false000175028400017502842024-09-272024-09-27
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): September 27, 2024 |
Olema Pharmaceuticals, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-39712 |
30-0409740 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
780 Brannan Street |
|
San Francisco, California |
|
94103 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 415 651-3316 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.0001 per share |
|
OLMA |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On September 27, 2024, Olema Pharmaceuticals, Inc. (the “Company” or “Olema”) posted a corporate presentation to its website that will be shared with investors and others from time to time. A copy of this presentation is being furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in Exhibit 99.1 attached hereto is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 8.01 Other Events.
The Company plans to present non-clinical data for OP-3136, the Company's development candidate targeting KAT6, at the EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics (“ENA”), which will be held October 23-25, 2024. An initial new drug (“IND”) application for OP-3136 is anticipated to be submitted in the fourth quarter of 2024 and the Phase 1/2 clinical study for OP-3136 is anticipated to begin in 2025.
The Company is also planning for a potential initiation of OPERA-02, a proposed Phase 3 clinical trial of palazestrant in combination with a CDK4/6 inhibitor, ribociclib, in 2025.
Forward Looking Statements
Statements contained in this Current Report on Form 8-K, including the exhibit furnished herewith, regarding matters that are not historical facts are “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Words such as “anticipate,” “expect,” “will,” “may,” “goal,” “potential” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. These forward-looking statements include, without limitation, statements regarding upcoming data presentations, the timing and likelihood of submitting an IND application for OP-3136, the timing of the commencement of a Phase 1/2 clinical study for OP-3136, and the timing and likelihood of initiation of OPERA-02. Because such statements deal with future events and are based on Olema’s current expectations, they are subject to various risks and uncertainties, and actual results, performance or achievements of Olema could differ materially from those described in or implied by the statements in this press release. These forward-looking statements are subject to risks and uncertainties, including, without limitation, those discussed in the section titled “Risk Factors” in Olema’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, and other filings and reports that Olema makes from time to time with the U.S. Securities and Exchange Commission. Except as required by law, Olema assumes no obligation to update these forward-looking statements, including in the event that actual results differ materially from those set forth in the forward-looking statements.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
OLEMA PHARMACEUTICALS, INC. |
|
|
|
|
Date: |
September 27, 2024 |
By: |
/s/ Shane Kovacs |
|
|
|
Shane Kovacs
Chief Operating and Financial Officer |

Advancing medicines for breast cancer �and beyond Corporate Overview September 2024

Forward-looking statements This presentation contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are forward-looking statements. Words such as “anticipate,” “believe,” “estimate,” “expect,” “goal,” “intend,” “may,” “plan,” “potential,” “project,” “will,” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. Such forward-looking statements include, without limitation, statements regarding our research and clinical development plans, the scope, progress, results and costs of developing our product candidates or any other future product candidates, strategy, market size and opportunity, clinical trial designs, regulatory matters, including the timing and likelihood of success of obtaining drug approvals, the timelines for potential clinical study results and clinical trials of palazestrant (OP-1250) as a monotherapy and in combination trials, including OPERA-01, the Company’s pivotal Phase 3 monotherapy clinical trial, and OPERA-02, the Company's potential pivotal Phase 3 clinical trial of palazestrant in combination with ribociclib, the timelines for potential commercial launch and related preparatory work, the sufficiency and potential beneficial characteristics, profile, safety, tolerability, efficacy and therapeutic effects of palazestrant as a monotherapy and in combination trials, the potential of palazestrant to become a therapeutic leader and a best-in-class treatment option for ER+/HER2- metastatic breast cancer and a backbone therapy for women living with breast cancer and beyond, the combinability of palazestrant with other drugs, the timelines for potential preclinical studies and clinical trials for our KAT6 inhibitor program, including OP-3136, the potential value and impact of a KAT6 inhibitor program, the best-in-class potential for OP-3136, the potential beneficial characteristics, profile, safety, efficacy, tolerability, and therapeutic effects of OP-3136, our ability to complete certain milestones, our financial condition, our opportunity in breast cancer and beyond, our ability to impact treatment for endocrine-driven cancers, cash position and runway and sufficiency of our financial resources, and the sufficiency and expertise of our management team. These forward-looking statements are based on the beliefs of the Company’s management as well as assumptions made by and information currently available to the Company. Such statements reflect the current views of the Company with respect to future events and are subject to known and unknown risks, including business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about the Company, including, without limitation, risks inherent in developing products and technologies, future results from the Company’s ongoing and planned clinical trials, the Company’s ability to obtain adequate financing to fund its planned clinical trials and other expenses, trends in the industry, the legal and regulatory framework for the industry and future expenditures, and other risks and uncertainties affecting the Company, including those described under the caption "Risk Factors" and elsewhere in the Company‘s Quarterly Reports on Form 10-Q, Annual Report on Form 10-K, and other filings and reports the Company files with the Securities and Exchange Commission from time to time. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The actual results may vary from the anticipated results and the variations may be material. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of the assumptions, fully stated in this presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this presentation is given. This presentation discusses product candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. This presentation incorporates publicly-available third-party data that we have not independently verified. There are risks inherent in conducting cross-trial comparisons and the results should be interpreted with caution. The presentation of such third-party data does not represent a head-to-head comparison of how palazestrant, in monotherapy or in combination, or OP-3136 performed against any other third-party drug candidate or study. Rather, such third-party data has been pulled by us from publicly-available sources for supplemental informational purposes, only. We caution you that any comparisons against third-party data set forth herein should not be viewed as a side-by-side comparison, and you should not rely on the completeness or accuracy of our presentation of the results of any third-party drug candidate in these slides, due to differences in study design, how other companies quantify or qualify eligibility criteria, and how results are recorded, among other distinguishing factors and uncertainties. Because we may be unaware of or may not adequately present various distinguishing factors and uncertainties, the comparisons set forth herein may not properly present such third-party data, which may differ materially from the data as presented here. Investors are encouraged to independently review third party data and should not rely on our presentation of such data (including any such data placed in comparison with the performance of palazestrant) as a single measure to evaluate our business. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products.

— We are on a mission to elevate patient care in breast cancer �and beyond Expertise in endocrine-driven cancers with mechanistically superior scientific approach that fully inactivates estrogen receptors OP-1250, a promising backbone therapy for ER+/HER2- breast cancer in late-stage clinical development, forms basis of breast cancer program Exciting new and potent KAT6 inhibitor with potential to significantly impact breast cancer treatment; IND filing expected in Q4 2024 Management and Board with deep expertise developing and commercializing oncology medicines Molecular Advantage Lead Asset – Palazestrant OP-3136 Expands Pipeline Proven Leadership

What drives us: we are all impacted by breast cancer The most common cancer diagnosed and the second leading cause of cancer death among women Women in the U.S. will be diagnosed with invasive breast cancer in her lifetime Estimated women in the U.S. that will be diagnosed with breast cancer in 2024 42k Estimated women in the U.S. will die �of metastatic breast cancer in 2024 311k 1 in 8 Source: American Cancer Society
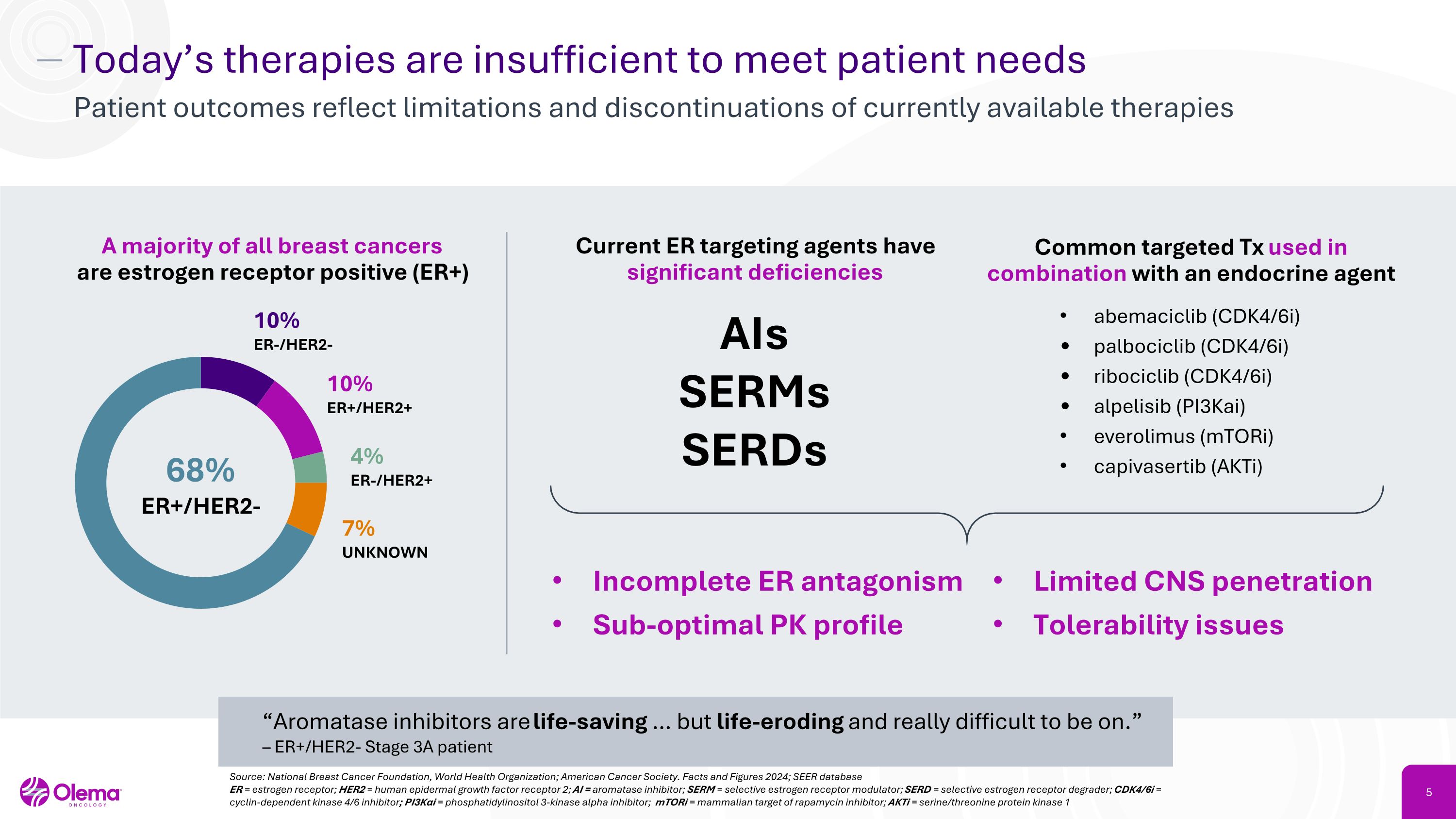
Today’s therapies are insufficient to meet patient needs Patient outcomes reflect limitations and discontinuations of currently available therapies Source: National Breast Cancer Foundation, World Health Organization; American Cancer Society. Facts and Figures 2024; SEER database ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; AI = aromatase inhibitor; SERM = selective estrogen receptor modulator; SERD = selective estrogen receptor degrader; CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; PI3Kαi = phosphatidylinositol 3-kinase alpha inhibitor; mTORi = mammalian target of rapamycin inhibitor; AKTi = serine/threonine protein kinase 1 A majority of all breast cancers�are estrogen receptor positive (ER+) Current ER targeting agents have significant deficiencies AIs SERMs SERDs Incomplete ER antagonism Sub-optimal PK profile Limited CNS penetration Tolerability issues “Aromatase inhibitors are life-saving … but life-eroding and really difficult to be on.” – ER+/HER2- Stage 3A patient Common targeted Tx used in combination with an endocrine agent abemaciclib (CDK4/6i) palbociclib (CDK4/6i) ribociclib (CDK4/6i) alpelisib (PI3Kai) everolimus (mTORi) capivasertib (AKTi) 68% ER+/HER2- 10% ER-/HER2- 10% ER+/HER2+ 4% ER-/HER2+ 7% UNKNOWN
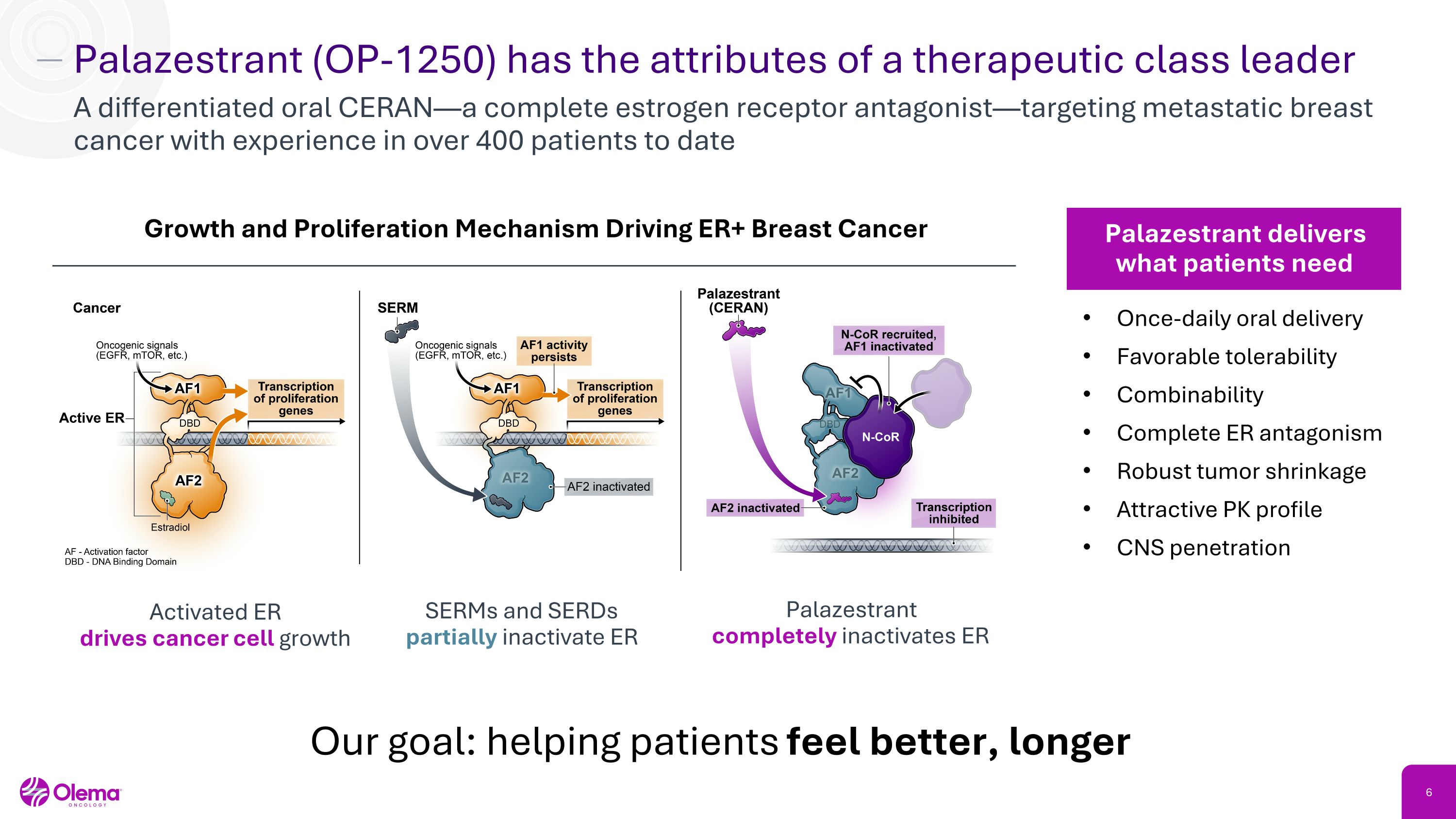
Palazestrant (OP-1250) has the attributes of a therapeutic class leader A differentiated oral CERAN—a complete estrogen receptor antagonist—targeting metastatic breast cancer with experience in over 400 patients to date Once-daily oral delivery Favorable tolerability Combinability Complete ER antagonism Robust tumor shrinkage Attractive PK profile CNS penetration Palazestrant delivers what patients need Growth and Proliferation Mechanism Driving ER+ Breast Cancer Our goal: helping patients feel better, longer Activated ER�drives cancer cell growth SERMs and SERDs partially inactivate ER Palazestrant �completely inactivates ER
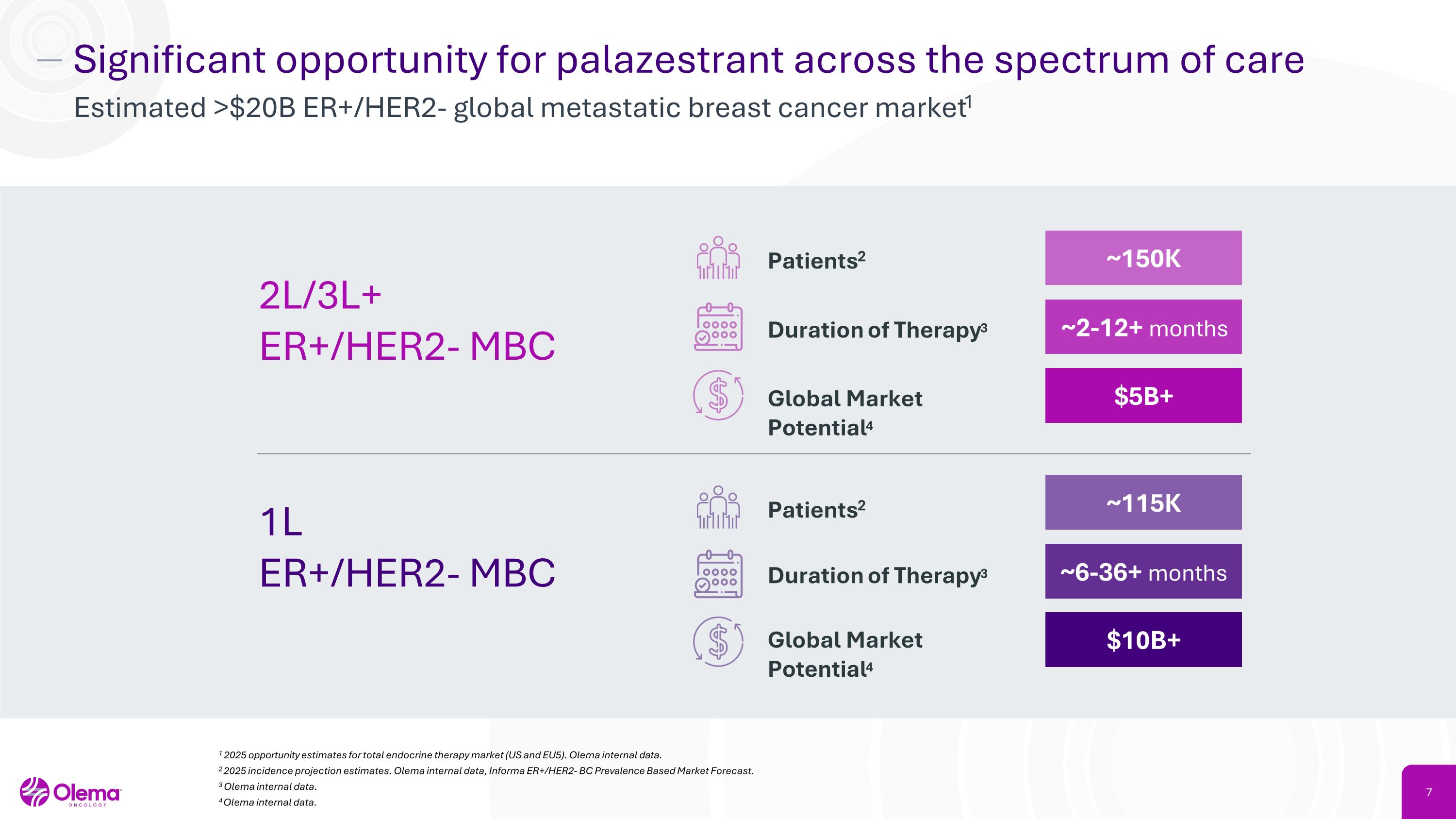
Significant opportunity for palazestrant across the spectrum of care Estimated >$20B ER+/HER2- global metastatic breast cancer market1 1 2025 opportunity estimates for total endocrine therapy market (US and EU5). Olema internal data. 2 2025 incidence projection estimates. Olema internal data, Informa ER+/HER2- BC Prevalence Based Market Forecast. 3 Olema internal data. 4Olema internal data. 2L/3L+�ER+/HER2- MBC 1L �ER+/HER2- MBC Patients2 ~150K Duration of Therapy3 ~2-12+ months Global Market Potential4 $5B+ Patients2 ~115K Duration of Therapy3 ~6-36+ months Global Market Potential4 $10B+
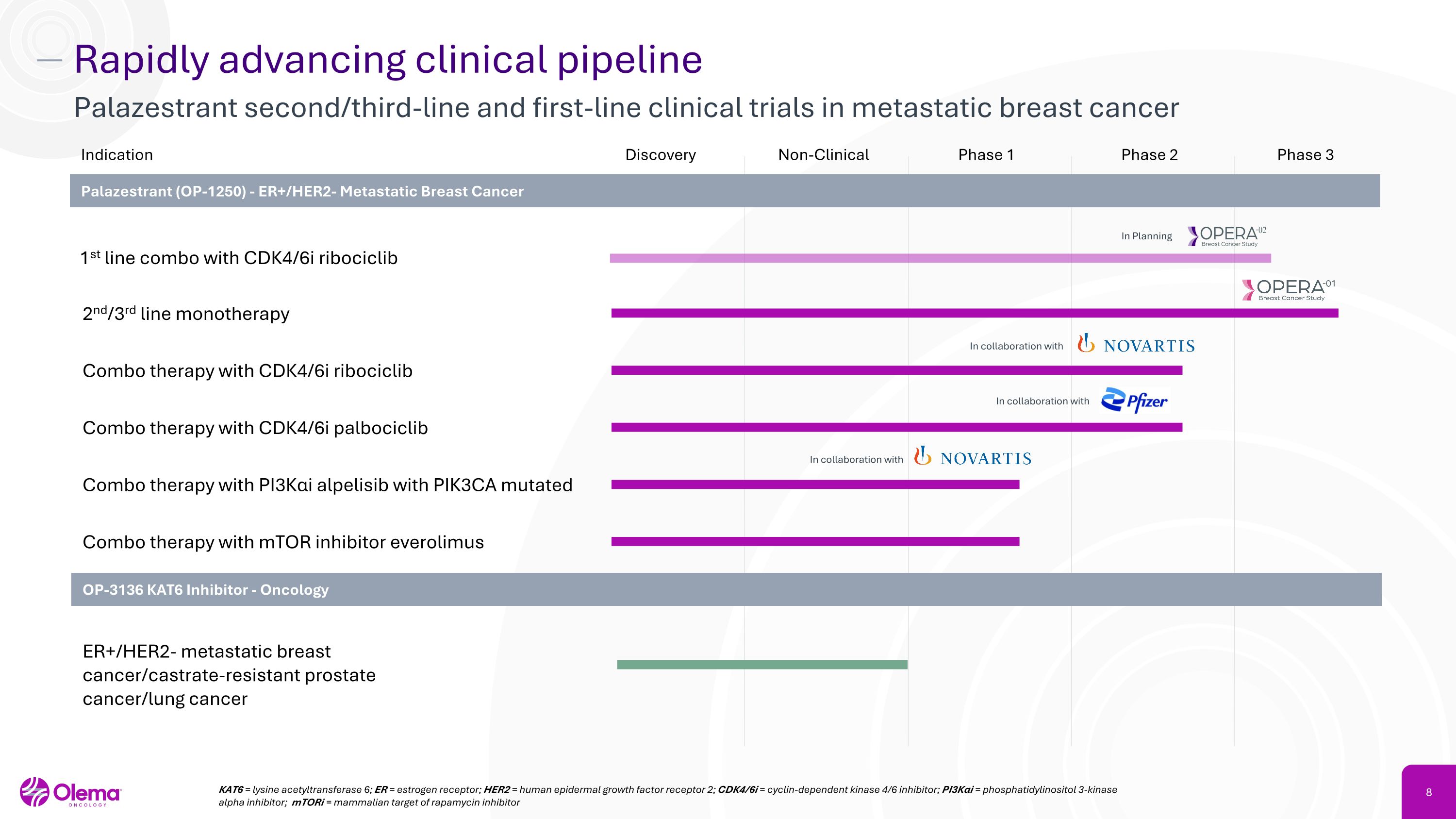
Rapidly advancing clinical pipeline Palazestrant second/third-line and first-line clinical trials in metastatic breast cancer Discovery Non-Clinical Phase 1 Phase 2 Phase 3 2nd/3rd line monotherapy Combo therapy with PI3Kαi alpelisib with PIK3CA mutated Combo therapy with mTOR inhibitor everolimus Indication Palazestrant (OP-1250) - ER+/HER2- Metastatic Breast Cancer Combo therapy with CDK4/6i ribociclib Combo therapy with CDK4/6i palbociclib OP-3136 KAT6 Inhibitor - Oncology ER+/HER2- metastatic breast cancer/castrate-resistant prostate cancer/lung cancer KAT6 = lysine acetyltransferase 6; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; PI3Kαi = phosphatidylinositol 3-kinase alpha inhibitor; mTORi = mammalian target of rapamycin inhibitor 1st line combo with CDK4/6i ribociclib In Planning In collaboration with In collaboration with In collaboration with

Strategy driven by leaders positioned to go the distance Oncology and industry experts with track record of advancing programs from clinical to commercial Executive Leadership Team Ian Clark�Chairman of the Board Sean Bohen, M.D., Ph.D.�President and CEO Sandra Horning, M.D., FACP, FASCO Cindy Butitta Scott Garland Cyrus Harmon, Ph.D. Gorjan Hrustanovic, Ph.D. Yi Larson Andy Rappaport Graham Walmsley, M.D., Ph.D. Sean Bohen, M.D., Ph.D.�President and CEO Shane Kovacs�Chief Operating and Financial Officer Naseem Zojwalla, M.D.�Chief Medical Officer David Myles, Ph.D.�Chief Discovery and Non-Clinical Development Officer Julie Dexter�Senior Vice President and Head of People Board of Directors Experience
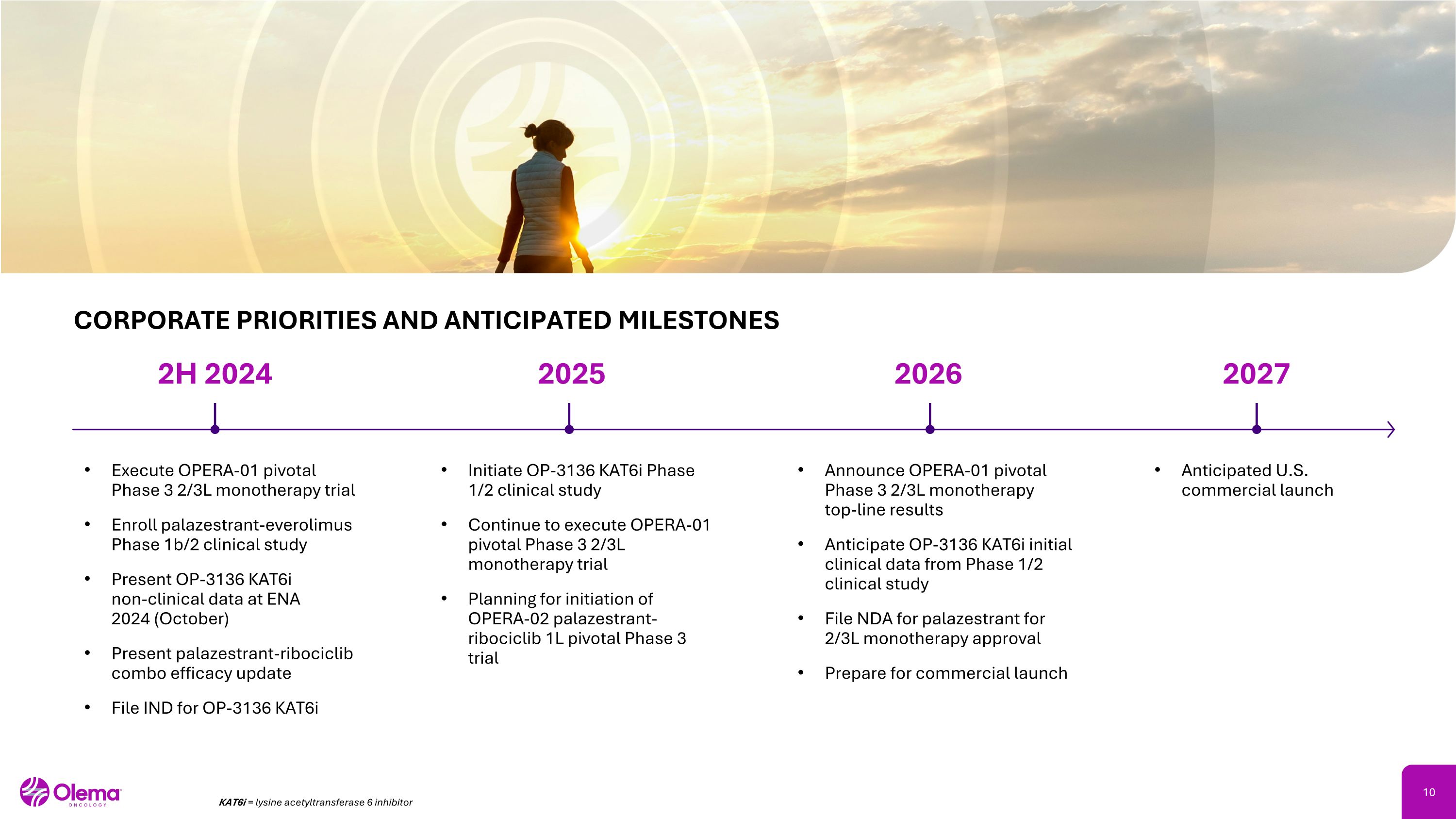
2H 2024 2025 2026 Execute OPERA-01 pivotal Phase 3 2/3L monotherapy trial Enroll palazestrant-everolimus Phase 1b/2 clinical study Present OP-3136 KAT6i �non-clinical data at ENA �2024 (October) Present palazestrant-ribociclib combo efficacy update File IND for OP-3136 KAT6i Initiate OP-3136 KAT6i Phase 1/2 clinical study Continue to execute OPERA-01 pivotal Phase 3 2/3L monotherapy trial Planning for initiation of OPERA-02 palazestrant-ribociclib 1L pivotal Phase 3 trial Announce OPERA-01 pivotal Phase 3 2/3L monotherapy �top-line results Anticipate OP-3136 KAT6i initial clinical data from Phase 1/2 clinical study File NDA for palazestrant for 2/3L monotherapy approval Prepare for commercial launch CORPORATE PRIORITIES AND ANTICIPATED MILESTONES Anticipated U.S. commercial launch 2027 KAT6i = lysine acetyltransferase 6 inhibitor

— Palazestrant OUR PHASE 3 ASSET “Palazestrant is not an endocrine therapy where you need to wait six months to see a patient begin to derive benefit. We’ve seen some not only nice responses, but pretty quick responses—significant reduction of disease burden. And you know, the patients feel great.” – Virginia Borges, M.D.�Professor, Medicine-Medical Oncology�University of Colorado�Principal Investigator
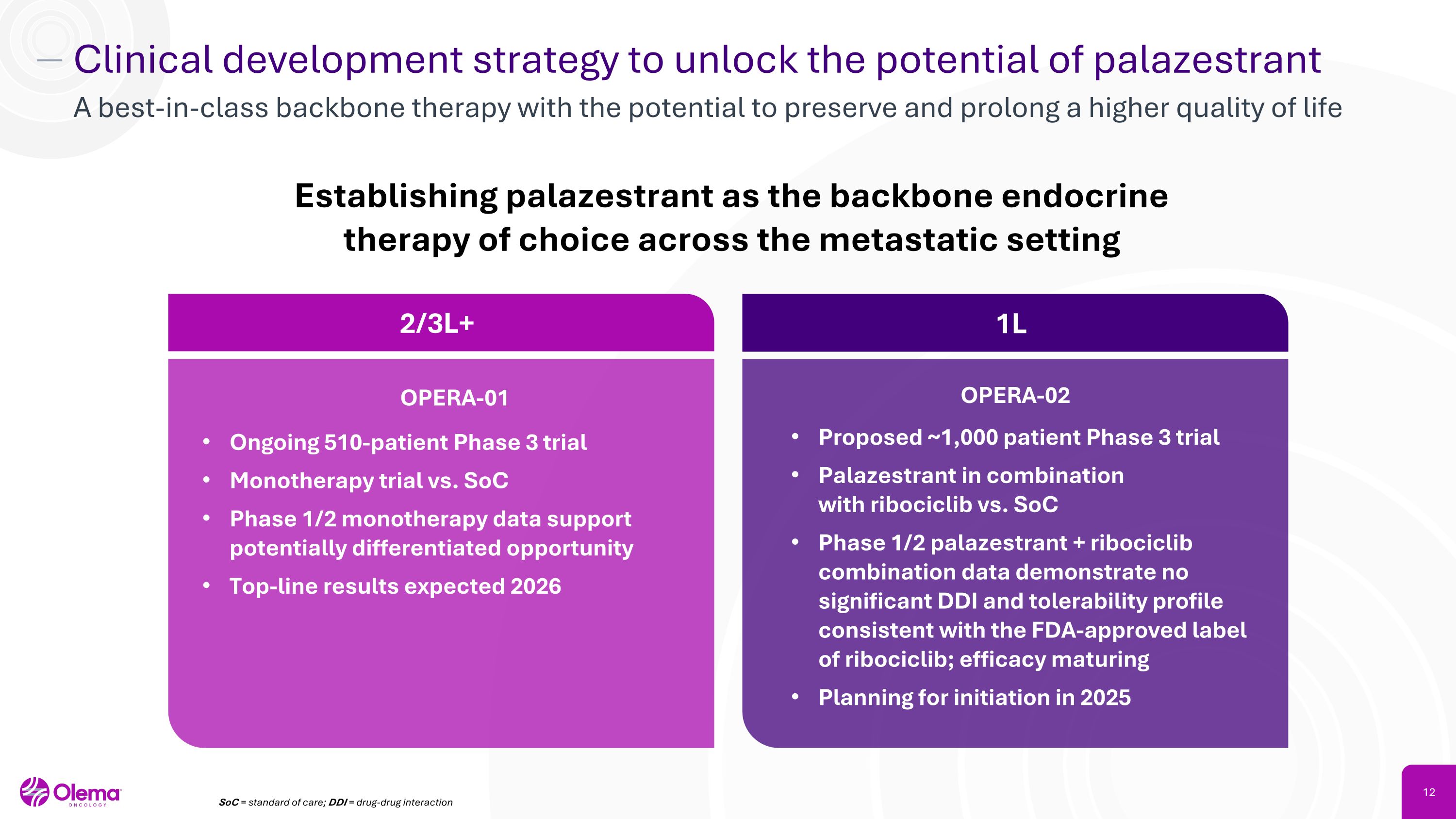
Clinical development strategy to unlock the potential of palazestrant A best-in-class backbone therapy with the potential to preserve and prolong a higher quality of life Establishing palazestrant as the backbone endocrine therapy of choice across the metastatic setting 2/3L+ 1L Ongoing 510-patient Phase 3 trial Monotherapy trial vs. SoC Phase 1/2 monotherapy data support potentially differentiated opportunity Top-line results expected 2026 OPERA-01 OPERA-02 Proposed ~1,000 patient Phase 3 trial Palazestrant in combination �with ribociclib vs. SoC Phase 1/2 palazestrant + ribociclib combination data demonstrate no significant DDI and tolerability profile consistent with the FDA-approved label �of ribociclib; efficacy maturing Planning for initiation in 2025 SoC = standard of care; DDI = drug-drug interaction
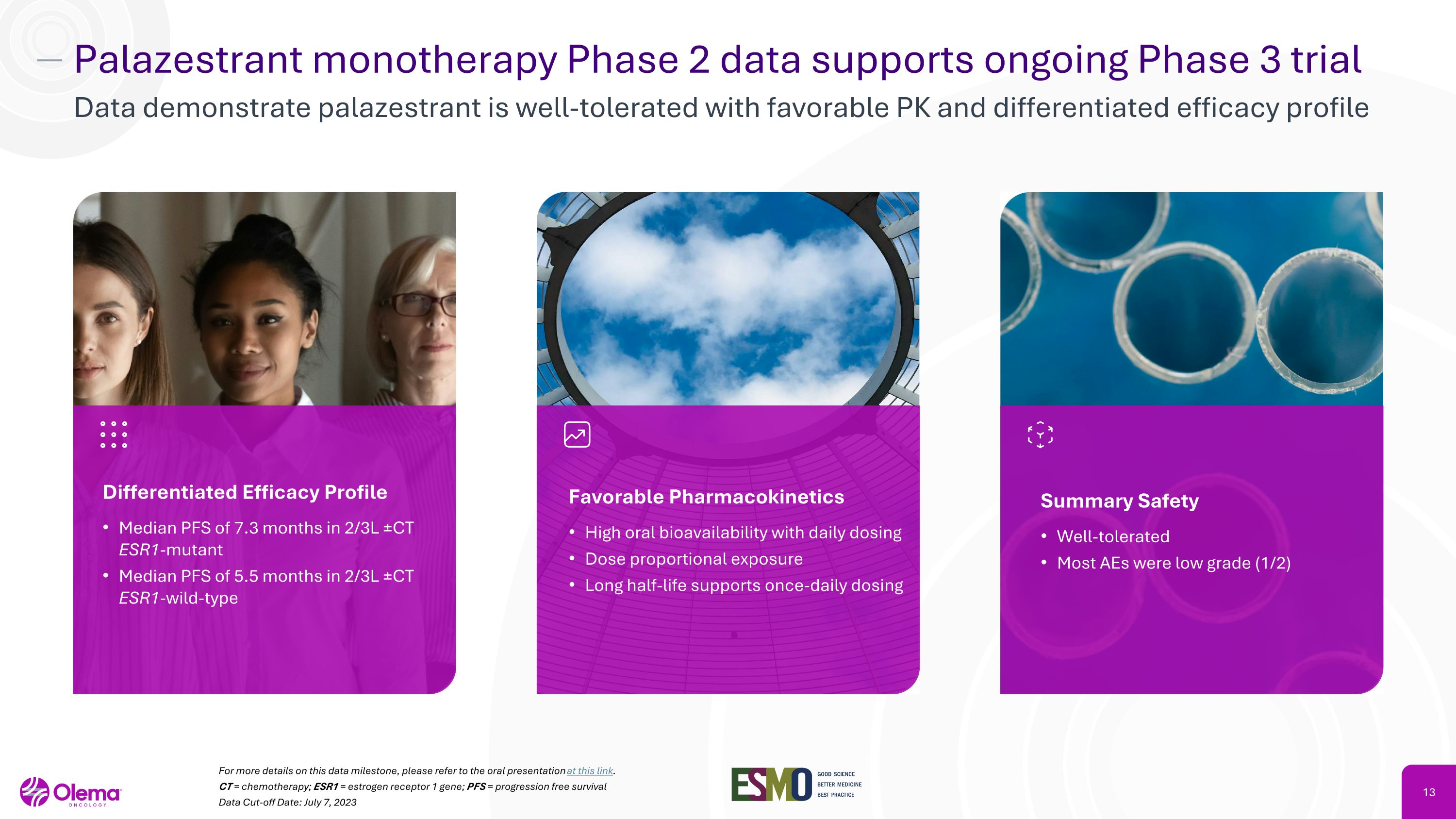
Palazestrant monotherapy Phase 2 data supports ongoing Phase 3 trial Data demonstrate palazestrant is well-tolerated with favorable PK and differentiated efficacy profile For more details on this data milestone, please refer to the oral presentation at this link. CT = chemotherapy; ESR1 = estrogen receptor 1 gene; PFS = progression free survival Data Cut-off Date: July 7, 2023 Summary Safety Well-tolerated Most AEs were low grade (1/2) Differentiated Efficacy Profile Median PFS of 7.3 months in 2/3L ±CT ESR1-mutant Median PFS of 5.5 months in 2/3L ±CT ESR1-wild-type Favorable Pharmacokinetics High oral bioavailability with daily dosing Dose proportional exposure Long half-life supports once-daily dosing
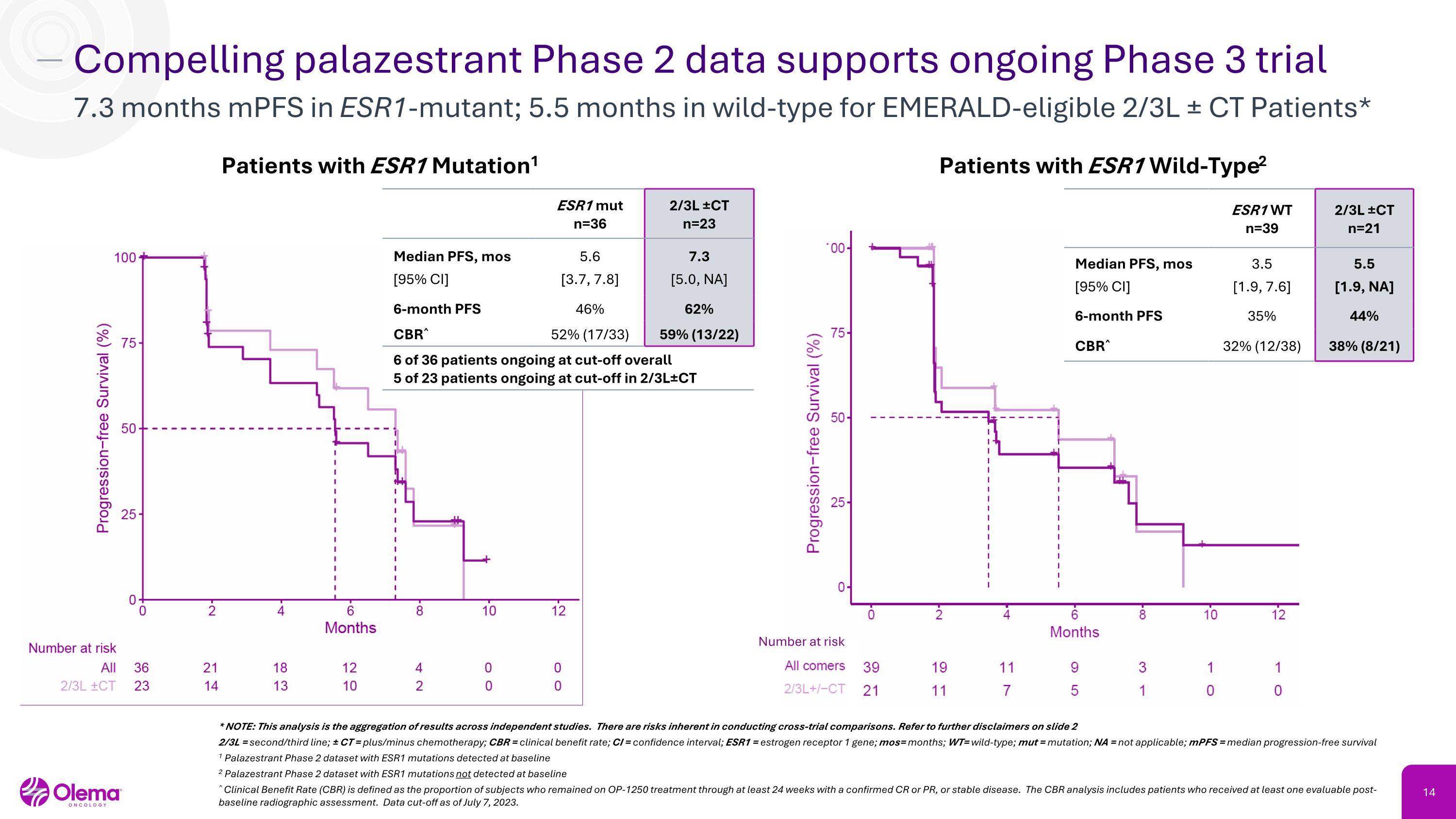
Compelling palazestrant Phase 2 data supports ongoing Phase 3 trial 7.3 months mPFS in ESR1-mutant; 5.5 months in wild-type for EMERALD-eligible 2/3L ± CT Patients* * NOTE: This analysis is the aggregation of results across independent studies. There are risks inherent in conducting cross-trial comparisons. Refer to further disclaimers on slide 2 2/3L = second/third line; ± CT = plus/minus chemotherapy; CBR = clinical benefit rate; CI = confidence interval; ESR1 = estrogen receptor 1 gene; mos= months; WT= wild-type; mut = mutation; NA = not applicable; mPFS = median progression-free survival 1 Palazestrant Phase 2 dataset with ESR1 mutations detected at baseline 2 Palazestrant Phase 2 dataset with ESR1 mutations not detected at baseline ^ Clinical Benefit Rate (CBR) is defined as the proportion of subjects who remained on OP-1250 treatment through at least 24 weeks with a confirmed CR or PR, or stable disease. The CBR analysis includes patients who received at least one evaluable post-baseline radiographic assessment. Data cut-off as of July 7, 2023. Patients with ESR1 Mutation1 ESR1 mut�n=36 2/3L ±CT�n=23 Median PFS, mos [95% CI] 5.6 [3.7, 7.8] 7.3 [5.0, NA] 6-month PFS 46% 62% CBR^ 52% (17/33) 59% (13/22) 6 of 36 patients ongoing at cut-off overall 5 of 23 patients ongoing at cut-off in 2/3L±CT Patients with ESR1 Wild-Type2 Number at risk ESR1 WT�n=39 2/3L ±CT�n=21 Median PFS, mos [95% CI] 3.5 [1.9, 7.6] 5.5 [1.9, NA] 6-month PFS 35% 44% CBR^ 32% (12/38) 38% (8/21)
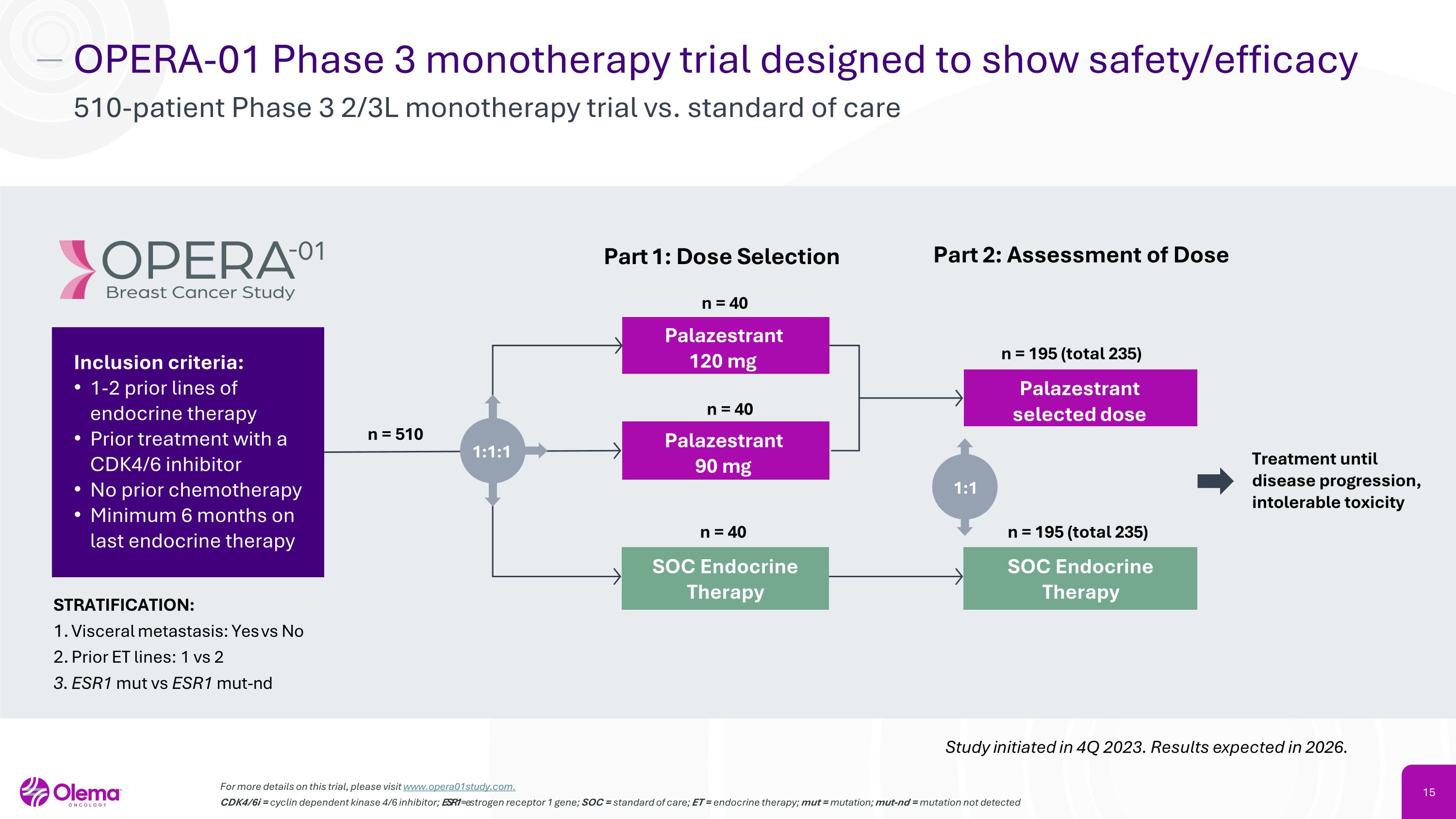
OPERA-01 Phase 3 monotherapy trial designed to show safety/efficacy 510-patient Phase 3 2/3L monotherapy trial vs. standard of care For more details on this trial, please visit www.opera01study.com. CDK4/6i = cyclin dependent kinase 4/6 inhibitor; ESR1 = estrogen receptor 1 gene; SOC = standard of care; ET = endocrine therapy; mut = mutation; mut-nd = mutation not detected Study initiated in 4Q 2023. Results expected in 2026. Palazestrant �120 mg STRATIFICATION: Visceral metastasis: Yes vs No Prior ET lines: 1 vs 2 ESR1 mut vs ESR1 mut-nd Palazestrant �90 mg n = 510 Palazestrant selected dose n = 40 Part 1: Dose Selection n = 40 Treatment until disease progression, intolerable toxicity n = 40 n = 195 (total 235) n = 195 (total 235) Part 2: Assessment of Dose 1:1:1 Inclusion criteria: 1-2 prior lines of endocrine therapy Prior treatment with a CDK4/6 inhibitor No prior chemotherapy Minimum 6 months on last endocrine therapy SOC Endocrine Therapy SOC Endocrine Therapy 1:1

— “Patients with metastatic breast cancer urgently await therapeutic advances that can be used effectively either as monotherapies or in combination with today’s standard of care to not only extend survival rates, but also deliver a better quality of life. With the body of evidence to date and our continued experience in clinical studies, palazestrant has strong potential to deliver on these persistent unmet needs.” Nancy Lin, M.D. Associate Chief, Division of Breast Oncology Dana-Farber Cancer Institute
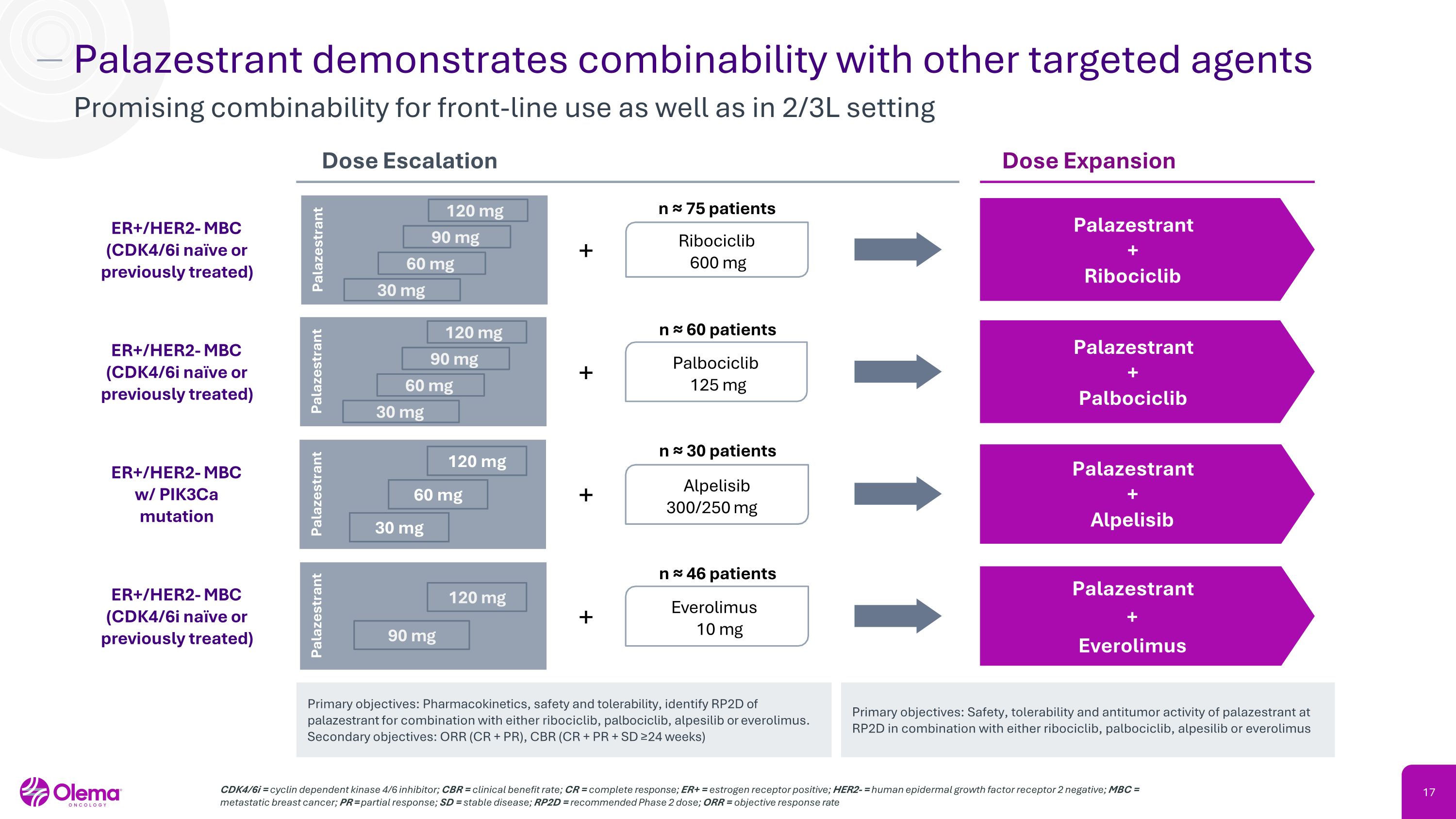
Palazestrant demonstrates combinability with other targeted agents Promising combinability for front-line use as well as in 2/3L setting CDK4/6i = cyclin dependent kinase 4/6 inhibitor; CBR = clinical benefit rate; CR = complete response; ER+ = estrogen receptor positive; HER2- = human epidermal growth factor receptor 2 negative; MBC = metastatic breast cancer; PR = partial response; SD = stable disease; RP2D = recommended Phase 2 dose; ORR = objective response rate + + ER+/HER2- MBC (CDK4/6i naïve or previously treated) ER+/HER2- MBC w/ PIK3Ca mutation Palazestrant + Ribociclib Palazestrant �+ �Alpelisib Ribociclib �600 mg Alpelisib 300/250 mg Dose Expansion Dose Escalation Primary objectives: Pharmacokinetics, safety and tolerability, identify RP2D of palazestrant for combination with either ribociclib, palbociclib, alpesilib or everolimus. Secondary objectives: ORR (CR + PR), CBR (CR + PR + SD ≥24 weeks) n ≈ 75 patients n ≈ 30 patients Palazestrant ER+/HER2- MBC (CDK4/6i naïve or previously treated) 90 mg 120 mg + Everolimus �10 mg n ≈ 46 patients + ER+/HER2- MBC (CDK4/6i naïve or previously treated) Palazestrant + Palbociclib Palbociclib �125 mg n ≈ 60 patients 30 mg 60 mg 120 mg 90 mg Palazestrant Palazestrant Primary objectives: Safety, tolerability and antitumor activity of palazestrant at RP2D in combination with either ribociclib, palbociclib, alpesilib or everolimus Palazestrant + Everolimus Palazestrant 120 mg 60 mg 30 mg 30 mg 60 mg 120 mg 90 mg Palazestrant
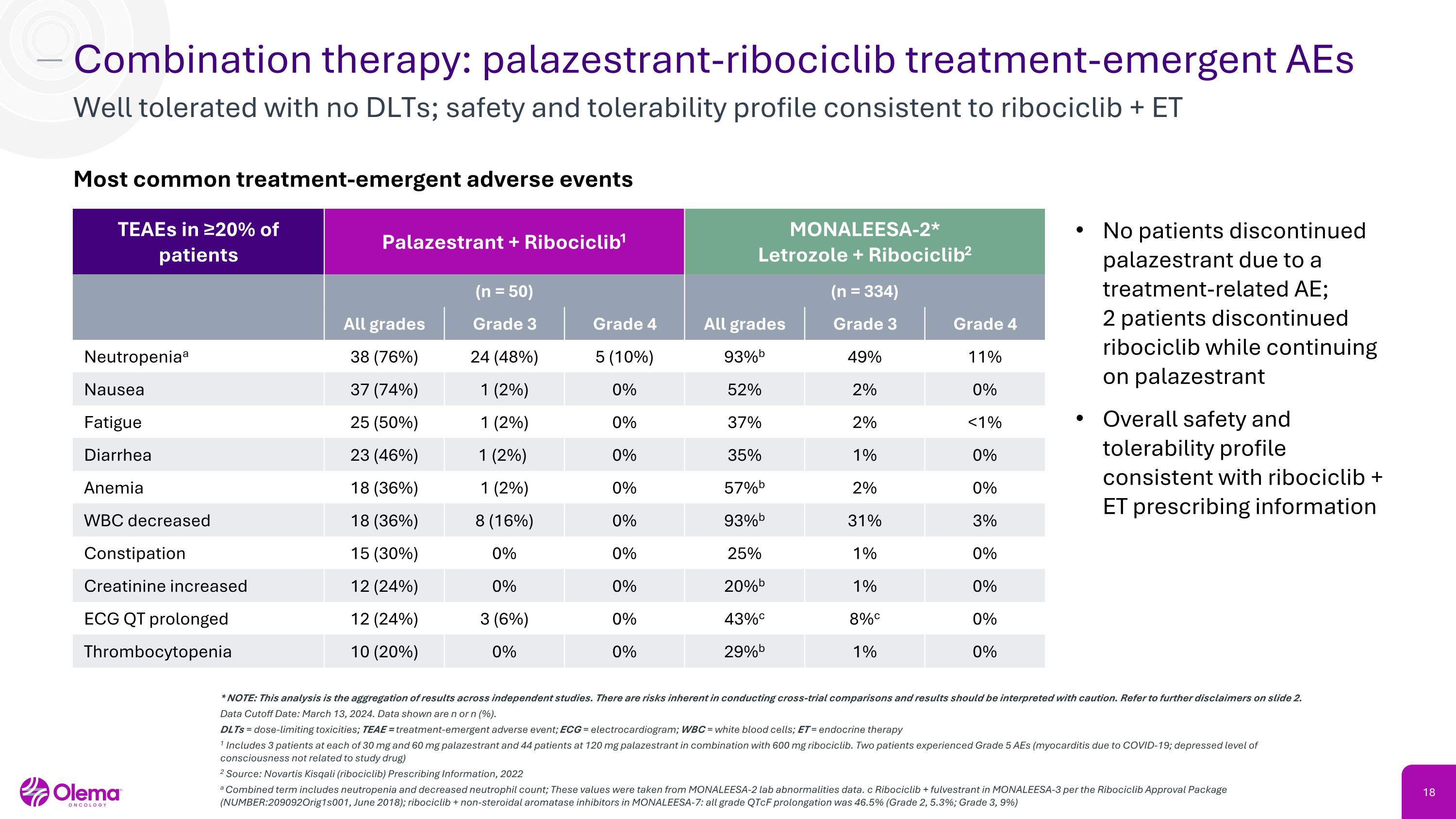
Combination therapy: palazestrant-ribociclib treatment-emergent AEs Well tolerated with no DLTs; safety and tolerability profile consistent to ribociclib + ET * NOTE: This analysis is the aggregation of results across independent studies. There are risks inherent in conducting cross-trial comparisons and results should be interpreted with caution. Refer to further disclaimers on slide 2. Data Cutoff Date: March 13, 2024. Data shown are n or n (%). DLTs = dose-limiting toxicities; TEAE = treatment-emergent adverse event; ECG = electrocardiogram; WBC = white blood cells; ET = endocrine therapy 1 Includes 3 patients at each of 30 mg and 60 mg palazestrant and 44 patients at 120 mg palazestrant in combination with 600 mg ribociclib. Two patients experienced Grade 5 AEs (myocarditis due to COVID-19; depressed level of consciousness not related to study drug) 2 Source: Novartis Kisqali (ribociclib) Prescribing Information, 2022 a Combined term includes neutropenia and decreased neutrophil count; These values were taken from MONALEESA-2 lab abnormalities data. c Ribociclib + fulvestrant in MONALEESA-3 per the Ribociclib Approval Package (NUMBER:209092Orig1s001, June 2018); ribociclib + non-steroidal aromatase inhibitors in MONALEESA-7: all grade QTcF prolongation was 46.5% (Grade 2, 5.3%; Grade 3, 9%) TEAEs in ≥20% of patients Palazestrant + Ribociclib1 MONALEESA-2*�Letrozole + Ribociclib2 (n = 50) (n = 334) All grades Grade 3 Grade 4 All grades Grade 3 Grade 4 Neutropeniaa 38 (76%) 24 (48%) 5 (10%) 93%b 49% 11% Nausea 37 (74%) 1 (2%) 0% 52% 2% 0% Fatigue 25 (50%) 1 (2%) 0% 37% 2% <1% Diarrhea 23 (46%) 1 (2%) 0% 35% 1% 0% Anemia 18 (36%) 1 (2%) 0% 57%b 2% 0% WBC decreased 18 (36%) 8 (16%) 0% 93%b 31% 3% Constipation 15 (30%) 0% 0% 25% 1% 0% Creatinine increased 12 (24%) 0% 0% 20%b 1% 0% ECG QT prolonged 12 (24%) 3 (6%) 0% 43%c 8%c 0% Thrombocytopenia 10 (20%) 0% 0% 29%b 1% 0% Most common treatment-emergent adverse events No patients discontinued palazestrant due to a treatment-related AE;�2 patients discontinued ribociclib while continuing on palazestrant Overall safety and tolerability profile consistent with ribociclib + ET prescribing information
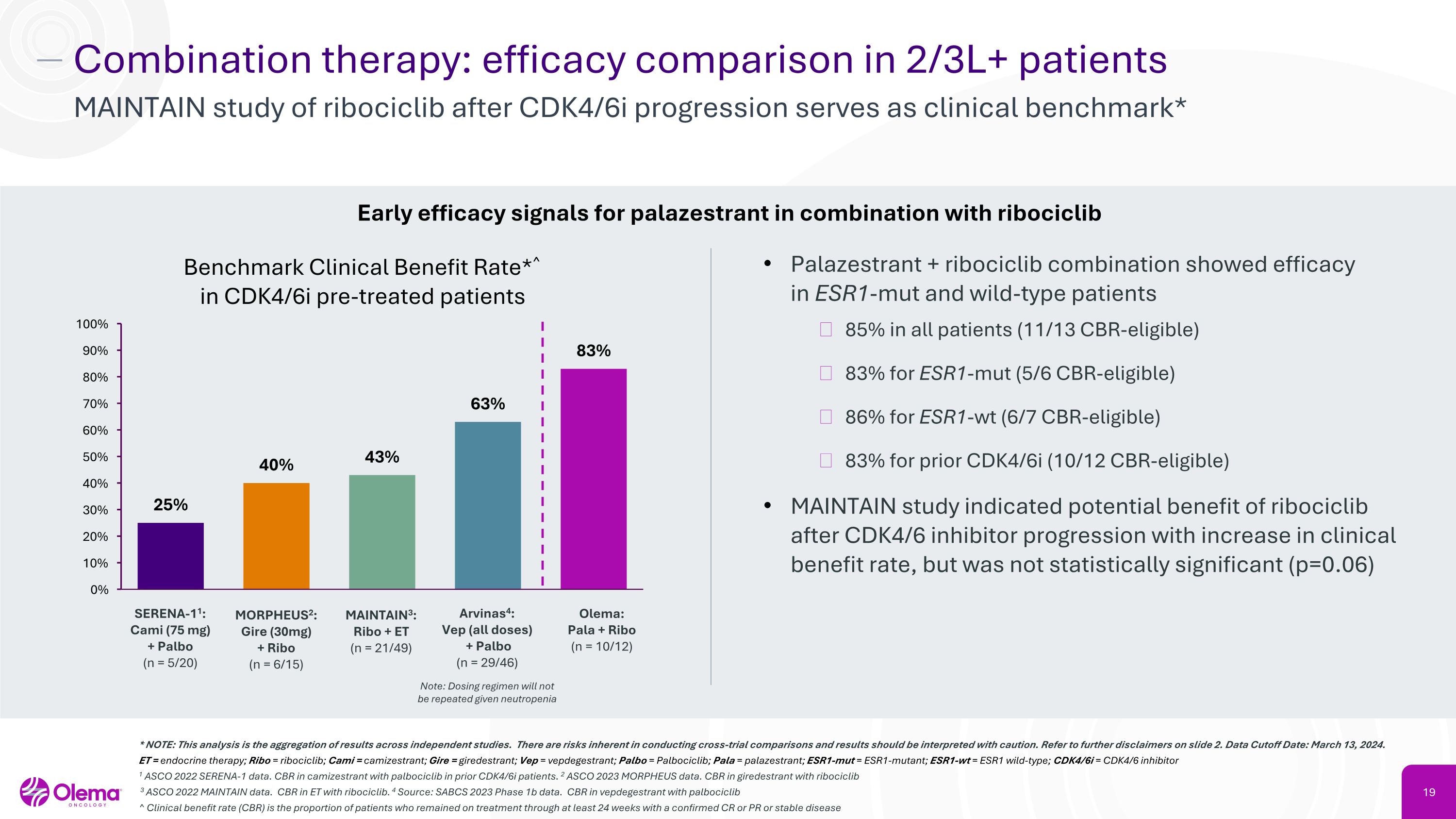
Combination therapy: efficacy comparison in 2/3L+ patients MAINTAIN study of ribociclib after CDK4/6i progression serves as clinical benchmark* Benchmark Clinical Benefit Rate*^�in CDK4/6i pre-treated patients Palazestrant + ribociclib combination showed efficacy �in ESR1-mut and wild-type patients 85% in all patients (11/13 CBR-eligible) 83% for ESR1-mut (5/6 CBR-eligible) 86% for ESR1-wt (6/7 CBR-eligible) 83% for prior CDK4/6i (10/12 CBR-eligible) MAINTAIN study indicated potential benefit of ribociclib after CDK4/6 inhibitor progression with increase in clinical benefit rate, but was not statistically significant (p=0.06) MAINTAIN3:�Ribo + ET�(n = 21/49) Olema: Pala + Ribo�(n = 10/12) Arvinas4: Vep (all doses)� + Palbo�(n = 29/46) Note: Dosing regimen will not be repeated given neutropenia Early efficacy signals for palazestrant in combination with ribociclib SERENA-11:�Cami (75 mg)�+ Palbo�(n = 5/20) MORPHEUS2:�Gire (30mg)�+ Ribo�(n = 6/15) * NOTE: This analysis is the aggregation of results across independent studies. There are risks inherent in conducting cross-trial comparisons and results should be interpreted with caution. Refer to further disclaimers on slide 2. Data Cutoff Date: March 13, 2024. ET = endocrine therapy; Ribo = ribociclib; Cami = camizestrant; Gire = giredestrant; Vep = vepdegestrant; Palbo = Palbociclib; Pala = palazestrant; ESR1-mut = ESR1-mutant; ESR1-wt = ESR1 wild-type; CDK4/6i = CDK4/6 inhibitor 1 ASCO 2022 SERENA-1 data. CBR in camizestrant with palbociclib in prior CDK4/6i patients. 2 ASCO 2023 MORPHEUS data. CBR in giredestrant with ribociclib 3 ASCO 2022 MAINTAIN data. CBR in ET with ribociclib. 4 Source: SABCS 2023 Phase 1b data. CBR in vepdegestrant with palbociclib ^ Clinical benefit rate (CBR) is the proportion of patients who remained on treatment through at least 24 weeks with a confirmed CR or PR or stable disease
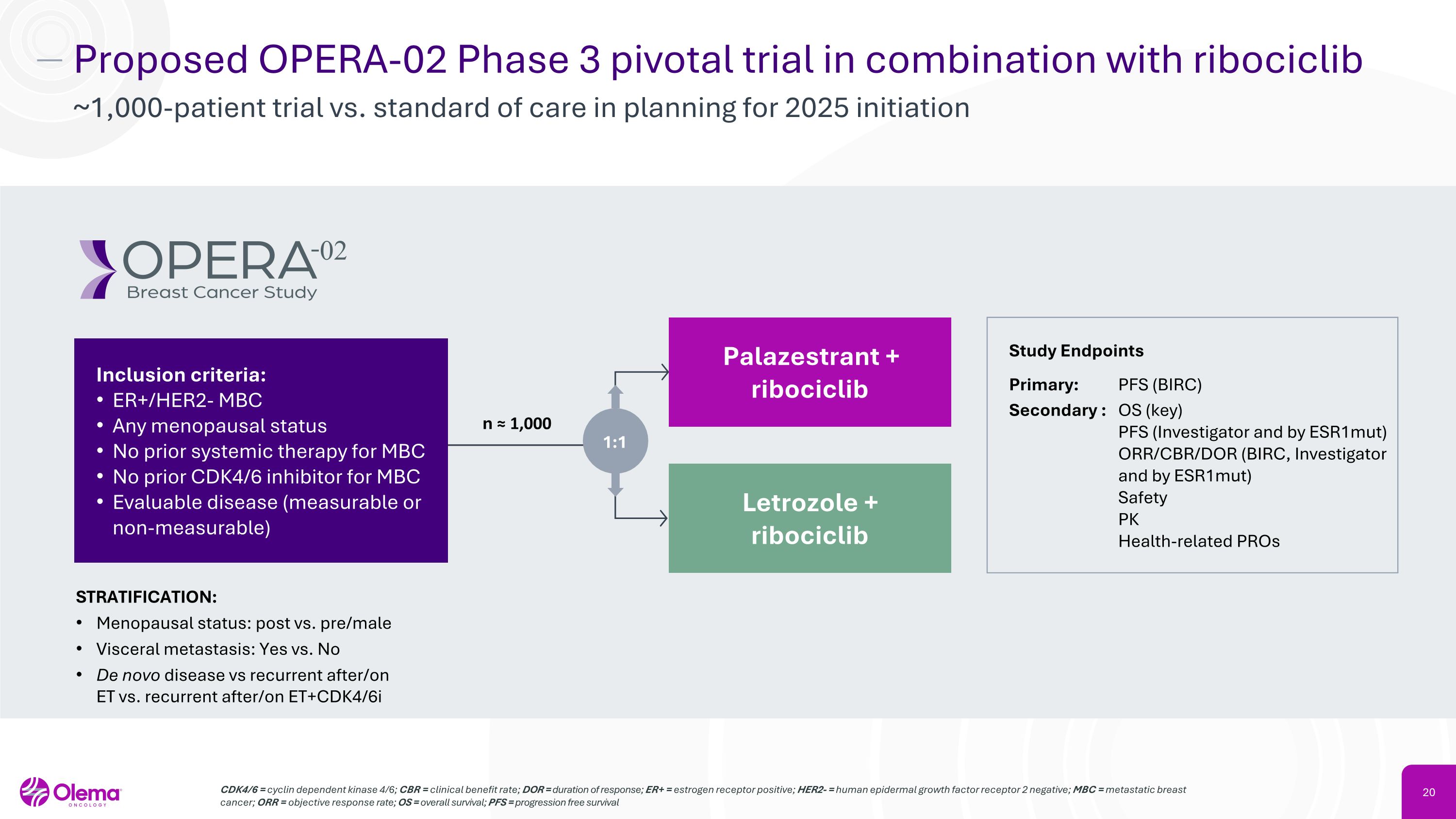
Proposed OPERA-02 Phase 3 pivotal trial in combination with ribociclib ~1,000-patient trial vs. standard of care in planning for 2025 initiation CDK4/6 = cyclin dependent kinase 4/6; CBR = clinical benefit rate; DOR = duration of response; ER+ = estrogen receptor positive; HER2- = human epidermal growth factor receptor 2 negative; MBC = metastatic breast cancer; ORR = objective response rate; OS = overall survival; PFS = progression free survival Study Endpoints Primary: PFS (BIRC) Secondary : OS (key) PFS (Investigator and by ESR1mut) ORR/CBR/DOR (BIRC, Investigator and by ESR1mut) Safety PK Health-related PROs STRATIFICATION: Menopausal status: post vs. pre/male Visceral metastasis: Yes vs. No De novo disease vs recurrent after/on ET vs. recurrent after/on ET+CDK4/6i n ≈ 1,000 Palazestrant +�ribociclib Letrozole +�ribociclib Inclusion criteria: ER+/HER2- MBC Any menopausal status No prior systemic therapy for MBC No prior CDK4/6 inhibitor for MBC Evaluable disease (measurable or non-measurable) 1:1
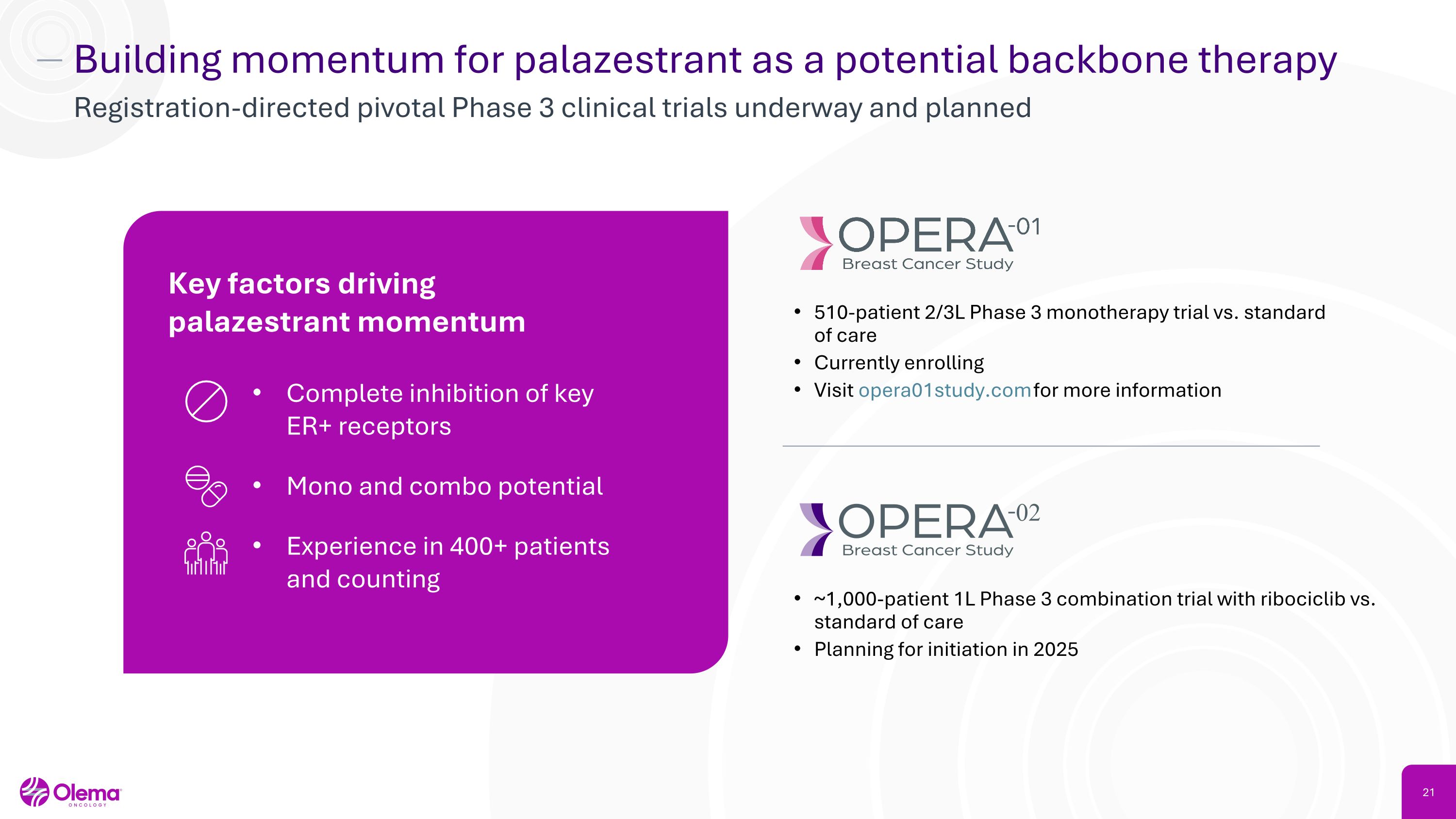
Building momentum for palazestrant as a potential backbone therapy Registration-directed pivotal Phase 3 clinical trials underway and planned 510-patient 2/3L Phase 3 monotherapy trial vs. standard of care Currently enrolling Visit opera01study.com for more information ~1,000-patient 1L Phase 3 combination trial with ribociclib vs. standard of care Planning for initiation in 2025 Key factors driving palazestrant momentum Complete inhibition of key �ER+ receptors Mono and combo potential Experience in 400+ patients �and counting
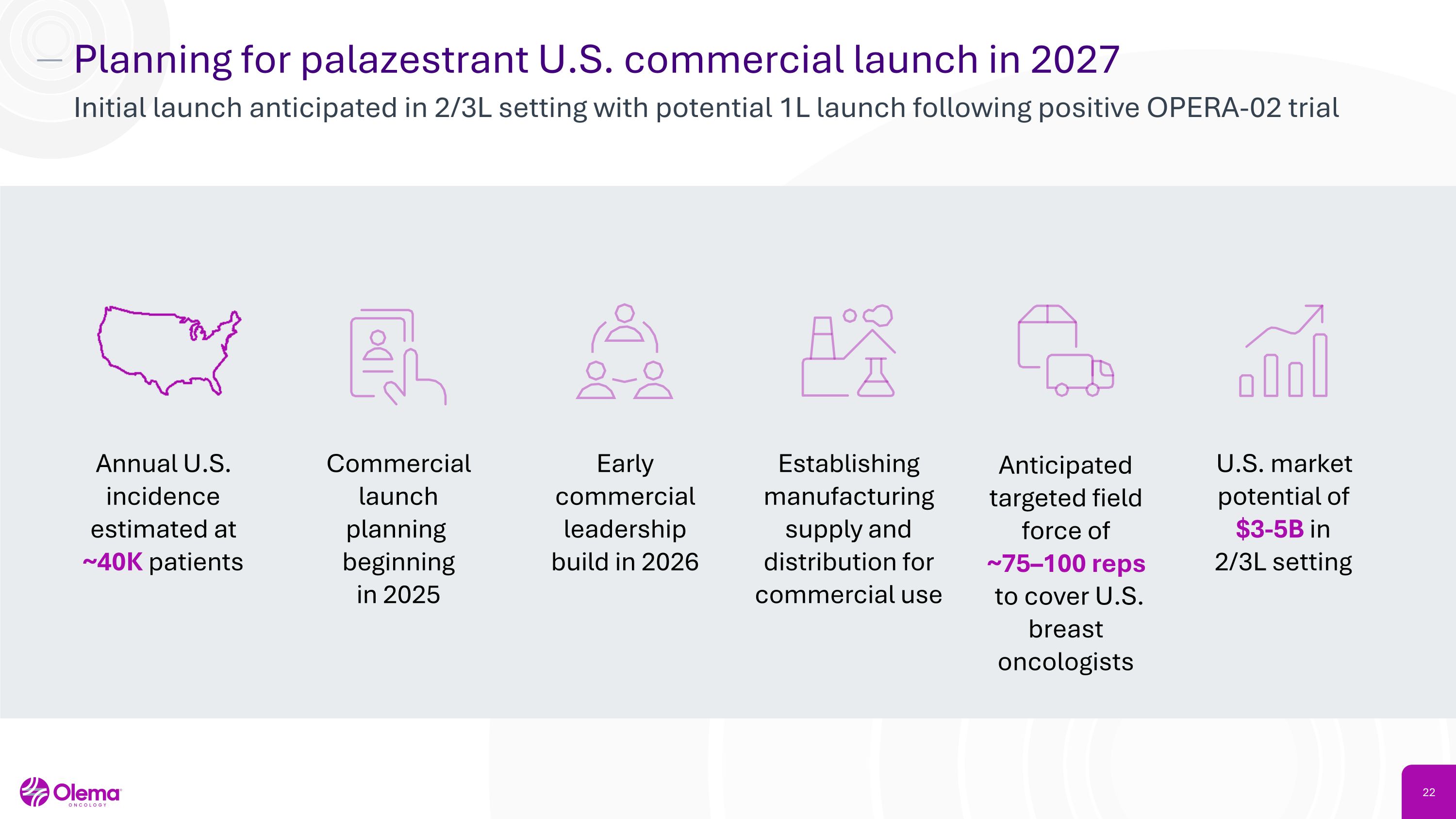
Planning for palazestrant U.S. commercial launch in 2027 Initial launch anticipated in 2/3L setting with potential 1L launch following positive OPERA-02 trial Annual U.S. incidence estimated at ~40K patients Commercial launch planning �beginning �in 2025 Early commercial leadership build in 2026 Establishing manufacturing supply and distribution for commercial use Anticipated targeted field force of �~75–100 reps to cover U.S. breast oncologists U.S. market potential of $3-5B in �2/3L setting

— KAT6 Inhibitor� (OP-3136)
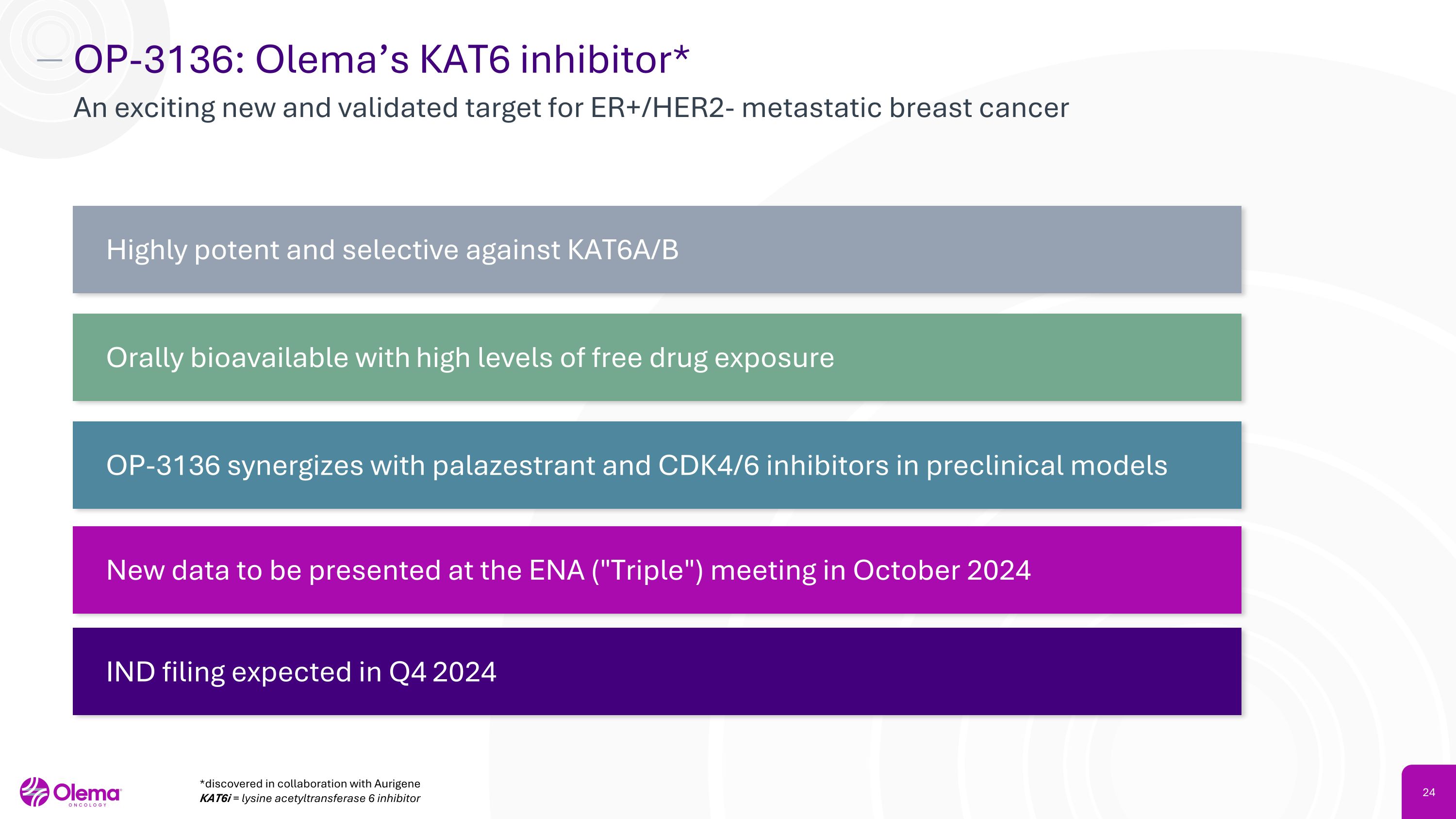
OP-3136: Olema’s KAT6 inhibitor* An exciting new and validated target for ER+/HER2- metastatic breast cancer Highly potent and selective against KAT6A/B Orally bioavailable with high levels of free drug exposure OP-3136 synergizes with palazestrant and CDK4/6 inhibitors in preclinical models New data to be presented at the ENA ("Triple") meeting in October 2024 IND filing expected in Q4 2024 *discovered in collaboration with Aurigene KAT6i = lysine acetyltransferase 6 inhibitor
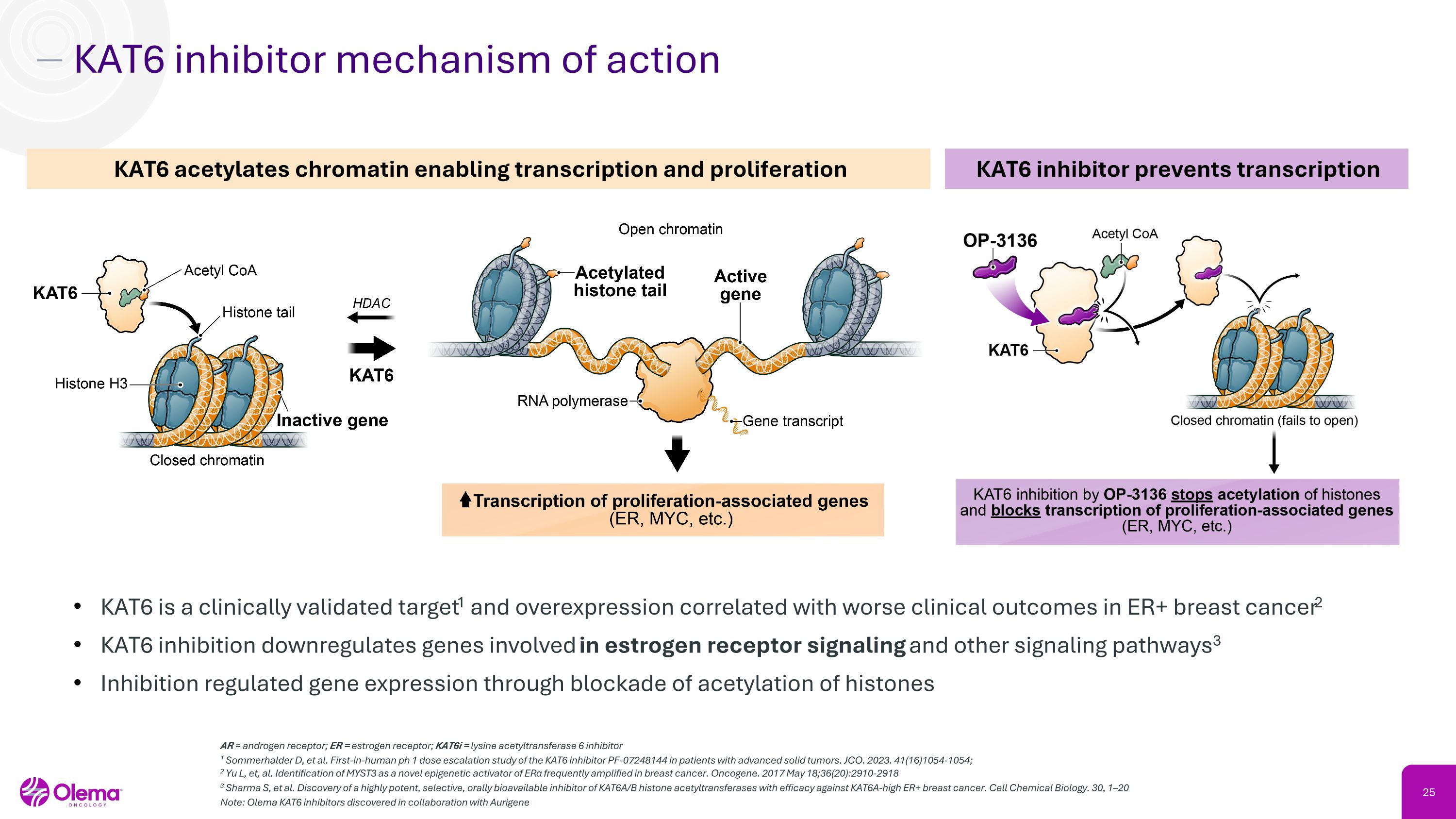
KAT6 inhibitor mechanism of action KAT6 is a clinically validated target1 and overexpression correlated with worse clinical outcomes in ER+ breast cancer2 KAT6 inhibition downregulates genes involved in estrogen receptor signaling and other signaling pathways3 Inhibition regulated gene expression through blockade of acetylation of histones KAT6 acetylates chromatin enabling transcription and proliferation KAT6 inhibitor prevents transcription AR = androgen receptor; ER = estrogen receptor; KAT6i = lysine acetyltransferase 6 inhibitor 1 Sommerhalder D, et al. First-in-human ph 1 dose escalation study of the KAT6 inhibitor PF-07248144 in patients with advanced solid tumors. JCO. 2023. 41(16)1054-1054; �2 Yu L, et, al. Identification of MYST3 as a novel epigenetic activator of ERα frequently amplified in breast cancer. Oncogene. 2017 May 18;36(20):2910-2918 3 Sharma S, et al. Discovery of a highly potent, selective, orally bioavailable inhibitor of KAT6A/B histone acetyltransferases with efficacy against KAT6A-high ER+ breast cancer. Cell Chemical Biology. 30, 1–20 Note: Olema KAT6 inhibitors discovered in collaboration with Aurigene
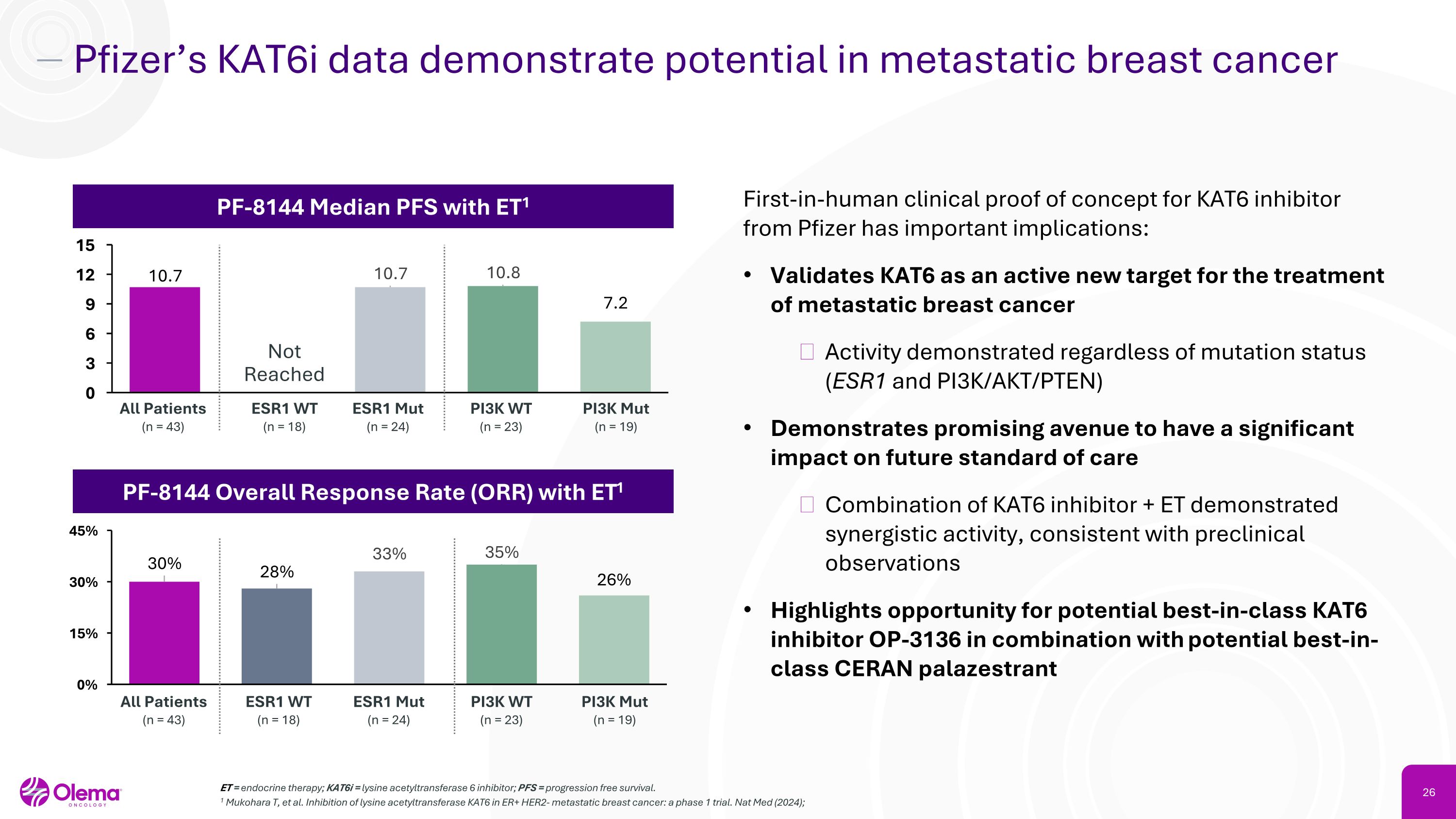
Pfizer’s KAT6i data demonstrate potential in metastatic breast cancer ET = endocrine therapy; KAT6i = lysine acetyltransferase 6 inhibitor; PFS = progression free survival. 1 Mukohara T, et al. Inhibition of lysine acetyltransferase KAT6 in ER+ HER2- metastatic breast cancer: a phase 1 trial. Nat Med (2024); PF-8144 Median PFS with ET1 ESR1 WT�(n = 18) All Patients�(n = 43) ESR1 Mut�(n = 24) PI3K WT�(n = 23) PI3K Mut�(n = 19) PF-8144 Overall Response Rate (ORR) with ET1 Not�Reached First-in-human clinical proof of concept for KAT6 inhibitor from Pfizer has important implications: Validates KAT6 as an active new target for the treatment of metastatic breast cancer Activity demonstrated regardless of mutation status �(ESR1 and PI3K/AKT/PTEN) Demonstrates promising avenue to have a significant impact on future standard of care Combination of KAT6 inhibitor + ET demonstrated synergistic activity, consistent with preclinical observations Highlights opportunity for potential best-in-class KAT6 inhibitor OP-3136 in combination with potential best-in-class CERAN palazestrant ESR1 WT�(n = 18) All Patients�(n = 43) ESR1 Mut�(n = 24) PI3K WT�(n = 23) PI3K Mut�(n = 19)
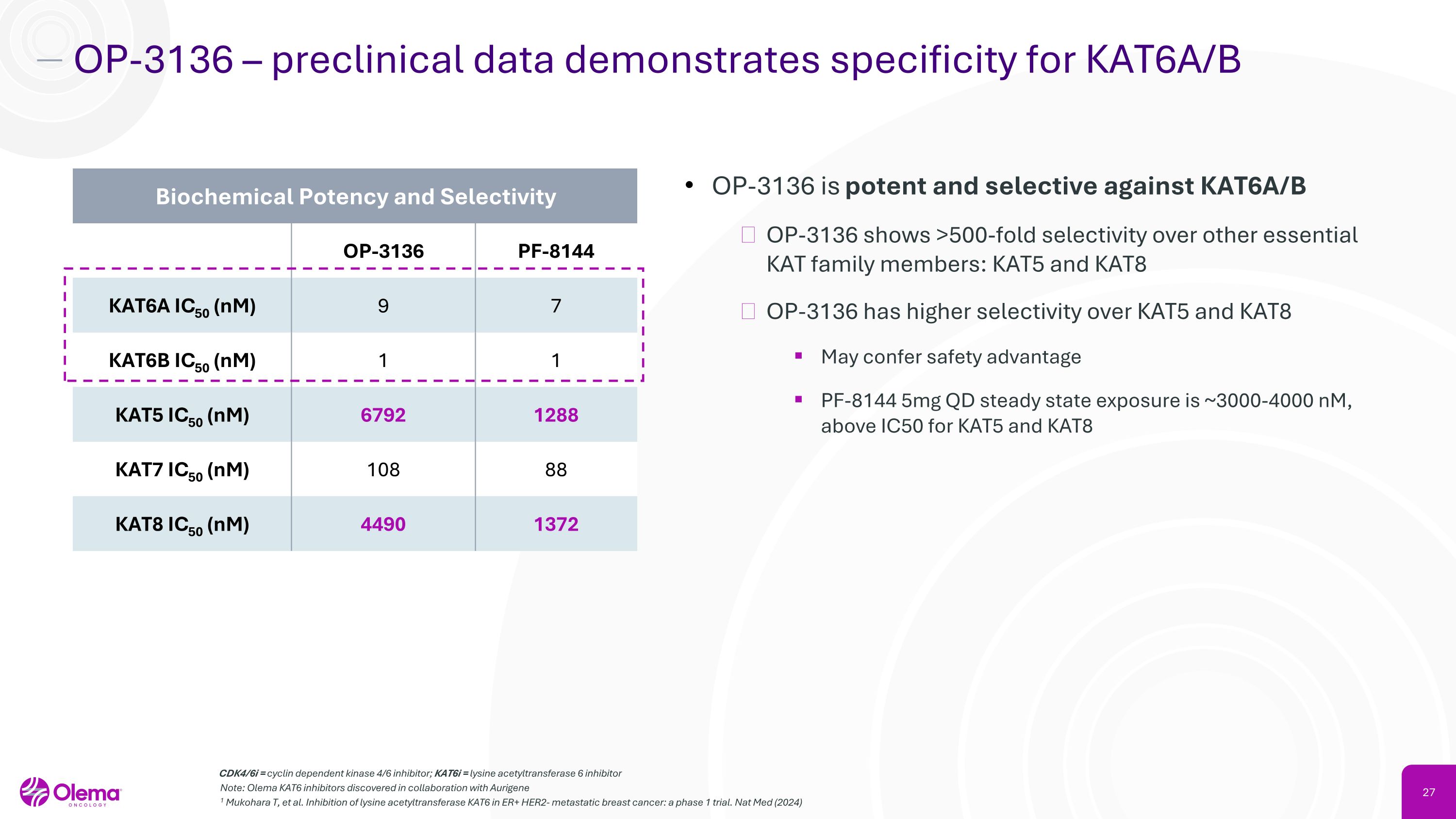
OP-3136 – preclinical data demonstrates specificity for KAT6A/B CDK4/6i = cyclin dependent kinase 4/6 inhibitor; KAT6i = lysine acetyltransferase 6 inhibitor Note: Olema KAT6 inhibitors discovered in collaboration with Aurigene 1 Mukohara T, et al. Inhibition of lysine acetyltransferase KAT6 in ER+ HER2- metastatic breast cancer: a phase 1 trial. Nat Med (2024) OP-3136 is potent and selective against KAT6A/B OP-3136 shows >500-fold selectivity over other essential KAT family members: KAT5 and KAT8 OP-3136 has higher selectivity over KAT5 and KAT8 May confer safety advantage PF-8144 5mg QD steady state exposure is ~3000-4000 nM, above IC50 for KAT5 and KAT8 Biochemical Potency and Selectivity OP-3136 PF-8144 KAT6A IC50 (nM) 9 7 KAT6B IC50 (nM) 1 1 KAT5 IC50 (nM) 6792 1288 KAT7 IC50 (nM) 108 88 KAT8 IC50 (nM) 4490 1372
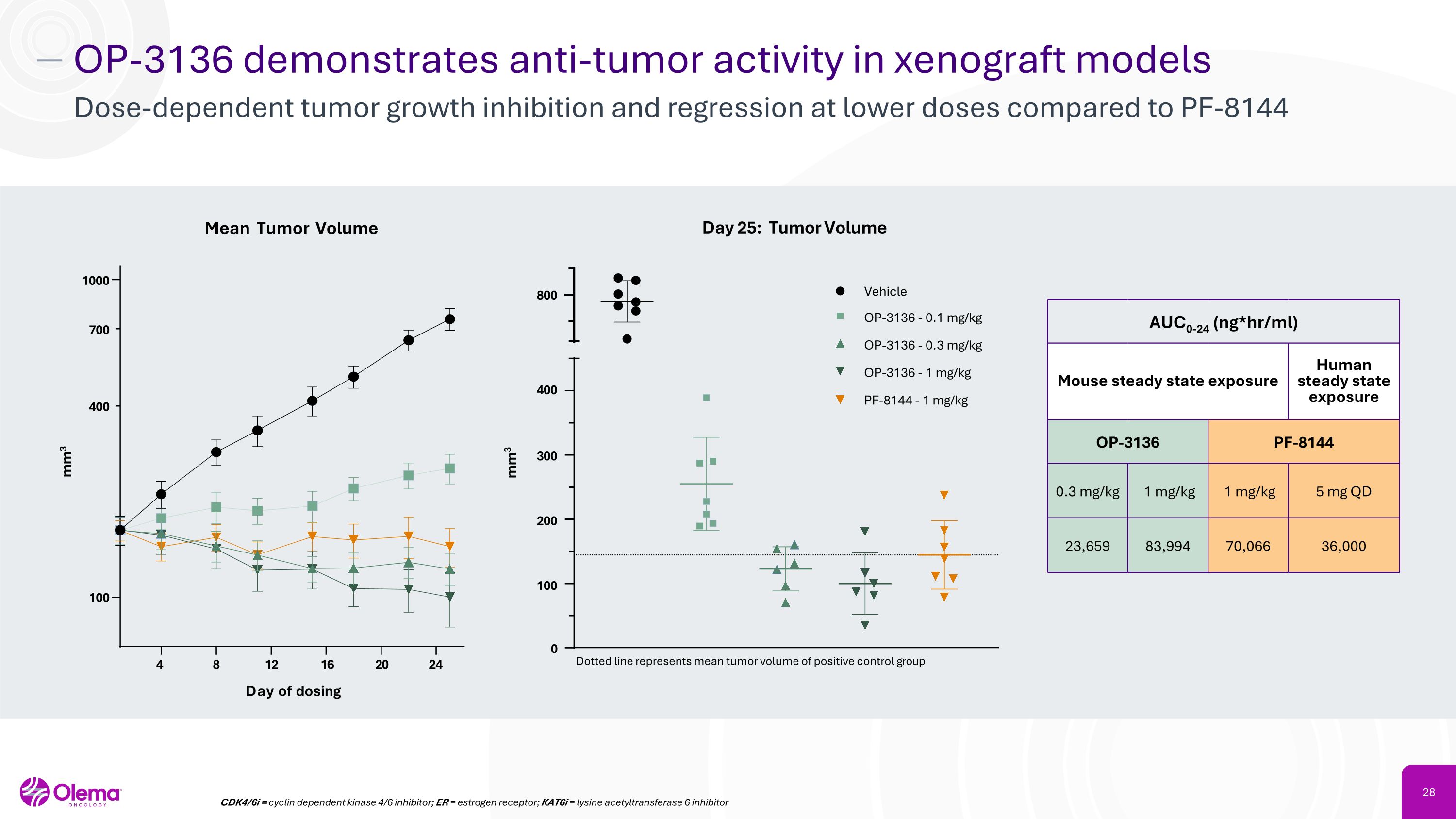
OP-3136 demonstrates anti-tumor activity in xenograft models Dose-dependent tumor growth inhibition and regression at lower doses compared to PF-8144 4 8 24 100 1000 Day of dosing mm3 Mean Tumor Volume 400 700 12 16 20 0 100 300 400 800 mm3 Day 25: Tumor Volume Vehicle OP-3136 - 0.1 mg/kg OP-3136 - 0.3 mg/kg OP-3136 - 1 mg/kg PF-8144 - 1 mg/kg Dotted line represents mean tumor volume of positive control group 200 CDK4/6i = cyclin dependent kinase 4/6 inhibitor; ER = estrogen receptor; KAT6i = lysine acetyltransferase 6 inhibitor AUC0-24 (ng*hr/ml) Mouse steady state exposure Human steady state exposure OP-3136 PF-8144 0.3 mg/kg 1 mg/kg 1 mg/kg 5 mg QD 23,659 83,994 70,066 36,000
Olema: a compelling late-stage opportunity in breast cancer and beyond Changing the treatment paradigm for endocrine-driven cancers 1 Cash position as of June 30, 2024, includes the Company’s cash, cash equivalents, and marketable securities. Palazestrant anchors Olema’s breast cancer program as a potential backbone therapy for metastatic breast cancer Highly differentiated as first oral CERAN endocrine agent Ongoing 2/3L OPERA-01 Phase 3 trial positioned for success based on strong Phase 2 data Planning 1L OPERA-02 Phase 3 trial in combination with ribociclib Go-to-market strategy for potential U.S. launch in 2027 OP-3136 expands pipeline with opportunity against novel and validated KAT6 target Well-capitalized with ~$239M of cash and cash equivalents as of June 30, 20241

— Advancing medicines for breast cancer and beyond
v3.24.3
Document And Entity Information
|
Sep. 27, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Sep. 27, 2024
|
| Entity Registrant Name |
Olema Pharmaceuticals, Inc.
|
| Entity Central Index Key |
0001750284
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-39712
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
30-0409740
|
| Entity Address, Address Line One |
780 Brannan Street
|
| Entity Address, City or Town |
San Francisco
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
94103
|
| City Area Code |
415
|
| Local Phone Number |
651-3316
|
| Entity Information, Former Legal or Registered Name |
N/A
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
OLMA
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Olema Pharmaceuticals (NASDAQ:OLMA)
Historical Stock Chart
From Oct 2024 to Nov 2024

Olema Pharmaceuticals (NASDAQ:OLMA)
Historical Stock Chart
From Nov 2023 to Nov 2024


