false 0001863127 0001863127 2024-10-24 2024-10-24
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 24, 2024
Tyra Biosciences, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
| |
| Delaware |
|
001-40800 |
|
83-1476348 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
|
| |
| 2656 State Street |
|
|
| Carlsbad, California |
|
92008 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (619) 728-4760
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
| |
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
TYRA |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 5.02 |
Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers. |
On October 24, 2024, Siddarth Subramony, Ph.D. resigned from the Board of Directors (the Board) of Tyra Biosciences, Inc. (Tyra or the Company) and the Compensation Committee of the Board (the Compensation Committee) effective immediately. Dr. Subramony’s resignation from the Board and the Compensation Committee was not made in connection with a disagreement with the Company on any matter relating to the Company’s operations, policies or practices.
| Item 7.01. |
Regulation FD Disclosure. |
On October 25, 2024, the Company will host a conference call and webcast to share interim clinical results of TYRA-300 from the SURF301 Phase 1/2 study in metastatic urothelial cancer (mUC). The event will begin at 8:00 a.m. Eastern Time and will be available via a live webcast accessible under the “For Investors” page of Tyra’s corporate website, at https://ir.tyra.bio. During the event, the Company will present the corporate slide presentation attached as Exhibit 99.1 to this Current Report on Form 8-K, which is incorporated herein by reference.
The information contained in this Item 7.01, including in Exhibit 99.1 hereto and on Tyra’s corporate website, is being “furnished” and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the Exchange Act), is not subject to the liabilities of that section and is not deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
On October 24, 2024, the Company announced interim clinical proof-of-concept data for TYRA-300 in patients with mUC from its ongoing SURF301 Phase 1/2 study.
As of August 15, 2024, the data cutoff date, 41 patients were enrolled in the Phase 1 portion of the SURF301 Phase 1/2 study. Eligible participants were adults with advanced malignancies with or without FGFR3 alterations, including those with prior treatment with erdafitinib. The enrolled patient population was heavily pre-treated, with 44% of patients receiving ≥ 3 lines of therapy prior to receiving TYRA-300, and 76% of FGFR3+ mUC patients receiving ≥ 3 lines of therapy. Treatment with TYRA-300 was evaluated across six dose levels, ranging from 10 mg-120 mg once daily (QD).
| |
• |
|
Preliminary PK/PD analysis in 41 patients as of the data cutoff date: TYRA-300 plasma concentrations indicate adequate target coverage at ≥90 mg QD, with further pharmacokinetic characterization ongoing. |
| |
• |
|
In patients with FGFR3+ mUC who received doses ≥ 90 mg QD, anti-tumor activity was observed in all patients: |
| |
o |
6 out of 11 (54.5%) patients at ≥ 90 mg QD achieved a confirmed partial response (PR), 3 of which are still ongoing. |
| |
o |
5 out of 10 (50%) patients at 90 mg QD achieved a PR. |
| |
o |
1 out of 1 (100%) patient at 120 mg QD achieved a PR. |
| |
o |
A 100% disease control rate (DCR) was achieved for all patients at ≥ 90 mg QD (PR + stable disease). |
| |
• |
|
TYRA-300 has demonstrated favorable interim safety results as of the data cutoff date: |
| |
o |
Preliminary data from SURF301 suggest TYRA-300 to be generally well-tolerated, with infrequent FGFR2- and FGFR1-associated toxicities. |
| |
o |
In doses from 10 mg up to 120 mg QD, there were 4 (10%) serious adverse events related to TYRA-300, 1 dose-limiting toxicity (DLT) of grade (Gr) 3 diarrhea at 90 mg QD, and 1 treatment-related adverse event (TRAE) leading to discontinuation of treatment (Gr3 ALT, 90 mg QD). |
| |
o |
There were no ≥ Gr4 TRAEs. |
| |
o |
The 120 mg QD dose was the highest dose evaluated with no DLTs reported. |
Forward-Looking Statements
Tyra cautions you that statements contained in this report regarding matters that are not historical facts are forward-looking statements. The forward-looking statements are based on our current beliefs and expectations and include, but are not limited to: the potential safety and therapeutic benefits of TYRA-300. Actual results may differ from those set forth in this report due to the risks and uncertainties inherent in our business, including, without limitation: interim results of a clinical trial are not necessarily indicative of final results and one or more of the clinical outcomes may materially change as patient enrollment continues, following more comprehensive reviews of the data, as follow-up on the outcome of any particular patient continues and as more patient or final data becomes available, including the risk that unconfirmed responses may not ultimately result in confirmed responses to treatment after follow-up evaluations; the potential for proof-of-concept results to fail to result in successful subsequent development of TYRA-300;
we are early in our development efforts, have only recently begun testing TYRA-300 and TYRA-200 for oncology in clinical trials and the approach we are taking to discover and develop drugs based on our SNÅP platform is novel and unproven and it may never lead to product candidates that are successful in clinical development or approved products of commercial value; potential delays in the commencement, enrollment, data readouts and completion of preclinical studies and clinical trials; results from preclinical studies or early clinical trials not necessarily being predictive of future results; our dependence on third parties in connection with manufacturing, research and preclinical testing; acceptance by the FDA of INDs or of similar regulatory submissions by comparable foreign regulatory authorities for the conduct of clinical trials of TYRA-300 in pediatric achondroplasia and hypochondroplasia; an accelerated development or approval pathway may not be available for TYRA-300 or other product candidates and any such pathway may not lead to a faster development process; later developments with the FDA may be inconsistent with the minutes from our prior meetings, including with respect to the proposed design of our planned Phase 2 study of TYRA-300 in ACH; unexpected adverse side effects or inadequate efficacy of our product candidates that may limit their development, regulatory approval, and/or commercialization; the potential for our programs and prospects to be negatively impacted by developments relating to our competitors, including the results of studies or regulatory determinations relating to our competitors; unfavorable results from preclinical studies; we may not realize the benefits associated with ODD, including that orphan drug exclusivity may not effectively protect a product from competition and that such exclusivity may not be maintained, or from the RPD Designation, including receipt of a Priority Review Voucher or any value therefrom; regulatory developments in the United States and foreign countries; our ability to obtain and maintain intellectual property protection for our product candidates and proprietary technologies; and other risks described in our prior filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in our annual report on Form 10-K and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and we undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
|
|
| |
| Exhibit
No. |
|
Description |
|
| |
| 99.1 |
|
Slide Presentation |
|
| |
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
| |
|
| |
| |
| |
TYRA BIOSCIENCES, INC. |
|
|
|
| |
| Date: October 25, 2024 |
|
| |
By: |
|
/s/ Ali Fawaz |
|
| |
| |
| |
Ali Fawaz General Counsel and Secretary |

Interim clinical proof-of-concept with
TYRA-300 in mUC (SURF301) October 25, 2024 Exhibit 99.1

Today’s participants and agenda
AGENDA Introduction Todd Interim SURF301 TYRA-300 results Doug Q&A: Perspective of a leading Urologist Gary Todd Harris, PhD Doug Warner, MD Gary Steinberg, MD CEO, TYRA CMO, TYRA Professor of Urology, Dept. of Urology, Rush University Medical
Center

Disclaimers We caution you that this
presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our business strategy, research and development plans, the anticipated timing
and phase of development, costs, design and conduct of our ongoing and planned preclinical studies and clinical trials for our product candidates, the timing and likelihood of regulatory filings and approvals for our product candidates, the
potential to develop product candidates and for them to be first-in-class, and the potential safety and therapeutic benefits of our product candidates, our ability to commercialize our product candidates, if approved, the pricing and reimbursement
of our product candidates, if approved, the timing and likelihood of success, plans and objectives of management for future operations, and future results of anticipated product development efforts, are forward-looking statements. In some cases, you
can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,”
“target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar
expressions. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties
inherent in our business, including, without limitation: interim results of a clinical trial are not necessarily indicative of final results and one or more of the clinical outcomes may materially change as patient enrollment continues, following
more comprehensive reviews of the data, as follow-up on the outcome of any particular patient continues and as more patient or final data becomes available, including the risk that unconfirmed responses may not ultimately result in confirmed
responses to treatment after follow-up evaluations; the potential for proof-of-concept results to fail to result in successful subsequent development of TYRA-300; competitors; unfavorable results from preclinical studies; we may not realize the
benefits (i) associated with orphan drug designation, including that orphan drug exclusivity may not effectively protect a product from competition and that such exclusivity may not be maintained or (ii) from the rare pediatric disease designation,
including potential to receive a Priority Review Voucher (PRV) or derive any value therefrom; regulatory developments in the United States and foreign countries; our ability to obtain and maintain intellectual property protection for our product
candidates and proprietary technologies; and other risks described in our prior filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in our annual report on Form 10-K and any subsequent
filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and we undertake no obligation to update such statements to reflect events that occur or circumstances
that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation
also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to
give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These
and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. we are early in our development efforts, have only recently begun testing TYRA-300 and TYRA-200 for oncology
in clinical trials and the approach we are taking to discover and develop drugs based on our SNÅP platform is novel and unproven and it may never lead to product candidates that are successful in clinical development or approved products of
commercial value; potential delays in the commencement, enrollment, data readouts, and completion of preclinical studies and clinical trials; results from preclinical studies or early clinical trials not necessarily being predictive of future
results; our dependence on third parties in connection with manufacturing, research and preclinical testing; we may expend our limited resources to pursue a particular product candidate and/or indication and fail to capitalize on product candidates
or indications with greater development or commercial potential; acceptance by the FDA of INDs or of similar regulatory submissions by comparable foreign regulatory authorities for the conduct of clinical trials of TYRA-300 in pediatric
achondroplasia or hypochondroplasia; an accelerated development or approval pathway may not be available for TYRA-300 or other product candidates and any such pathway may not lead to a faster development process; later developments with the FDA may
be inconsistent with the minutes from our prior meetings, including with respect to the design of our planned Phase 2 study of TYRA-300 in ACH; unexpected adverse side effects or inadequate efficacy of our product candidates that may limit their
development, regulatory approval, and/or commercialization; the potential for our programs and prospects to be negatively impacted by developments relating to our competitors, including the results of studies or regulatory determinations relating to
our Forward-looking statements and market data
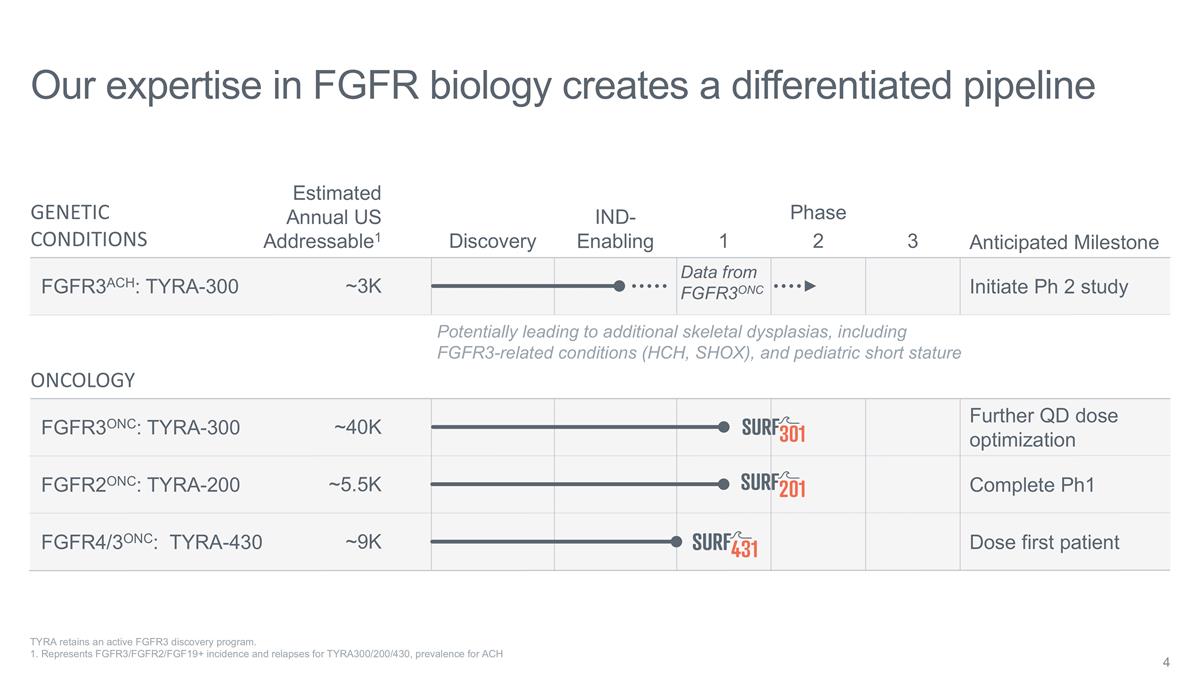
Our expertise in FGFR biology creates
a differentiated pipeline Discovery IND-Enabling 1 2 3 Phase Estimated Annual US Addressable1 GENETIC CONDITIONS FGFR3ACH: TYRA-300 ~3K Potentially leading to additional skeletal dysplasias, including FGFR3-related conditions (HCH, SHOX), and
pediatric short stature ONCOLOGY ~9K ~40K ~5.5K FGFR3ONC: TYRA-300 FGFR4/3ONC: TYRA-430 FGFR2ONC: TYRA-200 TYRA retains an active FGFR3 discovery program. 1. Represents FGFR3/FGFR2/FGF19+ incidence and relapses for TYRA300/200/430, prevalence for
ACH Data from FGFR3ONC Anticipated Milestone Initiate Ph 2 study Further QD dose optimization Complete Ph1 Dose first patient
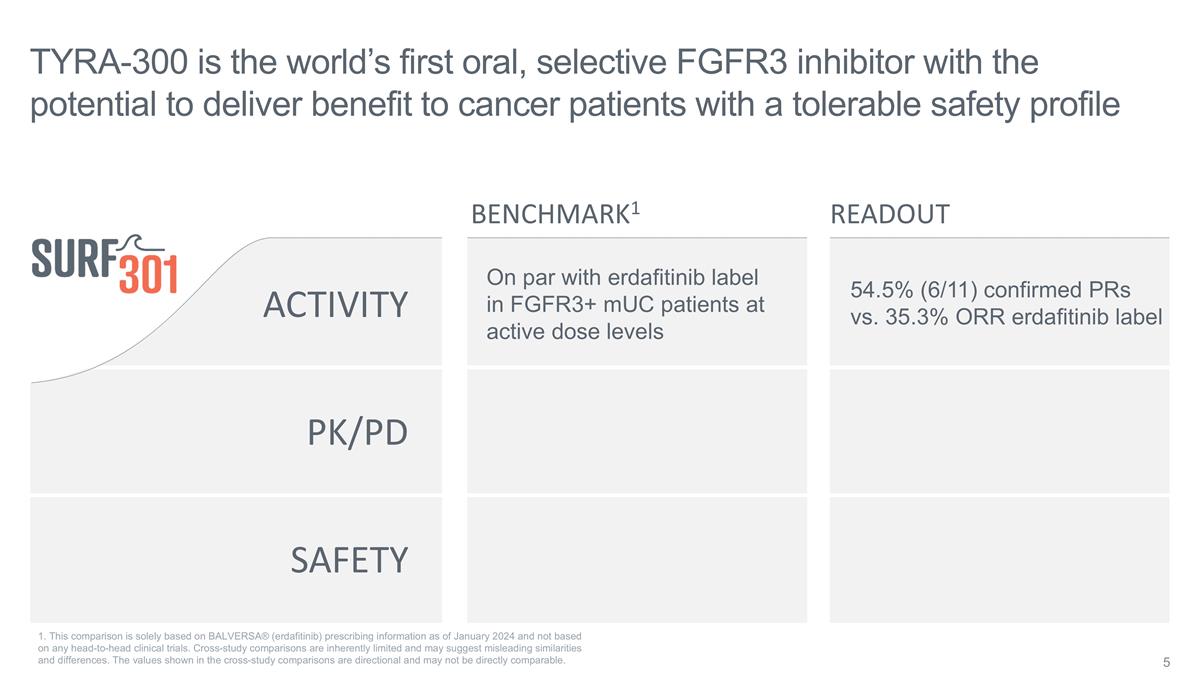
SAFETY ACTIVITY PK/PD On par with
erdafitinib label in FGFR3+ mUC patients at active dose levels TYRA-300 is the world’s first oral, selective FGFR3 inhibitor with the potential to deliver benefit to cancer patients with a tolerable safety profile BENCHMARK1 READOUT 54.5%
(6/11) confirmed PRs vs. 35.3% ORR erdafitinib label 1. This comparison is solely based on BALVERSA® (erdafitinib) prescribing information as of January 2024 and not based on any head-to-head clinical trials. Cross-study comparisons are
inherently limited and may suggest misleading similarities and differences. The values shown in the cross-study comparisons are directional and may not be directly comparable.
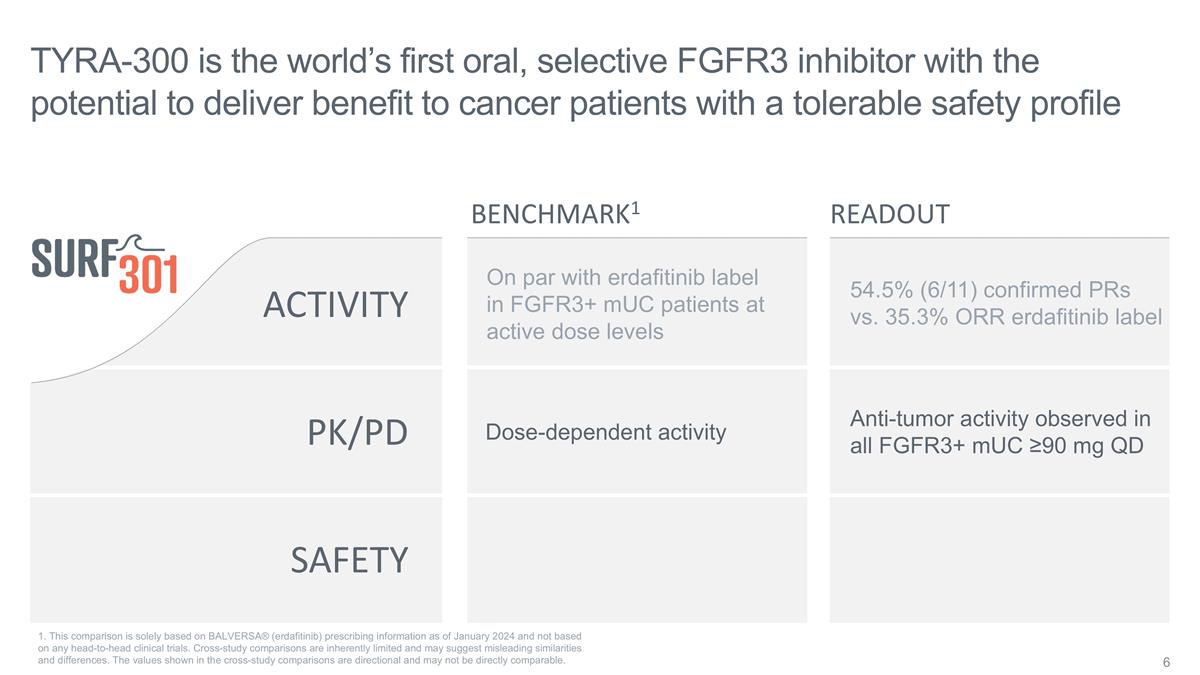
SAFETY ACTIVITY PK/PD On par with
erdafitinib label in FGFR3+ mUC patients at active dose levels Dose-dependent activity TYRA-300 is the world’s first oral, selective FGFR3 inhibitor with the potential to deliver benefit to cancer patients with a tolerable safety profile
Anti-tumor activity observed in all FGFR3+ mUC ≥90 mg QD BENCHMARK1 READOUT 54.5% (6/11) confirmed PRs vs. 35.3% ORR erdafitinib label 1. This comparison is solely based on BALVERSA® (erdafitinib) prescribing information as of January
2024 and not based on any head-to-head clinical trials. Cross-study comparisons are inherently limited and may suggest misleading similarities and differences. The values shown in the cross-study comparisons are directional and may not be directly
comparable.
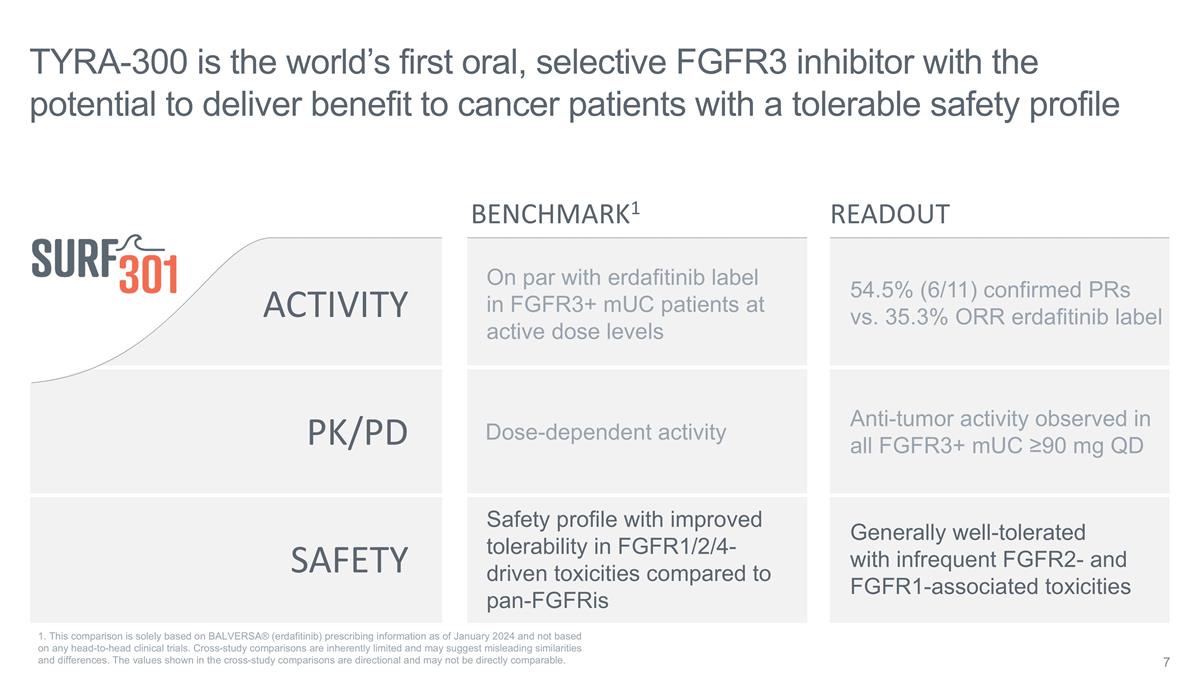
SAFETY ACTIVITY PK/PD On par with
erdafitinib label in FGFR3+ mUC patients at active dose levels Dose-dependent activity Safety profile with improved tolerability in FGFR1/2/4-driven toxicities compared to pan-FGFRis TYRA-300 is the world’s first oral, selective FGFR3
inhibitor with the potential to deliver benefit to cancer patients with a tolerable safety profile Anti-tumor activity observed in all FGFR3+ mUC ≥90 mg QD Generally well-tolerated with infrequent FGFR2- and FGFR1-associated toxicities
BENCHMARK1 READOUT 54.5% (6/11) confirmed PRs vs. 35.3% ORR erdafitinib label 1. This comparison is solely based on BALVERSA® (erdafitinib) prescribing information as of January 2024 and not based on any head-to-head clinical trials.
Cross-study comparisons are inherently limited and may suggest misleading similarities and differences. The values shown in the cross-study comparisons are directional and may not be directly comparable.

INTERIM PHASE 1 RESULTS ENA
2024
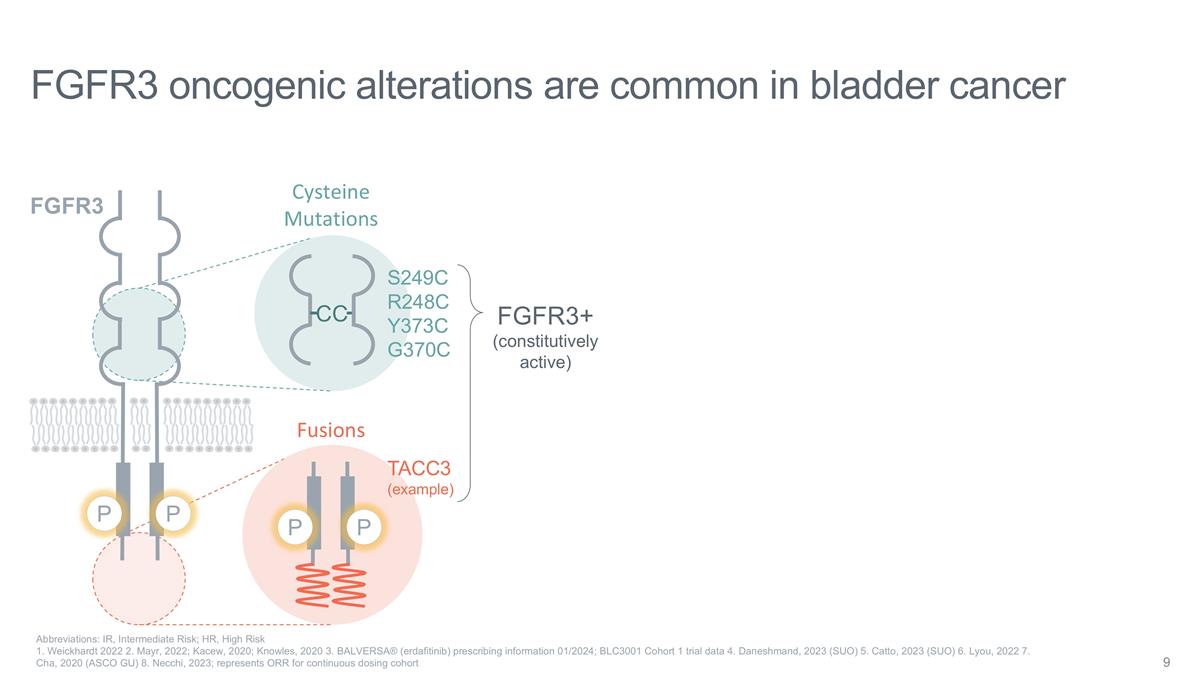
Cysteine Mutations CC S249C R248C
Y373C G370C P P Fusions TACC3 (example) FGFR3 oncogenic alterations are common in bladder cancer Abbreviations: IR, Intermediate Risk; HR, High Risk 1. Weickhardt 2022 2. Mayr, 2022; Kacew, 2020; Knowles, 2020 3. BALVERSA® (erdafitinib)
prescribing information 01/2024; BLC3001 Cohort 1 trial data 4. Daneshmand, 2023 (SUO) 5. Catto, 2023 (SUO) 6. Lyou, 2022 7. Cha, 2020 (ASCO GU) 8. Necchi, 2023; represents ORR for continuous dosing cohort P FGFR3 P FGFR3+ (constitutively
active)
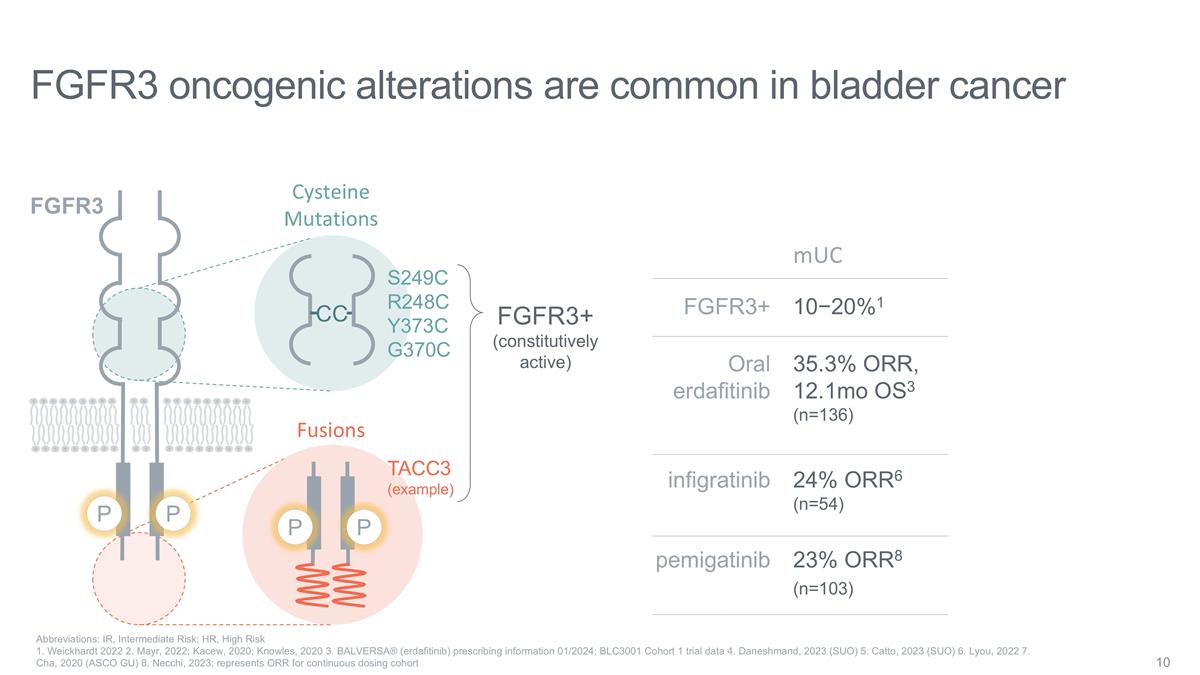
Cysteine Mutations CC S249C R248C
Y373C G370C P P Fusions TACC3 (example) FGFR3 oncogenic alterations are common in bladder cancer Abbreviations: IR, Intermediate Risk; HR, High Risk 1. Weickhardt 2022 2. Mayr, 2022; Kacew, 2020; Knowles, 2020 3. BALVERSA® (erdafitinib)
prescribing information 01/2024; BLC3001 Cohort 1 trial data 4. Daneshmand, 2023 (SUO) 5. Catto, 2023 (SUO) 6. Lyou, 2022 7. Cha, 2020 (ASCO GU) 8. Necchi, 2023; represents ORR for continuous dosing cohort P FGFR3 P FGFR3+ (constitutively active)
mUC FGFR3+ Oral erdafitinib 10−20%1 35.3% ORR, 12.1mo OS3 (n=136) pemigatinib infigratinib 23% ORR8 (n=103) 24% ORR6 (n=54)
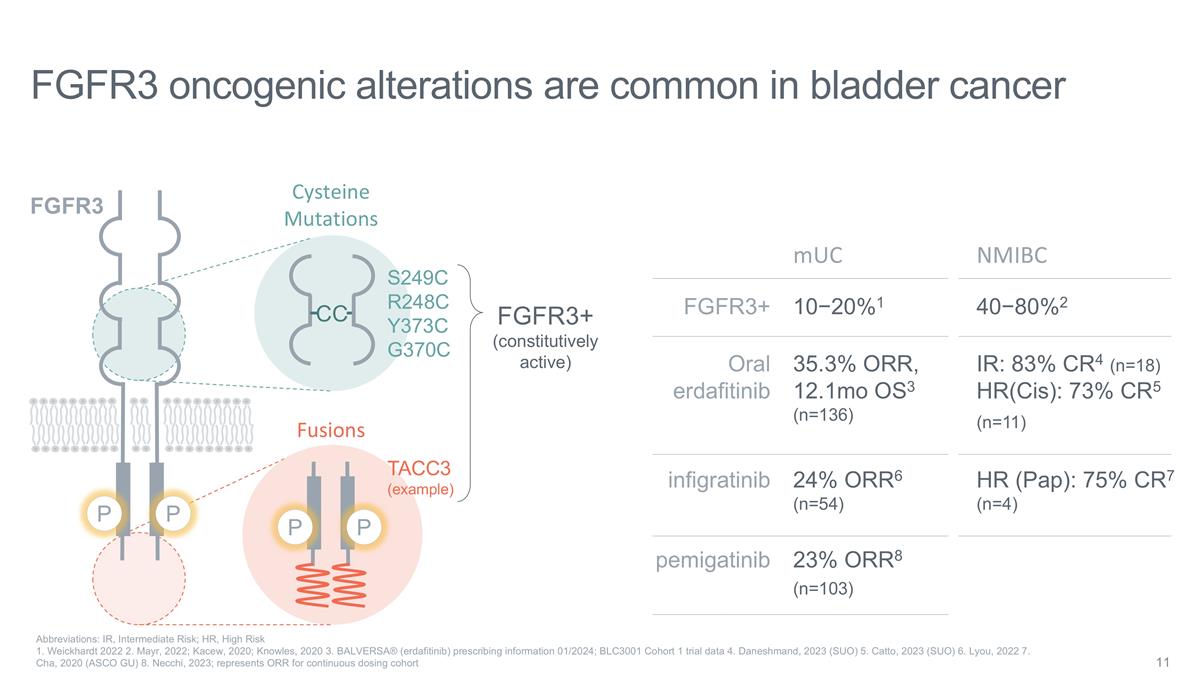
Cysteine Mutations CC S249C R248C
Y373C G370C P P Fusions TACC3 (example) FGFR3 oncogenic alterations are common in bladder cancer Abbreviations: IR, Intermediate Risk; HR, High Risk 1. Weickhardt 2022 2. Mayr, 2022; Kacew, 2020; Knowles, 2020 3. BALVERSA® (erdafitinib)
prescribing information 01/2024; BLC3001 Cohort 1 trial data 4. Daneshmand, 2023 (SUO) 5. Catto, 2023 (SUO) 6. Lyou, 2022 7. Cha, 2020 (ASCO GU) 8. Necchi, 2023; represents ORR for continuous dosing cohort P FGFR3 P FGFR3+ (constitutively active)
mUC FGFR3+ Oral erdafitinib 10−20%1 35.3% ORR, 12.1mo OS3 (n=136) pemigatinib infigratinib 23% ORR8 (n=103) 24% ORR6 (n=54) NMIBC 40−80%2 IR: 83% CR4 (n=18) HR(Cis): 73% CR5 (n=11) HR (Pap): 75% CR7 (n=4)
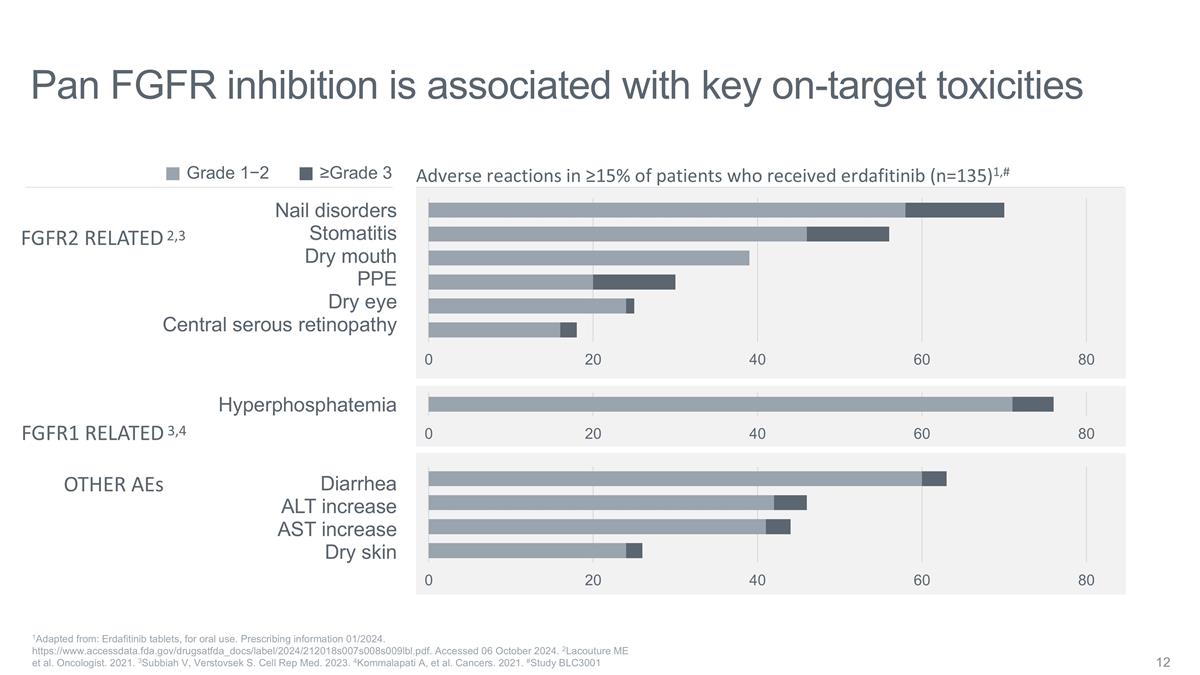
Pan FGFR inhibition is associated
with key on-target toxicities Nail disorders Stomatitis Dry mouth PPE Dry eye Central serous retinopathy Diarrhea ALT increase AST increase Dry skin Hyperphosphatemia Adverse reactions in ≥15% of patients who received erdafitinib (n=135)1,#
FGFR2 RELATED FGFR1 RELATED OTHER AEs ≥Grade 3 Grade 1−2 1Adapted from: Erdafitinib tablets, for oral use. Prescribing information 01/2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/212018s007s008s009lbl.pdf. Accessed 06
October 2024. 2Lacouture ME et al. Oncologist. 2021. 3Subbiah V, Verstovsek S. Cell Rep Med. 2023. 4Kommalapati A, et al. Cancers. 2021. #Study BLC3001 2,3 3,4
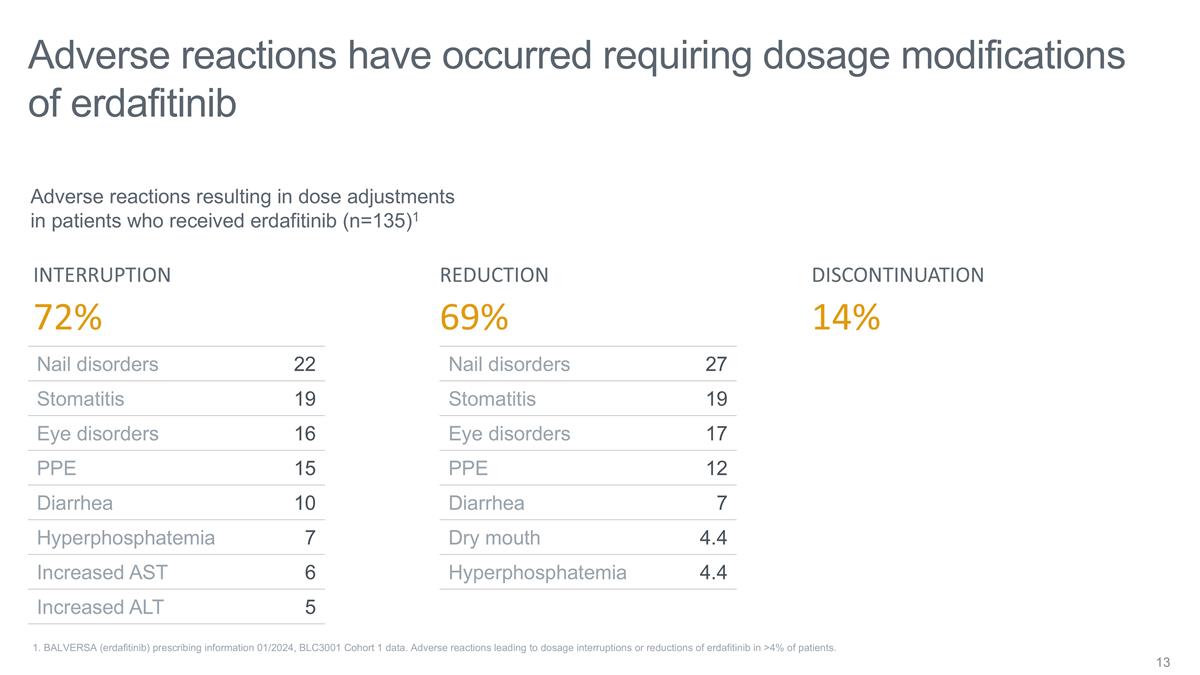
Adverse reactions have occurred
requiring dosage modifications of erdafitinib 1. BALVERSA (erdafitinib) prescribing information 01/2024, BLC3001 Cohort 1 data. Adverse reactions leading to dosage interruptions or reductions of erdafitinib in >4% of patients. Nail disorders 22
Stomatitis 19 Eye disorders 16 PPE 15 Diarrhea 10 Hyperphosphatemia 7 Increased AST 6 Increased ALT 5 Nail disorders 27 Stomatitis 19 Eye disorders 17 PPE 12 Diarrhea 7 Dry mouth 4.4 Hyperphosphatemia 4.4 DISCONTINUATION INTERRUPTION REDUCTION 14%
72% 69% Adverse reactions resulting in dose adjustments in patients who received erdafitinib (n=135)1
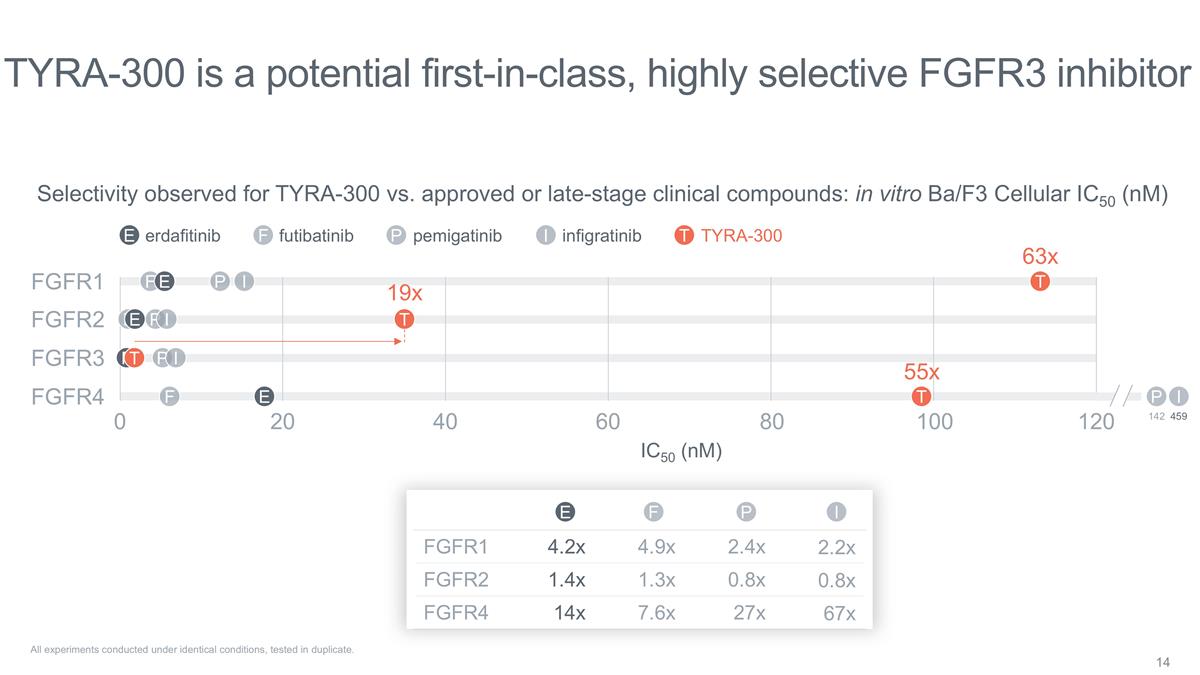
TYRA-300 is a potential
first-in-class, highly selective FGFR3 inhibitor All experiments conducted under identical conditions, tested in duplicate. 0 20 40 60 80 120 Selectivity observed for TYRA-300 vs. approved or late-stage clinical compounds: in vitro Ba/F3 Cellular
IC50 (nM) FGFR1 FGFR2 FGFR3 FGFR4 P F E I T P F E I T P E I P F E I T 459 142 F T T TYRA-300 I infigratinib erdafitinib E pemigatinib P futibatinib F 100 19x 63x 55x FGFR1 4.2x 4.9x 2.4x 2.2x FGFR2 1.4x 1.3x 0.8x 0.8x FGFR4 14x 7.6x 27x 67x I P F E
IC50 (nM)
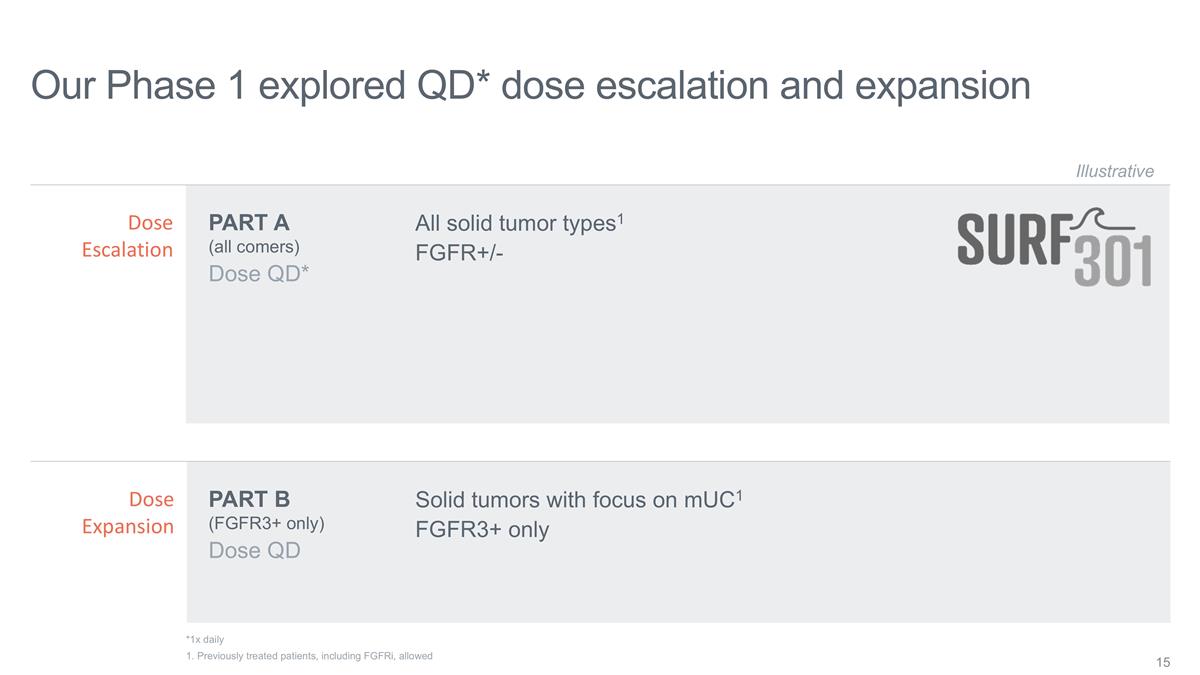
Our Phase 1 explored QD* dose
escalation and expansion Dose QD* Illustrative Dose QD PART A (all comers) PART B (FGFR3+ only) Dose Escalation Dose Expansion *1x daily All solid tumor types1 FGFR+/- Solid tumors with focus on mUC1 FGFR3+ only 1. Previously treated patients,
including FGFRi, allowed
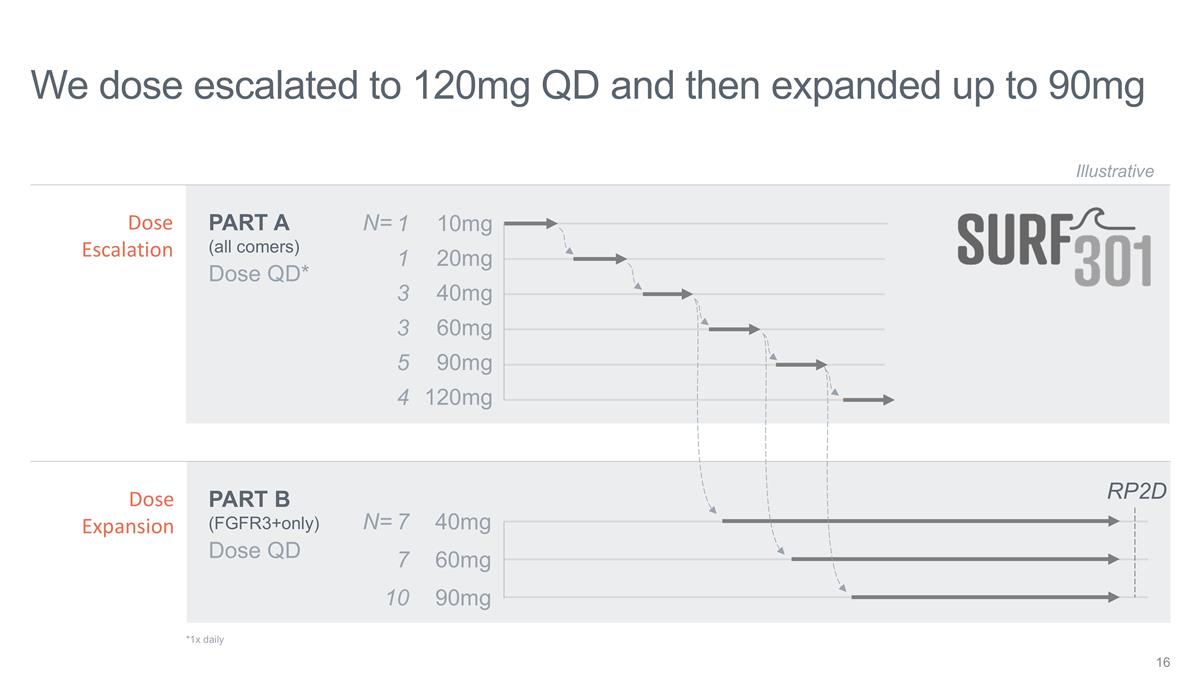
We dose escalated to 120mg QD and
then expanded up to 90mg Dose QD* 10mg 20mg 40mg 60mg Illustrative Dose QD 40mg 60mg PART A (all comers) PART B (FGFR3+only) 90mg RP2D Dose Escalation Dose Expansion 120mg 1 1 3 3 7 7 10 5 4 90mg N= N= *1x daily
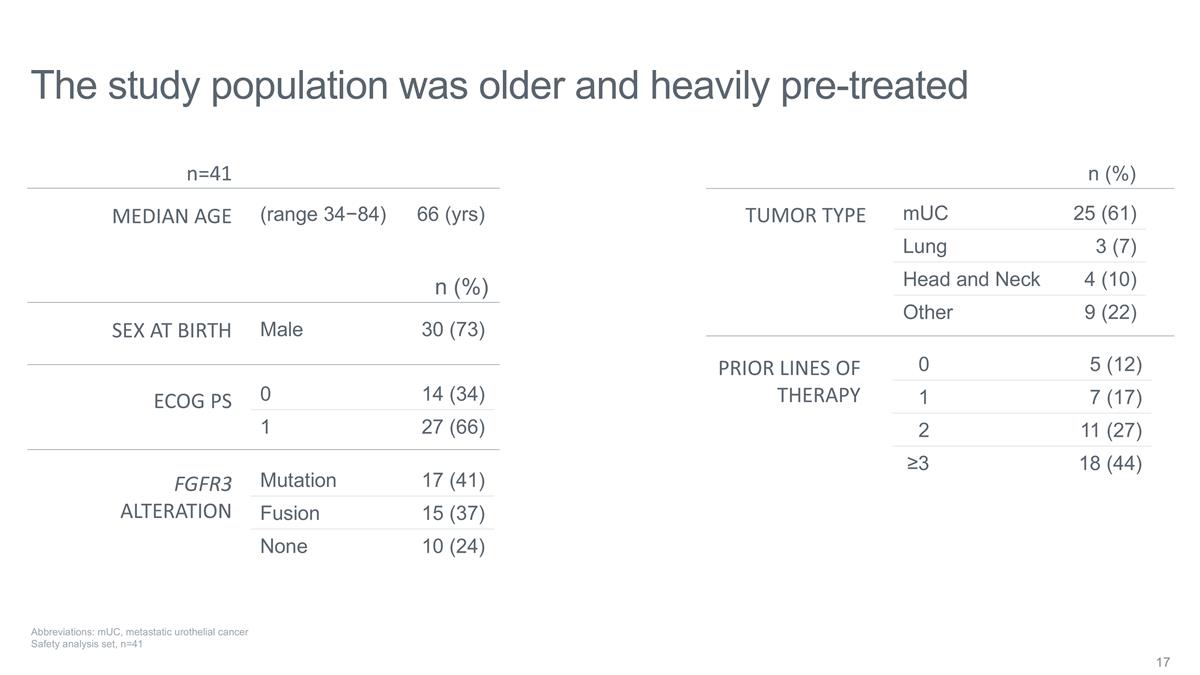
The study population was older and
heavily pre-treated mUC 25 (61) Lung 3 (7) Head and Neck 4 (10) Other 9 (22) Mutation 17 (41) Fusion 15 (37) None 10 (24) 0 5 (12) 1 7 (17) 2 11 (27) ≥3 18 (44) TUMOR TYPE FGFR3 ALTERATION PRIOR LINES OF THERAPY n=41 (range 34−84) 66
(yrs) MEDIAN AGE SEX AT BIRTH ECOG PS Male 30 (73) n (%) 0 14 (34) 1 27 (66) n (%) Abbreviations: mUC, metastatic urothelial cancer Safety analysis set, n=41
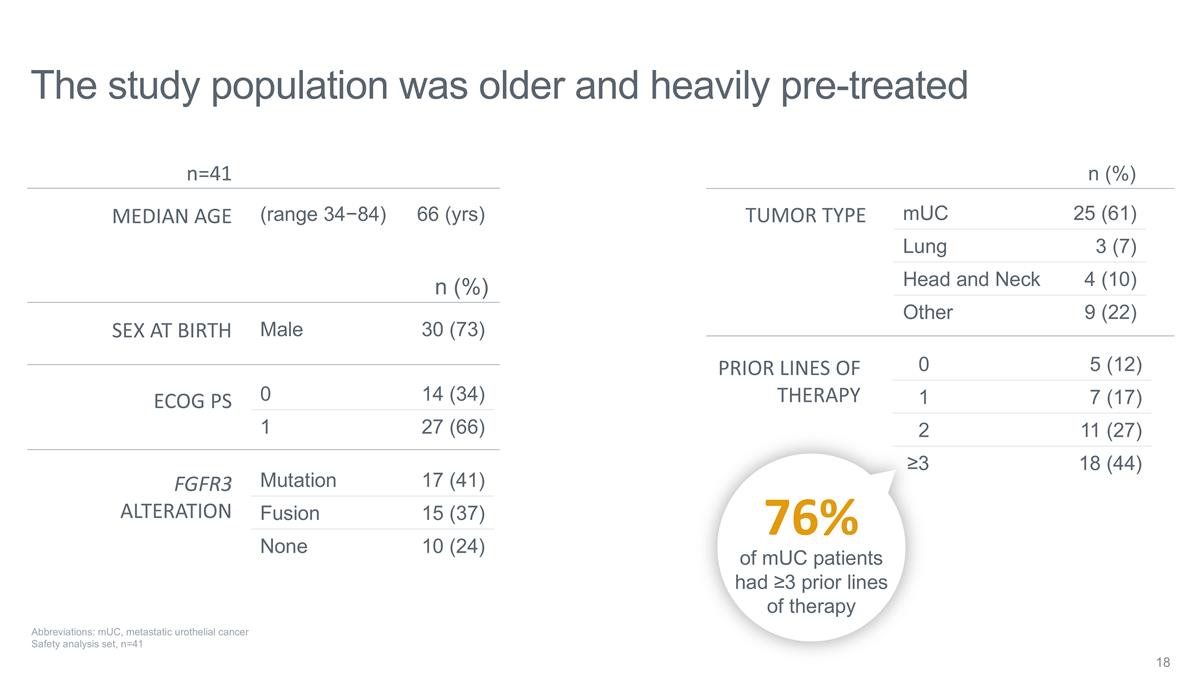
The study population was older and
heavily pre-treated mUC 25 (61) Lung 3 (7) Head and Neck 4 (10) Other 9 (22) Mutation 17 (41) Fusion 15 (37) None 10 (24) 0 5 (12) 1 7 (17) 2 11 (27) ≥3 18 (44) TUMOR TYPE FGFR3 ALTERATION PRIOR LINES OF THERAPY n=41 (range 34−84) 66
(yrs) MEDIAN AGE SEX AT BIRTH ECOG PS Male 30 (73) n (%) 0 14 (34) 1 27 (66) n (%) Abbreviations: mUC, metastatic urothelial cancer Safety analysis set, n=41 76% of mUC patients had ≥3 prior lines of therapy
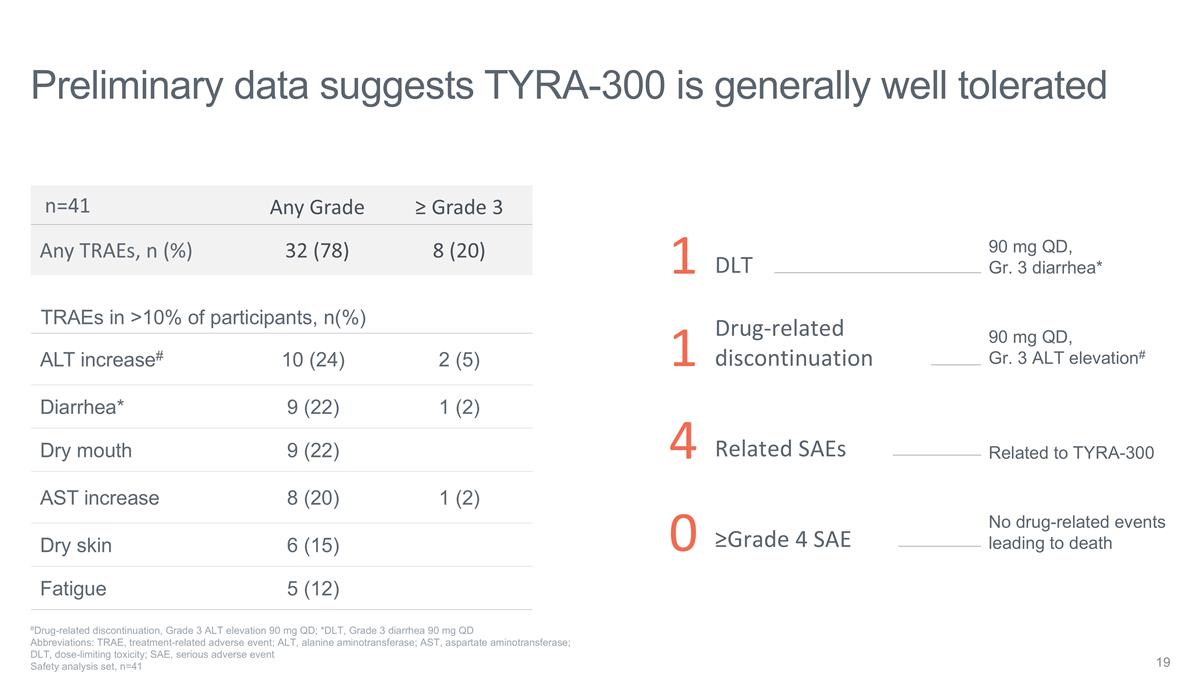
Preliminary data suggests TYRA-300
is generally well tolerated Any Grade ≥ Grade 3 Any TRAEs, n (%) 32 (78) 8 (20) TRAEs in >10% of participants, n(%) ALT increase# 10 (24) 2 (5) Diarrhea* 9 (22) 1 (2) Dry mouth 9 (22) AST increase 8 (20) 1 (2) Dry skin 6 (15) Fatigue 5 (12)
#Drug-related discontinuation, Grade 3 ALT elevation 90 mg QD; *DLT, Grade 3 diarrhea 90 mg QD Abbreviations: TRAE, treatment-related adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DLT, dose-limiting toxicity; SAE,
serious adverse event Safety analysis set, n=41 n=41 1 DLT 90 mg QD, Gr. 3 diarrhea* 1 Drug-related discontinuation 90 mg QD, Gr. 3 ALT elevation# 4 Related SAEs Related to TYRA-300 0 ≥Grade 4 SAE No drug-related events leading to
death
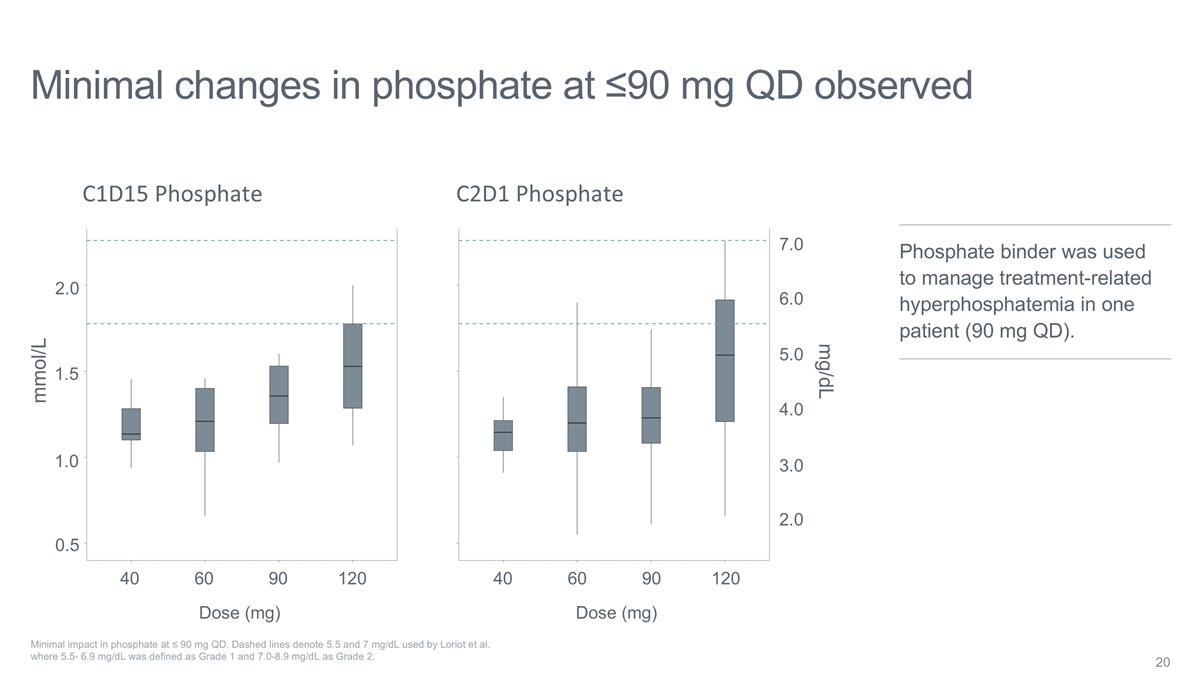
Minimal changes in phosphate at
≤90 mg QD observed Minimal impact in phosphate at ≤ 90 mg QD. Dashed lines denote 5.5 and 7 mg/dL used by Loriot et al. where 5.5- 6.9 mg/dL was defined as Grade 1 and 7.0-8.9 mg/dL as Grade 2. C1D15 Phosphate C2D1 Phosphate 2.0 1.5 1.0
0.5 40 60 90 120 40 60 90 120 mmol/L 2.0 3.0 4.0 5.0 6.0 7.0 mg/dL Dose (mg) Dose (mg) Phosphate binder was used to manage treatment-related hyperphosphatemia in one patient (90 mg QD).
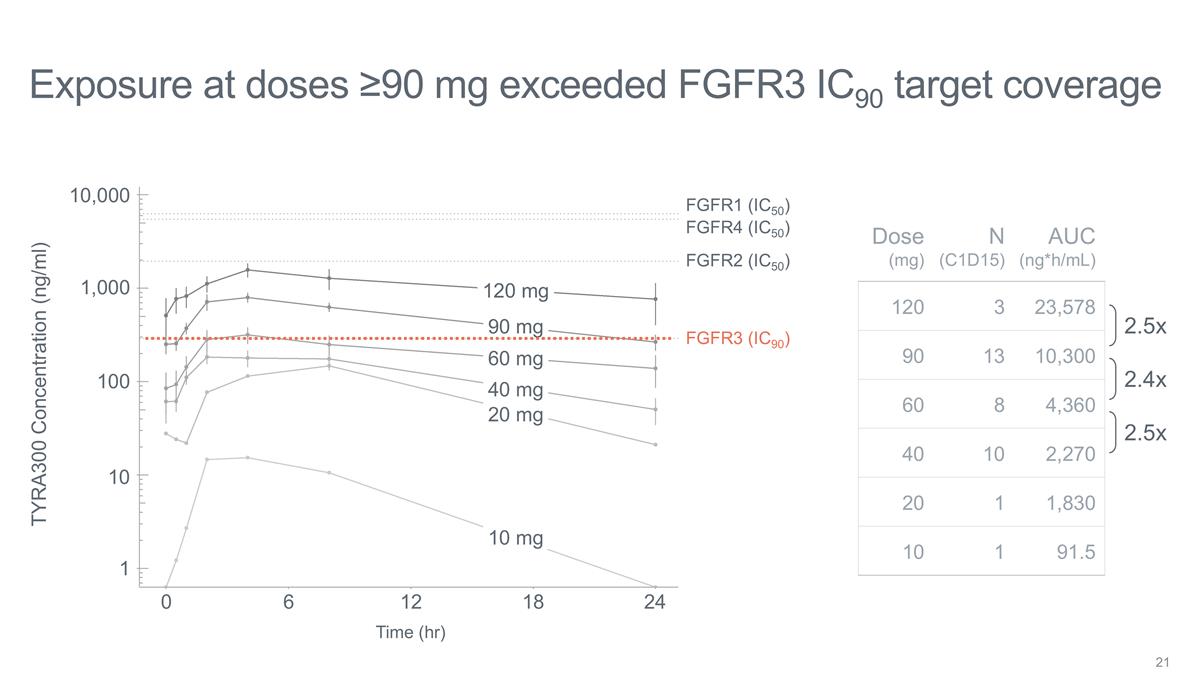
Exposure at doses ≥90 mg
exceeded FGFR3 IC90 target coverage 10,000 1,000 TYRA300 Concentration (ng/ml) 100 10 1 10 mg 20 mg 40 mg 60 mg 90 mg 120 mg 0 6 12 18 24 Time (hr) FGFR1 (IC50) FGFR4 (IC50) FGFR3 (IC90) FGFR2 (IC50) 2.5x 2.4x 2.5x Dose (mg) N (C1D15) AUC (ng*h/mL)
120 3 23,578 90 13 10,300 60 8 4,360 40 10 2,270 20 1 1,830 10 1 91.5
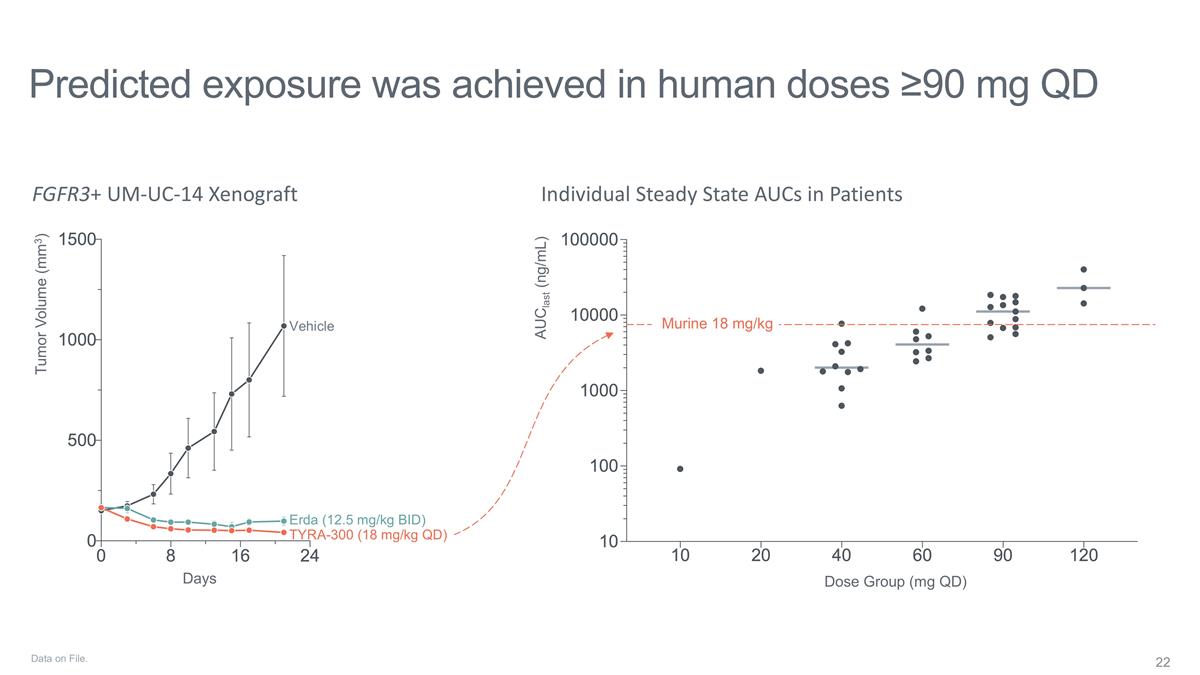
Predicted exposure was achieved in
human doses ≥90 mg QD FGFR3+ UM-UC-14 Xenograft Individual Steady State AUCs in Patients Murine 18 mg/kg Vehicle Erda (12.5 mg/kg BID) TYRA-300 (18 mg/kg QD) Days Dose Group (mg QD) AUClast (ng/mL) Tumor Volume (mm3) Data on File.
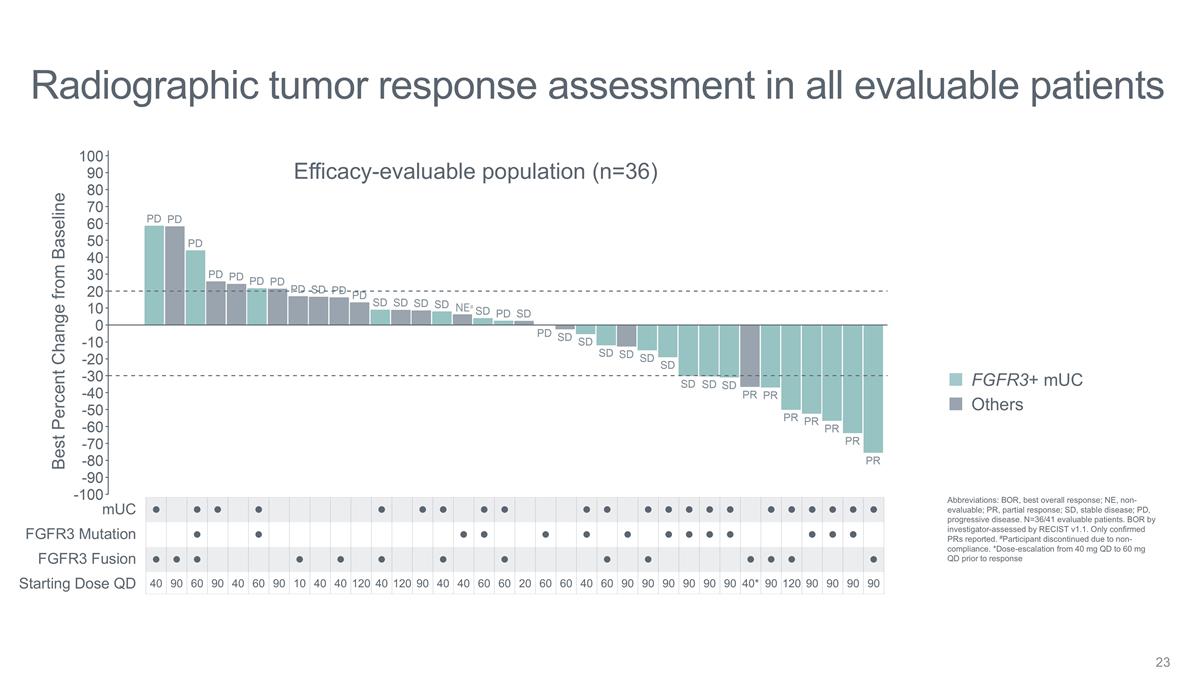
Radiographic tumor response
assessment in all evaluable patients Best Percent Change from Baseline Efficacy-evaluable population (n=36) FGFR3 Mutation FGFR3 Fusion mUC NE# FGFR3+ mUC Others Abbreviations: BOR, best overall response; NE, non-evaluable; PR, partial response; SD,
stable disease; PD, progressive disease. N=36/41 evaluable patients. BOR by investigator-assessed by RECIST v1.1. Only confirmed PRs reported. #Participant discontinued due to non-compliance. *Dose-escalation from 40 mg QD to 60 mg QD prior to
response Starting Dose QD l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l
l l l l l l l l l l l l l l l l l l 40 90 60 90 40 60 90 10 40 40 120 40 120 90 40 40 60 60 20 60 60 40 60 90 90 90 90 90 90 40* 90 120 90 90 90 90
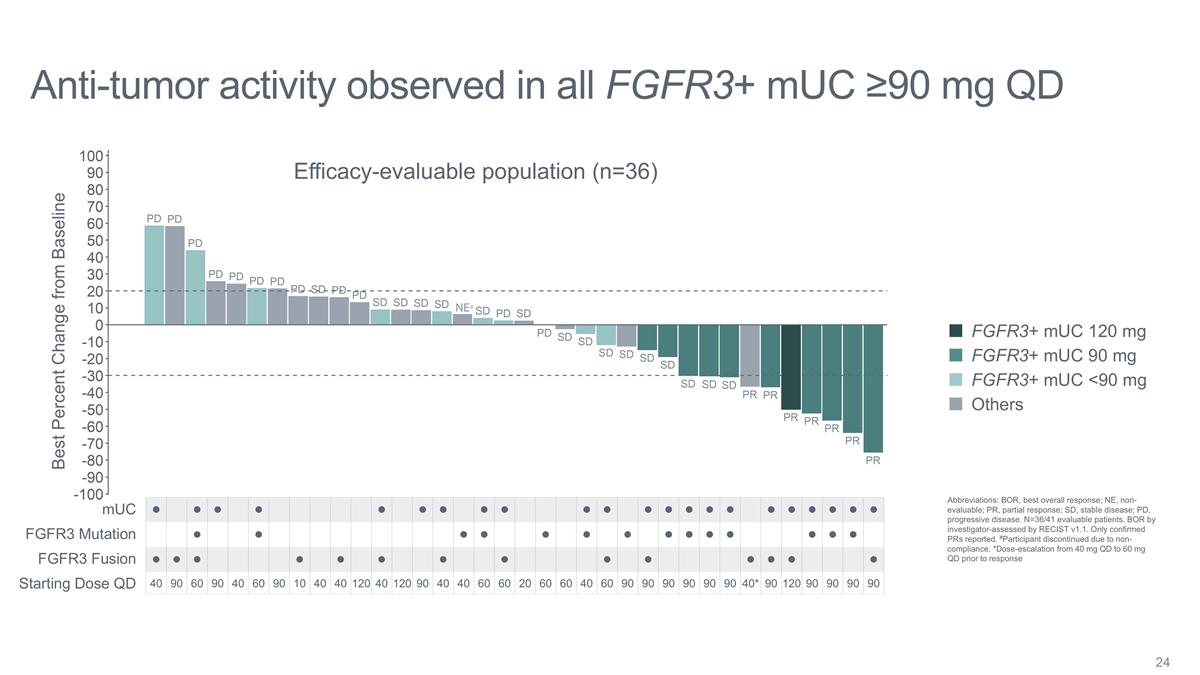
Anti-tumor activity observed in all
FGFR3+ mUC ≥90 mg QD Best Percent Change from Baseline l l l l l l l l l l l l l l l l l l l l l l l l l l l
l l l l l l l l l l l l l l l l l l l l l l l 40 90 60 90 40 60 90 10 40 40 120 40 120 90 40 40 60 60 20 60 60 40 60 90 90 90 90 90 90
40* 90 120 90 90 90 90 FGFR3 Mutation FGFR3 Fusion mUC Starting Dose QD Efficacy-evaluable population (n=36) FGFR3+ mUC <90 mg Others FGFR3+ mUC 90 mg FGFR3+ mUC 120 mg NE# Abbreviations: BOR, best overall response; NE, non-evaluable; PR, partial
response; SD, stable disease; PD, progressive disease. N=36/41 evaluable patients. BOR by investigator-assessed by RECIST v1.1. Only confirmed PRs reported. #Participant discontinued due to non-compliance. *Dose-escalation from 40 mg QD to 60 mg QD
prior to response
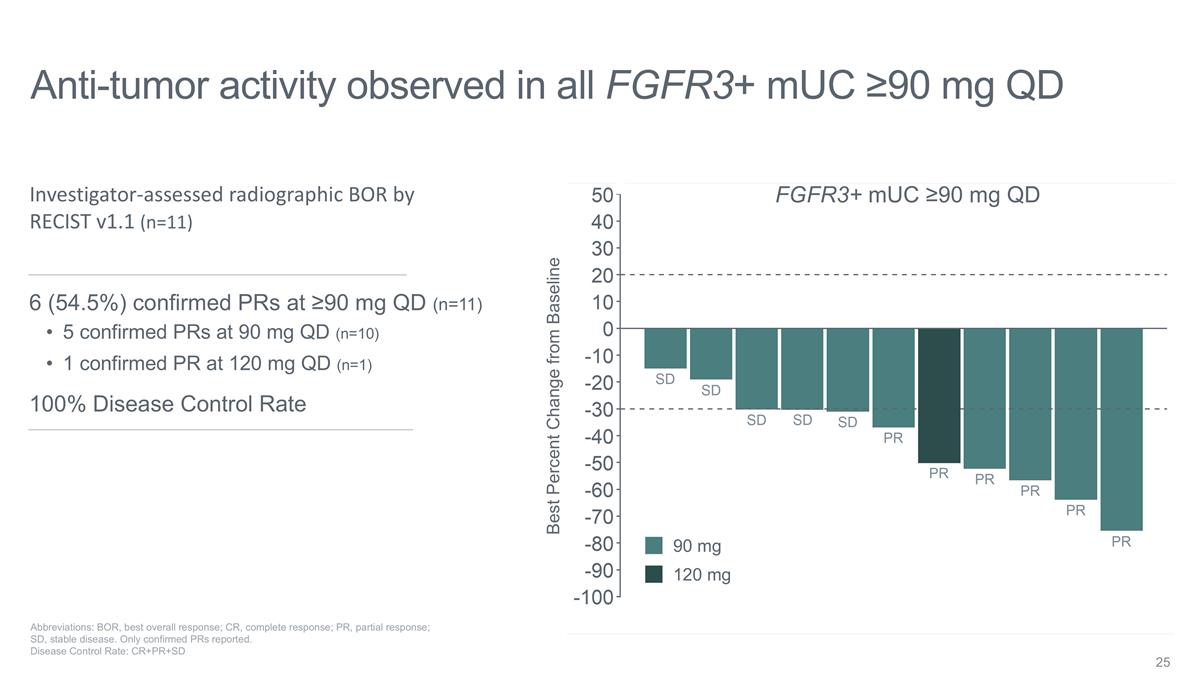
Anti-tumor activity observed in all
FGFR3+ mUC ≥90 mg QD Abbreviations: BOR, best overall response; CR, complete response; PR, partial response; SD, stable disease. Only confirmed PRs reported. Disease Control Rate: CR+PR+SD Best Percent Change from Baseline
Investigator-assessed radiographic BOR by RECIST v1.1 (n=11) 6 (54.5%) confirmed PRs at ≥90 mg QD (n=11) 5 confirmed PRs at 90 mg QD (n=10) 1 confirmed PR at 120 mg QD (n=1) 100% Disease Control Rate FGFR3+ mUC ≥90 mg QD 90 mg 120 mg
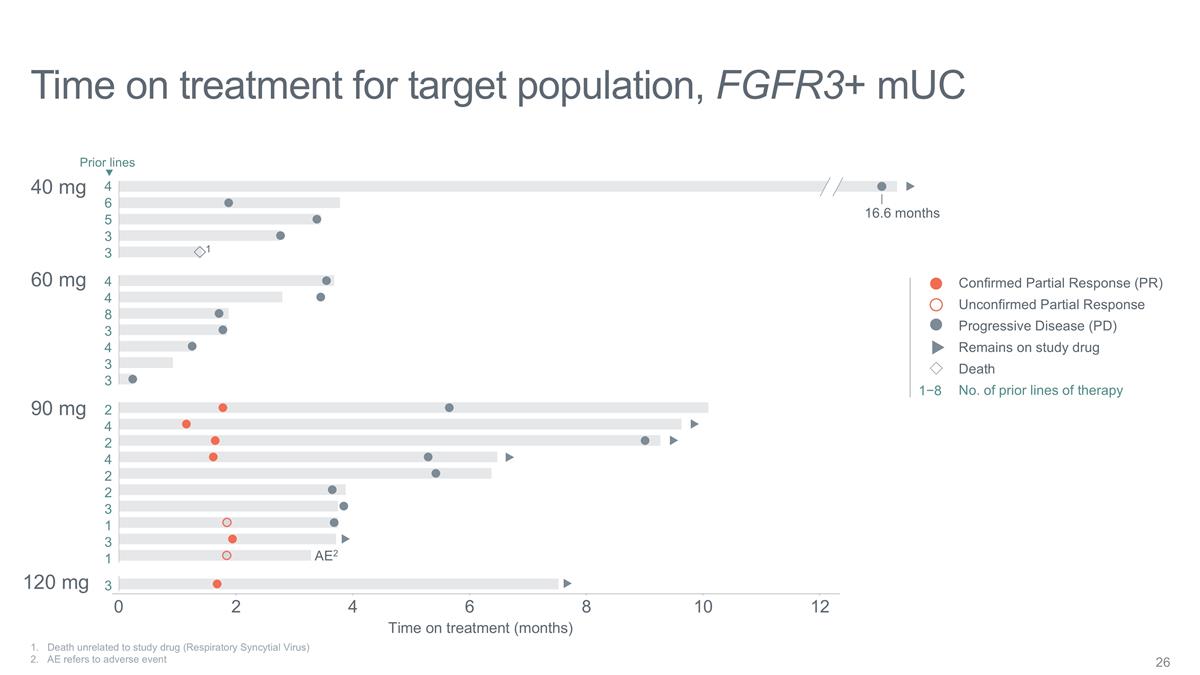
Time on treatment for target
population, FGFR3+ mUC Death unrelated to study drug (Respiratory Syncytial Virus) 2. AE refers to adverse event Time on treatment (months) Confirmed Partial Response (PR) Unconfirmed Partial Response Progressive Disease (PD) Remains on study drug
Death No. of prior lines of therapy 1−8 40 mg 60 mg 90 mg 120 mg 2 6 12 4 8 10 16.6 months AE2 0 4 6 5 3 3 4 4 8 3 4 2 4 2 4 2 2 3 1 3 1 3 3 3 Prior lines 1
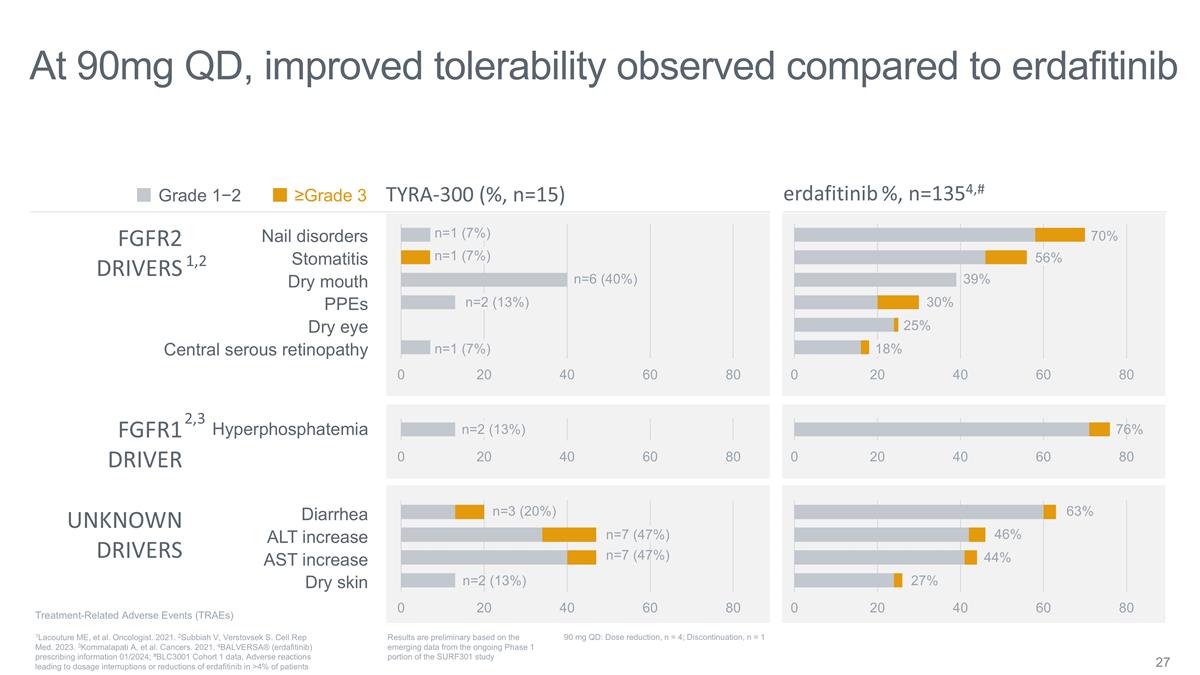
Nail disorders Stomatitis Dry mouth
PPEs Dry eye Central serous retinopathy Diarrhea ALT increase AST increase Dry skin Hyperphosphatemia erdafitinib %, n=1354,# TYRA-300 (%, n=15) FGFR2 DRIVERS FGFR1 DRIVER UNKNOWN DRIVERS ≥Grade 3 Grade 1−2 At 90mg QD, improved
tolerability observed compared to erdafitinib n=1 (7%) n=1 (7%) n=1 (7%) Treatment-Related Adverse Events (TRAEs) Results are preliminary based on the emerging data from the ongoing Phase 1 portion of the SURF301 study 1Lacouture ME, et al.
Oncologist. 2021. 2Subbiah V, Verstovsek S. Cell Rep Med. 2023. 3Kommalapati A, et al. Cancers. 2021. 4BALVERSA® (erdafitinib) prescribing information 01/2024; #BLC3001 Cohort 1 data, Adverse reactions leading to dosage interruptions or
reductions of erdafitinib in >4% of patients 1,2 2,3 90 mg QD: Dose reduction, n = 4; Discontinuation, n = 1 n=6 (40%) n=2 (13%) n=2 (13%) n=3 (20%) n=7 (47%) n=7 (47%) n=2 (13%) 56% 70% 18% 39% 30% 76% 63% 46% 44% 27% 25%
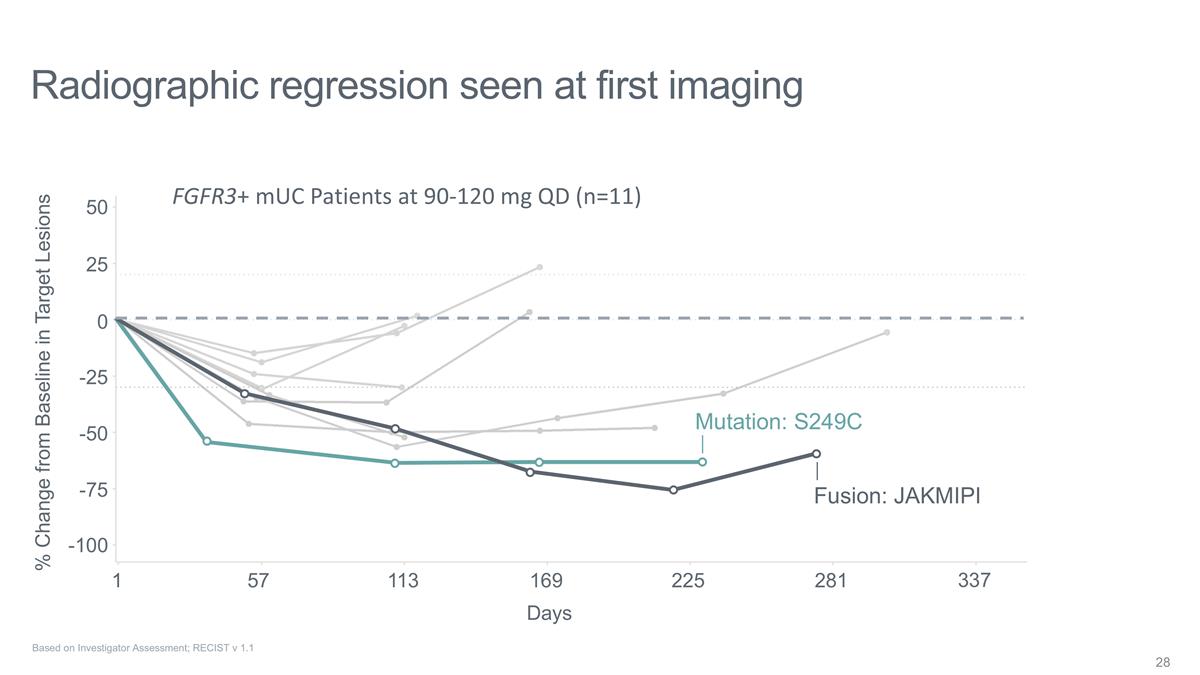
Radiographic regression seen at
first imaging Fusion: JAKMIPI Mutation: S249C FGFR3+ mUC Patients at 90-120 mg QD (n=11) 1 % Change from Baseline in Target Lesions 57 113 169 225 281 337 -100 -75 -50 -25 0 25 50 Days Based on Investigator Assessment; RECIST v 1.1
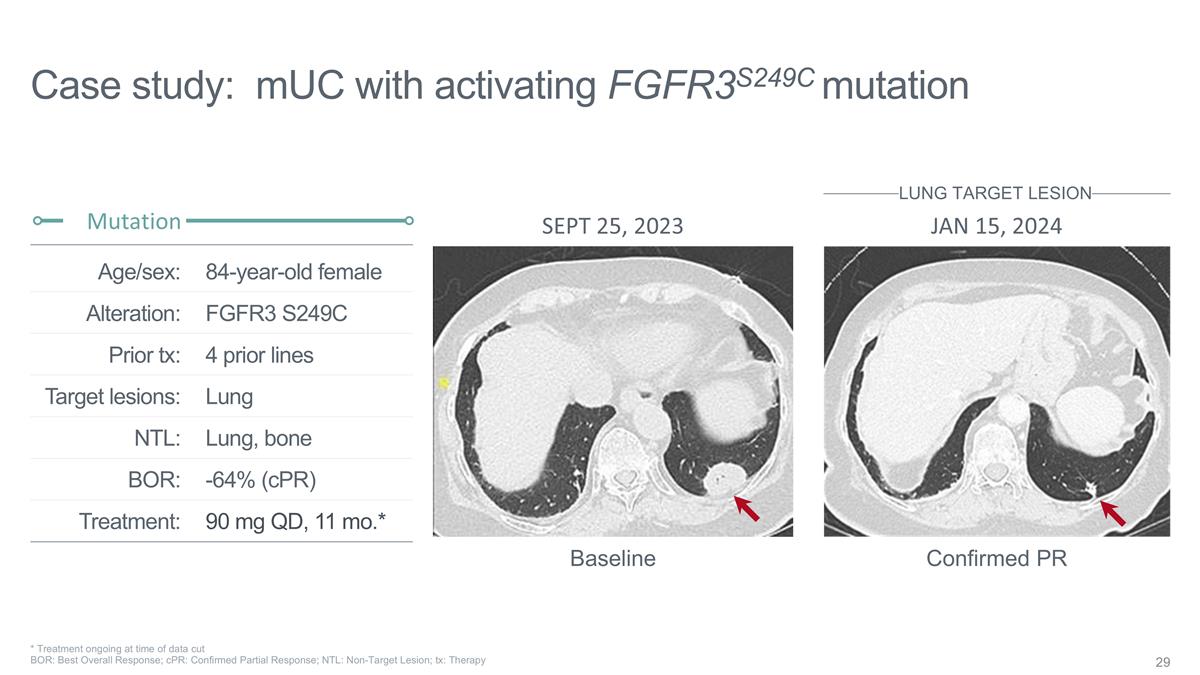
84-year-old female FGFR3 S249C 4
prior lines Lung Lung, bone -64% (cPR) 90 mg QD, 11 mo.* Age/sex: Alteration: Prior tx: Target lesions: NTL: BOR: Treatment: Case study: mUC with activating FGFR3S249C mutation Mutation * Treatment ongoing at time of data cut BOR: Best Overall
Response; cPR: Confirmed Partial Response; NTL: Non-Target Lesion; tx: Therapy Confirmed PR Baseline SEPT 25, 2023 JAN 15, 2024 LUNG TARGET LESION
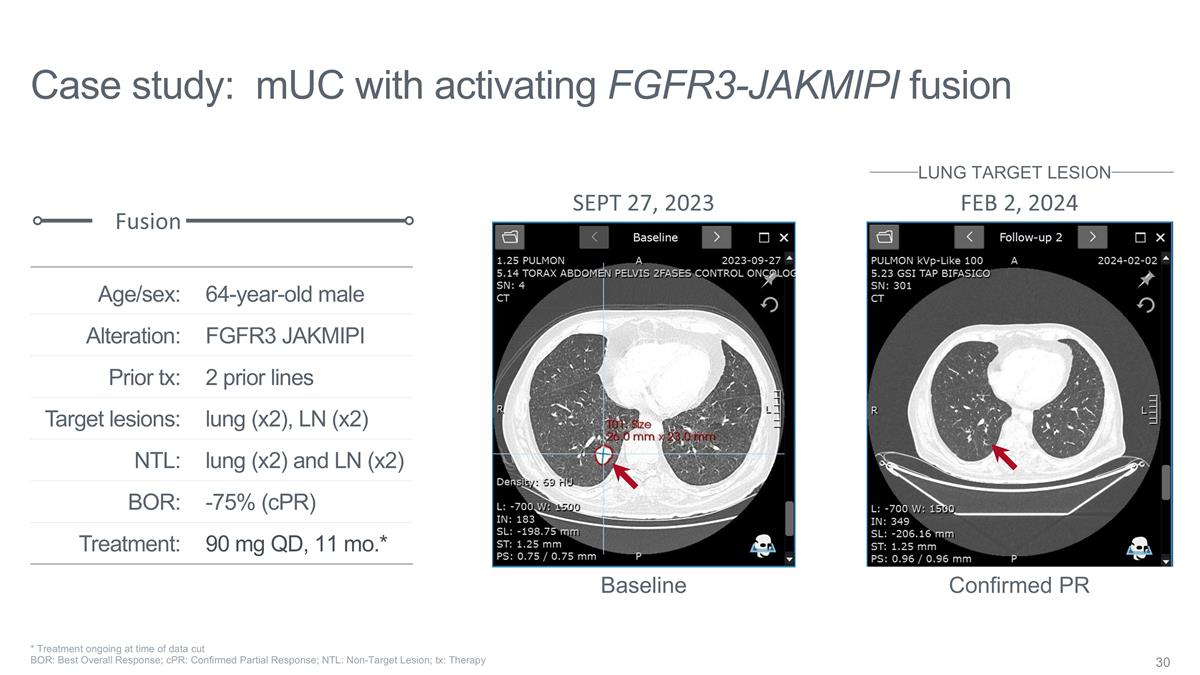
Case study: mUC with activating
FGFR3-JAKMIPI fusion 64-year-old male FGFR3 JAKMIPI 2 prior lines lung (x2), LN (x2) lung (x2) and LN (x2) -75% (cPR) 90 mg QD, 11 mo.* Age/sex: Alteration: Prior tx: Target lesions: NTL: BOR: Treatment: Fusion SEPT 27, 2023 FEB 2, 2024 Baseline
Confirmed PR LUNG TARGET LESION * Treatment ongoing at time of data cut BOR: Best Overall Response; cPR: Confirmed Partial Response; NTL: Non-Target Lesion; tx: Therapy
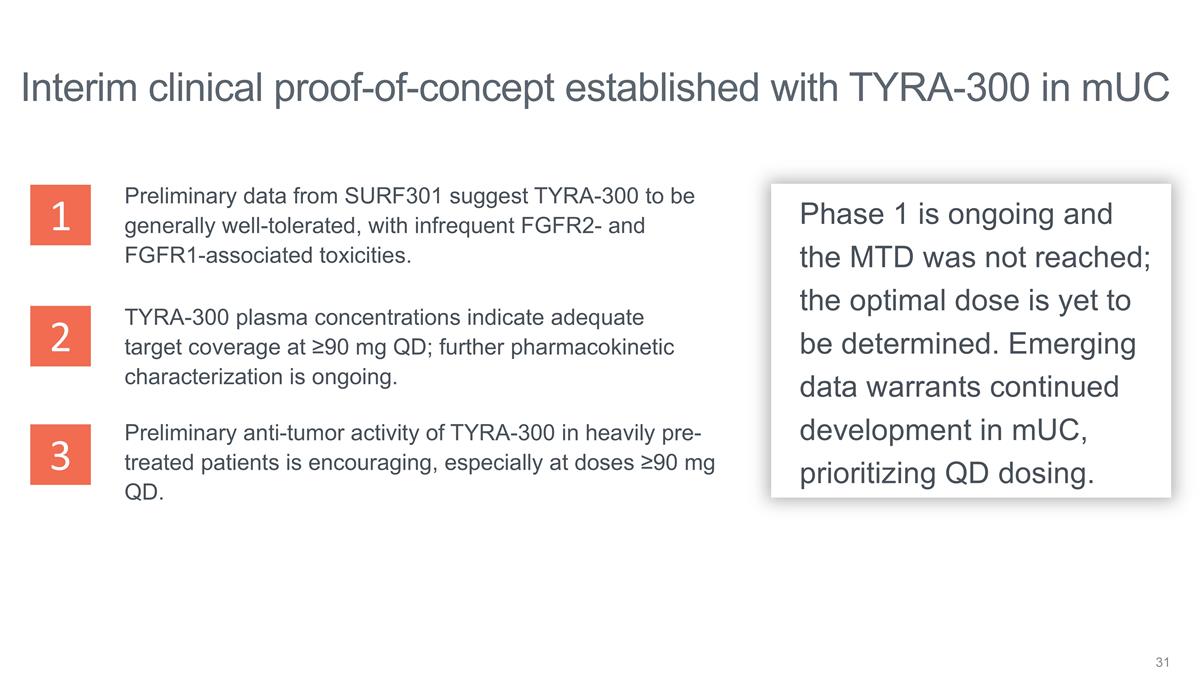
Interim clinical proof-of-concept
established with TYRA-300 in mUC TYRA-300 plasma concentrations indicate adequate target coverage at ≥90 mg QD; further pharmacokinetic characterization is ongoing. Preliminary anti-tumor activity of TYRA-300 in heavily pre-treated patients is
encouraging, especially at doses ≥90 mg QD. Preliminary data from SURF301 suggest TYRA-300 to be generally well-tolerated, with infrequent FGFR2- and FGFR1-associated toxicities. Phase 1 is ongoing and the MTD was not reached; the optimal dose
is yet to be determined. Emerging data warrants continued development in mUC, prioritizing QD dosing. 1 2 3

LOOKING AHEAD: TYRA-300 mUC
Improved toxicity profile NMIBC Patient-friendly oral ACH Differentiated efficacy
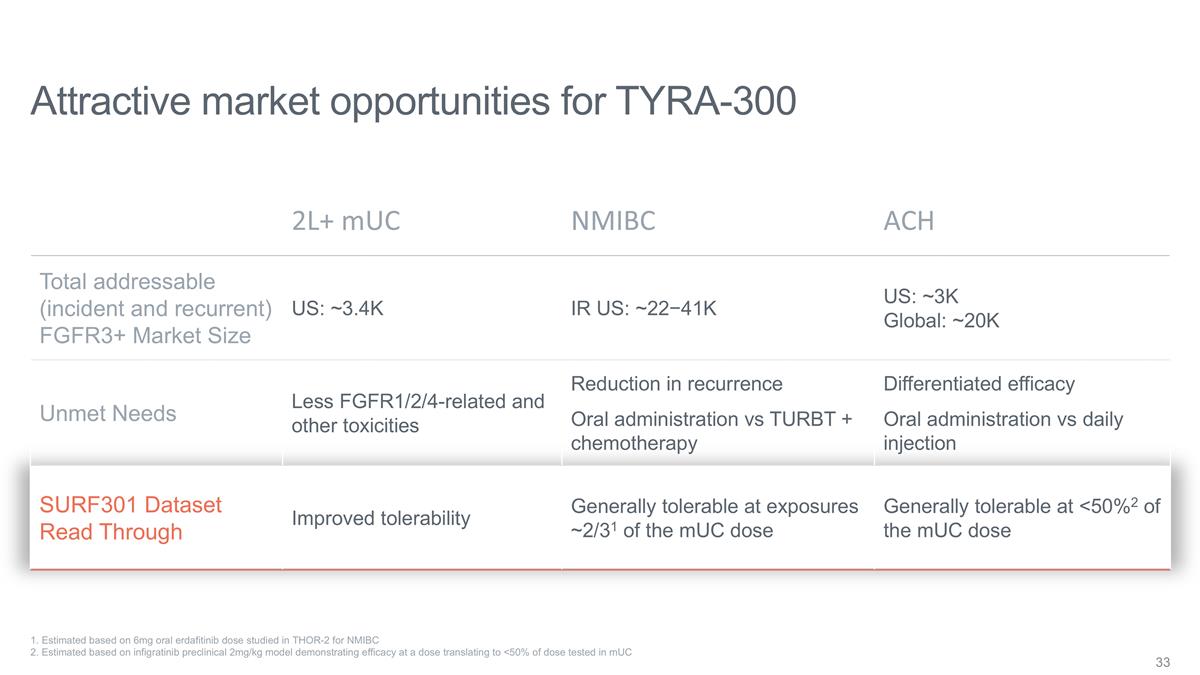
Attractive market opportunities for
TYRA-300 2L+ mUC NMIBC ACH Total addressable (incident and recurrent) FGFR3+ Market Size US: ~3.4K IR US: ~22−41K US: ~3K Global: ~20K Unmet Needs Less FGFR1/2/4-related and other toxicities Reduction in recurrence Oral administration vs TURBT
+ chemotherapy Differentiated efficacy Oral administration vs daily injection SURF301 Dataset Read Through Improved tolerability Generally tolerable at exposures ~2/31 of the mUC dose Generally tolerable at <50%2 of the mUC dose 1. Estimated
based on 6mg oral erdafitinib dose studied in THOR-2 for NMIBC 2. Estimated based on infigratinib preclinical 2mg/kg model demonstrating efficacy at a dose translating to <50% of dose tested in mUC
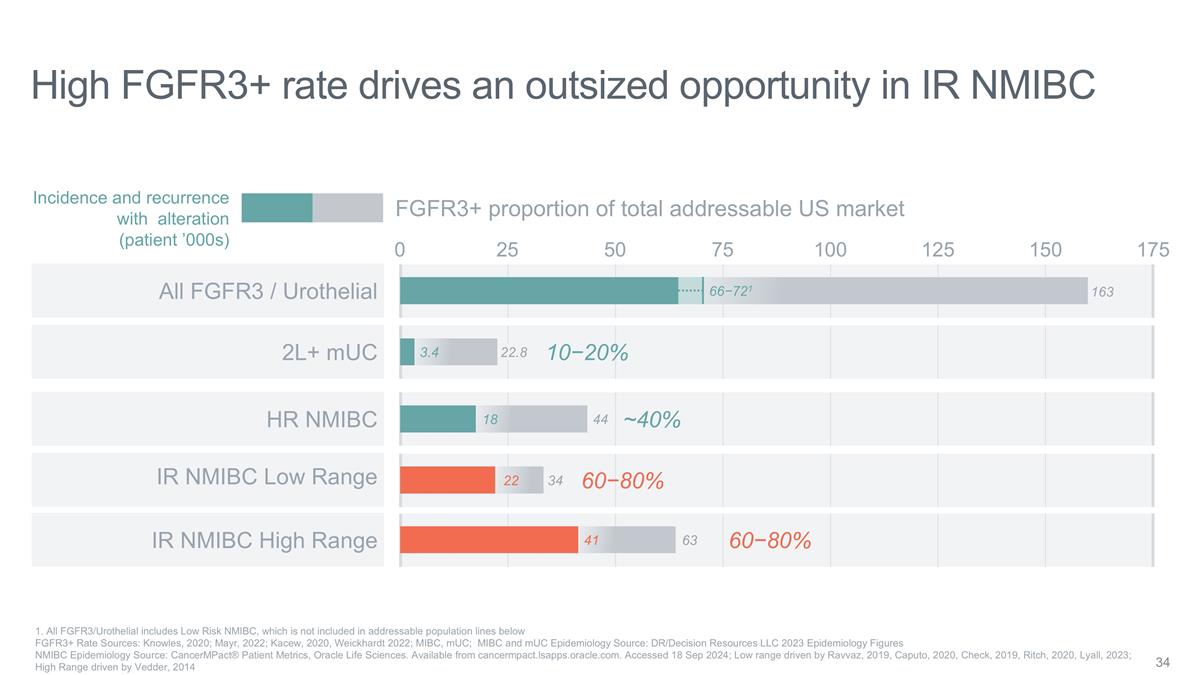
41 High FGFR3+ rate drives an
outsized opportunity in IR NMIBC 75 25 0 FGFR3+ proportion of total addressable US market HR NMIBC 2L+ mUC 175 50 100 125 150 34 44 22.8 10−20% ~40% 60−80% Incidence and recurrence with alteration (patient ’000s) 3.4 All FGFR3 /
Urothelial 163 66−721 22 18 63 60−80% IR NMIBC Low Range IR NMIBC High Range 1. All FGFR3/Urothelial includes Low Risk NMIBC, which is not included in addressable population lines below FGFR3+ Rate Sources: Knowles, 2020; Mayr, 2022;
Kacew, 2020, Weickhardt 2022; MIBC, mUC; MIBC and mUC Epidemiology Source: DR/Decision Resources LLC 2023 Epidemiology Figures NMIBC Epidemiology Source: CancerMPact® Patient Metrics, Oracle Life Sciences. Available from
cancermpact.lsapps.oracle.com. Accessed 18 Sep 2024; Low range driven by Ravvaz, 2019, Caputo, 2020, Check, 2019, Ritch, 2020, Lyall, 2023; High Range driven by Vedder, 2014
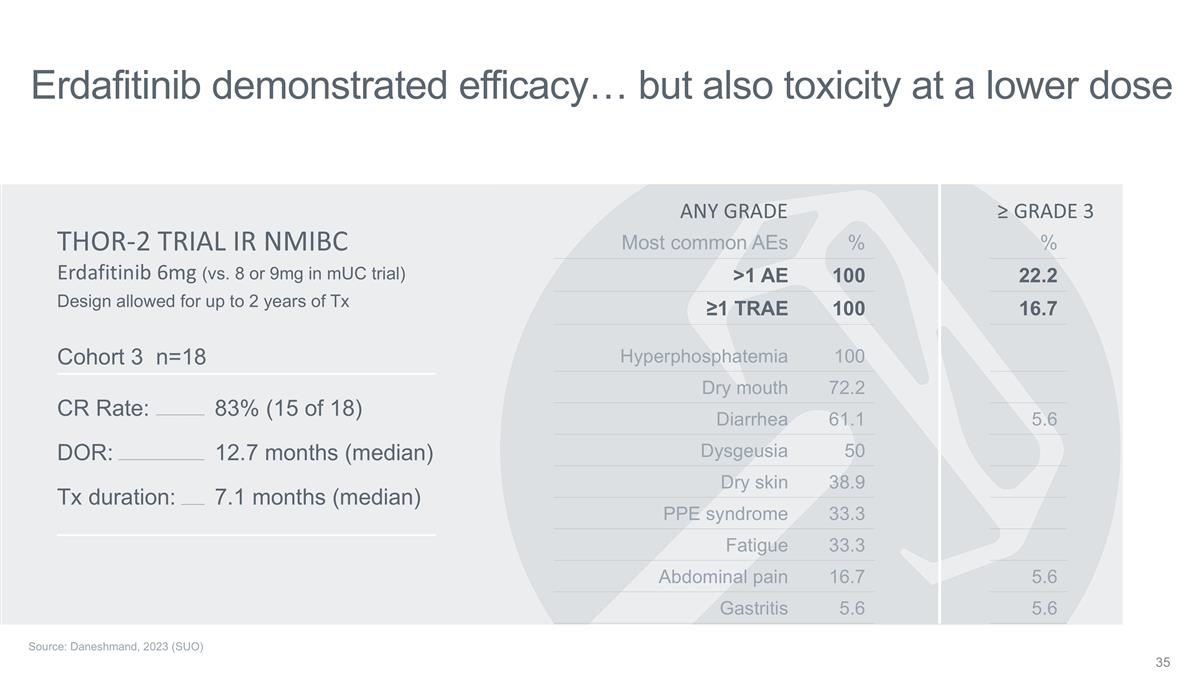
Erdafitinib demonstrated
efficacy… but also toxicity at a lower dose SG (spell out) Erdafitinib 6mg (vs. 8 or 9mg in mUC trial) THOR-2 TRIAL IR NMIBC Cohort 3 n=18 CR Rate: DOR: Tx duration: 83% (15 of 18) 12.7 months (median) 7.1 months (median) Hyperphosphatemia 100
Dry mouth 72.2 Diarrhea 61.1 Dysgeusia 50 Dry skin 38.9 PPE syndrome 33.3 Fatigue 33.3 Abdominal pain 16.7 Gastritis 5.6 Most common AEs % >1 AE 100 ≥1 TRAE 100 ANY GRADE 5.6 5.6 5.6 % 22.2 16.7 ≥ GRADE 3 Design allowed for up to 2
years of Tx Source: Daneshmand, 2023 (SUO)
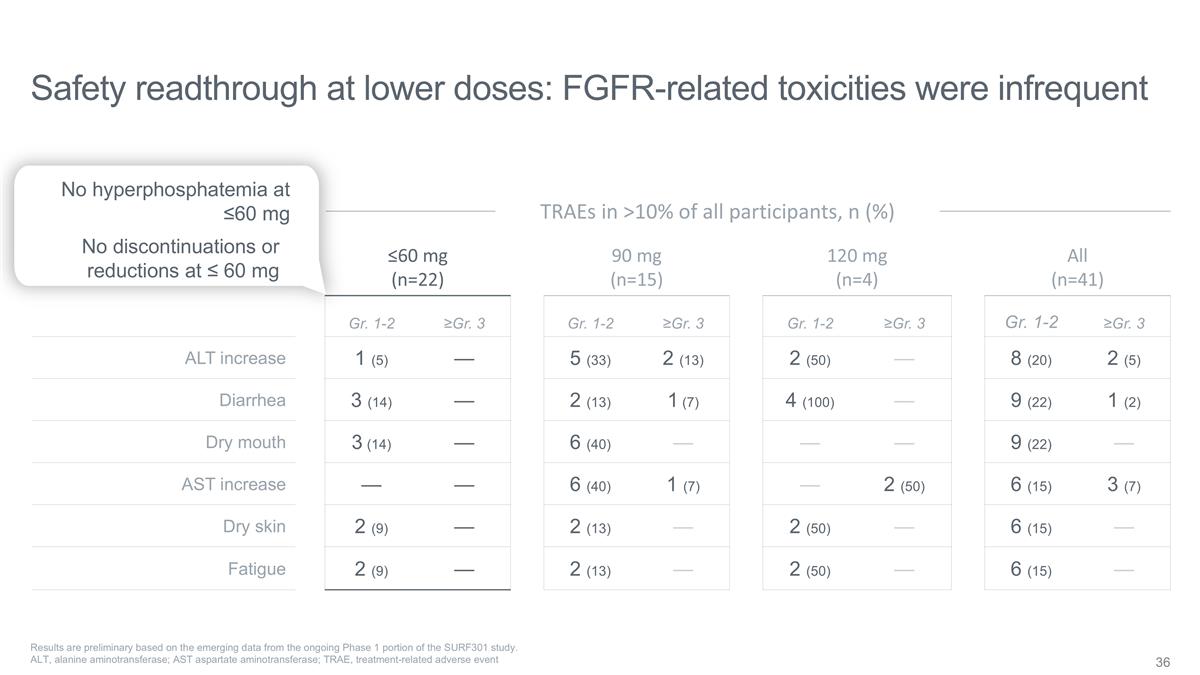
Safety readthrough at lower doses:
FGFR-related toxicities were infrequent ≤60 mg (n=22) 90 mg (n=15) 120 mg (n=4) All (n=41) Gr. 1-2 ≥Gr. 3 Gr. 1-2 ≥Gr. 3 Gr. 1-2 ≥Gr. 3 Gr. 1-2 ≥Gr. 3 ALT increase 1 (5) — 5 (33) 2 (13) 2 (50) — 8 (20) 2 (5)
Diarrhea 3 (14) — 2 (13) 1 (7) 4 (100) — 9 (22) 1 (2) Dry mouth 3 (14) — 6 (40) — — — 9 (22) — AST increase — — 6 (40) 1 (7) — 2 (50) 6 (15) 3 (7) Dry skin 2 (9) — 2 (13) — 2
(50) — 6 (15) — Fatigue 2 (9) — 2 (13) — 2 (50) — 6 (15) — Results are preliminary based on the emerging data from the ongoing Phase 1 portion of the SURF301 study. ALT, alanine aminotransferase; AST aspartate
aminotransferase; TRAE, treatment-related adverse event TRAEs in >10% of all participants, n (%) No hyperphosphatemia at ≤60 mg No discontinuations or reductions at ≤ 60 mg
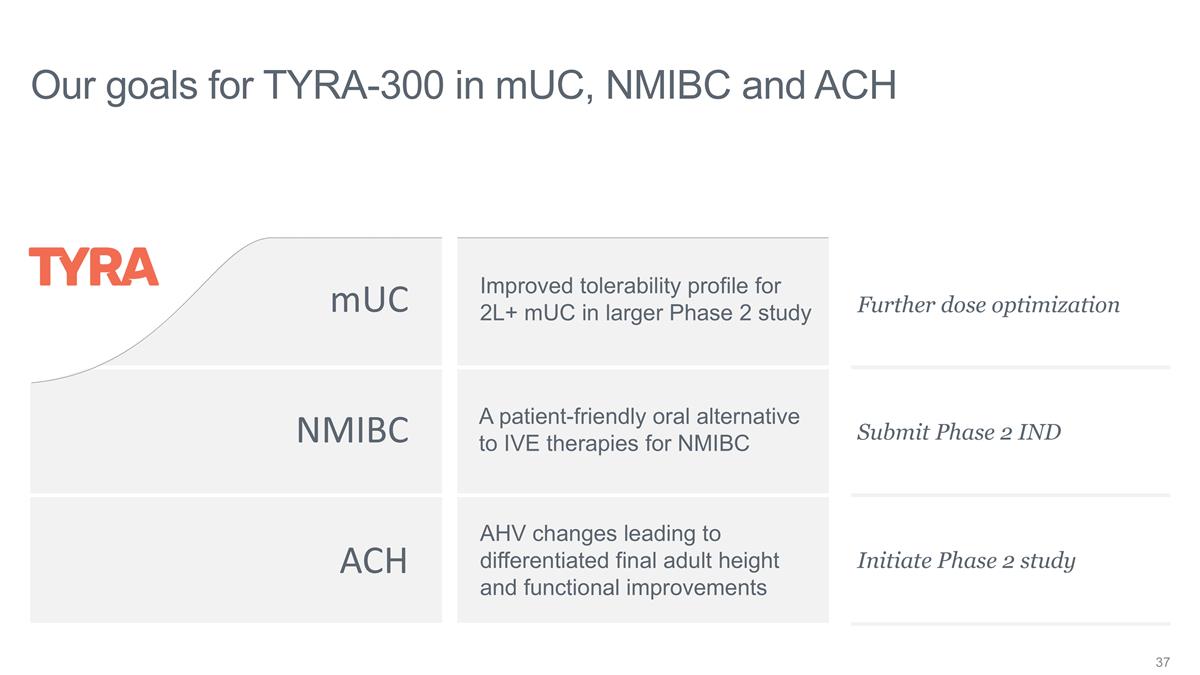
ACH mUC NMIBC Improved tolerability
profile for 2L+ mUC in larger Phase 2 study A patient-friendly oral alternative to IVE therapies for NMIBC AHV changes leading to differentiated final adult height and functional improvements Our goals for TYRA-300 in mUC, NMIBC and ACH Further dose
optimization Submit Phase 2 IND Initiate Phase 2 study

Q&A
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Tyra Biosciences (NASDAQ:TYRA)
Historical Stock Chart
From Feb 2025 to Mar 2025

Tyra Biosciences (NASDAQ:TYRA)
Historical Stock Chart
From Mar 2024 to Mar 2025
