
Kiora Pharmaceuticals, Inc. NASDAQ: KPRX H1 2024 | Corporate Overview

2 Forward Looking Statements Some of the statements in this presentation are "forward-looking" and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995. These "forward-looking" statements include statements relating to, among other things, the development and commercialization efforts and other regulatory or marketing approval efforts pertaining to Kiora's development- stage products, including KIO-301 and KIO-104, as well as the success thereof, with such approvals or success may not be obtained or achieved on a timely basis or at all, the potential ability of KIO-301 to restore vision in patients with RP, the expecting timing of enrollment, dosing and topline results for the ABACUS study, the ability to develop KIO-301 for Choroideremia and Stargardt Disease and KIO-104 for posterior non-infectious uveitis, the ability to utilize strategic relationships to develop certain product candidates, Kiora’s ability to maintain the listing of our common stock on a national securities exchange, and Kiora's ability to achieve the specific milestones described herein. These statements involve risks and uncertainties that may cause results to differ materially from the statements set forth in this presentation, including, among other things, the ability to conduct clinical trials on a timely basis, the ability to obtain any required regulatory approvals, market and other conditions and certain risk factors described under the heading "Risk Factors" contained in Kiora's Annual Report on Form 10-K filed with the SEC on March 23, 2023, or described in Kiora's other public filings. Kiora's results may also be affected by factors of which Kiora is not currently aware. The forward-looking statements in this presentation speak only as of the date of this presentation. Kiora expressly disclaims any obligation or undertaking to release publicly any updates or revisions to such statements to reflect any change in its expectations with regard thereto or any changes in the events, conditions, or circumstances on which any such statement is based, except as required by law.

3 Kiora is developing retinal therapeutics to improve sight in patients with severe vision loss due to inherited or age-related diseases Sharpened Focus on Orphan Retinal Diseases Patient Perspective Individual’s burden of disease Physicians Perspective Efficient, cost-effective treatments Societal Perspective Pharma industry obligation to help 70% of blind patients are unemployed

4 Why Retinal Diseases? “…the last light sensations faded and the dark discs had finally overwhelmed me. I had fought them bravely, as it seemed to me, for thirty-six years, but to no avail. It was then I began to sink into the deep ocean, and finally learn how to touch the rock on the far side of despair.” - John M. Hull, Touching the Rock JAMA Ophthalmology. Oct 2016. 134-10. 0 20 40 60 0 5 10 15 20 25 Re sp on de nt s (% ) Re sp on de nt s (% ) Conditions with Greatest Effect on Day-to-Day Life (n=2044) Rankings of Worst Conditions (n=2044)

5 Pipeline Product Route of Delivery Indication Development Stage Prevalence* Pre-clinical Phase 1 Phase 2 Phase 3 (US, EU5, JP) KIO-301 Intravitreal Retinitis Pigmentosa (Mutation Agnostic) 250,000 Choroideremia 16,000 Stargardt Disease 99,000 KIO-104 Intravitreal Posterior Non-Infectious Uveitis 180,000 Granted Orphan Drug Designation (USA) – Mar 2022 Granted Orphan Drug Designation (EU) – May 2015 * Approximate 2023 populations. Orpha.net, NORD, Ophthalmol Ther. 2021 Sep; 10(3).

6 Q4 ‘23 Q1 ‘24 Q2 ‘24 Q3 ‘24 Q4 ‘24 KIO-301 ABACUS-1 ✓ Top Line Data KIO-301 ABACUS-2 Ph2 Initiation KIO-301 ABACUS-2 Ph2 Top Line Data* KIO-301 Initiated Choroideremia ‘Extension’ KIO-301 Stargardt Ph2 Initiation KIO-301 ✓ FDA pIND RP KIO-301 EMA Meeting KIO-301 ODD Choroideremia KIO-301 ODD Stargardt Q1 ‘25 * Excludes open label extension RP – Retinitis Pigmentosa, PVR – Proliferative Vitreoretinopathy, PoC – Proof of Concept, ODD – Orphan Drug Designation, EMA – European Medicines Agency, IRD – Inherited Retinal Disease, PNIU – Posterior Noninfectious Uveitis Upcoming Clinical/Regulatory Milestones KIO-301 Choroideremia Ph2 Initiation KIO-301 EMA ODD IRD KIO-301 ABACUS-1 Full Data Q2 ‘25 KIO-104 PNIU Ph2b Initiation KIO-104 PVR PoC Preclin

7 Final Major Terms Territory Global less Asia Field of Use Ophthalmology (all indications) with development milestones for each indication (RP, CHM, SD) or others Development Responsibilities Kiora responsible until Ph3 (JSC) Development Costs for Ph2 Thea to reimburse Kiora for KIO-301 Research & Development Development Costs for Ph3 Thea to cover 100% Upfront Payment $16M Development & Commercial Milestones In aggregate Up to $285M Commercial Royalties Royalties on Net Sales Tiered up to low 20% Total Upfront and Milestones $301M (for RP and 2 add’l indications approved in USA and EU with net sales exceeding $1B) KIO-301 Partnership Non-Binding Term Sheet Signed 5Oct2023, 25Jan2024 for Definitive Agreement Execution Public announcement planned for after market 31Jan2024

8 Private, family owned, ophthalmic focused company Chibret family founded the French Society of Ophthalmology in 1883 >100 commercial ophthalmic products globally including: Azasite® Cosopt® Ivizia® Virgan® Zioptan® Other partnerships include: Théa products available in over 75 countries direct or through distributors Who is Théa?

9 KIO-301 Small Molecule Targeting Vision Restoration Retinitis Pigmentosa, Choroideremia, Stargardt Disease

10 Inherited Retinal Diseases Lead to Loss of Vision Healthy Vision Rods and cones, the photoreceptors of the retina, process light and relay an electrical signal to downstream cells. One of these cell types, retinal ganglion cells (RGCs), transmit the signal to the visual cortex. The visual cortex is the part of the brain where vision is perceived. Damage from Retinitis Pigmentosa Retinitis Pigmentosa (RP) results in progressive degeneration and loss of function of rods and cones. This causes continuous impairment of vision that often leads to blindness. Importantly, in RP and other inherited retinal diseases, the RGCs remain viable. Rods & Cones Light RGCs Rods & Cones Light RGCs

11 KIO-301 is a Molecular Photoswitch Designed to Restore Vision KIO-301 without Light When photoreceptors die, RGCs undergo some remodeling, including expressing specific proteins that allow KIO-301 to selectively enter the cell with ion channels. Without light, KIO-301 remains in its linear “off” (trans) position. KIO-301 with Light With light, KIO-301 is activated and bends into its “on” (cis) formation. This physically blocks ion channels and activates the cell to transmit signals to the visual cortex. Rods & Cones RGCs KIO-301 (trans) P2X7 Pore Ion Channel Rods & Cones Light RGCs KIO-301 (cis) P2X7 Pore Ion Channel

12 KIO-301 Reanimates the Retina & Changes Behaviour Extensive Validation in Preclinical Models Neuron. 81, 800-813 (2014). Behavioural study used a homologue molecule to KIO-301 API

13 Clinical Presentation Night blindness, reduced visual field range and eventual loss of central vision Visual acuity declines 50% of patients are not qualified to drive by age 37 and legally blind by 55 Etiology 50+ genetically distinct subtypes from 150+ mutations Inherited disease Market Opportunity ~100k patients in US (Provider: Retina Specialists [~3k]) Estimated total cost to US healthcare system in 2019: $3.7B Retinitis Pigmentosa A Disease with No Available Treatments Normal Vision Vision Declines over Time IOVS: Visual Field Progression in Retinitis Pigmentosa, American Academy of Ophthalmology, Clinical Ophthalmology 2021:15 2855–2866

14 KIO-301-1101: Phase 1b Study Design (ABACUS) Open Label, Single Ascending Dose Trial – 2 Sites (Australia) Two cohorts, non-randomized, open-label, single IVT injection per eye Cohort 1 – NLP/BLP patients; Cohort 2 – HM/CF patients Primary – AEs, PK & labs Secondary – Assessment days (shown only for Cohort 1 above) is repeated for each cohort per eye; intensity & contrast assessment, kinetic perimetry, functional MRI, etc. Safety review conducted by Investigators between after sentinel subject Study Design Endpoints Review N=6 3/cohort Binocular Day 90 Cohort 1: KIO-301 (7.5 µg) Day -30 to -2 Visit 1-Screening Day 29-30 Visit 5 Day 60 Day 120 Cohort 1: KIO-301 Contralateral (25 µg) Cohort 2: KIO-301 (25 µg) Cohort 2: KIO-301 Contralateral (50 µg) Day 1-3 Visit 2 Day 7-8 Visit 3 Day 14-15 Visit 4 Day -120 to -30 fMRI feasibility NLP – No Light Perception, BLP – Bare Light Perception, HM –Hand Motion, CF – Counting Fingers

15 Patient Testimonials Patient 1-02 Baseline VA: NLP Cohort 1 Patient 2-05 Baseline VA: CF Cohort 2 VA – Visual Acuity, NLP – No Light Perception, CF – Counting Fingers, HM – Hand Motion Patient 1-03 Baseline VA: HM Cohort 2

16 ABACUS-1 – Primary Endpoint Achieved Single Dose IVT KIO-301 is Safe & Well Tolerated @ 7.5μg, 25μg, 50μg MedDRA Term KIO-301 7.5µg (N=3); n (%) KIO-301 25µg (N=6); n (%) KIO-301 50µg (N=3); n (%) Severity Drug Related Total N=12; n (%) Ocular Hypertension 1 (33%) 0 (0%) 0 (0%) Mild Possible 1 (8.3%) Eye Swelling 0 (0%) 1 (17%) 0 (0%) Mild Unlikely 1 (8.3%) Eye Pain 0 (0%) 2 (33%) 0 (0%) Mild Unlikely 2 (17%) Anterior Chamber Cell 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Anterior Chamber Flare 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Vitreal Cells 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Retinitis 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Vasculitis 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Iritis 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Keratitic Precipitates 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Photophobia 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Photopsia 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Vitreous Floaters 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Punctate Keratitis 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Conjunctival Hyperemia 0 (0%) 0 (0%) 0 (0%) N/A N/A 0 (0%) Ocular AEs only, N = number of patients reporting event, n (%) = % of patients reporting event

17 Kinetic Visual Field (Goldmann Perimetry) Assessment & Insights: Applicability in this population Performed by experienced orthoptists Limited to 2-axis The patient is asked to acknowledge (using a buzzer) when light stimulus is visualized within the dome Method facilitates limitation of fixation > Proof-of-feasibility achieved > Will expand scope of evaluation to capture increased degrees Aim: Evaluate Peripheral Vision at a Basic Level

18 Kinetic Visual Field KIO-301 May Improve Visual Field 0 10 20 30 30 40 50 60 70 80 Kinetic Visual Field‡ LS Mean ± 80% CL Day D eg re es -20 -10 0 10 20 30 40 D2-BL D7-BL D14-BL D29-BL Kinetic Visual Field‡ Mean Change ± 80% CL Degrees of Change from Baseline * *p<0.05 ‡ Cohort 2 includes 3 patients (6 eyes) Goldmann perimetry Performed at baseline (BL), and each study visit Performed by same group of orthoptists to reduce variability Greater improvement observed in Cohort 2 Kinetic Visual Field

19 Visual Acuity — Berkeley Rudimentary Vision Test (BRVT) 0 10 20 30 1.50 1.75 2.00 2.25 2.50 Visual Acuity Mean ± SEM Day lo gM AR Off-Chart Counting Finger Hand Motion Optometry and Vision Science 2012:89(9), 1257-1264 Cohort 2 (3 patients, 3 eyes)

20 Light Perception (Intensity & Contrast Assessment) Assessment: Series of visual stimuli (a series of letters are presented on a screen to the patient via a rear projector) Binary outcome (yes/no) The subject is asked to acknowledge (verbally and/or physically) when a change in light is perceived Asked to also identify object, if possible Aim: Evaluate Light Perception at a Basic Level

21 Light Perception – Cohort 1 KIO-301 may improve light perception in the NLP/BLP Population Insights: Cohort 1 subjects demonstrate improved odds ratio on drug Odds Ratio – strength of association, OR=2 → 100% increase in the odds of an outcome > e.g., duration of diabetes mellitus (> 15 years) with diabetic retinopathy is >9.0* Cohort 2 subjects are existing light perception patients; therefore, expect little change Light Perception Cohort 1 includes 3 patients (6 eyes), NLP – No Light Perception, BLP – Bare Light Perception *Int J Retin Vitr 2016:2, 21 0 10 20 30 0 25 50 75 100 LSMean ± SEM Day Pr op or tio n of S uc ce ss (% ) 0.1 1 10 100 2 7 14 29 Odds Ratio (Change from Baseline, 80%CL) Odds Ratio Da y

22 2’x2’ tile Door Location Test Setup Functional Vision - Multiluminance Orientation & Mobility (MLOM) 2’x2’ tile Window Location Test Setup 2’x2’ tile Walking Direction Test Setup Start End 2’x2’ tile HCRE Course Setup Screen Screen Technician 1 Technician 2Door Window Left Center Right Left Center Right Seat Seat Seat

23 Functional Vision − Multiluminance Orientation & Mobility (MLOM) KIO-301 May Improve Functional Vision 0.1 1 10 100 Day 2 Day 7 Day 14 Day 29 Door Location Test* Change From Baseline ± 80% CL Odds Ratio 0.1 1 10 100 Day 2 Day 7 Day 14 Day 29 Walking Direction Test‡ Change From Baseline ± 80% CL Odds Ratio 0.1 1 10 100 Day 2 Day 7 Day 14 Day 29 Window Location Test§ Change From Baseline ± 80% CL Odds Ratio * Analysis of 4 patients (7 eyes) ‡ Analysis of 6 patients (10 eyes) § Analysis of 3 patients (5 eyes) Ora-MLOMTM Suite of Tests Overview First time used in ultra-low vision patients Question: “Is this test valuable?” Takeaways: Important aspect of documenting vision driven movement Not all “functional” tests relevant to the population tested One or two clinically meaningful functional tests will remain in Phase II Will incorporate light-level changes into Phase II MLOM 0 10 20 30 0 20 40 60 80 100 High Contrast Room Exit Test‡ Mean ± SEM Day R es po ns e R at e (% )

24 Visual Function Questionnaire (NEI VFQ-25) KIO-301 May Improve Patients’ Overall Quality of Life 0 30 35 40 45 50 Quality of Life Survey Day VF Q -2 5 C om po si te S co re n=12 *HMSA Medical Policy – Luxturna - 2016 National Eye Institute generated survey assess daily functions related to general health & vision, ocular pain, near & distance activities, social functioning, mental health, dependency, driving, color vision, and peripheral vision. 2-4 point increase is considered clinically meaningful* Quality of Life

25 Functional MRI Supportive of Cortical Activation NLP – No Light Perception, BLP – Bare Light Perception, CF – Counting Fingers

26 ABACUS-1 Takeaways Patients report improvements in vision Consistent with objective clinical assessments Follow-on study will include sham group Pilot study limitations Non-controlled Small sample size Approvable outcome assessments discussed with regulators Positive US FDA pIND meeting in Q4 2023 KIO-301 appears to reanimate the retina 4 3 2 1 No Safety & Tolerability Concerns

27 KIO-301-2101: Phase 2 Study Design (ABACUS-2) Randomized (3:1), Controlled*, Double Masked, Multiple Dose Study – 4 Sites (Australia) Cohort A: 16 patients with RP who have NLP (logMAR >3.0) Cohort B: 16 patients with RP who have ULV (logMAR > 1.6 and < 3.0 OU) Cohort 1A and Cohort 1B • 50µg KIO-301 OR • Control Cohort 2A and 2B: • 100µg KIO-301 OR • Control Cohort 1A and 1B: Open Label Extension Patients randomized to control will be eligible to receive 50ug KIO-301 OU monthly for 3 mo Cohort 2A and 2B: Open Label Extension Patients randomized to control will be eligible to receive 100ug KIO-301 OU monthly for 3 mo Primary: Safety & Tolerability o AEs, vitals ECG, chemistry and hematology, SD-OCT, FAF, slit lamp, IOP Secondary: Efficacy (change from baseline @ 11 weeks) o Visual acuity as measured by BRVT o Visual field as measured by automated Goldmann perimetry o Functional vision as measured by an orientation, mobility, & object identification test pIND feedback (12/23) supportive of p2 trial design FPFV planned for Q2 2024 & topline data Q2 2025 Randomized (3:1) to receive either: Randomized (3:1) to receive either: • IVT injection OU, monthly for 3 mo • Followed by 3 mo of follow-up visits • IVT injection OU, monthly for 3 mo • Followed by 3 mo of follow-up visits *IVT Saline Injection
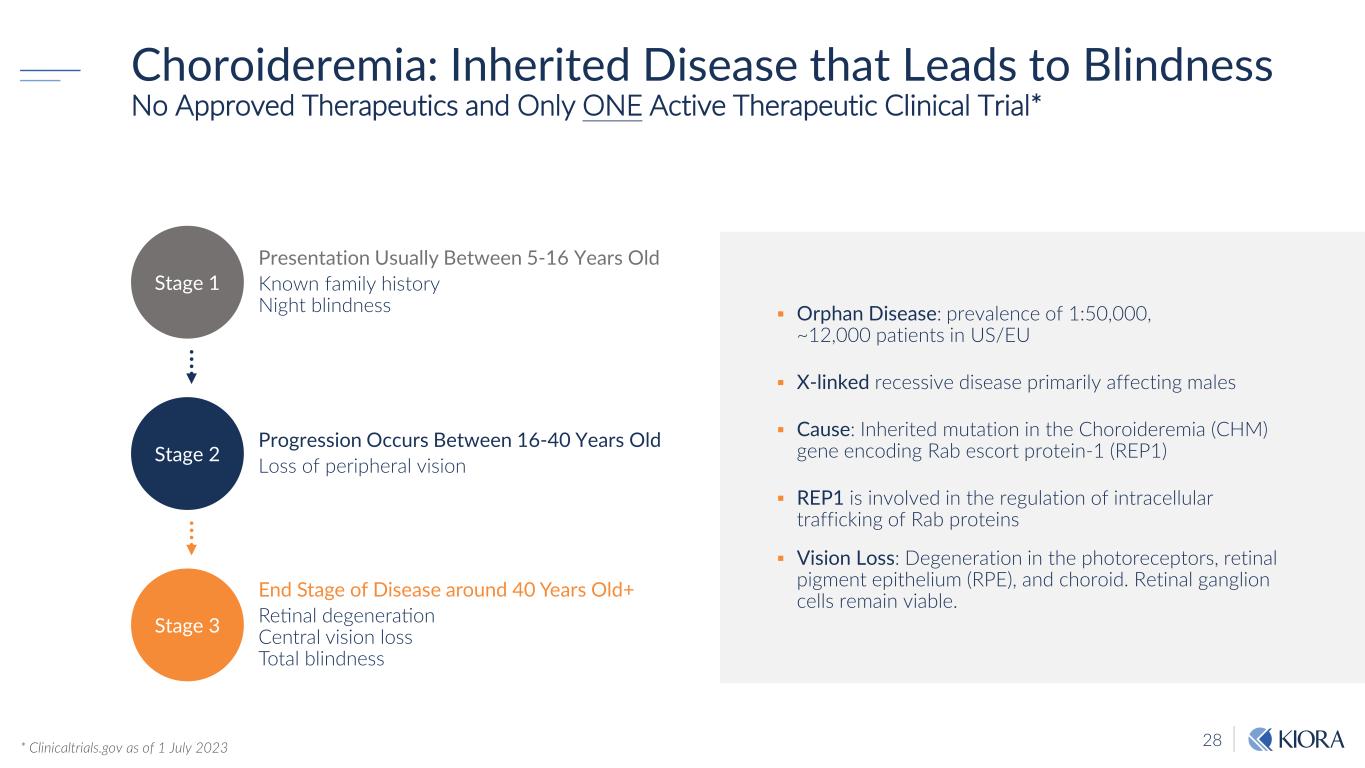
28 Orphan Disease: prevalence of 1:50,000, ~12,000 patients in US/EU X-linked recessive disease primarily affecting males Cause: Inherited mutation in the Choroideremia (CHM) gene encoding Rab escort protein-1 (REP1) REP1 is involved in the regulation of intracellular trafficking of Rab proteins Vision Loss: Degeneration in the photoreceptors, retinal pigment epithelium (RPE), and choroid. Retinal ganglion cells remain viable. Choroideremia: Inherited Disease that Leads to Blindness No Approved Therapeutics and Only ONE Active Therapeutic Clinical Trial* * Clinicaltrials.gov as of 1 July 2023 Stage 3 Presentation Usually Between 5-16 Years Old Known family history Night blindness Progression Occurs Between 16-40 Years Old Loss of peripheral vision End Stage of Disease around 40 Years Old+ Retinal degeneration Central vision loss Total blindness Stage 1 Stage 2

29 The Choroideremia Research Foundation (CRF) is the largest global not-for-profit organization focused on the search for a cure for Choroideremia (CHM). Partnership with the Choroideremia Research Foundation Education and Awareness of CHM and KIO-301 CHM KOL Network to Assist in Clinical Protocol Design CHM Patient Identification to Assist in Trial Enrollment Partnering on:

30 Stargardt Disease: No Approved Therapeutics Foundation Fighting Blindness, Makari Wellness Orphan Disease: prevalence of 1:10,000 ~30,000 patients in US Autosomal recessive disease inherited from parent carriers, typical onset in 2nd decade of life, vision loss in 4th-5th decade. Cause: Mutation in the ABCA4 or ELOVL4 gene Accumulation of lipofusion plaques in the retinal pigment epithelium (RPE), leading to inflammation and cell death. Vision Loss: Degeneration of the photoreceptors and RPE. Retinal ganglion cells remain viable. Often, some peripheral vision is retained.
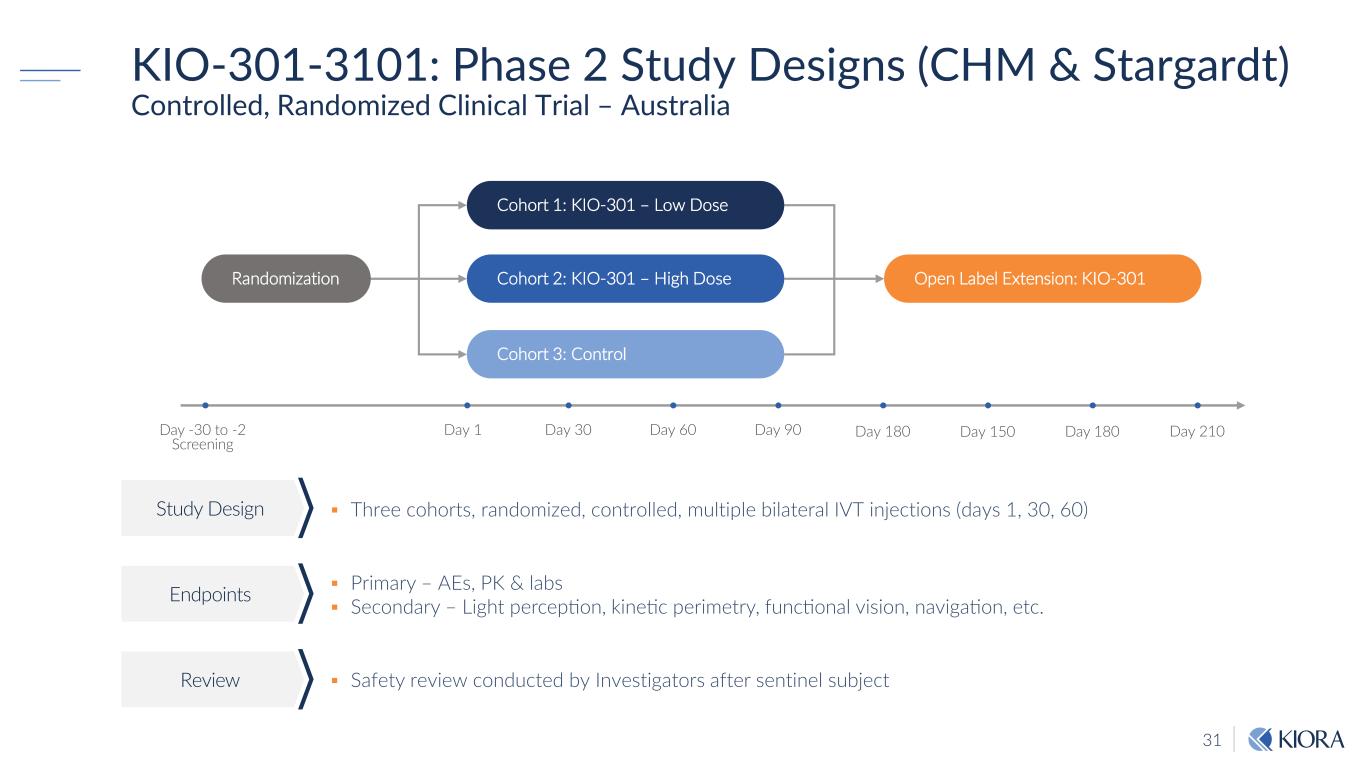
31 KIO-301-3101: Phase 2 Study Designs (CHM & Stargardt) Controlled, Randomized Clinical Trial – Australia Three cohorts, randomized, controlled, multiple bilateral IVT injections (days 1, 30, 60)Study Design Endpoints Review Safety review conducted by Investigators after sentinel subject Primary – AEs, PK & labs Secondary – Light perception, kinetic perimetry, functional vision, navigation, etc. Day 150 Cohort 1: KIO-301 – Low Dose Day -30 to -2 Screening Cohort 3: Control Day 1 Day 30 Randomization Open Label Extension: KIO-301 Day 60 Day 90 Day 180 Day 210Day 180 Cohort 2: KIO-301 – High Dose

32 KIO-104 Intravitreal Small Molecule DHODH Inhibitor Steroid Sparing Approach to Retinal Inflammation

33 KIO-104 is an intravitreal, non-steroidal, novel small molecule which mitigates: Metabolic activity and proliferation of T-cells Secretion of IL-17, VEGF and IFN-𝛾𝛾 KIO-104 Overview (DHODH Inhibitor) O F F F F F F F NH O S OH O Existing immunosuppressive agents have a fundamentally different mode of action on T-cells compared to KIO-104 KIO-104 is best-in-class inhibitor of DHODH (lowest IC50)* KIO-104 is first-in-class in ophthalmology *1,000x more potent than Teriflunomide (Aubagio© - Sanofi) DHODH - dihydroorotate dehydrogenase

34 Non-Infectious Uveitis Br J of Ophthalmol. 2004;88(9):1159-1162. Med Hypothesis Discov Innov Ophthalmol. 2013 Winter;2(4):113-120. Retina Today. 2016;47-51. Clin Ophthalmol. 2016;10:1983-2020. JAMA Ophthalmol. 2016 Nov 1;134(11):1237-1245. Uveitis Market Insights, Epidemiology & Market Forecast-2032 DelveInsights Redness and pain in the eye Sensitivity to light Blurred vision Dark floating spots in the vision Vision loss Clinical Symptoms Additional Statistics ~15% of all cases of legal blindness and visual handicap in the US and EU ~25% of all cases of blindness globally ~$55k annual tx cost of adalimumab (2nd line behind steroids) 6.9% CAGR 2020-2027 20-50 years old most common age affected in the United States Uveitis is a group of eye disorders affecting the uvea and characterized by intraocular inflammation that is often chronic, can flare up at any time, and can lead to visual impairment and vision loss. Significant unmet need for a steroid sparing approach 1.2 million patients in US + EU5

35 Day 28 Cohort 1: 0.3 µg N=4 Day -14 to 0 Screening Cohort 3: 1.2 µg N=4 Day 1 Day 7 Chronic Posterior Uveitis (N=12) Day 14 Day 21 Cohort 2: 0.6 µg N=4 KIO-104-1101: Phase 1 Study Design Study Design Endpoints Key Results Prospective, multi-center, open-label, dose ranging, single IVT injection in worse eye Visual acuity improved in all patients (in all dose groups) Anterior chamber inflammation and vitreous haze decreased Evidence of reduced macular edema No SAEs, excellent tolerability, no peripheral blood detection (at any timepoint) Primary – Ocular & systemic safety, pK, safety labs Secondary – Visual acuity, visual field, anterior chamber inflammation, vitreous haze

36 KIO-104 Improves VA and CME After Single IVT Dose * Historical Controls (Yeh et al, Retina 00, 1-9,2018; Suhler et al. Visual III, Ophthalmology 125, 7, 2018.) IVT - Intravitreal 0 2 4 6 8 10 12 14 16 0.3 μg 0.6 μg 1.2 μg Steroids* Humira*M ea n C ha ng e in L et te rs fr om B as el in e at D 28 N=4 N=4 N=4 Day 28 (421µm) Baseline (558µm) Visual Acuity Cystoid Macular Edema‡ ‡ 40% of eyes with vision threatening cystoid macular edema at baseline had clinically meaningful improvement

37 KIO-104 Path Forward Posterior Non-Infectious Uveitis ~$ 47 M • Ph2b Clinical Trial (EU): Q4 2024 – Q1 2026 • Non-Clinical, IND Enabling Studies: Q1 2025 – Q1 2026 • Ph3 Registration Study(s) in USA & EU: Q3 2026 – Q1 2028 • NDA: Late 2028 Proliferative Vitreoretinopathy and/or other retinal inflammatory conditions~$ 8M • PoC Non-Clinical Testing: Q2 2024 – Q1 2025 • Non-Clinical Dose Range Finding: Q4 2024 – Q2 2025 • Ph2 Clinical Trial (EU): Q3 2025 – Q3 2026

38 CORPORATE OVERVIEW
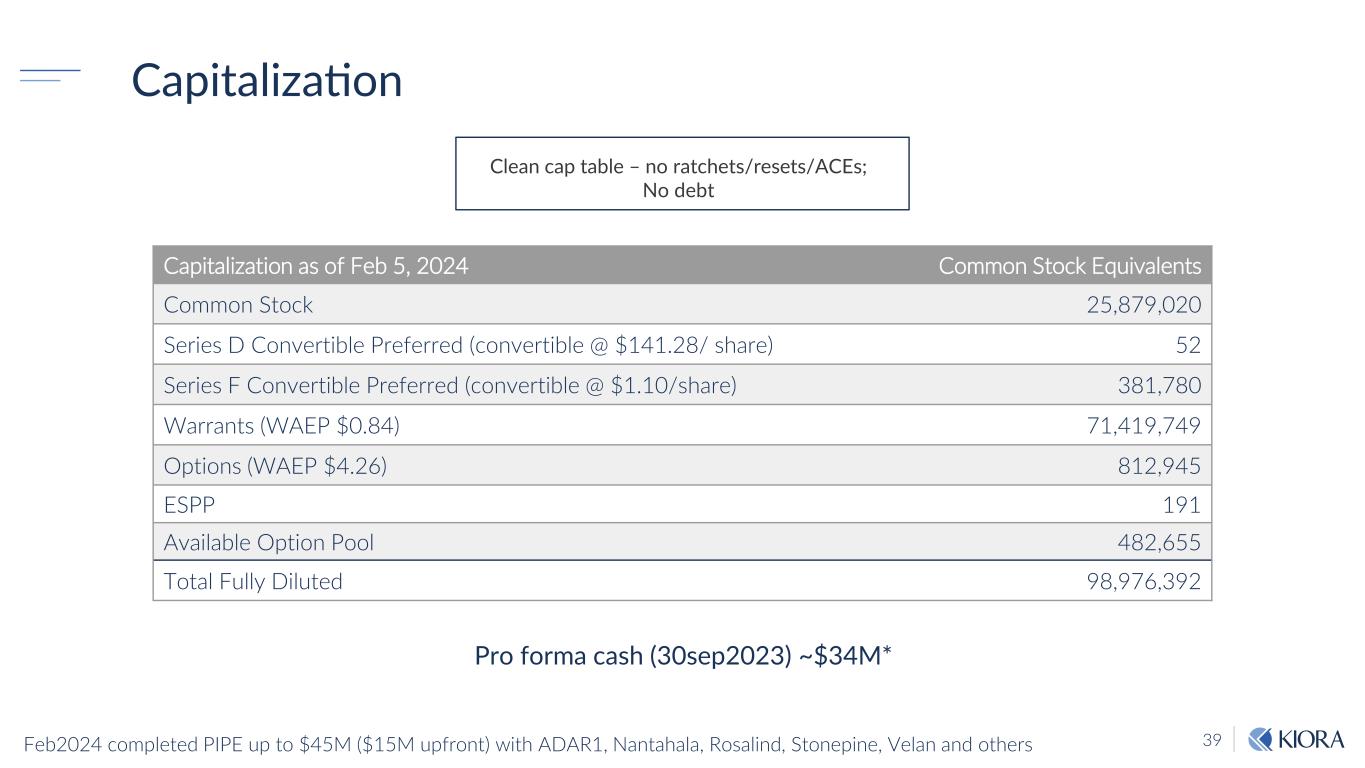
39 Capitalization Capitalization as of Feb 5, 2024 Common Stock Equivalents Common Stock 25,879,020 Series D Convertible Preferred (convertible @ $141.28/ share) 52 Series F Convertible Preferred (convertible @ $1.10/share) 381,780 Warrants (WAEP $0.84) 71,419,749 Options (WAEP $4.26) 812,945 ESPP 191 Available Option Pool 482,655 Total Fully Diluted 98,976,392 Clean cap table – no ratchets/resets/ACEs; No debt Pro forma cash (30sep2023) ~$34M* Feb2024 completed PIPE up to $45M ($15M upfront) with ADAR1, Nantahala, Rosalind, Stonepine, Velan and others

40 Brian M. Strem, PhD President & CEO Eric J. Daniels, MD Chief Development Officer Melissa Tosca, CPA EVP – Finance Stefan Sperl, PhD EVP – CMC & Operations Leadership Team

41 Board of Directors Brian M Strem, PhD President & CEO Aron Shapiro Ken Gayron Praveen Tyle, PhD Chairman David Hollander, MD, MBA Erin Parsons Carmine Stengone

42 Scientific Advisory Board Russel Van Gelder, MD, PhD Charlie Wykoff, MD, PhD Allen Ho, MD, PhD Christine Kay, MD, PhD Vitreo Retinal ASSOCIATES Mark Pennesi, MD, PhD

Contact: info@kiorapharma.com