offerings” as defined in Rule 415(a)(4) promulgated under the Securities
Act of 1933, as amended (the “July ATM Facility”) (see Note 7). Initially, the Company is eligible to sell up to $4,200,000
worth of shares of its common stock as the aggregate market value of the Company’s shares of common stock eligible for sale under
the July Sales Agreement is subject to the limitations of General Instruction I.B.6 of Form S-3 until such time that the Company’s
public float equals or exceeds $75.0 million. In the event the aggregate market value of the Company’s outstanding common stock
held by non-affiliates equals or exceeds $75.0 million, then the one-third limitation on sales set forth in General Instruction I.B.6
of Form S-3 shall not apply to additional sales made pursuant to the July Sales Agreement.
From
July 14, 2023 through February 5, 2024, the Company has sold 328,136 common shares pursuant to the July ATM Facility for net proceeds
of $1,091,887, after offering expenses. On February 5, 2024, the Company suspended, and is not offering any shares of its common stock
pursuant to, the prospectus supplement dated July 14, 2023, relating to the July Sales Agreement by and between the Company and the Sales
Agent. The Company will not make any sales of common stock pursuant to the July Sales Agreement unless and until a new prospectus supplement
is filed with the SEC; however, the July Sales Agreement remains in full force and effect.
In
February 2024, the Company conducted an underwritten public offering of 5,535,055 shares of its common stock at the public offering price
of $2.71 per share, for net proceeds of $13,565,760, after underwriter discounts and offering expenses (the “Offering”).
Pursuant to the underwriting agreement, the Company granted the underwriter a 30-day over-allotment option to purchase up to an additional
783,970 shares of the Company’s common stock, which was exercised in full on March 1, 2024, for net proceeds of $1,954,594, after
underwriting discounts and offering expenses (see Note 7).
On
July 25, 2024, the Company was awarded an $8
million grant from the California Institute for Regenerative Medicine to support the clinical development of chimeric antigen
receptor T-cell therapy NXC-201 for the treatment of relapsed/refractory AL Amyloidosis. The award is payable to the Company upon
achievement of milestones that are primarily based on patient enrollment in the Company’s clinical trials. Additionally, if
CIRM determines, in its sole discretion, that the Company has not complied with the terms and conditions of the grant, CIRM may
suspend or permanently cease disbursements. Funds received under this grant may only be used for allowable project costs
specifically identified with the CIRM-funded project. Such costs can include, but are not limited to, salary for personnel, itemized
supplies, consultants, and itemized clinical study costs. Under the terms of the grant, both CIRM and the Company will co-fund the
research project and the amount of the Company’s co-funding requirement is predetermined as a part of the award. The Company
signed the grant agreement in November 2024 and expects to begin receiving funds from the grant beginning in November of
2024.
The
Company has a history of, and expects to continue to report, negative cash flows from operations and net losses. We believe that our
existing cash, cash equivalents and restricted cash as of September 30, 2024 will enable us to fund our operating expenses and capital
expenditure requirements for at least the next 12 months.
|
| Concentration of Credit Risk |
Concentration
of Credit Risk – Periodically, the Company may carry cash and cash equivalents balances at financial institutions in excess
of the federally insured limit of $250,000, or the Australian insured limit of AUD 250,000. At times, deposits held with financial institutions
may exceed the amount of insurance provided. The Company has not experienced losses on these accounts and management believes that the
credit risk with regard to these deposits is not significant.
|
| Cash and Cash Equivalents |
Cash
and Cash Equivalents – The Company’s cash equivalents include short-term highly liquid investments with an original maturity
of 90 days or less when purchased and are carried at fair value.
|
| Fair Value of Financial Instruments |
Fair
Value of Financial Instruments – The carrying value of short-term instruments, including cash and cash equivalents, tax receivable,
accounts payable and accrued expenses, approximate fair value due to the relatively short period to maturity for these instruments.
Fair
value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal
or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The
Company utilizes a three-level valuation hierarchy for disclosures of fair value measurements, defined as follows:
Level
1 – inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level
2 – inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs
that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
Level
3 – inputs to the valuation methodology are unobservable and significant to the fair value.
The
following fair value hierarchy table presents information about the Company’s asset measured at fair value on a recurring basis:
Schedule of Asset Measured at Fair Value on a Recurring Basis
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at September 30, 2024 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets: | |
| | | |
| | | |
| | |
| Cash equivalents (money market funds) | |
$ | 18,650,711 | | |
$ | - | | |
$ | - | |
As
of September 30, 2024, the Company had no liabilities required to be measured at fair value on a recurring basis.
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at December 31, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets: | |
| | | |
| | | |
| | |
| Cash equivalents (money market funds) | |
$ | 16,113,006 | | |
$ | - | | |
$ | - | |
As
of December 31, 2023, the Company had no liabilities required to be measured at fair value on a recurring basis.
|
| Australian Tax Incentive |
Australian
Tax Incentive – IBAPL is eligible to receive a cash refund from the Australian Taxation Office for eligible research and development
(“R&D”) expenditures under the Australian R&D Tax Incentive Program (the “Australian Tax Incentive”).
The Australian Tax Incentive is recognized as a reduction to R&D expense when there is reasonable assurance that the relevant expenditure
has been incurred, the amount can be reliably measured and that the Australian Tax Incentive will be received. The Company recognized
additional R&D expense of $66,594 and reductions to R&D expense of $378,390 for the three months ended September 30, 2024 and
2023, respectively. The Company recognized reductions to R&D expense of $1,076,193 and $599,926 for the nine months ended September
30, 2024 and 2023, respectively.
|
| Deferred Offering Costs |
Deferred
Offering Costs – The Company had capitalized qualified legal, accounting and other direct costs related to its efforts to raise
capital through the sale of its common stock under the July ATM Facility. Deferred offering costs were deferred and being amortized ratably
upon sales under the July ATM Facility to additional paid-in capital as a reduction of the July ATM proceeds. As a result of the Company
pausing the July ATM Facility, all of the remaining deferred offering costs were immediately amortized to additional paid-in capital
as a reduction to the proceeds received in the nine months ended September 30, 2024. As of September 30, 2024, no remaining amounts of
deferred offering costs were capitalized related to the July ATM Facility.
|
| Stock-Based Compensation |
Stock-Based
Compensation – Stock-based compensation expense represents the estimated grant date fair value of the Company’s equity
awards, consisting of stock options issued under the Company’s stock option plan and restricted common stock (see Note 7). The
fair value of equity awards is recognized over the requisite service period of such awards (usually the vesting period) on a straight-line
basis. The Company estimates the fair value of stock options using the Black-Scholes option pricing model on the date of grant and recognizes
forfeitures as they occur. For stock awards for which vesting is subject to performance-based milestones, the expense is recorded over
the remaining service period after the point when the achievement of the milestone is probable, or the performance condition has been
achieved.
|
| Research and Development Costs |
Research
and Development Costs – R&D costs are expensed as incurred. R&D costs consist primarily of clinical research fees paid
to consultants and outside service providers, other expenses relating to design, development and testing of the Company’s therapy
candidates, and for license and milestone costs related to in-licensed products and technology. Costs incurred in obtaining technology
licenses are charged to R&D expense if the technology licensed has not reached commercial feasibility and has no alternative future
use. Such licenses purchased by the Company require substantial completion of research and development, regulatory and marketing approval
efforts in order to reach commercial feasibility and have no alternative future use.
Clinical
trial costs are a component of R&D expenses. The Company estimates expenses incurred for clinical trials that are in process based
on services performed under contractual agreements with clinical research organizations and actual clinical investigators. Included in
the estimates are (1) the fee per patient enrolled as specified in the clinical trial contract with each institution participating in
the clinical trial and (2) progressive data on patient enrollments obtained from participating clinical trial sites and the actual services
performed. Changes in clinical trial assumptions, such as the length of time estimated to enroll all patients, rate of screening failures,
patient drop-out rates, number and nature of adverse event reports, and the total number of patients enrolled can impact the average
and expected cost per patient and the overall cost of the clinical trial. The Company monitors the progress of the trials and their related
activities and adjusts expense accruals, when applicable. Adjustments to accruals are charged to expense in the period in which the facts
give rise to the adjustments become known.
|
| Other Comprehensive Income (Loss) |
Other
Comprehensive Income (Loss) – Other comprehensive income (loss) includes foreign currency translation gains and losses. The
cumulative amount of translation gains and losses are reflected as a separate component of stockholders’ equity in the condensed
consolidated balance sheets, as accumulated other comprehensive income.
|
| Foreign Currency Translation and Transaction Gains (Losses) |
Foreign
Currency Translation and Transaction Gains (Losses) – The Company, and its wholly-owned subsidiary Nexcella, maintain their
accounting records in U.S. Dollars. The Company’s operating subsidiary, IBAPL, is located in Australia and maintains its accounting
records in Australian Dollars, which is its functional currency. Assets and liabilities of the subsidiary are translated into U.S. dollars
at exchange rates at the balance sheet date, equity accounts are translated at historical exchange rate and revenues and expenses are
translated by using the average exchange rates for the period. Translation adjustments are reported as a separate component of other
comprehensive income (loss) in the consolidated statements of operations and comprehensive loss. Foreign currency denominated transactions
are translated at exchange rates approximating those in effect at the transaction dates. Gains (losses) resulting from foreign currency
transactions are included in general and administrative expenses in the accompanying condensed consolidated statements of operations
and comprehensive loss and were $(2,849) and $8,095 for the three months ended September 30, 2024 and 2023, respectively, and $(22,326)
and $6,372 for the nine months ended September 30, 2024 and 2023, respectively.
|
| Loss Per Common Share |
Loss
Per Common Share - Basic loss per common share is computed by dividing net loss attributable to common stockholders by the weighted-average
number of common shares outstanding during the period. Diluted loss per common share is determined using the weighted-average number
of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents. In periods when losses
are reported, the weighted-average number of common shares outstanding excludes common stock equivalents because their inclusion would
be anti-dilutive. Basic weighted average shares outstanding for the three and nine months ended September 30, 2024 include 1,913,661
shares underlying Pre-Funded warrants to purchase common shares (See Note 7). As the shares underlying these Pre-Funded warrants can
be issued for nominal consideration (an exercise price per share equal to $0.0001 per share), these shares are deemed to be issued for
purposes of basic loss per common share. For the three and nine months ended September 30, 2024 and 2023, the Company’s potentially
dilutive shares, which were not included in the calculation of net loss per share, included stock options and warrants exercisable for
4,408,488 and 2,911,412 shares of common stock, respectively.
|
| Property and Equipment |
Property
and Equipment - Included in property and equipment is construction-in-progress which consists of manufacturing space improvements
and includes the costs of construction, machinery and equipment, and any interest charges arising from borrowings used to finance these
assets during the period of construction or installation of the assets. No provision for depreciation is made on construction-in-progress
until such time as the relevant assets are completed and ready for their intended use.
Estimated
useful lives of the Company’s assets are as follows:
Schedule
of Property and Equipment Useful Lives
| | |
Useful Life |
| Operating equipment | |
3-10 years |
| Electronic equipment | |
3-5 years |
| Office equipment | |
3-5 years |
The
cost and related accumulated depreciation of assets sold or otherwise retired are eliminated from the accounts, and any gain or loss
are included in the Company’s results of operations. The costs of maintenance and repairs are recognized to expenses as incurred;
significant renewals and betterments are capitalized.
|
| Leases |
Leases
- At the inception of a contract the Company determines if the arrangement is, or contains a lease. Operating lease right-of-use
(“ROU”) assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent
its obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement
date based on the present value of the lease payments over the lease term. Lease expense is recognized on a straight-line basis over
the lease term.
The
Company has made certain accounting policy elections whereby it (i) does not recognize ROU assets or lease liabilities for short-term
leases (those with original terms of 12-months or less) and (ii) separates lease and non-lease elements of its operating leases as separate
lease components. As of September 30, 2024 and December 31, 2023, the Company did not have any finance leases.
|
| Recent Accounting Pronouncements |
Recent
Accounting Pronouncements
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”)
2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires disclosure
of incremental segment information on an annual and interim basis. This ASU is effective for fiscal years beginning after December 15,
2023, and interim periods within fiscal years beginning after December 15, 2024, on a retrospective basis. The Company has implemented
this ASU effective January 1, 2024, and determined no retrospective changes were necessary.
In
December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures,
which expands the disclosures required for income taxes. This ASU is effective for fiscal years beginning after December 15, 2024,
with early adoption permitted. The amendment should be applied on a prospective basis while retrospective application is permitted. The
Company is currently evaluating the effect of this pronouncement on its disclosures.
|
| X |
- DefinitionAustralian Tax Incentive [Policy Text Block]
| Name: |
IMMX_AustralianTaxIncentivePolicyTextBlock |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLiquidity And Going Concern [Policy Text Block]
| Name: |
IMMX_LiquidityAndGoingConcernPolicyTextBlock |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRisk And Uncertainties [Policy Text Block]
| Name: |
IMMX_RiskAndUncertaintiesPolicyTextBlock |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the basis of presentation and significant accounting policies concepts. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Accounting policies describe all significant accounting policies of the reporting entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
| Name: |
us-gaap_BasisOfPresentationAndSignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for salaries, bonuses, incentive awards, postretirement and postemployment benefits granted to employees, including equity-based arrangements; discloses methodologies for measurement, and the bases for recognizing related assets and liabilities and recognizing and reporting compensation expense. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_CompensationRelatedCostsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for comprehensive income.
| Name: |
us-gaap_ComprehensiveIncomePolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for credit risk. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 825
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478898/942-825-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
| Name: |
us-gaap_ConcentrationRiskCreditRisk |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1
| Name: |
us-gaap_ConsolidationPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for deferral and amortization of significant deferred charges. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DeferredChargesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for (1) transactions denominated in a currency other than the reporting enterprise's functional currency, (2) translating foreign currency financial statements that are incorporated into the financial statements of the reporting enterprise by consolidation, combination, or the equity method of accounting, and (3) remeasurement of the financial statements of a foreign reporting enterprise in a hyperinflationary economy. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/830/tableOfContent
| Name: |
us-gaap_ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for segment reporting. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 54
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-54
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 36
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-36
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
| Name: |
us-gaap_SegmentReportingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Summary of Significant Accounting Policies (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Schedule of Asset Measured at Fair Value on a Recurring Basis |
The
following fair value hierarchy table presents information about the Company’s asset measured at fair value on a recurring basis:
Schedule of Asset Measured at Fair Value on a Recurring Basis
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at September 30, 2024 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets: | |
| | | |
| | | |
| | |
| Cash equivalents (money market funds) | |
$ | 18,650,711 | | |
$ | - | | |
$ | - | |
As
of September 30, 2024, the Company had no liabilities required to be measured at fair value on a recurring basis.
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at December 31, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets: | |
| | | |
| | | |
| | |
| Cash equivalents (money market funds) | |
$ | 16,113,006 | | |
$ | - | | |
$ | - | |
|
| Schedule of Property and Equipment Useful Lives |
Estimated
useful lives of the Company’s assets are as follows:
Schedule
of Property and Equipment Useful Lives
| | |
Useful Life |
| Operating equipment | |
3-10 years |
| Electronic equipment | |
3-5 years |
| Office equipment | |
3-5 years |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets, including [financial] instruments measured at fair value that are classified in stockholders' equity, if any, by class that are measured at fair value on a recurring basis. The disclosures contemplated herein include the fair value measurements at the reporting date by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets (Level 1), significant other observable inputs (Level 2), and significant unobservable inputs (Level 3). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueAssetsMeasuredOnRecurringBasisTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Prepaid Expenses and Other Current Assets (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Prepaid Expenses And Other Current Assets |
|
| Schedule of Prepaid Expenses and Other Current Assets |
Prepaid
expenses and other current assets consist of the following as of September 30, 2024 and December 31, 2023:
Schedule of Prepaid Expenses and Other Current Assets
| | |
September 30, 2024 | | |
December 31, 2023 | |
| Prepaid research and development expenses | |
$ | 486,912 | | |
$ | 412,773 | |
| Prepaid insurance expense | |
| 73,813 | | |
| 263,927 | |
| Prepaid investor relations expense | |
| 492,391 | | |
| 384,494 | |
| Other current assets | |
| 36,521 | | |
| 44,582 | |
| Total prepaid expenses and other current assets | |
$ | 1,089,637 | | |
$ | 1,105,776 | |
|
| X |
- References
+ Details
| Name: |
IMMX_DisclosurePrepaidExpensesAndOtherCurrentAssetsAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrepaid Expenses and Other Current Assets Table [Text Block]
| Name: |
IMMX_PrepaidExpensesAndOtherCurrentAssetsTableTextBlock |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Accounts Payable and Accrued Expenses (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Payables and Accruals [Abstract] |
|
| Schedule of Accounts Payable and Accrued Expenses |
Accounts
payable and accrued expenses consist of the following as of September 30, 2024 and December 31, 2023:
Schedule of Accounts Payable and Accrued Expenses
| | |
September 30, 2024 | | |
December 31, 2023 | |
| Accounts payable | |
$ | 3,288,579 | | |
$ | 1,433,022 | |
| Accrued research and development expenses | |
| 2,878,386 | | |
| 1,571,261 | |
| Accrued professional services | |
| 93,385 | | |
| 38,639 | |
| Accrued compensation and related expenses | |
| 198,095 | | |
| 577,854 | |
| Other accrued expenses | |
| 43,341 | | |
| 101,007 | |
| Total accounts payable and accrued expenses | |
$ | 6,501,786 | | |
$ | 3,721,783 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the (a) carrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business (accounts payable); (b) other payables; and (c) accrued liabilities. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). An alternative caption includes accrued expenses.
| Name: |
us-gaap_ScheduleOfAccountsPayableAndAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Property and Equipment (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Property, Plant and Equipment [Abstract] |
|
| Schedule of Property and Equipment |
Property
and equipment at September 30, 2024 and December 31, 2023 consisted of:
Schedule
of Property and Equipment
| | |
September 30, 2024 | | |
December 31, 2023 | |
| Operating equipment | |
$ | 534,162 | | |
$ | 60,599 | |
| Office equipment | |
| 3,895 | | |
| 3,896 | |
| Total property and equipment, gross | |
| 538,057 | | |
| 64,495 | |
| Less: Accumulated depreciation | |
| (26,652 | ) | |
| (14,314 | ) |
| Property and equipment
excluding construction in progress | |
| 511,405 | | |
| 50,181 | |
| Construction in progress | |
| 844,019 | | |
| - | |
| Total property and equipment | |
$ | 1,355,424 | | |
$ | 50,181 | |
|
| X |
- DefinitionProperty And Equipment [Table Text Block]
| Name: |
IMMX_PropertyAndEquipmentTableTextBlock |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Stockholders’ Equity (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Schedule of Stock Option Activity |
The
following table summarizes the stock option activity for the nine months ended September 30, 2024:
Schedule of Stock Option Activity
| | |
Options | | |
Weighted- Average Exercise Price Per Share | |
| Outstanding, January 1, 2024 | |
| 2,512,561 | | |
$ | 1.92 | |
| Granted | |
| 1,517,176 | | |
$ | 2.21 | |
| Exercised | |
| (834 | ) | |
$ | 1.95 | |
| Forfeited | |
| (17,915 | ) | |
$ | 1.95 | |
| Expired | |
| - | | |
$ | - | |
| Outstanding and expected to vest, September 30, 2024 | |
| 4,010,988 | | |
$ | 2.03 | |
|
| Schedule of Stock Outstanding and Exercisable |
The
following table discloses information regarding outstanding and exercisable options at September 30, 2024:
Schedule of Stock Outstanding and Exercisable
| | | |
Outstanding | | |
Exercisable | |
| Exercise Price Range | | |
Number of Option Shares | | |
Weighted Average Exercise Price | | |
Weighted Average Remaining Life (Years) | | |
Number of Option Shares | | |
Weighted Average Exercise Price | |
| $ | 0.00-1.00 | | |
| 256,500 | | |
$ | 0.80 | | |
| 6.45 | | |
| 256,500 | | |
$ | 0.80 | |
| $ | 1.01-2.00 | | |
| 1,646,062 | | |
$ | 1.81 | | |
| 7.13 | | |
| 1,060,836 | | |
$ | 1.78 | |
| $ | 2.01-3.00 | | |
| 2,097,176 | | |
$ | 2.33 | | |
| 9.13 | | |
| 751,854 | | |
$ | 2.50 | |
| $ | 3.01-6.00 | | |
| 11,250 | | |
$ | 5.83 | | |
| 7.29 | | |
| 7,500 | | |
$ | 5.83 | |
| | | | |
| 4,010,988 | | |
$ | 2.03 | | |
| 8.13 | | |
| 2,076,690 | | |
$ | 1.93 | |
|
| Schedule of Stock Warrant Activity |
The
following table summarizes the stock warrant activity for the nine months ended September 30, 2024:
Schedule of Stock Warrant Activity
| | |
Warrants | | |
Weighted-Average
Exercise Price Per
Share | |
| Outstanding and exercisable, January 1, 2024 | |
| 2,311,161 | | |
$ | 0.71 | |
| Granted | |
| - | | |
$ | - | |
| Exercised | |
| - | | |
$ | - | |
| Forfeited | |
| - | | |
$ | - | |
| Expired | |
| - | | |
$ | - | |
| Outstanding and exercisable, September 30, 2024 | |
| 2,311,161 | | |
$ | 0.71 | |
|
| Nexcella [Member] |
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Schedule of Stock Option Activity |
The
following table summarizes the stock option activity for the nine months ended September 30, 2024 for Nexcella:
Schedule of Stock Option Activity
| | |
Options | | |
Weighted- Average Exercise Price Per Share | |
| Outstanding and exercisable, January 1, 2024 | |
| 186,528 | | |
$ | 6.49 | |
| Granted | |
| - | | |
$ | - | |
| Exercised | |
| - | | |
$ | - | |
| Forfeited | |
| (186,528 | ) | |
$ | 6.49 | |
| Expired | |
| - | | |
$ | - | |
| Outstanding and expected to vest, September 30, 2024 | |
| - | | |
$ | - | |
|
| Warrant [Member] |
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Schedule of Stock Outstanding and Exercisable |
The
following table discloses information regarding outstanding and exercisable warrants at September 30, 2024:
Schedule of Stock Outstanding and Exercisable
| | | |
Outstanding | | |
Exercisable | |
| Exercise Price | | |
Number of Warrant Shares | | |
Weighted Average Exercise Price | | |
Weighted Average Remaining Life (Years) | | |
Number of Warrant Shares | | |
Weighted Average Exercise Price | |
| $ | 0.0001 | | |
| 1,913,661 | | |
$ | 0.0001 | | |
| - | | |
| 1,913,661 | | |
$ | 0.0001 | |
| $ | 0.80 | | |
| 156,000 | | |
$ | 0.80 | | |
| 6.48 | | |
| 156,000 | | |
$ | 0.80 | |
| $ | 6.25 | | |
| 241,500 | | |
$ | 6.25 | | |
| 2.21 | | |
| 241,500 | | |
$ | 6.25 | |
| | | |
| 2,311,161 | | |
$ | 0.71 | | |
| 0.67 | | |
| 2,311,161 | | |
$ | 0.71 | |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-4
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-17
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of option exercise prices, by grouped ranges, including the upper and lower limits of the price range, the number of shares under option, weighted average exercise price and remaining contractual option terms. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of warrants or rights issued. Warrants and rights outstanding are derivative securities that give the holder the right to purchase securities (usually equity) from the issuer at a specific price within a certain time frame. Warrants are often included in a new debt issue to entice investors by a higher return potential. The main difference between warrants and call options is that warrants are issued and guaranteed by the company, whereas options are exchange instruments and are not issued by the company. Also, the lifetime of a warrant is often measured in years, while the lifetime of a typical option is measured in months. Disclose the title of issue of securities called for by warrants and rights outstanding, the aggregate amount of securities called for by warrants and rights outstanding, the date from which the warrants or rights are exercisable, and the price at which the warrant or right is exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-1
| Name: |
us-gaap_ScheduleOfStockholdersEquityNoteWarrantsOrRightsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=IMMX_NexcellaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Leases (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Leases |
|
| Schedule of Lease Cost for Operating Leases |
The
components of lease cost for operating leases, which are recorded in general and administrative expenses in the accompanying condensed
consolidated statement of operations, for the three and nine months ended September 30, 2024 were as follows:
Schedule of Lease Cost for Operating Leases
| | |
Three Months Ended September 30, 2024 | | |
Nine Months Ended September 30, 2024 | |
| Operating lease cost | |
$ | 42,150 | | |
$ | 126,450 | |
| Short-term lease cost | |
| 12,196 | | |
| 44,020 | |
| Total lease cost | |
$ | 54,346 | | |
$ | 170,470 | |
|
| Schedule of Lease Related Assets and Liabilities |
The
following table summarizes the lease-related assets and liabilities recorded in the consolidated balance sheets at September 30, 2024:
Schedule of Lease Related Assets and Liabilities
| | |
September 30, 2024 | |
| Operating Leases | |
| - | |
| Operating lease right-of-use assets | |
$ | 1,010,205 | |
| Right of use liability operating lease current portion | |
$ | 62,715 | |
| Right of use liability operating lease long term | |
| 1,026,340 | |
| Total operating lease liabilities | |
$ | 1,089,055 | |
|
| Schedule of Maturity Lease Liability |
The
following table provides the maturities of lease liabilities at September 30, 2024:
Schedule of Maturity Lease Liability
| | |
Operating | |
| | |
Leases | |
| 2024 (remaining 3 months) | |
$ | 35,700 | |
| 2025 | |
| 147,798 | |
| 2026 | |
| 152,971 | |
| 2027 | |
| 158,325 | |
| 2028 and thereafter | |
| 1,073,349 | |
| Total future undiscounted lease payments | |
| 1,568,143 | |
| Less: Interest | |
| (479,088 | ) |
| Present value of lease liabilities | |
$ | 1,089,055 | |
|
| X |
- References
+ Details
| Name: |
IMMX_DisclosureLeasesAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of components of income from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479773/842-30-50-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-6A
| Name: |
us-gaap_OperatingLeaseLeaseIncomeTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Schedule of Asset Measured at Fair Value on a Recurring Basis (Details) - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Fair Value, Inputs, Level 1 [Member] |
|
|
| Platform Operator, Crypto Asset [Line Items] |
|
|
| Cash equivalents (money market funds) |
$ 18,650,711
|
$ 16,113,006
|
| Fair Value, Inputs, Level 2 [Member] |
|
|
| Platform Operator, Crypto Asset [Line Items] |
|
|
| Cash equivalents (money market funds) |
|
|
| Fair Value, Inputs, Level 3 [Member] |
|
|
| Platform Operator, Crypto Asset [Line Items] |
|
|
| Cash equivalents (money market funds) |
|
|
| X |
- DefinitionFair value portion of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_CashAndCashEquivalentsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.3
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUseful life of long lived, physical assets used in the normal conduct of business and not intended for resale, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Examples include, but not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.
| Name: |
us-gaap_PropertyPlantAndEquipmentUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=IMMX_OperatingEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=IMMX_ElectronicEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_OfficeEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Summary of Significant Accounting Policies (Details Narrative)
|
|
|
1 Months Ended |
3 Months Ended |
7 Months Ended |
9 Months Ended |
|
|
|
|
Mar. 01, 2024
USD ($)
|
Jul. 14, 2023
USD ($)
|
Feb. 29, 2024
USD ($)
$ / shares
shares
|
Sep. 30, 2024
USD ($)
$ / shares
|
Mar. 31, 2024
USD ($)
|
Sep. 30, 2023
USD ($)
|
Feb. 05, 2024
USD ($)
shares
|
Sep. 30, 2024
USD ($)
Segment
$ / shares
shares
|
Sep. 30, 2023
USD ($)
shares
|
Sep. 30, 2024
AUD ($)
|
Jul. 25, 2024
USD ($)
|
Dec. 31, 2023
USD ($)
|
| Number of operating segments | Segment |
|
|
|
|
|
|
|
1
|
|
|
|
|
| Number of reportable segments | Segment |
|
|
|
|
|
|
|
1
|
|
|
|
|
| Shares issued |
|
|
|
|
$ 15,520,354
|
|
|
|
|
|
|
|
| Issuance of pubilc offering |
|
|
|
|
|
|
|
$ 2,489
|
|
|
|
|
| Stock option granted | shares |
|
|
|
|
|
|
|
1,517,176
|
|
|
|
|
| Grants receivable |
|
|
|
|
|
|
|
|
|
|
$ 8,000,000
|
|
| FDIC insured amount |
|
|
|
$ 250,000
|
|
|
|
$ 250,000
|
|
$ 250,000
|
|
|
| Liabilities measured at fair value |
|
|
|
0
|
|
|
|
0
|
|
|
|
$ 0
|
| Research and development expense |
|
|
|
4,445,528
|
|
$ 2,106,020
|
|
9,918,336
|
5,634,284
|
|
|
|
| Deferred offering costs |
|
|
|
0
|
|
|
|
0
|
|
|
|
|
| Foreign currency transaction gains and losses |
|
|
|
(2,849)
|
|
8,095
|
|
$ (22,326)
|
$ 6,372
|
|
|
|
| Stock Options and Warrants Exercisable [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Antidilutive securities excluded from computation of earnings per share, amount | shares |
|
|
|
|
|
|
|
4,408,488
|
2,911,412
|
|
|
|
| Australian Tax Incentive [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development expense |
|
|
|
$ 66,594
|
|
$ 378,390
|
|
$ 1,076,193
|
$ 599,926
|
|
|
|
| Common Stock [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Shares issued |
|
|
|
|
$ 632
|
|
|
|
|
|
|
|
| Number of shares sold in public offering | shares |
|
|
5,535,055
|
|
|
|
|
|
|
|
|
|
| Exercise price, per share | $ / shares |
|
|
$ 2.71
|
|
|
|
|
|
|
|
|
|
| Underwriter discounts and offering expenses |
$ 1,954,594
|
|
$ 13,565,760
|
|
|
|
|
|
|
|
|
|
| Stock option granted | shares |
|
|
783,970
|
|
|
|
|
|
|
|
|
|
| Common Stock [Member] | Prefunded Warrants [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Exercise price, per share | $ / shares |
|
|
|
$ 0.0001
|
|
|
|
$ 0.0001
|
|
|
|
|
| Underlying pre-funded warrants | shares |
|
|
|
|
|
|
|
1,913,661
|
|
|
|
|
| Scenario, Adjustment [Member] | July ATM Facility [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Sale of stock issued | shares |
|
|
|
|
|
|
328,136
|
|
|
|
|
|
| Net proceeds form sale of stock |
|
|
|
|
|
|
$ 1,091,887
|
|
|
|
|
|
| July Sales Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Shares issued |
|
$ 4,200,000
|
|
|
|
|
|
|
|
|
|
|
| Issuance of pubilc offering |
|
$ 75,000,000.0
|
|
|
|
|
|
|
|
|
|
|
| Immix Biopharma Australia Pty Ltd. [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-controlling interest rate |
|
|
|
100.00%
|
|
|
|
100.00%
|
|
100.00%
|
|
|
| X |
- DefinitionUnderwriter discounts and offering expenses.
| Name: |
IMMX_UnderwriterDiscountsAndOfferingExpenses |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of cash deposited in financial institutions as of the balance sheet date that is insured by the Federal Deposit Insurance Corporation.
| Name: |
us-gaap_CashFDICInsuredAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSpecific incremental costs directly attributable to a proposed or actual offering of securities which are deferred at the end of the reporting period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 5.A)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480341/340-10-S99-1
| Name: |
us-gaap_DeferredOfferingCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying amount as of the balance sheet date of amounts due under the terms of governmental, corporate, or foundation grants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_GrantsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value of financial and nonfinancial obligations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_LiabilitiesFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe parent entity's interest in net assets of the subsidiary, expressed as a percentage.
| Name: |
us-gaap_MinorityInterestOwnershipPercentageByParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of operating segments. An operating segment is a component of an enterprise: (a) that engages in business activities from which it may earn revenues and incur expenses (including revenues and expenses relating to transactions with other components of the same enterprise), (b) whose operating results are regularly reviewed by the enterprise's chief operating decision maker to make decisions about resources to be allocated to the segment and assess its performance, and (c) for which discrete financial information is available. An operating segment may engage in business activities for which it has yet to earn revenues, for example, start-up operations may be operating segments before earning revenues. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-18
| Name: |
us-gaap_NumberOfOperatingSegments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of segments reported by the entity. A reportable segment is a component of an entity for which there is an accounting requirement to report separate financial information on that component in the entity's financial statements. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 54
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-54
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-18
| Name: |
us-gaap_NumberOfReportableSegments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax, before reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-10A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-11
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-10A
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-10A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481839/830-10-45-9
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482014/830-20-35-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-12
| Name: |
us-gaap_OtherComprehensiveIncomeForeignCurrencyTransactionAndTranslationGainLossArisingDuringPeriodNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of units sold in a public offering of each class of partners' capital account. Units represent shares of ownership of the general, limited, and preferred partners. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 4.F)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-5
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_PartnersCapitalAccountUnitsSoldInPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCash received on stock transaction after deduction of issuance costs.
| Name: |
us-gaap_SaleOfStockConsiderationReceivedOnTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued or sold by the subsidiary or equity method investee per stock transaction.
| Name: |
us-gaap_SaleOfStockNumberOfSharesIssuedInTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares issued during the period upon the conversion of units. An example of a convertible unit is an umbrella partnership real estate investment trust unit (UPREIT unit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_StockIssuedDuringPeriodSharesConversionOfUnits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=IMMX_StockOptionsAndWarrantsExercisableMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=IMMX_AustralianTaxIncentiveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=IMMX_PrefundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementScenarioAxis=us-gaap_ScenarioAdjustmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_CreditFacilityAxis=IMMX_JulyATMFacilityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_JulySalesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=IMMX_ImmixBiopharmaAustraliaPtyLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Prior Agreements with Nexcella Subsidiary (Details Narrative) - USD ($)
|
May 20, 2024 |
Dec. 08, 2022 |
Sep. 30, 2024 |
Dec. 31, 2023 |
| Common stock par value, per share |
|
|
$ 0.0001
|
$ 0.0001
|
| Debt instrument maturity date |
|
Jan. 31, 2030
|
|
|
| Nexcella 2022 Equity Incentive Plan [Member] |
|
|
|
|
| Number of restricted stock awards |
$ 275,759
|
|
|
|
| Exercise price, per share |
$ 2.47
|
|
|
|
| Nexcella 2022 Equity Incentive Plan [Member] | Maximum [Member] |
|
|
|
|
| Number of options purchased |
595,676
|
|
|
|
| Nexcella Absorption [Member] |
|
|
|
|
| Common stock par value, per share |
$ 0.0001
|
|
|
|
| Capital stock shares outstanding, percentage |
100.00%
|
|
|
|
| Number of shares issued |
989,876
|
|
|
|
| Nexcella Absorption [Member] | Nexcella Inc [Member] |
|
|
|
|
| Equity ownership percentage |
95.00%
|
|
|
|
| Nexcella Founders Agreement [Member] |
|
|
|
|
| Loans payable |
|
$ 2,100,000
|
|
|
| Conversion interest rate |
|
7.875%
|
|
|
| Conversion price per share |
|
$ 2.00
|
|
|
| Agreement remaining contractual term |
|
15 years
|
|
|
| Preferred stock dividend rate percentage |
|
2.50%
|
|
|
| Conversion basis |
|
Each
share of Class A Common Stock was convertible, at the Company’s option, into one fully paid and nonassessable share of Nexcella’s
common stock, subject to certain adjustments. In addition, upon a Qualified IPO (as defined in the Nexcella COI) or Qualified Change
in Control (as defined in the Nexcella COI), each share of Class A Common Stock would automatically convert into one fully paid and nonassessable
share of Nexcella’s common stock; provided however, if at that time, the Class A Common Stock was not then convertible into a number
of shares of Nexcella common stock (or such other capital stock or securities at the time issuable upon the conversion of the Class A
Common Stock) that have a value of: (a) in the case of a Qualified IPO, at least $5,000,000 based on the initial offering price in such
initial public offering, or (b) in the case of a Qualified Change in Control, at least $5,000,000 in cash or at least $5,000,000 of equity
based on the implied value of a share of Nexcella common stock resulting from the price paid upon the consummation of such Qualified
Change of Control, the Class A Common Stock would automatically convert into such number of shares of Nexcella common stock (or such
other capital stock or securities at the time issuable upon the conversion of the Class A Common Stock) that have a value of $5,000,000
based on the initial offering price in such initial public offering or the implied value of a share of Nexcella common stock resulting
from the price paid upon the consummation of such Qualified Change of Control (or if such Qualified Change of Control results in the
Class A Shares being exchanged solely for cash, then $5,000,000 in cash).
|
|
|
| Business combination control obtained description |
|
(i) pay an equity fee in shares of common stock,
payable within five business days of the closing of any equity or debt financing for Nexcella or any of its respective subsidiaries that
occurs after the effective date of the Nexcella Founders Agreement and ending on the date when the Company no longer has majority voting
control in Nexcella’s voting equity, equal to 2.5% of the gross amount of any such equity or debt financing; and (ii) pay a cash
fee equal to 4.5% of Nexcella’s annual Net Sales (as defined in the Nexcella Founders Agreement), payable on an annual basis, within
90 days of the end of each calendar year. In the event of a Change of Control, Nexcella agreed to pay a one-time change in control fee
equal to five times the product of (A) Net Sales for the 12 months immediately preceding the Change of Control and (B) 4.5%
|
|
|
| Nexcella Founders Agreement [Member] | Preferred Class A [Member] |
|
|
|
|
| Number of shares issued |
|
250,000
|
|
|
| Nexcella Founders Agreement [Member] | Common Class A [Member] |
|
|
|
|
| Number of shares issued |
|
1,000,000
|
|
|
| Nexcella Founders Agreement [Member] | Common Stock [Member] |
|
|
|
|
| Number of shares issued |
|
5,000,000
|
|
|
| Nexcella Management Services Agreement [Member] |
|
|
|
|
| Management and consulting fee |
|
$ 500,000
|
|
|
| Gross proceeds |
|
$ 10,000,000
|
|
|
| X |
- DefinitionAgreement remaining contractual term.
| Name: |
IMMX_AgreementRemainingContractualTerm |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCapital stock shares outstanding percentage.
| Name: |
IMMX_CapitalStockSharesOutstandingPercentage |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDebt instrument convertible conversion percentage.
| Name: |
IMMX_DebtInstrumentConvertibleConversionPercentage |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionManagement and consulting fee.
| Name: |
IMMX_ManagementAndConsultingFee |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThis element represents a description of how the entity obtained control of the acquired entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479328/805-10-50-2
| Name: |
us-gaap_BusinessCombinationControlObtainedDescription |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of basis for conversion of convertible common stock. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockConversionBasis |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe price per share of the conversion feature embedded in the debt instrument. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-5
| Name: |
us-gaap_DebtInstrumentConvertibleConversionPrice1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDate when the debt instrument is scheduled to be fully repaid, in YYYY-MM-DD format. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
| Name: |
us-gaap_DebtInstrumentMaturityDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe percentage of ownership of common stock or equity participation in the investee accounted for under the equity method of accounting. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
| Name: |
us-gaap_EquityMethodInvestmentOwnershipPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIncluding the current and noncurrent portions, aggregate carrying value as of the balance sheet date of loans payable (with maturities initially due after one year or beyond the operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_LoansPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe percentage rate used to calculate dividend payments on preferred stock. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
| Name: |
us-gaap_PreferredStockDividendRatePercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ProceedsFromIssuanceOrSaleOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate value of stock related to Restricted Stock Awards issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueRestrictedStockAwardGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares that have been repurchased during the period and have not been retired and are not held in treasury. Some state laws may govern the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockRepurchasedDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=IMMX_Nexcella2022EquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_NexcellaAbsorptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ScheduleOfEquityMethodInvestmentEquityMethodInvesteeNameAxis=IMMX_NexcellaIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_NexcellaFoundersAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_PreferredClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_NexcellaManagementServicesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Schedule of Prepaid Expenses and Other Current Assets (Details) - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Prepaid Expenses And Other Current Assets |
|
|
| Prepaid research and development expenses |
$ 486,912
|
$ 412,773
|
| Prepaid insurance expense |
73,813
|
263,927
|
| Prepaid investor relations expense |
492,391
|
384,494
|
| Other current assets |
36,521
|
44,582
|
| Total prepaid expenses and other current assets |
$ 1,089,637
|
$ 1,105,776
|
| X |
- References
+ Details
| Name: |
IMMX_DisclosurePrepaidExpensesAndOtherCurrentAssetsAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrepaid investor relations expense.
| Name: |
IMMX_PrepaidInvestorRelationsExpense |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionPrepaid research and development expenses.
| Name: |
IMMX_PrepaidResearchAndDevelopmentExpenses |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of current assets classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for insurance that provides economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483032/340-10-45-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482955/340-10-05-5
| Name: |
us-gaap_PrepaidInsurance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.3
Schedule of Accounts Payable and Accrued Expenses (Details) - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Payables and Accruals [Abstract] |
|
|
| Accounts payable |
$ 3,288,579
|
$ 1,433,022
|
| Accrued research and development expenses |
2,878,386
|
1,571,261
|
| Accrued professional services |
93,385
|
38,639
|
| Accrued compensation and related expenses |
198,095
|
577,854
|
| Other accrued expenses |
43,341
|
101,007
|
| Total accounts payable and accrued expenses |
$ 6,501,786
|
$ 3,721,783
|
| X |
- DefinitionAccrued professional services.
| Name: |
IMMX_AccruedProfessionalServices |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAccrued research and development expenses.
| Name: |
IMMX_AccruedResearchAndDevelopmentExpenses |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities incurred and payable to vendors for goods and services received classified as other, and expenses incurred but not yet paid, payable within one year or the operating cycle, if longer.
| Name: |
us-gaap_OtherAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Schedule of Property and Equipment (Details) - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Property, Plant and Equipment [Line Items] |
|
|
| Total property and equipment, gross |
$ 538,057
|
$ 64,495
|
| Less: Accumulated depreciation |
(26,652)
|
(14,314)
|
| Property and equipment excluding construction in progress |
511,405
|
50,181
|
| Construction in progress |
844,019
|
|
| Total property and equipment |
1,355,424
|
50,181
|
| Operating Equipment [Member] |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Total property and equipment, gross |
534,162
|
60,599
|
| Office Equipment [Member] |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Total property and equipment, gross |
$ 3,895
|
$ 3,896
|
| X |
- DefinitionAmount, excluding lessor's underlying asset for which right to use has been conveyed to lessee under operating lease, of accumulated amortization, depreciation, depletion for physical asset used in normal conduct of business to create and distribute product and service. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479773/842-30-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentExcludingLessorAssetUnderOperatingLeaseAccumulatedDepreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after accumulated depreciation and excluding lessor's underlying asset for which right to use has been conveyed to lessee under operating lease, of physical asset used in normal conduct of business to create and distribute product and service. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479773/842-30-50-13
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentExcludingLessorAssetUnderOperatingLeaseAfterAccumulatedDepreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before accumulated depreciation and excluding lessor's underlying asset for which right to use has been conveyed to lessee under operating lease, of physical asset used in normal conduct of business to create and distribute product and service. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479773/842-30-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentExcludingLessorAssetUnderOperatingLeaseBeforeAccumulatedDepreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after accumulated depreciation, of lessor's underlying asset for which right to use has been conveyed to lessee under operating lease. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481501/840-20-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479773/842-30-50-13
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertySubjectToOrAvailableForOperatingLeaseNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=IMMX_OperatingEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_OfficeEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after accumulated depreciation, of lessor's underlying asset for which right to use has been conveyed to lessee under operating lease. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481501/840-20-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479773/842-30-50-13
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertySubjectToOrAvailableForOperatingLeaseNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.3
Schedule of Stock Option Activity (Details)
|
9 Months Ended |
|
Sep. 30, 2024
$ / shares
shares
|
|---|
| Subsidiary, Sale of Stock [Line Items] |
|
| Options, Outstanding and exercisable, Beginning |
2,512,561
|
| Weighted-Average Exercise Price Per Share, Outstanding and exercisable, Beginning | $ / shares |
$ 1.92
|
| Options, Granted |
1,517,176
|
| Weighted-Average Exercise Price Per Share, Granted | $ / shares |
$ 2.21
|
| Options, Exercised |
(834)
|
| Weighted-Average Exercise Price Per Share, Exercised | $ / shares |
$ 1.95
|
| Options, Forfeited |
(17,915)
|
| Weighted-Average Exercise Price Per Share, Forfeited | $ / shares |
$ 1.95
|
| Options, Expired |
|
| Weighted-Average Exercise Price Per Share, Expired | $ / shares |
|
| Options, Outstanding and expected to vest, Ending |
4,010,988
|
| Weighted-Average Exercise Price Per Share, Outstanding and expected to vest, Ending | $ / shares |
$ 2.03
|
| Options, Exercised |
834
|
| Nexcella [Member] |
|
| Subsidiary, Sale of Stock [Line Items] |
|
| Options, Outstanding and exercisable, Beginning |
186,528
|
| Weighted-Average Exercise Price Per Share, Outstanding and exercisable, Beginning | $ / shares |
$ 6.49
|
| Options, Granted |
|
| Weighted-Average Exercise Price Per Share, Granted | $ / shares |
|
| Options, Exercised |
|
| Weighted-Average Exercise Price Per Share, Exercised | $ / shares |
|
| Options, Forfeited |
(186,528)
|
| Weighted-Average Exercise Price Per Share, Forfeited | $ / shares |
$ 6.49
|
| Options, Expired |
|
| Weighted-Average Exercise Price Per Share, Expired | $ / shares |
|
| Options, Outstanding and expected to vest, Ending |
|
| Weighted-Average Exercise Price Per Share, Outstanding and expected to vest, Ending | $ / shares |
|
| Options, Exercised |
|
| X |
- DefinitionNumber of options or other stock instruments for which the right to exercise has lapsed under the terms of the plan agreements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExpirationsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options of the plan that expired. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExpirationsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SubsidiarySaleOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=IMMX_NexcellaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Schedule of Stock Outstanding and Exercisable (Details)
|
9 Months Ended |
|
Sep. 30, 2024
$ / shares
shares
|
|---|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Outstanding, Number of Option Shares | shares |
4,010,988
|
| Outstanding, Weighted Average Exercise Price |
$ 2.03
|
| Outstanding, Weighted Average Remaining Life (Years) |
8 years 1 month 17 days
|
| Exercisable, Number of Option Shares | shares |
2,076,690
|
| Exercisable, Weighted Average Exercise Price |
$ 1.93
|
| Warrant [Member] |
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Outstanding, Number of Option Shares | shares |
2,311,161
|
| Outstanding, Weighted Average Exercise Price |
$ 0.71
|
| Outstanding, Weighted Average Remaining Life (Years) |
8 months 1 day
|
| Exercisable, Number of Option Shares | shares |
2,311,161
|
| Exercisable, Weighted Average Exercise Price |
$ 0.71
|
| Range One [Member] |
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Outstanding, exercise price lower range limit |
0.00
|
| Outstanding, exercise price upper range limit |
$ 1.00
|
| Outstanding, Number of Option Shares | shares |
256,500
|
| Outstanding, Weighted Average Exercise Price |
$ 0.80
|
| Outstanding, Weighted Average Remaining Life (Years) |
6 years 5 months 12 days
|
| Exercisable, Number of Option Shares | shares |
256,500
|
| Exercisable, Weighted Average Exercise Price |
$ 0.80
|
| Range One [Member] | Warrant [Member] |
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Outstanding, Number of Option Shares | shares |
1,913,661
|
| Outstanding, Weighted Average Exercise Price |
$ 0.0001
|
| Exercisable, Number of Option Shares | shares |
1,913,661
|
| Exercisable, Weighted Average Exercise Price |
$ 0.0001
|
| Outstanding, Exercise Price |
0.0001
|
| Range Two [Member] |
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Outstanding, exercise price lower range limit |
1.01
|
| Outstanding, exercise price upper range limit |
$ 2.00
|
| Outstanding, Number of Option Shares | shares |
1,646,062
|
| Outstanding, Weighted Average Exercise Price |
$ 1.81
|
| Outstanding, Weighted Average Remaining Life (Years) |
7 years 1 month 17 days
|
| Exercisable, Number of Option Shares | shares |
1,060,836
|
| Exercisable, Weighted Average Exercise Price |
$ 1.78
|
| Range Two [Member] | Warrant [Member] |
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Outstanding, Number of Option Shares | shares |
156,000
|
| Outstanding, Weighted Average Exercise Price |
$ 0.80
|
| Outstanding, Weighted Average Remaining Life (Years) |
6 years 5 months 23 days
|
| Exercisable, Number of Option Shares | shares |
156,000
|
| Exercisable, Weighted Average Exercise Price |
$ 0.80
|
| Outstanding, Exercise Price |
0.80
|
| Range Three [Member] |
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Outstanding, exercise price lower range limit |
2.01
|
| Outstanding, exercise price upper range limit |
$ 3.00
|
| Outstanding, Number of Option Shares | shares |
2,097,176
|
| Outstanding, Weighted Average Exercise Price |
$ 2.33
|
| Outstanding, Weighted Average Remaining Life (Years) |
9 years 1 month 17 days
|
| Exercisable, Number of Option Shares | shares |
751,854
|
| Exercisable, Weighted Average Exercise Price |
$ 2.50
|
| Range Three [Member] | Warrant [Member] |
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Outstanding, Number of Option Shares | shares |
241,500
|
| Outstanding, Weighted Average Exercise Price |
$ 6.25
|
| Outstanding, Weighted Average Remaining Life (Years) |
2 years 2 months 15 days
|
| Exercisable, Number of Option Shares | shares |
241,500
|
| Exercisable, Weighted Average Exercise Price |
$ 6.25
|
| Outstanding, Exercise Price |
6.25
|
| Range Four [Member] |
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
| Outstanding, exercise price lower range limit |
3.01
|
| Outstanding, exercise price upper range limit |
$ 6.00
|
| Outstanding, Number of Option Shares | shares |
11,250
|
| Outstanding, Weighted Average Exercise Price |
$ 5.83
|
| Outstanding, Weighted Average Remaining Life (Years) |
7 years 3 months 14 days
|
| Exercisable, Number of Option Shares | shares |
7,500
|
| Exercisable, Weighted Average Exercise Price |
$ 5.83
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-4
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-17
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAgreed-upon price for the exchange of the underlying asset relating to the share-based payment award.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe floor of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeLowerRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares reserved for issuance pertaining to the outstanding exercisable stock options as of the balance sheet date in the customized range of exercise prices for which the market and performance vesting condition has been satisfied. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeNumberOfExercisableOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares reserved for issuance pertaining to the outstanding stock options as of the balance sheet date for all option plans in the customized range of exercise prices. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeNumberOfOutstandingOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe ceiling of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeUpperRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average exercise price as of the balance sheet date for those equity-based payment arrangements exercisable and outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeExercisableOptionsWeightedAverageExercisePrice1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted average price as of the balance sheet date at which grantees could acquire the underlying shares with respect to all outstanding stock options which are in the customized range of exercise prices. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeOutstandingOptionsWeightedAverageExercisePriceBeginningBalance1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining contractual term of outstanding stock options, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeOutstandingOptionsWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=IMMX_RangeOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=IMMX_RangeTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=IMMX_RangeThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=IMMX_RangeFourMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Schedule of Stock Warrant Activity (Details)
|
9 Months Ended |
|
Sep. 30, 2024
$ / shares
shares
|
|---|
| Equity [Abstract] |
|
| Warrants, Outstanding and exercisable, Beginning | shares |
2,311,161
|
| Weighted-Average Exercise Price Per Share, Outstanding and exercisable Beginning | $ / shares |
$ 0.71
|
| Warrants, Granted | shares |
|
| Weighted-Average Exercise Price Per Share, Granted | $ / shares |
|
| Warrants, Exercised | shares |
|
| Weighted-Average Exercise Price Per Share, Exercised | $ / shares |
|
| Warrants, Forfeited | shares |
|
| Weighted-Average Exercise Price Per Share, Forfeited | $ / shares |
|
| Warrants, Expired | shares |
|
| Weighted-Average Exercise Price Per Share, Expired | $ / shares |
|
| Warrants, Outstanding and exercisable, Ending | shares |
2,311,161
|
| Weighted-Average Exercise Price Per Share, Outstanding and exercisable Ending | $ / shares |
$ 0.71
|
| X |
- Definition
+ References
+ Details
| Name: |
IMMX_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityWeightedAverageExercisePriceExercised |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
IMMX_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityWeightedAverageExercisePriceExpirations |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
IMMX_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityWeightedAverageExercisePriceForfeitures |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
IMMX_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityWeightedAverageExercisePriceGranted |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of non-option equity instruments exercised by participants. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityInstrumentsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares under non-option equity instrument agreements for which rights to exercise lapsed. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityInstrumentsExpirations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares under non-option equity instrument agreements that were cancelled as a result of occurrence of a terminating event. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityInstrumentsForfeitures |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNet number of non-option equity instruments granted to participants. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityInstrumentsGranted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of equity instruments other than options outstanding, including both vested and non-vested instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityInstrumentsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.3
Stockholders’ Equity (Details Narrative) - USD ($)
|
|
|
|
|
|
|
1 Months Ended |
3 Months Ended |
9 Months Ended |
12 Months Ended |
|
|
|
Oct. 01, 2024 |
Feb. 08, 2024 |
Feb. 05, 2024 |
Jul. 14, 2023 |
Apr. 24, 2023 |
Mar. 13, 2023 |
Feb. 29, 2024 |
Sep. 30, 2024 |
Jun. 30, 2024 |
Mar. 31, 2024 |
Dec. 31, 2023 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Dec. 31, 2023 |
Dec. 31, 2021 |
Sep. 10, 2021 |
Dec. 31, 2016 |
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock, shares authorized |
|
|
|
|
|
|
|
200,000,000
|
|
|
200,000,000
|
|
200,000,000
|
|
200,000,000
|
|
|
|
| Preferred stock, shares authorized |
|
|
|
|
|
|
|
10,000,000
|
|
|
10,000,000
|
|
10,000,000
|
|
10,000,000
|
|
|
|
| Common stock par value, per share |
|
|
|
|
|
|
|
$ 0.0001
|
|
|
$ 0.0001
|
|
$ 0.0001
|
|
$ 0.0001
|
|
|
|
| Preferred stock, par value |
|
|
|
|
|
|
|
$ 0.0001
|
|
|
$ 0.0001
|
|
$ 0.0001
|
|
$ 0.0001
|
|
|
|
| Number of common stock issued |
|
|
|
|
|
|
|
|
|
$ 15,520,354
|
|
|
|
|
|
|
|
|
| Proceeds from issuance initial public offering |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2,489
|
|
|
|
|
|
| Deferred offering costs |
|
|
|
|
|
|
|
$ 0
|
|
|
|
|
0
|
|
|
|
|
|
| Net proceeds from issuance |
|
$ 13,565,760
|
|
|
|
|
|
|
|
|
|
|
$ 15,946,078
|
14,936,437
|
|
|
|
|
| Number of shares issued upon exercise |
|
|
|
|
|
|
|
|
|
|
|
|
834
|
|
|
|
|
|
| Debt instrument unamortized stock based compensation. |
|
|
|
|
|
|
|
14,876
|
|
|
|
|
$ 14,876
|
|
|
|
|
|
| Stock-based compensation remaining to be amortized |
|
|
|
|
|
|
|
0
|
|
|
|
|
0
|
|
|
|
|
|
| Stock based compensation |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2,314,920
|
1,514,900
|
|
|
|
|
| Options granted to purchase common stock |
|
|
|
|
|
|
|
|
|
|
|
|
1,517,176
|
|
|
|
|
|
| Exercise price per share |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2.21
|
|
|
|
|
|
| Unrecognized stock-based compensation expense |
|
|
|
|
|
|
|
3,153,545
|
|
|
|
|
$ 3,153,545
|
|
|
|
|
|
| Weighted-average vesting period |
|
|
|
|
|
|
|
|
|
|
|
|
2 years 7 months 9 days
|
|
|
|
|
|
| Intrinsic value option vested |
|
|
|
|
|
|
|
200,675
|
|
|
|
|
$ 200,675
|
|
|
|
|
|
| Intrinsic value of the options exercised |
|
|
|
|
|
|
|
3,069
|
|
|
|
|
$ 3,069
|
|
|
|
|
|
| Non Employee [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Options granted to purchase common stock |
|
|
|
|
|
|
|
|
|
|
|
|
198,000
|
|
|
|
|
|
| Management [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Options granted to purchase common stock |
|
|
|
|
|
|
|
|
|
|
|
|
680,000
|
|
|
|
|
|
| Options term |
|
|
|
|
|
|
|
|
|
|
|
|
10 years
|
|
|
|
|
|
| Exercise price per share |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2.04
|
|
|
|
|
|
| Vest over period |
|
|
|
|
|
|
|
|
|
|
|
|
vest over periods of 12 to 48 equal monthly installments
|
|
|
|
|
|
| Consultant [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Options granted to purchase common stock |
|
|
|
|
|
|
|
|
|
|
|
|
43,500
|
|
|
|
|
|
| Exercise price per share, lower range limit |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2.11
|
|
|
|
|
|
| Options term |
|
|
|
|
|
|
|
|
|
|
|
|
10 years
|
|
|
|
|
|
| Vest over period |
|
|
|
|
|
|
|
|
|
|
|
|
vest in 48 equal monthly installments
|
|
|
|
|
|
| Exercise price per share, upper range limit |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2.17
|
|
|
|
|
|
| Restricted Stock [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Restricted common stock shares |
|
|
|
|
|
|
|
|
|
|
|
|
93,895
|
|
|
|
|
|
| Unrecognized stock-based compensation expense |
|
|
|
|
|
|
|
417,163
|
|
|
|
|
$ 417,163
|
|
|
|
|
|
| Remaining vesting period |
|
|
|
|
|
|
|
|
|
|
|
|
7 months 13 days
|
|
|
|
|
|
| Stock options unvested |
|
|
|
|
|
|
|
|
|
|
|
|
181,864
|
|
|
|
|
|
| Vesting period |
|
|
|
|
|
|
|
|
|
|
|
|
7 months 13 days
|
|
|
|
|
|
| General and Administrative Expense [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock based compensation |
|
|
|
|
|
|
|
450,299
|
|
|
|
$ 171,454
|
$ 965,600
|
$ 507,017
|
|
|
|
|
| General and Administrative Expense [Member] | Restricted Stock [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Recognized stock-based compensation |
|
|
|
|
|
|
|
$ 0
|
|
|
|
|
402,163
|
|
|
|
|
|
| Stock based compensation |
|
|
|
|
|
|
|
|
|
|
|
|
$ 263,962
|
|
|
|
|
|
| Nexcella 2022 Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exercise price per share, lower range limit |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2.47
|
|
|
|
|
|
| 2016 Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of shares available for grant |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,761,120
|
|
417,120
|
| 2021 Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of shares available for grant |
|
|
|
|
|
|
|
2,265,757
|
|
|
|
|
2,265,757
|
|
|
|
900,000
|
|
| Amended and Restated 2021 Omnibus Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock number of shares issued |
|
|
|
|
1,034,561
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Share price percentage |
|
|
|
|
5.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Amended and Restated 2022 Omnibus Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock number of shares issued |
|
|
|
|
3,000,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total number of shares reserve |
|
|
|
|
4,934,561
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Second Amended And Restated Nexcella 2022 Equity Incentive Plan [Member] | Equity Option [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of stock issued |
|
|
|
|
|
|
|
|
|
|
|
|
375,000
|
|
|
|
|
|
| Second Amended And Restated Nexcella 2022 Equity Incentive Plan [Member] | Equity Option [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of stock issued |
|
|
|
|
|
|
|
|
|
|
|
|
607,640
|
|
|
|
|
|
| Third Amended And Restated 2022 Equity Incentive Plan [Member] | Equity Option [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of stock issued |
|
|
|
|
|
|
|
|
|
|
|
|
607,640
|
|
|
|
|
|
| Third Amended And Restated 2022 Equity Incentive Plan [Member] | Equity Option [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of stock issued |
|
|
|
|
|
|
|
|
|
|
|
|
800,000
|
|
|
|
|
|
| Common Stock [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of common stock issued |
|
|
|
|
|
|
|
|
|
$ 632
|
|
|
|
|
|
|
|
|
| Number of stock issued |
|
|
|
|
|
|
|
|
|
6,319,025
|
|
|
|
|
|
|
|
|
| Number of shares issued upon exercise |
|
|
|
|
|
|
|
|
|
1,251
|
|
|
|
|
|
|
|
|
| Number of shares issued for services |
|
|
|
|
|
|
|
51,378
|
42,901
|
85,486
|
|
|
|
|
|
|
|
|
| Options granted to purchase common stock |
|
|
|
|
|
|
783,970
|
|
|
|
|
|
|
|
|
|
|
|
| Common Stock [Member] | Nexcella 2022 Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of stock issued |
|
|
|
|
|
|
|
|
|
|
|
|
375,000
|
|
|
|
|
|
| Restricted common stock shares |
|
|
|
|
|
|
|
|
|
|
|
|
275,759
|
|
|
|
|
|
| Options granted to purchase common stock |
|
|
|
|
|
|
|
|
|
|
|
|
595,676
|
|
|
|
|
|
| Warrant [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Intrinsic value outstanding |
|
|
|
|
|
|
|
$ 2,958,804
|
|
|
|
|
$ 2,958,804
|
|
|
|
|
|
| July ATM Facility [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sale of stock issued |
|
|
|
|
|
|
|
|
|
|
|
|
68,302
|
|
|
|
|
|
| Sale of stock, consideration received on transaction |
|
|
|
|
|
|
|
|
|
|
|
|
$ 338,495
|
|
|
|
|
|
| Deferred offering costs |
|
|
|
|
|
|
|
87,229
|
|
|
|
|
$ 87,229
|
|
|
|
|
|
| Underwriting Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of stock issued |
|
|
5,535,055
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offering price per share |
|
|
$ 2.71
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sale of stock price per share |
|
|
$ 2.5203
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Underwiring discounts and offering expenses |
|
|
$ 1,954,594
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Over-Allotment Option [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of stock issued |
|
|
783,970
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Nexcella [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of shares issued upon exercise |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Options granted to purchase common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exercise price per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Nexcella [Member] | Equity Option [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Recognized stock-based compensation |
|
|
|
|
|
|
|
$ 0
|
|
|
|
|
$ 148,319
|
|
|
|
|
|
| July ATM Sales Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock par value, per share |
|
|
|
$ 0.0001
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of common stock issued |
|
|
|
$ 4,200,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Proceeds from issuance initial public offering |
|
|
|
$ 75,000,000.0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Commission percentage |
|
|
|
3.75%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Deposit expense |
|
|
|
$ 15,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Legal fees |
|
|
|
$ 50,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cost fees and expense |
|
|
|
|
|
|
|
|
|
|
$ 10,000
|
$ 7,500
|
|
|
|
|
|
|
| Marketing Service Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Restricted common stock shares |
|
|
|
|
|
|
|
|
|
|
|
|
104,758
|
|
|
|
|
|
| Restricted common stock value |
|
|
|
|
|
|
|
|
|
|
|
|
$ 317,500
|
|
|
|
|
|
| Marketing Service Agreement [Member] | July 25, 2023 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Restricted common stock shares |
27,062
|
|
|
|
|
|
|
|
|
|
|
|
75,007
|
|
|
|
|
|
| Restricted common stock value |
|
|
|
|
|
|
|
|
|
|
|
|
$ 202,500
|
|
|
|
|
|
| Marketing Services Agreement [Member] | Common Stock [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of shares issued upon exercise |
|
|
|
|
|
|
|
|
|
|
|
|
1,251
|
|
|
|
|
|
| Proceeds from stock options exercised |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2,489
|
|
|
|
|
|
| Number of shares issued for future services |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
122,300
|
|
|
|
| Number of shares issued for future services, value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 247,500
|
|
|
|
| Number of shares issued for services |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
122,300
|
|
|
|
| Recognized stock-based compensation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 232,624
|
|
|
|
| Founders Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of stock issued |
|
|
|
|
|
238,220
|
|
|
|
|
|
|
|
|
|
|
|
|
| X |
- DefinitionDebt instrument amortized stock based compensation.
| Name: |
IMMX_DebtInstrumentAmortizedStockBasedCompensation |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionDebt instrument unamortized stock based compensation.
| Name: |
IMMX_DebtInstrumentUnamortizedStockBasedCompensation |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionShare based compensation arrangement by share based payment award shares reserve.
| Name: |
IMMX_ShareBasedCompensationArrangementByShareBasedPaymentAwardSharesReserve |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of common stock to be issued
| Name: |
IMMX_ShareBasedCompensationArrangementByShareBasedPaymentAwardSharesToBeIssued |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period shares issued for future services.
| Name: |
IMMX_StockIssuedDuringPeriodSharesIssuedForFutureServices |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period value issued for future services.
| Name: |
IMMX_StockIssuedDuringPeriodValueIssuedForFutureServices |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionUnderwiring discounts and offering expenses
| Name: |
IMMX_UnderwiringDiscountsAndOfferingExpenses |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionSpecific incremental costs directly attributable to a proposed or actual offering of securities which are deferred at the end of the reporting period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 5.A)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480341/340-10-S99-1
| Name: |
us-gaap_DeferredOfferingCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cost not yet recognized for nonvested award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cost to be recognized for nonvested award under share-based payment arrangement. Excludes share and unit options. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedShareBasedAwardsOtherThanOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of expense provided in the period for legal costs incurred on or before the balance sheet date pertaining to resolved, pending or threatened litigation, including arbitration and mediation proceedings. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(6))
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_LegalFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of cash paid for deposits on goods and services during the period; excludes time deposits and deposits with other institutions, which pertain to financial service entities. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-17
| Name: |
us-gaap_PaymentsForDeposits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from exercise of option under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_ProceedsFromStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionA fee charged for services from professionals such as doctors, lawyers and accountants. The term is often expanded to include other professions, for example, pharmacists charging to maintain a medicinal profile of a client or customer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (k)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-3
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_ProfessionalFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCash received on stock transaction after deduction of issuance costs.
| Name: |
us-gaap_SaleOfStockConsiderationReceivedOnTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued or sold by the subsidiary or equity method investee per stock transaction.
| Name: |
us-gaap_SaleOfStockNumberOfSharesIssuedInTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of service or performance condition required to be met for earning right to award under share-based payment arrangement. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of equity-based payment instruments, excluding stock (or unit) options, that vested during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which current fair value of underlying stock exceeds exercise price of fully vested and expected to vest exercisable or convertible options. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestExercisableAggregateIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount by which current fair value of underlying stock exceeds exercise price of fully vested and expected to vest options outstanding. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingAggregateIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe floor of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeLowerRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe ceiling of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeUpperRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPeriod from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining contractual term for fully vested and expected to vest options outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue, after forfeiture, of shares granted under share-based payment arrangement. Excludes employee stock ownership plan (ESOP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 30
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480513/718-10-30-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 30
-Section 35
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480843/718-30-35-1
| Name: |
us-gaap_StockGrantedDuringPeriodValueSharebasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued in lieu of cash for services contributed to the entity. Number of shares includes, but is not limited to, shares issued for services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTotal number of shares issued during the period, including shares forfeited, as a result of Restricted Stock Awards. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesRestrictedStockAwardGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SubsidiarySaleOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=IMMX_NonEmployeeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_ManagementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=IMMX_ConsultantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=IMMX_NexcellaTwoThousandTwentyTwoEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=IMMX_TwoThousandSixteenPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=IMMX_TwoThousandTwentyOnePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=IMMX_AmendedAndRestatedTwoThousandTwentyOneOmnibusEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=IMMX_AmendedAndRestatedTwoThousandTwentyTwoOmnibusEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=IMMX_SecondAmendedAndRestatedNexcellaTwoThousandTwentyTwoEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_StockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=IMMX_ThirdAmendedAndRestatedTwoThousandTwentyTwoEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=IMMX_JulyATMFacilityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=IMMX_UnderwritingAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_OverAllotmentOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=IMMX_NexcellaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_JulyATMSalesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_MarketingServiceAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=IMMX_JulyTwentyFiveTwoThousandTwentyThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_MarketingServicesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_FoundersAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Licenses Acquired (Details Narrative) - USD ($)
|
|
1 Months Ended |
3 Months Ended |
9 Months Ended |
Dec. 08, 2022 |
Aug. 31, 2024 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
|
|
| Upfront license fee |
$ 1,500,000
|
|
|
|
|
|
| Quarterly payments description |
Additional quarterly payments totaling approximately $13 million related to the Company’s
ongoing support of the CAR-T clinical trials currently ongoing at HADASIT, are due through September 2026, along with an annual license
fee of $50,000
|
|
|
|
|
|
| Royality payment percentage |
5.00%
|
|
|
|
|
|
| Revenues |
$ 20,000,000
|
|
|
|
|
|
| Research and development expense |
|
|
$ 4,445,528
|
$ 2,106,020
|
$ 9,918,336
|
$ 5,634,284
|
| License Agreement [Member] |
|
|
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
|
|
| Installment due paymant |
|
$ 500,000
|
|
|
|
|
| Net licensed |
|
1,500,000
|
|
|
|
|
| License Agreement [Member] | Phase II Studies [Member] |
|
|
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
|
|
| Milestone payments |
|
2,000,000
|
|
|
|
|
| License Agreement [Member] | Phase III Studies [Member] |
|
|
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
|
|
| Milestone payments |
|
10,000,000
|
|
|
|
|
| License Agreement [Member] | Licensed Product [Member] |
|
|
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
|
|
| Milestone payments |
|
$ 13,500,000
|
|
|
|
|
| License Agreement Terms [Member] |
|
|
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
|
|
| Research and development expense |
|
|
|
|
$ 2,393,063
|
$ 1,929,601
|
| X |
- DefinitionRoyality payment percentage.
| Name: |
IMMX_RoyalityPaymentPercentage |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of the frequency of periodic payments, which may be presented in a variety of ways (for example, monthly, quarterly, annually). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityFrequencyOfPayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCash received from licensees for license fees during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_ProceedsFromLicenseFeesReceived |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for short-term and long-term debt. Excludes payment of lease obligation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477314/942-235-S99-1
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_LicenseAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=IMMX_PhaseIIStudiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=IMMX_PhaseIIIStudiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=IMMX_LicensedProductMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- References
+ Details
| Name: |
IMMX_DisclosureCirmGrantsAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount as of the balance sheet date of amounts due under the terms of governmental, corporate, or foundation grants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_GrantsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.3
| X |
- References
+ Details
| Name: |
IMMX_DisclosureLeasesAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lease cost recognized by lessee for lease contract. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of short-term lease cost, excluding expense for lease with term of one month or less. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_ShortTermLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.3
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingLeaseLiabilityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.3
Schedule of Maturity Lease Liability (Details)
|
Sep. 30, 2024
USD ($)
|
| Leases |
|
| 2024 (remaining 3 months) |
$ 35,700
|
| 2025 |
147,798
|
| 2026 |
152,971
|
| 2027 |
158,325
|
| 2028 and thereafter |
1,073,349
|
| Total future undiscounted lease payments |
1,568,143
|
| Less: Interest |
(479,088)
|
| Present value of lease liabilities |
$ 1,089,055
|
| X |
- References
+ Details
| Name: |
IMMX_DisclosureLeasesAbstract |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLessee operating lease liability payments due year four and thereafter.
| Name: |
IMMX_LesseeOperatingLeaseLiabilityPaymentsDueYearFourAndThereafter |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease having initial or remaining lease term in excess of one year to be paid in remainder of current fiscal year. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsRemainderOfFiscalYear |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.3
| X |
- DefinitionIncremental percentage increase (decrease) in the stated rate on a debt instrument.
| Name: |
us-gaap_DebtInstrumentInterestRateIncreaseDecrease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionCash payments to lessor's for use of assets under operating leases. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PaymentsForRent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Commitments and Contingencies (Details Narrative) - USD ($)
|
|
|
|
|
|
|
1 Months Ended |
Nov. 09, 2023 |
May 12, 2023 |
Mar. 07, 2023 |
Nov. 09, 2022 |
Jun. 18, 2021 |
Mar. 18, 2021 |
Dec. 31, 2021 |
| Employment Agreement [Member] | Dr. Rachman [Member] |
|
|
|
|
|
|
|
| Loss Contingencies [Line Items] |
|
|
|
|
|
|
|
| Annual base salary |
|
$ 446,000
|
$ 475,000
|
$ 425,000
|
$ 360,000
|
|
|
| Performance bonuses percentage |
|
|
|
|
100.00%
|
|
|
| Employment Agreement [Member] | Dr. Rachman [Member] | Maximum [Member] |
|
|
|
|
|
|
|
| Loss Contingencies [Line Items] |
|
|
|
|
|
|
|
| Performance bonuses percentage |
50.00%
|
|
|
|
|
|
|
| Management Sevices Agreement [Member] | Mr. Morris [Member] |
|
|
|
|
|
|
|
| Loss Contingencies [Line Items] |
|
|
|
|
|
|
|
| Annual base salary |
|
$ 446,000
|
$ 475,000
|
$ 425,000
|
|
$ 240,000
|
$ 120,000
|
| Performance bonuses percentage |
50.00%
|
|
|
|
|
100.00%
|
|
| Reimburesments and accured percentage |
|
|
|
|
|
150.00%
|
|
| X |
- DefinitionReimburesments and accured percentage.
| Name: |
IMMX_ReimburesmentsAndAccuredPercentage |
| Namespace Prefix: |
IMMX_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 720
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483359/720-20-50-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 27
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482395/460-10-55-27
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-9
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-4
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482425/460-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-1
| Name: |
us-gaap_LossContingenciesLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for salary and wage arising from service rendered by nonofficer employee. Excludes allocated cost, labor-related nonsalary expense, and direct and overhead labor cost included in cost of good and service sold. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SalariesAndWages |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_EmploymentAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=IMMX_DrRachmanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_ManagementSevicesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=IMMX_MrMorrisMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTotal number of shares issued during the period, including shares forfeited, as a result of Restricted Stock Awards. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesRestrictedStockAwardGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate value of stock related to Restricted Stock Awards issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueRestrictedStockAwardGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=IMMX_MarketingServiceAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=IMMX_JulyTwentyFiveTwoThousandTwentyThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Immix Biopharma (NASDAQ:IMMX)
Historical Stock Chart
From Nov 2024 to Dec 2024

Immix Biopharma (NASDAQ:IMMX)
Historical Stock Chart
From Dec 2023 to Dec 2024
