0001832168false00018321682024-08-012024-08-01
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 1, 2024
Longboard Pharmaceuticals, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
Delaware |
1-40192 |
84-5009619 |
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
4275 Executive Square, Suite 950 La Jolla, CA |
|
92037 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (858) 789-9283
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common stock, par value $0.0001 per share |
|
LBPH |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On August 1, 2024, Longboard Pharmaceuticals, Inc. ("Longboard") issued a press release announcing its financial results for the quarter ended June 30, 2024. A copy of the press release is attached hereto as Exhibit 99.1.
The information contained under this Item 2.02, including Exhibit 99.1 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or under the Securities Exchange Act of 1934, as amended, regardless of any general incorporation language in any such filing, unless Longboard expressly sets forth in such filing that such information is to be considered “filed” or incorporated by reference therein.
Item 7.01 Regulation FD Disclosure.
Included as Exhibit 99.2 to this Form 8-K is a corporate presentation dated August 1, 2024, that is incorporated herein by reference. We intend to utilize this presentation and its contents in various meetings with securities analysts, investors and others.
The information in this Item 7.01, including Exhibit 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended, nor shall it be incorporated by reference into any filing we make with the U.S. Securities and Exchange Commission, whether before or after the date hereof, regardless of any general incorporation language in such filing.
Item 8.01 Other Events.
On August 1, 2024, we announced LP659 Phase 1 single ascending dose (SAD) topline data from a study of 32 healthy volunteers.
LP659 was generally safe and well tolerated in this SAD study.
•Adverse events were mild
•No treatment emergent adverse events (TEAEs) leading to discontinuation
•No serious adverse events (SAEs) observed
•Impact on heart rate was low throughout the study with no first dose bradycardia
•No abnormal electrocardiograms, no atrioventricular (AV) block, and no abnormal echocardiograms
•No abnormal pulmonary/spirometry and ophthalmologic assessments
LP659 demonstrated a rapid reduction of lymphocytes in a dose- and formulation-dependent manner in this SAD study.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
Longboard Pharmaceuticals, Inc. |
|
|
|
|
Date: August 1, 2024 |
|
By: |
/s/ Kevin R. Lind |
|
|
|
Kevin R. Lind |
|
|
|
President and Chief Executive Officer |
Exhibit 99.1

Longboard Pharmaceuticals Reports Second Quarter 2024 Financial Results and Provides Corporate Updates
•Bexicaserin (LP352) received Breakthrough Therapy designation (BTD) for the treatment of seizures associated with Developmental and Epileptic Encephalopathies (DEEs) for patients two years of age or older
•Bexicaserin demonstrated a sustained, durable response in seizure reduction and a favorable safety and tolerability profile across a broad range of DEEs in an interim analysis of the Open-Label Extension (OLE) of the Phase 1b/2a PACIFIC Study
•Completed End of Phase 2 Meeting to discuss Longboard’s global Phase 3 program for bexicaserin, which is on track to initiate in H2 2024
•Longboard plans to host an Investor & Analyst Event on September 16 to highlight details of the global Phase 3 plan for bexicaserin
•LP659 demonstrated a rapid reduction of lymphocytes in a dose- and formulation-dependent manner and was generally safe and well tolerated in a first-in-human Phase 1 single-ascending dose (SAD) study
•Ended second quarter 2024 with $304.9 million in cash, cash equivalents and investments; cash runway is expected to support current planned operations into 2027
•Longboard to host conference call and webcast today at 4:30 PM ET
LA JOLLA, Calif., August 1, 2024 – Longboard Pharmaceuticals, Inc. (Nasdaq: LBPH), a clinical-stage biopharmaceutical company focused on developing novel, transformative medicines for neurological diseases, today provided a corporate update and reported second quarter 2024 financial results.
“The first half of the year has been monumental for Longboard and underpins the vision that we have been working towards since the inception of the Company, which is to help a broad range of patients living with devastating neurological conditions. With bexicaserin receiving Breakthrough Therapy designation for seizures associated with DEEs, it highlights the significant unmet need that still exists for so many patients. I want to thank the entire DEE community for this important milestone because this was the culmination of years of hard work and determination from families, physicians and advocates,” stated Kevin R. Lind, Longboard’s President and Chief Executive Officer. “We are proud to be the first company to generate a compelling clinical data set to evaluate DEE as a potential
indication, and I want to thank the FDA for their collaboration along the way. We look forward to sharing trial design and details of our highly anticipated global Phase 3 program at an R&D Day in September.”
Dr. Randall Kaye, Longboard’s Chief Medical Officer stated, “We are pleased to share topline SAD results today for LP659, our centrally acting, highly selective S1P receptor modulator. In this study, LP659 was generally safe and well tolerated and demonstrated a dose- and formulation-dependent lymphocyte reduction in healthy volunteers. We plan to provide additional updates this year related to our planned multiple-ascending dose trial following regulatory discussions. The depth of scientific data generated to date supports multiple potential attractive orphan CNS commercial opportunities.”
RECENT UPDATES AND UPCOMING MILESTONES:
Bexicaserin (LP352), an oral, centrally acting, 5-HT2C superagonist in development for the potential treatment of seizures associated with DEEs
•Longboard plans to host an Investor & Analyst Event on September 16 in New York, NY, to highlight details of the global Phase 3 plan for bexicaserin
•PACIFIC OLE interim analysis safety and tolerability data accepted as a late-breaker at the International League Against Epilepsy (ILAE) European Epilepsy Congress (ECC) 2024 in September
•U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy designation for bexicaserin for the treatment of seizures associated with DEEs for patients two years of age or older
•End of Phase 2 Meeting with U.S. Food and Drug Administration (FDA) has been completed, and alignment with other regulatory agencies is ongoing
•PACIFIC open-label extension (OLE) interim analysis results demonstrated a sustained, durable response in seizure reduction and a favorable safety and tolerability profile across a broad range of DEEs
•Phase 3 initiation expected in H2 2024
LP659, an oral, centrally acting, S1P receptor subtypes 1 and 5 (S1P1,5) modulator in development for the potential treatment of rare neuroinflammatory conditions
•Phase 1 SAD topline data
oLP659 was generally safe and well tolerated
▪Adverse events were mild
▪No treatment emergent adverse events (TEAEs) leading to discontinuation
▪No serious adverse events (SAEs) observed
▪Impact on heart rate was low throughout the study with no first dose bradycardia
▪No abnormal electrocardiograms, no atrioventricular (AV) block, or abnormal echocardiograms
▪No abnormal pulmonary/spirometry and ophthalmologic assessments
oLP659 demonstrated a rapid reduction of lymphocytes in a dose- and formulation-dependent manner
SECOND QUARTER 2024 FINANCIAL RESULTS:
Balance Sheet Highlights
At June 30, 2024, Longboard’s cash, cash equivalents and short-term investments were approximately $304.9 million.
Operating Results
Research and development expenses were $20.4 million for the three months ended June 30, 2024, an increase of $7.9 million, or 63%, compared to $12.5 million for the three months ended June 30, 2023. The net increase of $7.9 million is primarily related to increases of $3.8 million in clinical trial and preclinical expenses related to bexicaserin, $2.7 million in personnel-related expenses, $0.7 million in clinical trial and preclinical expenses related to LP659 and $0.7 million in other miscellaneous research and development related expenses.
General and administrative expenses were $5.2 million for the three months ended June 30, 2024, an increase of $2.1 million, or 67%, compared to $3.1 million for the three months ended June 30, 2023. The net increase of $2.1 million is primarily related to increases of $1.6 million in personnel-related expenses, $0.3 million in consulting and professional fees and $0.2 million of miscellaneous expenses.
Conference Call and Webcast Details
Longboard will host a conference call today at 4:30pm ET. Stockholders and other interested parties may participate in the call by following the instructions below. The live webcast can be accessed on the Events & Presentations portion of the investor page of Longboard’s website at https://ir.longboardpharma.com. A replay will be available on Longboard’s website shortly after completion of the event and will be archived for at least 30 days.
Participant Webcast Link: https://edge.media-server.com/mmc/p/qv83ogjy
Participant Dial-In:
USA & Canada - Toll-Free (800) 715-9871
United States - Toll (646) 307-1963
Conference ID: 3710661
ABOUT LONGBOARD PHARMACEUTICALS
Longboard Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company focused on developing novel, transformative medicines for neurological diseases. Longboard is working to advance a portfolio of centrally acting product candidates designed to be highly selective for specific G protein-coupled receptors (GPCRs). Longboard’s small molecule product candidates are based on more than 20 years of GPCR research. Longboard plans to advance bexicaserin (LP352), an oral, centrally acting 5-hydroxytryptamine 2C (5-HT2C) receptor superagonist, with no observed impact on 5-HT2B and 5-HT2A receptor subtypes, into a global Phase 3 program. The FDA has granted Breakthrough Therapy designation for bexicaserin for the treatment of seizures associated with Developmental and Epileptic Encephalopathies (DEEs) for patients two years of age and older. Earlier this year, Longboard reported
positive topline data from a Phase 1b/2a clinical trial (the PACIFIC Study) evaluating bexicaserin in participants with DEEs. Longboard is also evaluating LP659, an oral, centrally acting, sphingosine-1-phosphate (S1P) receptor subtypes 1 and 5 modulator, which is in development for the potential treatment of rare neuroinflammatory conditions. Longboard recently completed a Phase 1 single-ascending dose (SAD) clinical trial for LP659 in healthy volunteers.
Bexicaserin and LP659 are investigational compounds that are not approved for marketing by the FDA or any other regulatory authority.
FORWARD-LOOKING STATEMENTS
Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. In some cases, you can identify forward-looking statements by words such as “on track to”, “before”, “plan”, “expect”, “focused on”, “vision”, “potential”, “look forward”, “opportunity”, “upcoming”, “ongoing”, “working to”, “designed to”, the negative, plural or other tenses of these words, references to future dates or time periods, or other comparable language, and they may include, without limitation, statements about the following: Longboard’s clinical and preclinical product candidates, including plans, study design, and timing of study initiation of the planned global Phase 3 program for bexicaserin, the PACIFIC OLE study for bexicaserin and the complete data from such study, the Phase 1 SAD study for LP659 and plans for further development of LP659, their potential (including to be transformative, the number and type of conditions they may address, their ability to address an unmet need, and their commercial opportunity), and their design and characteristics; Longboard’s planned conference call and webcast, Investor & Analyst Event, and PACIFIC OLE interim analysis data presentation; Longboard’s cash position, expenses and runway to support operations; and Longboard’s focus and work. For such statements, Longboard claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Longboard’s expectations. Factors that could cause actual results to differ materially from those stated or implied by Longboard’s forward-looking statements include, but are not limited to, the following: risks related to Longboard’s limited operating history, financial position and need for additional capital; Longboard will need additional managerial and financial resources to advance all of its programs, and you and others may not agree with the manner Longboard allocates its resources; the standard for Breakthrough Therapy designation is not the same as the standard for drug approval, the clinical evidence supporting Breakthrough Therapy designation is preliminary, and not all drugs designated as Breakthrough Therapies ultimately will be shown to have substantial improvement over available therapies; the FDA may later decide to rescind a Breakthrough Therapy designation if it determines the designation is no longer supported by subsequent data; Longboard’s product candidates are in the early phases of a lengthy research and development process, the timing, manner and outcome of research, development and regulatory review is uncertain, and Longboard’s product candidates, including bexicaserin and LP659, may not advance in research or development or be approved for marketing; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; topline or interim data may not accurately reflect the complete results of a particular study or trial and remain subject to audit, and final data may differ materially from topline or interim data; risks related to the development and commercialization of Longboard’s product candidates; enrolling participants in clinical trials is competitive and challenging; risks related to unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Longboard or others, request
additional information, have additional recommendations or change their guidance or requirements before or after approval; risks related to relying on licenses or collaborative arrangements; other risks related to Longboard’s dependence on third parties; competition; product liability or other litigation or disagreements with others; government and third-party payor actions, including relating to reimbursement and pricing; risks related to regulatory compliance; and risks related to Longboard’s and third parties’ intellectual property rights. Additional factors that could cause actual results to differ materially from those stated or implied by Longboard’s forward-looking statements are disclosed in Longboard’s filings with the Securities and Exchange Commission (SEC). These forward-looking statements represent Longboard’s judgment as of the time of this release. Longboard disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
CORPORATE CONTACT:
Megan E. Knight
VP, Head of Investor Relations
IR@longboardpharma.com
858.789.9283
###
Financial Tables Follow
LONGBOARD PHARMACEUTICALS, INC.
CONDENSED BALANCE SHEETS
(unaudited)
|
|
|
|
|
|
|
|
|
|
|
June 30, |
|
|
December 31, |
|
(in thousands, except share and per share data) |
|
2024 |
|
|
2023 |
|
ASSETS |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
14,599 |
|
|
$ |
14,331 |
|
Short-term investments |
|
|
290,274 |
|
|
|
34,167 |
|
Prepaid expenses and other current assets |
|
|
3,932 |
|
|
|
1,723 |
|
Total current assets |
|
|
308,805 |
|
|
|
50,221 |
|
Right-of-use assets |
|
|
3,855 |
|
|
|
472 |
|
Property and equipment |
|
|
1 |
|
|
|
4 |
|
Other long-term assets |
|
|
244 |
|
|
|
— |
|
Total assets |
|
$ |
312,905 |
|
|
$ |
50,697 |
|
LIABILITIES AND EQUITY |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
2,475 |
|
|
$ |
1,001 |
|
Accrued research and development expenses |
|
|
9,347 |
|
|
|
4,556 |
|
Accrued compensation and related expenses |
|
|
2,070 |
|
|
|
3,374 |
|
Accrued other expenses |
|
|
551 |
|
|
|
368 |
|
Right-of-use liabilities, current portion |
|
|
294 |
|
|
|
475 |
|
Total current liabilities |
|
|
14,737 |
|
|
|
9,774 |
|
Right-of-use liabilities, net of current portion |
|
|
3,568 |
|
|
|
— |
|
Commitments and contingencies |
|
|
|
|
|
|
Stockholders' equity: |
|
|
|
|
|
|
Preferred stock, $0.0001 par value; authorized shares - 10,000,000 at June 30, 2024 and December 31, 2023; issued and outstanding shares - none at June 30, 2024 and December 31, 2023 |
|
|
— |
|
|
|
— |
|
Voting common stock, $0.0001 par value; authorized shares - 300,000,000 at June 30, 2024 and December 31, 2023; issued and outstanding shares - 33,613,299 and 22,096,494 at June 30, 2024 and December 31, 2023, respectively |
|
|
3 |
|
|
|
2 |
|
Non-voting common stock, $0.0001 par value; authorized shares - 10,000,000 at June 30, 2024 and December 31, 2023; issued and outstanding shares - 5,270,755 and 2,420,755 at June 30, 2024 and December 31, 2023, respectively |
|
|
— |
|
|
|
— |
|
Additional paid-in capital |
|
|
472,407 |
|
|
|
181,563 |
|
Accumulated other comprehensive loss |
|
|
(475 |
) |
|
|
(78 |
) |
Accumulated deficit |
|
|
(177,335 |
) |
|
|
(140,564 |
) |
Total stockholders' equity |
|
|
294,600 |
|
|
|
40,923 |
|
Total liabilities and stockholders' equity |
|
$ |
312,905 |
|
|
$ |
50,697 |
|
LONGBOARD PHARMACEUTICALS, INC.
CONDENSED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
(in thousands, except share and per share data) |
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
Research and development |
$ |
20,445 |
|
|
$ |
12,536 |
|
|
$ |
33,615 |
|
|
$ |
21,066 |
|
General and administrative |
|
5,198 |
|
|
|
3,106 |
|
|
|
10,138 |
|
|
|
6,538 |
|
Total operating expenses |
|
25,643 |
|
|
|
15,642 |
|
|
|
43,753 |
|
|
|
27,604 |
|
Loss from operations |
|
(25,643 |
) |
|
|
(15,642 |
) |
|
|
(43,753 |
) |
|
|
(27,604 |
) |
Interest income, net |
|
3,905 |
|
|
|
660 |
|
|
|
7,038 |
|
|
|
1,176 |
|
Other expense |
|
(47 |
) |
|
|
(17 |
) |
|
|
(56 |
) |
|
|
(27 |
) |
Net loss |
$ |
(21,785 |
) |
|
$ |
(14,999 |
) |
|
$ |
(36,771 |
) |
|
$ |
(26,455 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share, basic and diluted |
$ |
(0.56 |
) |
|
$ |
(0.65 |
) |
|
$ |
(0.99 |
) |
|
$ |
(1.22 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares outstanding, basic and diluted |
|
38,880,336 |
|
|
|
22,968,920 |
|
|
|
37,101,065 |
|
|
|
21,696,427 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
Net loss |
$ |
(21,785 |
) |
|
$ |
(14,999 |
) |
|
$ |
(36,771 |
) |
|
$ |
(26,455 |
) |
Unrealized gain (loss) on short-term investments |
|
(109 |
) |
|
|
131 |
|
|
|
(397 |
) |
|
|
402 |
|
Comprehensive loss |
$ |
(21,894 |
) |
|
$ |
(14,868 |
) |
|
$ |
(37,168 |
) |
|
$ |
(26,053 |
) |
|
|
|
|
|
|
|
|
|
|
|
|

Corporate Presentation August 1, 2024 Exhibit 99.2

Forward-Looking Statements and Other Legal Notices This presentation contains forward-looking statements about Longboard Pharmaceuticals, Inc. (“we,” “Longboard” or the “Company”), including statements regarding: our vision; commercial opportunities and analogs; the potential of bexicaserin (LP352) and LP659, including to be best-in-class, to treat indications, to have differentiated or next-generation design, selectivity, specificity and other characteristics, and to meet an unmet need; anticipated milestones and timing; the prevalence of, unmet need associated with, and market opportunity for, DEEs; the bexicaserin phase 3 global program design, initiation timing and DEE path forward; Breakthrough Therapy designation for bexicaserin; LP659’s broad applicability, predictive data, commercial opportunities, next generation and selectivity characteristics, mechanism of action and preclinical data, potential to limit off-target effects, potential neurodegenerative disease therapeutic areas, indications and opportunities, topline SAD data and Phase 1 MAD initiation timing; our intellectual property; our ability to obtain regulatory approval and commercialize our drug candidates (in the manner we may propose or at all); and other statements that are not historical facts, including statements that may include words such as “will”, “may”, “can”, “would”, “plan”, “anticipate”, “expect”, “believe”, “potential”, “goal”, “opportunity” and similar words. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, but not limited to: topline or interim data may not reflect the complete or final results of a particular study or trial, and are subject to change; nonclinical and clinical data are voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than us or others, request additional information, have additional recommendations or change their guidance or requirements before or after approval; we have a limited operating history, a history of incurring net losses and expectation that we will continue to incur net losses for the foreseeable future, and we may never be profitable; we will need additional capital to finance our operations; clinical and preclinical drug development involves lengthy and expensive processes with uncertain timing and outcomes; we have multiple product candidates with a variety of target indications, and we may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on a product candidate or indication that may be more profitable; the regulatory approval process in the U.S. and in other territories is lengthy, time consuming and inherently unpredictable, and we may not be able to obtain or maintain regulatory approval to conduct our clinical trials (in the manner we propose or at all) or, ultimately, to market our product candidates; receiving Breakthrough Therapy designation ma not lead to a faster development or regulator review or approval and does not mean bexicaserin will receive marketing approval for seizures associated with DEEs or for any other indication; risks relating to our ability to commercialize our product candidates and compete in the marketplace; risks regarding our license and dependencies on others; risks relating to our ability to obtain and maintain intellectual property protection and freedom to operate for our product candidates; risks relating to our ability to manage our growth; and other risks and factors disclosed in our filings with the U.S. Securities and Exchange Commission (the “SEC”). We operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. The forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Except as required by law, we assume no responsibility for the accuracy and completeness of the forward-looking statements, and we undertake no obligation to update any forward-looking statements after the date of this presentation to conform these statements to actual results or to changes in our expectations. Certain information contained in or that may orally accompany this presentation relate to or are based on studies, research, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, research, publications, surveys and other data to be reliable as of the date of this presentation, they have not been independently verified, and we make no representations as to the adequacy, fairness, accuracy or completeness of any information obtained from third-party sources. This presentation discusses product candidates, bexicaserin and LP659, that have not yet been approved for marketing by the U.S. Food and Drug Administration (the “FDA”) or any other regulatory authority.

Our Vision is �Backed by 20+ Years of World Class �GPCR Research Longboard Thesis Vision A world where devastating neurological conditions are no longer devastating 3 Longboard Pharmaceuticals Differentiated & innovative clinical approaches Relevant M&A analogs JAZZ - GW $7.2B PFE - ARNA $6.7B UCB - ZGNX $1.9B Bold & experienced leadership with expertise in CNS and rare disorders Pipeline with differentiated PK / PD and target engagement CNS programs with significant commercial opportunities Well-understood targets
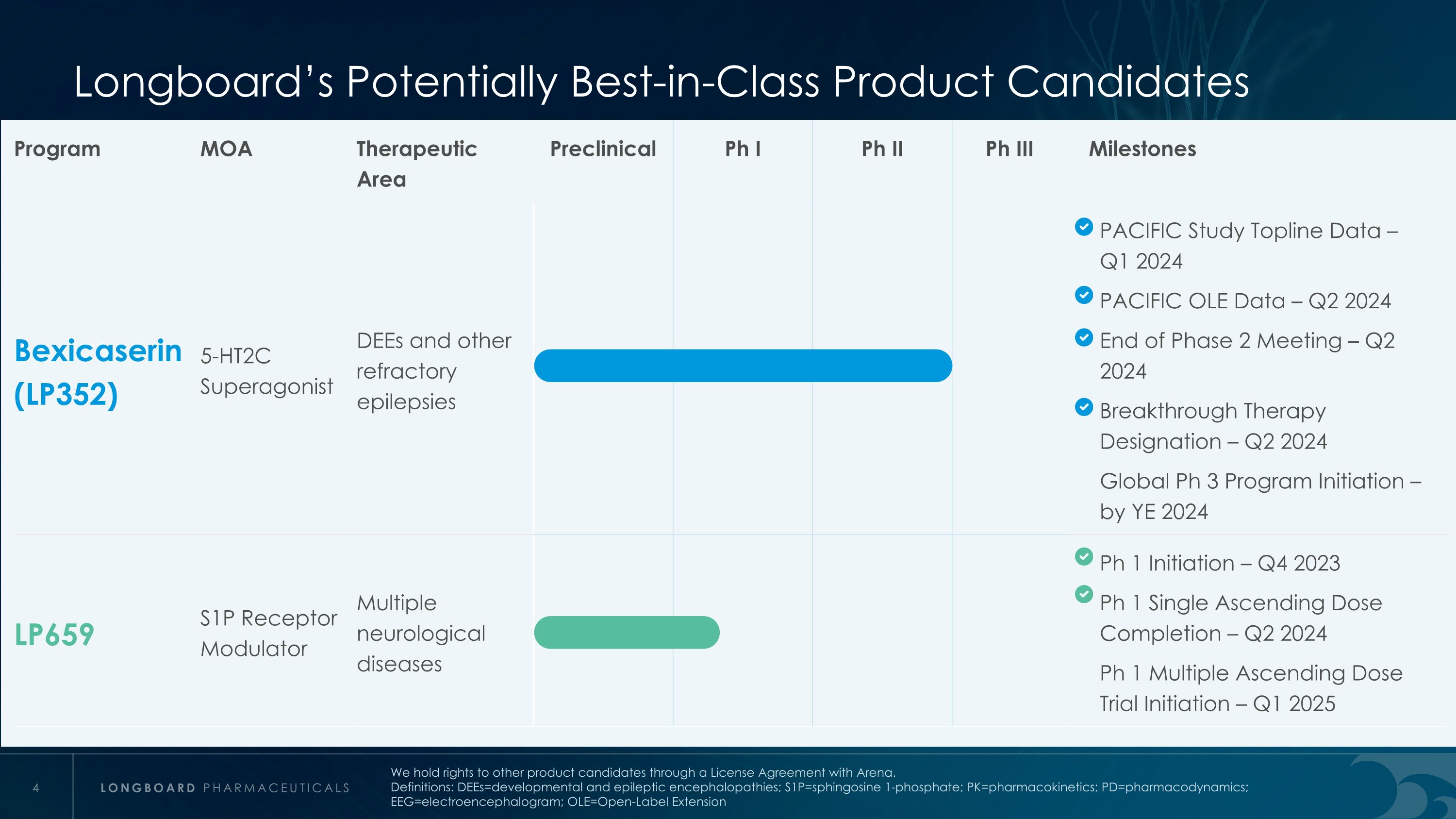
Longboard’s Potentially Best-in-Class Product Candidates Program MOA Therapeutic Area Preclinical Ph I Ph II Ph III Milestones Bexicaserin (LP352) 5-HT2C Superagonist DEEs and other refractory epilepsies PACIFIC Study Topline Data – Q1 2024 PACIFIC OLE Data – Q2 2024 End of Phase 2 Meeting – Q2 2024 Breakthrough Therapy Designation – Q2 2024 Global Ph 3 Program Initiation – by YE 2024 LP659 S1P Receptor Modulator Multiple neurological diseases Ph 1 Initiation – Q4 2023 Ph 1 Single Ascending Dose Completion – Q2 2024 Ph 1 Multiple Ascending Dose Trial Initiation – Q1 2025 We hold rights to other product candidates through a License Agreement with Arena. Definitions: DEEs=developmental and epileptic encephalopathies; S1P=sphingosine 1-phosphate; PK=pharmacokinetics; PD=pharmacodynamics; EEG=electroencephalogram; OLE=Open-Label Extension

Developmental & Epileptic Encephalopathies (DEE) Landscape
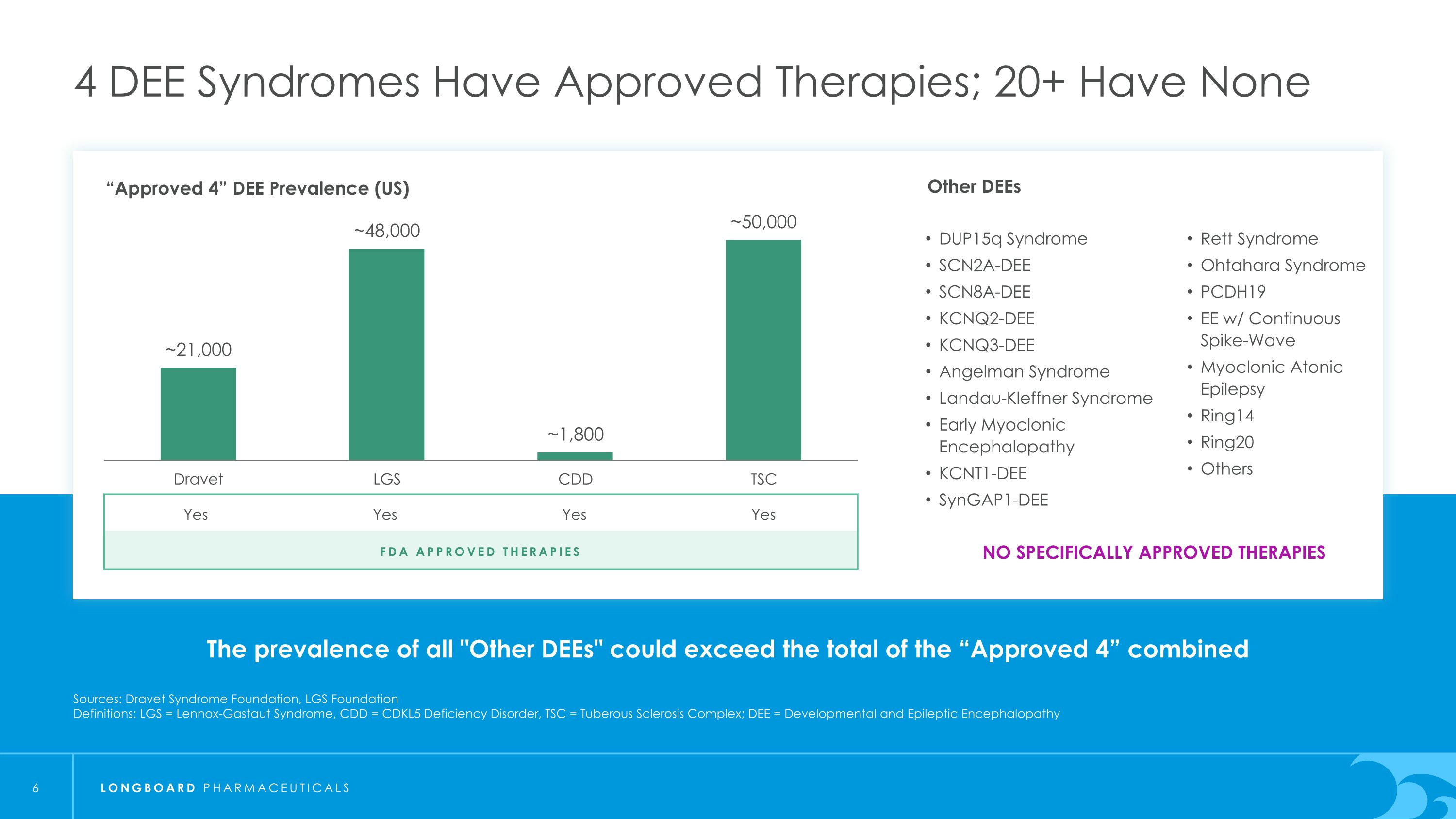
The prevalence of all "Other DEEs" could exceed the total of the “Approved 4” combined 4 DEE Syndromes Have Approved Therapies; 20+ Have None Sources: Dravet Syndrome Foundation, LGS Foundation Definitions: LGS = Lennox-Gastaut Syndrome, CDD = CDKL5 Deficiency Disorder, TSC = Tuberous Sclerosis Complex; DEE = Developmental and Epileptic Encephalopathy “Approved 4” DEE Prevalence (US) FDA Approved Therapies Yes Yes Yes Yes NO SPECIFICALLY APPROVED THERAPIES Other DEEs DUP15q Syndrome SCN2A-DEE SCN8A-DEE KCNQ2-DEE KCNQ3-DEE Angelman Syndrome Landau-Kleffner Syndrome Early Myoclonic Encephalopathy KCNT1-DEE SynGAP1-DEE Rett Syndrome Ohtahara Syndrome PCDH19 EE w/ Continuous Spike-Wave Myoclonic Atonic Epilepsy Ring14 Ring20 Others
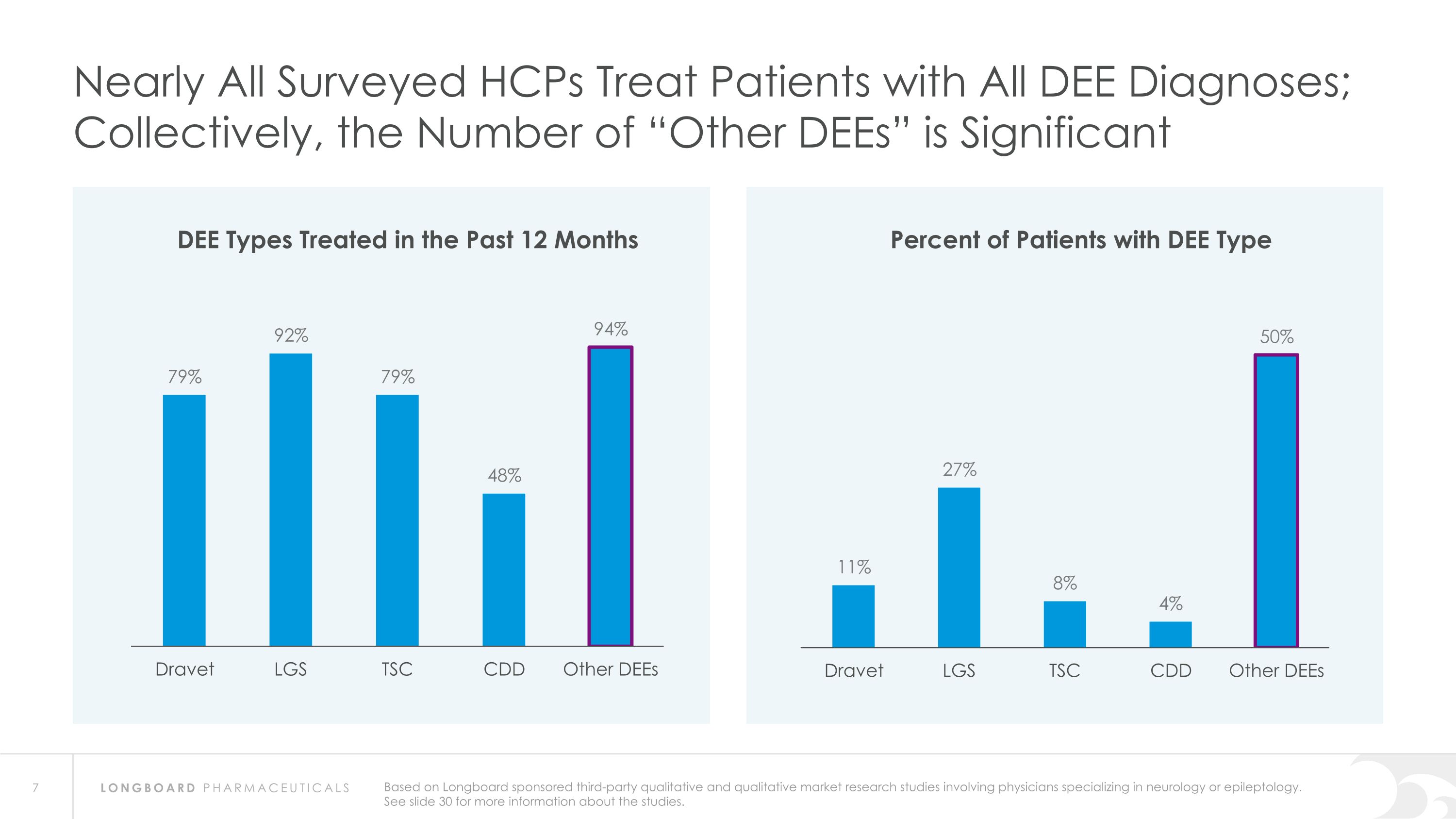
Nearly All Surveyed HCPs Treat Patients with All DEE Diagnoses; Collectively, the Number of “Other DEEs” is Significant DEE Types Treated in the Past 12 Months Percent of Patients with DEE Type Based on Longboard sponsored third-party qualitative and qualitative market research studies involving physicians specializing in neurology or epileptology. See slide 30 for more information about the studies.

Bexicaserin (LP352) Potential Best-in-Class 5-HT2C Superagonist - Entering a Ph 3 Program with the Goal of Treating a Broad Range of DEEs
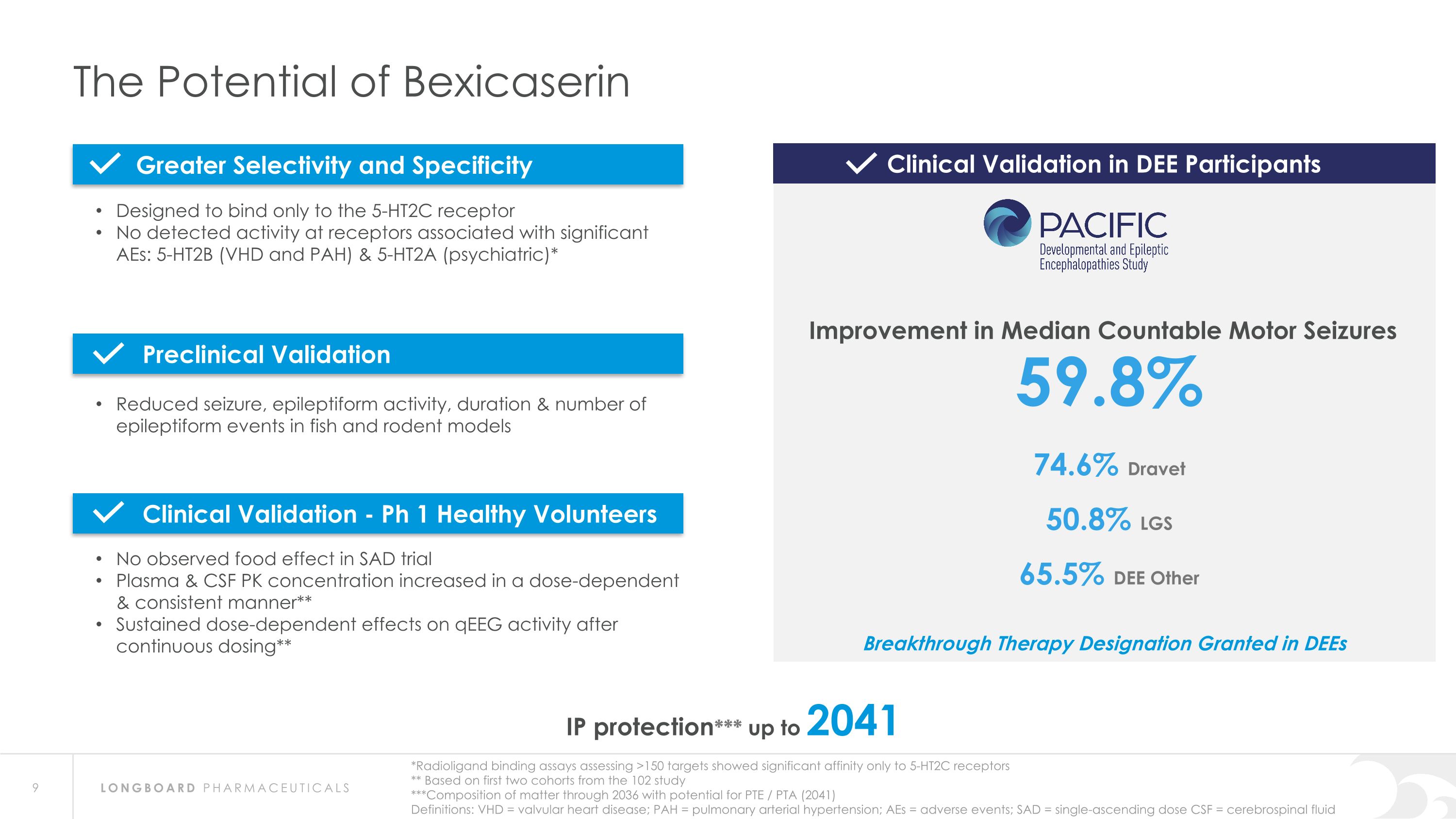
The Potential of Bexicaserin IP protection*** up to 2041 *Radioligand binding assays assessing >150 targets showed significant affinity only to 5-HT2C receptors ** Based on first two cohorts from the 102 study ***Composition of matter through 2036 with potential for PTE / PTA (2041) Definitions: VHD = valvular heart disease; PAH = pulmonary arterial hypertension; AEs = adverse events; SAD = single-ascending dose CSF = cerebrospinal fluid 59.8% 74.6% Dravet 50.8% LGS 65.5% DEE Other Designed to bind only to the 5-HT2C receptor No detected activity at receptors associated with significant AEs: 5-HT2B (VHD and PAH) & 5-HT2A (psychiatric)* Reduced seizure, epileptiform activity, duration & number of epileptiform events in fish and rodent models No observed food effect in SAD trial Plasma & CSF PK concentration increased in a dose-dependent & consistent manner** Sustained dose-dependent effects on qEEG activity after continuous dosing** Clinical Validation in DEE Participants Improvement in Median Countable Motor Seizures Greater Selectivity and Specificity Preclinical Validation Clinical Validation - Ph 1 Healthy Volunteers Breakthrough Therapy Designation Granted in DEEs
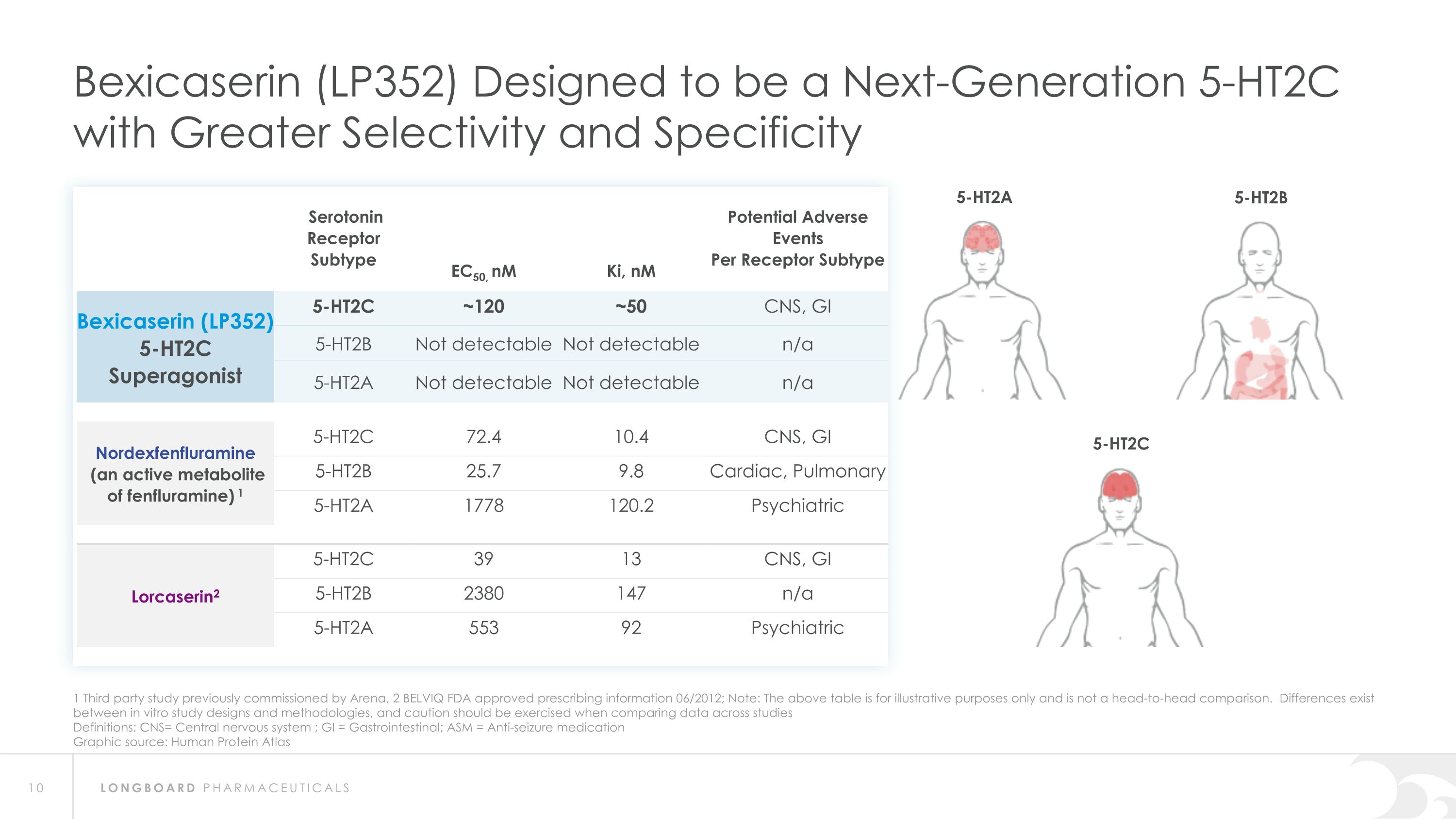
1 Third party study previously commissioned by Arena, 2 BELVIQ FDA approved prescribing information 06/2012; Note: The above table is for illustrative purposes only and is not a head-to-head comparison. Differences exist between in vitro study designs and methodologies, and caution should be exercised when comparing data across studies Definitions: CNS= Central nervous system ; GI = Gastrointestinal; ASM = Anti-seizure medication Graphic source: Human Protein Atlas Bexicaserin (LP352) Designed to be a Next-Generation 5-HT2C with Greater Selectivity and Specificity Serotonin �Receptor Subtype EC50, nM Ki, nM Potential Adverse Events Per Receptor Subtype Bexicaserin (LP352) 5-HT2C Superagonist 5-HT2C ~120 ~50 CNS, GI 5-HT2B Not detectable Not detectable n/a 5-HT2A Not detectable Not detectable n/a Nordexfenfluramine (an active metabolite �of fenfluramine) 1 5-HT2C 72.4 10.4 CNS, GI 5-HT2B 25.7 9.8 Cardiac, Pulmonary 5-HT2A 1778 120.2 Psychiatric Lorcaserin2 5-HT2C 39 13 CNS, GI 5-HT2B 2380 147 n/a 5-HT2A 553 92 Psychiatric 5-HT2A 5-HT2B 5-HT2C
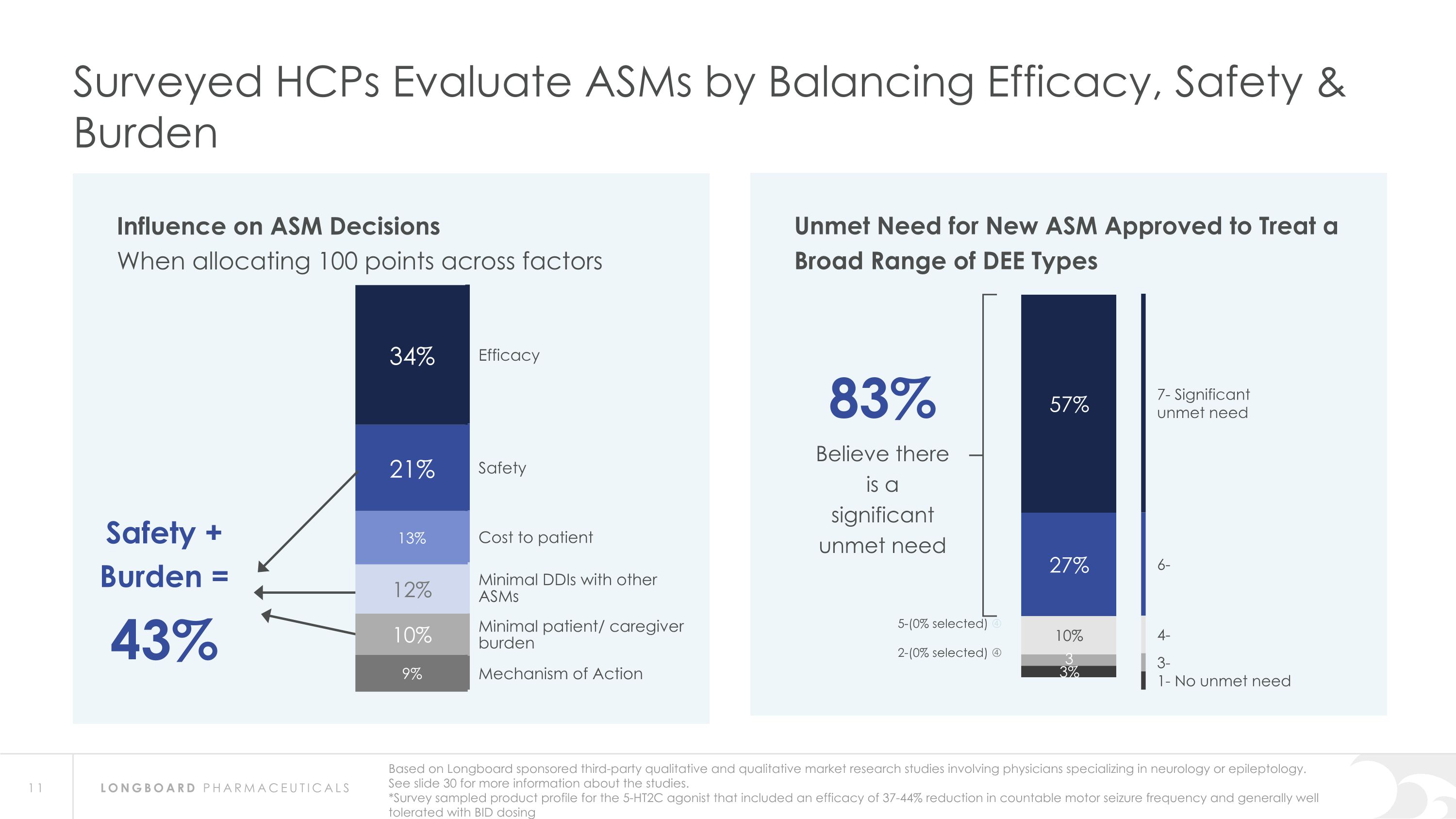
Influence on ASM Decisions When allocating 100 points across factors Surveyed HCPs Evaluate ASMs by Balancing Efficacy, Safety & Burden Efficacy Safety Cost to patient Minimal DDIs with other ASMs Minimal patient/ caregiver burden Mechanism of Action Safety + Burden = 43% Based on Longboard sponsored third-party qualitative and qualitative market research studies involving physicians specializing in neurology or epileptology. See slide 30 for more information about the studies. *Survey sampled product profile for the 5-HT2C agonist that included an efficacy of 37-44% reduction in countable motor seizure frequency and generally well tolerated with BID dosing Unmet Need for New ASM Approved to Treat a Broad Range of DEE Types 83% Believe there is a significant unmet need 7- Significant �unmet need 6- 4- 3- 1- No unmet need 5-(0% selected) 2-(0% selected)
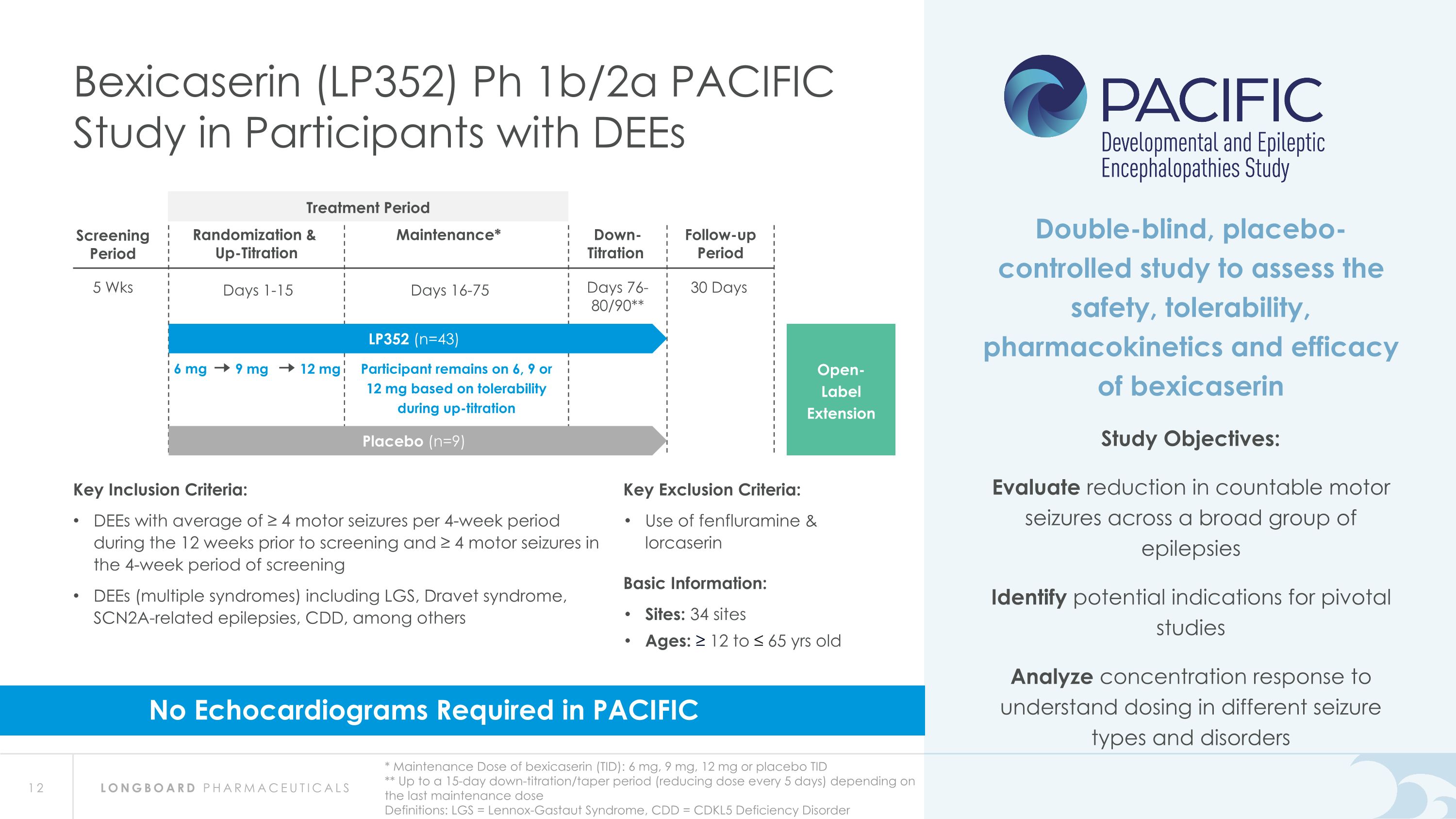
6 mg 9 mg 12 mg Participant remains on 6, 9 or 12 mg based on tolerability during up-titration 5 Wks Screening Period 30 Days Follow-up Period Randomization & Up-Titration Days 1-15 Maintenance* Days 16-75 Down-�Titration Days 76-�80/90** Bexicaserin (LP352) Ph 1b/2a PACIFIC Study in Participants with DEEs * Maintenance Dose of bexicaserin (TID): 6 mg, 9 mg, 12 mg or placebo TID �** Up to a 15-day down-titration/taper period (reducing dose every 5 days) depending on the last maintenance dose Definitions: LGS = Lennox-Gastaut Syndrome, CDD = CDKL5 Deficiency Disorder Open-�Label Extension Double-blind, placebo-controlled study to assess the safety, tolerability, pharmacokinetics and efficacy of bexicaserin Study Objectives: Evaluate reduction in countable motor seizures across a broad group of epilepsies Identify potential indications for pivotal studies Analyze concentration response to understand dosing in different seizure types and disorders Placebo (n=9) LP352 (n=43) Key Inclusion Criteria: DEEs with average of ≥ 4 motor seizures per 4-week period during the 12 weeks prior to screening and ≥ 4 motor seizures in the 4-week period of screening DEEs (multiple syndromes) including LGS, Dravet syndrome, SCN2A-related epilepsies, CDD, among others Key Exclusion Criteria: Use of fenfluramine & lorcaserin Basic Information: Sites: 34 sites Ages: ≥ 12 to ≤ 65 yrs old No Echocardiograms Required in PACIFIC Treatment Period
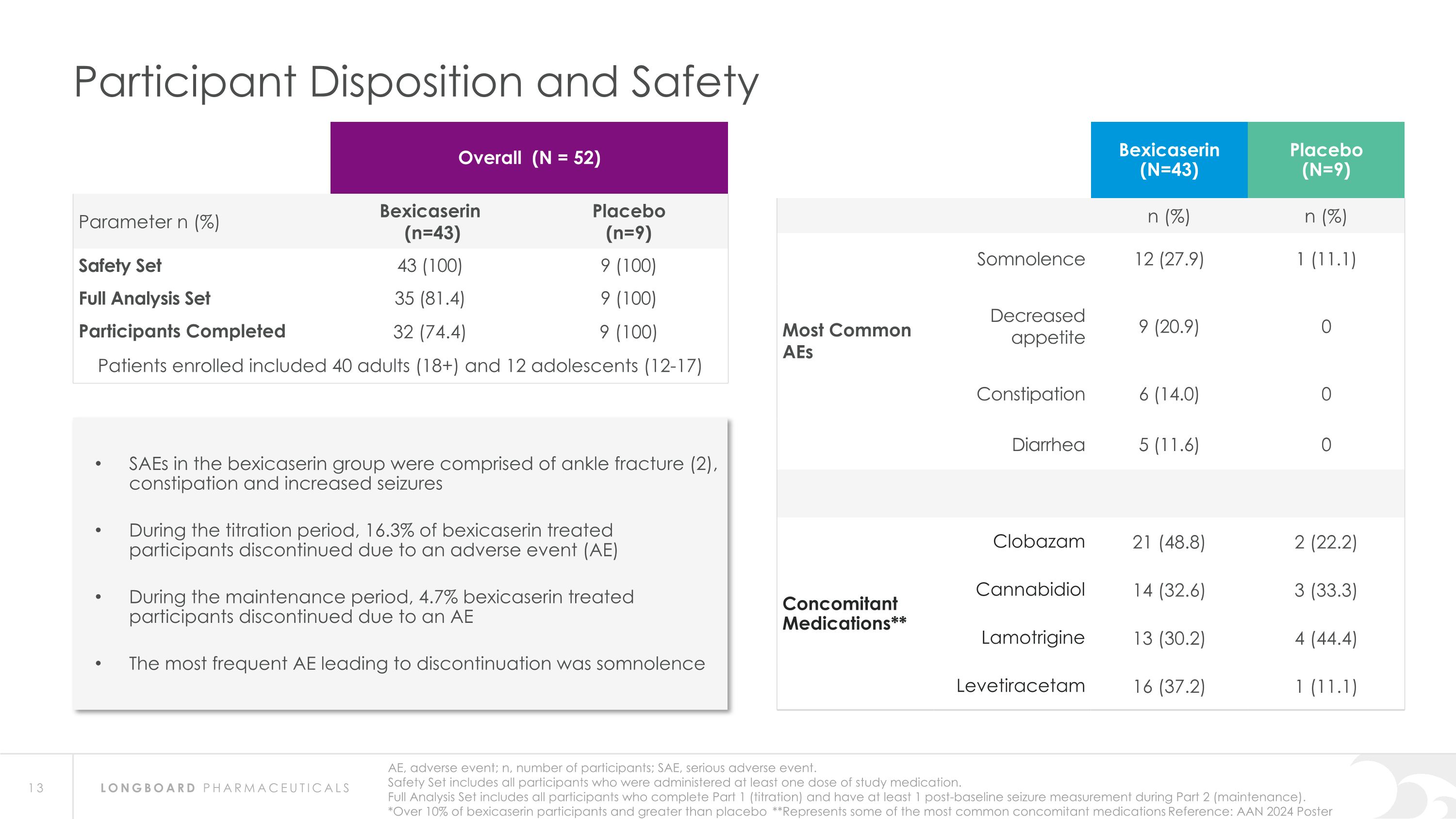
AE, adverse event; n, number of participants; SAE, serious adverse event. Safety Set includes all participants who were administered at least one dose of study medication. Full Analysis Set includes all participants who complete Part 1 (titration) and have at least 1 post-baseline seizure measurement during Part 2 (maintenance). *Over 10% of bexicaserin participants and greater than placebo **Represents some of the most common concomitant medications Reference: AAN 2024 Poster Participant Disposition and Safety Overall (N = 52) Parameter n (%) Bexicaserin (n=43) Placebo (n=9) Safety Set 43 (100) 9 (100) Full Analysis Set 35 (81.4) 9 (100) Participants Completed 32 (74.4) 9 (100) Patients enrolled included 40 adults (18+) and 12 adolescents (12-17) Bexicaserin (N=43) Placebo (N=9) n (%) n (%) Most Common AEs Somnolence 12 (27.9) 1 (11.1) Decreased appetite 9 (20.9) 0 Constipation 6 (14.0) 0 Diarrhea 5 (11.6) 0 Concomitant Medications** Clobazam 21 (48.8) 2 (22.2) Cannabidiol 14 (32.6) 3 (33.3) Lamotrigine 13 (30.2) 4 (44.4) Levetiracetam 16 (37.2) 1 (11.1) SAEs in the bexicaserin group were comprised of ankle fracture (2), constipation and increased seizures During the titration period, 16.3% of bexicaserin treated participants discontinued due to an adverse event (AE) During the maintenance period, 4.7% bexicaserin treated participants discontinued due to an AE The most frequent AE leading to discontinuation was somnolence
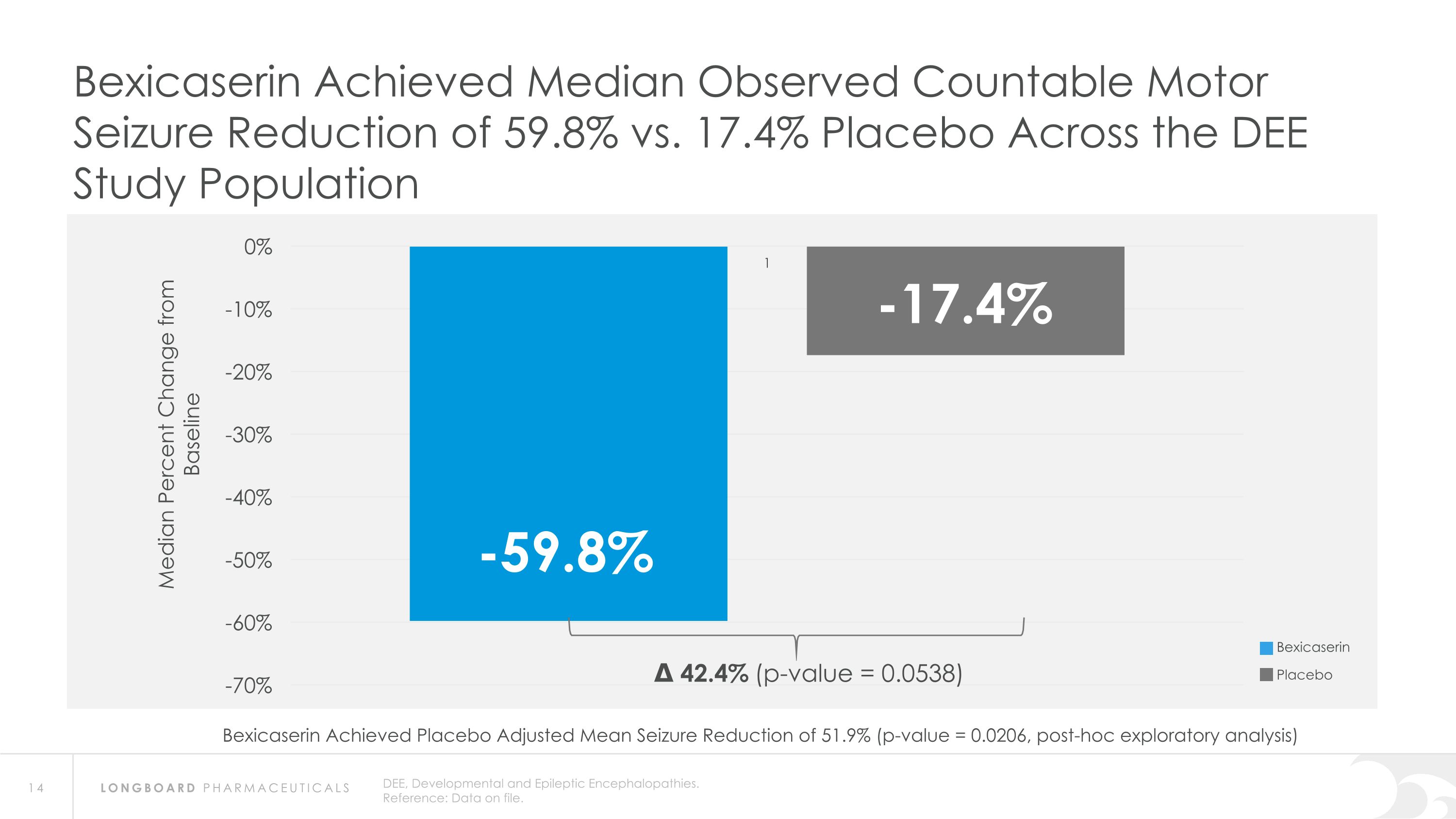
DEE, Developmental and Epileptic Encephalopathies. Reference: Data on file. Bexicaserin Achieved Median Observed Countable Motor Seizure Reduction of 59.8% vs. 17.4% Placebo Across the DEE Study Population Δ 42.4% (p-value = 0.0538) Bexicaserin Placebo Bexicaserin Achieved Placebo Adjusted Mean Seizure Reduction of 51.9% (p-value = 0.0206, post-hoc exploratory analysis)
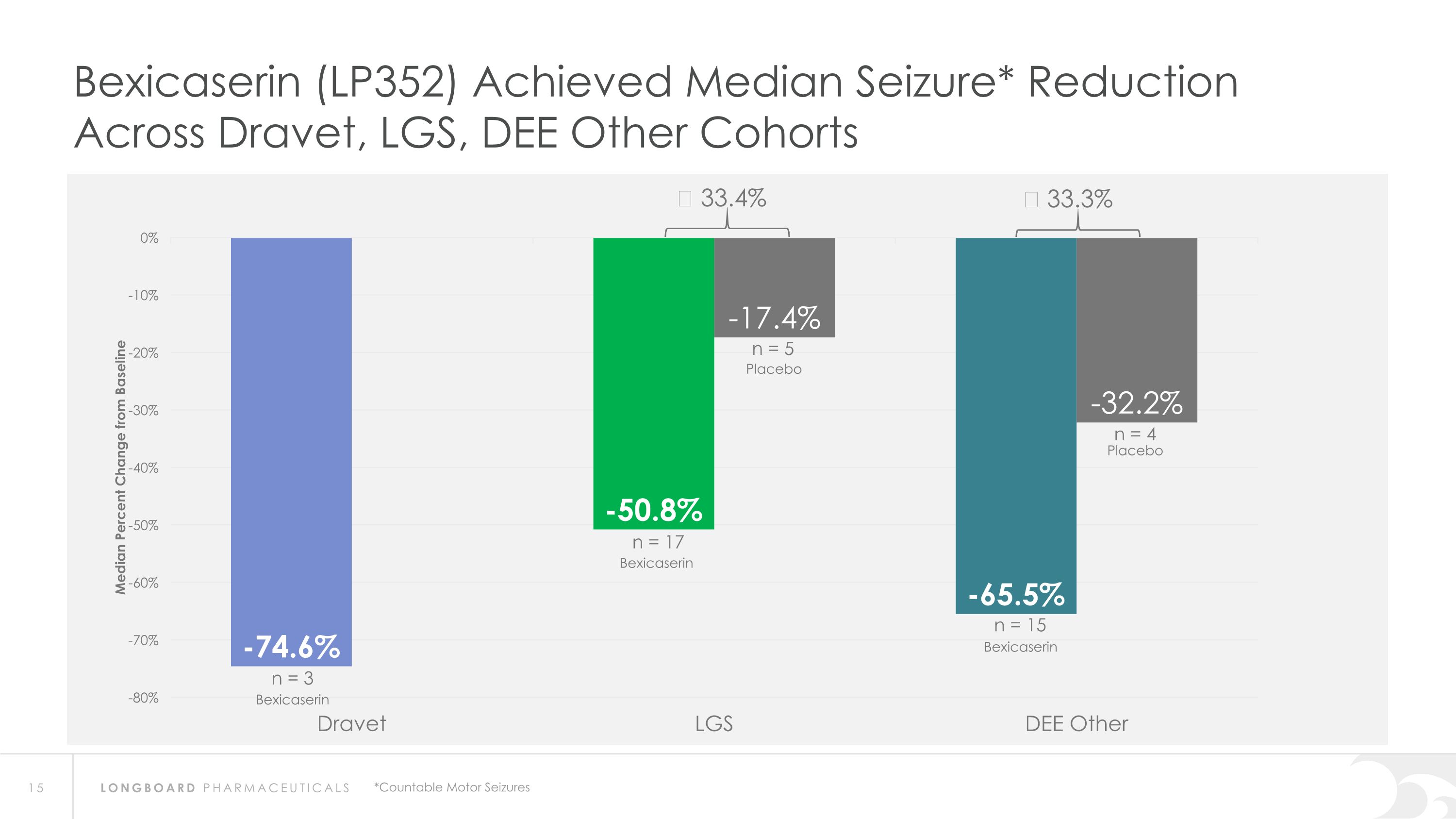
Bexicaserin (LP352) Achieved Median Seizure* Reduction Across Dravet, LGS, DEE Other Cohorts Δ 33.4% Δ 33.3% *Countable Motor Seizures n = 3 n = 5 n = 15 n = 4 Placebo Bexicaserin Placebo Bexicaserin Bexicaserin n = 17
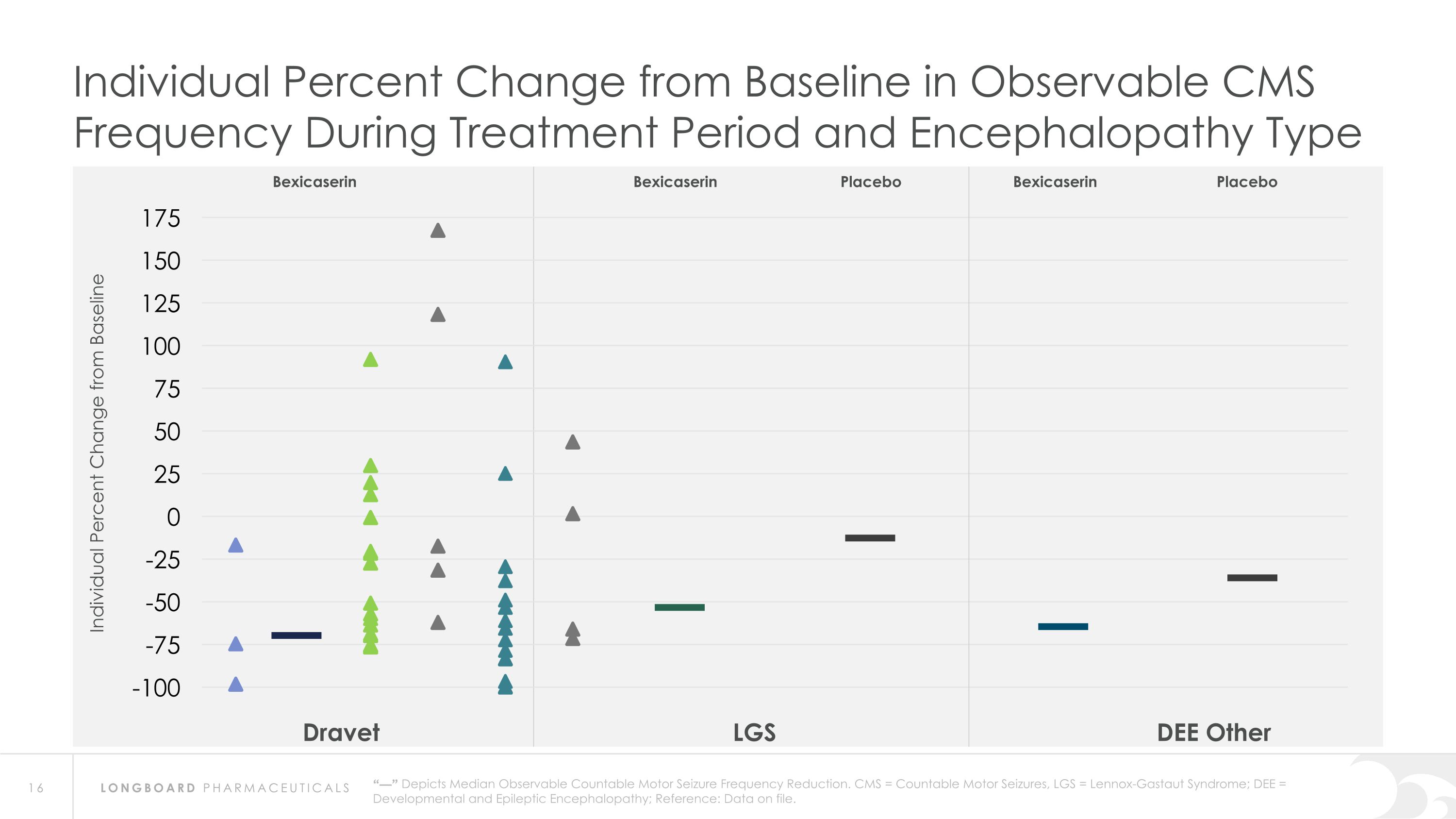
Dravet LGS DEE Other “—” Depicts Median Observable Countable Motor Seizure Frequency Reduction. CMS = Countable Motor Seizures, LGS = Lennox-Gastaut Syndrome; DEE = Developmental and Epileptic Encephalopathy; Reference: Data on file. Bexicaserin Placebo Bexicaserin Placebo Bexicaserin Individual Percent Change from Baseline in Observable CMS Frequency During Treatment Period and Encephalopathy Type
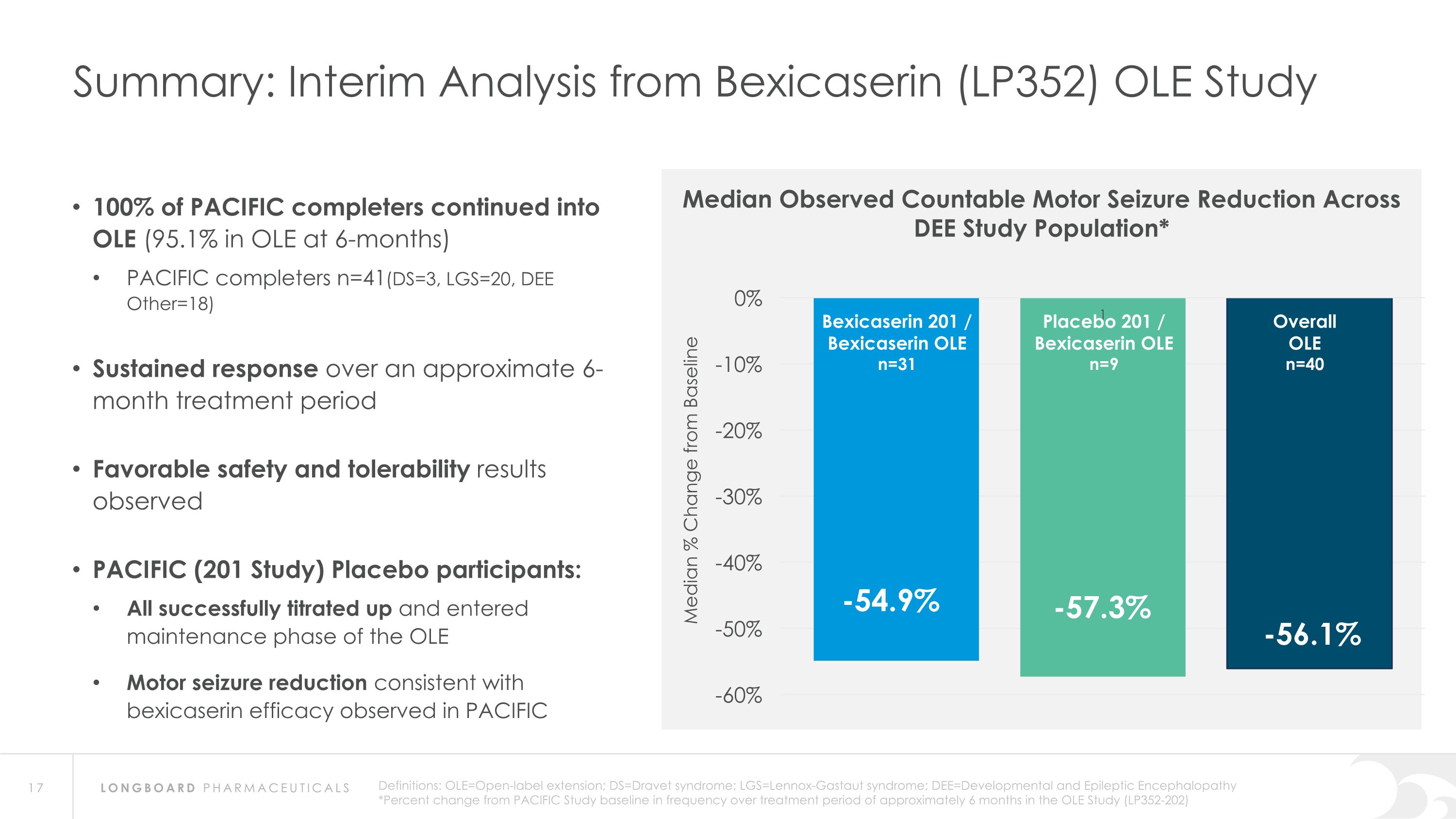
Summary: Interim Analysis from Bexicaserin (LP352) OLE Study Bexicaserin 201 / Bexicaserin OLE n=31 Placebo 201 / Bexicaserin OLE n=9 Overall OLE n=40 Median Observed Countable Motor Seizure Reduction Across DEE Study Population* 100% of PACIFIC completers continued into OLE (95.1% in OLE at 6-months) PACIFIC completers n=41(DS=3, LGS=20, DEE Other=18) Sustained response over an approximate 6-month treatment period Favorable safety and tolerability results observed PACIFIC (201 Study) Placebo participants: All successfully titrated up and entered maintenance phase of the OLE Motor seizure reduction consistent with bexicaserin efficacy observed in PACIFIC Definitions: OLE=Open-label extension; DS=Dravet syndrome; LGS=Lennox-Gastaut syndrome; DEE=Developmental and Epileptic Encephalopathy *Percent change from PACIFIC Study baseline in frequency over treatment period of approximately 6 months in the OLE Study (LP352-202)
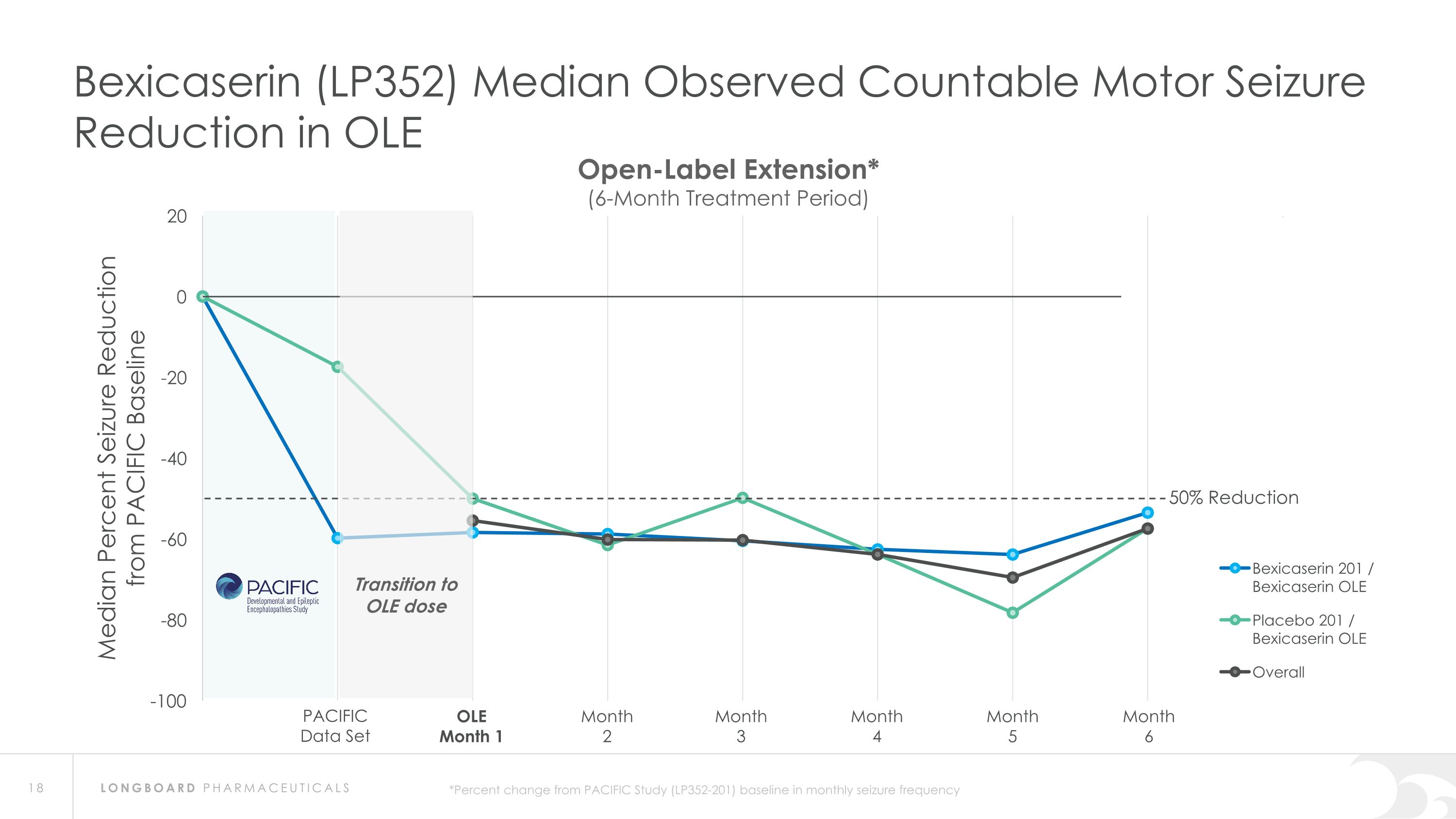
Bexicaserin (LP352) Median Observed Countable Motor Seizure Reduction in OLE PACIFIC Data Set OLE Month 1 Month 2 Month 3 Month 4 Month 5 Month 6 Open-Label Extension* (6-Month Treatment Period) 50% Reduction *Percent change from PACIFIC Study (LP352-201) baseline in monthly seizure frequency Transition to OLE dose

LGS = Lennox-Gastaut Syndrome Bexicaserin Phase 3 Global Program – DEE Path Forward�Subject to Ongoing Discussions with Regulatory Agencies Planned Study Parameters: Primary Endpoint: Reduction in Countable Motor Seizures Ages: ≥ 2 to ≤ 65 yrs old (weight-based dosing for pts of lower weight/age) Sites: Sites across the US, AUS, EU, other potential regions Open-Label Extension (OLE): Participants who complete either of the Ph 3 studies are eligible to enter a 52-week OLE Study 302: Dravet Syndrome Study 301: DEEs (LGS + Other DEEs) Expected to be run in parallel Breakthrough Therapy designation granted for bexicaserin for the treatment of seizures associated with Developmental and Epileptic Encephalopathies (DEEs) for patients ≥ 2 years of age

Global Phase 3 Program Expected to Initiate in 2024 Bexicaserin (LP352) achieved a median percent reduction from baseline in seizure frequency during the treatment period of: 59.8% in broad DEE population (42.4% placebo-adjusted) 74.6% in Dravet cohort 50.8% in LGS cohort (33.4% placebo-adjusted) 65.5% in DEE Other cohort (33.3% placebo-adjusted) Results were shown on top of a contemporary polytherapy background with multiple ASMs including cannabidiol (32.7% of participants were receiving cannabidiol) *Definitions: ASMs = Anti-Seizure Medications; UGT = Uridine Diphosphate Glucuronosyltransferase Favorable safety and tolerability results No echocardiograms required in PACIFIC study Metabolized via UGT pathway – potentially reduces risk of Drug-Drug Interactions 86% of participants achieved the highest dose of 12 mg of bexicaserin in the maintenance period 100% of PACIFIC participants who completed the study entered the Open Label Extension (OLE) Study OLE Interim Analysis Sustained response over an approximate 6-month treatment period Favorable safety and tolerability results observed All PACIFIC Placebo participants successfully titrated up and entered maintenance phase of the OLE Motor seizure reduction consistent with bexicaserin efficacy observed in PACIFIC

LP659 Centrally Acting, Highly Selective Sphingosine-1-Phosphate (S1P) Receptor Modulator Targeting Multiple Neurological Diseases
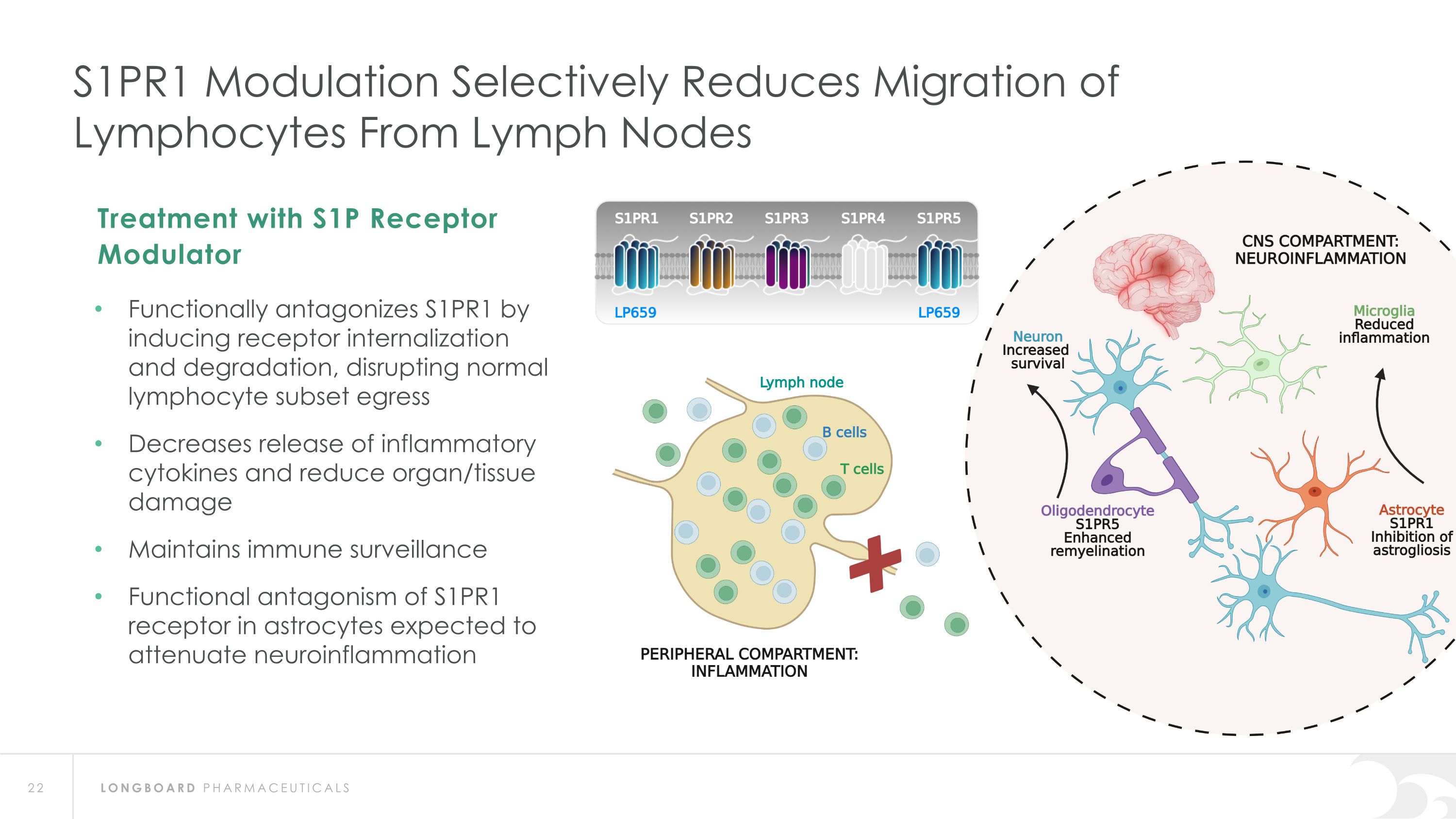
S1PR1 Modulation Selectively Reduces Migration of�Lymphocytes From Lymph Nodes Functionally antagonizes S1PR1 by inducing receptor internalization and degradation, disrupting normal lymphocyte subset egress Decreases release of inflammatory cytokines and reduce organ/tissue damage Maintains immune surveillance Functional antagonism of S1PR1 receptor in astrocytes expected to attenuate neuroinflammation Treatment with S1P Receptor Modulator
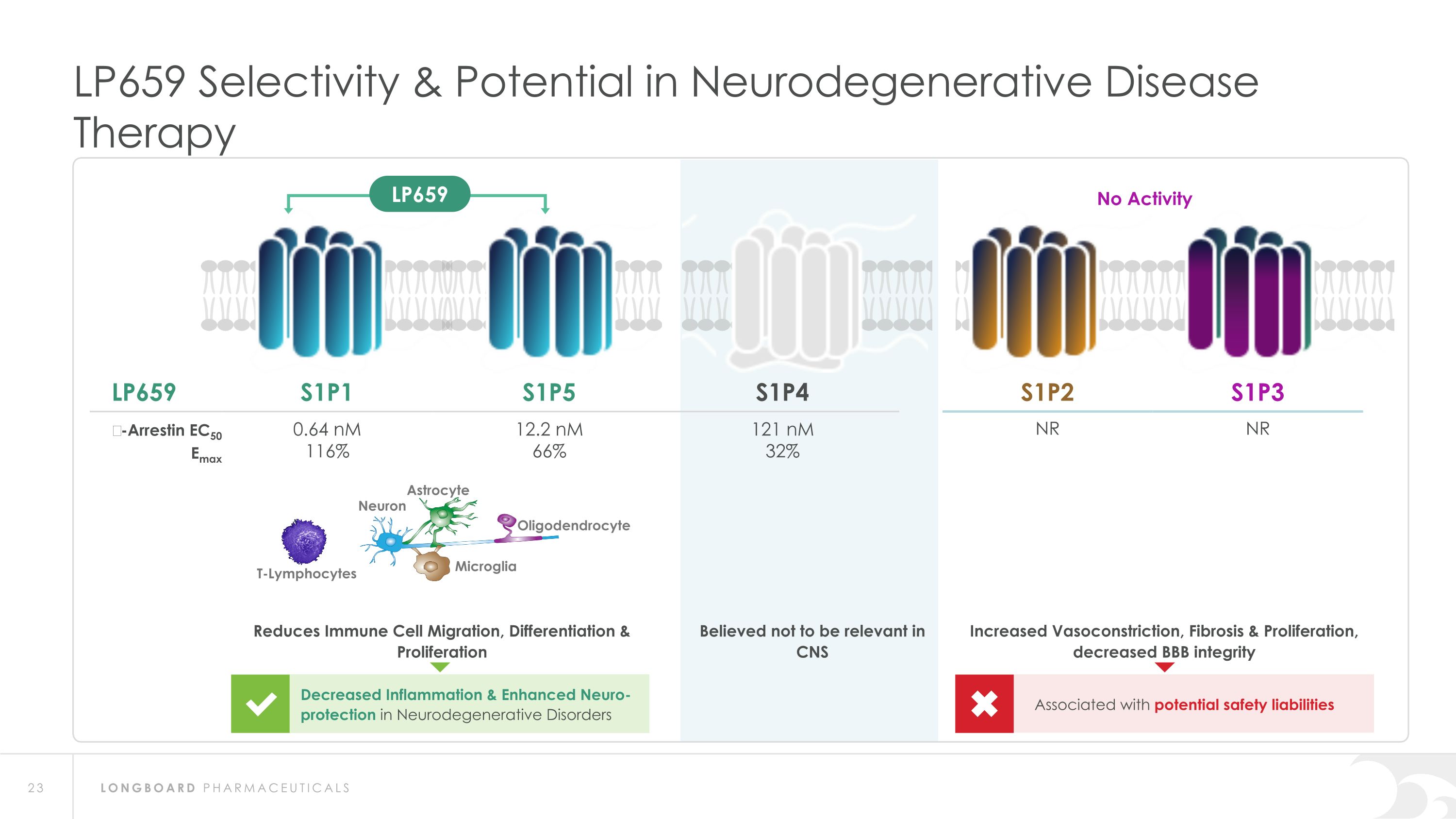
LP659 Selectivity & Potential in Neurodegenerative Disease Therapy LP659 S1P1 S1P5 S1P4 S1P2 S1P3 β-Arrestin EC50 Emax 0.64 nM 116% 12.2 nM 66% 121 nM 32% NR NR T-Lymphocytes Neuron Astrocyte Oligodendrocyte Microglia Decreased Inflammation & Enhanced Neuro-protection in Neurodegenerative Disorders Reduces Immune Cell Migration, Differentiation & Proliferation Associated with potential safety liabilities Increased Vasoconstriction, Fibrosis & Proliferation, decreased BBB integrity No Activity LP659 Believed not to be relevant in CNS
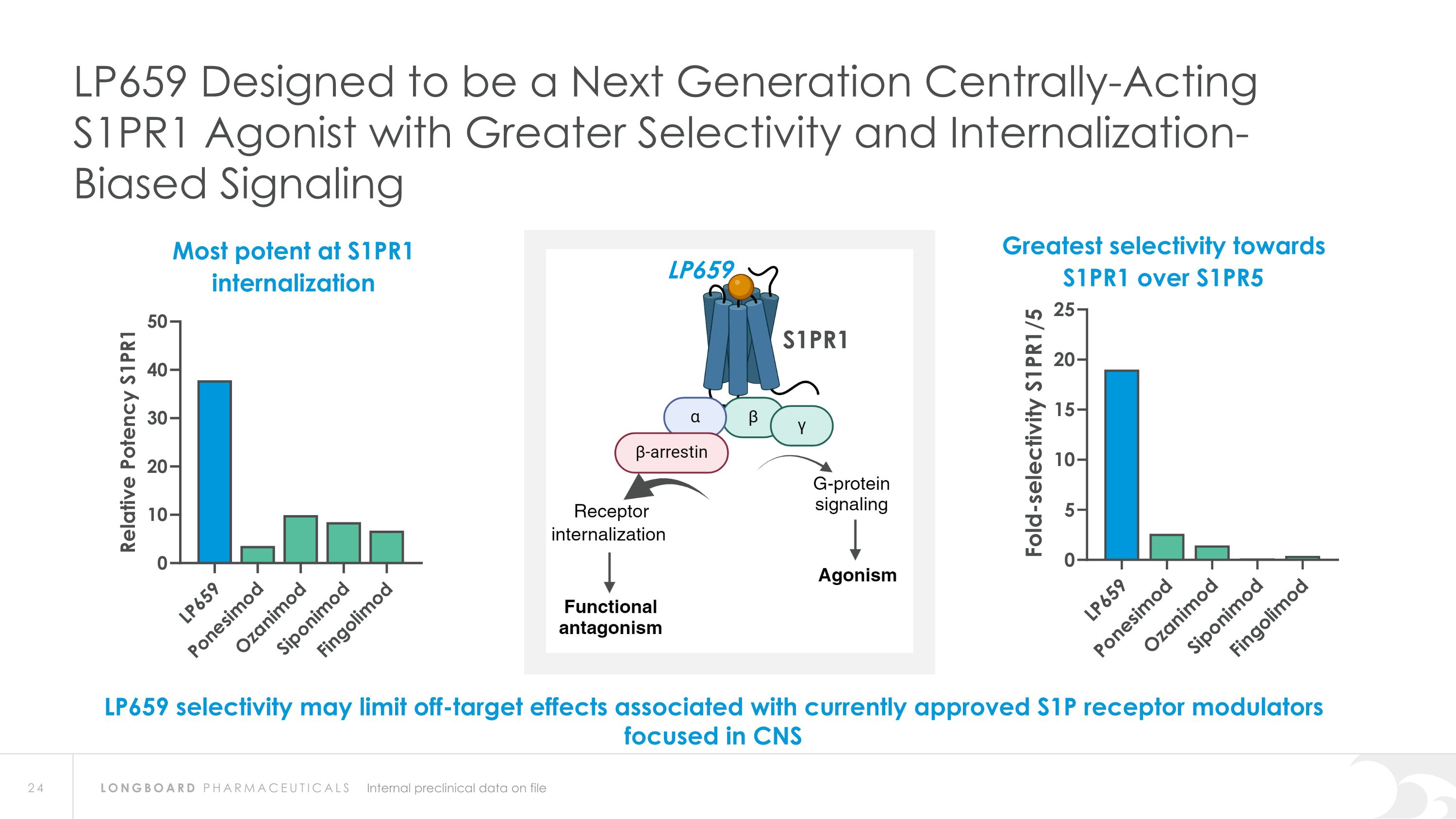
S1PR1 Internal preclinical data on file LP659 Designed to be a Next Generation Centrally-Acting S1PR1 Agonist with Greater Selectivity and Internalization-Biased Signaling LP659 selectivity may limit off-target effects associated with currently approved S1P receptor modulators focused in CNS Greatest selectivity towards S1PR1 over S1PR5 Most potent at S1PR1 internalization LP659
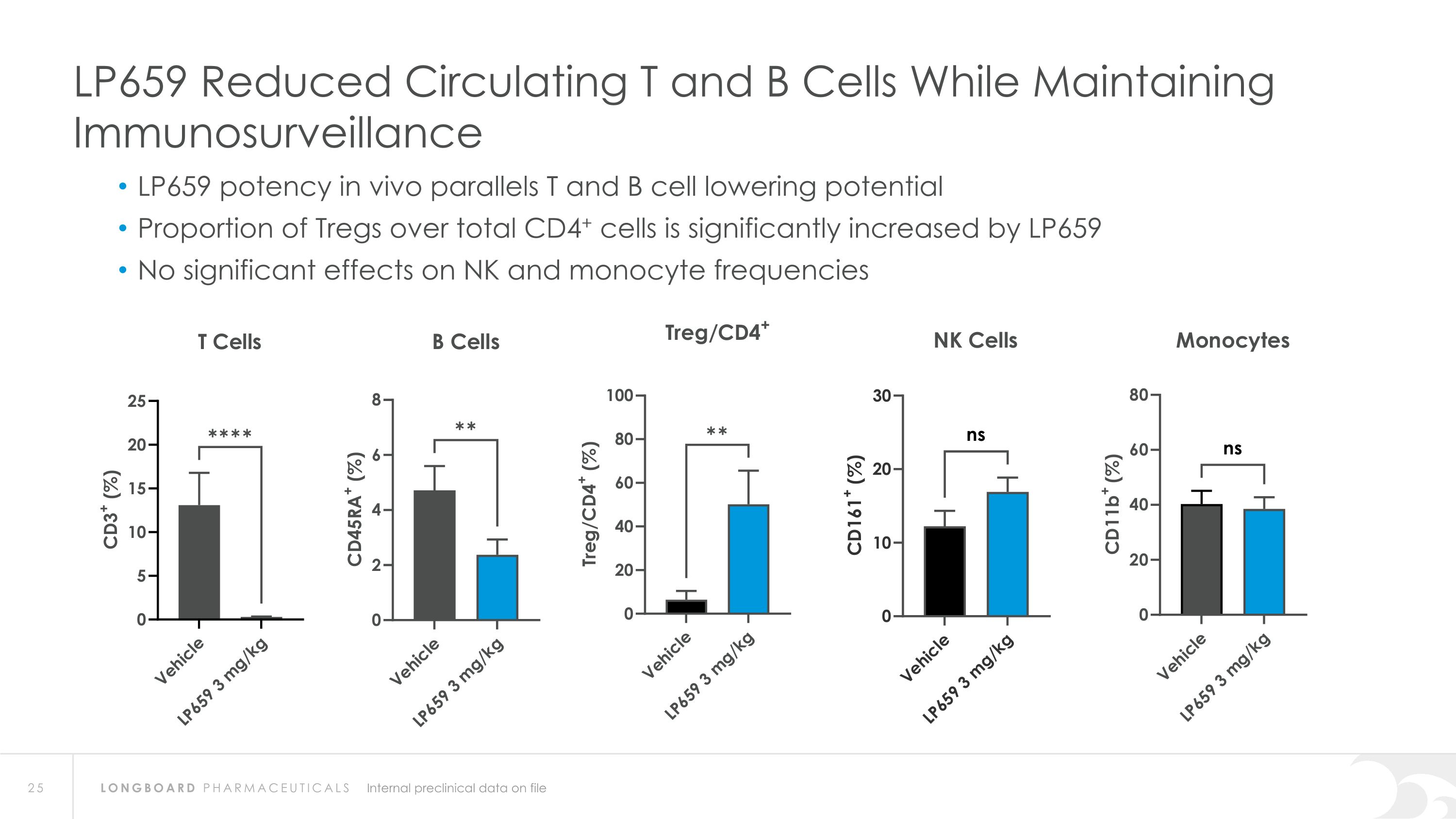
LP659 Reduced Circulating T and B Cells While Maintaining Immunosurveillance LP659 potency in vivo parallels T and B cell lowering potential Proportion of Tregs over total CD4+ cells is significantly increased by LP659 No significant effects on NK and monocyte frequencies Internal preclinical data on file

LP659 LP659 Phase 1 SAD Topline Data
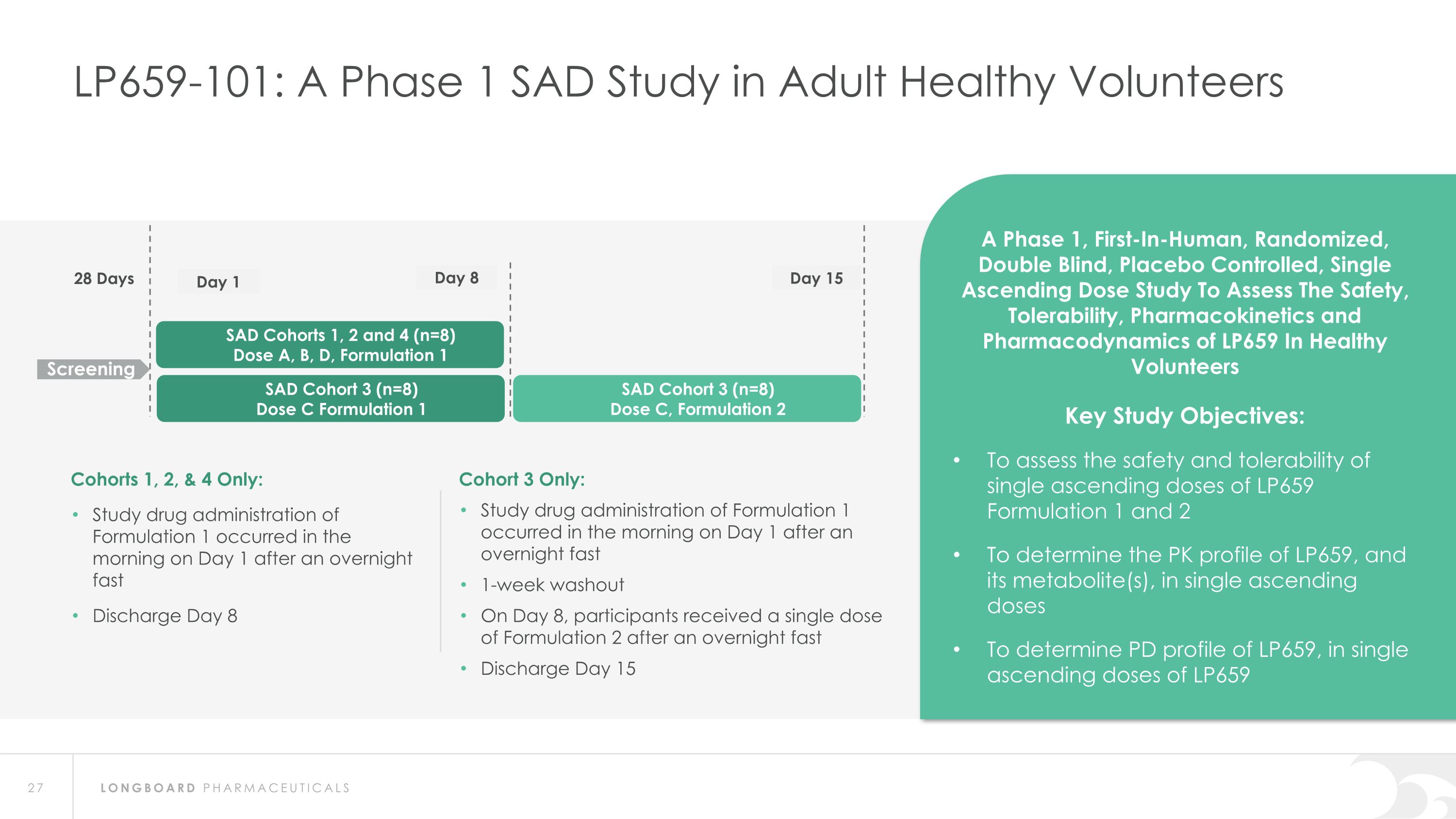
LP659-101: A Phase 1 SAD Study in Adult Healthy Volunteers SAD Cohorts 1, 2 and 4 (n=8) Dose A, B, D, Formulation 1 28 Days Screening A Phase 1, First-In-Human, Randomized, Double Blind, Placebo Controlled, Single Ascending Dose Study To Assess The Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of LP659 In Healthy Volunteers Key Study Objectives: To assess the safety and tolerability of single ascending doses of LP659 Formulation 1 and 2 To determine the PK profile of LP659, and its metabolite(s), in single ascending doses To determine PD profile of LP659, in single ascending doses of LP659 Day 1 Day 15 Cohorts 1, 2, & 4 Only: Study drug administration of Formulation 1 occurred in the morning on Day 1 after an overnight fast Discharge Day 8 Cohort 3 Only: Study drug administration of Formulation 1 occurred in the morning on Day 1 after an overnight fast 1-week washout On Day 8, participants received a single dose of Formulation 2 after an overnight fast Discharge Day 15 Day 8 SAD Cohort 3 (n=8) Dose C, Formulation 2 SAD Cohort 3 (n=8) Dose C Formulation 1

Adverse events were mild No TEAEs leading to discontinuation No SAEs observed Impact on HR was low throughout the study with no first dose bradycardia No abnormal ECGs (no AV block) or abnormal echocardiograms No abnormal pulmonary/spirometry and ophthalmologic assessments No infections Phase 1 Safety Results LP659 was generally safe and well tolerated ► Abbreviations: TEAEs = Treatment-Emergent Adverse Events; SAEs = serious adverse events, HR = Heart Rate, ECG = electrocardiogram, AV = atrioventricular
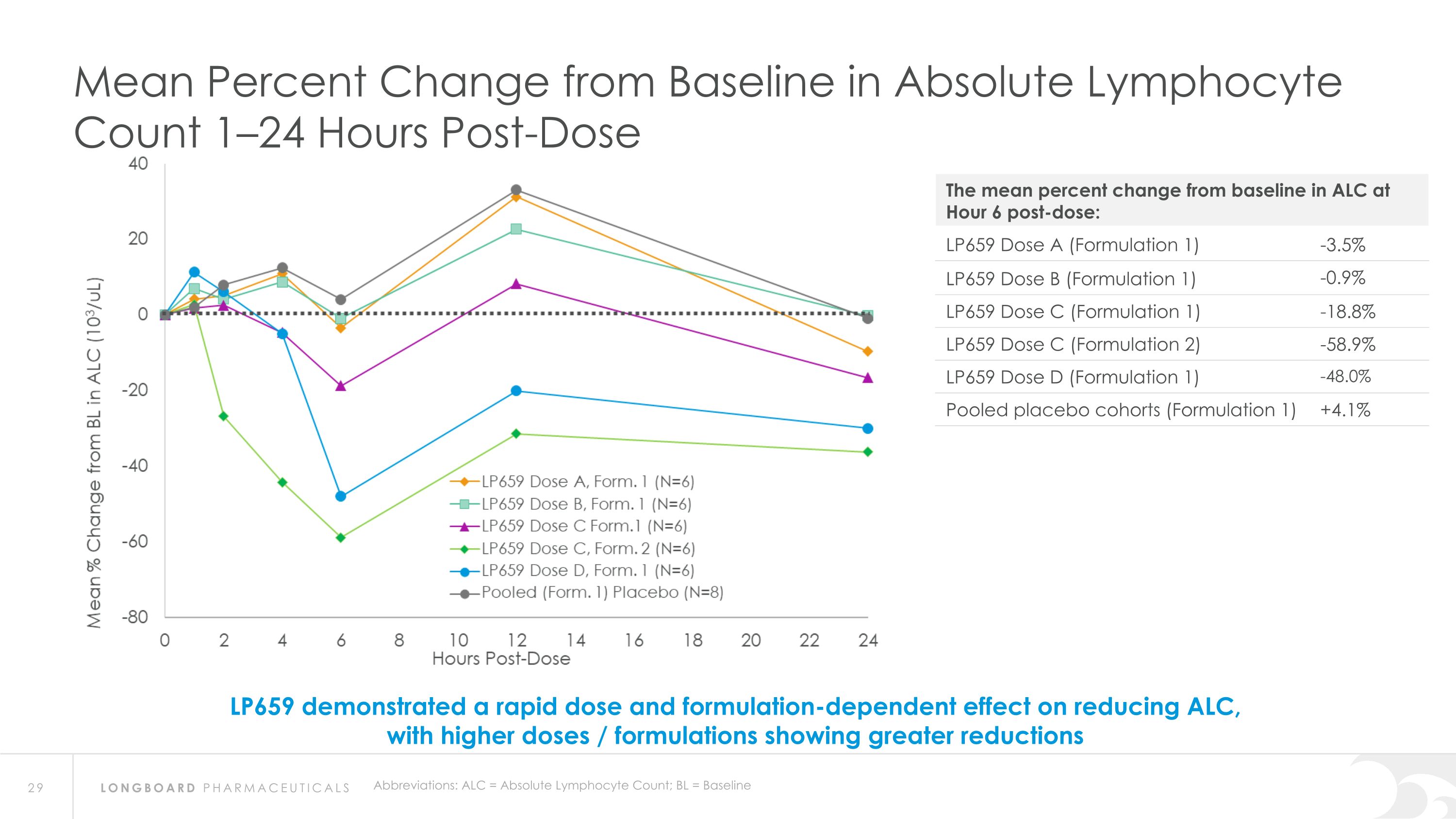
Abbreviations: ALC = Absolute Lymphocyte Count; BL = Baseline Mean Percent Change from Baseline in Absolute Lymphocyte Count 1–24 Hours Post-Dose LP659 demonstrated a rapid dose and formulation-dependent effect on reducing ALC, with higher doses / formulations showing greater reductions The mean percent change from baseline in ALC at Hour 6 post-dose: LP659 Dose A (Formulation 1) -3.5% LP659 Dose B (Formulation 1) -0.9% LP659 Dose C (Formulation 1) -18.8% LP659 Dose C (Formulation 2) -58.9% LP659 Dose D (Formulation 1) -48.0% Pooled placebo cohorts (Formulation 1) +4.1%
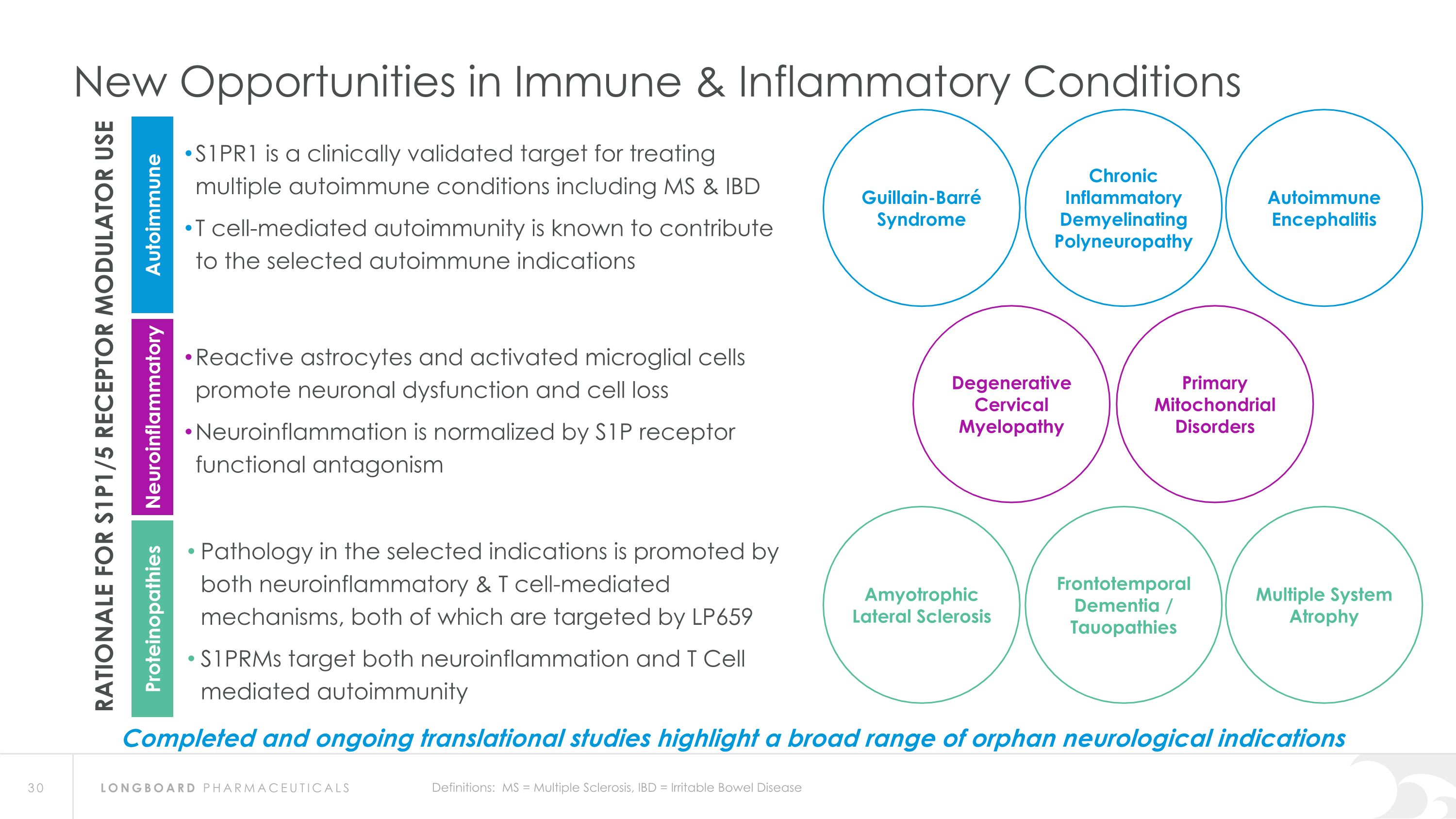
New Opportunities in Immune & Inflammatory Conditions S1PR1 is a clinically validated target for treating multiple autoimmune conditions including MS & IBD T cell-mediated autoimmunity is known to contribute to the selected autoimmune indications RATIONALE FOR S1P1/5 RECEPTOR MODULATOR USE Autoimmune Neuroinflammatory Proteinopathies Reactive astrocytes and activated microglial cells promote neuronal dysfunction and cell loss Neuroinflammation is normalized by S1P receptor functional antagonism Pathology in the selected indications is promoted by both neuroinflammatory & T cell-mediated mechanisms, both of which are targeted by LP659 S1PRMs target both neuroinflammation and T Cell mediated autoimmunity Degenerative Cervical Myelopathy Primary Mitochondrial Disorders Autoimmune Encephalitis Guillain-Barré Syndrome Chronic Inflammatory Demyelinating Polyneuropathy Definitions: MS = Multiple Sclerosis, IBD = Irritable Bowel Disease Multiple System Atrophy Amyotrophic Lateral Sclerosis Frontotemporal Dementia / Tauopathies Completed and ongoing translational studies highlight a broad range of orphan neurological indications
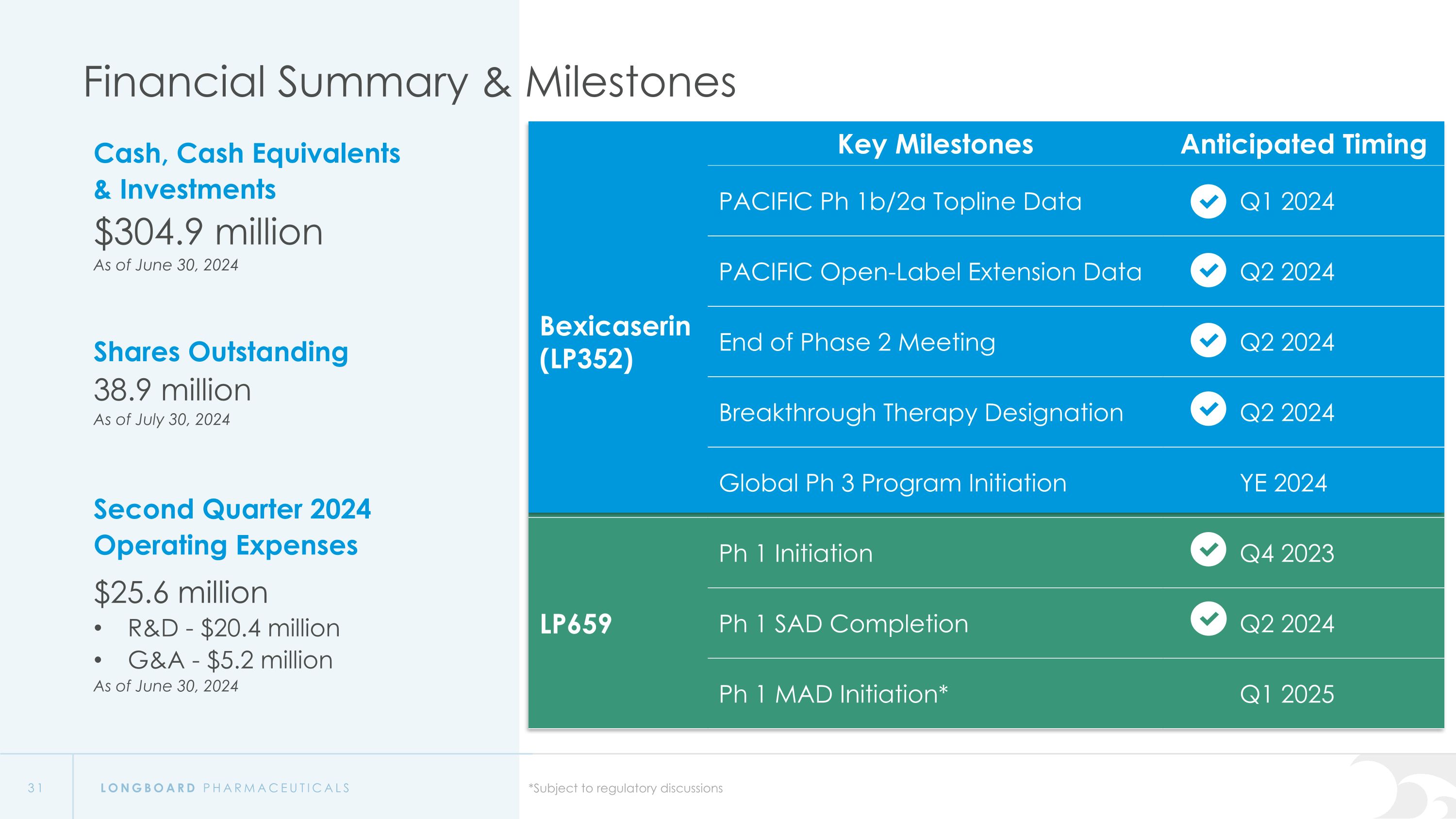
Financial Summary & Milestones Cash, Cash Equivalents �& Investments $304.9 million As of June 30, 2024 Shares Outstanding 38.9 million As of July 30, 2024 Second Quarter 2024 Operating Expenses $25.6 million R&D - $20.4 million G&A - $5.2 million As of June 30, 2024 Key Milestones Anticipated Timing Bexicaserin (LP352) PACIFIC Ph 1b/2a Topline Data Q1 2024 PACIFIC Open-Label Extension Data Q2 2024 LP352 End of Phase 2 Meeting Q2 2024 Breakthrough Therapy Designation Q2 2024 Global Ph 3 Program Initiation YE 2024 LP659 Ph 1 Initiation Q4 2023 Ph 1 SAD Completion Q2 2024 Ph 1 MAD Initiation* Q1 2025 *Subject to regulatory discussions

Thank you Nasdaq: LBPH IR@longboardpharma.com
v3.24.2.u1
Document and Entity Information
|
Aug. 01, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Aug. 01, 2024
|
| Entity Registrant Name |
Longboard Pharmaceuticals, Inc.
|
| Entity Central Index Key |
0001832168
|
| Entity Emerging Growth Company |
true
|
| Entity Ex Transition Period |
false
|
| Entity File Number |
1-40192
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
84-5009619
|
| Entity Address, Address Line One |
4275 Executive Square
|
| Entity Address, Address Line Two |
Suite 950
|
| Entity Address, City or Town |
La Jolla
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
92037
|
| City Area Code |
858
|
| Local Phone Number |
789-9283
|
| Entity Information, Former Legal or Registered Name |
N/A
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Trading Symbol |
LBPH
|
| Title of 12(b) Security |
Common stock, par value $0.0001 per share
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Longboard Pharmaceuticals (NASDAQ:LBPH)
Historical Stock Chart
From Feb 2025 to Mar 2025

Longboard Pharmaceuticals (NASDAQ:LBPH)
Historical Stock Chart
From Mar 2024 to Mar 2025
