false
0001558569
0001558569
2024-05-07
2024-05-07
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE
COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13
OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date
of earliest event reported): May 7, 2024
iSpecimen
Inc.
(Exact name of registrant
as specified in its charter)
| Delaware |
|
001-40501 |
|
27-0480143 |
(State
or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS
Employer
Identification No.) |
450
Bedford Street
Lexington,
MA
02420 |
| (Address of principal executive offices, including zip code) |
Registrant’s telephone
number, including area code: (781) 301-6700
Not Applicable
(Former name or former
address, if changed since last report)
Check the appropriate box below if the Form 8-K filing
is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications
pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant
to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications
pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications
pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
symbol(s) |
|
Name
of each exchange on which
registered |
| Common
Stock, par value $0.0001 per share |
|
ISPC |
|
The Nasdaq
Stock Market LLC |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of
the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company x
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 2.02. |
Results of Operations and Financial Condition. |
On May 7,
2024, iSpecimen Inc., a Delaware corporation (the “Company”), issued a press release (“Earnings Release”) announcing
its financial and operating results for the quarter ended March 31, 2024. A copy of the press release is furnished as Exhibit 99.1 to
this Current Report on Form 8-K. You are advised that financial information in the Earnings Release for the quarter ended March 31,
2024 is unaudited. The Company also filed its unaudited financial statements for the quarter ended March 31, 2024 with its Quarterly
Report on Form 10-Q on May 7, 2024.
The Earnings
Release contains certain statements and information that speak to the Company’s expectations or predictions of the future. These
statements and information may constitute “forward-looking statements” within the meaning of Section 27A of the Securities
Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the
“Exchange Act”). Such forward-looking statements are subject to risks and uncertainties, many of which are beyond the Company’s
control, which could cause the Company’s actual results to differ materially from those expressed in or implied by these statements.
Please see the Company’s disclosures regarding risk factors and forward-looking statements in its filings with the Securities and
Exchange Commission (the “SEC”) (including its Current Reports on Form 8-K, Quarterly Reports on Form 10-Q, and
most recent Annual Report on Form 10-K) for a discussion of the known material factors that could cause the Company’s actual
results to differ materially from those indicated or implied by such forward-looking statements.
The information
in this Item 2.02 and Exhibit 99.1 attached hereto will not be deemed “filed” for purposes of Section 18
of the Exchange Act or otherwise subject to the liabilities of that section, nor will it be deemed incorporated by reference in any filing
under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 7.01. |
Regulation FD Disclosure. |
The Company
also held a conference call and audio webcast at 8:30 a.m. Eastern Time on May 7, 2024 (“Earnings Call”) in which
it discussed its first quarter of 2024 results. The Company issued a press release on April 25, 2024, providing information on how
to access the Earnings Call. A replay of the Earnings Call is available through May 21, 2024, by calling 1+1-844-512-2921 (U.S. Toll
Free) or +1-412-317-6671 (International). An archived version of the Earnings Call will also be available on the Company’s Investor
Relations site at https://investors.ispecimen.com/presentations/. During the Earnings Call, the Company provided an investor
presentation, dated May 2024 (the “Presentation”), which is attached hereto as Exhibit 99.2 and
incorporated by reference herein. The Presentation will also be posted on the Company’s website and can be accessed at https://investors.ispecimen.com/.
The Company expressly disclaims any obligation to update the Presentation, or any other information posted on or available through its
website, and cautions that the information set forth therein is only accurate as of the date indicated on such materials. The inclusion
of any data or statements in the Presentation (or available on or through the Company’s website) does not signify that such information
is considered material.
The information
in this Item 7.01 and Exhibit 99.2 attached hereto will not be deemed “filed” for purposes of Section 18
of the Exchange Act or otherwise subject to the liabilities of that section, nor will it be deemed incorporated by reference in any filing
under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing.
Forward-Looking Statements
This Current
Report on Form 8-K may contain forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E
of the Exchange Act. Such forward-looking statements are characterized by future or conditional verbs such as “may,” “will,”
“expect,” “intend,” “anticipate,” “believe,” “estimate,” “continue”
or similar words. You should read statements that contain these words carefully because they discuss future expectations and plans, which
contain projections of future results of operations or financial condition or state other forward-looking information. Such statements
are only predictions and the Company’s actual results may differ materially from those anticipated in these forward-looking statements.
There may
be events in the future that the Company is not able to accurately predict or control. Factors that may cause such differences include,
but are not limited to, those discussed under risk factors in the Company’s Annual Report on Form 10-K for the year ended December 31,
2023 and other filings filed with the SEC, including the uncertainties associated with the Company’s lack of profitability, its
continued capital needs, its lack of a long operating history, its growth strategy, inflation and recession and its impact on the business,
Russia’s war with Ukraine and its impact on the operations, its technology development plans, and the regulatory environment in
which it operates. Forward-looking statements speak only as of the date they are made. The Company does not assume any obligation to update
forward-looking statements as circumstances change. The Company gives no assurance that it will achieve its expectations.
You may access
the Company’s SEC filings by visiting SEC’s website at http://www.sec.gov. This Current Report on Form 8-K
does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with the Company
or its affiliates. The information in this Current Report on Form 8-K is not targeted at the residents of any particular country
or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution
or use would be contrary to local law or regulation.
| Item 9.01. |
Financial Statements and Exhibits. |
Portions of this report may
constitute “forward-looking statements” as defined by federal law. Although the Company believes any such statements are based
on reasonable assumptions, there is no assurance that actual outcomes will not be materially different. Additional information about issues
that could lead to material changes in the Company’s performance is contained in the Company’s filings with the SEC.
SIGNATURE
Pursuant to
the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the
undersigned hereunto duly authorized.
Dated: May 7, 2024
| |
iSPECIMEN INC. |
| |
|
|
| |
By: |
/s/ Tracy Curley |
| |
Name: |
Tracy Curley |
| |
|
Title: Chief Executive Officer |
Exhibit 99.1
iSpecimen Reports First Quarter 2024 Results
LEXINGTON, Mass.,
May 7, 2024 – iSpecimen Inc. (Nasdaq: ISPC) (“iSpecimen” or the “Company”),
an online global marketplace that connects scientists requiring biospecimens for medical research with a network of healthcare specimen
providers, today reported its financial and operating results for the three-month period ended March 31, 2024.
“iSpecimen made tremendous progress during
the first quarter advancing our most promising operational initiative, Next Day Quotes, a program that expedites the biospecimen transaction
process, which has helped elevate our market position and is expected to contribute meaningfully to our growth in 2024 and beyond,”
said Tracy Curley, CEO of iSpecimen. “To transition our suppliers to Next Day Quotes, we have strategically refined our supplier
network, deeply aligning and effectively engaging with our strongest suppliers to maximize their operational capabilities, while terminating
relationships with suppliers unable to support the needs of our expanding customer base.”
“In the first quarter of 2024, 40% of all
quotes provided to customers qualified as Next Day Quotes, compared to 34% in the fourth quarter of 2023 and 25% in the third quarter
of 2023. This favorable quarter-over-quarter increase in Next Day Quotes was directly correlated to growth across all of our business
lines. During the first quarter of 2024, 91% of prospective Next Day Quotes dollars converted to purchase orders, compared to 32% in the
fourth quarter of 2023. As we continue to refine and strengthen our supplier network, I am confident transitioning a majority of
our business into Next Day Quotes will result in stronger positioning for iSpecimen and result in greatly improved top- and bottom-line
results over time,” concluded Ms. Curley.
First Quarter 2024 Highlights
| ● | As of March 31, 2024, iSpecimen had over 140 unique supplier organizations under contract, a reduction
of 103 from 243 suppliers at December 31, 2023, a planned reduction related to the Company’s focus on building a higher quality
of our supplier network; |
| ● | As of March 31, 2024, over 600 unique customer organizations purchased from iSpecimen, an increase
of 66 from 534 at the end of the first quarter 2023; |
| ● | iSpecimen Marketplace had nearly 7,564 registered research and supplier users as of March 31, 2024,
up approximately 9% from 6,918 as of March 31, 2023. |
Recent Corporate Updates
| ● | iSpecimen
and TriMetis Life Sciences Announce Strategic Partnership to help Transform Tissue-Based
Research |
Financial Results for First Quarter 2024
For the quarter ended March 31, 2024, revenue
was approximately $2.29 million, compared to approximately $2.95 million for the quarter ended March 31, 2023. The reduction was
primarily due to a decrease of 3,388 specimens, or approximately 39%, in specimen count, from 8,629 specimens during the quarter ended
March 31, 2023 to 5,241 specimens during the quarter ended March 31, 2024. The effect of the decrease in specimen count was
partially offset by an increase in the average selling price per specimen by approximately $95, or approximately 28%, from $342 in the
first quarter of 2023 to approximately $437 in the first quarter of 2024.
For the quarter ended March 31, 2024, cost
of revenue decreased by approximately $147,000, or approximately 13%, to approximately $1 million, compared to approximately $1.15 million
for the quarter ended March 31, 2023. The decrease in cost of revenue was attributable to a 39% decrease in the number of specimens
accessioned for the current period, compared to the same period in the prior year, partially offset by a $58, or 44%, increase in the
average cost per specimen.
For the quarter ended March 31, 2024, general
and administrative expenses increased by approximately $286,000 or approximately 16%, to approximately $2.10 million, compared to approximately
$1.82 million for the quarter ended March 31, 2023. The increase was attributable to increases in professional fees of approximately
$329,000, taxes and insurance of approximately $267,000, and bad debt expense of approximately $45,000, which were partially offset by
decreases in compensation costs of approximately $250,000, general operating expenses of approximately $78,000, depreciation and amortization
of approximately $23,000, and utilities and facilities expenses of approximately $4,000.
For the quarter ended March 31, 2024, the
net loss was approximately $2.90 million, or ($0.32) per share, compared to a net loss of approximately $2.43 million, or ($0.27) per
share, for the same period the prior quarter.
As of March 31, 2024, cash and cash equivalents,
along with available-for-sale securities, were approximately $2.56 million, compared to approximately $5.01 million as of December 31,
2023.
On March 5,
2024, iSpecimen entered into an At the Market Offering Agreement, under which iSpecimen may issue and sell shares of its common
stock from time to time for an aggregate offering price of up to $1.5 million, which shares, when issued, will be registered pursuant
to its shelf registration statement. The Company may seek additional funding through public equity or other sources to fund further capital
investments or for general corporate purposes.
Conference Call and Webcast Information
The Company will host a conference call and audio
webcast on Tuesday, May 7, 2024, at 8:30 a.m. Eastern Time featuring remarks by Tracy Curley, CEO.
| Event: |
iSpecimen First Quarter 2024 Results Conference Call |
| Date: |
Tuesday, May 7, 2024 |
| Time: |
8:30 a.m. Eastern Time |
| Dial in: |
1-800-717-1738 (U.S. Toll Free) or 1-646-307-1865 (International) |
| Webcast: |
https://viavid.webcasts.com/starthere.jsp?ei=1666705&tp_key=0ac1c8e17f |
For interested
individuals unable to join the conference call, a replay will be available through May 21, 2024, at +1-844-512-2921 (U.S. Toll Free)
or +1-412-317-6671 (International). Participants must use the following code to access the replay of the call: 1170104. An archived version
of the webcast will also be available on iSpecimen’s Investor Relations site: https://investors.ispecimen.com/presentations/.
About iSpecimen
iSpecimen (Nasdaq:
ISPC) offers an online marketplace for human biospecimens, connecting scientists in commercial and non-profit organizations with healthcare
providers that have access to patients and specimens needed for medical discovery. Proprietary, cloud-based technology enables scientists
to intuitively search for specimens and patients across a federated partner network of hospitals, labs, biobanks, blood centers and other
healthcare organizations. For more information, please visit www.ispecimen.com.
Forward Looking Statements
This press release may contain forward-looking
statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange
Act of 1934, as amended. Such forward-looking statements are characterized by future or conditional verbs such as "may," "will,"
"expect," "intend," "anticipate," “believe," "estimate," "continue" or similar
words. You should read statements that contain these words carefully because they discuss future expectations and plans, which contain
projections of future results of operations or financial condition or state other forward-looking information.
Forward-looking statements are predictions,
projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject
to risks and uncertainties. Many factors could cause actual future events to differ materially from the forward-looking statements in
this press release, including but not limited to the risk factors contained in the Company's filings with the U.S. Securities and Exchange
Commission, which are available for review at www.sec.gov. Forward-looking statements speak only as of the date they are made. New risks
and uncertainties arise over time, and it is not possible for the Company to predict those events or how they may affect the Company.
If a change to the events and circumstances reflected in the Company's forward-looking statements occurs, the Company's business, financial
condition and operating results may vary materially from those expressed in the Company's forward-looking statements.
Readers are cautioned not to put undue reliance
on forward-looking statements, and the Company assumes no obligation and does not intend to update or revise these forward-looking statements,
whether as a result of new information, future events or otherwise.
For further information, please contact:
Investor Contact
KCSA Strategic Communications
Phil Carlson / Erika Kay
iSpecimen@kcsa.com
iSpecimen Inc.
Condensed Balance Sheets
| | |
March 31,
2024 | | |
December 31,
2023 | |
| |
(Unaudited) | | |
| |
| ASSETS | |
| | |
| |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 2,089,891 | | |
$ | 2,343,666 | |
| Available-for-sale securities | |
| 466,493 | | |
| 2,661,932 | |
| Accounts receivable – unbilled | |
| 1,978,144 | | |
| 2,212,538 | |
| Accounts receivable, net of allowance for doubtful accounts of $718,821 and $520,897 at March 31, 2024 and December 31, 2023, respectively | |
| 437,424 | | |
| 728,388 | |
| Prepaid expenses and other current assets | |
| 313,999 | | |
| 292,079 | |
| Total current assets | |
| 5,285,951 | | |
| 8,238,603 | |
| Property and equipment, net | |
| 120,873 | | |
| 127,787 | |
| Internally developed software, net | |
| 6,065,770 | | |
| 6,323,034 | |
| Other intangible assets, net | |
| 860,366 | | |
| 908,255 | |
| Operating lease right-of-use asset | |
| 153,340 | | |
| 193,857 | |
| Security deposits | |
| 27,601 | | |
| 27,601 | |
| Total assets | |
$ | 12,513,901 | | |
$ | 15,819,137 | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 3,560,474 | | |
$ | 3,925,438 | |
| Accrued expenses | |
| 1,710,815 | | |
| 1,540,607 | |
| Operating lease current obligation | |
| 156,703 | | |
| 167,114 | |
| Deferred revenue | |
| 272,681 | | |
| 415,771 | |
| Total current liabilities | |
| 5,700,673 | | |
| 6,048,930 | |
| Operating lease long-term obligation | |
| — | | |
| 29,130 | |
| Total liabilities | |
| 5,700,673 | | |
| 6,078,060 | |
| | |
| | | |
| | |
| Commitments and contingencies (See Note 8) | |
| | | |
| | |
| | |
| | | |
| | |
| Stockholders’ equity | |
| | | |
| | |
| Common stock, $0.0001 par value, 200,000,000 shares authorized, 9,501,112 issued and 9,470,112 outstanding at March 31, 2024 and 9,114,371 issued and 9,083,371 outstanding at December 31, 2023 | |
| 947 | | |
| 908 | |
| Additional paid-in capital | |
| 69,079,341 | | |
| 69,104,313 | |
| Treasury stock, 31,000 shares at March 31, 2024 and December 31, 2023, at cost | |
| (172 | ) | |
| (172 | ) |
| Accumulated other comprehensive income | |
| 41 | | |
| 840 | |
| Accumulated deficit | |
| (62,266,929 | ) | |
| (59,364,812 | ) |
| Total stockholders’ equity | |
| 6,813,228 | | |
| 9,741,077 | |
| Total liabilities and stockholders’ equity | |
$ | 12,513,901 | | |
$ | 15,819,137 | |
iSpecimen Inc.
Condensed Statements of Operations and Comprehensive
Loss
(Unaudited)
| | |
Three Months Ended March 31, | |
| | |
2024 | | |
2023 | |
| Revenue | |
$ | 2,289,993 | | |
$ | 2,950,197 | |
| Operating expenses: | |
| | | |
| | |
| Cost of revenue | |
| 1,000,006 | | |
| 1,146,912 | |
| Technology | |
| 911,967 | | |
| 834,407 | |
| Sales and marketing | |
| 665,941 | | |
| 962,169 | |
| Supply development | |
| 197,839 | | |
| 275,246 | |
| Fulfillment | |
| 410,854 | | |
| 455,531 | |
| General and administrative | |
| 2,103,906 | | |
| 1,818,355 | |
| Total operating expenses | |
| 5,290,513 | | |
| 5,492,620 | |
| | |
| | | |
| | |
| Loss from operations | |
| (3,000,520 | ) | |
| (2,542,423 | ) |
| | |
| | | |
| | |
| Other income, net | |
| | | |
| | |
| Interest expense | |
| (4,465 | ) | |
| (3,535 | ) |
| Interest income | |
| 30,498 | | |
| 114,263 | |
| Other income (expense), net | |
| 72,370 | | |
| (117 | ) |
| Total other income, net | |
| 98,403 | | |
| 110,611 | |
| | |
| | | |
| | |
| Net loss | |
$ | (2,902,117 | ) | |
$ | (2,431,812 | ) |
| | |
| | | |
| | |
| Other comprehensive income (loss): | |
| | | |
| | |
| Net loss | |
$ | (2,902,117 | ) | |
$ | (2,431,812 | ) |
| Unrealized gain (loss) on available-for-sale securities | |
| (799 | ) | |
| 18,843 | |
| Total other comprehensive income (loss) | |
| (799 | ) | |
| 18,843 | |
| Comprehensive loss | |
$ | (2,902,916 | ) | |
$ | (2,412,969 | ) |
| | |
| | | |
| | |
| Net loss per share - basic and diluted | |
$ | (0.32 | ) | |
$ | (0.27 | ) |
| | |
| | | |
| | |
| Weighted average shares of common stock outstanding - basic and diluted | |
| 9,132,460 | | |
| 8,980,898 | |
Exhibit 99.2

| May 2024
Investor Presentation
Nasdaq: ISPC
The Online Marketplace for Human
Biospecimens
Transforming Biospecimen Procurement |

| This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Such forward-looking statements are
characterized by future or conditional verbs such as “may,” “will,” “expect,” “intend,” “anticipate,” “believe,” “estimate” and
“continue” or similar words. You should read statements that contain these words carefully because they discuss future
expectations and plans, which contain projections of future results of operations or financial condition or state other forward-looking information. Such statements are only predictions and our actual results may differ materially from those anticipated
in these forward-looking statements.
We believe that it is important to communicate future expectations to investors. However, there may be events in the future
that we are not able to accurately predict or control. Factors that may cause such differences include, but are not limited to,
those discussed under Risk Factors in our filings filed with the Securities and Exchange Commission (the "SEC"), including the
uncertainties associated with our lack of profitability, our continued capital needs, our lack of a long operating history, our
growth strategy, the uncertain effect of geopolitical developments, our technology development plans, and the regulatory
environment in which we operate. We do not assume any obligation to update forward-looking statements as circumstances
change.
Certain market data information in this presentation is based on management's estimates. We obtained the industry, market
and competitive position data used throughout this presentation from internal estimates and research as well as from industry
publications and research, surveys and studies conducted by third parties. We believe our estimates to be accurate as of the
date of this presentation. However, this information may prove to be inaccurate because of the method by which we obtained
some of the data for our estimates or because this information cannot always be verified due to the limits on the availability
and reliability of raw data, and the nature of the data gathering process.
You may access our SEC filings by visiting the SEC’s website at http://www.sec.gov. This presentation does not constitute an
offer or invitation for the sale or purchase of securities or to engage in any other transaction with us or our affiliates. The
information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for
distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law
or regulation.
2
Forward-Looking Statements |

| Our Vision
To transform the
biospecimen industry
with an online
marketplace connecting
researchers to patients,
biospecimens, and data,
through our global
network.
3 |

| 4
1. Similar to Amazon, iSpecimen has developed an
online marketplace to seamlessly connect
researchers with healthcare providers and supplier
organizations
2. First mover advantage that addresses an
inefficient and fragmented global biospecimen
supply chain that is poised to be disrupted by an
online solution
3. $3B-$4B global biospecimen market growing at
10-15% per year 1,2
4. Strong revenue growth with an 8-year CAGR of
39% fueled by precision and regenerative
medicine research
5. Plans to enhance existing platform and develop
new service offering to increase revenue
opportunities
Investor Highlights
>140
Healthcare Provider / Supplier
Organizations
>600
Customer
Organizations
1,2 “Sources Cited” page 31 |
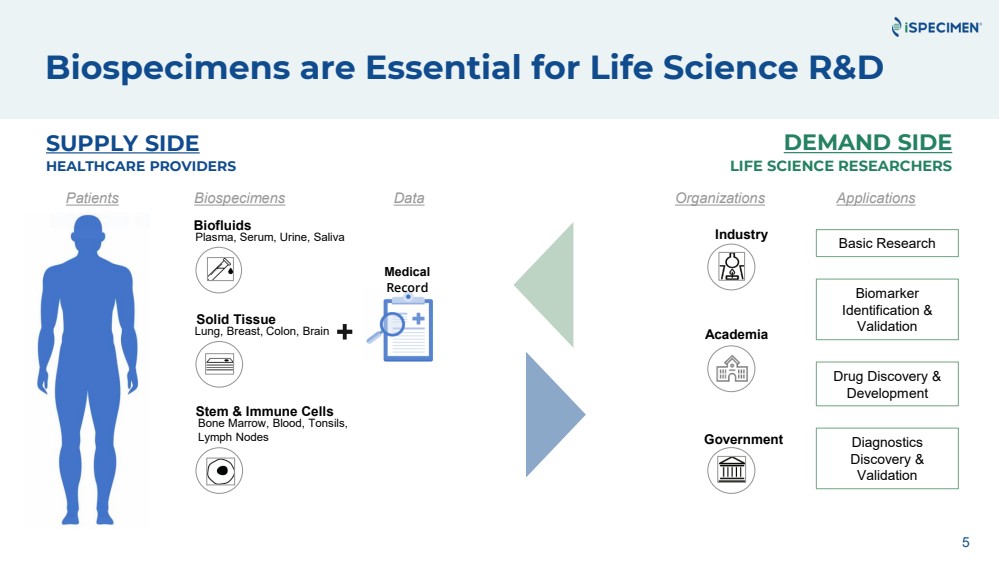
| Biospecimens are Essential for Life Science R&D
Basic Research
Biomarker
Identification &
Validation
Drug Discovery &
Development
Diagnostics
Discovery &
Validation
Biofluids
Solid Tissue
Stem & Immune Cells
Plasma, Serum, Urine, Saliva Industry
Academia
Government
5
Lung, Breast, Colon, Brain
Bone Marrow, Blood, Tonsils,
Lymph Nodes
Patients Applications
SUPPLY SIDE
HEALTHCARE PROVIDERS
DEMAND SIDE
LIFE SCIENCE RESEARCHERS
Biospecimens Data Organizations
Medical
Record |
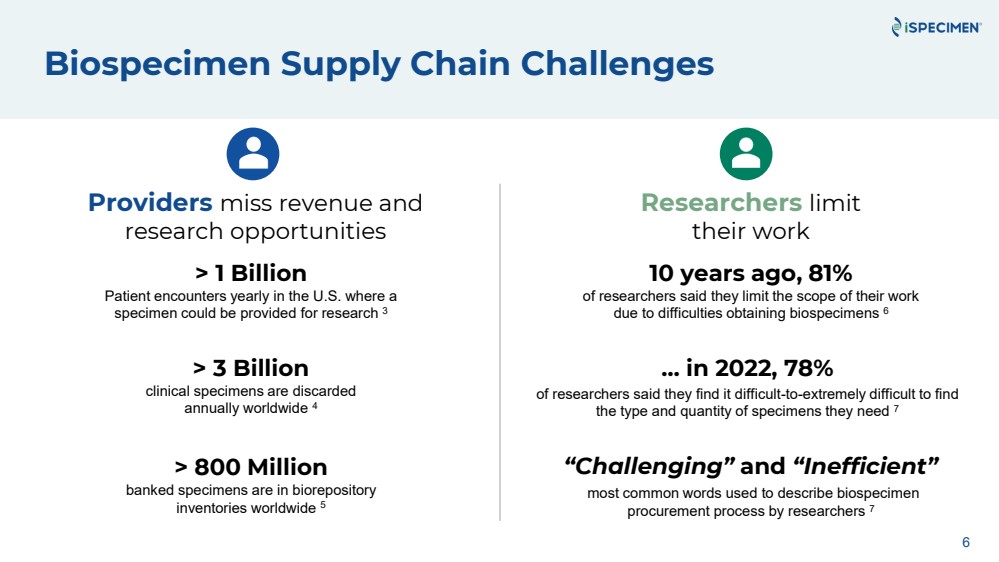
| Biospecimen Supply Chain Challenges
Providers miss revenue and
research opportunities
> 3 Billion
clinical specimens are discarded
annually worldwide 4
> 1 Billion
Patient encounters yearly in the U.S. where a
specimen could be provided for research 3
> 800 Million
banked specimens are in biorepository
inventories worldwide 5
6
Researchers limit
their work
… in 2022, 78%
of researchers said they find it difficult-to-extremely difficult to find
the type and quantity of specimens they need 7
“Challenging” and “Inefficient”
most common words used to describe biospecimen
procurement process by researchers 7
10 years ago, 81%
of researchers said they limit the scope of their work
due to difficulties obtaining biospecimens 6 |

| Biospecimen Supply Chain Challenges
7
-Difficult to Connect, Search and Compliantly Transact
-Average time to develop relationships is 6-12 months ᶟ
PROVIDERS CHALLENGE RESEARCHERS |

| iSpecimen Marketplace | Approach
Demand Side
Life Science Researchers
Supply Side
Providers |

| Full-Service Procurement Offering
-Connect Suppliers with Researchers
-Manage the Entire Procurement Process
SPECIMEN SEARCH & SELECTION
SPECIMEN LOGISTICS
SPECIMEN MANAGEMENT
DATA MANAGEMENT
STUDY/ORDER MANAGEMENT
SUPPLIER SITE TRAINING
COLLECTION KIT BUILDING
COMPLIANCE MANAGEMENT
STUDY DESIGN & SPECIFICATION
CONTRACTING
9
iSpecimen Services |

| Advance Scientific Discovery
Support research mission and advance
diagnostic, therapeutic and vaccine research
Monetize Banked Biospecimens
Instantly connect to a global network of
researchers in search for biospecimens
Ensure Compliance
Protect the privacy and security of patient
information
Accelerate Research
Search for specimens anytime, anywhere,
through easy-to-use online marketplace
Save Time and Money
Instantly connect to global network of
specimen providers
Reduce Risk
Manage contracting and regulatory compliance
Providers… Researchers…
10
iSpecimen Value Proposition |
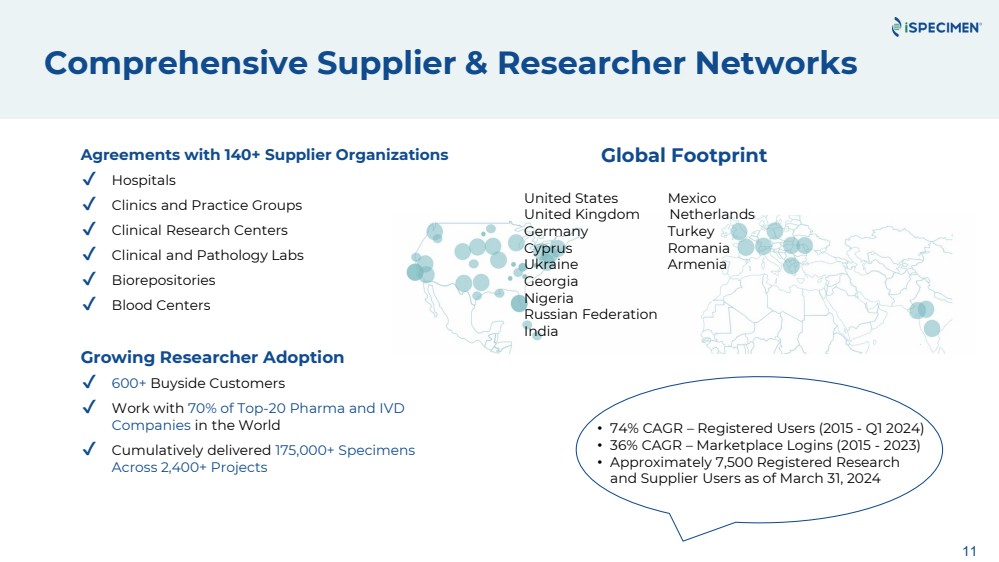
| 11
Agreements with 140+ Supplier Organizations
✔ Hospitals
✔ Clinics and Practice Groups
✔ Clinical Research Centers
✔ Clinical and Pathology Labs
✔ Biorepositories
✔ Blood Centers
Growing Researcher Adoption
✔ 600+ Buyside Customers
✔ Work with 70% of Top-20 Pharma and IVD
Companies in the World
✔ Cumulatively delivered 175,000+ Specimens
Across 2,400+ Projects
• 74% CAGR – Registered Users (2015 - Q1 2024)
• 36% CAGR – Marketplace Logins (2015 - 2023)
• Approximately 7,500 Registered Research
and Supplier Users as of March 31, 2024
Comprehensive Supplier & Researcher Networks
Global Footprint
United States Mexico
United Kingdom Netherlands
Germany Turkey
Cyprus Romania
Ukraine Armenia
Georgia
Nigeria
Russian Federation
India |

| Strategic Growth Initiatives
12 |

| Enhance iSpecimen Marketplace Technology
13
Increased investment and ongoing advancements of
iSpecimen Marketplace technology would enable:
● Improved integration with provider partners (our first patient data
electronic record integrations)
● Improved iSpecimen Marketplace search and request capabilities
● Updated back-end architecture to support growth and scale,
enhanced security, and us to prepare for a data-as-a-service pilot
● Utilization of insights provided by supplier and researcher
interactions to evolve matchmaking capabilities |
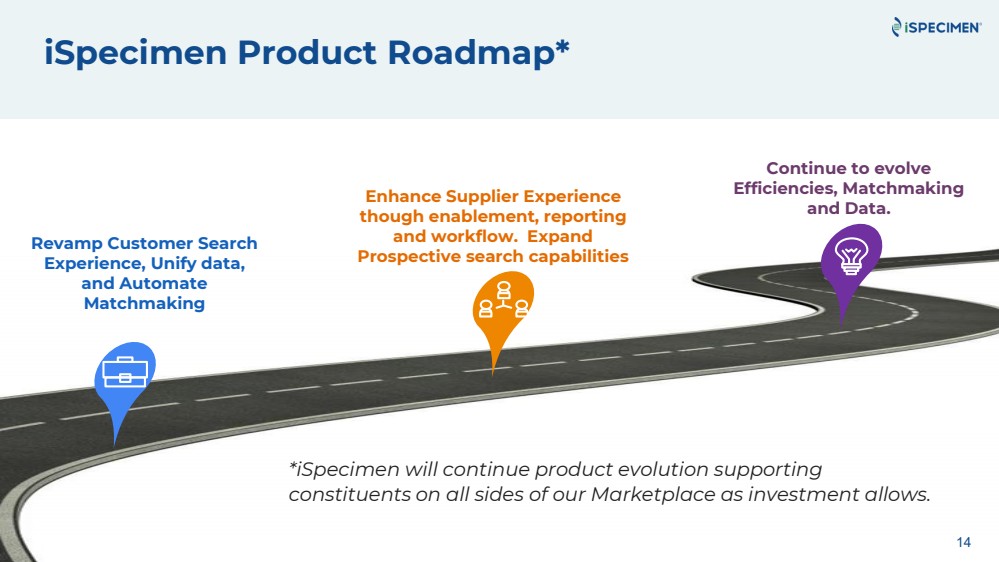
| iSpecimen Product Roadmap*
Revamp Customer Search
Experience, Unify data,
and Automate
Matchmaking
Enhance Supplier Experience
though enablement, reporting
and workflow. Expand
Prospective search capabilities
Continue to evolve
Efficiencies, Matchmaking
and Data.
*iSpecimen will continue product evolution supporting
constituents on all sides of our Marketplace as investment allows.
14 |
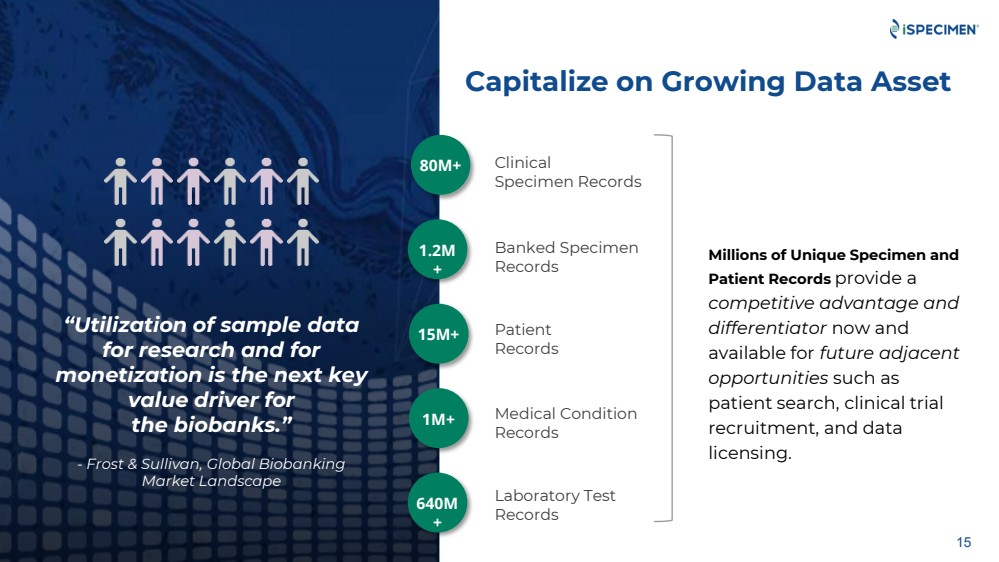
| “Utilization of sample data
for research and for
monetization is the next key
value driver for
the biobanks.”
- Frost & Sullivan, Global Biobanking
Market Landscape
Capitalize on Growing Data Asset
Banked Specimen
Records
Patient
Records
Medical Condition
Records
Clinical
Specimen Records
Laboratory Test
Records
Millions of Unique Specimen and
Patient Records provide a
competitive advantage and
differentiator now and
available for future adjacent
opportunities such as
patient search, clinical trial
recruitment, and data
licensing.
80M+
15
1.2M
+
15M+
1M+
640M
+ |

| Financial Overview
16 |
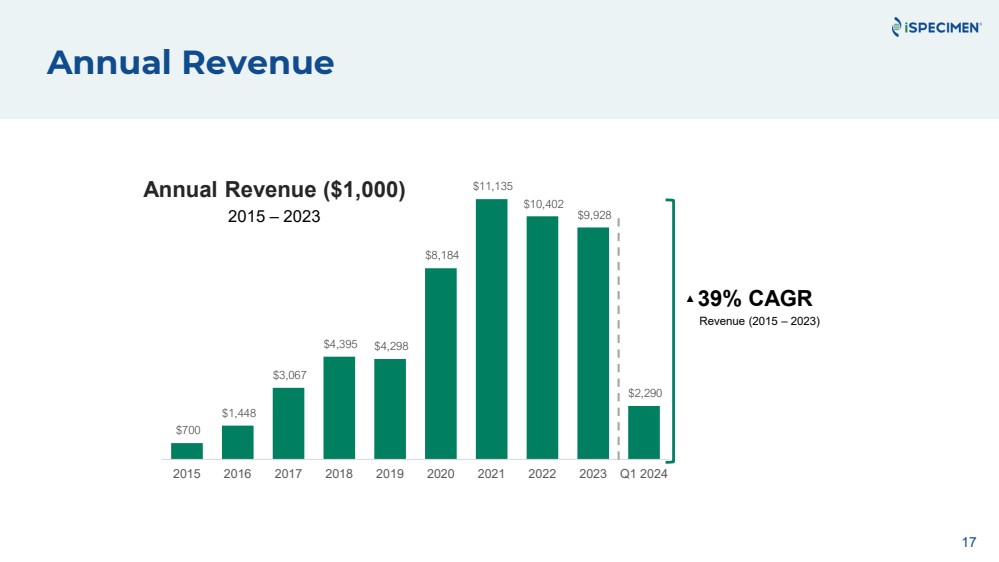
| $700
$1,448
$3,067
$4,395 $4,298
$8,184
$11,135
$10,402
$9,928
$2,290
2015 2016 2017 2018 2019 2020 2021 2022 2023 Q1 2024
Annual Revenue ($1,000)
2015 – 2023
▲ 39% CAGR
Revenue (2015 – 2023)
Annual Revenue
17 |
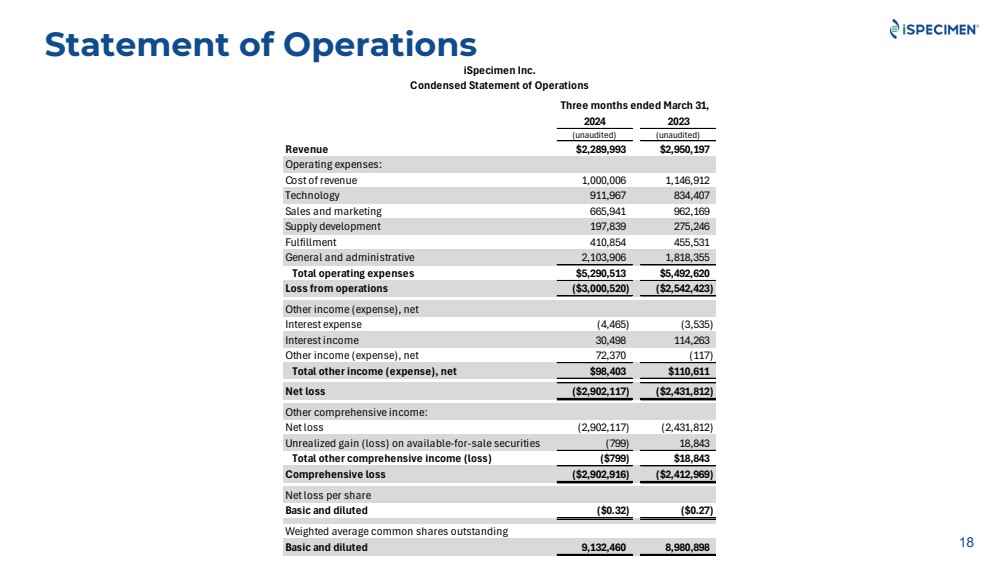
| Statement of Operations
18
iSpecimen Inc.
Condensed Statement of Operations
Three months ended March 31,
2024 2023
(unaudited) (unaudited)
Revenue $2,289,993 $2,950,197
Operating expenses:
Cost of revenue 1,000,006 1,146,912
Technology 911,967 834,407
Sales and marketing 665,941 962,169
Supply development 197,839 275,246
Fulfillment 410,854 455,531
General and administrative 2,103,906 1,818,355
Total operating expenses $5,290,513 $5,492,620
Loss from operations ($3,000,520) ($2,542,423)
Other income (expense), net
Interest expense (4,465) (3,535)
Interest income 30,498 114,263
Other income (expense), net 72,370 (117)
Total other income (expense), net $98,403 $110,611
Net loss ($2,902,117) ($2,431,812)
Other comprehensive income:
Net loss (2,902,117) (2,431,812)
Unrealized gain (loss) on available-for-sale securities (799) 18,843
Total other comprehensive income (loss) ($799) $18,843
Comprehensive loss ($2,902,916) ($2,412,969)
Net loss per share
Basic and diluted ($0.32) ($0.27)
Weighted average common shares outstanding
Basic and diluted 9,132,460 8,980,898 |
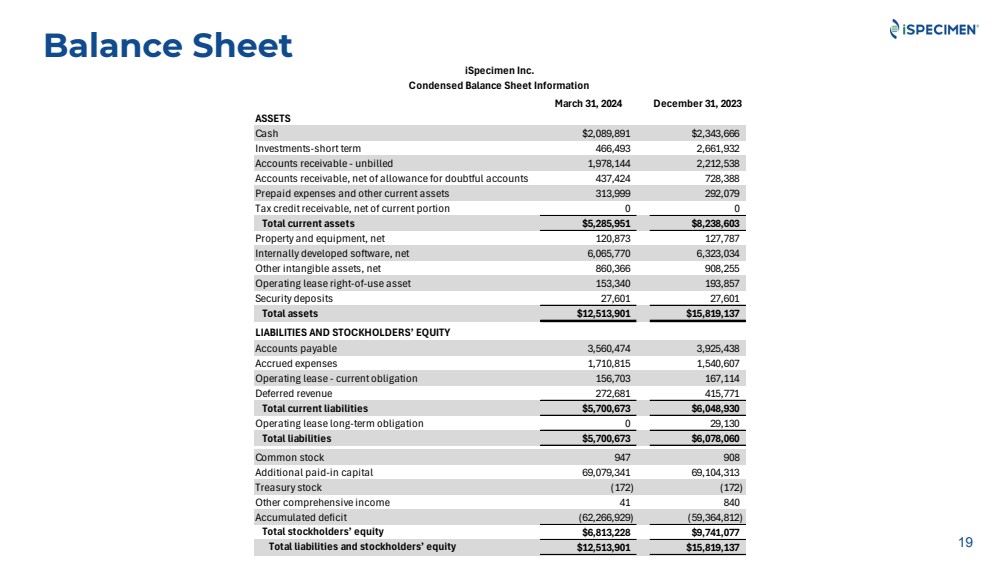
| Balance Sheet
19
iSpecimen Inc.
Condensed Balance Sheet Information
March 31, 2024 December 31, 2023
ASSETS
Cash $2,089,891 $2,343,666
Investments-short term 466,493 2,661,932
Accounts receivable - unbilled 1,978,144 2,212,538
Accounts receivable, net of allowance for doubtful accounts 437,424 728,388
Prepaid expenses and other current assets 313,999 292,079
Tax credit receivable, net of current portion 0 0
Total current assets $5,285,951 $8,238,603
Property and equipment, net 120,873 127,787
Internally developed software, net 6,065,770 6,323,034
Other intangible assets, net 860,366 908,255
Operating lease right-of-use asset 153,340 193,857
Security deposits 27,601 27,601
Total assets $12,513,901 $15,819,137
LIABILITIES AND STOCKHOLDERS’ EQUITY
Accounts payable 3,560,474 3,925,438
Accrued expenses 1,710,815 1,540,607
Operating lease - current obligation 156,703 167,114
Deferred revenue 272,681 415,771
Total current liabilities $5,700,673 $6,048,930
Operating lease long-term obligation 0 29,130
Total liabilities $5,700,673 $6,078,060
Common stock 947 908
Additional paid-in capital 69,079,341 69,104,313
Treasury stock (172) (172)
Other comprehensive income 41 840
Accumulated deficit (62,266,929) (59,364,812)
Total stockholders’ equity $6,813,228 $9,741,077
Total liabilities and stockholders’ equity $12,513,901 $15,819,137 |
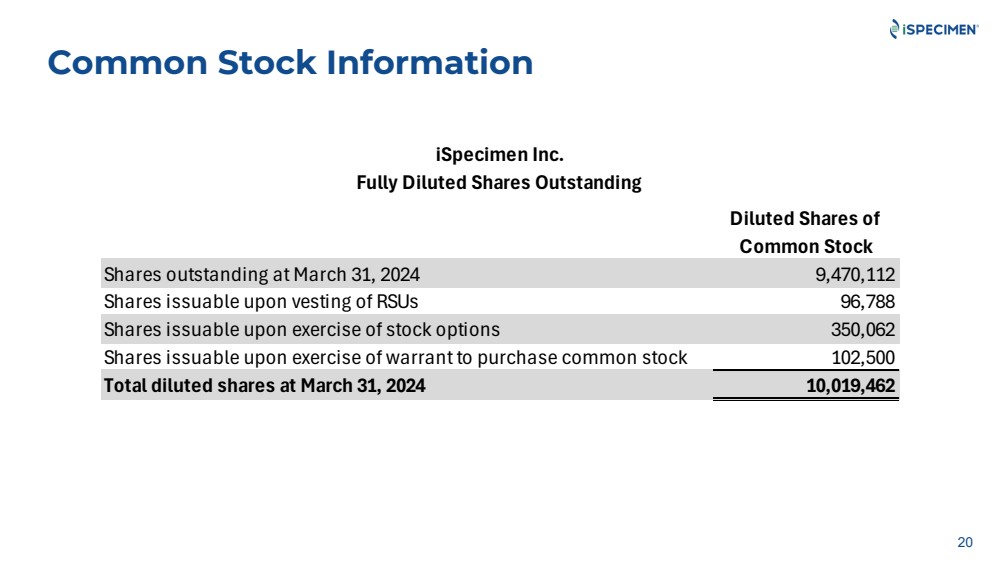
| Common Stock Information
20
iSpecimen Inc.
Fully Diluted Shares Outstanding
Diluted Shares of
Common Stock
Shares outstanding at March 31, 2024 9,470,112
Shares issuable upon vesting of RSUs 96,788
Shares issuable upon exercise of stock options 350,062
Shares issuable upon exercise of warrant to purchase common stock 102,500
Total diluted shares at March 31, 2024 10,019,462 |

| Tracy Curley | Chief Executive Officer, Chief Financial Officer and Treasurer
Ms. Curley brings three decades of experience in public accounting and corporate finance for both publicly traded companies and emerging
companies like iSpecimen and joined iSpecimen in August 2020. She came to iSpecimen after over a decade with national accounting firms such as
CohnReznick where she focused on serving clients in the middle markets. During her time as a partner in public accounting firms, she was
responsible for creating and leading teams to provide audit and consulting services to a growing clientele of private and public emerging growth
companies primarily in the technology and life sciences industries. Ms. Curley received her Master of Accountancy and Bachelor of Science in
Business Administration with a concentration in accounting from Kansas State University. She also attended the United States Military Academy.
She is a certified public accountant licensed in the Commonwealth of Massachusetts.
Benjamin Bielak | Chief Information Officer and Secretary
Mr. Bielak has been serving as our Chief Information Officer since June 2018. He served as the Chief Information Officer at GNS Healthcare (now
Aitia), a leading casual machine learning product and services company, from January 2017 to May 2018 and as Director of Academic Technology
at Harvard University, from February 2015 to January 2017. Prior to his work at GNS and Harvard, Mr. Bielak was the Chief Information Officer at
Dovetail Health from November 2006 to April 2014. He previously held roles as Manager of Development and Integration at Boston Medical Center
and Senior Manager of Technology at Sapient, a global services company, from December 1997 to July 2005. Mr. Bielak holds a Masters of
Business Administration degree from Bentley University, where his studies focused on change management, and a master’s degree from Boston
University in computer science.
Eric Langlois | Chief Revenue Officer
21
Management Team
9 “Sources Cited” page 31
Mr. Langlois has been serving as Chief Revenue Officer since January 2023, prior to which he served as iSpecimen’s SVP of Sales and Business
Development since arriving in January of 2016. Prior to joining iSpecimen, Mr. Langlois served as Global Head of Sales for The Reprocell Group
helping to integrate sales teams driven by the demands of integrating four portfolio companies with products and services in Cellular
Reprogramming, Stem Cells, 3D cell culture, genomics, and biospecimens. Prior to that, he held rapidly increasing positions from Regional Sales
Manager to VP of Sales with Genomics Collaborative, SeraCare Life Sciences, SeqWright, and BioServe. Mr. Langlois has amassed 25 years of
experience in life science research going from bench laboratory science into business development and executive management. Mr. Langlois
holds a BS in Biotechnology/Biochemistry from Worcester Polytechnic Institute. |

| 2
2
1. Similar to Amazon, iSpecimen has developed
an online marketplace to seamlessly connect
researchers with healthcare providers and
supplier organizations
2. First mover advantage that addresses an
inefficient and fragmented global biospecimen
supply chain that is poised to be disrupted by
an online solution
3. $3B-$4B global biospecimen market growing
at 10-15% per year
4. Strong revenue growth with an 8-year CAGR
of 39% fueled by precision and regenerative
medicine research
5. Plans to enhance existing platform and
develop new service offering to increase
revenue opportunities
Investor Highlights
>140
Healthcare Provider / Supplier
Organizations
>600
Customer
Organizations |

| Sign up for a free iSpecimen Marketplace account at iSpecimen.com
Contact us.
iSpecimen Inc.
450 Bedford Street
Lexington, MA 02420
investors@ispecimen.com
Be a part of the
biospecimen
revolution |

| Appendix
24 |

| PHASE 3
Expansion via the Online iSpecimen
Marketplace
START UP
Company Ideation and Self-Funding
PHASE 1
Proof of Concept
PHASE 2
Critical Mass of Suppliers and
Early Customer Adoption
🡡
🡡
2010 – 2011 2012 – 2013 2014 – 2017 2018 – 2024
0
Customers
1
Supplier Agreement
0
Specimen Records
0
Patient Records
0
FTEs
$500K
Friends and Family Covert. Debt
1
Customer
3
Supplier Agreements
2+ Million
Specimen Records
400+ Thousand
Patient Records
7
FTEs
$2.5M
Series A
140+
Customers
60+
Supplier Agreement
26+ Million
Specimen Records
5+ Million
Patient Records
33
FTEs
$8M
Series B
700
Customers
140
Supplier Agreement
80+ Million
Specimen Records
15+ Million
Patient Records
47
FTEs
$20.7M + $21M
IPO + Private Placement
25
Strategic Evolution |

| iSpecimen Marketplace UI/UX
Click Search to start searching for
specimens
26 |
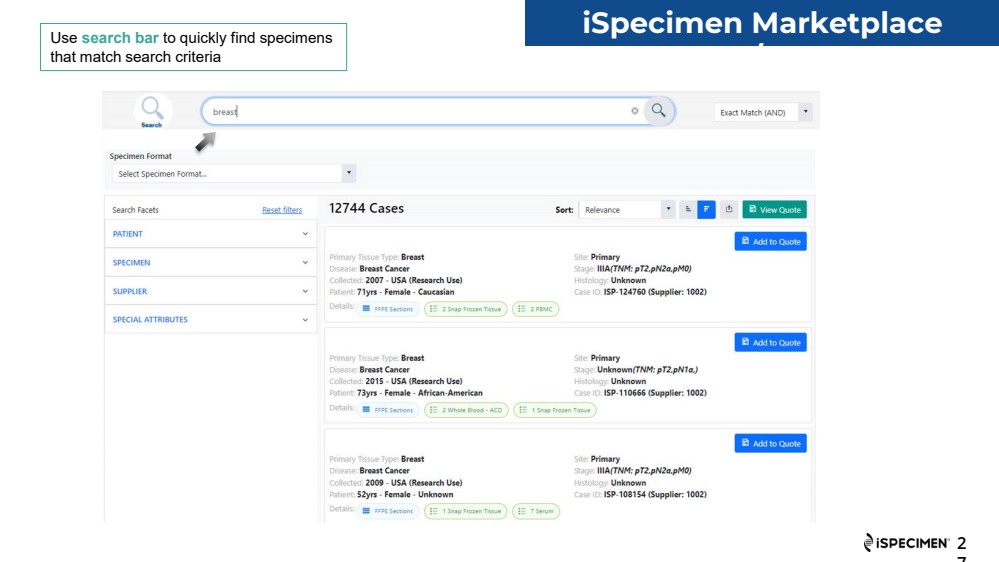
| 2
7
iSpecimen Marketplace
UI/UX Use search bar to quickly find specimens
that match search criteria |
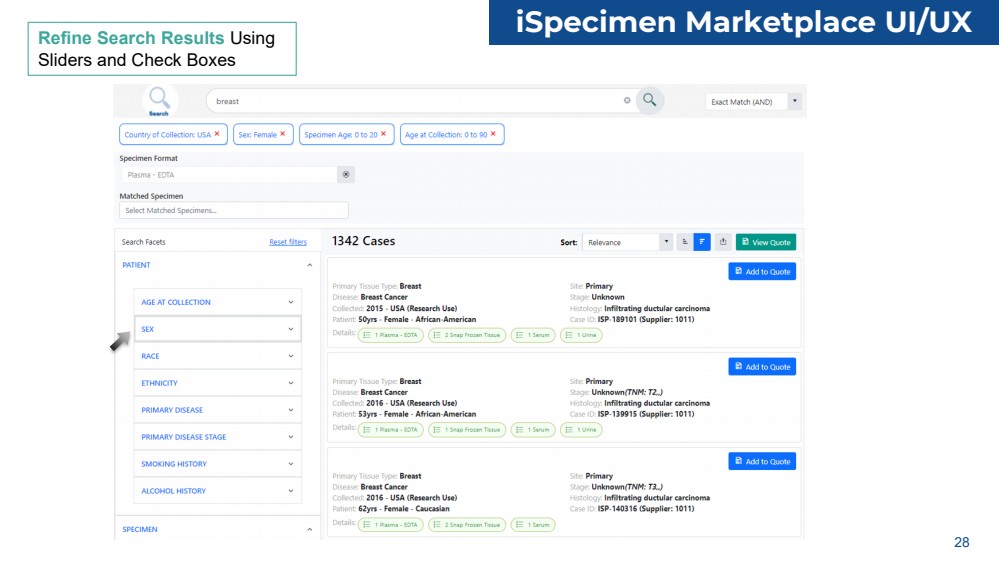
| Refine Search Results Using
Sliders and Check Boxes
iSpecimen Marketplace UI/UX
28 |

| View Quote Request and Add More
Specimens
Request a Quote
iSpecimen Marketplace UI/UX
29
Track and Manage Requests |

| 30
Page Note Source Cited
4 1 iSpecimen estimates based on In Vitro Diagnostics Market Size, Share & Trends Analysis Report By Product, By Application, By
Technology (Immunochemistry, Molecular Diagnostics) By End-use, By Region, And Segment Forecasts, 2021 – 2027. Grandview
Research, January 2021; and World Preview 2020,Outlook to 2026. EvaluatePharma, July 2020.
4 2 Global Marketing Insights, Precision Medicine Market Size By Technology (Big Data Analytics, Bioinformatics, Gene Sequencing, Drug
Discovery, Companion Diagnostics), By Application (Oncology, Immunology, CNS, Respiratory), By End-use (Pharmaceutical Companies,
Diagnostic Companies, Healthcare IT companies), Industry Analysis Report, Regional Outlook, Application Potential, Competitive Market
Share & Forecast, 2020-2026, Feb. 2020. and
Regenerative Medicine Market Size, Share and Industry Analysis by Product (Cell Therapy, Gene Therapy, Tissue Engineering, Platelet
Rich Plasma), By Application (Orthopaedics, Wound Care, Oncology), By Distribution Channel (Hospitals, Clinics) & Regional Forecast,
2019 – 2026. Fortune Business Insight, 2019.
6 3 Frost & Sullivan, Global Biobanking Market Landscape. May 20, 2020, page 357.
6 4 Centers for Disease Control, https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
6 5 iSpecimen estimates based upon American Association of Clinical Chemistry US lab test data cited at
https://www.360dx.com/research-funding/aacc-calls-congress-fund-clinical-lab-training-programs, 2020; and Clinical Lab Services,
Global Market Trajectory and Analytics; Global Industry Analysts report, 2020.
6 6 iSpecimen estimate based upon Henderson, G.E., Cadigan, R.J., Edwards, T.P. et al. Characterizing biobank organizations in the U.S.:
results from a national survey, 2013; and Puchois, Comprehensive Biomarker Discovery and Validation for Clinical Application, 2013.
6 7 Holly A. Massett et al. Assessing the need for a standardized cancer HUman Biobank (caHUB): findings from a national survey with
cancer researchers; JNCI Monographs, Volume 2011, Issue 42, June 2011.
7 8 Specimen Independent Researcher Survey, 2022.
22 9 All company names and logos appearing on this page are trademarks or registered® trademarks of their respective holders. Use
and appearance in this presentation of such trademarks (excluding iSpecimen®) do not imply or assert any form of affiliation with or
endorsement by those companies of iSpecimen or the contents of this presentation.
Sources Cited |
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
iSpecimen (NASDAQ:ISPC)
Historical Stock Chart
From Nov 2024 to Dec 2024

iSpecimen (NASDAQ:ISPC)
Historical Stock Chart
From Dec 2023 to Dec 2024
