UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended June 30, 2023
or
☐
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission File Number 001-41616
Lucy Scientific Discovery Inc.
(Exact Name of Registrant as Specified in Its Charter)
| British Columbia, Canada | | Not Applicable |
| (State or Other Jurisdiction of Incorporation or Organization) | | (I.R.S. Employer Identification No.) |
301-1321 Blanshard Street
Victoria, British Columbia, Canada V8W
0B6
(Address of Principal Executive Offices)
(778) 410-5195
(Registrant’s telephone number, including
area code)
Not applicable
(Former name, former address, and former fiscal
year, if changed since last report)
Securities registered pursuant to Section 12(b)
of the Act:
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, no par value | | LSDI | | The Nasdaq Stock Market LLC |
Securities registered under Section 12(g) of the
Exchange Act: None
Indicate by check mark if the registrant is a
well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
No ☒
Indicate by check mark if the registrant is not
required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐
No ☒
Indicate by check mark whether the registrant
has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial
reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or
issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether
any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the
registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the
registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to
such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the
registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T
(§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to
submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
| | Emerging growth company ☒ |
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
Indicate by check mark whether the
registrant is a shell company (as defined in rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The number of shares of the registrant’s
common stock outstanding as of October 13, 2023 was 17,646,296 shares.
The Registrant was not a public company as of the last business day
of its most recently completed second fiscal quarter (December 31, 2022) and therefore cannot calculate the aggregate market value of
the voting and non-voting common equity held by non-affiliates as of such date.
TABLE OF CONTENTS
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form
10-K (“Annual Report”) contains forward-looking statements within the meaning of the federal securities laws. All statements
contained in this Annual Report, other than statements of historical fact, including statements regarding our future operating results
and financial position, our business strategy and plans, potential growth or growth prospects, future research and development, sales
and marketing and general and administrative expenses, and our objectives for future operations, are forward-looking statements. Words
such as “believes,” “may,” “will,” “estimates,” “potential,” “continues,”
“anticipates,” “intends,” “expects,” “could,” “would,” “projects,”
“plans,” “targets,” and variations of such words and similar expressions are intended to identify forward-looking
statements. We have based these forward-looking statements largely on our current expectations and projections about future events and
trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business
operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions,
including those described in the “Risk Factors” in this Annual Report. Readers are urged to carefully review and consider
the various disclosures made in this Annual Report and in other documents we file from time to time with the Securities and Exchange Commission
(the “SEC”) that disclose risks and uncertainties that may affect our business. Moreover, we operate in a very competitive
and rapidly changing environment. New risks emerge from time to time. It is not possible for us to predict all risks, nor can we assess
the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ
materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties, and assumptions,
the future events and circumstances discussed in this Annual Report may not occur and actual results could differ materially and adversely
from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking
statements as predictions of future events. The events and circumstances reflected in the forward-looking statements may not be achieved
or occur. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future
results, performance, or achievements. In addition, the forward-looking statements in this Annual Report are made as of the date of this
filing, and we do not undertake, and expressly disclaim any duty, to update such statements for any reason after the date of this Annual
Report or to conform statements to actual results or revised expectations, except as required by law.
You should read this Annual
Report and the documents that we reference herein and have filed with the SEC as exhibits to this Annual Report with the understanding
that our actual future results, performance, and events and circumstances may be materially different from what we expect.
This Annual Report also contains
or may contain estimates, projections and other information concerning our industry, our business and the markets for our products, including
data regarding the estimated size of those markets and their projected growth rates. Information that is based on estimates, forecasts,
projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from
events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained these industry, business, market
and other data from reports, research surveys, studies and similar data prepared by third parties, industry and general publications,
government data and similar sources. In some cases, we do not expressly refer to the sources from which these data are derived.
PART I
ITEM 1. BUSINESS
Business Overview
Overview
We are an early-stage psychotropics
contract manufacturing company focused on becoming the premier contract research, development, and manufacturing organization for the
emerging psychotropics-based medicines industry. In August 2021, Health Canada’s Office of Controlled Substances granted us
a Controlled Drugs and Substances Dealer’s Licence under Part J of the Food and Drug Regulations promulgated under the Food
and Drugs Act (Canada), or a Dealer’s Licence. A Dealer’s Licence authorizes us to develop, sell, deliver, and manufacture
(through extraction or synthesis) certain pharmaceutical-grade active pharmaceutical ingredients, or APIs, used in controlled substances
and their raw material precursors. Since current Canadian regulations prohibit the commercial sales of APIs and other products we intend
to produce, APIs and such other products would only be authorized for sale in Canada for clinical testing purposes in an “institution,”
for the purpose of determining the hazards and efficacy of the drug, and for laboratory research in an institution by qualified investigators.
Our mission is to make our products and research services available to our clients for the development of medicines and experimental therapies
to address certain psychiatric health disorders and other medical needs. We cannot guarantee that we will receive further approvals from
Health Canada, and a failure to receive such approvals could have a material adverse effect on our business and result in an inability
to generate revenue from said products and services. Further, as of the date of this prospectus, we have not manufactured all of the psychedelics-based
products allowable under the Dealer’s Licence or generated any revenues from the sale of such psychedelics-based products.
The success of our business
plan is dependent on our activities being permissible under applicable laws and upon the occurrence of regulatory changes for psychotropics-based
medicines. In Canada, the psychedelic compounds that we are approved to produce under our Dealer’s Licence, psilocybin, psilocin,
lysergic acid diethylamide, or LSD, N,N-Dimethyltryptamine, or N,N-DMT, and 3,4-Methylenedioxymethamphetamine, or MDMA, and 4-Bromo-2,5-Dimethoxybenzeneethanamine,
or 2C-B, are regulated under the Controlled Drugs and Substances Act, or CDSA. Certain psychedelic substances, including psilocybin, psilocin,
mescaline and DMT, are classified as Schedule III drugs and the CDSA prohibits the possession of a Schedule III drug absent authorization
under the CDSA or a related regulation, and it is illegal to possess Schedule III substances without a prescription. In the United States,
these substances are classified under the Controlled Substances Act (21 U.S.C. § 811), or the CSA, and the Controlled Substances
Import and Export Act, or the CSIEA, and as such, medical and recreational use is illegal under the U.S. federal laws. Under the CSA,
the Drug Enforcement Agency, or DEA, regulates chemical compounds with a potential for abuse as Schedule I, II, III, IV or V substances.
Schedule I substances may not be prescribed, marketed or sold in the United States. Most, if not all, state laws in the United States
classify psilocybin, LSD, MDMA, DMT and 2C-B as Schedule I controlled substances. For any product containing any of these substances to
be available for commercial marketing in the United States, the applicable substance must be rescheduled, or the product itself must be
scheduled, by the DEA to Schedule II, III, IV or V. If the DEA does not reschedule psilocybin, LSD, MDMA, DMT and 2C-B as Schedule II,
III, IV or V, such substances will be subject to individually-allotted manufacturing and procurement quotas, which may have a material
adverse effect on our business and result in an inability to generate sufficient revenue from said substances to be profitable. Additionally,
regardless of the scheduling of a finished, approved therapeutic product, if the API used in the final dosage form is a Schedule I or
II controlled substance, it would be subject to such quotas as the API could remain listed on Schedule I or II. Moreover, even if the
finished dosage form of a psychedelics-based medicine developed by one of our clients is approved by the FDA, and if such product is listed
by the DEA as a Schedule II, III, or IV controlled substance, its manufacture, importation, exportation, domestic distribution, storage,
sale and legitimate use will continue to be subject to a significant degree of regulation by the DEA.
An increasing number of the
leading universities, hospitals and other public, private, and government institutions throughout the world have launched research programs
and are conducting clinical studies aimed at understanding the therapeutic potential of a range of psychedelic substances, including the
John Hopkins Center for Psychedelic and Conscious Research at Johns Hopkins University, the Imperial College London Centre for Psychedelic
Research, the Center for the Science of Psychedelics at the University of California, Berkeley, the Depression Evaluation Service at Columbia
University, the Center for Psychedelic Psychotherapy and Trauma Research at the Icahn School of Medicine at Mount Sinai Health System,
New York City’s largest academic medical system, and the Center for the Neuroscience of Psychedelics at Massachusetts General
Hospital, among many others.
To address mounting demands
for alternative therapies incorporating the use of psychedelics, we intend to leverage our 25,000 square foot facility located near Victoria,
British Columbia, for research, development, and large-scale production of high-quality biological raw materials, APIs, and finished biopharmaceutical
products. Supported by an executive leadership and advisory team consisting of highly experienced biotechnology and pharmaceutical industry
experts, we will seek to position our company to be at the forefront of new discovery in this rapidly emerging market.
Recent Developments
On January 16, 2023,
we entered into a strategic investment agreement, or the Strategic Investment Agreement, with Hightimes Holding Corp., or Hightimes, 1252240
BC LTD, a wholly owned subsidiary of Hightimes, and Trans-High Corporation, a wholly owned subsidiary of Hightimes, pursuant to which
Hightimes granted to us $833,333 of annual advertising and marketing credits, or Advertising Credits, for five consecutive years, in exchange
for 625,000 of our common shares. The Advertising Credits enable us to advertise (i) on all Hightimes publications, including the Hightimes
print and website publications, and (ii) at all festivals and events conducted by Hightimes. Unless earlier terminated pursuant to the
terms of the Strategic Investment Agreement, the Strategic Investment Agreement will terminate on December 31, 2025, which term may
be extended by the parties to the Strategic Investment Agreement upon such terms and conditions as the parties may mutually agree. Paul
Abramowitz, one of our directors, is the stepfather of the Executive Chairman of Hightimes. Mr. Abramowitz’s biological son
is a beneficial owner of Roma Ventures, LLC, or Roma Ventures, an entity that owns approximately 8.53% of our issued and outstanding common
shares. Benjamin Windle is the investment manager of Roma Ventures and has sole voting and investment control with respect to our common
shares held by the Roma Ventures. Each of the Executive Chairman of Hightimes, Mr. Abramowitz and Roma Ventures are shareholders
of Hightimes. The sale of the above common shares were deemed to be exempt from registration under the Securities Act in reliance upon
Section 4(a)(2) of the Securities Act as a transaction by an issuer not involving any public offering.
On February 13, 2023, we
completed our initial public offering (the “IPO”). Our registration statement on Form S-1 (File No. 333-262296) relating to
the IPO was declared effective by the SEC on February 8, 2023. We issued 1,875,000 common shares at a price of $4.00 per share for aggregate
net cash proceeds of $5.8 million, after deducting underwriting discounts and commissions and other offering related costs. None of the
expenses associated with the IPO were paid to directors, officers, persons owning 10% or more of any class of equity securities, or to
their associates, or to our affiliates. WestPark Capital, Inc. acted as sole book running manager of the offering and as representative
of the underwriters.
On February 16, 2023, we
filed an amendment with our current Dealer’s License to add coca leaves, ketamine, methamphetamine, methadone, buprenorphine, diacetylmorphine
(heroin), opium, thenaine (paramorphine), benzoylmethylecgonine (cocaine), fentanyl, hydromorphone, oxycodone, hydrocodone, morphine and
codine to the list of approved substances that it is authorized to manufacture. The shift toward a public health response to the drug
crisis should provide greater opportunities for people who use substances to connect with a growing range of harm reduction and treatment
options. Currently, we focus on the development and sale of psychedelic drugs for research purposes.
On February 27, 2023, we
agreed to our first commercial sale to the prestigious Hadassah BrainLabs - Center for Psychedelics Research, Hadassah Medical Center,
Hebrew University, Jerusalem, Israel. This first commercial sale of psilocybin, while modest in size, marks a key operational milestone
for the company as we shift from pre revenue to revenue producing. This transaction establishes our ability to supply the global psychedelic
community with compounds and services.
On March 20, 2023, we entered
into a definitive asset purchase agreement (the “APA”) with Wesana Health Holdings Inc. (“Wesana”) for the purchase
of Wesana’s SANA-013 intellectual property and related assets (the “Transaction”). The Transaction provides an opportunity
for the continued development of SANA-013 through the next phases of the US FDA regulatory process and for the Company to have economic
exposure to any positive advancements in any such future research and development efforts by Lucy. On June 30, 2023, the Company entered
into the First Amendment to the APA (the “First Amendment”). Pursuant to the First Amendment, the consideration to be paid
for these assets is: (a) $300,000 in cash to be paid within 24 hours of the signing of the First Amendment; (b) upon the closing of the
acquisition (the “Closing”), the Company will issue Wesana an aggregate of 1,000,000 shares of the Company’s common
stock (the “Shares”); (c) $177,973.99 in cash payable in the following 4 installments: (i) $100,000.00 due on or before July
1, 2023; (ii) $25,991.33 due on or before October 1, 2023; (iii) $25,991.33 due on or before January 1, 2024; and (iv) $25,991.33 due
on or before April 1, 2024, and (d) at the Closing, the Company will assume certain liabilities of Wesana which principally consists of
$92,026.01 of trade payables owed by Wesana to a law firm.
On March 23, 2023, we launched
a new line of unscheduled psychoactive compounds that are available for sale throughout the United States, and where permitted. This product
line is named Mindful by Lucy and is the first line in the new family of brands contains Amanita Muscaria mushrooms, a psychoactive adaptogen.
The product leverages the compounds of these mushrooms, and a proprietary blend of other natural functional ingredients, to create a transformative
experience for consumers. We aim to distribute and market Mindful by Lucy through Hightimes’ websites and social channels. Lucy
and High Times entered into a Strategic Investment Agreement in January 2023 whereby Lucy received $2.5 million in advertising credits
in exchange for 625,000 of our common shares that will help launch the new brand into market through High Times channels and experiential
events without cash outlays for marketing by Lucy. This new line is well-positioned to capitalize on the growing market for psychoactive
alternatives, which Forbes predicts will double to over $5 billion in gross sales by 2025.
On June 30, 2023, the Closing
of Wesana occurred. A total of $100,000 was paid by the Company to Wesana on July 5, 2023 and the Shares were issued on June 30, 2023.
On July 11, 2023, we announced
the launch of Twilight by Lucy, a blend of Amanita and Reishi mushrooms that include a variety of other nootropics promoting improved
cognitive function and enhanced sleep quality. This release comes on the heels of the recent launch of Mindful by Lucy. Both of these
products are now available for purchase on the company’s official online store, www.buytrippy.com, as well as through Hightimes.com
and other channels. Twilight by Lucy is a product designed to enhance and optimize consumer’s nightly sleep. The introduction of
Twilight alongside Mindful underscores Lucy’s dedication to providing solutions in the psychotropic marketplace.
On July 24, 2023, Christopher
McElvany resigned from his positions as the Company’s President and Chief Executive Officer and resigned as a member of the
Company’s Board of Directors (the “Board”). The Company and Mr. McElvany agreed that his last day of employment was
July 14, 2023. Mr. McElvany did not resign as a result of any disagreement with the Company on any matter relating to the Company’s
operations, policies or practices.
On July 24, 2023, the Board
ratified the appointment of Richard Nanula (a member of the Board since February 2022) as CEO.
On September 6, 2023, we entered
into a Stock Purchase Agreement (the “Stock Purchase Agreement”) with Hightimes to acquire the intellectual property of High
Times. Hightimes owns all of the issued and outstanding shares of common stock of HT-Lucy Acquisition Corp., a Delaware corporation. Pursuant
to the Stock Purchase Agreement, Hightimes agreed to sell to us all of the common stock of HT-Lucy Acquisition Corp. upon the terms and
subject to the conditions of the Stock Purchase Agreement. In exchange for the common stock of HT-Lucy Acquisition Corp., we shall pay
Hightimes as consideration (i) the number of shares of common stock of the Company that represents 19.9% of the total issued and outstanding
shares of the Company at the closing; and (ii) semi-annual earn-out payments (the “Hightimes Earn-Out Payments”) payable for
the five (5) consecutive fiscal years ending on June 30, 2029, in amounts equal to three (3) times the adjusted EBITDA of HT-Lucy Acquisition
Corp., calculated pursuant to the terms of the Stock Purchase Agreement. We have the discretion to pay the Hightimes Earn-Out Payments
with either Lucy common shares or cash. At the closing, we will also cause HT-Lucy Acquisition Corp. to enter into an intellectual property
license agreement pursuant to which HT-Lucy Acquisition Corp. will grant to an affiliate of Hightimes the exclusive right and license
to utilize certain intellectual property rights to operate retail stores and to manufacture and sell THC products in the United States
in return for a license fee of $1.0 million per year, increasing to $2.0 million per year upon Federal legalization.
On September 12, 2023, we entered
into an amalgamation agreement (the “Amalgamation Agreement”) with Bluesky Biologicals Inc. (“Bluesky”) to acquire
the Bluesky. Bluesky, through Bluesky Wellness Inc., owns a portfolio of plant-based wellness brands including Keoni, Keoni Sport, Blush
Wellness and AMMA Healing. Pursuant to the Amalgamation Agreement, Bluesky will amalgamate with a wholly-owned subsidiary of the Company
upon the terms and subject to the conditions of the Amalgamation Agreement. We shall pay Bluesky as consideration (i) the number of shares
of common stock of the Company that represents 19.9% of the total issued and outstanding shares of the Company at the closing; and (ii)
earn-out payments (the “Bluesky Earn-Out Payments”) payable for the four (4) consecutive fiscal years ending on June 30, 2028,
the six (6) month period ended June 30, 2024, and the six (6) month period ending December 31, 2028, in amounts equal to two and one half
(2.5) times the adjusted EBITDA of Bluesky., calculated pursuant to the terms of the Amalgamation Agreement. We have the discretion to
pay the Bluesky Earn-Out Payments with either Lucy common shares or cash.
Psychotropics: An Emerging Market Opportunity
Psychotropics are a broad classification
of chemical substances that can cause alterations in perception, mood, consciousness, cognition, or behavior through various interactions
with the nervous system. Psychedelics are a subclassification of psychotropics that interact primarily with serotonergic receptors in
the brain. Psychedelic compounds such as psilocybin, psilocin, LSD N,N-DMT, and MDMA, have become areas of interest for many new companies.
The psychedelic compounds we are approved to produce under our Dealer’s Licence — psilocybin, psilocin, N,N-DMT,
mescaline, MDMA, LSD, and 4-Bromo-2,5-Dimethoxybenzeneethanamine, or 2C-B — will represent our initial areas of focus
for our research, development and manufacturing efforts on behalf of our clients. In addition, subject to further approvals by Health
Canada with respect to the expansion of the scope of our Dealer’s Licence, we expect to extend our research and production efforts
to various non-serotonergic psychotropics, such as ketamine, as such compounds may provide significant future market opportunities for
us. Since current Canadian regulations prohibit the commercial sales of APIs and other products we intend to produce, APIs and such other
products would only be authorized for sale in Canada for clinical testing purposes in an “institution,” for the purpose of
determining the hazards and efficacy of the drug, and for laboratory research in an institution by qualified investigators. We cannot
guarantee we will receive such approvals, and a failure to receive further approvals would have a material adverse effect on our business
and result in an inability to generate revenue from said substances.
Clinical Trials and Studies Involving Psychotropics
To date, only a limited number
of psychotropic- and psychedelic-based medicines have been approved by Health Canada and the FDA. However, a number of studies have been
conducted in recent years to determine the efficacy of psychedelic therapies in patients suffering from various mental health and
addiction disorders, including the following:
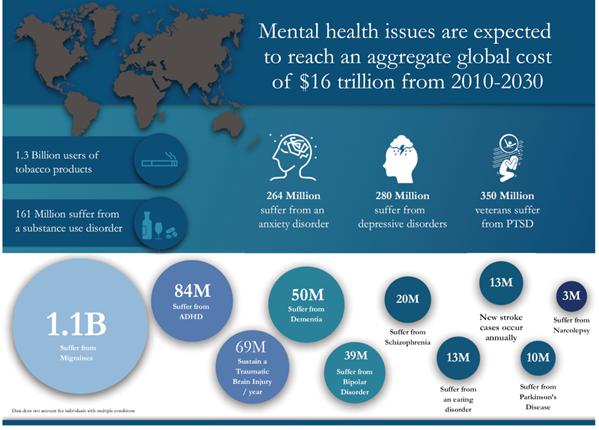
| ● | Depressive Disorders: An estimated
3.8% of the global population, or approximately 280 million people, suffer from depressive episodes. The bulk of these episodes
are part of major depressive disorder, or MDD, a mood disorder that causes a persistent feeling of sadness and loss of interest in normal
activities. MDD affects approximately 17.3 million adults or about 7.1% of the United States population age 18 and older in
a given year and an estimated 10% of the adult population of Canada will experience MDD at some point in their life. A small study of
adults with major depression conducted in November 2020, Johns Hopkins Medicine researchers report that two doses of the psychedelic
substance psilocybin, given with supportive psychotherapy, produced rapid and significant reductions in depressive symptoms, with most
participants showing improvement and half of study participants achieving remission through the four-week follow-up period. |
| ● | Anxiety Disorders: Anxiety
disorders are defined as frequent, intense, excessive, and/or persistent worry and fear about everyday situations, affecting an estimated
264 million individuals globally. Over 40 million U.S. adults and an estimated 3 million Canadians suffer from an anxiety
disorder. Anxiety disorders include generalized anxiety disorder, social anxiety disorder, specific phobias, separation anxiety disorder,
and many others. An individual may suffer from more than one anxiety disorder. A systematic literature review of 20 studies published
from 1940 to 2000 concluded that a combination of psychedelic drug administration and psychological therapy was most beneficial in treating
individuals suffering from anxiety disorders. |
| ● | Post-Traumatic Stress Disorder: Post-traumatic
stress disorder, or PTSD, is a disorder characterized by a person’s re-experiencing a past traumatic incident through flashbacks,
bad dreams, frightening thoughts and other manifestations. PTSD affects approximately 354 million war survivors worldwide, in addition
to others affected by traumatic events such as physical, sexual or psychological abuse. PTSD can result in avoidance of normal activities,
sleep disturbances, angry outbursts, and distorted feelings of guilt or blame, and it is often accompanied by depression and/or substance
abuse. According to the National Institute of Mental Health, or NIMH, about 6.8% of U.S. persons will experience PTSD in their lifetimes.
CNS Neuroscience & Therapeutics estimated the prevalence rate of lifetime PTSD in Canada to be 9.2% in spite of comparably low rates
of violent crime, a small military, and few natural disasters. A longitudinal pooled analysis of six Phase 2 clinical trials, published
in 2020 by the Medical University of South Carolina’s Dr. Michael Mithoefer and colleagues, considered the impact on PTSD
symptoms (measured using a clinician-administered assessment of known as CAPS-IV, a well-established means of assessing PTSD severity)
of administering two to three active doses of MDMA during psychotherapy sessions. The analysis showed a reduction in CAPS-IV total severity
scores from baseline to treatment exit (i.e., one to two months after the last active MDMA psychotherapy session) and when assessed
at least 12 months thereafter. From treatment exit to the 12 month follow-up, CAPS-IV scores continued to decrease (i.e., PTSD
symptoms became less severe) and the percentage of trial participants who no longer met PTSD criteria increased. |
| ● | Addiction: Substance addiction
and abuse represents a significant global problem, with approximately 1.3 billion people who are users of tobacco products, 107 million
people suffering from alcohol use disorder, and 36 million people impacted by drug use disorder. In Canada, it is estimated that
approximately 21% of the population, or about six million people, will meet the criteria for addiction in their lifetime. Approximately
21.2 million individuals in the U.S. have a substance abuse disorder, and in 2018, 11% of those patients received the treatment
for such substance abuse disorder. In a small study measured over the course of six months in 2014, researchers at Johns Hopkins
University reported an 80% smoking abstinence rate in patients participating in a treatment program involving psilocybin. In 2015, researchers
at the University of New Mexico treated a small population suffering from alcohol dependence with 1-2 supervised psilocybin treatment
sessions, resulting in immediate and sustained outcomes lasting 36 weeks. |
| ● | Other Potentially Applicable Conditions: According
to a study published in 2020 by Frontiers in Synaptic Neuroscience in the United Kingdom and reviewed by universities in the United States,
Brazil, and Switzerland, the renaissance in psychedelic research in recent years, in particular studies involving psilocybin and
LSD, coupled with anecdotal reports of cognitive benefits from micro-dosing, suggests that they may have a therapeutic role in a range
of psychiatric and neurological conditions due to their potential to enhance functional neuronal connectivity, stimulate neurogenesis,
restore brain plasticity, reduce inflammation, and enhance cognition. In 2021, scientists at the University of California at Los Angeles
made significant discoveries about the interaction of LSD with dopamine that they believe may lead to a better understanding and eventual
treatment of schizophrenia and that shows promise in the pursuit of treating physically crippling disorders such as Parkinson’s
disease. |
Many researchers actively conducting
studies today believe that a there is a significant opportunity for the continued discovery and refinement of alternative treatments using
psychotropics-based medicines for a variety of mental health and addiction disorders. A number of major academic institutions, including
those noted above, have established dedicated psychedelic research centers in the past two years. As of September 2021, clinicaltrials.gov
reports 146 registered clinical studies (including those not yet recruiting, enrolling by invitation, and active) involving psychedelic
compounds.
Mental Health and Addiction Disorders: Prevalence
and Costs
Public Support and Regulatory Change
Notable academic and clinical
research efforts, as well as broad support from both the psychiatric health community and the general public (according to an independent
study conducted by Prohibition Partners in 2020), have prompted U.S. and Canadian regulatory bodies to re-evaluate various psychedelic
compound classifications. In Canada, drug decriminalization is being strongly considered throughout the nation, government initiatives
such as Safe Supply and the Special Access Program for Drugs may provide opportunities for market growth. Similarly, in 2017, the U.S. Food
and Drug Administration, or the FDA, granted the Multidisciplinary Association for Psychedelic Studies, or MAPS, Breakthrough Therapy
Designation to MDMA-assisted psychotherapy for the treatment of PTSD on the basis of pooled analyses showing a large effect size for this
treatment. In 2018 and 2019, the FDA granted the Usona Institute Breakthrough Therapy Designation for psilocybin-assisted psychotherapy
for the treatment of MDD. In 2019, the FDA approved the use of S-ketamine nasal spray, in conjunction with an oral antidepressant, for
the treatment of TRD, which marked the first approval by the FDA of a psychedelics-based therapy. In February 2021, Oregon commenced
the first state-regulated psilocybin program, and concurrently, Washington, D.C. joined other cities including Oakland and Santa Cruz,
California, and Ann Arbor, Michigan in decriminalizing the cultivation and possession of all entheogenic plants and fungi. Decriminalize
Nature, an entheogenic educational campaign, currently has active lobbying campaigns ongoing in 42 cities in the United States to
decriminalize psychedelics. In January 2022, the Canada Gazette published a notice of amended regulations related to restricted drugs
that allow practitioners the ability to request access to restricted drugs through the Special Access Program for the emergency treatment
of patients with serious or life-threatening injuries.
Our Dealer’s Licence
Our Health Canada Dealer’s Licence, which we hold through our wholly
owned subsidiary, LSDI Manufacturing Inc., authorizes us to produce, sell, deliver, and conduct research using psilocybin, psilocin, N,N-DMT,
mescaline, MDMA, and LSD. Per current Canadian regulations, these APIs and other products we intend to produce would only be authorized
for sale in Canada for clinical testing purposes in an “institution,” for the purpose of determining the hazards and efficacy
of the drug, and for laboratory research in an institution by qualified investigators; sales of APIs in Canada for commercial purposes
are currently prohibited. We also anticipate submitting applications to Health Canada for additional approvals under our Dealer’s
Licence allowing us to produce and distribute ketamine. There is no guarantee that we will receive further approvals from the Office of
Controlled Substances in a timely manner or at all. A failure to receive such further approvals would have a material adverse effect on
our business and result in an inability to generate revenue from said substances.
Our Management Team
Mr. Nanula is our Chief Executive
Officer and has served as our Chair and a director since February 2022. Mr. Nanula is a highly experienced business advisor and senior
executive with more than 35 years of experience in corporate finance and strategy including tenure with the Walt Disney Company (Disney),
Starwood Hotels and Resorts, Amgen, and Colony Capital. Additionally, Mr. Nanula also served as a board member for Boeing Corporation
and Starwood Capital. Our management team also features Assad J. Kazeminy, Ph.D., our Chief Scientific Officer, who previously served
as Chief Executive Officer of Irvine Pharmaceutical Services Inc. and Avrio Biopharmaceutical LLC and has over 30 years of research
and development experience in the biopharmaceutical industry.
What Sets Us Apart
As a contract research and
manufacturing organization serving the emerging psychedelics-based medicines market, we believe that we can be distinguished from other
companies in the psychedelics market and we have a number of competitive advantages, as described below:
| ● | Strategic Approach Centered on Adaptability. We
believe that most other companies in the psychedelics market are centered around very specific drug targets with rigid production and
scaling plans that are heavily dependent on major regulatory changes. Our strategy has been designed to enable us to have agility and
scalability necessary to pursue groundbreaking research, advance with the changing regulatory landscape, and expand to meet future market
opportunities at scale. |
| ● | Executive Team and Board of Directors with Industry
Experience. Our management team consists of accomplished business entrepreneurs with deep knowledge of
agriculture, production, extraction, chemistry, research, medicine, and drug discovery. We intend to leverage our team’s experience
in an effort to target licensed organizations for contract manufacturing and research and development opportunities. |
| ● | Technologies, Processes, and Intellectual Property. Our
management team brings to market several key advantages, including the utilization of our TerraCube horticulture/fungiculture growth
chambers, which feature our patented downdraft technology, located on-site to our application of biosynthesis techniques. By leveraging
our processes and technologies, we will continually pursue new opportunities to obtain critical patents and other intellectual property
rights, and to develop advanced production methods and new enabling technologies in an effort to distinguish and position our company
in a rapidly evolving competitive landscape. Our management team intends to remain dedicated to ensuring the quality of our products
and forging a cooperative culture of perpetual discovery and advancement. |
Our History
We were initially founded in
2017 as Hollyweed North Cannabis, Inc., or HNCI. In May 2018, our newly-constructed facility was inspected by Health Canada, and we received
our Controlled Substances Dealer’s Licence in June of that year. Shortly thereafter, our wholly-owned subsidiary TerraCube was founded,
and the first TerraCube prototype was constructed. Later that same year, HNCI obtained a Health Canada Cannabis Standard Processing Licence.
In May of 2020, we submitted an application to Health Canada for a Controlled Substances Dealer’s Licence for the ability to produce
and conduct research using psilocybin, psilocin, N,N-DMT, and mescaline. In parallel, we began the process of rebranding to our current
name, Lucy Scientific Discovery, Inc. In February 2021, the Health Canada Office of Controlled Substances completed the inspection, and
the licence was obtained by Lucy in August 2021. In October 2021, we filed an amendment with Health Canada to add the ability to sell,
send, transport, and deliver the substances currently included on our licence and add MDMA, LSD, and 2C-B to our license, which was approved
on December 17, 2021.
Our Business Strategy
Our mission is to become the
premier research, development, and contract manufacturing organization in the emerging psychotropics-based medicines industry, while aggressively
working to pursue expanding global market frontiers. Leveraging our highly skilled and experienced management team, we have designed a
competitive business strategy centered around agility, speed, and innovation. We aim to first establish and secure base revenues by quickly
commencing production capabilities and partnerships, and to continually pursue new opportunities for growth in our market.
Secure Base Revenue
| ● | Leverage Assets to Facilitate Market Entry: Our
research, development, and manufacturing operations will be conducted at our 25,000 square foot facility near Victoria, British Columbia,
Canada. This facility was designed to optimize workflow and support industry-leading current good manufacturing practices, or cGMP, good
laboratory practices, or GLP, cultivation, processing, sanitation, and physical security standards. Featuring energy-efficient design
and equipment, compartmentalized production bays, testing and analytics laboratories, and dedicated office space, this complex will provide
our team of experts and research partners a premier venue for productivity and innovation. Our facility features multiple TerraCubes
for cultivating plant and fungi biomass, thereby minimizing reliance on external suppliers for naturally derived materials. |
| ● | Establish Ability to Rapidly Commence Contract Manufacturing: By
establishing the capability to produce APIs through various methods of cultivation, purification, advanced cell expression, and direct
synthesis, we believe that we will be able to quickly execute highly scalable, flexible, and efficient production operations while keeping
batch-manufacturing costs low. We plan to enter into supply agreements with institutions, clinicians, and licensed researchers throughout
the United States and Canada. To that end, we have already entered into a preliminary agreement for a project involving psychedelics
cultivation and supply, and we are currently engaged in discussions with counterparties for two additional projects. Our goal is to rapidly
commence scaled cGMP manufacturing capabilities for psilocybin, psilocin, N,N-DMT, MDMA, 2-CB and mescaline. We expect to be able to
commence additional production following minimal buildout and infrastructure acquisition. See “Use of Proceeds” for more
information. |
| ● | Facilitate and Conduct Contract Psychotropics Research: We
seek to serve as an incubator and facilitator for the advancement of the psychotropics-based medicines industry. We will pursue this
objective by building a comprehensive support suite with the means to provide research collaboration and contract research, production,
funding, data capture, intellectual property and IP-capture opportunities, quality assurance, and compliance capabilities. Our team brings
vast relevant experience to bear regarding the development and commercialization of APIs and finished products, and we intend to partner
with researchers in an effort to advance the market. We believe that acting as a contract research organization will provide diversified
revenue sources under a number of different partnerships in accordance with our project assessment and advancement pipeline, and that
these activities will lead to further opportunities as our clients develop and commercialize various psychotropics-based therapies. |
| ● | Achieve and Maintain Compliance Excellence: In
addition to maintaining necessary licensing for the production of APIs, we will uphold rigorous internal operating standards, employing
cGMPs for production and GLPs for testing and analysis. Our management team brings a wealth of relevant knowledge about, and extensive
experience with, regulatory compliance and quality controls, which we believe will enable us to comply with evolving legal frameworks. |
Pursue New Frontiers
| ● | Expand Market Access: We
plan to pave the way for growth into new and emerging market landscapes by designing and executing strategic market access initiatives.
These initiatives involve collaborating with regulators and participating in legislative study campaigns while strategically aligning
and optimizing our partnerships and capabilities to thrive in the regulated markets we intend to incubate. We believe this will be a
highly effective method of expanding the viability, access, and control of government regulated business and will provide us with substantial
advantages in both placement and speed-to-market. |
| ● | Meet Emerging Demands with Innovative Products: We
intend to develop raw materials, cGMP-grade APIs, and finished biopharmaceutical products in an ongoing effort to meet the needs of new
and evolving markets. Our management team aims to develop or acquire technology that could, for example, be applied to optimize the delivery
of drug compounds for use in conjunctive treatment therapy, ensuring a safer and more consistent dose. We may seek to add new compounds
to our Health Canada Dealer’s Licence through a 45-day application process, potentially facilitating the means to perpetually innovate
and adapt to the developing needs of the psychedelics industry and market. |
| ● | Develop and Acquire Intellectual Property Assets: Members
of our management and research and development teams have significant experience with establishing and protecting critical process, product,
and technological differentiators. We intend to actively pursue the direct development and acquisition of relevant intellectual property
related to the psychotropics-based medicines industry, with an initial focus on intellectual property that will support and enhance our
contract research and manufacturing capabilities. We expect that our extensive market and research knowledge will allow us to recognize
and define a number of opportunities to acquire and create intellectual property, and enable iterative process improvements, to maintain
a competitive advantage. |
| ● | Achieve Business and Technological Diversification: To
further capitalize on direct, indirect, and ancillary opportunities created by the market, we may further diversify by investing in and
acquiring additional biotechnology companies and/or specific technologies that are complementary to our products and business strategy
when suitable opportunities arise, subject to the availability of sufficient financial and other resources to enable us to make such
investments and acquisitions. These efforts are designed to support our goal of creating deeper levels of resilience and integration,
and to differentiate our company from our competitors. |
In an effort to actualize each
facet of our overall business strategy as outlined above, we have established the following three-phase plan:
| ● | Phase 1 — Commence operations (Complete): We
incurred costs of approximately $35,000 associated with Phase 1 of our business plan to procure general equipment to enable process development
for the production of key APIs from natural product extraction. Achieving this manufacturing capability allow us to fulfil supply agreements
with academic and research facilities or other companies as permitted by our licence, resulting in first revenue generation. |
| ● | Phase 2 — Complete construction of R&D labs
and initiate cGMP certification: In order to broaden our research capabilities and expand into lab-scale synthetic and biosynthetic
production, we will need to complete construction of R&D labs by acquiring equipment utilized in standard synthetic and biosynthetic
laboratories. We anticipate the costs associated with Phase 2 of our business plan to be approximately $700,000. We believe these expanded
capabilities will allow us to potentially generate more revenue contingent upon future supply agreements. In parallel, we intend to initiate
the process of obtaining cGMP certification of key processes involved in the production of APIs. At this time, we cannot estimate when
this phase will be completed. |
| ● | Phase 3 — Achieve production-scale manufacturing
capabilities and cGMP certification: Contingent upon market demands, we intend to expand to production-scale manufacturing capabilities
by procuring larger production-scale equipment. We also aim to obtain cGMP certification pursuant to our goal of becoming a preferred
supplier of cGMP-grade APIs and other compounds. We anticipate the costs associated with Phase 3 of our business plan to be approximately
$1,500,000. At this time, we cannot estimate when this phase will be completed. |
The timing of the target milestones
may be subject to change due to a variety of factors including the need to obtain additional financing through the issuance of debt or
equity securities. There can be no assurance that we will be able to obtain any such financing, if needed, upon commercially reasonable
terms or at all. The failure to obtain such financing, if needed, would have a material adverse effect on our business.
Production Program
Our goal is to position our
company as a premier contract manufacturer of high-quality biological raw materials, cGMP-grade APIs, and finished biopharmaceutical products,
utilizing various methods of scalable production capabilities, to meet the needs of the rapidly growing psychotropics-based medicines
market. Leveraging advanced and efficient systems and processes, we will seek to minimize production costs while maintaining the highest
standards in quality and safety. We believe that our purpose-built campus and use of state-of-the-art technology will facilitate a variety
of scaled production methods that adhere to cGMP pharmaceutical standards.
To meet immediate and anticipated
rising demands from researchers and clinicians, our initial focus of production will be centered around the classic serotonergic psychedelics:
psilocybin, psilocin, and N,N-DMT. These APIs are in increasingly high demand, and we believe that there are very few cGMP-compliant
sources that are currently available in the market.
Our Strategic Approach to Production
Recognizing the broad range
of product requirements needed to best support ongoing research, trials, and treatments, our production program will take a highly scalable
and tiered approach to manufacturing that we believe has the potential to secure a strong foundation for revenue and growth. This approach
will leverage three key methods of production, with the goal of achieving best-in-class quality and facilitating market penetration through
competitive pricing. Regardless of method, all production and formulation efforts will involve proper analytical procedures and quality
controls that are designed to ensure the highest standards of purity, quality, and safety.

| ● | Cultivation and Extraction: We
intend to utilize a full suite of cGMP-grade cultivation, extraction, and purification systems to fulfill biological raw material and
small volume API orders for a rapid market entry. Our state-of-the-art medicinal fungiculture and horticulture program, featuring our
TerraCube growth chambers, will be capable of facilitating the production of consistent and high-quality raw materials, from which we
may derive medicinally valuable key-compounds and minor constituent molecules. Utilizing this production suite, our team has the ability
to observe broad-spectrum compositions, advance superior trait lines, and support various research organizations with best-in-class raw
materials and APIs as well as crude extracts, single-molecule fractions, and targeted formulations as required. |
| ● | Biosynthesis: Through
the development or acquisition of transgenic yeast, bacteria, and/or other cell lines, we will employ lab and pilot-scale bioreactors
to produce a master repository of API expression systems which can be rapidly scaled to meet emerging market demands. Our biopharmaceutical-based
core manufacturing approach involves the use of designer expression cassettes genetically encoded to produce APIs of interest from host
cells such as yeast or bacteria. The APIs expressed in these cultures can be subsequently purified and characterized. This production
methodology will provide us with a far greater ability to control post-translational modifications and allows for rapid scalability and
precise manufacturing of cGMP grade APIs. |
| ● | Synthesis: To accommodate
the need for consistent and scalable production capabilities, we intend to employ direct chemical synthesis methods coupled with subsequent
chromatographic and crystallization techniques for the isolation and purification of APIs. In addition, we expect that our team’s
wealth of experience in industrial scale organic and pharmaceutical chemistry will allow us to apply process optimizing retrosynthetic
methodologies, a technique that involves the transformation and examination of target molecules into precursor molecules. These methods
will maximize safety, quality, and consistency while providing critical flexibility in production. |
Finished Product Development and Commercialization
Support
Moving beyond raw material
and single-molecule API manufacturing, our team intends to develop the capacity to support the development and production of finished
pharmaceutical products. In addition to contract-based projects for our customers, our team will independently pursue scientific breakthroughs
in optimized drug delivery and molecular enhancement for licensed application in finished drug products. The overall aim of these strategic
ventures is to expand our market reach and involvement while creating additional revenue streams. We have and will continue to build strong
relationships with pharmaceutical research and development groups and clinicians studying the efficacy of psychedelic and emerging psychotropic
compounds in an effort to ensure all product designs and applications will best achieve the desired outcome for patients.
Research and Development Program
Our mission is to become a
best-in-class producer of pharmaceutical-grade psychotropic APIs and finished products. Our research and development program will be established
with the goal of supporting this mission by providing better APIs, target formulations, and finished drug products faster and more affordably
to a broadening marketplace. Our employment of critical performance assessments, analyses, and improvement practices function with the
objective of optimizing production, maintaining high quality standards, and lowering costs. We expect that our projected revenue increases
will drive aggressive advancements in our production and formulation projects, as well as in intellectual property and critical patent
capture programs.
We expect that continuously
improving our product offerings and iterating our production processes will best enable us to support advancements in clinical research
and applied therapies. To that end, our team is committed to conducting research and development activities aimed at optimizing our production
program — from initial project selection through post-clinical commercialization — by strategically employing
enabling technologies and innovative processes to meet the rigors of the emerging psychotropics-based medicines industry.
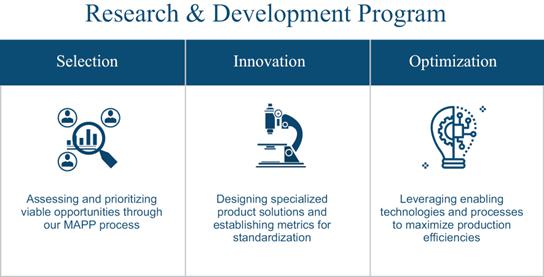
Market Assessment and Project Prioritization
Leveraging strategies from
the most successful growth companies in the biotechnology and pharmaceutical development industries, our Market Assessment and Project
Prioritization, or MAPP, process is designed to identify emerging market opportunities and direct our research and development pipeline.
The MAPP process quantifies and ranks opportunities in the following categories:
| ● | Potential for Treatment Efficacy |
| ● | Current & Forecasted Market Demand |
| ● | Market Regulation and Accessibility |
This data-driven approach is
designed to enable our team to determine key success and sustainability factors within each opportunity to inform selection, prioritization,
and resource allocation decisions. The intended outcome of the MAPP process is to support and pursue projects with the highest probabilities
of success and implement a consistent method to understand the value and risks associated with each R&D project candidate.
Project Selection and Advancement Pipeline
We seek to actively drive a
diversified research and development pipeline designed to accelerate potentially market-disrupting products from discovery through commercialization.
By combining powerful market opportunity analytics with well-established project selection and advancement processes, we believe that
our approach will maximize value-capture opportunities, success probabilities, and competitive advantages in new and emerging market spaces.
To best support these dynamic
project advancement efforts, our research and development teams will be well-equipped for success through the allocation of cutting-edge
technological systems and a centralized operational support network. These critical assets will facilitate project selection and advancement
decisions through:
| ● | Establishment of Success Metrics: A
key component of our pipeline process is a reliance on data to drive decisions. We will establish a set of measurable metrics and key
performance indicators, or KPIs, for all projects. We will track these KPIs and supporting metrics on a project dashboard to create transparency
and enable data-driven selection and advancement decisions. |
| ● | Prioritization of Functional Needs: To
facilitate effective functional support of all pipeline projects and the efficient use of assets, resources will be allocated to projects
determined to have the greatest impact and probability of success. Proper resource allocation and prioritization will enable our team
to support a broader range of projects appropriately and efficiently. |
| ● | Pipeline Advancement Decisions: Using
a structured process and well-defined advancement criteria our team will conduct periodic project reviews to assess KPIs, and success
probabilities. These reviews will consist of reports provided by project leadership and key personnel to a review panel of cross-functional
representatives from within the R&D organization. These process standards will be applied to all projects within the company’s
R&D pipeline, ensuring company assets are employed and redirected in accordance with company strategies. |
Furthermore, we intend to fully
leverage the broad network of collaborative relationships between the senior members of our management team and clinical research institutions,
contract research organizations, and licensed therapeutic clinicians to accurately assess emerging market needs and identify opportunities.
We believe that the value of our research and development program is enhanced by our commitment to explore and access enabling technologies
that can benefit and support our programs in a rapidly evolving emerging industry. We will seek to achieve sustainable revenue growth
by establishing and fostering a culture of continuous improvement and leveraging proven process and systems improvement philosophies.
Facilities
Our corporate headquarters
and operations are located near Victoria, British Columbia, Canada, where we currently lease approximately 25,000 square feet of laboratory
and office space. The property lease expires on July 31, 2027, at which point we may, at our option, either extend this lease for
an additional five-year term or purchase the facility. We have the option to purchase the property for CAD $14.5 million during the
lease term. Our facility has been designed to support key enabling technologies and production workflow, and feature two floors of compartmentalized
production bays, analytics laboratories, office space, and loading docks. With critical input from our highly experienced leadership team,
operators, and advisors, our facility was designed to maximize production while minimizing waste through the use of high-efficiency climate
and lighting systems. Furthermore, our security-by-design approach maintains high standards of safety and security, including expert implementation
of overlapping surveillance and monitoring systems, controlled access and alarms, and multiple Health Canada security level 8 vaults.
We believe that our current facilities are adequate to meet our ongoing needs, and that, if we require additional space, we will be able
to obtain additional facilities on commercially reasonable terms.
TerraCube Advanced Cultivation Module
Our TerraCube system, which
consists of climate-controlled agriculture/fungiculture growth chambers that employ our patented downdraft HEPA filtration technology,
is designed to provide our medicinal horticulture and fungiculture program leaders environmental control and manipulation capabilities.
These stackable and highly efficient systems are expected to facilitate multiple revenue-producing cultivation-based operations, adding
production scalability and sustainability to our program by allowing for modular and iterative expansion and project prioritization. From
cultivation of raw source materials and superior-trait psilocybe to contract ethnobotany research of rare medicinally valuable plants
from around the world, our TerraCube system will allow our team to rapidly begin researching and producing naturally derived products
and raw materials.
Each TerraCube module features
a patent-pending environmental control system drawing information from more than 100 data sensors. Each unit’s air-handling system
provides a positive pressure environment designed to ensure cultivars remain unadulterated by impurities or cross-contaminants. These
growth modules are expected to enable our team to conduct highly advanced genomic assessment and superior trait selection, intellectual
property capture, and Genetic Use Restriction Technology, or GURT, programs.

Bioprocess Development
Laboratory
Our bioprocess development
laboratory, or BDL, which supports API biosynthesis, is expected to enable the development of plasmids containing API expression cassettes
for insertion into host cell lines. The BDL will leverage both lab- and pilot-scale bioreactors in addition to necessary analytical and
supporting equipment. Stable API-expressing host cell lines will be banked in cryogenic freezers for subsequent transfer to quality control
and manufacturing processes.
Testing and Analysis Laboratory
Our testing and analysis
laboratory, or TAL, will utilize high-performance liquid chromatography with mass spectrophotometric detectors, or HPLC-MS, a proven
method for high purity quantification and low sensitivity detection of biopharmaceutical APIs. The HPLC-MS and supporting systems
are designed to ensure all APIs meet or exceed the standards for compliance and expectations of our customers. The TAL is capable of
facilitating various analytic functions in support of contract and partner research projects and serves as a final quality check for
production APIs prior to submission for third-party testing. The use of third-party laboratory testing is a requirement of good
clinical practices and GLP protocols.
Competition
The psychotropics-based product
manufacturing and contract research business is an emerging industry with increasing levels of competition and is subject to significant
technological change. We face substantial competition from other psychotropics-based product manufacturing companies and suppliers of
medical-grade psychedelic raw materials, APIs and finished drug products and/or contract research services. Our competitors are already
in the process of development and contract manufacturing of psychotropics-based products and providing contract research services in the
industry. Many of our competitors have substantially greater financial, technical and human resources, higher capitalization, a more experienced
management team, and a more mature business than us. These factors could prevent us from achieving our revenue, market share and growth
targets. Further, we may not be able to effectively manage our growth, if any, and operations, which could materially and adversely affect
our business. If we are not able to compete effectively against our current and future competitors, our business will not grow, and our
financial condition and operations will be materially and adversely affected.
Our potential competitors include
large and specialty pharmaceutical companies and biotechnology companies, academic research institutions and governmental agencies, and
public and private research institutions. Our plan to cultivate, extract and purify medical-grade psilocybin and other psychotropics-based
products and to offer them to appropriately licensed research institutions, biopharmaceutical companies and other parties who are engaged
in discovery and development with respect to psychotropics-based medicines, will compete with other entities that are developing or supplying
psychoactive compounds for use in medical research. Due to the depth and diversity of our intended product offerings, we may face competition
from a variety of companies, including:
| ● | Developers of psychotropics-based products: Companies
such as Mind Medicine (MindMed) Inc., a neuro-pharmaceutical drug development platform, Psygen Industries, Ltd., a manufacturer of pharmaceutical
grade psychedelic drug products for clinical research and therapeutic applications and Numinus Wellness Inc., a health care company focused
on creating wellness solutions centered on psychedelic therapies, and HAVN Life Sciences Inc., a biotechnology company pursuing standardized
extraction of psychoactive compounds, the development of natural health care products and mental health treatments. To the extent we
are unable to sell our products to these companies, our clients will face competition from them in the market for psychotropics-based
medicines. |
| ● | Contract research providers: Companies known
to provide contract research services to facilitate improved pharmaceutical and biotechnology product development, such as KGK Science
Inc. |
We expect to face increasing
competition as new APIs and other products enter the market and further advancements in technologies are made. We expect market adoption
of any products that we develop to be dependent on, among other things, purity, efficacy, and price.
Many of our current or potential
competitors, either alone or with their collaboration partners, have significantly greater financial resources and expertise in the development
and marketing of contract manufacturing and research services than we do. Mergers and acquisitions in the psychedelic and biotechnology
industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies
may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. As
attention on the emerging psychotropics-based medicines industry intensifies, we expect that additional competitors will enter the marketplace.
Government Regulation
We are focused on
developing and commercializing APIs comprising biologically sourced derivatives and synthetic compounds, primarily psychedelics,
with potential medicinal and therapeutic value as regulated medicines. In order for our APIs and other products to be developed into
regulated medicines, our process and our clients’ operations must be conducted in strict compliance with the regulations of
the regulatory agencies in the jurisdictions in which we and our clients operate or intend to operate, including the
United States and Canada, at the federal, state and (in the case of Canada) provincial level. These regulatory authorities
extensively regulate, among other things, the cultivation, manufacture, import, export, research, testing, quality control,
labelling, packaging, storage, record-keeping, promotion advertising, distribution, post-approval monitoring and reporting,
marketing, and export and import and commercialization of drugs and their APIs, such as those we are developing, in specific
jurisdictions under applicable laws and regulations.
We, along with our vendors
and research and commercial clients, will be required to navigate the various manufacturing, importation, exportation, preclinical, clinical,
and commercial approval requirements of the governing regulatory agencies of the countries in which we and our clients wish to manufacture,
test, store, seek approval and distribute our or our clients’ products and product candidates. The process of obtaining regulatory
approvals of drugs and their APIs and of ensuring subsequent compliance with appropriate federal, state, local and foreign statutes and
regulations requires the expenditure of substantial time and financial resources and may not be successful.
International Conventions Governing Controlled
Substances
Our business involves the use
of psychoactive compounds or materials that contain psychoactive compounds, including the manufacture, transportation, testing, storage
and sale of such compounds and products, and as such, will be subject to extensive regulation under international and national laws.
The current international drug
control system was established by three main international drug conventions: the 1961 United Nations, or UN, Single Convention on Narcotic
Drugs, or the Single Convention; the 1971 UN Convention on Psychotropic Substances, or the 1971 Convention; and the 1988 UN Convention
Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances, or the 1988 Convention. The Single Convention established the drug
scheduling system, which is also used in the 1971 Convention, to establish various degrees of control applicable to controlled substances,
or substances with a potential for abuse. The 1988 Convention focuses on criminal enforcement against illicit drug trafficking and money
laundering. All three conventions seek to restrict the use of controlled substances to legitimate purposes and avoid their diversion into
illicit markets through strict regulatory controls.
The 1971 Convention establishes
a regulatory framework and four schedules for psychotropic substances, which are substances that affect one’s mental state, including
hallucinogenic, or psychedelic, drugs. In addition to requiring controls on the manufacture, trade, distribution, and possession of psychotropic
substances, the 1971 Convention requires that signatories provide the International Narcotics Control Board, or INCB, with annual statistical
reports of quantities of psychotropic substances manufactured, exported from, or imported to their country. If, based on the information
provided, the INCB has reason to believe that the aims of the 1971 Convention are endangered, it can ask an endangering country to provide
explanations for its deviation from the 1971 Convention; call on the government to adopt remedial measures; or, bring the matter to the
attention of the greater UN and recommend that the country stop the export and/or import of a particular psychotropic substance. Regarding
substances in Schedule I of the convention, such as MDMA, DMT, and psilocybin, signatories are required to “prohibit all use
except for scientific and very limited medical purposes.”
The Single Convention requires
signatories to provide the INCB an annual estimate of the quantities of narcotic drugs to be used for medical and scientific purposes,
to be used in the manufacture of other drugs, and the stocks of narcotic drugs to be held by the signatory country. Signatories may not
exceed their submitted estimates without furnishing a supplementary estimate to the INCB explaining the need for the adjustment. The 1971
Convention does not establish the same type of ceiling on the manufacture and use of psychotropic substances.
The 1988 Convention outlines
a criminal enforcement framework for the manufacture, distribution, and sale of narcotic drugs or psychotropic substances in contravention
of the Single Convention and the 1971 Convention. It includes provision for the confiscation of proceeds from the illicit traffic of drugs
and creates a system for requesting extradition of guilty parties between signatory countries. In order to stem the international illicit
trade of narcotic drugs and psychotropic substances, all imports and exports of signatory countries — including the lawful
import and export of narcotics and psychotropics — must be properly documented and controlled.
As signatories to the Single
Convention, the 1971 Convention, and the 1988 Convention, Canada, our country of domicile, and the United States, one of our primary
markets, have based their domestic regulation of narcotics and psychotropic substances on the frameworks established in these conventions,
and they must comply with the conventions’ ongoing recordkeeping and reporting requirements.
Canada
Certain psychoactive compounds,
such as psilocybin, are considered controlled substances under Canada’s Controlled Drugs and Substances Act, or the CDSA. Specifically,
psilocin (3 — [2 — (dimethylamino)ethyl] — 4 — hydroxyindole) and any salt thereof
and psilocybin (3 — [2 — (dimethylamino)ethyl] — 4 — phosphoryloxyindole) and any
salt thereof, are listed under Schedule III of the CDSA. Psilocin and psilocybin are also restricted drugs under Part J
of the Food and Drug Regulations. In Canada, MDMA and ketamine are Schedule I controlled substances, while LSD is a Schedule III
controlled substance. The production, possession, obtaining, trafficking (including, among other things, sale, distribution, and administration),
importing or exporting of controlled substances and precursors is prohibited in Canada unless specifically permitted by applicable law.
Penalties for contravention of the CDSA related to Schedule I substances are the most punitive, with Schedule II being less
punitive than Schedule I, and Schedule III being less punitive than Schedule I and II. A party may seek government
approval for an exemption under Section 56(1) of the CDSA to allow for the possession, transport or production of a controlled
substance for medical or scientific purposes or if such usage is otherwise in the public interest.
A licence may be obtained
to produce, assemble, sell, provide, transport, send, deliver, import and export controlled substances and products that contain a controlled
substance. A party can apply for a Dealer’s Licence under the Canadian Food and Drug Regulations (Part J), which would permit
a party to perform authorized activities in relation to a restricted drug, such as psilocybin and psilocin. By law, a Dealer’s Licence
for a restricted drug may only be issued to eligible persons, which include: (i) an individual who ordinarily resides in Canada;
(ii) a corporation that has its head office in Canada or operates a branch office in Canada; or (iii) the holder of a position
that includes responsibility for restricted drugs on behalf of the Government of Canada or of a government of a province, a police force,
a hospital or a university in Canada.
To qualify for a Dealer’s
Licence, a party must meet all regulatory requirements, including having compliant facilities and security measures, compliant materials
and staff that meet the qualifications under the regulations. An applicant must designate a senior person in charge, who is responsible
for the management of the activities with respect to the restricted drugs subject to the licence application, and a qualified person in
charge, who is responsible for supervising activities with respect to the restricted drug. The qualified person in charge must meet prescribed
qualification requirements, in addition to working at the site specified in the Dealer’s Licence. The proposed qualified person
in charge must be a pharmacist or a practitioner of medicine, dentistry or veterinary medicine registered with a provincial professional
licensing authority, or hold a degree in an applicable science from a recognized Canadian University, or a foreign degree recognized by
a Canadian university or a Canadian professional association. Furthermore, an applicant must submit a criminal record check by a Canadian
police force evidencing that during the 10 years prior to the application, the senior person in charge, and the qualified person
in charge, was not convicted of a designated offenses as set out in Part J of the Food and Drug Regulations.
Licenced Dealers must have
a secure facility for the storage of controlled drugs and substances. There are 11 security levels applicable to controlled drugs and
substances, which are based upon the geographical location in Canada and the total value of controlled substances stored on the premises
at any given time. A physical security inspection is required as part of the application process.
Assuming compliance with all
relevant laws (Controlled Drugs and Substances Act, Food and Drugs Regulations) and subject to any restrictions placed on the licence
by Health Canada, an entity with a Dealer’s Licence may produce, assemble, sell, provide, transport, send, deliver, import or export
a restricted drug (as listed in Part J in the Food and Drugs Regulations, which includes psilocybin and psilocin) (see s. J.01.009(1) of
the Food and Drug Regulations). However, a licenced dealer may only import and export controlled substances and restricted drugs in accordance
with a permit from Health Canada, which must be obtained for each import or export.
There are a number of reasons
that Health Canada must refuse a licence under applicable law. For example, a licence must be refused if an applicant does not have prescribed
security measures in place, the applicant has submitted false or misleading information with respect to its licence application, or there
are reasonable grounds to believe that the issuance of the licence would likely create a risk to public health or safety, including the
risk of a restricted drug being diverted to an illicit market or use. Once issued, Health Canada has the authority to suspend or revoke
a Dealer’s Licence if it has reasonable grounds to believe that it is necessary to do so to protect public health or safety, which
includes preventing a restricted drug from being reverted to an illicit market or use.
Furthermore, permission to
export psychedelics is not guaranteed under a Dealer’s Licence, and there is risk that Health Canada may not issue an export permit
at each or any request. By law, an export permit must not be issued for a restricted drug where, among other things, the issuing body
has reasonable grounds to believe that the exportation would contravene an international obligation, or where there are reasonable grounds
to believe that the exportation would contravene the laws of the country of final destination or any country of transit or transhipment.
Therefore, even with a valid Dealer’s Licence issued for the production of psilocybin, legal export of APIs to customers outside
of Canada is not guaranteed.
Currently, a licenced dealer
may only sell psychedelics to an institution for clinical or research purposes. In Canada, an “institution” under Part J
of the Food and Drug Regulations is defined as any institution engaged in research on drugs and includes a hospital, a university in Canada
or a department or agency of the Government of Canada or of a government of a province or any part of them. Prior to the sale, the research
institution must obtain authorization for the sale from Health Canada.
In order to conduct research
or clinical trials with psychedelics in Canada, an institution must either hold its own Dealer’s Licence, or an exemption from Health
Canada under Section 56(1) of the CDSA, and a clinical trial authorization from Health Canada. Section 56(1) of the
CDSA allows for an exemption from the application of all or any of the provisions of the CDSA, and can permit the possession of a controlled
substance for medical or scientific purposes (for example, in clinical trials), or where it is in the public interest to grant and exemption.
The activities permitted under
a Section 56(1) exemption are not defined in the CDSA. An applicant seeking a Section 56(1) exemption for scientific
purposes must provide a project or study description, including a research protocol and approval by an animal care committee if applicable.
Administration to human subjects is not permitted under an exemption for scientific purposes, whereas administration to animals may be
permitted under certain conditions. An application for an exemption to use a controlled substance for clinical studies requires the submission
of a clinical trial protocol and an authorization from Health Canada to conduct a clinical trial (in the form of a No Objection Letter).
Physical security measures must be maintained at any facility in which research or clinical trials are conducted under a Section 56(1) exemption.
To our knowledge, the Canadian government has not yet granted a Section 56(1) exemption for the use of psychedelics.
Failure to comply with any
of the above applicable regulations, regulatory authorities or other requirements may result in civil or criminal penalties, recall or
seizure of products, partial or total suspension of production, or revocation of a licence or exemption.
Bringing New Drugs to Market
We expect that many of our
clients will purchase our products for purposes of conducting research and development, and ultimately obtaining regulatory approval for
and commercializing drug products designed to treat a range of mental health and cognitive conditions. In Canada, Health Canada regulates
drug products under the federal Food and Drugs Act, and its regulations. Failure to comply with applicable Health Canada requirements
at any time with respect to product development, clinical testing, approval or any other legal requirements relating to product manufacture,
processing, handling, storage, quality control, safety, marketing, advertising, promotion, packaging, labelling, export, import, distribution,
or sale may lead to administrative or judicial penalties or other consequences. These consequences could include, among other things,
Health Canada’s refusal to approve pending applications, suspension or revocation of approved applications, warning letters, recalls,
product seizures, relabelling or repackaging, total or partial suspensions of manufacturing or distribution, or prosecution. The sale
of pharmaceutical products may also be subject to other provincial regulations.
Before testing any drug in
humans, the product candidate must undergo rigorous preclinical testing. Preclinical studies include laboratory evaluations of drug chemistry,
formulation and stability, as well as in vitro and animal studies to assess safety and in some cases to establish the rationale for therapeutic
use. If preclinical tests indicate that a substance produces a desired effect and is not toxic, a sponsor may apply to the Health Canada
for authorization to conduct a clinical trial.
The clinical stage of development
involves the administration of the product candidate to healthy volunteers or patients under the supervision of qualified investigators
to research and gather information on a drug’s dose, effectiveness and safety in humans. Clinical trials are conducted in accordance
with good clinical practice, or GCP, requirements under protocols detailing, among other things, the objectives of the clinical trial,
administration procedures, subject selection and exclusion criteria and the parameters and criteria to be used in monitoring safety and
evaluating effectiveness. Each protocol, and any subsequent amendments to the protocol, must be submitted to Health Canada for approval.
Furthermore, each clinical trial must be reviewed and approved by a Research Ethics Board, or REB, for each site at which the clinical
trial will be conducted. The REB is not affiliated with the clinical trial sponsor, and its principal mandate is to approve the initiation
of, and conduct periodic reviews of, biomedical research involving human subjects in order to ensure the protection of subject rights,
safety and well-being. If the clinical studies demonstrate that the potential therapeutic benefits outweigh associated risks, a clinical
trial sponsor may file a New Drug Submission, or NDS, with Health Canada.
An NDS is a request for approval
to market a new drug in Canada, and it contains information gathered regarding the safety, efficacy and quality of a drug. The NDS includes
preclinical and clinical trial results, and information regarding therapeutic claims, side effects, production, packaging and labelling.
To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and efficacy of the
drug to the satisfaction of Health Canada. Health Canada must approve an NDS and issue a Notice of Compliance, or NOC, and a Drug Identification
Number, or DIN, before a drug may be marketed Canada.
Drugs approved for marketing
in Canada but remaining on Schedule III of the CDSA will still be subject to the restrictions contained therein. To date, no drugs
containing psilocybin or psilocin have been issued a NOC in Canada.
The United States
In the United States,
the Drug Enforcement Administration, or DEA, the Food and Drug Administration, or FDA, and other regulatory authorities at federal, state
and local levels, extensively regulate the research, development, testing, manufacture, quality control, import, export, safety, effectiveness,
labelling, packaging, storage, distribution, recordkeeping, approval, advertising, promotion, marketing, post-approval monitoring and
post-approval reporting of drugs and their APIs. Importantly, the United States’ federal Controlled Substances Act, or CSA,
the Controlled Substances Import Export Act, or the CSIEA, and their implementing regulations regulate the substances we intend to manufacture,
refine and export to the United States.
The Controlled Substances Act
Controlled substances are defined
as drugs or other substances that have a potential for abuse. The CSA imposes registration, security, recordkeeping and reporting, storage,
disposal and other requirements on any person or entity that manufactures, distributes, dispenses, imports, exports, or conducts research
with controlled substances. These requirements have been established to prevent the diversion of controlled substances to illicit channels
of commerce while providing for the legitimate medical and scientific needs of the United States. The United States Attorney
General has delegated responsibility for the regulation of controlled substances to the DEA.
The DEA categorizes controlled
substances into one of five schedules — Schedule I, II, III, IV or V — with varying qualifications
for listing in each schedule. Schedule I controlled substances are those that have a high potential for abuse, have no currently
accepted medical use in the United States and are not accepted as capable of being safely used under medical supervision. Substances
having a currently accepted medical use, including pharmaceutical products, may be listed as Schedule II, III, IV or V
controlled substances, with Schedule II controlled substances presenting the highest potential for abuse and physical or psychological
dependence, and Schedule V controlled substances presenting the lowest relative potential for abuse and dependence. The regulatory
requirements are more restrictive for handlers of Schedule II controlled substances than Schedule III-V controlled substances.
For example, all Schedule II drug prescriptions must be signed by a physician, physically presented to a pharmacist in most situations,
and cannot be refilled. Psychotropics such as psilocybin, psilocin, DMT and MDMA are regulated as Schedule I controlled substances,
and have the strictest controls imposed upon their use for any purpose.
Scheduling determinations by
the DEA are dependent on FDA approval of a substance or a specific formulation of a substance for medical use and marketing in the United States.
Therefore, while psilocybin and the other psychedelic substances we may cultivate and manufacture are primarily Schedule I controlled
substances, products approved by FDA for medical use and marketing in the United States that contain psilocybin or another such substance
would be placed in Schedules II-V, since approval by FDA satisfies the “accepted medical use” requirement. If and when
a product candidate developed by one of our clients receives FDA approval, the DEA will likely make a scheduling determination and place
it in a schedule other than Schedule I in order for it to be prescribed to patients in the United States.
Registration to Handle Schedule I Substances
Required by U.S. Facilities
While we are required to comply
with Canadian law governing manufacture and export of controlled substances, our U.S. clients will be required to comply with DEA
policy regarding the handling — including the import — of the U.S.-designated Schedule I substances they purchase from
us. Facilities conducting research, manufacturing, distribution, importation, exportation, or dispensing of any controlled substances
must register and receive a certificate of registration from the DEA. In order to obtain DEA registration, the facilities will first
need to register with the narcotics enforcement department of the state in which they are located. Once a facility receives a certificate
of registration from the DEA, it is referred to as a registrant. Registrants must have the security, control, recordkeeping, reporting,
and inventory mechanisms required by the DEA to prevent loss and diversion of any controlled substances.
Several categories of registrations
are available, depending on a registrant’s principal activity. These categories are:
| ● | Manufacturing (bulk or dosage form) |
| ● | Reverse Distributing (controlled substance waste disposal) |
| ● | Dispensing or Instructing (for medical practitioners, hospitals/clinics,
pharmacies and teaching institutions) |
| ● | Research with Schedule I substances |
| ● | Research with Schedule II through V substances |
| ● | Narcotic Treatment Program |
The certificate of registration
will specify the exact substances authorized to be used and the activities the registrant is authorized to engage in. Any facility that
engages in more than one group of independent activities must obtain a separate registration for each group of activities, unless the
additional activities are listed as “coincident activities” to the primary activity for which a registration is issued. For
instance, manufacturing registrants may, as an activity coincident to the primary activity of manufacturing, distribute the class of substance
for which registration was issued.
DEA registrations must be renewed
annually, except for dispensing facility registrations, which must be renewed every three years. The DEA conducts periodic inspections
of certain registered establishments that handle controlled substances. Our U.S. clients must receive certificates of registration
before they may apply to import our products.
A new application for registration
will include a DEA field investigation, analysis and review of the application at DEA headquarters, and a request for any other information
that the DEA deems necessary. Applications for importers or bulk manufacturers of controlled substances are published in the Federal Register,
and other registered bulk manufacturers may submit comments or objections to the new registration.
All applicants and registrants
must provide effective controls and procedures to guard against theft and diversion of controlled substances. In evaluating the overall
security system of a registrant or applicant, the DEA will consider, among other things, the adequacy of the applicant’s system
for monitoring the receipt, distribution, and disposition of controlled substances in its operations. The DEA may deny an application
for registration if it finds that the registration is inconsistent with the public interest, which is determined by considering:
| ● | maintenance of effective controls against diversion into
other than legitimate medical, scientific, research, or industrial channels by limiting the importation and bulk manufacturer of controlled
substances to establishments that can produce an adequate and uninterrupted supply of these substances under adequately competitive conditions
for legitimate medical, scientific, research, and industrial purposes; |
| ● | compliance with applicable state and local law; |
| ● | prior conviction records of applicants relating to the manufacture,
distribution, or dispensing of controlled substances; |
| ● | past experience in the applied for activity, and the existence
in the establishment of effective controls against diversion; |
| ● | for manufacturing registrants, promotion of technical advances
in the art of manufacturing the controlled substances and the development of new substances; and |
| ● | other factors as may be relevant to and consistent with the
public health and safety. |
Failure to comply with applicable
requirements of registration, particularly as manifested in the loss or diversion of controlled substances, can result in enforcement
action that could have a material adverse effect on a registrant’s business, operations and financial conditions. The DEA may seek
civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances,
violations could lead to criminal prosecution.
Registration Requirements for Research Facilities
We anticipate that some of
our clients will be research facilities at U.S. academic institutions or companies conducting research. Such research facilities
that wish to study Schedule I controlled substances must, in addition to meeting the applicable requirements described above, send
their research protocol to the DEA. The research protocol must include a statement of the purpose of the research project; the researchers’
institutional affiliation and qualifications; the name of the Schedule I substances involved and the amount of each needed; and the
source of the Schedule I substances, including whether they will be provided by a domestic or foreign manufacturer; a description
of the research to be conducted; a statement of the security provisions for storing and dispensing the substances in a way that prevents
diversion; and a statement of the quantity and sources of the substances to be manufactured or imported. All of the foregoing will be
submitted by the DEA to the Department of Health and Human Services (HHS) for approval. The research registrant must justify the need
for import of the substances, and HHS must approve the importation as part of the research protocol. If the registrant has already submitted
an Investigational New Drug, or IND, application to the FDA, proof of such application may be submitted to the DEA in lieu of the foregoing.
If a research registrant needs
to increase the quantity of a Schedule I substance used for an approved research project, a request must be submitted to the DEA,
which will forward the request to FDA for approval. Any change in the research protocol likewise must be submitted to the DEA. Facilities
registered to conduct research with Schedule I controlled substances may conduct research only with the substances for which the
facility’s research protocol was approved.
In September 2021, the U.S.
Office of National Drug Control Policy (ONDCP) issued a legislative proposal to the U.S. Congress to amend the process for obtaining a
DEA registration for research with Schedule I substances to align it more closely with Schedule II research registrations. If implemented,
the changes may shorten the timeline and simplify the paperwork required for U.S. research facilities to obtain registrations allowing
them to access Schedule I substances for scientific purposes. On December 2, 2021, the DEA expressed support for ONDCP’s
proposal via written testimony submitted to a House Energy and Commerce subcommittee.
U.S. Import Regulations Applicable to
Schedule I Substances
Once registered, our U.S. clients
must apply for permission from the DEA to import our APIs. Import of Schedule I substances into the United States is governed
by the CSIEA and its implementing regulations. Although it is generally unlawful to import Schedule I substances into the United States,
certain specified substances may be imported if the DEA finds such import would serve medical, scientific or other legitimate purposes.
Specifically, an application to import Schedule I substances may be authorized if competition among domestic manufacturers of the
controlled substance is inadequate and will not be rendered adequate by the registration of additional manufacturers, or, if the domestic
supply of any controlled substance is inadequate for scientific studies.
As described above, DEA registrations
comprise various categories based on the primary activity of the registrant. Only three of the DEA registration categories allow the registrant
to apply for authorization to import Schedule I substances: research, import, and chemical analysis. Registrants, such as manufacturers
and distributors, without the ability to import must obtain controlled substances from another entity registered under one of the following
categories:
| ● | “Research — Schedule I”
registration. The primary activity of this category of registrants is research with Schedule I substances. Coincident activities
include: manufacture or import of the “basic class” (i.e., encompassing all the chemical forms) of substance or substances
for which registration was issued (provided that such manufacture or import is set forth in the research protocol approved by FDA), and
distribution of such class to persons registered or authorized to conduct research with such class of substance or registered or authorized
to conduct chemical analysis with controlled substances. We anticipate that the majority of our prospective clients will pursue DEA registration
under this category. |
| ● | “Importing” registration. The primary
activity of this category of registrants is importation of controlled substances specified on the registration. Coincident activities
include distribution of that substance or class for which registration was issued. Importers may not distribute any substance or class
for which not they are not registered. Applications for import registrations are subject to a notice and comment period during which
bulk manufacturers of the affected basic classes may file written comments or objections to the issuance of the proposed registration. |
| ● | “Chemical Analysis” registration. The
primary activity of this category of registrants is analysis of controlled substances. Coincident activities include manufacture and
import controlled substances for analytical or instructional activities; distribution of such substances to persons registered or authorized
to conduct chemical analysis, instructional activities, or research with such substance; and the conduct of instructional activities
with controlled substances. |
In addition to needing a registration
for their primary activities, the above registrants must apply for import permits from the DEA to import particular shipments. A separate
permit is required for each shipment of a Schedule I substance to be imported. The DEA is authorized to issue an import permit if
it finds that the domestic supply of particular substance is inadequate for scientific studies or to meet the needs of an emergency, or,
in any case, if it finds that competition among domestic manufacturers of the controlled substance is inadequate and will not be rendered
adequate by the registration of additional manufacturers. U.S. policy favors domestic production of controlled substances. If there
are domestic manufacturers of the Schedule I substances, the registrant will have to justify the need for import. If an import permit
is approved, it must describe the precautions that the applicant will take to guard against storage or in-transit losses, such as ensuring
that shipping containers are unmarked. Applicants must indicate the source of the substances, the port of entry into the United States,
the name of the importing carrier or vessel, and the date the shipment will leave the foreign port or country. Registrants are responsible
for selecting common or contract carriers that can provide adequate security to guard against in-transit losses.
The DEA will send a copy of
the import permit to the Canadian authorities (the country of export) and our importing registrant-clients will be required to submit
a copy of the import permit and information about the transaction to the customs officer at the port of entry. If shipments are denied
release by a customs officer at the port of entry for any reason, the importer must submit a new application for an import permit. After
receipt of the Schedule I substance, registrants must submit a follow-up report to the DEA, confirming receipt and conformity of
the shipment to the import permit.
Some of our clients may wish
to register as importers, with importation as their primary activity and distribution as a coincident activity. The DEA grants an importer
registration if it determines that “such registration is consistent with the public interest and with United States obligations
under international treaties, conventions, or protocols.” The DEA is required to limit imports by registered importers to amounts
necessary to provide for the medical, scientific, or other legitimate needs of the United States in the case that competition among
domestic manufacturers of the controlled substance is inadequate and will not be rendered adequate by the registration of additional manufacturers.
In determining whether domestic competition is adequate, the DEA must consider price rigidity, conditions of supply and demand, and the
extent of service and quality competition among the domestic manufacturers. The fact that there are only a small number of registered
manufacturers for a particular controlled substance is not indicative of a lack of competition.
Customs Considerations
The importation of goods into
the United States is managed by U.S. Customs and Border Protection, or CBP. In addition to complying with DEA policy regarding
importation of Schedule I substances, our clients must also ensure that shipments comply with CBP laws and regulations regarding
the importation of merchandise from foreign countries. CBP requires the use of shipping manifests, bills of lading, inspection of merchandise,
payment of duties, if applicable, and reporting. The Toxic Substances Control Act, or TSCA, requires that importers of any chemicals include
a certification that the chemical either complies with the TSCA or is exempt from the TSCA. Failure to follow CBP and TSCA requirements
may result in detention or even destruction of shipments.
Procurement Quotas
Some of our clients may be
U.S. registered manufacturers who plan to convert our bulk APIs into dosage forms or into other substances. These U.S. registrants
must obtain procurement quotas from the DEA. Procurement quotas set a ceiling on the amount of a Schedule I or II controlled
substance a registered manufacturer may obtain for conversion into dosage form or other substances. A separate application for a procurement
quota must be submitted for each Schedule I or II substance a manufacturer wants to acquire. The applications must state the
purpose for which the substance is being acquired, and the quantity desired for that purpose during a calendar year. Procurement quota
applications must be submitted by April 1 of the year preceding the calendar year for which the procurement quota will apply. Only
manufacturers who plan to convert bulk quantities of controlled substances into dosage form or into other substances must apply for a
procurement quota; research registrants and chemical analysis registrants are not required to apply for procurement quotas.
Manufacturers that have been
issued a procurement quota may request an adjustment to the quota by applying with the DEA and showing the need for the adjustment. An
increase to a procurement quota is at the discretion of the DEA. If there exist sufficient domestically-produced quantities of a
certain Schedule I or II substance, the DEA may limit the issuance of import permits.
Production Quotas
Similar to procurement quotas,
production quotas set a ceiling on the amount of bulk Schedule I or II substances that may be manufactured in, or imported into,
the United States in any given year. The country’s aggregate production quota, or APQ, reflects the total quantity of each
basic class of controlled substances in Schedule I or II necessary to be manufactured in the United States in a given year
to provide for the estimated medical, scientific, research, and industrial needs of the country, for lawful export requirements, and for
the establishment and maintenance of reserve stocks. The APQ can be adjusted at any time, but only at the discretion of the DEA. The
APQ is divided among registered bulk manufacturers as individual manufacturing quotas. Registered bulk manufacturers wishing to manufacture
bulk quantities of Schedule I or II controlled substances in the United States must apply for individual manufacturing
quotas every year, and may not manufacture more than their assigned allotment without a modification to their manufacturing quota from
the DEA. The APQs and individual manufacturing quotas are established in terms of bulk quantities of each basic class of controlled
substances, and not in terms of individual pharmaceutical dosage forms prepared from, or containing a controlled substance. The DEA recently
increased the 2021 aggregate production quotas for psilocybin, psilocin, MDMA, and DMT. It has proposed increased 2022 aggregate production
quotas for psilocybin, psilocin, MDMA, DMT, LSD, mescaline, 5-MeO-DMT, and MDA.
State and Local Regulation of Psychedelics
Each state in the Unites States
also maintains separate controlled substance laws and regulations, including licensing, recordkeeping, security, distribution, and dispensing
requirements. State authorities, including Narcotics Control Boards and Boards of Pharmacy, regulate use of controlled substances in each
state. Though state-controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately
schedule a controlled substance or product containing a controlled substance. While some states automatically schedule a drug based on
federal action, other states schedule drugs through rule making or a legislative action. However, any state law in positive conflict with
the CSA is superseded by the CSA.
Nonetheless, beginning with
the state legalization of cannabis for medical use in California in 1996, U.S. jurisdictions have been modifying their own controlled
substance laws and criminal enforcement policies to allow for broader manufacture, distribution, dispensing, and possession of certain
controlled substances, in contravention of the CSA. While cannabis has been the most widespread example of this tension between state
and federal law, the therapeutic use of psychedelics is now gaining traction in U.S. cities and states. For instance, the city and
county of Denver voted in 2019 to make the enforcement of any laws imposing criminal penalties for the personal use and personal possession
of psilocybin mushrooms the lowest law enforcement priority in the city and county of Denver, and in Oregon, Measure 109 was passed in
November 2020 directing the Oregon Health Authority, or OHA, after a two-year development period, to license and regulate the manufacturing,
transportation, delivery, sale and purchase of psilocybin products and the provision of psilocybin services. Oakland and Santa Cruz, California,
Washington, D.C., and Ann Arbor, Michigan have declared the enforcement of laws that criminalize the non-commercial planting, cultivating,
purchasing, transporting, distributing, possessing or engaging in practices with entheogenic plants among their lowest law enforcement
priorities. Forty-one states and the U.S. Congress have adopted “right-to-try” laws, allowing patients with terminal
conditions to try investigational drugs which have passed Phase I clinical trials but have not yet been approved for general use.
The investigational drugs accessible via right-to-try laws include psychedelics-based drugs. In September 2021, the Washington State
attorney general argued in defense of the state’s right-to-try laws in a case challenging the DEA’s assertion that it has
no authority to help practitioners implement the laws. The case arose from a doctor’s request to give psilocybin to terminally ill
patients.
Although jurisdictions in the
United States have decriminalized or even legalized and regulated psychedelics to varying extents, employing varying regulatory frameworks,
participation in these state frameworks would be in violation of the CSA and could lead to federal prosecution, including seizure of assets
and criminal penalties. We will only be able to distribute our products to DEA-registered facilities and cannot participate in state-specific
psychedelics markets that are in contravention of U.S. federal law.
Bringing New Drugs to Market
We expect that many of our
clients will purchase our products for purposes of conducting research and development, and ultimately obtaining regulatory approval for
and commercializing, drug products designed to treat a range of mental health and cognitive conditions. In the United States, the
FDA regulates drug products under the Federal Food, Drug, and Cosmetic Act, as amended, or the FDCA, its implementing regulations and
other laws. Failure to comply with applicable FDA or other requirements at any time with respect to product development, clinical testing,
approval or any other legal requirements relating to product manufacture, processing, handling, storage, quality control, safety, marketing,
advertising, promotion, packaging, labelling, export, import, distribution, or sale may lead to administrative or judicial sanctions or
other legal consequences. These sanctions or consequences could include, among other things, the FDA’s refusal to approve pending
applications, issuance of clinical holds for ongoing studies, suspension or revocation of approved applications, warning or untitled letters,
product withdrawals or recalls, product seizures, relabeling or repackaging, total or partial suspensions of manufacturing or distribution,
injunctions, fines, civil penalties or criminal prosecution. Pharmaceutical products are also subject to other federal, state and local
statutes and regulations. A failure to comply with any requirements during the product development, approval, or post-approval periods,
may lead to administrative or judicial sanctions, which could include the imposition of a hold on clinical trials, refusal to approve
pending marketing applications or supplements, withdrawal of approval, warning letters, product recalls, product seizures, total or partial
suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution.
Before testing any drug in
humans, the product candidate must undergo rigorous preclinical testing. Preclinical studies include laboratory evaluations of drug chemistry,
formulation and stability, as well as in vitro and animal studies to assess safety and in some cases to establish the rationale for therapeutic
use. The conduct of preclinical studies is subject to federal and state regulation, including good laboratory practice, or GLP, requirements
for safety/toxicology studies and the Animal Welfare Act, which is enforced by the Department of Agriculture. The results of the preclinical
studies, together with manufacturing information and analytical data, must be submitted to FDA as part of an IND. An IND is a request
for authorization from FDA to administer an investigational product to humans and must become effective before clinical trials may begin.
The clinical stage of development
involves the administration of the product candidate to healthy volunteers or patients under the supervision of qualified investigators,
who generally are physicians not employed by or under the trial sponsor’s control, in accordance with good clinical practice, or
GCP, requirements, which include the requirements that all research subjects provide their informed consent for their participation in
any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial,
administration procedures, subject selection and exclusion criteria and the parameters and criteria to be used in monitoring safety and
evaluating effectiveness. Each protocol, and any subsequent amendments to the protocol, must be submitted to the FDA as part of the IND. Furthermore,
each clinical trial must be reviewed and approved by an institutional review board for each institution at which the clinical trial will
be conducted to ensure that the risks to individuals participating in the clinical trials are minimized and are reasonable compared to
the anticipated benefits.
Clinical trials to evaluate
therapeutic indications to support NDAs for marketing approval are typically conducted in three sequential phases, which may overlap.
| ● | Phase 1 — Phase 1 clinical
trials involve initial introduction of the investigational product into healthy human volunteers or patients with the target disease
or condition. These studies are typically designed to test the safety, dosage tolerance, absorption, metabolism and distribution of the
investigational product in humans, excretion, the side effects associated with increasing doses, and, if possible, to gain early evidence
of effectiveness. |
| ● | Phase 2 — Phase 2 clinical
trials typically involve administration of the investigational product to a limited patient population with a specified disease or condition
to evaluate the drug’s potential efficacy, to determine the optimal dosages and administration schedule and to identify possible
adverse side effects and safety risks. Phase 2 clinical trials are typically controlled and conducted in a limited patient population. |
| ● | Phase 3 — Phase 3 clinical
trials typically involve administration of the investigational product to an expanded patient population to further evaluate dosage,
to provide statistically significant evidence of clinical efficacy and to further test for safety, generally at multiple geographically
dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the investigational
product and to provide an adequate basis for product approval and physician labelling. In most (though not all) cases, FDA requires two
adequate and well controlled Phase 3 clinical trials to support approval of a drug. The Multidisciplinary Association for Psychedelic
Studies recently completed Phase 3 trials of a study evaluating MDMA-assisted therapy for severe PTSD. |
Assuming successful completion
of the required clinical testing, the results of the preclinical studies and clinical trials, together with detailed information relating
to the product’s chemistry, manufacture, controls and proposed labelling, among other things, are submitted to the FDA as part of
an NDA package requesting approval to market the product for one or more indications. An NDA is a request for approval to market a new
drug for one or more specified indications and must contain proof of the drug’s safety and efficacy for the requested indications.
The marketing application is required to include both negative and ambiguous results of preclinical studies and clinical trials, as well
as positive findings. Data may come from company-sponsored clinical trials intended to test the safety and efficacy of a product’s
use or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted
must be sufficient in quality and quantity to establish the safety and efficacy of the investigational product to the satisfaction of
the FDA. The FDA must approve an NDA before a drug may be marketed in the United States.
Drugs approved for marketing
in the United States but remaining on Schedule II will still be subject to the import permit requirements described herein.
If an approved nonnarcotic drug is listed on Schedule III, IV, or V in the United States, but remains listed on Schedule I
or II of the 1971 Convention, it will also continue to be subject to the import regulations described herein. If an approved nonnarcotic
drug in the United States is listed on Schedule III, IV, or V in the United States and is not listed on Schedule I
or II of the 1971 Convention, then it will be subject to modified import regulations. Specifically, this type of approved drug may
be imported pursuant to a controlled substances import declaration filed with the DEA no less than 15 days prior to the shipment’s
anticipated date of release by a custom’s officer. If we will be supplying the finished dosage form an approved Schedule III, IV,
or V drug which is not listed on Schedule I or II of the 1971 Convention, our products may be imported using an import
declaration. If, however, we will be supplying bulk APIs for use in manufacturing such drugs, and those APIs remain on Schedules I
or II in the United States, they will be subject to the import permit requirements described herein.
From time to time, legislation
is drafted, introduced and passed in the U.S. Congress that could significantly change the statutory provisions governing the approval,
manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations and policies are often
revised or reinterpreted by the agency in ways that may significantly affect our business and its product candidates.
Employees and Human Capital Resources
As of August 15, 2023,
we had 2 employees, being our Chief Executive Officer and Chief Financial Officer. All other of our executive officers provided services
to us as independent consultants. We have in the past, and may in the future, hire additional employees and engage consultants and advisors,
if and to the extent our management team determines that such actions would be helpful to implement our business plans and strategy. None
of our employees is represented by a labor union or covered under a collective bargaining agreement. We consider our relationship with
our employees and consultants to be good.
Our human capital resources
objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new employees, consultants
and advisors. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting
of stock-based and cash-based compensation awards, in order to increase stockholder value and the success of our company by motivating
such individuals to perform to the best of their abilities and achieve our objectives.
We recognize that our continued
ability to attract, retain and motivate exceptional employees is vital to ensuring our long-term competitive advantage. Our employees
are critical to our long-term success and are essential to helping us meet our goals. Among other things, we support and incentivize our
employees in the following ways:
| ● | Talent development, compensation, and retention. We
strive to provide our employees with a rewarding work environment, including the opportunity for success and a platform for personal
and professional development. We provide a competitive benefits package designed to attract and retain a skilled and diverse workforce.
We also offer employees a 401(k) plan. |
| ● | Health and safety. Employee health and safety
in the workplace is one of our core values. One of the ways in which we support the health and safety of our employees includes a generous
health insurance program. |
| ● | Inclusion and diversity. We are committed to
efforts to increase diversity and foster an inclusive work environment that supports our workforce. |
Our top priority during the
ongoing COVID-19 pandemic remains protecting the health and well-being of our employees, consultants, customers, partners and communities.
Since the onset of the COVID-19 pandemic, we have maintained a work-from-home policy for all our employees and consultants.
ITEM 1A. RISK FACTORS
Investing in our securities
involves a great deal of risk. Careful consideration should be made of the following factors as well as other information included in
this Annual Report before deciding to purchase our securities. There are many risks that affect our business and results of operations,
some of which are beyond our control. Our business, financial condition or operating results could be materially harmed by any of these
risks. This could cause the trading price of our securities to decline, and you may lose all or part of your investment. Additional risks
that we do not yet know of or that we currently think are immaterial may also affect our business and results of operations.
Risks Related to Our Financial Position, Limited
Operating History and Capital Requirements
We have incurred operating losses since
inception and anticipate that we may continue to incur operating losses. We may not achieve or maintain profitability in the foreseeable
future.
We have experienced operating
losses and cash outflows from operations since incorporation and will require ongoing financing to continue our research and development
and production activities. Our success is dependent upon our ability to finance our cash requirements to continue our activities. There
may be a risk of default on these liabilities and other liabilities of our business if we cannot raise additional funds through the issuance
of additional equity securities, through loan financing, or other means. Our comprehensive loss for the fiscal year ended June 30, 2023
was $8.7 million. As of June 30, 2023, we had an accumulated deficit of $44.4 million. We may incur operating losses for the next several
years, and we may not achieve or sustain profitability in the foreseeable future.
We anticipate that our expenses
will increase if, and as, we:
| |
● |
complete the build-out of our 25,000 square foot research and manufacturing facility; |
| |
|
|
| |
● |
engage in activities related to regulatory compliance in Canada, the United States and any other jurisdiction in which we may operate, which activities are likely to increase as we experience heightened regulatory scrutiny; |
| |
|
|
| |
● |
expand our infrastructure and facilities to accommodate our growing employee base, including adding equipment and physical infrastructure to support our research and development; |
| |
|
|
| |
● |
market and sell our products to academic researchers, biopharmaceutical companies and other eligible partners; |
| |
|
|
| |
● |
seek to identify and develop or in-license additional products or technologies; |
| |
|
|
| |
● |
maintain, expand and protect our intellectual property portfolio; and |
| |
|
|
| |
● |
add operational, financial and management information systems personnel to support our operations as a public company. |
To become and remain profitable,
we must succeed in successfully cultivating, synthesizing, extracting and purifying our products and eventually commercializing our products
in order to generate significant revenue. This will require us to be successful in a range of challenging activities, including manufacturing
our products at commercial scale, obtaining and maintaining compliance with all required regulatory permitting, and establishing brand
recognition in the industry. Our ability to become profitable will be dependent upon, in part and among other things, the size of the
market for our products, the number of competitors in such markets, the degree of market acceptance we achieve and the ability of our
clients to develop, obtain regulatory approval for and successfully commercialize psychedelics-based therapies.
Even if we do achieve profitability,
we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable may
decrease the value of our company and may impair our ability to raise capital, maintain our manufacturing operations, proceed with our
planned research and development efforts or expand our business. A decline in the value of our company may cause you to lose all or part
of your investment.
Our limited operating history may make it
difficult to evaluate our business to date and assess our future viability.
We have a limited history
of operations and will be in an early stage of development as we attempt to create an infrastructure to capitalize on the opportunity
for value creation in the psychedelics industry. Since our inception, we have focused our efforts on constructing our 25,000 square foot
manufacturing facility, developing our cultivation, extraction and purification processes, and building our executive management team.
We have not yet manufactured psychedelics-based products at commercial scale. The early stage of our cultivation, research and development
efforts makes it particularly uncertain whether any of our efforts will prove to be successful and meet the requirements of our customers,
and whether any of our products will be capable of being manufactured at a reasonable cost or be successfully marketed. We have no meaningful
operations upon which to evaluate our business and predictions about our future success or viability may not be as accurate as they could
be if we had a longer operating history or a history of successfully developing and commercializing active pharmaceutical ingredients
based on psychedelics. Accordingly, we are subject to many of the risks common to early-stage enterprises, including under-capitalization,
cash shortages, limitations with respect to personnel, financial and other resources and lack of revenue. The limited operating history
may also make it difficult for investors to evaluate our prospects for success. There is no assurance that we will be successful, and
our likelihood of success must be considered in light of our early stage of operations.
We may not be able to achieve
or maintain profitability and may incur losses in the future. In addition, we are expected to increase our capital investments as we implement
initiatives to grow our business. If our revenues do not increase to offset these expected increases, we may not generate positive cash
flow. There is no assurance that future revenues will be sufficient to generate the funds required to continue operations without external
funding. We may encounter unforeseen expenses, difficulties, complications, delays and other known or unknown factors in achieving our
business objectives, including with respect to our technology and products. We will eventually need to transition from a company with
a development focus to a company capable of supporting commercial activities. We may not be successful in such a transition. Our limited
operating history makes it more difficult for us to assess and plan for such unforeseen events.
We expect our financial condition
and operating results to continue to fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many
of which are beyond our control. Accordingly, you should not rely upon the results of any quarterly or annual periods as indications of
future operating performance.
We may require substantial additional funding
to finance our operations, and a failure to obtain this necessary funding when needed on acceptable terms, or at all, could force us to
delay, limit, reduce or terminate our manufacturing and commercialization efforts or other operations.
As of June 30, 2023, we had
cash and cash equivalents of approximately $1.7 million. We may need to raise additional capital, which cannot be assured. Moreover, our
operating plans may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned.
In addition, we may seek additional capital due to favourable market conditions or strategic considerations even if we believe we have
sufficient funds for our current or future operating plans.
Our future capital requirements
depend on many factors, including, but not limited to:
| ● | the scope, progress, results and
costs of researching and developing our products; |
| ● | the cost of manufacturing our products,
including costs associated with completing the build-out of our 25,000 square foot research and manufacturing facility; |
| ● | the effect of developments with
respect to the regulatory and competitive landscapes for psychedelics- and other psychotropics-based products and medicines; |
| ● | the number and scope of products
or technologies we decide to pursue; |
| ● | the cost of commercialization activities,
including marketing, sales and distribution costs; |
| ● | our ability to achieve revenue
growth; |
| ● | our ability to establish and maintain
strategic collaborations, licensing or other arrangements and the financial terms of any such agreements that we may enter into; |
| ● | whether we determine to acquire
or invest in complementary businesses or assets; |
| ● | the expenses needed to attract
and retain skilled personnel; |
| ● | our need to implement additional
internal systems and infrastructure, including financial and reporting systems associated with becoming a public company in the United
States; |
| ● | the costs involved in preparing,
filing, prosecuting, maintaining, defending and enforcing our intellectual property portfolio; and |
| ● | the continued impact of the COVID-19
pandemic on global social, political and economic conditions. |
Until we can generate sufficient
revenue to finance our cash requirements, which we may never do, we expect to finance our future cash needs through a combination of equity
offerings, debt offerings or financings, collaborations, strategic alliances or marketing, distribution or licensing arrangements with
third parties. The various ways we could raise additional capital carry potential risks. To the extent that we raise additional capital
by issuing equity securities, our existing stockholders may experience substantial dilution. Any preferred equity securities issued also
would likely provide for rights, preferences or privileges senior to those of holders of our common shares. If we raise funds by issuing
debt securities, those debt securities would have rights, preferences and privileges senior to those of holders of our common shares.
Debt financing and preferred equity financing, if available, may also involve agreements that include covenants restricting our ability
to take specific actions, such as incurring additional debt, selling or licensing our assets, making product acquisitions, making capital
expenditures, or declaring dividends. If we raise additional funds through collaborations, strategic alliances or marketing, distribution
or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research
programs or product candidates, or grant licenses on terms that may not be favourable to us.
Our ability to raise additional
funds will depend on financial, economic and market conditions and other factors, over which we may have no or limited control. Adequate
additional funds may not be available when we need them, on terms that are acceptable to us, or at all. In addition, heightened regulatory
scrutiny could have a negative impact on our ability to raise capital. If adequate funds are not available to us on a timely basis or
on attractive terms, we may be required to reduce our workforce, delay, limit, reduce or terminate our research and development activities
and commercialization efforts, or grant rights to develop and market products or technologies that we would otherwise develop and market
ourselves. In addition, attempting to secure additional financing may divert the time and attention of our management from daily activities
and distract from our research and development efforts.
Our commercial success depends on our technical
abilities to cultivate, extract or synthetically derive high quality psychotropic products, as well as on the acceptance of these products
by clients in our targeted markets.
We utilize advanced plant
and fungi cultivation technology along with various biotechnology and direct chemical synthesis, isolation, and purification systems to
produce high-quality, medical-grade psychotropic compounds to sale to appropriately licenced research institutions, biopharmaceutical
companies and other clients. Our clients, in turn, utilize our products for further research, development and potential commercialization
as therapies for a range of conditions. As a result, the quality and sophistication of our manufacturing processes and extraction and
purification techniques is critical to our ability to grow revenue, expand our operations and become profitable. In particular, our business
depends, among other things, on:
| ● | our ability to manufacture products
at commercial scale and on the desired timeframes that are set out by our clients; |
| ● | our ability to execute on our strategy
to enter into new arrangements with targeted clients and establish a robust sales pipeline for our products; |
| ● | our ability to increase awareness
in the market of our manufacturing capabilities and the benefits of our products; |
| ● | the rate of adoption of our products
by academic institutions, biopharmaceutical companies and others; |
| ● | if competitors develop a manufacturing
capacity or techniques that enable commercialization at a higher rate than us; |
| ● | the timing and scope of approvals
by Health Canada or the U.S. Food and Drug Administration, or FDA, or any other regulatory body for drugs that are developed by our clients
using products supplied by us; |
| ● | negative publicity regarding the
psychedelics industry or psychedelics-based medicines; and |
| ● | our ability to further validate
our manufacturing capabilities and technology through research and accompanying publications. |
There can be no assurance
that we will successfully address any of these or other factors that may affect the market acceptance of our products and techniques.
If we are unsuccessful in achieving and maintaining market acceptance of our platform, our business, financial condition, results of operations
and prospects could be adversely affected.
We have issued promissory notes or other
debt securities, and otherwise incurred substantial debt, which may adversely affect our financial condition and thus negatively impact
the value of our shareholders’ investment in us.
As of June 30, 2023, we have
entered into a credit facility pursuant to which we can borrow up to $5.0 million. As of June 30, 2023, no amounts had been borrowed under
the credit facility.
Our outstanding indebtedness
and any future indebtedness we may incur will result in increased fixed payment obligations. It could also result in certain restrictive
covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual
property rights and other operating restrictions that could adversely impact our ability to conduct our business and may result in liens
being placed on our assets and intellectual property. If we were to default on such indebtedness, we could lose such assets and intellectual
property. The incurrence of debt could have a variety of other negative effects, including:
| ● | default and foreclosure on our
assets if our operating revenues are insufficient to repay our debt obligations; |
| ● | acceleration of our obligations
to repay the indebtedness even if we make all principal and interest payments when due if we breach certain covenants that require the
maintenance of certain financial ratios or reserves without a waiver or renegotiation of that covenant; |
| ● | our immediate payment of all principal
and accrued interest, if any, if the debt security is payable on demand; |
| ● | our inability to obtain necessary
additional financing if the debt security contains covenants restricting our ability to obtain such financing while the debt security
is outstanding; |
| ● | our inability to pay dividends
on our common shares; |
| ● | using a substantial portion of
our cash flow to pay principal and interest on our debt, which will reduce the funds available for dividends on our ordinary shares if
declared, expenses, capital expenditures, acquisitions and other general corporate purposes; |
| ● | limitations on our flexibility
in planning for and reacting to changes in our business and in the industry in which we operate; |
| ● | increased vulnerability to adverse
changes in general economic, industry and competitive conditions and adverse changes in government regulation; and |
| ● | limitations on our ability to borrow
additional amounts for expenses, capital expenditures, acquisitions, debt service requirements, execution of our strategy and other purposes
and other disadvantages compared to our competitors who have less debt. |
In order to satisfy our current
and future debt service obligations, we will be required to raise funds from external sources. We may be unable to arrange for additional
financing to pay the amounts due under our existing debt. Funds from external sources may not be available on acceptable terms, if at
all. Our failure to satisfy our current and future debt obligations could adversely affect our business, financial condition and results
of operations.
Risks Related to our Business and the Psychedelics-Based
Medicines Industry
The psychedelics industry and market are
relatively new and the industry may not succeed in the long term.
We operate our business in
a relatively new industry and market. We believe that both regulators and the public have an increasing awareness and acceptance of this
field. Nevertheless, psychedelics remain a controlled substance in Canada, the United States and most other jurisdictions and their use
for research and therapeutic purposes remains highly regulated and narrow in scope. There is no assurance that the industry and market
will continue to grow as currently estimated or anticipated or function and evolve in the manner consistent with management’s expectations
and assumptions. Any event or circumstance that adversely affects the psychedelic manufacturing and medicines industry and market could
have a material adverse effect on our business, financial condition and results of operations. We have committed and expect to continue
committing significant resources and capital to develop our psychedelics manufacturing facilities, refine our product offerings and establish
our contract research services program. As a category of products and services, medical-grade psychedelics, raw materials and psychedelics-derived
active pharmaceutical ingredients, or API, and research into such substances represent relatively untested offerings in the marketplace,
and we cannot provide assurance that psychedelics as a category, or that our products and services in particular, will achieve market
acceptance. Moreover, as a relatively new industry, there are not many established players in the psychedelic-based medicines industry
whose business model we can emulate. Similarly, there is little information about comparable companies available for potential investors
to review in making a decision about whether to invest in our common shares.
Our business plan depends on the occurrence
of regulatory changes that may benefit the psychotropics-based medicines market and on determinations by U.S. and Canadian regulators
that are favorable to our company, and there can be no assurance that such changes or determinations will occur.
The strict regulatory environment
that governs our business activity has potential to severely limit our market opportunities both in Canada and the United States. Because
the APIs and other products we plan to produce are restricted drugs on the Schedule to Part J of the Canadian Food and Drug Regulations,
their sale in Canada will be authorized only for the purposes of clinical testing in an “institution” for the purpose of determining
the hazards and efficacy of the drug, and for laboratory research in the institution by qualified investigators. Sale of our APIs in Canada
for commercial purposes will be prohibited unless and until the substances we produce are removed from Part J of the Food and Drug Regulations.
This regulatory change may never happen, or it may not happen in time for our business to benefit from the change. Under the Food and
Drug Regulations, “institution” is defined as any institution engaged in research on drugs and includes a hospital, a university
in Canada or a department or agency of the Canadian government. While we believe that Health Canada is likely to interpret this definition
broadly to allow sales to private biopharmaceutical companies conducting research in this space, there remains a risk that Health Canada
may take a more restrictive view of which facilities qualify as “institutions” under the law. A restrictive interpretation
would limit our potential customers in Canada, even for clinical testing and laboratory research purposes. In the United States, where
most of the substances we intend to produce are currently listed on Schedule I of the Controlled Substances Act, the DEA will only approve
an import permit for our potential U.S. clients if U.S. domestic supply of the substance is found to be inadequate for scientific studies,
or if competition among domestic manufacturers of the substance is inadequate for medical or scientific needs and will not be rendered
adequate by the registration of additional U.S. domestic manufacturers. If U.S. manufacturers begin to produce the same APIs we produce,
and the DEA determines that U.S. domestic supply or competition is adequate, we may not be able to export to U.S. customers at all. Our
ability to sell our products on a commercial scale in the United States also depends on the substances being rescheduled to a schedule
that permits their use for commercial manufacture, as Schedule I substances can only be used for research purposes. Even if the substances
we produce are rescheduled to Schedule II, however, their use will still entail significant restrictions that may severely limit our market
potential in the United States. In order to sell our products in the United States, it is possible that we will have to establish a U.S.
manufacturing facility, which would be costly and time-consuming. All of the above are unknown variables and contingencies that affect
our ability to commercialize our products in Canada and the United States.
Unfavourable publicity or consumer perception
of psychedelic-based medicine may have an adverse impact on our client base, which in turn would have an adverse impact on our business,
financial condition and results of operations. Overcoming unfavourable publicity or consumer perception may entail extensive marketing
efforts.
Our ability to establish
and grow our business is substantially dependent on the success of the emerging market for psychedelics-based medicines, which will depend
upon, among other matters, pronounced and rapidly changing public preferences, factors which are difficult to predict and over which we
have little, if any, control. We and our clients will be highly dependent upon consumer perception of psychedelic-based therapies and
other products.
Therapies containing controlled
substances may generate public controversy. The public may associate such therapies and other products with illegal recreational drugs,
which are prohibited or controlled substances that could be associated with risks to health, safety and are potentially addictive. Political
and social pressures and adverse publicity could lead to delays in approval of, and increased expenses for, the therapeutic candidates
our clients may develop. Opponents of these therapies may seek restrictions on marketing and withdrawal of any regulatory approvals. In
addition, these opponents may seek to generate negative publicity in an effort to persuade the medical community to reject these therapies.
Anti-psychedelic protests have historically occurred and may occur in the future and generate media coverage. Political pressures and
adverse publicity could lead to delays in, and increased expenses for, and limit or restrict the introduction and marketing of, psychedelics-based
therapeutic candidates.
It will likely require significant
scientific evidence (including and possibly beyond that which our clients will have to produce in order to achieve regulatory approval)
to change public perception and consumers’ view that psychedelic-based therapies and other products are not harmful to physical
or social health or are not addictive. Even if our products conform to international safety and quality standards, sales could be adversely
affected if the public loses confidence in the safety, efficacy, and quality of psychedelics-based products, due to adverse events reported
in clinical trials or otherwise. Negative public perceptions could cause the market for such products to shrink and may compel regulators
to impose stringent requirements on the development of any such products. If such events were to occur, fewer academic institutions and
biopharmaceutical companies may seek to conduct research, develop and commercialize such products.
The psychedelics market will
face specific marketing challenges given the products’ status as a controlled substance, which resulted in past and current public
perception that the products have negative health and lifestyle effects and have the potential to cause physical and social harm due to
psychoactive and potentially addictive effects. Any marketing efforts we or our clients may undertake would need to overcome this perception
to build consumer confidence, brand recognition and goodwill. Consumer perception can be significantly influenced by scientific research
or findings regarding the consumption of psychedelic inspired products. There can be no assurance that such research or findings will
be favorable towards psychedelics-based products, or even if favorable, that such research or findings will be effective in convincing
a sufficient portion of the population that psychedelics-based therapies are safe and effective. Conversely, adverse publicity about psychedelics-based
therapies that we or our clients sell may discourage consumers from buying the therapies and other products that our clients may develop.
The expansion of the use of psychedelics
and other psychotropics in the medical industry may require new clinical research into effective medical therapies.
Research regarding the potential
medical benefits, viability, safety, efficacy, addictiveness, dosing and social acceptance of psychedelic and other psychotropic products
remains in early stages. There have been relatively few clinical trials on the benefits of such products. Although we believe that the
currently available studies support our beliefs regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance
of psychedelic and other psychotropic products, future research and clinical trials may prove such statements to be incorrect, or could
raise concerns regarding, and perceptions relating to, psychedelics-based raw material precursors and APIs. Given these risks, uncertainties
and assumptions, potential investors should understand that the breadth of application of psychedelics-based medicines may not be as expansive
as the existing research suggests. Future research studies and clinical trials may draw opposing conclusions to those stated in this Annual
Report or reach negative conclusions regarding the potential medical benefits, viability, safety, efficacy, dosing, social acceptance
or other facts and perceptions related to psychedelic and other psychotropic products, which could have a material adverse effect on the
demand for our products with the potential to lead to a material adverse effect on our business, financial condition and results of operations.
The sizes of the markets and forecasts of
market growth for the demand of our products and services and for psychedelics-based medicines generally are based on a number of complex
assumptions and estimates, and may be inaccurate.
We estimate annual total
addressable markets and forecasts of market growth for our products and services and for the psychedelics-based therapies that our clients
may develop. These estimates and forecasts are based on a number of complex assumptions, internal and third party estimates and other
business data, including assumptions and estimates relating to our ability to establish our business as a critical supplier of manufacturing
of medical-grade raw materials, API and finished drug products and pre-clinical research services within the psychedelics-based medicines
space; regulatory developments surrounding the use of psychedelics for research and therapeutic purposes; and the public’s acceptance
of such therapies, if approved; and our clients’ ability to develop, obtain regulatory approval for and successfully commercialize
their product candidates. While we believe our assumptions and the data underlying our estimates and key performance indicators are reasonable,
there are inherent challenges in measuring or forecasting such information. As a result, these assumptions and estimates may not be correct
and the conditions supporting our assumptions or estimates may change at any time, thereby reducing the predictive accuracy of these underlying
factors and indicators. As a result, our estimates of the annual total addressable market and our forecasts of market growth and future
revenue from technology access fees, discovery research fees, milestone payments or royalties may prove to be incorrect, and our key business
metrics may not reflect our actual performance. For example, if the annual total addressable market or the potential market growth for
our psychedelics-based products is smaller than we have estimated or if regulatory developments are adverse to this category of therapies
generally, it may impair our sales growth and have an adverse impact on our business, financial condition, results of operations and prospects.
Demand in the market for naturally derived
psychedelics products may not materialize.
Initially, we intend to cultivate,
extract and purify our psychedelics products, and our psilocybin API in particular, from naturally derived sources. We believe that this
approach, which facilitates the potential “entourage effect” provided by the synergistic interaction of the various compounds
within hallucinogenic plants, represents an advantage over the existing market for the manufacture of psychedelics-derived materials,
which relies predominantly on the synthetic manufacture of these materials and refinement into isolated single molecules (e.g., psilocybin).
However, we cannot provide assurances that the market for naturally derived psychedelics products will develop or that it will be as large
as we anticipate. There are multiple risks involved with this market strategy, including: our competitor’s synthetic manufacturing
processes may prove more cost-effective and efficient or may produce more consistent yields; key market participants we might otherwise
target as clients may already be more familiar and comfortable working with synthetically manufactured psychedelics products; regulatory
developments may favour synthetically derived psychedelics products; and psychedelics-based medicines developed with naturally derived
API or other materials may not provide the therapeutic benefits we anticipate. In the event that naturally derived psychedelics products
do not achieve the traction in the research and development market that we anticipate, such developments may have an adverse impact on
our business, financial condition and results of operations.
We believe that Canadian “safer supply
programs” and Special Access Program will expand the market for our products within Canada. Such programs, however, may not be used
for psychedelics products, may not provide the benefits we anticipate and may be terminated altogether.
The government of Canada
has established two programs which we believe may expand the market for our psychedelics-based products and those of our clients. The
Canadian government has created “safer supply programs,” or SSPs, pursuant to which a regulated supply of certain drugs will
be made available in order to combat the illegal drug supply and attendant risks of overdose and death. Additionally, Health Canada’s
Special Access Program for drugs, or SAP, enables drugs that are not marketed in Canada to be requested by practitioners for the treatment,
diagnosis, or prevention of serious or life-threatening conditions when conventional therapies have failed, are unsuitable, or unavailable.
Special access by Canadian health practitioners to unauthorized drugs is for serious or life-threatening conditions where conventional
therapies have failed, are unsuitable, or are unavailable either as marketed products, or through enrolment in clinical trials. We believe
that we or our clients may be able to utilize these programs to provide psychedelics-based therapies to consumers who might otherwise
face the risk of harm from the illegal drug supply or who would otherwise be unable to access potentially life-saving non-approved psychedelics-based
therapies.
However, there can be no
assurance that these programs continue or that they will provide the benefits that we anticipate. The SSPs are limited in scope and to
date have focused on providing a safer supply of opioids and other drugs that present a severe risk of overdose and death. To our knowledge,
the SSPs have not been used to prescribe medicinal psychedelics to consumers and may never be used for this purpose. With respect to the
SAP, the regulatory authority supporting the program is discretionary. In addition, access to restricted drugs, such as psychedelics,
through the SAP is prohibited. However, in December 2020, Health Canada, the body that administers the SAP, published its intention to
reverse the regulatory prohibition that prevents special access for restricted drugs. If and when that prohibition is removed the authorities
may still choose not to authorize psychedelics-based medicines through the program. A decision to authorize or deny a request is made
on a case-by-case basis by taking into consideration the nature of the applicable medical emergency, the availability of marketed alternatives
and the information provided in support of the request regarding the use, safety and efficacy of the drug. The SAP is not intended to
be a mechanism for circumventing drug clinical development or the regulatory review of a submission for marketing. Access to any drug
through the SAP is intended to be limited in duration and quantity to meet emergency needs only. In the event that a drug submission is
under regulatory review, access will be limited until that review is complete and the drug is marketed. Accordingly, psychedelics-based
medicines may not be authorized under the SAP, and even if they are, their availability under the program may be very limited, both in
terms of the breadth and duration of access. Moreover, our clients will be under no obligation to sell an unauthorized drug through the
SAP and Health Canada cannot compel a manufacturer to do so.
Additionally, the use of
the programs described above entails risks. Drugs accessed through the SAP do not undergo the scrutiny of a benefit-risk assessment that
is part of the regulatory framework for a new drug submission or a clinical trial application. These drugs are exempt from the Canadian
Food and Drugs Act and its regulations. The decisions to authorize a drug through the SAP are based on a practitioner’s rationale
about the use of the drug for the medical emergency and how it would benefit their patient based on the patient’s clinical history.
Accordingly, an authorization through the SAP does not constitute an opinion that a drug is safe, efficacious or of high quality. To the
extent that our clients have not completed the clinical development progress and they make drugs using our raw materials or API available
through the SAP, we may directly or indirectly face a greater than average risk of product liability exposure.
To the extent that the SSPs
or SAP do not provide the benefits to our business that we expect, such outcome may have a material adverse effect on our business, financial
condition and results of operations.
The manufacture of our psychotropics-based
products is complex. We may encounter various difficulties in production, which could delay or entirely halt our ability to supply raw
materials or API for research or clinical trials or finished drug products for commercial sale.
The process of manufacturing
API based on psychotropics materials is complex, highly regulated, and subject to multiple risks. As an organization, we have no experience
in cultivating and refining psychedelics-based products, we have not yet manufactured any such products and we may be unsuccessful in
our efforts to do so. We can make no assurances that our efforts will result in commercially viable products. Our manufacturing operations
will be susceptible to product loss due to contamination, equipment failure, improper installation or operation of equipment, vendor or
operator error, inconsistency in yields, variability in product characteristics and difficulties in scaling the production process. Even
minor deviations from normal manufacturing processes could result in reduced production yields, product defects, other supply disruptions
and higher costs. For example, if microbial, viral or other contaminations are discovered in our products or in the manufacturing facilities
in which our products are cultivated, extracted and purified, our manufacturing facilities may need to be closed for an extended period
of time to investigate and remedy the contamination.
In the event that one of
our clients begins preparation for later-stage clinical trials and potential commercialization, we will need to take steps to increase
the scale of production of our products. We have not yet scaled up the manufacturing process for any of our products. There are risks
associated with process development and large-scale manufacturing for clinical trials or commercial scale including, among others, cost
overruns, potential problems with process scale-up, process reproducibility, stability issues, compliance with current Good Manufacturing
Practices, or cGMP, requirements, lot consistency and timely availability of raw materials. The manufacturing of commercial quality drug
product has long lead times, is very expensive and requires significant efforts including, but not limited to, scale-up of production
to anticipated commercial scale, process characterization and validation, analytical method validation, identification of critical process
parameters and product quality attributes, and multiple process performance and validation runs. We may be unable to successfully increase
the manufacturing capacity for any of our products in a timely or cost-effective manner, or at all. In addition, quality issues may arise
during scale-up or commercial activities, including, for example, contaminations and crop failure.
Any performance failure on
our part could delay our client’s clinical development or receipt of marketing approval. If we cannot perform as agreed with our
clients, our clients may be compelled to terminate our relationship. The loss of client relationships or harm to our reputation from such
performance failures would have an adverse impact on our business, financial condition and results of operations.
We face multiple risks in establishing and
growing our contract research services offerings and we may not be successful in achieving profitability with respect to this aspect of
our business.
We intend to offer contract-based
drug discovery and research services to academic institutions and biopharmaceutical companies in the psychedelics space. We believe that
our management team and employees have the background and expertise necessary to engage in innovative research and collaborations with
key players to bring new psychedelics-based solutions into development and use. However, as an organization, we have no experience in
conducting research and development activities with respect to psychedelics-based products and we may be unsuccessful in our efforts to
do so. We face multiple risks in our efforts to establish and grow this aspect of our business. The economic factors and industry trends
that affect biopharmaceutical companies will also affect our contract research services business. Biopharmaceutical companies continue
to seek long-term strategic collaborations with global clinical research organizations with favorable pricing terms. Competition for these
collaborations is intense and we may decide to forego an opportunity or we may not be selected, in which case a competitor may enter into
the collaboration and our business with the client, if any, may be limited. In addition, if the biopharmaceutical industry reduces its
contract research services activities or reduces its outsourcing of research and development projects or such outsourcing fails to grow
at projected rates, our operations and financial condition could be materially and adversely affected. We may also be negatively impacted
by consolidation and other factors in the biopharmaceutical industry, which may slow decision making by our clients or result in the delay
or cancellation of research and development activities. Our commercial services may be affected by reductions in new drug launches and
increases in the number of drugs losing patent protection. All of these events could adversely affect our business, financial condition
or results of operations.
We expect that most of the
contracts we enter into with clients for our research services will be terminable by our clients upon a specified number of days’
notice. Our clients may delay, terminate or reduce the scope of our contracts for a variety of reasons beyond our control, including but
not limited to: lack of available financing, budgetary limits or changing priorities; actions by regulatory authorities; unexpected or
undesired clinical results for products; shift of business to a competitor or internal resources; and product withdrawal following market
launch. We also expect that most of our contacts will be either fee for service contracts or fixed-fee contracts. Our future financial
results may be adversely impacted if we initially under-price our contracts or otherwise overrun our cost estimates and are unable to
successfully negotiate a change order. Change orders typically occur when the scope of work we perform needs to be modified from that
originally contemplated by our contract with the client. Modifications can occur, for example, when there is a change in a key assumption
or parameter related to the research project or a significant change in timing. Where we are not successful in converting out-of-scope
work into change orders under our current contracts, we bear the cost of the additional work. Such under-pricing, significant cost overruns
or delay in documentation of change orders could have a material adverse effect on our business, financial condition and results of operations.
Biopharmaceutical drug development is inherently
uncertain. Even if we are able to sell our products and services to clients for research and development purposes, it is possible that
our clients will not be successful in developing and obtaining regulatory approval for psychedelics-based medicines. If they are unable
to do so, the market for our products and services will be limited.
We intend to cultivate, extract
and purify medical-grade psilocybin and other psychedelics-based products and to offer them to appropriately licensed research institutions,
biopharmaceutical companies and other parties who are engaged in discovery and development with respect to psychedelics-based medicines.
These clients may include universities, large cap pharmaceutical companies, biotechnology companies of all sizes and non-profit and government
organizations, and they may purchase our products in order to develop, obtain regulatory approval for and commercialize therapies for
a range of conditions, including but not limited to major depressive disorder, post-traumatic stress disorder, substance addiction, and
other conditions. While we believe that we will be able to obtain significant revenues from the sale of our products for research and
development purposes, we estimate that the vast majority of the economic value of the relationships we aim to establish with these potential
clients is in the downstream revenues that may result if they are successful in obtaining regulatory approval for and commercializing
psychedelics-based medicines. As a result, our future growth is dependent on the ability of our potential clients to successfully develop
and commercialize these therapies. Due to our reliance on the success of our client’s development and commercialization efforts,
the risks relating to product development, regulatory clearance, authorization or approval and commercialization apply to us derivatively
through the activities of our clients. We are making significant investments in our manufacturing capabilities and developing our extraction
and purification techniques because we believe in the vast potential of psychedelics-based medicines to treat a range of conditions. However,
there can be no assurance that our clients will successfully develop, secure marketing approvals for and commercialize any drug candidates
based on psychedelics. As a result, we may not realize the intended benefits of our investments in our business and may not be able to
sell sufficient quantities of our products to achieve and maintain profitability. To date, we have not yet sold any products and only
a limited number of psychedelics-based medicines have been approved by Health Canada and the FDA.
Due to the uncertain, time-consuming
and costly clinical development and regulatory approval process, our clients may not successfully develop any drug candidates with the
psychedelics-based materials or API that we provide, or our clients may choose to discontinue the development of these drug candidates
for a variety of reasons. Our clients’ ability to successfully develop psychedelics-based medicines will depend on many factors,
including:
| ● | their ability to raise required
capital on acceptable terms, or at all; |
| ● | timely completion of their preclinical
studies and clinical trials, which may be significantly slower or cost more than they anticipate; |
| ● | their ability to enroll subject
to their clinical studies, particularly given the untested nature of the product space, or their ability to retain subjects who have
enrolled in a clinical study; |
| ● | delays in developing and testing,
or inability to develop and test, any clinical outcome assessments to the extent necessary for the FDA and equivalent foreign regulatory
authorities to agree to their use as endpoints utilized in a clinical trial to support labelling claims; |
| ● | the prevalence, duration and severity
of potential side effects or other safety issues experienced with their psychedelics-based product candidates, if any, or experienced
by competitors who are developing psychedelics-based medicines or who are targeting the same indications in the mental health, addiction
or central nervous system disease spaces; |
| ● | determinations by regulators regarding
the potential for abuse of psychedelics-based medicines or products they contain; |
| ● | clinical trials of their product
candidates may produce negative or inconclusive results, and they may decide, or regulators may require them, to conduct additional clinical
trials or abandon drug development programs; |
| ● | our clients’ ability to demonstrate
to the satisfaction of Health Canada, the FDA or an equivalent regulatory authority that their psychedelics-based product candidates
are safe and effective for the requested indications; |
| ● | the timely receipt of necessary
marketing approvals from the FDA and equivalent foreign regulatory authorities; |
| ● | their ability to successfully develop
an effective commercial strategy in the psychedelics-based medicines and thereafter commercialize our product candidates in the United
States and internationally, if approved for marketing, reimbursement, sale and distribution in such countries and territories; |
| ● | acceptance by physicians, payors
and patients of the benefits, safety and efficacy of their psychedelics-based product candidates, if approved; |
| ● | obtainment and maintenance of coverage,
adequate pricing and adequate reimbursement from third-party payors, including government payors; |
| ● | their ability to establish and
enforce intellectual property rights in and to their product candidates; |
| ● | any adverse impacts to the U.S.
and global market for pharmaceutical products as a result of the COVID-19 pandemic; and |
| ● | business interruptions resulting
from geo-political actions, including war and terrorism, natural disasters including earthquakes, typhoons, floods and fires, pandemics,
or failures or significant downtime of our information technology systems resulting from cyber-attacks on such systems or otherwise. |
The risk of failure for our
clients’ psychedelics-based product candidates is high. The risk of failure is substantial with respect to any biopharmaceutical
development efforts, but risk may be exacerbated by the novel area in which we and our clients will work. Clinical development failure
can occur at any stage of testing, and there are any number of events that could delay or prevent our clients’ ability to receive
regulatory approval for their product candidates utilizing our psychedelics-based raw materials, APIs or finished drug products. If our
clients’ products entail serious side effects, they could limit the dosing of such products, limit their frequency of use, limit
the targeted patient population or abandon the development of such products altogether. Regulatory authorities could also require additional
warnings in the product labelling. We and our clients could be sued and held liable for harm caused to clinical trial subjects or patients.
Even if our clients eventually
complete clinical testing and receive approval from Health Canada, the FDA or other equivalent agencies for psychedelics-based medicines
that utilize our products, the applicable regulatory agency may grant approval or other marketing authorization contingent on the performance
of costly additional clinical trials, including post-market clinical trials. The applicable regulatory authority may also approve the
psychedelics-based product for a more limited indication or a narrower patient population than our client originally requested. Any such
determinations by the applicable regulatory authority would delay or limit our ability to sell commercial-scale quantities of our products.
Additionally, even if approved, clients will be subject to post-approval regulations, and any failure to remain in compliance with these
regulations may impair their ability to commercialize the applicable product candidate, which will in turn materially diminish the market
for our medical-grade psychedelics materials and APIs.
We and our clients are also
subject to industry-wide regulatory risk. The number of new drug applications, or NDAs, and biologics licence applications, or BLAs, approved
by Health Canada, the FDA and other equivalent agencies varies significantly over time and if there were to be an extended reduction in
the number of NDAs and BLAs approved, the industry would contract and our business would be materially harmed. These regulatory agencies
could also take an adverse position to the use of psychedelics-based therapies as a category, in which case our clients’ regulatory
pathway could narrow and our ability to commercialize our psychedelics-based raw materials, APIs and finished drug products could decline.
Our client’s failure
to effectively advance, market and sell suitable drug candidates with the psychedelics-based raw materials, APIs and finished drug products
we provide could have a material adverse effect on our business, financial condition, results of operations and prospects, and cause the
market price of our common shares to decline.
We face substantial competition, which may
result in others commercializing psychedelics-based products and services before or more successfully than we do, thus rendering our products
and services non-competitive, obsolete or reducing the size of our market. Our customers will also face significant competition from other
developers of psychedelics-based medicines and from companies pursing alternative treatments for the same indications.
The psychedelics-based product
manufacturing and contract research business is an emerging industry with increasing levels of competition. We believe that due to the
urgent need for new and innovative treatments for mental health conditions and the evidence-based studies showing the impact of psychedelics
as a treatment for mental health conditions, there is significant potential that psychedelics as a treatment for these conditions will
become more accepted in the medical community. As such, we expect to compete with other similar businesses who will are or will begin
to supply medical-grade psychedelic raw materials, APIs and finished drug products and/or contract research services to clients such as
universities and biopharmaceutical companies to formulate a wide range of products. We expect to face intense competition from new or
existing market participants, some of which may have greater financial resources. Increased competition by larger and better financed
competitors could materially and adversely affect our business, financial condition and results of operations.
We are aware of a number
of companies actively pursuing the development and contract manufacturing of psychedelics-based products and the provision of contract
research services in the psychedelics space. For example, Numinus Wellness Inc. is a Canada-based health care company focused on creating
wellness solutions centered on psychedelic therapies. Numinus is licenced in Canada to test, possess, buy and sell methylenedioxymethamphetamine,
or MDMA, psilocybin, psilocin, dimethyltryptamine, or DMT, and mescaline. Additionally, HAVN Life Sciences Inc. is a Canadian biotechnology
company pursuing standardized extraction of psychoactive compounds, the development of natural health care products and mental-health
treatments. These companies have greater experience than we do in the psychedelics manufacturing and research services industries and
as organizations they are more advanced in establishing and growing their businesses than we are. There can be no assurance that our competitors
are not currently developing, or will not in the future develop, products that are equally or more economically attractive as our products.
The emergence and licensing of additional U.S.-domiciled manufacturers of psychedelics-based raw ingredients or APIs may decrease our
clients’ ability to obtain import permits to import our raw ingredients or APIs. The success of our competitors and their products
and technologies relative to our technological capabilities and competitiveness, and the increase in the U.S. domestic supply of psychedelics-based
raw materials or APIs, could have a material adverse effect on our business, financial condition and results of operations.
Many other companies are
developing or commercializing therapies to treat the same diseases or indications for which our products may be useful. As a result, our
clients will face significant competition in their efforts to develop, obtain regulatory approval for and commercialize psychedelics-based
therapies. This competition will take the form of other companies pursuing similar psychedelics-based therapies, as well as from other
biopharmaceutical companies pursuing therapies for the same indications using alternative, more established approaches. For example, we
believe that psychedelics-based medicines may be effective in treating major depressive disorders. There are a number of companies that
currently market and sell products or therapies, or are pursuing the development of products or therapies, for the treatment of depression,
including antidepressants such as selective serotonin reuptake inhibitors and serotonergic norepinephrine reuptake inhibitors, antipsychotics,
cognitive behavioral therapy, or CBT, repeat transcranial magnetic stimulation, or rTMS, electroconvulsive therapy, or ECT, vagus nerve
stimulation, or VNS, and deep brain stimulation, or DBS, among others. Many of these pharmaceutical, biopharmaceutical and biotechnology
competitors have established markets for their therapies and have substantially greater financial, technical, human and other resources
than our clients do and may be better equipped than our clients to develop, manufacture and market superior products or therapies. In
addition, many of these competitors have significantly greater experience than our clients may have in undertaking preclinical studies
and human clinical trials of new therapeutic substances and in obtaining regulatory approvals of human therapeutic products. Accordingly,
competitors to our clients may develop therapies that are more effective, more convenient, more widely used and less costly or have a
better safety profile than our clients’ therapies and these competitors may also be more successful than our clients are in marketing
their therapies.
The biotechnology and pharmaceutical
industries are intensely competitive and subject to rapid and significant technological change. We expect that our and our client’s
competitors will include large, well-established pharmaceutical companies, natural health products companies, biotechnology companies,
and academic and research institutions. Many of these competitors may have greater name recognition and more extensive collaborative relationships
than we or our clients have. Smaller and earlier-stage companies may also prove to be significant competitors to us and/or our clients,
particularly through collaborative arrangements with large, established companies. Our competitors also compete with us in recruiting
and retaining qualified scientific, management and commercial personnel. If we are unable to compete effectively in the contract manufacturing
and services space against other companies providing such psychedelics-based products and services, or if our clients are unable to complete
effectively against other companies pursuing psychedelics-based medicines or other approaches to the treatment of the same indications
as our clients, then such failures would be likely to have a material impact on our business, financial condition and results of operations.
We face competition from unlicensed, unregulated
participants.
Despite Canadian federal
and state-level legalization of psychedelics for research purposes and the potential distribution of psychedelics through programs such
as the SSPs and SAP, illicit or “black-market” operations remain abundant and may present substantial competition to us and
our clients. In particular, illicit operations, despite being largely clandestine, are not required to comply with the extensive regulations
that we and our clients must comply with to conduct business, and accordingly may have significantly lower costs of operation. As a result,
we and our clients face competition from black market sources of psychedelics and psychedelics-based products, which are unlicensed and
unregulated, and which may sell products that are deemed more desirable than ours or our clients’ by certain consumers, including
products with higher concentrations of active ingredients or using delivery methods that we and our clients are not permitted to use.
Any inability or unwillingness of law enforcement authorities to enforce existing laws prohibiting the unlicensed cultivation and sale
of psychedelics and psychedelics-based products could result in the perpetuation of the black market for psychedelics and/or have a material,
adverse effect on the perception of psychedelics use. Any or all these events could have a material, adverse effect on our business, financial
condition and results of operations.
Our employees, independent contractors and
consultants may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements,
which could have a material adverse effect on our business.
We are exposed to the risk
that our employees, independent contractors and consultants may engage in fraudulent or other illegal activity or misconduct. Misconduct
by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities that violate, among
other things: (i) the terms and conditions of our Dealer’s Licence issued under Part J of the Food and Drug Regulations; (ii) other
government regulations; (iii) manufacturing standards; (iv) federal and provincial healthcare laws and regulations; or (v) laws that require
the true, complete and accurate reporting of financial information or data. In particular, sales, marketing and business arrangements
in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing, and other
abusive practices. Employee misconduct could also involve the improper use of information obtained in the course of our business, which
could result in regulatory sanctions and serious harm to our reputation. If any such actions are instituted against us, and we are not
successful in defending ourselves or asserting our rights, those actions could have a substantial impact on our business and results of
operations, including the imposition of substantial fines or other sanctions. We believe that the risk of employee misconduct is heightened
given that our operations will involve the cultivation or manufacture of psychedelics substantives, including initially the cultivation
of psychedelic mushrooms and products derived therefrom.
It is not always possible
for us to identify and deter misconduct by our employees and other associated persons, and the precautions taken by us to detect and prevent
this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations
or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. Additionally, we are subject to
the risk that a person could allege fraud or other misconduct by our employees and other associated persons, even if none occurred. If
actions by regulatory authorities are instituted against us with respect to fraud, kickbacks or other illegal practices, and we are not
successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including loss
our of Dealer’s Licence, the imposition of civil, criminal and administrative penalties, damages, monetary fines, contractual damages,
reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could have a material adverse
effect on our business, financial condition and results of operations.
If our operating facility becomes damaged
or inoperable or we are required to vacate our facility, our ability to conduct and pursue our research and development efforts may be
jeopardized.
We expect to derive the majority
of our revenue based upon production of psychedelics-based compounds, formulations and raw precursor materials, scientific and engineering
research and development and testing conducted at a single facility located outside of Victoria, British Columbia. Our facility and equipment
could be harmed or rendered inoperable or inaccessible by natural or man-made disasters or other circumstances beyond our control, including
fire, earthquake, power loss, communications failure, war or terrorism, or another catastrophic event, such as a pandemic or similar outbreak
or public health crisis, which may render it difficult or impossible for us to support our clients and conduct our manufacturing operations
for some period of time. The inability to address system issues could develop if our facility is inoperable or suffers a loss of utilization
for even a short period of time, may result in the loss of clients or harm to our reputation, and we may be unable to regain those clients
or repair our reputation in the future. Furthermore, our facility and the equipment we use to perform our cultivation, research and development
work could be unavailable or costly and time-consuming to repair or replace. It would be difficult, time-consuming and expensive to rebuild
our facility, to locate and qualify a new facility or license or transfer our proprietary technology to a third party. Even in the event
we are able to find a third party to assist in cultivation, research and development efforts, we may be unable to negotiate commercially
reasonable terms to engage with the third party. We carry insurance for damage to our property and the disruption of our business, but
this insurance may not cover all of the risks associated with damage or disruption to our business, may not provide coverage in amounts
sufficient to cover our potential losses and may not continue to be available to us on acceptable terms, if at all.
We and our clients may face risks due to the ongoing COVID-19
pandemic.
In December 2019, a novel
coronavirus, SARS-CoV-2, causing a respiratory disease known as COVID-19, emerged in Wuhan, China. On January 30, 2020, the World Health
Organization declared the outbreak a global health emergency, and on March 11, 2020, the spread of COVID-19 was declared a pandemic by
the World Health Organization. The pandemic has caused companies and various international jurisdictions to impose restrictions such as
quarantines, business closures and travel restrictions. While these effects are expected to be temporary and the administration of effective
vaccines has shown progress in some areas in significantly lowering the number of active infections, the duration of the business disruptions
internationally and related financial impact cannot be reasonably estimated at this time. Governments and central banks have reacted with
significant monetary and fiscal interventions designed to stabilize economic conditions. The duration of the COVID-19 outbreak is unknown
at this time, as is the efficacy of the government and central bank interventions. It is not possible to reliably estimate the length
and severity of these developments and the impact on the financial results and condition of our business. However, depending on the length
and severity of the pandemic, COVID-19 could impact our operations, could cause delays in our efforts to scale up our contract manufacturing
and research offerings, could postpone certain marketing activities, and could impair our ability to raise funds.
We have requested that most
of our employees, including all of our administrative employees, work remotely and have restricted on-site staff to only those personnel
who must perform essential on-site activities such as activities in our cultivation areas and research and development laboratories. Our
increased reliance on employees working from home may negatively impact productivity, or disrupt, delay, or otherwise adversely impact
our business. In addition, this could increase our cybersecurity risk, create data accessibility concerns, and make us more susceptible
to communication disruptions, any of which could adversely impact our business operations or delay necessary interactions with local and
federal regulators, ethics committees and other important agencies and contractors.
Our clients may face disruptions
resulting from the COVID-19 pandemic that could adversely impact their business and operations, including, among other things, their ability
to initiate and complete preclinical studies or clinical trials; their ability to procure items that are essential for their research
and development activities, such as, for example, laboratory supplies for their preclinical studies and planned clinical trials, or animals
that are used for preclinical testing; availability of clinical trial study personnel and site access; and their ability to successfully
commercialize our product candidates, if approved. With respect to our clients’ clinical trial activities, the COVID-19 pandemic
may result in the interruption or modification of clinical trial subject visits and study procedures, as well as confounding of efficacy
assessments or missing data as a result of direct patient infection, which may impact the integrity or acceptance by the Health Canada,
the FDA or other regulatory authorities of subject data, clinical study endpoints, and overall study interpretability. Any such disruptions
faced by our clients would be likely to have an adverse impact on our business, financial condition and results of operations.
We cannot be certain what
the overall impact of the COVID-19 pandemic will be on our business, and it has the potential to materially and adversely affect our business,
financial condition, results of operations and prospects. To the extent the COVID-19 pandemic adversely affects our business, financial
condition and results of operations, it may also have the effect of heightening many of the other risks described in this “Risk
Factors” section.
Our business could expose us to potential
product liability and other liability risks.
While we do carry product
liability insurance in Canada, we do not currently carry any product liability insurance coverage in the United States. Our business could
expose us to potential product liability, recalls and other liability risks that are inherent in the sale of pharmaceutical materials
and finished products. We can provide no assurance that such potential claims will not be asserted against us. A successful liability
claim or series of claims brought against us could have a material adverse effect on our business, financial condition and results of
operations. If we decide to obtain product liability insurance, we cannot provide any assurances that we will be able to obtain or maintain
adequate product liability insurance of on acceptable terms, if at all, or that such insurance will provide adequate coverage against
potential liabilities. Claims or losses in excess of any product liability cover that may be obtained by us could have a material adverse
effect on our business, financial condition and results of operations.
In addition, manufacturers
and distributors of pharmaceutical products are sometimes subject to the recall or return of their products for a variety of reasons,
including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety
and inadequate or inaccurate labelling disclosure. If any of our products are recalled due to an alleged product defect or for any other
reason, we could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with
the recall. We may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all. In
addition, product recall may require significant management attention. Although we will implement detailed procedures for testing our
products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product
recalls, regulatory action or lawsuits. A recall for any of the foregoing reasons could lead to decreased demand for our products and
could have a material adverse effect on the results of operations and financial condition of our business. Additionally, product recalls
may lead to increased scrutiny of our operations by regulatory agencies, requiring further management attention and potential legal fees
and other expenses.
We may expend our limited resources to pursue
a particular product and fail to capitalize on products that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial
and managerial resources, we will be compelled to focus our initial cultivation, research and development efforts on a limited number
of psychedelics-based products and research projects for our clients who are developing psychedelics-based medicines. As a result, we
may forego or delay pursuit of opportunities with other products or contract research offerings that later prove to have greater commercial
potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or services or profitable
market opportunities. Our spending on current and future manufacturing and contract research efforts may not yield any commercially viable
products or services. If we do not accurately evaluate the commercial potential or target market for a particular product or service offering,
we may relinquish valuable rights to related technology or intellectual property through collaboration, licensing or other royalty arrangements
in cases in which it would have been more advantageous for us to retain sole rights to such product or service. Failure to allocate resources
or capitalize on strategies in a successful manner will have an adverse impact on our business.
We may choose not to continue developing
or commercializing any of our product candidates at any time during development or after commercialization, which would reduce or eliminate
our potential return on investment for those product candidates.
At any time, we may decide
to discontinue the development or commercialization of any of our products or product candidates for a variety of reasons, including the
appearance of new technologies that render our product obsolete, competition from a competing product or changes in or inability to comply
with applicable regulatory requirements. If we terminate a program in which we have invested significant resources, we will not receive
any return on our investment and we will have missed the opportunity to allocate those resources to potentially more productive uses.
Risks Related to Government Regulation
The business to be conducted by us and our
clients will be subject to extensive governmental regulation, and our or our clients’ inability to comply with these regulations,
which are complex and relate to various jurisdictions and areas of law, would result in significant adverse consequences to our business.
Various Canadian and U.S.
federal, state, provincial and local laws govern our business in the jurisdictions in which we operate or currently plan to operate, and
to which we export or currently plan to export our products, including laws relating to health and safety, the conduct of our operations,
and the production, storage, sale and distribution of our products. Complying with these laws requires that we and our clients comply
concurrently with complex federal, state, foreign, provincial and/or local laws. These laws change frequently and may be difficult to
interpret and apply. To ensure our compliance with these laws, we will need to invest significant financial and managerial resources.
It is impossible for us to predict the cost of such laws or the effect they may have on our future operations. A failure to comply with
these laws could negatively affect our business and harm our reputation. Changes to these laws could negatively affect our competitive
position and the markets in which we operate, and there is no assurance that various levels of government in the jurisdictions in which
we operate will not pass legislation or regulation that adversely impacts our business.
In addition, even if we or
third parties were to conduct activities in compliance with Canadian laws, U.S. federal, state or local laws or the laws of other countries
and regions in which we conduct activities, certain violations of those laws may lead to enforcement proceedings that could involve significant
restrictions or criminal or civil penalties being imposed upon us or third parties, while diverting the attention of key executives. Such
proceedings could have a material adverse effect on our business, revenue, operating results and financial condition as well as on our
reputation and prospects, even if such proceedings conclude successfully in our favour. In the extreme case, such proceedings could ultimately
involve the criminal prosecution of our key executives, the seizure of corporate assets, and consequently, our inability to continue business
operations. Any such proceedings brought against us may adversely affect our operations and financial performance.
The psychedelic drug industry
is a fairly new industry and we cannot predict the impact of the ever-evolving compliance regime in respect of this industry. Similarly,
we cannot predict the time required to secure all appropriate regulatory approvals for future products, or the extent of testing and documentation
that may, from time to time, be required by governmental authorities. The impact of compliance regimes, any delays in obtaining, or failure
to obtain regulatory approvals as needed may significantly delay or impact the development of markets, its business and products, and
sales initiatives and could have a material adverse effect on our business, financial condition and results of operations.
Our products and services, and the product
candidates and approved products developed and marketed by our clients, will be subject to controlled substance laws and regulations in
the territories in which the product or service will be manufactured, developed, tested and marketed, and failure to comply with these
laws and regulations, or the cost of compliance with these laws and regulations, may adversely affect the results of our business operations,
both during clinical development and post approval, and our financial condition.
In Canada, certain psychotropic
drugs, including lysergic acid diethylamide, or LSD, MDMA, DMT and psilocybin, are regulated under the Controlled Drugs and Substances
Act, or CDSA. The CDSA classifies regulated drug substances into five schedules, with Schedule I containing the highest risk substances.
Certain psychedelic substances, including psilocybin, psilocin, mescaline and DMT, are classified as Schedule III drugs. The CDSA prohibits
the possession of a Schedule III drug absent authorization under the CDSA or a related regulation (either via a license or an authorized
exemption). Health Canada has not approved psilocybin as a drug for any indication and it is illegal to possess Schedule III substances
without a prescription. Under Section 56(1) of the CDSA, the Minister of Health has the ability to grant exemptions to these restrictions
if the Minister deems them necessary for a medical or scientific purpose, or otherwise in the public interest. It is not clear exactly
how and when the Section 56(1) exemption may be granted for psychedelics. To date, a limited number of Section 56 exemptions for psilocybin
access or research have been granted in Canada. Further, a Dealer’s Licence for psychedelic drugs can be obtained from Health Canada
under Part J of the Food and Drug Regulations allowing for the possession, processing, sending, sale, transportation and delivery of products
containing a controlled substance such as psilocybin. Only a very limited number of Dealer’s Licences for psychedelics have been
granted in Canada.
In the United States, these
substances are classified under the Controlled Substances Act (21 U.S.C. § 811), or the CSA, and the Controlled Substances Import
and Export Act, or the CSIEA, and as such, medical and recreational use is illegal under the U.S. federal laws. Under the CSA, the Drug
Enforcement Agency, or DEA, regulates chemical compounds with a potential for abuse as Schedule I, II, III, IV or V substances. Schedule
I substances by definition have a high potential for abuse, have no currently “accepted medical use” in the United States,
lack accepted safety for use under medical supervision, and may not be prescribed, marketed or sold in the United States. Pharmaceutical
products approved for use in the United States may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present
the highest potential for abuse or dependence and Schedule V substances the lowest relative risk of abuse among such substances. Schedule
I and II drugs are subject to the strictest controls under the CSA, including manufacturing and procurement quotas, security requirements
and criteria for importation. In addition, dispensing of Schedule II drugs is further restricted. For example, they may not be refilled
without a new prescription and may have a black box warning. Most, if not all, state laws in the United States classify psilocybin, LSD,
MDMA and DMT and as Schedule I controlled substances. For any product containing any of these substances to be available for commercial
marketing in the United States, the applicable substance must be rescheduled, or the product itself must be scheduled, by the DEA to Schedule
II, III, IV or V. Commercial marketing in the United States will also require scheduling-related legislative or administrative action.
Scheduling determinations
by the DEA are dependent on FDA approval of a substance or a specific formulation of a substance for medical use. Therefore, while psilocybin
and the other psychedelic substances we may cultivate and manufacture are Schedule I controlled substances, products developed by our
clients that are approved by the FDA for medical use in the United States that contain psilocybin or another such substance must be placed
in Schedules II-V prior to commercialization, since approval by the FDA satisfies the “accepted medical use” requirement.
If and when a product candidate developed by one of our clients receives FDA approval, the DEA will make a scheduling determination and
place it in a schedule other than Schedule I in order for it to be prescribed to patients in the United States. This scheduling determination
will be dependent on FDA approval and the FDA’s recommendation as to the appropriate schedule. During the review process, and prior
to approval, the FDA may determine that it requires additional data, either from non-clinical or clinical studies, including with respect
to whether, or to what extent, the substance has abuse potential. This may introduce a delay into the approval and any potential rescheduling
process. This scheduling determination will require the DEA to conduct notice and comment rule making including issuing an interim final
rule. Such action will be subject to public comment and requests for hearing which could affect the scheduling of these substances. There
can be no assurance that the DEA will make a favorable scheduling decision. Even assuming categorization as a Schedule II or lower controlled
substance (i.e., Schedule III, IV or V), at the federal level, such substances would also require scheduling determinations under state
laws and regulations. Even assuming that the applicable therapeutic candidate approved and scheduled by regulatory authorities to allow
their commercial marketing, the APIs in such therapeutic candidates would likely continue to be Schedule I, or the
state or foreign equivalent.
The laws and regulations
generally applicable to controlled substances may change in ways currently unforeseen. Any amendment to or replacement of existing laws
or regulations, including the classification or re-classification of the substances we are developing or working with, which are matters
beyond our control, may cause our business, financial condition, results of operations and prospects to be adversely affected or may cause
us to incur significant costs in complying with such changes or it may be unable to comply therewith.
Even if therapies containing psychedelics
substances receive scheduling determinations that allow them to be approved and commercialized, our raw materials and APIs and the finished
products into which they are incorporated will remain subject to extensive regulation as controlled substances.
Controlled substances are
subject to Health Canada and DEA regulations relating to manufacturing, storage, distribution and physician prescription procedures, which
regulations may be applicable to us or our clients. Moreover, even if the finished dosage form of a psychedelics-based medicine developed
by one of our clients is approved by the FDA, and if such product is listed by the DEA as a Schedule II, III, or IV controlled substance,
its manufacture, importation, exportation, domestic distribution, storage, sale and legitimate use will continue to be subject to a significant
degree of regulation by the DEA. The regulations that are relevant to our and our clients’ efforts to research, develop, obtain
approval for an commercialize psychedelics-based therapies in the United States include the following:
| ● | DEA registration and inspection
of facilities. Facilities conducting research, manufacturing, distributing, importing or exporting, or dispensing controlled
substances must be registered (licenced) to perform these activities and have the security, control, recordkeeping, reporting and inventory
mechanisms required by the DEA to prevent drug loss and diversion. All these facilities must renew their registrations annually, except
dispensing facilities, which must be renewed every three years. The registration process involves a written application and a field inspection
by the DEA. The DEA conducts periodic inspections of certain registered establishments that handle controlled substances. Our and our
client’s obtaining and maintaining the necessary registrations may result in delay of the importation, manufacturing or distribution
of the applicable raw materials, API or finished drug product. Furthermore, failure to maintain compliance with the CSA, particularly
noncompliance resulting in loss or diversion by us or our clients, can result in regulatory action that could have a material adverse
effect on our business, financial condition and results of operations. The DEA may seek civil penalties, refuse to renew necessary registrations,
or initiate proceedings to restrict, suspend or revoke those registrations. In certain circumstances, violations could lead to criminal
proceedings. |
| ● | State-controlled substances
laws. Individual U.S. states have also established controlled substance laws and regulations. Though state-controlled substances
laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule a controlled substance or
product containing a controlled substance. While some states automatically schedule a drug based on federal action, other states schedule
drugs through rule making or a legislative action. State scheduling may delay commercial sale of any product for which we obtain federal
regulatory approval and adverse scheduling could have a material adverse effect on the commercial attractiveness of such product. We
or our clients must also obtain separate state registrations, permits or licences in order to be able to obtain, handle, and distribute
controlled substances for clinical trials or commercial sale, and failure to meet applicable regulatory requirements could lead to enforcement
and sanctions by the states in addition to those from the DEA or otherwise arising under federal law. |
| ● | Clinical trials. To
the extent an investigational therapy contains a controlled substance, to conduct clinical trials in the United States prior to approval,
each of our clients’ research sites must submit a research protocol to the DEA and obtain and maintain a DEA researcher registration
that will allow those sites to handle and dispense the controlled substance and to obtain the product from us. The DEA submits research
protocols to the FDA for review and approval. The FDA may ask a research registrant to modify its research protocols in order to obtain
registration. If the DEA delays or denies the grant of a researcher registration to one or more research sites, or if the FDA delays,
denies or requests modifications to the research protocol, the clinical trial could be significantly delayed, and our clients could lose
clinical trial sites. |
| ● | Importation. The
DEA requires authorized registrants to obtain an import permit in order to import any substances on Schedules I and II for analytic,
research, or commercial purposes. The failure by our clients to obtain the necessary import authority, including specific quantities,
could have a material adverse effect on our business, results of operations and financial condition. In addition, an application for
a Schedule I or II importer registration must be published in the Federal Register, and there is a waiting period for third-party comments
to be submitted. It is possible that adverse comments may delay the grant of an importer registration. Our clients will not be allowed
to import the drug for commercial purposes unless the DEA determines that there is inadequate domestic competition among domestic manufacturers
for the substance as defined by the DEA. Moreover, the DEA has never permitted Schedule I controlled substances, including psilocybin
and psilocin, to be imported for commercial purposes, only for scientific and research needs. If, by the time a drug that incorporates
psychedelic substances is approved for commercial marketing in the United States, sufficient domestic manufacturers for the raw material
exist, our clients may not be authorized to import our APIs for conversion into therapeutic products for commercial purposes. |
| ● | Manufacture in the United
States. If, because of a Schedule II-V classification or voluntarily, we were to conduct manufacturing or repackaging/relabelling
in the United States, we would be subject to the DEA’s annual manufacturing and procurement quota requirements. Manufacturers that
seek to manufacture Schedule I or II controlled substances in bulk, and manufacturers that wish to convert bulk Schedule I or II controlled
substances into dosage form or other substances are required to comply with individually-allotted manufacturing and procurement quotas.
Additionally, regardless of the scheduling of a finished, approved therapeutic product, if the API used in the final dosage form is a
Schedule I or II controlled substance, it would be subject to such quotas as the API could remain listed on Schedule I or II. Although
the DEA increased the United States’ overall annual production quotas for certain psychedelic substances in 2022 and has proposed
increased national quotas for 2023, annual quotas allocated for our clients for the API in a particular therapeutic product may not be
sufficient to complete clinical trials or meet commercial demand. Consequently, any delay or refusal by the DEA in establishing or increasing
our clients’ procurement and/or production quotas for controlled substances could delay or stop our client’s clinical trials
or product launches, which could have a material adverse effect on our business, financial position and results of operations. |
| ● | Distribution in the United
States. If a particular approved therapy is scheduled as Schedule II, III, IV or V, our clients would also need to identify wholesale
distributors with the appropriate DEA registrations and authority to distribute the approved therapy. These distributors would need to
obtain Schedule II, III, IV or V distribution registrations. This limitation in the ability to distribute an approved therapy more broadly
may limit commercial uptake and could negatively impact our client’s prospects. The failure to obtain, or delay in obtaining, or
the loss of any of those registrations could result in increased costs to us. In addition, if an approved therapy is determined to have
a high potential for abuse, it could be required to be administered at clinical trial sites, which could limit commercial uptake. Furthermore,
state and federal enforcement actions, regulatory requirements, and legislation intended to reduce prescription drug abuse, such as the
requirement that physicians consult a state prescription drug monitoring program, may make physicians less willing to prescribe, and
pharmacies to dispense, Schedule II-V products. |
Violations of any federal,
state or foreign laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements
arising from civil proceedings conducted by either the federal government or private citizens, or criminal charges and penalties, including,
but not limited to, disgorgement of profits, cessation of business activities, divestiture, or prison time. This could have a material
adverse effect on us, including by impacting our or our clients’ reputation and ability to conduct business. Any such impact could
in turn adversely affect our financial position, operating results, profitability or liquidity or the market price of our common shares.
In addition, it is difficult for us to estimate the time or resources that would be needed for the investigation or defense of any such
matters or our final resolution because, in part, the time and resources that may be needed are dependent on the nature and extent of
any information requested by the applicable authorities involved, and such time or resources could be substantial. It is also illegal
to aid or abet such activities or to conspire or attempt to engage in such activities. An investor’s contribution to and involvement
in such activities may result in federal civil and/or criminal prosecution, including, but not limited to, forfeiture of his, her or its
entire investment, fines and/or imprisonment.
Our operations require that we receive and
maintain licensing from Health Canada.
To legally possess and conduct
anticipated activities with controlled substances in Canada, entities must first obtain a controlled substances Dealer’s Licence.
A Dealer’s Licence authorizes the holder to possess controlled substances and to conduct activities specified by the licence, such
as production, packaging, sale, sending, transportation, delivery, laboratory analysis, research and development, clinical studies, import/export
or distribution. Licence holders are responsible for compliance with licence specification, the CDSA and its regulations, as well as compliance
with other applicable federal, provincial, and territorial legislation and municipal by-laws. The issued licence dictates activities,
conditions, and restrictions for the licence holder depending on licence permissions, and the licence holder must strictly adhere to these
parameters.
A party can apply for a Dealer’s
Licence under the Food and Drug Regulations (Part J). In order to qualify as a licenced dealer, a party must meet all regulatory requirements
mandated by the regulations including having compliant facilities and security requirements, compliant materials and staff that meet the
qualifications under the regulations of a senior person in charge and a qualified person in charge. Assuming compliance with all relevant
laws (e.g., the CDSA, Food and Drug Regulations) and subject to any restrictions placed on the licence by Health Canada, an entity with
a Dealer’s Licence may produce, assemble, sell, provide, transport, send, deliver, import or export a restricted drug (as listed
in Part J in the Food and Drug Regulations), including, for example, psilocybin and psilocin.
There may be further changes
and amendments to the CDSA and the regulations regarding the issuance of Dealer Licences and the current regulatory landscape may be subject
to change at any time. We can provide no assurance that we will maintain a Dealer’s Licence, that it will permit us to undertake
all of the activities necessary to sell our products and become profitable, or that it will not be revoked.
Licensing programs relating
to controlled substances are strict and penalties for contravention of these laws could result in significant fines, penalties, administrative
sanctions, convictions or settlements arising from civil proceedings initiated by either government entities in the jurisdictions in which
we operate, or private citizens or criminal charges. The loss of these necessary licenses and permits would have a materially adverse
effect on our business, financial condition and results of operations.
Our potential clients in the United States
must register with the DEA in order to import, conduct research and develop new drugs using Schedule I or II controlled substances.
The cultivation, manufacture,
distribution and possession of U.S. Schedule I or II controlled substances violates federal law in the United States unless a U.S. federal
agency, such as the DEA, grants a registration for a specific use, such as import and/or research, of a specific controlled substance.
Significant regulatory disclosure, oversight, and reporting are required to possess these substances, both to test and conduct preclinical
and clinical trials and to develop and sell products whose active ingredients contain a controlled substance. U.S. manufacturers of Schedule
I or II controlled substances must apply for the issuance of procurement quotas in order to convert bulk substances on Schedule I or II
into finished dosage forms or other substances. The procurement quota establishes the maximum amount of a Schedule I or II substance that
a facility may procure in a given year, and that quota cannot be exceeded without an amendment to the quota from the DEA. Accordingly,
any U.S. manufacturers to which we sell our psychedelics-based raw materials or API, and who wish to convert these into finished dosage
form or other substances, must obtain and remain in compliance with these registration and quota requirements. These requirements may
sharply limit the available market in the United States for our products. If the U.S. market is smaller than we anticipate, or if U.S.
regulators determine to grant fewer registrations, impose more stringent requirements on existing registrants, or limit procurement quotas
for the controlled substances we manufacture, these events could have a material and adverse impact on our business, financial condition
and results of operations.
The registration of additional United States-based
manufacturers of the raw materials or APIs we create may hinder our ability to sell into the United States.
The United States has a policy
of prioritizing U.S. domestically-manufactured scheduled substances over foreign ones. The DEA establishes an aggregate production quota
for Schedule I or II controlled substances based on the amount of Schedule I or II controlled substances necessary to be manufactured
in or imported into the United States in a given year to provide for the estimated medical, scientific, research and industrial needs
of the United States, for lawful export requirements, and for the establishment and maintenance of reserve stocks. Individual manufacturing
quotas are issued to registered manufacturers who wish to manufacture a quantity of specific Schedule I or II controlled substances. Import
permits are only granted if the DEA finds that the United States’ domestic supply of any controlled substance is inadequate for
scientific studies or finds that competition among domestic manufacturers of the controlled substance is inadequate and will not be rendered
adequate by the registration of additional manufacturers. The aggregate U.S. production quotas for psilocybin, psilocin, MDMA, and DMT
among other psychedelics, were increased significantly in 2022. The DEA’s final aggregate production quotas for 2023 may be even
higher. As a result of the increased quotas, DEA may register additional U.S. domestic manufacturers of the raw materials or APIs we manufacture,
or increase individual manufacturing quotas for those raw materials or APIs. If DEA does increase U.S. domestic supply of the APIs we
manufacture, our market share in the United States may be significantly decreased or eliminated, which would have a material and adverse
impact on our business, financial condition and results of operations.
The import of our products into the United
States relies on the compliance of our clients abroad and the authorization of their governing jurisdictions.
Because we intend to manufacture
APIs for sale to clients conducting research and product development in jurisdictions foreign to Canada, we must rely on those foreign
clients to obtain the necessary approvals from their respective governing bodies in order to import our products to their facilities.
For instance, in the United States, only certain DEA registrants may apply for import permits related to Schedule I substances. Those
import permits may be subject to procurement quotas, which DEA has the full discretion to issue or increase. U.S. registrants must coordinate
with applicable ports of entry to notify border agents of incoming shipments of Schedule I substances and must also provide for the secure
transport of shipments of our products to their facilities. The DEA must approve our client’s security plans, including their provisions
related to secure transport. If a shipment is rejected by U.S. Customs for any reason, our U.S. client will have to re-apply for an import
permit for that shipment, possibly significantly delaying shipping times. Our clients’ inability to secure DEA authorization to
import our APIs, could have a material and adverse impact on our business, financial condition and results of operations. Delays in transport
of our products to their destinations may have a significant adverse impact on research protocols or clinical trials, potentially damaging
relationships with our customers, and having a material and adverse impact on our business, financial condition and results of operations.
Changes in the regulatory status of psychedelic
substances will present additional risks to our business and will create additional regulatory costs and challenges.
Any changes in applicable
laws and regulations could have an adverse effect on our operations. The psychedelic drug industry is a fairly new industry and we cannot
predict the impact of the ever-evolving compliance regime in respect of this industry. Similarly, we cannot predict the time required
to secure all appropriate regulatory approvals for future products and services, or the extent of testing and documentation that may,
from time to time, be required by governmental authorities. The impact of compliance regimes, any delays in obtaining, or failure to obtain
regulatory approvals may significantly delay or impact the development of markets, our business and products, and sales initiatives and
could have a material adverse effect on the business, financial condition and operating results of our business.
For example, if psilocybin
and/or psilocin is rescheduled under the CSA as a Schedule II or lower controlled substance (i.e., Schedule III, IV or V), the ability
to conduct research on psilocybin and psilocin would most likely be improved. However, rescheduling psilocybin and psilocin may materially
alter enforcement policies across many federal agencies, primarily the FDA and DEA. The FDA is responsible for ensuring public health
and safety through regulation of food, drugs, supplements, and cosmetics, among other products, through its enforcement authority pursuant
to the Federal Food, Drug, and Cosmetic Act, or the FDCA. The FDA’s responsibilities include regulating the ingredients as well
as the marketing and labelling of drugs sold in interstate commerce. Because it is currently illegal under federal law to produce and
sell psilocybin and psilocin, and because there are no federally recognized medical uses, the FDA has historically deferred enforcement
related to psilocybin and psilocin to the DEA. If psilocybin and psilocin were to be rescheduled to a federally controlled, yet legal,
substance, the FDA would likely play a more active regulatory role. The DEA would continue to be active in regulating manufacturing, distribution
and dispensing of such substances. The potential for multi-agency enforcement post-rescheduling could threaten or have a materially adverse
effect on our business.
Despite the current status
of psilocybin and psilocin as Schedule I controlled substances in the United States, there may be changes in the status of psilocybin
or psilocin under the laws of certain U.S. cities or states. For instance, the city and county of Denver voted in 2019 to make the enforcement
of any laws imposing criminal penalties for the personal use and personal possession of psilocybin mushrooms the lowest law enforcement
priority in the city and county of Denver, and in Oregon, Measure 109 was passed in November 2020 directing the Oregon Health Authority,
or OHA, after a two-year development period, to license and regulate the manufacturing, transportation, delivery, sale and purchase of
psilocybin products and the provision of psilocybin services. Other jurisdictions in Canada and the United States may proceed to authorize
decriminalization to varying extents and employing varying regulatory frameworks. The decriminalization of psilocybin or psilocin, or
other psychedelic substances, without regulatory oversight, or with inadequate or ineffective regulatory oversight, may lead to the setup
of clinics without proper therapeutic infrastructure or adequate clinical research, which could put patients at risk and bring reputational
and regulatory risk to the entire industry, making it harder for us to successfully operate our business. Furthermore, the legalization
of psilocybin or psilocin could also impact our commercial sales if our clients receive regulatory approval as it would reduce the barrier
to entry and could increase their competition.
The success of our business
is dependent on our activities being permissible under applicable laws and any reform of controlled substances laws or other laws may
have a material impact on our business and success. There is no assurance that activities of our business will continue to be legally
permissible.
We have to comply with current Good Manufacturing
Practices regulations applicable to our psychedelics-based products manufacturing operations.
Health Canada and the FDA
and other equivalent regulatory bodies in other jurisdictions ensure the quality of drug products by carefully monitoring drug manufacturers’
compliance with cGMP regulations. These regulations govern manufacturing processes and procedures (including record keeping) and the implementation
and operation of quality systems to control and assure the quality of investigational products and products approved for sale, and they
are enforced through Health Canada’s and the FDA’s inspection programs. If Health Canada or the FDA determines that we are
not in compliance with applicable laws and regulations, including those governing cGMPs, Health Canada or the FDA may not approve new
drug applications or submissions, or NDAs or NDSs, submitted by our clients and containing products manufactured by us until the deficiencies
are corrected. Correcting any such deficiencies may be costly and time-consuming, and it may harm our client relationships and status
in the marketplace. Moreover, our failure to comply with regulations application to our manufacturing facilities could result in sanctions
being imposed on us or our clients, including clinical holds, fines, injunctions, civil penalties, seizures or recalls of product candidates
or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect the demand for our
or our clients’ products. In addition, approved products and the facilities at which they are manufactured are required to maintain
ongoing compliance with extensive FDA requirements and the requirements of other similar agencies, including ensuring that quality control
and manufacturing procedures conform to cGMP requirements. As such, we are subject to continual review and periodic inspections to assess
compliance with cGMPs.
Even if therapeutic product candidates obtain
regulatory approval, our clients will be subject to ongoing obligations and continued regulatory review, which may result in significant
additional expense to them and may decrease the quantity of our products and services that they purchase. Additionally, any such therapeutic
candidates, if approved, could be subject to labelling and other restrictions and market withdrawal, which would also decrease the quantity
of our products and services that our clients purchase.
If Health Canada, the FDA
or another equivalent regulatory authority approves a client’s psychedelics-based therapeutic candidate, the manufacturing processes,
labelling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the therapy and underlying
therapeutic substance will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety
and other post-marketing information and reports, registration, as well as continued compliance with cGMPs and with good clinical practices,
or GCPs, for any clinical trials that our clients conduct post-approval, all of which may result in significant expense to them and limit
their ability to commercialize such therapies. Any such limits on their ability to commercialize approved therapies may cause them to
purchase fewer of our products and services, which will adversely impact our business, financial condition and results of operations.
Additionally, a company may not promote “off-label” uses for its drug products. An off-label use is the use of a product for
an indication that is not described in the product’s FDA-approved label in the United States or for uses in other jurisdictions
that differ from those approved by the applicable regulatory agencies. Physicians, on the other hand, may prescribe products for off-label
uses. Although the FDA and other regulatory agencies do not regulate a physician’s choice of drug treatment made in the physician’s
independent medical judgment, they do restrict promotional communications from companies or their sales force with respect to off-label
uses of products for which marketing clearance has not been issued.
Later discovery of previously
unknown problems with any approved therapeutic product candidate, including adverse events of unanticipated severity or frequency, or
with respect to a CMO’s manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| |
● |
restrictions on the labelling, distribution, marketing or manufacturing of an approved therapy or any of our client’s future therapeutic candidates, withdrawal of the product from the market, or product recalls; |
| |
|
|
| |
● |
untitled and warning letters, or holds on clinical trials; |
| |
● |
refusal by Health Canada, the FDA or other equivalent foreign regulatory authorities to approve pending applications or supplements to approved applications our clients filed or suspension or revocation of license approvals; |
| |
|
|
| |
● |
requirements to conduct post-marketing studies or clinical trials; |
| |
|
|
| |
● |
restrictions on coverage by third-party payors; |
| |
|
|
| |
● |
fines, restitution or disgorgement of profits or revenue; |
| |
|
|
| |
● |
suspension or withdrawal of marketing approvals; |
| |
|
|
| |
● |
product seizure or detention, or refusal to permit the import or export of the product; and |
| |
|
|
| |
● |
injunctions or the imposition of civil or criminal penalties. |
Any such outcomes would diminish
our client’s ability to successfully commercialize the applicable therapeutic products, which in turn would cause them to purchase
fewer of our products and services.
In addition, any regulatory
approvals that our clients receive for a therapeutic product candidate may also be subject to limitations on the approved indicated uses
for which the therapy may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing
testing, including Phase IV clinical trials, and surveillance to monitor the safety and efficacy of such therapeutic product candidates.
If there are changes in the
application of legislation, regulations or regulatory policies, or if problems are discovered with a client’s investigational therapy
or our manufacture of an underlying therapeutic substance, or if we, our client or one of their distributors, licensees or co-marketers
fails to comply with regulatory requirements, the regulators could take various actions. These include imposing fines on our client or
on us, if applicable, imposing restrictions on the therapeutic or its manufacture and requiring our client to recall or remove the therapeutic
from the market. The regulators could also suspend or withdraw marketing authorizations, requiring our client to conduct additional clinical
trials, change the therapeutic labelling or submit additional applications for marketing authorization. If any of these events occurs,
our client’s ability to sell the applicable therapeutic product may be impaired, and they may incur substantial additional expense
to comply with regulatory requirements. This could cause our client to purchase fewer of our products and services, which could materially
adversely affect our business, financial condition and results of operations.
We may become subject to U.S. federal and
state forfeiture laws which could negatively impact our business operations.
Violations of any U.S. federal
laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil
proceedings conducted by either the federal government or private citizens, or criminal charges, including, but not limited to, seizure
of assets, disgorgement of profits, cessation of business activities or divestiture. As an entity that conducts business involving psilocybin
and psilocin, we are potentially subject to federal and state forfeiture laws (criminal and civil) that permit the government to seize
the proceeds of criminal activity. Civil forfeiture laws could provide an alternative for the federal government or any state (or local
police force) that wants to discourage residents from conducting transactions with psilocybin- and psilocin-related businesses but believes
criminal liability is too difficult to prove beyond a reasonable doubt. Also, an individual can be required to forfeit property considered
to be the proceeds of a crime even if the individual is not convicted of the crime, and the standard of proof in a civil forfeiture matter
is lower than the standard in a criminal matter. Depending on the applicable law, whether federal or state, rather than having to establish
liability beyond a reasonable doubt, the federal government or the state, as applicable, may be required to prove that the money or property
at issue is proceeds of a crime only by either clear and convincing evidence or a mere preponderance of the evidence.
If our products are diverted
into criminal channels of commerce, investors located in jurisdictions where psychedelic substances remain illegal may be at risk of prosecution
under conspiracy, aiding and abetting, and money laundering statutes, and be at further risk of losing their investments or proceeds under
forfeiture statutes. Many jurisdictions remain fully able to take action to prevent the proceeds of psychedelics businesses from entering
their state. Our investors and prospective investors should be aware of these potentially relevant laws in considering whether to invest
in us.
Risks Related to Commercialization
Drug manufacturers who obtain FDA approval
for their new drugs must prove that domestic supplies are inadequate in order to import a foreign API on Schedule I or II to be used in
commercial drug manufacturing.
If a U.S. drug manufacturer
wishes to use our product as the API in an FDA-approved drug for commercial manufacture, it will need to obtain DEA approval for the importation
of our product. DEA will not approve an import permit request unless it is shown that the import is necessary to provide for the US’s
medical needs and competition among domestic manufacturers of the substance is inadequate. Depending on the existence, at that time, of
domestic registered manufacturers with the capability of producing the same APIs as us, DEA may not agree that domestic manufacture is
inadequate and may refuse our customers’ requests for import permits. In such cases, we may not be able to supply the drug manufacturer
APIs unless we were to open a U.S. manufacturing facility. Such an undertaking would require considerable additional time and resources
and may not materialize at all.
If we are unable to build a sales and marketing
team to reach our potential clients, our business may be adversely affected.
We do not currently have
a dedicated sales and marketing team. Our initial efforts to build brand and product awareness are expected to focus primarily on scientific
writing and publications. Subject to the easing of restrictions related to COVID-19, we may complement this strategy with research and
development staff attending a variety of scientific conferences in an effort bolster our business development pipeline. However, we may
need to expand our commercial organization in order to effectively market our products and services to new clients. Competition for employees
capable of negotiating and entering into contract manufacturing and supply agreements with pharmaceutical and biotechnology companies
is intense. We may not be able to attract and retain personnel or be able to build an efficient and effective sales organization, which
could negatively impact sales and market acceptance of our products and services and limit our revenue growth and potential profitability.
In addition, the time and cost of establishing a specialized sales, marketing and service force for a particular product or service may
be difficult to justify in light of the revenue generated or projected. Our expected future growth will impose significant added responsibilities
on members of management, including the need to identify, recruit, maintain and integrate additional employees. Our future financial performance
and our ability to successfully sell our programs and to compete effectively will depend, in part, on our ability to manage this potential
future growth effectively, without compromising quality.
Our psychedelics-based products and services
may not meet the expectations of our prospective clients, which means our business, financial condition, results of operations and prospects
could suffer.
Our success depends on, among
other things, the market’s confidence that our manufacturing operations are capable of producing high-end materials, APIs and finished
drug products in a cost-efficient manner and that our contract research services will facilitate improved pharmaceutical and biotechnology
product development in the psychedelics-based medicines space. To date, we have not yet cultivated significant quantities of psychedelic
mushrooms or produced a refined API or finished drug product, much less had a client’s product candidate using our materials receive
regulatory approval. We have also not yet undertaken a significant contract research project for a client. Accordingly, in order to successfully
commercialize our products and services we will need to build confidence in the market that we have the facility, equipment and expertise
to provide premium contract manufacturing and research services in the psychedelics space. There can be no guarantee that our product
and service offerings will meet the expectations of research institutions and of pharmaceutical and biotechnology companies. If we are
unable to effectively build client relationships and their confidence in our operations, our ability to commercialize or products and
services will be materially and adversely impacted.
If we are unable to support anticipated
growth in demand for our contract manufacturing and research services, including ensuring that we have adequate teams and facilities to
meet increased demand, or if we are unable to successfully manage our anticipated growth, our business could suffer.
We have only recently begun
initiating the development of our contract manufacturing and research services, and accordingly our personnel resources are currently
very limited. We anticipate significant growth in the number of programs under contract for which we are conducting manufacturing or research
discovery activities. As we secure additional programs under contract, our operational capacity to execute such manufacturing and research
activities may become strained. As a result, our strategy requires us to successfully scale our teams and facilities to meet future demand
for our solutions. Our ability to grow our capacity will depend on our ability to expand our workforce and our facilities, and increase
efficiency through automation and software solutions. We may also need to purchase additional equipment, some of which can take several
months or more to procure and set up. There is no assurance that any of these increases in scale, expansion of personnel, equipment, software
and computing capacities or process enhancements will be successfully implemented and in a timely manner. As limited facilities with appropriate
capabilities are available in British Columbia, such facilities require purpose-built buildings often with rezoning requirements. Such
projects are typically long in duration and subject to delays. Failure to manage this growth could result in delays, higher costs, declining
quality, and slower responses to competitive challenges. A failure in any one of these areas could make it difficult for us to meet market
expectations for our psychedelics-based products and services and could damage our reputation and the prospects for our business.
Even if our clients are successful in developing
and obtaining regulatory approval for their product candidates, they may not be as successful as we anticipate in commercializing psychedelics-based
medicines. If market acceptance of this class of products is limited, our business, financial results and operations may be adversely
affected.
In addition, even if these
product candidates receive regulatory approval in the United States, our clients may never obtain approval or commercialize such drugs
outside of the United States, which would limit their full market potential and therefore our ability to realize their potential downstream
value. Furthermore, approved drugs may not achieve broad market acceptance among physicians, patients, the medical community and third-party
payors, in which case revenue generated from their sales would be limited. Likewise, our clients have to make decisions about which clinical
stage and pre-clinical product candidates to develop and advance, and our clients may not have the resources to invest in all of the product
candidates that contain antibodies discovered using our platform, or clinical data and other development considerations may not support
the advancement of one or more drug candidates. Decision-making about which product candidates to prioritize involves inherent uncertainty,
and our clients’ development program decision-making and resource prioritization decisions, which are outside of our control, may
adversely affect the potential value of those client relationships. Additionally, if one more of our clients is involved in a business
combination, the client might deemphasize or terminate the development or commercialization of any product candidate that utilizes an
antibody that we have discovered. If one of our clients terminates its agreement with us, we may find it more difficult to attract new
clients.
Risks Related to Our Reliance on Third Parties
We face significant risks related to key
third-party relationships.
We plan to enter into agreements
with third parties with respect to our operations. Such relationships could present unforeseen obstacles or costs and may involve risks
that could adversely affect us, including significant amounts of management time that may be diverted from operations in order to pursue
and maintain such relationships. There can be no assurance that such third parties will achieve the expected benefits or that we will
be able to consummate any future relationships on satisfactory terms, or at all. Any of the foregoing could have a material adverse effect
on our business, financial condition and results of operations. Any violation of any applicable laws and regulations, such as the CDSA
and CSA, or of similar legislation in the jurisdictions in which it operates, could result in such third parties to suspend or withdraw
their services. The termination or cancellation of any such agreements or the failure of our business and/or the other parties to these
arrangements to fulfill their obligations could have a material adverse effect on our business, financial condition and results of operations.
In addition, disagreements between us and any of third parties could lead to delays or time consuming and expensive legal proceedings,
which could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Intellectual Property
Failure to obtain or register intellectual
property rights used or proposed to be used in our business could result in a material adverse impact on our business.
If we are unable to register
or, if registered, maintain effective patent rights for certain of our psychedelics-based products and proprietary cultivation and refinement
methods, we may not be able to effectively compete in the market. If we are not able to protect our proprietary information and know-how,
such proprietary information may be used by others to compete against us. We may not be able to identify infringements of our patents
(if and when granted), and, accordingly, the enforcement of our intellectual property rights may be difficult. Once such infringements
are identified, enforcement could be costly and time consuming. Third party claims of intellectual property infringement, whether or not
reasonable, may prevent or delay our development and commercialization efforts.
Our success will depend in
part upon our ability to protect our intellectual property and proprietary technologies and upon the nature and scope of the intellectual
property protection we receive. The ability to compete effectively and to achieve partnerships will depend on our ability to develop and
maintain proprietary aspects of our products and methods and to operate without infringing on the proprietary rights of others. The presence
of such proprietary rights of others could severely limit our ability to develop and commercialize our products and methods and to conduct
our existing research into psychedelics cultivation, extraction and purification, and could require financial resources to defend litigation,
which may be in excess of our ability to raise such funds. There is no assurance that our patent applications submitted, if any, or those
that we intend to acquire will be approved in a form that will be sufficient to protect our proprietary products and technology and gain
or keep any competitive advantage that we may have or, once approved, will be upheld in any post-grant proceedings brought by any third
parties.
The patent positions of biotechnology
companies can be highly uncertain and involve complex legal, scientific and factual questions for which important legal principles remain
unresolved. Patents that may be issued to us may be challenged, invalidated or circumvented. To the extent our intellectual property offers
inadequate protection, or is found to be invalid or unenforceable, we will be exposed to a greater risk of direct competition. If our
intellectual property does not provide adequate protection against our competitors, our competitive position could be adversely affected,
as could our business, financial condition and results of operations. Both the patent application process and the process of managing
patent disputes can be time consuming and expensive, and the laws of some foreign countries may not protect our intellectual property
rights to the same extent as do the laws of Canada and the United States. We will be able to protect our intellectual property from unauthorized
use by third parties only to the extent that our proprietary technologies, key products, and any future products are covered by valid
and enforceable intellectual property rights, including patents, or are effectively maintained as trade secrets, and provided we have
the funds to enforce our rights, if necessary.
Changes in patent law and its interpretation
could diminish the value of potential patents in general, thereby impairing our ability to protect our product candidates.
We may become dependent on
intellectual property rights. Obtaining and enforcing patents in our industry involves technological and legal complexity, and obtaining
and enforcing these potential patents is costly, time consuming and inherently uncertain. The U.S. Supreme Court has ruled on several
patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights
of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future,
this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the
U.S. Congress, the federal courts, and the United States Patent and Trademark Office the laws and regulations governing patents could
change in unpredictable ways that could weaken our ability to obtain new patents or to enforce existing patents.
Litigation regarding patents, patent applications,
and other proprietary rights may be expensive, time consuming and cause delays in the development of our proprietary products and methods.
To protect our competitive
position, we may from time to time need to resort to litigation in order to enforce or defend any patents or other intellectual property
rights owned by or licensed to us, or to determine or challenge the scope or validity of patents or other intellectual property rights
of third parties. Enforcement of intellectual property rights is difficult, unpredictable and expensive, and many of our adversaries in
these proceedings may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we can. We
may fail in enforcing our rights, in which case our competitors and other third parties may be permitted to use our proprietary products
and methods without payment to us.
In addition, litigation involving
our patents carries the risk that one or more of our patents will be subject to an adverse court ruling. Such an adverse court ruling
could allow third parties to commercialize our proprietary products and methods, and then compete directly with us, without payment to
us. Proceedings involving our patents or patent applications or those of others could result in adverse decisions regarding:
| |
● |
the patentability of our inventions relating to our products and methods; and |
| |
|
|
| |
● |
the enforceability, validity, or scope of protection offered by our patents relating to our products and methods. |
If we were to initiate legal
proceedings against a third party to enforce a patent covering one of our investigational therapies, the defendant could counterclaim
that our patent is invalid or unenforceable. In patent litigation in the United States or in Europe, defendant counterclaims alleging
invalidity or unenforceability are commonplace. A claim for a validity challenge may be based on failure to meet any of several statutory
requirements, for example, lack of novelty, obviousness or non-enablement. A claim for unenforceability assertion could be an allegation
that someone connected with prosecution of the patent withheld relevant information from the USPTO or made a misleading statement, during
prosecution. Third parties may also raise challenges to the validity of our patent claims before administrative bodies in the United States
or abroad, even outside the context of litigation. Such mechanisms include re-examination, post-grant review, inter partes review,
interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (i.e., opposition proceedings).
Such proceedings could result in the revocation of, cancellation of, or amendment to our patents in such a way that they no longer cover
our proprietary products or methods. The outcome following legal assertions of invalidity and unenforceability during patent litigation
or other proceedings is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating
prior art, of which we and the patent examiner were unaware during prosecution. If a defendant or third party were to prevail on a legal
assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our products or
methods. Such a loss of patent protection could have a material adverse impact on our business, financial condition, results of operations
and prospects.
If we are unable to avoid
infringing the patent rights of others, we may be required to seek a license, defend an infringement action, or challenge the validity
of the patents in court. Regardless of the outcome, patent litigation is costly and time consuming. In some cases, we may not have sufficient
resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, develop or obtain non-infringing
technology, fail to defend an infringement action successfully or have infringed patents declared invalid, we may:
| |
● |
incur substantial monetary damages; |
| |
|
|
| |
● |
encounter significant delays in bringing our key products and services to market; and |
| |
|
|
| |
● |
be precluded from participating in the manufacture, use or sale of our key products or methods requiring licenses. |
Even if we are successful
in these proceedings, we may incur substantial costs and divert management time and attention in pursuing these proceedings, which could
have a material adverse effect on our business.
Obtaining and maintaining our patent protection
depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent
agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance and
annuity fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the
patent. The USPTO and various foreign governmental patent agencies also require compliance with a number of procedural, documentary, fee
payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment
of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment
or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance
events that could result in abandonment or lapse of a patent or patent application include failure to respond to official actions within
prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we fail to maintain the patents
and patent applications covering our proprietary products and methods, third parties, including our competitors might be able to enter
the market with similar or identical products or methods, which would have a material adverse effect on our business, financial condition,
results of operations and prospects.
We may be subject to claims by third parties
asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own
intellectual property.
Many of our consultants,
advisors and employees, including our senior management, were previously employed at other biotechnology or pharmaceutical companies,
including our competitors and potential competitors. Some of these individuals executed proprietary rights, non-disclosure and non-competition
agreements in connection with such previous employment. Although we intend that our consultants, advisors and employees do not use proprietary
information or know-how of their former employers while working for us, we may be subject to claims that we or these individuals have
used or disclosed confidential information or intellectual property, including trade secrets or other proprietary information, of any
such individual’s former employer. Litigation may be necessary to defend against these claims.
If we fail in prosecuting
or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel or
sustain damages. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from
such third party to commercialize our therapies. Such a license may not be available on commercially reasonable terms or at all. Even
if we successfully prosecute or defend against such claims, litigation could result in substantial costs and distract our management from
its day-to-day activities.
In addition, while it is
our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute
agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact,
conceives or develops intellectual property that we regard as our own. The assignment of intellectual property rights may not be self-executing,
or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may
bring against us, to determine the ownership of what we regard as our intellectual property. Such claims could have a material adverse
effect on our business, financial condition and results of operations.
Confidentiality agreements with employees
and others may not adequately prevent disclosure of trade secrets and protect other proprietary information.
We consider proprietary trade
secrets, confidential know-how and unpatented know-how to be important to our business. We rely on trade secrets or confidential know-how
to protect our technology, especially where patent protection is believed to be of limited value. However, trade secrets and confidential
know-how are difficult to maintain as confidential.
To protect this type of information
against disclosure or appropriation by third parties and our competitors, our policy is to require our employees, consultants, contractors
and advisors to enter into confidentiality agreements with us. However, we cannot guarantee that we have entered into such agreements
with each party that may have or have had access to our trade secrets or confidential know-how. Also, current or former employees, consultants,
contractors and advisers may unintentionally or wilfully disclose our trade secrets and confidential know-how to our competitors and other
third parties or breach such agreements, and we may not be able to obtain an adequate remedy for such breaches. Enforcing a claim that
a third party obtained illegally and is using trade secrets or confidential know-how is difficult, expensive, time-consuming and unpredictable.
The enforceability of confidentiality agreements may vary from jurisdiction to jurisdiction. Furthermore, if a competitor or other third
party lawfully obtained or independently developed any of our trade secrets or confidential know-how, we would have no right to prevent
such competitor or other third party from using that technology or information to compete with us, which could harm our competitive position.
Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third
parties for misappropriating the trade secret. If any of our trade secrets were to be disclosed to or independently developed by a competitor
or other third party, our competitive position would be materially and adversely harmed.
Failure to obtain or maintain
trade secrets or confidential know-how trade protection could adversely affect our competitive position. Moreover, our competitors may
independently develop substantially equivalent proprietary information and may even apply for patent protection in respect of the same.
If successful in obtaining such patent protection, our competitors could limit our use of our trade secrets or confidential know-how.
If our trademarks and trade names are not
adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered
trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks.
We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition by potential partners
or clients in our markets of interest. If we are unable to establish name recognition based on our trademarks and trade names, then we
may not be able to compete effectively and our business may be adversely affected. If other entities use trademarks similar to ours in
different jurisdictions, or have senior rights to ours, it could interfere with our use of our current trademarks throughout the world.
Our reliance on third parties requires us
to share our trade secrets, which increases the possibility that a competitor will discover them.
Because we rely on third
parties, we may share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality
agreements and other similar agreements prior to disclosing proprietary information. These agreements typically restrict the ability to
publish data potentially relating to our trade secrets. Our academic and clinical collaborators typically have rights to publish data,
provided that we are notified in advance and may delay publication for a specified time in order to secure intellectual property rights
arising from the collaboration. In other cases, publication rights are controlled exclusively by us, although in some cases we may share
these rights with other parties. We may also conduct joint research and development programs which may require us to share trade secrets
under the terms of research and development collaborations or similar agreements. Despite our efforts to protect our trade secrets, our
competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information
including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication. A competitor’s
discovery of our trade secrets may impair its competitive position and could have a material adverse effect on our business and financial
condition.
Risks Related to Tax Laws
Changes in tax laws could have a material
adverse effect on our business.
There can be no assurance
that the Canadian and U.S. federal income tax treatment of our business or an investment in us will not be modified, prospectively or
retroactively, by legislative, judicial or administrative action, in a manner adverse to us or holders of common shares.
If we or one of our non-U.S. subsidiaries
is a CFC, there could be materially adverse U.S. federal income tax consequences to certain U.S. Holders of our common shares.
Each “Ten Percent Shareholder”
(as defined below) in a non-U.S. corporation that is classified as a controlled foreign corporation, or a CFC, for U.S. federal income
tax purposes generally may be required to include in income for U.S. federal tax purposes some or all of such Ten Percent Shareholder’s
pro rata share of the CFC’s income even if the CFC has made no distributions to its shareholders. In addition, a Ten Percent Shareholder
that realizes gain from the sale or exchange of shares in a CFC may be required to classify a portion of such gain as dividend income
rather than capital gain. A Ten Percent Shareholder in a CFC also has reporting obligations with respect to the ownership of the stock
in the CFC. Failure to comply with these reporting obligations may subject a Ten Percent Shareholder to significant monetary penalties
and may prevent the statute of limitations with respect to such Ten Percent Shareholder’s U.S. federal income tax return for the
year for which reporting was due from starting.
A non-U.S. corporation generally
will be classified as a CFC for U.S. federal income tax purposes if Ten Percent Shareholders own, directly or indirectly, more than 50%
of either the total combined voting power of all classes of stock of such corporation entitled to vote or of the total value of the stock
of such corporation. A “Ten Percent Shareholder” is a United States person (as defined by the Code) who owns or is considered
to own 10% or more of the total combined voting power of all classes of stock entitled to vote or 10% or more of the total value of all
classes of stock of such corporation.
The determination of CFC
status is complex and includes attribution rules, the application of which is not entirely certain. We cannot provide any assurances that
we will assist holders of our common shares in determining whether we or any of our non-U.S. subsidiaries are treated as a CFC or whether
any holder of the common shares is treated as a Ten Percent Shareholder with respect to any such CFC or furnish to any Ten Percent Shareholders
information that may be necessary to comply with the aforementioned reporting and tax payment obligations.
U.S. Holders should consult
their tax advisors with respect to the potential adverse U.S. tax consequences of becoming a Ten Percent Shareholder in a CFC.
Our U.S. shareholders may suffer adverse
tax consequences if we are characterized as a PFIC.
The rules governing passive
foreign investment companies, or PFICs, can have adverse effects on U.S. Holders (as defined under “Material U.S. Federal Income
Tax Considerations for U.S. Holders”) for U.S. federal income tax purposes. Generally, if, for any taxable year, at least 75% of
our gross income is passive income (such as interest income), or at least 50% of the gross value of our assets (determined on the basis
of a weighted quarterly average) is attributable to assets that produce passive income or are held for the production of passive income
(including cash), we would be characterized as a PFIC for U.S. federal income tax purposes. The determination of whether we are a PFIC,
which must be made annually after the close of each taxable year, depends on the particular facts and circumstances and may also be affected
by the application of the PFIC rules, which are subject to differing interpretations. Our status as a PFIC will depend on the composition
of our income and the composition and value of our assets (including goodwill and other intangible assets), which will be affected by
how, and how quickly, we spend any cash that is raised in any financing transaction. Moreover, our ability to earn specific types of income
that will be treated as non-passive for purposes of the PFIC rules is uncertain with respect to future years. Based upon the current and
expected composition of our income and assets, we believe that we were a PFIC for the taxable year ended June 30, 2022 and could be treated
as a PFIC for the current taxable year. The determination of whether we are a PFIC is a fact-intensive determination made on an annual
basis applying principles and methodologies that in some circumstances are unclear and subject to varying interpretation. Accordingly,
we cannot provide any assurances regarding our PFIC status for any current or future taxable years.
If we are a PFIC, a U.S.
Holder would be subject to adverse U.S. federal income tax consequences, such as ineligibility for certain preferred tax rates on capital
gains or on actual or deemed dividends, interest charges on certain taxes treated as deferred, and additional reporting requirements under
U.S. federal income tax laws and regulations. A U.S. Holder may in certain circumstances mitigate adverse tax consequences of the PFIC
rules by filing an election to treat the PFIC as a qualified electing fund, or QEF, or, if shares of the PFIC are “marketable stock”
for purposes of the PFIC rules, by making a mark-to-market election with respect to the shares of the PFIC. However, U.S. Holders should
be aware that there can be no assurance that we will satisfy the record keeping requirements that apply to a QEF, or that we will supply
U.S. Holders with information that such U.S. Holders require to report under the QEF election rules, in the event that we are a PFIC and
a U.S. Holder wishes to make a QEF election. Thus, U.S. Holders may not be able to make a QEF election with respect to their common shares.
For more information, see the discussion below under “Material U.S. Federal Income Tax Considerations for U.S. Holders – PFIC
Rules.” You are urged to consult your tax advisors regarding the potential consequences to you if we were or were to become a PFIC,
including the availability, and advisability, of, and procedure for making, any elections which may in certain circumstances mitigate
the adverse tax consequences of the PFIC rules.
Tax authorities may disagree with our positions
and conclusions regarding certain tax positions, resulting in unanticipated costs, taxes or non-realization of expected benefits.
A tax authority may disagree
with tax positions that we have taken, which could result in increased tax liabilities. For example, the Canadian tax authority, the IRS
or another tax authority could challenge our allocation of income by tax jurisdiction and the amounts paid between our affiliated companies
pursuant to an intercompany arrangement or a transfer pricing policy, including amounts paid with respect to our intellectual property
development. Similarly, a tax authority could assert that we are subject to tax in a jurisdiction where we believe we have not established
a taxable connection, often referred to as a “permanent establishment” under international tax treaties, and such an assertion,
if successful, could increase our expected tax liability in one or more jurisdictions. A tax authority may take the position that material
income tax liabilities, interest and penalties are payable by us, in which case, we expect that we might contest such assessment. Contesting
such an assessment may be lengthy and costly and if we were unsuccessful in disputing the assessment, the implications could increase
our anticipated effective tax rate, where applicable.
We are subject to certain tax risks and
treatments that could negatively impact our results of operations.
We may operate in the United
States or through a U.S. subsidiary. If we or our subsidiaries are subject to U.S. corporate income tax, Section 280E of the Internal
Revenue Code of 1986, as amended, or the Code, generally prohibits taxpayers from deducting or claiming tax credits with respect to expenses
paid or incurred in carrying on any trade or business if such trade or business (or the activities which comprise such trade or business)
consists of trafficking in controlled substances (within the meaning of Schedule I and II of the CSA) which is prohibited by U.S. federal
law or the law of any state in which such trade or business is conducted. The application of Code section 280E generally causes such businesses
to pay higher effective U.S. federal tax rates than similar businesses in other industries. Although the U.S. Internal Revenue Service,
or IRS, issued a clarification allowing the deduction of certain expenses, the scope of such items is interpreted very narrowly and the
bulk of operating costs and general administrative costs are not permitted to be deducted. There is no guarantee that any federal court
will issue an interpretation of Section 280E favorable to psilocybin and psilocin businesses.
Risks Related to Ownership of Our Common Shares
We may experience extreme stock price volatility
unrelated to our actual or expected operating performance, financial condition or prospects, making it difficult for prospective investors
to assess the rapidly changing value of our ordinary shares.
Recently, there have been
instances of extreme stock price run-ups followed by rapid price declines and strong stock price volatility with a number of recent initial
public offerings, especially among companies with relatively smaller public floats. As a relatively small-capitalization company with
relatively small public float, we may experience greater stock price volatility, extreme price run-ups, lower trading volume and less
liquidity than large-capitalization companies. In particular, our common shares may be subject to rapid and substantial price volatility,
low volumes of trades and large spreads in bid and ask prices. Such volatility, including any stock-run up, may be unrelated to our actual
or expected operating performance, financial condition or prospects, making it difficult for prospective investors to assess the rapidly
changing value of our common shares.
In addition, if the trading
volumes of our common shares are low, persons buying or selling in relatively small quantities may easily influence prices of our common
shares. This low volume of trades could also cause the price of our common shares to fluctuate greatly, with large percentage changes
in price occurring in any trading day session. Holders of our common shares may also not be able to readily liquidate their investment
or may be forced to sell at depressed prices due to low volume trading. Broad market fluctuations and general economic and political conditions
may also adversely affect the market price of our common shares. As a result of this volatility, investors may experience losses on their
investment in our common shares. A decline in the market price of our common shares also could adversely affect our ability to issue additional
shares of common shares or other securities and our ability to obtain additional financing in the future. No assurance can be given that
an active market in our common shares will develop or be sustained. If an active market does not develop, holders of our common shares
may be unable to readily sell the shares they hold or may not be able to sell their shares at all.
The market price of our common shares may
be volatile or may decline regardless of our operating performance.
The market price of our common
shares may fluctuate significantly in response to numerous factors, many of which are beyond our control, including:
| ● | actual or anticipated fluctuations
in our revenue and other operating results; |
| ● | the financial projections we
may provide to the public, any changes in these projections or our failure to meet these projections; |
| ● | actions of securities analysts
who initiate or maintain coverage of us, changes in financial estimates by any securities analysts who follow our company, or our failure
to meet these estimates or the expectations of investors; |
| ● | announcements by us or our
competitors of significant services or features, technical innovations, acquisitions, strategic partnerships, joint ventures, or capital
commitments; |
| ● | price and volume fluctuations
in the overall stock market, including as a result of trends in the economy as a whole; |
| ● | lawsuits threatened or filed
against us; and |
| ● | other events or factors, including
those resulting from war or incidents of terrorism, or responses to these events. |
In addition, the stock markets
have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities
of many companies. Stock prices of many companies have fluctuated in a manner unrelated or disproportionate to the operating performance
of those companies. In the past, shareholders have filed securities class action litigation following periods of market volatility. If
we were to become involved in securities litigation, it could subject us to substantial costs, divert resources and the attention of management
from our business, and adversely affect our business.
The prices at which the common shares will
trade cannot be predicted.
Securities will not necessarily
trade at values determined by reference to the underlying value of our business. The market price of the common shares could be subject
to significant fluctuations in response to a variety of factors, including the following: actual or anticipated fluctuations in our quarterly
results of operations; recommendations by securities research analysts; changes in the economic performance or market valuations of companies
in the industry in which we operate; additions or departures by our executive officers and other key personnel; significant acquisitions
or business combinations, strategic partnerships, joint ventures or capital commitments by or involving our business or our competitors;
operating and share price performance of other companies that investors deem comparable to us; fluctuations caused by COVID-19; and news
reports relating to trends, concerns, technological or competitive developments, regulatory changes and other related issues in our industry
or target markets.
The securities markets have
experienced significant price and volume fluctuations from time to time in recent years that often have been unrelated or disproportionate
to the operating performance of particular issuers. These broad fluctuations may adversely affect the market price of the common shares.
In addition, the market prices for securities of biopharmaceutical companies, in particular, have historically been volatile. Factors
such as industry related developments, the results of product development and commercialization, changes in government regulations, developments
concerning proprietary rights, the timing of costs for manufacturing, pre-clinical studies and clinical trials, the reporting of adverse
safety events involving our products and public rumors about such events and changes in the market prices of the securities of our competitors
may further influence the volatility in the trading price of the common shares.
The issuance of securities could result
in significant dilution in the equity interest of existing shareholders and adversely affect the marketplace of the securities.
The issuance of common shares
or other securities convertible into common shares could result in significant dilution in the equity interest of existing shareholders
and adversely affect the market price of the common shares. In addition, in the future, we may issue additional common shares or securities
convertible into common shares, which may dilute existing shareholders. Our Articles of Incorporation (“Articles”) permit
the issuance of an unlimited number of common shares and shareholders will have no pre-emptive rights in connection with such further
issuances.
The market price of the common
shares could decline as a result of future issuances, including issuance of shares issued in connection with strategic alliances, or sales
by our existing holders of common shares, or the perception that these sales could occur. Sales by shareholders might also make it more
difficult for us to sell equity securities at a time and price that it deems appropriate, which could reduce our ability to raise capital
and have an adverse effect on our business.
We have a material weakness in our internal
control over financing reporting. If we fail to establish and maintain proper and effective internal control over financial reporting,
our operating results and our ability to operate our business could be harmed.
Ensuring that we have adequate
internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis
is a costly and time-consuming effort that needs to be re-evaluated frequently. Our internal control over financial reporting is a process
designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements
in accordance with generally accepted accounting principles. Due to accounting resource constraints, we currently have a material weakness
in our internal control over financial reporting. Our control environment is currently oriented primarily towards business risks, rather
than financial reporting risks. We have not formally implemented risk assessment or monitoring controls, and information and communication
controls and certain review controls are not considered to be operating effectively. Resource constraints have also resulted in insufficient
segregation of duties in certain areas.
We intend to begin the process
of documenting, reviewing and improving our internal controls and procedures for compliance with Section 404 of the Sarbanes-Oxley Act
of 2002, as amended, or the Sarbanes-Oxley Act, and applicable Canadian laws, which will require annual management assessment of the effectiveness
of our internal control over financial reporting. We have begun recruiting additional finance and accounting personnel with certain skill
sets that we will need as a public company.
Implementing any appropriate
changes to our internal controls may distract our officers and employees, entail substantial costs to modify our existing processes, and
take significant time to complete. These changes may not, however, be effective in maintaining the adequacy of our internal controls,
and any failure to maintain that adequacy, or consequent inability to produce accurate financial statements on a timely basis, could increase
our operating costs and harm our business. In our efforts to maintain proper and effective internal control over financial reporting,
we may discover additional significant deficiencies or material weaknesses in our internal control over financial reporting, which we
may not successfully remediate on a timely basis or at all. Any failure to remediate any significant deficiencies or material weaknesses
identified by us or to implement required new or improved controls, or difficulties encountered in their implementation, could cause us
to fail to meet our reporting obligations or result in material misstatements in our financial statements. If we identify one or more
material weaknesses in the future, it could result in an adverse reaction in the financial markets due to a loss of confidence in the
reliability of our financial statements, which may harm the market price of our shares.
Future sales and issuances of our common
shares or rights to purchase common shares, including pursuant to our 2021 Equity Incentive Plan, or our 2021 Plan, could result in additional
dilution of the percentage ownership of our shareholders and could cause our share price to fall.
We expect that significant
additional capital will be needed in the future to continue our planned operations, including expanded research and development activities,
and costs associated with operating as a public company. To raise capital, we may sell common shares, convertible securities or other
equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common shares, convertible
securities or other equity securities, investors may be materially diluted by subsequent sales. Such sales may also result in material
dilution to our existing shareholders, and new investors could gain rights, preferences, and privileges senior to the holders of our common
shares.
Pursuant to the 2021 Plan
our management is authorized to grant share purchase options, restricted stock units or stock appreciation rights to our employees, directors
and consultants up to an amount of 2,172,279 common shares.
We do not intend to pay dividends on our
common shares, so any returns will be limited to the value of our common shares.
We currently anticipate that
we will retain future earnings for the development, operation, expansion and continued investment into our business and do not anticipate
declaring or paying any cash dividends for the foreseeable future. In addition, we may enter into agreements that prohibit us from paying
cash dividends without prior written consent from our contracting parties, or which other terms prohibiting or limiting the amount of
dividends that may be declared or paid on our common shares. Any return to shareholders will therefore be limited to the appreciation
of their common shares, which may never occur.
Our principal shareholders and management
own a significant percentage of our shares and will be able to exert significant influence over matters subject to shareholder approval.
Based on the number of shares
outstanding on a fully diluted basis as of June 30, 2023, our executive officers, directors and director nominees, and 5% shareholders
beneficially own approximately 16.97% of our common shares. Non-executive employees and consultants beneficially own an additional 0.15%
of our common shares on a fully diluted basis. Therefore, these shareholders will have the ability to influence us through this ownership
position. These shareholders may be able to determine all matters requiring shareholder approval. For example, these shareholders may
be able to control elections of directors, amendments of our organizational documents or approval of any merger, sale of assets or other
major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common shares that you
may feel are in your best interest as one of our shareholders.
We are an emerging growth company and a
smaller reporting company, and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies and
smaller reporting companies will make our common shares less attractive to investors.
We are an emerging growth
company, as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions
from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not
being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 reduced disclosure
obligations regarding executive compensation in our periodic reports and proxy statements, exemptions from the requirements of holding
nonbinding advisory votes on executive compensation and shareholder approval of any golden parachute payments not previously approved,
and an exemption from compliance with the requirement of the Public Accounting Oversight Board regarding the communication of critical
audit matters in the auditor’s report on the financial statements. We could be an emerging growth company for up to five years following
the year in which we complete our initial public offering, although circumstances could cause us to lose that status earlier. We will
remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the
date of the closing of our initial public offering, (b) in which we have total annual gross revenue of at least $1.235 billion or (c)
in which we are deemed to be a large accelerated filer, which requires the market value of our common shares that are held by non-affiliates
to exceed $700.0 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible
debt during the prior three-year period.
Further, even after we no
longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company,” which would allow us
to take advantage of many of the same exemptions from disclosure requirements, including reduced disclosure obligations regarding executive
compensation in our periodic reports and proxy statements. In addition, if we are a smaller reporting company with less than $100.0 million
in annual revenue, we would not be required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act,
or Section 404.
We cannot predict if investors
will find our common shares less attractive because we may rely on these exemptions. If some investors find our common shares less attractive
as a result, there may be a less active trading market for our common shares and our share price may be more volatile.
Sales of a substantial number of our common
shares by our existing shareholders in the public market could cause our share price to fall.
The lock-up agreements pertaining
to the IPO expired in August. If our existing shareholders sell, or indicate an intention to sell, substantial amounts of our common shares
in the public market, the trading price of our common shares could decline.
If our estimates or judgments relating to
our critical accounting policies prove to be incorrect or financial reporting standards or interpretations change, our results of operations
could be adversely affected.
The preparation of financial
statements in conformity with generally accepted accounting principles in the United States, or U.S. GAAP, requires management to make
estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. We base our
estimates on historical experience, known trends and events, and various other factors that we believe to be reasonable under the circumstances,
as provided in “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Critical Accounting
Policies and Estimates.” The results of these estimates form the basis for making judgments about the carrying values of assets
and liabilities that are not readily apparent from other sources. Significant assumptions and estimates used in preparing our consolidated
financial statements include share-based payments, provision for income taxes and useful lives of property, plant and equipment and intangibles.
Our results of operations may be adversely affected if our assumptions change or if actual circumstances differ from those in our assumptions,
which could cause our results of operations to fall below the expectations of securities analysts and investors, resulting in a decline
in the trading price of our common shares.
Additionally, we regularly
monitor our compliance with applicable financial reporting standards and review new pronouncements and drafts thereof that are relevant
to us. As a result of new standards, changes to existing standards and changes in their interpretation, we might be required to change
our accounting policies, alter our operational policies, and implement new or enhance existing systems so that they reflect new or amended
financial reporting standards, or we may be required to restate our published financial statements. Such changes to existing standards
or changes in their interpretation may have an adverse effect on our reputation, business, financial position, and profit.
Our disclosure controls and procedures may
not prevent or detect all errors or acts of fraud.
Our disclosure controls and
procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange
Act is accumulated and communicated to management, recorded, processed, summarized, and reported within the time periods specified in
the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter
how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because
of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or
more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements
or insufficient disclosures due to error or fraud may occur and not be detected.
If securities or industry analysts do not
publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our
common shares will depend in part on the research and reports that securities or industry analysts publish about us or our business. Securities
and industry analysts do not currently, and may never, publish research on our company. If no securities or industry analysts commence
coverage of our company, the trading price for our common shares would likely be negatively impacted. In the event securities or industry
analysts initiate coverage, if one or more of the analysts who cover us downgrades our common shares or publishes inaccurate or unfavorable
research about our business, our share price may decline. If one or more of these analysts ceases coverage of our company or fails to
publish reports on us regularly, demand for our shares could decrease, which might cause our share price and trading volume to decline.
Risks Related to Investment in a Canadian Company
We are governed by the corporate laws of
Canada which in some cases have a different effect on shareholders than the corporate laws of the United States.
We are governed by the Business
Corporations Act (British Columbia), or BCBCA, and other relevant federal and municipal laws, which may affect the rights of shareholders
differently than those of a company governed by the laws of a U.S. jurisdiction, and may, together with our charter documents, have the
effect of delaying, deferring or discouraging another party from acquiring control of our company by means of a tender offer, a proxy
contest or otherwise, or may affect the price an acquiring party would be willing to offer in such an instance. The material differences
between the BCBCA and Delaware General Corporation Law, or DGCL, that may have the greatest such effect include, but are not limited to,
the following: (i) for certain corporate transactions (such as mergers and amalgamations or amendments to our Articles) the BCBCA generally
requires the voting threshold to be a special resolution approved by 66 2/3% of shareholders, or as set out in the Articles, as applicable,
whereas DGCL generally only requires a majority vote; and (ii) under the BCBCA a holder of 5% or more of our common shares can requisition
a special meeting of shareholders, whereas such right does not exist under the DGCL. We cannot predict whether investors will find our
company and our common shares less attractive because we are governed by foreign laws.
Our Articles and certain Canadian legislation
contain provisions that may have the effect of delaying, preventing or making undesirable an acquisition of all or a significant portion
of our shares or assets or preventing a change in control.
Certain provisions of our
Articles and certain provisions under the BCBCA, together or separately, could discourage, delay or prevent a merger, acquisition or other
change in control of us that shareholders may consider favorable, including transactions in which they might otherwise receive a premium
for their common shares. These provisions include the establishment of a staggered board of directors, which divides the board into three
groups, with directors in each group serving a three-year term. The existence of a staggered board can make it more difficult for shareholders
to replace or remove incumbent members of our board of directors. As such, these provisions could also limit the price that investors
might be willing to pay in the future for our common shares, thereby depressing the market price of our common shares. In addition, because
our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any
attempts by our shareholders to replace or remove our current management by making it more difficult for shareholders to replace members
of our board of directors. Among other things, these provisions include the following:
| ● | shareholders cannot amend our
Articles unless such amendment is approved by shareholders holding at least 66 2/3% of the shares entitled to vote on such approval; |
| ● | our board of directors may,
without shareholder approval, issue preferred shares in one or more series having any terms, conditions, rights, preferences and privileges
as the board of directors may determine; and |
| ● | shareholders must give advance
notice to nominate directors or to submit proposals for consideration at shareholders’ meetings. |
A non-Canadian must file
an application for review with the Minister responsible for the Investment Canada Act and obtain approval of the Minister prior
to acquiring control of a “Canadian business” within the meaning of the Investment Canada Act, where prescribed financial
thresholds are exceeded. A reviewable acquisition may not proceed unless the Minister is satisfied that the investment is likely to be
of net benefit to Canada. If the applicable financial thresholds were exceeded such that a net benefit to Canada review would be required,
this could prevent or delay a change of control and may eliminate or limit strategic opportunities for shareholders to sell their common
shares. Furthermore, limitations on the ability to acquire and hold our common shares may be imposed by the Competition Act (Canada).
This legislation has a pre-merger notification regime and mandatory waiting period that applies to certain types of transactions that
meet specified financial thresholds, and permits the Commissioner of Competition to review any acquisition or establishment, directly
or indirectly, including through the acquisition of shares, of control over or of a significant interest in us.
Our Articles designate specific courts in
Canada and the United States as the exclusive forum for certain litigation that may be initiated by our shareholders, which could limit
our shareholders’ ability to obtain a favorable judicial forum for disputes with us.
Pursuant to our Articles,
unless we consent in writing to the selection of an alternative forum, the courts of the Province of British Columbia and the appellate
courts therefrom shall, to the fullest extent permitted by law, be the sole and exclusive forum for: (a) any derivative action or proceeding
brought on our behalf; (b) any action or proceeding asserting a claim of breach of fiduciary duty owed by any director, officer or other
employee of ours to us; (c) any action or proceeding asserting a claim arising out of any provision of the BCBCA or our Articles (as either
may be amended from time to time); or (d) any action or proceeding asserting a claim or otherwise related to our affairs, or the Canadian
Forum Provision. The Canadian Forum Provision will not apply to any causes of action arising under the Securities Act or the Exchange
Act. In addition, our Articles provide that unless we consent in writing to the selection of an alternative forum, the United States District
Court for the District of Delaware shall be the sole and exclusive forum for resolving any complaint filed in the United States asserting
a cause of action arising under the Securities Act, or the U.S. Federal Forum Provision. In addition, our Articles provide that any person
or entity purchasing or otherwise acquiring any interest in our common shares is deemed to have notice of and consented to the Canadian
Forum Provision and the U.S. Federal Forum Provision; provided, however, that shareholders cannot and will not be deemed to have waived
our compliance with the U.S. federal securities laws and the rules and regulations thereunder.
The Canadian Forum Provision
and the U.S. Federal Forum Provision in our Articles may impose additional litigation costs on shareholders in pursuing any such claims.
Additionally, the forum selection clauses in our amended Articles may limit our shareholders’ ability to bring a claim in a judicial
forum that they find favorable for disputes with us or our directors, officers or employees, which may discourage the filing of lawsuits
against us and our directors, officers and employees, even though an action, if successful, might benefit our shareholders. In addition,
while the Delaware Supreme Court ruled in March 2020 that federal forum selection provisions purporting to require claims under the Securities
Act be brought in federal court are “facially valid” under Delaware law, there is uncertainty as to whether other courts,
including courts in Canada and other courts within the United States, will enforce our U.S. Federal Forum Provision. If the U.S. Federal
Forum Provision is found to be unenforceable, we may incur additional costs associated with resolving such matters. The U.S. Federal Forum
Provision may also impose additional litigation costs on shareholders who assert that the provision is not enforceable or invalid. The
courts of the Province of British Columbia and the United States District Court for the District of Delaware may also reach different
judgments or results than would other courts, including courts where a shareholder considering an action may be located or would otherwise
choose to bring the action, and such judgments may be more or less favorable to us than our shareholders.
Because we are a Canadian company, it may
be difficult to serve legal process or enforce judgments against us.
We are incorporated and maintain
operations in Canada. In addition, while certain of our directors and officers reside in the United States, many of them reside outside
of the United States. Accordingly, service of process upon us may be difficult to obtain within the United States. Furthermore, because
substantially all of our assets are located outside the United States, any judgment obtained in the United States against us, including
one predicated on the civil liability provisions of the U.S. federal securities laws, may not be collectible within the United States.
Therefore, it may not be possible to enforce those actions against us.
In addition, it may be difficult
to assert U.S. securities law claims in original actions instituted in Canada. Canadian courts may refuse to hear a claim based on an
alleged violation of U.S. securities laws against us or these persons on the grounds that Canada is not the most appropriate forum in
which to bring such a claim. Even if a Canadian court agrees to hear a claim, it may determine that Canadian law and not U.S. law is applicable
to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming
and costly process. Certain matters of procedure will also be governed by Canadian law. Furthermore, it may not be possible to subject
foreign persons or entities to the jurisdiction of the courts in Canada. Similarly, to the extent that our assets are located in Canada,
investors may have difficulty collecting from us any judgments obtained in the U.S. courts and predicated on the civil liability provisions
of U.S. securities provisions.
We may be adversely affected by fluctuations
in the U.S. dollar relative to the Canadian dollar.
Our revenues and expenses
are expected to be primarily denominated in U.S. dollars, and therefore may be exposed to significant currency exchange fluctuations.
The Canadian dollar relative to the U.S. dollar or other foreign currencies is subject to fluctuations. Fluctuations in the exchange rate
between the U.S. dollar and the Canadian dollar may have a material adverse effect on our business, financial condition or results of
operations. We may, in the future, establish a program to hedge a portion of our foreign currency exposure with the objective of minimizing
the impact of adverse foreign currency exchange movements. However, even if we develop a hedging program, there can be no assurance that
it will effectively mitigate currency risks. Failure to adequately manage foreign exchange risk could therefore have a material adverse
effect on our business, financial condition or results of operations.
General Risks
We may expand our business through the acquisition
of companies or businesses or by entering into collaborations, each of which could disrupt our business and harm our financial condition
We may in the future seek
to expand our capabilities by acquiring one or more companies or businesses or entering into collaborations. Acquisitions and collaborations
involve numerous risks, including, but not limited to: substantial cash expenditures; technology development risks; potentially dilutive
issuances of equity securities; incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify
at the time of acquisition; difficulties in assimilating the operations of the acquired companies; potential disputes regarding contingent
consideration; diverting our management’s attention away from other business concerns; entering markets in which we have limited
or no direct experience; and potential loss of our key employees or key employees of the acquired companies or businesses.
Our management has experience
in making acquisitions and entering collaborations; however, we cannot provide assurance that any acquisition or collaboration will result
in short-term or long-term benefits to us. We may incorrectly judge the value or worth of an acquired company or business. In addition,
our future success would depend in part on our ability to manage the rapid growth associated with some of these acquisitions and collaborations.
We cannot provide assurance that we would be able to successfully combine our business with that of acquired businesses or manage a collaboration.
Furthermore, the development or expansion of our business may require a substantial capital investment by us.
We may be negatively impacted by challenging
global economic conditions.
Our business, financial condition,
results of operations and cash flow may be negatively impacted by challenging global economic conditions.
A global economic slowdown
would cause disruptions and extreme volatility in global financial markets, increased rates of default and bankruptcy and declining consumer
and business confidence, which can lead to decreased levels of consumer spending. These macroeconomic developments could negatively impact
our business, which depends on the general economic environment. As a result, we may not be able to maintain our existing clients or attract
new clients, or we may be forced to reduce the price of our products. We are unable to predict the likelihood of the occurrence, duration
or severity of such disruptions in the credit and financial markets or adverse global economic conditions. Any general or market-specific
economic downturn could have a material adverse effect on our business, financial condition and results of operations.
Additionally, the United
States has imposed and may impose additional quotas, duties, tariffs, retaliatory or trade protection measures or other restrictions or
regulations and may adversely adjust prevailing quota, duty or tariff levels, which can affect both the materials that we use to package
our products and the sale of finished products. Measures to reduce the impact of tariff increases or trade restrictions, including geographical
diversification of our sources of supply, adjustments in packaging design and fabrication or increased prices, could increase our costs,
delay our time to market and/or decrease sales. Other governmental action related to tariffs or international trade agreements has the
potential to adversely impact demand for our products and our costs, customers, suppliers and global economic conditions and cause higher
volatility in financial markets. While we actively review existing and proposed measures to seek to assess the impact of them on our business,
changes in tariff rates, import duties and other new or augmented trade restrictions could have a number of negative impacts on our business,
including higher prices and reduced demand for our products and higher input costs.
Our future growth and ability to compete
effectively depends on retaining our key personnel and recruiting additional qualified personnel, and on the key personnel employed by
our collaborative partners.
Our success depends upon
the continued contributions of our key management, scientific and technical personnel, many of whom have been instrumental for us and
have substantial experience with our therapies and related technologies. These key management individuals include the members of our board
of directors and certain executive officers. We do not currently maintain any key person insurance.
The loss of key managers
and senior scientists could delay our research and development activities. In addition, our ability to compete in the highly competitive
biotechnology industry depends upon our ability to attract and retain highly qualified management, scientific and medical personnel. Many
other companies and academic institutions that we compete against for qualified personnel have greater financial and other resources,
different risk profiles and a longer history in the industry than we do. Therefore, we might not be able to attract or retain these key
persons on conditions that are economically acceptable. Moreover, some qualified prospective employees may choose not to work for us due
to negative perceptions regarding the therapeutic use of psychedelic substances or other objections to the therapeutic use of a controlled
substance. Furthermore, we will need to recruit new managers and qualified scientific personnel to develop our business if we expand into
fields that will require additional skills. Our inability to attract and retain these key persons could prevent us from achieving our
objectives and implementing our business strategy, which could have a material adverse effect on our business and prospects.
We expect to experience significant
growth in the number of our employees and the scope of our operations, particularly in the area of research and development. To manage
our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our
facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources, we may not be able
to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations
may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay
the execution of our business plans or disrupt our operations.
We may be subject to growth-related risks including pressure
on our internal systems and controls.
Our ability to manage our
growth effectively will require us to continue to implement and improve our operational and financial systems and to expand, train and
manage our employee base. Our inability to deal with this growth could have a material adverse impact on our business, operations and
prospects. We may experience growth in the number of our employees and the scope of our operating and financial systems, resulting in
increased responsibilities for our personnel, the hiring of additional personnel and, in general, higher levels of operating expenses.
In order to manage our future growth effectively, we will also need to continue to implement and improve our operational, financial and
management information systems and to hire, train, motivate, manage and retain our employees. There can be no assurance that we will be
able to manage such growth effectively, that our management, personnel or systems will be adequate to support our operations or that we
will be able to achieve the increased levels of revenue commensurate with the increased levels of operating expenses associated with this
growth.
Security breaches, loss of data and other
disruptions could compromise sensitive information related to our business or prevent us from accessing critical information and expose
us to liability, which could adversely affect our business and our reputation.
In the ordinary course of
our business, we generate and store sensitive data, including research data, intellectual property and proprietary business information
owned or controlled by ourselves or our employees, partners and other parties. We manage and maintain our applications and data utilizing
a combination of on-site systems and cloud-based data centers. We utilize external security and infrastructure vendors to manage parts
of our data centers. These applications and data encompass a wide variety of business-critical information, including research and development
information, commercial information and business and financial information. We face a number of risks relative to protecting this critical
information, including loss of access risk, inappropriate use or disclosure, accidental exposure, unauthorized access, inappropriate modification
and the risk of our being unable to adequately monitor and audit and modify our controls over our critical information. This risk extends
to the third-party vendors and subcontractors we use to manage this sensitive data or otherwise process it on our behalf. Further, to
the extent our employees are working at home during the COVID-19 pandemic, additional risks may arise as a result of depending on the
networking and security put into place by the employees. The secure processing, storage, maintenance and transmission of this critical
information are vital to our operations and business strategy, and we devote significant resources to protecting such information. Although
we take reasonable measures to protect sensitive data from unauthorized access, use or disclosure, no security measures can be perfect
and our information technology and infrastructure may be vulnerable to attacks by hackers or infections by viruses or other malware or
breached due to employee erroneous actions or inactions by our employees or contractors, malfeasance or other malicious or inadvertent
disruptions. Any such breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized
parties, publicly disclosed, lost or stolen. Any such access, breach, or other loss of information could result in legal claims or proceedings.
Unauthorized access, loss or dissemination could also disrupt our operations and damage our reputation, any of which could adversely affect
our business.
Additionally, we do not currently
maintain cybersecurity insurance coverage. Even if we were to obtain such coverage, we cannot be certain that such coverage will be adequate
for data security liabilities actually incurred, will cover any indemnification claims against us relating to any incident, will continue
to be available to us on economically reasonable terms, or at all, or that any insurer will not deny coverage as to any future claim.
The successful assertion of one or more large claims against us that exceed available insurance coverage, or the occurrence of changes
in our insurance policies, including premium increases or the imposition of large deductible or co-insurance requirements, could adversely
affect our reputation, business, financial condition and results of operations.
In certain circumstances, our reputation
could be damaged.
Damage to our reputation
can be the result of the actual or perceived occurrence of any number of events, and could include any negative publicity, whether true
or not. The increased usage of social media and other web-based tools used to generate, publish and discuss user-generated content and
to connect with other users has made it increasingly easier for individuals and groups to communicate and share opinions and views regarding
us and our activities, whether true or not. Although we believe that we operate in a manner that is respectful to all stakeholders and
that we take care in protecting our image and reputation, we do not ultimately have direct control over how we are perceived by others.
Reputation loss may result in decreased investor confidence, increased challenges in developing and maintaining community relations and
an impediment to our overall ability to advance our projects, thereby having a material adverse impact on financial performance, financial
condition, cash flows and growth prospects.
We use biological and hazardous materials
that require considerable expertise and expense for handling, storage and disposal and may result in claims against us.
We work with materials, including
chemicals, biological agents and compounds that could be hazardous to human health and safety or the environment. Our operations also
produce hazardous and biological waste products. Federal, provincial, state and local laws and regulations govern the use, generation,
manufacture, storage, handling and disposal of these materials and wastes. We are subject to periodic inspections by Canadian provincial
and federal authorities to ensure compliance with applicable laws. Compliance with applicable environmental laws and regulations is expensive,
and current or future environmental laws and regulations may restrict our operations. If we do not comply with applicable regulations,
we may be subject to fines and penalties.
In addition, we cannot eliminate
the risk of accidental injury or contamination from these materials or wastes, which could cause an interruption of our commercialization
efforts, research and development programs and business operations, as well as environmental damage resulting in costly clean-up and liabilities
under applicable laws and regulations. In the event of contamination or injury, we could be liable for damages or penalized with fines
in an amount exceeding our resources and our operations could be suspended or otherwise adversely affected. Furthermore, environmental
laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes
and cannot be certain of our future compliance.
We will incur increased costs as a result
of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate
governance practices.
As a public company will
incur significant legal, accounting, and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, or
Sarbanes-Oxley, the Dodd-Frank Wall Street Reform, and Consumer Protection Act, the listing requirements of Nasdaq, and other applicable
securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective
disclosure and financial controls and corporate governance practices. We will have to hire additional accounting, finance, and other personnel
in connection with our efforts to comply with the requirements of being a public company and our management and other personnel devote
a substantial amount of time towards maintaining compliance with these requirements. These requirements will increase our legal and financial
compliance costs and make some activities more time-consuming and costly. These rules and regulations are often subject to varying interpretations,
in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is
provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs
necessitated by ongoing revisions to disclosure and governance practices.
In addition, Sarbanes-Oxley,
as well as rules subsequently adopted by the SEC and Nasdaq to implement provisions of Sarbanes-Oxley, impose significant requirements
on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate
governance practices. Further, pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, the SEC has adopted
additional rules and regulations in these areas, such as mandatory “say on pay” voting requirements that are applicable to
us. Stockholder activism, the current political environment, and the current high level of government intervention and regulatory reform
may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner
in which we operate our business in ways we cannot currently anticipate.
If these requirements divert
the attention of our management and personnel from other business concerns, they could have a material adverse effect on our business,
financial condition, and results of operations. The increased costs could impact our results of operations and may require us to reduce
costs in other areas of our business or increase the prices of our products or services. For example, these rules and regulations make
it more difficult and more expensive for us to obtain director and officer liability insurance. We cannot predict or estimate the amount
or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more
difficult for us to attract and retain qualified persons to serve on our Board of Directors, our board committees, or as executive officers.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
We
do not own any real property.
Our
principal executive office is located at 301-1321 Blanshard Street, Victoria, British Columbia, Canada V8W 0B6. Our research, development,
and manufacturing facility is located near Victoria, British Columbia, Canada. We rent approximately 25,000 square feet of space which
includes compartmentalized production bays, testing and analytics laboratories, and dedicated office space.
We
believe that our facilities are generally in good condition and suitable to carry on our business.
ITEM 3. LEGAL PROCEEDINGS
We are not aware of
any pending legal actions that would, if determined adversely to us, have a material adverse effect on our business and operations.
We may, from time to time, become involved in
disputes and proceedings arising in the ordinary course of business. In addition, as a public company, we are also potentially susceptible
to litigation, such as claims asserting violations of securities laws. Any such claims, with or without merit, if not resolved, could
be time-consuming and result in costly litigation. There can be no assurance that an adverse result in any future proceeding would not
have a potentially material adverse effect on our business, results of operations, and financial condition.
ITEM 4. MINE SAFETY DISCLOSURES
Not Applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON
EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
The Common Stock began trading
on the Nasdaq Capital Market under the symbol LSDI on February 9, 2023.
As of June 30, 2023, there
were approximately 258 holders of record of our Common Stock. Since certain shares of our Common Stock are held by brokers and other institutions
on behalf of stockholders, the foregoing number of holders of our Common Stock is not representative of the number of beneficial holders
of our Common Stock.
The last reported sales price
for our Common Stock as reported on the Nasdaq Capital Market on October 12, 2023 was $0.395.
Dividends
We have not declared or paid
any cash dividends on our Common Stock, and we do not anticipate declaring or paying cash dividends for the foreseeable future. We are
not subject to any legal restrictions respecting the payment of dividends, except that we may not pay dividends if the payment would render
us insolvent. Any future determination as to the payment of cash dividends on our Common Stock will be at the discretion of our Board
and will depend on our financial condition, operating results, capital requirements and other factors that the Board considers to be relevant.
Securities Authorized
for Issuance under Equity Compensation Plans
The table below sets forth
information with respect to compensation plans under which equity securities of the Company are authorized for issuance as of June 30,
2023:
| Plan Category | |
Number of
securities to be
issued upon
exercise of
outstanding
options and
rights (a) | | |
Weighted-average
exercise price of
outstanding
options and rights (b) | | |
Number of
securities
remaining
available for
future issuance
under equity
compensation
plans (excluding
securities
reflected in
column (a)(b) | |
| Equity compensation plans approved by shareholders | |
| 196,667 | | |
| 2.08 | | |
| — | |
| Equity compensation plans not approved by shareholders | |
| 394,448 | | |
| 2.51 | | |
| 2,028,329 | |
| Total | |
| 591,115 | | |
| 2.20 | | |
| 2,028,329 | |
Equity Compensation
Plans
2021 Equity Incentive Plan
Our Lucy Scientific Discovery
Inc. 2021 Equity Incentive Plan, or the 2021 Plan, became effective upon the effectiveness of the Registration Statement on February 18,
2023. A summary of the material terms of the 2021 Plan follows below.
The 2021 Plan authorizes the
award of both equity-based and cash-based incentive awards, including: (i) stock options (both incentive stock options and nonqualified
stock options), (ii) stock appreciation rights, or SARs, (iii) restricted stock awards, or RSAs, (iv) restricted stock units,
or RSUs, and (v) cash or other stock based awards. Incentive stock options may be granted only to employees. All other types of awards
may be issued to employees, directors, consultants and other service providers.
Shares Subject to 2021 Plan. We
have reserved 2,619,444 of our common shares for issuance under our 2021 Plan.
The following common shares
will be added (or added back) to the shares available for issuance under the 2021 Plan:
| ● | Common shares subject to 2019 Plan or 2021 Plan awards that
expire, terminate or are cancelled or forfeited for any reason after the effectiveness of the 2021 Plan; |
| ● | Common shares that after the effectiveness of the 2021 Plan
are withheld to satisfy the exercise price of an option issued under the 2019 Plan or the 2021 Plan; and |
| ● | Common shares that after the effectiveness of the 2021 Plan
are withheld to satisfy tax withholding obligations related to any award under the 2019 Plan or the 2021 Plan. |
However, the total number of
common shares underlying 2019 Plan awards that may be recycled into the 2021 Plan pursuant to the above-described rules will not exceed
shares.
Administration. We
expect that our 2021 Plan will be administered by our compensation committee. The administrator of the plan will have the authority to,
among other things, interpret the plan and award agreements, select grantees, determine the vesting, payment and other terms of awards,
and modify or amend awards. Our compensation committee may delegate to one or more of our officers the authority to issue awards under
the 2021 Plan to grantees who are not executive officers, subject to parameters established by the compensation committee.
Stock options. The
2021 Plan provides for the grant of both incentive stock options and non-qualified stock options to purchase our common shares at a stated
exercise price. The exercise price of stock options granted under the 2021 Plan must be at least equal to the fair market value of our
common shares on the date of grant. The maximum term of options granted under our 2021 Plan is ten years.
Our compensation committee
may provide in the terms of the applicable award agreement that the participant may exercise an unvested portion in exchange for restricted
stock subject to the same vesting terms as the option.
Stock appreciation rights. An
SAR provides for a payment, in cash or our common shares or a combination of both, to the holder based upon the difference between the
fair market value of our common shares on the date of exercise and a predetermined exercise price, multiplied by the number of shares.
The base price of a SAR must be at least the fair market value of a common share on the date of grant. SARs may not have a term that is
longer than ten years from the date of grant.
Adjustments. In
the event of certain corporate events or transactions (such as a merger, consolidation, reorganization, recapitalization, stock split,
reverse stock split, spin-off, stock dividend, or similar transaction or change in our capital structure), our compensation committee
will make adjustments or substitutions to the number and kind of shares that may be issued under the 2021 Plan, the number and kind of
shares subject to outstanding awards, the exercise price or base price of outstanding awards, and/or any other affected terms and conditions
of the 2021 Plan or outstanding awards, in each case as it deems appropriate and equitable.
Restricted stock awards. An
RSA is an issuance of our common shares subject to forfeiture restrictions that lapse based on the satisfaction of service and/or performance
conditions. The price, if any, of each share subject to an RSA will be determined by the compensation committee. During the vesting period,
a participant will have the right to vote and receive any dividends with respect to restricted stock, provided that our compensation committee
may specify that any such dividends are subject to the same vesting schedule as the shares to which they relate.
Restricted stock units. RSUs
represent the right to receive our common shares (or cash equal to the value of such shares) at a specified time in the future, following
the satisfaction of specified service and/or performance conditions.
Cash or other stock based
awards. Cash or other stock based awards (including awards to receive our unrestricted common shares or immediate
cash payments) may be granted to participants. Our compensation committee will determine the terms and conditions of each such award,
including, as applicable, the term, any exercise or purchase price, performance goals, vesting conditions, and other terms and conditions.
Payment in respect of a cash or other stock based award may be made in cash, our common shares, or a combination of both, at the discretion
of our compensation committee.
Change in control. Upon
or in anticipation of a change in control (which includes certain merger, asset or stock transactions, certain changes in our board composition
and any other event deemed by our board of directors to constitute a change in control), our compensation committee may take such actions
as it deems appropriate with respect to outstanding awards under the 2021 Plan. Such actions may include (among other things) the acceleration
of award vesting, the substitution of awards, the cancellation of unexercised or unvested awards and the redemption or cashout of awards.
In the discretion of our compensation committee, any cash or other substitute consideration payable upon redemption or cashout of an award
may be subjected to the same vesting terms that applied to the original award, or earn-out, escrow, holdback or similar arrangements comparable
to those applicable to stockholders in connection with the change in control. The compensation committee need not treat all outstanding
awards in an identical manner.
Repricing. The
compensation committee may in its discretion: (i) cancel options or stock appreciation rights outstanding under the 2021 Plan in
exchange for new options or stock appreciation rights with a lower exercise or base price per share; (ii) cancel underwater options
or stock appreciation rights outstanding under the 2021 Plan in exchange for consideration payable in our equity securities or cash; or
(iii) otherwise directly reduce the exercise or base price of options or stock appreciation rights outstanding under the 2021 Plan.
Clawback. Awards
under the 2021 Plan will be subject to clawback or recoupment pursuant to any applicable policy, law or exchange listing requirement in
effect from time to time.
Transferability. Except
for certain estate planning transfers authorized by the compensation committee, awards granted under the 2021 Plan are generally nontransferable
except by will or by the laws of descent and distribution.
Amendment and termination. Our
board of directors may amend our 2021 Plan at any time, subject to stockholder approval if required by applicable law or exchange listing
requirement. The 2021 Plan will terminate ten years after it becomes effective.
2019 Stock Option Plan
Our HNCI Stock Option Plan
was made effective May 27, 2019, or the 2019 Plan. Our Stock Plan was originally adopted to provide a share-related mechanism to
attract, retain and motivate directors, employees, officers and consultants, to reward such of those directors, employees, officers and
consultants as may be awarded options under the Stock Plan by the board from time to time for their contributions toward the long-term
goals of the company.
As noted above, we expect to
will cease granting awards under the 2019 Plan upon the effective date of our 2021 Plan (described above). Any outstanding awards will
continue to be subject to the terms of the 2019 Plan and the applicable award agreements, until such awards are exercised or settled,
or until they terminate or expire by their terms.
A summary of the material terms
of the 2019 Plan follows below.
Options. Under
the 2019 Plan, stock options to purchase our common shares may be granted to directors, officers, employees and consultants of the Company.
Administration. The
2019 Plan is administered by the Board, which may delegate some or all of its administrative duties thereunder.
Shares Subject to the Plan. The
aggregate number of shares that could be issued at any time under the 2019 Plan is equal to 10% of the then-issued and outstanding common
shares of the Company, on a rolling basis. The portion of options available for grants to directors is limited to 10% of the option under
the 2019 Plan available for grant at any time. Under the 2019 Plan, if any option is exercised, expires or otherwise terminates for any
reason, the number of common shares in respect of such option will again be available for the purposes of the 2019 Plan. As of March 15,
2021, the date on which the Board approved the 2021 Plan, there were 464,293 shares underlying options outstanding under the 2019 Plan.
Option Terms. Under
the 2019 Plan, the Board fixes the term of each option, which may be no longer than five years. The exercise price will generally
be no less than the fair market value of our common shares on the grant date. Unless otherwise determined by the Board, options granted
under the 2019 Plan will vest quarterly over a three-year period.
Post-Termination Exercisability. Unless
otherwise provided by the Board, options granted under the 2019 Plan remain exercisable following an option holder’s termination
of service in accordance with the terms described below.
In the event that an option
holder dies while his or her option remains outstanding, the 2019 Plan provides that the vested portion of the option will remain exercisable
until the earlier of the original expiration date and one year from the date of the option holder’s death. In the event that a director
terminates service for any reason other than death, the vested portion of his or her option will remain exercisable until the original
expiration date of the option. In the event that an option holder ceases to provide services as an officer, employee or consultant for
any reason other than death, the vested portion of the option will remain exercisable until the earlier of the original expiration date
and 90 days from the termination date. Notwithstanding the foregoing, if an option holder ceases to provide services due to a termination
by the Company for cause, the option will expire immediately.
Initial Public Offering. In
connection with an IPO, the board may in its sole discretion determine the treatment of outstanding awards in a manner it deems fair and
reasonable. This may include, without limitation, accelerating the vesting of options, requiring the option holder to exercise
the vested portion of the option prior to the IPO or automatically exercising such option through a cashless exercise if the option holder
fails to so exercise.
Triggering Event. If
we are subject to a “Triggering Event” which means generally an offer by a third party to acquire the equity securities of
the Company which at least 50% of the outstanding shares of the Company has agreed to accept; a merger or other consolidation after which
the voting securities of the Company outstanding immediately prior to the transaction represent less than 50% of the voting securities
after the transaction; or any other sale of the business of the company, as determined by the Board, the Board will determine in its sole
discretion how to treat outstanding awards under the 2019 Plan in a manner it deems fair and reasonable in light of the circumstances.
This may include, but is not limited to, one or more of the following: (i) acceleration of vesting; (ii) cancelling unvested
options and upon reasonable notice to the option holders, cancelling unexercised vested options; (iii) providing for the assumption
of or replacement of options with comparable stock options; (iv) cashing out in-the-money options; (v) automatically exercising
options through a cashless exercise; or (v) deeming an option to have been exercised in full without any payment by the option holder.
The board may also require the option holder to sell all of the common shares acquired upon the exercise of an option under the Triggering
Event.
Adjustments. In
the event that: (i) the common shares are changed into or exchanged for a different number or kind of shares of the company or securities
of another corporation, whether through an arrangement, amalgamation, recapitalization, subdivision or consolidation; (ii) a dividend
is paid in shares, other than in lieu of dividends paid in the ordinary course; or (iii) there is any other change that the Board,
in its sole discretion, determines equitably requires an adjustment to be make, then, subject to any required action by any of the shareholders
of the company, any term of the 2019 Plan or outstanding options that the Board determines requires adjustment will be adjusted by the
Board in the manner the Board deems appropriate and its determination will be final, binding and conclusive.
Transferability. Under
the 2019 Plan, options may generally not be assigned or transferred.
Plan Amendment and Termination. The
Board may generally terminate or amend the 2019 Plan at any time, provided that such amendment does not alter the terms or conditions
of any outstanding option without the option holder’s consent. The Board may in its discretion amend the terms of an outstanding
option, subject to the consent of the option holder.
Preferred Stock
As of June 30, 2023, the Company
does not have any shares of preferred stock outstanding.
Transfer Agent
The transfer agent of our
Common Stock is Vstock Transfer, LLC. Their address is 18 Lafayette Place, Woodmere, NY 11598.
Unregistered Sales of Equity Securities
We have previously disclosed
in our 10-Qs and 8-Ks filed in fiscal year 2023 all fiscal year 2023 sales of securities without registration under the Securities Act
of 1933 except the following. Unless otherwise noted, these shares were issued pursuant to the exemption
from the registration requirements of the Securities Act of 1933, as amended, afforded by Section 4(a)(2) thereof for the sale of securities
not involving a public offering.
On July 4, 2023, the
Company issued 100,000 common non-voting shares pursuant to a mutual settlement and release agreement.
On July 5, 2023, the Company
cancelled 104,167 common non-voting sha-res which had previously been issued pursuant to a donation to the Austin Community Foundation.
On August 1, 2023, the Company
issued 187,500 common non-voting shares to the former Chief Executive Officer.
ITEM 6.
[Reserved].
Not applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion
and analysis should be read in conjunction with our consolidated financial statements and related notes appearing elsewhere in this Annual
Report. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties,
and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain
factors, including but not limited to those set forth in “Part I – Item 1A. Risk Factors.”
Business Overview
We are an early-stage psychotropics
contract manufacturing company focused on becoming the premier contract research, development, and manufacturing organization for the
emerging psychotropics-based medicines industry. In August 2021, Health Canada’s Office of Controlled Substances granted us a Controlled
Drugs and Substances Dealer’s Licence under Part J of the Food and Drug Regulations promulgated under the Food and Drugs Act (Canada),
or a Dealer’s Licence. A Dealer’s Licence authorizes us to develop, sell, deliver, and manufacture (through extraction or
synthesis) certain pharmaceutical-grade active pharmaceutical ingredients, or APIs, used in controlled substances and their raw material
precursors. Our mission is to make our products and research services available to our clients for the development of medicines and experimental
therapies to address certain psychiatric health disorders and other medical needs. Since current Canadian regulations prohibit the commercial
sales of APIs and other products we intend to produce, APIs and such other products would only be authorized for sale in Canada for clinical
testing purposes in an “institution,” for the purpose of determining the hazards and efficacy of the drug, and for laboratory
research in an institution by qualified investigators. Our mission is to make our products and research services available to our clients
for the development of medicines and experimental therapies to address certain psychiatric health disorders and other medical needs. We
cannot guarantee that we will receive such further approvals from Health Canada, and a failure to receive such further approvals would
have a material adverse effect on our business and result in an inability to generate revenue from said substances. Further, as of the
date of this Annual Report, we have not manufactured all of the psychedelics-based products allowable under the Dealer’s Licence.
The success of our
business plan is dependent on our activities being permissible under applicable laws and upon the occurrence of regulatory changes
for psychotropics-based medicines. In Canada, the psychedelic compounds that we are approved to produce under our Dealer’s
Licence, psilocybin, psilocin, lysergic acid diethylamide, or LSD, N,N-Dimethyltryptamine, or N,N-DMT, and
3,4-Methylenedioxymethamphetamine, or MDMA, and 4-Bromo-2,5-Dimethoxybenzeneethanamine, or 2C-B, are regulated under the Controlled
Drugs and Substances Act, or CDSA. Certain psychedelic substances, including psilocybin, psilocin, mescaline and DMT, are classified
as Schedule III drugs and the CDSA prohibits the possession of a Schedule III drug absent authorization under the CDSA or a related
regulation, and are illegal to possess Schedule III substances without a prescription. In the United States, these substances are
classified under the Controlled Substances Act (21 U.S.C. § 811), or the CSA, and the Controlled Substances Import and Export
Act, or the CSIEA, and as such, medical and recreational use is illegal under the U.S. federal laws. Under the CSA, the Drug
Enforcement Agency, or DEA, regulates chemical compounds with a potential for abuse as Schedule I, II, III, IV or V substances.
Schedule I substances may not be prescribed, marketed or sold in the United States. Most, if not all, state laws in the United
States classify psilocybin, LSD, MDMA, DMT and 2C-B as Schedule I controlled substances. For any product containing any of these
substances to be available for commercial marketing in the United States, the applicable substance must be rescheduled, or the
product itself must be scheduled, by the DEA to Schedule II, III, IV or V. If the DEA does not reschedule psilocybin, LSD, MDMA, DMT
and 2C-B as II, III, IV or V, such substances will be subject to individually-allotted manufacturing and procurement quotas, which
may have a material adverse effect on our business and result in an inability to generate sufficient revenue from said substances to
be profitable. Additionally, regardless of the scheduling of a finished, approved therapeutic product, if the API used in the final
dosage form is a Schedule I or II controlled substance, it would be subject to such quotas as the API could remain listed on
Schedule I or II. Moreover, even if the finished dosage form of a psychedelics-based medicine developed by one of our clients is
approved by the FDA, and if such product is listed by the DEA as a Schedule II, III, or IV controlled substance, its manufacture,
importation, exportation, domestic distribution, storage, sale and legitimate use will continue to be subject to a significant
degree of regulation by the DEA.
An increasing number of the
leading universities, hospitals and other public, private, and government institutions throughout the world have launched research programs
and are conducting clinical studies aimed at understanding the therapeutic potential of a range of psychedelic substances, including the
John Hopkins Center for Psychedelic and Consciousness Research at Johns Hopkins University, the Imperial College of London Centre for
Psychedelic Research, the Center for the Science of Psychedelics at the University of California, Berkeley, the Depression Evaluation
Service at Columbia University, the Center for Psychedelic Psychotherapy and Trauma Research at the Icahn School of Medicine at Mount
Sinai Health System, New York City’s largest academic medical system, and the Center for the Neuroscience of Psychedelics at Massachusetts
General Hospital, among many others.
To address mounting demands
for alternative therapies incorporating the use of psychedelics and other psychotropics, we intend to leverage our 25,000 square foot
facility located near Victoria, British Columbia, for research, development, and large-scale production of high-quality biological raw
materials, APIs, and finished biopharmaceutical products. Supported by an executive leadership and advisory team consisting of highly
experienced biotechnology and pharmaceutical industry experts, we will seek to position our company to be at the forefront of new discovery
in this rapidly emerging market.
Since our inception, we have
devoted substantially all of our resources to establishing our 25,000 square foot manufacturing and research facilities, which is located
near Victoria, British Columbia, researching potential products related to psychotropics-based therapies, pursuing the approval of our
Dealer’s Licence from Health Canada, organizing and staffing our company, developing our business strategy, establishing our intellectual
property portfolio, raising capital and engaging in other general and administrative activities to support and expand these efforts. To
date, we have financed our operations primarily with proceeds from the sales of our common shares, convertible and non-convertible promissory
notes, and from a bridge loan agreement. Until such time as we can generate significant revenue from our contract manufacturing and research
services, as to which no assurance can be given, we expect to finance our cash needs through public or private equity or debt financings,
third-party funding and marketing and distribution arrangements, as well as other collaborations, strategic alliances and licensing arrangements,
or any combination of these approaches. However, we may be unable to raise additional funds or enter into such other arrangements when
needed or on commercially reasonable terms, or at all.
We have incurred net losses
in each year since inception. Our net loss was $8,988,456 for the year ended June 30, 2023. As of June 30, 2023, we had an accumulated
deficit of $44,415,798 and we had cash and cash equivalents of $1,673,874. Our net losses may fluctuate significantly from quarter to
quarter and year to year, depending on the timing of our research efforts, the expansion of our product and research offerings and the
timing of our other operating activities. Because of the numerous risks and uncertainties associated with our business, we are unable
to predict the timing or amount of increased expenses or when or if we will be able to achieve or maintain profitability. We expect to
incur increased expenses as we:
| ● | complete the buildout of our
manufacturing and research facilities; |
| ● | continue to establish our contract
manufacturing and research services; |
| ● | conduct research related to
potential API and finished product offerings in the psychotropics space; |
| ● | seek regulatory authorization
to distribute and export our product offerings; |
| ● | acquire or license products
or technologies; |
| ● | obtain, maintain, protect and
enforce our intellectual property portfolio; |
| ● | seek to attract and retain
new and existing skilled personnel; |
| ● | create additional infrastructure
to support our operations as a public company and incur increased legal, accounting, investor relations and other expenses; and |
| ● | experience delays or encounter
issues with any of the above. |
To the extent that that psychotropics-based
medicines receive approval from the FDA or Health Canada and the market for our products expands into commercial-scale projects, we expect
to incur significant additional expenses in connection with product manufacturing, marketing, and distribution.
Recent Developments
On January 16, 2023, we entered
into a strategic investment agreement, or the Strategic Investment Agreement, with Hightimes Holding Corp., or Hightimes, 1252240 BC LTD,
a wholly-owned subsidiary of Hightimes, and Trans-High Corporation, a wholly-owned subsidiary of Hightimes, pursuant to which Hightimes
granted to us $833,333 of annual advertising and marketing credits, or Advertising Credits, for three consecutive years, in exchange for
625,000 of our common shares. The Advertising Credits enable us to advertise (i) on all Hightimes publications, including the Hightimes
print and website publications, and (ii) at all festivals and events conducted by Hightimes. Unless earlier terminated pursuant to the
terms of the Strategic Investment Agreement, the Strategic Investment Agreement will terminate on December 31, 2025, which term may be
extended by the parties to the Strategic Investment Agreement upon such terms and conditions as the parties may mutually agree. Paul Abramowitz,
one of our directors, is the stepfather of the Executive Chairman of Hightimes. Mr. Abramowitz’s biological son is a beneficial
owner of Roma Ventures, LLC, or Roma Ventures, an entity that owns approximately 6.00% of our issued and outstanding common shares. Benjamin
Windle is the investment manager of Roma Ventures and has sole voting and investment control with respect to our common shares held by
the Roma Ventures. Each of the Executive Chairman of Hightimes, Mr. Abramowitz and Roma Ventures are shareholders of Hightimes.
On February 13, 2023, we
completed our IPO. Our registration statement on Form S-1 (File No. 333-262296) relating to the IPO was declared effective by the SEC
on February 8, 2023. We issued 1,875,000 common shares at a price of $4.00 per share for aggregate net cash proceeds of $5.8 million,
after deducting underwriting discounts and commissions and other offering related costs. None of the expenses associated with the IPO
were paid to directors, officers, persons owning 10% or more of any class of equity securities, or to their associates, or to our affiliates.
WestPark Capital, Inc. acted as sole book running manager of the offering and as representative of the underwriters.
On February 16, 2023, we
filed an amendment with our current Dealer’s License to add coca leaves, ketamine, methamphetamine, methadone, buprenorphine, diacetylmorphine
(heroin), opium, thenaine (paramorphine), benzoylmethylecgonine (cocaine), fentanyl, hydromorphone, oxycodone, hydrocodone, morphine and
codine to the list of approved substances that it is authorized to manufacture. The shift toward a public health response to the drug
crisis should provide greater opportunities for people who use substances to connect with a growing range of harm reduction and treatment
options. Currently, we focus on the development and sale of psychedelic drugs for research purposes.
On February 27, 2023, we
agreed to our first commercial sale to the prestigious Hadassah BrainLabs – Center for Psychedelics Research, Hadassah Medical Center,
Hebrew University, Jerusalem, Israel. This first commercial sale of psilocybin, while modest in size, marks a key operational milestone
for the company as we shift from pre revenue to revenue producing. This transaction establishes our ability to supply the global psychedelic
community with compounds and services.
On March 20, 2023, we entered
into adefinitive asset purchase agreement (the “APA”) with Wesana Health Holdings Inc. (“Wesana”) for the purchase
of Wesana’s SANA-013 intellectual property and related assets (the “Transaction”). The Transaction provides an opportunity
for the continued development of SANA-013 through the next phases of the US FDA regulatory process and for the Company to have economic
exposure to any positive advancements in any such future research and development efforts by Lucy. On June 30, 2023, the Company entered
into the First Amendment to the APA (the “First Amendment”). Pursuant to the First Amendment, the consideration to be paid
for these assets is: (a) $300,000 in cash to be paid within 24 hours of the signing of the First Amendment; (b) upon the closing of the
acquisition (the “Closing”), the Company will issue Wesana an aggregate of 1,000,000 shares of the Company’s common
stock (the “Shares”); (c) $177,973.99 in cash payable in the following 4 installments: (i) $100,000.00 due on or before July
1, 2023; (ii) $25,991.33 due on or before October 1, 2023; (iii) $25,991.33 due on or before January 1, 2024; and (iv) $25,991.33 due
on or before April 1, 2024, and (d) at the Closing, the Company will assume certain liabilities of Wesana which principally consists of
$92,026.01 of trade payables owed by Wesana to a law firm.
On March 23, 2023, we launched
a new line of unscheduled psychoactive compounds that are available for sale throughout the United States where permitted. This product
line is named Mindful by Lucy and is the first line in the new family of brands contains Amanita Muscaria mushrooms, a psychoactive adaptogen.
The product leverages the compounds of these mushrooms, and a proprietary blend of other natural functional ingredients, to create a transformative
experience for consumers. We aim to distribute and market Mindful by Lucy through Hightimes’ websites and social channels. Lucy
and High Times entered into a Strategic Investment Agreement in January 2023 whereby Lucy received $2.5 million in advertising credits
in exchange for 625,000 of our common shares that will help launch the new brand into market through High Times channels and experiential
events without cash outlays for marketing by Lucy. This new line is well-positioned to capitalize on the growing market for psychoactive
alternatives, which Forbes predicts will double to over $5 billion in gross sales by 2025.
On June 30, 2023, the Closing
of Wesana occurred. A total of $100,000 was paid by the Company to Wesana on July 5, 2023 and the Shares were issued on June 30, 2023.
On July 11, 2023, we announced
the launch of Twilight by Lucy, a blend of Amanita and Reishi mushrooms that include a variety of other nootropics promoting improved
cognitive function and enhanced sleep quality. This release comes on the heels of the recent launch of Mindful by Lucy. Both of these
products are now available for purchase on the company’s official online store, www.buytrippy.com, as well as through Hightimes.com
and other channels. Twilight by Lucy is a product designed to enhance and optimize consumer’s nightly sleep. The introduction of
Twilight alongside Mindful underscores Lucy’s dedication to providing solutions in the psychotropic marketplace.
On July 24, 2023, Christopher
McElvany resigned from his positions as the Company’s President and Chief Executive Officer and resigned as a member of the
Company’s Board. The Company and Mr. McElvany agreed that his last day of employment was July 14, 2023. Mr. McElvany did not resign
as a result of any disagreement with the Company on any matter relating to the Company’s operations, policies or practices.
On
July 24, 2023, the Board ratified the appointment of Richard Nanula (a member of the Board since February 2022) as CEO.
On September
6, 2023, we entered into a Stock Purchase Agreement (the “Stock Purchase Agreement”) with Hightimes to acquire the intellectual
property of High Times. Hightimes owns all of the issued and outstanding shares of common stock of HT-Lucy Acquisition Corp., a Delaware
corporation. Pursuant to the Stock Purchase Agreement, Hightimes agreed to sell to us all of the common stock of HT-Lucy Acquisition Corp.
upon the terms and subject to the conditions of the Stock Purchase Agreement. In exchange for the common stock of HT-Lucy Acquisition
Corp., we shall pay Hightimes as consideration (i) the number of shares of common stock of the Company that represents 19.9% of the total
issued and outstanding shares of the Company at the closing; and (ii) semi-annual earn-out payments (the “Hightimes Earn-Out Payments”)
payable for the five (5) consecutive fiscal years ending on June 30, 2029, in amounts equal to three (3) times the adjusted EBITDA of
HT-Lucy Acquisition Corp., calculated pursuant to the terms of the Stock Purchase Agreement. We have the discretion to pay the Hightimes
Earn-Out Payments with either Buyer Common Stock or cash. At the closing, we will also cause HT-Lucy Acquisition Corp. to enter into an
intellectual property license agreement pursuant to which HT-Lucy Acquisition Corp. will grant to an affiliate of Hightimes the exclusive
right and license to utilize certain intellectual property rights to operate retail stores and to manufacture and sell THC products in
the United States in return for a license fee of $1.0 million per year, increasing to $2.0 million per year upon Federal legalization.
On September 12, 2023, we entered into an amalgamation agreement (the “Amalgamation
Agreement”) with Bluesky Biologicals Inc. (“Bluesky”) to acquire the Bluesky. Bluesky, through Bluesky Wellness Inc.,
owns a portfolio of plant-based wellness brands including Keoni, Keoni Sport, Blush Wellness and AMMA Healing. Pursuant to the Amalgamation
Agreement, Bluesky will amalgamate with a wholly-owned subsidiary of the Company upon the terms and subject to the conditions of the Amalgamation
Agreement. We shall pay Bluesky as consideration (i) the number of shares of common stock of the Company that represents 19.9% of the
total issued and outstanding shares of the Company at the closing; and (ii) earn-out payments (the “Bluesky Earn-Out Payments”)
payable for the four (4) consecutive fiscal years ending on June 30, 2028, the six (6) month period ended June 30, 2024, and the six (6)
month period ending December 31, 2028, in amounts equal to two and one half (2.5) times the adjusted EBITDA of Bluesky., calculated pursuant
to the terms of the Amalgamation Agreement. We have the discretion to pay the Bluesky Earn-Out Payments with either Lucy common shares
or cash.
COVID-19 Impacts
We are continuing to closely
monitor the impact of the global COVID-19 pandemic on our business, and we are taking proactive efforts designed to protect the health
and safety of our employees and consultants and to maintain the continuity of our business. We believe that the measures we are implementing
are appropriate, and we will continue to monitor and seek to comply with guidance from governmental authorities and adjust our activities
as we deem appropriate.
While the COVID-19 pandemic
has not yet resulted in a significant impact to the development of our business and operations, as the pandemic continues, we could see
an impact on our ability to advance our manufacturing and research programs, obtain supplies from key suppliers or interact with regulators,
ethics committees or other important agencies due to limitations in regulatory authority, employee resources or otherwise. In any event,
if the COVID-19 pandemic continues and persists for an extended period of time, we could experience significant disruptions to our development
timelines, which would adversely affect our business, financial condition, results of operations, and growth prospects.
In addition, while the potential
economic impact brought by, and the duration of, the COVID-19 pandemic may be difficult to assess or predict, the pandemic could result
in significant and prolonged disruption of global financial markets, reducing our ability to access capital, which could in the future
negatively affect our liquidity. In addition, a recession or market correction resulting from the spread of COVID-19 could materially
affect our business and the potential value of our common shares.
The extent of the impact
of the COVID-19 pandemic on our efforts, our ability to raise sufficient additional capital on acceptable terms, if at all, and the future
value of and market for our common shares will depend on future developments that are highly uncertain and cannot be predicted with confidence
at this time, such as the ultimate duration of the pandemic, travel restrictions, quarantines, social distancing and business closure
requirements in Canada, the United States and in other countries, and the effectiveness of actions taken globally to contain and treat
COVID-19.
Components of Operating Results
Net Product Sales
Net product sales consist
primarily of sales of Mindful by Lucy which was launched in March 2023. The online
order and receipt of full payment creates the customer contract. Revenue is measured based on the amount of consideration that we receive
from customers when they place an order, reduced by estimates for return allowances. Performance obligation is the delivery of the ordered
product to the customer and the performance condition is satisfied, and revenue is recognized, when control of the goods is transferred
to the customer, which generally occurs upon our delivery to a third-party carrier.
Cost of Sales
Cost of sales primarily consists
of the purchase price of Mindful by Lucy, shipping costs, payment processing and related transaction costs, and applicable sales taxes.
Selling, General and Administrative Expenses
Selling, general and administrative
expense consists primarily of employee-related expenses, including salaries, share-based compensation expense, benefits, and travel for
our personnel in executive, finance and accounting, human resources, and other administrative functions, as well as fees paid for accounting,
legal and tax services, consulting fees and facilities costs. General and administrative expense also includes corporate facility costs,
including allocated rent and utilities, insurance premiums, legal fees related to corporate matters, and fees for auditing, accounting,
and other consulting services.
Impairment Loss
Impairment loss relates to the
Company’s equipment as fair market value exceeded the carrying amount.
We expect our selling, general
and administrative expenses to increase substantially in absolute dollars for the foreseeable future as we increase our headcount to grow
our business. Following the completion of our IPO on February 13, 2023, we anticipate that we will incur increased expenses as a result
of operating as a public company, including expenses related to audit, legal, regulatory and tax-related services associated with maintaining
compliance with SEC rules and regulations and those of any national securities exchange on which our securities are traded, additional
insurance expenses, investor relations activities and other administrative and professional services.
Interest Expense
Interest expense relates
to interest charges associated with indebtedness incurred under debt agreements, as well as charges associated with a debt modification
and certain lease liability. We anticipate that we will repay our outstanding debt obligations during the upcoming fiscal year, which
will result in a reduction of interest expense in future periods.
Change in fair value of warrant liability
Change in fair value of warrant
liability consist of a non-cash change in the fair value of 3,906,209 warrants. On January 22, 2021, the Company amended the warrants
whereby in the event that the Company effects a closing or closings of convertible notes is the minimum aggregate of (i) $1,000,000, the
exercise price of 1,111,112 warrants shall be adjusted to $0.015 (CAD$0.018), (ii) $2,000,000, the exercise price of 2,222,223 warrants
shall be adjusted to $0.015 (CAD$0.018), and (iii) $3,000,000, the exercise price of 3,333,334 warrants shall be adjusted to $0.015 (CAD$0.018).
The warrants were classified as a derivative liability due to the variable nature of the exercise price. On December 8, 2021, the Company
reclassified the 3,906,209 warrants valued at $6,392,476 from warrant liability to share capital as the exercise price became fixed for
the warrants outstanding, since the Company had successfully raised $3,000,000 in convertible notes, resolving the contingency affecting
the exercise price. Also, on December 8, 2021, the Company issued 3,477,919 common shares pursuant to the exercise of 3,477,919 warrants
with an exercise price of $0.015 (CAD $0.018) per warrant.
Loss on Debt Settlement
Loss on debt settlement relates
to the issuance of 613,513 common shares as settlement of trade payables on IPO and 461,213 common shares issued as settlement of due
to related parties on IPO. These common shares were issued at a 40% discount to IPO resulting in a non-cash loss on debt settlement.
Foreign Currency Translation Adjustment
The amount of foreign currency
translation adjustment will fluctuate from period to period with changes in foreign exchange rates between Canadian dollars and U.S. dollars.
Results of Operations
Comparison of the Years Ended June 30, 2023
and 2022
The following table summarizes
our results of operations for the periods indicated:
| For the years ended June 30: | |
2023 | | |
2022 | |
| | |
$ | | |
$ | |
| Net product sales | |
| 7,048 | | |
| — | |
| Cost of sales | |
| 4,500 | | |
| — | |
| Gross profit | |
| 2,548 | | |
| — | |
| Operating expenses | |
| | | |
| | |
| Selling, general and administrative expense | |
| 5,767,881 | | |
| 3,469,479 | |
| Impairment loss | |
| 78,850 | | |
| — | |
| Total operating expenses | |
| 5,846,731 | | |
| 3,469,479 | |
| Operating loss | |
| 5,844,183 | | |
| 3,469,479 | |
| | |
| | | |
| | |
| Non-operating expense (income) | |
| | | |
| | |
| Interest expense | |
| 1,966,143 | | |
| 2,064,547 | |
| Loss on debt settlement | |
| 1,178,183 | | |
| — | |
| Change in fair value of warrant liability | |
| — | | |
| 322,226 | |
| Other income | |
| (53 | ) | |
| (136 | ) |
| Net loss | |
| (8,988,456 | ) | |
| (5,856,116 | ) |
| Foreign currency translation adjustment | |
| 301,427 | | |
| 212,284 | |
| Comprehensive loss | |
| (8,687,029 | ) | |
| (5,643,832 | ) |
Net product sales. Net
product sales were $7,048 for the year ended June 30, 2023, compared to $nil for the year ended June 30, 2022. The increase is attributable
to the launch of Mindful by Lucy through www.buytrippy.com.
Cost of sales. Cost
of sales were $4,500 for the year ended June 30, 2023, compared to $nil for the year ended June 30, 2022. The increase is attributable
to the launch of Mindful by Lucy through www.buytrippy.com. Cost of sales include the
purchase price of our products, shipping costs, payment processing and related transaction costs, and applicable sales taxes.
Selling, general and administrative
expenses. Selling, general and administrative expenses were $5,767,881 for the year ended June 30, 2023, compared to $3,469,479 for
the year ended June 30, 2022. The increase is attributable to increased expenses as a result of operating as a public company, including
expenses related to audit, legal, regulatory and tax-related services associated with maintaining compliance with SEC rules and regulations
and those of any national securities exchange on which our securities are traded, additional insurance expenses, investor relations activities
and other administrative and professional services. Included in selling, general and administrative expenses for the year ended June 30,
2023 was $951,088 in non-cash expense related to the issuance of common shares for consulting services, donation to the Austin Community
Foundation, and shares to be issued to a consultant.
Impairment loss.
Impairment loss was $78,850 for the year ended June 30, 2023 related to the Company’s equipment as fair market value exceeded
the carrying amount, compared to impairment loss of $nil for the year ended June 30, 2022.
Interest expense. Interest
expense was $1,966,143 for the year ended June 30, 2023, compared to interest expense of $2,064,547 for the year ended June 30, 2022.
During the year ended June 30, 2023, interest expense included $1,547,104 related to the warrants issued in connection with the line of
credit. During the year ended June 30, 2022, interest expense included $1,627,181 related to the warrants issued in connection with the
line of credit.
Loss on debt settlement.
Loss on debt settlement was $1,178,183 for the year ended June 30, 2023 related to the issuance of 613,513 common shares as settlement
of trade payables on IPO and 461,213 common shares issued as settlement of due to related parties on IPO. These common shares were issued
at a 40% discount to IPO resulting in a non-cash loss on debt settlement. During the year ended June 30, 2022, loss on debt settlement
was $nil.
Change in fair value of
warrant liability. Change in fair value of warrant liability was $nil for the year ended June 30, 2023, compared to $322,226 for the
year ended June 30, 2022. On December 8, 2021, the Company reclassified the 3,906,209 warrants valued at $6,392,476 from warrant liability
to share capital as the exercise price became fixed for the warrants outstanding, since the Company had successfully raised $3,000,000
in convertible notes, resolving the contingency affecting the exercise price. Also on December 8, 2021, the Company issued 3,477,919 common
shares pursuant to the exercise of 3,477,919 warrants with an exercise price of $0.015 (CAD $0.018) per warrant.
Other income. Other
income was $53 for the year ended June 30, 2023, compared to other income of $136 for the year ended June 30, 2022.
Foreign Currency Translation
Adjustment. Foreign currency translation adjustment was income of $301,427 for the year ended June 30, 2023, compared to income of
$212,284 for the year ended June 30, 2022.
Liquidity and Capital Resources
Sources of Liquidity
Since inception, we have
incurred operating losses and negative cash flows from our operations. Our operations have been financed primarily by the sale and issuance
of our common shares, from the issuance of convertible and non-convertible promissory notes, and our IPO. We will continue to be dependent
upon equity and debt financings or collaborations or other forms of capital at least until we are able to generate positive cash flows
from product sales, if ever.
Our comprehensive loss was
$8,687,029 for the year ended June 30, 2023. As of June 30, 2023, we had an accumulated deficit of $44,415,798 and cash of $1,673,874.
The gross proceeds from the IPO were $7.5 million and the net proceeds were approximately $5.8 million, after deducting underwriting discounts
and commissions and other offering related expenses payable by the Company. Our primary use of cash is to fund operating expenses, which
consist primarily of selling, general and administrative expenditures and expenditures for research and development activities when liquidity
permits. Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in accounts
payable and accrued expenses. Our strategy for managing liquidity over the long-term is based on achieving positive cash flows from operations
to internally fund operating and capital requirements. We continually monitor factors that may affect our liquidity. These factors include
research and development costs, operating costs, capital costs, income tax refunds, foreign currency fluctuations, seasonality, market
immaturity and a highly fluid environment related to state and federal law passage and regulations.
Working Capital
At June 30, 2023 and 2022,
we had a working capital of $1,112,299 and a working capital deficiency of $3,911,421, respectively, as follows:
| As of: | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Cash | |
| 1,673,874 | | |
| 53,379 | |
| Prepaid expenses and deposits | |
| 1,219,180 | | |
| 185,723 | |
| Accounts receivable | |
| 7,048 | | |
| — | |
| Other assets – GST receivable | |
| 62,649 | | |
| 13,232 | |
| Other receivable | |
| 336,706 | | |
| — | |
| Digital assets | |
| — | | |
| 34,106 | |
| Deferred financing costs, current | |
| 523,041 | | |
| 1,612,228 | |
| Total current assets | |
| 3,822,498 | | |
| 1,898,668 | |
| Accounts payable and accrued liabilities | |
| 1,291,063 | | |
| 2,814,532 | |
| Convertible notes, current | |
| — | | |
| 825,707 | |
| Due to related parties | |
| 1,019,894 | | |
| 1,775,372 | |
| Notes payable – related parties | |
| — | | |
| 305,082 | |
| Notes payable, current | |
| 60,423 | | |
| — | |
| Lease liability, current | |
| 338,819 | | |
| 89,396 | |
| Total current liabilities | |
| 2,710,199 | | |
| 5,810,089 | |
| Working capital (deficiency) | |
| 1,112,299 | | |
| (3,911,421 | ) |
Cash Flows
Comparison of the Year Ended June 30, 2023
and 2022
The following table summarizes
our results of operations for the periods indicated:
| Net cash provided by (used in) | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Operating activities | |
| (4,266,353 | ) | |
| (2,418,422 | ) |
| Investing activities | |
| (265,894 | ) | |
| (34,106 | ) |
| Financing activities | |
| 6,192,288 | | |
| 2,250,238 | |
| Effect of exchange rate changes on cash | |
| (39,546 | ) | |
| 9,639 | |
| Cash, beginning of period | |
| 53,379 | | |
| 246,030 | |
| Cash, end of period | |
| 1,673,874 | | |
| 53,379 | |
Operating Activities
Cash used in operating activities
during the year ended June 30, 2023 was $4,266,353. The cash used in operating activities is attributable to the following:
| ● | Net loss of $8,988,456 due
primarily to spend on selling, general and administrative expenses, non-cash interest expense and non-cash loss on debt settlement related
to common shares issued on IPO as settlement of accounts payable and due to related parties. Included in net loss are non-cash items
of $4,579,379 for the year ended June 30, 2023. |
| ● | Movements in prepaid expenses
and deposits decreased cash by $81,289 related to the payment of insurance premiums. |
| ● | Movements in accounts receivable
which decreased cash by $7,048 due to timing of receipt from product sales. |
| ● | Movements in other assets including
GST receivable which decreased cash by $49,188 due to timing of receipt from the Canadian government. |
| ● | Movements in other receivable
which decreased cash by $332,762 due to timing of receipt of funds garnished
and paid into the British Columbia Supreme Court. These funds were subsequently received by the Company. |
| ● | Movements in accounts payable
and accrued liabilities which increased cash by $407,246 due to timing of payments to vendors. |
| ● | Movements in lease liability
which decreased cash by $305,452 due to contractual lease payments. |
| ● | Movements in due to related
parties which increased cash by $511,217 due to deferral of payments for various related parties. |
Cash used in operating activities
during the year ended June 30, 2022 was $2,418,422. The cash used in operating activities is attributable to the following:
| ● | Net loss of $5,846,116 due
primarily to spend on selling, general and administrative expenses and non-cash interest and change in fair value of warrant liability.
Included in net loss are non-cash items of $2,596,599 for the year ended June 30, 2022. |
| ● | Movements in prepaid expenses
and deposits increased cash by $6,899. |
| ● | Movements in other assets including
GST receivable which decreased cash by $97 due to timing of receipt from the Canadian government. |
| ● | Movements in accounts payable
and accrued liabilities which increased cash by $487,593 due to timing of payments to vendors. |
| ● | Movements in lease liability
which decreased cash by $226,217 due to contractual lease payments. |
| ● | Movements in due to related
parties which increased cash by $572,723 due to deferral of payments for various related parties. |
Investing Activities
Cash used in investing activities
during the year ended June 30, 2023 was $265,894 related to an initial $300,000 deposit on the Wesana Transaction which was partially
offset by the sale of digital assets.
Cash used in investing activities
during the year ended June 30, 2022 was $34,106 related to the purchase of digital assets through funds received on issuance of convertible
notes.
Financing Activities
Cash provided by financing
activities for the year ended June 30, 2023 was $6,192,288, which was the result of funds raised from the initial public offering and
issuance of convertible notes which were partially offset by deferred issuance costs and the repayment of related party notes payable.
Cash provided by financing
activities for the year ended June 30, 2022 was $2,250,238, which was the result of funds raised from the issuance of convertible notes,
exercise of warrants and exercise of options which was partially offset by deferred issuance costs.
Indebtedness
In February 2021, we issued
a convertible promissory note in the amount of $500,000 to Downwind Investments, LLC, (“Downwind”). Christopher McElvany,
our former President and Chief Executive Officer, is the principal owner of Downwind. The convertible promissory note bears an interest
rate of 8% per annum and matured on February 25, 2022 and maturity has been extended to the date of successful completion of our initial
public offering. The outstanding principal amount and accrued interest under this convertible promissory note is convertible at the option
of the holder into our common shares at a price of $1.58 per common share. On February 13, 2023, the closing date of our IPO, Mr. McElvany
converted the outstanding principal amount of $500,000 and accrued interest of $78,575 under, this convertible promissory note into 366,187
of our common shares and the Company has no continuing obligation with respect to the convertible promissory note.
In November 2020, we entered
into a credit agreement with Origo BC Holdings Ltd., (“the Origo Credit Agreement”). Under the Origo Credit Agreement, we
obtained a line of credit in an aggregate principal amount of up to $5,041,541, of which we can request an advance of up to $377,644 in
any calendar quarter. The Origo Credit Agreement has a term of three years, and all borrowings thereunder bear interest at a rate of 8%
per annum. In the event of default, all outstanding indebtedness under the Origo Credit Agreement will bear interest at a rate of 15%
per annum. As of June 30, 2023, there were no amounts outstanding under the Origo Credit Agreement. Pursuant to the Origo Credit Agreement,
the Company issued 3,906,209 warrants to purchase 3,906,209 common shares. As of June 30, 2023, 428,290 warrants remained outstanding.
For so long as any of the warrants are outstanding, Origo BC Holdings Ltd. shall have the right to appoint 40% of the members of the board
of directors of the Company.
In December 2018, we issued
a promissory note to Livio Susin, one of our directors, in the principal amount of $144,666, pursuant to which Mr. Susin loaned funds
to us through a series of advances. All indebtedness under this promissory note bears interest at a rate of 21% per annum. The maturity
date of this promissory note was December 31, 2021 and maturity has been extended to 90 days following to the successful completion of
an initial public offering or a reverse takeover transaction which results in our shares being listed on a public exchange. On February
13, 2022, the closing date of our IPO, the entire outstanding principal and interest of $88,707 was automatically converted into 36,962
of our common shares and the Company has no continuing obligation with respect to the promissory note.
In February 2019, we issued
a second promissory note to Mr. Susin in the principal amount of $245,768, pursuant to which he loaned funds to us through a series of
advances. Indebtedness under this promissory note bears interest at a rate of 2% per annum. The indebtedness under this promissory note
is unsecured, and is repayable 90 days following to the successful completion of an initial public offering or a reverse takeover transaction
which results in our shares being listed on a public exchange. In January 2021, Mr. Susin forgave $39,746 of indebtedness under this promissory
note in exchange for 13,889 shares of our common shares. On February 23, 2023, the Company repaid the entire outstanding principal and
interest of $207,266 and the Company has no continuing obligation with respect to the promissory note.
Funding Requirements
We have incurred significant
operating losses since our inception and expect to continue to incur significant operating losses for at least the next several years.
Moreover, we expect our losses to increase as we enhance our manufacturing and research facilities and product offerings. We may also
incur expenses in connection with the in-licensing or acquisition of additional product candidates. Furthermore, following the completion
of the IPO on February 13, 2023, we expect to incur additional costs associated with operating as a public company, including significant
legal, accounting, investor relations and other expenses that we did not incur as a private company. Our primary uses of capital are,
and we expect will continue to be, compensation and related expenses, manufacturing and development services, manufacturing costs, legal
and other regulatory expenses and general overhead costs.
At the time of issuance of
our financial statements as of and for the year ended June 30, 2023, we concluded that there was substantial doubt about our ability to
continue as a going concern for one year from the issuance of the consolidated financial statements. We have based our projections of
operating capital requirements on assumptions that may prove to be incorrect and we may use all of our available capital resources sooner
than we expect. Because of the numerous risks and uncertainties associated with our research and manufacturing efforts, we are unable
to estimate the exact amount of our operating capital requirements. Our future funding requirements depend on many factors, including,
but not limited to:
| ● | any
necessary enhancements to our manufacturing and research facilities; |
| |
● |
our need to purchase additional equipment; |
| |
● |
our acquisition or development of additional intellectual
property or technologies; |
| |
● |
the cost of commercialization activities, including
marketing, sales and distribution costs; |
| |
● |
our ability to establish and maintain strategic collaborations,
licensing or other arrangements and the financial terms of any such agreements that we may enter into; |
| |
● |
the expenses needed to attract and retain skilled
personnel; |
| |
● |
our need to implement additional internal systems
and infrastructure, including financial and reporting systems, and other costs associated with being a public company; |
| |
● |
the costs involved in preparing, filing, prosecuting,
maintaining, defending and enforcing our intellectual property portfolio; and |
| |
● |
the impact of the COVID-19 pandemic. |
Further, our development
and commercialization operating plans may change, and we may need additional funds to meet operational needs and capital requirements
for manufacturing or research and development activities and commercialization of our products. Because of the numerous risks and uncertainties
associated with the development, manufacturing and commercialization of our products, we are unable to estimate the amounts of increased
capital outlays and operating expenditures associated with our current and anticipated operations.
We may finance our cash
needs through public or private equity or debt offerings or other sources such as strategic collaborations. However, we may be unable
to raise additional funds or enter into such other arrangements when needed or on terms that are acceptable to us, or at all. To the
extent that we raise additional capital by issuing our equity securities, our existing stockholders may experience substantial dilution,
and the terms of these securities may include liquidation or other preferences that could harm the rights of a common shareholder. Any
agreements for future debt or preferred equity financings, if available, may involve covenants limiting or restricting our ability to
take specific actions, such as incurring additional indebtedness, making capital expenditures or declaring dividends. If we raise additional
funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be
required to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates, or grant
licenses on terms that may not be favorable to us. We may seek additional capital due to favorable market conditions or strategic considerations
even if we believe we have sufficient funds for our current or future operating plans.
Despite the risks and uncertainties,
management believes that we will have sufficient working capital to meet our liquidity needs through twelve months from the issuance
date of the financial statements included in this Annual Report.
Off-Balance Sheet Arrangements
During the periods presented
we did not have, nor do we currently have, any off-balance sheet arrangements as defined in the rules and regulations of the SEC.
Critical Accounting Policies and Estimates
Our management’s discussion
and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance
with generally accepted accounting principles in the United States. The preparation of these financial statements requires us to make
estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities
at the date of the financial statements, as well as the reported expenses incurred during the reporting periods. Our estimates are based
on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which
form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources.
Our actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting
policies are more fully described in the notes to our audited financial statements included elsewhere in this Annual Report, we believe
that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate
to the more significant areas involving management’s judgments and estimates.
Share-Based Payments
We account for our stock-based
compensation as expense in the statements of operations based on the awards’ grant date fair values. We account for forfeitures
as they occur by reversing any expense recognized for unvested awards.
We estimate the fair
value of options granted using the Black-Scholes option pricing model. The Black-Scholes option pricing model requires inputs based
on certain subjective assumptions, including (a) the expected stock price volatility, (b) the calculation of expected term of the
award, (c) the risk-free interest rate and (d) expected dividends. Due to the historical lack of a public market for our common
stock and a lack of company-specific historical and implied volatility data, we have based our estimate of expected volatility on
the historical volatility of a group of similar companies that are publicly traded. The historical volatility is calculated based on
a period of time commensurate with the expected term assumption. The computation of expected volatility is based on the historical
volatility of a representative group of companies with similar characteristics to us, including stage of product development and
life science industry focus. We use the simplified method as allowed by the Securities and Exchange Commission, or SEC, Staff
Accounting Bulletin, or SAB, No. 107, Share-Based Payment, to calculate the expected term for options granted to employees as we do
not have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term. The risk-free
interest rate is based on a treasury instrument whose term is consistent with the expected term of the share purchase options. The
expected dividend yield is assumed to be zero as we have never paid dividends and have no current plans to pay any dividends on our
common stock. The fair value of stock-based payments is recognized as expense over the requisite service period which is generally
the vesting period.
Common Stock Valuation
As there was no public
market for our common stock prior to February 13, 2023, the estimated fair value of our common stock has historically been
determined by our board of directors, with input from management based upon the most recent cash common share offering to
arms’ length parties. In addition to considering the most recent cash arms’ length third party offering, our board of
directors considered various objective and subjective factors to determine the fair value of our common stock as of each grant date,
including:
| |
● |
the progress of our research and development programs,
including the status and results of preclinical studies for our product candidates; |
| |
● |
our stage of development and commercialization and
our business strategy; |
| |
● |
external market conditions affecting the biotechnology
industry and trends within the biotechnology industry; |
| |
● |
our financial position, including cash on hand, and
our historical and forecasted performance and operating results; |
| |
● |
the lack of an active public market for our common
stock; |
| |
● |
the likelihood of achieving a liquidity event or sale
of our company in light of prevailing market conditions; and |
| |
● |
the analysis of initial public offerings and the market
performance of similar companies in the biotechnology industry. |
The assumptions underlying
these valuations represented management’s best estimate, which involved inherent uncertainties and the application of management’s
judgment. As a result, if we had used different assumptions or estimates, the fair value of our common stock and our stock-based compensation
expense could have been materially different.
Subsequent to February 13, 2023, the fair value of our common stock
has been determined based on the quoted market price of our common stock on the date of grant and discounted for any trading restrictions.
Income Taxes
Significant judgment is
required in determining the provision for income taxes. There are many transactions and calculations undertaken during the ordinary course
of business for which the ultimate tax determination is uncertain. We recognize liabilities and contingencies for anticipated tax audit
issues based on our current understanding of the tax law in the relevant jurisdiction. For matters where it is probable that an adjustment
will be made, we record our best estimate of the tax liability including the related interest and penalties in the current tax provision.
We believe that we have
adequately provided for the probable outcome of these matters; however, the outcome may result in a materially different outcome than
the amount included in the tax liabilities. In addition, we recognize deferred tax assets relating to tax losses carried forward only
to the extent that it is probable that taxable profit will be available against which a deductible temporary difference can be utilized.
This is deemed to be the case when there are sufficient taxable temporary differences relating to the same taxation authority and the
same taxable entity which are expected to reverse in the same year as the expected reversal of the deductible temporary difference, or
in years into which a tax loss arising from the deferred tax asset can be carried back or forward. However, utilization of the tax losses
also depends on the ability of the taxable entity to satisfy certain tests at the time the losses are recouped.
Useful Lives of Property, Plant and Equipment
and Intangibles
Property, plant, and equipment
and intangible assets are amortized or depreciated over their useful lives. Useful lives are based on management’s estimate of
the period that the assets will generate revenue, which are periodically reviewed for continued appropriateness. Changes to estimates
can result in significant variations in the carrying value and amounts charged to the statement of loss and other comprehensive loss
in specific periods.
Impairment
Long-lived assets, including
intangible assets are reviewed for indicators of impairment at each statement of financial position date or whenever events or changes
in circumstances indicate that the carrying amount of an asset exceeds its recoverable amount. For the purpose of impairment testing,
assets that cannot be tested individually are grouped together into the smallest group of assets that generates cash inflows from continuing
use that are largely independent of the cash inflows of other assets or groups of assets, or CGU. Judgments and estimates are required
in defining a CGU and determining the indicators of impairment and the estimates required to measure an impairment, if any.
Recently Adopted Accounting Pronouncements
See the section titled “Notes
to Consolidated Financial Statements — Note 2” included elsewhere in this Annual Report for additional information.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES
ABOUT MARKET RISK
Not applicable.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY
DATA
See Index to Consolidated Financial
Statements on page F-1 of this Annual Report.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH
ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
a) Evaluation of Disclosure Controls and
Procedures
Our
management, including our President and Chief Executive Officer (principal executive officer) and our Chief Financial Officer (principal
financial and accounting officer), do not expect that our disclosure controls or our internal control over financial reporting will prevent
all error and all fraud. Due to the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance
that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments
in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented
by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of
any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that
any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate
because of changes in conditions or deterioration in the degree of compliance with policies or procedures. Because of the inherent limitations
in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. In designing and evaluating the
disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated,
can provide only reasonable assurance of achieving the desired control objectives and our management necessarily was required to apply
its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Our
President and Chief Executive Officer and our Chief Financial Officer have evaluated the effectiveness of the design and operation of
our disclosure controls and procedures (as defined in Rule 13a-15(e) and Rule 15d-15(e) of the Exchange Act) as of the end of the period
covered by this Annual Report. Based on this evaluation, our President and Chief Executive Officer and our Chief Financial Officer concluded
that, as of the end of the period covered by this Annual Report, our disclosure controls and procedures were not effective to ensure that
information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated
to our management, including our President and Chief Executive Officer and our Chief Financial Officer, to allow for timely decisions
regarding required disclosures, and recorded, processed, summarized and reported within the time periods specified in the SEC’s
rules and forms.
The
Company has certain material weaknesses in internal controls as described below:
| |
● |
Company lacks an effective control environment; |
| |
● |
Company has not formally designed and implemented
risk assessment controls; |
| |
● |
Company has not formally designed and implemented
monitoring controls; and |
| |
● |
Company lacks segregation of duties in several areas,
and its review controls are not considered operating effectively due to historical misstatements. |
b) Management’s Annual Report on
Internal Control Over Financial Reporting
Our
management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f)
and 15d-15(f) under the Securities Exchange Act of 1934. Our internal control over financial reporting is a process designed by, or under
the supervision of, our chief executive officer and chief financial officer, or persons performing similar functions, and effected by
our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the
United States of America (GAAP). Our internal control over financial reporting includes those policies and procedures that: (i) pertain
to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and disposition of the assets
of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements
in accordance with GAAP and that receipts and expenditures of the Company are being made only in accordance with authorization of management
and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition,
use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Management
conducted an evaluation of the effectiveness of our control over financial reporting based on the 2013 framework in Internal Control
- Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, management
concluded that our internal control over financial reporting was not effective as of June 30, 2023 due to the material weaknesses identified
in part (a) of this Item 9A.
Pursuant
to Regulation S-K Item 308(b), as the Company is not an accelerated filer nor a large accelerated filer, this Annual Report does not
include an attestation report of our company’s registered public accounting firm regarding internal control over financial reporting.
Because
of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of
any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions
or that the degree of compliance with the policies or procedures may deteriorate. A control system, no matter how well designed and operated
can provide only reasonable, but not absolute, assurance that the control system’s objectives will be met. The design of a control
system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their cost.
c) Changes in Internal Control over Financial
Reporting
During
the year ended June 30, 2023, there were no changes in our internal controls over financial reporting, which were identified in connection
with our management’s evaluation required by paragraph (d) of rules 13a-15 and 15d-15 under the Exchange Act, that materially affected,
or is reasonably likely to have a materially affect, on our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE
REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not
applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND
CORPORATE GOVERNANCE
The
following table and biographical summaries set forth information, including principal occupation and business experience, about our directors
and executive officers as of June 30, 2023:
| Directors and Executive Officers |
|
Position/Title |
|
Age |
| |
|
|
|
|
| Richard Nanula |
|
Chief Executive Officer and Executive Chairman |
|
63 |
| Brian Zasitko CPA, CA |
|
Chief Financial Officer |
|
43 |
| Assad J. Kazeminy, Ph.D. |
|
Chief Scientific Officer |
|
72 |
| Paul Abramowitz(1)(2) |
|
Director |
|
67 |
| Brittany Kaiser(1)(2)(3) |
|
Director |
|
34 |
| Charles B. Nemeroff, M.D., Ph.D.(1)(2) |
|
Director |
|
73 |
| Scott M. Reeves |
|
Director |
|
54 |
| Livio Susin(3) |
|
Director |
|
68 |
| (1) | Member
of the Audit Committee. |
| (2) | Member
of the Compensation Committee. |
| (3) | Member
of the Nominating and Corporate Governance Committee. |
Executive Officers
Richard
Nanula has served as our Chair and a director since February 2022. Mr. Nanula is a highly experienced business advisor
and senior executive with more than 35 years of experience in corporate finance and strategy. After receiving his MBA from Harvard Business
School in 1986, he embarked on a 13-year tenure with the Walt Disney Company (Disney), serving 7 of those years as the corporation’s
Executive Vice President and Chief Financial Officer. While at Disney, Nanula led numerous successful and innovative finance dealings
including a first-ever 100-year bond issuance by an industrial company, a $20 billion acquisition of Capital Cities/ABC,
and more than $3 billion in film financing. His time at Disney saw growth in company revenues from $2 billion to more than
$20 billion and market capitalization from $3 billion to more than $40 billion. Upon leaving Disney, Nanula joined Starwood
Hotels and Resorts, serving as the company’s President and Chief Operating Officer from 1998 to 2000. During this time, he led
the integration of Starwood Hotels, Westin, and Sheraton ITT into a single $20 billion enterprise, forming the largest hospitality
company in the world. In 2001, Mr. Nanula began working for the major biotechnology firm Amgen. During his 7-year term with
the company serving as Executive Vice President of Finance and Strategy and Chief Financial Officer, he launched and successfully executed
5 acquisitions totalling $18 billion, including the largest in the history of the biotechnology industry at the time. Nanula also
led a series of low-cost funding deals and extremely successful stock buy-back initiatives for the company. In 2008, Mr. Nanula
joined Colony Capital as a Principal Officer responsible for global operations where he led the company’s acquisition of First
Republic Bank for $1.5 billion, and its $2.5 billion IPO just 9 months later. Additionally, Nanula successfully led Colony
Capital’s acquisition of Miramax Films for $650 million, a deal that was recently completed at a 3x equity multiple. In addition
to his executive leadership background, Mr. Nanula also served as a board member for Boeing Corporation and Starwood Capital where
he provided corporate guidance and oversight.
We
believe that Mr. Nanula is qualified to serve on our board of directors due to his wealth of knowledge in corporate finance and
transaction strategy as well as his experience in growth company leadership. His portfolio of professional successes can be attributed
to his drive for opportunistic growth, strategic planning abilities, and aggressive execution standards.
Brian
Zasitko, CPA, CA, has served as our Chief Financial Officer since January 1, 2023 and previously served as our Interim
Chief Financial Officer from November 2021 to December 31, 2022. Since December 2018, he has been a Director of Invictus Accounting
Group LLP, a professional services firm providing a host of finance, advisory and accounting services. From January 2020 to September
2023, he served as Chief Financial Officer of Lobe Sciences Ltd, a company developing psychedelic compounds as therapeutics for the treatment
of mild traumatic brain injuries and post-traumatic stress disorder. From May 2015 to June 2018, he was the treasurer of the Oppenheimer
Group, a worldwide marketer and distributer of fresh produce. He has an undergraduate degree from Simon Fraser University and a CPA (CA)
from Certified Professional Accountants, British Columbia.
Dr.
Assad J. Kazeminy, Ph.D. has served as our Chief Scientific Officer since February 2021. Since 2018, Dr. Kazeminy
has also served as the Chairman and Chief Executive Officer of AJK Biopharmaceutical, a drug development company. Dr. Kazeminy is
the founder of Partum Biopharma, which he started in October 2018, and the founder and director of TheraVida, Inc., a drug development
company, which he started in April 2005. Dr. Kazeminy also founded Irvine Pharmaceutical Services Inc., a premier contract
development and manufacturing organization providing support to the pharmaceutical, biopharmaceutical, and medical device industries,
and served as its Chief Executive Officer from 1988 to October 2016. He founded Avrio Biopharmaceutical LLC, a cGMP contract development
and manufacturing organization supporting the pharmaceutical and biopharmaceutical industries from Phase I through post-market life
cycle management, and served as its Chief Executive Officer from 2008 to October 2016. Both Irvine Pharmaceutical Services Inc.
and Avrio Biopharmaceutical LLC were acquired in October 2016 by Nitto Denko Avecia Inc., a recognized leader in therapeutic nucleic
acid manufacturing and development services. Dr. Kazeminy served as a member of the United States Pharmacopeia (USP) Expert
Committee from 2000 to 2020 and has served as a member of Dean Advisory Panel at Chapman University School of Pharmacy since 2014. He
has been appointed as Executive Industry Liaison and Adjunct Professor of Pharmacy at Chapman University School of Pharmacy and has served
as a board member of UCI Applied Innovation. Since September 2014, he serves as a Board Member of the Physical Science Dean’s
Leadership Council. He recently accepted an invitation to become board member of Dean’s Leadership Council at UCI School of Pharmaceutical
Science. Dr. Kazeminy has been awarded by United States Pharmacopeia a Winner for Innovative Responses to a Public Health Challenge
related to his outstanding work on a new General Chapter of USP on elemental Impurity. Dr. Kazeminy held various leadership roles
in national and local organizations, including as the Chairperson of the FDA Grass Roots, Pacific Region Importing Community Steering
Committee; the President of AOAC, International Southern California Section; the Executive Chairperson of the Southern California Pharmaceutical
Discussion Group; the President of American Chemical Society, Southern California Section; the Chairperson of the Pharmaceutical section
of American Council of Independent Laboratories; the President of Association of Iranian Pharmaceutical Scientists; and a Board Member
of AIHA Laboratory Accreditation Programs, LLC. Dr. Kazeminy received his Ph.D. in Pharmaceutical Science and Biochemistry
from Esfahan University in Esfahan, Iran and continued his graduate studies in Biochemistry at Colorado State University. He completed
a Post Doctorate course of study at the University of Southern California Medical School, Department of Pharmacology.
Non-Employee Directors
Paul
Abramowitz has served as a member of our board of directors since November 2021. He is a seasoned business strategist with
over 35 years of corporate finance and strategy experience across multiple industries. Since 1991, Mr. Abramowitz has served as a
Principal for Special Investments, Inc., a Seattle-based agency with an emphasis on providing operational and financial strategy
consulting services. From 2008 to present, he has served as President and Chief Executive Officer of Liquidity Capital Group, LLC, a private
company specializing in the purchase of illiquid assets. In 1983, he served as President and CEO at Infa Inc., a Las Vegas-based products
company, where he tripled sales by developing the core product and restructuring operations, marketing, and distribution. In 1988, Abramowitz
became President and CEO of Western Costume Co. in Los Angeles, the #1 theatrical costume company in the world. From 1991 until 1995,
he acted as Chief Restructuring Officer of DAK Industries, a $250 million direct marketing association in Chapter 11 at the time,
where he raised gross margins 23% through the implementation of various strategies. In 1995, Mr. Abramowitz founded National Claims
Management Corporation in Encino, CA as Principal, where he worked to penetrate the class action settlement script market by purchasing
coupons from corporations for resale to a leasing company for substantial profits. In 2003, Abramowitz was brought on as CEO of Experience
Learning Communities to develop and implement a strategy to reduce operating losses and install internal controls, resulting in a 40%
reduction in expenses. From 2006 to 2008, he served as President and CEO of Neah Power Systems Inc., where he financially revived the
organization after shutdown and engineered a reverse merger. He is also the creator of the silicone baby bottle nipple, an invention purchased
by Hasbro. Mr. Abramowitz has served as Board Member for the Technology Alliance and as President of Young Leadership, Israel Bonds
in Los Angeles. He is a member of Certified Public Accountants with a BS from Ohio State University and an MBA from the University of
Southern California.
We
believe that Mr. Abramowitz is qualified to serve on our board of directors because of his product development and intellectual
property protection knowledge and well as his demonstrable success in strategic repositioning and organizational transformation for over
20 enterprises.
Brittany
Kaiser has served as a member of our board of directors since November 2021. She is an entrepreneur, activist and expert
in data protection and privacy. Since August 2019, Ms. Kaiser has served as president and director of the Own Your Data Foundation,
an organization she co-founded that teaches digital literacy education and provides training to governments, corporates and families.
In February 2018, she co-founded the Digital Asset Trade Association (DATA) Technology for legal advocacy where she does legislative
drafting and lobbying on privacy and blockchain laws. She worked as director of program development of Cambridge Analytica from 2014
to January 2018 and its business development director from February 2017 to January 2018. She also worked as a director
of program development at SCL Group from February 2015 to January 2018. Ms. Kaiser sits on the board of many companies across
industries, including Gryphon Digital Mining since December 2020 and Achayot Partners, LLC since April 2019, working on data
ethics, compliance and privacy protocols. Ms. Kaiser has been a featured at events at the United Nations, the European and British Parliaments,
the G20, and WebSummit, as well as guest lecturing at universities such as Harvard, Oxford and Columbia. Ms. Kaiser received a Master
of Arts degree in International Relations from the University of Edinburgh, a Master of Laws degree in Human Rights from Birkbeck College,
University of London and a Master of Philosophy degree in International Law from Middlesex University.
We
believe that Ms. Kaiser is qualified to serve on our board of directors because of her varied experience in business operations, lobbying
and education efforts and product development, as well as her service on the boards of companies across various industries.
Charles
B. Nemeroff, M.D., Ph.D., has served as a member of our board of directors since November 2021. Since May 2019, Dr. Nemeroff
has served as the Chair of the Department of Psychiatry and Behavioral Sciences at the University of Texas, Austin, Dell Medical School
and he has been a professor in the same department since October 2018. From December 2009 to October 2018, he was a professor
and served as the Chair of the Department of Psychiatry and Behavioral Sciences at the University of Miami, Miller School of Medicine.
Since 2018, Dr. Nemeroff founded and served as the Chief Scientific Officer of EMA Wellness, a company focused on collection and
analysis of Ecological Momentary Assessments and digital biomarkers in clinical trials. In 2005, he also founded CeNeRx BioPharma, a
drug development company that engages in developing therapeutics to treat diseases related to the nervous system. Dr. Nemeroff previously
served on the board of directors of Cypress Bioscience and NovaDel Pharma Inc. Since January 2020, Dr. Nemeroff has served
as the elected president of Anxiety and Depression Association of America. He was elected to the National Academy of Medicine in 2002.
He has published more than 1,000 research reports and reviews, and his research is currently supported by grants from the National Institutes
of Health. He also served on the Mental Health Advisory Council of National Institute of Mental Health and the Biomedical Research Council
for NASA; is co-editor in chief (with Alan F. Schatzberg, M.D.) of the Textbook of Psychopharmacology, published by the APA
Press and now in its Fifth Edition; and is the co-editor in chief of a new journal published by Elsevier, Personalized Medicine
in Psychiatry. Dr. Nemeroff received a Bachelor of Science degree in Biology from the City College of New York, a Master of
Science degree in Biology from Northeastern University and medical degree and Ph.D. from University of North Carolina, Chapel Hill.
We
believe that Dr. Nemeroff is qualified to serve on our board of directors because of his expertise in psychiatry and behavioral
sciences and his extensive research experience in the medical field generally, and with psychiatry and behavior sciences specifically.
Scott
M. Reeves has served as a member of our board of directors since November 2021 and as our Corporate Secretary since
October 2019. Mr. Reeves has been a corporate securities lawyer based in Calgary, Alberta, Canada for 26 years and a partner
at TingleMerrett LLP, a law firm, since October 2003. He has acted as corporate and securities counsel to numerous Canadian, U.S. and
international public and private corporations, including pharma, oil and gas, technology, mining and industrial issuers, and has wide
experience in private and public debt and equity offerings, corporate acquisitions of assets and/or shares, corporate structuring and
debt financing. He is currently a director of several Canadian and U.S. public companies, including Radiko Holdings, Inc. since
February 2017, Tree of Knowledge International Corp. since May 2018, Navion Capital Corp. since May 2018, CBD Global Sciences
Inc. since July 2019, and Starrex International Ltd. since December 2019. Mr. Reeves previously served as a director of
Quattro Exploration and Production Ltd. from November 2011 to March 2017, Perisson Petroleum Corporation from November 2016
to September 2017, EastWest Biosciences Inc. from January 2015 to March 2019 and Canadabis Capital Inc. from May 2019
to January 2020. Mr. Reeves received a Bachelor of Commerce degree in Business Administration and Bachelor of Laws degree from
University of Alberta.
We
believe that Mr. Reeves is qualified to serve on our board of directors because of his expertise in corporate and securities law
and his experience as a director of various public companies.
Livio
Susin has served as a member of our board of directors since September 2017. In November 2017, Mr. Susin
founded Navion Capital Inc., a Capitol Pool Company listed on the TSX, and he currently serves as a member of its board of directors.
From June 2013 to June 2020, Mr. Susin operated two cafes under Rewind Coffee Co. Inc. He has been active in public markets
for over 40 years having been on the boards of numerous public companies, including Rock Tech Lithium Inc. and RNS Software, Inc.
He has significant experience in mining companies, early-stage start-ups, exploration financing, all aspects of corporate governance,
regulatory details and project management. Mr. Susin received a Bachelor of Science degree in Business Administration from the British
Columbia Institute of Technology.
We believe that Mr. Susin
is qualified to serve on our board of directors because of his experience with serving on the board of directors of numerous public companies
and his related knowledge about corporate governance.
None
of the above directors and executive officers has been involved in any legal proceedings as listed in Regulation S-K, Section 401(f)
material to an evaluation of the ability or integrity of any director or executive officer.
Family Relationships
There
are no family relationships among any of our directors or executive officers.
Board Meetings
Our
Board held 6 formal Board meetings during the year ended June 30, 2023 and 4 formal Board meetings during the year ended June 30, 2022.
For
the years June 30, 2023 and 2022, each incumbent director attended at least 75% of all meetings held by the Board and the committees
of the Board on which they served during each year.
Board Composition, Committees, and Independence
Our
board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee,
each of which have the composition and the responsibilities described below. In addition, from time to time, our board of directors may
establish other committees to facilitate the management of our business, when deemed necessary to address specific issues. Members serve
on these committees until their resignation or until otherwise determined by our board of directors.
Each
committee has adopted a written charter that satisfies the applicable rules and regulations of the SEC and the Nasdaq Listing Rules,
which are posted on our website at www.lucyscientific.com.
Audit Committee
Our
audit committee consists of Mr. Abramowitz, Dr. Nemeroff and Ms. Kaiser. Our board of directors has determined that each member
of our audit committee is independent under the Nasdaq Listing Rules and Rule 10A-3(b)(1) of the Exchange Act. The chair
of our audit committee is Mr. Abramowitz. Our board of directors has determined that each member of the audit committee satisfies the
financial literacy and sophistication requirements of the SEC and Nasdaq Listing Rules and that Mr. Abramowitz is an “audit committee
financial expert” as such term is currently defined in Item 407(d)(5)(ii) of Regulation S- K promulgated under the
Securities Act of 1933, as amended, or the Securities Act. This designation does not impose any duties, obligations or liabilities
that are greater than those generally imposed on members of our audit committee and our board of directors.
Our
audit committee is directly responsible for, among other things:
| ● | selecting
a firm to serve as the independent registered public accounting firm to audit our consolidated
financial statements; |
| ● | approving
or, as permitted, pre-approving all audit and non-audit services to be performed by the independent
registered public accounting firm; |
| ● | ensuring
the independence of the independent registered public accounting firm; |
| ● | discussing
the scope and results of the audit with the independent registered public accounting firm
and reviewing, with management and that firm, our interim and year-end operating results
and related disclosures as well as critical accounting policies and practices used by us; |
| ● | establishing
procedures for employees to anonymously submit concerns about questionable accounting or
audit matters; |
| ● | considering
the adequacy of our internal controls and internal audit function; |
| ● | preparing
and approving the audit committee report required to be included in our proxy statement relating
to our annual meeting of stockholders; |
| ● | reviewing
material related party transactions or those that require disclosure; and |
| ● | reviewing
quarterly earnings releases. |
Compensation Committee
Our compensation committee
consists of Mr. Abramowitz, Dr. Nemeroff and Ms. Kaiser. Our board of directors has determined that each member of this committee is a
non-employee director, as defined by Rule 16b-3 promulgated under the Exchange Act, and meets the requirements for independence
under the Nasdaq Listing Rules. Each member of this committee meets the requirements for independence under the current Nasdaq Listing
Rules. The chair of our compensation committee is Mr. Abramowitz.
Our
compensation committee is responsible for, among other things:
| ● | reviewing
and making recommendations to our board of directors as to our general compensation philosophy
and overseeing the development and implementation of an executive compensation program and
policies related to such program; |
| ● | annually
reviewing and recommending to the board of directors the corporate performance goals and
objectives relevant to the compensation of our Chief Executive Officer, and annually reviewing
the performance of our Chief Executive Officer and recommending to our board of directors
the compensation level for our Chief Executive Officer; |
| ● | reviewing
and approving the compensation of our other executive officers; |
| ● | reviewing
and recommending to our board of directors the compensation of our directors; |
| ● | overseeing
the administration of our equity incentive plans; |
| ● | reviewing
and approving, or making recommendations to our board of directors with respect to, incentive
compensation and equity plans; |
| ● | reviewing
and approving the retention or termination of any consulting firm or outside advisor to assist
in the evaluation of compensation matters; and |
| ● | evaluating
and assessing potential and current compensation advisors in accordance with the independence
standards identified in the applicable Nasdaq rules. |
Nominating and Corporate Governance Committee
Our
nominating and corporate governance committee consists of Ms. Kaiser and Mr. Susin. Our board of directors has determined that each member
of the nominating and corporate governance committee meets the requirements for independence under the Nasdaq Listing Rules. The chair
of our nominating and corporate governance committee is Ms. Kaiser.
Our
nominating and corporate governance committee is responsible for, among other things:
| ● | developing
criteria for the selection of new directors and committee membership, including policies
regarding the desired knowledge, experience, skills, independence, diversity, and other characteristics
of board and committee members; |
| ● | identifying,
reviewing and evaluating candidates for membership on our board of directors and making recommendations
to our board of directors regarding the size, composition and structure of our board of directors
and its committees; |
| ● | considering
proposals submitted by our shareholders and establishing any policies, requirements, criteria
and procedures to facilitate shareholder communications with our board of directors; |
| ● | annually
reviewing and recommending to our board of directors’ determinations with respect to
the independence of continuing and prospective directors within the meaning prescribed by
the SEC and Nasdaq; |
| ● | annually
reviewing and recommending to our board of directors (i) the assignment of directors
to serve on each of our board of directors committees, (ii) the chair of each committee
and (iii) the chair of our board of directors or lead independent director, as appropriate,
and recommending additional committee members to fill vacancies or as otherwise needed; |
| ● | developing,
recommending and overseeing the implementation of our corporate governance guidelines and
a code of business conduct and ethics; |
| ● | reviewing
proposed waivers of the corporate governance guidelines or the code of business conduct and
ethics for directors, executive officers and other senior financial officers; |
| ● | overseeing
the process of evaluating the performance of our board of directors and its committees; and |
| ● | assisting
our board of directors on corporate governance matters. |
Director Independence
The
Nasdaq Listing Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating
and governance committees be independent. Under the Nasdaq Listing Rules, a director will only qualify as an “independent director”
if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the
exercise of independent judgment in carrying out the responsibilities of a director. We currently satisfy all of the Nasdaq Listing Rules
regarding each member of our audit, compensation and nominating and governance committees being independent.
Audit
committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934,
as amended, or the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee
of a listed company may not, other than in his capacity as a member of the audit committee, the board of directors or any other board
committee: (i) accept, directly or indirectly, any consulting, advisory or other compensatory fee from the listed company or any
of its subsidiaries; or (ii) be an affiliated person of the listed company or any of its subsidiaries.
Additionally,
compensation committee members must not have a relationship with us that is material to the director’s ability to be independent
from management in connection with the duties of a compensation committee member.
Our
board of directors has undertaken a review of its composition, the composition of its committees and the independence of each director.
Based upon information provided by each director, our board of directors has determined that all of our directors, other than Mr. Nanula
and Mr. Reeves, qualify as “independent” directors as defined under the applicable rules and regulations of the Securities
and Exchange Commission, or SEC, and the Nasdaq Listing Rules. In making these determinations, our board of directors considered the
current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board
of directors deemed relevant in determining their independence, including the beneficial ownership of our shares by each non-employee
director, and the transactions involving them described in the section titled “Certain Relationships and Related Party Transactions.”
Mr. Nanula is not considered independent by virtue of his position as our Chief Executive Officer. Mr. Reeves is not considered
independent because the law firm in which he serves as a partner, Tingle Merrett LLP, in the current or any of the past three fiscal
years has received compensation in excess of the greater of 5% of the firm’s gross revenues or $200,000 with respect to legal services
provided to the Company.
Compensation Committee Interlocks and Insider
Participation
None
of the Company’s executive officers serves, or in the past has served, as a member of the Board of Directors or compensation committee,
or other committee serving an equivalent function, of any entity that has one or more executive officers who serve as members of the
Company’s Board or its Compensation Committee. None of the members of the Company’s Compensation Committee is, or has ever
been, an officer or employee of the company.
Code of Ethics
The
Board adopted a Code of Business Conduct and Ethics applicable to each officer, director, and employee of the Company. The full text
of our Code of Business Conduct and Ethics is posted on our website at www.ir.lucyscientific.com/corporate-governance/governance-overview.
We intend to disclose on our website any future amendments of our Code of Business Conduct and Ethics or waivers that exempt any principal
executive officer, principal financial officer, principal accounting officer or controller, persons performing similar functions or our
directors from provisions in the Code of Business Conduct and Ethics.
Term of Office
Our
board of directors is divided into three classes with staggered three-year terms. Only one class of directors will be elected at each
annual meeting of stockholders, with the other classes continuing for the remainder of their respective three-year terms. Our current
directors are divided among the three classes as follows:
| ● | the
Class I directors are Mr. Abramowitz and Mr. Susin, and their terms will expire
at the annual meeting of stockholders to be held in 2023; |
| ● | the
Class II directors are Ms. Kaiser, Mr. Nanula and Mr. Reeves, and their terms will
expire at the annual meeting of stockholders to be held in 2024; and |
| ● | the
Class III director is Dr. Nemeroff, and his terms will expire
at the annual meeting of stockholders to be held in 2025. |
At
each annual meeting of stockholders, upon the expiration of the term of a class of directors, the successor to each such director in
the class will be elected to serve from the time of election and qualification until the third annual meeting following his or her election
and until his or her successor is duly elected and qualified, in accordance with our Articles. Any additional directorships resulting
from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will
consist of one-third of our directors.
Delinquent Section 16(a) Reports
Section
16(a) of the Exchange Act requires our directors, executive officers and persons who beneficially own 10% or more of a class of securities
registered under Section 12 of the Exchange Act to file reports of beneficial ownership and changes in beneficial ownership with the
SEC. Directors, executive officers and greater than 10% stockholders are required by the rules and regulations of the SEC to furnish
us with copies of all reports filed by them in compliance with Section 16(a). To our knowledge, based solely on a review of reports furnished
to it, our officers, directors and ten percent holders have timely made the required filings other than each reporting person’s
Form 3, each of which was filed one day late.
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation Table
The
following table sets forth information concerning the compensation of our named executive officers for the years ended June 30,
2023 and 2022.
| Name and Principal Position | |
Year | |
Salary
($)(1) | | |
Option
Awards
($)(2) | | |
Total
($) | |
| Richard Nanula | |
2023 | |
| 41,665 | | |
| — | | |
| 41,665 | |
| Chief
Executive Officer(3) | |
2022 | |
| — | | |
| — | | |
| — | |
| Brian Zasitko | |
2023 | |
| 100,000 | | |
| — | | |
| 100,000 | |
| Chief Financial Officer(3) | |
2022 | |
| — | | |
| — | | |
| — | |
| Christopher McElvany | |
2023 | |
| 227,083 | | |
| — | | |
| 227,083 | |
| Chief
Executive Officer(3) (4) | |
2022 | |
| 175,000 | | |
| — | | |
| 175,000 | |
| Dr. Assad J. Kazeminy, Ph.D. | |
2023 | |
| 180,000 | | |
| — | | |
| 180,000 | |
| Chief
Scientific Officer(3) | |
2022 | |
| 180,000 | | |
| — | | |
| 180,000 | |
| (1) | The NEOs were compensated
in USD. |
| (2) | Amounts shown in this column represent the aggregate grant date fair
value for each option award granted in the fiscal year ending June 30, 202, as computed in accordance with Financial Accounting Standards
Board Accounting Standards Codification Topic 718. For a discussion of the assumptions and methodologies used to calculate the amounts
referred to above, please see the discussion of option awards contained in note 10 to the audited consolidated financial statements appearing
elsewhere in this prospectus. |
| (3) | Compensation
was paid to our executive officers personally and/or to certain entities on their behalf,
as described further in “— Narrative to Summary Compensation Table — Compensatory
Arrangements with our NEOs.” |
| (4) | On
July 24, 2023, Christopher McElvany resigned from his positions as the Company’s President
and Chief Executive Officer and resigned as a member of the Board. |
Narrative to Summary Compensation Table
Compensatory Arrangements with our NEOs
Richard D. Nanula — Employment Agreement
On
February 8, 2023, we entered into an employment agreement with Richard D. Nanula, our Executive Chair. Mr. Nanula, which we refer to
as the Nanula Employment Agreement. Under the Nanula Employment Agreement, Mr. Nanula will continue to serve as our Executive Chair.
As Executive Chair, Mr. Nanula will receive an initial annual base salary of $100,000, and he is eligible to receive an annual performance
cash bonus of up to 100% of his base salary, as determined by our board of directors in its discretion, based upon achievement of performance
goals to be established by the compensation committee of our board of directors, his overall personal performance and such other factors
as may be determined in the sole discretion of our board of directors. Additionally, the Board expects to grant to Mr. Nanula under the
2021 Plan, a stock option to purchase 668,836 common shares. The exercise price of this stock option will be $2.99. This stock option
will vest as to 25% of the underlying common shares on the grant date, and the balance of this stock option will vest and become exercisable
with respect to 13,934 common shares in 36 equal monthly installments commencing on the 13th month following the date of grant
and continuing until the 48th month following the date of grant, subject to Mr. Nanula’s continued employment with us
through each vesting date.
Under
the Nanula Employment Agreement, Mr. Nanula will be eligible to participate in certain pension, retirement, insurance and other employee
benefit plans maintained by us for our employees generally, subject to the eligibility provisions of these plans, and he will be entitled
to participate in all other bonus and benefit programs that we establish and make available to our employees, if any, to the extent his
position, tenure, salary, health and other qualifications make him eligible to participate in these programs. In addition, Mr. Nanula
is entitled to 20 business days of paid time off per annum (inclusive of personal and sick days), and to be reimbursed for all reasonable
travel, entertainment and other business expenses incurred or paid by him in connection with, or related to, the performance of his duties,
responsibilities or services, subject to, and in accordance with, our policies in effect from time to time.
Mr.
Nanula’s employment with us is “at will,” meaning that either he or we may terminate his employment with us at any
time, for any reason or no reason, and with or without Cause (as such term is described below). In the event that Mr. Nanula’s
employment with us is terminated without Cause or he resigns as an employee with Good Reason (as such term is described below), the portion
of his stock option that would have otherwise vested during the period that is 12 months following such termination or resignation will
accelerate and will vest in full, the portion of his stock option that has not vested as of the date of such termination or resignation
will expire on, and may no longer be exercised after, the effective date of such termination or resignation, and the portion of his stock
option that has vested prior to such termination or resignation may only be exercised for a period of 90 days following the effective
date of such termination or resignation, and will thereafter expire and may no longer be exercised after such 90-day period. If, however,
there is a change of control of our Company and Mr. Nanula is terminated without Cause or he resigns for Good Reason before his stock
option has vested in full, the vesting of Mr. Nanula’s stock option will accelerate in full and his stock option may thereafter
be exercised with respect to all of the underlying common shares for a period of 90 days following such termination or resignation. Further,
if Mr. Nanula’s employment with us is terminated for Cause or he resigns without Good Reason, or he is terminated or resigns as
a result of his Disability (as such term is described below), the portion of his stock option that has not vested as of the date of such
termination or resignation shall expire on the date of such termination or resignation, and the portion of his stock option that has
vested as of the date of such termination or resignation shall expire on the date that is 10 days following such termination or resignation.
If
Mr. Nanula’s employment with us is terminated by us without Cause or he resigns with Good Reason, he will be entitled to receive
cash payments, as severance, in an amount equal to (i) six months of his base salary plus (ii) one month of base salary in respect of
each full year that he has been employed by us prior to such termination or resignation, paid to him in six equal monthly installments
commencing 30 days following the effective date of such termination or resignation.
Under
the Nanula Employment Agreement, “Cause” means generally the occurrence of any of the following: (A) Mr. Nanula’s gross
negligence in connection with the performance of his duties that results in material injury to us or our affiliates; (B) his willful
and continued failure (except where due to a Disability) or refusal to perform substantially his duties; (C) any willful or intentional
act by Mr. Nanula that constitutes illegal conduct or gross misconduct and that materially injures our reputation or business; (D) the
material breach of his obligations under the Nanula Employment Agreement; (E) Mr. Nanula’s conviction of, or pleading nolo contendere
to, a felony, or a misdemeanor involving moral turpitude; (F) his commission of an act of fraud or embezzlement, or any other act that
involves the misappropriation of our material funds or assets; (G) chronic absenteeism (except where due to a Disability) or other dereliction
of duty; or (H) his failure to follow the reasonable and lawful instructions of the Board, subject to his ability to cure such breach
or conduct in certain of the foregoing instances.
“Good
Reason” means generally (A) a material reduction in Mr. Nanula’s base salary in then in effect; (B) a material diminution
in his duties, responsibility or authority; (C) a material change in the geographic location at which he is required to perform his services;
or (D) any material breach by us of the Nanula Employment Agreement, subject to our ability to cure such breach or conduct in certain
of the foregoing instances.
“Disability”
shall be deemed to occur generally if our board of directors determines that Mr. Nanula is unable to perform the essential functions
of his position, regardless of reason, with a reasonable accommodation, for a total (whether consecutive or cumulative) of 16 weeks in
any rolling 52-week period, or in the event we receive a medical certification that he will not be able to perform the essential functions
of his position permanently or for the indefinite future. Mr. Nanula’s resignation as an employee as a result of his Disability
will be treated for all purposes under his employment agreement as a resignation without Good Reason, and his death shall also be deemed
to constitute his Disability for purposes of under his employment agreement.
The
Nanula Employment Agreement includes customary confidentiality and invention assignment covenants, and an agreement by Mr. Nanula that
he will not solicit our current or former employees clients, customers or accounts either during the period of his employment with us
or the one year period after the termination or expiration of his employment with us.
Chris McElvany
On February 22, 2021,
we entered into an executive consulting agreement, which we refer to as the McElvany Agreement, with Supercritical Labs, LLC, a limited
liability company, the sole member of which is Christopher McElvany, our Chief Executive Officer. Pursuant to the McElvany Agreement,
Mr. McElvany serves as our President and Chief Executive Officer in exchange for compensation of $175,000 per annum. In addition,
Mr. McElvany may be granted annual or incentive bonuses under the McElvany Agreement, in an amount and on such terms and conditions
as the Compensation Committee may determine from time to time.
Under the McElvany Agreement,
Mr. McElvany will provide services for continuous annual terms, until terminated in accordance with the agreement. We are entitled
to terminate the McElvany Agreement at any time and for any reason. In the event we terminate the McElvany Agreement, we are required
to pay Supercritical Labs upon such termination, as severance, an amount equal to six months’ compensation, or $87,500.
Supercritical Labs may terminate
the McElvany Agreement by providing 90 days’ prior written notice to us. If Supercritical Labs terminates the agreement, we
are required to pay Mr. McElvany all unpaid compensation earned up to the date of termination, and we may either require Mr. McElvany
to continue performing his duties as our President and Chief Executive Officer for the entirety of the 90-day notice period, or dismiss
him any time after receiving notice and make a severance payment equal to two months’ compensation under the agreement. If
Supercritical Labs voluntarily terminates the McElvany Agreement for any reason other than the occurrence of a change of control of the
Company, then all vested and unvested portions of all stock options held by Supercritical Labs as of the date of termination shall be
cancelled.
Supercritical Labs may also
terminate the McElvany Agreement within 60 days following a change of control of the Company, in which case we (or our successor)
would be required to pay Supercritical Labs upon such termination, as severance, an amount equal to six months’ compensation,
or $87,500, plus the per month fees payable to Supercritical Labs under the agreement for the number of months remaining in the then-current
annual term of the agreement, with the aggregate severance payment not to exceed 12 months’ compensation, or $175,000. In addition,
in the event that Supercritical Labs terminates the McElvany Agreement following a change of control of the Company as described above,
the entire unvested portion of all stock options granted to Supercritical Labs (or Mr. McElvany) prior to such termination and then
held as of the date of termination will accelerate and vest in full, and may thereafter be exercised at any time and from time to time
during the six-month period following such termination.
Supercritical Labs, on its
behalf and on behalf of its affiliates, including Mr. McElvany, is required to release all claims it or he may have against us in
connection with, and as a condition to, the payment of the severance and other benefits described above, and is required to cooperate
with and assist us in connection with any change of control and the associated transitional period.
The McElvany Agreement also
provides for various customary confidentiality, non-competition and non-solicitation provisions. The non-competition and non-solicitation
provisions generally apply for a period of two-years following termination of the McElvany Agreement. In the event that we terminate the
agreement, or Supercritical Labs terminates the agreement within 60 days following a change of control, Supercritical Labs is generally
further restricted for an additional three months from engaging in certain activities relating to stock ownership and participation
in our governance and affairs. The McElvany Agreement also includes customary indemnification rights.
On
February 8, 2023, we entered into a new employment agreement with Mr. McElvany, which we refer to as the McElvany Employment Agreement.
Under the McElvany Employment Agreement, Mr. McElvany serve as Chief Executive Officer and Director. As Chief Executive Officer, Mr. McElvany
will receive an initial annual base salary of $300,000, which will increase to $400,000 on the first anniversary of the closing of the
IPO, and he is eligible to receive an annual performance cash bonus of up to 30% of his base salary, as determined by our board of directors
in its discretion, based upon achievement of performance goals to be established by the compensation committee of our board of directors,
his overall personal performance and such other factors as may be determined in the sole discretion of our board of directors.
Mr.
McElvany resigned as chief executive officer and director on July 24, 2023. In connection with his resignation, the Company agreed to
(1) the issuance of 187,500 common shares, (2) $161,507 in related to the McElvany Agreement and accrued salary, and (3) $10,502 in expenses.
Brian Zasitko — Employment Agreement
On
January 1, 2023, we entered into an employment agreement with Brian Zasitko, our Chief Financial Officer, which we refer to as the Zasitko
Employment Agreement. Under the Zasitko Employment Agreement, Mr. Zasitko will serve as our Chief Financial Officer. As Chief Financial
Officer, Mr. Zasitko will receive an initial annual base salary of $200,000, and he is eligible to receive an annual performance cash
bonus of up to 100% of his base salary, as determined by our board of directors in its discretion, based upon achievement of performance
goals to be established by the compensation committee of our board of directors, his overall personal performance and such other factors
as may be determined in the sole discretion of our board of directors. Additionally, the Board expects to grant to Mr. Zasitko under
the 2021 Plan, a stock option to purchase 307,332 common shares. The exercise price of this stock option will be $2.99. This stock option
will vest as to 25% of the underlying common shares on the grant date, and the balance of this stock option will vest and become exercisable
with respect to 8,537 common shares in 36 equal monthly installments commencing on the 13th month following the date of grant
and continuing until the 48th month following the date of grant, subject to Mr. Zasitko’s continued employment with
us through each vesting date.
Under
the Zasitko Employment Agreement, Mr. Zasitko will be eligible to participate in certain pension, retirement, insurance and other employee
benefit plans maintained by us for our employees generally, subject to the eligibility provisions of these plans, and he will be entitled
to participate in all other bonus and benefit programs that we establish and make available to our employees, if any, to the extent his
position, tenure, salary, health and other qualifications make him eligible to participate in these programs. In addition, Mr. Nanula
is entitled to 20 business days of paid time off per annum (inclusive of personal and sick days), and to be reimbursed for all reasonable
travel, entertainment and other business expenses incurred or paid by him in connection with, or related to, the performance of his duties,
responsibilities or services, subject to, and in accordance with, our policies in effect from time to time.
Mr.
Zasitko’s employment with us is “at will,” meaning that either he or we may terminate his employment with us at any
time, for any reason or no reason, and with or without Cause (as such term is described below). In the event that Mr. Zasitko’s
employment with us is terminated without Cause or he resigns as an employee with Good Reason (as such term is described below), the portion
of his stock option that would have otherwise vested during the period that is 12 months following such termination or resignation will
accelerate and will vest in full, the portion of his stock option that has not vested as of the date of such termination or resignation
will expire on, and may no longer be exercised after, the effective date of such termination or resignation, and the portion of his stock
option that has vested prior to such termination or resignation may only be exercised for a period of 90 days following the effective
date of such termination or resignation, and will thereafter expire and may no longer be exercised after such 90-day period. If, however,
there is a change of control of our Company and Mr. Zasitko is terminated without Cause or he resigns for Good Reason before his stock
option has vested in full, the vesting of Mr. Zasitko’s stock option will accelerate in full and his stock option may thereafter
be exercised with respect to all of the underlying common shares for a period of 90 days following such termination or resignation. Further,
if Mr. Zasitko’s employment with us is terminated for Cause or he resigns without Good Reason, or he is terminated or resigns as
a result of his Disability (as such term is described below), the portion of his stock option that has not vested as of the date of such
termination or resignation shall expire on the date of such termination or resignation, and the portion of his stock option that has
vested as of the date of such termination or resignation shall expire on the date that is 10 days following such termination or resignation.
If
Mr. Zasitko’s employment with us is terminated by us without Cause or he resigns with Good Reason, he will be entitled to receive
cash payments, as severance, in an amount equal to (i) six months of his base salary plus (ii) one month of base salary in respect of
each full year that he has been employed by us prior to such termination or resignation, paid to him in six equal monthly installments
commencing 30 days following the effective date of such termination or resignation.
Under
the Zasitko Employment Agreement, “Cause” means generally the occurrence of any of the following: (A) Mr. Zasitko’s
gross negligence in connection with the performance of his duties that results in material injury to us or our affiliates; (B) his willful
and continued failure (except where due to a Disability) or refusal to perform substantially his duties; (C) any willful or intentional
act by Mr. Zasitko that constitutes illegal conduct or gross misconduct and that materially injures our reputation or business; (D) the
material breach of his obligations under the Zasitko Employment Agreement; (E) Mr. Zasitko’s conviction of, or pleading nolo contendere
to, a felony, or a misdemeanor involving moral turpitude; (F) his commission of an act of fraud or embezzlement, or any other act that
involves the misappropriation of our material funds or assets; (G) chronic absenteeism (except where due to a Disability) or other dereliction
of duty; or (H) his failure to follow the reasonable and lawful instructions of the Board, subject to his ability to cure such breach
or conduct in certain of the foregoing instances.
“Good
Reason” means generally (A) a material reduction in Mr. Zasitko’s base salary in then in effect; (B) a material diminution
in his duties, responsibility or authority; (C) a material change in the geographic location at which he is required to perform his services;
or (D) any material breach by us of the Zasitko Employment Agreement, subject to our ability to cure such breach or conduct in certain
of the foregoing instances.
“Disability”
shall be deemed to occur generally if our board of directors determines that Mr. Zasitko is unable to perform the essential functions
of his position, regardless of reason, with a reasonable accommodation, for a total (whether consecutive or cumulative) of 16 weeks in
any rolling 52-week period, or in the event we receive a medical certification that he will not be able to perform the essential functions
of his position permanently or for the indefinite future. Mr. Zasitko’s resignation as an employee as a result of his Disability
will be treated for all purposes under his employment agreement as a resignation without Good Reason, and his death shall also be deemed
to constitute his Disability for purposes of under his employment agreement.
The
Zasitko Employment Agreement includes customary confidentiality and invention assignment covenants, and an agreement by Mr. Zasitko that
he will not solicit our current or former employees clients, customers or accounts either during the period of his employment with us
or the one year period after the termination or expiration of his employment with us.
Dr. Asad J. Kazeminy, Ph.D.
In
February 2021, we entered into an Executive Consulting Agreement, which we refer to as the Kazeminy Agreement, with AJK Biopharmaceutical
LLC — Canadian Consulting Series, a limited liability company, the principal for which is Dr. Assad J. Kazeminy,
Ph.D. Pursuant to the Kazeminy Agreement, Dr. Kazeminy serves as our Chief Scientific Officer in exchange for compensation
of $180,000 per annum for a term of three years. Dr. Kazeminy provides services to us as an independent contractor for up to
20 hours per week. The Kazeminy Agreement provides for the issuance of an option to purchase 166,667 shares of our common shares.
Accordingly, as required by Dr. Kazeminy’s Executive Consulting Agreement, the Board expects to grant to Dr. Kazeminy, under the
2021 Plan, a stock option to purchase 166,667 common shares. The exercise price of these stock options is $2.99. This stock option will
vest as to 25% of the underlying common shares on the grant date, and the balance of this stock option will vest and become exercisable
with respect to 3,472 common shares in 36 equal monthly installments commencing on the 13th month following the date of grant
and continuing until the 48th month following the date of grant, subject to Dr. Kazeminy’s continued employment with
us through each vesting date. If AJK Biopharmaceutical LLC terminates the Kazeminy Agreement for any reason other than a change of control
of the Company, Dr. Kazeminy is entitled to both the vested and unvested portion of all stock options held by AJK Biopharmaceutical LLC
as of the date of termination will be cancelled as of the termination date. Moreover, pursuant to the Kazeminy Agreement, Dr. Kazeminy
may be granted annual or incentive bonuses in an amount and on such terms and conditions as the Compensation Committee in its sole discretion
may determine from time to time.
The
Kazeminy Agreement also provides for various customary confidentiality, non-competition and non-solicitation provisions. The non-competition
provision only applies while the Kazeminy Agreement is in effect, whereas the non-solicit extends for two years following termination
of the agreement. Notwithstanding the non-compete provision, the Kazeminy Agreement permits Dr. Kazeminy to continue to carry out
any of his duties with Aingeal Therapeutics and AJK Biopharmaceutical LLC, other entities for which he provides services. Additionally,
in the event that we terminate the agreement or Dr. Kazeminy terminates the agreement within 60 days following a change of
control, Dr. Kazeminy is restricted for a further three months from certain activities relating to stock ownership and participation
in company governance and affairs. The Kazeminy Agreement provides for customary indemnification rights.
Outstanding Equity Awards at Fiscal Year-End
The
following table summarizes the number of common shares underlying outstanding equity incentive plan awards for each named executive officer
as of June 30, 2023.
| | |
| Option
Awards | | |
| Stock
Awards | |
| Name | |
| Grant
Date | | |
| Number
of
Securities
Underlying
Unexercised
Options (#)
Exercisable | | |
| Number
of
Securities
Underlying
Unexercised
Options (#)
Unexercisable | | |
| Equity
Incentive
Plan awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options | | |
| Option
Exercise
Price
($)(1) | | |
| Option
Expiration
Date | | |
| Number of
Shares of
Stock That
Have Not
Vested | | |
| Market
Value of
Shares of
Stock That
Have Not
Vested(2) | |
| Richard Nanula(1) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Brian Zasitko(2) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Christopher McElvany | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Dr. Assad Kazeminy(3) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| (1) | We expect to grant Richard
Nanula options to purchase 668,836 of our common shares. The exercise price of this stock
options will be $2.99. |
| (2) | We
expect to grant Brian Zasitko options to purchase 307,332 of our common shares. The exercise
price of this stock options will be $2.99. |
| (3) | We
expect to grant AJK Biopharmaceutical LLC. options to purchase 166,667 of our common shares.
The exercise price of this stock options will be $2.99. Dr. Assad Kazeminy is the principal
of AJK Biopharmaceutical LLC. |
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL
OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The
following table sets forth certain information known to us with respect to the beneficial ownership of our common shares, as of October
13, 2023.
We
have determined beneficial ownership in accordance with the rules of the Securities and Exchange Commission, and the information is not
necessarily indicative of beneficial ownership for any other purpose. These rules generally attribute beneficial ownership of securities
to persons who possess sole or shared voting power or investment power with respect to those securities as well as any common shares that
the person has the right to acquire within 60 days of October 13, 2023 through the exercise of stock options or other rights. These
shares are deemed to be outstanding and beneficially owned by the person holding those options for the purpose of computing the percentage
ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all
shares shown as beneficially owned by them.
| | |
Beneficially Ownership Common
Stock | |
| | |
Shares | | |
Percentage | |
| Name of beneficial owner | |
| | |
| |
| 5% shareholders: | |
| | |
| |
| Christopher
McElvany(1) | |
| 1,662,831 | | |
| 8.08 | % |
| Renee
Gagnon(2) | |
| 1,156,864 | | |
| 5.62 | % |
| Named executive officers and directors: | |
| | | |
| | |
| Richard D. Nanula | |
| 167,216 | | |
| * | |
| Brian Zasitko | |
| 76,833 | | |
| * | |
| Paul Abramowitz | |
| 25,000 | | |
| * | |
| Brittany Kaiser | |
| 25,000 | | |
| * | |
| Dr. Assad
J. Kazeminy(3) | |
| 126,667 | | |
| * | |
| Charles B. Nemeroff, M.D., Ph.D. | |
| 25,000 | | |
| * | |
| Scott
M. Reeves(4) | |
| 52,778 | | |
| * | |
| Livio
Susin(5) | |
| 188,700 | | |
| * | |
| All executive officers and directors
as a group (eight (8) persons) | |
| 687,194 | | |
| 3.34 | % |
| (1) | Consists
of (i) 990,741 common shares held by Mr. McElvany; (ii) 366,187 common shares held
by Downwind Investments, LLC (“Downwind Investments”), Mr. McElvany and
Ms. Sharon Lynn McElvany, his wife, are the sole members of Downwind Investments and have
shared voting and investment control with respect to common shares held by Downwind Investments;
(iii) 118,403 common shares held by Supercritical Labs, LLC (“Supercritical Labs”),
Mr. McElvany is the sole member of Supercritical Labs and has sole voting and investment
control with respect to common shares held by Supercritical Labs; and (iv) 187,500 common
shares to be issued to Mr. McElvany. |
| |
(2) |
Consists of (i) 423,955 common shares held by Ms. Gagnon; (ii) 125,001 common shares subject to options to purchase common shares exercisable within 60 days of June 30, 2023 held by Ms. Gagnon; (iii) 1,389 common shares held by Meagan Gagnon, Ms. Gagnon’s daughter; (iv) 1,389 common shares held by Fearon Gagnon, Ms. Gagnon’s son; (v) 280,834 common shares held by Heather Jennings, Ms. Gagnon’s spouse and our former employee; (vi) 83,334 common shares subject to options to purchase common shares exercisable within 60 days of June 30, 2023 held by Heather Jennings, Ms. Gagnon’s spouse and our former employee; (vii) 128,786 common shares held by 1118737 BC Ltd. Ms. Gagnon and Ms. Jennings are the sole shareholders of 1118737 BC Ltd and have shared voting and investment control with respect to common shares held by 1118737 BC Ltd; and (viii) 112,176 common shares held by Freedom Family Office Inc. Ms. Gagnon and Ms. Jennings are the sole shareholders of 1118737 BC Ltd and have shared voting and investment control with respect to common shares held by Freedom Family Office Inc. |
| (3) | Consists
of (i) 85,000 common shares held by AJK Biopharmaceutical LLC, a company controlled by Mr.
Kazeminy; and (2) 41,667 common shares issuable upon exercise of options within 60 days of
June 30, 2023 which are expected to be granted to Dr. Kazeminy. |
| (4) | Consists
of (i) 27,778 common shares held by Mr. Reeves; and (ii) 25,000 common shares issuable upon
exercise of options within 60 days of June 30, 2023, which are expected to be granted. |
| (5) | Consists
of (i) 134,809 common shares held by Mr. Susin; (ii) 50,000 common shares subject
to options held by Mr. Susin exercisable within 60 days of June 30, 2023; (iii) 2,223
common shares held by Ferruccio Susin, Mr. Susin’s brother; (iv) 278 shares held by
Serge Susin, Mr. Susin’s brother; (v) 834 common shares held by Rose-Marie Susin, Mr.
Susin’s sister; (vi) 278 common shares held by Darren Susin, Mr. Susin’s nephew;
and (vii) 278 common shares held by Scott Susin, Mr. Susin’s nephew. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED
TRANSACTIONS
The
following includes a summary of transactions since July 1, 2021, to which we have been a party in which the amount involved exceeded
or will exceed the lesser of (i) $120,000 and (ii) one percent (1%) of the average of our total assets at year-end for the
prior two fiscal years, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than
5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material
interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under
“Executive Compensation.” We also describe below certain other transactions with our directors, executive officers and stockholders.
McElvany Convertible Note
In
February 2021, we issued a convertible promissory note in the amount of $500,000 to Downwind Investments, LLC, or Downwind. Mr. McElvany
is the principal of Downwind. The convertible promissory note bears interest at the rate of 8% per annum and matures on February 25,
2022. There is no default rate of interest under the note. The outstanding principal amount and accrued interest under the note is convertible
at the option of the holder into our common shares at a price of $1.58 per common share. The convertible note contains customary events
of default, including for failure to pay amounts under the note when due; the entry of certain judgments or orders against us; loss,
theft, damage or description of our property in excess of specified amounts; the sale, transfer or other disposition of all or substantially
all of our assets; and certain events respect to insolvency or bankruptcy.
The
debt is secured pursuant to a general security agreement we entered into with Mr. McElvany, pursuant to which we pledged as collateral
for the loan certain of our assets, including, without limitation, all of our personal property including debts, accounts, claims, equipment,
inventory, documents of title, securities, money and intangible personal property such as licenses, contractual rights, patents, trademarks
and other intellectual property; all real property or leasehold property; fixtures; and investment property including capital of each
of our subsidiaries. The general security agreement contains customary affirmative and negative covenants undertaken by us, including
that we may not sell, lease or otherwise dispose of any of the collateral except in the ordinary course of business on commercially reasonable
terms. We are also restricted from permitting the collateral to become subject to any mortgage, charge, encumbrance or security interest,
except in the ordinary course of business.
On
February 13, 2023, the convertible note of $500,000 plus accrued interest of $78,575 were converted into 366,187 common shares. The Company
has no continuing obligation with respect to the convertible note.
Susin Promissory Notes
In
December 2018, we issued a promissory note to Livio Susin, one of our directors, in the principal amount of $144,666, pursuant to
which Mr. Susin loaned funds to us through a series of advances. All indebtedness under this promissory note bears interest at a
rate of 21% per annum. The indebtedness under this promissory note is unsecured, and repayable 90 days following to the successful completion
of an initial public offering or a reverse takeover transaction which results in our shares being listed on a public exchange. As of
September 30, 2022, the total outstanding principal amount of, and accrued interest under, this promissory note was $86,269. In November
2022, we entered into debt settlement and subscription agreement with Mr. Susin for the settlement of the promissory note, through the
issuance of common our shares at a 40% discount to the price of an initial public offering. The Company entered into a debt settlement
and subscription agreement with the director and stockholder under which the note payable was settled on February 13, 2023 through the
issuance of 36,962 common shares. The Company has no continuing obligation with respect to the note.
In
February 2019, we issued a second promissory note to Mr. Susin in the principal amount of $245,768, pursuant to which he loaned
funds to us through a series of advances. Indebtedness under this promissory note bears interest at a rate of 2% per annum. The indebtedness
under this promissory note is unsecured, and is repayable 90 days following to the successful completion of an initial public offering
or a reverse takeover transaction which results in our shares being listed on a public exchange. In January 2021, Mr. Susin
forgave $39,746 (CAD$50,000) of indebtedness under this promissory note in exchange for 13,889 shares of our Class B common shares.
During the year ended June 30, 2023, the Company made full repayment of the note of $207,236 (CAD$280,735). The Company has no
continuing obligation with respect to the note.
Susin Consulting Agreement
Pursuant
to a Consulting Agreement, dated December 16, 2020, Mr. Susin provides executive administration advisory and consulting services
to us and our two subsidiaries, LSDI Manufacturing Inc. and TerraCube, in exchange for CAD $12,500 per month.
The
Susin Consulting Agreement was terminated on May 31, 2022.
Strategic Investment Agreement
On
January 16, 2023, we entered into a strategic investment agreement, or the Strategic Investment Agreement, with Hightimes Holding Corp.,
or Hightimes, 1252240 BC LTD, a wholly owned subsidiary of Hightimes, and Trans-High Corporation, a wholly owned subsidiary of Hightimes,
pursuant to which Hightimes granted to us $833,333 of annual advertising and marketing credits, or Advertising Credits, for three consecutive
years, in exchange for 625,000 of our common shares. The Advertising Credits enable us to advertise (i) on all Hightimes publications,
including the Hightimes print and website publications, and (ii) at all festivals and events conducted by Hightimes. Unless earlier terminated
pursuant to the terms of the Strategic Investment Agreement, the Strategic Investment Agreement will terminate on December 31, 2025,
which term may be extended by the parties to the Strategic Investment Agreement upon such terms and conditions as the parties may mutually
agree. Paul Abramowitz, one of our directors, is the stepfather of the Executive Chairman of Hightimes. Mr. Abramowitz’s biological
son is a beneficial owner of Roma Ventures, LLC, or Roma Ventures, an entity that owns approximately 3.9% of our issued and outstanding
common shares. Benjamin Windle is the investment manager of Roma Ventures and has sole voting and investment control with respect to
our common shares held by the Roma Ventures. Each of the Executive Chairman of Hightimes, Mr. Abramowitz and Roma Ventures are shareholders
of Hightimes.
Policies and Procedures for Related Party Transactions
Our board of directors has
adopted a written related party transaction policy setting forth the policies and procedures for the review and approval or ratification
of related-party transactions. This policy covers any transaction, arrangement or relationship or any series of similar transactions,
arrangements or relationships, in which we were or are to be a participant and a related party had or will have a direct or indirect material
interest, as determined by the audit committee of our board of directors, including, without limitation, purchases of goods or services
by or from the related party or entities in which the related party has a material interest, and indebtedness, guarantees of indebtedness
or employment by us of a related party.
All related party transactions
described in this section occurred prior to adoption of this policy and as such, these transactions were not subject to the approval and
review procedures set forth in the policy. However, these transactions were reviewed and approved by our board of directors. Our board
of directors reviews and approves transactions with directors, officers and holders of five percent or more of our voting securities and
their affiliates, each a related party. Prior to this offering, the material facts as to the related party’s relationship or interest
in the transaction are disclosed to our board of directors prior to their consideration of such transaction, and the transaction is not
considered approved by our board of directors unless a majority of the directors who are not interested in the transaction approve the
transaction. Further, when our stockholders are entitled to vote on a transaction with a related party, the material facts of the related
party’s relationship or interest in the transaction are disclosed to the stockholders, who must approve the transaction in good
faith.
Disclosure of SEC Position on Indemnification of Securities Act
Liabilities
We
have agreed to indemnify each of our directors and certain officers against certain liabilities, including liabilities under the Securities
Act. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling
persons pursuant to the provisions described above, or otherwise, we have been advised that in the opinion of the SEC such indemnification
is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification
against such liabilities (other than our payment of expenses incurred or paid by our director, officer or controlling person in the successful
defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities
being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court
of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act
and will be governed by the final adjudication of such issue.
We
have been advised that in the opinion of the SEC indemnification for liabilities arising under the Securities Act is against public policy
as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities
is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, we will, unless
in the opinion of our legal counsel the matter has been settled by controlling precedent, submit the question of whether such indemnification
is against public policy to a court of appropriate jurisdiction. We will then be governed by the court’s decision.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Fees Billed for Audit and Non-Audit Services
The following table presents
for each of the last two fiscal years the aggregate fees billed in connection with the audits of our financial statements and other professional
services rendered by our independent registered public accounting firm Marcum LLP.
| | |
2023 | | |
2022 | |
| Audit Fees (1) | |
$ | 332,072 | | |
$ | 185,349 | |
| (1) | Audit Fees. These are
fees for professional services for the audit of our annual financial statements, and for the review of the financial statements included
in our filings on Form 10-K and Form 10-Q, and for services that are normally provided in connection with statutory and regulatory filings
or engagements. |
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| Exhibit |
|
|
|
Incorporated by Reference |
|
Filed or Furnished |
| Number |
|
Exhibit Description |
|
Form |
|
Exhibit |
|
Filing Date |
|
Herewith |
| 3.1 |
|
Amended and Restated Articles of Incorporation of the Registrant. |
|
S-1 |
|
3.1 |
|
01/21/2022 |
|
|
| 4.1 |
|
Form of Common Share Certificate. |
|
S-1 |
|
4.1 |
|
12/15/2022 |
|
|
| 4.2 |
|
Warrant to Purchase Common Shares, dated November 5, 2020, issued by the Registrant to Origo Holdings, Inc. |
|
S-1 |
|
4.3 |
|
01/21/2022 |
|
|
| 4.3 |
|
Form of Warrant to Purchase Common Shares, dated January 22, 2021, issued by the Registrant to Affiliates of Origo Holdings, Inc. |
|
S-1 |
|
4.4 |
|
01/21/2022 |
|
|
| 4.4 |
|
Description of Securities |
|
|
|
|
|
|
|
X |
| 10.1+ |
|
Form of Indemnification Agreement |
|
S-1/A |
|
10.1 |
|
12/15/2022 |
|
|
| 10.2+ |
|
2019 Stock Option Plan of the Registrant. |
|
S-1 |
|
10.2 |
|
01/21/2022 |
|
|
| 10.3+ |
|
Form of Option Certificate under the 2019 Stock Option Plan. |
|
S-1 |
|
10.3 |
|
01/21/2022 |
|
|
| 10.4+ |
|
2021 Equity Incentive Plan of the Registrant. |
|
S-1/A |
|
10.4 |
|
03/04/2022 |
|
|
| 10.5+ |
|
Form of Option Award under the 2021 Equity Incentive Plan. |
|
S-1/A |
|
10.5 |
|
03/04/2022 |
|
|
| 10.6+ |
|
Executive Consulting Agreement, dated February 22, 2021, by and between Supercritical Labs, LLC and the Registrant. |
|
S-1/A |
|
10.6 |
|
01/21/2022 |
|
|
| 10.7+ |
|
Form of Employment Agreement by and between Richard D. Nanula and the Registrant. |
|
S-1/A |
|
10.8 |
|
12/05/2022 |
|
|
| 10.8+ |
|
Form of Employment Agreement by and between Brian Zasitko and the Registrant. |
|
S-1/A |
|
10.9 |
|
12/05/2022 |
|
|
| 10.9+ |
|
Executive Consulting Agreement, dated February 22, 2021, by and between AJK Biopharmaceutical LLC — Canadian Consulting Series and the Registrant. |
|
S-1 |
|
10.8 |
|
01/21/2022 |
|
|
| 10.10+ |
|
Consulting Agreement, dated December 16, 2020, by and between Livio Susin and the Registrant. |
|
S-1 |
|
10.9 |
|
01/21/2022 |
|
|
| 10.11+ |
|
Consulting Agreement, dated September 30, 2020, by and between Renee Gagnon and the Registrant, as amended by the Amendment No. 1, dated December 21, 2020. |
|
S-1 |
|
10.10 |
|
01/21/2022 |
|
|
| 10.12+ |
|
Minutes of Settlement, dated April 20, 2020, by and among Mary Stipancic, Renee Gagnon, Livio Susin, Heather Jennings and the Registrant. |
|
S-1 |
|
10.11 |
|
01/21/2022 |
|
|
| 10.13+ |
|
First Amendment to Minutes of Settlement, dated October 23, 2020, by and among Mary Stipancic, Renee Gagnon, Livio Susin, Heather Jennings and the Registrant. |
|
S-1 |
|
10.12 |
|
01/21/2022 |
|
|
| 10.14+ |
|
Second Amendment to Minutes of Settlement, dated May 12, 2021, by and among Mary Stipancic, Renee Gagnon, Livio Susin, Heather Jennings and the Registrant. |
|
S-1 |
|
10.13 |
|
01/21/2022 |
|
|
| 10.15 |
|
Offer to Lease, dated March 22, 2017, Ark Holdings Ltd. and the Registrant, as amended. |
|
S-1 |
|
10.14 |
|
01/21/2022 |
|
|
| 10.16 |
|
Line of Credit Agreement, dated November 5, 2020, by and among Origo BC Holdings Ltd., its Participating Lenders, the Registrant and its subsidiaries, as amended. |
|
S-1 |
|
10.15 |
|
01/21/2022 |
|
|
| 10.17 |
|
Promissory Note, dated January 1, 2019, issued by the Registrant to Livio Susin. |
|
S-1 |
|
10.16 |
|
01/21/2022 |
|
|
| 10.18 |
|
Promissory Note, dated February 19, 2019, issued by the Registrant to Livio Susin. |
|
S-1 |
|
10.17 |
|
01/21/2022 |
|
|
| 10.19 |
|
Promissory Note, dated April 17, 2019, issued by the Registrant to 1118737 BC Ltd. |
|
S-1 |
|
10.18 |
|
01/21/2022 |
|
|
| 10.20 |
|
Promissory Note, dated February 25, 2021, issued by the Registrant to Downwind Investments LLC. |
|
S-1 |
|
10.19 |
|
01/21/2022 |
|
|
| 10.21 |
|
Asset Purchase Agreement, dated February 25, 2021, by and between the Registrant and Chris McElvany. |
|
S-1 |
|
10.20 |
|
01/21/2022 |
|
|
| 10.22 |
|
Strategic Investment Agreement, dated January 16, 2023 by and among Hightimes Holding Corp. 1252240 BC LTD., Trans-High Corporation and the Registrant. |
|
S-1/A |
|
10.23 |
|
01/18/2022 |
|
|
| 10.23 |
|
Definitive asset purchase agreement with Wesana Health Holdings Inc. |
|
10-Q |
|
10.2 |
|
05/10/2023 |
|
|
| + | Management contracts or compensatory plan. |
ITEM 16. FORM 10-K SUMMARY
Not applicable.
SIGNATURES
Pursuant to the requirements of Section 13 or
15(d) of the Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
Lucy Scientific Discovery Inc. |
| |
|
|
| Date: October 13, 2023 |
By: |
/s/ Richard D. Nanula |
| |
Name: |
Richard D. Nanula |
| |
Title: |
Chief Executive Officer |
Pursuant to the requirements of the Securities
Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and
on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/ Richard
D. Nanula |
|
Chief Executive Officer and Executive Chairman |
|
October 13, 2023 |
| Richard D. Nanula |
|
(Principal Executive Officer) |
|
|
| |
|
|
|
|
| /s/ Brian
Zasitko |
|
Chief Financial Officer |
|
October 13, 2023 |
| Brian Zasitko |
|
(Principal Financial
Officer and Principal Accounting Officer) |
|
|
| |
|
|
|
|
| /s/ Paul
Abramowitz |
|
Director |
|
October 13, 2023 |
| Paul Abramowitz |
|
|
|
|
| |
|
|
|
|
| /s/ Brittany
Kaiser |
|
Director |
|
October 13, 2023 |
| Brittany Kaiser |
|
|
|
|
| |
|
|
|
|
| /s/ Charles
B. Nemeroff |
|
Director |
|
October 13, 2023 |
| Charles B. Nemeroff,
M.D., Ph.D. |
|
|
|
|
| |
|
|
|
|
| /s/
Scott Reeves |
|
Director |
|
October 13, 2023 |
| Scott Reeves |
|
|
|
|
| |
|
|
|
|
| /s/
Livio Susin |
|
Director |
|
October 13, 2023 |
| Livio Susin |
|
|
|
|
LUCY SCIENTIFIC DISCOVERY INC.
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED JUNE 30, 2023 AND 2022
TABLE OF CONTENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
To the Stockholders and Board of Directors of
Lucy Scientific Discovery Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheets of Lucy Scientific Discovery Inc. (the “Company”) as of June 30, 2023 and 2022, the related consolidated statements
of operations and comprehensive loss, stockholders’ deficit and cash flows for each of the two years in the period ended June 30,
2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements
present fairly, in all material respects, the financial position of the Company as of June 30, 2023 and 2022, and the results of its operations
and its cash flows for each of the two years in the period ended June 30, 2023, in conformity with accounting principles generally accepted
in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying consolidated financial statements
have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 1, the Company has historically
had significant negative operating cash flows and net losses and needs to raise additional funds to meet its obligations and sustain its
operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in
regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might
result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We
are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are
required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and
regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding
of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal
control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Marcum LLP
Marcum LLP
We have served as the Company’s auditor since 2021.
Costa Mesa, CA
October 13, 2023
LUCY SCIENTIFIC DISCOVERY INC.
CONSOLIDATED BALANCE SHEETS
As of June 30, 2023 and 2022
(Expressed in US Dollars, except
share amounts)
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| ASSETS | |
| | |
| |
| Current assets | |
| | |
| |
| Cash | |
| 1,673,874 | | |
| 53,379 | |
| Prepaid expenses | |
| 1,219,180 | | |
| 185,723 | |
| Accounts receivable | |
| 7,048 | | |
| — | |
| Other assets – GST receivable | |
| 62,649 | | |
| 13,232 | |
| Other receivable | |
| 336,706 | | |
| — | |
| Digital assets | |
| — | | |
| 34,106 | |
| Deferred financing costs, current | |
| 523,041 | | |
| 1,612,228 | |
| Total current assets | |
| 3,822,498 | | |
| 1,898,668 | |
| | |
| | | |
| | |
| Non-current assets | |
| | | |
| | |
| Deferred financing costs, noncurrent | |
| — | | |
| 1,869,969 | |
| Property, plant, and equipment | |
| 764,650 | | |
| 843,500 | |
| Prepaid expenses, noncurrent | |
| 1,663,333 | | |
| — | |
| Right of use asset | |
| 1,025,033 | | |
| — | |
| Intangible assets | |
| 1,484,250 | | |
| — | |
| Long-term deposits | |
| 18,882 | | |
| 19,401 | |
| TOTAL ASSETS | |
| 8,778,646 | | |
| 4,631,538 | |
| | |
| | | |
| | |
| LIABILITIES | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Accounts payable and accrued liabilities | |
| 1,291,063 | | |
| 2,814,532 | |
| Convertible notes, current | |
| — | | |
| 825,707 | |
| Due to related parties | |
| 1,019,894 | | |
| 1,775,372 | |
| Notes payable – related parties | |
| — | | |
| 305,082 | |
| Notes payable, current | |
| 60,423 | | |
| — | |
| Lease liability, current | |
| 338,819 | | |
| 89,396 | |
| Total current liabilities | |
| 2,710,199 | | |
| 5,810,089 | |
| | |
| | | |
| | |
| Non-current liabilities | |
| | | |
| | |
| Convertible notes, noncurrent | |
| — | | |
| 2,972,161 | |
| Lease liability, noncurrent | |
| 1,389,558 | | |
| 571,062 | |
| Notes payable, noncurrent | |
| — | | |
| 56,176 | |
| TOTAL LIABILITIES | |
| 4,099,757 | | |
| 9,409,488 | |
| | |
| | | |
| | |
| STOCKHOLDERS’ EQUITY (DEFICIT) | |
| | | |
| | |
Common stock, no par value; unlimited shares authorized; 17,462,963 and 10,443,560 shares issued and outstanding as at June 30, 2023 and 2022, respectively | |
| 48,934,278 | | |
| 30,790,410 | |
| Accumulated deficit | |
| (44,415,798 | ) | |
| (35,427,342 | ) |
| Accumulated other comprehensive income (loss) | |
| 160,409 | | |
| (141,018 | ) |
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) | |
| 4,678,889 | | |
| (4,777,950 | ) |
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | |
| 8,778,646 | | |
| 4,631,538 | |
The accompanying notes are an integral part of
these consolidated financial statements.
LUCY SCIENTIFIC DISCOVERY INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars, except
share amounts)
| | |
2023 | | |
2022 | |
| | |
$ | | |
$ | |
| Net product sales | |
| 7,048 | | |
| — | |
| Cost of sales | |
| 4,500 | | |
| — | |
| Gross profit | |
| 2,548 | | |
| — | |
| Operating expenses | |
| | | |
| | |
| Selling, general and administrative expense | |
| 5,767,881 | | |
| 3,469,479 | |
| Impairment loss | |
| 78,850 | | |
| — | |
| Total operating expenses | |
| 5,846,731 | | |
| 3,469,479 | |
| Operating loss | |
| 5,844,183 | | |
| 3,469,479 | |
| | |
| | | |
| | |
| Non-operating expense (income) | |
| | | |
| | |
| Interest expense | |
| 1,966,143 | | |
| 2,064,547 | |
| Loss on debt settlement | |
| 1,178,183 | | |
| — | |
| Change in fair value of warrant liability | |
| — | | |
| 322,226 | |
| Other income | |
| (53 | ) | |
| (136 | ) |
| Total non-operating expense (income) | |
| 3,144,273 | | |
| 2,386,637 | |
| | |
| | | |
| | |
| Income tax expense | |
| — | | |
| — | |
| Net loss | |
| (8,988,456 | ) | |
| (5,856,116 | ) |
| Foreign exchange translation adjustment, net of tax of $nil | |
| 301,427 | | |
| 212,284 | |
| Comprehensive loss | |
| (8,687,029 | ) | |
| (5,643,832 | ) |
| | |
| | | |
| | |
| Net loss per common share | |
| | | |
| | |
Basic and diluted | |
| (0.71 | ) | |
| (0.68 | ) |
| | |
| | | |
| | |
| Weighted average number of common shares outstanding | |
| | | |
| | |
Basic and diluted | |
| 12,701,100 | | |
| 8,615,648 | |
The accompanying notes are an integral part of
these consolidated financial statements.
LUCY SCIENTIFIC DISCOVERY INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars, except
share amounts)
| | |
Class A voting
common shares | | |
Class B non-voting
common shares | | |
Common shares | | |
| | |
Accumulated
other | | |
Total | |
| | |
Number of
shares | | |
Paid-in
capital | | |
Number of
shares | | |
Paid-in
capital | | |
Number of
shares | | |
Paid-in
capital | | |
Accumulated
deficit | | |
comprehensive
income (loss) | | |
equity
(deficit) | |
| | |
| | |
$ | | |
| | |
$ | | |
| | |
$ | | |
$ | | |
$ | | |
$ | |
| Balance, June 30, 2021 | |
| 1 | | |
| — | | |
| 6,476,753 | | |
| 23,568,439 | | |
| — | | |
| — | | |
| (29,571,226 | ) | |
| (353,302 | ) | |
| (6,356,089 | ) |
| Share reorganization | |
| (1 | ) | |
| — | | |
| (6,476,753 | ) | |
| (23,568,439 | ) | |
| 6,476,753 | | |
| 23,568,439 | | |
| — | | |
| — | | |
| — | |
| Reclassification of warrants | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 6,392,476 | | |
| — | | |
| — | | |
| 6,392,476 | |
| Shares issued for exercise of warrants | |
| — | | |
| — | | |
| — | | |
| — | | |
| 3,477,919 | | |
| 48,866 | | |
| — | | |
| — | | |
| 48,866 | |
| Shares issued for conversion of convertible notes | |
| — | | |
| — | | |
| — | | |
| — | | |
| 185,138 | | |
| 314,016 | | |
| — | | |
| — | | |
| 314,016 | |
| Shares issued for consulting agreements | |
| — | | |
| — | | |
| — | | |
| — | | |
| 127,819 | | |
| 216,695 | | |
| — | | |
| — | | |
| 216,695 | |
| Shares issued for share purchase option exercise | |
| — | | |
| — | | |
| — | | |
| — | | |
| 175,931 | | |
| 39,346 | | |
| — | | |
| — | | |
| 39,346 | |
| Share purchase options issued | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 210,572 | | |
| — | | |
| — | | |
| 210,572 | |
| Foreign currency translation adjustment, net of tax of $nil | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 212,284 | | |
| 212,284 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (5,856,116 | ) | |
| — | | |
| (5,856,116 | ) |
| Balance, June 30, 2022 | |
| — | | |
| — | | |
| — | | |
| — | | |
| 10,443,560 | | |
| 30,790,410 | | |
| (35,427,342 | ) | |
| (141,018 | ) | |
| (4,777,950 | ) |
| Shares issued on initial public offering | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,875,000 | | |
| 7,500,000 | | |
| — | | |
| — | | |
| 7,500,000 | |
| Shares issued for conversion of convertible notes | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,932,006 | | |
| 4,307,115 | | |
| — | | |
| — | | |
| 4,307,115 | |
| Shares issued for settlement of notes payable - related party | |
| — | | |
| — | | |
| — | | |
| — | | |
| 36,962 | | |
| 88,707 | | |
| — | | |
| — | | |
| 88,707 | |
| Shares issued for settlement of accounts payable | |
| — | | |
| — | | |
| — | | |
| — | | |
| 635,065 | | |
| 2,169,171 | | |
| — | | |
| — | | |
| 2,169,171 | |
| Shares issued for settlement of due to related parties | |
| — | | |
| — | | |
| — | | |
| — | | |
| 561,203 | | |
| 1,715,712 | | |
| — | | |
| — | | |
| 1,715,712 | |
| Shares issued for consulting agreements | |
| — | | |
| — | | |
| — | | |
| — | | |
| 875,000 | | |
| 2,821,875 | | |
| — | | |
| — | | |
| 2,821,875 | |
| Shares issued as donation | |
| — | | |
| — | | |
| — | | |
| — | | |
| 104,167 | | |
| 257,032 | | |
| — | | |
| — | | |
| 257,032 | |
| Shares issued for IP acquisition | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,000,000 | | |
| 914,250 | | |
| — | | |
| — | | |
| 914,250 | |
| Shares to be issued for consulting agreement | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 457,844 | | |
| — | | |
| — | | |
| 457,844 | |
| Share issuance costs | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (2,253,764 | ) | |
| — | | |
| — | | |
| (2,253,764 | ) |
| Share purchase options | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 165,926 | | |
| — | | |
| — | | |
| 165,926 | |
| Foreign currency translation adjustment, net of tax of $nil | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 301,427 | | |
| 301,427 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (8,988,456 | ) | |
| — | | |
| (8,988,456 | ) |
| Balance, June 30, 2023 | |
| — | | |
| — | | |
| — | | |
| — | | |
| 17,462,963 | | |
| 48,934,278 | | |
| (44,415,798 | ) | |
| 160,409 | | |
| 4,678,889 | |
The accompanying notes are an integral part of
these consolidated financial statements.
LUCY SCIENTIFIC DISCOVERY INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
| | |
2023 | | |
2022 | |
| | |
$ | | |
$ | |
| Operating activities | |
| | |
| |
| Net loss | |
| (8,988,456 | ) | |
| (5,856,116 | ) |
| Items not involving cash: | |
| | | |
| | |
| Amortization expense | |
| 101,466 | | |
| — | |
| Interest expense | |
| 1,966,143 | | |
| 1,971,727 | |
| Amortization of debt discount | |
| 5,715 | | |
| 10,286 | |
| Loss on debt settlement | |
| 1,178,183 | | |
| — | |
| Shares issued for services | |
| 236,212 | | |
| 81,788 | |
| Shares issued as donation | |
| 257,032 | | |
| — | |
| Shares to be issued for consulting agreement | |
| 457,844 | | |
| — | |
| Share-based payments | |
| — | | |
| 210,572 | |
| Share purchase options | |
| 165,926 | | |
| — | |
| Impairment loss | |
| 78,850 | | |
| — | |
| Change in fair value of warrant liability | |
| — | | |
| 322,226 | |
| Unrealized foreign exchange transaction adjustment | |
| 132,008 | | |
| — | |
| Changes in non-cash working capital: | |
| | | |
| | |
| Prepaid expenses | |
| (81,289 | ) | |
| 6,899 | |
| Accounts receivable | |
| (7,048 | ) | |
| — | |
| Other assets – GST receivable | |
| (49,188 | ) | |
| 97 | |
| Other receivable | |
| (332,762 | ) | |
| — | |
| Accounts payable and accrued liabilities | |
| 407,246 | | |
| 487,593 | |
| Lease liability | |
| (305,452 | ) | |
| (226,217 | ) |
| Due to related parties | |
| 511,217 | | |
| 572,723 | |
| Net cash flows used in operating activities | |
| (4,266,353 | ) | |
| (2,418,422 | ) |
| | |
| | | |
| | |
| Investing activities | |
| | | |
| | |
| Purchase of digital assets | |
| — | | |
| (34,106 | ) |
| Sale of digital assets | |
| 34,106 | | |
| — | |
| Purchase of intangible assets | |
| (300,000 | ) | |
| — | |
| Net cash used in investing activities | |
| (265,894 | ) | |
| (34,106 | ) |
| | |
| | | |
| | |
| Financing activities | |
| | | |
| | |
| Proceeds from initial public offering | |
| 7,500,000 | | |
| — | |
| Net proceeds from Convertible Notes | |
| 340,000 | | |
| 2,829,500 | |
| Exercise of warrants | |
| — | | |
| 48,866 | |
| Exercising of options | |
| — | | |
| 39,346 | |
| Repayment of notes payable – related party | |
| (207,236 | ) | |
| — | |
| Deferred share issuance costs | |
| (1,440,476 | ) | |
| (667,474 | ) |
| Net cash flows provided by financing activities | |
| 6,192,288 | | |
| 2,250,238 | |
| Effect of foreign exchange on cash | |
| (39,546 | ) | |
| 9,639 | |
| Increase (decrease) in cash | |
| 1,620,495 | | |
| (192,651 | ) |
| Cash, beginning of year | |
| 53,379 | | |
| 246,030 | |
| Cash, end of year | |
| 1,673,874 | | |
| 53,379 | |
| | |
| | | |
| | |
| Supplemental disclosures of cash flow information: | |
| | | |
| | |
| Interest paid in cash | |
| — | | |
| — | |
| Income taxes paid in cash | |
| — | | |
| — | |
| | |
| | | |
| | |
| Non-Cash activities for financing activities: | |
| | | |
| | |
| Renewal of lease | |
| 1,144,349 | | |
| — | |
| Shares issued for settlement of accounts payable | |
| 2,169,171 | | |
| — | |
| Shares issued for settlement of due to related parties | |
| 1,715,711 | | |
| — | |
| Shares issued for conversion of convertible notes | |
| 4,307,115 | | |
| 314,016 | |
| Shares issued for conversion of notes payable – related parties | |
| 88,707 | | |
| — | |
| Shares issued for acquisition of intangible assets | |
| 914,250 | | |
| — | |
| Accounts payable incurred for acquisition of intangible assets | |
| 270,000 | | |
| — | |
| Shares issued for consulting agreements | |
| 2,821,875 | | |
| — | |
| Deferred offering costs accrued but unpaid | |
| — | | |
| 613,875 | |
| Reclassification of warrants to share capital | |
| — | | |
| 6,392,476 | |
The accompanying notes are an integral part of
these consolidated financial statements
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
NOTE 1 — NATURE OF THE ORGANIZATION
AND BUSINESS
Lucy Scientific Discovery Inc.
(“we,” “our,” “us,” or the “Company”) was incorporated under the Business Corporations
Act (British Columbia) on February 17, 2017. The Company previously specialized in developing supply chain products, services, and
distribution channels for the cannabis industry in the areas of cannabis production, cannabis extracts, edibles and other pharmaceutical
grade products. The Company changed its name from Hollyweed North Cannabis Inc. to Lucy Scientific Discovery Inc. and, under a new business
model, is engaged in the research, manufacturing and commercialization of psychedelic products. The Company’s registered office
is Suite 301 — 1321 Blanshard Street, Victoria, British Columbia, Canada.
Subsidiaries that are active
and wholly-owned by the Company to facilitate its business activities include:
| ● | TerraCube International Inc. — On October 4,
2017, the Company acquired control of TerraCube International Inc. (“TerraCube”), formerly Crop2Scale International Inc.
which was incorporated under the Business Corporations Act of British Columbia. TerraCube innovates, develops and produces
highly controlled agricultural grow environments for plant manufacturing and replication. |
| ● | LSDI Manufacturing Inc. — On June 29, 2017, the Company
incorporated LSDI Manufacturing Inc. (“LMI”), under the Business Corporations Act of British Columbia for the purposes of
cannabis extraction and manufacturing of adult-use and pharmaceutical products. LMI held a Health Canada Processor’s License under
the Cannabis Act but has never engaged in plant-touching activities up to the date the Board of Directors approved these consolidated
financial statements. On August 10, 2021, the Health Canada Standard Processor’s License was voluntarily withdrawn by LMI with
the revocation effective September 3, 2021. In August 2021, Health Canada’s Office of Controlled Substances granted us
a Controlled Drugs and Substances Dealer’s Licence under Part J of the Food and Drug Regulations promulgated under the Food
and Drugs Act (Canada), or a Dealer’s Licence. The Dealer’s Licence authorizes us to develop and produce (through cultivation,
extraction or synthesis) certain restricted substances. The company intends to develop and produce these restricted substances as pharmaceutical-grade
active pharmaceutical ingredients and their raw material. |
| ● | LSDI Retail Inc. — On June 5, 2023, the Company
incorporated LSDI Retail Inc. under the laws of the state of Delaware for the purpose of the sale of the Company’s products through
online distribution platform. |
| ● | Lucy Therapeutic Discoveries Inc. — On June
15, 2023, the Company incorporated LSDI Therapeutics Inc. under the laws of the state of Delaware to facilitate the acquisition of intellectual
property from Wesana Health on June 30, 2023, as further described in Note 7. |
| ● | Lucy Scientific Discovery USA Inc. — On November
17, 2022, the Company incorporated Lucy Scientific Discovery USA Inc. under the laws of the state of Delaware for the purpose of entering
into employment agreements with key executive officers of the Company. |
Impact of COVID-19
In March 2020, the World
Health Organization declared COVID-19 a global pandemic. This contagious disease outbreak, which has continued to spread, and any related
adverse public health developments, has adversely affected workforces, economies, and financial markets globally, and led to an economic
downturn. To date, COVID-19 has not had any material impact on the Company’s operations; however, it is possible that estimates
in these consolidated financial statements may change in the near term as a result of COVID-19 variants.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
Going Concern
The Company has incurred
net losses in recent periods and has accumulated a deficit of $44,415,798 as of June 30, 2023. The Company has funded operations
in the past primarily by the sale and issuance of our common shares, from the issuance of convertible and non-convertible promissory notes,
and our initial public offering (“IPO”). We will continue to be dependent upon equity and debt financings or collaborations
or other forms of capital at least until we are able to generate positive cash flows from product sales, if ever.
These consolidated financial
statements have been prepared on a going concern basis, which implies that the Company will continue to realize its assets and discharge
its liabilities in the normal course of business. The continuation of the Company as a going concern is dependent upon the continued financial
support from its management, its ability to identify future investment opportunities, to obtain the necessary debt or equity financing,
generating profitable operations from the Company’s future operations or the success of an initial public offering. These factors
raise substantial doubt regarding the Company’s ability to continue as a going concern during the next twelve months. These consolidated
financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification
of liabilities that might be necessary should the Company be unable to continue as a going concern.
NOTE 2 — SUMMARY OF SIGNIFICANT
ACCOUNTING POLICIES
Basis of Presentation and Principles of Consolidation
The consolidated financial
statements are prepared in accordance with accounting principles generally accepted in the United States of America and are expressed
in U.S. dollars. The consolidated financial statements include the accounts of the Company and our subsidiaries in which we have
controlling financial interest. All inter-company balances and transactions among the companies have been eliminated upon consolidation.
Use of Estimates
The preparation of the consolidated
financial statements requires management to make estimates and assumptions that affect the reported amounts of certain assets, liabilities,
revenue, and expenses as well as the related disclosures. The Company must often make estimates about effects of matters that are inherently
uncertain and will likely change in subsequent periods. Actual results could differ materially from those estimates.
Functional and Presentation Currency
The Company’s reporting
currency is the United States Dollar (“USD”). The Company’s functional currency is the local currency, Canadian
Dollar (“CAD”). Assets and liabilities of these operations are translated into USD at the end-of-period exchange rates; income
and expenses are translated using the average exchange rates for the reporting period. Resulting cumulative translation adjustments are
recorded as a component of stockholder’s equity (deficit) in the consolidated balance sheet in accumulated other comprehensive (loss).
Cash
Cash includes cash held with
Canadian financial institutions and cash held in trust with a law corporation, available upon demand.
Accounts Receivable
Included in “Accounts
receivable” on our consolidated balance sheets are amounts related to customers. As of June 30, 2023 and 2022, accounts receivable,
net, were $7,048 and $nil.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
We estimate losses on receivables
based on expected losses, including our historical experience of actual losses. Accounts receivable are considered impaired and written-off
when it is probable that all contractual payments due will not be collected in accordance with the terms of the agreement. The allowance
for doubtful accounts was $nil and $nil as of June 30, 2023 and 2022. Additions to the allowance were $nil and $nil, and deductions to
the allowance were $nil and $nil 2022 and 2023.
Digital assets
The
Company accounts for its digital assets, which are comprised solely of Tether, as indefinite-lived intangible assets in accordance with
Accounting Standards Codification (“ASC”) 350, Intangibles — Goodwill
and Other. The Company has ownership of and control over its Tether and uses third-party custodial services to store its Tether. The
Company’s digital assets are initially recorded at cost. Subsequently, they are measured at cost, net of any impairment losses incurred
since acquisition.
The Company determines the
fair value of its Tether on a nonrecurring basis in accordance with ASC 820, Fair Value Measurement, based on quoted (unadjusted)
prices on the Coinbase exchange, the active exchange that the Company has determined is its principal market for Tether (Level 1 inputs).
The Company performs an analysis each quarter to identify whether events or changes in circumstances, principally decreases in the quoted
(unadjusted) prices on the active exchange, indicate that it is more likely than not that any of the assets are impaired. In determining
if an impairment has occurred, the Company considers the lowest price of Tether quoted on the active exchange at any time since acquiring
the specific Tether held by the Company. If the carrying value of Tether exceeds that lowest price, an impairment loss has occurred with
respect to that Tether in the amount equal to the difference between its carrying value and such lowest price.
Impairment losses are recognized
as “Digital asset impairment losses” in the Company’s Consolidated Statements of Operations in the period in which the
impairment occurs. The impaired digital assets are written down to their fair value at the time of impairment and this new cost basis
will not be adjusted upward for any subsequent increase in fair value. Gains (if any) are not recorded until realized upon sale, at which
point they would be presented net of any impairment losses in the Company’s Consolidated Statements of Operations and Comprehensive
Loss. In determining the gain to be recognized upon sale, the Company calculates the difference between the sales price and carrying value
of the specific Tether sold immediately prior to sale.
See note 5, Digital Assets,
for further information regarding the Company’s purchase and sale of digital assets.
Property and Equipment
Property and equipment are
recorded at cost and presented net of accumulated depreciation. Depreciation is recognized on a straight-line basis over the estimated
useful lives of the related assets. The carrying value of property and equipment is periodically reviewed for recoverability when impairment
indicators are present. Such indicators include, among other factors, operating losses, unused capacity, market value declines, and obsolescence.
Intangible Assets
Intangible assets are recorded
at acquisition cost and presented net of accumulated amortization. Amortization is recognized on a straight-line basis over the estimated
useful lives of the related assets. The carrying value of intangible assets is periodically reviewed for recoverability when impairment
indicators are present. Such indicators include, among other factors, market value declines and the result of clinical trials.
Impairment of Long-Lived Assets
A review of long-lived assets
for impairment is performed when events or changes in circumstances indicate that the carrying value of such assets may not be recoverable.
If an indication of impairment is present, the Company compares the estimated undiscounted future cash flows to be generated by the asset
group to the asset group’s carrying amount. If the undiscounted future cash flows are less than the carrying amount of the asset
group, the Company records an impairment loss equal to the amount by which the asset group’s carrying amount exceeds its fair value.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
The fair value is determined based on valuation techniques such as a comparison to fair values of similar assets or a discounted cash
flow analysis.
Long lived assets that do
not have indefinite lives are amortized/depreciated over their useful lives and reviewed for impairment whenever events or changes in
circumstances indicate that the carrying amount of the asset may not be recoverable. The Company re-evaluates the useful life determinations
each year to determine whether events and circumstances warrant a revision to the remaining useful lives.
Financial Instruments
Financial instruments include
cash, evidence of ownership interests in an entity, and contracts that impose a contractual obligation either to deliver cash or another
financial instrument or to exchange other financial instruments on potentially unfavorable terms. Financial instruments are recorded initially
at fair value, which is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between
market participants at the measurement date. The Company calculates the estimated fair value of financial instruments using quoted market
prices whenever available. When quoted market prices are not available, the Company uses standard pricing models including the Black-Scholes
option pricing model. Subsequent measurement depends on how the financial instrument has been classified. The Company’s financial
instruments include cash, accounts receivable, other assets — GST receivable, other receivables, accounts payable and
accrued liabilities, notes payable, notes payable — related parties, convertible notes and lease liability.
The Company has established
a fair value hierarchy that reflects the significance of inputs of valuation techniques used in making fair value measurements as follows:
| ● | Level
1: quoted prices in active markets for identical assets or liabilities; |
| ● | Level
2: inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (i.e. as prices)
or indirectly (i.e. from derived prices); and |
| ● | Level
3: inputs for the asset or liability that are not based on observable market data. |
The Company’s financial
assets and financial liabilities are measured at amortized cost. As at June 30, 2023 and June 30, 2022 the carrying value of the cash,
accounts receivable, other assets – GST receivable, accounts payable and accrued liabilities and amounts due to related parties
approximates the fair value due to the short-term nature of these instruments.
The digital assets are categorized
as Level 1. If the carrying value of Tether exceeds that lowest price, as quoted on Coinbase, an impairment loss has occurred with respect
to that Tether in the amount equal to the difference between its carrying value and such lowest price. It is management’s opinion
that the Company is not exposed to significant interest or credit risks arising from this financial instrument.
The convertible notes, notes
payable, and notes payable — related parties are categorized as Level 2 and have been recorded at amortized cost. The
carrying value approximates its fair value due to its relatively short-term nature. It is management’s opinion that the Company
is not exposed to significant interest or credit risks arising from these financial instruments.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
Leases
In accordance with ASC 842,
Leases, operating leases are recognized as right-of-use assets and corresponding lease liabilities on the consolidated balance
sheet. Right-of-use assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation
to make lease payments arising from the lease. Operating lease right-of-use assets and liabilities are recognized at commencement date
based on the present value of lease payments over the lease term. Leases with an initial term of 12 months or less are not recorded
on our balance sheet; we recognize lease expense for these leases on a straight-line basis over the lease term. Our leases do not provide
an implicit lease rate, therefore, we utilize our incremental borrowing rate, as the basis to calculate the present value of future lease
payments, at lease commencement. Our incremental borrowing rate represents the rate that we would have to pay to borrow funds on a collateralized
basis over a similar term and in a similar economic environment.
We have lease agreements with
lease and non-lease components. At the adoption of ASC 842, we elected not to separate non-lease components from all classes of our
existing leases. The non-lease components have been accounted for as part of the single lease component to which they are related (See
Note 9).
Earnings per Share
The computation of diluted
earnings per share excludes the effect of the potential exercise of warrants and stock options when the average market price of the common
stock is lower than the exercise price of the respective warrant or stock option and when inclusion of these amounts would be anti-dilutive.
For the year ended June 30, 2023, the number of warrants and stock options excluded from the computation was 428,290 and 591,115,
respectively (year ended June 30, 2022 – 428,290 and 621,697, respectively).
Revenue
The Company recognizes revenue
under ASC 606, Revenue from Contracts with Customers. The Company determines revenue recognition through the following steps:
| ● | Identification of a contract with a customer; |
| ● | Identification of the performance obligations in the contract; |
| ● | Determination of the transaction price; |
| ● | Allocation of the transaction price to the performance obligations
in the contract; and |
| ● | Recognition of revenue when or as the performance obligations
are satisfied. |
We offer consumer products
online. The online order and receipt of full payment creates the customer contract. Revenue is measured based on the amount of consideration
that we receive from customers when they place an order, reduced by estimates for return allowances. Performance obligation is the delivery
of the ordered product to the customer and the performance condition is satisfied, and revenue is recognized, when control of the goods
is transferred to the customer, which generally occurs upon our delivery to a third-party carrier.
Return Allowances
Return allowances, which
reduce revenue and cost of sales, are estimated using historical experience. Liabilities for return allowances were $nil and $nil as of
June 30, 2023 and 2022.
Cost of Sales
Cost of sales primarily consists
of the purchase price of consumer products, incoming shipping costs, payment processing and related transaction costs, and applicable
sales taxes on our purchases of product.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
Selling, General and Administrative
Expenses
Selling, general and administrative
expenses include direct and indirect selling expenses such as marketing and business development and all general administrative expenses
of the Company such wages, benefits, travel costs, professional fees and other indirect expenses.
Share-based Compensation
Share-based compensation expense
consists of the Company’s share purchase option expense. Share purchase options granted to employees and consultants are measured based
on the grant-date fair value. Share-based compensation expense is generally recognized based on the straight-line basis over the requisite
service period. We account for forfeitures as they occur.
Income Taxes
The Company records deferred
tax assets (“DTAs”) and deferred tax liabilities (“DTLs”) based on differences between the book and tax bases
of assets and liabilities. The deferred tax assets and liabilities are calculated by applying enacted tax rates and laws to taxable years
in which such differences are expected to reverse. The Company continually reviews the need for, and the adequacy of, a valuation allowance
and recognizes the benefits from the Company’s deferred tax assets only when an analysis of both positive and negative factors indicate
that it is more likely than not that the benefits will be realized.
Recently Adopted Accounting Pronouncements
In August 2020, the Financial Accounting Standards Board (“FASB”)
issued Accounting Standards Update (“ASU”) 2020-06, Debt — Debt with Conversion and Other Options (Subtopic 470-20)
and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40). The ASU simplifies the accounting
for certain financial instruments with characteristics of liabilities and equity. The FASB reduced the number of accounting models for
convertible debt and convertible preferred stock instruments and made certain disclosure amendments to improve the information provided
to users. In addition, the FASB amended the derivative guidance for the own stock scope exception and certain aspects of the earnings-per-share
guidance. The amendments are effective for years beginning after December 15, 2021, including interim periods within such years,
with early adoption permitted for after December 15, 2020. The amendments were adopted on July 1, 2022. The adoption had no
impact on the consolidated financial statements.
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the FASB
or other standard setting bodies that the Company adopts as of the specified effective date. The Company is an emerging growth company,
as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, emerging growth
companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as
those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised
accounting standards that have different effective dates for public and private companies until the earlier of the date that it is (i) no
longer an emerging growth company or (ii) affirmatively and irrevocably opt out of the extended transition period provided in the
JOBS Act. As a result, these consolidated financial statements may not be comparable to companies that comply with the new or revised
accounting pronouncements as of public company effective dates.
In June 2022, the FASB issued ASU
2022-03, Fair Value Measurement (Topic 820). The amendments in this ASU clarify that a contractual restriction on the sale of an equity
security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value.
The amendments also clarify that an entity cannot, as a separate unit of account, recognize and measure a contractual sale restriction.
The amendments in this ASU also require additional disclosures for equity securities subject to contractual sale restrictions. The provisions
in this ASU are effective for fiscal years beginning after December 15, 2024. Early adoption is permitted. The Company does not expect
to early adopt this ASU. The Company is currently evaluating the impact of adopting this guidance on the consolidated financial statements.
The Company does not believe that any other recently issued, but not yet
effective, accounting pronouncements, if currently adopted, would have a material effect on the Company’s consolidated financial
statements.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
NOTE 3 — PREPAID EXPENSES
Prepaid expenses consist
of the following:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Advertising (a) | |
| 2,187,500 | | |
| — | |
| Consulting (b) | |
| 585,279 | | |
| 133,910 | |
| Insurance | |
| 65,140 | | |
| 8,020 | |
| Other | |
| 44,594 | | |
| 43,793 | |
| Total | |
| 2,882,513 | | |
| 185,723 | |
| Current portion | |
| 1,219,180 | | |
| 185,723 | |
| Non-current portion | |
| 1,663,333 | | |
| — | |
NOTE 4 — OTHER RECEIVABLE
During the year ended June
30, 2023, a past consultant of the Company obtained a garnishing order in an action against the Company whereby $336,706 (CAD$445,799)
of cash held at the Company’s bank was garnished and paid into the British Columbia Supreme Court. The amount was recorded as restricted
cash as at June 30, 2023 on the consolidated statement of financial position. On August 1, 2023 the British Columbia Supreme Court ordered
that the garnished funds be repaid to the Company. The Company has no ongoing obligation to the consultant and the legal action has been
concluded.
NOTE 5
— DIGITAL ASSETS
During the year ended June 30,
2023, the Company sold approximately 34,106 Tether for $34,106 in cash. The 34,106 Tether was acquired during the year ended June 30,
2022 for cash of $34,106.
NOTE 6 — PROPERTY, PLANT AND EQUIPMENT
On February 25, 2021,
the Company entered an agreement whereby the Company acquired certain equipment for consideration of 990,741 Class B common non-voting
shares with a fair value of $1,687,032 (CAD$2,140,000). At the time of acquisition, the equipment had a fair value of $843,500. The excess
of fair value of the Class B common non-voting shares above the fair value of the equipment of $843,532 was recorded as compensation
expense within selling, general and administrative expenses on the consolidated statement of operations and comprehensive loss during
the year ended June 30, 2021.
At June 30, 2023, the equipment
had a fair value of $765,650. The excess of carrying value above the fair value of the equipment of $78,850 was recorded as impairment
on the consolidated statement of operations and comprehensive loss during the year ended June 30, 2023.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
The equipment is not in
use and therefore no depreciation has been taken for the years ended June 30, 2023 and 2022. Property, plant and equipment
consist of the following:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Balance, beginning of year | |
| 843,500 | | |
| 843,500 | |
| Impairment loss | |
| 78,850 | | |
| — | |
| Balance, end of year | |
| 764,650 | | |
| 843,500 | |
NOTE 7 — INTANGIBLE ASSETS
On June 30, 2023, pursuant
to an asset purchase agreement dated June 30, 2023 (the “Agreement”), the Company closed on the acquisition of intellectual
property and related assets relating to Wesana Health Holdings Inc. (“Wesana”) psilocybin and cannabidiol combination investigational
therapy, SAN-013 (“Intellectual Property”) for consideration consisting of: (a) 1,000,000 shares of the Company’s common
stock with an aggregate issuance date fair value of $914,250, and (b) $570,000 in cash. The Company paid $300,000 on March 20, 2023 with
the remaining $270,000 due in the following 4 installments: (i) $123,000 due on or before July 1, 2023; (ii) $48,991 due on or before
October 1, 2023; (iii) $48,991 due on or before January 1, 2024; and (iv) $49,018 due on or before April 1, 2024.
Under the screen test requirements under ASC 805, Business Combinations,
the Company concluded that the Intellectual Property represented substantially all of the fair value of the gross assets acquired and,
accordingly, determined the set was not considered a business, such that the Company applied asset acquisition accounting and recorded
the acquisition of the Intellectual Property as an intangible asset in the amount of $1,484,250 that will be amortized on a straight-line
basis over the remaining weighted average useful life of 19.2 years.
The estimated future amortization
expense is as follows:
| Year ended June 30, | |
Amount | |
| 2024 | |
$ | 77,395 | |
| 2025 | |
| 77,395 | |
| 2026 | |
| 77,395 | |
| 2027 | |
| 77,395 | |
| 2028 | |
| 77,395 | |
| Thereafter | |
| 1,097,275 | |
| | |
$ | 1,484,250 | |
NOTE 8 — ACCOUNTS PAYABLE AND ACCRUED
LIABILITIES
Accounts payable and accrued
liabilities consist of the following:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Trade payables(a)(b) | |
| 1,291,063 | | |
| 2,419,118 | |
| Vacation accrual | |
| — | | |
| 23,225 | |
| Accrued liabilities | |
| — | | |
| 372,189 | |
| | |
| 1,291,063 | | |
| 2,814,532 | |
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
NOTE 9 — RIGHT OF USE ASSET AND LEASE LIABILITY
The lease liability relates
to a warehouse leased by the Company (the “Warehouse Lease”). The lease commenced on August 1, 2017 with an initial term of
5 years expiring on July 31, 2022. On August 1, 2022, the Company exercised its option to renew for 5 years. The new term starts on August
1, 2022 and ends on July 31, 2027, with an option to extend the lease for an additional five years. The renewal option needs to be exercised
no less than six months from the expiry date. As of lease renewal, the Company anticipated exercising the option to renew and as such
has determined the lease term to be 10 years in determining the lease liability. The discount rate used was 16%, equivalent to the interest
rate the Company would incur to borrow funds equal to the future lease payments on a collateralized basis over a similar term and in a
similar economic environment. As a result, the Company increased its right-of-use asset by $1,144,349 and lease liability by $1,144,349
related to the Warehouse Lease on August 1, 2022. During the year ended June 30, 2023, the Company recorded amortization expense of $101,466
(year ended June 30, 2022- $nil) with respect to the right of use asset.
Leases with an initial term
of less than 12 months are not recorded on the balance sheet. We recognize lease expense for these leases on a straight-line basis over
the lease term.
The long-term deposit of $18,882
(CAD$25,000) relates to a security deposit on the Warehouse Lease which is expected to be returned to the Company at the completion of
the lease, including renewal periods.
The right of use asset consists
of the following:
| |
|
June 30,
2023 |
|
|
June 30,
2022 |
|
| |
|
$ |
|
|
$ |
|
| Balance, beginning of the year |
|
|
— |
|
|
|
— |
|
| Addition on lease modification |
|
|
1,144,349 |
|
|
|
— |
|
| Amortization |
|
|
(101,466 |
) |
|
|
— |
|
| Unrealized foreign exchange loss |
|
|
(17,850 |
) |
|
|
— |
|
| Balance, end of year |
|
|
1,025,033 |
|
|
|
— |
|
The lease liability consists
of the following:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Balance, beginning of the year | |
| 660,458 | | |
| 771,976 | |
| Addition on lease modification | |
| 1,144,349 | | |
| — | |
| Payments | |
| (330,527 | ) | |
| (198,135 | ) |
| Interest expense | |
| 262,439 | | |
| 115,684 | |
| Unrealized foreign exchange gain | |
| (8,342 | ) | |
| (29,067 | ) |
| Balance, end of year | |
| 1,728,377 | | |
| 660,458 | |
| Current portion | |
| 338,819 | | |
| 89,396 | |
| Non-current portion | |
| 1,389,558 | | |
| 571,062 | |
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
The maturity of the lease liability is as follows:
| Year ended June 30, | |
Amount | |
| 2024 | |
$ | 338,819 | |
| 2025 | |
| 377,207 | |
| 2026 | |
| 417,265 | |
| 2027 | |
| 438,962 | |
| 2028 | |
| 330,474 | |
| Thereafter | |
| 1,308,542 | |
| Total lease payments | |
| 3,211,269 | |
| Less: Unamortized interest | |
| (1,482,892 | ) |
| Total lease liability | |
$ | 1,728,377 | |
NOTE 10 — NOTES PAYABLE AND NOTES
PAYABLE – RELATED PARTY
Following is a summary of the
Company’s notes payable and notes payable – related party:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Opening balance | |
| 361,258 | | |
| 352,464 | |
| Repayment of notes payable(a) | |
| (207,236 | ) | |
| — | |
| Settlement in common shares(b) | |
| (88,707 | ) | |
| — | |
| Interest expense(a)(b) | |
| 4,957 | | |
| 12,258 | |
| Amortization of debt discount(c)(d)(e)(f) | |
| 5,715 | | |
| 10,286 | |
| Foreign exchange gain | |
| (15,564 | ) | |
| (13,750 | ) |
| Balance, end of year | |
| 60,423 | | |
| 361,258 | |
| Current portion | |
| 60,423 | | |
| 305,082 | |
| Non-current portion | |
| — | | |
| 56,176 | |
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
The following table summarizes
the future principal repayments required on the Company’s notes payable:
| For the years ended June 30, | |
Amount | |
| 2024 | |
$ | 60,423 | |
| Total notes payable | |
$ | 60,423 | |
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
NOTE 11 — CONVERTIBLE NOTES
Following is a summary of the
Company’s convertible notes:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Opening balance | |
| 3,797,868 | | |
| 1,066,774 | |
| Convertible notes issued(a)(b)(c) | |
| 340,000 | | |
| 2,829,500 | |
| Conversion to common shares(a) (b) | |
| (4,307,115 | ) | |
| (314,016 | ) |
| Interest expense(a)(b)(c) | |
| 167,640 | | |
| 217,589 | |
| Foreign exchange loss (gain) | |
| 1,607 | | |
| (1,979 | ) |
| Balance, end of year | |
| — | | |
| 3,797,868 | |
| Current portion | |
| — | | |
| 825,707 | |
| Non-current portion | |
| — | | |
| 2,972,161 | |
NOTE 12 — LINE OF CREDIT
On November 5, 2020, the
Company established a line of credit of $5,041,541 (CAD$6,675,000). The line of credit is secured by the Company’s assets, bears
an interest rate of 8% per annum and matures on November 5, 2023. The Company may draw up to $377,644 (CAD$500,000) per quarter under
the line of credit beginning January 15, 2021. Pursuant to entering the line of credit, the Company issued the lender warrants to
purchase 3,906,209 common shares of the Company at an exercise price of $1.63 (CAD$2.16) per common share until November 5, 2025.
On January 22, 2021, the Company amended the warrants whereby in the event that the Company effects a closing or closings of convertible
notes is the minimum aggregate of (i) $1,000,000, the exercise price of 1,111,112 warrants shall be adjusted to $0.015 (CAD$0.018),
(ii) $2,000,000, the exercise price of 2,222,223 warrants shall be adjusted to $0.015 (CAD$0.018), and (iii) $3,000,000,
the exercise price of 3,333,334 warrants shall be adjusted to $0.015 (CAD$0.018).
The warrants were valued at
$4,775,535 and recorded as deferred financing costs to be recognized over the term of the line of credit. During the year ended June 30,
2023, the Company recorded interest expense of $1,547,104 (year ended June 30, 2022 — $1,627,181) related to the warrants.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
On January 22, 2021, pursuant
to the warrant amendment, the Company reclassified 3,906,209 warrants valued at $4,775,535 to warrant liability as the exercise price
became variable based on the amount of convertible notes payable raised. The incremental fair value resulting from the warrant amendment
of $1,079,468 was recorded as interest expense on the consolidated statement of operations and comprehensive loss.
On December 8, 2021, the
Company reclassified 3,906,209 warrants valued at $6,392,476 to share capital as the exercise price became fixed for the remaining
warrants outstanding since the Company had successfully raised $3,000,000 in convertible notes, resolving the contingency affecting the
exercise price.
Following is a summary of the
Company’s warrant liability for the year ended June 30:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Opening balance | |
| — | | |
| 6,192,883 | |
| Warrants reclassified to share capital | |
| — | | |
| (6,392,476 | ) |
| Change in fair value of warrant liability | |
| — | | |
| 322,226 | |
| Foreign exchange gain | |
| — | | |
| (122,633 | ) |
| Balance, end of year | |
| — | | |
| — | |
NOTE 13 — STOCKHOLDERS’ EQUITY
Share Capital
A summary of the Company’s
share capital is as follows:
Common Stock
The Company has authorized
an unlimited amount of common stock with no par value.
Stock Split
On December 1, 2021, the Company
authorized an 18:1 reverse stock split of its issued and outstanding Class B common stock. The effect of this reverse stock split
has been reflected retrospectively throughout the consolidated financial statements. Also on December 1, 2021, the Company amended
its Articles to create a single class of non-voting common shares and cancel the Class A voting common shares and Class B non-voting
common shares. Pursuant to the amendment, the Class A voting common shares and Class B non-voting common shares were converted
on a one-for-one basis into common shares of the Company.
On October 22, 2018, the Company authorized a 1:1.4 stock split of
its issued and outstanding Class B common stock resulting in an additional issuance of 23,187,182 shares of Class B common stock
to existing holders. The Company retrospectively adjusted common stock, stock option, warrants and per share amounts throughout the consolidated
financial statements for this 1:1.4 stock split.
Voting Rights
Holders of common stock have
no voting rights and are not entitled to receive notice of or to attend any annual or extraordinary general meeting of the stockholders
of the Company.
Dividends
The directors may declare dividends
on one or more class of shares to the exclusion of the others or declare dividends at different rates on different classes of shares,
at their discretion. No dividends shall be declared on the Class A common stock or any class of shares if to do so would reduce the
value of the net assets of the Company to less than the paid-up capital of the common stock.
No dividends have been declared by the Company for the year ended June 30, 2023.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
Liquidation
In the event of any liquidation,
dissolution or winding-up of the Company, whether voluntary or involuntary, the holders of the common stock shall be entitled to participate
equally in all of the profits and assets of the Company.
Redemption
The Company may redeem the
whole or any part of the common stock on payment for each share of common stock to be redeemed of an amount not exceeding the redemption
amount of each redeemed share, together with all non-cumulative accrued dividends declared.
Share Repurchase Program
On
April 6, 2023, the Company’s Board of Directors approved a share repurchase program with authorization to repurchase up to 500,000
common shares. The Company may repurchase common shares from time to time through open market purchases, in privately negotiated transactions
or by other means in accordance with federal securities laws. The exact number of shares of Common Stock to be repurchased by the Company
is not guaranteed, and the program may be suspended, modified, or discontinued at any time without prior notice.
Common Stock Issuances and Transfers
During the year ended June
30, 2023, the Company had the following common stock transactions:
On June 30, 2023, the Company
issued 1,000,000 common non-voting shares to Wesana pursuant to the Agreement.
On June 29, 2023, accounts
payable of $25,000 were settled through the issuance of 21,662 common non-voting shares.
On June 29, 2023, due to
related parties of $101,150 (CAD$136,059) were settled through the issuance of 100,000 common non-voting shares pursuant to a mutual settlement
and release agreement.
On January 16, 2023, we entered
into a strategic investment agreement with Hightimes , 1252240 BC LTD, a wholly owned subsidiary of Hightimes, and Trans-High Corporation,
a wholly-owned subsidiary of Hightimes, pursuant to which Hightimes granted to us $833,333 of annual advertising and marketing credits,
for three consecutive years, in exchange for 625,000 of our common non-voting shares.
On February 13, 2023, the
Company closed its IPO of 1,875,000 of the Company’s common non-voting shares at a public offering price of $4.00 per
share. The gross proceeds from the IPO were $5.8 million, after deducting underwriting discounts and commissions and other offering related
expenses payable by the Company.
On February 13, 2023, the
convertible notes in the aggregate principal amount of $4,307,115 were automatically converted into 1,932,006 common non-voting shares.
On February 13, 2023, the
related party notes payable in the aggregate amount of $88,707 were automatically converted into 36,962 common non-voting shares pursuant
to a settlement and subscription agreement.
On February 13, 2023, accounts
payable and due to certain related parties in the aggregate amount of $2,579,320 were automatically converted into 1,074,716 common non-voting
shares pursuant to settlement and subscription agreements.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
On February 13, 2023, the
Company issued 250,000 common non-voting shares pursuant to a two-year marketing agreement.
On February 13, 2023, the
Company issued 104,167 common non-voting shares pursuant to a donation to the Austin Community Foundation. The shares were subsequently
cancelled on July 5, 2023.
During the year ended June 30,
2022, the Company had the following common stock transactions:
On December 8, 2021, the
Company issued 3,477,919 common non-voting shares pursuant to the exercise of 3,477,919 warrants with an exercise price of $0.015
(CAD$0.018) per warrant.
On December 28, 2021,
the Company issued 185,138 common non-voting shares pursuant to the conversion of $300,000 of convertible notes plus $14,016 accrued interest
at a conversion price of $1.69 (CAD$2.16) per common non-voting share.
On February 15, 2022,
the Company issued 127,819 common non-voting shares with a fair value of $216,695 pursuant to consulting agreements.
On March 9, 2022, the
Company issued 175,931 common non-voting shares pursuant the exercise of share purchase options.
Share Purchase Options
The Company’s board of
directors adopted the Company’s 2021 Equity Incentive Plan (the “2021 Plan”), which became effective upon the effectiveness
of the registration statement filed in connection with the IPO on February 8, 2023. The maximum number of common shares that may be issued
in respect of awards under the 2021 Plan is equal to 2,172,279 or 15% of the common shares (on a fully diluted basis) on the day immediately
following the initial public offering of the Company. The Company may issue share purchase options, restricted stock units or stock appreciation
rights.
The following is a summary
of the changes in share purchase options during the year ended June 30, 2023 and 2022:
| | |
Number of
options | | |
Weighted average
exercise price
($) | | |
Weighted
average
remaining life
(years) | | |
Aggregate
intrinsic value
($) | |
| Balance at
June 30, 2021 | |
| 464,293 | | |
| 2.01(CAD2.59 | ) | |
| 2.63 | | |
| 276,294 | |
| Granted | |
| 333,335 | (1)(2) | |
| 1.68(CAD2.16 | ) | |
| 2.00 | | |
| — | |
| Exercised | |
| (175,931 | )(3) | |
| 0.22(CAD0.29 | ) | |
| — | | |
| (276,294 | ) |
| Balance
at June 30, 2022 | |
| 621,697 | | |
| 2.34(CAD3.01 | ) | |
| 1.91 | | |
| — | |
| Granted | |
| 30,000 | (4) | |
| 1.25 | | |
| 2.84 | | |
| — | |
| Expired | |
| (60,582 | )(5)(6) | |
| 2.50(CAD3.30 | ) | |
| — | | |
| — | |
| Balance
at June 30, 2023 | |
| 591,115 | | |
| 2.20(CAD2.91 | ) | |
| 1.74 | | |
| — | |
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
The following is a summary of the outstanding share
purchase options as at June 30, 2023:
| Exercise price ($) | |
Outstanding
at June 30,
2023 | | |
Weighted
average
remaining
contractual life | | |
Exercisable
at June 30,
2023 | | |
Weighted
average
remaining
contractual life | |
| 4.76 (CAD6.30) | |
| 111,112 | | |
| 1.00 | | |
| 111,112 | | |
| 1.00 | |
| 1.63 (CAD2.16) | |
| 166,668 | | |
| 1.00 | | |
| 166,668 | | |
| 1.00 | |
| 1.63 (CAD2.16) | |
| 166,667 | | |
| 2.26 | (1) | |
| 166,667 | | |
| 2.26 | (1) |
| 1.25 | |
| 30,000 | | |
| 2.50 | | |
| 30,000 | | |
| 2.50 | |
| 1.63 (CAD2.16) | |
| 116,668 | | |
| 2.84 | | |
| 116,668 | | |
| 2.84 | |
| | |
| 591,115 | | |
| 1.74 | | |
| 591,115 | | |
| 1.74 | |
During the year ended June 30,
2022, the Company recognized share-based payment expense of $165,926 (CAD$222,812) (year ended June 30, 2022 — $210,572
(CAD$266,045)) related to vested share purchase options. The Company has unrecognized stock-based compensation expense of $nil associated
with outstanding share purchase options.
The Company has computed the
fair value of share purchase options granted using the Black-Scholes option pricing model. The expected term used for options issued to
non-employees is the contractual life and the expected term used for options issued to employees and directors is the estimated period
of time that options granted are expected to be outstanding. The Company utilizes the “simplified” method to develop an estimate
of the expected term of “plain vanilla” employee share purchase option grants. The Company is utilizing an expected volatility
figure based on a review of the historical volatilities, over a period of time, equivalent to the expected life of the instrument being
valued, of similarly positioned public companies within its industry. The risk-free interest rate was determined from the implied yields
from U.S. Treasury zero-coupon bonds with a remaining term consistent with the expected term of the instrument being valued.
The Company applied the following
assumptions in the Black-Scholes option pricing model for the year ended June 30, 2023 and 2022:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| Expected life options (years) | |
| 1.19 | | |
| 1.00 | |
| Expected volatility | |
| 100 | % | |
| 100 | % |
| Expected dividend yield | |
| 0 | % | |
| 0 | % |
| Risk-free interest rate | |
| 3.79 | % | |
| 2.00 | % |
| Black-Scholes value of each option | |
$ | 0.74(CAD$0.98 | ) | |
$ | 0.95(CAD$1.22 | ) |
The Company plans to issue
1,642,861 share purchase options to various officers and the executive chairman. The exercise price of these share purchase options will
be the closing price of the Company’s common shares on the closing date of an IPO. These share purchase options will vest as to
25% of the underlying common shares on the grant date, and the balance of these share purchase options will vest and become exercisable
with respect to 45,635 common shares in 36 equal monthly instalments commencing on the 13th month following the date of grant
and continuing until the 48th month following the date of grant, subject to continued employment with us through each vesting
date. No expense has been recorded through June 30, 2023 with respect to these options.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
Warrants
The Company has computed the
fair value of warrants issued using a modified Black-Scholes option pricing model. The expected term used for warrants issued is the contractual
term. The Company is utilizing an expected volatility figure based on a review of the historical volatilities, over a period of time,
equivalent to the expected life of the instrument being valued, of similarly positioned public companies within its industry. The risk-free
interest rate was determined from the implied yields from U.S. Treasury zero-coupon bonds with a remaining term consistent with the
expected term of the instrument being valued.
Pursuant to entering the line
of credit, on January 15, 2021, the Company issued 3,906,209 warrants to purchase 3,906,209 common shares of the Company at
an exercise price of $1.63 (CAD$2.16) per common share until November 5, 2025. On January 22, 2021, the Company amended the
warrants whereby in the event that the Company effects a closing or closings of convertible notes is the minimum aggregate of (i) $1,000,000,
the exercise price of 1,111,112 warrants shall be adjusted to $0.015 (CAD$0.018), (ii) $2,000,000, the exercise price of 2,222,223 warrants
shall be adjusted to $0.015 (CAD$0.018), and (iii) $3,000,000, the exercise price of 3,333,334 warrants shall be adjusted to
$0.015 (CAD$0.018).
The following is a summary
of the warrants during the year ended June 30, 2023 and 2022:
| | |
Number of warrants | | |
Weighted average
exercise price
($) | | |
Weighted
average
remaining life
(years) | | |
Aggregate
intrinsic value | |
| Balance at June 30, 2021 | |
| 3,906,209 | | |
| 0.76(CAD0.94 | ) | |
| 4.35 | | |
| 5,709,315 | |
| Exercised(1) | |
| (3,477,919 | ) | |
| 0.015(CAD0.018 | ) | |
| — | | |
| (5,709,315 | ) |
| Balance at June 30, 2022 | |
| 428,290 | | |
| 1.68(CAD2.16 | ) | |
| 3.35 | | |
| — | |
| Balance at June 30, 2023 | |
| 428,290 | | |
| 1.63(CAD2.16 | ) | |
| 2.35 | | |
| — | |
On December 8, 2021 the
Company reclassified 3,906,209 warrants valued at $6,392,476 to share capital as the exercise price became fixed for the remaining
warrants outstanding since the Company had successfully raised $3,000,000 in convertible notes, resolving the contingency affecting the
exercise price.
The Company applied the following
assumptions in the Black-Scholes option pricing model:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| Expected life warrants (years) | |
| — | | |
| 3.91 | |
| Expected volatility | |
| — | | |
| 100 | % |
| Expected dividend yield | |
| — | | |
| 0 | % |
| Risk-free interest rate | |
| — | | |
| 0.34 | % |
| Black-Scholes value of each warrant | |
$ | — | | |
$ | 1.17(CAD$1.47 | ) |
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
NOTE 14 — INCOME TAXES
The domestic and foreign components
of loss before income taxes for the years ended June 30, 2023 and 2022 were as follows:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Domestic – Canada | |
| (8,708,905 | ) | |
| (5,856,116 | ) |
| Foreign – outside of Canada | |
| (279,551 | ) | |
| — | |
| Loss before provision for income taxes | |
| (8,988,456 | ) | |
| (5,856,116 | ) |
The components of the income
tax expense for the year ended June 30, 2023 and 2022 consisted of the following:
| | |
| June 30,
2023 | | |
| June 30,
2022 | |
| | |
| $ | | |
| $ | |
| Current income tax expense: | |
| | | |
| | |
| Domestic – Canada | |
| — | | |
| — | |
| Foreign – Outside of Canada | |
| — | | |
| — | |
| Total current tax expense | |
| — | | |
| — | |
| | |
| | | |
| | |
| Deferred income tax expense: | |
| | | |
| | |
| Domestic – Canada | |
| — | | |
| — | |
| Foreign – outside of Canada | |
| — | | |
| — | |
| Total deferred income tax expense | |
| — | | |
| — | |
| Total income tax expense | |
| — | | |
| — | |
A reconciliation of the Company’s effective tax rate to the statutory
Canada income tax rate for the year ended June 30, 2023 and 2022 were as follows:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Loss for the year before income taxes | |
| (8,988,456 | ) | |
| (5,856,116 | ) |
| Statutory rate | |
| 27 | % | |
| 27 | % |
| Expected income tax recovery | |
| (2,426,883 | ) | |
| (1,581,151 | ) |
| Non-deductible interest expense | |
| 418,701 | | |
| 443,108 | |
| Non-taxable loss on debt settlement | |
| 323,467 | | |
| — | |
| Non-deductible share-based payments | |
| 44,905 | | |
| 56,744 | |
| Non-deductible impairment loss | |
| 20,290 | | |
| — | |
| Other permanent differences | |
| 12,799 | | |
| (13,528 | ) |
| Prior year true-up | |
| 22,093 | | |
| (22,108 | ) |
| Other | |
| 93,472 | | |
| — | |
| Impact of foreign exchange on valuation allowance | |
| 179,049 | | |
| 206,639 | |
| Change in valuation allowance | |
| 1,312,107 | | |
| 910,296 | |
| Income tax expense | |
| — | | |
| — | |
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
The tax effects of temporary differences that give
rise to significant portions of the deferred tax assets and liabilities as at June 30, 2023 and 2022 were as follows:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| Deferred tax assets from: | |
| | |
| |
| Net operating loss carry forwards | |
| 5,805,330 | | |
| 4,278,122 | |
| Property and equipment | |
| 707,121 | | |
| 697,316 | |
| Right of use asset | |
| 276,759 | | |
| — | |
| Research and development tax credit carry-forward balances | |
| 433,168 | | |
| 445,068 | |
| Total deferred tax assets | |
| 7,222,378 | | |
| 5,420,506 | |
| | |
| | | |
| | |
| Deferred tax liabilities from: | |
| | | |
| | |
| Share issue costs | |
| (238,132 | ) | |
| (213,434 | ) |
| Lease liability | |
| (466,662 | ) | |
| — | |
| CEBA loans | |
| — | | |
| (1,595 | ) |
| Total deferred tax liabilities | |
| (704,794 | ) | |
| (215,029 | ) |
| Valuation allowance | |
| (6,517,584 | ) | |
| (5,205,477 | ) |
| Net deferred tax asset | |
| — | | |
| — | |
The Company must make judgements
as to the realization of deferred tax assets that are dependent upon a variety of factors, including the generation of future taxable
income, the reversal of deferred tax liabilities, and tax planning strategies. To the extent that the Company believes that recovery is
not likely, it must establish a valuation allowance. A valuation allowance has been established for deferred tax assets which the
Company does not believe meet the “more likely than not” criteria. The Company’s judgments regarding future taxable
income may change due to changes in market conditions, changes in tax laws, tax planning strategies or other factors. If the Company’s
assumptions and consequently its estimates change in the future, the valuation allowances it has established may be increased or decreased,
resulting in a respective increase or decrease in income tax expense. Based upon the Company’s historical operating losses and the
uncertainty of future taxable income, the Company has provided a valuation allowance primarily against its deferred tax assets up to the
deferred tax liabilities, as of June 30, 2023 and 2022.
The Company had non-capital losses carry forward balances of approximately
$21,700,000 (CAD$28,400,000) at June 30, 2023 and approximately $15,800,000 (CAD$20,400,000) at June 30, 2022 for which a deferred
tax asset has not been recognized. These losses may be carried forward to apply against future year income tax for Canadian income tax
purposes, subject to the final determination by taxation authorities, and expire through 2043. The utilization of the Company’s
non-capital losses carry-forward balances may be subject to limitation under the provisions of Canada Revenue Agency section 111(5.4).
The Company has not yet completed a study to determine if any losses are limited under section 111(5.4) as of June 30, 2023.
The Company recognizes the
tax benefit from uncertain tax positions only if it is “more likely than not” that the tax positions will be sustained on
examination by the tax authorities, based on the technical merits of the position. The tax benefit is measured based on the largest benefit
that has a greater than 50% likelihood of being realized upon ultimate settlement. The Company recognizes interest and penalties related
to income tax matters in income tax expense. The Company is also required to assess at each reporting date whether it is reasonably possible
that any significant increases or decreases to its unrecognized tax benefits will occur during the next 12 months.
The Company did not recognize any uncertain tax positions, or any accrued
interest and penalties associated with uncertain tax positions for the year ended June 30, 2023. The Company files tax returns in
Canada and the United States. The Company is generally subject to examination by income tax authorities for seven years from the
filing of a tax return, therefore, the federal and certain state returns from incorporation forward are subject to examination. The Company
currently is not under examination by any tax authority.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
In response to the COVID-19
pandemic, the COVID-19 Emergency Response Act was signed into law on March 25, 2020. The COVID-19 Emergency Response Act, among other
things, includes tax provisions relating to temporary wage subsidies and interest free business loans. The COVID-19 Emergency Response
Act did not have a material impact on the Company’s income tax provision for the years ended June 30, 2023 and 2021. The
Company will continue to evaluate the impact of the COVID-19 Emergency Response Act on its financial position, results of operations,
and cash flows.
NOTE 15 — RELATED PARTY TRANSACTIONS
Included under due to related
parties on our consolidated balance sheet as of June 30, 2023 is $1,019,894 (June 30, 2022 - $1,775,372) that relates to wages, short-term
benefits and contracted services for key management personnel. The amounts are unsecured and non-interest bearing. On February 13, 2023,
due to certain related parties in the aggregate amount of $1,106,877 were automatically converted into 461,203 common shares on completion
of the IPO pursuant to settlement and subscription agreements resulting in a non-cash loss on debt settlement of $507,324.
On December 31, 2018,
the Company issued a note payable of $144,666 (CAD$200,000) to a director and stockholder of the Company. The note bears interest of 21%
per annum, is unsecured and is repayable on December 31, 2021. Maturity was subsequently amended to 90 days subsequent to the
successful completion of an initial public offering or a reverse takeover transaction. During the year ended June 30, 2023, the Company
incurred interest expense of $2,577 (CAD$3,500) (June 30, 2022 — $8,190 (CAD$10,500)) with respect to the note payable. During
the year ended June 30, 2021, the Company made payments of $52,933 (CAD$67,999) with respect to the note. The Company entered into
debt settlement and subscription agreements with the director and stockholder under which the note payable with a face value of $88,707
(CAD$119,125) as at February 13, 2023 was settled upon IPO through the issuance of 36,962 common shares (Note 13). The Company has no
continuing obligation with respect to the note payable.
During the year ended June 30,
2019, the Company issued a series of notes payable totaling $245,768 (CAD $330,000) to a director and stockholder of the Company. On August 20,
2021, the Company issued an additional $2,273 (CAD$3,000) to a director and stockholder of the Company. The note bears interest of 2%
per annum, is unsecured and repayable 90 days subsequent to the successful completion of an initial public offering or a reverse
takeover transaction. During the year ended June 30, 2022, the Company incurred an interest expense of $2,380 (CAD$3,356) (June 30,
2022 — $4,068 (CAD$5,150)). During the year ended June 30, 2021, the Company issued 13,889 Class B non-voting common shares
as repayment of $39,746 (CAD$50,000). During the year ended June 30, 2023, the Company paid $207,236 (CAD$280,735) as repayment of
the note payable. The Company has no continuing obligation with respect to the note payable.
On
February 25, 2021, the Company issued a $500,000 convertible note at 8% interest rate to the CEO of the Company. The convertible note
matured on August 25, 2021. The note was modified subsequent to June 30, 2021 whereby the maturity was extended to February 25, 2022.
The note is convertible at the option of the holder into common shares at a conversion price of $1.59 (CAD$2.16) per share. During the
year ended June 30, 2022, the Company incurred an interest expense of $22,450 (June 30, 2022 — $42,621) with respect
to this note. On February 13, 2023, the convertible note of $500,000 plus accrued interest of $78,575 were converted into 366,187 common
shares. The Company has no continuing obligation with respect to the convertible note.
Following closing of the
IPO, the Company plans to issue to its Chief Executive Officer an award with respect to the number of the Company’s common shares
equal to the quotient obtained by dividing (x) $750,000 by (y) the closing price of the Company’s common shares on the
closing date of the IPO, which award shall be fully vested at the time of issuance.
LUCY SCIENTIFIC DISCOVERY INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2023 and 2022
(Expressed in US Dollars)
The Company plans to issue
1,642,861 share purchase options to various officers and the executive chairman. The exercise price of these share purchase options will
be the closing price of the Company’s common shares on the closing date of an IPO. These share purchase options will vest as to
25% of the underlying common shares on the grant date, and the balance of these share purchase options will vest and become exercisable
with respect to 45,635 common shares in 36 equal monthly instalments commencing on the 13th month following the date of grant
and continuing until the 48th month following the date of grant, subject to continued employment with us through each vesting
date. No expense has been recorded through June 30, 2023 with respect to these options.
NOTE 16 — SUBSEQUENT EVENTS
In connection with the preparation
of the consolidated financial statements, the Company evaluated subsequent events through October 13, 2023 which was the date the consolidated
financial statements were issued, and determined that the following subsequent events occurred as of that date:
Equity transactions
On July 4, 2023, the Company issued 100,000 common non-voting shares
and made a cash payment of $226,586 (CAD$300,000) pursuant to a mutual settlement and release agreement.
On July 5, 2023, the Company
cancelled 104,167 common non-voting shares which had previously been issued pursuant to a donation to the Austin Community Foundation.
On July 28, 2023, the Company
issued 93,750 share purchase warrants with an exercise price of $1.25 and a term of five years.
On August 1, 2023, the Company
issued 187,500 common non-voting shares to the former Chief Executive Officer.
Other
On September 6, 2023, we
entered into a Stock Purchase Agreement (the “Stock Purchase Agreement”) with Hightimes to acquire the intellectual property
of High Times. Hightimes owns all of the issued and outstanding shares of common stock of HT-Lucy Acquisition Corp., a Delaware corporation.
Pursuant to the Stock Purchase Agreement, Hightimes agreed to sell to us all of the common stock of HT-Lucy Acquisition Corp. upon the
terms and subject to the conditions of the Stock Purchase Agreement. In exchange for the common stock of HT-Lucy Acquisition Corp., we
shall pay Hightimes as consideration (i) the number of shares of common stock of the Company that represents 19.9% of the total issued
and outstanding shares of the Company at the closing; and (ii) semi-annual earn-out payments (the “Hightimes Earn-Out Payments”)
payable for the five (5) consecutive fiscal years ending on June 30, 2029, in amounts equal to three (3) times the adjusted EBITDA of
HT-Lucy Acquisition Corp., calculated pursuant to the terms of the Stock Purchase Agreement. We have the discretion to pay the Hightimes
Earn-Out Payments with either Lucy common shares or cash. At the closing, we will also cause HT-Lucy Acquisition Corp. to enter into an
intellectual property license agreement pursuant to which HT-Lucy Acquisition Corp. will grant to an affiliate of Hightimes the exclusive
right and license to utilize certain intellectual property rights to operate retail stores and to manufacture and sell THC products in
the United States in return for a license fee of $1.0 million per year, increasing to $2.0 million per year upon Federal legalization.
On September 12, 2023, we
entered into an amalgamation agreement (the “Amalgamation Agreement”) with Bluesky Biologicals Inc. (“Bluesky”)
to acquire the Bluesky. Bluesky, through Bluesky Wellness Inc., owns a portfolio of plant-based wellness brands including Keoni, Keoni
Sport, Blush Wellness and AMMA Healing. Pursuant to the Amalgamation Agreement, Bluesky will amalgamate with a wholly-owned subsidiary
of the Company upon the terms and subject to the conditions of the Amalgamation Agreement. We shall pay Bluesky as consideration (i) the
number of shares of common stock of the Company that represents 19.9% of the total issued and outstanding shares of the Company at the
closing; and (ii) earn-out payments (the “Bluesky Earn-Out Payments”) payable for the four (4) consecutive fiscal years ending
on June 30, 2028, the six (6) month period ended June 30, 2024, and the six (6) month period ending December 31, 2028, in amounts equal
to two and one half (2.5) times the adjusted EBITDA of Bluesky., calculated pursuant to the terms of the Amalgamation Agreement. We have
the discretion to pay the Bluesky Earn-Out Payments with either Lucy common shares or cash.
F-27
00-0000000
Unlimited
Unlimited
0.68
0.71
12701100
8615648
175931
60582
false
FY
0001865127
0
0001865127
2022-07-01
2023-06-30
0001865127
2023-10-13
0001865127
2022-12-31
0001865127
2023-06-30
0001865127
2022-06-30
0001865127
2021-07-01
2022-06-30
0001865127
lsdi:ClassAVotingCommonSharesMember
us-gaap:CommonStockMember
2021-06-30
0001865127
lsdi:ClassBCommonNonVotingSharesMember
us-gaap:CommonStockMember
2021-06-30
0001865127
us-gaap:CommonStockMember
2021-06-30
0001865127
us-gaap:RetainedEarningsMember
2021-06-30
0001865127
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-06-30
0001865127
2021-06-30
0001865127
lsdi:ClassAVotingCommonSharesMember
us-gaap:CommonStockMember
2021-07-01
2022-06-30
0001865127
lsdi:ClassBCommonNonVotingSharesMember
us-gaap:CommonStockMember
2021-07-01
2022-06-30
0001865127
us-gaap:CommonStockMember
2021-07-01
2022-06-30
0001865127
us-gaap:RetainedEarningsMember
2021-07-01
2022-06-30
0001865127
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-07-01
2022-06-30
0001865127
lsdi:ClassAVotingCommonSharesMember
us-gaap:CommonStockMember
2022-06-30
0001865127
lsdi:ClassBCommonNonVotingSharesMember
us-gaap:CommonStockMember
2022-06-30
0001865127
us-gaap:CommonStockMember
2022-06-30
0001865127
us-gaap:RetainedEarningsMember
2022-06-30
0001865127
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-06-30
0001865127
lsdi:ClassAVotingCommonSharesMember
us-gaap:CommonStockMember
2022-07-01
2023-06-30
0001865127
lsdi:ClassBCommonNonVotingSharesMember
us-gaap:CommonStockMember
2022-07-01
2023-06-30
0001865127
us-gaap:CommonStockMember
2022-07-01
2023-06-30
0001865127
us-gaap:RetainedEarningsMember
2022-07-01
2023-06-30
0001865127
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-07-01
2023-06-30
0001865127
lsdi:ClassAVotingCommonSharesMember
us-gaap:CommonStockMember
2023-06-30
0001865127
lsdi:ClassBCommonNonVotingSharesMember
us-gaap:CommonStockMember
2023-06-30
0001865127
us-gaap:CommonStockMember
2023-06-30
0001865127
us-gaap:RetainedEarningsMember
2023-06-30
0001865127
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-06-30
0001865127
us-gaap:AccountsReceivableMember
2022-06-30
0001865127
us-gaap:AccountsReceivableMember
2023-06-30
0001865127
lsdi:EarningsPerShareMember
2022-07-01
2023-06-30
0001865127
lsdi:EarningsPerShareMember
2021-07-01
2022-06-30
0001865127
lsdi:TetherMember
2022-07-01
2023-06-30
0001865127
lsdi:TetherMember
2021-07-01
2022-06-30
0001865127
lsdi:ClassBCommonNonVotingSharesMember
2021-02-01
2021-02-25
0001865127
lsdi:ClassBCommonNonVotingSharesMember
2021-02-25
0001865127
2021-02-25
0001865127
2023-03-01
2023-03-20
0001865127
us-gaap:IPOMember
2023-02-13
0001865127
us-gaap:IPOMember
2023-02-01
2023-02-13
0001865127
2023-02-13
0001865127
2023-02-01
2023-02-13
0001865127
2017-08-01
2017-08-01
0001865127
2022-08-01
2022-08-01
0001865127
2018-12-31
0001865127
2018-12-31
2018-12-31
0001865127
2020-07-01
2021-06-30
0001865127
2019-06-30
0001865127
2021-08-20
0001865127
2021-08-20
2021-08-20
0001865127
srt:MinimumMember
2021-07-01
2022-06-30
0001865127
srt:MaximumMember
lsdi:CanadianEmergencyBusinessAccountMember
2021-07-01
2022-06-30
0001865127
us-gaap:CommonClassBMember
2020-07-01
2021-06-30
0001865127
lsdi:CanadianEmergencyBusinessAccountMember
2020-04-20
2020-04-20
0001865127
srt:MinimumMember
srt:ScenarioForecastMember
lsdi:CanadianEmergencyBusinessAccountMember
2023-12-01
2023-12-31
0001865127
srt:ScenarioForecastMember
lsdi:CanadianEmergencyBusinessAccountMember
2023-12-01
2023-12-31
0001865127
srt:ScenarioForecastMember
lsdi:CanadianEmergencyBusinessAccountMember
2023-12-31
0001865127
srt:MaximumMember
srt:ScenarioForecastMember
lsdi:CanadianEmergencyBusinessAccountMember
2023-12-01
2023-12-31
0001865127
lsdi:CanadianEmergencyBusinessAccountMember
2023-06-30
0001865127
lsdi:CanadianEmergencyBusinessAccountMember
2022-07-01
2023-06-30
0001865127
currency:CAD
lsdi:CanadianEmergencyBusinessAccountMember
2022-07-01
2023-06-30
0001865127
lsdi:TerracubeMember
2020-04-20
2020-04-20
0001865127
lsdi:TerracubeMember
2020-04-20
0001865127
lsdi:TerracubeMember
2022-07-01
2023-06-30
0001865127
lsdi:TerracubeMember
2023-06-30
0001865127
lsdi:CanadianEmergencyBusinessAccountMember
lsdi:TerracubeMember
2021-07-01
2022-06-30
0001865127
lsdi:TerracubeMember
2020-12-31
2020-12-31
0001865127
srt:ScenarioForecastMember
lsdi:TerracubeMember
2023-12-31
2023-12-31
0001865127
srt:ScenarioForecastMember
lsdi:TerracubeMember
2023-12-31
0001865127
lsdi:TerracubeMember
2021-07-01
2022-06-30
0001865127
lsdi:TerracubeMember
2020-07-01
2021-06-30
0001865127
2021-02-18
2021-02-18
0001865127
srt:ScenarioForecastMember
2023-12-31
2023-12-31
0001865127
srt:ScenarioForecastMember
2023-12-31
0001865127
srt:ScenarioForecastMember
lsdi:CanadianEmergencyBusinessAccountMember
2023-12-31
2023-12-31
0001865127
srt:MinimumMember
lsdi:CanadianEmergencyBusinessAccountMember
2021-07-01
2022-06-30
0001865127
us-gaap:RelatedPartyMember
2022-06-30
0001865127
us-gaap:RelatedPartyMember
2021-06-30
0001865127
us-gaap:RelatedPartyMember
2022-07-01
2023-06-30
0001865127
us-gaap:RelatedPartyMember
2021-07-01
2022-06-30
0001865127
us-gaap:RelatedPartyMember
2023-06-30
0001865127
srt:ChiefExecutiveOfficerMember
2021-02-25
0001865127
lsdi:ConvertibleNotesMember
2023-06-30
0001865127
us-gaap:ConvertibleNotesPayableMember
2021-07-01
2022-06-30
0001865127
srt:ChiefExecutiveOfficerMember
2023-02-13
0001865127
2021-04-21
2021-05-28
0001865127
lsdi:ConvertibleNotesMember
2021-11-28
0001865127
2021-12-28
0001865127
lsdi:ConvertibleNotesMember
2021-12-01
2021-12-28
0001865127
us-gaap:ConvertibleNotesPayableMember
2023-02-13
0001865127
lsdi:ConvertibleNotesMember
2023-02-01
2023-02-13
0001865127
us-gaap:ConvertibleNotesPayableMember
2023-02-01
2023-02-13
0001865127
2021-06-29
2022-12-28
0001865127
us-gaap:ConvertibleNotesPayableMember
2023-06-30
0001865127
us-gaap:ConvertibleNotesPayableMember
2022-06-30
0001865127
us-gaap:ConvertibleNotesPayableMember
2022-07-01
2023-06-30
0001865127
2020-11-01
2020-11-05
0001865127
srt:ScenarioForecastMember
2023-11-01
2023-11-05
0001865127
2021-01-15
2021-01-15
0001865127
srt:ScenarioForecastMember
2025-11-01
2025-11-05
0001865127
us-gaap:LineOfCreditMember
2022-07-01
2023-06-30
0001865127
us-gaap:LineOfCreditMember
2021-07-01
2022-06-30
0001865127
2021-01-22
0001865127
2021-01-15
2021-01-22
0001865127
us-gaap:NoteWarrantMember
2021-01-15
2021-01-22
0001865127
2021-12-08
0001865127
2021-12-01
2021-12-08
0001865127
us-gaap:CommonStockMember
2022-07-01
2023-06-30
0001865127
us-gaap:CommonClassBMember
2022-07-01
2023-06-30
0001865127
us-gaap:CommonClassBMember
2018-10-22
0001865127
2023-04-06
0001865127
2023-06-29
0001865127
2023-06-29
2023-06-29
0001865127
us-gaap:RelatedPartyMember
2023-06-29
0001865127
2023-01-16
2023-01-16
0001865127
2021-12-28
2021-12-28
0001865127
2022-02-15
0001865127
2022-02-15
2022-02-15
0001865127
2022-03-09
0001865127
2021-10-01
0001865127
2021-10-01
2021-10-01
0001865127
2022-06-30
2022-06-30
0001865127
us-gaap:StockOptionMember
2022-03-09
0001865127
lsdi:NonvotingMember
2022-03-09
2022-03-09
0001865127
2023-05-01
0001865127
2023-05-01
2023-05-01
0001865127
2022-09-17
0001865127
2023-03-02
0001865127
2021-01-15
0001865127
us-gaap:WarrantMember
2021-12-08
0001865127
lsdi:SharePurchaseOptionsMember
2021-06-30
0001865127
lsdi:SharePurchaseOptionsMember
2021-07-01
2022-06-30
0001865127
lsdi:SharePurchaseOptionsMember
2022-06-30
0001865127
lsdi:SharePurchaseOptionsMember
2022-07-01
2023-06-30
0001865127
lsdi:SharePurchaseOptionsMember
2023-06-30
0001865127
lsdi:SharePurchaseOptionsOneMember
2022-07-01
2023-06-30
0001865127
lsdi:SharePurchaseOptionsOneMember
2023-06-30
0001865127
lsdi:SharePurchaseOptionsTwoMember
2022-07-01
2023-06-30
0001865127
lsdi:SharePurchaseOptionsTwoMember
2023-06-30
0001865127
lsdi:SharePurchaseOptionsThreeMember
2022-07-01
2023-06-30
0001865127
lsdi:SharePurchaseOptionsThreeMember
2023-06-30
0001865127
lsdi:SharePurchaseOptionsFourMember
2022-07-01
2023-06-30
0001865127
lsdi:SharePurchaseOptionsFourMember
2023-06-30
0001865127
lsdi:SharePurchaseOptionsFiveMember
2022-07-01
2023-06-30
0001865127
lsdi:SharePurchaseOptionsFiveMember
2023-06-30
0001865127
us-gaap:WarrantMember
2022-07-01
2023-06-30
0001865127
us-gaap:WarrantMember
2021-06-30
0001865127
us-gaap:WarrantMember
2021-07-01
2022-06-30
0001865127
us-gaap:WarrantMember
2022-06-30
0001865127
us-gaap:WarrantMember
2023-06-30
0001865127
us-gaap:IPOMember
2022-07-01
2023-06-30
0001865127
us-gaap:IPOMember
2018-12-31
0001865127
us-gaap:IPOMember
2021-07-01
2022-06-30
0001865127
2021-06-30
2021-06-30
0001865127
2023-02-13
2023-02-13
0001865127
us-gaap:IPOMember
2019-06-01
2019-06-30
0001865127
us-gaap:IPOMember
2023-06-30
0001865127
us-gaap:SubsequentEventMember
2023-07-04
0001865127
2023-07-04
2023-07-04
0001865127
us-gaap:SubsequentEventMember
2023-07-05
2023-07-05
0001865127
us-gaap:SubsequentEventMember
2023-07-28
0001865127
us-gaap:SubsequentEventMember
2023-08-01
0001865127
us-gaap:CommonStockMember
2023-09-06
0001865127
2023-09-06
2023-09-06
0001865127
2023-09-06
xbrli:shares
iso4217:USD
iso4217:USD
xbrli:shares
iso4217:CAD
xbrli:pure
iso4217:CAD
xbrli:shares
As of September 28, 2023, Lucy Scientific Discovery,
Inc. (the “Company”) has one class of securities registered under Section 12 of the Securities Exchange Act of 1934, as amended
(the “Exchange Act”): common shares, no par value.
The following description summarizes the most
important terms of our common shares. This summary does not purport to be complete and is qualified in its entirety by the provisions
of our amended and restated articles of incorporation a copy of which have been incorporated by reference as an exhibit to the Annual
Report on Form 10-K of which this Exhibit 4.4 is a part. For a complete description of our capital stock, you should refer to our amended
and restated articles of incorporation and to the applicable provisions of British Columbia law.
Our authorized share capital consists of an unlimited
number of common shares and an unlimited number of preferred shares, issuable in series, all of which preferred shares will be undesignated.
The outstanding common shares are fully paid and
nonassessable.
The holders of our common
shares are entitled to one vote for each share held on all matters submitted to a vote of the shareholders. Holders of our common shares
are entitled to receive ratably any dividends declared by our board of directors out of funds legally available for that purpose, subject
to any preferential dividend rights of any outstanding preferred shares. Our common shares have no pre-emptive rights, conversion rights
or other subscription rights or redemption or sinking fund provisions.
In the event of our liquidation,
dissolution or winding up, holders of our common shares will be entitled to share ratably in all assets remaining after payment of all
debts and other liabilities and any liquidation preference of any outstanding preferred shares. The shares to be issued by us in this
offering will be, when issued and paid for, validly issued, fully paid and non-assessable.
Lucy Scientific Discovery, Inc., a British
Columbia corporation, had the subsidiaries shown below as of September 28, 2023. Lucy Scientific Discovery, Inc. is not a subsidiary of
any other entity.
In connection with the annual report of Lucy Scientific
Discovery Inc. (the “Company”) on Form 10-K for the year end June 30, 2023, as filed with the Securities and Exchange Commission
on the date hereof (the “Report”), I, Richard Nanula, do hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant
to Section 906 of the Sarbanes-Oxley Act of 2002, to the best of my knowledge, that:
In connection with the annual report of Lucy Scientific
Discovery Inc. (the “Company”) on Form 10-K for the year ended June 30, 2023, as filed with the Securities and Exchange Commission
on the date hereof (the “Report”), I, Brian Zasitko, do hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant
to Section 906 of the Sarbanes-Oxley Act of 2002, to the best of my knowledge, that: