false
0001094038
0001094038
2024-01-08
2024-01-08
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
January 8, 2024
Date of Report (Date of earliest event reported)
MARKER THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware |
001-37939 |
45-4497941 |
| (State or other jurisdiction of
incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
9350 Kirby Drive, Suite 300
Houston, Texas |
77054 |
| (Address of principal executive offices) |
(Zip Code) |
(713) 400-6400
Registrant’s telephone number, including
area code
N/A
(Former name or former address, if changed since
last report)
Check the appropriate box below if the Form 8-K is intended to simultaneously
satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which registered |
| Common
Stock, par value $0.001 per share |
|
MRKR |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities
Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ¨
If an emerging
growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any
new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| | |
| Item 7.01. | Regulation FD Disclosure. |
As previously announced, the Marker Therapeutics, Inc. (the “Company”)
management team led by Chief Executive Officer, Juan Vera, plans to participate in the Biotech Showcase and present at the 19th
Annual Non-Dilutive Funding Summit held alongside the 42nd Annual J.P. Morgan Healthcare Conference 2024 (J.P. Morgan Week).
On January 8, 2024, the Company issued a press release announcing Clinical
Program Updates and Pipeline Prioritization. A copy of the press release is attached hereto as Exhibit 99.1 to this Current Report on
Form 8-K and is incorporated herein by reference.
In addition, on January 8, 2024, the Company updated its corporate
presentation for use in meetings with investors, analysts and others during J.P. Morgan Week and thereafter, as well as in connection
with the non-dilutive funding summit. The corporate presentation is available through the Company’s website and a copy is attached
hereto as Exhibit 99.2 to this Current Report on Form 8-K.
The information in this Item
7.01 of this Current Report on Form 8-K (including Exhibit 99.1 and 99.2) is being furnished and shall not be deemed “filed”
for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”),
or subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information shall
not be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly
set forth by specific reference in such a filing.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly
authorized.
| |
Marker Therapeutics, Inc. |
| |
|
|
| Dated: January 8, 2024 |
By: |
/s/ Juan Vera |
| |
|
Juan Vera |
| |
|
President and Chief Executive Officer |
Exhibit 99.1

Marker Therapeutics Announces Clinical Program
Updates and Pipeline Prioritization
Strategic prioritization of clinical programs
with focus on MT-601 in patients with lymphoma
MultiTAA-specific T cell therapies demonstrate
clinical safety and positive clinical data across multiple indications
Marker provides updates supporting the clinical
benefits of MT-401 in patients with measurable residual disease (MRD)
Houston, TX – January 8, 2024 –
Marker Therapeutics, Inc. (Nasdaq: MRKR), a clinical-stage immuno-oncology company focusing on developing next-generation T cell-based
immunotherapies for the treatment of hematological malignancies and solid tumors, today announced a restructuring of its clinical programs
and a strategic prioritization of its multi-tumor associated antigen (multiTAA)-specific T cell product pipeline. In addition, the Company
reported a clinical update on the Phase 2 ARTEMIS study investigating MT-401, a multiTAA-specific T cell product, for the treatment of
patients with acute myeloid leukemia (AML).
Following the non-dilutive transaction with Cell
Ready (Press Release, May 1, 2023), Marker has made significant progress on clinical and corporate restructuring with the objective
of accelerating the commercial development of our unique multiTAA technology. The Company today announced the prioritization of MT-601
in chimeric antigen receptor (CAR) relapse patients with lymphoma (APOLLO; clinicaltrials.gov identifier: NCT05798897). This strategic
decision was made based on 1) the Company’s promising non-clinical and clinical data using the multiTAA technology in lymphoma,
and 2) the lack of an approved treatment for patients who experience relapse after treatment with CD19 CAR T (up to 60% within a year;
Chong EA et al, N Engl J Med, 2021), which is a clear unmet medical need and provides an opportunity for accelerated product development.
Highlights from the Lymphoma Study
Clinical Efficacy in Patients with Lymphoma
in Previous Clinical Trial
| · | The multiTAA-specific T cell product targeting 5 TAAs was investigated in the TACTAL study, a Phase 1
trial conducted at Baylor College of Medicine. |
| · | The TACTAL study enrolled patients with Hodgkin’s and non-Hodgkin’s lymphoma and demonstrated
clinical safety and efficacy with durable clinical responses for 6 years (Vasileiou et al, J Clin Oncol, 2021). |
Non-Clinical Proof-of-Concept Data
| · | Marker
developed a long-term in vitro killing assay 1) to better understand resistance mechanisms
following CAR T cell treatment and 2) to determine if a product that is capable of targeting
6 TAAs (MT-601) will be able to kill CAR-resistant lymphoma cells (Press Release, May
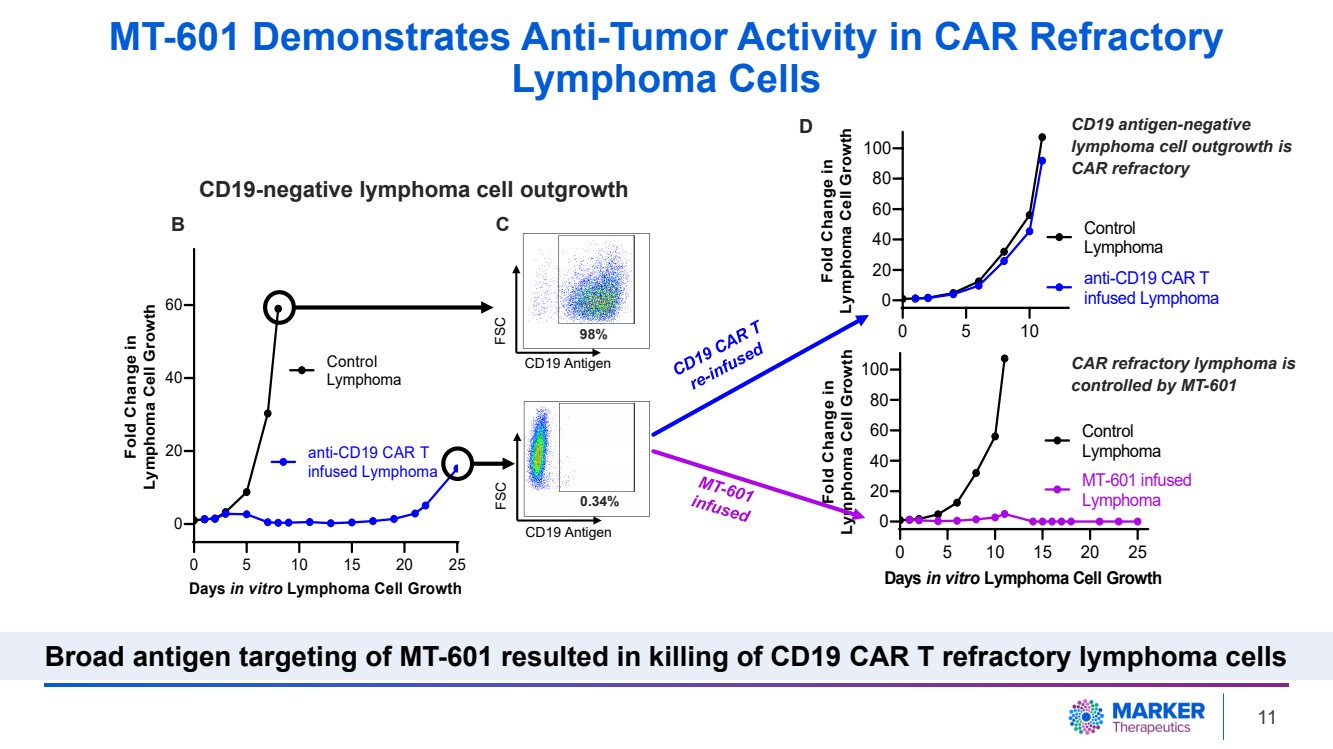
31, 2023). |
| · | After CD19-targeting CAR T cell treatment, 98% of lymphoma cells were eliminated
in vitro. Long-term follow-up (three weeks) demonstrated outgrowth of CD19-negative tumor cells. Additional anti-CD19 CAR T cell
treatment failed to inhibit tumor growth due to the lack of target antigen (CD19) expression on the tumor. |

| · | Treating
CAR-resistant lymphoma cells with MT-601 resulted in complete long-term growth inhibition
(over three weeks) highlighting that MT-601 has the potential to effectively treat CD19 CAR-resistant
tumors (Pre-Clinical Data in Lymphoma, May 31, 2023). |
Durable Response in CAR Relapsed Patient with
Lymphoma
| · | The Company-sponsored Phase 1 APOLLO study investigates the safety and efficacy
of MT-601 in patients with lymphoma who have failed or are ineligible to receive anti-CD19 CAR T cell therapy. |
| · | The
first study participant, a 57-year-old female with diffuse large B cell lymphoma (DLBCL),
was enrolled in the Phase 1 dose escalation stage of the trial after failing 4 prior lines
of therapy, including anti-CD19 CAR T cell therapy (Press Release, June 12, 2023).
The participant relapsed within 90 days of CAR T cell therapy, and was treated with MT-601
without prior lymphodepletion. |
| · | The
patient tolerated MT-601 well without treatment-related adverse events and achieved a complete
response eight weeks after the second infusion of MT-601 (Press Release, Sep 11, 2023). |
| · | Six
months following treatment with MT-601 the study participant has maintained a complete response
to treatment suggesting that MT-601 is more durable compared to CAR T cells in this study
participant (Press Release, Dec 11, 2023). |
CD19-targeting CAR T cell therapies are associated
with severe side effects and toxicities, and up to 60% of patients with DLBCL relapse within a year (Chong EA et al, N Engl J Med, 2021).
Additionally, the FDA is investigating CAR T therapies for the potential risk of inducing secondary cancers (U.S. Food and Drug Administration,
Nov 28, 2023). To date, multiTAA-specific T cell therapies have been well-tolerated in over 200 patients in clinical trials, and
Marker believes that, unlike CAR T cells, multiTAA-specific T cells could represent a safer therapeutic option due to their non-genetically
engineered approach that selectively expands tumor-specific T cells from a patient’s/donor’s blood without the risk of mutagenesis.
Promising Clinical Observations and New Directions
with MT-401 in Patients with MRD in AML
Today, Marker is providing a clinical update on
the Phase 2 ARTEMIS clinical study (clinicaltrials.gov identifier: NCT04511130), and the direction it will pursue. This multicenter study
is evaluating the safety and efficacy of MT-401 in patients with AML after allogeneic hematopoietic stem cell transplantation (HSCT).
A total of 8 patients with MRD+ AML after HSCT
were enrolled and treated with MT-401. None of the 8 treated patients experienced a drug related adverse event. Of the 8 treated patients,
4 experienced a clinical benefit, with 3 showing a conversion to MRD-negative, and one patient showing a partial response with a logarithmic
reduction of MRD levels by PCR. One patient has not yet had the first assessment post treatment. Of the 8 treated patients in the study,
only 1 patient had documented disease progression and was taken to a second transplant. The other 3 patients were taken off the study
for reasons unrelated to the clinical outcome.
Obtaining timely consent and re-accessing HSCT
donors for apheresis for the manufacture of MT-401 caused delayed patient accrual and patient eligibility issues. Consequently, the rapid
progression of disease contributed to some patients to withdraw from the study prior to administration of study product. Therefore, to
streamline resources and to reduce time to treatment, Marker intends to focus on a ready for use product from commercially available leukapheresis
material and will discontinue the patient-specific part (ARTEMIS) of the AML program.

“We are encouraged by the clinical observations
in patients with MRD in our AML study,” said Juan Vera, M.D., President and CEO of Marker Therapeutics. “The data demonstrate
the safety of MT-401 and provide evidence that MT-401 could benefit patients with MRD+ AML.”
Dr. Vera continued: “Decreasing the time
to treatment is critical when it comes to the treatment of patients that suffer from rapidly progressing cancers, such as patients with
MRD in AML, which typically advances rapidly into frank relapse with poor outcomes. We believe using commercial leukapheresis material
from healthy donors can bypass the bottleneck associated with donor identification and facilitate large-scale manufacturing. This approach
is expected to not only reduce manufacturing costs, but also expedite time to treatment to as little as 72 hours. We are currently working
to initiate the clinical study and anticipate that the first patient with AML will be treated with MT-401 manufactured from healthy donors
in the second half of 2024.”
The Company previously announced that the FDA
has cleared the clinical protocol to investigate a ready for use MT-401 product manufactured from healthy donors in patients with AML,
and a cellular inventory has been established with continuous efforts to expand this inventory (Press Release, Aug 7, 2023).
Marker has secured non-dilutive funding to support
the clinical investigation of a ready for use MT-401 product in patients with AML. Using these allocated funds will allow the Company
to proceed with the ready for use program without affecting the ongoing Phase 1 APOLLO study and the capital runway of the Company into
the fourth quarter of 2025.
In addition, the Company has an Investigational
New Drug (IND) application cleared by the U.S. FDA for a Phase 1 trial to investigate MT-601 in patients with pancreatic cancer in combination
with first-line chemotherapy. The clinical advancement of this multicenter study will be pending additional funding from non-dilutive
sources, including grant activities.
“The strategic restructure of our multiTAA
pipeline reflects our ongoing commitment to innovate and deliver groundbreaking treatments,” said Dr. Vera. “The decision
to shift our focus on MT-601 in patients with lymphoma is based on our promising non-clinical and clinical observations. Lymphoma is a
highly competitive landscape with numerous companies striving to compete with CAR T cell therapies. However, our approach differs by targeting
multiple antigens and focusing on a unique niche: patients who have experienced CAR T cell relapse or are ineligible for CAR T therapy.”
Dr. Vera continued: “We believe that MT-601
could address the unmet medical need in this patient population. Developing a product in this patient population is commercially attractive
due to the well understood natural history, the unmet medical need, and the lower number of competing trials. Assuming we continue to
see promising results in our APOLLO study, this would allow us to accelerate the development of MT-601 in CAR relapse patients with lymphoma.”

About multiTAA-specific T cells
The multi-tumor associated antigen (multiTAA)-specific
T cell platform is a novel, non-genetically modified cell therapy approach that selectively expands tumor-specific T cells from a patient's/donor’s
blood capable of recognizing a broad range of tumor antigens. Clinical trials that enrolled more than 200 patients with various hematological
malignancies and solid tumors showed that autologous and allogeneic multiTAA-specific T cell products were well tolerated and demonstrated
durable clinical responses, and consistent epitope spreading. The latter is typically not observed with other T cell therapies and enables
the potential contribution to a lasting anti-tumor effect.
About Marker Therapeutics, Inc.
Marker Therapeutics, Inc. is a clinical-stage
immuno-oncology company specializing in the development of next-generation T cell-based immunotherapies for the treatment of hematological
malignancies and solid tumor indications. The T cell therapy technology developed by Marker is based on the selective expansion of non-engineered,
tumor-specific T cells that recognize tumor associated antigens (i.e., tumor targets) and kill tumor cells expressing those targets. This
population of T cells is designed to attack multiple tumor targets following infusion into patients and to activate the patient’s
immune system to produce broad spectrum anti-tumor activity. Because Marker does not genetically engineer the T cells, Marker believes
that its product candidates will be easier and less expensive to manufacture, with reduced toxicities, compared to current engineered
CAR-T and TCR-based approaches, and may provide patients with meaningful clinical benefit. As a result, Marker believes its portfolio
of T cell therapies has a compelling product profile, as compared to current gene-modified CAR-T and TCR-based therapies.
To receive future press releases via email, please
visit: https://www.markertherapeutics.com/email-alerts.

Forward-Looking Statements
This release
contains forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
Statements in this news release concerning the Company’s expectations, plans, business outlook or future performance, and any other
statements concerning assumptions made or expectations as to any future events, conditions, performance or other matters, are “forward-looking
statements.” Forward-looking statements include statements regarding our intentions, beliefs, projections, outlook, analyses or
current expectations concerning, among other things: our research, development and regulatory activities and expectations relating to
our non-engineered multi-tumor antigen specific T cell therapies; the effectiveness of these programs or the possible range of application
and potential curative effects and safety in the treatment of diseases; the timing, conduct and success of our clinical trials of our
product candidates, including MT-401 for the treatment of patients with AML and MT-601 for the treatment of patients with lymphoma.
Forward-looking statements are by their nature subject to risks, uncertainties and other factors which could cause actual results to differ
materially from those stated in such statements. Such risks, uncertainties and factors include, but are not limited to the risks set forth
in the Company’s most recent Form 10-K, 10-Q and other SEC filings which
are available through EDGAR at WWW.SEC.GOV. The Company assumes no obligation to update
its forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release
except as may be required by law.
Contacts
TIBEREND STRATEGIC ADVISORS, INC.
Investors
Daniel Kontoh-Boateng
(862) 213-1398
dboateng@tiberend.com
Media
Casey McDonald
(646) 577-8520
cmcdonald@tiberend.com
Exhibit
99.2

| MARKER THERAPEUTICS
CORPORATE PRESENTATION
January 2024 |

| Forward Looking Statements
Certain statements contained herein are forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934,
as amended, and Section 27A of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended,
that involve risks and uncertainties. All statements other than statements relating to historical matters including statements to the effect that we
“believe”, “expect”, “anticipate”, “plan”, “target”, “intend” and similar expressions, including without limitation statements regarding Marker
Therapeutics, Inc.’s (“Marker” or the “Company”) intentions, beliefs, projections, outlook, analyses or current expectations concerning, among
other things: the Company’s research, development and regulatory activities and expectations relating to its non-engineered multi-tumor antigen
specific T cell therapies; the effectiveness of these programs or the possible range of application and potential curative effects and safety in the
treatment of diseases; the timing, conduct and success of the Company’s clinical trials of its product candidates, including MT-401 for the
treatment of MT-401 for the treatment of patients with Acute Myeloid Leukemia (“AML”), MT-401 Off-the-Shelf (“OTS”) for the treatment of patients
with AML and MT-601 for the treatment of patients with relapsed non-Hodgkin lymphoma; the Company’s long-term stability and cash runway; the
Company’s optimized manufacturing process; and the future development of multiTAA therapies. Forward-looking statements are by their nature
subject to risks, uncertainties and other factors which could cause actual results to differ materially from those stated in such statements. Such
risks, uncertainties and factors include, but are not limited to the risks set forth in the Company’s most recent Form 10-K, 10-Q and
other SEC filings which are available through EDGAR at WWW.SEC.GOV. No representation or warranty (expressed or implied) is made as to,
and no reliance should be placed on, the fairness, accuracy or completeness of the information contained herein. Accordingly, none of the
Company, or any of its principals, partners, subsidiaries or affiliates, or any of such person’s board members, officers or employees accepts any
liability whatsoever arising directly or indirectly from the use of this presentation. Certain information set forth herein includes estimates,
projections and targets and involves significant elements of subjective judgement and analysis, which may or may not be correct. No
representations are made as to the accuracy of such estimates, projections or targets or that all assumptions relating to such estimates,
projections or targets have been considered or stated or that such estimates, projections or targets will be realized. This presentation does not
purport to contain all of the information that may be required to evaluate the Company and any recipient hereof should conduct its own
independent analysis of the Company and the data and information contained herein. Any forward-looking statements are not guarantees of
future performance and actual results may differ materially from estimates in the forward-looking statements. Unless otherwise stated, all
information in this presentation is as of the date of the cover page of this presentation, and the Company undertakes no obligation to revise these
forward-looking statements to reflect events or circumstances that arise after the date hereof.
2 |
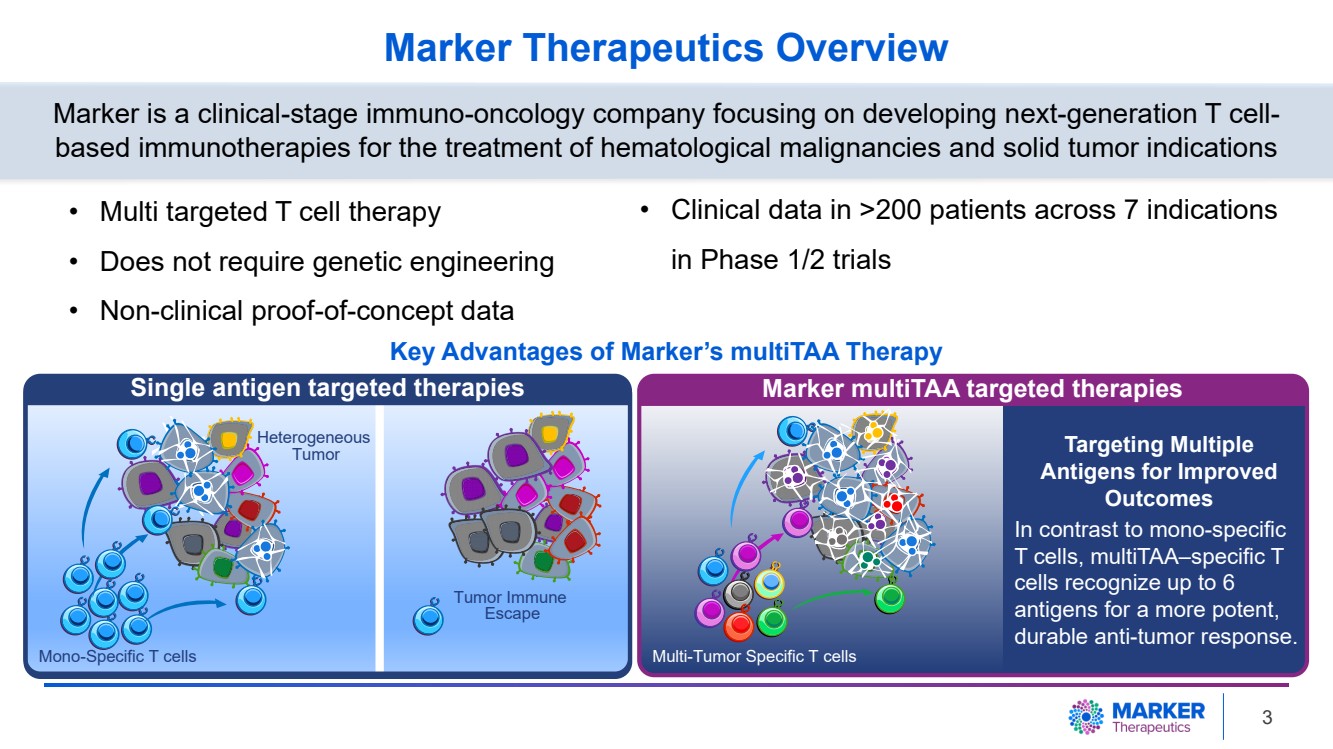
| Marker Therapeutics Overview
• Multi targeted T cell therapy
• Does not require genetic engineering
• Non-clinical proof-of-concept data
Marker is a clinical-stage immuno-oncology company focusing on developing next-generation T cell-based immunotherapies for the treatment of hematological malignancies and solid tumor indications
Mono-Specific T cells
Tumor Immune
Escape
Heterogeneous
Tumor
Multi-Tumor Specific T cells
Single antigen targeted therapies Marker multiTAA targeted therapies
Targeting Multiple
Antigens for Improved
Outcomes
In contrast to mono-specific
T cells, multiTAA–specific T
cells recognize up to 6
antigens for a more potent,
durable anti-tumor response.
• Clinical data in >200 patients across 7 indications
in Phase 1/2 trials
Key Advantages of Marker’s multiTAA Therapy
3 |
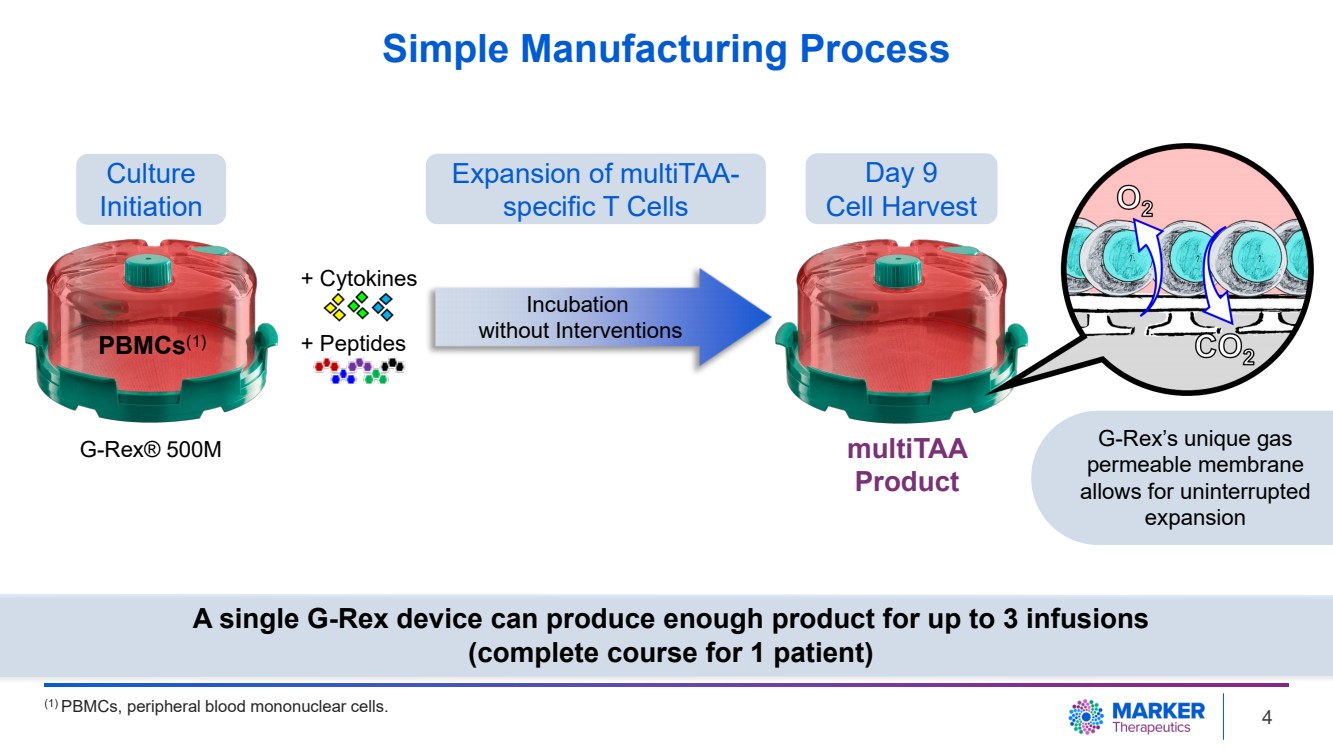
| Simple Manufacturing Process
G-Rex® 500M
Culture
Initiation
Expansion of multiTAA-specific T Cells
Day 9
Cell Harvest
multiTAA
Product
+ Cytokines
+ Peptides
Incubation
without Interventions PBMCs(1)
G-Rex’s unique gas
permeable membrane
allows for uninterrupted
expansion
A single G-Rex device can produce enough product for up to 3 infusions
(complete course for 1 patient)
(1) PBMCs, peripheral blood mononuclear cells. 4 |
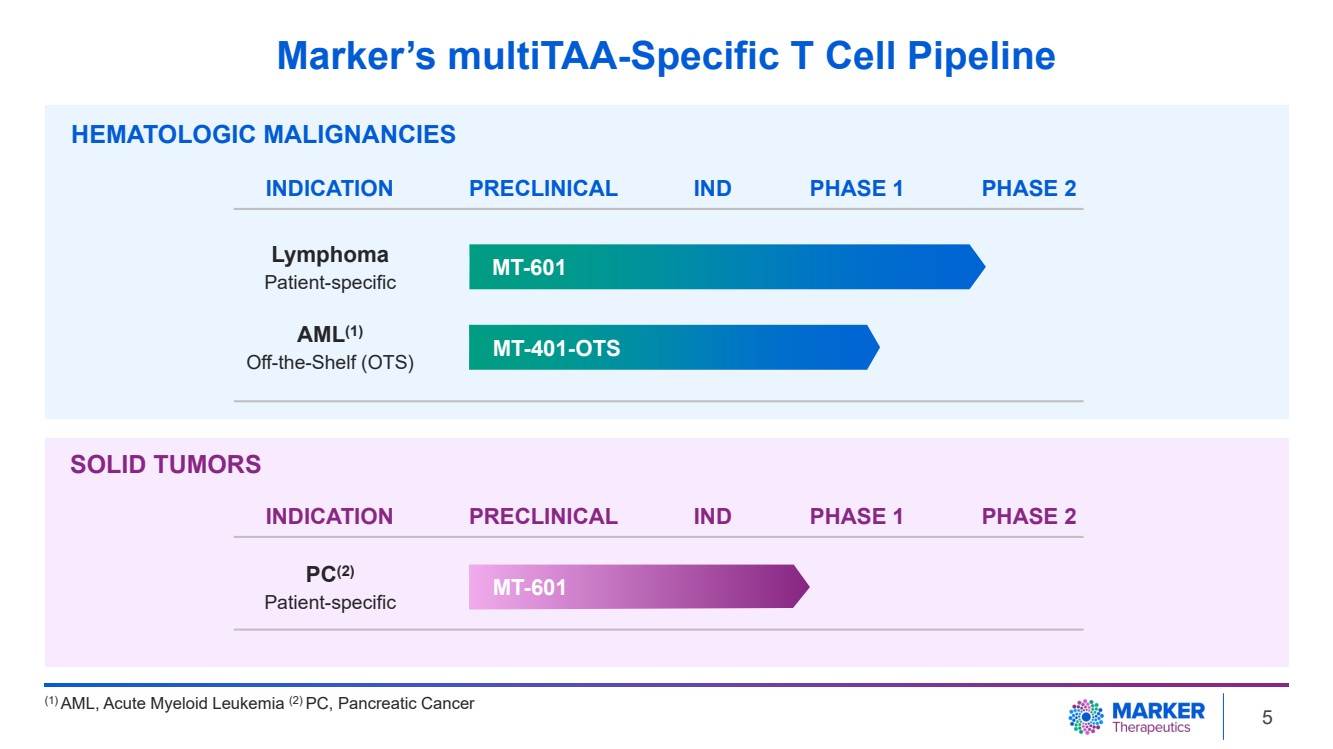
| Marker’s multiTAA-Specific T Cell Pipeline
HEMATOLOGIC MALIGNANCIES
MT-601
MT-601
MT-401-OTS
INDICATION PRECLINICAL PHASE 1 PHASE 2
AML(1)
Off-the-Shelf (OTS)
Lymphoma
Patient-specific
PC(2)
Patient-specific
(1) AML, Acute Myeloid Leukemia (2) PC, Pancreatic Cancer
IND
SOLID TUMORS
INDICATION PRECLINICAL IND PHASE 1 PHASE 2
5 |
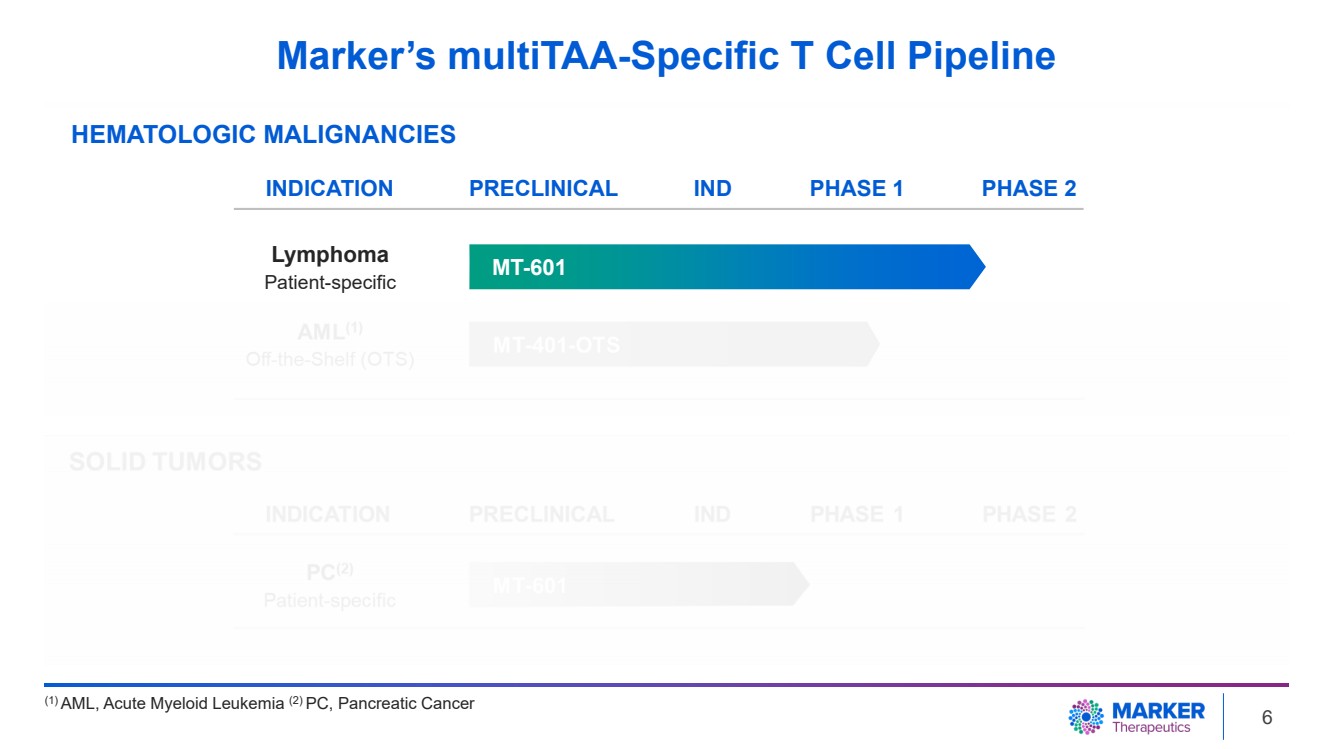
| Marker’s multiTAA-Specific T Cell Pipeline
(1) AML, Acute Myeloid Leukemia (2) PC, Pancreatic Cancer
HEMATOLOGIC MALIGNANCIES
MT-601
INDICATION PRECLINICAL PHASE 1 PHASE 2
Lymphoma
Patient-specific
IND
6 |
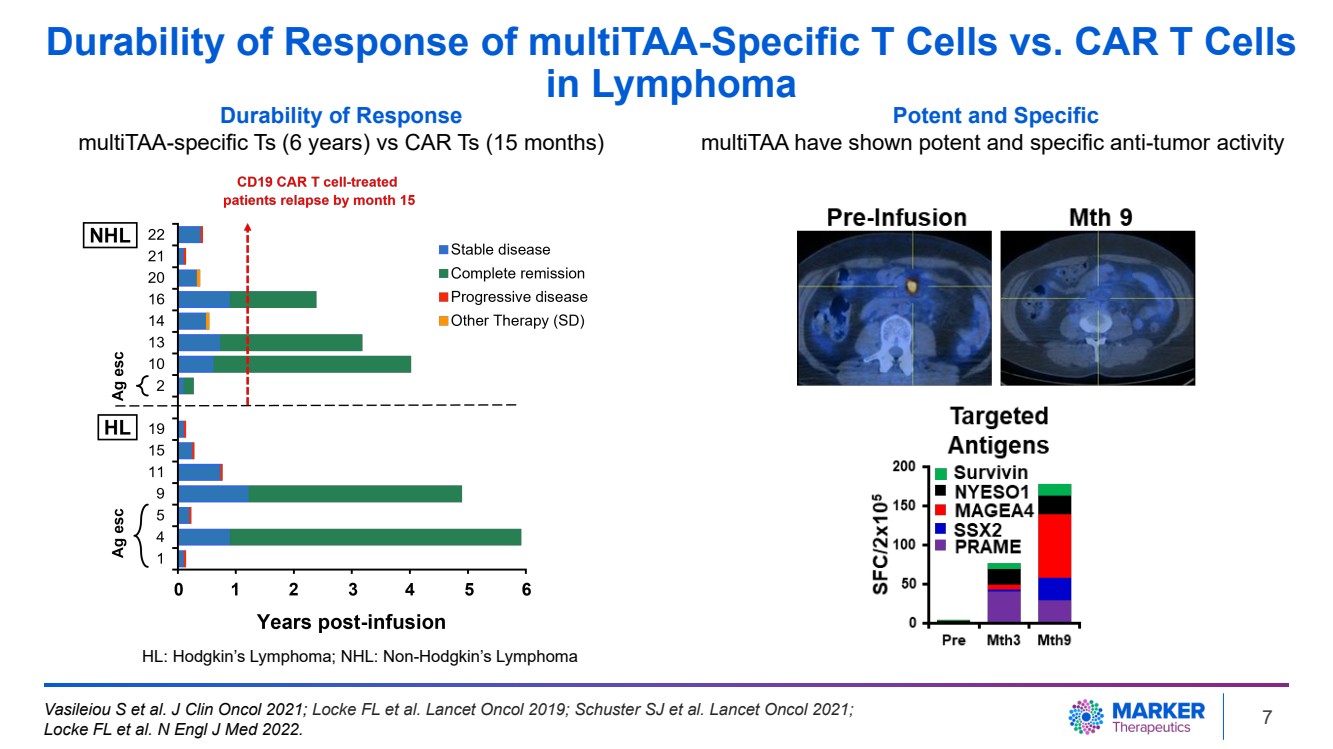
| Durability of Response of multiTAA-Specific T Cells vs. CAR T Cells
in Lymphoma
Durability of Response
multiTAA-specific Ts (6 years) vs CAR Ts (15 months)
Potent and Specific
multiTAA have shown potent and specific anti-tumor activity
HL: Hodgkin’s Lymphoma; NHL: Non-Hodgkin’s Lymphoma
Vasileiou S et al. J Clin Oncol 2021; Locke FL et al. Lancet Oncol 2019; Schuster SJ et al. Lancet Oncol 2021;
Locke FL et al. N Engl J Med 2022. 7 |
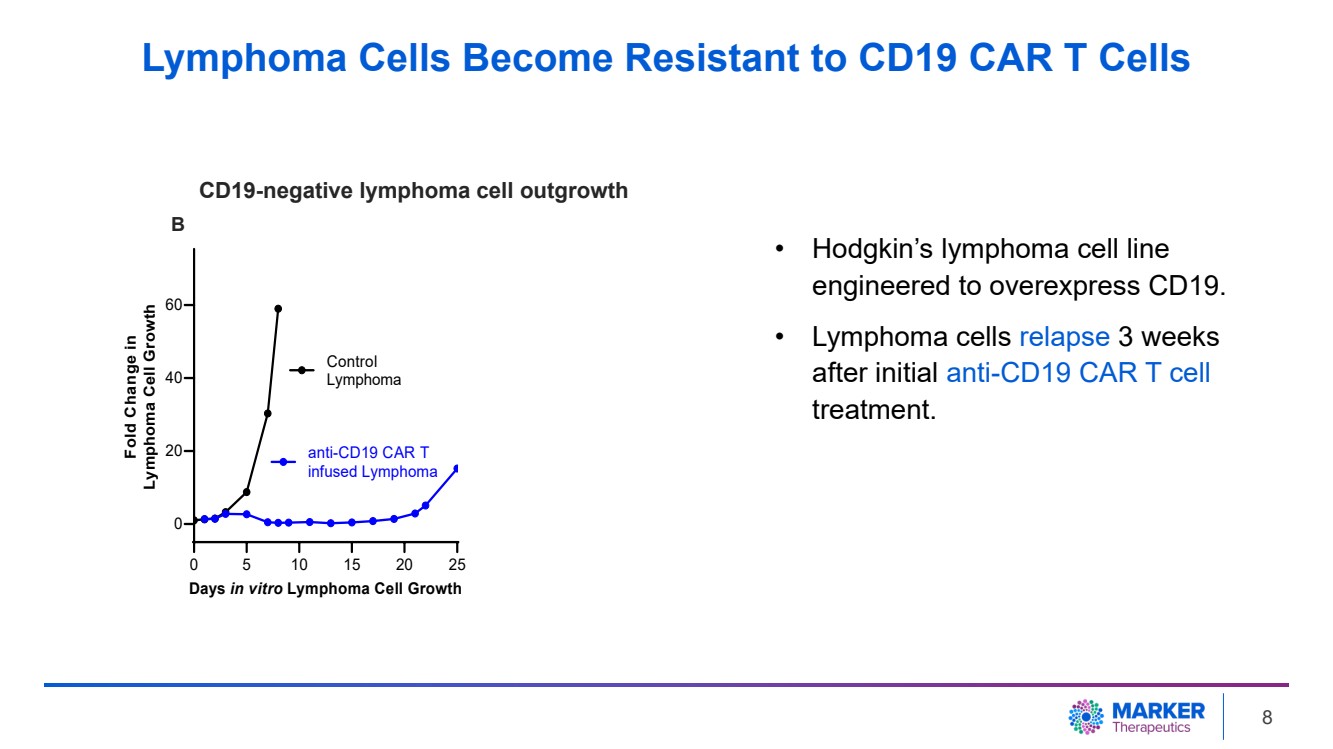
| • Hodgkin’s lymphoma cell line
engineered to overexpress CD19.
• Lymphoma cells relapse 3 weeks
after initial anti-CD19 CAR T cell
treatment.
0 5 10 15 20 25
0
20
40
60
Days in vitro Lymphoma Cell Growth
Fold Change in
Lymphoma Cell Growth
Control
Lymphoma
anti-CD19 CAR T
infused Lymphoma
B
CD19-negative lymphoma cell outgrowth
Lymphoma Cells Become Resistant to CD19 CAR T Cells
8 |
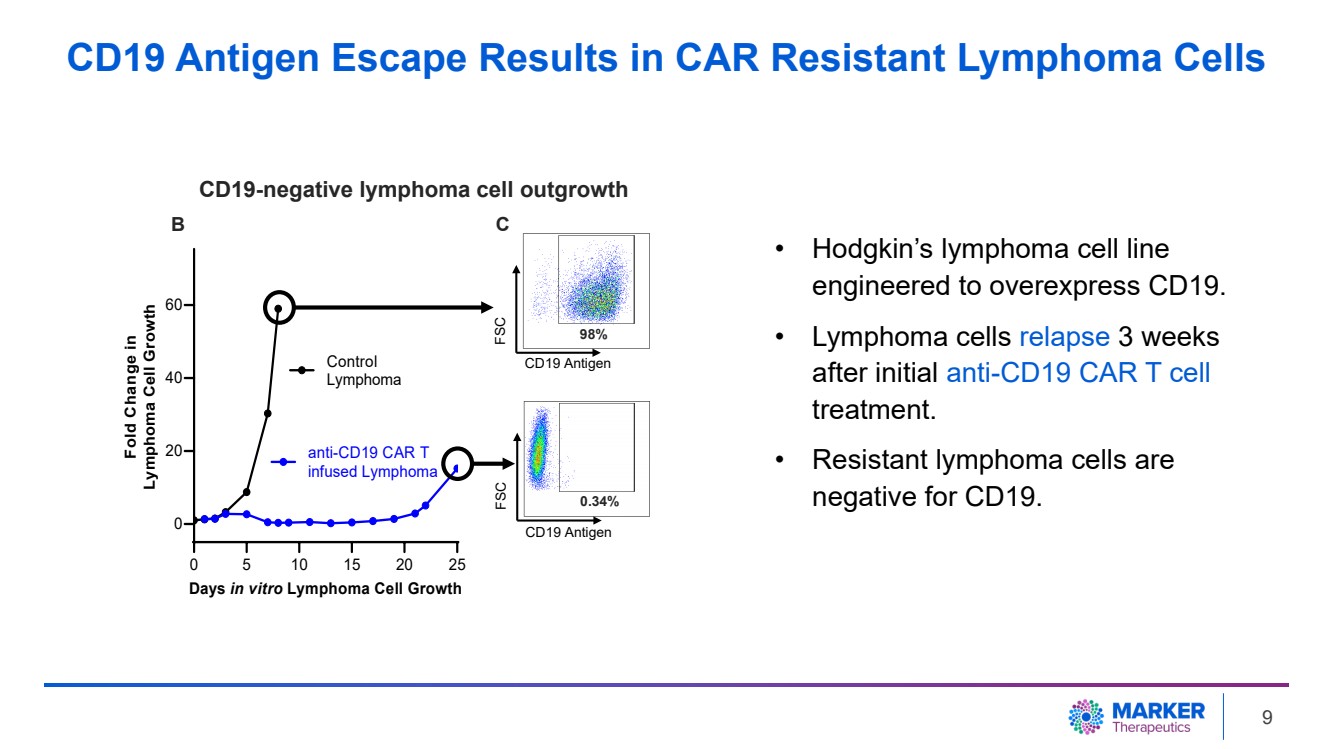
| • Hodgkin’s lymphoma cell line
engineered to overexpress CD19.
• Lymphoma cells relapse 3 weeks
after initial anti-CD19 CAR T cell
treatment.
• Resistant lymphoma cells are
negative for CD19.
0 5 10 15 20 25
0
20
40
60
Days in vitro Lymphoma Cell Growth
Fold Change in
Lymphoma Cell Growth
Control
Lymphoma
anti-CD19 CAR T
infused Lymphoma
CD19 Antigen
FSC
98%
0.34%
CD19 Antigen
FSC
B C
CD19-negative lymphoma cell outgrowth
CD19 Antigen Escape Results in CAR Resistant Lymphoma Cells
9 |
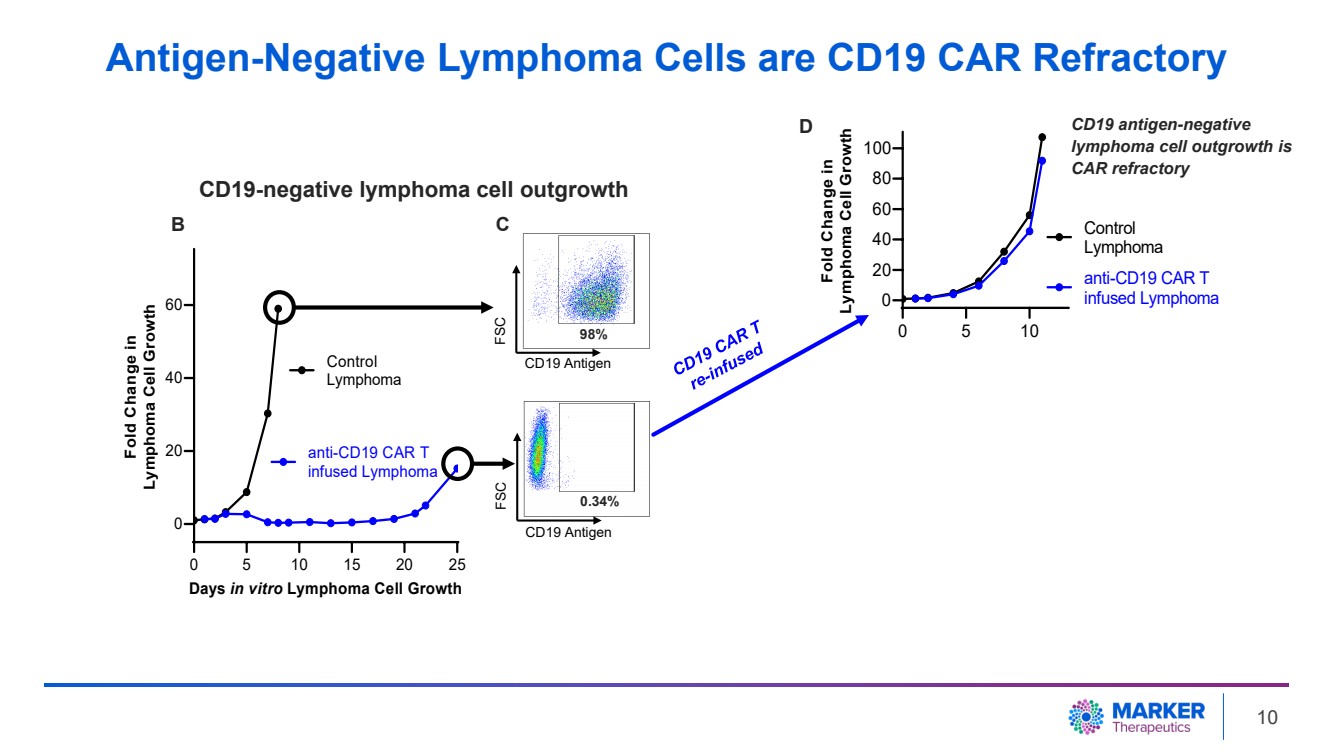
| Antigen-Negative Lymphoma Cells are CD19 CAR Refractory
0 5 10 15 20 25
0
20
40
60
Days in vitro Lymphoma Cell Growth
Fold Change in
Lymphoma Cell Growth
Control
Lymphoma
anti-CD19 CAR T
infused Lymphoma
0 5 10
0
20
40
60
80
100
Fold Change in
Lymphoma Cell Growth
Control
Lymphoma
anti-CD19 CAR T
infused Lymphoma
CD19 Antigen
FSC
98%
0.34%
CD19 Antigen
FSC
B C
CD19 antigen-negative
lymphoma cell outgrowth is
CAR refractory
D
CD19-negative lymphoma cell outgrowth
10 |
| 0 5 10 15 20 25
0
20
40
60
Days in vitro Lymphoma Cell Growth
Fold Change in
Lymphoma Cell Growth
Control
Lymphoma
anti-CD19 CAR T
infused Lymphoma
0 5 10
0
20
40
60
80
100
Fold Change in
Lymphoma Cell Growth
Control
Lymphoma
anti-CD19 CAR T
infused Lymphoma
CD19 Antigen
FSC
98%
0.34%
CD19 Antigen
FSC
B C
0 5 10 15 20 25
0
20
40
60
80
100
Days in vitro Lymphoma Cell Growth
Fold Change in
Lymphoma Cell Growth
Control
Lymphoma
MT-601 infused
Lymphoma
CD19 antigen-negative
lymphoma cell outgrowth is
CAR refractory
CAR refractory lymphoma is
controlled by MT-601
D
CD19-negative lymphoma cell outgrowth
MT-601 Demonstrates Anti-Tumor Activity in CAR Refractory
Lymphoma Cells
Broad antigen targeting of MT-601 resulted in killing of CD19 CAR T refractory lymphoma cells
11 |
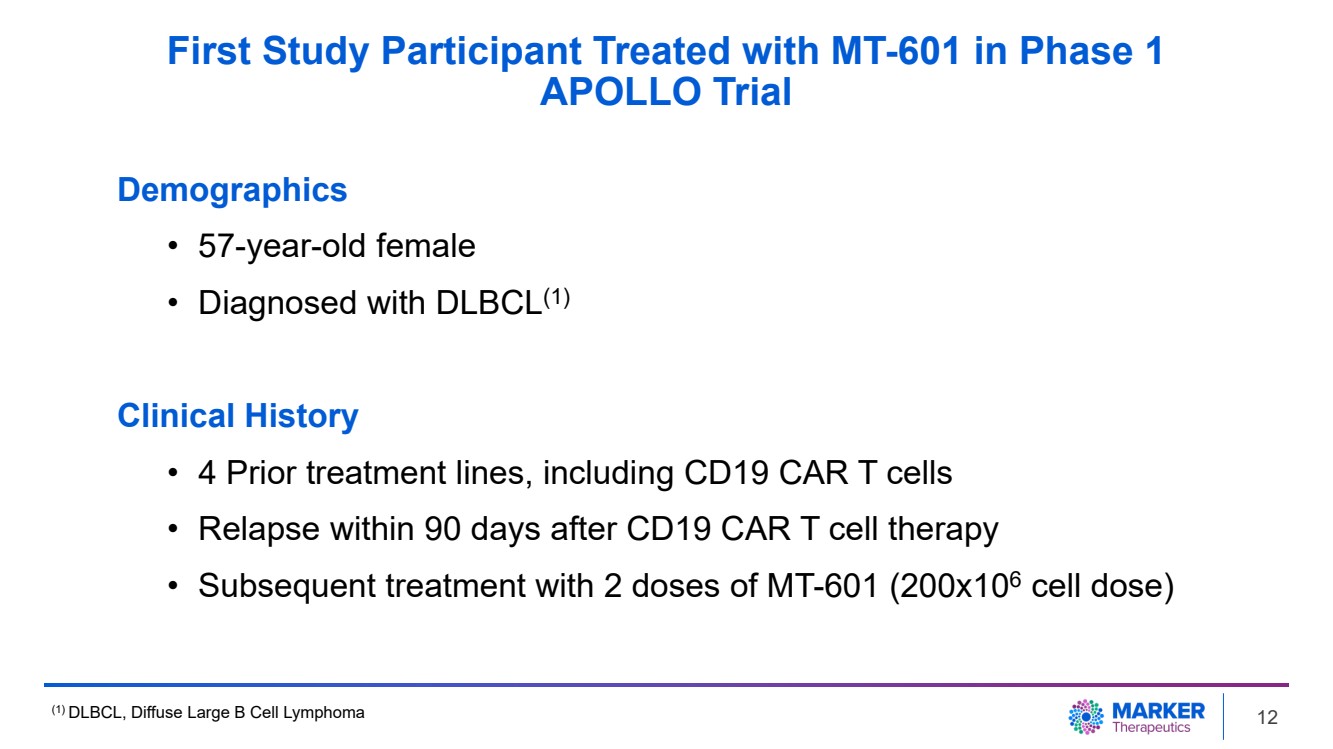
| First Study Participant Treated with MT-601 in Phase 1
APOLLO Trial
Demographics
• 57-year-old female
• Diagnosed with DLBCL(1)
Clinical History
• 4 Prior treatment lines, including CD19 CAR T cells
• Relapse within 90 days after CD19 CAR T cell therapy
• Subsequent treatment with 2 doses of MT-601 (200x106 cell dose)
(1) DLBCL, Diffuse Large B Cell Lymphoma 12 |

| Complete Response in Lymphoma Patient Treated with MT-601
after CAR T Relapse
MT-601 Clinical Safety
• MT-601 treatment was well tolerated
• No > Grade 1 treatment-related adverse events
Clinical Response
• Study participant achieved complete metabolic response 8 weeks
after 2nd infusion of MT-601
• Patient remains in complete response 6 months after MT-601 treatment
13 |
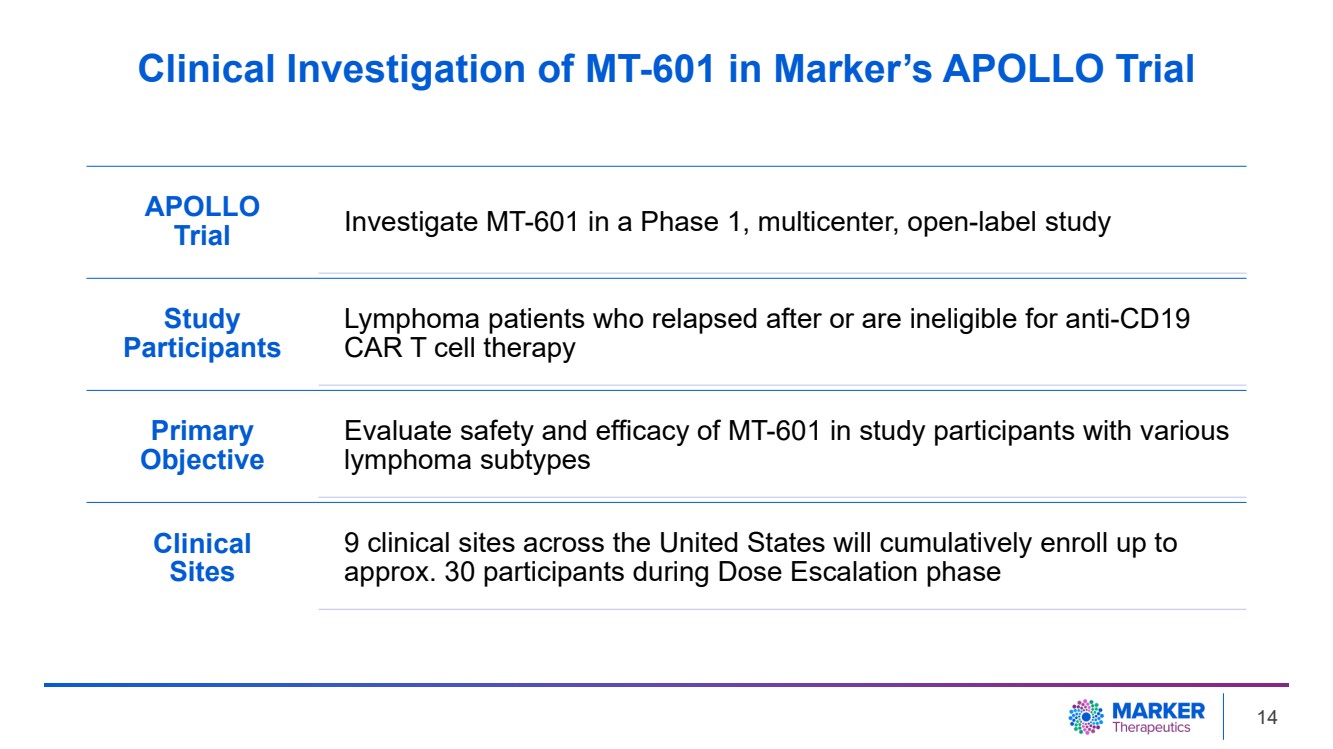
| Clinical Investigation of MT-601 in Marker’s APOLLO Trial
APOLLO
Trial Investigate MT-601 in a Phase 1, multicenter, open-label study
Study
Participants
Lymphoma patients who relapsed after or are ineligible for anti-CD19
CAR T cell therapy
Primary
Objective
Evaluate safety and efficacy of MT-601 in study participants with various
lymphoma subtypes
Clinical
Sites
9 clinical sites across the United States will cumulatively enroll up to
approx. 30 participants during Dose Escalation phase
14 |
| Future Developments |
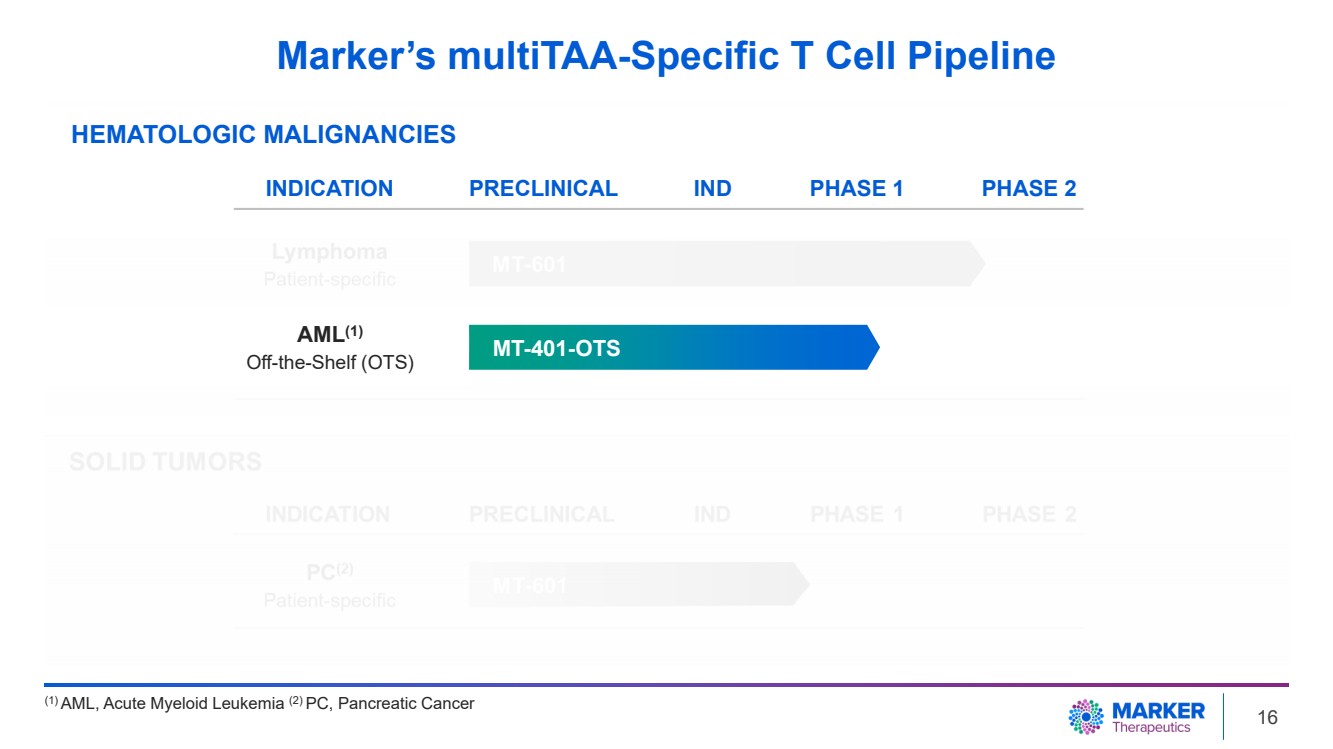
| Marker’s multiTAA-Specific T Cell Pipeline
(1) AML, Acute Myeloid Leukemia (2) PC, Pancreatic Cancer
HEMATOLOGIC MALIGNANCIES
INDICATION PRECLINICAL IND PHASE 1 PHASE 2
MT-401-OTS AML(1)
Off-the-Shelf (OTS)
16 |
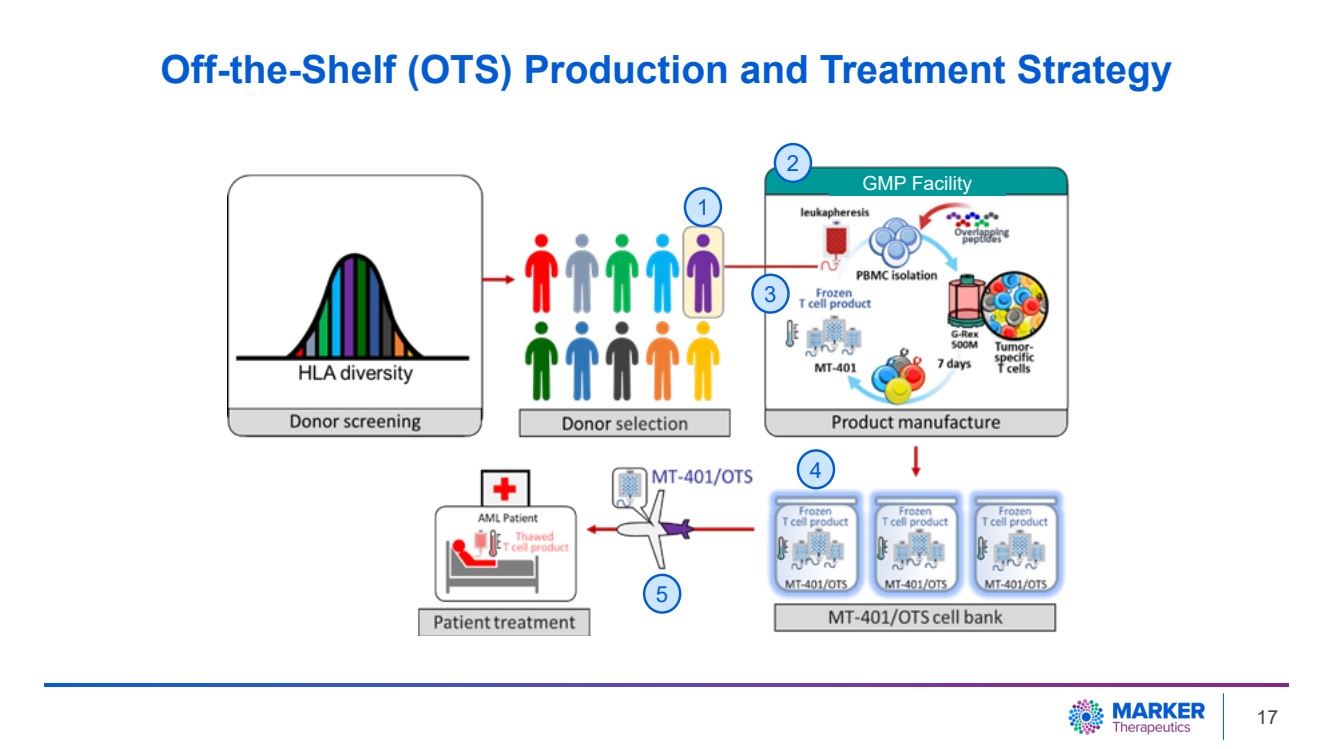
| Off-the-Shelf (OTS) Production and Treatment Strategy
2
GMP Facility
1
3
4
5
17 |
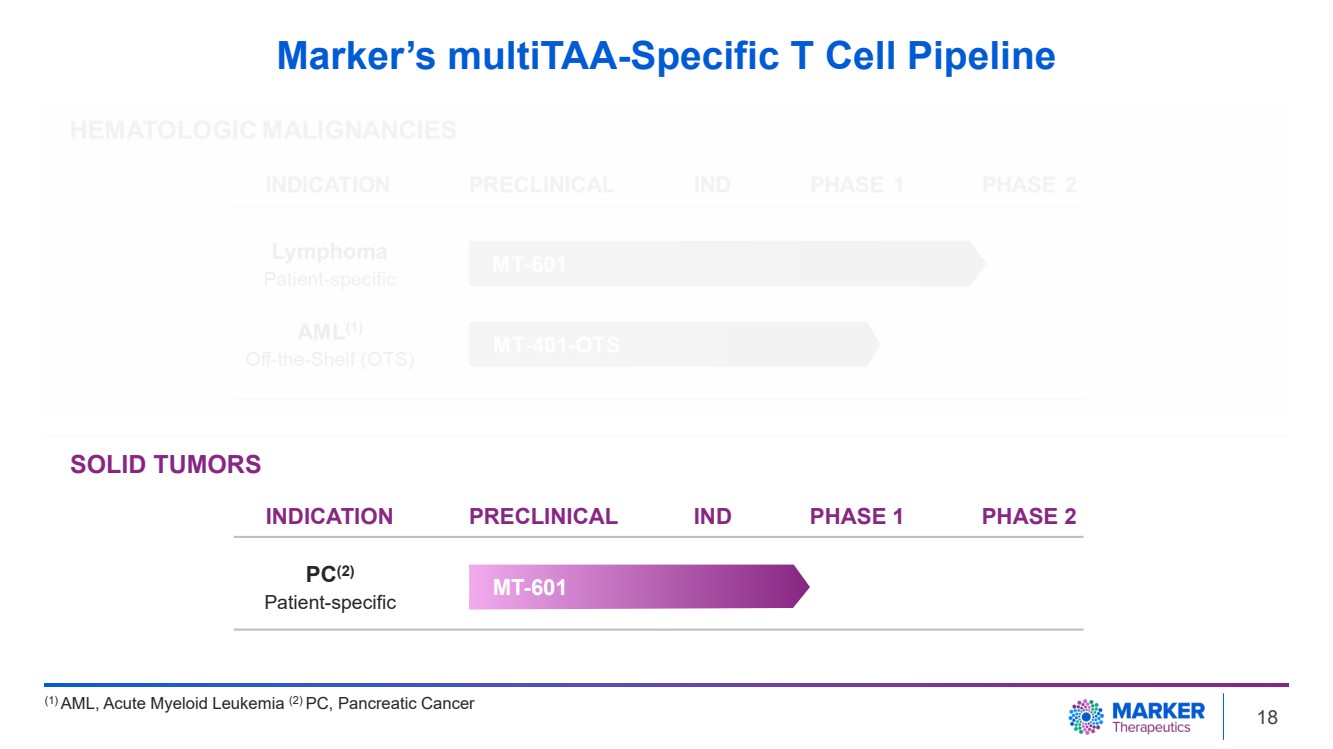
| Marker’s multiTAA-Specific T Cell Pipeline
(1) AML, Acute Myeloid Leukemia (2) PC, Pancreatic Cancer
MT-601 PC(2)
Patient-specific
SOLID TUMORS
INDICATION PRECLINICAL IND PHASE 1 PHASE 2
18 |
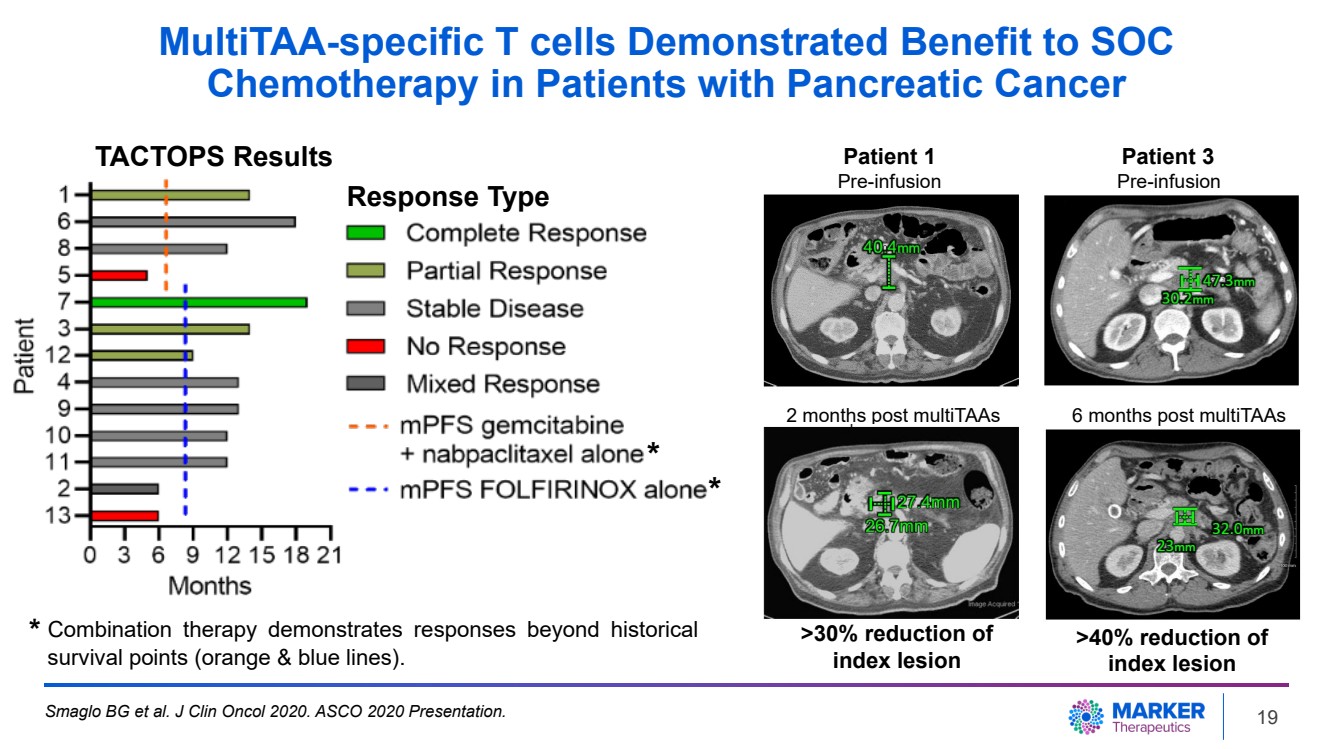
| MultiTAA-specific T cells Demonstrated Benefit to SOC
Chemotherapy in Patients with Pancreatic Cancer
Smaglo BG et al. J Clin Oncol 2020. ASCO 2020 Presentation.
* Combination therapy demonstrates responses beyond historical
survival points (orange & blue lines).
Patient 1 Patient 3
Pre-infusion Pre-infusion
2 months post multiTAAs 6 months post multiTAAs
>30% reduction of
index lesion
>40% reduction of
index lesion
*
*
TACTOPS Results
Response Type
19 |

| Corporate & Financial Highlights
Demonstrated clinical response in hematological malignancies and solid tumors
Favorable safety profile in clinical trials to date
3 FDA-approved INDs
Awarded over $17 million non-dilutive funding through grants
Cash & Cash Equivalents of $17.5 million(1)
Current cash runway expected through Q4 2025
(1) As of September 30, 2023. 20 |

| THANK YOU
21 |
Cover
|
Jan. 08, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 08, 2024
|
| Entity File Number |
001-37939
|
| Entity Registrant Name |
MARKER THERAPEUTICS, INC.
|
| Entity Central Index Key |
0001094038
|
| Entity Tax Identification Number |
45-4497941
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
9350 Kirby Drive, Suite 300
|
| Entity Address, City or Town |
Houston
|
| Entity Address, State or Province |
TX
|
| Entity Address, Postal Zip Code |
77054
|
| City Area Code |
713
|
| Local Phone Number |
400-6400
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common
Stock, par value $0.001 per share
|
| Trading Symbol |
MRKR
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Marker Therapeutics (NASDAQ:MRKR)
Historical Stock Chart
From Oct 2024 to Nov 2024

Marker Therapeutics (NASDAQ:MRKR)
Historical Stock Chart
From Nov 2023 to Nov 2024


