
Notice of Convocation of the 148th Ordinary General Meeting of Shareholders Date: June 26, 2024 (Wednesday), 10:00 a.m. Venue: Imperial Hotel, Osaka 3rd Floor Contents Notice of Convocation of the 148th Ordinary General Meeting of Shareholders ····· 1 Guidance Notes on the Exercise of Voting Rights via Electronic Means (e.g., the Internet, etc.)······································································ 4 Internet live stream and the advance questions ··············································· 5 Reference Document for the General Meeting of Shareholders ·························· 6 Business Report ························································································ 27 Consolidated Financial Statements ······························································· 79 Unconsolidated Financial Statements ···························································· 84 Audit Reports ···························································································· 88 Internet live stream will be delivered. Please refer to page 5. Takeda Pharmaceutical Company Limited TSE Code: 4502 Please note that the following is an English translation of the original Japanese version, prepared only for the convenience of shareholders residing outside Japan. In case of any discrepancy between the translation and the Japanese original, the latter shall prevail. TAKEDA PHARMACEUTICAL COMPANY LIMITED (“TAKEDA”) HEREBY DISCLAIMS ALL REPRESENTATIONS AND WARRANTIES WITH RESPECT TO THIS TRANSLATION, WHETHER EXPRESS OR IMPLIED INCLUDING, BUT WITHOUT LIMITATION TO, ANY REPRESENTATIONS OR WARRANTIES WITH RESPECT TO ACCURACY, RELIABILITY OR COMPLETENESS OF THIS TRANSLATION. IN NO EVENT SHALL TAKEDA BE LIABLE FOR ANY DAMAGES OF ANY KIND OR NATURE INCLUDING, BUT WITHOUT LIMITATION TO, DIRECT, INDIRECT, SPECIAL, PUNITIVE, CONSEQUENTIAL OR INCIDENTAL DAMAGES ARISING FROM OR IN CONNECTION WITH THIS TRANSLATION. This translation includes a translation of the audit report of the financial statements included in the original Japanese version, prepared by KPMG AZSA LLC, TAKEDA’s independent auditor. KPMG AZSA LLC has not audited and makes no warranty as to the accuracy or otherwise of the translation of the financial statements or other financial information included in this translation.

- 1 - TSE Code: 4502 June 4, 2024 Dear Shareholders Notice of Convocation of the 148th Ordinary General Meeting of Shareholders This is to inform you that TAKEDA PHARMACEUTICAL COMPANY LIMITED (the “Company” or “TAKEDA”) will be holding its 148th Ordinary General Meeting of Shareholders (the “Meeting”) as follows. For convening the Meeting, information contained in the Reference Document for General Meeting of Shareholders, etc. (matters subject to measures for electronic provision (“Electronic Provision Measures Matters”)) is provided electronically, and is posted on the Company’s website. Please go to the Company’s website below and review them. The Company’s website: https://www.takeda.com/investors/events In addition to the above, Electronic Provision Measures Matters are also available on the website of the Tokyo Stock Exchange (TSE). Please go to the TSE’s website below (Listed Company Search), enter the issue name (Takeda Pharmaceutical Company) or TSE code (4502), search for it, and select “Basic information” and “Documents for public inspection/PR information” to see them. The TSE’s website (Listed Company Search): https://www2.jpx.co.jp/tseHpFront/JJK020010Action.do?Show=Show If you are not attending the Meeting, you may exercise your voting rights via electronic means (e.g. the internet, etc.) or in writing. Please kindly go through the Reference Document for General Meeting of Shareholders described below and exercise your voting rights no later than 5:30 p.m. on June 25, 2024 (Tuesday). (The Internet live stream will be delivered so that you can view the Meeting at home or another remote location of your convenience as described in page 5. Please consider exercising voting rights in advance and viewing the internet live stream.) Exercise of Voting Rights via Electronic Means (e.g.: the Internet, etc.) Please refer to the “Guidance Notes on the Exercise of Voting Rights via Electronic Means (e.g., the Internet, etc.)” on page 4, and complete the entry of your approval or disapproval of the proposals in accordance with the instructions on the screen on or before the deadline below. Deadline for Exercise (completion of entry): 5:30 p.m. on June 25, 2024 (Tuesday) Exercise of Voting Rights in Writing Please indicate your approval or disapproval of the proposals on the enclosed “Voting Right Exercise Form” and send it back to reach us on or before the deadline below. Deadline for Exercise (arrival): 5:30 p.m. on June 25, 2024 (Tuesday) Yours faithfully, Christophe Weber President and Representative Director Takeda Pharmaceutical Company Limited 1-1, Doshomachi 4-chome Chuo-ku, Osaka 540-8645, Japan

- 2 - Details 1. Date: June 26, 2024 (Wednesday), 10:00 a.m. 2. Venue: Imperial Hotel, Osaka 3rd Floor 8-50, Temmabashi 1-Chome, Kita-ku, Osaka, Japan 3. Objectives of the Meeting: Matters to be reported: 1. Reports on the Business Report, Consolidated Financial Statements and Unconsolidated Financial Statements for the 147th fiscal year (from April 1, 2023 to March 31, 2024) 2. Reports on the Audit Reports on the Consolidated Financial Statements for the 147th fiscal year by the Accounting Auditor and Audit and Supervisory Committee Matters to be resolved: First Proposal: Appropriation of Surplus Second Proposal: Election of Ten (10) Directors who are not Audit and Supervisory Committee Members Third Proposal: Election of Four (4) Directors who are Audit and Supervisory Committee Members Fourth Proposal: Payment of Bonuses to Directors who are not Audit and Supervisory Committee Members ⚫ Please be so kind as to submit the enclosed Voting Right Exercise Form to a receptionist at the venue for attendance of the Meeting. ⚫ Please also be so kind to cooperate with measures that the Company or the hotel deem necessary for the safety of shareholders as a whole. ⚫ In case where the operation of the Meeting is significantly changed, those changes will be announced on our website (https://www.takeda.com/investors/events). Guidance Notes on the Treatment of Exercise of Voting Rights (1) If you exercise your voting rights both via electronic means (e.g., the Internet, etc.) and in writing, the Company will regard only the vote cast via electronic means (e.g., the Internet, etc.) as valid, regardless of the time and date the votes are received. (2) If you exercise your voting rights more than once via electronic means (e.g., the Internet, etc.), the Company will regard only your last vote as valid. (3) If you exercise your voting rights by proxy, you may delegate your voting rights to one shareholder who holds voting rights in the Company. However, please note that you are required to submit a document certifying the authority of such proxy. (4) If neither “for” nor “against” is marked on the submitted Voting Right Exercise Form, it will be treated as a consent for the relevant proposal(s).

- 3 - Other matters decided for convening the Meeting 1. Among the Electronic Provision Measures Matters, the following items are not included in the hardcopies of documents sent to shareholders who made a request for delivery of documents in accordance with relevant laws and regulations, as well as the Company’s Articles of Incorporation. Please note that Audit and Supervisory Committee and Accounting Auditor audited the documents which include the following items: 1) Following items of the Business Report Business Overview Business Performance for Fiscal 2023 Issues for the Takeda Group to Address Financial Position and Income Summary Main Businesses of the Takeda Group Major Offices of the Company Employees Principal lenders and loan amounts Common Stock of the Company Outline of the terms of the liability limitation agreement Outline of the terms of the company indemnification agreement Outlines of the terms of the directors & officers liability insurance External Directors (Major activities during this fiscal year and the summary of the duties which were conducted by the External Directors with regard to the roles which the Company had expected them to fulfill) Accounting Auditor Overview of the Systems to Ensure the Appropriateness of Operations of the Company and the Status of Implementation of such Systems 2) Consolidated Statement of Changes in Equity and Notes to the Consolidated Financial Statements 3) Unconsolidated Financial Statements (Unconsolidated Balance Sheet, Unconsolidated Statement of Operations, Unconsolidated Statements of Changes in Net Assets and Notes to the Unconsolidated Financial Statements) 2. Any modification made to the Electronic Provision Measures Matters will be communicated by posting a notification to that effect and the pre-modified versions of those matters on the Company’s website and TSE’s website. 3. The resolutions made at the 148th Ordinary General Meeting of Shareholders will be posted on our website after the completion thereof instead of sending the notice of resolutions in writing. Company’s website https://www.takeda.com/investors/events END OF DOCUMENT

- 4 - Guidance Notes on the Exercising of Voting Rights via Electronic Means (e.g., the Internet, etc.) (Not applicable for holders of American Depositary Shares) Website for exercising voting rights: https://evote.tr.mufg.jp/ You may exercise your voting rights via the Internet by accessing the website for exercising voting rights using a smartphone or a personal computer. Please exercise your voting rights following the instructions on the screen. ⚫ Please note that you will not be able to access the above URL from 2:30 a.m. to 4:30 a.m. each day. ⚫ Any Internet access fees or communication charges, etc., arising from access to the website for exercising voting rights shall be borne by the user. Method for Exercising Voting Rights by scanning QR code (QR Code is the registered trademark of DENSO WAVE INCORPORATED) Scan “QR Code for Login” provided in the right side of the enclosed “Voting Right Exercise Form” In exercising your voting rights by using a smartphone, neither “Login ID” nor “Tentative Password” is required. Method for Exercising Voting Rights by entering “Login ID” and “Tentative Password” (1) Access the website for exercising voting rights above by using a personal computer Click “Next Screen” (2) Enter “Login ID” and “Tentative Password” Enter “Login ID” and “Tentative Password” provided in the Voting Right Exercise Form (3) Login Click “Login” and enter your approval or disapproval of the proposals following the instructions on the screen. For inquiries with respect to systems, please contact: Mitsubishi UFJ Trust and Banking Corporation Corporate Agency Division (help desk) Telephone: 0120-173-027 (toll-free number) Operating Hours: 9:00 to 21:00 To Institutional Investors: “Electronic Voting Platform” is available as a method for exercising voting rights.

- 5 - <Internet live stream and the advance questions> The Internet live stream will be delivered so that you can view the Meeting at home or another remote location of your convenience, and post the video of the Meeting on the Company’s website available on demand at a later date of the Meeting. Please consider exercising voting rights in advance and viewing the internet live stream. Also, you can ask an advance question related to the objectives of the Meeting. Please refer to the enclosed “Guidance on Internet Live Stream of the 148th Ordinary General Meeting of Shareholders” for details such as the way of access. 1. For the Internet live stream and the advance questions Please access the URL below: https://web.lumiconnect.com/748524668 You will be able to access the website above once you scan the QR code indicated here using your smartphone or tablet. (QR Code is the registered trademark of DENSO WAVE INCORPORATED) Also, you will be able to access from the Company’s website (https://www.takeda.com/investors/events). 2. Internet Live Stream Date and time: From 10:00 a.m. to the end of the Meeting on June 26, 2024 (Wednesday) (You can access from 9:00 a.m. on June 26, 2024. Before that, you can conduct the test of access.) How to login: After accessing the URL above, please enter the “Login ID” and “Password” in accordance with the enclosed “Guidance on Internet Live Stream of the 148th Ordinary General Meeting of Shareholders.” Please note that the shareholders who are viewing the Meeting on the internet are not entitled to exercise their voting rights or ask questions during the Meeting. We will make free comments function available to you. However, please kindly understand that while we cannot answer to each comment, we will use it for the operation of the Meeting. 3. Acceptance of Advance Question via the Internet Acceptance period: From noon on June 5, 2024 (Wednesday) to 5:00 p.m. on June 18, 2024 (Tuesday) How to ask: After accessing the URL above, please enter the “Login ID” and “Password” in accordance with the enclosed “Guidance on Internet Live Stream of the 148th Ordinary General Meeting of Shareholders,” and fill out the advance question form. Please note that you can ask one question related to the objectives of the Meeting. Among such advance questions, the matters in which the shareholders are highly interested will be answered during the Meeting. However, please kindly understand that we cannot answer to each advance question. Notice regarding Digital Transition of Message from Christophe Weber, President & CEO The message from Christophe Weber, President & CEO, which to date had been sent together with the Notice of Convocation, will now be available on our website exclusively. This change reflects our commitment to protecting the planet. The message can be accessed via the following URL or QR code. (QR Code is the registered trademark of DENSO WAVE INCORPORATED) Our website: https://takeda.info/2024-shareholder-letter

- 6 - Reference Document for the General Meeting of Shareholders Proposals and Reference Matters: First Proposal: Appropriation of Surplus Guided by our vision to discover and deliver life-transforming treatments, and with a focus on maintaining solid investment grade credit ratings, we will allocate capital to deliver sustainable value to patients and attractive returns to our shareholders. The Company’s policy in the allocation of capital is as follows: • Invest in growth drivers; and • Shareholder returns. In respect of "Invest in growth drivers", the Company makes strategic investments in internal and external opportunities to enhance the pipeline, new product launches, and plasma-derived therapies. With regard to "Shareholder returns", the Company has adopted a progressive dividend policy of increasing or maintaining the annual dividend per share each year, alongside share buybacks when appropriate. The Company submits the following proposal with respect to the appropriation of surplus for this fiscal year: Year-end dividends (1) Type of dividend asset Cash (2) Allocation of dividend asset to shareholders and total amount of allocation 94 JPY per share of common stock; Total amount: 148,041,018,112 JPY (Reference) Combined with the interim dividend of 94 JPY per share, the annual dividend will be 188 JPY per share (an increase of 8 JPY per share over the previous fiscal year). (3) Effective date of distribution of the dividend June 27, 2024

- 7 - Second Proposal: Election of Ten (10) Directors who are not Audit and Supervisory Committee Members The term of office of the ten (10) Directors who are not Audit and Supervisory Committee (ASC) Members, namely, Christophe Weber, Andrew Plump, Costa Saroukos, Masami Iijima, Jean-Luc Butel, Ian Clark, Steven Gillis, John Maraganore, Michel Orsinger and Miki Tsusaka, will expire at the close of this General Meeting of Shareholders and Mr. Olivier Bohuon retired from his position at the time of his death on May 5, 2024. The Company therefore proposes the election of the ten (10) Directors who are not ASC Members, including the seven (7) External Directors. The 10 candidates for Directors who are not ASC Members including 2 female candidates are as follows: Candidate No. Name Current position and responsibilities Tenure as Director Number of Board of Directors meetings attended 1 Christophe Weber To be reelected President and Representative Director Chief Executive Officer 10 years 8/8 (100%) 2 Andrew Plump To be reelected Director President, Research and Development 9 years 8/8 (100%) 3 Milano Furuta To be newly elected Chief Financial Officer - - 4 Masami Iijima To be reelected as External Director Independent Director Director Chair of the Board of Directors meeting Chairperson of Nomination Committee 3 years 8/8 (100%) 5 Ian Clark To be reelected as External Director Independent Director Director Compensation Committee Member 5.5 years 7/8 (100%) 6 Steven Gillis To be reelected as External Director Independent Director Director Nomination Committee Member 5.5 years 8/8 (88%) 7 John Maraganore To be reelected as External Director Independent Director Director 2 years 8/8 (100%) 8 Michel Orsinger To be reelected as External Director Independent Director Director Nomination Committee Member Compensation Committee Member 8 years 8/8 (100%) 9 Miki Tsusaka To be reelected as External Director Independent Director Director 1 year 7/7 (100%)- 10 Emiko Higashi To be newly elected as External Director Independent Director Director (ASC Member) Chairperson of Compensation Committee 8 years 8/8 (100%) (Note) With regard to “Number of Board of Directors meetings attended,” the Board of Directors meetings which Ms. Miki Tsusaka, Director, was eligible to attend were those held on and after June 28, 2023 when she took office. <Reference> For the Board of Directors Skills Matrix in case the nominated directors proposed in the 2nd and 3rd proposals are elected, please access the following URL. https://takeda.info/skillmatrix_sm_148_en

- 8 - Candidate No.1 Christophe Weber Born on November 14, 1966 (57 years old) To be Reelected as Internal Director Tenure as Director 10 years Number of Board of Directors meetings attended 8/8 (100%) Number of Company Shares Held 785,900 shares Number of Company Shares to be provided 702,331 shares Number of Company American Depositary Shares (ADS) Held 0 share Number of Company ADS to be provided 211,756 shares Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held April 2012 President & General Manager, GlaxoSmithKline Vaccines April 2012 CEO, GlaxoSmithKline Biologicals April 2012 Member of GlaxoSmithKline Corporate Executive Team April 2014 Chief Operating Officer of the Company June 2014 President and Representative Director of the Company (to present) April 2015 Chief Executive Officer of the Company (to present) September 2020 Head of Global Business, Takeda Pharmaceuticals U.S.A., Inc. (to present) Rationale for Nomination as Candidate for Director Mr. Christophe Weber has more than 30 years of global experience in the pharmaceutical industry. Since 2014, he has demonstrated his strong leadership as President & CEO, transforming the Company into a truly global, values-based, R&D-driven, digital biopharmaceutical company through R&D transformation and a successful integration with Shire. He leads a diverse Takeda Executive Team consisting of 17 members of 9 different nationalities, who, together with our 50,000 global employees, are pursuing our vision of discovering and delivering life-transforming treatments, guided by our commitments to patients, our people and the planet. The Company nominates Mr. Weber as Director in the belief that it is necessary to continue to utilize his ability, experience, and leadership in the management of the Company.

- 9 - Candidate No.2 Andrew Plump Born on October 13, 1965 (58 years old) To be Reelected as Internal Director Tenure as Director 9 years Number of Board of Directors meetings attended 8/8 (100%) Number of Company Shares Held 0 share Number of Company Shares to be provided 0 share Number of Company American Depositary Shares (ADS) Held 276,845 shares Number of Company ADS to be provided 739,162 shares Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held January 2008 Vice President, Cardiovascular Disease Franchise, Worldwide Discovery Head, Merck & Co. March 2014 Senior Vice President & Deputy to the President for Research & Translational Medicine, Sanofi February 2015 Chief Medical & Scientific Officer Designate of the Company June 2015 Director of the Company (to present) June 2015 Chief Medical & Scientific Officer of the Company January 2019 President, Research & Development of the Company (to present) July 2021 President, Research & Development, Takeda Development Center Americas, Inc. (to present) Rationale for Nomination as Candidate for Director As President, Research & Development, Dr. Andrew Plump has been demonstrating his formidable initiative, guiding the R&D transformation and advancing measures to build the Company’s R&D pipeline, including driving innovative R&D agendas that leverage the Company’s expertise in core therapeutic areas. He has also enhanced the performance and culture of the R&D organization by strengthening internal R&D capabilities while cultivating external partnerships. The Company nominates Dr. Plump as Director in the belief that it is necessary to continue to utilize his ability and experience is essential for its management.

- 10 - Candidate No.3 Milano Furuta Born on February 26, 1978 (46 years old) To be newly elected as Internal Director Tenure as Director - Number of Board of Directors meetings attended - Number of Company Shares Held 13,200 shares Number of Company Shares to be provided 41,606 shares Number of Company American Depositary Shares (ADS) Held 0 share Number of Company ADS to be provided 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held April 2000 Joined The Industrial Bank of Japan, Limited (currently Mizuho Financial Group, Inc.) June 2006 Joined Taiyo Pacific Partners, USA July 2010 Joined the Company June 2017 Country Manager, Takeda Pharma AB (Sweden) January 2019 Corporate Strategy Officer & Chief of Staff of the Company April 2021 President, Japan Pharma Business Unit of the Company April 2024 Chief Financial Officer of the Company (to present) Rationale for Nomination as Candidate for Director Mr. Milano Furuta has expertise in finance and corporate management through investment and financing operations, and has accumulated experiences in business planning, sales and marketing, and business management related to pharmaceutical business in multiple countries at the Company. In recent years, Mr. Furuta, as a member of the Takeda Executive Team, served as Corporate Strategy Officer and President, Japan Pharma Business Unit, and currently serves as Chief Financial Officer. The Company nominates Mr. Furuta as Director in the belief that his extensive experience and competencies will contribute to the continuous growth and success of the Company as a global, values-based, and R&D- driven, digital biopharmaceutical leader.

- 11 - Candidate No.4 Masami Iijima Born on September 23, 1950 (73 years old) To be Reelected as External Director / Independent Director Tenure as Director 3 years Number of Board of Directors meetings attended 8/8 (100%) Number of Company Shares Held 300 shares Number of Company Shares to be provided 14,522 shares Number of Company American Depositary Shares (ADS) Held 0 share Number of Company ADS to be provided 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held June 2008 Representative Director, Executive Managing Officer, Mitsui & Co., Ltd October 2008 Representative Director, Senior Executive Managing Officer, Mitsui & Co., Ltd. April 2009 Representative Director, President and Chief Executive Officer, Mitsui & Co., Ltd. April 2015 Representative Director, Chairman of the Board of Directors, Mitsui & Co., Ltd. June 2018 External Director, SoftBank Group Corp. (to present) June 2019 Counsellor, Bank of Japan (to present) April 2021 Director, Mitsui & Co., Ltd. June 2021 Counselor, Mitsui & Co., Ltd. (to present) June 2021 External Director of the Company who is an ASC Member June 2022 External Director who is the Chair of the Board of Directors meeting of the Company (to present) June 2023 External Director, Kajima Corporation (to present) Rationale for Nomination as Candidate for External Director and Overview of Expected Role Mr. Masami Iijima served as Representative Director, President, and CEO of Mitsui & Co., Ltd., where he oversaw global management of the company. Later, as Chair of the Board and Representative Director and Chair of the Board meetings, he focused on management supervision and improving the effectiveness of the Board of Directors. During his career, he acquired extensive experience in various fields including corporate governance and risk management. In addition to active participation on the Company's Board of Directors as an External Director, he contributes to fair and impartial decision-making and ensuring the soundness of business activities by leading discussions at meetings of External Directors in addition to facilitating the proceedings of the Board of Directors as the Chair of the Board. He has been involved in the management of the Company as an External Director who is an ASC Member since June 2021, and as an External Director who is not an ASC Member and Chair of the Board of Directors meeting since June 2022. The Company nominates Mr. Iijima as an External Director because he is expected to continue to fulfill the important roles for the Company described above.

- 12 - Candidate No.5 Ian Clark Born on August 27, 1960 (63 years old) To be Reelected as External Director / Independent Director Tenure as Director 5.5 years Number of Board of Directors meetings attended 7/8 (88%) Number of Company Shares Held 0 share Number of Company Shares to be provided 16,878 shares Number of Company American Depositary Shares (ADS) Held 2,096 shares Number of Company ADS to be provided 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held January 2010 Director, Chief Executive Officer and Head of North American Commercial Operations, Genentech, Inc. January 2017 External Director, Shire plc January 2017 External Director, Corvus Pharmaceuticals, Inc. (to present) January 2017 External Director, Guardant Health, Inc. (to present) January 2019 External Director of the Company (to present) August 2020 External Director, Olema Pharmaceuticals, Inc. (to present) Rationale for Nomination as Candidate for External Director and Overview of Expected Role Mr. Ian Clark has experience as an External Director of Shire, bringing deep expertise to the Company’s portfolio and its related therapeutic areas. He has served in several pivotal positions at global healthcare companies in Europe and Canada. He has gained deep insights through his extensive experience in the management of global healthcare businesses, and has particular expertise in marketing in the oncology sector and in the management of biotechnology departments in healthcare companies. He has contributed to ensuring fair and appropriate decisions and actions of the Company through his active participation on the Board of Directors as an External Director. The Company nominates Mr. Clark as an External Director because he is expected to continue to fulfill the important roles for the Company described above.

- 13 - Candidate No.6 Steven Gillis Born on April 25, 1953 (71 years old) To be Reelected as External Director / Independent Director Tenure as Director 5.5 years Number of Board of Directors meetings attended 8/8 (100%) Number of Company Shares Held 0 share Number of Company Shares to be provided 16,878 shares Number of Company American Depositary Shares (ADS) Held 8,257 shares Number of Company ADS to be provided 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held August 1981 Founder, Director and Executive Vice President, Research and Development, Immunex Corporation (currently, Amgen, Inc.) May 1993 Chief Executive Officer, Immunex Corporation October 1994 Founder, Director and Chief Executive Officer, Corixa Corporation (currently, GlaxoSmithKline) January 1999 Director and Chairman, Corixa Corporation August 2005 Managing Director, ARCH Venture Partners (to present) October 2012 External Director, Shire plc October 2015 External Director and Chairman, Codiak BioSciences, Inc. (to present) May 2016 External Director and Chairman, VBI Vaccines, Inc. (to present) January 2019 External Director of the Company (to present) Rationale for Nomination as Candidate for External Director and Overview of Expected Role Dr. Steven Gillis has experience as an External Director of Shire, bringing deep expertise to the Company’s portfolio and its related therapeutic areas. He has a Ph.D. in Biology and has served in several pivotal positions at global healthcare companies in the U.S. and Europe. He also has extensive experience in global healthcare business management and significant expertise in immunology. He has contributed to ensuring fair and appropriate decisions and actions of the Company through his active participation on the Board of Directors as External Director. The Company nominates Dr. Gillis as External Director because he is expected to continue to fulfill the important roles for the Company described above.

- 14 - Candidate No.7 John Maraganore Born on October 11, 1962 (61 years old) To be Reelected as External Director / Independent Director Tenure as Director 2 years Number of Board of Directors meetings attended 8/8 (100%) Number of Company Shares Held 0 share Number of Company Shares to be provided 9,373 shares Number of Company American Depositary Shares (ADS) Held 0 share Number of Company ADS to be provided 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held April 2000 Senior Vice President, Strategic Product Development, Millennium Pharmaceuticals, Inc. December 2002 Director and Chief Executive Officer, Alnylam Pharmaceuticals, Inc. June 2017 Chairperson, Biotechnology Innovation Organization November 2021 External Director, Beam Therapeutics, Inc. (to present) February 2022 External Director, Kymera Therapeutics, Inc. (to present) June 2022 External Director of the Company (to present) July 2022 External Director, ProKidney Corporation (to present) Rationale for Nomination as Candidate for External Director and Overview of Expected Role Dr. John Maraganore is a pioneering executive with more than three decades of experience in the pharmaceutical industry. He served as the CEO and a Director of Alnylam Pharmaceuticals for nearly 20 years and retired at the end of 2021. Prior to Alnylam, he served as an officer and a member of the management team for Millennium. During his career, he has gained substantial experience in the pharmaceutical industry. He has contributed to ensuring fair and appropriate decisions and actions of the Company through his active participation on the Board of Directors as an External Director. The Company nominates Dr. Maraganore as an External Director because he is expected to continue to fulfill the important roles for the Company described above.

- 15 - Candidate No.8 Michel Orsinger Born on September 15, 1957 (66 years old) To be Reelected as External Director / Independent Director Tenure as Director 8 years Number of Board of Directors meetings attended 8/8 (100%) Number of Company Shares Held 0 share Number of Company Shares to be provided 21,054 shares Number of Company American Depositary Shares (ADS) Held 0 share Number of Company ADS to be provided 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held March 2001 Chief Executive Officer and President, OTC Division Worldwide, Consumer Health, Novartis AG April 2007 President and Chief Executive Officer, Synthes, Inc. (currently Johnson & Johnson) June 2012 Worldwide Chairman, Global Orthopedics Group, DePuy Synthes Companies, Johnson & Johnson June 2012 Member of Global Management Team, Johnson & Johnson June 2016 External Director of the Company June 2019 External Director of the Company who is an ASC Member June 2022 External Director of the Company (to present) Rationale for Nomination as Candidate for External Director and Overview of Expected Role Mr. Michel Orsinger has served in several pivotal positions at global healthcare companies in the U.S. and Europe. He has gained deep insights from extensive experience in global healthcare business management. He has contributed to ensuring fair and appropriate decisions and actions of the Company through his active participation on the Board of Directors as External Director. He has been involved in the management of the Company as an External Director who is not an ASC Member since June 2016, as an External Director who is an ASC Member since June 2019, and as an External Director who is not an ASC Member since June 2022. The Company nominates Mr. Orsinger as an External Director because he is expected to continue to fulfill the important roles for the Company described above.

- 16 - Candidate No.9 Miki Tsusaka Born on April 24, 1963 (61 years old) To be Reelected as External Director / Independent Director Tenure as Director 1 year Number of Board of Directors meetings attended 7/7 (100%) Number of Company Shares Held 0 share Number of Company Shares to be provided 4,252 shares Number of Company American Depositary Shares (ADS) Held 0 share Number of Company ADS to be provided 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held May 1995 Partner and Managing Director, Boston Consulting Group May 2003 Senior Partner and Managing Director, Boston Consulting Group May 2005 Global Leader, Marketing, Sales & Pricing Practice, Boston Consulting Group October 2011 Executive Committee Member, Boston Consulting Group June 2013 Chief Marketing Officer, Boston Consulting Group February 2023 President, Microsoft Japan Co., Ltd. (to present) June 2023 External Director of the Company (to present) Rationale for Nomination as Candidate for External Director and Overview of Expected Role Ms. Miki Tsusaka has exceptional leadership skills and substantial expertise in global business, strategy, and data & digital, and has deep insights into the leveraging of technology to drive innovation and create value. Having worked with companies across Asia, Europe, and North America, she brings deep insights and a wide variety of experiences relating to working in a global environment. She has contributed to ensuring fair and appropriate decisions and actions of the Company through her active participation on the Board of Directors as an External Director. The Company nominates Ms. Tsusaka as an External Director because she is expected to continue to fulfill the important roles for the Company described above.

- 17 - Candidate No.10 Emiko Higashi Born on November 6, 1958 (65 years old) To be newly elected as External Director / Independent Director Tenure as Director 8 years Number of Board of Directors meetings attended 8/8 (100%) Number of Company Shares Held 2,500 shares Number of Company Shares to be provided 21,054 shares Number of Company American Depositary Shares (ADS) Held 0 share Number of Company ADS to be provided 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held May 1994 Managing Director, Investment Banking, Merrill Lynch & Co. April 2000 CEO, Gilo Ventures, LLC January 2003 Managing Director, Tomon Partners, LLC (to present) November 2010 External Director, KLA-Tencor Corporation (currently KLA Corporation) (to present) June 2016 External Director of the Company May 2017 External Director, Rambus Inc. (to present) June 2019 External Director of the Company who is an ASC Member (to present) March 2023 External Director, Rapidus Corporation (to present) Rationale for Nomination as Candidate for External Director and Overview of Expected Role Ms. Emiko Higashi has experience in pivotal positions, such as CEO of investment funds mainly in the U.S., as well as experience in investment funds specializing in healthcare and technology. She has extensive knowledge and experience in finance and accounting, the financial industry, the healthcare industry, and data and technology. She has contributed to ensuring fair and appropriate decisions and actions of the Company through her active participation on the Board of Directors as External Director. She has been involved in the management of the Company as an External Director who is not an ASC Member since June 2016 and as an External Director who is an ASC Member since June 2019. The Company nominates Ms. Higashi as an External Director because she is expected to continue to fulfill the important roles for the Company described above.

- 18 - (Notes) 1. No special interests exist between the above candidates and the Company. 2. The number of Company shares held represents the number of common stocks held as of March 31, 2024. The number of Company shares to be provided represents the number of common stocks vested but undelivered and scheduled to be vested, including those granted to Directors based outside of Japan that will be converted to ADSs for settlement following vesting, under the Board Incentive Plan (“BIP”) for Directors (excluding Directors based outside of Japan who are not External Directors) and the Employee Stock Ownership Plan (“ESOP”), a stock grant plan for Company management in Japan (which relates to all of the Company Shares to be provided to Mr. Milano Furuta as described above). The number of Company shares to be provided to candidates (excluding candidates for Directors who are External Directors) pursuant to the BIP or ESOP is comprised of Restricted Stock Unit awards (“RSU awards”) and Performance Share Unit awards (“PSU awards”). The number of Company shares to be provided to candidates for External Directors (including the candidates for External Directors who are ASC Members) pursuant to the BIP is comprised only of RSU awards. RSU awards to be provided to candidates (excluding candidates for External Directors) vest one third each year over a three-year period and PSU awards vest three years from the date of grant. Included PSU awards to be vested in the future years represent the total number of shares to be issued assuming that relevant targets are met at the 100% level; the actual number of shares issued may be fewer or greater depending on the level at which targets are met. RSU awards to be provided to candidates for External Directors (including the candidates for External Directors who are ASC Members) will be provided or paid three years from the date of grant. In addition, with regard to the Company’s shares to be provided under the BIP or ESOP, the voting rights thereof may not be exercised before such shares are provided to each candidate. 3. The number of Company ADS held represents the number of American Depositary Shares held as of March 31, 2024 and is rounded to the nearest whole number. Each ADS represents one half of a common stock. The number of Company ADS to be provided represents the number of American Depositary Shares vested but undelivered and scheduled to be vested under Long-Term Incentive Plan for Company Group Employees Overseas (“LTIP”). The number of Company ADS to be provided pursuant to the LTIP is comprised of RSU awards and Performance Stock Unit awards (“PSU awards”). RSU awards vest one third each year over a three-year period and PSU awards vest three years from the date of grant. Included PSU awards to be vested in the future years represent the total number of ADS to be issued assuming that relevant targets are met at the 100% level; the actual number of ADS issued may be fewer or greater depending on the level at which targets are met. In addition, with regard to the ADS to be provided under the LTIP, the voting rights thereof may not be exercised before such shares are provided to each candidate. 4. Mr. Masami Iijima, Mr. Ian Clark, Dr. Steven Gillis, Dr. John Maraganore, Mr. Michel Orsinger, Ms. Miki Tsusaka and Ms. Emiko Higashi are candidates to become External Directors who are not ASC Members of the Company. The Company has set “Internal criteria for independence of external directors” (the contents of such criteria are as set forth on page 19.) and elected the External Directors based on such criteria. All of these 8 persons have met the requirements for Independent Directors based on the regulations of the financial instruments exchanges in Japan that the Company is listed on (e.g. Tokyo Stock Exchange, Inc.). The Company has appointed Mr. Masami Iijima, Mr. Ian Clark, Dr. Steven Gillis, Dr. John Maraganore, Mr. Michel Orsinger, Ms. Miki Tsusaka and Ms. Emiko Higashi as Independent Directors and submitted a notification to each of such exchanges. 5. The Company has entered into contracts with Mr. Masami Iijima, Mr. Ian Clark, Dr. Steven Gillis, Dr. John Maraganore, Mr. Michel Orsinger, Ms. Miki Tsusaka and Ms. Emiko Higashi limiting the maximum amount of their liability for the damages set forth in Article 423, Paragraph 1 of the Companies Act to the legally stipulated value. If the re-election of Mr. Masami Iijima, Mr. Ian Clark, Dr. Steven Gillis, Dr. John Maraganore, Mr. Michel Orsinger and Ms. Miki Tsusaka is approved, and if the election of Mr. Emiko Higashi as Director who is not an ASC Member is approved, the Company plans to continue the same contracts to limit their liability. 6. The Company has entered into company indemnification agreements with all of the candidates, who are Directors at present, as defined in Article 430-2, Paragraph 1 of the Companies Act, which provide that the Company shall indemnify expenses set forth in Article 430-2, Paragraph 1, Item 1 thereof, and damages set forth in Article 430-2, Paragraph 1, Item 2 thereof within the scope permitted by the laws and regulations. If re-election of Mr. Christophe Weber, Dr. Andrew Plump, Mr. Masami Iijima, Mr. Ian Clark, Dr. Steven Gillis, Dr. John Maraganore, Mr. Michel Orsinger and Ms. Miki Tsusaka is approved, and if the election of Mr. Emiko Higashi as Director who is not an ASC Member is approved, the Company plans to continue the same agreements. Also, if election of Mr. Milano Furuta is approved, the Company plans to conclude the same company indemnification agreement with him.

- 19 - 7. The Company has entered into directors & officers liability insurance contracts with insurance companies as defined in Article 430-3, Paragraph 1 of the Companies Act, under which Directors of the Company are insured. Such insurance covers damages which may arise from liability incurred by such insured persons in connection with the execution of their duties or claims made against such insured persons in relation to such liability. If re-election or election of the candidates is approved, such candidates will be insured under such insurance scheme. The insurance contracts are planned to be renewed during such candidates’ term of office. <Reference> Internal criteria for the independence of External Directors of the Company The Company will judge whether an External Director has sufficient independence against the Company with emphasis on his/her meeting the following quality requirements, on the premise that he/she meets the criteria for independence established by the financial instruments exchanges. The Company believes that such persons will truly meet the shareholders’ expectations as External Directors of the Company, i.e., persons who can exert a strong presence in a diverse group of people that comprise the directors of the Company by proactively continuing to inquire on the nature of, encourage improvement in, and make suggestions regarding the important matters of the Company doing a pharmaceutical business globally, for the purpose of facilitating an impartial and fair judgment of the Company’s business and securing the sound management of the Company. The Company requires that persons who will be external directors to meet two (2) or more items out of the following four (4) items of quality requirements: (1) He/She has advanced insight derived from experience in corporate management; (2) He/She has a high level of knowledge in areas requiring high expertise such as accounting and law; (3) He/She is well versed in the pharmaceutical and/or global business; and (4) He/She has advanced linguistic skills and/or broad experience, which enables him/her to understand diverse values and to actively participate in discussions with others.
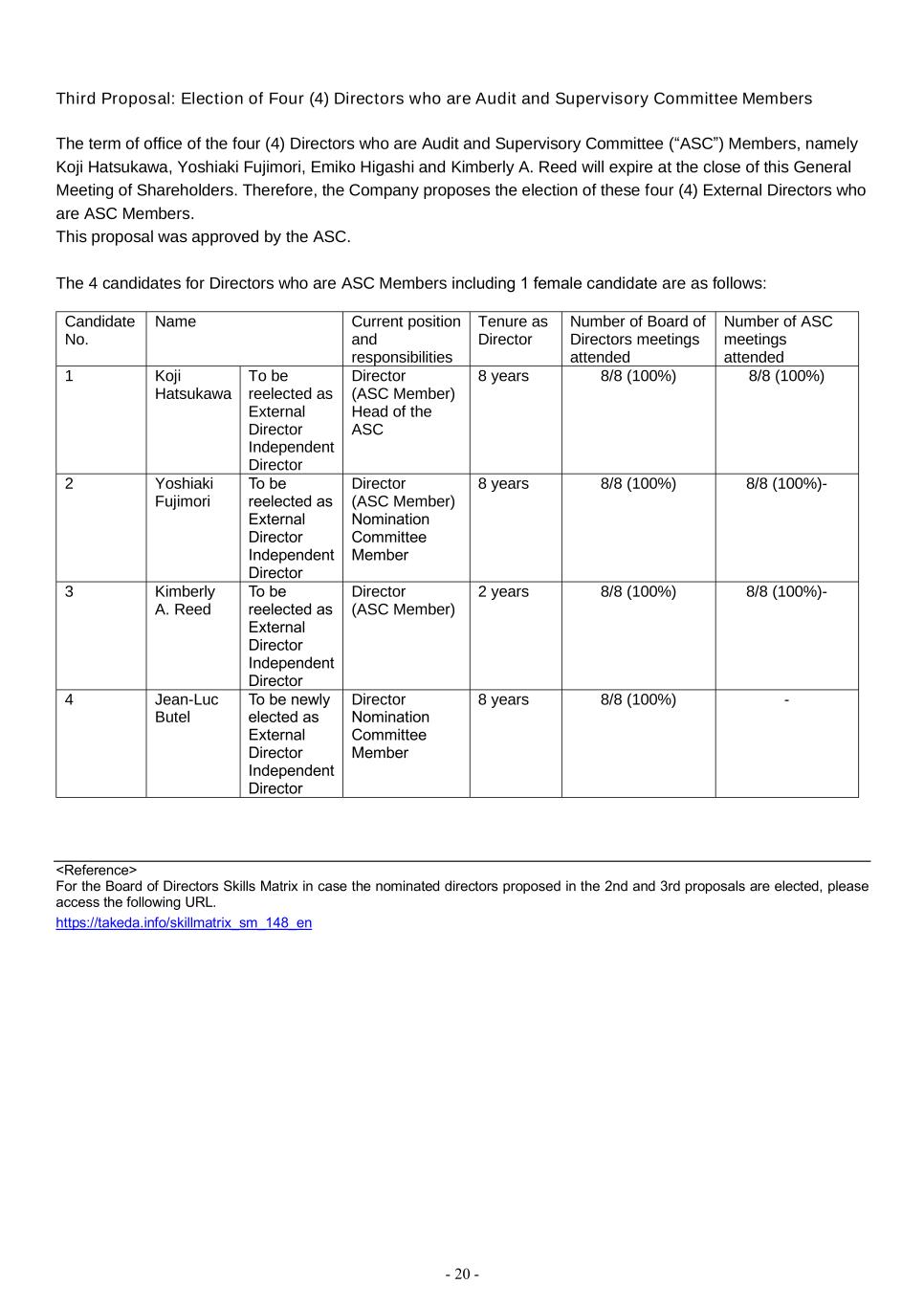
- 20 - Third Proposal: Election of Four (4) Directors who are Audit and Supervisory Committee Members The term of office of the four (4) Directors who are Audit and Supervisory Committee (“ASC”) Members, namely Koji Hatsukawa, Yoshiaki Fujimori, Emiko Higashi and Kimberly A. Reed will expire at the close of this General Meeting of Shareholders. Therefore, the Company proposes the election of these four (4) External Directors who are ASC Members. This proposal was approved by the ASC. The 4 candidates for Directors who are ASC Members including 1 female candidate are as follows: Candidate No. Name Current position and responsibilities Tenure as Director Number of Board of Directors meetings attended Number of ASC meetings attended 1 Koji Hatsukawa To be reelected as External Director Independent Director Director (ASC Member) Head of the ASC 8 years 8/8 (100%) 8/8 (100%) 2 Yoshiaki Fujimori To be reelected as External Director Independent Director Director (ASC Member) Nomination Committee Member 8 years 8/8 (100%) 8/8 (100%)- 3 Kimberly A. Reed To be reelected as External Director Independent Director Director (ASC Member) 2 years 8/8 (100%) 8/8 (100%)- 4 Jean-Luc Butel To be newly elected as External Director Independent Director Director Nomination Committee Member 8 years 8/8 (100%) - <Reference> For the Board of Directors Skills Matrix in case the nominated directors proposed in the 2nd and 3rd proposals are elected, please access the following URL. https://takeda.info/skillmatrix_sm_148_en

- 21 - Candidate No.1 Koji Hatsukawa Born on September 25, 1951 (72 years old) To be Reelected as External Director / Independent Director Tenure as Director 8 years Number of Board of Directors meetings attended 8/8 (100%) Number of ASC meetings attended 8/8 (100%) Number of Company Shares Held 10,000 shares Number of Company Shares to be provided 19,040 shares Number of Company American Depositary Shares (ADS) Held 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held March 1974 Joined Price Waterhouse Accounting Office July 1991 Representative Partner, Aoyama Audit Corporation October 2005 Director and Manager of International Operations, ChuoAoyama PricewaterhouseCoopers May 2009 CEO, PricewaterhouseCoopers Arata June 2013 External Audit & Supervisory Board Member, Fujitsu Limited (to present) June 2016 External Director of the Company who is an ASC Member (to present) June 2019 External Director of the Company who is the Head of the ASC (to present) Rationale for Nomination as Candidate for External Director (ASC Member) and Overview of Expected Role Mr. Koji Hatsukawa, a certified public accountant, has extensive experience and expertise in the areas of corporate finance and accounting. He also has experience as representative and CEO of an auditing firm. He has contributed to ensuring fair and appropriate decisions and actions of the Company through his active participation on the Board of Directors as External Director. He has been involved in the management of the Company as an External Director who is an ASC Member since June 2016 and as the Head of the ASC since June 2019. The Company nominates Mr. Hatsukawa as an External Director who is an ASC Member, because he is expected to continue to contribute to the realization of the ASC’s vision, which is to ensure the sound and continuous growth of the Company, to realize the creation of mid- and long-term corporate value, and to establish a robust corporate governance system worthy of public trust.

- 22 - Candidate No.2 Yoshiaki Fujimori Born on July 3, 1951 (72 years old) To be Reelected as External Director / Independent Director Tenure as Director 8 years Number of Board of Directors meetings attended 8/8 (100%) Number of ASC meetings attended 8/8 (100%) Number of Company Shares Held 12,500 shares Number of Company Shares to be provided 19,040 shares Number of Company American Depositary Shares (ADS) Held 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held May 2001 Senior Vice President, General Electric Company March 2011 Representative Director and Chairman, GE Japan Corporation August 2011 Representative Director, President and CEO, LIXIL Corporation August 2011 Director, Representative Executive Officer, President and CEO, LIXIL Group Corporation January 2016 Representative Director, Chairman and CEO, LIXIL Corporation June 2016 External Director of the Company July 2016 External Director, Boston Scientific Corporation (to present) February 2017 Senior Executive Advisor, CVC Asia Pacific (Japan) Kabushiki Kaisha (to present) August 2018 External Director and Chairman of the Board, Oracle Corporation Japan (to present) June 2019 External Director, Riraku K.K. (to present) June 2022 External Director of the Company who is an ASC Member (to present) Rationale for Nomination as Candidate for External Director (ASC Member) and Overview of Expected Role Mr. Yoshiaki Fujimori has served in pivotal positions, including CEO of a global U.S. company and its Japanese subsidiary, as well as at a Japanese company that was the first in its industry to pursue global expansion. During his career, he has acquired a wealth of insight based on his extensive experience in global corporate management and the healthcare industry. He has contributed to ensuring fair and appropriate decisions and actions of the Company through his active participation on the Board of Directors as External Director. He has been involved in the management of the Company as an External Director who is not an ASC Member since June 2016, and as an External Director who is an ASC Member since June 2022. The Company nominates Mr. Fujimori as an External Director who is an ASC Member, because he is expected to contribute to the realization of the ASC’s vision, which is to ensure the sound and continuous growth of the Company, to realize the creation of mid- and long-term corporate value, and to establish a robust corporate governance system worthy of public trust.

- 23 - Candidate No.3 Kimberly A. Reed Born on March 11, 1971 (53 years old) To be Reelected as External Director / Independent Director Tenure as Director 2 years Number of Board of Directors meetings attended 8/8 (100%) Number of ASC meetings attended 8/8 (100%) Number of Company Shares Held 0 share Number of Company Shares to be provided 9,373 shares Number of Company American Depositary Shares (ADS) Held 1,375 shares Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held October 1997 Counsel, United States House of Representatives May 2004 Senior Advisor to United States Secretaries of the Treasury, United States Department of the Treasury February 2007 Director and Chief Executive Officer, Community Development Financial Institutions Fund, United States Department of the Treasury December 2007 Vice President, Financial Markets Policy Relations, Lehman Brothers September 2009 President, International Food Information Council Foundation May 2019 Chairman of the Board of Directors, President, and Chief Executive Officer, Export- Import Bank of the United States February 2021 Distinguished Fellow, Council on Competitiveness (to present) August 2021 External Director, Momentus Inc. (to present) June 2022 External Director of the Company who is an ASC Member (to present) March 2023t External Director, Hannon Armstrong Sustainable Infrastructure Capital, Inc. (to present) Rationale for Nomination as Candidate for External Director (ASC Member) and Overview of Expected Role Ms. Kimberly A. Reed was the first woman to serve as Chairman of the Board of Directors, President, and CEO of the Export-Import Bank of the United States (EXIM)—the nation’s official export credit agency—where she helped companies succeed in the competitive global marketplace. She has extensive domestic and international experience in the field, having held pivotal positions at the International Foundation and Community Development Financial Institutions Fund in the U.S., and having served as a Senior Advisor of the U.S. Government and Counsel with U.S. Congressional Committees. Through her career, she has gained substantial leadership experience and wide expertise in global business; legal, regulation and public policy; and finance and accounting. She has contributed to ensuring fair and appropriate decisions and actions of the Company through her active participation on the Board of Directors as an External Director. The Company nominates Ms. Reed as an External Director who is an ASC Member because she is expected to contribute to the realization of the ASC’s vision, which is to ensure the sound and continuous growth of the Company, to realize the creation of mid- and long-term corporate value, and to establish a robust corporate governance system worthy of public trust.

- 24 - Candidate No.4 Jean-Luc Butel Born on November 8, 1956 (67 years old) To be newly elected as External Director / Independent Director Tenure as Director 8 years Number of Board of Directors meetings attended 8/8 (100%) Number of ASC meetings attended - Number of Company Shares Held 0 share Number of Company Shares to be provided 21,054 shares Number of Company American Depositary Shares (ADS) Held 0 share Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held January 1998 Corporate Officer, President, Worldwide Consumer Healthcare, Becton, Dickinson and Company November 1999 President, Independence Technology, Johnson & Johnson May 2008 Corporate Officer, Executive Committee Member, Executive Vice President and Group President, International, Medtronic, Inc. January 2015 President, International, Baxter International Inc. July 2015 Global Healthcare Advisor, President, K8 Global Pte. Ltd. (to present) June 2016 External Director of the Company who is an ASC Member September 2017 External Director, Novo Holdings A/S (to present) June 2019 External Director of the Company (to present) September 2021 External Director, Rani Therapeutics (to present) Rationale for Nomination as Candidate for External Director (ASC Member) and Overview of Expected Role Mr. Jean-Luc Butel has served in several pivotal positions at global healthcare companies in the U.S., Europe, and Asia. During his career, he has acquired deep insights as the result of extensive experience in global healthcare business management. He has contributed to ensuring fair and appropriate decisions and actions of the Company through his active participation on the Board of Directors as External Director. He has been involved in the management of the Company as an External Director who is an ASC Member of the Company since June 2016 and as an External Director who is not an ASC Member since June 2019. The Company nominates Mr. Butel as an External Director who is an ASC Member because he is expected to contribute to the realization of the ASC’s vision, which is to ensure the sound and continuous growth of the Company, to realize the creation of mid- and long-term corporate value, and to establish a robust corporate governance system worthy of public trust.

- 25 - (Notes) 1. No special interests exist between the above candidates and the Company. 2. With regard to the number of Company shares held and the number of Company shares to be provided, please refer to Note 2 in the 2nd Proposal. 3. The number of Company ADS held represents the number of American Depositary Shares held as of March 31, 2024 and is rounded to the nearest whole number. Each ADS represents one half of a common stock. 4. Mr. Koji Hatsukawa, Mr. Yoshiaki Fujimori, Ms. Kimberly A. Reed and Mr. Jean-Luc Butel are candidates to become External Directors of the Company who are ASC Members. The Company has set “Internal criteria for independence of External Directors of the Company” (The contents of such criteria are as set forth on page 19) and elected the External Directors based on such criteria. All of these 4 persons have met the requirements for Independent Directors based on the regulations of the financial instruments exchanges that the Company is listed on (e.g., Tokyo Stock Exchange, Inc.). The Company has appointed Mr. Koji Hatsukawa, Mr. Yoshiaki Fujimori, Ms. Kimberly A. Reed and Mr. Jean-Luc Butel as Independent Directors and submitted a notification to each exchange. 5. The Company has entered into contracts with Mr. Koji Hatsukawa, Mr. Yoshiaki Fujimori, Ms. Kimberly A. Reed and Mr. Jean-Luc Butel limiting the maximum amount of their liability for the damages set forth in Article 423, Paragraph 1 of the Companies Act to the legally stipulated value. If the re-election of Mr. Koji Hatsukawa, Mr. Yoshiaki Fujimori and Ms. Kimberly A. Reed is approved, and if the election of Mr. Jean-Luc Butel as Director who is an ASC Member is approved, the Company plans to continue the same contracts to limit their liability. 6. The Company has entered into company indemnification agreements with Mr. Koji Hatsukawa, Mr. Yoshiaki Fujimori, Ms. Kimberly A. Reed and Mr. Jean-Luc Butel, as defined in Article 430-2, Paragraph 1 of the Companies Act, which provide that the Company shall indemnify expenses set forth in Article 430-2, Paragraph 1, Item 1 thereof, and damages set forth in Article 430-2, Paragraph 1, Item 2 thereof within the scope permitted by the laws and regulations. If re-election of Mr. Koji Hatsukawa, Mr. Yoshiaki Fujimori and Ms. Kimberly A. Reed is approved, and if the election of Mr. Jean-Luc Butel as Director who is an ASC Member is approved, the Company plans to continue the same agreements. 7. The Company has entered into directors & officers liability insurance contracts with insurance companies as defined in Article 430-3, Paragraph 1 of the Companies Act, under which Directors of the Company are insured. Such insurance covers damages which may arise from liability incurred by such insured persons in connection with the execution of their duties or claims made against such insured persons in relation to such liability . If re-election or election of the candidates is approved, such candidates will be insured under such insurance scheme. The insurance contracts are planned to be renewed during such candidates’ term of office.

- 26 - Fourth Proposal: Payment of Bonuses to Directors who are not Audit and Supervisory Committee Members The Company proposes to pay bonuses up to the total amount of 500 million JPY to the two (2) Directors who are not Audit and Supervisory Committee Members (excluding Directors residing outside of Japan and External Directors) in office as of the end of this fiscal year, in keeping with the achievement of the key performance indicators such as the Total Core Revenue, Growth and Launch Product Incremental Core Revenue and Total Core Operating Profit set forth for this fiscal year. The contents of this proposal were deliberated upon at the Compensation Committee and the resolutions were approved by the Board of Directors based on the Director’s Compensation Policy, and the Company therefore considers this proposal as reasonable. END OF DOCUMENT <Reference> Please refer to “3. Executives of the Company (5) Compensation and related matters for Directors” of the Business Report for Director’s Compensation Policy described in the 4th proposal.

- 27 - Business Report (From April 1, 2023 to March 31, 2024) (1) Current State of the Takeda Group • Business Overview Takeda is a global, values-based, R&D-driven biopharmaceutical company with a diverse portfolio, engaged primarily in the research, development, production and global commercialization of pharmaceutical products. Takeda focuses on six key business areas: Gastroenterology (“GI”), Rare Diseases, Plasma-Derived Therapies (“PDT”)*1, Oncology, Vaccines*2 and Neuroscience. Our R&D efforts are focused on three core therapeutic areas: Gastrointestinal and Inflammation, Neuroscience and Oncology. We also make targeted R&D investments in PDT and Vaccines. We focus on developing innovative medicines that make a difference in people’s lives by advancing the frontier of new treatment options and leveraging our collaborative R&D engine and capabilities to create a robust, modality-diverse pipeline. We focus on the high unmet medical need, both in rare and more prevalent conditions, to deliver high-quality medicines and vaccines to patients and communities as quickly as possible. We have a presence in approximately 80 countries and regions, a network of manufacturing sites around the world, and major research centers in Japan and the United States. Over the past several years, we have extended our global reach, strengthened our presence in GI, Oncology and Neuroscience, and established a leading position in Rare Diseases and PDT, while adding potential best-in-class or first-in-class assets to our R&D pipeline. Commercially, we have significantly strengthened our presence in the United States, Europe, and Growth and Emerging Markets. We have also accelerated our focus on data, digital and technology to make our business operations more effective and efficient, increase innovation and better serve our stakeholders. *1 Starting from the fiscal year ending March 31, 2025 (FY2024), “Plasma-Derived Therapies” replaces the previous category of “PDT Immunology”, and now includes all plasma-derived products including those previously categorized within “Rare Diseases” (e.g., FEIBA, CINRYZE). *2 Starting from FY2024, “Vaccines” is now presented as a separate key business area (previously included in “Others”), reflecting the strategic focus on our dengue vaccine, QDENGA.

- 28 - (2) Business Performance for Fiscal 2023 (i) Consolidated Financial Results (April 1, 2023 to March 31, 2024) Billion JPY or percentage For the fiscal year ended March 31, Change versus the previous fiscal year AER CER 2023 2024 Amount of Change % Change % Change Revenue 4,027.5 4,263.8 236.3 5.9 % 1.5 % Cost of sales (1,244.1) (1,426.7) (182.6) 14.7 % 9.8 % Selling, general and administrative expenses (997.3) (1,053.8) (56.5) 5.7 % 0.9 % Research and development expenses (633.3) (729.9) (96.6) 15.3 % 8.4 % Amortization and impairment losses on intangible assets associated with products (542.4) (652.1) (109.7) 20.2 % 12.2 % Other operating income 25.4 19.4 (6.0) (23.8)% (26.3)% Other operating expenses (145.2) (206.5) (61.3) 42.2 % 34.5 % Operating profit 490.5 214.1 (276.4) (56.4)% (50.3)% Finance income and (expenses), net (106.8) (167.8) (61.0) 57.1 % 78.3 % Share of profit (loss) of investments accounted for using the equity method (8.6) 6.5 15.1 ― ― Profit before tax 375.1 52.8 (322.3) (85.9)% (84.1)% Income tax (expenses) benefit (58.1) 91.4 149.5 ― ― Net profit for the year 317.0 144.2 (172.8) (54.5)% (57.0)% In this section, when comparing results to the previous fiscal year, the amount of change and percentage change based on Actual Exchange Rates are presented in “AER” (which is presented in accordance with IFRS) and percentage change based on Constant Exchange Rate (which is a non-IFRS measure) is presented in “CER”. For additional information on CER %, please refer to (ii) Core Results (April 1, 2023 to March 31, 2024), Definition of Core financial measures and Constant Exchange Rate change. Revenue for the fiscal year ended March 31, 2024 was JPY 4,263.8 billion (JPY +236.3 billion and +5.9% AER, +1.5% CER). The increase is primarily attributable to favorable foreign exchange rates and growth from business momentum of Plasma-Derived Therapies (“PDT”) Immunology, Gastroenterology (“GI”), Rare Diseases and Oncology. The increase in these business areas was offset by the decrease in Neuroscience. Revenue outside of these key business areas decreased mainly due to the decline in sales of AZILVA (for hypertension), which were JPY 33.6 billion (JPY -39.3 billion and -53.9% AER, -53.9% CER) and impacted by generic entrants in Japan, as well as the lower revenue contribution from COVID-19 vaccines in Japan. Revenue by Geographic Region The following shows revenue by geographic region: Billion JPY or percentage For the fiscal year ended March 31, Change versus the previous fiscal year AER CER Revenue: 2023 2024 Amount of Change % Change % Change Japan 512.0 451.4 (60.7) (11.8) % (12.1)% United States 2,103.8 2,195.7 91.9 4.4 % (2.2) % Europe and Canada 842.7 966.8 124.2 14.7 % 4.5 % Asia (excluding Japan) 225.0 261.2 36.2 16.1 % 12.1 % Latin America 160.4 198.1 37.7 23.5 % 48.4 % Russia/CIS 88.4 72.6 (15.8) (17.9)% (6.5) % Other* 95.2 117.9 22.7 23.9 % 32.6 % Total 4,027.5 4,263.8 236.3 5.9 % 1.5 % * Other includes the Middle East, Oceania and Africa.

- 29 - Revenue by Business Area The following shows revenue by business area: Billion JPY or percentage For the fiscal year ended March 31, Change versus the previous fiscal year AER CER Revenue: 2023 2024 Amount of Change % Change % Change GI 1,094.5 1,216.2 121.7 11.1 % 4.7 % Rare Diseases * 723.4 770.7 47.3 6.5 % 4.1 % Rare Hematology 304.7 305.3 0.6 0.2 % (2.9) % Rare Genetics and Other 418.7 465.4 46.7 11.1 % 9.2 % PDT Immunology * 678.4 818.6 140.1 20.7 % 14.4 % Oncology 438.7 462.4 23.6 5.4 % 2.5 % Neuroscience 637.7 627.0 (10.7) (1.7) % (7.8) % Other * 454.6 368.9 (85.7) (18.8)% (17.7)% Total 4,027.5 4,263.8 236.3 5.9 % 1.5 % * Starting from the fiscal year ending March 31, 2025 (FY2024), “Plasma-Derived Therapies” replaces the previous category of “PDT Immunology”, and includes all plasma-derived products including those previously categorized within “Rare Diseases” (e.g., FEIBA, CINRYZE). “Vaccines” is presented as a separate key business area (previously included in “Others”), reflecting the strategic focus on our dengue vaccine, QDENGA. If the new categories are applied, revenue from “Rare Disease” is JPY 688.4 billion for FY2023 and JPY 639.8 billion for FY2022, revenue from “Plasma-Derived Therapies” is JPY 903.7 billion for FY2023 and JPY 765.4 billion for FY2022, revenue from “Vaccines” is JPY 50.4 bill ion for FY2023, and JPY 78.7 billion for FY2022, revenue from “Others” is JPY 315.7 billion for FY2023 and JPY 372.7 billion for FY2022. Year-on-year change in revenue for this fiscal year in each of our main business areas was primarily attributable to the following products: • In GI, revenue was JPY 1,216.2 billion (JPY +121.7 billion and +11.1% AER, +4.7% CER). Sales of ENTYVIO (for ulcerative colitis (“UC”) and Crohn’s disease) were JPY 800.9 billion (JPY +98.2 billion and +14.0% AER, +6.6% CER). Sales in the U.S. were JPY 546.1 billion (JPY +54.2 billion and +11.0% AER). The increase was due to favorable foreign exchange rates and demand in the first line biologic inflammatory bowel disease (“IBD”) population primarily in UC. Sales in Europe and Canada were JPY 195.8 billion (JPY +33.4 billion and +20.5% AER), supported by favorable foreign exchange rates and continued launches of the subcutaneous formulation. Sales of GATTEX/REVESTIVE (for short bowel syndrome) were JPY 119.3 billion (JPY +26.2 billion and +28.1% AER, +22.7% CER). The increase was primarily due to increased demand in the U.S., Europe and Japan, expansion activities (infant indication label expansion and geographic expansion), and favorable exchange rates. Sales of TAKECAB/VOCINTI (for acid-related diseases) were JPY 118.5 billion (JPY +9.8 billion and +9.0% AER, +8.2% CER). The increase was primarily due to increased sales in Japan and the Growth and Emerging Markets including Brazil and China. Sales of DEXILANT (for acid reflux disease) were JPY 45.3 billion (JPY -24.1 billion and -34.7% AER, -39.6% CER). The decrease was due to the loss of exclusivity and the termination of the authorized generics program in the U.S. • In Rare Diseases, revenue was JPY 770.7 billion (JPY +47.3 billion and +6.5% AER, +4.1% CER). Revenue of Rare Hematology was JPY 305.3 billion (JPY +0.6 billion and +0.2% AER, -2.9% CER). Sales of ADVATE (for hemophilia A) were JPY 122.9 billion (JPY +4.7 billion and +4.0% AER, +1.1% CER). The increase was attributable to favorable foreign exchange rates as well as sales increase in the Growth and Emerging Markets such as Brazil and China. Sales of VONVENDI (for von Willebrand disease) were JPY 16.2 billion (JPY +4.0 billion and +32.5% AER, +23.1% CER). The increase was primarily due to increased demand in the U.S. Sales of FEIBA (for hemophilia A and B) were JPY 40.5 billion (JPY -0.7 billion and -1.8% AER, -5.3% CER). The decrease was mainly due to competition in Brazil. Sales of RECOMBINATE (for hemophilia A) were JPY 12.1 billion (JPY -0.7 billion and -5.6% AER, -11.8% CER). The decrease was mainly due to weaker demand in the U.S. attributable to increased adoption of next generation therapies.

- 30 - Decrease in revenue of other rare hematology products largely offset the net increase of the above products. Revenue of Rare Genetics and Other was JPY 465.4 billion (JPY +46.7 billion and +11.1% AER, +9.2% CER). Sales of TAKHZYRO (for hereditary angioedema) were JPY 178.7 billion (JPY +26.9 billion and +17.7% AER, +11.6% CER). The continued growth was attributable to sustained launch momentum, expansion into new patient populations such as pediatrics, rising diagnosis rates, the growth of the prophylactic market, and favorable exchange rates. Sales of LIVTENCITY (for post-transplant cytomegalovirus (“CMV”) infection/disease) were JPY 19.1 billion (JPY +8.6 billion and +81.7% AER, +68.7% CER). The increase was primarily attributable to strong launch performance and fast uptake in the U.S., complemented by continued geographical expansion in Europe and positive market access trends. Sales of enzyme replacement therapy REPLAGAL (for fabry disease) were JPY 73.6 billion (JPY +6.8 billion and +10.2% AER, +15.1% CER). The increase was primary due to strong demand in the Growth and Emerging Markets. Sales of enzyme replacement therapy ELAPRASE (for Hunter syndrome) were JPY 91.6 billion (JPY +6.2 billion and +7.3% AER, +7.3% CER). The increase was primarily due to strong demand in the Growth and Emerging Markets. • In PDT Immunology, revenue was JPY 818.6 billion (JPY +140.1 billion and +20.7% AER, +14.4% CER). Aggregate sales of immunoglobulin products were JPY 644.6 billion (JPY +122.4 billion and +23.4% AER, +16.8% CER). Sales of each of our three global immunoglobulin brands marked double digit percentage of revenue growth, due to continued strong demand globally and growing supply, as well as favorable foreign exchange rates. Those include GAMMAGARD LIQUID/KIOVIG (for the treatment of primary immunodeficiency (“PID”) and multifocal motor neuropathy (“MMN”)), and subcutaneous immunoglobulin therapies (CUVITRU and HYQVIA) which are growing due to their benefit to patients and convenience in administration compared to intravenous therapies. Aggregate sales of albumin products including HUMAN ALBUMIN and FLEXBUMIN (both primarily used for hypovolemia and hypoalbuminemia) were JPY 134.0 billion (JPY +12.5 billion and +10.3% AER, +5.9% CER). The increase was primarily driven by strong albumin demand in China. • In Oncology, revenue was JPY 462.4 billion (JPY +23.6 billion and +5.4% AER, +2.5% CER). Sales of ADCETRIS (for malignant lymphomas) were JPY 109.4 billion (JPY +25.5 billion and +30.4% AER, +31.3% CER). The increase was led by strong growth in Growth and Emerging Markets and Europe. Sales of FRUZAQLA (for colorectal cancer), which newly launched in November 2023 in the U.S., were JPY 10.1 billion. Sales of ALUNBRIG (for non-small cell lung cancer) were JPY 28.5 billion (JPY +8.0 billion and +38.8% AER, +35.3% CER). The increase benefited from strong demand across all regions. Sales of ICLUSIG (for leukemia) were JPY 54.7 billion (JPY +7.5 billion and +15.9% AER, +7.5% CER). The increase was due to favorable foreign exchange rates and higher demand in the U.S. Sales of VELCADE (for multiple myeloma) were JPY 5.5 billion (JPY -22.2 billion and -80.0% AER, -81.3% CER). The decrease was due to generic erosion in the U.S. Sales of NINLARO (for multiple myeloma) were JPY 87.4 billion (JPY -5.3 billion and -5.7% AER, -9.2% CER). The decrease was due to intensified competition and decreased demand mainly in the U.S, partially aided by favorable foreign exchange rates. • In Neuroscience, revenue was JPY 627.0 billion (JPY -10.7 billion and -1.7% AER, -7.8% CER). Sales of VYVANSE/ELVANSE (for attention deficit hyperactivity disorder (“ADHD”)) were JPY 423.2 billion (JPY - 36.1 billion and -7.9% AER, -14.1% CER). The decrease was due to multiple generic entrants in the U.S. starting from August 2023, with the growth of the adult market in Europe and favorable foreign exchange rates partially offset the negative impacts. Sales of ADDERALL XR (for ADHD) were JPY 41.8 billion (JPY +13.2 billion and +46.0% AER, +36.6% CER). The increase was primarily due to a shortage of generic versions of the instant release formulation marketed by competitors in the U.S. and favorable foreign exchange rates. Sales of INTUNIV (for ADHD) were JPY 33.6 billion (JPY +17.2 billion and +105.2% AER, +100.8% CER). The increase was primarily due to the buy-back of full rights in Japan effective in April 2023.

- 31 - Cost of Sales Cost of Sales was JPY 1,426.7 billion (JPY +182.6 billion and +14.7% AER, +9.8% CER). The increase was primarily due to revenue growth in our key business areas with a change in product mix and the depreciation of Japanese yen as compared to the fiscal year ended March 31, 2023. This was partially offset by a decrease in non-cash charges related to the unwind of the fair value step up on acquired inventories recognized in connection with the acquisition of Shire plc (“Shire”). Selling, General and Administrative (SG&A) Expenses SG&A expenses were JPY 1,053.8 billion (JPY +56.5 billion and +5.7% AER, +0.9% CER). The increase was mainly due to the depreciation of Japanese yen and investments in Data, Digital and Technology ("DD&T") partially offset by various cost efficiencies. Research and Development (R&D) Expenses R&D expenses were JPY 729.9 billion (JPY +96.6 billion and +15.3% AER, +8.4% CER). The increase was mainly due to various investments in pipeline programs and the depreciation of Japanese yen. Amortization and Impairment Losses on Intangible Assets Associated with Products Amortization and Impairment Losses on Intangible Assets Associated with Products was JPY 652.1 billion (JPY +109.7 billion and +20.2% AER, +12.2% CER). The increase was mainly due to an increase in impairment charges for certain assets related to in-process R&D and marketed products and an increase of amortization expenses due to the depreciation of Japanese yen. JPY 130.6 billion impairment losses recorded in the fiscal year ended March 31, 2024 primarily includes JPY 74.0 billion impairment charges for ALOFISEL (for complex Crohn's perianal fistulas) following topline results of the phase 3 ADMIRE-CD II trial, JPY 28.5 billion impairment charges following a decision to voluntarily withdraw EXKIVITY (for non-small cell lung cancer) globally, and other impairment charges for certain in-process R&D assets including those related to TAK-007 and modakafusp alfa (TAK-573) in Oncology as results of decisions to terminate those programs. The increase was partially offset by a reversal of impairment loss of JPY 35.7 billion related to the approval of EOHILIA, a therapy for eosinophilic esophagitis (EoE), by the U.S. Food and Drug Administration (FDA) in February 2024. Other Operating Income Other Operating Income was JPY 19.4 billion (JPY -6.0 billion and -23.8% AER, -26.3% CER). Other Operating Expenses Other Operating Expenses were JPY 206.5 billion (JPY +61.3 billion and +42.2% AER, +34.5% CER). The increase was primarily driven by increases of restructuring expenses, additional losses recorded for the supply agreement litigation with AbbVie, Inc. ("AbbVie") in the fiscal year ended March 31,2024 and changes in the fair value of financial assets and liabilities associated with contingent consideration arrangements mainly from XIIDRA and EOHILIA. Operating Profit As a result of the above factors, Operating Profit was JPY 214.1 billion (JPY -276.4 billion and -56.4% AER, -50.3% CER). Net Finance Expenses Net Finance Expenses were JPY 167.8 billion (JPY +61.0 billion and +57.1% AER, +78.3% CER). The increase was primarily due to a decrease in financial income reflecting gains from acquisitions of prior equity method companies and a positive impact from the remeasurement of warrants to purchase stocks of the company held by Takeda recorded in the fiscal year ended March 31, 2023, as well as an increase in financial expenses in the fiscal year ended March 31, 2024 due to factors including interest recorded for the supply agreement litigation with AbbVie and increased expense on hyperinflation accounting. Share of Profit (Loss) of Investments Accounted for Using the Equity Method Share of Profit of Investments Accounted for Using the Equity Method was JPY 6.5 billion (JPY +15.1 billion, compared to Share of Loss of Investments Accounted for Using the Equity Method of JPY 8.6 billion in the fiscal year ended March 31, 2023).

- 32 - Income Tax (Expenses) Benefit Income Tax Benefit was JPY 91.4 billion (JPY +149.5 billion, compared to Income Tax Expenses of JPY 58.1 billion in the fiscal year ended March 31, 2023). The increase was primarily due to lower pretax earnings as well as a tax expense reduction of JPY 63.5 billion resulting from the reversal of the income taxes payable in excess of the settlement with the Irish Revenue Commissioners with respect to a tax assessment related to the treatment of an acquisition break fee Shire received from AbbVie in 2014 ("AbbVie Break Fee Settlement"). These increases were partially offset by the tax charges from legal entity restructuring and the reassessment of recoverability of deferred tax assets. Net Profit for the Year As a result of the above factors, Net Profit for the Year was JPY 144.2 billion (JPY -172.8 billion and -54.5% AER, - 57.0% CER).
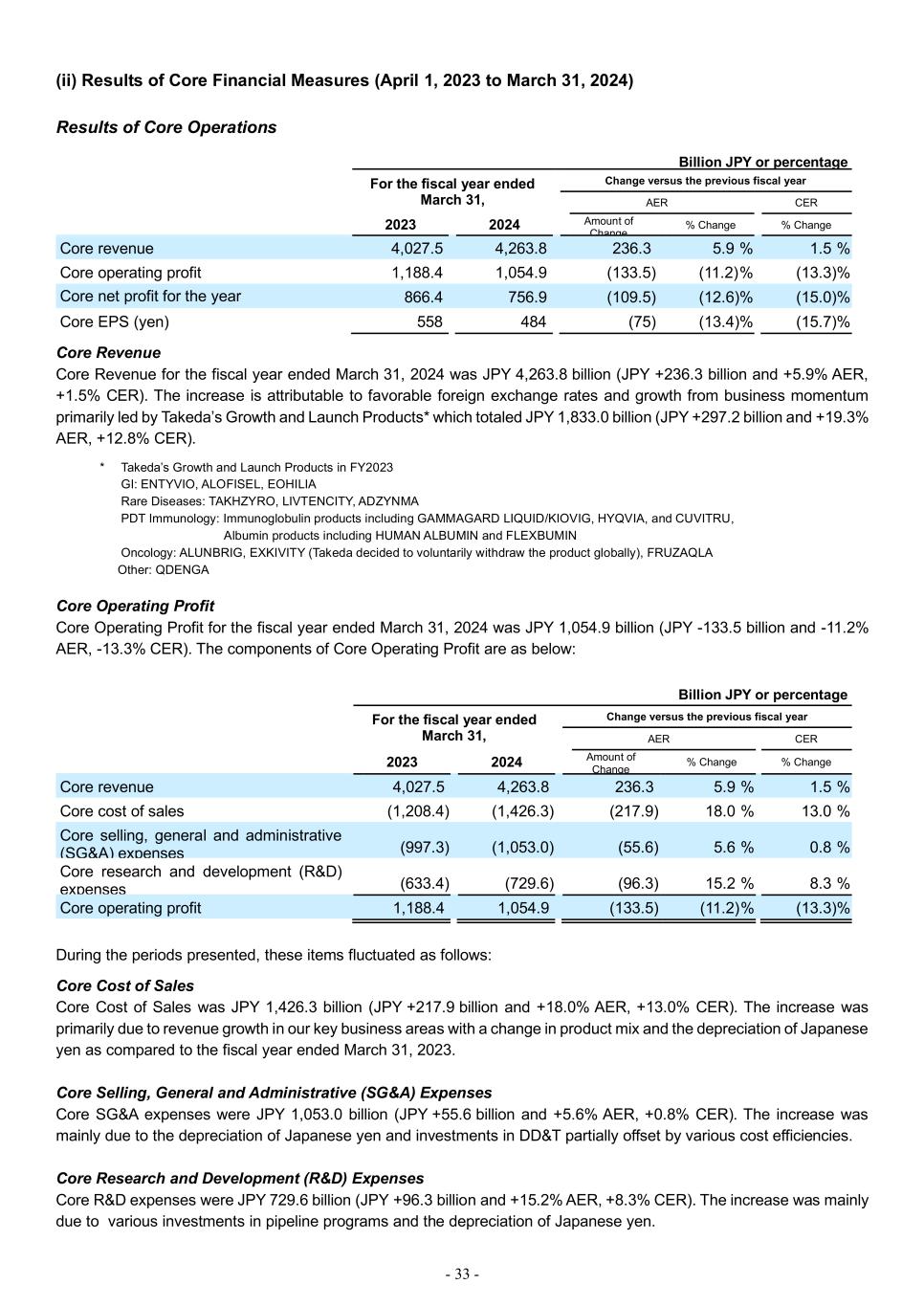
- 33 - (ii) Results of Core Financial Measures (April 1, 2023 to March 31, 2024) Results of Core Operations Billion JPY or percentage For the fiscal year ended March 31, Change versus the previous fiscal year AER CER 2023 2024 Amount of Change % Change % Change Core revenue 4,027.5 4,263.8 236.3 5.9 % 1.5 % Core operating profit 1,188.4 1,054.9 (133.5) (11.2) % (13.3)% Core net profit for the year 866.4 756.9 (109.5) (12.6)% (15.0)% Core EPS (yen) 558 484 (75) (13.4)% (15.7)% Core Revenue Core Revenue for the fiscal year ended March 31, 2024 was JPY 4,263.8 billion (JPY +236.3 billion and +5.9% AER, +1.5% CER). The increase is attributable to favorable foreign exchange rates and growth from business momentum primarily led by Takeda’s Growth and Launch Products* which totaled JPY 1,833.0 billion (JPY +297.2 billion and +19.3% AER, +12.8% CER). * Takeda’s Growth and Launch Products in FY2023 GI: ENTYVIO, ALOFISEL, EOHILIA Rare Diseases: TAKHZYRO, LIVTENCITY, ADZYNMA PDT Immunology: Immunoglobulin products including GAMMAGARD LIQUID/KIOVIG, HYQVIA, and CUVITRU, Albumin products including HUMAN ALBUMIN and FLEXBUMIN Oncology: ALUNBRIG, EXKIVITY (Takeda decided to voluntarily withdraw the product globally), FRUZAQLA Other: QDENGA Core Operating Profit Core Operating Profit for the fiscal year ended March 31, 2024 was JPY 1,054.9 billion (JPY -133.5 billion and -11.2% AER, -13.3% CER). The components of Core Operating Profit are as below: Billion JPY or percentage For the fiscal year ended March 31, Change versus the previous fiscal year AER CER 2023 2024 Amount of Change % Change % Change Core revenue 4,027.5 4,263.8 236.3 5.9 % 1.5 % Core cost of sales (1,208.4) (1,426.3) (217.9) 18.0 % 13.0 % Core selling, general and administrative (SG&A) expenses (997.3) (1,053.0) (55.6) 5.6 % 0.8 % Core research and development (R&D) expenses (633.4) (729.6) (96.3) 15.2 % 8.3 % Core operating profit 1,188.4 1,054.9 (133.5) (11.2) % (13.3)% During the periods presented, these items fluctuated as follows: Core Cost of Sales Core Cost of Sales was JPY 1,426.3 billion (JPY +217.9 billion and +18.0% AER, +13.0% CER). The increase was primarily due to revenue growth in our key business areas with a change in product mix and the depreciation of Japanese yen as compared to the fiscal year ended March 31, 2023. Core Selling, General and Administrative (SG&A) Expenses Core SG&A expenses were JPY 1,053.0 billion (JPY +55.6 billion and +5.6% AER, +0.8% CER). The increase was mainly due to the depreciation of Japanese yen and investments in DD&T partially offset by various cost efficiencies. Core Research and Development (R&D) Expenses Core R&D expenses were JPY 729.6 billion (JPY +96.3 billion and +15.2% AER, +8.3% CER). The increase was mainly due to various investments in pipeline programs and the depreciation of Japanese yen.

- 34 - Core Net Profit for the Year Core Net Profit for the Year was JPY 756.9 billion (JPY -109.5 billion and -12.6% AER, -15.0% CER) and is calculated from Core Operating Profit are as below: Billion JPY or percentage For the fiscal year ended March 31, Change versus the previous fiscal year AER CER 2023 2024 Amount of Change % Change % Change Core operating profit 1,188.4 1,054.9 (133.5) (11.2) % (13.3)% Core finance income and (expenses), net (126.6) (142.0) (15.4) 12.2 % 13.9 % Core share of profit of investments accounted for using the equity method 0.2 5.9 5.7 ― ― Core profit before tax 1,062.0 918.8 (143.2) (13.5)% (16.0)% Core income tax (expenses) benefit (195.6) (161.9) 33.7 (17.2)% (20.2)% Core net profit for the year 866.4 756.9 (109.5) (12.6)% (15.0)% During the periods presented, these items fluctuated as follows: Core Net Finance Expenses Core Net Finance Expenses were JPY 142.0 billion (JPY +15.4 billion and +12.2% AER, +13.9% CER). Core Share of Profit of Investments Accounted for Using the Equity Method Core Share of Profit of Investments Accounted for Using the Equity Method was JPY 5.9 billion (JPY +5.7 billion). Core Profit Before Tax Core Profit Before Tax was JPY 918.8 billion (JPY -143.2 billion and -13.5% AER, -16.0% CER). Core Income Tax (Expenses) Benefit Core Income Tax Expenses were JPY 161.9 billion (JPY -33.7 billion and -17.2% AER, -20.2% CER) and excludes the JPY 63.5 billion impact from AbbVie Break Fee Settlement in the fiscal year ended March 31, 2024. The decrease was mainly due to lower core pretax earnings. Core EPS Core EPS for the fiscal year ended March 31, 2024 was JPY 484 (JPY -75 and -13.4% AER, -15.7% CER). Definition of Core financial measures and Constant Exchange Rate change Takeda uses the concept of Core financial measures for measuring financial performance. These measures are not defined by International Financial Reporting Standards (IFRS). Core Financial Measures Takeda presents its Core Financial Measures, particularly Core Revenue, Core Operating Profit, Core Net Profit for the Year and Core EPS because Takeda believes that these measures are useful to understanding its business without the effect of items that Takeda considers to be unrelated to the underlying trends and business performance of its core operations, including items (i) which may vary significantly from year-to-year or may not occur in each year, or (ii) whose recognition Takeda believes is largely uncorrelated to trends in the underlying performance of our core business. Takeda believes that similar measures are frequently used by other companies in its industry, and that providing these measures helps investors evaluate Takeda’s performance against not only its performance in prior years but on a similar basis as its competitors. Takeda also presents Core Financial Measures because these measures are used by Takeda for budgetary planning and compensation purposes (i.e., certain targets for the purposes of Takeda’s Short-Term Incentive and Long-Term Incentive compensation programs, including incentive compensation of the CEO and CFO, are set in relation to the results of Takeda’s Core Financial Measures). Takeda’s Core Financial Measures exclude revenue from divestments, amortization and impairment losses on acquired intangible assets and other impacts unrelated to the underlying trends and business performance of Takeda’s core

- 35 - operations, such as non-recurring items, purchase accounting effects and transaction related costs. Core Revenue represents revenue adjusted to exclude significant revenue items unrelated to the underlying trends and business performance of Takeda’s core operations. Core Operating Profit represents operating profit adjusted to exclude other operating expenses and income, amortization and impairment losses on acquired intangible assets and other non-cash items or items unrelated to the underlying trends and business performance of Takeda’s core operations. Core EPS represents net profit adjusted to exclude the impact of items excluded in the calculation of Core Operating Profit, and other non-operating items (e.g. amongst other items, fair value adjustments and the imputed financial charge related to contingent consideration) that are unusual, non-recurring in nature or unrelated to the underlying trends and business performance of Takeda’s ongoing operations and the tax effect of each of the adjustments, divided by the average outstanding shares (excluding treasury shares) of the reporting periods presented. Constant Exchange Rate (CER) change eliminates the effect of foreign exchange rates from year-over-year comparisons by translating Reported or Core results for the current period using corresponding exchange rates in the same period of the previous fiscal year. Starting from the quarter ending June 30, 2024, we will cease adjustments for CER change for the results of operations of subsidiaries in countries experiencing hyperinflation and for which IAS29, Financial Reporting in Hyperinflation Economies, is applied, because of the increased impacts of hyperinflation in the calculation of CER change using corresponding exchange rates in the same period of the previous fiscal year, effectively keeping CER change for these subsidiaries unchanged from those reported with IAS29. (iii) Activities and Results of Research & Development Research and development expenses for the fiscal year ended March 31, 2024 were JPY 729.9 billion. Takeda does not report disaggregated R&D expenses, including by therapeutic area or clinical trial stage, as our R&D budget is determined on a company-wide basis and specific expenditures may be subject to re-allocation depending on development results and priorities. The research and development (R&D) of biopharmaceutical products is a lengthy and expensive process that can span more than 10 years. The process includes multiple studies to evaluate a product’s efficacy and safety, followed by submission to regulatory authorities who review the data and decide whether to grant marketing approval. Only a small number of therapeutic candidates pass such rigorous investigation and become available for use in clinical treatment. Once approved, there is ongoing R&D support for marketed products, including life-cycle management, medical affairs, and other investments. Clinical trials, which must comply with regional and international regulatory guidelines, generally take five to seven years or longer, and require substantial expenditures. In general, clinical trials are performed in accordance with the guidelines set by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. The relevant regional regulatory authorities are the Food and Drug Administration (FDA) for the United States, the European Medicines Agency (EMA) for the EU, the Ministry of Health, Labour and Welfare (MHLW) for Japan and National Medical Products Administration (NMPA) for China. The three phases of human clinical trials, which may overlap with each other, are as follows: Phase 1 (“P-1”) clinical trials Conducted using a small group of healthy adult volunteers in order to evaluate safety and absorption, distribution, metabolism and excretion of the drug. Phase 2 (“P-2”) clinical trials Conducted using a small group of patient volunteers in order to evaluate safety, efficacy, dosage and administration methods. P-2 clinical trials may be divided into two sub-categories, P-2a and P-2b. P-2a are usually pilot studies designed to demonstrate clinical efficacy or biological activity. P-2b studies look to find the optimum dose at which the drug shows biological activity with minimal side-effects. Phase 3 (“P-3”) clinical trials Conducted using a large number of patient volunteers in order to evaluate safety and efficacy in comparison to other medications already available or placebo. Of these three phases, Phase 3 requires the largest expenditures and thus the decision to proceed with Phase 3 testing is a critical business decision in the drug development process. For those drug candidates that pass Phase 3 clinical

- 36 - trials, a New Drug Application ("NDA"), Biologics License Application (“BLA”) or a Marketing Authorization Application (“MAA”) is submitted to the relevant governmental authorities for approval, which if granted permits the subsequent launch of the drug. The preparation of an NDA, BLA or MAA submission involves considerable data collection, verification, analysis and expense. Even after the launch of the product, health authorities require post-marketing surveillance of adverse events, and they may request a post-marketing study to provide additional information regarding the risks and benefits of the product. Takeda’s R&D engine is focused on translating science into highly innovative, life-transforming medicines that make a critical difference to patients. Takeda supports dedicated R&D efforts across three areas: Innovative Biopharma, Plasma- Derived Therapies (“PDT”) and Vaccines. The R&D engine for Innovative Biopharma is the largest component of our R&D investment and has produced exciting new molecular entities (“NMEs”) that represent potential best-in-class and/or first-in-class medicines in areas of high unmet medical need, both in rare and more prevalent conditions, across our core therapeutic areas (Gastrointestinal and inflammation, neuroscience, and oncology). Takeda is committed to rare diseases, and many of the life-transforming medicines we are pursuing will treat rare diseases in our core therapeutic areas as well as in PDT. We are working to harness the potential of cell and gene therapies by investing in new capabilities and next-generation platforms internally and through a network of partnerships. We are embracing data and digital technologies to improve the quality of innovation and accelerate execution. Takeda’s pipeline is positioned to support both the near-term and long-term sustained growth of the company. Once first approval of a product is achieved, Takeda R&D is equipped to support geographic expansions of such approval and approvals in additional indications, as well as post-marketing commitment and potential additional formulation work. Takeda’s R&D team works closely with the commercial functions to maximize the value of marketed products and reflect commercial insights in its R&D strategies and portfolio. In addition to our concentrated efforts to increase our in-house R&D capabilities, external partnerships with third-party partners are a key component of our strategy for enhancing our R&D pipeline. Our strategy to expand and diversify our external partnerships allows us to take part in research of a wide variety of new products and increases the chances that we will be able to take part in a major research-related breakthrough. Our key in-house R&D facilities include: • Greater Boston Area Research and Development Site: Our Boston R&D site is located in Cambridge, Massachusetts in the United States. It is the center of our global gastrointestinal and inflammation, oncology, and other rare diseases programs R&D, and also supports R&D in other areas including plasma-derived therapies and vaccines, as well as research in immunomodulation and biologics. The site is home to the Takeda Cell Therapy engine with a state-of-the-art cell therapy manufacturing facility. Furthermore, Takeda signed a 15- year lease for an approximately 600,000 square foot state-of-the-art R&D and office facility under construction in Kendall Square, which Takeda plans to occupy from 2026. • Shonan Health Innovation Park: Located in Fujisawa and Kamakura in Kanagawa Prefecture in Japan, the Shonan Health Innovation Park (“Shonan iPark”) was opened in 2018 when Takeda transformed its Shonan Research Center into the first pharma-led science park in Japan by opening its doors to external parties and is the primary location for Takeda’s neuroscience research. To attract more diverse partners and to further the success of the Shonan iPark, Takeda transferred ownership rights of Shonan iPark to a trustee in 2020 and transferred operation of Shonan iPark to a company established by Takeda in 2023. Takeda, as a flagship tenant, is committed to invigorating life science research in Japan. • San Diego Research and Development Site: Our R&D site located in San Diego, California in the United States supports R&D in the gastrointestinal and inflammation and neuroscience areas. The San Diego research center operates as a “biotech-like” site and leverages internal capabilities such as structural biology and biophysics to catalyze research internally and externally. • Vienna, Austria Research and Development Site: Our R&D site, located in Vienna, Austria, supports programs in R&D and in PDT. The research center focuses on biologics programs in R&D and contains manufacturing sites for plasma derived products. A new R&D laboratory is planned to be constructed in Vienna's Donaustadt district in 2026 as a “Green Building” and is designed to be certified as a Total Quality Building (TQB), which includes accessibility, comfort and adherence to environmental sustainability standards. Major progress on R&D events since April 2023 are listed as follows:

- 37 - R&D pipeline Gastrointestinal and Inflammation In Gastrointestinal and Inflammation, Takeda focuses on delivering innovative, life-changing therapeutics for patients with gastrointestinal diseases, including those of the liver as well as immune-mediated inflammatory diseases. Takeda is maximizing the potential of our inflammatory bowel disease (IBD) franchise around ENTYVIO, including development of a subcutaneous formulation and expansion into other indications such as active chronic pouchitis. Takeda is also expanding its position with GATTEX/REVESTIVE to support further potential geographic expansion. Furthermore, Takeda is progressing a pipeline built through in-house discovery, partnerships and business development, exploring opportunities in inflammatory diseases (specifically in gastric, dermatological and rheumatic disorders, along with select rare hematological & renal diseases (mezagitamab (TAK-079), etc.)), liver diseases, and neurogastric diseases. Zasocitinib (TAK-279) is an example of an acquisition through business development of a late-stage, potential best-in- class oral allosteric tyrosine kinase 2 (TYK2) inhibitor with potential to treat multiple immune-mediated inflammatory diseases. Fazirsiran (TAK-999) is an example of an addition through partnership and a potential first-in-class RNAi for alpha-1 antitrypsin-deficiency associated liver disease in late-stage development. Note: ADZYNMA (apadamtase alfa/cinaxadamtase alfa (recombinant) (Development code: TAK-755) ) and mezagitamab (TAK-079) have been developed in Gastrointestinal and Inflammation starting from FY2023 Q4. ENTYVIO / Generic name: vedolizumab – In April 2023, Takeda announced that the U.S. Food and Drug Administration (FDA) accepted for review its Biologics License Application (BLA) resubmission for the investigational subcutaneous (SC) administration of ENTYVIO for maintenance therapy in adults with moderately to severely active ulcerative colitis (UC) after induction therapy with ENTYVIO intravenous (IV). The resubmission was intended to address FDA feedback in a December 2019 Complete Response Letter (CRL). Since receiving the CRL Takeda worked closely with the FDA to address the Agency’s feedback; and this resubmission package included additional data collected to investigate the use of subcutaneous administration of ENTYVIO. The contents of the letter were unrelated to the IV formulation of ENTYVIO, the clinical safety and efficacy data, and conclusions from the pivotal VISIBLE 1 trial supporting the ENTYVIO SC BLA. VISIBLE 1 assessed the safety and efficacy of a SC formulation of ENTYVIO as maintenance therapy in 216 adult patients with moderately to severely active UC who achieved clinical response at week 6 following two doses of open-label ENTYVIO IV therapy at weeks 0 and 2. The primary endpoint was clinical remission at week 52, which was defined as a total Mayo score of ≤2 and no subscore >1. In September 2023, Takeda announced that the FDA approved a SC administration of ENTYVIO for maintenance therapy in adults with moderately to severely active UC after induction therapy with ENTYVIO IV. – In September 2023, Takeda announced that it received an approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for a partial change to the marketing authorization status of ENTYVIO Pens for SC Injection 108 mg /Syringes for SC Injection 108 mg (ENTYVIO SC) as a maintenance therapy for moderate to severe active Crohn's disease with inadequate response to conventional treatment. This approval is based on the results of the MLN0002SC-3031 and MLN0002SC-3030 clinical trials, which are international Phase 3 trials that evaluated the efficacy and safety of ENTYVIO SC as a maintenance therapy in moderate to severe active Crohn's disease. – In April 2024, Takeda announced that the FDA approved ENTYVIO SC administration for maintenance therapy in adults with moderately to severely active Crohn's disease after induction therapy with ENTYVIO IV. The approval is based on the VISIBLE 2 Study (SC CD Trial), a Phase 3, randomized, double-blind, placebo-controlled trial, which assessed the safety and efficacy of an SC formulation of ENTYVIO as maintenance therapy in total 409 adult patients with moderately to severely active Crohn's disease who had clinical response at week 6 following two doses of open-label ENTYVIO intravenous therapy at weeks 0 and 2. A statistically significant proportion of patients receiving ENTYVIO SC 108 mg maintenance therapy administered every 2 weeks achieved long-term clinical remission compared to patients receiving placebo (ENTYVIO SC: 48% vs. Placebo: 34%; p<0.01) at week 52. In clinical studies, the ENTYVIO SC safety profile was generally consistent with the known safety profile of ENTYVIO IV, with the addition of injection site reactions (including injection site erythema, rash, pruritus, swelling, bruising, hematoma, pain, urticaria and edema) as an adverse reaction for ENTYVIO SC. ALOFISEL / Generic name: darvadstrocel – In October 2023, Takeda announced that the Phase 3 ADMIRE-CD II study, assessing the efficacy and safety of ALOFISEL for the treatment of complex Crohn’s Perianal Fistulas (CPF), did not meet its primary endpoint of

- 38 - combined remission at 24 weeks, based on topline data. The safety profile for darvadstrocel was consistent with prior studies and there were no new safety signals identified. Full results of the study will be presented at a future medical meeting or published in a peer-reviewed journal. ALOFISEL is approved in the European Union (EU), Israel, Switzerland, Serbia, United Kingdom and Japan based on positive data from the previously completed ADMIRE-CD study. ADZYNMA / Generic name: apadamtase alfa/cinaxadamtase alfa (recombinant) (Development code: TAK-755) – In June 2023, Takeda presented favorable interim results from a global pivotal Phase 3 randomized, controlled, open-label, crossover trial evaluating the safety and efficacy of TAK-755 replacement therapy for the prophylactic treatment of congenital thrombotic thrombocytopenic purpura (cTTP), and pharmacokinetics (PK) characteristics of TAK-755, as well as long-term data on TAK-755 prophylaxis from a Phase 3b continuation study at the International Society on Thrombosis and Haemostasis (ISTH) 2023 Congress. In the pivotal trial, no patient had an acute TTP event while receiving TAK-755 prophylactic treatment. TAK-755 also reduced the incidence of thrombocytopenia by 60%, as compared to plasma-based therapy (hazard ratio [HR] 0.40; 95% confidence interval [CI]; 0.3- 0.7). Treatment-emergent adverse events (TEAEs) were reported in 10.3% of patients ages 12-68 receiving TAK-755 compared to 50% of patients receiving plasma-based therapy, demonstrating a favorable safety and tolerability profile with a potential safety advantage over plasma-based therapies. PK characteristics of ADAMTS13 after a single infusion (0-168 hours) were evaluated and compared to plasma-based therapy in 36 cTTP patients aged 12 and older. Patients receiving TAK-755 achieved a five-fold increase in their ADAMTS13 activity levels compared to those receiving plasma-based therapy (Cmax 100% activity for TAK-755 vs. 19% activity for plasma-based therapy) and lower variability (23.8% vs. 56% coefficient of variation [CV], respectively). Also, the results of an interim analysis of the Phase 3b continuation study, evaluating the safety and efficacy of long-term TAK-755 prophylaxis in 29 patients with cTTP, demonstrated a consistently favorable safety profile with TAK-755 prophylaxis and no development of neutralizing antibodies. Zero acute TTP events occurred during TAK-755 prophylaxis, and the incidence rates of subacute TTP events and TTP manifestations were comparable to those with TAK-755 prophylaxis in the pivotal study. – In November 2023, Takeda announced that the U.S. Food and Drug Administration (FDA) approved ADZYNMA for the prophylactic and on-demand treatment of adult and pediatric patients with cTTP. The FDA previously granted Fast Track Designation, Orphan Drug Designation, and Rare Pediatric Disease Designation in cTTP, as well as Priority Review for ADZYNMA’s Biologic License Application (BLA). The FDA granted the company a Rare Pediatric Disease Voucher for the approval of ADZYNMA. The FDA approval of ADZYNMA was supported by the totality of the evidence provided by the analysis of efficacy, pharmacokinetic, safety and tolerability data from the first randomized, controlled, open-label, crossover Phase 3 trial in cTTP as well as by data from the continuation trial. ADZYNMA is the first and only FDA-approved recombinant ADAMTS13 (rADAMTS13) designed to address an unmet medical need in people with cTTP by replacing the deficient ADAMTS13 enzyme. – In March 2024, Takeda announced that that the Japanese Ministry of Health, Labour and Welfare (MHLW) approved the use of ADZYNMA for the treatment of cTTP for individuals 12 years of age and older. The approval is supported by the totality of the evidence provided from an interim analysis of efficacy, pharmacokinetic, safety and tolerability data from the first randomized, controlled, open-label, crossover Phase 3 trial (281102) in cTTP patients primarily in ages 12-68, which includes five Japanese patients and supported by long-term safety and efficacy data from a continuation study (TAK-755-3002). EOHILIA / Generic name: budesonide (Development code: TAK-721) – In February 2024, Takeda announced that the U.S. Food and Drug Administration (FDA) approved EOHILIA (budesonide oral suspension) for 12 weeks of treatment in people 11 years and older with eosinophilic esophagitis (EoE). The FDA approval of EOHILIA 2 mg twice daily is based on efficacy and safety data from two multicenter, randomized, double-blind, parallel-group, placebo-controlled 12-week studies (Study 1 and Study 2) in patients (ages 11 to 56 and 11 to 42, respectively) with EoE. Development Code: TAK-279 / Generic name: zasocitinib – In November 2023, Takeda presented positive results from its randomized, double-blind, placebo-controlled, Phase 2b trial evaluating zasocitinib in patients with active psoriatic arthritis during a late-breaking session at the American College of Rheumatology (ACR) Convergence 2023. The study met its primary endpoint with a statistically significant proportion of patients, 53.3% (15 mg) and 54.2% (30 mg), treated once-daily with zasocitinib achieving at least an American College of Rheumatology 20 (ACR 20) response compared to 29.2% in the placebo arm at week 12 (p =

- 39 - 0.002). zasocitinib demonstrated improvements in key secondary endpoints and the safety and tolerability profile in the trial was consistent with that observed in the Phase 2b plaque psoriasis clinical study. Based on the Phase 2b results, Takeda intends to initiate a Phase 3 development program of zasocitinib in psoriatic arthritis. Takeda also initiated a Phase 3 development program of zasocitinib in plaque psoriasis in Q3 FY2023 and plans to evaluate zasocitinib in Crohn’s disease, ulcerative colitis and additional immune-mediated inflammatory diseases. Development code: TAK-079 / Generic name: mezagitamab – In March 2024, Takeda announced positive topline results from a Phase 2, randomized, double-blind, placebo- controlled study evaluating the safety, tolerability and efficacy of mezagitamab in patients with persistent or chronic primary immune thrombocytopenia (ITP). The Phase 2 trial (TAK-079-1004) evaluated three different doses of subcutaneous mezagitamab vs placebo, given once weekly for eight weeks in patients with chronic (more than one year in duration) or persistent (3-12 months in duration) primary ITP. An interim analysis of the ongoing Phase 2 study demonstrated positive safety and efficacy results. Mezagitamab has been generally safe and well tolerated across all three cohorts. All mezagitamab doses tested demonstrated a higher platelet response rate than placebo. The increases in platelet count were dose-dependent with the greatest platelet response observed at the highest dose tested. Platelet response in mezagitamab treated patients occurred rapidly and was maintained post-therapy. Based on these positive results, and following consultation with global health authorities, Takeda plans to initiate a global Phase 3 trial of mezagitamab in ITP in fiscal year 2024. Mezagitamab previously received Orphan Drug Designation for the treatment of ITP from the U.S. Food and Drug Administration (FDA) and the program received Fast Track Designation. Neuroscience In Neuroscience, Takeda is focusing its R&D investments on potentially transformative treatments for neurological and neuromuscular diseases of high unmet need and building its pipeline through a combination of in-house expertise and partnerships. By harnessing advances in disease biology understanding, translational tools, and innovative modalities, Takeda is primarily focusing on rare neurology, in particular, on potential investigative therapies for sleep-wake disorders such as narcolepsy and idiopathic hypersomnia with a franchise of orexin-2 receptor agonists (TAK-861, danavorexton (TAK-925), etc.), and rare epilepsies with soticlestat (TAK-935). Additionally, Takeda makes targeted investments to investigate well-defined segments of neuromuscular diseases, neurodegenerative diseases and movement disorders. Development Code: TAK-861 – In February 2024, Takeda announced positive topline results from a randomized, double-blind, placebo-controlled, multiple dose Phase 2b trial evaluating TAK-861 in patients with narcolepsy type 1 (NT1). Two separate Phase 2b studies were conducted in NT1 and narcolepsy type 2 (NT2). The NT1 trial (TAK-861-2001) evaluating TAK-861 in 112 patients demonstrated statistically significant and clinically meaningful improvement in objective and subjective measures of wakefulness compared to placebo at week 8 including on the primary endpoint Maintenance of Wakefulness Test (MWT) (p < 0.001). Improvements in key secondary endpoints including Epworth Sleepiness Scale (ESS) and Weekly Cataplexy Rate (WCR) were statistically significant and clinically meaningful, consistent with the primary endpoint. The majority of patients who completed the trial entered a long-term extension study. Based on these results, and in consultation with global health authorities, Takeda plans to initiate global Phase 3 trials of TAK-861 in NT1 rapidly in the first half of its fiscal year 2024. At this time, Takeda does not plan to advance TAK-861 in NT2. TAK-861 was generally safe and well tolerated in both NT1 and NT2 trials. No treatment related serious adverse events were reported. In addition, no cases of hepatotoxicity or visual disturbances were reported in the Phase 2b trials or in the ongoing TAK-861 long-term extension trial. Results from both trials will be presented at an upcoming scientific congress. Oncology In Oncology, we aspire to cure cancer, with inspiration from patients and innovation from everywhere. We are focused on: (1) building on our legacy in hematologic malignancies with marketed products (NINLARO, ADCETRIS, and ICLUSIG, etc.); (2) growing a solid tumor portfolio with marketed products (ALUNBRIG and FRUZAQLA [marketed in the U.S., development in other regions outside of mainland China, Hong Kong and Macau ongoing]); and (3) advancing a cutting-edge pipeline of highly innovative assets and platforms.

- 40 - CABOMETYX / Generic name: cabozantinib – In January 2024, Takeda announced that the detailed results from CONTACT-02, a phase 3 pivotal study led by Exelixis, evaluating CABOMETYX in combination with atezolizumab compared with a second novel hormonal therapy (NHT) in patients with metastatic castration-resistant prostate cancer (mCRPC) and measurable extra-pelvic soft tissue disease who have progressed on one prior NHT were presented during Oral Abstract Session at the American Society of Clinical Oncology 2024 Genitourinary Cancers Symposium (ASCO GU). For the primary endpoint of progression-free survival (PFS), at a median follow-up of 14.3 months for the PFS ITT (intent-to-treat) population (n=400), the hazard ratio (HR) was 0.65 (95% confidence interval [CI]: 0.50-0.84; p=0.0007); the median PFS (mPFS) was 6.3 months for CABOMETYX in combination with atezolizumab compared with 4.2 months for NHT. This was nearly identical to the PFS for the ITT population (n=507): HR was 0.64 (95% CI: 0.50-0.81, p=0.0002). At a median follow-up of 12.0 months for the ITT population, the median overall survival (OS), the other primary endpoint, was 16.7 months for CABOMETYX in combination with atezolizumab compared with 14.6 months for second NHT (HR: 0.79; 95% CI: 0.58-1.07; p=0.13), showing a trend toward OS improvement. The safety profiles of CABOMETYX and atezolizumab observed in this trial were consistent with their known safety profiles as monotherapies, and no new safety concerns were identified with the combination regimen. ADCETRIS / Generic name: brentuximab vedotin – In October 2023, Takeda announced that the European Commission (EC) approved ADCETRIS in combination with doxorubicin, vinblastine and dacarbazine (AVD) to treat adult patients with previously untreated CD30+ Stage III Hodgkin lymphoma. The decision follows a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) in September, 2023. The approval is based on the results of the randomized Phase 3 ECHELON-1 trial designed to compare ADCETRIS plus AVD to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as a therapy in adult patients with previously untreated Stage III or IV Hodgkin lymphoma. The trial met its primary endpoint of modified progression-free survival (PFS), as well as its key secondary endpoint of overall survival (OS), demonstrating a statistically significant improvement in OS in adult patients with previously untreated Stage III or IV classical Hodgkin lymphoma treated with ADCETRIS+AVD. The safety profile of ADCETRIS was consistent with previous studies, and no new safety signals were observed. – In November 2023, Takeda announced that it received an approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for a partial change in approved items of the manufacturing and marketing approval of ADCETRIS with the new indication of relapsed or refractory CD30-positive cutaneous T-cell lymphoma (CTCL). The approval is based on the results of the Phase 3 ALCANZA trial conducted outside of Japan as well as the Japanese Phase 2 investigator-initiated SGN-35-OU trial in patients with relapsed or refractory CD30-positive CTCL. NINLARO / Generic name: ixazomib – In September 2023, Takeda announced that it submitted a New Drug Application (NDA) to the Japanese Ministry of Health, Labour and Welfare (MHLW) for NINLARO capsules 0.5 mg as an additional dosage form of NINLARO (Capsules 2.3 mg/3 mg/4 mg). Aiming to achieve more appropriate dose adjustment in maintenance therapy for patients with multiple myeloma, Takeda filed this application to provide patients with a new treatment option (1.5 mg dose (0.5 mg/capsule x 3)) using a low-dose formulation of NINLARO. EXKIVITY / Generic name: mobocertinib – In October 2023, Takeda announced that, following discussions with the U.S. Food and Drug Administration (FDA), it will be working with the FDA towards a voluntary withdrawal of EXKIVITY in the U.S. for adult patients with epidermal growth factor receptor (EGFR) exon20 insertion mutation-positive (insertion+) locally advanced or metastatic non-small cell lung cancer (NSCLC) whose disease has progressed on or after platinum-based chemotherapy. Takeda intends to similarly initiate voluntary withdrawal globally where EXKIVITY is approved and is working with regulators in other countries where it is currently available on next steps. This decision was based on the outcome of the Phase 3 EXCLAIM-2 confirmatory trial, which did not meet its primary endpoint and thus did not fulfill the confirmatory data requirements of the accelerated approval granted by the U.S. FDA nor the conditional marketing approvals granted in other countries. The EXCLAIM-2 trial was a Phase 3, multicenter, open-label study designed to investigate the safety and efficacy of EXKIVITY as a monotherapy versus platinum-based chemotherapy in first-line EGFR exon20 insertion+ locally advanced or metastatic NSCLC. No new safety signals were observed

- 41 - in the EXCLAIM-2 trial. Full data from the trial will be presented at an upcoming medical meeting or published in a peer-reviewed journal. FRUZAQLA / Generic name: fruquintinib – In June 2023, Takeda and HUTCHMED (China) Limited announced that the European Medicines Agency (EMA) validated and accepted for regulatory review the marketing authorization application (MAA) for fruquintinib for the treatment of adult patients with previously treated metastatic colorectal cancer (mCRC). If approved, fruquintinib will be the first and only highly selective and potent inhibitor of vascular endothelial growth factor receptors (VEGFR) - 1, -2 and -3 approved in the European Union (EU) for previously treated mCRC. The MAA for fruquintinib includes results from the global Phase 3 FRESCO-2 clinical trial along with data from the Phase 3 FRESCO clinical trial. In April 2024,Takeda announced that the EMA’s Committee for Medicinal Products for Human Use (CHMP) recommended the approval of fruquintinib for the treatment of adult patients with previously treated mCRC. – In June 2023, Takeda and HUTCHMED (China) Limited announced that results of the Phase 3 FRESCO-2 study evaluating fruquintinib in patients with previously treated mCRC were published in The Lancet. FRESCO-2 is a global Phase 3 clinical trial (MRCT) conducted in the U.S., Europe, Japan and Australia investigating fruquintinib plus best supportive care (BSC) vs placebo plus BSC in patients with previously treated mCRC. The FRESCO-2 study met its primary and key secondary endpoints, demonstrating that treatment with fruquintinib resulted in a statistically significant and clinically meaningful improvement in overall survival (OS) and progression-free survival (PFS), respectively. The safety profile of fruquintinib in FRESCO-2 was consistent with previously reported fruquintinib studies. – In September 2023, Takeda announced that it submitted a New Drug Application (NDA) to the Japanese Ministry of Health, Labour and Welfare (MHLW) for fruquintinib for the treatment of previously treated mCRC. The NDA for fruquintinib is based on the global Phase 3 FRESCO-2 clinical trial and the Phase 3 FRESCO clinical trial. – In November 2023, Takeda announced that the U.S. Food and Drug Administration (FDA) approved FRUZAQLA for adults with mCRC who have been previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if RAS wild-type and medically appropriate, an anti-EGFR therapy. FRUZAQLA is the first and only selective inhibitor of all three VEGF receptor kinases approved in the U.S. for previously treated mCRC regardless of biomarker status. The approval of FRUZAQLA is based on data from two large Phase 3 trials: the global FRESCO-2 clinical trial along with the FRESCO clinical trial conducted in China. ICLUSIG / Generic name: ponatinib – In March 2024, Takeda announced that the U.S. Food and Drug Administration (FDA) approved the supplemental New Drug Application (sNDA) for ICLUSIG for the treatment of adult patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) in combination with chemotherapy. This indication is approved under accelerated approval based on minimal residual disease (MRD)-negative complete remission (CR) at the end of induction met by the global Phase 3 PhALLCON study in which ICLUSIG demonstrated superiority in MRD-negative complete remission rates to imatinib. In the trial, the safety profile of ICLUSIG was comparable to imatinib, and no new safety signals were identified. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. This accelerated approval application was granted Priority Review and evaluated under the Real-Time Oncology Review (RTOR) program, an FDA initiative designed to expedite the delivery of cancer medicines by allowing components of an application to be reviewed before submission of the complete application. VECTIBIX / Generic name: panitumumab – In February 2024, Takeda announced that the study on biomarker research analyzing circulating tumor DNA (ctDNA) obtained from patients participating in the PARADIGM trial, a Japanese Phase 3 clinical trial of VECTIBIX for the first-line treatment of unresectable advanced recurrent colorectal cancer, to investigate the correlation of baseline ctDNA with treatment efficacy was published in the biomedical journal Nature Medicine. The results of this follow- up analysis showed that, in a group who did not have 10 genetic mutations reported to be associated with resistance to anti-EGFR antibody drugs (KRAS, NRAS, BRAF (V600E), PTEN and EGFR extracellular domain mutations, HER2 and MET amplification, as well as ALK, RET, and NTRK1 fusions), overall survival was longer in the mFOLFOX6 + VECTIBIX combination therapy group than in the mFOLFOX6 + bevacizumab combination therapy group in both left and right sided tumors combined (VECTIBIX combination therapy group: 40.7 months, bevacizumab combination therapy group: 34.4 months, HR: 0.76 [95% CI: 0.62-0.92]). The safety profile of VECTIBIX in this analysis aligns with the findings reported in previously published clinical trial results. The results

- 42 - suggest that analysis of ctDNA extracted from patients' blood may identify patients who are more likely to benefit from treatment with panitumumab, rather than simply selecting the treatment by the site of the primary tumor. Other Rare Diseases programs Takeda’s R&D engine is focused on areas of high unmet medical need, both in rare and more prevalent conditions, across three core therapeutic areas (gastrointestinal and inflammation, neuroscience, and oncology). In other Rare Diseases programs, Takeda focuses on several areas of high unmet medical need. In hereditary angioedema, Takeda aspires to transform the treatment paradigm, including through TAKHZYRO, with continued investment in lifecycle management programs. In rare hematology, Takeda focuses on addressing today’s needs in the treatment of bleeding disorders, including through ADVATE and ADYNOVATE/ADYNOVI. In addition, Takeda aims to redefine the management of post-transplant cytomegalovirus (CMV) infection/disease with LIVTENCITY. Takeda commits to fulfilling our vision to deliver life-transforming medicines to patients with rare diseases. Takeda will continue to explore late-stage business development that may leverage our rare diseases capabilities as well as bolster our commitment and leadership in rare diseases. ADYNOVATE/ADYNOVI / Generic name: antihemophilic factor (recombinant), PEGylated – In June 2023, Takeda announced that it received an approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for a partial change in approved items of the manufacturing and marketing approval of ADYNOVATE for dosage and administration. This approval will contribute driving personalized treatments by adjusting dosage and administration including dosing amount and intervals, depending on individual patient’s clinical presentation and activity level. The approval is based primarily on the results of the global Phase 3 CONTINUATION study and Phase 3 PROPEL study conducted outside of Japan. OBIZUR / Generic name: Susoctocog Alfa (recombinant) – In March 2024, Takeda announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) approved OBIZUR, recombinant porcine coagulation factor VIII that is deficient in the glycosylated B domain, for the control of bleeding in patients with acquired hemophilia A (AHA). The approval is based primarily on a Phase 2/3 clinical trial in 5 Japanese patients aged 18 years and older with AHA and a Phase 2/3 clinical trial conducted outside of Japan in non-Japanese patients aged 18 years and older with AHA. LIVTENCITY / Generic name: maribavir – In November 2023, Takeda announced that it submitted a New Drug Application (NDA) to the Japanese Ministry of Health, Labour and Welfare (MHLW) for maribavir for the treatment of patients with post-transplant (including hematopoietic stem cell transplant) cytomegalovirus (CMV) infection/disease. The NDA is primarily based on the Japanese Phase 3 open-label trial in patients with CMV infection who underwent hematopoietic stem cell transplant (HSCT) or solid organ transplant (SOT), and the Phase 3 open-label SOLSTICE trial conducted outside of Japan in patients with CMV infection refractory or resistant to prior anti-CMV treatment who underwent HSCT or SOT. – In December 2023, Takeda announced that LIVTENCITY was approved by the National Medical Products Administration (NMPA) of China for the treatment of adult patients with post- HSCT or SOT CMV infection/disease that is refractory to treatment (with or without genotypic resistance) with ganciclovir, valganciclovir, cidofovir or foscarnet. The NMPA approval is based on the results of the Phase 3 SOLSTICE trial. LIVTENCITY was granted Breakthrough Therapy Designation by China Center for Drug Evaluation (CDE) in 2021. LIVTENCITY is the first and only inhibitor of CMV-specific UL97 protein kinase in China for this indication. Plasma-Derived Therapies (PDT) Takeda has created a dedicated PDT business unit with a focus on managing the business end-to-end, from plasma donation to manufacturing, R&D, and commercialization. In PDT, we aspire to develop life-saving plasma derived therapies, which are essential for patients with a variety of rare and complex chronic diseases. The dedicated R&D organization within PDT is charged with maximizing the value of existing therapies, identifying new targeted therapies, and optimizing efficiencies across the PDT value chain, from plasma donation to product manufacturing. Near-term, our priority is focused on delivering value from our broad immunoglobulin portfolio (HYQVIA, CUVITRU, GAMMAGARD LIQUID and GAMMAGARD S/D) through the pursuit of new indications, geographic expansions, and enhanced patient experience through integrated healthcare technologies. In our hematology and specialty care portfolio, our priority is

- 43 - pursuing new indication and formulation development opportunities for PROTHROMPLEX (4F-PCC), FEIBA and CEPROTIN. Additionally, we are developing next generation immunoglobulin products with 20% fSCIg (TAK-881) and liquid low IgA IG (TAK-880) and are pursuing other early stage opportunities (e.g. hypersialylated Immunoglobulin (hsIgG)) that would add to our diversified commercial portfolio of more than 20 therapeutic products distributed worldwide. HYQVIA / Generic name: Immunoglobulin (IG) Infusion 10% (Human) w/ Recombinant Human Hyaluronidase for subcutaneous administration (Development code: TAK-771) – In April 2023, Takeda announced that the U.S. Food and Drug Administration (FDA) approved a supplemental biologics license application (sBLA) to expand the use of HYQVIA to treat primary immunodeficiency (PI) in children 2-16 years old. The FDA approval of HYQVIA for the treatment of PI in pediatric patients was based on evidence from a pivotal, prospective, open-label, non-controlled Phase 3 clinical trial that included 44 PI patients between the ages of 2 and 16. During the 12-month trial period, HYQVIA was shown to be efficacious with respect to the occurrence of acute serious bacterial infections (aSBIs), a primary endpoint. The mean aSBI rate per year was 0.04 and was statistically significantly lower (with an upper 1-sided 99% confidence interval of 0.21, p<0.001) than the predefined success rate of less than one aSBI per subject per year, favoring efficacy of HYQVIA treatment in pediatric subjects with PI diseases. Results from the interim data analysis, where all subjects completed 12 months of participation (one year of observation period) in the study, indicated similar safety profiles to adults. – In June 2023, Takeda announced full results from the pivotal Phase 3 ADVANCE-CIDP 1 clinical trial investigating HYQVIA as maintenance therapy in adult patients with chronic inflammatory demyelinating polyneuropathy (CIDP). ADVANCE-CIDP 1 is a Phase 3, prospective, randomized, double-blind, multicenter, placebo-controlled study in which adults with stable CIDP on intravenous immunoglobulin (IVIG) were randomized 1:1 to be switched to HYQVIA (n=62) or placebo (n=70) and received their assigned treatment for six months or until relapse or study withdrawal. The primary endpoint was proportion of participants who experienced a relapse defined as worsening of CIDP symptoms as measured by Inflammatory Neuropathy Cause and Treatment (INCAT). Secondary endpoints included patient proportion experiencing functional worsening, time to relapse, change from pre-subcutaneous treatment baseline in Rasch-built Overall Disability Scale (R-ODS) centile score and safety. Results showed a clinically significant reduction in relapse rate with HYQVIA vs placebo (9.7% vs. 31.4%, respectively; p=0.0045) and other analysis showed delayed time to relapse with HYQVIA vs. placebo. Favorable data across other endpoints from the study and favorable tolerability were also observed. These findings were presented at the 2023 Peripheral Nerve Society (PNS) Annual Meeting in Denmark in June 2023, and simultaneously published in the Journal of the Peripheral Nervous System (JPNS). – In January 2024, Takeda announced that the FDA approved HYQVIA for the treatment of CIDP as maintenance therapy to prevent the relapse of neuromuscular disability and impairment in adult patients. The approval is based on results from ADVANCE-CIDP 1 clinical trial and ADVANCE-CIDP 3, a single-arm, open-label, extension study. HYQVIA is the only FDA-approved combination of immunoglobulin (IG) and hyaluronidase, which makes it a facilitated subcutaneous immunoglobulin (SCIG) infusion. For adults with CIDP, HYQVIA can be infused up to once monthly (every two, three or four weeks) due to the hyaluronidase component, which facilitates the dispersion and absorption of large IG volumes in the subcutaneous space between the skin and the muscle. Because it is delivered subcutaneously, HYQVIA can be administered by a healthcare professional in a medical office, infusion center or at a patient’s home. In addition, it can be self-administered after appropriate patient or caregiver training. – In January 2024, Takeda announced that the European Commission (EC) approved HYQVIA as maintenance therapy in patients of all ages with CIDP after stabilization with IVIG therapy. The approval is based on data from the pivotal Phase 3 ADVANCE-CIDP 1 clinical trial, which evaluated efficacy and safety of HYQVIA as maintenance therapy to prevent relapse in patients with CIDP. – In February 2024, Takeda announced that it submitted a New Drug Application (NDA) to the Japanese Ministry of Health, Labour and Welfare (MHLW) for manufacturing and marketing approval of TAK-771 for the treatment of agammaglobulinemia and hypogammaglobulinemia, disorders characterized by very low or absent levels of antibodies and an increased risk of serious recurring infection caused by primary immunodeficiency (PID) or secondary immunodeficiency (SID). The application is based primarily on a Phase 3 study (TAK-771-3004) in Japanese patients with primary immunodeficiency (PID) and three Phase 2/3 studies conducted outside of Japan in patients with PID (160603 study, 160902 study and 161503 study), which were conducted to evaluate efficacy, safety, tolerability, and pharmacokinetics.

- 44 - CUVITRU / Generic name: Immunoglobulin (IG) Infusion 20% (Human) for subcutaneous administration – In September 2023, Takeda announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) approved the use of CUVITRU in patients aged 2 years and older with agammaglobulinemia or hypogammaglobulinemia, disorders characterized by very low or absent levels of antibodies and an increased risk of serious recurring infection caused by primary immunodeficiency (PID) or secondary immunodeficiency (SID). The approval marks Takeda’s first subcutaneous immunoglobulin (SCIG) therapy for patients in Japan. The approval is based on results from a Phase 3 clinical trial that evaluated the efficacy, safety, tolerability and pharmacokinetics of CUVITRU in Japanese patients with PID, as well as two Phase 2/3 clinical trials conducted in patients with PID in North America and Europe. Results from the clinical trial in 17 patients in Japan confirmed its efficacy and safety profile. No serious or severe adverse events were reported, and CUVITRU was well-tolerated. The most frequently reported adverse reactions were headache and injection site swelling in four patients (23.5%) and injection site erythema in three patients (17.6%) during CUVITRU treatment. Previously reported clinical trial results also confirmed the efficacy and safety of CUVITRU. GAMMAGARD LIQUID / Generic name: Immunoglobulin (IG) Infusion 10% (Human) – In January 2024, Takeda announced that the U.S. Food and Drug Administration (FDA) approved GAMMAGARD LIQUID as an intravenous immunoglobulin (IVIG) therapy to improve neuromuscular disability and impairment in adults with chronic inflammatory demyelinating polyneuropathy (CIDP). It can be used as induction therapy, which includes an induction dose and maintenance doses. For treatment of CIDP, GAMMAGARD LIQUID has not been studied in immunoglobulin-naive patients nor as maintenance therapy for periods longer than 6 months. The approval is based on results from a prospective, open-label, single-arm, multicenter ADVANCE-CIDP 2 clinical trial that evaluated the efficacy and safety of GAMMAGARD LIQUID in adults with CIDP who developed a relapse in HYQVIA’s ADVANCE-CIDP 1 trial. CEPROTIN / Generic name: Human Dry Protein C Concentrate (Development code: TAK-662) – In March 2024, Takeda announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) approved CEPROTIN for the treatment of venous thromboembolism and purpura fulminans caused by congenital protein C deficiency, as well as for the prevention of thrombophilia. The approval is based primarily on a Phase 1/2 trial in five Japanese patients primarily in ages 4-27 with congenital protein C deficiency and two Phase 2/3 trials (IMAG-098 and 400101) conducted outside of Japan in non-Japanese patients with congenital protein C deficiency. Vaccine In Vaccines, Takeda is applying innovation to tackle some of the world’s most challenging infectious diseases such as dengue (QDENGA (development code: TAK-003)), COVID-19 (NUVAXOVID). To support the expansion of our pipeline and the development of our programs, we have entered into partnerships with government organizations in Japan and the U.S., and leading global institutions. Such partnerships have been essential in building the critical capabilities that will be necessary to deliver on our programs and realize their full potential. QDENGA / Generic name: Dengue tetravalent vaccine [live, attenuated] (Development code: TAK-003) – In July 2023, Takeda announced that it voluntarily withdrew the U.S. Biologics License Application (BLA) for TAK- 003, following discussions with the U.S. Food and Drug Administration (FDA) on aspects of data collection, which cannot be addressed within the current BLA review cycle. The future plan for TAK-003 in the U.S. will be further evaluated given the need for travelers and those living in dengue-endemic areas of the U.S., such as Puerto Rico. The efficacy and safety profiles of TAK-003 have been demonstrated through a robust clinical trial program, including a 4.5-year Phase 3 study of over 20,000 children and adolescents living in eight dengue endemic areas. The study was designed per World Health Organization (WHO) guidance for a second-generation dengue vaccine, and it considered the need to achieve high levels of subject retention and protocol compliance in endemic regions. The vaccine is approved in multiple endemic and non-endemic countries, with more approvals expected over the coming years. – In October 2023, Takeda announced that the WHO Strategic Advisory Group of Experts on Immunization (SAGE) shared recommendations for use of QDENGA. SAGE made the following recommendations:

- 45 - – The vaccine to be considered for introduction in settings with high dengue disease burden and high transmission intensity to maximize the public health impact and minimize any potential risk in seronegative persons. – The vaccine to be introduced to children aged 6 to 16 years of age. Within this age range, the vaccine should be introduced about 1-2 years prior to the age-specific peak incidence of dengue-related hospitalizations. The vaccine should be administered in a 2-dose schedule with a 3-month interval between doses. – The vaccine introduction should be accompanied by a well-designed communication strategy and community engagement. SAGE reviewed data across 19 Phase 1, 2 and 3 trials with more than 28,000 children and adults, including the pivotal Phase 3 Tetravalent Immunization against Dengue Efficacy Study (TIDES) trial, which was designed according to the WHO’s guidance for a second-generation dengue vaccine. The WHO will consider the SAGE recommendation and is expected to update its position paper on dengue vaccines to include final guidance on the use of QDENGA in public vaccination programs. Building a sustainable research platform / Enhancing R&D collaboration In addition to our concentrated efforts to increase our in-house R&D capabilities, external partnerships with third-party partners are a key component of our strategy for enhancing our R&D pipeline. Our strategy to expand and diversify our external partnerships allows us to take part in research of a wide variety of new products and increases the chances that we will be able to take part in a major research-related breakthrough. – In August 2023, Takeda announced that it entered into an exclusive licensing agreement with ImmunoGen, Inc. (ImmunoGen) to develop and commercialize mirvetuximab soravtansine-gynx (MIRV) for the Japanese market. MIRV is an intravenous injection antibody-drug conjugate (ADC), in which a microtubule inhibitor is linked to an anti- folate receptor-α (FRα) antibody. It is the first ADC developed for the treatment of ovarian cancer. MIRV is approved under accelerated approval (and was granted full approval thereafter) in the U.S. for the treatment of adult patients with FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have received one to three prior systemic treatment regimens. MIRV was the first medicine to show a significant prolongation of overall survival (OS) compared with conventional chemotherapy for the treatment of platinum- resistant relapsed or refractory ovarian cancer in a phase 3 MIRASOL study, conducted outside of Japan. In February 2024, ImmunoGen was acquired by AbbVie Inc. – In January 2024, Takeda and Protagonist Therapeutics, Inc. announced the signing of a worldwide license and collaboration agreement for the development and commercialization of rusfertide, an investigational injectable hepcidin mimetic peptide of the natural hormone hepcidin, currently in a pivotal Phase 3 trial, VERIFY, for the treatment of Polycythemia Vera (PV). Discovered through Protagonist's peptide technology platform, rusfertide’s mechanism of action is thought to regulate iron homeostasis and control the absorption, storage and distribution of iron in the body. The randomized portion of the Phase 2 REVIVE study of rusfertide in PV achieved its primary endpoint. The long-term follow-up data from the 2-year open label extension were presented at the American Society of Hematology 2023 Annual Meeting, which showed durable hematocrit control, decreased phlebotomy use, long- term tolerability and no new safety signals in patients with PV. Protagonist will remain responsible for research and development through the completion of the Phase 3 clinical trial and U.S regulatory approval. Takeda has rights for ex-U.S. development and is responsible for leading global commercialization activities. • In April 2024, Takeda and Japanese Foundation for Cancer Research (JFCR) announced that the signing of a partnership agreement with the goal to advance research and development in the field of oncology. Under the terms of this agreement, Takeda and JFCR will engage in mutual exchange utilizing each other's strengths for the purpose of advancing global early clinical trials and facilitating translational research based on this agreement. This will include necessary information exchanging and consultation regarding ongoing drug development. The partnership seeks to expedite the development of groundbreaking anti-cancer therapies and facilitate swift delivery to cancer patients and their families. • In April 2024, Takeda, Astellas Pharma Inc. (Astellas), and Sumitomo Mitsui Banking Corporation announced that three companies signed a master agreement to establish a joint venture company. The new company will be dedicated to the incubation of early drug discovery programs originating from Japan and toward the creation of innovative therapeutics. In addition to establishing the joint venture company, Takeda and Astellas will provide support to the joint venture company leveraging their expertise gained from global drug discovery research and

- 46 - development, aiming to accelerate open innovation in early-stage drug discovery, and toward the creation of start- up companies for the benefit of society. The joint venture company, once established, plans to begin incubation activities by collaboratively working with academia, pharmaceutical companies, and start-up companies across Japan to enable access to potentially transformative early drug discovery programs. (3) Facility Investment (Tangible assets) The total amount of investment in tangible assets (on an acquisition basis) during the current fiscal year was JPY 324.4 billion mainly for the new construction, expansion, and renewal of facilities, including plasma collection centers and manufacturing sites, as well as for the expansion of research sites and office. (4) Fund Procurement During the current fiscal year, Takeda redeemed USD 1,500 million in fixed rate unsecured senior notes on their maturity dates. Additionally, Takeda repaid JPY 100.0 billion in Syndicated Loans and efficiently refinanced them on a cost- effective basis with a new maturity of April 26, 2030. Takeda also had short term commercial paper drawings outstanding of JPY 317.0 billion as of March 31, 2024. The consolidated outstanding balances of bonds and loans as of March 31, 2024 were JPY 4,092.9 billion and JPY 750.9 billion respectively following the impact of the above noted debt repayment and refinancing activity during the fiscal year. (5) Issues for the Takeda Group to Address Takeda's Corporate Philosophy and Imperatives Our corporate philosophy tells the rich story of Takeda - who we are, what we do, how we do it, and why it matters. From our founding more than 240 years ago to today, we serve patients with integrity that also benefits society. Our imperatives - Patient-People-Planet, powered by Data, Digital and Technology (DD&T), direct where Takeda must focus to deliver on our purpose and vision, guided by our values. Our ambition is to be the most trusted, science-driven, digital biopharmaceutical company. Through our core business, Takeda creates long-term value for patients, shareholders and society while also sustaining positive impact for our people, communities, and the planet.

- 47 - Business Environment We believe that we need to navigate geopolitical uncertainty, rising healthcare costs, and rapid advancement of technology to ensure that we deliver on our vision. At the geopolitical level, risks are intensifying, with ongoing conflicts in Ukraine and the Middle East together with continued tensions between China and the U.S., EU and other countries creating an uncertain outlook for the global economy. As a global company, we need to be constantly attentive to the changing economic environment and attendant risks and adapt our business strategy accordingly. The business environment in which we operate is also influenced by government health care policies. While medical innovation in recent years has improved health care outcomes, spending on health care has for decades needed to rise faster than the gross domestic product and gross domestic incomes of developed countries due to aging populations, lifestyle changes and the availability of more advanced solutions for complex diseases. Consequently, payers are becoming increasingly selective in determining which treatments will be reimbursed. National governments are promoting generic and biosimilar alternatives and are increasing downward pressure on drug prices. In the United States, the Inflation Reduction Act (IRA), while offering some positives for Medicare patients such as greater predictability in out-of-pocket prescription expenses, establishes an unprecedented government price-setting system for medicines that could potentially result in declines in R&D investment in the country. Meanwhile, widening gaps in access to care further demonstrate the need for better access and policies to address health inequity. We believe that a transition away from the current prevailing fee-for-service model and toward value-based health care – an approach that pays for outcomes and care quality – could slow the pace of rising health care costs while expanding coverage and improving equity. The rapid advancement of technology must also be factored in to strategic planning. We believe that the pace of innovation in the global pharmaceutical industry continues to accelerate, enhanced by medical technologies such as immunotherapies in oncology and cell and gene therapy and, more recently, by the rapid adoption of artificial intelligence (AI). The potential to develop AI-enabled innovations to help individuals manage their disease and treatments is vast and we believe that this technology could transform how the pharmaceutical industry operates. With these and other factors influencing the external business environment, our commitment to patients and the work we do to support them is even more important. Patient We pursue life-transforming science and focus on the highest unmet medical need, both in rare and more prevalent conditions. Our research programs are based on targets with strong human validation and represent diverse modalities. We leverage DD&T broadly, from accelerating the pipeline to driving quality and efficiency in manufacturing, to enhancing interactions with health care practitioners and patients. AI is increasingly incorporated into the design of what we create to support patient experiences. Examples include our joint projects with Massachusetts Institute of Technology (MIT) to use AI to help accelerate diagnosis of rare diseases, such as Fabry disease; leveraging AI platforms to personalize the way we engage with physicians and improving diversity and data collection in clinical trials. We are intentional in looking at how we use technology in an ethical manner while trying to predict the regulatory environment in the future. We believe the potential to develop AI-enabled innovations to help individuals manage their disease and treatments is vast. In the fiscal year ended March 31, 2024 (FY2023), we received nine approvals from the U.S. Food and Drug Administration (FDA), including three new molecular entities: FRUZAQLA for the treatment of metastatic colorectal cancer; ADZYNMA for patients with congenital thrombotic thrombocytopenic purpura (cTTP); and EOHILIA for eosinophilic esophagitis. For more information on our major activities and progress on R&D from April 2023 to date, please see our discussion of Pipeline and R&D Activities in (2) Business Performance for Fiscal 2023 (iii) Activities and Results of Research & Development. We continue to see momentum in our Growth & Launch Product portfolio. ENTYVIO is our number-one product by revenue and we launched our subcutaneous administration in the U.S. for maintenance therapy in moderate-to-severely active ulcerative colitis and Crohn's disease, providing more flexibility and choice to patients. We are also encouraged by the global progress of our dengue vaccine QDENGA since first launching a little over a year ago. QDENGA is now available in more than 20 markets across the world, including many endemic countries where the need is highest. In 2023, there was an upsurge in dengue cases globally, with the disease spreading into previously unaffected regions. We are now working to expand production and ensure cooperation with communities worldwide who need QDENGA to combat the increase in dengue prevalence. To help us achieve our target to supply 100 million doses annually by 2030 we have entered into a manufacturing partnership agreement with Biological E. Limited (BE) in India that builds upon existing capabilities at our facility in

- 48 - Singen, Germany and our long-term contract manufacturing partnership with IDT Biologika GmbH in Germany. BE will manufacture up to 50 million doses of QDENGA per annum. People We recognize that no matter how far science and technology advance, meaningful change is always driven by people. Our intention is to create an inclusive workplace through diversity, equity, and inclusion (DE&I) initiatives, promote life- long learning, talent development and career growth, and reinforce our values-based culture, and prioritize employee well-being, which enable us to discover and deliver life-transforming treatments and vaccines for patients and communities. Our culture is one of belonging, engaging our people who originate from over 80 countries and who represent a wide range of backgrounds and experiences. Takeda embraces diversity and strives to provide equitable opportunities for patients and employees. Takeda has increased its investment in DE&I, including the expansion of the Global DE&I Council, which focuses on strategic direction, relationship-building, and efforts to address health disparities and inequities on a global scale. Life-long learning and career growth enhance employee motivation and expertise, leads to new ideas, and results in value creation for patients. We are upskilling employees and building in-house capabilities to create an agile and resilient organization that is positioned for long-term sustainable growth. Our new Career Navigator platform uses AI to show personalized internal positions and mentoring and learning opportunities so our people can reach their highest potential. We are also leveraging the rapid technological advancements shaping our sector today and investing in the digital skills of our people for the future of health care. As part of an initiative to improve work environment, we have transformed Takeda offices into ‘Takeda Community Spaces’ centered around employee well-being and learning. These spaces are designed for maximizing in-person interactions, where people can focus, collaborate and connect more closely in a sustainable environment. Takeda has partnered with Thrive, a behavioral health platform, to help our employees improve their overall well-being, build mental resilience and increase productivity. These components help us to build an exceptional people experience that promotes well-being and performance, embraces flexibility and emphasizes the value of regular face-to-face interactions. Planet The reality of climate change must now be factored into the decision-making processes of every business. Public health is integrally linked to the impacts of climate change and, as temperatures rise, there will be challenges related to climate- accelerated diseases and access to care for patients in impacted regions. Takeda is committed to delivering a high standard of environmental leadership, recognizing that climate change and pollution both impact human health. It is not enough to just work towards a healthier population – we need a healthier planet as well to realize our purpose. We are taking action to reduce our environmental impact on many fronts by prioritizing clean energy solutions, progressing toward net-zero targets and working to eliminate greenhouse gas (GHG) emissions from our entire value chain. While Takeda has maintained carbon neutrality through FY2022, in FY2024 we have transitioned away from carbon neutrality as a climate goal and are focusing resources on initiatives that advance our net-zero roadmap while continuing to invest in nature-based carbon removal projects in projects beyond our value chain. We are working to achieve net-zero GHG emissions in our operations by 2035 and across our value chain by 2040 in accordance with the Science Based Targets initiative's Corporate Net-Zero Standard, conserving natural resources, and designing our products with sustainability principles in mind. We continue to make notable progress towards our GHG emissions goals and have issued Environmental Sustainability Improvement Plans for several commercial products. For example, we are pioneering the use of CMYK (cyan, magenta, yellow and key plate (black)) printing in Japan and plan to roll out this program globally. This switch is expected to reduce waste of unused ink in the supplier’s printing process, as well as the amount of solvents necessary to clean the printing machine and the amount of waste generated during changeover between different packaging. Furthermore, 52% of all secondary packaging for our products is now made from Forest Stewardship Council-certified or recycled content paper or paperboard. In October 2023, we announced the opening of our BioLife plasma donation center in Linz, Austria, which is the first of our centers designed to operate as a zero-GHG emissions facility. Also in Austria, at our largest production site in Vienna we introduced a groundbreaking heat pump system that will reduce GHG emissions in the production area where it is installed by up to 90 percent.

- 49 - DD&T is also a key enabler of our environmental efforts. At our manufacturing site in Osaka we reduced distilled water consumption by approximately 460,000 liters per year, leading to a reduction of over two million liters in freshwater consumption annually, by installing sensors and monitors at every point of water use and analyzing the combined data to find ways to optimize water volumes and standardize best practices. Similar projects have been undertaken to reduce electricity consumption and increase our use of solar and other green energy sources. Financial Performance Takeda plans and manages financial profiles based on future forecasts and has a strong financial foundation that enhances inflation resilience and minimizes exposure to interest rate increases. Our financial foundation enables us to nurture a diverse pipeline with approximately 30 clinical stage medicines driven by our in-house R&D engine and through more than 200 partnerships. With our free cash flow, driven by financial discipline, we are also reinforcing our long-term growth potential through strategic investments in internal and external opportunities to strengthen the pipeline. Our R&D organization has delivered momentum across our mid- and late-stage pipeline with the approvals of FRUZAQLA, ADZYNMA and EOHILIA, and the advancement of our most highly prioritized programs, zasocitinib (TAK- 279) and TAK-861, which represent significant potential commercial opportunities. Zasocitinib is a highly selective, oral allosteric tyrosine kinase 2 (TYK2) inhibitor that has the potential to offer best-in- class treatment for patients with psoriasis and other immune-mediated inflammatory diseases, including psoriatic arthritis and inflammatory bowel disease (IBD). We continue to advance the development of zasocitinib, having initiated two Phase 3 psoriasis trials, and aim to file a regulatory submission in psoriasis between FY2026 and FY2027. TAK-861 is our lead orexin receptor 2 agonist, with the potential to address the underlying pathophysiology of narcolepsy. In February 2024, we made the decision to advance TAK-861 to Phase 3 development in narcolepsy type 1, further reinforcing our efforts to deliver growth into the next decade. While we are facing short-term headwinds primarily due to the loss of exclusivity for VYVANSE (for attention deficit hyperactivity disorder) in the U.S., we believe our Growth and Launch Products* will drive topline growth in the medium- to-long term. In 2023, we raised our peak sales estimate for ENTYVIO (for ulcerative colitis and Crohn’s disease) to USD 7.5 to 9.0 billion, based on its sustained global sales growth potential and our updated assumption for the timing of biosimilar competition. We expect that this momentum will be further boosted by new product launches. In the medium-to-long term, we aim to return to low to mid-30% Core Operating Profit margin and maintain strong cash flow generation. We plan to continue to allocate cash flow towards internal and external opportunities to enhance the pipeline, new product launches and PDT, and towards delivering on our commitment to shareholder returns. * Takeda’s Growth and Launch Products for FY2024: GI: ENTYVIO, EOHILIA Rare Diseases: TAKHZYRO, LIVTENCITY, ADZYNMA PDT: Immunoglobulin products including GAMMAGARD LIQUID/KIOVIG, HYQVIA, and CUVITRU, Albumin products including HUMAN ALBUMIN and FLEXBUMIN Oncology: ALUNBRIG, FRUZAQLA Vaccines: QDENGA Basic Policy for Profit Distribution Guided by our vision to discover and deliver life-transforming treatments, and with a focus on maintaining solid investment grade credit ratings, we will allocate capital to deliver sustainable value to patients and attractive returns to our shareholders. Takeda's policy in the allocation of capital is as follows:

- 50 - • Invest in growth drivers; and • Shareholder returns. In respect of "Invest in growth drivers", Takeda makes strategic investments in internal and external opportunities to enhance the pipeline, new product launches, and plasma-derived therapies. With regard to "Shareholder returns", Takeda has adopted a progressive dividend policy of increasing or maintaining the annual dividend per share each year, alongside share buybacks when appropriate.

- 51 - Financial Forecast for Fiscal 2024 Consolidated reported forecast for the fiscal year ending March 31, 2025 (FY2024)* is as below: *Financial Forecast for Fiscal 2024 is a matter to be approved in the board of director meeting scheduled on May 9, 2024 and does not constitute the contents of the business report. Consolidated Reported Forecast for the Fiscal Year Ending March 31, 2025 (FY2024) Billion JPY or percentage FY2023 Actual Results FY2024 Forecast Change versus the previous year Revenue 4,263.8 4,350.0 86.2 2.0 % Gross Profit 2,837.1 2,850.0 12.9 0.5 % Operating profit 214.1 225.0 10.9 5.1 % Profit before tax 52.8 55.0 2.2 4.2 % Net profit for the year (attributable to owners of the Company) 144.1 58.0 (86.1) (59.7)% EPS (JPY) 92.09 36.70 (55.39) (60.1)% Core Revenue 4,263.8 4,350.0 86.2 2.0 % Core Operating Profit 1,054.9 1,000.0 (54.9) (5.2)% Core EPS (JPY) 484 431 (53) (10.9)% [Revenue] Takeda expects FY2024 revenue to be JPY 4,350.0 billion, an increase of JPY 86.2 billion, or 2.0%, from FY2023. The continued decline of products experiencing generic competition, including VYVANSE in the U.S., is expected to be largely mitigated by expansion of Growth and Launch Products, including ENTYVIO, immunoglobulin products, and new products such as QDENGA, FRUZAQLA, and EOHILIA. In addition, the foreign exchange assumption rates for major currencies reflect the depreciation of the Japanese yen versus FY2023 actual rates, which results in a favorable year- on-year impact on revenue. Because Takeda does not expect any significant non-core items that require adjustment in its revenue forecast, the Core revenue forecast for FY2024 is the same as the reported revenue forecast. [Operating Profit] Operating Profit is expected to increase by JPY 10.9 billion, or 5.1%, to JPY 225.0 billion. While various cost efficiency initiatives will continue, we will also actively make investments for new product launches and in data, digital, and technology. There will also be a modest increase in R&D expenses to support our late-stage pipeline. Other operating expenses are expected to be JPY 200.0 billion, including JPY 140.0 billion of restructuring expense which is primarily related to the enterprise-wide efficiency program scheduled to start from FY2024. Operating Profit growth versus FY2023 also benefits from a lower assumption for impairment losses on intangible assets associated with products, with JPY 50.0 billion included in our FY2024 forecast compared to JPY 130.6 billion booked in FY2023. Core Operating Profit is expected to be JPY 1,000.0 billion, a decrease of JPY 54.9 billion JPY, or 5.2%. [Net profit for the year (attributable to owners of the Company)] Net profit for the year (attributable to owners of the Company) is expected to be JPY 58.0 billion, a decrease of JPY 86.1 billion, or 59.7%, mainly reflecting significant one-time tax expense reduction booked in FY2023 and resulting less tax benefit in FY2024 compared to FY2023. Profit Before Tax is expected to increase by JPY 2.2 billion, or 4.2%, to JPY 55.0 billion, reflecting an increase in net finance income and expenses, which partially offset the expected increase in Operating Profit of JPY 10.9 billion. Reported EPS is expected to be JPY 36.70, a decrease of JPY 55.39, or 60.1%, and Core EPS is expected to be JPY 431, a decrease of JPY 53, or 10.9%.

- 52 - Major assumptions used in preparing the FY2024 Reported Forecast Billion JPY or percentage FY2023 Actual Results FY2024 Forecast FX rates 1 USD = 144 JPY 1 Euro = 156 JPY 1 RUB = 1.6 JPY 1 CNY = 20.1 JPY 1 BRL = 29.1 JPY 1 USD = 150 JPY 1 Euro = 160 JPY 1 RUB = 1.6 JPY 1 CNY = 20.9 JPY 1 BRL = 30.4 JPY Cost of Sales (1,426.7) (1,500.0) SG&A Expenses (1,053.8) (1,080.0) R&D expenses (729.9) (770.0) Amortization of intangible assets associated with products (521.5) (540.0) Impairment of intangible assets associated with products*1 (130.6) (50.0) Other operating income 19.4 15.0 Other operating expenses*2 (206.5) (200.0) Other Core Operating Profit adjustments (1.5) — Finance income and (expenses), net (167.8) (172.0) Adjusted free cash flow*3 283.4 350.0 - 450.0 Capital expenditures (cash flow base) (480.7) (380.0 - 420.0) Depreciation and amortization (excluding intangible assets associated with products) (206.5) (205.0) Cash tax rate on adjusted EBITDA (excluding divestitures) ~15% Mid teen % *1 Includes in-process R&D. *2 JPY 140.0 billion of restructuring expense which is primarily related to the enterprise-wide efficiency program is included in FY2024 Forecast. *3 Starting from FY2024, we will i) change the title of free cash flow as currently represented to “Adjusted free cash flow” and ii) report “Free cash flow” as cash flows from operating activities less acquisition of property, plant and equipment. Management Guidance Takeda uses change in Core Revenue, Core Operating Profit and Core EPS at Constant Exchange Rate (CER) basis as its Management Guidance. FY2024 Management Guidance CER % Change*4 Core Revenue Flat to slightly declining Core Operating Profit Approx 10% decline Core EPS Mid-10s% decline *4 Please refer to 1. Current State of the Takeda Group, (2) Business Performance for Fiscal 2023, (ii) Core Results (April 1, 2023 to March 31, 2024), Definition of Core financial measures and Constant Exchange Rate change, for the definition. Other assumptions used in preparing the FY2024 Reported Forecast and the Management Guidance The FY2024 reported forecast and the management guidance assume global VYVANSE sales of JPY 225.0 billion, a year-on-year decline of JPY 198.2 billion (49% decline at CER). Forward looking statements All forecasts in this document are based on information currently available to management, and do not represent a promise or guarantee to achieve these forecasts. Various uncertain factors could cause actual results to differ, such as changes in the business environment and fluctuations in foreign exchange rates. Should any significant event occur which requires the forecast to be revised, the Company will disclose it in a timely manner.

- 53 - (6) Financial Position and Income Summary (i) Financial Position and Income Summary of the Takeda Group (Billion JPY, unless otherwise indicated) 144th fiscal year 145th fiscal year 146th fiscal year 147th fiscal year April 1, 2020 to March 31, 2021 April 1, 2021 to March 31, 2022 April 1, 2022 to March 31, 2023 April 1, 2023 to March 31, 2024 Revenue 3,197.8 3,569.0 4,027.5 4,263.8 Operating profit 509.3 460.8 490.5 214.1 Profit before income taxes 366.2 302.6 375.1 52.8 Net profit for the year 376.2 230.2 317.0 144.2 Net profit for the year attributable to the owners of the Company 376.0 230.1 317.0 144.1 Basic earnings per share (JPY) 240.72 147.14 204.29 92.09 Total assets 12,912.3 13,178.0 13,957.8 15,108.8 Total equity 5,177.2 5,683.5 6,354.7 7,274.0 (Note) Consolidated financial statements of the Takeda Group are prepared under the International Financial Reporting Standards (IFRS). (ii) Overseas Revenue of the Takeda Group (Billions JPY, unless otherwise indicated) 144th fiscal year 145th fiscal year 146th fiscal year 147th fiscal year April 1, 2020 to March 31, 2021 April 1, 2021 to March 31, 2022 April 1, 2022 to March 31, 2023 April 1, 2023 to March 31, 2024 Overseas revenue 2,638.1 2,910.0 3,515.4 3,812.4 Proportion of overseas revenue to the Takeda Group Revenue (%) 82.5 81.5 87.3 89.4 (iii) R&D Expenses of the Takeda Group (Billions JPY, unless otherwise indicated) 144th fiscal year 145th fiscal year 146th fiscal year 147th fiscal year April 1, 2020 to March 31, 2021 April 1, 2021 to March 31, 2022 April 1, 2022 to March 31, 2023 April 1, 2023 to March 31, 2024 R&D expenses 455.8 526.1 633.3 729.9 Ratio of R&D expenses to the Takeda Group Revenue (%) 14.3 14.7 15.7 17.1

- 54 - For your reference, the "Financial Position and Income Summary of the Company" is as follows: (Billions JPY, unless otherwise indicated) 144th fiscal year 145th fiscal year 146th fiscal year 147th fiscal year April 1, 2020 to March 31, 2021 April 1, 2021 to March 31, 2022 April 1, 2022 to March 31, 2023 April 1, 2023 to March 31, 2024 Net sales 602.6 764.3 632.1 595.6 Operating income 121.1 293.7 136.1 48.1 Ordinary income 50.0 550.9 340.1 286.4 Net income 247.5 324.5 330.6 338.9 Net income per share (JPY) 158.45 207.50 213.06 216.60 Total assets 10,856.5 9,641.6 9,407.3 9,756.3 Net assets 4,434.9 4,294.9 4,206.2 4,088.2 (7) Main Businesses of the Takeda Group (as of March 31, 2024) The main businesses of the Takeda Group are research, development, production and marketing of pharmaceuticals.

- 55 - (8) Principal Subsidiaries (as of March 31, 2024) Name of company (major offices) Capital stock Percentage of total shares (%) Principal business United States Takeda Pharmaceuticals U.S.A., Inc. (Head office: Cambridge, Massachusetts, U.S.) US$21 (¥3 thousand) 100.0 Sale of pharmaceuticals, holding intellectual properties and internal group finance ARIAD Pharmaceuticals, Inc. (Head office: Cambridge, Massachusetts, U.S.) US$6 (¥1 thousand) 100.0 R&D of pharmaceuticals and holding intellectual properties Takeda Vaccines, Inc. (Head office: Cambridge, Massachusetts, U.S.) US$1 100.0 R&D of pharmaceuticals Takeda Development Center Americas, Inc. (Head office: Cambridge, Massachusetts, U.S.) US$1 100.0 R&D of pharmaceuticals Baxalta Incorporated (Head office: Bannockburn, Illinois, U.S.) US$10 (¥2 thousand) 100.0 Holding Company Dyax Corp. (Head office: Lexington, Massachusetts, U.S.) US$215 (¥33 thousand) 100.0 R&D, sale of pharmaceuticals and holding intellectual properties Takeda Ventures, Inc. (Head office: Cambridge, Massachusetts, U.S.) US$2 100.0 Investment Company Baxalta US Inc. (Head office: Bannockburn, Illinois, U.S.) US$1 100.0 R&D, production, sale of pharmaceuticals, and holding intellectual properties Shire Human Genetic Therapies, Inc. (Head office: Lexington, Massachusetts, U.S.) US$10 (¥2 thousand) 100.0 R&D, production, sale of pharmaceuticals, and holding intellectual properties BioLife Plasma Services LP (Head office: Bannockburn, Illinois, U.S.) US$0 100.0 Plasma collection Takeda Manufacturing U.S.A., Inc. (Head office: Cambridge, Massachusetts, U.S.) US$9 thousand (¥1 million) 100.0 Production of pharmaceuticals Europe and Canada Takeda Pharmaceuticals International AG (Head office: Opfikon, Switzerland) €5 million (¥872 million) 100.0 R&D of pharmaceuticals, supervision of sale of pharmaceuticals for the areas other than Japan, holding intellectual properties, supervision of global manufacturing and product supply for all regions

- 56 - Name of company (major offices) Capital stock Percentage of total shares (%) Principal business Takeda GmbH (Head office: Konstanz, Germany) €11 million (¥1,779 million) 100.0 Production, sale of pharmaceuticals, and holding intellectual properties Takeda Italia S.p.A. (Head office: Rome, Italy) €11 million (¥1,836 million) 100.0 Sale of pharmaceuticals Takeda Austria GmbH (Head office, Factory: Linz, Austria) €15 million (¥2,426 million) 100.0 Production, sale of pharmaceuticals, and holding intellectual properties Takeda France S.A.S. (Head office: Paris, France) €3 million (¥528 million) 100.0 Sale of pharmaceuticals Takeda UK Limited (Head office: London, U.K.) £50 million (¥9,537 million) 100.0 Sale of pharmaceuticals Takeda Ireland Limited (Head office: Kilruddery, Ireland) (Factory: Bray and Grange Castle, Ireland) €396 million (¥64,639 million) 100.0 Production of pharmaceuticals and holding intellectual properties Shire Pharmaceuticals International Unlimited Company (Head office: Dublin, Ireland) US$6,892 million (¥1,043 billion) 100.0 Holding Company Shire Acquisitions Investments Ireland Designated Activity Company (Head office: Dublin, Ireland) US$20 (¥3 thousand) 100.0 Group finance and treasury Shire Ireland Finance Trading Limited (Head office: Dublin, Ireland) US$3,613 million (¥547,294 million) 100.0 Group finance and treasury Takeda Canada Inc. (Head office: Toronto, Canada) CAD41 million (¥4,580 million) 100.0 Sale of pharmaceuticals Takeda Farmaceutica Espana S.A. (Head office: Madrid, Spain) €2 million (¥254 million) 100.0 Sale of pharmaceuticals Takeda Manufacturing Austria AG (Head office: Vienna, Austria) €100 thousand (¥16 million) 100.0 Production of pharmaceuticals Baxalta Manufacturing, S.a.r.l. (Head office: Neuchatel, Switzerland) 3 million Swiss franc (¥481 million) 100.0 Production of pharmaceuticals and holding intellectual properties Baxalta Innovations GmbH (Head office: Vienna, Austria) €36 million (¥5,931 million) 100.0 R&D of pharmaceuticals Takeda Pharma AB (Head office: Stockholm, Sweden) 2 million Swedish krona (¥28 million) 100.0 Sale of pharmaceuticals Takeda Pharma AG (Head office: Opfikon, Switzerland) 550 thousand Swiss franc (¥92 million) 100.0 Sale of pharmaceuticals Takeda Nederland B.V. (Head office: Hoofddorp, Nederland) €5 million (¥751 million) 100.0 Sale of pharmaceuticals Russia Takeda Pharmaceuticals Limited Liability Company (Head office: Moscow, Russia) (Factory: Yaroslavl, Russia) 126 thousand Russian ruble (¥207 thousand) 100.0 Production and sale of pharmaceuticals Latin America Takeda Distribuidora Ltda. (Head office: São Paulo, Brazil) 140 million Brazilian real (¥4,245 million) 100.0 Sale of pharmaceuticals Takeda Mexico S.A.de C.V. (Head office: Naucalpan, Mexico) 820 million Mexican peso (¥7,481 million) 100.0 Production and sale of pharmaceuticals Takeda Pharma Ltda. (Head office: Jaguariúna, Brazil) 7 million Brazilian real (¥215 million) 100.0 Production and sale of pharmaceuticals

- 57 - Name of company (major offices) Capital stock Percentage of total shares (%) Principal business Takeda Argentina S.A. (Head office: Buenos Aires, Argentina) 853 million Argentine peso (¥151 million) 100.0 Sale of pharmaceuticals Asia Takeda (China) Holdings Co., Ltd. (Head office: Shanghai, China) US$192 million (¥29,007 million) 100.0 Holding company in China Takeda (China) International Trading Co., Ltd. (Head office: Shanghai, China) US$22 million (¥3,257 million) 100.0 Sale of pharmaceuticals Takeda Pharmaceuticals Korea Co., Ltd. (Head office: Seoul, Korea) 2,100 million Korean won (¥236 million) 100.0 Sale of pharmaceuticals Takeda Development Center Asia, Pte. Ltd. (Head office: Singapore) S$5 million (¥561 million) 100.0 R&D of pharmaceuticals Tianjin Takeda Pharmaceuticals Co., Ltd. (Head office: Tianjin, China) US$155 million (¥23,454 million) 100.0 Production and sale of pharmaceuticals Takeda Manufacturing Singapore Pte. Ltd. (Head office: Singapore) US$305 million (¥46,252 million) 100.0 Production of pharmaceuticals Takeda APAC Biopharmaceutical Research and Development Company Limited (Head office : Shanghai, China) CNY50 million (¥1,047 million) 100.0 R&D of pharmaceuticals (Notes) 1. The figures in parentheses under the column “Capital stock” show the Japanese yen equivalents, calculated using the exchange rates as of March 31, 2024. 2. The figures for “Percentage of total shares (%)” include shares that are held indirectly through subsidiaries. 3. As of March 31, 2024, the number of consolidated subsidiaries (including partnerships) was 169 and the number of equity method associates was 16. 4. No subsidiaries fall under "Specific Wholly Owned Subsidiary" as described in the Ordinance for Enforcement of the Companies Act.

- 58 - (9) Major Offices of the Company (as of March 31, 2024) Head Office 1-1, Doshomachi 4-chome, Chuo-ku, Osaka Global Headquarters 1-1, Nihonbashi-Honcho 2-chome, Chuo-ku, Tokyo Plants Osaka Plant (located in Osaka), Hikari Plant (located in Hikari, Yamaguchi) and Narita Plant (located in Narita. Chiba) (Notes) 1. The Sales division is engaged in its activities at the hubs established by the Company in the major cities in Japan. 2. The Company conducts research activities in Fujisawa, Kanagawa, in Narita, Chiba and in Hikari, Yamaguchi.. (10) Employees (as of March 31, 2024) (i) Number of employees of the Takeda Group Number of employees Increase (decrease) from the previous fiscal year end 49,281 186 (Note) The number of employees represents the number of working employees. (ii) Status of employees of the Company Number of employees Increase (decrease) from the previous fiscal year end Average age Average length of employment (years) 5,474 (12) 43.3 14.6 (Note) The number of employees represents the number of working employees. (11) Principal lenders and loan amounts (as of March 31, 2024) Lender Loan balance Syndicated loans JPY 540,518 million The Norinchukin Bank JPY 80,000 million Sumitomo Mitsui Trust Bank, Limited JPY 50,000 million Shinkin Central Bank JPY 50,000 million Mizuho Trust & Banking Co., Ltd. JPY 30,000 million (Note) The syndicated loans are joint financing by several lenders arranged by Sumitomo Mitsui Banking Corporation.

- 59 - 2. Common Stock of the Company (as of March 31, 2024) (1) Total number of shares authorized to be issued by the Company 3,500,000,000 shares (2) Total number of issued shares 1,582,418,725 shares (including 7,514,277 shares of treasury stock) (3) Number of shareholders 652,774 (4) Principal Shareholders Name of Shareholder Number of shares held (thousands) Percentage of total shares (%) The Master Trust Bank of Japan, Ltd. (Trust account) 261,696 16.62 Custody Bank of Japan, Ltd. (Trust account) 86,763 5.51 THE BANK OF NEW YORK MELLON AS DEPOSITARY BANK FOR DEPOSITARY RECEIPT HOLDERS 60,085 3.82 JP Morgan Chase Bank 385632 37,232 2.36 State Street Bank West Client-Treaty 505234 33,756 2.14 Nippon Life Insurance Company 24,752 1.57 JP Morgan Securities Japan Co., Ltd. 23,396 1.49 SMBC Nikko Securities Inc. 22,032 1.40 SSBTC CLIENT OMNIBUS ACCOUNT 21,344 1.36 JP Morgan Chase Bank 385781 21,118 1.34 (Note) The percentage of total shares is based on the number of shares (1,574,904,448 shares) calculated by subtracting the number of treasury stocks from the total number of issued shares. (5) Shares delivered to Directors of the Company during this fiscal year as a consideration for the execution of duties Number of shares Number of people Directors who are not Audit and Supervisory Committee Members (excluding External Directors) 217,500 shares 3 Directors External Directors who are not Audit and Supervisory Committee Members 7,500 shares 3 Directors Directors who are Audit and Supervisory Committee Members 9,100 shares 4 Directors (Note) Shares delivered to Directors who retired in this fiscal year and previous fiscal years are included. (6) Material items on the Common Stock of the Company other than the items mentioned above (i) The Company has introduced the BIP (Board Incentive Plan) trust compensation system for Directors (excluding Directors residing outside of Japan who are not External Directors), based on the resolutions of the General Meetings of Shareholders and the resolutions of the Board of Directors made in accordance with such shareholders̕ resolutions. The number of shares of the Company held by the trust account for the BIP trust is 2,258,019 shares as of March 31, 2024. (ii) The Company introduces a stock grant ESOP (Employee Stock Ownership Plan) trust for certain employees including members of senior management of the Company in Japan, based on the resolution of the Board of Directors. The number of shares of the Company held by the trust account for the stock grant ESOP trust is 3,630,339 shares as of March 31, 2024.

- 60 - 3. Executives of the Company (1) Status of Directors (as of March 31, 2024) The status of Directors as of the end of this fiscal year is as follows: The Company's Board of Directors is composed of 3 internal directors and 12 external directors, with one of the external directors chairing the Board of Directors meeting, ensuring a robust corporate governance with an Audit and Supervisory Committee (ASC) which consists entirely of external directors. Furthermore, all members of both the Nomination and Compensation Committees must be external directors to ensure the election of directors and the compensation for directors via a transparent process based on objective and reasonable standards. The Board composition achieves a balance of knowledge, experience and capabilities necessary for the management of the Company, given the nature of its global business. The Board of Directors, with its appropriate composition and size, decides on the most important matters for the business operation of group and supervises the execution of the business, which is delegated to the President and CEO and the Takeda Executive Team (TET). Name Position Duty Important Positions Held Concurrently Christophe Weber President & Representative Director Chief Executive Officer Head of Global Business, Takeda Pharmaceuticals U.S.A., Inc. Andrew Plump Director President, Research & Development President, Research & Development, Takeda Development Center Americas, Inc. Costa Saroukos Director Chief Financial Officer Masami Iijima Director Chair of the Board of Directors meeting Chairperson of Nomination Committee Counselor, Mitsui & Co., Ltd. Olivier Bohuon Director Compensation Committee Member Jean-Luc Butel Director Nomination Committee Member Ian Clark Director Compensation Committee Member Steven Gillis Director Nomination Committee Member Managing Director, ARCH Venture Partners John Maraganore Director Michel Orsinger Director Nomination Committee Member Compensation Committee Member *Miki Tsusaka Director President, Microsoft Japan Co., Ltd. Koji Hatsukawa Director who is an ASC Member Head of ASC Yoshiaki Fujimori Director who is an ASC Member Nomination Committee Member Senior Executive Advisor, CVC Asia Pacific (Japan) Kabushiki Kaisha Emiko Higashi Director who is an ASC Member Chairperson of Compensation Committee Managing Director, Tomon Partners, LLC Kimberly A. Reed Director who is an ASC Member

- 61 - (Notes)1. The duties of the following Director was revised as of April 1, 2024, as described below: Name New Old Costa Saroukos Director Director, Chief Financial Officer 2. The Director marked with an * was newly elected and took office at the 147th Ordinary General Meeting of Shareholders held on June 28, 2023. 3. The Director who retired from office during this fiscal year is as follows: Representative Director Masato Iwasaki (retired on June 28, 2023) 4. Directors, namely, Masami Iijima, Olivier Bohuon, Jean-Luc Butel, Ian Clark, Steven Gillis, John Maraganore, Michel Orsinger and Miki Tsusaka, as well as Directors who are ASC Members, namely, Koji Hatsukawa, Yoshiaki Fujimori, Emiko Higashi and Kimberly A. Reed, are External Directors as prescribed under Article 2, Item 15 of the Companies Act. 5. Mr. Koji Hatsukawa, Director who is an ASC Member, is a Certified Public Accountant and has expert knowledge in finance and accounting. 6. The ASC Office, which is an administrative section dedicated to the ASC, is established to assist ASC’s operations. The effectiveness of audit is ensured by conducting a systematic audit utilizing the internal control system as well as collection of information on a regular basis such as attendance at important meetings and review of important documents and periodical hearing of reports relating to the business performance of the division in charge of executing the business operation. Thus, a full-time ASC member is not appointed. 7. There are no relationships between the Company and the organizations in which the External Directors concurrently serve that should be noted. 8. The Company has set “Internal criteria for independence of external directors of the Company” and has elected the External Directors based on those criteria. Since all the External Directors (i.e., the Directors ,namely, Masami Iijima, Olivier Bohuon, Jean-Luc Butel, Ian Clark, Steven Gillis, John Maraganore, Michel Orsinger and Miki Tsusaka, and the Directors who are ASC Members, namely, Koji Hatsukawa, Yoshiaki Fujimori, Emiko Higashi and Kimberly A. Reed) have met the requirements for Independent Directors based on the regulations of the financial instruments exchanges in Japan that the Company is listed on (e.g., Tokyo Stock Exchange, Inc.), the Company has appointed all of them as Independent Directors and submitted notifications to each of such exchanges. (2) Outline of the terms of the liability limitation agreement The Company has executed agreements with Non-Executive Directors, namely, Masami Iijima, Olivier Bohuon, Jean-Luc Butel, Ian Clark, Steven Gillis, John Maraganore, Michel Orsinger, and Miki Tsusaka, and Non-Executive Directors who are Audit and Supervisory Committee Members, namely, Koji Hatsukawa, Yoshiaki Fujimori, Emiko Higashi and Kimberly A. Reed, stating that the maximum amount of their liabilities for damages as set forth in Article 423, Paragraph 1 of the Companies Act shall be the amount provided by law. (3) Outline of the terms of the company indemnification agreement The Company has executed company indemnification agreements as defined in Article 430-2, Paragraph 1 of the Companies Act with Directors, namely, Christophe Weber, Andrew Plump, Costa Saroukos, Masami Iijima, Olivier Bohuon, Jean-Luc Butel, Ian Clark, Steven Gillis, John Maraganore, Michel Orsinger and Miki Tsusaka, and Directors who are Audit and Supervisory Committee Members, namely, Koji Hatsukawa, Yoshiaki Fujimori, Emiko Higashi and Kimberly A. Reed, providing that the Company shall indemnify expenses set forth in Article 430-2, Paragraph 1, Item 1 thereof and damages set forth in Article 430-2, Paragraph 1, Item 2 thereof within the scope permitted by the laws and regulations. (4) Outlines of the terms of the directors & officers liability insurance The Company has executed directors & officers liability insurance contracts as defined in Article 430-3, Paragraph 1 of the Companies Act with insurance companies, under which directors, statutory auditors and employees in managerial or supervisory positions of the Company or the Company’s group are insured. Such insurance covers damages which may arise from liability incurred by such insured persons in connection with the execution of their duties or claims made against such insured persons in relation to such liability unless any exclusion stipulated in the insurance policy applies. The Company bears the full amount of the premium for such insurance and any insured person does not bear any substantial amount of the premium. (5) Compensation and related matters for Directors

- 62 - 1. Director’s Compensation Policy The Company has formulated the “Director’s Compensation Policy” set forth below based on the resolution by the Board of Directors. The Company determines the composition and level of compensation of the Directors in accordance with the concept and procedure of this Policy. Director’s Compensation Policy ■ Standard Compensation Mix Model for Internal Directors The following are the guiding principles of the Company's compensation system for Directors to achieve management objectives under the corporate governance code: ◆ To attract, retain and motivate managerial talent to realize our Vision ◆ To increase corporate value through optimization of the Company’s mid- and long-term performance, while reinforcing our patient first values ◆ To be closely linked with company performance, highly transparent and objective ◆ To support a strong alignment with the interests of shareholders and enhance shareholder - oriented management perspective ◆ To encourage Directors’ spirit of challenge aligned with the values of Takeda-ism, perseverance ◆ To establish transparent and appropriate governance of Directors' compensation to establish the credibility and support of our stakeholders We aim to be competitive in the global marketplace to attract and retain talent who will contribute to Takeda’s continued transformation into a Global, Values-based, R&D-driven Biopharmaceutical Leader. Directors' compensation is intended to be competitive in the global market consisting of major global companies. Specifically, the global market data includes compensation data from major global pharmaceutical companies with which we compete, and from other major companies in Japan, the U.S. and Switzerland. 3-1. Internal Directors who are not Audit & Supervisory Committee Members The compensation of Internal Directors who are not Audit & Supervisory Committee Members (Since there are no Internal Directors who are Audit & Supervisory Committee Members in the Company, they are referred to simply as "Internal Directors" hereinafter from page 62 to 69.) consists of "Basic Compensation"(Base Salary and other fixed compensation (if applicable)), which is paid at a fixed amount and "Performance-based Compensation", which is paid as a variable amount based on company and other performance factors. "Performance-based Compensation" consists of an annual "Bonus (short-term incentive compensation) " to be paid based on financial and other performance results for each fiscal year, and a "Long-term Incentive Plan (stock compensation)" linked with long-term company performance results over a 3-year period and with Takeda's share price. Both Bonus and Long-term incentives represent a significantly higher proportion of Total Director Compensation putting Internal Directors’ pay at risk in alignment with the Company’s performance. The ratio of Long-term Incentives is particularly high within Performance-based Compensation in order to ensure the alignment of the interests of Internal Directors and shareholders and drive mid-term and long-term company value creation. The targets range from 100%-250% of Base Salary for "Bonus" and range from 200% to 600% of Base Salary for "Long- term Incentive", reflecting the market practices of global companies. Fixed Performance-based Compensation *The ratio of Bonus and Long-term Incentives to Base Salary is determined according to the Internal Director’s posi tion. 3-2. External Directors who are not Audit & Supervisory Committee Members The compensation of External Directors who are not Audit & Supervisory Committee Members consists of Basic Compensation, which is paid as a fixed amount, and Long-term Incentive (stock compensation). As part of the Basic Compensation, Chair Retainers are paid for the chair of the board of directors meeting, chairperson of the Compensation Committee, and chairperson of the Nomination Committee, in addition to the Board Retainer. Bonus is not available for this category of Director. The current compensation mix is "Basic Compensation" and "Long-term Incentive", which is a maximum of 100% of the Board Retainer. 1. Guiding Principles 2. Level of Compensation 3. Compensation Mix Bonus 100%-250% of Base Salary* Basic Compensation Long-term Incentive Plan (stock compensation) 200% to 600% or more of Base Salary*

- 63 - ■ Standard Compensation Mix Model for External Directors who are not Audit & Supervisory Committee Members ■ Standard Compensation Mix Model for Directors who are Audit & Supervisory Committee Members The compensation of External Directors who are not Audit & Supervisory Committee Members based outside of Japan may be adjusted to account for the impact of foreign exchange rates. Fixed 3-3. Directors who are Audit & Supervisory Committee Members The compensation of Directors who are Audit & Supervisory Committee Members consists of Basic Compensation, which is paid as a fixed amount, and Long-term Incentive (stock compensation). As part of the Basic Compensation, Committee Retainer is paid for External Directors who are Audit & Supervisory Committee Members, and Chair Retainers are also paid for External Directors who are head of the Audit & Supervisory Committee, chairperson of the Compensation Committee, and chairperson of the Nomination Committee, in addition to the Board Retainer. Bonus is not available for this category of Director. The current compensation mix is "Basic Compensation" and "Long-term Incentive", which is a maximum of 100% of the Board Retainer. The compensation of External Directors who are Audit & Supervisory Committee Members based outside of Japan may be adjusted to account for the impact of foreign exchange rates. Fixed 4-1. Internal Directors For Internal Directors, the Company has introduced a Long-term Incentive Plan that is allocated as 60% for the plan designed based on Performance Share Units (Performance Share Unit awards) and 40% for the plan designed based on Restricted Stock Units (Restricted Stock Unit awards). Performance Share Unit awards are tied to company performance results to strengthen the link between compensation and company performance and share price, and to reinforce Internal Directors’ commitment to increasing corporate value in the mid- and long-term. Restricted Stock Unit awards are linked only to share price. Annual Performance Share Unit Awards Performance Share Unit awards, which fall under Performance-based Compensation, will be linked to the latest mid- to long-term key performance indicators (KPIs) over a three-year performance period. KPIs are intended to be transparent and objective and may include top line revenues, cash flow, indicators on profit, R&D metrics and other performance factors. The payout range for Performance Share Unit awards is from 0% to 200% (100% at target), based on performance achievement. For Long- term Incentive awarded in 2019 and after, a two year holding period will be mandated, and this includes Restricted Stock Unit awards if and when shares become vested. Annual Performance Share Unit Awards Image Special Performance Share Unit Awards In addition to regular stock compensation, the company may, from time to time, award one-time special Performance Share Unit awards which are directly linked to point-in-time corporate initiatives and which are aligned with shareholder expectations. Performance against established KPIs for one-time special Performance Share Unit awards are determined independently each year over a three-year period, with shares becoming vested after the relevant performance metric(s) are determined to have been achieved for the applicable period. There is no post-vesting holding period established for one-time special Performance Share Unit awards. 4. Performance- based Compensation Basic Compensation Chair Retainers are paid for chairs Long-term Incentive Plan (stock compensation) Maximum of 100% of Board Retainer Basic Compensation Committee Retainer is paid for External Directors and Chair Retainers are paid for External Directors who are chairs Long-term Incentive Plan (stock compensation) Maximum of 100% of Board Retainer

- 64 - Special Performance Share Unit Awards (stock compensation) Image Annual Bonus (Short-Term Incentive) Bonuses will be paid based on performance achievement of annual goals. Bonuses will be paid in the range of 0% to 200% (100% at target) in accordance with the achievement of KPIs, which may include top line revenues, indicators on profit, and other performance factors established for a single fiscal year. For President and CEO, the annual bonus is weighted as 100% to the achievement of the specified Corporate KPI(s). For other Internal Directors that have divisional responsibilities, 75% of their annual bonus opportunity is linked to the achievement of the specified Corporate KPI(s) to drive their commitment to group-wide goals, while 25% is linked to the achievement of the division KPI. 4-2. Directors who are Audit & Supervisory Committee Members and External Directors The Long-term Incentive Plan (stock compensation) for Directors who are Audit & Supervisory Committee Members and External Directors consists of Restricted Stock Unit awards linked only to share price and is not otherwise linked to company performance results. The stock compensation awarded in 2019 and after will vest three years after the award date of base points used for the calculation and Directors will be required to hold at least 75% of their vested share portion until they cease service as a director (however, stock compensation awarded in or before 2018 will vest and be paid after they cease service as a director). Bonuses are not available for these categories of Director. Whole Picture of Director’s Compensation Directors who are not Audit and Supervisory Committee Members Directors who are Audit and Supervisory Committee Members Internal Directors External Directors External Directors Basic Compensation Bonus 2 Long-term Incentive Plan (stock compensation) Performance based 1 3, 4 Not linked to performance results 4 5 5 1 Includes Special Performance Share Unit awards 2 Varies from 0% to 200% in accordance with the achievement of KPIs, which may include top line revenues, indicators on profit, and other performance factors established for a single fiscal year 3 Varies from 0% to 200% in accordance with the achievement of KPIs, which may include top line revenues, cash flow, indicators on profit, R&D metrics, and other performance factors over a three-year performance period 4 During term of office 5 Vest and paid three years after the award date of the base points used for the calculation are granted 5-1. Compensation Committee The Compensation Committee, with all the Committee members being External Directors, has been established to serve as an advisory body for the Board of Directors to ensure the appropriateness of Directors' compensation and the transparency in its decision-making process. 5. Compensation Governance

- 65 - The level of compensation, compensation mix and performance-based compensation (Long-term Incentives and Bonus programs) for Directors are reviewed by the Compensation Committee before resolution by the Board of Directors. The Company delegated to the Compensation Committee, by resolution of the Board of Directors, the authority to determine Internal Directors’ individual compensation in order to ensure the objectivity and transparency in the decision- making process. In order to enhance transparency of the Company’s corporate governance, the Company has externally disclosed the Compensation Committee Charter as a part of the Company’s corporate governance documents. The Director’s Compensation Policy may continue to evolve and be revised to guide the development of compensation programs that align with Directors' accountabilities and responsibilities, shareholder value creation and Takeda-ism. 5-2. Recoupment Policy The Compensation Committee and Board of Directors adopted a clawback policy in 2020 and amended that policy in 2023. The amended policy provides that, in the event of a restatement of financial results, Takeda will, in accordance with SEC and NYSE rules, recover from its executive officers any erroneously paid incentive compensation, which consists of incentive-based compensation for the applicable recovery period that would not have been granted absent the restatement (i.e., mandatory clawbacks). In addition, in the event of a restatement and/or significant misconduct, the independent External Directors may require Takeda to recoup additional incentive and other contingent compensation. This would include all or a portion of the incentive and other contingent compensation received by any Internal Director, any other member of the Takeda Executive Team (TET), and any other individual designated by the independent External Directors, within the fiscal year, and the three (3) prior fiscal years preceding the date of the Board of Directors’ determination of the restatement or the date that independent External Directors determines that significant misconduct occurred, as applicable. The amended policy became effective on October 2, 2023 and, with respect to mandatory clawbacks in the event of a restatement, applies to incentive compensation beginning in Fiscal Year 2023. 2. Total Amount of Compensation for Directors The total amounts of compensation by type for Directors for this fiscal year (not including the salaries and bonuses paid to the relevant Directors for their work as employees) are as follows. Notes: 1. Those aforementioned include 1 Director who retired from the office at the close of the 147th Ordinary General Meeting of Shareholders on June 28, 2023. 2. Bonus amounts above for Directors who are not ASC Members are reserved for Bonuses for directors based on the projected performance attainment. The actual bonus amounts in the previous fiscal year were 361 million JPY against the reserved bonus amounts 385 million JPY stated in the Business Report of the previous fiscal year. 3. Among the total amount of the Compensation, by type, amounts reported in the Performance Share Unit awards and Restricted Stock Unit awards are the amount of costs recorded in this fiscal year. Category Number of people Total amount of the Compensation Total amount of the Compensation by type Basic Compensation Performance-based Compensation Non-monetary Remuneration Bonus Performance Share Units awards Restricted Stock Units awards Directors who are not ASC members 12 2,942 million JPY 657 million JPY 436 million JPY 1,094 million JPY 755 million JPY (External Directors) (8) (306 million JPY) (156 million JPY) - (150 million JPY) Directors who are ASC members 4 167 million JPY 90 million JPY - 77 million JPY (External Directors) (4) (167 million JPY) (90 million JPY) - (77 million JPY)

- 66 - 4. Although Performance Share Unit awards are categorized as both Performance-based Compensation and Non-monetary Remuneration, Performance Share Unit awards are reported as Performance-based Compensation. 5. In addition to the above, to account for the impact of foreign exchange rates on compensation for the term of office for 2022 (from the close of the Ordinary General Meeting of Shareholders held on June 29, 2022, to the close of the Ordinary General Meeting of Shareholders held on June 28, 2023) for 8 External Directors residing outside of Japan (including 2 External Directors who are ASC Members), the total amount of 32 million yen (including 6.9 million yen for External Directors who are ASC Members) were paid within the scope for External Directors in the basic compensation per month for Directors who are not ASC Members and the basic compensation per month for Directors who are ASC Members, as per the resolution of the 140th Ordinary General Meeting of Shareholders held on June 29, 2016. Of this amount, 1.2 million yen (including 0.2 million yen for External Directors who are ASC Members) is compensation for this fiscal year. 3. Resolutions at General Meeting of Shareholders regarding Director Compensation etc., 1. Resolutions regarding Directors excluding ASC Members [1] The basic compensation is a fixed amount depending on each position, and its total amount per month is no more than 150 million JPY (within this amount, no more than 30 million JPY per month is for External Directors) (based on a resolution of the 140th Ordinary General Meeting of Shareholders held on June 29, 2016). There were 11 Directors, including 6 External Directors, related to this resolution as of the end of the Ordinary General Meeting of Shareholders. [2] Bonus for each fiscal year is resolved at the Ordinary General Meeting of Shareholders. [3] Stock compensation (Performance Share Unit awards and Restricted Stock Unit awards) is based on the resolution of the 143rd Ordinary General Meeting of Shareholders held on June 27, 2019. The upper limit of the amount contributed for that stock compensation and the number of shares to be granted is as follows (There were 11 Directors, including 8 External Directors, related to this resolution as of the end of the Ordinary General Meeting of Shareholders). (A) Stock compensation granted to Internal Directors (excluding Internal Directors residing outside of Japan): Upper limit of 4.5 billion JPY per year for three consecutive fiscal years (the upper limit of the number of stocks to be granted is calculated by dividing the amount of the above- mentioned upper limit by the closing price of the stocks of the Company at the Tokyo Stock Exchange on a predetermined day for this fiscal year) (B) Stock compensation granted to External Directors who are not ASC Members: Upper limit of 0.3 billion JPY for each fiscal year (the upper limit of the number of stocks to be granted is calculated by dividing the amount of the above-mentioned upper limit by the closing price of the stocks of the Company at the Tokyo Stock Exchange on a predetermined day for this fiscal year) 2. Resolutions regarding Directors (ASC Members) [1] The basic compensation is a fixed amount depending on each position, and its total amount per month is no more than 15 million JPY (based on a resolution of the 140th Ordinary General Meeting of Shareholders held on June 29, 2016). There were 4 Directors related to this resolution as of the end of the Ordinary General Meeting of Shareholders. [2] Stock compensation (Restricted Stock Unit awards) for Directors (ASC Members) is based on a resolution of the 143rd Ordinary General Meeting of Shareholders held on June 27, 2019, for which no more than 200 million JPY will be contributed for this fiscal year. The upper limit of the number of stocks to be granted is calculated by dividing the amount of the above-mentioned upper limit by the closing price of the stocks of the Company at the Tokyo Stock Exchange on a predetermined day for this fiscal year. There were 4 Directors related to this resolution as of the end of the Ordinary General Meeting of Shareholders. 4. Delegation of authority to make decisions on individual compensation for Directors As stated in the governance section of 1. the Director’s Compensation Policy (5. Compensation Governance), in order to ensure the appropriateness of Directors' compensation and transparency in its decision-making process,

- 67 - based on the resolution by the Board of Directors, the authority to determine individual compensation for Internal Directors has been delegated to the Compensation Committee. Through the procedures based on such governance, the Compensation Committee determined the amount of individual compensation for Internal Directors for this fiscal year. In this fiscal year, the Compensation Committee was comprised of the following members: Emiko Higashi (Chairperson and ASC member), Olivier Bohuon, Ian Clark and Michel Orsinger, all of whom are External Directors. 5. Performance-based Compensation The following sets forth the methodologies for determining performance-based compensation (Bonus (Short-Term Incentive (STI)) and the Performance Share Unit (PSU) awards as part of the Long-Term Incentives Plan) and key performance indicators (“KPIs”) for determining performance-based compensation for Directors, along with the rationale for each KPI, the weight of each KPI in the total score, the target goal, the result, the final performance scores and the payout rate based on the final performance scores. 1. Annual Bonus (STI) Annual STI cash payout is calculated as follows: Annual STI Payout Calculation for CEO Base Salary × STI Target % × STI Payout Multiple (based on Corporate KPI performance) = STI Payout Annual STI Payout Calculation for Internal Directors (other than CEO) Base Salary × STI Target % × STI Payout Multiple (based on 75% Corporate KPI performance + 25% Division KPI performance) = STI Payout The STI target range is from 100% to 250% of Base Salary for “Bonuses” and reflects the market practices of global companies. STI Payout Multiple (STI payout rate based on KPI performance) used for Bonuses varies from 0% to 200% in accordance with the achievement of KPIs, which may include top line revenues and indicators on profit, and other performance factors established for a single fiscal year. Payout Scores for specific Corporate KPIs are calculated and determined based on pre-established performance and payout ranges. The targets and the results of Corporate KPIs related to STI for the FY2023 are as follows: KPI Rationale Weight (A) Target Result Performance Achievement (% of Target) Payout Score (B) Weighted Payout Score (A) x (B) Total Core Revenue* • Key indicator of growth, including pipeline delivery • Important measure of success within the industry 45% 4,021.7 billion JPY 4,153.2 billion JPY 103.3% 165.4% 74.4% Growth and Launch Product Incremental Core Revenue • Growth Products: Emphasis on subset of revenue that is a key driver of future revenue growth • Launch Products: Key indicator of driving pipeline growth and commercial revenue success 15% 245.1 billion JPY 194.8 billion JPY 79.5% 0% 0%

- 68 - Total Core Operating Profit • Measure of margin achievement while ensuring expense discipline • Reflects synergy capture • Communicated to shareholders as a key measure of Takeda success post Shire acquisition 40% 1,073.5 billion JPY 1,127.2 billion JPY 105.0% 133.3% 53.3% Corporate KPI Payout Multiple 127.7% * In the FY2023, the payout score was reduced by an adjustment made to remove the effect of hyperinflation in certain countries. Division KPIs related to Bonuses for Internal Directors (other than the CEO) are set according to each division’s specific business and organizational goals which can clearly represent each division’s performance. The performance scores are expected to exceed 100%. Please refer to 1.(2)(ii) Core Results (April 1, 2023 to March 31, 2024) for definition of Core financial measures. 2. Long-Term Incentives (LTI) Plans The LTI framework aligns the long-term strategy with shareholder returns, while also promoting retention of critical global executive talent. Regarding PSU awards, which represent 60% of the standard points allocated to each Internal Director as part of the Long-Term Incentives Plan, the number of PSUs earned and granted to Internal Directors is calculated as follows: Target PSU Awards (Standard Points ((Target Number of Units)) × PSU Payout Multiple (based on KPI performance) = PSUs earned The PSU payout multiple ranges from 0% to 200%, based on performance of KPIs, such as top line revenues, cash flow, indicators on profit, R&D metrics, and other performance factors over a three-year performance period. The number of shares to be vested to Internal Directors based on the PSUs earned according to the achievement of company performance objectives are determined as one share per one unit. After a certain period after grant, 50% of the PSUs earned are vested as stock and the remaining are paid in cash. The targets and the results of KPIs related to PSU awards from FY2021 - 2023 are as follows: KPI*1 Weight (A) Target Result Performance Achievement (% of Target) Payout Score (B) Weighted Payout Score (A)x(B) 3-year Accumulated Underlying Revenue*2 25% 10,854.3 billion JPY 10,900.4 billion JPY 100.4% 108.5% 27.1% Aggregated FY21-23 Underlying Core Operating Profit Margin 25% 32.0% 28.0% 87.5% 0% 0% 3-year Accumulated Free Cash Flow*3 25% 2,100.9 billion JPY 2,212.7 billion JPY 105.3% 135.5% 33.9% R&D Pivotal Study Start and Approvals 25% - - 105.7% 112.6% 28.1% 3-year Relative TSR Modifier +/-20% points 0% point PSU Payout Multiple 89.1%

- 69 - *1 Each KPI has been set in order to align the long-term strategy with shareholder returns, while also promoting the retention of critical global executive talent. *2 In the FY2023, the payout score was reduced by an adjustment made to remove the effect of hyperinflation in certain countries. *3 Free cash flows excluding upfront payment related to the acquisition of TAK-279 were used for FY2022 and FY2023 to exclude the impact of a significant one-time event which was not predicted in the initial target from a consistent performance evaluation standpoint. 6. Non-monetary Remuneration Non-monetary Remuneration (Long-Term Incentive Plan) includes the following. With respect to Restricted Stock Unit (RSU) awards as part of the Long-Term Incentives Plan, based on the standard points determined according to the Director’s professional duties and responsibility, regardless of company performance, the share conversion units are calculated by multiplying the percentage for each Director below and are granted to the Directors. The number of shares to be vested to each Director is one share per one unit. Directors Percentage of RSU awards in Total LTI Internal Directors 40% External Directors who are not ASC members 100% Directors who are ASC members 100% Regarding the number of share conversion units to be vested in a certain period after the grant for Internal Directors, and 3 years after the grant of standard points for External Directors who are not ASC members and Directors who are ASC members, 50% of the share conversion units are vested as stock and the remaining are paid in cash. As for Performance Share Unit awards as part of Long-Term Incentives, please refer to 5.2 above. 7. Rationale that compensation for each Director (excluding ASC members) is in line with Director's Compensation Policy As stated in 5. Compensation Governance in section 1. Director’s Compensation Policy, in order to provide for objectivity and transparency in the compensation setting process, based on the resolution by the Board of Directors, the Compensation Committee has been delegated the authority to make decisions on individual compensation for Internal Directors. Individual compensation for External Directors who are not ASC members proposed by the Compensation Committee is approved by the Board of Directors. The level of compensation, compensation mix, and performance-based compensation (Short- and Long-term Incentive programs) for Directors is reviewed by the Compensation Committee from a multilateral perspective, consistent with the Director’s Compensation Policy stated above. Based on the resolution by the Board of Directors, the Compensation Committee was delegated authority to make decisions on individual compensation and determined the amount of individual compensation for Internal Directors for this fiscal year. The Compensation Committee proposed the amount of compensation for External Directors who are not ASC members to the Board of Directors. Therefore, after confirming the review of the process and the content of the proposal of the Compensation Committee, the Board of Directors believes that the individual compensation for Internal Directors and External Directors who are not ASC members is aligned with the Director’s Compensation Policy stated above.

- 70 - (6) External Directors Major activities during this fiscal year and the summary of the duties which were conducted by the External Directors with regard to the roles which the Company had expected them to fulfill. Name Number of meetings attended Major activities during this fiscal year and the summary of the duties which were conducted by the External Directors with regard to the roles which the Company had expected them to fulfill Board of Directors Audit and Supervisory Committee Directors Masami Iijima 8/8 - He actively participated in the discussions at the Board of Directors meetings by leveraging his deep insights from extensive experience in various fields including corporate governance and risk management as well as global management of the company. Also, he facilitated the Board of Directors meetings and Nomination Committee meetings as the chairperson as well as led meetings of External Directors, which contributed to the making of fair and appropriate decisions and securing sound management in the Company. Olivier Bohuon 7/8 - He actively participated in the discussions at the Board of Directors meetings and Compensation Committee meetings by leveraging his deep insights from extensive experience in the management of global pharmaceutical and healthcare businesses in the U.S. and Europe, and his remarkable expertise especially in the area of marketing in the overall healthcare business, which contributed to the making of fair and appropriate decisions and securing sound management in the Company. Jean-Luc Butel 8/8 - He actively participated in the discussions at the Board of Directors meetings and Nomination Committee meetings by leveraging his deep insights from extensive experience in the management of business at major global healthcare companies in the U.S., Europe and Asia, which contributed to the making of fair and appropriate decisions and securing sound management in the Company. Ian Clark 7/8 - He actively participated in the discussions at the Board of Directors meetings and Compensation Committee meetings by leveraging his deep insights from extensive experience in the management of global healthcare companies in Europe and Canada, and his remarkable expertise especially in marketing in the area of oncology and operations of the biotechnology division of a healthcare company, which contributed to the making of fair and appropriate decisions and securing sound management in the Company. Steven Gillis 8/8 - He has a Ph.D. in Biology and has served in several pivotal positions at global healthcare companies in the U.S. and Europe. He actively participated in the discussion at the Board of Directors meetings and Nomination Committee meetings leveraging such extensive experience and his remarkable expertise especially in the area of healthcare businesses for immunological therapy, which contributed to the making of fair and appropriate decisions and securing sound management in the Company. John Maraganore 8/8 - He actively participated in the discussions at the Board of Directors meetings by leveraging his deep insights from extensive experience in management of global business in the pharmaceutical industry, which contributed to the making of fair and appropriate decisions and securing sound management in the Company.

- 71 - Name Number of meetings attended Major activities during this fiscal year and the summary of the duties which were conducted by the External Directors with regard to the roles which the Company had expected them to fulfill Board of Directors Audit and Supervisory Committee Michel Orsinger 8/8 - He actively participated in the discussions at the Board of Directors meetings, Nomination Committee meetings and Compensation Committee meetings by leveraging his deep insights from extensive experience in the management of business at major healthcare companies in the U.S. and Europe, which contributed to the making of fair and appropriate decisions and securing sound management in the Company. Miki Tsusaka 7/7 - She actively participated in the discussions at the Board of Directors meetings by leveraging her wide expertise in global business, strategy and data & digital, which contributed to the making of fair and appropriate decisions and securing sound management in the Company. Directors who are Audit and Supervisory Committee Members Koji Hatsukawa 8/8 8/8 He has wide-ranging experience and expertise in the area of corporate finance and accounting as a certified public accountant. He contributed to the making of fair and appropriate decisions and securing sound management in the Company by actively participating in the discussions at the Board of Directors meetings based on such experience and expertise. He also contributed to the realization of the mission of Audit and Supervisory Committee: to ensure the sound and continuous growth of the Company, realize the creation of mid-and long- term corporate value, and establish a good corporate governance system that accommodates society’s trust through supervision and audit. Yoshiaki Fujimori 8/8 8/8 He actively participated in the discussions at the Board of Directors meetings and Nomination Committee meetings by leveraging his insights from extensive experience in global management of healthcare companies, which contributed to the making of fair and appropriate decisions and securing sound management in the Company. He also contributed to the realization of the mission of Audit and Supervisory Committee: to ensure the sound and continuous growth of the Company, realize the creation of mid-and long-term corporate value, and establish a good corporate governance system that accommodates society’s trust through supervision and audit. Emiko Higashi 8/8 8/8 She actively participated in the discussions at the Board of Directors meetings and Compensation Committee meetings by leveraging her extensive experience and wide expertise on healthcare, technology and financial industries, which contributed to the making of fair and appropriate decisions and securing sound management in the Company. She contributed to the realization of the mission of Audit and Supervisory Committee: to ensure the sound and continuous growth of the Company, realize the creation of mid-and long-term corporate value, and establish a good corporate governance system that accommodates society’s trust through supervision and audit.

- 72 - Name Number of meetings attended Major activities during this fiscal year and the summary of the duties which were conducted by the External Directors with regard to the roles which the Company had expected them to fulfill Board of Directors Audit and Supervisory Committee Kimberly A. Reed 7/7 8/8 She actively participated in the discussions at the Board of Directors meetings by leveraging her extensive U.S. domestic and international experience, leadership and wide expertise, which contributed to the making of fair and appropriate decisions and securing sound management in the Company. She contributed to the realization of the mission of Audit and Supervisory Committee: to ensure the sound and continuous growth of the Company, realize the creation of mid-and long- term corporate value, and establish a good corporate governance system that accommodates society’s trust through supervision and audit. (Notes) Ms, Miki Tsusaka, Director, was elected at the 147th Ordinary General Meeting of Shareholders held on June 28, 2023 and took office as the Director. Accordingly, the Board of Directors meetings to be attended by her are the meetings held after she took office as a Director.

- 73 - 4. Accounting Auditor (1) Name of Accounting Auditor KPMG AZSA LLC (2) Amount of fee, etc. of Accounting Auditor for this Fiscal Year (i) Amount of fee, etc. for this fiscal year JPY 1,370 million (ii) Total amount of cash and other financial benefits to be paid by the Company and its subsidiaries JPY 2,614 million (Notes) 1. As the audit agreement between the Company and its Accounting Auditor does not differentiate the amount of fee, etc. for audit under the Companies Act from those for audit under the Financial Instruments and Exchange Act and such differentiation is impossible in practice, the above amounts show the total fee, etc. for both audits. 2. The Audit and Supervisory Committee reviews and examines the audit plan of the Accounting Auditor, the status of audit by Accounting Auditor and the rationale for calculating the estimated audit fee based on the Guideline of Practice for Cooperation with Accounting Auditor published by Japan Audit & Supervisory Members Association. As a result of such review and examination, the Audit and Supervisory Committee agreed with the fee, etc. of the Accounting Auditor pursuant to Article 399, Paragraph 1 of the Companies Act. 3. As for the subsidiaries of the Company located overseas set forth in "1. Current State of the Takeda Group, (8) Principal Subsidiaries (as of March 31, 2024)", audit firms other than KPMG AZSA LLC perform audit for the financial statements. (3) Non-audit services The Company commissions to the Accounting Auditor the non-audit services which fall under services other than the services set forth in Article 2, Paragraph 1 of the Certified Public Accountants Act in respect of services for “Services for consent letter on Form S-8”. (4) Decision-Making Policy on Dismissal or Rejection of the Reappointment of Accounting Auditor If the Accounting Auditor is determined to fall under any of the events prescribed in each item of Article 340, Paragraph 1 of the Companies Act, or if an event which has a material adverse effect on the audit of the Company occurs, including, but not limited to, the case in which such Accounting Auditor’s auditing license is suspended, the Accounting Auditor shall be dismissed by the Audit and Supervisory Committee based on the approval of all members thereof. In addition, the Audit and Supervisory Committee, taking into consideration the audit quality, the quality control and independence of the Accounting Auditor and other factors, shall determine whether or not the Accounting Auditor will be reappointed.

- 74 - 5. Overview of the Systems to Ensure the Appropriateness of Operations of the Company and the Status of Implementation of such Systems (1) Overview of the systems to ensure the appropriateness of operations The Company regards internal control, together with risk management, as an important component of corporate governance and has developed its internal control system as described below. (i) Systems to ensure the appropriateness of operations in the Takeda Group • The Company’s “Corporate Philosophy,” consisting of its “Purpose,” “Values: Takeda-ism,” “Vision” and “Imperatives,” permeates the entire Takeda Group. These principles serve as the foundation of the Takeda corporate culture. In addition, the Company is continuously working to strengthen its compliance system through the dissemination of the "Takeda Global Code of Conduct" and by developing ethics and compliance programs. • As a “company with an Audit and Supervisory Committee (ASC),” the Company has established a system that enables the ASC to effectively perform its duties relating to audit and supervision, and has increased the proportion and diversity of External Directors in order to ensure the transparency and objectivity of the Board of Directors (BOD). • The Company has voluntarily established its Nomination Committee and Compensation Committee, as advisory bodies for the BOD. Both committees ensure objectivity and fairness in the selection and compensation of Directors by having only External Directors as committee members, including the Chairperson. • The Company has established the below management committees in order to properly deliberate and decide on important matters: ○ Business & Sustainability Committee: responsible for corporate/business and sustainability-related matters ○ Portfolio Review Committee: responsible for R&D and product related matters ○ Risk, Ethics & Compliance Committee: responsible for risk management, business ethics and compliance matters. • The Company has established the Takeda Executive Team (TET), which consists of the President & CEO and the heads of the divisions of the Takeda Group, in order to strengthen its global business management and deepen collaboration among various divisions. • The Company has established the “Takeda Group’s Management Policy (T-MAP),” which summarizes the Company’s business and operations, decision-making and reporting structure, important operational rules, and applies it to all divisions and subsidiaries of the Takeda Group. In addition, each TET member establishes rules for operations and delegation of authority in each division and subsidiary to ensure that operations are conducted appropriately. • The Company has developed a management system across the Takeda Group by establishing Global Policies such as business resilience, Environment, Health and Safety (EHS) and raising & handling concerns of potential misconduct. • The Company has established a Quality Management System (QMS), which includes documented requirements and procedures. The Company conducts audits of, and monitors, compliance with these documents. This helps to ensure proper operations in research and development, manufacturing and product quality, as well as compliance with the laws and regulations of the pharmaceutical industry (GxP). • The Company has established the Group Internal Audit (GIA), an independent assurance function within Takeda Group, to support the enhancement and protection of organizational value through its audit activities. The GIA department develops and maintains an audit quality assurance and improvement program and conducts internal audit activities in accordance with the “International Standards for the Professional Practice of Internal Auditing (IIA Standards)” issued by the Institute of Internal Auditors. (ii) System for retention and management of information concerning the execution of the duties of Directors • The Company has established the “Global Records and Information Management (RIM) Policy” and properly retains and manages the BOD meeting minutes, approvals of management decisions, and other information concerning the execution of the duties of Directors. (iii) Rules and other systems for managing the risk of loss • The Company has established an integrated system that brings together the three areas of enterprise risk management, business continuity management, and crisis management based on the “Global Business Resilience Policy.” ○ The Company conducts annual enterprise risk assessment for the identification, evaluation, and mitigation planning for prioritized risks. ○ The Company develops business continuity plans for major risks and essential business areas. ○ The Company formulates crisis management plans to identify, manage and recover from a crisis and responds to it by organizing a Crisis Management Committee according to the level of impact. • The Company has established the principles and processes to identify, monitor and report high-risk business activities based on the “Global Monitoring Policy.” • The Company has established a patient safety and quality management framework, under both normal state and crisis mode, to initiate necessary actions for patient safety and quality issues including product recall.

- 75 - (iv) System to ensure that the duties of Directors are executed efficiently • Under the provisions of its Articles of Incorporations, the Company has established a structure that delegates a certain degree of decision-making authorities with respect to business execution to certain Directors, which enables the BOD to focus more on business strategies, internal controls and other important business matters of the Takeda Group. • These matters delegated to certain Directors are discussed and decided at the appropriate management committees, to ensure an agile and effective decision-making process. • The Company has established delegation of authority and decision-making rules such as the "Board of Directors Charter" and "T-MAP" to ensure the duties of the Directors are executed in an appropriate and efficient manner. (v) Systems to ensure that Directors and employees comply with laws and regulations and the Company’s Articles of Incorporation in executing their duties • The Company has established a dedicated department responsible for business ethics and compliance in order to strengthen group-wide compliance systems. • The Company has established its Code of Conduct, global policies (prohibition of bribery, handling of personal information, prohibition of insider trading, etc.) and other compliance-related internal rules, and implements training programs throughout the Takeda Group. • The Company has established global policies and internal regulations for interactions with healthcare entities, patient organizations, and government entities to comply with laws and regulations, which are essential for pharmaceutical companies. • The Company has established guidelines for raising and handling concerns of potential misconduct and has procedures for employees to remain anonymous and ensure their confidentiality through the Takeda Ethics Line. (vi) System to ensure the reliability of financial reporting • The Company ensures the reliability of disclosed materials by establishing and implementing an internal control system for financial reporting based on the 2013 Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). (vii) Basic Views on Eliminating Anti-Social Forces • The Company’s basic policy is to eliminate any relationship, including normal transactions, with antisocial forces that pose a threat to the order or safety of civil society. The Company works to avert any damage from antisocial forces by maintaining close contact with the police, etc., collecting information, and providing the information and training opportunities internally. (viii) System to ensure that the audits by the Audit and Supervisory Committee are conducted effectively The Company has established the following system that defines the roles, authority, duties, etc. of the ASC through the “Audit and Supervisory Committee Charter,” as well as internal guidelines regarding the audit and supervision of the ASC. 1) Matters related to ensuring the independence from the Directors, of employees who assist the ASC, and the effectiveness of instructions given to such employees by the ASC: ・ The ASC Office is established, and dedicated staff members are appointed, in order to assist ASC in the execution of duties under the direction of the ASC. ・ The appointment, personnel changes, personnel evaluations and other matters related to the dedicated staff members require the consent of the ASC. 2) Structure for the Directors and employees to report to the ASC, and other reporting structures related to the ASC: ・ The ASC is informed on matters concerning the Company’s basic management policy and plans, and material matters including those related to subsidiaries and affiliates of the Company. ・ Any facts that could cause significant damage to the Takeda Group need to be immediately reported to the ASC. ・ The ASC can access the minutes and materials of important meetings at any time. ・ The Company has established a system to ensure that the Directors and employees, etc. would not be subject to any unfavorable treatment for reporting to the ASC. 3) Other systems to ensure that audits by the ASC are performed effectively: ・ The ASC can conduct systematic audits in cooperation with the internal audit division (to which the ASC is authorized to give instructions), the internal control promotion division and the accounting auditor. ・ Expenses necessary for the execution of duties by the ASC and the ASC members are borne by the Company.

- 76 - (2) Overview of the status of the implementation of systems to ensure the appropriateness of operations During this fiscal year, the Company made efforts to appropriately implement the systems described in (1) above. The major efforts made by the Company during this fiscal year that are considered important for internal control, are as follows: [Dissemination of the Company’s Corporate Philosophy] • TET members including the President & CEO, are working to permeate the Company’s Corporate Philosophy throughout the Takeda Group and to its employees. This philosophy includes the company's “Purpose,” “Values: Takeda-ism,” “Vision” and “Imperatives.” They are achieving this through various means such as delivering internal messages and holding town hall meetings. [Strengthening of the Corporate Governance Structure] • In 2016, the Company transitioned to a “company with an Audit and Supervisory Committee (ASC)” and has since increased the proportion and diversity of its External Directors. This was done to ensure that the Board of Directors (BOD) and the ASC can fulfill their respective roles more appropriately. As of the end of this fiscal year, the BOD consists of 15 members (including three female Directors), of which 12 are External Directors (with the three other Directors being referred to herein as “Internal Directors”). Five Directors are Japanese and ten are foreign nationals. All External Directors meet the applicable criteria of independence established by the financial instruments exchanges. • All ASC members, including the head, are External Directors. • The Company has voluntarily established the Nomination Committee and the Compensation Committee as advisory bodies for the BOD. All members of each Committee, including the Chairperson, are External Directors. [Status of the BOD] • The BOD held eight meetings during this fiscal year, chaired by an External Director. Each Director, drawing from their diverse backgrounds, made appropriate statements from their respective points of view. • As mentioned above, the BOD delegates the authority to decide on important business execution matters to the Internal Directors. This allows the BOD to allocate more time to deliberate on issues that can have a significant impact on the Takeda Group and its management strategies, and oversee the performance of the Internal Directors in executing the business. • Prior to each BOD meeting, External Directors receive an explanation of the meeting agenda from the Internal Directors . In addition, when new External Directors are appointed, the Company ensures that they thoroughly understand their legal obligations and provides them with information on the Company’s business environment, strategy, etc. to deepen their understanding. • During the BOD meetings, each External Director actively participates in the discussions and expresses their opinions on the agenda items. They provide valuable insights based on their broad experience in corporate management or their deep expertise in specialized areas such as accounting and law. • An evaluation of this fiscal year’s performance and effectiveness of the BOD was conducted by a third-party organization through individual interviews with all of the Directors. The interview focused on key evaluation items such as “Strategic Alignment & Engagement,” “Composition & Structure,” “Processes & Practices,” “Management Oversight,” and “Board Culture and Dynamics.” In addition, the Directors were also requested to make self- evaluations on the “oversight by ASC and Nomination Committee.” After incorporating the analysis and recommendations made by the third-party organization, the overall evaluation result was explained by the third-party organization and discussed by all Directors. The Compensation Committee members conducted a self-evaluation on the “Effectiveness of the Compensation Committee” through questionnaires which were created with support from a third-party organization. The Compensation Committee reported to the BOD about the results of the self-evaluation and actions for improvement. • Through these evaluation processes, it was concluded that the BOD was working effectively, confirming that (i) there were no new material concerns which were pointed out (ii) there is effective leadership in management and the Board and, (iii) governance is working robustly, especially as the reporting from the advisory committees to the BOD was strengthened. In addition, the BOD confirmed certain improvements from the previous fiscal year concerning “content of Board discussions and practice of Board meeting“ and “optimal Board composition.” • The BOD also confirmed the effectiveness of the ASC, Nomination Committee and Compensation Committee and their contributions to the robust corporate governance of the Company. [Efforts to develop the internal control system in the Takeda Group] • For matters other than those that need to be resolved by the Company’s decision-making bodies (specifically, the BOD, the Business & Sustainability Committee, the Portfolio Review Committee, and the Risk, Ethics & Compliance Committee), decision-making authority is delegated to the TET members which consists of the President & CEO and the heads of the Takeda Group. The delegation of authority from TET members to their subordinates is conducted based on the “Global Policy - Delegation of Authority.” • The Group Internal Audit (GIA) department conducted an internal audit of each business unit/function of the Company and each group company based on the “Group Internal Audit Charter,” and reported the results to the President & CEO, ASC, and BOD. In addition, the GIA department conducted verification procedures to assess the effectiveness of internal control systems for financial reporting and reported the results to the Global Finance

- 77 - division. • The Global Finance division confirmed the effectiveness of the internal controls of financial reporting of the Company’s business unit/function. This was confirmed based on (i) the results of its testing program, which evaluated the design and operating effectiveness of our controls, as well as (ii) answers to self-assessment through questionnaires received from the heads of each business unit/function of the Company. In addition, the Global Finance division reported the final assessment, including the results of the testing, to the Chief Financial Officer (CFO), President & CEO, ASC and BOD. • The Global Quality division maintained the Company’s commitment to, and vision for quality, and conducted global quality assurance for the Takeda Group based on the “Global Quality Policy.” • The Corporate EHS department confirmed the roles and responsibilities of its personnel to effectively monitor and execute the Company’s environmental, occupational health and safety management activities. Additionally, based on Takeda's "Global Environment, Health, and Safety Policy and Position" and other publicly available Takeda environmental positions, the Corporate EHS department sets specific targets and conducted internal audits of the Takeda group from the perspectives of environmental management, occupational health and safety, and compliance. [Efforts to promote compliance] • The Company monitored potentially high-risk business activities, and made continuous improvements based on identified root causes. • Takeda Group’s compliance-related issues were regularly reported to the Risk, Ethics & Compliance Committee and the ASC, and to the BOD and the TET in a timely manner. [Efforts relating to risk management] • The principal enterprise risks and their mitigation measures of this fiscal year were discussed and validated at the Risk, Ethics & Compliance Committee through an enterprise risk assessment report. • The enterprise risk assessment report was discussed and approved by the BOD. Responsibility for execution of the risk mitigation measures was delegated to TET risk owners. • Other concrete efforts relating to risk management for this fiscal year are as follows: ✓ Through the risk coordinator community within the Takeda Group, the Company promotes upskilling in risk management practices and knowledge sharing. The Company also uses a simple and user-friendly enterprise risk assessment tool, which facilitates a single view of risk across the Company. Based on this technology- based solution, the Company expects to promote efficiency and improve its ability to analyze risk data and trends, and take a more data-driven approach. ✓ In addition, the Company undertakes educational initiatives and simulations for the purpose of enhancing processes and level of proficiency associated with crisis management activities such as pandemic situations, shortages of critical therapies and market actions, natural disasters, and geopolitical risks. ✓ With respect to product quality risk, the Company integrates the identification, assessment and control of risks into its Quality Management System and provides risk management tools, training and support to employees who are involved in R&D, manufacturing and quality. ✓ The Company conducts various risk assessments and assurance activities in relation to data privacy and Artificial Intelligence (AI) risks. ✓ The Company conducted the following actions for cybersecurity: ◆ Since the Company recognized the critical role that cybersecurity plays in ensuring trusted digital interactions with the Company’s stakeholders, meetings of the Information & Digital Trust Governance Board continued to be held. The meeting of this Board was held every other month and on an ad hoc basis. This Board consists of representatives from all business units/functions of the Company and discussed relevant information risk topics and reviewed the status of actions taken to mitigate such risks. ◆ Mandatory online training, with the latest information concerning cyber threats in each business, was provided to all employees in order to strengthen cybersecurity awareness and address emerging threats. ◆ The Company continued to make investments to strengthen security in the process and technical aspects of the Company’s data and IT infrastructure. Insurance is held to cover certain costs related to significant cybersecurity events that the Company may face in the future. ✓ The company periodically conducts a crisis management exercise for TET without advance notice, in order to elate their crisis readiness and resilience. ✓ The meeting of the Regional Crisis Management Committee on COVID-19 was continued to be held, and provided appropriate training etc. to prepare the Company for potential future pandemic type crisis. ✓ The Global Crisis Management Committee concerning the situation in Ukraine and Gaza Strip continued to operate, and ensured the safety of employees by the swift and ongoing provision of safety confirmations and necessary support to employees. [Efforts by the Audit and Supervisory Committee] • The ASC meetings are chaired by the head of the ASC. The ASC held eight meetings during this fiscal year, and the members exchanged information and opinions relating to matters such as the agenda of the BOD meetings,

- 78 - status of the Director’s business executions and the status of the Company’s internal control system. The ASC members obtained information by attending important meetings, hearing periodic business reports from divisions executing the business and collaborating with the GIA department and the internal control promotion division to gather insights. This was done with the assistance of the ASC Office staff, who collect information on a regular basis. The ASC formulated their audit opinions by sharing this information amongst all of the ASC members. • The ASC reported on the result of the previous fiscal year’s activities and its activity policy and plan for this fiscal year, and exchanged opinions at the BOD meeting. As necessary, the ASC also gave its opinion on the Directors’ business execution. • The ASC had meetings to exchange opinions with the GIA department regularly or as necessary, and received reports related to the Company’s internal audit plan and audit results. The ASC effectively utilized these results for ASC’s audit after confirming the appropriateness of these reports. In addition, the ASC conducted a systematic audit while instructing or requesting an investigation as necessary to the GIA department and coordinating activities in their respective audit plans. • The appointed ASC Members attended the Nomination Committee and the Compensation Committee as members of those committees, and stated their opinions relating to the election of Directors who are not ASC Members and their compensation. Also, the information obtained from these committees was shared at the ASC, and through this and other relevant processes, the ASC formulated its opinion appropriately, and performed its duties of supervision. **************************************************************** [Note to Business Report] All monetary amounts indicated in the Business Report are rounded to the nearest unit.
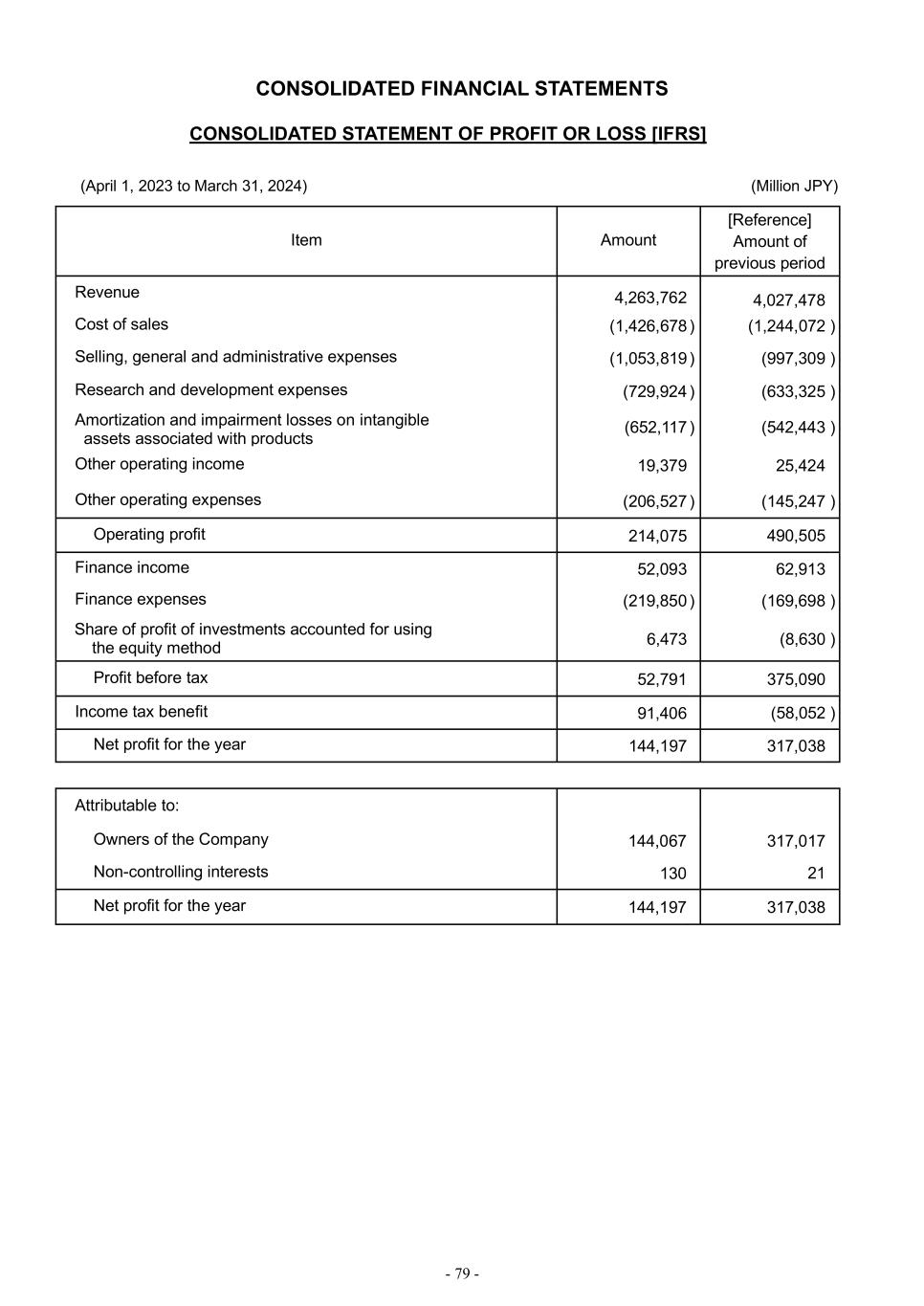
- 79 - CONSOLIDATED FINANCIAL STATEMENTS CONSOLIDATED STATEMENT OF PROFIT OR LOSS [IFRS] (April 1, 2023 to March 31, 2024) (Million JPY) Item Amount [Reference] Amount of previous period Revenue 4,263,762 4,027,478 Cost of sales (1,426,678 ) (1,244,072 ) Selling, general and administrative expenses (1,053,819 ) (997,309 ) Research and development expenses (729,924 ) (633,325 ) Amortization and impairment losses on intangible assets associated with products (652,117 ) (542,443 ) Other operating income 19,379 25,424 Other operating expenses (206,527 ) (145,247 ) Operating profit 214,075 490,505 Finance income 52,093 62,913 Finance expenses (219,850 ) (169,698 ) Share of profit of investments accounted for using the equity method 6,473 (8,630 ) Profit before tax 52,791 375,090 Income tax benefit 91,406 (58,052 ) Net profit for the year 144,197 317,038 Attributable to: 109,126 (112) Owners of the Company 144,067 317,017 Non-controlling interests 130 21 Net profit for the year 144,197 317,038

- 80 - [Reference] CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME [IFRS] (April 1, 2023 to March 31, 2024) (Million JPY) Item Amount [Reference] Amount of previous period Net profit for the year 144,197 317,038 Other comprehensive income (loss) Items that will not be reclassified to profit or loss: Changes in fair value of financial assets measured at fair value through other comprehensive income 2,309 (2,654 ) Remeasurement of defined benefit pension plans (5,002 ) 17,752 (2,693 ) 15,098 Items that may be reclassified subsequently to profit or loss: Exchange differences on translation of foreign operations 968,842 618,773 Cash flow hedges 23,456 (21,451 ) Hedging cost 7,197 (16,993 ) Share of other comprehensive loss of investments accounted for using the equity method (1,793 ) (892 ) 997,702 579,437 Other comprehensive income for the year, net of tax 995,009 594,535 Total comprehensive income for the year 1,139,206 911,574 Attributable to: Attributable to: Owners of the Company 1,139,033 911,529 Non-controlling interests 173 45 Total comprehensive income for the year 1,139,206 911,574 (Note) Consolidated Statement of Comprehensive Income is not required by the Companies Act and is not audited, but it is presented for the reference purpose.

- 81 - CONSOLIDATED STATEMENT OF FINANCIAL POSITION [IFRS] (As of March 31, 2024) (Million JPY) Item Amount [Reference] Amount of previous period Item Amount [Reference] Amount of previous period ASSETS 1,316,531 LIABILITIES 4,766,005 Non-current assets Non-current liabilities Property, plant and equipment 1,989,777 1,691,229 Bonds and loans 4,476,501 4,042,741 Goodwill 5,410,067 4,790,723 Other financial liabilities 687,833 534,269 Intangible assets 4,274,682 4,269,657 Net defined benefit liabilities 143,882 127,594 Investments accounted for using the equity method 89,831 99,174 Income taxes payable 4,381 24,558 Other financial assets 340,777 279,683 Provisions 14,373 55,969 Other non-current assets 51,214 63,325 Other non-current liabilities 80,938 65,389 Deferred tax assets 393,865 366,003 Deferred tax liabilities 113,777 270,620 Total non-current assets 12,550,212 11,559,794 Total non-current liabilities 5,521,684 5,121,138 Current assets Current liabilities Inventories 1,209,869 986,457 Bonds and loans 367,251 339,600 Trade and other receivables 668,403 649,429 Trade and other payables 547,521 649,233 Other financial assets 15,089 20,174 Other financial liabilities 143,421 185,537 Income taxes receivable 29,207 32,264 Income taxes payable 109,906 232,377 Other current assets 168,875 160,868 Provisions 524,420 508,360 Cash and cash equivalents 457,800 533,530 Other current liabilities 619,174 566,689 Assets held for sale 9,337 15,235 Liabilities held for sale 1,410 144 Total current assets 2,558,580 2,397,956 Total current liabilities 2,313,103 2,481,940 Total liabilities 7,834,788 7,603,078 EQUITY Share capital 1,676,596 1,676,345 Share premium 1,747,414 1,728,830 Treasury shares (51,259 ) (100,317 ) Retained earnings 1,391,203 1,541,146 Other components of equity 2,509,310 1,508,119 Equity attributable to owners of the company 7,273,264 6,354,122 Non-controlling interests 741 549 Total equity 7,274,005 6,354,672 TOTAL ASSETS 15,108,792 13,957,750 TOTAL LIABILITIES AND EQUITY 15,108,792 13,957,750

- 82 - CONSOLIDATED STATEMENT OF CHANGES IN EQUITY [IFRS] (April 1, 2023 to March 31, 2024) (Million JPY) Equity attributable to owners of the Company Share capital Share premium Treasury shares Retained earnings Other components of equity Exchange differences on translation of foreign operations Changes in fair value of financial assets measured at fair value through other comprehensive income As of April 1, 2023 1,676,345 1,728,830 (100,317) 1,541,146 1,606,128 12,470 Net profit for the year 144,067 Other comprehensive income (loss) 967,279 2,036 Comprehensive income (loss) for the year — — — 144,067 967,279 2,036 Transactions with owners: Issuance of new shares 251 251 Acquisition of treasury shares (2,367) Disposal of treasury shares 0 0 Dividends (287,785) Changes in ownership Transfers from other components of equity (6,226) 1,224 Share-based compensation 69,836 Exercise of share-based awards (51,503) 51,426 Total transactions with owners 251 18,584 49,059 (294,011) — 1,224 As of March 31, 2024 1,676,596 1,747,414 (51,259) 1,391,203 2,573,407 15,729

- 83 - Equity attributable to owners of the Company Non- controlling interests Total equity Other components of equity Total equity attributable to owners of the Company Cash flow hedges Hedging cost Remeasureme nts of defined benefit pension plans Total other components of equity As of April 1, 2023 (87,352) (23,127) — 1,508,119 6,354,122 549 6,354,672 Net profit for the year — 144,067 130 144,197 Other comprehensive income (loss) 23,456 7,197 (5,002) 994,966 994,966 44 995,009 Comprehensive income (loss) for the year 23,456 7,197 (5,002) 994,966 1,139,033 173 1,139,206 Transactions with owners: Issuance of new shares — 502 502 Acquisition of treasury shares — (2,367) (2,367) Disposal of treasury shares — 1 1 Dividends — (287,785) (287,785) Changes in ownership — — 18 18 Transfers from other components of equity 5,002 6,226 — — Share-based compensation — 69,836 69,836 Exercise of share-based awards — (77) (77) Total transactions with owners — — 5,002 6,226 (219,892) 18 (219,873) As of March 31, 2024 (63,896) (15,930) — 2,509,310 7,273,264 741 7,274,005

- 84 - UNCONSOLIDATED FINANCIAL STATEMENTS UNCONSOLIDATED BALANCE SHEET (As of March 31, 2024) (Million JPY) Item Amount [Reference] Amount of previous period Item Amount [Reference] Amount of previous period Current assets 730,761 862,669 Current liabilities 1,171,639 1,000,002 Cash and deposits 130,947 164,860 Accounts payable 71,654 54,471 Accounts receivable 47,917 59,765 Other payable 141,538 150,115 Securities 122,471 97,030 Accrued expenses 71,022 63,007 Merchandise and products 62,146 39,202 Income taxes payable 445 1,462 Work in process 38,541 46,094 Short-term loans 415,969 388,195 Raw materials and supplies 43,223 39,399 Current portion of bonds 317,000 106,715 Income taxes receivables 1,865 2,192 Current portion of long-term loans 50,000 100,000 Short-term loans receivable from subsidiaries and affiliates 179,261 275,053 Deposits received 69,157 92,025 Other 104,390 139,082 Reserve for employees’ bonuses 14,817 14,120 Allowance for doubtful accounts - (8 ) Reserve for share-based payments 3,171 3,281 ) Reserve for bonuses for directors and corporate auditors 436 385 Reserve for restructuring costs 1,022 2,020 Other 15,408 24,205 Non-current assets 9,025,558 8,544,633 Non-current liabilities 4,496,482 4,201,082 Tangible non-current assets 169,311 176,354 Bonds 3,016,582 2,787,470 Buildings and structures 81,261 85,059 Long-term loans 1,341,465 1,262,420 Machinery and equipment 21,668 17,276 Reserve for retirement benefits 7,789 7,047 Vehicles 45 35 Reserve for litigation 762 38,283 Tools and fixtures 10,837 8,492 Reserve for share-based payments 2,438 2,548 Land 35,043 39,794 Reserve for restructuring costs 452 2,219 Lease assets 1,211 1,300 Asset retirement obligations 1,832 1,893 Construction in progress 19,248 24,396 Long-term deferred income 12,880 12,486 Other 112,282 86,717 Intangible non-current assets 31,933 33,100 Total liabilities 5,668,121 5,201,084 Shareholders' equity 4,661,339 4,546,482 Investments and other assets 8,824,314 8,335,180 Share capital 1,676,596 1,676,345 Investment securities 37,044 32,854 Share premium 1,685,597 1,670,413 Investment in subsidiaries and affiliates 7,853,042 8,000,147 Additional paid-in capital 1,668,608 1,668,357 Investments in other securities of subsidiaries and affiliates - 5,031 Other share premium 16,989 2,055 Contributions to subsidiaries and affiliates 647,460 26,344 Retained earnings 1,350,375 1,300,012 Long-term deposits 5,913 6,743 Legal reserve 15,885 15,885 Prepaid pension costs 64,926 54,350 Other retained earnings 1,334,490 1,284,127 Deferred tax assets 123,639 165,410 Reserve for retirement benefits 5,000 5,000 Other 92,290 44,301 Reserve for dividends 11,000 11,000 Reserve for research and development 2,400 2,400 Reserve for capital improvements 1,054 1,054 Reserve for promotion of exports 434 434 Reserve for reduction of noncurrent assets 28,832 29,890 General reserve 814,500 814,500 Unappropriated retained earnings 471,270 419,850 Treasury shares (51,229 ) (100,288 ) Valuation and translation adjustments (574,252 ) (341,452 ) Unrealized gains on available-for-sale securities 11,031 8,584 Deferred gains on derivatives under hedge accounting (585,282 ) (350,036 ) Share acquisition rights 1,111 1,188 Total net assets 4,088,198 4,206,219 TOTAL ASSETS 9,756,319 9,407,303 TOTAL LIABILITIES AND NET ASSETS 9,756,319 9,407,303

- 85 - UNCONSOLIDATED STATEMENTS OF OPERATIONS (April 1, 2023 to March 31, 2024) (Million JPY) Item Amount [Reference] Amount of previous period Net sales 595,575 632,137 Cost of sales 245,505 214,973 Gross profit 350,070 417,164 Selling, general and administrative expenses 302,001 281,023 Operating income 48,070 136,140 Non-operating income 391,614 329,384 Interest and dividend income 306,382 276,023 Other 85,231 53,361 Non-operating expenses 153,285 125,403 Interest expenses 82,204 85,589 Other 71,081 39,814 Ordinary income 286,399 340,122 Extraordinary income 138,488 42,851 Gain on restructuring of subsidiaries and affiliates 138,488 42,851 Extraordinary loss Loss on Litigation 33,545 33,545 - - Income before income taxes 391,342 382,973 Income taxes – current 20,281 35,854 Income taxes – deferred 32,187 16,469 Net income 338,874 330,649

- 86 - UNCONSOLIDATED STATEMENTS OF CHANGES IN NET ASSETS (April 1, 2023 to March 31, 2024) (Million JPY) Shareholders’ equity Valuation and translation adjustments Share acquisition rights Total net assets Share capital Share premium Retained earnings Treasury shares Total shareholder s' equity Unrealized gains on available- for-sale securities Deferred gains on derivatives under hedge accounting Total valuation and translation adjustments Additional paid-in capital Other share premium Total share premium Legal reserve Other retained earnings (*) Total retained earnings As of April 1, 2023 1,676,345 1,668,357 2,055 1,670,413 15,885 1,284,127 1,300,012 (100,288) 4,546,482 8,584 (350,036) (341,452) 1,188 4,206,219 Changes of items during the fiscal year Issuance of new shares 251 251 251 — 502 — 502 Dividends — (288,512) (288,512) (288,512) — (288,512) Provision for reserve for reduction of noncurrent assets — — — — — Reversal of reserve for reduction of noncurrent assets — — — — — Net income — 338,874 338,874 338,874 — 338,874 Acquisition of treasury shares — — (2,367) (2,367) — (2,367) Disposal of treasury shares 14,933 14,933 — 51,426 66,359 — 66,359 Net change in items other than shareholders’ equity during the fiscal year — — — 2,447 (235,246) (232,800) (77) (232,877) Total changes of items during the fiscal year 251 251 14,933 15,184 — 50,363 50,363 49,059 114,856 2,447 (235,246) (232,800) (77) (118,021) As of March 31, 2024 1,676,596 1,668,608 16,989 1,685,597 15,885 1,334,490 1,350,375 (51,229) 4,661,339 11,031 (585,282) (574,252) 1,111 4,088,198

- 87 - *Breakdown of other retained earnings (Million JPY) Reserve for retirement benefits Reserve for dividends Reserve for research and development Reserve for capital improvements Reserve for promotion of exports Reserve for reduction of noncurrent assets General reserve Unappropriated retained earnings Total As of April 1, 2023 5,000 11,000 2,400 1,054 434 29,890 814,500 419,850 1,284,127 Changes of items during the fiscal year Issuance of new shares — Dividends (288,512) (288,512) Provision for reserve for reduction of noncurrent assets 773 (773) — Reversal of reserve for reduction of noncurrent assets (1,830) 1,830 — Net income 338,874 338,874 Acquisition of treasury shares — Disposal of treasury shares — Net change in items other than shareholders’ equity during the fiscal year — Total changes of items during the fiscal year — — — — — (1,057) — 51,420 50,363 As of March 31, 2024 5,000 11,000 2,400 1,054 434 28,832 814,500 471,270 1,334,490

88 [English Translation of the Accounting Auditor's Report Originally Issued in the Japanese Language] [Certified Copy of the Accounting Auditor’s Report related to the Consolidated Financial Statements] Independent Auditor’s Report May 8, 2024 The Board of Directors Takeda Pharmaceutical Company Limited KPMG AZSA LLC Tokyo Office Kotetsu Nonaka Designated Limited Liability Partner Engagement Partner Certified Public Accountant Masahiko Chino Designated Limited Liability Partner Engagement Partner Certified Public Accountant Hiroaki Namba Designated Limited Liability Partner Engagement Partner Certified Public Accountant Opinion We have audited the consolidated financial statements, comprising the consolidated statement of profit or loss, the consolidated statement of financial position, the consolidated statement of changes in equity and the related notes on the consolidated financial statements of Takeda Pharmaceutical Company Limited (“the Company”) as of March 31, 2024 and for the year from April 1, 2023 to March 31, 2024 in accordance with Article 444-4 of the Companies Act. In our opinion, the consolidated financial statements referred to above, which were prepared in accordance with the latter part of Article 120-1 of the Ordinance of Companies Accounting that prescribes some omissions of disclosure items required by International Financial Reporting Standards, present fairly, in all material respects, the financial position and the results of operations of the Company and its consolidated subsidiaries for the period, for which the consolidated financial statements were prepared. Basis for Opinion We conducted our audit in accordance with auditing standards generally accepted in Japan. Our responsibilities under those standards are further described in the "Auditor’s Responsibilities in Auditing the Consolidated Financial Statements" section of our report. We are independent from the Company and its consolidated subsidiaries and have fulfilled other ethical responsibilities as an auditor in accordance with Japan's professional ethics regulations. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.

89 Other Information The other information comprises the business report and its supplementary schedules. Management is responsible for the preparation and presentation of the other information. Audit and Supervisory Committee is responsible for overseeing the directors’ performance of their duties with regard to the design, implementation and maintenance of the reporting process for the other information. Our opinion on the consolidated financial statements does not cover the other information and we do not express any form of assurance conclusion thereon. In connection with our audit of the consolidated financial statements, our responsibility is to read the other information and, in doing so, consider whether the other information is materially inconsistent with the consolidated financial statements or our knowledge obtained in the audit, or otherwise appears to be materially misstated. If, based on the work we have performed, we conclude that there is a material misstatement of this other information, we are required to report that fact. We have nothing to report in this regard. Responsibilities of the Management and Audit and Supervisory Committee for the Consolidated Financial Statements Management is responsible for the preparation and fair presentation of the consolidated financial statements in accordance with the latter part of Article 120-1 of the Ordinance of Companies Accounting that prescribes some omissions of disclosure items required by International Financial Reporting Standards, and for such internal control as management determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatements, whether due to fraud or error. In preparing the consolidated financial statements, the management shall (i) evaluate whether or not it is appropriate to prepare the consolidated financial statements based on the premise of a going concern, unless the management intends to liquidate or suspend the business or there is no other practical alternative but to do so, and (ii) disclose matters relating to a going concern if it is necessary to do so in accordance with the provisions of the latter part of Article 120-1 of the Ordinance of Companies Accounting that prescribes some omissions of disclosure items required by International Financial Reporting Standards. Audit and Supervisory Committee is responsible for monitoring the performance of duties by directors including the design and implementation of the financial reporting process. Auditor’s Responsibilities in Auditing the Consolidated Financial Statements Our responsibility is to express an opinion on the consolidated financial statements based on our audit as independent auditor in the Auditor’s Report, obtaining reasonable assurance as to whether the consolidated financial statements as a whole are free of material misstatements, whether due to fraud or error. Misstatements may occur due to fraud or error, and if it is reasonably expected to affect the decision-making of users of the consolidated financial statements when individually or in the aggregate, it is judged to be material. In accordance with auditing standards generally accepted in Japan, we make judgment as a professional expert throughout the course of audit, maintain professional skepticism, and perform the following:

90 ⚫ We identify and assess the risks of material misstatements, whether due to fraud or error. Also, we design and implement audit procedures that address the risks of material misstatements. The selection and application of audit procedures is at our discretion. In addition, we obtain sufficient and appropriate audit evidence to form the basis of the opinion. ⚫ In making those risk assessments, we consider internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, while the objective of auditing the consolidated financial statements is not for the purpose of expressing an opinion on the effectiveness of the Company and its consolidated subsidiaries’ internal control. ⚫ We evaluate the appropriateness of the accounting policies adopted by management and the method of application thereof, as well as the reasonableness of accounting estimates made by management and the adequacy of related notes. ⚫ We conclude whether it is appropriate for management to prepare consolidated financial statements on the premise of a going concern, and whether there is significant uncertainty regarding events or circumstances that may cause significant doubts on the premise of a going concern based on the audit evidence obtained. We are required to draw attention to the notes on the consolidated financial statements in the Auditor’s Report if significant uncertainties regarding the premise of a going concern are observed, or to express a qualified opinion with a description of qualification if the notes on the consolidated financial statements regarding significant uncertainties are not appropriate. Though our conclusions are based on audit evidence obtained up to the date of the Auditor’s Report, future events and circumstances may prevent the the Company and its consolidated subsidiaries from continuing as a going concern. ⚫ We evaluate whether the presentation and notes of the consolidated financial statements comply with the provisions of the latter part of Article 120-1 of the Ordinance of Companies Accounting that prescribes some omissions of disclosure items required by International Financial Reporting Standards. In addition, we evaluate whether the presentation, composition and contents of the consolidated financial statements, including related notes, properly present the underlying transactions and accounting events. ⚫ We obtain sufficient and appropriate audit evidence regarding the financial information of the Company and its consolidated subsidiaries to express our opinion on the consolidated financial statements. We are responsible for directing, supervising and implementing the audit of the consolidated financial statements. We are solely responsible for our opinion. We report to the Audit and Supervisory Committee on the planned scope and timing of the audit, significant findings regarding the audit including significant deficiencies in internal controls identified during the audit process, and any other matters required by relevant audit standards. We report to the Audit and Supervisory Committee on our compliance with Japan's professional ethics regulations regarding independence, and communicate with them matters that could reasonably be considered to bear on our independence, and where applicable, measures taken to eliminate threats or safeguards applied. Interest required to be disclosed by the Certified Public Accountants Act of Japan Our firm and its designated engagement partners have no interest in the Company and its consolidated subsidiaries which should be disclosed pursuant to the provisions of the Certified Public Accountants Act of Japan. Notes to the Reader of Independent Auditor’s Report: The Independent Auditor’s Report herein is the English translation of the Independent Auditor’s Report as required by the Companies Act. End of Document

91 [English Translation of the Accounting Auditor's Report Originally Issued in the Japanese Language] [Certified Copy of the Accounting Auditor’s Report] Independent Auditor’s Report May 8, 2024 The Board of Directors Takeda Pharmaceutical Company Limited KPMG AZSA LLC Tokyo Office Kotetsu Nonaka Designated Limited Liability Partner Engagement Partner Certified Public Accountant Masahiko Chino Designated Limited Liability Partner Engagement Partner Certified Public Accountant Hiroaki Namba Designated Limited Liability Partner Engagement Partner Certified Public Accountant Opinion We have audited the financial statements, comprising the unconsolidated balance sheet, the unconsolidated statement of operations, the unconsolidated statement of changes in net assets and the related notes to the unconsolidated financial statements, as well as the supplementary schedules of Takeda Pharmaceutical Company Limited (“the Company”) as of March 31, 2024 and for the 147th fiscal year from April 1, 2023 to March 31, 2024 (“the Financial Statements and Others”) in accordance with Article 436-2-1 of the Companies Act. In our opinion, the Financial Statements and Others referred to above present fairly, in all material respects, the financial position and the results of operations of the Company for the period, for which the Financial Statements and Others were prepared, in accordance with accounting principles generally accepted in Japan. Basis for Opinion We conducted our audit in accordance with auditing standards generally accepted in Japan. Our responsibilities under those standards are further described in the "Auditor’s Responsibilities in Auditing the Financial Statements and Others" section of our report. We are independent from the Company and have fulfilled other ethical responsibilities as an auditor in accordance with Japan's professional ethics regulations. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.

92 Other Information The other information comprises the business report and its supplementary schedules. Management is responsible for the preparation and presentation of the other information. Audit and Supervisory Committee is responsible for overseeing the directors’ performance of their duties with regard to the design, implementation and maintenance of the reporting process for the other information. Our opinion on the financial statements and the accompanying supplementary schedules does not cover the other information and we do not express any form of assurance conclusion thereon. In connection with our audit of the financial statements and the accompanying supplementary schedules, our responsibility is to read the other information and, in doing so, consider whether the other information is materially inconsistent with the financial statements and the accompanying supplementary schedules or our knowledge obtained in the audit, or otherwise appears to be materially misstated. If, based on the work we have performed, we conclude that there is a material misstatement of this other information, we are required to report that fact. We have nothing to report in this regard. Responsibilities of the Management and Audit and Supervisory Committee for the Financial Statements and Others Management is responsible for the preparation and fair presentation of the Financial Statements and Others in accordance with accounting principles generally accepted in Japan, and for such internal control as management determines is necessary to enable the preparation of the Financial Statements and Others that are free from material misstatements, whether due to fraud or error. In preparing the Financial Statements and Others, the management shall (i) evaluate whether or not it is appropriate to prepare the Financial Statements and Others based on the premise of a going concern, and (ii) disclose matters relating to a going concern if it is necessary to do so in accordance with accounting principles generally accepted in Japan. Audit and Supervisory Committee is responsible for monitoring the performance of duties by directors including the design and implementation of the financial reporting process. Auditor’s Responsibilities in Auditing the Financial Statements and Others Our responsibilities are to express an opinion on the Financial Statements and Others based on our audit as independent auditor in the Auditor’s Report, obtaining reasonable assurance as to whether the Financial Statements and Others as a whole are free of material misstatements, whether due to fraud or error. Misstatements may occur due to fraud or error, and if it is reasonably expected to affect the decision-making of users of the Financial Statements and Others when individually or in the aggregate, it is judged to be material. In accordance with auditing standards generally accepted in Japan, we make judgment as a professional expert throughout the course of audit, maintain professional skepticism, and perform the following: ⚫ We identify and assess the risks of material misstatements, whether due to fraud or error. Also, we design and implement audit procedures that address the risks of material misstatements. The selection and application of audit procedures is at our discretion. In addition, we obtain sufficient and appropriate audit evidence to form the basis of the opinion.

93 ⚫ In making those risk assessments, we consider internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, while the objective of auditing the Financial Statements and Others is not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control. ⚫ We evaluate the appropriateness of the accounting policies adopted by management and the method of application thereof, as well as the reasonableness of accounting estimates made by management and the adequacy of related notes. ⚫ We conclude whether it is appropriate for management to prepare Financial Statements and Others on the premise of a going concern, and whether there is significant uncertainty regarding events or circumstances that may cause significant doubts on the premise of a going concern based on the audit evidence obtained. We are required to draw attention to the notes on the Financial Statements and Others in the Auditor’s Report if significant uncertainties regarding the premise of a going concern are observed, or to express a qualified opinion with a description of qualification if the notes on the Financial Statements and Others regarding significant uncertainties are not appropriate. Though our conclusions are based on audit evidence obtained up to the date of the Auditor’s Report, future events and circumstances may prevent the Company from continuing as a going concern. ⚫ We evaluate whether the presentation and notes of the Financial Statements and Others comply with accounting standards generally accepted in Japan. In addition, we evaluate whether the presentation, composition and contents of the Financial Statements and Others properly present the underlying transactions and accounting events. We report to the Audit and Supervisory Committee on the planned scope and timing of the audit, significant findings regarding the audit including significant deficiencies in internal controls identified during the audit process, and any other matters required by relevant audit standards. We report to the Audit and Supervisory Committee on our compliance with Japan's professional ethics regulations regarding independence, and communicate with them matters that could reasonably be considered to bear on our independence, and where applicable, measures taken to eliminate threats or safeguards applied. Interest required to be disclosed by the Certified Public Accountants Act of Japan Our firm and its designated engagement partners have no interest in the Company which should be disclosed pursuant to the provisions of the Certified Public Accountants Act of Japan. Notes to the Reader of Independent Auditor’s Report: The Independent Auditor’s Report herein is the English translation of the Independent Auditor’s Report as required by the Companies Act. End of Document

94 [English Translation of the Report of the Audit and Supervisory Committee Originally Issued in the Japanese Language] [Certified Copy of the Audit Report of the Audit and Supervisory Committee] Audit Report The Audit and Supervisory Committee has audited the Directors’ performance of their duties for the 147th business year from April 1, 2023 to March 31, 2024, and hereby reports the method and results of those audits, as follows: 1. Method and Contents of Audits (1) With regard to the content of the resolutions of the Board of Directors regarding the matters stated in Article 399-13, Paragraph (1), Items (i)(b) and (i)(c) of the Companies Act, as well as the systems developed pursuant to those resolutions (i.e., internal control systems), the Audit and Supervisory Committee periodically received reports from the Directors and employees, etc. regarding the status of the establishment and operation of those systems and, as necessary, requested explanations and expressed opinions with regard thereto. The Committee also received reports from Directors, etc. and KPMG AZSA LLC on the status of the evaluation and audit of the internal controls related to financial reporting and requested explanations as necessary. (2) The Audit and Supervisory Committee performed its duties based on the Audit and Supervisory Committee Charter determined by the Audit and Supervisory Committee. In accordance with the audit policies, audit plan and division of duties, etc., the Audit and Supervisory Committee attended important meetings, received reports from the Directors and employees, etc. regarding matters related to the performance of their duties, requested explanations as necessary, reviewed the important materials used for the deliberation and reporting, and inspected the status of operations and assets in cooperation with the internal audit division and the internal control promotion division to which the Audit and Supervisory Committee is authorized to give instructions. As for subsidiaries of the Company, the Audit and Supervisory Committee received reports on the audit results from the internal audit division, and, as necessary, received reports on the businesses of the subsidiaries from the Directors and employees, etc. of the subsidiaries and exchanged opinions with them. (3) The Audit and Supervisory Committee oversaw and verified whether the Accounting Auditor maintained an independent position and conducted an appropriate audit, received reports from the Accounting Auditor on the status of the performance of its duties, and requested explanations as necessary. Additionally, the Audit and Supervisory Committee received a notification from the Accounting Auditor that, in accordance with the “Quality Control Standard for Audits” (Business Accounting Council), etc., it had developed systems in order to ensure that its duties are appropriately performed (i.e., notification of the matters stated in the items under Article 131 of the Ordinance on Accounting of Companies) and requested explanations as necessary. Using the methods above, the Audit and Supervisory Committee examined the Business Report, the supplementary schedules thereto, the unconsolidated financial statements (i.e., the unconsolidated balance sheet, the unconsolidated statements of operations, the unconsolidated statements of changes in net assets, and the notes to the unconsolidated financial statements), the supplementary schedules to the unconsolidated financial statements, and the consolidated financial statements (i.e., the consolidated statement of financial position, consolidated statement of profit or loss, consolidated statement of changes in equity and the notes to the consolidated financial statements, which were prepared omitting the part of the items required to be disclosed using the International Financial Reporting Standards in accordance with the latter clause of Paragraph 1, Article 120 of the Ordinance on Accounting of Companies) for the business year. 2. Audit Results (1) Results of the audit of the Business Report, etc. (i) We find that the Business Report and the supplementary schedules thereto accurately present the status of the Company in accordance with laws, regulations, and the Articles of Incorporation. (ii) We do not find any misconduct or any material fact constituting a violation of any law,

95 regulation, or the Articles of Incorporation with respect to the Directors’ performance of their duties. (iii) We find the content of the resolutions of the Board of Directors regarding internal control systems to be reasonable. Additionally, we do not find any matters that should be commented upon with regard to the statement of Business Report or the Directors’ performance of their duties relating to the internal control systems, including the internal controls over financial reporting. (2) Results of the audit of the unconsolidated financial statements and the supplementary schedules thereto We find the methods and results of the audit by the Accounting Auditors, KPMG AZSA LLC to be reasonable. (3) Results of the audit of the consolidated financial statements We find the methods and the results of the audit by the Accounting Auditors, KPMG AZSA LLC to be reasonable. May 8, 2024 The Audit and Supervisory Committee of Takeda Pharmaceutical Company Limited Audit and Supervisory Committee Member: Koji Hatsukawa Audit and Supervisory Committee Member: Yoshiaki Fujimori Audit and Supervisory Committee Member: Emiko Higashi Audit and Supervisory Committee Member: Kimberly A. Reed Note: Audit and Supervisory Committee Members Koji Hatsukawa, Yoshiaki Fujimori, Emiko Higashi and Kimberly A. Reed are External Directors provided for in Article 2, Item15 and Article 331, Paragraph 6 of the Companies Act. END