false 0001827087 0001827087 2023-11-16 2023-11-16
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 16, 2023
VIGIL NEUROSCIENCE, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-41200 |
|
85-1880494 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
|
| Vigil Neuroscience, Inc. |
| 100 Forge Road, Suite 700 |
| Watertown, Massachusetts 02472 |
| (Address of principal executive offices, including zip code) |
(857) 254-4445
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trade Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.0001 par value per share |
|
VIGL |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
On November 16, 2023, Vigil Neuroscience, Inc. (the “Company”) issued a press release and held a webcast announcing interim data analyses of its Phase 2 IGNITE Clinical Trial evaluating iluzanebart (VGL101) as a treatment for adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (“ALSP”) and its ILLUMINATE natural history study of ALSP. A copy of the press release and a copy of the presentation are furnished herewith as Exhibits 99.1 and 99.2, respectively.
The information set forth under Item 7.01 and in Exhibits 99.1 and 99.2 attached hereto is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific reference in such filing.
On November 16, 2023, the Company announced an interim data analysis of its Phase 2 IGNITE Clinical Trial evaluating iluzanebart (formerly referred to as VGL101) as a treatment for ALSP representing the first six patients following six months of treatment with 20 mg/kg of iluzanebart (VGL101) – the announcement included:
| |
• |
|
Favorable safety and tolerability profile, including no hematologic adverse events. |
| |
• |
|
Predictable pharmacokinetic and brain penetration profile consistent with Phase 1 data in healthy volunteers. |
| |
• |
|
Clear target engagement, based on soluble TREM2 (“sTREM2”) levels, and downstream pharmacological activity, based on soluble colony stimulating factor 1 receptor (“sCSF1R”) and osteopontin levels, at 20 mg/kg, consistent with Phase 1 data in healthy volunteers. |
| |
• |
|
Directionally supportive changes in both neurofilament light (“NfL”) and magnetic resonance imaging (“MRI”) measurements on ventricular volume and gray matter volume in individual patients. |
| |
• |
|
The Company believes the quality and consistency of the interim data further support the continuation of IGNITE without modification. |
The Company also announced additional interim data at 12 months from ILLUMINATE, a natural history study of ALSP patients – the announcement included:
| |
• |
|
sCSF1R and NfL levels are remarkably altered in ALSP, supporting the Company’s hypothesis that these are key biomarkers of disease pathology. |
| |
• |
|
Totality of the data, including longitudinal progression observed on selected MRI measures and clinical endpoints, support engagement with regulatory authorities. |
| |
• |
|
The Company believes the quality and consistency of data in this interim analysis support chosen biomarkers for pharmacological activity. |
| |
• |
|
MRI measurements on ventricular volume and gray matter volume are emerging as key indicators of disease progression. |
| |
• |
|
Interim Montreal Cognitive Assessment and Cortical Basal Ganglia Functional Scale data support use as clinical endpoints in ALSP at 12 months. |
Forward-Looking Statements
The disclosure under this Item 8.01 contains “forward-looking statements” of the Company that are made pursuant to the safe harbor provisions of the federal securities laws, including, without limitation, express or implied statements regarding the potential of iluzanebart as a novel treatment option for patients with ALSP and the Company’s beliefs about TREM2 agonism’s importance in ALSP. Factors that could cause actual results to differ include the risks whether results from preclinical studies and interim data from clinical trials will be predictive of the
results of final data from clinical trials; as well as the risks and uncertainties identified in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2023 and in any subsequent filings it may make with the SEC. All disclosure under this Item 8.01 is as of the date of this Form 8-K, and the Company undertakes no duty to update this information unless required by law.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Vigil Neuroscience, Inc. |
|
|
|
|
| Date: November 16, 2023 |
|
|
|
By: |
|
/s/ Ivana Magovčević-Liebisch |
|
|
|
|
|
|
Ivana Magovčević-Liebisch |
|
|
|
|
|
|
President and Chief Executive Officer |
Exhibit 99.1

Vigil Neuroscience Reports Positive Interim Data from Phase 2 IGNITE Proof-of-Concept Clinical Trial Evaluating Iluzanebart (VGL101) as a Treatment for ALSP and from Ongoing Natural History Study ILLUMINATE
- Iluzanebart demonstrated favorable safety and tolerability, including no hematologic adverse events -
- Clear CNS target engagement and downstream pharmacological activity at 20 mg/kg consistent with Phase 1 data; Directionally supportive
changes in individual patients at 6 months on MRI and NfL biomarkers -
- Natural History Study continued to provide critical
insights on MRI and NfL biomarkers; sCSF1R emerging as key biomarker of ALSP disease pathology -
- Company expects to report Phase
2 IGNITE results from all patients in 20 mg/kg and 40 mg/kg cohorts at 6 months in third quarter of 2024 -
- Company to host
webinar today at 4:30 p.m. ET -
WATERTOWN, Mass. – November 16, 2023 – Vigil Neuroscience, Inc. (Nasdaq:
VIGL), a clinical-stage biotechnology company committed to harnessing the power of microglia for the treatment of neurodegenerative diseases, today announced positive interim data from the Company’s Phase 2 IGNITE
proof-of-concept clinical trial in patients with adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP). The interim data, representing the
first six patients following six months of treatment with 20 mg/kg of iluzanebart (formerly referred to as VGL101), further support the favorable safety and tolerability profile as was previously seen in healthy volunteers. In addition, these data
demonstrated clear target engagement and downstream pharmacological activity at 20 mg/kg consistent with the Company’s previously reported Phase 1 data in healthy volunteers. Directionally supportive changes at 6 months on magnetic resonance
imaging (MRI) and neurofilament light (NfL) biomarkers of disease progression in individual patients with ALSP were also observed.
The Company also
reported findings from its ongoing natural history study, ILLUMINATE, which continued to provide critical insights on MRI and NfL biomarkers and supports soluble colony stimulating factor 1 receptor (sCSF1R) as a potential key biomarker of ALSP
disease pathology.
“The positive interim results from our Phase 2 IGNITE trial represent the first clinical data reported from an
interventional study in patients with ALSP and reaffirm our belief in the potential of iluzanebart as a novel treatment option. Importantly, we are the first company to show clinical data on TREM2 agonism as a potential therapeutic approach in
patients with a neurodegenerative disease,” said Ivana Magovčević-
1
Liebisch, Ph.D., J.D., President and Chief Executive Officer of Vigil. “In the 6 patients observed in the interim analysis, iluzanebart demonstrated a favorable safety and tolerability
profile, compelling pharmacological activity, and positive quantifiable trends in NfL and MRI biomarkers in individual patients.”
“In addition,
our ongoing natural history study ILLUMINATE continues to provide critical insights into ALSP and biomarkers that we believe correlate with disease progression,” continued Dr. Magovčević-Liebisch. “We expect to use the
promising findings from IGNITE and ILLUMINATE to engage with the FDA to initiate discussions regarding a potential accelerated development pathway. We look forward to further executing on our precision medicine strategy in neurodegenerative diseases
– because patients cannot wait.”
Key Highlights from Phase 2 IGNITE Interim Data:
| |
• |
|
Favorable safety and tolerability profile, including no hematologic adverse events. |
| |
• |
|
Predictable pharmacokinetic and brain penetration profile consistent with Phase 1 data in healthy volunteers.
|
| |
• |
|
Clear target engagement, based on sTREM2 levels, and downstream pharmacological activity, based on sCSF1R and
osteopontin levels, at 20 mg/kg, consistent with Phase 1 data in healthy volunteers. |
| |
• |
|
Directionally supportive changes in both NfL and MRI measurements on ventricular volume and gray matter volume in
individual patients. |
| |
• |
|
We believe the quality and consistency of the interim data further support the continuation of IGNITE without
modification. |
Key Updates from Natural History Study ILLUMINATE:
| |
• |
|
sCSF1R and NfL levels are remarkably altered in ALSP, supporting our hypothesis that these are key biomarkers of
disease pathology. |
| |
• |
|
Totality of the data, including longitudinal progression observed on selected MRI measures and clinical
endpoints, support engagement with regulatory authorities. |
| |
• |
|
We believe the quality and consistency of data in this interim analysis support chosen biomarkers for
pharmacological activity. |
| |
• |
|
MRI measurements on ventricular volume and gray matter volume are emerging as key indicators of disease
progression. |
| |
• |
|
Interim Montreal Cognitive Assessment (MoCA) and Cortical Basal Ganglia Functional Scale data support use as
clinical endpoints in ALSP at 12 months. |
“ALSP is a rare, devastating and fast-progressing disease with no approved treatments
that target the underlying cause of the disease or slow its progression,” said Zbigniew Wszolek, M.D., Neurologist at Mayo Clinic. “These interim results are a great step forward for patients and caregivers impacted by ALSP and I am
encouraged by the progress the Vigil team has made developing a potential novel treatment option for this autosomal dominant disease. To date, iluzanebart has been well-tolerated, and the data seem to suggest that we are seeing directional changes
in CSF1R and positive trends in NfL and MRI measurements. This is an important milestone for the underserved ALSP community of patients and caregivers.”
2
“Today’s results are a reflection of the dedication and expertise of our incredible team who
brings an unwavering commitment to patients to their work,” concluded Dr. Magovčević-Liebisch. “We extend a sincere thank you to all who have contributed to this progress, including our trial participants, their caregivers,
and the clinical investigators. We look forward to sharing additional data from the IGNITE trial, including results from all patients in both the 20 mg/kg and 40 mg/kg cohorts at 6 months, in the third quarter of 2024.”
The Company also announced that it will host a virtual webinar to discuss the Phase 2 IGNITE interim data today, Thursday, November 16, 2023, from 4:30
p.m. to 5:30 p.m. ET.
The event will include prepared remarks by the Vigil management team who will be joined by David S. Lynch, M.D., Ph.D., Consultant
Neurologist National Hospital for Neurology & Neurosurgery, Queen Square & UCL Institute of Neurology, London & Clinical Lead, Adult Inherited White Matter Disorders Highly Specialist Service, NHS England.
To access the live webinar, please register here or visit “Events & Presentations” in the “Investors” section of
the Vigil website at www.vigilneuro.com. An archived replay of the webinar will be available for approximately 90 days following the event.
About
Phase 2 IGNITE Clinical Trial
IGNITE is a global Phase 2, open-label
proof-of-concept trial evaluating iluzanebart in approximately 15 patients with symptomatic ALSP who have a confirmed CSF1R gene mutation. The primary objective
of the IGNITE trial is to evaluate the safety and tolerability of iluzanebart. Secondary outcome measures include evaluating the effects of iluzanebart on target engagement and on MRI and NfL biomarkers of disease progression. Exploratory outcome
assessments include the evaluation of clinical efficacy measures using standard cognitive, motor and functional assessments of iluzanebart in patients with ALSP. Patients enrolled in the trial will receive an intravenous (IV) infusion of
iluzanebart at 20 mg/kg or 40 mg/kg approximately every four weeks for a treatment duration of one year.
About Iluzanebart (VGL101)
Iluzanebart, Vigil’s lead clinical candidate, is a fully human monoclonal antibody targeting human triggering receptor expressed on myeloid cells 2
(TREM2), which is responsible for maintaining microglial cell function. TREM2 deficiency is believed to be a driver of certain neurodegenerative diseases. Iluzanebart is in development for rare microgliopathies, such as ALSP, as well as other
neurodegenerative diseases for which TREM2 and/or microglia deficiency is believed to be a key driver of disease pathway.
About ALSP
ALSP is a rare, inherited, autosomal dominant neurological disease with high penetrance. It is caused by a mutation to the CSF1R gene and affects an estimated
10,000 people in the United States, with similar prevalence in Europe and Japan. The disease generally presents in adults in their forties, is diagnosed through genetic testing and established clinical/radiologic criteria and is characterized by
cognitive dysfunction, neuropsychiatric symptoms, and motor impairment. These symptoms typically exhibit rapid progression with a life expectancy of approximately six to seven years on average after diagnosis, causing significant patient and
caregiver burden. There are currently no approved therapies for the treatment of ALSP, underscoring the high unmet need in this rare indication.
3
About Vigil Neuroscience
Vigil Neuroscience is a clinical-stage biotechnology company focused on developing treatments for both rare and common neurodegenerative diseases by restoring
the vigilance of microglia, the sentinel immune cells of the brain. Vigil is utilizing the tools of modern neuroscience drug development across multiple therapeutic modalities in its efforts to develop precision-based therapies to improve the lives
of patients and their families. Iluzanebart, Vigil’s lead clinical candidate, is a fully human monoclonal antibody agonist targeting human triggering receptor expressed on myeloid cells 2 (TREM2) in people with adult-onset leukoencephalopathy
with axonal spheroids and pigmented glia (ALSP), a rare and fatal neurodegenerative disease. Vigil is also developing VG-3927, a novel small molecule TREM2 agonist, to treat common neurodegenerative diseases
associated with microglial dysfunction, with an initial focus on Alzheimer’s disease (AD) in genetically defined subpopulations.
Forward-Looking
Statements
This press release includes certain disclosures that contain “forward-looking statements” of Vigil Neuroscience
(“Vigil” or the “Company”) that are made pursuant to the safe harbor provisions of the federal securities laws, including, without limitation, express or implied statements regarding: the potential of iluzanebart as a novel
treatment option for patients with ALSP; Vigil’s beliefs about TREM2 agonism’s importance in Alzheimer’s disease and ALSP; the progress and timing of the clinical development of Vigil’s programs, including the availability of,
and expected timing for reporting, interim and final data from both the IGNITE Phase 2 clinical trial; and the success and timing of the Company’s interactions with regulatory authorities, including with the FDA regarding an accelerated
development pathway. Forward-looking statements are based on Vigil’s current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Factors that could cause actual results to differ include,
but are not limited to, risks and uncertainties related to uncertainties inherent in the identification and development of product candidates, including the conduct of research activities and the conduct of clinical trials; uncertainties as to the
availability and timing of results and data from clinical trials; whether results from preclinical studies and interim data from clinical trials will be predictive of the results of final data from clinical trials; the timing and content of
additional regulatory information from the FDA; whether Vigil’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; as well as the risks and uncertainties
identified in the Company’s filings with the Securities and Exchange Commission (SEC), including Vigil’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2023 and in any
subsequent filings Vigil makes with the SEC. Forward-looking statements contained in this announcement are made as of this date, and Vigil undertakes no duty to update such information except as required under applicable law. Readers should not rely
upon the information on this page as current or accurate after its publication date.
Internet Posting of Information
Vigil Neuroscience routinely posts information that may be important to investors in the “Investors” section of its website at
https://www.vigilneuro.com. The company encourages investors and potential investors to consult our website regularly for important information about Vigil Neuroscience.
4
Investor Contact:
Leah Gibson
Vice President, Investor Relations &
Corporate Communications
Vigil Neuroscience, Inc.
lgibson@vigilneuro.com
Media Contact:
Megan McGrath
MacDougall Advisors
mmcgrath@macdougall.bio
5

Interim Analyses of ILLUMINATE Natural
History Study & IGNITE Phase 2 Trial for Iluzanebart (VGL101) in ALSP November 16, 2023 Exhibit 99.2

Today’s Agenda 4:30 - 4:35 PM (5
min) Opening Remarks and Corporate Overview Ivana Magovčević-Liebisch, PhD, JD President & Chief Executive Officer, Vigil Neuroscience, Inc. 4:35 - 5:05 PM (30 min) Presentation of Iluzanebart (VGL101) Interim Data from Phase 2
IGNITE Study in ALSP & Ongoing ILLUMINATE Natural History Study David Gray, PhD Chief Science Officer, Vigil Neuroscience, Inc. 5:05 - 5:15 PM (10 min) ALSP Disease Overview & Perspective on Interim Results David S. Lynch, MD, PhD
Consultant Neurologist, National Hospital for Neurology & Neurosurgery, Queen Square & UCL Institute of Neurology, London Clinical Lead, Adult Inherited White Matter Disorders Highly Specialist Service, NHS England 5:15 - 5:30 PM (15
min) Closing Remarks and Q&A Session © Vigil Neuroscience, Inc. 2023. All rights reserved. © Vigil Neuroscience, Inc. 2023. All rights reserved.

Reminders Webinar scheduled to end at
5:30pm ET Presentation is available in investors section under Events & Presentations at www.vigilneuro.com Moderated Q&A session following prepared remarks To submit a written question, fill out form on webcast home page Webcast replay
available later today on Vigil website under "Events & Presentations" 3 © Vigil Neuroscience, Inc. 2023. All rights reserved.

FORWARD-LOOKING STATEMENTS This
presentation contains “forward-looking statements,” which are made pursuant to the safe harbor provisions of the federal securities laws, including the Private Securities Litigation Reform Act of 1995. Any statements that are not
statements of historical fact may be deemed to be forward-looking statements. Such statements may contain words such as “may,” “might,” “will,” “could,” “should,” “would,”
“expect,” “intend,” “plan,” “prepare,” “look,” “seek,” “anticipate,” “believe,” “estimate,” “predict,” “potential,”
“possible,” “continue,” “ongoing” or the negative of these terms, or other comparable words. These forward-looking statements include, among others, statements relating to: our plans to deliver precision-based
therapies to improve the lives of patients and their families and to target a broad range of neurodegenerative diseases; plans to discover and develop novel therapeutics to leverage microglial biology, such as VGL101, VG-3927 and current or future
product candidates, and to enable success in clinical development including demonstrating favorable safety and tolerability profiles and achieving therapeutic benefits for patients; beliefs about TREM2 agonism’s importance in ALSP and
Alzheimer’s disease; beliefs about the profiles and potential, including the commercial potential, of our pipeline and the strength of our intellectual property position; our analyses and beliefs about data, including pathology and disease
biomarkers and the signaling, engagement and potency as an agonism of TREM2 in microglia; plans and upcoming milestones, including estimated timelines, for our pipeline program development activities and pipeline expansion opportunities; expected
timing and next steps regarding data announcement, clinical trial design and activities, and regulatory filings and potential approvals; expectations regarding our ability to develop and advance our current and future product candidates and
discovery programs; and the belief that we are well-positioned to execute on our mission. These forward‐looking statements, which are only predictions, involve risks and uncertainties, many of which are beyond our control and are based on our
current beliefs, expectations and assumptions regarding our business. As such, you should not place undue reliance on any forward-looking statements because such risks and uncertainties could cause actual results, performance or achievement to
differ materially and adversely from those anticipated or implied in the forward-looking statements. Factors that could cause actual results to differ from those predicted in our forward-looking statements include, among others, risks and
uncertainties related to product development, including delays or challenges that may arise in the development and regulatory approval of our current and future product candidates or programs; uncertainties as to the availability and timing of
results and data from preclinical and clinical studies; the timing of our ability to submit and obtain regulatory clearance for investigational new drug applications and initiate additional clinical trials; our ability to initiate and complete our
current and expected clinical trials; our ability to establish and maintain collaborations, strategic relationships and supply arrangements, or that we will not realize the intended benefits from such relationships or arrangements; whether our cash
resources will be sufficient to fund our foreseeable and unforeseeable operating expenses and capital expenditure requirements; our ability to raise additional funding on favorable terms, or at all; the rate and degree of market acceptance and
clinical utility of our product candidates; the ability and willingness of our third-party collaborators to continue research and development activities relating to our product candidates; the accuracy of our data analyses or estimates for the
potential and market for our products; our ability, and the ability of our collaborators, to protect our intellectual property and to conduct activities for the development and commercialization of our candidates in view of third party intellectual
property positions; our financial performance; our ability to retain and recruit key personnel, as well as the potential contribution of our employees and board to our growth and success as a Company; developments and projections relating to our
competitors or our industry; our ability to work with the FDA to successfully remove the partial clinical hold on VG-3927; changes in general economic conditions and global instability, in particular economic conditions in the markets on which we or
our suppliers operate; changes in laws and regulations; and those risks and uncertainties identified in our filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in our most-recently filed
Annual Report on Form 10-K or Quarterly Report on Form 10-Q, and such other risks and uncertainties that may be described in subsequent filings we may make with the SEC. You should not rely upon forward-looking statements as predictions of future
events or performance, or as a representation or warranty (express or implied) by us or any other person that we will achieve our objectives and plans in any specified time frame, on such specified terms, or at all. Although our management believes
that the expectations reflected in our statements are reasonable, we cannot guarantee that the future results, performance or events and circumstances described in the forward-looking statements will be achieved or occur. These forward-looking
statements speak only as of the date such statements are made. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly
update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. 2 © Vigil Neuroscience, Inc. 2023. All rights reserved.

Corporate Overview Ivana
Magovčević-Liebisch, PhD, JD Chief Executive Officer Vigil Neuroscience, Inc. © Vigil Neuroscience, Inc. 2023. All rights reserved.
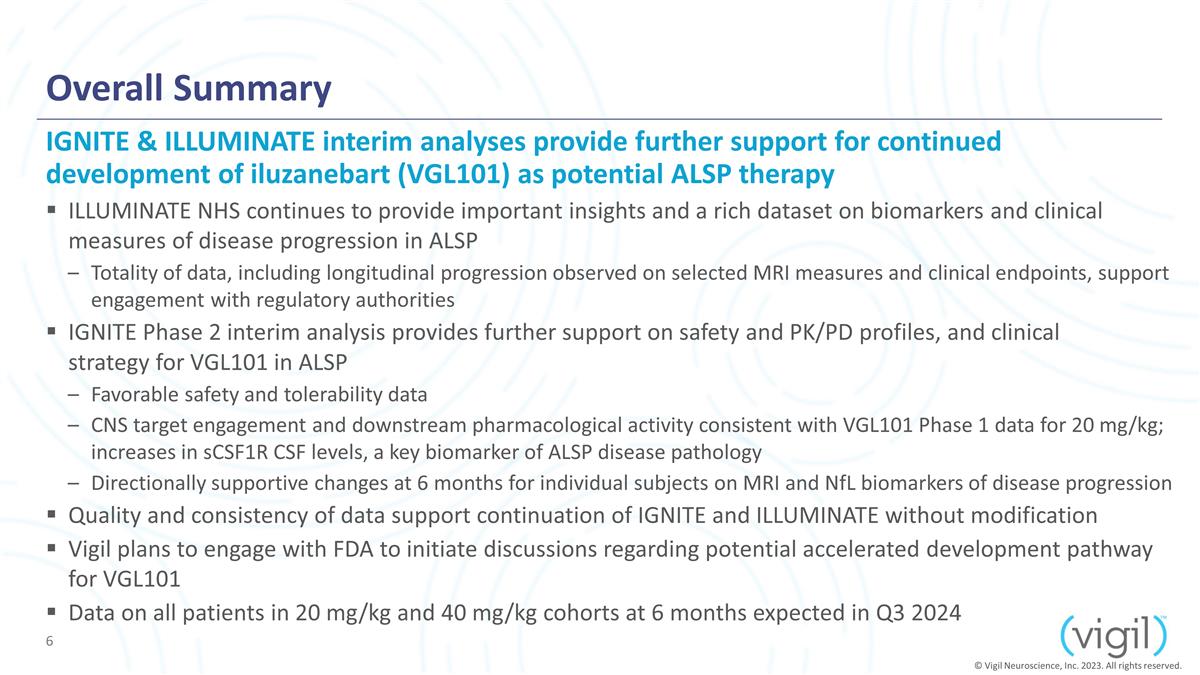
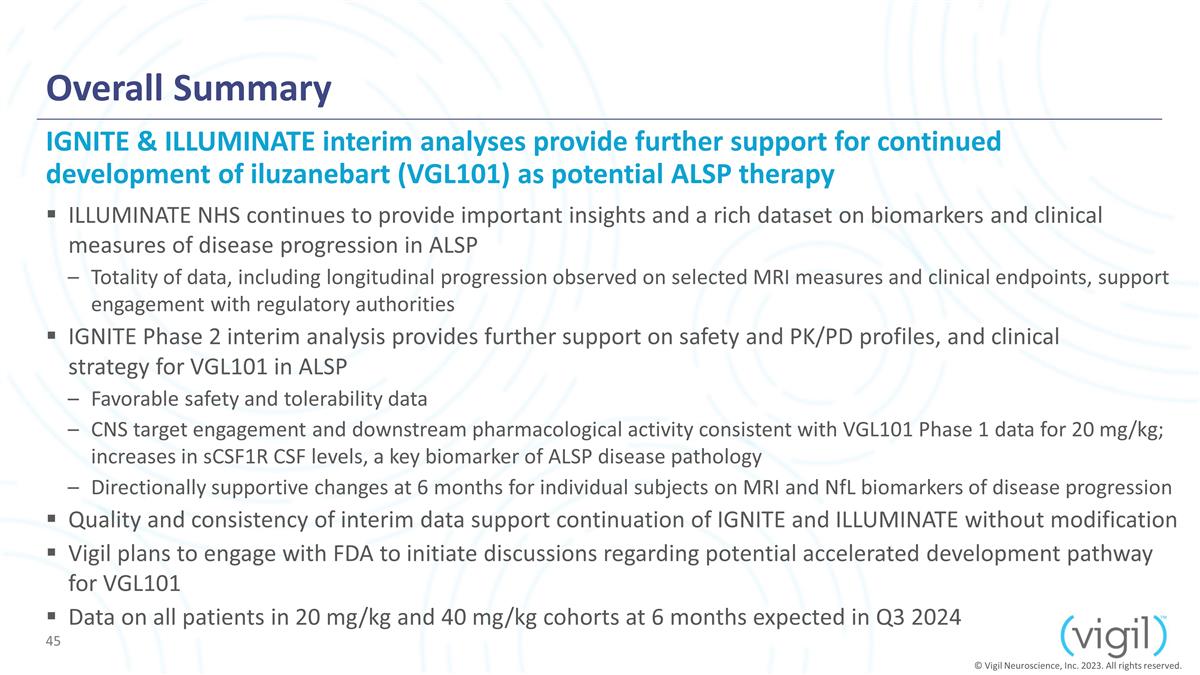
Overall Summary ILLUMINATE NHS
continues to provide important insights and a rich dataset on biomarkers and clinical measures of disease progression in ALSP Totality of data, including longitudinal progression observed on selected MRI measures and
clinical endpoints, support engagement with regulatory authorities IGNITE Phase 2 interim analysis provides further support on safety and PK/PD profiles, and clinical strategy for VGL101 in ALSP Favorable safety and tolerability data CNS target
engagement and downstream pharmacological activity consistent with VGL101 Phase 1 data for 20 mg/kg; increases in sCSF1R CSF levels, a key biomarker of ALSP disease pathology Directionally supportive changes at 6 months for individual subjects
on MRI and NfL biomarkers of disease progression Quality and consistency of data support continuation of IGNITE and ILLUMINATE without modification Vigil plans to engage with FDA to initiate discussions regarding potential accelerated
development pathway for VGL101 Data on all patients in 20 mg/kg and 40 mg/kg cohorts at 6 months expected in Q3 2024 IGNITE & ILLUMINATE interim analyses provide further support for continued development of iluzanebart (VGL101) as potential
ALSP therapy © Vigil Neuroscience, Inc. 2023. All rights reserved.

Vigil Neuroscience Vigil Neuroscience
is a clinical-stage microglia-focused therapeutics company Founded ~3 years ago in July 2020 Our purpose: to treat rare and common neurodegenerative diseases by restoring the vigilance of microglia, the brain’s sentinel
immune cells Precision-based strategy for developing microglia therapeutics Only company known to have 2 modalities for TREM2 agonism – monoclonal antibody and small molecule First company to show clinical data on TREM2 agonism as
potential therapeutic approach in patients with neurodegenerative disease >60 highly dedicated team members © Vigil Neuroscience, Inc. 2023. All rights reserved.

Featured Key Opinion Leader (KOL)
David S. Lynch, MD, PhD Consultant Neurologist, National Hospital for Neurology & Neurosurgery, Queen Square & UCL Institute of Neurology, London Clinical Lead, Adult Inherited White Matter Disorders Highly Specialist Service, NHS
England © Vigil Neuroscience, Inc. 2023. All rights reserved.

ILLUMINATE Natural History Study
Updated Interim Analysis
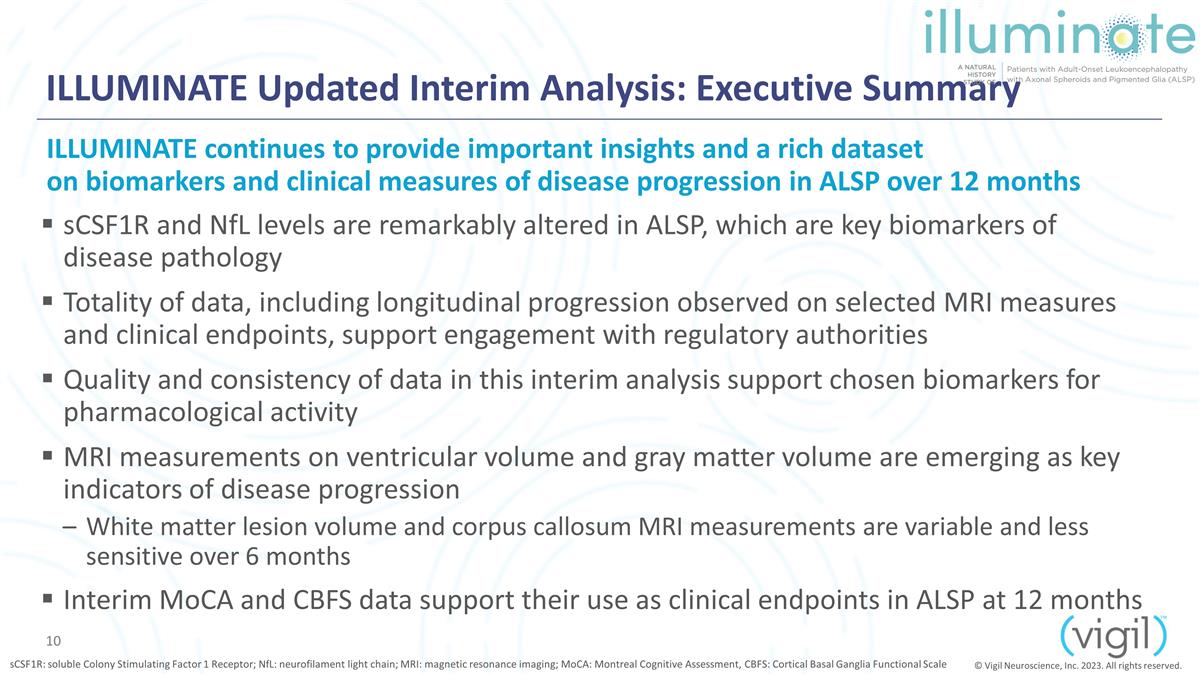
ILLUMINATE Updated Interim
Analysis: Executive Summary sCSF1R and NfL levels are remarkably altered in ALSP, which are key biomarkers of disease pathology Totality of data, including longitudinal progression observed on selected MRI measures and clinical
endpoints, support engagement with regulatory authorities Quality and consistency of data in this interim analysis support chosen biomarkers for pharmacological activity MRI measurements on ventricular volume and gray matter volume are emerging
as key indicators of disease progression White matter lesion volume and corpus callosum MRI measurements are variable and less sensitive over 6 months Interim MoCA and CBFS data support their use as clinical endpoints in ALSP at 12 months
ILLUMINATE continues to provide important insights and a rich dataset on biomarkers and clinical measures of disease progression in ALSP over 12 months sCSF1R: soluble Colony Stimulating Factor 1 Receptor;
NfL: neurofilament light chain; MRI: magnetic resonance imaging; MoCA: Montreal Cognitive Assessment, CBFS: Cortical Basal Ganglia Functional Scale © Vigil Neuroscience, Inc. 2023. All rights reserved.
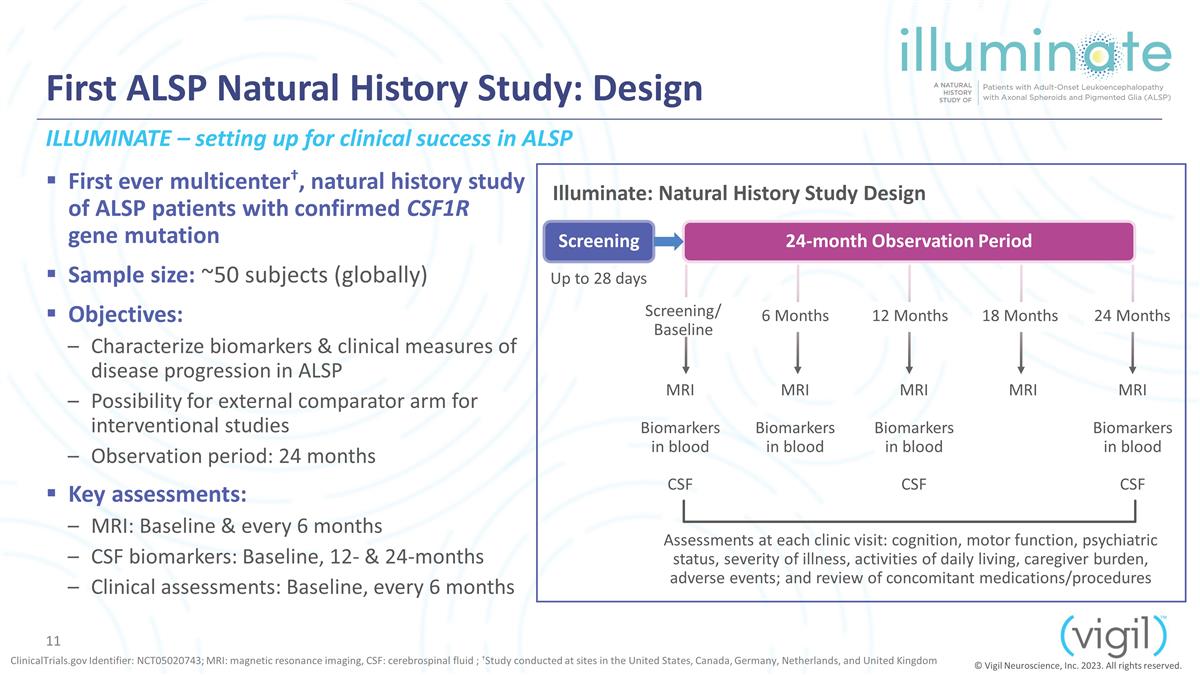
First ALSP Natural History Study:
Design First ever multicenter†, natural history study of ALSP patients with confirmed CSF1R gene mutation Sample size: ~50 subjects (globally) Objectives: Characterize biomarkers & clinical measures of disease progression in
ALSP Possibility for external comparator arm for interventional studies Observation period: 24 months Key assessments: MRI: Baseline & every 6 months CSF biomarkers: Baseline, 12- & 24-months Clinical assessments:
Baseline, every 6 months ClinicalTrials.gov Identifier: NCT05020743; MRI: magnetic resonance imaging, CSF: cerebrospinal fluid ; †Study conducted at sites in the United States, Canada, Germany, Netherlands, and United Kingdom Screening
24-month Observation Period Up to 28 days Screening/ Baseline 6 Months 12 Months 24 Months MRI Biomarkers in blood CSF MRI Biomarkers in blood MRI Biomarkers in blood CSF MRI Biomarkers in blood CSF Assessments at each clinic visit: cognition, motor
function, psychiatric status, severity of illness, activities of daily living, caregiver burden, adverse events; and review of concomitant medications/procedures MRI 18 Months Illuminate: Natural History Study Design ILLUMINATE – setting up
for clinical success in ALSP © Vigil Neuroscience, Inc. 2023. All rights reserved.
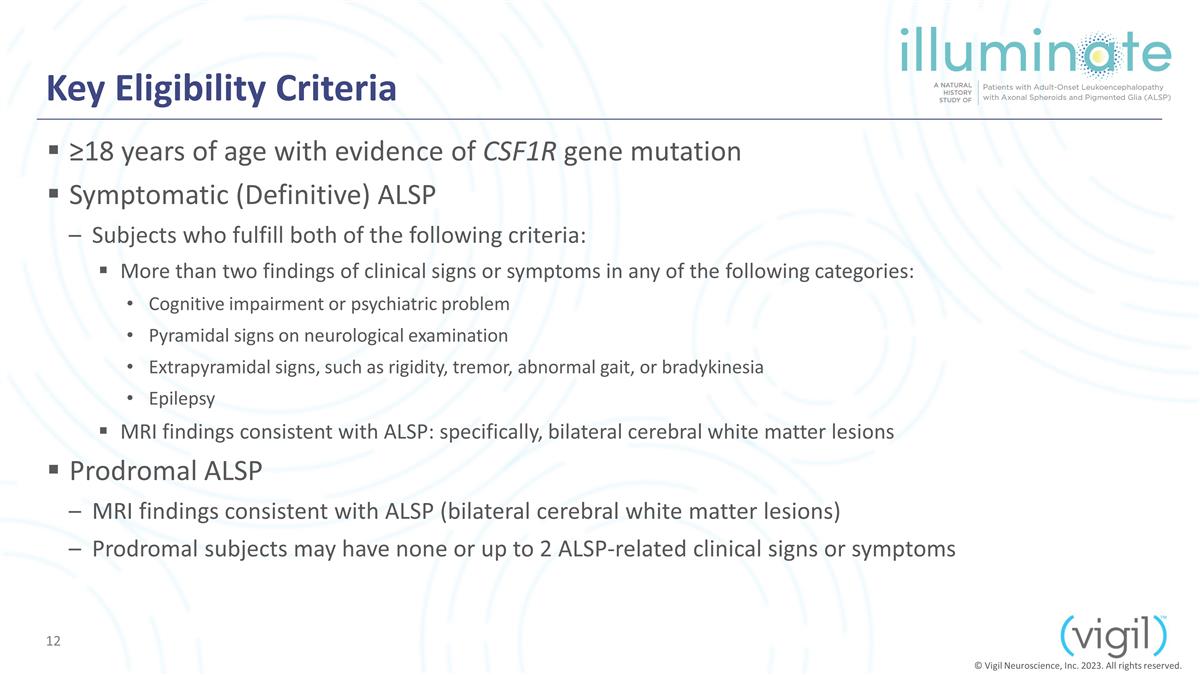
Key Eligibility Criteria ≥18
years of age with evidence of CSF1R gene mutation Symptomatic (Definitive) ALSP Subjects who fulfill both of the following criteria: More than two findings of clinical signs or symptoms in any of the following categories: Cognitive impairment or
psychiatric problem Pyramidal signs on neurological examination Extrapyramidal signs, such as rigidity, tremor, abnormal gait, or bradykinesia Epilepsy MRI findings consistent with ALSP: specifically, bilateral cerebral white matter lesions
Prodromal ALSP MRI findings consistent with ALSP (bilateral cerebral white matter lesions) Prodromal subjects may have none or up to 2 ALSP-related clinical signs or symptoms © Vigil Neuroscience, Inc. 2023. All rights reserved.

Natural History Study Baseline
Demography Prodromal Symptomatic (N=18) (N=14) Age at Screening (yrs.) number 18 14 Mean (SE) 45.7 (4.0) 44.1 (2.8) Median 40.5 44 Sex Female: number (%) 11 (61.1%) 8 (57.1%) Male:
number (%) 7 (38.9%) 6 (42.9%) Race White: number (%) 17 (94.4%) 14 (100.0%) Black: number (%) 1 (5.6%) 0 Ethnicity Hispanic or Latino: number (%) 2 (11.1%) 1 (7.1%) Not Hispanic or
Latino: number (%) 16 (88.9%) 12 (85.7%) Not Stated: number (%) 0 1 (7.1%) Time from Diagnosis (yrs.) number 9 14 Mean (SE) 0.86 (0.39) 0.76 (0.33) Median 0.17 0.38 © Vigil
Neuroscience, Inc. 2023. All rights reserved.

ILLUMINATE: Updated Interim
Analysis on Fluid Biomarkers
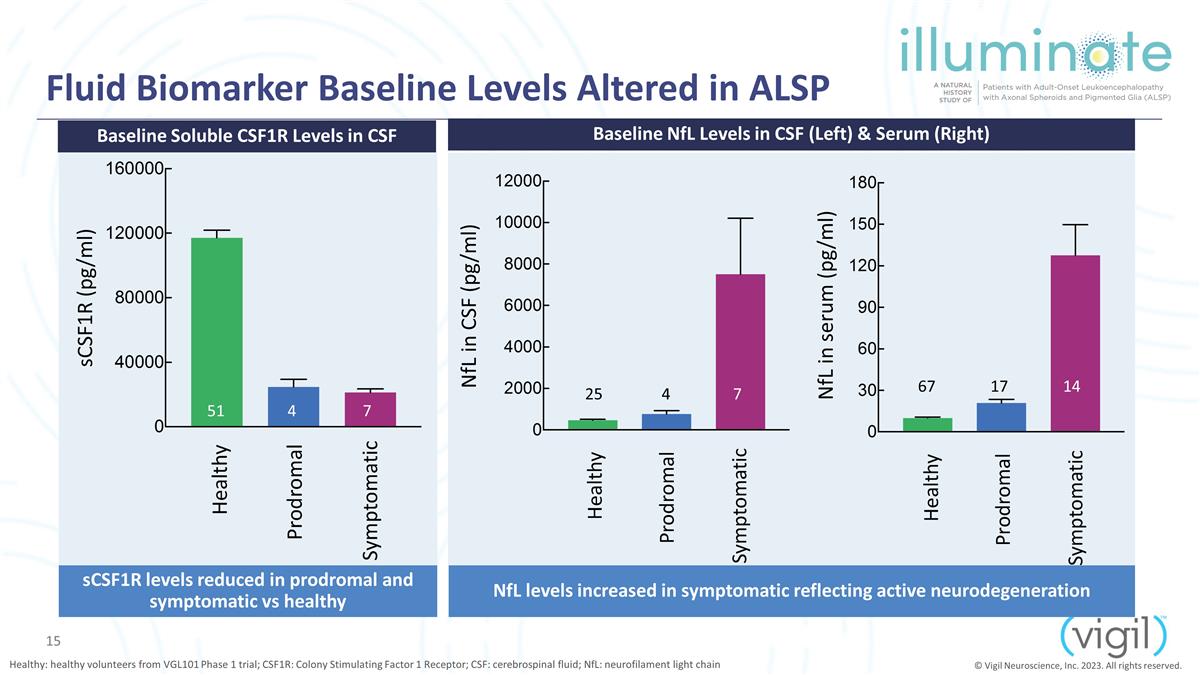
Fluid Biomarker Baseline Levels
Altered in ALSP Healthy: healthy volunteers from VGL101 Phase 1 trial; CSF1R: Colony Stimulating Factor 1 Receptor; CSF: cerebrospinal fluid; NfL: neurofilament light chain Baseline NfL Levels in CSF (Left) & Serum (Right) sCSF1R levels reduced
in prodromal and symptomatic vs healthy NfL levels increased in symptomatic reflecting active neurodegeneration Baseline Soluble CSF1R Levels in CSF © Vigil Neuroscience, Inc. 2023. All rights reserved. 51 4 7 sCSF1R (pg/ml)
25 4 7 NfL in CSF (pg/ml) 67
17 14 NfL in serum (pg/ml)
ILLUMINATE: Updated Interim
Analysis on MRI
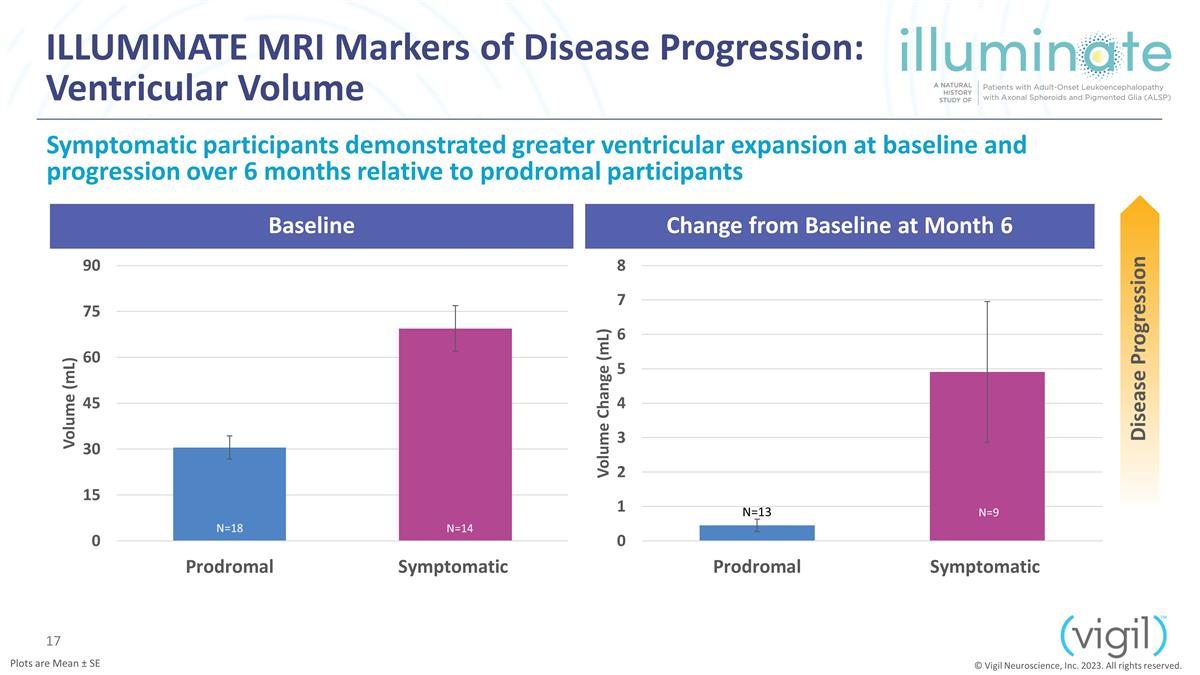
ILLUMINATE MRI Markers of Disease
Progression: Ventricular Volume Baseline Change from Baseline at Month 6 Symptomatic participants demonstrated greater ventricular expansion at baseline and progression over 6 months relative to prodromal participants Disease Progression Plots
are Mean ± SE N=14 © Vigil Neuroscience, Inc. 2023. All rights reserved. N=9
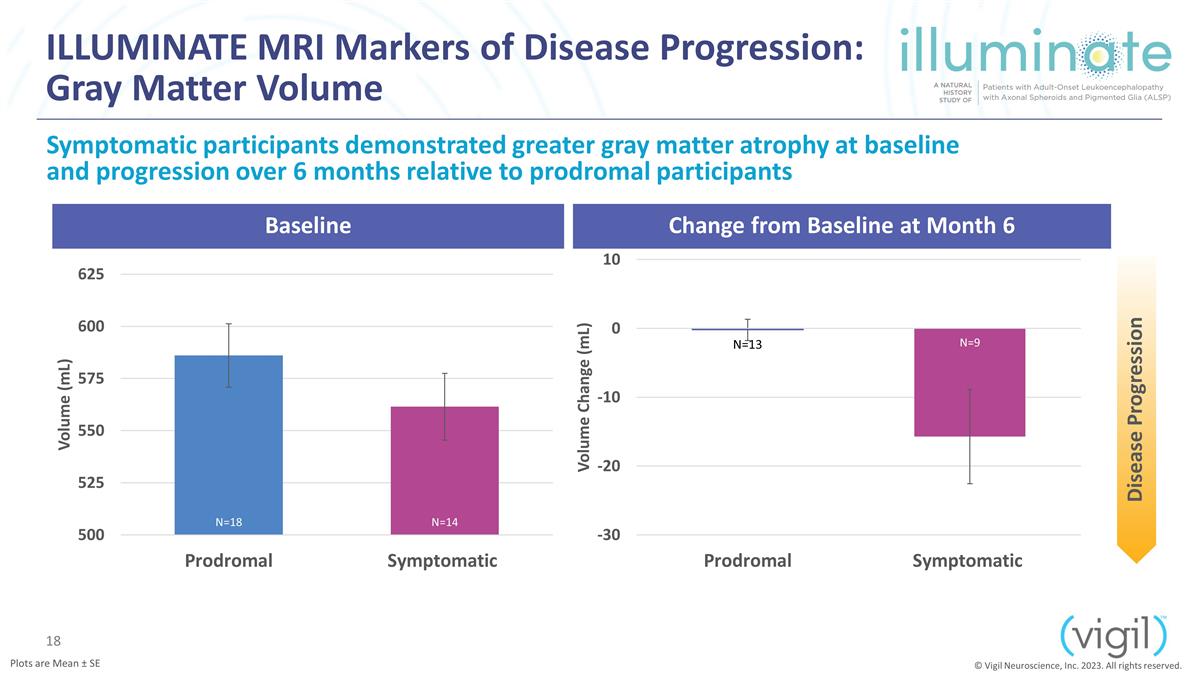
ILLUMINATE MRI Markers of Disease
Progression: Gray Matter Volume Baseline Change from Baseline at Month 6 Symptomatic participants demonstrated greater gray matter atrophy at baseline and progression over 6 months relative to prodromal participants Disease
Progression © Vigil Neuroscience, Inc. 2023. All rights reserved. N=9 Plots are Mean ± SE
ILLUMINATE: Interim Analysis on
Clinical Endpoints
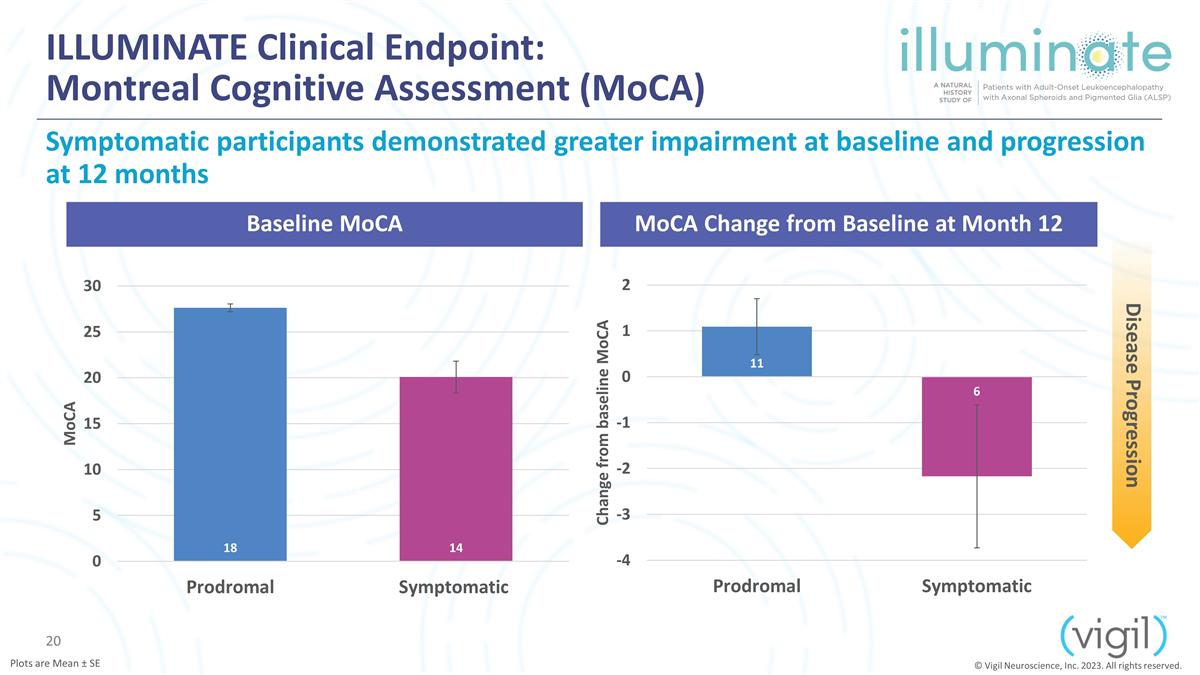
ILLUMINATE Clinical Endpoint:
Montreal Cognitive Assessment (MoCA) Symptomatic participants demonstrated greater impairment at baseline and progression at 12 months N=51 N=4 N=11 N=4 N=7 N=4 N=6 Baseline MoCA MoCA Change from Baseline at Month 12 Disease Progression © Vigil
Neuroscience, Inc. 2023. All rights reserved. Plots are Mean ± SE
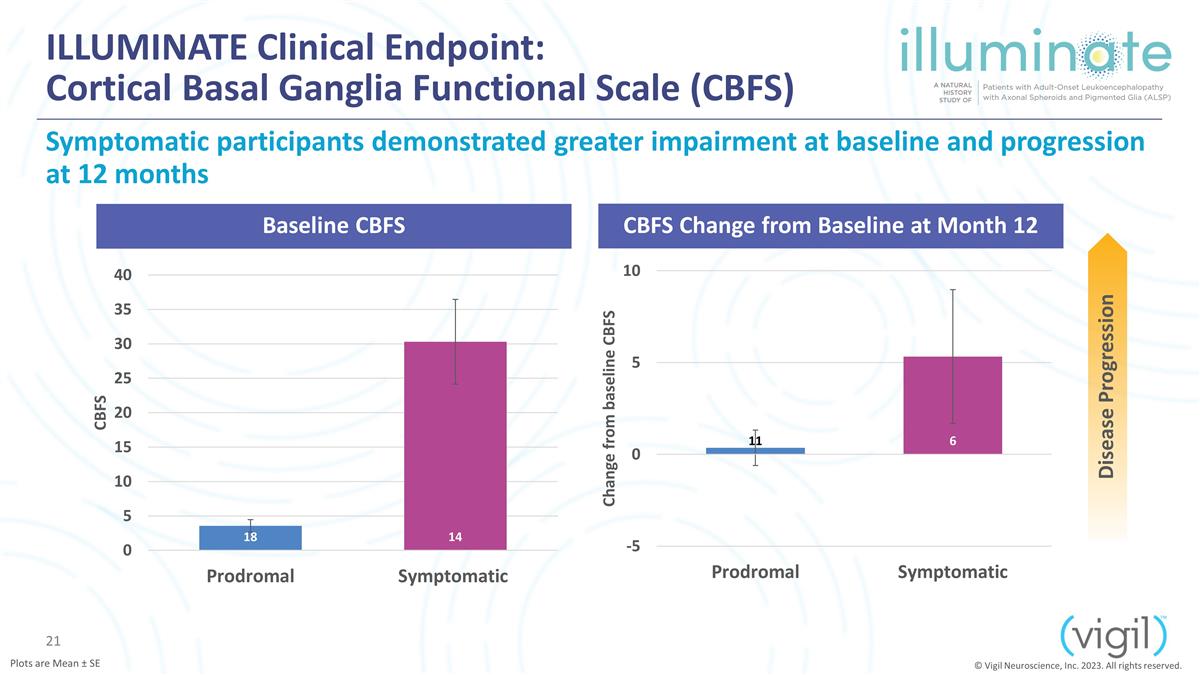
ILLUMINATE Clinical Endpoint:
Cortical Basal Ganglia Functional Scale (CBFS) Symptomatic participants demonstrated greater impairment at baseline and progression at 12 months Baseline CBFS CBFS Change from Baseline at Month 12 Disease Progression © Vigil Neuroscience, Inc.
2023. All rights reserved. Plots are Mean ± SE

IGNITE Phase 2 Proof-of-Concept
Trial of Iluzanebart (VGL101) in ALSP 1st Interim Analysis Iluzanebart (VGL101) is an investigational therapy and has not been reviewed or approved by any regulatory authority
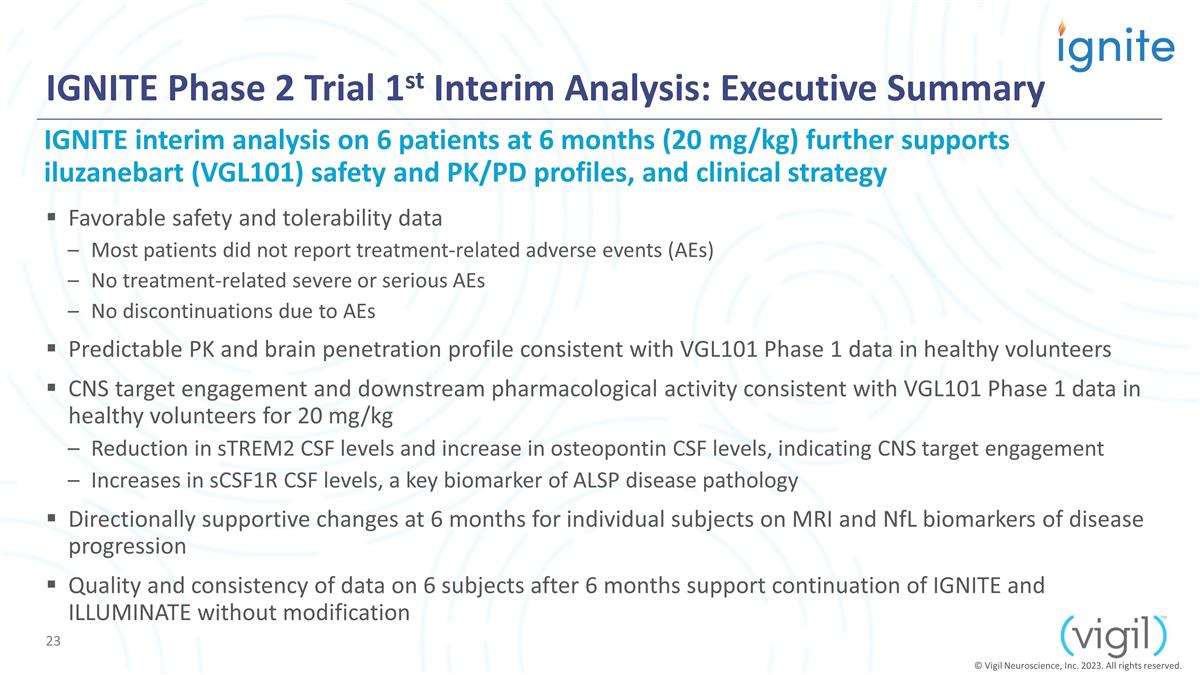
IGNITE Phase 2 Trial 1st Interim
Analysis: Executive Summary Favorable safety and tolerability data Most patients did not report treatment-related adverse events (AEs) No treatment-related severe or serious AEs No discontinuations due to AEs Predictable PK and brain penetration
profile consistent with VGL101 Phase 1 data in healthy volunteers CNS target engagement and downstream pharmacological activity consistent with VGL101 Phase 1 data in healthy volunteers for 20 mg/kg Reduction in sTREM2 CSF levels and increase
in osteopontin CSF levels, indicating CNS target engagement Increases in sCSF1R CSF levels, a key biomarker of ALSP disease pathology Directionally supportive changes at 6 months for individual subjects on MRI and NfL biomarkers of disease
progression Quality and consistency of data on 6 subjects after 6 months support continuation of IGNITE and ILLUMINATE without modification IGNITE interim analysis on 6 patients at 6 months (20 mg/kg) further supports iluzanebart (VGL101)
safety and PK/PD profiles, and clinical strategy © Vigil Neuroscience, Inc. 2023. All rights reserved.
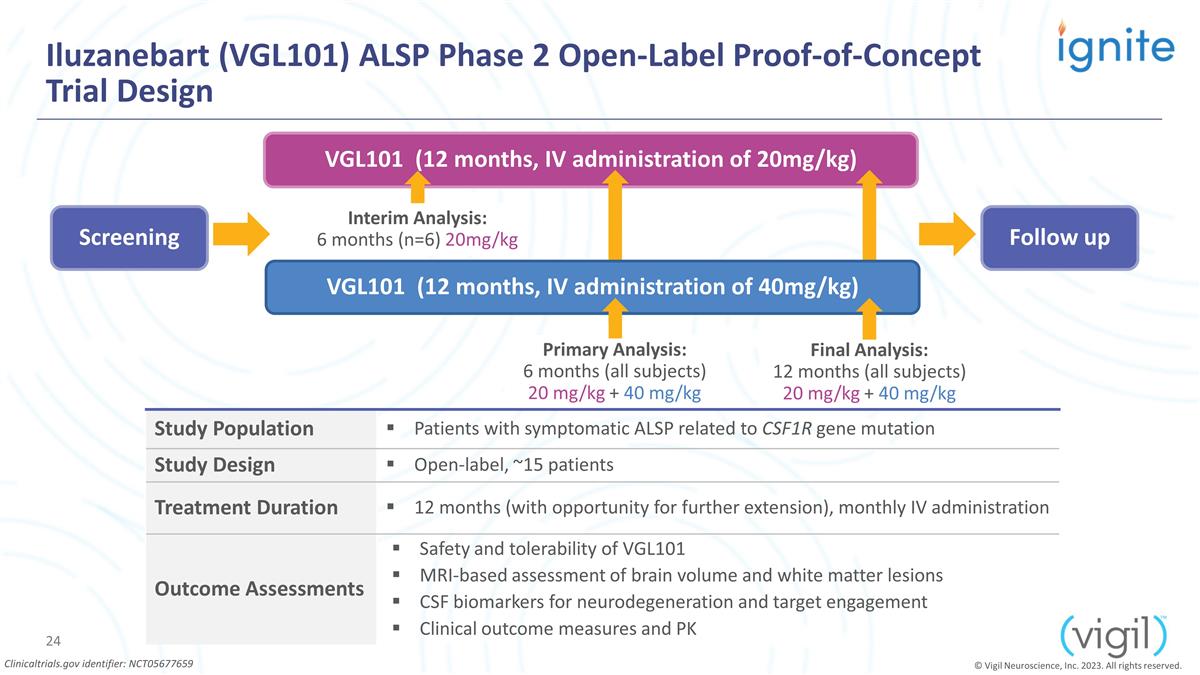
Iluzanebart (VGL101) ALSP Phase 2
Open-Label Proof-of-Concept Trial Design © Vigil Neuroscience, Inc. 2023. All rights reserved. VGL101 (12 months, IV administration of 20mg/kg) Screening Follow up Primary Analysis: 6 months (all subjects) 20 mg/kg + 40 mg/kg Final Analysis: 12
months (all subjects) 20 mg/kg + 40 mg/kg Interim Analysis: 6 months (n=6) 20mg/kg VGL101 (12 months, IV administration of 40mg/kg) Study Population Patients with symptomatic ALSP related to CSF1R gene mutation Study Design Open-label, ~15 patients
Treatment Duration 12 months (with opportunity for further extension), monthly IV administration Outcome Assessments Safety and tolerability of VGL101 MRI-based assessment of brain volume and white matter lesions CSF biomarkers for neurodegeneration
and target engagement Clinical outcome measures and PK Clinicaltrials.gov identifier: NCT05677659

Iluzanebart (VGL101) ALSP Phase 2
Patient Population Key Clinical Inclusion Criteria Key Clinical Exclusion Criteria Documentation of a CSF1R gene mutation Clinical symptoms consistent with ALSP MRI findings consistent with ALSP Mild and early-moderate stages defined by cognitive
and ambulation status Any neurological disease that poses a risk to the participant or produces symptoms like ALSP Patients unable to complete study procedures Comorbidities not permitting safe study participation © Vigil Neuroscience, Inc.
2022. All rights reserved.
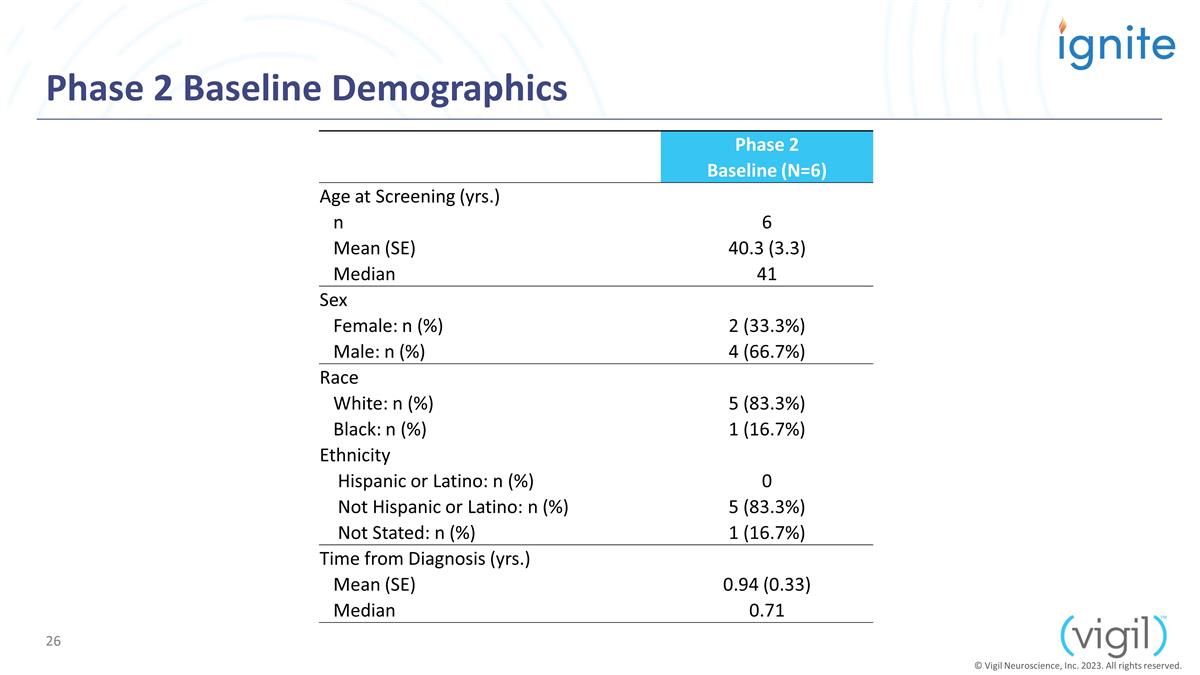
Phase 2 Baseline Demographics Phase
2 Baseline (N=6) Age at Screening (yrs.) n 6 Mean (SE) 40.3 (3.3) Median 41 Sex Female: n (%) 2 (33.3%) Male: n (%) 4 (66.7%) Race White: n (%) 5 (83.3%)
Black: n (%) 1 (16.7%) Ethnicity Hispanic or Latino: n (%) 0 Not Hispanic or Latino: n (%) 5 (83.3%) Not Stated: n (%) 1 (16.7%) Time from Diagnosis (yrs.) Mean (SE) 0.94 (0.33)
Median 0.71 © Vigil Neuroscience, Inc. 2023. All rights reserved.
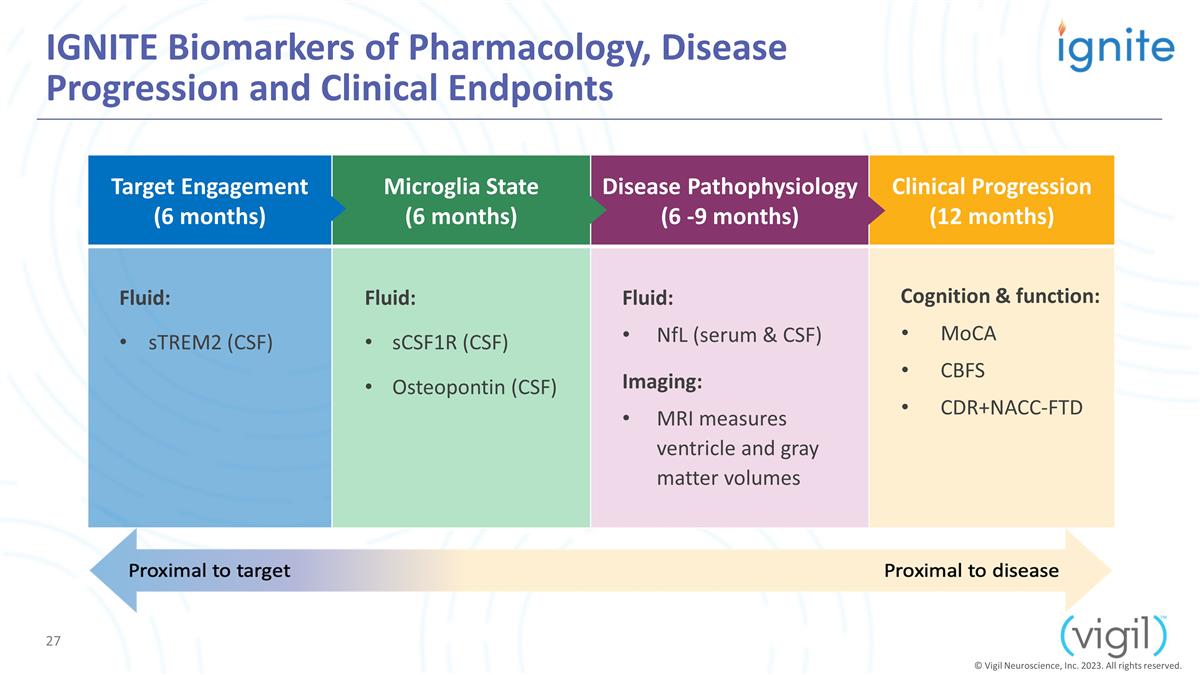
Target Engagement (6 months)
Microglia State (6 months) Disease Pathophysiology (6 -9 months) Clinical Progression (12 months) Fluid: sTREM2 (CSF) Fluid: sCSF1R (CSF) Osteopontin (CSF) Fluid: NfL (serum & CSF) Imaging: MRI measures ventricle and gray matter
volumes Cognition & function: MoCA CBFS CDR+NACC-FTD IGNITE Biomarkers of Pharmacology, Disease Progression and Clinical Endpoints © Vigil Neuroscience, Inc. 2023. All rights reserved.
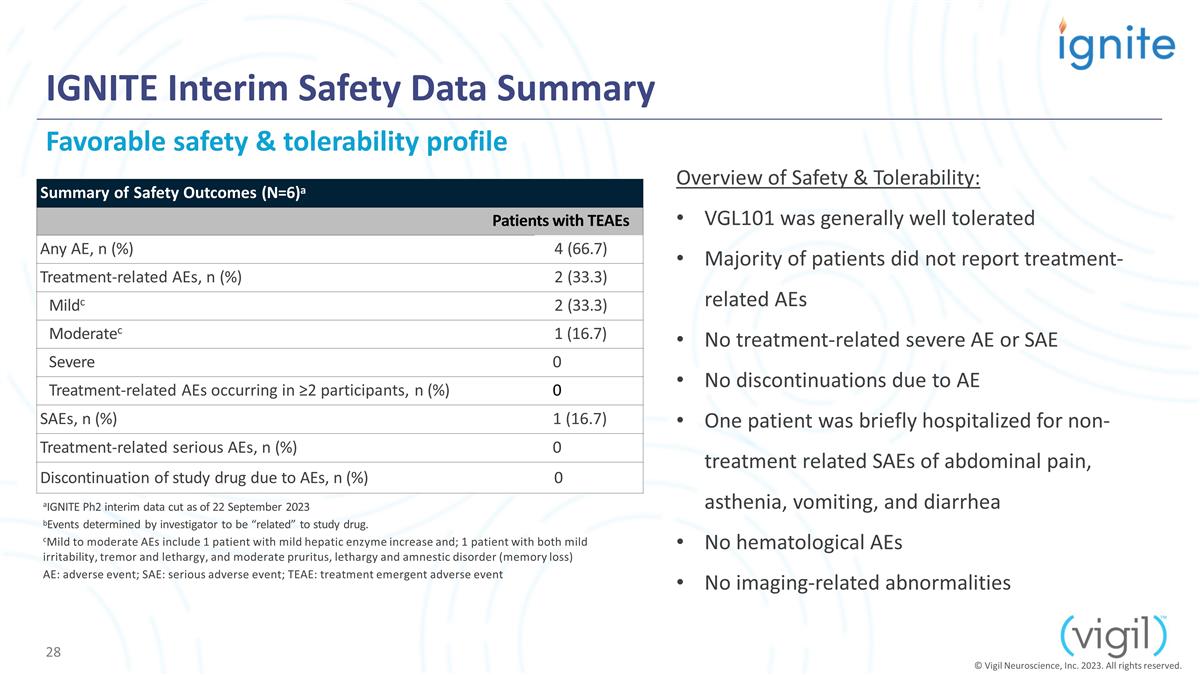
IGNITE Interim Safety Data Summary
Favorable safety & tolerability profile Summary of Safety Outcomes (N=6)a Patients with TEAEs Any AE, n (%) 4 (66.7) Treatment-related AEs, n (%) 2 (33.3) Mildc 2 (33.3) Moderatec 1 (16.7) Severe 0 Treatment-related AEs occurring in ≥2
participants, n (%) 0 SAEs, n (%) 1 (16.7) Treatment-related serious AEs, n (%) 0 Discontinuation of study drug due to AEs, n (%) 0 aIGNITE Ph2 interim data cut as of 22 September 2023 bEvents determined by investigator to be “related”
to study drug. cMild to moderate AEs include 1 patient with mild hepatic enzyme increase and; 1 patient with both mild irritability, tremor and lethargy, and moderate pruritus, lethargy and amnestic disorder (memory loss) AE: adverse event; SAE:
serious adverse event; TEAE: treatment emergent adverse event Overview of Safety & Tolerability: VGL101 was generally well tolerated Majority of patients did not report treatment-related AEs No treatment-related severe AE or SAE No
discontinuations due to AE One patient was briefly hospitalized for non-treatment related SAEs of abdominal pain, asthenia, vomiting, and diarrhea No hematological AEs No imaging-related abnormalities © Vigil Neuroscience, Inc. 2023. All rights
reserved.

Iluzanebart (VGL101) PK in IGNITE:
Predictable and Consistent with Phase 1 Simulated median (line) PK and 95 % prediction interval (shaded) based on HVs at 20 mg/kg monthly. Dots are mean (+/- SD) of observed PK in ALSP patients Brain penetration and achieving projected
CSF therapeutic exposures: ~0.5% CSF-to-serum ratio for ALSP patients (vs 0.1 – 0.2% for healthy subjects in Phase 1) © Vigil Neuroscience, Inc. 2023. All rights reserved. VGL101 concentration (µg/mL) Serum Time after dose
(week)
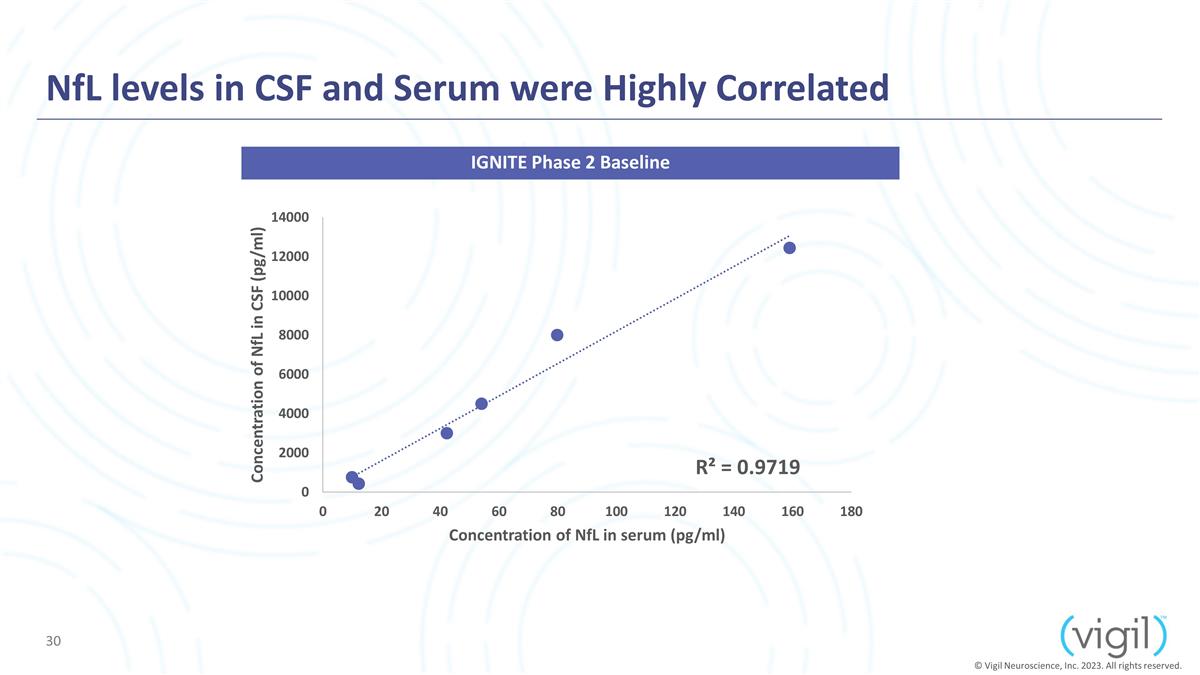
NfL levels in CSF and Serum were
Highly Correlated © Vigil Neuroscience, Inc. 2023. All rights reserved. IGNITE Phase 2 Baseline
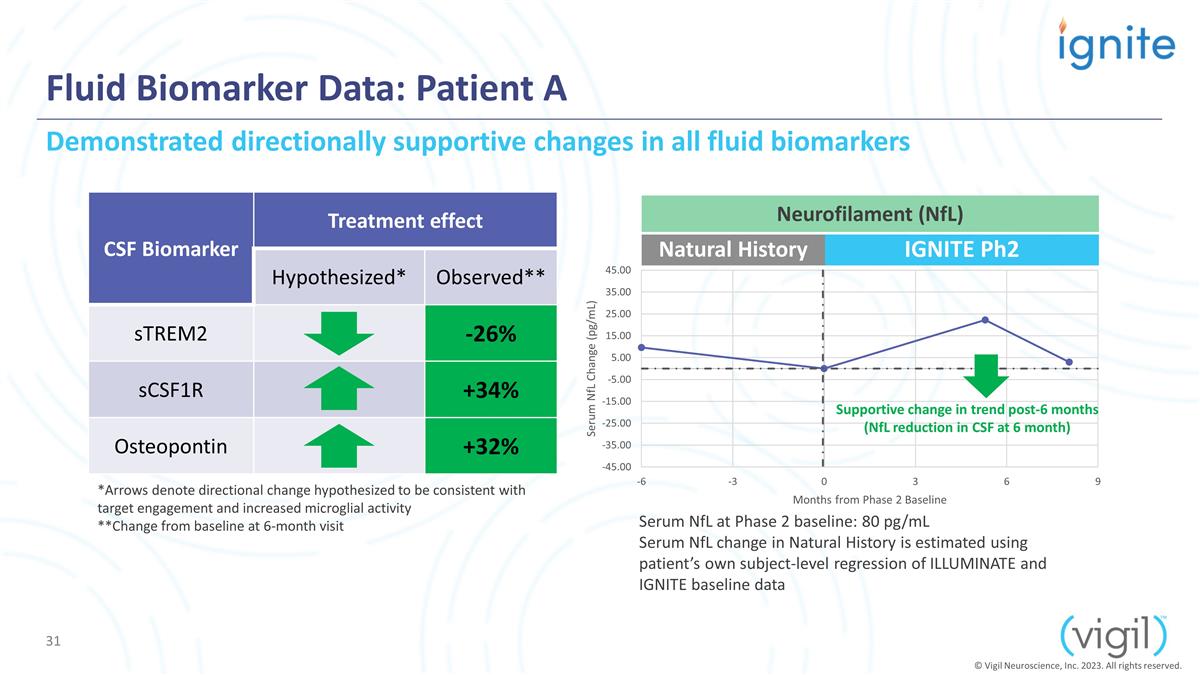
Fluid Biomarker Data: Patient A CSF
Biomarker Treatment effect Hypothesized* Observed** sTREM2 -26% sCSF1R +34% Osteopontin +32% Serum NfL at Phase 2 baseline: 80 pg/mL Serum NfL change in Natural History is estimated using patient’s own subject-level regression of ILLUMINATE
and IGNITE baseline data Natural History IGNITE Ph2 *Arrows denote directional change hypothesized to be consistent with target engagement and increased microglial activity **Change from baseline at 6-month visit Demonstrated directionally
supportive changes in all fluid biomarkers Supportive change in trend post-6 months (NfL reduction in CSF at 6 month) Neurofilament (NfL) © Vigil Neuroscience, Inc. 2023. All rights reserved.
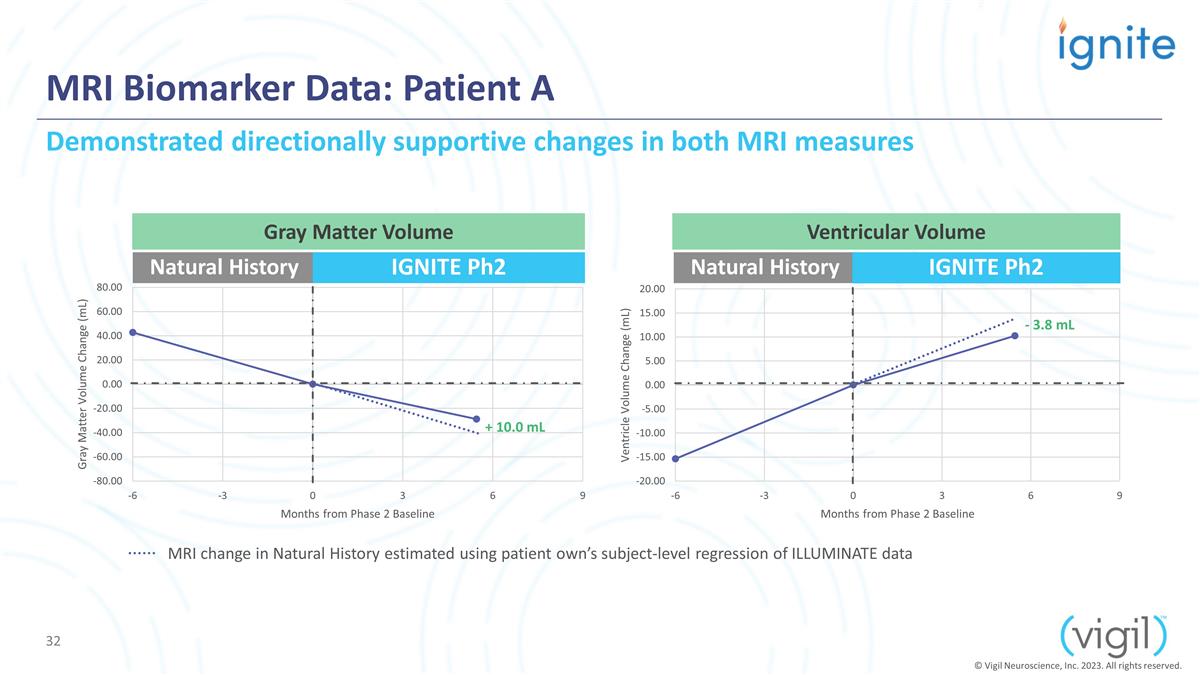
MRI Biomarker Data: Patient A Gray
Matter Volume Natural History IGNITE Ph2 Natural History IGNITE Ph2 + 10.0 mL - 3.8 mL Demonstrated directionally supportive changes in both MRI measures Ventricular Volume MRI change in Natural History estimated using patient own’s
subject-level regression of ILLUMINATE data © Vigil Neuroscience, Inc. 2023. All rights reserved.
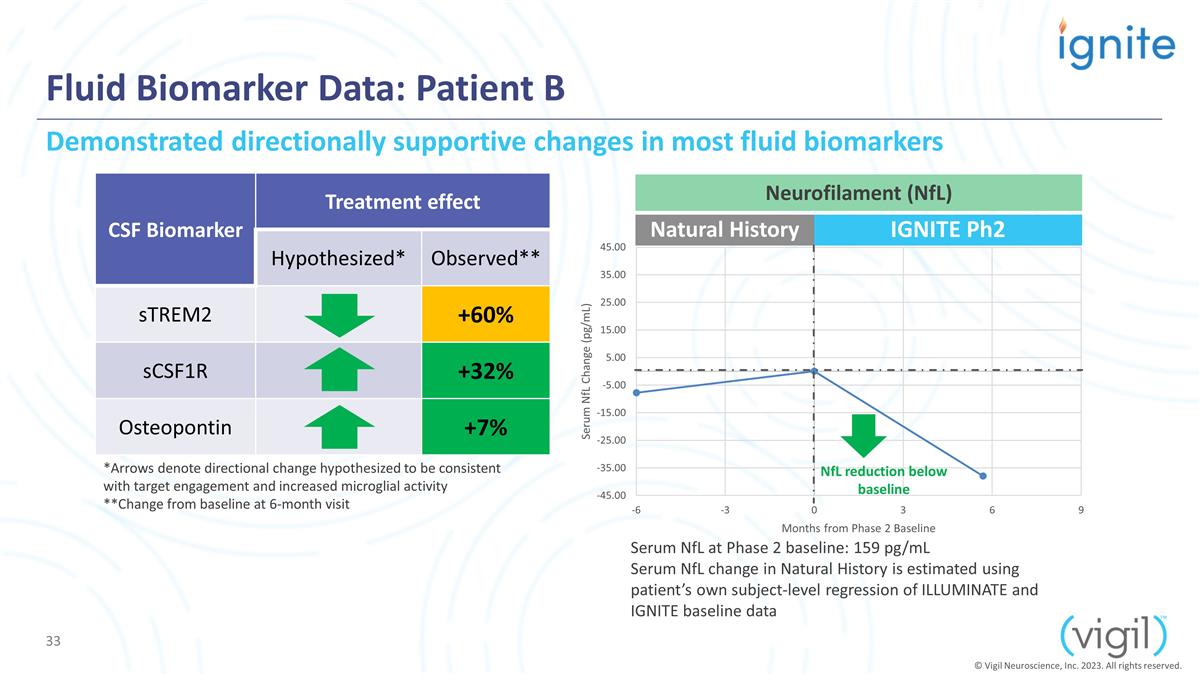
Fluid Biomarker Data: Patient B CSF
Biomarker Treatment effect Hypothesized* Observed** sTREM2 +60% sCSF1R +32% Osteopontin +7% Serum NfL at Phase 2 baseline: 159 pg/mL Serum NfL change in Natural History is estimated using patient’s own subject-level regression of ILLUMINATE
and IGNITE baseline data Natural History IGNITE Ph2 Demonstrated directionally supportive changes in most fluid biomarkers *Arrows denote directional change hypothesized to be consistent with target engagement and increased microglial activity
**Change from baseline at 6-month visit NfL reduction below baseline Neurofilament (NfL) © Vigil Neuroscience, Inc. 2023. All rights reserved.
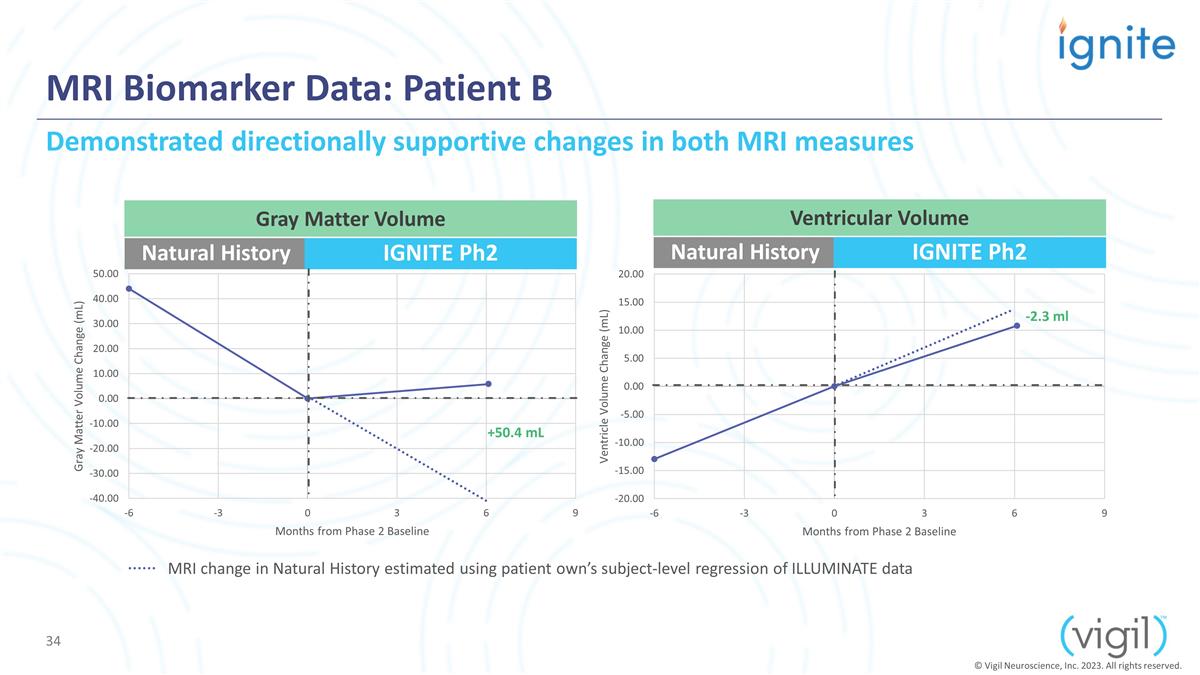
MRI Biomarker Data: Patient B
Natural History IGNITE Ph2 Natural History IGNITE Ph2 +50.4 mL Demonstrated directionally supportive changes in both MRI measures Gray Matter Volume Ventricular Volume -2.3 ml © Vigil Neuroscience, Inc. 2023. All rights reserved. MRI change in
Natural History estimated using patient own’s subject-level regression of ILLUMINATE data
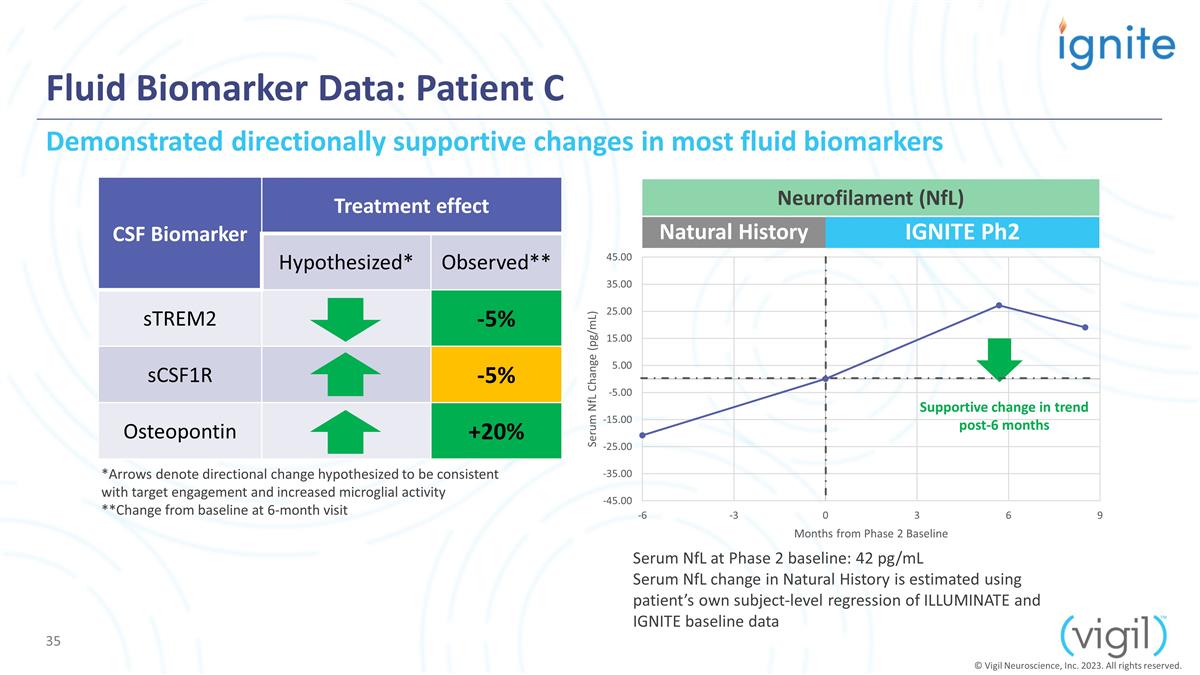
Fluid Biomarker Data: Patient C CSF
Biomarker Treatment effect Hypothesized* Observed** sTREM2 -5% sCSF1R -5% Osteopontin +20% Serum NfL at Phase 2 baseline: 42 pg/mL Serum NfL change in Natural History is estimated using patient’s own subject-level regression of ILLUMINATE and
IGNITE baseline data Natural History IGNITE Ph2 Demonstrated directionally supportive changes in most fluid biomarkers Neurofilament (NfL) Supportive change in trend post-6 months *Arrows denote directional change hypothesized to be consistent with
target engagement and increased microglial activity **Change from baseline at 6-month visit © Vigil Neuroscience, Inc. 2023. All rights reserved.
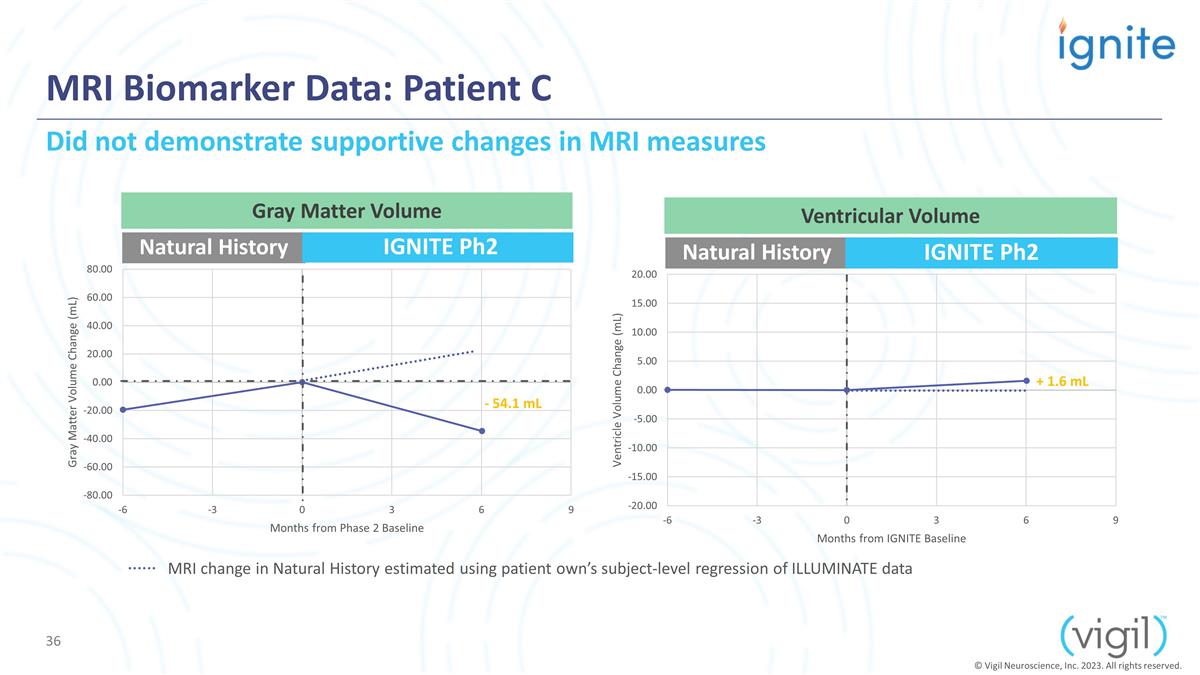
MRI Biomarker Data: Patient C
Natural History IGNITE Ph2 Natural History IGNITE Ph2 - 54.1 mL + 1.6 mL Gray Matter Volume Ventricular Volume Did not demonstrate supportive changes in MRI measures © Vigil Neuroscience, Inc. 2023. All rights reserved. MRI change in Natural
History estimated using patient own’s subject-level regression of ILLUMINATE data
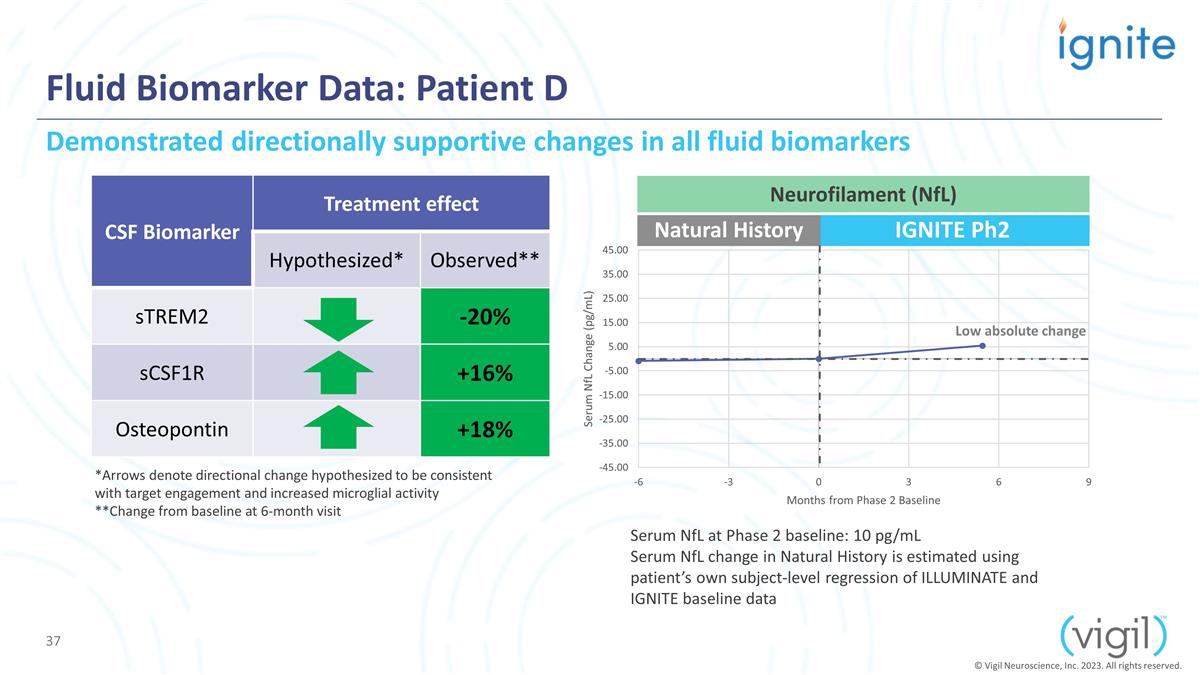
IGNITE Ph2 Fluid Biomarker Data:
Patient D CSF Biomarker Treatment effect Hypothesized* Observed** sTREM2 -20% sCSF1R +16% Osteopontin +18% Serum NfL at Phase 2 baseline: 10 pg/mL Serum NfL change in Natural History is estimated using patient’s own subject-level regression of
ILLUMINATE and IGNITE baseline data Natural History Demonstrated directionally supportive changes in all fluid biomarkers Low absolute change Neurofilament (NfL) *Arrows denote directional change hypothesized to be consistent with target engagement
and increased microglial activity **Change from baseline at 6-month visit © Vigil Neuroscience, Inc. 2023. All rights reserved.
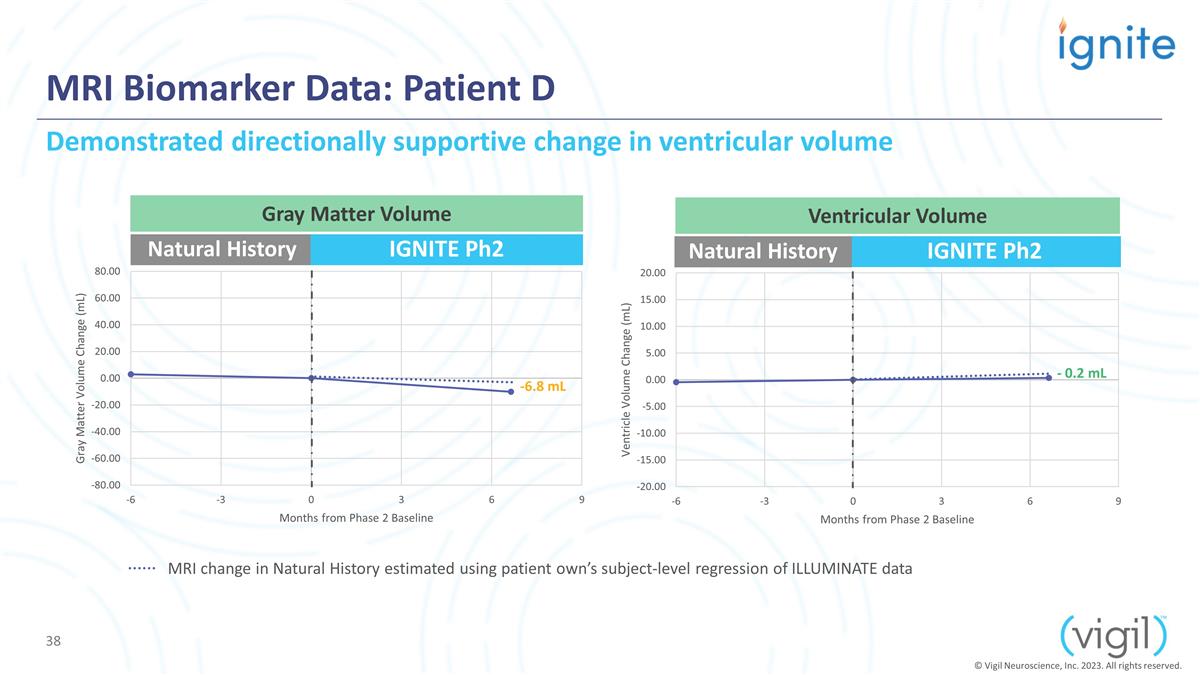
IGNITE Ph2 MRI Biomarker Data:
Patient D Natural History IGNITE Ph2 Natural History -6.8 mL - 0.2 mL Demonstrated directionally supportive change in ventricular volume Gray Matter Volume Ventricular Volume © Vigil Neuroscience, Inc. 2023. All rights reserved. MRI change in
Natural History estimated using patient own’s subject-level regression of ILLUMINATE data

Fluid Biomarker Data: Patient E CSF
Biomarker Treatment effect Hypothesized* Observed** sTREM2 -26% sCSF1R +0% Osteopontin +9% Serum NfL at Phase 2 baseline: 12 pg/mL Serum NfL change in Natural History is estimated using patient’s own subject-level regression of ILLUMINATE and
IGNITE baseline data IGNITE Ph2 Demonstrated directionally supportive changes in most fluid biomarkers *Arrows denote directional change hypothesized to be consistent with target engagement and increased microglial activity **Change from baseline at
6-month visit Neurofilament (NfL) Low absolute change © Vigil Neuroscience, Inc. 2023. All rights reserved. Natural History
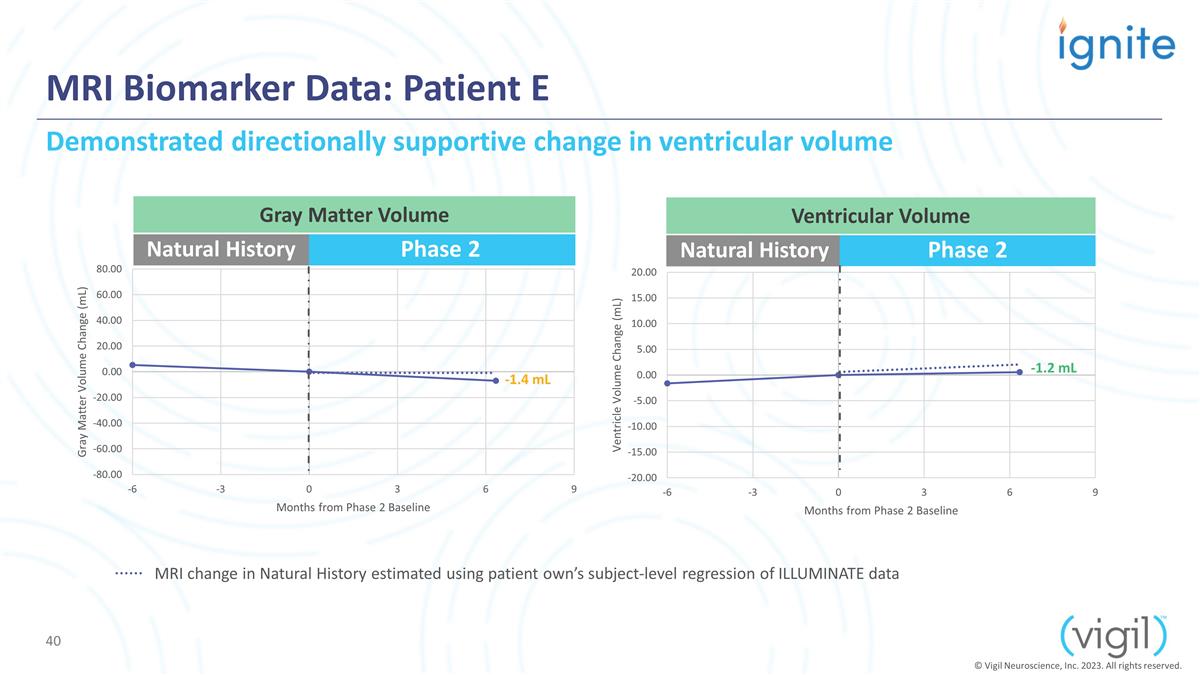
MRI Biomarker Data: Patient E Phase
2 Natural History Phase 2 -1.4 mL -1.2 mL Demonstrated directionally supportive change in ventricular volume Gray Matter Volume Ventricular Volume © Vigil Neuroscience, Inc. 2023. All rights reserved. Natural History MRI change in Natural
History estimated using patient own’s subject-level regression of ILLUMINATE data

Fluid Biomarker Data: Patient F CSF
Biomarker Treatment effect Hypothesized* Observed** sTREM2 +14% sCSF1R +24% Osteopontin +118% Serum NfL at Phase 2 baseline: 54 pg/mL Serum NfL change in Natural History is estimated using patient’s own subject-level regression of ILLUMINATE
and IGNITE baseline data IGNITE Ph2 Natural History Demonstrated directionally supportive changes in most fluid biomarkers Low absolute change (NfL reduction in CSF) Neurofilament (NfL) *Arrows denote directional change hypothesized to be consistent
with target engagement and increased microglial activity **Change from baseline at 6-month visit © Vigil Neuroscience, Inc. 2023. All rights reserved.
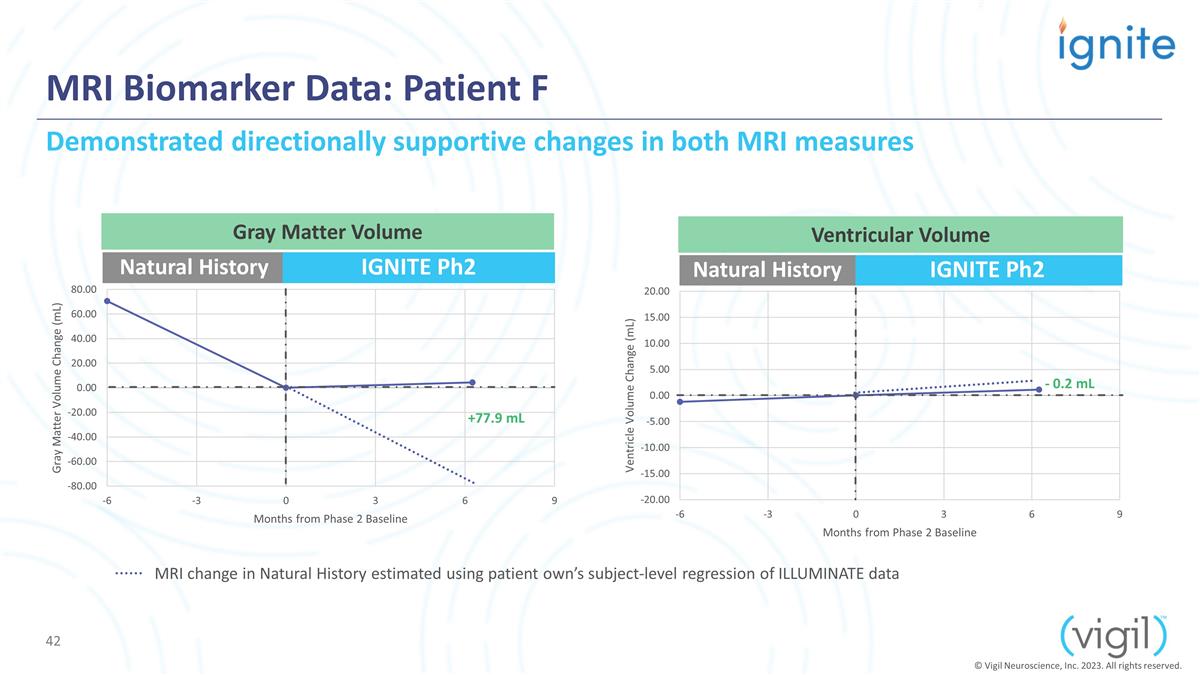
MRI Biomarker Data: Patient F
Natural History IGNITE Ph2 IGNITE Ph2 +77.9 mL Demonstrated directionally supportive changes in both MRI measures Gray Matter Volume Ventricular Volume - 0.2 mL © Vigil Neuroscience, Inc. 2023. All rights reserved. Natural History MRI change in
Natural History estimated using patient own’s subject-level regression of ILLUMINATE data

MRI Changes on Gray Matter and
Ventricular Volumes Directional change indicating reduced progression rate in ventricular expansion and gray matter atrophy in certain patients post-iluzanebart (VGL101) treatment vs. pre-treatment Region Rate of Progression Ventricles 5 out
of 6 patients had directional change supporting reduced rate of ventricular expansion1 Gray Matter Volume 3 out of 6 patients had directional change supporting reduced rate of atrophy2 © Vigil Neuroscience, Inc. 2023. All rights reserved. 1.
Patients A, B, D, E and F; 2. Patients A, B and F
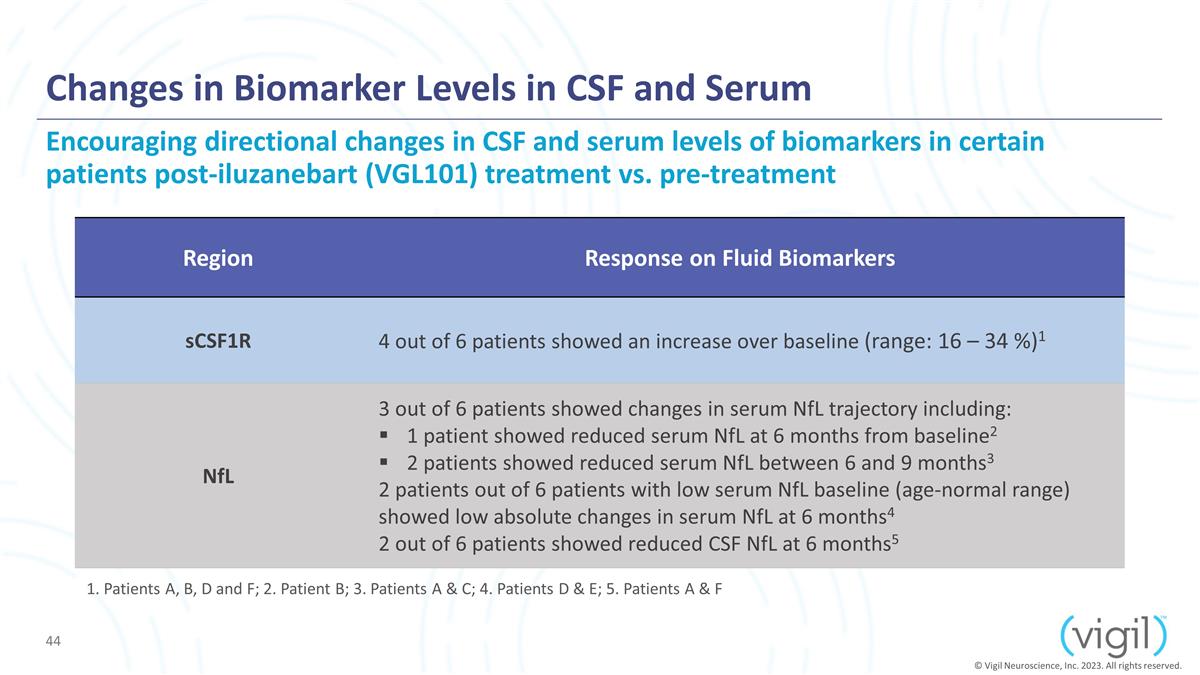
Changes in Biomarker Levels in CSF
and Serum Encouraging directional changes in CSF and serum levels of biomarkers in certain patients post-iluzanebart (VGL101) treatment vs. pre-treatment Region Response on Fluid Biomarkers sCSF1R 4 out of 6 patients showed an increase over
baseline (range: 16 – 34 %)1 NfL 3 out of 6 patients showed changes in serum NfL trajectory including: 1 patient showed reduced serum NfL at 6 months from baseline2 2 patients showed reduced serum NfL between 6 and 9 months3 2
patients out of 6 patients with low serum NfL baseline (age-normal range) showed low absolute changes in serum NfL at 6 months4 2 out of 6 patients showed reduced CSF NfL at 6 months5 © Vigil Neuroscience, Inc. 2023. All rights reserved. 1.
Patients A, B, D and F; 2. Patient B; 3. Patients A & C; 4. Patients D & E; 5. Patients A & F
Overall Summary ILLUMINATE NHS
continues to provide important insights and a rich dataset on biomarkers and clinical measures of disease progression in ALSP Totality of data, including longitudinal progression observed on selected MRI measures and
clinical endpoints, support engagement with regulatory authorities IGNITE Phase 2 interim analysis provides further support on safety and PK/PD profiles, and clinical strategy for VGL101 in ALSP Favorable safety and tolerability data CNS target
engagement and downstream pharmacological activity consistent with VGL101 Phase 1 data for 20 mg/kg; increases in sCSF1R CSF levels, a key biomarker of ALSP disease pathology Directionally supportive changes at 6 months for individual subjects
on MRI and NfL biomarkers of disease progression Quality and consistency of interim data support continuation of IGNITE and ILLUMINATE without modification Vigil plans to engage with FDA to initiate discussions regarding potential
accelerated development pathway for VGL101 Data on all patients in 20 mg/kg and 40 mg/kg cohorts at 6 months expected in Q3 2024 IGNITE & ILLUMINATE interim analyses provide further support for continued development of iluzanebart (VGL101)
as potential ALSP therapy © Vigil Neuroscience, Inc. 2023. All rights reserved.

ALSP Background & Perspectives
on ILLUMINATE & IGNITE Interim Data David S. Lynch, MD, PhD Consultant Neurologist National Hospital for Neurology & Neurosurgery, Queen Square & UCL Institute of Neurology, London Clinical Lead Adult Inherited White Matter Disorders
Highly Specialist Service, NHS England
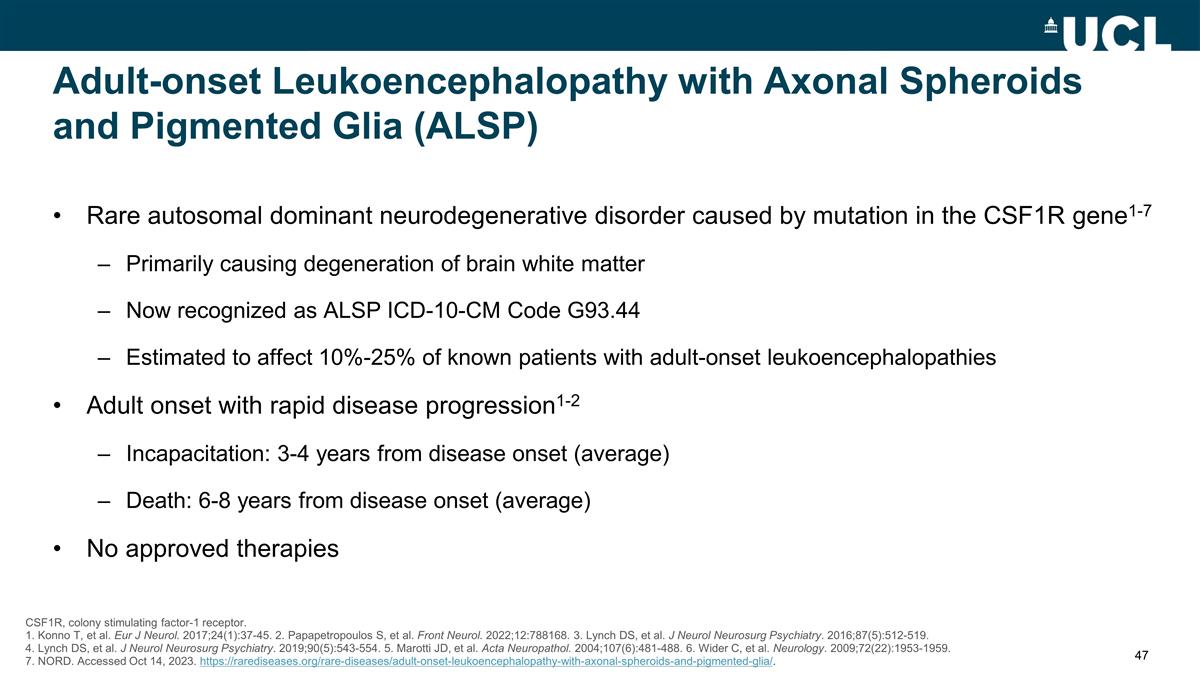
Adult-onset Leukoencephalopathy
with Axonal Spheroids and Pigmented Glia (ALSP) Rare autosomal dominant neurodegenerative disorder caused by mutation in the CSF1R gene1-7 Primarily causing degeneration of brain white matter Now recognized as ALSP ICD-10-CM Code G93.44 Estimated to
affect 10%-25% of known patients with adult-onset leukoencephalopathies Adult onset with rapid disease progression1-2 Incapacitation: 3-4 years from disease onset (average) Death: 6-8 years from disease onset (average) No approved therapies CSF1R,
colony stimulating factor-1 receptor. 1. Konno T, et al. Eur J Neurol. 2017;24(1):37-45. 2. Papapetropoulos S, et al. Front Neurol. 2022;12:788168. 3. Lynch DS, et al. J Neurol Neurosurg Psychiatry. 2016;87(5):512-519. 4. Lynch DS, et al. J Neurol
Neurosurg Psychiatry. 2019;90(5):543-554. 5. Marotti JD, et al. Acta Neuropathol. 2004;107(6):481-488. 6. Wider C, et al. Neurology. 2009;72(22):1953-1959. 7. NORD. Accessed Oct 14, 2023.
https://rarediseases.org/rare-diseases/adult-onset-leukoencephalopathy-with-axonal-spheroids-and-pigmented-glia/.
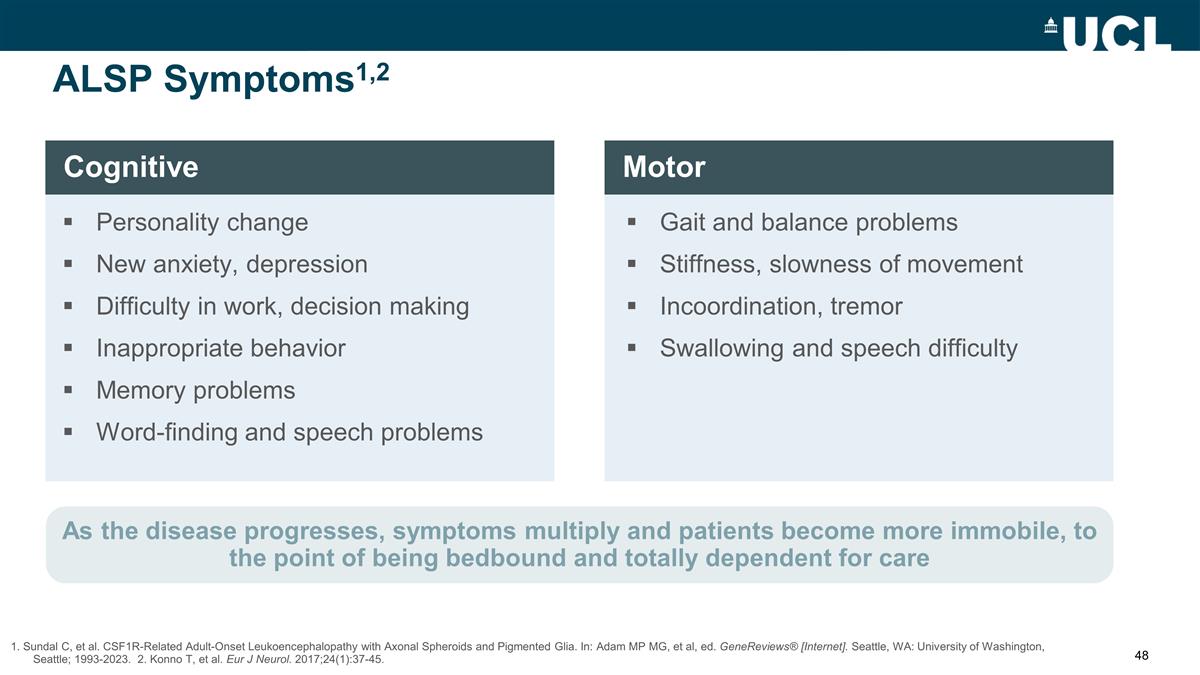
ALSP Symptoms1,2 1. Sundal C, et
al. CSF1R-Related Adult-Onset Leukoencephalopathy with Axonal Spheroids and Pigmented Glia. In: Adam MP MG, et al, ed. GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2023. 2. Konno T, et al. Eur J Neurol.
2017;24(1):37-45. Personality change New anxiety, depression Difficulty in work, decision making Inappropriate behavior Memory problems Word-finding and speech problems Cognitive As the disease progresses, symptoms multiply and patients become more
immobile, to the point of being bedbound and totally dependent for care Gait and balance problems Stiffness, slowness of movement Incoordination, tremor Swallowing and speech difficulty Motor
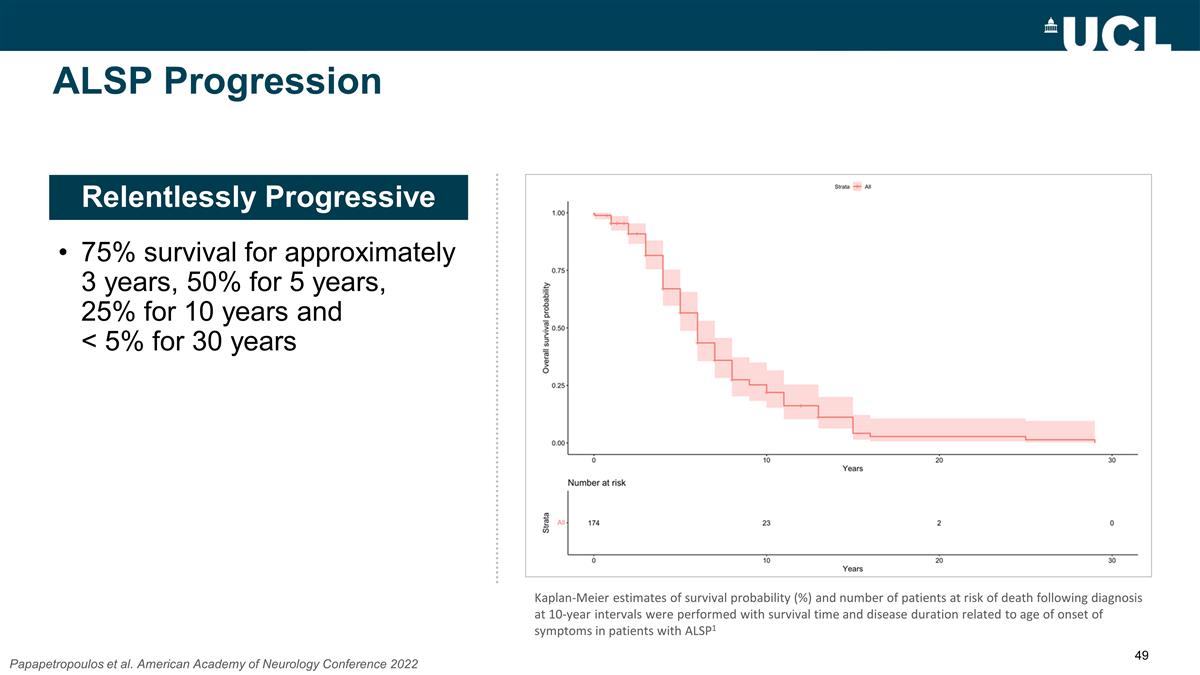
Relentlessly Progressive ALSP
Progression 75% survival for approximately 3 years, 50% for 5 years, 25% for 10 years and < 5% for 30 years Papapetropoulos et al. American Academy of Neurology Conference 2022 Kaplan-Meier estimates of survival probability (%) and number of
patients at risk of death following diagnosis at 10-year intervals were performed with survival time and disease duration related to age of onset of symptoms in patients with ALSP1
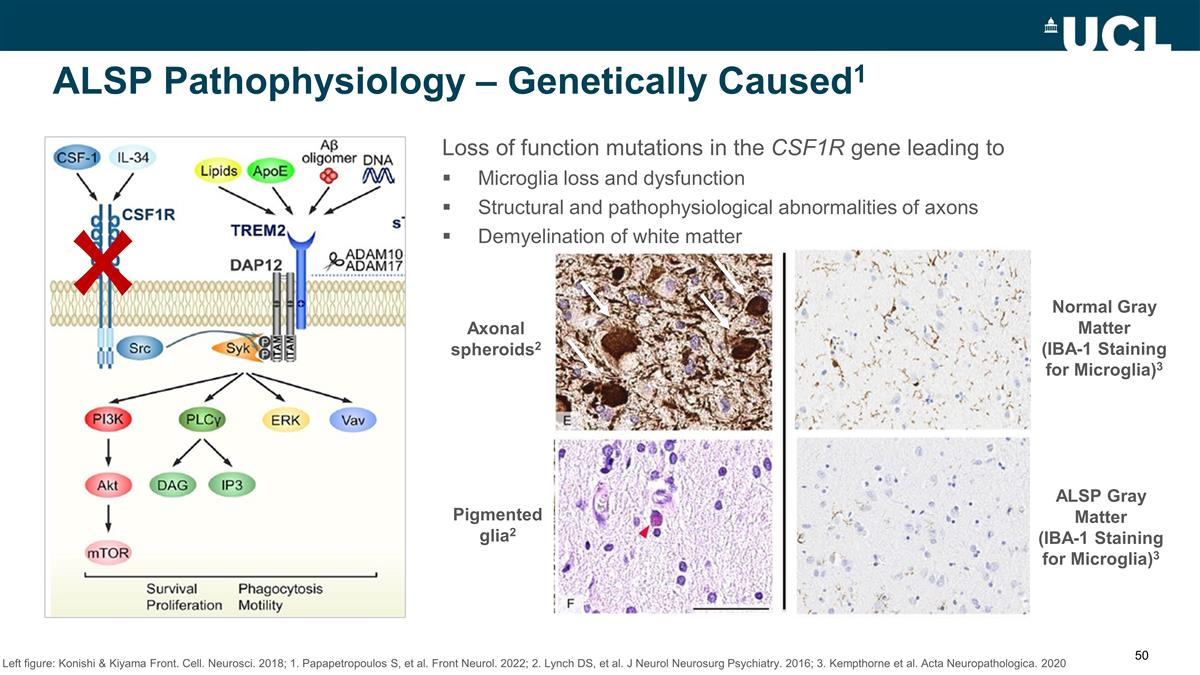
ALSP Pathophysiology –
Genetically Caused1 Loss of function mutations in the CSF1R gene leading to Microglia loss and dysfunction Structural and pathophysiological abnormalities of axons Demyelination of white matter Axonal spheroids2 Pigmented glia2 ALSP Gray Matter
(IBA-1 Staining for Microglia)3 Normal Gray Matter (IBA-1 Staining for Microglia)3 Left figure: Konishi & Kiyama Front. Cell. Neurosci. 2018; 1. Papapetropoulos S, et al. Front Neurol. 2022; 2. Lynch DS, et al. J Neurol Neurosurg Psychiatry.
2016; 3. Kempthorne et al. Acta Neuropathologica. 2020
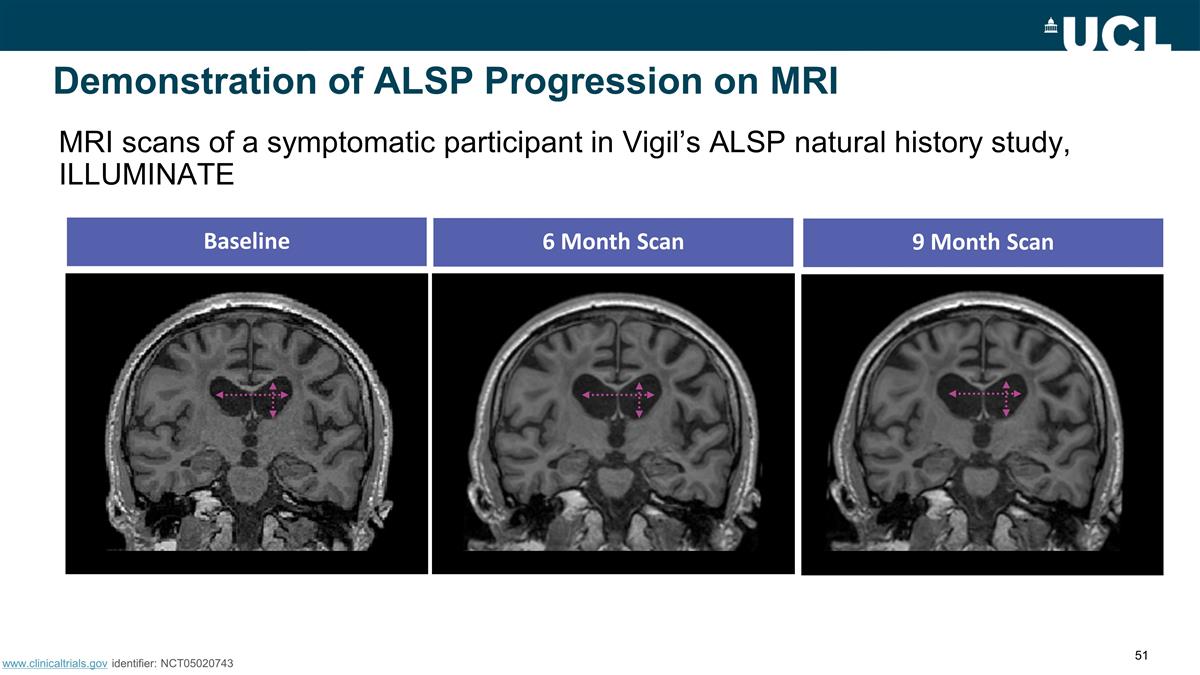
Demonstration of ALSP Progression
on MRI Baseline 6 Month Scan 9 Month Scan F MRI scans of a symptomatic participant in Vigil’s ALSP natural history study, ILLUMINATE www.clinicaltrials.gov identifier: NCT05020743
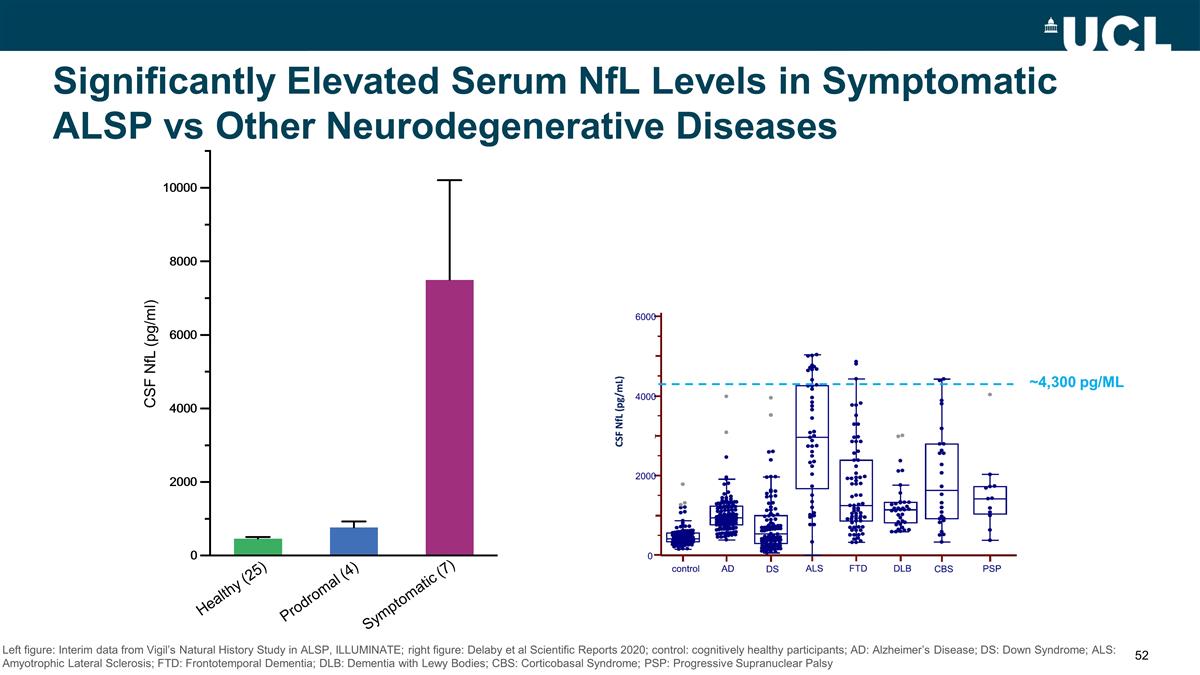
Significantly Elevated Serum NfL
Levels in Symptomatic ALSP vs Other Neurodegenerative Diseases Healthy (25) Prodromal (4) Symptomatic (7) CSF NfL (pg/ml) Left figure: Interim data from Vigil’s Natural History Study in ALSP, ILLUMINATE; right figure: Delaby et al Scientific
Reports 2020; control: cognitively healthy participants; AD: Alzheimer’s Disease; DS: Down Syndrome; ALS: Amyotrophic Lateral Sclerosis; FTD: Frontotemporal Dementia; DLB: Dementia with Lewy Bodies; CBS: Corticobasal Syndrome; PSP: Progressive
Supranuclear Palsy ~4,300 pg/ML
Vigil’s ILLUMINATE &
IGNITE Interim Data – Key Takeaways ILLUMINATE & IGNITE represent 1st ever trials of their kind in ALSP Increasing overall understanding of disease pathophysiology, and biomarkers for disease progression and treatment response Positive
interim data on VGL101’s potential as treatment for ALSP, a devastating disease with no approved therapy Favorable safety and tolerability with an accessible once monthly IV administration Observed differential sCSF1R response in ALSP patients
(vs HVs), indicating rescue of microglial activity in ALSP setting Very encouraging changes in MRI and NfL at 6 months compare favorably vs HSCT Indicating impact on disease progression 5 out of 6 patients showed reduction in rate of ventricular
expansion Slowing or reduction in ventricular expansion are clinically relevant Even when it works, HSCT needs at least 1 year for observable MRI changes with significant safety/tolerability risks 4 out of 6 patients showed reduction in NfL
trajectory in serum or CSF, indicating an impact on neuronal degeneration 1st targeted therapeutic candidate with clinical data in ALSP patients 1st data on TREM2 agonism as a therapeutic approach in a neurodegenerative disease setting Excited to
continue to participate in IGNITE to generate more data and further evaluate this candidate

Closing Remarks Ivana
Magovčević-Liebisch, PhD, JD Chief Executive Officer Vigil Neuroscience, Inc. © Vigil Neuroscience, Inc. 2023. All rights reserved.

Iluzanebart (VGL101): The Only
Targeted Therapeutic in Development for ALSP © Vigil Neuroscience, Inc. 2023. All rights reserved. First company to show clinical data on TREM2 agonism as a potential therapeutic approach in patients with a neurodegenerative disease
VGL101 demonstrated favorable safety and tolerability, including no hematologic adverse events Clear CNS target engagement and downstream pharmacological activity at 20 mg/kg consistent with Phase 1 data Directionally supportive changes in
individual patients at 6 months on MRI and NfL biomarkers Natural History Study continued to provide critical insights on MRI and NfL biomarkers; sCSF1R emerging as key biomarker of ALSP disease pathology Phase 2 IGNITE results from all
patients in 20 mg/kg and 40 mg/kg cohorts at 6 months expected in Q3 2024

Q&A VIGILANT FOR YOU is a
registered trademark of Vigil Neuroscience, Inc. VIGIL NEUROSCIENCE and the Vigil Neuro logo is a trademark of Vigil Neuroscience, Inc.
v3.23.3
Document and Entity Information
|
Nov. 16, 2023 |
| Cover [Abstract] |
|
| Amendment Flag |
false
|
| Entity Central Index Key |
0001827087
|
| Document Type |
8-K
|
| Document Period End Date |
Nov. 16, 2023
|
| Entity Registrant Name |
VIGIL NEUROSCIENCE, INC.
|
| Entity Incorporation State Country Code |
DE
|
| Entity File Number |
001-41200
|
| Entity Tax Identification Number |
85-1880494
|
| Entity Address, Address Line One |
Vigil Neuroscience, Inc.
|
| Entity Address, Address Line Two |
100 Forge Road
|
| Entity Address, Address Line Three |
Suite 700
|
| Entity Address, City or Town |
Watertown
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02472
|
| City Area Code |
(857)
|
| Local Phone Number |
254-4445
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre Commencement Tender Offer |
false
|
| Pre Commencement Issuer Tender Offer |
false
|
| Security 12b Title |
Common Stock, $0.0001 par value per share
|
| Trading Symbol |
VIGL
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Entity Ex Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 3 such as an Office Park
| Name: |
dei_EntityAddressAddressLine3 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Vigil Neuroscience (NASDAQ:VIGL)
Historical Stock Chart
From Oct 2024 to Nov 2024

Vigil Neuroscience (NASDAQ:VIGL)
Historical Stock Chart
From Nov 2023 to Nov 2024
