0001566044
false
0001566044
2023-10-27
2023-10-27
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported)
October 27, 2023
VYNE Therapeutics Inc.
(Exact name of registrant as specified
in its charter)
| Delaware |
|
001-38356 |
|
45-3757789 |
|
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification Number) |
685
Route 202/206 N., Suite 301
Bridgewater, New Jersey 08807
(Address of principal executive offices,
including Zip Code)
(800) 775-7936
(Registrant’s telephone number, including
area code)
Check the appropriate box below if the Form 8-K filing
is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b)
of the Act:
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common Stock, $0.0001 par value |
|
VYNE |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of
the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 13(a) of the Exchange Act.
Item 1.01 Entry into a Material Definitive Agreement.
Securities Purchase Agreement
On October 27, 2023, VYNE Therapeutics Inc. (the “Company”)
entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with certain institutional and other accredited
investors (the “Purchasers”), pursuant to which the Company agreed to sell and issue to the Purchasers in a private placement
transaction (the “Private Placement”) (i) 10,652,543 shares (the “Shares”) of the Company’s common
stock, par value $0.0001 per share (“Common Stock”), and (ii) with respect to certain Purchasers, pre-funded warrants
to purchase 28,614,437 shares of Common Stock (the “Pre-Funded Warrants”) in lieu of Shares. The purchase price per share
of Common Stock is $2.245 per share (the “Purchase Price”) and the purchase price for the Pre-Funded Warrants is the Purchase
Price minus $0.0001 per Pre-Funded Warrant. The Company anticipates receiving gross proceeds of $88.2 million from the Private
Placement, before deducting fees to the placement agent and offering expenses payable by the Company. The Private Placement is expected
to occur on or about November 1, 2023, subject to the satisfaction of customary closing conditions.
The Pre-Funded Warrants have a per share exercise price of $0.0001,
subject to proportional adjustments in the event of stock splits or combinations or similar events. The Pre-Funded Warrants will not expire
until exercised in full. The Pre-Funded Warrants may not be exercised if the aggregate number of shares of Common Stock beneficially owned
by the holder thereof immediately following such exercise would exceed a specified beneficial ownership limitation; provided, however,
that a holder may increase or decrease the beneficial ownership limitation by giving 60 days’ notice to the Company, but not to
exceed any percentage in excess of 19.99%.
The Securities Purchase Agreement contains customary representations,
warranties and covenants that were made solely for the benefit of the parties to the Securities Purchase Agreement. Such representations,
warranties and covenants (i) are intended as a way of allocating risk between the parties to the Securities Purchase Agreement and
not as statements of fact, and (ii) may apply standards of materiality in a way that is different from what may be viewed as material
by stockholders of, or other investors in, the Company. Accordingly, the Securities Purchase Agreement is included with this filing only
to provide investors with information regarding the terms of the transaction and not to provide investors with any other factual information
regarding the Company.
The foregoing description of the Securities Purchase Agreement
and Pre-Funded Warrants issued under the Securities Purchase Agreement does not purport to be complete and is qualified in its
entirety by references to the full text of (i) the form of Securities Purchase Agreement, which is filed as Exhibit 10.1
to this Current Report on Form 8-K and is incorporated by reference herein and (ii) the form of Pre-Funded Warrant issued
under the Securities Purchase Agreement, a copy of which is attached to this report as Exhibit 4.1 and is incorporated by
reference herein.
Registration Rights Agreement
In connection with the Private Placement, the Company also entered
into a Registration Rights Agreement, dated October 27, 2023 (the “Registration Rights Agreement”), with the Purchasers
requiring the Company to register the resale of the Shares and the shares underlying the Pre-Funded Warrants. The Company is required
to prepare and file an initial registration statement with the Securities and Exchange Commission (the “SEC”) as soon as reasonably
practicable, but in no event later than 30 calendar days following the date of the Securities Purchase Agreement (the “Filing Deadline”),
and to use best efforts to have the registration statement declared effective within 60 calendar days of the Filing Deadline, subject
to extension under the terms of the Registration Rights Agreement.
The foregoing description of the Registration Rights Agreement
does not purport to be complete and is qualified in its entirety by reference to the full text of the form of such agreement, which
is filed as Exhibit 10.2 to this Current Report on Form 8-K and is incorporated by reference herein.
On October 30, 2023, the Company issued a press release announcing
the Private Placement. A copy of the press release is attached as Exhibit 99.1 hereto and is incorporated by reference herein.
Item 1.02 Termination of a Material Definitive Agreement.
As previously disclosed, on March 15, 2022, the Company entered
into a purchase agreement (the “Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“Lincoln Park”), which
provided that, upon the terms and subject to the conditions and limitations set forth therein, the Company could sell to Lincoln Park
up to $30,000,000 of shares of its Common Stock from time to time over the 36-month term of the Purchase Agreement. On October 30,
2023, the Company delivered a notice of termination to Lincoln Park terminating the Purchase Agreement effective as of one business day
following delivery of notice. Prior to termination, the Company had not sold any shares of its Common Stock under the Purchase Agreement.
A copy of the Purchase Agreement was filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the
SEC on March 15, 2022. The description of the Purchase Agreement contained in this Current Report on Form 8-K does not purport
to be complete and is qualified in its entirety by reference to the copy of the Purchase Agreement filed as Exhibit 10.1 to the Form 8-K.
Item 2.02 Results of Operations and Financial Condition.
Based upon preliminary estimates and information available to the Company
as of the date of this Current Report, the Company expects to report that it had approximately $15.5 million in cash and cash equivalents
and restricted cash as of September 30, 2023. Therefore, when adding the expected gross proceeds of the Private Placement, the Company’s
cash and cash equivalents and restricted cash as of September 30, 2023 would have been approximately $103.7 million. The Company’s
condensed consolidated financial statements as of and for the three and nine months ended September 30, 2023 are not yet available
and the Company has not yet completed its quarterly financial closing processes for the period ended September 30, 2023. As such,
the foregoing information reflects the Company’s estimates with respect to cash and cash equivalents and restricted cash and is
based on currently available information, which is preliminary, unaudited and subject to change. The Company’s actual results may
differ from these estimates after the completion of financial closing procedures, final adjustments and review by the Company’s
staff and management. In addition, the Company’s actual results for the three and nine months ended September 30, 2023 may
differ from the Company’s preliminary estimates and are not indicative of the results to be expected for any future period. These
estimates should not be viewed as a substitute for full financial statements prepared in accordance with generally accepted accounting
principles in the U.S. and these estimates are not necessarily indicative of the results to be achieved for the stated period, or any
other period. The Company does not undertake any obligation to publicly update or revise these preliminary estimates, except as required
by law. Accordingly, undue reliance should not be placed on these preliminary financial results. See “Risk Factors,” “Special
Note Regarding Forward-Looking Statements” and “Management’s Discussion and Analysis of Financial Condition and Results
of Operations” in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and Quarterly
Report on Form 10-Q for the quarter ended June 30, 2023 for a discussion of certain factors that could result in differences
between these preliminary unaudited estimates and the actual results.
These preliminary unaudited estimates of the Company’s cash and
cash equivalents and restricted cash have been prepared by and are the responsibility of management. The Company’s independent registered
public accounting firm has not conducted a review of and does not express an opinion or any other form of assurance with respect to these
preliminary estimates. This information should be read in conjunction with the Company’s consolidated and combined financial statements
and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in
the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and Quarterly Report on Form 10-Q
for the quarter ended June 30, 2023.
Item 3.02 Unregistered Sales of Equity Securities.
The disclosure regarding the securities to be sold and issued under
the Securities Purchase Agreement as set forth under Item 1.01 of this report is incorporated by reference under this Item 3.02.
The securities described above under Item 1.01 have not been registered
under the Securities Act of 1933, as amended (the “Securities Act”). Based in part upon the representations of the Purchasers
in the Securities Purchase Agreement, the Company relied on the exemption afforded by Regulation D under the Securities Act, and corresponding
provisions of state securities or “blue sky” laws. Each of the Purchasers has represented that it is an “accredited
investor” as defined in Regulation D of the Securities Act and that it is acquiring the securities for investment only and not with
a view towards, or for resale in connection with, the public sale or distribution thereof, and appropriate legends will be affixed to
the securities. The sale of the securities did not involve a public offering and was made without general solicitation or general advertising.
Neither this Current Report on Form 8-K nor any exhibit attached
hereto is an offer to sell or the solicitation of an offer to buy any securities of the Company.
Item 5.08 Shareholder Director Nominations.
On October 27, 2023, the board of directors of the Company (the
“Board”) determined that the date of its 2023 Annual Meeting of Stockholders (the “2023 Annual Meeting”) will
be held at its corporate headquarters in Bridgewater, New Jersey on December 13, 2023. Additional details about the 2023 Annual Meeting
will be set forth in the Company’s Definitive Proxy Statement on Schedule 14A, which will be filed with the SEC prior to the 2023
Annual Meeting.
The record date for the determination of stockholders entitled to receive
notice of and to vote at the 2023 Annual Meeting will be November 3, 2023. Due to the fact that the meeting date for the 2023 Annual
Meeting is delayed more than 60 days following the anniversary of the Company’s stockholder meeting in 2022, the due dates for the
submission of any qualified stockholder proposal or qualified stockholder nominations under applicable SEC rules and the Company’s
Amended and Restated Bylaws (the “Bylaws”) listed in the Company’s 2022 Definitive Proxy Statement on Schedule 14A,
filed with the SEC on June 17, 2022, are no longer applicable. Such nominations or proposals, including any notice on Schedule 14N,
are now due to the Company no later than 10 calendar days following the date of this 8-K and must comply with all of the applicable requirements
set forth in the rules and regulations of the SEC, under the Securities Exchange Act of 1934,
as amended (the “Exchange Act”), and the Bylaws.
Item 7.01 Regulation FD Disclosure.
On October 30, 2023, the Company issued press releases entitled
(i) “VYNE Therapeutics Announces Positive Data from Phase 1b Trial for Novel BET Inhibitor VYN201 in Patients with Nonsegmental
Vitiligo” and (ii) “VYNE Therapeutics Reports Positive Results from Preclinical Models for Oral BD2-Selective BET Inhibitor
VYN202.” Copies of the press releases are attached as Exhibits 99.2 and 99.3, respectively, to this Current Report on Form 8-K.
In addition, the Company has updated its corporate presentation, which is attached as Exhibit 99.4 to this Current Report on Form 8-K.
The information in Items 2.02
and 7.01 of this Form 8-K and Exhibits 99.2, 99.3 and 99.4 attached hereto shall not be deemed “filed” for purposes
of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that Section, or incorporated by reference in any filing
under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such
a filing.
Item 8.01 Other Events.
Change to Board of Directors
In connection with the Private Placement, the Company intends to increase
the size of the Board from six to seven members and, subject to the recommendation of the Nominating and Corporate Governance Committee
of the Board (the “Nominating Committee”) and approval by the Board, to appoint one designee of Access Biotechnology, Inc.
to fill the resulting vacancy. Any such designee will be reasonably acceptable to the Nominating Committee and the Board and shall comply
with the requirements of the charter for, and related guidelines of, the Nominating Committee.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
The following exhibits are being filed herewith.
| Exhibit No. |
Description |
| |
|
| 4.1 |
Form of Pre-Funded Warrant to Purchase Common Stock. |
| |
|
| 10.1 |
Form of Securities Purchase Agreement, dated as of October 27, 2023, by and among VYNE Therapeutics Inc. and the Purchasers. |
| |
|
| 10.2 |
Form of Registration Rights Agreement, dated as of October 27, 2023, by and among VYNE
Therapeutics Inc. and the Purchasers. |
| |
|
| 99.1 |
Press release announcing the Private Placement, dated October 30, 2023. |
| |
|
| 99.2 |
Press release, dated October 30, 2023. |
| |
|
| 99.3 |
Press release, dated October 30, 2023. |
| |
|
| 99.4 |
Corporate Presentation, dated October 2023. |
| |
|
| 104 |
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
VYNE THERAPEUTICS INC. |
| |
|
|
| Date: October 30, 2023 |
By: |
/s/ Mutya Harsch |
| |
|
Mutya Harsch |
| |
|
Chief Legal Officer and General Counsel |
Exhibit 4.1
NEITHER THIS SECURITY NOR THE SECURITIES FOR WHICH THIS SECURITY
IS EXERCISABLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE
UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), AND, ACCORDINGLY,
MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE
EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE
STATE SECURITIES LAWS. THIS SECURITY AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS SECURITY MAY BE PLEDGED IN CONNECTION WITH
A BONA FIDE MARGIN ACCOUNT OR OTHER LOAN SECURED BY SUCH SECURITIES.
FORM OF PRE-FUNDED WARRANT TO PURCHASE
COMMON STOCK
Number of Shares: [ ]
(subject to adjustment)
| Warrant No. __ | Original Issue Date: [ ], 202[
] |
[INSERT ISSUER NAME]
VYNE Therapeutics Inc, a Delaware corporation (the “Company”),
hereby certifies that, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, [ ]
or its registered assigns (the “Holder”), is entitled, subject to the terms set forth below, to purchase from the Company
up to a total of [ ] shares of common stock, $0.0001 par value per share (the “Common Stock”),
of the Company (each such share, a “Warrant Share” and all such shares, the “Warrant Shares”) at
an exercise price per share equal to $0.0001 per share (as adjusted from time to time as provided in Section 9 herein, the
“Exercise Price”), upon surrender of this Warrant to Purchase Common Stock (including any Warrants to Purchase Common
Stock issued in exchange, transfer or replacement hereof, the “Warrant”) at any time and from time to time on or after
the date hereof (the “Original Issue Date”), subject to the following terms and conditions:
1. Definitions. For purposes of this Warrant, the following
terms shall have the following meanings:
(a) “Affiliate” means
any Person directly or indirectly controlled by, controlling or under common control with, a Holder, but only for so long as such control
shall continue. For purposes of this definition, “control” (including, with correlative meanings, “controlled by”,
“controlling” and “under common control with”) means, with respect to a Person, possession, direct or indirect,
of (a) the power to direct or cause direction of the management and policies of such Person (whether through ownership of securities
or partnership or other ownership interests, by contract or otherwise), or (b) at least 50% of the voting securities (whether directly
or pursuant to any option, warrant or other similar arrangement) or other comparable equity interests.
(b) “Attribution Parties”
means, collectively, the following Persons and entities: (i) any direct or indirect Affiliates of the Holder, (ii) any Person
acting or who could be deemed to be acting as a Section 13(d) “group” together with the Holder or any Attribution
Parties and (iii) any other Persons whose beneficial ownership of the Company’s Common Stock would or could be aggregated with
the Holder’s and/or any other Attribution Parties for purposes of Section 13(d) or Section 16 of the Exchange Act.
For clarity, the purpose of the foregoing is to subject collectively the Holder and all other Attribution Parties to the Maximum Percentage.
(c) “Commission” means
the United States Securities and Exchange Commission.
(d) “Closing Sale Price”
means, for any security as of any date, the last trade price for such security on the Principal Trading Market for such security, as reported
by Bloomberg Financial Markets, or, if such Principal Trading Market begins to operate on an extended hours basis and does not designate
the last trade price, then the last trade price of such security prior to 4:00 P.M., New York City time, as reported by Bloomberg Financial
Markets, or if the foregoing do not apply, the last trade price of such security in the over-the-counter market on the electronic bulletin
board for such security as reported by Bloomberg Financial Markets. If the Closing Sale Price cannot be calculated for a security on a
particular date on any of the foregoing bases, the Closing Sale Price of such security on such date shall be the fair market value as
mutually determined by the Company and the Holder. If the Company and the Holder are unable to agree upon the fair market value of such
security, then the Board of Directors of the Company shall use its good faith judgment to determine the fair market value. The Board of
Directors’ determination shall be binding upon all parties absent demonstrable error. All such determinations shall be appropriately
adjusted for any stock dividend, stock split, stock combination or other similar transaction during the applicable calculation period.
(e) “Exchange Act” means
the Securities Exchange Act of 1934, as amended.
(f) “Group” shall have
the meaning ascribed to it in Section 13(d) of the Exchange Act, and all related rules, regulations and jurisprudence.
(f) “Person” means an
individual, a limited liability company, a partnership, a joint venture, a corporation, a trust, an unincorporated organization, any other
entity and a government or any department or agency thereof.
(g) “Principal Trading Market”
means the national securities exchange or other trading market on which the Common Stock is primarily listed on and quoted for trading,
which, as of the Original Issue Date, shall be the [Nasdaq Capital Market].
(i) “Securities Act” means
the Securities Act of 1933, as amended.
(j) “Standard Settlement Period”
means the standard settlement period, expressed in a number of Trading Days, for the Principal Trading Market with respect to the Common
Stock that is in effect on the date of delivery of an applicable Exercise Notice, which as of the Issuance Date was “T+2.”
(k) “Trading Day” means
any weekday on which the Principal Trading Market is normally open for trading.
(l) “Transfer Agent” means
Equiniti Trust Company, LLC (formerly, American Stock Transfer & Trust Company, LLC), the Company’s transfer agent and
registrar for the Common Stock, and any successor appointed in such capacity.
2. Issuance of Securities; Registration of Warrants. The Company
shall register ownership of this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”),
in the name of the record Holder (which shall include the initial Holder or, as the case may be, any assignee to which this Warrant is
permissibly assigned hereunder) from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute
owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice
to the contrary.
3. Registration of Transfers. Subject to compliance with all
applicable securities laws, the Company shall, or will cause its Transfer Agent to, register the transfer of all or any portion of this
Warrant in the Warrant Register, upon surrender of this Warrant, and payment for all applicable transfer taxes (if any). Upon any such
registration or transfer, a new warrant to purchase Common Stock in substantially the form of this Warrant (any such new warrant, a “New
Warrant”) evidencing the portion of this Warrant so transferred shall be issued to the transferee, and a New Warrant evidencing
the remaining portion of this Warrant not so transferred, if any, shall be issued to the transferring Holder. The acceptance of the New
Warrant by the transferee thereof shall be deemed the acceptance by such transferee of all of the rights and obligations in respect of
the New Warrant that the Holder has in respect of this Warrant. The Company shall, or will cause its Transfer Agent to, prepare, issue
and deliver at the Company’s own expense any New Warrant under this Section 3. Until due presentment for registration
of transfer, the Company may treat the registered Holder hereof as the owner and holder for all purposes, and the Company shall not be
affected by any notice to the contrary.
4. Exercise of Warrants.
(a) All or any part of this Warrant shall
be exercisable by the registered Holder in any manner permitted by this Warrant (including Section 11) at any time and from time
to time on or after the Original Issue Date, and such rights shall not expire.
(b) The Holder may exercise this Warrant
by delivering to the Company (i) an exercise notice, in the form attached as Schedule 1 hereto (the “Exercise Notice”),
completed and duly signed, and (ii) payment of the Exercise Price for the number of Warrant Shares as to which this Warrant is being
exercised (which may take the form of a “cashless exercise” if so indicated in the Exercise Notice pursuant to Section 10
below), and the date on which the last of such items is delivered to the Company (as determined in accordance with the notice provisions
hereof) is an “Exercise Date.” The Holder shall not be required to deliver the original Warrant in order to effect
an exercise hereunder. Execution and delivery of the Exercise Notice shall have the same effect as cancellation of the original Warrant
and issuance of a New Warrant evidencing the right to purchase the remaining number of Warrant Shares, if any.
5. Delivery of Warrant Shares.
(a) Upon exercise of this Warrant, the Company
shall promptly (but in no event later than the number of Trading Days comprising the Standard Settlement Period following the Exercise
Date), upon the request of the Holder, credit such aggregate number of shares of Common Stock specified by the Holder in the Exercise
Notice and to which the Holder is entitled pursuant to such exercise (the “Exercise Shares”) to the Holder’s
or its designee’s balance account with The Depository Trust Company (“DTC”) through its Deposit Withdrawal At
Custodian system, or if the Transfer Agent is then a participant in the DTC Fast Automated Securities Transfer Program (the “FAST
Program”) and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares to
or the resale of such Warrant Shares by the Holder or (B) the Exercise Shares are eligible for resale by the Holder without volume
or manner-of-sale restrictions pursuant to Rule 144 promulgated under the Securities Act (assuming cashless exercise of this Warrant).
If the Transfer Agent is not a member of the FAST Program or if (A) and (B) above are not true, the Transfer Agent will either
(i) record the Exercise Shares in the name of the Holder or its designee on the certificates reflecting the Exercise Shares with
an appropriate legend regarding restriction on transferability, which shall be issued and dispatched by overnight courier to the address
as specified in the Exercise Notice, and on the Company’s share register or (ii) issue such Exercise Shares in the name of
the Holder or its designee in restricted book-entry form in the Company’s share register. The Holder, or any natural person or legal
entity (each, a “Person”) so designated by the Holder to receive Warrant Shares, shall be deemed to have become the
holder of record of such Warrant Shares as of the Exercise Date, irrespective of the date such Warrant Shares are credited to the Holder’s
DTC account, the date of the book entry positions or the date of delivery of the certificates evidencing such Exercise Shares, as the
case may be.
(b) If the Company fails to deliver to the
Holder or its designee Exercise Shares in the manner required pursuant to Section 5(a) within the Standard Settlement
Period following the Exercise Date and the Holder purchases (in an open market transaction or otherwise) shares of Common Stock to deliver
in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such exercise (a “Buy-In”)
but did not receive within the Standard Settlement Period, then the Company shall, within two (2) Trading Days after the Holder’s
request and in the Holder’s sole discretion, either (1) pay in cash to the Holder an amount equal to the Holder’s total
purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased, at which point the Company’s
obligation to issue and deliver such Exercise Shares shall terminate or (2) promptly honor its obligation to deliver to the Holder
or its designee the Exercise Shares pursuant to Section 5(a) and pay cash to the Holder in an amount equal to the excess
(if any) of Holder’s total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased
in the Buy-In, less the product of (A) the number of shares of Common Stock purchased in the Buy-In, times (B) the Closing Sale
Price of a share of Common Stock on the Exercise Date.
(c) To the extent permitted by law and subject
to Section 5(b), the Company’s obligations to issue and deliver Warrant Shares in accordance with and subject
to the terms hereof (including the limitations set forth in Section 11 below) are absolute and unconditional, irrespective
of any action or inaction by the Holder to enforce the same, any waiver or consent with respect to any provision hereof, the recovery
of any judgment against any Person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or termination,
or any breach or alleged breach by the Holder or any other Person of any obligation to the Company or any violation or alleged violation
of law by the Holder or any other Person, and irrespective of any other circumstance that might otherwise limit such obligation of the
Company to the Holder in connection with the issuance of Warrant Shares. Subject to Section 5(b), nothing herein shall
limit the Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation,
a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver shares of Common
Stock upon exercise of the Warrant as required pursuant to the terms hereof.
6. Charges, Taxes and Expenses. Issuance and delivery for shares
of Common Stock upon exercise of this Warrant shall be made without charge to the Holder for any issue or transfer tax, transfer agent
fee or other incidental tax or expense (excluding any applicable stamp duties) in respect of the issuance of such certificates, all of
which taxes and expenses shall be paid by the Company; provided, however, that the Company shall not be required to pay
any tax that may be payable in respect of any transfer involved in the registration of any Warrant Shares or the Warrants in a name other
than that of the Holder or an Affiliate thereof. The Holder shall be responsible for all other tax liability that may arise as a result
of holding or transferring this Warrant or receiving Warrant Shares upon exercise hereof.
7. Replacement of Warrant. If this Warrant is mutilated, lost,
stolen or destroyed, the Company shall issue or cause to be issued in exchange and substitution for and upon cancellation hereof, or in
lieu of and substitution for this Warrant, a New Warrant, but only upon receipt of evidence reasonably satisfactory to the Company of
such loss, theft or destruction (in such case) and, in each case, a customary and reasonable contractual indemnity, if requested by the
Company. If a New Warrant is requested as a result of a mutilation of this Warrant, then the Holder shall deliver such mutilated Warrant
to the Company as a condition precedent to the Company’s obligation to issue the New Warrant.
8. Reservation of Warrant Shares. The Company covenants that
it will, at all times while this Warrant is outstanding, reserve and keep available out of the aggregate of its authorized but unissued
and otherwise unreserved Common Stock, solely for the purpose of enabling it to issue Warrant Shares upon exercise of this Warrant as
herein provided, the number of Warrant Shares that are initially issuable and deliverable upon the exercise of this entire Warrant, free
from preemptive rights or any other contingent purchase rights of persons other than the Holder (taking into account the adjustments and
restrictions of Section 9). The Company covenants that all Warrant Shares so issuable and deliverable shall, upon issuance
and the payment of the applicable Exercise Price in accordance with the terms hereof, be duly and validly authorized, issued and fully
paid and non-assessable. The Company will take all such action as may be reasonably necessary to assure that such shares of Common Stock
may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of any securities exchange
or automated quotation system upon which the Common Stock may be listed. The Company further covenants that it will not, without the prior
written consent of the Holder, take any actions to increase the par value of the Common Stock at any time while this Warrant is outstanding.
9. Certain Adjustments. The Exercise Price and number of Warrant
Shares issuable upon exercise of this Warrant are subject to adjustment from time to time as set forth in this Section 9.
(a) Stock Dividends and Splits. If
the Company, at any time while this Warrant is outstanding, (i) pays a stock dividend on its Common Stock or otherwise makes a distribution
on any class of capital stock issued and outstanding on the Original Issue Date and in accordance with the terms of such stock on the
Original Issue Date or as amended, that is payable in shares of Common Stock, (ii) subdivides its outstanding shares of Common Stock
into a larger number of shares of Common Stock, (iii) combines its outstanding shares of Common Stock into a smaller number of shares
of Common Stock or (iv) issues by reclassification of shares of capital stock any additional shares of Common Stock of the Company,
then in each such case the Exercise Price shall be multiplied by a fraction, the numerator of which shall be the number of shares of Common
Stock outstanding immediately before such event and the denominator of which shall be the number of shares of Common Stock outstanding
immediately after such event. Any adjustment made pursuant to clause (i) of this paragraph shall become effective immediately after
the record date for the determination of stockholders entitled to receive such dividend or distribution, provided, however, that if such
record date shall have been fixed and such dividend is not fully paid on the date fixed therefor, the Exercise Price shall be recomputed
accordingly as of the close of business on such record date and thereafter the Exercise Price shall be adjusted pursuant to this paragraph
as of the time of actual payment of such dividends. Any adjustment pursuant to clause (ii) or (iii) of this paragraph shall
become effective immediately after the effective date of such subdivision or combination.
(b) Pro Rata Distributions. If, on
or after the Original Issue Date, the Company shall declare or make any dividend or other pro rata distribution of its assets (or rights
to acquire its assets) to holders of shares of Common Stock, by way of return of capital or otherwise (including, without limitation,
any distribution of cash, stock or other securities, property, options, evidence of indebtedness or any other assets by way of a dividend,
spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction, but, for the avoidance of doubt,
excluding any distribution of shares of Common Stock subject to Section 9(a), any distribution of Purchase Rights (as defined
below) subject to Section 9(c) and any Fundamental Transaction (as defined below) subject to Section 9(d))
(a “Distribution”) then, in each such case, the Holder shall be entitled to participate in such Distribution to the
same extent that the Holder would have participated therein if the Holder had held the number of shares of Common Stock acquirable upon
complete exercise of this Warrant (without regard to any limitations or restrictions on exercise of this Warrant, including without limitation,
the Maximum Percentage (as defined below)) immediately before the date on which a record is taken for such Distribution, or, if no such
record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the participation in such
Distribution (provided, that to the extent that the Holder’s right to participate in any such Distribution would result in the Holder
and the other Attribution Parties exceeding the Maximum Percentage, then the Holder shall not be entitled to participate in such Distribution
to such extent (and shall not be entitled to beneficial ownership of such shares of Common Stock as a result of such Distribution (and
beneficial ownership) to such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the Holder until
such time or times, if ever, as its right thereto would not result in the Holder and the other Attribution Parties exceeding the Maximum
Percentage, at which time or times the Holder shall be granted such Distribution (and any Distributions declared or made on such initial
Distribution or on any subsequent Distribution held similarly in abeyance) to the same extent as if there had been no such limitation).
(c) Purchase Rights. If at any time
on or after the Original Issue Date, the Company grants, issues or sells any Options, Convertible Securities or rights to purchase stock,
warrants, securities or other property, in each case pro rata to the record holders of any class of Common Stock (the “Purchase
Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase
Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise
of this Warrant (without regard to any limitations or restrictions on exercise of this Warrant, including without limitation, the Maximum
Percentage) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no
such record is taken, the date as of which the record holders of Common Stock are to be determined for the grant, issuance or sale of
such Purchase Rights (provided, that to the extent that the Holder’s right to participate in any such Purchase Right would result
in the Holder and the other Attribution Parties exceeding the Maximum Percentage, then the Holder shall not be entitled to participate
in such Purchase Right to such extent (and shall not be entitled to beneficial ownership of such Common Stock as a result of such Purchase
Right (and beneficial ownership) to such extent) and such Purchase Right to such extent shall be held in abeyance for the benefit of the
Holder until such time or times as its right thereto would not result in the Holder and the other Attribution Parties exceeding the Maximum
Percentage, at which time or times the Holder shall be granted such right (and any Purchase Right granted, issued or sold on such initial
Purchase Right or on any subsequent Purchase Right to be held similarly in abeyance) to the same extent as if there had been no such limitation).
As used in this Section 9(c), (i) “Options” means any rights, warrants or options to subscribe for or purchase shares
of Common Stock or Convertible Securities and (ii) “Convertible Securities” mean any stock or securities (other than
Options) directly or indirectly convertible into or exercisable or exchangeable for shares of Common Stock.
(d) Fundamental Transactions. If,
at any time while this Warrant is outstanding (i) the Company effects any merger or consolidation of the Company with or into another
Person, in which the Company is not the surviving entity or in which the stockholders of the Company immediately prior to such merger
or consolidation do not own, directly or indirectly, at least 50% of the voting power of the surviving entity immediately after such merger
or consolidation, (ii) the Company effects any sale to another Person of all or substantially all of its assets in one or a series
of related transactions, (iii) pursuant to any tender offer or exchange offer (whether by the Company or another Person), holders
of capital stock tender shares representing more than 50% of the voting power of the capital stock of the Company and the Company or such
other Person, as applicable, accepts such tender for payment, (iv) the Company consummates a stock purchase agreement or other business
combination (including, without limitation, a reorganization, recapitalization, spin-off or scheme of arrangement) with another Person
whereby such other Person acquires more than the 50% of the voting power of the capital stock of the Company (except for any such transaction
in which the stockholders of the Company immediately prior to such transaction maintain, in substantially the same proportions, the voting
power of such Person immediately after the transaction) or (v) the Company effects any reclassification of the Common Stock or any
compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or
property (other than as a result of a subdivision or combination of shares of Common Stock covered by Section 9(a) above)
(in any such case, a “Fundamental Transaction”), then following such Fundamental Transaction the Holder shall have
the right to receive, upon exercise of this Warrant, the same amount and kind of securities, cash or property as it would have been entitled
to receive upon the occurrence of such Fundamental Transaction if it had been, immediately prior to such Fundamental Transaction, the
holder of the number of Warrant Shares then issuable upon exercise in full of this Warrant without regard to any limitations on exercise
contained herein (the “Alternate Consideration”). The Company shall not effect any Fundamental Transaction in which
the Company is not the surviving entity or the Alternate Consideration includes securities of another Person unless (i) the Alternate
Consideration is solely cash and the Company provides for the simultaneous “cashless exercise” of this Warrant pursuant to
Section 10 below or (ii) prior to or simultaneously with the consummation thereof, any successor to the Company, surviving
entity or other Person (including any purchaser of assets of the Company) shall assume the obligation to deliver to the Holder such Alternate
Consideration as, in accordance with the foregoing provisions, the Holder may be entitled to receive, and the other obligations under
this Warrant. The provisions of this paragraph (c) shall similarly apply to subsequent transactions analogous of a Fundamental Transaction
type.
(e) Number of Warrant Shares. Simultaneously
with any adjustment to the Exercise Price pursuant to Section 9, the number of Warrant Shares that may be purchased upon exercise
of this Warrant shall be increased or decreased proportionately, so that after such adjustment the aggregate Exercise Price payable hereunder
for the increased or decreased number of Warrant Shares shall be the same as the aggregate Exercise Price in effect immediately prior
to such adjustment.
(f) Calculations. All calculations
under this Section 9 shall be made to the nearest one-millionth of one cent or the nearest share, as applicable.
(g) Notice of Adjustments. Upon the
occurrence of each adjustment pursuant to this Section 9, the Company at its expense will, at the written request of the Holder,
promptly compute such adjustment, in good faith, in accordance with the terms of this Warrant and prepare a certificate setting forth
such adjustment, including a statement of the adjusted Exercise Price and adjusted number or type of Warrant Shares or other securities
issuable upon exercise of this Warrant (as applicable), describing the transactions giving rise to such adjustments and showing in detail
the facts upon which such adjustment is based. Upon written request, the Company will promptly deliver a copy of each such certificate
to the Holder and to the Company’s transfer agent.
(h) Notice of Corporate Events. If,
while this Warrant is outstanding, the Company (i) declares a dividend or any other distribution of cash, securities or other property
in respect of its Common Stock, including, without limitation, any granting of rights or warrants to subscribe for or purchase any capital
stock of the Company or any subsidiary, (ii) authorizes or approves, enters into any agreement contemplating or solicits stockholder
approval for any Fundamental Transaction or (iii) authorizes the voluntary dissolution, liquidation or winding up of the affairs
of the Company, then, except if such notice and the contents there of shall be deemed to constitute material non-public information (in
which case the Company shall ask Holder whether Holder wishes to receive such information prior to conveying the substance of such information),
the Company shall deliver to the Holder a notice of such transaction at least ten (10) days prior to the applicable record or effective
date on which a Person would need to hold Common Stock in order to participate in or vote with respect to such transaction; provided,
however, that the failure to deliver such notice or any defect therein shall not affect the validity of the corporate action required
to be described in such notice. In addition, if while this Warrant is outstanding, the Company authorizes or approves, enters into any
agreement contemplating or solicits stockholder approval for any Fundamental Transaction contemplated by Section 9(d), other
than a Fundamental Transaction under clause (iii) of Section 9(d), the Company shall deliver to the Holder a notice of
such Fundamental Transaction at least thirty (30) days prior to the date such Fundamental Transaction is consummated. Holder agrees
to maintain any information disclosed pursuant to this Section 9(h) in confidence until such information is publicly available,
and shall comply with applicable law with respect to trading in the Company’s securities following receipt any such information.
10. Payment of Exercise Price. Notwithstanding anything contained
herein to the contrary, the Holder may, in its sole discretion, satisfy its obligation to pay the Exercise Price through a “cashless
exercise”, in which event the Company shall issue to the Holder the number of Warrant Shares in an exchange of securities effected
pursuant to Section 3(a)(9) of the Securities Act, as determined as follows:
X = Y [(A-B)/A]
where:
“X” equals the number of Warrant Shares
to be issued to the Holder;
“Y” equals the total number of Warrant
Shares with respect to which this Warrant is then being exercised;
“A” equals the Closing Sale Prices
of the shares of Common Stock (as reported by Bloomberg Financial Markets) as of the Trading Day on the date immediately preceding the
Exercise Date; and
“B” equals the Exercise Price then
in effect for the applicable Warrant Shares at the time of such exercise.
For purposes of Rule 144 promulgated under the Securities Act,
it is intended, understood and acknowledged that the Warrant Shares issued in a “cashless exercise” transaction shall be deemed
to have been acquired by the Holder, and the holding period for the Warrant Shares shall be deemed to have commenced, on the date this
Warrant was originally issued (provided that the Commission continues to take the position that such treatment is proper at the time of
such exercise). In the event that a registration statement registering the issuance of Warrant Shares is, for any reason, not effective
at the time of exercise of this Warrant, then the Warrant may only be exercised through a cashless exercise, as set forth in this Section 10.
Except as set forth in Section 5(b) (Buy-In remedy) and Section 12 (payment of cash in lieu of fractional shares), in no
event will the exercise of this Warrant be settled in cash.
11. Limitations on Exercise.
(a) Notwithstanding anything to the contrary
contained herein, the Company shall not effect the exercise of any portion of this Warrant, and the Holder of the Warrant shall not have
the right to exercise any portion of the Warrant, and any such exercise shall be null and void ab initio and treated as if the exercise
had not been made, to the extent that immediately prior to or following such exercise, the Holder, together with the Attribution Parties,
beneficially owns or would beneficially own as determined in accordance with Section 13(d) of the Exchange Act and the rules promulgated
thereunder, in excess of [4.99%] (the “Maximum Percentage”) of the Common Stock that would be issued and outstanding
following such exercise. For purposes of calculating beneficial ownership for determining whether the Maximum Percentage is or will be
exceeded, the aggregate number of shares of Common Stock held and/or beneficially owned by the Holder together with the Attribution Parties,
shall include the number of shares of Common Stock held and/or beneficially owned by the Holder together with the Attribution Parties
plus the number of shares of Common Stock issuable upon exercise of the relevant Warrant with respect to which the determination is being
made but shall exclude the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, unexercised
Warrant held and/or beneficially owned by the Holder or the Attribution Parties and (ii) exercise or conversion of the unexercised
or unconverted portion of any other securities of the Company held and/or beneficially owned by such Holder or any Attribution Party (including,
without limitation, any convertible notes, convertible stock or warrants) that are subject to a limitation on conversion or exercise analogous
to the limitation contained herein. For purposes of this Paragraph 11(a), beneficial ownership of the Holder or the Attribution Parties
shall, except as set forth in the immediately preceding sentence, be calculated and determined in accordance with Section 13(d) of
the Exchange Act and the rules promulgated thereunder. For purposes of the Warrant, in determining the number of outstanding shares
of Common Stock, a Holder of the Warrant may rely on the number of outstanding shares of Common Stock as reflected in (1) the Company’s
most recent Form 10-K, Form 10-Q, Current Report on Form 8-K or other public filing with the Securities and Exchange Commission,
as the case may be, (2) a more recent public announcement by the Company or (3) any other notice by the Company or the Company’s
transfer agent setting forth the number of shares of Common Stock outstanding (such issued and outstanding shares, the “Reported
Outstanding Share Number”). For any reason at any time, upon the written request of the Holder, the Company shall within one
(1) Business Day confirm in writing or by electronic mail to the Holder the number of shares of Common Stock then outstanding. The
Holder shall disclose to the Company the number of shares of Common Stock that it, together with the Attribution Parties holds and/or
beneficially owns and has the right to acquire through the exercise of derivative securities and any limitations on exercise or conversion
analogous to the limitation contained herein contemporaneously or immediately prior to submitting an Exercise Notice for the relevant
Warrant. If the Company receives an Exercise Notice from the Holder at a time when the actual number of outstanding shares of Common Stock
is less than the Reported Outstanding Share Number, the Company shall (i) notify the Holder in writing of the number of shares of
Common Stock then outstanding and, to the extent that such Exercise Notice would otherwise cause the Holder’s, together with the
Attribution Parties’, beneficial ownership, as determined pursuant to this Section 11(a), to exceed the Maximum Percentage,
the Holder must notify the Company of a reduced number of Warrant Shares to be purchased pursuant to such Exercise Notice (the number
of shares by which such purchase is reduced, the “Reduction Shares”) and (ii) as soon as reasonably practicable, the
Company shall return to the Holder any exercise price paid by the Holder for the Reduction Shares. In any case, the number of outstanding
shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this
Warrant, by the Holder and the Attribution Parties since the date as of which the Reported Outstanding Share Number was reported. In the
event that the issuance of Common Stock to the Holder upon exercise of this Warrant results in the Holder, together with the Attribution
Parties, being deemed to beneficially own, in the aggregate, more than the Maximum Percentage of the number of outstanding shares of Common
Stock (as determined under Section 13(d) of the Exchange Act), the number of shares so issued by which the Holder’s, together
with the Attribution Parties’, aggregate beneficial ownership exceeds the Maximum Percentage (the “Excess Shares”) shall
be deemed null and void and shall be cancelled ab initio, and the Holder and/or the Attribution Parties shall not have the power to vote
or to transfer the Excess Shares. As soon as reasonably practicable after the issuance of the Excess Shares has been deemed null and void,
the Company shall return to the Holder the exercise price paid by the Holder for the Excess Shares. By written notice to the Company,
a Holder of the Warrant may from time to time increase or decrease the Maximum Percentage to any other percentage not in excess of 19.99%
specified in such notice; provided that any increase in the Maximum Percentage will not be effective until the sixty-first (61st) day
after such notice is delivered to the Company and shall not negatively affect any partial exercise effected prior to such change.
(b) This
Section 11 shall not restrict the number of shares of Common Stock which a Holder or the Attribution Parties may receive or
beneficially own in order to determine the amount of securities or other consideration that such Holder or the Attribution Parties may
receive in the event of a Fundamental Transaction as contemplated in Section 9(d) of this Warrant. For purposes
of clarity, the shares of Common Stock issuable pursuant to the terms of this Warrant in excess of the Maximum Percentage shall not be
deemed to be beneficially owned by the Holder or the Attribution Parties for any purpose including for purposes of Section 13(d) of
the Exchange Act and the rules promulgated thereunder or Section 16 of the Exchange Act Rule 16a-1(a)(1) promulgated
thereunder. No prior inability to exercise this Warrant pursuant to this paragraph shall have any effect on the applicability of the provisions
of this paragraph with respect to any subsequent determination of exercisability. The provisions of this paragraph shall be construed
and implemented in a manner otherwise than in strict conformity with the terms of this Section 11(a) to the extent necessary
to correct this paragraph or any portion of this paragraph which may be defective or inconsistent with the intended beneficial ownership
limitation contained in this Section 11(a) or to make changes or supplements necessary or desirable to properly give
effect to such limitation. The limitation contained in this paragraph may not be waived and shall apply to a successor holder of this
Warrant.
12. No Fractional Shares. No fractional Warrant Shares will
be issued in connection with any exercise of this Warrant. In lieu of any fractional shares that would otherwise be issuable, the number
of Warrant Shares to be issued shall be rounded down to the next whole number and the Company shall pay the Holder in cash the fair market
value (based on the Closing Sale Price) for any such fractional shares.
13. Notices. Any and all notices or other communications or
deliveries hereunder (including, without limitation, any Exercise Notice) shall be in writing and shall be deemed given and effective
on the earliest of (i) the date of transmission, if such notice or communication is delivered via confirmed e-mail at the e-mail
address specified in the books and records of the Transfer Agent prior to 5:30 P.M., New York City time, on a Trading Day, (ii) the
next Trading Day after the date of transmission, if such notice or communication is delivered via confirmed e-mail at the e-mail address
specified in the books and records of the Transfer Agent on a day that is not a Trading Day or later than 5:30 P.M., New York City time,
on any Trading Day, (iii) the Trading Day following the date of mailing, if sent by nationally recognized overnight courier service
specifying next business day delivery, or (iv) upon actual receipt by the Person to whom such notice is required to be given, if
by hand delivery.
14. Warrant Agent. The Company shall initially serve as warrant
agent under this Warrant. Upon thirty (30) days’ notice to the Holder, the Company may appoint a new warrant agent. Any corporation
into which the Company or any new warrant agent may be merged or any corporation resulting from any consolidation to which the Company
or any new warrant agent shall be a party or any corporation to which the Company or any new warrant agent transfers substantially all
of its corporate trust or shareholders services business shall be a successor warrant agent under this Warrant without any further act.
Any such successor warrant agent shall promptly cause notice of its succession as warrant agent to be mailed (by first class mail, postage
prepaid) to the Holder at the Holder’s last address as shown on the Warrant Register.
15. Miscellaneous.
(a) No Rights as a Stockholder. Except
as otherwise expressly set forth in this Warrant, the Holder, solely in such Person’s capacity as a holder of this Warrant, shall
not be entitled to vote or receive dividends or be deemed the holder of share capital of the Company for any purpose, nor shall anything
contained in this Warrant be construed to confer upon the Holder, solely in such Person’s capacity as the Holder of this Warrant,
any of the rights of a stockholder of the Company or any right to vote, give or withhold consent to any corporate action (whether any
reorganization, issue of stock, reclassification of stock, consolidation, merger, amalgamation, conveyance or otherwise), receive notice
of meetings, receive dividends or subscription rights, or otherwise, prior to the issuance to the Holder of the Warrant Shares which such
Person is then entitled to receive upon the due exercise of this Warrant. In addition, nothing contained in this Warrant shall be construed
as imposing any liabilities on the Holder to purchase any securities (upon exercise of this Warrant or otherwise) or as a stockholder
of the Company, whether such liabilities are asserted by the Company or by creditors of the Company.
(b) Authorized Shares. (i) Except
and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending
its certificate or articles of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue
or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this
Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may
be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality
of the foregoing, the Company will (a) not increase the par value of any Warrant Shares above the amount payable therefor upon such
exercise immediately prior to such increase in par value, (b) take all such action as may be necessary or appropriate in order that
the Company may validly and legally issue fully paid and non-assessable Warrant Shares upon the exercise of this Warrant, and (c) use
commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction
thereof as may be necessary to enable the Company to perform its obligations under this Warrant.
(ii) Before taking any action which would result in an adjustment
in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations
or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
(c) Successors and Assigns. Subject
to compliance with applicable securities laws, this Warrant may be assigned by the Holder. This Warrant may not be assigned by the Company
without the written consent of the Holder, except to a successor in the event of a Fundamental Transaction. This Warrant shall be binding
on and inure to the benefit of the Company and the Holder and their respective successors and assigns. Subject to the preceding sentence,
nothing in this Warrant shall be construed to give to any Person other than the Company and the Holder any legal or equitable right, remedy
or cause of action under this Warrant. This Warrant may be amended only in writing signed by the Company and the Holder, or their successors
and assigns.
(d) Amendment and Waiver. Except as
otherwise provided herein, the provisions of the Warrants may be amended and the Company may take any action herein prohibited, or omit
to perform any act herein required to be performed by it, only if the Company has obtained the written consent of the Holder.
(e) Acceptance. Receipt of this Warrant
by the Holder shall constitute acceptance of and agreement to all of the terms and conditions contained herein.
(f) Governing Law; Jurisdiction. ALL
QUESTIONS CONCERNING THE CONSTRUCTION, VALIDITY, ENFORCEMENT AND INTERPRETATION OF THIS WARRANT SHALL BE GOVERNED BY AND CONSTRUED AND
ENFORCED IN ACCORDANCE WITH THE LAWS OF THE STATE OF NEW YORK WITHOUT REGARD TO THE PRINCIPLES OF CONFLICTS OF LAW THEREOF. EACH OF THE
COMPANY AND THE HOLDER HEREBY IRREVOCABLY SUBMITS TO THE EXCLUSIVE JURISDICTION OF THE STATE AND FEDERAL COURTS SITTING IN THE CITY OF
NEW YORK, BOROUGH OF MANHATTAN, FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR IN CONNECTION HEREWITH OR WITH ANY TRANSACTION CONTEMPLATED
HEREBY OR DISCUSSED HEREIN (INCLUDING WITH RESPECT TO THE ENFORCEMENT OF ANY OF THE TRANSACTION DOCUMENTS), AND HEREBY IRREVOCABLY WAIVES,
AND AGREES NOT TO ASSERT IN ANY SUIT, ACTION OR PROCEEDING, ANY CLAIM THAT IT IS NOT PERSONALLY SUBJECT TO THE JURISDICTION OF ANY SUCH
COURT. EACH OF THE COMPANY AND THE HOLDER HEREBY IRREVOCABLY WAIVES PERSONAL SERVICE OF PROCESS AND CONSENTS TO PROCESS BEING SERVED IN
ANY SUCH SUIT, ACTION OR PROCEEDING BY MAILING A COPY THEREOF VIA REGISTERED OR CERTIFIED MAIL OR OVERNIGHT DELIVERY (WITH EVIDENCE OF
DELIVERY) TO SUCH PERSON AT THE ADDRESS IN EFFECT FOR NOTICES TO IT AND AGREES THAT SUCH SERVICE SHALL CONSTITUTE GOOD AND SUFFICIENT
SERVICE OF PROCESS AND NOTICE THEREOF. NOTHING CONTAINED HEREIN SHALL BE DEEMED TO LIMIT IN ANY WAY ANY RIGHT TO SERVE PROCESS IN ANY
MANNER PERMITTED BY LAW. EACH OF THE COMPANY AND THE HOLDER HEREBY WAIVES ALL RIGHTS TO A TRIAL BY JURY.
(g) Headings. The headings herein
are for convenience only, do not constitute a part of this Warrant and shall not be deemed to limit or affect any of the provisions hereof.
(h) Severability. In case any one
or more of the provisions of this Warrant shall be invalid or unenforceable in any respect, the validity and enforceability of the remaining
terms and provisions of this Warrant shall not in any way be affected or impaired thereby, and the Company and the Holder will attempt
in good faith to agree upon a valid and enforceable provision which shall be a commercially reasonable substitute therefor, and upon so
agreeing, shall incorporate such substitute provision in this Warrant.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
IN WITNESS WHEREOF, the Company has caused this
Warrant to be duly executed by its authorized officer as of the date first indicated above.
| |
VYNE THERAPEUTICS INC. |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
SCHEDULE 1
FORM OF EXERCISE NOTICE
[To be executed by the Holder to purchase shares
of Common Stock under the Warrant]
Ladies and Gentlemen:
(1) The undersigned is the Holder of Warrant No. __ (the
“Warrant”) issued byVYNE Therapeutics Inc., a Delaware corporation (the “Company”). Capitalized terms used
herein and not otherwise defined herein have the respective meanings set forth in the Warrant.
(2) The undersigned hereby exercises its right to purchase Warrant
Shares pursuant to the Warrant.
(3) The Holder intends that payment of the Exercise Price shall
be made as (check one):
| |
¨ |
“Cashless Exercise” under Section 10 of the Warrant |
(4) If the Holder has elected a Cash Exercise, the Holder shall
pay the sum of $ in immediately available funds to the Company in accordance with the terms of the Warrant.
(5) Pursuant to this Exercise Notice, the Company shall deliver
to the Holder Warrant Shares determined in accordance with the terms of the Warrant.
(6) By its delivery of this Exercise Notice, the undersigned represents
and warrants to the Company that in giving effect to the exercise evidenced hereby the Holder will not beneficially own in excess of the
number of shares of Common Stock (as determined in accordance with Section 13(d) of the Securities Exchange Act of 1934, as
amended) permitted to be owned under Section 11(a) of the Warrant to which this notice relates.
| Dated: |
|
|
| Name of Holder: |
|
|
| By: |
|
|
| Name: |
|
|
| Title: |
|
|
(Signature must conform in all respects to name
of Holder as specified on the face of the Warrant)
Exhibit 10.1
SECURITIES
PURCHASE AGREEMENT
This
Securities Purchase Agreement (this “Agreement”) is dated as of October 27, 2023, between VYNE
Therapeutics Inc., a Delaware corporation (the “Company”), and each purchaser identified on the signature
pages hereto (each, including its successors and assigns, a “Purchaser” and collectively, the “Purchasers”).
Whereas,
subject to the terms and conditions set forth in this Agreement and pursuant to Section 4(a)(2) of the Securities Act (as defined
below) and/or Rule 506 of Regulation D promulgated thereunder, the Company desires to issue and sell to each Purchaser, and each
Purchaser, severally and not jointly, desires to purchase from the Company, securities of the Company as more fully described in this
Agreement.
Now,
Therefore, In Consideration of the mutual covenants contained in this Agreement, and for other good and valuable consideration,
the receipt and adequacy of which are hereby acknowledged, the Company and each Purchaser agree as follows:
Article I
DEFINITIONS
1.1 Definitions.
In addition to the terms defined elsewhere in this Agreement, for all purposes of this Agreement, the following terms have the meanings
set forth in this Section 1.1:
“Action”
shall have the meaning ascribed to such term in Section 3.1(j).
“Affiliate”
means any Person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control
with a Person, as such terms are used in and construed under Rule 405 under the Securities Act.
“Board of Directors”
means the board of directors of the Company.
“Business Day”
means any day other than Saturday, Sunday or other day (excluding Lincoln’s Birthday (February 12) and Election Day) on which
commercial banks in The City of New York are authorized or required by law or other governmental action to remain closed; provided,
however, for clarification, commercial banks shall not be deemed to be authorized or required by law to remain closed due to “stay
at home”, “shelter-in-place”, “non-essential employee” or any other similar orders or restrictions or the
closure of any physical branch locations at the direction of any governmental authority so long as the electronic funds transfer systems
(including for wire transfers) of commercial banks in The City of New York are generally open for use by customers on such day.
“Closing”
means the closing of the purchase and sale of the Securities pursuant to Section 2.1.
“Closing Date”
means the Trading Day on which all of the Transaction Documents have been executed and delivered by the applicable parties thereto, and
all conditions precedent to (i) the Purchasers’ obligations to pay the Subscription Amount and (ii) the Company’s
obligations to deliver the Securities, in each case, have been satisfied or waived.
“Commission”
means the United States Securities and Exchange Commission.
“Common Stock”
means the common stock of the Company, par value $0.0001 per share, and any other class of securities into which such securities may hereafter
be reclassified or changed.
“Common Stock
Equivalents” means any securities of the Company or the Subsidiaries which would entitle the holder thereof to acquire at
any time Common Stock, including any debt, preferred stock, right, option, warrant or other instrument that is at any time convertible
into or exercisable or exchangeable for, or otherwise entitles the holder thereof to receive, Common Stock.
“Company Counsel”
means Cooley LLP, with offices located at One Freedom Square, Reston Town Center, 11951 Freedom Drive, Reston, VA 20190-5656.
“Disclosure Time”
means, (i) if this Agreement is signed on a day that is not a Trading Day or after 9:00 a.m. (New York City time) and before
midnight (New York City time) on any Trading Day, 9:01 a.m. (New York City time) on the Trading Day immediately following the date
hereof, unless otherwise instructed as to an earlier time by the Placement Agent, and (ii) if this Agreement is signed between midnight
(New York City time) and 9:00 a.m. (New York City time) on any Trading Day, no later than 9:01 a.m. (New York City time) on
the date hereof, unless otherwise instructed as to an earlier time by the Placement Agent.
“Exchange Act”
means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“FCPA”
means the Foreign Corrupt Practices Act of 1977, as amended.
“FDA”
shall have the meaning ascribed to such term in Section 3.1(jj).
“GAAP”
shall have the meaning ascribed to such term in Section 3.1(h).
“Indebtedness”
shall have the meaning ascribed to such term in Section 3.1(bb).
“Intellectual
Property” shall have the meaning ascribed to such term in Section 3.1(p).
“Liens”
means a lien, charge, pledge, security interest, encumbrance, right of first refusal, preemptive right or other restriction.
“Material Adverse
Effect” shall have the meaning assigned to such term in Section 3.1(b).
“Per Share Purchase
Price” equals $2.245 (less $0.0001 for each Pre-Funded Warrant issued at Closing), subject to adjustment for reverse
and forward stock splits, stock dividends, stock combinations and other similar transactions of the Common Stock that occur after the
date of this Agreement and prior to the Closing Date.
“Material Contract”
means any contract, instrument or other agreement to which the Company is a party or by which it is bound which is material to the business
of the Company, including those that have been filed as an exhibit to the SEC Reports pursuant to Item 601(b)(10) of Regulation S-K.
“Officer’s
Certificate” means a certificate signed by the Chief Executive Officer and either the General Counsel or the Chief Financial
Officer of the Company to the effect that (i) the representations and warranties of the Company in Section 3.1 hereof are true
and correct as if made on the Closing Date, (ii) all obligations, covenants and agreements to be performed or complied with by the
Company at or prior to the Closing have been performed or complied with by it, and (iii) all of the conditions set forth in Section 2.3
have been satisfied.
“Person”
means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company,
joint stock company, government (or an agency or subdivision thereof) or other entity of any kind.
“Placement Agent”
means LifeSci Capital LLC.
“Pre-Funded Warrants”
means, collectively, the pre-funded Common Stock purchase warrants delivered to the Purchasers at the Closing in accordance with Section 2.2(a) hereof
and the pre-funded Common Stock purchase warrants issuable in accordance with Section 2(a) of the agreement governing such warrants,
in the form of Exhibit A attached hereto.
“Pre-Funded Warrant
Shares” means the shares of Common Stock issuable upon exercise of the Pre-Funded Warrants.
“Proceeding”
means an action, claim, suit, arbitration, hearing, investigation,
notice of violation, inquiry or proceeding (including an informal investigation or partial proceeding, such as a deposition), whether
commenced or threatened in writing or (to the Company’s knowledge) otherwise.
“Purchaser Party”
shall have the meaning ascribed to such term in Section 4.8.
“Registration
Rights Agreement” means the Registration Rights Agreement, dated as of the date hereof, among the Company and the Purchasers,
in the form of Exhibit B attached hereto.
“Registration
Statement” means a registration statement meeting the requirements set forth in the Registration Rights Agreement and covering
the resale by the Purchasers of the Shares and the Pre-Funded Warrant Shares, subject to any cutbacks permitted under the Registration
Rights Agreement.
“Required Approvals”
shall have the meaning ascribed to such term in Section 3.1(e).
“Rule 144”
means Rule 144 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from
time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect
as such Rule.
“SEC Reports”
shall have the meaning ascribed to such term in Section 3.1(h).
“Securities”
means the Shares, the Pre-Funded Warrants and the Pre-Funded Warrant Shares.
“Securities Act”
means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Secretary’s
Certificate” means a certificate signed by the Secretary of the Company, certifying the resolutions of the Board of Directors
of the Company or a duly authorized committee thereof approving this Agreement and all of the transactions contemplated hereunder.
“Shares”
means the shares of Common Stock issued or issuable to each Purchaser pursuant to this Agreement, but excluding the Pre-Funded Warrant
Shares.
“Short Sales”
means all “short sales” as defined in Rule 200 of Regulation SHO under the Exchange Act (but shall not be deemed to include
locating and/or borrowing shares of Common Stock).
“Subscription
Amount” means, as to each Purchaser, the aggregate amount to be paid for Shares and Pre-Funded Warrants purchased hereunder
as specified below such Purchaser’s name on the signature page of this Agreement and next to the heading “Subscription
Amount,” in United States dollars and in immediately available funds (minus, if applicable, a Purchaser’s aggregate exercise
price of the Pre-Funded Warrants, which amounts shall be paid as and when such Pre-Funded Warrants are exercised for cash).
“Subsidiary”
means any subsidiary of the Company as set forth in its SEC Reports and shall, where applicable, also include any direct or indirect subsidiary
of the Company formed or acquired after the date hereof.
“Trading Day”
means a day on which the principal Trading Market is open for trading.
“Trading Market”
means any of the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the
NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, or the New York Stock Exchange (or
any successors to any of the foregoing).
“Transaction Documents”
means this Agreement, the Registration Rights Agreement, all exhibits and schedules thereto and hereto and any other documents or agreements
executed in connection with the transactions contemplated hereunder and thereunder.
“Transfer Agent”
means Equiniti Trust Company, LLC (formerly, American Stock Transfer & Trust Company, LLC), the current transfer agent of the
Company, with a mailing address of 6201 15th Avenue, Brooklyn, NY 11219 and a facsimile number of (718) 921-8367, and any successor transfer
agent of the Company.
Article II
PURCHASE
AND SALE
2.1 Closing.
On the Closing Date, upon the terms and subject to the conditions set forth herein, substantially concurrent with the execution and delivery
of this Agreement by the parties hereto, the Company agrees to sell, and the Purchasers, severally and not jointly, agree to purchase,
an aggregate of $88,151,508.66 of Shares and Pre-Funded Warrants. Notwithstanding anything herein to the contrary, to the extent that a
Purchaser determines, in its sole discretion, that such Purchaser’s Subscription Amount (together with such Purchaser’s Affiliates
and any Person acting as a group together with such Purchaser or any of such Purchaser’s Affiliates) would cause such Purchaser’s
beneficial ownership of the shares of Common Stock to exceed the Beneficial Ownership Limitation, or as such Purchaser may otherwise choose,
such Purchaser may elect to purchase Pre-Funded Warrants in lieu of the Shares as determined pursuant to Section 2.2(a). The “Beneficial
Ownership Limitation” shall be 4.99% (or, at the election of the Purchaser at Closing, 9.99%) of the number of shares of the Common
Stock outstanding immediately after giving effect to the issuance of the Securities on the Closing Date. In each case, any Purchaser’s
election to receive Pre-Funded Warrants is solely at the option of such Purchaser. Unless otherwise instructed by the Placement Agent,
each Purchaser shall deliver to the Company, via wire transfer, immediately available funds equal to such Purchaser’s Subscription
Amount as set forth on the signature page hereto executed by such Purchaser. The Company shall deliver to each Purchaser its respective
Shares and Pre-Funded Warrants as determined pursuant to Section 2.2(a), and the Company and each Purchaser shall deliver the other
items set forth in Section 2.2 deliverable at the Closing. Upon satisfaction of the covenants and conditions set forth in Sections
2.2 and 2.3, the Closing shall occur at the offices of Company Counsel or such other location (including remotely by electronic transmission)
as the parties shall mutually agree. In the event the Closing does not occur within five Business Days after the expected Closing Date,
unless otherwise agreed by the Company and such Purchaser, the Company shall promptly (but not later than one Business Day thereafter)
return the previously wired Purchaser’s Subscription Amount to each respective Purchaser by wire transfer of United States dollars
in immediately available funds to the account specified by each Purchaser, and any book entries for the Shares and Pre-Funded Warrants
shall be deemed cancelled. Notwithstanding anything to the contrary in this Agreement or otherwise, the Company shall not be required
to consummate any sale or issuance of Shares, Pre-Funded Warrants or Pre-Funded Warrant Shares to a Purchaser if the Company determines
in good faith that such sale or issuance would result in requiring a vote of the Company’s stockholders pursuant to the applicable
rules of the Nasdaq Stock Market, including because such sale or issuance would result in such Purchaser (together with such Purchaser’s
Affiliates and any Person acting as a group together with such Purchaser or any of such Purchaser’s Affiliates) beneficially owning
(1) in excess of 19.99% of the number of shares of Common Stock outstanding and (2) the largest ownership position of the Company
immediately after giving effect to such sale or issuance.
2.2 Deliveries.
(a) On
or prior to the Closing Date, the Company shall deliver or cause to be delivered to each Purchaser the following:
| (i) | this Agreement duly executed by the Company; |
| (ii) | a legal opinion of Company Counsel, dated as of the Closing Date, substantially in the form of Exhibit C
attached hereto; |
| (iii) | an Officer’s Certificate in the form of Exhibit D attached hereto; |
| (iv) | a Secretary Certificate in the form of Exhibit E attached hereto; |
(v) a
copy of the irrevocable instructions to the Transfer Agent instructing the Transfer Agent to deliver, on an expedited basis, a certificate
evidencing a number of Shares equal to such Purchaser’s Subscription Amount divided by the Per Share Purchase Price, registered
in the name of such Purchaser, or, at the election of the Company, evidence of the issuance of such Purchaser’s Shares hereunder
as held in DRS book-entry form by the Transfer Agent and registered in the name of such Purchaser, which evidence shall be reasonably
satisfactory to such Purchaser;
(vi) the
Company shall have provided each Purchaser with the Company’s wire instructions;
(vii) if
applicable, a Pre-Funded Warrant registered in the name of such Purchaser to purchase up to a number of shares of Common Stock equal to
the portion of such Purchaser’s Subscription Amount applicable to the Pre-Funded Warrant divided by the Per Share Purchase
Price with an exercise price equal to $0.0001 per share of Common Stock, subject to adjustment therein;
(viii) the
Registration Rights Agreement duly executed by the Company; and
(ix) a
lock-up agreement executed by each of the Company’s executive officers and directors (in their capacities in such positions) restricting
sales for a period not to exceed 30 days from the Closing Date.
(b) On
or prior to the Closing Date, each Purchaser, severally and not jointly, shall deliver or cause to be delivered to the Company, the following:
(i) this
Agreement duly executed by such Purchaser;
(ii) such
Purchaser’s Subscription Amount by wire transfer; and
(iii) the
Registration Rights Agreement duly executed by such Purchaser.
2.3 Closing
Conditions.
(a) The
obligations of the Company hereunder in connection with the Closing are subject to the following conditions being satisfied or waived
by the Company:
(i) the
accuracy in all material respects (or, to the extent representations or warranties are qualified by materiality, in all respects) on the
Closing Date of the representations and warranties of the Purchasers contained herein (unless as of a specific date therein in which case
they shall be accurate in all material respects (or, to the extent representations or warranties are qualified by materiality, in all
respects) as of such date);
(ii) all
obligations, covenants and agreements of each Purchaser required to be performed at or prior to the Closing Date shall have been performed;
and
(iii) the
delivery by each Purchaser of the items set forth in Section 2.2(b) of this Agreement.
(b) The
respective obligations of the Purchasers hereunder in connection with the Closing are subject to the following conditions being satisfied
or, severally and not jointly, waived by each Purchaser:
(i) the
accuracy in all material respects (or, to the extent representations or warranties are qualified by materiality or Material Adverse Effect,
in all respects) when made and on the Closing Date of the representations and warranties of the Company contained herein (unless as of
a specific date therein in which case they shall be accurate in all material respects (or, to the extent representations or warranties
are qualified by materiality or Material Adverse Effect, in all respects) as of such date);
(ii) all
obligations, covenants and agreements of the Company required to be performed at or prior to the Closing Date shall have been performed;
(iii) the
delivery by the Company of the items set forth in Section 2.2(a) of this Agreement;
(iv) there
shall have been no Material Adverse Effect with respect to the Company since the date hereof; and
(v) from
the date hereof to the Closing Date, trading in the Common Stock shall not have been suspended by the Commission or the Company’s
principal Trading Market, and, at any time prior to the Closing Date, trading in securities generally as reported by Bloomberg L.P. shall
not have been suspended or limited, or minimum prices shall not have been established on securities whose trades are reported by such
service, or on any Trading Market, nor shall a banking moratorium have been declared either by the United States or New York State authorities
nor shall there have occurred any material outbreak or escalation of hostilities or other national or international calamity of such magnitude
in its effect on, or any material adverse change in, any financial market which, in each case, in the reasonable judgment of such Purchaser,
makes it impracticable or inadvisable to purchase the Securities at the Closing.
Article III
REPRESENTATIONS
AND WARRANTIES
3.1 Representations
and Warranties of the Company. Except as set forth in the SEC Reports, which SEC Reports shall be deemed a part hereof and shall qualify
any representation or warranty otherwise made herein to the extent of the disclosure contained in the SEC Reports, the Company hereby
makes the following representations and warranties to each Purchaser:
(a) Subsidiaries.
All of the direct and indirect subsidiaries of the Company are set forth in the SEC Reports. The Company owns, directly or indirectly,
all of the capital stock or other equity interests of each Subsidiary free and clear of any Liens, and all of the issued and outstanding
shares of capital stock of each Subsidiary are validly issued and are fully paid, non-assessable and free of preemptive and similar rights
to subscribe for or purchase securities.
(b) Organization
and Qualification. The Company and each of the Subsidiaries is an entity duly incorporated or otherwise organized, validly existing
and in good standing under the laws of the jurisdiction of its incorporation or organization, with the requisite power and authority to
own and use its properties and assets and to carry on its business as currently conducted. Neither the Company nor any Subsidiary is in
violation or default of any of the provisions of its respective certificate or articles of incorporation, bylaws or other organizational
or charter documents. Each of the Company and the Subsidiaries is duly qualified to conduct business and is in good standing as a foreign
corporation or other entity in each jurisdiction in which the nature of the business conducted or property owned by it makes such qualification
necessary, except where the failure to be so qualified or in good standing, as the case may be, could not have or reasonably be expected
to result in: (i) a material adverse effect on the legality, validity or enforceability of any Transaction Document, (ii) a
material adverse effect on the results of operations, assets, business, prospects or condition (financial or otherwise) of the Company
and the Subsidiaries, taken as a whole, or (iii) a material adverse effect on the Company’s ability to perform in any material
respect on a timely basis its obligations under any Transaction Document (any of (i), (ii) or (iii), a “Material Adverse
Effect”); provided that changes in the market price or trading volume of the Common Stock shall not, in and of itself,
constitute a Material Adverse Effect. No Proceeding has been instituted in any such jurisdiction revoking, limiting or curtailing or seeking
to revoke, limit or curtail such power and authority or qualification.
(c) Authorization;
Enforcement. The Company has the requisite corporate power and authority to enter into and to consummate the transactions contemplated
by this Agreement and each of the other Transaction Documents and otherwise to carry out its obligations hereunder and thereunder. The
execution and delivery of this Agreement and each of the other Transaction Documents by the Company and the consummation by it of the
transactions contemplated hereby and thereby have been duly authorized by all necessary action on the part of the Company and no further
action is required by the Company, the Board of Directors or the Company’s stockholders in connection herewith or therewith other
than in connection with the Required Approvals. This Agreement and each other Transaction Document to which the Company is a party has
been (or upon delivery will have been) duly executed by the Company and, when delivered in accordance with the terms hereof and thereof,
will constitute the valid and binding obligation of the Company enforceable against the Company in accordance with its terms, except (i) as
limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application
affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance,
injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be limited by
applicable law.
(d) No
Conflicts. The execution, delivery and performance by the Company of this Agreement and the other Transaction Documents to which it
is a party, the issuance and sale of the Securities and the consummation by it of the transactions contemplated hereby and thereby do
not and will not (i) conflict with or violate any provision of the Company’s or any Subsidiary’s certificate or articles
of incorporation, bylaws or other organizational or charter documents, or (ii) conflict with, or constitute a default (or an event
that with notice or lapse of time or both would become a default) under, result in the creation of any Lien upon any of the properties
or assets of the Company or any Subsidiary, or give to others any rights of termination, amendment, anti-dilution or similar adjustments,
acceleration or cancellation (with or without notice, lapse of time or both) of, any agreement, credit facility, debt or other instrument
(evidencing a Company or Subsidiary debt or otherwise) or other understanding to which the Company or any Subsidiary is a party or by
which any property or asset of the Company or any Subsidiary is bound or affected, or (iii) subject to the Required Approvals, conflict
with or result in a violation of any law, rule, regulation, order, judgment, injunction, decree or other restriction of any court or governmental
authority to which the Company or a Subsidiary is subject (including federal and state securities laws and regulations), or by which any
property or asset of the Company or a Subsidiary is bound or affected, or give to others any rights of termination, amendment, acceleration
or cancellation (with or without notice, lapse of time or both) of, any Material Contract; except in the case of each of clauses (ii) and
(iii), such as could not have or reasonably be expected to result in a Material Adverse Effect.
(e) Filings,
Consents and Approvals. The Company is not required to obtain any consent, waiver, authorization or order of, give any notice to,
or make any filing or registration with, any court or other federal, state, local or other governmental authority or other Person in connection
with the execution, delivery and performance by the Company of the Transaction Documents, other than: (i) the filings required pursuant
to Section 4.4 of this Agreement, (ii) the filing(s) with the Commission pursuant to the Registration Rights Agreement,
(iii) the notice and/or application(s) to each applicable Trading Market for the issuance and sale of the Securities and the
listing of the Shares and Pre-Funded Warrant Shares for trading thereon in the time and manner required thereby and (iv) the filing
of Form D with the Commission and such filings as are required to be made under applicable state securities laws (collectively, the
“Required Approvals”).
(f) Issuance
of the Securities. The Securities are duly authorized and, when issued and paid for in accordance with the applicable Transaction
Documents, will be duly and validly issued, fully paid and nonassessable, free and clear of all Liens imposed by the Company other than
restrictions on transfer provided for in the Transaction Documents. The Company has reserved from its duly authorized capital stock the
maximum number of shares of Common Stock issuable pursuant to this Agreement.
(g) Capitalization.
The capitalization of the Company is as set forth in the SEC Reports as of the date reported therein. The Company has not issued any capital
stock since its most recently filed periodic report under the Exchange Act, other than pursuant to the exercise or settlement of employee
stock options or other awards under the Company’s stock option plans and pursuant to the conversion or exercise of Common Stock
Equivalents outstanding as of the date of the most recently filed periodic report under the Exchange Act. No Person has any right of first
refusal, preemptive right, right of participation, or any similar right to participate in the transactions contemplated by the Transaction
Documents. Except as a result of the purchase and sale of the Securities as contemplated hereby, there are no outstanding options, warrants,
scrip rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities, rights or obligations convertible
into or exercisable or exchangeable for, or giving any Person any right to subscribe for or acquire, any shares of Common Stock or the
capital stock of any Subsidiary, or contracts, commitments, understandings or arrangements by which the Company or any Subsidiary is or
may become bound to issue additional shares of Common Stock or Common Stock Equivalents or capital stock of any Subsidiary. The issuance
and sale of the Securities will not obligate the Company or any Subsidiary to issue shares of Common Stock or other securities to any
Person (other than the Purchaser). There are no outstanding securities or instruments of the Company or any Subsidiary with any provision
that adjusts the exercise, conversion, exchange or reset price of such security or instrument upon an issuance of securities by the Company
or any Subsidiary. There are no outstanding securities or instruments of the Company or any Subsidiary that contain any redemption or
similar provisions, and there are no contracts, commitments, understandings or arrangements by which the Company or any Subsidiary is
or may become bound to redeem a security of the Company or such Subsidiary. The Company does not have any stock appreciation rights or
“phantom stock” plans or agreements or any similar plan or agreement. All of the outstanding shares of capital stock of the
Company are duly authorized, validly issued, fully paid and nonassessable, have been issued in compliance with all applicable federal
and state securities laws, and none of such outstanding shares was issued in violation of any preemptive rights,
rights of first refusal or other or similar rights to subscribe for or
purchase securities. Except for the rights provided in the Transaction Documents, there are no stockholders agreements, voting agreements
or other similar agreements with respect to the Company’s capital stock to which the Company is a party or, to the knowledge of
the Company, between or among any of the Company’s stockholders.
(h) SEC
Reports; Financial Statements. The Company has filed or furnished all reports, schedules, forms, statements and other documents required
to be filed or furnished by the Company under the Securities Act and the Exchange Act, including pursuant to Section 13(a) or
15(d) thereof, for the three years preceding the date hereof (or such shorter period as the Company was required by law or regulation
to file such material) (the foregoing materials, including all exhibits thereto and documents incorporated by reference therein, and including
all registration statements and prospectuses filed with the SEC, being collectively referred to herein as the “SEC Reports”),
all of which are available on the Commission’s EDGAR system, on a timely basis or has received a valid extension of such time of
filing or furnishing and has filed or furnished any such SEC Reports prior to the expiration of any such extension. As of their respective
dates, the SEC Reports (including any audited or unaudited financial statements and any notes thereto or schedules included therein) complied
in all material respects with the requirements of the Securities Act and the Exchange Act, as applicable, and none of the SEC Reports,
when filed or furnished, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein
or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. There
are no material outstanding or unresolved comments in comment letters from the staff of the Division of Corporation Finance of the SEC
with respect to any of the SEC Reports as of the date hereof. The Company meets the requirements for use of Form S-3 under the 1933
Act. The financial statements (including the notes thereto) of the Company included in the SEC Reports complied in all material respects
with the Securities Act and the Exchange act, as applicable, applicable accounting requirements and the rules and regulations of
the Commission with respect thereto as in effect at the time of filing. Such financial statements have been prepared in accordance with
United States generally accepted accounting principles applied on a consistent basis during the periods involved (“GAAP”),
except as may be otherwise specified in such financial statements or the notes thereto and except that unaudited financial statements
may not contain all footnotes required by GAAP, and fairly present in all material respects the financial position of the Company and
its consolidated Subsidiaries as of and for the dates thereof and the results of operations and cash flows for the periods then ended,
subject, in the case of unaudited statements, to normal, immaterial, year-end audit adjustments. Except as set forth in the financial
statements of the Company included in the SEC Documents prior to the date hereof, the Company has not incurred any liabilities, contingent
or otherwise, except for those incurred in the ordinary course of business, consistent (as to amount and nature) with past practices since
the date of such financial statements, none of which, individually or in the aggregate, have had or would reasonably be expected to have
a Material Adverse Effect.
(i) Material
Changes; Undisclosed Events, Liabilities or Developments. Since the date of the latest financial statements included within the SEC
Reports, (i) there has been no event, occurrence or development that has had or that would reasonably be expected to result in a
Material Adverse Effect, (ii) the Company has not incurred any material liabilities (contingent or otherwise) other than (A) trade
payables and accrued expenses incurred in the ordinary course of business consistent with past practice and (B) liabilities not required
to be reflected in the Company’s financial statements pursuant to GAAP or disclosed in filings made with the Commission, (iii) the
Company has not materially altered its method of accounting, (iv) the Company has not declared or made any dividend or distribution
of cash or other property to its stockholders or purchased, redeemed or made any agreements to purchase or redeem any shares of its capital
stock and (v) the Company has not issued any equity securities to any officer, director or Affiliate, except pursuant to existing
Company stock option plans or other equity plans. The Company does not have pending before the Commission any request for confidential
treatment of information. Except for the issuance of the Securities contemplated by this Agreement, no event, liability, fact, circumstance,
occurrence or development has occurred or exists with respect to the Company or its Subsidiaries or their respective businesses, prospects,
properties, operations, assets, financial condition or results of operations (each a “Subsequent Event”), that
would be required to be disclosed by the Company under applicable securities laws at the time this representation is made or deemed made
or thereafter that will not be publicly disclosed by or before the Disclosure Time.
(j) Litigation.
There is no Proceeding against the Company, any Subsidiary or any of their respective properties before or by any court, arbitrator, governmental
or administrative agency or regulatory authority (federal, state, county, local or foreign) (each, an “Action”)
that, (i) adversely affects or challenges the legality, validity or enforceability of any of the Transaction Documents or the Securities
or (ii) would, if there were an unfavorable decision, reasonably be expected to result in a Material Adverse Effect. Neither the
Company nor any Subsidiary, nor, to the Company’s knowledge, any director or officer thereof, is or has been the subject of any
Action involving a claim of violation of or liability under federal or state securities laws or a claim of breach of fiduciary duty. There
has not been, and to the knowledge of the Company, there is not pending or contemplated, any investigation by the Commission involving
the Company or any current or former director or officer of the Company (other than requests for documentation or information from the
Commission as have been disclosed to the Purchasers). The Commission has not issued any stop order or other order suspending the effectiveness
of any registration statement filed by the Company or any Subsidiary under the Exchange Act or the Securities Act.
(k) Labor
Relations. No labor dispute exists or, to the knowledge of the Company, is imminent with respect to any of the employees of the Company,
which would reasonably be expected to result in a Material Adverse Effect. None of the Company’s or its Subsidiaries’ employees
is a member of a union that relates to such employee’s relationship with the Company or such Subsidiary, and neither the Company
nor any of its Subsidiaries is a party to a collective bargaining agreement, and the Company and its Subsidiaries believe that their relationships
with their employees are good. To the knowledge of the Company, no executive officer of the Company or any Subsidiary, is, or is now expected
to be, in violation of any material term of any employment contract, confidentiality, disclosure or proprietary information agreement
or non-competition agreement, or any other contract or agreement or any restrictive covenant in favor of any third party, and the continued
employment of each such executive officer does not subject the Company or any of its Subsidiaries to any liability with respect to any
of the foregoing matters. The Company and its Subsidiaries are in compliance with all applicable U.S. federal, state, local and foreign
laws and regulations relating to employment and employment practices, terms and conditions of employment and wages and hours, except where
the failure to be in compliance would not, individually or in the aggregate, reasonably be expected to have or result in a Material Adverse
Effect.
(l) Compliance.
Neither the Company nor any Subsidiary: (i) is in default under or in violation of (and no event has occurred that has not been waived
that, with notice or lapse of time or both, would result in a default by the Company or any Subsidiary under), nor has the Company or
any Subsidiary received written (or, to the knowledge of the Company, any other) notice of a claim that it is in default under or that
it is in violation of, any indenture, loan or credit agreement or any other agreement or instrument to which it is a party or by which
it or any of its properties is bound (whether or not such default or violation has been waived), (ii) is in violation of any judgment,
decree or order of any court, arbitrator or other governmental authority or (iii) is or has been in violation of any statute, rule,
ordinance or regulation of any governmental authority, including all foreign, federal, state and local laws relating to taxes, environmental
protection, occupational health and safety, product quality and safety and employment and labor matters, except in each case as would
not reasonably be expected to result in a Material Adverse Effect.
(m) Environmental
Laws. The Company and its Subsidiaries (i) are in compliance with any and all applicable foreign, federal, state and local laws
and regulations relating to the protection of human health and safety, the environment or hazardous or toxic substances or wastes, pollutants
or contaminants (“Environmental Laws”), (ii) have received all permits, licenses or other approvals required
of them under applicable Environmental Laws to conduct their respective businesses and (iii) are in compliance with all terms and
conditions of any such permit, license or approval, except where, in each of the three foregoing clauses, the failure to so comply would
not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect.
(n) Regulatory
Permits. The Company and its Subsidiaries possess all material certificates, authorizations and permits issued by the appropriate
federal, state, local or foreign
regulatory authorities necessary to conduct their respective businesses as currently conducted, except where the failure to possess such
certificates, authorizations, or permits would not reasonably be expected to have a Material Adverse Effect, and neither the Company nor
any such Subsidiary has received any written notice of proceedings relating to the revocation or modification of any such material certificate,
authorization or permit.
(o) Title
to Assets. The Company and the Subsidiaries have good and marketable title in fee simple to all real property owned by them. The Company
and the Subsidiaries have good and marketable title in all personal property owned by them that is material to the business of the Company
and the Subsidiaries, in each case free and clear of all Liens, except for (i) Liens as do not materially affect the value of such
property and do not materially interfere with the use made and proposed to be made of such property by the Company and the Subsidiaries,
(ii) Liens for the payment of federal, state or other taxes, for which appropriate reserves have been made therefor in accordance
with GAAP and, the payment of which is neither delinquent nor subject to penalties, and (iii) Liens that would not, individually
or in the aggregate, have or reasonably be expected to have a Material Adverse Effect. Any real property and facilities held under lease
by the Company and the Subsidiaries are held by them under valid, subsisting and enforceable leases with which the Company or the Subsidiaries,
as the case may be, are in compliance in all material respects.
(p) Intellectual
Property. The Company and its Subsidiaries own or possess adequate rights or licenses to use all trademarks, trade names, service
marks, service mark registrations, service names, patents, patent rights, copyrights, inventions, licenses, approvals, governmental authorizations,
trade secrets and other intellectual property rights (the “Intellectual Property”) necessary to conduct their
respective businesses as now conducted or as proposed to be conducted (other than the Pending Patents), except as such failure to own,
possess or acquire such rights would not reasonably be expected, individually or in the aggregate, to result in a Material Adverse Effect.
The Company and its Subsidiaries have applied for all patents necessary to conduct its business as proposed to be conducted (the “Pending
Patents”), except where the failure to acquire such patents would not reasonably be expected, individually or in the aggregate,
to result in a Material Adverse Effect. None of the Company’s Intellectual Property rights have expired or terminated, or, by the
terms and conditions thereof, could expire or terminate within two years from the date of this Agreement, except as would not reasonably
be expected, individually or in the aggregate, to have a Material Adverse Effect. The Company and its Subsidiaries do not have any knowledge
of any infringement by the Company or its Subsidiaries of any trademark, trade name rights, patents, patent rights, copyrights, inventions,
licenses, service names, service marks, service mark registrations, trade secret or other similar rights of others, or of any such development
of similar or identical trade secrets or technical information by others, and there is no claim, action or proceeding that has been brought
against, or to the Company’s knowledge, being threatened against, the Company or its Subsidiaries regarding trademark, trade name,
patents, patent rights, invention, copyright, license, service names, service marks, service mark registrations, trade secret or other
infringement, which would reasonably be expected to have a Material Adverse Effect. The Company and its Subsidiaries have taken reasonable
security measures to protect the secrecy and confidentiality of the Intellectual Property (excluding any patents or patent applications
that have or will become public) except where failure to do so would not reasonably be expected to have a Material Adverse Effect. Furthermore,
(i) no present or former employee, officer, or director of the Company, or agent or outside contractor or consultant of the Company,
holds any right, title or interest, directly or indirectly, in whole or in part, in or to any Intellectual Property owned, purported to
be owned, or licensed by the Company; (ii) each Company employee involved with the development of Intellectual Property has entered
into an invention assignment agreement with the Company and (iii) no employee of the Company has misappropriated any trade secrets
or other confidential information of any other person in the course of the performance of his or her duties as an employee of the Company.
(q) Insurance.
The Company and the Subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such
amounts as are prudent and customary for companies of the Company’s size and in the businesses in which the Company and the Subsidiaries
are engaged, including directors and officers insurance coverage. Neither the Company nor any Subsidiary has any reason to believe that
it will not be able to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar
insurers as may be necessary to continue its business without a significant increase in cost.
(r) Transactions
with Affiliates and Employees. To the Company’s knowledge, none of the Company’s stockholders, officers or directors or
any family member or affiliate of any of the foregoing, has either directly or indirectly an interest in, or is a party to, any transaction
that would be required to be disclosed as a related party transaction pursuant to Item 404 of Regulation S-K promulgated under the Securities
Act.
(s) Sarbanes-Oxley.
The Company is and has been in material compliance with all provisions of the Sarbanes-Oxley Act of 2002, as amended, applicable to it
and the rules and regulations promulgated in connection therewith.
(t) Certain
Fees. Except for fees payable by the Company to the Placement Agent, no brokerage or finder’s fees or commissions are or will
be payable by the Company or any Subsidiary to any broker, financial advisor or consultant, finder, placement agent, investment banker,
bank or other Person with respect to the transactions contemplated by the Transaction Documents. None of the Purchasers shall have any
obligation with respect to any fees or with respect to any claims made by or on behalf of other Persons for fees of a type contemplated
in this Section that may be due in connection with the transactions contemplated by the Transaction Documents.
(u) Private
Placement. Assuming the accuracy of the Purchasers’ representations and warranties set forth in Section 3.2, no registration
under the Securities Act is required for the offer and sale of the Securities by the Company to the Purchasers as contemplated hereby.
The issuance and sale of the Securities hereunder does not contravene the rules and regulations of the Trading Market.
(v) Investment
Company. The Company is not required to be registered as, and immediately after receipt of payment for the Securities will not be
required to be registered as, an “investment company” within the meaning of the Investment Company Act of 1940, as amended.
(w) Registration
Rights. Other than the Purchasers, no Person has any right to cause the Company or any Subsidiary to effect the registration under
the Securities Act of the offer or sale of any securities of the Company or any Subsidiary.
(x) Listing
and Maintenance Requirements. The Common Stock is registered pursuant to Section 12(b) or 12(g) of the Exchange Act,
and the Company has taken no action designed to, or which to its knowledge is likely to have the effect of, terminating the registration
of the Common Stock under the Exchange Act nor has the Company received any notification that the Commission is contemplating terminating
such registration. The Company has not, in the 12 months preceding the date hereof, received notice from any Trading Market on which the
Common Stock is or has been listed, quoted to the effect that the Company is not in compliance with the listing or maintenance requirements
of such Trading Market or other exchange or market. The Company is, and has no reason to believe that it will not in the foreseeable future
continue to be, in compliance with the listing and maintenance requirements of the Trading Market or any other exchange or market on which
the Common Stock is or has been listed or quoted (other than the potential of failing to satisfy the minimum bid price requirement). The
Common Stock is currently eligible for electronic transfer through the Depository Trust Company or another established clearing corporation
and the Company is current in payment of the fees to the Depository Trust Company (or such other established clearing corporation) in
connection with such electronic transfer.
(y) Application
of Takeover Protections. The Company and the Board of Directors have taken all necessary action, if any, in order to render inapplicable
any control share acquisition, business combination, poison pill (including any distribution under a rights agreement) or other similar
anti-takeover provision under the Company’s certificate of incorporation (or similar charter documents) or the laws of its state
of incorporation that is or could become applicable to any Purchaser as a result of such Purchaser and the Company fulfilling their respective
obligations or exercising their respective rights under the Transaction Documents, including as a result of the Company’s issuance
of the Securities and the Purchaser’s ownership of the Securities.
(z) Disclosure.
The written materials delivered to the Purchasers in connection with the transactions contemplated by the Transaction Documents, when
considered together with the SEC filings, do not contain any untrue statement of a material fact or omit to state a material fact necessary
in order to make the statements contained therein, in light of the circumstances under which they were made, not misleading. The Company
understands and confirms that the Purchasers will rely on the foregoing representations in effecting transactions in securities of the
Company.
(aa) No
Integrated Offering. Assuming the accuracy of the Purchasers’ representations and warranties set forth in Section 3.2,
neither the Company, nor any of its Affiliates, nor, to the Company’s knowledge, any Person acting on its or their behalf has,
directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that
would cause the offering of the Securities contemplated hereunder to be integrated with prior offerings by the Company for purposes of
(i) the Securities Act which would require the registration of the offer or sale of any such securities under the Securities Act,
or (ii) any applicable stockholder approval provisions of any Trading Market on which any of the securities of the Company are listed,
quoted or designated for trading.
(bb) Solvency.
Based on the consolidated financial condition of the Company as of the Closing Date, after giving effect to the receipt by the Company
of the proceeds from the sale of the Securities hereunder, the fair saleable value of the Company’s assets exceeds the amount that
will be required to be paid on or in respect of the Company’s existing debts and other liabilities (including known contingent liabilities)
as they mature. The Company does not intend to incur debts beyond its ability to pay such debts as they mature (taking into account the
timing and amounts of cash to be payable on or in respect of its debt). The Company has no knowledge of any facts or circumstances which
lead it to believe that it will file for reorganization or liquidation under the bankruptcy or reorganization laws of any jurisdiction
within one year from the Closing Date. The SEC Reports set forth as of the date hereof all outstanding secured and unsecured Indebtedness
of the Company or any Subsidiary, or for which the Company or any Subsidiary has commitments. For the purposes of this Agreement, “Indebtedness”
means (x) any liabilities for borrowed money or amounts owed (other than trade accounts payable incurred in the ordinary course of
business), (y) all guaranties, endorsements and other contingent obligations in respect of indebtedness of others, whether or not
the same are or should be reflected in the Company’s consolidated balance sheet (or the notes thereto), except guaranties by endorsement
of negotiable instruments for deposit or collection or similar transactions in the ordinary course of business; and (z) the present
value of any lease payments due under leases required to be capitalized in accordance with GAAP and disclosed or required to be disclosed
in the SEC Reports filed as of the date hereof. Neither the Company nor any Subsidiary is in default with respect to any Indebtedness.
(cc) Tax
Status. Except for matters that would not, individually or in the aggregate, have or reasonably be expected to result in a Material
Adverse Effect, the Company and its Subsidiaries each (i) has made or filed all required United States federal, state and local income
and all foreign income and franchise tax returns, reports and declarations required by any jurisdiction to which it is subject, (ii) has
paid all taxes and other governmental assessments and charges that are material in amount, shown or determined to be due on such returns,
reports and declarations, except those being contested in good faith, with respect to which adequate reserves in accordance with GAAP
have been set aside on the books of the Company, and (iii) has set aside on its books provision reasonably adequate for the payment
of all material taxes for periods subsequent to the periods to which such returns, reports or declarations apply. There are no unpaid
taxes in any material amount claimed to be due by the taxing authority of any jurisdiction, and the officers of the Company or of any
Subsidiary know of no basis for any such claim. The Company is classified as a Subchapter C corporation for U.S. federal tax purposes.
(dd) No
General Solicitation. Neither the Company, nor any of its Affiliates nor any Person acting on behalf of the Company has offered or
sold any of the Securities by any form of general solicitation or general advertising. The Company has offered the Securities for sale
only to the Purchasers and certain other “accredited investors” within the meaning of Rule 501 under the Securities Act.
(ee) Foreign
Corrupt Practices. Neither the Company nor any Subsidiary nor, to the knowledge of the Company, any agent or other person acting on
behalf of the Company or any Subsidiary, has (i) directly or indirectly, used any funds for unlawful contributions, gifts, entertainment
or other unlawful expenses related to foreign or domestic political activity, (ii) made any unlawful payment to foreign or domestic
government officials or employees or to any foreign or domestic political parties or campaigns from corporate funds, (iii) failed
to disclose fully any contribution made by the Company or any Subsidiary (or made by any person acting on its behalf of which the Company
is aware) which is in violation of law or (iv) violated in any material respect any provision of FCPA.
(ff) Accountants.
The Company’s accounting firm is set forth in the SEC Reports. To the knowledge and belief of the Company, such accounting firm
(i) is a registered public accounting firm as required by the Exchange Act and (ii) shall express its opinion with respect to
the financial statements to be included in the Company’s Annual Report for the fiscal year ending December 31, 2023.
(gg) Acknowledgment
Regarding Purchasers’ Purchase of Securities. The Company acknowledges and agrees that each Purchaser is acting solely in the
capacity of an arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated thereby. The Company
further acknowledges that no Purchaser is acting as a financial advisor or fiduciary of the Company (or in any similar capacity) with
respect to the Transaction Documents and the transactions contemplated thereby and any advice given by any Purchaser or any of its respective
representatives or agents in connection with the Transaction Documents and the transactions contemplated thereby is merely incidental
to the Purchasers’ purchase of the Securities. The Company further represents to each Purchaser that the Company’s decision
to enter into this Agreement and the other Transaction Documents has been based solely on the independent evaluation of the transactions
contemplated hereby by the Company and its representatives.
(hh) Regulation
M Compliance. The Company, and to its knowledge anyone acting on its behalf (i) has not taken and will not take, directly or
indirectly, any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company
to facilitate the sale or resale of any of the Securities, (ii) has not sold, bid for, purchased, or paid, and will not sell, bid
for, purchase or pay any compensation for soliciting purchases of, any of the Securities, and (iii) has not paid or agreed to pay,
and will not pay or agree to pay to any Person any compensation for soliciting another to purchase any other securities of the Company.
(ii) Tests
and Preclinical and Clinical Trials. The studies, tests and preclinical and clinical trials conducted by or on behalf of the Company
that are described in, or the results of which are referred to in, the Company’s SEC Reports were and, if still pending, are being,
conducted in all material respects in compliance with the protocols, procedures and controls submitted to the U.S. Food and Drug Administration
(the “FDA”) or any foreign governmental body exercising comparable authority, procedures and controls pursuant
to, where applicable, accepted professional and scientific standards, and all applicable laws and regulations; the descriptions of the
studies, tests and preclinical and clinical trials conducted by or, to the knowledge of the Company, on behalf of the Company, and the
results thereof, contained in the SEC Reports are accurate and complete in all material respects; the Company is not aware of any other
studies, tests or preclinical and clinical trials, the results of which call into question the results described in the SEC Reports; and
the Company has not received any notices or correspondence from the FDA, any foreign, state or local governmental body exercising comparable
authority or any Institutional Review Board requiring the termination, suspension, material modification or clinical hold of any studies,
tests or preclinical or clinical trials conducted by or on behalf of the Company. Each description of such studies, tests and preclinical
and clinical trials and the results thereof that are described in the SEC Reports is complete in all material respects and fairly presents
the data derived from such studies, tests and preclinical and clinical trials. The Company has made all such filings and obtained all
such approvals as may be required by the FDA or the U.S. Department of Health and Human Services or any committee thereof, from any other
U.S. or non-U.S. government or drug or medical device regulatory agency, or health care facility Institutional Review Board, or from any
other regulatory authority.
(jj) Stock
Option Plans. Each stock option granted by the Company under the Company’s stock option plan or other equity plan was granted
(i) in accordance with the terms of the Company’s stock option plan or other equity plan and (ii) with an exercise price
at least equal to the fair market value of the Common Stock on the date such stock option would be considered granted under GAAP and applicable
law. No stock option granted under the Company’s stock option plan or other equity plan has been backdated. The Company has not
knowingly granted, and there is no and has been no Company policy or practice to knowingly grant, stock options prior to, or otherwise
knowingly coordinate the grant of stock options with, the release or other public announcement of material information regarding the Company
or its Subsidiaries or their financial results or prospects.
(kk) Cybersecurity.
(i) To the Company’s knowledge, there has been no security breach or other compromise of or relating to any of the Company’s
or any Subsidiary’s information technology and computer systems, networks, hardware, software, data (including the data of their
respective customers, employees, suppliers, vendors and any third party data maintained by or on behalf of them), equipment or technology
(collectively, “IT Systems and Data”) and (ii) the Company and the Subsidiaries have not been notified
of, and have no knowledge of any event or condition that would reasonably be expected to result in, any security breach or other compromise
to their IT Systems and Data. The Company and the Subsidiaries are presently in compliance with all applicable laws or statutes and all
judgments, orders, rules and regulations of any court or arbitrator or governmental or regulatory authority, internal policies and
contractual obligations relating to the privacy and security of IT Systems and Data and to the protection of such IT Systems and Data
from unauthorized use, access, misappropriation or modification, except as would not, individually or in the aggregate, have a Material
Adverse Effect. The Company and the Subsidiaries have implemented and maintained commercially reasonable safeguards to maintain and protect
their material confidential information and the integrity, continuous operation, redundancy and security of all IT Systems and Data. The
Company and the Subsidiaries have implemented backup and recovery technology consistent with industry standards and practices. The Company’s
IT Systems and Data are sufficient for the conduct of the Company’s business in all material respects as now conducted.
(ll)
Office of Foreign Assets Control. Neither the Company nor any Subsidiary nor, to the
Company’s knowledge, any director, officer, agent, employee or affiliate of the Company or any Subsidiary is currently subject
to any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S. Treasury Department
(“OFAC”).
(mm) U.S.
Real Property Holding Corporation. The Company is not and has never been a U.S. real property holding corporation within the meaning
of Section 897 of the Internal Revenue Code of 1986, as amended, and the Company shall so certify upon Purchaser’s request.
(nn) Bank
Holding Company Act. Neither the Company nor any of its Subsidiaries or Affiliates is subject to the Bank Holding Company Act of
1956, as amended (the “BHCA”) or to regulation by the Board of Governors of the Federal Reserve System (the
“Federal Reserve”). Neither the Company nor any of its Subsidiaries or Affiliates owns or controls, directly
or indirectly, five percent (5%) or more of the outstanding shares of any class of voting securities or twenty-five percent (25%) or
more of the total equity of a bank or any entity that is subject to the BHCA and to regulation by the Federal Reserve. Neither the Company
nor any of its Subsidiaries or Affiliates exercises a controlling influence over the management or policies of a bank or any entity that
is subject to the BHCA and to regulation by the Federal Reserve.
(oo) Money
Laundering. The operations of the Company and its Subsidiaries are and have been conducted at all times in compliance with applicable
financial record-keeping and reporting requirements of the Currency and Foreign Transactions Reporting Act of 1970, as amended, applicable
money laundering statutes and applicable rules and regulations thereunder (collectively, the “Money Laundering Laws”),
and no Action or Proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company or
any Subsidiary with respect to the Money Laundering Laws is pending or threatened in writing or (to the knowledge of the Company or any
Subsidiary) otherwise.
(pp) Shell
Company Status. The Company is not, and has never been, an issuer identified in Rule 144(i)(1).
3.2 Representations
and Warranties of the Purchasers. Each Purchaser, for itself and for no other Purchaser, hereby represents and warrants as of the
date hereof and as of the Closing Date to the Company as follows (except in the case of representations and warranties that speak as of
a specific date, in which case such Purchaser represents and warrants as of such date):
(a) Organization;
Authority. Such Purchaser is either an individual or an entity duly incorporated or formed, validly existing and in good standing
under the laws of the jurisdiction of its incorporation or formation with full right, corporate, partnership, limited liability company
or similar power and authority to enter into and to consummate the transactions contemplated by the Transaction Documents and otherwise
to carry out its obligations hereunder and thereunder. The execution and delivery of the Transaction Documents and performance by such
Purchaser of the transactions contemplated by the Transaction Documents have been duly authorized by all necessary corporate, partnership,
limited liability company or similar action, as applicable, on the part of such Purchaser. Each Transaction Document to which it is a
party has been duly executed by such Purchaser, and when delivered by such Purchaser in accordance with the terms hereof, will constitute
the valid and legally binding obligation of such Purchaser, enforceable against it in accordance with its terms, except (i) as limited
by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application
affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance,
injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be limited by
applicable law.
(b) Understandings
or Arrangements. Such Purchaser is acquiring the Securities as principal for its own account and has no direct or indirect arrangement
or understandings with any other persons to distribute or regarding the distribution of such Securities or any part thereof in violation
of the Securities Act or any applicable securities laws (this representation and warranty not limiting such Purchaser’s right to
sell the Securities pursuant to the Registration Statement or otherwise in compliance with applicable federal and state securities laws).
Such Purchaser is acquiring the Securities hereunder in the ordinary course of its business.
(c) Own
Account. Such Purchaser understands that the Securities are “restricted securities” and the offer and sale thereof have
not been registered under the Securities Act or any applicable state securities law and is acquiring the Securities as principal for its
own account and not with a view to or for distributing or reselling such Securities or any part thereof in violation of the Securities
Act or any applicable state securities law, has no present intention of distributing any of such Securities in violation of the Securities
Act or any applicable state securities law and has no direct or indirect arrangement or understandings with any other persons to distribute
or regarding the distribution of such Securities in violation of the Securities Act or any applicable state securities law (this representation
and warranty not limiting such Purchaser’s right to sell the Securities pursuant to the Registration Statement or otherwise in compliance
with applicable federal and state securities laws). Such Purchaser has independently made its own analysis and decision to invest in the
Securities. Nothing contained herein shall constitute a representation or warranty by such Purchaser to hold the Securities for any period
of time.
(d) Purchaser
Status. At the time such Purchaser was offered the Shares or Pre-Funded Warrants, it was, and
as of the date hereof it is, and on each date on which it exercises any Pre-Funded Warrants, it will be an “accredited investor”
as defined in Rule 501(a)(1), (a)(2), (a)(3), (a)(7), (a)(8), (a)(9) or (a)(12) under the Securities Act.
(e) Experience
of Such Purchaser. Such Purchaser, either alone or together with its representatives, has such knowledge, sophistication and experience
in business and financial matters so as to be capable of evaluating the merits and risks of the prospective investment in the Securities,
and has so evaluated the merits and risks of such investment. Such Purchaser is able to bear the economic risk of an investment in the
Securities and, at the present time, is able to afford a complete loss of such investment.
(f) General
Solicitation. Such Purchaser is not, to such Purchaser’s knowledge, purchasing the Securities as a result of any advertisement,
article, notice or other communication regarding the Securities published in any newspaper, magazine or similar media or broadcast over
television or radio or presented at any seminar or, to the knowledge of such Purchaser, any other general solicitation or general advertisement
as such terms are used in Regulation D of the Securities Act.
(g) Access
to Information. Such Purchaser acknowledges that it has had the opportunity to review the Transaction Documents (including all exhibits
and schedules thereto) and the SEC Reports and has been afforded (i) the opportunity to ask such questions as it has deemed necessary
of, and to receive answers from, representatives of the Company concerning the terms and conditions of the offering of the Securities
and the merits and risks of investing in the Securities; (ii) access to information about the Company and its financial condition,
results of operations, business, properties, management and prospects sufficient to enable it to evaluate its investment; and (iii) the
opportunity to obtain such additional information that the Company possesses or can acquire without unreasonable effort or expense that
is necessary to make an informed investment decision with respect to the investment. Such Purchaser acknowledges and agrees that neither
the Placement Agent nor any Affiliate of the Placement Agent has provided such Purchaser with any information or advice with respect to
the Securities nor is such information or advice necessary or desired. Neither the Placement Agent nor any Affiliate has made or makes
any representation as to the Company or the quality of the Securities and the Placement Agent and any Affiliate may have acquired non-material,
non-public information with respect to the Company which such Purchaser agrees need not be provided to it. In connection with the issuance
of the Securities to such Purchaser, neither the Placement Agent nor any of its Affiliates has acted as a financial advisor or fiduciary
to such Purchaser. Each Purchaser acknowledges that it is not relying upon, and has not relied upon, any statement, representation or
warranty made by the Placement Agent or any of its Affiliates or any of its or their control persons, officers, directors and employees,
in making its investment or decision to invest in the Company. Each Purchaser agrees that, except to the extent that any waiver, or disclaimer
of any claim, may not be waived under applicable law, none of the Placement Agent, its affiliates or any of its or their control persons,
officers, directors or employees, shall be liable to any Purchaser for any action heretofore or hereafter taken or omitted to be taken
in good faith by any of them in connection with any Purchaser’s purchase of the Securities, unless such action or omission results
from such party’s gross negligence, willful misconduct or bad faith.
(h) Certain
Transactions and Confidentiality. Other than consummating the transactions contemplated hereunder, such Purchaser has not, nor has
any Person acting on behalf of or pursuant to any understanding with such Purchaser, directly or indirectly, executed any purchases or
sales, including Short Sales, of the securities of the Company during the period commencing as of the time that such Purchaser first received
a term sheet (written or oral) from the Company or any other Person representing the Company setting forth the material terms of the transactions
contemplated hereunder and ending immediately prior to the execution hereof. Notwithstanding the foregoing, in the case of a Purchaser
that is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of such Purchaser’s assets
and the portfolio managers have no direct knowledge of the investment decisions made by the portfolio managers managing other portions
of such Purchaser’s assets, the representation set forth above shall only apply with respect to the portion of assets managed by
the portfolio manager that made the investment decision to purchase the Securities covered by this Agreement. Other than to other Persons
party to this Agreement or to such Purchaser’s representatives, including its officers, directors, partners, legal and other advisors,
employees, and agents and Affiliates,
such Purchaser has maintained the confidentiality of all disclosures made to it in connection with this transaction (including the existence
and terms of this transaction).
(i) Residency.
Such Purchaser’s residence (if an individual) or offices in which its investment decision with respect to the Securities was made
(if an entity) are located at the address immediately below such Purchaser’s name on its signature page hereto, except as otherwise
communicated by such Purchaser to the Company.
(j) Bad
Actor Status. If the Purchaser is a person listed in the first paragraph of Rule 506(d)(1) with respect to the Company as
an “issuer” for purposes of Rule 506 promulgated under the Securities Act, such Purchaser represents that such Purchaser
is not a person of the type described in Section 506(d) of Regulation D under the Securities Act that would disqualify the Company
from engaging in a transaction pursuant to Section 506 of Regulation D under the Securities Act.
The Company acknowledges and
agrees that the representations contained in this Section 3.2 shall not modify, amend or affect such Purchaser’s right to rely
on the Company’s representations and warranties contained in this Agreement or any representations and warranties contained in any
other Transaction Document or any other document or instrument executed and/or delivered in connection with this Agreement or the consummation
of the transactions contemplated hereby.
Article IV
OTHER
AGREEMENTS OF THE PARTIES
4.1 Transfer
Restrictions.
(a) The
Securities may only be disposed of in compliance with state and federal securities laws. In connection with any transfer of Securities
other than pursuant to an effective registration statement or Rule 144, to the Company or to an Affiliate of a Purchaser, the Company
may require the transferor thereof to provide to the Company customary representations and other documentation (which shall not include
a legal opinion) , the form and substance of which shall be reasonably satisfactory to the Company, to enable the Company and its counsel
to conclude that such transfer does not require registration of such transferred Securities under the Securities Act. As a condition of
transfer, any such transferee shall agree in writing to be bound by the terms of this Agreement and the Registration Rights Agreement
and shall have the rights and obligations of a Purchaser under this Agreement and the Registration Rights Agreement.
(b) The
Purchasers agree to the imprinting, so long as is required by this Section 4.1, of a legend on any of the Securities in the following
form:
THE OFFER
AND SALE OF The securities represented hereby have not been registered with the Securities and Exchange Commission or the securities commission
of any state in reliance upon an exemption from registration under the Securities Act of 1933, as amended (TOGETHER WITH THE RULES AND
REGULATIONS THEREUNDER, THE “SECURITIES ACT”), and, accordingly, may not be transferred unless (i) THE OFFER AND SALE
OF such securities have been registered pursuant to the Securities Act, (ii) such securities may be sold pursuant to Rule 144
OR OTHER APPLICABLE EXEMPTION FROM THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT, or (iii) the Company has received an opinion
of ITS counsel that such transfer may lawfully be made without registration under the Securities..
(c) In
connection with any sale or disposition of the Securities by a Purchaser pursuant to Rule 144 or pursuant to any other exemption
under the Securities Act such that the purchaser acquires freely tradable shares and upon compliance by the Purchaser with the requirements
of this Agreement, if requested by the Purchaser, the Company shall cause the Transfer Agent to timely remove any restrictive legends
related to the book entry account holding such Securities and make a new, unlegended entry for such book entry Securities sold or disposed
of without restrictive legends, provided that the Company has received customary representations and other documentation (which shall
not include a legal opinion) reasonably acceptable to the Company in connection therewith. Subject to receipt by the Company of customary
representations and other documentation (which shall not include a legal opinion) reasonably acceptable to the Company in connection therewith,
upon the earlier of such time as the Securities (i) have been sold or transferred pursuant to an effective registration statement,
(ii) such time as the Securities have been sold pursuant to Rule 144, (iii) are eligible for resale pursuant to an effective
registration statement or (iv) are eligible for resale under Rule 144(b)(1) or any successor provision, the Company shall
(A) deliver to the Transfer Agent irrevocable instructions that the Transfer Agent shall make a new, unlegended entry for such book
entry Securities, and (B) cause its counsel to deliver to the Transfer Agent one or more opinions to the effect that the removal
of such legends in such circumstances may be effected under the Securities Act. The Company shall be responsible for the fees of its Transfer
Agent and all DTC fees associated with such issuance.
(d) Each
Purchaser, severally and not jointly with the other Purchasers, agrees with the Company that such Purchaser will sell any Securities pursuant
to either the registration requirements of the Securities Act, including any applicable prospectus delivery requirements, or an exemption
therefrom, and that if Securities are sold pursuant to a Registration Statement, they will be sold in compliance with the plan of distribution
set forth therein, and acknowledges that the removal of the restrictive legend from certificates representing Securities as set forth
in this Section 4.1 is predicated upon the Company’s reliance upon this understanding.
4.2 Furnishing
of Information; Public Information.
(a) Until
the time that no Purchaser owns Securities, the Company covenants to maintain the registration of the Common Stock under Section 12(b) or
12(g) of the Exchange Act.
4.3 Integration.
The Company shall not sell, offer for sale or solicit offers to buy or otherwise negotiate in respect of any security (as defined in Section 2
of the Securities Act) that would be integrated with the offer or sale of the Securities in a manner that would require the registration
under the Securities Act of the sale of the Securities or that would be integrated with the offer or sale of the Securities for purposes
of the rules and regulations of any Trading Market such that it would require stockholder approval prior to the closing of such other
transaction unless stockholder approval is obtained before the closing of such subsequent transaction.
4.4 Securities
Laws Disclosure; Publicity. The Company shall (a) by the Disclosure Time, issue one or more press releases disclosing the material
terms of the transactions contemplated hereby and all other material non-public information provided to the Purchasers, including any
Subsequent Event that is required to be disclosed by the Company under applicable securities laws as of the date hereof but has not been
publicly disclosed, and (b) file a Current Report on Form 8-K, including the Transaction Documents as exhibits thereto, with
the Commission within the time required by the Exchange Act. The Company covenants to the Purchasers that, from and after the issuance
of such press release, the Company represents to the Purchasers that it shall have publicly disclosed all material, non-public information
delivered to any of the Purchasers (by the Company or any of its Subsidiaries, or any of their respective officers, directors, employees,
Affiliates or agents (including the Placement Agents), or anyone acting on behalf of the foregoing, in connection with the transactions
contemplated by the Transaction Documents). In addition, other than with respect to any Purchaser and the Placement Agent that has executed
a confidentiality or non-disclosure agreement with the Company, effective upon the earlier of (i) the earlier of the issuance of
such press release or the filing of such Form 8-K and (ii) the Disclosure Time, the Company acknowledges and agrees that any
and all confidentiality or similar obligations under any agreement, whether written or oral, between the Company, any of its Subsidiaries
or any of their respective officers, directors, agents, employees, Affiliates or agents on the one hand, and any of the Purchasers or
any of their respective officers, directors, agents, employees, investment advisers or Affiliates on the other hand, shall terminate and
be of no further force or effect. The Company understands and confirms that each Purchaser shall be relying on the foregoing covenant
in effecting transactions in securities of the Company. The Company and each Purchaser shall consult with each other in issuing any other
press releases with respect to the transactions contemplated hereby, and neither the Company nor any Purchaser shall issue any such press
release nor otherwise make any such public statement without the prior consent of the Company or any Purchaser identified therein, with
respect to any press release of any Purchaser, or without the prior consent of each Purchaser, with respect to any press release of the
Company, which consent shall not unreasonably be withheld or delayed, except if such disclosure is required by law, in which case the
disclosing party shall promptly provide the other party (or parties) with prior notice of such public statement or communication. Notwithstanding
the foregoing, the Company shall not publicly disclose the name of any Purchaser, or include the name of any Purchaser in any filing with
the Commission or any regulatory agency or Trading Market, without the prior written consent of such Purchaser, except (a) as required
by federal securities law in connection with (i) any registration statement contemplated by the Registration Rights Agreement and
(ii) the filing of final Transaction Documents with the Commission and (b) to the extent such disclosure is required by law
or Trading Market regulations, in which case the Company shall provide the Purchasers with prior notice of such disclosure permitted under
this clause (b) and reasonably cooperate with such Purchaser regarding such disclosure.
4.5 Stockholder
Rights Plan. The Company does not and as of the Closing Date will not have outstanding stockholder purchase rights or “poison
pill” or any similar arrangement in effect giving any Person the right to purchase any equity interest in the Company upon the occurrence
of certain events.
4.6 Non-Public
Information. Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents,
which shall be disclosed pursuant to Section 4.4, the Company covenants and agrees that neither it, nor any other Person acting on
its behalf will provide any Purchaser or its agents or counsel with any information that constitutes, or the Company reasonably believes
constitutes, material non-public information, unless prior to disclosure of such information the Company identifies such information as
being material nonpublic information and provides the Purchaser, and its advisors and representatives with the opportunity to accept or
refuse to accept such material nonpublic information for review and such Purchaser shall have accepted, and any Purchaser wishing to obtain
such information enters into an appropriate confidentiality agreement with the Company with respect thereto. The Company understands and
confirms that each Purchaser shall be relying on the foregoing covenant in effecting transactions in securities of the Company. To the
extent that the Company delivers any material, non-public information to a Purchaser without such Purchaser’s consent, the Company
hereby covenants and agrees that such Purchaser shall not have any duty of confidentiality to the Company or any of its officers, directors,
agents, employees or Affiliates, or a duty to the Company or any of its officers, directors, agents, employees or Affiliates not to trade
on the basis of such material, non-public information, provided that the Purchaser shall remain subject to applicable law. To the extent
that any notice provided pursuant to any Transaction Document constitutes or contains material, non-public information regarding the Company,
the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K. The Company understands
and confirms that each Purchaser shall be relying on the foregoing covenant in effecting transactions in securities of the Company.
4.7 Use
of Proceeds. The net proceeds of the sale of the Securities hereunder shall be used by the Company for working capital and general
corporate purposes.
4.8 Indemnification
of Purchasers. Subject to the provisions of this Section 4.8, the Company will indemnify and hold each Purchaser and their Affiliates
and each of their respective directors, officers, equity holders (regardless of whether such interests are held directly or indirectly),
members, partners, principals, managers, portfolio managers, investment advisors, employees and agents, attorneys and advisors (and any
other Persons with a functionally equivalent role of a Person holding such titles notwithstanding a lack of such title or any other title),
each Person who controls such Purchaser (within the meaning of Section 15 of the Securities Act and Section 20 of the Exchange
Act), and directors, officers, equity holders (regardless of whether such interests are held directly or indirectly), members, partners,
principals, managers, portfolio managers, investment advisors, employees and agents, attorneys and advisors (and any other Persons with
a functionally equivalent role of a Person holding such titles notwithstanding a lack of such title or any other title) of such controlling
persons (each, a “Purchaser Party”) harmless, to the fullest extent permitted by applicable law, from and against
any and all losses, liabilities, obligations, claims, contingencies, damages, costs, actions, suits, proceedings, investigations, inquiries,
and expenses, including all judgments, amounts paid in settlements, court costs, reasonable attorneys’ fees and other documented
out of pocket expenses and costs of investigation (collectively, “Losses”) that any such Purchaser Party may
suffer or incur as a result of, arising out of, or relating to (a) any breach of any of the representations, warranties, covenants
or agreements made by the Company in this Agreement or in the other Transaction Documents, (b) any action instituted against the
Purchaser Parties in any capacity, or any of them or their respective Affiliates, by any stockholder of the Company who is not an Affiliate
of such Purchaser Party, with respect to any of the transactions contemplated by the Transaction Documents (unless such action is solely
based upon a material breach of such Purchaser Party’s representations, warranties or covenants under the Transaction Documents
or any agreements or understandings such Purchaser Party may have with any such stockholder or any violations by such Purchaser Party
of state or federal securities laws or any conduct by such Purchaser Party which is finally judicially determined to constitute fraud,
gross negligence or willful misconduct), (c) any untrue or alleged untrue statement of a material fact contained in such registration
statement, any prospectus or any form of prospectus or in any amendment or supplement thereto or in any preliminary prospectus, or arising
out of or relating to any omission or alleged omission of a material fact required to be stated therein or necessary to make the statements
therein (in the case of any prospectus or supplement thereto, in the light of the circumstances under which they were made) not misleading,
except to the extent, but only to the extent, that such untrue statements or omissions are based solely upon information regarding such
Purchaser Party furnished in writing to the Company by such Purchaser Party expressly for use therein, or (d) any violation or alleged
violation by the Company of the Securities Act, the Exchange Act or any state securities law, or any rule or regulation thereunder
in connection therewith, and the Company shall promptly reimburse the Purchaser Party for any legal or other documented expenses reasonably
incurred in investigating, defending, preparing to defend, providing evidence in, preparing to serve or serving as a witness with respect
to, settling, compromising or paying any such Loss in connection with any such action, proceeding or claim. If any action shall be brought
against any Purchaser Party in respect of which indemnity may be sought pursuant to this Agreement, such Purchaser Party shall promptly
notify the Company in writing, and, the Company shall have the right to assume the defense thereof with counsel of its own choosing reasonably
acceptable to the Purchaser Party. Any Purchaser Party shall have the right to employ separate counsel in any such action and participate
in the defense thereof, but the fees and expenses of such counsel shall be at the expense of such Purchaser Party except to the extent
that (i) the employment thereof has been specifically authorized by the Company in writing, (ii) the Company has failed after
a reasonable period of time to assume such defense and to employ counsel or (iii) in such action there is, in the reasonable opinion
of counsel a material conflict on any material issue between the position of the Company and the position of such Purchaser Party, in
which case the Company shall be responsible for the reasonable fees and expenses of no more than one such separate counsel. The Company
will not be liable to any Purchaser Party under this Agreement (i) for any settlement by a Purchaser Party effected without the Company’s
prior written consent, which shall not be unreasonably withheld, conditioned or delayed; or (ii) to the extent, but only to the extent
that a loss, claim, damage or liability is attributable to any Purchaser Party’s breach of any of the representations made by such
Purchaser Party in the Transaction Documents. The Company shall not, without the prior written consent of the Purchaser Party, consent
to entry of any judgment or enter into any settlement or other compromise unless such judgement, settlement or compromise (i) includes
as an unconditional term thereof the claimant or plaintiff providing to such Purchaser Party a release from all liability in respect to
such action, claim or proceeding, (ii) imposes no obligation or liability on the Purchaser Party, and (iii) does not include
any admission as to fault, culpability, wrongdoing or malfeasance on the part of the Purchaser Party. The indemnification required by
this Section 4.8 shall be made by periodic payments of the amount thereof during the course of the investigation or defense, as and
when bills are received or are incurred. The indemnity agreements contained herein shall be in addition to any cause of action or similar
right of any Purchaser Party against the Company or others and any liabilities the Company may be subject to pursuant to law.
4.9 Reservation
of Common Stock. As of the date hereof, the Company has reserved and the Company shall continue to reserve and keep available at all
times, free of preemptive rights, a sufficient number of shares of Common Stock for the purpose of enabling the Company to issue Shares
pursuant to this Agreement and Pre-Funded Warrant Shares pursuant to any exercise of the Pre-Funded Warrants.
4.10 Listing
of Common Stock. The Company hereby agrees to use reasonable best efforts to maintain the listing or quotation of the Common Stock
on the Trading Market on which it is currently listed, and concurrently with the Closing, the Company shall apply to list or quote all
of the Shares and Pre-Funded Warrant Shares on such Trading Market and promptly secure the listing of all of the Shares and Pre-Funded
Warrant Shares on such Trading Market. The Company further agrees, if the Company applies to have the Common Stock traded on any other
Trading Market, it will then include in such application all of the Shares and Pre-Funded Warrant Shares, and will take such other action
as is necessary to cause all of the Shares and Pre-Funded Warrant Shares to be listed or quoted on such other Trading Market as promptly
as possible. The Company will then take all action reasonably necessary to continue the listing and trading of its Common Stock on a Trading
Market and will comply in all material respects with the Company’s reporting, filing and other obligations under the bylaws or rules of
the Trading Market. For so long as the Company maintains a listing or quotation of the Common Stock on a Trading Market, the Company agrees
to maintain the eligibility of the Common Stock for electronic transfer through the Depository Trust Company or another established clearing
corporation, including by timely payment of fees to the Depository Trust Company or such other established clearing corporation in connection
with such electronic transfer.
4.11 Certain
Transactions and Confidentiality. Each Purchaser, severally and not jointly with the other Purchasers, covenants that neither it,
nor any Affiliate acting on its behalf or pursuant to any understanding with it will execute any purchases or sales, including Short Sales,
of any of the Company’s securities during the period commencing with the execution of this Agreement and, other than with respect
to any Purchaser that has executed a confidentiality or non-disclosure agreement with the Company, ending at such time that the transactions
contemplated by this Agreement are first publicly announced pursuant to the initial press release as described in Section 4.4. Each
Purchaser, severally and not jointly with the other Purchasers, covenants that until the earlier of (i) such time as the transactions
contemplated by this Agreement are publicly disclosed by the Company pursuant to the initial press release as described in Section 4.4
and (ii) the Disclosure Time, such Purchaser will maintain the confidentiality of the existence and terms of this transaction (other
than as disclosed to its legal and other representatives). Notwithstanding the foregoing and notwithstanding anything contained in this
Agreement to the contrary, the Company expressly acknowledges and agrees that (i) no Purchaser makes any representation, warranty
or covenant hereby that it will not engage in effecting transactions in any securities of the Company after the time that the transactions
contemplated by this Agreement are first publicly announced pursuant to the initial press release as described in Section 4.4, (ii) no
Purchaser shall be restricted or prohibited from effecting any transactions in any securities of the Company in accordance with applicable
securities laws from and after the time that the transactions contemplated by this Agreement are first publicly announced pursuant to
the initial press release as described in Section 4.4 and (iii) no Purchaser shall have any duty not to trade in the securities
of the Company to the Company, any of its Subsidiaries, or any of their respective officers, directors, employees, Affiliates or agent,
after the earlier of (x) issuance of the initial press release as described in Section 4.4 and (ii) the Disclosure Time.
Notwithstanding the foregoing, in the case of a Purchaser that is a multi-managed investment vehicle whereby separate portfolio managers
manage separate portions of such Purchaser’s assets, the covenant set forth above shall only apply with respect to the portion of
assets managed by the portfolio manager that made the investment decision to purchase the Securities covered by this Agreement.
4.12 Blue
Sky Filings. The Company shall take such action as the Company shall reasonably determine is necessary in order to obtain an exemption
for the sale of the Securities or to qualify the Securities for sale to the Purchasers at the Closing under applicable securities or “Blue
Sky” laws of the states of the United States, and shall provide evidence of such actions promptly upon request of any Purchaser.
Article V
MISCELLANEOUS
5.1 Fees
and Expenses. Except as expressly set forth in the Transaction Documents to the contrary, each party shall pay the fees and expenses
of its advisers, counsel, accountants and other experts, if any, and all other expenses incurred by such party incident to the negotiation,
preparation, execution, delivery and performance of this Agreement. The Company shall pay all Transfer Agent fees (including any fees
required for same-day processing of any instruction letter delivered by the Company and any exercise notice delivered by a Purchaser),
stamp taxes and other taxes and duties levied in connection with the delivery of any Securities to the Purchasers.
5.2 Entire
Agreement. The Transaction Documents, together with the exhibits and schedules thereto, contain the entire understanding of the parties
with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, oral or written, with respect
to such matters, which the parties acknowledge have been merged into such documents, exhibits and schedules.
5.3 Notices.
Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be in writing and shall
be deemed given and effective on the earliest of: (a) the time of transmission, if such notice or communication is delivered via
email attachment at the email address as set forth on the signature pages attached hereto at or prior to 5:30 p.m. (New York
City time) on a Trading Day, with no rejection notice received, (b) the next Trading Day after the date of transmission, if such
notice or communication is delivered via email attachment at the email address as set forth on the signature pages attached hereto
on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, with no rejection notice received,
(c) the second (2nd) Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier
service or (d) upon actual receipt by the party to whom such notice is required to be given. The address for such notices and communications
shall be as set forth on the signature pages attached hereto.
5.4 Amendments;
Waivers. No provision of this Agreement may be waived, modified, supplemented or amended except in a written instrument signed, in
the case of a supplement, amendment or modification, by the Company and Purchasers which purchased at least 50.1% in interest of the Shares
and Pre-Funded Warrants based on the initial Subscription Amounts hereunder (or, prior to the Closing, the Company and each Purchaser)
or, in the case of a waiver, by the party against whom enforcement of any such waived provision is sought, provided that if any
amendment, modification or waiver disproportionately and adversely impacts a Purchaser (or subset of Purchasers), the consent of such
disproportionately impacted Purchaser (or each member of such subset of Purchasers) shall also be required. No waiver of any default with
respect to any provision, condition or requirement of this Agreement shall be deemed to be a continuing waiver in the future or a waiver
of any subsequent default or a waiver of any other provision, condition or requirement hereof, nor shall any delay or omission of any
party to exercise any right hereunder in any manner impair the exercise of any such right. Any proposed amendment or waiver that disproportionately,
materially and adversely affects the rights and obligations of any Purchaser (or subset of Purchasers) relative to the comparable rights
and obligations of the other Purchasers shall require the prior written consent of such adversely affected Purchaser (or each member of
such subset of Purchasers). Any amendment effected in accordance with this Section 5.5 shall be binding upon each Purchaser and holder
of Securities and the Company.
5.5 Headings.
The headings herein are for convenience only, do not constitute a part of this Agreement and shall not be deemed to limit or affect any
of the provisions hereof.
5.6 Successors
and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their successors and permitted assigns.
The Company may not assign this Agreement or any rights or obligations hereunder without the prior written consent of each Purchaser (other
than by merger). Any Purchaser may assign any or all of its rights under this Agreement to any Person to whom such Purchaser assigns or
transfers any Securities, provided that such transferee agrees in writing to be bound, with respect to the transferred Securities,
by the provisions of the Transaction Documents that apply to the “Purchasers.”
5.7 No
Third-Party Beneficiaries. The Placement Agent shall be the third party beneficiary of the representations and warranties of the Company
in Section 3.1 and the representations and warranties of the Purchasers in Section 3.2. This Agreement is intended for the benefit
of the parties hereto and their respective successors and permitted assigns and is not for the benefit of, nor may any provision hereof
be enforced by, any other Person, except as otherwise set forth in Section 4.8 and this Section 5.8.
5.8 Governing
Law. All questions concerning the construction, validity, enforcement and interpretation of the Transaction Documents shall be governed
by and construed and enforced in accordance with the internal laws of the State of New York. Each party agrees that all legal Proceedings
concerning the interpretations, enforcement and defense of the transactions contemplated by this Agreement and any other Transaction Documents
(whether brought against a party hereto or its respective affiliates, directors, officers, stockholders, partners, members, employees
or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York, Borough of Manhattan. Each
party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough
of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed
herein (including with respect to the enforcement of any of the Transaction Documents), and hereby irrevocably waives, and agrees not
to assert in any Action or Proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such Action
or Proceeding is improper or is an inconvenient venue for such Proceeding. Each party hereby irrevocably waives personal service of process
and consents to process being served in any such Action or Proceeding by mailing a copy thereof via registered or certified mail or overnight
delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Agreement and agrees that such
service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit
in any way any right to serve process in any other manner permitted by law. If any party shall commence an Action or Proceeding to enforce
any provisions of the Transaction Documents, then, in addition to the obligations of the Company under Section 4.8, the prevailing
party in such Action or Proceeding shall be reimbursed by the non-prevailing party for its reasonable attorneys’ fees and other
costs and expenses incurred with the investigation, preparation and prosecution of such Action or Proceeding.
5.9 Survival.
The representations and warranties contained herein shall survive the Closing and the delivery of the Securities.
5.10 Execution.
This Agreement may be executed in two or more counterparts, all of which when taken together shall be considered one and the same agreement
and shall become effective when counterparts have been signed by each party and delivered to each other party, it being understood that
the parties need not sign the same counterpart. In the event that any signature is delivered by e-mail delivery of a “.pdf”
format data file, such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature
is executed) with the same force and effect as if such “.pdf” signature page were an original thereof. Counterparts may
be delivered via facsimile, electronic mail (including any electronic signature covered by the U.S. federal ESIGN Act of 2000, Uniform
Electronic Transactions Act, the Electronic Signatures and Records Act or other applicable law, e.g., www.docusign.com) or other transmission
method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
5.11 Severability.
If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, illegal,
void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force
and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their commercially reasonable efforts
to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision,
covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would have executed the remaining
terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or unenforceable.
5.12 Rescission
and Withdrawal Right. Notwithstanding anything to the contrary contained in (and without limiting any similar provisions of) any of
the other Transaction Documents, whenever any Purchaser exercises a right, election, demand or option under a Transaction Document and
the Company does not timely perform its related obligations within the periods therein provided, then such Purchaser may rescind or withdraw,
in its sole discretion from time to time upon written notice to the Company, any relevant notice, demand or election in whole or in part
without prejudice to its future actions and rights.
5.13 Replacement
of Securities. If any certificate or instrument evidencing any Securities is mutilated, lost, stolen or destroyed, the Company shall
issue or cause to be issued in exchange and substitution for and upon cancellation thereof (in the case of mutilation), or in lieu of
and substitution therefor, a new certificate or instrument, but only upon receipt of evidence reasonably satisfactory to the Company of
such loss, theft or destruction. The applicant for a new certificate or instrument under such circumstances shall also pay any reasonable
third-party costs (including customary indemnity) associated with the issuance of such replacement Securities.
5.14 Remedies.
In addition to being entitled to exercise all rights provided herein or granted by law, including recovery of damages, each of the Purchasers
and the Company will be entitled to specific performance and injunctive or other equitable relief under the Transaction Documents, without
the necessity of posting a bond. The parties agree that monetary damages may not be adequate compensation for any loss incurred by reason
of any breach of obligations contained in the Transaction Documents and hereby agree to waive and not to assert in any Action for specific
performance of any such obligation the defense that a remedy at law would be adequate. Except as expressly provided in this Agreement
or the other Transaction Documents, each party hereto agrees that it shall not have a remedy of punitive or consequential damages against
the other and hereby waives any right or claim to punitive or consequential damages it may now have or may arise in the future.
5.15 Payment
Set Aside. To the extent that the Company makes a payment or payments to any Purchaser pursuant to any Transaction Document or a Purchaser
enforces or exercises its rights thereunder, and such payment or payments or the proceeds of such enforcement or exercise or any part
thereof are subsequently invalidated, declared to be fraudulent or preferential, set aside, recovered from, disgorged by or are required
to be refunded, repaid or otherwise restored to the Company, a trustee, receiver or any other Person under any law (including any bankruptcy
law, state or federal law, common law or equitable cause of action), then to the extent of any such restoration the obligation or part
thereof originally intended to be satisfied shall be revived and continued in full force and effect as if such payment had not been made
or such enforcement or setoff had not occurred.
5.16 Independent
Nature of Purchasers’ Obligations and Rights. The obligations of each Purchaser under any Transaction Document are several and
not joint with the obligations of any other Purchaser, and no Purchaser shall be responsible in any way for the performance or non-performance
of the obligations of any other Purchaser under any Transaction Document. Nothing contained herein or in any other Transaction Document,
and no action taken by any Purchaser pursuant hereto or thereto, shall be deemed to constitute the Purchasers as a partnership, an association,
a joint venture or any other kind of entity, or create a presumption that the Purchasers are in any way acting in concert or as a group
(including a “group” within the meaning of Section 13(d)(3) of the Exchange Act) with respect to such obligations
or the transactions contemplated by the Transaction Documents, and the Company acknowledges that, to the knowledge of the Company, the
Purchasers are not acting in concert or as a group. Each Purchaser shall be entitled to independently protect and enforce its rights including
the rights arising out of this Agreement or out of the other Transaction Documents, and it shall not be necessary for any other Purchaser
to be joined as an additional party in any Proceeding for such purpose. Each Purchaser has been represented by its own separate legal
counsel in its review and negotiation of the Transaction Documents. The Company has elected to provide all Purchasers with the same terms
and Transaction Documents for the convenience of the Company and not because it was required or requested to do so by any of the Purchasers.
It is expressly understood and agreed that each provision contained in this Agreement and in each other Transaction Document is between
the Company and a Purchaser, solely, and not between the Company and the Purchasers collectively and not between and among the Purchasers.
5.17 Saturdays,
Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or
granted herein shall not be a Business Day, then such action may be taken or such right may be exercised on the next succeeding Business
Day.
5.18 Construction.
The parties agree that each of them or their respective counsel have reviewed and had an opportunity to revise the Transaction Documents
and, therefore, the normal rule of construction to the effect that any ambiguities are to be resolved against the drafting party
shall not be employed in the interpretation of the Transaction Documents or any amendments thereto. In addition, each and every reference
to share prices and shares of Common Stock in any Transaction Document shall be subject to adjustment for reverse and forward stock splits,
stock dividends, stock combinations and other similar transactions of the Common Stock that occur after the date of this Agreement. Furthermore,
wherever required by the context of this Agreement, the singular shall include the plural and vice versa, and the masculine gender shall
include the feminine and neuter genders and vice versa, and references to any agreement, document or instrument shall be deemed to refer
to such agreement, document or instrument as amended, supplemented or modified from time to time. All article, section, paragraph or clause
references not attributed to a particular document shall be references to such parts of this Agreement, and all exhibit, annex, letter
and schedule references not attributed to a particular document shall be references to such exhibits, annexes, letters and schedules to
this Agreement. In addition, the word “or” is not exclusive; the words “including,” “includes,” “included”
and “include” are deemed to be followed by the words “without limitation”; the terms “herein,” “hereof”
and “hereunder” and other words of similar import refer to this Agreement as a whole and not to any particular section, paragraph
or subdivision; and the phrase “to the knowledge of the Company” are deemed to be followed by the words “following due
inquiry based on the Company’s current controls”.
5.19 WAIVER
OF JURY TRIAL. IN ANY ACTION, SUIT, OR PROCEEDING IN ANY JURISDICTION BROUGHT BY ANY PARTY AGAINST ANY OTHER PARTY, THE PARTIES EACH
KNOWINGLY AND INTENTIONALLY, TO THE GREATEST EXTENT PERMITTED BY APPLICABLE LAW, HEREBY ABSOLUTELY, UNCONDITIONALLY, IRREVOCABLY
AND EXPRESSLY WAIVES FOREVER TRIAL BY JURY.
(Signature Pages Follow)
In
Witness Whereof, the parties hereto have caused this Securities Purchase Agreement to be duly executed by their respective
authorized signatories as of the date first indicated above.
| |
VYNE Therapeutics
Inc. |
| |
|
| |
By: |
|
| |
|
| |
Name: |
|
| |
|
| |
Title: |
|
| |
|
| |
Date: |
|
| |
Email: |
|
| |
|
| |
With a copy to (which shall not constitute notice): |
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK
SIGNATURE PAGE FOR PURCHASER FOLLOWS]
[Signature
Page to VYNE Securities Purchase Agreement]
In
Witness Whereof, the undersigned have caused this Securities Purchase Agreement to be duly executed by their respective authorized
signatories as of the date first indicated above.
| Signature of Authorized Signatory of Purchaser: |
|
| Name of Authorized Signatory: |
|
| Title of Authorized Signatory: |
|
| Email Address of Authorized Signatory: |
|
Address for Notice to Purchaser:
Address for Delivery of Securities to Purchaser (if not same as address
for notice):
Subscription Amount: $_________________
Beneficial Ownership Blocker ¨ 4.99% or ¨ 9.99% or ¨
Other ____________
Shares: _________________
Pre-Funded Warrant Shares: __________________
EIN Number: _______________________
Account Type (options listed below):
¨ Partnership
¨ S-Corporation
¨ Bank
¨ Broker
¨ Nominee
¨ Corporation
¨ Non-Profit Organization
¨ Other: __________
[Signature
Page to VYNE Securities Purchase Agreement]
Exhibit A
Form of Pre-Funded Warrant
Exhibit B
Form of Registration Rights Agreement
Exhibit C
Form of Company Counsel Legal Opinion
Exhibit D
Form of Officer’s Certificate
Exhibit E
Form of Secretary Certificate
Exhibit 10.2
REGISTRATION RIGHTS AGREEMENT
This
Registration Rights Agreement (this “Agreement”) is made and entered into as of October 27,
2023, between VYNE Therapeutics Inc., a Delaware corporation (the “Company”), and each of the several purchasers
signatory hereto (each such purchaser, a “Purchaser” and, collectively, the “Purchasers”).
This Agreement is made pursuant
to the Securities Purchase Agreement, dated as of the date hereof, between the Company and each Purchaser (the “Purchase Agreement”).
The Company and each Purchaser
hereby agrees as follows:
1. Definitions.
Capitalized terms used
and not otherwise defined herein that are defined in the Purchase Agreement shall have the meanings given such terms in the Purchase Agreement.
As used in this Agreement, the following terms shall have the following meanings:
“Advice”
shall have the meaning set forth in Section 7(b).
"Cutback Effectiveness
Date" means the date a Cutback Registration Statement is declared effective by the Commission.
"Cutback Effectiveness
Deadline" means, as to a Cutback Registration Statement, 120 days following the filing of such Cutback Registration Statement.
"Cutback Filing Deadline"
means, if Cutback Shares are required to be included in a Cutback Registration Statement, the date that is the earlier of (i) the
later of (A) six months from the Effectiveness Date or the then-most recent Cutback Effectiveness Date, as applicable, and (B) 60
days after the Company has been informed that substantially all of the Registrable Securities held by the Purchasers included in any Registration
Statements previously declared effective hereunder have been sold in accordance therewith, or (ii) 30 days from the first date on
which the Company is then permitted by the Commission to register such Cutback Shares.
"Cutback Registrable
Securities" means, (i) any Cutback Shares not previously included in a Registration Statement, and (ii) any shares
of capital stock of the Company (or any successor or assign of the Company, whether by merger, reorganization, consolidation, sale of
assets or otherwise) which may be issued or issuable with respect to, in exchange for, or in substitution of the Cutback Shares, as a
result of any stock split, stock dividend, recapitalization, exchange or similar event or otherwise; provided, however, that
any Cutback Registrable Securities shall cease to be Cutback Registrable Securities when (a) a Registration Statement with respect
to the sale of such securities has become effective under the Securities Act and such securities are disposed of in accordance with such
Registration Statement, or (b) such securities are sold in accordance with Rule 144, or (c) all of such securities are
eligible to be sold by the holder thereof pursuant to Rule 144 without limitation, restriction or condition (including any current
public information requirement) thereunder, or (d) when such securities are sold to the Company.
"Cutback Registration
Statement" means a registration statement or registration statements of the Company filed under the Securities Act covering any
Cutback Registrable Securities, including (in each case) the Prospectus, amendments and supplements to any such registration statement
or Prospectus, including pre- and post-effective amendments, all exhibits thereto, and all material incorporated by reference or deemed
to be incorporated by reference in any such registration statement.
"Cutback Required
Registration Amount" means the lesser of (i) any Cutback Shares not previously included in a Registration Statement, and
(ii) such number of Registrable Securities as the Company is then permitted by the Commission to register pursuant to Rule 415.
"Cutback Shares"
means, at any time on or after the Effectiveness Date, any of the Registrable Securities not included in all Registration Statements previously
declared effective hereunder as a result of a limitation on the maximum number of shares of Common Stock permitted by the Commission to
be registered pursuant to Rule 415.
“Effectiveness
Date” means, with respect to the Initial Registration Statement required to be filed hereunder, the 60th
calendar day following the date hereof (or, in the event of a review by the Commission, the 90th
calendar day following the date hereof); provided, however, that in the event the Company is notified by the
Commission that the Initial Registration Statement will not be reviewed or is no longer subject to further review and comments, the Effectiveness
Date as to such Registration Statement shall be the fifth Trading Day following the date on which the Company is so notified if such date
precedes the dates otherwise required above, provided, further, if such Effectiveness Date falls on a day that is not a
Trading Day, then the Effectiveness Date shall be the next succeeding Trading Day; provided, further, that if the Commission is closed
for operations due to a government shutdown or lapse in appropriations, the Effectiveness Date shall be extended by the same number of
days that the Commission remains closed for operations.
“Effectiveness
Period” shall have the meaning set forth in Section 2(a).
“Event”
shall have the meaning set forth in Section 2(c).
“Event Date”
shall have the meaning set forth in Section 2(c).
“Filing Date”
means, with respect to the Initial Registration Statement required hereunder, no later than the 30th calendar day following
the date hereof.
“Holder”
or “Holders” means the holder or holders, as the case may be, from time to time of Registrable Securities.
“Indemnified Party”
shall have the meaning set forth in Section 6(c).
“Indemnifying
Party” shall have the meaning set forth in Section 6(c).
“Initial Registration
Statement” means the initial Registration Statement filed pursuant to this Agreement.
“Losses”
shall have the meaning set forth in Section 6(a).
“Plan of Distribution”
shall have the meaning set forth in Section 6(a).
“Prospectus”
means the prospectus included in a Registration Statement (including a prospectus that includes any information previously omitted from
a prospectus filed as part of an effective registration statement in reliance upon Rule 430A or Rule 430B promulgated by the
Commission pursuant to the Securities Act), as amended or supplemented by any prospectus supplement, with respect to the terms of the
offering of any portion of the Registrable Securities covered by a Registration Statement, and all other amendments and supplements to
the Prospectus, including pre- and post-effective amendments, and all material incorporated by reference or deemed to be incorporated
by reference in such Prospectus.
“Registrable Securities”
means, as of any date of determination, (a) all Shares, (b) all Pre-Funded Warrant Shares then issued and issuable upon exercise
of the Pre-Funded Warrants (assuming on such date the Pre-Funded Warrants are exercised in full without regard to any exercise limitations
in the Transaction Documents), (c) any additional shares of Common Stock issued and issuable in connection with any anti-dilution
provisions in the Pre-Funded Warrants (in each case, without giving effect to any limitations on exercise set forth in the Transaction
Documents), and (d) any securities issued or then issuable upon any stock split, dividend or other distribution, recapitalization
or similar event with respect to the foregoing; provided, however, that any such Registrable Securities shall cease to be
Registrable Securities (and the Company shall not be required to maintain the effectiveness of any, or file another, Registration Statement
hereunder with respect thereto) for so long as (a) a Registration Statement with respect to the sale of such Registrable Securities
is declared effective by the Commission under the Securities Act and such Registrable Securities have been sold or disposed of by the
Holder in accordance with such effective Registration Statement (in which case, only any such security sold or disposed of by the holder
shall cease to be a Registrable Security), (b) such Registrable Securities have been previously sold in accordance with Rule 144
(in which case, only any such security sold or disposed of by the holder shall cease to be a Registrable Security), (c) such securities
become eligible for resale without volume or manner-of-sale restrictions and without the requirement for the Company to be in compliance
with the current public information requirement under Rule 144, (d) such securities shall have ceased to be outstanding, or
(e) such securities have been sold to, or through, a broker, dealer or underwriter in a public distribution or other public securities
transaction.
“Registration
Statement” means any registration statement required to be filed hereunder pursuant to Section 2(a) and any additional
registration statements contemplated by Section 2(b) or Section 4(c), including (in each case) the Prospectus, amendments
and supplements to any such registration statement or Prospectus, including pre- and post-effective amendments, all exhibits thereto,
and all material incorporated by reference or deemed to be incorporated by reference in any such registration statement.
“Rule 415”
means Rule 415 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from
time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect
as such Rule.
“Rule 424”
means Rule 424 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from
time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect
as such Rule.
“Selling Stockholder
Questionnaire” shall have the meaning set forth in Section 4(a).
“SEC Guidance”
means (i) any publicly-available written or oral guidance of the Commission staff, or any comments, requirements or requests of the
Commission staff and (ii) the Securities Act.
“Securities Act”
means the Securities Act of 1933, as amended from time to time.
“Underwriters’
Maximum Number” means, with respect to an underwritten offering, the lesser of (a) the number of Registrable Securities
that can be sold in such offering and (b) the maximum number of Registrable Securities that may be included in such offering without
adversely affecting the price per share of the Company’s equity securities to be sold in such offering.
2. Resale
Registration.
(a) On
or prior to the Filing Date, the Company shall prepare and file with the Commission a Registration Statement covering the resale of all
of the Registrable Securities that are not then registered on an effective Registration Statement for an offering to be made on a continuous
basis pursuant to Rule 415 or, if Rule 415 is not available for offers and sales of the Registrable Securities, by such other
means of distribution of Registrable Securities as the Holders may reasonably specify. Each Registration Statement filed hereunder shall
be on Form S-3, or any successor short form registration statement available or resale that permits importation by reference at least
to the same extent as such form (except if the Company is not then eligible to register the resale of the Registrable Securities on Form S-3,
subject to the provisions of Section 2(d)) and shall contain (unless otherwise directed by at least 85% in interest of the Holders)
substantially the “Plan of Distribution” attached hereto as Annex A and substantially the “Selling
Stockholder” section attached hereto as Annex B; provided, however, that no Holder
shall be required to be named as an “underwriter” without such Holder’s express prior written consent. Subject to the
terms of this Agreement, the Company shall use best efforts to cause a Registration Statement filed under this Agreement (including under
Section 4(c)) to be declared effective under the Securities Act as promptly as possible after the filing thereof, but in any event
no later than the applicable Effectiveness Date (including filing with the Commission a request for acceleration of effectiveness in accordance
with Rule 461), and shall use best efforts to keep such Registration Statement continuously effective under the Securities Act until
the date that all Registrable Securities covered by such Registration Statement (i) have been sold, thereunder or pursuant to Rule 144
or any other rule of similar effect, or (ii) may be sold without volume or manner of sale restrictions pursuant to Rule 144
and without the requirement for the Company to be in compliance with the current public information requirement under Rule 144 (the
“Effectiveness Period”). The Company shall telephonically request effectiveness of a Registration Statement
as of 4:00 p.m. (New York City time) on a Trading Day. The Company shall promptly notify the Holders via e-mail of the effectiveness
of a Registration Statement or any post-effective amendment thereto on the same Trading Day that the Commission confirms effectiveness
with the Company, which shall be the date requested for effectiveness of such Registration Statement. The Company shall, by 9:30 a.m. (New
York City time) on the Trading Day after the effective date of such Registration Statement, file a final Prospectus with the Commission
as required by Rule 424 and provide the Holders with copies of the final Prospectus to be used in connection with the sale or other
disposition of the securities covered thereby (unless such Prospectus is available on the Commission’s EDGAR system).
(b) Notwithstanding
the registration obligations set forth in Section 2(a), if the Commission informs the Company that the resale of all of the Registrable
Securities cannot, as a result of the application of Rule 415, be registered as a secondary offering on a single registration statement,
the Company agrees to promptly inform each of the Holders thereof and shall prepare, and, as soon as reasonably practicable, but in no
event later than each Cutback Filing Deadline, file with the Commission a Cutback Registration Statement on Form S-3, or any successor
short form registration statement available for such resale that permits incorporation by reference at least to the same extent as such
form (except if the Company is not then eligible to register the resale of the Registrable Securities on Form S-3, subject to the
provisions of Section 2(d)) to effect a registration for resale of the Registrable Securities covering the resale of the number of
Cutback Registrable Securities equal to the Cutback Required Registration Amount. To the extent the staff of the Commission does not permit
all of the Cutback Registrable Securities to be registered on a Cutback Registration Statement, the Company shall file Cutback Registration
Statements successively trying to register on each such Cutback Registration Statement the maximum number of remaining Cutback Registrable
Securities until all of the Cutback Registrable Securities have been registered with the Commission. Each Cutback Registration Statement
prepared pursuant hereto shall register for resale at least that number of shares of Common Stock equal to the Cutback Required Registration
Amount as of the date such Cutback Registration Statement is initially filed with the Commission. The Company shall use commercially reasonable
efforts to have each Cutback Registration Statement declared effective by the Commission as soon as reasonably practicable, but in no
event later than the Cutback Effectiveness Deadline and shall use commercially reasonable efforts to keep the Cutback Registration Statement
continuously effective under the Securities Act until such date on which there are no longer any Registrable Securities covered by such
Cutback Registration Statement. Prior to filing the first Cutback Registration Statement, the Company shall be obligated to use commercially
reasonable efforts to advocate with the Commission for the registration of all of the Registrable Securities in accordance with the SEC
Guidance, including Compliance and Disclosure Interpretation 612.09. In the event of a cutback hereunder, unless otherwise directed in
writing by a Holder as to its Registrable Securities, the number of Registrable Securities to be registered on the Initial Registration
Statement and any subsequent Cutback Registration Statement requiring a cutback will be reduced as follows:
a. First,
the Company shall reduce or eliminate any securities to be included other than Registrable Securities;
b. Second,
the Company shall reduce Registrable Securities represented by Shares and Pre-Funded Warrants (applied, in the case that some Shares and
Pre-Funded Warrant Shares may be registered, to the Holders on a pro rata basis based on the total number of such restricted Shares and
Pre-Funded Warrants held by such Holders).
(c) If:
(i) a Registration Statement covering Registrable Securities and required to be filed by the Company pursuant to Section 2(a) or
Section 2(b) of this Agreement is not (a) filed with the Commission on or before the Filing Date or Cutback Filing Deadline,
as applicable, (a "Filing Failure") or (b) declared effective by the Commission on or before the Effectiveness
Date or Cutback Effectiveness Deadline, as applicable, (an "Effectiveness Failure") or (ii) on any day after
a Registration Statement has been declared effective by the Commission, sales of all the Registrable Securities required to be included
on such Registration Statement cannot be made for more than ten consecutive calendar days or more than an aggregate of 15 calendar days
(which need not be consecutive calendar days) during any 12 month period pursuant to such Registration Statement for any reason (including
by reason of a stop order or because of a failure to keep such Registration Statement effective by disclosing such information as is necessary
for sales to be made pursuant to such Registration Statement) (a "Maintenance Failure," and each of a Filing Failure,
an Effectiveness Failure and a Maintenance Failure being referred to as an "Event", and for purposes of clause
(i), the date on which such Event occurs, and for purposes of clause (ii), the date on which such ten or 15 calendar day period, as applicable,
is exceeded, being referred to as “Event Date”), then, in addition to any other rights the Holders may have
hereunder or under applicable law, on each such Event Date and on each monthly anniversary of each such Event Date (pro rata for any portion
thereof) (if the applicable Event shall not have been cured by such date) until the applicable Event is cured, the Company shall pay to
each Holder an amount in cash, as partial liquidated damages and not as a penalty, equal to 1.0% of the aggregate amount invested by such
Holder for the Registrable Securities pursuant to the Purchase Agreement held by such Holder as of the Event Date; provided, that the
aggregate liquidated damages payable hereunder shall not exceed, in the aggregate, 6.0% of the aggregate amount invested by such Holder
for the Registrable Securities; and provided further that in the event of a cutback limitation as described in Section 2(b), liquidated
damages hereunder shall only accrue and be payable with respect to Cutback Shares such that the liquidated damages shall be reduced in
the same proportion as the total number of Shares and Pre-Funded Warrant Shares required to be registered hereunder, less
the Cutback Shares, bear to the total number of Shares and Pre-Funded Warrant Shares required to be registered hereunder. By way of example,
if the total number of Shares and Pre-Funded Warrant Shares required to be registered hereunder is 1,000,000 and the number of Cutback
Shares is 250,000, then the liquidated damages shall be reduced by 75%. If the Company fails to pay any partial liquidated damages pursuant
to this Section in full within ten (10) Business Days after the date payable, the Company will pay interest thereon at a rate
of 12.0% per annum (or such lesser maximum amount that is permitted to be paid by applicable law) to the Holder, accruing daily from the
date such partial liquidated damages are due until such amounts, plus all such interest thereon, are paid in full. The partial liquidated
damages pursuant to the terms hereof shall apply on a daily pro rata basis for any portion of a month prior to the cure of an Event. Notwithstanding
anything contained herein, no liquidated damages shall accrue or be payable under this Section 2(c) on any securities that are
not Registrable Securities.
(d) If
Form S-3 is not available for the registration of the resale of Registrable Securities hereunder, the Company shall (i) register
the resale of the Registrable Securities on another appropriate form and (ii) undertake to register the Registrable Securities on
Form S-3 as soon as such form is available, provided that the Company shall maintain the effectiveness of the Registration Statement
then in effect until such time as a Registration Statement on Form S-3 covering the Registrable Securities has been declared effective
by the Commission.
(e) Notwithstanding
anything to the contrary contained herein, in no event shall the Company name or identify any Holder or affiliate of a Holder as a statutory
“underwriter”; provided, that if the Commission requires that a Holder be identified as a statutory underwriter in a Registration
Statement, such Holder will have the option, in its sole and absolute discretion, to either (i) withdraw from such Registration Statement
upon its prompt written request to the Company or (ii) be included as such in the Registration Statement.
3. Piggyback
Registration.
(a) If,
at any time while there still remain Registrable Securities, the Company proposes to file a new registration statement or a supplement
or amendment to an existing registration statement under the Securities Act with respect to an offering of Common Stock for (i) the
Company’s own account (other than a registration statement on Form S-4 or S-8 (or any substitute form that may be adopted by
the Commission) or with respect to a Company at-the-market offering program (“ATM Program”) or Company dividend
reinvestment plans) or (ii) the account of any holder of Common Stock (other than the Holders), then the Company shall give written
notice of such proposed filing to the Holders as soon as reasonably practicable (but in no event less than ten (10) Business Days
before the anticipated filing date of such new registration statement), which notice shall describe the type and amount of securities
to be included in such offering, the intended method of distribution and the name of the proposed managing underwriter, if any, in such
offering . Upon a written request, given by Holders to the Company within three (3) Business Days after delivery of any such notice
by the Company, to include Registrable Securities in such Registration (which request shall specify the number of Registrable Securities
proposed to be included in such new registration statement if such registration statement is not a “pay as you go” Automatic
Shelf Registration Statement), the Company shall include all such requested Registrable Securities in such new registration statement
on the same terms and conditions as applicable to the Company’s or such holder’s Common Stock (a “Piggyback Registration”).
Notwithstanding the foregoing, if at any time after giving written notice of such proposed filing and prior to the effective date of such
new registration statement, the Company or such holders shall determine for any reason not to proceed with the proposed filing of the
new registration statement, then the Company may, at its election, give written notice of such determination to the Holders and, thereupon,
will be relieved of its obligation to register the resale of any Registrable Securities in connection with such new registration statement.
(b) The
Holders of Registrable Securities shall be permitted to withdraw all or any part of their shares from any Piggyback Registration at any
time on or before (i) the second (2nd) Business Day prior to the planned effective
date of such Piggyback Registration or (ii) if such Piggyback Registration is an underwritten offering the second (2nd)
Business Day prior to the anticipated filing date of the preliminary prospectus supplement with respect to an underwritten offering by
the Company, except as otherwise provided in any written agreement with the Company’s underwriter(s), if any, establishing the terms
and conditions under which such Holders would be obligated to sell such securities in such Piggyback Registration.
(c) If
a Piggyback Registration is an underwritten offering on behalf of the Company, and the managing underwriter(s) advise the Company
that in its or their reasonable opinion the number of securities proposed to be included in such underwritten offering exceeds the Underwriters’
Maximum Number, then the Company shall include in such underwritten offering (i) first, the number of shares of Common Stock proposed
to be offered by the Company, (ii) second, the Registrable Securities of the Holders that have requested to participate in such underwritten
offering, allocated pro rata among such Holders on the basis of the percentage of the Registrable Securities requested to be included
in such offering by such Holders (or allocated among such Holders as such Holders shall mutually agree in writing to the Company) and
(iii) third, any other securities that have been requested to be so included by any other person.
(d) If
a Piggyback Registration is an underwritten offering initiated by one or more other holders of Common Stock that have the contractual
right to initiate such an underwritten offering (a “Third Party Holder”), and the managing underwriter(s) advise
the Company that in its or their reasonable opinion the number of securities proposed to be included in such registration exceeds the
Underwriters’ Maximum Number, then the Company shall include in such registration (i) first, the securities held by the Third
Party Holders that have requested, and have a contractual right, to participate in such underwritten offering, allocated pro rata among
such Third Party Holders on the basis of the percentage of the Registrable Securities of the Third Party Holders requested to be included
in such offering by such Third Party Holders, (ii) second, the Registrable Securities of the Holders that have requested to participate
in such underwritten offering, allocated pro rata among such Holders on the basis of the percentage of the Registrable Securities by such
Holders (or allocated among such Holders as such Holders shall mutually agree in writing to the Company) and (iii) third, any other
securities that have been requested to be so included by any other person.
(e) In
any Piggyback Registration that is an underwritten offering pursuant to this Section 3, (i) except as set forth in an agreement
with any other holder of the Company’s securities, the Company shall have the right to select the underwriter(s) for such underwritten
offering and (ii) the Company shall not be required to include any Registrable Securities in such underwritten offering unless such
selling Holders accept the terms of the underwritten offering as agreed upon by the Company and the underwriter(s).
(f) The
Company shall not grant to any person the right to request the Company to register the resale of any shares of Company unless such rights
are not more favorable than the provisions of Section 3.
4. Registration
Procedures.
In connection with the Company’s
registration obligations hereunder, the Company shall:
(a) Not
less than five (5) Trading Days prior to the filing of each Registration Statement and not less than one (1) Trading Day prior
to the filing of any related Prospectus or any amendment or supplement thereto (including any document that would be incorporated or deemed
to be incorporated therein by reference), the Company shall (i) furnish to each Holder copies of all such documents proposed to be
filed, which documents (other than those incorporated or deemed to be incorporated by reference) will be subject to the review of such
Holders, and (ii) respond and cause its officers and directors, counsel and independent registered public accountants to respond
to such inquiries as shall be necessary, in the reasonable opinion of respective counsel to each Holder, to conduct a reasonable investigation
within the meaning of the Securities Act. The Company shall not file a Registration Statement or any such Prospectus or any amendments
or supplements thereto to which the Holders of a majority of the Registrable Securities (or, in the case of a Piggyback Registration Statement,
the Holders of a majority of the Registrable Securities to be included in such Piggyback Registration Statement) shall reasonably object
in good faith, provided that, the Company is notified of such objection in writing no later than five (5) Trading Days after the
Holders have been so furnished copies of a Registration Statement or one (1) Trading Day after the Holders have been so furnished
copies of any related Prospectus or amendments or supplements thereto. Each Holder agrees to furnish to the Company a completed questionnaire
in the form attached to this Agreement as Annex B (a “Selling Stockholder Questionnaire”) on or
prior to the later of (x) a date that is not less than three (3) Trading Days prior to the Filing Date and (y) the end
of the fourth (4th) Trading Day following the date on which such Holder receives draft materials in accordance with this Section.
Each Holder shall provide any additional information as may be reasonably requested by the Company and is necessary for purposes of complying
with requirements under applicable securities laws and regulations or rules of any applicable stock exchange where the Common Stock
is then listed.
(b) (i) Prepare
and file with the Commission such amendments, including post-effective amendments, and supplements, to each Registration Statement and
each Prospectus used in connection therewith as may be necessary to keep such Registration Statement continuously effective as to the
applicable Registrable Securities for the Effectiveness Period and prepare and file with the Commission such additional Registration Statements
in order to register the resale under the Securities Act of all of the Registrable Securities, (ii) cause the related Prospectus
to be amended or supplemented by any required prospectus supplement (subject to the terms of this Agreement), and, as so supplemented
or amended, to be filed pursuant to Rule 424, (iii) respond as promptly as reasonably possible to any comments received from
the Commission with respect to a Registration Statement or any amendment thereto and provide as promptly as reasonably possible to the
Holders true and complete copies of all correspondence from and to the Commission relating to a Registration Statement (provided that,
the Company shall excise any information contained therein that would constitute material non-public information regarding the Company
or any of its Subsidiaries), and (iv) comply in all material respects with the applicable provisions of the Securities Act and the
Exchange Act with respect to the disposition of all Registrable Securities covered by a Registration Statement during the applicable period
in accordance (subject to the terms of this Agreement) with the intended methods of disposition by the Holders thereof set forth in such
Registration Statement as so amended or in such Prospectus as so supplemented.
(c) If
during the Effectiveness Period, the number of Registrable Securities at any time exceeds 100% of the number of shares of Common Stock
then registered in a Registration Statement, then the Company shall file as soon as reasonably practicable, but in any case prior to the
applicable Filing Date, an additional Registration Statement that, together with the existing Registration Statements, covers the resale
by the Holders of not less than the number of such Registrable Securities at such time, and thereafter use commercially reasonable efforts
to have such additional Registration Statement declared effective under the Securities Act.
(d) Notify
the Holders of Registrable Securities to be sold (which notice, if given pursuant to clauses (iii) through (vi) hereof, shall
be accompanied by an instruction to suspend the use of the Prospectus until the requisite changes have been made) as promptly as reasonably
possible (and, in the case of (i)(A) below, not less than one (1) Trading Day prior to such filing) and (if requested by any
such Person) confirm such notice in writing no later than one (1) Trading Day following the day (i) of any of the following
(A) when a Prospectus or any prospectus supplement or post-effective amendment to a Registration Statement is proposed to be filed,
(B) when the Commission notifies the Company whether there will be a “review” of such Registration Statement and whenever
the Commission comments in writing on such Registration Statement (in which case the Company shall provide to each of the Holders true
and complete copies of all comments that pertain (x) to such Holder as a “Selling Stockholder” or (y) to the “Plan
of Distribution” and all written responses thereto, but not information that the Company believes would constitute material, non-public
information, and (C) with respect to a Registration Statement or any post-effective amendment, when the same has become effective,
(ii) of any request by the Commission or any other federal or state governmental authority for amendments or supplements to a Registration
Statement or Prospectus or for additional information, (iii) of the issuance by the Commission or any other federal or state governmental
authority of any stop order suspending the effectiveness of a Registration Statement covering any or all of the Registrable Securities
or the initiation of any Proceedings for that purpose, (iv) of the receipt by the Company of any notification with respect to the
suspension of the qualification or exemption from qualification of any of the Registrable Securities for sale in any jurisdiction, or
the initiation or threatening of any Proceeding for such purpose, (v) of the occurrence of any event or passage of time that makes
the financial statements included or incorporated by reference in a Registration Statement ineligible for inclusion or incorporation by
reference therein or any statement made in a Registration Statement or Prospectus or any document incorporated or deemed to be incorporated
therein by reference untrue in any material respect or that requires any revisions to a Registration Statement, Prospectus or other documents
so that, in the case of a Registration Statement or the Prospectus, as the case may be, it will not contain any untrue statement of a
material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein, in light of
the circumstances under which they were made, not misleading, and (vi) of the occurrence or existence of any pending corporate development
with respect to the Company that the Company believes may be material and that, in the determination of the Company, makes it not in the
best interest of the Company to allow continued availability of a Registration Statement or Prospectus; provided, however,
that in no event shall any such notice contain any information that would constitute material, non-public information regarding the Company
or any of its Subsidiaries.
(e) Use
commercially reasonable efforts to avoid the issuance of, or, if issued, obtain the withdrawal of (i) any order stopping or suspending
the effectiveness of a Registration Statement, or (ii) any suspension of the qualification (or exemption from qualification) of any
of the Registrable Securities for sale in any jurisdiction, at the earliest practicable moment.
(f) Furnish
to each Holder, without charge, at least one conformed copy of each such Registration Statement and each amendment thereto, including
financial statements and schedules, all documents incorporated or deemed to be incorporated therein by reference to the extent requested
by such Person, and all exhibits to the extent requested by such Person (including those previously furnished or incorporated by reference)
promptly after the filing of such documents with the Commission, provided that any such item that is available on the EDGAR system (or
successor thereto) need not be furnished in physical form.
(g) Subject
to the terms of this Agreement, the Company hereby consents to the use of such Prospectus and each amendment or supplement thereto by
each of the selling Holders in connection with the offering and sale of the Registrable Securities covered by such Prospectus and any
amendment or supplement thereto, except after the giving of any notice pursuant to Section 4(d).
(h) Prior
to any resale of Registrable Securities by a Holder, use its commercially reasonable efforts to register or qualify or cooperate with
the selling Holders in connection with the registration or qualification (or exemption from the registration or qualification) of the
resale of such Registrable Securities (or, in the case of qualification, of such Registrable Securities for the resale) by the Holder
under the securities or Blue Sky laws of such jurisdictions within the United States as any Holder reasonably requests in writing, to
keep each registration or qualification (or exemption therefrom) effective during the Effectiveness Period and to do any and all other
acts or things reasonably necessary to enable the disposition in such jurisdictions of the Registrable Securities covered by each Registration
Statement, provided that the Company shall not be required to qualify generally to do business in any jurisdiction where it is not then
so qualified, subject the Company to any material taxation in any such jurisdiction where it is not then so subject or file a general
consent to service of process in any such jurisdiction.
(i) If
requested by a Holder, promptly (and in any event within two (2) Trading Days of such request), at the Company’s sole expense,
cooperate with such Holder to facilitate the timely preparation and delivery of certificates or book entry statements, as applicable,
representing Registrable Securities to be delivered to a transferee pursuant to a Registration Statement, which certificates shall be
free, to the extent permitted by the Purchase Agreement, of all restrictive legends, and to enable such Registrable Securities to be in
such denominations and registered in such names as any such Holder may request.
(j) Upon
the occurrence of any event contemplated by Section 4(d)(iii) through (vi)), as promptly as reasonably possible under the circumstances
taking into account the Company’s good faith assessment of any adverse consequences to the Company and its stockholders of the premature
disclosure of such event, prepare a supplement or amendment, including a post-effective amendment, to a Registration Statement or a supplement
to the related Prospectus or any document incorporated or deemed to be incorporated therein by reference, and file any other required
document so that, as thereafter delivered, neither a Registration Statement nor such Prospectus will contain an untrue statement of a
material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in light of
the circumstances under which they were made, not misleading. If the Company notifies the Holders in accordance with clauses (iii) through
(vi) of Section 4(d) above to suspend the use of any Prospectus until the requisite changes to such Prospectus have been
made, then the Holders shall suspend use of such Prospectus. The Company will use commercially reasonable efforts to ensure that the use
of the Prospectus may be resumed as promptly as is practicable. The Company shall be entitled to exercise its right under this Section 4(j) to
suspend the availability of a Registration Statement and Prospectus, subject to the payment of partial liquidated damages otherwise required
pursuant to Section 2(c), on up to two (2) occasions in any 12-month period for a period not to exceed 45 consecutive calendar
days or a total of ninety calendar days, in each case in any such 12-month period. In the event the Company files a Registration Statement
on a form other than Form S-3, as permitted hereunder, the Company’s rights under this Section 3(j) shall include
suspensions of availability arising from the filing of a post-effective amendment to a Registration Statement to update the Prospectus
therein to include the information contained in the Company’s Annual Report on Form 10-K, which suspensions may extend for
the amount of time reasonably required to respond to any comments of the staff of the Commission on such amendment.
(k) Otherwise
use commercially reasonable efforts to comply with all applicable rules and regulations of the Commission under the Securities Act
and the Exchange Act, including Rule 172 under the Securities Act, file any final Prospectus, including any supplement or amendment
thereof, with the Commission pursuant to Rule 424 under the Securities Act, promptly inform the Holders in writing if, at any time
during the Effectiveness Period, the Company does not satisfy the conditions specified in Rule 172 and, as a result thereof, the
Holders are required to deliver a Prospectus in connection with any disposition of Registrable Securities and take such other actions
as may be reasonably necessary to facilitate the registration of the resale of the Registrable Securities.
(l) The
Company may require each selling Holder to furnish to the Company a certified statement as to the number of shares of Common Stock beneficially
owned by such Holder and, if required by the Commission, the natural persons thereof that have voting and dispositive control over the
shares. During any periods that the Company is unable to meet its obligations hereunder with respect to the registration of the Registrable
Securities solely because any Holder fails to furnish such information within three Trading Days of the Company’s request, any liquidated
damages that are accruing at such time as to such Holder only shall be tolled and any Event that may otherwise occur solely because of
such delay shall be suspended as to such Holder only, until such information is delivered to the Company.
(m) The
Company shall use its commercially reasonable efforts to cause all Registrable Securities to be listed on each securities exchange or
market, if any, on which the shares of Common Stock are listed.
(n) The
Company shall use commercially reasonable efforts to maintain eligibility for use of Form S-3 (or any successor form thereto) for
the registration of the resale of the Registrable Securities.
5. Registration
and Underwritten Offering Expenses. All fees and expenses incident to the performance of or compliance with, this Agreement by the
Company shall be borne by the Company whether or not any Registrable Securities are sold pursuant to a Registration Statement. The fees
and expenses referred to in the foregoing sentence shall include (i) all registration and filing fees (including fees, expenses and
disbursements of the Company’s counsel and independent registered public accountants) (A) with respect to filings made with
the Commission, (B) with respect to filings required to be made with any Trading Market on which the Common Stock is then listed
or designated for trading, and (C) in compliance with applicable state securities or Blue Sky laws reasonably agreed to by the Company
in writing (including fees, expenses and disbursements of counsel for the Company in connection with Blue Sky qualifications or exemptions
of the Registrable Securities or the resale thereof), (ii) printing expenses (including expenses of printing certificates for Registrable
Securities), (iii) messenger, telephone and delivery expenses, (iv) fees, expenses and disbursements of counsel for the Company,
(v) Securities Act liability insurance, if the Company so desires such insurance, and (vi) fees and expenses of all other Persons
retained by the Company in connection with the consummation of the transactions contemplated by this Agreement, including the Company’s
transfer agent. In addition, the Company shall be responsible for all of its internal expenses incurred in connection with the registration
and consummation of the transactions contemplated by this Agreement (including all salaries and expenses of its officers and employees
performing legal or accounting duties), the expense of any annual audit and the fees and expenses incurred in connection with the listing
of the Registrable Securities on any securities exchange as required hereunder. In no event shall the Company be responsible for any broker
or similar commissions or any underwriter commissions or expenses of any Holder or, except to the extent provided for in the Transaction
Documents, any legal fees or other costs or expenses of the Holders.
6. Indemnification.
(a) Indemnification
by the Company. The Company shall, notwithstanding any termination of this Agreement, indemnify, defend and hold harmless each Holder
and its Affiliates and each of their respective officers, directors, members, partners, agents and other representatives, brokers, employees,
shareholders, equity holders (regardless of whether such interests are held directly or indirectly), principals, managers, portfolio managers,
trustees, investment advisors, predecessors, successors and assigns, subsidiaries, attorneys and advisors (and any other Persons with
a functionally equivalent role of a Person holding such titles, notwithstanding a lack of such title or any other title), each Person
who controls such any such Holder or any Affiliate thereof (within the meaning of Section 15 of the Securities Act or Section 20
of the Exchange Act) and the officers, directors, members, partners, agents and other representatives, brokers, employees, shareholders,
equity holders (regardless of whether such interests are held directly or indirectly), principals, managers, portfolio managers, trustees,
investment advisors, predecessors, successors and assigns, subsidiaries, attorneys and advisors (and any other Persons with a functionally
equivalent role of a Person holding such titles, notwithstanding a lack of such title or any other title) of each such controlling Person
(each a “Holder Indemnified Person”), to the fullest extent permitted by applicable law, from and against any
and all losses, damages, liabilities, costs (including all judgments, amounts paid in settlements, court costs and reasonable attorneys'
fees), expenses, obligations, contingencies and Proceedings (collectively, “Losses”), joint or several, that
any Holder Indemnified Person may suffer or incur, as incurred, in connection with, arising out of, as a result of, relating to or based
upon (1) any untrue or alleged untrue statement of a material fact contained in a Registration Statement, any Prospectus or any form
of prospectus or in any amendment or supplement thereto or in any preliminary prospectus, or arising out of or relating to any omission
or alleged omission of a material fact required to be stated therein or necessary to make the statements therein (in the case of any Prospectus,
form of prospectus or supplement thereto, in light of the circumstances under which they were made) not misleading or (2) any violation
or alleged violation by the Company or its agents of the Securities Act, the Exchange Act or any state securities law, or any rule or
regulation thereunder, in connection with the performance or non-performance of its obligations under this Agreement or any action or
inaction required of the Company in connection with any registration, except to the extent, that (i) such untrue statements, alleged
untrue statements or omissions are based solely upon information regarding such Holder furnished in writing to the Company by such Holder
expressly for use therein, or to the extent that such information relates to such Holder or such Holder’s proposed method of distribution
of Registrable Securities and was reviewed and expressly approved in writing by such Holder expressly for use in a Registration Statement,
such Prospectus or in any amendment or supplement thereto (it being understood that the Holder has approved Annex A hereto for this purpose)
or (ii) in the case of an occurrence of an event of the type specified in Section 4(d)(iii)-(vi), the use by such Holder of
an outdated, defective or otherwise unavailable Prospectus after the Company has notified such Holder in writing that the Prospectus is
outdated, defective or otherwise unavailable for use by such Holder and prior to the receipt by such Holder of the Advice contemplated
in Section 7(b) and will reimburse such Holder Indemnified Person for legal and other expenses reasonably incurred as such expenses
are incurred by such Holder Indemnified Person in connection with investigating, defending, preparing to defend, providing evidence in,
preparing to serve or serving as a witness with respect to, settling, compromising or paying such Loss. The Company shall notify the Holders
promptly of the institution, threat or assertion of any Proceeding in connection with, arising out of, as a result of, relating to or
based upon the transactions contemplated by this Agreement of which the Company is aware. Such indemnity shall remain in full force and
effect regardless of any investigation made by or on behalf of such indemnified person and shall survive the transfer of any Registrable
Securities by any of the Holders in accordance with Section 7(e).
(b) Indemnification
by Holders. Each Holder shall, severally and not jointly, indemnify, defend and hold harmless the Company and its officers, directors,
members, partners, agents and other representatives, brokers, employees, shareholders, equity holders (regardless of whether such interests
are held directly or indirectly), principals, managers, portfolio managers, trustees, investment advisors, predecessors, successors and
assigns, subsidiaries, attorneys and advisors (and any other Persons with a functionally equivalent role of a Person holding such titles,
notwithstanding a lack of such title or any other title) of each of them, each Person who controls such any such Holder or any Affiliate
thereof (within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act) and the officers, directors,
members, partners, agents and other representatives, brokers, employees, shareholders, equity holders (regardless of whether such interests
are held directly or indirectly), principals, managers, portfolio managers, trustees, investment advisors, predecessors, successors and
assigns, subsidiaries, attorneys and advisors (and any other Persons with a functionally equivalent role of a Person holding such titles,
notwithstanding a lack of such title or any other title) of each such controlling Person (each a “Company Indemnified Person”),
to the fullest extent permitted by applicable law, from and against all Losses, as incurred, to the extent in connection with, arising
out of, as a result of, relating to or based solely upon: any untrue or alleged untrue statement of a material fact contained in any Registration
Statement, any Prospectus, or in any amendment or supplement thereto or in any preliminary prospectus, or arising out of or relating to
any omission or alleged omission of a material fact required to be stated therein or necessary to make the statements therein (in the
case of any Prospectus or supplement thereto, in light of the circumstances under which they were made) not misleading (i) to the
extent that such untrue statement or omission is contained in any information regarding such Holder so furnished in writing by such Holder
to the Company expressly for inclusion in such Registration Statement or such Prospectus or (ii) to the extent that such information
relates to such Holder’s information provided in the Selling Stockholder Questionnaire or the proposed method of distribution of
Registrable Securities and that was reviewed and expressly approved in writing by such Holder expressly for use in a Registration Statement
(it being understood that the Holder has approved Annex A hereto for this purpose), such Prospectus or in any amendment or supplement
thereto. In no event shall the aggregate liability of a selling Holder under this Section 6(b) and Section 6(d) be
greater in amount than the dollar amount of the proceeds (net of all expenses paid by such Holder in connection with any claim relating
to this Section 6 and the amount of any damages such Holder has otherwise been required to pay by reason of such untrue statement
or omission) received by such Holder upon the sale of the Registrable Securities included in the Registration Statement giving rise to
such indemnification obligation.
(c) Conduct
of Indemnification Proceedings. If any Proceeding shall be brought or asserted against, or any circumstance shall exist which would
reasonably be expected to give rise to a demand or claim or the commencement of any Proceeding against, any Person entitled to indemnity
hereunder (an “Indemnified Party”), such Indemnified Party shall promptly notify the Person from whom indemnity
is sought (the “Indemnifying Party”) in writing, and the Indemnifying Party shall assume the defense thereof,
including the employment of counsel reasonably satisfactory to the Indemnified Party and the payment of all fees and expenses incurred
relating to or in connection with defense thereof, provided that the failure of any Indemnified Party to give such notice shall not relieve
the Indemnifying Party of its obligations or liabilities pursuant to this Agreement, except to the extent that it shall be finally determined
by a court of competent jurisdiction (which determination is not subject to appeal or further review) that such failure shall have materially
and adversely prejudiced the Indemnifying Party.
An Indemnified Party shall
have the right to employ separate counsel in any such Proceeding and to participate in the defense thereof, but the fees, expenses and
disbursements of such counsel shall be at the expense of such Indemnified Party or Parties unless: (1) the Indemnifying Party has
agreed in writing to pay such fees, expenses and disbursements, (2) the Indemnifying Party shall have failed promptly to assume the
defense of such Proceeding and to employ counsel reasonably satisfactory to such Indemnified Party in any such Proceeding, or (3) in
the reasonable judgment of counsel to such Indemnified Party, representation of both parties by the same counsel would be inappropriate
due to actual or potential differing interests between them. In the event of the circumstances described in the foregoing clauses (1)-(3),
the fees, expenses and disbursements of such separate counsel and other expenses related to such participation shall be reimbursed by
the Indemnifying Party as incurred (in which case, if such Indemnified Party notifies the Indemnifying Party in writing that it elects
to employ separate counsel at the expense of the Indemnifying Party, the Indemnifying Party shall not have the right to assume the defense
thereof and the reasonable fees and expenses of no more than one separate counsel shall be at the expense of the Indemnifying Party).
The Indemnifying Party shall not be liable for any settlement of or entry of any judgment with respect to any such Proceeding effected
without its written consent, which consent shall not be unreasonably withheld or delayed. No Indemnifying Party shall, without the prior
written consent of the Indemnified Party, effect any settlement of or consent to the entry of any judgment with respect to any pending
Proceeding in respect of which any Indemnified Party is or could have been a party and indemnity could have been sought hereunder by such
Indemnified Party, unless such settlement or judgment (i) imposes no liability or obligation on the Indemnified Party, (ii) includes
an unconditional release from the party bringing such indemnified claims of such Indemnified Party from all liability in respect of or
arising out of on such claims or Proceedings or claims or Proceedings that are the subject matter of such Proceeding and (iii) does
not include any admission of fault, culpability, wrongdoing or malfeasance by or on behalf of the Indemnified Party.
Subject to the terms of this
Agreement, all reasonable fees and expenses of the Indemnified Party (including reasonable fees and expenses to the extent incurred in
connection with investigating or preparing to defend such Proceeding in a manner not inconsistent with this Section) shall be paid to
the Indemnified Party, as incurred, within ten Trading Days of written notice thereof to the Indemnifying Party, provided that the Indemnified
Party shall promptly reimburse the Indemnifying Party for that portion of such fees and expenses applicable to such actions for which
such Indemnified Party is finally determined by a court of competent jurisdiction (which determination is not subject to appeal or further
review) not to be entitled to indemnification hereunder.
(d) Contribution.
If the indemnification under Section 6(a) or 6(b) is unavailable to an Indemnified Party or insufficient to hold an Indemnified
Party harmless for any Losses, then each Indemnifying Party shall contribute to the amount paid or payable by such Indemnified Party as
a result of such Loss in such proportion as is appropriate to reflect the relative fault of the Indemnifying Party and Indemnified Party
in connection with the actions, statements or omissions that resulted in such Losses, as well as any other relevant equitable considerations.
The relative fault of such Indemnifying Party and Indemnified Party shall be determined by reference to, among other things, whether any
action in question, including any untrue or alleged untrue statement of a material fact or omission or alleged omission of a material
fact, has been taken or made by (or not taken or made by, in the case of an omission), or relates to information supplied by (or not supplied
by, in the case of an omission), or on behalf of, such Indemnifying Party or Indemnified Party, and the parties’ relative intent,
knowledge, access to information and opportunity to correct or prevent such action, statement or omission; provided, however, that
no Person guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities Act) will be entitled
to contribution from any Person who was not guilty of such fraudulent misrepresentation. The amount paid or payable by a party as a result
of any Losses shall be deemed to include, subject to the limitations set forth in this Agreement, any reasonable attorneys’ or other
fees, charges or expenses incurred by such party in connection with any Proceeding to the extent such party would have been indemnified
for such fees or expenses if the indemnification provided for in this Section was available to such party in accordance with its
terms.
The parties hereto agree that
it would not be just and equitable if contribution pursuant to this Section 6(d) were determined by pro rata allocation or by
any other method of allocation that does not take into account the equitable considerations referred to in the immediately preceding paragraph.
In no event shall the contribution obligation of a Holder of Registrable Securities be greater in amount than the dollar amount of the
proceeds (net of all expenses paid by such Holder in connection with any claim relating to this Section 6 and the amount of any damages
such Holder has otherwise been required to pay by reason of such untrue or alleged untrue statement or omission or alleged omission) received
by it upon the sale of the Registrable Securities giving rise to such contribution obligation.
The indemnity and contribution
agreements contained in this Section 6(d) are in addition to any liability that the Indemnifying Parties may have to the Indemnified
Parties and are not in diminution or limitation of the indemnification provisions under the Purchase Agreement. Notwithstanding the foregoing
sentence, in no event shall the aggregate liability of a selling Holder under Section 6(b) and this Section 6(d) be
greater in amount than the dollar amount of the proceeds (net of all expenses paid by such Holder in connection with any claim relating
to this Section 5 and the amount of any damages such Holder has otherwise been required to pay by reason of such untrue statement
or omission) received by such Holder upon the sale of the Registrable Securities included in the Registration Statement giving rise to
such indemnification obligation.
7. Miscellaneous.
(a) Remedies.
In addition to being entitled to exercise all rights provided herein or granted by law, including recovery of damages, each of the Holders
and the Company will be entitled to specific performance and injunctive or other equitable relief under this Agreement, without the necessity
of posting a bond. The parties agree that monetary damages may not be adequate compensation for any loss incurred by reason of any breach
of obligations contained in this Agreement and hereby agree to waive and not to assert in any Action for specific performance of any such
obligation the defense that a remedy at law would be adequate. Except as expressly provided in this Agreement, each party hereto agrees
that it shall not have a remedy of punitive or consequential damages against the other and hereby waives any right or claim to punitive
or consequential damages it may now have or may arise in the future.
(b) Discontinued
Disposition. By its acquisition of Registrable Securities, each Holder agrees that, upon receipt of a notice from the Company of the
occurrence of any event of the kind described in Section 4(d)(iii) through (vi), such Holder will, subject to the limitations
on suspension of the use of a Registration Statement or Prospectus contained therein set forth in Section 4, forthwith discontinue
disposition of such Registrable Securities under a Registration Statement until it is advised in writing (the “Advice”)
by the Company that the use of the applicable Prospectus (as it may have been supplemented or amended) may be resumed. The Company will
use commercially reasonable efforts to ensure that the use of the Prospectus may be resumed as promptly as is practicable. The Company
agrees and acknowledges that any periods during which the Holder is required to discontinue the disposition hereunder shall be subject
to the provisions of Section 2(c).
(c) Amendments
and Waivers. No provision of this Agreement may be waived, modified, supplemented or amended except in a written instrument signed,
in the case of a supplement, amendment or modification, by the Company and Holders of 50.1% or more of the then outstanding Registrable
Securities (including Registrable Securities issuable upon exercise or conversion of any Security) (or, prior to the Closing, the Company
and each Purchaser) or, in the case of a waiver, by the party against whom enforcement of any such waived provision is sought, provided
that if any amendment, modification or waiver disproportionately and adversely impacts a Holder (or subset of Holders), the consent of
such disproportionately impacted Holder (or each member of such subset of Holders) shall also be required. No waiver of any default with
respect to any provision, condition or requirement of this Agreement shall be deemed to be a continuing waiver in the future or a waiver
of any subsequent default or a waiver of any other provision, condition or requirement hereof, nor shall any delay or omission of any
party to exercise any right hereunder in any manner impair the exercise of any such right. Any proposed amendment or waiver that disproportionately,
materially and adversely affects the rights and obligations of any Holder (or subset of Holders) relative to the comparable rights and
obligations of the other Holders shall require the prior written consent of such adversely affected Holder (or each member of such subset
of Holders). Any amendment effected in accordance with this Section 7(c) shall be binding upon each Holder. If a Registration
Statement does not register all of the Registrable Securities pursuant to a waiver or amendment done in compliance with the previous sentence,
then the number of Registrable Securities to be registered for each Holder shall be reduced pro rata among all Holders and each Holder
shall have the right to designate which of its Registrable Securities shall be omitted from such Registration Statement. Notwithstanding
the foregoing, a waiver or consent to depart from the provisions hereof with respect to a matter that relates exclusively to the rights
of a Holder or some Holders and that does not directly or indirectly affect the rights of other Holders may be given only by such Holder
or Holders of all of the Registrable Securities to which such waiver or consent relates; provided, however, that the provisions
of this sentence may not be amended, modified, or supplemented except in accordance with the provisions of the first sentence of this
Section 7(c). No consideration shall be offered or paid to any Person to amend or consent to a waiver or modification of any provision
of this Agreement unless the same consideration is also offered to all of the parties to this Agreement.
(d) Notices.
Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be delivered as set forth
in the Purchase Agreement.
(e) Successors
and Assigns. This Agreement shall inure to the benefit of and be binding upon and enforceable by the successors and permitted assigns
of each of the parties and shall inure to the benefit of each Holder. The Company may not assign (except by merger) its rights or obligations
hereunder without the prior written consent of all of the Holders of the then outstanding Registrable Securities. Each Holder may assign
their respective rights hereunder in the manner and to the Persons as permitted under Section 5.7 of the Purchase Agreement. This
Agreement shall not inure to the benefit of or be enforceable by any other person.
(f) Execution.
This Agreement may be executed in two or more counterparts, all of which when taken together shall be considered one and the same agreement
and shall become effective when counterparts have been signed by each party and delivered to each other party, it being understood that
the parties need not sign the same counterpart. In the event that any signature is delivered by e-mail delivery of a “.pdf”
format data file, such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature
is executed) with the same force and effect as if such “.pdf” signature page were an original thereof. Counterparts may
be delivered via facsimile, electronic mail (including any electronic signature covered by the U.S. federal ESIGN Act of 2000, Uniform
Electronic Transactions Act, the Electronic Signatures and Records Act or other applicable law, e.g., www.docusign.com) or other transmission
method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
(g) Governing
Law. All questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be determined in
accordance with the provisions of the Purchase Agreement and Sections 5.8 and 5.19 of the Purchase Agreement are hereby incorporated herein
mutatis mutandi.
(h) Cumulative
Remedies. The remedies provided herein are cumulative and not exclusive of any other remedies provided by law.
(i) Severability.
If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, illegal,
void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force
and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their commercially reasonable efforts
to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision,
covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would have executed the remaining
terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or unenforceable.
(j) Headings.
The headings herein are for convenience only, do not constitute a part of this Agreement and shall not be deemed to limit or affect any
of the provisions hereof.
(k) Independent
Nature of Holders’ Obligations and Rights. The obligations of each Holder hereunder are several and not joint with the obligations
of any other Holder hereunder, and no Holder shall be responsible in any way for the performance or non-performance of the obligations
of any other Holder hereunder. Nothing contained herein or in any other agreement or document delivered at any closing, and no action
taken by any Holder pursuant hereto or thereto, shall be deemed to constitute the Holders as a partnership, an association, a joint venture
or any other kind of group or entity, or create a presumption that the Holders are in any way acting in concert or as a group (including
a “group” within the meaning of Section 13(d)(3) of the Exchange Act) or entity with respect to such obligations
or the transactions contemplated by this Agreement or any other matters, and the Company acknowledges that the Holders are not acting
in concert or as a group, and the Company shall not assert any such claim, with respect to such obligations or transactions. Each Holder
shall be entitled to protect and enforce its rights, including the rights arising out of this Agreement, and it shall not be necessary
for any other Holder to be joined as an additional party in any proceeding for such purpose. Each Holder has been represented by its own
separate legal counsel in its review and negotiation of the Transaction Documents. The Company has elected to provide all Holders with
the same terms and Agreement for the convenience of the Company and not because it was required or requested to do so by any of the Holders.
It is expressly understood and agreed that each provision contained in this Agreement is between the Company and a Holder, solely, and
not between the Company and the Holders collectively and not between and among Holders.
(l) No
Inconsistent Agreements. Neither the Company nor any of its subsidiaries has entered, as of the date hereof, nor shall the Company
or any of its subsidiaries, on or after the date of this Agreement, enter into any agreement with respect to its securities, that would
have the effect of impairing the rights granted to the Holders in this Agreement or otherwise conflicts with the provisions hereof. Neither
the Company nor any of its subsidiaries has previously entered into any agreement granting any registration rights with respect to any
of its securities to any Person that have not been satisfied in full.
(m) Construction.
Wherever required by the context of this Agreement, the singular shall include the plural and vice versa, and the masculine gender shall
include the feminine and neuter genders and vice versa, and references to any agreement, document or instrument shall be deemed to refer
to such agreement, document or instrument as amended, supplemented or modified from time to time. All article, section, paragraph or clause
references not attributed to a particular document shall be references to such parts of this Agreement, and all exhibit, annex, letter
and schedule references not attributed to a particular document shall be references to such exhibits, annexes, letters and schedules to
this Agreement. In addition, the word “or” is not exclusive; the words “including,” “includes,” “included”
and “include” are deemed to be followed by the words “without limitation”; and the terms “herein,”
“hereof” and “hereunder” and other words of similar import refer to this Agreement as a whole and not to any particular
section, paragraph or subdivision.
(n) Current
Public Information. With a view to making available to the Holders the benefits of Rule 144 (or its successor rule) and any other
rule or regulation of the Commission that may at any time permit the Holders to sell shares of Common Stock to the public without
registration, the Company covenants and agrees to: (i) make and keep adequate current public information available, as those terms
are understood and defined in Rule 144, until the earlier of (A) six months after the date as all of the Registrable Securities
may be sold without restriction by the holders hereof pursuant to Rule 144 or any other rule of similar effect and (B) such
date as there are no longer Registrable Securities; (ii) file with the Commission in a timely manner all reports and other documents
required of the Company under the Exchange Act; and (iii) furnish electronically to each Holder upon request, as long as such Holder
owns any Registrable Securities, (A) a written statement by the Company that it has complied with the reporting requirements of the
Exchange Act, (B) a copy of or electronic access to the Company’s most recent Annual Report on Form 10-K or Quarterly
Report on Form 10-Q, and (C) such other information as may be reasonably requested in order to avail such Holder of any rule or
regulation of the Commission that permits the selling of any such Registrable Securities without registration.
(Signature
Pages Follow)
In
Witness Whereof, the parties have executed this Registration Rights Agreement as of the date first written above.
| |
VYNE Therapeutics
Inc. |
| |
|
| |
|
| |
By: |
|
| |
Name: |
| |
Title: |
[SIGNATURE PAGE OF HOLDERS FOLLOWS]
[SIGNATURE PAGE OF HOLDERS TO RRA]
| Signature of Authorized Signatory of Holder: |
|
| Name of Authorized Signatory: |
|
| Title of Authorized Signatory: |
|
Annex A
Plan of Distribution
Each selling stockholder of
the securities and any of their pledgees, assignees, donees, transferees or other successors-in-interest (each, a “Selling
Stockholder” and collectively, the “Selling Stockholders”) may, from time to time, sell, transfer
or otherwise dispose of any or all of their securities covered hereby on the principal Trading Market or any other stock exchange, market
or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. A
Selling Stockholder may use any one or more of the following methods when selling securities:
| · | ordinary brokerage transactions and transactions
in which the broker-dealer solicits purchasers; |
| · | block trades in which the broker-dealer will
attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases by a broker-dealer as principal and
resale by the broker-dealer for its account; |
| · | to or through underwriters; |
| · | an exchange distribution in accordance with the
rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | settlement of short sales entered into after
the effective date of the registration statement of which this prospectus is a part; |
| · | in transactions through broker-dealers that agree
with the Selling Stockholders to sell a specified number of such securities at a stipulated price per security; |
| · | through the writing or settlement of options
or other hedging transactions, whether through an options exchange or otherwise; |
| · | through the distribution of the securities by
any Selling Stockholder to its partners, members or stockholders; |
| · | directly to one or more purchasers; |
| · | through delayed delivery requirements; |
| · | by pledge to secured debts and other obligations
or any transfer upon the foreclosure under such pledges; |
| · | a combination of any such methods of sale; or |
| · | any other method permitted pursuant to applicable
law. |
The Selling Stockholders may
also sell securities under Rule 144 or any other exemption from registration under the Securities Act of 1933, as amended (the “Securities
Act”), if available, rather than under this prospectus.
Broker-dealers engaged by
the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts
from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts
to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a
customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown
in compliance with FINRA Rule 2121.
In connection with the sale
of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial
institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling
Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities
to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with
broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer
or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution
may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction). The Selling Stockholders also may transfer
the securities in other circumstances in which the transferees, pledgees, donees or other successors-in-interest will be the selling beneficial
owners for purposes of this prospectus.
The Selling Stockholders and
any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning
of the Securities Act in connection with such sales (it being understood that the Selling Stockholders shall not be deemed to be underwriters
solely as a result of their participation in this offering). In such event, any commissions received by such broker-dealers or agents
and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities
Act. Each Selling Stockholder has informed the Company that it does not have any written or oral agreement or understanding, directly
or indirectly, with any person to distribute the securities.
The Company is required to
pay certain fees and expenses incurred by the Company incident to the registration of the securities. The Company has agreed to indemnify
the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep this prospectus
effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders without registration
and without regard to any volume or manner-of-sale limitations by reason of Rule 144, or (ii) all of the securities have been
sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities
will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in
certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable
state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and
regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market
making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement
of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the Exchange Act and the rules and
regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the common stock by the Selling Stockholders
or any other person. We will make copies of this prospectus available to the Selling Stockholders and have informed them of the need to
deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under
the Securities Act).
SELLING STOCKHOLDERS
The common stock being offered
by the selling stockholders are those previously issued to the Selling Stockholders, and those issuable to the Selling Stockholders, upon
exercise of the warrants. For additional information regarding the issuances of those shares of common stock and warrants, see “Private
Placement of Common Shares and Warrants” above. We are registering the offer and resale of the shares of common stock in order to
permit the Selling Stockholders to offer the shares for resale from time to time. Except for the ownership of the shares of common stock
and the warrants, the Selling Stockholders have not had any material relationship with us within the past three years.
The table below lists the
Selling Stockholders and other information regarding the beneficial ownership of the shares of common stock by each of the Selling Stockholders.
The second column lists the number of shares of common stock beneficially owned by each Selling Stockholder, based on its ownership of
the shares of common stock and warrants, as of ________, 2023, assuming exercise of the warrants held by the Selling Stockholders on that
date.
The third column lists the
shares of common stock being offered by this prospectus by the Selling Stockholders.
In accordance with the terms
of a registration rights agreement with the Selling Stockholders, this prospectus generally covers the resale of the sum of (i) the
number of shares of common stock issued to the Selling Stockholders in the “Private Placement of Common Shares and Warrants”
described above and (ii) the maximum number of shares of common stock issuable upon exercise of the related warrants, determined
as if the outstanding warrants were exercised in full as of the trading day immediately preceding the date this registration statement
was initially filed with the SEC, each as of the trading day immediately preceding the applicable date of determination and all subject
to adjustment as provided in the registration right agreement, without regard to any limitations on the exercise of the warrants. The
fourth reflects the number of shares of common stock beneficially owned by each Selling Stockholder, assuming the sale of all of the shares
offered by the Selling Stockholders pursuant to this prospectus.
Under the terms of the warrants,
a Selling Stockholders may not exercise the warrants to the extent such exercise would cause such Selling Stockholders, together with
its affiliates and attribution parties, to beneficially own a number of shares of common stock which would exceed 4.99% or 9.99%, as applicable,
of our then outstanding common stock following such exercise, excluding for purposes of such determination shares of common stock issuable
upon exercise of such warrants which have not been exercised. The number of shares in the second and fourth columns do not reflect this
limitation. The Selling Stockholders may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
|
Name of Selling
Stockholders |
|
Number of
shares of
Common Stock
Owned Prior to
Offering |
|
Maximum Number
of
shares of Common
Stock to be Sold
Pursuant to this
Prospectus |
|
Number of
shares of
Common Stock
Owned After Offering |
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
Annex B
VYNE THERAPEUTICS INC.
Selling Stockholder Notice and Questionnaire
The undersigned beneficial
owner of Common Stock (the “Registrable Securities”) of VYNE Therapeutics Inc., a Delaware corporation (the
“Company”), understands that the Company has filed or intends to file with the Securities and Exchange Commission
(the “Commission”) a registration statement (the “Registration Statement”) for the
registration under Rule 415 of the Securities Act of 1933, as amended (the “Securities Act”), of the resale
of the Registrable Securities, in accordance with the terms of the Registration Rights Agreement, dated as of October 27, 2023 to
which the Company and the undersigned are parties (the “Registration Rights Agreement”). A copy of the Registration
Rights Agreement is available from the Company upon request at the address set forth below. All capitalized terms not otherwise defined
herein shall have the meanings ascribed thereto in the Registration Rights Agreement.
Certain legal consequences
arise from being named as a selling stockholder in the Registration Statement and the related prospectus. Accordingly, holders and beneficial
owners of Registrable Securities are advised to consult their own securities law counsel regarding the consequences of being named or
not being named as a selling stockholder in the Registration Statement and the related prospectus.
NOTICE
The undersigned beneficial
owner (the “Selling Stockholder”) of Registrable Securities hereby elects to include the Registrable Securities
owned by it in the Registration Statement.
The undersigned hereby provides
the following information to the Company and represents and warrants that such information is accurate as of the date hereof:
QUESTIONNAIRE
| (a) | Full Legal Name of Selling Stockholder |
| | | |
| | | |
| (b) | Full Legal Name of Registered Holder (if not the same as (a) above) through which Registrable Securities
are held: |
| | | |
| | | |
| (c) | Full Legal Name of Natural Control Person (which means a natural person who directly or indirectly alone
or with others has power to vote or dispose of the securities covered by this Questionnaire): |
| | | |
| | | |
| 2. | Address for Notices to Selling Stockholder: |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
Telephone: |
| |
|
| |
|
| |
E-mail: |
| |
|
| |
|
| |
Contact Person: |
| (a) | Are you a broker-dealer? |
Yes ¨ No ¨
| (b) | If “yes” to Section 3(a), did you receive your Registrable Securities as compensation
for investment banking services to the Company? |
Yes ¨ No ¨
| Note: | If “no” to Section 3(b), the Commission’s staff has indicated that you should be
identified as an underwriter in the Registration Statement. |
| (c) | Are you an affiliate of a broker-dealer? |
Yes ¨ No ¨
| (d) | If you are an affiliate of a broker-dealer, do you certify that you purchased the Registrable Securities
in the ordinary course of business, and at the time of the purchase of the Registrable Securities to be resold, you had no agreements
or understandings, directly or indirectly, with any person to distribute the Registrable Securities? |
Yes ¨ No ¨
| Note: | If “no” to Section 3(d), the Commission’s staff has indicated that you should be
identified as an underwriter in the Registration Statement. |
| 4. | Beneficial Ownership of Securities of the Company Owned by
the Selling Stockholder. |
Except as set forth below in this
Item 4, the undersigned is not the beneficial or registered owner of any securities of the Company other than the securities issuable
pursuant to the Purchase Agreement.
| (a) | Type and Amount of other Company securities beneficially owned by the Selling Stockholder: |
| | | |
| | | |
| 5. | Relationships with the Company: |
Except as set forth below, the undersigned
has not held any position or office or had any other material relationship with the Company (or its predecessors or affiliates) during
the past three years.
State any exceptions here:
The undersigned agrees to
promptly notify the Company of any material inaccuracies or changes in the information provided herein that may occur subsequent to the
date hereof at any time while the Registration Statement remains effective; provided, that the undersigned shall not be required to notify
the Company of any changes to the number of securities held or owned by the undersigned or its affiliates.
By signing below, the undersigned
consents to the disclosure of the information contained herein in its answers to Items 1 through 5 and the inclusion of such information
in the Registration Statement and the related prospectus and any amendments or supplements thereto, to the extent (but only to the extent)
required by Regulation S-K. The undersigned understands that such information will be relied upon by the Company in connection with the
preparation or amendment of the Registration Statement and the related prospectus and any amendments or supplements thereto.
In
Witness Whereof the undersigned, by authority duly given, has caused this Notice and Questionnaire to be executed and delivered
either in person or by its duly authorized agent.
| Date: |
|
|
Beneficial Owner: |
| |
|
|
|
| |
|
|
| |
|
By: |
|
| |
|
Name: |
|
| |
|
|
Title: |
|
| |
|
|
|
|
| |
|
|
|
|
PLEASE EMAIL A .PDF COPY
OF THE COMPLETED AND EXECUTED NOTICE AND QUESTIONNAIRE TO:
Exhibit 99.1

VYNE Therapeutics Announces Private Placement
of $88 Million
| · | Transaction provides $88 million to fund VYNE’s clinical development programs for VYN201 and VYN202 through the end of 2025 |
BRIDGEWATER, N.J., October 30, 2023 -- VYNE Therapeutics Inc.
(Nasdaq: VYNE) (“VYNE” or the “Company”), a clinical-stage biopharmaceutical company developing proprietary, innovative
and differentiated therapies for the treatment of immuno-inflammatory conditions, today announced that the Company has signed a securities
purchase agreement with certain healthcare-focused institutional investors for a private placement financing (the “PIPE”)
that is expected to result in gross proceeds of $88 million, before deducting placement agent fees and offering expenses. The PIPE was
led by Access Biotechnology, with participation from Eventide Asset Management, Cormorant Asset Management, Acorn Bioventures, Parkman
Healthcare Partners, Surveyor Capital (a Citadel company), Soleus Capital, Palo Alto Investors LP, and other undisclosed investors.
“We are proud to partner with leading fundamental healthcare
investors in this transformative financing of our company to advance our InhiBET™ platform,” said David Domzalski, President
and Chief Executive Officer of VYNE. “This is an exciting time at VYNE as we announce positive clinical data from our Phase 1b trial
evaluating VYN201 in patients with nonsegmental vitiligo. We expect the net proceeds from this financing will fund us through critical
milestones, including the advancement of VYN201 into a Phase 2b clinical trial in vitiligo and Phase 1 trials for VYN202, our BD2-selective
BET inhibitor.”
In the PIPE, VYNE is selling an aggregate of 10,652,543 shares of its
common stock at a price of $2.245 per share and, in lieu of common stock to certain investors, pre-funded warrants to purchase up to an
aggregate of 28,614,437 shares of common stock at a purchase price of $2.2449 per pre-funded warrant. Each pre-funded warrant will have
an exercise price of $0.0001 per share of common stock and will be immediately exercisable and will remain exercisable until exercised
in full. The PIPE was priced to satisfy the “Minimum Price” requirement of an “at the market” price as set forth
in the Nasdaq listing rules. The PIPE is expected to close on November 1, 2023, subject to customary closing conditions.
LifeSci Capital LLC is acting as the exclusive placement agent for
the PIPE.
The securities to be sold in the PIPE, including the shares of common
stock underlying the pre-funded warrants, have not been registered under the Securities Act of 1933, as amended, or applicable state securities
laws, and may not be offered or sold in the United States except pursuant to an effective registration statement or an applicable exemption
from the registration requirements. As part of the PIPE, VYNE has agreed to file a registration statement with the Securities and Exchange
Commission within 30 days of the closing for the purpose of registering the resale of the shares of common stock issued in the PIPE and
the shares of common stock underlying the pre-funded warrants issued in the PIPE.
This press release is issued pursuant to Rule 135c of the Securities
Act and does not constitute an offer to sell or the solicitation of an offer to buy any securities described herein, nor shall there be
any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration
or qualification under the securities laws of any such state or jurisdiction. Any offering of the securities under the resale registration
statement will only be by means of a prospectus.
About VYNE Therapeutics Inc.
VYNE’s mission is to improve the lives of patients by
developing proprietary, innovative and differentiated therapies for the treatment of immuno-inflammatory conditions. The
Company’s unique and proprietary bromodomain & extra-terminal (BET) domain inhibitors, which comprise its
InhiBET™ platform, include a locally administered pan-BD BET inhibitor (VYN201) and an orally available BD2-selective BET
inhibitor (VYN202) that were licensed from Tay Therapeutics Limited.
Cautionary Statement Regarding
Forward-Looking Statements
This release includes forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements related
to whether and when the PIPE will close, expected gross proceeds to be received in the PIPE, the forecast of cash runway and VYNE’s
expectations regarding funding, operating and working capital expenditures and expected use of proceeds. All statements in this press
release which are not historical facts are forward-looking statements. Any forward-looking statements are based on VYNE’s current
knowledge and its present beliefs and expectations regarding possible future events and are subject to risks, uncertainties and assumptions
that could cause actual results to differ materially and adversely from those set forth or implied by such forward-looking statements.
These risks and uncertainties include, but are not limited to, VYNE’s inability, or the inability of the investors in the PIPE,
to satisfy the conditions to closing for the PIPE and other important factors discussed in the section titled “Risk Factors”
in VYNE’s Annual Report on Form 10-K for the year ended December 31, 2022, Quarterly Report on Form 10-Q for the
quarter ended June 30, 2023, and VYNE’s other filings from time to time with the U.S. Securities and Exchange Commission. Although
VYNE believes these forward-looking statements are reasonable, they speak only as of the date of this announcement and VYNE undertakes
no obligation to update publicly such forward-looking statements to reflect subsequent events or circumstances, except as otherwise required
by law. Given these risks and uncertainties, you should not rely upon forward-looking statements as predictions of future events.
Investor Relations:
John Fraunces
LifeSci Advisors, LLC
917-355-2395
jfraunces@lifesciadvisors.com
Tyler Zeronda
VYNE Therapeutics Inc.
908-458-9106
Tyler.Zeronda@vynetx.com
Exhibit 99.2

VYNE Therapeutics Announces Positive Data from
Phase 1b Trial for Novel BET Inhibitor VYN201 in Patients with Nonsegmental Vitiligo
| · | Clinical proof-of-concept achieved with statistically significant dose dependent reduction in F-VASI score from baseline for the
VYN201 1.0% and 2.0% cohorts compared to the 0.5% cohort |
| · | VYN201 was generally well-tolerated with no clinically relevant treatment emergent adverse events across all dose cohorts |
| · | Clinical response and rapid onset of action suggest VYN201 has the potential to be the category leader in the treatment of nonsegmental
vitiligo |
| · | VYN201 is expected to advance into a Phase 2b clinical trial in the first half of 2024 |
BRIDGEWATER, N.J., October 30, 2023 -- VYNE Therapeutics Inc.
(Nasdaq: VYNE) (“VYNE” or the “Company”), a clinical-stage biopharmaceutical company developing proprietary, innovative
and differentiated therapies for the treatment of immuno-inflammatory conditions, today announced positive data from its Phase 1b trial
evaluating once-daily dosing of VYN201 in patients with nonsegmental vitiligo.
The Phase 1b trial is a 16-week open-label trial assessing the safety,
tolerability and pharmacokinetics of once-daily topical VYN201 in 29 patients across three dose cohorts (0.5%, 1.0% and 2.0% strengths).
Exploratory efficacy of VYN201 was also evaluated, including VYN201’s ability to arrest the progression of skin depigmentation and
support skin repigmentation in patients with active disease, through changes in the facial vitiligo area scoring index (“F-VASI”).
“We are encouraged by these data from our Phase 1b trial that
demonstrate proof-of-concept in nonsegmental vitiligo, which we believe is the first clinical evidence of a BET inhibitor’s effect
in autoimmune disease. We believe these data support the continued advancement of VYN201 as a potential category leading therapy in the
treatment of vitiligo and also more broadly validates our InhiBETTM platform as we work to develop BET inhibitors for the treatment
of immuno-inflammatory diseases,” said David Domzalski, President and CEO of VYNE. “We look forward to discussing the final
results from the trial at an R&D Day event on November 9, where we will also provide our plans for a Phase 2b clinical trial.”
“Vitiligo patients with active disease face an uphill battle
compared to those with stable vitiligo and may require aggressive treatment in order to avoid potentially permanent loss of pigmentation,”
said Amit Pandya, M.D., former President of the Global Vitiligo Foundation and Staff Dermatologist, Department of Dermatology, Palo Alto
Foundation Medical Group. “VYN201’s ability to halt the progression of depigmentation and demonstrate repigmentation in these
difficult-to-treat patients as observed in this trial is very promising. These data merit the continued investigation of VYN201’s
potential as a differentiated therapy for vitiligo.”
Key results include:
| · | Proof-of-concept achieved: |
| o | Significant clinical improvement observed in 1% and 2% cohorts with rapid onset of action and a dose-dependent response. Mean percentage
reduction in F-VASI score from baseline after 16 weeks of treatment was: |
| § | 7.5% for the 0.5% cohort (n=10), with 20% of patients achieving a ≥25% improvement in F-VASI score from baseline (“F-VASI25”) |
| § | 30.3% for the 1.0% cohort (n=10), with 50% of patients achieving F-VASI25 and 30% of patients achieving a ≥50% improvement in F-VASI
score from baseline (“F-VASI50”) |
| § | 39.0% for the 2.0% cohort (n=9), with 67% of patients achieving F-VASI25 and 33% of patients achieving F-VASI50, including one patient
who achieved a ≥75% improvement in F-VASI score from baseline |
| § | The mean percentage reduction in F-VASI score from baseline for each of the 1.0% and 2.0% cohorts was statistically significant (p
= 0.0137 and p = 0.00771, respectively) compared to the 0.5% cohort at week 16, indicative of a dose response |
| · | Safety, Tolerability and Pharmacokinetics: Topical VYN201 was generally well-tolerated with a favorable safety profile in vitiligo
patients at all dose levels with no serious adverse events reported and no abnormal laboratory values suggestive of low or reducing platelet
counts. All treatment emergent adverse events have been classified as mild or moderate with no dose-dependent trends observed to date.
All mean local skin tolerability assessment scores were less than 0.30 on a severity scale 0 (“None”) to 3 (“Severe”). |
Next Steps
| · | R&D
Day Event: VYNE will host an R&D Day event on Thursday, November 9, 2023 at
1:30 p.m. ET. VYNE management will review the final results from the Phase 1b trial
for VYN201. The event will feature a presentation from Dr. Amit Pandya, former President
of the Global Vitiligo Foundation and Staff Dermatologist, Department of Dermatology, Palo
Alto Foundation Medical Group. The VYNE team will also discuss the Company’s development
plans for VYN201 and its oral BET inhibitor VYN202. Register Here |
| · | Initiate Phase 2b Trial: The Company intends to begin Phase 2b preparatory activities and expects to advance VYN201 into a
longer duration Phase 2b trial to evaluate optimal dosing and peak efficacy in patients with active and stable nonsegmental vitiligo in
1H 2024. |
About Vitiligo
Vitiligo is a chronic autoimmune depigmenting disorder of the skin,
characterized by the loss of pigment producing cells known as melanocytes. Vitiligo is the most common depigmenting skin condition, with
a prevalence estimated at 0.5-2% of the world population.2 There is currently only one FDA-approved product for the treatment
of vitiligo. Non-segmental vitiligo is the most common type of vitiligo.
About VYN201
VYN201 is a pan-bromodomain BET
inhibitor designed to be locally-administered as a “soft” drug to address diseases involving multiple, diverse inflammatory
cell signaling pathways while providing low systemic exposure. To date, VYN201 has produced consistent reductions in pro-inflammatory
and disease-related biomarkers and improvements in disease severity, and demonstrated local activity in several preclinical models (using
several different routes of administration).
About BET Inhibitors
BET proteins play a key role in regulating
gene transcription via epigenetic interactions (“reading”), and recent research has determined a key role for these proteins
in regulating B cell and T cell activation and subsequent inflammatory processes. As epigenetic readers, BET proteins regulate the recruitment
of transcriptional factors that are key to the production of several pro-inflammatory cytokines. BET inhibitors have the potential to
treat a range of immuno-inflammatory and fibrotic diseases by blocking pro-inflammatory cytokine transcription with additional potential
in myeloproliferative neoplastic disorders.
1. Percentage change from baseline one-tailed T-test based on latest
observed case.
2. Rosmarin et al, Lancet (2020);396:110-120.
About VYNE Therapeutics Inc.
VYNE’s mission is to improve the lives of patients by
developing proprietary, innovative and differentiated therapies for the treatment of immuno-inflammatory conditions. The
Company’s unique and proprietary bromodomain & extra-terminal (BET) domain inhibitors, which comprise its
InhiBET™ platform, include a locally administered pan-BD BET inhibitor (VYN201) and an orally available BD2-selective BET
inhibitor (VYN202) that were licensed from Tay Therapeutics Limited.
For
more information about VYNE Therapeutics Inc. or its product candidates, visit www.vynetherapeutics.com. VYNE may use its
website to comply with its disclosure obligations under Regulation FD. Therefore, investors should monitor VYNE’s website in addition
to following its press releases, filings with the U.S. Securities and Exchange Commission, public conference calls, and webcasts.
Cautionary Statement Regarding
Forward-Looking Statements
This release includes forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding
VYNE’s plans and development timelines for VYN201, the release of complete topline results for the Phase 1b trial of VYN201, the
potential of VYN201 and VYNE’s InhiBET™ platform, VYNE’s intentions regarding a Phase 2b trial of VYN201 and other statements
regarding the future expectations, plans and prospects of VYNE. All statements in this press release which are not historical facts are
forward-looking statements. Any forward-looking statements are based on VYNE’s current knowledge and its present beliefs and expectations
regarding possible future events and are subject to risks, uncertainties and assumptions that could cause actual results to differ materially
and adversely from those set forth or implied by such forward-looking statements. These risks and uncertainties include, but are not limited
to: VYNE’s ability to successfully develop its product candidates; the timing of commencement of future preclinical studies and
clinical trials; VYNE’s ability to complete and receive favorable results in, clinical trials for its product candidates; VYNE’s
intentions and its ability to obtain additional funding, either through equity or debt financing transactions or collaboration arrangements;
VYNE’s ability to comply with various regulations applicable to its business; VYNE’s ability to create intellectual property
and the scope of protection it is able to establish and maintain for intellectual property rights covering its product candidates, including
the projected terms of patent protection; risks that any of VYNE’s patents may be held to be narrowed, invalid or unenforceable
or one or more of VYNE’s patent applications may not be granted and potential competitors may also seek to design around VYNE’s
granted patents or patent applications; estimates of VYNE’s expenses, capital requirements, its needs for additional financing and
its ability to obtain additional capital on acceptable terms or at all; VYNE’s expectations regarding licensing, business transactions
and strategic operations; VYNE’s future financial performance and liquidity; and volatility in VYNE’s stock price may result
in rapid and substantial increases or decreases in the stock price that may or may not be related to VYNE’s operating performance
or prospects. For a discussion of other risks and uncertainties, and other important factors, any of which could cause VYNE’s actual
results to differ from those contained in the forward-looking statements, see the section titled “Risk Factors” in VYNE’s
Annual Report on Form 10-K for the year ended December 31, 2022, Quarterly Report on Form 10-Q for the quarter ended June 30,
2023, and VYNE’s other filings from time to time with the U.S. Securities and Exchange Commission. Although VYNE believes these
forward-looking statements are reasonable, they speak only as of the date of this announcement and VYNE undertakes no obligation to update
publicly such forward-looking statements to reflect subsequent events or circumstances, except as otherwise required by law. Given these
risks and uncertainties, you should not rely upon forward-looking statements as predictions of future events.
This release includes preliminary
clinical data from VYNE’s Phase 1b clinical trial evaluating topical VYN201 in patients with nonsegmental vitiligo. These preliminary
data remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary
data previously provided. As a result, data should be viewed with caution until the final data are available. This preliminary data is
not intended to convey conclusions about the complete efficacy or safety results from the trial.
Investor Relations:
John Fraunces
LifeSci Advisors, LLC
917-355-2395
jfraunces@lifesciadvisors.com
Tyler Zeronda
VYNE Therapeutics Inc.
908-458-9106
Tyler.Zeronda@vynetx.com
Exhibit 99.3

VYNE Therapeutics Reports Positive Results from
Preclinical Models for Oral BD2-Selective BET Inhibitor VYN202
| · | Treatment with VYN202 resulted in significant inhibition of key inflammatory biomarkers and substantial resolution of the signs
and symptoms of psoriasis and rheumatoid arthritis |
| · | Following completion of recent financing, VYNE expects to initiate a Phase 1a SAD/MAD clinical trial in Q1 2024, followed by Phase
1b trials in moderate-to-severe plaque psoriasis and in moderate-to-severe adult-onset rheumatoid arthritis in the second half of 2024 |
| · | Preclinical data and clinical programs to be discussed at R&D Day event on November 9, 2023 |
BRIDGEWATER, N.J., October 30, 2023 -- VYNE Therapeutics Inc.
(Nasdaq: VYNE) (“VYNE” or the “Company”), a clinical-stage biopharmaceutical company developing proprietary, innovative
and differentiated therapies for the treatment of immuno-inflammatory conditions, today announced new preclinical data showing the positive
effect of its oral small molecule BD2-selective BET inhibitor, VYN202, in preclinical models of psoriasis and rheumatoid arthritis.
“We believe VYN202 has the potential to be the most potent and
selective BET inhibitor in development and has consistently demonstrated its ability to improve signs and symptoms of disease with marked
inhibition of key inflammatory cytokines implicated in the pathology of several chronic autoimmune diseases in preclinical models,”
said David Domzalski, President and Chief Executive Officer of VYNE. “These compelling preclinical data in skin inflammation and
joint disease models highlight VYN202’s potential for therapeutic utility in both psoriasis and rheumatoid arthritis. We look forward
to advancing these programs into the clinic next year and updating investors on our progress.”
Key findings from preclinical in vivo models:
| · | Psoriasis: In a well-established model, a psoriasis phenotype was induced in BALB-C mice. Treatment was then administered intraperitoneally
with either VYN202, deucravacitinib (an allosteric TYK2 inhibitor), or placebo. VYN202 and deucravacitinib at equivalent dosing demonstrated
comparable onset of action and efficacy. |
| o | Mice receiving VYN202 3mg/kg had approximately 95% mean reduction in PASI score from baseline by day 7 of treatment, which was consistent
with the results in the deucravacitinib 3mg/kg group. |
| o | Treatment with VYN202 3mg/kg reduced the expression of IL-17A (a major effector cytokine involved in the pathogenesis of psoriasis),
by >93% compared to placebo. Treatment with VYN202 at all doses also resulted in a marked reduction of other disease-related cytokines
(IL-1β, IL-6, IL-22, IL-23, and TNF-α) compared to the placebo group. |
| · | Rheumatoid Arthritis: In a 21-day collagen-induced arthritis model, signs and symptoms of inflammatory arthritis were induced
in Lewis rats. Each treatment group orally received either placebo, GSK620 (an early generation BD2-selective BET inhibitor) at 10 mg/kg,
or VYN202 at three different dose strengths (1, 3, or 10 mg/kg). |
| o | Treatment with VYN202 10mg/kg QD resulted in a 71% reduction in the overall signs and symptoms of rheumatoid arthritis at day 21,
compared to mice receiving placebo. |
| o | Treatment with VYN202 10mg/kg QD resulted in a 79% lower paw volume (a measure of swelling) compared to mice receiving placebo. |
| o | 75% of the animals treated with the highest dose of VYN202 presented with normal joint histopathology at the end of the study, whereas
animals treated with placebo experienced marked inflammatory cell infiltrate, granulation tissue, bone erosion and cartilagenous ulceration. |
| o | Treatment with VYN202 10mg/kg achieved a 98% lower expression of Immunoglobin G1, a biomarker associated with rheumatoid arthritis,
compared to treatment with placebo. |
| o | Treatment with VYN202 at 3 and 10mg/kg demonstrated a statistically-significant superior anti-inflammatory effect compared to GSK620
10mg/kg for all study efficacy endpoints. |
Next Steps
| · | R&D Day Event:
VYNE will host an R&D Day event on Thursday, November 9, 2023 at 1:30 p.m. ET.
VYNE management will discuss these data as well as the final data from VYNE’s Phase
1b trial evaluating VYN201 in vitiligo. The Company will also discuss its development plans
for VYN201 and VYN202. Register Here |
| · | IND Submission: VYNE expects to submit its IND for VYN202 by year-end 2023 and commence a first-in-human Phase 1a single ascending
dose/multiple ascending dose trial in the first quarter of 2024. If successful, VYNE anticipates commencing Phase 1b trials in moderate-to-severe
plaque psoriasis and in moderate-to-severe adult-onset rheumatoid arthritis in the second half of 2024. |
About VYN202
VYN202 is an oral small molecule BD2-selective
BET inhibitor that has been designed to achieve class-leading selectivity (BD2 vs. BD1), maximum potency versus BD2 and optimal oral bioavailability.
By maximizing BD2 selectivity, VYNE believes VYN202 has the potential to be a more conveniently-administered non-biologic treatment option
for both acute control and chronic management of immuno-inflammatory indications, where the damaging effects of unrestricted inflammatory
signaling activity is common. VYN202 is structurally distinct from VYNE’s pan-BET inhibitor (VYN201) and covered by distinct PCT
and provisional composition of matter patent applications directed to new chemical entities and their uses.
About BET Inhibitors
BET proteins play a key role in regulating
gene transcription via epigenetic interactions (“reading”), and recent research has determined a key role for these proteins
in regulating B cell and T cell activation and subsequent inflammatory processes. As epigenetic readers, BET proteins regulate the recruitment
of transcriptional factors that are key to the production of several pro-inflammatory cytokines. BET inhibitors have the potential to
treat a range of immuno-inflammatory and fibrotic diseases by blocking pro-inflammatory cytokine transcription with additional potential
in myeloproliferative neoplastic disorders.
About VYNE Therapeutics Inc.
VYNE’s mission is to improve the lives of patients by
developing proprietary, innovative and differentiated therapies for the treatment of immuno-inflammatory conditions. The
Company’s unique and proprietary bromodomain & extra-terminal (BET) domain inhibitors, which comprise its
InhiBET™ platform, include a locally administered pan-BD BET inhibitor (VYN201) and an orally available BD2-selective BET
inhibitor (VYN202) that were licensed from Tay Therapeutics Limited.
For more information about VYNE Therapeutics Inc. or its product candidates,
visit www.vynetherapeutics.com. VYNE may use its website to comply with its disclosure obligations under Regulation FD. Therefore,
investors should monitor VYNE’s website in addition to following its press releases, filings with the U.S. Securities and Exchange
Commission, public conference calls, and webcasts.
Cautionary Statement Regarding
Forward-Looking Statements
This release includes forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding
VYNE’s plans and development timelines for VYN202, the potential of VYN202 and VYNE’s InhiBET™ platform, VYNE’s
intentions regarding a Phase 1a and Phase 1b trials of VYN202, the granting or conversion of certain patent applications, and other statements
regarding the future expectations, plans and prospects of VYNE. All statements in this press release which are not historical facts are
forward-looking statements. Any forward-looking statements are based on VYNE’s current knowledge and its present beliefs and expectations
regarding possible future events and are subject to risks, uncertainties and assumptions that could cause actual results to differ materially
and adversely from those set forth or implied by such forward-looking statements. These risks and uncertainties include, but are not limited
to: VYNE’s ability to successfully develop its product candidates; the timing of commencement of future preclinical studies and
clinical trials; VYNE’s ability to complete and receive favorable results in, clinical trials for its product candidates; VYNE’s
ability to comply with various regulations applicable to its business; VYNE’s ability to create intellectual property and the scope
of protection it is able to establish and maintain for intellectual property rights covering its product candidates, including the projected
terms of patent protection; risks that any of VYNE’s patents may be held to be narrowed, invalid or unenforceable or one or more
of VYNE’s patent applications may not be granted and potential competitors may also seek to design around VYNE’s granted patents
or patent applications; estimates of VYNE’s expenses, capital requirements, its needs for additional financing and its ability to
obtain additional capital on acceptable terms or at all; VYNE’s expectations regarding licensing, business transactions and strategic
operations; VYNE’s future financial performance and liquidity; and volatility in VYNE’s stock price may result in rapid and
substantial increases or decreases in the stock price that may or may not be related to VYNE’s operating performance or prospects.
For a discussion of other risks and uncertainties, and other important factors, any of which could cause VYNE’s actual results to
differ from those contained in the forward-looking statements, see the section titled “Risk Factors” in VYNE’s Annual
Report on Form 10-K for the year ended December 31, 2022, Quarterly Report on Form 10-Q for the quarter ended June 30,
2023, and VYNE’s other filings from time to time with the U.S. Securities and Exchange Commission. Although VYNE believes these
forward-looking statements are reasonable, they speak only as of the date of this announcement and VYNE undertakes no obligation to update
publicly such forward-looking statements to reflect subsequent events or circumstances, except as otherwise required by law. Given these
risks and uncertainties, you should not rely upon forward-looking statements as predictions of future events.
Investor Relations:
John Fraunces
LifeSci Advisors, LLC
917-355-2395
jfraunces@lifesciadvisors.com
Tyler Zeronda
VYNE Therapeutics Inc.
908-458-9106
Tyler.Zeronda@vynetx.com
Exhibit 99.4

| Corporate Presentation
October 2023 |

| Forward Looking Statements and Important Notes This presentation by VYNE Therapeutics Inc ..(" includes forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 including, but not limited to, statements regarding VYNE's plans, regulatory filings and development timelines for VYN 201 and VYN 202 VYNE's InhiBET platform VYNE's cash runway through the end of 2025 and other statements regarding the future expectations, plans and prospects of VYNE All statements in this presentation which are not historical facts are forward looking statements Any forward looking statements are based on VYNE's current knowledge and its present beliefs and expectations regarding possible future events and are subject to risks, uncertainties and assumptions that could cause actual results to differ materially and adversely from those set forth or implied by such forward looking statements These risks and uncertainties include, but are not limited to VYNE's ability to successfully develop its product candidates the timing of commencement of future preclinical studies and clinical trials VYNE's ability to enroll patients and successfully progress, complete, and receive favorable results in, clinical trials for its product candidates VYNE's ability to comply with various regulations applicable to its business VYNE's ability to create intellectual property and the scope of protection it is able to establish and maintain for intellectual property rights covering its product candidates, including the projected terms of patent protection risks that any of VYNE's patents may be held to be narrowed, invalid or unenforceable or one or more of VYNE's patent applications may not be granted and potential competitors may also seek to design around VYNE's granted patents or patent applications estimates of VYNE's expenses, capital requirements, its needs for additional financing and its ability to obtain additional capital on acceptable terms or at all VYNE's expectations regarding licensing, business transactions and strategic operations VYNE's future financial performance and liquidity and volatility in VYNE's stock price may result in rapid and substantial increases or decreases in the stock price that may or may not be related to VYNE's operating performance or prospects For a discussion of other risks and uncertainties, and other important factors, any of which could cause VYNE's actual results to differ from those contained in the forward looking statements, see the section titled "Risk Factors" in VYNE's Annual Report on Form 10 K for the year ended December 31 2022 and Quarterly Report on Form 10 Q for the period ended June 30 2023 as well as discussions of potential risks, uncertainties, and other important factors in VYNE's subsequent filings with the U S Securities and Exchange Commission Although VYNE believes these forward looking statements are reasonable, they speak only as of the date of this presentation and VYNE undertakes no obligation to update publicly such forward looking statements to reflect subsequent events or circumstances, except as otherwise required by law Given these risks and uncertainties, you should not rely upon forward looking statements as predictions of future events This presentation includes preliminary clinical data from VYNE's Phase 1 b clinical trial evaluating topical VYN 201 in patients with nonsegmental vitiligo These preliminary data remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data included herein As a result, data should be viewed with caution until the final data are available in November 2023 Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third party sources and VYNE's own internal estimates and research While VYNE believes these third party sources to be reliable as of the date of this presentation, VYNE has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third party sources In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions Finally, while VYNE believes its own internal research is reliable, such research has not been verified by any independent source You are cautioned not to give undue weight to any such information, projections and estimates The trademarks included herein are the property of the owners thereof and are used for reference purposes only This presentation concerns product candidates that are under clinical investigation None of such product candidates have been approved for marketing by the FDA or the EMA, and such product candidates are currently limited to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated. 2 |

| Investment Highlights (NASDAQ: VYNE)
Advancing novel BET inhibitor platform to improve the lives of patients
suffering from immuno-inflammatory conditions
Potential for
Multiple Clinical
Catalysts
• VYN201: Phase 1b PoC data in vitiligo suggest VYN201 has the potential to be category leader
• VYN202: Supported by robust preclinical data across multiple diverse models of autoimmune disease,
Phase 1a SAD/MAD expected to be initiated in Q1 2024 following IND clearance
Clinical Stage
Pipeline
Experienced
Team & Strong
Balance Sheet
• Seasoned leadership team with demonstrated track record of progressing programs through regulatory approval
• Pro forma cash: $103.7M1; No debt
Recent financing with syndicate of leading healthcare investors provides cash runway through the end of 2025
Innovative
Target &
Approach
• BET inhibition represents a novel target for the treatment of autoimmune diseases: Addressing the complex
signaling of immuno-inflammatory diseases by disrupting inflammatory gene transcription in T cells
• Potential across broad range of immune-mediated diseases representing multi-billion-dollar opportunities
• VYN201: Phase 2b trial expected to be initiated in 1H 2024 targeting top-line results in mid-2025
• VYN202: Phase 1a SAD/MAD read-out anticipated mid-2024
• (2) Phase 1b PoC studies in plaque psoriasis and rheumatoid arthritis planned with targeted top-line
results expected in mid-2025
1. As of September 30, 2023, reflecting gross proceeds from $88 million PIPE financing in October 2023; Pro forma shares outstanding: ~14.0M; Pro forma fully-diluted shares outstanding: ~42.8M excluding treasury stock method; unaudited 3 |

| 4 Based on current estimates.
Building a Leading Early-Stage Pipeline to Address Unmet Needs in I&I Conditions
Program Indication(s) Route of
Administration
Current Stage Status / Next
Anticipated Milestones Rights Preclinical IND-Enabling Phase 1 Phase 2
InhiBETTM Platform - Library of NCE BET Inhibitors for Any Indication Worldwide
VYN201
Soft pan-BD
BET inhibitor
Nonsegmental Vitiligo Topical
• Phase 1 completed
• 1H 2024: Initiate P2b
Worldwide
VYN202
BD2-selective
BET inhibitor
Moderate-to-Severe
Plaque Psoriasis
Oral
• IND-enabling studies
ongoing
• Q1 2024: Initiate P1
SAD/MAD
Worldwide
Moderate-to-Severe
Rheumatoid Arthritis
Ongoing evaluation for other autoimmune and fibro-inflammatory diseases |
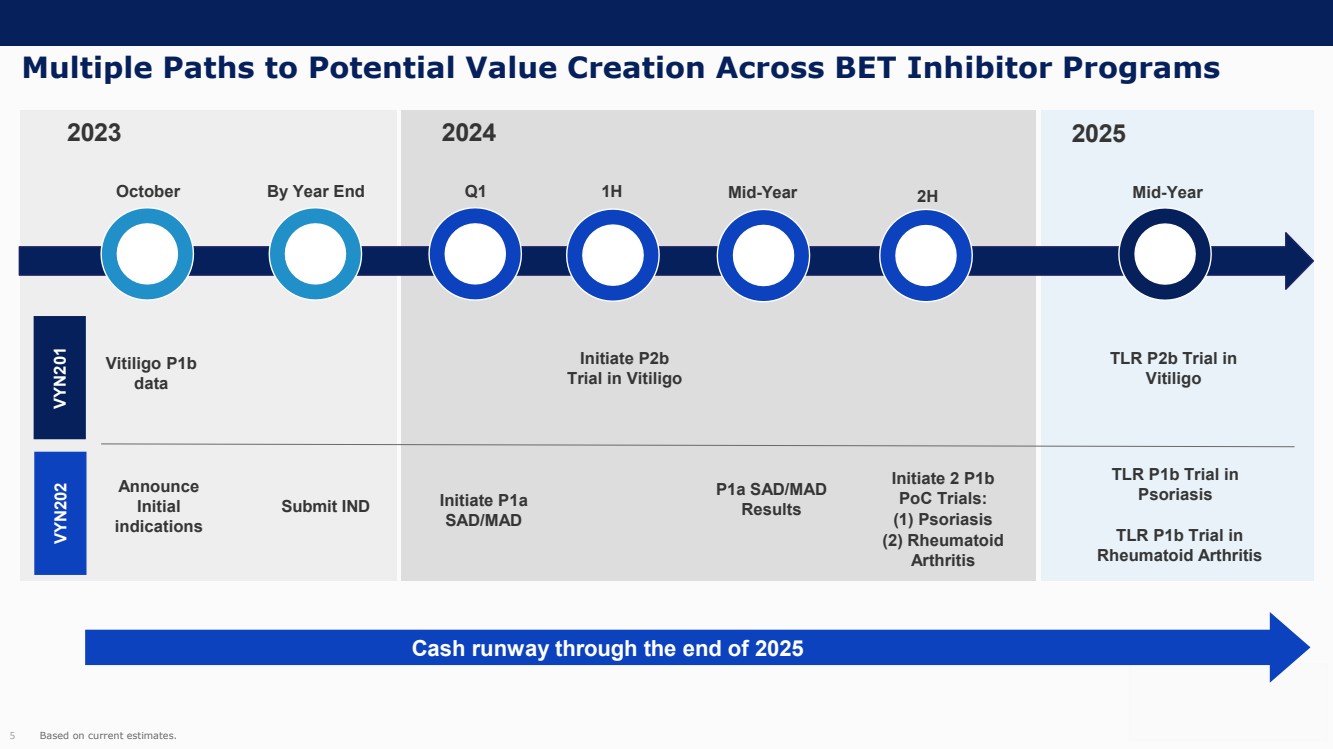
| 5
Multiple Paths to Potential Value Creation Across BET Inhibitor Programs
2023
October
Vitiligo P1b
data
Q1
Initiate P1a
SAD/MAD
1H
Initiate P2b
Trial in Vitiligo
2025
Mid-Year
TLR P2b Trial in
Vitiligo
Cash runway through the end of 2025
By Year End
Submit IND
2024
Mid-Year 2H
P1a SAD/MAD
Results
Initiate 2 P1b
PoC Trials:
(1) Psoriasis
(2) Rheumatoid
Arthritis
Announce
Initial
indications
VYN201 VYN202
TLR P1b Trial in
Psoriasis
TLR P1b Trial in
Rheumatoid Arthritis
Based on current estimates. |

| InhiBET™ BET Inhibitor Platform
Bromodomain & Extra-Terminal Domain (BET) Inhibition for
Immunology and Inflammatory Diseases
6 |
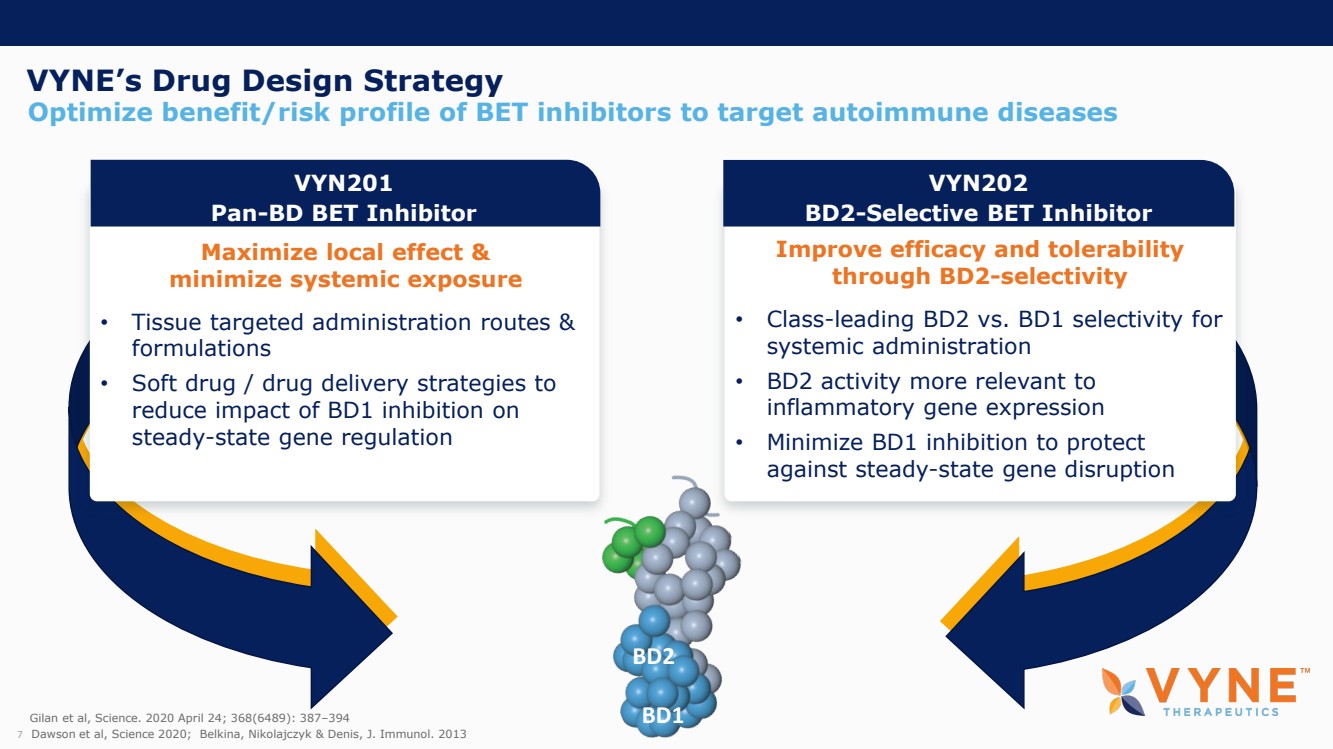
| Improve efficacy and tolerability
through BD2-selectivity
• Class-leading BD2 vs. BD1 selectivity for
systemic administration
• BD2 activity more relevant to
inflammatory gene expression
• Minimize BD1 inhibition to protect
against steady-state gene disruption
VYNE’s Drug Design Strategy
7 Dawson et al, Science 2020; Belkina, Nikolajczyk & Denis, J. Immunol. 2013
Optimize benefit/risk profile of BET inhibitors to target autoimmune diseases
VYN201
Pan-BD BET Inhibitor
Maximize local effect &
minimize systemic exposure
• Tissue targeted administration routes &
formulations
• Soft drug / drug delivery strategies to
reduce impact of BD1 inhibition on
steady-state gene regulation
VYN202
BD2-Selective BET Inhibitor
BD1
BD2
Gilan et al, Science. 2020 April 24; 368(6489): 387–394 |

| VYN201: Vitiligo |
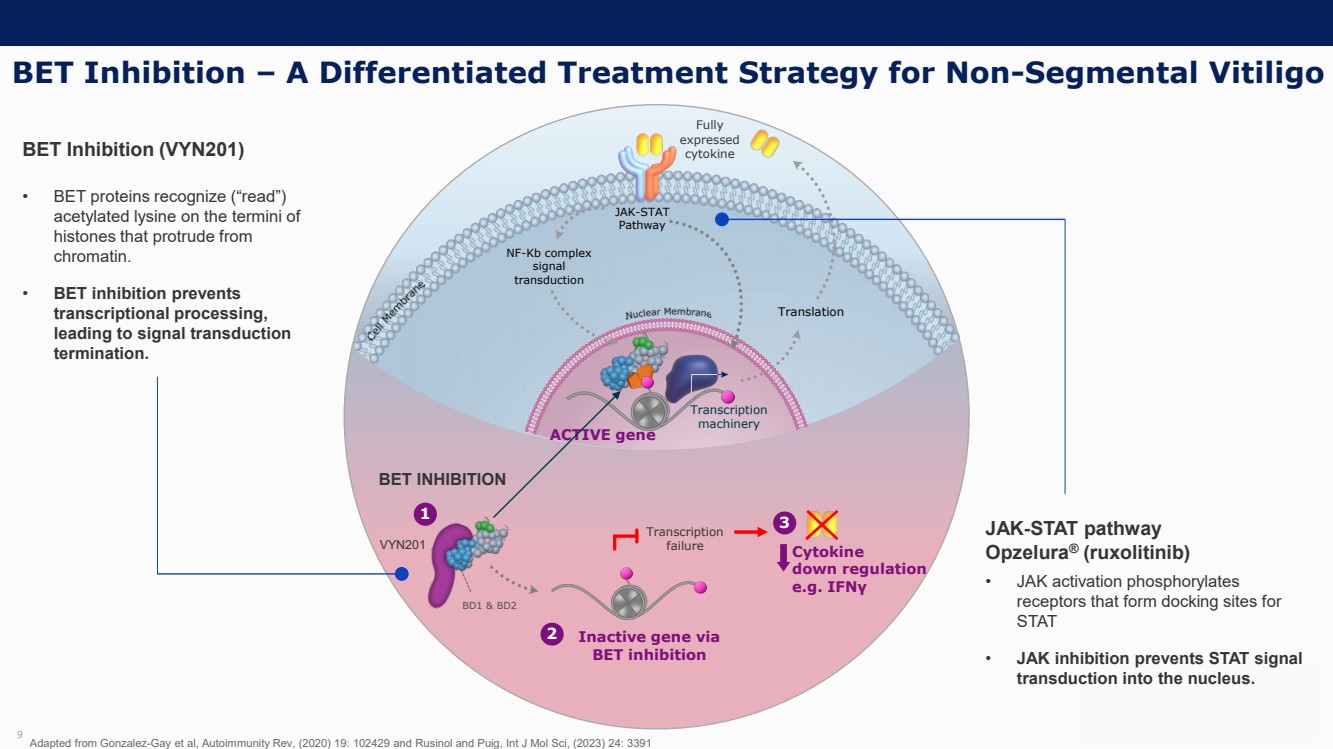
| 9
BET Inhibition – A Differentiated Treatment Strategy for Non-Segmental Vitiligo
ACTIVE gene
VYN201
Transcription
machinery
NF-Κb complex
signal
transduction
Translation
Transcription
failure
Fully
expressed
cytokine
Cytokine
down regulation
e.g. IFNγ
3 1
BD1 & BD2
Inactive gene via
BET inhibition
2
BET INHIBITION
JAK-STAT
Pathway
JAK-STAT pathway
Opzelura® (ruxolitinib)
• JAK activation phosphorylates
receptors that form docking sites for
STAT
• JAK inhibition prevents STAT signal
transduction into the nucleus.
BET Inhibition (VYN201)
• BET proteins recognize (“read”)
acetylated lysine on the termini of
histones that protrude from
chromatin.
• BET inhibition prevents
transcriptional processing,
leading to signal transduction
termination.
Adapted from Gonzalez-Gay et al, Autoimmunity Rev, (2020) 19: 102429 and Rusinol and Puig, Int J Mol Sci, (2023) 24: 3391 |
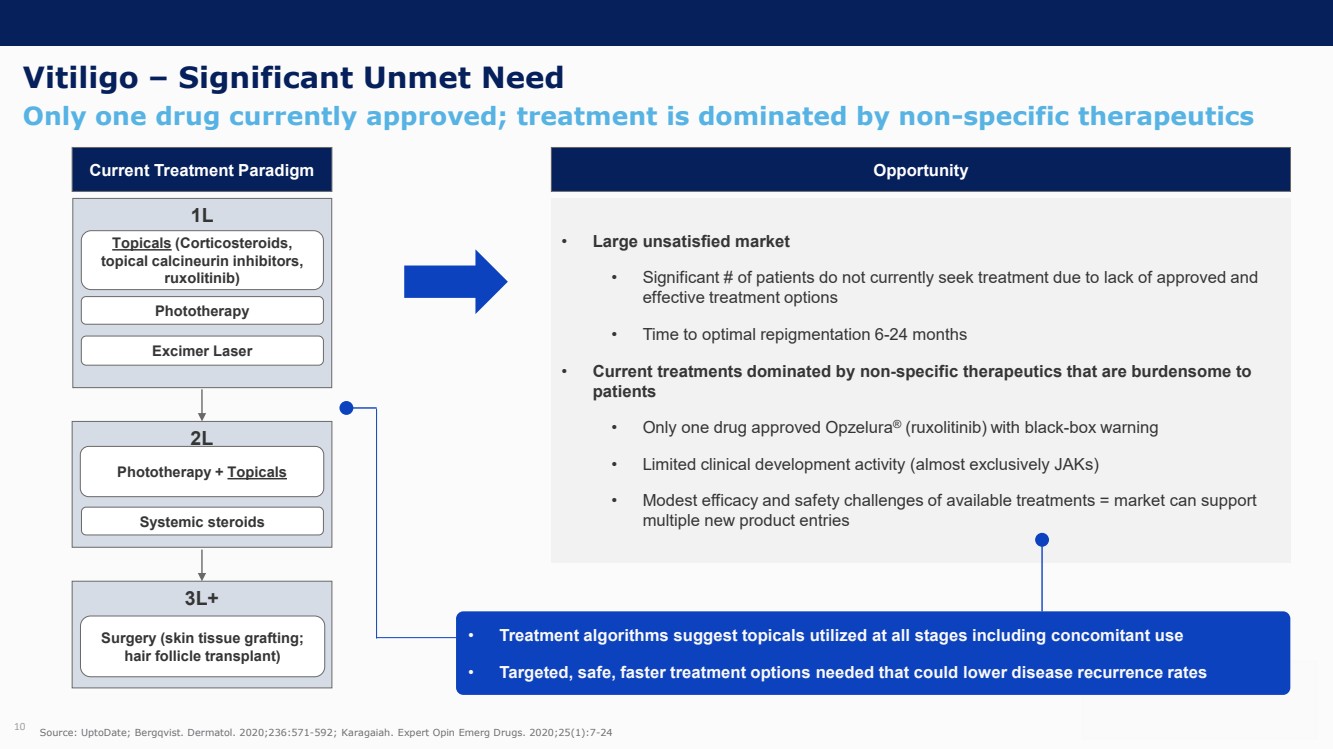
| Vitiligo – Significant Unmet Need
Only one drug currently approved; treatment is dominated by non-specific therapeutics
10
1L
Topicals (Corticosteroids,
topical calcineurin inhibitors,
ruxolitinib)
Phototherapy
Excimer Laser
2L
Phototherapy + Topicals
3L+
Surgery (skin tissue grafting;
hair follicle transplant)
Source: UptoDate; Bergqvist. Dermatol. 2020;236:571-592; Karagaiah. Expert Opin Emerg Drugs. 2020;25(1):7-24
Systemic steroids
• Large unsatisfied market
• Significant # of patients do not currently seek treatment due to lack of approved and
effective treatment options
• Time to optimal repigmentation 6-24 months
• Current treatments dominated by non-specific therapeutics that are burdensome to
patients
• Only one drug approved Opzelura® (ruxolitinib) with black-box warning
• Limited clinical development activity (almost exclusively JAKs)
• Modest efficacy and safety challenges of available treatments = market can support
multiple new product entries
Current Treatment Paradigm Opportunity
• Treatment algorithms suggest topicals utilized at all stages including concomitant use
• Targeted, safe, faster treatment options needed that could lower disease recurrence rates |

| Vitiligo Represents a Large and Growing Market Opportunity
11
Source: Gandhi et al. JAMA Dermatol. 2022;158(1):43-50; Kruger. 2012;51(10):1206-1212; Rangu. J Clin Dermatol Ther. 2021;7:070; Pandya. AAD 2023 Presentation; Incyte Corporate Pres. Aug. 2023; TD
Cowen research dated Sept. 2023; Piper research dated Jan. 2023; Citi research dated July 2023
1. Opzelura pricing: $2k per 60g tube*10 tubes per patient per year less GTN discount of 50% per Incyte mgmt.; 2. Q2’23 net sales $80mm: Vitiligo represents ~35% of TRx per Incyte mgmt.
1.9M
<0.2M
U.S. Diagnosed Vitiligo Patients Patients Currently Seeking Treatment
Addressable Market
2022 acquisition of Villaris for preclinical vitiligo asset auremolimab underscores attractive opportunity for VYN201
($70M upfront / $1.4B total deal value)
$112
$550
$553
$725
Q2 '23
Annualized
Vitiligo Net
Sales
Cowen Citi Piper
Analyst 2030 Vitiligo U.S. Net Sales Estimates
Annualized vitiligo net
sales >$100M within
3 qtrs of launch2
Est. 0.3M to 0.45M patients seeking treatment
• Market penetration: Opzelura is the
only product approved for treatment
of vitiligo
• Patient activation represents large
long-term growth opportunity:
• Significant # of patients do not
currently seek treatment due to
lack of approved and effective
treatment options
• Annual net sales of ~$10K / patient1
$ in millions |

| VYN201: Preclinical Data |
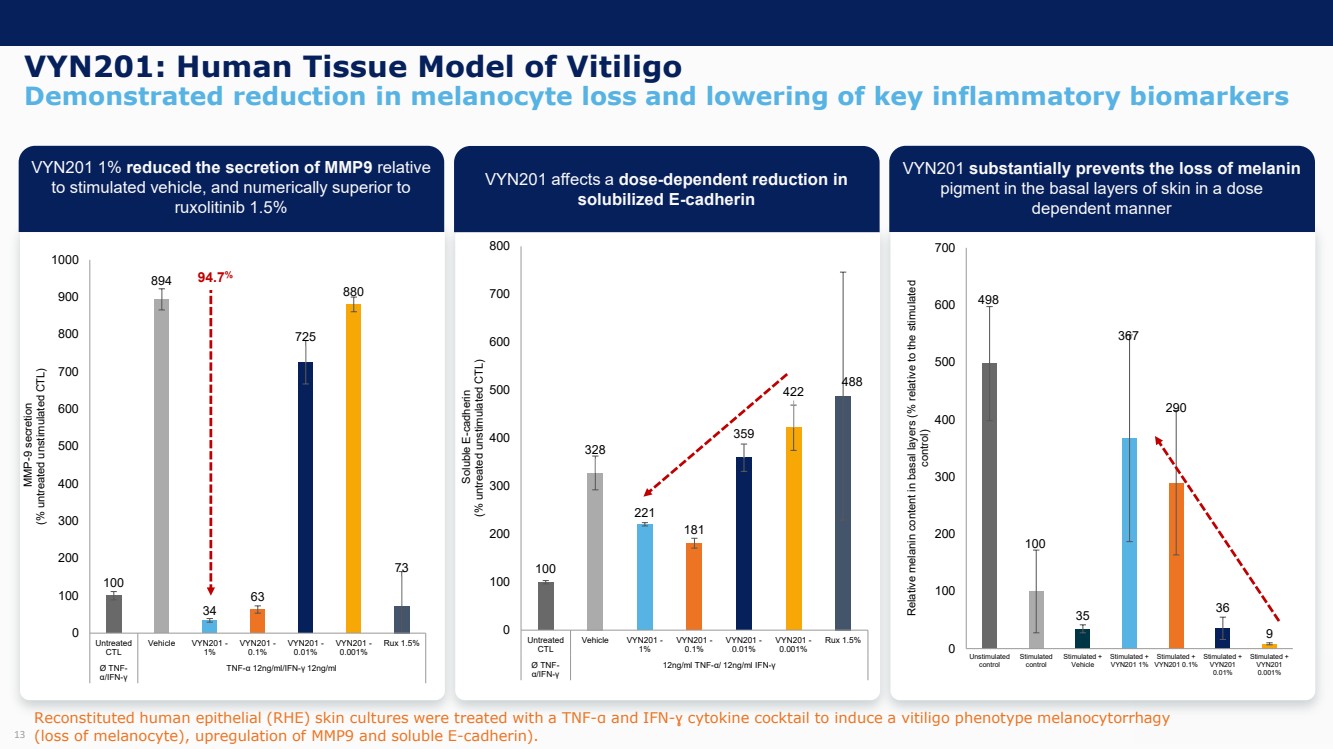
| 13
VYN201: Human Tissue Model of Vitiligo
Demonstrated reduction in melanocyte loss and lowering of key inflammatory biomarkers
100
894
34
63
725
880
73
0
100
200
300
400
500
600
700
800
900
1000
Untreated
CTL
Vehicle VYN201 -
1%
VYN201 -
0.1%
VYN201 -
0.01%
VYN201 -
0.001%
Rux 1.5%
Ø TNF-α/IFN-γ
TNF-α 12ng/ml/IFN-γ 12ng/ml
MMP-9 secretion
(% untreated unstimulated CTL)
Reconstituted human epithelial (RHE) skin cultures were treated with a TNF-α and IFN-ɣ cytokine cocktail to induce a vitiligo phenotype melanocytorrhagy
(loss of melanocyte), upregulation of MMP9 and soluble E-cadherin).
94.7%
498
100
35
367
290
36
9
0
100
200
300
400
500
600
700
Unstimulated
control
Stimulated
control
Stimulated +
Vehicle
Stimulated +
VYN201 1%
Stimulated +
VYN201 0.1%
Stimulated +
VYN201
0.01%
Stimulated +
VYN201
0.001%
Relative melanin content in basal layers (% relative to the stimulated
control)
100
328
221
181
359
422
488
0
100
200
300
400
500
600
700
800
Untreated
CTL
Vehicle VYN201 -
1%
VYN201 -
0.1%
VYN201 -
0.01%
VYN201 -
0.001%
Rux 1.5%
Ø TNF-α/IFN-γ
12ng/ml TNF-α/ 12ng/ml IFN-γ
Soluble E-cadherin
(% untreated unstimulated CTL)
VYN201 1% reduced the secretion of MMP9 relative
to stimulated vehicle, and numerically superior to
ruxolitinib 1.5%
VYN201 affects a dose-dependent reduction in
solubilized E-cadherin
VYN201 substantially prevents the loss of melanin
pigment in the basal layers of skin in a dose
dependent manner |
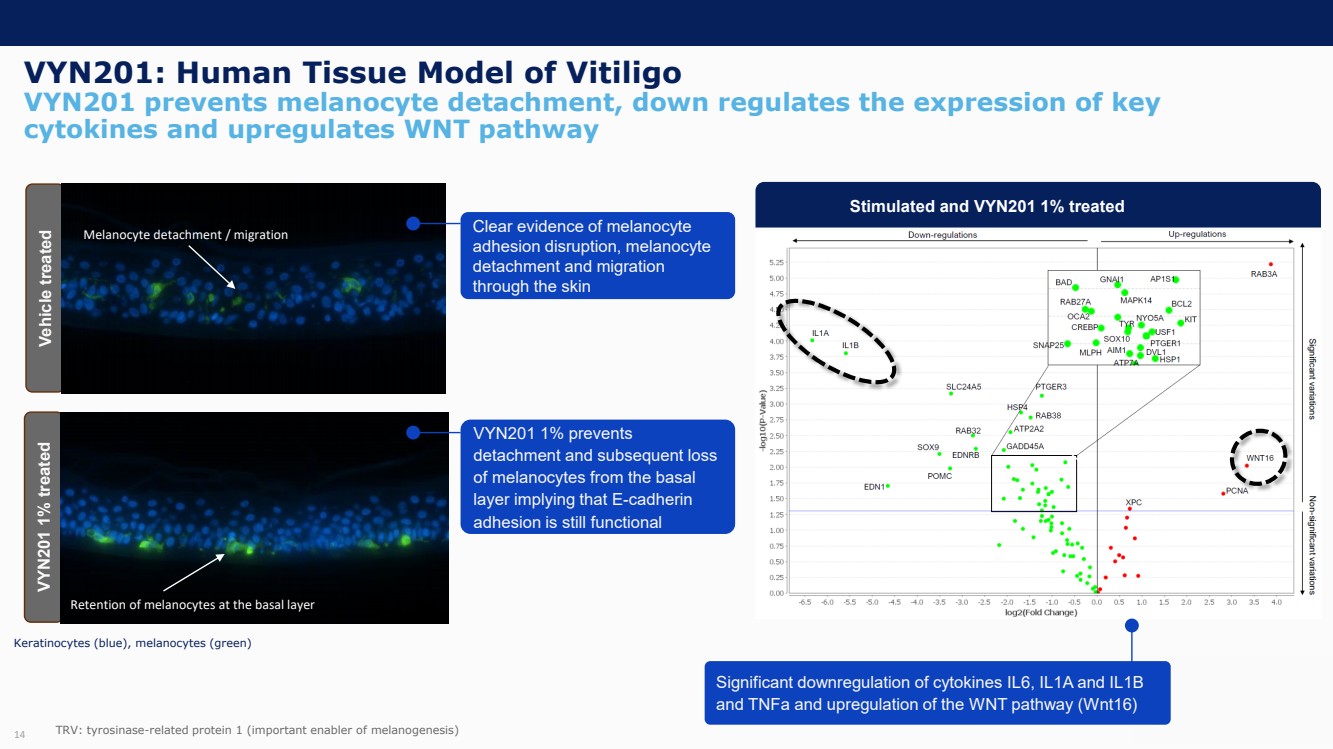
| VYN201 1% treated Vehicle treated
TRV: tyrosinase-related protein 1 (important enabler of melanogenesis)
Keratinocytes (blue), melanocytes (green)
Melanocyte detachment / migration
Retention of melanocytes at the basal layer
VYN201: Human Tissue Model of Vitiligo
VYN201 prevents melanocyte detachment, down regulates the expression of key
cytokines and upregulates WNT pathway
14
Clear evidence of melanocyte
adhesion disruption, melanocyte
detachment and migration
through the skin
Melanocyte migration
Stimulated and VYN201 1% treated
VYN201 1% prevents
detachment and subsequent loss
of melanocytes from the basal
layer implying that E-cadherin
adhesion is still functional
Significant downregulation of cytokines IL6, IL1A and IL1B
and TNFa and upregulation of the WNT pathway (Wnt16) |

| VYN201: Phase 1 Proof-of-Concept |
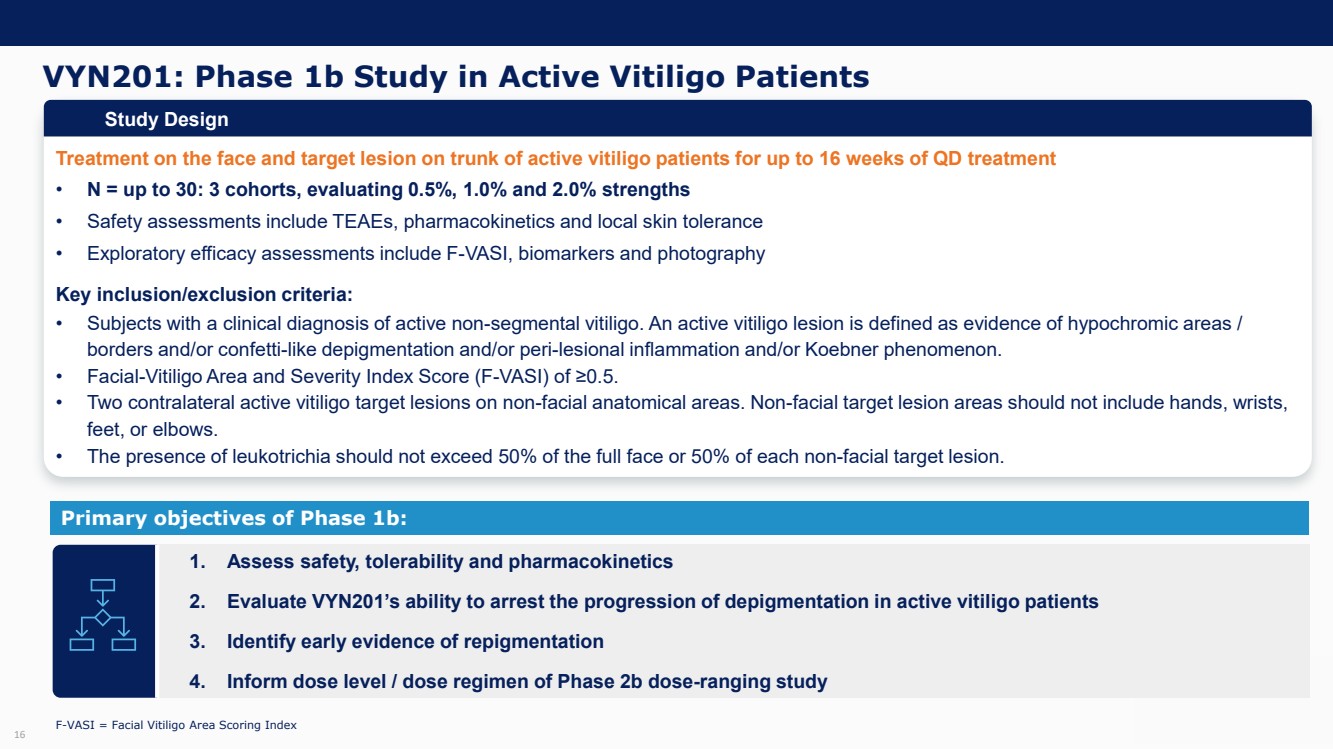
| VYN201: Phase 1b Study in Active Vitiligo Patients
16
F-VASI = Facial Vitiligo Area Scoring Index
Study Design:
Treatment on the face and target lesion on trunk of active vitiligo patients for up to 16 weeks of QD treatment
• N = up to 30: 3 cohorts, evaluating 0.5%, 1.0% and 2.0% strengths
• Safety assessments include TEAEs, pharmacokinetics and local skin tolerance
• Exploratory efficacy assessments include F-VASI, biomarkers and photography
Key inclusion/exclusion criteria:
• Subjects with a clinical diagnosis of active non-segmental vitiligo. An active vitiligo lesion is defined as evidence of hypochromic areas /
borders and/or confetti-like depigmentation and/or peri-lesional inflammation and/or Koebner phenomenon.
• Facial-Vitiligo Area and Severity Index Score (F-VASI) of ≥0.5.
• Two contralateral active vitiligo target lesions on non-facial anatomical areas. Non-facial target lesion areas should not include hands, wrists,
feet, or elbows.
• The presence of leukotrichia should not exceed 50% of the full face or 50% of each non-facial target lesion.
Primary objectives of Phase 1b:
1. Assess safety, tolerability and pharmacokinetics
2. Evaluate VYN201’s ability to arrest the progression of depigmentation in active vitiligo patients
3. Identify early evidence of repigmentation
4. Inform dose level / dose regimen of Phase 2b dose-ranging study
Study Design |
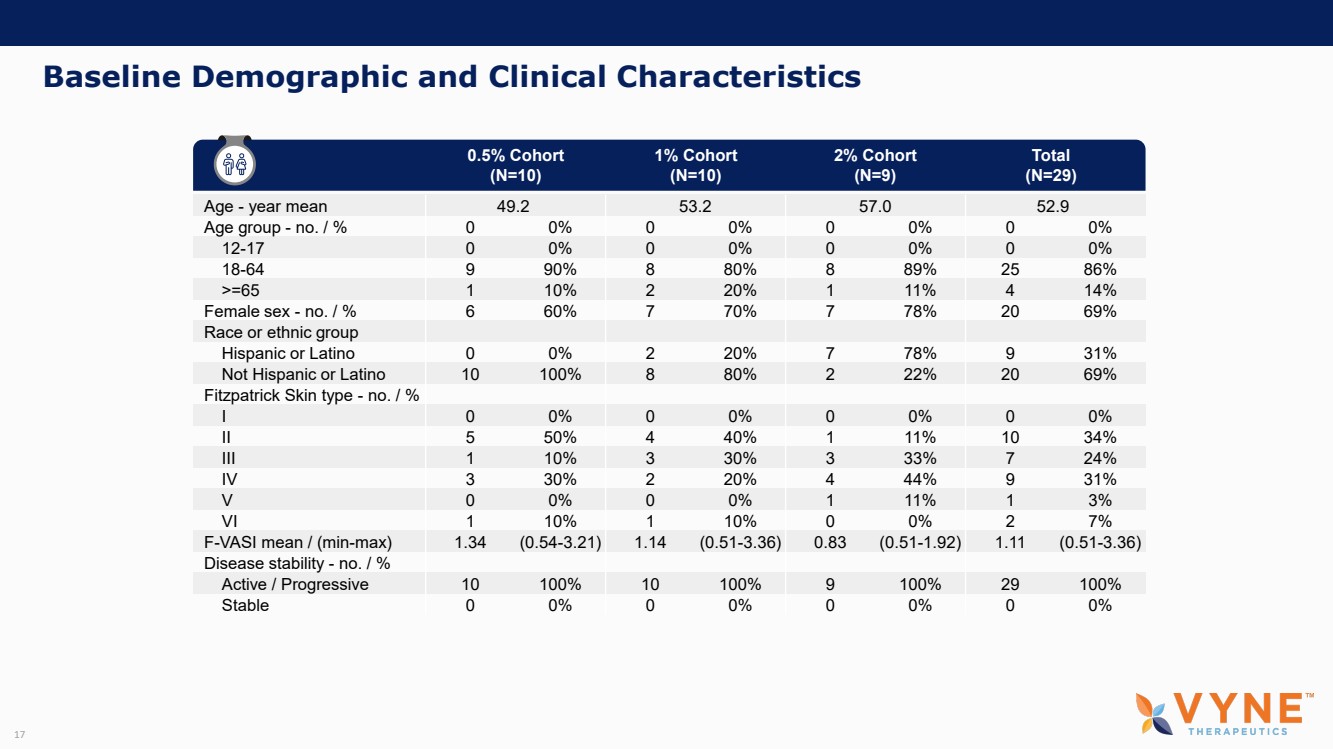
| Age - year mean 49.2 53.2 57.0 52.9
Age group - no. / % 0 0% 0 0% 0 0% 0 0%
12-17 0 0% 0 0% 0 0% 0 0%
18-64 9 90% 8 80% 8 89% 25 86%
>=65 1 10% 2 20% 1 11% 4 14%
Female sex - no. / % 6 60% 7 70% 7 78% 20 69%
Race or ethnic group
Hispanic or Latino 0 0% 2 20% 7 78% 9 31%
Not Hispanic or Latino 10 100% 8 80% 2 22% 20 69%
Fitzpatrick Skin type - no. / %
I 0 0% 0 0% 0 0% 0 0%
II 5 50% 4 40% 1 11% 10 34%
III 1 10% 3 30% 3 33% 7 24%
IV 3 30% 2 20% 4 44% 9 31%
V 0 0% 0 0% 1 11% 1 3%
VI 1 10% 1 10% 0 0% 2 7%
F-VASI mean / (min-max) 1.34 (0.54-3.21) 1.14 (0.51-3.36) 0.83 (0.51-1.92) 1.11 (0.51-3.36)
Disease stability - no. / %
Active / Progressive 10 100% 10 100% 9 100% 29 100%
Stable 0 0% 0 0% 0 0% 0 0%
Baseline Demographic and Clinical Characteristics
17
0.5% Cohort
(N=10)
1% Cohort
(N=10)
2% Cohort
(N=9)
Total
(N=29) |
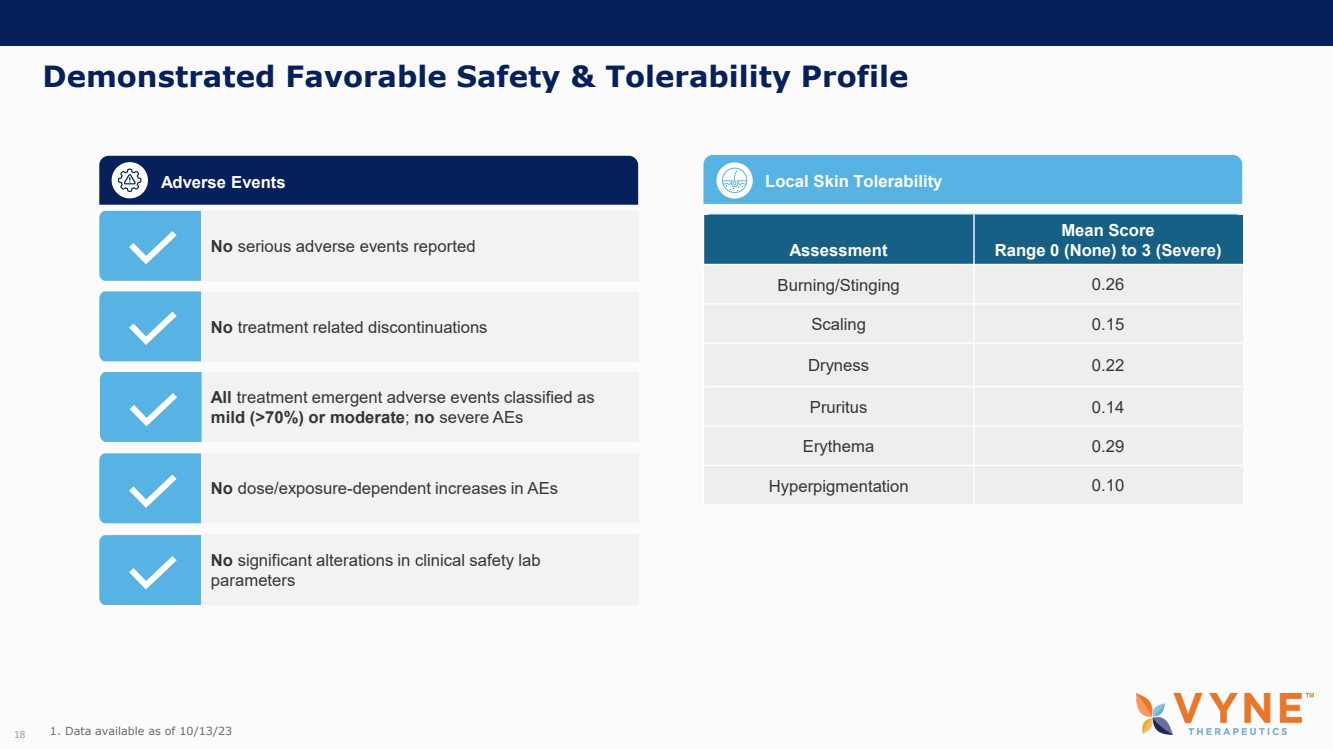
| Demonstrated Favorable Safety & Tolerability Profile
18 1. Data available as of 10/13/23
Adverse Events
No serious adverse events reported
No treatment related discontinuations
All treatment emergent adverse events classified as
mild (>70%) or moderate; no severe AEs
No dose/exposure-dependent increases in AEs
No significant alterations in clinical safety lab
parameters
Assessment
Mean Score
Range 0 (None) to 3 (Severe)
Burning/Stinging 0.26
Scaling 0.15
Dryness 0.22
Pruritus 0.14
Erythema 0.29
Hyperpigmentation 0.10
Local Skin Tolerability |
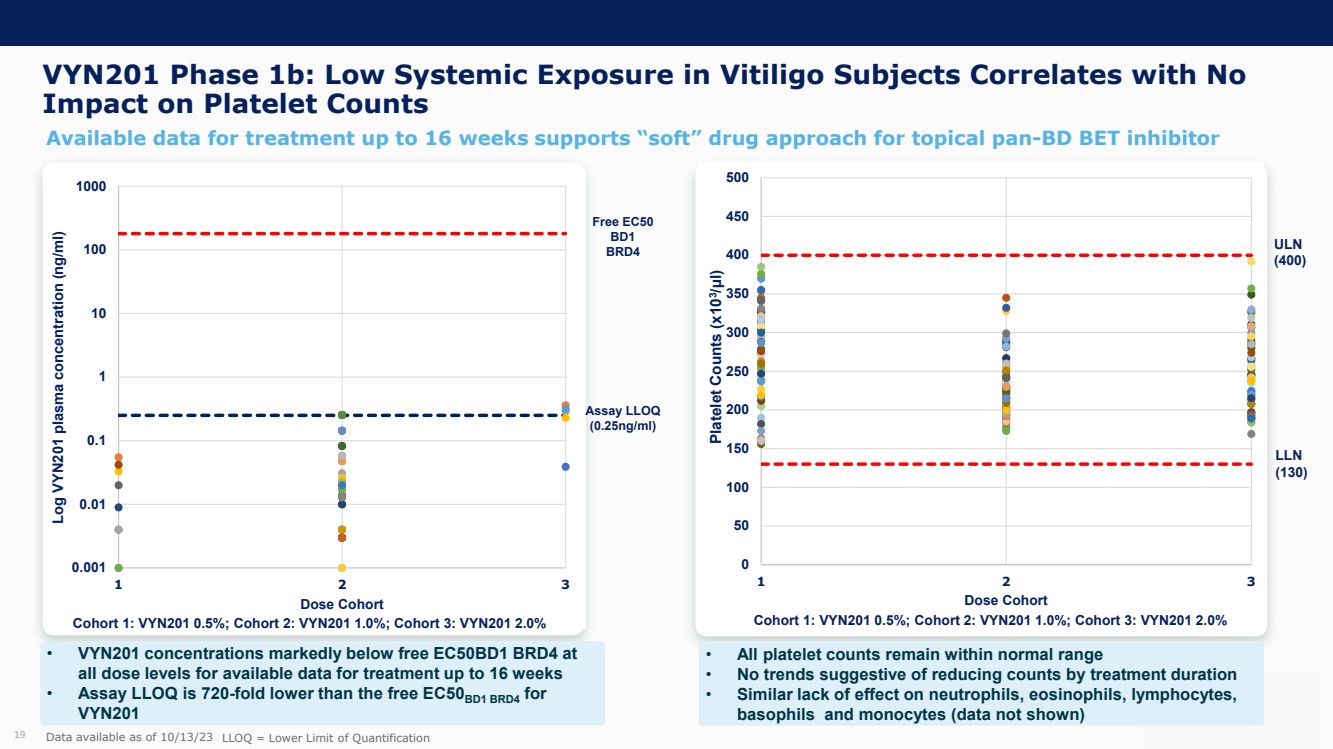
| VYN201 Phase 1b: Low Systemic Exposure in Vitiligo Subjects Correlates with No
Impact on Platelet Counts
19
Available data for treatment up to 16 weeks supports “soft” drug approach for topical pan-BD BET inhibitor
LLOQ = Lower Limit of Quantification
0.001
0.01
0.1
1
10
100
1000
1 2 3
Log VYN201 plasma concentration (ng/ml)
Dose Cohort
Cohort 1: VYN201 0.5%; Cohort 2: VYN201 1.0%; Cohort 3: VYN201 2.0%
Data available as of 10/13/23
Assay LLOQ
(0.25ng/ml)
Free EC50
BD1
BRD4
• VYN201 concentrations markedly below free EC50BD1 BRD4 at
all dose levels for available data for treatment up to 16 weeks
• Assay LLOQ is 720-fold lower than the free EC50BD1 BRD4 for
VYN201
0
50
100
150
200
250
300
350
400
450
500
1 2 3
Platelet Counts (x103/µl)
Dose Cohort
Cohort 1: VYN201 0.5%; Cohort 2: VYN201 1.0%; Cohort 3: VYN201 2.0%
ULN
(400)
LLN
(130)
• All platelet counts remain within normal range
• No trends suggestive of reducing counts by treatment duration
• Similar lack of effect on neutrophils, eosinophils, lymphocytes,
basophils and monocytes (data not shown) |

| Vitiligo Disease Activity within PoC Study Designs
20
Patients with Active Disease Will Continue to Depigment
in Areas that Show High Activity without Therapy5
VYNE expects to enroll patients with active and stable
disease in P2b study
Patient Population and Asset Development Status
Fig 1:
Vitiligo of the hand
with confetti-like
depigmentation
Fig 2:
16 weeks later
Showing extension
of depigmentation
and new areas of
confetti-like
depigmentation
Disease Activity Status
VYN201 P1b
(VYNE) • Active Only • PoC achieved
• Phase 1b completed
Litfulo® (ritlecitinib) P2b1
(Pfizer) • Active Only
• Pfizer currently
enrolling P3 study
evaluating 50 mg QD
dose in active & stable
disease
Povorcitinib P2b2
(Incyte)
• Active & Stable • Incyte preparing for
Phase 3 program
Opzelura® (ruxolitinib) P2b3
(Incyte) • Active & Stable
• 1.5% BID dose
approved in the U.S.
(July 2022) and EU
(April 2023)
Rinvoq® (upadacitinib) P2b4
(AbbVie) • Active & Stable • Phase 2b completed
Physicians expect patients with active disease
to be more difficult to treat Source: Data on file; 1. NCT03715829; 2. NCT04818346; 3. NCT03099304; 4. NCT04927975;
5. Sosa et al, J Am Acad Dermatol 2015 |
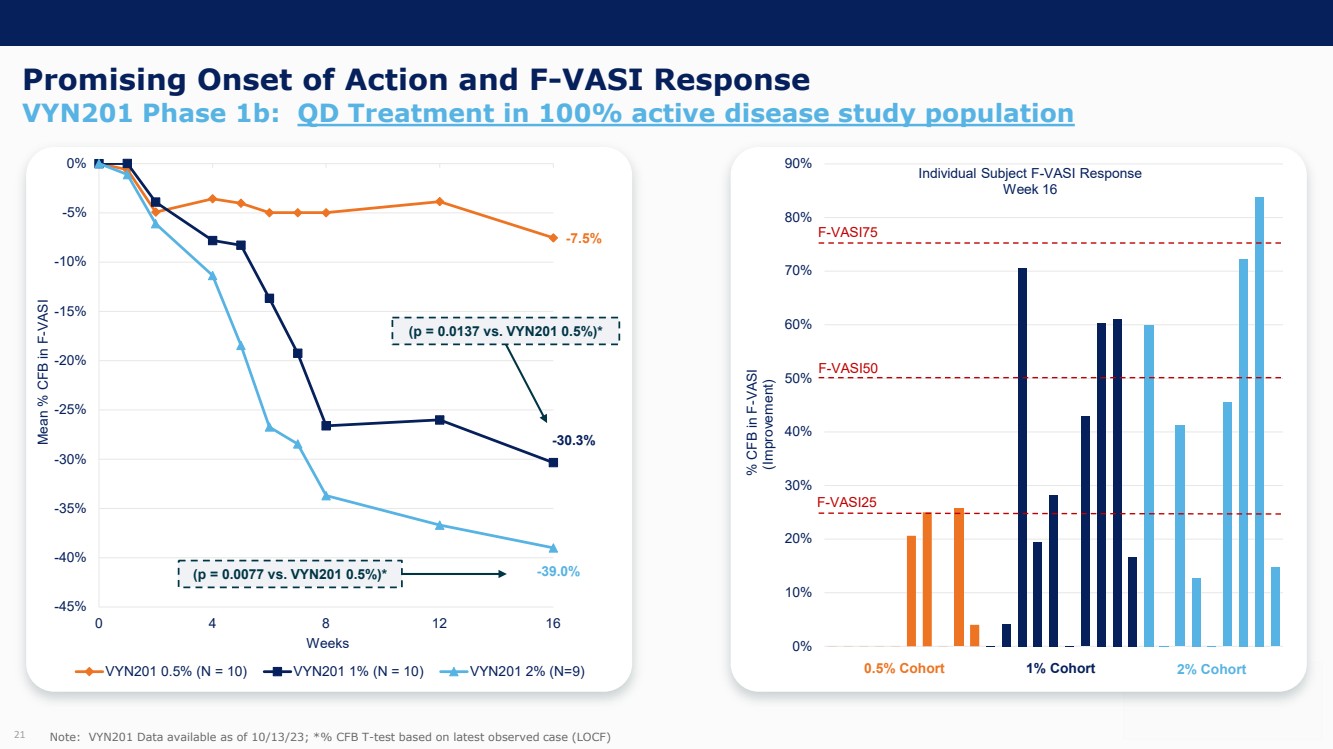
| Promising Onset of Action and F-VASI Response
VYN201 Phase 1b: QD Treatment in 100% active disease study population
21
-7.5%
-30.3%
-39.0%
-45%
-40%
-35%
-30%
-25%
-20%
-15%
-10%
-5%
0%
0 4 8 12 16
Mean % CFB in F-VASI
Weeks
VYN201 0.5% (N = 10) VYN201 1% (N = 10) VYN201 2% (N=9)
(p = 0.0137 vs. VYN201 0.5%)*
Note: VYN201 Data available as of 10/13/23; *% CFB T-test based on latest observed case (LOCF)
(p = 0.0077 vs. VYN201 0.5%)*
2% Cohort
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
% CFB in F-VASI
(Improvement)
Individual Subject F-VASI Response
Week 16
0.5% Cohort 1% Cohort
F-VASI50
F-VASI25
F-VASI75 |
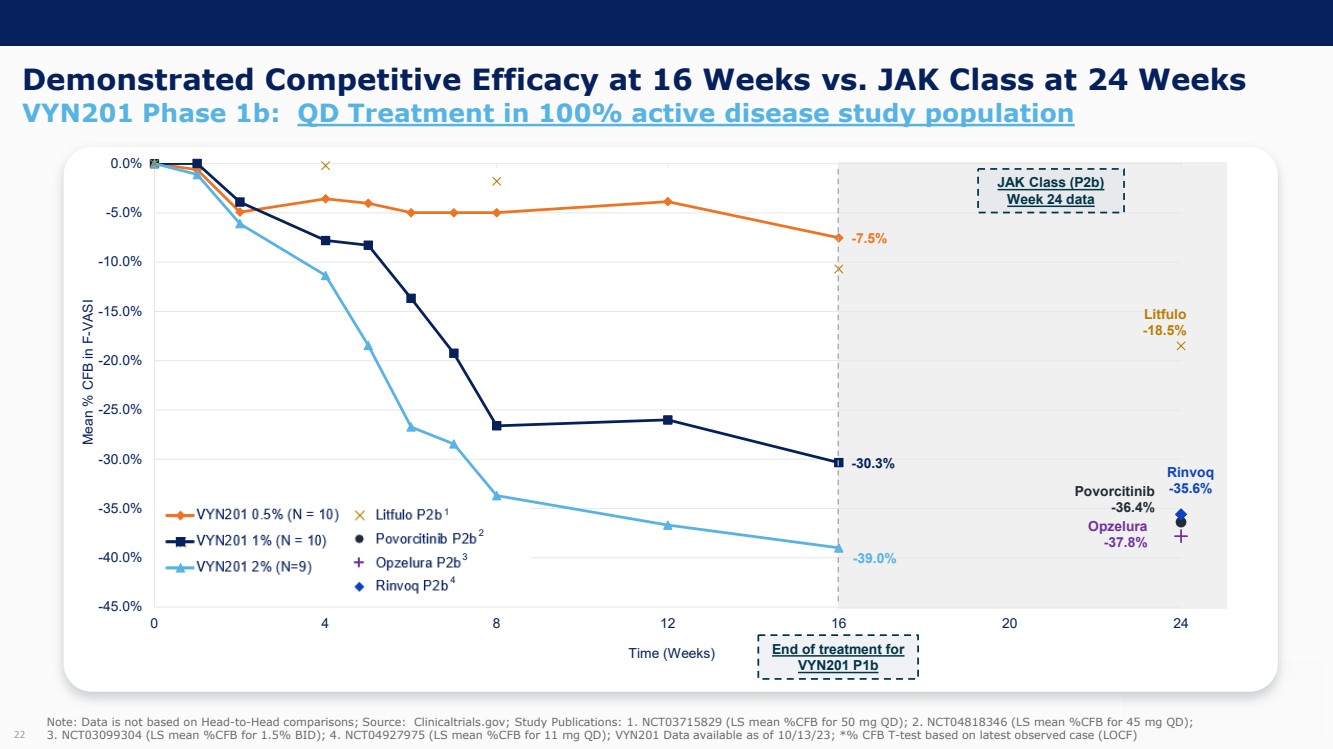
| -7.5%
-30.3%
-39.0%
Litfulo
-18.5%
Povorcitinib
-36.4%
Opzelura
-37.8%
Rinvoq
-35.6%
-45.0%
-40.0%
-35.0%
-30.0%
-25.0%
-20.0%
-15.0%
-10.0%
-5.0%
0.0%
0 4 8 12 16 20 24
Mean % CFB in F-VASI
Time (Weeks)
JAK Class (P2b)
Week 24 data
End of treatment for
VYN201 P1b
Demonstrated Competitive Efficacy at 16 Weeks vs. JAK Class at 24 Weeks
VYN201 Phase 1b: QD Treatment in 100% active disease study population
22
Note: Data is not based on Head-to-Head comparisons; Source: Clinicaltrials.gov; Study Publications: 1. NCT03715829 (LS mean %CFB for 50 mg QD); 2. NCT04818346 (LS mean %CFB for 45 mg QD);
3. NCT03099304 (LS mean %CFB for 1.5% BID); 4. NCT04927975 (LS mean %CFB for 11 mg QD); VYN201 Data available as of 10/13/23; *% CFB T-test based on latest observed case (LOCF)
1
2
3
4 |
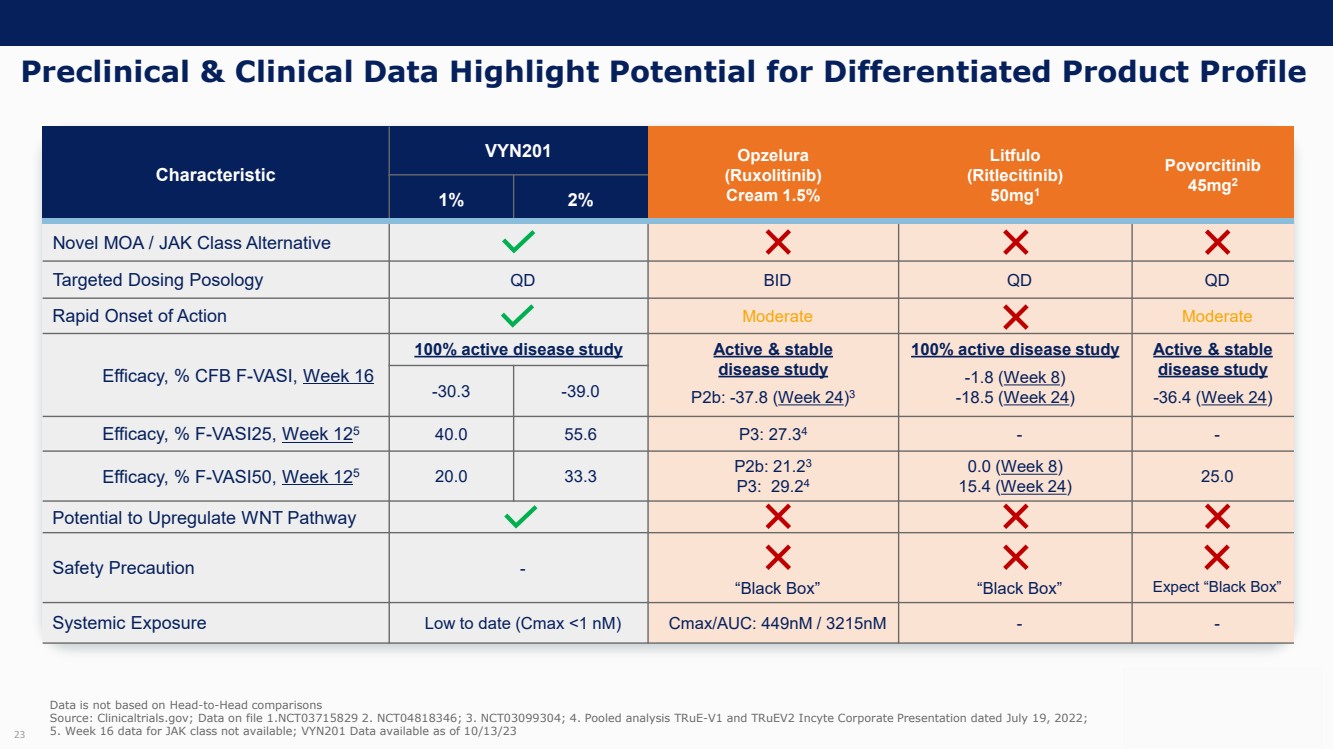
| Characteristic
VYN201 Opzelura
(Ruxolitinib)
Cream 1.5%
Litfulo
(Ritlecitinib)
50mg1
Povorcitinib
45mg2
1% 2%
Novel MOA / JAK Class Alternative
Targeted Dosing Posology QD BID QD QD
Rapid Onset of Action Moderate Moderate
Efficacy, % CFB F-VASI, Week 16
100% active disease study Active & stable
disease study
P2b: -37.8 (Week 24)3
100% active disease study
-1.8 (Week 8)
-18.5 (Week 24)
Active & stable
disease study
-36.4 (Week 24) -30.3 -39.0
Efficacy, % F-VASI25, Week 125 40.0 55.6 P3: 27.34 - -
Efficacy, % F-VASI50, Week 125 20.0 33.3 P2b: 21.23
P3: 29.24
0.0 (Week 8)
15.4 (Week 24) 25.0
Potential to Upregulate WNT Pathway
Safety Precaution -
“Black Box” “Black Box” Expect “Black Box”
Systemic Exposure Low to date (Cmax <1 nM) Cmax/AUC: 449nM / 3215nM - -
Preclinical & Clinical Data Highlight Potential for Differentiated Product Profile
23
Data is not based on Head-to-Head comparisons
Source: Clinicaltrials.gov; Data on file 1.NCT03715829 2. NCT04818346; 3. NCT03099304; 4. Pooled analysis TRuE-V1 and TRuEV2 Incyte Corporate Presentation dated July 19, 2022;
5. Week 16 data for JAK class not available; VYN201 Data available as of 10/13/23 |
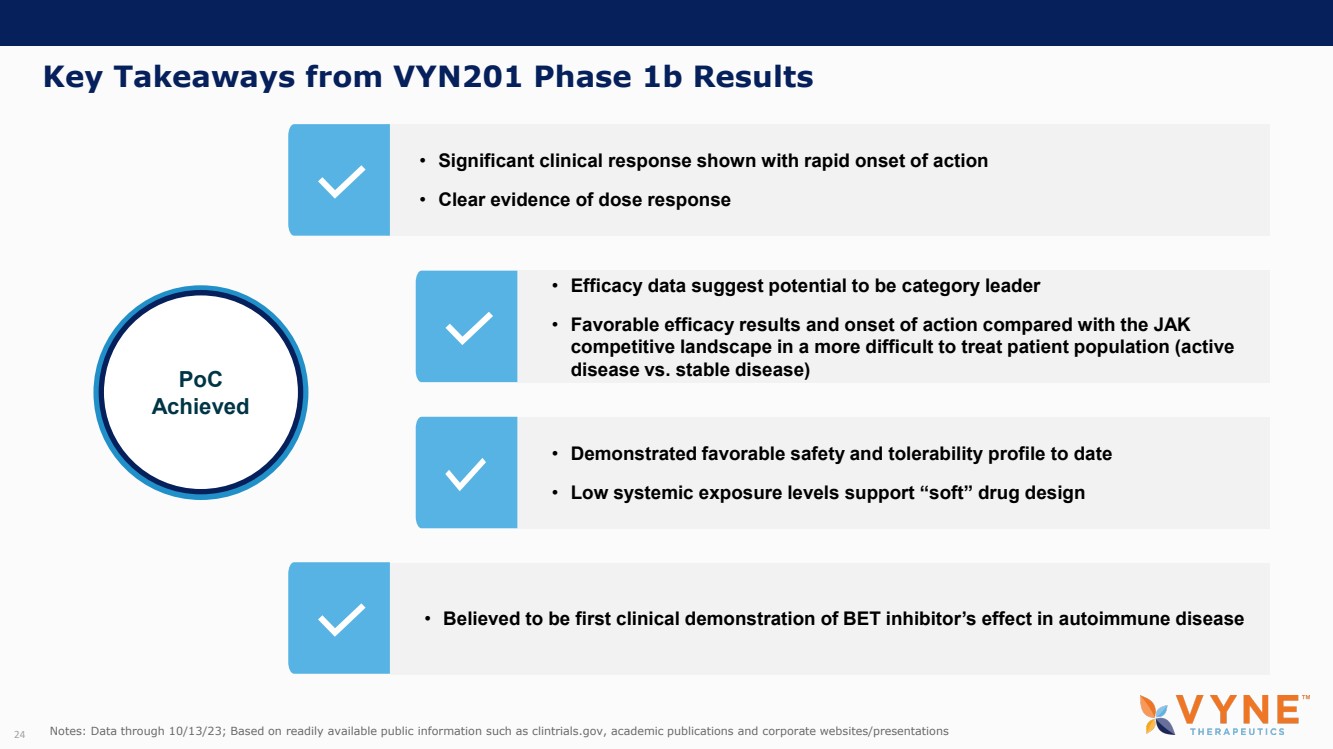
| Key Takeaways from VYN201 Phase 1b Results
24 Notes: Data through 10/13/23; Based on readily available public information such as clintrials.gov, academic publications and corporate websites/presentations
• Significant clinical response shown with rapid onset of action
• Clear evidence of dose response
PoC
Achieved
• Efficacy data suggest potential to be category leader
• Favorable efficacy results and onset of action compared with the JAK
competitive landscape in a more difficult to treat patient population (active
disease vs. stable disease)
• Believed to be first clinical demonstration of BET inhibitor’s effect in autoimmune disease
• Demonstrated favorable safety and tolerability profile to date
• Low systemic exposure levels support “soft” drug design |

| VYN202: BD2 selective BET inhibitor |
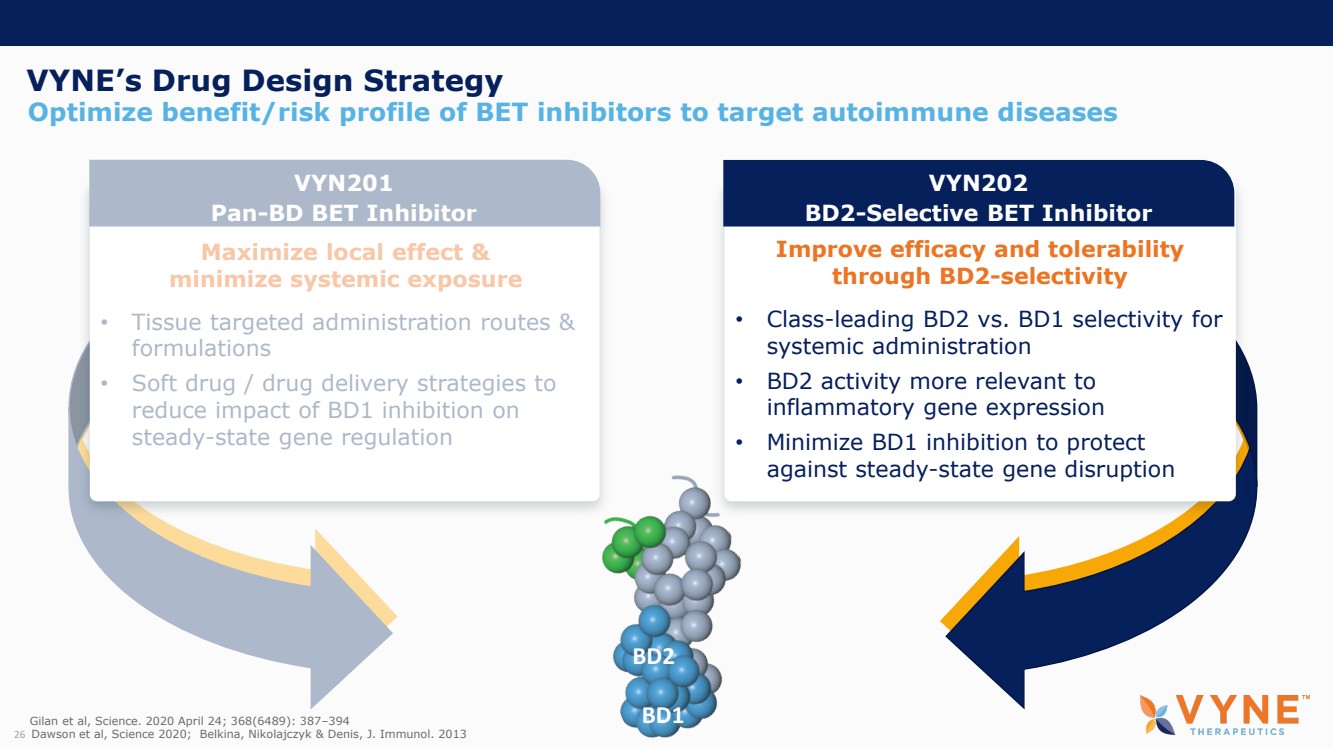
| Improve efficacy and tolerability
through BD2-selectivity
• Class-leading BD2 vs. BD1 selectivity for
systemic administration
• BD2 activity more relevant to
inflammatory gene expression
• Minimize BD1 inhibition to protect
against steady-state gene disruption
VYNE’s Drug Design Strategy
26 Dawson et al, Science 2020; Belkina, Nikolajczyk & Denis, J. Immunol. 2013
Optimize benefit/risk profile of BET inhibitors to target autoimmune diseases
VYN201
Pan-BD BET Inhibitor
Maximize local effect &
minimize systemic exposure
• Tissue targeted administration routes &
formulations
• Soft drug / drug delivery strategies to
reduce impact of BD1 inhibition on
steady-state gene regulation
VYN202
BD2-Selective BET Inhibitor
BD1
BD2
Gilan et al, Science. 2020 April 24; 368(6489): 387–394 |

| Maximizing On-target Potency vs. BD2 and Minimizing Affinity to BD1 May
Be The Key to Optimizing the Benefit/Risk Profile of BET Inhibitors for
Autoimmune Diseases
27
Compound ID Potency vs. BD2*
(nM)
Selectivity**
(BD1/BD2)
VYN202 (VYNE) < >>>>
NUV-868 (Nuvation)2 2 1,460x (FRET)
ABBV-744 (AbbVie)3 28 753x (FRET)
GSK620 (GSK)4 79 220x
Pelabresib (Constellation)2 17 5x (FRET)
ABBV-0753 13 2.6x
MK-8628/OTX-0155 26 1.5x
BI-8949996 41 0.1x
*Lower number denotes higher potency
**Higher number denotes higher selectivity. Data based on nanoBRET assay unless otherwise indicated.
1. Based on readily available public information such as clintrials.gov, academic publications and corporate websites/presentations. 2. Nuvation corporate presentation (May 2023); 3. Faivre et al 2020; 4. Delmont et al 2020; 5.
Wang et al 2017 6. Kraut et al 2018; Data on file
VYN202 is believed to be the most potent and BD2-selective BET Inhibitor in development1 which is designed to improve efficacy and tolerability
BD1
BD2
EXT
BD1 regulates “housekeeping”
gene activity
BD2 activity is associated with
inflammatory gene induction
that drives inflammatory
responses in autoimmune disease |

| Strategies to Control Dysregulated TH17 Immune Cell Activity
Inhibitors of receptor signal transduction
(JAK/TYK2 inhibitors via IL23/TNFα mediation:
tofacitinib, upadacitinib, deucravacitinib, TAK-279)
Inhibitors of TH17 cell differentiation
(risankizumab, tildrakizumab, guselkumab, ustekinumab)
IL-6, TGF-β1, IL-1, IL-23
Inhibitors of IL-17A & IL-17A-F
(secukinumab, ixekizumab, bimekizumab, DC-806)
Inhibitors of TNFα
(adalimumab, etanercept, infliximab)
Inhibitors of IL-17RA
(brodalumab)
IL-21 IL-22
IL-17
TNFR2
Naïve
TH0 cell TH17 cell
Existing strategies target:
• Extracellular cytokines
• Cytokine receptor
inhibition or
• Inhibition of trans-cellular inflammatory
signal propagation
28 Data on file; Not exhaustive |

| 29
BET Inhibitors Disrupt Inflammatory Gene Transcription in T Cells to Directly
Address the Complex Signaling of Immuno- & Fibro-inflammatory Diseases
ACTIVE gene
VYN202
Transcription
machinery
NF-Κb complex
signal
transduction
Translation
Transcription
failure
Fully
expressed
cytokine
Cytokine
down regulation
3 1
BD2
Inactive gene via
BET inhibition
2
BET INHIBITION
Clinical PoC of BET
inhibition in I&I disease
demonstrated in VYN201
P1b vitiligo trial
Adapted from Gonzalez-Gay et al, Autoimmunity Rev, (2020) 19: 102429 and Rusinol and Puig, Int J Mol Sci, (2023) 24: 3391 |
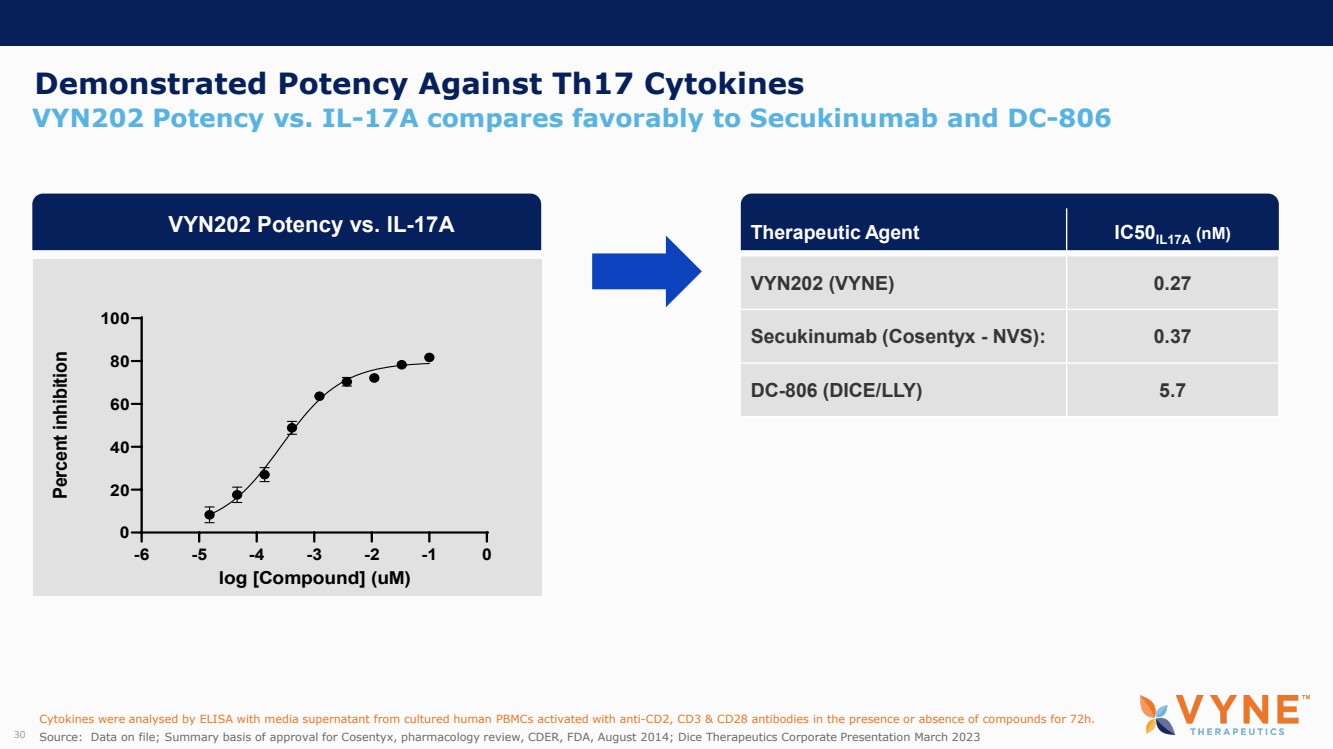
| -6 -5 -4 -3 -2 -1 0
0
20
40
60
80
100
BA83_IL17
log [Compound] (uM)
Percent inhibition
Demonstrated Potency Against Th17 Cytokines
30 Source: Data on file; Summary basis of approval for Cosentyx, pharmacology review, CDER, FDA, August 2014; Dice Therapeutics Corporate Presentation March 2023
VYN202 Potency vs. IL-17A compares favorably to Secukinumab and DC-806
Cytokines were analysed by ELISA with media supernatant from cultured human PBMCs activated with anti-CD2, CD3 & CD28 antibodies in the presence or absence of compounds for 72h.
VYN202 Potency vs. IL-17A Therapeutic Agent IC50IL17A (nM)
VYN202 (VYNE) 0.27
Secukinumab (Cosentyx - NVS): 0.37
DC-806 (DICE/LLY) 5.7 |

| Cytokine VYN202 IC50 (nM) Selectivity vs. CXCL10
IL-17A 0.27 >13,000
IL-22 0.21 >17,000
CXCL10 3637 -
Minimal Inhibition on Key Innate Immune Chemokine Demonstrates
Selectivity of Cytokine Expression (IL-17A and IL-22 vs. CXCL10)
31 Source: Data on file
Analysed by ELISA with media supernatant from human PBMCs stimulated with IFN-γ for 24h.
• VYN202 significantly more potent against Th17
Cytokines vs. CXCL10
• CXCL10 interacts with CXCR3 to attract Th1 cells,
eosinophils, monocytes, and NK cells to sites of
inflammation and is known to be an important part of host
defense mechanism |

| Preclinical Safety Data |

| Dog Toxicokinetics & Effect on Hematopoietic system
• VYN202 exposure significantly above free EC50BD2 BRD4 at 1,3 & 10mg/kg QD for 24 hours
• No effect on platelet counts
33
1
10
100
1000
10000
100000
0.5 1 2 4 8 24
Log VYN202 plasma concentration (ng/ml)
Timepoint (Hours)
1mg/kg/day
3mg/kg/day
10mg/kg/day
EC50BD1 BRD4
EC50BD2 BRD4
0
50
100
150
200
250
300
350
400
450
500
0 1 2 3 4 5 6 7 8 9 10
Mean platelet count (x103/µl)
VYN202 (mg/kg/day QD PO)
Max
count
Min
count
VYN202 Exposure after 14 Days of Treatment
at 1, 3 and 10mg/kg QD PO
Effect of VYN202 on Platelet Counts
(Day 15)
• VYN202 exposure above free EC50BD2 BRD4 at all dose for 24hrs
• No exposure differences between males and females
• No evidence of reduced or reducing platelet counts |

| Effect on Gastrointestinal (GI) System
34
VYN202 (BD2 Selective)
Vehicle
ABBV-075 (Pan-BD BET inhibitor)
Depleted goblet
cells
VYN202 (BD2 Selective)
10mg/kg
Goblet cells
GI villus
Images for VYN202 representative from histopathology of the duodenum (H&E stain). Comparable results obtained for ileum and jejunum.
GI villus
Goblet cells
* Faivre EJ et al, Nature, 578, 306-310 (2020)
Oral pan-BD BET inhibitors (like ABBV-075) are
known to negatively impact GI mucosa via goblet
cell toxicity, leading to related gastrointestinal
adverse events in the clinic*
Treatment with BD2 selective VYN202 had no effect on GI villus morphology or goblet cell number
3mg/kg QD PO 28d (rat)*
QD PO 15d (dog) QD PO 15d (dog)
Compared to pan-BD BET inhibitors |

| Preclinical Efficacy Models |

| VYN202: Psoriasis Model |
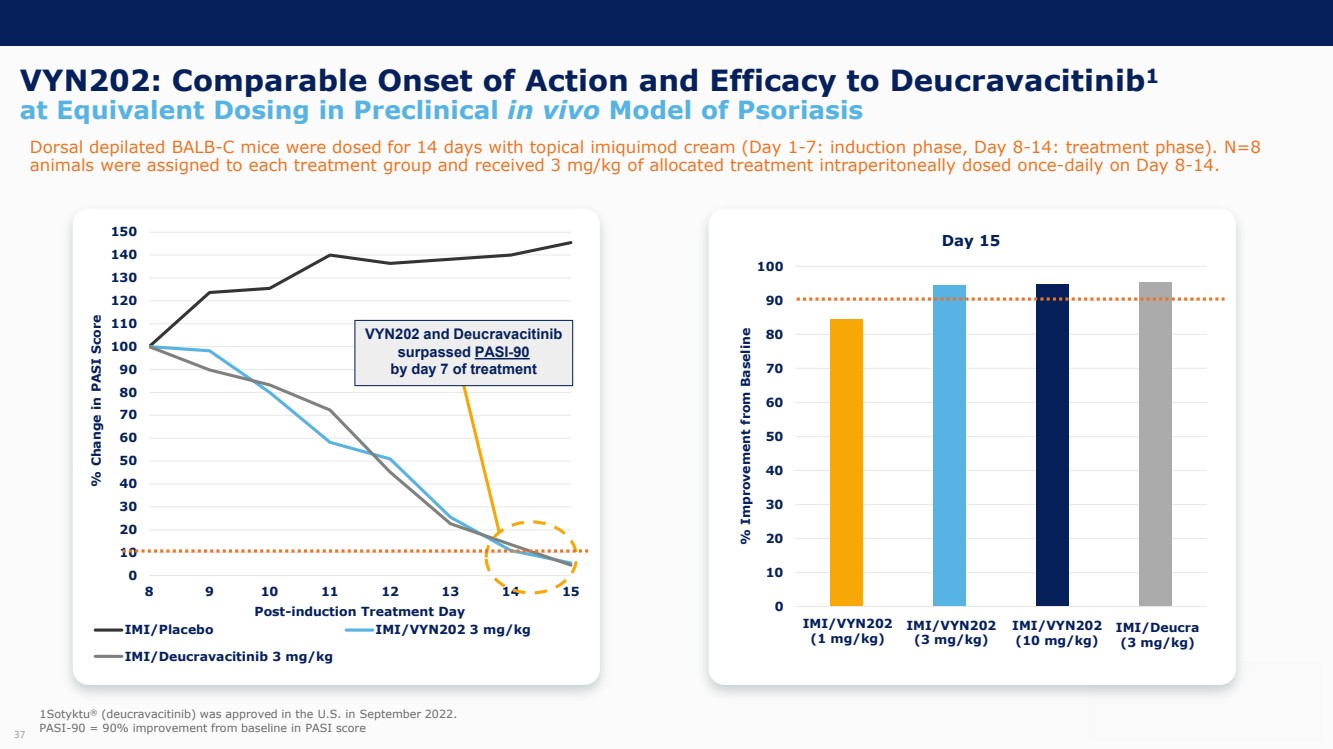
| 1Sotyktu® (deucravacitinib) was approved in the U.S. in September 2022.
PASI-90 = 90% improvement from baseline in PASI score
Dorsal depilated BALB-C mice were dosed for 14 days with topical imiquimod cream (Day 1-7: induction phase, Day 8-14: treatment phase). N=8
animals were assigned to each treatment group and received 3 mg/kg of allocated treatment intraperitoneally dosed once-daily on Day 8-14.
37
0
10
20
30
40
50
60
70
80
90
100
110
120
130
140
150
8 9 10 11 12 13 14 15
% Change in PASI Score
Post-induction Treatment Day
IMI/Placebo IMI/VYN202 3 mg/kg
IMI/Deucravacitinib 3 mg/kg
VYN202 and Deucravacitinib
surpassed PASI-90
by day 7 of treatment
VYN202: Comparable Onset of Action and Efficacy to Deucravacitinib1
at Equivalent Dosing in Preclinical in vivo Model of Psoriasis
0
10
20
30
40
50
60
70
80
90
100
% Improvement from Baseline
Day 15
IMI/VYN202
(1 mg/kg)
IMI/VYN202
(3 mg/kg)
IMI/VYN202
(10 mg/kg)
IMI/Deucra
(3 mg/kg) |

| VYN202 Reduced Key Cytokines Associated with Pathogenesis of Psoriasis
in Preclinical in vivo Model
38
-
10
20
30
40
50
60
70
80
IL-17 IL-22
Pg/ml
IMI/Placebo
IMI/VYN202
(1mg/kg)
IMI/VYN202
(3mg/kg)
IMI/VYN202
(10mg/kg)
IMI/Deucra
(3mg/kg)
-
5
10
15
IL-1β IL-6 TNF-α IL-23
Pg/ml
IMI/Placebo
IMI/VYN202
(1mg/kg)
IMI/VYN202
(3mg/kg)
IMI/VYN202
(10mg/kg)
IMI/Deucra
(3mg/kg)
>93% lower expression of IL-17
for VYN202 vs. Placebo
IMI = imiquimod
• >93% lower expression of IL-17 at all VYN202 doses
compared to placebo • Marked reduction of other disease related Th17 and Th1
cytokines |
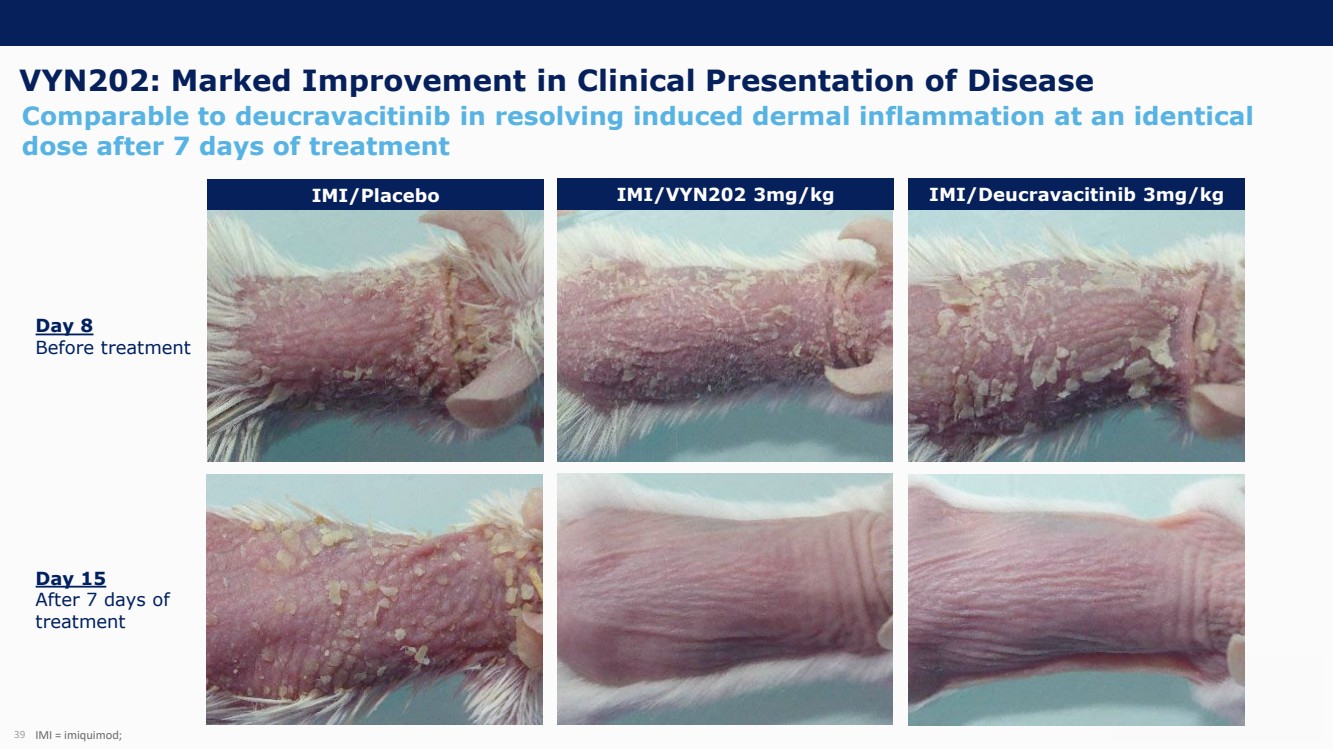
| VYN202: Marked Improvement in Clinical Presentation of Disease
IMI/Placebo IMI/VYN202 3mg/kg
39
Day 8
Before treatment
Day 15
After 7 days of
treatment
IMI/Deucravacitinib 3mg/kg
IMI = imiquimod;
Comparable to deucravacitinib in resolving induced dermal inflammation at an identical
dose after 7 days of treatment |

| VYN202: Rheumatoid Arthritis Model |
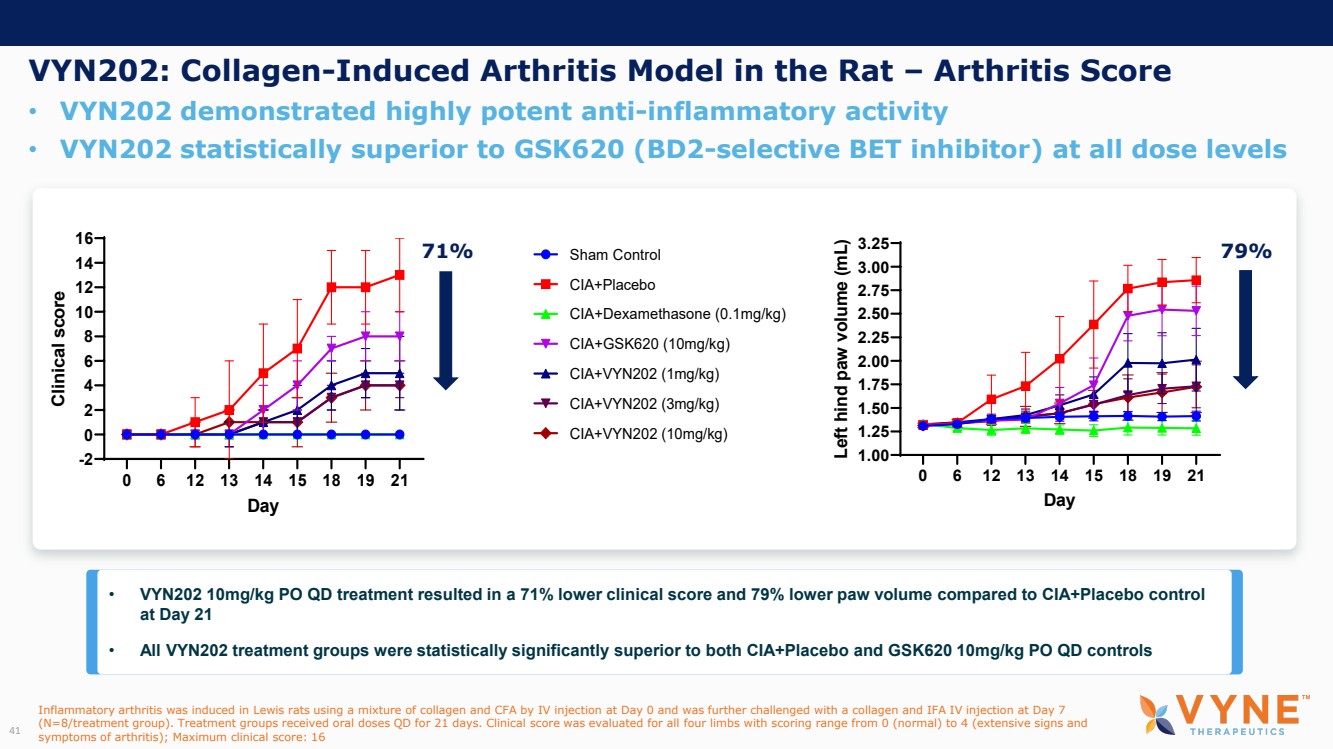
| VYN202: Collagen-Induced Arthritis Model in the Rat – Arthritis Score
• VYN202 demonstrated highly potent anti-inflammatory activity
• VYN202 statistically superior to GSK620 (BD2-selective BET inhibitor) at all dose levels
41
Inflammatory arthritis was induced in Lewis rats using a mixture of collagen and CFA by IV injection at Day 0 and was further challenged with a collagen and IFA IV injection at Day 7
(N=8/treatment group). Treatment groups received oral doses QD for 21 days. Clinical score was evaluated for all four limbs with scoring range from 0 (normal) to 4 (extensive signs and
symptoms of arthritis); Maximum clinical score: 16
0 6 12 13 14 15 18 19 21
-2
0
2
4
6
8
10
12
14
16
Day
Clinical score
Sham Control
CIA+Placebo
CIA+Dexamethasone (0.1mg/kg)
CIA+GSK620 (10mg/kg)
CIA+VYN202 (1mg/kg)
CIA+VYN202 (3mg/kg)
CIA+VYN202 (10mg/kg)
0 6 12 13 14 15 18 19 21
1.00
1.25
1.50
1.75
2.00
2.25
2.50
2.75
3.00
3.25
Day
Left hind paw volume (mL)
71% 79%
• VYN202 10mg/kg PO QD treatment resulted in a 71% lower clinical score and 79% lower paw volume compared to CIA+Placebo control
at Day 21
• All VYN202 treatment groups were statistically significantly superior to both CIA+Placebo and GSK620 10mg/kg PO QD controls |
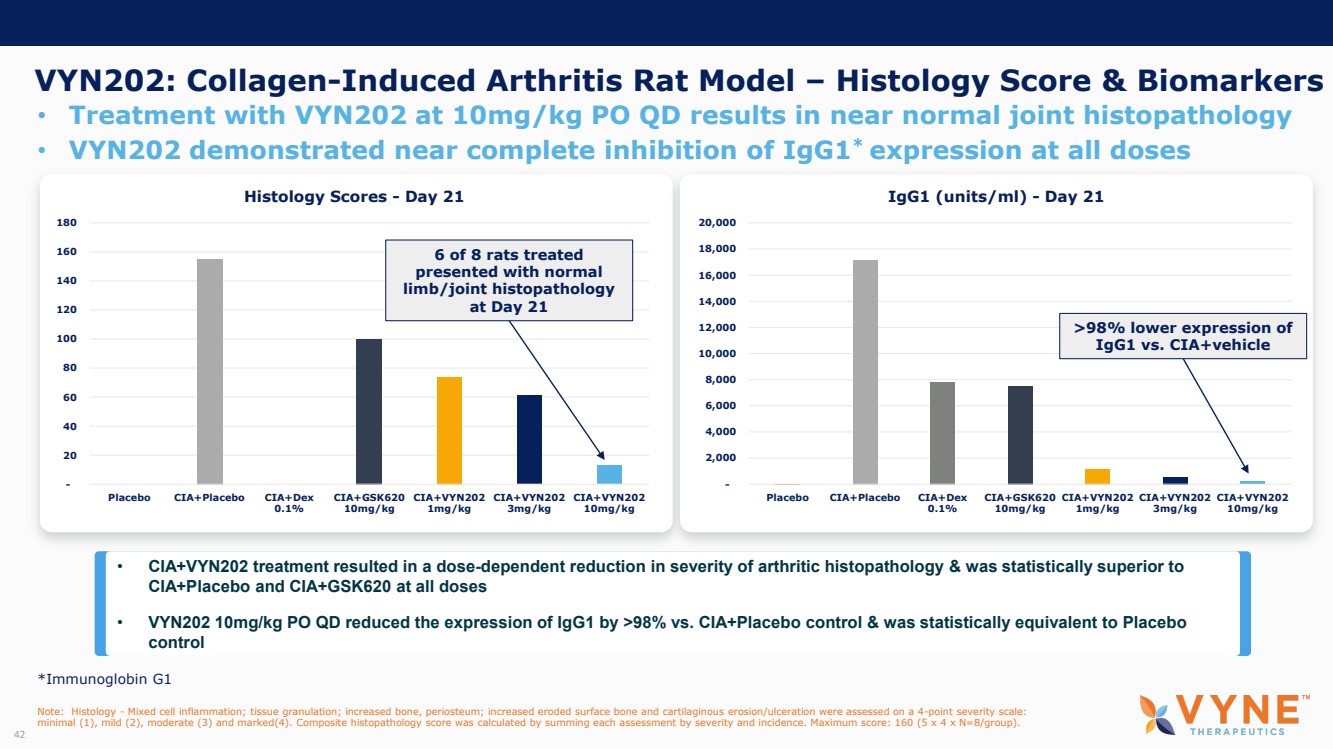
| -
2,000
4,000
6,000
8,000
10,000
12,000
14,000
16,000
18,000
20,000
Placebo CIA+Placebo CIA+Dex
0.1%
CIA+GSK620
10mg/kg
CIA+VYN202
1mg/kg
CIA+VYN202
3mg/kg
CIA+VYN202
10mg/kg
IgG1 (units/ml) - Day 21
VYN202: Collagen-Induced Arthritis Rat Model – Histology Score & Biomarkers
42
• Treatment with VYN202 at 10mg/kg PO QD results in near normal joint histopathology
• VYN202 demonstrated near complete inhibition of IgG1* expression at all doses
*Immunoglobin G1
Note: Histology - Mixed cell inflammation; tissue granulation; increased bone, periosteum; increased eroded surface bone and cartilaginous erosion/ulceration were assessed on a 4-point severity scale:
minimal (1), mild (2), moderate (3) and marked(4). Composite histopathology score was calculated by summing each assessment by severity and incidence. Maximum score: 160 (5 x 4 x N=8/group).
>98% lower expression of
IgG1 vs. CIA+vehicle
-
20
40
60
80
100
120
140
160
180
Placebo CIA+Placebo CIA+Dex
0.1%
CIA+GSK620
10mg/kg
CIA+VYN202
1mg/kg
CIA+VYN202
3mg/kg
CIA+VYN202
10mg/kg
Histology Scores - Day 21
6 of 8 rats treated
presented with normal
limb/joint histopathology
at Day 21
• CIA+VYN202 treatment resulted in a dose-dependent reduction in severity of arthritic histopathology & was statistically superior to
CIA+Placebo and CIA+GSK620 at all doses
• VYN202 10mg/kg PO QD reduced the expression of IgG1 by >98% vs. CIA+Placebo control & was statistically equivalent to Placebo
control |
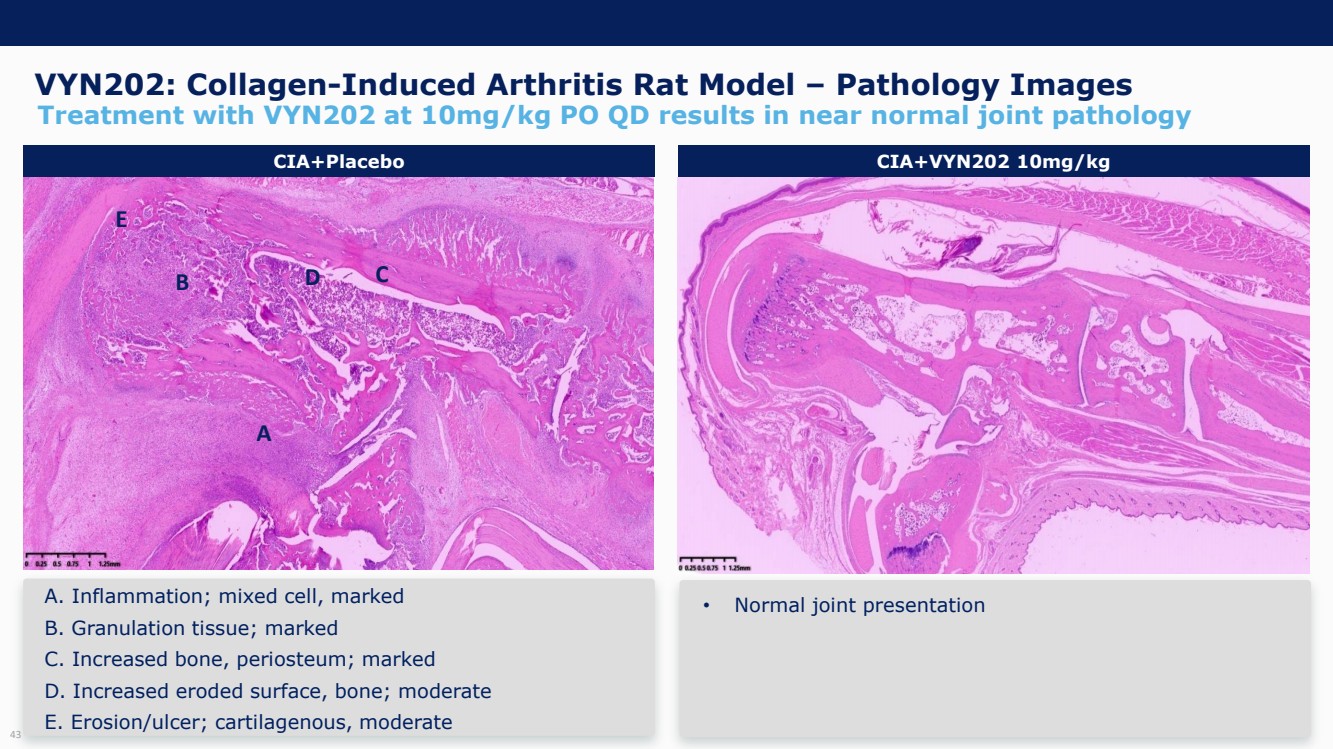
| VYN202: Collagen-Induced Arthritis Rat Model – Pathology Images
43
A. Inflammation; mixed cell, marked
B. Granulation tissue; marked
C. Increased bone, periosteum; marked
D. Increased eroded surface, bone; moderate
E. Erosion/ulcer; cartilagenous, moderate
• Normal joint presentation
B
A
D C
E
CIA+Placebo CIA+VYN202 10mg/kg
Treatment with VYN202 at 10mg/kg PO QD results in near normal joint pathology |
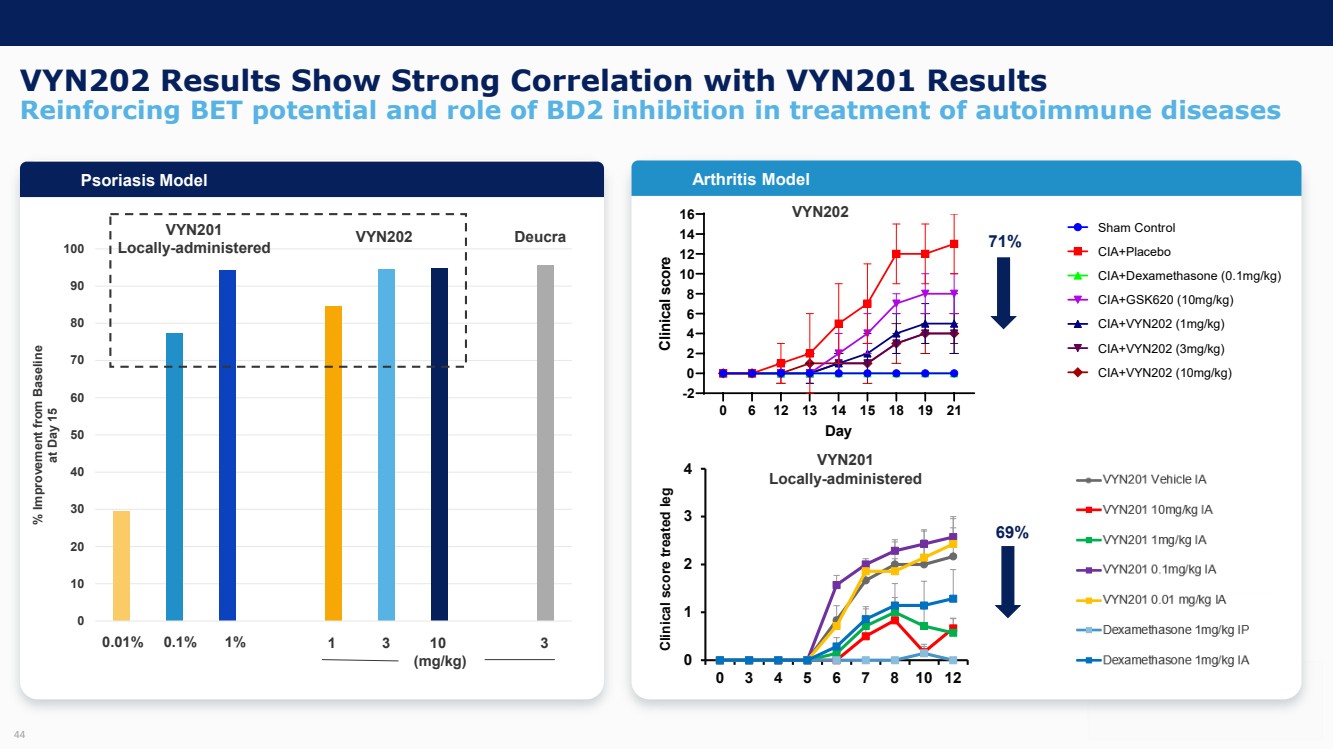
| 0 6 12 13 14 15 18 19 21
-2
0
2
4
6
8
10
12
14
16
Day
Clinical score
Sham Control
CIA+Placebo
CIA+Dexamethasone (0.1mg/kg)
CIA+GSK620 (10mg/kg)
CIA+VYN202 (1mg/kg)
CIA+VYN202 (3mg/kg)
CIA+VYN202 (10mg/kg)
44
VYN202 Results Show Strong Correlation with VYN201 Results
Reinforcing BET potential and role of BD2 inhibition in treatment of autoimmune diseases
0
10
20
30
40
50
60
70
80
90
100
% Improvement from Baseline
at Day 15
Psoriasis Model
VYN201
Locally-administered
0.01% 0.1% 1% 1
(mg/kg)
3 10 3
VYN202 Deucra 71%
0
1
2
3
4
0 3 4 5 6 7 8 10 12
Clinical score treated leg
69%
VYN201
Locally-administered
VYN202
Psoriasis Model Arthritis Model |

| VYN202 Clinical Development Plan
1. Moderate-to-Severe Psoriasis
2. Moderate-to-Severe Active Rheumatoid Arthritis |
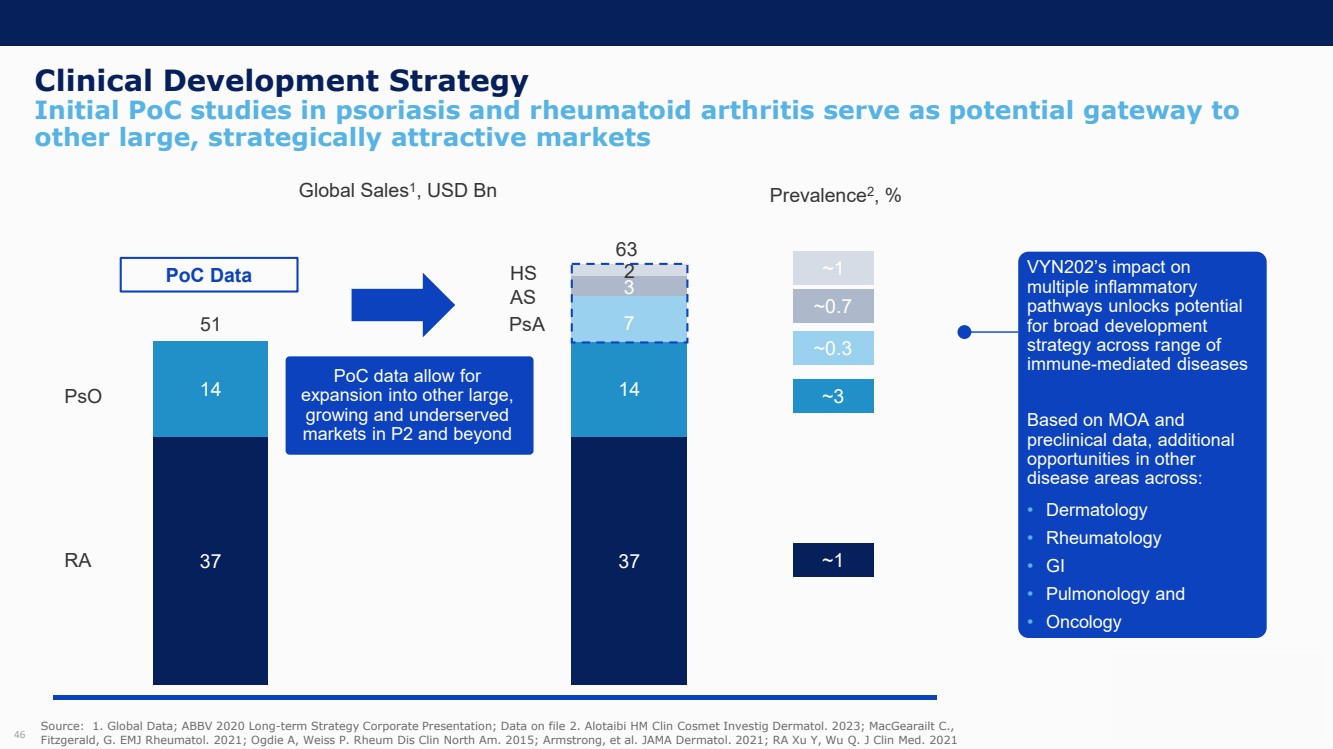
| 37
14
37
14
7
3
2
46 Source: 1. Global Data; ABBV 2020 Long-term Strategy Corporate Presentation; Data on file 2. Alotaibi HM Clin Cosmet Investig Dermatol. 2023; MacGearailt C.,
Fitzgerald, G. EMJ Rheumatol. 2021; Ogdie A, Weiss P. Rheum Dis Clin North Am. 2015; Armstrong, et al. JAMA Dermatol. 2021; RA Xu Y, Wu Q. J Clin Med. 2021
Clinical Development Strategy
63
RA
PsO
PsA
AS
HS
Global Sales1, USD Bn Prevalence2, %
~1
~3
~0.7
~1
~0.3
51
PoC data allow for
expansion into other large,
growing and underserved
markets in P2 and beyond
PoC Data VYN202’s impact on
multiple inflammatory
pathways unlocks potential
for broad development
strategy across range of
immune-mediated diseases
Based on MOA and
preclinical data, additional
opportunities in other
disease areas across:
• Dermatology
• Rheumatology
• GI
• Pulmonology and
• Oncology
Initial PoC studies in psoriasis and rheumatoid arthritis serve as potential gateway to
other large, strategically attractive markets |
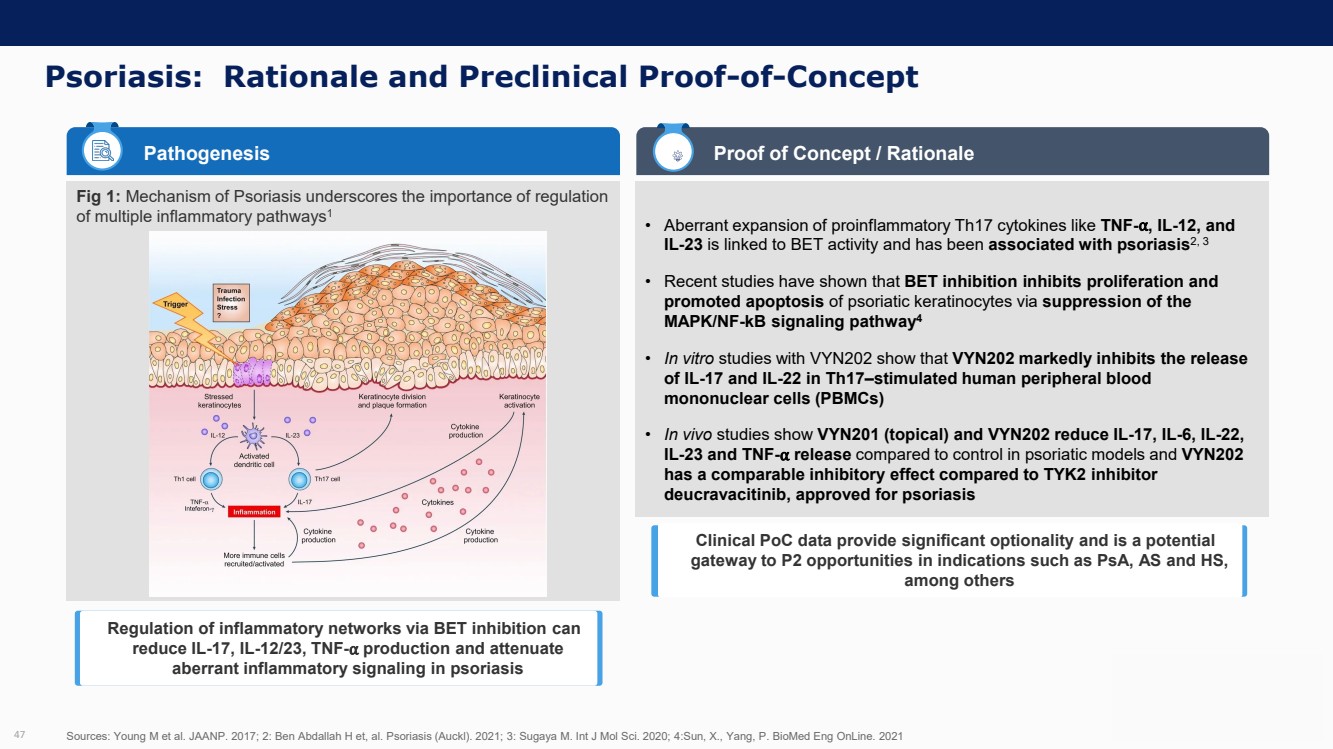
| Psoriasis: Rationale and Preclinical Proof-of-Concept
47 Sources: Young M et al. JAANP. 2017; 2: Ben Abdallah H et, al. Psoriasis (Auckl). 2021; 3: Sugaya M. Int J Mol Sci. 2020; 4:Sun, X., Yang, P. BioMed Eng OnLine. 2021
Proof of Concept / Rationale
Fig 1: Mechanism of Psoriasis underscores the importance of regulation
of multiple inflammatory pathways1
Regulation of inflammatory networks via BET inhibition can
reduce IL-17, IL-12/23, TNF-⍺ production and attenuate
aberrant inflammatory signaling in psoriasis
Pathogenesis
• Aberrant expansion of proinflammatory Th17 cytokines like TNF-⍺, IL-12, and
IL-23 is linked to BET activity and has been associated with psoriasis2, 3
• Recent studies have shown that BET inhibition inhibits proliferation and
promoted apoptosis of psoriatic keratinocytes via suppression of the
MAPK/NF-kB signaling pathway4
• In vitro studies with VYN202 show that VYN202 markedly inhibits the release
of IL-17 and IL-22 in Th17–stimulated human peripheral blood
mononuclear cells (PBMCs)
• In vivo studies show VYN201 (topical) and VYN202 reduce IL-17, IL-6, IL-22,
IL-23 and TNF-⍺ release compared to control in psoriatic models and VYN202
has a comparable inhibitory effect compared to TYK2 inhibitor
deucravacitinib, approved for psoriasis
Clinical PoC data provide significant optionality and is a potential
gateway to P2 opportunities in indications such as PsA, AS and HS,
among others |
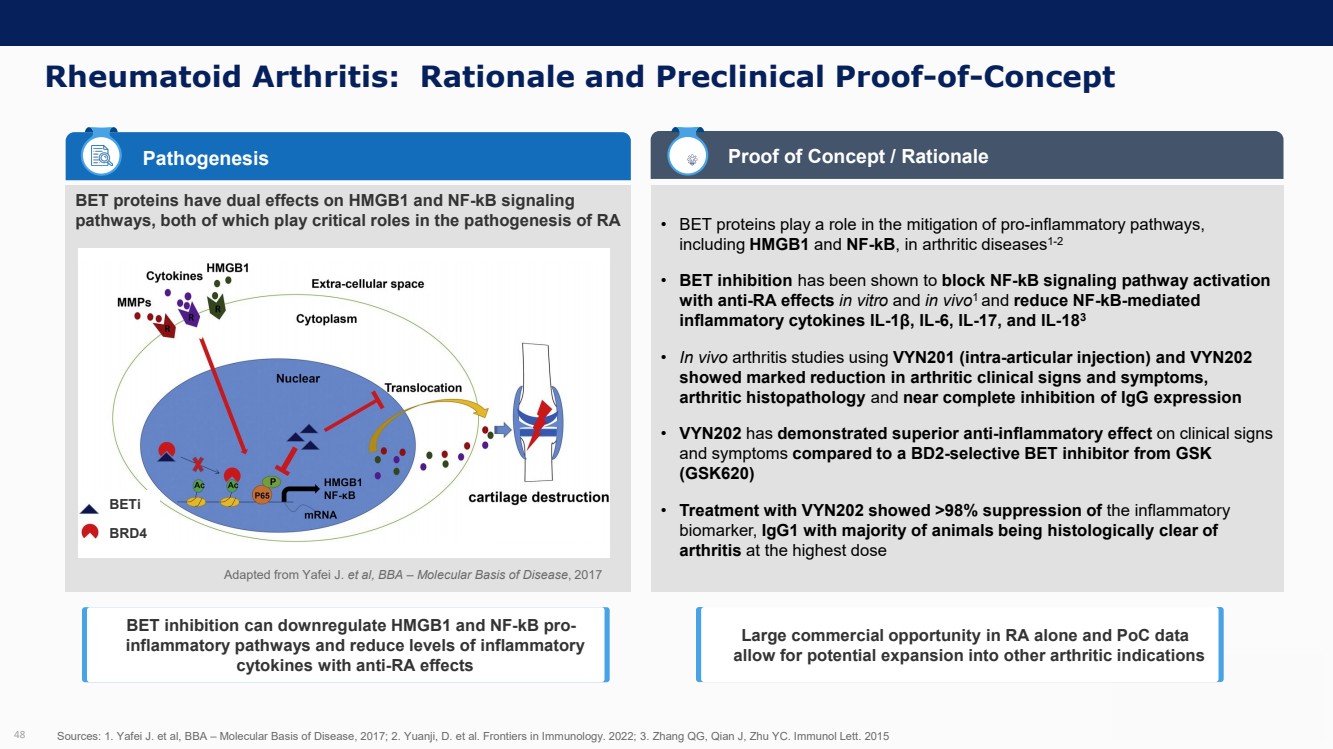
| Rheumatoid Arthritis: Rationale and Preclinical Proof-of-Concept
48 Sources: 1. Yafei J. et al, BBA – Molecular Basis of Disease, 2017; 2. Yuanji, D. et al. Frontiers in Immunology. 2022; 3. Zhang QG, Qian J, Zhu YC. Immunol Lett. 2015
BET proteins have dual effects on HMGB1 and NF-kB signaling
pathways, both of which play critical roles in the pathogenesis of RA
BET inhibition can downregulate HMGB1 and NF-kB pro-inflammatory pathways and reduce levels of inflammatory
cytokines with anti-RA effects
Pathogenesis
Adapted from Yafei J. et al, BBA – Molecular Basis of Disease, 2017
BETi
BRD4
Proof of Concept / Rationale
• BET proteins play a role in the mitigation of pro-inflammatory pathways,
including HMGB1 and NF-kB, in arthritic diseases1-2
• BET inhibition has been shown to block NF-kB signaling pathway activation
with anti-RA effects in vitro and in vivo1 and reduce NF-kB-mediated
inflammatory cytokines IL-1β, IL-6, IL-17, and IL-183
• In vivo arthritis studies using VYN201 (intra-articular injection) and VYN202
showed marked reduction in arthritic clinical signs and symptoms,
arthritic histopathology and near complete inhibition of IgG expression
• VYN202 has demonstrated superior anti-inflammatory effect on clinical signs
and symptoms compared to a BD2-selective BET inhibitor from GSK
(GSK620)
• Treatment with VYN202 showed >98% suppression of the inflammatory
biomarker, IgG1 with majority of animals being histologically clear of
arthritis at the highest dose
Large commercial opportunity in RA alone and PoC data
allow for potential expansion into other arthritic indications |
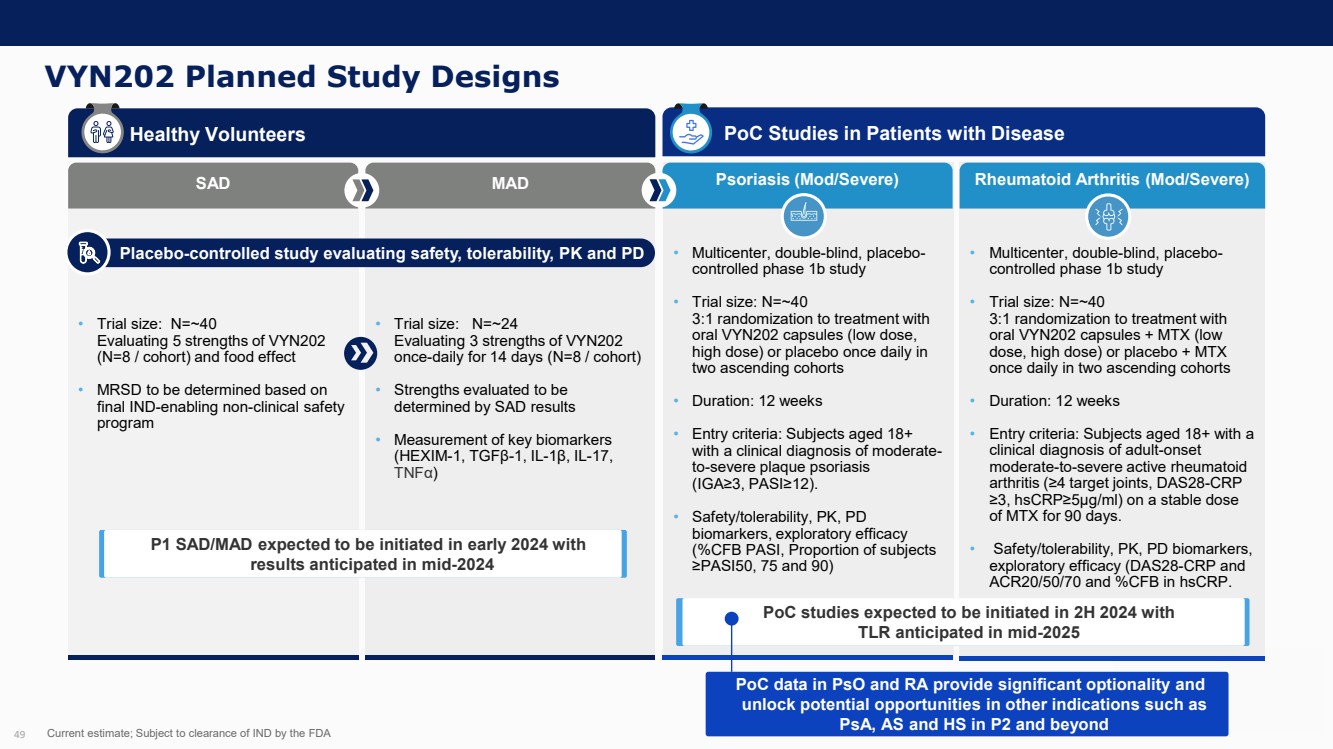
| • Trial size: N=~40
Evaluating 5 strengths of VYN202
(N=8 / cohort) and food effect
• MRSD to be determined based on
final IND-enabling non-clinical safety
program
VYN202 Planned Study Designs
49
Healthy Volunteers
• Multicenter, double-blind, placebo-controlled phase 1b study
• Trial size: N=~40
3:1 randomization to treatment with
oral VYN202 capsules (low dose,
high dose) or placebo once daily in
two ascending cohorts
• Duration: 12 weeks
• Entry criteria: Subjects aged 18+
with a clinical diagnosis of moderate-to-severe plaque psoriasis
(IGA≥3, PASI≥12).
• Safety/tolerability, PK, PD
biomarkers, exploratory efficacy
(%CFB PASI, Proportion of subjects
≥PASI50, 75 and 90)
• Multicenter, double-blind, placebo-controlled phase 1b study
• Trial size: N=~40
3:1 randomization to treatment with
oral VYN202 capsules + MTX (low
dose, high dose) or placebo + MTX
once daily in two ascending cohorts
• Duration: 12 weeks
• Entry criteria: Subjects aged 18+ with a
clinical diagnosis of adult-onset
moderate-to-severe active rheumatoid
arthritis (≥4 target joints, DAS28-CRP
≥3, hsCRP≥5µg/ml) on a stable dose
of MTX for 90 days.
• Safety/tolerability, PK, PD biomarkers,
exploratory efficacy (DAS28-CRP and
ACR20/50/70 and %CFB in hsCRP.
• Trial size: N=~24
Evaluating 3 strengths of VYN202
once-daily for 14 days (N=8 / cohort)
• Strengths evaluated to be
determined by SAD results
• Measurement of key biomarkers
(HEXIM-1, TGFβ-1, IL-1β, IL-17,
TNFα)
SAD MAD Psoriasis (Mod/Severe) Rheumatoid Arthritis (Mod/Severe)
Placebo-controlled study evaluating safety, tolerability, PK and PD
PoC Studies in Patients with Disease
P1 SAD/MAD expected to be initiated in early 2024 with
results anticipated in mid-2024
PoC studies expected to be initiated in 2H 2024 with
TLR anticipated in mid-2025
Current estimate; Subject to clearance of IND by the FDA
PoC data in PsO and RA provide significant optionality and
unlock potential opportunities in other indications such as
PsA, AS and HS in P2 and beyond |

| VYN202 Program Summary
50
• Novel, highly differentiated MOA - acting at the point of gene transcription in T Cells to directly address the complex
signaling of immuno- & fibro-inflammatory diseases
• BET Inhibition’s impact on multiple inflammatory pathways unlocks potential across broad range of immune-mediated
diseases
• Historical pan-BD BET safety concerns addressed via potential class-leading selectivity and potency vs. BD2 binding
domain and supported by completed and on-going nonclinical toxicity studies
• Strong preclinical data across multiple diverse models of autoimmune disease demonstrating significant down
regulation of key pro-inflammatory & disease-related biomarkers with corresponding improvements in disease
severity
• VYN202 preclinical efficacy results show strong correlation with VYN201 results reinforcing drug design thesis and
BET inhibition potential in psoriasis, rheumatoid arthritis, and other TH17 driven diseases |
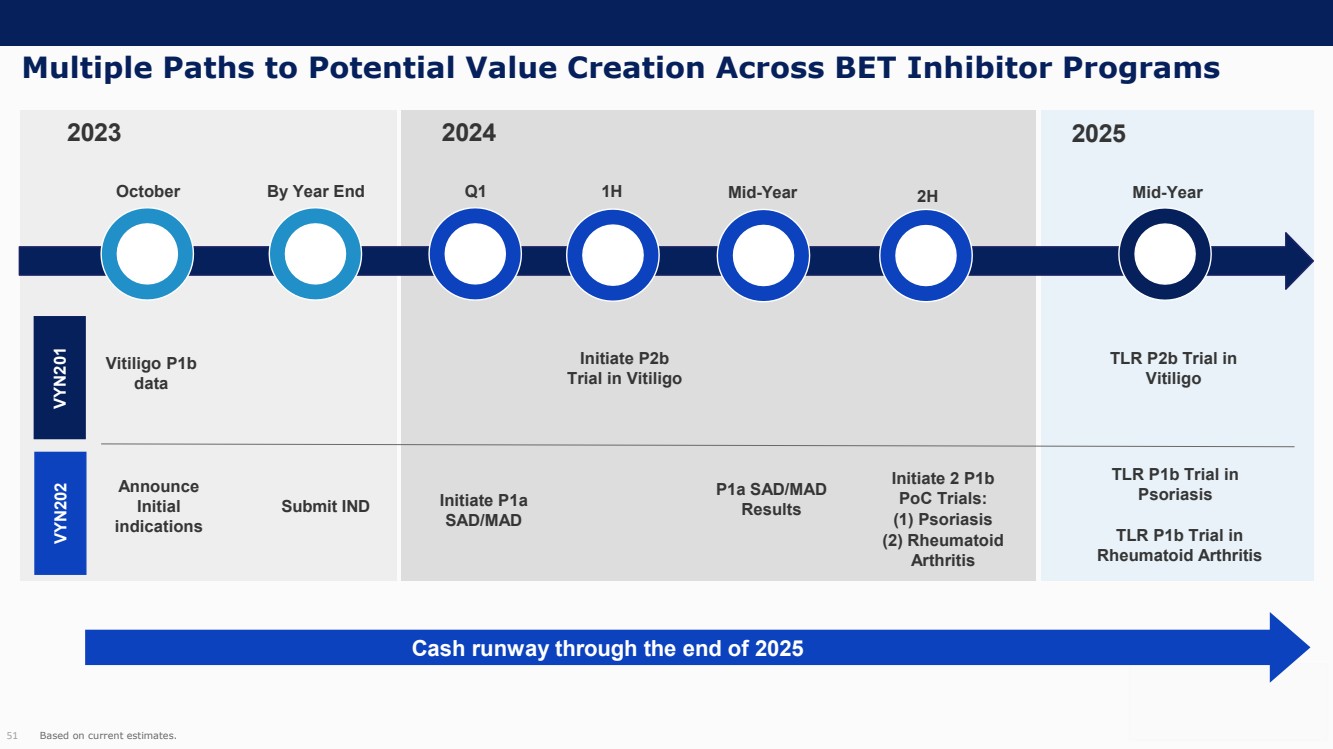
| 51
Multiple Paths to Potential Value Creation Across BET Inhibitor Programs
2023
October
Vitiligo P1b
data
Q1
Initiate P1a
SAD/MAD
1H
Initiate P2b
Trial in Vitiligo
2025
Mid-Year
TLR P2b Trial in
Vitiligo
Cash runway through the end of 2025
By Year End
Submit IND
2024
Mid-Year 2H
P1a SAD/MAD
Results
Initiate 2 P1b
PoC Trials:
(1) Psoriasis
(2) Rheumatoid
Arthritis
Announce
Initial
indications
VYN201 VYN202
TLR P1b Trial in
Psoriasis
TLR P1b Trial in
Rheumatoid Arthritis
Based on current estimates. |

| NASDAQ: VYNE |
Cover
|
Oct. 27, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Oct. 27, 2023
|
| Entity File Number |
001-38356
|
| Entity Registrant Name |
VYNE Therapeutics Inc.
|
| Entity Central Index Key |
0001566044
|
| Entity Tax Identification Number |
45-3757789
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
685
Route 202/206 N.
|
| Entity Address, Address Line Two |
Suite 301
|
| Entity Address, City or Town |
Bridgewater,
|
| Entity Address, State or Province |
NJ
|
| Entity Address, Postal Zip Code |
08807
|
| City Area Code |
800
|
| Local Phone Number |
775-7936
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.0001 par value
|
| Trading Symbol |
VYNE
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
true
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
VYNE Therapeutics (NASDAQ:VYNE)
Historical Stock Chart
From Oct 2024 to Nov 2024

VYNE Therapeutics (NASDAQ:VYNE)
Historical Stock Chart
From Nov 2023 to Nov 2024
