0001382101false00013821012024-01-042024-01-04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 4, 2024 |
SUTRO BIOPHARMA, INC.
(Exact name of registrant as specified in its charter)
|
|
|
Delaware |
001-38662 |
47-0926186 |
(State or other jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
111 Oyster Point Blvd.
South San Francisco, California, 94080
(Address of principal executive offices) (Zip Code)
(650) 881-6500
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common stock, $0.001 par value |
|
STRO |
|
The NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On January 8, 2024, Sutro Biopharma, Inc. (the “Company”) will be disclosing certain financial information about the Company’s estimated cash balance and estimated fair value of its Vaxcyte common stock holdings as of December 31, 2023. The Company will report the preliminary, unaudited amount of the Company’s cash, cash equivalents and marketable securities as of December 31, 2023, as approximately $333 million, and that the Company held approximately 0.7 million shares of Vaxcyte common stock with an estimated fair value of approximately $42 million as of December 31, 2023, which together the Company expects will enable it to fund its operations into the second half of 2025, based on current business plans and assumptions. The amounts are preliminary, unaudited and may change, were prepared by management and were based on the most current information available to management, and are subject to completion by management of the financial statements as of and for the year ended December 31, 2023, including performance of the Company’s financial closing procedures, any final adjustments and other developments that may arise between now and the time the financial results for this period are finalized, and the completion of the external audit of such financial statements. The Company’s independent registered public accounting firm has not audited, reviewed, compiled, or applied agreed-upon procedures with respect to the preliminary financial data included herein. Accordingly, the Company’s independent registered public accounting firm does not express an opinion or any other form of assurance with respect thereto.
Item 7.01 Regulation FD Disclosure.
On January 8, 2024, the Company will be disclosing an updated corporate presentation. A copy of the corporate presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K. The corporate presentation will also be available on the Company’s website in the Investors section at https://www.sutrobio.com/corporate-presentation/.
The information in this Item 2.02 and Item 7.01 of this report, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (“Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 8.01 Other Events.
Additionally, on January 4, 2024, the Company announced updated data and certain milestones for luveltamab tazevibulin (luvelta), a novel folate receptor-α (FolRα) targeting antibody drug conjugate (“ADC”).
luvelta FolRα-targeting ADC Franchise Upcoming Milestones:
•The registration-directed trial, REFRαME-O1, in platinum-resistant ovarian cancer, is enrolling with 26 active sites across 5 countries and an anticipated approximately 140 sites in approximately 20 countries by the end of 2024. Part 1 of the trial is expected to be completed in the first half of 2024.
•Initiation of REFRαME-P1, a registration-enabling trial for pediatric patients with CBF/GLIS AML, is planned for the first half of 2024.
•An Investigational New Drug application submission is planned in non-small cell lung cancer (“NSCLC”) in the first half of 2024.
•Continued clinical development is planned in endometrial cancer and in combination with bevacizumab for the treatment of ovarian cancer.
Updated luvelta Data:
•An aggregated analysis of nearly 100 women with ovarian cancer from Company’s Phase 1 program led to the following observations:
•Treatment with luvelta demonstrated improved clinical outcomes and tolerability compared to historical results with standard of care chemotherapy in an evaluable patient population matching the eligibility criteria for the REFRαME-O1 trial.
•The safety profile across the aggregated analysis remained consistent with previously reported data.
•Safety data from an additional cohort with prophylactic G-CSF treatment showed significant reduction of neutropenia and resulting dose delays.
•New data in combination with bevacizumab demonstrated clinical activity in treated patients regardless of FolRα expression level.
•Preclinical data in a model of NSCLC demonstrated that a single dose of luvelta produced potent anti-tumor activity and that the combination of luvelta and PD-1 blockade (avelumab) demonstrated benefit and complete tumor regression.
•Promising clinical data in late-stage endometrial cancer and CBF/GLIS AML have been presented at ESMO and ASH in 2023.
This Current Report on Form 8-K contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to, anticipated preclinical and clinical development activities, including enrollment and site activation; timing of announcements of clinical results, trial initiation, and regulatory filings; outcome of regulatory decisions; the Company’s expectations about its cash runway; potential benefits of luvelta and the Company’s other product candidates and platform; potential expansion into other indications and combinations, including the timing and development activities related to such expansion; and potential market opportunities for luvelta and the Company’s other product candidates. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, the Company cannot guarantee future events, results, actions, levels of activity, performance or achievements, and the timing and results of biotechnology development and potential regulatory approval is inherently uncertain. Forward-looking statements are subject to risks and uncertainties that may cause the Company’s actual activities or results to differ significantly from those expressed in any forward-looking statement, including risks and uncertainties related to the Company’s ability to advance its product candidates, the receipt and timing of potential regulatory designations, approvals and commercialization of product candidates and the Company’s ability to successfully leverage Fast Track designation, the market size for the Company’s product candidates to be smaller than anticipated, clinical trial sites, supply chain and manufacturing facilities, the Company’s ability to maintain and recognize the benefits of certain designations received by product candidates, the timing and results of preclinical and clinical trials, the Company’s ability to fund development activities and achieve development goals, the Company’s ability to protect intellectual property, the value of the Company’s holdings of Vaxcyte common stock, and the Company’s commercial collaborations with third parties and other risks and uncertainties described under the heading “Risk Factors” in documents the Company files from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this press release, and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof.
|
|
Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
|
|
|
Exhibit Number |
|
Description |
99.1 |
|
Corporate Presentation |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Sutro Biopharma, Inc. |
|
|
|
|
Date: |
January 8, 2024 |
By: |
/s/ Edward Albini |
|
|
|
Edward Albini
Chief Financial Officer |
Company Overview January 2024 Sutro Biopharma�NASDAQ: STRO Exhibit 99.1

Forward-Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance; business plans and objectives; anticipated preclinical and clinical development activities, including enrollment and site activation; timing of announcements of clinical results, trial initiation, and regulatory filings; outcome of regulatory decisions; our expectations about our cash runway; potential benefits of luvelta and our other product candidates and platform; potential expansion into other indications and combinations, including the timing and development activities related to such expansion; potential growth opportunities, financing plans, potential future milestone and royalty payments, competitive position, industry environment and potential market opportunities for the Company’s product candidates. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors, including risks and uncertainties related to our cash forecasts, our and our collaborators’ ability to advance our product candidates, the receipt, feedback and timing of potential regulatory submissions, designations, approvals and commercialization of product candidates, the design, timing and results of preclinical and clinical trials, and the expected impact of the COVID-19 pandemic on our operations. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that may be described in greater detail under the heading “Risk Factors” contained in our most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and other reports the company files from time to time with the Securities and Exchange Commission, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we nor our management assume responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to publicly update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
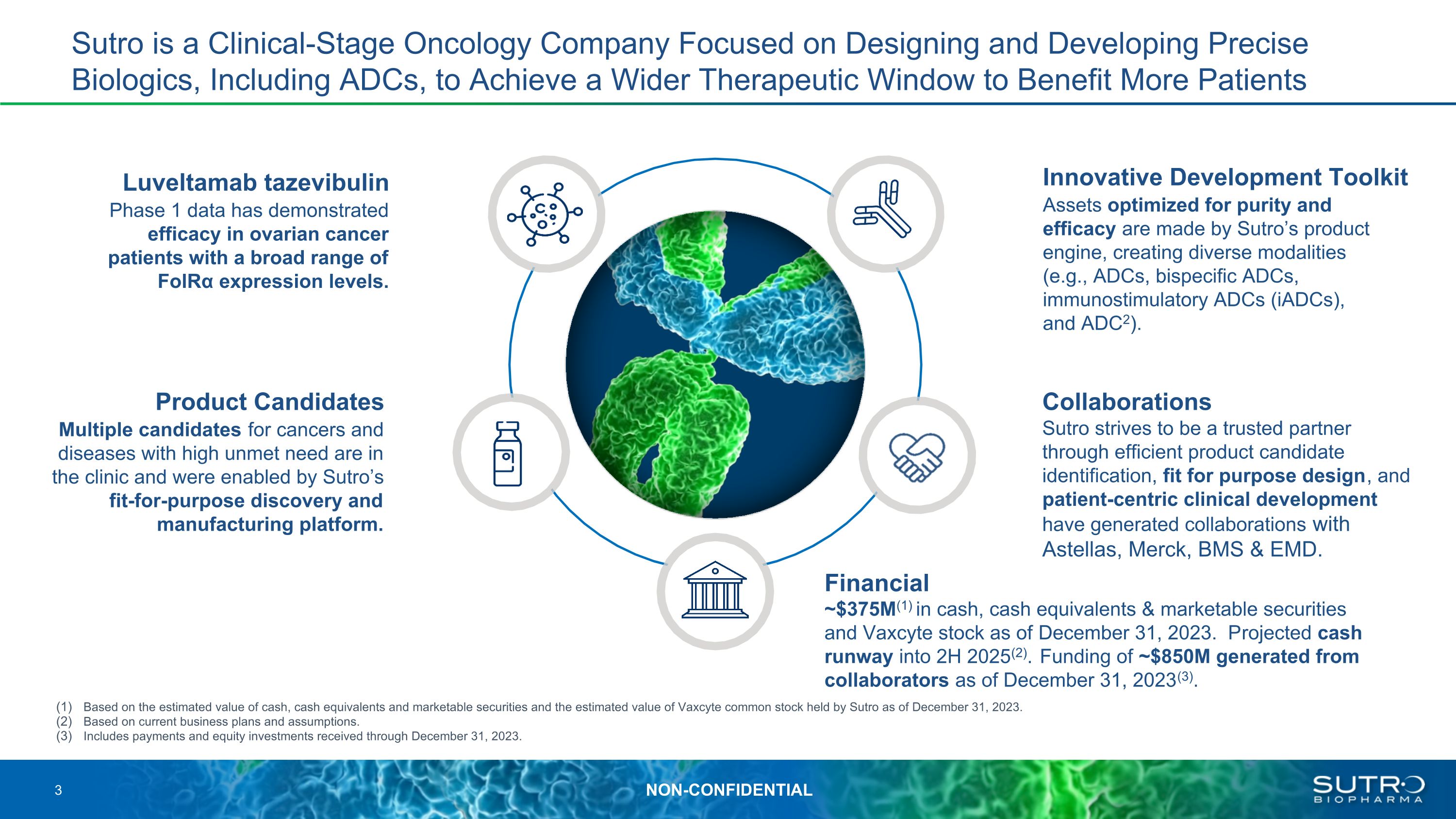
Luveltamab tazevibulin Phase 1 data has demonstrated efficacy in ovarian cancer patients with a broad range of FolRα expression levels. Innovative Development Toolkit Assets optimized for purity and efficacy are made by Sutro’s product engine, creating diverse modalities (e.g., ADCs, bispecific ADCs, immunostimulatory ADCs (iADCs), and ADC2). Financial Product Candidates Multiple candidates for cancers and diseases with high unmet need are in the clinic and were enabled by Sutro’s fit-for-purpose discovery and manufacturing platform. Sutro is a Clinical-Stage Oncology Company Focused on Designing and Developing Precise Biologics, Including ADCs, to Achieve a Wider Therapeutic Window to Benefit More Patients Collaborations Sutro strives to be a trusted partner through efficient product candidate identification, fit for purpose design, and patient-centric clinical development have generated collaborations with Astellas, Merck, BMS & EMD. ~$375M(1) in cash, cash equivalents & marketable securities and Vaxcyte stock as of December 31, 2023. Projected cash runway into 2H 2025(2). Funding of ~$850M generated from collaborators as of December 31, 2023(3). Based on the estimated value of cash, cash equivalents and marketable securities and the estimated value of Vaxcyte common stock held by Sutro as of December 31, 2023. Based on current business plans and assumptions. Includes payments and equity investments received through December 31, 2023.

Sutro’s Robust Pipeline of Product Candidates Demonstrates our Innovative Processes and Designed Intentionally to Expand Patient Benefit in Areas of High Unmet Need PROGRAM MODALITY/TARGET INDICATION DISCOVERY PRECLINICAL PHASE 1/1B PHASE 2/3 WORLDWIDE OR GEOGRAPHIC PARTNER SUTRO-LED PROGRAMS Luveltamab �tazevibulin (Luvelta,�STRO-002) FolRα Antibody-Drug Conjugate (ADC) Ovarian Cancer Ovarian Cancer �(bevacizumab combo) Endometrial Cancer CBF/GLIS2 Pediatric AML Adenocarcinoma, NSCLC STRO-001(1) CD74 ADC B-cell Malignancies STRO-003 ROR1 ADC Solid Tumors & Hematological Cancers STRO-004 Tissue Factor ADC Solid Tumors PARTNER PROGRAMS VAX-24 24-Valent Conjugate Vaccine Invasive Pneumococcal Disease VAX-31 31-Valent Conjugate Vaccine Invasive Pneumococcal Disease MK-1484 Selective IL-2 Agonist Advanced or Metastatic �Solid Tumors Undisclosed Programs Immunostimulatory ADCs (iADCs) Cancers (Greater China Rights) (Greater China Rights) Fast Track Designation Orphan Drug Designation Orphan Drug & Rare Pediatric Disease Designation Multiple Programs 1. Phase 1 dose escalation has completed in the U.S., and clinical development is ongoing in Greater China led by BioNova 4
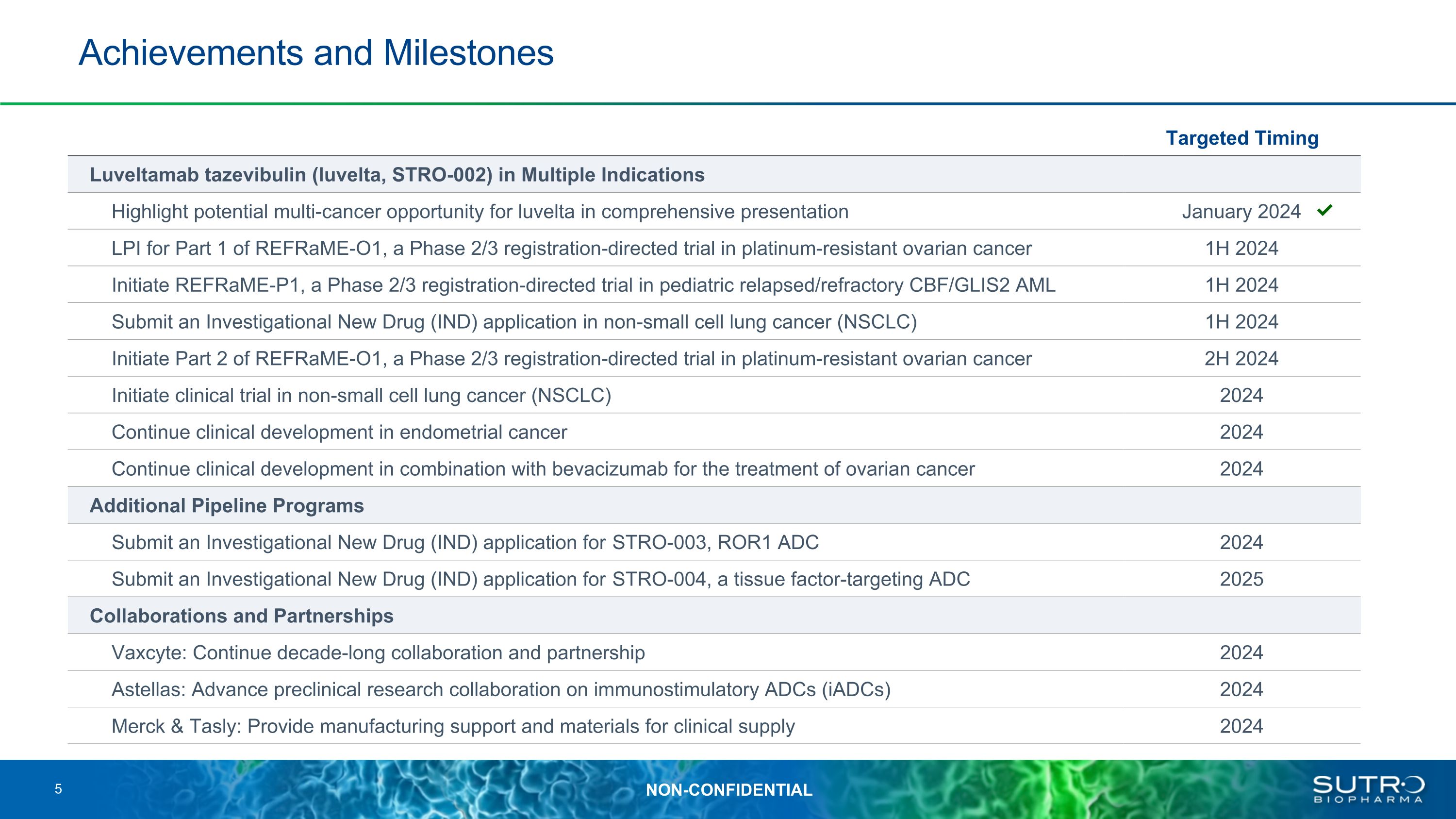
Achievements and Milestones 5 Targeted Timing Luveltamab tazevibulin (luvelta, STRO-002) in Multiple Indications Highlight potential multi-cancer opportunity for luvelta in comprehensive presentation January 2024 LPI for Part 1 of REFRaME-O1, a Phase 2/3 registration-directed trial in platinum-resistant ovarian cancer 1H 2024 Initiate REFRaME-P1, a Phase 2/3 registration-directed trial in pediatric relapsed/refractory CBF/GLIS2 AML 1H 2024 Submit an Investigational New Drug (IND) application in non-small cell lung cancer (NSCLC) 1H 2024 Initiate Part 2 of REFRaME-O1, a Phase 2/3 registration-directed trial in platinum-resistant ovarian cancer 2H 2024 Initiate clinical trial in non-small cell lung cancer (NSCLC) 2024 Continue clinical development in endometrial cancer 2024 Continue clinical development in combination with bevacizumab for the treatment of ovarian cancer 2024 Additional Pipeline Programs Submit an Investigational New Drug (IND) application for STRO-003, ROR1 ADC 2024 Submit an Investigational New Drug (IND) application for STRO-004, a tissue factor-targeting ADC 2025 Collaborations and Partnerships Vaxcyte: Continue decade-long collaboration and partnership 2024 Astellas: Advance preclinical research collaboration on immunostimulatory ADCs (iADCs) 2024 Merck & Tasly: Provide manufacturing support and materials for clinical supply 2024 ✓

Luveltamab Tazevibulin –�A Pipeline in a Drug ��(Luvelta, STRO-002)
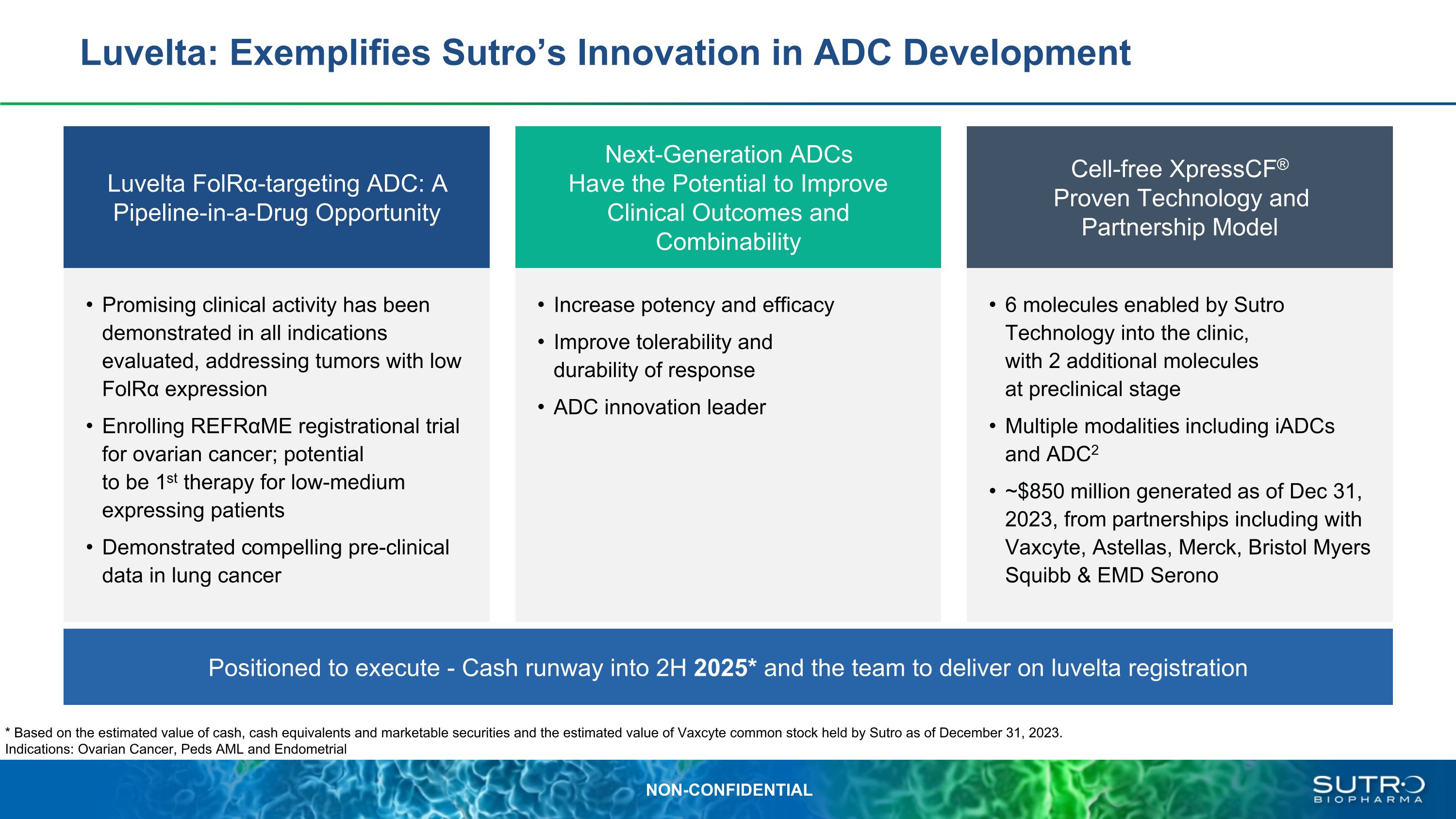
Luvelta: Exemplifies Sutro’s Innovation in ADC Development Cell-free XpressCF®�Proven Technology and �Partnership Model Luvelta FolRα-targeting ADC: A�Pipeline-in-a-Drug Opportunity Next-Generation ADCs �Have the Potential to Improve Clinical Outcomes and Combinability Positioned to execute - Cash runway into 2H 2025* and the team to deliver on luvelta registration 6 molecules enabled by Sutro Technology into the clinic, �with 2 additional molecules at preclinical stage Multiple modalities including iADCs and ADC2 ~$850 million generated as of Dec 31, 2023, from partnerships including with Vaxcyte, Astellas, Merck, Bristol Myers Squibb & EMD Serono Promising clinical activity has been demonstrated in all indications evaluated, addressing tumors with low FolRα expression Enrolling REFRαME registrational trial for ovarian cancer; potential �to be 1st therapy for low-medium �expressing patients Demonstrated compelling pre-clinical data in lung cancer Increase potency and efficacy Improve tolerability and �durability of response ADC innovation leader * Based on the estimated value of cash, cash equivalents and marketable securities and the estimated value of Vaxcyte common stock held by Sutro as of December 31, 2023. Indications: Ovarian Cancer, Peds AML and Endometrial
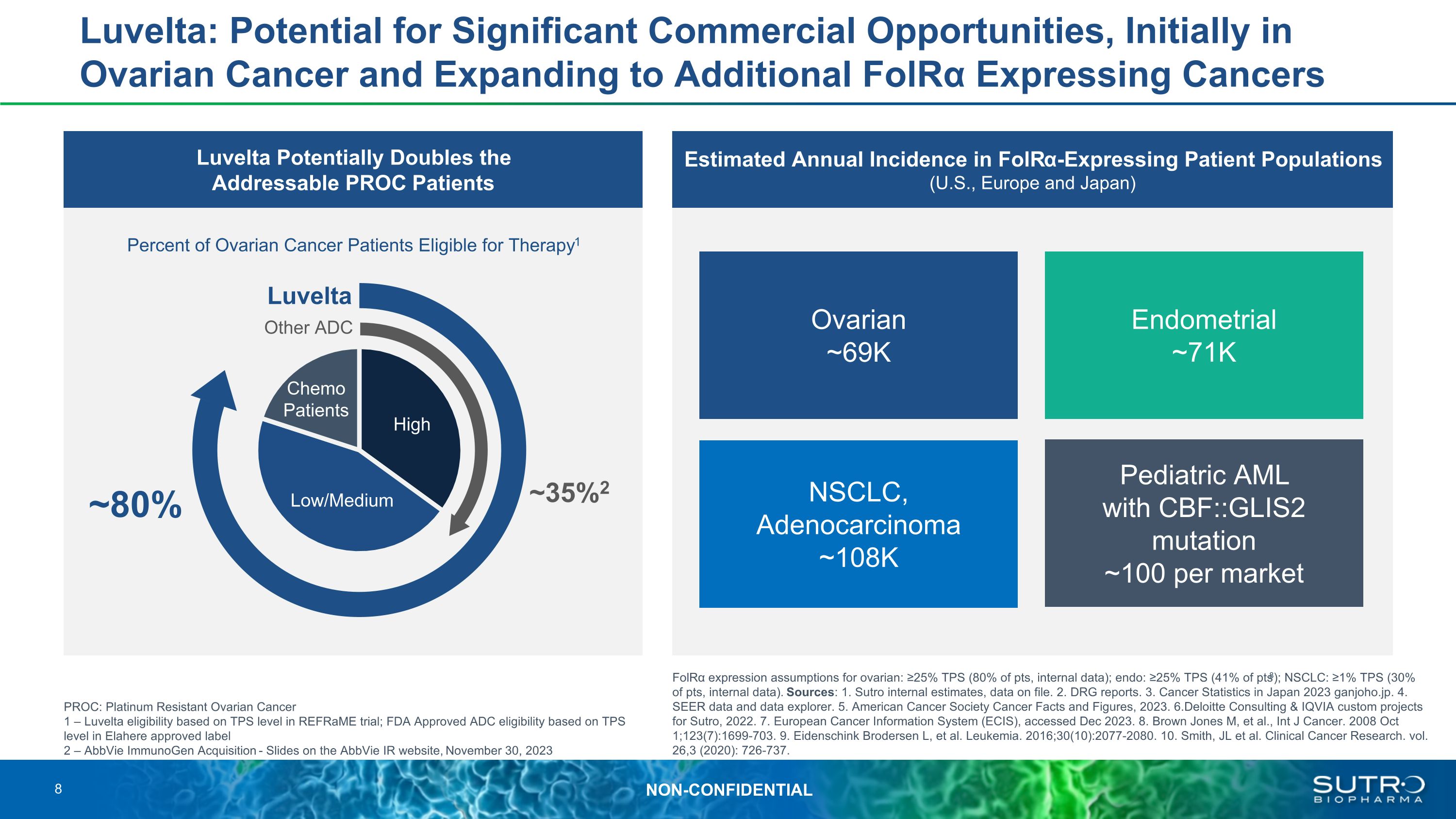
Luvelta: Potential for Significant Commercial Opportunities, Initially in Ovarian Cancer and Expanding to Additional FolRα Expressing Cancers Luvelta Potentially Doubles the Addressable PROC Patients Estimated Annual Incidence in FolRα-Expressing Patient Populations �(U.S., Europe and Japan) PROC: Platinum Resistant Ovarian Cancer 1 – Luvelta eligibility based on TPS level in REFRaME trial; FDA Approved ADC eligibility based on TPS level in Elahere approved label 2 – AbbVie ImmunoGen Acquisition - Slides on the AbbVie IR website, November 30, 2023 FolRα expression assumptions for ovarian: ≥25% TPS (80% of pts, internal data); endo: ≥25% TPS (41% of pts8); NSCLC: ≥1% TPS (30% of pts, internal data). Sources: 1. Sutro internal estimates, data on file. 2. DRG reports. 3. Cancer Statistics in Japan 2023 ganjoho.jp. 4. SEER data and data explorer. 5. American Cancer Society Cancer Facts and Figures, 2023. 6.Deloitte Consulting & IQVIA custom projects for Sutro, 2022. 7. European Cancer Information System (ECIS), accessed Dec 2023. 8. Brown Jones M, et al., Int J Cancer. 2008 Oct 1;123(7):1699-703. 9. Eidenschink Brodersen L, et al. Leukemia. 2016;30(10):2077-2080. 10. Smith, JL et al. Clinical Cancer Research. vol. 26,3 (2020): 726-737. Percent of Ovarian Cancer Patients Eligible for Therapy1 Ovarian ~69K NSCLC, Adenocarcinoma ~108K Pediatric AML with CBF::GLIS2 mutation ~100 per market Endometrial ~71K Other ADC Luvelta High Low/Medium Chemo Patients ~35%2 ~80% 8
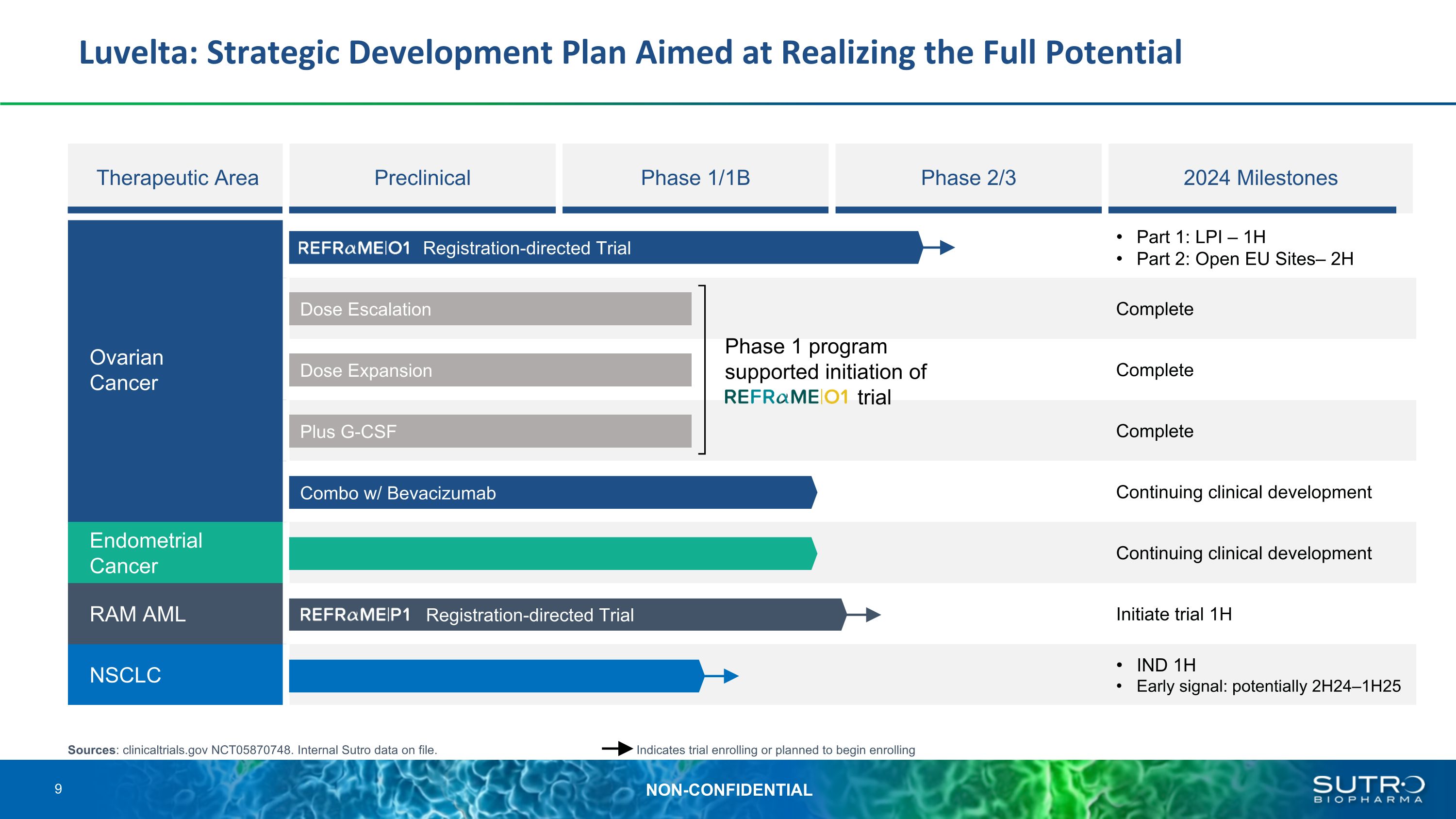
Luvelta: Strategic Development Plan Aimed at Realizing the Full Potential Therapeutic Area Preclinical Phase 1/1B Phase 2/3 2024 Milestones Ovarian �Cancer Part 1: LPI – 1H Part 2: Open EU Sites– 2H Ovarian Cancer Complete Complete Complete Continuing clinical development Endometrial Cancer Continuing clinical development RAM AML Initiate trial 1H NSCLC IND 1H Early signal: potentially 2H24–1H25 Plus G-CSF Dose Expansion Dose Escalation Combo w/ Bevacizumab Sources: clinicaltrials.gov NCT05870748. Internal Sutro data on file. Indicates trial enrolling or planned to begin enrolling Phase 1 program supported initiation of trial REFRαME-P1 Registration-directed Trial REFRαME-O1 Registration-directed Trial 9

Luvelta: Peds RAM-AML Strategically Positioned for Potential PRV and Accelerates Market Entry and Commercial Readiness for OC Eligibility Dose Finding Dose Expansion Key Endpoints Relapsed/Refractory CBFA2T3::GLIS2 AML ≥ 5% Bone Marrow Involvement with Leukemic Blasts Complete remission (CR) rate Measurable residual disease (MRD)-negative response rate Complete remission with partial hematologic recovery (CRh) rate EFS, RFS and OS Safety, PK Selected Dose N = ~18 PRV: Pediatric Review Voucher Sources: clinicaltrials.gov NCT05870748. Internal Sutro data on file. Eligibility Phase 2: �Dose Finding Key Endpoints Phase 3: �Randomized Trial Platinum Resistant Ovarian Cancer to 1st platinum or progression ≤ 6m to last platinum 1-3 prior lines ECOG PS 0-1 Exclude primary platinum refractory FolR1 expression ≥25% Final analysis for full approval: PFS, OS Interim analysis planned to support accelerated approval: ORR, DOR Safety, QoL, PK Optimized Dose Regimen Investigator’s Choice Chemotherapy R 1:1 N = ~258 N = ~258 Dose A: �5.2 mg/kg�IV q3w + prophylactic G CSF 4.3 mg/kg after 2 cycles Dose B: �4.3 mg/kg�IV q3w R 1:1 N = 25 N = 25 3.5 mg/kg IV q2w Lead in Dose 4.3 mg/kg IV q2w R 1:1 N = 3-6 N = 3-6 10
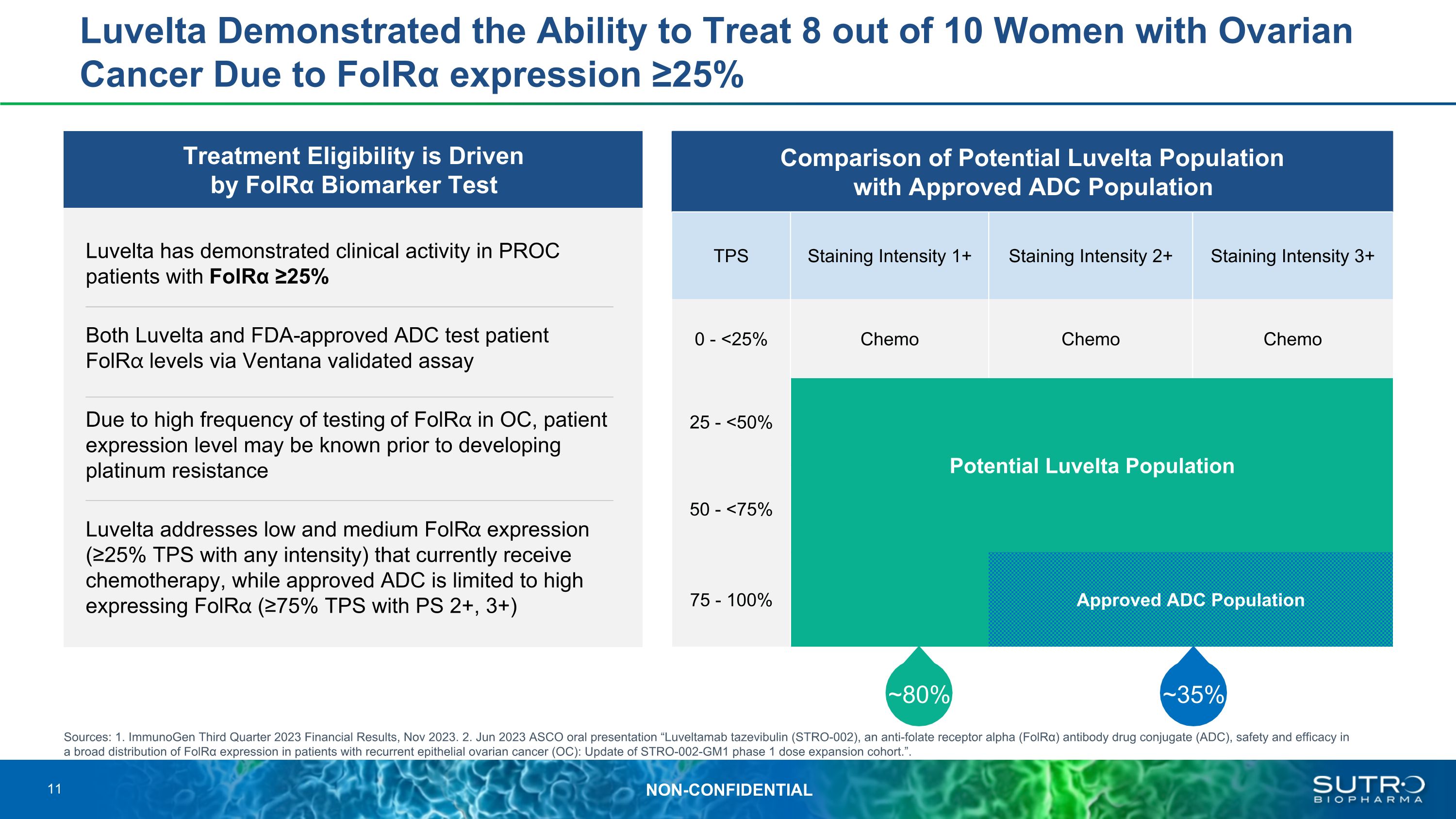
Treatment Eligibility is Driven �by FolRα Biomarker Test Luvelta has demonstrated clinical activity in PROC patients with FolRα ≥25% Both Luvelta and FDA-approved ADC test patient FolR⍺ levels via Ventana validated assay Due to high frequency of testing of FolR⍺ in OC, patient expression level may be known prior to developing platinum resistance Luvelta addresses low and medium FolR⍺ expression (≥25% TPS with any intensity) that currently receive chemotherapy, while approved ADC is limited to high expressing FolR⍺ (≥75% TPS with PS 2+, 3+) Luvelta Demonstrated the Ability to Treat 8 out of 10 Women with Ovarian Cancer Due to FolRα expression ≥25% Comparison of Potential Luvelta Population �with Approved ADC Population TPS Staining Intensity 1+ Staining Intensity 2+ Staining Intensity 3+ 0 - <25% Chemo Chemo Chemo 25 - <50% Potential Luvelta Population Luvelta 50 - <75% Luvelta Luvelta 75 - 100% Approved ADC Population Luvelta/ Approved ADC ~80% ~35% Sources: 1. ImmunoGen Third Quarter 2023 Financial Results, Nov 2023. 2. Jun 2023 ASCO oral presentation “Luveltamab tazevibulin (STRO-002), an anti-folate receptor alpha (FolRα) antibody drug conjugate (ADC), safety and efficacy in a broad distribution of FolRα expression in patients with recurrent epithelial ovarian cancer (OC): Update of STRO-002-GM1 phase 1 dose expansion cohort.”. 11
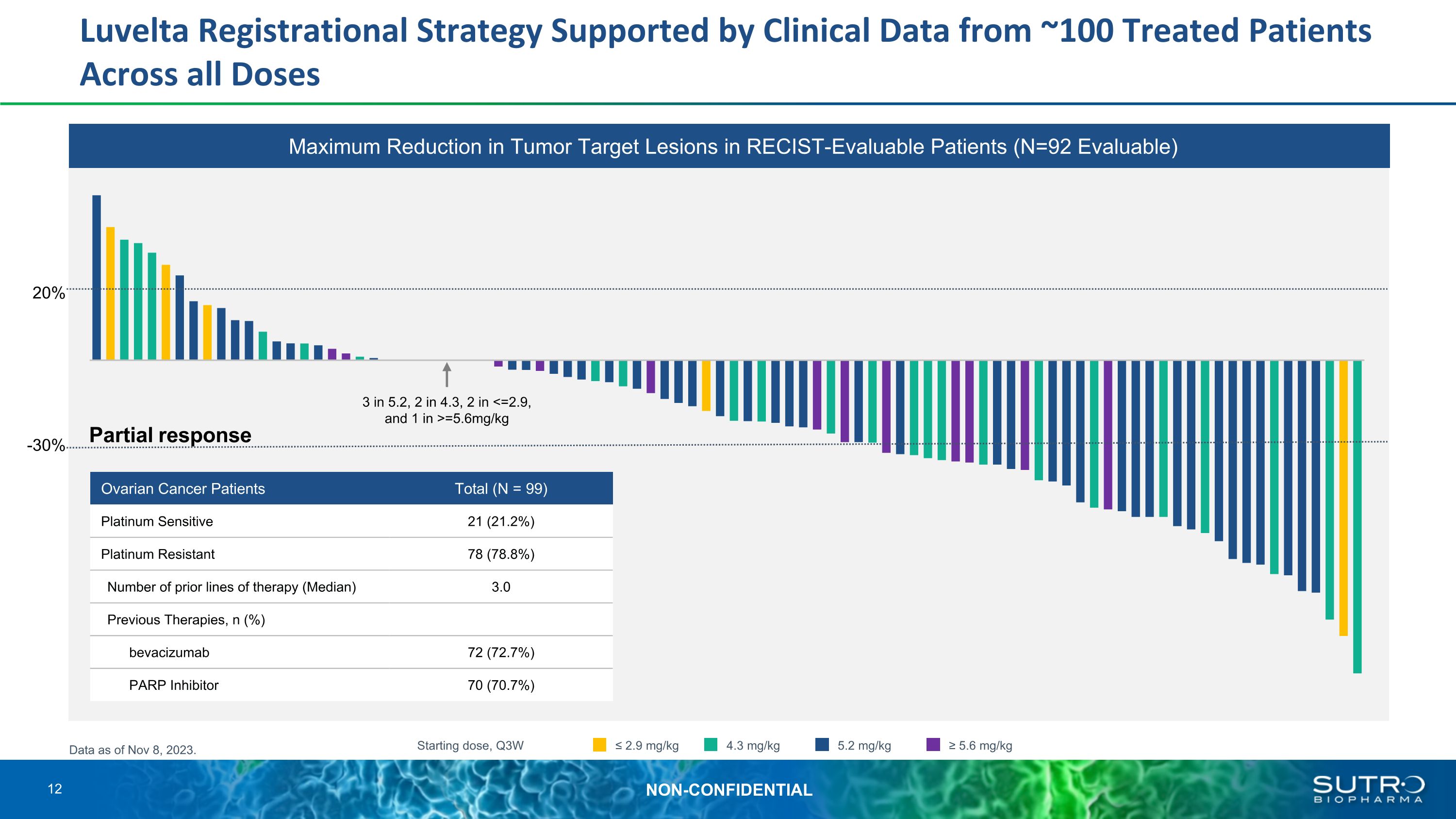
Luvelta Registrational Strategy Supported by Clinical Data from ~100 Treated Patients Across all Doses Partial response 3 in 5.2, 2 in 4.3, 2 in <=2.9, and 1 in >=5.6mg/kg Starting dose, Q3W ≤ 2.9 mg/kg 4.3 mg/kg 5.2 mg/kg ≥ 5.6 mg/kg 20% -30% Data as of Nov 8, 2023. Ovarian Cancer Patients Total (N = 99) Platinum Sensitive 21 (21.2%) Platinum Resistant 78 (78.8%) Number of prior lines of therapy (Median) 3.0 Previous Therapies, n (%) bevacizumab 72 (72.7%) PARP Inhibitor 70 (70.7%) Maximum Reduction in Tumor Target Lesions in RECIST-Evaluable Patients (N=92 Evaluable) 12

Phase 1: �Dose Expansion Signal Seeking Plus G-CSF (Neutropenia Mgt) N = 44 N = 16 Established FolRα ≥25% PROC Reduced high-grade neutropenia Luvelta Demonstrated Compelling Anti-Tumor Activity and Tolerable Safety Broadly in Ovarian Cancer Phase 1: �Dose Escalation Escalation Combo w/ Bevacizumab N = 39 N = 18 Optimal dose range Tolerable and active Aggregated Analysis of Ovarian Cancer Patients Improved clinical outcome vs. SoC chemotherapy (historical) Improved tolerability profile vs. SoC chemotherapy (historical) Clinical benefit shown in unmet need low-medium expressing patients 13
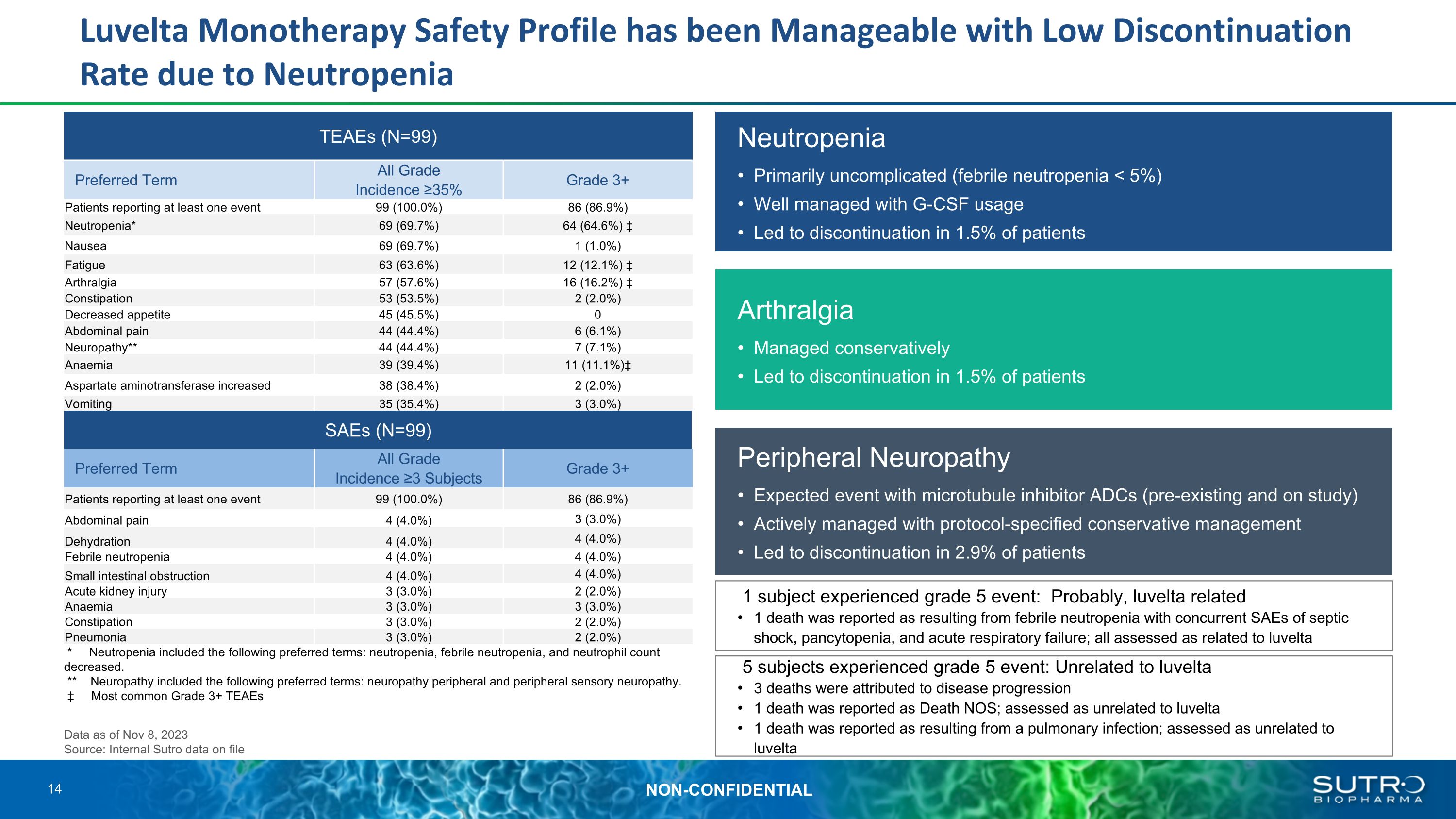
Luvelta Monotherapy Safety Profile has been Manageable with Low Discontinuation Rate due to Neutropenia TEAEs (N=99) TEAEs (N=18) Preferred Term All Grade�Incidence ≥35% Grade 3+ Patients reporting at least one event 99 (100.0%) 86 (86.9%) Neutropenia* 69 (69.7%) 64 (64.6%) ‡ Nausea 69 (69.7%) 1 (1.0%) Fatigue 63 (63.6%) 12 (12.1%) ‡ Arthralgia 57 (57.6%) 16 (16.2%) ‡ Constipation 53 (53.5%) 2 (2.0%) Decreased appetite 45 (45.5%) 0 Abdominal pain 44 (44.4%) 6 (6.1%) Neuropathy** 44 (44.4%) 7 (7.1%) Anaemia 39 (39.4%) 11 (11.1%)‡ Aspartate aminotransferase increased 38 (38.4%) 2 (2.0%) Vomiting 35 (35.4%) 3 (3.0%) SAEs (N=99) TEAEs (N=18) Preferred Term All Grade�Incidence ≥3 Subjects Grade 3+ Patients reporting at least one event 99 (100.0%) 86 (86.9%) Abdominal pain 4 (4.0%) 3 (3.0%) Dehydration 4 (4.0%) 4 (4.0%) Febrile neutropenia 4 (4.0%) 4 (4.0%) Small intestinal obstruction 4 (4.0%) 4 (4.0%) Acute kidney injury 3 (3.0%) 2 (2.0%) Anaemia 3 (3.0%) 3 (3.0%) Constipation 3 (3.0%) 2 (2.0%) Pneumonia 3 (3.0%) 2 (2.0%) * Neutropenia included the following preferred terms: neutropenia, febrile neutropenia, and neutrophil count decreased. ** Neuropathy included the following preferred terms: neuropathy peripheral and peripheral sensory neuropathy. ‡ Most common Grade 3+ TEAEs Peripheral Neuropathy Expected event with microtubule inhibitor ADCs (pre-existing and on study) Actively managed with protocol-specified conservative management Led to discontinuation in 2.9% of patients Neutropenia Primarily uncomplicated (febrile neutropenia < 5%) Well managed with G-CSF usage Led to discontinuation in 1.5% of patients Arthralgia Managed conservatively Led to discontinuation in 1.5% of patients Data as of Nov 8, 2023�Source: Internal Sutro data on file 1 subject experienced grade 5 event: Probably, luvelta related 1 death was reported as resulting from febrile neutropenia with concurrent SAEs of septic shock, pancytopenia, and acute respiratory failure; all assessed as related to luvelta 5 subjects experienced grade 5 event: Unrelated to luvelta 3 deaths were attributed to disease progression 1 death was reported as Death NOS; assessed as unrelated to luvelta 1 death was reported as resulting from a pulmonary infection; assessed as unrelated to luvelta 14

Luvelta: Demonstrated Compelling Anti-Tumor Activity and Manageable Safety Profile In Lower and/or Variable FolRα Expression Tumors Additional Indications Phase 1: �Dose Expansion (all single agent) Endometrial RAM AML1 NSCLC N = 17 N = 25 Preclinical Evidence of anti-tumor activity No new safety signals observed Continuing clinical development Meaningful clinical responses, including complete remission and prolonged overall survival Well tolerated and can be given as out-patient Positioned for registration-enabling trial Single dose and combination with PD-1 blockade demonstrated anti-tumor activity IND 1H 2024 5 15 1 2 99 6 25 45 15 35 8 45 75 18 70 30 2 pts had Δ 0% Partial Response 20% -30% TPS (%) Treatment ongoing TPS >25% ≤25% Luvelta EAP mOS not reached 1.0 0.8 0.6 0.4 0.2 0.0 0 2 4 6 8 10 12 14 Survival Time Since The First Dose of IP or Relapse (Months) Survival Probability Historic Luvelta Historical (N=26) mOS = 6.1 months Maximum Reduction in Target Lesions* Overall Survival for Children who Received Luvelta as Non-Fractionated Dosing Regimen (N=21) NSCLC PDX model with single dose Luvelta monotherapy Data cutoff: 04 August 2023. *n=16 response evaluable patients. PR, partial response; TPS, tumor proportion score. 1 - These data were generated by the treating physicians and collected and enabled for presentation by Sutro. Endometrial source: Oct 2023 ESMO mini-oral presentation “741MO - Luveltamab tazevibulin (STRO-002), an anti-folate receptor alpha (FolRα) antibody drug conjugate (ADC), demonstrates clinical activity in recurrent/progressive epithelial endometrial cancer (EEC): STRO-002-GM1 phase I dose expansion.” RAM AML source: Dec 2023 ASH poster “Anti-leukemic Activity of Luveltamab Tazevibulin (LT, STRO-002), a Novel Folate Receptor-α (FR-α)-targeting Antibody Drug Conjugate (ADC) in Relapsed/Refractory CBFA2T3::GLIS2 AML.” NSCLC source: Internal Sutro preclinical data on file. censored Study Days 800 600 400 200 0 0 20 40 60 80 100 Tumor size (mm3) Vehicle 5 mg/kg luvelta (single dose) 10 mg/kg luvelta (single dose) 15
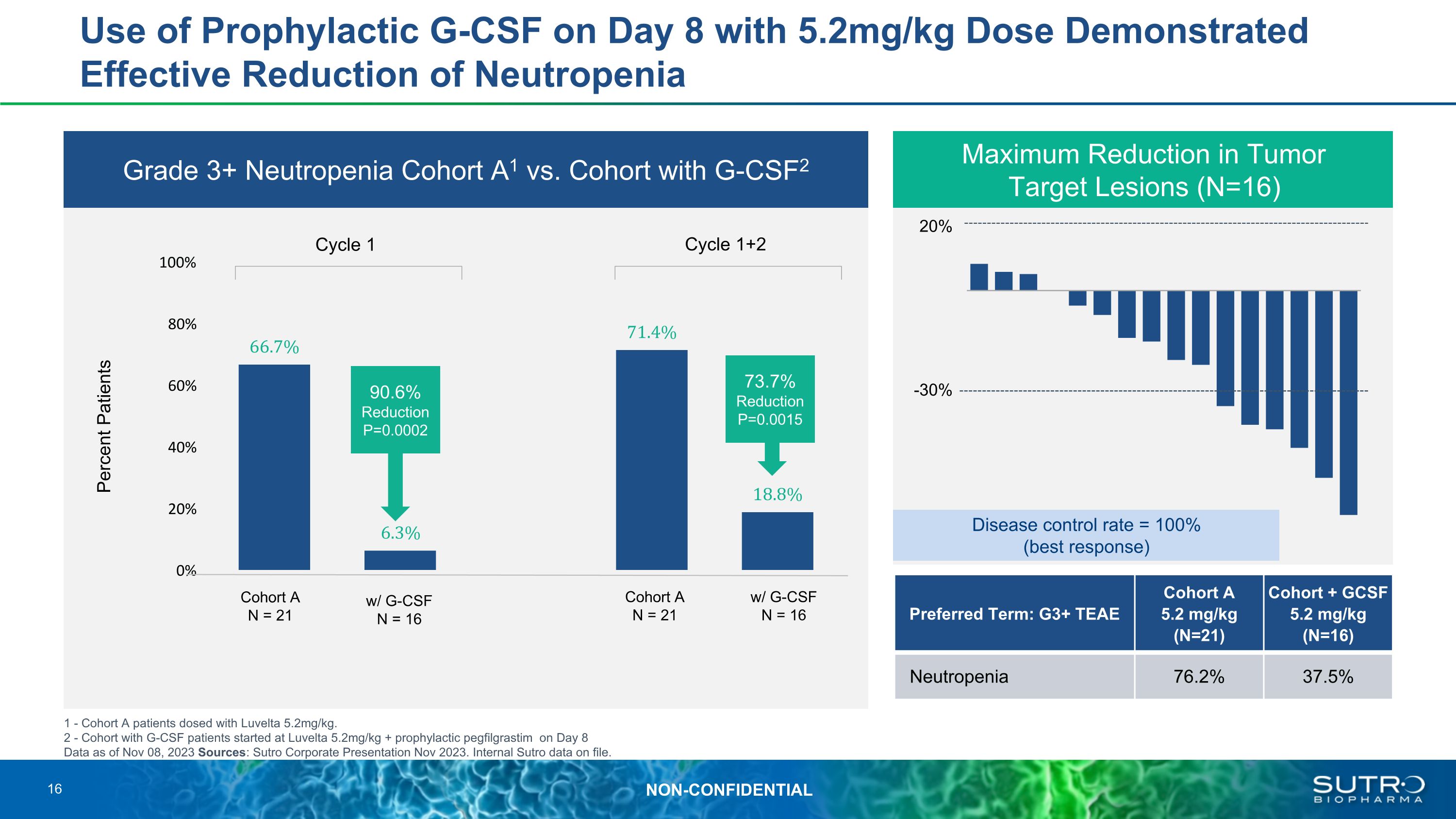
Use of Prophylactic G-CSF on Day 8 with 5.2mg/kg Dose Demonstrated Effective Reduction of Neutropenia Grade 3+ Neutropenia Cohort A1 vs. Cohort with G-CSF2 1 - Cohort A patients dosed with Luvelta 5.2mg/kg. 2 - Cohort with G-CSF patients started at Luvelta 5.2mg/kg + prophylactic pegfilgrastim on Day 8 Data as of Nov 08, 2023 Sources: Sutro Corporate Presentation Nov 2023. Internal Sutro data on file. Maximum Reduction in Tumor�Target Lesions (N=16) 20% -30% Cohort A N = 21 w/ G-CSF N = 16 Cohort A N = 21 w/ G-CSF N = 16 Cycle 1 Cycle 1+2 Percent Patients 90.6% Reduction P=0.0002 73.7% Reduction P=0.0015 Disease control rate = 100% (best response) Preferred Term: G3+ TEAE Cohort A 5.2 mg/kg (N=21) Cohort + GCSF 5.2 mg/kg (N=16) Neutropenia 76.2% 37.5% 16

Luvelta Showed Evidence of Anti-tumor Activity in FolRα Expressing Endometrial Cancer: Data Presented at ESMO 2023 Maximum Reduction in Target Lesions* Consistent Safety Signals Observed 2 pts had Δ 0% Partial Response 20% -30% TPS (%) 5 15 1 2 99 6 25 45 15 35 8 45 75 18 70 30 Median �exposure (range): �12 (3–53) weeks Median �follow-up: �10.1 months Treatment ongoing TPS >25% ≤25% TEAEs, n (%) Most Common Events Total (N = 17) AEs, n (%) Any grade* Grade ≥3 Patients reporting at least 1 event 17 (100.0) 15 (82.2) Anemia 13 (76.5) 4 (23.5) Arthralgia 12 (70.6) 3 (17.6) Neutropenia† 11 (64.7) 9 (52.9) Nausea 10 (58.8) 1 (5.9) Decreased appetite 10 (58.8) 0 5 of 17 (29%) patients received �≥5 cycles Data cutoff: 04 August 2023. *n=16 response evaluable patients. DCR, disease control rate; EC, endometrial cancer; PR, partial response; Q3W, every 3 weeks; TPS, tumor proportion score. †Neutropenia included the following preferred terms: neutropenia, febrile neutropenia, and neutrophil count decreased. �Source: Oct 2023 ESMO mini-oral presentation “741MO - Luveltamab tazevibulin (STRO-002), an anti-folate receptor alpha (FolRα) antibody drug conjugate (ADC), demonstrates clinical activity in recurrent/progressive epithelial endometrial cancer (EEC): STRO-002-GM1 phase I dose expansion.” ORR 29% in TPS >25% patients 17
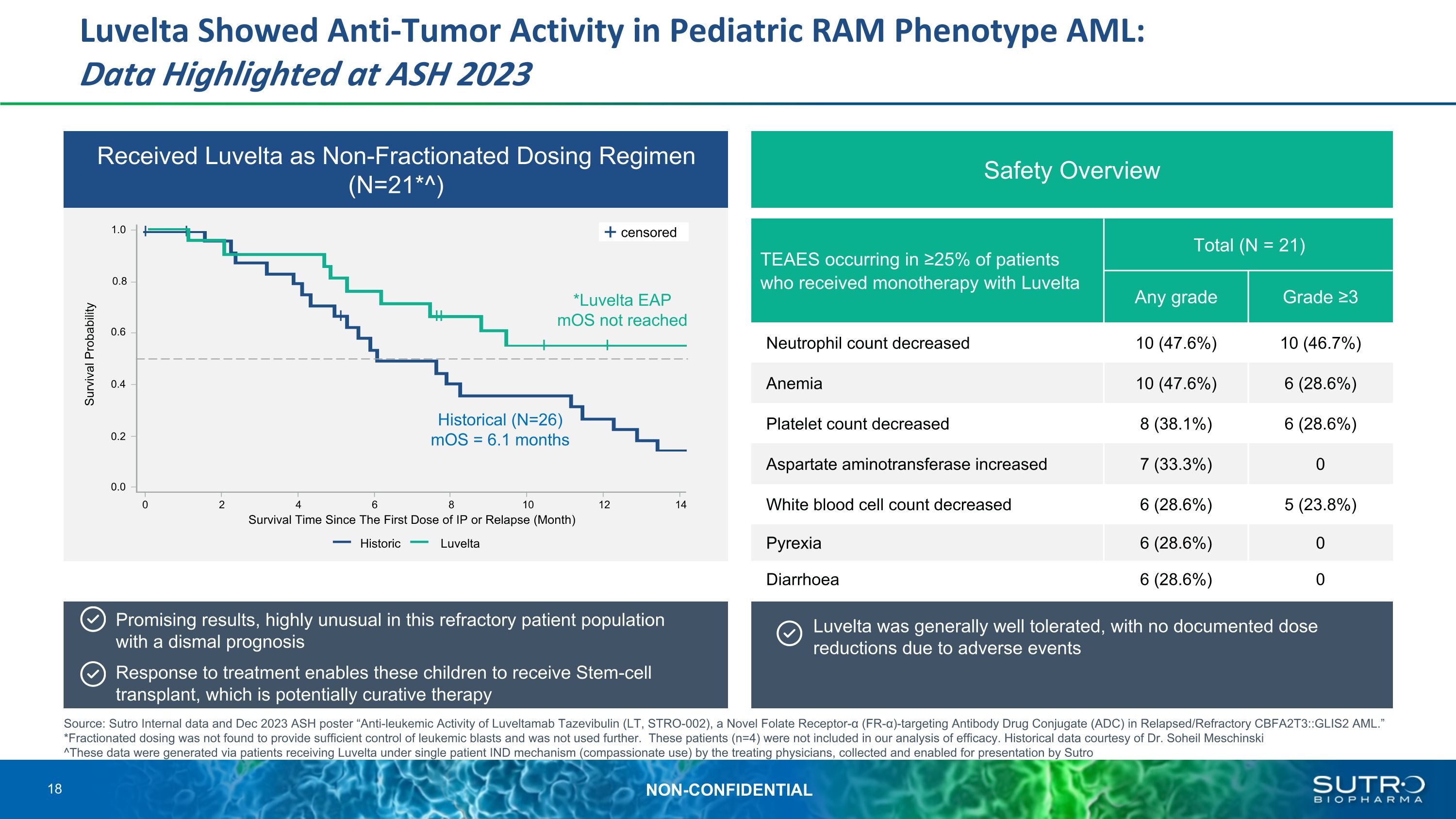
Luvelta Showed Anti-Tumor Activity in Pediatric RAM Phenotype AML: �Data Highlighted at ASH 2023 Received Luvelta as Non-Fractionated Dosing Regimen (N=21*^) Safety Overview TEAES occurring in ≥25% of patients who received monotherapy with Luvelta Total (N = 21) AEs, n (%) Any grade Grade ≥3 Neutrophil count decreased 10 (47.6%) 10 (46.7%) Anemia 10 (47.6%) 6 (28.6%) Platelet count decreased 8 (38.1%) 6 (28.6%) Aspartate aminotransferase increased 7 (33.3%) 0 White blood cell count decreased 6 (28.6%) 5 (23.8%) Pyrexia 6 (28.6%) 0 Diarrhoea 6 (28.6%) 0 Promising results, highly unusual in this refractory patient population �with a dismal prognosis Response to treatment enables these children to receive Stem-cell transplant, which is potentially curative therapy Luvelta was generally well tolerated, with no documented dose reductions due to adverse events *Luvelta EAP mOS not reached 1.0 0.8 0.6 0.4 0.2 0.0 0 2 4 6 8 10 12 14 Survival Time Since The First Dose of IP or Relapse (Month) Survival Probability Historic Luvelta Historical (N=26) mOS = 6.1 months Source: Sutro Internal data and Dec 2023 ASH poster “Anti-leukemic Activity of Luveltamab Tazevibulin (LT, STRO-002), a Novel Folate Receptor-α (FR-α)-targeting Antibody Drug Conjugate (ADC) in Relapsed/Refractory CBFA2T3::GLIS2 AML.” *Fractionated dosing was not found to provide sufficient control of leukemic blasts and was not used further. These patients (n=4) were not included in our analysis of efficacy. Historical data courtesy of Dr. Soheil Meschinski ^These data were generated via patients receiving Luvelta under single patient IND mechanism (compassionate use) by the treating physicians, collected and enabled for presentation by Sutro censored 18
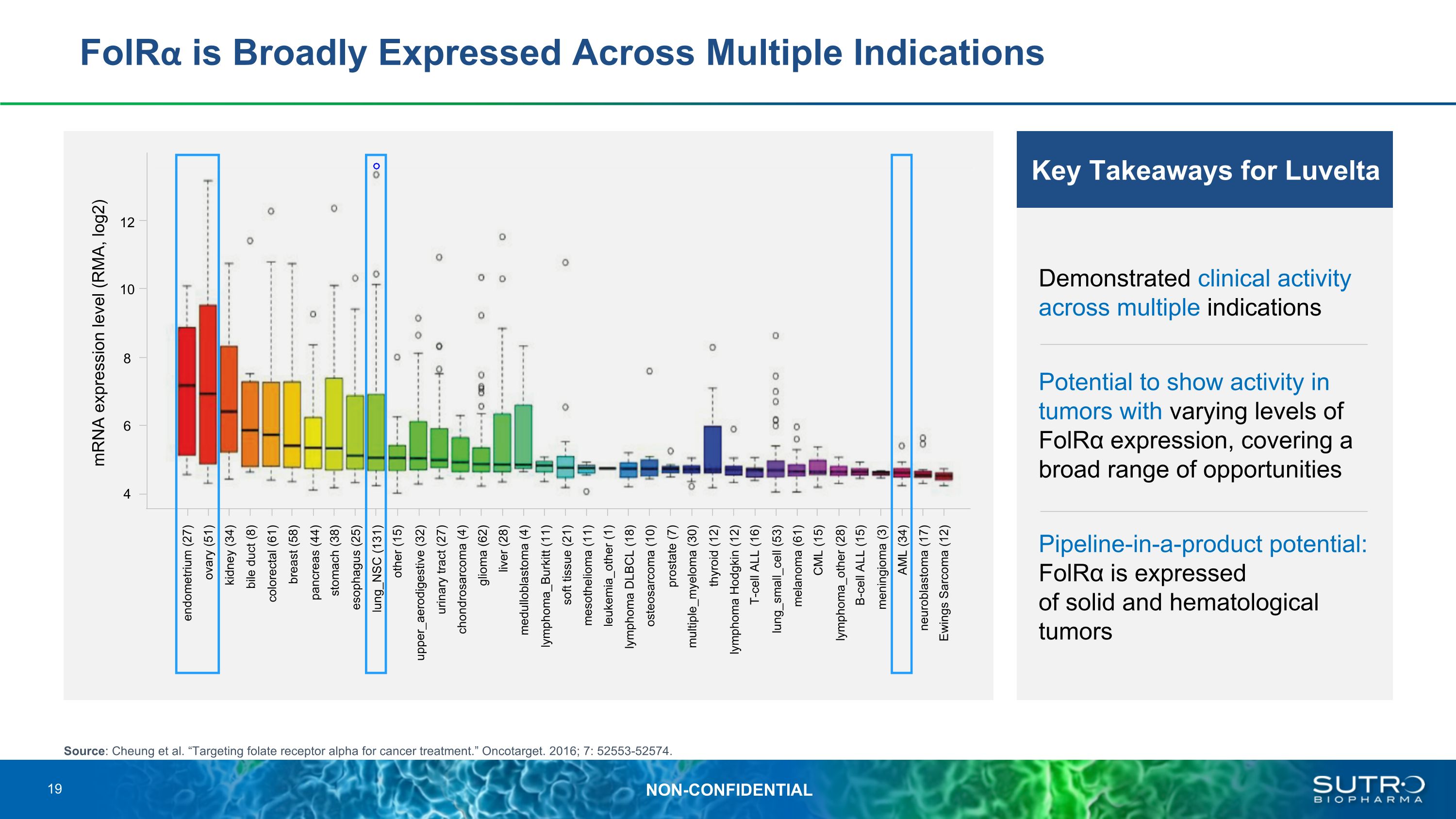
FolRα is Broadly Expressed Across Multiple Indications Source: Cheung et al. “Targeting folate receptor alpha for cancer treatment.” Oncotarget. 2016; 7: 52553-52574. Key Takeaways for Luvelta Demonstrated clinical activity across multiple indications Potential to show activity in tumors with varying levels of FolRα expression, covering a broad range of opportunities Pipeline-in-a-product potential: FolRα is expressed�of solid and hematological tumors mRNA expression level (RMA, log2) 12 10 8 6 4 endometrium (27) ovary (51) kidney (34) bile duct (8) colorectal (61) breast (58) pancreas (44) stomach (38) esophagus (25) lung_NSC (131) upper_aerodigestive (32) other (15) urinary tract (27) chondrosarcoma (4) glioma (62) liver (28) medulloblastoma (4) lymphoma_Burkitt (11) soft tissue (21) mesothelioma (11) leukemia_other (1) lymphoma DLBCL (18) osteosarcoma (10) prostate (7) multiple_myeloma (30) thyroid (12) lymphoma Hodgkin (12) T-cell ALL (16) lung_small_cell (53) melanoma (61) CML (15) lymphoma_other (28) B-cell ALL (15) meningioma (3) AML (34) neuroblastoma (17) Ewings Sarcoma (12) 19

STRO-003�and Emerging Research Portfolio

2. Single or Dual Conjugations � of Different Mechanisms 1. Mono- or Bispecific TAA Targeting Sutro’s Flexibility in Design and Innovative Toolkit Provide the Potential for Superior Solutions and the Opportunity for an Improved Patient Experience Enhanced tumor targeting with cytotoxic payloads Bispecific ADC Novel payloads for better targeting Tumor Antigen Dual Tumor Antigens Conjugated Antibody Tumor Antigen MODALITY DRUG PROPERTIES TARGET STRUCTURE ADC / ISAC iADC / ADC2 Site-specific dual drug conjugate with complementary modalities Our ADC design process delivers optimized and consistent product candidates, designed to benefit broader patient populations and provide a solution for unwanted ADC class effects Toolkit of Fit-to-Purpose Linker-Payloads DNA targeting / tubulin targeting cytotoxins Immune modulators Other mechanistically synergistic payloads Proprietary cleavable / non-cleavable linkers
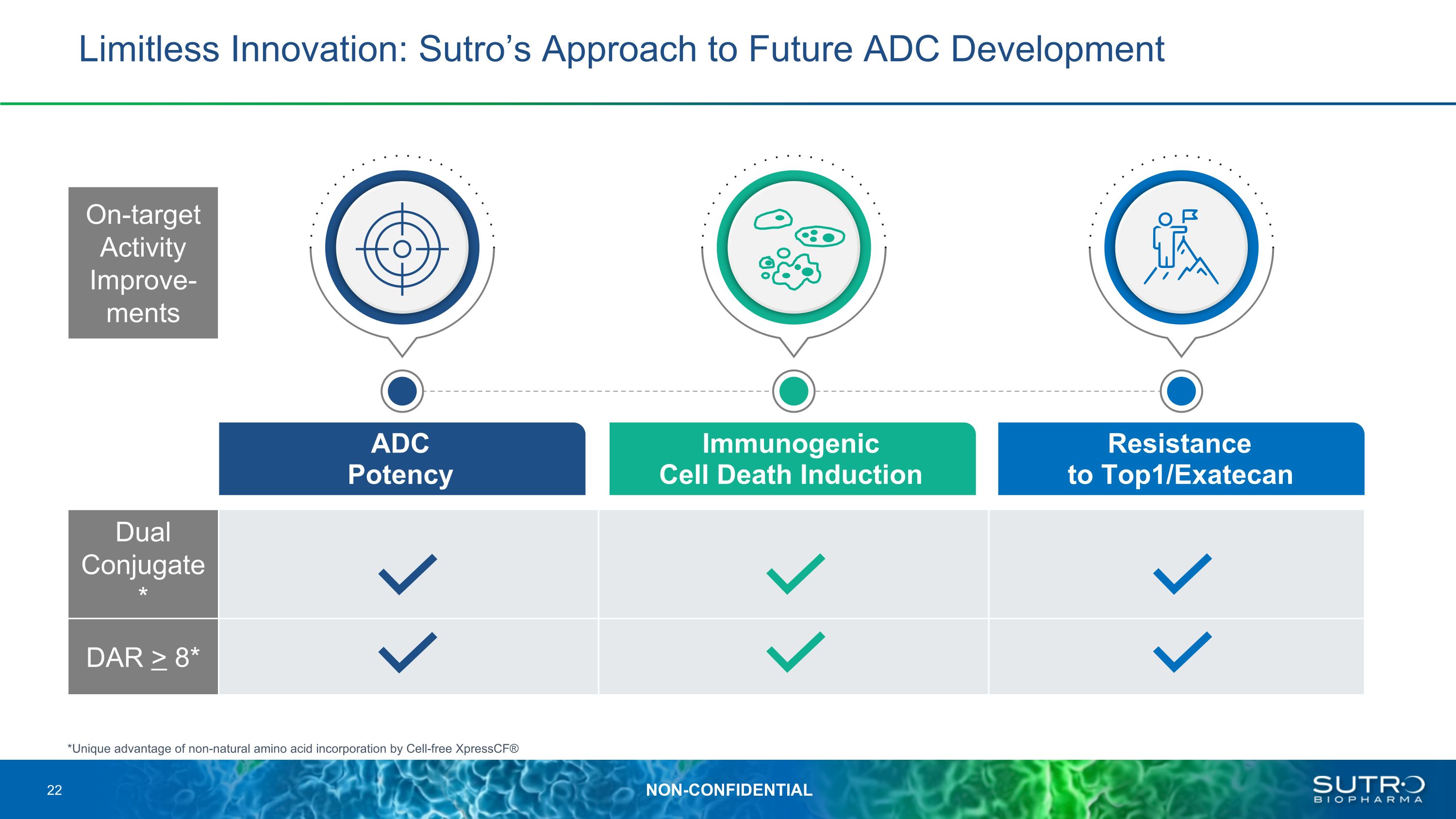
Limitless Innovation: Sutro’s Approach to Future ADC Development ADC �Potency Immunogenic �Cell Death Induction Resistance �to Top1/Exatecan *Unique advantage of non-natural amino acid incorporation by Cell-free XpressCF® Dual Conjugate* DAR > 8* On-target Activity Improve-ments
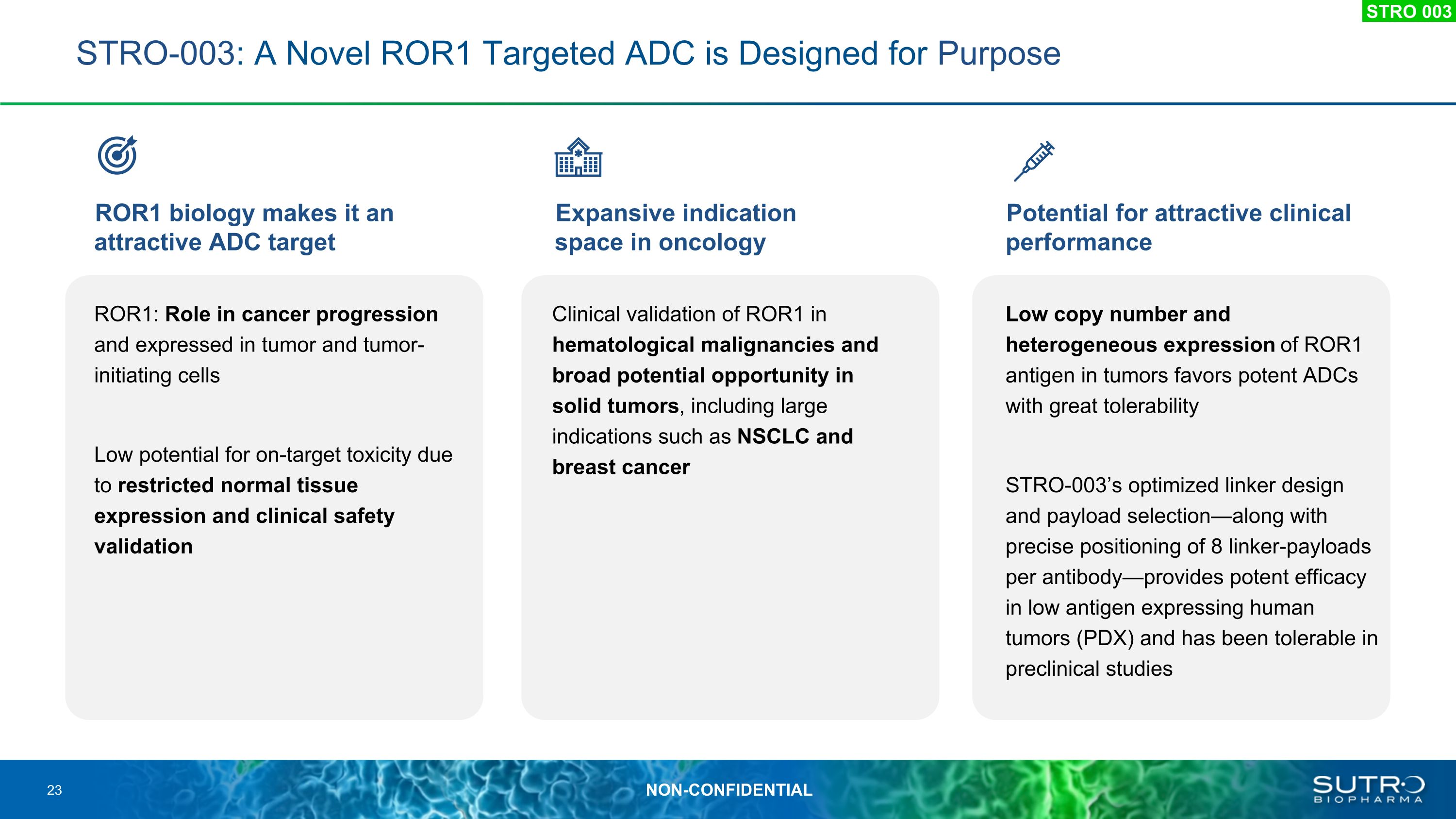
STRO-003: A Novel ROR1 Targeted ADC is Designed for Purpose ROR1 biology makes it an attractive ADC target Expansive indication space in oncology Potential for attractive clinical performance ROR1: Role in cancer progression and expressed in tumor and tumor- initiating cells Low potential for on-target toxicity due to restricted normal tissue expression and clinical safety validation Clinical validation of ROR1 in hematological malignancies and broad potential opportunity in solid tumors, including large indications such as NSCLC and breast cancer Low copy number and heterogeneous expression of ROR1 antigen in tumors favors potent ADCs with great tolerability STRO-003’s optimized linker design and payload selection—along with precise positioning of 8 linker-payloads per antibody—provides potent efficacy in low antigen expressing human tumors (PDX) and has been tolerable in preclinical studies STRO 003
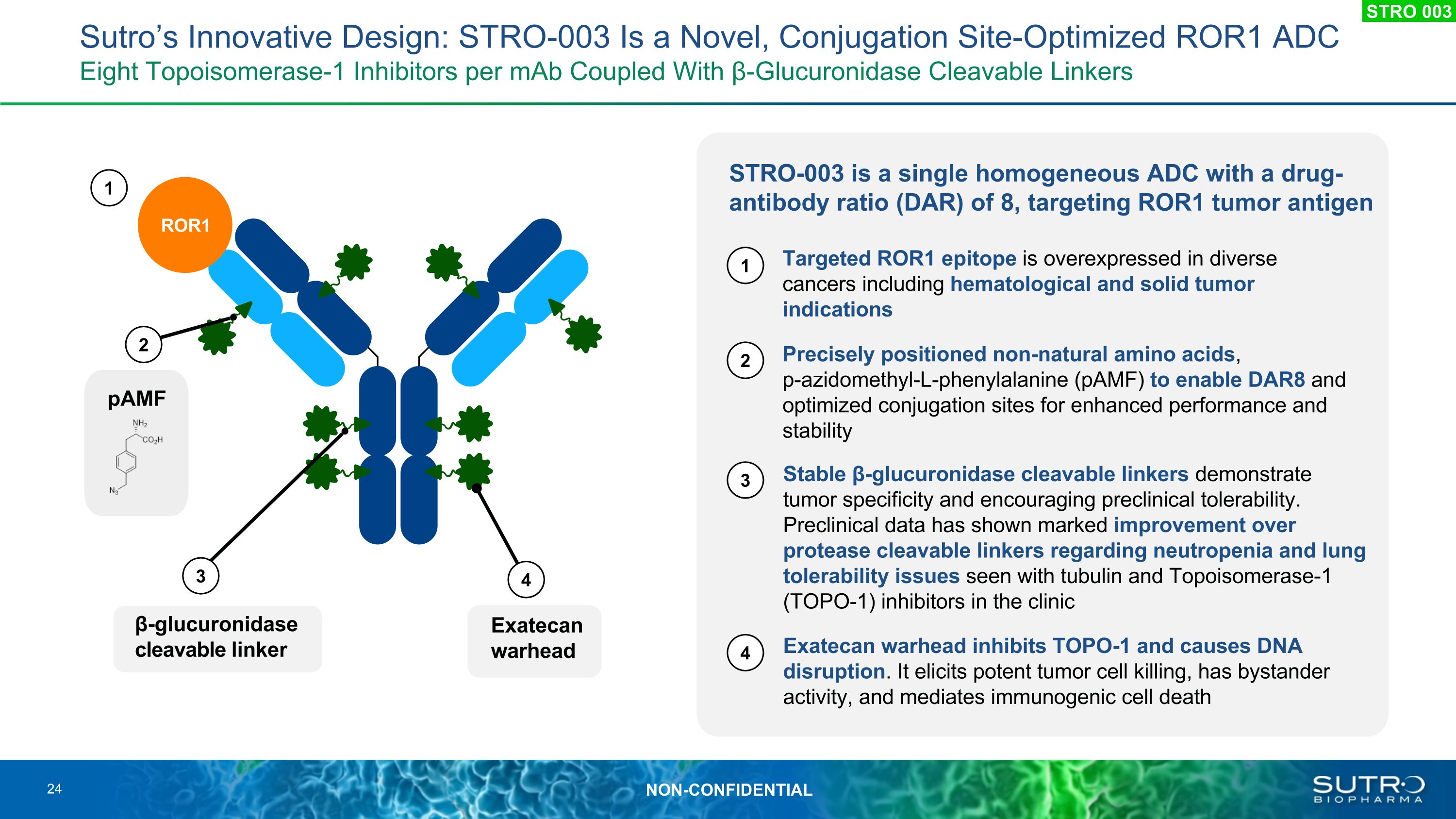
STRO-003 is a single homogeneous ADC with a drug-antibody ratio (DAR) of 8, targeting ROR1 tumor antigen pAMF ROR1 Targeted ROR1 epitope is overexpressed in diverse cancers including hematological and solid tumor indications Precisely positioned non-natural amino acids, �p-azidomethyl-L-phenylalanine (pAMF) to enable DAR8 and optimized conjugation sites for enhanced performance and stability Stable β-glucuronidase cleavable linkers demonstrate tumor specificity and encouraging preclinical tolerability. Preclinical data has shown marked improvement over protease cleavable linkers regarding neutropenia and lung tolerability issues seen with tubulin and Topoisomerase-1 (TOPO-1) inhibitors in the clinic Exatecan warhead inhibits TOPO-1 and causes DNA disruption. It elicits potent tumor cell killing, has bystander activity, and mediates immunogenic cell death Exatecan�warhead β-glucuronidase cleavable linker Sutro’s Innovative Design: STRO-003 Is a Novel, Conjugation Site-Optimized ROR1 ADC�Eight Topoisomerase-1 Inhibitors per mAb Coupled With β-Glucuronidase Cleavable Linkers 24 STRO 003 4 3 2 1 1 2 3 4
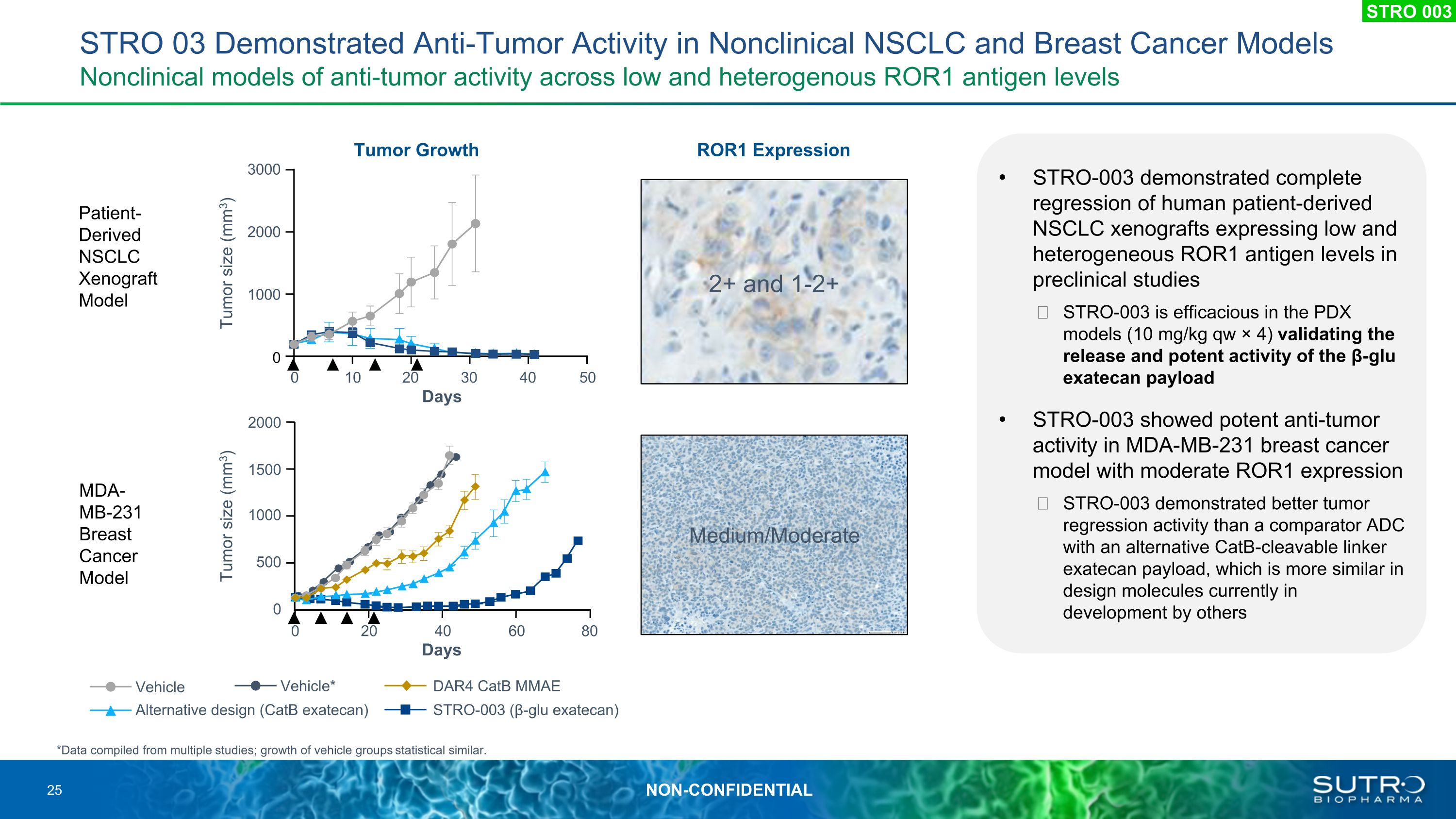
STRO 03 Demonstrated Anti-Tumor Activity in Nonclinical NSCLC and Breast Cancer Models�Nonclinical models of anti-tumor activity across low and heterogenous ROR1 antigen levels STRO-003 demonstrated complete regression of human patient-derived NSCLC xenografts expressing low and heterogeneous ROR1 antigen levels in preclinical studies STRO-003 is efficacious in the PDX models (10 mg/kg qw × 4) validating the release and potent activity of the β-glu exatecan payload STRO-003 showed potent anti-tumor activity in MDA-MB-231 breast cancer model with moderate ROR1 expression STRO-003 demonstrated better tumor regression activity than a comparator ADC with an alternative CatB-cleavable linker exatecan payload, which is more similar in design molecules currently in development by others Patient-Derived NSCLC Xenograft Model MDA-MB-231 Breast Cancer Model ROR1 Expression Tumor Growth 2+ and 1-2+ Medium/Moderate 2000 1500 1000 0 0 20 40 60 80 Tumor size (mm3) Days 500 3000 2000 1000 0 0 10 20 30 40 50 Tumor size (mm3) Days *Data compiled from multiple studies; growth of vehicle groups statistical similar. STRO-003 (β-glu exatecan) DAR4 CatB MMAE Vehicle Alternative design (CatB exatecan) Vehicle* STRO 003
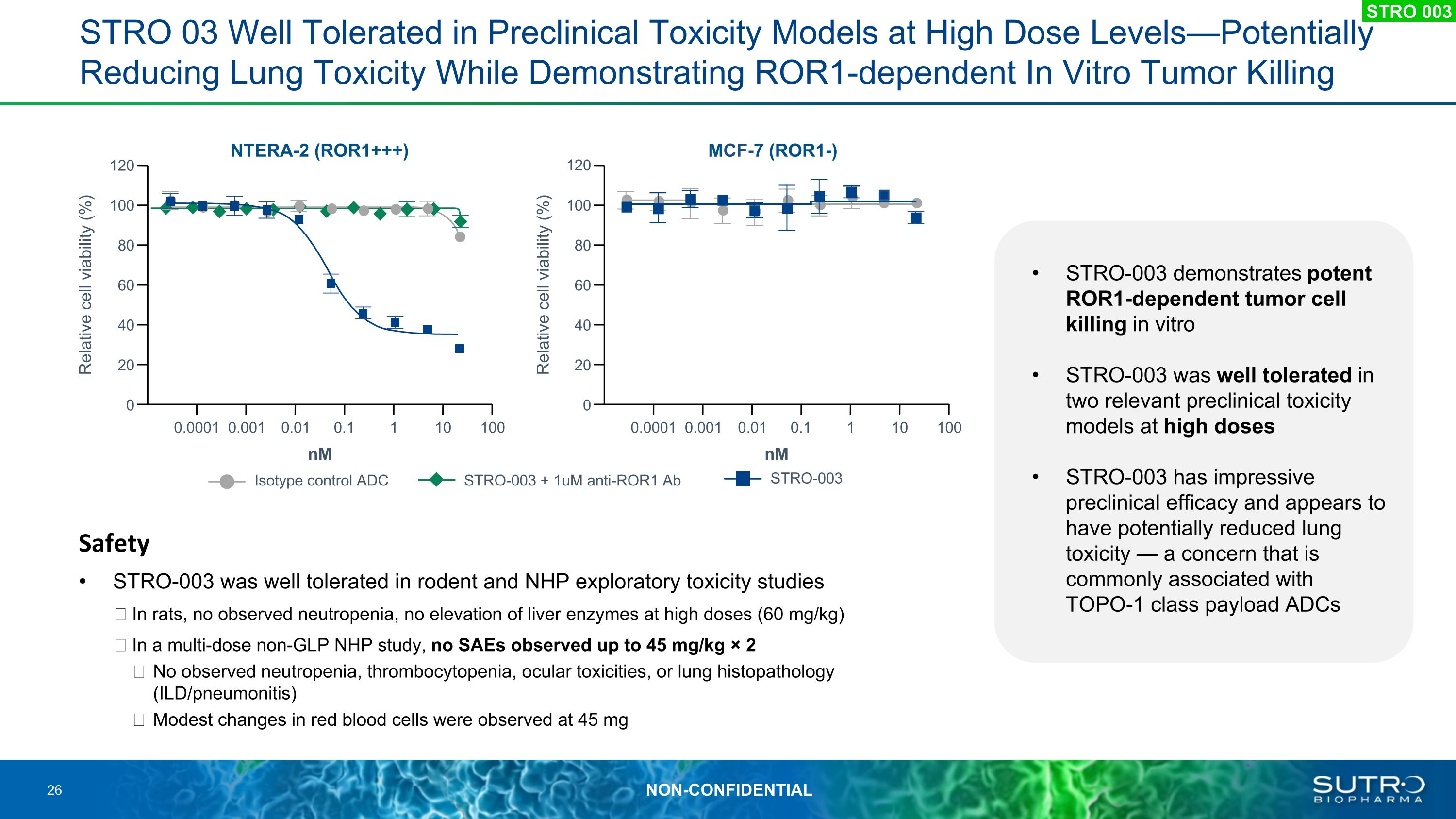
STRO 03 Well Tolerated in Preclinical Toxicity Models at High Dose Levels—Potentially Reducing Lung Toxicity While Demonstrating ROR1-dependent In Vitro Tumor Killing Safety STRO-003 was well tolerated in rodent and NHP exploratory toxicity studies In rats, no observed neutropenia, no elevation of liver enzymes at high doses (60 mg/kg) In a multi-dose non-GLP NHP study, no SAEs observed up to 45 mg/kg × 2 No observed neutropenia, thrombocytopenia, ocular toxicities, or lung histopathology (ILD/pneumonitis) Modest changes in red blood cells were observed at 45 mg STRO-003 Isotype control ADC STRO-003 + 1uM anti-ROR1 Ab NTERA-2 (ROR1+++) nM 120 60 40 0 0.0001 Relative cell viability (%) 100 80 20 0.001 0.01 0.1 1 10 100 MCF-7 (ROR1-) 60 40 0 0.0001 Relative cell viability (%) nM 100 80 20 0.001 0.01 0.1 1 10 100 120 STRO 003 STRO-003 demonstrates potent ROR1-dependent tumor cell killing in vitro STRO-003 was well tolerated in two relevant preclinical toxicity models at high doses STRO-003 has impressive preclinical efficacy and appears to have potentially reduced lung toxicity — a concern that is commonly associated with TOPO-1 class payload ADCs

$90M upfront to develop iADCs for up to three targets Research activities are being conducted for two targets, representing two distinct programs $422.5M in development, regulatory and commercial milestones for each product candidate, plus tiered royalties ranging from low-double digit to mid-teen percentages Builds on success of Sutro’s ADC platform and engineering expertise Leverages Astellas’ primary focus on immuno-oncology Sutro has the option to share costs/profits for U.S. product development Sutro retained option to develop iADCs outside of/beyond this collaboration in other targets Strategic iADC Collaboration Initiated on June 27, 2022 New Modality for Cold Tumors: Immunostimulatory Antibody Drug Conjugate (iADC)�Featuring dual drug conjugation technology with both cytotoxin and immune modulator 27

~$375M(1) in cash, cash equivalents & marketable securities and Vaxcyte stock Projected cash runway into 2H 2025, based on current business plans and assumptions ~0.7M shares �of Vaxcyte (Nasdaq: PCVX) included in the $ amount above Funding generated from our collaborators of ~$850M(2) 1. Based on the estimated value of cash, cash equivalents and marketable securities and the estimated value of Vaxcyte common stock held by Sutro as of December 31, 2023. 2. Includes payments and equity investments received through December 31, 2023. Financial Overview – December 31, 2023�Well-capitalized through multiple funding sources 28
Experienced Leadership Team Lynx Neurex 29 Anne Borgman, MD Chief Medical Officer Venkatesh Srinivasan, PhD Chief Technical Operations Officer Linda Fitzpatrick Chief People and Communications Officer Nicki Vasquez, PhD Chief Portfolio Strategy and Alliance Officer Jane Chung, RPh President and �Chief Operating Officer Ed Albini, MBA Chief Financial Officer Hans-Peter Gerber, PhD Chief Scientific Officer William Newell, JD �Chief Executive Officer and Member of the Board of Directors
v3.23.4
Document and Entity Information
|
Jan. 04, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 04, 2024
|
| Entity Registrant Name |
SUTRO BIOPHARMA, INC.
|
| Entity Central Index Key |
0001382101
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-38662
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
47-0926186
|
| Entity Address, Address Line One |
111 Oyster Point Blvd.
|
| Entity Address, City or Town |
South San Francisco
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
94080
|
| City Area Code |
(650)
|
| Local Phone Number |
881-6500
|
| Entity Information, Former Legal or Registered Name |
Not Applicable
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common stock, $0.001 par value
|
| Trading Symbol |
STRO
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Sutro Biopharma (NASDAQ:STRO)
Historical Stock Chart
From Oct 2024 to Nov 2024

Sutro Biopharma (NASDAQ:STRO)
Historical Stock Chart
From Nov 2023 to Nov 2024
