UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
SCHEDULE
14C
Information
Statement Pursuant to Section 14(c) of the
Securities
Exchange Act of 1934
Check
the appropriate box:
| ☒ |
Preliminary
Information Statement |
| ☐ |
Confidential,
for Use of the Commission Only (as permitted by Rule 14c-5(d)(2)) |
| ☐ |
Definitive
Information Statement |
TRxADE
HEALTH, INC.
(Exact
name of Registrant as specified in its charter)
Payment
of Filing Fee (Check all boxes that apply):
| ☒ |
No
fee required |
| ☐ |
Fee
paid previously with preliminary materials |
| ☐ |
Fee
computed on table in exhibit required by Item 25(b) of Schedule 14A (17 CFR 240.14a-101) per Item 1 of this Schedule and Exchange
Act Rules 14c-5(g) and 0-11 |

TRxADE
HEALTH, INC.
6308
Benjamin Rd, Suite 708
Tampa,
Florida 33634
NOTICE
OF STOCKHOLDER ACTION BY WRITTEN CONSENT
WE
ARE NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND US A PROXY
THIS
IS NOT A NOTICE OF A MEETING OF STOCKHOLDERS AND NO STOCKHOLDERS’ MEETING WILL BE HELD TO CONSIDER ANY MATTER DESCRIBED HEREIN.
THIS INFORMATION STATEMENT IS BEING FURNISHED TO YOU SOLELY FOR THE PURPOSE OF INFORMING YOU OF THE MATTERS DESCRIBED HEREIN.
Dear
Stockholders:
This
information statement (the “Information Statement”) has been filed with the Securities and Exchange Commission (the “SEC”)
and is being mailed or otherwise furnished to the holders of record of the common stock, par value $0.00001 per share, of TRxADE HEALTH,
INC., a Delaware corporation (the “Company,” “us” or “we”), on or about August [●], 2024. Only
stockholders of record as of the close of business on July 25, 2024 (the “Record Date”) are entitled to receive this Information
Statement.
The
purpose of this notice and the accompanying Information Statement is to notify you that on July 25, 2024, in connection with the Company’s
previously announced transaction with Scienture, Inc., a Delaware corporation (“Scienture”), a majority of the holders of
the Company’s common stock executed a written consent in lieu of a meeting of stockholders (the “Stockholder Consent”),
which (i) approved the conversion of the Company’s Series X Non-Voting Convertible Preferred Stock, par value $0.00001 per share
(the “Series X Preferred Stock”) into shares of the Company’s common stock (the “Preferred Stock Conversion”),
(ii) authorized the Company’s board of directors (the “Board”) to change the Company’s name to “Scienture
Holdings, Inc.” (the “Name Change”), and (iii) approved an increase in the number of shares available to be awarded
under the Company’s Second Amended and Restated 2019 Equity Incentive Plan, as amended, to five million shares of the Company’s
common stock (the “Incentive Plan Share Increase” and, together with the Preferred Stock Conversion and the Name Change,
the “Stockholder Approval Matters”).
The
Stockholder Consent was entered into in connection with the Agreement and Plan of Merger (the “Merger Agreement”), which
was entered into and closed on July 25, 2024, by and among the Company, MEDS Merger Sub I, Inc., a Delaware corporation and wholly
owned subsidiary of the Company (“Merger Sub I”), MEDS Merger Sub II, LLC, a Delaware limited liability company and wholly
owned subsidiary of the Company (“Merger Sub II”), and Scienture, Inc., a Delaware corporation (“Scienture”).
Pursuant to the Merger Agreement, on July 25, 2024, (i) Merger Sub I merged with and into Scienture (the “First Merger”),
with Scienture continuing as the surviving entity and a wholly owned subsidiary of the Company, and (ii) Scienture merged with and into
Merger Sub II (the “Second Merger” and, together with the First Merger, the “Mergers”), with Merger Sub II continuing
as the surviving entity and Merger Sub II changed its name to “Scienture, LLC”. The Board approved the Merger
Agreement and the related transactions, and the consummation of the Mergers was not subject to approval of the Company’s stockholders.
As
consideration for the Mergers, at the effective time of the First Merger (the “First Effective Time”), the shares of Scienture’s
common stock issued and outstanding immediately prior to the First Effective Time were converted into the right to receive, in the aggregate,
(i) 291,555 shares of the Company’s common stock, which represents 19.99% of the number of shares of the Company’s common
stock issued and outstanding immediately prior to the First Effective Time, and (ii) 6,826,713 shares of Series X Preferred Stock, each
share of which is convertible into one share of the Company’s common stock, subject to certain conditions.
In
accordance with Rule 14c-2 of the Securities Exchange Act of 1934, as amended, and the rules promulgated
by the SEC thereunder, the Information Statement is being furnished to our stockholders solely for the purpose of informing our stockholders
of the corporate actions taken in the Stockholder Consent, which will be effective on September [●], 2024, twenty (20) days
after we mail the Information Statement to stockholders of record.
August
[●], 2024
| |
By
order of the Board, |
| |
|
| |
/s/
Suren Ajjarapu |
| |
Suren
Ajjarapu |
| |
Chairman
of the Board |

TRxADE
HEALTH, INC.
6308
Benjamin Rd, Suite 708
Tampa,
Florida 33634
INFORMATION
STATEMENT
PURSUANT
TO SECTION 14(C) OF THE
SECURITIES
EXCHANGE ACT OF 1934
WE
ARE NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND US A PROXY
TABLE
OF CONTENTS
ABOUT
THIS INFORMATION STATEMENT
TRxADE
HEALTH, INC. and its consolidated subsidiaries are referred to herein as “the Company,” “we,” “us”
and “our,” unless the context indicates otherwise.
This
Information Statement (the “Information Statement”) is being furnished to the Company’s stockholders of record
as of July 25, 2024 (the “Record Date”) in the manner required by Section 14(c) of the Securities Exchange Act of 1934,
as amended (the “Exchange Act”), and is being furnished to notify stockholders of the action taken by written consent of
a majority of the Company’s stockholders (the “Majority Stockholders”).
On
July 25, 2024, in connection with the Company’s previously announced transaction with Scienture, Inc., a Delaware corporation (“Scienture”),
the Majority Stockholders executed a written consent in lieu of a meeting of stockholders (the “Stockholder Consent”), which
(i) approved the conversion of the Company’s Series X Non-Voting Convertible Preferred Stock, par value $0.00001 per share (the
“Series X Preferred Stock”) into shares of the Company’s common stock (the “Preferred Stock Conversion”),
(ii) authorized the Company’s board of directors (the “Board”) to change the Company’s name to “Scienture
Holdings, Inc.” (the “Name Change”), and (iii) approved an increase in the number of shares available to be awarded
under the Company’s Second Amended and Restated 2019 Equity Incentive Plan, as amended (the “Incentive Plan”), to five
million shares of the Company’s common stock (the “Incentive Plan Share Increase” and, together with the Preferred
Stock Conversion and the Name Change, the “Stockholder Approval Matters”).
The
Stockholder Consent was entered into in connection with the Agreement and Plan of Merger (the “Merger Agreement”), attached
hereto as Annex A, which was entered into and closed on July 25, 2024, by and among the Company, MEDS Merger Sub I, Inc.,
a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub I”), MEDS Merger Sub II, LLC, a Delaware limited
liability company and wholly owned subsidiary of the Company (“Merger Sub II”), and Scienture. Pursuant to the Merger Agreement,
on July 25, 2024, (i) Merger Sub I merged with and into Scienture (the “First Merger”), with Scienture continuing as the
surviving entity and a wholly owned subsidiary of the Company, and (ii) Scienture merged with and into Merger Sub II (the “Second
Merger” and, together with the First Merger, the “Mergers”), with Merger Sub II continuing as the surviving entity
and Merger Sub II changed its name to “Scienture, LLC”. The Board approved the Merger Agreement and the related
transactions, and the consummation of the Mergers was not subject to approval of the Company’s stockholders.
As
consideration for the Mergers, at the effective time of the First Merger (the “First Effective Time”), the shares of Scienture’s
common stock issued and outstanding immediately prior to the First Effective Time were converted into the right to receive, in the aggregate,
(i) 291,555 shares of the Company’s common stock, which represents 19.99% of the number of shares of the Company’s common
stock issued and outstanding immediately prior to the First Effective Time, and (ii) 6,826,713 shares of Series X Preferred Stock, each
share of which is convertible into one share of the Company’s common stock, subject to certain conditions.
The
Company’s common stock is listed on the Nasdaq Stock Market LLC (“Nasdaq”) and the Company is subject to Nasdaq’s
rules and regulations, including (i) Nasdaq Rule 5635(a), which requires stockholder approval prior to the issuance of securities in
connection with the acquisition of the stock or assets of another company if such securities represent, or will represent upon issuance,
twenty percent (20%) or more of the outstanding common stock or voting power of a company prior to the issuance of the securities
in connection with the acquisition, (ii) Nasdaq Rule 5635(c), which requires stockholder approval prior to issuing securities when a
stock option or purchase plan, or other equity compensation arrangement, pursuant to which stock may be acquired by officers, directors,
employees, or consultants (subject to certain exceptions) is materially amended, and (iii) Nasdaq Rule 5635(d), which requires stockholder
approval prior to the issuance of securities in a transaction (other than a public offering) of common stock (or securities convertible
into or exercisable for common stock) equal to 20% or more of the outstanding common stock or 20% or more of the voting power of a company
for a purchase price that is lower than (a) the Nasdaq Official Closing Price (as reflected on Nasdaq.com) immediately preceding the
signing of a binding agreement, or (b) the average Nasdaq Official Closing Price of the common stock (as reflected on Nasdaq.com) for
the five trading days immediately preceding the signing of the binding agreement (such lower amount, the “Minimum Price”).
The
approval of (i) the Preferred Stock Conversion, for purposes of Nasdaq Rule 5635(a) and Nasdaq Rule 5635(d), and
(ii) the Incentive Plan Share Increase, for purposes of Nasdaq Rule 5635(c), was taken by written consent pursuant to Section 228
of the General Corporation Law of the State of Delaware, which provides that any action that may be taken at any annual or special meeting
of stockholders may be taken without a meeting, without prior notice and without a vote, if a consent or consents in writing, setting
forth the action so taken, shall be signed by the holders of outstanding common stock having not less than the minimum number of votes
that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted.
On
July 25, 2024, the Board adopted resolutions approving the Merger Agreement and the transactions contemplated thereby, including the
Stockholder Approval Matters. In connection with the adoption of these resolutions, the Board had been informed that the Majority Stockholders
were in favor of the proposals and would enter into a written consent approving each of the Stockholder Approval Matters. Thereafter,
on July 25, 2024, the Majority Stockholders executed the Stockholder Consent to consent in writing to each of the Stockholder Approval
Matters.
Accordingly,
all necessary corporate approvals in connection with the transactions have been obtained and this Information Statement is being furnished
to stockholders solely for purposes of informing the stockholders of the actions in the manner required under the Exchange Act.
The
Company knows of no other matters other than those described in this Information Statement which have been recently approved or considered
by the holders of the Company’s issued and outstanding voting securities.
Available
Information
We
file annual, quarterly, and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the
public over the Internet at the SEC’s website at http://www.sec.gov. Copies of documents filed by us with the SEC are also
available from us without charge, upon oral or written request to our Secretary, who can be contacted at the corporate address and telephone
number set forth in this Information Statement. Our website address is https://trxadehealth.com/. The information on, or
that may be accessed through, our websites not incorporated by reference into this Information Statement and should not be considered
a part of this Information Statement.
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This
Information Statement contains statements that constitute forward-looking statements which are subject to the safe-harbor provisions
of the Private Securities Litigation Reform Act of 1995. Statements that are not historical are forward-looking statements within the
meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Exchange Act. Some of the statements in this Information Statement constitute forward-looking statements because they relate
to future events or our future performance or future financial condition. These forward-looking statements are not historical facts,
but rather are based on current expectations, estimates and projections about our company, our industry, our beliefs and our assumptions.
Our forward-looking statements include, but are not limited to, statements regarding our or our management team’s expectations,
hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other
characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. In some cases,
you can identify forward-looking statements by the following words: “anticipate,” “believe,” “continue,”
“could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,”
“potential,” “predict,” “project,” “should,” or the negative of these terms or other
similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
The
forward-looking statements contained in this Information Statement are based on our current expectations and beliefs concerning future
developments and their potential effects on us. There can be no assurance that future developments affecting us will be those that we
have anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or
other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking
statements. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual
results may vary in material respects from those projected in these forward-looking statements. We undertake no obligation to update
or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required
under applicable securities laws.
Although
we believe that the assumptions on which these forward-looking statements are based are reasonable, any of those assumptions could prove
to be inaccurate, and as a result, the forward-looking statements based on those assumptions also could be inaccurate. In light of these
and other uncertainties, the inclusion of a projection or forward-looking statements in this Information Statement should not be regarded
as a representation by us that our plans and objectives will be achieved.
We
have based the forward-looking statements included in this Information Statement on information available to us on the date of this Information
Statement, and we assume no obligation to update any such forward-looking statements. Although we undertake no obligation to revise
or update any forward-looking statements in this Information Statement, whether as a result of new information, future events or otherwise,
you are advised to consult any additional disclosures that we may make directly to you or through reports that we may file in the future
with the SEC, including Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K.
SUMMARY
TERM SHEET
This
summary highlights selected information from this Information Statement and may not contain all of the information that is important
to you to understand the Mergers and related transactions. For a more complete description of the legal terms of the Mergers,
you should carefully read this entire Information Statement, the annexes attached to this Information Statement and the documents referred
to or incorporated by reference in this Information Statement. Any document or agreement referred to in this Information Statement is
qualified in its entirety by reference to the full text of such document or agreement. All references in this Information Statement to
terms defined in the notice to which this information statement is attached have the meanings provided in that notice. All references
to capitalized terms not defined herein or in the notice to which this Information Statement is attached have the meanings ascribed to
them in the Merger Agreement.
| |
● |
On
July 25, 2024, the Company entered into the Merger Agreement with Merger Sub I, Merger Sub II, and Scienture. |
| |
|
|
| |
● |
The
Board approved the Merger Agreement and the related transactions on July 25, 2024, and the consummation of the Mergers was not subject
to approval of the Company’s stockholders. |
| |
|
|
| |
● |
The
Majority Stockholders executed the Stockholder Consent on July 25, 2024, following the execution of the Merger Agreement. |
| |
|
|
| |
● |
Also
on July 25, 2024, the parties completed the Mergers, pursuant to which (i) Merger Sub I merged with and into Scienture, with Scienture
continuing as the surviving entity and a wholly owned subsidiary of the Company, and (ii) Scienture merged with and into Merger Sub
II, with Merger Sub II continuing as the surviving entity and Merger Sub II changed its name to “Scienture, LLC”.
|
| |
|
|
| |
● |
The
aggregate merger consideration paid in connection with the Mergers consisted of: (i) 291,555 shares of the Company’s common
stock, which represents 19.99% of the number of shares of the Company’s common stock issued and outstanding immediately prior
to the First Effective Time, and (ii) 6,826,713 shares of Series X Preferred Stock, each share of which is convertible into one share
of the Company’s common stock, subject to certain conditions. |
| |
|
|
| |
● |
The
Board considered various factors in determining whether to approve the Merger Agreement and the transactions contemplated
thereby, including the Mergers. For more information about the reasons for approving the Merger Agreement and the transactions, see
“The Transactions – Reasons for Entering into the Merger Agreement.” |
| |
|
|
| |
● |
Each
party’s obligation to complete the Mergers was subject to the satisfaction or waiver by each of the parties of various conditions,
which include, but were not limited to, the following: |
| |
● |
Scienture
having obtained its required stockholder vote; |
| |
|
|
| |
● |
the
Company having received approval for the listing of additional shares of the Company’s common stock on Nasdaq; and |
| |
|
|
| |
● |
the
Company having filed the Certificate of Designation of Preferences, Rights and Limitations of Series X Preferred Stock dated July
25, 2024 (the “Series X Certificate of Designation”) with the Secretary of State of the State of Delaware. |
| |
● |
Pursuant
to the Merger Agreement, on July 25, 2024, the Company entered into lock-up agreements with each of the directors and officers of
the Company and Scienture as well as certain of the stockholders of each of the Company and Scienture. For more information
see “The Transactions – Related Agreements – Lock-Up Agreements.” |
| |
|
|
| |
● |
Pursuant
to the Merger Agreement, on July 25, 2024, the Company entered into consulting agreements with each of Suren Ajjarapu and Prashant
Patel, the material terms of which will become effective when Mr. Ajjarapu or Mr. Patel, as applicable, are no longer employed by
the Company for any reason. For more information see “The Transactions – Related Agreements – Consulting Agreements.” |
| |
|
|
| |
● |
Separately,
in connection with the transactions, the Company intends to enter into a registration rights agreement. For more information see
“The Transactions – Registration Rights Agreement.” |
THE
PARTIES
TRxADE
Health, Inc.
We
historically focused on health services IT assets and operations aimed at digitalizing the retail pharmacy experience via an online pharmaceutical
marketplace. Our current primary operations are conducted through our wholly-owned subsidiary, Integra Pharma Solutions, LLC (“IPS”),
which is a licensed pharmaceutical wholesaler and sells brand, generic and non-drug products to customers. IPS customers include all
healthcare markets including government organizations, hospitals, clinics and independent pharmacies nationwide.
We
began operations as Trxade Group, Inc., a Nevada corporation (“Trxade Nevada”) in August of 2010 and spent over two years
creating and enhancing our web-based services. The Company changed its name on June 1, 2021, from “Trxade Group, Inc” to
“TRxADE HEALTH, INC.” Our services provided pricing transparency, purchasing capabilities and other value-added services
on a single platform focused on serving the nation’s approximately 19,397 independent pharmacies with annual purchasing power of
$67.1 billion (according to the National Community of Pharmacists Association’s 2021 Digest). Our national wholesale supply partners
and manufacturers were able to fulfill orders on our platform in real-time and provide pharmacies and wholesale suppliers with cost-saving
payment terms and next-day delivery capabilities in unrestrictive states. We expanded significantly since 2015 and served approximately
14,400+ registered members on our sales platform.
Trxade.com
previously operated the Company’s web-based pharmaceutical marketplace engaged in promoting and enabling commerce among independent
pharmacies, small chains, hospitals, clinics, and alternate dispensing sites with large pharmaceutical suppliers nationally. That marketplace
had over 60 national and regional pharmaceutical suppliers providing over 120,000 branded and generic drugs, including over-the-counter
drugs (OTCs), and drugs available for purchase by pharmacists. We served approximately 14,400+ registered members, providing access to
Trxade’s proprietary pharmaceutical database and data analytics regarding medication pricing. We generated revenue from these services
by charging a transaction fee to the seller of the products for sales conducted via the Trxade platform. The buyers did not bear the
cost of transaction fees for the purchases that they made, nor did they pay a fee to join or register with our platform. In February
2024 we divested substantially all of our assets related to our web-based pharmaceutical marketplace previously operated through TRxADE,
Inc. Substantially all of our revenues during Fiscal 2023, Fiscal 2022, and Fiscal 2021 were from platform revenue generated on www.rx.trxade.com,
product sales through Integra Pharma Solutions, LLC, and prescription sales through Community Specialty Pharmacy, LLC.
We
previously had a number of products and services focused on the US market in operation and business assets, which are described below.
Integra
Pharma Solutions, LLC. IPS is intended to serve as our logistics company for pharmaceutical distribution. We currently distribute
through our manufacturer and strategic distribution partners prescription medication, medical devices and over the counter medication
to over 1,600 pharmacies and medical clinics across 38 states.
Trxade
Prime. Trxade Prime previously allowed pharmacy members on the Trxade platform to process, consolidate and ship purchase orders that
were placed directly with Trxade suppliers via Trxade Prime. This service was provided at no cost, with the goal of offering a single
tool with one low order minimum, one invoice, one package and one delivery from multiple quality wholesalers and distributors. Revenue
had been generated from this service through our IPS subsidiary, which provided the consolidation of the orders.
Bonum
Health Application. The “Bonum Health app,” previously provided an overall healthcare experience comparable to a primary
care practitioner, and an online portal as a personal electronic medical record and scheduling system was available on a subscription
basis, primarily as a stand-alone telehealth software application that could be licensed on a business-to-business (B2B) model to clients
as an employment health benefit for the clients’ employees. Revenue was generated from this service through our Bonum subsidiary.
Bonum+
Business to Business (B2B). Bonum+ previously bundled telehealth, a COVID-19 risk assessment tool and a Personal Protective Equipment
(PPE) purchasing tool, through a secure mobile dashboard for corporate clients. The B2B platform eased pressure on employees who were
required to report any relevant health issues daily, centralizing communication and contact tracing to deliver risk scores. This allowed
employers to monitor employee COVID-19 risk profiles and streamlined the ordering of new PPE as needed. An integrated artificial intelligence
(AI) tool offered health recommendations and connects employees with board certified physicians, as needed. No revenue was generated
from this product.
SOSRx,
LLC. On February 15, 2022, the Company entered into a relationship with Exchange Health, LLC (“Exchange Health”), a technology
company providing an online platform for manufacturers and suppliers to sell and purchase pharmaceuticals. SOSRx, LLC (“SOSRx”)
was formed, which was owned 51% by the Company and 49% by Exchange Health. SOSRx did not generate material revenue and in February of
2023, the Company voluntarily withdrew from the joint venture agreement.
Superlatus.
As of December 31, 2023, Superlatus, Inc. (“Superlatus”) was a wholly owned subsidiary of the Company as a result of
a merger transaction that closed in July 2023. Superlatus is a diversified food technology company with distribution capabilities and
systems to optimize food security and population health via innovative Consumer Packaged Goods products, agritech, foodtech, plant-based
proteins and alt-protein and includes wholly-owned subsidiary, Sapientia, Inc., a food tech business. Subsequent to December 31, 2023,
the Company divested its entire interest in Superlatus.
Scienture,
Inc. (n/k/a Scienture, LLC)
Overview
Scienture
is a specialty pharmaceutical company focused on developing and commercializing products for the treatment of central nervous system
(“CNS”) and cardiovascular (“CVS”) diseases. Scienture is developing a broad range of novel product candidates
including new potential treatments for hypertension, migraine, pain and thrombosis and other related disorders
Scienture
was originally incorporated in Delaware and commenced operations in 2019.In connection with its acquisition by the Company in July 2024,
Scienture became a wholly owned subsidiary of the Company and merged with and into Merger Sub II, continuing as a limited liability company.
As described in this Information Statement, the Company will change its name to “Scienture Holdings, Inc.” immediately following
the effectiveness of the Stockholder Consent. Scienture’s principal executive offices are located in Commack, New York.
Scienture’s
Strategy
Scienture’s
mission is to improve the lives of patients suffering from CNS and CVS diseases. Scienture’s vision is to be a leader in the industry
by developing and commercializing new medicines for the treatment of CNS and CVS diseases. Key elements of Scienture’s strategy
to achieve this vision include:
| | ● | Advance product
candidates through clinical studies and toward commercialization. Scienture is in various stages of clinical development for the
product candidates in its pipeline, and it intends to move these programs efficiently toward being commercially available to patients,
subject to approval by the U. S. Food and Drug Administration (the “FDA”). Scienture is working to obtain regulatory approval
of its first product candidate, SCN-102. |
| | | |
| | ● | Drive
growth and profitability. Using dedicated sales and marketing resources in the U.S., which Scienture is in the process of building,
Scienture will seek to drive the revenue growth of its product candidates approved for marketing by the FDA. |
| | | |
| | ● | Continue
to grow pipeline. Scienture will continue to evaluate and seek to develop additional product candidates that Scienture believes have
significant commercial potential through Scienture’s internal research and development efforts. |
| | | |
| | ● | Target
strategic business development opportunities. Scienture is exploring a broad range of strategic opportunities. This may include in-licensing
products and entering into co-promotion and co-development partnerships for Scienture’s product candidates, although no agreements
have been reached. |
Research
and Development and Product Portfolio
Scienture
is committed to the development of innovative product candidates in the CNS and CVS therapeutic areas, including the following:
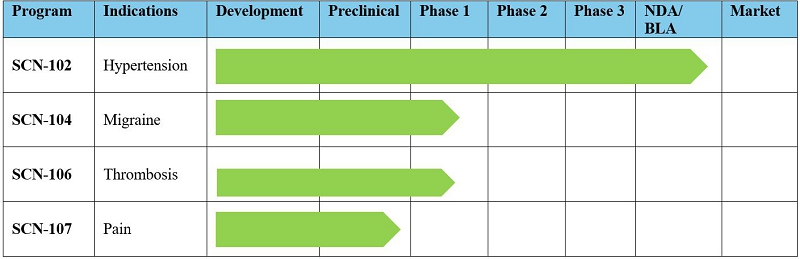
Scienture
does not have any product candidates approved for sale and has not generated any revenue from product sales. Scienture will not generate
revenue from product sales unless and until it successfully obtains regulatory approval for its product candidates. Scienture
is engaged in a variety of research and development efforts including development of a pipeline of novel product candidates for the treatment
of various disease conditions. Scienture has devoted and will continue to devote significant resources to research and development activities.
Scienture expects to incur significant expenses as Scienture continues advancing its product candidates towards FDA approval and expanding
product indications for approved products and its intellectual property portfolio. Scienture’s expectations regarding its research
and development programs are subject to risks, including the risk that Scienture’s financial condition and results of operations
for fiscal year 2024 and beyond may be materially and adversely affected by delays and failures in the completion of clinical development
of its product candidates, which could increase its costs or delay or limit our ability to generate revenues.
SCN-102
(ARBLITM - Losartan Oral Suspension)
SCN-102
is an oral liquid formulation of losartan potassium in development under the 505(b)(2) pathway, for (i) treatment of hypertension, to
lower blood pressure in adults and children greater than 6 years old, (ii) reduction of the risk of stroke in patients with hypertension
and left ventricular hypertrophy, and (iii) treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria in patients
with type 2 diabetes and a history of hypertension. Currently, there are no FDA-approved liquid formulations of losartan potassium. SCN-102
has shown close comparability to the immediate-release tablet and, if approved, would be the first FDA approved oral liquid formulation
of losartan on the market.
Scienture
submitted an Investigational New Drug (“IND”) application to the FDA in September 2022. Multiple human pharmacokinetics
studies were performed, showing close comparability with the oral solid dosage form. In October 2023, Scienture submitted a New Drug
Application (“NDA”) to the FDA. In December 2023, the FDA accepted the NDA for review and assigned a Prescription Drug
User Fee Act (“PDUFA”) target action date of August 19, 2024. During the FDA’s review, Scienture has responded to information requests related to chemistry and manufacturing controls (“CMC”), pharmacovigilance, clinical, microbiology and labeling. If Scienture receives
approval on the PDUFA target action date, Scienture anticipates commercially launching this product in the first quarter of
2025.
SCN-104
(Multi-dose Dihydroergotamine (DHE) Mesylate injection pen)
The
SCN-104 injection pen is a disposable, multiple fixed dose, single entity combination product comprised of a small molecule drug, SCN-104,
which is administered using a customized injection pen. The SCN-104 injection pen is being developed via the 505(b)(2) regulatory pathway.
The SCN-104 injection pen is in development for the acute treatment of migraine headaches with or without aura and the acute treatment
of cluster headache episodes.
As
shown in third party studies of DHE, SCN-104’s mechanism of action for its antimigraine effect is due to its potential action as
an agonist at the serotonin 5-HT1D receptors. SCN-104 is intended for subcutaneous administration. SCN-104 is also
intended for acute use and is not intended for chronic administration.
Scienture
believes the SCN-104 injection pen may offer a significant improvement, in terms of usability and patient acceptability, on the current
standard of care in the market (ampoules for injection). The intended pen delivery system was designed with patients in mind to carry
multiple doses, have a lower volume of injection, and utilize shielded needles to avoid unnecessary exposure.
Scienture
has had initial discussions with the FDA to align on a path forward for this development program. The formulation has been scaled up
to enable future commercial scale production and the pen has been optimized for commercial use. Several pharmacokinetics studies
have shown comparability between SCN-104 and the currently available marketed injection product. Scienture is initiating
manufacturing activities and planning to conduct bioequivalence studies. Scienture plans to initiate a Phase 1 single dose study in
healthy adults in 2025, following submission of an IND, if the IND is cleared by the FDA.
SCN-106
(Potential Biosimilar)
Scienture
is developing a potential biosimilar, SCN-106, based on a reference product that is a thrombolytic agent that binds to fibrin in clots
and converts entrapped plasminogen to plasmin. SCN-106 is a sterile, purified glycoprotein that is synthesized using the complementary
DNA for natural human tPA obtained from a Chinese hamster ovary cell-line.
Scienture
is working with an external partner to develop a biosimilar product that utilizes the same mechanism(s) of action for the proposed condition
of use, and has the same route of administration, dosage form, and strength as the reference product.
The
CMC development program is focused on establishing the analytical similarity of SCN-106 to the reference product. Multiple clones of
CHO cells have been produced to synthesize lots of SCN-106 which were screened for similarity to the reference product for several key
biochemical quality attributes as well as overall protein yield and finalization of a lead clone.
Scienture
completed a Biosimilar Initial Advisory meeting with the FDA in June 2023 to discuss the CMC, non-clinical, and clinical studies required
for regulatory approval.
SCN-107
(Bupivacaine Long-Acting Injection)
SCN-107
is a long acting injection suspension formulation of a non-opioid analgesic that is indicated for postsurgical local and regional analgesia.
Scienture’s long-acting
formulation, SCN-107, is a novel microsphere-based formulation of bupivacaine that comprises the drug in polymer-based microspheres and
is intended to provide pain management over a period of 5-7 days. The product candidate is designed to potentially provide longer term
post-surgical pain relief compared to the currently available products in the market.
Based
on initial discussions with FDA regarding this program, Scienture believes this product candidate would require at least one Phase 3
clinical trial to support submission of a marketing application.
Scienture
anticipates submitting an IND and, if cleared by the FDA, initiating a Phase 1 single dose study in healthy adults in 2025 to conduct
an initial assessment of safety and tolerability of SCN-107.
Sales
and Marketing
Scienture
intends to market its products through its own sales forces in the U.S. and seek strategic collaborations with other pharmaceutical
companies to commercialize its products outside of the U.S. Scienture is in the process of building a commercial sales and marketing
operation in the U.S., through a
partnership with a Contract Sales Organization, to support sales of Scienture’s products. Once
approved, this sales and marketing organization will include a combination of field teams, virtual sales representatives and
omnichannel marketing to effectively reach Health Care Providers (“HCPs”) and offer patient education. Scienture’s promotional
efforts are expected to further include developing a market access strategy to obtain commercial and government payor coverage for
its products. In addition, Scienture intends to partner with a third party logistics
provider (“3PL”) and have internal sales operations and analytics teams to provide state-of-the-art distribution capabilities to
wholesalers, pharmacies, institutional buying groups and hospitals. Scienture believes
its commercial operations infrastructure, once established, will enable it to effectively target healthcare providers to support and
grow its products subsequent to market entry.
Customers
The
majority of Scienture’s product sales, if its products are approved by the FDA, are expected to be to pharmaceutical
wholesalers, specialty pharmacies, and distributors who, in turn, would sell such products to pharmacies, hospitals, long term care
institutions and other customers, potentially including federal and state
entities.
Market
and Competition
Scienture
is engaged in segments of the pharmaceutical
industry that are highly competitive and rapidly changing. Many large pharmaceutical and biotechnology companies, academic institutions,
governmental agencies, and other public and private research organizations are commercializing or pursuing the development of products
utilizing the same molecules or compounds or for the same indications that Scienture is currently pursuing or may target in the future.
Hypertension
Hypertension
(high blood pressure) is a CVS condition, when the pressure in the blood vessels is too high (140/90 mmHg or higher). According to the
CDC, hypertension, or high blood pressure, affects nearly half of adults in the United States, or 119.9 million people. Hypertension
is defined as a systolic blood pressure of 140 mmHg or higher, and diastolic blood pressure of 90 mmHg or higher. Hypertension is a risk
factor for stroke and heart disease, which are leading causes of death in the U.S. Factors that increase the risk of having high blood
pressure include: older age, genetics, being overweight or obese, not being physically active, high-salt diet and drinking too much alcohol.
Hypertension is clinically diagnosed if, when blood pressure is measured on two different days, the systolic blood pressure readings
on both days is ≥140 mmHg and/or the diastolic blood pressure readings on both days is ≥ 90 mmHg.
The
hypertension market has increased with the commercial launch of several branded products in recent years, as well as the launch of generic
versions of branded drugs, such as Prinvil, Lotensin, Cozaar, Cardizem, Apresoline, Nitrostat and Toprol-XL. Treatment options for hypertension
in the U.S. market can be broadly classified across the following product classes, Angiotensin-converting enzyme (ACE) inhibitors, Angiotensin
II receptor blockers (ARBs), Beta-Blockers, Diuretics and Calcium Channel Blockers.
Scienture’s
product candidate SCN-102, ARBLITM (Losartan Oral Suspension 10mg/mL), is a ready to use oral suspension of losartan for increased
patient convenience and ease of dosing. Losartan is classified as an ARB for treating hypertension and is one of the highest prescribed
molecules for this indication. Current products in the market containing losartan are available only as oral solids, which can be further
compounded to a liquid formulation. Scienture believes that ARBLITM, if approved by the FDA, would be the first liquid formulation
of losartan on the market that does not require compounding and has reduced dosing volume and long term shelf life at room temperature
storage.
Migraine
Migraine
is a painful, complex neurological disorder consisting of recurring painful attacks that can significantly impact quality of life. Migraine
headaches are often characterized by throbbing pain, extreme sensitivity to light or sound, and potentially nausea and vomiting. The
World Health Organization categorizes migraine as one of the most disabling medical illnesses worldwide. The American Research Foundation
categorizes migraine as the third most prevalent illness in the world, and nearly 1 in 4 U.S. households includes someone with migraines.
Migraine is estimated to affect over 39 million individuals in the U.S.
Current
products in the market that are available to treat migraine headaches, include CGRP antagonists (calcitonin gene related peptide), which
is a class of products first introduced in 2018 (Nurtec, Ubrelvy), Botox, branded and generic versions of triptans (Imitrex, Maxalt,
Relpax), and ergot alkaloids (Ergotamine and Dihydroergotamine (DHE)).
Scienture’s
product candidate, SCN-104, is supplied in a multi-dose pen-based delivery system for self-injection and increased patient convenience.
The product candidate is in development for the acute treatment of migraine headaches with or without aura and the acute treatment of
cluster headache episodes.
Thrombotically
Occluded Catheter (CVAD) Management
Catheters,
which are a type of a Central Venous Access Device (“CVAD”), are employed to deliver life-sustaining therapies. They can be used for short-term
or long-term infusion of antibiotics, parenteral nutrition, chemotherapy, blood and blood products in patients with limited peripheral
access. More than 7 million CVADs are inserted each year in patients in the United States. Occlusion of catheters while in use can complicate
patient care by interrupting the administration of medications and solutions, delaying or disrupting therapies and leading to additional
procedures such as catheter replacement. Occlusion is the most common noninfectious complication in the long-term use of CVADs and may
occur soon after insertion of a device or develop at any time. About 58% of catheter occlusions are thrombotic, resulting from the formation
of a thrombus within, surrounding, or at the tip of the catheter.
Scienture’s
product candidate, SCN-106, is a thrombolytic agent currently in development. Scienture plans to develop SCN-106 through the
FDA’s 351(k) pathway for biosimilars.
Postoperative
Pain
Post-surgery
pain, also known as postoperative pain, is pain that a patient experiences after a surgical procedure. Pain can be caused by a number
of factors, including: the type of procedure, the size of the operation, and medications used during surgery. Chronic pain can negatively
impact a patient’s rehabilitation, quality of life, and the results of the procedure.
Current
drug product treatments available in the market for treating postoperative pain include IV and oral opioids, injectable local
anesthetics, and steroidal and non-steroidal analgesics. Marketed products include branded and generic versions of Celebrex,
Ketalar, Exparel, Lyrica, Neurontin and Astromorph.
Scienture’s
product candidate, SCN-107, is a microsphere based long-acting injection of Bupivacaine, a local anesthetic, in development for postsurgical
analgesia. SCN-107 is designed to be a non-opioid treatment regimen with rapid onset of action and analgesia that is intended to provide
coverage over a period of 5-7 days.
Manufacturing
Scienture
currently depends on third-party commercial manufacturing organizations (“CMOs”) for all manufacturing
operations, including the production of raw materials, finished dosage form product, and product packaging for both its planned commercial
scale manufacturer and the products used in its preclinical and clinical research. Scienture does not own or operate manufacturing facilities
for the production of any of its product candidates nor does Scienture have plans to develop its own manufacturing operations in the
foreseeable future to support clinical trials or commercial production. Scienture currently employs internal resources to manage its manufacturing
contractors.
Scienture
is in discussion with CMOs headquartered in North America, Europe and Asia for its pipeline product candidates. These CMOs offer a comprehensive
range of commercial contract manufacturing and packaging services.
If
Scienture fails to produce its products and product candidates in the volumes that Scienture requires on a timely basis, or fails to comply
with stringent regulations applicable to pharmaceutical drug manufacturers, Scienture may face delays in the development and commercialization
of its products and product candidates or be required to withdraw its products from the market for
risks associated with manufacturing and supply of its products and product candidates.
License Agreements
On May 26, 2020, Scienture
entered into Feasibility Study and Animal Trial Material Manufacturing Agreement with Innocore Technologies, B.V. (“Innocore”),
as amended on December 2, 2022 (the “Innocore License”), for certain intellectual property rights. Under the Innocore License,
Innocore granted Scienture a worldwide exclusive, milestone, royalty-bearing and sublicensable license to certain patent rights for the
research and development of SCN-107 in postsurgical local and regional analgesia. Pursuant to the Innocore License, Scienture is required
to make low single-digit percentage royalty payments based on annual net sales of licensed products for the first three years of sales
on a country-by-country basis, subject to a low single digit increase as of the fourth year of sales on a country-by-country basis. Scienture
is required to remunerate Innocore for the development of the licensed product, subject to a limit of $0.4 million for certain safety
and toxicity studies which will be deducted from certain development and regulatory milestones as described below. Scienture is required
to make development and regulatory milestone payments up to €2.7 million in the aggregate, commercial sale milestone payments
of up to €18.875 million in the aggregate, and maintenance fees of €0.25 million annually, subsequent to the first regulatory
filing, until the date that Scienture begins making royalty payments based on annual net sales, up to €0.5 million of which may
be credited toward the regulatory milestone payments.
Intellectual
Property
Overview
Scienture continues to build its intellectual
property portfolio to provide protection for its technologies, products, and product candidates. Scienture seeks patent protection,
where appropriate, both in the U.S. and internationally for products and product candidates.
Scienture’s intended objective is to protect its innovations and proprietary
products by, among other things, filing patent applications in the U.S. and abroad, including Europe, Canada, and other countries when
appropriate. Scienture also relies on trade secrets, know-how, proprietary knowledge, continuing technological innovation, and in-licensing
opportunities to develop and maintain its proprietary position. Scienture cannot be sure that patents will be granted with respect to
its pending patent applications or with respect to any patent applications filed by it in the future, nor can Scienture be sure that any
of its existing patents or any patents that may be granted to it in the future will be commercially useful in protecting its technology
or its products. Scienture cannot be sure that any patents, if granted, will sustain a legal challenge.
Patent
Portfolio
SCN-102
SCN-102 will soon have two orange book listable formulation composition
and method of use patents in the U.S. One of them is already issued (Patent #: 11,890,273, Issue Date: February 6, 2024, titled “Losartan
Liquid Formulations and Methods of Use”) and the other patent application is allowed, with the issue fee paid on July 24,
2024 (Appl. No. 18/421,405; Filing Date: January 24, 2024, titled “LOSARTAN LIQUID FORMULATIONS AND METHODS OF USE”). A third
application is pending (Appl. No. 18/061,819, Filing Date: December 5, 2022).
SCN-104
SCN-104 has a formulation composition and method of use application pending
in the U.S. (Appl. No. 17/757,924; Filing Date: June 23, 2022).
SCN-107
SCN-107 has a formulation composition and method of use application pending
in the U.S. (Appl. No. 17/996,995; Filing Date: October 24, 2022). Applications in Canada and Europe are currently pending.
Collaborations
and Licensing Arrangements
Scienture entered into exclusive license and commercial agreements on August
28, 2022 and April 24, 2023, with Kesin Pharma Corporation (“Kesin”), a related party, pursuant to which Scienture granted
the exclusive license rights to commercialize SCN-102 and SCN-104, respectively to Kesin for use in the United States of America (together,
the “Kesin Agreement”). In consideration of the rights granted, Scienture received milestone payments and reimbursement of
costs actually incurred related to SCN-102 and SCN-104.
On March 13, 2024, the parties terminated the Kesin Agreement by entering
a Confidential Termination Agreement (the “Kesin Termination Agreement”), and the parties agreed that Scienture would pay
Kesin a total gross amount of $1.285 million upon commercialization of either SCN-102 or SCN-104 via a royalty arrangement. The Kesin
Termination Agreement also requires that if the full $1.285 million has not been repaid within two years of the earlier of (i) commercial
launch of a product or (ii) 120 days after FDA approval of a product, then interest will accrue prospectively at a rate of 8% annually
on the unpaid balance.
In
August 2024, Kesin demanded immediate payment of the full amount under the Kesin Termination Agreement, alleging the full amount is payable
in connection with the consummation Scienture’s business combination with the Company. Scienture has disputed that the amount is
now payable, and the parties are in discussions to resolve the issue. There can be no assurance that an amicable resolution will be obtained.
If Kesin brings a legal action, Scienture will vigorously defend it.
Government
Regulation
U.S.
Drug Development Process
In
the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act (the
“FDCA”) and other federal and state statutes and regulations, govern, among other things, the research, development, testing,
manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting,
sampling, and import and export of pharmaceutical products. Scienture, along with third-party contractors, will be required to navigate
the various preclinical, clinical and commercial approval requirements of the governing regulatory authorities of the countries in which
Scienture wishes to conduct studies or seek approval of its product candidates. Failure to comply with applicable United States requirements
may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending applications, withdrawal
of an approval, warning or untitled letters, clinical holds, product recalls or withdrawals from the market, product seizures, total
or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement
of profits, civil penalties, and criminal prosecution.
FDA
approval is required before any new unapproved product or a product with certain changes to a previously approved product, including
a new use of a previously approved drug, can be marketed in the United States. The steps required to be completed by the FDA before a
drug may be marketed in the United States generally include the following:
| ● | completion
of preclinical laboratory tests, animal studies, and formulation studies performed in accordance
with the FDA’s Good Laboratory Practice (“GLP”) regulations; |
| | | |
| ● | submission
to the FDA of an IND application for human clinical testing, which
must become effective before human clinical trials may begin and must be updated annually
or when significant changes are made; |
| | | |
| ● | approval
by an independent institutional review board (“IRB”) or ethics committee at each
clinical site before the clinical trial is commenced; |
| | | |
| ● | performance
of adequate and well-controlled human clinical trials in accordance with applicable IND regulations,
good clinical practices (“GCPs”) requirements and other clinical-trial related regulations to establish the safety and
efficacy of the proposed drug for each indication; |
| | | |
| ● | preparation
and submission to the FDA of a NDA or biologics license application (“BLA”),
after completion of all pivotal clinical trials, which includes not only the results of the
clinical trials, but also, detailed information on the chemistry, manufacture and quality
controls for the product candidate and proposed labeling; |
| | | |
| ● | satisfactory
completion of an FDA Advisory Committee review, if applicable; |
| | | |
| ● | a
determination by the FDA within 60 days of its receipt of an NDA or BLA to file the application
for review; |
| | | |
| ● | satisfactory
completion of an FDA pre-approval inspection of the manufacturing facility or facilities
at which the proposed drug is produced to assess compliance with current good manufacturing practices (“GMPs”) regulations
and of selected clinical trial sites to assess compliance with GCPs; and |
| | | |
| ● | FDA
review and approval of the NDA or BLA to permit commercial marketing of the product for particular
indications for use in the United States. |
Satisfaction
of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the
type, complexity, and novelty of the product or disease.
Preclinical
and Clinical Development
Preclinical
tests include laboratory evaluation of product chemistry, formulation, and toxicity, as well as animal trials to assess the characteristics
and potential safety and efficacy of the product candidate. The conduct of the preclinical tests must comply with federal regulations
and requirements, including GLP. The results of preclinical testing are submitted to the FDA as part of an IND application along with
other information, including information about the product candidate, chemistry, manufacturing and controls, any available human data
or literature to support the use of the product candidate and a proposed clinical trial protocol. Long term preclinical tests, such as
animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
An
IND application must become effective before human clinical trials may begin. The IND application automatically becomes effective 30
days after receipt by the FDA, unless the FDA, within the 30-day period, raises safety concerns or questions relating to one or more
proposed clinical trials and places the clinical trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any
outstanding concerns or questions before the clinical trial can begin. The FDA may also impose clinical holds on a product candidate
at any time before or during clinical trials due to safety concerns, non-compliance or other issues affecting the integrity of the trial.
Accordingly, submission of an IND application may or may not result in the FDA allowing clinical trials to commence and, once begun,
issues may arise that could cause the trial to be suspended or terminated.
Clinical
trials involve the administration of the investigational drug product to human subjects under the supervision of a qualified investigator.
Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with GCP, an international standard
meant to protect the rights and health of clinical research participants and to define the roles of clinical trial sponsors, administrators,
and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety,
and the effectiveness criteria to be evaluated. Each protocol involving testing on United States patients and subsequent protocol amendments
must be submitted to the FDA as part of the IND. Furthermore, an independent IRB or ethics committee for each site proposing to conduct
the clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins
at that site, and must monitor the study until completed. An IRB is charged with protecting the welfare and rights of trial participants
and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in
relation to anticipated benefits.
Regulatory
authorities, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects
are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objects. The FDA may order the temporary,
or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial is not being conducted in accordance with FDA requirements. Further, an IRB may also require the clinical trial at the site to be halted,
either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions. Some trials
also include oversight by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring
board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain
data from the study and may recommend a clinical trial to be halted if it determines that there is an unacceptable safety risk for subjects
or other grounds, such as futility.
Clinical
trials to support an NDA or BLA for marketing approval are typically conducted in three sequential phases, but the phases may overlap
or be combined. In Phase 1 clinical trials, the investigational product is typically introduced into a limited population of healthy
human subjects or patients with the target disease or condition. These trials are designed to test the safety, dosage tolerance, pharmacokinetics
and pharmacological actions of the investigational product, to identify side effects associated with increasing doses, and, if possible,
to gain early evidence on effectiveness. Phase 2 clinical trials usually involve administering the investigational product to a limited
patient population with the specified disease or condition to evaluate the preliminarily efficacy, dosage tolerance, and optimum dosage,
and to identify possible adverse effects and safety risks. Phase 3 clinical trials are typically undertaken in a larger number of patients,
typically at geographically dispersed clinical trial sites, to provide substantial evidence of clinical efficacy and to further test
for safety in an expanded and diverse patient population. These clinical trials are intended to permit the FDA to evaluate the overall
benefit-risk relationship of the investigational product and to provide adequate information for the labeling of the product candidate.
In
reviewing an NDA or BLA, the FDA will consider all information submitted in the application, including the results of all clinical trials
conducted. In some cases, the FDA may require, or companies may voluntarily pursue, additional clinical trials after a product is approved
to gain more information about the product. These so-called Phase 4 studies may be made a condition to approval of the NDA or BLA. These
trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication and further document
clinical benefit in the case of drugs approved under accelerated approval regulations. Failure to exhibit due diligence with regard to
conducting Phase 4 clinical trials could result in the withdrawal of approval for products.
Concurrent
with clinical trials, companies may complete additional animal studies and develop additional information about the biological characteristics
of the product candidate, and must finalize a process for manufacturing the product in commercial quantities in accordance with current
GMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among
other things, must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate
packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo
unacceptable deterioration over its shelf life.
During
all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical
data, and clinical study investigators. Progress reports detailing the results of the clinical trials, among other information, must
be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and the investigators for serious
and unexpected suspected adverse events, findings from other studies suggesting a significant risk to humans exposed to the product candidate,
findings from animal or in vitro testing that suggest a significant risk for human subjects, and any clinically important increase in
the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure.
NDA
and BLA Submission and Review
Assuming
successful completion of the required clinical testing in accordance with all applicable regulatory requirements, an NDA or BLA application
which includes, among other information, the results of product development, preclinical studies and clinical trials is submitted to
the FDA. FDA approval of the application is required before marketing of the product may begin in the United States. The application
must include, among other things, the results of all trials and preclinical testing, and other testing and a compilation of data relating
to the product’s pharmacology, chemistry, manufacture, controls and proposed labeling. The cost of preparing and submitting an
NDA or BLA is substantial.
The
FDA has 60 days from its receipt of an NDA or BLA to either issue a Refuse to File Letter or accept the NDA or BLA for filing, indicating
that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth
review. The FDA has agreed to certain performance goals in the review of NDAs and BLAs. Under applications subject to the performance
goals of the PDUFA, the FDA has a goal of responding to standard review NDAs and BLAs within ten months after
it accepts the application for filing, or, if the application qualifies for priority review, six months after the FDA accepts the application
for filing, but this timeframe can be extended, such as by the submission of major amendments by applicants during the review period.
The FDA reviews an application to determine, among other things, whether the product is safe and effective and the facility in which
it is manufactured, processed, packed or held meets standards designed to assure the product’s continued safety, purity and potency.
The
FDA may refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an advisory
committee—typically a panel that includes clinicians and other experts—for review, evaluation, and a recommendation as to
whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows
such recommendations. Before approving an application, the FDA will typically inspect one or more clinical sites to assure compliance
with GCPs. Additionally, the FDA will inspect the facility or the facilities at which the proposed product is manufactured. If the FDA
determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies
in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional
information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
After
the FDA evaluates the application and conducts inspections of the manufacturing facilities where the investigational product and/or its
drug substance will be produced, it issues either an approval letter or a Complete Response Letter. An approval letter authorizes commercial
marketing of the drug with approved prescribing information for specific indications. A Complete Response Letter indicates that the review
cycle of the application is complete and the application is not ready for approval. A Complete Response Letter generally outlines the
deficiencies in the submission, except that where the FDA determines that the data supporting the application are inadequate to support
approval, the FDA may issue the Complete Response Letter without first conducting required inspections or reviewing proposed labeling.
In issuing the Complete Response Letter, the FDA may require substantial additional clinical data and/or other significant, expensive,
and time-consuming requirements related to clinical trials, preclinical studies and/or manufacturing. If a Complete Response Letter is
issued, the applicant may either resubmit the NDA or BLA, addressing all of the deficiencies identified in the letter, withdraw the application
or request a hearing. The FDA has committed to reviewing resubmissions of the NDA or BLA addressing such deficiencies in two or six months
depending on the type of information included. Even if such data are submitted, however, the FDA may ultimately decide that the NDA or
BLA does not satisfy the criteria for approval.
If
regulatory approval of a product is granted, such approval will be granted for a particular indication(s) and may include
limitations on the indicated use(s) for which such product may be marketed. Further, the FDA may require that certain
contraindications, warnings or precautions be included in the product labeling or may condition the approval of the application on
other changes to the proposed labeling, development of adequate controls and specifications, or a commitment to conduct post-market
testing or clinical trials and surveillance to monitor the effects of approved products. As a condition of NDA or BLA approval, the
FDA may require a risk evaluation and mitigation strategy (“REMS”) to help ensure that the benefits of the drug outweigh
the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure
safe use (“ETASU”). ETASU can include, but are not limited to, special training or certification for prescribing or
dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. The requirement for
REMS can materially affect the potential market and profitability of the product. Moreover, product approval may also be conditioned
on substantial post-approval testing, such as Phase 4 post-market studies, and surveillance to monitor the product’s safety or
efficacy, and the FDA may limit further marketing of the product based on the results of these post-approval studies. Once granted,
product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following
initial marketing.
Changes
to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes
or facilities, require submission and FDA approval of a new NDA or BLA, or NDA or BLA supplement before the change can be implemented.
An NDA or BLA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA
uses the same procedures and actions in reviewing NDA and BLA supplements as it does in reviewing NDAs and BLAs. As with new NDAs and
BLAs, the review process is often significantly extended by requests for additional information or clarification.
505(b)(2)
NDA Approval Process
Section
505(b)(2) of the FDCA provides an alternate regulatory pathway for the FDA to approve a new product and permits reliance for such approval
on published literature or an FDA finding of safety and effectiveness for a previously approved drug product. Specifically, section 505(b)(2)
permits the filing of an NDA where one or more of the investigations relied upon by the applicant for approval were not conducted by
or for the applicant and for which the applicant has not obtained a right of reference. Typically, 505(b)(2) applicants must perform
additional trials to support the change from the previously approved drug and to further demonstrate the new product’s safety and
effectiveness. The FDA may then approve the new product candidate for all or some of the labeled indications for which the referenced
product has been approved, as well as for any new indication sought by the section 505(b)(2) applicant.
Regulation
of Combination Products in the United States
Certain
products may be comprised of components, such as drug components and device components, that would normally be regulated under different
types of regulatory authorities, and frequently by different centers at the FDA. These products are known as combination products. Specifically,
under regulations issued by the FDA, a combination product may be:
| ● | a
product comprised of two or more regulated components that are physically, chemically, or
otherwise combined or mixed and produced as a single entity; |
| | | |
| ● | two
or more separate products packaged together in a single package or as a unit and comprised
of drug and device products, device and biological products, or biological and drug products; |
| | | |
| ● | a
drug, or device, or biological product packaged separately that according to its investigational
plan or proposed labeling is intended for use only with an approved individually specified
drug, or device, or biological product where both are required to achieve the intended use,
indication, or effect and where upon approval of the proposed product the labeling of the
approved product would need to be changed, e.g., to reflect a change in intended use, dosage
form, strength, route of administration, or significant change in dose; or |
| | | |
| ● | any
investigational drug, or device, or biological product packaged separately that according
to its proposed labeling is for use only with another individually specified investigational
drug, device, or biological product where both are required to achieve the intended use,
indication, or effect. |
Under
the FDCA and its implementing regulations, the FDA is charged with assigning a center with primary jurisdiction, or a lead center, for
review of a combination product. The designation of a lead center generally eliminates the need to receive approvals from more than one
FDA component for combination products, although it does not preclude consultations by the lead center with other components of FDA.
The determination of which center will be the lead center is based on the “primary mode of action” of the combination product.
Thus, if the primary mode of action of a drug-device combination product is attributable to the drug product, the FDA center responsible
for premarket review of the drug product would have primary jurisdiction for the combination product. The FDA has also established an
Office of Combination Products to address issues surrounding combination products and provide more certainty to the regulatory review
process. That office serves as a focal point for combination product issues for agency reviewers and industry. It is also responsible
for developing guidance and regulations to clarify the regulation of combination products, and for assignment of the FDA center that
has primary jurisdiction for review of combination products where the jurisdiction is unclear or in dispute.
A
combination product with a drug primary mode of action generally would be reviewed and approved pursuant to the drug approval processes
under the FDCA. In reviewing the NDA application for such a product, however, FDA reviewers in the drug center could consult with their
counterparts in the device center to ensure that the device component of the combination product met applicable requirements regarding
safety, effectiveness, durability and performance. In addition, under FDA regulations, combination products are subject to current GMP
requirements applicable to both drugs and devices, including the Quality System regulations applicable to medical devices.
Post-Approval
Requirements
Once
an NDA or BLA is approved, a product will be subject to pervasive and continuing regulation by the FDA including, among other things,
requirements relating to current GMPs, quality controls, record-keeping, reporting of adverse experiences, periodic reporting, product
sampling and distribution, and advertising and promotion of the product. For instance, the FDA closely regulates the post-approval marketing
and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored
scientific and educational activities and promotional activities involving the internet. Drugs may be marketed only for the approved
indications and in accordance with the provisions of the approved labeling. Failure to comply with these requirements can result in adverse
publicity, warning letters, corrective advertising, and potential civil and criminal penalties. Physicians may prescribe legally available
products for uses that are not described in the product’s labeling and that differ from those tested by Scienture and approved
by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment
for many patients in varied circumstances. The FDA does not regulate the practice of medicine by physicians or their choice of treatments.
The FDA does, however, regulate manufacturer’s communications on the subject of off-label use of their products.
In
addition, quality control, drug manufacture, packaging, and labeling procedures must continue to conform to current GMPs after approval.
Drug manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies,
and are subject to periodic unannounced inspections by the FDA, and certain state agencies for compliance with current GMPs, which impose
certain organizational, procedural and documentation requirements with respect to manufacturing and quality assurance activities. Changes
to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval
before being implemented. FDA regulations also require investigation and correction of any deviations from current GMPs and impose reporting
requirements upon Scienture and any third-party manufacturers that Scienture may decide to use. NDA or BLA holders using contract manufacturers,
laboratories or packagers are responsible for the selection and monitoring of qualified firms, and, in certain circumstances, qualified
suppliers to these firms. Drug manufacturers and other parties involved in the drug supply chain for prescription drug products must
also comply with product tracking and tracing requirements and notify the FDA of counterfeit, diverted, stolen and intentionally
adulterated products or products that are otherwise unfit for distribution in the United States. The discovery of violative conditions,
including failure to conform to current GMPs, could result in enforcement actions that interrupt the operation of any such facilities
or the ability to distribute products manufactured, processed or tested by them. Accordingly, manufacturers must continue to expend time,
money, and effort in the areas of production and quality-control to maintain compliance with current GMPs.
The
FDA may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards or is not maintained,
if problems occur following initial marketing, or if previously unrecognized problems are subsequently discovered. Later discovery of
previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes,
or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition
of post-market studies or clinical trials to assess new safety risks; or imposition of distribution restrictions or other restrictions
under a REMS program. Other potential consequences include, among other things:
| ● | restrictions
on the marketing or manufacturing of a product, complete withdrawal of the product from the
market or product recalls; |
| | | |
| ● | fines,
warning letters or holds on post-approval clinical trials; |
| | | |
| ● | refusal
of the FDA to approve pending applications or supplements to approved applications, or suspension
or revocation of existing product approvals; |
| | | |
| ● | product
seizure or detention, or refusal of the FDA to permit the import or export of products; |
| | | |
| ● | consent
decrees, corporate integrity agreements, debarment or exclusion from federal healthcare programs; |
| | | |
| ● | mandated
modification of promotional materials and labeling and the issuance of corrective information; |
| | | |
| ● | the
issuance of safety alerts, Dear Healthcare Provider letters, press releases and other communications
containing warnings or other safety information about the product; or |
| | | |
| ● | injunctions
or the imposition of civil or criminal penalties. |
U.S.
Patent Term Restoration
Depending
upon the timing, duration and specifics of the potential FDA approval of Scienture’s product candidates, some of its U.S. patents
may be eligible for limited patent term extension. The Hatch-Waxman Amendments permit a patent restoration term, often referred to as
patent term extension, of up to five years as compensation for patent term lost during product development and the FDA regulatory review
process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s
approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission
date of an NDA plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to
an approved drug is eligible for the extension and the application for the extension must be submitted prior to the expiration of the
patent. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves or denies the application for any patent
term extension or restoration.
U.S.
Marketing Exclusivity
Market
exclusivity provisions under the FDCA can also delay the submission or the approval of certain marketing applications, including 505(b)(2)
applications. The FDA provides three years of marketing exclusivity for an NDA (including a 505(b)(2) application), or supplement to
an existing NDA, if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant
are deemed by the FDA to be essential to the approval of the application. Three-year exclusivity is typically awarded to innovative changes
to a previously-approved drug product, such as new indications, dosage forms or strengths. This three-year exclusivity covers only the
modification for which the drug received approval on the basis of the new clinical investigations and does not prohibit the FDA from
approving applications for drugs that do not have the innovative change, such as generic copies of the original, unmodified drug product.
Three-year exclusivity blocks approval of 505(b)(2) applications and Abbreviated New Drug Applications (“ANDAs”), but will not delay
the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right
of reference to all of the nonclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Orphan drug exclusivity, as described above, may offer a seven-year period of marketing exclusivity, except in certain circumstances.
Pediatric exclusivity is another type of regulatory market exclusivity in the United States. Pediatric exclusivity, if granted, adds
six months to existing exclusivity periods, including exclusivity attaching to certain patent certifications. This six-month exclusivity,
which runs from the end of other exclusivity protection and patent terms, may be granted based on the voluntary completion of a pediatric
trial in accordance with an FDA-issued “Written Request” for such a trial, provided that at the time pediatric exclusivity
is granted there is not less than nine months of term remaining.
Biosimilars
and Exclusivity
The
Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the
“ACA”), which was signed into law in March 2010, included a subtitle called the Biologics Price Competition and Innovation
Act of 2009 (the “BPCIA”). The BPCIA established a regulatory scheme authorizing the FDA to approve biosimilars and interchangeable
biosimilars. A biosimilar is a biological product that is highly similar to an existing FDA-licensed “reference product.”
The FDA has issued multiple guidance documents outlining an approach to review and approval of biosimilars. Under the BPCIA, a manufacturer
may submit an application for licensure of a biologic product that is “biosimilar to” or “interchangeable with”
a previously approved biological product or “reference product.” In order for the FDA to approve a biosimilar product, it
must find that there are no clinically meaningful differences between the reference product and proposed biosimilar product in terms
of safety, purity and potency. For the FDA to approve a biosimilar product as interchangeable with a reference product, the agency must
find that the biosimilar product can be expected to produce the same clinical results as the reference product, and (for products administered
multiple times) that the biologic and the reference biologic may be switched after one has been previously administered without increasing
safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic.
Under
the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date of approval of
the reference product. The FDA may not approve a biosimilar product until 12 years from the date on which the reference product was approved.
Even if a product is considered to be a reference product eligible for exclusivity, another company could market a competing version
of that product if the FDA approves a full BLA for such product containing the sponsor’s own preclinical data and data from adequate
and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. The BPCIA also created certain exclusivity
periods for biosimilars approved as interchangeable products. Since the passage of the BPCIA, many states have passed laws or amendments
to laws, including laws governing pharmacy practices, which are state regulated, to regulate the use of biosimilars.
Orphan
Drug Designation
Under
the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition—generally
a disease or condition with either a patient population that affects fewer than 200,000 individuals in the United States or a patient
population greater than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing and
making available the drug will be recovered from sales of the drug in the United States. Orphan drug designation must be requested before
submitting an NDA or BLA. After the FDA grants orphan drug designation, the generic identity of the product and its potential orphan
use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory
review and approval process.
The
first applicant to receive FDA approval for a particular active ingredient to treat a particular disease with FDA orphan drug designation
is entitled to a seven-year exclusive marketing period in the United States for that product, for that indication. During the seven-year
exclusivity period, the FDA may not approve any other applications to market the same product for the same disease, except in limited
circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or if the FDA finds that the holder
of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet
the needs of the patients with the disease or condition for which the product was designated. Orphan drug exclusivity does not prevent
the FDA from approving a different product for the same disease or condition, or the same product for a different disease or condition.
Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA or BLA application user
fee.
A
designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which
it received orphan drug designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA
later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities
of the product to meet the needs of patients with the rare disease or condition.
Fast
Track Designation and Breakthrough Therapy Designation
The
FDA is required to facilitate the development, and expedite the review, of drugs that are intended for the treatment of a serious or
life-threatening disease or condition which demonstrate the potential to address unmet medical needs for the condition, and accordingly,
the FDA has established the fast track designation and breakthrough therapy designation programs.
A
product candidate is eligible for fast track designation if it is intended to treat a serious or life-threatening disease or condition
and demonstrates the potential to address unmet medical needs for such disease or condition. Fast track designation applies to the combination
of the product and the specific indication for which it is being studied. Under the fast track program, the sponsor of a drug candidate
may request that the FDA designate the candidate for a specific indication as a fast track product concurrent with, or after, the filing
of the IND for the candidate. The FDA must determine if the product candidate qualifies for fast track designation within 60 days of
receipt of the sponsor’s request. Fast track designation provides increased opportunities for sponsor interactions with the FDA
during preclinical and clinical development, in addition to the potential for rolling review of sections of the applicant’s NDA
or BLA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule
for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA’s time period goal
for reviewing an application does not begin until the last section of the application is submitted. Additionally, the fast track designation
may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Under
the FDA’s breakthrough therapy program, a sponsor may seek FDA designation of its product candidate as a breakthrough therapy if
the product candidate is intended, alone or in combination with one or more other drugs or biologics, to treat a serious or life-threatening
disease or condition and preliminary clinical evidence indicates that it may demonstrate substantial improvement over existing therapies
on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Breakthrough
therapy designation comes with all of the benefits of fast track designation. The FDA may take other actions appropriate to expedite
the development and review of the product candidate, including intensive guidance on an efficient product development program beginning
as early as Phase 1, and FDA organizational commitment to expedited development, including involvement of senior managers and experienced
review staff in a cross-disciplinary review, where appropriate.
Priority
Review
A
product is eligible for priority review if it has the potential to provide a significant improvement in safety or effectiveness in the
treatment, diagnosis or prevention of a serious disease or condition. A priority review means that the goal for the FDA to review an
application is six months, rather than the standard review of ten months under current PDUFA guidelines. Under the current PDUFA agreement,
these six and ten month review periods are measured from the “filing” date rather than the receipt date for NDAs for new
molecular entities, which typically adds approximately two months to the timeline for review and decision from the date of submission.
Most products that are eligible for fast track designation are also likely to be considered appropriate to receive a priority review.
Pediatric
Information
Under
the Pediatric Research Equity Act (the “PREA”), NDAs and BLAs, or supplements to NDAs and BLAs, must contain data to assess
the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and
administration for each pediatric subpopulation for which the drug is safe and effective. The FDA may grant full or partial waivers,
or deferrals, for submission of data. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for
which orphan designation has been granted.
Disclosure
of Clinical Trial Information
Sponsors
of clinical trials of FDA-regulated products, including drugs and combination products, are required to register and disclose certain
clinical trial information. Information related to the product, patient population, phase of investigation, trial sites and investigators,
and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to disclose the
results of their clinical trials after completion. Competitors may use this publicly available information to gain knowledge regarding
the progress of development programs. Disclosure of the results of these trials can be delayed until the new product or new indication
being studied has been approved. Failure to timely register a covered clinical study or to submit study results as provided for in the
law can give rise to civil monetary penalties and also prevent the non-compliant party from receiving future grant funds from the federal
government. The Final Rule on ClinicalTrials.gov registration and reporting requirements became effective in 2017, and both the National
Institutes of Health and the FDA have signaled the government’s willingness to begin enforcing those requirements against non-compliant
clinical trial sponsors.
Other
Regulatory Requirements
Health
Care Laws
Pharmaceutical
companies are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states
and foreign jurisdictions in which they conduct their business that may constrain the financial arrangements and relationships through
which Scienture researches,
as well as sell, market and distribute any products for which Scienture obtains marketing
authorization. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, and transparency
laws and regulations related to drug pricing and payments and other transfers of value made to physicians and other healthcare providers.
If Scienture’s operations are found to be in violation of any of such laws or any
other governmental regulations that apply, Scienture may be subject to penalties, including,
without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of operations,
integrity oversight and reporting obligations, exclusion from participation in federal and state healthcare programs and responsible
individuals may be subject to imprisonment. Scienture may be subject to:
| ● | The
federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from
knowingly and willfully soliciting, receiving, offering or paying any remuneration (including
any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in
kind, to induce, or in return for, the purchase, lease, order, arrangement, or recommendation
of any good, facility, item or service for which payment may be made, in whole or in part,
under a federal healthcare program, such as the Medicare and Medicaid programs. A person
or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or
specific intent to violate it to have committed a violation. Violations are subject to civil
and criminal fines and penalties for each violation, plus up to three times the remuneration
involved, imprisonment, and exclusion from government healthcare programs. In addition, the
government may assert that a claim including items or services resulting from a violation
of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes
of the federal False Claims Act or federal civil monetary penalties; |
| | | |
| ● | The
federal civil and criminal false claims laws and civil monetary penalty laws, such as the
federal False Claims Act, which impose criminal and civil penalties and authorize civil whistleblower
or qui tam actions, against individuals or entities for, among other things: knowingly presenting,
or causing to be presented, to the federal government, claims for payment that are false
or fraudulent; knowingly making, using or causing to be made or used, a false statement of
record material to a false or fraudulent claim or obligation to pay or transmit money or
property to the federal government or knowingly concealing or knowingly and improperly avoiding
or decreasing an obligation to pay money to the federal government. Manufacturers can be
held liable under the federal False Claims Act even when they do not submit claims directly
to government payors if they are deemed to “cause” the submission of false or
fraudulent claims. The federal False Claims Act also permits a private individual acting
as a “whistleblower” to bring actions on behalf of the federal government alleging
violations of the federal False Claims Act and to share in any monetary recovery; |
| | | |
| ● | The
federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”),
which created new federal criminal statutes that prohibit a person from knowingly and willfully
executing, or attempting to execute, a scheme to defraud any healthcare benefit program or
obtain, by means of false or fraudulent pretenses, representations or promises, any of the
money or property owned by, or under the custody or control of, any healthcare benefit program,
regardless of the payor (e.g., public or private) and knowingly and willfully falsifying,
concealing or covering up by any trick or device a material fact or making any materially
false, fictitious, or fraudulent statements or representations in connection with the delivery
of, or payment for, healthcare benefits, items or services relating to healthcare matters;
similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual
knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| | | |
| ● | HIPAA,
as amended by the Health Information Technology for Economic and Clinical Health Act of 2009
(“HITECH”) and their respective implementing regulations, including the Final
Omnibus Rule published in January 2013, which impose requirements on certain covered healthcare
providers, health plans, and healthcare clearinghouses as well as their respective business
associates, independent contractors or agents of covered entities, that perform services
for them that involve the creation, maintenance, receipt, use, or disclosure of, individually
identifiable health information relating to the privacy, security and transmission of individually
identifiable health information. HITECH also created new tiers of civil monetary penalties,
amended HIPAA to make civil and criminal penalties directly applicable to business associates,
and gave state attorneys general new authority to file civil actions for damages or injunctions
in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs
associated with pursuing federal civil actions. In addition, there may be additional
federal, state and non-U.S. laws which govern the privacy and security of health and other
personal information in certain circumstances, many of which differ from each other in significant
ways and may not have the same effect, thus complicating compliance efforts; |
| | | |
| ● | The
U.S. federal transparency requirements under the ACA, including the provision commonly referred to as the Physician Payments
Sunshine Act, and its implementing regulations, which requires applicable manufacturers of drugs, devices, biologics and medical
supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program to report annually
to the Centers for Medicare & Medicaid Services (“CMS”), information related to payments or other transfers of value made to
physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other licensed health care
practitioners and teaching hospitals, as well as ownership and investment interests held by the physicians described above and their
immediate family members; |
| | | |
| ● | Federal
price reporting laws, which require manufacturers to calculate and report complex pricing
metrics to government programs, where such reported prices may be used in the calculation
of reimbursement and/or discounts on approved products; and |
| | | |
| ● | Federal
consumer protection and unfair competition laws, which broadly regulate marketplace activities
and activities that potentially harm consumers. |
Additionally,
Scienture is subject to state and foreign equivalents of each of the healthcare laws and regulations described above, among others,
some of which may be broader in scope and may apply regardless of the payor. Many U.S. states have adopted laws similar to the
federal Anti-Kickback Statute and False Claims Act, and may apply to Scienture’s business practices, including, but not
limited to, research, distribution, sales or marketing arrangements and claims involving healthcare items or services reimbursed by
non-governmental payors, including private insurers. In addition, some states have passed laws that require pharmaceutical companies
to comply with the April 2003 Office of Inspector General Compliance Program Guidance for Pharmaceutical Manufacturers and/or the
Pharmaceutical Research and Manufacturers of America’s Code on Interactions with Healthcare Professionals. Several states also
impose other marketing restrictions or require pharmaceutical companies to make marketing or price disclosures to the state and
require the registration of pharmaceutical sales representatives. There are ambiguities as to what is required to comply with these
state requirements and if Scienture fails to comply with an applicable state law requirement Scienture could be subject to
penalties. State and foreign laws, including for example the European Union General Data Protection Regulation, which became
effective in May 2018, also govern the privacy and security of health information in some circumstances, many of which differ from
each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. There are ambiguities as
to what is required to comply with these state requirements and if Scienture fails to comply with an applicable state law
requirement Scienture could be subject to penalties.
The
scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform.
Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare
providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry.
Ensuring
that Scienture’s internal operations and future business arrangements with third parties comply with applicable healthcare laws
and regulations will involve substantial costs. It is possible that governmental authorities will conclude that Scienture’s business
practices do not comply with current or future statutes, regulations, agency guidance or case law involving applicable fraud and abuse
or other healthcare laws and regulations. If Scienture’s operations are found to be in violation of any of the laws described above
or any other governmental laws and regulations that may apply to us, Scienture may be subject to significant penalties, including administrative,
civil and criminal penalties, damages, fines, disgorgement, the exclusion from participation in federal and state healthcare programs,
individual imprisonment, reputational harm, and the curtailment or restructuring of Scienture’s operations, as well as additional
reporting obligations and oversight if Scienture becomes subject to a corporate integrity agreement or other agreement to resolve allegations
of non-compliance with these laws. Further, defending against any such actions can be costly and time consuming, and may require significant
financial and personnel resources. Therefore, even if Scienture is successful in defending against any such actions that may be brought
against it, its business may be impaired. If any of the physicians or other providers or entities with whom Scienture expects to do business
are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including
exclusions from government funded healthcare programs and imprisonment. If any of the above occur, Scienture’s ability to operate
its business and its results of operations could be adversely affected.
Healthcare
Reform
Payors,
whether domestic or foreign, or governmental or private, are developing increasingly sophisticated methods of controlling healthcare
costs and those methods are not always specifically adapted for new technologies such as gene therapy and therapies addressing rare diseases
such as those Scienture is
developing. In both the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory changes
to the health care system that could impact Scienture’s ability to sell its products
profitably. In particular, in 2010, the ACA was enacted, which, among other things, subjected biologic products to potential competition
by lower-cost biosimilars; increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program;
extended the Medicaid Drug Rebate program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations;
subjected manufacturers to new annual fees and taxes for certain branded prescription drugs; created a Medicare Part D coverage gap discount
program, in which manufacturers must agree to offer 70% point-of-sale discounts off negotiated prices of applicable brand drugs
to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered
under Medicare Part D; and provided incentives to programs that increase the federal government’s comparative effectiveness research.
In
addition, other legislative and regulatory changes have been proposed and adopted in the United States since the ACA was enacted.
| ● | The
Budget Control Act of 2011 and subsequent legislation, among other things, created
measures for spending reductions by Congress that include aggregate reductions of Medicare
payments to providers of 2% per fiscal year, which remain in effect through 2031. Due to
the Statutory Pay-As-You-Go Act of 2010, estimated budget deficit increases resulting from
the American Rescue Plan Act of 2021, and subsequent legislation, Medicare payments to providers
will be further reduced starting in 2025 absent further legislation. The U.S.
American Taxpayer Relief Act of 2012 further reduced Medicare payments to several types
of providers and increased the statute of limitations period for the government to recover
overpayments to providers from three to five years. |
| | | |
| ● | On
April 13, 2017, CMS published a final rule that gives states greater flexibility in setting
benchmarks for insurers in the individual and small group marketplaces, which may have the
effect of relaxing the essential health benefits required under the ACA for plans sold through
such marketplaces. |
| | | |
| ● | On
May 30, 2018, the Right to Try Act, was signed into law. The law, among other things, provides
a federal framework for certain patients to access certain investigational new drug products
that have completed a Phase 1 clinical trial and that are undergoing investigation for FDA
approval. Under certain circumstances, eligible patients can seek treatment without enrolling
in clinical trials and without obtaining FDA permission under the FDA expanded access program.
There is no obligation for a pharmaceutical manufacturer to make its drug products available
to eligible patients as a result of the Right to Try Act. |
| | | |
| ● | On May
23, 2019, CMS published a final rule to allow Medicare Advantage Plans the option of using
step therapy for Part B drugs. |
Additionally,
there has been increasing legislative and enforcement interest in the United States with respect to drug pricing practices. Specifically,
there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which
has resulted in several U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other
things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, and review the relationship between
pricing and manufacturer patient programs.
In
August 2022, the Inflation Reduction Act of 2022 (the “IRA”), was signed into law. The IRA includes several provisions that
may impact Scienture’s business, depending on how various aspects of the IRA are implemented. Provisions that may impact Scienture’s
business include a $2,000 out-of-pocket cap for Medicare Part D beneficiaries, the imposition of new manufacturer financial liability
on most drugs in Medicare Part D, permitting the U.S. government to negotiate Medicare Part B and Part D pricing for certain high-cost
drugs and biologics without generic or biosimilar competition, requiring companies to pay rebates to Medicare for drug prices that increase
faster than inflation, and delay until January 1, 2032 the implementation of the U.S. Department of Health and Human
Services (“HHS”) rebate rule that would have limited the fees
that pharmacy benefit managers can charge. Further, under the IRA, orphan drugs are exempted from the Medicare drug price negotiation
program, but only if they have one orphan designation and for which the only approved indication is for that disease or condition.
If a product receives multiple orphan designations or has multiple approved indications, it may not qualify for the orphan drug exemption.
The implementation of the IRA is currently subject to ongoing litigation challenging the constitutionality of the IRA’s Medicare
drug price negotiation program. The effects of the IRA on Scienture’s
business and the healthcare industry in general is not yet known.
President
Biden has issued multiple executive orders that have sought to reduce prescription drug costs. In February 2023, HHS also issued
a proposal in response to an October 2022 executive order from President Biden that includes a proposed prescription drug pricing model
that will test whether targeted Medicare payment adjustments will sufficiently incentivize manufacturers to complete confirmatory trials
for drugs approved through FDA’s accelerated approval pathway. Although a number of these and other proposed measures may require
authorization through additional legislation to become effective, and the Biden administration may reverse or otherwise change these
measures, both the Biden administration and Congress have indicated that they will continue to seek new legislative measures to control
drug costs.
Scienture
expects that additional U.S. federal healthcare reform measures will be adopted in the future,
any of which could limit the amounts that the U.S. Federal Government will pay for healthcare drugs and services, which could result
in reduced demand for Scienture’s drug candidates or additional pricing pressures.
Individual
states in the United States have also become increasingly active in passing legislation and implementing regulations designed to control
pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain
drug access and marketing cost disclosure and transparency measures, and designed to encourage importation from other countries and bulk
purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm Scienture’s
business, financial condition, results of operations and prospects. In addition, regional healthcare authorities and individual hospitals
are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription
drug and other healthcare programs. This could reduce the ultimate demand for Scienture’s drugs or put pressure on its drug pricing,
which could negatively affect its business, financial condition, results of operations and prospects.
Pharmaceutical
Coverage, Pricing, and Reimbursement
The
success of Scienture’s product candidates, if approved, depends on the availability of coverage and adequate reimbursement from
third-party payors. Scienture cannot be sure that coverage and reimbursement will be available for, or accurately estimate the potential
revenue from, its product candidates or assure that coverage and reimbursement will be available for any product that it may develop.
Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the
costs associated with their treatment. Coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and
Medicaid, and commercial payors is critical to new product acceptance.
Government
authorities and other third-party payors, such as private health insurers and health maintenance organizations, decide which drugs and
treatments they will cover and the amount of reimbursement. Coverage and reimbursement by a third-party payor may depend upon a number
of factors, including the third-party payor’s determination that use of a product is:
| ● | a
covered benefit under its health plan; |
| | | |
| ● | safe,
effective and medically necessary; |
| | | |
| ● | appropriate
for the specific patient; |
| | | |
| ● | cost-effective;
and |
| | | |
| ● | neither
experimental nor investigational. |
In
the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors. As a result, obtaining
coverage and reimbursement approval of a product from a government or other third-party payor is a time-consuming and costly process
that could require Scienture to provide to each payor supporting scientific, clinical and cost-effectiveness data for the use of Scienture’s
products on a payor-by-payor basis, with no assurance that coverage and adequate reimbursement will be obtained. In the United States,
the principal decisions about reimbursement for new medicines are typically made by CMS. CMS decides whether and to what extent a new
medicine will be covered and reimbursed under Medicare and private payors tend to follow CMS to a substantial degree. Even if Scienture
obtains coverage for a given product, the resulting reimbursement payment rates might not be adequate for Scienture to achieve or sustain
profitability or may require co-payments that patients find unacceptably high. Additionally, third-party payors may not cover, or provide
adequate reimbursement for, long-term follow-up evaluations required following the use of product candidates, once approved. Patients
are unlikely to use Scienture’s product candidates, once approved, unless coverage is provided and reimbursement is adequate to
cover a significant portion of their cost. There is significant uncertainty related to insurance coverage and reimbursement of newly
approved products. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement
for Scienture’s product candidates.
Net
prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by
any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in
the United States. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from
list prices and are challenging the prices charged for medical products. Scienture cannot be sure that reimbursement will be available
for any product candidate that it commercializes and, if reimbursement is available, the level of reimbursement. In addition, many pharmaceutical
manufacturers must calculate and report certain price reporting metrics to the government, such as average sales price and best price.
Penalties may apply in some cases when such metrics are not submitted accurately and timely. Further, these prices for drugs may be reduced
by mandatory discounts or rebates required by government healthcare programs. Payment methodologies may be subject to changes in healthcare
legislation and regulatory initiatives.
Scienture
expects that healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria and in additional
downward pressure on the price that Scienture receives for any approved product. The implementation of cost containment measures or other
healthcare reforms may prevent Scienture from being able to generate revenue, attain profitability, or commercialize Scienture’s
products. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional
activities for pharmaceutical products. Scienture cannot be sure whether additional legislative changes will be enacted, or whether existing
regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals or clearances
of Scienture’s product candidates, if any, may be.
In
addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements
governing drug pricing vary widely from country to country. For example, the European Union provides options for its Member States to
restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices
of medicinal products for human use. To obtain reimbursement or pricing approval, some of these countries may require the completion
of clinical trials that compare the cost effectiveness of a particular product candidate to currently available therapies. A Member State
may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability
of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement
limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of Scienture’s product
candidates. Historically, products launched in the European Union do not follow price structures of the United States and generally prices
tend to be significantly lower.
Environmental
Matters
Scienture’s
operations and those of its third-party manufacturers and suppliers are subject to national, state and local environmental laws. Scienture
has made, and intends to continue to make, expenditures and undertake efforts to comply with applicable laws. Scienture believes the safety
procedures utilized by it for the handling and disposing hazardous materials comply with the standards prescribed by applicable laws
and regulations.
Human
Capital
Scienture’s
success begins and ends with our people. Scienture’s solid progress to date reflects the talent and hard work of all of its employees.
Scienture considers the intellectual capital of its employees to be an essential driver of its business and key to its future prospects.
Attracting, developing, and retaining talented people in technical, marketing, sales, research, and other positions is crucial to executing
its strategy and its ability to compete effectively.
Talent
Acquisition, Retention and Development
Scienture’s
key human capital objectives are to attract, retain and develop the highest quality talent. Scienture
employs various human resource programs in support of these objectives. Scienture’s
ability to recruit and retain such talent depends on a number of factors, including compensation
and benefits, talent development and career opportunities, and the work environment.
Scienture
attracts and rewards its employees by providing market competitive compensation and benefit packages,
including incentives and recognition plans that extend to all levels in its organization.
To that end, Scienture offers a comprehensive total rewards program aimed at health, home-life,
and financial needs of its employees. Scienture’s total rewards package includes market-competitive
pay, broad-based stock grants, bonuses, healthcare benefits, retirement savings plans, paid time off and family leave, an Employee Assistance
Program, and mental health services.
Scienture
is committed to the safety, health, and security of its employees. Scienture believes
a hazard-free environment is critical for the success of its business. Throughout Scienture’s operations,
Scienture strives to ensure that all its employees have access to safe workplaces that allow
them to succeed in their jobs. Scienture’s experience and continuing focus on workplace
safety has enabled it to preserve business continuity without sacrificing its commitment to keeping its colleagues and workplace visitors
safe.
Inclusion
and Diversity
Scienture
places a strong value on collaboration, inclusion, and diversity, and believes that working together
leads to better outcomes for its customers. This extends to the way Scienture employees treat
each other as team members. Scienture strives to create an environment where innovative
ideas can flourish by demonstrating respect for each other and valuing the diverse opinions, backgrounds, and viewpoints of employees.
Scienture believes a diverse and inclusive workplace results in business growth and encourages
increased innovation, retention of talent, and a more engaged workforce.
Facilities
Scienture’s
corporate headquarters is located at 20 Austin Blvd, Commack, NY 11725, which is 2,000 square feet of office space with a lease termination
date of July 31, 2026. Scienture believe its facilities are sufficient to meet its current needs for the foreseeable future.
Legal
Proceedings
From
time to time, Scienture may be involved in various claims and legal proceedings. Scienture is not currently a party to any material legal
proceedings.
THE
TRANSACTIONS
The
Merger Agreement
As
described above, on July 25, 2024, the Company, entered into and closed the Merger Agreement with Merger Sub I, Merger Sub II, and Scienture,
pursuant to which the parties consummated the Mergers. In connection with the transactions, Merger Sub II, as the surviving entity of
the Mergers, changed its name from “MEDS Merger Sub II, LLC” to “Scienture, LLC”. Following the Preferred Stock
Conversion, the Company will change its name from “TRxADE HEALTH, INC.” to “Scienture Holdings, Inc.” The Board
approved the Merger Agreement and the related transactions, and the consummation of the Mergers was not subject to approval of the Company’s
stockholders.
Merger
Consideration
As
consideration for the Mergers, at the First Effective Time, the shares of Scienture’s common stock issued and outstanding immediately
prior to the First Effective Time were converted into the right to receive, in the aggregate, (i) 291,555 shares of the Company’s
common stock, which represents 19.99% of the number of shares of the Company’s common stock issued and outstanding immediately
prior to the First Effective Time, and (ii) 6,826,713 shares of the Company’s Series X Preferred Stock, each share of which is
convertible into one share of the Company’s common stock, subject to certain conditions.
Stockholder
Approvals
Pursuant
to the Merger Agreement, on July 25, 2024, following the execution of the Merger Agreement, the Majority Stockholders entered
into the Stockholder Consent, which approved (i) the Preferred Stock Conversion, (ii) the Name Change, and (iii) the Incentive Plan Share
Increase. In addition, the Company agreed to, as promptly as practicable after the closing, prepare and file with the Securities and
Exchange Commission (the “SEC”) this Information Statement relating to the Stockholder Approval Matters. The Stockholder
Consent will be deemed effective on the 20th calendar day following the mailing of this Information Statement to the Company’s
stockholders of record as of the Record Date. The parties agreed that immediately following the effectiveness of the Stockholder
Consent (a) the Preferred Stock Conversion will occur, (b) the Name Change will occur, and (c) the Incentive Plan Share Increase will
become effective.
Representations
and Warranties
The
Merger Agreement contains a number of representations and warranties made by each of the Company and Scienture as of the date of the
Merger Agreement or other specified dates. Certain of the representations and warranties are qualified by materiality and/or information
provided in the disclosure schedules to the Merger Agreement.
No
Survival
The
representations and warranties of the parties contained in the Merger Agreement terminated at the First Effective Time, and only
the covenants or agreements that by their terms survive the First Effective Time, or those covenants or agreements to be performed in
whole or in part after the First Effective Time, survived the First Effective Time.
Covenants
of the Parties
Each
of the Company and Scienture agreed to use its commercially reasonable efforts to consummate the transactions contemplated by the Merger
Agreement and the Mergers were completed on July 25, 2024. The Merger Agreement also contains certain customary covenants by each of
the parties.
The
parties agreed that the post-closing board of directors will consist of seven directors, comprised of the Company’s five continuing
directors and two directors designated by Scienture. The parties further agreed that the individuals serving as the chief executive officer
and chief financial officer of the Company immediately after the closing would be the same individuals as that of the Company immediately
prior to the closing.
Closing
Conditions
The
Merger Agreement contains customary conditions to closing, including the following mutual conditions of the parties: (i) no temporary
restraining order, preliminary or permanent injunction or other order preventing the consummation of the transactions contemplated by
the Merger Agreement shall have been issued by any court of competent jurisdiction or other governmental authority of competent jurisdiction
and remain in effect and there shall not be any law which has the effect of making the consummation of the transactions contemplated
by the Merger Agreement illegal; (ii) Scienture shall have obtained its required stockholder vote; (iii) the Company shall have received
approval for the listing of additional shares of the Company’s common stock on Nasdaq; (iv) the Company shall have satisfied
its transaction expenses and net cash requirement as set forth in the Merger Agreement; (v) the Company shall have filed the Series X
Certificate of Designation with the Secretary of State of the State of Delaware; and (vi) as of the Closing, the Company shall have entered
into an exchange agent agreement pertaining to the exchange of shares of Scienture’s common stock for shares of the Company’s
common stock and Series X Preferred Stock as contemplated by the Merger Agreement.
Related
Agreements
Lock-Up
Agreements
Pursuant
to the Merger Agreement, on July 25, 2024, the Company entered into lock-up agreements (the “Lock-Up Agreements”) with each
of the directors and officers of the Company and Scienture as well as certain of the shareholders of each of the Company and Scienture
(each, a “Locked-Up Party”) with respect to all of the Company’s securities held by such Locked-Up Parties (the “Lock-Up
Securities”) immediately following the closing. Pursuant to the Lock-Up Agreements, each Locked-Up Party agreed not to transfer
any Lock-Up Securities during the period commencing on the date of the Preferred Stock Conversion and ending on the earliest of (x) one
hundred eighty (180) days after the date of the Preferred Stock Conversion or (y) the date after the closing on which the Company
completes a liquidation, merger, stock exchange, or other similar transaction with an unaffiliated third party resulting in all of its
stockholders having the right to exchange their Lock-Up Securities for cash, securities, or other property.
Consulting
Agreements
Pursuant
to the Merger Agreement, on July 25, 2024, the Company entered into consulting agreements with each of Suren Ajjarapu and Prashant Patel
(each a “Consulting Agreement” and collectively the “Consulting Agreements”), the material terms of which will
become effective upon Mr. Ajjarapu or Mr. Patel, as applicable, are no longer employed by the Company for any reason. Each Consulting
Agreement will enable the Company to continue to receive critical support and management-related services from Mr. Ajjarapu and Mr. Patel
for up to a period of two years after either Mr. Ajjarapu or Mr. Patel, as applicable, is no longer employed by the Company. Specifically,
the Consulting Agreements state that the duties of Mr. Ajjarapu and Mr. Patel may include, but not necessarily be limited to (i) assisting
with the development of the Company’s corporate strategies, organizational design, research and development, product commercialization,
and such matters otherwise requested by Company officers; (ii) assisting with the ideation and analysis of financial structuring and
accounting approaches and alternatives the Company should consider and can implement in the course of raising money, financing and funding
its operations and initiatives, and optimizing its cost efficiencies and effectiveness; (iii) assisting with the creation and dissemination
of corporate and financial information regarding the Company to the investment and financial community and public at large as requested
by the Company through its authorized personnel, pursuant to applicable company policies; and (iv) other such consultation the Company’s
officers deem useful to the Company’s management and within the scope of their expertise.
As
consideration for Mr. Ajjarapu providing services under his Consulting Agreement, the Company has agreed (i) to reimburse Mr. Ajjarapu
for reasonable and necessary costs and expenses associated with Mr. Ajjarapu’s services to the Company, including travel costs,
research expenses, copy and production charges, and courier fees, as substantiated by statements submitted to and approved by the Company
and (ii) to issue Mr. Ajjarapu 702,086 shares of the Company’s common stock (subject to certain equitable adjustments) in eight
installments beginning on the date that Mr. Ajjarapu’s employment with the Company terminates for any reason. On such date, that
certain Executive Employment Agreement, dated April 14, 2020, as amended on May 5, 2020, August 29, 2022 and January 17, 2023, referenced
on the Current Reports on Form 8-K filed on April 16, 2020, May 7, 2020, September 1, 2020, and January 20, 2023, will terminate. In
the event that Mr. Ajjarapu terminates his Consulting Agreement or the Company terminates his Consulting Agreement for cause, then the
Company will owe no further compensation to him.
As
consideration for Mr. Patel providing services under his Consulting Agreement, the Company has agreed (i) to reimburse Mr. Patel for
reasonable and necessary costs and expenses associated with Mr. Patel’s services to the Company, including travel costs, research
expenses, copy and production charges, and courier fees, as substantiated by statements submitted to and approved by the Company and
(ii) to issue Mr. Patel 614,325 shares of the Company’s common stock (subject to certain equitable adjustments) in eight installments
beginning on the date that Mr. Patel’s employment with the Company terminates for any reason. On such date, that certain Executive
Employment Agreement, dated May 24, 2013, as amended on August 29, 2022 and January 17, 2023, referenced in the Current Reports on Form
8-K filed on July 24, 2014, September 1, 2020, and January 20, 2023, will terminate. In the event that Mr. Patel terminates his Consulting
Agreement or the Company terminates his Consulting Agreement for cause, then the Company will owe no further compensation to him.
Registration
Rights Agreement
Separately,
the Company intends to enter into a Registration Rights Agreement (the “Registration Rights Agreement”), which contemplates
that the Company will prepare and file a resale registration statement with the SEC upon receiving a request from the Initiating Holders
(as defined in the Registration Rights Agreement) in compliance with the terms of the Registration Rights Agreement. The Company anticipates
that it will agree to use commercially reasonable efforts to cause this resale registration statement to become effective.
Under
the Registration Rights Agreement, the Company will agree to, among other things, indemnify the selling Holder (as defined in the Registration
Rights Agreement), and the partners, members, directors, officers and stockholders of each such Holder; legal counsel and accountants
for each such Holder; any underwriter (as defined in the Securities Act) for each such Holder; and each individual, corporation, partnership,
trust, limited liability company, association or other entity, if any, who controls such Holder or underwriter within the meaning of
the Securities Act or the Exchange Act against any Damages (as defined in the Registration Rights Agreement).
Past
Contacts, Transactions, or Negotiations
In
the past two years there have been no negotiations, transactions or material contracts between the Company and Scienture, except for
negotiations related to the Mergers, as discussed in this Information Statement. Aside from the Merger Agreement and mutual nondisclosure
agreement described in this Information Statement, there are no present or proposed material agreements, arrangement, understandings
or relationships between the Company (or any of its executive officers, directors, controlling persons or subsidiaries) and Scienture
(or any of its executive officers, directors, controlling persons or subsidiaries).
Background
of the Merger Agreement
The
Company is listed on Nasdaq and operates its pharmaceutical distribution business through its IPS subsidiary. In 2023, the Board decided
to look for complimentary businesses to merge with in similar industries, which could provide growth and support the Company’s
existing business operations. Prior to entering into the Merger Agreement with Scienture, the Company conducted a thorough search for
potential target companies, utilizing the network and investing and operating experience of our management team and Board. The terms
of the Merger Agreement with Scienture were the result of thorough negotiations between the representatives of the Company and Scienture,
based on diligence efforts of management, with the support of advisors, as further described below.
Through
the date of the execution of the Merger Agreement, the Company’s management and Board evaluated and considered a number of potential
target companies as candidates for a possible transaction. Representatives of the Company contacted and were contacted by a number of
individuals and entities who offered to present potential acquisition opportunities to the Company across a wide array of industries.
The Company compiled a list of high priority potential targets and updated and supplemented such list from time to time. The Company
made decisions on how to prioritize targets according to size, profitability, cash requirements, readiness, and willingness of the target
to move quickly.
During
that period, the Company and its representatives identified and evaluated potential acquisition target companies and participated in
in-person or telephonic discussions with representatives of potential acquisition targets. The Company reviewed the potential acquisition
opportunities based on criteria that were the same or similar to the criteria that the Board used in evaluating the transaction with
Scienture, which included, among other criteria, the cash requirements at closing, the readiness and willingness of potential target
companies to become public, the markets in which potential target companies operate and their competitive positions and “track
records” within such markets, the experience of the potential target companies’ management teams and the potential for revenue
and earnings growth and strong free cash flow generation.
In
March 2024, the Company and Scienture commenced high level discussions for the possible acquisition of Scienture by the Company. Scienture
was introduced to the Company’s management on or around March 13, 2024. On March 26, 2024, the parties entered into a mutual
nondisclosure agreement and Scienture sent a letter of intent to the Company outlining terms of a potential transaction. The Board
met on April 8, 2024 to discuss, among other topics, the Scienture letter of intent and also evaluated other potential merger targets.
The parties continued negotiations until early April 2024 but then negotiations broke off and each explored other options. The Company
considered other merger targets.
The
Board met again on May 13, 2024 to discuss merger target options and instructed management to reengage with Scienture. The parties
reengaged with negotiations in late May 2024, the Board met again on May 28, 2024 to approve the Scienture letter of intent, and
on June 6, 2024, the parties executed a letter of intent. The Company commenced legal, financial and operational due diligence
review of Scienture immediately after execution of the letter of intent. The parties held weekly (or sometimes more frequent) calls with
the working group including the companies, lawyers, auditors and/or accountants. The Company engaged outside accounting professionals
to preform analysis on the proposed transaction structure and ultimately produced a memorandum supporting the determination that the
Company would be the accounting acquirer if the transaction were consummated.
In
late June 2024, the Company circulated an initial draft of the Merger Agreement to Scienture. The Board met on July 8,
2024 to discuss the Merger Agreement. Based on that meeting the management of the Company was instructed by the Board to
proceed with the transaction.
The
Company’s legal counsel contacted the Company’s Nasdaq representative on July 10, 2024, and submitted its Listing
of Additional Shares Notification Form on the same day. On July 12, 2024, the Company’s legal counsel spoke with the Company’s
Nasdaq representative and, on July 17, 2024, received Nasdaq’s written confirmation that the transaction structure and additional
shares notification met with Nasdaq’s requirements.
Throughout
the month of July the parties completed their respective due diligence reviews and finalized the Merger Agreement, culminating
in the simultaneous signing and closing of the Merger Agreement on July 25, 2024.
On
July 25, 2024, the Board approved the Merger Agreement, the Mergers, the issuance of the Series X Preferred Stock, the Preferred Stock
Conversion and the other transactions contemplated in the Merger Agreement by unanimous written consent, with Suren Ajjarapu and Prashant
Patel abstaining from all matters related to their respective Consulting Agreements described in this Information Statement.
Reasons
for Entering into the Merger Agreement
In
the course of its evaluation of the transactions, the Merger Agreement and related agreements, the Board held numerous meetings,
consulted with the Company’s management, legal counsel, and financial advisors and reviewed a significant amount of information
and, in reaching its decision to approve the transactions and the Merger Agreement, the Board considered a number of factors,
including, among others, the following factors:
| |
● |
the
Board believes that, as a result of arm’s length negotiations with Scienture, the Company negotiated a favorable equity
split for the Company’s stockholders, and that the terms of the Merger Agreement include the most favorable terms to the Company
in the aggregate that were mutually agreeable to Scienture; |
| |
|
|
| |
● |
the
Board believes, after a thorough review of strategic alternatives and discussions with the Company’s senior management,
advisors and legal counsel, that the transactions are more favorable to the Company’s stockholders than the potential value
that might have resulted from other strategic options available to the Company; |
| |
|
|
| |
● |
the
Board believes, based in part on a scientific and business diligence and analysis process conducted by the Company’s
management and reviewed with the Board, that Scienture’s assets and programs represent a sizeable potential market opportunity,
and may thereby create value for the stockholders of the combined organization and an opportunity for the Company’s stockholders
to participate in the potential growth of the combined company; and |
| |
|
|
| |
● |
the
Board also considered the possibility that the combined company would be able to take advantage of the potential benefits
resulting from the combination of the technological platforms and infrastructure of both the Company and Scienture. |
The
Board also reviewed various reasons impacting the financial condition, results of operations and prospects of the Company, including:
| |
● |
the
Board having undertaken a comprehensive and thorough process of reviewing and analyzing the potential merger transaction to
identify the opportunity that would, in the board of directors’ opinion, create the most value for the Company’s
stockholders; |
| |
|
|
| |
● |
the
Board’s belief that, as a result of arm’s length negotiations with Scienture, the Company negotiated a favorable
equity split for the Company’s stockholders, and that the terms of the Merger Agreement include the most favorable terms to
the Company in the aggregate that were mutually agreeable to Scienture; and |
| |
|
|
| |
● |
the
Board’s belief, after a thorough review of strategic alternatives and the Company’s discussions with its financial
advisors and legal counsel, as well as the Company’s management’s discussions with Scienture’s senior management,
that, compared to the transactions, no alternatives or other strategic options that may have been available to the Company were reasonably
likely to create greater value for the Company’s stockholders. |
The
foregoing information and factors considered by the Board are not intended to be exhaustive but are believed to include all of
the material factors considered by the Board. In view of the wide variety of reasons considered in connection with its evaluation
of the transactions and the complexity of these matters, the Board did not find it useful to attempt, and did not attempt, to
quantify, rank or otherwise assign relative weights to these reasons. In considering the reasons described above, individual members
of the Board may have given different weight to different reasons. The Board conducted an overall analysis of the factors
described above, including thorough discussions with, and questioning of, the Company’s management team, the legal and financial
advisors of the Company, and considered the reasons overall to be favorable to, and to support, its determination.
Regulatory
Approvals
Other
than the filing and mailing of this Information Statement, filing the certificate of amendment to the Company’s Second Amended
and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware effecting the Name Change and notifying
Nasdaq of the Name Change there are no federal or state regulatory requirements that must be complied with or approval that must
be obtained in connection with the Stockholder Approval Matters.
Accounting
Treatment
The
Mergers were accounted for as a “business combination” in accordance with U.S. GAAP. Scienture is treated as a “business”
and the Company is treated as the “accounting acquirer.” Consequently, the Company applied acquisition accounting to the
assets and liabilities of Scienture that were acquired or assumed upon the consummation of the Mergers. The historical financial statements
of the Company for the periods ended prior to closing of the Mergers reflect only the operations and financial condition of the Company.
Subsequent to closing of the Mergers, the financial statements of the Company include the combined operations and financial condition
of the Company and remaining Scienture operations.
Certain
U.S. Federal Income Tax Consequences
The
following discussion is a summary of certain U.S. federal income tax consequences of the Mergers generally applicable to a U.S.
holder (as defined below) of Scienture, Inc. common stock or Scienture, Inc. preferred stock (collectively “Scienture Stock”).
This discussion applies only to a U.S. holder that holds its Scienture Stock as a capital asset for U.S. federal income tax purposes
(generally, property held for investment). This discussion is a summary only and does not discuss all aspects of U.S. federal income
taxation that may be relevant to U.S. holders in light of their particular circumstances or U.S. holders with special status, including,
but not limited to:
| |
● |
brokers,
dealers or traders in securities or foreign currencies, banks, insurance companies, other financial institutions or mutual
funds; |
| |
|
|
| |
● |
real
estate investment trusts; regulated investment companies; tax-exempt organizations or governmental organizations; |
| |
|
|
| |
● |
pass-through
entities such as partnerships, S corporations, disregarded entities for federal income tax purposes and limited liability companies
(and investors therein); |
| |
|
|
| |
● |
persons
that have a functional currency other than the U.S. dollar; |
| |
|
|
| |
● |
taxpayers
that are subject to or use the mark-to-market accounting rules; |
| |
|
|
| |
● |
“controlled foreign corporations,” “passive
foreign investment companies” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| |
|
|
| |
● |
persons
who hold their Scienture Stock that constitutes “qualified small business stock” under Section 1202 of the Code or as
“Section 1244 stock” for purposes of Section 1244 of the Code; |
| |
|
|
| |
● |
persons
that hold their Scienture Stock as part of a straddle, constructive sale, hedging, conversion or other integrated or similar transaction; |
| |
|
|
| |
● |
persons
who exercise dissenters’ rights; |
| |
|
|
| |
● |
persons
who acquired their shares of Scienture Stock pursuant to the exercise of options or otherwise as compensation or through a tax-qualified
retirement plan or through the exercise of a warrant or conversion rights under convertible instruments; |
| |
|
|
| |
● |
persons subject to special tax accounting rules as a
result of any item of gross income with respect to the stock being taken into account in an applicable financial statement; |
| |
|
|
| |
● |
persons
who acquired their Scienture Stock in a transaction subject to the gain rollover provisions of Section 1045 of the Code; and |
| |
|
|
| |
● |
expatriates
or former citizens or long-term residents of the United States. |
This
discussion is based on the Internal Revenue Code of 1986, as amended (the “Code”), proposed, temporary and final Treasury
Regulations promulgated under the Code, and judicial and administrative interpretations thereof, all as of the date of this Information
Statement and all of which are subject to change, which change could apply retroactively and could affect the tax consequences described
in this Information Statement. This discussion does not address (i) the tax consequences of the Mergers under U.S. federal non-income
tax law (including estate, gift, or other non-income taxes), (ii) the tax consequences of the Mergers under state, local or non-U.S.
tax laws, (iii) the impact of the alternative minimum tax provisions of the Code (including the 15% minimum tax applicable to the adjusted
financial statement income of certain corporations) or the Medicare contribution tax on net investment income, (iv) the tax consequences
of transactions effectuated before, subsequent to or concurrently with the Mergers (whether or not any such transactions are consummated
in connection with the Mergers), including any transaction in which shares of Scienture Stock are acquired, or (v) the tax consequences
to holders of options, warrants, or similar rights to acquire Scienture Stock.
Scienture
has not and does not intend to seek any rulings from the Internal Revenue Service (the “IRS”) regarding the tax consequences
of the Mergers. There can be no assurance that the IRS will not take positions inconsistent with the consequences discussed below
or that any such positions would not be sustained by a court.
If
any entity or arrangement classified as a partnership for U.S. federal income tax purposes holds Scienture Stock, the tax treatment of
such partnership and any person treated as a partner of such partnership will generally depend on the status and activities of the partner
and the activities of the partnership. Each partnership holding any Scienture Stock and each person that is treated as a partner of such
partnership should consult its tax advisor as to the particular U.S. federal income tax consequences of the Mergers.
As
used herein, a “U.S. holder” is a beneficial owner of Scienture Stock that is, for U.S. federal income tax purposes:
| |
● |
an
individual citizen or resident of the United States; |
| |
|
|
| |
● |
a
corporation (or other entity that is classified as a corporation for U.S. federal income tax purposes) that is created or organized
(or treated as created or organized) in or under the laws of the United States, any state thereof, or the District of Columbia or
otherwise treated as a U.S. tax resident for U.S. federal income tax purposes; |
| |
|
|
| |
● |
an
estate whose income is subject to U.S. federal income tax regardless of its source; or |
| |
|
|
| |
● |
a
trust if (1) a U.S. court can exercise primary supervision over the administration of such trust and one or more U.S. persons have
the authority to control all substantial decisions of the trust or (2) it has a valid election in place to be treated as a U.S. person. |
WE
URGE YOU TO CONSULT YOUR TAX ADVISORS AS TO THE SPECIFIC TAX CONSEQUENCES TO YOU OF THE MERGER, INCLUDING THE APPLICABILITY AND EFFECT
OF U.S. FEDERAL, STATE, LOCAL AND NON-U.S. INCOME AND OTHER TAX LAWS IN LIGHT OF YOUR PARTICULAR CIRCUMSTANCES.
THIS
DISCUSSION IS FOR INFORMATION PURPOSES ONLY AND IS NOT TAX ADVICE. INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION
OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE MERGER ARISING UNDER THE U.S.
FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME
TAX TREATY.
Effects
of the Merger
Neither
the Company nor Scienture intends to request any ruling from the IRS as to the U.S. federal income tax considerations of the Mergers.
Consequently, no assurance can be given that the IRS will not challenge the conclusions reflected below or that a court would not sustain
such a challenge.
Based
on the assumptions, qualifications and limitations described herein, the Mergers will qualify as a “reorganization” within
the meaning of Section 368(a) of the Code. As a result, a U.S. holder of Scienture Stock generally will not recognize any gain or loss
for U.S. federal income tax purposes upon receipt of the Company’s common stock or Series X Preferred Stock in the Mergers. Each
U.S. holder’s aggregate tax basis in the Company’s common stock or the Series X Preferred Stock received in connection with
the Mergers will equal such U.S. holder’s aggregate adjusted tax basis in the Scienture Stock surrendered in the Mergers. The holding
period of the Company’s common stock and the Series X Preferred Stock received by a U.S. holder in connection with the Mergers
will include such U.S. holder’s holding period for the Scienture Stock surrendered in connection with the Mergers. If a U.S. holder
holds different blocks of Scienture Stock (generally, Scienture Stock acquired on different dates or at different prices), such U.S.
holder is urged to consult his, her or its tax advisor with respect to the determination of the tax bases and/or holding periods of the
shares of the Company’s common stock or Series X Preferred Stock received in connection with the Mergers.
Information
Reporting
Each
U.S. holder who receives the Company’s common stock or Series X Preferred Stock in the Mergers is required to retain permanent
records pertaining to the Mergers and make such records available to any authorized IRS officers and employees. Such records should specifically
include information regarding the amount, basis, and fair market value of all transferred property, and relevant facts regarding any
liabilities assumed or extinguished as part of such reorganization. Each U.S. holder who owned immediately before the Mergers at least
one percent (by vote or value) of the total outstanding stock of Scienture or owned securities of Scienture that had an adjusted tax
basis of $1,000,000 or more is required to attach a statement to its tax return for the year in which the Mergers are consummated
that contains the information listed in Treasury Regulation Section 1.368-3(b). Such statement must include the U.S. holder’s tax
basis in such U.S. holder’s Scienture Stock surrendered in connection with the Mergers, the fair market value of such Scienture
Stock, the date of the Mergers and the name and employer identification number of each of Scienture and the Company. Each U.S. holder
is urged to consult with its tax advisor to comply with these rules.
This
discussion of U.S. federal income tax considerations of the Mergers is for general information purposes only and is not intended to be,
and should not be construed as, tax advice. Determining the actual tax consequences of the Mergers to you may be complex and will depend
on your specific situation and on factors that are not within the Company’s knowledge or control. You should consult your tax advisor
with respect to the application of U.S. federal income tax laws to your specific situation as well as any tax consequences arising under
the U.S. federal estate or gift tax rules or under the laws of any state, local, non-U.S. or other taxing jurisdiction.
UNAUDITED
PRO FORMA FINANCIAL INFORMATION
The
following unaudited pro forma combined financial information presents the unaudited pro forma combined balance sheet and statement of
operations based upon the combined historical financial statements of the Company and Scienture after giving effect to the Mergers and
the adjustments described in the accompanying notes.
The
unaudited pro forma combined balance sheets of the Company and Scienture as of and for the six months ended June 30, 2024, have
been prepared to reflect the effects of the Mergers as if they occurred on January 1, 2024. The unaudited pro forma combined statements
of operations for the Company and Scienture as of and for the six months ended June 30, 2024 combine the historical results and
operations of the Company and Scienture giving effect to the Mergers as if they occurred on January 1, 2024.
The
unaudited pro forma combined financial information should be read in conjunction with the audited and unaudited historical financial
statements of the Company and Scienture and the notes thereto. Additional information about the basis of presentation of this information
is provided in Note 2 below.
The
unaudited pro forma combined financial information was prepared in accordance with Article 11 of Regulation S-X. The unaudited pro forma
adjustments reflecting the transaction have been prepared in accordance with business combination accounting guidance as provided in
Accounting Standards Codification Topic 805, Business Combinations and reflect the preliminary allocation of the purchase price
to the acquired assets and liabilities based upon the preliminary estimate of fair values, using the assumptions set forth in the notes
to the unaudited pro forma combined financial information.
The
unaudited pro forma combined financial information is provided for informational purposes only and is not necessarily indicative of the
operating results or financial position that would have occurred if the transaction had been completed as of the dates set forth above,
nor is it indicative of the future results or financial position of the combined company. In connection with the pro forma financial
information, the Company allocated the purchase price using its best estimates of fair value. Accordingly, the pro forma acquisition
price adjustments are preliminary and subject to further adjustments as additional information becomes available and as additional analyses
are performed. The unaudited pro forma combined financial information also does not give effect to the potential impact of current financial
conditions, any anticipated synergies, operating efficiencies or cost savings that may result from the transaction or any integration
costs. Furthermore, the unaudited pro forma combined statements of operations do not include certain nonrecurring charges and the related
tax effects which result directly from the transaction as described in the notes to the unaudited pro forma combined financial information.
Unaudited
Proforma Combined Statement of Operations for the Six Months Ended June 30, 2024
| | |
| | |
| | |
Pro Forma | | |
Pro Forma | |
| | |
TRxADE | | |
Scienture | | |
Adjustments | | |
Combined | |
| | |
| | |
| | |
| | |
| |
| Revenues | |
$ | 18,699 | | |
$ | - | | |
$ | - | | |
$ | 18,699 | |
| Cost of sales | |
| 19,402 | | |
| - | | |
| - | | |
| 19,402 | |
| Gross profit | |
| (703 | ) | |
| - | | |
| - | | |
| (703 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Wage and salary expense | |
| 534,644 | | |
| - | | |
| - | | |
| 534,644 | |
| Professional fees | |
| 688,689 | | |
| - | | |
| - | | |
| 688,689 | |
| Accounting and legal expense | |
| 510,755 | | |
| - | | |
| - | | |
| 510,755 | |
| Technology expense | |
| 138,289 | | |
| - | | |
| - | | |
| 138,289 | |
| Research and development | |
| - | | |
| 1,520,947 | | |
| - | | |
| 1,520,947 | |
| General and administrative | |
| 5,115,582 | | |
| 1,413,893 | | |
| 1,495,525 | (b) | |
| 8,025,000 | |
| Termination fee | |
| - | | |
| 1,285,000 | | |
| - | | |
| 1,285,000 | |
| Total operating expenses | |
| 6,987,959 | | |
| 4,219,840 | | |
| 1,495,525 | | |
| 12,703,325 | |
| Operating loss | |
| (6,988,662 | ) | |
| (4,219,840 | ) | |
| (1,495,525 | ) | |
| (12,704,028 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | | |
| | | |
| | |
| Change in fair value of warrant liability | |
| (895,021 | ) | |
| - | | |
| - | | |
| (895,021 | ) |
| Interest and other income | |
| 103,952 | | |
| 11,931 | | |
| - | | |
| 115,883 | |
| Loss on disposal of asset | |
| (374,968 | ) | |
| - | | |
| - | | |
| (374,968 | ) |
| Interest expense | |
| (103,464 | ) | |
| (227,905 | ) | |
| - | | |
| (331,369 | ) |
| Total non-operating income (expense) | |
| (1,269,501 | ) | |
| (215,974 | ) | |
| - | | |
| (1,485,475 | ) |
| Net loss from continuing operations | |
| (8,258,163 | ) | |
| (4,435,814 | ) | |
| (1,495,525 | ) | |
| (14,189,502 | ) |
| Net income on discontinued operations | |
| 27,670,294 | | |
| - | | |
| - | | |
| 27,670,294 | |
| Net income/(loss) | |
$ | 19,412,131 | | |
$ | (4,435,814 | ) | |
$ | (1,495,525 | ) | |
$ | 13,480,792 | |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss per common share from continuing operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (6.75 | ) | |
| | | |
| | | |
$ | (9.36 | ) |
| Diluted | |
$ | (6.75 | ) | |
| | | |
| | | |
$ | (9.36 | ) |
| Net income per common share from discontinued operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | 22.60 | | |
| | | |
| | | |
$ | 18.25 | |
| Diluted | |
$ | 19.02 | | |
| | | |
| | | |
$ | 15.85 | |
| Net income/(loss) | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | 15.86 | | |
| | | |
| | | |
$ | 8.89 | |
| Diluted | |
$ | 13.35 | | |
| | | |
| | | |
$ | 7.72 | |
| Weighted average common shares outstanding | |
| | | |
| | | |
| | | |
| | |
| Basic | |
| 1,224,337 | | |
| | | |
| | | |
| 1,515,892 | |
| Diluted | |
| 1,454,558 | | |
| | | |
| | | |
| 1,746,113 | |
Unaudited Proforma Combined Statement of Operations
for the Year Ended December 31, 2023
| | |
| | |
| | |
Pro Forma | | |
Pro Forma | |
| | |
TRxADE | | |
Scienture | | |
Adjustments | | |
Combined | |
| | |
| | |
| | |
| | |
| |
| Revenues | |
$ | 8,272,214 | | |
$ | 800,000 | | |
$ | - | | |
$ | 9,072,214 | |
| Cost of sales | |
| 5,673,957 | | |
| - | | |
| - | | |
| 5,673,957 | |
| Gross profit | |
| 2,598,257 | | |
| 800,000 | | |
| - | | |
| 3,398,257 | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Wage and salary expense | |
| 2,698,178 | | |
| - | | |
| - | | |
| 2,698,178 | |
| Professional fees | |
| 1,466,567 | | |
| - | | |
| - | | |
| 1,466,567 | |
| Accounting and legal expense | |
| 1,534,377 | | |
| - | | |
| - | | |
| 1,534,377 | |
| Technology expense | |
| 1,376,908 | | |
| - | | |
| - | | |
| 1,376,908 | |
| Research and development | |
| - | | |
| 2,029,812 | | |
| - | | |
| 2,029,812 | |
| General and administrative | |
| 2,785,633 | | |
| 719,398 | | |
| 5,982,100 | (b) | |
| 9,487,131 | |
| Total operating expenses | |
| 9,861,663 | | |
| 2,749,210 | | |
| 5,982,100 | | |
| 18,592,973 | |
| Operating loss | |
| (7,263,406 | ) | |
| (1,949,210 | ) | |
| (5,982,100 | ) | |
| (15,194,716 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | | |
| | | |
| | |
| Change in fair value of warrant liability | |
| (148,420 | ) | |
| - | | |
| - | | |
| (148,420 | ) |
| Interest and other income | |
| 18,741 | | |
| 20,798 | | |
| - | | |
| 39,539 | |
| Goodwill impairment | |
| (5,129,115 | ) | |
| - | | |
| - | | |
| (5,129,115 | ) |
| Interest expense | |
| (1,198,346 | ) | |
| (312,577 | ) | |
| - | | |
| (1,510,923 | ) |
| Total non-operating income (expense) | |
| (6,457,140 | ) | |
| (291,779 | ) | |
| - | | |
| (6,748,919 | ) |
| Net loss from continuing operations | |
| (13,720,546 | ) | |
| (2,240,989 | ) | |
| (5,982,100 | ) | |
| (21,943,635 | ) |
| Net loss on discontinued operations | |
| (4,123,028 | ) | |
| - | | |
| - | | |
| (4,123,028 | ) |
| Net loss | |
$ | (17,843,574 | ) | |
$ | (2,240,989 | ) | |
$ | (5,982,100 | ) | |
$ | (26,066,663 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss per common share from continuing operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (17.96 | ) | |
| | | |
| | | |
$ | (20.79 | ) |
| Diluted | |
$ | (5.76 | ) | |
| | | |
| | | |
$ | (8.21 | ) |
| Net loss per common share from discontinued operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (5.40 | ) | |
| | | |
| | | |
$ | (3.91 | ) |
| Diluted | |
$ | (1.73 | ) | |
| | | |
| | | |
$ | (1.54 | ) |
| Net loss | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (23.35 | ) | |
| | | |
| | | |
$ | (24.69 | ) |
| Diluted | |
$ | (7.49 | ) | |
| | | |
| | | |
$ | (9.75 | ) |
| Weighted average common shares outstanding | |
| | | |
| | | |
| | | |
| | |
| Basic | |
| 764,058 | | |
| | | |
| | | |
| 1,055,613 | |
| Diluted | |
| 2,381,443 | | |
| | | |
| | | |
| 2,672,998 | |
Unaudited Proforma Combined Balance Sheet as of
June 30, 2024
| | |
| | |
| | |
Pro Forma | | |
Pro Forma | |
| | |
TRxADE | | |
Scienture | | |
Adjustments | | |
Combined | |
| ASSETS | |
| | | |
| | | |
| | | |
| | |
| Current assets: | |
| | | |
| | | |
| | | |
| | |
| Cash | |
$ | 7,719,993 | | |
$ | 114,210 | | |
$ | - | | |
$ | 7,834,203 | |
| Accounts receivable, net | |
| 13,091 | | |
| - | | |
| - | | |
| 13,091 | |
| Inventory | |
| 6,439 | | |
| - | | |
| - | | |
| 6,439 | |
| Prepaid expenses | |
| 797,383 | | |
| - | | |
| - | | |
| 797,383 | |
| Notes receivable - related party | |
| 1,300,000 | | |
| - | | |
| - | | |
| 1,300,000 | |
| Other receivables | |
| 2,230,797 | | |
| 485 | | |
| - | | |
| 2,231,282 | |
| Deferred offering costs | |
| 69,444 | | |
| - | | |
| - | | |
| 69,444 | |
| Current assets of discontinued operations | |
| 7,297 | | |
| - | | |
| | | |
| 7,297 | |
| Total current assets | |
| 12,144,444 | | |
| 114,695 | | |
| - | | |
| 12,259,139 | |
| Property plant and equipment, net | |
| 6,500 | | |
| - | | |
| - | | |
| 6,500 | |
| Deposits | |
| 22,039 | | |
| - | | |
| - | | |
| 22,039 | |
| Deferred offering costs | |
| - | | |
| - | | |
| - | | |
| - | |
| Goodwill | |
| - | | |
| - | | |
| 23,928,400 | (a) | |
| 23,928,400 | |
| Intangible assets, net | |
| - | | |
| - | | |
| 70,289,675 | (a) | |
| 70,289,675 | |
| Investments | |
| 2,500,000 | | |
| - | | |
| - | | |
| 2,500,000 | |
| Operating lease right-of-use assets | |
| 175,550 | | |
| 61,579 | | |
| - | | |
| 237,129 | |
| Total assets | |
$ | 14,848,533 | | |
$ | 176,273 | | |
$ | 94,218,075 | | |
$ | 109,242,882 | |
| | |
| | | |
| | | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | |
| | | |
| | | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | | |
| | | |
| | |
| Accounts payable | |
$ | 726,266 | | |
$ | 884,581 | | |
$ | - | | |
$ | 1,610,847 | |
| Accrued liabilities | |
| 500,454 | | |
| 1,198,822 | | |
| - | | |
| 1,699,276 | |
| Other current liabilities | |
| 5,441 | | |
| - | | |
| - | | |
| 5,441 | |
| Lease liability - current | |
| 32,608 | | |
| 22,567 | | |
| - | | |
| 55,175 | |
| Warrant liability | |
| 1,631,974 | | |
| - | | |
| - | | |
| 1,631,974 | |
| Current liabilities of discontinued operations | |
| 5,346 | | |
| | | |
| | | |
| 5,346 | |
| Total current liabilities | |
| 2,902,089 | | |
| 2,105,970 | | |
| - | | |
| 5,008,059 | |
| Long-term convertible notes, net of debt discount | |
| - | | |
| 1,734,661 | | |
| - | | |
| 1,734,661 | |
| Lease liability | |
| 160,996 | | |
| 39,319 | | |
| - | | |
| 200,315 | |
| Development agreement liability | |
| - | | |
| 1,285,000 | | |
| - | | |
| 1,285,000 | |
| Total liabilities | |
| 3,063,085 | | |
| 5,164,950 | | |
| - | | |
| 8,228,035 | |
| | |
| | | |
| | | |
| | | |
| | |
| Stockholders’ equity (deficit): | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| 68 | (a) | |
| | |
| Preferred stock | |
| - | | |
| 337 | | |
| (337 | )(a) | |
| 68 | |
| Common stock | |
| 14 | | |
| 500 | | |
| 3 | (a) | |
| 17 | |
| | |
| | | |
| | | |
| (500 | )(a) | |
| - | |
| Additional paid-in capital | |
| 38,290,315 | | |
| 10,835,257 | | |
| 90,724,853 | (a) | |
| 129,015,168 | |
| | |
| | | |
| | | |
| (10,835,257 | )(a) | |
| - | |
| Accumulated deficit | |
| (26,504,881 | ) | |
| (15,824,770 | ) | |
| 15,824,770 | (a) | |
| (28,000,406 | ) |
| | |
| | | |
| | | |
| (1,495,525 | )(b) | |
| - | |
| Total stockholders’ equity | |
| 11,785,448 | | |
| (4,988,676 | ) | |
| 94,218,075 | | |
| 101,014,847 | |
| Total liabilities and stockholders’ equity | |
$ | 14,848,533 | | |
$ | 176,273 | | |
$ | 94,218,075 | | |
$ | 109,242,882 | |
(a)
To record the purchase price allocation of the pro forma acquisition, including the recognition of goodwill and intangible assets, purchase
price consideration by the Company, and elimination of Scienture’s equity.
(b)
To record amortization on the intangible assets recorded as a result of the acquisitions.
Description
of Transactions
As
consideration for the Mergers, at the First Effective Time, the shares of Scienture
common stock issued and outstanding immediately prior to the First Effective Time were converted into the right to receive, in the aggregate,
(i) 291,555 shares of the Company’s common stock, par value $0.00001 per share representing 19.99% of the number of shares of the
Company’s common stock issued and outstanding immediately prior to the effective time of the First Merger, and (ii) 6,826,713 shares
of the Series X Preferred Stock, each share of which is convertible into one share of the Company’s common stock.
Basis
of Presentation
The
historical financial information has been adjusted to give pro forma effect to events that are (i) directly attributable to the transaction,
(ii) factually supportable, and (iii) with respect to the unaudited proforma combined balance sheets and unaudited pro forma combined
statements of operations, expected to have a continuing impact on the combined results.
The
transaction was accounted for as a business acquisition wherein Scienture is the accounting acquiree and the Company is the accounting
acquirer.
Consideration
Transferred
The
Company issued 291,555 shares of its common stock and 6,826,713 shares of Series X Preferred Stock in connection with the Mergers. The
total fair value of the initial purchase price consideration associated with the Mergers was determined as follows:
| Common stock issued | |
$ | 3,390,785 | |
| Preferred stock issued | |
| 87,334,139 | |
| Total purchase price | |
$ | 90,724,924 | |
The
following table shows the preliminary allocation of the purchase price for Scienture to the acquired net identifiable assets and pro
forma goodwill:
| Assets acquired | |
$ | 176,273 | |
| Goodwill | |
| 23,928,400 | |
| Intangible assets | |
| 71,785,200 | |
| Liabilities assumed | |
| (5,164,950 | ) |
| | |
$ | 90,724,924 | |
The
Company will record $23,816,798 in pro forma goodwill representing the remaining excess purchase price of the fair value of net
assets acquired and liabilities assumed. The Company expects to identify intangible assets such as developed technology and customer
relationships upon the business combination.
APPROVAL
OF THE preferred stock conversion
Overview
As
of July 24, 2024, the Company was authorized to issue up to 10,000,000 in three series of preferred stock. Specifically, the Board had
authorized the Company to issue up to 9,211,246 shares of the Company’s Series A preferred stock, par value $0.00001 par value
(“Series A Preferred Stock”), 787,754 shares of the Company’s Series B preferred stock, par value $0.00001 par value,
and 1,000 shares of the Company’s Series C preferred stock, par value $0.00001 par value (“Series C Preferred Stock”).
On July 25, 2024, in anticipation of closing the Mergers and given that no shares of the Series A Preferred Stock were then outstanding,
the Board revoked the authorization to issue any shares of Series A Preferred Stock and simultaneously authorized the issuance of up
to 9,211,246 shares of Series X Preferred Stock.
Upon
closing of the Mergers, all shares of Scienture were converted into the right to receive, in the aggregate, (i) 291,555 shares of the
Company’s common stock, which represents 19.99% of the number of shares of the Company’s common stock issued and outstanding
immediately prior to the First Effective Time, and (ii) 6,826,713 shares of Series X Preferred Stock, each share of which is convertible
into one share of the Company’s common stock, subject to the terms and conditions set forth in the Series X Certificate of Designation,
attached hereto as Annex B. The Series X Certificate of Designation specifies that, among other things, each share
of Series X Preferred Stock will automatically convert into one share of the Company’s common stock as of the earliest date permitted
by the listing rules of Nasdaq after approval by the Company’s stockholders of the Preferred Stock Conversion.
Nasdaq
Rules
As
described above, our common stock is listed on Nasdaq, and as a result, we are subject to Nasdaq’s Listing Rules, including Nasdaq
Rule 5635(a) and Nasdaq Rule 5635(d).
Pursuant
to Nasdaq Rule 5635(a), an issuer must obtain stockholder approval prior to the issuance of securities in connection with the acquisition
of the stock or assets of another company if such securities represent, or will represent upon issuance, twenty percent (20%) or more
of the outstanding common stock or voting power of the issuer prior to the issuance of the securities in connection with the acquisition.
The number of shares of common stock to be issued upon conversion of the Series X Preferred Stock will result in the issuance of a number
of shares exceeding twenty percent (20%) of the outstanding common stock and voting power of the Company prior to the Preferred Stock
Conversion. To ensure compliance with Nasdaq Rule 5635(a), the Majority Stockholders approved the Preferred Stock Conversion.
In
addition, pursuant to Nasdaq Rule 5635(d), if an issuer intends to issue securities in a transaction which could result in the issuance
of 20% or more of the issued and outstanding shares of the issuer’s common stock on a pre-transaction basis for less than the Minimum
Price for such stock, the issuer generally must obtain the prior approval of its stockholders. The number of shares of common stock to
be issued in the transaction upon conversion of the Series X Preferred Stock will result in the issuance of a number of shares exceeding
the threshold and pricing for which stockholder approval is required under Nasdaq Rule 5635(d). To ensure compliance with Nasdaq Rule
5635(d), the Majority Stockholders approved the Preferred Stock Conversion.
Effect
of the Preferred Stock Conversion on Existing Stockholders
The
issuance of securities in connection with the Preferred Stock Conversion will have a dilutive effect on the Company’s existing
stockholders. The Preferred Stock Conversion will reduce each existing stockholder’s proportionate ownership in the Company’s
common stock and reduce the voting power of the existing stockholders. The Company’s existing stockholders do not have
preemptive rights to subscribe to additional shares that may be issued by us upon the Preferred Stock Conversion in order to maintain
their proportionate ownership of the Company’s common stock. The Preferred Stock Conversion could also dilute the voting
power of a person seeking control of the Company, thereby deterring or rendering more difficult a merger, tender offer, proxy contest
or an extraordinary corporate transaction opposed by the Company. In addition, upon the Preferred Stock Conversion, there will be a greater
number of shares of the Company’s common stock eligible for sale in the public markets. Any such sales, or the anticipation
of the possibility of such sales, represents an overhang on the market and could depress the market price of the Company’s
common stock.
Stockholder
Approval of the Preferred Stock Conversion
The
approval of the Preferred Stock Conversion, including for purposes of Nasdaq Rule 5635(a) and Nasdaq Rule 5635(d), required the affirmative
approval of a majority of the holders of the outstanding shares of the Company’s common stock that were entitled to approve the
Preferred Stock Conversion.
As
of the Record Date, the Company had 1,458,506 shares of common stock issued and outstanding and the Majority Stockholders held 737,708
shares of common stock as of the Record Date, representing approximately 50.58% of the voting power of all shares of the Company’s
common stock. The Majority Stockholders approved the Preferred Stock Conversion upon execution of the Stockholder Consent on July 25,
2024, following execution of the Merger Agreement.
This
Information Statement is first being mailed on or about August [●], 2024 to the Company’s stockholders of record as of the
Record Date. The corporate action shall be effective on or about September [●], 2024, or approximately 20 days after we
mail this Information Statement.
Notice
Pursuant to Section 228 of the General Corporation Law of the State of Delaware
Pursuant
to Section 228 of the Delaware General Corporation Law (the “DGCL”), we are required to provide prompt notice of the taking
of corporate action by written consent to our stockholders who have not consented in writing to such action. This Information Statement
serves as the notice required by Section 228 of the DGCL.
APPROVAL
OF The name change
Overview
On
July 25, 2024, the Board and the Majority Stockholders approved an amendment to the Company’s Second Amended and Restated Certificate
of Incorporation, which will effect a change in the Company’s name from “TRxADE HEALTH, INC.” to “Scienture Holdings,
Inc.” The Name Change will become effective upon the filing of a certificate of amendment with the Secretary of State of the State
of Delaware, which will be filed following the effectiveness of the Stockholder Consent. The certificate of amendment as filed will be
in substantially the form of Annex G, subject to non-material technical, administrative, or similar changes and modifications
in order to comply with Delaware law.
Reasons
for the Name Change
On
February 16, 2024, the Company completed the sale of substantially all of the assets of Trxade, Inc., a wholly owned subsidiary of the
Company, pursuant to an asset purchase agreement. In connection with the asset sale, the Company agreed to cease all use of the “Trxade”
name and to not use or adopt any name confusingly similar to “Trxade.” Further, the Merger Agreement requires the Company
to complete the Name Change.
Stockholder
Approval of the Name Change
The
approval of the Name Change required the affirmative approval of a majority of the holders of the outstanding shares of the Company’s
common stock that were entitled to approve the Name Change.
As
of the Record Date, the Company had 1,458,506 shares of common stock issued and outstanding and the Majority Stockholders held 737,708
shares of common stock as of the Record Date, representing approximately 50.58% of the voting power of all shares of the Company’s
common stock. The Majority Stockholders approved the Name Change upon execution of the Stockholder Consent on July 25, 2024, following
execution of the Merger Agreement.
This
Information Statement is first being mailed on or about August [●], 2024 to the Company’s stockholders of record as of the
Record Date. The corporate action shall be effective on or about September [●], 2024, or approximately 20 days after we
mail this Information Statement.
APPROVAL
OF the incentive plan share increase
Overview
On
July 25, 2024, the Board and the Majority Stockholders approved an amendment to the Company’s Incentive Plan resulting in the Incentive
Plan Share Increase. The Incentive Plan Share Increase was necessary as the Incentive Plan did not have a sufficient number of authorized
shares to be issued under the Incentive Plan in the event that the Company is required to issue Mr. Ajjarapu or Mr. Patel shares of the
Company’s common stock pursuant the Consulting Agreements. The Board did not make any further changes to the Incentive Plan other
than effecting the Incentive Plan Share Increase.
Key
Provisions of the Incentive Plan
The
following is a summary of the principal features of the Incentive Plan, which does not purport to be a complete description of all of
the provisions of the Incentive Plan. This summary is qualified in its entirety by reference to the full text of the Incentive Plan, which is attached as Exhibit 10.1 to the Current Report on Form 8-K filed with the SEC on May 28, 2021.
In making such determinations,
the Board (or the Compensation Committee) may take into account the nature of the services rendered by such person, his or her present
and potential future contribution to the Company’s success, and such other factors as the Board (or the Compensation Committee)
in its discretion shall deem relevant. Incentive stock options granted under the Incentive Plan are intended to qualify as “incentive
stock options” within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended (the “Code”). Nonqualified
(non-statutory stock options) granted under the Incentive Plan are not intended to qualify as incentive stock options under the
Code.
Purpose.
The Incentive Plan is intended to secure for
the Company the benefits arising from ownership of the Company’s common stock by the employees, officers, directors and consultants
of the Company, all of whom are and will be responsible for the Company’s future growth. The Incentive Plan is designed to help
attract and retain for the Company, qualified personnel for positions of exceptional responsibility, to reward employees, officers, directors,
and consultants for their services to the Company and to motivate such individuals through added incentives to further contribute to
the success of the Company.
Effective
Date. The Board adopted the Incentive Plan on October 9, 2019 (the “Effective Date”). The Incentive Plan was later amended
on April 8, 2020. The stockholders of the Company approved and ratified a further amendment to the Incentive Plan on May 27, 2021. Unless
sooner terminated as provided in the Incentive Plan, the Incentive Plan will terminate upon the close of business on the day next preceding
the tenth (10th) anniversary of the Effective Date.
Administration.
The Board or the Compensation Committee (the “Administrator”) is responsible for the administration of the Incentive Plan.
Eligibility.
The Administrator may designate any employees, officers, directors, non-employee directors (as defined in Rule 16b-3 of the Exchange
Act) of, and consultants rendering bona fide consulting or advisory services to, the Company or its affiliates are eligible to participate
in the Incentive Plan. In making determinations of eligibility, the Administrator may take into account the nature of the services rendered
by a participant, the present and potential future contribution to the Company’s success by a participant, and any other relevant
factors.
Awards.
The Administrator has the authority to make the following type of equity-based awards to participants under the Incentive Plan (subject
to any limitations provided by federal or state securities laws): (i) incentive stock options (to eligible employees only); (ii) nonqualified
stock options; (iii) restricted stock; (iv) stock awards; (v) performance shares; or (vi) any combination of the foregoing.
Available
Shares. Prior to the Incentive Plan Share
Increase the maximum aggregate number of shares of common stock available for issue under the Incentive Plan—subject to adjustment
in connection with the payment of a stock dividend, a stock split or subdivision or combination of the shares of common stock, or a reorganization
or reclassification of the Company’s common stock—was (i) two million (2,000,000) shares of common stock, and (ii) an annual
increase on April 1st of each calendar year, beginning in 2021 (provided that no increase was approved in 2021 or 2022) and ending in
2029 (each a “Date of Determination”), in each case subject to the approval and determination of the Board (or the Compensation
Committee) on or prior to the applicable Date of Determination, equal to the lesser of (A) ten percent (10%) of the total shares of common
stock of the Company outstanding on the last day of the immediately preceding fiscal year and (B) such smaller number of shares as determined
by the Board (or the Compensation Committee) (the “Share Limit”), also known as an “evergreen” provision. Notwithstanding
the above, no more than 25 million shares of shares of common stock may be issuable upon exercise of incentive stock options granted
under the plan.
In
June 2023 our shareholders approved an amendment to the Incentive Plan to increase the number of shares of common stock reserved
under the Incentive Plan by 2,000,000 additional shares. The shares reserved under the Incentive Plan (and outstanding
awards under the Incentive Plan) were proportionally reduced to give effect to the 1-for-15 reverse stock split effected by the
Company in June 2023.
After
accounting for the Incentive Plan Share Increase, as of the date of this Information Statement, a total of 3,038,763 shares of
common stock remain available for awards under the Incentive Plan.
Stock
Options. The Administrator may grant participants incentive stock options within the meaning of Section 422 of the Code or nonqualified
stock options that fail to meet one or more requirements of an incentive stock option. The exercise price for each option must not be
less than 100% of the market value of a share of the Company’s common stock on the grant date (110% of the market value of a share
of the Company’s common stock on the grant date for incentive stock options awarded to persons holding more than 10% of the total
combined voting power of all classes of stock of the Company or its affiliates (each, a “Ten Percent Stockholder”). Incentive
stock options granted to persons other than Ten Percent Stockholders under the Incentive Plan may be exercised within 10 years from the
grant date or such shorter period as determined by the Administrator. Incentive stock options granted to Ten Percent Stockholders under
the Incentive Plan may be exercised within 5 years from the grant date or such shorter period as determined by the Administrator. Incentive
stock options lapse and cease to be exercisable upon the grantee’s termination or service with the Company or its affiliates, unless
otherwise determined by the Administrator or as otherwise set forth in the Incentive Plan. Nonqualified stock options lapse and cease
to be exercisable upon a termination of service or within such period following a termination of service as determined by the Administrator.
The maximum number of shares of the Company’s common stock that may be issued pursuant to the exercise of incentive stock options
granted under the Incentive Plan is 25,000,000 shares, subject to adjustment for increases or decreases of the number of outstanding
shares of the Company’s common stock resulting from dividend payments, stock splits, subdivisions, or combinations, reorganizations
or reclassifications of, or any other change in the structure of the Company’s common stock. Participants awarded stock options
have no rights as a stockholder of the Company with respect to any shares covered by the stock options (including the right to vote or
receive any dividend or other distribution).
Restricted
Stock. The Administrator may award shares of the Company’s common stock under a restricted stock grant. The grant will
set forth the purchase price (if any), the duration and terms of a restriction period whereby the grantee is restricted or limited as
to selling, transferring, pledging, or assigning the restricted stock, whether restricted stock is subject to repurchase a right of first
refusal, or a forfeiture provision, and whether dividends and other distributions related to the restricted stock will be paid or withheld.
During this restricted period, the grantee will generally have all the rights of a stockholder, including the right to vote the shares
and receive dividends. The Company will issue a certificate or certificates representing the shares of restricted stock that will be
registered in the name of the participant and, unless otherwise determined by the Administrator, such certificate or certificates will
be held in custody by the Company until (i) expiration of the restricted period or (ii) a prior forfeiture by the participant.
Performance
Shares. A performance share is the equivalent of one share of the Company’s common stock and serves as an incentive for
the performance of future services that will contribute materially to the successful operation of the Company and its affiliates. The
Administrator establishes the purchase price, if any, to be paid for performance shares as well as the performance objectives and the
length of the performance period to attain such objectives at the time a performance share is awarded. The Administrator may prescribe
different conditions for different participants. Unless waived by the Administrator, all performance unearned shares held by a participant
who terminates from service with the Company or its affiliates will be forfeited.
Change
of Control. Upon a change of control of the Company (as defined in the Incentive Plan), (i) all outstanding stock options become
immediately exercisable in full, subject to any appropriate adjustments in the number of shares subject to the stock option and price,
and remain exercisable for the remaining option period; (ii) all outstanding performance shares with existing performance periods become
immediately payable as if the applicable performance objectives had been met and the applicable performance period satisfied in an amount
determined by the Administrator or set forth in the applicable award agreement multiplied by a fraction, the numerator of which is the
number of full calendar months of the applicable performance period that have elapsed prior to occurrence of the change of control, and
the denominator of which is the total number of months in the original performance period; and (iii) all outstanding shares of restricted
stock will no longer be deemed restricted.
Amendment
and Termination. The Board may amend or terminate the Incentive Plan at any time, but the Company’s stockholder must approve
any amendment that (i) materially alters the group of persons eligible to participate in the Incentive Plan, (ii) generally changes the
maximum aggregate number of shares of the Company’s common stock that are available for awards; or (iii) alters the class of individuals
eligible to receive an incentive stock option or increase the limit on incentive stock options set forth in the Incentive Plan or the
value of shares of the Company’s common stock for which an eligible employee may be granted an incentive stock option. Furthermore,
no amendment or termination may, without the consent of the impacted participant, adversely affect any award granted under the Incentive
Plan despite that the Administrator may annul an award to a participant who is terminated for cause as well as convert any outstanding
incentive stock option to a nonqualified stock option. Upon a change of control, no amendment or termination may impair the rights of
any person with respect to an outstanding award.
Federal
Income Tax Consequences of Incentive Plan Awards
The
following summary is intended only as a general guide to the U.S. federal income tax consequences under current law of equity-based awards
that may be granted under the Incentive Plan of the Company. It does not attempt to describe all possible federal or other tax consequences
of participation in the Incentive Plan or tax consequences based on particular circumstances. The exact federal income tax treatment
of transactions under the Incentive Plan will vary depending upon the specific facts and circumstances involved and participants (as
such term is defined in the Incentive Plan) are advised to consult their personal tax advisors with regard to all consequences arising
from the grant or exercise of awards and the disposition of any acquired shares.
Incentive
Stock Options
There
will be no federal income tax consequences to either the Company or the participant upon the grant of an incentive stock option. Upon
exercise of the option, the excess of the fair market value of the stock over the exercise price, or the “spread,” will be
added to the alternative minimum tax base of the recipient unless a disqualifying disposition is made in the year of exercise. A disqualifying
disposition is the sale of the stock prior to the expiration of two years from the date of grant and one year from the date of exercise.
If the shares of common stock are disposed of in a disqualifying disposition, the participant will realize taxable ordinary income in
an amount equal to the spread at the time of exercise, and the Company will be entitled (subject to the requirement of reasonableness,
the provisions of Section 162(m) of the Code and the satisfaction of a tax reporting obligation) to a federal income tax deduction equal
to such amount. If the recipient sells the shares of common stock after the specified periods, the gain or loss on the sale of the shares
will be long-term capital gain or loss and the Company will not be entitled to a federal income tax deduction.
Nonqualified
Stock Options and Restricted Stock Awards
Nonqualified
stock options and restricted stock awards granted under the Incentive Plan generally have the following federal income tax consequences.
There
are no tax consequences to the participant or the Company by reason of the grant. Upon acquisition of the stock, the participant will
recognize taxable ordinary income equal to the excess, if any, of the stock’s fair market value on the acquisition date over the
purchase price. However, to the extent the stock is subject to “a substantial risk of forfeiture” (as defined in Section
83 of the Code), the taxable event will be delayed until the forfeiture provision lapses unless the Participant elects to be taxed on
receipt of the stock by making a Section 83(b) election within 30 days of receipt of the stock. If such election is not made, the recipient
generally will recognize income as and when the forfeiture provision lapses, and the income recognized will be based on the fair market
value of the stock on such future date. On that date, the recipient’s holding period for purposes of determining the long-term
or short-term nature of any capital gain or loss recognized on a subsequent disposition of the stock will begin. If a participant makes
a Section 83(b) election, the participant will recognize ordinary income equal to the difference between the stock’s fair market
value and the purchase price, if any, as of the date of receipt and the holding period for purposes of characterizing as long-term or
short-term any subsequent gain or loss will begin at the date of receipt.
Stock
Awards.
A
participant who receives a stock payment in lieu of a cash payment that would otherwise have been made will generally be taxed as if
the cash payment has been received, and the Company generally will be entitled to a deduction for the same amount.
Performance
Awards.
A
participant who has been granted a performance award generally will not recognize taxable income at the time of grant, and the Company
will not be entitled to a deduction at that time. When an award is paid, whether in cash or common stock, the participant generally will
recognize ordinary income, and the Company will be entitled to a corresponding deduction.
Potential
Limitation on Company Deductions
Section
162(m) of the Code denies a deduction to any publicly held corporation for compensation paid to certain senior executives of the Company
(a “covered employee”) in a taxable year to the extent that compensation to such employees exceeds $1,000,000. It is possible
that compensation attributable to awards, when combined with all other types of compensation received by a covered employee from the
Company, may cause this limitation to be exceeded in any particular year.
Forfeiture
or Cancellation of Awards
Awards
will be subject to such generally applicable policies as to forfeiture, cancellation and recoupment as may be adopted by the Administrator.
Tax
Withholding Adjustments
To
the extent a participant is required to pay to the Company an amount with respect to income and employment tax withholding obligations
in connection with (i) the exercise of a nonqualified stock option, (ii) certain dispositions of common stock acquired upon the exercise
of an Incentive Stock Option, or (iii) the receipt of common stock pursuant to any other award, a participant may satisfy any federal,
state or local tax withholding obligation relating to the exercise of such award, or award by a cash payment upon exercise, or in the
discretion of the Administrator, by authorizing the Company to withhold a portion of the stock otherwise issuable to the participant,
by (i) tendering a cash payment; (ii) authorizing the Company to withhold shares of common stock from the shares of common stock otherwise
issuable to the participant as a result of the exercise or acquisition of common stock under the award, provided, however, that no shares
of common stock are withheld with a value exceeding the minimum amount of tax required to be withheld by law; or (iii) delivering to
the Company owned and unencumbered shares of common stock.
Section
409A of the Code.
The
Incentive Plan and each award agreement is intended to be exempt from Section 409A of the Code. To the extent that any award hereunder
could be subject to Section 409A of the Code, it will be structured to comply with Section 409A of the Code, and, to the extent not so
exempt, in compliance with Section 409A of the Code.
Section
162(m) of the Code
The
federal legislation informally known as the Tax Reform and Jobs Act of 2017 (“Tax Act”) generally eliminated the ability
to deduct compensation qualifying for the “performance-based compensation” exception under Section 162(m) of the Code for
tax years commencing after December 31, 2017. Section 162(m) of the Code imposes a $1 million limit on the amount that a public company
may deduct for compensation paid to anyone who has ever been the Company’s chief executive officer, chief financial officer or
one of the three highest compensated officers in any fiscal year beginning after December 31, 2016 (i.e., a “covered employee”).
For 2017 and prior taxable years, an exception to this deduction limit applied to “performance-based compensation,” such
as stock options and other equity awards that satisfied certain criteria. Under the Tax Act, the performance-based pay exception to Section
162(m) was eliminated, but a transition rule may allow the exception to continue to apply to certain performance-based compensation payable
under written binding contracts that were in effect on November 2, 2017. The Board and the Compensation Committee intend to consider
the potential impact of Section 162(m) on grants made under the Incentive Plan, but reserve the right to approve grants of options and
other awards for an executive officer that exceeds the deduction limit of Section 162(m).
Securities
Issued Pursuant to Incentive Plan
The
following table provides information as of December 31, 2024, with respect to securities that may be issued under the Incentive
Plan.
| Plan
Category |
|
Number
of
securities to be
issued upon
exercise of
outstanding options,
warrants and rights |
|
|
Weighted-average
exercise
price of outstanding options,
warrants and rights |
|
|
Number
of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a)) |
|
| |
|
(a) |
|
|
(b) |
|
|
(c) |
|
| Equity
compensation plans approved by security holders |
|
|
10,942 |
|
|
$ |
85.95 |
|
|
|
3,038,763 |
|
| Equity
compensation plans not approved by security holders |
|
|
- |
|
|
$ |
[0.00] |
|
|
|
- |
|
| Total |
|
|
10,942 |
|
|
$ |
85.95 |
|
|
|
3,038,763 |
|
Nasdaq
Rules
As
described above, we are subject to Nasdaq’s Listing Rules, including Nasdaq Rule 5635(c). Pursuant to Nasdaq Rule 5635(c), an issuer
must obtain stockholder approval prior to the issuing securities when a stock option or purchase plan, or other equity compensation arrangement,
pursuant to which stock may be acquired by officers, directors, employees, or consultants (subject to certain exceptions) is materially
amended. Nasdaq considers any material increase in the number of shares to be issued under a plan or arrangement (other than to reflect
a reorganization, stock split, merger, spinoff or similar transaction) to be a material amendment. Prior to the Incentive Share Plan
Increase, 2,000,000 shares of the Company’s common stock were available to be issued pursuant to the Incentive Plan. Thus, the
Incentive Plan Share Increase resulted in a material increase in the number of shares available for issuance under the Incentive Plan.
Given this material increase, the Majority Shareholders approved the Incentive Plan Share Increase to comply with Nasdaq Rule 5635(c).
Stockholder
Approval of the Incentive Plan Share Increase
The
approval of the Incentive Plan Share Increase, including for purposes of Nasdaq Rule 5635(c), required the affirmative approval of a
majority of the holders of the outstanding shares of the Company’s common stock that were entitled to approve the Incentive Plan
Share Increase.
As
of the Record Date, the Company had 1,458,506 shares of the Company’s common stock issued and outstanding and the Majority Stockholders
held 737,708 shares of the Company’s common stock as of the Record Date, representing approximately 50.58% of the voting power
of all shares of the Company’s common stock. The Majority Stockholders approved the Incentive Plan Share Increase upon execution
of the Stockholder Consent on July 25, 2024, following execution of the Merger Agreement.
This
Information Statement is first being mailed on or about August [●], 2024 to the Company’s stockholders of record as of the
Record Date. The corporate action shall be effective on or about September [●], 2024, or approximately 20 days after we
mail this Information Statement.
SCIENTURE’S
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You
should read the following discussion and analysis of Scienture’s financial condition and results of operations together with Scienture’s
consolidated financial statements and related notes thereto, appearing elsewhere in this information statement. In addition to historical
information, some of the information in this discussion and analysis contains forward-looking statements reflecting our current expectations
and involving risk and uncertainties. For example, statements regarding our expectations as to our plans and strategy for our business,
future financial performance, expense levels, and liquidity sources are forward-looking statements. Our actual results and the timing
of those events could differ materially from those discussed in our forward-looking statements because of many factors, including those
set forth under the “Risk Factors” section and elsewhere in this information statement.
Unless
the content requires otherwise, the words “Scienture,” “we,” and “our” in this discussion and analysis
refer to Scienture, LLC and/or one or more of its subsidiaries, as the case may be. These terms are used solely for the convenience of
the reader. Scienture and each of its subsidiaries are distinct legal entities.
Overview
Scienture
is a specialty pharmaceutical company focused on developing and commercializing products for the treatment of central nervous system
(CNS) and cardiovascular (CVS) diseases. Scienture is developing a broad range of novel product candidates including new potential treatments
for hypertension, migraine, pain and thrombosis and other related disorders.
Research
and Development and Product Portfolio
Scienture
is committed to the development of innovative product candidates in the CNS and CVS therapeutic areas, including the following:
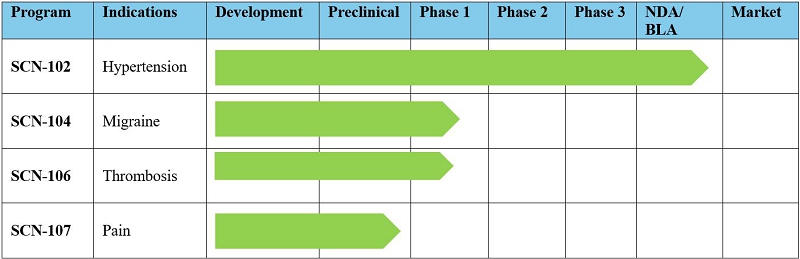
Scienture
does not have any product candidates approved for sale and has not generated any revenue from product sales. Scienture will not generate
revenue from product sales unless and until it successfully obtains regulatory approval for its product candidates. We
are engaged in a variety of research and development efforts including development of a pipeline of novel product candidates for the
treatment of various disease conditions. We have devoted and will continue to devote significant resources to research and development
activities. Scienture expects to incur significant expenses as we continue advancing our product candidates towards FDA approval; and
expanding product indications for approved products and our intellectual property portfolio. Scienture’s expectations regarding
our research and development programs are subject to risks, including the risk that Scienture’s financial condition and results
of operations for fiscal year 2024 and beyond may be materially and adversely affected by delays and failures in the completion of clinical
development of our product candidates, which could increase our costs or delay or limit our ability to generate revenues.
SCN-102
(ARBLITM - Losartan Oral Suspension)
SCN-102
is an oral liquid formulation of losartan potassium, in development, under the 505(b)(2) pathway, for (i) treatment of hypertension,
to lower blood pressure in adults and children greater than 6 years old, (ii) reduction of the risk of stroke in patients with hypertension
and left ventricular hypertrophy, and (iii) treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria in patients
with type 2 diabetes and a history of hypertension. Currently, there are no FDA-approved liquid formulations of losartan potassium. SCN-102
has shown close comparability to the immediate-release tablet and, if approved, would be the first FDA-approved oral liquid formulation
of losartan on the market.
Scienture submitted an
IND application to the FDA in September 2022. Multiple human pharmacokinetics studies were performed, showing close comparability
with the oral solid dosage form. In October 2023, we submitted a NDA to the FDA. In December 2023, the FDA accepted the NDA for review
and assigned a PDUFA target action date of August 19, 2024. During the FDA’s review, we have responded to information
requests related to CMC, pharmacovigilance, clinical, microbiology and labeling. If we receive approval on the PDUFA target action date,
we anticipate commercially launching this product in the first quarter of 2025.
SCN-104
(Multi-dose Dihydroergotamine (DHE) Mesylate injection pen)
The
SCN-104 injection pen is a disposable, multiple fixed dose, single entity combination product comprised of a small molecule drug, SCN-104,
which is administered using a customized injection pen. The SCN-104 injection pen is being developed via the 505(b)(2) regulatory pathway.
The SCN-104 injection pen is in development for the acute treatment of migraine headaches with or without aura and the acute treatment
of cluster headache episodes.
As
shown in third party studies of DHE, SCN-104’s mechanism of action for its antimigraine effect is due to its potential action as
an agonist at the serotonin 5-HT1D receptors. SCN-104 is intended for subcutaneous administration. SCN-104 is also intended for
acute use and is not intended for chronic administration.
We
believe the SCN-104 injection pen may offer a significant improvement, in terms of usability and patient acceptability, on the current
standard of care in the market (ampoules for injection). The intended pen delivery system was designed with patients in mind to carry
multiple doses, have a lower volume of injection, and utilize shielded needles to avoid unnecessary exposure.
Scienture
has had initial discussions with the FDA to align on a path forward for this development program. The formulation has been scaled up
to enable future commercial scale production and the pen has been optimized for commercial use. Several pharmacokinetics studies have
shown comparability between SCN-104 and the currently available marketed injection product. Scienture is initiating manufacturing
activities and planning to conduct bioequivalence studies. Scienture plans to initiate a Phase 1 single dose study in healthy adults
in 2025 following submission of an IND, if the IND is cleared by the FDA.
SCN-106
(Potential Biosimilar)
Scienture
is developing a potential biosimiliar SCN-106, based on a reference product that is a thrombolytic agent that binds to fibrin in clots
and converts entrapped plasminogen to plasmin. SCN-106 is a sterile, purified glycoprotein that is synthesized using the complementary
DNA for natural human tPA obtained from a Chinese hamster ovary cell-line.
Scienture
is working with an external partner to develop a biosimilar product that utilizes the same mechanism(s) of action for the proposed condition
of use, and has the same route of administration, dosage form, and strength as the reference product.
The
CMC development program is focused on establishing the analytical similarity of SCN-106 to the reference product. Multiple clones of
CHO cells have been produced to synthesize lots of SCN-106 which were screened for similarity to the reference product for several key
biochemical quality attributes as well as overall protein yield and finalization of a lead clone.
Scienture
completed a Biosimilar Initial Advisory meeting with the FDA in June 2023 to discuss the CMC, non-clinical, and clinical studies required
for regulatory approval.
SCN-107
(Bupivacaine Long-Acting Injection)
SCN-107
is a long acting injection suspension formulation of a non-opioid analgesic that is indicated for postsurgical local and regional analgesia.
Scienture’s long-acting formulation, SCN-107, is a novel microsphere-based formulation of bupivacaine that comprises the drug in
polymer-based microspheres and is intended to provide pain management over a period of 5-7 days. The product candidate is designed to
potentially provide longer term post-surgical pain relief compared to the currently available products in the market.
Based
on initial discussions with the FDA regarding this program, Scienture believes this product candidate would require at least one Phase
3 clinical trial to support submission of a marketing application.
Scienture
anticipates submitting an IND and, if cleared by the FDA, initiating a Phase 1 single dose study in healthy adults in 2025 to conduct
an initial assessment of safety and tolerability of SCN-107.
Collaborations
On
May 26, 2020, Scienture entered into Feasibility Study and Animal Trial Material Manufacturing Agreement with Innocore Technologies,
B.V. (“Innocore”), as amended on December 2, 2022 (the “Innocore License”), for certain intellectual property
rights. Under the Innocore License, Innocore granted Scienture a worldwide exclusive, milestone, royalty-bearing and sublicensable license
to certain patent rights for the research and development of SCN-107 in postsurgical local and regional analgesia. Pursuant to the Innocore
License, Scienture and Innocore are required to jointly research, develop and manufacture the licensed product, including by adhering
to a development and manufacturing plan, and Scienture must launch and market the licensed product as soon as commercially feasible.
Critical
Accounting Estimates
The
significant accounting policies and basis of presentation for Scienture’s consolidated financial statements are described in Note
2, Summary of Significant Accounting Policies, in the Notes to Scienture’s Consolidated Financial Statements, included elsewhere
herein. Scienture’s consolidated financial statements are prepared in accordance with the U.S. generally accepted accounting principles
(“U.S. GAAP”), requiring Scienture to make estimates, judgments, and assumptions that affect the reported amounts of assets,
liabilities, revenues, and expenses, and other related disclosures. Some judgments can be subjective and complex, and therefore, actual
results could differ materially from those estimates under different assumptions or conditions.
The
preparation of Scienture’s financial statements in conformity with U.S. GAAP requires Scienture to make estimates and assumptions
that affect the reported amounts of certain assets and liabilities; the reported amounts of revenues and expenses for the periods covered
and certain amounts disclosed in the notes to the financial statements. To the extent there are material differences between Scienture’s
estimates and the actual results, Scienture’s future consolidated results of operation may be affected. Areas requiring significant
estimates and assumptions by Scienture include, but are not limited to:
| ● | fair
value of long-term convertible debt and warrants issued in connection with such debt; |
| | | |
| ● | accruals
for estimated liabilities; |
| | | |
| ● | lease
term, |
| | | |
| ● | the
valuation of stock-based compensation awards; and |
| | | |
| ● | provisions
for income taxes and related valuation allowances and tax uncertainties. |
Warrant
Valuation
Stock
warrant valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards is estimated
using the Black-Scholes option model with a volatility figure derived from an average of historical stock prices for comparable entities.
Scienture accounts for the expected life based on the contractual life of the warrants. The risk-free interest rate is determined from
the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the warrants.
Scienture
entered into a Consent and Waiver on July 25, 2024 (the “NVK Consent and Waiver”), regarding that certain Loan and
Security Agreement dated September 8, 2023, by and between NVK Finance LLC, a Nebraska Limited Liability Company (“NVK”)
and Scienture (the “NVK Loan Agreement”) in connection with the business combination with the Company. The NVK Loan
Agreement granted 4% warrants on a fully diluted basis to NVK to purchase common stock of Scienture. Under the NVK Consent and
Waiver, these warrants were converted into 5.25% warrants on a fully diluted basis, equaling 500,526 shares of outstanding common
stock of Scienture and placed in escrow.
Scienture also issued
2% warrants on a fully diluted basis to purchase common stock of Scienture to Nanocapital II, LLC, in connection with the closing of
the NVK Loan Agreement. As a condition to the closing of the merger in July 2024, these warrants were converted into 190,677 shares of
outstanding common stock of Scienture. Scienture no longer has any warrants outstanding. NVK is entitled to receive warrants to purchase
0.5 shares of Company common stock with respect to each share of Company common stock issued upon any conversion of Scienture’s
loan agreement with NVK discussed below.
Stock-Based
Compensation
Scienture’s
stock-based compensation expense relates to stock options. Stock compensation expense is based on their grant date fair value. The fair
values of stock-based compensations are recognized as compensation expense on a straight-line basis over the requisite service period
in which the awards are expected to vest. Scienture estimates the fair value of stock option awards on the grant date using the Black-Scholes
option-pricing model. The Black-Scholes model considers several variables and assumptions in estimating the fair value of stock-based
awards. These variables include the per share fair value of the underlying common stock, exercise price, expected term, risk-free interest
rate, expected annual dividend yield and the expected stock price volatility over the expected term. Scienture has estimated volatility
by reference to the historical volatilities of Scienture and those of similar publicly traded peer companies. The risk-free interest
rate is based on the yield available on U.S. Treasury zero-coupon issues similar in duration to the expected term of the equity-settled
award.
Deferred
Tax Assets and Liabilities
Deferred
tax assets and liabilities are determined based on the differences between the financial statement and the tax basis of assets and liabilities.
Realization of the future tax benefits related to the net deferred tax assets is dependent on many factors including Scienture’s
ability to generate taxable income. Management believes that, at a minimum, it is more likely than not that future taxable income may
not be sufficient to realize the recorded assets.
Revenue
Recognition
Scienture’s
main revenue source has been milestone payments and reimbursement of costs related to the products. Revenue has been recognized when
such development milestone events take place, and the amounts are due to be received. The Kesin Agreement under which Scienture has recognized
revenue in the past was terminated in March 2024.
Revenue
is recognized when control of the promised services is transferred to customers, at an amount that reflects the consideration to which
the entity expects to be entitled in exchange for those services. Scienture adopted FASB ASU No. 2014-09, Revenue from Contracts with
Customers and the related amendments, which are codified into ASC 606, which establishes a broad principle that requires entities to
assess the products or services promised in contracts with customers at contract inception to determine the appropriate unit at which
to record revenues, which is referred to as a performance obligation. Revenue is recognized when control of the promised products or
services is transferred to customers, at an amount that reflects the consideration to which the entity expects to be entitled in exchange
for those products or services.
To
determine revenue recognition for arrangements that Scienture determines are within the scope of ASC 606, Scienture performs the following
five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii) determine
the transaction price; (iv) allocate the transaction price to the performance obligation(s) in the contract; and (v) recognize revenue
when (or as) the entity satisfies a performance obligation. Scienture only applies the five-step model to contracts when it is probable
that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the customer.
At contract inception, once the contract was determined to be within the scope of ASC 606, Scienture assessed the goods or services promised
within each contract and determined those that were performance obligations and assessed whether each promised good or service was distinct.
Scienture then recognizes as revenue the transaction price allocated to the respective performance obligation when (or as) the performance
obligation is satisfied.
Results
of Operations
Six
Months ended June 30, 2024 and Six Months Ended June 30, 2023
Revenues
Scienture
recognized revenues of $0.8 million in the six months ended June 30, 2023. There was no revenue recorded in the six months ended June 30, 2024;
this is the result of the termination of the Kesin Agreement, under which Scienture had recognized revenue related primarily to milestone
payments and reimbursement of costs related to the products in the U.S. in prior periods.
Research
and Development Expense (“R&D”)
R&D
expenses were $1.5 million and $1.0 million for the six months ended June 30, 2024 and 2023, respectively. This increase is a result
of the timing of activities with the contract manufacturing organization related to SCN-102 (product stability and regulatory activities)
and SCN-104 (device development, assembly set-up, and scale-up).
Termination
Fee
Termination
Fee is a charge of $1.285 million and $0.0 in the six months ended June 30, 2023 and 2024, respectively, to record an obligation to Kesin
upon entry into the Kesin Termination Agreement. This activity is associated with R&D and is reported separately from R&D due
to materiality.
General
and Administrative Expense (“G&A”)
G&A
expenses were $1.4 million and $0.3 for the six months ended June 30, 2024 and 2023, respectively. This increase of $1.1 million is primarily
a result of external legal and professional fees to support of the business combination that occurred in July 2024. Remaining costs are
primarily internal employee-related costs and office-related charges.
Other
Income (Expense)
Other
income includes primarily interest and dividends earned from cash, cash equivalents, and marketable securities holdings. Interest
expense is associated with unpaid interest on convertible debt, which was converted into Scienture equity, and in turn Company
common stock, in the merger, and a loan agreement with NVK.
Income
Tax Expense
As
Scienture continues to operate at a loss, no
provision for federal or state income tax has been recognized.
Net
Earnings
Scienture continues to operate at a net loss which amounted to $4.4 million
and $0.5 million for the six months ended June 30, 2024 and 2023, respectively. This increase in loss of $3.0 million is a result of the
costs associated with development work for SCN-102 and SCN-104, entry into the Kesin Termination Agreement and legal and professional
fees for the merger that occurred in July 2024.
Year
ended December 31, 2023 compared to year ended December 31, 2022
Revenues
Revenues
consist primarily of milestone payments and reimbursement of costs related to the product candidates in the U.S. under the agreement
with Kesin, which was subsequently terminated. Scienture recognized revenues of $0.8 million and $0.3 million in 2023 and 2022, respectively.
The increase in revenue of $0.5 million or 167% was a result of an increase in development work on SCN-104 that was reimbursed by Kesin
as well as achievement of a regulatory milestone on SCN-102 under Scienture’s agreement with Kesin which was terminated in May
2024.
Research
and Development Expense (“R&D”)
R&D
expenses were $2.0 million and $3.1 million for the twelve months ended December 31, 2023 and 2022, respectively. This decrease in expense
is a result of the submission of SCN-102 to the FDA in the fourth quarter of 2023. Scienture was incurring significant spend on two projects,
SCN-102 and SCN-104, in 2022 with SCN-102 being submitted to the FDA 2023.
General
and Administrative Expense (“G&A”)
G&A
expenses were $0.7 million and $0.9 million for the twelve months ended December 31, 2023 and 2022, respectively. This decrease of $0.2
million, or 22%, is a result of lower employee-related costs which was a reduction in variable compensation to employees. Scienture also
experienced a decrease in insurance related costs.
Other
Income (Expense)
Interest
expense is associated with unpaid interest on convertible debt and a loan agreement with NVK. The interest expense increase is a result
of the NVK loan agreement only starting in the third quarter of 2023 and a second convertible note issued in 2023. Other income includes primarily interest and dividends earned from cash, cash equivalents, and marketable securities holdings.
Miscellaneous income is from funds received in association with the Employee Retention Tax Credit (“ERC”) that the federal
government made available to Scienture.
Income
Tax Expense
As
Scienture continues to operate at a loss, no provision for federal or state income tax has been recognized.
Net
Earnings
Scienture
continues to operate at a net loss which amounted to $2.2 million and $3.7 million for the twelve months ended December 31, 2023 and
2022, respectively. This reduction in loss of $1.5 million, or 41%, is a result of the reduction in R&D spend with the filing of
SCN-102 combined with the increase in outstanding debt to fund operations.
Cash
Flows
Six
Months Ended June 30, 2024 and Six Months ended June 30, 2023
Operating
Activities
Net
cash used in operating activities is comprised of two components: cash used in operating loss; and cash used from changes in working
capital. The net cash used in operating activities was $1.0 million for the six months ended June 30, 2024. This use of cash was for
the payment of employees and employee-related expenses, and external spend with contract research/manufacturing organizations to support
SCN-002 and SCN-004.
Net cash used in
operating activities was $0.6 million for the six months ended June 30, 2023, primarily driven by our Scienture’s net loss of $0.5
million and cash used from changes in working capital of $0.2 million.
Investing
& Financing Activities
Scienture
had no investing activities in the six months ended June 30, 2024 or 2023.
Scienture
had no financing activities in the six months ended June 30, 2024. During the six months ended June 30, 2023, Scienture received $0.4
million from the proceeds of convertible notes.
Year
ended December 31, 2023 and year ended December 31, 2022
Net
change in cash and cash equivalents generated $1.1 million and $0.6 million for the twelve months ended December 31, 2023 and 2022, respectively.
This increase of $0.5 million or 83% is a result of issuance of debt securities to finance operations of the company.
Operating
Activities
Net
cash used by operating activities is comprised of two components: cash used in operating loss and cash used from changes in working capital.
The net cash used in operating activities was $2.2 million and $3.4 million for the twelve months ended December 31, 2023 and 2022, respectively.
The decrease of $1.2 million or 35% is primarily a result of submission of SCN-102 to FDA in the third quarter of 2023. This use of cash
was for the payment of employees and employee-related expenses, and external spend with contract research/manufacturing organizations
to support SCN-002 and SCN-004.
Investing
Activities
Scienture
had no investing activities for the years ended December 31, 2023 and 2024.
Financing
Activities
Scienture
had cash from financing activities representing inflows of $2.7 million and $0.9 million for the twelve months ended December 31, 2023
and 2022, respectively. These funds were the result of the issuance of short-term convertible securities and longer-term debt discussed
above. These funds were primarily used to finance ongoing development activities.
Liquidity
and Capital Resources
Scienture’s
cash and cash equivalents and money market securities are as follows (dollars in thousands) as of June 30, 2024:
| | |
June 30, 2024 | |
| Cash and cash equivalents | |
$ | 109 | |
| Money market securities | |
$ | 5 | |
| Total | |
$ | 114 | |
Since
inception Scienture has generated losses. Scienture has financed its operations primarily with cash raised through equity or debt financing.
Scienture expects that proceeds from equity and/or debt financings will constitute a significant component of the funding for Scienture’s
operations, particularly before it is able to generate revenues, which will require obtaining FDA approval of a product candidate or
entering collaboration, out-license or similar agreements.
Scienture’s
ability to continue as a going concern in the next twelve months is dependent upon its ability to produce revenues and/or obtain financing
sufficient to meet current and future obligations and deploy such to produce profitable operating results. Following the completion of
the merger in July 2024, Scienture expects its current cash resources, together with those of its parent company, to be sufficient to
fund its operations through September 2024. Management intends to seek to raise capital through equity and/or debt issuances, and to
seek to generate revenue after any FDA approval of its product candidates.
Scienture
expects to consider raising additional capital through financings or equity securities of the Company, and/or debt, new collaborative
arrangements; strategic alliances; or financing from other sources, including in conjunction with opportunistic business development
initiatives. Scienture will continue to actively manage its capital structure and to consider all financing opportunities that could
strengthen its long-term financial profile. There can be no assurance that any such financing opportunities will be available on acceptable
terms, if at all.
If
additional funds are raised through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third
parties, Scienture may be required to relinquish valuable rights to its technologies, future revenue streams, research programs or product
candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise capital when needed or on acceptable
terms, it would have a negative impact on our financial condition and we could be forced to delay, reduce or eliminate our research,
clinical trials, product development or future commercialization efforts.
Scienture’s
material cash requirements include the following contractual and other obligations.
Outstanding
Debt
As
of June 30, 2024, Scienture had $2,000,000 of debt outstanding, none of the principal of which is payable within 12 months.
In
September 2023, Scienture entered a loan agreement with NVK for $2,000,000, which bears interest at a rate of Prime + 7.0% per annum,
which, as of June 30, 2024, was 15.50% per annum, payable in cash at maturity This debt is due upon maturity, together with all unpaid
interest expense, in September 2025. The outstanding balance under the NVK debt is convertible at the option of NVK at any time into
common stock of the combined company equivalent to a fully-diluted valuation of Scienture of $60,000,000. In addition, NVK shall receive 0.5 warrants (at a basis of $0.0001) for
each share issued to them at the time of conversion. Scienture’s
obligations under the loan agreement with NVK are secured by a first priority security interest in all of its assets, including its intellectual
property rights.
On July 1, 2024, Scienture
issued a Demand Promissory Note to Pushpa Shankar in the amount of $215,000. Interest will accrue immediately, computed daily, at the
rate per annum equal to minimum applicable federal rate.
On July 10, 2024, Scienture issued a Demand Promissory
Note to Srivatsav, LLC in the amount of $50,000. Interest will accrue immediately, computed daily, at the rate per annum equal to minimum
applicable federal rate.
Contract
Termination Obligation
In
March 2024, Kesin and Scienture terminated the Kesin Agreement, and the parties agreed that Scienture would pay Kesin a total gross amount
of $1.285 million upon commercialization of SCN-102 or SCN-104 via a royalty arrangement. This agreement also requires that if the full
$1.285 million has not been repaid within two years of the earlier of (i) commercial launch or (ii) 120 days from FDA approval, then interest
will accrue prospectively at a rate of 8% annually on the unpaid balance.
In
August 2024, Kesin demanded immediate payment of the full amount under this agreement, alleging it is payable in connection with the
consummation of Scienture’s business combination with the Company. Scienture has disputed that the amount is now payable, and the
parties are in discussions to resolve the issue. There can be no assurance that an amicable resolution will be obtained. If Kesin brings
a legal action, Scienture will vigorously defend it.
Leases
Scienture’s
operating lease commitments for administrative office continues through December 31, 2026, with fixed payments of $0.1 million, with
$0.03 million payable within 12 months of June 30, 2024.
Funding
Requirements
We
expect our operating expenses to increase substantially in future years in connection with ongoing activities, particularly as we continue
the research and development of, continue or initiate clinical trials of, and seek marketing approval for any current and future product
candidates, including SCN-102, which has a PDUFA target action date in August 2024. In addition, we have begun to incur costs for pre-commercial
preparatory activities and, if marketing approval is obtained for any product candidates, we expect to incur significant commercialization
expenses related to product sales, marketing, manufacturing and distribution. In addition, inflation may affect our use of capital resources
by increasing our cost of labor, research and clinical trial expenses. Accordingly, there will be a need to obtain substantial additional
funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would
be forced to delay, reduce or eliminate research and development programs or future commercialization efforts.
We
anticipate that our expenses will increase substantially as we:
| ● | seek
to develop current and future clinical and preclinical product candidates; |
| ● | scale
up clinical and regulatory capabilities; |
| ● | adapt
regulatory compliance efforts to incorporate requirements applicable to marketed products; |
| ● | establish
a sales, marketing and distribution capabilities and scale up external manufacturing capabilities
to commercialize any product candidates for which regulatory approval may be obtained, including
SCN-102 which has a PDUFA target action date in August 2024; |
| ● | maintain,
expand and protect the intellectual property portfolio; |
| ● | hire
additional internal or external clinical, manufacturing and scientific personnel or consultants; |
| ● | add
operational, financial and management information systems and personnel, including personnel
to support product development efforts; and |
| ● | incur
additional legal, accounting and other expenses in operating as part of a public company. |
Because
of the numerous risks and uncertainties associated research, development and commercialization of product candidates, we are unable to
estimate the exact amount of its working capital requirements. Future funding requirements will depend on and could increase significantly
as a result of many factors, including:
| ● | the
scope, progress, results and costs of preclinical studies and clinical trials; |
| ● | the
scope, prioritization and number of research and development programs; |
| ● | the
costs, timing and outcome of regulatory review of product candidates; |
| ● | the
ability to establish and maintain collaborations on favorable terms, if at all; |
| ● | the
extent to which obligations to reimburse exist, or entitled to reimbursement of, clinical
trial costs under collaboration agreements, if any; |
| ● | the
costs of preparing, filing and prosecuting patent applications, maintaining and enforcing
intellectual property rights and defending intellectual property-related claims; |
| ● | the
costs of securing manufacturing arrangements for commercial production; and |
| ● | the
costs of establishing or contracting for sales and marketing capabilities if regulatory approvals
are obtained to market product candidates. |
Identifying
potential product candidates and conducting preclinical studies and clinical trials is a time- consuming, expensive and uncertain process
that takes many years to complete, and may never generate the necessary data or results required to obtain marketing approval and achieve
product sales. In addition, product candidates, if approved, may not achieve commercial success. Commercial revenues, if any, will be
derived from sales of product candidates that we do not expect to be commercially available for the next couple of years, if at all,
other than SCN-102, which potentially could be commercially available in 2025, if approved by the FDA on its PDUFA target action date
in August 2024. Accordingly, the need to continue to rely on additional financing to achieve our business objectives will exist. Adequate
additional financing may not be available on acceptable terms, or at all.
INTERESTS
OF CERTAIN PERSONS IN THE MATTERS TO BE ACTED UPON
As
it relates to the Stockholder Approval Matters, stockholders should be aware that certain of the Company’s officers and directors
have interests in such matters that may be different from, or in addition to, the interests of other Company stockholders. The Board
was made aware of, and considered these interests during its deliberations when it considered and approved the Merger Agreement, the
Mergers, and matters in connection therewith, including the matters comprising the Stockholder Approval Matters, and when it determined
to recommend to the Company’s stockholders that they vote in favor of the Stockholder Approval Matters. The Company’s
stockholders who executed and delivered the Stockholder Consent were made aware of these interests prior to their execution of the
Stockholder Consent.
As
described in this Information Statement, pursuant to the Merger Agreement on July 25, 2024, each of Mr. Suren Ajjarapu (the Company’s
Chief Executive Officer and Chairman) and Prashant Patel (the Company’s interim Chief Financial Officer and a director) entered
into a Consulting Agreement. Each Consulting Agreement is effective upon the termination of Mr. Ajjarapu’s and Mr. Patel’s
employment with the Company, as applicable. The terms of each Consulting Agreement provide for the issuance of Company common stock to
Mr. Ajjarapu and Mr. Patel, with those shares to be delivered in eight installments (the “Stock Consideration”). However,
each Consulting Agreement provides that the applicable Stock Consideration in any given calendar year will not be issued if, in any given
calendar year, in an amount greater than the amount of Company common stock then available to be issued under the Company’s executive
plans. As of the date of the Consulting Agreements there were an insufficient number of shares of Company common stock reserved and available
for issuance under 2019 EIP to permit the Company to deliver the aggregate Stock Consideration. As a result, Mr. Ajjarapu and Mr. Patel
have an interest in the Incentive Plan Share Increase.
EXECUTIVE
AND DIRECTOR COMPENSATION
2023
Summary Compensation Table
The
following table sets forth certain information concerning compensation earned by or paid to certain persons who we refer to as our “Named
Executive Officers” for services provided for the fiscal years ended December 31, 2023 and 2022.
| Name
and Principal Position |
|
Year |
|
|
Salary
($) |
|
|
Bonus
($) |
|
|
Stock
Awards
($)* |
|
|
Option
Awards
($)* |
|
|
All
Other Compensation
($) |
|
|
Total
($) |
|
| Suren
Ajjarapu |
|
|
2023 |
|
|
$ |
360,000 |
|
|
|
- |
|
|
|
243,075 |
|
|
$ |
- |
|
|
|
24,934 |
(3) |
|
$ |
628,009 |
|
| Chairman
of the Board, Chief Executive Officer, and Secretary |
|
|
2022 |
|
|
$ |
354,231 |
(1) |
|
|
- |
|
|
|
60,000 |
|
|
$ |
- |
|
|
|
16,647 |
(4) |
|
$ |
414,230 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Prashant
Patel |
|
|
2023 |
|
|
$ |
150,000 |
|
|
|
- |
|
|
|
43,650 |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
193,650 |
|
| President,
Chief Operating Officer, Interim Principal Financial/ Accounting Officer and Director |
|
|
2022 |
|
|
$ |
147,038 |
(2) |
|
|
- |
|
|
|
10,000 |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
157,038 |
|
| * |
Amounts
in this column represent the aggregate grant date fair value of awards computed in accordance with Financial Accounting Standards
Board Accounting Standard Codification Topic 718. Such grant date fair value does not take
into account any estimated forfeitures. The assumptions used in calculating the grant date fair value of restricted shares and option
awards are set forth in the Critical Accounting Estimates as disclosed in our Consolidated Financial Statements for the year ended
December 31, 2023. The amount reported in this column reflects the accounting cost for these awards and does not correspond to the
actual economic value that may be received by the officer upon the vesting of the restricted shares, the exercise of the stock options,
or any sale of the underlying shares of common stock. |
| |
|
| (1) |
The
amount shown reflects compensation under an employment agreement with the Company. |
| |
|
| (2) |
The
amount shown reflects compensation under an at will employment agreement with the Company.
|
| (3) |
Represents
the car allowance of $1,000 per month and a disability insurance policy paid for by the Company. |
| |
|
| (4) |
Represents
the car allowance of $1,000 per month and a disability insurance policy paid for by the Company. |
Narrative
Disclosure to 2023 Summary Compensation Table
Elements
of Compensation
The
compensation of our named executive officers generally consists of base salary and long-term incentive
compensation in the form of equity awards and other benefits, as described below.
2022
Reduced Officer Compensation
Effective
September 1, 2022, the Board and Compensation Committee, with the approval of each of the following officers, agreed to reduce the annual
cash compensation payable to Suren Ajjarapu, Prashant Patel, and Janet Huffman, in an effort to conserve cash.
Specifically,
effective beginning on September 1, 2022, the cash salaries of the officers set forth below were reduced in the following amounts, applied
pro rata for the 2022 fiscal year, which reductions in salary remained in place until January 1, 2023 (further described below):
| Officer |
|
Position
with Company |
|
Reduced
Cash Salary |
|
|
Shares
of the Company’s Common Stock In Lieu of Reduced Cash Salary |
|
| Suren
Ajjarapu |
|
Chief
Executive Officer and Secretary |
|
$ |
60,000 |
|
|
|
51,724 |
|
| Prashant
Patel |
|
President,
Chief Operating Officer and Interim Principal Financial/ Accounting Officer |
|
$ |
10,000 |
|
|
|
8,620 |
|
| Janet
Huffman(1) |
|
Former
Chief Financial Officer |
|
$ |
25,000 |
|
|
|
21,551 |
|
| (1) |
Effective
February 27, 2023, Ms. Janet Huffman, the Company’s former Chief Financial Officer notified the Company of the termination
of her Offer Letter dated February 3, 2022. Effective March 1, 2023, Ms. Huffman also transitioned from Chief Financial Officer to
a consulting relationship with the Company. Effective March 6, 2023, Prashant Patel, a member of the Board, the President and the
Chief Operating Officer of the Company, was appointed as Interim Principal Financial/Accounting Officer of the Company. |
In
lieu of the reduced cash salary payable to each officer, the Board and Compensation Committee agreed to issue such officers shares of
the Company’s common stock as set forth in the table above under “Shares of the Company’s Common Stock In Lieu of
Reduced Cash Salary,” equal to the amount of reduced cash salary set forth in the table above, divided by the closing sales
price of the Company’s common stock on Nasdaq on August 31, 2022, the date approved by the Board.
The
shares of common stock issuable to the officers, vested at the rate of 1/4th of such shares on each of September 30, 2022, October 31,
2022, November 30, 2022, and December 31, 2022, subject to each applicable officer’s continued service to the Company on such dates
and subject to the restricted stock award agreements entered into evidence such awards.
The
reductions in officer compensation were documented by amendments to the employment agreements with each officer. Mr. Ajjarapu’s
agreement also clarified that any severance payment paid to Mr. Ajjarapu under the terms of his employment agreement, described above,
would reduce any Change of Control Payment payable to Mr. Ajjarapu under the terms of his agreement, as amended.
The
reductions in cash salary discussed above were implemented in order for the Company to conserve cash and reduce its cash operating expenses.
All
of the awards discussed above were issued under the Incentive Plan and all restricted stock awards discussed above were evidenced by
restricted stock grant agreements.
2023
Increased Officer Compensation
Effective
January 1, 2023, the Board and the Compensation Committee, increased the annual salaries of each of Mr. Ajjarapu, Mr. Patel and Ms. Huffman
to the levels of their salaries prior to the September 1, 2022 decreases discussed above. Mr. Ajjarapu’s annual salary was increased
back to $360,000 per year, Mr. Patel’s annual salary was increased back to $150,000 per year. There were no changes made to terms
of the restricted stock shares discussed above.
The
increases in officer salaries were documented by amendments to the employment agreements with each officer. The amendments also clarified
that the equity compensation issuable to each officer was additional compensation and not specifically a result of the reduction in salaries
effective on September 1, 2022, and that the amount of reduced salary from September 1, 2022 to December 31, 2022 was forgiven by each
officer.
Compensation
Recovery and Clawback Policies
The
Board has adopted a Clawback Policy (the “Clawback Policy”) designed to comply with Section 10D of the Exchange Act of 1934,
the rules promulgated thereunder, and the listing standards of the national securities exchange on which the Company’s securities
are listed. The Clawback Policy is attached as Exhibit 97.1 to the Company’s Annual Report on Form 10-K/A filed with the SEC on
May 3, 2024.
Compensation
Risk Assessment
The
Compensation Committee has reviewed the relationship between our risk management policies and compensation policies and practices and
concluded that we do not have any compensation policies or practices that expose us to risks that are reasonably likely to have a material
adverse effect on the Company.
Policy
on Equity Ownership
The
Company does not have a policy on equity ownership at this time. However, all Named Executive Officers and directors are beneficial owners
of stock of the Company.
Rule
10b5-1 Trading Plans
Our
executive officers and directors are encouraged to conduct purchase or sale transactions under a trading plan established pursuant to
Rule 10b5-1 under the Exchange Act. Through a Rule 10b5-1 trading plan, the executive officer or director contracts with a broker to
buy or sell shares of our common stock on a periodic basis. The broker then executes trades pursuant to parameters established by the
executive officer or director when entering into the plan, without further direction from them. The executive officer or director may
amend or terminate the plan in specified circumstances.
Pledging
of Shares
Employees,
officers and directors of the Company are prohibited from pledging the Company’s securities as collateral for a loan. Additionally,
shares of the Company’s stock may not be held in a margin account.
Outstanding
Equity Awards At Fiscal Year-End
The
following table sets forth information as of December 31, 2023 concerning unexercised options, unvested stock and equity incentive plan
awards for each of the executive officers named in the Summary Compensation Table.
| Name |
|
Grant
Date |
|
Number
of Securities Underlying Unexercised Options (#) Exercisable |
|
|
Number
of Securities Underlying Unexercised Options (#) Unexercisable |
|
|
Equity
Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options |
|
|
Option
Exercise Price ($) |
|
Option
Expiration Date |
|
| Suren
Ajjarapu |
|
5/13/2019 |
|
|
1,111 |
|
|
|
- |
|
|
|
- |
|
|
$ |
39.60 |
|
05/13/2029 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Prashant
Patel |
|
5/13/2019 |
|
|
1,111 |
|
|
|
- |
|
|
|
- |
|
|
$ |
39.60 |
|
05/13/2029 |
|
Employment
Agreements with Our Named Executive Officers
Suren
Ajjarapu, Chief Executive Officer and Secretary
Effective
on April 14, 2020, we entered into an employment agreement with Mr. Suren Ajjarapu, our Chief Executive Officer, which replaced and superseded
his prior employment agreement with the Company.
The
agreement, which provides for Mr. Ajjarapu to serve as our Chief Executive Officer, has a term extending through December 31, 2025, provided
that the agreement automatically extends for additional one-year terms thereafter in the event neither party provides the other at least
60 days prior notice of their intention not to renew the terms of the agreement. The agreement also requires the Board, subject to certain
exceptions, to nominate Mr. Ajjarapu to serve on the Board at each stockholders’ meeting which occurs during the term of the agreement
and to serve as the Chairman of the Board.
Pursuant
to the terms of the agreement, Mr. Ajjarapu’s annual compensation package includes (1) a base salary of $360,000 per year ($300,000
for the 2020 fiscal year), subject to annual increases as determined in the sole discretion of the Compensation Committee of the Board
(the “Compensation Committee”), and as discussed below (the “Base Salary”), and (2) a performance bonus equal
to up to 100% of his Base Salary each year, based on the Company meeting certain performance metrics as determined from time to time
by the Compensation Committee and Mr. Ajjarapu (“Performance Metrics”). Additionally, in the event that Mr. Ajjarapu meets
at least 70% of the requirements for any annual performance bonus, as determined in the reasonable discretion of the Compensation Committee
of the Board (which requirement was met for the 2020 fiscal year, and which salary was automatically increased), Mr. Ajjarapu’s
Base Salary is increased by 20%. Mr. Ajjarapu is eligible for the Base Salary increase on an annual basis, with such increases being
cumulative. Such increases in Base Salary do not require an amendment to the agreement. Mr. Ajjarapu’s performance bonus metrics
include specific company performance goals and objectives, including revenue goals, app downloads, and net operating income milestones,
as may be modified or added to from time to time with the mutual approval of Mr. Ajjarapu and the Compensation Committee. The determination
of whether the Performance Metrics have been met are determined in the reasonable discretion of the Compensation Committee, no later
than 90 days after (a) December 31, 2020, in connection with the 2020 Performance Metrics; and (b) the end of such calendar year for
subsequent years. For the year ended December 31, 2020, Mr. Ajjarapu was awarded 49,020 shares of restricted common stock (the “2020
Restricted Stock”), valued at $372,062, based on the closing sales price of the Company’s common stock on the effective date
of grant, which vested in full. Mr. Ajjarapu may also receive additional bonuses awarded from time to time in the discretion of the Board
and/or Compensation Committee and the Board (in cash, options or other forms of equity) or the Compensation Committee may waive or change
the performance metrics associated with his performance bonus in their discretion. Mr. Ajjarapu’s compensation under his employment
agreement may be increased from time to time, by the Compensation Committee, or the Board (with the recommendation of the Compensation
Committee), which increases do not require the entry into an amended employment agreement. Mr. Ajjarapu is also paid an automobile allowance
of $1,000 per month during the term of the agreement and is eligible to participate in our stock option plan and other benefit plans.
The
agreement requires Mr. Ajjarapu to devote at least 75% of his business time and efforts to Company business. The agreement also prohibits
Mr. Ajjarapu from competing against us during the term of the agreement and for a period of twelve months after the termination of the
agreement in any state and any other geographic area in which we or our subsidiaries provide Restricted Services or Restricted Products,
directly or indirectly, during the twelve months preceding the date of the termination of the agreement. “Restricted Services”
means the manufacture, distribution, wholesale and sale of Restricted Products, healthcare services and any other services that we or
our subsidiaries have provided or are researching, developing, performing and/or providing at any time during the two years immediately
preceding the date of termination, or which Mr. Ajjarapu has obtained any trade secret or other confidential information about at any
time during the two years immediately preceding the date of termination of the agreement. “Restricted Products” means pharmaceutical
drugs and other healthcare products and any other product, that we or our subsidiaries have provided or are researching, developing,
manufacturing, distributing, purchasing, selling and/or providing at any time during the two years immediately preceding the date the
agreement is terminated, or which Mr. Ajjarapu obtained any trade secret or other confidential information in connection with at any
time during the two years immediately preceding the date of termination of the agreement.
We
may terminate Mr. Ajjarapu’s employment (a) for “cause” (which is defined to include, a material breach of the agreement
by Mr. Ajjarapu, any act of misappropriation of funds or embezzlement by Mr. Ajjarapu, Mr. Ajjarapu committing any act of fraud, or Mr.
Ajjarapu being indicted of, or pleading guilty or nolo contendere with respect to, theft, fraud, a crime involving moral turpitude, or
a felony under federal or applicable state law); (b) in the event Mr. Ajjarapu suffers a physical or mental disability which renders
him unable to perform his duties and obligations for either 90 consecutive days or 180 days in any 12-month period; (c) for any reason
without “cause”; or (d) upon expiration of the initial term of the agreement (or any renewal) upon notice as provided above.
The agreement also automatically terminates upon the death of Mr. Ajjarapu.
Mr.
Ajjarapu may terminate his employment (a) for “good reason” (i.e., (i) if his position or duties are modified to such an
extent that his duties are no longer consistent with the position of CEO of the Company, (ii) there has been a material breach by us
of a material term of the agreement or Mr. Ajjarapu reasonably believes that we are violating any law which would have a material adverse
effect on our operations and such violation continues uncured thirty days after such breach and after notice thereof has been provided
to us by Mr. Ajjarapu, (iii) Mr. Ajjarapu’s compensation is reduced without his consent, or we fail to pay to Mr. Ajjarapu any
compensation due to him upon five days written notice from Mr. Ajjarapu informing us of such failure, or (iv) if Mr. Ajjarapu is also
then serving as a member of the Board and is not re-nominated by the Board to serve as a member of the Board at any annual meeting of
stockholders of the Company; provided, however, prior to any such termination by Mr. Ajjarapu for “good reason”, Mr. Ajjarapu
must first advise us in writing (within 15 days of the occurrence of such event) and provide us 15 days to cure (5 days in connection
with the reduction of Mr. Ajjarapu’s salary or the failure to pay amounts owed to him)); (b) for any reason without “good
reason”; and (c) upon expiration of the initial term of the agreement (or any renewal) upon notice as provided above.
In
the event that Mr. Ajjarapu’s employment is terminated for any reason (not including, however, a termination by us for “cause”
or a termination as a result of Mr. Ajjarapu’s death or disability) during the twelve month period following a Change of Control
(a “Change of Control Termination”) or in anticipation of a Change of Control, we are required to pay Mr. Ajjarapu, within
60 days following the later of (i) the date of such Change of Control Termination; and (ii) the date of such Change of Control, a cash
severance payment in a lump sum in an amount equal to 3.0 times the sum of his current base salary and the amount of the last bonus payable
to Mr. Ajjarapu (the “Change of Control Payment”), which amount is due within 60 days of the later of (i) the date of such
Change of Control Termination; and (ii) the date of such Change of Control. If Mr. Ajjarapu’s employment terminates due to a Change
of Control Termination within six (6) months prior to a Change of Control, it will be deemed to be “in anticipation of a Change
of Control” for all purposes. In addition, in the event of a Change of Control, all of Mr. Ajjarapu’s equity-based compensation
immediately vests to Mr. Ajjarapu and any outstanding stock options held by Mr. Ajjarapu can be exercised by Mr. Ajjarapu until the earlier
of (A) one (1) year from the date of termination and (B) the latest date upon which such stock options would have expired by their original
terms under any circumstances, provided that if Mr. Ajjarapu’s employment ends in anticipation of a Change of Control and such
equity-based compensation awards or stock options have previously expired pursuant to their terms, the Company is required to pay Mr.
Ajjarapu a lump sum payment, payable on the same date as the Change of Control Payment, equal to the Black Scholes value of the expired
and unexercised equity compensation awards and stock options held by Mr. Ajjarapu on the date of termination, based on the value of such
awards had they been exercisable through the end of their stated term and had not previously expired. “Change of Control”
for the purposes of the agreement means: (a) any person obtaining beneficial ownership representing more than 50% of the total voting
power represented by our then outstanding voting securities without the approval of not fewer than two-thirds of our Board; (b) a merger
or consolidation of us whether or not approved by our Board, other than a merger or consolidation that would result in our voting securities
immediately prior thereto continuing to represent at least 50% of the total voting power outstanding immediately after such merger or
consolidation, (c) our stockholders approving a plan of complete liquidation or an agreement for the sale or disposition by us of all
or substantially all of our assets, or (d) as a result of the election of members to our Board, a majority of the Board consists of persons
who are not members of the Board on April 14, 2020, except in the event that such slate of directors is proposed by a committee of the
Board or the Board; provided that if the definition of “Change of Control” in our Stock Incentive Plans or Equity Compensation
Plans is more favorable than the definition above, then such definition shall be controlling.
If
Mr. Ajjarapu’s employment is terminated pursuant to his death, disability, the end of the initial term (or any renewal term), without
“good reason” by Mr. Ajjarapu, or by us for “cause”, Mr. Ajjarapu is entitled to all salary accrued through the
termination date and no other benefits other than as required under the terms of employee benefit plans in which Mr. Ajjarapu was participating
as of the termination date. Additionally, any unvested stock options or equity compensation held by Mr. Ajjarapu immediately terminate
and are forfeited (unless otherwise provided in the applicable award) and any previously vested stock options (or if applicable equity
compensation) are subject to the terms and conditions set forth in the applicable Stock Incentive Plan or Equity Compensation Plan, or
award agreement, as such may describe the rights and obligations upon termination of employment of Mr. Ajjarapu.
If
Mr. Ajjarapu’s employment is terminated by Mr. Ajjarapu for “good reason”, or by us without “cause”, Mr.
Ajjarapu is entitled to continue to receive the salary due pursuant to the terms of the agreement at the rate in effect upon the termination
date for eighteen (18) months, plus the pro rata amount of any discretionary bonus and performance bonus he would have been due for the
following eighteen (18) months (with any metrics being extrapolated based on the last four (4) full prior quarters of the Company’s
operations prior to termination). Additionally, unvested benefits (whether equity or cash benefits and bonuses) will vest immediately
upon such termination and any outstanding stock options previously granted to Mr. Ajjarapu will vest immediately upon such termination
and will be exercisable until the earlier of (A) one year from the date of termination and (B) the latest date upon which such stock
options would have expired by their original terms under any circumstances. Mr. Ajjarapu is also to receive, if he elects, continued
health insurance under COBRA, paid for by the Company, for eighteen (18) months following the termination date (subject to certain rights
which reduce such obligation if Mr. Ajjarapu is covered by health insurance with a substantially similar level of insurance as prior
to the termination).
The
agreement contains standard assignment of inventions, indemnification and confidentiality provisions. Further, Mr. Ajjarapu is subject
to non-solicitation covenants during the term of the agreement.
Although
Mr. Ajjarapu will be prohibited from competing with us while he is employed with us, he will only be prohibited from competing for twelve
months after his employment with us ends pursuant to the agreement.
See
also “2022 Reduced Officer Compensation” and “2023 Increased Officer Compensation” above.
Prashant
Patel, President, Chief Operating Officer and Interim Principal Financial/Accounting Officer
Effective
March 31, 2024, we entered into an employment agreement with Mr. Prashant Patel, our President, Chief Operating Officer and Interim Principal
Financial/Accounting Officer, which replaced and superseded his prior employment agreement with the Company.
The
agreement, which provides for Mr. Patel to serve as our President Chief Operating Officer, has a term extending through December 31,
2025, provided that the agreement automatically extends for additional one-year terms thereafter in the event neither party provides
the other at least 60 days prior notice of their intention not to renew the terms of the agreement. The agreement also requires the Board,
subject to certain exceptions, to nominate Mr. Patel to serve on the Board at each stockholders’ meeting which occurs during the
term of the agreement.
Pursuant
to the terms of the agreement, Mr. Patel’s annual compensation package includes (1) a base salary of $350,000 per year, subject
to annual increases as determined in the sole discretion of the Chief Executive Officer, and as discussed below (the “Base Salary”),
and (2) a performance bonus equal to up to 100% of his Base Salary each year, based on the Company meeting certain performance metrics
as determined from time to time by the Compensation Committee of the Board (“Performance Metrics”). Additionally, in the
event that Mr. Patel meets at least 70% of the requirements for any annual performance bonus, as determined in the reasonable discretion
of the Compensation Committee of the Board (the “Compensation Committee”), Mr. Patel’s Base Salary will increase by
20%. Mr. Patel is eligible for the Base Salary increase on an annual basis, with such increases being cumulative. Such increases in Base
Salary do not require an amendment to the agreement. Mr. Patel’s performance bonus metrics are to be added to the agreement and
the Company currently contemplates that such metrics will include specific company performance goals and objectives, including revenue
goals, app downloads, and net operating income milestones, as may be modified or added to from time to time with the mutual approval
of Mr. Patel and the Compensation Committee. The determination of whether the Performance Metrics have been met are determined in the
reasonable discretion of the Compensation Committee, no later than 90 days after the end of such calendar year. Mr. Patel may also receive
additional bonuses awarded from time to time in the discretion of the Compensation Committee (in cash, options or other forms of equity).
Mr. Patel is also paid an automobile allowance of $1,000 per month during the term of the agreement and is eligible to participate in
our stock option plan and other benefit plans.
The
agreement requires Mr. Patel to devote at least 75% of his business time and efforts to Company business. The agreement also prohibits
Mr. Patel from competing against us during the term of the agreement and for a period of twelve months after the termination of the agreement
in any state and any other geographic area in which we or our subsidiaries provide Restricted Services or Restricted Products, directly
or indirectly, during the twelve months preceding the date of the termination of the agreement. “Restricted Services” means
the manufacture, distribution, wholesale and sale of Restricted Products, healthcare services and any other services that we or our subsidiaries
have provided or are researching, developing, performing and/or providing at any time during the two years immediately preceding the
date of termination, or which Mr. Patel has obtained any trade secret or other confidential information about at any time during the
two years immediately preceding the date of termination of the agreement. “Restricted Products” means pharmaceutical drugs
and other healthcare products and any other product, that we or our subsidiaries have provided or are researching, developing, manufacturing,
distributing, purchasing, selling and/or providing at any time during the two years immediately preceding the date the agreement is terminated,
or which Mr. Patel obtained any trade secret or other confidential information in connection with at any time during the two years immediately
preceding the date of termination of the agreement.
We
may terminate Mr. Patel’s employment (a) for “cause” (which is defined to include, a material breach of the agreement
by Mr. Patel, any act of misappropriation of funds or embezzlement by Mr. Patel, any act of fraud by Mr. Patel, or Mr. Patel being indicted
of, or pleading guilty or nolo contendere with respect to, theft, fraud, a crime involving moral turpitude, or a felony under federal
or applicable state law); (b) in the event Mr. Patel suffers a physical or mental disability which renders him unable to perform his
duties and obligations for either 90 consecutive days or 180 days in any 12-month period; (c) for any reason without “cause”;
or (d) upon expiration of the initial term of the agreement (or any renewal) upon notice as provided above. The agreement also automatically
terminates upon the death of Mr. Patel.
Mr.
Patel may terminate his employment (a) for “good reason” (i.e., (i) if his position or duties are modified to such an extent
that his duties are no longer consistent with the position of Chief Compliance Officer of the Company, (ii) there has been a material
breach by us of a material term of the agreement or Mr. Patel reasonably believes that we are violating any law which would have a material
adverse effect on our operations and such violation continues uncured thirty days after such breach and after notice thereof has been
provided to us by Mr. Patel, (iii) Mr. Patel’s compensation is reduced without his consent, or we fail to pay to Mr. Patel any
compensation due to him upon five days written notice from Mr. Patel informing us of such failure, or (iv) if Mr. Patel is also then
serving as a member of the Board and is not re-nominated by the Board to serve as a member of the Board at any annual meeting of stockholders
of the Company; provided, however, prior to any such termination by Mr. Patel for “good reason”, Mr. Patel must first advise
us in writing (within 15 days of the occurrence of such event) and provide us 15 days to cure (5 days in connection with the reduction
of Mr. Patel’s salary or the failure to pay amounts owed to him)); (b) for any reason without “good reason”; and (c)
upon expiration of the initial term of the agreement (or any renewal) upon notice as provided above.
In
the event that Mr. Patel’s employment is terminated for any reason (not including, however, a termination by us for “cause”
or a termination as a result of Mr. Patel’s death or disability) during the twelve month period following a Change of Control (a
“Change of Control Termination”) or in anticipation of a Change of Control, we are required to pay Mr. Patel, within 60 days
following the later of (i) the date of such Change of Control Termination; and (ii) the date of such Change of Control, a cash severance
payment in a lump sum in an amount equal to 3.0 times the sum of his current base salary and the amount of the last bonus payable to
Mr. Patel (the “Change of Control Payment”), which amount is due within 60 days of the later of (i) the date of such Change
of Control Termination; and (ii) the date of such Change of Control. If Mr. Patel’s employment terminates due to a Change of Control
Termination within six (6) months prior to a Change of Control, it will be deemed to be “in anticipation of a Change of Control”
for all purposes. In addition, in the event of a Change of Control, all of Mr. Patel’s equity-based compensation immediately vests
to Mr. Patel and any outstanding stock options held by Mr. Patel can be exercised by Mr. Patel until the earlier of (A) one (1) year
from the date of termination and (B) the latest date upon which such stock options would have expired by their original terms under any
circumstances, provided that if Mr. Patel’s employment ends in anticipation of a Change of Control and such equity-based compensation
awards or stock options have previously expired pursuant to their terms, the Company is required to pay Mr. Patel a lump sum payment,
payable on the same date as the Change of Control Payment, equal to the Black Scholes value of the expired and unexercised equity compensation
awards and stock options held by Mr. Patel on the date of termination, based on the value of such awards had they been exercisable through
the end of their stated term and had not previously expired. “Change of Control” for the purposes of the agreement means:
(a) any person obtaining beneficial ownership representing more than 50% of the total voting power represented by our then outstanding
voting securities without the approval of not fewer than two-thirds of our Board; (b) a merger or consolidation of us whether or not
approved by our Board, other than a merger or consolidation that would result in our voting securities immediately prior thereto continuing
to represent at least 50% of the total voting power outstanding immediately after such merger or consolidation, (c) our stockholders
approving a plan of complete liquidation or an agreement for the sale or disposition by us of all or substantially all of our assets,
or (d) as a result of the election of members to our Board, a majority of the Board consists of persons who are not members of the Board
on March 31, 2024, except in the event that such slate of directors is proposed by a committee of the Board or the Board; provided that
if the definition of “Change of Control” in our Stock Incentive Plans or Equity Compensation Plans is more favorable than
the definition above, then such definition shall be controlling.
If
Mr. Patel’s employment is terminated pursuant to his death, disability, the end of the initial term (or any renewal term), without
“good reason” by Mr. Patel, or by us for “cause”, Mr. Patel is entitled to all salary accrued through the termination
date and no other benefits other than as required under the terms of employee benefit plans in which Mr. Patel was participating as of
the termination date. Additionally, any unvested stock options or equity compensation held by Mr. Patel immediately terminate and are
forfeited (unless otherwise provided in the applicable award) and any previously vested stock options (or if applicable equity compensation)
are subject to the terms and conditions set forth in the applicable stock incentive plan or equity compensation plan, or award agreement,
as such may describe the rights and obligations upon termination of employment of Mr. Patel.
If
Mr. Patel’s employment is terminated by Mr. Patel for “good reason”, or by us without “cause”, Mr. Patel
is entitled to continue to receive the salary due pursuant to the terms of the agreement at the rate in effect upon the termination date
for eighteen (18) months, plus the pro rata amount of any discretionary bonus and performance bonus he would have been due for the following
eighteen (18) months (with any metrics being extrapolated based on the last four (4) full prior quarters of the Company’s operations
prior to termination). Additionally, unvested benefits (whether equity or cash benefits and bonuses) will vest immediately upon such
termination and any outstanding stock options previously granted to Mr. Patel will vest immediately upon such termination and will be
exercisable until the earlier of (A) one year from the date of termination and (B) the latest date upon which such stock options would
have expired by their original terms under any circumstances. Mr. Patel is also to receive, if he elects, continued health insurance
under COBRA, paid for by the Company, for eighteen (18) months following the termination date (subject to certain rights which reduce
such obligation if Mr. Patel is covered by health insurance with a substantially similar level of insurance as prior to the termination).
The
agreement contains standard assignment of inventions, indemnification and confidentiality provisions. Further, Mr. Patel is subject to
non-solicitation covenants during the term of the agreement.
Although
Mr. Patel will be prohibited from competing with us while he is employed with us, he will only be prohibited from competing for twelve
months after his employment with us ends pursuant to the agreement.
See
also “2022 Reduced Officer Compensation” and “2023 Increased Officer Compensation” above.
Director
Compensation
Summary
Independent Director Compensation Table
The
following table provides information regarding all compensation awarded to, earned by or paid to each person who served as a non-executive
director of the Company for some portion or all of 2023. Other than as set forth in the table and described more fully below, the Company
did not pay any fees, make any equity or non-equity awards, or pay any other compensation, to its non-employee directors. All compensation
paid to its employee directors is set forth in the tables summarizing executive officer compensation above.
| Name |
|
Fees
Earned or
paid
in cash |
|
|
Stock
Awards* |
|
|
Option
Awards** |
|
|
All
Other Compensation |
|
|
Total |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Donald
G. Fell(1) |
|
$ |
48,677 |
|
|
$ |
100,000 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
148,677 |
|
| Charles
L. Pope(2) |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
- |
|
| Jeff
Newell (3) |
|
$ |
30,375 |
|
|
$ |
108,250 |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
138,625 |
|
| Michael
L. Peterson (4) |
|
$ |
41,250 |
|
|
$ |
100,000 |
|
|
$ |
55,000 |
|
|
|
- |
|
|
$ |
196,250 |
|
| Candice
Beaumont (5) |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
- |
|
*
Amounts in this column represent the aggregate grant date fair value of awards computed in accordance with Financial Accounting Standards
Board Accounting Standard Codification Topic 718. Such grant date fair value does not take into
account any estimated forfeitures. The assumptions used in calculating the grant date fair value of restricted shares and option awards
are set forth in the Critical Accounting Estimates as disclosed in our Consolidated Financial Statements for the year ended December
31, 2023. The amount reported in this column reflects the accounting cost for these awards and does not correspond to the actual economic
value that may be received by the director upon the vesting of the restricted shares, the exercise of the stock options, or any sale
of the underlying shares of common stock.
**
Amounts in this column represent the aggregate grant date fair value of awards computed in accordance with the Black-Scholes option pricing
model. The Black-Scholes model considers several variables and assumptions in estimating the fair value of stock-based awards.
These variables include the per share fair value of the underlying common stock, exercise price, expected term, risk-free interest rate,
expected annual dividend yield and the expected stock price volatility over the expected term. The Company estimates volatility by reference
to the historical volatilities of the Company. The risk-free interest rate is based on the yield available on U.S. Treasury zero-coupon
issues similar in duration to the expected term of the equity-settled award.
(1)
As of December 31, 2023. Mr. Fell had been awarded $215,178 in vested stock options and $100,000 in vested
common stock of the Company.
(2)
Mr. Pope resigned from the Board effective January 3, 2023. Mr. Pope did not receive any fees or other compensation for serving
on the Board during a portion of 2023.
(3)
As of December 31, 2023, Mr. Newell had been awarded $108,250 in vested stock awards.
(4)
As of December 31, 2023, Mr. Peterson had been awarded $159,650 in stock options and $100,000 in common stock of
the Company.
(5)
Ms. Beaumont was appointed to the Board on July 31, 2023 and then resigned from the Board effective April 10, 2024. Ms. Beaumont
did not receive any fees or other compensation for serving on the Board during a portion of 2023.
Independent
Director Compensation Policy
Each
independent member of the Board is to receive an annual grant of restricted common stock of the Company equal to $55,000 in value, on
April 1st of each year (or such date thereafter as the awards are approved by the Board), and valued on such same date, based on the
closing sales price on such date (or the first business day thereafter), which restricted stock awards will vest at the rate of 1/4th
of such awards over the following four calendar quarters, subject to such directors continued service to the Company.
The
Company has also entered into an indemnification agreement with each member of the Board.
2023
Independent Director Compensation
Effective
on August 13, 2023, the Board approved the issuance of 12,222 shares of the Company’s common stock to each of Mr. Fell and
Mr. Peterson for services rendered to the Company during fiscal 2023, which shares were valued at $110,000. The Board also approved the
issuance of 14,056 shares of the Company’s common stock to Jeff Newell for services rendered during fiscal 2023. The shares vest
at the rate of 1/4th of such shares immediately on the grant date, and 1/4th of such shares on each of October 1, 2023, January 1, 2024
and April 1, 2024, subject to each applicable independent director’s continued service to the Company on such dates. Additionally,
the Board approved 10,000 shares with immediate vesting to each Board member to recognize the significant additional work for various
financing, sales, acquisitions, operations restructuring.
All
of the awards discussed above were issued under the Incentive Plan and all restricted stock awards discussed above were evidenced by
Restricted Stock Grant Agreements.
Changes
in Independent Director Cash Compensation
Also
on August 31, 2022, in an effort to conserve cash for operations, the Board approved a reduction in the annual cash retainer payable
to independent members of the Board from $35,000 per year to $26,750 per year, effective as of September 1, 2022. However, effective
January 1, 2023, the annual cash retainer payable to each independent member of the Board was increased back to $35,000.
Compensation
Committee Interlocks and Insider Participation
No
member of the Compensation Committee is an employee or a former employee of the Company. During 2023, none of our executive officers
(A) served as a member of the Compensation Committee (or other Board committee performing equivalent functions or, in the
absence of any such committee, the entire Board) of another entity, one of whose executive officers served on the Compensation
Committee (or other Board committee performing equivalent functions or, in the absence of any such committee, the entire Board)
of the Company; (B) served as a director of another entity, one of whose executive officers served on the Compensation Committee
(or other Board committee performing equivalent functions or, in the absence of any such committee, the entire Board)
of the Company; or (C) served as a member of the Compensation Committee (or other Board committee performing equivalent
functions or, in the absence of any such committee, the entire Board) of another entity, one of whose executive officers served
as a director of the Company.
Additionally,
no Compensation Committee member (1) was, during the fiscal year, an officer or employee of the registrant; (2) was formerly an officer
of the registrant (except as discussed above); or (3) had any relationship requiring disclosure by the Company under Section 404 of Regulation
S-K.
Accordingly,
the Compensation Committee members have no interlocking relationships required to be disclosed under SEC rules and regulations.
BENEFICIAL
OWNERSHIP OF SECURITIES
The
following table sets forth certain information regarding the beneficial ownership of the Company’s common stock as of July 25,
2024 by (i) each Named Executive Officer, (ii) each member of our Board, (iii) each person deemed to be the beneficial owner of more
than five percent (5%) of the Company’s common stock, and (iv) all of our executive officers and directors as a group. Unless otherwise
indicated, each person named in the following table is assumed to have sole voting power and investment power with respect to all shares
of the Company’s common stock listed as owned by such person. The address of each person is deemed to be the address of the Company
unless otherwise noted.
Beneficial
ownership is determined in accordance with the rules of the SEC and includes voting and/or investing power with respect to securities.
These rules generally provide that shares of the Company’s common stock subject to options, warrants or other convertible securities
that are currently exercisable or convertible, or exercisable or convertible within 60 days of July 25, 2024, are deemed to be outstanding
and to be beneficially owned by the person or group holding such options, warrants or other convertible securities for the purpose of
computing the percentage ownership of such person or group, but are not treated as outstanding for the purpose of computing the percentage
ownership of any other person or group. The percentages are based upon 1,458,506 shares of the Company’s common stock outstanding
as of July 25, 2024.
Beneficial
ownership as set forth below is based on our review of our record stockholders list and public ownership reports filed by certain stockholders
of the Company and may not include certain securities held in brokerage accounts or beneficially owned by the Company’s stockholders
described below.
To
our knowledge, except as indicated in the footnotes to this table and pursuant to applicable community property laws, as of July 25,
2024, (a) the persons named in the table have sole voting and investment power with respect to all shares of the Company’s common
stock shown as beneficially owned by them, subject to applicable community property laws; and (b) no person owns more than 5% of our
common stock. Unless otherwise indicated, the address for each of the officers or directors listed in the table below is 6308 Benjamin
Rd, Suite 708, Tampa, FL 33634. All of the securities reported below are shares of the Company’s common stock.
| Name
and Address of Beneficial Owner |
|
Amount
and Nature of Beneficial Ownership |
|
|
Percentage
of Class |
|
|
|
|
|
|
|
|
| Directors
and Named Executive Officers: |
|
|
|
|
|
|
|
|
| Suren
Ajjarapu, Chairman, CEO (1) |
|
|
211,214 |
|
|
|
14.5 |
% |
| Prashant
Patel, Interim CFO, Director, COO, and President (2) |
|
|
177,798 |
|
|
|
12.2 |
% |
| Donald
G. Fell, Director (3) |
|
|
37,407 |
|
|
|
2.6 |
% |
| Mayur
Doshi, Director |
|
|
— |
|
|
|
— |
|
| Subbarao
Jayanthi, Director |
|
|
— |
|
|
|
— |
|
| All
executive officers, directors and director nominees as a Group (six persons) |
|
|
426,419 |
|
|
|
29.1 |
% |
| |
|
|
|
|
|
|
|
|
| Greater
than 5% Stockholders: |
|
|
|
|
|
|
|
|
| Annapurna
Gundlapalli |
|
|
95,000 |
|
|
|
6.5 |
% |
| Nitesh
Patel |
|
|
87,265 |
|
|
|
6.0 |
% |
*
Less than 1%.
(1)
Includes (i) 86,092 shares of the Company’s common stock owned directly by Mr. Ajjarapu, (ii) 34,844 shares of the Company’s
common stock owned by the Surendra Ajjarapu Revocable Trust of 2007, which Mr. Ajjarapu claims beneficial ownership of, as Trustee, (iii)
89,167 shares of the Company’s common stock owned by the Sandhya Ajjarapu Revocable Trust of 2007, which shares Mr. Ajjarapu is therefore deemed to beneficially own, and (iv) options to purchase 1,111 shares
of the Company’s common stock granted in 2019, that are exercisable within 60 days of July 25, 2024.
(2)
Includes (i) 112,242 shares of the Company’s common stock owned directly by Mr. Patel, (ii) 27,778 shares of the Company’s
common stock owned by Rina Patel, Mr. Patel’s wife, which Mr. Patel claims beneficial ownership of, (iii) 36,667 shares of the
Company’s common stock owned by the Patel Trust 2010, which Mr. Patel claims beneficial ownership of, as Trustee; and (iv)
options to purchase 1,111 shares of the Company’s common stock granted in 2019, that are exercisable within 60 days of July 25,
2024.
(3)
Includes (i) 35,163 shares of the Company’s common stock owned by DG Fell Consulting which Mr. Fell claims beneficial
ownership of, and (ii) 2,244 shares of the Company’s common stock issuable upon the exercise of stock options that are
exercisable within 60 days of July 25, 2024.
DESCRIPTION
OF SECURITIES
The
following summary describes the common stock of the Company, which common stock is registered pursuant to Section 12 of the Exchange
Act. Only the Company’s common stock is registered under Section 12 of the Exchange Act.
The
following description of our common stock is a summary and is qualified in its entirety by reference to our Certificate of Incorporation,
as amended and our Bylaws, as amended, which are incorporated by reference herein, and by applicable law. For purposes of this description,
references to the “Company,” “we,” “our” and “us” refer only to the Company and not to
its subsidiaries.
Authorized
Capitalization
As
of August [●], 2024, the Company has authorized 100,000,000 shares of the Company’s common stock and 10,000,000 shares of
preferred stock, including 9,211,246 shares of Series X Preferred Stock. As of that same date, the Company had [1,750,042] shares
of its common stock issued and outstanding and 6,826,753 shares of Series X Preferred Stock outstanding.
Company’s
Common Stock
Voting
Rights. Each share of our common stock is entitled to one vote on all stockholder matters. Shares of our common stock do not
possess any cumulative voting rights.
Except
for the election of directors, if a quorum is present, an action on a matter is approved if it receives the affirmative vote of the holders
of a majority of the voting power of the shares of capital stock present in person or represented by proxy at the meeting and entitled
to vote on the matter, unless otherwise required by applicable law, Delaware law, our Certificate of Incorporation, as amended or Bylaws,
as amended. The election of directors will be determined by a plurality of the votes cast in respect of the shares present in person
or represented by proxy at the meeting and entitled to vote, meaning that the nominees with the greatest number of votes cast, even if
less than a majority, will be elected. The rights, preferences and privileges of holders of common stock are subject to, and may be impacted
by, the rights of the holders of shares of any series of preferred stock that we have designated, or may designate and issue in the future.
Dividend
Rights. Each share of our common stock is entitled to equal dividends and distributions per share with respect to the common
stock when, as and if declared by our Board, subject to any preferential or other rights of any outstanding preferred stock.
Liquidation
and Dissolution Rights. Upon liquidation, dissolution or winding up, our common stock will be entitled to receive pro rata on
a share-for-share basis, the assets available for distribution to the stockholders after payment of liabilities and payment of preferential
and other amounts, if any, payable on any outstanding preferred stock.
Fully
Paid Status. All outstanding shares of the Company’s common stock are validly issued, fully paid and non-assessable.
Listing.
Our common stock is listed and traded on Nasdaq under the symbol “MEDS”.
Other
Matters. Shares of our Series X Preferred Stock will automatically convert as of the earliest date permitted by the listing rules
of Nasdaq because the Majority Stockholders have approved the Preferred Stock Conversion.
Series
X Preferred Stock
Voting
Rights. Holders of our shares of Series X Preferred Stock generally are not entitled to vote on stockholder matters, unless required
by the DGCL or the Series X Certificate of Designation.
Dividend
Rights. Holders of our shares of Series X Preferred Stock are entitled to participate in dividends or to the same extent as holders
of the Company’s common stock, but are limited to receiving such amounts based on the amount of shares of the Company’s common
stock that holders of our Series X Preferred Stock would receive upon conversion of the Series X Preferred Stock into shares of the Company’s
common stock.
Liquidation
and Dissolution Rights. The Series X Preferred Stock ranks on parity with of the Company’s common stock as to distributions
of assets upon the Company’s liquidation, dissolution, or winding up. In the event of a liquidation, dissolution, or winding up
of the Company, holders of our Series X Preferred Stock are entitled to receive the same amount that a holder of the Company’s
common stock would receive if the Series X Preferred Stock were fully converted to the Company’s common stock, plus unpaid dividends.
This payment is to be paid to holders of the Series X Preferred Stock and the Company’s common stock on a pari passu basis.
Fully
Paid Status. All shares of the Company’s common stock to be issued as a the result of the conversion of the Series X Preferred
Stock will be validly issued, fully paid and non-assessable.
Listing.
Our Series X Preferred Stock is not listed or traded on a stock exchange.
Other
Matters. Shares of our Series X Preferred Stock will automatically convert into shares of the Company’s common stock as
of the earliest date permitted by the listing rules of Nasdaq because the Majority Shareholders have approved the Preferred Stock Conversion.
Anti-Takeover
Effects Under Section 203 of Delaware General Corporation Law, our Certificate of Incorporation and Bylaws
Section
203 of the DGCL prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period
of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
| |
● |
before
such date, the Board approved either the business combination or the transaction that resulted in
the stockholder becoming an interested stockholder; |
| |
|
|
| |
● |
upon
completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned
at least 85 percent of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes
of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares
owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have
the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or an exchange offer;
or |
| |
|
|
| |
● |
on
or after such date, the business combination is approved by our Board and authorized at an annual or a special meeting
of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3 percent of the outstanding voting stock
that is not owned by the interested stockholder. |
In
general, Section 203 defines “business combination” to include the following:
| |
● |
any
merger or consolidation involving the corporation or any direct or indirect majority owned subsidiary of the corporation and the
interested stockholder or any other corporation, partnership, unincorporated association, or other entity if the merger or consolidation
is caused by the interested stockholder and as a result of such merger or consolidation the transaction is not excepted as described
above; |
| |
|
|
| |
● |
any
sale, transfer, pledge, or other disposition (in one transaction or a series) of 10% or more of the assets of the corporation involving
the interested stockholder; |
| |
|
|
| |
● |
subject
to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation
to the interested stockholder; |
| |
● |
any
transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series
of the corporation beneficially owned by the interested stockholder; or |
| |
|
|
| |
● |
the
receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges, or other financial benefits by or
through the corporation. |
In
general, Section 203 defines an “interested stockholder” as an entity or a person who, together with the person’s affiliates
and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own,
15 percent or more of the outstanding voting stock of the corporation.
A
Delaware corporation may “opt out” of these provisions with an express provision in its Certificate of Incorporation. Our
Certificate of Incorporation provides that we shall not be governed by Section 203 of DGCL and as a result, Section 203 of DGCL does
not apply to us.
Our
Certificate of Incorporation does not provide that our Board will be classified. As a result, a person can gain control of our
board only by successfully engaging in a proxy contest at one annual meeting.
Our
authorized but unissued common stock and preferred stock are available for future issuances without stockholder approval and could be
utilized for a variety of corporate purposes, including future offerings to raise additional capital, acquisitions and employee benefit
plans. The existence of authorized but unissued and unreserved common stock and preferred stock could render more difficult or discourage
an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Exclusive
forum for certain lawsuits
Our
Certificate of Incorporation requires, that unless the Company consents in writing to an alternative forum, the Court of Chancery of
the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for (a) any derivative action or
proceeding brought on behalf of the Company; (b) any action asserting a claim of breach of fiduciary duty owed by, or other wrongdoing
by, any director, officer, employee or agent of the Company to the Company or the Company’s stockholders; (c) any action asserting
a claim arising pursuant to any provision of the DGCL or the Certificate of Incorporation or Bylaws of the Company; (d) any action to
interpret, apply, enforce or determine the validity of the Certificate of Incorporation or Bylaws of the Company; or (e) any action asserting
a claim governed by the internal affairs doctrine, in each case subject to said Court of Chancery having personal jurisdiction over the
indispensable parties named as defendants therein (or such indispensable parties consenting to the personal jurisdiction of the Court
of Chancery within 10 days following any determination by the Court of Chancery that an indispensable party is not subject to such personal
jurisdiction); provided that, if and only if the Court of Chancery of the State of Delaware dismisses any action for lack of subject
matter jurisdiction, such action may be brought in another state or federal court sitting in the State of Delaware.
Notwithstanding
any other provisions of law, the Certificate of Incorporation or the Bylaws of the Company, and notwithstanding the fact that a lesser
percentage may be specified by law, the affirmative vote of the holders of at least two-thirds in voting power of the outstanding shares
of capital stock of the Company entitled to vote thereon shall be required to amend or repeal, or to adopt any provision inconsistent
with the exclusive forum requirements in our Certificate of Incorporation. If any provision or provision of the exclusive forum requirements
in our Amended and Restated Certificate of Incorporation shall be held to be invalid, illegal or unenforceable as applied to any person
or entity or circumstance for any reason whatsoever, then, to the fullest extent permitted by law, the validity, legality and enforceability
of such provisions in any other circumstance and of the remaining provisions and the application of such provision to other persons or
entities and circumstances shall not in any way be affected or impaired thereby.
As
a result of the above, our Certificate of Incorporation provides that the exclusive forum provision will be applicable to the fullest
extent permitted by applicable law, subject to certain exceptions. However, Section 27 of the Exchange Act creates exclusive federal
jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder.
As a result, the exclusive forum provision will not apply to suits brought to enforce any duty or liability created by the Exchange Act
or any other claim for which the federal courts have exclusive jurisdiction. We also note that investors cannot waive compliance with
the federal securities laws and the rules and regulations thereunder. Section 22 of the Securities Act of 1933, as amended (“Securities
Act”), creates concurrent jurisdiction for state and federal courts over all suits brought to enforce any duty or liability created
by the Securities Act or the rules and regulations thereunder.
Special
meeting of stockholders
Our
Bylaws provide that special meetings of our stockholders may be called only by the chairperson of the Board, the chief executive
officer or president (in the absence of a chief executive officer). Because our stockholders do not have the right to call a special
meeting, a stockholder could not force stockholder consideration of a proposal over the opposition of our Board by calling a special
meeting of stockholders prior to such time as the chairperson of the Board, the chief executive officer or president (in the absence
of a chief executive officer) believed the matter should be considered or until the next annual meeting provided that the requestor met
the notice requirements. The restriction on the ability of stockholders to call a special meeting means that a proposal to replace our
Board also could be delayed until the next annual meeting.
Advance
notice requirements for stockholder proposals and director nominations
Our
Bylaws provide that stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates for election
as directors at our annual meeting of stockholders, must provide timely notice of their intent in writing. Separately, pursuant to Rule
14a-8 of the Exchange Act, proposals seeking inclusion in our annual proxy statement must comply with the notice periods contained therein.
Our Bylaws also specify certain requirements as to the form and content of a stockholders’ meeting. These provisions may preclude
our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual
meeting of stockholders and may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the
acquirer’s own slate of directors or otherwise attempting to obtain control of our company.
Action
by written consent
Any
action required or permitted to be taken by our common stockholders may be effected by written consent of the stockholders having not
less than the minimum percentage of the vote required by DGCL for the proposed corporate action.
Vacancies
on the Board of Directors
Our
Bylaws provide that, subject to the rights of the holders of any outstanding series of preferred stock and unless otherwise required
by law or resolution of our Board, vacancies on the Board arising through death, resignation, retirement, disqualification
or removal, an increase in the number of directors or otherwise may be filled by a majority of the directors then in office, though less
than a quorum.
Amendment
to Bylaws by Stockholders
Subject
to certain limitations preventing amendments which decrease or diminish indemnification rights provided for in our Bylaws, our Bylaws
provide that any amendment to such Bylaws undertaken solely by our stockholders requires the affirmative vote of at least two-thirds
in voting power of the outstanding shares of capital stock of the Company.
CHANGE
IN AUDITOR
Dismissal
of MaloneBailey, LLP
On
September 14, 2023, the Company dismissed MaloneBailey, LLP (“MaloneBailey”) as its independent registered public accounting
firm to audit the Company’s financial statements, effective as of such date. The dismissal of MaloneBailey was approved by the
Audit Committee. MaloneBailey’s audit report on the Company’s financial statements for each of the fiscal years ended December
31, 2022 and 2021 did not contain an adverse opinion or a disclaimer of opinion, nor was it qualified or modified as to uncertainty,
audit scope, or accounting principles.
During
the Company’s two most recent fiscal years and the subsequent interim period through June 30, 2023, there were no (i) disagreements
(as defined in Item 304(a)(1)(iv) of Regulation S-K under the Exchange Act and the related instructions to that Item) with MaloneBailey
on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreement,
if not resolved to the satisfaction of MaloneBailey would have caused it to make reference to the subject matter of the disagreement
in connection with its report, or (ii) “reportable events” as that term is defined in Item 304(a)(1)(v) of Regulation S-K
under the Exchange Act.
Engagement
of CM3 Advisory
On
September 14, 2023, the Company engaged CM3 Advisory as its new independent registered public accounting firm of the Company. The engagement
of CM3 Advisory was approved by Audit Committee.
During
the Company’s two most recent fiscal years and the subsequent interim period through June 30, 2023, neither the Company nor anyone
on its behalf consulted with CM3 Advisory regarding: (i) the application of accounting principles to a specified transaction, either
completed or proposed, (ii) the type of audit opinion that might be rendered on the Company’s financial statements, and neither
a written report nor oral advice was provided to the Company that CM3 Advisory concluded was an important factor considered by the Company
in reaching a decision as to an accounting, auditing or financial reporting issue, or (iii) any matter that was either the subject of
a disagreement (as defined in Item 304(a)(1)(iv) of Regulation S-K under the Exchange Act and the related instructions to that Item)
or a reportable event (as described in Item 304(a)(1)(v) of Regulation S-K under the Exchange Act).
information
incorporated by reference
The
SEC allows us to “incorporate by reference” information that we file with them. Incorporation by reference allows us to disclose
important information to you by referring you to those other documents. The information incorporated by reference is an important part
of this Information Statement, and information that we file later with the SEC will automatically update and supersede this information.
The documents we are incorporating by reference into this Information Statement are:
| |
● |
our
Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed on April 22, 2024, as amended by our Amendment No.
1 to Form 10-K filed on May 3, 2024; |
| |
|
|
| |
● |
our
Quarterly Report on Form 10-Q for the period ended March 31, 2024, filed on June 26, 2024; |
| |
|
|
| |
● |
our Quarterly Report on Form 10-Q for the period ended
June 30, 2024, filed on August 9, 2024; and |
| |
|
|
| |
● |
our
Current Reports on Form 8-K (other than portions thereof furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits accompanying
such reports that relate to such items) filed on January 17, 2024; February 16, 2024; March 6, 2024; May 23, 2024; May 30, 2024;
June 20, 2024; July 9, 2024; and July 31, 2024. |
Any
statement contained herein or in a document incorporated or deemed to be incorporated by reference into this Information Statement will
be deemed to be modified or superseded for purposes of the document to the extent that a statement contained in this Information Statement
or any other subsequently filed document that is deemed to be incorporated by reference into this document modifies or supersedes the
statement.
We
will provide without charge to each person, including any beneficial owner, to whom this Information Statement is delivered, upon written
or oral request, a copy of any or all documents that are incorporated by reference into this Information Statement, but not delivered
with the prospectus, other than exhibits to such documents unless such exhibits are specifically incorporated by reference into the documents
that this Information Statement incorporates. You should direct oral or written requests to our corporate secretary, who can be contacted
at 6308 Benjamin Rd, Suite 708, Tampa, Florida 33634 or (866) 468-6535. You may also access these documents, free of charge on the SEC’s
website at www.sec.gov. Our website address is https://trxadehealth.com/. The information found on our website, or that may
be accessed by links on our website, is not part of this Information Statement. We have included our website address solely as an inactive
textual reference. Investors should not rely on any such information in deciding whether to purchase our common stock.
DELIVERY
OF DOCUMENTS TO SECURITY HOLDERS SHARING AN ADDRESS
Regulations
regarding the delivery of copies of information statements to stockholders permit us, banks, brokerage firms and other nominees to send
one information statement to multiple stockholders who share the same address under certain circumstances. This practice is known as
“householding.” Stockholders who hold their shares through a bank, broker or other nominee may have consented to reducing
the number of copies of materials delivered to their address. In the event that a stockholder wishes to revoke a “householding”
consent previously provided to a bank, broker or other nominee, the stockholder must contact the bank, broker or other nominee, as applicable,
to revoke such consent. If a stockholder wishes to receive a separate information statement, we will promptly deliver a separate copy
to such stockholder that contacts us at 6308 Benjamin Rd, Suite 708, Tampa, Florida 33634; or by telephone at: (866) 468-6535. Any stockholders
of record sharing an address who now receive multiple copies of our annual reports, proxy statements and information statements, and
who wish to receive only one copy of these materials per household in the future should also contact the Company’s Secretary by
mail or telephone as instructed above. Any stockholders sharing an address whose shares of our common stock are held by a bank, broker
or other nominee who now receive multiple copies of our annual reports, proxy statements and information statements, and who wish to
receive only one copy of these materials per household, should contact the bank, broker or other nominee to request that only one set
of these materials be delivered in the future.
WHERE
YOU CAN FIND MORE INFORMATION
We
file annual, quarterly, and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the
public over the Internet at the SEC’s website at http://www.sec.gov. Copies of documents filed by us with the SEC are also
available from us without charge, upon oral or written request to our Secretary, who can be contacted at 6308 Benjamin Rd, Suite 708,
Tampa, Florida 33634 or (866) 468-6535. Our website address is https://trxadehealth.com/. Information on, or that may be accessed through, our websites is not incorporated by reference into this Information Statement and should
not be considered a part of this Information Statement.
INDEX
TO FINANCIAL STATEMENTS
TRxADE
HEALTH, INC. Financial Statements
Scienture,
Inc. (n/k/a Scienture, LLC) Financial Statements
TRxADE
HEALTH, INC.
Condensed
Consolidated Balance Sheets
June
30, 2024 and December 31, 2023
(Unaudited)
| | |
June
30, | | |
December
31, | |
| | |
2024 | | |
2023 | |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash | |
$ | 7,719,993 | | |
$ | 314 | |
| Accounts receivable,
net | |
| 13,091 | | |
| - | |
| Inventory | |
| 6,439 | | |
| 968 | |
| Prepaid expenses | |
| 797,383 | | |
| 50,724 | |
| Notes receivable, related
party | |
| 1,300,000 | | |
| 1,300,000 | |
| Other receivables | |
| 2,230,797 | | |
| 1,224,702 | |
| Deferred offering costs | |
| 69,444 | | |
| - | |
| Current
assets of discontinued operations | |
| 7,297 | | |
| 176,355 | |
| Total current assets | |
| 12,144,444 | | |
| 2,753,063 | |
| Property, plant and equipment, net | |
| 6,500 | | |
| 7,500 | |
| Deposits | |
| 22,039 | | |
| 10,531 | |
| Investments | |
| 2,500,000 | | |
| - | |
| Operating lease right-of-use assets | |
| 175,550 | | |
| 191,216 | |
| Noncurrent assets
of discontinued operations | |
| - | | |
| 9,570,603 | |
| Total
assets | |
$ | 14,848,533 | | |
$ | 12,532,913 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’
EQUITY (DEFICIT) | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 726,266 | | |
$ | 1,463,014 | |
| Accrued liabilities | |
| 500,454 | | |
| 160,214 | |
| Other current liabilities | |
| 5,441 | | |
| 67,831 | |
| Contingent funding liabilities | |
| - | | |
| 1,246,346 | |
| Lease liability, current | |
| 32,608 | | |
| 32,595 | |
| Warrant liability | |
| 1,631,974 | | |
| 736,953 | |
| Current
liabilities of discontinued operations | |
| 5,346 | | |
| 7,849,402 | |
| Total current liabilities | |
| 2,902,089 | | |
| 11,556,355 | |
| Lease liability, net of current portion | |
| 160,996 | | |
| 176,909 | |
| Noncurrent liabilities
of discontinued operations | |
| - | | |
| 257,296 | |
| Total
liabilities | |
| 3,063,085 | | |
| 11,990,560 | |
| | |
| | | |
| | |
| Commitments and contingencies (Note 16) | |
| | | |
| | |
| | |
| | | |
| | |
| Stockholders’ equity (deficit): | |
| | | |
| | |
| Series A preferred stock, $0.00001 par
value; 9,211,246 shares authorized; none issued and outstanding as of both June 30, 2024 and December 31, 2023 | |
| - | | |
| - | |
| Series B preferred stock, $0.00001 par
value; 787,754 shares authorized; 15,759 shares issued and outstanding as of both June 30, 2024 and December 31, 2023 | |
| - | | |
| - | |
| Series C preferred stock, $0.00001 par
value; 1,000 shares authorized; 290 shares issued and outstanding as of both June 30, 2024 and December 31, 2023 | |
| - | | |
| - | |
| Preferred stock, value | |
| - | | |
| - | |
| Common stock, $0.00001 par value; 100,000,000
shares authorized; 1,406,348 and 905,008 shares issued and outstanding as of June 30, 2024 and December 31, 2023, respectively | |
| 14 | | |
| 9 | |
| Additional paid-in capital | |
| 38,290,315 | | |
| 33,788,284 | |
| Accumulated
deficit | |
| (26,504,881 | ) | |
| (33,245,940 | ) |
| Total
stockholders’ equity | |
| 11,785,448 | | |
| 542,353 | |
| Total
liabilities and stockholders’ equity | |
$ | 14,848,533 | | |
$ | 12,532,913 | |
The
accompanying notes are an integral part of the unaudited consolidated financial statements.
TRxADE
HEALTH, INC.
Condensed
Consolidated Statements Of Operations
For
the Three and Six Months Ended June 30, 2024 and 2023
(Unaudited)
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| | |
Three
Months Ended | | |
Six
Months Ended | |
| | |
June
30, | | |
June
30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Revenues | |
$ | 18,699 | | |
$ | 366,526 | | |
$ | 18,699 | | |
$ | 842,882 | |
| Cost of sales | |
| 19,402 | | |
| 299,387 | | |
| 19,402 | | |
| 719,484 | |
| Gross (loss) profit | |
| (703 | ) | |
| 67,139 | | |
| (703 | ) | |
| 123,398 | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Wage and salary expense | |
| 312,049 | | |
| 156,300 | | |
| 534,644 | | |
| 337,893 | |
| Professional fees | |
| 509,136 | | |
| 188,343 | | |
| 688,689 | | |
| 324,297 | |
| Accounting and legal expense | |
| 171,708 | | |
| 124,799 | | |
| 510,755 | | |
| 373,015 | |
| Technology expense | |
| 86,674 | | |
| 27,579 | | |
| 138,289 | | |
| 52,875 | |
| General and administrative | |
| 415,421 | | |
| 169,900 | | |
| 5,115,582 | | |
| 416,494 | |
| Total
operating expenses | |
| 1,494,988 | | |
| 666,921 | | |
| 6,987,959 | | |
| 1,504,574 | |
| Operating loss | |
| (1,495,691 | ) | |
| (599,782 | ) | |
| (6,988,662 | ) | |
| (1,381,176 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | | |
| | | |
| | |
| Change in fair value
of warrant liability | |
| (165,132 | ) | |
| (1,448,519 | ) | |
| (895,021 | ) | |
| (1,368,628 | ) |
| Interest income | |
| 41,031 | | |
| - | | |
| 103,952 | | |
| 4,198 | |
| Loss on disposal of
asset | |
| - | | |
| - | | |
| (374,968 | ) | |
| (352,244 | ) |
| Interest
expense | |
| (4,949 | ) | |
| (180,734 | ) | |
| (103,464 | ) | |
| (243,126 | ) |
| Total
non-operating expense | |
| (129,050 | ) | |
| (1,629,253 | ) | |
| (1,269,501 | ) | |
| (1,959,800 | ) |
| Net loss from continuing operations | |
| (1,624,741 | ) | |
| (2,229,035 | ) | |
| (8,258,163 | ) | |
| (3,340,976 | ) |
| Net (loss) income
from discontinued operations | |
| (209,161 | ) | |
| 254,157 | | |
| 27,670,294 | | |
| 688,145 | |
| Net (loss) income | |
$ | (1,833,902 | ) | |
$ | (1,974,878 | ) | |
$ | 19,412,131 | | |
$ | (2,652,831 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss per common share from continuing
operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (1.16 | ) | |
$ | (3.27 | ) | |
$ | (6.75 | ) | |
$ | (4.95 | ) |
| Diluted | |
$ | (1.16 | ) | |
$ | (3.27 | ) | |
$ | (6.75 | ) | |
$ | (4.95 | ) |
| Net (loss) income per common share from
discontinued operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (0.15 | ) | |
$ | 0.37 | | |
$ | 22.60 | | |
$ | 1.02 | |
| Diluted | |
$ | (0.15 | ) | |
$ | 0.37 | | |
$ | 19.02 | | |
$ | 1.02 | |
| Net (loss) income per common share | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (1.30 | ) | |
$ | (2.90 | ) | |
$ | 15.86 | | |
$ | (3.93 | ) |
| Diluted | |
$ | (1.30 | ) | |
$ | (2.90 | ) | |
$ | 13.35 | | |
$ | (3.93 | ) |
| Weighted average common shares outstanding | |
| | | |
| | | |
| | | |
| | |
| Basic | |
| 1,406,348 | | |
| 681,199 | | |
| 1,224,337 | | |
| 675,143 | |
| Diluted | |
| 1,406,348 | | |
| 681,199 | | |
| 1,454,558 | | |
| 675,143 | |
The
accompanying notes are an integral part of the unaudited consolidated financial statements.
TRxADE
HEALTH, INC.
Condensed
Consolidated Statements of Changes in Stockholders’ Equity
(Unaudited)
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Subsidiaries | | |
Equity | |
| | |
Series
B | | |
Series
C | | |
Common | | |
Additional | | |
| | |
Non-controlling | | |
Total | |
| | |
Preferred Stock | | |
Preferred
Stock | | |
Stock | | |
Paid-in | | |
Accumulated | | |
Interests
in | | |
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Subsidiaries | | |
Equity | |
| Balances at December 31, 2022 | |
| - | | |
$ | - | | |
| - | | |
$ | - | | |
| 626,247 | | |
$ | 6 | | |
$ | 20,482,666 | | |
$ | (19,719,536 | ) | |
$ | (420,269 | ) | |
$ | 342,867 | |
| Common stock issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| 14,362 | | |
| - | | |
| 63,486 | | |
| - | | |
| - | | |
| 63,486 | |
| Disposition of assets, related party | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 492,030 | | |
| 420,269 | | |
| 912,299 | |
| Warrants exercised for cash | |
| - | | |
| - | | |
| - | | |
| - | | |
| 40,116 | | |
| 1 | | |
| 6 | | |
| - | | |
| - | | |
| 7 | |
| Options expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 14,434 | | |
| - | | |
| - | | |
| 14,434 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (677,954 | ) | |
| - | | |
| (677,953 | ) |
| Balances at March 31, 2023 | |
| - | | |
| - | | |
| - | | |
| - | | |
| 680,725 | | |
| 7 | | |
| 20,560,592 | | |
| (19,905,459 | ) | |
| - | | |
| 655,140 | |
| Common stock issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 15,813 | | |
| - | | |
| - | | |
| 15,813 | |
| Warrants exercised for cash | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,795 | | |
| - | | |
| 1,615 | | |
| - | | |
| - | | |
| 1,615 | |
| Options expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 7,783 | | |
| - | | |
| - | | |
| 7,783 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,974,878 | ) | |
| - | | |
| (1,974,878 | ) |
| Balances at June
30, 2023 | |
| - | | |
$ | - | | |
| - | | |
$ | - | | |
| 682,520 | | |
$ | 7 | | |
$ | 20,585,803 | | |
$ | (21,880,337 | ) | |
$ | - | | |
$ | (1,294,527 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balances at December 31, 2023 | |
| 15,759 | | |
$ | - | | |
| 290 | | |
$ | - | | |
| 905,008 | | |
$ | 9 | | |
$ | 33,788,284 | | |
| (33,245,940 | ) | |
$ | - | | |
$ | 542,353 | |
| Cash dividends paid ($8 per share) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (12,671,072 | ) | |
| - | | |
| (12,671,072 | ) |
| Common stock issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| 470,482 | | |
| 5 | | |
| 4,450,914 | | |
| - | | |
| - | | |
| 4,450,919 | |
| Options exercised for cash | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,371 | | |
| - | | |
| 9,840 | | |
| - | | |
| - | | |
| 9,840 | |
| Warrants exercised for cash | |
| - | | |
| - | | |
| - | | |
| - | | |
| 28,487 | | |
| - | | |
| 16,567 | | |
| - | | |
| - | | |
| 16,567 | |
| Options expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 24,266 | | |
| - | | |
| - | | |
| 24,266 | |
| Net income | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 21,246,033 | | |
| - | | |
| 21,246,033 | |
| Balances at March 31, 2024 | |
| 15,759 | | |
| - | | |
| 290 | | |
| - | | |
| 1,406,348 | | |
| 14 | | |
| 38,289,871 | | |
| (24,670,979 | ) | |
| - | | |
| 13,618,906 | |
| Balance | |
| 15,759 | | |
| - | | |
| 290 | | |
| - | | |
| 1,406,348 | | |
| 14 | | |
| 38,289,871 | | |
| (24,670,979 | ) | |
| - | | |
| 13,618,906 | |
| Options expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 444 | | |
| - | | |
| - | | |
| 444 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,833,902 | ) | |
| - | | |
| (1,833,902 | ) |
| Net income (loss) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,833,902 | ) | |
| - | | |
| (1,833,902 | ) |
| Balances at June
30, 2024 | |
| 15,759 | | |
$ | - | | |
| 290 | | |
$ | - | | |
| 1,406,348 | | |
$ | 14 | | |
$ | 38,290,315 | | |
$ | (26,504,881 | ) | |
$ | - | | |
$ | 11,785,448 | |
| Balance | |
| 15,759 | | |
$ | - | | |
| 290 | | |
$ | - | | |
| 1,406,348 | | |
$ | 14 | | |
$ | 38,290,315 | | |
$ | (26,504,881 | ) | |
$ | - | | |
$ | 11,785,448 | |
The
accompanying notes are an integral part of the unaudited consolidated financial statements
TRxADE
HEALTH, INC.
Condensed
Consolidated Statements of Cash Flows
For
The Six Months Ended June 30, 2024 and 2023
(Unaudited)
| | |
2024 | | |
2023 | |
| | |
Six
Months Ended | |
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| Cash flows from operating
activities: | |
| | | |
| | |
| Net loss from continuing operations | |
$ | (8,258,163 | ) | |
$ | (3,340,976 | ) |
Adjustments to reconcile net loss to net
cash used in
operating activities: | |
| | | |
| | |
| Depreciation expense | |
| 1,000 | | |
| 5,643 | |
| Change in fair value of warrant liability | |
| 895,021 | | |
| 1,368,628 | |
| Options expense | |
| 24,710 | | |
| 22,217 | |
| Common stock issued
for services | |
| 4,450,919 | | |
| 79,299 | |
| Amortization of right-of-use
assets | |
| 15,666 | | |
| 100,197 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable,
net | |
| (13,091 | ) | |
| (38,761 | ) |
| Prepaid expenses and
deposits | |
| (758,167 | ) | |
| (3,766 | ) |
| Inventory | |
| (5,471 | ) | |
| (41,677 | ) |
| Other receivables | |
| (1,006,095 | ) | |
| - | |
| Lease liability | |
| (15,900 | ) | |
| (95,915 | ) |
| Accounts payable | |
| (736,748 | ) | |
| 180,926 | |
| Accrued liabilities | |
| 270,796 | | |
| (219,853 | ) |
| Current liabilities | |
| (62,390 | ) | |
| 724,561 | |
| Net
cash used in operating activities from continuing operations | |
| (5,197,913 | ) | |
| (1,259,477 | ) |
| Net
cash (used in) provided by operating activities from discontinued operations | |
| (769,805 | ) | |
| 656,512 | |
| Net
cash used in operating activities | |
| (5,967,718 | ) | |
| (602,965 | ) |
| Cash flows from investing
activities: | |
| | | |
| | |
| Investment in capitalized software | |
| - | | |
| (138,875 | ) |
| Investment in securities | |
| (2,500,000 | ) | |
| - | |
| Net cash used in
investing activities from continuing operations | |
| (2,500,000 | ) | |
| (138,875 | ) |
| Net cash provided
by investing activities from discontinued operations | |
| 29,931,815 | | |
| 420,269 | |
| Net
cash provided by investing activities | |
| 27,431,815 | | |
| 281,394 | |
| Cash flows from financing
activities: | |
| | | |
| | |
| Repayment of contingent liability | |
| (1,246,346 | ) | |
| (870,646 | ) |
| Cash dividends paid | |
| (12,671,072 | ) | |
| - | |
| Proceeds from sale of future revenue | |
| - | | |
| 825,000 | |
| Proceeds from exercise of warrants | |
| 16,567 | | |
| 1,622 | |
| Proceeds from exercise of options | |
| 9,840 | | |
| - | |
| Net cash used in
financing activities from continuing operations | |
| (13,891,011 | ) | |
| (44,024 | ) |
| Net cash used in
financing activities from discontinued operations | |
| (5,000 | ) | |
| - | |
| Net
cash used in financing activities | |
| (13,896,011 | ) | |
| (44,024 | ) |
| Net change in cash | |
| 7,568,086 | | |
| (365,595 | ) |
| Cash at beginning of period | |
| 151,907 | | |
| 1,111,156 | |
| Cash at end of period | |
$ | 7,719,993 | | |
$ | 745,561 | |
| | |
| | | |
| | |
| Supplemental disclosure
of cash flow information: | |
| | | |
| | |
| Cash paid for interest | |
$ | - | | |
$ | 243,126 | |
| Cash paid for taxes | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Non-Cash Transactions | |
| | | |
| | |
| Insurance premium financed | |
$ | 198,245 | | |
$ | 306,152 | |
| Deferred offering costs included in accrued expenses | |
$ | 69,444 | | |
$ | - | |
| Note issued as SOSRx contribution | |
$ | - | | |
$ | 500,000 | |
| Disposition of assets, related party | |
$ | - | | |
$ | 492,030 | |
The
accompanying notes are an integral part of the unaudited consolidated financial statements.
NOTE
1 – ORGANIZATION AND BASIS OF PRESENTATION
Overview
TRxADE
HEALTH, INC. (“we”, “our”, “Trxade”, and the “Company”) owns,
as of June 30, 2024, 100% of Trxade, Inc., Integra Pharma Solutions, LLC and Bonum Health, LLC
During
the year ended December 31, 2023 and a portion of the quarter ended March 31, 2024, Trxade, Inc., operated a web-based market platform
that enabled commerce among healthcare buyers and sellers of pharmaceuticals, accessories and services.
Integra
Pharma Solutions, LLC (“IPS” d.b.a. Trxade Prime), is a licensed pharmaceutical wholesaler and sells brand, generic and non-drug
products to customers. IPS customers include all healthcare markets including government organizations, hospitals, clinics and independent
pharmacies nationwide.
On
January 20, 2023, the Company entered into Membership Interest Purchase Agreements to sell 100% of the outstanding membership interests
of the Company’s former subsidiaries, Community Specialty Pharmacy, LLC (“CSP”) and Alliance Pharma Solutions, LLC
(“APS” d.b.a DelivMeds). The Company also agreed to enter into a Master Service Agreement to operate the businesses prior
to closing. Additional amounts owed to the Company as a result of this Master Service Agreement totaled $1,075,000 as of the closing
date of August 22, 2023 (see Note 3 and Note 7).
Bonum
Health, LLC (“Bonum Health”), was formed to hold certain telehealth assets acquired in October 2019. The “Bonum Health
Hub” was launched in February 2020; however, the Company does not anticipate installations moving forward. The Company is in the
process of dissolving Bonum Health, Inc. and Bonum Health, LLC and those entities are each dissolved in the second quarter of 2024.
Superlatus
Merger
On
July 14, 2023, the Company entered into an Amended and Restated Agreement and Plan of Merger (the “Merger Agreement”) with
Superlatus, Inc., a U.S.-based holding company of food products and distribution capabilities (“Superlatus”) and Foods Merger
Sub, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub”).
On
July 31, 2023, the Company completed its acquisition of Superlatus in accordance with the terms and conditions of the Merger Agreement
(the “Superlatus Merger”), pursuant to which the Company acquired Superlatus by way of a merger of the Merger Sub with and
into Superlatus, with Superlatus being a wholly owned subsidiary of the Company and the surviving entity in the Superlatus Merger.
Under
the terms of the Merger Agreement, at the closing of the Superlatus Merger (the “Closing”), shareholders of Superlatus received
in aggregate 136,441 shares of common stock of the Company, representing 19.99% of the then total issued and outstanding common stock
of the Company after the consummation of the Superlatus Merger and 306,855 shares of Company’s Series B Preferred Stock, par value
$0.00001 per share (the “Series B Preferred Stock”), with a conversion ratio of 100 shares of Series B Preferred Stock to
one share of common stock. At Closing, the value of the common stock was $7.30 per share, resulting in a total value of $225,000,169.
Upon consummation of the Superlatus Merger, the Company continued to trade under the current ticker symbol “MEDS.”
Not
all of the closing conditions of the Merger Agreement were met. As a result, the Company entered into Amendment No. 1 to the Amended
and Restated Agreement and Plan of Merger (the “Amendment”) on January 8, 2024. Under the terms of the Amendment, the merger
consideration to the shareholders of Superlatus was adjusted to the aggregate of 136,441 shares of common stock of the Company, representing
19.99% of the total issued and outstanding common stock of the Company after the consummation of the Superlatus Merger and 15,759 shares
of Company’s Series B Preferred Stock, with a conversion ratio of 100 shares of Series B Preferred Stock to one share of common
stock. At Closing, the value of the common stock was $7.30 per share, resulting in a total value of $12,500,089. Additionally, the shareholders
of Superlatus agreed to surrender back to the Company 291,096 shares of the Company’s Series B Preferred Stock. As described below,
in March 2024 the Company divested of its interest in Superlatus.
Dispositions
On
February 16, 2024, the Company, together with Trxade, Inc., and Micro Merchant Systems, Inc. (“MMS”) entered into an asset
purchase agreement (the “APA”) under which MMS agreed to purchase for cash substantially all of the assets of Trxade, Inc.
On February 16, 2024, the parties consummated the closing of the transactions contemplated by the APA. Trxade, Inc. operated a web-based
market platform designed to enable trading among healthcare buyers and sellers of pharmaceuticals, accessories and services. The purchase
price paid at closing was $22,660,182. Pursuant to the terms and conditions of the APA, because MMS received $1,600,000 or greater in
certain collections from third parties resulting from any products or services sold, or provided, by the business assets and operations
acquired from Trxade, Inc. during the period ending on the four-month anniversary of the closing date, Trxade, Inc. was due an additional
$7,500,000 payment from MMS. The Company received the $7,500,000 in May 2024.
On
March 5, 2024, the Company entered in a Stock Purchase Agreement (“SPA”) with Superlatus Foods Inc. (the “Buyer”).
Pursuant to the SPA, the Company sold all of the issued and outstanding stock of Superlatus, to the Buyer. The $1.00 purchase price for
the stock was delivered to the Company at the closing, which occurred simultaneously with the execution of the SPA. As a result of the
transaction Superlatus ceased to be a subsidiary of the Company, and the rights and assets of Superlatus together with various liabilities
and obligations that were specific to Superlatus became rights and obligations of Buyer.
See
Note 3 for further detail on the dispositions.
Basis
of Presentation and Principles of Consolidation
The
accompanying unaudited interim condensed consolidated financial statements of TRxADE HEALTH, Inc. have been prepared in accordance with
accounting principles generally accepted in the United States of America (“U.S. GAAP”) and the rules of the SEC and should
be read in conjunction with the audited financial statements and notes thereto contained in the Company’s Annual Report on Form
10-K for the year ended December 31, 2023, as filed with the SEC on April 22, 2024.
In
the opinion of management, all adjustments, consisting of normal recurring adjustments, necessary for a fair presentation of financial
position and the results of operations for the interim periods presented have been reflected herein. All significant intercompany balances
and transactions have been eliminated in consolidation. The results of operations for the interim periods are not necessarily indicative
of the results to be expected for the full year. Notes to the financial statements that would substantially duplicate the disclosures
contained in the audited financial statements for the year ended December 31, 2023, as reported in the Company’s Annual Report
on Form 10-K have been omitted.
Use
of Estimates
The
preparation of condensed consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions
that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue
and expenses in the reporting period. The Company bases its estimates and assumptions on current facts, historical experience and various
other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about
the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources.
The actual results experienced by the Company may differ materially and adversely from its estimates. To the extent there are material
differences between estimates and the actual results, future results of operations will be affected. Significant estimates for the six
months ended June 30, 2024 and 2023 include the valuation of intangible assets, including goodwill, and gain (losses) on dispositions.
Fair
Value of Financial Instruments
The
carrying amounts for cash, accounts receivable, accounts payable, accrued liabilities, and other current liabilities approximate their
fair value because of their short-term maturity.
Stock
Split
Effective
June 21, 2023, the Company executed a 1:15 reverse stock split for stockholders of record on that date. This was executed to comply with
the Nasdaq Listing Rule 5550(a)(2) to have the price of the stock above $1.00.
Recently
Issued Accounting Pronouncements
In
November 2023, the FASB issued ASU 2023-07 Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. The
new guidance requires enhanced disclosure of significant expenses that are regularly reported to the chief operating decision maker and
the nature of segment expense information used to manage operations. The new guidance is effective for all public companies for annual
reporting periods beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early
adoption is permitted. The Company will adopt the new standard in annual reporting period beginning after December 15, 2023 and is currently
evaluating the impacts of the new guidance on its disclosure within the financial statements.
In
December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. The new guidance
requires disaggregated information about the effective tax rate reconciliation and additional information on taxes paid that meet a quantitative
threshold. The new guidance is effective for public companies for annual reporting periods beginning after December 15, 2024, and for
non-public companies for annual reporting periods beginning after December 15, 2025, with early adoption permitted for both. The Company
will adopt the new standard in annual reporting period beginning after December 15, 2025, and is currently evaluating the impacts of
the new guidance on its disclosures within the consolidated financial statements.
Accounts
Receivable, net
On
January 1, 2023, the Company adopted ASU 2016-13 “Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses
on Financial Instruments” and its related amendments using the prospective method. The new standard requires the use of a current
expected credit loss impairment model to develop and recognize credit losses for financial instruments at amortized cost when the asset
is first originated or acquired, and each subsequent reporting period.
The
Company’s receivables are from customers and are typically collected within 90 days. The Company determines the allowance based
on known troubled accounts, historical experience, and other currently available evidence.
Other
Receivables
As
of June 30, 2024 and December 31, 2023, other receivables are $2,230,797 and $1,224,702. As of June 30, 2024, other receivables primarily
consist of short-term advances to Danam Health, APS and CSP.
Deferred Offering Costs
The Company complies
with the requirements of ASC 340-10-S99-1 with regards to offering costs. Prior to the completion of an offering, offering costs are
capitalized. The deferred offering costs are charged to additional paid-in capital or as a discount to debt, as applicable, upon the
completion of an offering or to expense if the offering is not completed. As of June 30, 2024, the Company has $69,444 capitalized deferred
offering costs.
Acquisitions
The
Company accounts for acquisitions and investments in businesses as business combinations if the target meets the definition of a business
and (a) the target is a variable interest entity (“VIE”) and the Company is the target’s primary beneficiary, and therefore
the Company must consolidate its financial statements, or (b) the Company acquires more than 50% of the voting interest of the target
and it was not previously consolidated. The Company records business combinations using the acquisition method of accounting, which requires
all the assets acquired and liabilities assumed to be recorded at fair value as of the acquisition date. The excess of the purchase price
over the estimated fair values of the net tangible and intangible assets acquired is recorded as goodwill.
The
application of the acquisition method of accounting for business combinations requires management to make significant estimates and assumptions
in the determination of the fair value of assets acquired and liabilities assumed in order to properly allocate purchase price consideration
between assets that are depreciated and amortized from goodwill. The fair value assigned to tangible and intangible assets acquired and
liabilities assumed are based on management’s estimates and assumptions, as well as other information compiled by management, including
valuations that utilize customary valuation procedures and techniques. Significant assumptions and estimates include, but are not limited
to, the cash flows that an asset is expected to generate in the future, the appropriate weighted-average cost of capital, and the cost
savings expected to be derived from acquiring an asset, if applicable.
If
the actual results differ from the estimates and judgments used in these estimates, the amounts recorded in the Company’s financial
statements may be exposed to potential impairment of the intangible assets and goodwill.
If
the Company’s investment involves the acquisition of an asset or group of assets that does not meet the definition of a business,
the transaction is accounted for as an asset acquisition. An asset acquisition is recorded at cost, which includes capitalizing transaction
costs, and does not result in the recognition of goodwill.
Intangible
Assets and Goodwill
The
Company tests indefinite-lived intangible assets for impairment on an annual basis or whenever events or changes occur that would more-likely-than
not reduce the fair value of the indefinite-lived intangible asset below its carrying value between annual impairment tests. Any indefinite-lived
intangible asset assessment is performed at the Company level.
The
Company did not record an indefinite-lived intangible asset impairment charge for the three or six months ended June 30, 2024 and 2023.
Investments
The
Company accounts for investments that it does not control using the cost method, equity method or fair value method, as applicable. Investments
in companies in which the Company owns less than a 20% equity interest and where it does not exercise significant influence over the
operating and financial policies of the investee are accounted for using the cost method of accounting. The Company periodically reviews
the carrying value of these investments to determine if there has been an other-than-temporary decline in fair value below carrying value.
A variety of factors are considered when determining if a decline in fair value below carrying value is other-than-temporary, including,
among others, the financial condition and business prospects of the investee, as well as the Company’s investment intent. Cost
method investments are carried at cost, which approximates or is less than fair value. Dividends received by the Company are recognized
in equity (losses) earnings of affiliates, net of tax on the consolidated statements of operations.
On
February 29, 2024, the Company’s wholly owned subsidiary Trxade, Inc. entered into a Subscription Agreement (the “Subscription
Agreement”) with Lafayette Energy Corp., a Delaware corporation (“Lafayette”). Pursuant to the Subscription Agreement,
Trxade, Inc. will, in two equal tranches, invest a total of up to $5,000,000 in Lafayette in exchange for up to 2,000,000 shares of Lafayette’s
Series A Convertible Preferred Stock, with the second tranche becoming payable only upon Trxade, Inc.’s receipt of notice that
Lafayette has successfully drilled its first oil and gas well and produced at least one hundred (100) barrels of oil.
As
of June 30, 2024, the Company’s investment in Lafayette was $2,500,000. The Company determined there was no impairment necessary
as of June 30, 2024.
Income
(loss) Per Common Share
Basic
net income per common share is computed by dividing net income available to common stockholders by the weighted average number of common
shares outstanding. Diluted net income per common share is computed similar to basic net income per common share except that the denominator
is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been
issued and if the additional common shares were dilutive. The dilutive effect of the Company’s options and warrants is computed
using the treasury stock method. As of June 30, 2024, we had 190,242 outstanding warrants to purchase shares of common stock, 15,759
shares of Series B preferred stock, 290 shares of Series C preferred stock and 23,930 options to purchase shares of common stock.
The
following table sets forth the computation of basic and diluted loss per share:
SCHEDULE
OF BASIC AND DILUTIVE LOSS PER SHARE
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| | |
Three
Months Ended | | |
Six
Months Ended | |
| | |
June
30, | | |
June
30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Numerator: | |
| | | |
| | | |
| | | |
| | |
| Net loss from continuing operations | |
$ | (1,624,741 | ) | |
$ | (2,229,035 | ) | |
$ | (8,258,163 | ) | |
$ | (3,340,976 | ) |
| Net (loss) income
on discontinued operations | |
| (209,161 | ) | |
| 254,157 | | |
| 27,670,294 | | |
| 688,145 | |
| Net (loss) income | |
$ | (1,833,902 | ) | |
$ | (1,974,878 | ) | |
$ | 19,412,131 | | |
$ | (2,652,831 | ) |
| Denominator: | |
| | | |
| | | |
| | | |
| | |
| Denominator for EPS – weighted average shares | |
| | | |
| | | |
| | | |
| | |
| Basic | |
| 1,406,348 | | |
| 681,199 | | |
| 1,224,337 | | |
| 675,143 | |
| Diluted | |
| 1,406,348 | | |
| 681,199 | | |
| 1,454,558 | | |
| 675,143 | |
| Net loss per common share from continuing
operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (1.16 | ) | |
$ | (3.27 | ) | |
$ | (6.75 | ) | |
$ | (4.95 | ) |
| Diluted | |
$ | (1.16 | ) | |
$ | (3.27 | ) | |
$ | (6.75 | ) | |
$ | (4.95 | ) |
| Net loss per common share from discontinued
operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (0.15 | ) | |
$ | 0.37 | | |
$ | 22.60 | | |
$ | 1.02 | |
| Diluted | |
$ | (0.15 | ) | |
$ | 0.37 | | |
$ | 19.02 | | |
$ | 1.02 | |
| Net (loss) income per common share | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (1.30 | ) | |
$ | (2.90 | ) | |
$ | 15.86 | | |
$ | (3.93 | ) |
| Diluted | |
$ | (1.30 | ) | |
$ | (2.90 | ) | |
$ | 13.35 | | |
$ | (3.93 | ) |
Income
taxes
The
Company’s provision for income taxes was $0 for the three and six months ended June 30, 2024 and 2023. The income tax provisions
for these six-month periods are based upon estimates of annual income (loss), annual permanent differences and statutory tax rates in
the various jurisdictions in which the Company operates. For all periods presented, the Company utilized net operating loss carryforwards
to offset the impact of any taxable income. The Company’s tax rate differs from the applicable statutory rates due primarily to
the establishment of a valuation allowance, utilization of deferred and the effect of permanent differences and adjustments.
NOTE
2 – GOING CONCERN
The
accompanying interim consolidated financial statements have been prepared assuming that the Company will continue as a going concern,
which contemplates realization of assets and the satisfaction of liabilities in the normal course of business within one year after the
date the consolidated financial statements are issued. In accordance with Financial Accounting Standards Board, or the FASB, Accounting
Standards Update No. 2014-15, Presentation of Financial Statements - Going Concern (Subtopic 205-40), our management evaluates whether
there are conditions or events, considered in aggregate, that raise substantial doubt about our ability to continue as a going concern
within one year after the date that the financial statements are issued.
As
of June 30, 2024, the Company had an accumulated deficit of $26,504,881. As a result of the receipt of consideration from the asset disposition
by Trxade, Inc. to MMS completed in February 2024 and the receipt of the Milestone Payment in May, the Company had $7,719,993 in cash
as of June 30, 2024. In July 2024, the Company paid a cash dividend of $1.50 per share, or $2,187,759 in the aggregate. As of the issuance
date of these consolidated financial statements, the Company has approximately $2,295,000 in cash.
We
will need to raise additional capital or secure debt funding to support on-going operations, and to fund the assets and operations of
any businesses or assets we acquire. The sources of this capital are expected to be the sale of equity and debt, which may not be available
on favorable terms, if at all, and may, if sold, cause significant dilution to existing stockholders. If we are unable to access additional
capital moving forward, it may hurt our ability to grow and to generate future revenues, our financial position, and liquidity. These
factors raise substantial doubt about the ability of the Company to continue as a going concern. Unless Management is able to obtain
additional financing, it is unlikely that the Company will be able to meet its funding requirements during the next 12 months. The financial
statements do not include any adjustments that might result from the outcome of this uncertainty.
NOTE
3 – ACQUISITIONS AND DISPOSITIONS
Acquisitions
Superlatus,
Inc.
On
July 31, 2023, the Company entered into the Merger Agreement (see Note 1) with Superlatus (“Seller”) whereby the Company
acquired 100% of the stock of the Seller (the “Acquisition”). Superlatus includes a wholly-owned subsidiary, Sapientia. Consideration
for the Acquisition consisted of (i) 136,441 shares of the Company’s common stock at a fair value of $7.30 per share, representing
19.99% of the total issued and outstanding share of the Company’s common stock at Closing, and (ii) 306,855 shares of the Company’s
Series B Preferred Stock, a new class of the Company’s non-voting convertible preferred stock with a conversion ratio of 100 to
one. The total fair value of the common stock and Series B Preferred Stock on the Closing Date was $225,000,169 (“Purchase Price”).
On January 8, 2024, the Company entered into Amendment No. 1 to the Agreement and Plan of Merger (the “Amendment”). Under
the terms of the Amendment, the merger consideration to the shareholders of Superlatus was adjusted to an aggregate of 136,441 shares
of common stock of the Company, representing 19.99% of the total issued and outstanding common stock of the Company after the consummation
of the Merger and 15,759 shares of Company’s Series B Preferred Stock, par value $0.00001 per share, with a conversion ratio of
100 shares of Series B Preferred Stock to one share of common stock. The total fair value of the common stock and Series B Preferred
Stock on the Closing Date was adjusted to $12,500,089 (“Amended Purchase Price”). Additionally, the shareholders of Superlatus
agreed to surrender back to the Company 289,731 shares of the Company’s Series B Preferred Stock received before the Amendment.
The
acquisition of Superlatus was accounted for as a business combination using the acquisition method pursuant to FASB ASC Topic 805. As
the acquirer for accounting purposes, the Company had estimated the Purchase Price, assets acquired and liabilities assumed as of the
acquisition date, with the excess of the Purchase Price over the fair value of net assets acquired recognized as goodwill. An independent
valuation expert assisted the Company in determining these fair values.
Accounting
guidance provides that an acquirer must recognize adjustments to provisional amounts that are identified during the measurement period,
which runs through July 31, 2024, in the measurement period in which the adjustment amounts are determined. The acquirer must record
in the financial statements, the effect on earnings of changes in depreciation, amortization or other income effects, if any, as a result
of the changes to the provisional amounts, calculated as if the accounting had been completed at the acquisition date. Items that could
be subject to adjustment include credit fair value adjustments on loans, core deposit intangible and the deferred income tax assets resulting
from the acquisition.
The
Amended Purchase Price allocation as of the acquisition date is presented as follows:
SCHEDULE
OF PURCHASE PRICE ALLOCATION
| | |
July
31, 2023 | |
| Purchase consideration: | |
| | |
| Common Stock,
at fair value | |
$ | 996,019 | |
| Series
B Preferred Stock, at fair value | |
| 11,504,070 | |
| Total
purchase consideration | |
$ | 12,500,089 | |
| | |
| | |
| Purchase price allocation: | |
| | |
| Cash | |
$ | 5,546 | |
| Prepaid expenses | |
| 3,705 | |
| Inventory | |
| 122,792 | |
| Intangible assets, net | |
| 9,777,479 | |
| Goodwill | |
| 5,129,115 | |
| Assets acquired | |
| 15,038,637 | |
| Accounts payable and
other current liabilities | |
| (283,548 | ) |
| Purchase price payable | |
| (350,000 | ) |
| Notes
payable | |
| (1,905,000 | ) |
| Liabilities assumed | |
| (2,538,548 | ) |
| Net
assets acquired | |
$ | 12,500,089 | |
The
Urgent Company, Inc.
On
September 27, 2023, the Company entered into an Asset Purchase Agreement (“APA”) with The Urgent Company, Inc. (“TUC”)
and its wholly owned subsidiaries, pursuant to which, the Company was assigned certain inventory and property and equipment and assumed
certain operating leases for consideration of $4,400,000 in promissory notes (“Purchase Price”, see Note 11). Subsequent
to December 31, 2023, we divested our interest in TUC.
The
transaction was accounted for as an asset acquisition pursuant to FASB ASC Topic 805. As the acquirer for accounting purposes, the Company
allocated the cost of the asset acquisition to the assets acquired and liabilities assumed as of the acquisition date based on their
respective relative fair value as of the date of the transaction.
The
following summarizes the provisional relative fair values of the assets acquired as of the acquisition date based on the allocation of
the cost of the asset acquisition:
SCHEDULE
OF FAIR VALUES OF ASSETS ACQUIRED
| | |
September
27, 2023 | |
| Purchase consideration: | |
| | |
| Promissory
note | |
$ | 4,400,000 | |
| Total
purchase consideration | |
$ | 4,400,000 | |
| | |
| | |
| Allocation of cost of assets acquired: | |
| | |
| Inventory | |
$ | 4,168,830 | |
| Property and equipment | |
| 231,170 | |
| Assets acquired | |
| 4,400,000 | |
| Net
assets acquired | |
$ | 4,400,000 | |
Dispositions
and Divestitures
Alliance
Pharma Solutions, LLC and Community Specialty Pharmacy, LLC
On
August 22, 2023, the Company and Wood Sage, LLC (“Wood Sage”) entered into (i) a Membership Interest Purchase Agreement,
pursuant to which the Company sold its 100% membership interest in Alliance Pharma Solutions, LLC (“ASP MIPA”) for consideration
of a $125,000 promissory note (“ASP Sale Price”) and (ii) a Membership Interest Purchase Agreement, pursuant to which the
Company sold 100% of the membership interest in Community Specialty Pharmacy, LLC (“CSP MIPA”) in exchange for a $100,000
promissory note (“CSP Sale Price”). As a result, the results of APS and CSP were classified as discontinued operations in
our condensed statements of operations and excluded from both continuing operations and segment results for the three months ended March
31, 2023.
As
part of recognizing the business as held for sale in accordance with U.S. GAAP, the Company was required to measure APS and CSP at the
lower of its carrying amount or fair value less cost to sell. As a result of this analysis, during the year ended December 31, 2023,
the Company recognized a non-cash, pre-tax loss on disposal of $3,300,225. The loss is included in “Net loss from discontinued
operations” in the consolidated statements of operations. The loss was determined by comparing the fair value of the consideration
received for the sale of a 100% interest in APS and CSP with the net assets of APS and CSP, respectively, immediately prior to the transaction.
As
a result of the transactions, the following assets and liabilities of APS and CSP were transferred to Wood Sage as of August 22, 2023:
SCHEDULE
OF ASSETS AND LIABILITIES
| | |
Alliance
Pharma
Solutions, LLC | | |
Community
Specialty
Pharmacy, LLC | |
| Cash | |
$ | 1,050 | | |
$ | 61,988 | |
| Accounts receivable, net | |
| - | | |
| 101,901 | |
| Inventory | |
| - | | |
| 123,230 | |
| Prepaid assets | |
| - | | |
| 525 | |
| Intangible assets and capitalized software,
net | |
| 739,337 | | |
| - | |
| Accounts payable | |
| (23,982 | ) | |
| (231,876 | ) |
| Accrued liabilities | |
| - | | |
| (10,182 | ) |
| Net assets sold | |
$ | 716,405 | | |
$ | 45,586 | |
Trxade,
Inc.
On
February 16, 2024, the Company, together with Trxade, Inc., a wholly owned subsidiary of the Company, and MMS entered into an asset purchase
agreement (the “APA”) under which MMS agreed to purchase for cash substantially all of the assets of Trxade, Inc. On February
16, 2024, the parties consummated the closing of the transactions contemplated by the APA. The purchase price paid at closing was $22,660,182.
Subject to the terms and conditions of the APA, if, during the period beginning on the closing date and ending on the four-month anniversary
of the closing date, MMS received $1,600,000 or greater in certain collections from third parties resulting from any products or services
sold, or provided, by the business assets and operations acquired from Trxade, Inc., Trxade, Inc. would receive an additional $7,500,000
payment from MMS (the “Milestone Payment”). The Company received the Milestone Payment in May 2024.
The
APA was accounted for a business disposition in accordance with ASC 810-40-40-3A. As of February 16, 2024, the Company no longer consolidated
the assets, liabilities, revenues and expenses of Trxade, Inc. The components of the disposition are as follows:
SCHEDULE
OF BUSINESS ACQUISITIONS ASSETS AND LIABILITIES
| | |
| | |
| Cash received from MMS | |
$ | 22,660,182 | |
| | |
| - | |
| Other receivable
from MMS | |
| 7,500,000 | |
| Total
fair value of consideration received | |
$ | 30,160,182 | |
| | |
| | |
| Carrying amount
of assets and liabilities | |
| | |
| Cash | |
$ | 76,821 | |
| Accounts receivable, net | |
| 719,876 | |
| Prepaid expenses | |
| 55,397 | |
| Property, plant and equipment, net | |
| 45,655 | |
| Intangible assets, net | |
| | |
| Operating lease right-of-use assets | |
| 12,277 | |
| Purchase price payable | |
| | |
| Accounts payable | |
| (347,000 | ) |
| Accrued liabilities | |
| (5,269 | ) |
| Other current liabilities | |
| (26,244 | ) |
| Lease liability, current | |
| (1,556 | ) |
| Notes payable, current portion | |
| (45,000 | ) |
| Lease liability,
net of current portion | |
| (10,720 | ) |
| Notes payable | |
| | |
| Total
carrying amount of assets and liabilities | |
| 474,236 | |
| | |
| | |
| Gain on disposition
of business | |
$ | 29,685,946 | |
The
gain on disposition of business of $29,685,946 was included in income from discontinued operations, net of tax in the consolidated statements
of operations.
Superlatus
Inc.
On
March 5, 2024, the Company entered in a Stock Purchase Agreement (“SPA”) with Superlatus Foods Inc. (the “Buyer”).
Pursuant to the SPA, the Company sold all of the issued and outstanding stock (the “Stock”) of Superlatus to the Buyer. The
purchase price for the stock was $1.00 which was delivered to the Company at the closing, which occurred simultaneously with the execution
of the SPA. As a result of the transaction Superlatus is no longer a subsidiary of the Company, and the rights and assets of Superlatus
together with various liabilities and obligations that were specific to Superlatus became rights and obligations of Buyer.
The
transaction was accounted for a business disposition in accordance with ASC 810-40-40-3A. As of March 5, 2024, the Company no longer
consolidated the assets, liabilities, revenues and expenses of Superlatus. The components of the disposition are as follows:
SCHEDULE
OF BUSINESS ACQUISITIONS ASSETS AND LIABILITIES
| | |
| | |
| Fair value of consideration
received | |
$ | 1 | |
| Total
fair value of consideration received | |
$ | 1 | |
| | |
| | |
| Carrying amount
of assets and liabilities | |
| | |
| Cash | |
$ | 151,546 | |
| Property, plant and equipment, net | |
| 223,080 | |
| Intangible assets, net | |
| 8,962,688 | |
| Operating lease right-of-use assets | |
| 325,995 | |
| Purchase price payable | |
| (350,000 | ) |
| Accounts payable | |
| (224,137 | ) |
| Accrued liabilities | |
| (173,436 | ) |
| Notes payable, current portion | |
| (6,480,000 | ) |
| Lease liability - current | |
| (105,567 | ) |
| Lease liability - net of current portion
| |
| (221,428 | ) |
| Notes payable | |
| (25,000 | ) |
| Total
carrying amount of assets and liabilities | |
| 2,083,743 | |
| | |
| | |
| Loss on disposition
of business | |
$ | (2,083,742 | ) |
The
loss of disposition of business of $2,083,742 was included in income from discontinued operations, net of tax in the consolidated statements
of operations.
Discontinued
Operations
In
accordance with the provisions of ASC 205-20, the Company has excluded the results of discontinued operations from its results of continuing
operations in the accompanying consolidated statements of operations for the three and six months ended June 30, 2024 and 2023. The results
of the discontinued operations for the three and six months ended June 30, 2024 and 2023 consist of the following:
SCHEDULE
OF DISCONTINUED OPERATIONS
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| | |
TRX | | |
Bonum | | |
Superlatus | | |
SOSRx | | |
CSP | | |
APS | | |
Total | |
| | |
Three
Months
Ended | | |
Three
Months
Ended | | |
Three Months
Ended | | |
Three Months
Ended | | |
Three Months
Ended | | |
Three Months
Ended | | |
Three Months
Ended | |
| | |
June
30, | | |
June
30, | | |
June
30, | | |
June
30, | | |
June
30, | | |
June
30, | | |
June
30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Revenues | |
$ | - | | |
$ | 1,556,843 | | |
$ | - | | |
$ | 1,896 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 325,811 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 1,884,550 | |
| Cost of sales | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 306,962 | | |
| - | | |
| - | | |
| - | | |
| 306,962 | |
| Gross profit | |
| - | | |
| 1,556,843 | | |
| - | | |
| 1,896 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 18,849 | | |
| - | | |
| - | | |
| - | | |
| 1,577,588 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Wage and salary expense | |
| 161,038 | | |
| 470,002 | | |
| - | | |
| 20,300 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 174,354 | | |
| - | | |
| - | | |
| 161,038 | | |
| 664,656 | |
| Professional fees | |
| 46,775 | | |
| 38,462 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,444 | | |
| - | | |
| 1,375 | | |
| 46,775 | | |
| 41,281 | |
| Technology expense | |
| - | | |
| 328,373 | | |
| - | | |
| 19,795 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 65,965 | | |
| - | | |
| 68,314 | | |
| - | | |
| 482,447 | |
| General and administrative | |
| 1,348 | | |
| 118,766 | | |
| - | | |
| 1,138 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 13,303 | | |
| - | | |
| 1,839 | | |
| 1,348 | | |
| 135,046 | |
| Total
operating expenses | |
| 209,161 | | |
| 955,603 | | |
| - | | |
| 41,234 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 255,066 | | |
| - | | |
| 71,528 | | |
| 209,161 | | |
| 1,323,431 | |
| Operating
income (loss) | |
| (209,161 | ) | |
| 601,239 | | |
| - | | |
| (39,337 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (236,217 | ) | |
| - | | |
| (71,528 | ) | |
| (209,161 | ) | |
| 254,157 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Gain (loss) on dispositions | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total non-operating income (expense) | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net income (loss)
on discontinued operations | |
$ | (209,161 | ) | |
$ | 601,239 | | |
$ | - | | |
$ | (39,337 | ) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | (236,217 | ) | |
$ | - | | |
$ | (71,528 | ) | |
$ | (209,161 | ) | |
$ | 254,157 | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| | |
TRX | | |
Bonum | | |
Superlatus | | |
SOSRx | | |
CSP | | |
APS | | |
Total | |
| | |
Six Months
Ended | | |
Six Months
Ended | | |
Six Months
Ended | | |
Six Months
Ended | | |
Six Months
Ended | | |
Six Months
Ended | | |
Six Months
Ended | |
| | |
June
30, | | |
June
30, | | |
June
30, | | |
June
30, | | |
June
30, | | |
June
30, | | |
June
30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Revenues | |
$ | 970,808 | | |
$ | 3,000,020 | | |
$ | - | | |
$ | 18,856 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 637,068 | | |
$ | - | | |
$ | - | | |
$ | 970,808 | | |
$ | 3,655,944 | |
| Cost of sales | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 577,535 | | |
| - | | |
| - | | |
| - | | |
| 577,535 | |
| Gross profit | |
| 970,808 | | |
| 3,000,020 | | |
| - | | |
| 18,856 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 59,533 | | |
| - | | |
| - | | |
| 970,808 | | |
| 3,078,408 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Wage and salary expense | |
| 713,021 | | |
| 999,329 | | |
| 578 | | |
| 42,109 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 347,525 | | |
| - | | |
| - | | |
| 713,599 | | |
| 1,388,963 | |
| Professional fees | |
| 62,160 | | |
| 39,695 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,168 | | |
| - | | |
| 3,125 | | |
| 62,160 | | |
| 44,988 | |
| Technology expense | |
| 86,660 | | |
| 509,197 | | |
| 2,245 | | |
| 38,216 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 69,532 | | |
| - | | |
| 73,491 | | |
| 88,905 | | |
| 690,436 | |
| General and administrative | |
| 37,377 | | |
| 232,008 | | |
| 678 | | |
| 2,564 | | |
| - | | |
| - | | |
| - | | |
| 146 | | |
| - | | |
| 27,529 | | |
| - | | |
| 3,629 | | |
| 38,055 | | |
| 265,876 | |
| Total
operating expenses | |
| 899,218 | | |
| 1,780,229 | | |
| 3,500 | | |
| 82,889 | | |
| - | | |
| - | | |
| - | | |
| 146 | | |
| - | | |
| 446,754 | | |
| - | | |
| 80,245 | | |
| 902,719 | | |
| 2,390,264 | |
| Operating income (loss) | |
| 71,590 | | |
| 1,219,790 | | |
| (3,500 | ) | |
| (64,033 | ) | |
| - | | |
| - | | |
| - | | |
| (146 | ) | |
| - | | |
| (387,221 | ) | |
| - | | |
| (80,245 | ) | |
| 68,089 | | |
| 688,144 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Gain (loss) on dispositions | |
| 29,685,946 | | |
| - | | |
| - | | |
| - | | |
| (2,083,742 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 27,602,204 | | |
| - | |
| Total
non-operating income (expense) | |
| 29,685,946 | | |
| - | | |
| - | | |
| - | | |
| (2,083,742 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 27,602,204 | | |
| - | |
| Net income (loss)
on discontinued operations | |
$ | 29,757,536 | | |
$ | 1,219,790 | | |
$ | (3,500 | ) | |
$ | (64,033 | ) | |
$ | (2,083,742 | ) | |
$ | - | | |
$ | - | | |
$ | (146 | ) | |
$ | - | | |
$ | (387,221 | ) | |
$ | - | | |
$ | (80,245 | ) | |
$ | 27,670,294 | | |
$ | 688,145 | |
In
the second quarter of 2024, the Company determined to dissolve the business of Bonum, and have presented the results of operations in
net income (loss) from discontinued operations.
NOTE
4- RELATED PARTY TRANSACTIONS
On
November 21, 2023, but effective September 14, 2023, the Company issued a promissory note to Danam Health, Inc. (the “Danam Note”)
in the amount of $300,000. Danam Health, Inc. prepaid $250,000 prior to the execution date. The Danam Note did not accrue interest. As
of December 31, 2023, the balance of the Danam Note was $50,000. The Danam Note was fully paid off in February 2024.
On
February 29, 2024, the Company’s wholly owned subsidiary Trxade, Inc. entered into a Subscription Agreement (the “Subscription
Agreement”) with Lafayette. Pursuant to the Subscription Agreement, Trxade, Inc. will, in two equal tranches, invest a total of
up to $5,000,000 in Lafayette in exchange for up to 2,000,000 shares of Lafayette’s newly created Series A Convertible Preferred
Stock, with the second tranche becoming payable only upon Trxade, Inc.’s receipt of notice that Lafayette has successfully drilled
its first oil and gas well and produced at least one hundred (100) barrels of oil.
As
of June 30, 2024, other receivables includes a $997,500 receivable from Danam Health Inc. and $1,203,682 receivable from APS and CSP.
See
Note 7 for note receivable from Wood Sage, LLC.
NOTE
5 – REVENUE RECOGNITION
The
Company derives revenue from two primary sources—product revenue and service revenue.
Product
revenue consists of shipments of:
| |
● |
Resale
of pharmaceutical products to pharmacies; and |
| |
|
|
| |
● |
Revenues
for our products are recognized and invoiced when the product is shipped to the customer. |
Service
revenue consists primarily of:
| |
● |
Transaction
fees from the facilitation of buyer generated purchase orders to suppliers, billed monthly; |
| |
|
|
| |
● |
Data
service fees associated with providing vendors of pharmaceutical products with data analysis of their catalogues and branding of
their products or company to the Company’s registered buyers, billed monthly or as a one-time fee; and |
| |
|
|
| |
● |
Software-as-a-Service
(“SaaS”) fees for a platform for virtual healthcare provider visits, billed monthly. |
Revenues
for the Company’s services that are billed monthly are recognized and invoiced at the beginning of the month. Revenues for one-time
services are recognized at the point in time when services are rendered.
Payment
terms for products and services are generally 0 to 60 days and the Company has no contract assets or liabilities.
The
following table presents disaggregated revenue by major product categories during the three and six months ended June 30, 2024 and 2023:
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| | |
Three
Months Ended | | |
Six
Months Ended | |
| | |
June
30, | | |
June
30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Product revenues | |
| | | |
| | | |
| | | |
| | |
| Pharmaceutical
product resale | |
$ | 18,699 | | |
$ | 366,526 | | |
$ | 18,699 | | |
$ | 842,882 | |
| Total
product revenue | |
$ | 18,699 | | |
$ | 366,526 | | |
$ | 18,699 | | |
$ | 842,882 | |
| Total
revenue | |
$ | 18,699 | | |
$ | 366,526 | | |
$ | 18,699 | | |
$ | 842,882 | |
NOTE
6 – INVENTORY
Inventory
value is determined using the weighted average cost method and is stated at the lower of cost or net realizable value. As of June 30,
2024 and December 31, 2023, inventory was comprised of the following:
| | |
June
30, | | |
December
31, | |
| | |
2024 | | |
2023 | |
| Raw materials | |
$ | - | | |
$ | - | |
| Finished
goods | |
| 6,439 | | |
| 968 | |
| Inventory | |
$ | 6,439 | | |
$ | 968 | |
NOTE
7 – NOTES RECEIVABLE – RELATED PARTY
On
August 22, 2023, the Company received a Promissory Note (the “Wood Sage Note”) in the amount of $1,300,000 from Wood Sage,
LLC and entered into the APS MIPA and CSP MIPA for the Company to sell APS and CSP and entered into a Master Service Agreement (“Wood
Sage MSA”). The Wood Sage Note bears no interest and is due and payable within thirty days of a change in control, as defined by
the Wood Sage Note, of the borrower. As of both June 30, 2024 and December 31, 2023, the outstanding balance of the Wood Sage Note was
$1,300,000, respectively.
NOTE
8 – INTANGIBLE ASSETS
The
intangible assets were sold to Superlatus on March 5, 2024 per the Stock Purchase Agreement (see Note 3).
NOTE
9 – OTHER CURRENT LIABILITIES
As
of June 30, 2024 and December 31, 2023, other current liabilities consisted of the following:
SCHEDULE
OF OTHER CURRENT LIABILITIES
| | |
June 30, | | |
December
31, | |
| | |
2024 | | |
2023 | |
| Insurance refunds payable | |
$ | - | | |
$ | 62,390 | |
| Other payables | |
| 5,441 | | |
| 5,441 | |
| Other
current liabilities | |
$ | 5,441 | | |
$ | 67,831 | |
NOTE
10 – CONTINGENT FUNDING LIABILITIES
On
December 13, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables
(the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $150,000
to purchase $214,500 of future receivables. The Company also paid $7,500 as a one-time origination fee in connection with the Receivables
Agreement. This agreement was fully paid off in February 2024.
On
November 22, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables
(the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $275,000
to purchase $393,250 of future receivables. The Company also paid $13,750 as a one-time origination fee in connection with the Receivables
Agreement. This agreement was fully paid off in February 2024.
On
October 25, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables
(the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $1,200,000
to purchase $1,728,000 of future receivables. The Company also paid $60,000 as a one-time origination fee in connection with the Receivables
Agreement. This agreement was fully paid off in February 2024.
The
Company’s relationship with the funding source meets the criteria in ASC 470-10-25 – Sales of Future Revenues or Various
Other Measures of Income (“ASC 470”), which relates to cash received from a funding source in exchange for a specified percentage
or amount of revenue or other measure of income of a particular product line, business segment, trademark, patent or contractual right
for a defined period. Under this guidance, the Company recognized the fair value of its contingent obligation to the funding source,
as of the acquisition date, as a current liability in its consolidated balance sheet.
Under
ASC 470, amounts recorded as debt are to be amortized under the interest method. The Company made an accounting policy election to utilize
the prospective method when there is a change in the estimated future cash flows, whereby a new effective interest rate is determined
based on the revised estimate of remaining cash flows. The new rate is the discount rate that equates the present value of the revised
estimate of remaining cash flows with the carrying amount of the debt, and it will be used to recognize interest expense for the remaining
period. Under this method, the effective interest rate is not constant, and any change in expected cash flows is recognized prospectively
as an adjustment to the effective yield. As of June 30, 2024, and December 31, 2023, the total contingent funding liability was $0 and
$1,246,346 respectively, and the effective interest rate was approximately 0% and 31%, respectively. This rate represents the discount
rate that equates the estimated future cash flows with the fair value of the debt and is used to compute the amount of interest to be
recognized each period. Any future payments made to the funding source will decrease the contingent funding liability balance accordingly.
NOTE
11 – NOTES PAYABLE
On
November 17, 2023, the Company issued promissory notes to Moku Foods, Inc. (the “Moku Foods November 2023 Note”) in the amount
of $50,000. The promissory note accrues interest at 11.5% per annum, compounded monthly and is payable upon demand at any time after
November 30, 2023. As of December 31, 2023, the balance of the Moku Foods November 2023 Note was $50,000. The Company has accrued interest
of $945 as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc. whereby
the Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
On
October 16, 2023, the Company issued promissory notes to Moku Foods, Inc. (the “Moku Foods October 2023 Note”) in the amount
of $150,000. The promissory note accrues interest at 11.5% per annum, compounded monthly and is payable upon demand at any time after
October 31, 2023. As of December 31, 2023, the balance of the Moku Foods October 2023 Note was $150,000. The Company has accrued interest
of $4,300 as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc. whereby
the Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
On
September 27, 2023, the Company issued promissory notes to Perfect Day, Inc. (the “Perfect Day Note”) in the amount of $4,400,000
as consideration for the TUC APA (see Note 3). The promissory notes do not accrue interest and are payable upon demand at any time after
October 31, 2023. The entire aggregate, unpaid principal sum of the note is immediately due and payable upon the occurrence of a change
in control, as defined in the agreement. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc.
whereby the Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
On
September 14, 2023, the Company issued a promissory note to Danam Health, Inc. (the “Danam Note”) in the amount of $300,000.
The Company received a deposit of $200,000 on September 14, 2023, and an additional deposit of $100,000 on October 13, 2023. The Danam
Note accrues interest at 0% per annum and is due and payable no later than 30 days after a change in control of borrower, as defined
in the note agreement. As of December 31, 2023, the balance of the Danam Note was $50,000. The Danam Note was fully paid off in February
2024.
On
June 16, 2023, the Company issued a secured debenture to Eat Well Investment Group, Inc. (the “Eat Well June 2023 Note”)
in the amount of $1,150,000 for the purchase of Sapientia, a wholly-owned subsidiary of Superlatus. The Eat Well June 2023 Note is secured
by 100% of the membership interests in Sapientia. The Eat Well June 2023 Note began accruing interest at 12% per annum, compounded monthly,
as of October 31, 2023. The Eat Well June 2023 Note matured on December 31, 2023. As of December 31, 2023, the balance of the Eat Well
June 2023 Note was $1,150,000. The Company has accrued interest of $23,063 as of December 31, 2023. On March 5, 2024, the Company entered
into Stock Purchase Agreement with Superlatus Foods, Inc. whereby the Company sold of its entire interest in Superlatus to Superlatus
Foods, Inc. thereby transferring all assets and liabilities.
On
February 8, 2023, Sapientia, a wholly-owned subsidiary of Superlatus, entered into a Loan Agreement with Eat Well Investment Group, Inc.
(the “Eat Well February 2023 Note”) in the amount of $25,000. The Eat Well February 2023 Note is unsecured, accrues interest
at a rate of 1.87% per annum, and matures February 7, 2025. As of December 31, 2023, the balance of the Eat Well February 2023 Note was
$25,000. The Company has accrued interest of $418 as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase
Agreement with Superlatus Foods, Inc. whereby the Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby
transferring all assets and liabilities.
On
September 14, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well September 2022
Note”) in the amount of $50,000. The Eat Well September 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum,
and matures September 13, 2024. As of December 31, 2023, the balance of the Eat Well September 2022 Note was $50,000. The Company has
accrued interest of $1,212 as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus
Foods, Inc. whereby the Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and
liabilities.
On
July 26, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well July 26, 2022 Note”)
in the amount of $35,000. The Eat Well July 26, 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures July
25, 2024. As of December 31, 2023, the balance of the Eat Well July 26, 2022 Note was $35,000. The Company has accrued interest of $938
as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc. whereby the
Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
On
July 12, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well July 12, 2022 Note”)
in the amount of $25,000. The Eat Well July 12, 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures July
11, 2024. As of December 31, 2023, the balance of the Eat Well July 12, 2022 Note was $25,000. The Company has accrued interest of $688
as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc. whereby the
Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
On
March 15, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well March 2022 Note”)
in the amount of $100,000. The Eat Well March 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures March
14, 2024. As of December 31, 2023, the balance of the Eat Well March 2022 Note was $100,000. The Company has accrued interest of $3,361
as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc. whereby the
Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
On
February 1, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well February 2022 Note”)
in the amount of $100,000. The Eat Well February 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures February
1, 2024. As of December 31, 2023, the balance of the Eat Well February 2022 Note was $100,000. The Company has accrued interest of $3,576
as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc. whereby the
Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
On
January 20, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well January 2022 Note”)
in the amount of $20,000. The Eat Well January 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures January
20, 2024. As of December 31, 2023, the balance of the Eat Well January 2022 Note was $20,000. The Company has accrued interest of $728
as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc. whereby the
Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
On
December 24, 2021, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well December 2021 Note”)
in the amount of $100,000. The Eat Well December 2021 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matured December
24, 2023. As of December 31, 2023, the balance of the Eat Well December 2021 Note was $100,000. The Company has accrued interest of $3,776
as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc. whereby the
Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
On
November 10, 2021, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well November 2021 Note”)
in the amount of $50,000. The Eat Well November 2021 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matured November
10, 2023. As of December 31, 2023, the balance of the Eat Well November 2021 Note was $50,000. The Company has accrued interest of $2,001
as of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc. whereby the
Company sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
On
August 18, 2021, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well August 2021 Note”)
in the amount of $250,000. The Eat Well August 2021 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matured August
18, 2023. As of December 31, the balance of the Eat Well August 2021 Note was $250,000. The Company has accrued interest of $11,079 as
of December 31, 2023. On March 5, 2024, the Company entered into Stock Purchase Agreement with Superlatus Foods, Inc. whereby the Company
sold of its entire interest in Superlatus to Superlatus Foods, Inc. thereby transferring all assets and liabilities.
NOTE
12 – STOCKHOLDERS’ EQUITY
Designation
of Series C Preferred Stock
Effective
October 4, 2023, the Company filed a Certificate of Designation, Preferences, Rights and Limitations of the Series C Preferred Stock
with the Secretary of the State of Delaware which designated 1,000 shares of the Company’s authorized and unissued preferred stock
as convertible Series C Preferred Stock at a par value of $0.00001 per share.
Hudson
Global Ventures Stock Purchase Agreement
On
October 4, 2023, the Company entered into a Securities Purchase Agreement (“Agreement”, or “SPA”) with Hudson
Global Ventures, LLC (“Hudson”). Under the terms of the Agreement, the Company agreed to sell, and Hudson agreed to purchase,
Two Hundred Ninety (290) shares of Series C Preferred Stock (the “Purchased Shares”) at a price of $1,000 per share and a
Warrant to purchase up to 41,193 shares of Common Stock. Additionally, pursuant to the Agreement, 40,000 shares of Common Stock were
issued to Hudson upon closing for a commitment fee. The Company received $250,000 in exchange for the Purchased Shares, Common Stock,
and Warrants, net of issuance costs.
Designation
of Series B Preferred Stock
Effective
June 26, 2023, the Company filed a Certificate of Designation, Preferences, Rights and Limitations of the Series B Preferred Stock with
the Secretary of the State of Delaware which designated 787,754 shares of the Company’s authorized and unissued preferred stock
as convertible Series B Preferred Stock at a par value of $0.00001 per share.
2023
1:15 Stock Split
Effective
June 21, 2023, the Company executed a 1:15 reverse stock split for stockholders of record on that date. This was executed to comply with
the Nasdaq Listing Rule 5550(a)(2) to have the price of the stock above $1.
Common
Stock
During
the six months ended June 30, 2024, the Company issued 470,482 shares of common stock for services. The fair value of shares issued for
services was $4,450,919 and was included in general and administrative expenses in the consolidated statements of operations.
During
the six months ended June 30, 2024, a warrants holder exercised a warrant and acquired 28,487 shares of common stock for $16,567 in proceeds
(see Note 14).
During
the six months ended June 30, 2024, an options holder exercised an option and acquired 2,371 shares of common stock for $9,840 in proceeds
(see Note 15).
Special
Cash Dividend
On
March 6, 2024, the Company announced the declaration of a special cash dividend of eight dollars ($8.00) per share of common stock, payable
to stockholders of record as of March 18, 2024, with the dividend being paid on March 22, 2024. The special dividend of $12,671,072 (in
the aggregate) was paid using a portion of the proceeds from the closing of the sale of the Trxade assets to MMS.
On
July 9, 2024, the Company announced the declaration of a special cash dividend of one dollar and fifty cents ($1.50) per share of common
stock, payable to stockholders of record as of July 19, 2024, with the dividend being paid on or about July 24, 2024. The special dividend
of $2,187,759 was paid using a portion of the proceeds received in May 2024 in connection with the sale of the Trxade assets to MMS.
Equity
Compensation Awards
Each
independent member of the Board is to receive an annual grant of restricted common stock of the Company equal to $55,000 in value on
April 1st of each year (or such date thereafter as the awards are approved by the Board), and valued on such same date, based on the
closing sales price on such date (or the first business day thereafter), which restricted stock awards will vest at the rate of 1/4th
of such awards over the following four calendar quarters, subject to such directors continued service to the Company.
Effective
on August 13, 2023, the Board approved the issuance of 24,444 shares of common stock of the Company to each of Mr. Fell and Mr. Peterson
(who each at the time of issuance were members of the Board of Directors) for services rendered to the Company during fiscal 2023, which
shares were valued at $110,000. The Board also approved the issuance of 14,056 shares of common stock of the Company to Jeff Newell (who,
at the time of issuance was a member of the Board of Directors) for services rendered during fiscal 2023, which were valued at $63,250
based on the most recent close price of the Company’s common stock on the date approved by the Board. The shares vest at the rate
of 1/4th of such shares immediately on the grant date, and 1/4th of such shares on each of October 1, 2023, January 1, 2024 and April
1, 2024, subject to each applicable independent director’s continued service to the Company on such dates. Additionally, the Board
approved 10,000 shares with immediate vesting to each Board member to recognize the significant additional work for various financing,
sales, acquisitions, operations restructuring.
All
of the awards discussed above were issued under the Company’s Second Amended and Restated 2019 Equity Incentive Plan (the “Plan”)
and all restricted stock awards discussed above were evidenced by Restricted Stock Grant Agreements.
The Company’s
board of directors and stockholders approved an amendment to the Company’s Second Amended and Restated 2019 Equity Incentive Plan
(the “2019 EIP”) increasing the available shares under the 2019 EIP to 5,000,000 shares of the Common Stock as such common
stock existed on July 24, 2024 (see Note 19).
NOTE
13 – PREFUNDED AND PRIVATE PLACEMENT WARRANTS
On
October 4, 2022 the Company entered into a securities purchase agreement (the “Purchase Agreement”) with institutional
investor (the “Purchaser”) which provided for the sale and issuance by the Company of (i) the Company’s common stock
(the “Common Stock”), (ii) pre-funded warrants (the “Pre-Funded Warrants”) and (iii) warrants (the “Private
Placement Warrants” and, together with the Shares and the Pre-Funded Warrants, the “Securities”).
On
January 4, 2023, the investor exercised the Pre-Funded Warrants for a purchase price of $6.02. The investor was issued the shares on
this date. Each Private Placement Warrant has an exercise price of $22.50 per share and is exercisable following the Stockholder
Approval obtained in December 2022, and will expire on the fifth anniversary of the date on which the Private Placement Warrants became
exercisable. The Private Placement Warrants contain standard adjustments to the exercise price including for stock splits, stock dividend,
rights offerings and pro rata distributions, and include full ratchet anti-dilutive rights in the event the Company issues shares of
Common Stock or Common Stock equivalents within fifteen months of the initial exercise date, with a value less than the then exercise
price of such Private Placement Warrants, subject to certain customary exceptions, and further subject to a minimum exercise price of
$3.48 per share. The Private Placement Warrants also include certain rights upon ‘fundamental transactions’ as described
in the Private Placement Warrants, including allowing the holders thereof to require that the Company re-purchase such Private Placement
Warrants at the Black Scholes Value of such securities.
NOTE
14 – WARRANTS
During
the six months ended June 30, 2024, 28,487 warrants to purchase shares of common stock were exercised for a total purchase price of $16,567
(see Note 12).
The
Company uses the Black-Scholes pricing model to estimate the fair value of stock-based awards on the date of the grant.
There
was no compensation cost related to the warrants for the six months ended June 30, 2024, and 2023, respectively.
As
of June 30, 2024, the Company remeasured the fair value of warrants outstanding at $1,631,974. In connection with remeasurement of warrants,
a $165,132 and $895,021 expense was recognized during the three and six months ended June 30, 2024, respectively, as the change in fair
value of warrant liability.
The
Company’s outstanding and exercisable warrants As of June 30, 2024, are presented below:
SCHEDULE
OF OUTSTANDING AND EXERCISABLE WARRANTS
| | |
Number
Outstanding | | |
Weighted
Average
Exercise
Price | | |
Contractual
Life In Years | | |
Intrinsic
Value | |
| Warrants outstanding as of December
31, 2023 | |
| 218,729 | | |
| 19.62 | | |
| 3.95 | | |
| - | |
| Warrants granted | |
| - | | |
| - | | |
| - | | |
| - | |
| Warrants forfeited, expired, cancelled | |
| - | | |
| - | | |
| - | | |
| - | |
| Warrants exercised | |
| (28,487 | ) | |
| 7.14 | | |
| - | | |
| - | |
| Warrants outstanding
as of June 30, 2024 | |
| 190,242 | | |
| 21.48 | | |
| 3.34 | | |
| 141,926 | |
| Warrants exercisable as of June 30, 2024 | |
| 190,242 | | |
| 21.48 | | |
| 3.34 | | |
| 141,926 | |
NOTE
15 – OPTIONS
The
Company maintains stock option plans under which certain employees are awarded option grants based on a combination of performance and
tenure. The stock option plans provide for the grant of up to 155,556 shares, and the Company’s Second Amended and Restated 2019
Equity Incentive Plan provides for automatic increases in the number of shares available under such plan (currently 133,333 shares) on
April 1st of each calendar year, beginning in 2021 and ending in 2029 (each a “Date of Determination”), in each
case subject to the approval and determination of the administrator of the plan (the Board of Directors or Compensation Committee) on
or prior to the applicable Date of Determination, equal to the lesser of (A) ten percent (10%) of the total shares of common stock of
the Company outstanding on the last day of the immediately preceding fiscal year and (B) such smaller number of shares as determined
by the administrator. The administrator as a result of the annual meeting shareholder vote increased the number of shares available to
grant to employees under the 2019 incentive plan by 2,000,000. The administrator did not approve an increase in the number of shares
covered under the plan as of April 1, 2022.
For
the six-month period ended June 30, 2024, no options to purchase shares were granted. For the six-month period ended June 30, 2024, 2,371
options to purchase shares of common stock were exercised for $9,840 in cash (see Note 12).
Total
compensation cost related to stock options granted was $444 and $7,783 for the three months ended June 30, 2024, and 2023, respectively.
Total compensation cost related to stock options granted was $24,710 and $22,217 for the six months ended June 30, 2024 and 2023, respectively.
The
following table represents stock option activity for the six-month period ended June 30, 2024:
SCHEDULE
OF STOCK OPTION ACTIVITY
| | |
Number
Outstanding | | |
Weighted-
Average
Exercise
Price | | |
Weighted-
Average
Contractual
Life in Years | | |
Intrinsic
Value | |
| Options outstanding as of December 31, 2023 | |
| 26,229 | | |
$ | 43.04 | | |
| 3.70 | | |
$ | - | |
| Options exercisable as of December 31, 2023 | |
| 16,141 | | |
| 60.75 | | |
| 3.64 | | |
| - | |
| Options granted | |
| - | | |
| - | | |
| - | | |
| - | |
| Options adjusted | |
| 72 | | |
| - | | |
| - | | |
| - | |
| Options expired | |
| - | | |
| - | | |
| - | | |
| - | |
| Options exercised | |
| (2,371 | ) | |
| 53.29 | | |
| 3.32 | | |
| - | |
| Options outstanding as of June 30,
2024 | |
| 23,930 | | |
| 40.78 | | |
| 3.18 | | |
| 46,125 | |
| Options exercisable as of June 30, 2024 | |
| 23,930 | | |
| 42.16 | | |
| 2.28 | | |
| 46,125 | |
NOTE
16 – CONTINGENCIES
Studebaker
Defense Group, LLC
In
July 2020, the Company’s wholly-owned subsidiary, IPS, entered into an agreement with Studebaker Defense Group, LLC (“Studebaker”)
wherein IPS would pay Studebaker a down payment of $550,000 and Studebaker would deliver 180,000 boxes of nitrile gloves by August 14,
2020. IPS wired the $550,000 to Studebaker, but to date, Studebaker has not delivered the gloves or provided a refund of the deposit.
In December 2020, the Company filed a complaint against Studebaker in Florida state court, Case No. 20-CA-010118 in the Circuit Court
for the Thirteenth Judicial Circuit in Hillsborough County, for among other things, breach of contract. Studebaker did not answer the
complaint, nor did counsel for Studebaker file an appearance. Accordingly, in February 2021, the Company filed for a default judgment;
however, on March 22, 2021, counsel for Studebaker filed an appearance and shortly thereafter filed a motion to vacate the default judgment
and dismiss the complaint on jurisdictional grounds. The court granted Studebaker’s motion to set aside the default judgment but
denied the motion to dismiss. At June 30, 2021, the $500,000 was recorded as Loss on Inventory Investment. The Company won this case
but has not collected any settlement yet, another lawsuit was filed to collect.
On
April 13, 2023, a settlement was reached in the Studebaker and IPS legal case. The court found in favor of IPS and ordered Studebaker
to pay $550,000 to IPS. The payments were to commence on May 1, 2023 and continue monthly in 17 installments until the full amount is
paid in full but as of the filing date, no payment has been received by IPS.
GSG
PPE, LLC
On
November 19, 2021, IPS filed a complaint against GSG PPE, LLC (“GSG”) and Gary Waxman (“Waxman”), the owner,
alleging three counts of breach of contract for a purchase agreement, a promissory note, and a personal guaranty. Collectively, the company
alleges that GSG and Waxman have materially breached all three contracts. In late 2020, GSG and IPS executed a valid initial contract
setting the terms of a business transaction. GSG failed to pay IPS approximately 75% of the amount owed to IPS. GSG acknowledged it owed
the money and executed a promissory note in favor of IPS in the amount of $630,000 which matured on September 30, 2021. The note provides
for attorney fees and interest in addition to the $630,000. Waxman’s personal guaranty confirmed that GSG owed IPS $630,000. On
September 30, 2021, the $630,000 was recorded as Bad Debt Expense. A settlement was entered into between the parties in June 2022, whereby
GSG and Waxman agreed to pay $743,000 which included attorney fees and interest, which is required to be paid to the Company in monthly
installments over 17 months. The Company received additional monthly installment payments as part of the agreement through January 2023.
As of June 30, 2024, and through the date of this filing, the Company has not received the monthly installment payments due to the Company
from GSG since January of 2023.
NOTE
17 – LEASES
The
Company has one operating lease for a corporate office as of June 30, 2024. The following table outlines the details of the leases:
SCHEDULE
OF OPERATING LEASES
| | |
Lease
1 | | |
Lease
2 | | |
Lease
3 | |
| Initial lease term | |
| January
2021 to December 2021 | | |
| October
2018 to November 2023 | | |
| October
2023 to September 2026 | |
| New initial lease term | |
| January
2022 to December 2026 | | |
| November
2023 to October 2028 | | |
| | |
| Initial recognition of right
of use assets at January 1, 2019 | |
$ | 534,140 | | |
$ | 313,301 | | |
$ | - | |
| New initial recognition of right of use
assets at December 31, 2021 | |
$ | 977,220 | | |
$ | - | | |
$ | - | |
| New initial recognition of right of use
assets at December 31, 2023 | |
$ | - | | |
$ | - | | |
$ | 351,581 | |
| Incremental borrowing rate | |
| 10 | % | |
| 10 | % | |
| 10 | % |
The
Company entered into a new corporate office lease (Lease 1) in January 2022. At inception, the Company determined that the new lease
required remeasurement of the lease liability resulting in the increase of the right-of-use asset and the associated lease liability
by $977,220. The Company and the Lessor agreed to terminate the lease and vacate the premises in November 2023. The termination resulted
in the surrender of the Company’s security deposit of $38,500. The related right-of-use assets of $642,887 and lease liabilities
of $664,992 were removed from the balance sheet as of December 31, 2023.
The
Company entered into a lease agreement (Lease 2) for the period of October 2018 to November 2023. At inception, management had included
the renewal period from November 2023 to November 2028 within the initial recognition of the related right of use assets and lease liabilities,
as it was reasonably expected, at the time, that the renewal option would be exercised. The Company determined that the new lease required
measurement and recognition of the lease liability and right-of-use assets of $313,301. The lease is classified as an operating lease.
No incentives were included in the lease.
The
Company entered into a new warehouse lease (Lease 3) October 2023. The Company determined that the new lease required measurement and
recognition of the lease liability and right-of-use assets of $351,581. The lease is classified as an operating lease. No incentives
were included in the lease.
In
the first quarter of 2024, the Company sold assets and liabilities of Trxade, Inc. and Superlatus. including the related right of use
assets and liabilities. The Company has only Lease 2 active and continuing in the condensed consolidated balance sheet as of June 30,
2024.
The
table below reconciles the fixed component of the undiscounted cash flows for Lease 2 of the first five years and the total remaining
years to the lease liabilities recorded in the Consolidated Balance Sheet as of June 30, 2024.
SCHEDULE
OF FUTURE MINIMUM PAYMENTS FOR OPERATING LEASE LIABILITIES
| Future
lease obligations | |
| | |
| 2024 remaining | |
$ | 26,174 | |
| 2025 | |
| 53,652 | |
| 2026 | |
| 55,261 | |
| 2027 | |
| 56,919 | |
| 2028 | |
| 48,612 | |
| Total minimum lease payments | |
| 240,618 | |
| Less: effect of discounting | |
| (47,014 | ) |
| Present value of future minimum lease payments | |
| 193,604 | |
| Less: current obligation
under lease | |
| 32,608 | |
| Long-term lease obligations | |
$ | 160,996 | |
For
the three months ended June 30, 2024, and 2023, total operating lease expense was $12,840 and $75,496, which is included in general and
administrative expenses in the condensed consolidated statements of operations, as well as $62,656 from discontinued operations, respectively.
For
the six months ended June 30, 2024, and 2023, total operating lease expense was $25,681 and $150,992, which is included in general and
administrative expenses in the condensed consolidated statements of operations, as well as $125,312 from discontinued operations, respectively.
For the three
months ended June 30, 2024, and 2023, total short-term lease expense was $2,143 and $8,511, which is included in general and administrative
expenses in the condensed consolidated statements of operations, respectively.
For the six months
ended June 30, 2024, and 2023, total short-term lease expense was $10,228 and $14,039, which is included in general and administrative
expenses in the condensed consolidated statements of operations, respectively.
NOTE
18 – SEGMENT REPORTING
Operating
segments are defined as the components of an enterprise about which separate financial information is available that is evaluated regularly
by the chief operating decision makers in deciding how to allocate resources and in assessing performance. The Company’s chief
operating decision makers direct the allocation of resources to operating segments based on the profitability, cash flows, and growth
opportunities of each respective segment.
The
Company classifies its business interests into reportable segments which are:
| |
● |
IPS
- Integra Pharma, LLC - Licensed wholesaler of brand, generic and non-drug products – B2B sales |
| |
● |
Unallocated
- Other – corporate overhead expense and discontinued operations. |
| Three Months Ended June 30, 2024 | |
Integra | | |
Unallocated | | |
Total | |
| Revenue | |
$ | 18,699 | | |
$ | - | | |
$ | 18,699 | |
| Gross Profit | |
| (703 | ) | |
| - | | |
| (703 | ) |
| Segment Assets | |
| 9,477,347 | | |
| 5,371,187 | | |
| 14,848,533 | |
| Segment Profit/Loss | |
| (346,431 | ) | |
| (1,487,471 | ) | |
| (1,833,902 | ) |
| Cost of Sales | |
$ | 19,402 | | |
$ | - | | |
$ | 19,402 | |
| Three Months Ended June 30, 2023 | |
Integra | | |
Unallocated | | |
Total | |
| Revenue | |
$ | 366,526 | | |
$ | - | | |
$ | 366,526 | |
| Gross Profit | |
| 67,139 | | |
| - | | |
| 67,139 | |
| Segment Assets | |
| 343,873 | | |
| 5,328,587 | | |
| 5,672,460 | |
| Segment Profit/Loss | |
| (103,219 | ) | |
| (1,871,659 | ) | |
| (1,974,878 | ) |
| Cost of Sales | |
$ | 299,387 | | |
$ | 306,962 | | |
$ | 606,349 | |
| Six
Months Ended June 30, 2024 | |
Integra | | |
Unallocated | | |
Total | |
| Revenue | |
$ | 18,699 | | |
$ | - | | |
$ | 18,699 | |
| Gross Profit | |
| (703 | ) | |
| - | | |
| (703 | ) |
| Segment Assets | |
| 9,477,347 | | |
| 5,371,187 | | |
| 14,848,533 | |
| Segment Profit/Loss | |
| (585,086 | ) | |
| 19,997,217 | | |
| 19,412,131 | |
| Cost of Sales | |
$ | 19,402 | | |
$ | - | | |
$ | 19,402 | |
| Six Months
Ended June 30, 2023 | |
Integra | | |
Unallocated | | |
Total | |
| Revenue | |
$ | 842,882 | | |
$ | - | | |
$ | 842,882 | |
| Gross Profit | |
| 123,398 | | |
| - | | |
| 123,398 | |
| Segment Assets | |
| 343,873 | | |
| 5,328,587 | | |
| 5,672,460 | |
| Segment Profit/Loss | |
| (208,086 | ) | |
| (2,444,746 | ) | |
| (2,652,831 | ) |
| Cost of Sales | |
$ | 719,484 | | |
$ | 577,535 | | |
$ | 1,297,019 | |
NOTE
19 – SUBSEQUENT EVENTS
On
July 9, 2024, the Company announced the declaration of a special cash dividend of one dollar and fifty cents ($1.50) per share of common
stock, payable to stockholders of record as of July 19, 2024, with the dividend being paid on or about July 24, 2024. The special dividend
was $2,187,759 paid using a portion of the proceeds received in May 2024 in connection with the prior sale of the Company’s web-based
market platform assets.
On
July 12, 2024, the Company converted 290 shares of Series C Preferred Stock into 52,158 shares of common stock at the election of the
holder.
On July 25, 2024,
the Company entered into and closed an Agreement and Plan of Merger (the “Scienture Merger Agreement”) with MEDS Merger Sub
I, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub I”), MEDS Merger Sub II, LLC, a Delaware
limited liability company and wholly owned subsidiary of the Company (“Merger Sub II” and, together with Merger Sub I, the
“Merger Subs”), and Scienture, Inc., a Delaware corporation (“Scienture”). Pursuant to the Merger Agreement,
(i) Merger Sub I merged with and into Scienture (the “First Merger”), with Scienture continuing as the surviving entity and
a wholly owned subsidiary of the Company, and (ii) Scienture merged with and into Merger Sub II (the “Second Merger” and,
together with the First Merger, the “Mergers”), with Merger Sub II continuing as the surviving entity. In connection with
the transactions, the Company will change its name from “TRxADE HEALTH, INC.” to “Scienture Holdings, Inc.” and
Merger Sub II, as the surviving entity of the Mergers, will change its name from “MEDS Merger Sub II, LLC” to “Scienture
LLC”. Scienture is a pharmaceutical company based in Commack, New York, and focuses on developing unique specialty product concepts
and solutions that bring enhanced value to patients and healthcare systems. Scienture is in the process of developing various assets
across therapeutics areas, indications and cater to different market segments.
As consideration
for the Mergers, at the effective time of the First Merger (the “First Effective Time”), the shares of Scienture common stock
issued and outstanding immediately prior to the First Effective Time were converted into the right to receive, in the aggregate, (i)
291,555 shares of the Company’s common stock which represents 19.99% of the number of shares of common stock issued and outstanding
immediately prior to the effective time of the First Merger, and (ii) 6,826,713 shares of the Company’s Series X Non-Voting Convertible
Preferred Stock, par value $0.00001 per share (the “Series X Preferred Stock”), each share of which is convertible into one
share of common stock. See below for description of the Series X Preferred Stock.
On July 25, 2024,
the Company revoked the authorization to issue shares of the Company’s Series A Preferred Stock, par value $0.00001 per share (the
“Series A Preferred Stock”). Concurrently with revoking the Company’s authority to issue Series A Preferred Stock,
the Company authorized the issuance of up to 9,211,246 shares of the Series X Preferred Stock, a new class of preferred stock.
All issued and
outstanding shares of Scienture, Inc.’s common stock were converted into the right to receive a combination of shares of Series
X Preferred Stock and shares of the Common Stock in connection with the Merger. Specifically, former shareholders of Scienture, Inc.
collectively have the right to obtain such shareholders’ pro rata share of 291,555 shares of Common Stock and 6,826,713 shares
of Series X Preferred Stock. Shares of Common Stock and Series X Preferred Stock will be issued to former stockholders of Scienture,
Inc. upon the exchange agent receiving the former stockholder’s executed letter of transmittal and such other documents reasonably
required by the exchange agent or the Company.
The Certificate
of Designation provides that, subject to any beneficial ownership limitations designated by former Scienture, Inc. stockholders, the
shares of Series X Preferred Stock will automatically convert into shares of Common Stock at a 1:1 conversion ratio upon the earliest
date permitted by the listing rules of the Nasdaq Stock Market following the date that the Company’s stockholders approve the Preferred
Stock Conversion (the “Eligible Conversion Date”). Holders of the Series X Preferred Stock may convert, at any time after
the Eligible Conversion Date, shares of the Series X Preferred Stock into shares of the Common Stock.
Holders of the
Series X Preferred Stock are entitled to receive dividends on shares of the Series X Preferred Stock on an as-if-converted-to-Common-Stock
basis, without regard to any beneficial ownership limitation described in a letter of transmittal, equal to and in the same form and
manner as dividends are paid to holders of the shares of Common Stock. Subject to any requirements of the General Corporation Law of
the State of Delaware, the Series X Preferred Stock has no voting rights. The Series X Preferred Stock ranks on parity with shares of
Common Stock as to distributions of assets upon liquidation, dissolution, or winding up of the Company.
In connection
with the consummation of the Mergers, on July 25, 2024, the Company’s Board appointed Shankar Hariharan and Narasimhan Mani to
the Board. It has not yet been determined on which committees of the Board either Dr. Hariharan or Dr. Mani will serve.
The Company’s
board of directors and stockholders approved an amendment to the Company’s Second Amended and Restated 2019 Equity Incentive Plan
(the “2019 EIP”) increasing the available shares under the 2019 EIP to 5,000,000 shares of the Common Stock as such common
stock existed on July 24, 2024.
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Board of Directors and
Stockholders
of TRxADE HEALTH Inc.
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheet of TRxADE Health, Inc. (the Company) as of December 31, 2023, and the related
consolidated statements of operations, stockholders’ equity, and cash flows for the year then ended, and the related notes (collectively
referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial
position of the Company as of December 31, 2023, and the results of its operations and its cash flows for the year ended then ended,
in conformity with accounting principles generally accepted in the United States of America.
Basis
for Opinion
These
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s
financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board
(United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities
laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We
conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company
is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit,
we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or
fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides
a reasonable basis for our opinion.
Emphasis
of a matter – Going concern
The
accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note
2 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raises
substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described
in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Critical
Audit Matters
The
critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated
or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial
statements and (2) involved challenging, subjective, or complex judgments. The communication of critical audit matters does not alter
in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below,
providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Fair
value of acquired intangible assets
Description
of the matter
As
discussed in Note 1 and Note 3 to the consolidated financial statements, on July 31, 2023, the Company acquired Superlatus, Inc. in a
transaction accounted for as a business combination. As a result of the transaction, the Company recognized acquired technology associated
with the generation of future income. The acquisition-date fair value of the acquired technology was $9.8 million.
We
identified the evaluation of the acquisition-date fair value of the acquired technology as a critical audit matter. A high degree of
subjective auditor judgment was required to evaluate the key assumptions within the discounted cash flows model used to estimate the
acquisition-date fair value of the acquired technology, specifically the revenue growth rate, margin, and discount rate. There was limited
observable market information related to these assumptions and the estimated acquisition-date fair value of the acquired technology was
sensitive to minor changes in such assumptions.
How
We Addressed the Matter in our Audit
The
following are the primary procedures we performed to address this critical audit matter.
| |
● |
We
evaluated the Company’s revenue growth rate and margin assumptions by comparing them to the pre-acquisition budget and the
Company’s historical financial results. |
| |
|
|
| |
● |
We
evaluated the discount rate used by comparing it to a discount rate that was developed using publicly available market data for comparable
entities. |
| |
|
|
| |
● |
We
compared the revenue growth rate, margin, to those of comparable entities |
| |
|
|
| |
● |
We
validated the mathematical accuracy of the management’s calculations. |
Impairment
of Goodwill
Description
of the Matter
As
reflected in the Company’s consolidated financial statements at December 31, 2023, the Company impaired all goodwill as of December
31, 2023. As disclosed in Notes 1 to the consolidated financial statements, goodwill is tested for impairment at least annually or more
frequently if indicators of impairment require the performance of an interim impairment assessment. As a result of these assessments,
management concluded that there was an impairment to goodwill for the year ended December 31, 2023, in the amount of $5.1 million.
Auditing
management’s impairment tests of goodwill is complex and highly judgmental due to the significant measurement uncertainty in determining
the fair values of the reporting units. In particular, the fair value estimates of the reporting units were sensitive to changes in significant
assumptions such as discount rates, revenue growth rates, operating margins, estimated spending on capital expenditures, terminal growth
rates, and comparable company specific information. These assumptions are affected by current and expected future market or economic
conditions.
How
We Addressed the Matter in our Audit
Our
audit procedures related to the selection of the discount rates used and forecasts of future net sales, operating margins, operating
expenses, and other market and economic data of the reporting units, involved:
| |
● |
Obtaining
an understanding of the Company’s process and related controls to evaluate goodwill for impairment. |
| |
|
|
| |
● |
Evaluating
the reasonableness of managements forecasts of future net sales, operating margins, and operating expenses by comparing the forecasts
to historical results, marketing plans relevant economic factors, and other comparable company and industry information. |
/s/
CM3 Advisory
We
have served as the Company’s auditor since 2023
San
Diego, California
April
22, 2024
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Shareholders and Board of Directors of
TRxADE
HEALTH, INC.
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheet of TRxADE HEALTH, INC. (the “Company”) as of December 31, 2022,
and the related statements of operations, stockholders’ equity, and cash flows for the year then ended, and the related notes (collectively
referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects,
the financial position of the Company as of December 31, 2022, and the results of its operations and its cash flows for the year then
ended, in conformity with accounting principles generally accepted in the United States of America.
Going
Concern Matter
The
accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note
2 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raises
substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described
in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis
for Opinion
These
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s
financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board
(United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal
securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We
conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company
is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit
we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or
fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides
a reasonable basis for our opinion.
/s/
MaloneBailey, LLP
www.malonebailey.com
We
have served as the Company’s auditor from 2013 to 2023.
Houston,
Texas
March
27, 2023
TRxADE
HEALTH, INC.
Consolidated
Balance Sheets
December
31, 2023 and 2022
| | |
December 31, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Assets | |
| | | |
| | |
| Current Assets | |
| | | |
| | |
| Cash | |
$ | 151,908 | | |
$ | 1,094,894 | |
| Accounts receivable, net | |
| 821,804 | | |
| 629,921 | |
| Inventory | |
| 968 | | |
| 65,523 | |
| Prepaid assets | |
| 107,774 | | |
| 104,461 | |
| Notes receivable | |
| 1,300,000 | | |
| - | |
| Other receivables | |
| 370,608 | | |
| - | |
| Current assets of discontinued operations | |
| - | | |
| 198,324 | |
| Total Current Assets | |
| 2,753,062 | | |
| 2,093,123 | |
| | |
| | | |
| | |
| Property, plant and equipment, net | |
| 277,009 | | |
| 65,214 | |
| Intangible assets and capitalized software, net | |
| 8,962,688 | | |
| - | |
| Security deposits | |
| 10,531 | | |
| 49,029 | |
| Operating lease right-of-use assets | |
| 529,623 | | |
| 1,051,815 | |
| Noncurrent assets of discontinued operations | |
| - | | |
| 450,845 | |
| Total Assets | |
$ | 12,532,913 | | |
$ | 3,710,026 | |
| | |
| | | |
| | |
| Liabilities and Shareholders’ Equity | |
| | | |
| | |
| Current Liabilities | |
| | | |
| | |
| Accounts payable | |
| 2,082,054 | | |
| 527,984 | |
| Accrued liabilities | |
| 400,987 | | |
| 271,230 | |
| Other current liabilities | |
| 70,310 | | |
| 67,517 | |
| Contingent funding liabilities | |
| 1,246,346 | | |
| 108,036 | |
| Lease liabilities – current portion | |
| 139,705 | | |
| 196,872 | |
| Notes payable – current portion | |
| 6,530,000 | | |
| 166,667 | |
| Warrant liability | |
| 736,953 | | |
| 588,533 | |
| Purchase price payable | |
| 350,000 | | |
| - | |
| Current liabilities of discontinued operations | |
| - | | |
| 219,952 | |
| Total Current liabilities | |
| 11,556,355 | | |
| 2,146,791 | |
| | |
| | | |
| | |
| Long Term Liabilities | |
| | | |
| | |
| Lease liabilities – net of current portion | |
| 409,205 | | |
| 887,035 | |
| Notes payable | |
| 25,000 | | |
| 333,333 | |
| | |
| | | |
| | |
| Total Liabilities | |
| 11,990,560 | | |
| 3,367,159 | |
| | |
| | | |
| | |
| Stockholders’ Equity | |
| | | |
| | |
| | |
| | | |
| | |
| Series A preferred stock, $0.00001 par value; 9,211,246 shares authorized; none
issued and outstanding as of December 31, 2023 and December 31, 2022 | |
| - | | |
| - | |
| Series B preferred stock, $0.00001 par value; 787,754 shares authorized; 15,759
outstanding as of December 31, 2023, and none as December 31, 2022 | |
| - | | |
| - | |
| Series C preferred stock, $0.00001 par value; 1,000 shares authorized; 290 issued
and outstanding as of December 31, 2023, and none as of December 31, 2022 | |
| - | | |
| - | |
| Preferred stock value | |
| | | |
| | |
| Common stock, $0.00001 par value; 100,000,000 shares authorized; 905,008, and 626,247 shares issued
and outstanding as of December 31, 2023 and December 31, 2022, respectively | |
| 9 | | |
| 6 | |
| Additional paid-in capital | |
| 33,788,284 | | |
| 20,482,666 | |
| Retained deficit | |
| (33,245,940 | ) | |
| (19,719,536 | ) |
| Total TRxADE Health, Inc stockholders’ equity | |
| 542,353 | | |
| 763,136 | |
| Non-controlling interest in subsidiary | |
| - | | |
| (420,269 | ) |
| Total stockholders’ equity | |
| 542,353 | | |
| 342,867 | |
| | |
| | | |
| | |
| Total Liabilities and Stockholders’
Equity | |
$ | 12,532,913 | | |
$ | 3,710,026 | |
The
accompanying notes are an integral part of the consolidated financial statements.
TRxADE
HEALTH, INC.
Consolidated
Statements of Operations
Years
Ended December 31, 2023 and 2022
| | |
2023 | | |
2022 | |
| | |
Years Ended December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Revenues | |
$ | 8,272,214 | | |
$ | 10,250,168 | |
| Cost of sales | |
| 5,673,957 | | |
| 4,730,897 | |
| Gross Profit | |
| 2,598,257 | | |
| 5,519,271 | |
| | |
| | | |
| | |
| Operating Expenses: | |
| | | |
| | |
| Loss on inventory investment | |
| - | | |
| 875,250 | |
| Wage and salary expense | |
| 2,698,178 | | |
| 3,581,089 | |
| Professional fees | |
| 1,466,567 | | |
| 466,735 | |
| Accounting and legal expense | |
| 1,534,377 | | |
| 829,751 | |
| Technology expense | |
| 1,376,908 | | |
| 993,185 | |
| General and administrative | |
| 2,785,633 | | |
| 1,689,230 | |
| Total operating expenses | |
| 9,861,663 | | |
| 8,435,240 | |
| Operating Loss | |
| (7,263,406 | ) | |
| (2,915,969 | ) |
| | |
| | | |
| | |
| Nonoperating Income (Expense) | |
| | | |
| | |
| Change in fair value of warrant liability | |
| (148,420 | ) | |
| 825,544 | |
| Interest income | |
| 4,198 | | |
| 20,989 | |
| Goodwill impairment | |
| (5,129,115 | ) | |
| - | |
| Gain on disposal of asset | |
| - | | |
| 2,200 | |
| Other income | |
| 14,543 | | |
| - | |
| Interest expense | |
| (1,198,346 | ) | |
| (336,206 | ) |
| Total nonoperating income (expense) | |
| (6,457,140 | ) | |
| 512,527 | |
| Net loss from continuing operations | |
| (13,720,546 | ) | |
| (2,403,442 | ) |
| | |
| | | |
| | |
| Net loss on discontinued operations | |
| (4,123,028 | ) | |
| (1,506,426 | ) |
| Net Loss | |
| (17,843,574 | ) | |
| (3,909,868 | ) |
| | |
| | | |
| | |
| Net loss attributable to TRxADE Health, Inc. | |
| (17,843,574 | ) | |
| (3,472,099 | ) |
| Net loss attributable to non-controlling interests | |
| - | | |
| (437,769 | ) |
| Net loss per common share from continuing operations | |
| | | |
| | |
| Basic | |
$ | (17.96 | ) | |
$ | (3.48 | ) |
| Diluted | |
$ | (5.76 | ) | |
$ | (3.47 | ) |
| Net loss per common share from discontinued operations | |
| | | |
| | |
| Basic | |
$ | (5.40 | ) | |
$ | (2.67 | ) |
| Diluted | |
$ | (1.73 | ) | |
$ | (2.66 | ) |
| Net loss attributable to common stockholders | |
| | | |
| | |
| Basic | |
$ | (23.35 | ) | |
$ | (6.15 | ) |
| Diluted | |
$ | (7.49 | ) | |
$ | (6.13 | ) |
| Weighted average common shares outstanding | |
| | | |
| | |
| Basic | |
| 764,058 | | |
| 564,862 | |
| Diluted | |
| 2,381,443 | | |
| 566,609 | |
The
accompanying notes are an integral part of the consolidated financial statements.
TRxADE
HEALTH, INC.
Consolidated
Statements of Changes in Stockholders’ Equity
Years
Ended December 31, 2023 and 2022
| | |
| | |
| |
|
|
|
|
|
|
|
| |
| | |
| | |
| | |
| | |
| | |
| |
| | |
Series
B Preferred Stock | |
|
|
Series
C Preferred
Stock
|
| |
Common Stock | | |
Additional | | |
| | |
Non-Controlling | | |
Total | |
| | |
Shares | | |
$ Amount | |
|
|
Shares |
|
|
|
$
Amount |
| |
Shares | | |
$ Amount | | |
Paid-in Capital | | |
Accumulated
Deficit | | |
Interest
in Subsidiary | | |
Stockholders’
Equity | |
| Balance at December 31, 2021 | |
| - | | |
$ | - | |
|
|
- |
|
|
$ |
- |
| |
| 544,430 | | |
$ | 5 | | |
$ | 20,017,605 | | |
$ | (16,247,437 | ) | |
$ | - | | |
$ | 3,770,173 | |
| Capital Contributions | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| - | | |
| - | | |
| - | | |
| - | | |
| 792,500 | | |
| 792,500 | |
| Capital Distribution | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| - | | |
| - | | |
| - | | |
| - | | |
| (775,000 | ) | |
| (775,000 | ) |
| Common stock issued for services | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| 19,511 | | |
| - | | |
| 254,106 | | |
| - | | |
| - | | |
| 254,106 | |
| Common stock issued for placement, net issuance costs | |
| - | | |
| | |
|
|
- |
|
|
|
- |
| |
| 61,334 | | |
| 1 | | |
| 130,917 | | |
| - | | |
| - | | |
| 130,918 | |
| Warrants exercised for cash | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| 972 | | |
| - | | |
| 875 | | |
| - | | |
| - | | |
| 875 | |
| Options expense | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| - | | |
| - | | |
| 79,163 | | |
| - | | |
| - | | |
| 79,163 | |
| Net loss | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| - | | |
| - | | |
| - | | |
| (3,472,099 | ) | |
| (437,769 | ) | |
| (3,909,868 | ) |
| Balance at December 31, 2022 | |
| - | | |
$ | - | |
|
|
- |
|
|
$ |
- |
| |
| 626,247 | | |
$ | 6 | | |
$ | 20,482,666 | | |
$ | (19,719,536 | ) | |
$ | (420,269 | ) | |
$ | 342,867 | |
| Balance | |
| - | | |
$ | - | |
|
|
|
|
|
|
|
| |
| 626,247 | | |
$ | 6 | | |
$ | 20,482,666 | | |
$ | (19,719,536 | ) | |
$ | (420,269 | ) | |
$ | 342,867 | |
| Common stock issued for services | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| 38,480 | | |
| - | | |
| 257,772 | | |
| - | | |
| - | | |
| 257,772 | |
| Warrants exercised for cash | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| 41,911 | | |
| 1 | | |
| 1,621 | | |
| - | | |
| - | | |
| 1,622 | |
| Options expense | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| - | | |
| - | | |
| 29,738 | | |
| - | | |
| - | | |
| 29,738 | |
| Reverse split rounding adjustment | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| 21,929 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Disposition of assets | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| - | | |
| - | | |
| - | | |
| 4,317,170 | | |
| 420,269 | | |
| 4,737,439 | |
| Shares issued pursuant to merger agreement | |
| 15,759 | | |
| - | |
|
|
- |
|
|
|
- |
| |
| 136,441 | | |
| 1 | | |
| 12,500,088 | | |
| - | | |
| - | | |
| 12,500,089 | |
| Shares issued pursuant to securities purchase agreement | |
| - | | |
| - | |
|
|
290 |
|
|
|
- |
| |
| 40,000 | | |
| 1 | | |
| 516,399 | | |
| - | | |
| - | | |
| 516,400 | |
| Net loss | |
| - | | |
| - | |
|
|
- |
|
|
|
- |
| |
| - | | |
| - | | |
| - | | |
| (17,843,574 | ) | |
| - | | |
| (17,843,574 | ) |
| Balance at December 31, 2023 | |
| 15,759 | | |
$ | - | |
|
|
290 |
|
|
$ |
- |
| |
| 905,008 | | |
$ | 9 | | |
$ | 33,788,284 | | |
$ | (33,245,940 | ) | |
$ | - | | |
$ | 542,353 | |
| Balance | |
| 15,759 | | |
$ | - | |
|
|
290 |
|
|
|
- |
| |
| 905,008 | | |
$ | 9 | | |
$ | 33,788,284 | | |
$ | (33,245,940 | ) | |
$ | - | | |
$ | 542,353 | |
The
accompanying notes are an integral part of the consolidated financial statements.
TRxADE
HEALTH, INC.
Consolidated
Statements of Cash Flows
Years
ended December 31, 2023 and 2022
| | |
2023 | | |
2022 | |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (13,720,546 | ) | |
$ | (2,403,442 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Depreciation expense | |
| 19,375 | | |
| 13,486 | |
| Options expense | |
| 29,738 | | |
| 79,163 | |
| Common stock issued for services | |
| 257,772 | | |
| 254,106 | |
| Bad debt expense | |
| - | | |
| (246,683 | ) |
| Loss on write-off of intangible asset | |
| - | | |
| 792,500 | |
| Loss on inventory investment | |
| - | | |
| 875,250 | |
| Goodwill impairment | |
| 5,129,115 | | |
| - | |
| Loss on inventory investments | |
| - | | |
| - | |
| Gain on sale of asset | |
| - | | |
| (2,200 | ) |
| Amortization of right-of-use assets | |
| 215,665 | | |
| 181,218 | |
| Amortization of intangible assets | |
| 814,790 | | |
| - | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable, net | |
| (293,784 | ) | |
| 369,932 | |
| Prepaid assets and deposits | |
| 38,367 | | |
| 335,066 | |
| Inventory | |
| 4,232,947 | | |
| (51,737 | ) |
| Other receivables | |
| (254,924 | ) | |
| (875,250 | ) |
| Right-of-use assets | |
| 306,527 | | |
| - | |
| Lease liability | |
| (534,997 | ) | |
| (164,618 | ) |
| Accounts payable | |
| 1,607,625 | | |
| 199,833 | |
| Accrued liabilities | |
| 58,692 | | |
| (211,694 | ) |
| Purchase price payable | |
| 350,000 | | |
| - | |
| Current liabilities | |
| 2,794 | | |
| 67,517 | |
| Warrant liability | |
| 148,420 | | |
| 588,533 | |
| Net cash used in operating activities
from continuing operations | |
| (1,592,424 | ) | |
| (199,020 | ) |
| Net cash used in operating activities
from discontinued operations | |
| (481,177 | ) | |
| (1,365,648 | ) |
| | |
| | | |
| | |
| Cash flows from investing activities: | |
| | | |
| | |
| Funds acquired through acquisitions | |
| (344,454 | ) | |
| - | |
| Proceeds from sale of fixed assets | |
| - | | |
| 749 | |
| Investment in capitalized software | |
| - | | |
| - | |
| Net cash (used in) investing activities
from continuing operations | |
| (344,454 | ) | |
| 749 | |
| Net cash provided by investing activities
from discontinued operations | |
| 68,737 | | |
| (428,594 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from the issuance of debt | |
| 400,000 | | |
| - | |
| Repayment of debt | |
| (150,000 | ) | |
| - | |
| Repayment of contingent liability | |
| (1,043,107 | ) | |
| (716,964 | ) |
| Proceeds from sale of future revenue | |
| 2,181,417 | | |
| 825,000 | |
| Proceeds from exercise of stock options | |
| - | | |
| - | |
| Proceeds from exercise of warrants | |
| 1,622 | | |
| 875 | |
| Proceeds from securities purchase agreement | |
| 516,400 | | |
| - | |
| Proceeds from issuance of common stock, net of issuance
costs | |
| - | | |
| 130,918 | |
| Net cash provided by (used in) financing
activities from continuing operations | |
| 1,906,332 | | |
| 239,829 | |
| Net cash (used in) financing activities
from discontinued operations | |
| (500,000 | ) | |
| (275,000 | ) |
| | |
| | | |
| | |
| Net decrease in cash | |
| (942,986 | ) | |
| (2,027,684 | ) |
| Cash at beginning of the year | |
| 1,094,894 | | |
| 3,122,578 | |
| Cash at end of the period | |
$ | 151,908 | | |
$ | 1,094,894 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information | |
| | | |
| | |
| Cash paid for interest, net | |
$ | 733,694 | | |
$ | 336,206 | |
| Cash paid for income taxes | |
$ | - | | |
$ | - | |
| Non-Cash Transactions | |
| | | |
| | |
| Insurance premium financed | |
$ | 306,152 | | |
$ | 220,354 | |
| Note issued as SOSRx contribution | |
| - | | |
$ | 500,000 | |
| Not cancelled from SORx agreement termination | |
$ | 500,000 | | |
| - | |
| Intangible asset contribution from non-controlling interest | |
$ | - | | |
$ | 792,500 | |
| Disposition of assets, related party | |
$ | 492,030 | | |
| - | |
| Issuance of note receivable | |
$ | 1,300,000 | | |
$ | - | |
The
accompanying notes are an integral part of the consolidated financial statements.
TRxADE
HEALTH, INC.
Notes
to Consolidated Financial Statements
For
the years ended December 31, 2023 and 2022
NOTE
1 – ORGANIZATION AND BASIS OF PRESENTATION
Overview
TRxADE
HEALTH, INC. (“we”, “our”, “Trxade”, and the “Company”) owns
as of December 31, 2023, 100% of Trxade, Inc. and Integra Pharma Solutions, LLC, Bonum Health, LLC, Superlatus, Inc. and its wholly-owned
subsidiaries, Sapientia Technologies, LLC (“Sapientia”), Superlatus Food Service Holding Company, Superlatus PD Holding Company,
and The Urgent Company, Inc. On July 31, 2023, the Company completed a merger transaction that resulted in with Superlatus, Inc. becoming
a wholly owned subsidiary of the Company (see “Merger”, below). On September 27, 2023, the Company acquired The Urgent Company,
Inc. and its related subsidiaries (see Note 3).
During
the year ended December 31, 2023, Trxade, Inc., operated a web-based market platform that enables commerce among healthcare buyers and
sellers of pharmaceuticals, accessories and services.
Integra
Pharma Solutions, LLC (“IPS”, d.b.a. Trxade Prime), is a licensed pharmaceutical wholesaler and sells brand, generic and
non-drug products to customers. IPS customers include all healthcare markets including government organizations, hospitals, clinics and
independent pharmacies nationwide.
Community
Specialty Pharmacy, LLC, (“CSP”) is an accredited independent retail pharmacy with a focus on a community-based model offering
home delivery services to patients.
Alliance
Pharma Solutions, LLC (“APS”, d.b.a. DelivMeds) is currently being rebranded and the consumer-based app is still being developed.
To date, the Company has not generated any revenue from this product.
On
January 20, 2023, the Company entered into Membership Interest Purchase Agreements to sell 100% of the outstanding membership interests
of the Company’s subsidiaries, CSP and APS. The Company will receive consideration in the amount of $125,000 for APS and $100,000
for CSP. The Company also agreed to enter into a Master Service Agreement to operate the businesses prior to closing. Additional amounts
owed to the Company as a result of this Master Service Agreement totaled $1,075,000 as of the closing date of August 22, 2023 (see Note
3 and Note 7).
Bonum
Health, LLC (“Bonum Health”), was formed to hold certain telehealth assets acquired in October 2019. The “Bonum Health
Hub” was launched in February 2020; however, the Company does not anticipate installations moving forward. The Bonum Health mobile
application is available on a subscription basis, primarily as a stand-alone telehealth software application that can be licensed on
a business-to-business (B2B) model to clients as an employment health benefit for the clients’ employees.
SOSRx,
LLC (“SOSRx”) was formed on February 15, 2022. The Company entered into a relationship with Exchange Health, LLC (“Exchange
Health”), a technology company providing an online platform for manufacturers and suppliers to sell and purchase pharmaceuticals.
SOSRx, a Delaware limited liability company, was formed, which was owned 51% by the Company and 49% by Exchange Health. SOSRx did not
generate material revenue and in February of 2023, the Company voluntarily withdrew from the joint venture agreement. As part of the
voluntary withdrawal the Company has recorded a loss of $352,244 from disposal of assets, which is included in net loss on discontinued
operations in the audited consolidated statement of operations in the amount of for the year ended December 31, 2023.
Merger
On
July 14, 2023, the Company entered into an Amended and Restated Agreement and Plan of Merger (the “Merger Agreement”) with
Superlatus, Inc., a U.S.-based holding company of food products and distribution capabilities (“Superlatus”) and Foods Merger
Sub, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub”).
Superlatus
is a diversified food technology company with distribution capabilities and systems to optimize food security and population health via
innovative Consumer Packaged Goods (“CPG”) products, agritech, foodtech, plant-based proteins and alt-protein and includes
wholly-owned subsidiary, Sapientia, Inc. (“Sapientia”), a food tech business.
On
July 31, 2023 (the “Closing Date”), the Company completed its acquisition of Superlatus in accordance with the terms and
conditions of the Merger Agreement (the “Merger”), pursuant to which the Company acquired Superlatus by way of a merger of
the Merger Sub with and into Superlatus, with Superlatus being a wholly owned subsidiary of the Company and the surviving entity in the
Merger.
Under
the terms of the Merger Agreement, at the closing of the Merger (the “Closing”), shareholders of Superlatus received in aggregate
136,441 shares of common stock of the Company, representing 19.99% of the then total issued and outstanding common stock of the Company
after the consummation of the Merger and 306,855 shares of Company’s Series B Preferred Stock, par value $0.00001 per share (the
“Series B Preferred Stock”), with a conversion ratio of 100 shares of Series B Preferred Stock to one share of common stock.
At Closing, the value of the common stock was $7.30 per share, resulting in a total value of $225,000,169. Upon consummation of the Merger,
the Company continued to trade under the current ticker symbol “MEDS.”
As
a condition and inducement to Superlatus’ willingness to enter into the Merger Agreement, on June 28, 2023, Suren Ajjarapu and
Prashant Patel (the “Principal Stockholders”) entered into an agreement with TRxADE (the “Stock Swap Agreement”),
pursuant to which, TRxADE was to transfer all of the shares or membership interest of the operating subsidiaries currently owned by TRxADE
to Principal Stockholders, in exchange for Suran Ajjarapu to surrender 85,000 share of common stock of TRxADE and Prashant Patel to surrender
81,666 shares of the common stock of TRxADE (the “Stock Swap Transaction”). The closing of the Stock Swap Transaction was
to take place simultaneously with the approval of TRxADE stockholders of the conversion of the Series B preferred stock into common stock.
As of the date of this filing, TRxADE stockholders have not approved the conversion.
In
connection with the Merger, effective one (1) business day immediately prior to the Closing Date (the “MEDS Rights Record Date”),
the Company issued to the shareholders of the Company as of the MEDS Rights Record Date, including the independent directors who are
entitled to certain amount of common stock of the Company in connection with their 2023 annual compensation and regardless of whether
the common stock has been issued or vest before the MEDS Rights Records Date (collectively, the “MEDS Rights Shareholders”)
a non-transferrable right to receive one share of common stock of the Company at no cost (the “MEDS Rights”), with seven
(7) MEDS Rights issued per share of common stock of the Company held as of the MEDS Rights Record Date, conditioned upon their execution
of a Registration Rights Agreement. Such issuances will be made in reliance on the exemption from registration pursuant to Section 3(a)(9)
or Section 4(a)(2) of the Securities Act, Regulation D under the Securities Act promulgated thereunder, and corresponding provisions
of state securities or “blue sky” laws. The MEDS Rights are not actionable or transferable until registration; provided they
become transferable one year after the date of the Merger if no registration has occurred. As of the date of this filing, no MEDS Rights
shares have been issued.
Not
all of the closing conditions of the Merger Agreement were met. As a result, the Company entered into Amendment No. 1 to the Amended
and Restated Agreement and Plan of Merger (the “Amendment”) on January 8, 2024. Under the terms of the Amendment, the merger
consideration to the shareholders of Superlatus was adjusted to the aggregate of 136,441 shares of common stock of the Company, representing
19.99% of the total issued and outstanding common stock of the Company after the consummation of the Merger and 15,759 shares of Company’s
Series B Preferred Stock, par value $0.00001 per share (the “Series B Preferred Stock”), with a conversion ratio of 100 shares
of Series B Preferred Stock to one share of common stock. At Closing, the value of the common stock was $7.30 per share, resulting in
a total value of $12,500,089. Additionally, the shareholders of Superlatus agreed to surrender back to the Company 291,096 shares of
the Company’s Series B Preferred Stock. In March 2024 the Company divested of its interest in Superlatus and, among other things,
the Stock Swap Transaction in not expected to occur.
Basis
of Presentation and Principles of Consolidation
The
Company’s consolidated financial statements include the accounts of TRxADE HEALTH, INC., Trxade, Inc., Integra Pharma Solutions,
Inc., Bonum Health, LLC, Superlatus, Inc., Sapientia Technologies, LLC and The Urgent Company, Inc. The accompanying consolidated financial
statements of TRxADE HEALTH, Inc. have been prepared in accordance with accounting principles generally accepted in the United States
of America (“U.S. GAAP”) and the rules of the SEC. All significant intercompany accounts and transactions have been eliminated.
Use
of Estimates
The
preparation of condensed consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions
that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue
and expenses in the reporting period. The Company bases its estimates and assumptions on current facts, historical experience and various
other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about
the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources.
The actual results experienced by the Company may differ materially and adversely from its estimates. To the extent there are material
differences between estimates and the actual results, future results of operations will be affected. Significant estimates for the years
ended December 31, 2023 and 2022 include the valuation of intangible assets, including goodwill.
Fair
value of financial instruments
The
carrying amounts for cash, accounts receivable, accounts payable, accrued liabilities, and other current liabilities approximate their
fair value because of their short-term maturity.
Stock
Split
Effective
June 21, 2023, the Company executed a 1:15 reverse stock split for stockholders of record on that date. This was executed to comply with
the Nasdaq Listing Rule 5550(a)(2) to have the price of the stock above $1.00.
Recently
Issued Accounting Pronouncements
In
June 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-13,
“Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments” (“ASU 2016-13”).
ASU 2016-13 requires companies to measure credit losses utilizing a methodology that reflects expected credit losses and requires a consideration
of a broader range of reasonable and supportable information to inform credit loss estimates. ASU 2016-13 is effective for fiscal years
beginning after December 15, 2022, including interim periods within those fiscal years. The Company adopted ASU 2016-13 effective January
1, 2023. The Company determined that the update applied to trade receivables, but that there was no material impact to the consolidated
financial statements from the adoption of ASU 2016-13.
In
August 2020, the FASB issued ASU 2020-06, “Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and
Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40)”. This ASU reduces the number of accounting models for convertible
debt instruments and convertible preferred stock and amends the guidance for the derivatives scope exception for contracts in an entity’s
own equity to reduce form-over-substance-based accounting conclusions. In addition, this ASU improves and amends the related earnings
per share guidance. This standard is effective for us on January 1, 2022, including interim periods within those fiscal years. Adoption
is either a modified retrospective method or a fully retrospective method of transition. The adoption of ASU 2020-06 did not have a material
impact on the consolidated financial statements.
Accounts
Receivable, net
On
January 1, 2023, the Company adopted ASU 2016-13 “Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses
on Financial Instruments” and its related amendments using the prospective method. The new standard requires the use of a current
expected credit loss impairment model to develop and recognize credit losses for financial instruments at amortized cost when the asset
is first originated or acquired, and each subsequent reporting period.
The
Company’s receivables are from customers and are typically collected within 90 days. The Company determines the allowance based
on known troubled accounts, historical experience, and other currently available evidence.
The
Company had an account receivable with a single customer, GSG PPE, LLC (“GSG”), for the amount of $630,000, which was past
due. The Company had obtained a Note Receivable which was due on September 30, 2021 and remained unpaid. The Company did not believe
the amount to be collectible without legal actions, and therefore, recorded bad debt expense reflected on the consolidated statement
of operations during the year ended December 31, 2021. The note was not paid pursuant to its terms and the Company had filed a suit to
collect on the note and the personal guaranty securing the note. The Company settled the lawsuit in June of 2022. During the years ended
December 31, 2023, and 2022, there was a bad debt recovery from the GSG lawsuit of $32,074 and $98,841 respectively.
Other
Receivables, net
The
Company’s other receivables balance is from one vendor. On May 20, 2022, effective as of May 18, 2022, Community Specialty Pharmacy,
LLC (“CSP”) entered into an agreement to acquire COVID-19 testing kits from a third-party vendor for an aggregate of $1,200,000,
of which $875,000 was paid on May 23, 2022. The Company received the COVID-19 testing kits in July 2022. On August 18, 2022, the Company
was informed by the vendor that the vendor had received a letter from the U.S. Food and Drug Administration (“FDA”) that
the COVID-19 test kits were misbranded under Section 502(o) of the Federal Food, Drug, and Cosmetic Act (“FDC Act”) (21 USC
352(o)) and adulterated under Section 501(f) of the FDC Act (21 USC 351(f)). Furthermore, the vendor informed the Company that the letter
from the FDA also stated that because of the FDA’s prohibition on the distribution of adulterated and/or misbranded devices applies
to all parties along the distribution chain, the FDA was advising the vendor against furthering the distribution of the COVID-19 test
kits in interstate commerce. The company wrote the amount off as a loss of inventory as of December 31, 2022. As of December 31, 2023,
and December 31, 2022, the balance of this receivable was $0.
On
August 22, 2023, the Company completed the sale of CSP and APS (see Note 3). The net balance due to the Company from these entities,
in excess of the Note Receivable (see Note 6), was $370,608 as of December 31, 2023.
Acquisitions
The
Company accounts for acquisitions and investments in businesses as business combinations if the target meets the definition of a business
and (a) the target is a variable interest entity (“VIE”) and the Company is the target’s primary beneficiary, and therefore
the Company must consolidate its financial statements, or (b) the Company acquires more than 50% of the voting interest of the target
and it was not previously consolidated. The Company records business combinations using the acquisition method of accounting, which requires
all the assets acquired and liabilities assumed to be recorded at fair value as of the acquisition date. The excess of the purchase price
over the estimated fair values of the net tangible and intangible assets acquired is recorded as goodwill.
The
application of the acquisition method of accounting for business combinations requires management to make significant estimates and assumptions
in the determination of the fair value of assets acquired and liabilities assumed in order to properly allocate purchase price consideration
between assets that are depreciated and amortized from goodwill. The fair value assigned to tangible and intangible assets acquired and
liabilities assumed are based on management’s estimates and assumptions, as well as other information compiled by management, including
valuations that utilize customary valuation procedures and techniques. Significant assumptions and estimates include, but are not limited
to, the cash flows that an asset is expected to generate in the future, the appropriate weighted-average cost of capital, and the cost
savings expected to be derived from acquiring an asset, if applicable.
If
the actual results differ from the estimates and judgments used in these estimates, the amounts recorded in the Company’s financial
statements may be exposed to potential impairment of the intangible assets and goodwill.
If
the Company’s investment involves the acquisition of an asset or group of assets that does not meet the definition of a business,
the transaction is accounted for as an asset acquisition. An asset acquisition is recorded at cost, which includes capitalizing transaction
costs, and does not result in the recognition of goodwill.
Intangible
Assets and Goodwill
The
Company tests indefinite-lived intangible assets for impairment on an annual basis or whenever events or changes occur that would more-likely-than
not reduce the fair value of the indefinite-lived intangible asset below its carrying value between annual impairment tests. Any indefinite-lived
intangible asset assessment is performed at the Company level.
The
Company recognized a goodwill impairment loss of $5,129,115 for the year ended December 31, 2023. The goodwill resulted from the acquisition
of Superlatus and was subsequently determined to be impaired based on the facts and circumstances surrounding the sale of Superlatus
on March 5, 2024. See Note 20.
Income
(loss) Per Common Share
Basic
net income per common share is computed by dividing net income available to common stockholders by the weighted average number of common
shares outstanding. Diluted net income per common share is computed similar to basic net income per common share except that the denominator
is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been
issued and if the additional common shares were dilutive. The dilutive effect of the Company’s options and warrants is computed
using the treasury stock method. As of December 31, 2023, we had 218,729 outstanding warrants to purchase shares of common stock and
26,229 options to purchase shares of common stock. As part of the termination of the White Lion deal, White Lion was issued 50,000 shares
of stock per the agreement on March 1, 2023. Armistice Capital executed its pre-funded warrants on January 4, 2023, and purchased 601,740
shares (40,116 shares after the effect of the 1:15 reverse stock split on June 21, 2023, see Note 13) of stock with a purchase price
of $6.02.
The
following table sets forth the computation of basic and diluted loss per share:
| | |
| | | |
| | |
| | |
For the Years Ended
December 31, | |
| | |
2023 | | |
2022 | |
| Numerator: | |
| | | |
| | |
| Net loss from continuing operations | |
$ | (13,720,546 | ) | |
$ | (2,403,442 | ) |
| Net loss attributable to noncontrolling interest | |
| - | | |
| (437,769 | ) |
| Net loss from continuing operations available to common stockholders | |
| (13,720,546 | ) | |
| (1,965,673 | ) |
| Net loss from discontinued operations | |
| (4,123,028 | ) | |
$ | (1,506,426 | ) |
| Numerator for basic and diluted EPS - income available to common stockholders | |
| (17,843,574 | ) | |
$ | (3,472,099 | ) |
| Denominator: | |
| | | |
| | |
| Denominator for EPS – weighted average shares | |
| | | |
| | |
| Basic | |
| 764,058 | | |
| 564,862 | |
| Diluted | |
| 2,381,443 | | |
| 566,609 | |
| Net loss per common share attributable to common stockholders | |
| | | |
| | |
| Basic | |
$ | (23.35 | ) | |
$ | (6.15 | ) |
| Diluted | |
$ | (7.49 | ) | |
$ | (6.13 | ) |
| Net loss per common share from continuing operations | |
| | | |
| | |
| Basic | |
$ | (17.96 | ) | |
$ | (3.48 | ) |
| Diluted | |
$ | (5.76 | ) | |
$ | (3.47 | ) |
| Net loss per common share from discontinued operations | |
| | | |
| | |
| Basic | |
$ | (5.40 | ) | |
$ | (2.67 | ) |
| Diluted | |
$ | (1.73 | ) | |
$ | (2.66 | ) |
Income
taxes
The
Company’s provision for income taxes was $0 for the year ended December 31, 2023, and $0 for the year ended December 31, 2022,
respectively. The income tax provisions for the twelve-month periods are based upon estimates of annual income (loss), annual permanent
differences and statutory tax rates in the various jurisdictions in which the Company operates. For all periods presented, the Company
utilized net operating loss carryforwards to offset the impact of any taxable income. The Company’s tax rate differs from the applicable
statutory rates due primarily to the establishment of a valuation allowance, utilization of deferred and the effect of permanent differences
and adjustments.
NOTE
2 – GOING CONCERN
The
accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates
realization of assets and the satisfaction of liabilities in the normal course of business within one year after the date the consolidated
financial statements are issued. In accordance with Financial Accounting Standards Board, or the FASB, Accounting Standards Update No.
2014-15, Presentation of Financial Statements - Going Concern (Subtopic 205-40), our management evaluates whether there are conditions
or events, considered in aggregate, that raise substantial doubt about our ability to continue as a going concern within one year after
the date that the financial statements are issued.
As
of December 31, 2023, the Company had an accumulated deficit of $33,245,940. The Company has limited financial resources. As of December
31, 2023, the Company had a working capital deficit of $8,803,293 and a cash balance of $151,908. The Company will need to raise additional
capital or secure debt funding to support on-going operations. The sources of this capital are expected to be the sale of equity and
debt, which may not be available on favorable terms, if at all, and may, if sold, cause significant dilution to existing stockholders.
If the Company is unable to access additional capital moving forward, it may hurt the Company’s ability to grow and to generate
future revenues, financial position, and liquidity. These factors raise substantial doubt about the ability of the Company to continue
as a going concern. Unless Management is able to obtain additional financing, it is unlikely that the Company will be able to meet its
funding requirements during the next 12 months. The financial statements do not include any adjustments that might result from the outcome
of this uncertainty.
NOTE
3 – ACQUISITIONS AND DISPOSITIONS
Acquisitions
Superlatus,
Inc.
On
July 31, 2023, the Company entered into the Merger Agreement (see Note 1) with Superlatus (“Seller”) whereby the Company
acquired 100% of the stock of the Seller (the “Acquisition”). Superlatus includes a wholly-owned subsidiary, Sapientia. Consideration
for the Acquisition consisted of (i) 136,441 shares of the Company’s common stock at a fair value of $7.30 per share, representing
19.99% of the total issued and outstanding share of the Company’s common stock at Closing, and (ii) 306,855 shares of the Company’s
Series B Preferred Stock, a new class of the Company’s non-voting convertible preferred stock with a conversion ratio of 100 to
one. The total fair value of the common stock and Series B Preferred Stock on the Closing Date was $225,000,169 (“Purchase Price”).
On January 8, 2024, the Company entered into Amendment No. 1 to the Agreement and Plan of Merger (the “Amendment”). Under
the terms of the Amendment, the merger consideration to the shareholders of Superlatus was adjusted to an aggregate of 136,441 shares
of common stock of the Company, representing 19.99% of the total issued and outstanding common stock of the Company after the consummation
of the Merger and 15,759 shares of Company’s Series B Preferred Stock, par value $0.00001 per share, with a conversion ratio of
100 shares of Series B Preferred Stock to one share of common stock. The total fair value of the common stock and Series B Preferred
Stock on the Closing Date was adjusted to $12,500,089 (“Amended Purchase Price”). Additionally, the shareholders of Superlatus
agreed to surrender back to the Company 291,096 shares of the Company’s Series B Preferred Stock previously received before the
Amendment.
The
acquisition of Superlatus was accounted for as a business combination using the acquisition method pursuant to FASB ASC Topic 805. As
the acquirer for accounting purposes, the Company had estimated the Purchase Price, assets acquired and liabilities assumed as of the
acquisition date, with the excess of the Purchase Price over the fair value of net assets acquired recognized as goodwill. An independent
valuation expert assisted the Company in determining these fair values.
The
Amended Purchase Price allocation as of the acquisition date is presented as follows:
| | |
July 31, 2023 | |
| Purchase consideration: | |
| | |
| Common Stock, at fair value | |
$ | 996,019 | |
| Series B Preferred Stock, at fair value | |
| 11,504,070 | |
| Total purchase consideration | |
$ | 12,500,089 | |
| | |
| | |
| Purchase price allocation: | |
| | |
| Cash | |
$ | 5,546 | |
| Prepaid expenses | |
| 3,705 | |
| Inventory | |
| 122,792 | |
| Intangible assets, net | |
| 9,777,479 | |
| Goodwill | |
| 5,129,115 | |
| Assets acquired | |
| 15,038,637 | |
| Accounts payable and other current liabilities | |
| (283,548 | ) |
| Purchase price payable | |
| (350,000 | ) |
| Notes payable | |
| (1,905,000 | ) |
| Liabilities assumed | |
| (2,538,548 | ) |
| Net assets acquired | |
$ | 12,500,089 | |
The
Urgent Company, Inc.
On
September 27, 2023, the Company entered into an Asset Purchase Agreement (“APA”) with The Urgent Company, Inc. (“TUC”)
and its wholly owned subsidiaries, pursuant to which, the Company was assigned certain inventory and property and equipment and assumed
certain operating leases for consideration of $4,400,000 in promissory notes (“Purchase Price”, see Note 11). This acquisition
is expected to enhance the Company’s production of sustainable food products and enable the expansion of market share.
The
transaction was accounted for as an asset acquisition pursuant to FASB ASC Topic 805. As the acquirer for accounting purposes, the Company
allocated the cost of the asset acquisition to the assets acquired and liabilities assumed as of the acquisition date based on their
respective relative fair value as of the date of the transaction.
The
following summarizes the relative fair values of the assets acquired as of the acquisition date based on the allocation of the cost of
the asset acquisition:
| | |
September 27, 2023 | |
| Purchase consideration: | |
| | |
| Promissory note | |
$ | 4,400,000 | |
| Total purchase consideration | |
$ | 4,400,000 | |
| | |
| | |
| Allocation of cost of assets acquired: | |
| | |
| Inventory | |
$ | 4,168,830 | |
| Property and equipment | |
| 231,170 | |
| Assets acquired | |
| 4,400,000 | |
| Net assets acquired | |
$ | 4,400,000 | |
Dispositions
and Divestitures
SOSRx,
LLC
Effective
on, February 1, 2023, the Company, Exchange Health and SOSRx, entered into a Voluntary Withdrawal and Release Agreement, which was replaced
in its entirety, corrected, and became effective on February 4, 2023 (as replaced and corrected, the “Release Agreement”).
As
part of the Release Agreement, a note payable to Exchange Health was forgiven in the amount of $500,000 and $15,000 in accounts payable
was waived. Effective February 4, 2023, the operations of SOSRx were discontinued and operations were shut down. As a result of this,
the assets and liabilities of SOSRx have been reflected as assets and liabilities of discontinued operations in the Company’s consolidated
balance sheets. As of December 31, 2023 and December 31, 2022 as follows:
| | |
December 31,
2023 | | |
December 31,
2022 | |
| | |
| | |
| |
| Cash | |
$ | - | | |
$ | 22,474 | |
| Accounts receivable | |
| - | | |
| 363 | |
| Total assets of discontinued operations | |
$ | - | | |
$ | 22,837 | |
| | |
| | | |
| | |
| Accounts payable | |
$ | - | | |
$ | 46,500 | |
| Total liabilities of discontinued operations | |
$ | - | | |
$ | 46,500 | |
The
terms of the Release Agreement qualify the transaction as a discontinued operation in accordance with U.S. GAAP. As a result, operating
results and cash flows related to the SOSRx operations have been reflected as discontinued operations in the Company’s consolidated
statements of operations, consolidated statements of cash flows and consolidated statements of shareholders’ equity.
Alliance
Pharma Solutions, LLC and Community Specialty Pharmacy, LLC
On
August 22, 2023, the Company and Wood Sage, LCC (“Wood Sage”) entered into a Membership Interest Purchase Agreement, pursuant
to which the Company sold 100% of the membership interest in Alliance Pharma Solutions, LLC (“ASP MIPA”) for consideration
of a $125,000 promissory note (“ASP Sale Price”) and a Membership Interest Purchase Agreement, pursuant to which the Company
sold 100% of the membership interest in Community Specialty Pharmacy, LLC (“CSP MIPA”) in exchange for a $100,000 promissory
note (“CSP Sale Price”).
The
divestiture of APS and CSP represented an intended strategic shift in the Company’s operations and will allow the Company to become
focused on food technology As a result, the results of APS and CSP were classified as discontinued operations in our condensed statements
of operations and excluded from both continuing operations and segment results for the years ended December 31, 2023 and 2022.
As
part of recognizing the business as held for sale in accordance with U.S. GAAP, the Company was required to measure APS and CSP at the
lower of its carrying amount or fair value less cost to sell. As a result of this analysis, during the year ended December 31, 2023,
the Company recognized a non-cash, pre-tax loss on disposal of $3,300,225.42. The loss is included in “Net loss from discontinued
operations” in the consolidated statements of operations. The loss was determined by comparing the fair value of the consideration
received for the sale of a 100% interest in APS and CSP with the net assets of APS and CSP, respectively, immediately prior to the transaction.
As
a result of the transactions, the following assets and liabilities of APS and CSP were transferred to Wood Sage as of August 22, 2023:
| | |
Alliance
Pharma
Solutions, LLC | | |
Community
Specialty
Pharmacy, LLC | |
| Cash | |
$ | 1,050 | | |
$ | 61,988 | |
| Accounts receivable, net | |
| - | | |
| 101,901 | |
| Inventory | |
| - | | |
| 123,230 | |
| Prepaid assets | |
| - | | |
| 525 | |
| Intangible assets and capitalized software, net | |
| 739,337 | | |
| - | |
| Accounts payable | |
| (23,982 | ) | |
| (231,876 | ) |
| Accrued liabilities | |
| - | | |
| (10,182 | ) |
| Net assets sold | |
$ | 716,405 | | |
$ | 45,586 | |
Discontinued
Operations
The
results of operations from discontinued operations for the years ended December 31, 2023 and 2022, have been reflected as discontinued
operations in the consolidated statements of operations and consist of the following:
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
SOSRx | | |
APS | | |
CPS | | |
Total | |
| | |
Years
ended December 31, | | |
Years
ended December 31, | | |
Years
ended December 31, | | |
Years
ended December 31, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Revenue | |
$ | - | | |
$ | 22,623 | | |
$ | - | | |
$ | - | | |
$ | 851,755 | | |
$ | 1,175,474 | | |
$ | 851,755 | | |
$ | 1,198,097 | |
| Cost of sales | |
| - | | |
| - | | |
| - | | |
| - | | |
| 705,206 | | |
| 1,266,152 | | |
| 705,206 | | |
| 1,266,152 | |
| Gross Profit | |
| - | | |
| 22,623 | | |
| - | | |
| - | | |
| 146,549 | | |
| (90,678 | ) | |
| 146,549 | | |
| (68,055 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Operating Expenses | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Impairment of intangible asset | |
| | | |
| 792,500 | | |
| | | |
| - | | |
| | | |
| - | | |
| - | | |
| 792,500 | |
| Wage and salary expense | |
| - | | |
| 55,439 | | |
| - | | |
| - | | |
| 456,297 | | |
| 304,947 | | |
| 456,297 | | |
| 360,386 | |
| Professional fees | |
| - | | |
| - | | |
| 3,125 | | |
| 46,787 | | |
| 20,246 | | |
| 6,120 | | |
| 23,371 | | |
| 52,907 | |
| Accounting and legal expense | |
| - | | |
| - | | |
| 7,773 | | |
| 104 | | |
| 63,000 | | |
| 500 | | |
| 70,773 | | |
| 604 | |
| Technology expense | |
| - | | |
| 63,160 | | |
| 20,611 | | |
| 86,688 | | |
| 9,464 | | |
| 17,823 | | |
| 30,075 | | |
| 167,671 | |
| General and Administrative | |
| - | | |
| 4,931 | | |
| 3,762 | | |
| 11,562 | | |
| 32,830 | | |
| 49,710 | | |
| 36,592 | | |
| 66,203 | |
| Total operating expense | |
| - | | |
| 916,030 | | |
| 35,271 | | |
| 145,141 | | |
| 581,837 | | |
| 379,100 | | |
| 617,108 | | |
| 1,440,271 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Operating income (loss) from discontinued operations | |
| - | | |
| (893,407 | ) | |
| (35,271 | ) | |
| (145,141 | ) | |
| (435,288 | ) | |
| (469,778 | ) | |
| (470,559 | ) | |
| (1,508,326 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Other income (expense) | |
| - | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Gain (loss) on asset sale | |
| - | | |
| - | | |
| - | | |
| 1,900 | | |
| - | | |
| - | | |
| - | | |
| 1,900 | |
| Total other income (expense) | |
| - | | |
| - | | |
| - | | |
| 1,900 | | |
| - | | |
| - | | |
| - | | |
| 1,900 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) from discontinued operations | |
$ | - | | |
$ | (893,407 | ) | |
$ | (35,271 | ) | |
$ | (143,241 | ) | |
$ | (435,288 | ) | |
$ | (469,778 | ) | |
$ | (470,559 | ) | |
$ | (1,506,426 | ) |
NOTE
4 - RELATED PARTY TRANSACTIONS
On
April 1, 2023 and July 1, 2023 the Company entered into a relationship with Scietech, LLC (“Scietech”) in an independent
contractor agreement to consult on increasing sales on the IPS and Trxade Inc. platforms. The agreement was for an annual fee of $400,000
to be split equally between IPS and Trxade Inc. A 31% investor in Scietech is the spouse of the interim CFO, Prashant Patel, which qualifies
as a related party. The company was chosen because they were the most qualified to perform the desired qualifications.
On
February 15, 2022, the Company entered into a relationship with Exchange Health, a technology company providing an online platform for
manufacturers and suppliers to sell and purchase pharmaceuticals. In connection therewith, SOSRx was formed in February 2022, which is
owned 51% by the Company and 49% by Exchange Health. On February 15, 2022, the Company contributed cash to SOSRx in the amount of $325,000,
issued a promissory note to SOSRx in the amount of $500,000, which was immediately assigned to Exchange Health (the “Promissory
Note”), and agreed to make an earn out payment of up to $400,000, payable, at the Company’s discretion, in cash or common
stock of the Company, based on SOSRx achieving certain revenue targets of SOSRx (the “Earn Out Payments”); and entered into
a Distribution Services Agreement with SOSRx (the “Distribution Agreement”). Exchange Health contributed $792,000 in software
and contracts which was recorded as an intangible asset on the balance sheet of SOSRx. The intangible asset was determined to be impaired
and was written off on December 31, 2022.
At
December 31, 2023, total related party debt was $0.
On
and effective on, February 1, 2023, the Company, Exchange Health and SOSRx, entered into a Voluntary Withdrawal and Release Agreement,
which was replaced in its entirety and corrected on February 4, 2023, and effective February 4, 2023 (as replaced and corrected, the
“Release Agreement”). Pursuant to the Release Agreement, the Company voluntarily withdrew as a member of SOSRx pursuant to
the terms of the Operating Agreement of SOSRx, which provided that the Company would withdraw from SOSRx if certain revenue targets were
not met, which targets have not been met.
Also
pursuant to the Release Agreement, (a) the Company agreed to the termination of its interests in SOSRx and its withdrawal as a member
thereof for no consideration (the “Withdrawal”); (b) the Promissory Note, and all of the Company’s obligations under
such Promissory Note were terminated; and (c) the parties agreed that no Earn Out Payments will be due. The Release Agreement also (i)
provides that all accumulated losses of SOSRx through December 20, 2022, will be allocated 51% to the Company and 49% to Exchange Health;
(ii) provides for a total of approximately $15,000 in outstanding invoices owed by the Company to SOSRx to be waived; (iii) includes
certain indemnification obligations of SOSRx and Exchange Health; (iv) requires SOSRx to pay certain pre-agreed outstanding invoices
of SOSRx; (v) includes mutual releases of the Company and SOSRx and Exchange Health; and (vi) includes customary representations and
warranties of the parties.
NOTE
5 – REVENUE RECOGNITION
The
Company derives revenue from two primary sources—product revenue and service revenue.
Product
revenue consists of shipments of:
| |
● |
Resale
of pharmaceutical products to pharmacies; and
|
| |
● |
Revenues for our products are recognized and invoiced
when the product is shipped to the customer. |
Service
revenue consists primarily of:
| |
● |
Transaction
fees from the facilitation of buyer generated purchase orders to suppliers, billed monthly; |
| |
● |
Data
service fees associated with providing vendors of pharmaceutical products with data analysis of their catalogues and branding of
their products or company to the Company’s registered buyers, billed monthly or as a one-time fee; and |
| |
● |
Software-as-a-Service
(“SaaS”) fees for a platform for virtual healthcare provider visits, billed monthly.
|
Revenues
for the Company’s services that are billed monthly are recognized and invoiced when the at the beginning of the month. Revenues
for one-time services are recognized at the point in time when services are rendered.
Payment
terms for products and services are generally 0 to 60 days and the Company has no contract assets or liabilities.
The
following table presents disaggregated revenue by major product and service categories during the years ended December 31, 2023, and
2022:
| Years ended December 31, | |
2023 | | |
2022 | |
| Product revenues | |
| | | |
| | |
| Pharmaceutical product resale | |
$ | 1,363,830 | | |
$ | 4,754,067 | |
| Packaged food resale | |
| 487,021 | | |
| - | |
| Total product revenue | |
$ | 1,850,851 | | |
$ | 4,754,067 | |
| | |
| | | |
| | |
| Service revenues | |
| | | |
| | |
| Transaction fee income | |
$ | 6,200,334 | | |
$ | 5,347,401 | |
| Data service fee income | |
| 201,825 | | |
| 88,413 | |
| SaaS fee income | |
| 19,204 | | |
| 60,287 | |
| Total service revenue | |
$ | 6,421,363 | | |
| 5,496,101 | |
| | |
| | | |
| | |
| Total revenues | |
$ | 8,272,214 | | |
$ | 10,250,168 | |
NOTE
6 – INVENTORY
Inventory
value is determined using the weighted average cost method and is stated at the lower cost or net realizable value. As of December 31,
2023, and 2022, inventory was comprised of the following:
| As of December 31, | |
2023 | | |
2022 | |
| Raw materials | |
$ | - | | |
$ | 65,523 | |
| Finished goods | |
| 968 | | |
| - | |
| Inventory | |
$ | 968 | | |
$ | 65,523 | |
NOTE
7 – NOTES RECEIVABLE
On
August 22, 2023, the Company received a Promissory Note (the “Wood Sage Note”) in the amount of $1,300,000 from Wood Sage,
LLC and entered into the APS MIPA and CSP MIPA for the Company to sell APS and CSP and entered into a Master Service Agreement (“Wood
Sage MSA”). The Wood Sage Note bears no interest and is due and payable within thirty days of a change in control, as defined by
the Wood Sage Note, of the borrower. As of December 31, 2023, the outstanding balance of the Wood Sage Note was $1,300,000.
NOTE
8 – INTANGIBLE ASSETS
As
of December 31, 2023, intangible assets, net consisted of the following:
| | |
Weighted
Average | | |
| | |
| | |
| |
| | |
Useful Life | | |
| | |
Accumulated | | |
| |
| | |
(years) | | |
Cost | | |
Amortization | | |
Net | |
| Developed technology | |
| 5.0 | | |
$ | 9,777,478 | | |
$ | (814,790 | ) | |
$ | 8,962,688 | |
| | |
December 31, 2023 | | |
December 31, 2022 | |
| Amortization expense | |
$ | 814,790 | | |
$ | - | |
| Total Amortization Expense | |
$ | 814,790 | | |
$ | - | |
NOTE
9 – OTHER CURRENT LIABILITIES
As
of December 31, 2023 and December 31, 2022, other current liabilities consisted of the following:
| | |
December 31,
2023 | | |
December 31,
2022 | |
| Insurance refunds payable | |
$ | 62,390 | | |
$ | 62,390 | |
| Deferred revenue | |
| - | | |
| 5,127 | |
| Other payables | |
| 7,920 | | |
| - | |
| Other current liabilities | |
$ | 70,310 | | |
$ | 67,517 | |
NOTE
10 – CONTINGENT FUNDING LIABILITIES
On
December 13, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables
(the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $150,000
to purchase $214,500 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables
of Trxade Inc. The Company also paid $7,500 as a one-time origination fee in connection with the Receivables Agreement. The Receivables
Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events
of default. As of December 31, 2023, the balance of the payable balance is $144,231.
On
November 22, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables
(the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $275,000
to purchase $393,250 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables
of Trxade Inc. The Company also paid $13,750 as a one-time origination fee in connection with the Receivables Agreement. The Receivables
Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events
of default. As of December 31, 2023, the balance of the payable balance is $222,115.
On
October 25, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables
(the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $1,200,000
to purchase $1,728,000 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables
of Trxade Inc. The Company also paid $60,000 as a one-time origination fee in connection with the Receivables Agreement. The Receivables
Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events
of default. As of December 31, 2023, the balance of the payable balance is $880,000.
On
June 27, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables
(the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $1,250,000
to purchase $1,800,000 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables
of Trxade Inc. The Company also paid $62,500 as a one-time origination fee in connection with the Receivables Agreement. The Receivables
Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events
of default. This agreement was fully paid off in October 2023.
On
March 14, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables
(the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $875,000
to purchase $1,224,000 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables
of Trxade Inc. The Company also paid $42,500 as a one-time origination fee in connection with the Receivables Agreement. The Receivables
Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events
of default. This agreement was fully paid off in June 2023.
On
September 14, 2022, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future
receivables (the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company
$275,000 to purchase $396,000 of future receivables. Under the funding agreement, the third-party receives a priority interest in the
receivables of Trxade Inc. The Company also paid $15,000 as a one-time origination fee in connection with the Receivables Agreement.
The Receivables Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes
customary events of default. This agreement was fully paid off in January 2023.
On
June 27, 2022, the Company entered into a non-recourse funding agreement with a third-party funder for the purchase and sale of future
receivables. Pursuant to the Receivables Agreement, the third party agreed to fund the Company $550,000 to purchase $792,000 of future
receivables. Under the funding agreement, the third-party receives a priority interest in the receivables of Trxade Inc. The Company
also paid $27,500 as a one-time origination fee in connection with the Receivables Agreement. The Receivables Agreement also allows for
the third-party funder to file UCCs securing their interest in the receivables and includes customary events of default. This agreement
was fully paid off in January 2023.
The
Company’s relationship with the funding source meets the criteria in ASC 470-10-25 – Sales of Future Revenues or Various
Other Measures of Income (“ASC 470”), which relates to cash received from a funding source in exchange for a specified percentage
or amount of revenue or other measure of income of a particular product line, business segment, trademark, patent or contractual right
for a defined period. Under this guidance, the Company recognized the fair value of its contingent obligation to the funding source,
as of the acquisition date, as a current liability in its consolidated balance sheet.
Under
ASC 470, amounts recorded as debt are to be amortized under the interest method. The Company made an accounting policy election to utilize
the prospective method when there is a change in the estimated future cash flows, whereby a new effective interest rate is determined
based on the revised estimate of remaining cash flows. The new rate is the discount rate that equates the present value of the revised
estimate of remaining cash flows with the carrying amount of the debt, and it will be used to recognize interest expense for the remaining
period. Under this method, the effective interest rate is not constant, and any change in expected cash flows is recognized prospectively
as an adjustment to the effective yield. As of December 31, 2023, and December 31, 2022, the total contingent funding liability was $1,246,346
and $108,036, respectively, and the effective interest rate was approximately 31% and 31%, respectively. This rate represents the discount
rate that equates the estimated future cash flows with the fair value of the debt and is used to compute the amount of interest to be
recognized each period. Any future payments made to the funding source will decrease the contingent funding liability balance accordingly.
NOTE
11 – NOTES PAYABLE
On
November 17, 2023, the Company issued promissory notes to Moku Foods, Inc. (the “Moku Foods November 2023 Note”) in the amount
of $50,000. The promissory note accrues interest at 11.5% per annum, compounded monthly and is payable upon demand at any time after
November 30, 2023. As of December 31, 2023, the balance of the Moku Foods October 2023 Note is $50,000. The Company has accrued interest
of $945 as of December 31, 2023.
On
October 16, 2023, the Company issued promissory notes to Moku Foods, Inc. (the “Moku Foods October 2023 Note”) in the amount
of $150,000. The promissory note accrues interest at 11.5% per annum, compounded monthly and is payable upon demand at any time after
October 31, 2023. As of December 31, 2023, the balance of the Moku Foods October 2023 Note is $150,000. The Company has accrued interest
of $4,300 as of December 31, 2023.
On
September 27, 2023, the Company issued promissory notes to Perfect Day, Inc. (the “Perfect Day Note”) in the amount of $4,400,000
as consideration for the TUC APA (see Note 3). The promissory notes do not accrue interest and are payable upon demand at any time after
October 31, 2023. The entire aggregate, unpaid principal sum of the note is immediately due and payable upon the occurrence of a change
in control, as defined in the agreement.
On
September 14, 2023, the Company issued a promissory note to Danam Health, Inc. (the “Danam Note”) in the amount of $300,000.
The Company received a deposit of $200,000 on September 14, 2023, and an additional deposit of $100,000 on October 13, 2023. The Danam
Note accrues interest at 0% per annum and is due and payable no later than 30 days after a change in control of borrower, as defined
in the note agreement. As of December 31, 2023, the balance of the Danam Note is $50,000.
On
June 16, 2023, the Company issued a secured debenture to Eat Well Investment Group, Inc. (the “Eat Well June 2023 Note”)
in the amount of $1,150,000 for the purchase of Sapientia, a wholly-owned subsidiary of Superlatus. The Eat Well June 2023 Note is secured
by 100% of the membership interests in Sapientia. The Eat Well June 2023 Note began accruing interest at 12% per annum, compounded monthly,
as of October 31, 2023. The Eat Well June 2023 matured on December 31, 2023. As of December 31, 2023, the balance of the Eat Well June
2023 Note is $1,150,000. The Company has accrued interest of $23,063 as of December 31, 2023. As of the date of this filing, the parties
are working on an amendment for an extension.
On
February 8, 2023, Sapientia, a wholly-owned subsidiary of Superlatus, entered into a Loan Agreement with Eat Well Investment Group, Inc.
(the “Eat Well February 2023 Note”) in the amount of $25,000. The Eat Well February 2023 Note is unsecured, accrues interest
at a rate of 1.87% per annum, and matures February 7, 2025. As of December 31, 2023, the balance of the Eat Well February 2023 Note is
$25,000. The Company has accrued interest of $418 as of December 31, 2023.
On
September 14, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well September 2022
Note”) in the amount of $50,000. The Eat Well September 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum,
and matures September 13, 2024. As of December 31, 2023, the balance of the Eat Well September 2022 Note is $50,000. The Company has
accrued interest of $1,212 as of December 31, 2023.
On
July 26, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well July 26, 2022 Note”)
in the amount of $35,000. The Eat Well July 26, 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures July
25, 2024. As of December 31, 2023, the balance of the Eat Well July 26, 2022 Note is $35,000. The Company has accrued interest of $938
as of December 31, 2023.
On
July 12, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well July 12, 2022 Note”)
in the amount of $25,000. The Eat Well July 12, 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures July
11, 2024. As of December 31, 2023, the balance of the Eat Well July 12, 2022 Note is $25,000. The Company has accrued interest of $688
as of December 31, 2023.
On
March 15, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well March 2022 Note”)
in the amount of $100,000. The Eat Well March 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures March
14, 2024. As of December 31, 2023, the balance of the Eat Well March 2022 Note is $100,000. The Company has accrued interest of $3,361
as of December 31, 2023.
On
February 1, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well February 2022 Note”)
in the amount of $100,000. The Eat Well February 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures February
1, 2024. As of December 31, 2023, the balance of the Eat Well February 2022 Note is $100,000. The Company has accrued interest of $3,576
as of December 31, 2023.
On
January 20, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well January 2022 Note”)
in the amount of $20,000. The Eat Well January 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures January
20, 2024. As of December 31, 2023, the balance of the Eat Well January 2022 Note is $20,000. The Company has accrued interest of $728
as of December 31, 2023.
On
December 24, 2021, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well December 2021 Note”)
in the amount of $100,000. The Eat Well December 2021 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matured December
24, 2023. As of December 31, 2023, the balance of the Eat Well December 2021 Note is $100,000. The Company has accrued interest of $3,776
as of December 31, 2023. As of the date of this filing, the parties are working on an amendment for an extension.
On
November 10, 2021, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well November 2021 Note”)
in the amount of $50,000. The Eat Well November 2021 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matured November
10, 2023. As of December 31, 2023, the balance of the Eat Well November 2021 Note is $50,000. The Company has accrued interest of $2,001
as of December 31, 2023. As of the date of this filing, the parties are working on an amendment for an extension.
On
August 18, 2021, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well August 2021 Note”)
in the amount of $250,000. The Eat Well August 2021 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matured August
18, 2023. As of December 31, the balance of the Eat Well August 2021 Note is $250,000. The Company has accrued interest of $11,079 as
of December 31, 2023. As of the date of this filing, the parties are working on an amendment for an extension.
The
following table summarizes notes payable balances as of December 31, 2023:
| | |
Current | | |
Noncurrent | | |
| | |
Accrued | |
| | |
Portion | | |
Portion | | |
Total | | |
Interest | |
| | |
Current Portion | | |
Non current Portion | | |
Note Payable Total | | |
Accrued Interest | |
| Perfect Day Notes | |
$ | 4,400,000 | | |
$ | - | | |
$ | 4,400,000 | | |
$ | - | |
| Danam Note | |
| 50,000 | | |
| - | | |
| 50,000 | | |
| - | |
| Moku Foods November 2023 Note | |
| 50,000 | | |
| - | | |
| 50,000 | | |
| 945 | |
| Moku Foods October 2023 Note | |
| 150,000 | | |
| - | | |
| 150,000 | | |
| 4,300 | |
| Eat Well June 2023 Note | |
| 1,150,000 | | |
| - | | |
| 1,150,000 | | |
| 57,847 | |
| Eat Well February 2023 Note | |
| - | | |
| 25,000 | | |
| 25,000 | | |
| 418 | |
| Eat Well September 2022 Note | |
| 50,000 | | |
| - | | |
| 50,000 | | |
| 1,212 | |
| Eat Well July 26, 2022 Note | |
| 35,000 | | |
| - | | |
| 35,000 | | |
| 938 | |
| Eat Well July 12, 2022 Note | |
| 25,000 | | |
| - | | |
| 25,000 | | |
| 688 | |
| Eat Well March 2022 Note | |
| 100,000 | | |
| - | | |
| 100,000 | | |
| 3,361 | |
| Eat Well February 2022 Note | |
| 100,000 | | |
| - | | |
| 100,000 | | |
| 3,576 | |
| Eat Well January 2022 Note | |
| 20,000 | | |
| - | | |
| 20,000 | | |
| 728 | |
| Eat Well December 2021 Note | |
| 100,000 | | |
| - | | |
| 100,000 | | |
| 3,776 | |
| Eat Well November 2021 Note | |
| 50,000 | | |
| - | | |
| 50,000 | | |
| 2,001 | |
| Eat Well August 2021 Note | |
| 250,000 | | |
| - | | |
| 250,000 | | |
| 11,079 | |
| | |
$ | 6,530,000 | | |
$ | 25,000 | | |
$ | 6,555,000 | | |
$ | 90,869 | |
NOTE 12 – INCOME TAXES
The
provision for income taxes on income from operations for fiscal 2023 and 2022 consists of the following:
| | |
2023 | | |
2022 | |
| Federal: | |
| | | |
| | |
| Current | |
| - | | |
| - | |
| Deferred | |
| - | | |
| - | |
| | |
| | | |
| | |
| State | |
| | | |
| | |
| Current | |
| - | | |
| - | |
| Deferred | |
| - | | |
| - | |
| | |
| | | |
| | |
| Total | |
| - | | |
| - | |
Income
(loss) before income taxes for the years ended December 31, 2023 and 2022 consisted of the following:
| For
the year ended December 31, | |
2023 | | |
2022 | |
| | |
| | |
| |
| US | |
| (17,843,574 | ) | |
| (3,909,868 | ) |
As
a result of the full net valuation allowance position, the Company did not recognize any U.S. federal income tax expense or tax benefit
on any components of continuing or discontinued operations.
| | |
2023 | | |
2022 | |
| Deferred
Tax Assets | |
| | | |
| | |
| Net
operating Losses | |
| 5,800,214 | | |
| 4,030,755 | |
| Purchased
Intangibles | |
| 151,877 | | |
| - | |
| Lease
Liability | |
| 127,896 | | |
| - | |
| | |
| | | |
| | |
| Total
Deferred Tax Assets | |
| 6,079,987 | | |
| 4,030,755 | |
| | |
| | | |
| | |
| Deferred
Tax Liabilities | |
| | | |
| | |
| Purchased
Goodwill | |
| (15,534 | ) | |
| - | |
| Right
to Use Assets | |
| (127,896 | ) | |
| - | |
| | |
| | | |
| | |
| Total
Deferred Tax Liabilities | |
| (143,430 | ) | |
| - | |
| | |
| | | |
| | |
| Valuation
Allowance | |
| (5,936,557 | ) | |
| (4,030,755 | ) |
| | |
| | | |
| | |
| Net
Deferred Taxes | |
| - | | |
| - | |
The
Company has established a valuation allowance equal to the full amount of the deferred tax asset primarily due to uncertainty in the
utilization of the net operating loss carry forwards.
The
estimated net operating loss carry forwards of approximately $24,893,624 will be available based on the new carryover rules in section
172(a) passed with the Tax Cuts and Jobs Acts.
NOTE
13 – STOCKHOLDERS’ EQUITY
Designation of Series C Preferred Stock
Effective October 4, 2023, the Company filed a
Certificate of Designation, Preferences, Rights and Limitations of the Series C Preferred Stock with the Secretary of the State of Delaware
which designated 1,000 shares of the Company’s authorized and unissued preferred stock as convertible Series C Preferred Stock
at a par value of $0.00001 per share.
Hudson
Global Ventures Stock Purchase Agreement
On October 4, 2023, the Company entered into a
Securities Purchase Agreement (“Agreement”, or “SPA”) with Hudson Global Ventures, LLC (“Hudson”).
Under the terms of the Agreement, the Company agreed to sell, and Hudson agreed to purchase, Two Hundred Ninety (290) shares of Series
C Preferred Stock (the “Purchased Shares”) at a price of $1,000 per share and a Warrant to purchase up to 41,193 shares of
Common Stock. Additionally, pursuant to the Agreement, 40,000 shares of Common Stock were issued to Hudson upon closing for a commitment
fee. The Company received $250,000 in exchange for the Purchased Shares, Common Stock, and Warrants, net of issuance costs.
Designation
of Series B Preferred Stock
Effective
June 26, 2023, the Company filed a Certificate of Designation, Preferences, Rights and Limitations of the Series B Preferred Stock with
the Secretary of the State of Delaware which designated 787,754 shares of the Company’s authorized and unissued preferred stock
as convertible Series B Preferred Stock at a par value of $0.00001 per share.
2023
1:15 Stock Split
Effective
June 21, 2023, the Company executed a 1:15 reverse stock split for stockholders of record on that date. This was executed to comply with
the Nasdaq Listing Rule 5550(a)(2) to have the price of the stock above $1.
2022
Equity Compensation Awards
Effective
September 1, 2022, the Board of Directors and Compensation Committee of the Company, with the approval of each of the following officers,
agreed to reduce the annual cash compensation payable to Suren Ajjarapu, the Company’s Chief Executive Officer; Prashant Patel,
the Company’s President and Chief Operating Officer and Janet Huffman, the Company’s former Chief Financial Officer, in an
effort to conserve cash.
In
lieu of the reduced cash salary payable to each officer, the Board and Compensation Committee agreed to issue such officers shares of
the Company’s common stock equal to the amount of reduced cash salary, divided by the closing sales price of the Company’s
common stock on the NASDAQ Capital Market on August 31, 2022, the date approved by the Board of Directors. The total amount of shares
of common stock issued on August 31, 2022, to the officers was 5,460.
The
shares of common stock issuable to the officers vested at the rate of 1/4th of such shares on each of September 30, 2022, October 31,
2022, November 30, 2022, and December 31, 2022, subject to each applicable Officer’s continued service to the Company on such dates
and subject to the restricted stock award agreements entered into as evidence of such awards.
Separately,
certain employees of the Company agreed to reduce their cash salaries by an aggregate of $37,000 in consideration for an aggregate of
2,126 shares of the Company’s restricted common stock, with the same vesting terms as the officer shares discussed above.
Effective
on August 31, 2022, the Board of Directors approved the issuance of 3,635 shares of common stock of the Company to each independent member
of the Board of Directors, for services rendered to the Company during fiscal 2022, which shares were valued at $63,250, based on the
closing sales price of the Company’s common stock on the date approved by the Board of Directors. The shares vested at the rate
of 1/4th of such shares immediately on the grant date, and 1/4th of such shares on each of October 1, 2022, January 1, 2023, and April
1, 2023, subject to each applicable independent director’s continued service to the Company on such dates.
All
of the awards discussed above were issued under the Company’s Second Amended and Restated 2019 Equity Incentive Plan (the “Plan”)
and all restricted stock awards discussed above were evidenced by Restricted Stock Grant Agreements.
NOTE
14 – PREFUNDED AND PRIVATE PLACEMENT WARRANTS
On
October 4, 2022 the Company entered into a securities purchase agreement (the “Purchase Agreement”) with a certain institutional
investor (the “Purchaser”) which provided for the sale and issuance by the Company of (i) the Company’s common stock
(the “Common Stock”), (ii) pre-funded warrants (the “Pre-Funded Warrants”) and (iii) warrants (the “Private
Placement Warrants” and, together with the Shares and the Pre-Funded Warrants, the “Securities”). The Private Placement
Warrants were sold in a concurrent private placement (the “Private Placement”).
Simultaneously
with the closing of the stock placement, the investor pre-purchased 40,116 Private Warrants at a purchase price of $17.25 per warrant.
The Pre-Funded Warrants are immediately exercisable into one share of common stock per warrant, have an exercise price of $0.00015 per
share, and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. On January 4, 2023, the investor
exercised the 40,116 warrants for a purchase price of $6.02. The investor was issued the shares on this date. Each Private Warrant has
an exercise price of $22.50 per share, will be exercisable following Stockholder Approval, which was obtained in December 2022, and will
expire on the fifth anniversary of the date on which the Private Warrants become exercisable. The Private Warrants contain standard adjustments
to the exercise price including for stock splits, stock dividend, rights offerings and pro rata distributions, and include full ratchet
anti-dilutive rights in the event the Company issues shares of Common Stock or Common Stock equivalents within fifteen months of the
initial exercise date, with a value less than the then exercise price of such Private Warrants, subject to certain customary exceptions,
and further subject to a minimum exercise price of $3.48 per share. The Private Warrants also include certain rights upon ‘fundamental
transactions’ as described in the Private Warrants, including allowing the holders thereof to require that the Company re-purchase
such Private Warrants at the Black Scholes Value of such securities.
NOTE
15 – WARRANTS
During
the year ended December 31, 2023, 41,193 warrants were granted, and none expired. During the year ended December 31, 2023, 40,116 prefunded
warrants and 1,795 granted warrants to purchase shares of common stock were exercised for a total purchase price of $1,621. See Note
13 for further description.
The
Company uses the Black-Scholes pricing model to estimate the fair value of stock-based awards on the date of the grant.
There
was no compensation cost related to the warrants for the years ended December 31, 2023, and 2022, respectively.
The
following table summarizes the assumptions used to estimate the fair value of the outstanding warrants during the years ended December
31, 2023, and 2022.
| | |
2023 | | |
2022 | |
| Expected dividend yield | |
| 0 | % | |
| 0 | % |
| Weighted-average expected volatility | |
| 165 | % | |
| 86 | % |
| Weighted-average risk-free interest rate | |
| 3.9 | % | |
| 4.3 | % |
| Warrants, measurement input | |
| 3.9 | % | |
| 4.3 | % |
| Expected life of warrants | |
| 3.8
years | | |
| 5
years | |
The
Company’s outstanding and exercisable warrants as of December 31, 2023 and 2022 are presented below:
| | |
Number
Outstanding | | |
Weighted
Average
Exercise Price | | |
Contractual
Life In Years | | |
Intrinsic
Value | |
| Warrants outstanding as of December 31, 2021 | |
| 2,969 | | |
$ | 4.82 | | |
| 0.95 | | |
$ | 11,135 | |
| Warrants granted | |
| 177,536 | | |
| 22.50 | | |
| 4.77 | | |
| - | |
| Warrants forfeited, expired, cancelled | |
| (202 | ) | |
| 3.90 | | |
| - | | |
| - | |
| Warrants exercised | |
| (972 | ) | |
| 0.06 | | |
| - | | |
| - | |
| Warrants outstanding as of December 31, 2022 | |
| 179,331 | | |
| 22.50 | | |
| 4.72 | | |
| 6,731 | |
| Warrants granted | |
| 41,193 | | |
| 7.20 | | |
| 4.76 | | |
| - | |
| Warrants forfeited, expired, cancelled | |
| - | | |
| - | | |
| - | | |
| - | |
| Warrants exercised | |
| (1,795 | ) | |
| 0.90 | | |
| - | | |
| - | |
| Warrants outstanding as of December 31, 2023 | |
| 218,729 | | |
| 19.62 | | |
| 3.95 | | |
$ | - | |
| Warrants exercisable as of December 31, 2023 | |
| 218,279 | | |
| 19.62 | | |
| 3.95 | | |
$ | - | |
NOTE
16 – OPTIONS
The
Company maintains stock option plans under which certain employees are awarded option grants based on a combination of performance and
tenure. The stock option plans provide for the grant of up to 155,556 shares, and the Company’s Second Amended and Restated 2019
Equity Incentive Plan provides for automatic increases in the number of shares available under such plan (currently 133,333 shares) on
April 1st of each calendar year, beginning in 2021 and ending in 2029 (each a “Date of Determination”), in each
case subject to the approval and determination of the administrator of the plan (the Board of Directors or Compensation Committee) on
or prior to the applicable Date of Determination, equal to the lesser of (A) ten percent (10%) of the total shares of common stock of
the Company outstanding on the last day of the immediately preceding fiscal year and (B) such smaller number of shares as determined
by the administrator. The administrator as a result of the annual meeting shareholder vote increased the number of shares available to
grant to employees under the 2019 incentive plan by 2 million. The administrator did not approve an increase in the number of shares
covered under the plan as of April 1, 2022.
For
the year ended December 31, 2023, 9,053 options to purchase shares were granted, 140 options to purchase shares were forfeited and 2,393
options expired. For the year ended December 31, 2023, no options to purchase shares of common stock were exercised.
Total
compensation cost related to stock options granted was $29,738 and $79,163 for the years ended December 31, 2023, and 2022, respectively.
The
following table represents stock option activity for the year ended December 31, 2023:
| | |
Number
Outstanding | | |
Weighted-Average
Exercise Price | | |
Weighted-Average
Contractual
Life in Years | | |
Intrinsic
Value | |
| | |
| | |
| | |
| | |
| |
| Options outstanding as of December 31, 2021 | |
| 27,398 | | |
$ | 4.78 | | |
| 4.67 | | |
$ | 368,417 | |
| Options exercisable as of December 31, 2021 | |
| 20,146 | | |
| 4.88 | | |
| 4.38 | | |
| 257,186 | |
| Options granted | |
| - | | |
| - | | |
| - | | |
| - | |
| Options forfeited | |
| (1,234 | ) | |
| 87.37 | | |
| 4.91 | | |
| - | |
| Options expired | |
| (6,456 | ) | |
| 86.03 | | |
| 2.66 | | |
| - | |
| Options exercised | |
| - | | |
| - | | |
| - | | |
| - | |
| Options outstanding as of December 31, 2022 | |
| 19,708 | | |
| 66.00 | | |
| 3.92 | | |
| - | |
| Options exercisable as of December 31, 2022 | |
| 17,167 | | |
| 66.30 | | |
| 3.89 | | |
| - | |
| Options granted | |
| 9,053 | | |
| 6.08 | | |
| 4.25 | | |
| - | |
| Options forfeited | |
| (140 | ) | |
| 82.33 | | |
| 1.75 | | |
| - | |
| Options expired | |
| (2,392 | ) | |
| 89.89 | | |
| 0.06 | | |
| - | |
| Options exercised | |
| - | | |
| - | | |
| - | | |
| - | |
| Options outstanding as of December 31, 2023 | |
| 26,229 | | |
$ | 43.04 | | |
| 3.70 | | |
$ | - | |
| Options exercisable as of December 31, 2023 | |
| 16,141 | | |
$ | 60.75 | | |
| 3.64 | | |
$ | - | |
NOTE
17 – CONTINGENCIES
Studebaker
Defense Group, LLC
In
July 2020, the Company’s wholly-owned subsidiary, IPS, entered into an agreement with Studebaker Defense Group, LLC (“Studebaker”)
wherein IPS would pay Studebaker a down payment of $500,000 and Studebaker would deliver 180,000 boxes of nitrile gloves by August 14,
2020. IPS wired the $500,000 to Studebaker, but to date, Studebaker has not delivered the gloves or provided a refund of the deposit.
In December 2020, the Company filed a complaint against Studebaker in Florida state court, Case No. 20-CA-010118 in the Circuit Court
for the Thirteenth Judicial Circuit in Hillsborough County, for among other things, breach of contract. Studebaker did not answer the
complaint, nor did counsel for Studebaker file an appearance. Accordingly, in February 2021, the Company filed for a default judgment;
however, on March 22, 2021, counsel for Studebaker filed an appearance and shortly thereafter filed a motion to vacate the default judgment
and dismiss the complaint on jurisdictional grounds. The court granted Studebaker’s motion to set aside the default judgment but
denied the motion to dismiss. At June 30, 2021, the $500,000 was recorded as Loss on Inventory Investment. The Company won this case
but has not collected any settlement yet, another lawsuit was filed to collect.
On
April 13, 2023, a settlement was reached in the Studebaker and IPS legal case. The court found in favor of IPS and ordered Studebaker
to pay $550,000 to IPS. The payments were to commence on May 1, 2023 and continue monthly in 17 installments until the full amount is
paid in full but as of the filing date, no payment has been received by IPS.
Sandwave
Group Dsn Bhd and Crecom Burj Group SDN BHD
In
August 2020, IPS entered into an agreement with Sandwave Group Dsn Bhd (“Sandwave”), wherein IPS would pay Sandwave a down
payment of $581,250 and Sandwave’s supplier, Crecom Burj Group SDN BHD (“Crecom”), would deliver 150,000 boxes of nitrile
gloves within 45 days. IPS wired the $581,250 to Sandwave, which in turn wired the purchase price to Crecom, which Crecom accepted; however,
to date, Crecom has not delivered the nitrile gloves. IPS demanded return of its $581,250 and Crecom acknowledged that IPS was entitled
to a refund. As of February 2021, Crecom had not returned any funds and IPS filed a complaint against Crecom in Malaysia: Case No. WA-22NCC-55-02/2021
in the High Court of Malaysia at Kuala Lumpur in the Federal Territory, Malaysia for the Malaysian equivalent of breach of contract.
On September 1, 2022 counsel for Crecom informed the court that Crecom had been wound up on August 23, 2022; under Section 471 of the
Malaysian Companies Act 2016, the suit filed by IPS was stayed until leave of the court is obtained to proceed. Given this new information
regarding Crecom the Company has decided at this time to stop its pursuit of this lawsuit until or unless additional information is obtained
by counsel for IPS. At June 30, 2021, the $581,250 was recorded as Loss on Inventory Investment.
GSG
PPE, LLC
On
November 19, 2021, IPS filed a complaint against GSG PPE, LLC (“GSG”) and Gary Waxman (“Waxman”), the owner,
alleging three counts of breach of contract for a purchase agreement, a promissory note, and a personal guaranty. Collectively, the company
alleges that GSG and Waxman have materially breached all three contracts. In late 2020, GSG and IPS executed a valid initial contract
setting the terms of a business transaction. GSG failed to pay IPS approximately 75% of the amount owed to IPS. GSG acknowledged it owed
the money and executed a promissory note in favor of IPS in the amount of $630,000 which matured on September 30, 2021. The note provides
for attorney fees and interest in addition to the $630,000. Waxman’s personal guaranty confirmed that GSG owed IPS $630,000. On
September 30, 2021, the $630,000 was recorded as Bad Debt Expense. A settlement was entered into between the parties in June 2022, whereby
GSG and Waxman agreed to pay $743,000 which included attorney fees and interest, which is required to be paid to the Company in monthly
installments over 17 months. The Company received additional monthly installment payments as part of the agreement through January 2023.
As of December 31, 2023, and through the date of this filing, the Company has not received the monthly installment payments due to the
Company from GSG since January of 2023.
NOTE
18 – LEASES
The
Company has two operating leases for corporate offices as of December 31, 2023. The following table outlines the details of the leases:
| | |
Lease 1 | | |
Lease 2 | | |
Lease 3 | |
| Initial Lease Term | |
| January
2021 to December 2021 | | |
| October
2018 to November 2023 | | |
| October
2023 to September 2026 | |
| New Initial Lease Term | |
| January
2022 to December 2026 | | |
| November 2023 to October 2028 | | |
| - | |
| Initial Recognition of Right of use assets at January 1, 2019 | |
$ | 534,140 | | |
$ | 313,301 | | |
| - | |
| New Initial Recognition of Right of use Assets at December 31, 2021 | |
$ | 977,220 | | |
$ | - | | |
| - | |
| New Initial Recognition of Right of use Assets at December 31, 2023 | |
| | | |
| | | |
| 351,581 | |
| Incremental Borrowing Rate | |
| 10 | % | |
| 10 | % | |
| 10 | % |
The
Company entered into a new corporate office lease (Lease 1) in January 2022. At inception, the Company determined that the new lease
required remeasurement of the lease liability resulting in the increase of the right-of-use asset and the associated lease liability
by $977,220. The Company and the Lessor agreed to terminate the lease and vacate the premises in November 2023. The termination resulted
in the surrender of the Company’s security deposit of $38,500. The related right-of-use assets of $642,887 and lease liabilities
of $664,992 were removed from the balance sheet as of December 31, 2023.
The
Company entered into a lease agreement (Lease 2) for the period of October 2018 to November 2023. At inception, management had included
the renewal period from November 2023 to November 2028 within the initial recognition of the related right of use assets and lease liabilities,
as it was reasonably expected, at the time, that the renewal option would be exercised. The Company determined that the new lease required
measurement and recognition of the lease liability and right-of-use assets of $313,301. The lease is classified as an operating lease.
No incentives were included in the lease.
The
Company entered into a new warehouse lease (Lease 3) October 2023. The Company determined that the new lease required measurement and
recognition of the lease liability and right-of-use assets of $351,581. The lease is classified as an operating lease. No incentives
were included in the lease.
The
table below reconciles the fixed component of the undiscounted cash flows for each of the first five years and the total remaining years
to the lease liabilities recorded in the Consolidated Balance Sheet as of December 31, 2023.
| Future lease obligations | |
| |
| 2024 | |
| 187,935 | |
| 2025 | |
| 193,487 | |
| 2026 | |
| 163,146 | |
| 2027 | |
| 58,347 | |
| 2028 | |
| 48,612 | |
| Thereafter | |
| - | |
| Total minimum lease payments | |
| 651,527 | |
| Less: effect of discounting | |
| (102,617 | ) |
| Present value of future minimum lease payments | |
| 548,910 | |
| Less: current obligations under leases | |
| 139,705 | |
| Long-term lease obligations | |
$ | 409,205 | |
| Weighted Average Discount Rate | |
| 10 | % |
| Weighted Average Term Remaining | |
| 3.6
Years | |
| Short-Term Lease Expense Remaining | |
$ | 187,361 | |
For
the years ended December 31, 2023, and 2022, total lease expense was $385,977 and $344,525, respectively.
For
the years ended December 31, 2023, and 2022, amortization of right-of-use assets was $215,665 and $181,218, respectively.
For
the years ended December 31, 2023, and 2022, net operating lease liabilities settled was $195,475 and $164,618, respectively.
NOTE
19 – SEGMENT REPORTING
Operating
segments are defined as the components of an enterprise about which separate financial information is available that is evaluated regularly
by the chief operating decision makers in deciding how to allocate resources and in assessing performance. The Company’s chief
operating decision makers direct the allocation of resources to operating segments based on the profitability, cash flows, and growth
opportunities of each respective segment.
The
Company classifies its business interests into reportable segments which are:
| |
● |
Trxade,
Inc. - Web based pharmaceutical marketplace platform – B2B sales |
| |
● |
IPS
- Integra Pharma, LLC - Licensed wholesaler of brand, generic and non-drug products – B2B sales |
| |
● |
Superlatus
– holds Sapientia’s intellectual property for advanced food extrusion technology and The Urgent Company – Manufacturer
of ice cream that is animal product-free, vegan, lactose-free, and made with plants – B2B sales |
| |
● |
Unallocated
- Other – corporate overhead expense, discontinued operations and Bonum Health, LLC. |
| Years Ended December 31, 2023 | |
Trxade, Inc. | | |
Integra | | |
Superlatus | | |
Unallocated | | |
Total | |
| Revenue | |
| 6,402,159 | | |
| 1,363,830 | | |
| 487,021 | | |
| 19,204 | | |
| 8,272,214 | |
| Gross Profit | |
| 6,402,159 | | |
| 49,030 | | |
| (3,872,136 | ) | |
| 19,204 | | |
| 2,598,257 | |
| Segment Assets | |
| 1,375,109 | | |
| 220,634 | | |
| 9,663,310 | | |
| 1,273,860 | | |
| 12,532,913 | |
| Segment Profit/Loss | |
| 2,325,175 | | |
| (668,625 | ) | |
| (10,416,347 | ) | |
| (9,083,777 | ) | |
| (17,843,574 | ) |
| Cost of Sales | |
| - | | |
| 1,314,800 | | |
| 4,359,157 | | |
| - | | |
| 5,673,957 | |
| Years Ended December 31, 2022 | |
Trxade, Inc. | | |
Integra | | |
Superlatus | | |
Unallocated | | |
Total | |
| Revenue | |
| 5,435,814 | | |
| 4,754,067 | | |
| - | | |
| 60,287 | | |
| 10,250,168 | |
| Gross Profit | |
| 5,433,641 | | |
| 25,343 | | |
| - | | |
| 60,287 | | |
| 5,519,271 | |
| Segment Assets | |
| 1,877,881 | | |
| 445,264 | | |
| - | | |
| 1,386,881 | | |
| 3,710,026 | |
| Segment Profit (Loss) | |
| 1,924,355 | | |
| (545,557 | ) | |
| - | | |
| (5,288,666 | ) | |
| (3,909,868 | ) |
| Cost of Sales | |
| 2,173 | | |
| 4,728,724 | | |
| - | | |
| - | | |
| 4,730,897 | |
NOTE
20 – SUBSEQUENT EVENTS
Asset
Purchase Agreement
On
February 16, 2024, the Company, together with Trxade, Inc., a wholly owned subsidiary of the Company, and Micro Merchant Systems, Inc.
(“MMS”) entered into an asset purchase agreement (the “APA”) under which MMS agreed to purchase for cash substantially
all of the assets of Trxade, Inc. On February 16, 2024, the parties consummated the closing of the transactions contemplated by the APA.
Trxade, Inc. operated a web-based market platform designed to enable trading among healthcare buyers and sellers of pharmaceuticals,
accessories and services. The purchase price paid at closing was $22.5 million, subject to customary adjustments for cash, indebtedness,
working capital and transaction expenses. Subject to the terms and conditions of the APA, if, during the period beginning on the closing
date and ending on the four-month anniversary of the closing date, MMS receives $1.6 million or greater in certain collections from third
parties resulting from any products or services sold, or provided, by the business assets and operations acquired from Trxade, Inc.,
Trxade, Inc. will be due an additional $7.5 million payment from MMS.
Subscription
Agreement
On
February 29, 2024, the Company’s wholly owned subsidiary Trxade, Inc. entered into a Subscription Agreement (the “Subscription
Agreement”) with Lafayette Energy Corp., a Delaware corporation (“Lafayette”). Pursuant to the Subscription Agreement,
Trxade, Inc. will, in two equal tranches, invest a total of up to $5.0 million in Lafayette in exchange for up to 2,000,000 shares of
Lafayette’s newly created Series A Convertible Preferred Stock, with the second tranche becoming payable only upon Trxade, Inc.’s
receipt of notice that Lafayette has successfully drilled its first oil and gas well and produced at least one hundred (100) barrels
of oil. Mr. Michael Peterson is a director of the Company as well as the CEO of Lafayette and a member of Lafayette’s board of
directors. This relationship was disclosed to the Company’s Board of Directors and the audit committee of the Board of Directors
prior to, and at the time that the terms of the Subscription Agreement and the transaction effected thereby were approved by the Board
of Directors as a whole and the members of the audit committee.
Stock
Purchase Agreement
On
March 5, 2024, the Company entered in a Stock Purchase Agreement (“SPA”) with Superlatus Foods Inc. (the “Buyer”).
Pursuant to the SPA, the Company sold all of the issued and outstanding stock (the “Stock”) of Superlatus Inc., a Delaware
corporation and wholly-owned subsidiary of the Company (“Superlatus”), to the Buyer. The purchase price for the Stock was
$1.00 which was delivered to the Company at the closing, which occurred simultaneously with the execution of the SPA. As a result of
the transaction Superlatus is no longer a subsidiary of the Company, and the rights and assets of Superlatus together with various liabilities
and obligations that were specific to Superlatus became rights and obligations of Buyer.
Special
Cash Dividend
On
March 6, 2024, the Company announced the declaration of a special cash dividend of eight dollars ($8.00) per share of common stock, payable
to stockholders of record as of March 18, 2024, with the dividend being paid on or about March 22, 2024. The special dividend was paid
using a portion of the proceeds from the closing of the sale of the Company’s web-based market platform assets.
Scienture
Inc.
Balance
Sheets
Unaudited
| | |
June 30, | | |
December
31, | |
| | |
2024 | | |
2023 | |
| Assets | |
|
|
|
|
| |
| Current Assets: | |
| | | |
| | |
| Cash and
cash equivalents | |
$ | 114,210 | | |
$ | 1,123,878 | |
| Accounts receivable | |
| - | | |
| 66,414 | |
| Other
receivables | |
| 485 | | |
| 485 | |
| Total
Current Assets | |
| 114,695 | | |
| 1,190,777 | |
| Operating lease,
right of use asset | |
| 61,579 | | |
| 64,091 | |
| Total
Assets | |
$ | 176,273 | | |
$ | 1,254,868 | |
| | |
| | | |
| | |
| Liabilities and Stockholders’
Equity | |
| | | |
| | |
| Current Liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 884,581 | | |
$ | 107,175 | |
| Accrued expenses and
other liabilities | |
| 1,198,822 | | |
| 332,212 | |
| Convertible notes | |
| - | | |
| 3,665,220 | |
| Operating
lease liability | |
| 22,567 | | |
| 21,403 | |
| Total
Current Liabilities | |
| 2,105,970 | | |
| 4,126,010 | |
| Long-term convertible notes, net of debt
discount | |
| 1,734,661 | | |
| 1,625,117 | |
| Operating Lease Liability, non current | |
| 39,319 | | |
| 42,893 | |
| Development agreement
liability | |
| 1,285,000 | | |
| - | |
| Total
Liabilities | |
| 5,164,950 | | |
| 5,794,020 | |
| | |
| | | |
| | |
| Commitments and contingencies (Refer Note
8) | |
| | | |
| | |
| Stockholders’ Deficit: | |
| | | |
| | |
| Preferred stock, $.0001
par value, 3,365,657 authorized, issued and outstanding | |
| 337 | | |
| 240 | |
| Common stock, $0.0001
par value, 10,000,000 authorized, 5,000,000 issued and outstanding | |
| 500 | | |
| 500 | |
| Additional paid-in capital | |
| 10,835,257 | | |
| 6,849,064 | |
| Accumulated
deficit | |
| (15,824,770 | ) | |
| (11,388,956 | ) |
| Total
stockholders’ deficit | |
| (4,988,676 | ) | |
| (4,539,152 | ) |
| Total
Liabilities and Stockholders’ Deficit | |
$ | 176,273 | | |
$ | 1,254,868 | |
Scienture
Inc.
Statements
of Operations and Comprehensive Loss
Unaudited
| | |
Six
Months Ended June 30, | |
| | |
2024 | | |
2023 | |
| Revenue | |
$ | - | | |
$ | 800,000 | |
| | |
| | | |
| | |
| Operating Expenses: | |
| | | |
| | |
| Research and development | |
| 1,520,947 | | |
| 946,435 | |
| General and administrative | |
| 1,413,893 | | |
| 267,236 | |
| Termination
fee | |
| 1,285,000 | | |
| - | |
| Total
operating expenses | |
| 4,219,841 | | |
| 1,213,670 | |
| | |
| | | |
| | |
| Loss from Operations | |
| (4,219,841 | ) | |
| (413,670 | ) |
| | |
| | | |
| | |
| Other Income (Expense) | |
| | | |
| | |
| Other income | |
| 11,931 | | |
| 18,304 | |
| Interest
expense | |
| (227,905 | ) | |
| (76,203 | ) |
| Total
other expense | |
| (215,974 | ) | |
| (57,900 | ) |
| | |
| | | |
| | |
| Net
Loss | |
$ | (4,435,814 | ) | |
$ | (471,570 | ) |
| | |
| | | |
| | |
| Net loss per share - basic and diluted | |
$ | (0.89 | ) | |
$ | (0.09 | ) |
| Weighted-average shares used to compute
net loss per share - diluted | |
| 5,000,000 | | |
| 5,000,000 | |
Scienture
Inc.
Statements
of Stockholders’ Deficit
Unaudited
| | |
Preferred
Stock | | |
Common
Stock | | |
Additional
Paid-In | | |
Accumulated | | |
Total Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Deficit | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance at December 31, 2021 | |
| 2,400,000 | | |
$ | 240 | | |
| 4,850,000 | | |
$ | 485 | | |
$ | 6,111,783 | | |
$ | (5,439,589 | ) | |
$ | 672,920 | |
| Common stock issued for services | |
| - | | |
| - | | |
| 150,000 | | |
| 15 | | |
| 145,485 | | |
| - | | |
| 145,500 | |
| Stock-based compensation expenses | |
| - | | |
| - | | |
| - | | |
| - | | |
| 68,087 | | |
| - | | |
| 68,087 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (3,708,378 | ) | |
| (3,708,378 | ) |
| Balance at December 31, 2022 | |
| 2,400,000 | | |
$ | 240 | | |
| 5,000,000 | | |
$ | 500 | | |
$ | 6,325,355 | | |
$ | (9,147,967 | ) | |
$ | (2,821,872 | ) |
| Stock-based compensation expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| 39,724 | | |
| - | | |
| 39,724 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (471,570 | ) | |
| (471,570 | ) |
| Balance at June
30, 2023 | |
| 2,400,000 | | |
$ | 240 | | |
| 5,000,000 | | |
$ | 500 | | |
$ | 6,365,079 | | |
$ | (9,619,537 | ) | |
$ | (3,253,718 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance at December 31, 2023 | |
| 2,400,000 | | |
$ | 240 | | |
| 5,000,000 | | |
$ | 500 | | |
$ | 6,849,064 | | |
$ | (11,388,956 | ) | |
$ | (4,539,152 | ) |
| Conversion of notes into preferred stock | |
| 965,657 | | |
| 97 | | |
| - | | |
| - | | |
| 3,941,356 | | |
| - | | |
| 3,941,453 | |
| Stock-based compensation expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| 44,837 | | |
| - | | |
| 44,837 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (4,435,814 | ) | |
| (4,435,814 | ) |
| Balance at June
30, 2024 | |
| 3,365,657 | | |
$ | 337 | | |
| 5,000,000 | | |
$ | 500 | | |
$ | 10,835,257 | | |
$ | (15,824,770 | ) | |
$ | (4,988,676 | ) |
Scienture
Inc.
Statements
of Cash Flows
Unaudited
| | |
Six
Months Ended June 30, | |
| | |
2024 | | |
2023 | |
| Cash flows from operating
activities: | |
| | | |
| | |
| Net loss | |
$ | (4,435,814 | ) | |
$ | (471,570 | ) |
| Adjustments to reconcile Net loss to net
cash used in operating activities: | |
| | | |
| | |
| Amortization of debt discount | |
| 109,544 | | |
| - | |
| Stock-based compensation expense | |
| 44,837 | | |
| 39,724 | |
| Changes in operating
assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| 66,414 | | |
| (300,000 | ) |
| Accounts payable | |
| 777,406 | | |
| 29,729 | |
| Accrued expenses and other liabilities | |
| 1,142,843 | | |
| 76,212 | |
| Development agreement liability | |
| 1,285,000 | | |
| - | |
| Operating lease liability,
Net | |
| 103 | | |
| - | |
| Net
cash used in operating activities | |
| (1,009,668 | ) | |
| (625,905 | ) |
| | |
| | | |
| | |
| Cash flows from financing
activities: | |
| | | |
| | |
| Proceeds from the
issuance of convertible notes | |
| | | |
| 400,000 | |
| Net
cash used in financing activities | |
| - | | |
| 400,000 | |
| | |
| | | |
| | |
| Net change in cash and cash equivalents | |
| (1,009,668 | ) | |
| (225,905 | ) |
| Cash and cash equivalents
at beginning of period | |
| 1,123,878 | | |
| 604,813 | |
| Cash
and cash equivalents at end of period | |
$ | 114,210 | | |
$ | 378,908 | |
| | |
| | | |
| | |
| Supplemental disclosure
of cash flow information: | |
| | | |
| | |
| Cash paid for interest | |
$ | - | | |
$ | - | |
| Supplemental disclosure
of non-cash financing activities: | |
| | | |
| | |
| Conversion of notes and accrued interest
into preferred stock | |
$ | 3,941,453 | | |
$ | - | |
Note
1 Organization Overview and Basis of Presentation
Nature
of Operations
Scienture
Inc. (“the Company”) is a pharmaceutical research company which is engaged in the research and development of branded pharmaceutical
products. The IP application process of the company initiated in November 2019 and commenced the product development activities from
January 2020. The Company also plans to foray into commercialization of innovative and branded pharmaceutical products in the US market.
The
Company was incorporated in the state of Delaware in June 2019. The Company is headquartered in Hauppauge, New York, United States of
America.
Basis
of Presentation
The
Company’s fiscal year ends on December 31.
The
accompanying financial statements for the periods ending June 30, 2024 and 2023 have been prepared in accordance with accounting principles
generally accepted in the United States (“U.S.GAAP”).
Note
2 Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of the Company’s financial statements in conformity with U.S.GAAP requires the Company to make estimates and assumptions
that affect the reported amounts of certain assets and liabilities; the reported amounts of revenues and expenses for the periods covered
and certain amounts disclosed in the notes to the financial statements. These estimates are based on information available through the
date of the issuance of the financial statements and actual results could differ from those estimates. To the extent there are material
differences between the Company’s estimates and the actual results, the Company’s future consolidated results of operation
may be affected. Areas requiring significant estimates and assumptions by the Company include, but are not limited to:
| ● | fair
value of long-term convertible debt and warrants issued in connection with such debt; |
| ● | accruals
for estimated liabilities; |
| ● | the
valuation of stock-based compensation awards ; and |
| ● | provisions
for income taxes and related valuation allowances and tax uncertainties. |
Unaudited
Interim Financial Information
The
unaudited condensed consolidated interim financial statements and related notes have been prepared in accordance with U.S. GAAP for interim
financial information, within the rules and regulations of the United States Securities and Exchange Commission (the “SEC”).
Certain information and disclosures normally included in the annual financial statements prepared in accordance with U.S. GAAP have been
condensed or omitted pursuant to such rules and regulations. The unaudited interim financial statements have been prepared on a basis
consistent with the audited financial statements and in the opinion of management, reflect all adjustments, consisting of only normal
recurring adjustments, necessary for the fair presentation of the results for the interim periods presented and of the financial condition
as of the date of the interim balance sheet. The financial data and the other information disclosed in these notes to the interim financial
statements related to the six-month periods are unaudited. Unaudited interim results are not necessarily indicative of the results for
the full fiscal year.
The
accompanying unaudited interim condensed consolidated financial statements should be read in conjunction with the Company’s audited
financial statements and the notes thereto for the year ended December 31, 2023.
Liquidity
The
entity has just commenced operations and is expected to be funded by the stockholders for liquidity purposes. The liquidity position
of the entity is also dependent on the fundings by the additional development partners.
Comprehensive
Loss
Comprehensive
loss includes net loss as well as other changes in stockholder’s equity that result from transactions and economic events other
than those with stockholders. There was no difference between net loss and comprehensive loss presented in the financial statements for
the six months ended June 30, 2024 and 2023.
Segment
Reporting
The
Company’s chief operating decision-maker is its Chief Executive Officer, who makes resource allocation decisions and assesses performance
based on financial information presented on an aggregate basis. There are no segment managers who are held accountable by the chief operating
decision-maker, or anyone else, for any planning, strategy and key decision-making regarding operations. Accordingly, the Company has
a single reportable segment and operating segment structure.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash
equivalents. Cash equivalents consist of amounts invested in money market funds and are stated at fair value.
Accounts
Receivable
Accounts
receivable consist of milestone payments due from development partners as a consideration for the rights granted for the commercialization
of the products to be developed. The Company reviews its accounts receivable and provides allowances of specific amounts if collectability
is no longer reasonably assured based on historical experience and specific collection issues. The allowance for doubtful accounts was
$0 as of June 30, 2024 and December 31, 2023, respectively.
Revenue
Recognition
Revenue
is recognized when control of the promised services is transferred to customers, at an amount that reflects the consideration to which
the entity expects to be entitled in exchange for those services.
The
Company adopted FASB ASU No. 2014-09, Revenue from Contracts with Customers and the related amendments, which are codified into ASC 606,
which establishes a broad principle that requires entities to assess the products or services promised in contracts with customers at
contract inception to determine the appropriate unit at which to record revenues, which is referred to as a performance obligation. Revenue
is recognized when control of the promised products or services is transferred to customers, at an amount that reflects the consideration
to which the entity expects to be entitled in exchange for those products or services.
To
determine revenue recognition for arrangements that the Company determines are within the scope of ASC 606, the Company performs the
following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii)
determine the transaction price; (iv) allocate the transaction price to the performance obligation(s) in the contract; and (v) recognize
revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it
is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the
customer. At contract inception, once the contract was determined to be within the scope of ASC 606, the Company assessed the goods or
services promised within each contract and determined those that were performance obligations, and assessed whether each promised good
or service was distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective
performance obligation when (or as) the performance obligation is satisfied.
A
performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in
ASC 606. The Company recognizes revenue at the point of sale of service.
Exclusive
License and Commercial Agreements
The
Company entered into an exclusive license and commercial agreement with Kesin Pharma Corporation, a related party where the Company granted
the exclusive license rights to commercialize SCN-102 in 2022 and SCN-104 in 2023 to Kesin (SCN-102 and SCN-104 are together referred
to as “the Products”) for use in the United States of America. In consideration of the rights granted, the Company is in
receipt of milestone payments and reimbursement of costs actually incurred related to the products. Revenue has been recognized when
such development milestone events take place and the amounts are due to be received. The Company recognized $800,000 for the six months
ended June 30, 2023, at the point when the development milestone events occurred.
In
March 2024, the parties have terminated the agreement, and the parties agreed that, Scienture shall pay Kesin a total gross amount of
$1,285,000 upon commercialization of product via a royalty arrangement.
This
agreement also requires that if the full $1,285,00 has not been repaid within two years of the early of i) commercial launch or ii) 120
from FDA approval, then interest will accrue prospectively at a rate of 8% annually on unpaid balance. Accordingly, the Company recorded
a $1,285,000 termination fee liability. As of the date of issue of financial statements, the entire amount is outstanding.
Fair
Value of Financial Instruments
Fair
value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction
between market participants at the measurement date. A hierarchy has been established for inputs used in measuring fair value that maximizes
the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available.
Observable inputs are inputs that market participants would use in pricing the asset or liability and are developed based on market data
obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions of what
market participants would use in pricing the asset or liability based on the best information available in the circumstances. The financial
and nonfinancial assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurement.
The hierarchy is presented down into three levels based on the reliability of the inputs.
| |
Level 1 |
Quoted prices are available
in active markets for identical assets or liabilities. |
| |
|
|
| |
Level 2 |
Observable inputs other than quoted prices in active markets for identical assets and liabilities,
quoted prices for identical or similar assets or liabilities in inactive markets, or other inputs that are observable or can be corroborated
by observable market data for substantially the full term of the assets or liabilities. |
| |
|
|
| |
Level 3 |
Unobservable pricing inputs that are generally less observable from objective sources, such as discounted
cash flow models or valuations. |
The
carrying amounts of cash, accounts receivable, accounts payable, accrued liabilities and short-term convertible notes approximate their
fair value because of the short-term nature of these instruments. The carrying amount of long-term convertible debt approximate the fair
value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Concentration
of Credit Risks and Major Customers
Financial
instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and receivables. The
Company places its cash and cash equivalents with financial institutions. During the six months ended June 30, 2024, the Company
had one development partner that accounted for the entire revenue recognized in the Statement of Comprehensive Loss.
Research
& Development Expenses
Research
and development costs are expensed in the period incurred in accordance with ASC 730. Research and development expenses consist of independent
contractor costs , costs for outsourced analytical research and development activities, batch manufacturing cost and, advisory costs
as a part of research, market research costs and other regulatory consulting costs.
Stock-Based
Compensation
The
Company’s stock-based compensation expense relates to stock options. Stock-based compensation expense for its stock-based awards
is based on their grant date fair value. The Company estimates the fair value of stock option awards on the grant date using the Black-Scholes
option-pricing model. The Black-Scholes model considers several variables and assumptions in estimating the fair value of stock-based
awards. These variables include the per share fair value of the underlying common stock, exercise price, expected term, risk-free interest
rate, expected annual dividend yield and the expected stock price volatility over the expected term. The Company has estimated volatility
by reference to the historical volatilities of the Company and that of similar publicly traded peer companies. The risk-free interest
rate is based on the yield available on U.S. Treasury zero-coupon issues similar in duration to the expected term of the equity-settled
award.
Warrant
Valuation
Stock
warrant valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards is estimated
using the Black-Scholes option model with a volatility figure derived from an average of historical stock prices for comparable entities.
The Company accounts for the expected life based on the contractual life of the warrants. The risk-free interest rate is determined from
the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the warrants.
Net
Loss per share
Basic
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during the period,
adjusted for outstanding shares that are subject to repurchase.
For
the calculation of diluted net loss per share, basic net loss per share is adjusted by the effect of dilutive securities if any. Diluted
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding and potential
common stock outstanding, if dilutive. For periods in which the Company reports net losses, diluted net loss per share is the same as
basic net loss per share because potentially dilutive shares of common stock are not assumed to have been issued if their effect is anti-dilutive.
Accounting
Pronouncements Not Yet Adopted
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2023-07, “Segment
Reporting (Topic 280): Improvements to Reportable Segment Disclosures” (“ASU 2023-07”), which requires additional operating
segment disclosures in annual and interim consolidated financial statements. ASU 2023-07 is effective for annual periods beginning after
December 15, 2023 and for interim periods beginning after December 15, 2024 on a retrospective basis, with early adoption permitted.
The Company is evaluating the effect of adopting ASU 2023-07.
In
December 2023, the FASB issued Accounting Standards Update No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”
(“ASU 2023-09”), which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components
of the effective tax rate reconciliation and modifies other income tax-related disclosures. ASU 2023-09 is effective for annual periods
beginning after December 15, 2024 on a retrospective or prospective basis. The Company is evaluating the effect of adopting ASU 2023-09.
Note
3 Going Concern
The
Company has a net loss of ($4,435,814) for the six months ended June 30, 2024 and stockholders’ deficit of ($4,988,676) as of June
30, 2024. The Company’s situation raises a substantial doubt on whether the entity can continue as a going concern in the next
twelve months.
The
Company’s ability to continue as a going concern in the next twelve months following the date the financial statements were available
to be issued is dependent upon its ability to produce revenues and/or obtain financing sufficient to meet current and future obligations
and deploy such to produce profitable operating results.
Management
has evaluated these conditions and plans to generate revenues and raise capital as needed to satisfy its capital needs. During the next
twelve months, the Company intends to fund its operations through debt and/or equity financing.
There
are no assurances that management will be able to raise capital on terms acceptable to the Company. If it is unable to obtain sufficient
amount of additional capital, it may be required to reduce the scope of its planned development, which could harm its business, financial
condition, and operating results. The accompanying financial statements do not include any adjustments that might result from these uncertainties.
The
Company has evaluated whether there are certain conditions and events, considered in the aggregate, that raise substantial doubt about
the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued.
Note
4 Cash and Cash Equivalents
Cash
and cash equivalents consist of the following:
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Balances with banks | |
$ | 116,107 | | |
$ | 31,943 | |
| Money market securities(Highly
liquid investments) | |
| - | | |
| 1,091,935 | |
| Total
Cash and Cash Equivalents | |
$ | 116,106 | | |
$ | 1,123,879 | |
Money
market securities were considered a Level 1 financial instrument.
Note
5 Convertible Notes
The
carrying value of the convertible notes approximate their fair value because of the short-term nature of these instruments. The convertible
notes issued bear an interest at a rate of 8% per annum and certain notes issued prior to 2022 bear an interest at a rate of 2% per annum.
As of December 31, 2023, there $3,665,220 in outstanding principal. All the short-term convertible notes matured during the period of
December 2023. In March 2024, the Company had converted the outstanding principal of $3,665,220 and the accrued interest through the
date of conversion amounting to $276,233 into an aggregate of 965,567 shares of preferred stock of the Company.
Note
6 Long-Term Convertible Debt, net of debt discount
In
September 2023, the Company entered into a loan agreement with NVK Finance LLC, a Nebraska Limited Liability Company (‘NVK”)
for $2,000,000. The Board Member of the Company has significant influence in the decision making in NVK and hence considered as a related
party. The debt shall accrue interest at a per annum rate equal to Prime Rate plus 7 percent and the prime rates shall be adjusted quarterly
commencing on December 2023. As of June 30, 2024 and December 31, 2023, the interest rate was 15.50%. The debt is collateralized by all
of the Company’s receivables, cash and cash equivalents and the title in Intellectual Property Rights and all proceeds thereof.
The principal is entirely repayable on the maturity date i.e. September 2025 and interest shall be paid monthly upon a Qualified Financing
as defined in the Loan Agreement. Interest expense related to the debt amounted to $95,583 for the year ended December 31, 2023 and the
principal amount is entirely outstanding as at December 31,2023. The outstanding balance under the NVK debt is convertible into common
stock of the Company at a fully-diluted Company valuation of $60,000,000.
In
connection with the NVK debt, the Company granted 509,014 warrants to purchase common stock. The fair value of the warrants was $444,260
using Black-Scholes option pricing model, which will be amortized to interest expense over the life of the notes. During the six months
ended June 30, 2024, the Company amortized $109,544 of the debt discount to interest expense.
Long-term
convertible debt, net of debt discount, consisted of the following:
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Principal | |
$ | 2,000,000 | | |
$ | 2,000,000 | |
| Less: Unamortized
debt discount | |
| 265,339 | | |
| 374,883 | |
| Long-term convertible
notes, net of debt discount | |
$ | 1,734,661 | | |
$ | 1,625,117 | |
Maturities
of the outstanding notes are as follows:
| Years Ending December 31 | |
| |
| 2024 | |
$ |
- | |
| 2025 | |
| 2,000,000 | |
| | |
$ | 2,000,000 | |
Note
7 Fair Value of Financial Instruments
The
carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and short-term convertible notes
approximate their fair value because of the short-term nature of these instruments. The carrying amount of long-term debt approximates
fair value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Note
8 Commitment and Contingencies
The
Company, in conjunction with its legal counsel, assesses the need to record a liability for litigation or loss contingencies. A liability
is recorded when and if it is determined that such a liability for litigation or loss contingencies is both probable and estimable. The
Company does not record any anticipated gains relating to its litigation or legal claims. The gains are only recorded upon receipt of
the settlement.
Although
the results of legal proceedings and claims cannot be predicted with certainty, the Company is not currently a party to any legal proceedings,
which would, individually or in the aggregate, have a material adverse effect on its results of operations, cash flows, or financial
position.
In
August 2024, Kesin demanded immediate payment of the full amount under the Kesin Termination Agreement, alleging the full amount is payable
in connection with the consummation Scienture’s business combination with the Company. Scienture has disputed that the amount is
now payable, and the parties are in discussions to resolve the issue. There can be no assurance that an amicable resolution will be obtained.
If Kesin brings a legal action, Scienture will vigorously defend it.
Note
9 Related Party Transactions
The
Company had entered into an Exclusive and Commercial agreement with Kesin Pharma Corporation (“Kesin”), in which one of the
company’s board member is the President and CEO, and had a significant influence in the decision making, which makes it a related
party. Sales made to Kesin as a part of milestone structure for the six months ended June 30, 2024 and 2023 were $0 and $800,000, respectively.
The Company terminated the agreement with Kesin in March 2024, and recorded a termination fee and related liability of $1,285,000 as
of June 30, 2024.
The
Company has leased its office from Saptalis Pharmaceuticals LLC (“Saptalis”) , in which one of the Company’s Director
is the President and CEO. Lease payments made during the six months ended June 30, 2024 and 2023 are $14,440 and $0, respectively. The Company
has also engaged Saptalis to provide development services and conduct testing and studies for the products under development by the Company.
Expenses incurred towards such testing and studies which is included in the Research and Development Expenses in the Statement of Comprehensive
Loss for the six months ended June 30, 2024 and 2023 amounted to $106,539 and $189,027, respectively.
During
the six months ended June 30, 2023, a related party to a director issued a convertible note to the Company for $250,000.
In
July 2024, officers of the company provided a short term promissory note to the Company for $265,000
Note
10 Scienture Inc. 2020 Stock Option and Grant Plan
The
Stock Option and Grant Plan allows for the issuance of incentive stock options (“ISOs”), non-statutory stock options (“NSOs”).
ISOs may be granted only to the Company’s employees (including officers and directors who are also considered employees) and ex-employees.
NSOs may be granted to the Company’s employees and service providers such as advisors etc. Options under the Stock Option and Grant
Plan have a contractual term of not more than 10 years.
A
summary of the Company’s stock option activity under the Plans is as follows:
| | |
Outstanding
Options | | |
Weighted-
Average
Exercise
Price | | |
Weighted-
Average
Remaining
Term
(Years) | |
| Balance as of December 31,
2023 | |
| 655,000 | | |
$ | 0.90 | | |
| 7.56 | |
| Granted | |
| 142,199 | | |
| 1.13 | | |
| | |
| Exercised | |
| - | | |
| - | | |
| | |
| Cancelled and forfeited | |
| - | | |
| - | | |
| | |
| Balance as of June
20, 2024 | |
| 797,199 | | |
$ | 0.94 | | |
| 6.21 | |
| | |
| | | |
| | | |
| | |
| Vested and exercisable
as of June 30, 2024 | |
| 499,089 | | |
$ | 0.84 | | |
| 5.62 | |
The
weighted-average grant date fair value of options granted during the six months ended June 30, 2024 was $0.76 per share.
The
Company recorded stock-based compensation expense in the Statement of Operations and Comprehensive Loss for the periods presented as
follows:
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| General and Administrative Expenses | |
$ | 44,837 | | |
$ | 39,724 | |
| Total stock-based compensation
expense | |
$ | 44,837 | | |
$ | 39,724 | |
Stock
Option Valuation Assumptions
The
fair value of each employee option grant was estimated on the date of grant using the Black-Scholes option pricing model and the following
assumptions for the periods indicated:
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| Expected volatility | |
| 72 | % | |
| 65%
- 75 | % |
| Risk-free interest rate | |
| 4.1%
- 4.4 | % | |
| 0.5%
- 3.5 | % |
| Expected term | |
| 5.7
- 6.1 years | | |
| 5.9
- 6.0 years | |
| Expected dividend | |
| 0 | % | |
| 0 | % |
Note
11 Warrants
As
of June 30, 2024, there were 509,014 warrants outstanding and exercisable with an exercise price of $0.01 per share. The warrants were
granted in connection with the NVK debt (Refer - Note 6 - Long-Term Convertible Debt, net of debt discount).
Note
12 Net Loss per Share
Stock
options to purchase 799,199 and 655,000 shares of common stock, warrants to purchase 509,014 and 0 common stock, convertible preferred
stock and convertible notes to purchase 3,365,669 and 3,195,911 common stock and long-term convertible debt to purchase 0 and 3,350,000
shares common stock were outstanding at June 30, 2024 and 2023, respectively, that were not included in the computation of diluted weighted
average common shares outstanding because their effect would have been anti-dilutive.
Note
13 Leases
The
Company determines if an arrangement is a lease at inception. Operating leases are included in Operating lease, Right of Use asset, Operating
Lease Liability (Current and Non-Current) in the Company’s balance sheets. The ROU assets represent the Company’s right to
use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising
from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments
over the lease term. As most of the leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate
at commencement date. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain
that the Company will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
As a practical expedient, the Company elected, for all office and facility leases, not to separate non-lease components from lease components
and instead to account for each separate lease component and its associated non-lease components as a single lease component. The Company
made an accounting policy election by class of underlying asset not to recognize the lease liability and related right-of-use asset for
leases with a term of one year or less.
The
Company has an operating lease for administrative office. The lease has remaining lease term around three years.
The
components of lease expense were as follows:
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| Operating lease costs | |
| | | |
| | |
| Amortization of ROU Assets | |
$ | 2,152 | | |
$ | - | |
| Interest on Lease
Liabilities | |
$ | 1,190 | | |
$ | - | |
| Short term lease
costs | |
$ | 17,010 | | |
$ | - | |
Supplemental
balance sheet information related to leases was as follows:
| | |
June 30, | | |
December
31, | |
| | |
2024 | | |
2023 | |
| Operating
Leases | |
| | | |
| | |
| Right of Use Assets | |
$ | 61,579 | | |
$ | 64,091 | |
| | |
| | | |
| | |
| Short term Lease
libilities | |
$ | 22,567 | | |
$ | 21,403 | |
| Long term Lease libilities | |
$ | 39,319 | | |
$ | 42,893 | |
| | |
$ | 61,886 | | |
$ | 64,296 | |
| | |
| | | |
| | |
| Weighted Average Remaining Lease Term (in
years) | |
| 2.33 | | |
| - | |
| | |
| | | |
| | |
| Weighted Average Discount Rate | |
| 15.50 | % | |
| - | |
Note
14 Subsequent Events
In
July 2024, the executives of the Company issued a short-term loan to Company for an aggregate amount of $250,000.
In
July 2024, all of the unvested options per the Company’s Stock Option and Grant Plan became vested.
Business
Combination
On July 25, 2024, Scienture, Inc.
(the “Company”) entered into and closed an Agreement and Plan of Merger (the “Merger Agreement”) with MEDS, MEDS
Merger Sub I, Inc., a Delaware corporation and wholly owned subsidiary of MEDS (“Merger Sub I”) and MEDS Merger Sub II, LLC,
a Delaware limited liability company and wholly owned subsidiary of the Company (“Merger Sub II”). Pursuant to the Merger
Agreement, (i) Merger Sub I merged with and into the Company, with the Company continuing as the surviving entity and a wholly owned
subsidiary of MEDS, and (ii) the Company merged with and into Merger Sub II, with Merger Sub II continuing as the surviving entity.
Management
has evaluated subsequent events through August 15, 2024, the date the financial statements were available to be issued.
Report of Independent Registered Public Accounting Firm
To
the Stockholders and Board of Directors of Scienture Inc.
Opinion
on the Financial Statements
We have audited the accompanying balance sheets of Scienture Inc. (the “Company”)
as of December 31, 2023 and 2022, the related statements of operations and comprehensive loss, statements of stockholders’ deficit
and statements of cash flows, and the related notes collectively referred to as the “financial statements” for each of the
two years in the period ended December 31, 2023. In our opinion, the financial statements present fairly, in all material respects, the
financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the
two years in the period ended December 31, 2023, in conformity with Generally Accepted Accounting Principles of United States of America.
We were appointed as the independent auditors of Scienture Inc. since 2022.
Matters
related to Going Concern
The
accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note
3 to the financial statements, if the Company is unable to raise additional funds to alleviate liquidity needs, it may be required to
reduce the scope of its planned development. The company has suffered losses from operations and has a net capital deficiency that raise
substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described
in Note 3 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of
this uncertainty.
Responsibilities
of the Management for the Financial Statements
These
financial statements are the responsibility of the Company’s management. In preparing the financial statements, management is required
to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s
ability to continue as a going concern for one year after the date that the financial statements are available to be issued. Our responsibility
is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered
with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect
to the Company in accordance with the applicable rules and regulations of PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The company
is not required to have, nor we have engaged to perform, an audit of its internal control over financial reporting. As part of our audit,
we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or
fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides
a reasonable basis for our opinion.
Critical
Audit Matter
Critical
audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be
communicated to the audit committee and that:
| (1) |
relate
to accounts or disclosures that are material to the financial statements and |
| |
|
| (2)
|
involved
our especially challenging, subjective, or complex judgments. |
We
determined that there are no critical audit matters.
We
have served as the Company’s auditors since 2022.
/s/ Suri & Co., Chartered Accountants
Date:
July 31,2024
Place:
Chennai, India
Scienture Inc.
Balance
Sheets
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Assets | |
| | |
| |
| Current Assets: | |
| | |
| |
| Cash and cash equivalents | |
$ | 1,123,878 | | |
$ | 604,813 | |
| Accounts receivable | |
| 66,414 | | |
| - | |
| Other receivables | |
| 485 | | |
| 485 | |
| Total Current Assets | |
| 1,190,777 | | |
| 605,298 | |
| Operating lease, right of use asset | |
| 64,091 | | |
| - | |
| Total Assets | |
$ | 1,254,868 | | |
$ | 605,298 | |
| | |
| | | |
| | |
| Liabilities and Stockholders’ Deficit | |
| | | |
| | |
| Current Liabilities: | |
| | | |
| | |
| Accounts payable | |
| 107,175 | | |
| 393,676 | |
| Accrued expenses and other liabilities | |
| 332,211 | | |
| 83,494 | |
| Convertible notes | |
| 3,665,220 | | |
| 2,950,000 | |
| Operating lease liability | |
| 21,404 | | |
| - | |
| Total Current Liabilities | |
| 4,126,010 | | |
| 3,427,170 | |
| Long-term convertible debt, net of debt discount | |
| 1,625,117 | | |
| - | |
| Operating lease liability, non current | |
| 42,893 | | |
| - | |
| Total Liabilities | |
| 5,794,020 | | |
| 3,427,170 | |
| | |
| | | |
| | |
| Commitments and contingencies (Refer Note 8) | |
| | | |
| | |
| Stockholders’ Deficit: | |
| | | |
| | |
| Preferred stock, $.0001 par value, 2,400,000 authorized,
issued and outstanding | |
| 240 | | |
| 240 | |
| Common stock, $0.0001 par value, 10,000,000 authorized,
5,000,000 issued and outstanding | |
| 500 | | |
| 500 | |
| Additional paid-in capital | |
| 6,849,064 | | |
| 6,325,355 | |
| Accumulated deficit | |
| (11,388,956 | ) | |
| (9,147,967 | ) |
| Total stockholders’ deficit | |
| (4,539,152 | ) | |
| (2,821,872 | ) |
| Total Liabilities and Stockholders’
Deficit | |
$ | 1,254,868 | | |
$ | 605,298 | |
Scienture Inc.
Statements
of Operations and Comprehensive Loss
| | |
Year Ended December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Net revenue | |
$ | 800,000 | | |
$ | 300,000 | |
| | |
| | | |
| | |
| Operating Expenses: | |
| | | |
| | |
| Research and development | |
| 2,029,812 | | |
| 3,061,492 | |
| General and administrative expenses | |
| 719,318 | | |
| 880,110 | |
| Total operating expenses | |
| 2,749,210 | | |
| 3,941,602 | |
| | |
| | | |
| | |
| Loss from Operations | |
| (1,949,210 | ) | |
| (3,641,602 | ) |
| | |
| | | |
| | |
| Other Income (Expense) | |
| | | |
| | |
| Dividend income | |
| 2,401 | | |
| - | |
| Interest income (expense), net | |
| (312,577 | ) | |
| (76,351 | ) |
| Miscellaneous income | |
| 18,397 | | |
| 9,574 | |
| Total other expense | |
| (291,779 | ) | |
| (66,777 | ) |
| | |
| | | |
| | |
| Net Loss | |
$ | (2,240,989 | ) | |
$ | (3,708,378 | ) |
| | |
| | | |
| | |
| Net loss per share - basic and diluted | |
| (0.45 | ) | |
| (0.74 | ) |
| Weighted-average shares used to compute net loss per share - basic and diluted | |
| 5,000,000 | | |
| 5,000,000 | |
Scienture Inc.
Statements
of Stockholders’ Deficit
| | |
Preferred Stock | | |
Common Stock | | |
Additional Paid-In | | |
Accumulated | | |
Total Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Deficit | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance at December 31, 2021 | |
| 2,400,000 | | |
$ | 240 | | |
| 4,850,000 | | |
$ | 485 | | |
$ | 6,111,783 | | |
$ | (5,439,589 | ) | |
$ | 672,919 | |
| Common stock issued for services | |
| - | | |
| - | | |
| 150,000 | | |
| 15 | | |
| 145,485 | | |
| - | | |
| 145,500 | |
| Stock-based compensation expenses | |
| - | | |
| - | | |
| - | | |
| - | | |
| 68,087 | | |
| - | | |
| 68,087 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (3,708,378 | ) | |
| (3,708,378 | ) |
| Balance at December 31, 2022 | |
| 2,400,000 | | |
| 240 | | |
| 5,000,000 | | |
| 500 | | |
| 6,325,355 | | |
| (9,147,967 | ) | |
| (2,821,872 | ) |
| Warrants issued in connection with long-term convertible debt | |
| - | | |
| - | | |
| - | | |
| - | | |
| 444,260 | | |
| - | | |
| 444,260 | |
| Stock-based compensation expenses | |
| - | | |
| - | | |
| - | | |
| - | | |
| 79,449 | | |
| - | | |
| 79,449 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (2,240,989 | ) | |
| (2,240,989 | ) |
| Balance at December 31, 2023 | |
| 2,400,000 | | |
$ | 240 | | |
| 5,000,000.00 | | |
$ | 500 | | |
$ | 6,849,064 | | |
$ | (11,388,956 | ) | |
$ | (4,539,152 | ) |
Scienture Inc.
Statements
of Cash Flows
| | |
Year Ended December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (2,240,989 | ) | |
$ | (3,708,378 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Common stock issued for services | |
| - | | |
| 145,500 | |
| Amortization of debt discount | |
| 69,378 | | |
| - | |
| Stock-based compensation expenses | |
| 79,449 | | |
| 68,087 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts payable | |
| (286,501 | ) | |
| 65,394 | |
| Accrued expenses and other liabilities | |
| 248,716 | | |
| 76,705 | |
| Operating lease liability, net | |
| 206 | | |
| - | |
| Accounts receivable | |
| (66,414 | ) | |
| - | |
| Net cash used in operating activities | |
| (2,196,155 | ) | |
| (3,352,693 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from the issuance of convertible notes | |
| 715,220 | | |
| 850,000 | |
| Proceeds from the issuance of long-term convertible debt | |
| 2,000,000 | | |
| - | |
| Net cash used in financing activities | |
| 2,715,220 | | |
| 850,000 | |
| | |
| | | |
| | |
| Net change in cash and cash equivalents | |
| 519,065 | | |
| (2,502,693 | ) |
| Cash and cash equivalents at beginning of year | |
| 604,813 | | |
| 3,107,506 | |
| Cash and cash equivalents at end of year | |
$ | 1,123,878 | | |
$ | 604,813 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | |
| Cash paid for interest | |
$ | - | | |
$ | - | |
| Supplemental disclosure of non-cash financing activities: | |
| | | |
| | |
| Warrants issued in connection with long-term convertible debt | |
$ | 444,260 | | |
$ | - | |
Note
1 Organization Overview and Basis of Presentation
Nature
of Operations
Scienture
Inc. (“the Company”) is a pharmaceutical research company which is engaged in the research and development of branded pharmaceutical
products. The IP application process of the company initiated in November 2019 and commenced the product development activities from
January 2020. The Company also plans to foray into commercialization of innovative and branded pharmaceutical products in the US market.
The
Company was incorporated in the state of Delaware in June 2019. The Company is headquartered in Hauppauge, New York, United States of
America.
Basis
of Presentation
The
Company’s fiscal year ends on December 31.
The
accompanying financial statements for the period ending December 31, 2023 and 2022 have been prepared in accordance with accounting principles
generally accepted in the United States (“U.S.GAAP”).
Note
2 Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of the Company’s financial statements in conformity with U.S.GAAP requires the Company to make estimates and assumptions
that affect the reported amounts of certain assets and liabilities; the reported amounts of revenues and expenses for the periods covered
and certain amounts disclosed in the notes to the financial statements. These estimates are based on information available through the
date of the issuance of the financial statements and actual results could differ from those estimates. To the extent there are material
differences between the Company’s estimates and the actual results, the Company’s future consolidated results of operation
may be affected. Areas requiring significant estimates and assumptions by the Company include, but are not limited to:
| |
● |
fair
value of long-term convertible debt and warrants issued in connection with such debt; |
| |
● |
accruals
for estimated liabilities; |
| |
● |
lease
term |
| |
● |
the
valuation of stock-based compensation awards ; and |
| |
● |
provisions
for income taxes and related valuation allowances and tax uncertainties. |
Liquidity
The
entity has just commenced operations and is expected to be funded by the stockholders for liquidity purposes. The liquidity position
of the entity is also dependent on the fundings by the additional development partners.
Comprehensive
Loss
Comprehensive
loss includes net loss as well as other changes in stockholder’s equity that result from transactions and economic events other
than those with stockholders. There was no difference between net loss and comprehensive loss presented in the financial statements for
the years ended December 31, 2023 and 2022.
Segment
Reporting
The
Company’s chief operating decision-maker is its Chief Executive Officer, who makes resource allocation decisions and assesses performance
based on financial information presented on an aggregate basis. There are no segment managers who are held accountable by the chief operating
decision-maker, or anyone else, for any planning, strategy and key decision-making regarding operations. Accordingly, the Company has
a single reportable segment and operating segment structure.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash
equivalents. Cash equivalents consist of amounts invested in money market funds and are stated at fair value.
Accounts
Receivable
Accounts
receivable consist of milestone payments due from development partners as a consideration for the rights granted for the commercialization
of the products to be developed. The Company reviews its accounts receivable and provides allowances of specific amounts if collectability
is no longer reasonably assured based on historical experience and specific collection issues. The allowance for doubtful accounts was
$0 as of December 31, 2023 and 2022, respectively.
(Refer
– Note 14– Subsequent Events – Termination of Exclusive License and Commercial Agreement).
Revenue
Recognition
Revenue
is recognized when control of the promised services is transferred to customers, at an amount that reflects the consideration to which
the entity expects to be entitled in exchange for those services.
The
Company adopted FASB ASU No. 2014-09, Revenue from Contracts with Customers and the related amendments, which are codified into ASC 606,
which establishes a broad principle that requires entities to assess the products or services promised in contracts with customers at
contract inception to determine the appropriate unit at which to record revenues, which is referred to as a performance obligation. Revenue
is recognized when control of the promised products or services is transferred to customers, at an amount that reflects the consideration
to which the entity expects to be entitled in exchange for those products or services.
To
determine revenue recognition for arrangements that the Company determines are within the scope of ASC 606, the Company performs the
following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii)
determine the transaction price; (iv) allocate the transaction price to the performance obligation(s) in the contract; and (v) recognize
revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it
is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the
customer. At contract inception, once the contract was determined to be within the scope of ASC 606, the Company assessed the goods or
services promised within each contract and determined those that were performance obligations, and assessed whether each promised good
or service was distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective
performance obligation when (or as) the performance obligation is satisfied.
A
performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in
ASC 606. The Company recognizes revenue at the point of sale of service.
Exclusive
License and Commercial Agreements
The
Company entered into an exclusive license and commercial agreement with Kesin Pharma Corporation, a related party where the Company granted
the exclusive license rights to commercialize SCN-102 in 2022 and SCN-104 in 2023 to Kesin (SCN-102 and SCN-104 are together referred
to as “the Products”) for use in the United States of America. In consideration of the rights granted, the Company is in
receipt of milestone payments and reimbursement of costs actually incurred related to the products. Revenue has been recognized when
such development milestone events take place and the amounts are due to be received. The Company recognized $800,000 and $300,000, respectively,
during the years ended December 31, 2023 and 2022 at the point when the development milestone events occurred. (Refer – Note 14–
Subsequent Events – Termination of Exclusive License and Commercial Agreement).
Fair
Value of Financial Instruments
Fair
value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction
between market participants at the measurement date. A hierarchy has been established for inputs used in measuring fair value that maximizes
the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available.
Observable inputs are inputs that market participants would use in pricing the asset or liability and are developed based on market data
obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions of what
market participants would use in pricing the asset or liability based on the best information available in the circumstances. The financial
and nonfinancial assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurement.
The hierarchy is presented down into three levels based on the reliability of the inputs.
| |
Level
1 |
Quoted
prices are available in active markets for identical assets or liabilities. |
| |
|
|
| |
Level
2 |
Observable
inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets
or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially
the full term of the assets or liabilities. |
| |
|
|
| |
Level
3 |
Unobservable
pricing inputs that are generally less observable from objective sources, such as discounted cash flow models or valuations. |
The
carrying amounts of cash, accounts receivable, accounts payable, accrued liabilities and short-term convertible notes approximate their
fair value because of the short-term nature of these instruments. The carrying amount of long-term convertible debt approximate the fair
value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Concentration
of Credit Risks and Major Customers
Financial
instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and receivables. The
Company places its cash and cash equivalents with financial institutions. During the years ended December 31, 2023, and 2022, the company
had one development partner that accounted for the entire revenue recognized in the Statement of Comprehensive Loss.
Research
& Development Expenses
Research
and development costs are expensed in the period incurred in accordance with ASC 730. Research and development expenses consist of independent
contractor costs , costs for outsourced analytical research and development activities, batch manufacturing cost and, advisory costs
as a part of research, market research costs and other regulatory consulting costs.
Stock-Based
Compensation
The
Company’s stock-based compensation expense relates to stock options. Stock-based compensation expense for its stock-based
awards is based on their grant date fair value. The fair values of stock-based compensations are recognized as compensation expense
on a straight-line basis over the requisite service period in which the awards are expected to vest. The Company estimates the fair
value of stock option awards on the grant date using the Black-Scholes option-pricing model. The Black-Scholes model considers
several variables and assumptions in estimating the fair value of stock-based awards. These variables include the per share fair
value of the underlying common stock, exercise price, expected term, risk-free interest rate, expected annual dividend yield and the
expected stock price volatility over the expected term. The Company has estimated volatility by reference to the historical
volatilities of the Company and that of similar publicly traded peer companies. The risk-free interest rate is based on the yield
available on U.S. Treasury zero-coupon issues similar in duration to the expected term of the equity-settled award.
Warrant
Valuation
Stock
warrant valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards is estimated
using the Black-Scholes option model with a volatility figure derived from an average of historical stock prices for comparable entities.
The Company accounts for the expected life based on the contractual life of the warrants. The risk-free interest rate is determined from
the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the warrants.
Income
Taxes
State
Income Tax:
The
Company is incorporated in Delaware and headquartered in New York where the state tax is 8.70% and 7.25% respectively. However, due to
losses for the years ended December 31, 2023 and 2022, no provision on state income tax has been recognized.
Federal
Income Tax:
The
Company is a C Corporation for tax purposes, filing Form 1120 annually. Profits are not being passed through to owners. The company records
income taxes pursuant to the liability method. The Company has a loss before tax of ($2,240,989) and ($3,708,378) for years ended December
31, 2023 and 2022 respectively. Therefore, no provision for federal income tax has been recognized.
Deferred
Tax Assets and Liabilities
Deferred
tax assets and liabilities are determined based on the differences between the financial statement and the tax basis of assets and liabilities.
Realization of the future tax benefits related to the net deferred tax assets is dependent on many factors including the Company’s
ability to generate taxable income. Management believes that, at a minimum, it is more likely than not that future taxable income may
not be sufficient to realize the recorded assets.
The Company has recorded a deferred tax asset related to its net operating
loss carryforwards, timing difference between written down value of assets, and unutilized R&D credit, which are expected to reduce
future taxable income. The company has assessed the likelihood of realizing the deferred tax assets and determined that it is more likely
than not that a portion of the assets may not be realized. Therefore, a valuation allowance has been created to account for 100% of the
deferred tax assets to its expected realizable value.
The
impact of the deferred tax assets and related valuation allowance on the Company’s financial statements is as follows:
| | |
Year Ended December 31, | |
| | |
2023 | | |
2022 | |
| Deferred Tax Assets | |
| | | |
| | |
| Net operating loss carryforwards | |
$ | 3,173,840 | | |
$ | 2,491,667 | |
| Research and development tax credits | |
| 6,635 | | |
| 2,705 | |
| Property and equipment and operating lease liability | |
| 49,220 | | |
| 53,960 | |
| Valuation allowance | |
| (3,229,695 | ) | |
| (2,548,331 | ) |
| Net deferred tax assets | |
$ | - | | |
$ | - | |
Net
Loss per share
Basic
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during the period,
adjusted for outstanding shares that are subject to repurchase.
For
the calculation of diluted net loss per share, basic net loss per share is adjusted by the effect of dilutive securities if any. Diluted
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding and potential
common stock outstanding, if dilutive. For periods in which the Company reports net losses, diluted net loss per share is the same as
basic net loss per share because potentially dilutive shares of common stock are not assumed to have been issued if their effect is anti-dilutive.
Recent
Accounting Pronouncements
Accounting
Pronouncements Recently Adopted
The
Company has implemented all new relevant accounting pronouncements that are in effect through the date of these financial statements.
The pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not
believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial
position or results of operations.
In
June 2016, the FASB issued ASU No. 2016-13, Financial Instruments—Credit Losses (ASC 326), which provides guidance on measurement
of credit losses on financial instruments. This ASU adds a current expected credit loss impairment model to U.S.GAAP that is based on
expected losses rather than incurred losses whereby a broader range of reasonable and supportable information is required to be utilized
in order to derive credit loss estimates. The effective date of the new guidance as amended by ASU No. 2019-10 is fiscal years beginning
after December 15, 2022, including interim periods within those fiscal years. The Company adopted ASU 2016-13 effective January 1, 2023
the company determined that the update applied to trade receivables, but that there no material impact to the financial statements from
the adoption of ASU 2016-13.
In
February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standard Update (“ASU”) No.
2016-2, Leases, to provide guidance for the accounting for leasing transactions. The standard requires the lessee to recognize a lease
liability along with a right-of-use asset for all leases with a term longer than one year. A lessee is permitted to make an accounting
policy election by class of underlying asset to not recognize the lease liability and related right-of-use asset for leases with a term
of one year or less. The provisions of this standard also apply to situations where the Company is the lessor. In March 2019, the FASB
issued ASU 2019-01, “Lease (842): Codification improvements.” This updated clarified that entities were exempt from disclosing
the effect of the change on income from continuing operations, net income, and related per-share amounts, if applicable, for interim
periods after the adoption of Accounting Standards Codification (“ASC”) 842.
The
standard was initially effective for annual and interim reporting periods beginning after December 15, 2019. However, in November 2019,
the FASB issued ASU 2019-10, “Financial Instruments Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases
(Topic 842): Effective Dates”, which deferred the effective date of ASU 2016-02 by an additional year. At its April 8, 2020, meeting,
the FASB voted to defer the effective date for ASC 842 another year. As such, the Company is required to adopt the new leases standard
for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022. The Company
adopted this new guidance effective January 1, 2022.
Accounting
Pronouncements Not Yet Adopted
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2023-07, “Segment
Reporting (Topic 280): Improvements to Reportable Segment Disclosures” (“ASU 2023-07”), which requires additional operating
segment disclosures in annual and interim consolidated financial statements. ASU 2023-07 is effective for annual periods beginning after
December 15, 2023 and for interim periods beginning after December 15, 2024 on a retrospective basis, with early adoption permitted.
The Company is evaluating the effect of adopting ASU 2023-07.
In
December 2023, the FASB issued Accounting Standards Update No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”
(“ASU 2023-09”), which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components
of the effective tax rate reconciliation and modifies other income tax-related disclosures. ASU 2023-09 is effective for annual periods
beginning after December 15, 2024 on a retrospective or prospective basis. The Company is evaluating the effect of adopting ASU 2023-09.
Note
3 Going Concern
The
Company has a net loss of ($2,240,989) for the year ended December 31,2023 and stockholders’ deficit of ($4,539,152) as of December
31, 2023. The Company’s situation raises a substantial doubt on whether the entity can continue as a going concern in the next
twelve months.
The
Company’s ability to continue as a going concern in the next twelve months following the date the financial statements were available
to be issued is dependent upon its ability to produce revenues and/or obtain financing sufficient to meet current and future obligations
and deploy such to produce profitable operating results.
Management
has evaluated these conditions and plans to generate revenues and raise capital as needed to satisfy its capital needs. During the next
twelve months, the Company intends to fund its operations through debt and/or equity financing.
There
are no assurances that management will be able to raise capital on terms acceptable to the Company. If it is unable to obtain sufficient
amount of additional capital, it may be required to reduce the scope of its planned development, which could harm its business, financial
condition, and operating results. The accompanying financial statements do not include any adjustments that might result from these uncertainties.
The
Company has evaluated whether there are certain conditions and events, considered in the aggregate, that raise substantial doubt about
the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued.
Note
4 Cash and Cash Equivalents
Cash
and cash equivalents consist of the following:
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Balances with banks | |
$ | 31,943 | | |
$ | 604,813 | |
| Money market securities (Highly liquid investments) | |
| 1,091,935 | | |
| - | |
| Total Cash and Cash Equivalents | |
$ | 1,123,878 | | |
$ | 604,813 | |
Money
market securities were considered a Level 1 financial instrument.
Note
5 Convertible Notes
The
carrying value of the convertible notes approximate their fair value because of the short-term nature of these instruments. The convertible
notes issued bear an interest at a rate of 8% per annum and certain notes issued prior to 2022 bear an interest at a rate of 2% per annum.
As of December 31, 2023 and 2022, there were $3,665,220 and $2,950,000 in outstanding principal. All such notes have matured on December
31,2023. Interest expenses recognized for the years ended December 31, 2023 and 2022 amounted to $152,423 and $76,705 respectively. (Refer
– Note 14– Subsequent Events – Short-Term Convertible Notes)
Note
6 Long-Term Convertible Debt, net of debt discount
In
September 2023, the Company entered into a loan agreement with NVK Finance LLC, a Nebraska Limited Liability Company (‘NVK”)
for $2,000,000. The Board Member of the Company has significant influence in the decision making in NVK and hence considered as a related
party. The debt shall accrue interest at a per annum rate equal to Prime Rate plus 7 percent and the prime rates shall be adjusted quarterly
commencing on December 2023. As of December 31, 2023, the interest rate was 15.50%. The debt is collateralized by all of the Company’s
receivables, cash and cash equivalents and the title in Intellectual Property Rights and all proceeds thereof. The principal is entirely
repayable on the maturity date i.e. September 2025 and interest shall be paid monthly upon a Qualified Financing as defined in the Loan
Agreement. Interest expense related to the debt amounted to $95,583 for the year ended December 31, 2023 and the principal amount is
entirely outstanding as at December 31,2023. The outstanding balance under the NVK debt is convertible into common stock of the Company
at a fully-diluted Company valuation of $60,000,000.
In
connection with the NVK debt, the Company granted 509,014 warrants to purchase common stock. The fair value of the warrants was $444,260
using Black-Scholes option pricing model, which will be amortized to interest expense over the life of the notes. During the year ended
December 31, 2023, the Company amortized $69,377 of the debt discount to interest expense. (Refer – Note 11– Warrants)
Long-term
convertible debt, net of debt discount, consisted of the following:
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Principal | |
$ | 2,000,000 | | |
$ | - | |
| Less: Unamortized debt discount | |
| 374,883 | | |
| - | |
| Long-term convertible debt, net of debt discount | |
$ | 1,625,117 | | |
$ | - | |
Maturities
of the outstanding debt are as follows:
| Years Ending December 31 | |
| |
| 2024 | |
$ | - | |
| 2025 | |
| 2,000,000 | |
| | |
$ | 2,000,000 | |
Note
7 Fair Value of Financial Instruments
The
carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and short-term convertible notes
approximate their fair value because of the short-term nature of these instruments. The carrying amount of long-term debt approximates
fair value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Note
8 Commitment and Contingencies
The
Company, in conjunction with its legal counsel, assesses the need to record a liability for litigation or loss contingencies. A liability
is recorded when and if it is determined that such a liability for litigation or loss contingencies is both probable and estimable. The
Company does not record any anticipated gains relating to its litigation or legal claims. The gains are only recorded upon receipt of
the settlement.
Although
the results of legal proceedings and claims cannot be predicted with certainty, the Company is not currently a party to any legal proceedings,
which would, individually or in the aggregate, have a material adverse effect on its results of operations, cash flows, or financial
position.
Note
9 Related Party Transactions
The
Company had entered into an Exclusive and Commercial agreement with Kesin Pharma Corporation (“Kesin”), in which one of the
company’s board member is the President and CEO, and had a significant influence in the decision making, which makes it a related
party. Sales made to Kesin as a part of milestone structure for the years ended December 31,2023 and 2022 amounted to $800,000 and $300,000
respectively.
The
Company has leased its office from Saptalis Pharmaceuticals LLC (“Saptalis”) , in which one of the Company’s Director
is the President and CEO. Lease payments made during the year ended December 31, 2023 and 2022 are $ 7200 and $0 respectively. The company
has also engaged Saptalis to provide development services and conduct testing and studies for the products under development by the Company.
Expenses incurred towards such testing and studies which is included in the Research and Development Expenses in the Statement of Comprehensive
Loss for the year ended December 31, 2023 and December 31,2022 amounted to $355,124 and $647,566 respectively.
Relatives
or related parties to directors purchased securities from the Company on the same terms as unrelated parties as set forth below:
| Name of the Related Party | |
Nature of transaction | |
Transactions
during the year
ended
December 31 | | |
Balances as at
December 31 | |
| | |
| |
2023 | | |
2022 | | |
2022 | | |
2023 | |
| Ms. Pushpa Shankar | |
Issue of Preferred Stock | |
$ | - | | |
$ | - | | |
$ | 750,000 | | |
$ | 750,000 | |
| Ms. Pushpa Shankar | |
Issue of Convertible notes | |
| 400,000 | | |
| 150,000 | | |
| 550,000 | | |
| 400,000 | |
| Ms. Yogita Desai | |
Issue of Preferred Stock | |
| - | | |
| - | | |
| 500,000 | | |
| 500,000 | |
| Ms. Yogita Desai | |
Issue of Convertible notes | |
| - | | |
| - | | |
| 100,000 | | |
| 100,000 | |
| Mr. Sandeep Gupta | |
Issue of Convertible notes | |
| - | | |
| 50,000 | | |
| 50,000 | | |
| - | |
| Total | |
| |
$ | 400,000 | | |
$ | 200,000 | | |
$ | 1,950,000 | | |
$ | 1,750,000 | |
Note
10 Scienture Inc. 2020 Stock Option and Grant Plan
The
Stock Option and Grant Plan allows for the issuance of incentive stock options (“ISOs”), non-statutory stock options (“NSOs”).
ISOs may be granted only to the Company’s employees (including officers and directors who are also considered employees) and ex-employees.
NSOs may be granted to the Company’s employees and service providers such as advisors etc. Options under the Stock Option and Grant
Plan have a contractual term of not more than 10 years.
A
summary of the Company’s stock option activity under the Plans is as follows:
| | |
Outstanding
Options | | |
Weighted-
Average
Exercise
Price | | |
Weighted-
Average
Remaining
Term
(Years) | |
| Balance as of December 31, 2022 | |
| 560,000 | | |
$ | 0.83 | | |
| 7.95 | |
| Granted | |
| 185,000 | | |
| 1.13 | | |
| | |
| Exercised | |
| - | | |
| - | | |
| | |
| Cancelled and forfeited | |
| (90,000 | ) | |
| - | | |
| | |
| Balance as of December 31, 2023 | |
| 655,000 | | |
$ | 0.90 | | |
| 7.56 | |
| | |
| | | |
| | | |
| | |
| Vested and exercisable as of December 31, 2023 | |
| 410,065 | | |
$ | 0.84 | | |
| 6.84 | |
The
weighted-average grant date fair value of options granted during the years ended December 31, 2023 and 2022 are $ 0.55 and $ 0.52 per
share respectively.
The
Company recorded stock-based compensation expense in the Statement of Operations and Comprehensive Loss for the periods presented as
follows:
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| General and Administrative Expenses | |
$ | 79,449 | | |
$ | 68,087 | |
| Total stock-based compensation expense | |
$ | 79,449 | | |
$ | 68,087 | |
Stock
Option Valuation Assumptions
The
fair value of each employee option grant was estimated on the date of grant using the Black-Scholes option pricing model and the following
assumptions for the periods indicated:
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Expected volatility | |
| 65%
- 75% | | |
| 65%
- 75% | |
| Risk-free interest rate | |
| 0.5%
- 3.5% | | |
| 0.5%
- 2.8% | |
| Expected term | |
| 5.9
- 6.0 years | | |
| 5.9
- 6.0 years | |
| Expected dividend | |
| 0 | % | |
| 0 | % |
Note
11 Warrants
As
of December 31, 2023, there were 509,014 warrants outstanding and exercisable with an exercise price of $0.01 per share. The warrants
were granted in connection with the NVK debt (Refer - Note 6 - Long-Term Convertible Debt, net of debt discount).
The
following table summarizes the assumptions used to estimate the fair value of the outstanding warrants during the years ended December
31, 2023, and 2022:
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Expected dividend yield | |
| 0 | % | |
| - | |
| Weighted-average expected volatility | |
| 70.52 | % | |
| - | |
| Weighted-average risk-free interest rate | |
| 4.50 | % | |
| - | |
| Expected life of warrants | |
| 5
years | | |
| - | |
Note
12 Net Loss per Share
Stock
options to purchase 655,000 and 560,000 common stock, warrants to purchase 509,014 and 0 common stock, convertible preferred stock and
convertible notes to purchase 3,365,669 and 3,195,911 common stock and long-term convertible debt to purchase 9,529,683 and 0 common
stock were outstanding at December 31, 2023 and 2022, respectively, that were not included in the computation of diluted weighted average
common shares outstanding because their effect would have been anti-dilutive.
Note
13 Leases
The
Company determines if an arrangement is a lease at inception. Operating leases are included in Operating lease, Right of Use asset, Operating
Lease Liability (Current and Non-Current) in the Company’s balance sheets. The ROU assets represent the Company’s right to
use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising
from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments
over the lease term. As most of the leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate
at commencement date. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain
that the Company will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
As a practical expedient, the Company elected, for all office and facility leases, not to separate non-lease components from lease components
and instead to account for each separate lease component and its associated non-lease components as a single lease component. The Company
made an accounting policy election by class of underlying asset not to recognize the lease liability and related right-of-use asset for
leases with a term of one year or less.
The
Company has an operating lease for administrative office. The lease has remaining lease term around three years.
The
components of lease expense were as follows:
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Operating lease costs | |
| | | |
| | |
| Amortization of ROU Assets | |
$ | 5,025 | | |
$ | - | |
| Interest on Lease Liabilities | |
$ | 2,380 | | |
$ | - | |
| Short term lease costs | |
$ | 34,021 | | |
$ | 32,677 | |
Supplemental
cash flow information related to leases was as follows:
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Cash paid for accounts included in the measurements of lease liabilities | |
| | |
| |
| Operating cash flows for Operating leases | |
$ | 7,200 | | |
$ | - | |
| Right of Use Assets obtained in exchange for new Lease Liabilities | |
$ | 205 | | |
$ | - | |
Supplemental
balance sheet information related to leases was as follows:
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Operating Leases | |
| | | |
| | |
| Right of Use Assets | |
$ | 64,091 | | |
$ | - | |
| | |
| | | |
| | |
| Short term Lease liabilities | |
$ | 21,403 | | |
$ | - | |
| Long term Lease liabilities | |
$ | 42,893 | | |
$ | - | |
| Total Lease Liabilities | |
$ | 64,296 | | |
$ | - | |
| | |
| | | |
| | |
| Weighted Average Remaining Lease Term (in years) | |
| 2.83 | | |
| - | |
| | |
| | | |
| | |
| Weighted Average Discount Rate | |
| 15.50 | % | |
| - | |
Maturities
of lease liabilities were as follows at December 31, 2023:
| December 31, 2023 | |
| | |
| 2024 | |
$ | 29,017 | |
| 2025 | |
| 29,887 | |
| 2026 | |
| 17,823 | |
| Total lease payments | |
| 76,727 | |
| Less: Imputed interest | |
| 12,430 | |
| Total | |
$ | 64,297 | |
Note
14 Subsequent Events
Short-Term
Convertible Notes
All
the short-term convertible notes matured during the period of December 2023. The Company has not paid the amounts due including the principal
and the accrued interest. However, in March 2024, the Company had converted the outstanding principal of $3,665,220 and the accrued interest
till the date of conversion amounting to $276,233 into an aggregate of 965,568 preferred stock of the company.
Business Combination
On July 25, 2024, Scienture, Inc. (the “Company”)
entered into and closed an Agreement and Plan of Merger (the “Merger Agreement”) with MEDS, MEDS Merger Sub I, Inc., a Delaware
corporation and wholly owned subsidiary of MEDS (“Merger Sub I”) and MEDS Merger Sub II, LLC, a Delaware limited liability
company and wholly owned subsidiary of the Company (“Merger Sub II”). Pursuant to the Merger Agreement, (i) Merger Sub I
merged with and into the Company, with the Company continuing as the surviving entity and a wholly owned subsidiary of MEDS, and (ii)
the Company merged with and into Merger Sub II, with Merger Sub II continuing as the surviving entity.
Termination
of Exclusive License and Commercial Agreement:
Scienture
Inc. (Scienture) and Kesin had entered into two exclusive license commercial agreements where Scienture had granted Kesin the rights
to commercialize the products. In March 2024, the parties have terminated the agreement, and the parties agreed that, Scienture shall
pay Kesin a total gross amount of $1,285,000 upon commercialization of product via a royalty arrangement.
This
agreement also requires that if the full $1,285,000 has not been repaid within two years of the early of i) commercial launch or ii)
120 days from FDA approval, then interest will accrue prospectively at a rate of 8% annually on unpaid balance. As of the date of
issue of financial statements, the entire amount is outstanding.
Management
has evaluated subsequent events through July 31, 2024, the date the financial statements were available to be issued.
ANNEX
A
Execution
Version
AGREEMENT
AND PLAN OF MERGER
among:
TRXADE
HEALTH, INC.;
MEDS
MERGER SUB I, INC.;
MEDS
MERGER SUB II, LLC; and
SCIENTURE,
INC.
Dated
as of July 25, 2024
| TABLE
OF CONTENTS |
| |
|
|
| Section
1. |
Definitions
and Interpretative Provisions. |
6 |
| |
|
|
| 1.1 |
Definitions. |
6 |
| |
|
|
| 1.2 |
Other
Definitional and Interpretative Provisions. |
16 |
| |
|
|
| Section
2. |
Description
of Transaction. |
17 |
| |
|
|
| 2.1 |
The
Merger. |
17 |
| |
|
|
| 2.2 |
Effects
of the Merger |
17 |
| |
|
|
| 2.3 |
Closing;
First Effective Time; Second Effective Time. |
17 |
| |
|
|
| 2.4 |
Organizational
Documents; Directors and Officers. |
17 |
| |
|
|
| 2.5 |
Conversion
of Company Common Stock. |
18 |
| |
|
|
| 2.6 |
Dissenting
Shares. |
20 |
| |
|
|
| 2.7 |
Closing
of the Company’s Transfer Books. |
20 |
| |
|
|
| 2.8 |
Surrender
of Company Common Stock. |
20 |
| |
|
|
| 2.9 |
Calculation
of Net Cash and Company Valuation. |
21 |
| |
|
|
| 2.10 |
Further
Action. |
21 |
| |
|
|
| 2.11 |
Tax
Consequences. |
21 |
| |
|
|
| 2.12 |
Withholding. |
21 |
| |
|
|
| Section
3. |
Representations
and Warranties of the Company. |
22 |
| |
|
|
| 3.1 |
Due
Organization; Subsidiaries. |
22 |
| |
|
|
| 3.2 |
Organizational
Documents. |
22 |
| |
|
|
| 3.3 |
Authority;
Binding Nature of Agreement |
22 |
| |
|
|
| 3.4 |
Vote
Required |
22 |
| |
|
|
| 3.5 |
Non-Contravention;
Consents. |
23 |
| |
|
|
| 3.6 |
Capitalization. |
23 |
| |
|
|
| 3.7 |
Financial
Statements. |
24 |
| |
|
|
| 3.8 |
Absence
of Changes. |
25 |
| |
|
|
| 3.9 |
Absence
of Undisclosed Liabilities |
26 |
| |
|
|
| 3.10 |
Title
to Assets |
26 |
| |
|
|
| 3.11 |
Real
Property; Leasehold |
27 |
| |
|
|
| 3.12 |
Intellectual
Property. |
27 |
| |
|
|
| 3.13 |
Agreements,
Contracts and Commitments. |
29 |
| |
|
|
| 3.14 |
Compliance;
Permits; Restrictions. |
30 |
| |
|
|
| 3.15 |
Legal
Proceedings; Orders. |
32 |
| |
|
|
| 3.16 |
Tax
Matters. |
32 |
| |
|
|
| 3.17 |
Employee
and Labor Matters; Benefit Plans. |
33 |
| |
|
|
| 3.18 |
Environmental
Matters. |
34 |
| |
|
|
| 3.19 |
Insurance. |
35 |
| 3.20 |
No
Financial Advisors. |
35 |
| |
|
|
| 3.21 |
Transactions
with Affiliates. |
35 |
| |
|
|
| 3.22 |
Privacy
and Data Security. |
35 |
| |
|
|
| 3.23 |
Accredited
Investor Status |
36 |
| |
|
|
| 3.24 |
No
Other Representations or Warranties |
36 |
| |
|
|
| Section
4. |
Representations
and Warranties of MEDS and Merger Subs. |
36 |
| |
|
|
| 4.1 |
Due
Organization; Subsidiaries. |
36 |
| |
|
|
| 4.2 |
Organizational
Documents. |
37 |
| |
|
|
| 4.3 |
Authority;
Binding Nature of Agreement |
37 |
| |
|
|
| 4.4 |
Vote/Consent
Required. |
37 |
| |
|
|
| 4.5 |
Non-Contravention;
Consents. |
37 |
| |
|
|
| 4.6 |
Capitalization. |
38 |
| |
|
|
| 4.7 |
SEC
Filings; Financial Statements. |
39 |
| |
|
|
| 4.8 |
Absence
of Changes |
41 |
| |
|
|
| 4.9 |
Absence
of Undisclosed Liabilities. |
42 |
| |
|
|
| 4.10 |
Title
to Assets |
43 |
| |
|
|
| 4.11 |
Real
Property; Leasehold. |
43 |
| |
|
|
| 4.12 |
Intellectual
Property. |
43 |
| |
|
|
| 4.13 |
Agreements,
Contracts and Commitments. |
45 |
| |
|
|
| 4.14 |
Compliance;
Permits; Restrictions. |
47 |
| |
|
|
| 4.15 |
Legal
Proceedings; Orders. |
48 |
| |
|
|
| 4.16 |
Tax
Matters. |
49 |
| |
|
|
| 4.17 |
Employee
and Labor Matters; Benefit Plans. |
50 |
| |
|
|
| 4.18 |
Environmental
Matters |
52 |
| |
|
|
| 4.19 |
Insurance. |
52 |
| |
|
|
| 4.20 |
Transactions
with Affiliates. |
53 |
| |
|
|
| 4.21 |
No
Financial Advisors |
53 |
| |
|
|
| 4.22 |
Valid
Issuance; No Bad Actor |
53 |
| |
|
|
| 4.23 |
Privacy
and Data Security |
53 |
| |
|
|
| 4.24 |
No
Other Representations or Warranties. |
53 |
| |
|
|
| Section
5. |
Agreements
of the Parties. |
53 |
| |
|
|
| 5.1 |
Information
Statement. |
53 |
| |
|
|
| 5.2 |
Conversion,
Name Change and Stock Plan Share Increase. |
54 |
| |
|
|
| 5.3 |
Employment
and Benefit Matters. |
54 |
| |
|
|
| 5.4 |
Indemnification
of Officers and Directors. |
55 |
| |
|
|
| 5.5 |
Tax
Matters. |
56 |
| |
|
|
| 5.6 |
Legends. |
56 |
| |
|
|
| 5.7 |
Officers
and Directors. |
56 |
| |
|
|
| 5.8 |
Section
16 Matters. |
56 |
| 5.9 |
Allocation
Certificate |
56 |
| |
|
|
| 5.10 |
Subsequent
Financings |
57 |
| |
|
|
| 5.11 |
Obligations
of Merger Subs. |
57 |
| |
|
|
| 5.12 |
Transfer
of Funds. |
57 |
| |
|
|
| 5.13 |
Shares
under 2019 Stock Plan |
57 |
| |
|
|
| 5.14 |
Reservation
of Shares for NVK Conversion |
57 |
| |
|
|
| 5.15 |
Conversion
of Series B Preferred Stock |
57 |
| |
|
|
| Section
6. |
Conditions
Precedent to Obligations of Each Party. |
57 |
| |
|
|
| 6.1 |
No
Restraints. |
57 |
| |
|
|
| 6.2 |
Company
Stockholder Approval. |
57 |
| |
|
|
| 6.3 |
Listing |
57 |
| |
|
|
| 6.4 |
MEDS
Cash |
57 |
| |
|
|
| 6.5 |
Certificate
of Designation |
58 |
| |
|
|
| 6.6 |
Exchange
Agent Agreement |
58 |
| |
|
|
| Section
7. |
Closing
Deliveries of the Company. |
58 |
| |
|
|
| Section
8. |
Closing
Deliveries of MEDS. |
58 |
| |
|
|
| Section
9. |
Miscellaneous
Provisions. |
58 |
| |
|
|
| 9.1 |
Non-Survival
of Representations and Warranties. |
58 |
| |
|
|
| 9.2 |
Amendment. |
59 |
| |
|
|
| 9.3 |
Waiver. |
59 |
| |
|
|
| 9.4 |
Fees
and Expenses |
59 |
| |
|
|
| 9.5 |
Entire
Agreement; Counterparts; Exchanges by Electronic Transmission or Facsimile. |
59 |
| |
|
|
| 9.6 |
Applicable
Law; Jurisdiction |
59 |
| |
|
|
| 9.7 |
Assignability |
59 |
| |
|
|
| 9.8 |
Notices. |
60 |
| |
|
|
| 9.9 |
Cooperation. |
60 |
| |
|
|
| 9.10 |
Severability. |
60 |
| |
|
|
| 9.11 |
Other
Remedies; Specific Performance. |
61 |
| |
|
|
| 9.12 |
No
Third-Party Beneficiaries. |
61 |
| |
|
|
| 9.13 |
Waiver
of Jury Trial. |
61 |
Exhibits:
| Exhibit
A |
|
Form
of Lock-Up Agreement |
| |
|
|
| Exhibit
B |
|
Form
of Certificate of Designation |
AGREEMENT
AND PLAN OF MERGER
THIS
AGREEMENT AND PLAN OF MERGER (this “Agreement”) is made and entered into as of July 25, 2024, by and among TRxADE Health
INC., a Delaware corporation (“MEDS”), MEDS MERGER SUB I, Inc., a Delaware corporation and wholly owned subsidiary
of MEDS (“Merger Sub I”), MEDS MERGER SUB II, LLC, a Delaware limited liability company and wholly owned subsidiary of
MEDS (“Merger Sub II” and together with Merger Sub I, “Merger Subs”), and Scienture, Inc., a Delaware corporation
(the “Company”). Certain capitalized terms used in this Agreement are defined in Section 1.
RECITALS
A.
MEDS and the Company intend to effect a merger of Merger Sub I with and into the Company (the “First Merger”) in accordance with
this Agreement and the DGCL. Upon consummation of the Merger, Merger Sub will cease to exist and the Company will become a wholly owned
subsidiary of MEDS.
B.
Immediately following the First Merger and as part of the same overall transaction as the First Merger, the Company will merge with and
into Merger Sub II (the “Second Merger” and, together with the First Merger, the “Merger”), with Merger Sub II being the
surviving entity of the Second Merger.
C.
The Parties intend that the First Merger and Second Merger, taken together, will constitute an integrated transaction described in Rev.
Rul. 2001-46, 2001-2 C.B. 321 that qualifies as a “reorganization” within the meaning of Section 368(a) of the Code and the Treasury
Regulations, and that this Agreement be, and hereby is, adopted as a “plan of reorganization” for the purposes of Section 368 of the
Code and Treasury Regulations Section 1.368-2(g).
D.
The MEDS Board has (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of MEDS and its
stockholders, (ii) approved and declared advisable this Agreement and the Contemplated Transactions, including the issuance of shares
of MEDS Capital Stock to the shareholders of the Company pursuant to the terms of this Agreement and (iii) determined to recommend, upon
the terms and subject to the conditions set forth in this Agreement, that the stockholders of MEDS consent to the conversion of the MEDS
Preferred Stock issued pursuant to this Agreement into shares of MEDS Common Stock (the “Conversion”), authorize the MEDS Board
to change MEDS’ name to “Scienture Holdings, Inc.” subsequent to the Conversion (the “Name Change”), and consent to increasing
the number of shares available to be awarded under the 2019 Stock Plan to five million (5,000,000) shares of MEDS Common Stock (the “Stock
Plan Share Increase”).
E.
The Merger Sub I Board has (i) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of Merger
Sub I and its sole shareholder, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined
to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the shareholders of Merger Sub I votes to
adopt this Agreement and thereby approve the Contemplated Transactions.
F.
The Merger Sub II Board has (i) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of Merger
Sub II and its sole member, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined
to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the member of Merger Sub II votes to adopt
this Agreement and thereby approve the Contemplated Transactions.
G.
The Company Board has (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of the Company
and its shareholders, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined to recommend,
upon the terms and subject to the conditions set forth in this Agreement, that the shareholders of the Company vote to adopt this Agreement
and thereby approve the Contemplated Transactions.
H.
Concurrently with the execution and delivery of this Agreement and as a condition and inducement to MEDS’s and the Company’s willingness
to enter into this Agreement, all of the executive officers and members of the MEDS Board following the approval of the Conversion, as
well as certain affiliates of the same, all of which are listed on Section A of the Company Disclosure Schedule, are executing
lock-up agreements in substantially the form attached hereto as Exhibit A (the “Lock-Up Agreement,” and collectively, the
“Lock-Up Agreements”).
I.
Concurrently with the execution and delivery of this Agreement, the shareholders of the Company sufficient to adopt and approve this
Agreement and the First Merger as required under the DGCL and the Company’s Organizational Documents are executing and delivering an
action by written consent in form and substance reasonably acceptable to MEDS in order to obtain the Required Company Shareholder Vote
(each, a “Company Shareholder Written Consent” and collectively, the “Company Shareholder Written Consents”).
J.
Concurrently with the execution and delivery of this Agreement, the shareholders of MEDS sufficient to approve the Conversion, the Name
Change and the Stock Plan Share Increase as required under the DGCL and MEDS’ Organizational Documents are executing and delivering the
Required MEDS Stockholder Consent or satisfying the Required MEDS Stockholder Vote, each, as defined below.
K.
Concurrently with the execution and delivery of this Agreement, MEDS shall enter into consulting agreements with Suren Ajjarapu and Prashant
Patel, the terms of which are reasonably acceptable to the Company (the “Consulting Agreements”).
L.
In preparation for consummating this Agreement and the Contemplated Transactions, the Company consummated the Company Series Seed Preferred
Conversion, as defined below, resulting in Company Common Stock being the sole remaining issued and outstanding Company Capital Stock,
and consummated the Company Warrant Termination, as defined below, resulting in the termination of all issued and outstanding warrants
in exchange for shares of Company Capital Stock.
AGREEMENT
The
Parties, intending to be legally bound, agree as follows:
Section
1. Definitions and Interpretative Provisions.
1.1
Definitions.
(a)
For purposes of the Agreement (including this Section 1):
“Affiliate”
shall have the meaning given to such term in Rule 145 under the Securities Act.
“Affordable
Care Act” means the Patient Protection and Affordable Care Act.
“Allocation
Certificate” shall have the meaning set forth in Section 5.9.
“Business
Day” means any day other than a day on which banks in the State of New York are authorized or obligated to be closed.
“Cash
and Cash Equivalents” means all (a) cash and cash equivalents and (b) marketable securities, in each case determined in accordance
with GAAP.
“Certificate
of Designation” means a certificate of designation for MEDS Preferred Stock in the form attached hereto as Exhibit B.
“COBRA”
means the Consolidated Omnibus Budget Reconciliation Act of 1985, as set forth in Section 4980B of the Code and Part 6 of Title I of
ERISA.
“Code”
means the Internal Revenue Code of 1986, as amended.
“Company
Board” means the board of directors (as such term is used in the DGCL) of the Company.
“Company
Capital Stock” means the Company Common Stock and Series Seed Preferred Stock.
“Company
Common Stock” means the common stock, $0.001 par value per share, of the Company.
“Company
Contract” means any Contract: (i) to which the Company is a Party, (ii) by which the Company or any Company IP Rights or other asset
of the Company is or may become bound or under which the Company has, or may become subject to, any obligation, or (iii) under which
the Company has or may acquire any right or interest.
“Company
IP Rights” means all Intellectual Property owned, licensed, or controlled by the Company that is necessary for, or used or held for
use in, the operation of the business of the Company as presently conducted.
“Company
IP Rights Agreement” means any Contract governing, related to or pertaining to any Company IP Rights other than any confidential
information provided under confidentiality agreements.
“Company
Material Adverse Effect” means any Effect that, considered together with all other Effects that have occurred prior to the date of
determination of the occurrence of a Company Material Adverse Effect, (i) has or would reasonably be expected to have a material adverse
effect on the business, financial condition or results of operations of the Company or (ii) materially impairs the ability of the Company
to consummate the Merger or any of the Contemplated Transactions; provided, however, that, in the case of clause (i) Effects
arising or resulting from the following shall not be taken into account in determining whether there has been a Company Material Adverse
Effect: (a) the announcement of the Agreement or the pendency of the Contemplated Transactions, (b) the taking of any action, or the
failure to take any action, by the Company that is required to comply with the terms of the Agreement, (c) any natural disaster or epidemics,
pandemics or other force majeure events, or any act or threat of terrorism or war, any armed hostilities or terrorist activities (including
any escalation or general worsening of any of the foregoing) anywhere in the world or any governmental or other response or reaction
to any of the foregoing, (d) any change in GAAP or applicable Law or the interpretation thereof, (e) general economic or political conditions
or conditions generally affecting the industries in which the Company operates, or the economy or financial, debt, banking, capital,
credit or securities markets, in the United States, including effects on such industries, economy or markets resulting from any regulatory
and political conditions or developments in general, or (f) any change in the cash position of the Company which results from operations
in the Ordinary Course of Business; except in each case with respect to clauses (c), (d) and (e), to the extent disproportionately affecting
the Company, relative to other similarly situated companies in the industries in which the Company operates.
“Company
Series Seed Preferred Stock” means the Series Seed Preferred Stock, $0.0001 par value per share, of the Company.
“Company
Series Seed Preferred Conversion” means that certain event that occurred on the date hereof, in which all of the issued and outstanding
shares of Company Series Seed Preferred Stock were converted to shares of Company Common Stock pursuant to the organizational documents
and the proper voting procedures.
“Company
Shareholder Written Consent” shall have the meaning set forth in the recitals.
“Company
Merger Shares” means, subject to Section 2.5(e), the product determined by multiplying
(i) the Post-Closing MEDS Shares by (ii) the Company Allocation Percentage,
in which:
| |
● |
“Aggregate
Valuation” means the sum of (i) the Company Valuation, plus (ii) the MEDS Valuation. |
| |
● |
“Company
Allocation Percentage” means the quotient (rounded to four decimal places) determined by dividing (i) the Company
Valuation by (ii) the Aggregate Valuation. |
| |
● |
“Company
Valuation” means $83,670,000. |
| |
● |
“Lower
MEDS Net Cash Amount” means, if MEDS Net Cash is less than the Target MEDS Net Cash, then the amount, if any, that the Target
MEDS Net Cash exceeds the MEDS Net Cash. |
| |
● |
“Post-Closing
MEDS Shares” means the quotient determined by dividing (i) the MEDS Outstanding Shares by (ii) the
MEDS Allocation Percentage. |
| |
● |
“MEDS
Allocation Percentage” means the quotient (rounded to four decimal places) determined by dividing (i) the MEDS
Valuation by (ii) the Aggregate Valuation. |
| |
● |
“MEDS
Equity Value” means $20,000,000. |
| |
● |
“MEDS
Outstanding Shares” means, subject to Section 2.5(e), the total number of shares of MEDS Common Stock outstanding immediately
prior to the First Effective Time expressed on a fully-diluted basis, and assuming, without limitation or duplication, the issuance
of shares of MEDS Common Stock in respect of all MEDS Options, warrants or other rights to receive shares, whether conditional or
unconditional, that will be outstanding as of immediately prior to the First Effective Time. Notwithstanding any of the foregoing,
(i) MEDS Options with an exercise price greater than $30.00 per share (as adjusted for any stock splits or reverse stock splits as
of the date hereof) and (ii) and (ii) MEDS Series B Preferred Stock shall not be included in the total number of shares of MEDS Common
Stock outstanding for purposes of determine the MEDS Outstanding Shares. |
| |
|
Set
forth on Section 1.1(a)(i) of the MEDS Disclosure Schedule is the calculation of “MEDS Outstanding Shares.” |
| |
● |
“MEDS
Valuation” means (i) MEDS Equity Value minus (ii) the Lower MEDS Net Cash Amount (if any) plus (iii) the Upper
MEDS Net Cash Amount (if any). |
| |
● |
“Target
MEDS Net Cash” means $2,000,000, plus an amount equal to a good faith estimate of expected MEDS operating costs for forty-five
(45) days post-closing (including any additional transaction costs related to SEC and Nasdaq matters related to the Contemplated
Transactions). |
| |
● |
“Upper
MEDS Net Cash Amount” means, if MEDS Net Cash is greater than the Target MEDS Net Cash, then the amount, if any, that the MEDS
Net Cash exceeds the Target MEDS Net Cash. |
For
the avoidance of doubt, set forth in Section 1.1(a)(ii) of the MEDS Disclosure Schedule is an illustrative example of the calculation
of “Company Merger Shares.”
“Company
Registered IP” means all Company IP Rights that are owned or exclusively licensed by the Company that are registered, filed or issued
under the authority of, with or by any Governmental Authority, including all patents, registered copyrights and registered trademarks
and all applications and registrations for any of the foregoing.
“Company
Unaudited Interim Balance Sheet” means the estimated unaudited statement of assets, liabilities and partner’s capital of the Company
for the period since January 1, 2024 through June 30, 2024.
“Company
Warrant Termination” means that certain event that occurred on the date hereof, in which all of the issued and outstanding warrants
for Company Capital Stock were terminated pursuant to the terms hereof in exchange for the issuance of shares of Company Common Stock.
“Confidentiality
Agreement” means the Confidentiality Agreement dated March 26, 2024, between the Company and MEDS.
“Consent”
means any approval, consent, ratification, permission, waiver or authorization (including any Governmental Authorization).
“Contemplated
Transactions” means the Merger and the other transactions contemplated by the Agreement, including the Conversion, the Name Change
and the Stock Plan Share Increase.
“Contract”
means, with respect to any Person, any written or oral bond, debenture, note, indenture, guarantee, lease (whether for real or personal
property), mortgage, license, purchase or sale order, or other legally binding contract, commitment, agreement, instrument, obligation,
arrangement, understanding, undertaking, permit, concession or franchise of any nature to which such Person is a party or by which such
Person or any of its assets are bound or affected under applicable Law.
“DGCL”
means the General Corporation Law of the State of Delaware.
“DLLCA”
means the Delaware Limited Liability Company Act.
“Effect”
means any effect, change, event, circumstance, or development.
“Employee
Plan” means (i) an “employee benefit plan” within the meaning of Section 3(3) of ERISA whether or not subject to ERISA; (ii) other
plan, program, policy or arrangement providing for stock options, stock purchases, equity-based compensation, bonuses (including any
annual bonuses and retention bonuses) or other incentives, severance pay, deferred compensation, employment, compensation, change in
control or transaction bonuses, supplemental, vacation, retirement benefits (including post-retirement health and welfare benefits),
pension benefits, profit-sharing benefits, fringe benefits, life insurance benefits, perquisites, health benefits, medical benefits,
dental benefits, vision benefits, and all other employee benefit plans, agreements, and arrangements, not described in (i) above; and
(iii) all other plans, programs, policies or arrangements providing compensation to employees, consultants and non-employee directors.
“Encumbrance”
means any lien, pledge, hypothecation, charge, mortgage, security interest, lease, exclusive license, option, easement, reservation,
servitude, adverse title, claim, infringement, interference, option, right of first refusal, preemptive right, community property interest
or restriction or encumbrance of any nature (including any restriction on the voting of any security, any restriction on the transfer
of any security or other asset, any restriction on the receipt of any income derived from any asset, any restriction on the use of any
asset and any restriction on the possession, exercise or transfer of any other attribute of ownership of any asset).
“Enforceability
Exceptions” means the (i) Laws of general application relating to bankruptcy, insolvency and the relief of debtors and (ii) rules
of law governing specific performance, injunctive relief and other equitable remedies.
“Entity”
means any corporation (including any nonprofit corporation), partnership (including any general partnership, limited partnership or limited
liability partnership), joint venture, estate, trust, company (including any company limited by shares, limited liability company or
joint stock company), firm, society or other enterprise, association, organization or entity, and each of its successors.
“Environmental
Law” means any federal, state, local or foreign Law relating to pollution or protection of human health or the environment (including
ambient air, surface water, ground water, land surface or subsurface strata), including any law or regulation relating to emissions,
discharges, releases or threatened releases of Hazardous Materials, or otherwise relating to the manufacture, processing, distribution,
use, treatment, storage, disposal, transport or handling of Hazardous Materials.
“ERISA”
means the Employee Retirement Income Security Act of 1974, as amended.
“ERISA
Affiliate” means, with respect to any Entity, any other Person that would be treated as a single employer with such Entity or part
of the same “controlled group” as such Entity under Sections 414(b), (c), (m), or (o) of the Code.
“Exchange
Act” means the Securities Exchange Act of 1934, as amended.
“Governmental
Authority” means any: (i) nation, state, commonwealth, province, territory, county, municipality, district or other jurisdiction
of any nature, (ii) federal, state, local, municipal, foreign, supra-national or other government, (iii) governmental or quasi-governmental
authority of any nature (including any governmental division, department, agency, commission, bureau, instrumentality, official, ministry,
fund, foundation, center, organization, unit, body or Entity and any court or other tribunal, and for the avoidance of doubt, any taxing
authority) or (iv) self-regulatory organization (including Nasdaq).
“Governmental
Authorization” means any: (i) permit, license, certificate, franchise, permission, variance, exception, order, approval, clearance,
registration, qualification or authorization issued, granted, given or otherwise made available by or under the authority of any Governmental
Authority or pursuant to any Law or (ii) right under any Contract with any Governmental Authority.
“Hazardous
Materials” means any pollutant, chemical, substance and any toxic, infectious, carcinogenic, reactive, corrosive, ignitable or flammable
chemical, or chemical compound, or hazardous substance, material or waste, whether solid, liquid or gas, that is subject to regulation,
control or remediation under any Environmental Law, including without limitation, crude oil or any fraction thereof, and petroleum products
or by-products.
“Intellectual
Property” means: (i) United States, foreign and international patents, patent applications, including all provisionals, nonprovisionals,
substitutions, divisionals, continuations, continuations-in-part, reissues, extensions, supplementary protection certificates, reexaminations,
term extensions, certificates of invention and the equivalents of any of the foregoing, statutory invention registrations, invention
disclosures and inventions (collectively, “Patents”), (ii) trademarks, service marks, trade names, domain names, corporate names,
brand names, URLs, trade dress, logos and other source identifiers, including registrations and applications for registration thereof
and goodwill associated therewith, (iii) copyrights, including registrations and applications for registration thereof, (iv) software,
including all source code, object code and related documentation, (v) formulae, customer lists, trade secrets, know-how, confidential
information and other proprietary rights and intellectual property, whether patentable or not, and (vi) all United States and foreign
rights arising under or associated with any of the foregoing.
“IRS”
means the United States Internal Revenue Service.
“Key
Employee” means (i) an executive officer of MEDS; and (ii) any employee of MEDS that reports directly to the board of directors of
MEDS or to an executive officer of MEDS.
“Knowledge”
means, with respect to an individual, that such individual is actually aware of the relevant fact or such individual would reasonably
be expected to know or discover or otherwise become aware of such fact in the ordinary course of the performance of such individual’s
employment responsibilities or conducting a reasonably comprehensive investigation, consistent with such individual’s title or responsibilities,
concerning the existence of the relevant matter. Any Person that is an Entity shall have Knowledge if any executive officer or director
of such Person as of the date such knowledge is imputed has or should reasonably be expected to have Knowledge of such fact or other
matter. With respect to any matters relating to Intellectual Property, such awareness or reasonable expectation to have knowledge does
not require any such individual to conduct or have conducted or obtain or have obtained any freedom to operate opinions of counsel or
any Intellectual Property rights clearance searches.
“Law”
means any federal, state, national, supra-national, foreign, local or municipal or other law, statute, constitution, principle of common
law, resolution, ordinance, code, edict, decree, rule, regulation, ruling or requirement issued, enacted, adopted, promulgated, implemented
or otherwise put into effect by or under the authority of any Governmental Authority (including under the authority of Nasdaq or the
Financial Industry Regulatory Authority).
“Legal
Proceeding” means any action, suit, litigation, arbitration, proceeding (including any civil, criminal, administrative, investigative
or appellate proceeding), hearing, inquiry, audit, examination or investigation commenced, brought, conducted or heard by or before,
or otherwise involving, any court or other Governmental Authority or any arbitrator or arbitration panel.
“MEDS
Associate” means any current or former employee, independent contractor, officer or director of MEDS or any of its Subsidiaries
“MEDS
Balance Sheet” means the audited balance sheet of MEDS as of December 31, 2023, included in MEDS’s Report on Form 10-K for the year
ended December 31, 2023, as filed with the SEC.
“MEDS
Board” means the board of directors of MEDS.
“MEDS
Capital Stock” means the MEDS Common Stock and MEDS Preferred Stock.
“MEDS
Common Stock” means the common stock, $0.0001 par value per share, of MEDS.
“MEDS
Contract” means any Contract: (i) to which MEDS or any of its Subsidiaries is a party, (ii) by which MEDS or any of its Subsidiaries
or any MEDS IP Rights or any other asset of MEDS or its Subsidiaries is or may become bound or under which MEDS has, or may become subject
to, any obligation, or (iii) under which MEDS or any of its Subsidiaries has or may acquire any right or interest.
“MEDS
Covered Person” means, with respect to MEDS as an “issuer” for purposes of Rule 506 promulgated under the Securities Act, any Person
listed in the first paragraph of Rule 506(d)(1).
“MEDS
Employee Plan” means any Employee Plan that MEDS or any of its Subsidiaries (i) sponsors, maintains, administers, or contributes
to, or (ii) provides benefits under or through, or (iii) has any obligation to contribute to or provide benefits under or through, or
(iv) may reasonably be expected to have any Liability, or (v) utilizes to provide benefits to or otherwise cover any current or former
employee, officer, director or other service provider of MEDS or any of its Subsidiaries (or their spouses, dependents, or beneficiaries).
“MEDS
IP Rights” means all Intellectual Property owned, licensed or controlled by MEDS that is necessary for, or used or held for use in,
the operation of the business of MEDS as presently conducted.
“MEDS
IP Rights Agreement” means any Contract governing, related or pertaining to any MEDS IP Rights.
“MEDS
Material Adverse Effect” means any Effect that, considered together with all other Effects that have occurred prior to the date of
determination of the occurrence of the MEDS Material Adverse Effect, (i) has or would reasonably be expected to have a material adverse
effect on the business, financial condition, assets, liabilities or results of operations of MEDS or any of its Subsidiaries, taken as
a whole or (ii) materially impairs the ability of MEDS to consummate the Merger or any of the Contemplated Transactions; provided,
however, that, in the case of clause (i); provided, however, that Effects arising or resulting from the following
shall not be taken into account in determining whether there has been a MEDS Material Adverse Effect: (a) the announcement of the Agreement
or the pendency of the Contemplated Transactions, (b) any change in the stock price or trading volume of MEDS Common Stock (it being
understood, however, that any Effect causing or contributing to any change in stock price or trading volume of MEDS Common Stock may
be taken into account in determining whether a MEDS Material Adverse Effect has occurred, unless such Effects are otherwise excepted
from this definition), (c) the taking of any action, or the failure to take any action, by MEDS that is required to comply with the terms
of the Agreement, (d) any natural disaster or epidemics, pandemics or other force majeure events, or any act or threat of terrorism or
war, any armed hostilities or terrorist activities (including any escalation or general worsening of any of the foregoing) anywhere in
the world, or any governmental or other response or reaction to any of the foregoing, (e) any change in GAAP or applicable Law or the
interpretation thereof or (f) general economic or political conditions or conditions generally affecting the industries in which MEDS
or any of its Subsidiaries operates, or the economy or financial, debt, banking, capital, credit or securities markets, in the United
States, including effects on such industries, economy or markets resulting from any regulatory and political conditions or developments
in general; except, in each case with respect to clauses (d), (e) and (f), to the extent materially and disproportionately affecting
MEDS and any its Subsidiaries, taken as a whole, relative to other similarly situated companies in the industries in which MEDS or any
of its Subsidiaries operates. Notwithstanding the above, a delisting of MEDS Common Stock on Nasdaq shall constitute a MEDS Material
Adverse Effect, provided that the Company has not refused or unreasonably delayed its consent to reasonable actions by MEDS to maintain
the listing of MEDS Common Stock on Nasdaq.
“MEDS
Net Cash” means the amount, whether positive or negative, without duplication, (i) MEDS’s Cash and Cash Equivalents determined, to
the extent in accordance with GAAP, in a manner consistent with the manner in which such items were historically determined and in accordance
with the financial statements (including any related notes) contained or incorporated by reference in the MEDS SEC Documents and the
MEDS Balance Sheet, minus (ii) the sum of MEDS’s consolidated short-term and long-term contractual obligations accrued
at the Closing Date (but excluding deferred revenue), in each case determined in accordance with GAAP and, to the extent in accordance
with GAAP, in a manner consistent with the manner in which such items were historically determined in accordance with the financial statements
(including any related notes) contained or incorporated by reference in the MEDS SEC Documents and the MEDS Balance Sheet, minus
(iii) fees and expenses of MEDS incurred in connection with the Contemplated Transactions, including for the avoidance of doubt,
Transaction Expenses of MEDS to the extent unpaid as of the Closing, minus (iv) any and all Liabilities of MEDS (a) to
any current or former officer, director, employee, consultant or independent contractor (including change of control payments, retention
payments, severance and other employee-, consultant- or independent contractor-related termination costs, or other payments) of MEDS
or any of its Subsidiaries, or (b) pursuant to any MEDS Employee Plan, including deferred compensation, accrued but unpaid bonuses and
accrued but unpaid vacation or paid time off (including related employer employment taxes on all the foregoing), minus
(v) all Liabilities related to MEDS’s or any of its Subsidiaries’ lease obligations, plus (vi) all costs and expenses relating
to the winding down of MED’s or any of its Subsidiaries’ prior research and development activities, plus (vii) all prepaid
expenses set forth on Section 1.1(a)(iii) of the MEDS Disclosure Schedule, plus (viii) expenses paid, or Liabilities
incurred, prior to Closing, that are approved in writing to be covered by MEDS’s D&O insurance in excess of the deductible and within
overall policy limits, minus (ix) any deductibles paid under applicable insurance policies taken out by MEDS or any of
its Subsidiaries, plus (x) deposits set forth on Section 1.1(a)(iv) of the MEDS Disclosure Schedule, and minus
(xi) any unpaid Taxes of MEDS and its Subsidiaries for Tax periods (or pre-Closing portions thereof) ending on or before the
Closing Date determined in a manner consistent with past practice (to the extent such past practice is consistent with applicable law),
including any payroll Taxes payable as a result of the vesting of each outstanding and unvested MEDS Restricted Stock Unit pursuant to
Section 5.5). For avoidance of doubt, the Cash and Cash Equivalents received in Subsequent Financings will be excluded from the
calculation of MEDS Net Cash.
“MEDS
Options” means options or other rights to purchase shares of MEDS Common Stock granted by MEDS, including pursuant to any MEDS Stock
Plan or as an “inducement” award.
“MEDS
Preferred Stock” means the Series X Non-Voting Convertible Preferred Stock, $0.00001 par value per share, of MEDS.
“MEDS
Registered IP” means all MEDS IP Rights that are owned or exclusively licensed by MEDS that are registered, filed or issued under
the authority of, with or by any Governmental Authority, including all patents, registered copyrights and registered trademarks and all
applications for any of the foregoing.
“MEDS
Restricted Stock Units” means any equity award with respect to MEDS Common Stock that represents the right to receive in the future
shares of MEDS Common Stock pursuant to any MEDS Stock Plan.
“MEDS
Stockholder Support Agreements” shall have the meaning set forth in the recitals.
“Merger
Sub I Board” means the board of directors of Merger Sub I.
“Merger
Sub II Board” means the board of directors of Merger Sub II.
“Multiemployer
Plan” means a “multiemployer plan,” as defined in Section 3(37) or 4001(a)(3) of ERISA.
“Multiple
Employer Plan” means a “multiple employer plan” within the meaning of Section 413(c) of the Code or Section 3(40) of ERISA.
“Multiple
Employer Welfare Arrangement” means a “multiple employer welfare arrangement” within the meaning of Section 3(40) of ERISA.
“Nasdaq”
means The Nasdaq Stock Market.
“Order”
means any judgment, order, writ, injunction, ruling, decision or decree of (that is binding on a Party), or any plea agreement, corporate
integrity agreement, resolution agreement, or deferred prosecution agreement with, or any settlement under the jurisdiction of, any court
or Governmental Authority.
“Ordinary
Course of Business” means, in the case of each of the Company and MEDS, such actions taken in the ordinary course of its normal operations
and consistent with its past practices.
“Organizational
Documents” means, with respect to any Person (other than an individual), (i) the certificate or articles of association or incorporation
or organization or limited partnership or limited liability company, and any joint venture, limited liability company, operating or partnership
agreement and other similar documents adopted or filed in connection with the creation, formation or organization of such Person and
(ii) all bylaws, regulations and similar documents or agreements relating to the organization or governance of such Person, in each case,
as amended or supplemented.
“Party”
or “Parties” means the Company, Merger Sub I, Merger Sub II and MEDS.
“Permitted
Encumbrance” means (i) any statutory liens for current Taxes not yet due and payable or for Taxes that are being contested in good
faith and for which adequate reserves have been made on the Company Unaudited Interim Balance Sheet or the MEDS Unaudited Interim Balance
Sheet, as applicable, in accordance with GAAP, (ii) minor liens that have arisen in the Ordinary Course of Business and that do not (in
any case or in the aggregate) materially detract from the value of the assets subject thereto or materially impair the operations of
the Company or MEDS, as applicable, (iii) statutory liens to secure obligations to landlords, lessors or renters under leases or rental
agreements, (iv) deposits or pledges made in connection with, or to secure payment of, workers’ compensation, unemployment insurance
or similar programs mandated by Law, (v) statutory liens in favor of carriers, warehousemen, mechanics and materialmen, to secure claims
for labor, materials or supplies and (vi) liens arising under applicable securities Law.
“Person”
means any individual, Entity or Governmental Authority.
“Personal
Information” means data and information concerning an identifiable natural person.
“Privacy
Laws” mean Laws relating to privacy, security and/or collection, use or other processing of Personal Information.
“Representatives”
means directors, officers, employees, agents, attorneys, accountants, investment bankers, advisors and representatives.
“Sarbanes-Oxley
Act” means the Sarbanes-Oxley Act of 2002.
“SEC”
means the United States Securities and Exchange Commission.
“Securities
Act” means the Securities Act of 1933, as amended.
An
Entity shall be deemed to be a “Subsidiary” of a Person if such Person directly or indirectly owns or purports to own, beneficially
or of record, (i) an amount of voting securities or other interests in such Entity that is sufficient to enable such Person to elect
at least a majority of the members of such entity’s board of directors or other governing body or (ii) at least 50% of the outstanding
equity, voting, beneficial or financial interests in such Entity.
“Tax”
means (i) any federal, state, local, foreign or other tax, including any income tax, franchise tax, capital gains tax, gross receipts
tax, value-added tax, surtax, estimated tax, unemployment tax, national health insurance tax, excise tax, ad valorem tax, transfer tax,
stamp tax, sales tax, use tax, property tax, business tax, withholding tax, payroll tax, customs duty, alternative or add-on minimum
or other tax or similar charge (whether imposed directly or through withholding and whether or not disputed), and including any fine,
penalty, addition to tax, interest or additional amount imposed by a Governmental Authority with respect thereto (or attributable to
the nonpayment thereof) and (ii) any liability for payment of amounts described in clause (i) whether as a result of transferee or successor
liability, of being a member of an affiliated, consolidated, combined or unitary group for any period, pursuant to a Contract, through
operation of Law or otherwise.
“Tax
Return” means any return (including any information return), report, statement, declaration, claim or refund, estimate, schedule,
notice, notification, form, election, certificate or other document or information, and any amendment or supplement to any of the foregoing,
filed or required to be filed with any Governmental Authority (or provided to a payee) in connection with the determination, assessment,
collection or payment of any Tax or in connection with the administration, implementation or enforcement of or compliance with any Law
relating to any Tax.
“Transaction
Expenses” means, with respect to a Party, the aggregate amount (without duplication) of all costs, fees and expenses incurred by
such Party or any of its Subsidiaries (including Merger Subs), or for which such Party or any of its Subsidiaries are or may become liable
in connection with the Contemplated Transactions and the negotiation, preparation and execution of this Agreement or any other agreement,
document, instrument, filing, certificate, schedule, exhibit, letter or other document prepared or executed in connection with the Contemplated
Transactions, including (i) any fees and expenses of legal counsel and accountants, the maximum amount of fees and expenses payable to
financial advisors, investment bankers, brokers, consultants, tax advisors, transfer agents, proxy solicitor and other advisors of such
Party; and (ii) any bonus, retention payments, severance, change-in-control payments or similar payment obligations (including payments
with “single-trigger” provisions triggered at and as of the consummation of the Contemplated Transactions) that become due or payable
to any director, officer, employee or consultant in connection with the consummation of the Contemplated Transactions, together with
any payroll Taxes associated therewith.
“Treasury
Regulations” means the United States Treasury regulations promulgated under the Code.
(b)
Each of the following terms is defined in the Section set forth opposite such term:
Term |
|
Section |
| Agreement |
|
Preamble |
| Allocation
Certificate |
|
5.9 |
| Capitalization
Date |
|
4.6(a) |
| Cash
Determination Time |
|
2.9 |
| Certifications |
|
4.7(a) |
| Closing
Date |
|
2.3 |
| Company |
|
Preamble |
| Company
Employees |
|
5.3(b) |
| Company
Material Contract |
|
3.13(a) |
| Company
Permits |
|
3.14(b) |
| Company
Product Candidates |
|
3.14(d) |
| Company
Real Estate Leases |
|
3.11 |
| Company
Regulatory Permits |
|
3.14(d) |
| Company
Shareholder Written Consent |
|
Recitals |
| Consulting
Agreements |
|
Recitals |
| Conversion |
|
Recitals |
| D&O
Indemnified Parties |
|
5.4(a) |
| Dissenting
Shares |
|
2.6 |
| Disqualifying
Event |
|
4.22 |
| Drug
Regulatory Agency |
|
3.14(c) |
| Exchange
Agent |
|
2.8 |
| Exchange
Fund |
|
2.8 |
| Excluded
Shares |
|
2.5(b) |
| FDA |
|
3.14(c) |
| FDCA |
|
3.14(c) |
| First
Certificate of Merger |
|
2.3 |
| First
Effective Time |
|
2.3 |
| First
Merger |
|
Recitals |
| First
Step Surviving Company |
|
2.1 |
| Information
Statement |
|
5.1(a) |
| Lock-Up
Agreements |
|
Recitals |
| Loan
Agreement |
|
5.15 |
| MEDS |
|
Preamble |
| MEDS
2013 Plan |
|
4.6(c) |
| MEDS
2014 Plan |
|
4.6(c) |
| MEDS
2019 Plan |
|
4.6(c) |
| MEDS
Common Stock Consideration Cap |
|
2.5(g) |
| MEDS
Grant Date |
|
4.6(f) |
| MEDS
Material Contracts |
|
4.13(a) |
| MEDS
Net Cash Calculation |
|
2.9 |
| MEDS
Net Cash Schedule |
|
2.9 |
| MEDS
Product Candidates |
|
4.14(d) |
| MEDS
Regulatory Permits |
|
4.14(d) |
| MEDS
SEC Documents |
|
4.7(a) |
| MEDS
Stock Plans |
|
4.6(c) |
| Merger |
|
Recitals |
| Merger
Consideration |
|
2.5(c) |
| Merger
Sub I |
|
Preamble |
| Merger
Sub II |
|
Preamble |
| Merger
Subs |
|
Preamble |
| Name
Change |
|
Recitals |
| NVK |
|
5.15 |
| NVK
Reserved Shares |
|
5.15 |
| PHSA |
|
3.14(c) |
| Privacy
Policies |
|
3.22 |
| Required
Company Stockholder Vote |
|
3.4 |
| Required
MEDS Stockholder Consent |
|
4.4 |
| Required
MEDS Stockholder Vote |
|
4.4 |
| Second
Certificate of Merger |
|
2.3 |
| Second
Effective Time |
|
2.3 |
| Second
Merger |
|
Recitals |
| Series
B Preferred Stock |
|
4.6 |
| Subsequent
Financing |
|
5.10 |
| Surviving
Company |
|
2.1 |
| Stock
Plan Share Increase |
|
Recitals |
| Transfer |
|
5.12 |
| WARN
Act |
|
3.17(f) |
| Withholding
Agent |
|
2.12 |
| 409A
Plan |
|
4.17(j) |
1.2
Other Definitional and Interpretative Provisions. The words “hereof,” “herein” and “hereunder” and words of like import used in
this Agreement shall refer to this Agreement as a whole and not to any particular provision of this Agreement. The captions herein are
included for convenience of reference only and shall be ignored in the construction or interpretation hereof. References to Sections,
Exhibits and Schedules are to Sections, Exhibits and Schedules of this Agreement unless otherwise specified. Any capitalized terms used
in any Exhibit or Schedule but not otherwise defined therein shall have the meaning as defined in this Agreement. Any singular term in
this Agreement shall be deemed to include the plural, and any plural term the singular, the masculine gender shall include the feminine
and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter gender shall include masculine
and feminine gender. Whenever the words “include,” “includes” or “including” are used in this Agreement, they shall be deemed to be followed
by the words “without limitation,” whether or not they are in fact followed by those words or words of like import. The word “or” is
not exclusive. “Writing,” “written” and comparable terms refer to printing, typing and other means of reproducing words (including electronic
media) in a visible form. References to any agreement or Contract are to that agreement or Contract as amended, modified or supplemented
from time to time in accordance with the terms hereof and thereof. References to any Person include the successors and permitted assigns
of that Person. References to any statute are to that statute and to the rules and regulations promulgated thereunder, in each case as
amended, modified, re-enacted thereof, substituted, from time to time. References to “$” and “dollars” are to the currency of the United
States. All accounting terms used herein will be interpreted, and all accounting determinations hereunder will be made, in accordance
with GAAP unless otherwise expressly specified. References from or through any date shall mean, unless otherwise specified, from and
including or through and including, respectively. All references to “days” shall be to calendar days unless otherwise indicated as a
“Business Day.” Except as otherwise specifically indicated, for purposes of measuring the beginning and ending of time periods in this
Agreement (including for purposes of “Business Day” and for hours in a day or Business Day), the time at which a thing, occurrence or
event shall begin or end shall be deemed to occur in the Eastern time zone of the United States. The Parties agree that any rule of construction
to the effect that ambiguities are to be resolved against the drafting Party shall not be applied in the construction or interpretation
of this Agreement. The Parties agree that the Company Disclosure Schedule or MEDS Disclosure Schedule shall be arranged in sections and
subsections corresponding to the numbered and lettered sections and subsections contained in Section 3 or Section 4, respectively.
The disclosures in any section or subsection of the Company Disclosure Schedule or the MEDS Disclosure Schedule shall qualify other sections
and subsections in Section 3 or Section 4, respectively, to the extent it is readily apparent from a reading of the disclosure
that such disclosure is applicable to such other sections and subsections. The words “delivered” or “made available” mean, with respect
to any documentation, (a) that prior to 5:00 p.m. (New York City time) on the date that is the day prior to the date of this Agreement,
a copy of such material has been posted to and made available by a Party to the other Party and its Representatives in the electronic
data room maintained by such disclosing Party for the purposes of the Contemplated Transactions or (b) delivered by or on behalf of a
Party or its Representatives to the other Party or its Representatives via electronic mail or in hard copy form prior to the execution
of this Agreement.
Section
2. Description of Transaction.
2.1
The Merger. Upon the terms and subject to the conditions set forth in this Agreement and in accordance with the DGCL, at the First
Effective Time, Merger Sub I shall be merged with and into the Company, and the separate existence of Merger Sub I shall cease. As a
result of the First Merger, the Company will continue as the surviving company of the First Merger (the “First Step Surviving Company”).
Upon the terms and subject to the conditions set forth in this Agreement and in accordance with the DGCL and DLLCA, at the Second Effective
Time, the First Step Surviving Company will merge with and into Merger Sub II, and the separate existence of the First Step Surviving
Company shall cease. As a result of the Second Merger, Merger Sub II will continue as the surviving company in the Second Merger (the
“Surviving Company”).
2.2
Effects of the Merger. At and after the First Effective Time, the First Merger shall have the effects set forth in this Agreement
and in the applicable provisions of the DGCL. Without limiting the generality of the foregoing and subject thereto, at the First Effective
Time, all the property, rights, privileges, powers and franchises of the Company and Merger Sub I shall vest in the First Step Surviving
Company, and all debts, liabilities and duties of the Company and Merger Sub I shall become the debts, liabilities and duties of the
First Step Surviving Company. As a result of the First Merger, the First Step Surviving Company will become a wholly owned subsidiary
of MEDS. At and after the Second Effective Time, the Second Merger shall have the effects set forth in this Agreement and in the applicable
provisions of the DGCL and the DLLCA. Without limiting the generality of the foregoing and subject thereto, at the Second Effective Time,
all the property, rights, privileges, powers and franchises of the First Step Surviving Company and Merger Sub II shall vest in the Surviving
Company, and all debts, liabilities and duties of the First Step Surviving Company and Merger Sub II shall become the debts, liabilities
and duties of the Surviving Company.
2.3
Closing; First Effective Time; Second Effective Time. Subject to the satisfaction or waiver of the conditions set forth in Section
7, Section 8, and Section 9, the consummation of the Merger (the “Closing”) shall take place remotely, on the
date of this Agreement, or at such other time, date and place as MEDS and the Company may mutually agree in writing. The date on which
the Closing actually takes place is referred to as the “Closing Date.” At the Closing, (i) the Parties shall cause the First Merger
to be consummated by executing and filing with the Secretary of State of the State of Delaware a certificate of merger with respect to
the First Merger, satisfying the applicable requirements of the DGCL and in form and substance to be agreed upon by the Parties (the
“First Certificate of Merger”) and (ii) the Parties shall cause the Second Merger to be consummated by executing and filing with
the Secretary of State of the State of Delaware a certificate of merger with respect to the Second Merger, satisfying the applicable
requirements of the DGCL and the DLLCA and in form and substance to be agreed upon by the Parties (the “Second Certificate of Merger”).
The First Merger shall become effective at the time of the filing of such First Certificate of Merger with the Secretary of State of
the State of Delaware or at such later time as may be specified in such First Certificate of Merger or such other time as MEDS and the
Company shall agree in writing and shall specify in the First Certificate of Merger (the time as of which the First Merger becomes effective
being referred to as the “First Effective Time”). The Second Merger shall become effective at the time of the filing of such Second
Certificate of Merger with the Secretary of State of the State of Delaware or at such later time as MEDS and the Company shall agree
in writing and shall specify in the Second Certificate of Merger (the time as of which the Second Merger becomes effective being referred
to as the “Second Effective Time”).
2.4
Organizational Documents; Directors and Officers.
(a)
As of the First Effective Time:
(i)
the certificate of incorporation of the First Step Surviving Company shall be amended and restated in its entirety as the certificate
of incorporation of Merger Sub I (except that references to the name of Merger Sub I shall be replaced by references to the name of the
First Step Surviving Company), until thereafter amended as provided by the DGCL and the First Step Surviving Company’s Organizational
Documents;
(ii)
the certificate of incorporation and bylaws of MEDS shall be identical to the certificate of incorporation and bylaws of MEDS immediately
prior to the First Effective Time, until thereafter amended as provided by the DGCL and MED’s Organizational Documents;
(iii)
the bylaws of the First Step Surviving Company shall be amended and restated in its entirety as the bylaws of Merger Sub I (except that
references to the name of Merger Sub I shall be replaced with references to the name of the First Step Surviving Company), until thereafter
amended as provided by the DGCL and the First Step Surviving Company’s Organizational Documents; and
(iv)
the directors and officers of the First Step Surviving Company, each to hold office in accordance with the certificate of incorporation
and bylaws of the First Surviving Company, shall be as set forth in Section 5.7.
(b)
As of the Second Effective Time:
(i)
the manager of the Surviving Company in accordance with the certificate of formation and limited liability agreement of the Surviving
Company, shall be MEDS;
(ii)
the limited liability company agreement of the Surviving Company shall be identical to the limited liability company agreement of Merger
Sub II as in effect immediately prior to the Second Effective Time, until thereafter amended as provided by the DLLCA and such limited
liability company agreement; provided that (A) the limited liability company agreement of the Surviving Company shall comply with Section
5.4 and (B) all references to Merger Sub II in the limited liability company agreement of the Surviving Company shall be changed
to reference to Scienture LLC; and
(iii)
the certificate of formation of the Surviving Company shall be identical to the certificate of formation of Merger Sub II as in effect
immediately prior to the Second Effective Time, except all references to the name Merger Sub II shall be replaced by references to the
name Scienture LLC” until thereafter further amended in accordance with DLLCA and as provided in the Surviving Company’s Organizational
Documents.
(iv)
the Parties shall take all action necessary (including, to the extent necessary, procuring the resignation of any directors on the MEDS
Board immediately prior the Second Effective Time) so that, as of the Second Effective Time, the number of directors that comprise the
full Board of Directors of MEDS shall be seven (7) (or such other number of directors and MEDS and the Company may mutually agree), and
such Board of Directors shall upon the Second Effective Time initially consist of the Persons set forth in Section 2.4(b)(iv)
of the MEDS Disclosure Schedules.
2.5
Conversion of Company Common Stock.
(a)
At the First Effective Time, by virtue of the First Merger and without any further action on the part of MEDS, Merger Subs, the Company
or any stockholder of the Company or stockholder of MEDS, subject to Section 2.5(c) and Section 2.5 (g), the shares of
Company Common Stock issued and outstanding immediately prior to the First Effective Time (other than any Excluded Shares or Dissenting
Shares), shall be converted solely into the right to receive a number of shares of MEDS Capital Stock equal to the amount of Company
Merger Shares (as set forth on the Allocation Certificate), (the “Merger Consideration”). As of the First Effective Time, all
such shares of Company Common Stock shall no longer be outstanding and shall automatically be cancelled and cease to exist, and shall
thereafter only represent the right to receive the Merger Consideration.
(b)
Each share of Company Common Stock held in the treasury of the Company or owned, directly or indirectly, by Parent or Merger Sub I immediately
prior to the First Effective Time (collectively, “Excluded Shares”) shall automatically be cancelled and shall cease to exist,
and no consideration shall be delivered in exchange therefor.
(c)
If any shares of Company Common Stock outstanding immediately prior to the First Effective Time are unvested or are subject to a repurchase
option or a risk of forfeiture under any applicable restricted stock agreement, option award agreement or other similar agreement with
the Company relating to Company Common Stock or options to purchase Company Common Stock, then the shares of MEDS Common Stock issued
in exchange for such Company Common Stock will to the same extent be unvested and subject to the same repurchase option or risk of forfeiture,
and such shares of MEDS Common Stock issued upon conversion of the Company Common Stock shall accordingly be marked with appropriate
legends. The Company shall take all actions that may be necessary to ensure that, from and after the First Effective Time, MEDS is entitled
to exercise any such repurchase option or other right set forth in any such restricted stock agreement, option award agreement or other
similar agreement relating to Company Common stock or options to purchase Company Common Stock.
(d)
No fractional shares of MEDS Common Stock shall be issued in connection with the First Merger, and no certificates or scrip for any such
fractional shares shall be issued, with no cash being paid for any fractional share eliminated by such rounding. Any fractional shares
of MEDS Common Stock a holder of Company Common Stock would otherwise be entitled to receive shall be aggregated together first prior
to eliminating any remaining fractional share.
(e)
At the First Effective Time, by virtue of the First Merger and without any further action on the part of MEDS, Merger Subs, the Company
or any stockholder of the Company or stockholder of MEDS, each share of Merger Sub I Common Stock issued and outstanding immediately
prior to the Effective Time shall be converted into and exchanged for one share of the First Step Surviving Company Common Stock. If
applicable, each stock certificate of Merger Sub I evidencing ownership of any such Merger Sub I Common Stock shall, as of the First
Effective Time, evidence ownership of such First Surviving Company Common Stock.
(f)
If, between the date of this Agreement and the First Effective Time, the outstanding Company Common Stock or MEDS Common Stock shall
have been changed into, or exchanged for, a different number of shares or a different class of Company Capital Stock or MEDS Capital
Stock, respectively, by reason of any stock dividend, subdivision, reclassification, recapitalization, split, combination or exchange
of shares or other like change, the Company Merger Shares issued shall, to the extent necessary, be proportionally adjusted to reflect
such change to the extent necessary to provide the holders of Company Common Stock and MEDS Common Stock with the same economic effect
as contemplated by this Agreement prior to such stock dividend, subdivision, reclassification, recapitalization, split, combination or
exchange of shares or other like change; provided, however, that nothing herein will be construed to permit the Company
or MEDS to take any action with respect to Company Common Stock or MEDS Common Stock, respectively, that is prohibited or not expressly
permitted by the terms of this Agreement.
(g)
Notwithstanding anything to the contrary, the aggregate number of shares of MEDS Common Stock issued pursuant to Section 2.5(a)
to any stockholder of the Company shall not result in the acquisition of beneficial ownership of MEDS in excess of 19.99% of the total
number of shares of MEDS Common Stock outstanding immediately prior to the First Effective Time (the “MEDS Common Stock Consideration
Cap”). In the event the aggregate number of shares of MEDS Common Stock issued pursuant to Section 2.5(a) to any stockholder
of the Company at Closing would result in the issuance of shares of MEDS Common Stock in an amount in excess of the MEDS Common Stock
Consideration Cap, MEDS shall issue to such holders of such Company Common Stock shares of MEDS Common Stock up to the MEDS Common Stock
Consideration Cap and shall issue the remaining balance of such holder’s Company Common Stock in shares of MEDS Preferred Stock, in each
case, in accordance with the applicable stockholder’s ownership of Company Common Stock as set forth on the Allocation Certificate.
(h)
At the Second Effective Time, by virtue of the Second Merger and without any action on the part of MEDS, the First Step Surviving Company,
Merger Sub II or their respective members, each First Step Surviving Company Common Stock issued and outstanding immediately prior to
the Second Effective Time shall be canceled and extinguished without any conversion thereof and no payment or distribution shall be made
with respect thereto.
2.6
Dissenting Shares. Notwithstanding anything in this Agreement to the contrary, shares of Company Common Stock (other than Excluded
Shares) that are issued and outstanding immediately prior to the First Effective Date and that are held by a stockholder of the Company
who is entitled to demand and who has properly exercised appraisal rights available under Section 262 of the DGCL (8 Del. C. § 262) (the
“Dissenting Shares”) shall not be converted into or be exchangeable for the right to receive the Merger Consideration unless and
until such holder fails to perfect or withdraws or otherwise loses such holder’s right to appraisal and payment under the DGCL. If, after
the First Effective Time, any such holder shall fail to perfect or otherwise shall waive, withdraw or otherwise lose the right to appraisal
under Section 262 of the DGCL, such holder’s shares shall be deemed to have been converted at the First Effective Date into the right
to receive the portion of the Merger Consideration, if any, to which such holder is entitled to pursuant to Section 2.5(a), without any
interest thereon. The Company shall give prompt written notice to MEDS of any demands for appraisal of any shares of Company Common Stock,
withdrawals of any such demands and any other related instruments served pursuant to the DGCL, and MEDS shall have the right to participate
in and direct all negotiations and proceedings with respect to such demands. The Company shall not, except with the prior written consent
of MEDS, voluntarily make or agree to make any payment with respect to any demands for appraisals of Company Common Stock, offer to settle
or settle any such demands or approve any withdrawal of any such demands.
2.7
Closing of the Company’s Transfer Books. At the First Effective Time: (a) all Company Common Stock outstanding immediately prior
to the First Effective Time shall be treated in accordance with Section 2.5(a), and all holders of certificates representing Company
Common Stock that were outstanding immediately prior to the First Effective Time shall cease to have any rights as stockholders of the
Company and (b) the transfer books of the Company shall be closed with respect to all Company Common Stock outstanding immediately prior
to the First Effective Time. No further transfer of any such Company Common Stock shall be made on such transfer books after the First
Effective Time.
2.8
Surrender of Company Common Stock.
(a)
On or prior to the Closing Date, MEDS and the Company shall jointly select a reputable bank, transfer agent or trust company to act as
exchange agent in the Merger (the “Exchange Agent”). At the First Effective Time, MEDS shall deposit (or cause to be deposited)
with the Exchange Agent, in trust for the benefit of holders of shares of Company Common Stock immediately prior to the First Effective
Time (other than holders to the extent they hold Excluded Shares or Dissenting Shares) evidence of book-entry shares representing the
shares of MEDS Capital Stock issuable pursuant to Section 2.5(a) in exchange for Company Common Stock. In addition, MEDS shall
make available by depositing with the Exchange Agent, as necessary from time to time after the First Effective Time, any dividends or
distributions payable under Section 2.8(c). All certificates representing shares of MEDS Capital Stock, dividends, distributions
and cash deposited with the Exchange Agent are hereinafter referred to as the “Exchange Fund”.
(b)
Promptly after the First Effective Time, and in any event not later than the third (3rd) Business Day thereafter, the Parties
shall cause the Exchange Agent to mail to the Persons who were record holders of Company Common Stock that were converted into the right
to receive the Merger Consideration, and any dividends or distributions payable under Section 2.8(c): (i) a form of letter of
transmittal in customary form and containing such provisions as MEDS or the Exchange Agent may reasonably specify and (ii) instructions
for use in effecting the surrender of Company Common Stock in exchange for the Merger Consideration, and any dividends or distributions
payable under Section 2.8(c). Upon surrender of a duly executed letter of transmittal and such other documents as may be reasonably
required by the Exchange Agent or MEDS, the holder of such Company Common Stock (other than Excluded Shares or Dissenting Shares) shall
be entitled to receive in exchange therefor book-entry shares representing a number of whole shares of MEDS Capital Stock that such holder
has the right to receive pursuant to the provisions of Section 2.5(a) and any dividends or distributions payable under Section
2.8(c).
(c)
No dividends or other distributions declared or made with respect to MEDS Capital Stock with a record date after the First Effective
Time shall be paid to the holder of any Company Common Stock with respect to the shares of MEDS Capital Stock that such holder has the
right to receive in the Merger until such holder delivers a duly executed letter of transmittal (at which time (or, if later, on the
applicable payment date) such holder shall be entitled, subject to the effect of applicable abandoned property, escheat or similar Laws,
to receive all such dividends and distributions, without interest).
(d)
Any portion of the Exchange Fund deposited with the Exchange Agent that remain undistributed to holders of Company Common Stock as of
the date that is 180 days after the Closing Date shall be delivered to the Surviving Company upon demand, and any holders of Company
Common Stock (except to the extent representing Excluded Shares or Dissenting Shares) who have not theretofore delivered a duly executed
letter of transmittal in accordance with this Section 2.8 shall thereafter look only to the Surviving Company for satisfaction
of their claims for the Merger Consideration and any dividends or distributions with respect to shares of MEDS Capital Stock.
(e)
No Party shall be liable to any holder of any Company Common Stock or to any other Person with respect to any shares of MEDS Capital
Stock (or dividends or distributions with respect thereto) or for any cash amounts delivered to any public official pursuant to any applicable
abandoned property Law, escheat Law or similar Law.
2.9
Calculation of Net Cash and Company Valuation.
(a)
No later than the Closing Date, MEDS will deliver to the Company a schedule (the “MEDS Net Cash Schedule”) setting forth, in reasonable
detail, MEDS’s good faith, estimated calculation of MEDS Net Cash, including each component thereof (the “MEDS Net Cash Calculation”
as of 11:59 p.m. on the last Business Day prior to the Closing Date (the “Cash Determination Time”) prepared and certified by
MEDS’s chief financial officer (or if there is no chief financial officer at such time, the principal financial and accounting officer
for MEDS). MEDS shall make available to the Company (electronically to the greatest extent possible), as reasonably requested by the
Company, the work papers and back-up materials used or useful in preparing the MEDS Net Cash Schedule and, if reasonably requested by
the Company, MEDS’s accountants and counsel at reasonable times and upon reasonable notice. The MEDS Net Cash Calculation shall include
MEDS’s determination, as of the Cash Determination Time, of the defined terms in Section 1.1(a) necessary to calculate the Company
Merger Shares.
2.10
Further Action. If, at any time after the First Effective Time, any further action is determined by the Surviving Company to be
necessary or desirable to carry out the purposes of this Agreement or to vest the Surviving Company with full right, title and possession
of and to all rights and property of the Company, then the officers and manager of the Surviving Company shall be fully authorized, and
shall use their and its commercially reasonable efforts (in the name of the Company, in the name of Merger Subs, in the name of the Surviving
Company and otherwise) to take such action.
2.11
Tax Consequences. The Parties acknowledge and agree that, for U.S. federal (and applicable state and local), income tax purposes
the First Merger and the Second Merger, taken together, are intended to qualify as a reorganization within the meaning of Section 368(a)
of the Code. The Parties adopt this Agreement as a “plan of reorganization” within the meaning of Treasury Regulations Section 1.368-2(g).
2.12
Withholding. Each of the Exchange Agent, MEDS, and the First Step Surviving Company (each, a “Withholding Agent”) shall
be entitled to deduct and withhold from any consideration deliverable pursuant to this Agreement (including the Closing Distribution)
such amounts as are required to be deducted or withheld from such consideration under the Code or under any other applicable Law; provided
that if a Withholding Agent determines that any payment to any member of the Company hereunder is subject to deduction and/or withholding,
then, except with respect to compensatory payments or as a result of a failure to deliver the certificate described in Section 5.5(c),
such Withholding Agent shall (i) provide notice to such member as soon as reasonably practicable after such determination and (ii) use
commercially reasonable efforts to cooperate with such member prior to Closing to reduce or eliminate any such deduction and/or withholding.
To the extent such amounts are so deducted or withheld, and remitted to the appropriate taxing authority, such amounts shall be treated
for all purposes under this Agreement as having been paid to the Person to whom such amounts would otherwise have been paid.
Section
3. Representations and Warranties of the Company.
Subject
to Section 3, except as set forth in the written disclosure schedule delivered by the Company to MEDS (the “Company Disclosure
Schedule”), the Company represents and warrants to MEDS and Merger Subs as of the date hereof follows:
3.1
Due Organization; Subsidiaries.
(a)
The Company is a corporation or other legal entity duly incorporated or otherwise organized, validly existing and in good standing under
the Laws of the jurisdiction of Delaware and has all necessary power and authority: (i) to conduct its business in the manner in which
its business is currently being conducted, (ii) to own or lease and use its property and assets in the manner in which its property and
assets are currently owned or leased and used and (iii) to perform its obligations under all Contracts by which it is bound.
(b)
The Company is duly licensed and qualified to do business, and is in good standing (to the extent applicable in such jurisdiction), under
the Laws of all jurisdictions where the nature of its business in the manner in which its business is currently being conducted requires
such licensing or qualification other than in jurisdictions where the failure to be so qualified individually or in the aggregate would
not be reasonably expected to have a Company Material Adverse Effect. Such jurisdictions are set forth on Section 3.1(b) of the
Company Disclosure Schedule.
(c)
Except as set forth on Section 3.1(c) of the Company Disclosure Schedules, (i) the Company has no Subsidiaries and the Company
does not own any capital stock or membership interests of, or any equity, ownership or profit sharing interest of any nature in, or controls
directly or indirectly, any other Entity; (ii) the Company is not and has never otherwise been, directly or indirectly, a party to, member
of or participant in any partnership, joint venture or similar business entity; (iii) the Company has not agreed or is obligated to make,
or is bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any other
Entity; and (iv) the Company has not, at any time, been a general partner of, or has otherwise been liable for any of the debts or other
obligations of, any general partnership, limited partnership or other Entity.
3.2
Organizational Documents. The Company has delivered to MEDS accurate and complete copies of the Organizational Documents of the
Company. The Company is not in breach or violation of its Organizational Documents in any material respect.
3.3
Authority; Binding Nature of Agreement. The Company has all necessary corporate power and authority to enter into and to
perform its obligations under this Agreement and to consummate the Contemplated Transactions. The Company Board has (i) determined that
the Contemplated Transactions are fair to, advisable and in the best interests of the Company and its stockholders, (ii) approved and
declared advisable this Agreement and the Contemplated Transactions and (iii) determined to recommend, upon the terms and subject to
the conditions set forth in this Agreement, that the stockholders of the Company vote to adopt this Agreement and thereby approve the
Contemplated Transactions. This Agreement has been duly executed and delivered by the Company and assuming the due authorization, execution
and delivery by MEDS and Merger Subs, constitutes the legal, valid and binding obligation of the Company, enforceable against the Company
in accordance with its terms, subject to the Enforceability Exceptions.
3.4
Vote Required. The affirmative vote of the holders of a majority of the outstanding shares of Company Common Stock (voting as
a single class) (the “Required Company Stockholder Vote”), are the only votes of the holders of any Company Capital Stock necessary
to adopt and approve this Agreement and approve the Contemplated Transactions. The Company has obtained approval by written consent from
Company stockholders sufficient for the Required Company Stockholder Vote in lieu of a meeting pursuant to Section 228 of the DGCL, for
purposes of adopting and approving this Agreement and the Contemplated Transactions.
3.5
Non-Contravention; Consents.
(a)
Subject to obtaining the Required Company Stockholder Vote, the filing of the First Certificate of Merger required by the DGCL and the
filing of the Second Certificate of Merger required by the DGCL and the DLLCA, neither (x) the execution, delivery or performance of
this Agreement by the Company, nor (y) the consummation of the Contemplated Transactions, will directly or indirectly (with or without
notice or lapse of time):
(i)
contravene, conflict with or result in a violation of any of the provisions of the Company’s Organizational Documents;
(ii)
contravene, conflict with or result in a material violation of, or give any Governmental Authority or other Person the right to challenge
the Contemplated Transactions or to exercise any remedy or obtain any relief under, any Law or any Order by which the Company, or any
of the assets owned or used by the Company, is subject;
(iii)
contravene, conflict with or result in a material violation of any of the terms or requirements of, or give any Governmental Authority
the right to revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by the Company;
(iv)
contravene, conflict with or result in a violation or breach of, or result in a default under, any provision of any Company Material
Contract, or give any Person the right to: (A) declare a default or exercise any remedy under any Company Material Contract, (B) any
material payment, rebate, chargeback, penalty or change in delivery schedule under any Company Material Contract, (C) accelerate the
maturity or performance of any Company Material Contract or (D) cancel, terminate or modify any term of any Company Material Contract,
except in the case of any nonmaterial breach, default, penalty or modification; or
(v)
result in the imposition or creation of any Encumbrance upon or with respect to any asset owned or used by the Company (except for Permitted
Encumbrances).
(b)
Except for (i) the Required Company Stockholder Vote, (ii) the filing of the First Certificate of Merger with the Secretary of State
of the State of Delaware pursuant to the DGCL (iii) the filing of the Second Certificate of Merger with the Secretary of State of Delaware
pursuant to the DLLCA, and (iv) such consents, waivers, approvals, orders, authorizations, registrations, declarations and filings as
may be required under applicable federal and state securities laws, the Company was not, is not, nor will be required to make any filing
with or give any notice to, or to obtain any Consent from, any Person in connection with (x) the execution, delivery or performance of
this Agreement or (y) the consummation of the Contemplated Transactions.
(c)
The Company Board has taken and will take all actions necessary to ensure that the restrictions applicable to business combinations contained
in Section 203 of the DGCL, to the extent applicable to the Company, are, and will be, inapplicable to the execution, delivery and performance
of this Agreement and to the consummation of the Contemplated Transactions. No other state takeover statute or similar Law applies or
purports to apply to the Merger, this Agreement or any of the Contemplated Transactions.
3.6
Capitalization.
(a)
Section 3.6(a) of the Company Disclosure Schedule sets forth an accurate and complete capitalization table of the Company as of
the date of this Agreement. There are no shares of Company Capital Stock issued and outstanding other than Company Common Stock.
(b)
All of the outstanding Company Common Stock as set out in Section 3.6(a) of the Company Disclosure Schedule have been duly authorized
and validly issued, and are fully paid and nonassessable and are free of any Encumbrances other than Encumbrances set forth in the Organizational
Documents or under applicable securities Laws. None of the outstanding Company Common Stock is entitled or subject to any preemptive
right, right of participation, right of maintenance or any similar right and none of the outstanding Company Common Stock is subject
to any right of first refusal in favor of the Company. Except as contemplated herein, there is no Company Contract relating to the voting
or registration of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting any option or
similar right with respect to), any Company Capital Stock. The Company is not under any obligation, nor is it bound by any Contract pursuant
to which it may become obligated, to repurchase, redeem or otherwise acquire any outstanding Company Common Stock or other securities.
Section 3.1(b) of the Company Disclosure Schedule accurately and completely lists all repurchase rights held by the Company with
respect to Company Common Stock (including shares issued pursuant to the exercise of options) and specifies which of those repurchase
rights are currently exercisable.
(c)
Except as set forth in Section 3.6(c) of the Company Disclosure Schedule, the Company has no option plan or any other plan, program,
agreement or arrangement providing for an equity-based compensation for any Person.
(d)
Except as set forth in Section 3.6(d) of the Company Disclosure Schedule, there is no: (i) outstanding subscription, option, call,
warrant or right (whether or not currently exercisable) to acquire any Company Common Stock or other securities of the Company, (ii)
outstanding security, instrument or obligation that is or may become convertible into or exchangeable for any shares of the capital stock
or other securities of the Company, (iii) stockholder rights plan (or similar plan commonly referred to as a “poison pill”) or Contract
under which the Company is or may become obligated to sell or otherwise issue any equity interest or any other securities or (iv) condition
or circumstance that could be reasonably likely to give rise to or provide a basis for the assertion of a claim by any Person to the
effect that such Person is entitled to acquire or receive any shares of capital stock or other securities of the Company, and there are
no outstanding or authorized stock appreciation, phantom stock, profit participation or other similar rights with respect to the Company.
(e)
All outstanding Company Common Stock and other securities of the Company have been issued and granted in material compliance with (i)
all applicable securities laws and other applicable Law and (ii) all requirements set forth in applicable Contracts.
3.7
Financial Statements.
(a)
Section 3.7(a) of the Company Disclosure Schedule includes true and complete copies of the Company Unaudited Interim Balance Sheet,
and the Company’s unaudited estimated statement of income, cash flow and changes in partners’ capital for the six months ended June 30,
2024 (collectively, the “Company Financials”). The Company Financials (A) were prepared in accordance with United States generally
accepted accounting principles (“GAAP”) (except that the Company Financials may not have notes thereto and other presentation
items that may be required by GAAP and are subject to normal and recurring year-end adjustments that are not reasonably expected to be
material in amount) applied on a consistent basis unless otherwise noted therein throughout the periods indicated and (B) fairly present,
in all material respects, the financial position and operating results of the Company as of the dates and for the periods indicated therein.
(b)
The Company maintains a system of internal accounting controls designed to provide reasonable assurance that: (i) transactions are executed
in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation
of the financial statements of the Company in conformity with GAAP and to maintain accountability of the Company’s assets, (iii) access
to the Company’s assets is permitted only in accordance with management’s general or specific authorization and (iv) the recorded accountability
for the Company’s assets is compared with the existing assets at regular intervals and appropriate action is taken with respect to any
differences. The Company maintains internal controls consistent with the practices of similarly situated private companies over financial
reporting that provides reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements
for external purposes.
(c)
Section 3.7(c) of the Company Disclosure Schedule lists, and the Company has delivered to MEDS accurate and complete copies of
the documentation creating or governing, all securitization transactions and “off-balance sheet arrangements” (as defined in Item 303(c)
of Regulation S-K under the Exchange Act) effected by the Company since January 1, 2021.
(d)
Since January 1, 2021, there have been no formal internal investigations regarding financial reporting or accounting policies and practices
discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer or general counsel
of the Company, the Company Board or any committee thereof. Since January 1, 2021, neither the Company nor its independent auditors have
identified (i) any significant deficiency or material weakness in the design or operation of the system of internal accounting controls
utilized by the Company, (ii) any fraud, whether or not material, that involves the Company, the Company’s management or other employees
who have a role in the preparation of financial statements or the internal accounting controls utilized by the Company or (iii) any claim
or allegation regarding any of the foregoing.
3.8
Absence of Changes. Except as set forth on Section 3.8 of the Company Disclosure Schedule, between January 1, 2024 and
the date of this Agreement, the Company has conducted its business only in the Ordinary Course of Business (except for the execution
and performance of this Agreement and the discussions, negotiations and transactions related thereto) and there has not been any (x)
Company Material Adverse Effect or (y) actions to do any of the following:
(a)
declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of capital stock; or repurchase,
redeem or otherwise reacquire any shares of its capital stock or other securities (except for Company Common Stock from terminated employees,
directors or consultants of the Company in accordance with agreements in effect on the date of this Agreement providing for the repurchase
of shares at no more than the purchase price thereof in connection with any termination of services to MEDS or any of its Subsidiaries);
(b)
except as required to give effect to anything in contemplation of the Closing, amend any of its Organizational Documents, or effect or
be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split,
reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(c)
sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing actions with respect to: (i) any capital
stock or other security of the Company, (ii) any option, warrant or right to acquire any capital stock or any other security or (iii)
any instrument convertible into or exchangeable for any capital stock or other security of the Company;
(d)
form any Subsidiary or acquire any equity interest or other interest in any other Entity or enter into a joint venture with any other
Entity;
(e)
(i) lend money to any Person, (ii) incur or guarantee any indebtedness for borrowed money, (iii) guarantee any debt securities of others
or (iv) make any capital expenditure or commitment;
(f)
other than as required by applicable Law or any Employee Plan: (i) adopt, establish or enter into any Employee Plan, including, for the
avoidance of doubt, any equity awards plans, (ii) cause or permit any Employee Plan to be amended other than as required by law or in
order to make amendments for the purposes of Section 409A of the Code, (iii) pay any bonus or make any profit-sharing or similar payment
to (except with respect to obligations in place on the date of this Agreement pursuant to any Employee Plan), or increase the amount
of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its directors, officers or
employees, (iv) increase the severance or change of control benefits offered to any current or new employees, directors or consultants,
or (v) hire any officer, employee or consultant;
(g)
enter into any material transaction outside the Ordinary Course of Business;
(h)
acquire any material asset or sell, lease, license or otherwise irrevocably dispose of any of its assets or properties, or grant any
Encumbrance with respect to such assets or properties;
(i)
sell, assign, transfer, license, sublicense or otherwise dispose of any material Company IP Rights (other than pursuant to nonexclusive
licenses in the Ordinary Course of Business);
(j)
make (other than consistent with past practice), change or revoke any material Tax election; file any material amendment to any Tax Return;
settle or compromise any material Tax claim; waive or extend any statute of limitations in respect of a period within which an assessment
or reassessment of material Taxes may be issued (other than any extension pursuant to an extension to file any Tax Return); enter into
any “closing agreement” as described in Section 7121 of the Code (or any similar Law) with any Governmental Authority; or adopt or change
any material accounting method in respect of Taxes;
(k)
waive, settle or compromise any pending or threatened Legal Proceeding against the Company, other than waivers, settlements or agreements
(i) for an amount not in excess of $100,000 in the aggregate (excluding amounts to be paid under existing insurance policies or renewals
thereof) and (ii) that do not impose any material restrictions on the operations or businesses of the Company or any equitable relief
on, or the admission of wrongdoing by the Company;
(l)
delay or fail to repay when due any material obligation, including accounts payable and accrued expenses, other than in the Ordinary
Course of Business;
(m)
forgive any loans to any Person, including its employees, officers, directors or Affiliate;
(n)
sell, assign, transfer, license, sublicense or otherwise dispose of any material MEDS IP Rights (other than in the Ordinary Course of
Business);
(o)
terminate or modify in any material respect, or fail to exercise renewal rights with respect to, any material insurance policy;
(p)
enter into, amend, terminate, or waive any material option or right under, any Company Material Contract;
(q)
(i) materially change pricing or royalties or other payments set or charged by the Company to its customers or licensees or (ii) agree
to materially change pricing or royalties or other payments set or charged by Persons who have licensed Intellectual Property to the
Company; or
(r)
agree, resolve or commit to do any of the foregoing.
3.9
Absence of Undisclosed Liabilities. The Company does not have any liability, indebtedness, obligation, expense, claim, deficiency,
guaranty or endorsement of any kind, whether accrued, absolute, contingent, matured, unmatured or otherwise (each a “Liability”), in
each case, of a type required to be reflected or reserved for on a balance sheet prepared in accordance with GAAP, except for: (a) Liabilities
disclosed, reflected or reserved against in the Company Unaudited Interim Balance Sheet, (b) normal and recurring current Liabilities
that have been incurred by the Company since the date of the Company Unaudited Interim Balance Sheet in the Ordinary Course of Business
(none of which relates to any breach of contract, breach of warranty, tort, infringement, or violation of Law), (c) Liabilities for performance
of obligations of the Company under Company Contracts, (d) Liabilities incurred in connection with the Contemplated Transactions and
(e) Liabilities listed in Section 3.9 of the Company Disclosure Schedule.
3.10
Title to Assets. The Company has good and valid title to, or, in the case of leased properties and assets, valid leasehold interests
in, all tangible properties or tangible assets and equipment used or held for use in its business or operations or purported to be owned
by it, including: (a) all tangible assets reflected on the Company Unaudited Interim Balance Sheet and (b) all other tangible assets
reflected in the books and records of the Company as being owned by the Company. All of such assets are owned or, in the case of leased
assets, leased by the Company free and clear of any Encumbrances, other than Permitted Encumbrances.
3.11
Real Property; Leasehold. The Company does not own nor has ever owned any real property. The Company has made available to MEDS
(a) an accurate and complete list of all real properties with respect to which the Company directly or indirectly holds a valid leasehold
interest as well as any other real estate that is in the possession of or leased by the Company and (b) copies of all leases under which
any such real property is possessed (the “Company Real Estate Leases”), each of which is in full force and effect, with no existing
material default thereunder. Section 3.11(a) of the Company Disclosure Schedule lists all of the Company Real Estate Leases.
3.12
Intellectual Property.
(a)
Section 3.12(a) of the Company Disclosure Schedule is an accurate, true and complete listing of all Company Registered IP.
(b)
Section 3.12(b) of the Company Disclosure Schedule accurately identifies (i) all Company Contracts pursuant to which any Company
IP Rights are licensed to the Company (other than (A) any non-customized software that (1) is so licensed solely in executable or object
code form pursuant to a nonexclusive, internal use software license and other Intellectual Property associated with such software and
(2) is not incorporated into, or material to the development, manufacturing, or distribution of, any of the Company’s products or services,
(B) any Intellectual Property licensed on a nonexclusive basis ancillary to the purchase or use of equipment, reagents or other materials,
(C) any confidential information provided under confidentiality agreements and (D) agreements between Company and its employees in Company’s
standard form thereof), (ii) the corresponding Company Contract pursuant to which such Company IP Rights are licensed to the Company
and (iii) whether the license or licenses granted to the Company are exclusive or nonexclusive.
(c)
Section 3.12(c) of the Company Disclosure Schedule accurately identifies each Company Contract pursuant to which any Person has
been granted any license or covenant not to sue under, or otherwise has received or acquired any right (whether or not currently exercisable)
or interest in, any Company IP Rights (other than (i) any confidential information provided under confidentiality agreements and (ii)
any Company IP Rights nonexclusively licensed to academic collaborators, suppliers or service providers for the sole purpose of enabling
such academic collaborator, supplier or service providers to provide services for the Company’s benefit).
(d)
Except as set forth in Section 3.12(d) of the Company Disclosure Schedule, the Company is not bound by, and no Company IP Rights
are subject to, any Contract containing any covenant or other provision that in any way limits or restricts the ability of the Company
to use, exploit, assert, or enforce any Company IP Rights anywhere in the world.
(e)
The Company exclusively owns all right, title, and interest to and in Company IP Rights (other than (i) Company IP Rights licensed to
the Company, or co-owned rights each as identified in Section 3.12(c) of the Company Disclosure Schedule, (ii) any non-customized
software that (A) is licensed to the Company solely in executable or object code form pursuant to a nonexclusive, internal use software
license and other Intellectual Property associated with such software and (B) is not incorporated into, or material to the development,
manufacturing, or distribution of, any of the Company’s products or services and (iii) any Intellectual Property licensed on a nonexclusive
basis ancillary to the purchase or use of equipment, reagents or other materials), in each case, free and clear of any Encumbrances (other
than Permitted Encumbrances). Without limiting the generality of the foregoing:
(i)
All documents and instruments necessary to register or apply for or renew registration of Company Registered IP have been validly executed,
delivered, and filed in a timely manner with the appropriate Governmental Authority.
(ii)
Except as set forth in Section 3.12(e)(ii) of the Company Disclosure Schedule, each Person who is or was an employee or contractor
of the Company and who is or was involved in the creation or development of any Intellectual Property for the Company has signed a valid,
enforceable agreement containing a present assignment of such Intellectual Property to the Company and confidentiality provisions protecting
trade secrets and confidential information of the Company.
(iii)
To the Knowledge of the Company, no current or former member, officer, director, or employee of the Company has any claim, right (whether
or not currently exercisable), or interest to or in any Company IP Rights purported to be owned by the Company. To the Knowledge of the
Company, no employee of the Company is (a) bound by or otherwise subject to any Contract restricting him or her from performing his or
her duties for the Company or (b) in breach of any Contract with any former employer or other Person concerning Company IP Rights purported
to be owned by the Company or confidentiality provisions protecting trade secrets and confidential information comprising Company IP
Rights purported to be owned by the Company.
(iv)
No funding, facilities, or personnel of any Governmental Authority were used, directly or indirectly, to develop or create, in whole
or in part, any Company IP Rights in which the Company has an ownership interest.
(v)
The Company has taken reasonable steps to maintain the confidentiality of and otherwise protect and enforce its rights in all proprietary
information that the Company holds, or purports to hold, as confidential or a trade secret.
(vi)
The Company has not assigned or otherwise transferred ownership of, or agreed to assign or otherwise transfer ownership of, any Company
IP Rights to any other Person.
(vii)
To the Knowledge of the Company, the Company IP Rights constitute all Intellectual Property necessary for the Company to conduct its
business as currently conducted; provided, however, that the foregoing representation is not a representation with respect
to noninfringement of Intellectual Property.
(f)
The Company has delivered or made available to MEDS, a complete and accurate copy of all Company IP Rights Agreements. With respect to
each of the Company IP Rights Agreements: (i) each such agreement is valid and binding on the Company and in full force and effect, (ii)
the Company has not received any written notice of termination or cancellation under such agreement, or received any written notice of
breach or default under such agreement, which breach has not been cured or waived and (iii) the Company, and to the Knowledge of the
Company, no other party to any such agreement, is not in breach or default thereof in any material respect.
(g)
The manufacture, marketing, sale, offering for sale, importation, use or intended use or other disposal of any product as currently sold
or under development by the Company does not violate any license or agreement between the Company and any other third party, and, to
the Knowledge of the Company, does not infringe or misappropriate any valid and issued Patent right or other Intellectual Property of
any other Person, which infringement or misappropriation would reasonably be expected to have a Company Material Adverse Effect. To the
Knowledge of the Company, no third party is infringing upon any Patents owned by Company within the Company IP Rights, or otherwise violating
any Company IP Rights Agreement.
(h)
As of the date of this Agreement, Company is not a party to any Legal Proceeding (including, but not limited to, opposition, interference
or other proceeding in any patent or other government office) contesting the validity, enforceability, claim construction, ownership
or right to use, sell, offer for sale, license or dispose of any Company IP Rights. The Company has not received any written notice asserting
that any Company IP Rights or the proposed use, sale, offer for sale, license or disposition of products, methods, or processes claimed
or covered thereunder infringes or misappropriates or violates the rights of any other Person or that the Company has otherwise infringed,
misappropriated or otherwise violated any Intellectual Property of any Person. None of the Company IP Rights is subject to any outstanding
order of, judgment of, decree of or agreement with any Governmental Authority that limits the ability of the Company to exploit any Company
IP Rights.
(i)
Each item of Company Registered IP is and at all times has been filed and maintained in compliance in all material respects with all
applicable Law and all filings, payments, and other actions required to be made or taken to maintain such item of Company Registered
IP in full force and effect have been made by the applicable deadline. To the Knowledge of the Company, all Company Registered IP that
is issued or granted is valid and enforceable.
(j)
To the Knowledge of the Company, no trademark (whether registered or unregistered) or trade name owned, used, or applied for by the Company
conflicts or interferes with any trademark (whether registered or unregistered) or trade name owned, used, or applied for by any other
Person. None of the goodwill associated with or inherent in any trademark (whether registered or unregistered) in which the Company has
or purports to have an ownership interest has been impaired as determined by the Company in accordance with GAAP.
(k)
Except as set forth in Sections 3.12(b) or 3.12(c) of the Company Disclosure Schedule or as contained in license, distribution
or service agreements entered into in the Ordinary Course of Business by the Company (i) the Company is not bound by any Contract to
indemnify, defend, hold harmless, or reimburse any other Person with respect to any Intellectual Property infringement, misappropriation,
or similar claim which is material to the Company, taken as a whole and (ii) the Company has never assumed, or agreed to discharge or
otherwise take responsibility for, any existing or potential liability of another Person for infringement, misappropriation, or violation
of any Intellectual Property right, which assumption, agreement or responsibility remains in force as of the date of this Agreement.
(l)
The Company is not party to any Contract that, as a result of such execution, delivery and performance of this Agreement, will cause
the grant of any license or other right to any Company IP Rights, result in breach of, default under or termination of such Contract
with respect to any Company IP Rights, or impair the right of the Company or the Surviving Company and its Subsidiaries to use, sell
or license or enforce any Company IP Rights or portion thereof, except for the occurrence of any such grant or impairment that would
not individually or in the aggregate, reasonably be expected to result in a Company Material Adverse Effect.
3.13
Agreements, Contracts and Commitments.
(a)
Section 3.13(a) of the Company Disclosure Schedule lists the following Company Contracts in effect as of the date of this Agreement
(each, a “Company Material Contract” and collectively, the “Company Material Contracts”), excluding any Employee Plan:
(i)
each Company Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business;
(ii)
each Company Contract containing (A) any covenant limiting the freedom of the Company or the Surviving Company to engage in any line
of business or compete with any Person, or limiting the development, manufacture, or distribution of the Company’s products or services
(B) any most-favored pricing arrangement, (C) any exclusivity provision or (D) any non-solicitation provision, in the case of the foregoing
that restricts the activities of the Company (and excluding, for the avoidance of doubt, such provisions for the benefit of the Company);
(iii)
each Company Contract (A) pursuant to which any Person granted the Company an exclusive license under any Intellectual Property, or (B)
pursuant to which the Company granted any Person an exclusive license under any Company IP Rights;
(iv)
each Company Contract relating to capital expenditures and requiring payments after the date of this Agreement in excess of $100,000
pursuant to its express terms and not cancelable without penalty;
(v)
each Company Contract relating to the disposition or acquisition of material assets or any ownership interest in any Entity;
(vi)
each Company Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other agreements
or instruments relating to the borrowing of money or extension of credit in excess of $100,000 or creating any material Encumbrances
with respect to any assets of the Company or any loans or debt obligations with officers or directors of the Company;
(vii)
each Company Contract requiring payment by or to the Company after the date of this Agreement in excess of $100,000 pursuant to its express
terms relating to: (A) any distribution agreement (identifying any that contain exclusivity provisions), (B) any agreement involving
provision of services or products with respect to any pre-clinical or clinical development activities of the Company, (C) any dealer,
distributor, joint marketing, alliance, joint venture, cooperation, development or other agreement currently in force under which the
Company has continuing obligations to develop or market any product, technology or service, or any agreement pursuant to which the Company
has continuing obligations to develop any Intellectual Property that will not be owned, in whole or in part, by the Company or (D) any
Contract to license any patent, trademark registration, service mark registration, trade name or copyright registration to or from any
third party to manufacture or produce any product, service or technology of the Company or any Contract to sell, distribute or commercialize
any products or service of the Company, in each case, except for Company Contracts entered into in the Ordinary Course of Business;
(viii)
each Company Contract with any Person, including any financial advisor, broker, finder, investment banker or other Person, providing
advisory services to the Company in connection with the Contemplated Transactions;
(ix)
each Company Contract to which the Company is a party or by which any of its assets and properties is currently bound, which involves
annual obligations of payment by, or annual payments to, the Company in excess of $100,000;
(x)
a Company Real Estate Lease; or
(xi)
any other Company Contract that is not terminable at will (with no penalty or payment) by the Company, and (A) which involves payment
or receipt by the Company after the date of this Agreement under any such agreement, contract or commitment of more than $100,000 in
the aggregate, or obligations after the date of this Agreement in excess of $100,000 in the aggregate or (B) that is material to the
business or operations of the Company taken as a whole.
(b)
The Company has delivered or made available to MEDS accurate and complete copies of all Company Material Contracts, including all amendments
thereto. There are no Company Material Contracts that are not in written form. The Company has not, nor to the Company’s Knowledge, as
of the date of this Agreement has any other party to a Company Material Contract, breached, violated or defaulted under, or received
notice that it breached, violated or defaulted under, any of the terms or conditions of any Company Material Contract in such manner
as would permit any other party to cancel or terminate any such Company Material Contract, or would permit any other party to seek damages
which would reasonably be expected to have a Company Material Adverse Effect. As to the Company, as of the date of this Agreement, each
Company Material Contract is valid, binding, enforceable and in full force and effect, subject to the Enforceability Exceptions. No Person
is renegotiating, or has a right pursuant to the terms of any Company Material Contract to change, any material amount paid or payable
to the Company under any Company Material Contract or any other material term or provision of any Company Material Contract.
3.14
Compliance; Permits; Restrictions.
(a)
The Company is, and since January 1, 2021 has been, in material compliance with all applicable Laws. No investigation, claim, suit, proceeding,
audit, Order, or other action by any Governmental Authority is pending or, to the Knowledge of the Company, threatened against the Company.
There is no agreement or Order binding upon the Company which (i) has or would reasonably be expected to have the effect of prohibiting
or materially impairing any business practice of the Company, any acquisition of material property by the Company or the conduct of business
by the Company as currently conducted, (ii) is reasonably likely to have an adverse effect on the Company’s ability to comply with or
perform any covenant or obligation under this Agreement or (iii) is reasonably likely to have the effect of preventing, delaying, making
illegal or otherwise interfering with the Contemplated Transactions.
(b)
The Company holds all required Governmental Authorizations which are material to the operation of the business of the Company as currently
conducted (the “Company Permits”). Section 3.14(b) of the Company Disclosure Schedule identifies each Company Permit. The
Company is in material compliance with the terms of the Company Permits. No Legal Proceeding is pending or, to the Knowledge of the Company,
threatened, which seeks to revoke, substantially limit, suspend, or materially modify any Company Permit. The rights and benefits of
each Company Permit will be available to the Surviving Company or its Subsidiaries, as applicable, immediately after the Second Effective
Time on terms substantially identical to those enjoyed by the Company as of the date of this Agreement and immediately prior to the First
Effective Time.
(c)
There are no Legal Proceedings pending or, to the Knowledge of the Company, threatened with respect to an alleged material violation
by the Company of the Federal Food, Drug, and Cosmetic Act (“FDCA”), the Public Health Service Act (“PHSA”), Food and Drug
Administration (“FDA”) regulations adopted thereunder, the Controlled Substances Act or any other similar Law promulgated by the
FDA or other comparable Governmental Authority responsible for regulation of the development, testing, manufacturing, processing, storage,
labeling, sale, marketing, advertising, distribution and importation or exportation of drug products (“Drug Regulatory Agency”).
(d)
The Company holds all required Governmental Authorizations issuable by any Drug Regulatory Agency necessary for the conduct of the business
of the Company as currently conducted, and the development, testing, manufacturing, processing, storage, labeling, distribution and importation
or exportation, as currently conducted, of any of its product candidates (the “Company Product Candidates”) (collectively, the
“Company Regulatory Permits”) and no such Company Regulatory Permit has been (i) revoked, withdrawn, suspended, cancelled or terminated
or (ii) modified in any adverse manner, other than immaterial adverse modifications. The Company is in compliance in all material respects
with the Company Regulatory Permits and has not, since January 1, 2021, received any written notice or other written communication from
any Drug Regulatory Agency regarding (A) any material violation of or failure to comply materially with any term or requirement of any
Company Regulatory Permit or (B) any revocation, withdrawal, suspension, cancellation, termination or adverse material modification of
any Company Regulatory Permit. The Company has made available to MEDS all information requested by MEDS in the Company’s possession or
control relating to the Company Product Candidates and the development, testing, manufacturing, processing, storage, labeling, sale,
marketing, advertising, distribution and importation or exportation of the Company Product Candidates, including but not limited to complete
copies of the following (to the extent there are any): (x) adverse event reports; summaries of material study data; inspection reports,
notices of adverse findings, untitled letters, warning letters and other material written correspondence to and from any Drug Regulatory
Agency; and meeting minutes with any Drug Regulatory Agency and (y) similar reports, material study data, notices, letters, material
correspondence and meeting minutes with any other Governmental Authority. All such information is accurate and complete in all material
respects.
(e)
All clinical, preclinical and other studies and tests conducted by or on behalf of, or sponsored by, the Company, or in which the Company
or its current products or product candidates, including the Company Product Candidates, have participated, were and, if still pending,
are being conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance
in all material respects with the applicable regulations of the Drug Regulatory Agencies and other applicable Law, including 21 C.F.R.
Parts 50, 54, 56, 58 and 312. The Company has not received any written notices, correspondence, or other communications from any Drug
Regulatory Agency requiring, or to the Knowledge of the Company threatening to initiate, any action to place a clinical hold order on,
or otherwise terminate, delay, or suspend any clinical studies conducted by or on behalf of, or sponsored by, the Company or in which
the Company or its current products or product candidates, including the Company Product Candidates, have participated. Further, no clinical
investigator, researcher, or clinical staff participating in any clinical study conducted by or, to the Knowledge of the Company, on
behalf of the Company has been disqualified from participating in studies involving the Company Product Candidates, and to the Knowledge
of the Company, no such administrative action to disqualify such clinical investigators, researchers or clinical staff has been threatened
in writing or is pending.
(f)
The Company is not, and to the Knowledge of the Company, no contract manufacturer with respect to any Company Product Candidate, is the
subject of any pending investigation in respect of its business or products by the FDA pursuant to its “Fraud, Untrue Statements of Material
Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto.
The Company has not committed any acts, made any statement, or failed to make any statement, in each case in respect of its business
or products that would violate the FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy,
and any amendments thereto. None of the Company, and to the Knowledge of the Company, any contract manufacturer with respect to any Company
Product Candidate, or any of their respective officers, employees or agents has been convicted of any crime or engaged in any conduct
that could result in a debarment or exclusion under (i) 21 U.S.C. Section 335a or (ii) any similar applicable Law. To the Knowledge of
the Company, no debarment or exclusionary claims, actions, proceedings or investigations in respect of their business or products are
pending or threatened against the Company, and to the Knowledge of the Company, any contract manufacturer with respect to any Company
Product Candidate, or any of their respective officers, employees or agents.
(g)
All manufacturing operations conducted by, or to the Knowledge of the Company, for the benefit of, the Company in connection with any
Company Product Candidate, since January 1, 2021, have been and are being conducted in compliance in all material respects with applicable
Laws, including the FDA’s standards for current good manufacturing practices, including applicable requirements contained in 21 C.F.R.
Parts 210 and 211, and the respective counterparts thereof promulgated by Governmental Authorities in countries outside the United States.
(h)
No laboratory or manufacturing site owned by the Company, and to the Knowledge of the Company, no manufacturing site of a contract manufacturer
or laboratory, with respect to any Company Product Candidate, (i) is subject to a Drug Regulatory Agency shutdown or import or export
prohibition or (ii) has received any Form FDA 483, notice of violation, warning letter, untitled letter, or similar correspondence or
notice from the FDA or other Governmental Authority alleging or asserting noncompliance with any applicable Law, in each case, that have
not been complied with or closed to the satisfaction of the relevant Governmental Authority.
3.15
Legal Proceedings; Orders.
(a)
There is no pending Legal Proceeding and, to the Knowledge of the Company, no Person has threatened in writing to commence any Legal
Proceeding: (i) that involves the Company or any of the material assets owned or used by the Company or (ii) that challenges, or that
may have the effect of preventing, delaying, making illegal or otherwise interfering with, the Contemplated Transactions.
(b)
There is no Order to which the Company, or any of the material assets owned or used by the Company, is subject.
3.16
Tax Matters.
(a)
The Company has timely filed all income Tax Returns and all other material Tax Returns that they were required to file under applicable
Law. All such Tax Returns were correct and complete in all material respects and have been prepared in material compliance with all applicable
Law. Subject to exceptions as would not be material, no claim has ever been made by a Governmental Authority in a jurisdiction where
the Company does not file Tax Returns that the Company is subject to taxation by that jurisdiction.
(b)
All material amounts of Taxes due and owing by (or on behalf of) the Company (whether or not shown on any Tax Return) have been timely
paid. The unpaid Taxes of the Company for periods (or portions thereof) ending on or prior to the date of the Company Unaudited Interim
Balance Sheet do not materially exceed the accruals for current Taxes set forth on the Company Unaudited Interim Balance Sheet. Since
the date of the Company Unaudited Interim Balance Sheet, the Company has not incurred any material Liability for Taxes outside the Ordinary
Course of Business or otherwise inconsistent with past custom and practice.
(c)
The Company has withheld and paid to the appropriate Governmental Authority all material Taxes required to have been withheld and paid
in connection with any amounts paid or owing to any employee, independent contractor, creditor, member, or other third party.
(d)
There are no Encumbrances for Taxes (other than Encumbrances described in clause (a) of the definition of “Permitted Encumbrances”) upon
any of the assets of the Company.
(e)
No deficiencies for a material amount of Taxes with respect to the Company have been claimed, proposed or assessed by any Governmental
Authority in writing that have not been timely paid in full. There are no pending (or, based on written notice, threatened) material
audits, assessments, examinations or other actions for or relating to any liability in respect of Taxes of the Company. The Company (or
any of its predecessors) has not waived any statute of limitations in respect of material Taxes or agreed to any extension of time with
respect to a material Tax assessment or deficiency.
(f)
The Company has not been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code in the
last five years.
(g)
The Company is not a party to any Tax allocation, Tax sharing or similar agreement (including indemnity arrangements), other than customary
indemnification provisions in commercial Contracts entered into in the Ordinary Course of Business with vendors, customers, lenders,
or landlords (an “Ordinary Course Agreement”).
(h)
The Company has never been a member of an affiliated group filing a consolidated U.S. federal income Tax Return (other than a group the
common parent of which is the Company). The Company has no material Liability for the Taxes of any Person (other than the Company) under
Treasury Regulations Section 1.1502-6 (or any similar provision of state, local, or foreign law), as a transferee or successor, by Contract
(other than an Ordinary Course Agreement) or otherwise.
(i)
The Company has not entered into any transaction identified as a “reportable transaction” for purposes of Treasury Regulations Sections
1.6011-4(b)(2) or 301.6111-2(b)(2).
(j)
The Company will not be required to include any item of income in, or exclude any item of deduction from, taxable income for any taxable
period (or portion thereof) ending after the Closing Date as a result of any: (i) change in, or use of improper, method of accounting
for a taxable period ending on or prior to the Closing Date; (ii) “closing agreement” as described in Section 7121 of the Code (or any
corresponding or similar provision of state, local or foreign income Tax law) executed on or prior to the Closing Date; (iii) installment
sale or open transaction disposition made on or prior to the Closing Date; (iv) prepaid amount, advance payments or deferred revenue
received or accrued on or prior to the Closing Date other than in respect of such amounts reflected in the Company Unaudited Interim
Balance Sheet or received in the Ordinary Course of Business since the date of the Company Unaudited Interim Balance Sheet; or (v) intercompany
transaction or excess loss amount described in Treasury Regulations under Section 1502 of the Code (or any corresponding or similar provision
of state, local or foreign income Tax Law).
(k)
The Company has never made an election to be classified as an S corporation for U.S. federal income tax purposes.
(l)
The Company is not aware of any facts and has not knowingly taken or agreed to take any action, in each case, that would reasonably be
expected to prevent or impede the Merger from qualifying as a “reorganization” within the meaning of Section 368(a) of the Code.
3.17
Employee and Labor Matters; Benefit Plans.
(a)
Section 3.17(a)(i) of the Company Disclosure Schedule contains a complete and accurate list of the following information, as applicable,
for each current employee of the Company, including each employee on leave of absence or other non-active status: name, employing entity,
workplace location, job title, date of hire, service reference date (if different from date of hire), exempt or non-exempt classification
under the Fair Labor Standards Act, active or non-active status (and the reason for such non-active status and expected return date,
if known), work visa status, current base salary or wage rate, prior year base salary, or wage rate, current incentive compensation target,
prior year incentive compensation target, prior year incentive compensation earned, current commission rate, and commissions earned year
to date, prior year commission rate, and prior year commissions earned, accrued but unused paid time off. Section 3.17(a)(ii)
of the Company Disclosure Schedule contains a complete and accurate list of all individuals who perform services for the Company (A)
under a leasing, contract worker, or similar arrangement with a third-party employer, or (B) as an independent contractor (excluding
accounting, tax, legal, and similar service providers), along with, for each Person described in clauses (A) and (B), such Person’s current
compensation or fee, date of engagement, workplace location, and the nature of the services they perform in respect of the Company.
(b)
To the Company’s Knowledge, no employee or independent contractor performing services for the Company is bound by any contract that purports
to limit the ability of such Person to engage in any activity, services, duties, or practice on behalf of the Company. No employee holding
a management or executive position has notified the Company of an intention to resign, retire, or otherwise terminate his or her employment
prior to the Closing or within six (6) months of the Closing.
(c)
No current or former employees of the Company are or have been represented by a union or similar employee organization with respect to
such employment. The Company is not a party to, bound by, or subject to, or is currently negotiating in connection with entering into,
any collective bargaining agreement or understanding with a labor union or organization. To the Knowledge of the Company, there is not
now, and during the past three (3) years there has not been, any activity or proceeding by a labor union or representative thereof to
organize any employees of the Company. During the last three (3) years, there have not been any strikes, material slowdowns, work stoppages
or, to the Knowledge of the Company threats thereof, by or with respect to the employees of the Company. There is no charge or complaint
pending (or, to the Knowledge of the Company, threatened) before the National Labor Relations Board or other Governmental Authority of
any unfair labor practice in respect of any employees of the Company, nor is the Company subject to any existing order, judgment, or
decision regarding an unfair labor practice claim.
(d)
The Company has, at all times during the past three (3) years, complied in all material respects with all applicable Laws concerning
labor and employment and the terms of each applicable employment or services agreement in respect of all of their respective current
and former employees and independent contractors, including without limitation such Laws relating to wages, hours, discrimination in
employment, whistleblower protections, retaliation, worker classification, workplace safety and health, immigration, employee data privacy
and security, tax withholding and reporting, workers’ compensation, unemployment insurance and employment termination. Except as described
in Section 3.17(d) of the Company Disclosure Schedule, within the past three (3) years, the Company has not (i) has received written
nor, to the Knowledge of the Company, oral notice of any actual or alleged violation of any such Law or breach of any such agreement,
and, to the Knowledge of the Company, there are no grounds therefor, or (ii) has been subject to or received notice of an audit or investigation
by any Governmental Authority relating to any employment-related matter.
(e)
The Company is not delinquent in payments that have become due and payable to any employee or other individual who has performed services
for the Company for wages, salaries, commissions, bonuses, fees, or other compensation for any services performed.
(f)
In the past twelve (12) months, there has been no “mass layoff” or “plant closing” as defined by the Worker Adjustment and Retraining
Notification Act of 1988 (the “WARN Act”) in respect of the Company and the Company has not engaged in layoffs or employment terminations
sufficient in number to trigger application of any state, local, or foreign law or regulation which is similar to the WARN Act.
(g)
Except as set forth on Section 3.17(g) of the Company Disclosure Schedule, neither the Company, nor any of its ERISA Affiliates,
has ever sponsored, contributed to, or provided benefits under or through, or had any obligation to contribute to or provide benefits
under or through any Employee Plan.
3.18
Environmental Matters. Since January 1, 2021, the Company has complied with all applicable Environmental Laws, which compliance
includes the possession by the Company of all permits and other Governmental Authorizations required under applicable Environmental Laws
and compliance with the terms and conditions thereof, except for any failure to be in compliance that, individually or in the aggregate,
would not result in a Company Material Adverse Effect. The Company has not received, since January 1, 2021, any written notice or other
communication (in writing or otherwise), whether from a Governmental Authority, citizens group, employee or otherwise, that alleges that
the Company is not in compliance with any Environmental Law and, to the Knowledge of the Company, there are no circumstances that may
prevent or interfere with the Company’s compliance with any Environmental Law in the future, except where such failure to comply would
not reasonably be expected to have a Company Material Adverse Effect. To the Knowledge of the Company: (a) no current or prior owner
of any property leased or controlled by the Company has received, since January 1, 2021, any written notice or other communication relating
to property owned or leased at any time by the Company, whether from a Governmental Authority, citizens group, employee or otherwise,
that alleges that such current or prior owner or the Company is not in compliance with or violated any Environmental Law relating to
such property and (b) the Company has no material liability under any Environmental Law.
3.19
Insurance. The Company has delivered to MEDS accurate and complete copies of all material insurance policies and all material
self-insurance programs and arrangements relating to the business, assets, liabilities and operations of the Company. Each of such insurance
policies is in full force and effect and the Company is in compliance in all material respects with the terms thereof. Other than customary
end of policy notifications from insurance carriers, since January 1, 2022, the Company has not received any notice or other communication
regarding any actual or possible: (i) cancellation or invalidation of any insurance claim under any insurance policy or (ii) refusal
or denial of any coverage, reservation of rights or rejection of any material claim under any insurance policy. The Company has provided
timely written notice to the appropriate insurance carrier(s) of each Legal Proceeding pending against the Company, and no such carries
has issued a denial of coverage or a reservation of rights with respect to any such Legal Proceeding, or informed the Company of its
intent to do so.
3.20
No Financial Advisors. Except as set forth on Section 3.20 of the Company Disclosure Schedule, no broker, finder or investment
banker is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection
with the Contemplated Transactions based upon arrangements made by or on behalf of the Company.
3.21
Transactions with Affiliates. Section 3.21 of the Company Disclosure Schedule describes any material transactions or relationships,
in the past three (3) years, between, on one hand, the Company and, on the other hand, any (a) executive officer or director of the Company
or any of such executive officer’s or director’s immediate family members, (b) owner of more than five percent (5%) of the voting power
of the outstanding Company Capital Stock or (c) to the Knowledge of the Company, any “related person” (within the meaning of Item 404
of Regulation S-K under the Securities Act) of any such officer, director or owner (other than the Company) in the case of each of (a),
(b) or (c) that is of the type that would be required to be disclosed under Item 404 of Regulation S-K under the Securities Act.
3.22
Privacy and Data Security. The Company has complied with all applicable Privacy Laws and the applicable terms of any Company Contracts
relating to privacy, security, collection or use of Personal Information of any individuals (including clinical trial participants, patients,
patient family members, caregivers or advocates, physicians and other health care professionals, clinical trial investigators, researchers,
pharmacists) that interact with the Company in connection with the operation of the Company’s business, except for such noncompliance
as has not had, and would not reasonably be expected to have, individually or in the aggregate, a Company Material Adverse Effect. To
the Knowledge of the Company, the Company has implemented and maintains reasonable written policies and procedures, satisfying the requirements
of applicable Privacy Laws, concerning the privacy, security, collection and use of Personal Information (the “Privacy Policies”)
and has complied with the same, except for such noncompliance as has not to the Knowledge of the Company had, and would not reasonably
be expected to have, individually or in the aggregate, a Company Material Adverse Effect. To the Knowledge of the Company, as of the
date hereof, no claims have been asserted or threatened against the Company by any Person alleging a violation of Privacy Laws, Privacy
Policies and/or the applicable terms of any Company Contracts relating to privacy, security, collection or use of Personal Information
of any individuals. To the Knowledge of the Company, there have been no data security incidents, personal data breaches or other adverse
events or incidents related to Personal Information or Company data in the custody or control of the Company or any service provider
acting on behalf of the Company, in each case where such incident, breach or event would result in a notification obligation to any Person
under applicable law or pursuant to the terms of any Company Contract.
3.23
Accredited Investor Status. Prior to the date of this Agreement each holder of Company Common Stock has previously represented
to the Company that it is an “accredited investor” within the meaning of Regulation D, Rule 501(a), promulgated by the SEC under the
Securities Act or is not a “U.S. person” within the meaning of Regulation S, Rule 902, promulgated by the SEC under the Securities Act.
3.24
No Other Representations or Warranties. The Company hereby acknowledges and agrees that, except for the representations and warranties
contained in this Agreement, neither MEDS nor any other person on behalf of MEDS makes any express or implied representation or warranty
with respect to MEDS or with respect to any other information provided to the Company, any of its members or any of their respective
Affiliates in connection with the Contemplated Transactions, and (subject to the express representations and warranties of MEDS set forth
in Section 4 (in each case as qualified and limited by the MEDS Disclosure Schedule)) none of the Company, or any of its Representatives
or stockholders, has relied on any such information (including the accuracy or completeness thereof).
Section
4. Representations and Warranties of MEDS and Merger Subs.
Except
(a) as set forth in the written disclosure schedule delivered by MEDS to the Company (the “MEDS Disclosure Schedule”) or (b) as
disclosed in the MEDS SEC Documents filed with the SEC prior to the date hereof and publicly available on the SEC’s Electronic Data Gathering
Analysis and Retrieval system (but (i) without giving effect to any amendment thereof filed with, or furnished to the SEC on or after
the date hereof and (ii) excluding any disclosures contained under the heading “Risk Factors” and any disclosure of risks included in
any “forward-looking statements” disclaimer or in any other section to the extent they are forward-looking statements or cautionary,
predictive or forward-looking in nature), it being understood that any matter disclosed in the MEDS SEC Documents (x) shall not be deemed
disclosed for purposes of Sections 4.1(a), 4.1(b), 4.3, 4.4, 4.5, or 4.6 and (y) shall be deemed
to be disclosed in a section of the MEDS Disclosure Schedule only to the extent that is readily apparent from a reading of such MEDS
SEC Documents that is applicable to such section or subsection of the MEDS Disclosure Schedule, MEDS and Merger Subs represent and warrant
to the Company as follows:
4.1
Due Organization; Subsidiaries.
(a)
Each of MEDS and its Subsidiaries (including Merger Subs) is a corporation or limited liability company duly incorporated or formed,
validly existing and in good standing under the Laws of the jurisdiction of its incorporation or organization and has all necessary corporate
power and authority: (i) to conduct its business in the manner in which its business is currently being conducted, (ii) to own or lease
and use its property and assets in the manner in which its property and assets are currently owned or leased and used and (iii) to perform
its obligations under all Contracts by which it is bound. Since the date of their formation, Merger Subs have not engaged in any activities
other than in connection with or as contemplated by this Agreement. Except as set forth on Section 4.1(a) of the MEDS Disclosure
Schedule, all of MEDS’s Subsidiaries are wholly owned by MEDS.
(b)
Each of MEDS and its Subsidiaries is licensed and qualified to do business, and is in good standing (to the extent applicable in such
jurisdiction), under the Laws of all jurisdictions where the nature of its business in the manner in which its business is currently
being conducted requires such licensing or qualification other than in jurisdictions where the failure to be so qualified individually
or in the aggregate would not be reasonably expected to have a MEDS Material Adverse Effect. Such jurisdictions are set forth on Section
4.1(b) of the MEDS Disclosure Schedule.
(c)
Except as set forth on Section 4.1(c) of the MEDS Disclosure Schedule: (i) MEDS has no Subsidiaries other than Merger Subs and
MEDS does not own any capital stock of, or any equity ownership or profit sharing interest of any nature in, or control directly or indirectly,
any other Entity other than Merger Subs, (ii) MEDS is not and has not otherwise been, directly or indirectly, a party to, member of or
participant in any partnership, joint venture or similar business entity, and (iii) MEDS has not agreed and is not obligated to make,
nor is MEDS bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any
other Entity. MEDS has not, at any time, been a general partner of, and has not otherwise been liable for any of the debts or other obligations
of, any general partnership, limited partnership or other Entity.
4.2
Organizational Documents. MEDS has delivered to the Company accurate and complete copies of MEDS’s Organizational Documents. MEDS
is not in breach or violation of its Organizational Documents in any material respect.
4.3
Authority; Binding Nature of Agreement. Each of MEDS and Merger Subs has all necessary corporate power and authority to enter
into and to perform its obligations under this Agreement and to consummate the Contemplated Transactions. The MEDS Board (at meetings
duly called and held) has: (a) determined that the Contemplated Transactions are fair to, advisable and in the best interests of MEDS
and its stockholders, (b) approved and declared advisable this Agreement and the Contemplated Transactions, including the issuance of
shares of MEDS Capital Stock to the stockholders of the Company pursuant to the terms of this Agreement and (c) determined to recommend,
upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of MEDS vote to approve the Conversion,
the Name Change and the Stock Plan Share Increase (or consent via a Required MEDS Stockholder Consent) pursuant to the terms of this
Agreement. The Merger Sub I Board (by unanimous written consent) has: (x) determined that the Contemplated Transactions are fair to,
advisable, and in the best interests of Merger Sub I and its sole stockholder, (y) deemed advisable and approved this Agreement and the
Contemplated Transactions and (z) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement,
that the stockholder of Merger Sub I vote to adopt this Agreement and thereby approve the Contemplated Transactions. The Merger Sub II
Board (by unanimous written consent) has: (x) determined that the Contemplated Transactions are fair to, advisable, and in the best interests
of Merger Sub II and its sole member, (y) deemed advisable and approved this Agreement and the Contemplated Transactions and (z) determined
to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the member of Merger Sub II vote to adopt
this Agreement and thereby approve the Contemplated Transactions. This Agreement has been duly executed and delivered by Utah and Merger
Subs and, assuming the due authorization, execution and delivery by the Company, constitutes the legal, valid and binding obligation
of MEDS and Merger Subs, enforceable against each of MEDS and Merger Subs in accordance with its terms, subject to the Enforceability
Exceptions.
4.4
Vote/Consent Required. The affirmative vote of a majority of (a) the votes cast at the MEDS Stockholder Meeting is the only vote
of the holders of any class or series of MEDS’s capital stock necessary to approve the Conversion, the Name Change and the Stock Plan
Share Increase (the “Required MEDS Stockholder Vote”), and the Required MEDS Stockholder Vote is the only vote of the holders
of any class or series of MEDS Capital Stock necessary to approve the Conversion, the Name Change and the Stock Plan Share Increase.
Furthermore, the Required MEDS Stockholder Vote is not required solely in the event a fully executed written consent from MEDS stockholders
holding a majority of the shares of MEDS Common Stock entitled to vote thereon is obtained (the “Required MEDS Stockholder Consent”).
4.5
Non-Contravention; Consents.
(a)
Subject to obtaining the Required MEDS Stockholder Vote (or the Required MEDS Stockholder Consent) and the filing of the First Certificate
of Merger required by the DGCL, neither (x) the execution, delivery or performance of this Agreement by MEDS or Merger Subs, nor (y)
the consummation of the Contemplated Transactions, will directly or indirectly (with or without notice or lapse of time):
(i)
contravene, conflict with or result in a violation of any of the provisions of the Organizational Documents of MEDS or its Subsidiaries;
(ii)
contravene, conflict with or result in a material violation of, or give any Governmental Authority or other Person the right to challenge
the Contemplated Transactions or to exercise any remedy or obtain any relief under, any Law or any Order to which MEDS or its Subsidiaries
or any of the assets owned or used by MEDS or its Subsidiaries, is subject;
(iii)
contravene, conflict with or result in a material violation of any of the terms or requirements of, or give any Governmental Authority
the right to revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by MEDS or its Subsidiaries
or that otherwise relates to the business of MEDS, or any of the assets owned, leased or used by MEDS;
(iv)
contravene, conflict with or result in a violation or breach of, or result in a default under, any provision of any MEDS Material Contract,
or give any Person the right to: (A) declare a default or exercise any remedy under any MEDS Material Contract, (B) any material payment,
rebate, chargeback, penalty or change in delivery schedule under any such MEDS Material Contract, (C) accelerate the maturity or performance
of any MEDS Material Contract or (D) cancel, terminate or modify any term of any MEDS Material Contract, except in the case of any nonmaterial
breach, default, penalty or modification; or
(v)
result in the imposition or creation of any Encumbrance upon or with respect to any asset owned or used by MEDS or its Subsidiaries (except
for Permitted Encumbrances).
(b)
Except for (i) any Consent set forth on Section 4.5 of the MEDS Disclosure Schedule under any MEDS Contract, (ii) the Required
MEDS Stockholder Vote (or the Required MEDS Stockholder Consent), (iii) the filing of the First Certificate of Merger with the Secretary
of State of the State of Delaware pursuant to the DGCL, (iv) the filing of the Second Certificate of Merger with the Secretary of State
of the State of Delaware pursuant to the DLLCA and (v) such consents, waivers, approvals, orders, authorizations, registrations, declarations
and filings as may be required under applicable federal and state securities laws, neither MEDS nor any of its Subsidiaries was, is or
will be required to make any filing with or give any notice to, or to obtain any Consent from, any Person in connection with (x) the
execution, delivery or performance of this Agreement or (y) the consummation of the Contemplated Transactions.
(c)
The MEDS Board, the Merger Sub I Board, and the Merger Sub II Board have taken and will take all actions necessary to ensure that the
restrictions applicable to business combinations contained in Section 203 of the DGCL are, and will be, inapplicable to the execution,
delivery and performance of this Agreement and to the consummation of the Contemplated Transactions. No other state takeover statute
or similar Law applies or purports to apply to the Merger, this Agreement or any of the other Contemplated Transactions.
4.6
Capitalization.
(a)
The authorized capital stock of MEDS consists of (i) 100,000,000 shares of MEDS Common Stock of which 1,458,506 shares have been issued
and are outstanding as of July 23, 2024 (the “Capitalization Date”) and (ii) 10,000,000 shares of preferred stock, par value $0.00001
per share ( 787,754 of Series B preferred stock (the “Series B Preferred Stock”), 1,000 of Series C preferred stock and 9,211,246
shares of Series X non-noting convertible preferred stock), of which 15,759 shares of Series B preferred stock, 0 shares of Series C
preferred stock and 0 shares of Series X non-noting convertible preferred stock have been issued and are outstanding as of the Capitalization
Date. MEDS does not hold any shares of its capital stock in its treasury.
(b)
All of the outstanding shares of MEDS Common Stock have been duly authorized and validly issued, and are fully paid and nonassessable
and are free of any Encumbrances. None of the outstanding shares of MEDS Common Stock is entitled or subject to any preemptive right,
right of participation, right of maintenance or any similar right. None of the outstanding shares of MEDS Common Stock is subject to
any right of first refusal in favor of MEDS. Except as contemplated herein, there is no MEDS Contract relating to the voting or registration
of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting any option or similar right with
respect to), any shares of MEDS Common Stock. MEDS is not under any obligation, nor is MEDS bound by any Contract pursuant to which it
may become obligated, to repurchase, redeem or otherwise acquire any outstanding shares of MEDS Common Stock or other securities. Section
4.6(b) of the MEDS Disclosure Schedule accurately and completely describes all repurchase rights held by MEDS with respect to shares
of MEDS Common Stock (including shares issued pursuant to the exercise of stock options) and specifies which of those repurchase rights
are currently exercisable.
(c)
Except for the MEDS 2013 Equity Incentive Plan, as amended (the “Meds 2013 Plan”), the MEDS 2014 Equity Incentive Plan, as amended
(the “MEDS 2014 Plan”) and the MEDS Second Amended and Restated 2019 Equity Incentive Plan, as amended (the “MEDS 2019 Plan”
and, together with the MEDS 2013 Plan and MEDS 2014 Plan, the “MEDS Stock Plans”), and except as set forth on Section 4.6(c)
of the MEDS Disclosure Schedule, MEDS does not have any stock option plan or any other plan, program, agreement or arrangement providing
for any equity-based compensation for any Person. The MEDS 2014 Plan initially authorized 2,000,000 shares of MEDS Common Stock. The
MEDS 2019 Plan initially authorized 2,000,000 shares of MEDS Common Stock, subsequently increased the number of available shares by 2,000,000
in June of 2023, and later reduced by a 1-for-15 reverse stock split in June of 2023. As of the date of this Agreement, no shares of
MEDS Common Stock are available for awards under the MEDS 2014 Plan, and 123,094 shares remain available for future issuance pursuant
to the MEDS 2019 Plan. As of the date of this Agreement, MEDS has reserved 23,930 shares for issuance upon exercise or settlement of
MEDS Options granted under the MEDS Stock Plans. Section 4.6(c) of the MEDS Disclosure Schedule sets forth the following information
with respect to each MEDS Option and MEDS Restricted Stock Unit outstanding as of the date of this Agreement, as applicable: (i) the
name of the holder, (ii) the number of shares of MEDS Common Stock subject to such MEDS Option and MEDS Restricted Stock Units at the
time of grant, (iii) the number of shares of MEDS Common Stock subject to such MEDS Option and MEDS Restricted Stock Units as of the
date of this Agreement, (iv) the exercise price of such MEDS Option, (v) the date on which such MEDS Option and MEDS Restricted Stock
Units was granted, (vi) the applicable vesting schedule, including any acceleration provisions and the number of vested and unvested
shares as of the date of this Agreement, (vii) the date on which such MEDS Option expires, (viii) whether such MEDS Option is intended
to be an “incentive stock option” (as defined in the Code) or a nonqualified stock option and (ix) in the case of a MEDS Option, the
plan pursuant to which such MEDS Option was granted. MEDS has made available to the Company accurate and complete copies of equity incentive
plans pursuant to which MEDS has equity-based awards, the forms of all award agreements evidencing such equity-based awards and evidence
of board and stockholder approval of the MEDS Stock Plans and any amendments thereto.
(d)
Except for the outstanding MEDS Options and MEDS Restricted Stock Units or as set forth on Section 4.6(d) of the MEDS Disclosure
Schedule, there is no: (i) outstanding subscription, option, call, warrant or right (whether or not currently exercisable) to acquire
any shares of the capital stock or other securities of MEDS, (ii) outstanding security, instrument or obligation that is or may become
convertible into or exchangeable for any shares of the capital stock or other securities of MEDS, (iii) stockholder rights plan (or similar
plan commonly referred to as a “poison pill”) or Contract under which MEDS is or may become obligated to sell or otherwise issue any
shares of its capital stock or any other securities or (iv) condition or circumstance that may give rise to or provide a basis for the
assertion of a claim by any Person to the effect that such Person is entitled to acquire or receive any shares of capital stock or other
securities of MEDS. There are no outstanding or authorized stock appreciation, phantom stock, profit participation or other similar rights
with respect to MEDS.
(e)
All outstanding shares of MEDS Common Stock, MEDS Options, MEDS Restricted Stock Units and other securities of MEDS have been issued
and granted in compliance with (i) all applicable securities laws and other applicable Law and (ii) all requirements set forth in applicable
Contracts.
(f)
With respect to MEDS Options and MEDS Restricted Stock Units granted pursuant to the MEDS Stock Plans, (i) each grant of a MEDS Option
or MEDS Restricted Stock Unit was duly authorized no later than the date on which the grant of such MEDS Option and MEDS Restricted Stock
Unit was by its terms to be effective (the “MEDS Grant Date”) by all necessary corporate action, including, as applicable, approval
by the MEDS Board (or a duly constituted and authorized committee thereof) and any required stockholder approval by the necessary number
of votes or written consents, and the award agreement governing such grant (if any) was duly executed and delivered by each party thereto,
(ii) each MEDS Option and MEDS Restricted Stock Unit grant was made in accordance with the terms of the MEDS Stock Plan pursuant to which
it was granted and all other applicable Law and regulatory rules or requirements, and (iii) the per share exercise price of each MEDS
Option was not less than the fair market value of a share of MEDS Common Stock on the applicable MEDS Grant Date.
4.7
SEC Filings; Financial Statements.
(a)
Except as set forth in Section 4.7(a) of the MEDS Disclosure Schedule, MEDS has filed or furnished, as applicable, on a timely
basis all forms, statements, certifications, reports and documents required to be filed or furnished by it with the SEC under the Exchange
Act or the Securities Act since January 1, 2022 (the “MEDS SEC Documents”). As of the time it was filed with the SEC (or, if amended
or superseded by a filing prior to the date of this Agreement, then on the date of such filing), each of the MEDS SEC Documents complied
in all material respects with the applicable requirements of the Securities Act or the Exchange Act (as the case may be) and as of the
time they were filed, none of the MEDS SEC Documents contained any untrue statement of a material fact or omitted to state a material
fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they
were made, not misleading. The certifications and statements required by (i) Rule 13a-14 under the Exchange Act and (ii) 18 U.S.C. §1350
(Section 906 of the Sarbanes-Oxley Act) relating to the MEDS SEC Documents (collectively, the “Certifications”) are accurate and
complete and comply as to form and content with all applicable Laws. As used in this Section 4.7, the term “file” and variations
thereof shall be broadly construed to include any manner in which a document or information is furnished, supplied or otherwise made
available to the SEC.
(b)
The financial statements (including any related notes) contained or incorporated by reference in the MEDS SEC Documents: (i) complied
as to form in all material respects with the Securities Act and the Exchange Act, as applicable, and the published rules and regulations
of the SEC applicable thereto, (ii) were prepared in accordance with GAAP (except as may be indicated in the notes to such financial
statements or, in the case of unaudited financial statements, as permitted by Form 10-Q of the SEC, and except that the unaudited financial
statements may not contain footnotes and are subject to normal and recurring year-end adjustments that are not reasonably expected to
be material in amount) applied on a consistent basis unless otherwise noted therein throughout the periods indicated and (iii) fairly
present, in all material respects, the financial position of MEDS as of the respective dates thereof and the results of operations and
cash flows of MEDS for the periods covered thereby. Other than as expressly disclosed in the MEDS SEC Documents filed prior to the date
hereof, there has been no material change in MEDS’s accounting methods or principles that would be required to be disclosed in MEDS’s
financial statements in accordance with GAAP. The books of account and other financial records of MEDS and each of its Subsidiaries are
true and complete in all material respects.
(c)
MEDS’s auditor has at all times since the date of enactment of the Sarbanes-Oxley Act been: (i) a registered public accounting firm (as
defined in Section 2(a)(12) of the Sarbanes-Oxley Act), (ii) to the Knowledge of MEDS, “independent” with respect to MEDS within the
meaning of Regulation S-X under the Exchange Act and (iii) to the Knowledge of MEDS, in compliance with subsections (g) through (l) of
Section 10A of the Exchange Act and the rules and regulations promulgated by the SEC and the Public Company Accounting Oversight Board
thereunder.
(d)
Except as set forth on Section 4.7(d) of the MEDS Disclosure Schedule, MEDS has not received any comment letter from the SEC or
the staff thereof or any correspondence from Nasdaq or the staff thereof relating to the delisting or maintenance of listing of the MEDS
Common Stock on Nasdaq. MEDS has not disclosed any unresolved comments in the MEDS SEC Documents.
(e)
There have been no formal internal investigations regarding financial reporting or accounting policies and practices discussed with,
reviewed by or initiated at the direction of the chief executive officer, chief financial officer, or general counsel of MEDS, the MEDS
Board or any committee thereof, other than ordinary course audits or reviews of accounting policies and practices or internal controls
required by the Sarbanes-Oxley Act.
(f)
Except as set forth on Section 4.7(f) of the MEDS Disclosure Schedule, MEDS is in compliance in all material respects with the
applicable provisions of the Sarbanes-Oxley Act, the Exchange Act and the applicable listing and governance rules and regulations of
Nasdaq.
(g)
MEDS maintains a system of internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act)
that is sufficient to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with GAAP, including policies and procedures sufficient to provide reasonable assurance
(i) that MEDS maintains records that in reasonable detail accurately and fairly reflect MEDS’s transactions and dispositions of assets,
(ii) that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, (iii) that receipts
and expenditures are made only in accordance with authorizations of management and the MEDS Board and (iv) regarding prevention or timely
detection of the unauthorized acquisition, use or disposition of MEDS’s assets that could have a material effect on MEDS’s financial
statements. MEDS has evaluated the effectiveness of MEDS’s internal control over financial reporting and, to the extent required by applicable
Law, presented in any applicable MEDS SEC Document that is a report on Form 10-K or Form 10-Q (or any amendment thereto) its conclusions
about the effectiveness of the internal control over financial reporting as of the end of the period covered by such report or amendment
based on such evaluation. MEDS has disclosed to MEDS’s auditors and the Audit Committee of the MEDS Board (and made available to the
Company a summary of the significant aspects of such disclosure) (A) all significant deficiencies and material weaknesses in the design
or operation of internal control over financial reporting that are reasonably likely to adversely affect MEDS’s ability to record, process,
summarize and report financial information and (B) any fraud, whether or not material, that involves management or other employees who
have a significant role in MEDS’s or its Subsidiaries’ internal control over financial reporting. Except as disclosed in the MEDS SEC
Documents filed prior to the date hereof, MEDS’s internal control over financial reporting is effective and MEDS has not identified any
material weaknesses in the design or operation of MEDS’s internal control over financial reporting.
(h)
MEDS’s “disclosure controls and procedures” (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act) are designed to ensure
that all information (both financial and nonfinancial) required to be disclosed by MEDS in the reports that it files or submits under
the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC,
and that all such information is accumulated and communicated to MEDS’s principal executive officer and principal financial officer as
appropriate to allow timely decisions regarding required disclosure and to make the Certifications and such disclosure controls and procedures
are effective. MEDS has carried out evaluation of the effectiveness of its disclosure controls and procedures as required by Rule 13a-15
of the Exchange Act.
(i)
MEDS has not been and is not currently a “shell company” as defined under Section 12b-2 of the Exchange Act.
4.8
Absence of Changes. Except as set forth on Section 4.8 of the MEDS Disclosure Schedule, between January 1, 2024
and the date of this Agreement, MEDS has conducted its business only in the Ordinary Course of Business (except for the execution and
performance of this Agreement and the discussions, negotiations and transactions related thereto) and there has not been any (a) MEDS
Material Adverse Effect or (b) actions to do any of the following:
(a)
declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase,
redeem or otherwise reacquire any shares of its capital stock or other securities (except for shares of MEDS Common Stock from terminated
employees, directors or consultants of MEDS in accordance with agreements in effect on the date of this Agreement providing for the repurchase
of shares at no more than the purchase price thereof in connection with any termination of services to MEDS or any of its Subsidiaries);
(b)
sell, issue, grant, pledge or otherwise dispose of or encumber or authorize the issuance of: (A) any capital stock or other security
(except for MEDS Common Stock issued upon the valid exercise or settlement of outstanding MEDS Options or MEDS Restricted Stock Units
as applicable), (B) any option, warrant or right to acquire any capital stock or any other security or (C) any instrument convertible
into or exchangeable for any capital stock or other security;
(c)
except as required to give effect to anything in contemplation of the Closing, amend any of its Organizational Documents, or effect or
be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split,
reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(d)
form any Subsidiary or acquire any equity interest or other interest in any other Entity or enter into a joint venture with any other
Entity;
(e)
(i) lend money to any Person, (ii) incur or guarantee any indebtedness for borrowed money, (iii) guarantee any debt securities of others
or (iv) make any capital expenditure or commitment;
(f)
other than as required by applicable Law or the terms of any MEDS Employee Plan in effect as of the date of this Agreement: (i) adopt,
establish or enter into any MEDS Employee Plan, including, for the avoidance of doubt, any equity awards plans, (ii) cause or permit
any MEDS Employee Plan to be amended other than as required by law or in order to make amendments for the purposes of Section 409A of
the Code, (iii) pay any bonus or make any profit-sharing or similar payment to (except with respect to obligations in place on the date
of this Agreement pursuant to any MEDS Employee Plan), or increase the amount of the wages, salary, commissions, fringe benefits or other
compensation or remuneration payable to, any of its employees, directors or consultants, (iv) increase the severance or change of control
benefits offered to any current or new employees, directors or consultants, or (v) hire any officer, employee or consultant;
(g)
enter into any material transaction outside the Ordinary Course of Business;
(h)
acquire any material asset or sell, lease, license or otherwise irrevocably dispose of any of its assets or properties, or grant any
Encumbrance with respect to such assets or properties;
(i)
make (other than consistent with past practice), change or revoke any material Tax election; file any material amendment to any Tax Return;
settle or compromise any material Tax claim; waive or extend any statute of limitations in respect of a period within which an assessment
or reassessment of material Taxes may be issued (other than any extension pursuant to an extension to file any Tax Return); enter into
any “closing agreement” as described in Section 7121 of the Code (or any similar Law) with any Governmental Authority; or adopt or change
any material accounting method in respect of Taxes;
(j)
waive, settle or compromise any pending or threatened Legal Proceeding against MEDS or any of its Subsidiaries, other than waivers, settlements
or agreements (i) for an amount not in excess of $100,000 in the aggregate (excluding amounts to be paid under existing insurance policies
or renewals thereof) and (ii) that do not impose any material restrictions on the operations or businesses of MEDS or its Subsidiaries,
taken as a whole, or any equitable relief on, or the admission of wrongdoing by MEDS or any of its Subsidiaries;
(k)
delay or fail to repay when due any material obligation, including accounts payable and accrued expenses, other than in the Ordinary
Course of Business;
(l)
forgive any loans to any Person, including its employees, officers, directors or Affiliate;
(m)
sell, assign, transfer, license, sublicense or otherwise dispose of any material MEDS IP Rights (other than in the Ordinary Course of
Business);
(n)
terminate or modify in any material respect, or fail to exercise renewal rights with respect to, any material insurance policy;
(o)
enter into, amend, terminate, or waive any material option or right under, any MEDS Material Contract;
(p)
(i) materially change pricing or royalties or other payments set or charged by MEDS or any of Subsidiaries to its customers or licensees
or (ii) agree to materially change pricing or royalties or other payments set or charged by Persons who have licensed Intellectual Property
to MEDS or any of Subsidiaries; or
(q)
agree, resolve or commit to do any of the foregoing.
4.9
Absence of Undisclosed Liabilities. Neither MEDS nor any of its Subsidiaries has any Liability of a type required to be reflected
or reserved for on a balance sheet prepared in accordance with GAAP, except for: (a) Liabilities disclosed, reflected or reserved against
in the MEDS Unaudited Interim Balance Sheet, (b) normal and recurring current Liabilities that have been incurred by MEDS or its Subsidiaries
since the date of the MEDS Unaudited Interim Balance Sheet in the Ordinary Course of Business (none of which relates to any breach of
contract, breach of warranty, tort, infringement, or violation of Law), (c) Liabilities for performance of obligations of MEDS or any
of its Subsidiaries under MEDS Contracts, (d) Liabilities incurred in connection with the Contemplated Transactions and (e) described
in Section 4.9 of the MEDS Disclosure Schedule.
4.10
Title to Assets. Each of MEDS and its Subsidiaries owns, and has good and valid title to, or, in the case of leased properties
and assets, valid leasehold interests in, all tangible properties or tangible assets and equipment used or held for use in its business
or operations or purported to be owned by it, including: (a) all tangible assets reflected on the MEDS Unaudited Interim Balance Sheet
and (b) all other tangible assets reflected in the books and records of MEDS as being owned by MEDS. All of such assets are owned or,
in the case of leased assets, leased by MEDS or any of its Subsidiaries free and clear of any Encumbrances, other than Permitted Encumbrances.
4.11
Real Property; Leasehold. Neither MEDS nor any of its Subsidiaries owns or has ever owned any real property. MEDS has made available
to the Company (a) an accurate and complete list of all real properties with respect to which MEDS directly or indirectly holds a valid
leasehold interest as well as any other real estate that is in the possession of or leased by MEDS or any of its Subsidiaries and (b)
copies of all leases under which any such real property is possessed (the “MEDS Real Estate Leases”), each of which is in full
force and effect, with no existing material default thereunder.
4.12
Intellectual Property.
(a)
Section 4.12(a) of the MEDS Disclosure Schedule is an accurate, true and complete listing of all MEDS Registered IP.
(b)
Section 4.12(b) of the MEDS Disclosure Schedule accurately identifies (i) all MEDS Contracts pursuant to which any MEDS IP Rights
are licensed to MEDS (other than (A) any non-customized software that (1) is so licensed solely in executable or object code form pursuant
to a nonexclusive, internal use software license and other Intellectual Property associated with such software and (2) is not incorporated
into, or material to the development, manufacturing, or distribution of, any of MEDS products or services, (B) any Intellectual Property
licensed on a nonexclusive basis ancillary to the purchase or use of equipment, reagents or other materials, (C) any confidential information
provided under confidentiality agreements and (D) agreements between MEDS and its employees in MEDS’s standard form thereof) and (ii)
whether the license or licenses granted to MEDS are exclusive or nonexclusive.
(c)
Section 4.12(c) of the MEDS Disclosure Schedule accurately identifies each MEDS Contract pursuant to which any Person has been
granted any license under, or otherwise has received or acquired any right (whether or not currently exercisable) or interest in, any
MEDS IP Rights (other than (i) any confidential information provided under confidentiality agreements and (ii) any MEDS IP Rights nonexclusively
licensed to academic collaborators, suppliers or service providers for the sole purpose of enabling such academic collaborator, supplier
or service providers to provide services for MEDS’s benefit).
(d)
Neither MEDS not any of its Subsidiaries is bound by, and no MEDS IP Rights are subject to, any Contract containing any covenant or other
provision that in any way limits or restricts the ability of MEDS or any of its Subsidiaries to use, exploit, assert, or enforce any
MEDS IP Rights anywhere in the world.
(e)
MEDS or one of its Subsidiaries exclusively owns all right, title, and interest to and in the MEDS IP Rights (other than (i) MEDS IP
Rights licensed to the Company, or co-owned rights each as identified in Section 4.12(c) of the MEDS Disclosure Schedule, (ii)
any non-customized software that (A) is licensed to the Company solely in executable or object code form pursuant to a nonexclusive,
internal use software license and other Intellectual Property associated with such software and (B) is not incorporated into, or material
to the development, manufacturing, or distribution of, any of MEDS or its Subsidiaries’ products or services and (iii) any Intellectual
Property licensed on a nonexclusive basis ancillary to the purchase or use of equipment, reagents or other materials), in each case,
free and clear of any Encumbrances (other than Permitted Encumbrances). Without limiting the generality of the foregoing:
(i)
All documents and instruments necessary to register or apply for or renew registration of MEDS Registered IP have been validly executed,
delivered, and filed in a timely manner with the appropriate Governmental Authority.
(ii)
Each Person who is or was an employee or contractor of MEDS or any of its Subsidiaries and who is or was involved in the creation or
development of any Intellectual Property for MEDS or any of its Subsidiaries has signed a valid, enforceable agreement containing a present
assignment of such Intellectual Property to MEDS or such Subsidiary and confidentiality provisions protecting trade secrets and confidential
information of MEDS and its Subsidiaries.
(iii)
To the Knowledge of MEDS, no current or former member, officer, director, or employee of MEDS or any of its Subsidiaries has any claim,
right (whether or not currently exercisable), or interest to or in any MEDS IP Rights purported to be owned by MEDS. To the Knowledge
of MEDS, no employee of MEDS or any of its Subsidiaries is (a) bound by or otherwise subject to any Contract restricting him or her from
performing his or her duties for MEDS or such Subsidiary or (b) in breach of any Contract with any former employer or other Person concerning
MEDS IP Rights purported to be owned by MEDS or such Subsidiary or confidentiality provisions protecting trade secrets and confidential
information comprising MEDS IP Rights purported to be owned by MEDS or such Subsidiary.
(iv)
No funding, facilities, or personnel of any Governmental Authority were used, directly or indirectly, to develop or create, in whole
or in part, any MEDS IP Rights in which MEDS or any of its Subsidiaries has an ownership interest.
(v)
MEDS and each of its Subsidiaries has taken reasonable steps to maintain the confidentiality of and otherwise protect and enforce its
rights in all proprietary information that MEDS or such Subsidiary holds, or purports to hold, as confidential or a trade secret.
(vi)
MEDS or any of its Subsidiaries has not assigned or otherwise transferred ownership of, or agreed to assign or otherwise transfer ownership
of, any MEDS IP Rights to any other Person.
(vii)
To the Knowledge of MEDS, the MEDS IP Rights constitute all Intellectual Property necessary for MEDS to conduct its business as currently
conducted; provided, however, that the foregoing representation is not a representation with respect to non-infringement
of Intellectual Property.
(f)
MEDS has delivered, or made available to the Company, a complete and accurate copy of all material MEDS IP Rights Agreements.
(g)
The manufacture, marketing, offering for sale, sale, importation, use or intended use or other disposal of any product as currently sold
or under development by MEDS does not violate any license or agreement between MEDS or its Subsidiaries and any third party in any material
respect, and, to the Knowledge of MEDS, does not infringe or misappropriate any valid and issued Patent right or other Intellectual Property
of any other Person, which infringement or misappropriation would reasonably be expected to have a MEDS Material Adverse Effect. To the
Knowledge of MEDS, no third party is infringing upon any Patents owned by MEDS within the MEDS IP Rights, or violating any MEDS IP Rights
Agreement.
(h)
As of the date of this Agreement, MEDS is not a party to any Legal Proceeding (including, but not limited to, opposition, interference
or other proceeding in any patent or other government office) contesting the validity, ownership or right to use, sell, offer for sale,
license or dispose of any MEDS IP Rights. MEDS has not received any written notice asserting that any MEDS Registered IP or the proposed
use, sale, offer for sale, license or disposition of any products, methods, or processes claimed or covered thereunder infringes or misappropriates
or violates the rights of any other Person or that MEDS or any of its Subsidiaries have otherwise infringed, misappropriated or otherwise
violated any Intellectual Property of any Person.
(i)
To the Knowledge of MEDS, no trademark (whether registered or unregistered) or trade name owned, used, or applied for by MEDS conflicts
or interferes with any trademark (whether registered or unregistered) or trade name owned, used, or applied for by any other Person except
as would not have a MEDS Material Adverse Effect. None of the goodwill associated with or inherent in any trademark (whether registered
or unregistered) in which MEDS has or purports to have an ownership interest has been impaired as determined by MEDS in accordance with
GAAP.
(j)
Except as may be set forth in the Contracts listed on Section 4.12(b) or 4.12(c) of the MEDS Disclosure Schedule or as
contained in license, distribution or service agreements entered into in the Ordinary Course of Business by MEDS (i) MEDS is not bound
by any Contract to indemnify, defend, hold harmless, or reimburse any other Person with respect to any Intellectual Property infringement,
misappropriation, or similar claim which is material to MEDS taken as a whole and (ii) MEDS has never assumed, or agreed to discharge
or otherwise take responsibility for, any existing or potential liability of another Person for infringement, misappropriation, or violation
of any Intellectual Property right, which assumption, agreement or responsibility remains in force as of the date of this Agreement.
(k)
Neither MEDS nor any of its Subsidiaries is party to any Contract that, as a result of such execution, delivery and performance of this
Agreement, will cause the grant of any license or other right to any MEDS IP Rights, result in breach of, default under or termination
of such Contract with respect to any MEDS IP Rights, or impair the right of MEDS or the Surviving Company and its Subsidiaries to use,
sell or license or enforce any MEDS IP Rights or portion thereof, except for the occurrence of any such grant or impairment that would
not individually or in the aggregate, reasonably be expected to result in a MEDS Material Adverse Effect.
4.13
Agreements, Contracts and Commitments.
(a)
Section 4.13(a) of the MEDS Disclosure Schedule identifies each MEDS Contract that is in effect as of the date of this Agreement
(each, an “MEDS Material Contract” and collectively, the “MEDS Material Contracts”), excluding any Employee Plan:
(i)
each MEDS Contract relating to any material bonus, deferred compensation, severance, incentive compensation, pension, profit-sharing
or retirement plans, or any other employee benefit plans or arrangements;
(ii)
each MEDS Contract requiring payments by MEDS after the date of this Agreement in excess of $100,000 pursuant to its express terms relating
to the employment of, or the performance of employment-related services by, any Person, including any employee, consultant or independent
contractor, or Entity providing employment related, consulting or independent contractor services, not terminable by MEDS on ninety (90)
calendar days’ or less notice without liability, except to the extent general principles of wrongful termination Law may limit MEDS’s,
or such successor’s ability to terminate employees at will;
(iii)
each MEDS Contract relating to any agreement or plan, including any option plan, stock appreciation right plan or stock purchase plan,
any of the benefits of which will be increased, or the vesting of benefits of which will be accelerated, by the occurrence of any of
the Contemplated Transactions (either alone or in conjunction with any other event, such as termination of employment), or the value
of any of the benefits of which will be calculated on the basis of any of the Contemplated Transactions;
(iv)
each MEDS Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business;
(v)
each MEDS Contract containing (A) any covenant limiting the freedom of MEDS or any of its Subsidiaries to engage in any line of business
or compete with any Person, or limiting the development, manufacture, or distribution of the MEDS’s products or services (B) any most-favored
pricing arrangement, (C) any exclusivity provision or (D) any non-solicitation provision;
(vi)
each MEDS Contract (A) pursuant to which any Person granted MEDS an exclusive license under any Intellectual Property, or (B) pursuant
to which MEDS granted any Person an exclusive license under any MEDS IP Rights;
(vii)
each MEDS Contract relating to capital expenditures and requiring payments after the date of this Agreement in excess of $100,000 pursuant
to its express terms and not cancelable without penalty;
(viii)
each MEDS Contract relating to the disposition or acquisition of material assets or any ownership interest in any Entity, in each case,
involving payments in excess of $100,000 after the date of this Agreement;
(ix)
each MEDS Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other agreements
or instruments relating to the borrowing of money or extension of credit in excess of $100,000 or creating any material Encumbrances
with respect to any assets of MEDS or any loans or debt obligations with officers or directors of MEDS;
(x)
each MEDS Contract requiring payment by or to the Company after the date of this Agreement in excess of $100,000 pursuant to its express
terms relating to: (A) any distribution agreement (identifying any that contain exclusivity provisions), (B) any agreement involving
provision of services or products with respect to any pre-clinical or clinical development activities of the Company, (C) any dealer,
distributor, joint marketing, alliance, joint venture, cooperation, development or other agreement currently in force under which MEDS
or any of its Subsidiaries has continuing obligations to develop or market any product, technology or service, or any agreement pursuant
to which MEDS or any of its Subsidiaries has continuing obligations to develop any Intellectual Property that will not be owned, in whole
or in part, by MEDS or such Subsidiary or (D) any Contract to license any patent, trademark registration, service mark registration,
trade name or copyright registration to or from any third party to manufacture or produce any product, service or technology of MEDS
or any of its Subsidiaries or any Contract to sell, distribute or commercialize any products or service of MEDS or any of its Subsidiaries,
in each case, except for MEDS Contracts entered into in the Ordinary Course of Business;
(xi)
each MEDS Contract with any Person, including any financial advisor, broker, finder, investment banker or other Person, providing advisory
services to MEDS in connection with the Contemplated Transactions;
(xii)
each MEDS Contract to which MEDS or any of its Subsidiaries is a party or by which any of their assets and properties is currently bound,
which involves annual obligations of payment by, or annual payments to, MEDS or such Subsidiary in excess of $100,000;
(xiii)
a MEDS Real Estate Lease;
(xiv)
a Contract disclosed in or required to be disclosed in Section 4.12(b) or Section 4.12(c) of the MEDS Disclosure Schedule;
or
(xv)
any other MEDS Contract that is not terminable at will (with no penalty or payment) by MEDS or any of its Subsidiaries, and (A) which
involves payment or receipt by MEDS or such Subsidiary after the date of this Agreement under any such agreement, contract or commitment
of more than $100,000 in the aggregate, or obligations after the date of this Agreement in excess of $100,000 in the aggregate or (B)
that is material to the business or operations of MEDS and its Subsidiaries taken as a whole.
(b)
MEDS has delivered or made available to the Company accurate and complete copies of all MEDS Material Contracts, including all amendments
thereto. There are no MEDS Material Contracts that are not in written form. MEDS has not nor, to MEDS’s Knowledge as of the date of this
Agreement, has any other party to a MEDS Material Contract, breached, violated or defaulted under, or received notice that it breached,
violated or defaulted under, any of the terms or conditions of any MEDS Material Contract in such manner as would permit any other party
to cancel or terminate any such MEDS Material Contract, or would permit any other party to seek damages which would reasonably be expected
to have a MEDS Material Adverse Effect. As to MEDS and its Subsidiaries, as of the date of this Agreement, each MEDS Material Contract
is valid, binding, enforceable and in full force and effect, subject to the Enforceability Exceptions. No Person is renegotiating, or
has a right pursuant to the terms of any MEDS Material Contract to change, any material amount paid or payable to MEDS under any MEDS
Material Contract or any other material term or provision of any MEDS Material Contract.
4.14
Compliance; Permits; Restrictions.
(a)
MEDS and each of its Subsidiaries is, and since January 1, 2020, has been in material compliance with all applicable Laws. No investigation,
claim, suit, proceeding, audit, Order, or other action by any Governmental Authority is pending or, to the Knowledge of MEDS, threatened
against MEDS or any of its Subsidiaries. There is no agreement or Order binding upon MEDS or any of its Subsidiaries which (i) has or
could reasonably be expected to have the effect of prohibiting or materially impairing any business practice of MEDS or any of its Subsidiaries,
any acquisition of material property by MEDS or any of its Subsidiaries or the conduct of business by MEDS or any of its Subsidiaries
as currently conducted, (ii) is reasonably likely to have an adverse effect on MEDS’s ability to comply with or perform any covenant
or obligation under this Agreement or (iii) is reasonably likely to have the effect of preventing, delaying, making illegal or otherwise
interfering with the Contemplated Transactions.
(b)
Each of MEDS and its Subsidiaries holds all required Governmental Authorizations that are material to the operation of the business of
MEDS and Merger Subs as currently conducted (collectively, the “MEDS Permits”). Section 4.12(b) of the MEDS Disclosure
Schedule identifies each MEDS Permit. Each of MEDS and its Subsidiaries is in material compliance with the terms of the MEDS Permits.
No Legal Proceeding is pending or, to the Knowledge of MEDS, threatened, which seeks to revoke, substantially limit, suspend, or materially
modify any MEDS Permit. The rights and benefits of each MEDS Permit will be available to MEDS and Surviving Company immediately after
the Second Effective Time on terms substantially identical to those enjoyed by MEDS and its Subsidiaries as of the date of this Agreement
and immediately prior to the First Effective Time.
(c)
There are no Legal Proceedings pending or, to the Knowledge of MEDS, threatened with respect to an alleged material violation by MEDS
or any of its Subsidiaries of the FDCA, PHSA, FDA regulations adopted thereunder, the Controlled Substances Act or any other similar
Law promulgated by a Drug Regulatory Agency.
(d)
Each of MEDS and its Subsidiaries holds all required Governmental Authorizations issuable by any Drug Regulatory Agency necessary for
the conduct of the business of MEDS and Merger Subs as currently conducted, and, as applicable, the development, testing, manufacturing,
processing, storage, labeling, sale, marketing, advertising, distribution and importation or exportation, as currently conducted, of
any of its product candidates (the “MEDS Product Candidates”) (the “MEDS Regulatory Permits”) and no such MEDS Regulatory
Permit has been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified in any adverse manner other than immaterial
adverse modifications. MEDS has timely maintained and is in compliance in all material respects with the MEDS Regulatory Permits and
neither MEDS nor or any of its Subsidiaries has, since January 1, 2022, received any written notice or other written communication from
any Drug Regulatory Agency regarding (A) any material violation of or failure to comply materially with any term or requirement of any
MEDS Regulatory Permit or (B) any revocation, withdrawal, suspension, cancellation, termination or material modification of any MEDS
Regulatory Permit. Except for the information and files identified in Section 4.14(d) of the MEDS Disclosure Schedule, MEDS has
made available to the Company all information requested by the Company in MEDS’s or its Subsidiaries’ possession or control relating
to the MEDS Product Candidates and the development, testing, manufacturing, processing, storage, labeling, sale, marketing, advertising,
distribution and importation or exportation of the MEDS Product Candidates, including, but not limited to, complete copies of the following
(to the extent there are any): (x) adverse event reports; pre-clinical, clinical and other study reports and material study data; inspection
reports, notices of adverse findings, untitled letters, warning letters, filings and letters and other written correspondence to and
from any Drug Regulatory Agency; and meeting minutes with any Drug Regulatory Agency and (y) similar reports, material study data, notices,
letters, filings, correspondence and meeting minutes with any other Governmental Authority. All such information are accurate and complete
in all material respects.
(e)
All clinical, pre-clinical and other studies and tests conducted by or on behalf of, or sponsored by, MEDS or its Subsidiaries, in which
MEDS or its Subsidiaries or their respective product candidates, including the MEDS Product Candidates, have participated were and, if
still pending, are being conducted in all material respects in accordance with standard medical and scientific research procedures and
in compliance in all material respects with the applicable regulations of the Drug Regulatory Agencies and other applicable Law, including,
without limitation, 21 C.F.R. Parts 50, 54, 56, 58 and 312. Other than as set forth on Section 4.14(e) of the MEDS Disclosure
Schedule, neither MEDS nor any of its Subsidiaries has received any written notices, correspondence, or other communications from any
Drug Regulatory Agency requiring or, to the Knowledge of MEDS, any action to place a clinical hold order on, or otherwise terminate,
delay, or suspend any clinical studies conducted by or on behalf of, or sponsored by, MEDS or any of its Subsidiaries or in which MEDS
or any of its Subsidiaries or its current product candidates, including the MEDS Product Candidates, have participated. Further, no clinical
investigator, researcher, or clinical staff participating in any clinical study conducted by or, to the Knowledge of MEDS, on behalf
of MEDS or any of its Subsidiaries has been disqualified from participating in studies involving the MEDS Product Candidates, and to
the Knowledge of MEDS, no such administrative action to disqualify such clinical investigators, researchers or clinical staff has been
threatened or is pending.
(f)
Neither MEDS nor any of its Subsidiaries and, to the Knowledge of MEDS, any contract manufacturer with respect to any MEDS Product Candidate
is the subject of any pending or, to the Knowledge of MEDS, threatened investigation in respect of its business or products by the FDA
pursuant to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg.
46191 (September 10, 1991) and any amendments thereto. To the Knowledge of MEDS, neither MEDS nor any of its Subsidiaries and no contract
manufacturer with respect to any MEDS Product Candidate has committed any acts, made any statement, or failed to make any statement,
in each case in respect of its business or products that would violate FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and
Illegal Gratuities” Final Policy, and any amendments thereto. None of MEDS, any of its Subsidiaries, and to the Knowledge of MEDS, any
contract manufacturer with respect to any MEDS Product Candidate, or any of their respective officers, employees or agents has been convicted
of any crime or engaged in any conduct that could result in a material debarment or exclusion under (i) 21 U.S.C. Section 335a or (ii)
any similar applicable Law. To the Knowledge of MEDS, no material debarment or exclusionary claims, actions, proceedings or investigations
in respect of their business or products are pending or threatened against MEDS, any of its Subsidiaries, and to the Knowledge of the
MEDS, any contract manufacturer with respect to any MEDS Product Candidate, or any of its officers, employees or agents.
(g)
All manufacturing operations conducted by, or to the Knowledge of MEDS, for the benefit of, MEDS or its Subsidiaries in connection with
any MEDS Product Candidate, since January 1, 2021, have been and are being conducted in compliance in all material respects with applicable
Laws, including the FDA’s standards for current good manufacturing practices, including applicable requirements contained in 21 C.F.R.
Parts 210 and 211, and the respective counterparts thereof promulgated by Governmental Authorities in countries outside the United States.
(h)
No laboratory or manufacturing site owned by MEDS or its Subsidiaries, and to the Knowledge of MEDS, no manufacturing site of a contract
manufacturer or laboratory, with respect to any MEDS Product Candidate, (i) is subject to a Drug Regulatory Agency shutdown or import
or export prohibition or (ii) has received any Form FDA 483, notice of violation, warning letter, untitled letter, or similar correspondence
or notice from the FDA or other Governmental Authority alleging or asserting noncompliance with any applicable Law, in each case, that
have not been complied with or closed to the satisfaction of the relevant Governmental Authority, and, to the Knowledge of MEDS, neither
the FDA nor any other Governmental Authority is considering such action.
4.15
Legal Proceedings; Orders.
(a)
Except as set forth in Section 4.15(a) of the MEDS Disclosure Schedule, there is no pending Legal Proceeding and, to the Knowledge
of MEDS, no Person has threatened in writing to commence any Legal Proceeding: (i) that involves MEDS or any of its Subsidiaries or any
MEDS Associate (in his or her capacity as such) or any of the material assets owned or used by MEDS or any of its Subsidiaries or (ii)
that challenges, or that may have the effect of preventing, delaying, making illegal or otherwise interfering with, the Contemplated
Transactions.
(b)
There is no Order to which MEDS or any of its Subsidiaries, or any of the material assets owned or used by MEDS or any of its Subsidiaries
is subject. To the Knowledge of MEDS, no officer or other Key Employee of MEDS or any of its Subsidiaries is subject to any Order that
prohibits such officer or employee from engaging in or continuing any conduct, activity or practice relating to the business of MEDS
or any of its Subsidiaries or to any material assets owned or used by MEDS or any of its Subsidiaries.
4.16
Tax Matters.
(a)
Each of MEDS and each of its Subsidiaries has timely filed all income Tax Returns and all other material Tax Returns that were required
to be filed by or with respect to it under applicable Law. All such Tax Returns were correct and complete in all material respects and
have been prepared in material compliance with all applicable Law. Subject to exceptions as would not be material, no claim has ever
been made by a Governmental Authority in a jurisdiction where MEDS or any of its Subsidiaries does not file Tax Returns that MEDS or
any of its Subsidiaries is subject to taxation by that jurisdiction.
(b)
All material amounts of Taxes due and owing by MEDS and each of its Subsidiaries (whether or not shown on any Tax Return) have been timely
paid. The unpaid Taxes of MEDS and each of its Subsidiaries for periods (or portions thereof) ending on or prior to the date of the MEDS
Unaudited Interim Balance Sheet do not materially exceed the accruals for current Taxes set forth on the MEDS Unaudited Interim Balance
Sheet. Since the date of the MEDS Unaudited Interim Balance Sheet, neither MEDS nor any of its Subsidiaries has incurred any material
Liability for Taxes outside the Ordinary Course of Business or otherwise inconsistent with past custom and practice.
(c)
Each of MEDS and each of its Subsidiaries has (i) withheld and paid to the appropriate Governmental Authority all material Taxes required
to have been withheld and paid in connection with any amounts paid or owing to any employee, independent contractor, creditor, stockholder,
or other third party.
(d)
There are no Encumbrances for material Taxes (other Encumbrances described in clause (a) of the definition of “Permitted Encumbrances”)
upon any of the assets of MEDS or any of its Subsidiaries.
(e)
No deficiencies for a material amount of Taxes with respect to MEDS or any of its Subsidiaries have been claimed, proposed or assessed
by any Governmental Authority in writing that have not been timely paid in full. There are no pending (or, based on written notice, threatened)
material audits, examinations assessments or other actions for or relating to any liability in respect of Taxes of MEDS or any of its
Subsidiaries. Neither MEDS nor any of its Subsidiaries has waived any statute of limitations in respect of material Taxes or agreed to
any extension of time with respect to a material Tax assessment or deficiency.
(f)
Neither MEDS nor any of its Subsidiaries is a party to any Tax allocation, Tax sharing or similar agreement (including indemnity arrangements),
other than Ordinary Course Agreements.
(g)
Neither MEDS nor any of its Subsidiaries has been a member of an affiliated group filing a consolidated U.S. federal income Tax Return
(other than a group the common parent of which is MEDS). Neither MEDS nor any of its Subsidiaries has any material Liability for the
Taxes of any Person (other than MEDS and Merger Subs) under Treasury Regulations Section 1.1502-6 (or any similar provision of state,
local, or foreign law), as a transferee or successor, by Contract (other than an Ordinary Course Agreement) or otherwise.
(h)
Neither MEDS nor any of its Subsidiaries has distributed stock of another Person, or had its stock distributed by another Person, in
a transaction that was purported or intended to be governed in whole or in part by Section 355 of the Code or Section 361 of the Code.
(i)
Neither MEDS nor any of its Subsidiaries has entered into any transaction identified as a “reportable transaction” for purposes of Treasury
Regulations Sections 1.6011-4(b)(2) or 301.6111-2(b)(2).
(j)
Neither MEDS nor any of its Subsidiaries will be required to include any item of income in, or exclude any item of deduction from, taxable
income for any taxable period (or portion thereof) ending after the Closing Date as a result of any: (i) change in, or use of improper,
method of accounting for a taxable period ending on or prior to the Closing Date; (ii) “closing agreement” as described in Section 7121
of the Code (or any corresponding or similar provision of state, local or foreign income Tax law) executed on or prior to the Closing
Date; (iii) installment sale or open transaction disposition made on or prior to the Closing Date; (iv) prepaid amount, advance payments
or deferred revenue received or accrued on or prior to the Closing Date other than in respect of such amounts reflected in the MEDS Balance
Sheet or received in the Ordinary Course of Business since the date of the MEDS Balance Sheet; or (v) intercompany transaction or excess
loss amount described in Treasury Regulations under Section 1502 of the Code (or any corresponding or similar provision of state, local
or foreign income Tax Law).
(k)
Section 4.16(k) of the MEDS Disclosure Schedule sets forth the entity classification of MEDS and each of its Subsidiaries for
U.S. federal income tax purposes. Neither MEDS nor any of its Subsidiaries has made an election or taken any other action to change its
federal and state income tax classification from such classification.
(l)
Neither MEDS nor any of its Subsidiaries is aware of any facts or has knowingly taken or agreed to take any action, in each case, that
would reasonably be expected to prevent or impede the Merger from qualifying as a “reorganization” within the meaning of Section 368(a)
of the Code.
(m)
The membership interests in each of Merger Sub II are directly and wholly owned by MEDS, and Merger Sub II is, and has been since formation,
disregarded as an entity (within the meaning of Section 301.7701-3 of the Treasury Regulations) separate from MEDS for United States
federal income tax purposes.
4.17
Employee and Labor Matters; Benefit Plans.
(a)
Section 4.17(a) of the MEDS Disclosure Schedule sets forth, for each Person who is currently an employee of MEDS or any of its
Subsidiaries, such employee’s name, employer, title, hire date, location, whether full- or part-time, whether active or on leave (and,
if on leave, the expected return), whether exempt from the Fair Labor Standards Act, annual salary and wage rate, most recent annual
bonus received and current annual bonus opportunity. Section 4.17(a) of the MEDS Disclosure Schedule separately sets forth, for
each Person who currently is an individual independent contractor engaged by MEDS or any of its Subsidiaries, such contractor’s name,
duties and rate of compensation. No Key Employee has indicated to MEDS or any of its Subsidiaries that he or she intends to resign or
retire as a result of the transactions contemplated by this Agreement or otherwise.
(b)
The employment of MEDS’s employees is terminable by MEDS at will. MEDS has made available to the Company accurate and complete copies
of all employee manuals and handbooks, disclosure materials, policy statements and other materials relating to the employment of MEDS
employees to the extent currently effective and material.
(c)
MEDS is not a party to, bound by the terms of, and does not have a duty to bargain under, any collective bargaining agreement or other
Contract with a labor organization representing any of its employees, and there are no labor organizations representing or, to the Knowledge
of MEDS, purporting to represent or seeking to represent any employees of MEDS.
(d)
Section 4.17(d) of the MEDS Disclosure Schedule lists all MEDS Employee Plans (other than employment arrangements which are terminable
“at will” without any contractual obligation on the part of MEDS or any of its Subsidiaries to make any severance, termination, change
in control or similar payment and that are substantively identical to the employment arrangements made available to the Company).
(e)
Each MEDS Employee Plan that is intended to be qualified under Section 401(a) of the Code has received a favorable determination or opinion
letter with respect to such qualified status from the IRS. To the Knowledge of MEDS, nothing has occurred that would reasonably be expected
to adversely affect the qualified status of any such MEDS Employee Plan or the exempt status of any related trust.
(f)
Each MEDS Employee Plan has been established, maintained and operated in compliance, in all material respects, with its terms all applicable
Law, including, without limitation, the Code, ERISA and the Affordable Care Act. No Legal Proceeding (other than those relating to routine
claims for benefits) is pending or, to the Knowledge of MEDS, threatened with respect to any MEDS Employee Plan. All payments and/or
contributions required to have been made with respect to all MEDS Employee Plans either have been made or have been accrued in accordance
with the terms of the applicable MEDS Employee Plan and applicable Law.
(g)
Neither MEDS nor any of its ERISA Affiliates maintains, contributes to or is required to contribute to, or has, in the past six (6) years,
maintained, contributed to, or been required to contribute to (i) any “employee benefit plan” that is or was subject to Title IV or Section
302 of ERISA or Section 412 of the Code, (ii) a Multiemployer Plan, (iii) any funded welfare benefit plan within the meaning of Section
419 of the Code, (iv) any Multiple Employer Plan, or (v) any Multiple Employer Welfare Arrangement. Neither MEDS nor any of its ERISA
Affiliates has ever incurred any liability under Title IV of ERISA.
(h)
No MEDS Employee Plan provides for medical or other welfare benefits to any service provider beyond termination of service or retirement,
other than (1) pursuant to COBRA or an analogous state law requirement or (2) continuation coverage through the end of the month in which
such termination or retirement occurs. MEDS does not sponsor or maintain any self-funded medical or long-term disability benefit plan.
(i)
No MEDS Employee Plan is subject to any law of a foreign jurisdiction outside of the United States.
(j)
Each MEDS Employee Plan that constitutes in any part a “nonqualified deferred compensation plan” (as such term is defined under Section
409A(d)(1) of the Code and the guidance thereunder) (each, a “409A Plan”) has been operated and maintained in all material respects
in operational and documentary compliance with the requirements of Section 409A of the Code and the applicable guidance thereunder. No
payment to be made under any 409A Plan is or, when made in accordance with the terms of the 409A Plan, will be subject to the penalties
of Section 409A(a)(1) of the Code.
(k)
MEDS is, and at all times during the past three (3) years has been, in compliance in all material respects with all applicable federal,
state and local laws, rules and regulations respecting employment, employment practices, terms and conditions of employment, worker classification,
tax withholding, prohibited discrimination, harassment, equal employment, fair employment practices, meal and rest periods, immigration
status, employee safety and health, wages (including overtime wages), compensation, and hours of work, and in each case, with respect
to the employees of MEDS: (i) has withheld and reported all amounts required by law or by agreement to be withheld and reported with
respect to wages, salaries and other payments to employees, (ii) is not liable for any arrears of wages, severance pay or any Taxes or
any penalty for failure to comply with any of the foregoing and (iii) is not liable for any payment to any trust or other fund governed
by or maintained by or on behalf of any Governmental Authority, with respect to unemployment compensation benefits, social security or
other benefits or obligations for employees (other than routine payments to be made in the Ordinary Course of Business). There are no,
and during the past three (3) years there have been no, actions, suits, claims or administrative matters pending or, to the Knowledge
of MEDS, threatened or reasonably anticipated against MEDS relating to any employee, independent contractor, director, employment agreement
or MEDS Employee Plan (other than routine claims for benefits). To the Knowledge of MEDS, there are no pending or threatened or reasonably
anticipated claims or actions against MEDS, any MEDS trustee or any trustee of any Subsidiary under any workers’ compensation policy
or long-term disability policy. MEDS is not a party to a conciliation agreement, consent decree or other agreement or Order with any
federal, state, or local agency or Governmental Authority with respect to employment practices.
(l)
MEDS has no material Liability with respect to any misclassification within the past four (4) years of: (i) any Person as an independent
contractor rather than as an employee, (ii) any employee leased from another employer or (iii) any employee currently or formerly classified
as exempt from overtime wages. MEDS has not taken any action which would constitute a “plant closing” or “mass layoff” within the meaning
of the WARN Act or similar state or local law, issued any notification of a plant closing or mass layoff required by the WARN Act or
similar state or local law (nor has MEDS been under any requirement or obligation to issue any such notification), or incurred any liability
or obligation under WARN or any similar state or local law that remains unsatisfied.
(m)
There has never been, nor has there been any threat of, any strike, slowdown, work stoppage, lockout, job action, union, organizing activity,
question concerning representation or any similar activity or dispute, affecting MEDS. No event has occurred within the past six months,
and no condition or circumstance exists, that might directly or indirectly be likely to give rise to or provide a basis for the commencement
of any such strike, slowdown, work stoppage, lockout, job action, union organizing activity, question concerning representation or any
similar activity or dispute.
(n)
MEDS is not, nor has MEDS been, engaged in any unfair labor practice within the meaning of the National Labor Relations Act. There is
no, and during the past three (3) years there has been no, Legal Proceeding, claim, labor dispute or grievance pending or, to the Knowledge
of MEDS, threatened or reasonably anticipated relating to any employment contract, privacy right, labor dispute, wages and hours, leave
of absence, plant closing notification, workers’ compensation policy, long-term disability policy, harassment, retaliation, immigration,
employment statute or regulation, safety or discrimination matter involving any MEDS Associate, including charges of unfair labor practices
or discrimination complaints.
(o)
There is no contract, agreement, plan or arrangement to which MEDS or any of its Subsidiaries is a party or by which it is bound to compensate
any of its employees for excise taxes paid pursuant to the Code, including, but not limited to, Section 4999 or Section 409A of the Code.
(p)
Neither MEDS nor any of its Subsidiaries is a party to any Contract that as a result of the execution and delivery of this Agreement,
the shareholder approval of this Agreement, nor the consummation of the transactions contemplated hereby, could (either alone or in conjunction
with any other event) (i) result in the payment of any “parachute payment” within the meaning of Section 280G of the Code or (ii) result
in, or cause the accelerated vesting, payment, funding or delivery of, or increase the amount or value of, any payment or benefit to
any employee, officer, director or other service provider of MEDS or any of its Subsidiaries.
4.18
Environmental Matters. Since January 1, 2020, MEDS and each of its Subsidiaries has complied with all applicable Environmental
Laws, which compliance includes the possession by MEDS of all permits and other Governmental Authorizations required under applicable
Environmental Laws and compliance with the terms and conditions thereof, except for any failure to be in compliance that, individually
or in the aggregate, would not result in a MEDS Material Adverse Effect. Neither MEDS nor any of its Subsidiaries has received since
January 1, 2020, any written notice or other communication (in writing or otherwise), whether from a Governmental Authority, citizens
group, employee or otherwise, that alleges that MEDS or any of its Subsidiaries is not in compliance with any Environmental Law, and,
to the Knowledge of MEDS, there are no circumstances that may prevent or interfere with MEDS’s or any of its Subsidiaries’ compliance
with any Environmental Law in the future, except where such failure to comply would not reasonably be expected to have a MEDS Material
Adverse Effect. To the Knowledge of MEDS: (i) no current or prior owner of any property leased or controlled by MEDS or any of its Subsidiaries
has received since January 1, 2021, any written notice or other communication relating to property owned or leased at any time by MEDS
or any of its Subsidiaries, whether from a Governmental Authority, citizens group, employee or otherwise, that alleges that such current
or prior owner or MEDS or any of its Subsidiaries is not in compliance with or violated any Environmental Law relating to such property
and (ii) neither MEDS nor any of its Subsidiaries has any material liability under any Environmental Law.
4.19
Insurance. MEDS has made available to the Company accurate and complete copies of all material insurance policies and all material
self-insurance programs and arrangements relating to the business, assets, liabilities and operations of MEDS and its Subsidiaries (including
Merger Subs). Each of such insurance policies is in full force and effect and MEDS and its Subsidiaries (including Merger Subs) are in
compliance in all material respects with the terms thereof. Other than customary end of policy notifications from insurance carriers,
since January 1, 2020, neither MEDS nor any of its Subsidiaries has received any notice or other communication regarding any actual or
possible: (i) cancellation or invalidation of any insurance policy or (ii) refusal or denial of any coverage, reservation of rights or
rejection of any material claim under any insurance policy. Each of MEDS and its Subsidiaries (including Merger Subs) has provided timely
written notice to the appropriate insurance carrier(s) of each Legal Proceeding pending against MEDS or such Subsidiary for which MEDS
or such Subsidiary has insurance coverage, and no such carrier has issued a denial of coverage or a reservation of rights with respect
to any such Legal Proceeding, or informed MEDS or any of its Subsidiaries of its intent to do so.
4.20
Transactions with Affiliates. Except as set forth in the MEDS SEC Documents filed prior to the date of this Agreement, since the
date of MEDS’s last proxy statement filed in 2020 with the SEC, no event has occurred that would be required to be reported by MEDS pursuant
to Item 404 of Regulation S-K promulgated by the SEC. Section 4.20 of the MEDS Disclosure Schedule identifies each Person who
is (or who may be deemed to be) an Affiliate of MEDS as of the date of this Agreement.
4.21
No Financial Advisors. Except as set forth on Section 4.21 of the MEDS Disclosure Schedule, no broker, finder or investment
banker is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection
with the Contemplated Transactions based upon arrangements made by or on behalf of MEDS.
4.22
Valid Issuance; No Bad Actor. The MEDS Capital Stock to be issued in the First Merger will, when issued in accordance with the
provisions of this Agreement, be validly issued, fully paid and nonassessable. To the Knowledge of MEDS as of the date of this Agreement
and as of the Closing, no “bad actor” disqualifying event described in Rule 506(d)(1)(i)-(viii) of the Securities Act (a “Disqualifying
Event”) is applicable to MEDS or, to MEDS’s Knowledge, any MEDS Covered Person, except for a Disqualifying Event as to which Rule
506(d)(2)(ii-iv) or (d)(3) of the Securities Act is applicable.
4.23
Privacy and Data Security. MEDS and its Subsidiaries have complied with all applicable Privacy Laws and the applicable terms of
any MEDS Contracts relating to privacy, security, collection or use of Personal Information of any individuals (including clinical trial
participants, patients, patient family members, caregivers or advocates, physicians and other health care professionals, clinical trial
investigators, researchers, pharmacists) that interact with MEDS or any of its Subsidiaries in connection with the operation of MEDS’s
and its Subsidiaries’ business, except for such noncompliance as has not had, and would not reasonably be expected to have, individually
or in the aggregate, a MEDS Material Adverse Effect. To the Knowledge of MEDS, MEDS has implemented and maintains reasonable Privacy
Policies and has complied with its Privacy Policies, except for such noncompliance as has not to the Knowledge of the MEDS had, and would
not reasonably be expected to have, individually or in the aggregate, a MEDS Material Adverse Effect. To the Knowledge of MEDS, as of
the date hereof, no claims have been asserted or threatened against MEDS by any Person alleging a violation of Privacy Laws, Privacy
Policies and/or the applicable terms of any MEDS Contracts relating to privacy, security, collection or use of Personal Information of
any individuals. To the Knowledge of MEDS, there have been no data security incidents, personal data breaches or other adverse events
or incidents related to Personal Information or MEDS data in the custody or control of MEDS or any service provider acting on behalf
of MEDS, in each case where such incident, breach or event would result in a notification obligation to any Person under applicable law
or pursuant to the terms of any MEDS Contract.
4.24
No Other Representations or Warranties. MEDS hereby acknowledges and agrees that, except for the representations and warranties
contained in this Agreement, neither the Company nor any of its Subsidiaries nor any other person on behalf of the Company or its Subsidiaries
makes any express or implied representation or warranty with respect to the Company or its Subsidiaries or with respect to any other
information provided to MEDS, Merger Subs or stockholders or any of their respective Affiliates in connection with the Contemplated Transactions,
and (subject to the express representations and warranties of the Company set forth in Section 3 (in each case as qualified and
limited by the Company Disclosure Schedule)) none of MEDS, Merger Sub or any of their respective Representatives, stockholders or members,
has relied on any such information (including the accuracy or completeness thereof).
Section
5. Agreements of the Parties.
5.1
Information Statement.
(a)
As promptly as practicable after the Closing Date, MEDS shall prepare and file with the SEC an information statement on Schedule 14C
relating to the Required MEDS Stockholder Consent regarding the Conversion, the Name Change and the Stock Plan Share Increase (together
with any amendments thereof or supplements thereto, the “Information Statement”). MEDS shall use its commercially reasonable efforts
to (i) cause the Information Statement to comply with applicable rules and regulations promulgated by the SEC and the guidance of the
staff of the SEC and (ii) respond promptly to any comments or requests of the SEC or its staff related to the Information Statement.
(b)
MEDS covenants and agrees that the Information Statement (and the letter to stockholders included therewith) will (i) comply as to form
in all material respects with the requirements of applicable U.S. federal securities laws and the DGCL, and (ii) will not contain any
untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the
statements made therein, in light of the circumstances under which they were made, not misleading.
(c)
MEDS shall use commercially reasonable efforts to cause the Information Statement to be mailed to MEDS’s stockholders as promptly as
practicable after the Information Statement has been filed with the SEC and either (i) the SEC has indicated that it does not intend
to review the Information Statement or that its review of the Information Statement has been completed or (ii) at least ten (10) days
shall have passed since the Information Statement was filed with the SEC without receiving any correspondence from the SEC commenting
upon, or indicating that it intends to review, the Information Statement, all in compliance with applicable U.S. federal securities laws
and the DGCL. If MEDS, either of the Merger Subs or the Company become aware of any event or information that, pursuant to the Securities
Act or the Exchange Act, should be disclosed in an amendment or supplement to the Information Statement, as the case may be, then such
Party, as the case may be, shall promptly inform the other Parties thereof and shall cooperate with such other Parties in MEDS filing
such amendment or supplement with the SEC and, if appropriate, in mailing such amendment or supplement to the MEDS stockholders.
5.2
Conversion, Name Change and Stock Plan Share Increase. The Required MEDS Stockholder Consent shall be deemed effective on the
twentieth (20th) calendar day following the mailing of the Information Statement to MEDS stockholders pursuant to Section
5.1(c) above. The Conversion shall occur immediately following the effectiveness of the Required MEDS Stockholder Consent, the Name
Change shall occur within a reasonable time following the Conversion, as determined in the MEDS Board’s discretion, and the Stock Plan
Share Increase shall be effective immediately following the effectiveness of the Required MEDS Stockholder Consent.
5.3
Employment and Benefit Matters.
(a)
MEDS shall comply with the terms of any employment, severance, retention, change of control, or similar agreement specified on Section
4.17(d) of the MEDS Disclosure Schedule, subject to the provisions of such agreements.
(b)
As of the Second Effective Time, MEDS agrees to cause the surviving corporation to maintain through December 31, 2024, the compensation
and benefit levels, including base salary, annual cash incentive opportunities, retirement benefits, and health and welfare benefits
for the employees of the Company who remain employed after the Second Effective Time (the “Company Employees”) at levels which
are, in the aggregate, no less favorable to those in effect for the Company Employees immediately prior to the Second Effective Time.
(c)
Nothing in this Agreement shall confer upon any Company Employee any right to continue in the employ or service of MEDS or any affiliate
of MEDS, or shall interfere with or restrict in any way the rights of MEDS, which rights are hereby expressly reserved, to discharge
or terminate the services of any Company Employee at any time for any reason whatsoever, with or without cause. Notwithstanding any provision
in this Agreement to the contrary, nothing in this Section 5.3 shall (i) be deemed or construed to be an amendment or other modification
of any Employee Plan or MEDS employee benefit plan, or (ii) create any third party rights in any current or former employee, director
or other service provider of MEDS, the Company or any of their respective affiliates (or any beneficiaries or dependents thereof).
5.4
Indemnification of Officers and Directors.
(a)
From the First Effective Time through the sixth anniversary of the date on which the First Effective Time occurs, each of MEDS and the
Surviving Company shall indemnify and hold harmless each person who is now, or has been at any time prior to the date hereof, or who
becomes prior to the First Effective Time, a director or officer of MEDS or the Company, respectively (the “D&O Indemnified Parties”),
against all claims, losses, liabilities, damages, judgments, fines and reasonable fees, costs and expenses, including attorneys’ fees
and disbursements (collectively, “Costs”), incurred in connection with any claim, action, suit, proceeding or investigation, whether
civil, criminal, administrative or investigative, arising out of or pertaining to the fact that the D&O Indemnified Party is or was
a director or officer of MEDS or of the Company, whether asserted or claimed prior to, at or after the First Effective Time, in each
case, to the fullest extent permitted under the DGCL and the DLLCA. Each D&O Indemnified Party will be entitled to advancement of
expenses incurred in the defense of any such claim, action, suit, proceeding or investigation from each of MEDS and the Surviving Company,
jointly and severally, upon receipt by MEDS or the Surviving Company from the D&O Indemnified Party of a request therefor; provided
that any such person to whom expenses are advanced provides an undertaking to MEDS, to the extent then required by the DGCL and the DLLCA,
to repay such advances if it is ultimately determined that such person is not entitled to indemnification.
(b)
The provisions of the certificate of incorporation and bylaws of MEDS with respect to indemnification, advancement of expenses and exculpation
of present and former directors and officers of MEDS that are presently set forth in the certificate of incorporation and bylaws of MEDS
shall not be amended, modified or repealed for a period of six years from the First Effective Time in a manner that would adversely affect
the rights thereunder of individuals who, at or prior to the First Effective Time, were officers or directors of MEDS, unless such modification
is required by applicable Law. The certificate of formation and limited liability company agreement of the Surviving Company shall contain,
and MEDS shall cause the certificate of formation and limited liability company agreement of the Surviving Company to so contain, provisions
no less favorable with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers
as those presently set forth in the certificate of incorporation and bylaws of MEDS.
(c)
From and after the First Effective Time, (i) the Surviving Company shall fulfill and honor in all respects the obligations of the Company
to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification provisions under the Company’s
Organizational Documents and pursuant to any indemnification agreements between the Company and such D&O Indemnified Parties, with
respect to claims arising out of matters occurring at or prior to the First Effective Time and (ii) MEDS shall fulfill and honor in all
respects the obligations of MEDS to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification
provisions under MEDS’s Organizational Documents and pursuant to any indemnification agreements between MEDS and such D&O Indemnified
Parties, with respect to claims arising out of matters occurring at or prior to the First Effective Time.
(d)
From and after the First Effective Time, MEDS shall maintain directors’ and officers’ liability insurance policies, with an effective
date as of the Closing Date, on commercially available terms and conditions and with coverage limits customary for U.S. public companies
similarly situated to MEDS.
(e)
From and after the First Effective Time, MEDS shall pay all expenses, including reasonable attorneys’ fees, that are incurred by the
persons referred to in this Section 5.4 in connection with their enforcement of the rights provided to such persons in this Section
5.4.
(f)
The provisions of this Section 5.4 are intended to be in addition to the rights otherwise available to the current and former
officers and directors of MEDS and the Company by Law, charter, statute, bylaw or agreement, and shall operate for the benefit of, and
shall be enforceable by, each of the D&O Indemnified Parties, their heirs and their Representatives.
(g)
In the event MEDS or the Surviving Company or any of their respective successors or assigns (i) consolidates with or merges into any
other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger or (ii) transfers all
or substantially all of its properties and assets to any Person, then, and in each such case, proper provision shall be made so that
the successors and assigns of MEDS or the Surviving Company, as the case may be, shall succeed to the obligations set forth in this Section
5.4. MEDS shall cause the Surviving Company to perform all of the obligations of the Surviving Company under this Section 5.4.
5.5
Tax Matters.
(a)
Each of MEDS and the Company shall use reasonable best efforts (and cause its Affiliates) to (i) cause the First Merger and the Second
Merger, taken together, to constitute an integrated transaction described in Rev. Rul. 2001-46, 2001-2 C.B. 321 that qualifies as a “reorganization”
within the meaning of Section 368(a) of the Code, and (ii) not take any actions, or fail to take or cause to be taken any action, which
action or failure to act would reasonably be expected to prevent or impede the First Merger and Second Merger, taken together, from constituting
an integrated transaction described in Rev. Rul. 2001-46, 2001-2 C.B. 321 that qualifies as a “reorganization” within the meaning of
Section 368(a) of the Code. The Parties shall not file any U.S. federal, state or local Tax Return in a manner that is inconsistent with
the treatment of the Merger as a “reorganization” within the meaning of Section 368(a) of the Code for U.S. federal, state income and
other relevant Tax purposes, and shall not take any inconsistent position during the course of any audit, litigation or other proceeding
with respect to Taxes, in each case, unless otherwise required by a determination within the meaning of Section 1313(a) of the Code.
(b)
All transfer, documentary, sales, use, stamp, registration, excise, recording, registration value added and other such similar Taxes
and fees (including any penalties and interest) that become payable in connection with or by reason of the execution of this Agreement
and the transactions contemplated hereby (collectively, “Transfer Taxes”) shall be borne and paid by MEDS. Unless otherwise required
by applicable law, MEDS shall timely file any Tax Return or other document with respect to such Taxes or fees (and the Company shall
reasonably cooperate with respect thereto as necessary).
(c)
At the Closing, the Company shall deliver to MEDS a certificate pursuant to Treasury Regulations Sections 1.1445-2(c) and 1.897-2(h),
together with a form of notice to the IRS in accordance with the requirements of Treasury Regulations Section 1.897-2(h), in each case,
in form and substance reasonably acceptable to MEDS.
5.6
Legends. MEDS shall be entitled to place appropriate legends on the book entries and/or certificates evidencing any shares of
MEDS Capital Stock to be received in the First Merger by equityholders of the Company who may be considered “affiliates” of MEDS for
purposes of Rules 144 and 145 under the Securities Act reflecting the restrictions set forth in Rules 144 and 145 and to issue appropriate
stop transfer instructions to the transfer agent for MEDS Capital Stock.
5.7
Officers and Directors. Until successors are duly elected or appointed and qualified in accordance with applicable Law, the Parties
shall use commercially reasonable efforts and take all necessary action so that the Persons listed on Section 5.7 of the MEDS
Disclosure Schedule are elected or appointed, as applicable, to the positions of officers, directors and managers of MEDS and the Surviving
Company, as set forth therein, to serve in such positions effective as of the First Effective Time. The Parties shall use reasonable
best efforts to have each of the Persons that will serve as directors and officers of the MEDS following the Closing to execute and deliver
a Lock-Up Agreement prior to Closing.
5.8
Section 16 Matters. Prior to the Effective Time, MEDS shall take all such steps as may be required to cause any acquisitions of
MEDS Common Stock (including derivative securities with respect to such MEDS Common Stock) resulting from the Contemplated Transactions,
by each individual who is reasonably expected to become subject to the reporting requirements of Section 16(a) of the Exchange Act with
respect to MEDS, to be exempt under Rule 16b-3 promulgated under the Exchange Act.
5.9
Allocation Certificate. The Company will prepare and deliver to MEDS prior to the Closing a certificate signed by the Company’s
chief executive officer in a form reasonably acceptable to MEDS setting forth (as of immediately prior to the First Effective Time) (a)
each holder of Company Common Stock, (b) such holder’s name and address, (c) the number or Company Common Stock held as of the Closing
Date for each such holder and (d) the number of shares of MEDS Common Capital Stock to be issued to such holder pursuant to this Agreement
in respect of the Company Common Stock held by such holder as of immediately prior to the First Effective Time (the “Allocation Certificate”).
5.10
Subsequent Financings. MEDS shall use commercially reasonably efforts to take such actions and cause certain investors to enter
into and consummate subscription agreements with investors totaling, in the aggregate, not less than $15,000,000 relating to private
investments in MEDS following the Closing Date for MEDS Preferred Stock at a minimum pre-money valuation of $110,000,000 (each a “Subsequent
Financing” and collectively “Subsequent Financings”). MEDS and the Company shall, and shall cause their respective Representatives
to, cooperate with each other and their respective Representatives in connection with such Subsequent Financings and use their respective
commercially reasonable efforts to cause Subsequent Financings of at least $8,000,000 in the aggregate to occur within 45 days following
the Closing Date, and additional Subsequent Financings of up to $7,000,000 in the aggregate to occur by December 31, 2024 (including
having the Company’s senior management participate in any investor meetings and roadshows as reasonably requested by MEDS).
5.11
Obligations of Merger Subs. MEDS will take all action necessary to cause Merger Subs to perform their obligations under this Agreement
and to consummate the Merger on the terms and conditions set forth in this Agreement.
5.12
Transfer of Funds. At or prior to Closing, MEDS shall transfer $2,000,000 to the Surviving Company (the “Transfer”).
5.13
Shares under 2019 Stock Plan. The MEDS Board shall take such action such that, following the the effectiveness of the Stock Plan
Share Increase, ten-percent (10%) of the shares available to be awarded under the MEDS Stock Plans shall be reserved for issuance to
the officers of MEDS that will be appointed following the Conversion.
5.14
Reservation of Shares for NVK Conversion. The MEDS Board shall take such action such that, at Closing, MEDS shall executing such
documents and instruments and taking such actions as are reasonably required to assume the Company’s obligations under the Loan and Security
Agreement, dated as of September 8, 2023, by and between the Company and NVK Finance, LLC (“NVK”) (as may be amended from time
to time the “Loan Agreement”). Furthermore, the MEDS Board shall take such action such that, following the Closing, 484,756 shares
of MEDS Common Stock shall be reserved to satisfy NVK’s conversion rights pursuant the Loan Agreement (“NVK Reserved Shares”).
Immediately upon the earlier of (a) NVK’s conversion pursuant to the Loan Agreement or (b) the first calendar day following the maturity
date of the Loan Agreement, any NVK Reserved Shares not issued to NVK pursuant to a conversion exercised by NVK pursuant to the Loan
Agreement shall automatically cease to be reserved and shall return to MEDS’s treasury.
5.15
Conversion of Series B Preferred Stock. MEDS shall not call a meeting of its stockholders to vote upon, nor solicit proxies for,
the conversion of the Series B Preferred Stock.
Section
6. Conditions Precedent to Obligations of Each Party.
The
obligations of each Party to effect the Merger and otherwise consummate the Contemplated Transactions to be consummated at the Closing
are subject to the satisfaction or, to the extent permitted by applicable law, the written waiver by each of the Parties, at or prior
to the Closing, of each of the following conditions:
6.1
No Restraints. No temporary restraining order, preliminary or permanent injunction or other Order preventing the consummation
of the Contemplated Transactions shall have been issued by any court of competent jurisdiction or other Governmental Authority of competent
jurisdiction and remain in effect and there shall not be any Law which has the effect of making the consummation of the Contemplated
Transactions illegal.
6.2
Company Stockholder Approval. The Company shall have obtained the Required Company Stockholder Vote.
6.3
Listing. The approval of the listing of the additional shares of MEDS Common Stock on Nasdaq shall have been obtained and the
shares of MEDS Common Stock to be issued in the First Merger pursuant to this Agreement shall have been approved for listing (subject
to official notice of issuance) on Nasdaq.
6.4
MEDS Cash. MEDS shall have satisfied its Transaction Expenses and the MEDS Net Cash, as set forth in the MEDS Net Cash Schedule,
is not less than $2,000,000, plus an amount equal to a good faith estimate of expected MEDS operating costs for forty-five (45) days
post-closing (including any additional transaction costs related to SEC and Nasdaq matters related to the Contemplated Transactions).
6.5
Certificate of Designation. MEDS shall have filed the Certificate of Designation with the Secretary of State of the State of Delaware.
6.6
Exchange Agent Agreement. As of the Closing, MEDS shall have entered into an exchange agent agreement with the Exchange Agent
pertaining to the exchange of shares of Company Common Stock for shares of MEDS Capital Stock as contemplated hereby, including a form
of letter of transmittal, in form and substance reasonably satisfactory to the Company.
Section
7. Closing Deliveries of the Company.
The
obligations of MEDS and Merger Subs to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are
subject to MEDS receiving the following documents, each of which shall be in full force and effect, or the written waiver by MEDS of
delivery:
| |
(a) |
The Company Lock-Up Agreements;
and |
| |
|
|
|
|
|
| |
(b) |
The Allocation Certificate. |
Section
8. Closing Deliveries of MEDS.
The
obligations of the Company to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject
to the Company receiving the following documents, each of which shall be in full force and effect, or the written waiver by the Company
of delivery:
| |
(a) |
A fully-executed Required
MEDS Stockholder Consent shall be in full force and effect; |
| |
|
|
| |
(b) |
a copy of the Certificate
of Designation, certified by the Secretary of State of the State of Delaware; |
| |
|
|
| |
(c) |
the MEDS Net Cash Schedule
which shall reflect that the MEDS Net Cash is not less than $2,000,000, plus an amount equal to a good faith estimate of expected MEDS
operating costs for forty-five (45) days post-closing (including any additional transaction costs related to SEC and Nasdaq matters
related to the Contemplated Transactions).; |
| |
|
|
| |
(d) |
written resignations in forms
satisfactory to the Company, dated as of the Closing Date and effective as of the Closing executed by the officers and directors of
MEDS who are not to continue as officers or directors of MEDS pursuant to Section 5.7 hereof; |
| |
|
|
| |
(e) |
Fully-executed Consulting
Agreements; and |
| |
|
|
| |
(f) |
Evidence that the Transfer
has been completed. |
Section
9. Miscellaneous Provisions.
9.1
Non-Survival of Representations and Warranties. The representations and warranties of the Company, MEDS and Merger Subs contained
in this Agreement or any certificate or instrument delivered pursuant to this Agreement shall terminate at the First Effective Time,
and only the covenants or agreements that by their terms survive the First Effective Time and this Section 9, or those covenants
or agreements to be performed in whole or in part after the First Effective Time, shall survive the First Effective Time.
9.2
Amendment. This Agreement may be amended, modified or supplemented by the parties with the approval of the respective boards of
directors of the Company, Merger Subs and MEDS at any time (whether before or after the Required Company Stockholder vote, the Required
MEDS Stockholder Vote (or the Required MEDS Stockholder Consent)); provided, however, that after any such approval of this
Agreement by a Party’s stockholders, no amendment shall be made which by Law requires further approval of such stockholders of the applicable
Party without the further approval or adoption of such stockholders. This Agreement may not be amended, modified or supplemented in any
manner, whether by course of conduct or otherwise, except by an instrument in writing specifically designated as an amendment hereto,
signed on behalf of each of the Company, Merger Subs and MEDS.
9.3
Waiver. The parties may, by action taken or authorized with the approval of the respective boards of directors of the Company,
Merger Subs and MEDS at any time, to the extent permitted by applicable Law, waive compliance with any of the agreements or conditions
of the other parties contain herein; provided, however, that after the Required Company Stockholder vote, the Required
MEDS Stockholder Vote (or the Required MEDS Stockholder Consent) has been obtained, no waiver may be made that pursuant to applicable
Law requires further approval or adoption by the stockholders of the Company or MEDS, as applicable, without such further approval or
adoption. Any agreement on the part of a party to any such waiver shall be valid only if set forth in a written instrument executed and
delivered by a duly authorized officer on behalf of such party. No failure or delay of any party in exercising any right or remedy hereunder
shall operate as a waiver thereof, nor shall any single or partial exercise of any such right or power, or any abandonment or discontinuance
of steps to enforce such right or power, or any course of conduct, preclude any other or further exercise thereof or the exercise of
any other right or power. The rights and remedies of the parties hereunder are cumulative and are not exclusive of any rights or remedies
which they would otherwise have hereunder.
9.4
Fees and Expenses. Except as otherwise set forth in this Agreement, all fees and expenses incurred in connection with this Agreement,
the Merger and the other Contemplated Transactions shall be paid by the party incurring such fees or expenses.
9.5
Entire Agreement; Counterparts; Exchanges by Electronic Transmission or Facsimile. This Agreement and the other schedules, exhibits,
certificates, instruments and agreements referred to in this Agreement constitute the entire agreement and supersede all prior agreements
and understandings, both written and oral, among or between any of the Parties with respect to the subject matter hereof and thereof;
provided, however, that the Confidentiality Agreement shall not be superseded and shall remain in full force and effect
in accordance with its terms. This Agreement may be executed in several counterparts, each of which shall be deemed an original and all
of which shall constitute one and the same instrument. The exchange of a fully executed Agreement (in counterparts or otherwise) by all
Parties by facsimile or electronic transmission in .PDF format shall be sufficient to bind the Parties to the terms and conditions of
this Agreement.
9.6
Applicable Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the laws of the State of
Delaware, regardless of the laws that might otherwise govern under applicable principles of conflicts of laws. In any action or proceeding
between any of the Parties arising out of or relating to this Agreement or any of the Contemplated Transactions, each of the Parties:
(a) irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State
of Delaware or, to the extent such court does not have subject matter jurisdiction, the Superior Court of the State of Delaware or the
United States District Court for the District of Delaware, (b) agrees that all claims in respect of such action or proceeding shall be
heard and determined exclusively in accordance with clause (a) of this Section 9.5, (c) waives any objection to laying venue in
any such action or proceeding in such courts, (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction
over any Party, (e) agrees that service of process upon such Party in any such action or proceeding shall be effective if notice is given
in accordance with Section 9.8 of this Agreement and (f) irrevocably and unconditionally waives the right to trial by jury.
9.7
Assignability. This Agreement shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the Parties
and their respective successors and permitted assigns; provided, however, that neither this Agreement nor any of a Party’s
rights or obligations hereunder may be assigned or delegated by such Party without the prior written consent of the other Party, and
any attempted assignment or delegation of this Agreement or any of such rights or obligations by such Party without the other Party’s
prior written consent shall be void and of no effect.
9.8
Notices. All notices and other communications hereunder shall be in writing and shall be deemed to have been duly delivered and
received hereunder (a) one Business Day after being sent for next Business Day delivery, fees prepaid, via a reputable international
overnight courier service, (b) upon delivery in the case of delivery by hand or (c) on the date delivered in the place of delivery if
sent by email or facsimile (with a written or electronic confirmation of delivery) prior to 6:00 p.m. New York City time, otherwise on
the next succeeding Business Day, in each case to the intended recipient as set forth below:
if
to MEDS or Merger Subs:
TRxADE
Health, Inc.
6308
Benjamin Rd, Suite 708
Tampa,
Florida 33634
Attention:
Suren Ajjarapu, Chief Executive Officer
Email:
suren@rxintegra.com
with
a copy to (which shall not constitute notice):
Dykema
Gossett PLLC
111
E. Kilbourn Ave – Suite 1050
Milwaukee,
WI 53202
Attention:
Kate Bechen, Andrew Frost
Email:
kbechen@dykema.com, afrost@dykema.com
if
to the Company:
Scienture,
Inc.
20
Austin Blvd, Commack, NY 11725
Attention:
Shankar Hariharan, Ph.D., President and Chief Executive Officer
Email:
shankar.hariharan@scienture.com
with
a copy to (which shall not constitute notice):
Goodwin
Procter LLP
The
New York Times Building
620
Eighth Avenue
New
York, NY 10018
Attention:
Stephen Davis, Michael R. Patrone
Email:
sdavis@goodwinlaw.com, mpatrone@goodwinlaw.com
9.9
Cooperation. Each Party agrees to cooperate fully with the other Party and to execute and deliver such further documents, certificates,
agreements and instruments and to take such other actions as may be reasonably requested by the other Party to evidence or reflect the
Contemplated Transactions and to carry out the intent and purposes of this Agreement.
9.10
Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall
not affect the validity or enforceability of the remaining terms and provisions of this Agreement or the validity or enforceability of
the offending term or provision in any other situation or in any other jurisdiction. If a final judgment of a court of competent jurisdiction
declares that any term or provision of this Agreement is invalid or unenforceable, the Parties agree that the court making such determination
shall have the power to limit such term or provision, to delete specific words or phrases or to replace such term or provision with a
term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term
or provision, and this Agreement shall be valid and enforceable as so modified. In the event such court does not exercise the power granted
to it in the prior sentence, the Parties agree to replace such invalid or unenforceable term or provision with a valid and enforceable
term or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable
term or provision.
9.11
Other Remedies; Specific Performance. Except as otherwise provided herein, any and all remedies herein expressly conferred upon
a Party will be deemed cumulative with and not exclusive of any other remedy conferred hereby, or by law or equity upon such Party, and
the exercise by a Party of any one remedy will not preclude the exercise of any other remedy. The Parties agree that irreparable damage
for which monetary damages, even if available, would not be an adequate remedy, would occur in the event that any of the provisions of
this Agreement were not performed in accordance with their specific terms (including failing to take such actions as are required of
it hereunder to consummate this Agreement) or were otherwise breached. It is accordingly agreed that the Parties shall be entitled to
an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof in any
court of the United States or any state having jurisdiction, this being in addition to any other remedy to which they are entitled at
law or in equity, and each of the Parties waives any bond, surety or other security that might be required of any other Party with respect
thereto. Each of the Parties further agrees that it will not oppose the granting of an injunction, specific performance or other equitable
relief on the basis that any other Party has an adequate remedy at law or that any award of specific performance is not an appropriate
remedy for any reason at law or in equity.
9.12
No Third-Party Beneficiaries.
(a)
Nothing in this Agreement, express or implied, is intended to or shall confer upon any Person (other than the Parties and the D&O
Indemnified Parties to the extent of their respective rights pursuant to Section 5.4) any legal or equitable right, benefit or
remedy of any nature whatsoever under or by reason of this Agreement.
(b)
The representations and warranties in this Agreement are the product of negotiations among the parties hereto and are for the sole benefit
of the parties hereto. Any inaccuracies in such representations and warranties are subject to waiver by the parties hereto in accordance
with Section 9.3 without notice or liability to any other Person. In some instances, the representations and warranties in this
Agreement may represent an allocation among the parties hereto of risks associated with particular matters regardless of the knowledge
of any of the parties hereto. Consequently, Persons other than the parties hereto may not rely upon the representations and warranties
in this Agreement as characterizations of actual facts or circumstances as of the date of this Agreement or as of any other date.
9.13
Waiver of Jury Trial. EACH OF THE PARTIES TO THIS AGREEMENT HEREBY IRREVOCABLY WAIVES ALL RIGHT TO A TRIAL BY JURY IN ANY ACTION,
PROCEEDING OR COUNTERCLAIM ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY.
[Remainder
of page intentionally left blank]
IN
WITNESS WHEREOF, the Parties have caused this Agreement to be executed as of the date first above written.
| |
By: |
/s/
Suren Ajjarapu |
| |
|
Suren Ajjarapu, Chief Executive Officer |
| |
MERGER SUB
I: |
| |
|
|
| |
MEDS MERGER
SUB I, INC. |
| |
|
|
| |
By: |
/s/
Suren Ajjarapu |
| |
|
Suren Ajjarapu, Chief Executive
Officer |
| |
MERGER SUB
II: |
| |
|
| |
MEDS
MERGER SUB II, LLC |
| |
By: |
TRxADE
Health Inc., |
| |
|
its Manager |
| |
By: |
/s/
Suren Ajjarapu |
| |
|
Suren Ajjarapu, |
| |
|
Chief Executive Officer |
[Signature
Page to Agreement and Plan of Merger]
IN
WITNESS WHEREOF, the Parties have caused this Agreement to be executed as of the date first above written.
| |
By: |
/s/
Shankar Hariharan |
| |
|
Shankar Hariharan, Ph.D.,
President and Chief Executive Officer |
[Signature
Page to Agreement and Plan of Merger]
ANNEX
B













ANNEX
C
LOCK-UP
AGREEMENT
THIS
LOCK-UP AGREEMENT (this “Agreement”) is made and entered into as of July 25, 2024, by and between (i) TRxADE
HEALTH, INC., a Delaware corporation (including any successor entity thereto, “MEDS”), and (ii) ______________________
(the “Subject Party”). Any capitalized term used but not defined in this Agreement will have the meaning
ascribed to such term in the Merger Agreement (as defined below).
WHEREAS,
on July 25, 2024 (i) MEDS, (ii) MEDS MERGER SUB I, Inc., a Delaware corporation and wholly owned subsidiary of MEDS (“Merger
Sub I”), MEDS MERGER SUB II, LLC, a Delaware limited liability company and wholly owned subsidiary of MEDS (“Merger
Sub II” and together with Merger Sub I, “Merger Subs”), and Scienture, Inc., a Delaware corporation
(the “Company”) entered into that certain Agreement and Plan of Merger (as amended from time to time in accordance
with the terms thereof, the “Merger Agreement”), pursuant to which: (a) Merger Sub I will merge with and into
the Company (the “First Merger”), with the Company surviving the First Merger as a wholly-owned subsidiary
of MEDS (the “Surviving Corporation”); and (b) immediately following the First Merger and as part of the same
overall transaction as the First Merger, the Surviving Corporation will merge with and into the Merger Sub II (the “Second
Merger” and together with the First Merger, the “Mergers”), with Merger Sub II being the surviving
entity of the Second Merger (the “Surviving Entity”), as a result of which all of the issued and outstanding
capital stock of the Company immediately prior to the First Effective Time shall be canceled and converted into the right to receive
the Merger Consideration, all upon the terms and subject to the conditions set forth in this Agreement;
WHEREAS,
pursuant to the Merger Agreement, and in view of the valuable consideration to be received by the Subject Party pursuant to the Merger
Agreement, all of the shares of MEDS Common Stock and all of the shares of MEDS Preferred Stock (including, without limitation, the underlying
shares of MEDS Common Stock upon conversion and any MEDS Common Stock or such other securities which may be deemed beneficially owned
by the Subject Party in accordance with the rules and regulations of the SEC and securities of MEDS which may be issued upon exercise
of an option to purchase MEDS Common Stock or warrant or settlement of any equity interests in MEDS) that are currently or hereinafter
held by the Subject Party (all such securities, together with any securities paid as dividends or distributions with respect to such
securities or into which such securities are exchanged or converted, the “Restricted Securities”), shall become
subject to limitations on disposition as set forth in this Agreement.
NOW,
THEREFORE, in consideration of the premises set forth above, which are incorporated into this Agreement as if fully set forth below,
and intending to be legally bound hereby, the parties hereby agree as follows:
1.
Lock-Up Provisions.
(a)
The Subject Party hereby agrees not to, during the period commencing from the date of the Conversion (as defined in the Merger Agreement)
and ending on the earliest of (x) one hundred eighty (180) days after the date of the Conversion or (y) the date after the Closing on
which MEDS completes a liquidation, merger, stock exchange, or other similar transaction with an unaffiliated third party that results
in all of MEDS’s stockholders having the right to exchange their Restricted Securities for cash, securities, or other property
(the “Lock-Up Period”):
(i)
lend, offer, pledge, hypothecate, encumber, donate, assign, sell, contract to sell, sell any option or contract to purchase, purchase
any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly,
any Restricted Securities,
(ii)
enter into any swap, short sale, hedge or other arrangement that transfers to another, in whole or in part, any of the economic consequences
of ownership of the Restricted Securities regardless of whether any such transaction described in clause (i) above or this clause (ii)
is to be settled by delivery of Restricted Securities or other securities, in cash or otherwise, or
(iii)
publicly disclose the intention to do any of the foregoing, whether any such transaction described in clauses (i) or (ii) above is to
be settled by delivery of Restricted Securities or other securities, in cash or otherwise (any of the foregoing described in clauses
(i), (ii), or (iii), a “Prohibited Transfer”).
(b)
The foregoing shall not apply to the transfer of any or all of the Restricted Securities in connection with any Permitted Transfer; provided,
however, that it shall be a condition to such transfer that such transfer complies with the Securities Act, and other applicable law,
and that the transferee executes and delivers to MEDS an agreement stating that the transferee is receiving and holding the Restricted
Securities subject to the provisions of this Agreement applicable to the Subject Party, and there shall be no further transfer of such
Restricted Securities except in accordance with this Agreement. As used in this Agreement, the term “Permitted Transfer”
shall mean:
(1)
if the Subject Party is a natural person, (A) to any person who is an immediate family member of the Subject Party (for purposes
of this Agreement, “immediate family” shall mean any relationship by blood, marriage, domestic partnership or adoption, not
more remote than a first cousin) (a “Family Member”), or to a trust formed for the direct or indirect benefit
of the Subject Party or any of the Subject Party’s Family Members, (B) to the Subject Party’s estate, following the death
of the Subject Party, by will, intestacy or other operation of Law, (C) as a bona fide gift or a charitable contribution, as such term
is described in Section 501(c)(3) of the Internal Revenue Code of 1986, as amended, (D) by operation of Law pursuant to a court order,
qualified domestic order or in connection with a divorce settlement or (E) to any partnership, corporation or limited liability company
which, directly or indirectly, controls or manages, is controlled or managed by, or is under common control or management with, MEDS,
the Subject Party and/or by any such Family Member(s);
(2)
if the Subject Party is a corporation, partnership, limited liability company or other entity, (A) to another corporation, partnership,
limited liability company or other entity that is an affiliate (as defined under Rule 12b-2 of the Exchange Act) of the Subject Party,
including investment funds or other entities under common control or management or advisement with the Subject Party (including, for
the avoidance of doubt, where the Subject Party is a partnership, to its general partner or a successor partnership or fund, or any other
funds managed by such partnership), (B) as a distribution or dividend to equity holders, including, without limitation, current or former
general or limited partners, members or managers (or to the estates of any of the foregoing), as applicable, of the Subject Party (including
upon the liquidation and dissolution of the Subject Party pursuant to a plan of liquidation approved by the Subject Party’s equity
holders), (C) as a bona fide gift or a charitable contribution, as such term is described in Section 501(c)(3) of the Internal Revenue
Code of 1986, as amended, (D) transfer or dispositions not involving a change in beneficial ownership or (E) with prior written consent
of MEDS;
(3)
if the Subject Party is a trust, to any grantors or beneficiaries of the trust;
(4)
to MEDS or its officers or directors;
(5)
a pledge of a portion of the MEDS Common Stock by the Subject Party, in the amounts and as described in Annex A hereto; or
(6)
transfers by virtue of the laws of the State of Delaware.
The
Subject Party further agrees to execute such agreements as may be reasonably requested by MEDS that are consistent with the foregoing
or that are necessary to give further effect to any Permitted Transfer, and such Permitted Transfer is not for value and each donee,
heir, beneficiary or other transferee or distributee shall enter into a written agreement agreeing to be bound by the restrictions contained
herein with respect to such Restricted Securities or such other securities that have been so transferred or distributed.
(c)
If any Prohibited Transfer is made or attempted contrary to the provisions of this Agreement, such purported Prohibited Transfer shall
be null and void ab initio, and MEDS shall refuse to recognize any such purported transferee of the Restricted Securities as one of its
equity holders for any purpose, and shall refuse to record any such purported transfer of the Restricted Securities in the books of MEDS.
In order to enforce this Section 1, MEDS may impose stop-transfer instructions with respect to the Restricted Securities of the
Subject Party (and Permitted Transferees and assigns thereof) until the end of the Lock-Up Period.
(d)
During the Lock-Up Period, each certificate evidencing any Restricted Securities shall be stamped or otherwise imprinted with a legend
in substantially the following form, in addition to any other applicable legends:
“THE
SECURITIES REPRESENTED BY THIS CERTIFICATE ARE SUBJECT TO RESTRICTIONS ON TRANSFER SET FORTH IN A LOCK-UP AGREEMENT, DATED AS OF JULY
25, 2024, BY AND AMONG THE ISSUER OF SUCH SECURITIES (THE “ISSUER”) AND THE ISSUER’S SECURITY HOLDER NAMED THEREIN,
AS AMENDED. A COPY OF SUCH LOCK-UP AGREEMENT WILL BE FURNISHED WITHOUT CHARGE BY THE ISSUER TO THE HOLDER HEREOF UPON WRITTEN REQUEST.”
(e)
For the avoidance of any doubt, the Subject Party shall retain all of its rights as a stockholder of MEDS during the Lock-Up Period,
including the right to vote any Restricted Securities.
(f)
The Subject Party hereby represents and warrants that the undersigned has full power and authority to enter into this Agreement. All
authority herein conferred or agreed to be conferred and any obligations of the undersigned shall be binding upon the successors, assigns,
heirs or personal representatives of the undersigned.
(g)
The Subject Party understands that if the Merger Agreement is terminated for any reason, the Subject Party shall be released from all
obligations under this Agreement. The undersigned understands that MEDS and the Company are proceeding with the Contemplated Transactions
in reliance upon this Agreement.
2.
Miscellaneous; No Third-Party Beneficiaries.
(a)
Effective Date; Termination of Merger Agreement. This Agreement shall be binding upon Subject Party upon Subject Party’s
execution and delivery of this Agreement, but this Agreement shall only become effective upon the Closing. Notwithstanding anything to
the contrary, if the Merger Agreement is terminated in accordance with its terms prior to the Closing, this Agreement shall automatically
terminate and become null and void, and the parties shall not have any rights or obligations related to this Agreement.
(b)
Binding Effect; Assignment. This Agreement and all of the provisions herein shall be binding upon and inure to the benefit of
the parties hereto and their respective permitted successors and assigns. This Agreement and all rights and obligations of a party are
personal and may not be transferred or delegated at any time. Notwithstanding the foregoing, MEDS may freely assign any or all of its
rights under this Agreement, in whole or in part, to any successor entity (whether by merger, consolidation, equity sale, asset sale,
or otherwise) without obtaining the consent or approval of the Subject Party. This Agreement is intended for the benefit of the parties
hereto and their respective successors and permitted assigns and is not for the benefit of, nor may any provision herein be enforced
by, any other person.
(c)
Third Parties. Nothing contained in this Agreement or in any instrument or document executed by any party in connection with the
transactions contemplated hereby shall create any rights in, or be deemed to have been executed for the benefit of, any person or entity
that is not a party hereto or thereto or a successor or permitted assign of such a party.
(d)
Governing Law; Jurisdiction. This Agreement and any dispute or controversy arising out of or relating to this Agreement shall
be governed by and construed in accordance with the laws of the State of Delaware, without regard to the conflict of law principles thereof.
All Actions arising out of or relating to this Agreement shall be heard and determined exclusively in any state or federal court located
in Wilmington, Delaware (or in any appellate courts thereof) (the “Specified Courts”). Each party hereto hereby
(i) submits to the exclusive jurisdiction of any Specified Court for the purpose of any Action arising out of or relating to this Agreement
brought by any party hereto and (ii) irrevocably waives, and agrees not to assert by way of motion, defense, or otherwise, in any such
Action, any claim that it is not subject personally to the jurisdiction of the above-named courts, that its property is exempt or immune
from attachment or execution, that the Action is brought in an inconvenient forum, that the venue of the Action is improper, or that
this Agreement or the transactions contemplated hereby may not be enforced in or by any Specified Court. Each party agrees that a final
judgment in any Action shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner
provided by Law. Each party irrevocably consents to the service of the summons and complaint and any other process in any other action
or proceeding relating to the transactions contemplated by this Agreement, on behalf of itself, or its property, by personal delivery
of copies of such process to such party at the applicable address set forth in Section 2(f). Nothing in this Section shall affect
the right of any party to serve legal process in any other manner permitted by applicable law.
(e)
WAIVER OF JURY TRIAL. EACH OF THE PARTIES HERETO HEREBY WAIVES TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW ANY RIGHT IT
MAY HAVE TO A TRIAL BY JURY WITH RESPECT TO ANY ACTION DIRECTLY OR INDIRECTLY ARISING OUT OF, UNDER OR IN CONNECTION WITH THIS AGREEMENT
OR THE TRANSACTIONS CONTEMPLATED HEREBY. EACH PARTY HERETO (i) CERTIFIES THAT NO REPRESENTATIVE OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY
OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF ANY ACTION, SEEK TO ENFORCE THAT FOREGOING WAIVER AND (ii) ACKNOWLEDGES
THAT IT AND THE OTHER PARTIES HERETO HAVE BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS
IN THIS SECTION.
(f)
Interpretation. The titles and subtitles used in this Agreement are for convenience only and are not to be considered in construing
or interpreting this Agreement. In this Agreement, unless the context otherwise requires: (i) any pronoun used in this Agreement shall
include the corresponding masculine, feminine, or neuter forms, and the singular form of nouns, pronouns, and verbs shall include the
plural and vice versa; (ii) “including” (and with correlative meaning “include”) means including without limiting
the generality of any description preceding or succeeding such term and shall be deemed in each case to be followed by the words “without
limitation”; (iii) the words “herein,” “hereto,” and “hereby” and other words of similar import
in this Agreement shall be deemed in each case to refer to this Agreement as a whole and not to any particular section or other subdivision
of this Agreement; and (iv) the term “or” means “and/or”. The parties have participated jointly in the negotiation
and drafting of this Agreement. Consequently, in the event an ambiguity or question of intent or interpretation arises, this Agreement
shall be construed as if drafted jointly by the parties hereto, and no presumption or burden of proof shall arise favoring or disfavoring
any party by virtue of the authorship of any provision of this Agreement.
(g)
Notices. All notices, consents, waivers, and other communications hereunder shall be in writing and shall be deemed to have been
duly given when delivered (i) in person, (ii) by facsimile or other electronic means, with affirmative confirmation of receipt, (iii)
one Business Day after being sent, if sent by reputable, nationally recognized overnight courier service, or (iv) three (3) Business
Days after being mailed, if sent by registered or certified mail, pre-paid and return receipt requested, in each case to the applicable
party at the following addresses (or at such other address for a party as shall be specified by like notice):
If
to MEDS, prior to the Closing Date to:
TRxADE
Health, Inc.
6308
Benjamin Rd, Suite 708
Tampa,
Florida 33634
Attention:
Suren Ajjarapu, Chief Executive Officer
Email:
suren@rxintegra.com
If
to the Company, or to MEDS after the Closing Date to:
Scienture,
Inc.
20
Austin Blvd
Commack,
NY 11725
Attention:
Shankar Hariharan, Ph.D., President and
Chief Executive Officer
Email:
shankar.hariharan@scienture.com |
|
with
copies to (which shall not constitute notice):
Dykema
Gossett PLLC
111
E Kilbourn Ave, Suite 1050
Milwaukee,
WI 53202
Attn:
Kate Bechen, Andrew Frost
Facsimile
No.: (866) 945-9792
Telephone
No.: (414) 488-7333
Email:
kbechen@dykema.com;
afrost@dykema.com
with
copies to (which shall not constitute notice):
Goodwin
Procter LLP
The
New York Times Building
620
Eighth Avenue
New
York, NY 10018
Attention:
Stephen Davis, Michael R. Patrone
Email:
sdavis@goodwinlaw.com; mpatrone@goodwinlaw.com |
If
to the Subject Party, to: the address set forth below the Subject Party’s name on the signature page to this Agreement.
(h)
Amendments and Waivers. Any term of this Agreement may be amended and the observance of any term of this Agreement may be waived
(either generally or in a particular instance, and either retroactively or prospectively) only with the written consent of MEDS and the
Subject Party. No failure or delay by a party in exercising any right hereunder shall operate as a waiver thereof. No waivers of or exceptions
to any term, condition, or provision of this Agreement, in any one or more instances, shall be deemed to be or construed as a further
or continuing waiver of any such term, condition, or provision.
(i)
Authorization on Behalf of MEDS. The parties acknowledge and agree that notwithstanding anything to the contrary contained in
this Agreement, any and all determinations, actions, or other authorizations under this Agreement on behalf of MEDS, including enforcing
MEDS’s rights and remedies under this Agreement, or providing any waivers with respect to the provisions hereof, shall solely be
made, taken, and authorized by majority of the disinterested independent directors of MEDS’s board of directors. In the event that
MEDS at any time does not have any disinterested directors, so long as the Subject Party has any remaining obligations under this Agreement,
MEDS will promptly appoint one in connection with this Agreement. Without limiting the foregoing, in the event that an affiliate of a
Subject Party serves as a director, officer, employee, or other authorized agent of MEDS or any of its current or future affiliates,
neither the Subject Party nor its affiliate shall have authority, express or implied, to act or make any determination on behalf of MEDS
or any of its current or future affiliates in connection with this Agreement or any dispute or Action with respect hereto.
(j)
Severability. In case any provision in this Agreement shall be held invalid, illegal, or unenforceable in a jurisdiction, such
provision shall be modified or deleted, as to the jurisdiction involved, only to the extent necessary to render the same valid, legal,
and enforceable, and the validity, legality, and enforceability of the remaining provisions hereof shall not in any way be affected or
impaired thereby nor shall the validity, legality, or enforceability of such provision be affected thereby in any other jurisdiction.
Upon such determination that any term or other provision is invalid, illegal, or incapable of being enforced, the parties will substitute
for any invalid, illegal, or unenforceable provision a suitable and equitable provision that carries out, so far as may be valid, legal,
and enforceable, the intent and purpose of such invalid, illegal, or unenforceable provision.
(k)
Specific Performance. Each party acknowledges that its obligations under this Agreement are unique, recognizes and affirms that,
in the event of a breach of this Agreement, money damages will be inadequate and there will be no adequate remedy at law, and agrees
that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed in accordance with their
specific terms or were otherwise breached. Accordingly, the adversely affected party or parties shall be entitled to an injunction or
restraining order to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof, without the requirement
to post any bond or other security, this being in addition to any other right or remedy available under this Agreement, at law or in
equity.
(l)
Entire Agreement. This Agreement constitutes the full and entire understanding and agreement among the parties with respect to
the subject matter hereof, and any other written or oral agreement relating to the subject matter hereof existing between the parties
is expressly canceled; provided, that, for the avoidance of doubt, the foregoing shall not affect the rights and obligations of
the parties under the Merger Agreement or any Ancillary Agreements. Notwithstanding the foregoing, nothing in this Agreement shall limit
any of the rights or remedies or any of the obligations of the parties hereto under any other agreement between a Subject Party and MEDS
or any certificate or instrument delivered in connection with the Purchase, and nothing in any other agreement, certificate, or instrument
shall limit any of the rights or remedies or any of the obligations under this Agreement.
(m)
Further Assurances. From time to time, at another party’s request and without further consideration (but at the requesting
party’s reasonable cost and expense), each party shall execute and deliver such additional documents and take all such further
action as may be reasonably necessary to consummate the transactions contemplated by this Agreement.
(n)
Counterparts; Facsimile. This Agreement may also be executed and delivered by facsimile signature or by email in portable document
format in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the
same instrument.
[Remainder
of Page Intentionally Left Blank; Signature Pages Follow]
IN
WITNESS WHEREOF, the parties have executed this Lock-Up Agreement as of the date first written above.
| |
MEDS: |
| |
|
|
| |
TRxADE HEALTH INC. |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
{Additional
Signatures on the Following Pages}
[Signature
Page to Lock-Up Agreement (___________________)]
The
Subject Party:
By:
_____________________________________________
Number
of and Type of Restricted Securities:
MEDS
Common Stock: ______________________________
MEDS
Preferred Stock: ______________________________
MEDS
Common Stock subject to options: ________________
MEDS
Common Stock subject to warrants: _______________
Address
for Notice:
| _________________________________________________ |
|
| _________________________________________________ |
|
| Email:
___________________________________________ |
|
ANNEX
D
CONSULTING
AGREEMENT
This
Consulting Agreement (this “Agreement”) is made and entered into as of this 25th day of July, 2024, between TRxADE
HEALTH, INC., a Delaware corporation (as the name may be changed in accordance with the provisions of the DGCL, the “Company”)
and Surendra K. Ajjarapu (“Consultant”). The Company and Consultant are referred to herein individually as
a “Party,” or collectively as the “Parties.” The Executive Employment Agreement, dated April 14,
2020, as amended on May 5, 2020, August 29, 2022 and January 17, 2023, shall be terminated immediately upon the Service Start Date (as
defined below) of this Agreement (the “Executive Employment Agreement”).
The
Parties agree as follows:
1.
SERVICES. Beginning on the Service Start Date (as defined below) and continuing until the termination
of this Agreement pursuant to Section 3, the Consultant will provide the Company with regular and customary general business and financial
consulting advice as the Company reasonably requests. In performing these duties, the Consultant shall take into account public market
considerations and provide the Company with the benefits of his best judgment and efforts. Consultant shall perform the Services in a
timely and workmanlike manner, in accordance with the highest applicable professional standards and practices and all applicable law.
The Consultant’s duties may include, but not necessarily be limited to:
(a)
Assisting with the development of the Company’s corporate strategies, organizational design, research and development, product
commercialization, and such matters otherwise requested by Company officers;
(b)
Assisting with the ideation and analysis of financial structuring and accounting approaches and alternatives the Company should consider
and can implement in the course of raising money, financing and funding its operations and initiatives, and optimizing its cost efficiencies
and effectiveness; and,
(c)
Assisting with the creation and dissemination of corporate and financial information regarding the Company to the investment and financial
community and public at large as requested by the Company through its authorized personnel, pursuant to applicable company policies;
and,
(d)
Other such consultation the Company’s officers deem useful to the Company’s management and within the scope of Consultant’s
expertise.
2.
COMPENSATION AND EXPENSE PAYMENTS.
(a)
Stock Compensation. As consideration for the services provided herein, beginning on the first calendar quarter following the Service
Start Date, and on each of the following seven (7) calendar quarters thereafter (for a total of eight (8) times), the Company shall issue
Consultant from a Company executive equity plan 702,086 shares (subject to equitable adjustment as a result of stock splits, reverse
stock splits or other adjustments to capitalization occurring after the date hereof) of Company common stock (the “Stock Compensation”).
However, in no event shall issuance of Stock Compensation in any given calendar year be greater than the amount of Company common stock
available to be issued under the Company’s executive equity plans. Any issuance of Stock Compensation unable to be issued due to
a lack of available shares of Company common stock in the Company’s executive equity plans shall roll over quarter-to-quarter until
the Stock Compensation has been issued in full. The common stock issued to the Consultant as Stock Compensation will be unregistered
stock, but shall be subject to registration on the Company’s first S-1 filing immediately following the issuance.
(b)
Expenses. Company shall reimburse Consultant for reasonable and necessary costs and expenses associated with the Consultant’s
Services, including travel costs, research expenses, copy and production charges, and courier fees, in each case only when substantiated
statements have been submitted to and approved by Company.
3.
SERVICE START DATE, TERM AND TERMINATION.
(a)
Service Start Date. Consultant shall begin providing services pursuant to this Agreement upon Consultant’s termination of
employment (for any reason) with the Company (the “Service Start Date”).
(b)
Term. Unless otherwise terminated pursuant to Sections 3(c), 3(d) or 3(e) of this Agreement, this Agreement terminates on the
second anniversary of the Service Start Date.
(c)
Termination by Consultant. Consultant may terminate this Agreement sixty (60) days after written notice from Consultant to the
Company. Upon a termination by Consultant, the Company will owe no further Stock Compensation to Consultant.
(d)
Termination by the Company without Cause. The Company may terminate this Agreement without Cause (as defined below) upon sixty
(60) days’ written notice to Consultant; however, the Company’s obligation to pay Consultant the Stock Compensation shall
continue until paid in full.
(e)
Termination by Company for Cause. Notwithstanding anything to the contrary in this Section 3, the Company may terminate this Agreement
for Cause (as defined below) at any time and with immediate effect, without advance notice to Consultant or penalty to the Company. Upon
a termination for Cause, the Company will owe no further Stock Compensation to Consultant. The occurrence of one of the following events
shall constitute a termination for “Cause”:
(i)
The Consultant engages in fraud, embezzlement or misappropriation of funds or property or commits or engages in a felony, breach of trust
in connection with Consultant’s Services, sexual or other unlawful harassment or abuse, discrimination or retaliation, illegal
drug usage, misrepresentation, dishonesty, disloyalty or any act involving moral turpitude, or other similar cause;
(ii)
Any material breach by the Consultant of Consultant’s obligations under this Agreement and such breach is not cured within thirty
(30) days following written notice to the Consultant describing the breach. For the avoidance of doubt, this right to cure such breach
under this Section 3(e)(ii) does not apply to Section 3(e)(i).
(f)
Effect of Termination. The Consultant’s and Company’s rights and obligations under Sections 3, 5, 6, 7, 8, 9, 10 and
11 shall survive the Agreement’s termination, expiration, or cancellation.
(g)
Return of Materials. Upon the Agreement’s termination, expiration, cancellation, or Company’s request, Consultant
shall immediately deliver to Company all documents, records, or other materials relating to the Services performed and/or containing
Confidential Information or Work Product, as those terms are defined as part of this Agreement. This includes optical, magnetic, or other
electronic media, documentation or other materials, along with a written list of all uncompleted Services pertaining to this Agreement,
specifically identifying the uncompleted Service’s status.
4.
GENERAL RELEASE OF CLAIMS. In consideration for, among other terms, the Company’s execution of
this Agreement and the benefits provided to Consultant pursuant to Section 2, on the Service Start Date Consultant shall execute and
deliver to the Company the general release of claims attached as Exhibit B hereto.
5.
INDEPENDENT CONTRACTOR. Consultant is an independent contractor and not an employee. Without limiting
the generality of the foregoing, (i) neither the Company nor any of its affiliates are responsible to Consultant or any governmental
body for any payroll-related taxes, excise taxes (including, without limitation under Section 280G or 4999 of the Code), any penalty
taxes or any other taxes relating to Consultant’s services or the amounts provided hereunder and (ii) Consultant is solely responsible
for all matters relating compliance with worker’s compensation, unemployment, disability insurance, social security withholding,
and all other federal, state and local laws, rules and regulations. Consultant shall indemnify and hold Company harmless from any causes
of action or claims arising from this Section 5. This Agreement is not a partnership or joint venture. Neither Party is liable for any
obligations incurred by the other Party. If Company deems necessary or appropriate, Company may report Consultants income to the Internal
Revenue Service on IRS Form 1099. Consultant shall comply promptly with Company’s reasonable requests for information the Internal
Revenue Service or any other governmental agency requires.
6.
WORK OWNERSHIP. All right, title, and interest in and to all materials, products, and work Consultant
produces that is related in any way to the Services performed under this Agreement (the “Work Product”), including
the rights to ideas or inventions and rights under patent, copyright, trademark, trade secret and other applicable laws, belong exclusively
to Company and are works made for hire in the course of the Services performed under this Agreement. Consultant irrevocably assigns all
right, title, and interest in the Work Product to Company without further consideration and free from any claim, lien, or right. Company
has the right to obtain and to hold all copyright, patent, registration, or other protection for the Work Product as Company may require.
Consultant agrees to execute any further documents or instruments Company deems necessary to perfect the Company’s rights set forth
in this Section 6. Consultant grants to Company, or any person designated by Company, a limited power of attorney to execute the documents
or instruments if Consultant is unable or unwilling to do so.
7.
NO CONFLICT. Consultant represents and warrants that (i) Consultant’s execution and delivery
of this Agreement and Consultant’s performance and obligations in this Agreement do not, and will not, violate any other contract,
agreement, or arrangement, whether written or oral, that Consultant is a party or otherwise subject to; and (ii) there is no conflict
of interest between this Agreement’s performance by Consultant and any performance of services by Consultant for any other party.
In the event Consultant believes any conflict may arise during the Agreement’s term, Consultant shall immediately notify Company
and Company may, at its sole and absolute discretion, terminate this Agreement.
8.
CONFIDENTIALITY. Consultant will sign the Confidentiality and Non-Disclosure Agreement in Exhibit
A at the same time as this Agreement and the Confidentiality and Non-Disclosure Agreement is incorporated into this Agreement by
reference herein.
9.
REASONABLENESS OF SCOPE; REMEDIES. Consultant acknowledges and agrees that Consultant’s services
to Company are of a special character with unique value to Company and that the confidentiality and other covenants set forth in this
Agreement are reasonably necessary to protect Company’s legitimate business interests and are valid in all respects. Consultant
further acknowledges and agrees that a breach by Consultant of the Agreement’s provisions is likely to cause Company serious, immediate,
and irreparable injury and damage that cannot be reasonably or adequately compensated by damages at law. Consultant therefore agrees
that Company is entitled to immediate injunctive or other equitable relief (including temporary restraining orders or preliminary or
permanent injunctions) to prevent a breach, continued breach, or anticipated breach of this Agreement, without the necessity of posting
bond, in addition to all other remedies available to it. Consultant agrees to pay any and all reasonable costs and expenses, including
attorneys’ fees and costs, Company incurs in enforcing any provision in the Agreement.
10.
WAIVER OF SEVERANCE. As an inducement for the Company entering into this Agreement, in the event Consultant’s
employment with the Company is terminated for any reason, Consultant hereby waives any right to any severance compensation owed to Consultant
by the Company under any contractual obligation or otherwise. For the avoidance of doubt, with the exception of accrued but unpaid salary,
in no event shall any amount be payable pursuant to the Executive Employment Agreement whether in connection with the execution of this
Agreement or any subsequent termination of this Agreement.
11.
INDEMNIFICATION.
(a)
If Consultant is made a party to any Proceeding (as defined below) by a third party (excluding the Company and its Affiliates) in connection
with Consultant’s Services hereunder, then the Company shall indemnify and hold Consultant harmless against any and all reasonable
and documented costs, expenses, liabilities, and losses (including, without limitation, reasonable attorneys’ fees and charges)
incurred or suffered by Consultant in connection therewith (“Losses”), except, in each case, to the extent such Losses
arise out of or are related to Consultant’s fraud, bad faith, willful misconduct or gross negligence.
(b)
For purposes of this Agreement, the following terms shall have the following meanings: “Affiliate” of a Person shall mean
any Person that directly or indirectly controls, is controlled by, or is under common control with, such Person; “Person”
shall mean any individual, corporation, partnership, limited liability company, joint venture, trust, estate, board, committee, agency,
body, employee benefit plan, or other person or entity; and “Proceeding” shall mean any action, suit, or proceeding, whether
civil, criminal, administrative, or appellate.
12.
GENERAL PROVISIONS.
(a)
Governing Law. The Agreement shall be construed, interpreted, and performed in accordance with the laws of the State of Delaware,
without reference to any conflicts of law provisions.
(b)
Assignment. Neither Party may assign this Agreement without the other Party’s prior written consent. Any assignment attempted
or made by one Party without the other Party’s prior written consent is void and of no force or effect.
(c)
Notice. Any notice required or desired to be given under this Agreement is deemed given if in writing and sent by certified mail
to Company at the address in this Agreement.
(d)
Headings, Gender, Interpretation. Headings or titles contained in this Agreement are used for convenience only and are not be
used in the Agreement’s construction or in interpretating the Agreement. All pronouns used in this Agreement include masculine,
feminine, and neuter forms. Any singular number includes the plural and any plural number includes the singular. Unless otherwise specified,
references to Sections or Exhibits are to the Sections or Exhibits in this Agreement and all of the foregoing is incorporated in this
Agreement by reference. The term “including” is not solely exclusive and shall mean “including, but not limited to.”
(e)
No Party Considered Drafter. Despite the possibility that one Party may have prepared the Agreement’s initial draft or played
a greater role in subsequent draft’s physical preparation, the Parties agree that neither of them are the Agreement’s drafter
and that, in construing this Agreement in case of any claim that any provision hereof may be ambiguous, no such provision shall be construed
in favor of one Party on the ground that another Party drafted the provision.
(f)
Publicity. Consultant shall not use Company’s name in any news release, public announcement, advertisement, or other form
of publicity without the Company’s prior written consent.
(g)
Severability. All covenants and provisions contained herein are severable. In the event that any court of competent jurisdiction
holds covenant or provision invalid, this Agreement shall be construed as if such invalid covenant or provision did not exist. In the
event that any covenant or provision of this section is broader or of greater scope as to time, territory, products, services, or customers
than any court of competent jurisdiction will enforce, the Parties hereto intend that the court may enforce the covenants and provisions
to the greatest extent permitted by law and modify the covenants and provisions accordingly.
(h)
No Waiver. The Company failure to exercise, and no delay to exercise, any right in the Agreement shall operate as a waiver of
that right, nor shall any single or partial exercise of any right preclude further exercise of the same right or the exercise of any
other right by Company.
(i)
Opportunity to Review. Each party agrees that this is a legally binding agreement and acknowledges and agrees that it or he has
had the opportunity, if desired, to consult with legal counsel of its or his own choice.
(j)
Entire Agreement. This Agreement supersedes all previous agreements between the Parties and contains the entire agreement between
them related to the Agreement’s subject matter provided herein (including the Executive Employment Agreement, but excluding any
indemnification obligations the Company has to Consultant). No other representations, promises, conditions, warranties, or understandings,
whether expressed or implied, are binding upon either Party, and no provision in this Agreement may be waived, altered, or amended except
by a writing signed by Consultant and Company that specifically identifies the Section of this Agreement to be waived, altered or amended.
[Signature
page follows.]
IN
WITNESS WHEREOF, the Parties executed this Agreement on the day written below.
| TRxADE
HEALTH, INC. |
|
SURENDRA
K. AJJARAPU |
| |
|
|
|
|
| By:
|
/s/
Prashant Patel |
|
By: |
/s/
Surendra K. Ajjarapu |
| |
|
|
|
|
| Name:
|
Prashant
Patel |
|
Date: |
July
25, 2024 |
| |
|
|
|
|
| Title:
|
Chief
Financial Officer |
|
|
|
| |
|
|
|
|
| Date:
|
July
25, 2024 |
|
|
|
EXHIBIT
A
Confidentiality
and Non-Disclosure Agreement (the “Agreement”)
In
connection with your consulting services with TRxADE HEALTH, INC. (the “Company,” which term shall include the Company
and its subsidiaries) you will have access to certain information regarding the Company which is non-public, confidential and/or proprietary
in nature. In consideration of, and as a condition to, furnishing you with such information and any other information (whether communicated
in writing or orally) delivered to you by the Company or its directors, officers, employees, advisors (including without limitation financial
advisors, counsel and accountants), agents or controlling persons (such affiliates and other persons being herein referred to collectively
as “Representatives”), including, but not limited to, trade secrets, technical data (e.g., computer software, drawings, processes,
patents, procedures, inventions, designs, production methods, techniques, know-how), business and financial information, correspondence,
written or oral representations, memoranda, reports, records, or other information, including any other information or notes you derived
from any such information (such information being herein referred to as “Confidential Information”), the Company hereby requests
your agreement as follows:
1.
The Confidential Information will be used solely for purposes related to the Services detailed in the Consulting Agreement and not in
a manner in any way detrimental to the Company, and you will, at all times including following the termination of the Services, keep
the Confidential Information confidential. You agree to take all reasonable steps to ensure that the Confidential Information is kept
confidential, including, but not limited to, properly and securely storing all written Confidential Information and the marking of
all reports, summaries, records or other material relating thereto prepared by you as confidential. You shall not copy, abstract, reverse
engineer or disclose any Confidential Information to any other person, firm, corporation, or other entity.
2.
The term “Confidential Information” does not include any information which (i) at the time of disclosure or thereafter is
generally available to and known by the public (other than as a result of its disclosure by you), (ii) was available to you on a non-confidential
basis prior to disclosure by the Company or its Representatives, as evidenced by your written records, or (iii) becomes available to
you on a non-confidential basis from a person who is not otherwise bound by a confidentiality agreement with the Company or its Representatives,
or by any other obligation of secrecy, or is not otherwise prohibited from transmitting the information to you. As used in this Agreement,
the term “person” shall be broadly interpreted to include, without limitation, any corporation, company, partnership and
individual.
3.
In the event that you receive a request to disclose all or any part of the information contained in the Confidential Information under
the terms of a valid and effective subpoena or order issued by a court of competent jurisdiction, you agree to (i) immediately notify
the Company of the existence, terms and circumstances surrounding such a request, (ii) consult with the Company on the advisability of
taking legally available steps to resist or narrow such request, and (iii) if disclosure of such information is required, upon request
by the Company, cooperate with the Company at the Company’s expense in obtaining an order or other reliable assurance that confidential
treatment will be accorded to such portion of the information which the Company so designates.
4.
You will return to the Company all copies of the Confidential Information in your possession and you will destroy all copies of any analyses,
compilations, studies or other documents prepared by you or for your internal use which reflect the Confidential Information, promptly
upon the Consulting Agreement’s termination or when the Company so requests such return and destruction of the Confidential Information.
Notwithstanding the foregoing, the aforementioned date(s) and timing of requisite return and destruction may be extended if mutually
agreed upon in a separate writing signed by both parties to this Agreement. You shall keep a record, in reasonable detail, of the
Confidential Information provided to you, the location of such Confidential Information and all persons to whom you furnish any Confidential
Information, and will make such record available to the Company promptly upon its request.
5.
You hereby acknowledge that you have received a copy of the Company’s Insider Trading Compliance Policy (the “ITCP”)
and that you are included within the ITCP’s scope of “Company Personnel” and are hereby being designated by the Company
as an “Insider” under the ITCP during the course of this Agreement, and you are therefore subject to and agree to comply
with the conditions and restrictions applicable to Company Personnel and Insiders outlined in the ITCP. Further, you acknowledge that
you are aware, that the United States securities laws prohibit any person who has received from an issuer material, non-public information
from purchasing or selling securities of such issuer (and options, warrants and rights relating thereto) or from communicating such information
to any other person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such securities.
You hereby agree that you will not use or communicate any Confidential Information in violation of these laws.
7.
No license or conveyance of any rights under any discoveries, inventions, patents, trade secrets, copyrights or other form of intellectual
property is granted or implied by the provision of Confidential Information to you. Any and all documents containing Confidential Information
produced or delivered to you shall remain the property of the Company. You understand and acknowledge that the Company and its Representatives
make no representation or warranty, express or implied, as to the accuracy or completeness of the Confidential Information or freedom
from defect of any kind, including freedom from any patent, copyright, or trademark infringement which may result from the use of such
Confidential Information. Neither the Company, its affiliates or Representatives, nor any of their officers, directors, employees, agents
or controlling persons (within the meaning of the Securities Exchange Act of 1934) shall have any liability to you or any other person
resulting from your use of the Confidential Information. Any and all representations and warranties shall be made solely by the Company
and shall be set forth in a signed agreement and then be subject to the provisions thereof.
8.
You agree to reimburse, indemnify and hold harmless the Company and its Representatives from any damage, loss or expense incurred by
them as a result of the use of the Confidential Information contrary to the terms of this Agreement. You understand that any breach of
this Agreement may cause the Company and its Representatives to suffer irreparable harm for which monetary damages would not be sufficient.
Without prejudice to the rights and remedies at law or in equity otherwise available to the Company and its Representatives, the Company
shall be entitled to equitable relief by way of specific performance or injunction if you breach or threaten to breach any of the provisions
of this Agreement. You also agree to waive the requirement for bond in conjunction with such remedy.
9.
You understand and agree that no failure or delay by the Company or its Representatives in exercising any right, power or privilege hereunder
shall operate as a waiver thereof, nor shall any single or partial exercise thereof preclude any other or further exercise thereof or
the exercise of any right, power or privilege hereunder.
10.
This Agreement is for the benefit of the Company and its Representatives, and shall be governed by the laws (excluding the conflicts
of laws rules) of the State of Delaware and subject to the exclusive jurisdiction of the federal and state courts located in Delaware,
and you agree not to commence any action, suit or proceeding relating to this Agreement except in such courts.
11.
This Agreement represents the entire understanding and agreement of the parties hereto and may be modified or waived only by a separate
writing executed by the Company and you expressly so modifying or waiving such Agreement. This Agreement is in addition to, and does
not supersede or replace, any other obligations of confidentiality, assignment of inventions, or restrictive covenants between you and
the Company or any Representative.
12.
If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, void or
unenforceable, the remainder of the terms, provisions, covenants and restrictions of this Agreement shall remain in full force and effect.
13.
You hereby acknowledge that you are aware that the United States securities laws and other laws prohibit any person who has material,
non-public information concerning an entity from purchasing or selling securities of that entity or from communicating such information
to any other person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such securities.
the Company hereby advises you and you hereby acknowledge that Confidential Information may contain material non-public information relating
to the Company and its affiliates, customers and vendors. Without limiting the foregoing, you hereby agree that you will only use the
Confidential Information in accordance with all applicable laws.
[Signature
page follows.]
| TRxADE
HEALTH, INC. |
|
SURENDRA
K. AJJARAPU |
| |
|
|
|
|
| By:
|
|
|
By:
|
|
| |
|
|
|
|
| Name:
|
|
|
Date:
|
|
| |
|
|
|
|
| Title:
|
|
|
|
|
| |
|
|
|
|
| Date:
|
|
|
|
|
EXHIBIT
B
General
Release of Claims
This
General Release of Claims (this “Release”) is entered into by and between Surendra K. Ajjarapu (the “Executive”)
and Scienture Holdings, Inc. (the “Company”) in connection with that certain Consulting Agreement (the “Consulting
Agreement”) between the Executive and the Company, to which this Release is attached. Capitalized terms that are not otherwise
defined in this Release have the meanings set forth in the Employment Agreement.
| 1. | Tender
of Release. This Release is automatically tendered to
the Executive upon the Service Start Date, if the Consulting Agreement remains effective
as of such date. |
| 2. | General
Release of Claims. In consideration for, among other
terms, the Company’s execution of the Consulting Agreement and the benefits provided
to the Executive thereunder, to which the Executive acknowledges the Executive would otherwise
not be entitled, the Executive, on behalf of the Executive and the Executive’s heirs,
administrators, representatives and executors, voluntarily releases and forever discharges
the Company, its affiliated and related entities, their respective predecessors, successors
and assigns, their respective employee benefit plans and fiduciaries of such plans, and the
current and former officers, directors, shareholders, employees, attorneys, accountants and
agents of each of the foregoing in their official and personal capacities (collectively referred
to as the “Releasees”) generally
from all claims, demands, debts, damages and liabilities of every name and nature, known
or unknown (“Claims”) that, as
of the Service Start Date, the Executive has, ever had, now claim to have or ever claimed
to have had against any or all of the Releasees relating to the Executive’s employment
by and termination of employment with the Company, including but not limited to (a) wrongful
discharge or violation of public policy; (b) breach of contract; (c) defamation or other
torts; (d) intentional or negligent infliction of emotional distress; of retaliation, harassment,
or discrimination under federal, state or local law (including, without limitation, Claims
of discrimination, harassment, or retaliation under Americans with Disabilities Act, and
Title VII of the Civil Rights Act of 1964); (e) Claims under any other federal or state statute
(including, without limitation, Claims under the Worker Adjustment and Retraining Notification
Act (“WARN”), any state mini-WARN laws, and the Fair Labor Standards Act);
(f) Claims under any federal, district, state or local statutes, including, without limitation,
any and all claims under the Florida Civil Rights Act (FCRA), Florida Whistleblower Protection
Act (FWA), Florida Workers’ Compensation Law Retaliation Act (FWCA), Florida Wage Discrimination
Law, Florida Minimum Wage Act, Florida Equal Pay Law, Florida AIDS Act, Florida Discrimination
on the Basis of Sickle Cell Trait Law, Florida OSHA, the Florida Constitution, the Florida
Fair Housing Act (FHA), Miami-Dade County Code, Chapter 11A, Broward County Human Rights
Act, and Palm Beach County Code, Article VI, all including any amendments and their respective
implementing regulations; (g) Claims for wages, bonuses, incentive compensation, commissions,
stock, stock options, vacation pay or any other compensation or benefits; and for damages
or other remedies of any sort, including, without limitation, compensatory damages, punitive
damages, injunctive relief and attorney’s fees. |
Notwithstanding
the foregoing, this release shall not (a) affect the Executive’s rights under this Release of the Consulting Agreement, (b) apply
to rights or claims that cannot be waived as a matter of law, or (c) affect the Executive’s vested rights, if any, under the Company’s
Section 401(k) plan.
This
Release is intended to be effective as a general release of and bar to all Claims, including unknown Claims.
As
a material inducement to the Company to enter into this Release, the Executive represents that the Executive has not assigned any Claim
to any third party. The Executive acknowledges and agrees that the Executive is not entitled to any wages, salary, commissions, vacation,
equity, bonuses, or any other compensation or benefits from the Company or any of its affiliates, except as is expressly set forth in
the Consulting Agreement.
| 3. | Non-Disparagement.
Subject to Section 6 of this Release, the Executive
agrees not to make any disparaging, critical or detrimental statements (whether written,
oral, through social or electronic media or otherwise) concerning the Company, the Releasees
or any of its or their products or services provided or to be provided. |
| 4. | Protected
Disclosures and Other Protected Actions. Nothing contained
in this Release, any other agreement with the Company, or any Company policy limits the Executive’s
ability, with or without notice to the Company, to: (i) file a charge or complaint with any
federal, state or local governmental agency or commission (a “Government
Agency”), including without limitation, the Equal
Employment Opportunity Commission, the National Labor Relations Board or the Securities and
Exchange Commission (the “SEC”);
(ii) communicate with any Government Agency or otherwise participate in any investigation
or proceeding that may be conducted by any Government Agency, including by providing non-privileged
documents or information; (iii) discuss or disclose information about unlawful acts in the
workplace, such as harassment or discrimination or any other conduct that the Executive has
reason to believe is unlawful; or (v) testify truthfully in a legal proceeding. Any such
communications and disclosures must not violate applicable law and the information disclosed
must not have been obtained through a communication that was subject to the attorney-client
privilege (unless disclosure of that information would otherwise be permitted consistent
with such privilege or applicable law). If a Government Agency or any other third party pursues
any Claim on the Executive’s behalf, the Executive waives any right to monetary or
other individualized relief (either individually or as part of any collective or class action),
but the Company will not limit any right the Executive may have to receive an award pursuant
to the whistleblower provisions of any applicable law or regulation for providing information
to the SEC or any other Government Agency. |
| 5. | Defend
Trade Secrets Act. Pursuant to the federal Defend Trade
Secrets Act of 2016, you shall not be held criminally or civilly liable under any federal
or state trade secret law or under this Release or any other agreement for the disclosure
of a trade secret that (a) is made (i) in confidence to a federal, state, or local government
official, either directly or indirectly, or to an attorney; and (ii) solely for the purpose
of reporting or investigating a suspected violation of law; or (b) is made in a complaint
or other document filed in a lawsuit or other proceeding, if such filing is made under seal. |
(a)
Absence of Reliance. In signing this Release, the Executive is not relying upon any promises or representations made by anyone
at or on behalf of the Company.
(b)
Non-Admission. The Executive understands that the Company is not admitting in any way that it violated any legal obligation that
it owed to the Executive.
(c)
Enforceability. If any portion or provision of this Release (including, without limitation, any portion or provision of any section
of this Release) shall to any extent be declared illegal or unenforceable by a court of competent jurisdiction, then the remainder of
this Release, or the application of such portion or provision in circumstances other than those as to which it is so declared illegal
or unenforceable, shall not be affected thereby, and each portion and provision of this Release shall be valid and enforceable to the
fullest extent permitted by law.
(d)
Waiver. No waiver of any provision of this Release shall be effective unless made in writing and signed by the waiving party.
The failure of a party to require the performance of any term or obligation of this Release, or the waiver by a party of any breach of
this Release, shall not prevent any subsequent enforcement of such term or obligation or be deemed a waiver of any subsequent breach.
(e)
Governing Law; Interpretation. This Release shall be interpreted and enforced under the laws of the state of Florida, without
regard to conflict of law principles. In the event of any dispute, this Release is intended by the parties to be construed as a whole,
to be interpreted in accordance with its fair meaning, and not to be construed strictly for or against either the Executive or the Company
or the “drafter” of all or any portion of this Release.
(f)
Entire Agreement. This Release, together with the Consulting Agreement, constitutes the entire agreement between the Executive
and the Company regarding the subject matter hereof (including the Executive Employment Agreement). This Release, together with the Consulting
Agreement, supersedes any previous agreements or understandings between the Executive and the Company, except any other obligations specifically
preserved in this Release or the Consulting Agreement.
(g)
Time for Consideration; Effective Date. The Executive acknowledges that the Executive has been given the opportunity to consider
this Release for five business days before signing it (the “Consideration Period”) and that the Executive has knowingly
and voluntarily entered into this Release. To accept this Release, the Executive must return a signed copy of this Release so that it
is received by the Company at or before the expiration of the Consideration Period. If the Executive signs this Release before the end
of the Consideration Period, the Executive acknowledges by signing this Release that such decision was entirely voluntary and that the
Executive had the opportunity to consider this Release for the entire Consideration Period. This Release shall become effective on the
date it becomes fully executed (the “Effective Date”).
(h)
Counterparts. This Release may be executed and delivered in separate counterparts, including by facsimile or other electronic
means. When both counterparts are signed, they shall be treated together as one and the same document.
[Signature
page follows]
| SCIENTURE
HOLDINGS, INC. |
|
Surendra
K. Ajjarapu |
| |
|
|
|
|
| By:
|
|
|
By:
|
|
| |
|
|
|
|
| Name:
|
|
|
Date:
|
|
| |
|
|
|
|
| Title:
|
|
|
|
|
| |
|
|
|
|
| Date:
|
|
|
|
|
ANNEX
E
CONSULTING
AGREEMENT
This
Consulting Agreement (this “Agreement”) is made and entered into as of this 25th day of July, 2024, between TRxADE
HEALTH, INC., a Delaware corporation (as the name may be changed in accordance with the provisions of the DGCL, the “Company”)
and Prashant Patel (“Consultant”). The Company and Consultant are referred to herein individually as a “Party,”
or collectively as the “Parties.” The Executive Employment Agreement, dated April 14, 2020, as amended on May 5, 2020,
August 29, 2022 and January 17, 2023, shall be terminated immediately upon the Service Start Date (as defined below) of this Agreement
(the “Executive Employment Agreement”).
The
Parties agree as follows:
1.
SERVICES. Beginning on the Service Start Date (as defined below) and continuing until the termination
of this Agreement pursuant to Section 3, the Consultant will provide the Company with regular and customary general business and financial
consulting advice as the Company reasonably requests. In performing these duties, the Consultant shall take into account public market
considerations and provide the Company with the benefits of his best judgment and efforts. Consultant shall perform the Services in a
timely and workmanlike manner, in accordance with the highest applicable professional standards and practices and all applicable law.
The Consultant’s duties may include, but not necessarily be limited to:
(a)
Assisting with the development of the Company’s corporate strategies, organizational design, research and development, product
commercialization, and such matters otherwise requested by Company officers;
(b)
Assisting with the ideation and analysis of financial structuring and accounting approaches and alternatives the Company should consider
and can implement in the course of raising money, financing and funding its operations and initiatives, and optimizing its cost efficiencies
and effectiveness; and,
(c)
Assisting with the creation and dissemination of corporate and financial information regarding the Company to the investment and financial
community and public at large as requested by the Company through its authorized personnel, pursuant to applicable company policies;
and,
(d)
Other such consultation the Company’s officers deem useful to the Company’s management and within the scope of Consultant’s
expertise.
2.
COMPENSATION AND EXPENSE PAYMENTS.
(a)
Stock Compensation. As consideration for the services provided herein, beginning on the first calendar quarter following the Service
Start Date, and on each of the following seven (7) calendar quarters thereafter (for a total of eight (8) times), the Company shall issue
Consultant from a Company executive equity plan 614,325 shares (subject to equitable adjustment as a result of stock splits, reverse
stock splits or other adjustments to capitalization occurring after the date hereof) of Company common stock (the “Stock Compensation”).
However, in no event shall issuance of Stock Compensation in any given calendar year be greater than the amount of Company common stock
available to be issued under the Company’s executive equity plans. Any issuance of Stock Compensation unable to be issued due to
a lack of available shares of Company common stock in the Company’s executive equity plans shall roll over quarter-to-quarter until
the Stock Compensation has been issued in full. The common stock issued to the Consultant as Stock Compensation will be unregistered
stock, but shall be subject to registration on the Company’s first S-1 filing immediately following the issuance.
(b)
Expenses. Company shall reimburse Consultant for reasonable and necessary costs and expenses associated with the Consultant’s
Services, including travel costs, research expenses, copy and production charges, and courier fees, in each case only when substantiated
statements have been submitted to and approved by Company.
3.
SERVICE START DATE, TERM AND TERMINATION.
(a)
Service Start Date. Consultant shall begin providing services pursuant to this Agreement upon Consultant’s termination of
employment (for any reason) with the Company (the “Service Start Date”).
(b)
Term. Unless otherwise terminated pursuant to Sections 3(c), 3(d) or 3(e) of this Agreement, this Agreement terminates on the
second anniversary of the Service Start Date.
(c)
Termination by Consultant. Consultant may terminate this Agreement sixty (60) days after written notice from Consultant to the
Company. Upon a termination by Consultant, the Company will owe no further Stock Compensation to Consultant.
(d)
Termination by the Company without Cause. The Company may terminate this Agreement without Cause (as defined below) upon sixty
(60) days’ written notice to Consultant; however, the Company’s obligation to pay Consultant the Stock Compensation shall
continue until paid in full.
(e)
Termination by Company for Cause. Notwithstanding anything to the contrary in this Section 3, the Company may terminate this Agreement
for Cause (as defined below) at any time and with immediate effect, without advance notice to Consultant or penalty to the Company. Upon
a termination for Cause, the Company will owe no further Stock Compensation to Consultant. The occurrence of one of the following events
shall constitute a termination for “Cause”:
(i)
The Consultant engages in fraud, embezzlement or misappropriation of funds or property or commits or engages in a felony, breach of trust
in connection with Consultant’s Services, sexual or other unlawful harassment or abuse, discrimination or retaliation, illegal
drug usage, misrepresentation, dishonesty, disloyalty or any act involving moral turpitude, or other similar cause;
(ii)
Any material breach by the Consultant of Consultant’s obligations under this Agreement and such breach is not cured within thirty
(30) days following written notice to the Consultant describing the breach. For the avoidance of doubt, this right to cure such breach
under this Section 3(e)(ii) does not apply to Section 3(e)(i).
(f)
Effect of Termination. The Consultant’s and Company’s rights and obligations under Sections 3, 5, 6, 7, 8, 9, 10 and
11 shall survive the Agreement’s termination, expiration, or cancellation.
(g)
Return of Materials. Upon the Agreement’s termination, expiration, cancellation, or Company’s request, Consultant
shall immediately deliver to Company all documents, records, or other materials relating to the Services performed and/or containing
Confidential Information or Work Product, as those terms are defined as part of this Agreement. This includes optical, magnetic, or other
electronic media, documentation or other materials, along with a written list of all uncompleted Services pertaining to this Agreement,
specifically identifying the uncompleted Service’s status.
4.
GENERAL RELEASE OF CLAIMS. In consideration for, among other terms, the Company’s execution of
this Agreement and the benefits provided to Consultant pursuant to Section 2, on the Service Start Date Consultant shall execute and
deliver to the Company the general release of claims attached as Exhibit B hereto.
5.
INDEPENDENT CONTRACTOR. Consultant is an independent contractor and not an employee. Without limiting
the generality of the foregoing, (i) neither the Company nor any of its affiliates are responsible to Consultant or any governmental
body for any payroll-related taxes, excise taxes (including, without limitation under Section 280G or 4999 of the Code), any penalty
taxes or any other taxes relating to Consultant’s services or the amounts provided hereunder and (ii) Consultant is solely responsible
for all matters relating compliance with worker’s compensation, unemployment, disability insurance, social security withholding,
and all other federal, state and local laws, rules and regulations. Consultant shall indemnify and hold Company harmless from any causes
of action or claims arising from this Section 5. This Agreement is not a partnership or joint venture. Neither Party is liable for any
obligations incurred by the other Party. If Company deems necessary or appropriate, Company may report Consultants income to the Internal
Revenue Service on IRS Form 1099. Consultant shall comply promptly with Company’s reasonable requests for information the Internal
Revenue Service or any other governmental agency requires.
6.
WORK OWNERSHIP. All right, title, and interest in and to all materials, products, and work Consultant
produces that is related in any way to the Services performed under this Agreement (the “Work Product”), including
the rights to ideas or inventions and rights under patent, copyright, trademark, trade secret and other applicable laws, belong exclusively
to Company and are works made for hire in the course of the Services performed under this Agreement. Consultant irrevocably assigns all
right, title, and interest in the Work Product to Company without further consideration and free from any claim, lien, or right. Company
has the right to obtain and to hold all copyright, patent, registration, or other protection for the Work Product as Company may require.
Consultant agrees to execute any further documents or instruments Company deems necessary to perfect the Company’s rights set forth
in this Section 6. Consultant grants to Company, or any person designated by Company, a limited power of attorney to execute the documents
or instruments if Consultant is unable or unwilling to do so.
7.
NO CONFLICT. Consultant represents and warrants that (i) Consultant’s execution and delivery
of this Agreement and Consultant’s performance and obligations in this Agreement do not, and will not, violate any other contract,
agreement, or arrangement, whether written or oral, that Consultant is a party or otherwise subject to; and (ii) there is no conflict
of interest between this Agreement’s performance by Consultant and any performance of services by Consultant for any other party.
In the event Consultant believes any conflict may arise during the Agreement’s term, Consultant shall immediately notify Company
and Company may, at its sole and absolute discretion, terminate this Agreement.
8.
CONFIDENTIALITY. Consultant will sign the Confidentiality and Non-Disclosure Agreement in Exhibit
A at the same time as this Agreement and the Confidentiality and Non-Disclosure Agreement is incorporated into this Agreement by
reference herein.
9.
REASONABLENESS OF SCOPE; REMEDIES. Consultant acknowledges and agrees that Consultant’s services
to Company are of a special character with unique value to Company and that the confidentiality and other covenants set forth in this
Agreement are reasonably necessary to protect Company’s legitimate business interests and are valid in all respects. Consultant
further acknowledges and agrees that a breach by Consultant of the Agreement’s provisions is likely to cause Company serious, immediate,
and irreparable injury and damage that cannot be reasonably or adequately compensated by damages at law. Consultant therefore agrees
that Company is entitled to immediate injunctive or other equitable relief (including temporary restraining orders or preliminary or
permanent injunctions) to prevent a breach, continued breach, or anticipated breach of this Agreement, without the necessity of posting
bond, in addition to all other remedies available to it. Consultant agrees to pay any and all reasonable costs and expenses, including
attorneys’ fees and costs, Company incurs in enforcing any provision in the Agreement.
10.
WAIVER OF SEVERANCE. As an inducement for the Company entering into this Agreement, in the event Consultant’s
employment with the Company is terminated for any reason, Consultant hereby waives any right to any severance compensation owed to Consultant
by the Company under any contractual obligation or otherwise. For the avoidance of doubt, with the exception of accrued but unpaid salary,
in no event shall any amount be payable pursuant to the Executive Employment Agreement whether in connection with the execution of this
Agreement or any subsequent termination of this Agreement.
11.
INDEMNIFICATION.
(a)
If Consultant is made a party to any Proceeding (as defined below) by a third party (excluding the Company and its Affiliates) in connection
with Consultant’s Services hereunder, then the Company shall indemnify and hold Consultant harmless against any and all reasonable
and documented costs, expenses, liabilities, and losses (including, without limitation, reasonable attorneys’ fees and charges)
incurred or suffered by Consultant in connection therewith (“Losses”), except, in each case, to the extent such Losses
arise out of or are related to Consultant’s fraud, bad faith, willful misconduct or gross negligence.
(b)
For purposes of this Agreement, the following terms shall have the following meanings: “Affiliate” of a Person shall mean
any Person that directly or indirectly controls, is controlled by, or is under common control with, such Person; “Person”
shall mean any individual, corporation, partnership, limited liability company, joint venture, trust, estate, board, committee, agency,
body, employee benefit plan, or other person or entity; and “Proceeding” shall mean any action, suit, or proceeding, whether
civil, criminal, administrative, or appellate.
12.
GENERAL PROVISIONS.
(a)
Governing Law. The Agreement shall be construed, interpreted, and performed in accordance with the laws of the State of Delaware,
without reference to any conflicts of law provisions.
(b)
Assignment. Neither Party may assign this Agreement without the other Party’s prior written consent. Any assignment attempted
or made by one Party without the other Party’s prior written consent is void and of no force or effect.
(c)
Notice. Any notice required or desired to be given under this Agreement is deemed given if in writing and sent by certified mail
to Company at the address in this Agreement.
(d)
Headings, Gender, Interpretation. Headings or titles contained in this Agreement are used for convenience only and are not be
used in the Agreement’s construction or in interpretating the Agreement. All pronouns used in this Agreement include masculine,
feminine, and neuter forms. Any singular number includes the plural and any plural number includes the singular. Unless otherwise specified,
references to Sections or Exhibits are to the Sections or Exhibits in this Agreement and all of the foregoing is incorporated in this
Agreement by reference. The term “including” is not solely exclusive and shall mean “including, but not limited to.”
(e)
No Party Considered Drafter. Despite the possibility that one Party may have prepared the Agreement’s initial draft or played
a greater role in subsequent draft’s physical preparation, the Parties agree that neither of them are the Agreement’s drafter
and that, in construing this Agreement in case of any claim that any provision hereof may be ambiguous, no such provision shall be construed
in favor of one Party on the ground that another Party drafted the provision.
(f)
Publicity. Consultant shall not use Company’s name in any news release, public announcement, advertisement, or other form
of publicity without the Company’s prior written consent.
(g)
Severability. All covenants and provisions contained herein are severable. In the event that any court of competent jurisdiction
holds covenant or provision invalid, this Agreement shall be construed as if such invalid covenant or provision did not exist. In the
event that any covenant or provision of this section is broader or of greater scope as to time, territory, products, services, or customers
than any court of competent jurisdiction will enforce, the Parties hereto intend that the court may enforce the covenants and provisions
to the greatest extent permitted by law and modify the covenants and provisions accordingly.
(h)
No Waiver. The Company failure to exercise, and no delay to exercise, any right in the Agreement shall operate as a waiver of
that right, nor shall any single or partial exercise of any right preclude further exercise of the same right or the exercise of any
other right by Company.
(i)
Opportunity to Review. Each party agrees that this is a legally binding agreement and acknowledges and agrees that it or he has
had the opportunity, if desired, to consult with legal counsel of its or his own choice.
(j)
Entire Agreement. This Agreement supersedes all previous agreements between the Parties and contains the entire agreement between
them related to the Agreement’s subject matter provided herein (including the Executive Employment Agreement, but excluding any
indemnification obligations the Company has to Consultant). No other representations, promises, conditions, warranties, or understandings,
whether expressed or implied, are binding upon either Party, and no provision in this Agreement may be waived, altered, or amended except
by a writing signed by Consultant and Company that specifically identifies the Section of this Agreement to be waived, altered or amended.
[Signature
page follows.]
IN
WITNESS WHEREOF, the Parties executed this Agreement on the day written below.
| TRxADE HEALTH, INC. |
|
Prashant
Patel |
| |
|
|
|
|
| By: |
/s/
Surendra Ajjarapu |
|
By: |
/s/
Prashant Patel |
| |
|
|
|
|
| Name: |
Surendra
Ajjarapu |
|
Date: |
July 25,
2024 |
| |
|
|
|
|
| Title: |
Chief
Executive Officer |
|
|
|
| |
|
|
|
|
| Date: |
July
25, 2024 |
|
|
|
EXHIBIT
A
Confidentiality
and Non-Disclosure Agreement (the “Agreement”)
In
connection with your consulting services with TRxADE HEALTH, INC. (the “Company,” which term shall include the Company
and its subsidiaries) you will have access to certain information regarding the Company which is non-public, confidential and/or proprietary
in nature. In consideration of, and as a condition to, furnishing you with such information and any other information (whether communicated
in writing or orally) delivered to you by the Company or its directors, officers, employees, advisors (including without limitation financial
advisors, counsel and accountants), agents or controlling persons (such affiliates and other persons being herein referred to collectively
as “Representatives”), including, but not limited to, trade secrets, technical data (e.g., computer software, drawings, processes,
patents, procedures, inventions, designs, production methods, techniques, know-how), business and financial information, correspondence,
written or oral representations, memoranda, reports, records, or other information, including any other information or notes you derived
from any such information (such information being herein referred to as “Confidential Information”), the Company hereby requests
your agreement as follows:
1.
The Confidential Information will be used solely for purposes related to the Services detailed in the Consulting Agreement and not in
a manner in any way detrimental to the Company, and you will, at all times including following the termination of the Services, keep
the Confidential Information confidential. You agree to take all reasonable steps to ensure that the Confidential Information is kept
confidential, including, but not limited to, properly and securely storing all written Confidential Information and the marking of
all reports, summaries, records or other material relating thereto prepared by you as confidential. You shall not copy, abstract, reverse
engineer or disclose any Confidential Information to any other person, firm, corporation, or other entity.
2.
The term “Confidential Information” does not include any information which (i) at the time of disclosure or thereafter is
generally available to and known by the public (other than as a result of its disclosure by you), (ii) was available to you on a non-confidential
basis prior to disclosure by the Company or its Representatives, as evidenced by your written records, or (iii) becomes available to
you on a non-confidential basis from a person who is not otherwise bound by a confidentiality agreement with the Company or its Representatives,
or by any other obligation of secrecy, or is not otherwise prohibited from transmitting the information to you. As used in this Agreement,
the term “person” shall be broadly interpreted to include, without limitation, any corporation, company, partnership and
individual.
3.
In the event that you receive a request to disclose all or any part of the information contained in the Confidential Information under
the terms of a valid and effective subpoena or order issued by a court of competent jurisdiction, you agree to (i) immediately notify
the Company of the existence, terms and circumstances surrounding such a request, (ii) consult with the Company on the advisability of
taking legally available steps to resist or narrow such request, and (iii) if disclosure of such information is required, upon request
by the Company, cooperate with the Company at the Company’s expense in obtaining an order or other reliable assurance that confidential
treatment will be accorded to such portion of the information which the Company so designates.
4.
You will return to the Company all copies of the Confidential Information in your possession and you will destroy all copies of any analyses,
compilations, studies or other documents prepared by you or for your internal use which reflect the Confidential Information, promptly
upon the Consulting Agreement’s termination or when the Company so requests such return and destruction of the Confidential Information.
Notwithstanding the foregoing, the aforementioned date(s) and timing of requisite return and destruction may be extended if mutually
agreed upon in a separate writing signed by both parties to this Agreement. You shall keep a record, in reasonable detail, of the
Confidential Information provided to you, the location of such Confidential Information and all persons to whom you furnish any Confidential
Information, and will make such record available to the Company promptly upon its request.
5.
You hereby acknowledge that you have received a copy of the Company’s Insider Trading Compliance Policy (the “ITCP”)
and that you are included within the ITCP’s scope of “Company Personnel” and are hereby being designated by the Company
as an “Insider” under the ITCP during the course of this Agreement, and you are therefore subject to and agree to comply
with the conditions and restrictions applicable to Company Personnel and Insiders outlined in the ITCP. Further, you acknowledge that
you are aware, that the United States securities laws prohibit any person who has received from an issuer material, non-public information
from purchasing or selling securities of such issuer (and options, warrants and rights relating thereto) or from communicating such information
to any other person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such securities.
You hereby agree that you will not use or communicate any Confidential Information in violation of these laws.
7.
No license or conveyance of any rights under any discoveries, inventions, patents, trade secrets, copyrights or other form of intellectual
property is granted or implied by the provision of Confidential Information to you. Any and all documents containing Confidential Information
produced or delivered to you shall remain the property of the Company. You understand and acknowledge that the Company and its Representatives
make no representation or warranty, express or implied, as to the accuracy or completeness of the Confidential Information or freedom
from defect of any kind, including freedom from any patent, copyright, or trademark infringement which may result from the use of such
Confidential Information. Neither the Company, its affiliates or Representatives, nor any of their officers, directors, employees, agents
or controlling persons (within the meaning of the Securities Exchange Act of 1934) shall have any liability to you or any other person
resulting from your use of the Confidential Information. Any and all representations and warranties shall be made solely by the Company
and shall be set forth in a signed agreement and then be subject to the provisions thereof.
8.
You agree to reimburse, indemnify and hold harmless the Company and its Representatives from any damage, loss or expense incurred by
them as a result of the use of the Confidential Information contrary to the terms of this Agreement. You understand that any breach of
this Agreement may cause the Company and its Representatives to suffer irreparable harm for which monetary damages would not be sufficient.
Without prejudice to the rights and remedies at law or in equity otherwise available to the Company and its Representatives, the Company
shall be entitled to equitable relief by way of specific performance or injunction if you breach or threaten to breach any of the provisions
of this Agreement. You also agree to waive the requirement for bond in conjunction with such remedy.
9.
You understand and agree that no failure or delay by the Company or its Representatives in exercising any right, power or privilege hereunder
shall operate as a waiver thereof, nor shall any single or partial exercise thereof preclude any other or further exercise thereof or
the exercise of any right, power or privilege hereunder.
10.
This Agreement is for the benefit of the Company and its Representatives, and shall be governed by the laws (excluding the conflicts
of laws rules) of the State of Delaware and subject to the exclusive jurisdiction of the federal and state courts located in Delaware,
and you agree not to commence any action, suit or proceeding relating to this Agreement except in such courts.
11.
This Agreement represents the entire understanding and agreement of the parties hereto and may be modified or waived only by a separate
writing executed by the Company and you expressly so modifying or waiving such Agreement. This Agreement is in addition to, and does
not supersede or replace, any other obligations of confidentiality, assignment of inventions, or restrictive covenants between you and
the Company or any Representative.
12.
If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, void or
unenforceable, the remainder of the terms, provisions, covenants and restrictions of this Agreement shall remain in full force and effect.
13.
You hereby acknowledge that you are aware that the United States securities laws and other laws prohibit any person who has material,
non-public information concerning an entity from purchasing or selling securities of that entity or from communicating such information
to any other person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such securities.
the Company hereby advises you and you hereby acknowledge that Confidential Information may contain material non-public information relating
to the Company and its affiliates, customers and vendors. Without limiting the foregoing, you hereby agree that you will only use the
Confidential Information in accordance with all applicable laws.
[Signature
page follows.]
| TRxADE HEALTH, INC. |
|
Prashant
Patel |
| |
|
|
|
|
| By: |
|
|
By: |
|
| |
|
|
|
|
| Name: |
|
|
Date: |
|
| |
|
|
|
|
| Title: |
|
|
|
|
| |
|
|
|
|
| Date: |
|
|
|
|
EXHIBIT
B
General
Release of Claims
This
General Release of Claims (this “Release”) is entered into by and between Prashant Patel (the “Executive”)
and Scienture Holdings, Inc. (the “Company”) in connection with that certain Consulting Agreement (the “Consulting
Agreement”) between the Executive and the Company, to which this Release is attached. Capitalized terms that are not otherwise
defined in this Release have the meanings set forth in the Employment Agreement.
| 1. | Tender
of Release. This Release is automatically tendered to
the Executive upon the Service Start Date, if the Consulting Agreement remains effective
as of such date. |
| 2. | General
Release of Claims. In consideration for, among other
terms, the Company’s execution of the Consulting Agreement and the benefits provided
to the Executive thereunder, to which the Executive acknowledges the Executive would otherwise
not be entitled, the Executive, on behalf of the Executive and the Executive’s heirs,
administrators, representatives and executors, voluntarily releases and forever discharges
the Company, its affiliated and related entities, their respective predecessors, successors
and assigns, their respective employee benefit plans and fiduciaries of such plans, and the
current and former officers, directors, shareholders, employees, attorneys, accountants and
agents of each of the foregoing in their official and personal capacities (collectively referred
to as the “Releasees”) generally
from all claims, demands, debts, damages and liabilities of every name and nature, known
or unknown (“Claims”) that, as
of the Service Start Date, the Executive has, ever had, now claim to have or ever claimed
to have had against any or all of the Releasees relating to the Executive’s employment
by and termination of employment with the Company, including but not limited to (a) wrongful
discharge or violation of public policy; (b) breach of contract; (c) defamation or other
torts; (d) intentional or negligent infliction of emotional distress; of retaliation, harassment,
or discrimination under federal, state or local law (including, without limitation, Claims
of discrimination, harassment, or retaliation under Americans with Disabilities Act, and
Title VII of the Civil Rights Act of 1964); (e) Claims under any other federal or state statute
(including, without limitation, Claims under the Worker Adjustment and Retraining Notification
Act (“WARN”), any state mini-WARN laws, and the Fair Labor Standards Act);
(f) Claims under any federal, district, state or local statutes, including, without limitation,
any and all claims under the Florida Civil Rights Act (FCRA), Florida Whistleblower Protection
Act (FWA), Florida Workers’ Compensation Law Retaliation Act (FWCA), Florida Wage Discrimination
Law, Florida Minimum Wage Act, Florida Equal Pay Law, Florida AIDS Act, Florida Discrimination
on the Basis of Sickle Cell Trait Law, Florida OSHA, the Florida Constitution, the Florida
Fair Housing Act (FHA), Miami-Dade County Code, Chapter 11A, Broward County Human Rights
Act, and Palm Beach County Code, Article VI, all including any amendments and their respective
implementing regulations; (g) Claims for wages, bonuses, incentive compensation, commissions,
stock, stock options, vacation pay or any other compensation or benefits; and for damages
or other remedies of any sort, including, without limitation, compensatory damages, punitive
damages, injunctive relief and attorney’s fees. |
Notwithstanding
the foregoing, this release shall not (a) affect the Executive’s rights under this Release of the Consulting Agreement, (b) apply
to rights or claims that cannot be waived as a matter of law, or (c) affect the Executive’s vested rights, if any, under the Company’s
Section 401(k) plan.
This
Release is intended to be effective as a general release of and bar to all Claims, including unknown Claims.
As
a material inducement to the Company to enter into this Release, the Executive represents that the Executive has not assigned any Claim
to any third party. The Executive acknowledges and agrees that the Executive is not entitled to any wages, salary, commissions, vacation,
equity, bonuses, or any other compensation or benefits from the Company or any of its affiliates, except as is expressly set forth in
the Consulting Agreement.
| 3. | Non-Disparagement.
Subject to Section 6 of this Release, the Executive
agrees not to make any disparaging, critical or detrimental statements (whether written,
oral, through social or electronic media or otherwise) concerning the Company, the Releasees
or any of its or their products or services provided or to be provided. |
| 4. |
Protected Disclosures
and Other Protected Actions. Nothing contained in this Release, any other agreement with the Company,
or any Company policy limits the Executive’s ability, with or without notice to the Company, to: (i) file a charge or complaint
with any federal, state or local governmental agency or commission (a “Government Agency”),
including without limitation, the Equal Employment Opportunity Commission, the National Labor Relations Board or the Securities and
Exchange Commission (the “SEC”); (ii) communicate with any Government Agency or
otherwise participate in any investigation or proceeding that may be conducted by any Government Agency, including by providing non-privileged
documents or information; (iii) discuss or disclose information about unlawful acts in the workplace, such as harassment or discrimination
or any other conduct that the Executive has reason to believe is unlawful; or (v) testify truthfully in a legal proceeding. Any such
communications and disclosures must not violate applicable law and the information disclosed must not have been obtained through a
communication that was subject to the attorney-client privilege (unless disclosure of that information would otherwise be permitted
consistent with such privilege or applicable law). If a Government Agency or any other third party pursues any Claim on the Executive’s
behalf, the Executive waives any right to monetary or other individualized relief (either individually or as part of any collective
or class action), but the Company will not limit any right the Executive may have to receive an award pursuant to the whistleblower
provisions of any applicable law or regulation for providing information to the SEC or any other Government Agency. |
| 5. | Defend
Trade Secrets Act. Pursuant to the federal Defend Trade
Secrets Act of 2016, you shall not be held criminally or civilly liable under any federal
or state trade secret law or under this Release or any other agreement for the disclosure
of a trade secret that (a) is made (i) in confidence to a federal, state, or local government
official, either directly or indirectly, or to an attorney; and (ii) solely for the purpose
of reporting or investigating a suspected violation of law; or (b) is made in a complaint
or other document filed in a lawsuit or other proceeding, if such filing is made under seal. |
(a)
Absence of Reliance. In signing this Release, the Executive is not relying upon any promises or representations made by anyone
at or on behalf of the Company.
(b)
Non-Admission. The Executive understands that the Company is not admitting in any way that it violated any legal obligation that
it owed to the Executive.
(c)
Enforceability. If any portion or provision of this Release (including, without limitation, any portion or provision of any section
of this Release) shall to any extent be declared illegal or unenforceable by a court of competent jurisdiction, then the remainder of
this Release, or the application of such portion or provision in circumstances other than those as to which it is so declared illegal
or unenforceable, shall not be affected thereby, and each portion and provision of this Release shall be valid and enforceable to the
fullest extent permitted by law.
(d)
Waiver. No waiver of any provision of this Release shall be effective unless made in writing and signed by the waiving party.
The failure of a party to require the performance of any term or obligation of this Release, or the waiver by a party of any breach of
this Release, shall not prevent any subsequent enforcement of such term or obligation or be deemed a waiver of any subsequent breach.
(e)
Governing Law; Interpretation. This Release shall be interpreted and enforced under the laws of the state of Florida, without
regard to conflict of law principles. In the event of any dispute, this Release is intended by the parties to be construed as a whole,
to be interpreted in accordance with its fair meaning, and not to be construed strictly for or against either the Executive or the Company
or the “drafter” of all or any portion of this Release.
(f)
Entire Agreement. This Release, together with the Consulting Agreement, constitutes the entire agreement between the Executive
and the Company regarding the subject matter hereof (including the Executive Employment Agreement). This Release, together with the Consulting
Agreement, supersedes any previous agreements or understandings between the Executive and the Company, except any other obligations specifically
preserved in this Release or the Consulting Agreement.
(g)
Time for Consideration; Effective Date. The Executive acknowledges that the Executive has been given the opportunity to consider
this Release for five business days before signing it (the “Consideration Period”) and that the Executive has knowingly
and voluntarily entered into this Release. To accept this Release, the Executive must return a signed copy of this Release so that it
is received by the Company at or before the expiration of the Consideration Period. If the Executive signs this Release before the end
of the Consideration Period, the Executive acknowledges by signing this Release that such decision was entirely voluntary and that the
Executive had the opportunity to consider this Release for the entire Consideration Period. This Release shall become effective on the
date it becomes fully executed (the “Effective Date”).
(h)
Counterparts. This Release may be executed and delivered in separate counterparts, including by facsimile or other electronic
means. When both counterparts are signed, they shall be treated together as one and the same document.
[Signature
page follows]
| SCIENTURE HOLDINGS, INC. |
|
PRASHANT PATEL |
| |
|
|
|
|
| By: |
|
|
By: |
|
| |
|
|
|
|
| Name: |
|
|
Date: |
|
| |
|
|
|
|
| Title: |
|
|
|
|
| |
|
|
|
|
| Date: |
|
|
|
|
ANNEX
F
REGISTRATION
RIGHTS AGREEMENT
This
Registration Rights Agreement (this “Agreement”) is dated as of ____________, 2024, by and among TRxADE Health, Inc.,
a Delaware corporation (the “Company”), and the several former stockholders of Scienture, Inc. signatory hereto (each,
including its successors and assigns, a “Holder” and collectively, the “Holders”).
This
Agreement is made in connection with the Agreement and Plan of Merger, dated as of July 25, 2024, among Scienture, Inc., MEDS Merger
Sub I, Inc., MEDS Merger Sub II LLC, and the Company (the “Merger Agreement”).
NOW,
THEREFORE, IN CONSIDERATION of the mutual covenants contained in this Agreement, and for other good and valuable consideration, the receipt
and adequacy of which are hereby acknowledged, the Company and each of the Holders agree as follows:
1.
Definitions. For purposes of this Agreement:
1.1
“Affiliate” means, with respect to any specified Person, any other Person who, directly or indirectly, controls, is
controlled by, or is under common control with such Person, including without limitation any general partner, managing member, officer,
director or trustee of such Person, or any venture capital fund or registered investment company now or hereafter existing that is controlled
by one or more general partners, managing members or investment adviser of, or shares the same management company or investment adviser
with, such Person.
1.2
“Board of Directors” means the board of directors of the Company.
1.3
“Common Stock” means shares of the Company’s common stock, par value $0.0001 per share, and stock of any other
class of securities into which such securities may hereafter be reclassified or changed.
1.4
“Damages” means any loss, damage, claim or liability (joint or several) to which a party hereto may become subject
under the Securities Act, the Exchange Act, or other federal or state law, insofar as such loss, damage, claim or liability (or any action
in respect thereof) arises out of or is based upon: (i) any untrue statement or alleged untrue statement of a material fact contained
in any registration statement of the Company, including any preliminary prospectus or final prospectus contained therein or any amendments
or supplements thereto; (ii) an omission or alleged omission to state therein a material fact required to be stated therein, or necessary
to make the statements therein not misleading; or (iii) any violation or alleged violation by the indemnifying party (or any of its agents
or Affiliates) of the Securities Act, the Exchange Act, any state securities law, or any rule or regulation promulgated under the Securities
Act, the Exchange Act, or any state securities law.
1.5
“Derivative Securities” means any securities or rights convertible into, or exercisable or exchangeable for (in each
case, directly or indirectly), Common Stock, including options and warrants.
1.6
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
1.7
“Form S-1” means such form under
the Securities Act as in effect on the date hereof or any successor registration form under the Securities Act subsequently adopted by
the SEC.
1.8
“Form S-3” means such form under the Securities Act as in effect on the date hereof or any registration form under
the Securities Act subsequently adopted by the SEC that permits forward incorporation of substantial information by reference to other
documents filed by the Company with the SEC.
1.9
“Holder” means any holder
of shares of Registrable Securities who is a party to this Agreement.
1.10
“Immediate Family Member” means
a child, stepchild, grandchild, parent, stepparent, grandparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law,
brother-in-law, or sister-in-law, including, adoptive relationships, of a natural person referred to herein.
1.11
“Initiating Holders” means,
collectively, Holders who properly initiate a registration request under this Agreement.
1.12
“Person”
means any individual, corporation, partnership, trust, limited liability company, association or other entity.
1.13
“Registrable Securities” means (i) shares of Common Stock issuable or issued upon conversion of shares of the Preferred
Stock; (ii) any shares of Common Stock, or any shares of Common Stock issued or issuable (directly or indirectly) upon conversion and/or
exercise of any other securities of the Company, acquired by the Investors after the date hereof; and (iii) any Common Stock issued as
(or issuable upon the conversion or exercise of any warrant, right or other security that is issued as) a dividend or other distribution
with respect to, or in exchange for or in replacement of, the shares referenced in clauses (i) and (ii) above; excluding in all cases,
however, any Registrable Securities sold by a Person in a transaction in which the applicable rights under this Agreement are not assigned
pursuant to Subsection 3.1.
1.14
“Registrable Securities then outstanding” means the number of shares determined by adding the number of shares of
outstanding Common Stock that are Registrable Securities and the number of shares of Common Stock issuable (directly or indirectly) pursuant
to then exercisable and/or convertible securities that are Registrable Securities.
1.15
“SEC” means the Securities and Exchange Commission.
1.16
“SEC Rule 144” means Rule 144 promulgated by the SEC under the Securities Act.
1.17
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
1.18
“Selling Expenses” means all underwriting discounts, selling commissions, and stock transfer taxes applicable to the
sale of Registrable Securities, and fees and disbursements of counsel for any Holder, except for the fees and disbursements of the Selling
Holder Counsel borne and paid by the Company as provided in Subsection 2.6.
2.
Registration Rights. The Company covenants and agrees as follows:
2.1
Demand Registration.
(a)
Form S-1 Demand. If, at any time after November 22, 2024, the Company receives a request from Holders of at least forty percent
(40%) of the Registrable Securities then outstanding that the Company file a Form S-1 registration statement with respect to at least
forty percent (40%) of the Registrable Securities then outstanding (or a lesser percent if the anticipated aggregate offering price,
net of Selling Expenses, would exceed $10,000,000), then the Company shall (x) within ten (10) days after the date such request is given,
give notice thereof (the “Demand Notice”) to all Holders other than the Initiating Holders; and (y) as soon as practicable,
and in any event within sixty (60) days after the date such request is given by the Initiating Holders, file a Form S-1 registration
statement under the Securities Act covering all Registrable Securities that the Initiating Holders requested to be registered and any
additional Registrable Securities requested to be included in such registration by any other Holders, as specified by notice given by
each such Holder to the Company within twenty (20) days of the date the Demand Notice is given, and in each case, subject to the limitations
of Subsections 2.1(c) and 2.3.
(b)
Form S-3 Demand. If, at any time when it is eligible to use a Form S-3 registration statement, the Company receives a request
from Holders of at least thirty percent (30%) of the Registrable Securities then outstanding that the Company file a Form S-3 registration
statement with respect to outstanding Registrable Securities of such Holders having an anticipated aggregate offering price, net of Selling
Expenses, of at least $5,000,000, then the Company shall (i) within ten (10) days after the date such request is given, give a Demand
Notice to all Holders other than the Initiating Holders; and (ii) as soon as practicable, and in any event within forty-five (45) days
after the date such request is given by the Initiating Holders, file a Form S-3 registration statement under the Securities Act covering
all Registrable Securities requested to be included in such registration by any other Holders, as specified by notice given by each such
Holder to the Company within twenty (20) days of the date the Demand Notice is given, and in each case, subject to the limitations of
Subsections 2.1 (c) and 2.3.
(c)
Notwithstanding the foregoing obligations, if
the Company furnishes to Holders requesting a registration pursuant to this Subsection 2.1 a certificate signed by the Company’s
Chief Executive Officer stating that, in the good faith judgment of the Board, it would be materially detrimental to the Company and
its stockholders for such registration statement to either become effective or remain effective for as long as such registration statement
otherwise would be required to remain effective, because such action would (i) materially interfere with a significant acquisition, corporate
reorganization or other similar transaction involving the Company; (ii) require premature disclosure of material information that the
Company has a bona fide business purpose for preserving as confidential; or (iii) render the Company unable to comply with requirements
under the Securities Act or the Exchange Act, then the Company shall have the right to defer taking action with respect to such filing,
and any time periods with respect to filing or effectiveness thereof shall be tolled correspondingly, for a period of not more than ninety
(90) days after the request of the Initiating Holders is given; provided, however, that the Company may not invoke this right
more than twice in any twelve (12) month period.
(d)
The Company shall not be obligated to effect, or to take any action to effect, any registration pursuant to Subsection 2.1(a)(i)
during the period that is sixty (60) days before the Company’s good faith estimate of the date of filing of, and ending on a date
that is one hundred eighty (180) days after the effective date of, a Company-initiated registration; provided that the Company
is actively employing in good faith commercially reasonable efforts to cause such registration statement to become effective; (ii) after
the Company has effected one registration pursuant to Subsection 2.1(a); or (iii) if the Initiating Holders propose to dispose
of shares of Registrable Securities that may be immediately registered on Form S-3 pursuant to a request made pursuant to Subsection
2.1(b). The Company shall not be obligated to effect, or to take any action to effect, any registration pursuant to Subsection
2.1(b) (i) during the period that is thirty (30) days before the Company’s good faith
estimate of the date of filing of, and ending on a date that is ninety (90) days after the effective date of, a Company-initiated registration,
provided that the Company is actively employing in good faith commercially reasonable efforts to cause such registration statement
to become effective; or (ii) if the Company has effected two registrations pursuant to Subsection 2.1(b) within the twelve (12)
month period immediately preceding the date of such request. A registration shall not be counted as “effected” for the purposes
of this Subsection 2.1(d) until such time as the applicable registration statement has been declared effective by the SEC, unless
the Initiating Holders withdraw their request for such registration, elect not to pay the registration expenses therefor, and forfeit
their right to one demand registration statement pursuant to Subsection 2.6, in which case such withdrawn registration statement
shall be counted as “effected” for the
purposes of this Subsection 2.1(d); provided that if such withdrawal is during a period the Company has deferred taking
action pursuant to Subsection 2.1(c), then the Initiating Holders may withdraw their request for registration and such registration
will not be counted as “effected” for the purposes of this Subsection 2.1(d).
2.2
Company Registration. If the Company proposes to register (including, for this purpose, a registration effected by the Company for
stockholders of the Company other than the Holders) any of its securities under the Securities Act in connection with the public offering
of such securities solely for cash, the Company shall, at such time, promptly give each Holder notice of such registration. Upon the
request of each Holder given within twenty (20) days after such notice is given by the Company, the Company shall, subject to the provisions
of Subsection 2.3, cause to be registered all of the Registrable Securities that each such Holder has requested to be included
in such registration. The Company shall have the right to terminate or withdraw any registration initiated by it under this Subsection
2.2 before the effective date of such registration, whether or not any Holder has elected to include Registrable Securities in such
registration. The expenses (other than Selling Expenses) of such withdrawn registration shall be borne by the Company in accordance with
Subsection 2.6.
2.3
Underwriting Requirements.
(a)
If, pursuant to Subsection 2.1, the Initiating Holders intend to distribute the Registrable Securities covered by their request
by means of an underwriting, they shall so advise the Company as a part of their request made pursuant to Subsection 2.1, and
the Company shall include such information in the Demand Notice. The underwriter(s) will be selected by the Board and shall be reasonably
acceptable to a majority in interest of the Initiating Holders. In such event, the right of any Holder to include such Holder’s
Registrable Securities in such registration shall be conditioned upon such Holder’s participation in such underwriting and the
inclusion of such Holder’s Registrable Securities in the underwriting to the extent provided herein. All Holders proposing to distribute
their securities through such underwriting shall (together with the Company as provided in Subsection 2.4(e)) enter into an underwriting
agreement in customary form with the underwriter(s) selected for such underwriting. Notwithstanding any other provision of this Subsection
2.3, if the underwriter(s) advise(s) the Initiating Holders in writing that marketing factors require a limitation on the number
of shares to be underwritten, then the Initiating Holders shall so advise all Holders of Registrable Securities that otherwise would
be underwritten pursuant hereto, and the number of Registrable Securities that may be included in the underwriting shall be allocated
among such Holders of Registrable Securities, including the Initiating Holders, in proportion (as nearly as practicable) to the number
of Registrable Securities owned by each Holder or in such other proportion as shall mutually be agreed to by all such selling Holders;
provided, however, that the number of Registrable Securities held by the Holders to be included in such underwriting shall not
be reduced unless all other securities are first entirely excluded from the underwriting. To facilitate the allocation of shares in accordance
with the above provisions, the Company or the underwriters may round the number of shares allocated to any Holder to the nearest one
hundred (100) shares.
(b)
In connection with any offering involving an underwriting of shares of the Company’s capital stock pursuant to Subsection 2.2,
the Company shall not be required to include any of the Holders’ Registrable Securities in such underwriting unless the Holders
accept the terms of the underwriting as agreed upon between the Company and its underwriters, and then only in such quantity as the underwriters
in their sole discretion determine will not jeopardize the success of the offering by the Company. If the total number of securities,
including Registrable Securities, requested by stockholders of the Company to be included in such offering exceeds the number of securities
to be sold (other than by the Company) that the underwriters in their reasonable discretion determine is compatible with the success
of the offering, then the Company shall be required to include in the offering only that number of such securities, including Registrable
Securities, which the underwriters and the Company in their sole discretion determine will not jeopardize the success of the offering.
If the underwriters determine that less than all of the Registrable Securities requested to be registered can be included in such offering,
then the Registrable Securities that are included in such offering shall be allocated among the selling Holders in proportion (as nearly
as practicable to) the number of Registrable Securities owned by each selling Holder or in such other proportions as shall mutually be
agreed to by all such selling Holders. To facilitate the allocation of shares in accordance with the above provisions, the Company or
the underwriters may round the number of shares allocated to any Holder to the nearest one hundred (100) shares. Notwithstanding the
foregoing, in no event shall (i) the number of Registrable Securities included in the offering be reduced unless all other securities
(other than securities to be sold by the Company) are first entirely excluded from the offering, or (ii) the number of Registrable Securities
included in the offering be reduced below thirty percent (30%) of the total number of securities included in such offering, in which
case the selling Holders may be excluded further if the underwriters make the determination described above and no other stockholder’s
securities are included in such offering. For the purposes of the provision in this Subsection 2.3 (b) concerning apportionment,
for any selling Holder that is a partnership, limited liability company or corporation, the partners, members, retired partners, retired
members, stockholders and Affiliates of such Holder, or the estates and Immediate Family Members of any such partners, retired partners,
members and retired members and any trusts for the benefit of any of the foregoing Persons, shall be deemed to be a single “selling
Holder,” and any pro rata reduction with respect to such “selling Holder” shall be based upon the aggregate number
of Registrable Securities owned by all Persons included in such “selling Holder,” as defined in this sentence.
2.4
Obligations of the Company. Whenever required under this Section 2 to effect the registration of any Registrable Securities,
the Company shall, as expeditiously as reasonably possible:
(a)
prepare and file with the SEC a registration statement with respect to such Registrable Securities and use its commercially reasonable
efforts to cause such registration statement to become effective and, upon the request of the Holders of a majority of the Registrable
Securities registered thereunder, keep such registration statement effective for a period of up to one hundred twenty (120) days or,
if earlier, until the distribution contemplated in the registration statement has been completed; provided, however, that (i)
such one hundred twenty (120) day period shall be extended for a period of time equal to the period the Holder refrains, at the request
of an underwriter of Common Stock (or other securities) of the Company, from selling any securities included in such registration, and
(ii) in the case of any registration of Registrable Securities on Form S-3 that are intended to be offered on a continuous or delayed
basis, subject to compliance with applicable SEC rules, such one hundred twenty (120) day period shall be extended for up to ninety (90)
days, if necessary, to keep the registration statement effective until all such Registrable Securities are sold;
(b)
prepare and file with the SEC such amendments and supplements to such registration statement, and the prospectus used in connection with
such registration statement, as may be necessary to comply with the Securities Act in order to enable the disposition of all securities
covered by such registration statement;
(c)
furnish to the selling Holders such numbers of copies of a prospectus, including a preliminary prospectus, as required by the Securities
Act, and such other documents as the Holders may reasonably request in order to facilitate their disposition of their Registrable Securities;
(d)
use its commercially reasonable efforts to register and qualify the securities covered by such registration statement under such other
securities or blue-sky laws of such jurisdictions as shall be reasonably requested by the selling Holders; provided that the Company
shall not be required to qualify to do business or to file a general consent to service of process in any such states or jurisdictions,
unless the Company is already subject to service in such jurisdiction and except as may be required by the Securities Act;
(e)
in the event of any underwritten public offering, enter into and perform its obligations under an underwriting agreement, in usual and
customary form, with the underwriter(s) of such offering;
(f)
use its commercially reasonable efforts to cause all such Registrable Securities covered by such registration statement to be listed
on a national securities exchange or trading system and each securities exchange and trading system (if any) on which similar securities
issued by the Company are then listed;
(g)
provide a transfer agent and registrar for all Registrable Securities registered pursuant to this Agreement and provide a CUSIP number
for all such Registrable Securities, in each case not later than the effective date of such registration;
(h)
promptly make available for inspection by the selling Holders, any underwriter(s) participating in any disposition pursuant to such registration
statement, and any attorney or accountant or other agent retained by any such underwriter or selected by the selling Holders, all financial
and other records, pertinent corporate documents and properties of the Company, and cause the Company’s directors, officers, employees
and independent accountants to supply all information reasonably requested by any such seller, underwriter, attorney, accountant or agent,
in each case, as necessary or advisable to verify the accuracy of the information in such registration statement and to conduct appropriate
due diligence in connection therewith;
(i)
notify each selling Holder, promptly after the Company receives notice thereof, of the time when such registration statement has been
declared effective or a supplement to any prospectus forming a part of such registration statement has been filed; and
(j)
after such registration statement becomes effective, notify each selling Holder of any request by the SEC that the Company amend or supplement
such registration statement or prospectus.
In
addition, the Company shall ensure that, at all times after any registration statement covering a public offering of securities of the
Company under the Securities Act shall have become effective, its insider trading policy shall provide that the Company’s directors
may implement a trading program under Rule 10b5-1 of the Exchange Act.
2.5
Furnish Information. It shall be a condition precedent to the obligations of the Company to take any action pursuant to this Section
2 with respect to the Registrable Securities of any selling Holder that such Holder shall furnish to the Company such information
regarding itself, the Registrable Securities held by it, and the intended method of disposition of such securities as is reasonably required
to effect the registration of such Holder’s Registrable Securities.
2.6
Expenses of Registration. All expenses (other than Selling Expenses) incurred in connection with registrations, filings or qualifications
pursuant to Section 2, including all registration, filing and qualification fees; printers’ and accounting fees; fees and
disbursements of counsel for the Company; and the reasonable fees and disbursements, not to exceed $25,000, of one counsel for the selling
Holders (“Selling Holder Counsel”),
shall be borne and paid by the Company; provided, however, that the Company shall not be required to pay for any expenses of any
registration proceeding begun pursuant to Subsection 2.1 if the registration request is subsequently withdrawn at the request
of the Holders of a majority of the Registrable Securities to be registered (in which case all selling Holders shall bear such expenses
pro rata based upon the number of Registrable Securities that were to be included in the withdrawn registration), unless the Holders
of a majority of the Registrable Securities agree to forfeit their right to one registration pursuant to Subsections 2.1(a) or
2.1(b), as the case may be; provided further that if, at the time of such withdrawal, the Holders shall have learned of
a material adverse change in the condition, business, or prospects of the Company from that known to the Holders at the time of their
request and have withdrawn the request with reasonable promptness after learning of such information, then the Holders shall not be required
to pay any of such expenses and shall not forfeit their right to one registration pursuant to Subsections 2.1(a) or 2.1(b).
All Selling Expenses relating to Registrable Securities registered pursuant to this Section 2 shall be borne and paid by the Holders
pro rata on the basis of the number of Registrable Securities registered on their behalf.
2.7
Delay of Registration. No Holder shall have any right to obtain or seek an injunction restraining or otherwise delaying any registration
pursuant to this Agreement as the result of any controversy that might arise with respect to the interpretation or implementation of
this Section 2.
2.8
Indemnification. If any Registrable Securities are included in a registration statement under this Section 2:
(a)
To the extent permitted by law, the Company will indemnify and hold harmless each selling Holder, and the partners, members, directors,
officers and stockholders of each such Holder; legal counsel and accountants for each such Holder; any underwriter (as defined in the
Securities Act) for each such Holder; and each Person, if any, who controls such Holder or underwriter within the meaning of the Securities
Act or the Exchange Act, against any Damages, and the Company will pay to each such Holder, underwriter, controlling Person or other
aforementioned Person any legal or other expenses reasonably incurred thereby in connection with investigating or defending any claim
or proceeding from which Damages may result, as such expenses are incurred; provided, however, that the indemnity agreement contained
in this Subsection 2.8 (a) shall not apply to amounts paid in settlement of any such claim or proceeding if such settlement is
effected without the consent of the Company, which consent shall not be unreasonably withheld, nor shall the Company be liable for any
Damages to the extent that they arise out of or are based upon actions or omissions made in reliance upon and in conformity with written
information furnished by or on behalf of any such Holder, underwriter, controlling Person or other aforementioned Person expressly for
use in connection with such registration.
(b)
To the extent permitted by law, each selling Holder, severally and not jointly, will indemnify and hold harmless the Company, and each
of its directors, each of its officers who has signed the registration statement, each Person (if any), who controls the Company within
the meaning of the Securities Act, legal counsel and accountants for the Company, any underwriter (as defined in the Securities Act),
any other Holder selling securities in such registration statement, and any controlling Person of any such underwriter or other Holder,
against any Damages, in each case only to the extent that such Damages arise out of or are based upon actions or omissions made in reliance
upon and in conformity with written information furnished by or on behalf of such selling Holder expressly for use in connection with
such registration; and each such selling Holder will pay to the Company and each other aforementioned Person any legal or other expenses
reasonably incurred thereby in connection with investigating or defending any claim or proceeding from which Damages may result, as such
expenses are incurred; provided, however, that the indemnity agreement contained in this Subsection 2.8 (b) shall not apply
to amounts paid in settlement of any such claim or proceeding if such settlement is effected without the consent of the Holder, which
consent shall not be unreasonably withheld; provided further that in no event shall the aggregate amounts payable by any Holder
by way of indemnity or contribution under Subsections 2.8 (b) and 2.8(d) exceed the proceeds from the offering received
by such Holder (net of any Selling Expenses paid by such Holder), except in the case of fraud or willful misconduct by such Holder.
(c)
Promptly after receipt by an indemnified party under this Subsection 2.8 of notice of the commencement of any action (including
any governmental action) for which a party may be entitled to indemnification hereunder, such indemnified party will, if a claim in respect
thereof is to be made against any indemnifying party under this Subsection 2.8, give the indemnifying party notice of the commencement
thereof. The indemnifying party shall have the right to participate in such action and, to the extent the indemnifying party so desires,
participate jointly with any other indemnifying party to which notice has been given, and to assume the defense thereof with counsel
mutually satisfactory to the parties; provided, however, that an indemnified party (together with all other indemnified parties
that may be represented without conflict by one counsel) shall have the right to retain one separate counsel, with the fees and expenses
to be paid by the indemnifying party, if representation of such indemnified party by the counsel retained by the indemnifying party would
be inappropriate due to actual or potential differing interests between such indemnified party and any other party represented by such
counsel in such action. The failure to give notice to the indemnifying party within a reasonable time of the commencement of any such
action shall relieve such indemnifying party of any liability to the indemnified party under this Subsection 2.8, to the extent
that such failure materially prejudices the indemnifying party’s ability to defend such action. The failure to give notice to the
indemnifying party will not relieve it of any liability that it may have to any indemnified party otherwise than under this Subsection
2.8.
(d)
To provide for just and equitable contribution to joint liability under the Securities Act in any case in which either: (i) any party
otherwise entitled to indemnification hereunder makes a claim for indemnification pursuant to this Subsection 2.8 but it is judicially
determined (by the entry of a final judgment or decree by a court of competent jurisdiction and the expiration of time to appeal or the
denial of the last right of appeal) that such indemnification may not be enforced in such case, notwithstanding the fact that this Subsection
2.8 provides for indemnification in such case, or (ii) contribution under the Securities Act may be required on the part of any party
hereto for which indemnification is provided under this Subsection 2.8, then, and in each such case, such parties will contribute
to the aggregate losses, claims, damages, liabilities or expenses to which they may be subject (after contribution from others) in such
proportion as is appropriate to reflect the relative fault of each of the indemnifying party and the indemnified party in connection
with the statements, omissions or other actions that resulted in such loss, claim, damage, liability or expense, as well as to reflect
any other relevant equitable considerations. The relative fault of the indemnifying party and of the indemnified party shall be determined
by reference to, among other things, whether the untrue or allegedly untrue statement of a material fact, or the omission or alleged
omission of a material fact, relates to information supplied by the indemnifying party or by the indemnified party and the parties’
relative intent, knowledge, access to information, and opportunity to correct or prevent such statement or omission; provided, however,
that, in any such case (x) no Holder will be required to contribute any amount in excess of the public offering price of all such Registrable
Securities offered and sold by such Holder pursuant to such registration statement, and (y) no Person guilty of fraudulent misrepresentation
(within the meaning of Section 11(f) of the Securities Act) will be entitled to contribution from any Person who was not guilty of such
fraudulent misrepresentation; provided further that in no event shall a Holder’s liability pursuant to this Subsection
2.8 (d), when combined with the amounts paid or payable by such Holder pursuant to Subsection 2.8 (b), exceed the proceeds
from the offering received by such Holder (net of any Selling Expenses paid by such Holder), except in the case of willful misconduct
or fraud by such Holder.
(e)
Notwithstanding the foregoing, to the extent that the provisions on indemnification and contribution contained in the underwriting agreement
entered into in connection with the underwritten public offering are in conflict with the foregoing provisions, the provisions in the
underwriting agreement shall control.
(f)
Unless otherwise superseded by an underwriting agreement entered into in connection with the underwritten public offering, the obligations
of the Company and Holders under this Subsection 2.8 shall survive the completion of any offering of Registrable Securities in
a registration under this Section 2, and otherwise shall survive the termination of this Agreement.
2.9
Reports Under Exchange Act. With a view to making available to the Holders the benefits of SEC Rule 144 and any other rule or regulation
of the SEC that may at any time permit a Holder to sell securities of the Company to the public without registration or pursuant to a
registration on Form S-3, the Company shall:
(a)
make and keep available adequate current public information, as those terms are understood and defined in SEC Rule 144, at all times
after the effective date of this Agreement;
(b)
use commercially reasonable efforts to file with the SEC in a timely manner all reports and other documents required of the Company under
the Securities Act and the Exchange Act (at any time after the Company has become subject to such reporting requirements); and
(c)
furnish to any Holder, so long as the Holder owns any Registrable Securities, forthwith upon request (i) to the extent accurate, a written
statement by the Company that it has complied with the reporting requirements of SEC Rule 144, the Securities Act and the Exchange Act
(at any time after the Company has become subject to such reporting requirements), or that it qualifies as a registrant whose securities
may be resold pursuant to Form S-3 (at any time after the Company so qualifies); and (ii) such other information as may be reasonably
requested in availing any Holder of any rule or regulation of the SEC that permits the selling of any such securities without registration
(at any time after the Company has become subject to the reporting requirements under the Exchange Act) or pursuant to Form S-3 (at any
time after the Company so qualifies to use such form).
2.10
“Market Stand-off” Agreement. Each Holder hereby agrees that it will not, without the prior written consent of the
managing underwriter, during the period commencing on the date of the final prospectus relating to the registration by the Company for
its own behalf of shares of its Common Stock or any other equity securities under the Securities Act on a registration statement on Form
S-1 or Form S-3, and ending on the date specified by the Company and the managing underwriter (such period not to exceed one hundred
eighty (180) days), (i) lend; offer; pledge; sell; contract to sell; sell any option or contract to purchase; purchase any option or
contract to sell; grant any option, right, or warrant to purchase; or otherwise transfer or dispose of, directly or indirectly, any shares
of Common Stock or any securities convertible into or exercisable or exchangeable (directly or indirectly) for Common Stock (whether
such shares or any such securities are then owned by the Holder or are thereafter acquired) or (ii) enter into any swap or other arrangement
that transfers to another, in whole or in part, any of the economic consequences of ownership of such securities, whether any such transaction
described in clause (i) or (ii) above is to be settled by delivery of Common Stock or other securities, in cash, or otherwise. The foregoing
provisions of this Subsection 2.11 shall not apply to the sale of any shares to an underwriter pursuant to an underwriting agreement,
or the transfer of any shares to any trust for the direct or indirect benefit of the Holder or the immediate family of the Holder, provided
that the trustee of the trust agrees to be bound in writing by the restrictions set forth herein, and provided further that
any such transfer shall not involve a disposition for value, and shall be applicable to the Holder only if all officers and directors
are subject to the same restrictions and the Company uses commercially reasonable efforts to obtain a similar agreement from all stockholders
individually owning more than one percent (1%) of the Company’s outstanding Common Stock (after giving effect to conversion into
Common Stock of all outstanding Preferred Stock). The underwriters in connection with such registration are intended third-party beneficiaries
of this Subsection 2.11 and shall have the right, power and authority to enforce the provisions hereof as though they were a party
hereto. Each Holder further agrees to execute such agreements as may be reasonably requested by the underwriters in connection with such
registration that are consistent with this Subsection 2.11 or that are necessary to give further effect thereto. Any discretionary
waiver or termination of the restrictions of any or all of such agreements by the Company or the underwriters shall apply pro rata to
all Company stockholders that are subject to such agreements, based on the number of shares subject to such agreements.
3.
Miscellaneous.
3.1
Successors and Assigns. The rights under this Agreement may be assigned (but only with all related obligations) by a Holder to
a transferee of Registrable Securities that (i) is an Affiliate of a Holder; (ii) is a Holder’s
Immediate Family Member or trust for the benefit of an individual Holder or one or more of such a Holder’s
Immediate Family Members; or (iii) after such transfer, holds at least 100,000 shares of Registrable Securities (subject to appropriate
adjustment for stock splits, stock dividends, combinations, and other recapitalizations); provided, however, that (x) the
Company is, within a reasonable time after such transfer, furnished with written notice of the name and address of such transferee and
the Registrable Securities with respect to which such rights are being transferred; and (y) such transferee agrees in a written instrument
delivered to the Company to be bound by and subject to the terms and conditions of this Agreement, including the provisions of Subsection
2.11. For the purposes of determining the number of shares of Registrable Securities held by a transferee, the holdings of a transferee
(1) that is an Affiliate or stockholder of a Holder; (2) who is a Holder’s Immediate Family
Member; or (3) that is a trust for the benefit of an individual Holder or such Holder’s
Immediate Family Member shall be aggregated together and with those of the transferring Holder; provided further that all transferees
who would not qualify individually for assignment of rights shall, as a condition to the applicable transfer, establish a single attorney-in-fact
for the purpose of exercising any rights, receiving notices, or taking any action under this Agreement. The terms and conditions of this
Agreement inure to the benefit of and are binding upon the respective successors and permitted assignees of the parties. Nothing in this
Agreement, express or implied, is intended to confer upon any party other than the parties hereto or their respective successors and
permitted assignees any rights, remedies, obligations or liabilities under or by reason of this Agreement, except as expressly provided
herein.
3.2
Governing Law. This Agreement shall be governed by the internal law of the State of Delaware, without regard to conflict of law principles
that would result in the application of any law other than the law of the State of Delaware.
3.3
Counterparts. This Agreement may be executed in two (2) or more counterparts, each of which shall be deemed an original, but all
of which together shall constitute one and the same instrument. Counterparts may be delivered via electronic mail (including pdf or any
electronic signature complying with the U.S. federal ESIGN Act of 2000, e.g., www.docusign.com) or other transmission method and
any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
3.4
Titles and Subtitles. The titles and subtitles used in this Agreement are for convenience only and are not to be considered in construing
or interpreting this Agreement.
3.5
Notices.
(a)
All notices and other communications given or made pursuant to this Agreement shall be in writing and shall be deemed effectively given
upon the earlier of actual receipt or (i) personal delivery to the party to be notified; (ii) when sent, if sent by electronic mail during
the recipient’s normal business hours, and if not sent during normal business hours, then on the recipient’s next business
day; (iii) five (5) days after having been sent by registered or certified mail, return receipt requested, postage prepaid; or (iv) one
(1) business day after the business day of deposit with a nationally recognized overnight courier, freight prepaid, specifying next-day
delivery, with written verification of receipt. All communications shall be sent to the respective parties at their addresses as set
forth on the signature pages hereto, or to the principal office of the Company and to the attention of the Chief Executive Officer, in
the case of the Company, or to such email address or address as subsequently modified by written notice given in accordance with this
Subsection 3.5. If notice is given to the Company, a copy shall also be sent to Goodwin Procter LLP, 620 Eighth Avenue, New York,
NY 10018, Attention: Stephen Davis, E-mail: sdavis@goodwinlaw.com.
(b)
Consent to Electronic Notice. Each Investor consents to the delivery of any stockholder notice pursuant to the Delaware General
Corporation Law (the “DGCL”), as amended or superseded from time to time, by electronic transmission pursuant to Section
232 of the DGCL (or any successor thereto) at the electronic mail address as on the books of the Company. Each Investor agrees to promptly
notify the Company of any change in such stockholder’s electronic mail address, and that failure to do so shall not affect the
foregoing.
3.6
Amendments and Waivers. Any term of this Agreement may be amended, modified or terminated and the observance of any term of this
Agreement may be waived (either generally or in a particular instance, and either retroactively or prospectively) only with the written
consent of the Company and the holders of a majority of the Registrable Securities then outstanding; provided that any provision
hereof may be waived by any waiving party on such party’s own behalf, without the consent
of any other party. Notwithstanding the foregoing, (a) this Agreement may not be amended, modified or terminated and the observance of
any term hereof may not be waived with respect to any Investor without the written consent of such Investor, unless such amendment, modification,
termination, or waiver applies to all Investors in the same fashion. Any amendment, modification, termination, or waiver effected in
accordance with this Subsection 3.6 shall be binding on all parties hereto, regardless of whether any such party has consented
thereto. No waivers of or exceptions to any term, condition, or provision of this Agreement, in any one or more instances, shall be deemed
to be or construed as a further or continuing waiver of any such term, condition, or provision.
3.7
Severability. In case any one or more of the provisions contained in this Agreement is for any reason held to be invalid, illegal
or unenforceable in any respect, such invalidity, illegality, or unenforceability shall not affect any other provision of this Agreement,
and such invalid, illegal, or unenforceable provision shall be reformed and construed so that it will be valid, legal, and enforceable
to the maximum extent permitted by law.
3.8
Aggregation of Stock. All Registrable Securities held or acquired by Affiliates shall be aggregated together for the purpose of determining
the availability of any rights under this Agreement and such Affiliates may apportion such rights as among themselves in any manner they
deem appropriate.
3.9
Entire Agreement. This Agreement (including any Schedules and Exhibits hereto) constitutes the full and entire understanding and
agreement among the parties with respect to the subject matter hereof, and any other written or oral agreement relating to the subject
matter hereof existing between the parties is expressly canceled.
3.10
Dispute Resolution. The parties (a) hereby irrevocably and unconditionally submit to the jurisdiction of the state courts of the
State of New York and to the jurisdiction of the United States District Court for the Southern District of New York for the purpose of
any suit, action or other proceeding arising out of or based upon this Agreement, (b) agree not to commence any suit, action or other
proceeding arising out of or based upon this Agreement except in the state courts of the State of New York or the United States District
Court for the Southern District of New York, and (c) hereby waive, and agree not to assert, by way of motion, as a defense, or otherwise,
in any such suit, action or proceeding, any claim that it is not subject personally to the jurisdiction of the above-named courts, that
its property is exempt or immune from attachment or execution, that the suit, action or proceeding is brought in an inconvenient forum,
that the venue of the suit, action or proceeding is improper or that this Agreement or the subject matter hereof may not be enforced
in or by such court.
Waiver
of Jury Trial: EACH PARTY HEREBY WAIVES ITS RIGHTS
TO A JURY TRIAL OF ANY CLAIM OR CAUSE OF ACTION BASED UPON OR ARISING OUT OF THIS AGREEMENT, THE OTHER TRANSACTION DOCUMENTS, THE SECURITIES
OR THE SUBJECT MATTER HEREOF OR THEREOF. THE SCOPE OF THIS WAIVER IS INTENDED TO BE ALL-ENCOMPASSING OF ANY AND ALL DISPUTES THAT MAY
BE FILED IN ANY COURT AND THAT RELATE TO THE SUBJECT MATTER OF THIS TRANSACTION, INCLUDING, WITHOUT LIMITATION, CONTRACT CLAIMS, TORT
CLAIMS (INCLUDING NEGLIGENCE), BREACH OF DUTY CLAIMS, AND ALL OTHER COMMON LAW AND STATUTORY CLAIMS. THIS SECTION HAS BEEN FULLY DISCUSSED
BY EACH OF THE PARTIES HERETO AND THESE PROVISIONS WILL NOT BE SUBJECT TO ANY EXCEPTIONS. EACH PARTY HERETO HEREBY FURTHER WARRANTS AND
REPRESENTS THAT SUCH PARTY HAS REVIEWED THIS WAIVER WITH ITS LEGAL COUNSEL, AND THAT SUCH PARTY KNOWINGLY AND VOLUNTARILY WAIVES ITS
JURY TRIAL RIGHTS FOLLOWING CONSULTATION WITH LEGAL COUNSEL.
3.11
Delays or Omissions. No delay or omission to exercise any right, power, or remedy accruing to any party under this Agreement,
upon any breach or default of any other party under this Agreement, shall impair any such right, power, or remedy of such nonbreaching
or nondefaulting party, nor shall it be construed to be a waiver of or acquiescence to any such breach or default, or to any similar
breach or default thereafter occurring, nor shall any waiver of any single breach or default be deemed a waiver of any other breach or
default theretofore or thereafter occurring. All remedies, whether under this Agreement or by law or otherwise afforded to any party,
shall be cumulative and not alternative.
[Remainder
of Page Intentionally Left Blank]
IN
WITNESS WHEREOF, the parties have executed this Registration Rights Agreement as of the date first written above.
| |
TRXADE
HEALTH, INC. |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title:
|
|
[REMAINDER
OF PAGE INTENTIONALLY LEFT BLANK]
IN
WITNESS WHEREOF, the parties have executed this Registration Rights Agreement as of the date first written above.
| |
NAME
OF HOLDER |
| |
|
|
| |
|
| |
|
|
| |
AUTHORIZED
SIGNATORY |
| |
|
|
| |
By:
|
|
| |
Name: |
|
| |
Title: |
|
| |
|
|
| |
ADDRESS
FOR NOTICE |
| |
|
|
| |
c/o:
|
|
| |
Street:
|
|
| |
City/State/Zip:
|
|
| |
Attention:
|
|
| |
Tel: |
|
| |
Fax: |
|
| |
Email: |
|
ANNEX
G
CERTIFICATE
OF AMENDMENT
TO
THE SECOND AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF
TRxADE
HEALTH, INC.
TRxADE
HEALTH, INC. (the “Corporation”), a corporation organized and existing under the General Corporation Law of the State of
Delaware (the “General Corporation Law”), DOES HEREBY CERTIFY:
First:
The name of the Corporation is Scienture Holdings, Inc.
Second:
The foregoing amendment was duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State
of Delaware.
Third:
That this Certificate of Amendment to the Second Amended and Restated Certificate of Incorporation shall be effective as of [●]
on the [●] day of [●], 2024.
IN
WITNESS WHEREOF, the Corporation has caused this Certificate of Amendment to the Second Amended and Restated Certificate of Incorporation
to be signed by a duly authorized officer of the Corporation on this [●] day of [●], 2024.
| |
|
TRxADE
HEALTH, INC. |
| |
|
|
| |
By: |
[●] |
| |
|
[●] |
| |
|
[●] |
TRxADE Health (NASDAQ:MEDS)
Historical Stock Chart
From Nov 2024 to Dec 2024

TRxADE Health (NASDAQ:MEDS)
Historical Stock Chart
From Dec 2023 to Dec 2024
