Form 8-K - Current report
January 11 2024 - 5:25PM
Edgar (US Regulatory)
false
0001855644
0001855644
2024-01-11
2024-01-11
0001855644
us-gaap:CommonClassAMember
2024-01-11
2024-01-11
0001855644
us-gaap:WarrantMember
2024-01-11
2024-01-11
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
Current Report
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
January 11, 2024
Date of Report (Date of earliest event reported)
Zura
Bio Limited
(Exact Name of Registrant
as Specified in its Charter)
| Cayman Islands |
|
001-40598 |
|
98-1725736 |
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(I.R.S. Employer
Identification No.) |
| 1489 W. Warm Springs Rd. #110 |
|
|
| Henderson, Nevada |
|
89014 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant's telephone number,
including area code: (702) 757-6133
N/A
(Former name or former address,
if changed since last report)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written
communications pursuant to Rule 425 under the Securities Act |
| ¨ | Soliciting
material pursuant to Rule 14a-12 under the Exchange Act |
| ¨ | Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act |
| ¨ | Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on
which registered |
| Class A Ordinary Shares, par value $0.0001 per share |
|
ZURA |
|
The Nasdaq Stock Market |
| Warrants, each whole warrant exercisable for one Class A Ordinary Share at an exercise price of $11.50 per share |
|
ZURAW |
|
The Nasdaq Stock Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 13(a) of the Exchange Act.
| Item 7.01 |
Regulation FD Disclosure. |
On January
11, 2024, representatives of Zura Bio Limited, a Cayman Islands exempted company (the “Company”), began making presentations
to banks and analysts using slides containing the information attached to this Current Report on Form 8-K as Exhibit 99.1 (the “Investor
Presentation”), which is incorporated herein by reference. The Company has filed the Investor Presentation on its website and expects
to use the Investor Presentation, in whole or in part, and possibly with modifications, in connection with presentations to investors,
analysts and others during the fiscal year ending December 31, 2024.
By filing
this Current Report on Form 8-K and furnishing the information contained herein, the Company makes no admission as to the materiality
of any information in this report that is required to be disclosed solely by reason of Regulation FD.
The information
contained in the Investor Presentation is summary information that is intended to be considered in the context of the Company’s
Securities and Exchange Commission (“SEC”) filings and other public announcements that the Company may make, by press release
or otherwise, from time to time. The Company undertakes no duty or obligation to publicly update or revise the information contained in
this report, although it may do so from time to time as its management believes is warranted. Any such updating may be made through the
filing of other reports or documents with the SEC, through press releases or through other public disclosure.
The information
presented in Item 7.01 of this Current Report on Form 8-K and Exhibit 99.1 shall not be deemed to be “filed” for purposes
of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities
of that section, unless the Company specifically states that the information is to be considered “filed” under the Exchange
Act or specifically incorporates it by reference into a filing under the Securities Act of 1933, as amended, or the Exchange Act.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of
the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
Dated: January 11, 2024
| ZURA BIO LIMITED |
|
| |
|
| By: |
/s/ Kim Davis |
|
| |
Kim Davis |
|
| |
Chief Legal Officer |
|
Exhibit 99.1

| ©2024 Zura Bio Ltd.
Nasdaq Ticker: ZURA
Building the Next
Immunology Leader
42nd Annual J.P. Morgan Healthcare Conference
January 11, 2024
San Francisco, California |

| ©2024 Zura Bio Ltd. 2
Forward Looking Statements Disclaimer
This communication includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995.
Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believe,” “predict,” “potential,” “continue,”
“strategy,” “future,” “opportunity,” “would,” “seem,” “seek,” “outlook” and similar expressions are intended to identify such forward-looking statements. Forward-looking
statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are
subject to risks and uncertainties that could cause the actual results to differ materially from the expected results. These statements are based on various
assumptions, whether or not identified in this communication. These forward-looking statements are provided for illustrative purposes only and are not intended to
serve as, and must not be relied on by an investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability.
Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. You should carefully consider the risks and uncertainties
described in the “Risk Factors” sections of Zura Bio’s recent filings with the SEC. These filings would identify and address other important risks and uncertainties that
could cause actual events and results to differ materially from those contained in the forward-looking statements. Many of these factors are outside Zura Bio’s control
and are difficult to predict. Many factors could cause actual future events to differ from the forward-looking statements in this communication, including but not
limited to: (1) the outcome of any legal proceedings that may be instituted against Zura Bio; (2) volatility in the price of Zura Bio’s securities; (3) the ability of Zura Bio to
successfully conduct research and development activities, grow and manage growth profitably, maintain relationships with customers and suppliers, and retain key
employees; (4) costs related to financing transactions and the ongoing costs relating to operating as a public company; (5) changes in the applicable laws or
regulations; (6) the possibility that Zura Bio may be adversely affected by other economic, business, and/or competitive factors; (7) the risk of downturns and a
changing regulatory landscape in the highly competitive industry in which Zura Bio operates; (8) the impact of the global COVID-19 pandemic; (9) the potential
inability of Zura Bio to raise additional capital needed to pursue its business objectives or to achieve efficiencies regarding other costs; (10) the enforceability of Zura
Bio’s intellectual property, including its patents, and the potential infringement on the intellectual property rights of others, cyber security risks or potential breaches
of data security; and (11) other risks and uncertainties described in the Registration Statement and such other documents filed by Zura Bio from time to time with
the SEC. These risks and uncertainties may be amplified by the COVID-19 pandemic or other unanticipated global disruption events, which may continue to cause
economic uncertainty. Zura Bio cautions that the foregoing list of factors is not exclusive or exhaustive and not to place undue reliance upon any forward-looking
statements, including projections, which speak only as of the date made. Zura Bio gives no assurance that it will achieve its expectations.
Zura Bio does not undertake or accept any obligation to publicly provide revisions or updates to any forward-looking statements, whether as a result of new
information, future developments or otherwise, or should circumstances change, except as otherwise required by securities and other applicable laws. |

| © 2024 Zura Bio Ltd. ©2024 Zura Bio Ltd. 3
Began trading on
Nasdaq under
“ZURA” ticker
ZB-106 in-licensed from Eli
Lilly and Company and
PIPE announced
$80M
PIPE
closed
Entered into sponsored research
agreement with Benaroya
Research Institute
Arnout Ploos van Amstel
joined Board
Rob Lisicki and Dr. Kiran Nistala join
Zura Bio Executive Leadership
Inclusion into the Russell
2000® and 3000® Indexes
2023 2024
In 2023, Zura established a strong foundation to enable
focused execution into 2024
2024 Key Objectives:
On time clinical trial execution
Build leadership team with specific expertise
Translational excellence & validating external clinical readouts
COVERING ANALYSTS (as of 08-Jan): Daniil Gataulin, PhD, Chardan; Yatin Suneja, Guggenheim Securities;
Aydin Huseynov, MD, CFA, Ladenburg Thalmann & Co. Inc.; Justin Kim, Oppenheimer; Steven Seedhouse, PhD, Raymond James |

| ©2024 Zura Bio Ltd. 4
Experienced management team with proven ability to
successfully execute and build a leading market position
Nasdaq: ZURA
Board of Directors
Executive Team
Amit Munshi Arnout Ploos van Amstel Jennifer Jarrett Neil Graham, M.D. Parvinder Thiara Sandeep Kulkarni, M.D. Someit Sidhu, M.D. Steve Schoch
Chairman Independent Director Independent Director Independent Director Independent Director Independent Director Director Independent Director
Mike Howell Ph.D.
Chief Scientific Officer and
Head of Translational Medicine
Kim Davis
Chief Legal Officer
Kiran Nistala M.D., Ph.D.
Chief Medical Officer and
Head of Development
Gary Whale Ph.D.
Chief Technology Officer
Someit Sidhu M.D.
Founder, Chief Executive Officer and
Director
Verender Badial
Chief Financial Officer
Robert Lisicki
President and
Chief Operating Officer |
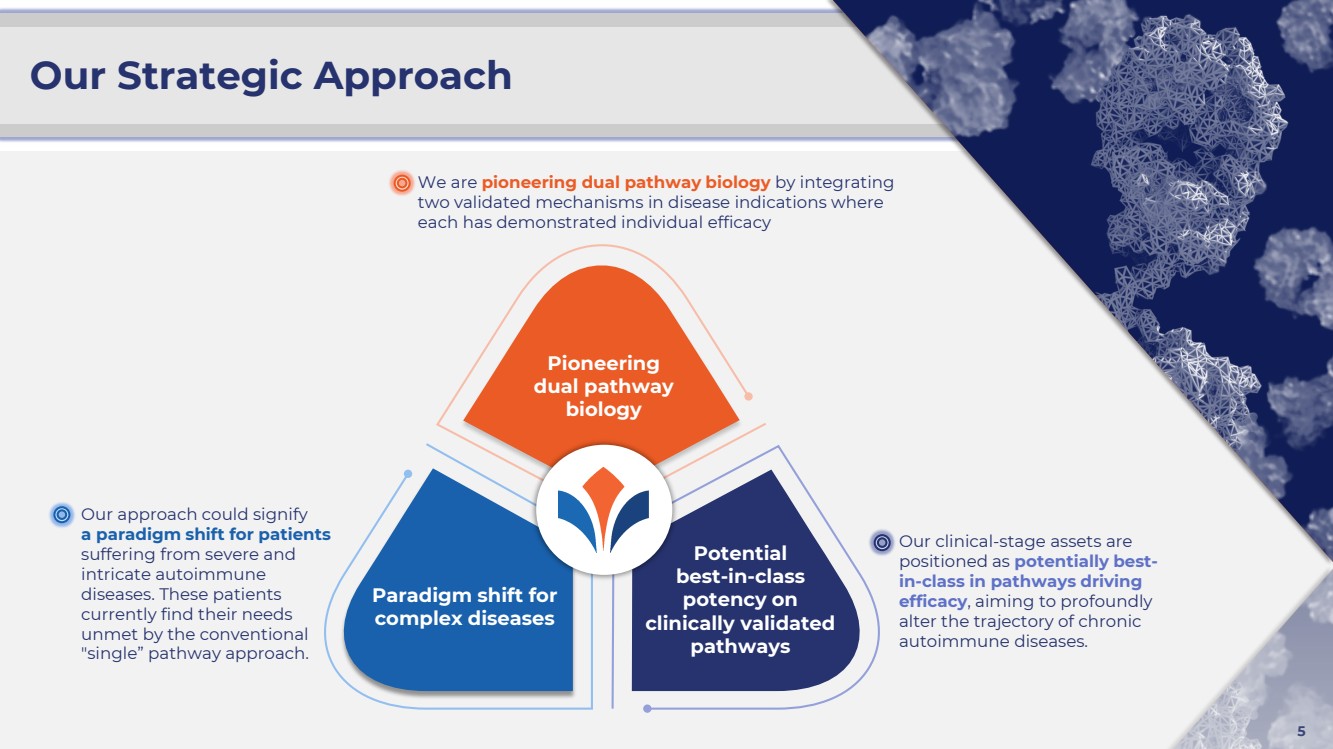
| ©2024 Zura Bio Ltd. 5
Our Strategic Approach
Our approach could signify
a paradigm shift for patients
suffering from severe and
intricate autoimmune
diseases. These patients
currently find their needs
unmet by the conventional
"single” pathway approach.
We are pioneering dual pathway biology by integrating
two validated mechanisms in disease indications where
each has demonstrated individual efficacy
Our clinical-stage assets are
positioned as potentially best-in-class in pathways driving
efficacy, aiming to profoundly
alter the trajectory of chronic
autoimmune diseases.
Pioneering
dual pathway
biology
Potential
best-in-class
potency on
clinically validated
pathways
Paradigm shift for
complex diseases |
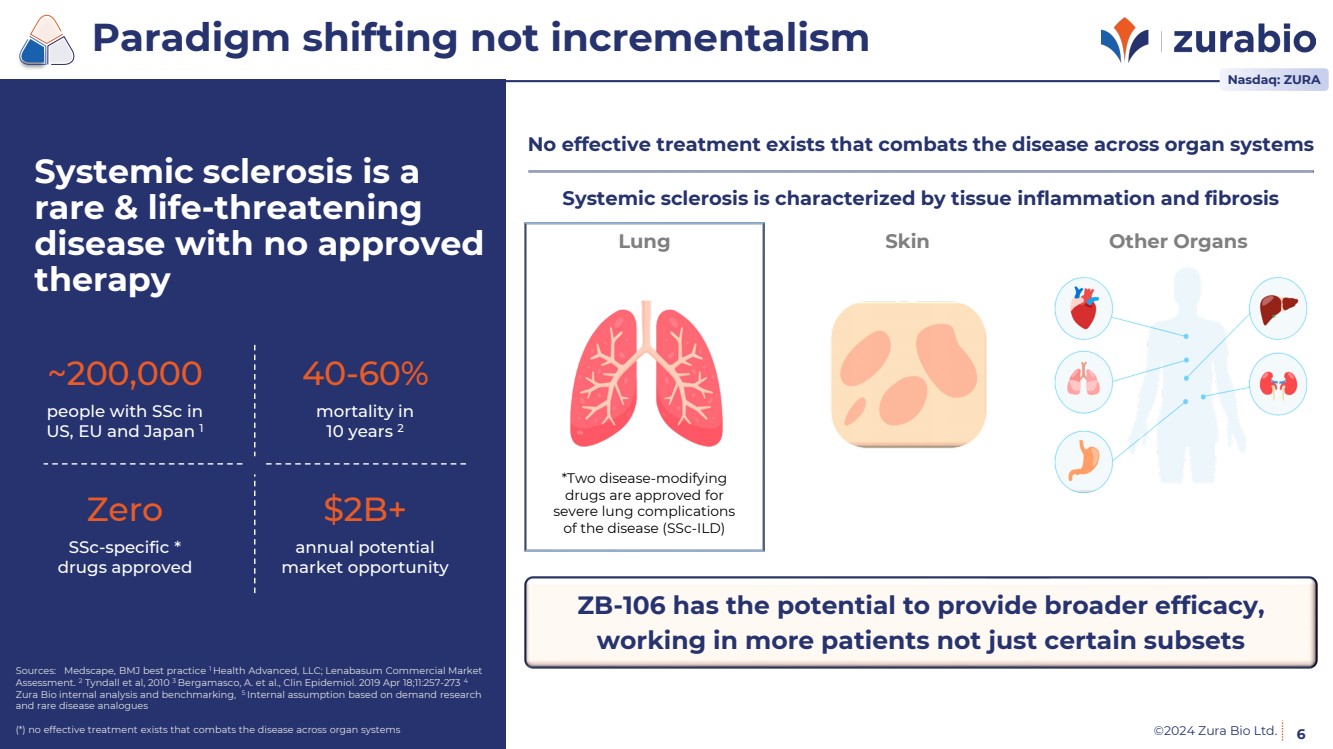
| ©2024 Zura Bio Ltd. 6
Systemic sclerosis is a
rare & life-threatening
disease with no approved
therapy
~200,000
people with SSc in
US, EU and Japan 1
40-60%
mortality in
10 years 2
Zero
SSc-specific *
drugs approved
$2B+
annual potential
market opportunity
Sources: Medscape, BMJ best practice 1 Health Advanced, LLC; Lenabasum Commercial Market
Assessment. 2 Tyndall et al, 2010 3 Bergamasco, A. et al., Clin Epidemiol. 2019 Apr 18;11:257-273 4
Zura Bio internal analysis and benchmarking, 5 Internal assumption based on demand research
and rare disease analogues
(*) no effective treatment exists that combats the disease across organ systems
Paradigm shifting not incrementalism
Nasdaq: ZURA
Systemic sclerosis is characterized by tissue inflammation and fibrosis
No effective treatment exists that combats the disease across organ systems
Lung Skin Other Organs
*Two disease-modifying
drugs are approved for
severe lung complications
of the disease (SSc-ILD)
ZB-106 has the potential to provide broader efficacy,
working in more patients not just certain subsets |

| ©2024 Zura Bio Ltd.
Pioneering Dual Pathway Biology
7
Sources: 1 Liu, Ling, et al. Journal of Inflammation Research, doi:10.2147/jir.s100940. 2 Manetta, Joseph et al. Journal of Inflammation Research, doi:10.2147/jir.s67751. 3 Benschop, Robert J., et al. mAbs, doi:10.1080/19420862.2019.1624463.
(*) Figure Generated with BioRender
Nasdaq: ZURA
ZB-106 is an IgG-scFv engineered by the fusion of ixekizumab (TALTZ®) and tabalumab 1, 2, 3
BAFF IL-17
Anti-IL-17 scFv
TALTZ®
Anti-BAFF Ab
tabalumab
tibulizumab
Anti BAFF x IL-17
ZB-106 neutralizes IL-17A or
BAFF regardless of whether
the other binding sites are
occupied
ZB-106 binds in the same way
as TALTZ® and tabalumab
with the same number of
binding sites
Activity is mediated through
direct target engagement and
not ADCC
t1/2 is 26.9 days
* * tabalumab
tabalumab + IL- 17A
ZB-106
ZB-106 + IL- 17A
TALTZ®
TALTZ® + BAFF
ZB-106
ZB-106 + BAFF
ZB-106 inhibits BAFF-mediated proliferation in
T1165 cells in an IL-17 independent manner 3
ZB-106 inhibits IL-17 mediated CXCL1 in epithelial
cells in a BAFF independent manner 3
nM |

| ©2024 Zura Bio Ltd.
Anti-IL-17 scFv
TALTZ®
Anti-BAFF Ab
tabalumab
BAFF
IL-17
tibulizumab: BAFF / IL-17 ZB-168: IL-7R / TSLP torudokimab: IL-33 / RAGE
Potent molecules with highly validated pathways
Nasdaq: ZURA
78 Participants Dosed Across Three Ph1/1b studies
57 participants with single dose
21 participants with multiple dose up to 12 weeks
93 Participants Dosed
60 participants with single dose
33 participants with multiple doses up to 12 weeks
244 Participants Dosed
81 participants with single dose
163 participants with multiple doses up to 52 weeks
IL-17 binds to IL-17A preventing IL-17A/A and IL-17A/F
heterodimerization1
• ZB-168 is nearly 10-fold more
potent than AZ/AMG’s
tezepelumab, and
tezepelumab does not inhibit
IL-7 signaling
• ZB-168 is >300-fold more potent
than Q32Bio’s bempikibart in
TSLP-induced markers, but
similar in IL-7-induced pSTA58
Torudokimab was 2.9 and 5.5-fold more potent than etokimab and
itepekimab, respectively, inhibiting IL-33-induced GM-CSF production
by human mast cells
POTENCY
DOSING TO DATE 2
half-life
( t1/2 )
tibulizumab 26.9 days
sonelokimab 11-12 days
izokibep ~11 days
Sources: 1 Liu, Ling, et al. Journal of Inflammation Research, doi:10.2147/jir.s100940. 2 IB and CSR, 3 Manetta, Joseph et al. Journal of Inflammation Research, doi:10.2147/jir.s67751. 4 Benschop, Robert J., et al. mAbs, doi:10.1080/19420862.2019.1624463. 5 Zura Internal Data. 6 Herold, Kevan C., et al. JCI Insight, doi:10.1172/jci.insight.126054. 7 Numazaki, Mako, et al. Journal of Pharmacology and Experimental Therapeutics, doi:10.1124/jpet.121.000686. 8.BMS patent https://patents.google.com/patent/WO2020154293A1/en
UPB-101
(α-TSLPR)
tezepelumab
(TSLP)
bempikibart
(IL-7Rα)
ZB-168
(IL-7Rα)
α-TSLPR mAb TSLP mAb IL-7Rα mAb IL-7Rα mAb
TSLP-Induced
Signals
16.1 ng / ml /
0.1nM (CCL17)(7)
67 ng / ml /
0.44nM (CCL17)(7)
24 nM
(CCL2)(8)
7.5 ng / ml / 0.05nM (CCL17)(5)
11 ng / ml / 0.07nM (CCL22)(5)
0.08 nM (CCL2)(8)
IL-7-Induced
Signals Neg Neg
0.6 nM
(IL-7 at 0.25ng/ml)(8)
2.1 nM
(IL-7 at 2.5ng/ml)(8)
0.46nM (pSTAT5)(6)
Antibody kon (M-1
s-1
) koff (s-1
) kd (pM) torudokimab
Potency
torudokimab (LY3375880) 1.7 x 106 6.7 x 10-5 39
etokimab (AnaptysBio) 9.4 x 105 1.2 x 10-4 112 2.9x
itepekimab (Regeneron) 7.6 x 105 1.6 x 10-4 215 5.5x
IL-7R
γc TSLPR
8 |
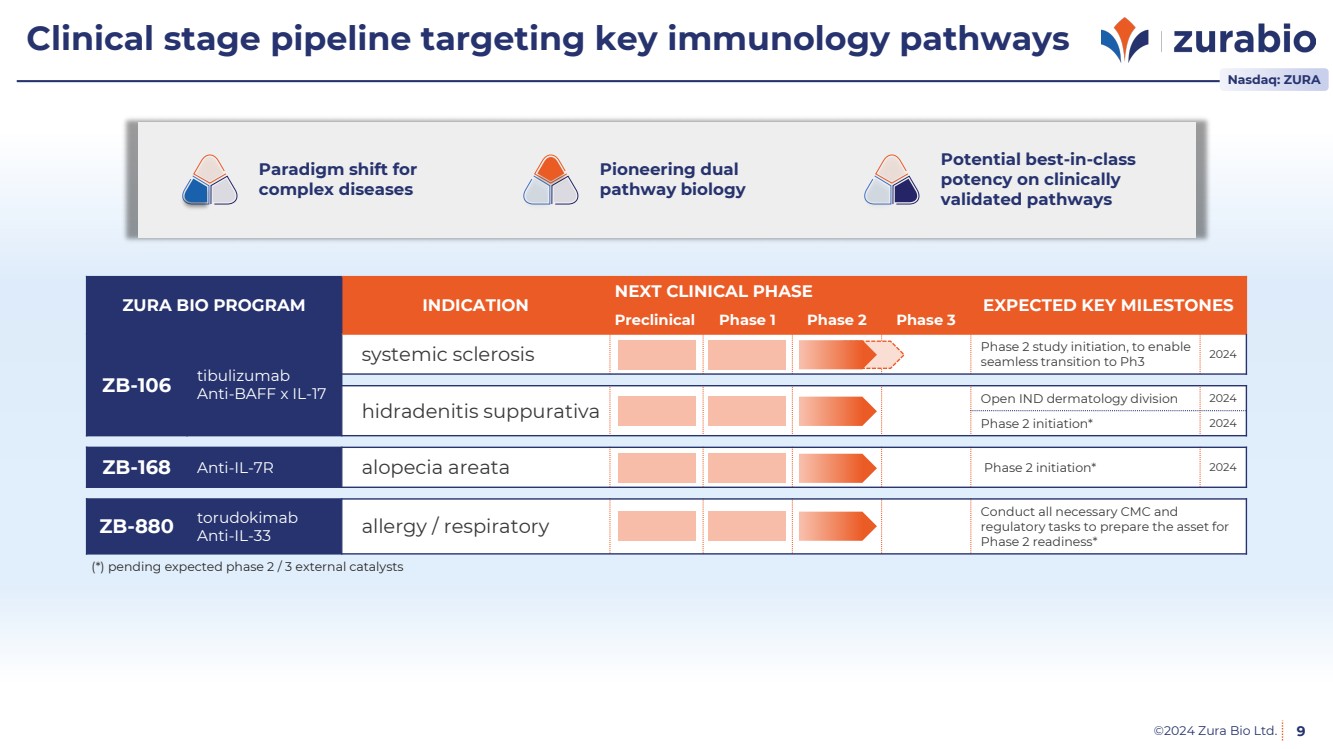
| ©2024 Zura Bio Ltd.
Clinical stage pipeline targeting key immunology pathways
9
Pioneering dual
pathway biology
Paradigm shift for
complex diseases
Potential best-in-class
potency on clinically
validated pathways
ZURA BIO PROGRAM INDICATION
NEXT CLINICAL PHASE
EXPECTED KEY MILESTONES
Preclinical Phase 1 Phase 2 Phase 3
ZB-106 tibulizumab
Anti-BAFF x IL-17
systemic sclerosis Phase 2 study initiation, to enable
seamless transition to Ph3 2024
hidradenitis suppurativa Open IND dermatology division 2024
Phase 2 initiation* 2024
ZB-168 Anti-IL-7R alopecia areata Phase 2 initiation* 2024
ZB-880 torudokimab
Anti-IL-33 allergy / respiratory Conduct all necessary CMC and
regulatory tasks to prepare the asset for
Phase 2 readiness*
(*) pending expected phase 2 / 3 external catalysts
Nasdaq: ZURA |
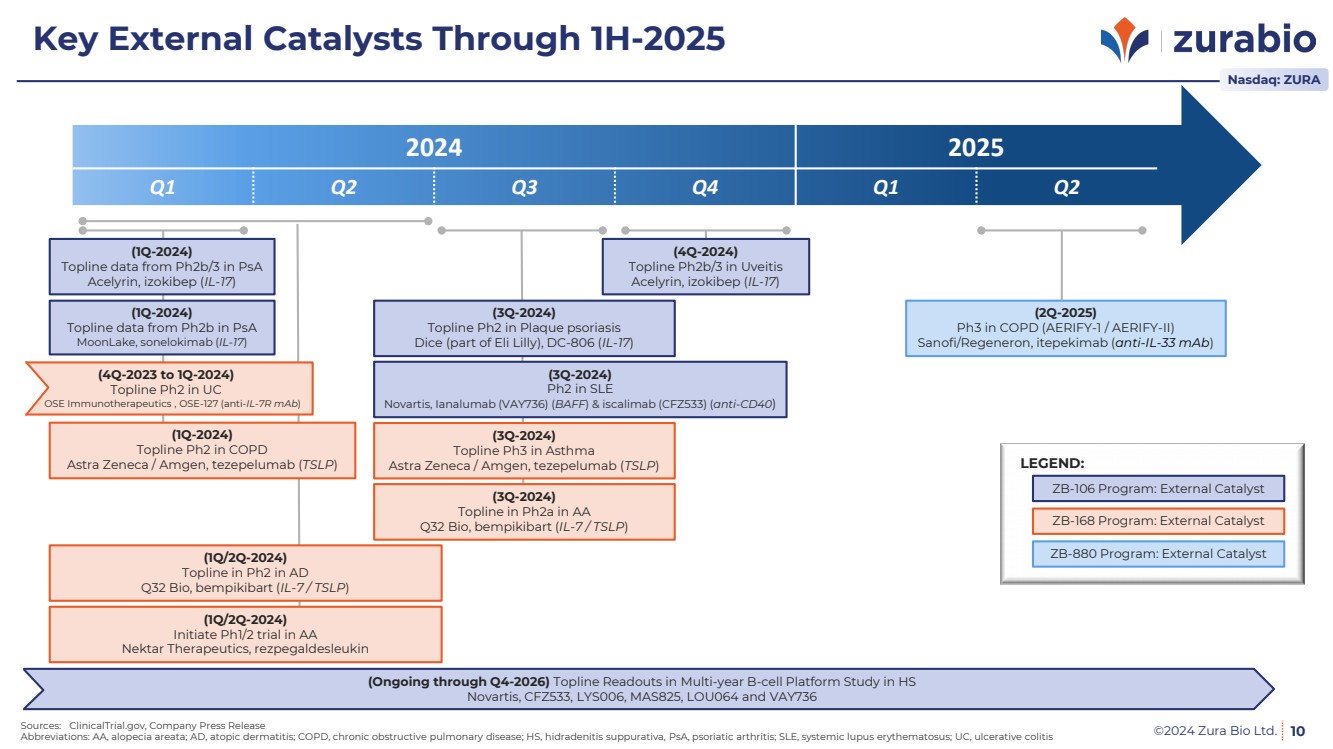
| ©2024 Zura Bio Ltd. 10
ZB-106 Program: External Catalyst
ZB-168 Program: External Catalyst
ZB-880 Program: External Catalyst
LEGEND:
Sources: ClinicalTrial.gov, Company Press Release
Abbreviations: AA, alopecia areata; AD, atopic dermatitis; COPD, chronic obstructive pulmonary disease; HS, hidradenitis suppurativa, PsA, psoriatic arthritis; SLE, systemic lupus erythematosus; UC, ulcerative colitis
2024 2025
Q1 Q2 Q3 Q4 Q1 Q2
(1Q-2024)
Topline data from Ph2b/3 in PsA
Acelyrin, izokibep (IL-17)
(4Q-2024)
Topline Ph2b/3 in Uveitis
Acelyrin, izokibep (IL-17)
(3Q-2024)
Topline Ph2 in Plaque psoriasis
Dice (part of Eli Lilly), DC-806 (IL-17)
(3Q-2024)
Topline Ph3 in Asthma
Astra Zeneca / Amgen, tezepelumab (TSLP)
(3Q-2024)
Ph2 in SLE
Novartis, Ianalumab (VAY736) (BAFF) & iscalimab (CFZ533) (anti-CD40)
(1Q-2024)
Topline Ph2 in COPD
Astra Zeneca / Amgen, tezepelumab (TSLP)
(1Q-2024)
Topline data from Ph2b in PsA
MoonLake, sonelokimab (IL-17)
(3Q-2024)
Topline in Ph2a in AA
Q32 Bio, bempikibart (IL-7 / TSLP)
(1Q/2Q-2024)
Topline in Ph2 in AD
Q32 Bio, bempikibart (IL-7 / TSLP)
(1Q/2Q-2024)
Initiate Ph1/2 trial in AA
Nektar Therapeutics, rezpegaldesleukin
(2Q-2025)
Ph3 in COPD (AERIFY-1 / AERIFY-II)
Sanofi/Regeneron, itepekimab (anti-IL-33 mAb)
Nasdaq: ZURA
Key External Catalysts Through 1H-2025
(4Q-2023 to 1Q-2024)
Topline Ph2 in UC
OSE Immunotherapeutics , OSE-127 (anti-IL-7R mAb)
(Ongoing through Q4-2026) Topline Readouts in Multi-year B-cell Platform Study in HS
Novartis, CFZ533, LYS006, MAS825, LOU064 and VAY736 |

| ©2024 Zura Bio Ltd.
Potential First-in-Class, Dual
Antagonist Combining
tabalumab and TALTZ®
systemic sclerosis (SSc)
ZB-106
tibulizumab
Anti-BAFF x IL-17 |
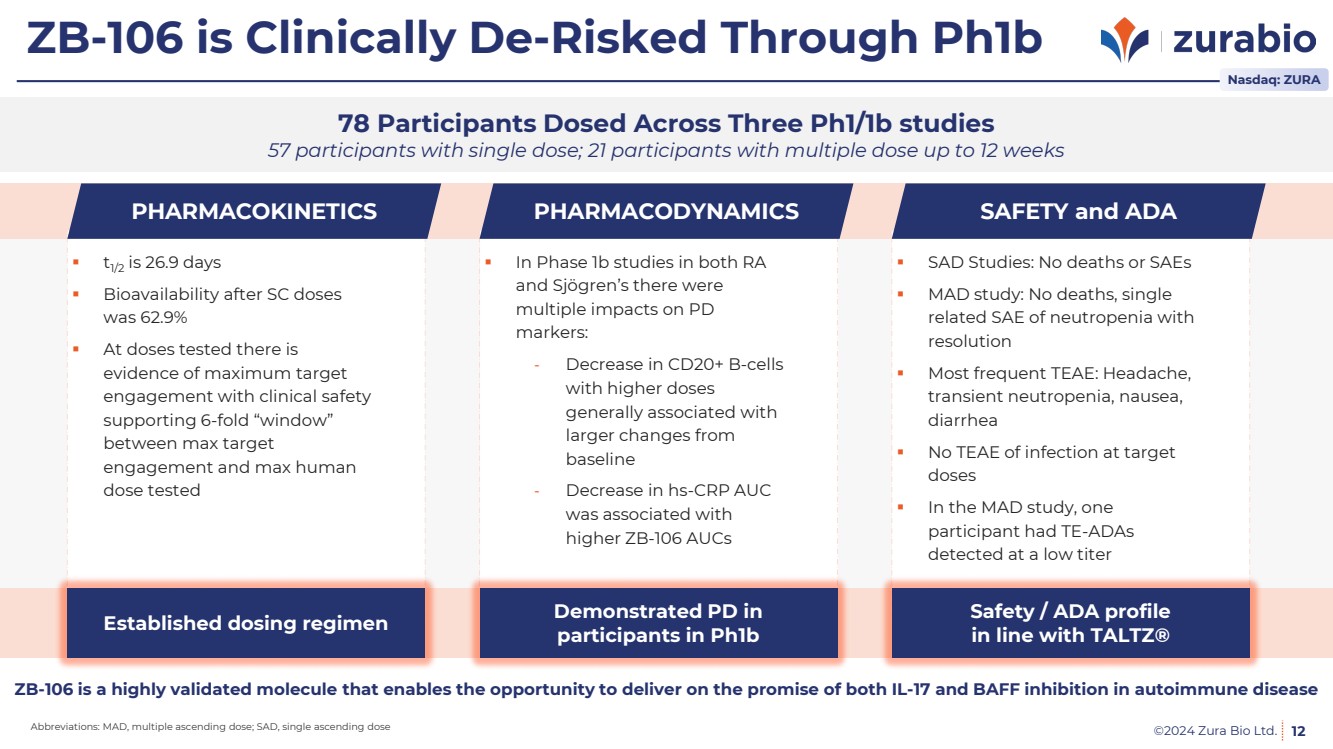
| ©2024 Zura Bio Ltd.
ZB-106 is Clinically De-Risked Through Ph1b
12
78 Participants Dosed Across Three Ph1/1b studies
57 participants with single dose; 21 participants with multiple dose up to 12 weeks
SAD Studies: No deaths or SAEs
MAD study: No deaths, single
related SAE of neutropenia with
resolution
Most frequent TEAE: Headache,
transient neutropenia, nausea,
diarrhea
No TEAE of infection at target
doses
In the MAD study, one
participant had TE-ADAs
detected at a low titer
SAFETY and ADA
t1/2 is 26.9 days
Bioavailability after SC doses
was 62.9%
At doses tested there is
evidence of maximum target
engagement with clinical safety
supporting 6-fold “window”
between max target
engagement and max human
dose tested
PHARMACOKINETICS
Established dosing regimen
In Phase 1b studies in both RA
and Sjögren’s there were
multiple impacts on PD
markers:
- Decrease in CD20+ B-cells
with higher doses
generally associated with
larger changes from
baseline
- Decrease in hs-CRP AUC
was associated with
higher ZB-106 AUCs
PHARMACODYNAMICS
Demonstrated PD in
participants in Ph1b
Safety / ADA profile
in line with TALTZ®
ZB-106 is a highly validated molecule that enables the opportunity to deliver on the promise of both IL-17 and BAFF inhibition in autoimmune disease
Nasdaq: ZURA
Abbreviations: MAD, multiple ascending dose; SAD, single ascending dose |
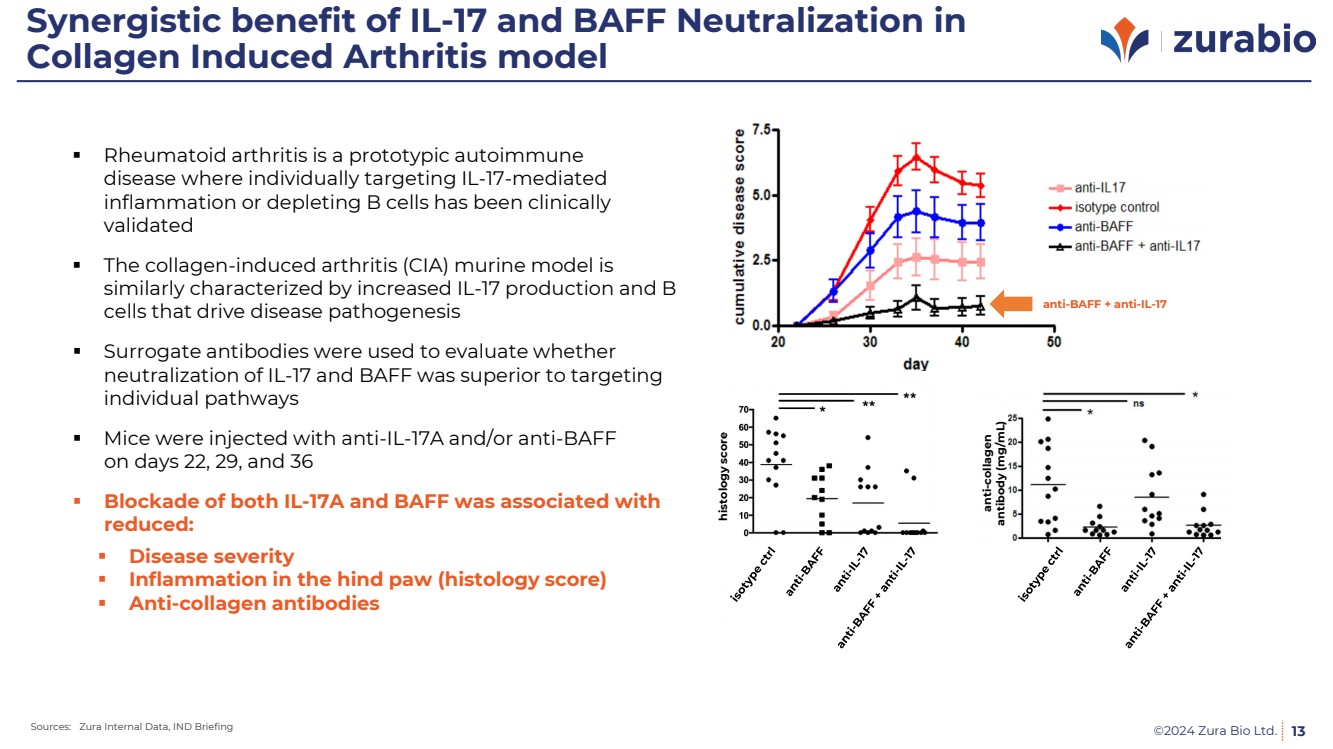
| ©2024 Zura Bio Ltd.
Synergistic benefit of IL-17 and BAFF Neutralization in
Collagen Induced Arthritis model
13
Rheumatoid arthritis is a prototypic autoimmune
disease where individually targeting IL-17-mediated
inflammation or depleting B cells has been clinically
validated
The collagen-induced arthritis (CIA) murine model is
similarly characterized by increased IL-17 production and B
cells that drive disease pathogenesis
Surrogate antibodies were used to evaluate whether
neutralization of IL-17 and BAFF was superior to targeting
individual pathways
Mice were injected with anti-IL-17A and/or anti-BAFF
on days 22, 29, and 36
Blockade of both IL-17A and BAFF was associated with
reduced:
Disease severity
Inflammation in the hind paw (histology score)
Anti-collagen antibodies
histology score
anti-collagen
antibody (mg/mL)
anti-BAFF + anti-IL-17
Sources: Zura Internal Data, IND Briefing |
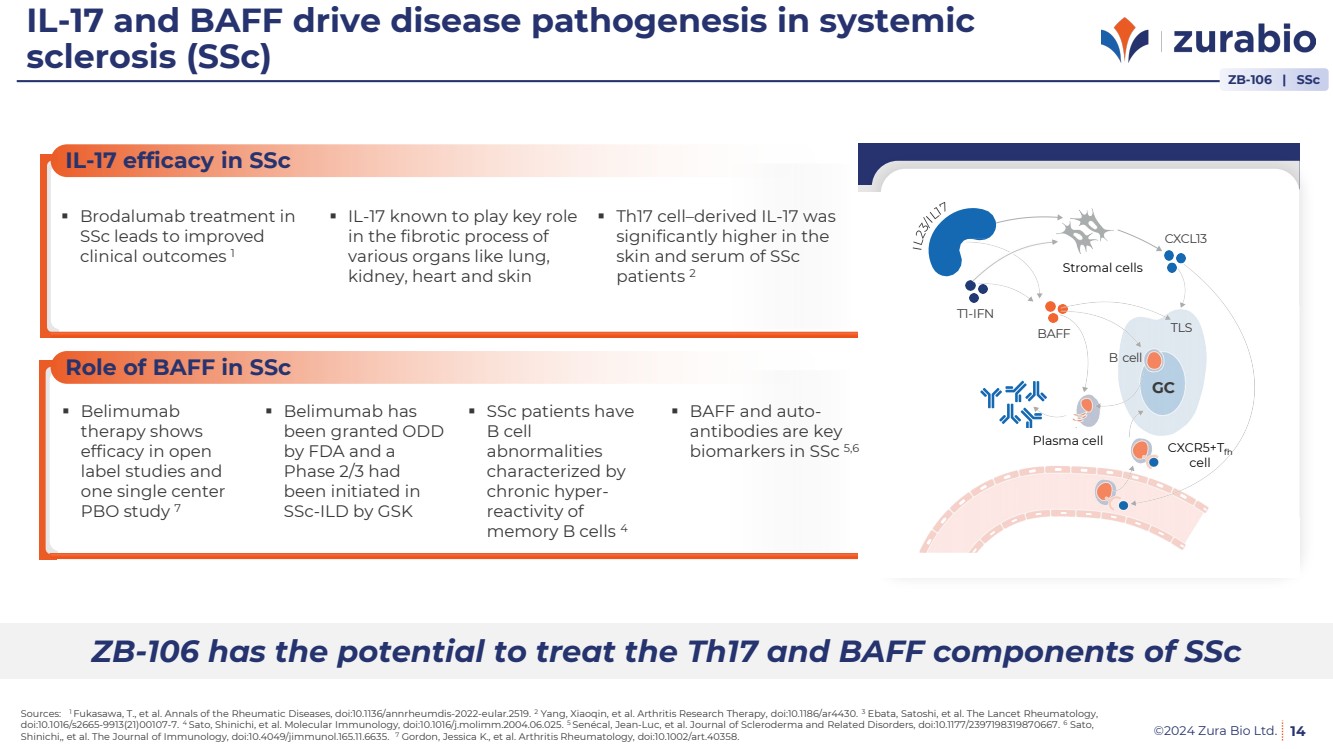
| ©2024 Zura Bio Ltd.
IL-17 efficacy in SSc
Role of BAFF in SSc
IL-17 and BAFF drive disease pathogenesis in systemic
sclerosis (SSc)
14
Strom
al
cells
T1-IFN
BAFF
Plasm
a cell CXCR5+
Tfh cell
TLS
GC
B cell
CXCL13
Brodalumab treatment in
SSc leads to improved
clinical outcomes 1
IL-17 known to play key role
in the fibrotic process of
various organs like lung,
kidney, heart and skin
Th17 cell–derived IL-17 was
significantly higher in the
skin and serum of SSc
patients 2
Sources: 1 Fukasawa, T., et al. Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2022-eular.2519. 2 Yang, Xiaoqin, et al. Arthritis Research Therapy, doi:10.1186/ar4430. 3 Ebata, Satoshi, et al. The Lancet Rheumatology,
doi:10.1016/s2665-9913(21)00107-7. 4 Sato, Shinichi, et al. Molecular Immunology, doi:10.1016/j.molimm.2004.06.025. 5 Senécal, Jean-Luc, et al. Journal of Scleroderma and Related Disorders, doi:10.1177/2397198319870667. 6 Sato,
Shinichi,, et al. The Journal of Immunology, doi:10.4049/jimmunol.165.11.6635. 7 Gordon, Jessica K., et al. Arthritis Rheumatology, doi:10.1002/art.40358.
Belimumab
therapy shows
efficacy in open
label studies and
one single center
PBO study 7
Belimumab has
been granted ODD
by FDA and a
Phase 2/3 had
been initiated in
SSc-ILD by GSK
SSc patients have
B cell
abnormalities
characterized by
chronic hyper-reactivity of
memory B cells 4
BAFF and auto-antibodies are key
biomarkers in SSc 5,6
ZB-106 has the potential to treat the Th17 and BAFF components of SSc
ZB-106 | SSc
Stromal cells
Plasma cell CXCR5+Tfh
cell |

| ©2024 Zura Bio Ltd.
IL-17 and BAFF Inhibition Have Shown Efficacy in Placebo
Controlled Trials in systemic sclerosis (SSc)
15
ZB-106 | SSc
Brodalumab
IL-17 receptor antagonist
Achieved primary endpoint of treatment difference of least
square mean: −21.2 [95% CI -23.9, 18.5]; P<0.0001), and
demonstrated a rapid, sustained reduction in mRSS over 52
weeks1
Demonstrated therapeutic effects on lung/respiratory
functions, digital ulcers, the symptoms of gastroesophageal
reflux disease, and QOL without noteworthy safety concerns
Belimumab
BAFF antagonist
52-week, investigator initiated, single center, double blind,
placebo-controlled pilot study in 20 participants with dcSSC
on MMF
Both treatment groups experienced improvements in mRSS
favoring belimumab (-10 vs -3; p=NS)
All secondary endpoints favored belimumab with statistical
significance in 2 endpoints: SHAQ DI and VAS Raynaud’s
phenomenon
Sources: 1 Fukasawa, T., et al. Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2022-eular.2519. 2 Gordon, Jessica K., et al. Arthritis Rheumatology, doi:10.1002/art.40358.
CLINICAL PRECEDENT
Phase 2 belimumab IIT study
- 15
- 10
- 5
0
5
10
Δ
mRSS
mRSS
Belimumab
n = 9
-10.0
Placebo
n = 9
-3.0
Δ +7.0 points
Δ FVC
FVC (% predicted)
Belimumab
n = 9
5.0
Placebo
n = 9
-2.0
Δ +7.0%
- 15
- 10
- 5
0
5
10 |
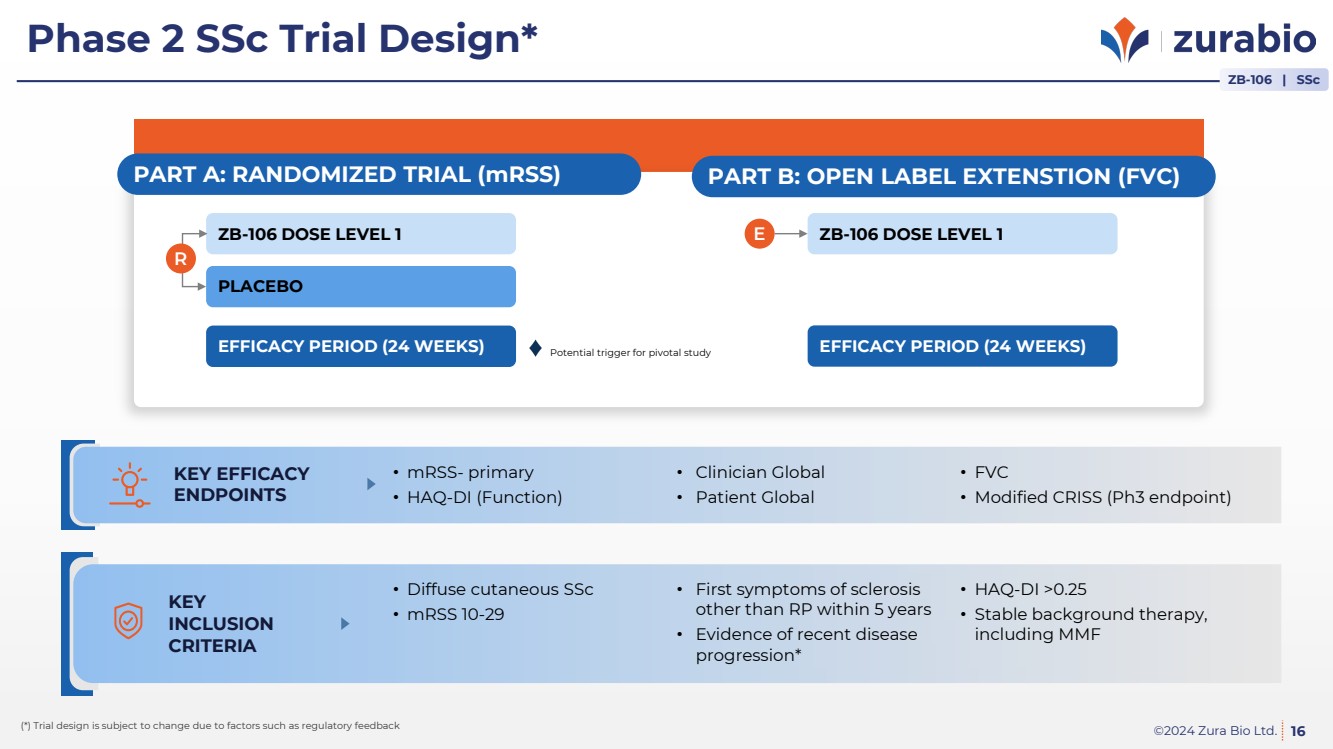
| ©2024 Zura Bio Ltd.
Phase 2 SSc Trial Design*
16
ZB-106 | SSc
KEY EFFICACY
ENDPOINTS
• mRSS- primary
• HAQ-DI (Function)
• Clinician Global
• Patient Global
• FVC
• Modified CRISS (Ph3 endpoint)
(*) Trial design is subject to change due to factors such as regulatory feedback
PART A: RANDOMIZED TRIAL (mRSS)
EFFICACY PERIOD (24 WEEKS)
PLACEBO
ZB-106 DOSE LEVEL 1
R
PART B: OPEN LABEL EXTENSTION (FVC)
EFFICACY PERIOD (24 WEEKS)
E ZB-106 DOSE LEVEL 1
KEY
INCLUSION
CRITERIA
• Diffuse cutaneous SSc
• mRSS 10-29
• First symptoms of sclerosis
other than RP within 5 years
• Evidence of recent disease
progression*
• HAQ-DI >0.25
• Stable background therapy,
including MMF
Potential trigger for pivotal study |
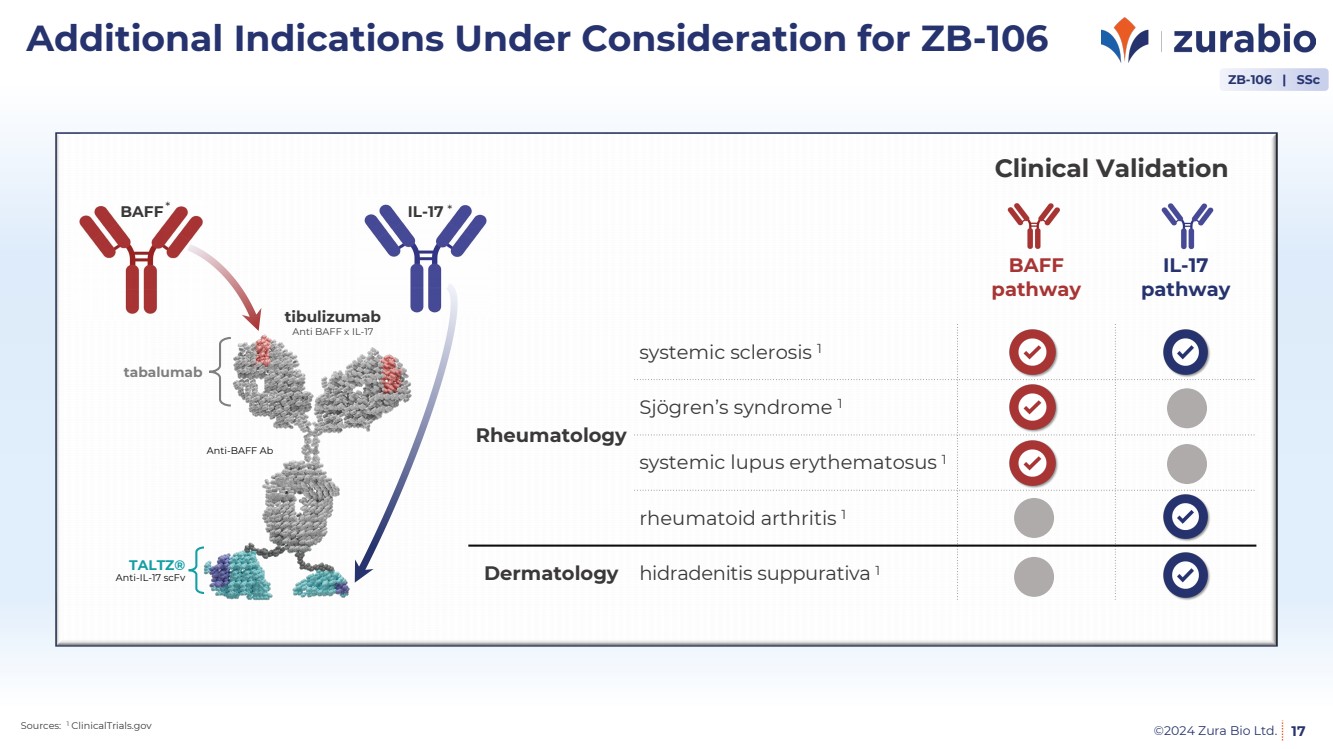
| ©2024 Zura Bio Ltd.
Additional Indications Under Consideration for ZB-106
17
ZB-106 | SSc
BAFF
pathway
IL-17
pathway
Rheumatology
systemic sclerosis 1
Sjögren’s syndrome 1
systemic lupus erythematosus 1
rheumatoid arthritis 1
Dermatology hidradenitis suppurativa 1
Sources: 1 ClinicalTrials.gov
BAFF IL-17
Anti-IL-17 scFv
TALTZ®
Anti-BAFF Ab
tabalumab
tibulizumab
Anti BAFF x IL-17
* *
Clinical Validation |

| ©2024 Zura Bio Ltd. 18
Development opportunities exist across
Rheumatology, Respiratory and Dermatology
ZURA
ASSETS
Rheumatology
systemic sclerosis, Sjögren's
syndrome, lupus, rheumatoid
arthritis
Respiratory
asthma, chronic obstructive
pulmonary disease
Dermatology
alopecia areata, hidradenitis
suppurativa, atopic dermatitis
ZB-168
Anti-IL-7R
ZB-106
tibulizumab
Anti-BAFF x IL-17
ZB-880
torudokimab
Anti-IL-33
ZB-106 is Zura’s lead asset and a
key value driver in rheumatology
ZB-168 and ZB-880 have potential
in respiratory and dermatology |

| Thank you to J.P. Morgan Healthcare for hosting
Nasdaq Ticker: ZURA |
Cover
|
Jan. 11, 2024 |
| Document Information [Line Items] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 11, 2024
|
| Entity File Number |
001-40598
|
| Entity Registrant Name |
Zura
Bio Limited
|
| Entity Central Index Key |
0001855644
|
| Entity Tax Identification Number |
98-1725736
|
| Entity Incorporation, State or Country Code |
E9
|
| Entity Address, Address Line One |
1489 W. Warm Springs Rd.
|
| Entity Address, Address Line Two |
#110
|
| Entity Address, City or Town |
Henderson
|
| Entity Address, State or Province |
NV
|
| Entity Address, Postal Zip Code |
89014
|
| City Area Code |
702
|
| Local Phone Number |
757-6133
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| Common Class A [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Class A Ordinary Shares, par value $0.0001 per share
|
| Trading Symbol |
ZURA
|
| Security Exchange Name |
NASDAQ
|
| Warrant [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Warrants, each whole warrant exercisable for one Class A Ordinary Share at an exercise price of $11.50 per share
|
| Trading Symbol |
ZURAW
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Zura Bio (NASDAQ:ZURA)
Historical Stock Chart
From Nov 2024 to Dec 2024

Zura Bio (NASDAQ:ZURA)
Historical Stock Chart
From Dec 2023 to Dec 2024
