false 0001845337 0001845337 2024-01-08 2024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): (January 8, 2024)
DAY ONE BIOPHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-40431 |
|
83-2415215 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 2000 Sierra Point Parkway, Suite 501 |
|
|
| Brisbane, California |
|
94005 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (650) 484-0899
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
DAWN |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
As previously announced, on January 8, 2024, Day One Biopharmaceuticals, Inc. (the “Company”) will participate in the 42nd Annual J.P. Morgan Healthcare Conference (the “JPM Conference”). A copy of the Company’s presentation materials for the JPM Conference is attached hereto as Exhibit 99.1 and is incorporated herein by reference.
Also on January 8, 2024, the Company updated its corporate presentation in connection with its participation in the JPM Conference. A copy of the updated corporate presentation materials is attached hereto as Exhibit 99.2 and is incorporated herein by reference.
The information in this Item 7.01, including Exhibits 99.1 and 99.2 to this report, shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”). The information contained in this Item 7.01 and in the accompanying Exhibits 99.1 and 99.2 shall not be incorporated by reference into any other filing under the Exchange Act or under the Securities Act, except as shall be expressly set forth by specific reference in such filing.
| Item 9.01 |
Financial Statements and Exhibits. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
DAY ONE BIOPHARMACEUTICALS, INC. |
|
|
|
|
| Date: January 8, 2024 |
|
|
|
By: |
|
/s/ Charles N. York II, M.B.A. |
|
|
|
|
|
|
Charles N. York II, M.B.A. |
|
|
|
|
|
|
Chief Operating Officer and Chief Financial Officer |

Exhibit 99.1 Nasdaq: DAWN Day One Biopharmaceuticals Targeted Therapies
for People of All Ages nd 42 Annual J.P. Morgan Healthcare Conference January 2024 1

Disclaimer This presentation and the accompanying oral commentary
contain forward-looking statements that are based on our management’s beliefs and assumptions and on information currently available to our management. Forward-looking statements are inherently subject to risks and uncertainties, some of which
cannot be predicted or quantified. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expect, ” “plan, ”
anticipate, ” “believe, ” “estimate,” “predict,” “intend,” “potential,” “would,” “continue,” “ongoing” or the negative of these terms or other
comparable terminology. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, including the sufficiency of our
cash, cash equivalents and short-term investments to fund our operations, business plans and objectives, timing and success of our planned nonclinical and clinical development activities, the results of any of our strategic collaborations, including
the potential achievement of milestones and provision of royalty payments thereunder, timing and results of nonclinical studies and clinical trials, efficacy and safety profiles of our product candidates, execution of the Phase 2 and Phase 3
clinical trials for tovorafenib and the Phase 1b/2 clinical trial for tovorafenib and pimasertib as designed, any expectations about safety, efficacy, timing and ability to complete clinical trials and to obtain regulatory approvals for tovorafenib
and other candidates in development, the ability of tovorafenib to treat pediatric low-grade glioma (pLGG) or related indications, the potential therapeutic benefits and economic value of our product candidates, potential growth opportunities,
competitive position, industry environment and potential market opportunities, our ability to protect intellectual property and the impact of global business or macroeconomic conditions, including as a result of inflation, rising interest rates,
cybersecurity incidents, instability in the global banking system, government shutdowns, uncertainty with respect to the federal budget and global regional conflicts, on our business and operations. Forward-looking statements are subject to known
and unknown risks, uncertainties, assumptions and other factors. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors,
may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that are described under the heading “Risk Factors” contained in our most recent
Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (“SEC”) and other documents we file from time to time with the SEC, may cause our actual results, performance or achievements to differ materially and
adversely from those anticipated or implied by our forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon
information available to us as of the date of this presentation, and although we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate
that we have conducted a thorough inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements. Furthermore, if our
forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any
other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise,
except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions
and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to
a high degree of uncertainty and risk. 2

3 Sawyer, age 7, lives with pLGG.

Cancer Therapies for People of All Ages Our Approach • Develop
medicines for genomically-defined cancers • Establish first-in-class position through rapid registration pathways • Expand to adolescent and adult populations in parallel and pursue those opportunities with the same commitment we do for
children IPO: 2021 Financial Position: Runway into 2026 Founded: 2018 Nasdaq: DAWN 4

FIREFLY-1: Relapsed or Progressive pLGG • NDA initiated in May
2023 • Clinical data presented in oral presentation at ASCO in June 2023 • FDA acceptance of NDA and priority review granted in October 2023 • Data published in Nature Medicine and oral presentation at SNO in November 2023 2023 Key
• PDUFA target action date of April 30, 2024 Accomplishments FIREFLY-2: Frontline pLGG • Dosed the first patient in March 2023 Business Development • Research collaboration and license agreement for preclinical program targeting
VRK1 in August 2023 Financials • $405.5 million in cash, cash equivalents and short-term investments as of September 30, 2023 • Cash runway into 2026 5

Priorities as we Expand into a Commercial-Stage Company Launch
Tovorafenib Advance Portfolio Expand Pipeline • Secure the first FDA-approved • FIREFLY-2: Study tovorafenib as a • Grow Day One into a leading, targeted therapy for pLGG with frontline therapy for treatment- biopharmaceutical
company that is BRAF fusions and point mutations naive patients with pLGG the partner of choice for oncology that have relapsed or progressed drug development • FIRELIGHT-1: Evaluate • Expand awareness amongst tovorafenib in combination
with • Explore selective partnerships as a physicians and establish broad pimasertib in adolescent and source of capital and risk sharing coverage to enable patient access adult populations • Further invest in business • Following
approval, establish • Advance early stage VRK1 development activities to expand our tovorafenib as the standard of program to clinical development multiple asset portfolio for both care for relapsed or progressive children and adults pLGG
6

Tovorafenib (DAY101) Type II RAF Inhibitor 7

Kids like Sawyer spend most of their childhood as patients rather than
children 3 (1-9) 51% Median (range) number of lines Percentage of patients who 1 of prior systemic therapy had greater than or equal to 3 1 lines of prior systemic therapy 1 8 Sawyer, lives with pLGG. Figures come from FIREFLY-1 baseline patient
characteristics with a June 5, 2023 data cutoff. Total patients enrolled in arm 1 of the FIREFLY-1 trial was 77.

Pediatric Low-Grade Glioma (pLGG): The Most Common Type Of Brain Tumor
In Children pLGGs are chronic and relentless, with patients suffering profound tumor and treatment- 6 associated morbidity that can impact their life trajectory over the long term 7 A Serious and Life-Threatening Disease Disease Symptoms Cerebellar
• An estimated 26,000 children/young adults are living with Cerebral gliomas: gliomas: 1,2 BRAF-altered pLGGs in the U.S. today Seizures, muscle Impaired balance, weakness, behavioral coordination or • For the majority of patients in the
relapsed setting, there is changes depth perception no standard of care and no approved therapies Hypothalamic gliomas: • Surgery plays a significant role in treatment, but vast Endocrine dysfunction 3,4 majority of patients require systemic
therapy and visual deficits Brain stem gliomas: • ~70% of pLGGs have BRAF alterations, which means ~60% Optic pathway gliomas: Difficulty swallowing of pLGGs are BRAF fusions and ~10% are BRAF V600E Decreased vision (acuity or with speech, 5
mutations and/or fields), bulging or abnormal breathing misalignment of eyes 1 2 CBTRUS, Qaddoumi et al 2009, Schreck et al 2019, ClearView Analysis; SEER US complete prevalence counts of patients aged under 25 with Brain and Other Nervous Systems
tumors as of January 1, 2017. Estimated prevalence are Day 3 4 5 6 One calculations based on publicly available data. Ostrum QT et al., Neuro Oncol. 2015; 16(Suppl 10):x1-x36; De Blank P. et al., Curr Opin Pediatr. 2019 Feb; 31(1):21-27. Jones DTW
et al., Cancer Res. 2008; 68:8673–77. Traunwieser T 9 7 et al., Neurooncol Adv. 2020; 2:vdaa094. Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24(11):1397-1408. doi:10.1177/0883073809342005.

Tumor Response To Tovorafenib (DAY101) in FIREFLY-1 is Well Aligned
with pLGG Treatment Goals RANO-HGG Overall Response Rate Clinically meaningful responses observed across all three assessment criteria, 67% ORR by RANO-HGG, 53% ORR by RANO-LGG, 51% ORR by RAPNO-LGG. Clinical Benefit Rate RANO-LGG Responses seen in
a heavily-pretreated population, 93% CBR by RANO-HGG, 83% ORR by RANO-LGG, 82% ORR by RAPNO-LGG. Duration of Treatment Median duration of tovorafenib treatment of 15.8 months as of June 5, 2023, with 66% of patients remaining on treatment. RAPNO-LGG
Safety Safety and tolerability profile indicating monotherapy tovorafenib to be generally well-tolerated 10 June 5, 2023 data cutoff. CBR, clinical benefit rate; CR, complete response; DOR, duration of response; HGG, high-grade glioma; LGG,
low-grade glioma; MR, minor response; ORR, overall response rate; PD, progressive disease; PR, partial response; RANO, Response Assessment in Neuro-Oncology; RAPNO, Response Assessment in Pediatric Neuro-Oncology; SD, stable disease; ORR and CBR for
RAPNO-LGG and RANO-LGG included MRs. Maximum change in Maximum change in Maximum change in tumor size (%) tumor size (%) tumor size (%)

Estimated BRAF-Altered pLGG Patient Population In The U.S. ~26,000
Prevalence of Systemically- ~2,000-3,000 Treated Patients 1-5 ~1,100 Under 25 Recurrent/Progressive Total Addressable Incidence of Patient Population Treatment- 6 per Year Eligible Frontline 4,5 Patients The estimated addressable pool of recurrent
or 6 progressive pLGG patients is ~2,000-3,000 per year at steady state* 1 2 Selt F, van Tilburg CM, Bison B, et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neurooncol. 2020;149(3):499-510.
doi:10.1007/s11060-020-03640-3. Ryall S, Tabori U, Hawkins C. Pediatric low- 3 grade glioma in the era of molecular diagnostics. Acta Neuropathol Commun. 2020;8(1):30. doi:10.1186/s40478-020-00902-z. SEER US complete prevalence counts of patients
aged under 25 with Brain and Other Nervous Systems tumors 11 4 5 as of January 1, 2017. CBTRUS, Qaddoumi et al 2009, Schreck et al 2019, ClearView Analysis. US Census. Estimated annual incidence, estimated prevalence, and estimated
recurrent/progressive total addressable patient population 6 are Day One calculations based on publicly available data. Source: Internal market research conducted by EpidStrategies, A Division of ToxStrategies, Inc. on behalf of Day One , * The
estimated addressable pool of recurrent or progressive pLGG patients is based on progression free survival curves modeled from published literature.

* Preparing for a Successful Launch Key Factors Priorities •
Communicate strong clinical profile for Drive FIREFLY-1 Trial and Physician tovorafenib without significant disruption to Awareness childhood • Enable patient access through establishing Build momentum with pediatric broad coverage and patient
support programs oncologists at the ~200 U.S. Centers of Excellence • Experienced, fully dedicated field sales force (18 U.S. Account Managers) • Positive patient experience, drug profile Enable unrestricted patient access consisting of
once-weekly dosing (oral tablet or liquid formulation) * 12 Pending FDA Approval

FIREFLY-2 Pivotal Phase 3 Trial of Tovorafenib (DAY101) in Frontline
pLGG 13

Expansion into Frontline Treatment Represents a Meaningful Expansion
Opportunity for Tovorafenib in pLGG Disease Overview Phase 3 Trial Summary • pLGG is the most common brain tumor diagnosed in • Randomized, global, registrational Phase 3 trial of children monotherapy tovorafenib (DAY101) vs SoC
chemotherapy • pLGG can be diagnosed at any age in pediatric patients from • Eligibility: Patients aged 6 months to <25 years with LGG infancy to young adults harboring a RAF alteration and requiring first-line systemic therapy
• Estimated U.S. treatment eligible incidence of ~1,100 annually • Primary endpoint: ORR based on RANO-LGG criteria, assessed by blinded independent central review • Conventional treatment options are surgery, chemotherapy,
radiation or targeted therapy • Key secondary endpoints: PFS and DoR by RANO criteria, ORR by RAPNO criteria • High unmet need for an effective therapy for the majority of pLGG patients that is minimally disruptive to their lives ~80
sites activated globally * COG or SIOPe-LGG regimen. Abbreviations: CHG, chiasmatic, hypothalamic glioma; DoR, duration of response; LGG, low-grade glioma; ORR, objective response rate; QoL, quality of life; QW, once weekly; SoC, 1 standard of care.
Primary endpoint of FIREFLY-2 will be ORR by RANO-LGG (2017) following full approval by FDA on March 16, 2023 of dabrafenib with trametinib in pediatric patients with low-grade glioma with a 14 BRAF V600E mutation who require systemic therapy based
on a study with the same primary endpoint.

FIRELIGHT-1 Phase 1b/2 Trials Evaluating Tovorafenib (DAY101) as a
Combination with Pimasertib 15

Tovorafenib (DAY101) / Pimasertib Combination In Solid Tumors
(FIRELIGHT-1) Combination with tovorafenib (DAY101) and other targeted therapies may unlock the full value of pimasertib in advanced solid tumors Pimasertib Overview Phase 1b/2 Trial Summary • Pimasertib is an investigational
orally-bioavailable, selective, • Combination dose escalation, global phase 1b/2 trial, Phase 1b, non-competitive MEK1/2 inhibitor in-licensed from Merck KGaA BOIN (adaptive), n = ~10/cohort, Phase 2, Simon 2-stage, n = in February 2021
~25/cohort • Nearly three-fold higher CNS penetration than other MEKi • Eligibility: Patients aged 12 years and older, dose escalation will inhibitors (trametinib or selumetinib) be performed in advanced solid tumor patients with any
MAPK alteration. Expansion cohorts will focus on indications with a • Pimasertib showed monotherapy clinical activity, including an potential path to accelerated approval improvement in median PFS versus dacarbazine in NRAS mutant melanoma
• Endpoints: Phase 1b: PK, PD and Safety, MTD/RP2D, Phase 2: Efficacy (ORR, DOR) • Patient populations targeted in expansion phase will include NRAS mutant melanoma including BRAF-altered tumors of class 1 non-EK, class 2 BRAF
alternations and fusion patients Anticipate recommended Phase 2 dose and schedule in 2H 2024 * COG or SIOPe-LGG regimen. Abbreviations: CHG, chiasmatic, hypothalamic glioma; DoR, duration of response; LGG, low-grade glioma; ORR, objective response
rate; QoL, quality of life; QW, once weekly; SoC, 1 standard of care. Primary endpoint of FIREFLY-2 will be ORR by RANO-LGG (2017) following full approval by FDA on March 16, 2023 of dabrafenib with trametinib in pediatric patients with low-grade
glioma with a 16 BRAF V600E mutation who require systemic therapy based on a study with the same primary endpoint.

Pillars for Sustainable Growth to Drive Long-Term Success Tovorafenib
Launch Pipeline Capital People • Secure FDA approval; • Continue enrollment into • Thoughtful, data-driven • Deeply experienced in PDUFA target action date frontline pLGG trial approach to capital oncology, pediatric, and of
April 30, 2024 (FIREFLY-2) driving allocation driving rare disease disciplined investment in development, meaningful expansion • Institute broad coverage clinical development and registration and and growth opportunity to drive patient access
pipeline expansion commercialization • Initiate combination • Following approval, cohorts with pimasertib • PRV eligible with establish tovorafenib as in adolescent and adult approval of tovorafenib the standard of care in in
relapsed or populations relapsed or progressive progressive pLGG pLGG • Advance early stage VRK1 program to clinical • Cash runway into 2026 development 17

Q&A 18

Exhibit 99.2 Day One Biopharmaceuticals Targeted Therapies for People of
All Ages January 2024 1

Disclaimer This presentation and the accompanying oral commentary
contain forward-looking statements that are based on our management’s beliefs and assumptions and on information currently available to our management. Forward-looking statements are inherently subject to risks and uncertainties, some of which
cannot be predicted or quantified. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expect, ” “plan, ”
anticipate, ” “believe, ” “estimate,” “predict,” “intend,” “potential,” “would,” “continue,” “ongoing” or the negative of these terms or other
comparable terminology. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, including the sufficiency of our
cash, cash equivalents and short-term investments to fund our operations, business plans and objectives, timing and success of our planned nonclinical and clinical development activities, the results of any of our strategic collaborations, including
the potential achievement of milestones and provision of royalty payments thereunder, timing and results of nonclinical studies and clinical trials, efficacy and safety profiles of our product candidates, execution of the Phase 2 and Phase 3
clinical trials for tovorafenib and the Phase 1b/2 clinical trial for tovorafenib and pimasertib as designed, any expectations about safety, efficacy, timing and ability to complete clinical trials and to obtain regulatory approvals for tovorafenib
and other candidates in development, the ability of tovorafenib to treat pediatric low-grade glioma (pLGG) or related indications, the potential therapeutic benefits and economic value of our product candidates, potential growth opportunities,
competitive position, industry environment and potential market opportunities, our ability to protect intellectual property and the impact of global business or macroeconomic conditions, including as a result of inflation, rising interest rates,
cybersecurity incidents, instability in the global banking system, government shutdowns, uncertainty with respect to the federal budget and global regional conflicts, on our business and operations. Forward-looking statements are subject to known
and unknown risks, uncertainties, assumptions and other factors. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors,
may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that are described under the heading “Risk Factors” contained in our most recent
Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (“SEC”) and other documents we file from time to time with the SEC, may cause our actual results, performance or achievements to differ materially and
adversely from those anticipated or implied by our forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon
information available to us as of the date of this presentation, and although we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate
that we have conducted a thorough inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements. Furthermore, if our
forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any
other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise,
except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions
and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to
a high degree of uncertainty and risk. 2

Cancer Therapies for People of All Ages Our Approach • Develop
medicines for genomically-defined cancers • Establish first-in-class position through rapid registration pathways • Expand to adolescent and adult populations in parallel and pursue those opportunities with the same commitment we do for
children IPO: 2021 Financial Position: Runway into 2026 Founded: 2018 Nasdaq: DAWN 3
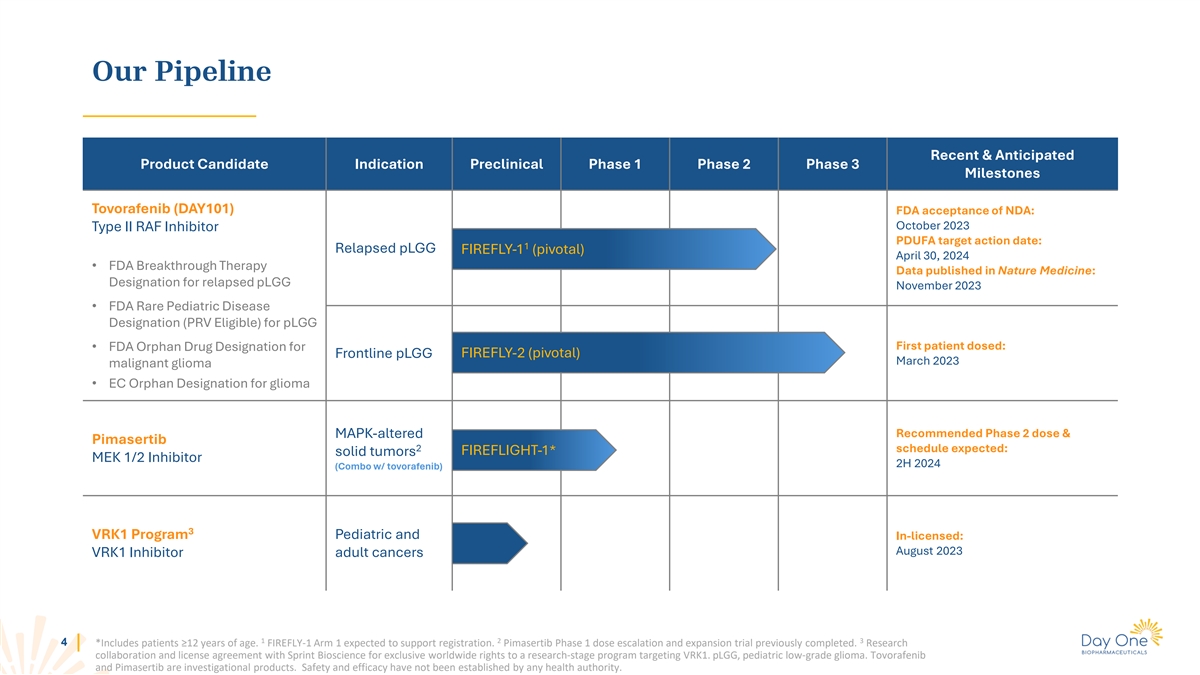
Our Pipeline Recent & Anticipated Product Candidate Indication
Preclinical Phase 1 Phase 2 Phase 3 Milestones Tovorafenib (DAY101) FDA acceptance of NDA: October 2023 Type II RAF Inhibitor PDUFA target action date: 1 Relapsed pLGG FIREFLY-1 (pivotal) April 30, 2024 • FDA Breakthrough Therapy Data
published in Nature Medicine: Designation for relapsed pLGG November 2023 • FDA Rare Pediatric Disease Designation (PRV Eligible) for pLGG First patient dosed: • FDA Orphan Drug Designation for FIREFLY-2 (pivotal) Frontline pLGG March
2023 malignant glioma • EC Orphan Designation for glioma Recommended Phase 2 dose & MAPK-altered Pimasertib 2 schedule expected: FIREFLIGHT-1* solid tumors MEK 1/2 Inhibitor 2H 2024 (Combo w/ tovorafenib) 3 VRK1 Program Pediatric and
In-licensed: August 2023 VRK1 Inhibitor adult cancers 1 2 3 4 *Includes patients ≥12 years of age. FIREFLY-1 Arm 1 expected to support registration. Pimasertib Phase 1 dose escalation and expansion trial previously completed. Research
collaboration and license agreement with Sprint Bioscience for exclusive worldwide rights to a research-stage program targeting VRK1. pLGG, pediatric low-grade glioma. Tovorafenib and Pimasertib are investigational products. Safety and efficacy have
not been established by any health authority.

Tovorafenib (DAY101) Type II RAF Inhibitor 5

Kids like Sawyer spend most of their childhood as patients rather than
children 3 (1-9) 51% Median (range) number of lines Percentage of patients who 1 of prior systemic therapy had greater than or equal to 3 1 lines of prior systemic therapy 1 6 Sawyer, lives with pLGG. Figures come from FIREFLY-1 baseline patient
characteristics with a June 5, 2023 data cutoff. Total patients enrolled in arm 1 of the FIREFLY-1 trial was 77.

Pediatric Low-Grade Glioma (pLGG): The Most Common Type Of Brain Tumor
In Children pLGGs are chronic and relentless, with patients suffering profound tumor and treatment- 6 associated morbidity that can impact their life trajectory over the long term 7 A Serious and Life-Threatening Disease Disease Symptoms Cerebellar
• An estimated 26,000 children/young adults are living with Cerebral gliomas: gliomas: 1,2 BRAF-altered pLGGs in the U.S. today Seizures, muscle Impaired balance, weakness, behavioral coordination or • For the majority of patients in the
relapsed setting, there is changes depth perception no standard of care and no approved therapies Hypothalamic gliomas: • Surgery plays a significant role in treatment, but vast Endocrine dysfunction 3,4 majority of patients require systemic
therapy and visual deficits Brain stem gliomas: • ~70% of pLGGs have BRAF alterations, which means ~60% Optic pathway gliomas: Difficulty swallowing of pLGGs are BRAF fusions and ~10% are BRAF V600E Decreased vision (acuity or with speech, 5
mutations and/or fields), bulging or abnormal breathing misalignment of eyes 1 2 CBTRUS, Qaddoumi et al 2009, Schreck et al 2019, ClearView Analysis; SEER US complete prevalence counts of patients aged under 25 with Brain and Other Nervous Systems
tumors as of January 1, 2017. Estimated prevalence are Day 3 4 5 6 One calculations based on publicly available data. Ostrum QT et al., Neuro Oncol. 2015; 16(Suppl 10):x1-x36; De Blank P. et al., Curr Opin Pediatr. 2019 Feb; 31(1):21-27. Jones DTW
et al., Cancer Res. 2008; 68:8673–77. Traunwieser T 7 7 et al., Neurooncol Adv. 2020; 2:vdaa094. Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24(11):1397-1408. doi:10.1177/0883073809342005.

Conventional Treatments Can Be Disruptive To Childhood and Can Have
Significant Long-Term Consequences Surgery Chemotherapy Radiation • Significant recovery times • Requirement for indwelling • Risk of secondary malignancy catheter and weekly infusions • Risks of complications • Risk of
malignant transformation • Risk of neutropenia, • Resection may be limited by • Risk of vascular proliferation and hypersensitivity reactions, location of tumor stroke nausea and vomiting and • Potential for functional
deficits • Neurocognitive impact, peripheral neuropathy based on location of tumor depending on location of tumor and extent of resection and radiation field Clear need for an effective therapy for the majority of pLGG relapsed or progressive
patients that is minimally disruptive to their lives. Source: 1. Heitzer AM, Raghubar K, Ris MD, et al. Neuropsychological functioning following surgery for pediatric low-grade glioma: a prospective longitudinal study. J Neurosurg Pediatr. 2019;1-9.
doi:10.3171/2019.9.PEDS19357. 2. Bryant R. Managing side effects of childhood cancer treatment. J Pediatr Nurs. 2003;18(2):113-125. doi:10.1053/jpdn.2003.11. 3. Zahnreich S, Schmidberger H. Childhood cancer: occurrence, treatment and risk of second
primary malignancies. Cancers (Basel). 2021;13(11):2607. doi:10.3390/cancers/13112607. 4. National Cancer Institute. Fertility issues in girls and women with cancer. http://www.cancer.gov. Accessed June 13, 2022. 5. Alessi I., Caroleo A.M., de Palma
L., Mastronuzzi A., Pro S., Colafati G.S., Boni A., Della Vecchia N., Velardi M., Evangelisti M., et al. Short and Long-Term Toxicity 8 in Pediatric Cancer Treatment: Central Nervous System Damage. Cancers. 2022;14:1540. doi:
10.3390/cancers14061540.

Tovorafenib (DAY101) Inhibits Both BRAF Fusions And BRAF V600 Mutations
MAPK pathway RAS-independent activation of the MAPK pathway Tovorafenib (DAY101) is an investigational, oral, selective, CNS- penetrant, type II RAF inhibitor that was RAS designed to inhibit both monomeric and dimeric RAF kinase • Activity in
tumors driven by both RAF fusions RAF RAF RAF Tovorafenib and BRAF V600E mutations mutation fusion • Tablet and pediatric-friendly liquid suspension • Once weekly dosing MEK Currently approved type I BRAF inhibitors are indicated for use
in patients with tumors bearing BRAF V600E mutations ERK • Type I BRAF inhibitors cause paradoxical MAPK activation in the setting of wild-type RAF, increasing the risk of tumor growth in Proliferation and survival Proliferation and survival
Proliferation and survival BRAF fusion-driven 9 Source: 1. Sun Y et al., Neuro Oncol. 2017; 19: 774–85; 2. Sievart AJ et al., PNAS. 2013; 110:5957-62; 3. Karajannis MA et al., Neuro Oncol 2014;16(10):1408-16.

Pivotal Phase 2 Trial Of Monotherapy Tovorafenib (DAY101) In Relapsed
Or Progressive pLGG (FIREFLY-1) – Fully Enrolled & Data Accepted by FDA Trial Design Endpoints (Pivotal Arm 1) 1 • Three arm, open-label, global registrational phase 2 trial • Primary endpoint: ORR based on RANO-HGG , assessed
by blinded independent central review • Pivotal Arm 1 (recurrent/progressive pLGG, n=77): harboring a KIAA1549- BRAF fusion or BRAF V600E mutation 2 • Secondary endpoints: ORR by RAPNO-LGG assessed by blinded independent • Arm 2
(expanded access recurrent/progressive LGG, n=60): harboring an central review; PFS, DoR; TTR, CBR; safety activating RAF alteration 3 • Exploratory analyses: ORR and CBR by RANO-LGG assessed by blinded • Arm 3 (extracranial solid
tumors): harboring an activating RAF fusion independent central review rd Clinical and radiological evaluations at baseline, and every 3 Key Inclusion Criteria nd cycle for pLGG and every 2 cycle for solid tumors • 6 months – 25 years of
age Day –28 to 0 • RAF-altered tumor Enrollment/ • ≥1 prior line of systemic Screening Baseline End of Trial Study Drug Administration After Cycle 27: patients may either C27D1 therapy with radiographic (C1D1) 2 continue
treatment or enter drug 420mg/m QW (not to exceed 600mg), progression holiday period at any time (at QW in 28-day cycles discretion of investigator) • Prior use of MAPK pathway targeted therapy was permitted Eligibility evaluation Treatment
period: minimum of 2 years or until progression or toxicity/intolerability 1 2 3 June 5, 2023 data cutoff. Wen PY, et al. J Clin Oncol. 2010;28(11):1963-1972. Fangusaro J, et al. Lancet Oncol. 2020;21(6):e305–316. van den Bent MJ, et al.
Lancet Oncol. 2011;12(6):583-593. Abbreviations: CBR, clinical benefit rate; IRC, independent review committee; C, cycle; 10 D, day; LGG, low-grade glioma; ORR, objective response rate; PFS, progression-free survival; DoR, duration of response; QW,
once weekly; TTR, time to response; RANO, Response Assessment in Neuro-Oncology; RAPNO, Response Assessment in Pediatric Neuro-Oncology; MAPK, mitogen-activated protein kinase. For more information, please refer to NCT04775485

Data from Pivotal Phase 2 FIREFLY-1 Trial June 5, 2023 data cutoff
11

FIREFLY-1 Baseline Patient Characteristics Characteristic Arm 1 (n=77)
Location (n=77) Deep midline structures Optic pathway Median age, years (range) 8 (2-21) 12% 51% Sex, n (%) Other Male 40 (52) † 16% Female 37 (48) Cerebral hemisphere 8% Race, n (%) Cerebellum Brain stem White 41 (53) 6% 8% Asian 5 (6) Black
2 (3) Multiple 3 (4) Other 6 (8) BRAF alteration (n=77) Not specified 20 (26) Number of lines of prior systemic therapy 17% Median (range) 3 (1-9) 1, n (%) 17 (22) 2, n (%) 21 (27) ≥3, n (%) 39 (51) 83% Prior MAPK pathway targeted therapy, n
(%) Prior MEK inhibitor 43 (56) Prior BRAF inhibitor 8* (10) ‡ Prior BRAF and MEK inhibitors 5 (7) BRAF V600E BRAF Fusion* Any MAPK inhibitor 46 (60) * † June 5, 2023 data cutoff. Includes 6 patients with BRAF duplication and 2 with BRAF
rearrangement per fluorescence in situ hybridization or in situ hybridization. Includes tumors that were 12 extending into multiple regions of the brain, leptomeningeal disease, and/or spinal disease. ‡The 5 patients that had previously
received both a MEK inhibitor and also a BRAF inhibitor are recorded in both the “Prior MEK inhibitor” and “Prior BRAF inhibitor” groups. MAPK, mitogen-activated protein kinase.

Tumor Response To Tovorafenib (DAY101) Using RAPNO-LGG, RANO-LGG and
RANO-HGG RAPNO-LGG RAPNO-LGG RANO-LGG RANO-HGG Response (IRC) n=76 N=76 N=69 ORR,* n (%) 39 (51) 40 (53) 46 (67) 95% CI 40-63 41-64 54-78 CBR,* n (%) 62 (82) 63 (83) 64 (93) SD of any length of time 43 (57) 46 (61) 54 (78) SD ≥12 months
RANO-LGG BOR,* n (%) 0 0 12 (17) CR 28 (37) 20 (26) 34 (49) PR 11 (14) 20 (26) n/a MR 23 (30) 23 (30) 18 (26) SD 19 (25) 17 (22) 10 (14) SD <12 months 4 (5) 6 (8) 8 (12) SD ≥12 months 13 (17) 11 (14) 4 (6) PD 1 (1) 2 (3) 1 (1) NE RANO-HGG
Median DOR, months 13.8 14.4 16.6 95% CI 11.3-NR 11.0-NR 11.6-NR Median TTR, months 5.3 5.5 3.0 Range 1.6-11.2 1.6-11.3 2.6-16.6 13 June 5, 2023 data cutoff. BOR, best overall response; CBR, clinical benefit rate; CI, confidence interval; CR,
complete response; DOR, duration of response; HGG, high-grade glioma; IRC, independent radiology review committee; LGG, low-grade glioma; MR, minor response; n/a, not applicable; NE, not evaluable; NR, not reached; ORR, overall response rate; PD,
progressive disease; PR, partial response; RANO, Response Assessment in Neuro-Oncology; RAPNO, Response Assessment in Pediatric Neuro-Oncology; SD, stable disease; TTR, time to response. * ORR, CBR and BOR for RAPNO-LGG and RANO-LGG included MRs.
Maximum change in Maximum change in Maximum change in tumor size (%) tumor size (%) tumor size (%)

Duration Of Tovorafenib (DAY101) Therapy For All Patients With
RAPNO-LGG Evaluable Lesions 5.3 Median time to response Months Median duration of 13.8 treatment Months Overall treatment duration (months) 14 June 5, 2023 data cutoff. Patients

Tumor Kinetics In Patients With Best Response Of Progressive Disease
According To RAPNO-LGG The majority of patients who had radiographic progression by RAPNO-LGG at their initial disease assessment had subsequent prolonged reductions in the size of their tumor with continued treatment. Months 15 June 5, 2023 data
cutoff. Change in tumor size (%)

Tumor Response To Tovorafenib (DAY101) According To RAPNO-LGG In
Subgroups Defined By Baseline Characteristics Male (n=40) Female (n=36) 6 months-<2 years of age (n=0) 2-<6 years of age (n=14) 6-<12 years of age (n=40) Analysis of response data across various subgroups shows no 12-<16 years of age
(n=16) significant differences in 16-25 years of age (n=6) response rate by RAPNO-LGG. White (n=40) Other (n=16) Not reported (n=20) US (n=25) Ex-US (n=51) 0 10 20 30 40 50 60 70 80 90 100 Overall response rate, % (95% confidence interval) 16 June
5, 2023 data cutoff. Other races included Asian (n=5), Black (n=2), Multiple (n=3),and Other (n=6). There were no Native Hawaiian or other Pacific Islander or American Indian or Alaska Native. No race information was missing. Ex, External to; US,
United States.

Tumor Response To Tovorafenib (DAY101) Across Three Assessment Criteria
Were Consistent Across BRAF Fusion And Mutation Patients, and Patients With Prior MAPK Treatment 2 3,4 1 RAPNO-LGG RANO-LGG RANO-HGG Response (IRC) n n n ORR,* n (%) 76 39 (51) 76 40 (53) 69 46 (67) BRAF fusion 64 33 (52) 64 33 (52) 59 41 (69) BRAF
mutation 12 6 (50) 12 7 (58) 10 5 (50) Prior MAPKi 45 22 (49) 45 23 (51) 41 29 (71) MAPKi-naive 31 17 (55) 31 17 (55) 28 17 (61) CBR,* n (%) (SD of any length of time) 76 62 (82) 76 63 (83) 69 64 (93) BRAF fusion 64 53 (83) 64 53 (83) 59 55 (93)
BRAF mutation 12 9 (75) 12 10 (83) 10 9 (90) Prior MAPKi 45 38 (84) 45 38 (84) 41 37 (90) MAPKi-naive 31 24 (77) 31 25 (81) 28 27 (96) CBR,* n (%) (SD ≥12 months) 76 43 (57) 76 46 (61) 69 54 (78) BRAF fusion 64 37 (58) 64 39 (61) 59 49 (83)
BRAF mutation 12 6 (50) 12 7 (58) 10 5 (50) Prior MAPKi 45 25 (56) 45 26 (58) 41 33 (80) MAPKi-naive 31 18 (58) 31 20 (65) 28 21 (75) Median DOR, months (95% CI)** 39 13.8 (11.3-NR) 40 14.4 (11.0-NR) 46 16.6 (11.6-NR) BRAF fusion 33 13.8 (11.3-NR)
33 16.3 (11.0-NR) 41 16.8 (11.6-NR) BRAF mutation 6 NR (8.4-NR) 7 12.0 (8.4-NR) 5 15.1 (8.3-NR) Prior MAPKi 22 13.8 (11.3-NR) 23 12.0 (8.5-NR) 29 15.1 (9.0-16.8) MAPKi-naive 17 NR (8.4-NR) 17 16.3 (8.4-NR) 17 NR (11.6-NR) 1 2 3 June 5, 2023 data
cutoff. Fangusaro J, et al. Lancet Oncol. 2020;21(6):e305–316. Fangusaro J, et al. Lancet Oncol. 2020;21(6):e305–316. van den Bent MJ, et al. Lancet Oncol. 2011;12(6):583- 17 593. 4. Wen PY, et al. J. Clin Oncol. 2017;35(21),2439-2449. *
ORR, CBR for RAPNO-LGG and RANO-LGG included MRs. ** the 95% CI were calculated using Kaplan-Meier method.

Tovorafenib (DAY101) Safety Data (n=137) TEAEs TRAEs Preferred Term, n
(%) Any Grade Grade ≥3 Any Grade Grade ≥3 Any AE 137 (100) 86 (63) 134 (98) 58 (42) Hair color changes 104 (76) 0 104 (76) 0 • The most common reasons Anemia 81 (59) 15 (11) 67 (49) 14 (10) Elevated CPK 80 (58) 16 (12) 77 (56) 16
(12) for discontinuation were Fatigue 76 (55) 6 (4) 60 (44) 6 (4) tumor hemorrhage (3 Vomiting 68 (50) 6 (4) 28 (20) 3 (2) patients) and decrease in Hypophosphatemia 64 (47) 0 48 (35) 0 growth velocity (2 patients) Headache 61 (45) 2 (1) 29 (21) 0
Maculo-papular rash 60 (44) 11 (8) 56 (41) 11 (8) • 33 patients (24%) had TRAEs Pyrexia 53 (39) 5 (4) 17 (12) 1 (1) leading to dose reduction; 50 Dry skin 49 (36) 0 45 (33) 0 patients (37%) had TRAEs Elevated LDH 48 (35) 0 42 (31) 0 Increased
AST 47 (34) 4 (3) 41 (30) 4 (3) leading to dose interruption Constipation 45 (33) 0 31 (23) 0 • Median duration of dose Nausea 45 (33) 0 25 (18) 0 Upper RTI 43 (31) 2 (1) 2 (1) 0 interruption was 2 weeks Dermatitis acneiform 42 (31) 1 (1) 41
(30) 1 (1) Epistaxis 42 (31) 1 (1) 27 (20) 0 • 9 patients (7%) had TRAEs Decreased appetite 39 (28) 5 (4) 28 (20) 4 (3) leading to discontinuation Paronychia 36 (26) 2 (1) 32 (23) 2 (1) Pruritus 35 (26) 1 (1) 32 (23) 1 (1) COVID-19 34 (25) 0 0
0 June 5, 2023 data cutoff. Treatment-emergent AEs ≥25% any grade in arms 1 & 2. AE, adverse event; ALT, Alanine transaminase; AST, aspartate aminotransferase; COVID-19, Coronavirus 18 disease 2019; CPK, creatine phosphokinase; LDH,
lactate dehydrogenase; RTI, respiratory tract infection; TEAEs, treatment-emergent adverse events; TRAEs, treatment-related adverse events.
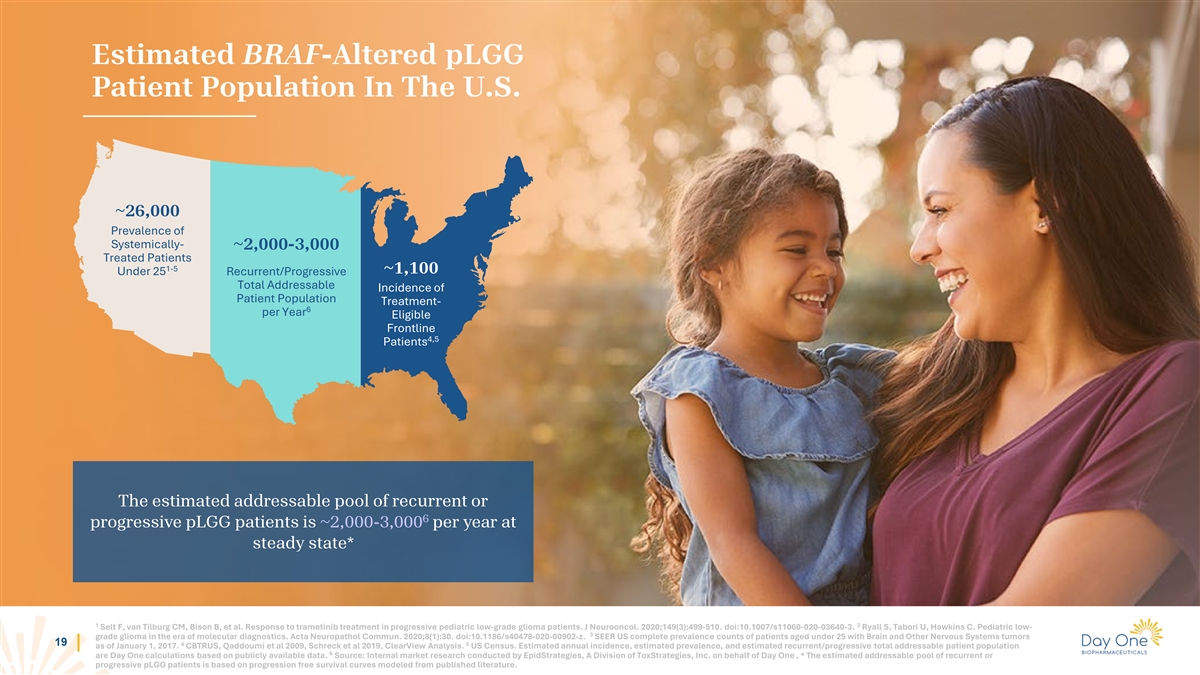
Estimated BRAF-Altered pLGG Patient Population In The U.S. ~26,000
Prevalence of Systemically- ~2,000-3,000 Treated Patients 1-5 ~1,100 Under 25 Recurrent/Progressive Total Addressable Incidence of Patient Population Treatment- 6 per Year Eligible Frontline 4,5 Patients The estimated addressable pool of recurrent
or 6 progressive pLGG patients is ~2,000-3,000 per year at steady state* 1 2 Selt F, van Tilburg CM, Bison B, et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neurooncol. 2020;149(3):499-510.
doi:10.1007/s11060-020-03640-3. Ryall S, Tabori U, Hawkins C. Pediatric low- 3 grade glioma in the era of molecular diagnostics. Acta Neuropathol Commun. 2020;8(1):30. doi:10.1186/s40478-020-00902-z. SEER US complete prevalence counts of patients
aged under 25 with Brain and Other Nervous Systems tumors 19 4 5 as of January 1, 2017. CBTRUS, Qaddoumi et al 2009, Schreck et al 2019, ClearView Analysis. US Census. Estimated annual incidence, estimated prevalence, and estimated
recurrent/progressive total addressable patient population 6 are Day One calculations based on publicly available data. Source: Internal market research conducted by EpidStrategies, A Division of ToxStrategies, Inc. on behalf of Day One , * The
estimated addressable pool of recurrent or progressive pLGG patients is based on progression free survival curves modeled from published literature.

* Preparing for a Successful Launch Key Factors Priorities •
Communicate strong clinical profile for Drive FIREFLY-1 Trial and Physician tovorafenib without significant disruption to Awareness childhood • Enable patient access through establishing Build momentum with pediatric broad coverage and patient
support programs oncologists at the ~200 U.S. Centers of Excellence • Experienced, fully dedicated field sales force (18 U.S. Account Managers) • Positive patient experience, drug profile Enable unrestricted patient access consisting of
once-weekly dosing (oral tablet or liquid formulation) * 20 Pending FDA Approval

Key Takeaways From FIREFLY-1 Data And Next Steps • Response rate
is clinically meaningful from FIREFLY-1 for pediatric patients with recurrent or progressive LGG harboring BRAF fusions or BRAF V600E mutations (“BRAF-altered”) • 67% ORR by RANO-HGG • 51% ORR by RAPNO-LGG • 53% ORR by
RANO-LGG • Deepening of responses observed in patients from December 2022 to June 2023 data cutoffs across all three assessment criteria • Meaningful duration of response as of data cutoff (median times: 16.6 months with RANO-HGG, 13.8
months with RAPNO-LGG, and 14.4 months with RANO-LGG)* • Responses were observed in patients with either BRAF fusion or BRAF V600E mutations • Responses seen in a heavily-pretreated population where the majority (60%) of patients
progressed on or after one or more prior MAPK inhibitors • Safety and tolerability profile indicating monotherapy tovorafenib to be generally well-tolerated • FDA Rare Pediatric Disease Designation for pLGG, eligible for Priority Review
Voucher Next Steps: Priority review granted with PDUFA target action date of April 30, 2024 21 June 5, 2023 data cutoff. * RANO-HGG 95% CI: 11.6-NR, RAPNO-LGG 95% CI: 11.3-NR, RANO-LGG 95% CI: 11.0-NR.

FIREFLY-2 / LOGGIC Pivotal Phase 3 Trial of Tovorafenib (DAY101) in
Frontline pLGG 22

FIREFLY-2/LOGGIC Pivotal Phase 3 Trial Of Tovorafenib (DAY101) In
Frontline pLGG Trial Design Endpoints • Randomized, global, registrational Phase 3 trial of monotherapy tovorafenib • Primary endpoint: ORR based on RANO-LGG criteria, assessed by 1 (DAY101) vs SoC chemotherapy blinded independent
central review ‒ The ORR primary analysis is expected to occur ~12 months after the • Eligibility: Patients aged 6 months to <25 years with LGG harboring a RAF alteration and requiring first-line systemic therapy last patient
randomized • Tovorafenib (DAY101) available as tablets and pediatric-friendly liquid • Key secondary endpoints: PFS and DoR by RANO criteria, ORR by RAPNO suspension criteria • Patients who progress after stopping tovorafenib
(DAY101) may be re- • Other secondary endpoints: changes in neurological and visual function, challenged safety, and tolerability • Patients who progress in the SoC arm during or post-treatment may cross- • Key exploratory
objectives: QoL and health utilization measures over to receive tovorafenib 2 Tovorafenib, 420mg/m QW Non-resectable or (not to exceed 600 mg) Stratified by sub-total resected LGG Long-term AND • Location of tumor follow-up Requiring
first-line • Genomic alteration systemic therapy Investigator's choice of • CDKN2A status (48 months) vincristine/carboplatin* or N ≈ 400 • Infant CHG diagnosis vinblastine * COG or SIOPe-LGG regimen. Abbreviations: CHG,
chiasmatic, hypothalamic glioma; DoR, duration of response; LGG, low-grade glioma; ORR, objective response rate; QoL, quality of life; QW, once weekly; SoC, 1 standard of care. Primary endpoint of FIREFLY-2 will be ORR by RANO-LGG (2017) following
full approval by FDA on March 16, 2023 of dabrafenib with trametinib in pediatric patients with low-grade glioma with a 23 BRAF V600E mutation who require systemic therapy based on a study with the same primary endpoint. 1:1 Randomization

FIRELIGHT-1 Phase 1b/2 Trials Evaluating Tovorafenib (DAY101) as a
Combination with Pimasertib 24

Pimasertib: Investigational Allosteric MEK1/2 Inhibitor With
Demonstrated Activity In MAPK-Driven Solid Tumors • Pimasertib is an investigational orally-bioavailable, selective, non- competitive MEK1/2 inhibitor in-licensed from Merck KGaA in February 2021 • Extensive non-clinical and clinical
development work through Phase 2, including a solid tumor trial in Japan and combinations with other MOAs • Main AEs typical for all in-class allosteric MEK inhibitors (GI, CPK elevation, skin rash, visual disturbances) • Nearly
three-fold higher CNS penetration than other MEKi inhibitors (trametinib or selumetinib) • Pimasertib showed monotherapy clinical activity, including an improvement in median PFS versus dacarbazine in NRAS mutant melanoma • Combination
with tovorafenib (DAY101) and other targeted therapies may unlock the full value of pimasertib in advanced solid tumors 25 Sources: Pimasertib Investigator Brochure, v12, 2019; de Gooijer et al., Int J Cancer, 2018; Shaw et al., AACR LB-456, 2012;
Lebbe et al., Cancers, 2020.

Vertical MAPK Pathway Inhibition With Tovorafenib (DAY101) And
Pimasertib May Unlock Potential Synergy For Adult Solid Tumors BRAF KRAS or NRAS non-V600 mutant mutant MEK RAF/ PI3K/m PI3K/m MEK/ERK TOR TOR ERK Proliferation, survival Proliferation, survival Type II RAFi + MEKI Type II RAFi + MEKI A Type II RAFi
+ MEKi is synergistic in BRAF fusion melanoma PDX model ex vivo (internal data) Non V600 BRAF dimers are Targeting multiple nodes of effectively inhibited by type II MAPK pathway will drive deeper Sensitivity of KRAS Q61 mutant cells to pimasertib
is enhanced when cells are treated with the type II B RAFi , but not type I BRAFi and more durable response BRAF inhibitor BGB-283 (Yuan et al., Mol Onc 2020) Tovorafenib (DAY101) + MEK inhibitor is synergistic in KRAS G12C and Q61 mutant tumor
cells C (Venetsanakos et al., 2021 AACR poster presentation) 26

Tovorafenib (DAY101) / Pimasertib Combination In Solid Tumors
(FIRELIGHT-1) 1 Trial Design Endpoints 2 • Combination dose escalation, global phase 1b/2 trial • Phase 1b: PK, PD and Safety, MTD/RP2D • Phase 1b, BOIN (adaptive), n = 10/cohort (approximately) • Phase 2: Efficacy (ORR, DOR)
• Phase 2, Simon 2-stage, n = 25/cohort (approximately) • Eligibility: Patients aged 12 years and older, dose escalation will be performed in advanced solid tumor patients with any MAPK alteration. Expansion cohorts will focus on
indications with a potential path to accelerated approval Phase 1b Phase 2* NRASmut Selected tumors DAY101 + Tumors with MAPK Pimasertib Safety BRAF Class 1 (non-E/K) and Class 2 mutant tumors pathway alterations until disease Follow Up 3
progression BRAF-fusion selected tumors Pre-identified patients with advanced solid tumors and available clinical molecular profiling information. *Additional biomarker-selected cohorts may be pursued based on developing data 1 Abbreviations: BOIN,
Bayesian Optimal Interval Design; BRAF, B-Raf proto-oncogene, serine/threonine kinase; MAPK, mitogen-activated protein kinase; NRAS, neuroblastoma rat sarcoma viral oncogene. Umbrella 2 master study – DAY101-102 (main protocol) DAY101 and MAPK
pathway aberration, Sub-study 1 monotherapy (DAY101-102a), Sub-study 2 MEK combo (DAY101-102b). Intend to open U.S. and ex-U.S. clinical sties. 27 3 DAY101 + Pimasertib until disease progression, intolerable toxicity, withdrawal of consent, or
death

Summary 28
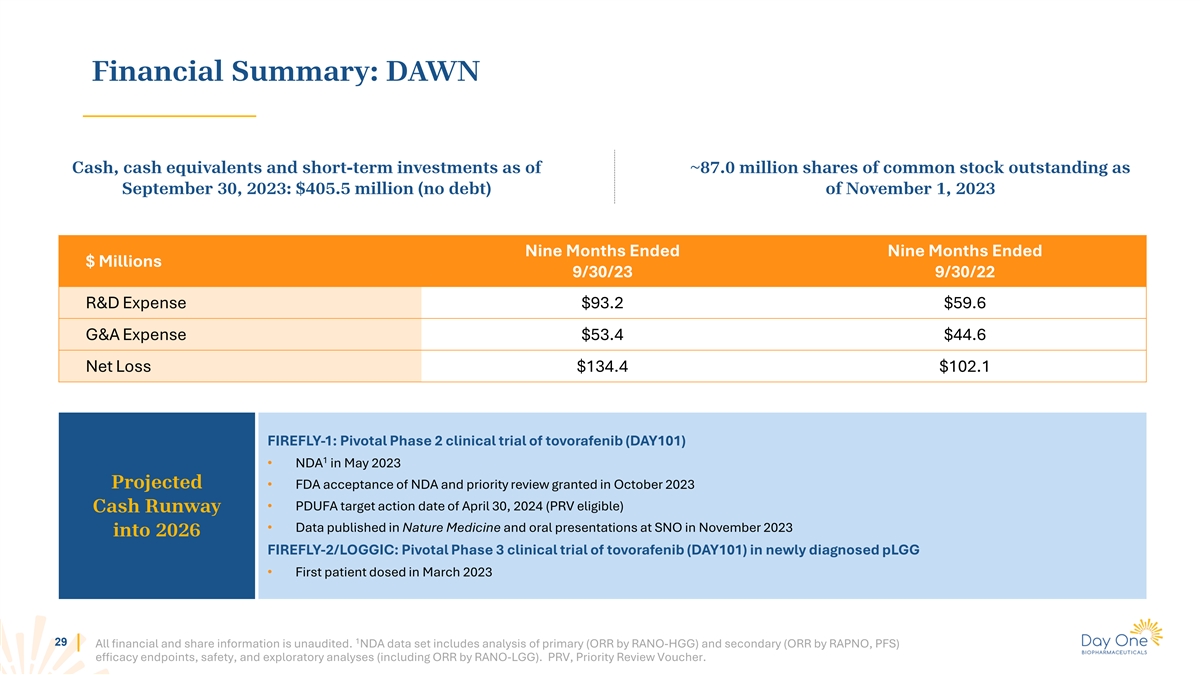
Financial Summary: DAWN Cash, cash equivalents and short-term
investments as of ~87.0 million shares of common stock outstanding as September 30, 2023: $405.5 million (no debt) of November 1, 2023 Nine Months Ended Nine Months Ended $ Millions 9/30/23 9/30/22 R&D Expense $93.2 $59.6 G&A Expense $53.4
$44.6 Net Loss $134.4 $102.1 FIREFLY-1: Pivotal Phase 2 clinical trial of tovorafenib (DAY101) 1 • NDA in May 2023 Projected • FDA acceptance of NDA and priority review granted in October 2023 • PDUFA target action date of April
30, 2024 (PRV eligible) Cash Runway • Data published in Nature Medicine and oral presentations at SNO in November 2023 into 2026 FIREFLY-2/LOGGIC: Pivotal Phase 3 clinical trial of tovorafenib (DAY101) in newly diagnosed pLGG • First
patient dosed in March 2023 1 29 All financial and share information is unaudited. NDA data set includes analysis of primary (ORR by RANO-HGG) and secondary (ORR by RAPNO, PFS) efficacy endpoints, safety, and exploratory analyses (including ORR by
RANO-LGG). PRV, Priority Review Voucher.

FIREFLY-1: Relapsed or Progressive pLGG • NDA initiated in May
2023 • Clinical data presented in oral presentation at ASCO in June 2023 • FDA acceptance of NDA and priority review granted in October 2023 • Data published in Nature Medicine and oral presentation at SNO in November 2023 2023 Key
• PDUFA target action date of April 30, 2024 Accomplishments FIREFLY-2: Frontline pLGG • Dosed the first patient in March 2023 Business Development • Research collaboration and license agreement for preclinical program targeting
VRK1 in August 2023 Financials • $405.5 million in cash, cash equivalents and short-term investments as of September 30, 2023 • Cash runway into 2026 30

Priorities as we Expand into a Commercial-Stage Company Launch
Tovorafenib Advance Portfolio Expand Pipeline • Secure the first FDA-approved • FIREFLY-2: Study tovorafenib as a • Grow Day One into a leading, targeted therapy for pLGG with frontline therapy for treatment- biopharmaceutical
company that is BRAF fusions and point mutations naive patients with pLGG the partner of choice for oncology that have relapsed or progressed drug development • FIRELIGHT-1: Evaluate • Expand awareness amongst tovorafenib in combination
with • Explore selective partnerships as a physicians and establish broad pimasertib in adolescent and source of capital and risk sharing coverage to enable patient access adult populations • Further invest in business • Following
approval, establish • Advance early stage VRK1 development activities to expand our tovorafenib as the standard of program to clinical development multiple asset portfolio for both care for relapsed or progressive children and adults pLGG
31

Appendix 32

Duration Of Tovorafenib (DAY101) Therapy For All Patients With RANO-HGG
Evaluable Lesions 3.0 Median time to response Months Median duration of 16.6 treatment Months Overall treatment duration (months) 33 June 5, 2023 data cutoff. Patients

Duration Of Tovorafenib (DAY101) Therapy For All Patients With RANO-LGG
Evaluable Lesions 5.5 Median time to response Months Median duration of 14.4 treatment Months Overall treatment duration (months) 34 June 5, 2023 data cutoff. Patients

Case Study: Activity Of Tovorafenib (DAY101) In KIAA1549-BRAF Fusion
Optic Pathway Glioma A 7-years-old female child with an optic pathway glioma, with very poor vision, entropion, folliculitis, eczema, mouth ulceration and xerosis -36 -30 -24 -18 -12 -6 0 6 Months PR 1L: Vincristine/carboplatin Diagnostic biopsy 2L:
Trametinib 3L: Tovorafenib Best response SD Best response PD Baseline After 6 Cycles Tumor kinetics (RANO) • PR (-58%) and improvement in vision reported at cycle 3 • AEs included grade 3 erythematous rash requiring dose interruption and
dose reduction (400 mg QW to 300 mg Tovorafenib QW in cycle 1), and grade 2 eczema and maculopapular rash • Patient continues to receive weekly tovorafenib 35 Apr 14, 2022 data cutoff. Tovorafenib is an investigational agent. Safety and
efficacy have not been established by any health authority.

Case Study: Activity Of Tovorafenib (DAY101) In KIAA1549-BRAF Fusion
Posterior Fossa Pilocytic Astrocytoma An 8-years-old female child with a posterior fossa pilocytic astrocytoma, eczema, nausea and constipation -84 -72 -60 -48 -36 -24 -12 0 12 Months PR Suboccipital craniotomies 1L: Carboplatin 2L:
Carboplatin/vincristine 3L: Vinblastine 4L: Trametinib 5L: Tovorafenib Best response SD Best response PD Best response PR Best response SD • PR (-69%) at cycle 3 with 500 mg QW tovorafenib, with a deepening of response (80% and 91% in cycles 6
and 9, respectively) over time • AEs included grade 2 decrease in neutrophil count, pustular rash, and upper respiratory infection • Patient continues to receive weekly tovorafenib Baseline After 3 Cycles After 6 Cycles After 9 Cycles
Tumor kinetics Tovorafenib 36 Apr 14, 2022 data cutoff. Tovorafenib is an investigational agent. Safety and efficacy have not been established by any health authority.

Case Study: Activity Of Tovorafenib (DAY101) In BRAF V600E Mutation
Deep Midline Astrocytoma A 9-year-old female child with deep midline BRAF V600E-mutant astrocytoma with precocious puberty -60 -48 -36 -24 -12 0 12 Months PR Subtotal 1L: Carboplatin/vincristine 2L: Dabrafenib 3L: Tovorafenib Best response PR
resection Best response PR • PR (-74%) at cycle 3, with a deepening of response (-94%) at cycle 6 • AEs included grade 3 maculopapular rash and increased CPK, requiring drug interruption and dose reduction (500 mg QW to 400 mg QW in
cycle 1) • Tovorafenib dose was re-escalated back to 500 mg QW in cycle 4; patient continues on treatment Baseline After 3 Cycles After 6 Cycles Tumor kinetics Tovorafenib 37 Apr 14, 2022 data cutoff. Tovorafenib is an investigational agent.
Safety and efficacy have not been established by any health authority.

Case Study: Activity Of Tovorafenib (DAY101) In KIAA1549-BRAF Fusion
Posterior Fossa Pilocytic Astrocytoma 8-year-old boy with relapsed pilomyxoid astrocytoma of the optic pathway, with visual loss in right eye, visual field loss in left eye, fatigue, intermittent nausea/vomiting, intermittent headaches, anorexia,
and temperature regulation disorder Months -60 -48 -36 -24 -12 0 12 24 Tovorafenib VC Binimetinib Trametinib PR • Initiated treatment with tovorafenib 400 mg/QW following 3 prior therapies, including binimetinib and trametinib, which were
discontinued due to PD • At cycle 3, PR (-88%) per RANO-HGG, and MR (-32% and -40%) per RAPNO-LGG and RANO-LGG, respectively − Sustained improvements in visual acuity reported; logMAR change 0.2 → 0 − PD criteria met (-94% to
-91%) with RANO-HGG at cycle 15; continued treatment as investigator deemed no radiographic progression with subsequent reduction in target lesion (-97%) • AEs were G2 (drug eruption, elevated CPK) and G1 (hair color change, paronychia, growth
retardation) T1 + C (baseline and post cycle 3) T2 (baseline and post cycles 3 & 12) Tumor kinetics Dec 22, 2022, data cut-off. AEs, adverse events; C, contrast; CPK, creatine phosphokinase; G, grade; HGG, high-grade glioma; LGG, low-grade
glioma; logMAR, Logarithm of the Minimum Angle of Resolution; MR, minor 38 response; PD, progressive disease; PR, partial response; QW, once weekly; RANO, Response Assessment in Neuro-Oncology; RAPNO, Response Assessment in Pediatric Neuro-Oncology;
VC, vincristine-carboplatin..

FIREFLY-2/LOGGIC: Pivotal Phase 3 Study Of Tovorafenib (DAY101) In
Newly Diagnosed pLGG • Collaboration between Day One Approximately 100 potential sites (~65 from the LOGGIC consortium) and the LOGGIC consortium, internationally recognized experts in pLGG research • Coupled with the LOGGIC-CORE
molecular diagnostic program ~20 ~65 • Worked jointly on the study Sites Sites design and discussions with the ~5 U.S. and EU regulatory authorities Sites ~10 Sites 39
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Day One Biopharmaceuticals (NASDAQ:DAWN)
Historical Stock Chart
From Oct 2024 to Nov 2024

Day One Biopharmaceuticals (NASDAQ:DAWN)
Historical Stock Chart
From Nov 2023 to Nov 2024
