0001445283falseKINETA, INC./DENASDAQ00014452832023-11-062023-11-06
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 6, 2023
KINETA, INC.
(Exact name of registrant as specified in its charter)
|
|
|
Delaware |
001-37695 |
20-8436652 |
(State or other jurisdiction |
(Commission |
(IRS Employer |
of incorporation) |
File Number) |
Identification No.) |
219 Terry Ave. N., Suite 300 |
|
|
Seattle, WA |
|
98109 |
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (206) 378-0400
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|
|
☐ |
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
Title of each class |
Trading |
Name of each exchange |
|
|
Symbol(s) |
on which registered |
|
Common Stock, par value $0.001 per share |
|
KA |
|
The Nasdaq Capital Market |
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
Press Release
On November 6, 2023, Kineta, Inc. (the “Company”) issued a press release announcing positive new data in its ongoing Phase 1/2 VISTA-101 clinical trial to evaluate the safety and tolerability of the Company’s VISTA blocking immunotherapy, KVA12123, at the Society for Immunotherapy of Cancer’s 38th Annual Meeting. A copy of the press release is attached hereto as Exhibit 99.1.
Corporate Presentation
On November 6, 2023, the Company updated its corporate presentation (the “Corporate Presentation”), which it intends to use at various meetings with investors, investment banks and investment bank analysts. The Corporate Presentation is attached hereto as Exhibit 99.2.
The information in this Item 7.01, including Exhibits 99.1 and 99.2 attached hereto, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
|
|
Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Date: November 6, 2023
Kineta, Inc.
|
|
By: |
/s/ Shawn Iadonato |
Name: |
Shawn Iadonato |
Title: |
Chief Executive Officer and Director |
Exhibit 99.1

Kineta Unveils Positive New Data from VISTA-101 Clinical Trial of KVA12123 at the Society for Immunotherapy of Cancer’s (SITC) 38th Annual Meeting
Favorable Clinical Safety and Tolerability Profile Observed with No Evidence of CRS-associated Cytokines
Achieved Greater Than Dose-Proportional Pharmacokinetics
Biomarker Results Demonstrate Robust VISTA Target Engagement
Additional Monotherapy and Combination Therapy Clinical Data Anticipated in Q2 2024
SEATTLE — (November 6, 2023) Kineta, Inc. (Nasdaq: KA), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology that address cancer immune resistance, announced today the presentation of new positive data from its ongoing VISTA-101 Phase 1/2 clinical trial evaluating KVA12123, the company’s VISTA blocking immunotherapy, in patients with advanced solid tumors at the Society for Immunotherapy of Cancer’s (SITC) 38th Annual Meeting. Thierry Guillaudeux, Ph.D., Chief Scientific Officer of Kineta, presented the company’s poster unveiling the new clinical data.
“We are excited to unveil these promising findings from our ongoing VISTA-101 clinical trial. VISTA blocking immunotherapy KVA12123 has performed remarkably well in demonstrating excellent safety and tolerability in the clinic. Notably, the pharmacokinetic data and biomarker assessments demonstrate the drug's potential efficacy and confirm its mechanism of action,” said Shawn Iadonato, Ph.D., Chief Executive Officer of Kineta. “We continue to advance KVA12123 to the higher monotherapy dose levels, as well as evaluate it in combination with pembrolizumab, where we recently announced the first patient dosed. Kineta is committed to advancing KVA12123 as a potential new immunotherapy for cancer patients in need of new treatment options.”
The Phase 1/2 VISTA-101 trial (NCT05708950) enrolled 11 patients with advanced solid tumors in the first three monotherapy dose-escalation cohorts, where subjects received either 3, 10 or 30 mg of KVA12123 by intravenous (IV) infusion every two weeks. Primary objectives of the Phase 1/2 study are to evaluate the safety and tolerability of KVA12123 and to determine the recommended Phase 2 dose (RP2D). Patients enrolled in the study were heavily pretreated with multiple prior lines of therapy including chemotherapy, radiation and immunotherapy. Additional monotherapy and initial combination therapy data are anticipated in the second quarter of 2024.
Key highlights from the poster presentation:
Safety
Eleven patients were dosed and cleared the first three monotherapy cohorts. KVA12123 was well tolerated at all doses and no dose limiting toxicities (DLT) were observed. All KVA12123 treatment
emergent adverse events were grades 1-2. Furthermore, no evidence of cytokine release syndrome (CRS) or associated cytokines including IL-6, TNFα & IL-10 were detected at any of the dose levels.
Pharmacokinetics and VISTA Receptor Occupancy (RO)
KVA12123 exhibited a greater than dose-proportional pharmacokinetic profile in drug exposure across all evaluated doses, consistent with target-mediated drug disposition at lower doses. To guide the RP2D decision, Kineta developed a proprietary assay to evaluate VISTA RO on immune cells from patients treated with KVA12123. KVA12123 achieved a greater than 90% VISTA RO at the 30 mg dose indicating that KVA12123 may be approaching an optimal clinical dose.
Biomarkers
KVA12123 demonstrated dose-proportional on-target biomarker immune responses involved in anti-tumor activity. KVA12123 demonstrated significant efficacy-related cytokine induction of CXCL10, MCP1, MIP1α and MIP1β, which are involved in immune cell activation and recruitment to the tumor microenvironment. Additionally, increases in anti-tumor immune cell subpopulations including nonclassical monocytes, NK cells, CD4+ T cells and CD8+ T cells were observed during treatment. Changes in these key biomarkers and immune cell populations are indicative of the anti-tumor effects of blocking VISTA.
The poster presentation is available for viewing under Publications in the KVA12123 section of the company's website at www.kinetabio.com .
SITC Presentation Details:
Title: VISTA-101 – A phase 1/2 clinical trial of KVA12123, an engineered IgG1 targeting VISTA, alone and in combination with pembrolizumab in advanced solid tumors
Abstract Number: 780
Date / Time: Saturday, November 4 at 9:00 A.M. – 7:00 P.M. Pacific Time
Location: Exhibit Halls A and B1 - San Diego Convention Center
Kineta is advancing the VISTA-101 trial to higher monotherapy dose levels and recently initiated Part B of the study evaluating KVA12123 in combination with pembrolizumab. Additional monotherapy safety and efficacy data and combination therapy clinical data are anticipated in the second quarter of 2024.
About Kineta
Kineta (Nasdaq: KA) is a clinical-stage biotechnology company with a mission to develop next-generation immunotherapies that transform patients’ lives. Kineta has leveraged its expertise in innate immunity and is focused on discovering and developing potentially differentiated immunotherapies that address the major challenges with current cancer therapy. The company’s immuno-oncology pipeline includes KVA12123, a novel VISTA blocking immunotherapy currently in a Phase 1/2 clinical trial in patients with advanced solid tumors, and a preclinical monoclonal antibody targeting CD27. For more information on Kineta, please visit www.kinetabio.com, and follow Kineta on Twitter, LinkedIn and Facebook.
KVA12123 is a VISTA blocking immunotherapy in development as a twice weekly infusion. The drug is being evaluated in a Phase 1/2 clinical trial for patients with advanced solid tumors. Competitive therapies targeting VISTA have demonstrated either poor monotherapy anti-tumor activity in preclinical models or induction of cytokine release syndrome (CRS) in human clinical trials. Through the combination of unique epitope binding and an optimized IgG1 Fc region, KVA12123 demonstrates strong monotherapy tumor growth inhibition in preclinical models without evidence of CRS in clinical trial participants. KVA12123 effectively de-risks the VISTA target and provides a novel approach to address immune suppression in the tumor microenvironment (TME) with a mechanism of action that is
differentiated and complementary to T cell focused therapies. KVA12123 may be an effective immunotherapy for many types of cancer including non-small cell lung (NSCLC), colorectal, renal cell carcinoma, head and neck, and ovarian cancer.
VISTA (V-domain Ig suppressor of T cell activation) is a negative immune checkpoint that suppresses T cell function in a variety of solid tumors. High VISTA expression in tumor correlates with poor survival in cancer patients and has been associated with a lack of response to other immune checkpoint inhibitors. Blocking VISTA induces an efficient polyfunctional immune response to address immunosuppression and drives anti-tumor responses.
Cautionary Statements Regarding Forward-Looking Statements:
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. The use of words such as, but not limited to, “believe,” “expect,” “estimate,” “project,” “intend,” “future,” “potential,” “continue,” “may,” “might,” “plan,” “will,” “should,” “seek,” “anticipate,” or “could” and other similar words or expressions are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Kineta’s current beliefs, expectations and assumptions regarding the future of Kineta’s business, future plans and strategies, clinical results and other future conditions. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements.
Such forward-looking statements are subject to a number of material risks and uncertainties including, but not limited to: the adequacy of Kineta’s capital to support its future operations (including its ability to complete the second tranche of the previously disclosed contemplated private placement) and its ability to successfully initiate and complete clinical trials; the difficulty in predicting the time and cost of development of Kineta’s product candidates; Kineta’s plans to research, develop and commercialize its current and future product candidates, including, but not limited to, KVA12123; the timing and anticipated results of Kineta’s planned pre-clinical studies and clinical trials and the risk that the results of Kineta’s pre-clinical studies and clinical trials may not be predictive of future results in connection with future studies or clinical trials; the timing of the availability of data from Kineta’s clinical trials; the timing of any planned investigational new drug application or new drug application; the risk of cessation or delay of any ongoing or planned clinical trials of Kineta or its collaborators; the clinical utility, potential benefits and market acceptance of Kineta’s product candidates; Kineta’s commercialization, marketing and manufacturing capabilities and strategy; developments and projections relating to Kineta’s competitors and its industry; the impact of government laws and regulations; the timing and outcome of Kineta’s planned interactions with regulatory authorities; Kineta’s ability to protect its intellectual property position; Kineta’s estimates regarding future revenue, expenses, capital requirements and need for additional financing; the intended use of proceeds from the registered direct offerings completed in April 2023 and October 2023; and those risks set forth under the caption “Risk Factors” in the company’s most recent Annual Report on Form 10-K filed with the SEC on March 31, 2023, and Quarterly Reports on Form 10-Q filed with the SEC on May 11, 2023 and August 11, 2023, as well as discussions of potential risks, uncertainties and other important factors in Kineta’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Except as required by law, Kineta undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Kineta, Inc. :
Jacques Bouchy
EVP Investor Relations & Business Development
+1 206-378-0400
jbouchy@kineta.us
Investor Relations:
John Mullaly
LifeSci Advisors, LLC
jmullaly@lifesciadvisors.com
Source: Kineta, Inc.

Developing next-generation immunotherapies that address cancer immune resistance KA (Nasdaq) November 2023 Exhibit 99.2

Disclaimers and other information Cautionary Statements Regarding Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. The use of words such as, but not limited to, “believe,” “expect,” “estimate,” “project,” “intend,” “future,” “potential,” “continue,” “may,” “might,” “plan,” “will,” “should,” “seek,” “anticipate,” or “could” and other similar words or expressions are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Kineta’s current beliefs, expectations and assumptions regarding the future of Kineta’s business, future plans and strategies, clinical results and other future conditions. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Such forward-looking statements are subject to a number of material risks and uncertainties including, but not limited to: the adequacy of Kineta’s capital to support its future operations (including its ability to complete the second tranche of the previously disclosed contemplated private placement) and its ability to successfully initiate and complete clinical trials; the difficulty in predicting the time and cost of development of Kineta’s product candidates; Kineta’s plans to research, develop and commercialize its current and future product candidates, including, but not limited to, KVA12123; the timing and anticipated results of Kineta’s planned pre-clinical studies and clinical trials and the risk that the results of Kineta’s pre-clinical studies and clinical trials may not be predictive of future results in connection with future studies or clinical trials; the timing of the availability of data from Kineta’s clinical trials; the timing of any planned investigational new drug application or new drug application; the risk of cessation or delay of any ongoing or planned clinical trials of Kineta or its collaborators; the clinical utility, potential benefits and market acceptance of Kineta’s product candidates; Kineta’s commercialization, marketing and manufacturing capabilities and strategy; developments and projections relating to Kineta’s competitors and its industry; the impact of government laws and regulations; the timing and outcome of Kineta’s planned interactions with regulatory authorities; Kineta’s ability to protect its intellectual property position; Kineta’s estimates regarding future revenue, expenses, capital requirements and need for additional financing; the intended use of proceeds from the registered direct offerings completed in April 2023 and October 2023; and those risks set forth under the caption “Risk Factors” in Kineta’s most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on March 31, 2023 and Quarterly Reports on Form 10-Q filed with the SEC on May 11, 2023 and August 11, 2023, as well as discussions of potential risks, uncertainties and other important factors in Kineta’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Except as required by law, Kineta undertakes no obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise.
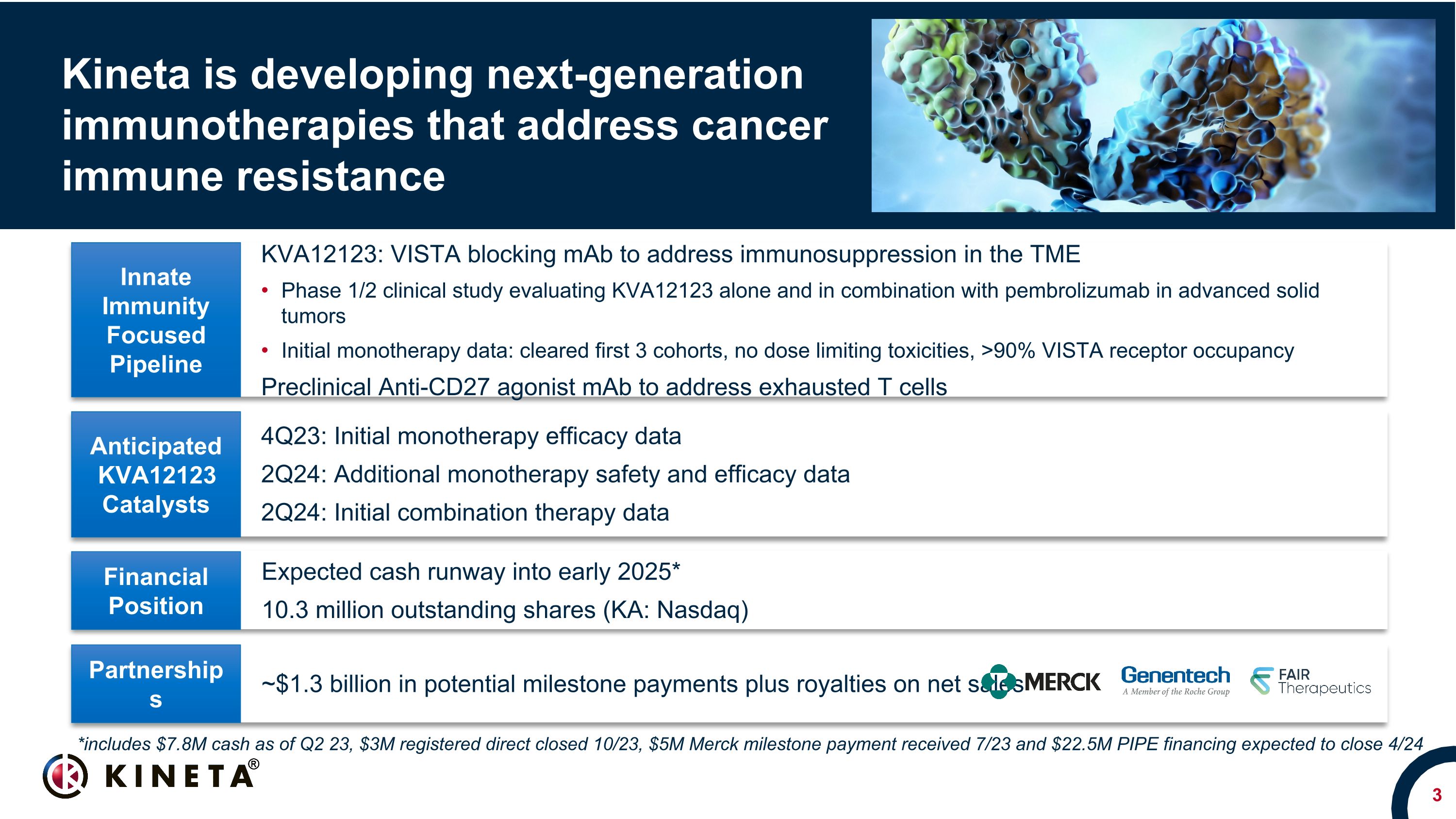
KVA12123: VISTA blocking mAb to address immunosuppression in the TME Phase 1/2 clinical study evaluating KVA12123 alone and in combination with pembrolizumab in advanced solid tumors Initial monotherapy data: cleared first 3 cohorts, no dose limiting toxicities, >90% VISTA receptor occupancy Preclinical Anti-CD27 agonist mAb to address exhausted T cells Expected cash runway into early 2025* 10.3 million outstanding shares (KA: Nasdaq) 4Q23: Initial monotherapy efficacy data 2Q24: Additional monotherapy safety and efficacy data 2Q24: Initial combination therapy data Innate Immunity Focused Pipeline Anticipated KVA12123 Catalysts Financial Position Kineta is developing next-generation immunotherapies that address cancer immune resistance ~$1.3 billion in potential milestone payments plus royalties on net sales Partnerships *includes $7.8M cash as of Q2 23, $3M registered direct closed 10/23, $5M Merck milestone payment received 7/23 and $22.5M PIPE financing expected to close 4/24
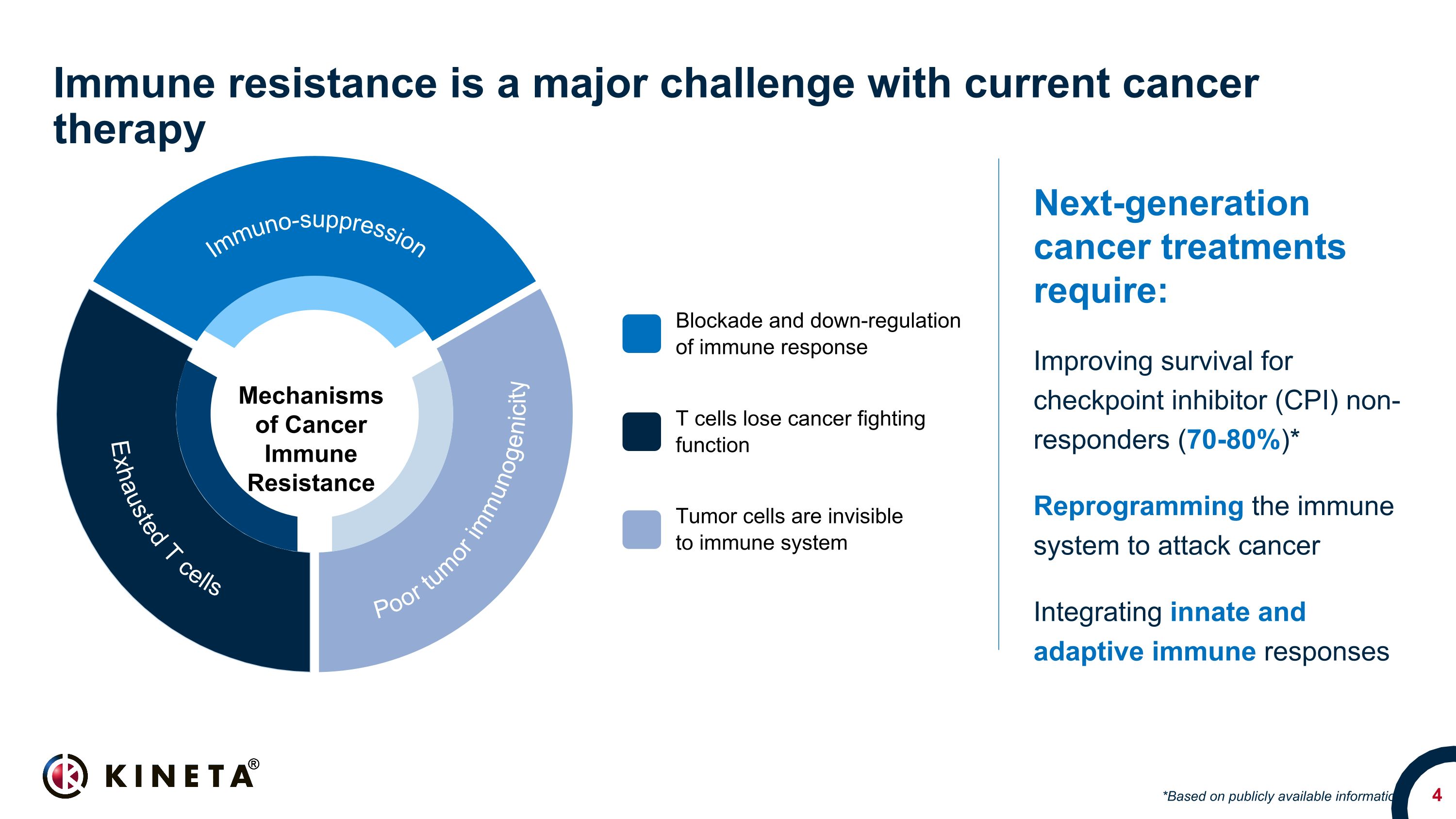
Immune resistance is a major challenge with current cancer therapy Next-generation cancer treatments require: Improving survival for checkpoint inhibitor (CPI) non-responders (70-80%)* Reprogramming the immune system to attack cancer Integrating innate and adaptive immune responses *Based on publicly available information Blockade and down-regulation of immune response T cells lose cancer fighting function Mechanisms of Cancer Immune Resistance Tumor cells are invisible to immune system Exhausted T cells Poor tumor immunogenicity Immuno-suppression

Kineta pipeline integrates innate and adaptive immunity to address mechanisms of cancer resistance Innate immunity Involved in early response to cancer Necessary driver for appropriate adaptive immunity Significant cause of cancer resistance Adaptive immunity Most competitor drug development is focused only on T cell adaptive immunity Innate Immunity Adaptive Immunity Dendritic cell Macrophage Mast cell Eosinophil Basophil Neutrophil Natural Killer cell Granulocytes gd T cell Natural killer T cell B cell Antibodies CD4 T cell CD8 T cell ab T cell
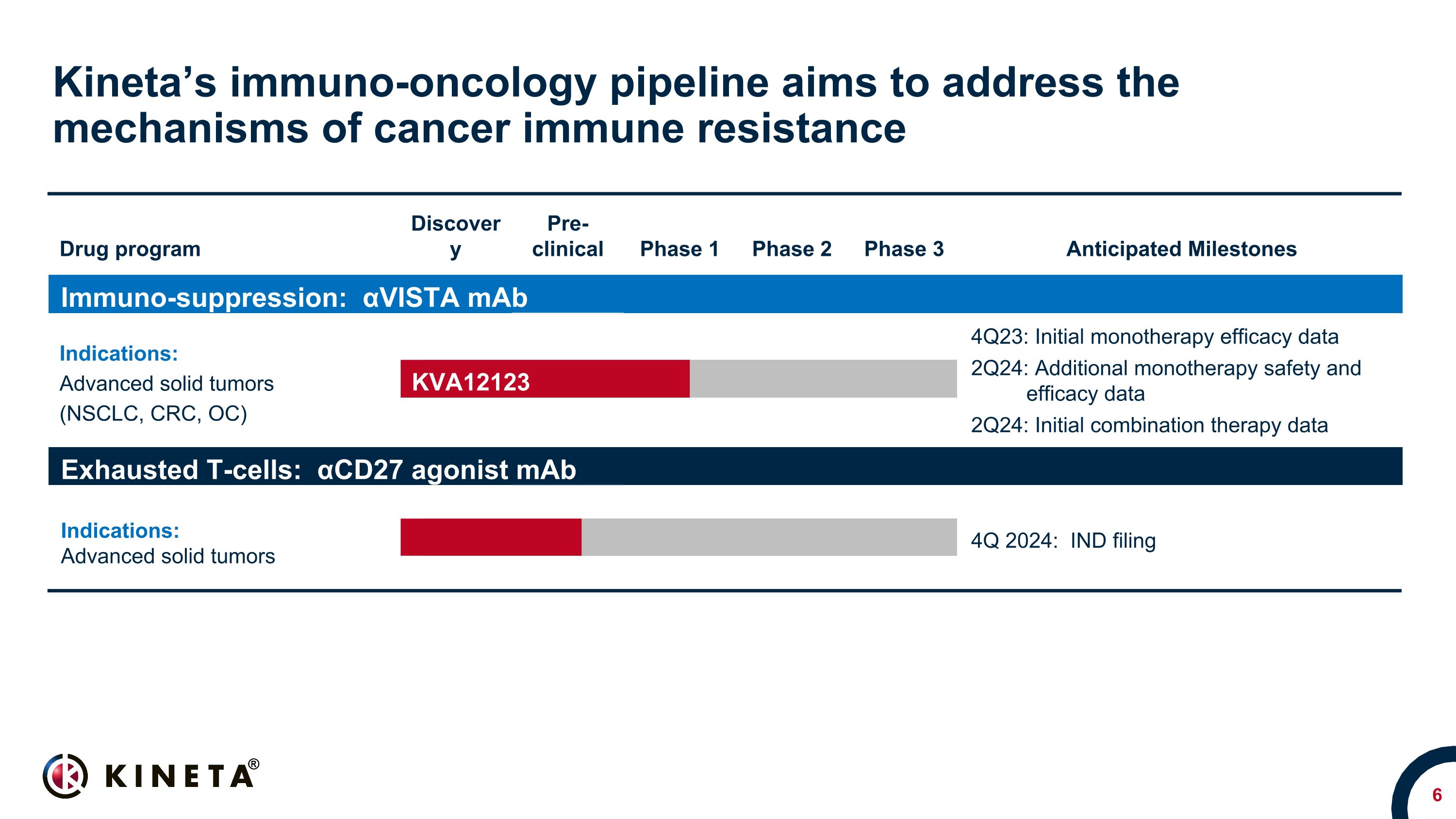
Kineta’s immuno-oncology pipeline aims to address the mechanisms of cancer immune resistance Drug program Discovery Pre- clinical Phase 1 Phase 2 Phase 3 Anticipated Milestones Immuno-suppression: αVISTA mAb Indications: Advanced solid tumors (NSCLC, CRC, OC) Advanced solid tumors NSCLC, CRC, OC, RCC & SCCHN* 4Q23: Initial monotherapy efficacy data 2Q24: Additional monotherapy safety and efficacy data 2Q24: Initial combination therapy data Exhausted T-cells: αCD27 agonist mAb Indications: Advanced solid tumors Advanced solid tumors 4Q 2024: IND filing KVA12123

KVA12123��Potentially differentiated �VISTA blocking immunotherapy

VISTA is a key driver of immunosuppression in the tumor microenvironment Immunosuppressive protein expressed on myeloid cells Highly expressed in cold tumors including lung, colon and ovarian cancers Correlates with poor outcomes in cancer patients Up-regulated after CPI therapy and associated with treatment failure VISTA Negative VISTA Positive Brown staining in human tumors indicates VISTA expression Melanoma patient survival by VISTA expression in tumor-infiltrating immune cells 1 VISTA expression increases in melanoma patient during pembrolizumab relapse/progression 2 References: 1. Kuklinski et al. 2018; 2. Kakavand et al. 2017
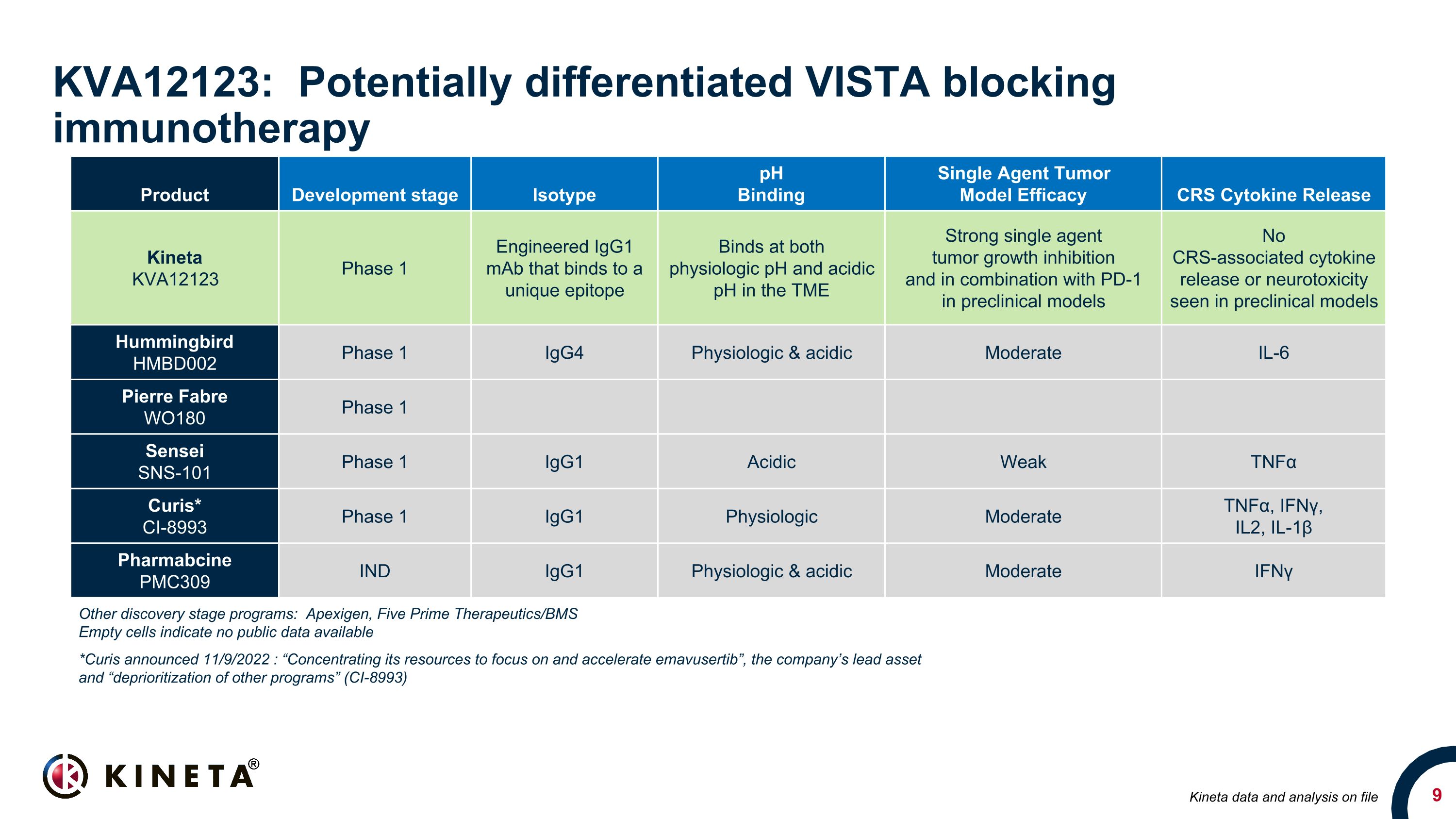
KVA12123: Potentially differentiated VISTA blocking immunotherapy Product Development stage Isotype pH Binding Single Agent Tumor Model Efficacy CRS Cytokine Release Kineta KVA12123 Phase 1 Engineered IgG1 mAb that binds to a unique epitope Binds at both physiologic pH and acidic pH in the TME Strong single agent tumor growth inhibition and in combination with PD-1 in preclinical models No CRS-associated cytokine release or neurotoxicity seen in preclinical models Hummingbird HMBD002 Phase 1 IgG4 Physiologic & acidic Moderate IL-6 Pierre Fabre WO180 Phase 1 Sensei SNS-101 Phase 1 IgG1 Acidic Weak TNFα Curis* CI-8993 Phase 1 IgG1 Physiologic Moderate TNFα, IFNγ, IL2, IL-1β Pharmabcine PMC309 IND IgG1 Physiologic & acidic Moderate IFNγ Other discovery stage programs: Apexigen, Five Prime Therapeutics/BMS Empty cells indicate no public data available *Curis announced 11/9/2022 : “Concentrating its resources to focus on and accelerate emavusertib”, the company’s lead asset and “deprioritization of other programs” (CI-8993) Kineta data and analysis on file
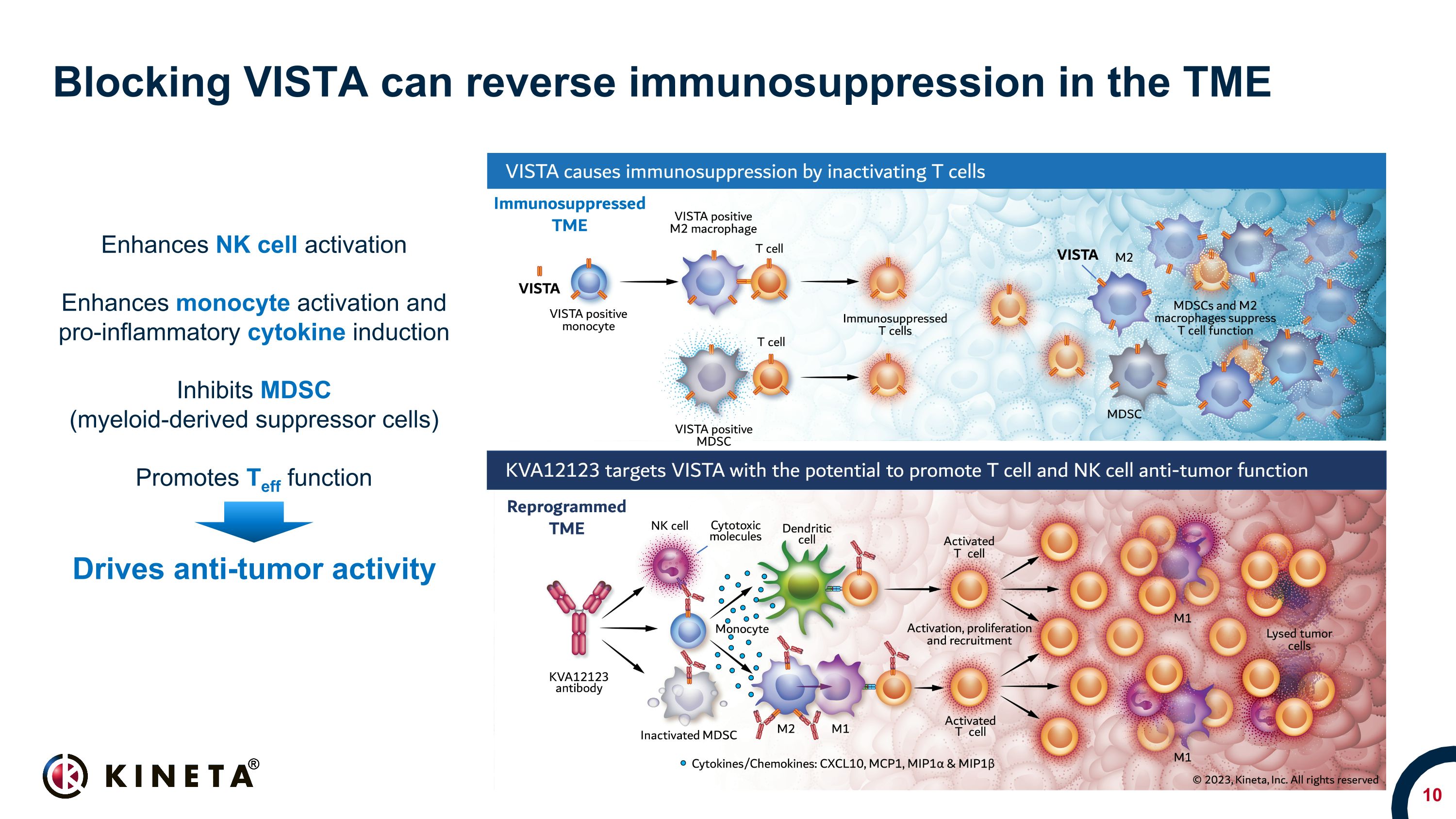
Blocking VISTA can reverse immunosuppression in the TME Enhances NK cell activation Enhances monocyte activation and pro-inflammatory cytokine induction Inhibits MDSC (myeloid-derived suppressor cells) Promotes Teff function Drives anti-tumor activity
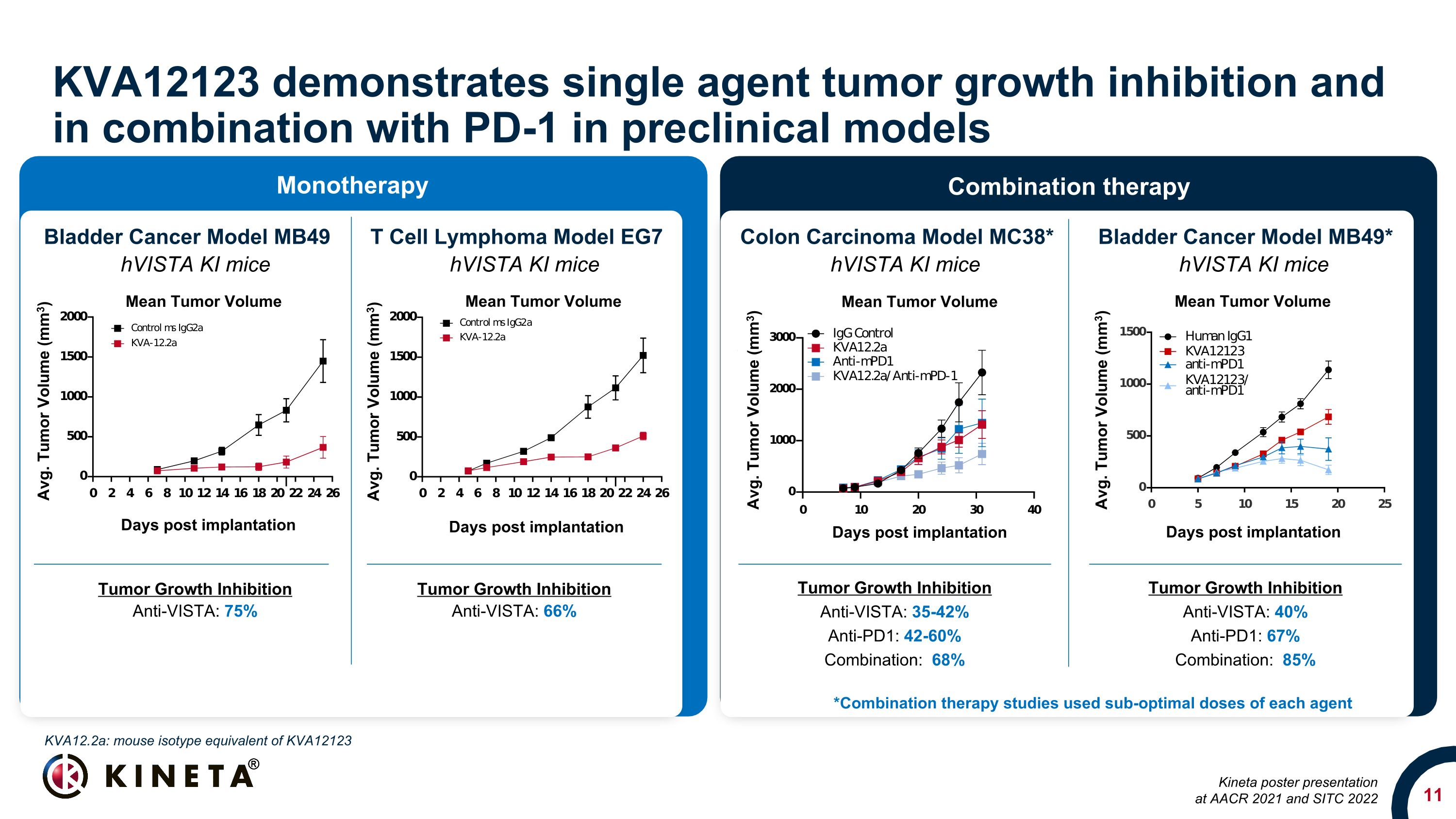
KVA12123 demonstrates single agent tumor growth inhibition and in combination with PD-1 in preclinical models Tumor Growth Inhibition Anti-VISTA: 35-42% Anti-PD1: 42-60% Combination: 68% Colon Carcinoma Model MC38* Bladder Cancer Model MB49 T Cell Lymphoma Model EG7 hVISTA KI mice hVISTA KI mice hVISTA KI mice Bladder Cancer Model MB49* hVISTA KI mice Tumor Growth Inhibition Anti-VISTA: 40% Anti-PD1: 67% Combination: 85% Tumor Growth Inhibition Anti-VISTA: 75% Tumor Growth Inhibition Anti-VISTA: 66% *Combination therapy studies used sub-optimal doses of each agent KVA12.2a: mouse isotype equivalent of KVA12123 Kineta poster presentation at AACR 2021 and SITC 2022 Monotherapy Combination therapy Mean Tumor Volume Days post implantation Avg. Tumor Volume (mm3) Mean Tumor Volume Days post implantation Avg. Tumor Volume (mm3) Avg. Tumor Volume (mm3) Days post implantation Avg. Tumor Volume (mm3) Mean Tumor Volume Days post implantation Avg. Tumor Volume (mm3) Mean Tumor Volume
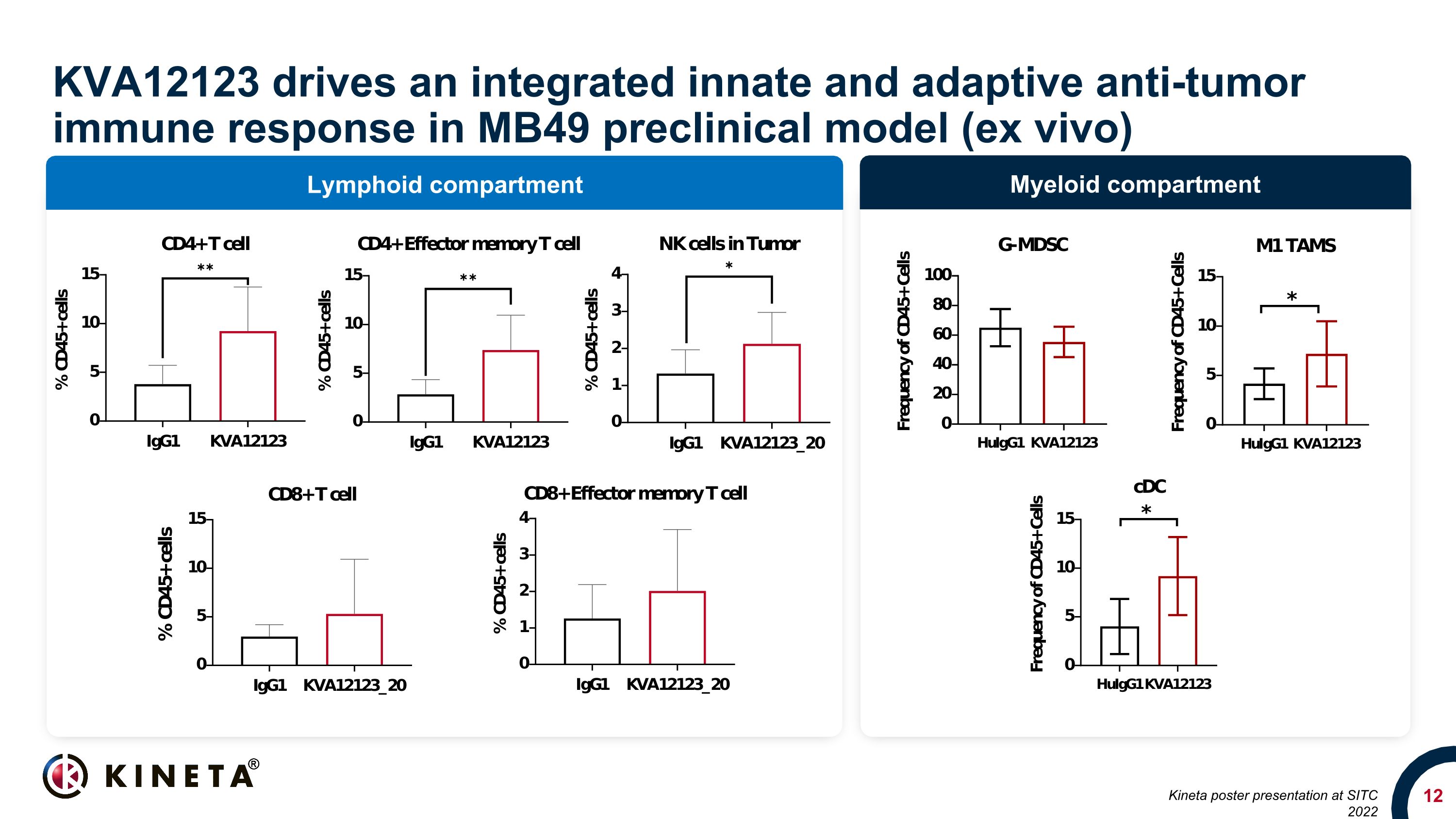
Myeloid compartment Lymphoid compartment KVA12123 drives an integrated innate and adaptive anti-tumor immune response in MB49 preclinical model (ex vivo) Kineta poster presentation at SITC 2022
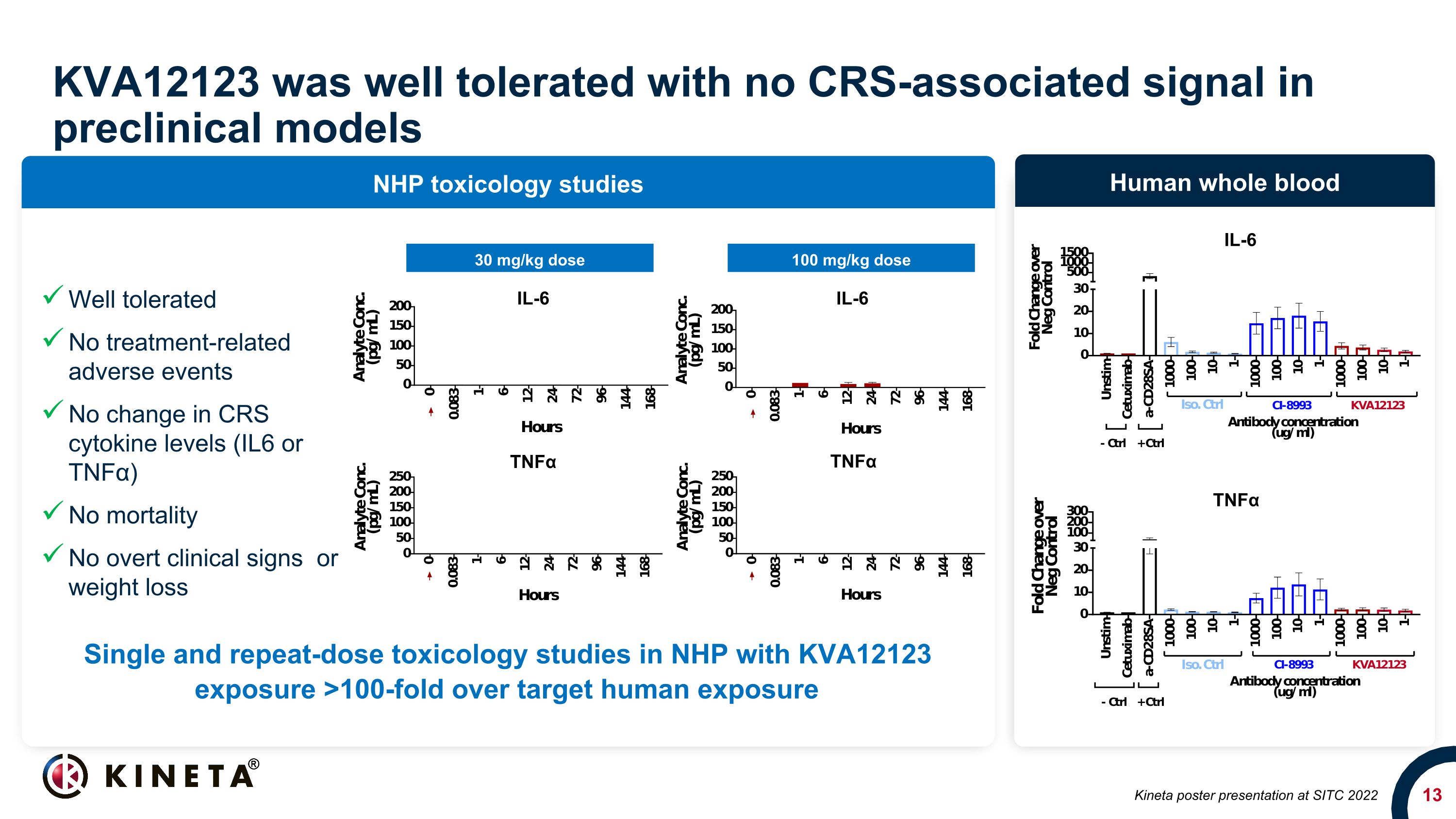
KVA12123 was well tolerated with no CRS-associated signal in preclinical models NHP toxicology studies Human whole blood 30 mg/kg dose 100 mg/kg dose TNFα IL-6 Kineta poster presentation at SITC 2022 Single and repeat-dose toxicology studies in NHP with KVA12123 exposure >100-fold over target human exposure Well tolerated No treatment-related adverse events No change in CRS cytokine levels (IL6 or TNFα) No mortality No overt clinical signs or weight loss IL-6 IL-6 TNFα TNFα
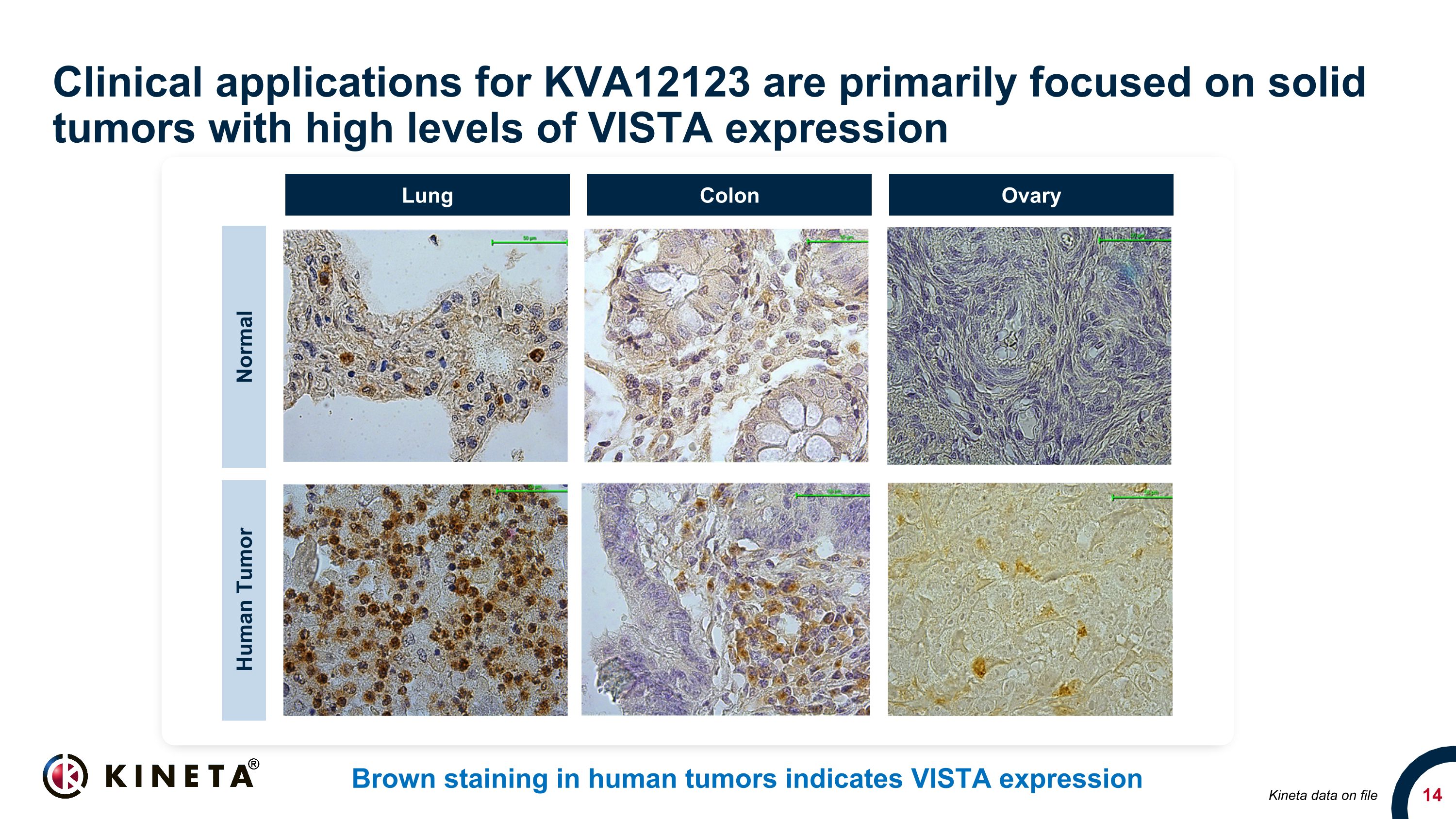
Clinical applications for KVA12123 are primarily focused on solid tumors with high levels of VISTA expression Brown staining in human tumors indicates VISTA expression 20x 20x 20x Normal Human Tumor Lung Colon Ovary Kineta data on file
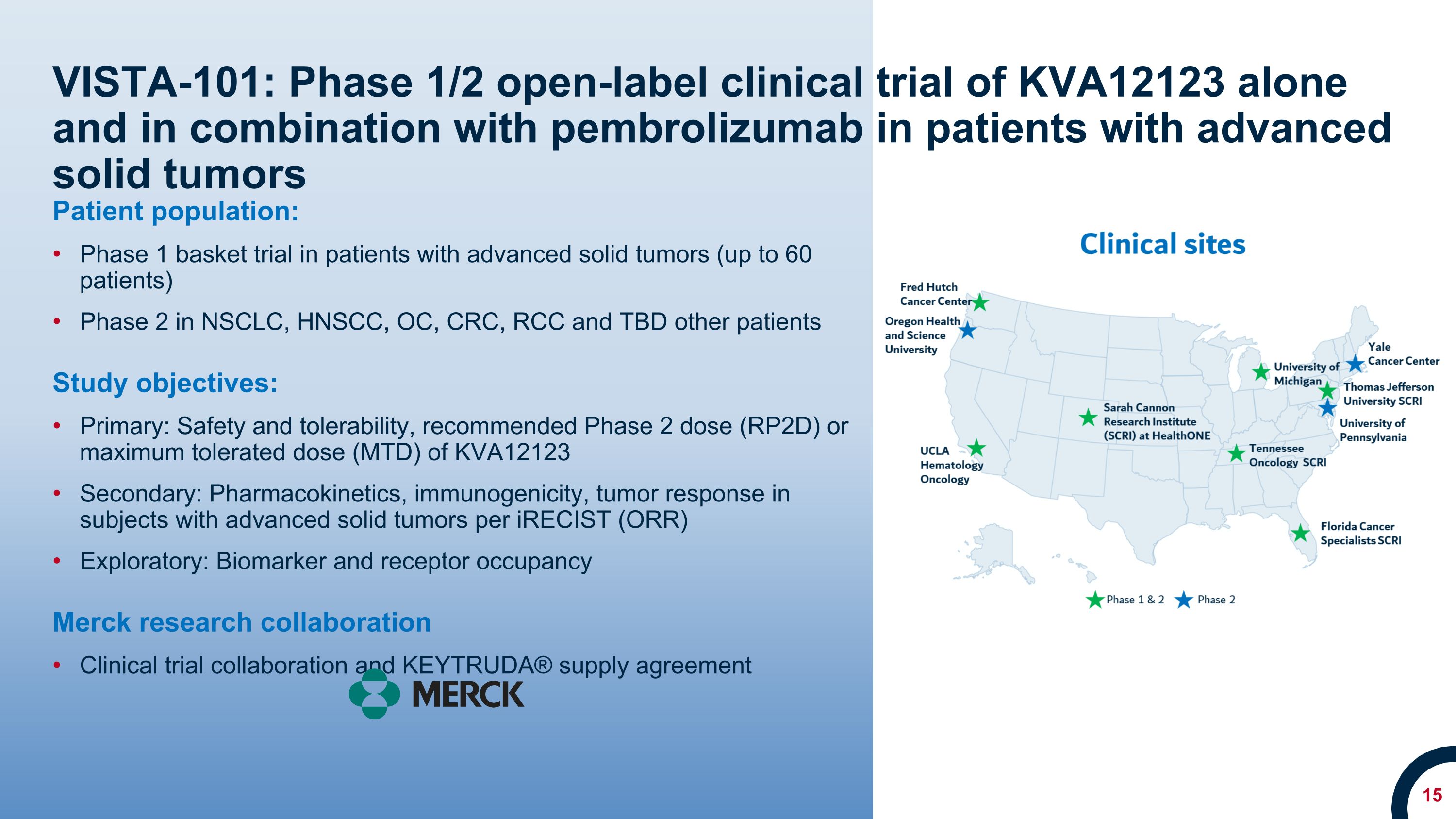
VISTA-101: Phase 1/2 open-label clinical trial of KVA12123 alone and in combination with pembrolizumab in patients with advanced solid tumors Patient population: Phase 1 basket trial in patients with advanced solid tumors (up to 60 patients) Phase 2 in NSCLC, HNSCC, OC, CRC, RCC and TBD other patients Study objectives: Primary: Safety and tolerability, recommended Phase 2 dose (RP2D) or maximum tolerated dose (MTD) of KVA12123 Secondary: Pharmacokinetics, immunogenicity, tumor response in subjects with advanced solid tumors per iRECIST (ORR) Exploratory: Biomarker and receptor occupancy Merck research collaboration Clinical trial collaboration and KEYTRUDA® supply agreement
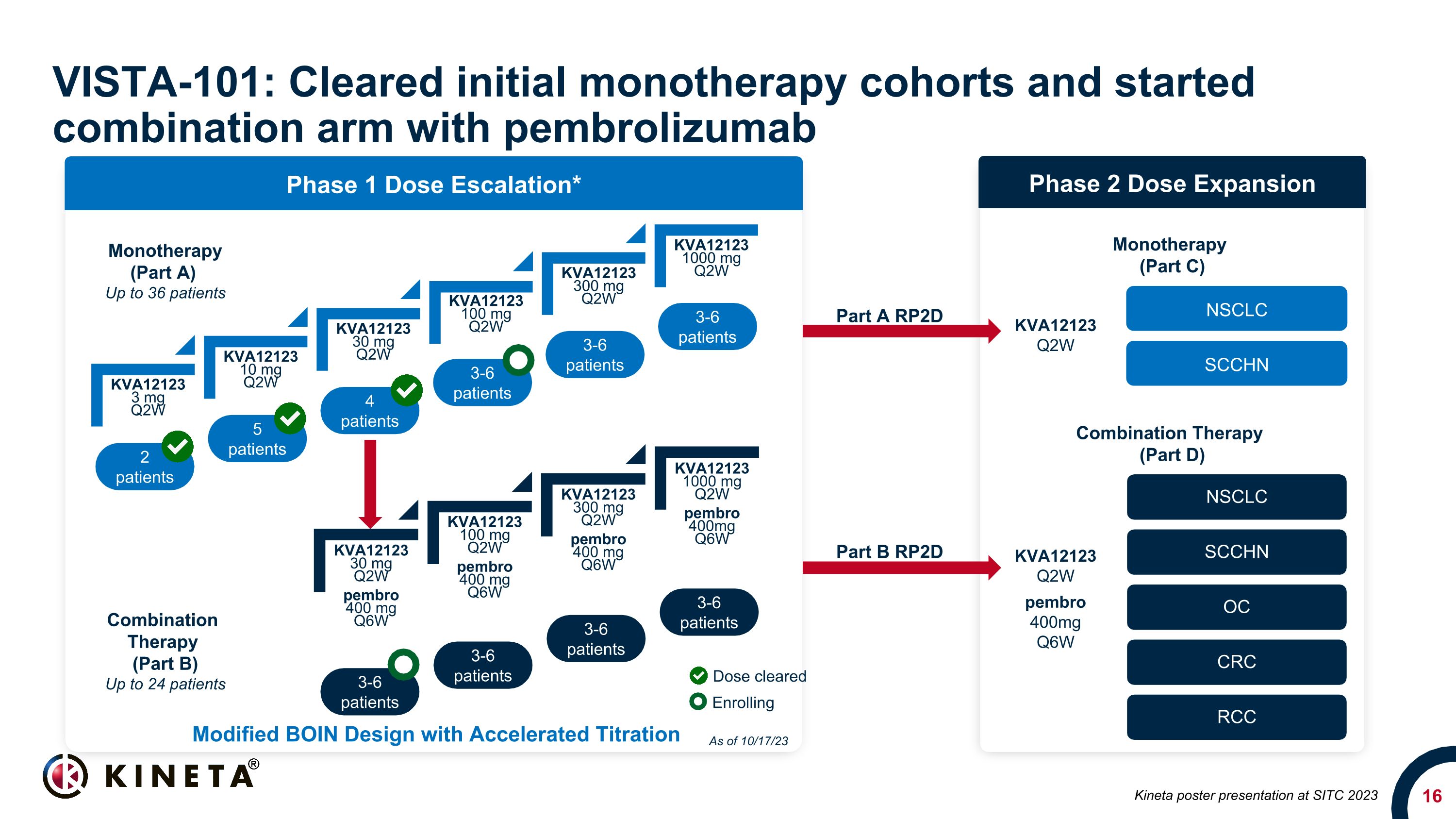
KVA12123 3 mg Q2W KVA12123 10 mg Q2W KVA12123 30 mg Q2W KVA12123 100 mg Q2W KVA12123 300 mg Q2W KVA12123 1000 mg Q2W VISTA-101: Cleared initial monotherapy cohorts and started combination arm with pembrolizumab 2 patients 5 patients 3-6 patients 4 patients 3-6 patients 3-6 patients Phase 1 Dose Escalation* 3-6 patients 3-6 patients 3-6 patients 3-6 patients Phase 2 Dose Expansion NSCLC SCCHN OC CRC RCC NSCLC SCCHN Monotherapy (Part A) Up to 36 patients Combination Therapy (Part B) Up to 24 patients Monotherapy (Part C) Combination Therapy (Part D) Part A RP2D Part B RP2D KVA12123 Q2W pembro 400mg Q6W KVA12123 Q2W Modified BOIN Design with Accelerated Titration Dose cleared Enrolling KVA12123 30 mg Q2W pembro 400 mg Q6W KVA12123 100 mg Q2W pembro 400 mg Q6W KVA12123 300 mg Q2W pembro 400 mg Q6W KVA12123 1000 mg Q2W pembro 400mg Q6W As of 10/17/23 Kineta poster presentation at SITC 2023
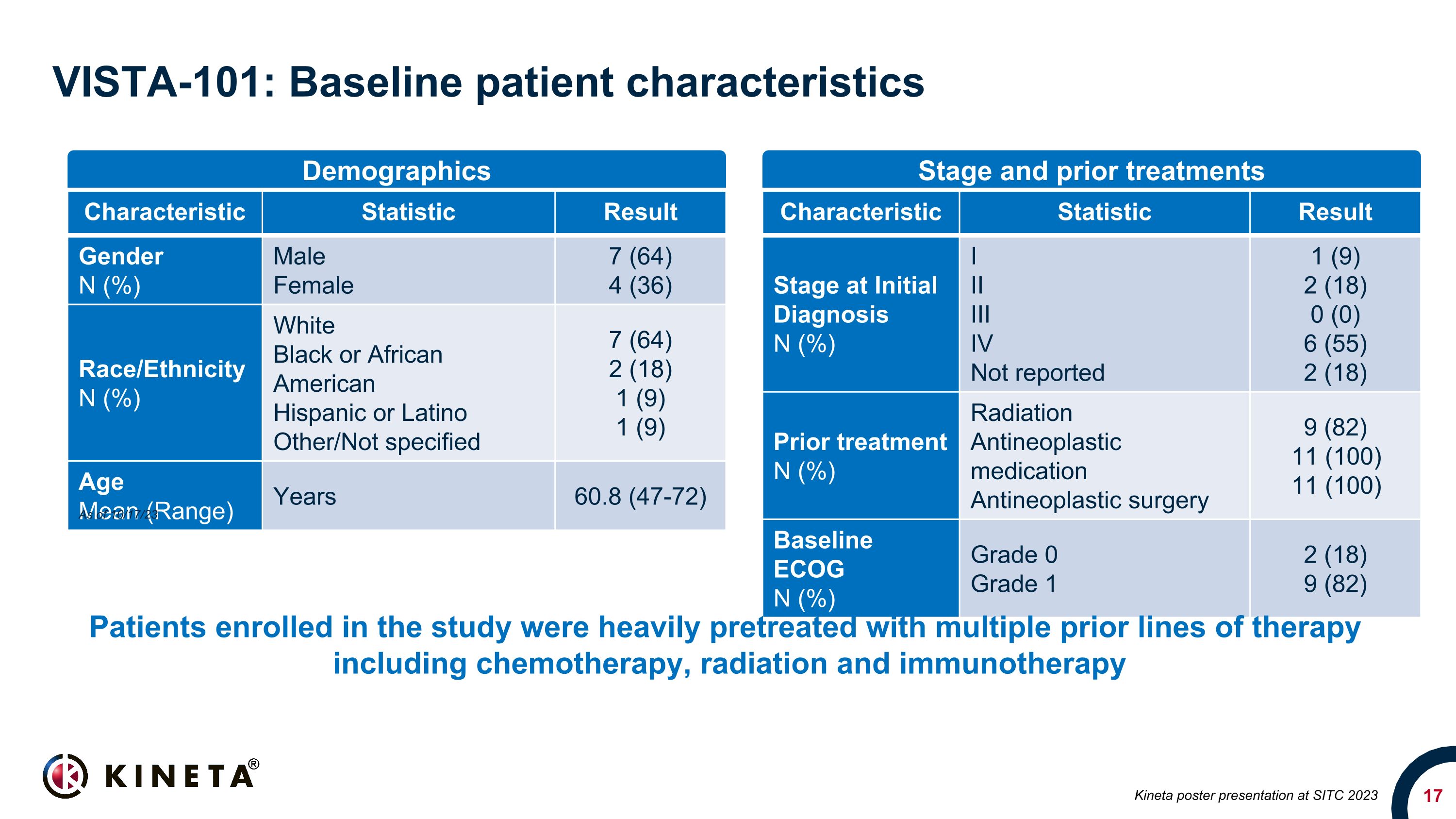
VISTA-101: Baseline patient characteristics Characteristic Statistic Result Gender N (%) Male Female 7 (64) 4 (36) Race/Ethnicity N (%) White Black or African American Hispanic or Latino Other/Not specified 7 (64) 2 (18) 1 (9) 1 (9) Age Mean (Range) Years 60.8 (47-72) Characteristic Statistic Result Stage at Initial Diagnosis N (%) I II III IV Not reported 1 (9) 2 (18) 0 (0) 6 (55) 2 (18) Prior treatment N (%) Radiation Antineoplastic medication Antineoplastic surgery 9 (82) 11 (100) 11 (100) Baseline ECOG N (%) Grade 0 Grade 1 2 (18) 9 (82) Demographics Stage and prior treatments Kineta poster presentation at SITC 2023 Patients enrolled in the study were heavily pretreated with multiple prior lines of therapy including chemotherapy, radiation and immunotherapy As of 10/17/23
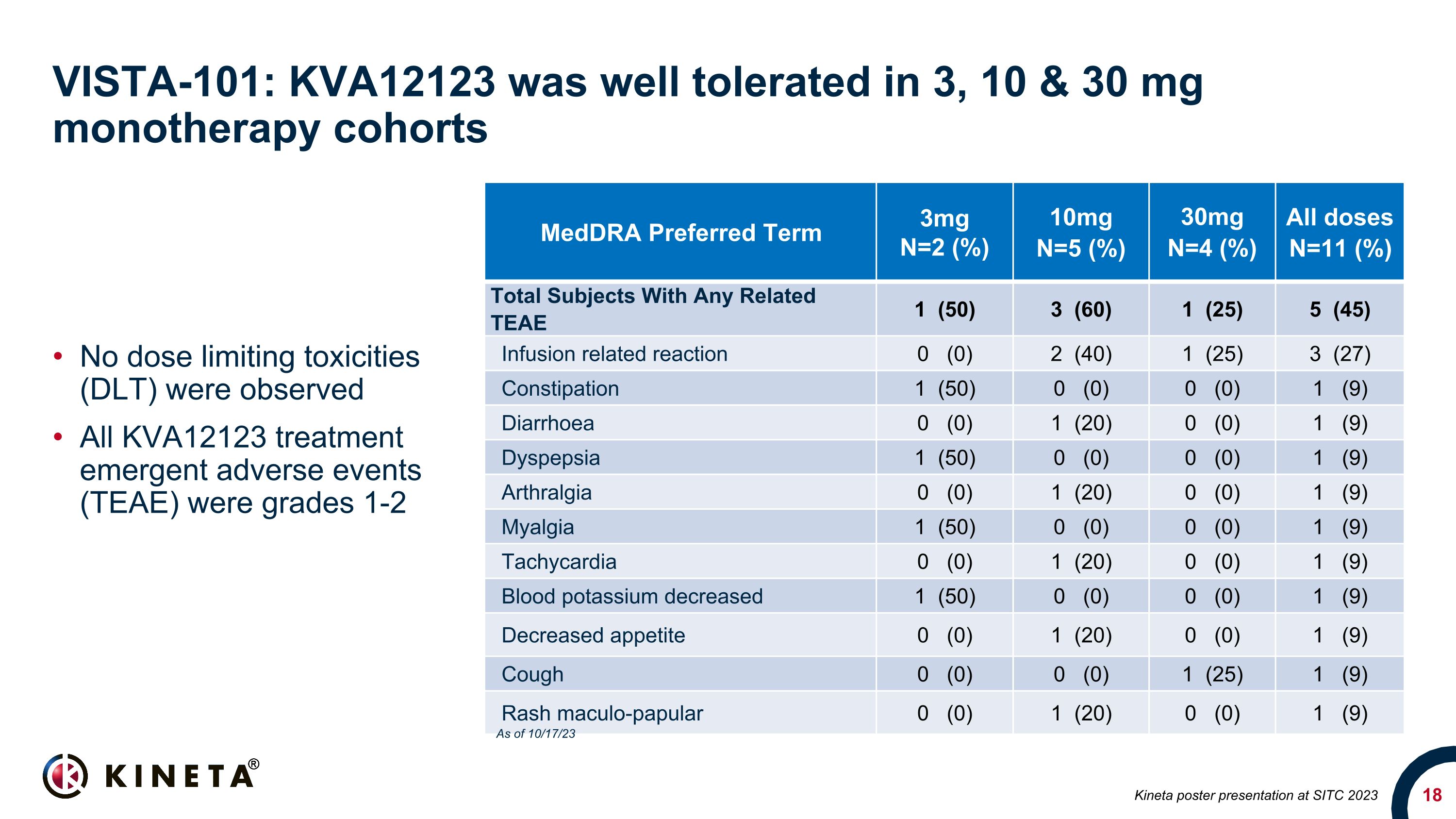
VISTA-101: KVA12123 was well tolerated in 3, 10 & 30 mg �monotherapy cohorts No dose limiting toxicities (DLT) were observed All KVA12123 treatment emergent adverse events (TEAE) were grades 1-2 MedDRA Preferred Term 3mg N=2 (%) 10mg�N=5 (%) 30mg �N=4 (%) All doses�N=11 (%) Total Subjects With Any Related TEAE 1 (50) 3 (60) 1 (25) 5 (45) Infusion related reaction 0 (0) 2 (40) 1 (25) 3 (27) Constipation 1 (50) 0 (0) 0 (0) 1 (9) Diarrhoea 0 (0) 1 (20) 0 (0) 1 (9) Dyspepsia 1 (50) 0 (0) 0 (0) 1 (9) Arthralgia 0 (0) 1 (20) 0 (0) 1 (9) Myalgia 1 (50) 0 (0) 0 (0) 1 (9) Tachycardia 0 (0) 1 (20) 0 (0) 1 (9) Blood potassium decreased 1 (50) 0 (0) 0 (0) 1 (9) Decreased appetite 0 (0) 1 (20) 0 (0) 1 (9) Cough 0 (0) 0 (0) 1 (25) 1 (9) Rash maculo-papular 0 (0) 1 (20) 0 (0) 1 (9) Kineta poster presentation at SITC 2023 As of 10/17/23
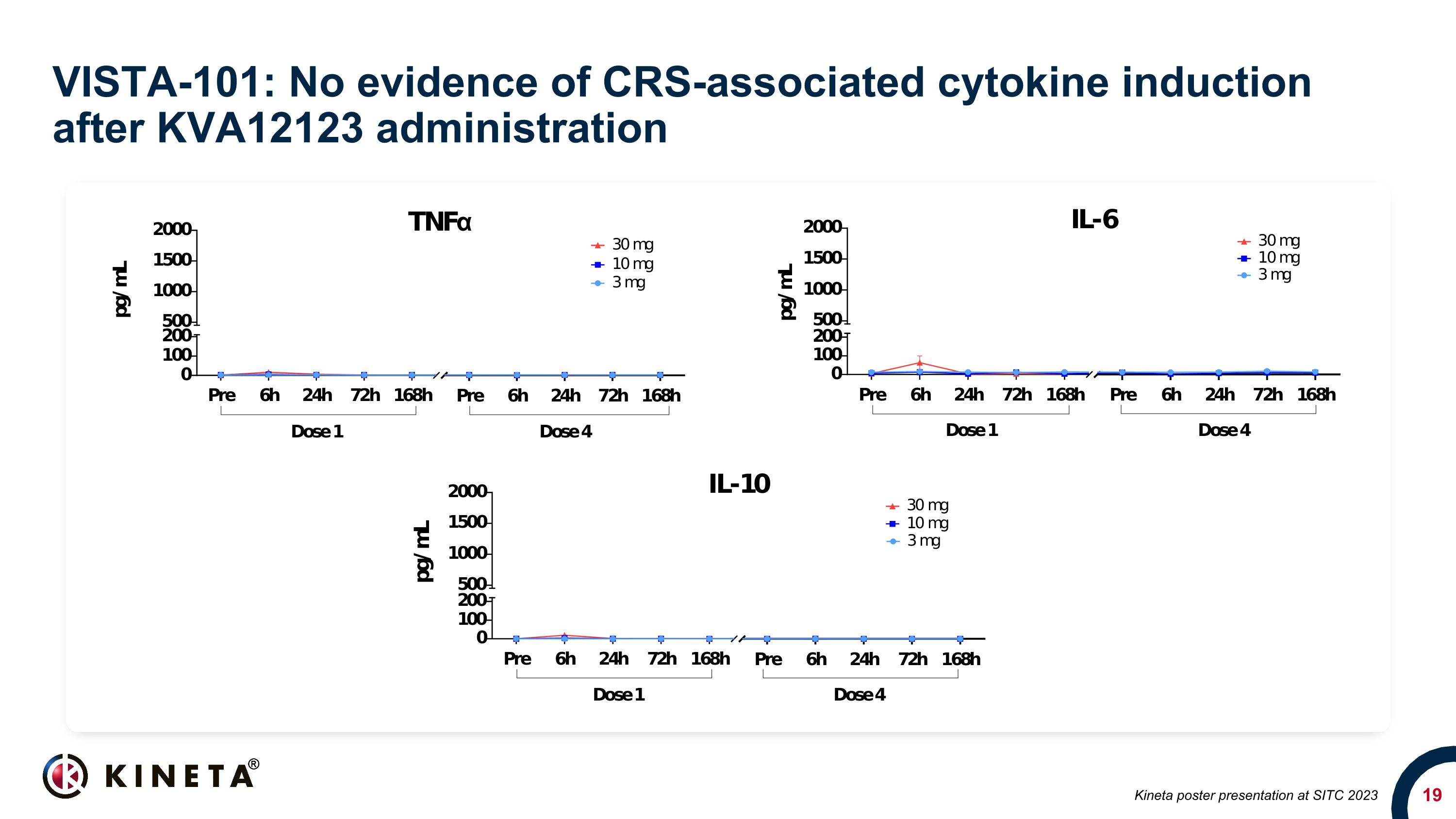
VISTA-101: No evidence of CRS-associated cytokine induction after KVA12123 administration Kineta poster presentation at SITC 2023

VISTA-101: KVA12123 exhibited a greater than dose-proportional PK profile and achieved >90% VISTA RO across patients in the 30 mg cohort VISTA Receptor Occupancy (RO)* Pharmacokinetics *The VISTA-receptor occupancy on circulating monocytes in 11 patients after a single administration of KVA12123. Kineta utilizes a proprietary method to evaluate VISTA receptor occupancy. *** p<0.001 Kineta poster presentation at SITC 2023 *not evaluable
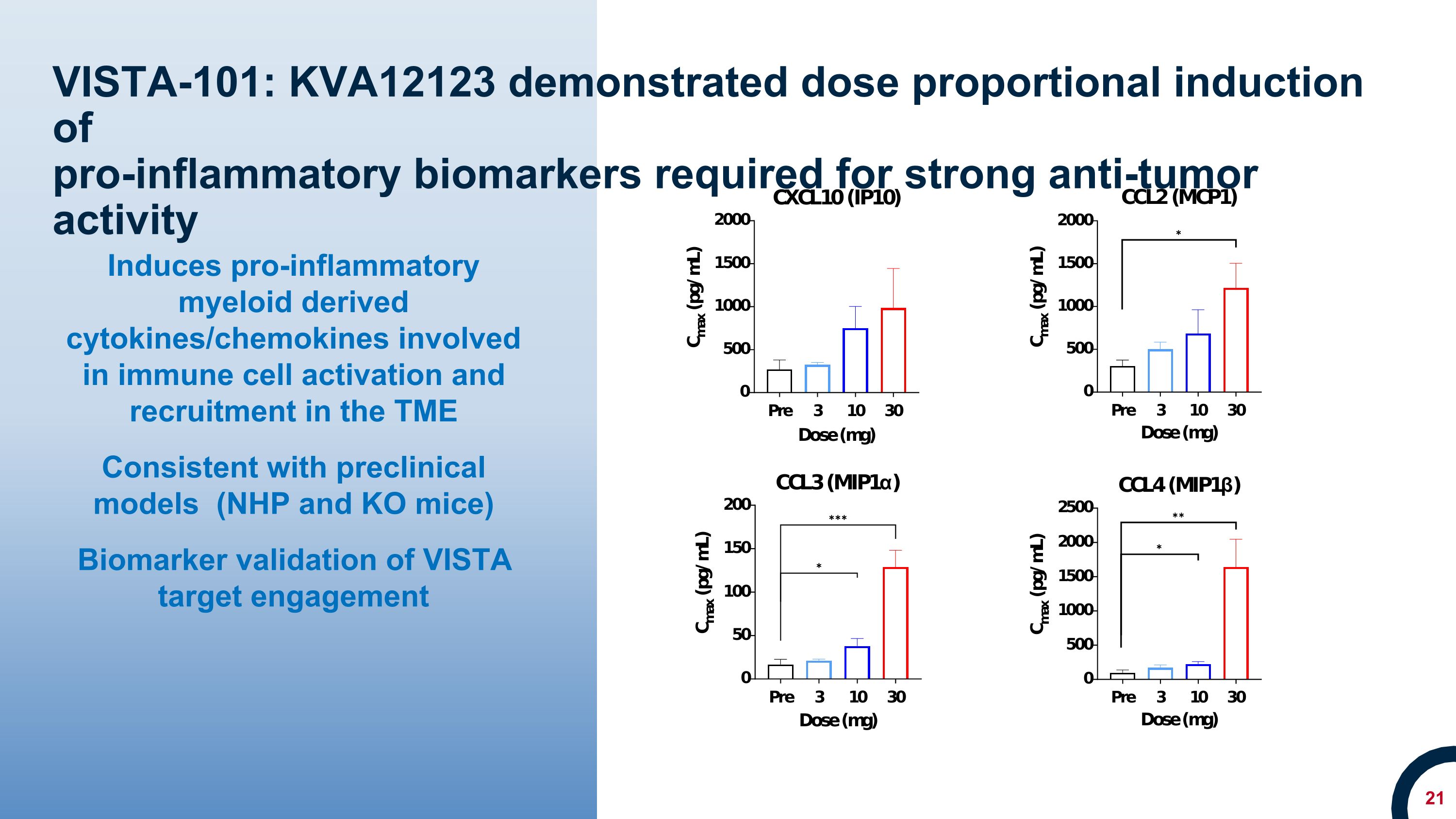
VISTA-101: KVA12123 demonstrated dose proportional induction of �pro-inflammatory biomarkers required for strong anti-tumor activity Induces pro-inflammatory myeloid derived cytokines/chemokines involved in immune cell activation and recruitment in the TME Consistent with preclinical models (NHP and KO mice) Biomarker validation of VISTA target engagement
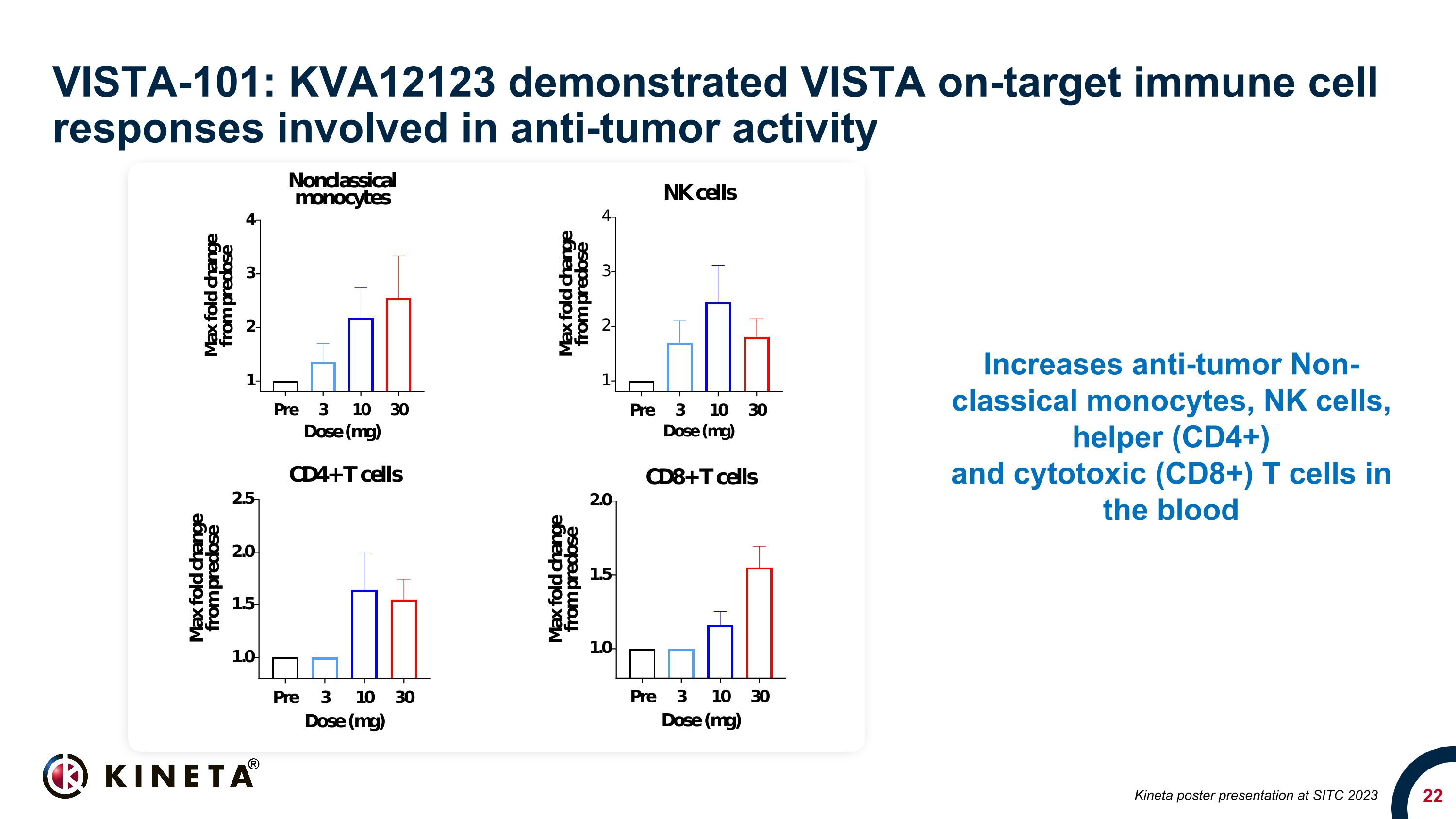
VISTA-101: KVA12123 demonstrated VISTA on-target immune cell responses involved in anti-tumor activity Increases anti-tumor Non-classical monocytes, NK cells, helper (CD4+) and cytotoxic (CD8+) T cells in the blood Kineta poster presentation at SITC 2023
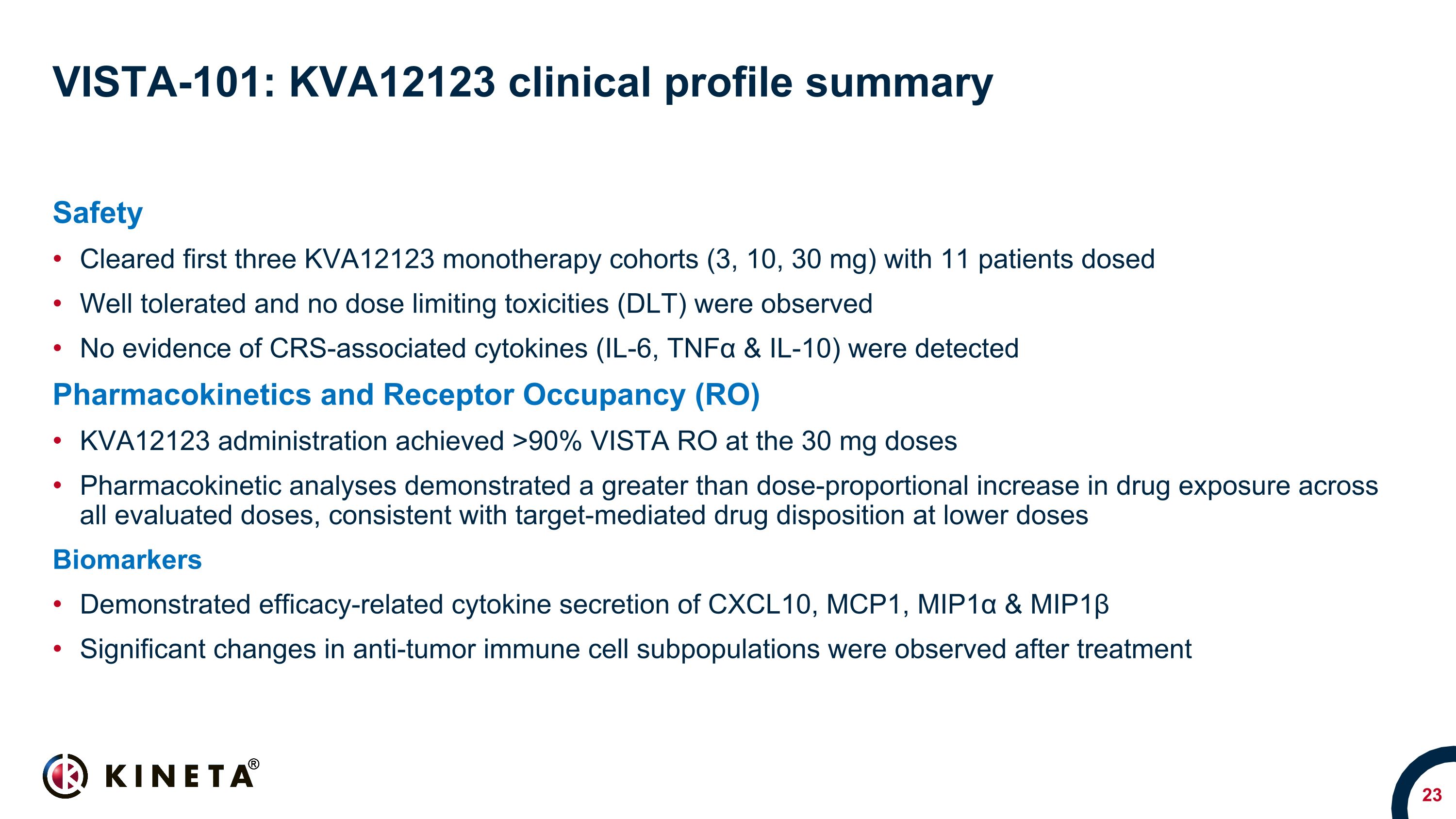
VISTA-101: KVA12123 clinical profile summary Safety Cleared first three KVA12123 monotherapy cohorts (3, 10, 30 mg) with 11 patients dosed Well tolerated and no dose limiting toxicities (DLT) were observed No evidence of CRS-associated cytokines (IL-6, TNFα & IL-10) were detected Pharmacokinetics and Receptor Occupancy (RO) KVA12123 administration achieved >90% VISTA RO at the 30 mg doses Pharmacokinetic analyses demonstrated a greater than dose-proportional increase in drug exposure across all evaluated doses, consistent with target-mediated drug disposition at lower doses Biomarkers Demonstrated efficacy-related cytokine secretion of CXCL10, MCP1, MIP1α & MIP1β Significant changes in anti-tumor immune cell subpopulations were observed after treatment
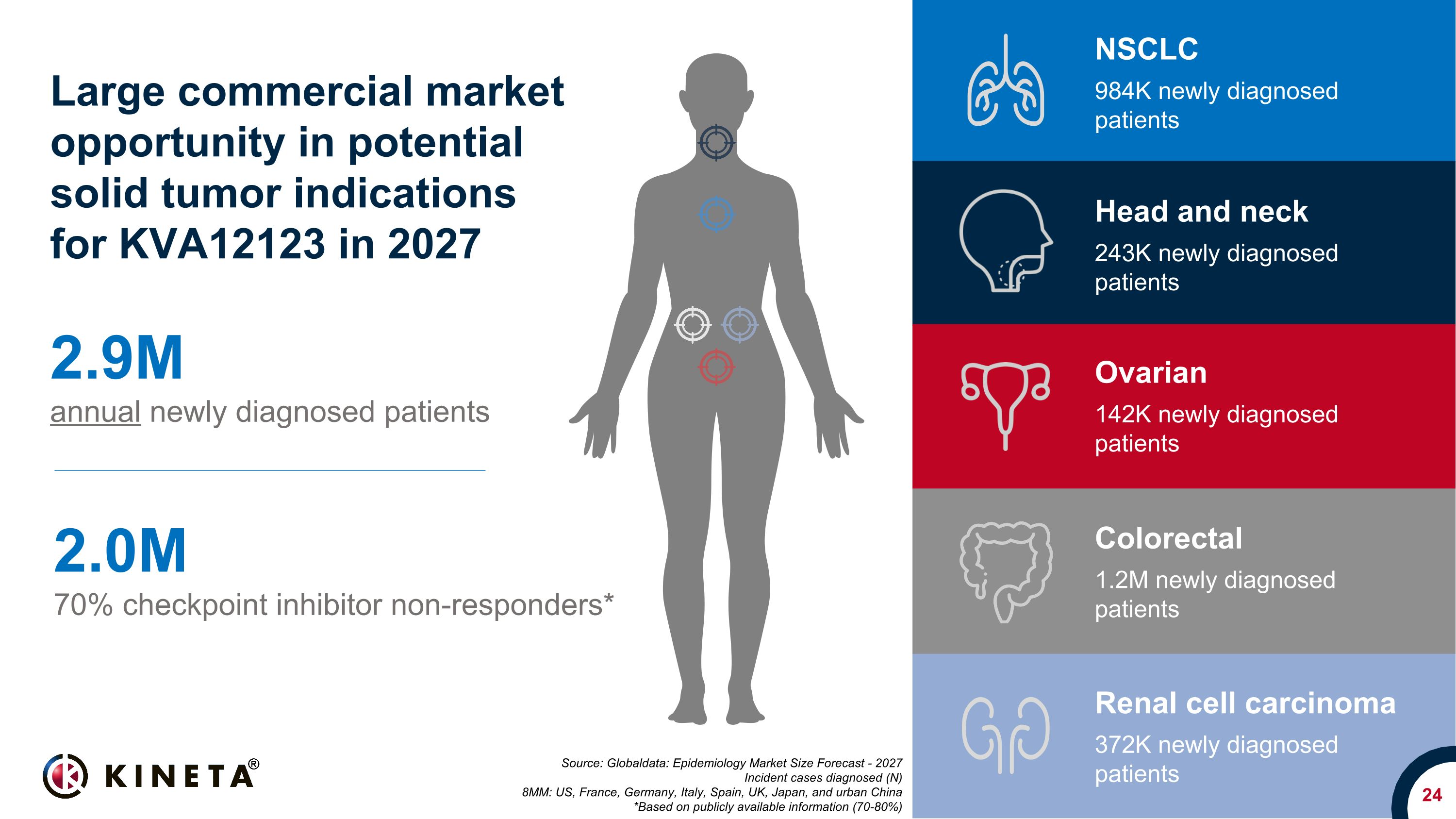
24 Source: Globaldata: Epidemiology Market Size Forecast - 2027 Incident cases diagnosed (N) 8MM: US, France, Germany, Italy, Spain, UK, Japan, and urban China *Based on publicly available information (70-80%) 2.9M annual newly diagnosed patients 2.0M 70% checkpoint inhibitor non-responders* Large commercial market opportunity in potential solid tumor indications�for KVA12123 in 2027 NSCLC 984K newly diagnosed patients Colorectal 1.2M newly diagnosed patients Ovarian 142K newly diagnosed patients Head and neck 243K newly diagnosed patients Renal cell carcinoma 372K newly diagnosed patients
Anti-CD27 agonist �mAb immunotherapy
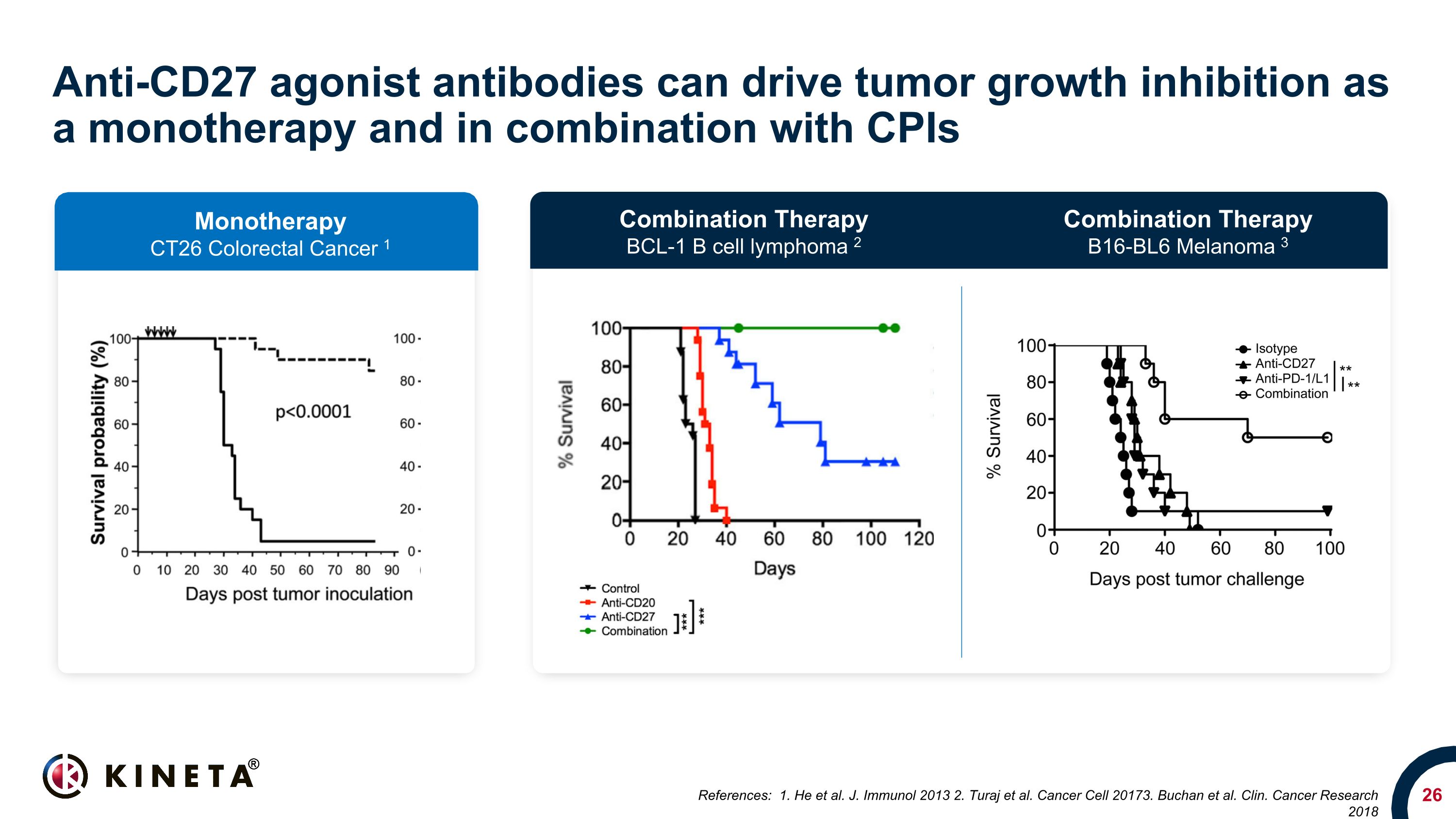
Anti-CD27 agonist antibodies can drive tumor growth inhibition as a monotherapy and in combination with CPIs References: 1. He et al. J. Immunol 2013 2. Turaj et al. Cancer Cell 20173. Buchan et al. Clin. Cancer Research 2018 Monotherapy CT26 Colorectal Cancer 1 Combination Therapy BCL-1 B cell lymphoma 2 Combination Therapy B16-BL6 Melanoma 3
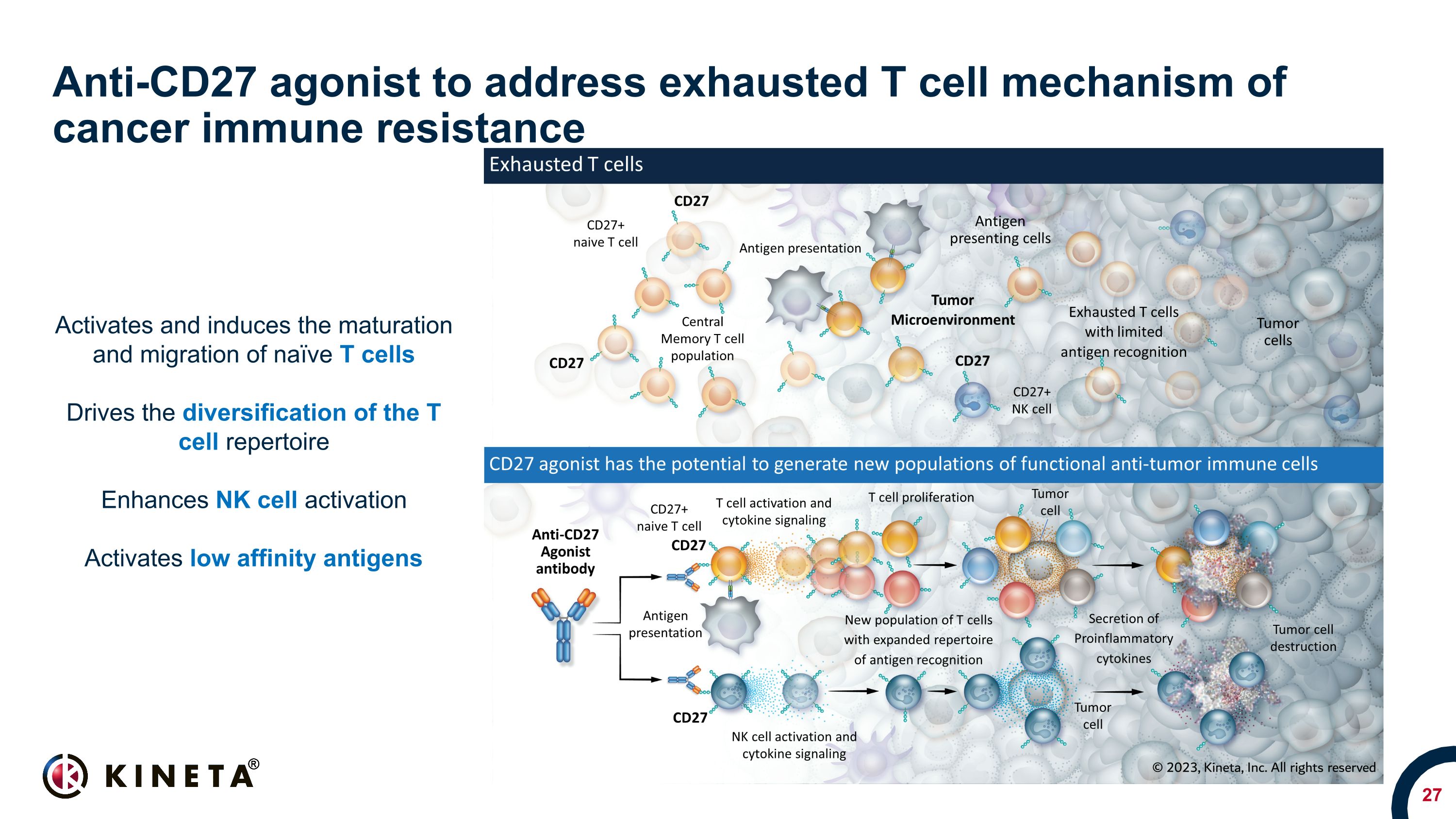
Anti-CD27 agonist to address exhausted T cell mechanism of cancer immune resistance Activates and induces the maturation and migration of naïve T cells Drives the diversification of the T cell repertoire Enhances NK cell activation Activates low affinity antigens
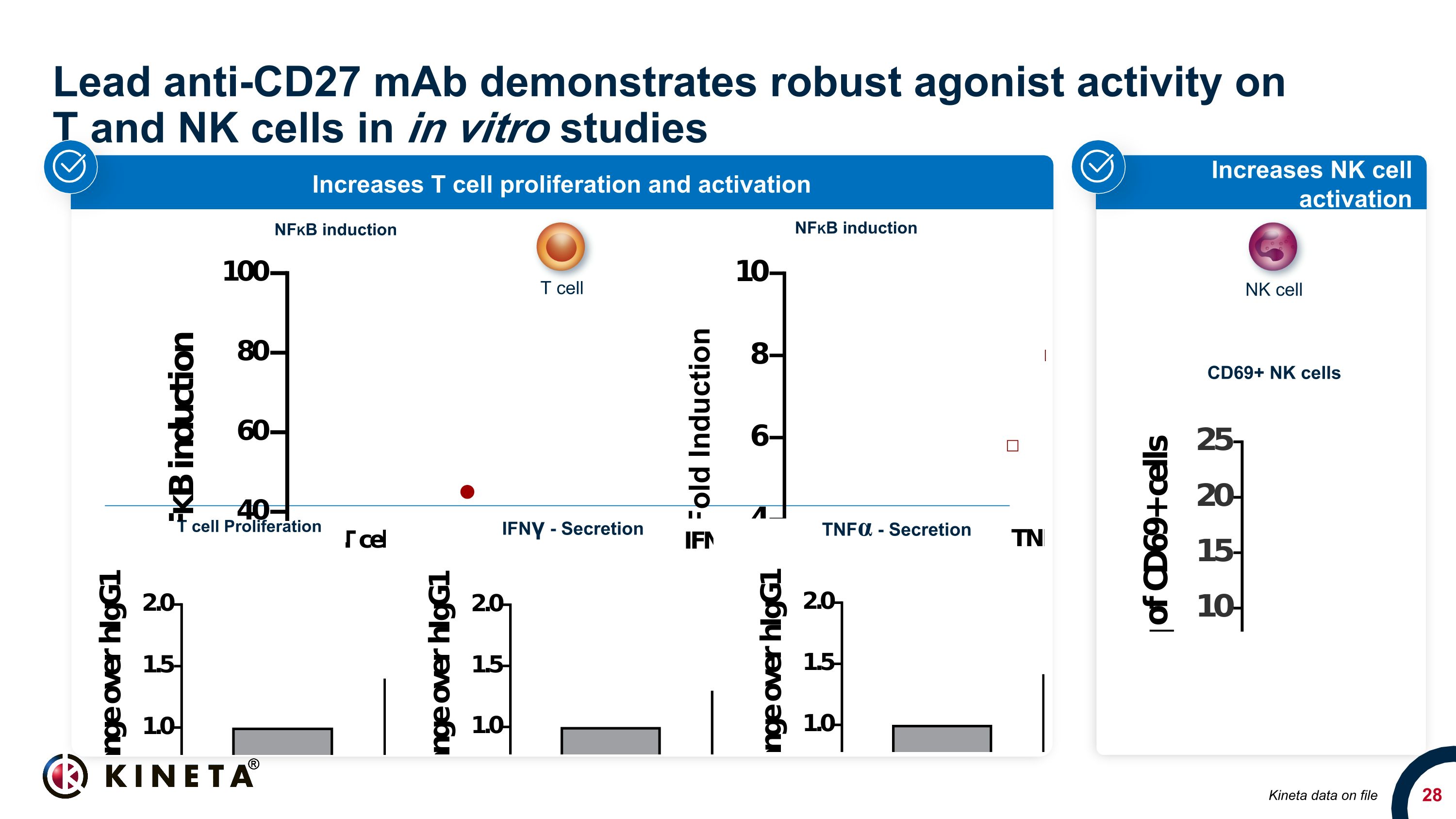
Lead anti-CD27 mAb demonstrates robust agonist activity on �T and NK cells in in vitro studies Increases NK cell activation Increases T cell proliferation and activation T cell Proliferation IFNγ - Secretion TNFα - Secretion Kineta data on file CD69+ NK cells T cell NK cell NFKB induction NFKB induction
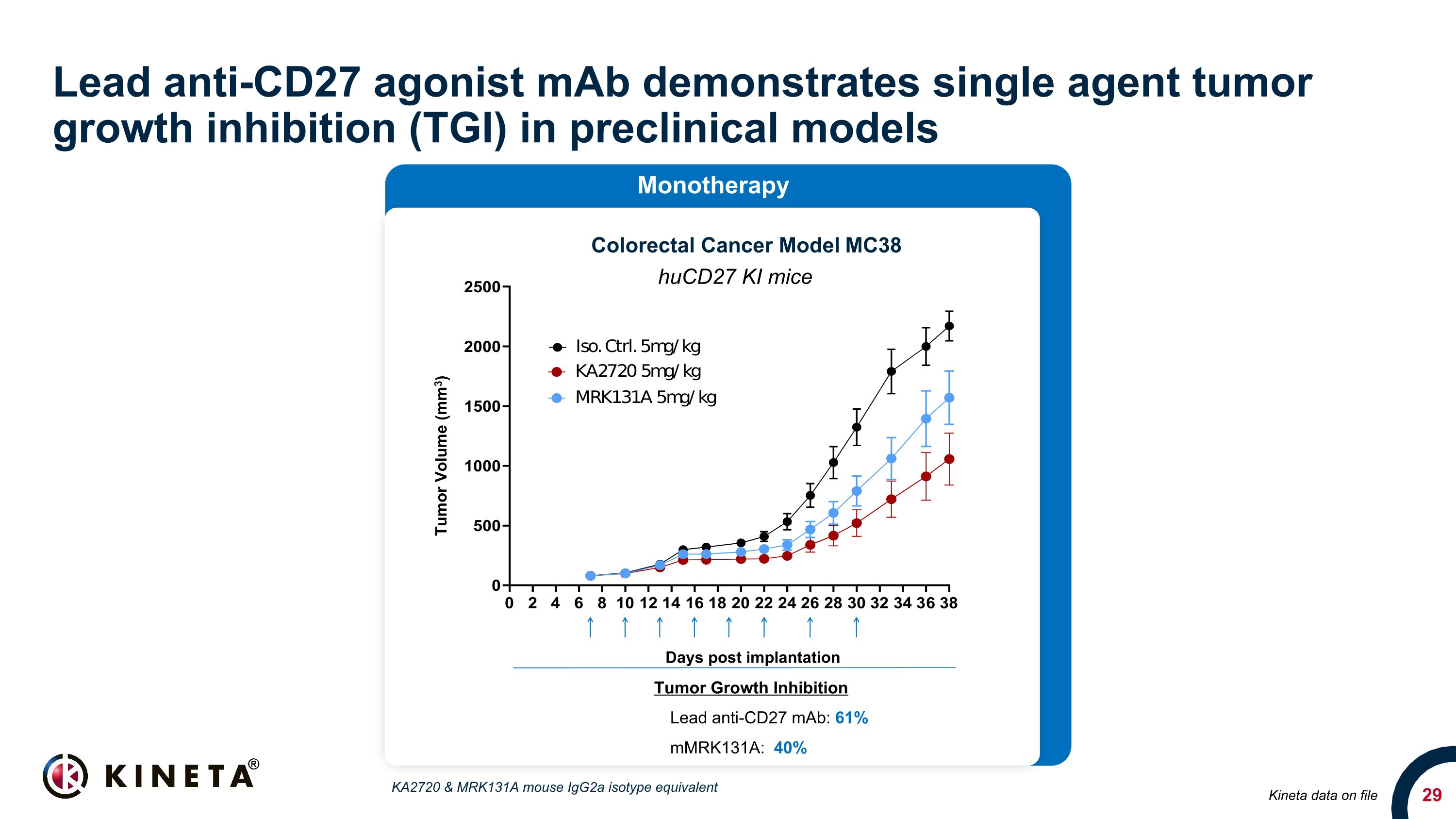
Lead anti-CD27 agonist mAb demonstrates single agent tumor growth inhibition (TGI) in preclinical models Kineta data on file Colorectal Cancer Model MC38 huCD27 KI mice Tumor Growth Inhibition Lead anti-CD27 mAb: 61% mMRK131A: 40% Monotherapy Days post implantation Tumor Volume (mm3) KA2720 & MRK131A mouse IgG2a isotype equivalent
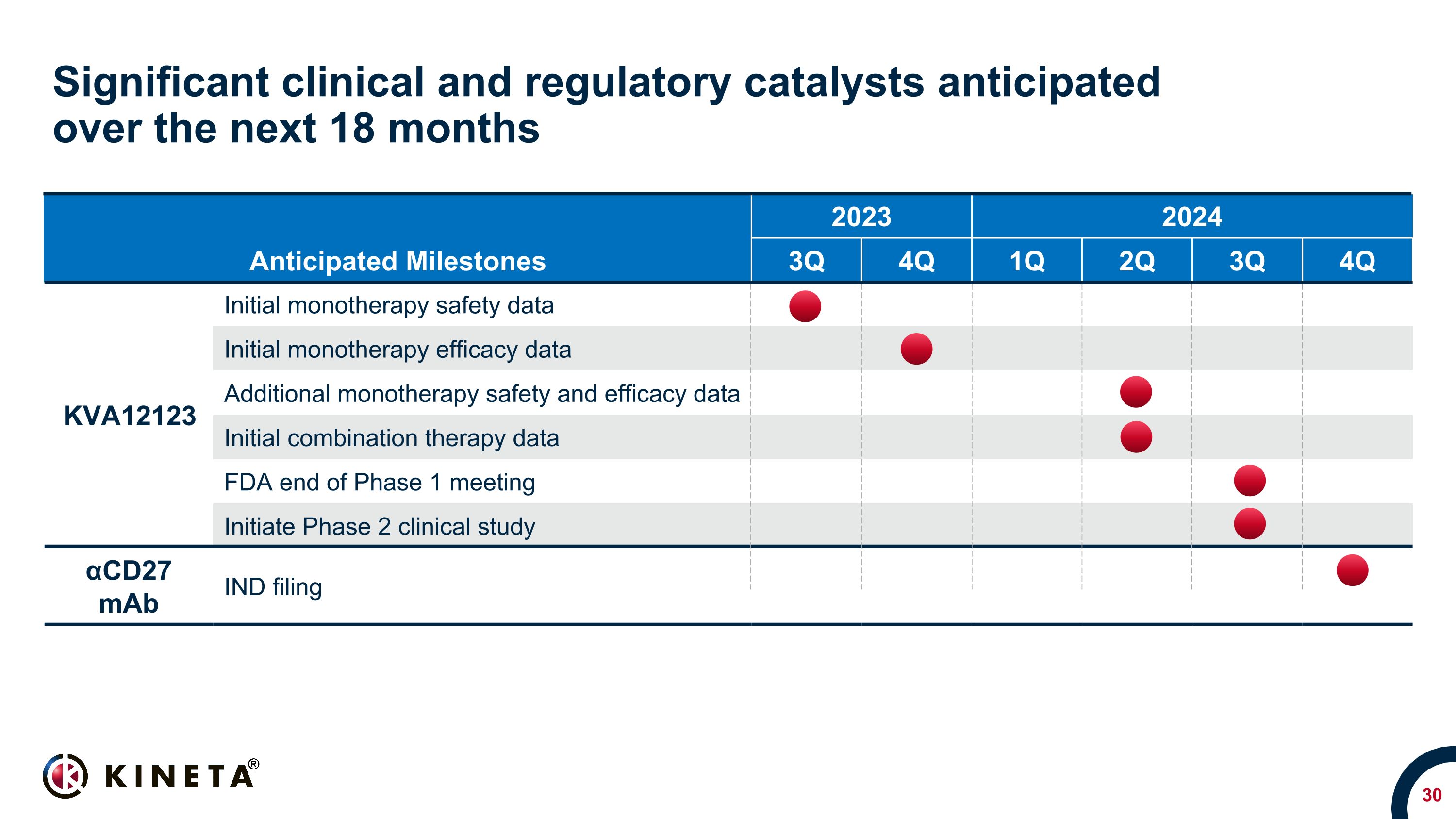
Significant clinical and regulatory catalysts anticipated �over the next 18 months Anticipated Milestones 2023 2024 2024 3Q 4Q 1Q 2Q 3Q 4Q KVA12123 Initial monotherapy safety data Initial monotherapy efficacy data Additional monotherapy safety and efficacy data Initial combination therapy data FDA end of Phase 1 meeting Initiate Phase 2 clinical study αCD27 mAb IND filing

Program Neuromuscular diseases-ALS Oncology Cystic fibrosis Partner Key deal terms Received $5M milestone payment in July 2023 Up to $255M in milestones Royalties on net sales Up to $96M in milestones Royalties on net sales Up to $965M in commercial only milestones Royalties on net sales Revenue share on sub-license payments ~$1.3 billion in potential milestone payments plus royalties on net sales License Agreements

Shawn Iadonato, PhD Chief Executive Officer Vinny Hayreh, MD VP Clinical Research Thierry Guillaudeux, PhD Chief Scientific Officer Jacques Bouchy EVP Investor Relations & Business Development Experienced leadership team Craig Philips President Keith Baker Chief Financial Officer Pauline Kenny General Counsel
KVA12123: VISTA blocking mAb to address immunosuppression in the TME Phase 1/2 clinical study evaluating KVA12123 alone and in combination with pembrolizumab in advanced solid tumors Initial monotherapy data: cleared first 3 cohorts, no dose limiting toxicities, >90% VISTA receptor occupancy Preclinical Anti-CD27 agonist mAb to address exhausted T cells Expected cash runway into early 2025* 10.3 million outstanding shares (KA: Nasdaq) 4Q23: Initial monotherapy efficacy data 2Q24: Additional monotherapy safety and efficacy data 2Q24: Initial combination therapy data Innate Immunity Focused Pipeline Anticipated KVA12123 Catalysts Financial Position Kineta is developing next-generation immunotherapies that address cancer immune resistance ~$1.3 billion in potential milestone payments plus royalties on net sales Partnerships *includes $7.8M cash as of Q2 23, $3M registered direct closed 10/23, $5M Merck milestone payment received 7/23 and $22.5M PIPE financing expected to close 4/24

Developing next generation immunotherapies for cancer patients�www.kinetabio.com

Appendix
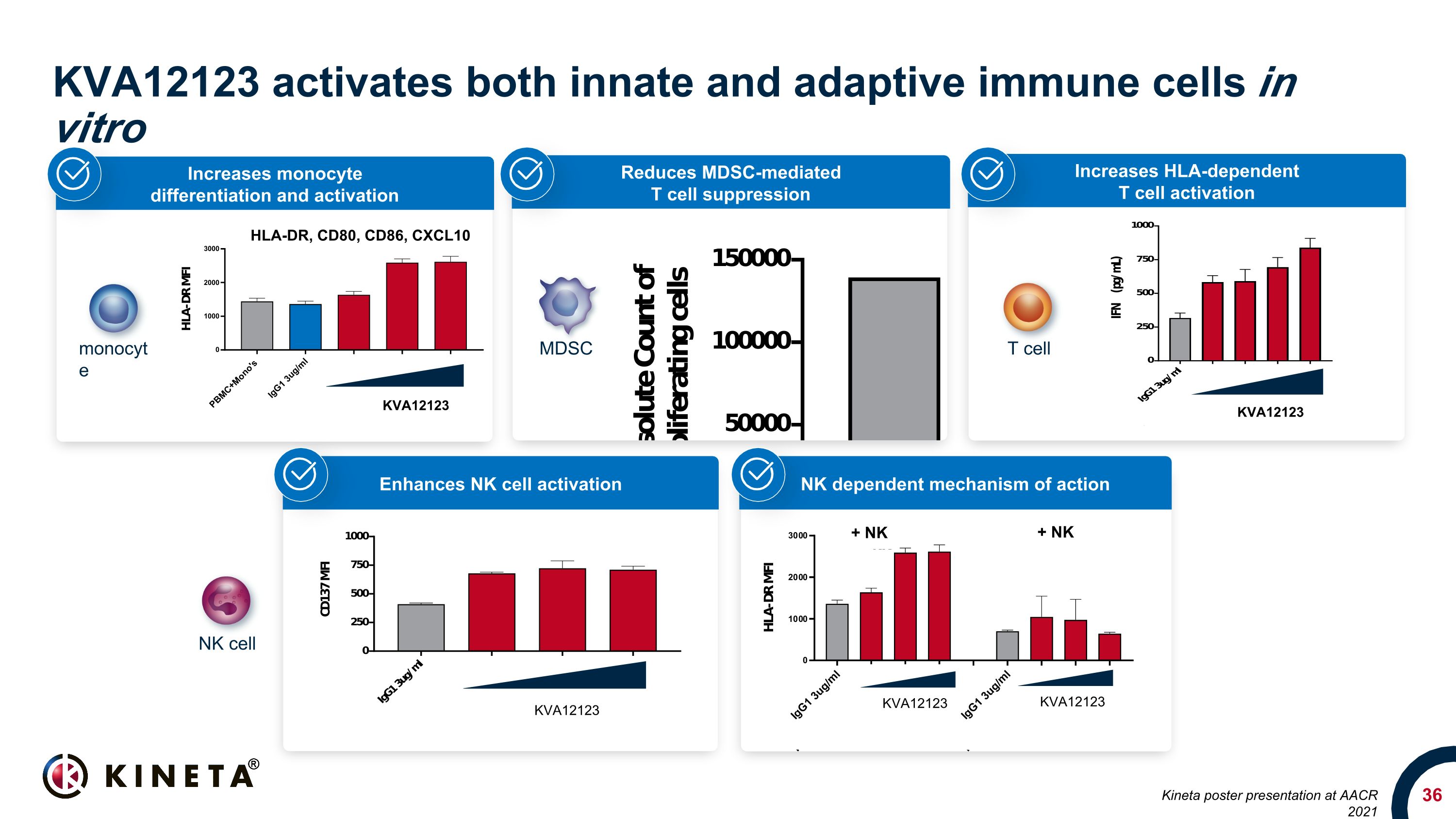
Increases HLA-dependent T cell activation Reduces MDSC-mediated T cell suppression KVA12123 activates both innate and adaptive immune cells in vitro HLA-DR, CD80, CD86, CXCL10 KVA12123 Increases monocyte differentiation and activation KVA12123 KVA12123 NK dependent mechanism of action Enhances NK cell activation MDSC T cell NK cell monocyte KVA12123 KVA12123 + NK + NK Kineta poster presentation at AACR 2021

KVA12123 binds at physiologic and acidic pH Binding studies by ELISA and Octet demonstrate rapid on-rate and slow off-rate from pH 7.4 to pH 6.0 ELISA Octet Kineta data on file 0.5 0.4 0.1 0.3 0.2 0 nm 0 50 100 150 200 250 300 Time (s) 350 400 450 500 550 600 KVA12123 pH 6.0 KVA12123 pH 6.5 KVA12123 pH 7.0 KVA12123 Ph 7.4 Various pH Association and Dissociation pH 7.0 pH 6.0 pH 6.5 pH 7.4 KVA12123 Binding KVA Concentration (ng/mL) OD 450
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Kineta (NASDAQ:KA)
Historical Stock Chart
From Nov 2024 to Dec 2024

Kineta (NASDAQ:KA)
Historical Stock Chart
From Dec 2023 to Dec 2024
