false
0001580149
0001580149
2023-11-29
2023-11-29
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
8-K
CURRENT
REPORT PURSUANT
TO SECTION 13 OR 15(d) OF
THE SECURITIES EXCHANGE ACT OF 1934
Date
of Report (Date of earliest event reported) November 29, 2023
BioVie Inc.
(Exact Name of Registrant as Specified in Its
Charter)
| Nevada |
|
001-39015 |
|
46-2510769 |
| (State
or Other Jurisdiction of Incorporation) |
|
(Commission
File Number) |
|
(I.R.S.
Employer Identification No.) |
680 W Nye Lane Suite 201
Carson City, NV |
|
89703 |
| (Address
of Principal Executive Offices) |
|
(Zip
Code) |
| |
|
|
(775)
888-3162
(Registrant’s Telephone Number, Including Area Code)
(Former
Name or Former Address, if Changed Since Last Report)
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
Trading
Symbol(s) |
Name
of each exchange on which registered |
| Class
A Common Stock, par value $0.0001 per share |
BIVI |
The
Nasdaq Stock Market, LLC |
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions (see General Instruction A.2. below):
| |
☐ |
Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
☐ |
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
☐ |
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate
by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933
(§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ☐
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
On November 29, 2023, BioVie Inc.
(the “Company”) issued a press release and posted on its website at https://bioviepharma.com/ an investor presentation disclosing
top line data from its clinical trial of NE3107 in the treatment of mild to moderate Alzheimer’s Disease. Copies of the press release
and the presentation are filed herewith as Exhibits 99.1 and 99.2, respectively, and are incorporated by reference herein.
| Item
9.01 |
Financial
Statements and Exhibits |
(d)
Exhibits.
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
Dated:
November 29, 2023
| |
BIOVIE
INC. |
|
| |
|
|
|
| |
By: |
/s/
Joanne Wendy Kim |
|
| |
Name:
|
Joanne
Wendy Kim |
|
| |
Title: |
Chief
Financial Officer |
|
BioVie Announces EfficacyData from Phase 3 Trial
of NE3107
in Patientswith Mild to Moderate Alzheimer’s Disease
Positive TrendingData from 57 Per-Protocol PatientsSuggest
NE3107 is Biologically Active
and May Have Impact on Cognitive, Functional, and Biomarker Endpoints
Sponsor Identified Issues Relating to SignificantGCPViolations
and Protocol Deviations, Which Allowed for Data from Only a Subset of Enrolled Patients tobe Included in the Efficacy Analysis;Sites Suspectedof
Improprieties Have BeenReferred to FDA
Due to Exclusions, the Primary Efficacy EndpointMissedStatistical
Significance;Adaptive Feature of Trial May Allow the Company to Continue Enrolling Patients to Reach Statistical Significancefor Registrational
Purposes
Management to Host Conference Call at 8:30 AMET
Today to Discuss Data
Carson City NV,November 29,– BioVie
Inc., (NASDAQ: BIVI) (“BioVie” or the “Company”) a clinical-stage company developing innovative drug therapies
for the treatment of neurological and neurodegenerative disorders and advanced liver disease,today announced positiveanalysisofunblinded,topline
efficacy data from its Phase 3 clinical trial (NCT04669028) of NE3107 in
the treatment of mild to moderate Alzheimer’s Disease (AD).
Data from evaluable patients show NE3107’s
treatment advantage compared to placebo may be equal to or greater than the benefit from approved AD monoclonal antibodies. NE3107-treated
patients also experienced a 4.66-year advantage in age deceleration vs. placebo as measured by epigenetics/DNA methylation Skin Blood
Clock.
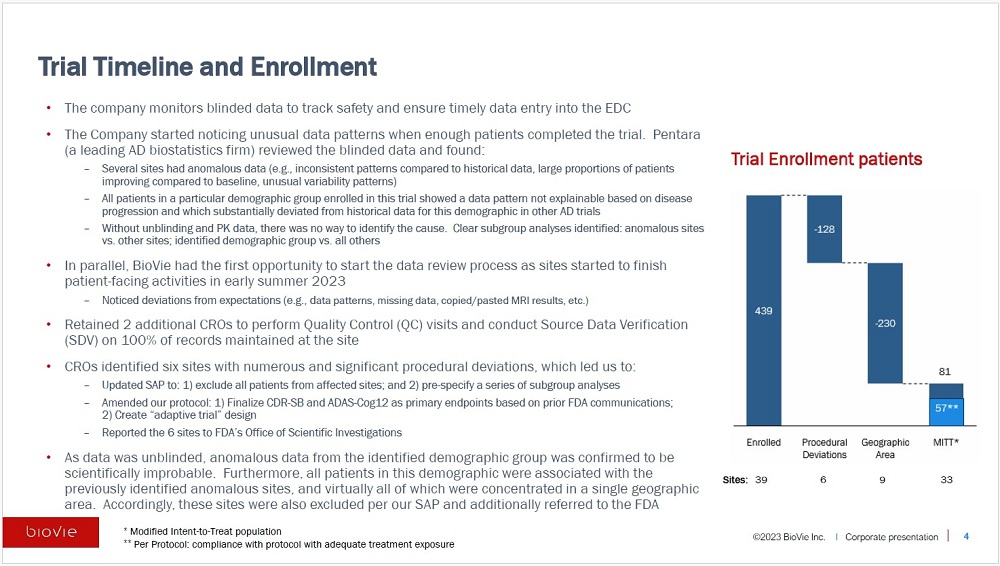
The trial started during the COVID-19 pandemic when
access to clinical sites was limited and enrolled a total of 439 patients through 39 sites. Upon trial completion, the Company found significant
deviation from protocol and Good Clinical Practice (GCP) violations at 15 sites (virtually all of which were from one geographic area).
This highly unusual level of suspected improprieties led the Companyto exclude all patients from these sites and to refer them to the
U.S. Food and Drug Administration(FDA) Office of Scientific Investigations (OSI) for further action. After these exclusions,81 patientsremained
in our Modified Intent to Treat (MITT) population, 57 of whom were in the Per-Protocol populationwhich included those who completed the
trial and wereverified to take study drug from pharmacokinetic(PK) data.
“These data show NE3107’s treatment
advantage over placebo to potentially be equal to or greater than data reported from clinical trials for the approved medications
for AD without the associated safety concerns,” said Cuong Do, BioVie’s President and CEO. “The adaptive trial
design givesus the flexibility to continue patient enrollmentin the advancement of this potentially important treatment for AD, and
we look forward to discussing our findingsof NE3107’s magnitude of therapeutic impact with our potentialpartners. I am also
very proud of the integrity our team displayed in taking immediate action to identify and report the potentially problematic sites
to the FDA for independent investigation.Importantly,we recognize that along with the development of new and innovative
therapeutics, our foremost responsibility in clinical testing is to protect the rights and well-being of study patients and the
integrity of the clinical research process.”
Key Findings
| · | Patients treated with NE3107 showedimproved performance
compared to placebo on all cognitive and functional assessments commonly used in the prior approval of amyloid beta (Aβ)-based AD
therapies, although the data missed statistically significance due to site exclusions. Placebo-treated patients significantly worsened
on virtually all assessments as expected from natural history of the disease. By contrast, NE3107-treated patients experienced a treatment
advantage after 6 months that was equal to or greaterthan results reported from clinical trials for the approved Aβ monoclonal antibody
treatments after 18 months.

1 Lecanamab
after 18 months; van Dyck et al. N Engl J Med.2023;388:9-2.
2 Aducunumab
after 18 months; Haeberlein et al. J Prev Alz Dis. 2022;2(9):197-210. |
| | · | Improvements in Clinical Dementia Rating-Sum of Boxes
(CDR-SB)among NE3107-treated patients appear correlated to changes in tumor necrosis factor alpha (TNFα),plasma phosphorylated tau
(p-tau),and the ratio of Aβ42to Aβ40 |
| | | |
| | |  |
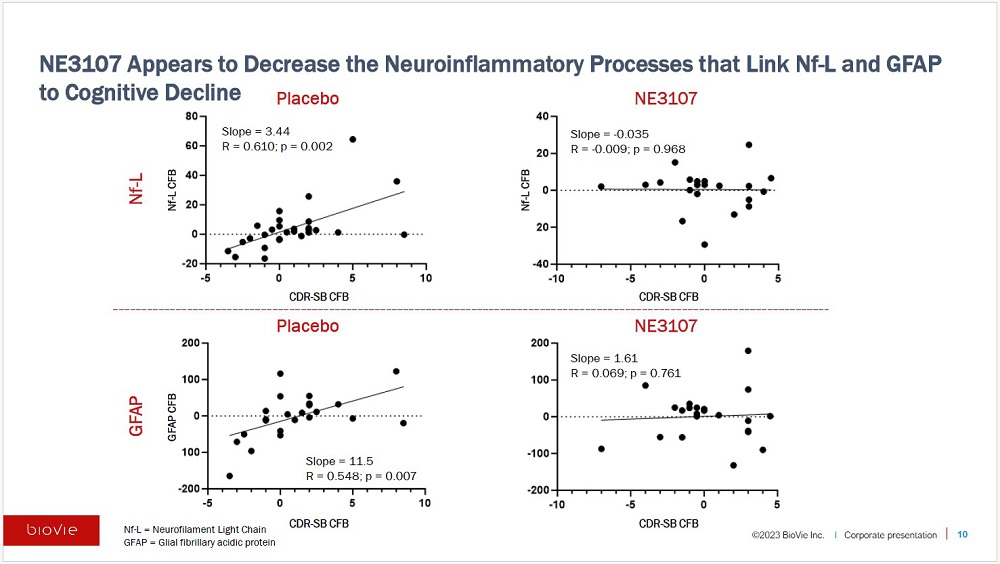
| | · | NE3107 appearedto decrease neuroinflammatory processes
that linkNeurofilament Light Chain (Nf-L) and Glial fibrillary acidic protein (GFAP),both considered biomarkers ofneurodegeneration and
cognitive decline. |
| | | |
| | |  |
| | · | Patients treated with NE3107 saw an average of -5.66
years of age deceleration of the DNA methylation advantage compared to those on placebo. Age deceleration is the difference between the
patient’s biological age as measured by the Horvath DNA methylation Skin Blood clock less the actual chronological age. NE3107 is
believed to be the first drug candidate to demonstrate this impact on DNA methylation and the aging process in a double-blinded, placebo-controlled
clinical. |
| | | |
| | |  |
“The unblinded topline efficacy data from
57 per-protocol participants reaffirmed what has been seen in previous studies of NE3107 – which is that patients treated with
this molecule appear to experience cognitive and functional improvements as measured by multiple assessment tools,” said Dr.
Joseph Palumbo, Executive Vice President, R&D and Chief Medical Officer. “This data reinvigorates our ambition to further
evaluate NE3107 and bring the Alzheimer’s community a differentiated treatment that is safe and has a meaningful impact on
cognition.”
“The unblinding of topline efficacy data from
the trialconfirmed an unusualpattern we saw with the blinded data – that patients in a particular demographic group within the trial
seemed tohave a data pattern different from historical evidence forthis demographic group.Patients from this demographic group in this
trialreportedly experienced cognitive improvements that were improbable scientifically, and inconsistent with the pathology of this disease,”
stated Suzanne Hendrix, Founder & CEO of Pentara, a specialized biostatistics consulting firm that has assisted dozens of AD clinical
trials. “When sensitivity analyses were performed, we determined that the anomalous demographic datawere associated with the previously
identified anomalous sites located in the same geographic area.”
Conference Call & Webcast
| Date |
November 29, 2023 |
| Time |
8:30am Eastern Time |
| Conference Call Details |
1-877-407-3982 - Investors Dial (US)
1-201-493-6780 - Investors Dial (Ex US)
13742846 - Conference ID#
|
| Webcast Link |
Click Here |
| Call MeTM Link |
Click Here |
The NCT04669028 Trial – Background &
Corrective Actions
The NCT04669028 trial is a Phase 3, double-blind,
randomized, placebo-controlled, parallel group, multicenter study of NE3107 in patients who have probable mild- to moderate-AD withscores
on CDR 1-2 and MMSE 14-24. The study has co-primary endpoints looking at cognition using the Alzheimer’s Disease Assessment Scale-Cognitive
Scale (ADAS-Cog 12) and function using theClinical Dementia Rating-Sum of Boxes (CDR-SB). Patients went through two weeks each of 5 mg
and 10 mg BID dose titration followed by 26 weeks of 20 mg twice daily vs. placebo, randomized 1:1.
Enrollment
The trial started enrolling patients in August 2021when COVID-19 pandemic
restrictions provided limited access to clinical sites,and the last patient’s last treatment visit was completed in September 2023.
A total of 439 patientswere enrolled in the trial. BioVie monitored blinded data over the course of the trial to tracksafety and ensure
timely entry of data from the clinical sites into the Electronic Data Capture (EDC, the official database that is submitted to the FDA
for registrational purposes) by the Company’s Clinical Research Organization (CRO). The CRO manages the EDC, and each clinical site
can enter data only from its patientswhile BioVie has read-only access to review blinded data.
Data Review and Audit
The Company retained three independent biostatistical consulting
firms to analyze the data, including Pentara, which has helped pharmaceutical companies assess dozens of AD trials. Pentara reviewed
the blinded data when enough patients completed the trial and identified: 1) several sites in a singlegeographic area were anomalous
(inconsistent data patterns compared to historical data, large proportions of patientsimproving compared to baseline, unusual data
variability); and 2) all patientsin a particular demographic group enrolled in this trial seemed to havea data pattern not
explainable based on established disease progression and which substantially deviated from historical data for this demographic in
other AD trials. Without unblinding and PK data, there was no way to identify the causeof the pattern. Thus,Pentara recommended a
subgroup analysis of the identified demographic group vs.all others and anomalous sites vs. others when the study becomes
unblinded.
In parallel, some sites started to complete their patient-facing activities
in early summer 2023, which created the first opportunity for BioVie to start the data verification and assessment process. The processsurfaced
unusual data patterns and deviations from expectations (missing data, suspectedcopied/pasted MRI results, etc.), which ledthe Company
to retain two new CROsto conduct a multi-step process that entailed quality control (QC) visits at all sites, performingsource data verification
(SDV) on 100% of the documents used in the clinical sites to ensure what was notated on paper during patient visits was accurately reflected
in the EDC, and auditing the sites. This extensive, multi-monthprocess concluded when the Company received the final report identifying
six sites that appeared to havea large number ofdeviations from the study protocol and Good Clinical Practices (GCP).
Corrective Actions
Based on the reportedfindings, and to act responsibly with an abundance
of caution, the Company undertook the following before the EDC containing cognitive and functional assessments from the clinical sites
was frozen:
| 1. | Modifiedthe trial’s Statistical Analysis Plan (SAP)
to exclude the 6 sites with GCP and/or protocol deviation from the analysis; |
| 2. | Amended
the studyprotocol to finalize the primary endpoints to be CDR-SB and ADAS-Cog121
based on
our interactions with the FDA and to allow for us to work with FDA to employ the “adaptive
design” feature of the trial whereby we mightbe able to continue enrolling additional
patients into the trial should we miss statistical significance due to exclusion of patients
from the affected sites; and |
| 3. | Notified the FDA’s Office of Scientific Investigation
(OSI) of the potential issues at the multiple sites with GCP and/or protocol deviation. |
As the top-line efficacy data was unblinded and PK data became available,
the pre-specified demographic subgroup analyses showed that patients in the identified demographic on placebo significantly improved cognitively
without any intervention – a finding that cannot be explained scientifically. Furthermore, the pre-specified anomalous sites vs.
others revealed a similar scientifically improbable and that these 9 sites are in the same single geographic area.It turned out that virtually
all of the patients in the identified demographic group were associated with the 9 anomalous sites. Consistent with our pre-specified
statistical plan, these 9 additional sites were also excluded to arrive at our Modified Intent to Treat population, which became underpowered
with just 81 subjects. Out of an abundance of caution, we also referred these 9 additional sites to the FDA’s OSI. It should be
noted that virtually all of the 15sites referred to the FDA were in the same geographic area.
1 CDR-SB
= Clinical Dementia Rating-Sum of Boxes;ADAS-Cog12 = Alzheimer’s Disease Assessment Scale-Cognitive
Adaptive Design & Next Steps
The trial was originally designed to be 80% powered with 125 patients
in each of the treatment and placebo arms. The unplanned exclusion of so many patients has left the trial unpowered for the primary endpoints.
Based on the efficacy signal seen in this trial, the Company intends to work with the FDA to potentially employ the adaptive trial feature
of the protocol to continue enrolling patients to achieve statistical significance. The Company has retained a new CRO for future trials.
About NE3107
NE3107 is an oral, small molecule, blood-brain permeable
anti-inflammatory insulin sensitizer that binds extracellular signal-regulated kinase.BioVie’s Phase 3 trial is the largest study
to date to evaluate the safety and efficacy of NE3107 in patients with AD. NE3107 is the only anti-inflammatory agent currently in phase
3 development for AD. Consistent with the proposed anti-inflammatory and insulin-sensitizing properties of NE3107, this phase 3 study
was designed to confirm the efficacy and safety of NE3107 treatment in patients with probable AD.
About BioVie
BioVie Inc. (NASDAQ: BIVI) is a clinical-stage company
developing innovative drug therapies for the treatment of neurological and neurodegenerative disorders and advanced liver disease. In
neurodegenerative disease, the Company’s drug candidate NE3107 inhibits inflammatory activation of ERK and NFkB (e.g., TNF signaling)
that leads to neuroinflammation and insulin resistance, but not their homeostatic functions (e.g., insulin signaling and neuron growth
and survival). Both are drivers of Alzheimer’s and Parkinson’s diseases. The Company is conducting a Phase 3 randomized, double-blind,
placebo-controlled, parallel-group, multicenter study to evaluate NE3107 in patients who have mild to moderate Alzheimer's disease (NCT04669028).
Results of a Phase 2 investigator-initiated trial (NCT05227820) showing NE3107-treated patients experienced improved cognition and biomarker
levels were presented at the Clinical Trial in Alzheimer’s Disease (CTAD) annual conference in December 2022. An estimated six million
Americans suffer from Alzheimer’s. A Phase 2 study of NE3107 in Parkinson’s disease (NCT05083260) has completed, and data
presented at the International Conference on Alzheimer's and Parkinson's Disease and Related Neurological Disorders conference in Gothenburg,
Sweden in March 2023 showed significant improvements in “morning on” symptoms and clinically meaningful improvement in motor
control in patients treated with a combination of NE3107 and levodopa vs. patients treated with levodopa alone, and no drug-related adverse
events. In liver disease, the Company’s Orphan drug candidate BIV201 (continuous infusion terlipressin), with FDA Fast Track status,
is being evaluated anddiscussed with guidance received from the FDA regarding the design of Phase 3 clinical testing of BIV201 for the
treatment of ascites due to chronic liver cirrhosis. The active agent is approved in the U.S. and in about 40 countries for related complications
of advanced liver cirrhosis. For more information, visit http://www.bioviepharma.com/.
Forward-Looking Statements
This press release contains forward-looking
statements, which may be identified by words such as "expect," "look forward to," "anticipate" "intend,"
"plan," "believe," "seek," "estimate," "will," "project" or words of similar
meaning. Although BioVie Inc. believes such forward-looking statements are based on reasonable assumptions, it can give no assurance
that its expectations will be attained. Actual results may vary materially from those expressed or implied by the statements herein due
tothe risk that unblinded data is not consistent with blinded data, the Company's ability to successfully raise sufficient capital on
reasonable terms or at all, available cash on hand and contractual and statutory limitations that could impair our ability to pay future
dividends, our ability to complete our pre-clinical or clinical studies and to obtain approval for our product candidates,
our ability to successfully defend potential future litigation, changes in local or national economic conditions as
well as various additional risks, many of which are now unknown and generally out of the Company's control, and which are detailed from
time to time in reports filed by the Company with the SEC, including quarterly reports on Form 10-Q, reports on Form 8-K and annual reports
on Form 10-K. BioVie Inc. does not undertake any duty to update any statements contained herein (including any forward-looking statements),
except as required by law.
For Investor Relations Inquiries:
Contact:
Bruce Mackle
Managing Director,LifeSci Advisors, LLC
bmackle@lifesciadvisors.com |
|
ForMedia Relations Inquiries:
Melyssa Weible
Managing Partner, Elixir Health Public Relations
mweible@elixirhealthpr.com
Exhibit 99.2











v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
BioVie (NASDAQ:BIVI)
Historical Stock Chart
From Oct 2024 to Nov 2024

BioVie (NASDAQ:BIVI)
Historical Stock Chart
From Nov 2023 to Nov 2024
